Abstract
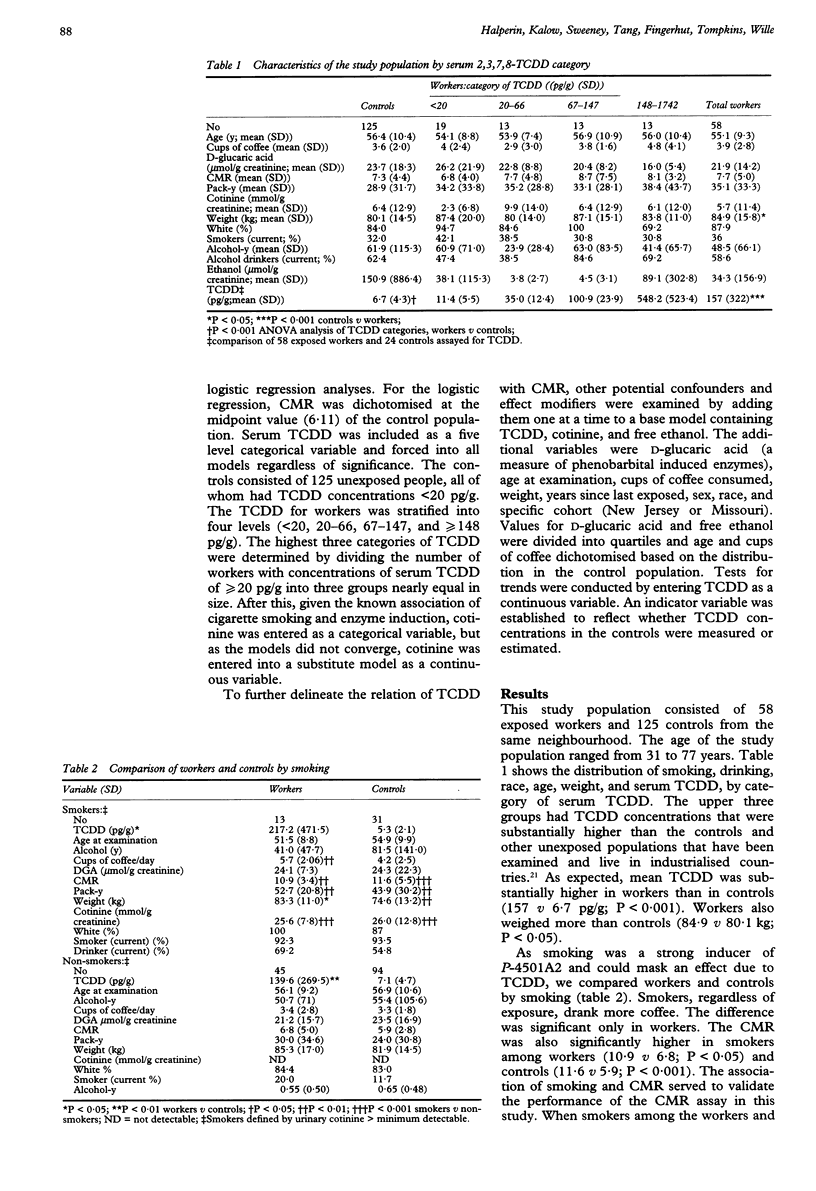
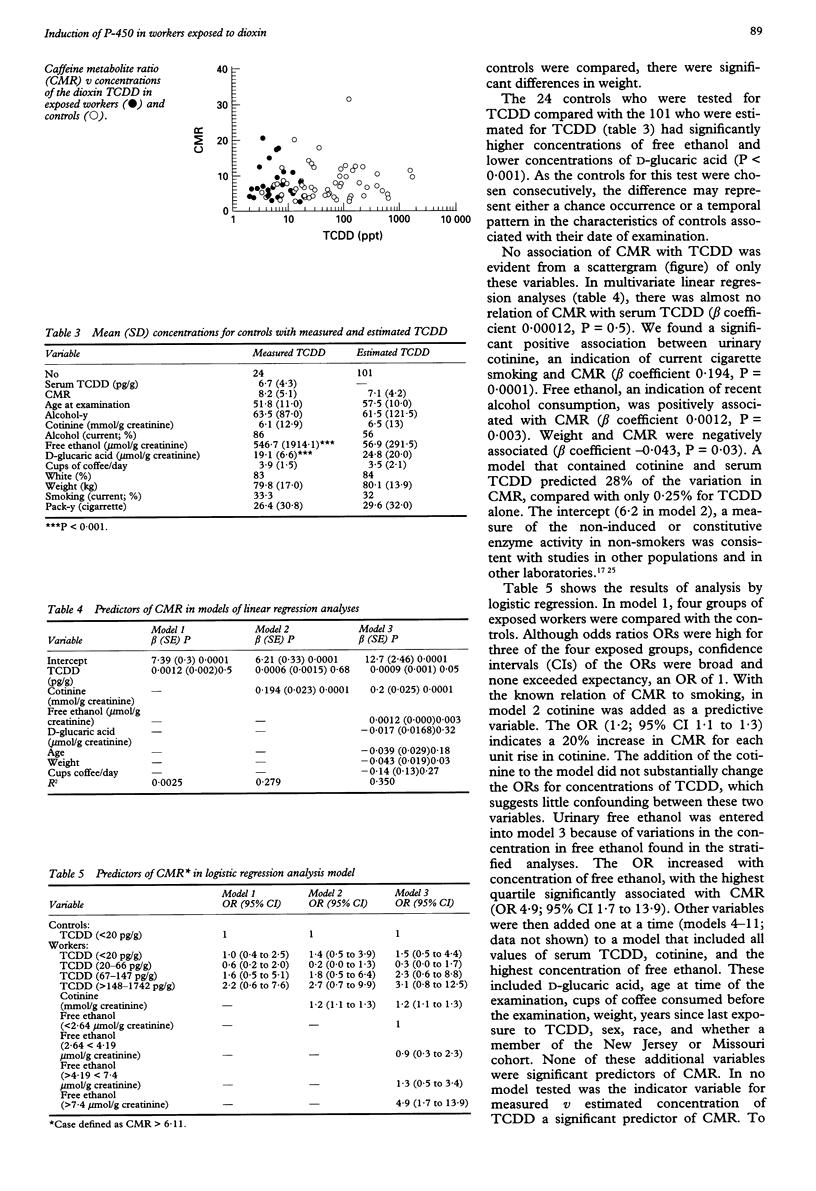
OBJECTIVES--To examine the effects of occupational exposure to substances contaminated with 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) on cytochrome P-4501A2 activity in a cross sectional medical survey. METHODS--The exposed workers had been employed at two chemical plants > 15 years earlier in the manufacture of 2,4, 5-trichlorophenol and its derivatives. The control group consisted of people with no occupational exposure to phenoxy herbicides and who lived within the communities of the exposed workers. A total of 58 workers and 125 unexposed controls participated in the analysis. Cytochrome P-450 activity was assessed with test that measures caffeine metabolites in the urine. A ratio of metabolites of caffeine (CMR) constituted a measure of P-4501A2 activity. RESULTS--Compared with the control group in multivariate logistic regression, raised non-significant associations were found for three of four categories of TCDD in exposed workers (TCDD < 20 pg/g, odds ratio (OR) 1.7, 95% confidence interval (95% CI) 0.6 to 5.0, TCDD 20-66, OR 0.3, 95% CI 0.0 to 1.7; TCDD 67-147, OR 2.3, 95% CI 0.6 to 8.8; TCDD > or = 148, OR 3.1, 95% CI 0.8 to 12.5). We found a strongly significant association of CMR and urinary cotinine, a measure of smoking, and urinary free ethanol. We found weak non-significant associations between P-4501A2 activity and increased serum TCDD among workers. CONCLUSIONS--The absence of an association between serum TCDD and cytochrome P-4501A2 may be due to the size of the study, insensitivity of the CMR to assess cytochrome P-4501A2 activity, or inadequate levels of exposure, although these were among the highest in human groups tested.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Butler M. A., Iwasaki M., Guengerich F. P., Kadlubar F. F. Human cytochrome P-450PA (P-450IA2), the phenacetin O-deethylase, is primarily responsible for the hepatic 3-demethylation of caffeine and N-oxidation of carcinogenic arylamines. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7696–7700. doi: 10.1073/pnas.86.20.7696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler M. A., Lang N. P., Young J. F., Caporaso N. E., Vineis P., Hayes R. B., Teitel C. H., Massengill J. P., Lawsen M. F., Kadlubar F. F. Determination of CYP1A2 and NAT2 phenotypes in human populations by analysis of caffeine urinary metabolites. Pharmacogenetics. 1992 Jun;2(3):116–127. doi: 10.1097/00008571-199206000-00003. [DOI] [PubMed] [Google Scholar]
- Campbell M. E., Grant D. M., Inaba T., Kalow W. Biotransformation of caffeine, paraxanthine, theophylline, and theobromine by polycyclic aromatic hydrocarbon-inducible cytochrome(s) P-450 in human liver microsomes. Drug Metab Dispos. 1987 Mar-Apr;15(2):237–249. [PubMed] [Google Scholar]
- Campbell M. E., Spielberg S. P., Kalow W. A urinary metabolite ratio that reflects systemic caffeine clearance. Clin Pharmacol Ther. 1987 Aug;42(2):157–165. doi: 10.1038/clpt.1987.126. [DOI] [PubMed] [Google Scholar]
- Fingerhut M. A., Halperin W. E., Marlow D. A., Piacitelli L. A., Honchar P. A., Sweeney M. H., Greife A. L., Dill P. A., Steenland K., Suruda A. J. Cancer mortality in workers exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin. N Engl J Med. 1991 Jan 24;324(4):212–218. doi: 10.1056/NEJM199101243240402. [DOI] [PubMed] [Google Scholar]
- Grant D. M., Tang B. K., Kalow W. A simple test for acetylator phenotype using caffeine. Br J Clin Pharmacol. 1984 Apr;17(4):459–464. doi: 10.1111/j.1365-2125.1984.tb02372.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu L., Gonzalez F. J., Kalow W., Tang B. K. Biotransformation of caffeine, paraxanthine, theobromine and theophylline by cDNA-expressed human CYP1A2 and CYP2E1. Pharmacogenetics. 1992 Apr;2(2):73–77. doi: 10.1097/00008571-199204000-00004. [DOI] [PubMed] [Google Scholar]
- Ikeya K., Jaiswal A. K., Owens R. A., Jones J. E., Nebert D. W., Kimura S. Human CYP1A2: sequence, gene structure, comparison with the mouse and rat orthologous gene, and differences in liver 1A2 mRNA expression. Mol Endocrinol. 1989 Sep;3(9):1399–1408. doi: 10.1210/mend-3-9-1399. [DOI] [PubMed] [Google Scholar]
- Kalow W. Genetic variation in the human hepatic cytochrome P-450 system. Eur J Clin Pharmacol. 1987;31(6):633–641. doi: 10.1007/BF00541288. [DOI] [PubMed] [Google Scholar]
- Kalow W., Tang B. K. The use of caffeine for enzyme assays: a critical appraisal. Clin Pharmacol Ther. 1993 May;53(5):503–514. doi: 10.1038/clpt.1993.63. [DOI] [PubMed] [Google Scholar]
- Kalow W., Tang B. K. Use of caffeine metabolite ratios to explore CYP1A2 and xanthine oxidase activities. Clin Pharmacol Ther. 1991 Nov;50(5 Pt 1):508–519. doi: 10.1038/clpt.1991.176. [DOI] [PubMed] [Google Scholar]
- Lambert G. H., Schoeller D. A., Humphrey H. E., Kotake A. N., Lietz H., Campbell M., Kalow W., Spielberg S. P., Budd M. The caffeine breath test and caffeine urinary metabolite ratios in the Michigan cohort exposed to polybrominated biphenyls: a preliminary study. Environ Health Perspect. 1990 Nov;89:175–181. doi: 10.1289/ehp.9089175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okey A. B. Enzyme induction in the cytochrome P-450 system. Pharmacol Ther. 1990;45(2):241–298. doi: 10.1016/0163-7258(90)90030-6. [DOI] [PubMed] [Google Scholar]
- Okey A. B., Roberts E. A., Harper P. A., Denison M. S. Induction of drug-metabolizing enzymes: mechanisms and consequences. Clin Biochem. 1986 Apr;19(2):132–141. doi: 10.1016/s0009-9120(86)80060-1. [DOI] [PubMed] [Google Scholar]
- Patterson D. G., Jr, Fingerhut M. A., Roberts D. W., Needham L. L., Sweeney M. H., Marlow D. A., Andrews J. S., Jr, Halperin W. E. Levels of polychlorinated dibenzo-p-dioxins and dibenzofurans in workers exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Am J Ind Med. 1989;16(2):135–146. doi: 10.1002/ajim.4700160205. [DOI] [PubMed] [Google Scholar]
- Poland A., Glover E. Chlorinated dibenzo-p-dioxins: potent inducers of delta-aminolevulinic acid synthetase and aryl hydrocarbon hydroxylase. II. A study of the structure-activity relationship. Mol Pharmacol. 1973 Nov;9(6):736–747. [PubMed] [Google Scholar]
- Poland A., Knutson J. C. 2,3,7,8-tetrachlorodibenzo-p-dioxin and related halogenated aromatic hydrocarbons: examination of the mechanism of toxicity. Annu Rev Pharmacol Toxicol. 1982;22:517–554. doi: 10.1146/annurev.pa.22.040182.002505. [DOI] [PubMed] [Google Scholar]
- Sherson D., Sigsgaard T., Overgaard E., Loft S., Poulsen H. E., Jongeneelen F. J. Interaction of smoking, uptake of polycyclic aromatic hydrocarbons, and cytochrome P450IA2 activity among foundry workers. Br J Ind Med. 1992 Mar;49(3):197–202. doi: 10.1136/oem.49.3.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silbergeld E. K., Gasiewicz T. A. Dioxins and the Ah receptor. Am J Ind Med. 1989;16(4):455–474. doi: 10.1002/ajim.4700160411. [DOI] [PubMed] [Google Scholar]
- Tang B. K., Kadar D., Kalow W. An alternative test for acetylator phenotyping with caffeine. Clin Pharmacol Ther. 1987 Nov;42(5):509–513. doi: 10.1038/clpt.1987.189. [DOI] [PubMed] [Google Scholar]
- Vistisen K., Poulsen H. E., Loft S. Foreign compound metabolism capacity in man measured from metabolites of dietary caffeine. Carcinogenesis. 1992 Sep;13(9):1561–1568. doi: 10.1093/carcin/13.9.1561. [DOI] [PubMed] [Google Scholar]


