Abstract
Purpose
The objective of this study was to investigate the effects of agrimonolide (AM) on mice with dextran sulfate sodium (DSS)-induced colitis and elucidate its protective mechanisms.
Methods
A 3 % DSS solution was used to induce colitis, and intragastric administration of AM at doses of 25 and 50 mg/kg was performed. A comprehensive assessment was conducted to evaluate inflammatory responses and mucosal integrity in the colon. Inflammatory factors were quantified using enzyme-linked immunosorbent assay (ELISA). The proportions of T helper cell 17 (Th17) and regulatory T cells (Treg) cells in mesenteric lymph nodes (MLNs) was analyzed through RT-qPCR and flow cytometry. Proteins associated with the Notch and JAK2/STAT3 pathways were examined via RT-qPCR, western blotting, and immunofluorescence. Additionally, the impact of AM on Treg and Th17 cell differentiation was investigated in vitro.
Results
Pre-treatment with AM significantly alleviated colon inflammation in mice, as evidenced by reduced body weight loss, shorter colon length, lower disease activity index (DAI) score, and decreased myeloperoxidase (MPO) content. Notably, AM pre-treatment attenuated the production of pro-inflammatory cytokines, including interleukin (IL)-1β, tumor necrosis factor (TNF)-α, and IL-6, in mice with DSS-induced colitis. Additionally, AM pre-treatment significantly enhanced the expression of tight junction proteins (Occludin and ZO-1), thereby preserving gut barrier function. Moreover, we observed that AM administration decreased the ratio of Th17 cells while increasing the frequency of colonic Treg cells, thus modulating the Th17/Treg balance both in vivo and in vitro. Furthermore, in the AM-treated group, the expression of Notch-1, Jagged1, delta like 4 (DLL4), phospho-janus kinases 2 (p-JAK2)/JAK2, and p-signal transducer and activator of transcription 3 (STAT3)/STAT3 in colonic tissue was reduced compared to the DSS group. Remarkably, the therapeutic effects of AM in colitis mice were blocked by a Notch activator.
Conclusion
These findings underscore the effectiveness of AM in alleviating symptoms and pathological damage in DSS-induced colitis mice by rebalancing Th17/Treg cell homeostasis through modulation of the Notch and JAK2/STAT3 signaling pathways. These insights into AM's mechanisms of action offer potential avenues for novel therapeutic strategies.
Keywords: Agrimonolide, Ulcerative colitis, Th17/Treg, Notch, JAK2/STAT3
1. Introduction
In recent years, there has been a notable increase in the incidence of inflammatory bowel disease (IBD), including ulcerative colitis (UC) and Crohn's disease (CD) [1,2]. UC, characterized by superficial ulcers in the mucosa of the rectum and colon, poses a significant risk of colon cancer if left untreated [3,4]. However, the clinical management of UC presents a complex challenge. Current treatments often involve the use of medications such as 5-aminosalicylic acid (5-ASA), glucocorticoids, or immunomodulators. Despite their widespread use, these drugs have limitations in terms of efficacy and safety for long-term use, often failing to achieve consistent remission or cure and leading to serious side effects. Given the inadequate outcomes associated with traditional therapies for UC, there is an urgent need to explore new alternative approaches to its treatment.
Dysregulation of intestinal immune homeostasis caused by over-activation of T cells is a key feature of UC, leading to the secretion of pro-inflammatory mediators, aberrant immune cells aggregation, and disruption of intestinal mucosal tissue. CD4+ T helper cells plays a pivotal role in the adaptive immune response, contributing to immune defense, immune surveillance, and immune homeostasis [5,6]. In response to immune signals, naïve CD4+ T lymphocytes differentiate into mature effector T lymphocytes, including regulatory T (Treg) cells and T helper 17 (Th17) cells [7,8]. Th17 cells are associated with the pathogenesis of many common autoimmune diseases, such as rheumatoid arthritis (RA) and IBD [[9], [10], [11]]. In recent decades, targeting and regulating Th17 and Treg cell differentiation has become a crucial strategy for the treatment or prevention of UC.
The Notch signaling pathway is a conserved and crucial mechanism for maintaining immune homeostasis by regulating cell differentiation and modulating the inflammatory response to cell fate decision [12]. In mammals, this pathway comprises Notch receptors (Notch1-4), ligands such as Jagged1, Jagged2, Delta1, Delta3, and Delta4, and downstream signaling components. Notch signaling is involved in various aspects of T cell development and differentiation, including the formation of different effector T cell subpopulations [13,14]. Pre-clinical model studies have highlighted that targeting the Notch pathway can enhance the therapeutic potential of immunotherapy, emphasizing the importance of elucidating how the Notch signaling pathway modulates T cell differentiation and function. These findings suggest that Notch blockade could be a potential therapeutic target for UC.
Agrimonia Pilosa Ledeb, a traditional Chinese medicine (TCM), is used for hemostasis, antidiarrhea, and detoxication in China. Agrimonolide (AM), identified as a main active compound of Agrimonia Pilosa Ledeb, has demonstrated anti-inflammatory, antioxidative, and anticancer properties, indicating significant pharmacological value [[15], [16], [17]]. Additionally, the protective effects of Agrimonia Pilosa Ledeb in DSS-induced colitis have been documented [18]. However, the molecular mechanisms underlying the anti-UC activity of its bioactive components remain unexplored.
This research aimed to elucidate the protective effects of AM in the progression of DSS-induced colitis and to determine its underlying mechanism. The results revealed that AM effectively alleviated DSS-treated colitis by modulating the Th17/Treg balance through inhibition of Notch activation and the JAK2/STAT3 signaling pathway. This study aims to identify potential therapeutic Chinese herb medicines for the treatment of UC.
2. Materials and methods
2.1. Chemicals and material
Agrimonolide (purity >98 %, Fig. 1A) was procured from MedChemExpress. The antibodies, including anti-mouse CD45 PC7, anti-mouse CD3 FITC, anti-mouse CD4 PE, anti-mouse interleukin (IL)-17A APC, anti-mouse CD25 PB-A, and anti-mouse Foxp3 PC5.5, were purchased from Biolegend (MA, USA). Sulfasalazine (SASP) was obtained from Sigma (MA, USA).
Fig. 1.
a.m. alleviated DSS-induced colitis in mice induced by DSS. (A) the chemical structure of AM. (B) Schematic diagram of the animal experiment. (C) Body weight of mice. n = 6 each group. (C) Representative colon images. n = 6 each group (D) Colon length. n = 6 each group. (E) Disease activity index of mice. n = 6 each group. (F) Representative images of H&E-stained colon sections in different groups (Scale bar = 100 μm). n = 4 each group. (G) MPO activity in the serum. n = 4 each group. The values are expressed as the mean ± SD, *P < 0.01, **P < 0.001.
2.2. DSS-induced colitis mouse model and treatment
6-week-old male C57BL/6J mice were used in this study. All mice were obtained from Nanjing University of Chinese Medicine (Jiangsu, China) and acclimatized for 7 days before the initiation of experiments. The animal experiment was approved by the Ethics Committee of Nanjing University Of Chinese Medicine (Approval No. 2022042501). Mice were randomly divided into five groups: Control (0.5 % sodium carboxymethyl cellulose [CMC-Na]), DSS, low-dose AM (25 mg/kg/day, ig), high-dose AM (50 mg/kg/day, ig), and salicylazosulfapyridine (SASP, positive control). Mice in the AM groups received 25 mg/kg or 50 mg/kg of the AM solution via gavage, respectively. Colitis was induced by administering 3 % dextran sodium sulfate (DSS) dissolved in the drinking water of C57BL/6J mice. Fig. 1B illustrated the detailed grouping and treatment of mice. For the experiment involving activation of the Notch signaling pathway, a solution of Jagged1 (MedChemExpress, MCE) at a dose of 25 mg/kg was intraperitoneally injected once every other day [19,20]. Finally, all mice were euthanized by cervical dislocation immediately following blood collection. In addition, distal colon tissues were collected for histological examination, and the remaining of the colon tissue was stored at −80 °C for further analysis.
2.3. Disease activity index (DAI)
The DAI is calculated based on body weight loss, stool consistency, and rectal bleeding, as previously described with a few modifications [21]. The scoring system is as follows: weight loss (0–0%; 1–1% ∼ 5 %; 2–5% ∼ 10 %; 3–10 % ∼ 20 %; 4–20 %), diarrheas (0—normal; 2—loose stools; 4—diarrhea), and rectal bleeding (0—normal; 4—gross bleeding).
2.4. Myeloperoxidase (MPO) activity in the colon
The MPO activity assay was performed per manufacturer's instructions using a kit form Nanjing Jiancheng Co., Ltd (Nanjing, China). In brief, mouse colon tissues were weighed and homogenized, followed by incubation at 37 °C for 15 min. The homogenate was then mixed with a chromogenic agent and incubated at 37 °C for 30 min. Afterward, it was combined with the detection reagent and incubated at 60 °C for 10 min. The absorbance was measured at 460 nm.
2.5. Hematoxylin and eosin (H&E) staining
The colonic tissue was sliced to a thickness of 4 μm. The section was then deparaffinized, rehydrated and stained with eosin and hematoxylin. Finally, the sections were observed under a microscope (BX61VS, Olympus, Japan).
2.6. Immunohistochemistry staining
Immunohistochemistry staining was performed on formaldehyde-fixed and paraffin-embedded tissue sections per manufacturer's instructions. Briefly, tissue sections were incubated at 4 °C overnight with primary antibodies against Occludin (1:200) and ZO-1 (1:200), followed by incubation with HRP-conjugated secondary antibodies. Finally, the samples were observed using a light microscope (BX61VS, Olympus, Japan).
2.7. Enzyme-linked immunosorbent assay (ELISA)
The colonic samples were weighed and homogenized in cell extraction buffer at a ratio of 100 mg tissue per 1 mL of buffer. The homogenates were then centrifuged at 18000 rpm for 20 min, and the protein concentration of the samples was normalized. The levels of IL-1β (detection range: 1.56 pg/mL-100 pg/mL), tumor necrosis factor (TNF)-α (detection range: 46.88 pg/mL-3000 pg/mL) and IL-6 (detection range: 15.6 pg/mL-1000 pg/mL) in murine colonic tissues homogenates were assessed using an ELISA kit (Abcam, MA, USA) per manufacturer's instructions. Absorbance per well was measured at 450 nm.
2.8. Measurement of the colonic permeability
Colonic permeability was detected using Fluorescein isothiocyanate-dextran 4 kDa (Sigma, USA). Blood samples were collected 4 h after mice received 60 mg/kg FD4 via intragastric administration. Serum was separated and examined for FD4 concentration. A fluorescence spectrophotometer (excitation 485 nm, emission 520 nm) was subjected to analyze serum FD4 concentrations.
2.9. In vitro Treg and Th17 cell differentiation
Spleens were harvested from C57BL/6J mice and processed into a single-cell suspension. Naïve CD4+ T cells were purified from C57BL/6J mice per manufacturer's instructions, utilizing a naïve CD4+ T cell isolation kit (Biolegend, MA, USA). These naïve CD4+ T cells were cultured in Iscove's Modified Dulbecco Medium (IMDM) supplemented with 20 % foetal bovine serum (FBS). For Th17 cells differentiation, naïve T cells were cultured for 4 days with plate-bound anti-CD3ε (10 μg/mL), anti-CD28 (1 μg/mL), anti-mouse CD25, recombinant mouse IL-6, recombinant human TGF-β1, recombinant mouse IL-23, anti-mouse IL-4 and anti-mouse IFN-γ. To induce Treg cell differentiation, naïve T cells were cultured for 4 days with plate-bound anti-mouse-CD3ε (5 μg/mL), anti-mouseCD28, recombinant mouse IL-2 (200 U/mL), and recombinant human TGF-β1. The frequencies of Th17 and Treg cells were determined by flow cytometry.
2.10. Real-time fluorescence quantitative polymerase chain reaction (RT-qPCR) assays
Total RNA was extracted from tissues and cells using TRIzol reagent (Invtrogen, USA), followed by reverse transcription of 1000 ng RNA into cDNA using PrimeScript™ RT. Amplification reactions were carried out with specific primer pairs and qPCR SYBR Green Master Mix. The fold induction of target gene levels was determined using the comparison method and normalized to the internal control β-actin. The primer sequence used in this study are listed in Table 1.
Table 1.
Primer sequences for RT-qPCR amplification.
| Gene | Sequences (5’→3′) | Ref. | |
|---|---|---|---|
| RORγt | F | CGCCTCACCTGACCTACCC | [22] |
| R | TGGCTGTCTGGACCCTGTTC | ||
| Foxp3 | F | TGCAGTTCCTTTGTGTCCGA | [22] |
| R | ATAGTCACCCCAACACAGCG | ||
| IL-17A | F | TACCTCAACCGTTCCACGTC | [22] |
| R | TTTCCCAACCGCATTGACACA | ||
| TNF-α | F | ACTCCAGGCGGTGCCTATGT | [23] |
| R | GTGAGGGTCTGGGCCATAGAA | ||
| IL-1β | F | TCCAGGATGAGGACATGAGCAC | [23] |
| R | GAACGTCACACACCAGCAGGTTA | ||
| IL-6 | F | CCACTTCACAAGTCGGAGGCTTA | [24] |
| R | TGCAAGTGCATCATCGTTGTTC | ||
| β-actin | F | CTGAGAGGGAAATCGTGCGT | [25] |
| R | CCACAGGATTCCATACCCAAGA | ||
| Notch 1 | F | ACAGTAACCCCTGCATCCAC | [26] |
| R | GGTTGGACTCACACTCGTTG | ||
| Jagged1 | F | CCAGCCAGTGAAGACCAAGT | [26] |
| R | CAATTCGCTGCAAATGTGTT | ||
| Delta-like ligand 4 (DDL4) | F | GGAACCTTCTCACTCAACATCC | [26] |
| R | CTCGTCTGTTCGCCAAATCT |
2.11. Flow cytometry
Treg and Th17 cells were detected using flow cytometry. Initially, mesenteric lymph nodes (MLNs) and spleens were stained with anti-CD4, anti-CD25, and anti-Foxp3 antibodies to identify Treg cells. For the identification of Th17 cells, lymphocytes were stimulated with a Cell Stimulation Cocktail (plus protein transport inhibitors) containing phorbol 12-myristate 13-acetate (PMA), ionomycin, brefeldin A for 6 h in 5 % CO2 at 37 °C. Subsequently, the cells were stained with a Zombie Fixable Viability Kit for 15 min at RT in the dark. Then, the cells were stained with anti-CD45, anti-CD4 and anti-IL-17 antibodies. Cells were initially stained using the Zombie Fixable Viability Kit. Anti-CD45 and anti-CD4 antibodies were then applied for a 30-min incubation at 4 °C in darkness. Following this, the cells were fixed and permeabilized using a Foxp3/Transcription staining buffer set for 30 min at room temperature in darkness. The percentages of CD4+CD25+Foxp3+ (Treg) cells and CD4+IL-17+ (Th17) cells were analyzed using FlowJo V.10 software. The gating strategies for the FACS analysis of the percentages of Th17 cells and Treg cells were shown in Supplementary Fig. 1 in the Supplemental information.
2.12. Western blotting (WB)
Colonic tissue was lysed on ice for 30 min in radio immunoprecipitation assay (RIPA) buffer and collected using a cell scraper. Subsequently, 30–40 μg of protein from each sample was separated using 10 % (w/v) sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene fluoride (PVDF) membranes (Millipore, USA). Next, the membrane was blocked with 5 % BSA for 1 h and incubated overnight with primary antibodies, including ZO-1 (1:1000, #5406, Cell Signaling Technology, CST), Occludin (1:1000, #91131, CST), phospho (p)-IKKα/β (1:1000, #2697, CST), IKKα (1:1000, #2682, CST), p-IκBα (1:1000, #2859, CST), IκBα (1:1000, #4812, CST), p-p65 (1:1000, #3033, CST), p65 (1:1000, #8242, CST), Notch1 (1:1000, #3608, CST), Jagged1 (1:1000, #70109, CST), DLL4 (1:1000, #2589, CST), p-JAK2 (1:1000, #3776, CST), JAK2 (1:1000, #3230, CST), p-STAT3 (1:1000, #9145, CST), STAT3 (1:1000, #30835, CST), and GAPDH (1:1000, #5174, CST). After incubation, the membrane was probed with a secondary antibody (1:5000, ab6721, Abcam, MA, USA) for 1 h. Protein bands were visualized using enhanced chemiluminescence (ECL) reagents (Beyotime, China), and the integrated density was quantified using ImageJ software.
2.13. Statistical analysis
GraphPad Prism 8 were employed to analyze the experimental data. One-way ANOVA was subjected to analyze differences between groups. Data were presented as the means ± SD, and significance was determined at P < 0.05.
3. Results
3.1. Agrimonolide alleviated DSS-induced colitis in mice
A mouse model of DSS-induced colitis was employed to evaluate the efficacy, safety, and mechanism of AM. Administration of AM led to an increase in body weight compared to the DSS group (Fig. 1C). Colon shortening is a key macroscopic indicator of intestinal damage, and both dose of AM and SASP effectively restored colon length in colitis mice (Fig. 1D and E). The severity of colitis was further assessed by DAI score, which was significantly elevated in DSS-treated mice but significantly reduced with AM pretreatment (Fig. 1F). Histological analysis revealed alleviation of mucosal damage, inflammatory cell infiltration, and crypt structure destruction in the AM-treated mice group (Fig. 1G). After 10 days of AM administration, symptoms and colon damage were notably mitigated compared to the DSS group. MPO activity, indicative of neutrophile granulocyte infiltration, was significantly increased in DSS-treated colitis, but AM pretreatment effectively suppressed MPO activity in colon tissues (Fig. 1H). Overall, pretreatment with AM effectively attenuated the symptoms of DSS-induced colitis.
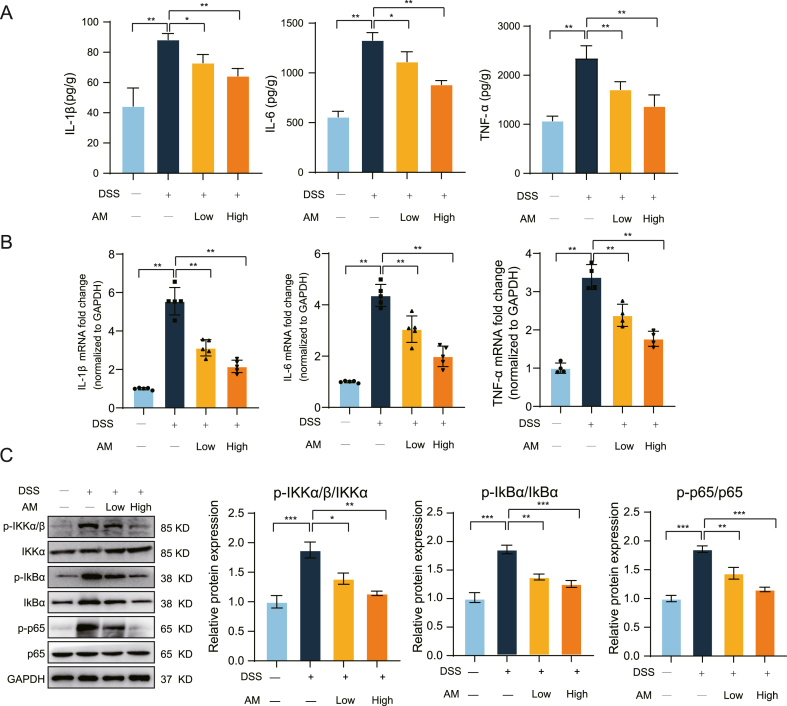
3.2. Agrimonolide suppressed the production of immune-inflammatory cytokines in mice induced by DSS
Inflammatory cytokines, including IL-1β, TNF-α, and IL-6, play a crucial role in the progression of colitis pathogenesis [27]. To assess the inflammatory response in colonic tissue treated with AM, ELISA and RT-qPCR assays were conducted. As shown in Fig. 2A, DSS induction obviously increased the secretion of IL-1β, TNF-α, and IL-6 compared to the control group. However, AM pretreatment notably suppressed the upregulated of these inflammatory factors in the colonic tissue of DSS-induced colitis mice. Similar results were observed in RT-qPCR experiments (Fig. 2B). The activation of the nuclear factor-κB (NF-κB) signaling pathway is closely associated with the inflammatory response, and our findings indicated that AM pretreatment significantly reduced the phosphorylation levels of IKKα/β, IκBα, and p65, key molecules in the NF-κB pathway (Fig. 2C). These findings support the conclusion that AM effectively inhibited the levels of immune-inflammatory cytokines, thereby alleviating colonic inflammatory damage in mice with UC.
Fig. 2.
a.m. inhibited the secretion of inflammatory cytokines in mice with colitis. (A and B) The concentration and mRNA expression levels of IL-6, TNF-α, and IL-1β in the colon tissue of mice. n = 5 each group. (C) The NF-κB signaling pathway was examined using Western blotting. n = 5 each group. The values are expressed as the mean ± SD, *P < 0.01, **P < 0.001.
3.3. Agrimonolide enhanced the recovery of the intestinal mucosal barrier in mice with colitis
During the progression of UC, abnormal changes in the permeability of the intestinal mucosa led to bacteria translocation and aberrant inflammation of the colon [28]. Initially, we conducted immunohistochemistry and Western blot analysis to evaluate the expression of tight junction (TJ) proteins in colon tissue. As shown in Fig. 3A and B, the expression of TJ proteins, including Occludin and ZO-1, was markedly decreased following DSS administration compared to the control group. However, AM treatment significantly increased the protein expression levels of Occludin and ZO-1. To further assess intestinal permeability, we used FITC-dextran. The results indicated that AM had a protective effect on the intestinal barrier damage induced by DSS (Fig. 3C).
Fig. 3.
a.m. improved recovery of intestinal mucosal barrier in mice with colitis. (A) The colonic protein levels of Occludin and ZO-1 in each group were examined by Western blotting. n = 4 each group. (B) Representative images of immunohistochemistry staining for Occludin and ZO-1 in different groups. (Scale bar = 50 μm). n = 4 each group. (C) The serum FITC-dextran concentrations of mice in each group. n = 5 each group. The values are expressed as the mean ± SD, *P < 0.01, **P < 0.001.
3.4. Effect of agrimonolide on the proportions of Th17 and Treg cells in mice induced by DSS
It is well recognized that the imbalance of Th17/Treg cells in the intestinal mucosa plays a crucial role in the pathogenesis of UC [29,30]. To investigate the effect of AM pretreatment on Treg/Th17 homeostasis, we characterized T cell responses in the MLNs using flow cytometry. We observed a significant increase in the absolute number of CD4+IL-17A+ Th17 cells within the CD3+ T cell compartment in the MLNs of DSS-treated mice compared to the control group (Fig. 4A). However, this increase was markedly reversed by AM pretreatment. Additionally, AM pretreatment elevated the percentage of CD4+CD25+Foxp3+ Treg cells within the CD4+T cells compartment compared to the DSS group (Fig. 4B). To further explore the regulatory effect of AM on Th17/Treg cell imbalance, we examined Th17 and Treg-specific transcription factors ROPγt and Foxp3 using RT-qPCR and Western blot assays. As presented in Fig. 4C and D, there were significant changes in the protein and mRNA levels of ROPγt and Foxp3 in the colonic tissues of AM-treated mice compared to the DSS group. A similar trend was observed in the spleen (Fig. 4E and F).
Fig. 4.
a.m. suppressed Th17/Treg cell differentiation in intestinal microenvironment. (A) Representative plots of CD4+IL-17A+Th17 cells and (B) CD4+CD25+Foxp3+Treg cells in the MLNs of DSS and DSS+AM group. n = 4 each group. (C) The mRNA level of Foxp3 and RORγt were determined using RT-qPCR. n = 4 each group. (D) The protein expression of Foxp3 and RORγt were tested using Western blot. n = 4 each group. (E) Representative plots of CD4+IL-17A+Th17 cells and (F) CD4+CD25+Foxp3+Treg cells in the spleen of mice. n = 4 each group. The values are expressed as the mean ± SD, *P < 0.01, **P < 0.001.
In summary, these results indicate that AM effectively repressed the imbalance between Th17 and Treg cells in the MLNs and spleen of DSS-induced mice.
3.5. Agrimonolide preserved the balance of Th17/Treg cells in mice induced by DSS through modulation of the Notch and JAK2/STAT3 signaling pathway
As previously mentioned, AM modulated the hyperimmunity in DSS-treated colitis via skewing the imbalance in Th17 and Treg differentiation. Aberrant activation of the Notch signaling pathway can disturb Th17/Treg cell homeostasis [31]. Considering these findings, we evaluated whether AM modulates this cell balance via the Notch pathway in DSS mice. The expression levels of Notch-1, Jagged-1, and DLL4 were measured using RT-qPCR and Western blotting assays. As shown in Fig. 5A, the mRNA level of Notch-1, Jagged-1, and DLL4 were markedly increased in the colonic tissues of DSS-treated mice. Furthermore, Western blot results indicated that the protein expression levels of Notch-1, Jagged-1, and DLL4 were higher in DSS-induced mice than in the control group (Fig. 5B). Notably, AM pre-conditioning significantly repressed the Notch signaling pathway in colonic tissue, as evidenced by a significant reduction in mRNA levels and protein levels of Notch-1, Jagged-1, and DLL4. The JAK2/STAT3 pathway has a potential role in modulating the balance of Th17/Treg. The expression of p-JAK2 and p-STAT3 in the colon of the DSS group were obviously higher than those in the control group. However, pretreatment with AM in DSS mice inhibited the phosphorylation of JAK2 and STAT3 (Fig. 5C). As illustrated in Fig. 5D, immunofluorescence assay further confirmed these results, showing reduced protein expression of Notch1 and STAT3.
Fig. 5.
a.m. inhibited Notching pathway signaling in DSS-treated mice. (A) The mRNA level of Notch 1, Jagged1 and DDL4 in mice colonic tissue were examined using RT-qPCR. n = 4 each group. (B) Western blotting was employed to detect the protein expression of Notch 1, Jagged1 and DDL4 in mice colonic tissue. n = 4 each group. (C) Western blotting was employed to detect the protein expression of p-JAK2, JAK2, p-STAT3 and STAT3 in mice colonic tissue. n = 4 each group. (D) Immunofluorescence was used to measure the expression of Notch1 and STAT3. n = 4 each group. The values are expressed as the mean ± SD, *P < 0.01, **P < 0.001.
In conclusion, these findings indicate that alterations in the Notch and JAK2/STAT3 signaling pathways partially account for the effects of AM activity on Th17/Treg responses in mice with DSS-induced colitis.
3.6. Agrimonolide inhibited Th17 cell differentiation and promoted Treg cell differentiation in vitro
To corroborate our in vivo findings, we conducted in vitro experiments to assess the effect of AM on the modulation of Th17/Treg cells. As presented in Fig. 6A, the proportion of CD4+IL17A+Th17 T cells was elevated in the Th17 group, but this increase was reduced following AM treatment. Additionally, the inhibitory effects of AM on gene expression of RORγt were determined by RT-qPCR (Fig. 6B). Flow cytometry analysis and RT-qPCR further demonstrated significant changes in the frequency of CD25+Foxp3+ Treg cells and the mRNA level of Foxp3 after AM intervention compared to the Treg group (Fig. 6C and D). In conclusion, AM decreased the number of Th17 cells and increased the proportion of Treg cells in vitro.
Fig. 6.
a.m. repressed Th17 cell differentiation and promoted Treg production in vitro. (A) Representative plots of CD4+IL-17A+Th17 cells in CD4+ cells. n = 5 each group. (B) RT-qPCR assay was subjected to examine the mRNA level of RORγt. n = 5 each group (C) Representative plots of CD4+CD25+Foxp3+Treg cells in CD4+ cells. n = 5 each group. (D) RT-qPCR assay was subjected to examine the mRNA level of Foxp3. n = 5 each group. The values are expressed as the mean ± SD, *P < 0.01, **P < 0.001.
3.7. The protective effect of AM against DSS-induced colitis relied on the Notch signaling pathway
To further investigate the role of Notch signaling in AM-alleviated colitis in mice, we used the Notch activator Jagged1. Colitis mice were administrated Jagged1 with or without AM. Notably, Jagged1 significantly diminished the therapeutic effects of AM in DSS-induced mice, as evidenced by changes in colon length (Fig. 7A). Additionally, Jagged1 treatment increased the percentage of CD4+IL-17A+ Th17 cells and decreased the proportion of CD4+CD25+Foxp3+ Treg cells in MLNs (Fig. 7B). Taken together, these findings suggest that the Notch signaling pathway plays a crucial role in AM-mediated restoration of the Th17/Treg balance.
Fig. 7.
Protective effect of AM against DSS-induced colitis depended on Notch signaling pathway. (A) Colon length of mice. n = 5 each group. (B) Representative plots of CD4+IL-17A+Th17 cells and CD4+CD25+Foxp3+Treg cells. n = 5 each group. The values are expressed as the mean ± SD, *P < 0.01, **P < 0.001.
4. Discussion
The incidence of UC has been steadily rising globally [32]. Patients with UC often suffer from both mental and physical challenges, including symptoms like diarrhea, bloody stool, mucosal injury, and colonic ulcers, and are at a higher risk of developing colorectal cancer compared to non-UC individuals [33]. Currently, therapeutic strategies for UC focus on modulating the inflammatory milieu and promoting intestinal barrier repair. In this study, established a mouse model of DSS-induced colitis to investigate the therapeutic potential and underlying mechanisms of AM against UC. Our initial findings confirmed that AM preconditioning significantly ameliorated DSS-induced colitis, as evidenced by body weight, colon length, DAI scores, and pathological features. Additionally, we observed a decreased in MPO activity in the colon tissue of DSS-induced colitis mice treated with AM, indicative of reduced inflammation and potentially decreased immune cell infiltration.
Given that increased levels of proinflammatory cytokines IL-1β, TNF-α, and IL-6 are known to drive intestinal inflammation, we selected these cytokines as targets to explore the impact of AM on DSS-induced colitis. As anticipated, AM demonstrated inhibitory effects on the aberrant levels of IL-1β, TNF-α, and IL-6 in DSS-induced colitis mice. The NF-κB pathway is activated in DSS-induced colitis mice, leading to the overproduction of inflammatory factors. These cytokines further promote the infiltration of inflammatory cells and the development of intestinal inflammation. Additionally, AM has been shown to attenuate DSS-induced colitis by inhibiting inflammation through the inactivation of the NF-κB pathway. As crucial constituents of the TJ complex, transmembrane proteins such as Occludin and ZO-1 play a vital role in preserving barrier integrity [34]. Therefore, we confirmed that AM significantly restored the expression of Occludin and ZO-1 in the mice with UC, potentially enhancing intestinal mucosal barrier integrity.
The onset of UC is closely associated with an overactive Th17 immune response and impaired functioning of Treg functioning [35,36]. Research has shown that the NF-κB signaling pathway plays a significant role in maintaining the balance between these two immune cell types. The transcription of IL-17 is regulated by the Th17-specific transcription factor retinoic acid-related orphan receptor γt (RORγt) [35,36], while Foxp3 is a Treg-specific transcription factor crucial for the differentiation and normal physiological function of Treg cells [37]. Our study found that AM inhibited the differentiation of IL-17-producing cell subsets and increased the number of Treg cells in the MLNs and spleen. Consistent with in vivo results, AM treatment significantly inhibited Th17 cell differentiation and promoted Treg cell production in vitro. These findings suggest that AM improves DSS-induced colitis by correcting the imbalance between Th17 and Treg cells.
Emerging evidence indicates that the Notch signaling pathway is implicated in numerous inflammation-related diseases, including intestinal inflammation. Notably, this pathway induces the differentiation of naïve CD4+ T cells into Th17 and Treg cells and facilitates their interconversion [38]. Clinical evidence has shown that molecules associated with Notch signaling are highly activated in patients with UC, correlating with elevated Th17 responses [[39], [40], [41]]. Given these findings, we investigated whether AM modulates Notch signaling to alleviate colitis symptoms. Our research highlights the significant role of the Notch signaling pathway, demonstrating that the therapeutic effects of AM and its regulation of Th17 and Treg cells in colitis mice are mediated through this pathway. Additionally, existing evidence has shown that the Janus kinase 2 (JAK2) and signal transducer and activators of transcription 3 (STAT3) are tightly linked to the severity of UC clinical features and play distinct roles in T cell proliferation and differentiation. Research suggests that excessive activation of the STAT3 pathway can drive a Th17-like conversion of Tregs, leading to an escalation of the immune response. Our experimental results indicate that AM modulates the Notch and JAK2/STAT3 signaling pathways to reshape the balance of Th17/Treg cells, thereby treating colitis.
In conclusion, our study demonstrates that AM effectively attenuated clinical symptoms, reduced inflammatory cytokine expression, and repaired the intestinal mucosal barrier in DSS-induced colitis mice. Mechanistically, AM corrected the imbalance of Th17/Treg cells by inhibiting the Notch and JAK/STAT3 signaling pathways. These findings provide a solid theoretical basis for the application of AM in treating patients with UC. However, further preclinical studies are necessary to validate these results and explore the therapeutic potential of AM.
Funding
This study was financially supported by the Jiangsu Province Traditional Chinese medicine science and technology development program (MS2021058), Natural Science Foundation of Nanjing University of Chinese Medicine (XZR2020062) for their great supports in financing this research.
Consent for publication
Not applicable.
Ethics statement
The animal experiments were approved by the Ethics Committee of Nanjing University Of Chinese Medicine, Approval number: 2022042501.
Data availability statement
Data will be made available on request.
CRediT authorship contribution statement
Jie Jiang: Writing – original draft, Conceptualization. Yuxiang Sheng: Data curation. Zheng Zheng: Formal analysis, Data curation. Fuhao Qin: Investigation, Data curation. Bin Jiang: Writing – review & editing, Funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Not applicable.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e33803.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
References
- 1.F. D'Amico, L. Peyrin-Biroulet, S. Danese, G. Fiorino, New drugs in the pipeline for the treatment of inflammatory bowel diseases: what is coming? Curr. Opin. Pharmacol. 552020) 141-150. [DOI] [PubMed]
- 2.Ramos G.P., Papadakis K.A. Mechanisms of disease: inflammatory bowel diseases. Mayo Clin. Proc. 2019;94(1):155–165. doi: 10.1016/j.mayocp.2018.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Larrosa P.F., Bondesson E., Schelin M., Joud A. A diagnosis of rheumatoid arthritis, endometriosis or ibd is associated with later onset of fibromyalgia and chronic widespread pain. Eur. J. Pain. 2019;23(8):1563–1573. doi: 10.1002/ejp.1432. [DOI] [PubMed] [Google Scholar]
- 4.Neurath M.F. Targeting immune cell circuits and trafficking in inflammatory bowel disease. Nat. Immunol. 2019;20(8):970–979. doi: 10.1038/s41590-019-0415-0. [DOI] [PubMed] [Google Scholar]
- 5.Dimitrijevic M., Arsenovic-Ranin N., Kosec D., et al. Sexual dimorphism in th17/treg axis in lymph nodes draining inflamed joints in rats with collagen-induced arthritis. Brain Behav. Immun. 2019;76:198–214. doi: 10.1016/j.bbi.2018.11.311. [DOI] [PubMed] [Google Scholar]
- 6.Rezalotfi A., Solgi G., Ebrahimi M. Gastrospheres as a model of gastric cancer stem cells skew th17/treg balance toward antitumor th17 cells. J Immunol. Res. 2020;2020 doi: 10.1155/2020/6261814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu Y.J., Tang B., Wang F.C., et al. Parthenolide ameliorates colon inflammation through regulating treg/th17 balance in a gut microbiota-dependent manner. Theranostics. 2020;10(12):5225–5241. doi: 10.7150/thno.43716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang G., Xia Y., Ren W. Glutamine metabolism in th17/treg cell fate: applications in th17 cell-associated diseases. Sci. China Life Sci. 2021;64(2):221–233. doi: 10.1007/s11427-020-1703-2. [DOI] [PubMed] [Google Scholar]
- 9.Chang Y., Zhai L., Peng J., Wu H., Bian Z., Xiao H. Phytochemicals as regulators of th17/treg balance in inflammatory bowel diseases. Biomed. Pharmacother. 2021;141 doi: 10.1016/j.biopha.2021.111931. [DOI] [PubMed] [Google Scholar]
- 10.Lee G.R. The balance of th17 versus treg cells in autoimmunity. Int. J. Mol. Sci. 2018;19(3) doi: 10.3390/ijms19030730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shan J., Jin H., Xu Y. T cell metabolism: a new perspective on th17/treg cell imbalance in systemic lupus erythematosus. Front. Immunol. 2020;11:1027. doi: 10.3389/fimmu.2020.01027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mirandola L., Comi P., Cobos E., Kast W.M., Chiriva-Internati M., Chiaramonte R. Notch-ing from t-cell to b-cell lymphoid malignancies. Cancer Lett. 2011;308(1):1–13. doi: 10.1016/j.canlet.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 13.Garcia E.G., Veloso A., Oliveira M.L., et al. Prl3 enhances t-cell acute lymphoblastic leukemia growth through suppressing t-cell signaling pathways and apoptosis. Leukemia. 2021;35(3):679–690. doi: 10.1038/s41375-020-0937-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paiva R.A., Sousa A., Ramos C.V., et al. Self-renewal of double-negative 3 early thymocytes enables thymus autonomy but compromises the beta-selection checkpoint. Cell Rep. 2021;35(2) doi: 10.1016/j.celrep.2021.108967. [DOI] [PubMed] [Google Scholar]
- 15.Y. Liu, X. Liu, H. Wang, P. Ding, C. Wang, Agrimonolide inhibits cancer progression and induces ferroptosis and apoptosis by targeting scd1 in ovarian cancer cells, Phytomedicine 1012022) 154102. [DOI] [PubMed]
- 16.Teng H., Chen L., Song H. The potential beneficial effects of phenolic compounds isolated from a. Pilosa ledeb on insulin-resistant hepatic hepg2 cells. Food Funct. 2016;7(10):4400–4409. doi: 10.1039/c5fo01067e. [DOI] [PubMed] [Google Scholar]
- 17.Teng H., Huang Q., Chen L. Inhibition of cell proliferation and triggering of apoptosis by agrimonolide through map kinase (erk and p38) pathways in human gastric cancer ags cells. Food Funct. 2016;7(11):4605–4613. doi: 10.1039/c6fo00715e. [DOI] [PubMed] [Google Scholar]
- 18.Li C., Wang M., Sui J., Zhou Y., Chen W. Protective mechanisms of agrimonia pilosa ledeb in dextran sodium sulfate-induced colitis as determined by a network pharmacology approach. Acta Biochim. Biophys. Sin. 2021;53(10):1342–1353. doi: 10.1093/abbs/gmab116. [DOI] [PubMed] [Google Scholar]
- 19.Xiang Y., Tan M., Ning Z., Zhang Y. Anti-cerebral ischemic neuronal injury mechanism of zhenlong xingnao capsules: role of the notch/nf-kappab signaling pathway. Am. J. Transl. Res. 2023;15(7):4587–4599. [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou Y., Liu D., Li H. Fgl1 promotes tumor immune escape in stomach adenocarcinoma via the notch signaling pathway. Mol. Biotechnol. 2023 doi: 10.1007/s12033-023-00928-3. [DOI] [PubMed] [Google Scholar]
- 21.Guo H., Guo H., Xie Y., et al. Mo(3)se(4) nanoparticle with ros scavenging and multi-enzyme activity for the treatment of dss-induced colitis in mice. Redox Biol. 2022;56 doi: 10.1016/j.redox.2022.102441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao X., Yi Y., Jiang C., et al. Gancao fuzi decoction regulates the th17/treg cell imbalance in rheumatoid arthritis by targeting foxp3 via mir-34a. J. Ethnopharmacol. 2023;301 doi: 10.1016/j.jep.2022.115837. [DOI] [PubMed] [Google Scholar]
- 23.Li J., Yan S., Han W., et al. Phospholipid-grafted plla electrospun micro/nanofibers immobilized with small extracellular vesicles from rat adipose mesenchymal stem cells promote wound healing in diabetic rats. Regen. Biomater. 2022;9 doi: 10.1093/rb/rbac071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang X., Sun H., Liu H., et al. Microrna-181b-5p modulates tumor necrosis factor-alpha-induced inflammatory responses by targeting interleukin-6 in cementoblasts. J. Cell. Physiol. 2019;234(12):22719–22730. doi: 10.1002/jcp.28837. [DOI] [PubMed] [Google Scholar]
- 25.Gao C., Liu Y., Jiang C., et al. Intensive running enhances nf-kappab activity in the mice liver and the intervention effects of quercetin. Nutrients. 2020;12(9) doi: 10.3390/nu12092770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Batista M.R., Diniz P., Murta D., Torres A., Lopes-Da-Costa L., Silva E. Balanced notch-wnt signaling interplay is required for mouse embryo and fetal development. Reproduction. 2021;161(4):385–398. doi: 10.1530/REP-20-0435. [DOI] [PubMed] [Google Scholar]
- 27.Friedrich M., Pohin M., Powrie F. Cytokine networks in the pathophysiology of inflammatory bowel disease. Immunity. 2019;50(4):992–1006. doi: 10.1016/j.immuni.2019.03.017. [DOI] [PubMed] [Google Scholar]
- 28.P. Li, N. Xiao, L. Zeng, et al., Structural characteristics of a mannoglucan isolated from Chinese yam and its treatment effects against gut microbiota dysbiosis and dss-induced colitis in mice, Carbohydr. Polym. 2502020) 116958. [DOI] [PubMed]
- 29.Wang D., Huang S., Yuan X., et al. The regulation of the treg/th17 balance by mesenchymal stem cells in human systemic lupus erythematosus. Cell. Mol. Immunol. 2017;14(5):423–431. doi: 10.1038/cmi.2015.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.M. Xu, X.Y. Duan, Q.Y. Chen, et al., Effect of compound sophorae decoction on dextran sodium sulfate (dss)-induced colitis in mice by regulating th17/treg cell balance, Biomed. Pharmacother. 1092019) 2396-2408. [DOI] [PubMed]
- 31.Marquez-Exposito L., Rodrigues-Diez R.R., Rayego-Mateos S., et al. Deletion of delta-like 1 homologue accelerates renal inflammation by modulating the th17 immune response. Faseb. J. 2021;35(1) doi: 10.1096/fj.201903131R. [DOI] [PubMed] [Google Scholar]
- 32.Tang S., Liu W., Zhao Q., et al. Combination of polysaccharides from astragalus membranaceus and codonopsis pilosula ameliorated mice colitis and underlying mechanisms. J. Ethnopharmacol. 2021;264 doi: 10.1016/j.jep.2020.113280. [DOI] [PubMed] [Google Scholar]
- 33.Wang K., Jin X., Li Q., et al. Propolis from different geographic origins decreases intestinal inflammation and bacteroides spp. Populations in a model of dss-induced colitis. Mol. Nutr. Food Res. 2018;62(17) doi: 10.1002/mnfr.201800080. [DOI] [PubMed] [Google Scholar]
- 34.Niu W., Chen X., Xu R., et al. Polysaccharides from natural resources exhibit great potential in the treatment of ulcerative colitis: a review. Carbohydr. Polym. 2021;254 doi: 10.1016/j.carbpol.2020.117189. [DOI] [PubMed] [Google Scholar]
- 35.Saez A., Gomez-Bris R., Herrero-Fernandez B., Mingorance C., Rius C., Gonzalez-Granado J.M. Innate lymphoid cells in intestinal homeostasis and inflammatory bowel disease. Int. J. Mol. Sci. 2021;22(14) doi: 10.3390/ijms22147618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao Y., Luan H., Jiang H., et al. Gegen qinlian decoction relieved dss-induced ulcerative colitis in mice by modulating th17/treg cell homeostasis via suppressing il-6/jak2/stat3 signaling. Phytomedicine. 2021;84 doi: 10.1016/j.phymed.2021.153519. [DOI] [PubMed] [Google Scholar]
- 37.Huang J., Yang Z., Li Y., et al. Lactobacillus paracasei r3 protects against dextran sulfate sodium (dss)-induced colitis in mice via regulating th17/treg cell balance. J. Transl. Med. 2021;19(1):356. doi: 10.1186/s12967-021-02943-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yu S., Liu C., Li L., et al. Inactivation of notch signaling reverses the th17/treg imbalance in cells from patients with immune thrombocytopenia. Lab. Invest. 2015;95(2):157–167. doi: 10.1038/labinvest.2014.142. [DOI] [PubMed] [Google Scholar]
- 39.H. Li, L. Wang, Y. Pang, et al., In patients with chronic aplastic anemia, bone marrow-derived mscs regulate the treg/th17 balance by influencing the notch/rbp-j/foxp3/rorgammat pathway, Sci Rep 72017) 42488. [DOI] [PMC free article] [PubMed]
- 40.Melnik B.C., John S.M., Chen W., Plewig G. T helper 17 cell/regulatory t-cell imbalance in hidradenitis suppurativa/acne inversa: the link to hair follicle dissection, obesity, smoking and autoimmune comorbidities. Br. J. Dermatol. 2018;179(2):260–272. doi: 10.1111/bjd.16561. [DOI] [PubMed] [Google Scholar]
- 41.Fasching P., Stradner M., Graninger W., Dejaco C., Fessler J. Therapeutic potential of targeting the th17/treg axis in autoimmune disorders. Molecules. 2017;22(1) doi: 10.3390/molecules22010134. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.