Abstract
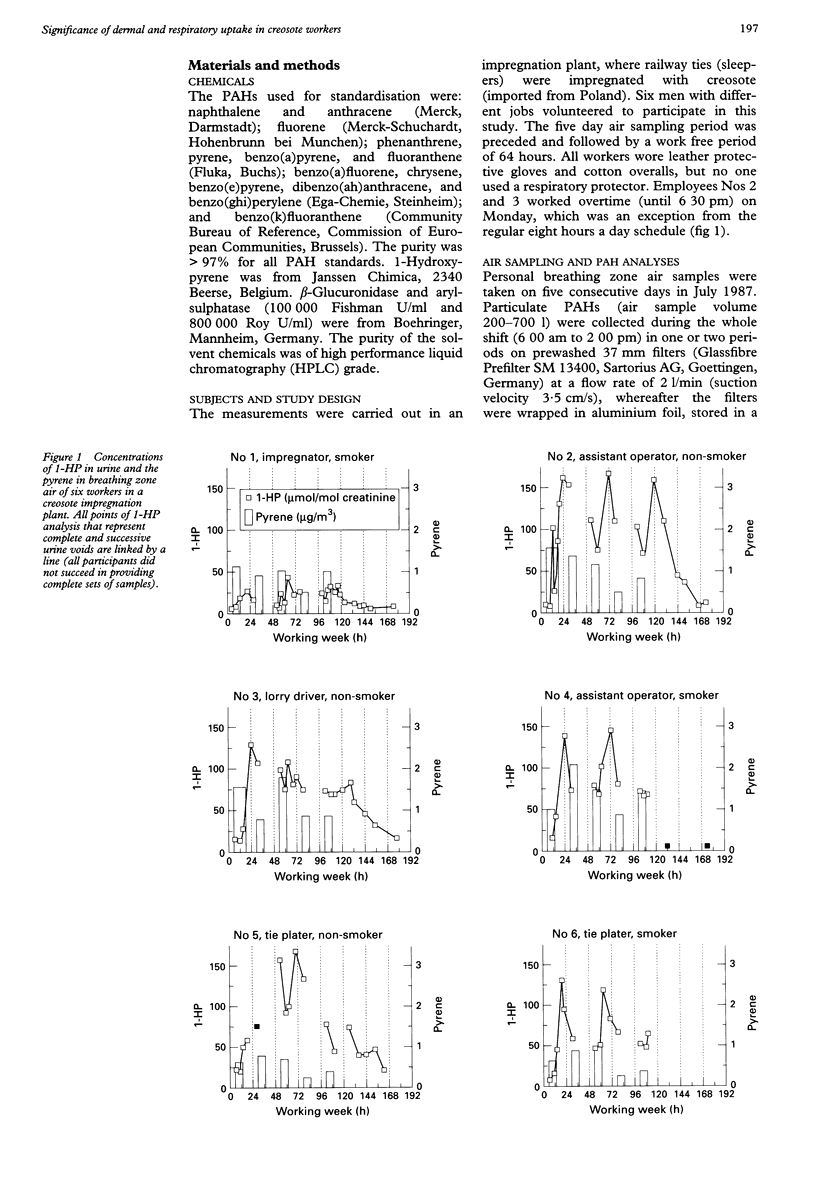
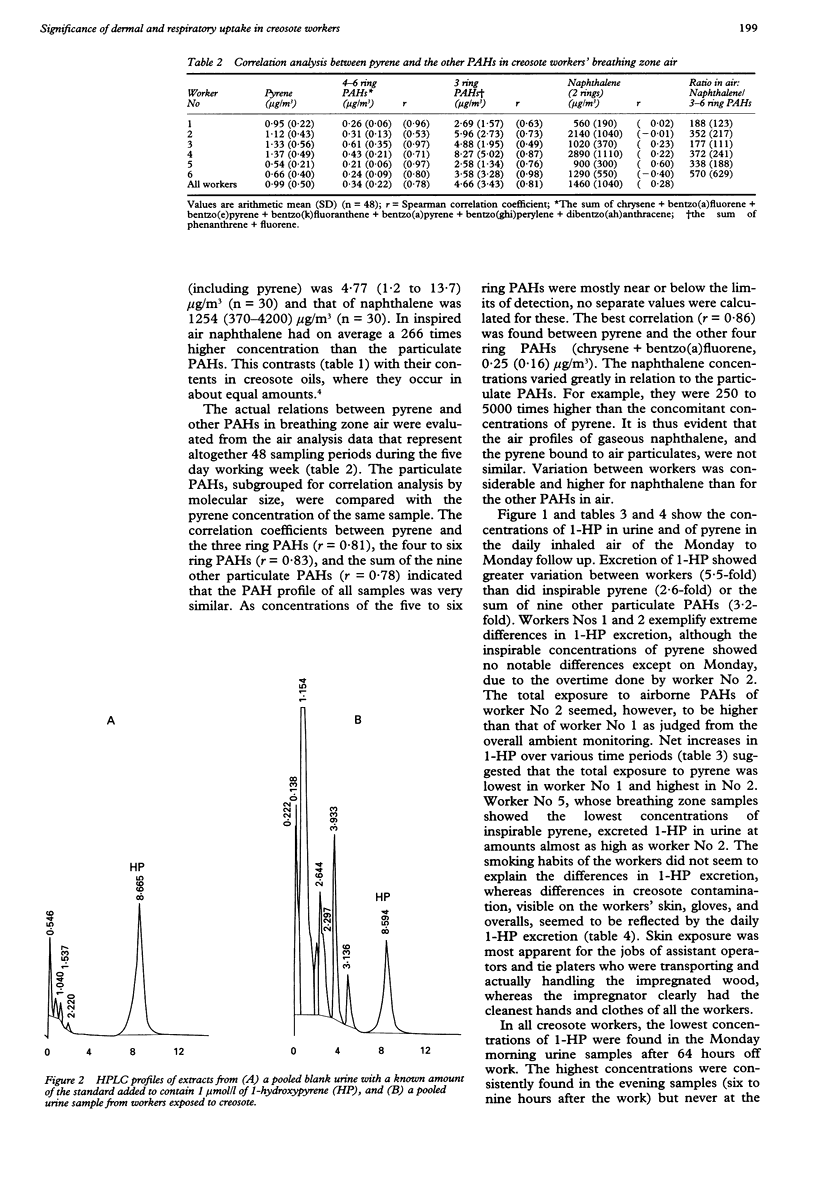
OBJECTIVES--To evaluate workers' exposure in a creosote impregnation plant by means of ambient and biological monitoring. METHODS--Naphthalene (vapour phase) and 10 large molecular polycyclic aromatic hydrocarbons (PAHs) (particulate phase) were measured in the breathing zone air during an entire working week. 1-Hydroxypyrene (1-HP) was measured in 24 hour urine as a metabolite of the pyrene found in neat (dermal exposure) and airborne creosote. RESULTS--Naphthalene (0.4-4.2 mg/m3) showed 1000 times higher concentrations in air than did the particulate PAHs. In total, the geometric mean (range) of three to six ring PAHs was 4.8 (1.2-13.7) micrograms/m3; pyrene 0.86 (0.23-2.1) micrograms/m3, and benzo(a)pyrene 0.012 (0.01-0.05) micrograms/m3. There was no correlation between pyrene and gaseous naphthalene. The correlations between pyrene and the other nine particulate PAHs were strong, and gave a PAH profile that was similar in all air samples: r = 0.83 (three to six ring PAHs); r = 0.81 (three ring PAHs); r = 0.78 (four to six ring PAHs). Dermal exposure was probably very high in all workers, because the daily output of urinary 1-HP exceeded the daily uptake of inhaled pyrene by < or = 50-fold. Urinary 1-HP concentrations were very high, even on Monday mornings, when they were at their lowest (4-22 mumol/mol creatinine). 1-HP seldom showed any net increase over a workshift (except on Monday) due to its high concentrations (16 to 120 mumol/mol creatinine) in the morning samples. 1-HP was always lower at the end of the shift (19 to 85 mumol/mol creatinine) than in the evening (27 to 122), and the mean (SD) change over the working week (47 (18)) was greater than the change over Monday (35 (32)). The timing of 1-HP sampling is therefore very important. CONCLUSIONS--Urinary 1-HP proved to be a good biomarker of exposure to three to six ring PAHs but not to airborne naphthalene. Hence, biomonitoring based on 1-HP has to be completed with exposure assessment for naphthalene as a marker for creosote volatiles that mainly enter the body through the lungs.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexandrov K., Rojas M., Geneste O., Castegnaro M., Camus A. M., Petruzzelli S., Giuntini C., Bartsch H. An improved fluorometric assay for dosimetry of benzo(a)pyrene diol-epoxide-DNA adducts in smokers' lung: comparisons with total bulky adducts and aryl hydrocarbon hydroxylase activity. Cancer Res. 1992 Nov 15;52(22):6248–6253. [PubMed] [Google Scholar]
- Bertrand J. P., Chau N., Patris A., Mur J. M., Pham Q. T., Moulin J. J., Morviller P., Auburtin G., Figueredo A., Martin J. Mortality due to respiratory cancers in the coke oven plants of the Lorraine coalmining industry (Houillères du Bassin de Lorraine). Br J Ind Med. 1987 Aug;44(8):559–565. doi: 10.1136/oem.44.8.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley T. J., Lioy P. J. An examination of the time course from human dietary exposure to polycyclic aromatic hydrocarbons to urinary elimination of 1-hydroxypyrene. Br J Ind Med. 1992 Feb;49(2):113–124. doi: 10.1136/oem.49.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgaz S., Borm P. J., Jongeneelen F. J. Evaluation of urinary excretion of 1-hydroxypyrene and thioethers in workers exposed to bitumen fumes. Int Arch Occup Environ Health. 1992;63(6):397–401. doi: 10.1007/BF00386935. [DOI] [PubMed] [Google Scholar]
- Caporaso N., Hayes R. B., Dosemeci M., Hoover R., Ayesh R., Hetzel M., Idle J. Lung cancer risk, occupational exposure, and the debrisoquine metabolic phenotype. Cancer Res. 1989 Jul 1;49(13):3675–3679. [PubMed] [Google Scholar]
- Clonfero E., Zordan M., Venier P., Paleologo M., Levis A. G., Cottica D., Pozzoli L., Jongeneelen F. J., Bos R. P., Anzion R. B. Biological monitoring of human exposure to coal tar. Urinary excretion of total polycyclic aromatic hydrocarbons, 1-hydroxypyrene and mutagens in psoriatic patients. Int Arch Occup Environ Health. 1989;61(6):363–368. doi: 10.1007/BF00381025. [DOI] [PubMed] [Google Scholar]
- Cosma G. N., Toniolo P., Currie D., Pasternack B. S., Garte S. J. Expression of the CYP1A1 gene in peripheral lymphocytes as a marker of exposure to creosote in railroad workers. Cancer Epidemiol Biomarkers Prev. 1992 Jan-Feb;1(2):137–142. [PubMed] [Google Scholar]
- Granella M., Clonfero E. Urinary excretion of 1-pyrenol in automotive repair workers. Int Arch Occup Environ Health. 1993;65(4):241–245. doi: 10.1007/BF00381197. [DOI] [PubMed] [Google Scholar]
- Grimmer G., Dettbarn G., Jacob J. Biomonitoring of polycyclic aromatic hydrocarbons in highly exposed coke plant workers by measurement of urinary phenanthrene and pyrene metabolites (phenols and dihydrodiols). Int Arch Occup Environ Health. 1993;65(3):189–199. doi: 10.1007/BF00381155. [DOI] [PubMed] [Google Scholar]
- Hansen A. M., Poulsen O. M., Menné T. Longitudinal study of excretion of metabolites of polycyclic aromatic hydrocarbons in urine from two psoriatic patients. Acta Derm Venereol. 1993 Jun;73(3):188–190. doi: 10.2340/0001555573188190. [DOI] [PubMed] [Google Scholar]
- Heikkilä P. R., Hämeilä M., Pyy L., Raunu P. Exposure to creosote in the impregnation and handling of impregnated wood. Scand J Work Environ Health. 1987 Oct;13(5):431–437. doi: 10.5271/sjweh.2017. [DOI] [PubMed] [Google Scholar]
- Jacob J., Brune H., Gettbarn G., Grimmer D., Heinrich U., Mohtashamipur E., Norpoth K., Pott F., Wenzel-Hartung R. Urinary and faecal excretion of pyrene and hydroxypyrene by rats after oral, intraperitoneal, intratracheal or intrapulmonary application. Cancer Lett. 1989 Jul 1;46(1):15–20. doi: 10.1016/0304-3835(89)90209-7. [DOI] [PubMed] [Google Scholar]
- Jongeneelen F. J., Anzion R. B., Henderson P. T. Determination of hydroxylated metabolites of polycyclic aromatic hydrocarbons in urine. J Chromatogr. 1987 Jan 23;413:227–232. doi: 10.1016/0378-4347(87)80230-x. [DOI] [PubMed] [Google Scholar]
- Jongeneelen F. J., Anzion R. B., Leijdekkers C. M., Bos R. P., Henderson P. T. 1-hydroxypyrene in human urine after exposure to coal tar and a coal tar derived product. Int Arch Occup Environ Health. 1985;57(1):47–55. doi: 10.1007/BF00383545. [DOI] [PubMed] [Google Scholar]
- Jongeneelen F. J., Anzion R. B., Scheepers P. T., Bos R. P., Henderson P. T., Nijenhuis E. H., Veenstra S. J., Brouns R. M., Winkes A. 1-Hydroxypyrene in urine as a biological indicator of exposure to polycyclic aromatic hydrocarbons in several work environments. Ann Occup Hyg. 1988;32(1):35–43. doi: 10.1093/annhyg/32.1.35. [DOI] [PubMed] [Google Scholar]
- Jongeneelen F. J. Biological exposure limit for occupational exposure to coal tar pitch volatiles at cokeovens. Int Arch Occup Environ Health. 1992;63(8):511–516. doi: 10.1007/BF00386338. [DOI] [PubMed] [Google Scholar]
- Jongeneelen F. J., van Leeuwen F. E., Oosterink S., Anzion R. B., van der Loop F., Bos R. P., van Veen H. G. Ambient and biological monitoring of cokeoven workers: determinants of the internal dose of polycyclic aromatic hydrocarbons. Br J Ind Med. 1990 Jul;47(7):454–461. doi: 10.1136/oem.47.7.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulin J. J., Wild P., Mantout B., Fournier-Betz M., Mur J. M., Smagghe G. Mortality from lung cancer and cardiovascular diseases among stainless-steel producing workers. Cancer Causes Control. 1993 Mar;4(2):75–81. doi: 10.1007/BF00053147. [DOI] [PubMed] [Google Scholar]
- Nylund L., Heikkilä P., Hämeilä M., Pyy L., Linnainmaa K., Sorsa M. Genotoxic effects and chemical compositions of four creosotes. Mutat Res. 1992 Feb;265(2):223–236. doi: 10.1016/0027-5107(92)90051-3. [DOI] [PubMed] [Google Scholar]
- Seto H., Ohkubo T., Kanoh T., Koike M., Nakamura K., Kawahara Y. Determination of polycyclic aromatic hydrocarbons in the lung. Arch Environ Contam Toxicol. 1993 May;24(4):498–503. doi: 10.1007/BF01146169. [DOI] [PubMed] [Google Scholar]
- Sherson D., Sigsgaard T., Overgaard E., Loft S., Poulsen H. E., Jongeneelen F. J. Interaction of smoking, uptake of polycyclic aromatic hydrocarbons, and cytochrome P450IA2 activity among foundry workers. Br J Ind Med. 1992 Mar;49(3):197–202. doi: 10.1136/oem.49.3.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjoe Ny E., Heederik D., Kromhout H., Jongeneelen F. The relationship between polycyclic aromatic hydrocarbons in air and in urine of workers in a Söderberg potroom. Am Ind Hyg Assoc J. 1993 Jun;54(6):277–284. doi: 10.1080/15298669391354685. [DOI] [PubMed] [Google Scholar]
- Tokiwa H., Sera N., Horikawa K., Nakanishi Y., Shigematu N. The presence of mutagens/carcinogens in the excised lung and analysis of lung cancer induction. Carcinogenesis. 1993 Sep;14(9):1933–1938. doi: 10.1093/carcin/14.9.1933. [DOI] [PubMed] [Google Scholar]
- Van Hummelen P., Gennart J. P., Buchet J. P., Lauwerys R., Kirsch-Volders M. Biological markers in PAH exposed workers and controls. Mutat Res. 1993 Aug;300(3-4):231–239. doi: 10.1016/0165-1218(93)90055-i. [DOI] [PubMed] [Google Scholar]
- VanRooij J. G., Bodelier-Bade M. M., Jongeneelen F. J. Estimation of individual dermal and respiratory uptake of polycyclic aromatic hydrocarbons in 12 coke oven workers. Br J Ind Med. 1993 Jul;50(7):623–632. doi: 10.1136/oem.50.7.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanrooij J. G., Bodelier-Bade M. M., De Looff A. J., Dijkmans A. P., Jongeneelen F. J. Dermal exposure to polycyclic aromatic hydrocarbons among primary aluminium workers. Med Lav. 1992 Sep-Oct;83(5):519–529. [PubMed] [Google Scholar]
- Verma D. K., Julian J. A., Roberts R. S., Muir D. C., Jadon N., Shaw D. S. Polycyclic aromatic hydrocarbons (PAHs): a possible cause of lung cancer mortality among nickel/copper smelter and refinery workers. Am Ind Hyg Assoc J. 1992 May;53(5):317–324. doi: 10.1080/15298669291359717. [DOI] [PubMed] [Google Scholar]
- Vähäkangas K., Pyy L., Yrjänheikki E. Assessment of PAH-exposure among coke oven workers. Pharmacogenetics. 1992 Dec;2(6):304–308. [PubMed] [Google Scholar]
- Zhao Z. H., Quan W. Y., Tian D. H. Urinary 1-hydroxypyrene as an indicator of human exposure to ambient polycyclic aromatic hydrocarbons in a coal-burning environment. Sci Total Environ. 1990 Mar;92:145–154. doi: 10.1016/0048-9697(90)90326-p. [DOI] [PubMed] [Google Scholar]


