Abstract
Background:
Abdominal pain due to menses (primary dysmenorrhea) is an extremely pervasive and debilitating symptom affecting up to 90% of menstruating individuals.
Objective:
The objective of this randomized control trial was to investigate the effect of a commercial transcutaneous electrical nerve stimulation unit, Therabody PowerDot® (Therabody Inc., Los Angeles) on dysmenorrhea compared with non-steroidal anti-inflammatory drug use.
Design:
This was a randomized cross-over study.
Methods:
A total of 47 participants agreed to participate in the study, with 34 completing it. Participants completed treatments across three consecutive menstrual cycles in randomized order: single-unit transcutaneous electrical nerve stimulation (Uno), dual unit transcutaneous electrical nerve stimulation (Duo), and non-steroidal anti-inflammatory drug use (Control). Upon onset of dysmenorrhea, participants applied transcutaneous electrical nerve stimulation to their abdomen for a minimum of 30 min. Control participants were instructed to take non-steroidal anti-inflammatory drugs as needed. Surveys were used to record pain before and after treatment. We hypothesized that the PowerDot would decrease self-reported pain scores, and decrease non-steroidal anti-inflammatory drug consumption during menses.
Results:
Participants experienced a statistically and clinically significant reduction in pain during the Control (−3.52 ± 1.9), Uno (−2.10 ± 1.6), and Duo (−2.19 ± 1.7) cycles (p < 0.001). The doses of non-steroidal anti-inflammatory drugs consumed during the Control cycle (3.5 ± 2.6), was significantly different as compared with that of Uno (1.5 ± 3.0), or Duo (1.1 ± 2.6) (p = 0.004).
Conclusions:
Use of a commercial transcutaneous electrical nerve stimulation unit results in significant decrease in pain. Although not as robust as the relief in pain induced by non-steroidal anti-inflammatory drugs, the adverse events of transcutaneous electrical nerve stimulation are minimal in comparison. Therefore, transcutaneous electrical nerve stimulation appears to be a viable alternative to pain relief from dysmenorrhea.
Clinical Trial Registration:
Keywords: dysmenorrhea, menstruation, transcutaneous electrical nerve stimulation
Plain Language Summary
The role of electrical signals for period pain relief
Menstruation, also known as the period, is a cyclicly occurring event in people who are assigned female at birth. Often, the period is associated with abdominal pain that can be debilitating for many. This abdominal pain is typically treated using over-the-counter medications, such as ibuprofen; however, there several noted side effects that can arise from use of such medication. As such, this study aimed to understand if a device (Therabody PowerDot®; Therabody Inc., Los Angeles) that sends an electrical current to pads placed over the abdomen, much like a heating pad, could be used to decrease pain during the period to a similar level as medication. The research team studied three consecutive periods with differing setups: a single, elongated pad, placed on the lower abdomen (Uno), two circular pads placed on the lower abdomen (Duo), or no use of the device, only medication (Control). The researchers analyzed data from 34 individuals. It was found that all three cycles experienced a significant decrease in pain, with the control cycle having a greater decrease in pain than both the Uno and Duo. This study suggests that the electrical stimulation used here can greatly decrease pain during the period, though not as substantial as medication.
Introduction
Primary dysmenorrhea (PD) refers to painful abdominal cramping in the absence of a gynecological pathology that accompanies menses. 1 The physiological process that results in PD involves a complex interplay of hormonal, inflammatory, and muscular factors. If pregnancy is not achieved, the female sex hormones, estrogen and progesterone, rapidly decline triggering an inflammatory response involving a variety of cytokines, chemokines, and prostaglandins. 2 It is suggested that this inflammatory influx causes vasoconstriction and ischemia. 3 This hypoxic environment, when coupled with myometrial contractions, is thought to be the cause of uncomfortable cramping and pain.
While only one of many complaints that coincide with the cyclical recurrence of menstruation, PD has dramatic impacts on many socioeconomic, productivity, and quality of life variables of menstruating individuals. A 2019 survey by Schoep et al. found that approximately 14% of individuals abstained from work due to PD, and over 80% suffered from presenteeism (i.e., a loss of productivity while in the workplace). 4 In addition, dysmenorrhea is associated with lower physical and social activity, and overall lower acute quality of life. 5 Estimated to affect between 45% and 95% of individuals, PD has a dramatic impact on the lives of many. 5
Generally, over-the-counter medicine, such as non-steroidal anti-inflammatory drugs (NSAIDs) are recommended for those that suffer from PD. Interrupting the cyclooxygenase (COX) pathways, NSAIDs offer relief from the abundance of prostaglandins behind menstrual pain. 6 However helpful they may be, NSAIDs may be accompanied by a variety of adverse effects, including gastric upset, headaches, dizziness, rash, drowsiness, and ringing in the ears. 7 More serious side effects have been noted including liver and kidney failure, ulcers, increased bleeding time, and cardiovascular risk. When used over a longer period, such as with cyclical pain associated with menses, NSAIDs pose risks to the renal, hepatic, and cardiovascular systems. 7
Transcutaneous electrical nerve stimulation (TENS) may provide a noninvasive and safe alternative to these over-the-counter medicines. TENS is proposed to provide analgesic relief through three predominant pathways. First, TENS activates large diameter afferent fibers to close the pain gate in the dorsal horn of the spinal cord. 8 In addition, high frequency (HF)-TENS is purported to stimulate the release of endogenous opiates into the bloodstream. 9 Finally, HF-TENS may reduce excitability of afferent receptors in the spinal cord, as well as peripheral excitability of nociceptors. 9 Regardless of the pathway, HF-TENS should provide relief from dysmenorrhea.
The objective of the present study was to investigate the effect of a commercial HF-TENS device, the Therabody PowerDot® (Therabody, Inc., Los Angeles, CA) on PD compared with NSAID use. The efficacy of such a device outside of a laboratory setting could provide non-medicinal relief to those who experience dysmenorrhea at home. We expected that the use of the TENS device would decrease self-reported pain scores associated with menses. Furthermore, we expected that the two-unit TENS sessions, covering a greater surface area of the lower abdomen, would provide a greater decrease in pain, as compared with the single-unit sessions. Finally, we postulated that using the TENS device would decrease analgesic pill consumption during menses compared with no use of TENS. Secondary outcomes investigated include the use of time and number of uses on alleviation of pain. We hypothesized that as the number of uses and amount of time the device is worn increase, the amount of pain reduction will increase.
Materials and methods
Experimental design
Study design
This study was approved by the Institutional Review Board at the University of Southern California and registered on ClinicalTrials.gov (NCT05178589). All data were collected through the Clinical Exercise Research Center (CERC) on the Health Sciences Campus at the University of Southern California. Data were collected between February 2022 and January 2023. The study was completed upon reaching an adequate sample size. The duration of the study for each participant was three menstrual cycles. After receiving their equipment, participants conducted research at their discretion outside of the lab. The preparation of the article followed CONSORT guidelines.
An initial visit was scheduled to explain the study, and to allow participants to review and sign the informed consent. Participants were then familiarized with the Therabody PowerDot, asked to complete a demographic questionnaire, and shown the daily survey, which was delivered via email.
The device of interest was the Therabody PowerDot, a rechargeable, class II medical device. When connected, via Bluetooth, to a phone, the device itself supplies electrical current through lead cables that attach to electrode pads. When the device is fully charged, the PowerDot can provide 6 h of continuous stimulation. 10 The treatment can be administered via a single pod (Uno), or through use of both pods (Duo), as discussed later in this section. Participants can choose the duration of their treatment session, ranging from 30 up to 90 min. Intensity can be controlled through the app and can be modified throughout treatment. Participants were instructed to select the “Period Pain Relief” program in the app. This program delivers continuous HF (50–100 Hz) electrical stimulation with a phase duration of 100 µS. 1 This frequency and pulse width is consistent with the International Association for the Study of Pain (ISAP) definition of conventional TENS. 11 The aim of this type of treatment is to stimulate afferent neurons to inhibit the transmission of pain in the central nervous system. 12
Participants completed three consecutive menstrual cycles with the treatments assigned in randomized order for each participant. In the cross-over design, participants would use the PowerDot or Control as assigned for the duration of menses and the remainder of their menstrual cycle was used as the washout period, with no use of the PowerDot permitted. It was expected that participants complete all three cycles. However, as will be discussed later in this article, a number of individuals did not experience menstrual pain during one or more cycle, and therefore did not use the treatment as assigned.
Participants
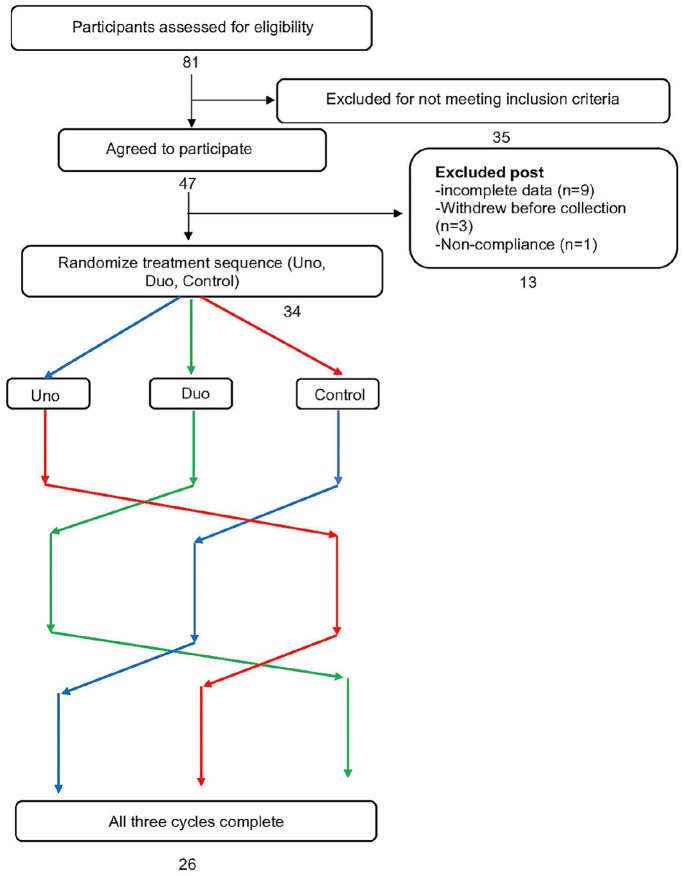
A priori power analysis was conducted using G*Power to estimate sample size required. 13 The sample size was calculated using a previously conducted study with an effect size of 0.29. 14 Based on a significance level of 0.05 and a power of 95%, it was determined that a total sample size of 33 was sufficient. A total of 47 menstruating individuals were recruited to account for an estimated 20% attrition rate. Data from 9 participants were incomplete, 3 participants withdrew before beginning any treatment, and 1 participant was excluded because of non-compliance. Therefore, data from 34 participants (24.6 ± 3.4 years old; 23.05 ± 3.11 kg/m2) were used in this cross-over analysis. A flow chart of participant inclusion is shown in Figure 1. As mentioned above, some participants did not complete all three cycles: 26 individuals completed all assignments, 5 participants completed only two assignments, and 3 participants only completed one of the three assignments.
Figure 1.
Cross-over flow chart.
Participants were asked to complete an initial questionnaire to assess eligibility to take part in the study. To qualify for the study, individuals were required (a) to be between the ages of 18 and 35 years; 15 (b) have a history of lower moderate-to-severe abdominal pain for more than six consecutive menstrual cycles with a pain rating greater than 5 out of 10; 16 (c) refrained from hormonal birth control for at least 6 months prior to participating in the study; 15 and (d) to have a body mass index (BMI) of 18.5–29.9. 17 Individuals were excluded if they (a) were amenorrheic or oligomenorrheic; (b) suffered from secondary dysmenorrhea and/or other gynecological disorders; 18 (c) had a history of surgery over the abdominal area; (d) suffered from cardiac disease, uncontrolled hemorrhage, blood clots, pacemakers,; (e) had an allergy to NSAIDs; or (f) were pregnant or planning to become pregnant. 14
After initial screening, participants were asked to complete an additional survey to collect menstrual history and demographic data. BMI was calculated using self-reported height and weight. Demographic and self-reported menstrual pain is shown in Table 1.
Table 1.
Characteristics of the analyzed study population.
| Characteristic | n = 34 |
|---|---|
| Age | 24.65 (3.31) |
| BMI (kg/m2) | 23.05 (3.11) |
| Race | |
| White | 38.23% |
| Black or African American | 11.76% |
| Hispanic or Latino | 11.76% |
| Asian or Pacific Islander | 11.76% |
| Biracial or Multiracial | 20.59% |
| Other | 5.88% |
| Self-reported pain during menses (0–10 numeric rating scale) | 6.76 (1.56) |
Numbers represent mean (SD).
The three treatments were as follows: Duo, Uno, and NSAIDs (control). The order of treatment for each participant was randomized using an online random number generator. The randomized assignment, implementation, and enrollment of participants was conducted by the principal investigator. The treatment order was not blinded to participant or investigator.
Intervention
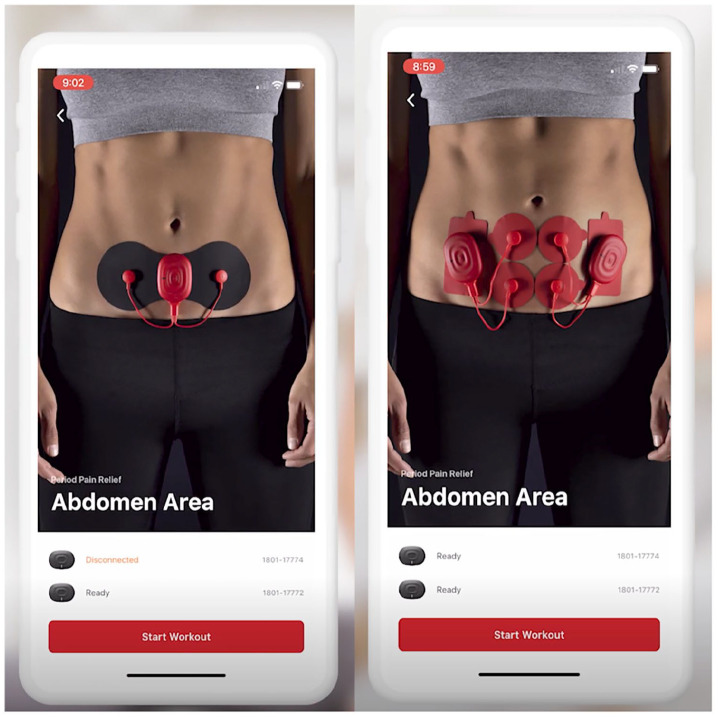
Participants rated all dysmenorrhea on an 11-point Numerical Rating Scale (NRS) available through the app before and after treatment (0 = “no pain”; 10 = “worst possible”). The use of NRS has a long history in understanding pain, with high validity and sensitivity to pain among patients that are able to use them.16,19 Depending on the order of treatment, participants were instructed to use the PowerDot setup assigned or NSAIDs upon pain onset. During the Uno/Duo cycles, pads were to be placed over the location of pain on the abdomen. 1 For the Uno assignment, only one device was connected to a “butterfly” pad that extended to two electrodes. During the Duo assignment, two devices were attached to four electrodes on two separate pad setups. The adhesive pads are reusable and reportedly last for up to 25 sessions. If the electrical intervention failed to alleviate pain, participants were instructed to report their level of pain after use, and take NSAIDs as needed. The NSAID use was to be recorded, and no further PowerDot usage was allowed for the remainder of the day. Figure 2 shows examples of how participants arranged the pads on their abdomen.
Figure 2.
Examples of a setup using the PowerDot TENS device. Uno device setup (left) uses a single device, attached to two electrodes on a “butterfly” pad. Duo device setup (right) uses both devices, attached to four electrodes on four separate pads. Copyright permission to use the above image was provided by Therabody, Inc.
The intensity of the device was to be set to the highest tolerable sensation. As the central nervous system acclimated to the stimulation, participants were instructed to increase the intensity. Sessions on the app varied in length, with a minimum duration of 30 min. There was no limit to the number of sessions that individuals were able to use, and the session lengths could change depending on the participant needs.
If no relief was experienced after use of the PowerDot, participants were free to use their NSAID of choice, so long as it was documented. NSAIDs were only to be taken after a trial session with the PowerDot, so as not to convolute the pain response. Other pain-relieving practices, such as use of a heating pad, were not allowed during the study, to limit the potential analgesic effect of other methods.
During the control cycle, participants were instructed to use their NSAID of choice as frequently as needed to ease dysmenorrheal symptoms. It was recommended that the dose in a 24-h period not exceed the manufacturer recommendation of 1600 mg. However, to maintain transparency, all doses were to be recorded on the daily survey truthfully. NSAID use for other purposes (i.e. headaches) was prohibited while menstruating. As this was an investigation on the acute effects of treatment, no patient follow-up was performed.
Statistical analyses
Participants were asked to record the number of uses, the length of treatment, and the pre- and post-pain scores for each treatment on a daily survey delivered via SurveyMonkey (San Mateo, CA). Data were exported from the SurveyMonkey website to a .csv file. All statistical tests were run using R Version 4.2.3 (R Foundation for Statistical Computing; Vienna, Austria). For the initial intent to treat analysis, which included all participants randomized to the intervention (n = 34), a Kruskal–Wallis test was used to detect differences between treatment groups. A Wilcoxon rank-sum test was used for pairwise comparisons.
To investigate the change in pain score on those that completed all three cycles (n = 26), a repeated measures ANOVA was performed. A paired t-test with Benjamini–Hochberg correction was used to assess difference between PowerDot assignment and the control. A repeated measures ANOVA was also conducted to investigate the change in NSAID consumption across cycles. A multivariable linear mixed effects regression was used to perform sub-analyses (i.e. assessing the relationship between change in pain score and other variables) on those that completed all cycles (n = 26). For any repeated measures analysis, participants with missing group data were excluded. The significance level was set at 0.05.
Results
Initial intent to treat analysis is shown in Table 2. The intent to treat analysis included all participants that completed the study (n = 34), including those that did not complete one or more assignments, as will be discussed later. This initial analysis showed that the change in pain score was significantly different in the Control (−3.6 ± 2.0 units) as compared with the Uno (−1.9 ± 1.9 units) and Duo (−2.2 ± 1.8 units) assignments (p < 0.001). In addition, after controlling for baseline differences, the pain score prior to each use (pre-pain) was different in the Control (5.8 ± 1.2 units) when compared with the Uno (4.7 ± 1.6 units) and Duo (4.5 ± 1.7 units) assignments (p < 0.001). Alternatively, pain score after each treatment (post-use) were not significantly different between Control (2.2 ± 1.9), Uno (2.8 ± 1.9 units), and Duo (2.4 ± 1.7 units) assignments (p = 0.157). These statistical trends hold true for those that completed all three cycles as well, shown in Table 3. Further analysis of the initial participant pool revealed that all three treatments showed a significant difference in pain score from pre- to post-use (p < 0.001).
Table 2.
Pain score variables by intervention group in initial intent to treat analysis.
| Variable | Group assignment | p-value | ||
|---|---|---|---|---|
| Control (n = 34) |
Uno (n = 34) |
Duo (n = 34) |
||
| Change in score | −3.6 (2.0) (min =−7, max = 3) |
−1.9Ɨ (1.9) (min = −7, max = 3) |
−2.2Ɨ (1.8) (min =-7, max = 3) |
<0.001* |
| Pre-use score | 5.8 (1.2) (min = 3, max = 8) |
4.7Ɨ (1.6) (min = 1, max = 8) |
4.5Ɨ (1.7) (min = 1, max = 8) |
<0.001* |
| Post-use score | 2.2 (1.9) (min = 0, max = 10) |
2.8 (1.9) (min = 0, max = 8) |
2.4 (1.7) (min = 0, max = 7) |
0.157 |
| p-value (pre vs post) | <0.001 + | <0.001 + | <0.001 + | |
Please note that the number of treatments may differ between participants (i.e. participant 1 may have used the PowerDot four times while menstruating, while participant 2 may have only used it once). Numbers represent mean (SD) (min, max).
Significant at p < 0.05 (Kruskal–Wallis); Ɨsignificantly different from control (Wilcoxon sum-rank test); +Significant at p < 0.05 (paired t-test).
Table 3.
Pain score variables by intervention group of individuals who completed all three assignments.
| Variable | Group assignment | p-value | ||
|---|---|---|---|---|
| Control (n = 26) |
Uno (n = 26) |
Duo (n = 26) |
||
| Change in score | −3.52 (1.9) (6.2, 2.0) |
−2.10Ɨ (1.6) (−5.5, 0.6) |
−2.19Ɨ (1.7) (−6.3, 2.0) |
0.007* |
| Pre-use pain score | 5.86 (0.9) (3.7, 7.5) |
4.72Ɨ (1.1) (2.5, 7.0) |
4.65Ɨ (1.2) (3, 7.3) |
<0.001* |
| Post-use pain score | 2.37 (1.7) (0, 8) |
2.64 (1.4) (0.4, 6) |
2.46 (1.4) (0, 5.5) |
0.797 |
| p-value (pre vs post) | <0.001 + | <0.001 + | <0.001 + | |
Numbers represent mean (SD) (min, max).
Significant at p < 0.05 (repeated measures ANOVA); significantly different from control (paired t-test w/ Benjamini-Hochberg correction).
Significant at p < 0.05 (paired t-test).
It is important to note that the number of participants analyzed in the intent to treat analysis differed from those that completed all three cycles of treatment. This is due to the dynamic nature of the menstrual cycle at large, but particularly in the differing severity of dysmenorrhea. Previous work has revealed that pain due to menses tends to differ in severity between cycles. 20 To make this study as realistic as possible, participants were advised that if they did not feel that they needed the PowerDot and/or NSAIDs for pain relief, then they were not required to use either. Given these instructions, of the total 34 participants only 26 completed all three treatments (ie, Duo, Uno, and Control). The data presented throughout the remainder of this section reflect those that completed all three treatments.
Between group analyses, shown in Table 3, indicate that the change in pain score during the Control cycle (−3.52 ± 1.9 units) was significantly different than that of both the Uno (−2.10 ± 1.6 units) and Duo (−2.19 ± 1.7 units) (p = 0.007). One important factor to note is that, after controlling for baseline differences, the pre-use pain score of Control (5.86 ± 0.9 units) was significantly higher than that of both Uno (4.72 ± 1.1 units) and Duo (4.65 ± 1.2 units) (p < 0.001). Meanwhile, the “post-use pain score” was not significantly different between Uno (2.64 ± 1.4 units), Duo (2.46 ± 1.4 units), and Control (2.37 ± 1.7 units) (p = 0.797). This large discrepancy in pre-, but not post-treatment pain had a large impact on the significant difference in change scores between groups (p = 0.007). The dynamic nature of the menses pain is likely responsible for this discrepancy in the pre-pain score. There were eight participants that had an average pre-pain score during Control that was ranked as a 7 or higher, versus only one in the Uno cycle and one in the Duo cycle. This large cohort of individuals that happened to experience higher pain during their Control cycle likely drew the pre-pain score average higher.
Table 3 illustrates that there was a significant difference in pre versus post pain scores for Control (Pre: 5.81 ± 0.9 units; Post: 2.37 ± 1.7 units), Uno (Pre: 4.72 ± 1.1 units; Post: 2.64 ± 1.4 units), and Duo (Pre: 4.65 ± 1.2 units; Post: 2.46 ± 1.4 units) (p < 0.001). In addition, all treatments experienced a decrease in pain score that was greater than the criteria for clinical significance, which is a decline in 2 points on an 11-point scale. 21
NSAID consumption during Uno (1.5 ± 3.0 doses per day) and Duo (1.1 ± 2.6 doses per day) cycles were significantly different as compared with that of the Control (3.5 ± 2.6 doses per day) (p = 0.004). Another important note is that while all 26 individuals consumed NSAIDs for relief during their Control cycle, only 9 individuals consumed NSAIDs during their Uno cycle, and 6 individuals during their Control cycle.
After adjusting for the total number of uses, as shown in Table 4, the Control group experienced a greater reduction in pain compared with both Uno and Duo. In other words, the use of Control decreased pain, on average, 1.247 units more than Uno (p = 0.004). Meanwhile, the use of Control decreased pain, on average, 1.149 units more than Duo (p = 0.008). Moreover, both the Uno and Duo were less effective at decreasing pain, regardless of the number of uses.
Table 4.
Linear mixed effects regression with average change in pain score as outcome.
| Variable | Estimate (95% confidence interval) | p-value |
|---|---|---|
| Intervention assignment | ||
| Control | Reference | |
| Uno | 1.247 (0.442–2.062) | 0.004* |
| Duo | 1.149 (0.344–1.965) | 0.008* |
| Total uses during assignment | 0.158 (−0.042–0.356) | 0.122 |
Significant at p < 0.05.
Additional analyses were conducted to investigate the effect of supplemental variables including the duration of use, the setup assigned (ie, Uno vs Duo), the number of uses, and NSAIDs used in addition to the PowerDot. A multivariable linear effects regression was conducted on cycles in which participants were assigned to Uno or Duo treatment by the PowerDot (Table 5). There was no difference in Uno versus Duo setup for pain relief (p = 0.711). Additional PowerDot uses did not decrease pain score (p = 0.350). Participants that used NSAIDs during PowerDot assignment experienced significantly less relief due to the treatment, as compared with those that did not take additional medication (p < 0.001). This is unsurprising, as participants were instructed to only use NSAIDs if the PowerDot did not provide relief. In addition, time of use had no effect on pain relief (p = 0.908). When controlling for the assignment (i.e., Uno vs Duo), there was also no significant effect of number of uses (p = 0.667). In other words, using the PowerDot more frequently, regardless of assignment, did not have a significant effect on change in pain score. Furthermore, when treating time as a continuous variable, each additional 10 min, from 30 to 90 min, had no effect on pain score for increased duration (p = 0.908).
Table 5.
Linear mixed effects regression with change in pain score as outcome—PowerDot uses only.
| Variable | Estimate (95% confidence interval) | p-value |
|---|---|---|
| PowerDot Assignment | ||
| Uno | Reference | 0.711 |
| Duo | −0.234 (−1.387–0.956) | |
| Additional NSAID use | ||
| No | Reference | <0.001* |
| Yes | 1.886 (0.997–2.814) | |
| Duration of PowerDot use (10-unit change) | 0.013 (−0.235–0.253) | 0.908 |
| Number of uses | 0.120 (−0.112–0.360) | 0.350 |
| Assignment: Number of uses interaction | ||
| Uno: Use number | Reference | 0.667 |
| Duo: Use number | 0.065 (−0.221–0.338) | |
Significant at p < 0.05.
No serious adverse events have been reported as a result of TENS, including throughout the present study; however, more benign reactions have been recorded. Previous studies have reported that TENS has resulted in muscular discomfort (i.e., tightness), headache, and skin irritation from the pads.22,23 One participant in the present study reported hiccups during use on numerous occasions. There were no other adverse events reported.
Discussion
To our knowledge, this is the first study to investigate the efficacy of a Bluetooth TENS device to decrease dysmenorrhea outside of a clinical setting. It can be concluded, from the present study, that HF-TENS delivered via the Therabody PowerDot resulted in a clinically and statistically significant decrease in pain using both Uno and Duo setups. There was no notable difference in the amount of pain reduction that was experienced between the PowerDot intervention groups, as was originally thought. The results did support the initial hypothesis that use of the TENS device would lead to a decrease in NSAID consumption. Finally, it was also revealed that additional treatments and longer durations did not result in an increase in pain relief, as was initially believed.
The present study investigated the effectiveness of HF, instead of low frequency (LF)-TENS, because of its more pleasant, and equally effective analgesic effect. LF-TENS, or acupuncture-like TENS, delivers pulses at a frequency of 1–4 Hz, known to result in intense muscle twitches. 12 It has been suggested that, because of the physical unpleasantness of this treatment, it may inhibit participants from carrying out day-to-day activities.18,24 In addition, LF-TENS has not been reported to be more effective than HF-TENS for dysmenorrheal relief. 18 In fact, one review reported that HF-TENS was more effective than placebo at reducing dysmenorrhea, while LF-TENS was no more effective than placebo at reducing pain. 18 Therefore, HF-TENS, such as was used in this study, seems to be a more realistic treatment for PD.
The present study expands on previous investigations into the effect of HF-TENS on dysmenorrhea. First, previous studies have required treatment to be completed in a laboratory setting. 25 The present study was conducted in a more ecological setting, allowing for a more realistic view of how TENS would be used outside of a clinical space. Second, our study builds on previous investigations that relied on a single TENS intervention as compared with a sham. 26 Using a dual versus single unit arrangement, we were able to conclude that the surface area covered by the device was not as important as originally believed. This is likely due to the fact that the butterfly pad, as was used in the Uno assignment, as well as the dual pad arrangement, covered the entirety of the area of discomfort. Previous work has noted that HF-TENS relief is most effective when placed directly over the area(s) of pain, which in this case was the uterus. 1 Finally, previous studies have limited the duration and/or number of uses that participants were allowed to use the TENS device.26,27 The current study allowed participants to use the TENS device as many times and for as long as they felt was helpful. This helps navigate the transient relief provided by TENS. 28 Furthermore, we believe that this is more representative of how the device would be used in real life.
While the use of TENS for treatment of pain, particularly that associated with menses, is not novel, the expansion of this work into an ecological setting is. As noted numerous times throughout this article, treatment of PD using TENS is often restricted to a laboratory environment. However, it is highly unlikely that, at the onset of dysmenorrhea, individuals will seek out treatment from medical professionals; due in part to the inconvenience of traveling for relief, but also in part to the historical minimization of women’s pain.29 –31 Furthermore, supporting the use of at-home treatment for dysmenorrhea provides those experiencing PD with an alternative to traditional medicinal interventions, without requiring them to seek external interventions that may invalidate their pain or requiring them to risk experiencing harmful side effects associated with medication.
The greater decrease in pain exhibited by the Control/NSAID group corroborates previous findings that investigated TENS versus NSAIDs. 32 We were able to further build on these findings by exhibiting that the PowerDot resulted in a significant decrease in pain, and decreased reliance on NSAIDs. This is particularly valuable given that the adverse events of TENS are limited and relatively benign compared with those of NSAIDs. 7 In addition, long-term use of NSAIDs, such as when taken for cyclic pain associated with dysmenorrhea, have been shown to worsen symptoms, particularly those relating to the kidneys, liver, and the gastrointestinal (GI) tract. 7
It should be noted that, although the enrolled participants satisfied the initial power analysis, not all participants used their assigned method of pain relief for each cycle. It is important to keep in mind that the severity of dysmenorrhea fluctuates across menstrual cycles. In fact, previous work has revealed that symptom presentation and severity differed within each cycle, and across cycles. 15 Therefore, while cycle regularity was controlled for, it is near impossible to control for dysmenorrheal symptoms. It is from this inconsistency in pain that eight participants did not complete all three cycles. It is possible that, had the sample size been larger, the significantly higher pre-pain score seen in the Control versus the Uno and Duo cycles could have been minimized, or eliminated altogether. However, because the decrease in pain was well above what was needed for clinical significance, we are confident that the decrease in pain seen as a result of PowerDot use is valid.
There are several limitations of the current study. When discussing design, we relied solely on subjective metrics of pain (i.e., self-reported pain), rather than using objective inflammation metrics. In addition, while there were benefits to the ecological nature of the study, it also introduced several areas of weaknesses. For example, participants were asked to self-administer their treatment, creating between-participant variability. Elboim-Gabyzon noted that intensity was one of the most influential factors in the magnitude and duration of effect from TENS. 1 In addition, the program in the PowerDot app allowed participants to self-select a duration of treatment, and self-select how many times they were able to use the device. This could have influence on the magnitude of the results. However, it should be noted that the sub-analyses performed noted that the duration and number of uses did not have a significant effect on pain reduction. While allowing participants to deliver their own treatment could impact the results, we argue that this study represents a more realistic representation of how a commercial TENS device will be used for dysmenorrheal relief. Furthermore, participants were asked to use retrospective recall for their pain scores. This method of recording has the potential to skew pain scores higher than what was experienced in-the-moment. 33
Conclusion
The current study provides encouraging results as to the use of TENS in the modulation of PD outside of the laboratory setting. The results confirm the original hypothesis that HF-TENS delivered via the Therabody PowerDot decreased self-reported pain due to menses, regardless of Uno or Duo use. This study also found that there was a significant reduction in NSAID consumption during cycles in which the PowerDot was used, as compared with Control.
Acknowledgments
None.
Footnotes
ORCID iD: Bailey McLagan  https://orcid.org/0009-0003-1055-3792
https://orcid.org/0009-0003-1055-3792
Supplemental material: Supplemental material for this article is available online.
Declarations
Ethical approval and consent to participate: This study was approved by the Institutional Review Board at the University of Southern California and registered on ClinicalTrials.gov (NCT05178589). Written informed consent was obtained from all participants.
Consent for publication: Written informed consent stating that the participant’s data may be used for publication was obtained from all participants.
Author contribution(s): Bailey McLagan: Investigation (lead); Formal analysis (lead); Writing – original draft (lead); Writing – review & editing (lead).
Joshua Dexheimer: Methodology (lead); Conceptualization (lead); Writing – review & editing (equal).
Nicole Strock: Methodology (supporting); Conceptualization (supporting); Writing – review & editing (equal).
Shayna Goldstein: Investigation (equal);
Stephanie Guzman: Investigation (equal);
David Erceg: Writing – review & editing (equal);
E Todd Schroeder: Supervision; Writing – review & editing (equal).
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Departmental funds from the University of Southern California, Department of Biokinesiology and Physical Therapy. Product support provided by Therabody, Inc. (Los Angeles, CA).
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: N.S. served as a consultant that helped in study design and manuscript editing, but did not have access to raw data/analysis.
Availability of data and materials: Data are available on request from the authors.
References
- 1. Elboim-Gabyzon M, Kalichman L. Transcutaneous electrical nerve stimulation (TENS) for primary dysmenorrhea: an overview. Int J Womens Health 2020; 12: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ferries-Rowe E, Corey E, Archer JS. Primary dysmenorrhea: diagnosis and therapy. Obstet Gynecol 2020; 136: 1047–1058. [DOI] [PubMed] [Google Scholar]
- 3. Evans J, Salamonsen LA. Inflammation, leukocytes and menstruation. Rev Endocr Metab Disord 2012; 13(4): 277–288. [DOI] [PubMed] [Google Scholar]
- 4. Schoep ME, Adang EMM, Maas JWM, et al. Productivity loss due to menstruation-related symptoms: a nationwide cross-sectional survey among 32 748 women. BMJ Open 2019; 9: e026186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Iacovides S, Avidon I, Baker FC. What we know about primary dysmenorrhea today: a critical review. Hum Reprod Update 2015; 21(6): 762–778. [DOI] [PubMed] [Google Scholar]
- 6. Ghlichloo I, Gerriets V. Nonsteroidal anti-inflammatory drugs (NSAIDs). Treasure Island, FL: StatPearls Publishing, 2022, https://www.ncbi.nlm.nih.gov/books/NBK547742/ [PubMed] [Google Scholar]
- 7. Zahradnik HP, Hanjalic-Beck A, Groth K. Nonsteroidal anti-inflammatory drugs and hormonal contraceptives for pain relief from dysmenorrhea: a review. Contraception 2010; 81(3): 185–196. [DOI] [PubMed] [Google Scholar]
- 8. Melzack R, Wall PD. Pain mechanisms: a new theory: a gate control system modulates sensory input from the skin before it evokes pain perception and response. Science 1965; 150: 971–979. [DOI] [PubMed] [Google Scholar]
- 9. Vance CGT, Dailey DL, Rakel BA, et al. Using TENS for pain control: the state of the evidence. Pain Manag 2014; 4: 197–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. PowerDot M2: instruction manual, https://www.therabody.com/on/demandware.static/-/Library-Sites-TheragunSharedLibrary/default/dw7e95d917/pdf/coa/PD-Manual-Blue-V2.pdf (accessed 13 May 2024).
- 11. Charlton J. Core curriculum for professional education in pain. Washington, DC: IASP, 2005, pp. 93–96. [Google Scholar]
- 12. Johnson M. Transcutaneous electrical nerve stimulation: mechanisms, clinical application and evidence. Rev Pain 2007; 1(1): 7–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Faul F, Erdfelder E, Buchner A, et al. Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav Res Methods 2009; 41(4): 1149–1160. [DOI] [PubMed] [Google Scholar]
- 14. Bai HY, Bai HY, Yang ZQ. Effect of transcutaneous electrical nerve stimulation therapy for the treatment of primary dysmenorrheal. Medicine (Baltimore) 2017; 96(36): e7959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Williams NI, Mallinson RJ, De Souza MJ. Rationale and study design of an intervention of increased energy intake in women with exercise-associated menstrual disturbances to improve menstrual function and bone health: the REFUEL study. Contemp Clin Trials Commun 2019; 14: 100325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Williamson A, Hoggart B. Pain: a review of three commonly used pain rating scales. J Clin Nurs 2005; 14(7): 798–804. [DOI] [PubMed] [Google Scholar]
- 17. Bull JR, Rowland SP, Scherwitzl EB, et al. Real-world menstrual cycle characteristics of more than 600,000 menstrual cycles. NPJ Digit Med 2019; 2: 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Proctor M, Farquhar C, Stones W, et al. Transcutaneous electrical nerve stimulation for primary dysmenorrhoea. Cochrane Database Syst Rev 2002; 2002: CD002123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Atisook R, Euasobhon P, Saengsanon A, et al. Validity and utility of four pain intensity measures for use in international research. J Pain Res 2021; 14: 1129–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chen CX, Draucker CB, Carpenter JS. What women say about their dysmenorrhea: a qualitative thematic analysis. BMC Womens Health 2018; 18: 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Farrar JT, Young JP, Jr, LaMoreaux L, et al. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain 2001; 94(2): 149–158. [DOI] [PubMed] [Google Scholar]
- 22. Wang S-F, Lee J-P, Hwa H-L. Effect of transcutaneous electrical nerve stimulation on primary dysmenorrhea. Neuromodulation 2009; 12: 302–309. [DOI] [PubMed] [Google Scholar]
- 23. Johnson MI, Paley CA, Jones G, et al. Efficacy and safety of transcutaneous electrical nerve stimulation (TENS) for acute and chronic pain in adults: a systematic review and meta-analysis of 381 studies (the meta-TENS study). BMJ Open 2022; 12: e051073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Johnson MI. Acupuncture-like transcutaneous electrical nerve stimulation (AL-TENS) in the management of pain. Phys Ther Rev 1998; 3: 73–93. [Google Scholar]
- 25. Rodrigues JC, Avila MA, Driusso P. Transcutaneous electrical nerve stimulation for women with primary dysmenorrhea: study protocol for a randomized controlled clinical trial with economic evaluation. PLoS ONE 2021; 16(5): e0250111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Guy M, Foucher C, Juhel C, et al. Transcutaneous electrical neurostimulation relieves primary dysmenorrhea: a randomized, double-blind clinical study versus placebo. Prog Urol 2022; 32(7): 487–497. [DOI] [PubMed] [Google Scholar]
- 27. Tugay N, Akbayrak T, Demirtürk F, et al. Effectiveness of transcutaneous electrical nerve stimulation and interferential current in primary dysmenorrhea. Pain Med 2007; 8(4): 295–300. [DOI] [PubMed] [Google Scholar]
- 28. Buonocore M, Camuzzini N. Increase of the heat pain threshold during and after high-frequency transcutaneous peripheral nerve stimulation in a group of normal subjects. Eura Medicophys 2006; 43: 155–160. [PubMed] [Google Scholar]
- 29. Zhang L, Losin EAR, Ashar YK, et al. Gender biases in estimation of others’ pain. J Pain 2021; 22(9): 1048–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wandner LD, Heft MW, Lok BC, et al. The impact of patients’ gender, race, and age on health care professionals’ pain management decisions: an online survey using virtual human technology. Int J Nurs Stud 2014; 51(5): 726–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chen EH, Shofer FS, Dean AJ, et al. Gender disparity in analgesic treatment of emergency department patients with acute abdominal pain. Acad Emerg Med 2008; 15(5): 414–418. [DOI] [PubMed] [Google Scholar]
- 32. Dawood MY, Ramos J. Transcutaneous electrical nerve stimulation (TENS) for the treatment of primary dysmenorrhea: a randomized crossover comparison with placebo TENS and ibuprofen. Obstet Gynecol 1990; 75(4): 656–660. [PubMed] [Google Scholar]
- 33. Haase I. Accuracy of retrospective pain measurement in patients with chronic pain. Med Int (London) 2023; 3(4): 35. [DOI] [PMC free article] [PubMed] [Google Scholar]