Abstract
The clinical management of malignant tumours is challenging, often leading to severe adverse effects and death. Drug resistance (DR) antagonises the effectiveness of treatments, and increasing drug dosage can worsen the therapeutic index (TI). Current efforts to overcome DR predominantly involve the use of drug combinations, including applying multiple anti-cancerous drugs, employing drug sensitisers, which are chemical agents that enhance pharmacokinetics (PK), including the targeting of cellular pathways and regulating pertinent membrane transporters. While combining multiple compounds may lead to drug–drug interactions (DDI) or polypharmacy effect, the use of drug sensitisers permits rapid attainment of effective treatment dosages at the disease site to prevent early DR and minimise side effects and will reduce the chance of DDI as lower drug doses are required. This review highlights the essential use of TI in evaluating drug dosage for cancer treatment and discusses the lack of a unified standard for TI within the field. Commonly used benefit–risk assessment criteria are summarised, and the critical exploration of the current use of TI in the pharmaceutical industrial sector is included. Specifically, this review leads to the discussion of drug sensitisers to facilitate improved ratios of effective dose to toxic dose directly in humans. The combination of drug and sensitiser molecules might see additional benefits to rekindle those drugs that failed late-stage clinical trials by the removal of detrimental off-target activities through the use of lower drug doses. Drug combinations and employing drug sensitisers are potential means to combat DR. The evolution of drug combinations and polypharmacy on TI are reviewed. Notably, the novel binary weapon approach is introduced as a new opportunity to improve TI. This review emphasises the urgent need for a criterion to systematically evaluate drug safety and efficiency for practical implementation in the field.
Keywords: therapeutic index, cancer, drug interaction, drug combination, polypharmacy, drug sensitisers, binary weapon, precision medicine
1. Therapeutic Index and Its Use in the Pharmaceutical Sector
The pharmaceutical sector continues to face significant challenges in drug development. Approximately 90% of drug candidates do not achieve approval, especially failing during clinical trial stages III and IV, which is primarily attributed to insufficient clinical efficacy (40–50%), excessive toxicity (30%), unfavourable drug properties (10–15%), and commercial considerations (10%) [1,2,3,4]. Early identification of unsuitable drug candidates could mitigate costly late-stage clinical trial withdrawal, therefore, favourably shifting the balance towards drug safety and efficacy [5,6,7].
Drugs can have potential side effects [8], with drug dosage being positively linked to increased off-target (toxic) interactions. Despite this situation, drugs with benefits that outweigh the known risks, sometimes with caution, receive approval [9]. The Food and Drug Administration (FDA) has not prescribed any specific criteria for assessing drug efficacy and safety. Instead, the case-specific evaluation for each drug application via a benefit–risk framework (BRF) is usually conducted [10,11,12]. In addition, the FDA has recently been encouraging the quantitative benefit–risk assessment (qBRA). In this context, the multi-criteria decision analysis (MCDA) method, suggested by the European Medicine Agency (EMA), is used to support decision-making especially when traditional BRA is marginal [13]. While indexes represent straightforward approaches for quantifying both drug safety and efficacy, as shown in Table 1, the pharmaceutical industry still relies on these measures, with the therapeutic index (TI) being prominent.
Table 1.
Criteria used to assess drug safety and efficacy.
| Criteria | Equation | Pros and Cons |
|---|---|---|
| Half-Maximal Effective Dose (ED50) | ED50 = Dose at which the drug produces a half-maximal effect | Reflects the relationship between therapeutic effect and acute toxicity but not chronic toxicity and allergenicity. |
| Lethal Dose (LD50) | LD50 = Dose that is lethal to 50% of the test population | Can evaluate the acute toxicity but does not reflect the chronic toxicity and carcinogenicity. LD50 may vary due to varying testing conditions. |
| Therapeutic Index (TI) | TI = LD50/ED50 = TD50/ED50 | Widely used safety index but is not applicable to very rare or idiosyncratic adverse drug reactions. |
| Maximum Tolerated Dose (MTD) | The highest dose of a drug that can be administered without unacceptable toxicity | Usually used to assess chemotherapy drugs. |
| Therapeutic Window (TW) | TW = minimum toxic concentration (MTC)/minimum effective concentration (MEC) | Related to TI and is more flexible but lacks formal definition. |
| Margin of Safety (MoS)/Certain safety factor (CSF) | MoS/CSF = TD1/ED99 | Patients will not be exposed to high risks. Can be seldom achieved in clinic. |
The therapeutic index, also known as the therapeutic ratio, describes a range of drug doses that show higher efficacy of drug medication with no more than acceptable adverse effects [14]. With largely academic research-based origins, TI identifies lethal drug doses in animal models or toxicity in clinical studies as a therapeutic effect [15]. In general, TI is calculated by dividing TD50 (toxic dose) by ED50 (effective dose). TD50 is the dose of the target drug that will cause unacceptable effects in 50% of the population and replaced the ethically unacceptable lethal dose 50 (LD50) measurements in animal models. ED50 represents the dose of the target drug that produces a therapeutic effect in 50% of the population [16]. Establishing the likely TI could help reduce drug failure by eliminating unsuitable drug candidates at an earlier stage [15].
The FDA does not have criteria for TI; however, a high-value TI (above 10) is preferable for selecting universal drugs, and TI greater than 5 serves as a criterion for considering a drug candidate for further preclinical study. Nevertheless, a low TI (below 2), known as a narrow therapeutic index drug (NTI), is also available occasionally due to limited choices for some severe diseases [17,18], such as Warfarin. The therapeutic index quantifies a drug’s selectivity for its intended target and plays a vital role in determining the necessity for therapeutic drug monitoring (TDM) and dosage intervals, particularly in the case of NTI, like anti-cancer medications [19,20]. Antibiotics such as beta-lactams, or remifentanil (TI = 33,000), have a wide margin of safety and do not require precise drug monitoring [21,22]. Despite the potential extensive application of TI in assessing drug candidates and informing decision-making, the complexities of TI are often underestimated due to the varying interpretations of TI among different disciplines within drug development. For instance, TI calculations differ between preclinical animal and human tests, with TD50 replacing LD50 in clinical trials. Furthermore, pharmacological effects and toxicity are determined by the drug pharmacokinetics (PK), including absorption, distribution, metabolism, and excretion (ADME), and exposure of the specific tissue to the drug rather than the dosage; hence, plasma exposure is usually used as a surrogate for tissue exposure in calculating TI. Moreover, patients will usually be treated with multiple dosages; hence, TI sometimes is calculated using the drug exposure at a steady state rather than after the first dosage [23,24,25].
Several factors can affect the TI including the following: (1) Drug delivery methods and drug formulation. Both can significantly influence drug uptake, which in turn affects drug efficacy and toxicity. (2) Interindividual variability. This includes age, gender, genetics, weight, ethnicity, and disease history which affect drug metabolism and elimination. (3) Environmental factors including daily lifestyle, nutritional status, disease status, existence of co-morbidities, and diet can also impact drug metabolism and excretion, hence varying TI. (4) Drug interactions can lead to variation in PK and pharmacodynamics (PD), subsequently affecting the TI value [15,26,27].
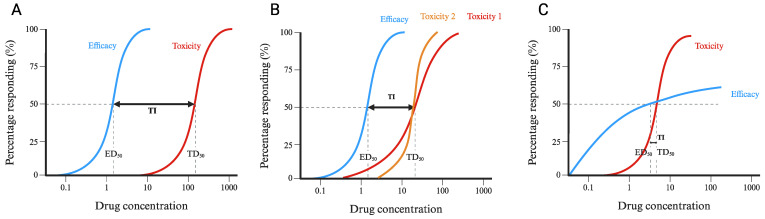
However, in a practical clinical context, all these factors and intraindividual variation make comprehensive utility difficult to achieve in practice. Moreover, several limitations are associated with TI calculations. Firstly, measuring TD50 can be challenging (Figure 1A). Toxicity may be unpredictable, delayed, or unrelated to the dose, rendering comparisons between different drugs difficult due to differing endpoints. Secondly, assessing drug exposure in human tissues is typically challenging, ergo plasma exposure is often utilised as a surrogate for tissue exposure. However, this approach is limited to drugs with adequate membrane permeability. Nonetheless, it is not frequently employed as a guide for clinical therapy, primarily due to cost and resource constraints and because it may not consistently align with drug efficacy, particularly when considering interindividual variability. Mostly, drugs are assessed through direct and simple monitoring of the objective endpoints, subjective endpoints, and adverse reactions, rather than relying on TI measurements (Figure 1B). Thirdly, the applicability of TI is restricted by its inability to account for rare but severe toxicities that are detectable only in large populations and, therefore, only present in late-stage clinical trials, such as the withdrawn drug pergolide to treat Parkinson’s disease [28]. Additionally, if the quantitative efficacy curve of a drug does not align with its dosage-toxicity curve, the TI value may not fully reflect the drug’s safety (Figure 2). Lastly, TI does not consider drug interactions or synergistic effects, which may be relevant in real-life clinical scenarios.
Figure 1.
Challenges of measuring TI and current drug safety assessment methods. To provide a suitable drug administration to patients, physicians make several safety assessments. (A) However, there are some challenges to measuring TI due to unpredicted TD50, tissue exposure, limitations in detecting severe and rare toxicity, and unknown drug interactions. (B) Instead of using a specific criterion, drug safety is assessed by using an objective endpoint, subjective endpoint, and adverse event.
Figure 2.
Dose-response curves used for TI calculations. The blue curve represents therapeutic efficacy, while the red and orange curves represent toxic effects. (A) represents the ideal dose-response curve. Drug concentration between the two curves, labelled as TI, are appropriate dosages that make the drug efficient but without severe side effects. The therapeutic index is then calculated using ED50 and TD50. In (B), toxicity curve 1 kills approximately 10% of the population at ED50, while toxicity curve 2 does not cause any deaths at ED50 but then rapidly becomes lethal after TD50. (C) shows when the efficacy curve does not align with the toxicity curve, and TI is not reliable.
A similar criterion, the therapeutic window (TW), is also widely used, which is affected by several factors: (1) the dosage range of drugs between a minimum dose of effective concentration and minimum toxic concentration, (2) the ability to distinguish the main targets and the non-targets, (3) the quality of the manufactured drug, (4) drug PK, and (5) individual cases. It can be applied in generic drugs, immunosuppressants, transplantation, cytotoxic drugs with antibody conjugates, and CRISPR/Cas9 technology [29,30].
Overall, the evaluation and application of TI in drug development are critical and complex. Achieving a balance between drug safety and efficacy requires an understanding of the limitations and complexities in TI calculations [5]. While in practice, the drug BRA often becomes a unique and case-specific undertaking, the specific clinical behaviour of drugs, which can also be a function of disease, has hampered the widespread adoption of TI. A new strategy of applying TI in practice is suggested to improve the specificity and efficacy of clinical medications.
The primary medical models in use include evidence-based medicine, translational medicine, and precision medicine [31]. The implementation of precision medicine relies on the methods of evidence-based medicine, grounded in the principles of systems biology and multi-omics, with increasing input from engineering and modelling via synthetic biology [31,32,33,34]. Drug treatments are applied in a personalised manner, tailored to the individual’s genetic profile, living environment, and lifestyle. Precision medicine is currently focused on complex polygenic diseases such as cancer and diabetes, as well as rare diseases. It provides solutions to issues including uncharacterised targets and a lack of drug specificity in cancer immunotherapy [35,36,37,38,39]. Currently, several achievements have been made in implementing precision cancer medicine including the identification of new diagnostic biomarkers, integration of multi-omics, increased drug portfolio, long-term cost reduction of sequencing, data harmonization, and setup of prospective registry trials [40,41,42,43]. Given the complexity of the widespread utility of TI, its application in precision medicine is considered to be more accurate than using a universal standard for every patient, since the genetic profile and individual information including body weight, lifestyle, and disease history can then be applied to TI calculation, and the precise dosing regimen specified for the individual will lead to better clinical outcomes. However, challenges in precision medicine include difficulties in follow-up monitoring, the requirement of highly dynamic diagnostic technologies, increasing complexity, and analysis of real-world data proving too complex for detailed analyses [44].
2. Strategies to Improve Therapeutic Index in Cancer Treatment
Cancers affect around 50% of the UK population and are the focus of study for disease mechanisms in academia and efficacious product development within the pharmaceutical industry [45,46]. Both sectors converge on clinical trials and the identification of clinical staging that relates the presentation of the disease to risk and disease severity. However, drug resistance (DR) presents a significant challenge in cancer treatment and impairs clinical benefit [47]. The drug-resistant phenotype usually occurs in cancer cells due to (1) active expression of efflux transporters (drug efflux) [48], (2) low expression of influx transporters (drug influx) [49], (3) alteration of drug targets [50], (4) inhibition of related pathways [49], and (5) interference in the interaction between drugs and their targets [51]. Several strategies to overcome DR include refining drug delivery systems (DDS) [49,52], applying multiple anti-cancerous drugs (drug combination) [53,54], and employing drug sensitisers [55]. This section aims to review procedures developed to counteract mechanisms that limit drug uptake and action.
2.1. Modification of Drug Delivery System
Drug delivery is a process of administrating pharmaceutical materials to reach designated therapeutic targets [56]. A drug delivery system (DDS) is a combination of physical/chemical/biological techniques to direct the controlled release and delivery of compounds, including antibodies, vaccines, drugs, peptides, proteins, and enzymes, aiming to minimise the off-target localisations and increase drug durability during transportation [57].
As shown in Table 2, several DDSs have been well established, with oral pills and injections being the most predominant methods of administration. Data from the US Food and Drug Administration (FDA) indicate annual sales of US dollar 2.45 billion for aspirin tablets and over 10 billion injections administered worldwide. Oral drugs are widely used due to their convenience and ease of use. To improve drug PK and therapeutic outcomes, various DDSs are applied to treat various diseases. Nevertheless, imprecise delivery that leads to side effects poses a significant challenge for clinical treatment. Therefore, effective increases in drug specificity via an appropriate delivery system are essential [58].
Table 2.
Comparison of different drug delivery systems ranked by clinical application prevalence.
| Routes of Drug Administration | Working Model | Advantages | Disadvantages | Current Drugs | Refs. |
|---|---|---|---|---|---|
| Oral drug delivery | 1. Small molecule delivery 2. Patient self-administration |
1. Sustained and easy administration 2. Major method to establish patient compliance 3. Large surface area for mucosal layer attachment |
1. Need to pass through GI system (multiple barriers) 2. Slow absorption 3. Degradation problems |
1. Venlafaxine hydrochloride 2. Diltiazem 3. Indomethacin 4. Heparin |
[59,60,61] |
| Injectable drug delivery | 1. Protein and peptide delivery 2. Intravenous (IV), intramuscular (IM), intranasal (IN) 3. Induce immune response mechanisms |
1. The highest bioavailability and the fastest effect 2. Acute and emergency responses |
1. Needle-related pain, wounds, phobia | 1. Glucagon-like peptide-1 2. Insulin 3. Superoxide dismutase 4. Hydrocodone (Vicodin) |
[62,63,64,65] |
| Transdermal patch drug delivery | 1. From skin layers to the blood circulatory system | 1. Direct treatment away from GI system 2. Can maintain sustained drug level 3. Readily administered |
1. Lower drug absorption level | 1. Nitroglycerin 2. Nicotine 3. Scopolamine 4. Clonidine 5. Fentanyl 6. Testosterone |
[66,67,68] |
| Ocular drug delivery | 1. Deliver drugs to eyes against disorders related to vision 2. Formation: eye drop, eye implant |
1. Easy administration and preparation 2. High patient convenience and compliance |
1. Poor bioavailability 2. Low retention time 3. Side effect caused by high-frequency administration 4. Instability for dissolved drugs |
1. Ocusert® Pilo-20 2. Pilo-40 Ocular system |
[69,70] |
| Pulmonary drug delivery | 1. Inhalation of drugs via nebulisers or inhalers 2. Drugs that target the lungs |
1. Rapid effects 2. High therapeutics due to large surface of lungs |
1. Drug irritation to the lung 2. Limited drug dissolution 3. High drug clearance |
1. Nebulisers 2. Pressurised metered dose inhalers (pMDIs) 3. Soft-mist inhalers 4. Dry powder inhalers (DPIs) |
[71,72] |
| Implantable drug delivery | 1. A reservoir surrounded by polymers or drug–polymer mixture 2. Passive delivery: use diffusion, osmosis, or gradients to control drug release 3. Active delivery: activate a pump to release drugs |
1. Reduce dosing frequency 2. Increase patient compliance |
1. Lack of systemic treatment 2. Undegradable, need a removal process |
1. Vitrasert 2. Norplant® |
[73,74,75] |
| Antibody drug conjugate delivery | 1. Combination of clonal antibodies and drugs 2. Conjugation between target sites and antibodies |
1. Highly toxic drug delivery (high specificity, low off-targets) | 1. Poor tumour penetration (ex: hypoxic area) 2. Side effects in the non-target sites 3. Undesirable immune response (ex: Fc interaction) |
1. Brentuximab vedotin 2. Trastuzumab emtansine (Kadcyla) |
[76,77,78] |
| Polysaccharide-based hydrogel drug delivery | 1. Use natural polymers to build beads 2. Suitable for peptides, proteins, DNA, and RNA |
1. High biocompatibility and biodegradability 2. Cost efficiency 3. Ease of surface modification 4. Low toxicity 5. Rapid drug release |
1. The quantity and homogeneity of drugs are limited (hydrophobic drugs) 2. Unable to bind with hard tissues (bone) 3. Difficult to sterilise |
1. Poly (ethylene glycol)-diacrylate (PEGDA) | [56,79] |
2.2. Administration of Multiple Anti-Cancerous Drugs
Several methods have been developed to combat cancer, including surgery, radiation therapy, cryotherapy, and the use of anti-cancer drugs. Among these, drug treatment emerges as the leading approach, classified into four main categories: (1) chemotherapy, (2) targeted therapy, (3) hormonal therapy, and (4) immunotherapy [80,81], as indicated in Table 3.
Table 3.
Traditional and new classified anti-cancer drugs with clinical examples.
| Classification of Anti-Cancer Drugs | Principle | Subtypes | Examples | Ref. |
|---|---|---|---|---|
| Chemotherapy | Interfere with tumour cell cycle, cell proliferation, and replication | (1) Alkylating agents | (1) Cyclophosphamide, chlormethine | [82,83] |
| (2) Anti-metabolites | (2) 5-FU, 6-mercaptopurine, gemcitabine | [84] | ||
| (3) Anti-tumour antibodies | (3) Atezolizumab, trastuzumab-deruxtecan | [85,86] | ||
| (4) Topoisomerase inhibitors | (4) TOPI: camptothecin TOPII: doxorubicin | [87] | ||
| (5) Tubulin-binding drugs | (5) Microtubule-stabilising: taxanes Microtubule-destabilising: vincristine | [88] | ||
| (6) Antibiotics | (6) Bleomycin, daunorubicin, doxorubicin | [89,90] | ||
| (7) Mitosis inhibitors | (7) Alisertib, ispinesib, GSK461364 | [91] | ||
| Targeted therapy | Target specific proteins or genes related to cancer growth | (1) Receptor tyrosine kinase inhibitors | (1) Erlotinib, gefitnib, lapatnib, afatinib | [92,93] |
| (2) Intracellular tyrosine inhibitors | (2) Imatnib, nilotnib, everlimus | [92,94] | ||
| (3) DNA/RNA synthesis inhibitors | (3) Capecitabine, oxaliplatin | [95] | ||
| (4) Topoisomerase I inhibitors | (4) Irinotecan, belotecan, topotecan | [96,97] | ||
| (5) Proteasome inhibitors | (5) Bortzomib, ixazomib, carfilzomib | [98] | ||
| Hormonal therapy | Inhibit tumour growth dependent on hormones | (1) Steroids | (1) Dexamethasone, methylprednisolone | [99,100] |
| (2) Anti-estrogens | (2) Tamoxifen, raloxifene, toremifene | [101,102,103] | ||
| (3) Anti-androgens | (3) Bicalutamide, enzalutamide | [104] | ||
| (4) LHRH conjugated drugs | (4) LHRH-paclitaxel, LHRH-prodigiosin | [105] | ||
| (5) Anti-aromatase agents | (5) Exemestane, anastrozole, | [106,107] | ||
| Immunotherapy | Induce anti-tumour responses from the immune system | (1) Interferon | (1) IFNα-1a, IFNα-1b | [108] |
| (2) Interleukin 2 | (2) Aldesleukin | [109] | ||
| (3) Vaccines | (3) Sipuleucel-T | [110] | ||
| (4) Oncolytic virus therapy | (4) T-VEC | [111] | ||
| Others | (1) Disrupt energy production, essential cellular processes in mitochondria (2) Induce apoptosis pathways |
Mitochondria-targeted anti-cancer drugs (mitocans) | ||
| (1) Hexokinase inhibitors | (1) 2-deoxyglucose, 3-bromopyruvate | [112,113] | ||
| (2) Bcl-2/Bcl-xL mimetics | (2) Antimycin A, Gossypol, ABT-263 | [114] | ||
| (3) Thiol redox inhibitors | (3) Dichloroacetate, isothiocyanates | [115] | ||
| (4) VDAC/ANT targeting drugs | (4) CD437, lonidamine | [116,117] | ||
| (5) Electron transport chain targeting drugs | (5) Tamoxifen, MitoVES | [118,119] | ||
| (6) Lipophilic cations targeting inner membrane | (6) MKT-077, Rhodamine-123 | [120,121] | ||
| (7) Drug targeting TCA cycle | (7) DCA, 3-bromopyruvate | [122] | ||
| (8) Drug targeting mtDNA | (8) Vitamin K3, Mito VES | [123] | ||
| Induce cells to produce specific proteins | mRNA drugs | |||
| (1) Vaccines | (1) mRNA-4157, pembrolizumab | [124] | ||
| (2) Antibodies | (2) Anti-HER2 | [125] | ||
| (3) Antigen receptors | (3) Chimeric antigen receptor T cell therapy | [126] |
From the various anti-cancer drug types summarised above, single-drug treatments that only target one pathway usually show limited drug efficiency and rapidly trigger DR [127,128]. These therapies are invariably insufficient for treating complex and multifactorial diseases such as malignant tumours, central nervous system (CNS) disorders, and immune disorders [129]. This contributes to high attrition rates and reduced cost recovery in drug discovery. Drug combination therapy was first established by Emil Frei et al. who conducted drug combination studies with anti-leukaemic agents in paediatric acute leukaemia patients [130,131]. Nowadays, the utilisation of multiple drugs is a common approach when addressing complex diseases or combating DR. This includes drug synergism when drugs that individually yield similar effects exhibit significantly enhanced outcomes [132].
There are several advantages of employing drug combinations, including maximising drug efficacy by targeting different mechanisms, reducing DR, and increasing the cost effectiveness of the drug development process, particularly when repurposing FDA-approved drugs for cancer treatment [133,134,135].
Several methods to conduct multiple drug combinations have been developed and applied to medical and surgical treatments:
2.2.1. Combinations of Drugs Targeting Different Pathways
To enhance drug efficiency, multiple drugs that target different disease-related proteins and pathways are applied to patients. Nevertheless, the treatment may also simultaneously lead to additional toxicity and adverse side effects. Various anti-cancer agents designed on natural compounds have demonstrated multiple functions across different pathways due to their inherent characteristics. For instance, (1) ruscogenin, an anti-inflammatory and anti-thrombotic steroid found in Ruscus aculeatus (a traditional medicine used as diuretics and treatment for urinary system disorders) [136,137], shows a decreased pattern in regulating MMP2, MMP9, VEGF, and HIF-1α that could suppress the metastasis of hepatocellular carcinoma [138], and (2) plumbagin, an extract from the medicinal plant Plumbago zeylanica (one of the oldest herbs in Ayurveda including functions such as anti-inflammatory and anti-tumour [139]) can down-regulate tumour cell growth by inhibiting the mTOR/PI3K/Akt pathway and EMT transition in prostate cancer [140], in addition, inducing ROS production for specific cell apoptosis [141,142]. Some advanced computational-based methods (such as chemogenomics, proteochemometric modelling, target fishing, and system pharmacology) are also applied for further investigation [143].
2.2.2. Modification of Drug Delivery into a Multi-Agent Delivery System
DDS can be categorised according to different methods or materials. By increasing the specificity of the delivery system, drug efficiency is considered the main factor. Hence, most DDS are designed to be single-drug delivery, which is not fully relevant for combination drug delivery. The development of advanced equipment and compounds as a multi-drug delivery system (MDDS) compensates for the disadvantage of a single-drug delivery approach which often has an uncontrollable drug release and ergo overdose that could cause adverse effects. For instance, the titania nanotube (TNT) array is a prototype for self-automated electrochemical equipment suitable for implantable drug delivery with its sequential drug release [144]; in addition, biophysical materials such as polymer micelles, polymer-drug conjugates, polymeric nanoparticles, hydrogels, and liposomes are also designed for drug delivery for complicating diseases [145].
2.2.3. Combination of Different Therapies
Even if monotherapy is commonly used to treat numerous diseases, especially malignant cancers, the complexity of the human body can rarely be treated via a single therapy [146]. To overcome the challenges caused by drug resistance and low drug efficiency, therapies such as immunotherapy, radiotherapy, surgery, and photodynamic therapy are usually conducted together to treat one single disorder [147].
2.2.4. Drug Interactions and Polypharmacy in Cancer
Despite the benefits of higher efficacy by combining drugs, drug interactions can arise when multiple drugs [148,149] are prescribed to a patient. The potential drug interactions can profoundly influence TI by altering both drug efficacy and toxicity, and can be classified into PK interactions, PD interactions, pharmaceutical interactions, and herbal interactions [150].
Pharmacokinetic Interactions
Cancer treatment, especially chemotherapy and radiotherapy, can significantly weaken the immune system, making patients more susceptible to fungal infections [151]. Hence, antifungal drugs such as ketoconazole may be used in co-treatment with the chemotherapy drug, cyclophosphamide. However, this potent inhibitor of the CYP3A4 enzyme suppresses the concentration of the cyclophosphamide metabolite by inhibiting both CYP2C9 and p-glycoprotein, leading to an elevated plasma drug level and subsequently higher toxicity [152]. This interaction is known as a PK interaction which is defined as the alteration of drug ADME, with the subsequent effect on drug plasma levels, drug efficacy, and toxicity profiles.
Pharmacodynamic Interactions
Drugs masking each other’s effects directly at the site of action or through common physiological pathways are termed PD interactions. For example, patients undergoing cancer treatment often suffer from body pain as a common side effect; hence, pain relief is commonly co-administrated with anti-cancerous drugs. The methotrexate used in leukaemia treatment can lead to high-risk interactions when combined with non-steroidal anti-inflammation drugs (NSAIDs). NSAIDs, often used as pain relief, compete with methotrexate for the same protein binding sites. The enhanced plasma level of active methotrexate often subsequently results in side effects including liver injury and severe bone marrow suppression [153].
Other Interactions
Except for PK and PD interactions, pharmaceutical interactions may occur when drugs influence each other’s physical or chemical properties at the level of drug formulation. For example, Erlotinib, a tyrosine kinase inhibitor used for treating non-small cell lung cancer, has its absorption affected by proton pump inhibitors (PPIs), through increased gastric pH and reduced drug solubility [154]. In addition, the rising use of natural supplements may heighten the significance of interactions between herbal medicines and drugs. For instance, St. John’s Wort (SJW), a herbal treatment for depression, can interfere with imatinib, a tyrosine kinase inhibitor used to manage chronic myeloid leukaemia. SJW enhances the activity of the enzyme CYP3A4, which metabolises imatinib, resulting in faster clearance of imatinib from the body [155].
However, polypharmacy can arise when multiple drugs, usually above five [148,149], are prescribed to a patient. This scenario often occurs in the elderly since they often suffer from age-related cancers, decreased renal and hepatic functions, and geriatric syndromes which increases the risk of multimorbidity and mortality [156]. According to a study in 2020, the prevalence of polypharmacy was 44% among 1,742,336 elderly adults tested [157]; therefore, improvements of TI in multiple drug treatments are essential to decrease the appearance of polypharmacy.
Chemotherapeutic agents often require extra supportive components to manage the therapy process [158]. According to the reports from Globocan data in 2020, cancer has affected more than 60% of the elderly people in Europe [159]. Adverse events from chemotherapy agents such as nausea, vomiting, malnutrition, and myelosuppression would also increase the appearance of polypharmacy [160]. Polypharmacy is highly associated with chronic diseases such as hypertension (85%), diabetes (86.7%), and cardiovascular-related issues in elderly patients with cancer [161], largely due to their correlation with ageing. The primary concern with polypharmacy arises from inappropriate medication use, multiple prescriptions, low adherence, and underuse of drugs. Multiple drug interactions raise the risk of altering serum albumin levels, essential for drug delivery and efficacy in the circulatory blood system, eventually leading to cytotoxicity and new symptoms due to adverse events [162,163].
2.3. Drug Repurposing
Drug repurposing is a method that seeks new uses for existing drugs, which can be rapidly validated in clinical trials due to their well-studied functional activities and toxicity. Mostly, these drugs when used in isolation failed to reach satisfying clinical outcomes due to the lack of efficiency. Candidate drugs can re-enter clinical trials by repurposing the drugs to bind with more specific targets [164]. In the last decade, around one-third of the current drugs involved in drug repurposing generated 25% of the annual income for the pharmaceutical sector [165,166]. Tumours often involve multiple symptomatic phenotypes and pathogenetic mechanisms, making combined drug repurposing a promising approach that has been developed and applied in anti-cancer drug research.
Key examples of drug repurposing are as follows: (1) the use of mebendazole, an anti-hookworm medication, now recognised as a multi-targeted kinase inhibitor potentially more effective and with a preferred side effect profile for cancer treatment than existing kinase inhibitors; (2) sildenafil was repurposed into a pulmonary hypertension drug when it was originally designed for hypertension [167]; (3) tadalafil can inhibit myeloid-derived suppressor cells in patients when it used to be a PDR-5 inhibitor for erectile dysfunction; and (4) propranolol, inhibiting the metastasis and proliferation angiosarcoma cells, was a hypertension beta-blocker [168]. Three strategies for repurposing current drugs are shown in Table 4, including knowledge-based, signature-based, and phenotype-based repurposing [169].
Table 4.
Three strategies for repurposing drugs.
| Strategy | Concept | Pros and Cons | Examples | Refs. |
|---|---|---|---|---|
| Knowledge-based repurposing | Use the properties of drugs to predict the possibility of treating diseases: (1) Target-based repurposing (2) Pathway-based repurposing (3) Target-based repurposing |
Pros: 1. Large scale prediction 2. Precise prediction 3. Time-efficient 4. Cost-effective Cons: 1. Only positive data can be found in knowledge database |
1. CAS biomedical knowledge graph for COVID-19 2. L-type calcium channel blockers for cryptococcosis |
[170,171,172,173] |
| Signature-based repurposing | A method to discover new off-targets or pathways. Genetic and molecular mechanisms are highly involved in the analysis. | Pros: 1. Identify new mechanisms of drugs Cons: 1. Only provide data for preliminary analysis |
1. New candidates for atypical meningioma 2. New candidates for inflammatory bowel disease |
[174,175] |
| Phenotype-based repurposing | A method for systemic approaches to detect human diseases, multiple independent screens for similar compounds and their potential for repurposing. | Pros: 1. Can predict extra adverse events Cons: 1. Might lead to compounds with poor pharmacokinetics |
1. Electronic health records (EHRs) define the use of metformin in cancer treatment 2. Killing ability towards Toxoplasma by Pimozide and tamoxifen |
[167,176] |
2.4. Application of Drug Sensitising Agents
Unlike the repurposing of drugs, a means to improve TI with a reduced drug dose and to simultaneously resurrect drugs that previously failed because of a too-high ED50 is through the application of drug synergy agents [177]. Drug synergy agents act to increase patient sensitivity to a drug and do not have direct biological activity to treat disease but can help enhance drug efficacy, reduce toxicity, and overcome drug resistance. This approach maximises therapeutic benefits while minimising adverse effects and primarily aims to increase the TI of drug treatments. Table 5 indicates increasing (⬆️) and decreasing (⬇️) impacts on TI.
2.4.1. Piperine
Piperine is an insoluble solid alkaloid produced by Piper species such as Piper nigrum L. (black pepper) and Piper longum L. (long pepper) [178] and has been demonstrated to enhance the bioavailability and pharmacokinetic properties when co-administrating with anti-tumour drugs. For instance, (1) a combination of rapamycin and piperine is proven to increase its bioavailability by 60% to cure human tuberculosis by enhancing autophagy activity. Via inhibition of Ser/Thr kinase mammalian target of rapamycin (mTOR) and maturation of the mycobacterial phagosome, the survival of Mycobacterium tuberculosis is inhibited [179,180]. (2) Nevirapine is a potent non-nucleoside inhibitor of HIV-1 reverse transcriptase mainly applied to HIV-1 infection. The co-administration of piperine and Nevirapine increases the bioavailability of Nevirapine with very few adverse effects [181]. (3) 5-FU, a common analogue of fluoropyrimidine chemotherapy drug inactivating thymidylate synthase (TS), leads to the inhibition of DNA replication and RNA synthesis in tumour cells [182]. Piperine increases the toxicity and bioavailability of 5-FU to suppress tumour growth by decreasing the half-life of drugs and expression of related efflux transporters (P-gp, MRP1, and BCRP) [183].
2.4.2. Amifostine
Amifostine (WR-2721), a broad-spectrum cytoprotective agent, protects against mutagenic and carcinogenic damage by chemotherapy and radiation therapy in normal tissues without diminishing the anti-tumour activity [184]. Amifostine could also minimise the side effects by using higher antineoplastic doses of cisplatin chemotherapy in patients. The cytoprotecting mechanism of amifostine is by its active metabolite WR-1065, a thiol compound with scavenger activity against free radicals:
-
(1)
Radiation therapy: The oxidation tension and haemoglobin saturation caused by radiation therapy induce the oxidation of WR-1065 and HIF activation in normal tissues, activating the cytoprotection [185].
-
(2)
Chemotherapy: Several clinical studies suggest that amifostine does not interfere with the antineoplastic efficacy of different chemotherapeutic agents. The main function of amifostine is to protect the normal tissues under high doses of cisplatin in melanoma patients [185]. In addition, another study also points out that amifostine could further prolong the half-life of platinum-based chemotherapy which could increase the exposure of tumours to chemotherapeutic agents [186].
2.4.3. Tariquidar
Tariquidar, a P-gp drug efflux pump inhibitor, works as an adjuvant against multidrug resistance in cancer undergoing clinical research [187]. With high potency and specificity to P-gp and low impact on common chemotherapeutic agents, tariquidar may result in accumulating intracellular concentrations of anti-cancer drugs [188].
Docetaxel/Paclitaxel: The combination of docetaxel/paclitaxel and tariquidar can increase the therapeutic efficacy of docetaxel/paclitaxel by increasing the specificity and the water solubility of various drugs and reducing drug resistance by efflux transporters in breast, non-small cell lung, ovarian, and prostate cancers [189,190].
2.4.4. Binary Weapon
Numerous drugs have failed clinical trials due to their off-target effects and low drug efficiency; hence, there is a growing interest in optimising those validated drugs but with poor TIs. Here, a new approach termed binary weapons (BWs) is introduced which works similarly to drug sensitisers. Binary weapons lack toxicity yet show potential to enhance gemcitabine killing in pancreatic cancer cells via co-treatment [177]. In addition, the efflux transporter ABCC10 was found to be down-regulated with the addition of BW, highlighting the potential of BWs to inhibit efflux transporters [191]. Similar criteria for selecting BWs are established to develop new drugs for malaria [192]. According to the BW approach, the application of a small chemical fragment can potentially revive drugs that previously failed due to off-target effects and high toxicity. This strategy contributes to the development of more effective therapies, ultimately benefiting patients [191].
Table 5.
The use of compound synergy to increase chemosensitivity in cancer therapy.
| Drug Sensitiser | Co-Treated Drug | Description | Current Clinical Status | Functions | Effect on TI | Refs. |
|---|---|---|---|---|---|---|
| Piperine | (1) Rapamycin | (1) Activate the autophagy pathway. | (1) FDA-approved | Bioavailability enhancers | ED50 ⬇️ | [180,193] |
| (2) Nevirapine | (2) Inhibit CYP450 and UDP glucuronyl transferase. | (2) Not approved due to limited clinical trials; few ongoing clinical trials | [194] | |||
| (3) 5-FU | (3) Shorten the half-life of the drug. | (3) Under clinical investigation | [195] | |||
| Amifostine | Cisplatin | Cytoprotective agent used in chemotherapy. | FDA-approved, and it is used in clinic with cisplatin | Toxicity reducers | TD50 ⬆️ | [196,197] |
| Tariquidar | Docetaxel, Paclitaxel | Inhibit P-gp efflux transporter to enhance drug efficacy. | Under clinical investigation | Efflux inhibitors | ED50 ⬇️ | [187] |
| Binary weapon | Gemcitabine | Enhances the gemcitabine toxicity to pancreatic cancer cells by co-treatment but non-toxic by sole treatment. | Laboratory evidence | Enhance anti-cancer drug efficacy and specific to cancer cells | ED50 ⬇️ | [191] |
| Bovine lactoferrin | Cisplatin | Sensitise Cis-anti-neoplastic potency. | No clinical data | Enhance drug efficacy, immunomodulators | ED50 ⬇️ | [198] |
| Fedratinib | Vincristine | Fedratinib is a JAK2 inhibitor that sensitises P-gp-overexpressing drug-resistant cancer cells. | FDA-approved | Inhibits P-gp activity, inducing cytotoxicity and apoptosis in drug-resistant cancer cells | ED50 ⬇️ | [199] |
| MG132 | Idarubicin | Inhibit NF-κB regulations. | Under clinical investigation | Inhibition of NF-κB induces apoptosis of leukaemic stem cells and leaves normal cells viable | ED50 ⬇️ | [200,201] |
| Urolithin A (UroA)/UAS03 (UroA analogue) | 5-FU | Inhibit cancer cell viability, proliferation, and invasion in colon cancer cells. | Laboratory evidence | Enhance drug efficiency and inhibit the expression of related efflux transporters | ED50 ⬇️ | [202] |
2.5. Educating Patients
Patient behaviour has a significant impact on the TI, particularly for drugs with NTIs. Adherence, which refers to the extent to which a patient’s actions follow prescriber instructions, is essential for achieving optimal clinical outcomes [203]. It is reported that patients with good adherence to treatment have a lower rate of mortality and fewer hospitalisations compared to those who do not [204]. Moreover, it is found that adherence is significantly correlated with positive treatment outcomes, which makes the outcome predictable [205]. Non-adherence can manifest as frequent dosing, leading to toxic drug levels and adverse effects or missing doses, resulting in reduced drug efficacy and disease progression, and hence, lowering TI. Therefore, the development of pharmaceuticals within a DDS [206] offers a promising solution to many common impediments to adherence. An example is transdermal patches, which provide continuous release of medication over an extended period, eliminating the need for daily manual drug administration. This approach is widely used for hormone replacement therapy and the treatment in psychiatric illness [207]. Ensuring that patients understand their medications, maintaining open communication between patients and prescribers, and educating patients about their treatment are crucial for optimizing therapeutic outcomes and improving TI. Simplifying drug regimens and involving regular monitoring and follow-up can enhance the quality of care for patients receiving complex medications.
3. Conclusions and Future Perspectives
The therapeutic index has recently attracted much attention as it aims to evaluate drug efficiency and safety more accurately. Nevertheless, its implementation in cancer treatments can be challenging due to the difficulties in measuring TD50 and tissue exposure, detection of severe and rare toxicity, and unknown drug-drug interactions. To improve the efficiency of most failed drugs (improve TI), (1) modification of drug delivery systems, (2) multiple drug combinations, (3) drug repurposing, and (4) educating patients are continuously applied during drug developments. Moreover, the field is moving towards using simulation to predict drug behaviour. Machine learning algorithms are being reported to have the potential to predict drug efficacy and toxicity by building a predictive model, allowing for better personalised treatment [208].
From our previous studies [191], BWs are new materials with no known pharmacological activities and only show the advantage of increasing drug efficiency and specificity and lowering the drug dose during co-administrations. Given the reduced off-target potential with associated increased drug specificity, BWs could be the newest strategy to improve TI value for drug development and lessen drug-drug interactions through reduced drug dosage. In addition, reducing the number of drugs consumed and the use of polypills could also minimise the risks of toxicity and unknown DDI in polypharmacy and drug combination therapies.
A long-term goal for drug development is to build precision medicine portfolios for different diseases, and the improvement of TI by BWs has increased the opportunities for more customised medication to help cancer patients receive more targeted clinical benefits in the future (Figure 3).
Figure 3.
The advantages of applying binary weapons in drug administrations. The diagram describes the current solutions to improve low drug efficiency and the future perspectives of co-administrating BWs in clinical treatments. BWs could improve TI by increasing drug efficiency and reducing toxicity, leading to reduced drug intake, increased drug specificity, and overcoming drug resistance by inhibiting efflux transporters. The application of BWs provides opportunities for drugs that previously failed to re-enter clinical trials. Furthermore, using a reduced drug dosage while maintaining drug efficacy can pave the way for precision medicine, offering highly targeted treatment for a broad range of disease risk groups.
Author Contributions
Conception: P.J.D.; design: Y.-S.C., E.J. and P.J.D.; writing of the manuscript: Y.-S.C. and E.J. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
P.J.D. thanks the Biotechnology and Biological Sciences Research Council for supporting this work (BBSRC grants BB/K019783/1 and BB/M017702/1).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Dowden H., Munro J. Trends in clinical success rates and therapeutic focus. Nat. Rev. Drug Discov. 2019;18:495–496. doi: 10.1038/d41573-019-00074-z. [DOI] [PubMed] [Google Scholar]
- 2.Harrison R.K. Phase II and phase III failures: 2013–2015. Nat. Rev. Drug Discov. 2016;15:817–818. doi: 10.1038/nrd.2016.184. [DOI] [PubMed] [Google Scholar]
- 3.Takebe T., Imai R., Ono S. The current status of drug discovery and development as originated in United States academia: The influence of industrial and academic collaboration on drug discovery and development. Clin. Transl. Sci. 2018;11:597–606. doi: 10.1111/cts.12577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sun D., Gao W., Hu H., Zhou S. Why 90% of clinical drug development fails and how to improve it? Acta Pharm. Sin. B. 2022;12:3049–3062. doi: 10.1016/j.apsb.2022.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Watkins P.B. Drug safety sciences and the bottleneck in drug development. Clin. Pharmacol. Ther. 2011;89:788–790. doi: 10.1038/clpt.2011.63. [DOI] [PubMed] [Google Scholar]
- 6.Ray A. Beyond debacle and debate: Developing solutions in drug safety. Nat. Rev. Drug Discov. 2009;8:775–779. doi: 10.1038/nrd2988. [DOI] [PubMed] [Google Scholar]
- 7.de Boisferon M.H., Benzaid I., Chatel E.M.D., Hoffmann N., Littlefield B. Development of a panel of breast cancer patient-derived xenograft models (PDX) with estrogen independence and/or acquired resistance to endocrine treatment. Cancer Res. 2020;80:5281. doi: 10.1158/1538-7445.AM2020-5281. [DOI] [Google Scholar]
- 8.O’Donovan B., Rodgers R.M., Cox A.R., Krska J. ‘You feel like you haven’t got any control’: A qualitative study of side effects from medicines. J. Patient Saf. Risk Manag. 2019;24:13–24. doi: 10.1177/2516043518821499. [DOI] [Google Scholar]
- 9.Rudmann D.G. On-target and off-target-based toxicologic effects. Toxicol. Pathol. 2013;41:310–314. doi: 10.1177/0192623312464311. [DOI] [PubMed] [Google Scholar]
- 10.Lackey L., Thompson G., Eggers S. FDA’s Benefit–Risk Framework for Human Drugs and Biologics: Role in Benefit–Risk Assessment and Analysis of Use for Drug Approvals. Ther. Innov. Regul. Sci. 2021;55:170–179. doi: 10.1007/s43441-020-00203-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Juhaeri J. Benefit–risk evaluation: The past, present and future. Ther. Adv. Drug Saf. 2019;10:2042098619871180. doi: 10.1177/2042098619871180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith M.Y., Benattia I., Strauss C., Bloss L., Jiang Q. Structured benefit-risk assessment across the product lifecycle: Practical considerations. Ther. Innov. Regul. Sci. 2017;51:501–508. doi: 10.1177/2168479017696272. [DOI] [PubMed] [Google Scholar]
- 13.Smith M.Y., van Til J., DiSantostefano R.L., Hauber A.B., Marsh K. Quantitative Benefit-Risk Assessment: State of the Practice Within Industry. Ther. Innov. Regul. Sci. 2021;55:415–425. doi: 10.1007/s43441-020-00230-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tamargo J., Le Heuzey J.Y., Mabo P. Narrow therapeutic index drugs: A clinical pharmacological consideration to flecainide. Eur. J. Clin. Pharmacol. 2015;71:549–567. doi: 10.1007/s00228-015-1832-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muller P.Y., Milton M.N. The determination and interpretation of the therapeutic index in drug development. Nat. Rev. Drug Discov. 2012;11:751–761. doi: 10.1038/nrd3801. [DOI] [PubMed] [Google Scholar]
- 16.Wang E., Cesano A., Butterfield L.H., Marincola F. Improving the therapeutic index in adoptive cell therapy: Key factors that impact efficacy. J. Immunother. Cancer. 2020;8:e001619. doi: 10.1136/jitc-2020-001619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blix H.S., Viktil K.K., Moger T.A., Reikvam A. Drugs with narrow therapeutic index as indicators in the risk management of hospitalised patients. Pharm Pr. 2010;8:50–55. doi: 10.4321/S1886-36552010000100006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tyson R.J., Park C.C., Powell J.R., Patterson J.H., Weiner D., Watkins P.B., Gonzalez D. Precision Dosing Priority Criteria: Drug, Disease, and Patient Population Variables. Front. Pharmacol. 2020;11:420. doi: 10.3389/fphar.2020.00420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Habet S. Narrow Therapeutic Index drugs: Clinical pharmacology perspective. J. Pharm. Pharmacol. 2021;73:1285–1291. doi: 10.1093/jpp/rgab102. [DOI] [PubMed] [Google Scholar]
- 20.Cheifetz A. Overview of Therapeutic Drug Monitoring of Biologic Agents in Patients With Inflammatory Bowel Disease. Gastroenterol. Hepatol. 2017;13:556–559. [PMC free article] [PubMed] [Google Scholar]
- 21.Zylbersztajn B., Barraza M., Torres J.P., Morales J. Therapeutic monitoring of antimicrobial agents in pediatrics. Review based on Latin American experiences. Rev. Chil. Infectol. 2018;35:22–28. doi: 10.4067/s0716-10182018000100022. [DOI] [PubMed] [Google Scholar]
- 22.Stanley T.H. Anesthesia for the 21st century. Bayl. Univ. Med. Cent. Proc. 2000;13:7–10. doi: 10.1080/08998280.2000.11927635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Filozof C., Chow S.C., Dimick-Santos L., Chen Y.F., Williams R.N., Goldstein B.J., Sanyal A. Clinical endpoints and adaptive clinical trials in precirrhotic nonalcoholic steatohepatitis: Facilitating development approaches for an emerging epidemic. Hepatol. Commun. 2017;1:577–585. doi: 10.1002/hep4.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Cacqueray N., Boujaafar S., Bille E., Moulin F., Gana I., Benaboud S., Hirt D., Beranger A., Toubiana J., Renolleau S., et al. Therapeutic Drug Monitoring of Antibiotics in Critically Ill Children: An Observational Study in a Pediatric Intensive Care Unit. Ther. Drug Monit. 2022;44:319–327. doi: 10.1097/FTD.0000000000000918. [DOI] [PubMed] [Google Scholar]
- 25.Bottino D.C., Patel M., Kadakia E., Zhou J., Patel C., Neuwirth R., Iartchouk N., Brake R., Venkatakrishnan K., Chakravarty A. Dose Optimization for Anticancer Drug Combinations: Maximizing Therapeutic Index via Clinical Exposure-Toxicity/Preclinical Exposure-Efficacy Modeling. Clin. Cancer Res. 2019;25:6633–6643. doi: 10.1158/1078-0432.CCR-18-3882. [DOI] [PubMed] [Google Scholar]
- 26.He H., Yuan D., Wu Y., Cao Y. Pharmacokinetics and pharmacodynamics modeling and simulation systems to support the development and regulation of liposomal drugs. Pharmaceutics. 2019;11:110. doi: 10.3390/pharmaceutics11030110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gummadi A.C., Guddati A.K. Genetic polymorphisms in pharmaceuticals and chemotherapy. World J. Oncol. 2021;12:149. doi: 10.14740/wjon1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Capulli A.K., MacQueen L.A., O’Connor B.B., Dauth S., Parker K.K. Acute pergolide exposure stiffens engineered valve interstitial cell tissues and reduces contractility in vitro. Cardiovasc. Pathol. 2016;25:316–324. doi: 10.1016/j.carpath.2016.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ericson J.E., Zimmerman K.O., Gonzalez D., Melloni C., Guptill J.T., Hill K.D., Wu H., Cohen-Wolkowiez M. A Systematic Literature Review Approach to Estimate the Therapeutic Index of Selected Immunosuppressant Drugs After Renal Transplantation. Ther. Drug Monit. 2017;39:13–20. doi: 10.1097/FTD.0000000000000364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schmelas C., Grimm D. Split Cas9, Not Hairs—Advancing the Therapeutic Index of CRISPR Technology. Biotechnol. J. 2018;13:e1700432. doi: 10.1002/biot.201700432. [DOI] [PubMed] [Google Scholar]
- 31.Hartl D., de Luca V., Kostikova A., Laramie J., Kennedy S., Ferrero E., Siegel R., Fink M., Ahmed S., Millholland J., et al. Translational precision medicine: An industry perspective. J. Transl. Med. 2021;19:245. doi: 10.1186/s12967-021-02910-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Subbiah V. The next generation of evidence-based medicine. Nat. Med. 2023;29:49–58. doi: 10.1038/s41591-022-02160-z. [DOI] [PubMed] [Google Scholar]
- 33.De Maria Marchiano R., Di Sante G., Piro G., Carbone C., Tortora G., Boldrini L., Pietragalla A., Daniele G., Tredicine M., Cesario A., et al. Translational Research in the Era of Precision Medicine: Where We Are and Where We Will Go. J. Pers. Med. 2021;11:216. doi: 10.3390/jpm11030216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stalidzans E., Zanin M., Tieri P., Castiglione F., Polster A., Scheiner S., Pahle J., Stres B., List M., Baumbach J. Mechanistic modeling and multiscale applications for precision medicine: Theory and practice. Netw. Syst. Med. 2020;3:36–56. doi: 10.1089/nsm.2020.0002. [DOI] [Google Scholar]
- 35.Faulkner E., Holtorf A.-P., Liu C.Y., Lin H., Biltaj E., Brixner D., Barr C., Oberg J., Shandhu G., Siebert U. Being precise about precision medicine: What should value frameworks incorporate to address precision medicine? A report of the personalized precision medicine special interest group. Value Health. 2020;23:529–539. doi: 10.1016/j.jval.2019.11.010. [DOI] [PubMed] [Google Scholar]
- 36.Naithani N., Sinha S., Misra P., Vasudevan B., Sahu R. Precision medicine: Concept and tools. Med. J. Armed Forces India. 2021;77:249–257. doi: 10.1016/j.mjafi.2021.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.MacEachern S.J., Forkert N.D. Machine learning for precision medicine. Genome. 2021;64:416–425. doi: 10.1139/gen-2020-0131. [DOI] [PubMed] [Google Scholar]
- 38.Suwinski P., Ong C., Ling M.H., Poh Y.M., Khan A.M., Ong H.S. Advancing personalized medicine through the application of whole exome sequencing and big data analytics. Front. Genet. 2019;10:49. doi: 10.3389/fgene.2019.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hassan M., Awan F.M., Naz A., deAndres-Galiana E.J., Alvarez O., Cernea A., Fernandez-Brillet L., Fernandez-Martinez J.L., Kloczkowski A. Innovations in Genomics and Big Data Analytics for Personalized Medicine and Health Care: A Review. Int. J. Mol. Sci. 2022;23:4645. doi: 10.3390/ijms23094645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sisodiya S.M. Precision medicine and therapies of the future. Epilepsia. 2021;62((Suppl. S2)):S90–S105. doi: 10.1111/epi.16539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lander E.S. Cutting the Gordian helix—Regulating genomic testing in the era of precision medicine. N. Engl. J. Med. 2015;372:1185–1186. doi: 10.1056/NEJMp1501964. [DOI] [PubMed] [Google Scholar]
- 42.Nomura S., Komuro I. Precision medicine for heart failure based on molecular mechanisms: The 2019 ISHR Research Achievement Award Lecture. J. Mol. Cell. Cardiol. 2021;152:29–39. doi: 10.1016/j.yjmcc.2020.11.010. [DOI] [PubMed] [Google Scholar]
- 43.Wong S.H., Marquet P., Cattaneo D., Svinarov D., Bergan S., Walson P., Vinks A.A., Shipkova M. Therapeutic Drug Monitoring in the Era of Precision Medicine: Achievements, Gaps, and Perspectives-An Interview in Honor of Professor Charles Pippenger. Ther. Drug Monit. 2021;43:719–727. doi: 10.1097/FTD.0000000000000932. [DOI] [PubMed] [Google Scholar]
- 44.Edsjö A., Holmquist L., Geoerger B., Nowak F., Gomon G., Alix-Panabières C., Staaf J., Ploeger C., Lassen U., Le Tourneau C. Precision cancer medicine: Concepts, current practice, and future developments. J. Intern. Med. 2023;294:455–481. doi: 10.1111/joim.13709. [DOI] [PubMed] [Google Scholar]
- 45.Torjesen I. Half of the UK population can expect a diagnosis of cancer. BMJ. 2015;350:h614. doi: 10.1136/bmj.h614. [DOI] [PubMed] [Google Scholar]
- 46.Ahmad A.S., Ormiston-Smith N., Sasieni P.D. Trends in the lifetime risk of developing cancer in Great Britain: Comparison of risk for those born from 1930 to 1960. Br. J. Cancer. 2015;112:943–947. doi: 10.1038/bjc.2014.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vasan N., Baselga J., Hyman D.M. A view on drug resistance in cancer. Nature. 2019;575:299–309. doi: 10.1038/s41586-019-1730-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pote M.S., Gacche R.N. ATP-binding cassette efflux transporters and MDR in cancer. Drug Discov. Today. 2023;28:103537. doi: 10.1016/j.drudis.2023.103537. [DOI] [PubMed] [Google Scholar]
- 49.Haider T., Pandey V., Banjare N., Gupta P.N., Soni V. Drug resistance in cancer: Mechanisms and tackling strategies. Pharmacol. Rep. 2020;72:1125–1151. doi: 10.1007/s43440-020-00138-7. [DOI] [PubMed] [Google Scholar]
- 50.Nussinov R., Tsai C.J., Jang H. Anticancer drug resistance: An update and perspective. Drug Resist. Updat. 2021;59:100796. doi: 10.1016/j.drup.2021.100796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alfarouk K.O., Stock C.M., Taylor S., Walsh M., Muddathir A.K., Verduzco D., Bashir A.H., Mohammed O.Y., Elhassan G.O., Harguindey S., et al. Resistance to cancer chemotherapy: Failure in drug response from ADME to P-gp. Cancer Cell Int. 2015;15:71. doi: 10.1186/s12935-015-0221-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yao Y., Zhou Y., Liu L., Xu Y., Chen Q., Wang Y., Wu S., Deng Y., Zhang J., Shao A. Nanoparticle-Based Drug Delivery in Cancer Therapy and Its Role in Overcoming Drug Resistance. Front. Mol. Biosci. 2020;7:193. doi: 10.3389/fmolb.2020.00193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Su Z., Dong S., Zhao S.C., Liu K., Tan Y., Jiang X., Assaraf Y.G., Qin B., Chen Z.S., Zou C. Novel nanomedicines to overcome cancer multidrug resistance. Drug Resist. Updat. 2021;58:100777. doi: 10.1016/j.drup.2021.100777. [DOI] [PubMed] [Google Scholar]
- 54.Wang L., Wang H., Song D., Xu M., Liebmen M. New strategies for targeting drug combinations to overcome mutation-driven drug resistance. Semin. Cancer Biol. 2017;42:44–51. doi: 10.1016/j.semcancer.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 55.Thorat S.S., Gujar K.N., Karale C.K. Bioenhancers from mother nature: An overview. Future J. Pharm. Sci. 2023;9:20. doi: 10.1186/s43094-023-00470-8. [DOI] [Google Scholar]
- 56.Pushpamalar J., Meganathan P., Tan H.L., Dahlan N.A., Ooi L.-T., Neerooa B.N.H.M., Essa R.Z., Shameli K., Teow S.-Y. Development of a Polysaccharide-Based Hydrogel Drug Delivery System (DDS): An Update. Gels. 2021;7:153. doi: 10.3390/gels7040153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Anselmo A.C., Mitragotri S. An overview of clinical and commercial impact of drug delivery systems. J. Control Release. 2014;190:15–28. doi: 10.1016/j.jconrel.2014.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rosen H., Abribat T. The rise and rise of drug delivery. Nat. Rev. Drug Discov. 2005;4:381–385. doi: 10.1038/nrd1721. [DOI] [PubMed] [Google Scholar]
- 59.Alqahtani M.S., Kazi M., Alsenaidy M.A., Ahmad M.Z. Advances in oral drug delivery. Front. Pharmacol. 2021;12:618411. doi: 10.3389/fphar.2021.618411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jain S., Datta M. Montmorillonite-alginate microspheres as a delivery vehicle for oral extended release of venlafaxine hydrochloride. J. Drug Deliv. Sci. Technol. 2016;33:149–156. doi: 10.1016/j.jddst.2016.04.002. [DOI] [Google Scholar]
- 61.Beckwith M.C., Feddema S.S., Barton R.G., Graves C. A guide to drug therapy in patients with enteral feeding tubes: Dosage form selection and administration methods. Hosp. Pharm. 2004;39:225–237. doi: 10.1177/001857870403900308. [DOI] [Google Scholar]
- 62.Bruno M.C., Cristiano M.C., Celia C., d’Avanzo N., Mancuso A., Paolino D., Wolfram J., Fresta M. Injectable drug delivery systems for osteoarthritis and rheumatoid arthritis. ACS Nano. 2022;16:19665–19690. doi: 10.1021/acsnano.2c06393. [DOI] [PubMed] [Google Scholar]
- 63.Huynh D.P., Nguyen M.K., Pi B.S., Kim M.S., Chae S.Y., Lee K.C., Kim B.S., Kim S.W., Lee D.S. Functionalized injectable hydrogels for controlled insulin delivery. Biomaterials. 2008;29:2527–2534. doi: 10.1016/j.biomaterials.2008.02.016. [DOI] [PubMed] [Google Scholar]
- 64.Cicero T.J., Ellis M.S., Kasper Z.A. Relative preferences in the abuse of immediate-release versus extended-release opioids in a sample of treatment-seeking opioid abusers. Pharmacoepidemiol. Drug Saf. 2017;26:56–62. doi: 10.1002/pds.4078. [DOI] [PubMed] [Google Scholar]
- 65.Li Z., Wang F., Roy S., Sen C.K., Guan J. Injectable, highly flexible, and thermosensitive hydrogels capable of delivering superoxide dismutase. Biomacromolecules. 2009;10:3306–3316. doi: 10.1021/bm900900e. [DOI] [PubMed] [Google Scholar]
- 66.Walters K. Routes of Drug Administration: Topics in Pharmacy. Volume 2. Wright; Bristol, UK: 2013. Transdermal drug delivery; p. 78. [Google Scholar]
- 67.Østergaard J., Meng-Lund E., Larsen S.W., Larsen C., Petersson K., Lenke J., Jensen H. Real-time UV imaging of nicotine release from transdermal patch. Pharm. Res. 2010;27:2614–2623. doi: 10.1007/s11095-010-0257-9. [DOI] [PubMed] [Google Scholar]
- 68.Murthy S.N. Transdermal Drug Delivery: Approaches and Significance. Taylor & Francis; New York, NY, USA: 2012. pp. 1–2. [Google Scholar]
- 69.Patel A., Cholkar K., Agrahari V., Mitra A.K. Ocular drug delivery systems: An overview. World J. Pharmacol. 2013;2:47. doi: 10.5497/wjp.v2.i2.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nayak K., Choudhari M.V., Bagul S., Chavan T.A., Misra M. Drug Delivery Devices and Therapeutic Systems. Elsevier; Amsterdam, The Netherlands: 2021. Ocular drug delivery systems; pp. 515–566. [Google Scholar]
- 71.Nelson H.S. Inhalation devices, delivery systems, and patient technique. Ann. Allergy Asthma Immunol. 2016;117:606–612. doi: 10.1016/j.anai.2016.05.006. [DOI] [PubMed] [Google Scholar]
- 72.Sorino C., Negri S., Spanevello A., Visca D., Scichilone N. Inhalation therapy devices for the treatment of obstructive lung diseases: The history of inhalers towards the ideal inhaler. Eur. J. Intern. Med. 2020;75:15–18. doi: 10.1016/j.ejim.2020.02.023. [DOI] [PubMed] [Google Scholar]
- 73.Rajgor N., Patel M., Bhaskar V. Implantable Drug Delivery Systems: An Overview. Syst. Rev. Pharm. 2011;2:473–511. doi: 10.4103/0975-8453.86297. [DOI] [Google Scholar]
- 74.Yasin M.N., Svirskis D., Seyfoddin A., Rupenthal I.D. Implants for drug delivery to the posterior segment of the eye: A focus on stimuli-responsive and tunable release systems. J. Control. Release. 2014;196:208–221. doi: 10.1016/j.jconrel.2014.09.030. [DOI] [PubMed] [Google Scholar]
- 75.Nitsch M.J., Banakar U.V. Implantable drug delivery. Adv. Control. Deliv. Drugs. 2022;8:21–58. [Google Scholar]
- 76.Arslan F.B., Ozturk K., Calis S. Antibody-mediated drug delivery. Int. J. Pharm. 2021;596:120268. doi: 10.1016/j.ijpharm.2021.120268. [DOI] [PubMed] [Google Scholar]
- 77.Bradley A.M., Devine M., DeRemer D. Brentuximab vedotin: An anti-CD30 antibody–drug conjugate. Am. J. Health-Syst. Pharm. 2013;70:589–597. doi: 10.2146/ajhp110608. [DOI] [PubMed] [Google Scholar]
- 78.Kim M.T., Chen Y., Marhoul J., Jacobson F. Statistical modeling of the drug load distribution on trastuzumab emtansine (Kadcyla), a lysine-linked antibody drug conjugate. Bioconjugate Chem. 2014;25:1223–1232. doi: 10.1021/bc5000109. [DOI] [PubMed] [Google Scholar]
- 79.Auriemma G., Russo P., Del Gaudio P., García-González C.A., Landín M., Aquino R.P. Technologies and formulation design of polysaccharide-based hydrogels for drug delivery. Molecules. 2020;25:3156. doi: 10.3390/molecules25143156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sugimura T. The principles of cancer treatment—Changes in chemotherapy. Gan Kagaku Ryoho. 2002;29:1263–1278. [PubMed] [Google Scholar]
- 81.Espinosa E., Zamora P., Feliu J., Gonzalez Baron M. Classification of anticancer drugs—A new system based on therapeutic targets. Cancer Treat. Rev. 2003;29:515–523. doi: 10.1016/S0305-7372(03)00116-6. [DOI] [PubMed] [Google Scholar]
- 82.McClean S., Costelloe C., Denny W.A., Searcey M., Wakelin L.P. Sequence selectivity, cross-linking efficiency and cytotoxicity of DNA-targeted 4-anilinoquinoline aniline mustards. Anticancer. Drug Des. 1999;14:187–204. [PubMed] [Google Scholar]
- 83.Lajous H., Lelievre B., Vauleon E., Lecomte P., Garcion E. Rethinking Alkylating(-Like) Agents for Solid Tumor Management. Trends Pharmacol. Sci. 2019;40:342–357. doi: 10.1016/j.tips.2019.03.003. [DOI] [PubMed] [Google Scholar]
- 84.Kaye S.B. New antimetabolites in cancer chemotherapy and their clinical impact. Br. J. Cancer. 1998;78((Suppl. S3)):1–7. doi: 10.1038/bjc.1998.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rassy E., Rached L., Pistilli B. Antibody drug conjugates targeting HER2: Clinical development in metastatic breast cancer. Breast. 2022;66:217–226. doi: 10.1016/j.breast.2022.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zahavi D., Weiner L. Monoclonal Antibodies in Cancer Therapy. Antibodies. 2020;9:34. doi: 10.3390/antib9030034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Burgess D.J., Doles J., Zender L., Xue W., Ma B., McCombie W.R., Hannon G.J., Lowe S.W., Hemann M.T. Topoisomerase levels determine chemotherapy response in vitro and in vivo. Proc. Natl. Acad. Sci. USA. 2008;105:9053–9058. doi: 10.1073/pnas.0803513105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kavallaris M. Microtubules and resistance to tubulin-binding agents. Nat. Rev. Cancer. 2010;10:194–204. doi: 10.1038/nrc2803. [DOI] [PubMed] [Google Scholar]
- 89.Bhattacharya B., Mukherjee S. Cancer therapy using antibiotics. J. Cancer Ther. 2015;6:849. doi: 10.4236/jct.2015.610093. [DOI] [Google Scholar]
- 90.Madabhavi I., Modi G., Patel A., Anand A., Panchal H., Parikh S. Pulmonary toxicity following bleomycin use: A single-center experience. J. Cancer Res. Ther. 2017;13:466–470. doi: 10.4103/0973-1482.204887. [DOI] [PubMed] [Google Scholar]
- 91.Yan V.C., Butterfield H.E., Poral A.H., Yan M.J., Yang K.L., Pham C.D., Muller F.L. Why Great Mitotic Inhibitors Make Poor Cancer Drugs. Trends Cancer. 2020;6:924–941. doi: 10.1016/j.trecan.2020.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Parichatikanond W., Luangmonkong T., Mangmool S., Kurose H. Therapeutic targets for the treatment of cardiac fibrosis and cancer: Focusing on TGF-β signaling. Front. Cardiovasc. Med. 2020;7:34. doi: 10.3389/fcvm.2020.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yamaoka T., Kusumoto S., Ando K., Ohba M., Ohmori T. Receptor Tyrosine Kinase-Targeted Cancer Therapy. Int. J. Mol. Sci. 2018;19:3491. doi: 10.3390/ijms19113491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Alves R., Gonçalves A.C., Rutella S., Almeida A.M., De Las Rivas J., Trougakos I.P., Sarmento Ribeiro A.B. Resistance to tyrosine kinase inhibitors in chronic myeloid leukemia—From molecular mechanisms to clinical relevance. Cancers. 2021;13:4820. doi: 10.3390/cancers13194820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sullivan D.C., Kelloff G. Seeing into cells: The promise of in vivo molecular imaging in oncology. EMBO Rep. 2005;6:292–296. doi: 10.1038/sj.embor.7400382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hevener K., Verstak T.A., Lutat K.E., Riggsbee D.L., Mooney J.W. Recent developments in topoisomerase-targeted cancer chemotherapy. Acta Pharm. Sin. B. 2018;8:844–861. doi: 10.1016/j.apsb.2018.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Talukdar A., Kundu B., Sarkar D., Goon S., Mondal M.A. Topoisomerase I inhibitors: Challenges, progress and the road ahead. Eur. J. Med. Chem. 2022;236:114304. doi: 10.1016/j.ejmech.2022.114304. [DOI] [PubMed] [Google Scholar]
- 98.Manasanch E.E., Orlowski R.Z. Proteasome inhibitors in cancer therapy. Nat. Rev. Clin. Oncol. 2017;14:417–433. doi: 10.1038/nrclinonc.2016.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dietrich J., Rao K., Pastorino S., Kesari S. Corticosteroids in brain cancer patients: Benefits and pitfalls. Expert. Rev. Clin. Pharmacol. 2011;4:233–242. doi: 10.1586/ecp.11.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Goodman R.S., Johnson D.B., Balko J.M. Corticosteroids and Cancer Immunotherapy. Clin. Cancer Res. 2023;29:2580–2587. doi: 10.1158/1078-0432.CCR-22-3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.van Weelden W.J., Massuger L., Enitec, Pijnenborg J.M.A., Romano A. Anti-estrogen Treatment in Endometrial Cancer: A Systematic Review. Front. Oncol. 2019;9:359. doi: 10.3389/fonc.2019.00359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mallick S., Benson R., Julka P.K. Breast cancer prevention with anti-estrogens: Review of the current evidence and future directions. Breast Cancer. 2016;23:170–177. doi: 10.1007/s12282-015-0647-2. [DOI] [PubMed] [Google Scholar]
- 103.Gu R., Jia W., Zeng Y., Rao N., Hu Y., Li S., Wu J., Jin L., Chen L., Long M., et al. A comparison of survival outcomes and side effects of toremifene or tamoxifen therapy in premenopausal estrogen and progesterone receptor positive breast cancer patients: A retrospective cohort study. BMC Cancer. 2012;12:161. doi: 10.1186/1471-2407-12-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lin T.H., Lee S.O., Niu Y., Xu D., Liang L., Li L., Yeh S.D., Fujimoto N., Yeh S., Chang C. Differential androgen deprivation therapies with anti-androgens casodex/bicalutamide or MDV3100/Enzalutamide versus anti-androgen receptor ASC-J9(R) Lead to promotion versus suppression of prostate cancer metastasis. J. Biol. Chem. 2013;288:19359–19369. doi: 10.1074/jbc.M113.477216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Obayemi J., Salifu A., Eluu S., Uzonwanne V., Jusu S., Nwazojie C., Onyekanne C., Ojelabi O., Payne L., Moore C.M. LHRH-conjugated drugs as targeted therapeutic agents for the specific targeting and localized treatment of triple negative breast cancer. Sci. Rep. 2020;10:8212. doi: 10.1038/s41598-020-64979-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ghosh N., Chaki R., Mandal V., Mandal S.C. COX-2 as a target for cancer chemotherapy. Pharmacol. Rep. 2010;62:233–244. doi: 10.1016/S1734-1140(10)70262-0. [DOI] [PubMed] [Google Scholar]
- 107.Sood A., Lang D.K., Kaur R., Saini B., Arora S. Relevance of Aromatase Inhibitors in Breast Cancer Treatment. Curr. Top. Med. Chem. 2021;21:1319–1336. doi: 10.2174/1568026621666210701143445. [DOI] [PubMed] [Google Scholar]
- 108.Dyavar S.R., Singh R., Emani R., Pawar G.P., Chaudhari V.D., Podany A.T., Avedissian S.N., Fletcher C.V., Salunke D.B. Role of toll-like receptor 7/8 pathways in regulation of interferon response and inflammatory mediators during SARS-CoV2 infection and potential therapeutic options. Biomed. Pharmacother. 2021;141:111794. doi: 10.1016/j.biopha.2021.111794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Amaria R.N., Reuben A., Cooper Z.A., Wargo J.A. Update on use of aldesleukin for treatment of high-risk metastatic melanoma. Immunotargets Ther. 2015;4:79–89. doi: 10.2147/ITT.S61590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wei X.X., Fong L., Small E.J. Prostate cancer immunotherapy with sipuleucel-T: Current standards and future directions. Expert Rev. Vaccines. 2015;14:1529–1541. doi: 10.1586/14760584.2015.1099437. [DOI] [PubMed] [Google Scholar]
- 111.Lawler S.E., Chiocca E.A. Oncolytic virus-mediated immunotherapy: A combinatorial approach for cancer treatment. J. Clin. Oncol. 2015;33:2812–2814. doi: 10.1200/JCO.2015.62.5244. [DOI] [PubMed] [Google Scholar]
- 112.Ralph S.J., Low P., Dong L., Lawen A., Neuzil J. Mitocans: Mitochondrial targeted anti-cancer drugs as improved therapies and related patent documents. Recent Pat. Anti-Cancer Drug Discov. 2006;1:327–346. doi: 10.2174/157489206778776952. [DOI] [PubMed] [Google Scholar]
- 113.Nikravesh H., Khodayar M.J., Behmanesh B., Mahdavinia M., Teimoori A., Alboghobeish S., Zeidooni L. The combined effect of dichloroacetate and 3-bromopyruvate on glucose metabolism in colorectal cancer cell line, HT-29; the mitochondrial pathway apoptosis. BMC Cancer. 2021;21:903. doi: 10.1186/s12885-021-08564-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mani S., Swargiary G., Singh K.K. Natural Agents Targeting Mitochondria in Cancer. Int. J. Mol. Sci. 2020;21:6992. doi: 10.3390/ijms21196992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chen G., Chen Z., Hu Y., Huang P. Inhibition of mitochondrial respiration and rapid depletion of mitochondrial glutathione by β-phenethyl isothiocyanate: Mechanisms for anti-leukemia activity. Antioxid. Redox Signal. 2011;15:2911–2921. doi: 10.1089/ars.2011.4170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.D’Souza G.G., Wagle M.A., Saxena V., Shah A. Approaches for targeting mitochondria in cancer therapy. Biochim. Et Biophys. Acta (BBA)-Bioenerg. 2011;1807:689–696. doi: 10.1016/j.bbabio.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 117.Guo L., Shestov A.A., Worth A.J., Nath K., Nelson D.S., Leeper D.B., Glickson J.D., Blair I.A. Inhibition of mitochondrial complex II by the anticancer agent lonidamine. J. Biol. Chem. 2016;291:42–57. doi: 10.1074/jbc.M115.697516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wang J., Li J., Xiao Y., Fu B., Qin Z. TPP-based mitocans: A potent strategy for anticancer drug design. RSC Med. Chem. 2020;11:858–875. doi: 10.1039/C9MD00572B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Dong L., Gopalan V., Holland O., Neuzil J. Mitocans revisited: Mitochondrial targeting as efficient anti-cancer therapy. Int. J. Mol. Sci. 2020;21:7941. doi: 10.3390/ijms21217941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Koya K., Li Y., Wang H., Ukai T., Tatsuta N., Kawakami M., Shishido T., Chen L.B. MKT-077, a novel rhodacyanine dye in clinical trials, exhibits anticarcinoma activity in preclinical studies based on selective mitochondrial accumulation. Cancer Res. 1996;56:538–543. [PubMed] [Google Scholar]
- 121.Heise N.V., Hoenke S., Serbian I., Csuk R. An improved partial synthesis of corosolic acid and its conversion to highly cytotoxic mitocans. Eur. J. Med. Chem. Rep. 2022;6:100073. doi: 10.1016/j.ejmcr.2022.100073. [DOI] [Google Scholar]
- 122.Neuzil J., Dong L.-F., Rohlena J., Truksa J., Ralph S.J. Classification of mitocans, anti-cancer drugs acting on mitochondria. Mitochondrion. 2013;13:199–208. doi: 10.1016/j.mito.2012.07.112. [DOI] [PubMed] [Google Scholar]
- 123.Chen G., Wang F., Trachootham D., Huang P. Preferential killing of cancer cells with mitochondrial dysfunction by natural compounds. Mitochondrion. 2010;10:614–625. doi: 10.1016/j.mito.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Carvalho T. Personalized anti-cancer vaccine combining mRNA and immunotherapy tested in melanoma trial. Nat. Med. 2023;29:2379–2380. doi: 10.1038/d41591-023-00072-0. [DOI] [PubMed] [Google Scholar]
- 125.Rybakova Y., Kowalski P.S., Huang Y., Gonzalez J.T., Heartlein M.W., DeRosa F., Delcassian D., Anderson D.G. mRNA Delivery for Therapeutic Anti-HER2 Antibody Expression In Vivo. Mol. Ther. 2019;27:1415–1423. doi: 10.1016/j.ymthe.2019.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Soundara Rajan T., Gugliandolo A., Bramanti P., Mazzon E. In Vitro-Transcribed mRNA Chimeric Antigen Receptor T Cell (IVT mRNA CAR T) Therapy in Hematologic and Solid Tumor Management: A Preclinical Update. Int. J. Mol. Sci. 2020;21:6514. doi: 10.3390/ijms21186514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wijdeven R.H., Pang B., Assaraf Y.G., Neefjes J. Old drugs, novel ways out: Drug resistance toward cytotoxic chemotherapeutics. Drug Resist. Updat. 2016;28:65–81. doi: 10.1016/j.drup.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 128.Meng J., Guo F., Xu H., Liang W., Wang C., Yang X.D. Combination Therapy using Co-Encapsulated Resveratrol and Paclitaxel in Liposomes for Drug Resistance Reversal in Breast Cancer Cells in vivo. Sci. Rep. 2016;6:22390. doi: 10.1038/srep22390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Tan Z., Chaudhai R., Zhang S. Polypharmacology in Drug Development: A Minireview of Current Technologies. ChemMedChem. 2016;11:1211–1218. doi: 10.1002/cmdc.201600067. [DOI] [PubMed] [Google Scholar]
- 130.Frei E., III, Karon M., Levin R.H., Freireich E.J., Taylor R.J., Hananian J., Selawry O., Holland J.F., Hoogstraten B., Wolman I.J., et al. The effectiveness of combinations of antileukemic agents in inducing and maintaining remission in children with acute leukemia. Blood. 1965;26:642–656. doi: 10.1182/blood.V26.5.642.642. [DOI] [PubMed] [Google Scholar]
- 131.Ayoub N.M. Editorial: Novel Combination Therapies for the Treatment of Solid Cancers. Front. Oncol. 2021;11:708943. doi: 10.3389/fonc.2021.708943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wang X., Zhang H., Chen X. Drug resistance and combating drug resistance in cancer. Cancer Drug Resist. 2019;2:141–160. doi: 10.20517/cdr.2019.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sun W., Sanderson P.E., Zheng W. Drug combination therapy increases successful drug repositioning. Drug Discov. Today. 2016;21:1189–1195. doi: 10.1016/j.drudis.2016.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Schein C.H. Repurposing approved drugs on the pathway to novel therapies. Med. Res. Rev. 2020;40:586–605. doi: 10.1002/med.21627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Shankar E., Subramaniam V., Allimuthu D. Editorial: Adopting drug repurposing to overcome drug resistance in cancer. Front. Cell Dev. Biol. 2023;11:1191682. doi: 10.3389/fcell.2023.1191682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ercan G., Ilbar Tartar R., Solmaz A., Gulcicek O.B., Karagulle O.O., Meric S., Cayoren H., Kusaslan R., Kemik A., Gokceoglu Kayali D., et al. Potent therapeutic effects of ruscogenin on gastric ulcer established by acetic acid. Asian J. Surg. 2020;43:405–416. doi: 10.1016/j.asjsur.2019.07.001. [DOI] [PubMed] [Google Scholar]
- 137.Rodrigues J.P.B., Fernandes A., Dias M.I., Pereira C., Pires T., Calhelha R.C., Carvalho A.M., Ferreira I., Barros L. Phenolic Compounds and Bioactive Properties of Ruscus aculeatus L. (Asparagaceae): The Pharmacological Potential of an Underexploited Subshrub. Molecules. 2021;26:1882. doi: 10.3390/molecules26071882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hua H., Zhu Y., Song Y.H. Ruscogenin suppressed the hepatocellular carcinoma metastasis via PI3K/Akt/mTOR signaling pathway. Biomed. Pharmacother. 2018;101:115–122. doi: 10.1016/j.biopha.2018.02.031. [DOI] [PubMed] [Google Scholar]
- 139.Roy A., Bharadvaja N. A review on pharmaceutically important medical plant: Plumbago zeylanica. J. Ayurvedic Herb. Med. 2017;3:225–228. doi: 10.31254/jahm.2017.3411. [DOI] [Google Scholar]
- 140.Aziz M.H., Dreckschmidt N.E., Verma A.K. Plumbagin, a medicinal plant–derived naphthoquinone, is a novel inhibitor of the growth and invasion of hormone-refractory prostate cancer. Cancer Res. 2008;68:9024–9032. doi: 10.1158/0008-5472.CAN-08-2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zhang R., Wang Z., You W., Zhou F., Guo Z., Qian K., Xiao Y., Wang X. Suppressive effects of plumbagin on the growth of human bladder cancer cells via PI3K/AKT/mTOR signaling pathways and EMT. Cancer Cell Int. 2020;20:520. doi: 10.1186/s12935-020-01607-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Chen S.M., Feng J.N., Zhao C.K., Yao L.C., Wang L.X., Meng L., Cai S.Q., Liu C.Y., Qu L.K., Jia Y.X., et al. A multi-targeting natural product, aiphanol, inhibits tumor growth and metastasis. Am. J. Cancer Res. 2022;12:4930–4953. [PMC free article] [PubMed] [Google Scholar]
- 143.Méndez-Lucio O., Naveja J.J., Vite-Caritino H., Prieto-Martínez F.D., Medina-Franco J.L. One drug for multiple targets: A computational perspective. J. Mex. Chem. Soc. 2016;60:168–181. doi: 10.29356/jmcs.v60i3.100. [DOI] [Google Scholar]
- 144.Aw M.S., Addai-Mensah J., Losic D. A multi-drug delivery system with sequential release using titania nanotube arrays. Chem. Commun. 2012;48:3348–3350. doi: 10.1039/c2cc17690d. [DOI] [PubMed] [Google Scholar]
- 145.Tabakoglu S., Kolbuk D., Sajkiewicz P. Multifluid electrospinning for multi-drug delivery systems: Pros and cons, challenges, and future directions. Biomater. Sci. 2022;11:37–61. doi: 10.1039/D2BM01513G. [DOI] [PubMed] [Google Scholar]
- 146.Bayat Mokhtari R., Homayouni T.S., Baluch N., Morgatskaya E., Kumar S., Das B., Yeger H. Combination therapy in combating cancer. Oncotarget. 2017;8:38022–38043. doi: 10.18632/oncotarget.16723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Zhang H., Wang G., Yang H. Drug delivery systems for differential release in combination therapy. Expert Opin. Drug Deliv. 2011;8:171–190. doi: 10.1517/17425247.2011.547470. [DOI] [PubMed] [Google Scholar]
- 148.Davies L.E., Spiers G., Kingston A., Todd A., Adamson J., Hanratty B. Adverse Outcomes of Polypharmacy in Older People: Systematic Review of Reviews. J. Am. Med. Dir. Assoc. 2020;21:181–187. doi: 10.1016/j.jamda.2019.10.022. [DOI] [PubMed] [Google Scholar]
- 149.Masnoon N., Shakib S., Kalisch-Ellett L., Caughey G.E. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017;17:230. doi: 10.1186/s12877-017-0621-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sun L., Mi K., Hou Y., Hui T., Zhang L., Tao Y., Liu Z., Huang L. Pharmacokinetic and Pharmacodynamic Drug-Drug Interactions: Research Methods and Applications. Metabolites. 2023;13:897. doi: 10.3390/metabo13080897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Viscoli C., Castagnola E., Machetti M. Antifungal treatment in patients with cancer. J. Intern. Med. 1997;242:89–94. doi: 10.1111/joim.1997.242.s740.89. [DOI] [PubMed] [Google Scholar]
- 152.Nikulin S., Tonevitsky E., Poloznikov A. Effect of ketoconazole on the transport and metabolism of drugs in the human liver cell model. Russ. Chem. Bull. 2017;66:150–155. doi: 10.1007/s11172-017-1713-z. [DOI] [Google Scholar]
- 153.Svanström H., Lund M., Melbye M., Pasternak B. Concomitant use of low-dose methotrexate and NSAIDs and the risk of serious adverse events among patients with rheumatoid arthritis. Pharmacoepidemiol. Drug Saf. 2018;27:885–893. doi: 10.1002/pds.4555. [DOI] [PubMed] [Google Scholar]
- 154.Lee C.-H., Shen M.-C., Tsai M.-J., Chang J.-S., Huang Y.-B., Yang Y.-H., Hsieh K.-P. Proton pump inhibitors reduce the survival of advanced lung cancer patients with therapy of gefitinib or erlotinib. Sci. Rep. 2022;12:7002. doi: 10.1038/s41598-022-10938-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Frye R.F., Fitzgerald S.M., Lagattuta T.F., Hruska M.W., Egorin M.J. Effect of St John’s wort on imatinib mesylate pharmacokinetics. Clin. Pharmacol. Ther. 2004;76:323–329. doi: 10.1016/j.clpt.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 156.Whitman A., Erdeljac P., Jones C., Pillarella N., Nightingale G. Managing Polypharmacy in Older Adults with Cancer Across Different Healthcare Settings. Drug Heal. Patient Saf. 2021;13:101–116. doi: 10.2147/DHPS.S255893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Khezrian M., McNeil C.J., Murray A.D., Myint P.K. An overview of prevalence, determinants and health outcomes of polypharmacy. Ther. Adv. Drug Saf. 2020;11:2042098620933741. doi: 10.1177/2042098620933741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Goh I., Lai O., Chew L. Prevalence and risk of polypharmacy among elderly cancer patients receiving chemotherapy in ambulatory oncology setting. Curr. Oncol. Rep. 2018;20:38. doi: 10.1007/s11912-018-0686-x. [DOI] [PubMed] [Google Scholar]
- 159.Couderc A.L., Boisseranc C., Rey D., Nouguerede E., Greillier L., Barlesi F., Duffaud F., Deville J.L., Honore S., Villani P., et al. Medication Reconciliation Associated with Comprehensive Geriatric Assessment in Older Patients with Cancer: ChimioAge Study. Clin. Interv. Aging. 2020;15:1587–1598. doi: 10.2147/CIA.S262209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Chopra D., Rehan H.S., Sharma V., Mishra R. Chemotherapy-induced adverse drug reactions in oncology patients: A prospective observational survey. Indian. J. Med. Paediatr. Oncol. 2016;37:42–46. doi: 10.4103/0971-5851.177015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Alwhaibi M., AlRuthia Y., Alhawassi T.M., Almalag H., Alsalloum H., Balkhi B. Polypharmacy and comorbidities among ambulatory cancer patients: A cross-sectional retrospective study. J. Oncol. Pharm. Pract. 2020;26:1052–1059. doi: 10.1177/1078155219880255. [DOI] [PubMed] [Google Scholar]
- 162.Larsen M.T., Kuhlmann M., Hvam M.L., Howard K.A. Albumin-based drug delivery: Harnessing nature to cure disease. Mol. Cell Ther. 2016;4:3. doi: 10.1186/s40591-016-0048-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Munger M.A. Polypharmacy and combination therapy in the management of hypertension in elderly patients with co-morbid diabetes mellitus. Drugs Aging. 2010;27:871–883. doi: 10.2165/11538650-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 164.Mittal N., Mittal R. Repurposing old molecules for new indications: Defining pillars of success from lessons in the past. Eur. J. Pharmacol. 2021;912:174569. doi: 10.1016/j.ejphar.2021.174569. [DOI] [PubMed] [Google Scholar]
- 165.Naylor D.M., Kauppi D., Schonfeld J. Therapeutic drug repurposing, repositioning and rescue. Drug Discov. 2015;16:57–63. [Google Scholar]
- 166.Pushpakom S., Iorio F., Eyers P.A., Escott K.J., Hopper S., Wells A., Doig A., Guilliams T., Latimer J., McNamee C., et al. Drug repurposing: Progress, challenges and recommendations. Nat. Rev. Drug Discov. 2019;18:41–58. doi: 10.1038/nrd.2018.168. [DOI] [PubMed] [Google Scholar]
- 167.Parvathaneni V., Kulkarni N.S., Muth A., Gupta V. Drug repurposing: A promising tool to accelerate the drug discovery process. Drug Discov. Today. 2019;24:2076–2085. doi: 10.1016/j.drudis.2019.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Correia A.S., Gartner F., Vale N. Drug combination and repurposing for cancer therapy: The example of breast cancer. Heliyon. 2021;7:e05948. doi: 10.1016/j.heliyon.2021.e05948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Park K. A review of computational drug repurposing. Transl. Clin. Pharmacol. 2019;27:59–63. doi: 10.12793/tcp.2019.27.2.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Zhu Y., Che C., Jin B., Zhang N., Su C., Wang F. Knowledge-driven drug repurposing using a comprehensive drug knowledge graph. Health Inform. J. 2020;26:2737–2750. doi: 10.1177/1460458220937101. [DOI] [PubMed] [Google Scholar]
- 171.Al-Saleem J., Granet R., Ramakrishnan S., Ciancetta N.A., Saveson C., Gessner C., Zhou Q. Knowledge Graph-Based Approaches to Drug Repurposing for COVID-19. J. Chem. Inf. Model. 2021;61:4058–4067. doi: 10.1021/acs.jcim.1c00642. [DOI] [PubMed] [Google Scholar]
- 172.Sahoo B.M., Ravi Kumar B.V.V., Sruti J., Mahapatra M.K., Banik B.K., Borah P. Drug Repurposing Strategy (DRS): Emerging Approach to Identify Potential Therapeutics for Treatment of Novel Coronavirus Infection. Front. Mol. Biosci. 2021;8:628144. doi: 10.3389/fmolb.2021.628144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Truong M., Monahan L.G., Carter D.A., Charles I.G. Repurposing drugs to fast-track therapeutic agents for the treatment of cryptococcosis. PeerJ. 2018;6:e4761. doi: 10.7717/peerj.4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Barrett T., Suzek T.O., Troup D.B., Wilhite S.E., Ngau W.-C., Ledoux P., Rudnev D., Lash A.E., Fujibuchi W., Edgar R. NCBI GEO: Mining millions of expression profiles—Database and tools. Nucleic Acids Res. 2005;33:D562–D566. doi: 10.1093/nar/gki022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Zador Z., King A.T., Geifman N. New drug candidates for treatment of atypical meningiomas: An integrated approach using gene expression signatures for drug repurposing. PLoS ONE. 2018;13:e0194701. doi: 10.1371/journal.pone.0194701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Xu H., Aldrich M.C., Chen Q., Liu H., Peterson N.B., Dai Q., Levy M., Shah A., Han X., Ruan X., et al. Validating drug repurposing signals using electronic health records: A case study of metformin associated with reduced cancer mortality. J. Am. Med. Inf. Assoc. 2015;22:179–191. doi: 10.1136/amiajnl-2014-002649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Morsi D., Salem M.L., Ibrahim H., Osman G., Mohamed A., Nofal A. Synergistic and chemosensitizing effects of bovine lactoferrin or muramyl dipeptide in Ehrlich solid tumor-bearing mice treated with cisplatin. Int. J. Cancer Biomed. Res. 2021;5:75–94. [Google Scholar]
- 178.Brahmachari G. Discovery and Development of Neuroprotective Agents from Natural Products. Elsevier; Amsterdam, The Netherlands: 2017. [Google Scholar]
- 179.Tatiraju D.V., Bagade V.B., Karambelkar P.J., Jadhav V.M., Kadam V. Natural bioenhancers: An overview. J. Pharmacogn. Phytochem. 2013;2:55–60. [Google Scholar]
- 180.Tasneen R., Mortensen D.S., Converse P.J., Urbanowski M.E., Upton A., Fotouhi N., Nuermberger E., Hawryluk N. Dual mTORC1/mTORC2 inhibition as a host-directed therapeutic target in pathologically distinct mouse models of tuberculosis. Antimicrob. Agents Chemother. 2021;65:e0025321. doi: 10.1128/AAC.00253-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Bang J.S., Oh D.H., Choi H.M., Sur B.J., Lim S.J., Kim J.Y., Yang H.I., Yoo M.C., Hahm D.H., Kim K.S. Anti-inflammatory and antiarthritic effects of piperine in human interleukin 1beta-stimulated fibroblast-like synoviocytes and in rat arthritis models. Arthritis Res. Ther. 2009;11:R49. doi: 10.1186/ar2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Therizols G., Bash-Imam Z., Panthu B., Machon C., Vincent A., Ripoll J., Nait-Slimane S., Chalabi-Dchar M., Gaucherot A., Garcia M. Alteration of ribosome function upon 5-fluorouracil treatment favors cancer cell drug-tolerance. Nat. Commun. 2022;13:173. doi: 10.1038/s41467-021-27847-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Bhattacharjya D., Sivalingam N. Mechanism of 5-fluorouracil induced resistance and role of piperine and curcumin as chemo-sensitizers in colon cancer. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2024 doi: 10.1007/s00210-024-03189-2. [DOI] [PubMed] [Google Scholar]
- 184.Taylor C., Wang L., List A., Fernandes D., Paine-Murrieta G., Johnson C., Capizzi R. Amifostine protects normal tissues from paclitaxel toxicity while cytotoxicity against tumour cells is maintained. Eur. J. Cancer. 1997;33:1693–1698. doi: 10.1016/S0959-8049(97)00221-9. [DOI] [PubMed] [Google Scholar]
- 185.Koukourakis M.I. Amifostine in clinical oncology: Current use and future applications. Anticancer. Drugs. 2002;13:181–209. doi: 10.1097/00001813-200203000-00001. [DOI] [PubMed] [Google Scholar]
- 186.Ekborn A., Hansson J., Ehrsson H., Eksborg S., Wallin I., Wagenius G., Laurell G. High-dose Cisplatin with amifostine: Ototoxicity and pharmacokinetics. Laryngoscope. 2004;114:1660–1667. doi: 10.1097/00005537-200409000-00030. [DOI] [PubMed] [Google Scholar]
- 187.Fox E., Bates S.E. Tariquidar (XR9576): A P-glycoprotein drug efflux pump inhibitor. Expert Rev. Anticancer Ther. 2007;7:447–459. doi: 10.1586/14737140.7.4.447. [DOI] [PubMed] [Google Scholar]
- 188.Matzneller P., Kussmann M., Eberl S., Maier-Salamon A., Jäger W., Bauer M., Langer O., Zeitlinger M., Poeppl W. Pharmacokinetics of the P-gp inhibitor tariquidar in rats after intravenous, oral, and intraperitoneal administration. Eur. J. Drug Metab. Pharmacokinet. 2018;43:599–606. doi: 10.1007/s13318-018-0474-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Kim C.H., Lee T.H., Kim B.D., Kim H.K., Lyu M.J., Jung H.M., Goo Y.T., Kang M.J., Lee S., Choi Y.W. Co-administration of tariquidar using functionalized nanostructured lipid carriers overcomes resistance to docetaxel in multidrug resistant MCF7/ADR cells. J. Drug Deliv. Sci. Technol. 2022;71:103323. doi: 10.1016/j.jddst.2022.103323. [DOI] [Google Scholar]
- 190.Patel N.R., Rathi A., Mongayt D., Torchilin V.P. Reversal of multidrug resistance by co-delivery of tariquidar (XR9576) and paclitaxel using long-circulating liposomes. Int. J. Pharm. 2011;416:296–299. doi: 10.1016/j.ijpharm.2011.05.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Grixti J.M., O’Hagan S., Day P.J., Kell D.B. Enhancing drug efficacy and therapeutic index through cheminformatics-based selection of small molecule binary weapons that improve transporter-mediated targeting: A cytotoxicity system based on gemcitabine. Front. Pharmacol. 2017;8:155. doi: 10.3389/fphar.2017.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Rollando R., Maulada F., Afthoni M.H., Monica E., Yuniati Y., Nugraha A.T. Screening Carica Papaya Compounds as an Antimalarial Agent: In Silico Study. Trop. J. Nat. Prod. Res. 2023;7:2895–2903. [Google Scholar]
- 193.Stojanović-Radić Z., Pejčić M., Dimitrijević M., Aleksić A., V Anil Kumar N., Salehi B., C Cho W., Sharifi-Rad J. Piperine-a major principle of black pepper: A review of its bioactivity and studies. Appl. Sci. 2019;9:4270. doi: 10.3390/app9204270. [DOI] [Google Scholar]
- 194.Kasibhatta R., Naidu M. Influence of piperine on the pharmacokinetics of nevirapine under fasting conditions: A randomised, crossover, placebo-controlled study. Drugs R. D. 2007;8:383–391. doi: 10.2165/00126839-200708060-00006. [DOI] [PubMed] [Google Scholar]
- 195.Bezerra D.P., de Castro F.O., Alves A.P., Pessoa C., de Moraes M.O., Silveira E.R., Lima M.A., Elmiro F.J., de Alencar N.M., Mesquita R.O., et al. In vitro and in vivo antitumor effect of 5-FU combined with piplartine and piperine. J. Appl. Toxicol. 2008;28:156–163. doi: 10.1002/jat.1261. [DOI] [PubMed] [Google Scholar]
- 196.Culy C.R., Spencer C.M. Amifostine: An update on its clinical status as a cytoprotectant in patients with cancer receiving chemotherapy or radiotherapy and its potential therapeutic application in myelodysplastic syndrome. Drugs. 2001;61:641–684. doi: 10.2165/00003495-200161050-00012. [DOI] [PubMed] [Google Scholar]
- 197.King M., Joseph S., Albert A., Thomas T.V., Nittala M.R., Woods W.C., Vijayakumar S., Packianathan S. Use of amifostine for cytoprotection during radiation therapy: A review. Oncology. 2020;98:61–80. doi: 10.1159/000502979. [DOI] [PubMed] [Google Scholar]
- 198.Ibrahim H.M., Mohamed A.H., Salem M.L., Osman G.Y., Morsi D.S. Anti-neoplastic and immunomodulatory potency of co-treatment based on bovine lactoferrin and/or muramyl dipeptide in tumor-bearing mice. Toxicol. Res. 2020;9:137–147. doi: 10.1093/toxres/tfaa012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Oh Y., Lee J.S., Lee J.S., Park J.H., Kim H.S., Yoon S. JAK2 Inhibitor, Fedratinib, Inhibits P-gp Activity and Co-Treatment Induces Cytotoxicity in Antimitotic Drug-Treated P-gp Overexpressing Resistant KBV20C Cancer Cells. Int. J. Mol. Sci. 2022;23:4597. doi: 10.3390/ijms23094597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Guzman M.L., Swiderski C.F., Howard D.S., Grimes B.A., Rossi R.M., Szilvassy S.J., Jordan C.T. Preferential induction of apoptosis for primary human leukemic stem cells. Proc. Natl. Acad. Sci. USA. 2002;99:16220–16225. doi: 10.1073/pnas.252462599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Rodrigues A.C.B.d.C., Costa R.G., Silva S.L., Dias I.R., Dias R.B., Bezerra D.P. Cell signaling pathways as molecular targets to eliminate AML stem cells. Crit. Rev. Oncol. /Hematol. 2021;160:103277. doi: 10.1016/j.critrevonc.2021.103277. [DOI] [PubMed] [Google Scholar]
- 202.Ghosh S., Singh R., Vanwinkle Z.M., Guo H., Vemula P.K., Goel A., Haribabu B., Jala V.R. Microbial metabolite restricts 5-fluorouracil-resistant colonic tumor progression by sensitizing drug transporters via regulation of FOXO3-FOXM1 axis. Theranostics. 2022;12:5574–5595. doi: 10.7150/thno.70754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Wilhelmsen N.C., Eriksson T. Medication adherence interventions and outcomes: An overview of systematic reviews. Eur. J. Hosp. Pharm. 2019;26:187–192. doi: 10.1136/ejhpharm-2018-001725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Granger B.B., Swedberg K., Ekman I., Granger C.B., Olofsson B., McMurray J.J., Yusuf S., Michelson E.L., Pfeffer M.A., CHARM investigators Adherence to candesartan and placebo and outcomes in chronic heart failure in the CHARM programme: Double-blind, randomised, controlled clinical trial. Lancet. 2005;366:2005–2011. doi: 10.1016/S0140-6736(05)67760-4. [DOI] [PubMed] [Google Scholar]
- 205.Tjelle K., Opstad H.B., Solem S., Launes G., Hansen B., Kvale G., Hagen K. Treatment adherence as predictor of outcome in concentrated exposure treatment for obsessive-compulsive disorder. Front. Psychiatry. 2021;12:667167. doi: 10.3389/fpsyt.2021.667167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Baryakova T.H., Pogostin B.H., Langer R., McHugh K.J. Overcoming barriers to patient adherence: The case for developing innovative drug delivery systems. Nat. Rev. Drug Discov. 2023;22:387–409. doi: 10.1038/s41573-023-00670-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Isaac M., Holvey C. Transdermal patches: The emerging mode of drug delivery system in psychiatry. Ther. Adv. Psychopharmacol. 2012;2:255–263. doi: 10.1177/2045125312458311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Badwan B.A., Liaropoulos G., Kyrodimos E., Skaltsas D., Tsirigos A., Gorgoulis V.G. Machine learning approaches to predict drug efficacy and toxicity in oncology. Cell Rep. Methods. 2023;3:100413. doi: 10.1016/j.crmeth.2023.100413. [DOI] [PMC free article] [PubMed] [Google Scholar]