Abstract
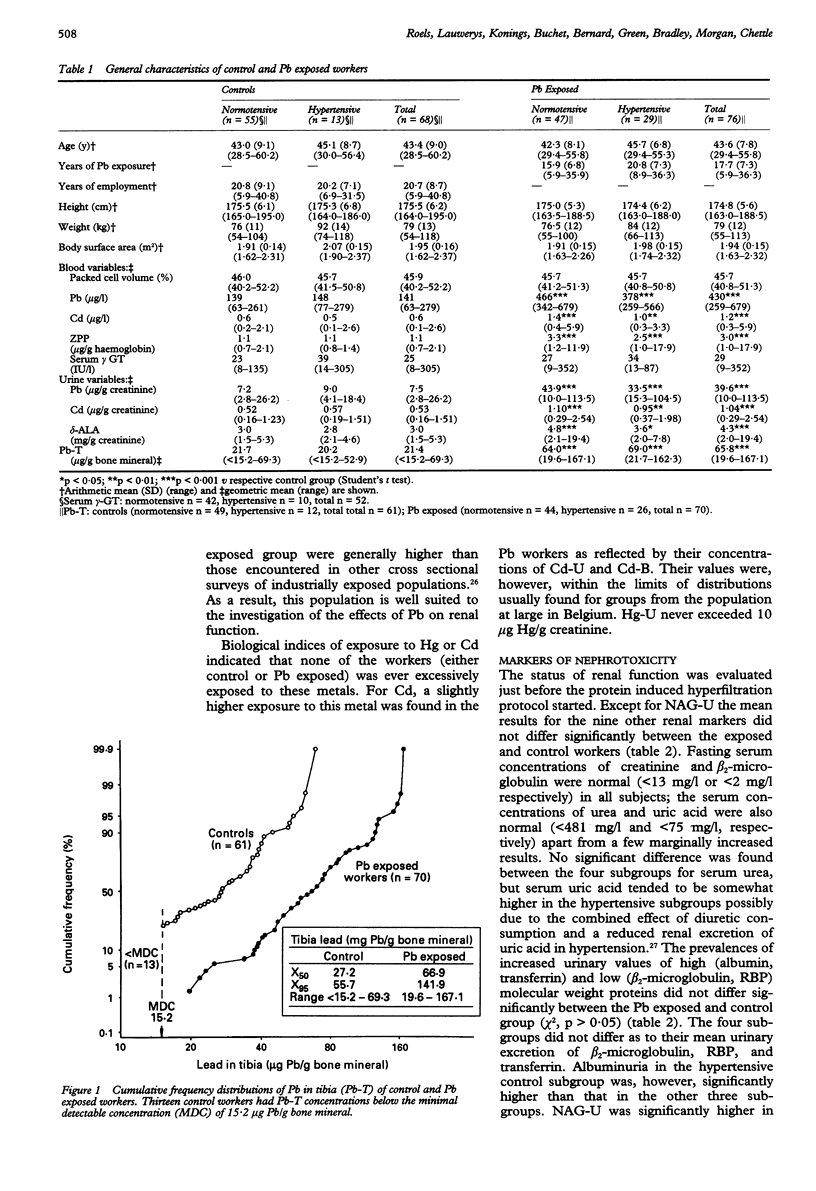
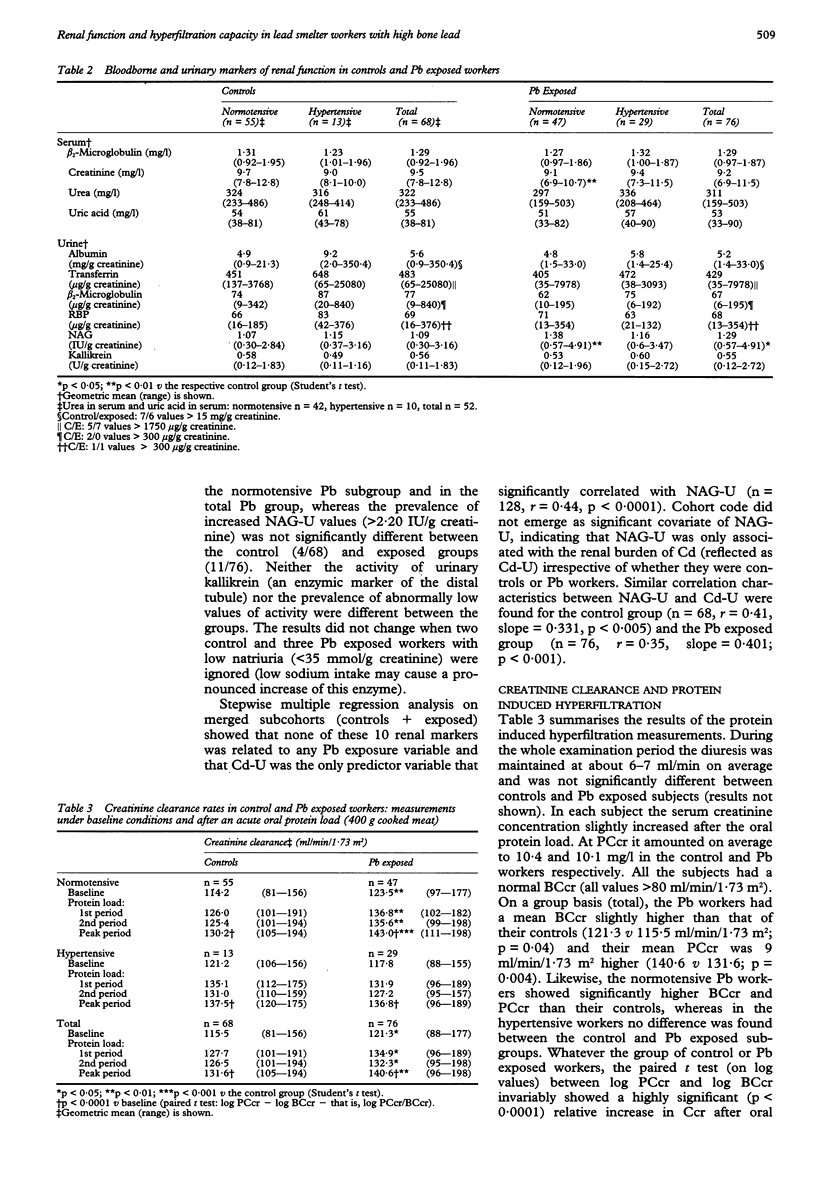
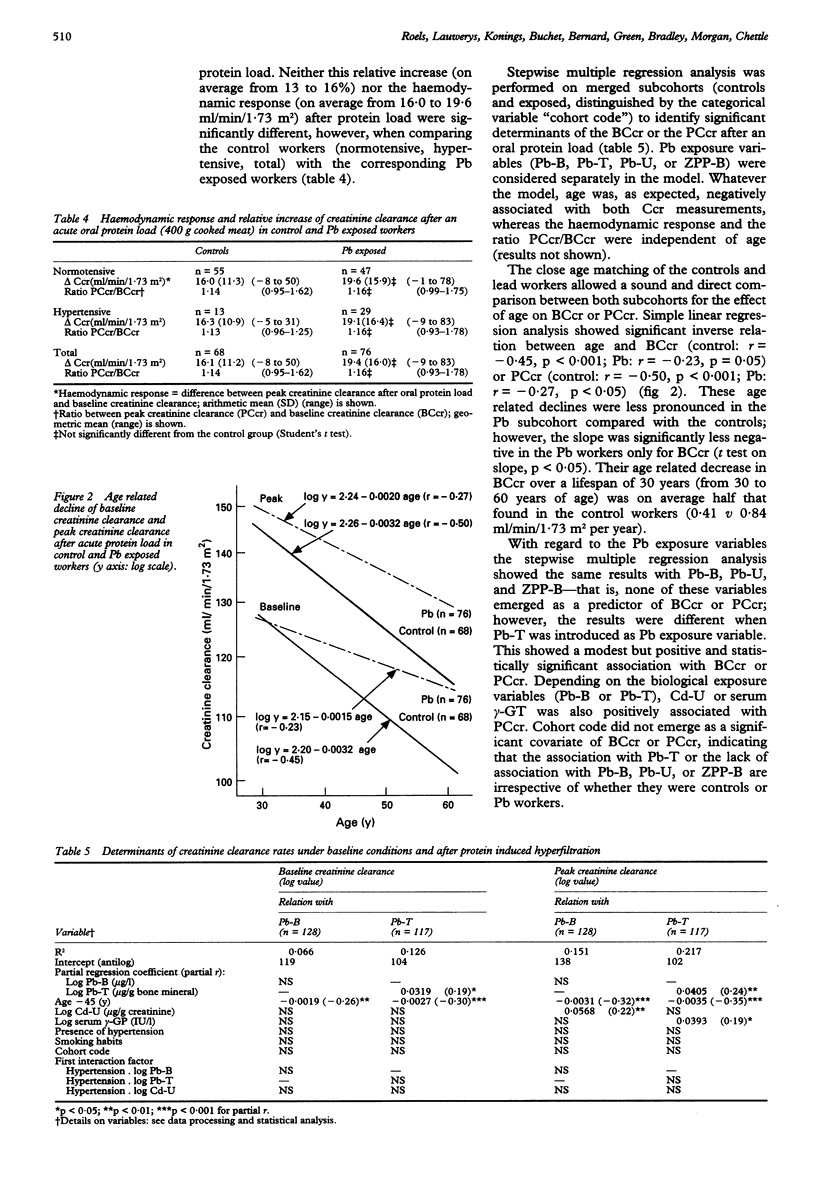
OBJECTIVE--The study was undertaken to assess whether the changes in urinary excretion of eicosanoids (a decrease of 6-keto-PGF1 alpha and PGF2 and an increase of thromboxane) previously found in lead (Pb) exposed workers may decrease the renal haemodynamic response to an acute oral protein load. METHODS--The renal haemodynamic response was estimated by determining the capacity of the kidney to increase the glomerular filtration rate (in terms of creatinine clearance) after an acute consumption of cooked red meat (400 g). A cross sectional study was carried out in 76 male Pb workers (age range 30 to 60 years) and 68 controls matched for age, sex, socioeconomic state, general environment (residence), and workshift characteristics. RESULTS--The Pb workers had been exposed to lead on average for 18 (range 6-36) years and showed a threefold higher body burden of Pb than the controls as estimated by in vivo measurements of tibial Pb concentration (Pb-T) (geometric mean 66 v 21 micrograms Pb/g bone mineral). The geometric mean concentrations of Pb in blood (Pb-B) and Pb in urine (Pb-U) were also significantly higher in the Pb group (Pb-B: 430 v 141 micrograms Pb/l; Pb-U: 40 v 7.5 micrograms Pb/g creatinine). These conditions of chronic exposure to Pb did not entail any significant changes in the concentration of blood borne and urinary markers of nephrotoxicity, such as urinary low and high molecular weight plasma derived proteins (beta 2-microglobulin, retinol binding protein, albumin, transferrin), urinary activities of N-acetyl-beta-D-glucosaminidase and kallikrein, and serum concentrations of creatinine, beta 2-microglobulin, urea, and uric acid. All participants also had normal baseline creatinine clearances (> 80 ml/min/1.73 m2) amounting on average to 115.5 in the controls v 121.3 ml/min/1.73 m2 in the Pb group. Both control and Pb exposed workers showed a significant increment in creatinine clearance (on average 15%) after oral protein load suggesting that the previously found changes in secretion of urinary eicosanoids apparently has no deleterious effect on renal haemodynamics in the examined Pb workers. CONCLUSIONS--The finding that both baseline and stimulated creatinine clearance rates were not only significantly higher in the Pb workers but also positively correlated with Pb-T, suggests that moderate exposure to Pb may be associated with a slight hyperfiltration state, which has been found to attenuate the age related decline in baseline creatinine clearance by a factor of two. Although the relevance of this effect for the worker's health is unknown, it can be concluded that adverse renal changes are unlikely to occur in most adult male Pb workers when their blood Pb concentration is regularly kept below 700 micrograms Pb/l. One should, however, be cautious in extra-polating this conclusion to the general population because of pre-employment screening of the Pb workers for the absence of renal risk factors.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett W. M. Lead nephropathy. Kidney Int. 1985 Aug;28(2):212–220. doi: 10.1038/ki.1985.143. [DOI] [PubMed] [Google Scholar]
- Bernard A. M., Lauwerys R. R. Continuous-flow system for automation of latex immunoassay by particle counting. Clin Chem. 1983 Jun;29(6):1007–1011. [PubMed] [Google Scholar]
- Bosch J. P., Lauer A., Glabman S. Short-term protein loading in assessment of patients with renal disease. Am J Med. 1984 Nov;77(5):873–879. doi: 10.1016/0002-9343(84)90529-1. [DOI] [PubMed] [Google Scholar]
- Bosch J. P., Saccaggi A., Lauer A., Ronco C., Belledonne M., Glabman S. Renal functional reserve in humans. Effect of protein intake on glomerular filtration rate. Am J Med. 1983 Dec;75(6):943–950. doi: 10.1016/0002-9343(83)90873-2. [DOI] [PubMed] [Google Scholar]
- Buchet J. P., Roels H., Bernard A., Lauwerys R. Assessment of renal function of workers exposed to inorganic lead, calcium or mercury vapor. J Occup Med. 1980 Nov;22(11):741–750. [PubMed] [Google Scholar]
- Chettle D. R., Scott M. C., Somervaille L. J. Lead in bone: sampling and quantitation using K X-rays excited by 109Cd. Environ Health Perspect. 1991 Feb;91:49–55. doi: 10.1289/ehp.919149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper W. C. Deaths from chronic renal disease in U.S. battery and lead production workers. Environ Health Perspect. 1988 Jun;78:61–63. doi: 10.1289/ehp.887861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cárdenas A., Roels H., Bernard A. M., Barbon R., Buchet J. P., Lauwerys R. R., Roselló J., Ramis I., Mutti A., Franchini I. Markers of early renal changes induced by industrial pollutants. II. Application to workers exposed to lead. Br J Ind Med. 1993 Jan;50(1):28–36. doi: 10.1136/oem.50.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gennart J. P., Bernard A., Lauwerys R. Assessment of thyroid, testes, kidney and autonomic nervous system function in lead-exposed workers. Int Arch Occup Environ Health. 1992;64(1):49–57. doi: 10.1007/BF00625951. [DOI] [PubMed] [Google Scholar]
- Gerhardsson L., Attewell R., Chettle D. R., Englyst V., Lundström N. G., Nordberg G. F., Nyhlin H., Scott M. C., Todd A. C. In vivo measurements of lead in bone in long-term exposed lead smelter workers. Arch Environ Health. 1993 May-Jun;48(3):147–156. doi: 10.1080/00039896.1993.9940813. [DOI] [PubMed] [Google Scholar]
- Gerhardsson L., Chettle D. R., Englyst V., Nordberg G. F., Nyhlin H., Scott M. C., Todd A. C., Vesterberg O. Kidney effects in long term exposed lead smelter workers. Br J Ind Med. 1992 Mar;49(3):186–192. doi: 10.1136/oem.49.3.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green S., Bradley D. A., Palethorpe J. E., Mearman D., Chettle D. R., Lewis A. D., Mountford P. J., Morgan W. D. An enhanced sensitivity K-shell x-ray fluorescence technique for tibial lead determination. Phys Med Biol. 1993 Mar;38(3):389–396. doi: 10.1088/0031-9155/38/3/006. [DOI] [PubMed] [Google Scholar]
- Green S., Bradley D. A., Roels H. A., Mountford P. J., Morgan W. D., Chettle D. R., Konings J. F., Palethorpe J. E., Mearman D. H., Lauwerys R. R. Development and calibration of an in vivo bone lead measurement system, and its application to an industrially exposed population. Basic Life Sci. 1993;60:295–298. doi: 10.1007/978-1-4899-1268-8_65. [DOI] [PubMed] [Google Scholar]
- Hu H. A 50-year follow-up of childhood plumbism. Hypertension, renal function, and hemoglobin levels among survivors. Am J Dis Child. 1991 Jun;145(6):681–687. doi: 10.1001/archpedi.1991.02160060099029. [DOI] [PubMed] [Google Scholar]
- Huang J. X., He F. S., Wu Y. G., Zhang S. C. Observations on renal function in workers exposed to lead. Sci Total Environ. 1988 Jun 1;71(3):535–537. doi: 10.1016/0048-9697(88)90230-6. [DOI] [PubMed] [Google Scholar]
- Khalil-Manesh F., Gonick H. C., Cohen A. H., Alinovi R., Bergamaschi E., Mutti A., Rosen V. J. Experimental model of lead nephropathy. I. Continuous high-dose lead administration. Kidney Int. 1992 May;41(5):1192–1203. doi: 10.1038/ki.1992.181. [DOI] [PubMed] [Google Scholar]
- Koster J., Erhardt A., Stoeppler M., Mohl C., Ritz E. Mobilizable lead in patients with chronic renal failure. Eur J Clin Invest. 1989 Apr;19(2):228–233. doi: 10.1111/j.1365-2362.1989.tb00222.x. [DOI] [PubMed] [Google Scholar]
- Lauwerys R., Delbroeck R., Vens M. D. Automated analysis of delta-aminolaevulinic acid in urine. Clin Chim Acta. 1972 Sep;40(2):443–447. doi: 10.1016/0009-8981(72)90356-7. [DOI] [PubMed] [Google Scholar]
- Levine M. M., Kirschenbaum M. A., Chaudhari A., Wong M. W., Bricker N. S. Effect of protein on glomerular filtration rate and prostanoid synthesis in normal and uremic rats. Am J Physiol. 1986 Oct;251(4 Pt 2):F635–F641. doi: 10.1152/ajprenal.1986.251.4.F635. [DOI] [PubMed] [Google Scholar]
- Maggioni A. P., Franzosi M. G., Santoro E., White H., Van de Werf F., Tognoni G. The risk of stroke in patients with acute myocardial infarction after thrombolytic and antithrombotic treatment. Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto Miocardico II (GISSI-2), and The International Study Group. N Engl J Med. 1992 Jul 2;327(1):1–6. doi: 10.1056/NEJM199207023270101. [DOI] [PubMed] [Google Scholar]
- Magos L., Clarkson T. W. Atomic absorption determination of total, inorganic, and organic mercury in blood. J Assoc Off Anal Chem. 1972 Sep;55(5):966–971. [PubMed] [Google Scholar]
- Meyer B. R., Fischbein A., Rosenman K., Lerman Y., Drayer D. E., Reidenberg M. M. Increased urinary enzyme excretion in workers exposed to nephrotoxic chemicals. Am J Med. 1984 Jun;76(6):989–998. doi: 10.1016/0002-9343(84)90847-7. [DOI] [PubMed] [Google Scholar]
- Nilsson U., Attewell R., Christoffersson J. O., Schütz A., Ahlgren L., Skerfving S., Mattsson S. Kinetics of lead in bone and blood after end of occupational exposure. Pharmacol Toxicol. 1991 Jun;68(6):477–484. doi: 10.1111/j.1600-0773.1991.tb01273.x. [DOI] [PubMed] [Google Scholar]
- Omae K., Sakurai H., Higashi T., Muto T., Ichikawa M., Sasaki N. No adverse effects of lead on renal function in lead-exposed workers. Ind Health. 1990;28(2):77–83. doi: 10.2486/indhealth.28.77. [DOI] [PubMed] [Google Scholar]
- Roels H. A., Lauwerys R. R., Bernard A. M., Buchet J. P., Vos A., Oversteyns M. Assessment of the filtration reserve capacity of the kidney in workers exposed to cadmium. Br J Ind Med. 1991 Jun;48(6):365–374. doi: 10.1136/oem.48.6.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roels H. A., Lauwerys R. R., Buchet J. P., Bernard A. M., Lijnen P., Van Houte G. Urinary kallikrein activity in workers exposed to cadmium, lead, or mercury vapour. Br J Ind Med. 1990 May;47(5):331–337. doi: 10.1136/oem.47.5.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roels H., Abdeladim S., Braun M., Malchaire J., Lauwerys R. Detection of hand tremor in workers exposed to mercury vapor: a comparative study of three methods. Environ Res. 1989 Aug;49(2):152–165. doi: 10.1016/s0013-9351(89)80060-x. [DOI] [PubMed] [Google Scholar]
- Roels H., Abdeladim S., Ceulemans E., Lauwerys R. Relationships between the concentrations of mercury in air and in blood or urine in workers exposed to mercury vapour. Ann Occup Hyg. 1987;31(2):135–145. doi: 10.1093/annhyg/31.2.135. [DOI] [PubMed] [Google Scholar]
- Roels H., Van de Voorde R., Vargas V. M., Lauwerys R. Relationship between atmospheric and urinary nickel in workers manufacturing electrical resistances using nickel oxide: role of the bioavailability of nickel. Occup Med (Lond) 1993 May;43(2):95–104. doi: 10.1093/occmed/43.2.95. [DOI] [PubMed] [Google Scholar]
- Rosenberg M. E., Swanson J. E., Thomas B. L., Hostetter T. H. Glomerular and hormonal responses to dietary protein intake in human renal disease. Am J Physiol. 1987 Dec;253(6 Pt 2):F1083–F1090. doi: 10.1152/ajprenal.1987.253.6.F1083. [DOI] [PubMed] [Google Scholar]
- Selevan S. G., Landrigan P. J., Stern F. B., Jones J. H. Mortality of lead smelter workers. Am J Epidemiol. 1985 Oct;122(4):673–683. doi: 10.1093/oxfordjournals.aje.a114146. [DOI] [PubMed] [Google Scholar]
- Slemenda C. W., Christian J. C., Reed T., Reister T. K., Williams C. J., Johnston C. C., Jr Long-term bone loss in men: effects of genetic and environmental factors. Ann Intern Med. 1992 Aug 15;117(4):286–291. doi: 10.7326/0003-4819-117-4-286. [DOI] [PubMed] [Google Scholar]
- Tucker S. M., Boyd P. J., Thompson A. E., Price R. G. Automated assay of N-acetyl-beta-glucosaminidase in normal and pathological human urine. Clin Chim Acta. 1975 Jul 23;62(2):333–339. doi: 10.1016/0009-8981(75)90245-4. [DOI] [PubMed] [Google Scholar]
- Vanrenterghem Y. F., Verberckmoes R. K., Roels L. M., Michielsen P. J. Role of prostaglandins in protein-induced glomerular hyperfiltration in normal humans. Am J Physiol. 1988 Apr;254(4 Pt 2):F463–F469. doi: 10.1152/ajprenal.1988.254.4.F463. [DOI] [PubMed] [Google Scholar]
- Verschoor M., Wibowo A., Herber R., van Hemmen J., Zielhuis R. Influence of occupational low-level lead exposure on renal parameters. Am J Ind Med. 1987;12(4):341–351. doi: 10.1002/ajim.4700120402. [DOI] [PubMed] [Google Scholar]
- Wittmers L. E., Jr, Aufderheide A. C., Wallgren J., Rapp G., Jr, Alich A. Lead in bone. IV. Distribution of lead in the human skeleton. Arch Environ Health. 1988 Nov-Dec;43(6):381–391. doi: 10.1080/00039896.1988.9935855. [DOI] [PubMed] [Google Scholar]
- Zinterhofer L. J., Jatlow P. I., Fappiano A. Atomic absorption determination of lead in blood and urine in the presence of EDTA. J Lab Clin Med. 1971 Oct;78(4):664–674. [PubMed] [Google Scholar]


