Abstract
In recent years, the exploration of the therapeutic potential of Salvia has gained considerable attention, leading to a growing number of scientific studies emphasizing its pharmacological properties. Despite this, therapeutic applications of Salvia remain underexploited, requiring further investigation. Iran is a major center for sage diversity in Asia, boasting 60 Salvia species, 17 of which are unique to the area. This study aimed to comprehensively explore and compare the extracts of 102 Salvia samples belonging to 20 distinct Salvia species from Iran, providing a deeper understanding of their specific polyphenol content and, consequently, their antioxidant capabilities and potential therapeutic uses. All samples were analyzed to determine the contents of total phenolics, total flavonoids, total tannin, photosynthetic pigments, and ascorbic acid, along with their antioxidant activity. These data were then combined with the forty distinct chemical fingerprints identified by ultrafast high-pressure liquid chromatography coupled with high-resolution mass spectrometry. Multivariate data analysis was employed to find correlations and differences among the huge number of data obtained and to identify Salvia species with similar phytochemical and/or antioxidant properties. The results show that each Salvia species is characterized by a distinct class of polyphenols recognized for their antidiabetic, anti-inflammatory, cardioprotective and neuroprotective properties. Overall, our findings reveal the potential of some Salvia species for targeted therapeutic applications and provide a rational basis for the development of Salvia-derived nutraceuticals, ultimately improving the prospects for the use of Salvia in medicine.
Keywords: Salvia, Antioxidants, Polyphenolic composition, UHPLC-HRMS, Principal component analysis
Subject terms: Natural variation in plants, Secondary metabolism
Introduction
The Lamiaceae family is composed of approximately 250 genera and 7000 species worldwide. Salvia is one of the largest and most valuable genera of the Lamiaceae family, with over 1000 species distributed worldwide1,2. The Salvia genus (sage) is widely recognized as one of the most important and widespread medicinal and aromatic groups within the Lamiaceae family. Sages have traditionally been used for various medicinal purposes, including as spasmolytics, antiseptics, antihidrotics, anti-inflammatories, and in the treatment of mental conditions and nervous disorders3,4.
Phytochemical analysis of Salvia species has revealed the presence of various compounds with a significant antioxidant role, as reported by Kahnamoei et al.5 and Kan et al.6. Major phytochemicals identified within Salvia species include flavonoids, anthocyanins, phenolic acids, phenolic glycosides, polysaccharides, terpenoids, coumarins, and essential oils7,8. Among these, terpenoids and polyphenols stand out as the most prominent secondary metabolites in Salvia species. In sage plants, among flavonoids, flavones like luteolin and apigenin, along with their corresponding flavonols and 6-hydroxylated derivatives, represent the most abundant compounds. Caffeic acid, found in various Salvia species, serves as a fundamental component for various metabolites, spanning from simple monomers to oligomers. Notably, rosmarinic acid emerges as the predominant dimer of caffeic acid identified in sage plants6. Additionally, trimers derived from caffeic acid, such as salvianolic, lithospermic, and yunnaneic acids, as well as sage-coumarin also exhibits a significant presence9–11.
A positive correlation was observed between phenolic content and antioxidant activity, as reported by Kamatou et al.12. Notably, species with high phenolic content demonstrated the most promising antioxidant activity12. Phenolic compounds are extensively distributed in Salvia species13, and studies by Kamatou et al.12, Farhat et al.14, and Tepe et al.15 have reported the potent radical scavenging abilities of various Salvia species.
Salvia species are known to contain a wide range of terpenoid compounds. As an example, borneol, camphor, caryophyllene, and α- and β-thujone are widely mentioned in essential oil of Salvia species, and other characteristic diterpenoid quinones have been found in Salvia extracts, including tanshinone I, tanshinone IIA, cryptotanshinone and miltirone. These compounds, together with polyphenolics (such as rosmarinic acid, caffeic acid and its metabolites, yunnaneic and salvianolic acids) and flavonoids (like luteolin, apigenin, and kaempferol), contribute to a variety of bioactivities. Studies by Wu et al.8, Lu and Foo9, Ghorbani and Esmaeilizadeh16, Jassbi et al.17, Sharifi-Rad et al.18, Topçu19 and Fierascu20 confirm the presence of various bioactive compounds in Salvia plants.
However, the specific types and concentrations of phytochemicals can vary significantly among different species of Salvia, contributing to their diverse pharmacological properties, which include antioxidant, anti-inflammatory, antidiabetic, and antimicrobial effects, among others. Moreover, the qualitative and quantitative composition of phytochemicals in sage plants, which is crucial for its beneficial properties, is strongly influenced by a multitude of factors, including genetics and specie variations, environmental factors, cultivation techniques, and post-harvest processing. Environmental factors, like temperature and rainfall, play a significant role in the biosynthesis of phenolic compounds in sage leaves. Changes in these environmental conditions can impact the metabolic pathways within the plant, influencing the production of phenolic compounds. For instance, certain phenolic compounds might be produced in higher quantities in response to specific temperature ranges. Rainfall or moisture levels in the soil can also impact the plant’s stress levels, triggering the production of secondary metabolites, like phenolic compounds, as a defense mechanism. Therefore, understanding how environmental factors impact the growth, yield, and physiological processes of alternative crops like Salvia, is crucial for effective cultivation and optimizing the production of beneficial phytochemicals. The studies by Cardile et al.21, Mancini et al.22, Canzoneri et al.23, and Tenore et al.24 have explored these impacts in detail.
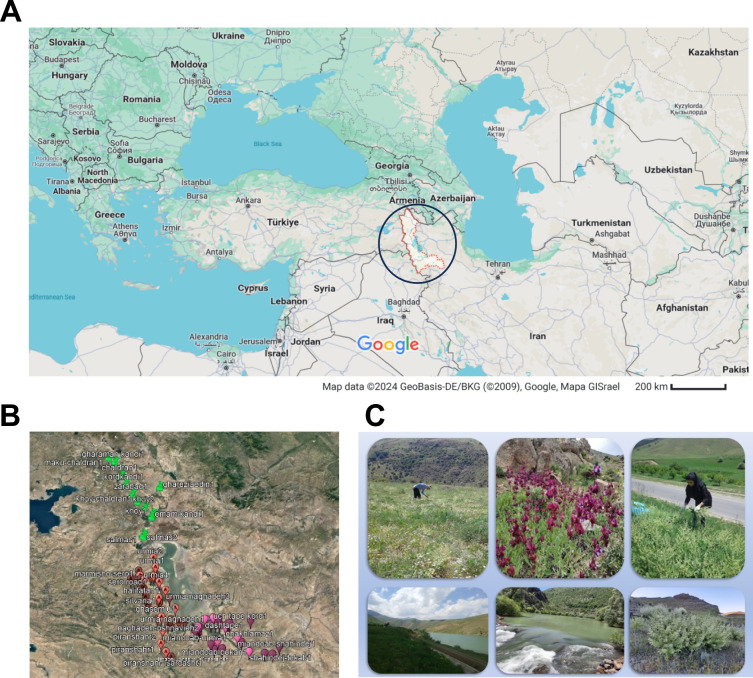
Investigating the phytochemical profile and antioxidant activity of Salvia is of paramount importance due to the growing interest in natural antioxidants and their potential health benefits. In the present study, 102 Salvia samples belonging to 20 different species (Fig. 1, Table 1), collected from various locations in the Iranian province of the West Azerbaijan (Fig. 2) were examined and their phytochemical properties were compared to select the superior species based on phytochemical markers. For this purpose, the contents of total phenolics, total flavonoids, total tannin, photosynthetic pigments, and ascorbic acid were analyzed, along with the antioxidant activity. These data were combined with the chemical fingerprints determined by ultrafast high-pressure liquid chromatography coupled with high-resolution mass spectrometry (UHPLC-HRMS) and then analyzed by employing multivariate data analysis. This approach enables simultaneous analysis of multiple variables, providing a more comprehensive understanding of the complex relationships within the data. Unlike many existing studies in the field, our research takes a broader approach by examining correlations and differences between a wide range of phytochemical and antioxidant properties among various Salvia species. Rather than quantifying individual phytomarkers, our goal is to identify similarities and differences between species. This holistic approach not only provides valuable insights into the intricate interplay of phytochemicals, but also holds promise for guiding the selection of Salvia species for targeted therapeutic applications. Through its comprehensive analysis and nuanced understanding of phytochemical profiles, this study paves the way for more effective and tailored applications of Salvia in medicine. Furthermore, the regional significance of this study is crucial. Salvia species are an integral part of Iran's rich botanical heritage, underscoring the importance of conserving and utilizing these plants for their medicinal properties. By specifically examining Salvia species from Iran, we offer valuable insights into the diversity and bioactivity of the flora in this region.
Figure 1.
Pictures of the 20 Salvia species investigated in this study (all pictures were taken by A. Moshari-Nasirkandi and A. Alirezalu).
Table 1.
List of species investigated along with the relative number of samples and collecting areas.
| Species | Number of samples | Collecting areas |
|---|---|---|
| S. multicaulis | 14 | Silvana; Mavana; Aghbolagh; Hesarlu; Saqqez; Qasemlu; Tekab; Urmia-Tabriz; Nokhtalu; Khalifatan; Khaneqah Sorkh; Marmisho; Marmisho-Serow; Oshnavieh-Piranshahr |
| S. limbata | 15 | Talebin village; Aghbolagh; Bukan; Qasemlu; Marmisho; Serow; Qurdik-Khoy; Shahindej-Tekab; Takhte-Soleiman; Gareziaedin; Nokhtalu; Daryan; Bukan-Mahabad; Mahabad-Miandoab; Chaldoran |
| S. syriaca | 16 | Shiban village; Baruq town; Shahindej-Tekab; Naghadeh-Piranshahr; Saqqez; Qushchi; Qasemlu; Marmisho; Serow; Takhte-Soleiman; Nokhtalu; Daryan; Bukan-Mahabad; Salmas-Khoy; Marmisho-Serow; Oshnavieh-Piranshahr |
| S. verticillata | 6 | Mavana; Qasemlu; Marmisho; Qurdik-Khoy; Daryan; Marmisho |
| S. nemorosa | 17 | Silvana; Aghbolagh; Mahabad-Urmia; Miandoab; Utmish village; Noshin Shahr; Qasemlu; Qasemlu; Anhar road; Marmisho; Serow; Khoy; Daryan; Tasuj; Maku; Piranshahr-Sardasht; Noshin Shahr |
| S. officinalis | 2 | Urmia university |
| S. atropatana | 4 | Sirdaghi; Pardanan jungle; Qushchi; Mishodagh |
| S. sclarea | 3 | Urmia university; Marmisho; Piranshahr-Sardasht |
| S. bracteata | 2 | Pardanan jungle; Piranshahr-Sardasht |
| S. spinosa | 6 | Bukan; Saqqez; Mahabad-miandoab; Qushchi; Urmia-Tabriz; Cheshmeh Kanan |
| S. Staminea | 1 | Qahreman Kandi |
| S. ceratophylla | 5 | Qushchi; Daryan; Bukan road; Khalifatan; Marmisho-Serow |
| S. macrochlamys | 2 | Marmisho; Marmisho-Serow |
| S. candidissima | 1 | Marmisho |
| S. aethiopis | 2 | Dalamper; Khoy |
| S. sahendica | 1 | Tabriz |
| Salvia sp. | 1 | Tupchi |
| S. grossheimii | 1 | Khaneqah Sorkh |
| S. poculata | 1 | Qushchi |
| S. hydrangea | 2 | Qushchi; Marmisho-Serow |
Figure 2.
(A) Geographical location of West Azerbaijan. (B) Satellite image of West Azerbaijan province showing identification of 3 distinct collection areas where Salvia samples were picked. (C) Illustrative shots of both the handpicking process of sampling and the different collecting landscapes. Images in (A,B) were extracted from Google Earth Pro V 7.3.6.9796. Pictures in (C) were taken by A. Moshari-Nasirkandi and A. Alirezalu.
Results and discussion
Total phenolic content (TPC)
Due to the multitude of potential health advantages and industrial applications, total phenolic content serves as a valuable parameter in various fields, including food science, nutrition, and pharmaceuticals25. TPC is extensively used in scientific research to study the bioactivity and potential health benefits of natural compounds and extracts26. In fact, phenolic compounds are renowned for their antioxidant properties, reducing cellular oxidative stress and lowering risks linked to chronic diseases like cardiovascular diseases and certain cancers27. Consequently, TPC proves instrumental in assessing the potential health advantages of herbal medicines and natural remedies.
In this study, TPC levels of the aerial parts extracts of Salvia plants were determined using the Folin-Ciocalteu method. Significant differences in total phenolic content were observed among the extracts (p < 0.01). Table 2 shows the TPC values for the 102 Salvia plants, ranging from 12.67 to 62.46 mg GAE/g DW, with S. ceratophylla and S. limbata exhibiting the highest and lowest total phenol content, respectively. Previous studies showed that the total phenolic content of S. officinalis ranged from 2.02 to 184 mg GAE/g DW. Alizadeh and Shaabani reported a TPC content of 25.13 mg GAE/g DW for Iranian S. officinalis28. Similar ranges of phenolic amounts were obtained for some Salvia species such as S. lanceolata, S. dolomitica, and S. garepensis (54.2, 53.0, and 45.6 mg GAE/g DW, respectively)12. TPC variations within species suggest that weather, geographic location, and genetic factors may influence total phenol content. Previous studies have also demonstrated the crucial role of intrinsic and extrinsic factors, such as genotype, climatic conditions, growing location, developmental stage, and extraction or calibration methods used in determining the TPC29,30. In these literature studies, by carefully considering sample size and representativeness, it was possible to obtain accurate insights into the phytochemical diversity of Salvia populations. This is essential for various purposes such as conservation, medicinal research, and breeding programs. Therefore, conducting multi-site studies encompassing different ecological conditions and habitats could provide a more comprehensive understanding distribution and characteristics of Salvia species within Iran.
Table 2.
Phytochemical content (TPC, TFC, TTC and AAC) determined for the 102 Salvia extracts studied.
| Sample code | Species | TPC (mg GAE/g DW) | TFC (mg QUE/g DW) | TTC (mg TAE/100 g DW) | AAC (mg AA/g DW) |
|---|---|---|---|---|---|
| S1 | S. multicaulis | 52.46 ± 0.01 | 11.72 ± 0.74 | 15.59 ± 0.27 | 41.05 ± 1.69 |
| S2 | S. limbata | 17.72 ± 0.04 | 9.58 ± 0.13 | 12.62 ± 0.20 | 39.96 ± 1.24 |
| S3 | S. syriaca | 42.81 ± 0.81 | 13.18 ± 0.63 | 18.83 ± 0.17 | 41.97 ± 1.60 |
| S4 | S. multicaulis | 39.47 ± 0.07 | 10.92 ± 0.12 | 14.06 ± 0.16 | 39.96 ± 0.86 |
| S5 | S. verticillata | 49.25 ± 0.25 | 11.23 ± 0.39 | 7.60 ± 0.68 | 39.07 ± 1.44 |
| S6 | S. nemorosa | 49.70 ± 1.70 | 11.96 ± 0.85 | 24.22 ± 0.65 | 37.38 ± 0.38 |
| S7 | S. officinalis | 54.31 ± 2.31 | 13.63 ± 0.37 | 123.87 ± 5.87 | 40.47 ± 0.41 |
| S8 | S. officinalis | 57.55 ± 4.55 | 11.75 ± 0.50 | 45.74 ± 1.60 | 37.76 ± 0.26 |
| S9 | S. atropatana | 48.97 ± 0.22 | 11.87 ± 0.74 | 57.50 ± 0.16 | 41.02 ± 1.85 |
| S10 | S. sclarea | 52.26 ± 0.06 | 11.82 ± 0.27 | 85.43 ± 2.15 | 40.44 ± 1.85 |
| S11 | S. syriaca | 48.91 ± 0.01 | 12.24 ± 0.49 | 46.80 ± 2.34 | 40.76 ± 1.66 |
| S12 | S. multicaulis | 54.04 ± 0.04 | 10.88 ± 0.09 | 46.13 ± 0.87 | 41.24 ± 2.27 |
| S13 | S. nemorosa | 32.44 ± 1.22 | 10.10 ± 0.21 | 9.04 ± 0.03 | 41.59 ± 0.83 |
| S14 | S. limbata | 23.00 ± 0.50 | 10.49 ± 0.34 | 22.38 ± 0.59 | 41.37 ± 0.86 |
| S15 | S. multicaulis | 41.20 ± 0.20 | 11.63 ± 0.41 | 27.97 ± 0.97 | 41.37 ± 2.14 |
| S16 | S. syriaca | 40.29 ± 0.03 | 11.59 ± 0.94 | 46.80 ± 2.10 | 43.83 ± 1.91 |
| S17 | S. nemorosa | 46.17 ± 0.07 | 11.58 ± 0.30 | 33.71 ± 0.29 | 36.93 ± 0.57 |
| S18 | S. syriaca | 27.28 ± 0.28 | 11.45 ± 0.12 | 24.89 ± 2.51 | 40.60 ± 0.67 |
| S19 | S. atropatana | 49.84 ± 3.84 | 12.52 ± 0.55 | 29.64 ± 1.24 | 39.58 ± 0.73 |
| S20 | S. bracteata | 48.17 ± 3.17 | 10.72 ± 0.26 | 56.33 ± 0.63 | 43.12 ± 2.11 |
| S21 | S. nemorosa | 51.57 ± 1.07 | 11.49 ± 0.73 | 51.80 ± 1.40 | 39.36 ± 0.96 |
| S22 | S. bracteata | 38.19 ± 1.00 | 10.31 ± 0.31 | 46.60 ± 2.40 | 40.44 ± 3.45 |
| S23 | S. nemorosa | 42.25 ± 1.61 | 10.50 ± 0.22 | 22.30 ± 0.40 | 41.56 ± 0.54 |
| S24 | S. limbata | 30.09 ± 1.09 | 12.07 ± 0.78 | 138.06 ± 5.03 | 44.02 ± 0.57 |
| S25 | S. spinosa | 42.56 ± 0.56 | 12.43 ± 0.12 | 56.76 ± 3.32 | 44.40 ± 0.26 |
| S26 | S. spinosa | 20.61 ± 0.61 | 10.30 ± 0.22 | 16.41 ± 0.41 | 39.90 ± 0.10 |
| S27 | S. syriaca | 45.10 ± 0.90 | 13.53 ± 0.53 | 38.32 ± 1.60 | 39.33 ± 3.22 |
| S28 | S. Staminea | 34.51 ± 1.00 | 11.15 ± 0.06 | 56.84 ± 4.18 | 41.18 ± 0.03 |
| S29 | S. multicaulis | 57.52 ± 1.22 | 12.63 ± 3.81 | 46.25 ± 4.92 | 42.13 ± 2.46 |
| S30 | S. nemorosa | 30.63 ± 0.63 | 10.11 ± 0.17 | 24.18 ± 0.35 | 42.13 ± 0.35 |
| S31 | S. spinosa | 32.81 ± 0.70 | 11.47 ± 0.05 | 21.41 ± 1.72 | 40.60 ± 0.99 |
| S32 | S. spinosa | 23.60 ± 0.20 | 9.96 ± 0.16 | 8.93 ± 0.53 | 40.44 ± 0.57 |
| S33 | S. syriaca | 35.72 ± 0.02 | 12.96 ± 0.93 | 29.53 ± 0.53 | 40.25 ± 0.26 |
| S34 | S. ceratophylla | 62.46 ± 0.03 | 16.04 ± 0.86 | 66.76 ± 1.76 | 41.18 ± 0.61 |
| S35 | S. atropatana | 28.44 ± 0.44 | 10.35 ± 0.14 | 38.63 ± 1.63 | 41.66 ± 1.40 |
| S36 | S. nemorosa | 36.34 ± 0.04 | 10.66 ± 0.28 | 21.56 ± 0.56 | 36.61 ± 0.61 |
| S37 | S. verticillata | 43.80 ± 0.05 | 11.67 ± 0.15 | 19.92 ± 0.47 | 40.92 ± 1.12 |
| S38 | S. nemorosa | 35.55 ± 0.15 | 10.35 ± 0.18 | 21.13 ± 0.13 | 42.29 ± 1.66 |
| S39 | S. limbata | 27.00 ± 0.20 | 11.04 ± 0.25 | 63.71 ± 1.91 | 38.62 ± 1.44 |
| S40 | S. syriaca | 28.59 ± 0.19 | 11.28 ± 0.32 | 25.90 ± 0.90 | 42.55 ± 1.79 |
| S41 | S. nemorosa | 31.87 ± 0.04 | 11.16 ± 0.10 | 31.25 ± 0.45 | 39.61 ± 1.91 |
| S42 | S. multicaulis | 45.72 ± 1.02 | 11.03 ± 0.73 | 21.80 ± 0.80 | 42.58 ± 2.46 |
| S43 | S. nemorosa | 54.51 ± 0.49 | 11.99 ± 0.19 | 39.18 ± 0.18 | 41.62 ± 1.76 |
| S44 | S. sclarea | 32.93 ± 0.07 | 10.06 ± 0.01 | 26.91 ± 0.66 | 40.41 ± 0.35 |
| S45 | S. macrochlamys | 39.13 ± 0.13 | 10.91 ± 0.44 | 121.86 ± 3.14 | 40.67 ± 0.41 |
| S46 | S. syriaca | 36.76 ± 0.20 | 12.17 ± 0.72 | 13.63 ± 0.42 | 41.91 ± 1.40 |
| S47 | S. verticillata | 48.36 ± 0.03 | 10.77 ± 0.23 | 10.02 ± 0.53 | 40.16 ± 2.20 |
| S48 | S. nemorosa | 43.94 ± 0.05 | 11.10 ± 0.42 | 14.14 ± 0.16 | 39.64 ± 3.16 |
| S49 | S. candidissima | 47.47 ± 1.32 | 15.96 ± 0.77 | 80.08 ± 2.99 | 41.21 ± 1.09 |
| S50 | S. limbata | 18.76 ± 0.76 | 9.95 ± 0.14 | 14.57 ± 0.57 | 39.64 ± 2.07 |
| S51 | S. syriaca | 22.76 ± 0.04 | 11.05 ± 0.23 | 15.70 ± 0.82 | 40.92 ± 2.27 |
| S52 | S. limbata | 31.92 ± 0.08 | 11.30 ± 0.33 | 41.99 ± 2.55 | 42.10 ± 0.38 |
| S53 | S. nemorosa | 33.28 ± 0.28 | 9.80 ± 0.11 | 11.95 ± 0.93 | 41.50 ± 1.63 |
| S54 | S. verticillata | 44.76 ± 0.76 | 10.54 ± 0.51 | 9.34 ± 0.12 | 41.88 ± 0.35 |
| S55 | S. limbata | 23.13 ± 0.03 | 10.19 ± 0.27 | 42.50 ± 2.11 | 40.73 ± 1.69 |
| S56 | S. limbata | 12.67 ± 1.67 | 9.72 ± 0.16 | 27.87 ± 0.98 | 44.05 ± 1.18 |
| S57 | S. multicaulis | 53.66 ± 0.2 | 12.40 ± 0.94 | 31.45 ± 1.37 | 49.70 ± 4.09 |
| S58 | S. syriaca | 36.93 ± 0.93 | 11.92 ± 0.1 | 43.91 ± 3.13 | 41.40 ± 1.02 |
| S59 | S. nemorosa | 51.03 ± 0.97 | 12.68 ± 0.95 | 67.07 ± 1.93 | 41.97 ± 2.87 |
| S60 | S. aethiopis | 52.56 ± 1.44 | 12.05 ± 1.00 | 126.62 ± 4.38 | 45.23 ± 0.64 |
| S61 | S. limbata | 32.07 ± 2.04 | 11.29 ± 0.55 | 120.61 ± 2.88 | 40.76 ± 0.06 |
| S62 | S. sahendica | 33.38 ± 0.30 | 11.02 ± 0.29 | 59.80 ± 0.98 | 41.97 ± 0.26 |
| S63 | S. nemorosa | 41.23 ± 0.12 | 10.69 ± 0.34 | 19.84 ± 0.39 | 42.13 ± 0.35 |
| S64 | S. verticillata | 55.77 ± 0.37 | 16.26 ± 0.66 | 23.55 ± 0.55 | 39.96 ± 1.50 |
| S65 | S. aethiopis | 59.33 ± 0.27 | 12.80 ± 0.30 | 68.71 ± 2.20 | 43.67 ± 0.48 |
| S66 | S. spinosa | 34.54 ± 1.54 | 11.16 ± 0.38 | 9.44 ± 0.94 | 44.88 ± 2.07 |
| S67 | S. multicaulis | 56.14 ± 0.14 | 11.99 ± 0.42 | 32.85 ± 0.20 | 43.60 ± 1.63 |
| S68 | S. limbata | 38.07 ± 1.41 | 10.37 ± 0.09 | 29.69 ± 0.69 | 39.26 ± 2.65 |
| S69 | S. syriaca | 45.30 ± 1.30 | 12.24 ± 0.94 | 27.50 ± 0.50 | 40.67 ± 0.03 |
| S70 | S. multicaulis | 50.09 ± 0.09 | 10.72 ± 0.14 | 13.48 ± 0.20 | 42.74 ± 1.09 |
| S71 | S. limbata | 26.54 ± 1.04 | 10.07 ± 0.21 | 14.49 ± 0.82 | 43.25 ± 0.26 |
| S72 | S. syriaca | 54.34 ± 0.09 | 18.66 ± 0.27 | 114.68 ± 1.68 | 43.09 ± 1.18 |
| S73 | S. limbata | 56.86 ± 0.86 | 13.61 ± 0.61 | 245.02 ± 4.12 | 43.76 ± 2.11 |
| S74 | S. ceratophylla | 45.23 ± 0.23 | 11.64 ± 0.20 | 34.88 ± 2.07 | 42.87 ± 1.53 |
| S75 | S. spinosa | 53.13 ± 0.13 | 12.08 ± 0.10 | 31.60 ± 0.37 | 44.81 ± 2.78 |
| S76 | Salvia sp. | 43.52 ± 0.52 | 11.67 ± 0.13 | 37.73 ± 2.66 | 42.10 ± 0.89 |
| S77 | S. nemorosa | 56.91 ± 0.61 | 12.34 ± 0.81 | 37.81 ± 0.93 | 41.85 ± 0.83 |
| S78 | S. ceratophylla | 34.02 ± 0.24 | 10.14 ± 0.22 | 18.59 ± 0.41 | 43.41 ± 1.56 |
| S79 | S. syriaca | 23.10 ± 0.52 | 10.98 ± 0.14 | 23.52 ± 0.52 | 41.37 ± 0.86 |
| S80 | S. limbata | 19.99 ± 1.11 | 10.38 ± 0.19 | 123.67 ± 3.67 | 40.38 ± 2.11 |
| S81 | S. limbata | 21.10 ± 1.10 | 10.91 ± 0.59 | 134.73 ± 4.73 | 41.81 ± 0.73 |
| S82 | S. poculata | 33.50 ± 1.05 | 10.34 ± 0.27 | 15.63 ± 0.08 | 40.41 ± 0.73 |
| S83 | S. hydrangea | 54.88 ± 2.38 | 12.06 ± 0.57 | 38.24 ± 0.54 | 40.16 ± 2.65 |
| S84 | S. syriaca | 33.67 ± 1.14 | 11.71 ± 0.09 | 31.68 ± 0.68 | 44.27 ± 0.57 |
| S85 | S. ceratophylla | 53.77 ± 3.77 | 13.63 ± 0.63 | 48.98 ± 1.19 | 41.02 ± 1.02 |
| S86 | S. limbata | 25.35 ± 0.35 | 10.37 ± 0.11 | 22.19 ± 0.81 | 40.41 ± 1.12 |
| S87 | S. multicaulis | 52.86 ± 2.85 | 12.21 ± 0.21 | 33.83 ± 0.93 | 43.28 ± 4.12 |
| S88 | S. grossheimii | 54.59 ± 1.50 | 13.42 ± 0.42 | 66.56 ± 2.89 | 42.04 ± 0.77 |
| S89 | S. multicaulis | 53.30 ± 0.08 | 12.24 ± 0.50 | 24.53 ± 0.53 | 45.55 ± 0.77 |
| S90 | S. multicaulis | 50.66 ± 1.66 | 11.40 ± 0.36 | 11.12 ± 0.06 | 40.06 ± 0.38 |
| S91 | S. verticillata | 57.13 ± 1.02 | 16.85 ± 0.86 | 14.88 ± 0.98 | 45.23 ± 2.43 |
| S92 | S. syriaca | 34.17 ± 1.12 | 12.30 ± 0.34 | 32.30 ± 0.40 | 44.56 ± 0.99 |
| S93 | S. multicaulis | 46.71 ± 1.01 | 12.19 ± 0.93 | 16.25 ± 0.02 | 46.73 ± 1.63 |
| S94 | S. aethiopis | 30.36 ± 0.36 | 10.53 ± 0.28 | 23.13 ± 0.31 | 44.97 ± 1.34 |
| S95 | S. hydrangea | 40.12 ± 0.12 | 11.07 ± 0.36 | 23.71 ± 0.71 | 44.66 ± 1.09 |
| S96 | S. macrochlamys | 30.93 ± 0.10 | 9.92 ± 0.13 | 33.75 ± 1.25 | 42.13 ± 2.14 |
| S97 | S. ceratophylla | 58.83 ± 0.83 | 13.24 ± 0.34 | 40.35 ± 1.05 | 42.36 ± 3.64 |
| S98 | S. atropatana | 34.39 ± 0.31 | 11.15 ± 0.23 | 43.16 ± 1.84 | 45.61 ± 1.15 |
| S99 | S. sclarea | 44.09 ± 0.09 | 11.60 ± 0.16 | 57.42 ± 2.66 | 45.10 ± 1.47 |
| S100 | S. nemorosa | 45.45 ± 1.11 | 10.21 ± 0.19 | 15.70 ± 0.28 | 38.78 ± 1.15 |
| S101 | S. syriaca | 29.08 ± 0.31 | 11.45 ± 0.46 | 20.08 ± 0.23 | 41.88 ± 1.50 |
| S102 | S. multicaulis | 48.56 ± 0.56 | 11.83 ± 0.58 | 60.39 ± 3.14 | 43.83 ± 1.53 |
| Significant levels | ** | ** | ** | ** | |
TPC total phenolic content, TFC total flavonoid content, TTC total tannin content, AAC ascorbic acid content.
**Indicates significant difference at 1% confidence levels.
Total flavonoid content (TFC)
Total flavonoid content holds significance across various fields due to the potential health benefits of flavonoids, wide industrial applications, and roles in diet and environmental analysis31. Flavonoids are among the most diverse and widespread natural compounds and considered the most important among natural phenolics. These compounds exhibit a wide spectrum of chemical and biological activities31. Flavonoids are renowned for their antioxidant properties. Indeed, they are capable of neutralizing free radicals and reducing oxidative stress, potentially decreasing the risk of chronic diseases. Some flavonoids also exhibit anti-inflammatory properties and the potential to inhibit cancer cell proliferation9. Furthermore, consuming flavonoid-rich foods is linked to improved cardiovascular health as it lowers blood pressure and reduces the risk of heart disease. Certain flavonoids also demonstrate neuroprotective properties, beneficial for conditions such as Alzheimer’s disease.
Therefore, identifying sage species with high TFC is crucial for developing nutraceuticals to prevent and support treatment for various health issues and metabolic disorders. Significant variation in TFC (p < 0.01) was observed among the studied plants. TFC ranged from 9.58 to 18.66 mg QUE/g DW (Table 2), with the highest and lowest levels exhibited by S. syriaca and S. limbata, respectively. A previous study identified the three Salvia species with the highest total flavonoids content as S. hierosolymitana (770.85 mg QUE/g DW), S. eigii (520.60 mg QUE/g DW), and S. viridis (311.36 mg QUE/g DW)32. While the amount of flavonoid content in methanolic extracts of S. chudaei and S. officinalis L. was reported as 4.68 and 9.1 mg QUE/g, respectively33,34. The variations in TFC may be due to seasonal changes. This and several other studies have not taken this effect in account. Different seasons and years can influence the synthesis and accumulation of secondary metabolites in plants. To mitigate this limitation, future research could consider conducting multiple sampling rounds across different seasons to capture seasonal variations in plant chemistry and antioxidant activity.
Total tannin content (TTC)
A statistically significantly difference in total tannin content (TTC) was also observed between the extracts (p < 0.01). TTC ranged from 7.60 to 245.02 mg TAE/100 g DW (Table 2), with S. limbata and S. verticillata extracts showing the highest and lowest values, respectively. In a previous study, the TTC content ranged from 0.33 to 6.49 mg TAE/100 g DW, with S. macrochlamys and S. verticillata displaying the highest and lowest values, respectively35. Variations in species, extraction methods, solvents, and analytical techniques can lead to differences in the measured TTC.
The higher content of TTC compared to TPC may be due to factors related to the chemical nature and measurement methods of tannins and phenolics. For instance, the Folin-Ciocalteu method for TPC is known to not react equally with all phenolics. TTC measurements, specifically targeting tannins, may be more sensitive. In addition, different solvents and extraction methods affect yields, and tannins, with multiple phenolic groups per molecule, may exhibit higher TTC, even if fewer in number. Sample matrix interferences could also reduce TPC more than TTC. Phenolics are prone to degradation, reducing TPC, while larger, more complex tannins are more stable, leading to higher TTC.
Tannins are polyphenolic substances with different molecular weights and complexity36,37. Tannins are secondary metabolites that play a primary role in plant defense mechanisms38. Tannins possess various properties, including anti-inflammatory, regeneration, anticatarrhal, antimicrobial and soothing effects39. Tannins have been used in traditional medicines to treat various diseases. Epidemiological data suggest that tannin intake may inhibit the onset of chronic diseases40. Tannins are also renowned to possess antimicrobial and antioxidant properties that are significant in addressing skin disorders and wounds41. Traditional medicines rich in tannins have been reported to be used for treating wounds, burns, inflammation, and other medical ailments42. Tannins can generate smaller phenolic compounds, such as pyrogallol, catechol, and ellagic acid, which are known for their bactericidal activity. Tannins also perform many other biologically significant functions, including protection against oxidative stress and degenerative diseases39,42.
Ascorbic acid content (AAC)
Significant differences in ascorbic acid content were observed among the investigated samples (p < 0.01), as shown in Table 2. Salvia multicaulis extracts showed the highest AAC value, while S. nemorosa extracts the lowest (49.70 and 36.61 mg AA/100 g DW, respectively). In the investigation of the amount of ascorbic acid in 20 different species of the Lamiaceae family, values ranged from 0.58 to 1.15 mg AA/g DW, with sage and Stachys sp. showing the highest and lowest amount of ascorbic acid, respectively35. Ascorbic acid is susceptible to rapid decomposition, and improper storage and processing of plant samples can result in the degradation of phytochemicals and antioxidants, thus affecting the accuracy of analysis. Establishing standardized protocols for sample handling and storage is crucial to mitigate these issues.
The action of ascorbic acid (AA) is essential for normal wound healing43. Ascorbic acid acts as a hydrogen atom donor to lipid radicals, quenches singlet oxygen, and removes molecular oxygen44. The biochemical function of AA is often associated with its redox potential45,46. In other words, AA has the characteristic of being easily oxidized by releasing electrons in an aqueous solution, making it a powerful water-soluble antioxidant that reacts with reactive oxygen species (ROS) or free radicals47.
Photosynthetic pigments content
Among the investigated plants, significant differences in the levels of chlorophyll a and b (Ca and Cb, respectively), total carotenoid concentration (Cx+c), and β-carotene were observed (p < 0.01). However, in this case it was not possible to identify a Salvia species with the highest and lowest pigment concentration (Table 3). The extracts from S. spinosa (S31) showed the highest content of Ca and Cb (41.78 mg/100 g DW and 18.25 mg/100 g DW, respectively), while S. atropatana (S35), showed the highest Cx+c and β-carotene content (38.13 mg/g DW and 2.98 mg/100 g DW, respectively). Conversely, the lowest values of Ca and Cb were displayed by S. verticillata (S64) and S. multicaulis (S42) (5.13 mg/100 g DW and 2.04 mg/100 g DW, respectively), while S. spinosa (S66) exhibited the lowest content of both Cx+c and β-carotene (5.25 mg/g DW and 0.40 mg/100 g DW, respectively). Previous studies reported a chlorophyll a content in S. sclarea, Origanum vulgare L., Mentha and Thymus vulgaris L. of 1.56, 10.42, 14.23 and 2.89 mg/g fresh weight (FW), respectively48–50. Also, the content of chlorophyll b in S. sclarea, Origanum vulgare L., Mentha and Thymus vulgaris L. were reported as 0.43, 4.66, 5.78 and 1.38 mg/g FW, respectively48,49. The content of total carotenoids in S. sclarea and Thymus vulgaris L. were reported as 0.45 and 0.56 mg/g FW, respectively48,49. Various factors, including light conditions, ozone stress, drought, salinity, and temperature, can help to explain some of the observed variations among the samples. In fact, these factors are known to influence carotenoid content in sage51–53. Carotenoids are antioxidants that function to protect membranes against damage from free radicals and play a significant role in plant reproduction. Along with phenolic compounds, they are responsible for the vibrant colors of plants54. Carotenoids play a role in supporting cellular structures by dissipating excess energy that reaches the chloroplast and inhibiting the formation and/or scavenging of singlet oxygen, which can lead to lipid peroxidation in photosynthetic membranes55.
Table 3.
Content of photosynthetic pigments for the 102 Salvia species studied.
| Sample code | Species | Ca (mg/100 g Dw) | Cb (mg/100 g Dw) | Cx+c (TCC) (mg/g) | β-Carotene (mg/100 g Dw) |
|---|---|---|---|---|---|
| S1 | S. multicaulis | 9.88 ± 0.09 | 5.71 ± 0.05 | 9.54 ± 0.74 | 0.73 ± 0.02 |
| S2 | S. limbata | 16.04 ± 1.04 | 5.52 ± 0.02 | 12.60 ± 0.26 | 0.94 ± 0.01 |
| S3 | S. syriaca | 19.06 ± 1.41 | 5.41 ± 0.06 | 13.71 ± 0.03 | 1.07 ± 0.02 |
| S4 | S. multicaulis | 25.09 ± 0.02 | 9.59 ± 0.24 | 22.12 ± 0.02 | 1.71 ± 0.02 |
| S5 | S. verticillata | 25.75 ± 0.84 | 8.77 ± 0.07 | 22.43 ± 0.43 | 1.75 ± 0.05 |
| S6 | S. nemorosa | 19.78 ± 0.88 | 6.73 ± 0.18 | 12.89 ± 0.89 | 0.97 ± 0.08 |
| S7 | S. officinalis | 24.74 ± 0.07 | 9.78 ± 0.28 | 24.16 ± 0.16 | 1.88 ± 0.07 |
| S8 | S. officinalis | 25.41 ± 0.94 | 11.00 ± 0.18 | 26.56 ± 0.16 | 2.07 ± 0.02 |
| S9 | S. atropatana | 30.26 ± 0.09 | 11.40 ± 0.01 | 23.51 ± 0.24 | 1.80 ± 0.09 |
| S10 | S. sclarea | 10.46 ± 0.12 | 5.16 ± 0.57 | 12.98 ± 0.03 | 1.02 ± 0.02 |
| S11 | S. syriaca | 12.56 ± 0.86 | 4.17 ± 0.01 | 10.96 ± 0.96 | 0.86 ± 0.01 |
| S12 | S. multicaulis | 26.63 ± 0.63 | 9.61 ± 0.61 | 20.52 ± 0.86 | 1.57 ± 0.10 |
| S13 | S. nemorosa | 25.60 ± 0.01 | 9.99 ± 0.81 | 20.94 ± 0.94 | 1.60 ± 0.01 |
| S14 | S. limbata | 11.77 ± 0.96 | 3.78 ± 0.30 | 7.45 ± 0.29 | 0.56 ± 0.03 |
| S15 | S. multicaulis | 20.51 ± 0.17 | 7.45 ± 0.90 | 7.69 ± 0.17 | 0.80 ± 0.03 |
| S16 | S. syriaca | 18.71 ± 0.09 | 5.22 ± 0.87 | 20.06 ± 0.50 | 1.31 ± 0.04 |
| S17 | S. nemorosa | 28.24 ± 0.27 | 11.74 ± 0.31 | 20.87 ± 0.54 | 1.57 ± 0.02 |
| S18 | S. syriaca | 21.35 ± 0.85 | 6.74 ± 0.25 | 17.01 ± 0.50 | 1.32 ± 0.05 |
| S19 | S. atropatana | 39.88 ± 1.88 | 16.14 ± 0.14 | 33.94 ± 0.72 | 2.60 ± 0.09 |
| S20 | S. bracteata | 9.25 ± 0.20 | 4.20 ± 0.09 | 10.28 ± 0.50 | 0.80 ± 0.04 |
| S21 | S. nemorosa | 28.91 ± 0.10 | 12.49 ± 0.11 | 25.70 ± 0.90 | 1.97 ± 0.07 |
| S22 | S. bracteata | 15.59 ± 0.46 | 6.70 ± 0.02 | 20.84 ± 0.7 | 1.34 ± 0.04 |
| S23 | S. nemorosa | 18.46 ± 0.05 | 6.57 ± 0.07 | 18.41 ± 0.05 | 1.20 ± 0.03 |
| S24 | S. limbata | 18.77 ± 0.97 | 6.43 ± 0.18 | 15.08 ± 0.34 | 1.17 ± 0.08 |
| S25 | S. spinosa | 15.88 ± 0.63 | 3.23 ± 0.05 | 8.79 ± 0.19 | 0.85 ± 0.02 |
| S26 | S. spinosa | 11.00 ± 0.19 | 4.29 ± 0.81 | 10.08 ± 0.34 | 0.78 ± 0.02 |
| S27 | S. syriaca | 23.80 ± 1.15 | 7.34 ± 0.35 | 24.35 ± 0.03 | 1.94 ± 0.04 |
| S28 | S. Staminea | 23.17 ± 0.37 | 8.13 ± 0.05 | 19.82 ± 0.37 | 1.54 ± 0.02 |
| S29 | S. multicaulis | 28.19 ± 0.01 | 10.62 ± 0.62 | 20.10 ± 0.77 | 1.52 ± 0.03 |
| S30 | S. nemorosa | 29.86 ± 0.48 | 11.66 ± 0.66 | 23.63 ± 0.63 | 1.80 ± 0.05 |
| S31 | S. spinosa | 41.78 ± 0.50 | 18.25 ± 0.61 | 32.22 ± 0.75 | 2.48 ± 0.02 |
| S32 | S. spinosa | 11.04 ± 0.34 | 4.28 ± 0.08 | 8.83 ± 0.09 | 0.67 ± 0.01 |
| S33 | S. syriaca | 16.62 ± 0.05 | 4.81 ± 0.02 | 10.78 ± 0.16 | 0.83 ± 0.01 |
| S34 | S. ceratophylla | 20.41 ± 0.03 | 7.70 ± 0.33 | 19.00 ± 0.09 | 1.48 ± 0.02 |
| S35 | S. atropatana | 40.73 ± 0.02 | 14.61 ± 0.17 | 38.13 ± 0.63 | 2.98 ± 0.04 |
| S36 | S. nemorosa | 26.37 ± 0.37 | 9.73 ± 0.96 | 22.85 ± 0.95 | 1.77 ± 0.08 |
| S37 | S. verticillata | 25.14 ± 0.09 | 8.96 ± 1.97 | 18.95 ± 0.27 | 1.48 ± 0.07 |
| S38 | S. nemorosa | 19.07 ± 1.08 | 6.83 ± 0.28 | 17.52 ± 0.25 | 1.13 ± 0.03 |
| S39 | S. limbata | 16.11 ± 0.01 | 6.17 ± 0.20 | 15.00 ± 0.79 | 1.01 ± 0.01 |
| S40 | S. syriaca | 17.50 ± 0.39 | 7.89 ± 0.17 | 15.19 ± 0.61 | 1.18 ± 0.02 |
| S41 | S. nemorosa | 33.79 ± 0.79 | 12.35 ± 0.35 | 27.11 ± 0.11 | 2.08 ± 0.08 |
| S42 | S. multicaulis | 5.19 ± 0.03 | 2.04 ± 0.48 | 6.76 ± 0.27 | 0.53 ± 0.03 |
| S43 | S. nemorosa | 22.48 ± 0.86 | 8.53 ± 0.06 | 18.58 ± 0.70 | 1.42 ± 0.07 |
| S44 | S. sclarea | 23.38 ± 0.58 | 10.01 ± 0.68 | 23.29 ± 0.81 | 1.80 ± 0.08 |
| S45 | S. macrochlamys | 7.96 ± 0.37 | 3.09 ± 0.05 | 7.18 ± 0.02 | 0.55 ± 0.06 |
| S46 | S. syriaca | 9.38 ± 0.14 | 3.38 ± 0.03 | 8.93 ± 0.03 | 0.70 ± 0.02 |
| S47 | S. verticillata | 21.31 ± 0.51 | 7.77 ± 0.77 | 20.48 ± 0.74 | 1.60 ± 0.08 |
| S48 | S. nemorosa | 12.22 ± 0.22 | 4.53 ± 0.00 | 10.08 ± 0.33 | 0.78 ± 0.02 |
| S49 | S. candidissima | 12.48 ± 0.58 | 7.13 ± 0.13 | 20.51 ± 0.77 | 1.62 ± 0.04 |
| S50 | S. limbata | 11.64 ± 0.12 | 4.47 ± 0.03 | 9.68 ± 0.45 | 0.73 ± 0.01 |
| S51 | S. syriaca | 12.48 ± 0.68 | 4.61 ± 0.02 | 9.12 ± 0.10 | 0.69 ± 0.01 |
| S52 | S. limbata | 28.14 ± 0.06 | 9.42 ± 0.04 | 21.66 ± 0.03 | 1.67 ± 0.02 |
| S53 | S. nemorosa | 21.42 ± 1.09 | 8.14 ± 0.15 | 22.02 ± 0.05 | 1.73 ± 0.05 |
| S54 | S. verticillata | 19.99 ± 1.11 | 8.19 ± 0.91 | 25.61 ± 0.06 | 2.03 ± 0.01 |
| S55 | S. limbata | 20.04 ± 0.54 | 7.61 ± 0.61 | 16.87 ± 0.33 | 1.30 ± 0.02 |
| S56 | S. limbata | 13.44 ± 0.70 | 6.09 ± 0.05 | 15.29 ± 0.10 | 1.19 ± 0.01 |
| S57 | S. multicaulis | 16.60 ± 1.16 | 6.20 ± 0.53 | 13.96 ± 0.33 | 1.08 ± 0.03 |
| S58 | S. syriaca | 31.64 ± 0.03 | 10.77 ± 0.04 | 25.95 ± 0.01 | 2.02 ± 0.02 |
| S59 | S. nemorosa | 22.16 ± 0.51 | 8.50 ± 0.09 | 20.95 ± 0.46 | 1.63 ± 0.01 |
| S60 | S. aethiopis | 30.80 ± 0.80 | 14.53 ± 0.42 | 28.78 ± 0.30 | 2.19 ± 0.05 |
| S61 | S. limbata | 24.27 ± 0.53 | 8.18 ± 0.14 | 19.19 ± 0.29 | 1.48 ± 0.08 |
| S62 | S. sahendica | 17.59 ± 0.41 | 6.44 ± 0.07 | 14.22 ± 0.45 | 0.96 ± 0.06 |
| S63 | S. nemorosa | 27.29 ± 0.31 | 11.13 ± 0.44 | 25.26 ± 0.78 | 1.95 ± 0.06 |
| S64 | S. verticillata | 5.13 ± 0.03 | 2.48 ± 0.26 | 5.92 ± 0.06 | 0.46 ± 0.08 |
| S65 | S. aethiopis | 20.67 ± 0.20 | 8.18 ± 0.12 | 18.73 ± 0.73 | 1.45 ± 0.09 |
| S66 | S. spinosa | 5.36 ± 0.04 | 2.34 ± 0.60 | 5.25 ± 0.01 | 0.40 ± 0.02 |
| S67 | S. multicaulis | 23.86 ± 0.86 | 8.46 ± 0.56 | 18.01 ± 0.53 | 1.38 ± 0.05 |
| S68 | S. limbata | 13.36 ± 0.43 | 4.74 ± 0.21 | 13.84 ± 0.84 | 1.09 ± 0.09 |
| S69 | S. syriaca | 21.04 ± 0.36 | 6.45 ± 0.95 | 14.30 ± 0.45 | 1.10 ± 0.09 |
| S70 | S. multicaulis | 21.27 ± 0.55 | 6.94 ± 0.03 | 14.54 ± 0.04 | 1.11 ± 0.02 |
| S71 | S. limbata | 11.66 ± 0.85 | 4.11 ± 0.01 | 8.04 ± 0.10 | 0.61 ± 0.02 |
| S72 | S. syriaca | 38.24 ± 1.00 | 14.30 ± 0.94 | 28.22 ± 0.07 | 2.14 ± 0.01 |
| S73 | S. limbata | 12.59 ± 0.02 | 4.70 ± 0.22 | 9.37 ± 0.07 | 0.71 ± 0.02 |
| S74 | S. ceratophylla | 14.49 ± 0.58 | 5.61 ± 0.03 | 12.00 ± 0.34 | 0.92 ± 0.03 |
| S75 | S. spinosa | 23.97 ± 0.97 | 7.89 ± 0.92 | 19.40 ± 0.91 | 1.51 ± 0.06 |
| S76 | Slvia sp. | 20.21 ± 0.04 | 10.26 ± 0.06 | 23.14 ± 0.57 | 1.78 ± 0.08 |
| S77 | S. nemorosa | 22.14 ± 0.59 | 7.60 ± 0.07 | 16.53 ± 0.53 | 1.27 ± 0.09 |
| S78 | S. ceratophylla | 13.99 ± 0.22 | 6.83 ± 0.07 | 20.61 ± 0.02 | 1.63 ± 0.02 |
| S79 | S. syriaca | 28.61 ± 1.58 | 9.55 ± 0.84 | 20.65 ± 0.29 | 1.58 ± 0.01 |
| S80 | S. limbata | 6.72 ± 0.05 | 3.03 ± 0.19 | 6.71 ± 0.07 | 0.51 ± 0.02 |
| S81 | S. limbata | 14.08 ± 0.08 | 5.18 ± 0.18 | 10.22 ± 0.02 | 0.77 ± 0.02 |
| S82 | S. poculata | 30.78 ± 0.47 | 11.85 ± 0.85 | 26.75 ± 0.05 | 2.07 ± 0.07 |
| S83 | S. hydrangea | 13.54 ± 0.73 | 5.31 ± 0.18 | 12.69 ± 0.02 | 0.98 ± 0.02 |
| S84 | S. syriaca | 22.93 ± 0.07 | 6.92 ± 0.01 | 16.93 ± 0.06 | 1.31 ± 0.02 |
| S85 | S. ceratophylla | 15.70 ± 0.07 | 7.18 ± 0.33 | 21.20 ± 0.58 | 1.68 ± 0.04 |
| S86 | S. limbata | 20.78 ± 0.13 | 6.93 ± 0.93 | 19.25 ± 0.25 | 1.50 ± 0.01 |
| S87 | S. multicaulis | 30.25 ± 0.42 | 11.76 ± 0.06 | 25.03 ± 0.05 | 1.92 ± 0.07 |
| S88 | S. grossheimii | 20.00 ± 1.01 | 7.18 ± 0.18 | 17.06 ± 0.02 | 1.32 ± 0.02 |
| S89 | S. multicaulis | 15.60 ± 0.73 | 5.56 ± 0.56 | 13.86 ± 0.02 | 1.08 ± 0.02 |
| S90 | S. multicaulis | 13.30 ± 0.80 | 5.18 ± 0.91 | 12.37 ± 0.63 | 0.96 ± 0.07 |
| S91 | S. verticillata | 18.81 ± 0.27 | 7.02 ± 0.02 | 18.54 ± 0.09 | 1.45 ± 0.03 |
| S92 | S. syriaca | 12.66 ± 0.67 | 4.34 ± 0.04 | 11.73 ± 0.81 | 0.92 ± 0.05 |
| S93 | S. multicaulis | 17.81 ± 0.69 | 6.76 ± 0.20 | 16.67 ± 0.67 | 1.30 ± 0.10 |
| S94 | S. aethiopis | 25.49 ± 0.77 | 8.99 ± 0.14 | 18.37 ± 0.96 | 1.40 ± 0.08 |
| S95 | S. hydrangea | 29.56 ± 0.65 | 12.35 ± 0.71 | 27.09 ± 0.79 | 2.09 ± 0.06 |
| S96 | S. macrochlamys | 11.90 ± 0.03 | 4.24 ± 0.22 | 9.48 ± 0.18 | 0.72 ± 0.02 |
| S97 | S. ceratophylla | 12.61 ± 0.61 | 4.60 ± 0.55 | 15.40 ± 0.66 | 1.23 ± 0.06 |
| S98 | S. atropatana | 39.01 ± 0.14 | 13.58 ± 0.67 | 28.37 ± 0.89 | 2.17 ± 0.10 |
| S99 | S. sclarea | 15.50 ± 0.16 | 6.05 ± 0.18 | 13.38 ± 0.75 | 1.03 ± 0.06 |
| S100 | S. nemorosa | 23.51 ± 0.51 | 8.58 ± 0.25 | 18.57 ± 0.57 | 1.43 ± 0.10 |
| S101 | S. syriaca | 14.19 ± 0.28 | 5.86 ± 0.21 | 11.13 ± 0.13 | 0.84 ± 0.05 |
| S102 | S. multicaulis | 9.05 ± 0.15 | 3.67 ± 0.25 | 8.05 ± 0.28 | 0.62 ± 0.02 |
| Significant levels | ** | ** | ** | ** | |
Ca Chlorophyll a content, Cb Chlorophyll b content, Cx + c Cartenoid content, β-Carotene β-Carotene content.
**Indicates significant difference at 1% confidence levels.
Chlorophyll is typically synthesized and photo-oxidized in the presence of light. Rezai et al. investigated the carotenoid and chlorophyll contents in S. officinalis grown in a semi-arid region of Iran under different light conditions, by quantifying the effects of different shade levels (30%, 50%, or 70%) on plant morphology56.
Our samples were collected from an environment with limited shade, which could explain the lower carotenoid content observed, primarily in high shade conditions.
Antioxidant activity
The antioxidant capacities of the 102 plant extracts studied were examined using two free radical scavenging methods represented by the DPPH and FRAP assays. Due to its simplicity, speed, reproducibility and low cost, the DPPH assay has been used most frequently to evaluate the antioxidant potential of hydrophilic extracts (alcoholic, hydroalcoholic) taken from the above-ground parts of a number of Salvia species, including two known medicinal species as S. miltiorrhiza and S. officinalis57,58. The DPPH (1,1-diphenyl-2-picrylhydrazyl) is a stable free radical that can be reduced by transferring a hydrogen from other compounds. The FRAP assay is based on the presence of antioxidant substances in plant extracts, mainly polyphenolic compounds, which convert the ferric (Fe3+) complex to the ferrous (Fe2+) form. The decrease in Fe3+ in the solution indicates the potent reducing power of the plant extracts. Iron and copper ions are well known as effective pro-oxidant agents, and polyphenolic compounds can chelate metal ions, preventing free radical formation59. Polyphenols are natural antioxidants and have a correlation with ROS scavenging capacity helping to reduce the oxidative stress at product60,61.
In this study, a significant difference (p < 0.01) in antioxidant activity was observed among the investigated plants (Table 4). Antioxidant activity values, measured using the DPPH method, ranged from 4.58 to 68.93 µg AAE/mL DW, with the highest and lowest values observed for S. limbata and S. ceratophylla extracts, respectively. Results from the FRAP assay indicate that the highest values were observed for a sample of S. limbata (S56) and one of S. aethiopis (S60) (1090.55 and 1071.79 μmol Fe2+/g DW, respectively), while the lowest values were observed for another sample of S. limbata (S2) and one of S. nemorosa (S53) (64.28 and 73.06 μmol Fe2+/g DW, respectively). The differences observed in antioxidant activity could be attributed to the diverse methods used for evaluation62. Each method has distinct strengths and limitations. Depending on the assay chosen, some aspects of antioxidant capacity may be overlooked. To address this limitation, future research should consider employing multiple antioxidant assays, each targeting different mechanisms or aspects of antioxidant activity. This approach would provide a more comprehensive assessment of antioxidant capacity and improve understanding of the overall antioxidant potential of the compounds or samples studied.
Table 4.
Antioxidant activity determined for the 102 Salvia species by DPPH and FRAP assays.
| Sample code | Species | Antioxidant activity (DPPH) (µg AAE/mL) | Antioxidant activity (FRAP) (μmol Fe2+/g DW) |
|---|---|---|---|
| S1 | S. multicaulis | 66.63 ± 0.72 | 524.35 ± 6.26 |
| S2 | S. limbata | 35.72 ± 1.52 | 64.28 ± 3.40 |
| S3 | S. syriaca | 48.29 ± 2.99 | 231.59 ± 4.83 |
| S4 | S. multicaulis | 60.86 ± 0.99 | 165.08 ± 1.11 |
| S5 | S. verticillata | 67.73 ± 0.39 | 178.73 ± 6.16 |
| S6 | S. nemorosa | 67.85 ± 0.21 | 274.80 ± 5.68 |
| S7 | S. officinalis | 67.85 ± 0.75 | 997.09 ± 5.27 |
| S8 | S. officinalis | 67.46 ± 0.06 | 375.98 ± 5.88 |
| S9 | S. atropatana | 66.96 ± 0.27 | 252.63 ± 2.63 |
| S10 | S. sclarea | 67.70 ± 0.48 | 395.31 ± 5.22 |
| S11 | S. syriaca | 54.77 ± 3.61 | 258.88 ± 4.11 |
| S12 | S. multicaulis | 66.90 ± 0.87 | 839.85 ± 2.52 |
| S13 | S. nemorosa | 57.40 ± 0.51 | 81.51 ± 1.51 |
| S14 | S. limbata | 38.56 ± 1.91 | 124.62 ± 1.01 |
| S15 | S. multicaulis | 63.79 ± 0.57 | 290.15 ± 2.05 |
| S16 | S. syriaca | 15.95 ± 1.10 | 382.39 ± 3.73 |
| S17 | S. nemorosa | 66.06 ± 0.87 | 234.44 ± 2.00 |
| S18 | S. syriaca | 39.75 ± 2.81 | 206.01 ± 3.00 |
| S19 | S. atropatana | 67.70 ± 0.90 | 361.77 ± 4.10 |
| S20 | S. bracteata | 67.79 ± 0.45 | 196.92 ± 3.92 |
| S21 | S. nemorosa | 66.39 ± 0.54 | 224.20 ± 6.20 |
| S22 | S. bracteata | 63.25 ± 0.75 | 236.14 ± 2.02 |
| S23 | S. nemorosa | 63.31 ± 0.99 | 95.42 ± 4.42 |
| S24 | S. limbata | 40.59 ± 1.13 | 1090.55 ± 3.55 |
| S25 | S. spinosa | 60.30 ± 0.12 | 689.78 ± 1.78 |
| S26 | S. spinosa | 41.37 ± 0.96 | 255.47 ± 5.47 |
| S27 | S. syriaca | 50.59 ± 5.35 | 349.83 ± 5.18 |
| S28 | S. Staminea | 44.11 ± 0.18 | 497.14 ± 5.06 |
| S29 | S. multicaulis | 66.99 ± 0.54 | 713.08 ± 3.08 |
| S30 | S. nemorosa | 58.03 ± 1.91 | 104.06 ± 4.06 |
| S31 | S. spinosa | 46.86 ± 2.98 | 365.18 ± 4.18 |
| S32 | S. spinosa | 46.53 ± 0.33 | 97.70 ± 4.70 |
| S33 | S. syriaca | 45.10 ± 0.75 | 211.70 ± 1.70 |
| S34 | S. ceratophylla | 68.93 ± 0.57 | 544.25 ± 6.25 |
| S35 | S. atropatana | 49.64 ± 1.16 | 345.29 ± 4.19 |
| S36 | S. nemorosa | 63.22 ± 2.21 | 167.93 ± 1.93 |
| S37 | S. verticillata | 68.54 ± 0.06 | 260.02 ± 5.14 |
| S38 | S. nemorosa | 57.70 ± 0.51 | 180.43 ± 3.38 |
| S39 | S. limbata | 39.66 ± 3.79 | 414.07 ± 2.41 |
| S40 | S. syriaca | 37.63 ± 1.64 | 220.79 ± 0.89 |
| S41 | S. nemorosa | 63.49 ± 0.93 | 119.68 ± 1.68 |
| S42 | S. multicaulis | 64.03 ± 1.88 | 287.30 ± 3.30 |
| S43 | S. nemorosa | 67.17 ± 0.02 | 238.98 ± 3.98 |
| S44 | S. sclarea | 53.07 ± 0.78 | 81.61 ± 1.61 |
| S45 | S. macrochlamys | 65.49 ± 1.31 | 411.80 ± 4.72 |
| S46 | S. syriaca | 47.28 ± 1.19 | 188.39 ± 1.85 |
| S47 | S. verticillata | 68.12 ± 1.61 | 278.21 ± 1.21 |
| S48 | S. nemorosa | 66.72 ± 0.45 | 200.90 ± 1.90 |
| S49 | S. candidissima | 68.09 ± 0.21 | 810.86 ± 1.86 |
| S50 | S. limbata | 40.35 ± 3.11 | 114.97 ± 1.97 |
| S51 | S. syriaca | 34.32 ± 0.42 | 90.69 ± 1.71 |
| S52 | S. limbata | 48.35 ± 1.55 | 406.11 ± 6.11 |
| S53 | S. nemorosa | 58.80 ± 1.85 | 73.06 ± 1.86 |
| S54 | S. verticillata | 67.67 ± 0.51 | 120.86 ± 1.98 |
| S55 | S. limbata | 37.24 ± 0.02 | 205.44 ± 5.44 |
| S56 | S. limbata | 4.58 ± 1.08 | 237.28 ± 4.28 |
| S57 | S. multicaulis | 46.89 ± 2.36 | 559.60 ± 1.60 |
| S58 | S. syriaca | 17.92 ± 0.09 | 129.95 ± 2.95 |
| S59 | S. nemorosa | 68.12 ± 0.42 | 398.72 ± 0.72 |
| S60 | S. aethiopis | 66.84 ± 0.33 | 1071.79 ± 4.79 |
| S61 | S. limbata | 20.67 ± 0.15 | 910.91 ± 1.91 |
| S62 | S. sahendica | 21.63 ± 1.46 | 313.45 ± 0.68 |
| S63 | S. nemorosa | 60.60 ± 0.18 | 210.56 ± 3.57 |
| S64 | S. verticillata | 68.21 ± 0.21 | 747.76 ± 3.42 |
| S65 | S. aethiopis | 67.20 ± 0.22 | 571.61 ± 2.97 |
| S66 | S. spinosa | 47.82 ± 0.36 | 312.69 ± 1.67 |
| S67 | S. multicaulis | 64.81 ± 0.81 | 296.40 ± 3.40 |
| S68 | S. limbata | 45.70 ± 0.45 | 82.20 ± 1.23 |
| S69 | S. syriaca | 45.16 ± 3.08 | 265.21 ± 3.86 |
| S70 | S. multicaulis | 59.49 ± 0.63 | 186.12 ± 0.56 |
| S71 | S. limbata | 34.71 ± 0.15 | 79.96 ± 2.42 |
| S72 | S. syriaca | 60.84 ± 1.73 | 453.86 ± 4.86 |
| S73 | S. limbata | 65.67 ± 0.54 | 995.02 ± 4.24 |
| S74 | S. ceratophylla | 63.13 ± 0.63 | 257.17 ± 2.17 |
| S75 | S. spinosa | 62.87 ± 0.24 | 473.19 ± 3.19 |
| S76 | Salvia sp. | 56.48 ± 0.54 | 173.04 ± 2.38 |
| S77 | S. nemorosa | 64.84 ± 0.48 | 382.24 ± 2.24 |
| S78 | S. ceratophylla | 43.25 ± 0.09 | 161.67 ± 5.78 |
| S79 | S. syriaca | 18.70 ± 0.39 | 102.07 ± 3.73 |
| S80 | S. limbata | 25.90 ± 0.90 | 690.35 ± 1.35 |
| S81 | S. limbata | 23.21 ± 0.22 | 780.16 ± 1.01 |
| S82 | S. poculata | 39.66 ± 0.99 | 93.41 ± 1.52 |
| S83 | S. hydrangea | 64.24 ± 0.48 | 376.55 ± 2.55 |
| S84 | S. syriaca | 28.05 ± 1.13 | 219.66 ± 5.16 |
| S85 | S. ceratophylla | 65.82 ± 0.39 | 559.03 ± 0.94 |
| S86 | S. limbata | 31.96 ± 0.45 | 88.11 ± 3.11 |
| S87 | S. multicaulis | 63.82 ± 0.60 | 439.65 ± 1.76 |
| S88 | S. grossheimii | 64.12 ± 0.84 | 700.58 ± 6.25 |
| S89 | S. multicaulis | 61.55 ± 1.02 | 298.10 ± 0.10 |
| S90 | S. multicaulis | 60.27 ± 0.63 | 320.84 ± 2.84 |
| S91 | S. verticillata | 67.08 ± 0.87 | 374.85 ± 4.85 |
| S92 | S. syriaca | 32.56 ± 0.21 | 225.34 ± 5.56 |
| S93 | S. multicaulis | 60.27 ± 0.69 | 494.23 ± 4.23 |
| S94 | S. aethiopis | 41.93 ± 0.93 | 183.27 ± 5.62 |
| S95 | S. hydrangea | 40.29 ± 0.06 | 280.48 ± 2.93 |
| S96 | S. macrochlamys | 40.83 ± 2.15 | 179.86 ± 3.41 |
| S97 | S. ceratophylla | 66.03 ± 0.54 | 451.59 ± 6.04 |
| S98 | S. atropatana | 43.52 ± 0.66 | 287.87 ± 6.22 |
| S99 | S. sclarea | 56.95 ± 0.90 | 346.42 ± 0.92 |
| S100 | S. nemorosa | 48.59 ± 0.42 | 158.26 ± 4.60 |
| S101 | S. syriaca | 19.45 ± 0.18 | 95.36 ± 2.36 |
| S102 | S. multicaulis | 59.19 ± 0.33 | 719.91 ± 1.03 |
| Significant levels | ** | ** | |
AAE ascorbic acid equivalents.
**Indicates significant difference at 1% confidence levels.
Primary antioxidants found in sage plants, such as Salvia species, often include polyphenols, flavonoids, and other phytochemicals. Polyphenols represent potent antioxidants primarily due to the presence and specific positioning of hydroxyl (–OH) and carboxyl (–COOH) groups on their aromatic rings. These functional groups make polyphenols effective in scavenging free radicals and neutralizing oxidative reactions, which is why they are considered important contributors to the antioxidant properties of various foods, plants, and dietary compounds. On the other hand, the differences in the free radical scavenging activity of flavonoids can be attributed to their structural variations, as flavonoids typically possess three different rings, labeled as A, B, and C63. The specific arrangement and types of substitutions on these rings can significantly impact their antioxidant properties and biological activities. Primary antioxidants also include hindered phenols and secondary aromatic amines64, which play a crucial role in preventing the oxidation of molecules by scavenging free radicals and stabilizing reactive intermediates that are formed during oxidative reactions. Evidence supporting the role of these compounds as primary antioxidants in the aerial parts of S. viridis has been previously provided65,66.
Identification of metabolites
UHPLC-HRMS was used to determine the chemical compounds present in the extracts of the 20 selected Salvia species. Representative chromatograms for a sample of each of the 20 species studied are included in Figs. S1–S20. The compounds identified in the samples mainly belong to phenolic and flavonoid groups, with one from the fatty acyl glycoside group, as listed in Table 5.
Table 5.
General classification of the chemical compounds identified by UHPLC-HRMS.
| Phenolic acids | Flavonoids | Fatty acyl glicosides |
|---|---|---|
| Syringic acid | Myricitrin | Tuberonic acid glucosides |
| Caffeic acid hexosides | Eriodictyol-O-glucuronide | |
| Yunnaneic acid F | Hyperoside | |
| Isoverbascoside | 6-Hydroxyluteolin 7-O-glucuronide | |
| Yunnaneic acid E | Lipedoside A | |
| Rosmarinic acid | Luteolin-glucoside | |
| Sagerinic acid | Luteolin-glucuronide | |
| Salvianolic acid K | Luteolin | |
| Martynoside/isomartynoside | Apigenin 7-O-glucoside | |
| Caffeoyl malic acid | Genistein | |
| Sagecoumarin | Hispidulin | |
| Salvianolic acid A | Hispidulin glucuronide | |
| Salvianolic acid B | Kaempferol-glucoside | |
| Salvianolic acid C | Kaempferol-glucuronide | |
| Salvianolic acid F isomers | Apigenin acetyl glucoside | |
| Ferulic acid | Luteolin acetyl glucoside | |
| Lityhospermic acid isomers | Kaempferol | |
| Caffeoylquinic acids | Apigenin | |
| Verbascoside | Kaempferide | |
| Forsythoside A |
The identified compounds were classified into nine other subgroups (Table 6) to better represent their structural heterogeneity. These compounds are known to have different antioxidant activities and pharmacological properties.
Table 6.
Detailed classification of compounds identified by UHPLC-HRMS.
| Phenolic acids | ||
|---|---|---|
| 2-Arylbenzofuran propanoids | Phenyletanoids | Hydroxycinnamic acids |
| Salvianolic acid isomers | Isoverbascoside | Caffeic acid hexosides |
| Salvianolic acid A | Verbascoside | Ferulic acid |
| Salvianolic acid B | Martynoside | Rosmarinic acid |
| Salvianolic acid C | Phenylpropanoids | Caffeoyl malic acid |
| Lithyospermic acid isomers | Caffeoyl quinic acids | |
| Benzoic acids | Coumarin | Lignans |
| Syringic acid | Sagecoumarin | Yunnaneic acid F |
| Flavonoids | Yunnaneic acid E | |
| Myricitrin | Genistein | Sagerinic acid |
| Forsythoside A | Hispidulin | Salvianolic acid K |
| Eriodictyol-O-glucuronide | Hispidulin-glucuronide | |
| Hyperoside | Kaempferol-glucoside | |
| 6-Hydroxyluteolin-7-O-glucuronide | Kaempferol-glucuronide | Fatty acyl glycoside |
| Lipedoside_A | Apigenin acetyl_glucoside | Tuberonic acid glucosides |
| Luteolin-glucoside | Luteolin acetyl_glucoside | |
| Luteolin-glucuronide | Kaempferol | |
| Luteolin | Apigenin | |
| Apigenin-7-O-glucoside | Kaempferide | |
Previous studies have already revealed the presence of both flavonoids and phenolic acids in Salvia species67. Flavonoids are categorized into six subclasses including flavones, flavonols, isoflavones, flavanols, flavanones, and anthocyanins9. Phenolic acids have diverse structures, ranging from monomers to dimers, trimers, tetramers, and multimers, and are present in high concentrations in sage, with significant taxonomic implications as observed for Chinese Salvia species68. Phenolic acids have been shown to play a central role in mediating the major pharmacological activities of Chinese Salvia species, particularly their antioxidant effects68. Several studies have indicated that S. officinalis and other plants exhibit strong antioxidant and free radical scavenging activities, including the study by Nickavar et al.69. The ethanol extract of S. officinalis contains both phenolic compounds and terpenoids, such as carnosic acid (phenolic diterpene), carnosol (ortho-diphenolic diterpene and an oxidative derivative of carnosic acid), rosmarinic acid (phenolic compound), rosmanol (phenolic diterpene), rosmadial (diterpene lactone), methyl carnosate (diterpene), epirosmanol (diterpene lactone), luteolin-7-O-betaglucopyranoside (flavonoid)70, and caffeic acid (polyphenol)71,72. Aqueous extracts of Salvia africana, Salvia officinalis ‘Icterina’, and Salvia mexicana were found to contain distinct phenolic compounds, with S. africana exhibiting the highest total phenol content level (231.6 mg GAE/g DW) in agreement with its potent antioxidant potential, as also reported by Afonso et al.73. Various phenolic compounds, such as caffeic, chlorogenic and rosmarinic acids, as well as flavonoid compounds such as luteolin (flavone), apigenin (flavone) and their glycosides were identified as the most important polyphenols in hydroalcoholic extracts of S. hortensis20. Rosmarinic acid, which is one of the most abundant phenolic acids, is commonly found in many species of Salvia6,35. Carnosic acid, caffeic acid, oleanolic acid (pentacyclic triterpenoid), ursolic acid (pentacyclic triterpenoid), kaempferol (flavonoid), and rosmarinic acid were identified in 17 extracts of indigenous Salvia species (obtained with a mixture of methanol/chloroform 1:1 v/v), as reported by Kamatou et al.74. Around 160 polyphenolic compounds have been identified from sage leaves, encompassing a wide range of different flavonoids and phenolic acids. The six most significant flavonoids found in sage plants are luteolin, apigenin, hispidulin, 7-O-glucoside, kaempferol, cirsimaritin, and quercetin9. Numerous flavones were found in S. disermas, while a few were noted in S. aurita, S. africana-caerulea, S. dolomitica, S. runcinata, S. stenophylla, S. garipensis, S. lanceolata, S. namaensis, S. chamelaeagnea, S. radula, S. schlechteri and S. repens12. Polyphenol analysis of S. officinalis leaf extracts has also revealed the presence of various phenolic compounds, such as salvianolic acids, caffeic acid and its derivatives, rosmarinic acid, lithospermic acids, sagerinic acid, yunnaneic acids and sage coumarin. Caffeic acid derivatives (trans-verbascoside, cis-verbascoside, leucosceptoside A, martynoside, caffeic acid, 6-O-caffeoyl-glucose, rosmarinic acid and salidroside) are considered as the primary phenolic acids in Salvia species, serving as building blocks for various plant metabolites. In the biochemistry of the Lamiaceae family, caffeic acid plays a central role and remains in subdued form as rosmarinic acid. According to a study by Skendi et al.75 and Moshari Nasirkandi et al.76, both rosmarinic acid and caffeic acid were identified as the main polyphenolic compounds in extracts obtained from plants of the Lamiaceae family.
Chemometric analysis
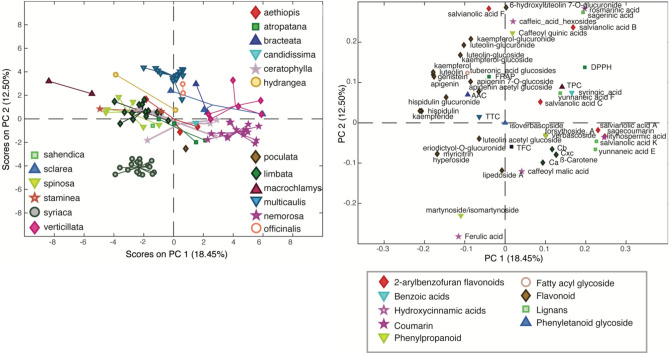
Principal Component Analysis (PCA) is one of the most commonly used statistical techniques in metabolomics77,78. The primary goal of PCA is to reduce the dimensionality of a dataset consisting of many interrelated variables, thus providing a visual representation of the major variance in the data, emphasizing variation and bringing out strong patterns in a dataset. PCA achieves this by converting the original correlated variables into a smaller set of new orthogonal (uncorrelated) variables, called principal components (PCs). PCs can be considered as the axes of a new coordinate system, where the greatest variance lies on the first coordinate (principal component), the second greatest variance on the second coordinate, and so on. The PCs are displayed in two plots, called “scores plot”, where the samples appear close to each other when they are similar and distant when they are dissimilar, and “loadings plot”, which highlights the variables responsible for the separation of the samples along each PC. PCA is an unsupervised method commonly used in several research fields, including agricultural science79,80. In the current study, PCA was employed due to the extensive volume of analyzed data/variables (102 samples × 50 variables). The aim was to enhance result interpretation and find potential relationships between them. In this sense, PCA was performed using data from the 102 Salvia samples, i.e., the results of TPC, TFC, TTC, AAC, photosynthetic pigment content (chlorophyll a and b, total carotenoid concentration, and β-carotene), and antioxidant activity (FRAP and DPPH), combined with those from the quantification of 40 polyphenols via UHPLC-HRMS (for a total of 50 variables). In particular, a relative quantification of polyphenols was carried out, as it is commonly considered sufficient in metabolomics to explore similarities and differences among analyzed samples through Principal Component Analysis, which operates by comparing samples. Notably, a distinct separation was observed when samples were classified by species (Fig. 3), with the first two principal components (PCs) explaining 30.95% of the total variance (PC1: 18.45%, and PC2: 12.50%). These components were instrumental in visualizing the sample relationships. No significant separation was observed when samples were classified according to different area and altitude classes (Fig. S21).
Figure 3.
PCA scores plot (left) and loadings plot (right) results colored by species, obtained from the data of the 10 colorimetric determinations (TPC, TFC, TTC, AAC, FRAP, DPPH, Ca, Cb, Cxc, and β-carotene) and the 40 chemical compounds identified for the 102 Salvia extracts.
The results of the PCA analysis revealed distinct characteristics among various Salvia species. For instance, the S. multicaulis group, positioned in the upper part of the scores plot (Fig. 3), showed high levels of the flavonoid 6-hydroxyluteolin-7-glucuronide. This group also contained notable quantities of several phenolic acids known for their potent antioxidant activity, such as salvianolic acid F isomer, rosmarinic and sagerinic acids, caffeic acid hexoside, caffeoyl quinic acid, and salvianolic acid B. Notably, these compounds exhibit connections; for instance, sagerinic acid is believed to be derived from rosmarinic acid through a photochemical cyclization and salvianolic acid B is a part of caffeic acid tetramers originating from the dimerization of rosmarinic acid81,82.
The S. nemorosa samples, situated in the bottom right quadrant of the scores plot, showed higher levels of caffeic acid trimers, including salvianolic acid A and K, sagecoumarin, lityhospermic acid isomers, and yunnaneic acid E. These compounds correspondingly appear in the right section of the loadings plot. In contrast, the S. macrochlamys group, positioned in an opposing direction to S. nemorosa, displayed lower concentrations of these caffeic acid trimers but presented higher levels of various flavonoids: luteolin, apigenin, hispidulin, and hispidulin-glucuronide among the flavones and their glucuronides; kaempherol, kaempheride, myricitrin, kaempferol-glucoside and hyperoside among flavonols and their glycoside derivatives; genistein and eriodictyol-O-glucuronide among isoflavones and flavanone glucuronides.
S. syriaca showed considerable differences from other sage species and was notably distanced from all other sample groups in the scores plot. The loadings plot attributes this to its high content of ferulic acid and martynoside. Conversely, species like S. verticillata, S. bracteata, and S. officinalis, located in the top right panel of the scores plot, had lower concentrations of these compounds. These species displayed increased levels of syringic acid, yunnaneic acid F, and salvianolic acid C, and higher values of TPC and DPPH. Additionally, the species belonging to S. limbata, S. spinosa, S. staminea, S. aethiopis, and S. atropatana groups, clustered at the center of the graph, showed the highest levels of TTC and TFC. Among the latter, especially the flavonoid glycosides and glucuronides were notably abundant.
Potential applications of different Salvia species in treating different diseases
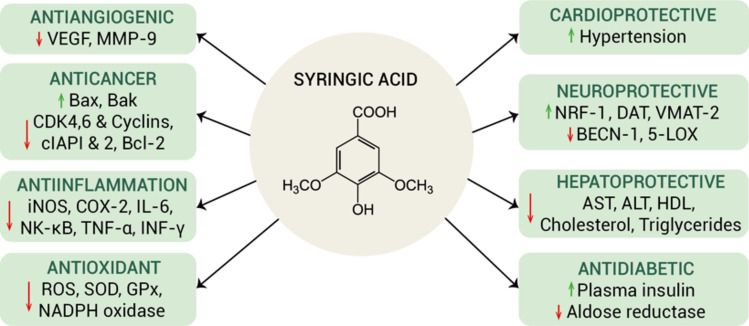
Considering that each Salvia species is distinguished by high levels of compounds of the same chemical class (Table 6) or sharing the same biological pathways, the observed separations suggest that each species serves as a distinct representative of a particular class of polyphenols. These differences in phenolic composition may hold potential for the treatment of various diseases. For instance, S. verticillata and S. bracteata species, characterized by increased antioxidant activity and a high content of polyphenols, including a high abundance of syringic acid, could face conditions that require robust free radical scavenging. Syringic acid, with its distinctive phenolic structure featuring methoxy moieties at positions 3 and 5, exhibits multifaceted properties (Fig. 4). Notably, it acts as a powerful antioxidant, helping to reduce oxidative stress and neuronal degeneration, while also contributing to cardioprotection by regulating blood pressure, lipid peroxides, and improving nitric oxide and antioxidant levels83. Furthermore, syringic acid also possesses antimicrobial properties by inhibiting various bacteria, including drug-resistant strains of Staphylococcus aureus and Salmonella typhi84. Moreover, it has been shown to induce apoptosis in tumor cells by modulating gene expression85. Other wide-ranging effects of syringic acid encompass antidiabetic, hepatoprotective, antihyperlipidemic, and anti-inflammatory actions, which contribute to insulin regulation, liver health, lipid profiles, and immune responses83,86.
Figure 4.
Biomedical applications of syringic acid.
On the contrary, the extracts of S. nemorosa, rich in salvianolic and lithyospermic acids, exhibit promise in combating conditions related to excessive oxidative stress, such as endothelial dysfunction, pulmonary arterial hypertension, and cardiac fibrosis. These compounds, particularly salvianolic acid A, have shown chelating abilities, inhibiting Cu2+-mediated oxidation and leading to the formation of oxidized LDL, associated with atherosclerosis87. Their antioxidative effects are attributed to multiple pathways, involving the downregulation of NADPH oxidase 4 (NOX4) and upregulation of Nrf2/heme oxygenase-1 (HO-1) signaling pathway, significantly impacting vascular dysfunction and inflammation87. Other evidence shows that salvianolic acids A, B and C, along with lithyospermic and rosmarinic acids may have great potential for the treatment of Alzheimer’s disease by inhibiting the glycogen synthase kinase 3β (GSK-3β), considered a strategic therapeutic target against Alzheimer’s disease88.
S. multicaulis and S. officinalis species, showing higher levels of salvianolic acid B, could also yield similar health benefits. Furthermore, the high sagerinic acid content observed for them holds promise due to its recognized anti-inflammatory and neuromodulatory properties, interacting with various families of proteins, including oxidoreductases, hydrolases, and enzymes like MAO-A, MAO-B, COX-2, tyrosinase, and cholinesterase89.
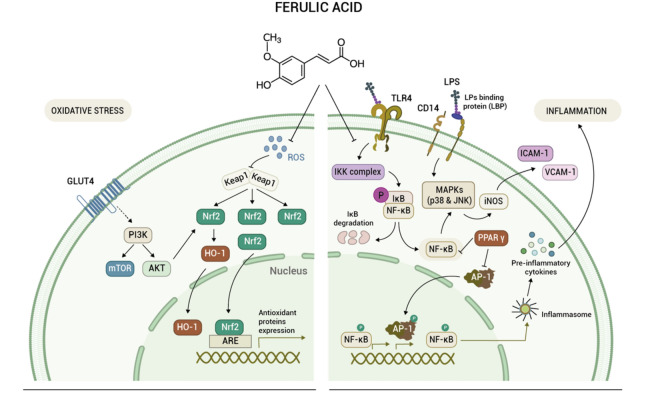
Instead, S. syriaca stands out for its particularly high content of ferulic acid, renowned for its antioxidant role in the elimination of oxidative free radicals, inhibiting the generation of reactive oxygen species (ROS), and for its involvement in multiple signaling pathways90. A detailed illustration of the molecular mechanisms underlying the antioxidant and anti-inflammatory activities of ferulic acid is reported in Fig. 5.
Figure 5.
Molecular pathways involved in the antioxidant (left) and anti-inflammatory (right) activities of ferulic acid (adapted from Li et al.81).
Ferulic acid has been reported to exert diverse effects such as reducing ROS production in glomerular podocytes, inhibiting advanced glycation end products and xanthine oxidase, and promoting glucose uptake by activating the PI3K/Akt signaling pathway (Fig. 5, left panel)91. It also exhibits anti-inflammatory properties in various diseases by suppressing the secretion and expression of inflammatory factors through multiple pathways (Fig. 5, right panel)90,91. Moreover, ferulic acid is known for its cardioprotective, anti-fibrotic, anti-apoptotic, and anti-platelet activities. It helps regulate vascular function, endothelial cell health, and prevents excessive collagen accumulation, particularly beneficial in liver, kidney, and lung fibrosis90,91.
Among the studied species, S. limbata, S. spinosa, and S. staminea exhibit the highest levels of flavonoid glycosides and glucuronides. Flavonoids possess a spectrum of biological activities, and O-glycosylation appears to enhance specific bioactivities, including anti-HIV, anti-rotavirus, anti-stress, anti-obesity, tyrosinase inhibition, anticholinesterase potential, anti-adipogenicity, anti-allergic effects, and utility for treatment of chronic kidney disease92. Flavonoid glycosides, especially administered orally, showed enhanced antidiabetic, anti-inflammatory, anti-degranulation, anti-stress, and anti-allergic activities compared to their aglycone counterparts92. Indeed, flavonoid glycosides exhibit prolonged presence in the bloodstream and higher plasma concentrations, also impacting cardiovascular health through metal chelation and reduction of oxidative processes92,93. Flavonoid glycosides interact with enzymes like α-amylases and α-glucosidases, crucial in diabetes management. The glycosides of kaempferol and quercetin display higher inhibitory activity against α-glucosidase and aldose reductase92,94–96. The latter, involved in the polyol pathway, has implications in diabetic complications by causing sorbitol accumulation in organs97. Flavonoids are being explored for various diseases, including obesity, viral and bacterial infections, inflammatory conditions, neurodegenerative diseases, and cancer treatments92,98,99.
Conclusions
In recent years, the exploration of the therapeutic potential of Salvia has gained considerable attention, leading to an increasing number of scientific studies emphasizing its pharmacological properties. Despite this, therapeutic applications of Salvia remain underexploited, necessitating further investigation. In this study, a comprehensively exploration and comparison of the extracts of 102 samples from 20 distinct Salvia species from Iran was performed, providing a deeper understanding of their specific polyphenol content and, consequently, their antioxidant capabilities and potential therapeutic uses. All samples were analyzed to determine the contents of total phenolics, total flavonoids, total tannin, photosynthetic pigments, and ascorbic acid, followed by the evaluation of their antioxidant activity in vitro. These data were then combined with those from the UHPLC-HRMS analysis, which identified forty distinct chemical compounds in the analyzed samples. The large number of data obtained was subjected to multivariate data analysis with the purpose of finding similarities and differences among the investigated species. Results indicate that each species represents a distinct class of polyphenols due to their unique phenolic compositions. The variations in phenolic profiles suggest diverse potential applications of different Salvia species in treating a wide array of diseases. More specifically, S. verticillata and S. bracteata stand out for their high syringic acid content, known for various activities like cardioprotection, hepatoprotection, antidiabetic, antimicrobial, anti-inflammatory, and pro-apoptotic effects, warranting further investigation in these fields. On the other hand, S. nemorosa, particularly rich in salvianolic and lithyospermic acids, holds promise for treating endothelial dysfunction, pulmonary arterial hypertension, and cardiac fibrosis. Furthermore, combining extracts from species with a high content of specific compounds, like salvianolic acid A (abundant in S. nemorosa) and salvianolic acid B (in S. multicaulis and S. officinalis), along with rosmarinic acid and salvianolic acid C (in S. verticillata and S. bracteata) may have great potential for Alzheimer’s treatment. The elevated ferulic acid content in the extracts of S. syriaca is also interesting for its cardioprotective, anti-fibrotic, anti-apoptotic, and anti-platelet activities. Extracts of species like S. macrochlamys, S. staminea, S. limbata, and S. spinosa, rich in flavonoid glycosides and glucuronides, could exhibit antidiabetic, anti-inflammatory, anti-stress, and antiallergic activities, and be potentially useful as anti-viral, anti-bacterial, and even as antiproliferative agents.
This study employed a targeted approach focused on the analysis of specific phytochemicals or antioxidant compounds. An untargeted approach, on the other hand, may offer a broader and more exploratory analysis, enabling the discovery of novel metabolites or unexpected chemical entities that may not have been previously characterized. Integrating both approaches in future studies will provide complementary insights into the metabolome of a sample, combining the specificity of targeted analysis with the exploratory nature of untargeted analysis to enhance metabolite discovery and characterization.
In conclusion, the Salvia species investigated in this study show significant potential for the development of innovative nutraceuticals, filling a gap in the current limited range of Salvia-derived products available on the market. Current offerings, often composed of individual Salvia species in forms like leaf extracts, tinctures, or tablets, address limited health concerns such as digestive issues or specific afflictions, such as headaches and rheumatism. Our findings highlight particularly effective species (S. verticillata, S. bracteata, S. nemorosa, S. multicaulis, S. officinalis, and S. syriaca), suggesting their collective use in the creation of new nutraceuticals. Combining two or more of these species could generate synergistic effects, broadening their medical applications with various health benefits.
Materials and methods
Chemicals and reagents
1,1-Diphenyl-2-picryl hydrazyl (DPPH), 2,4,6-tripyridyl-S-triazine (TPTZ), and quercetin were purchased from Sigma Chemical Co. (St., Louis, USA). Gallic acid, Folin Ciocalteu reagent, sulfuric acid (H2SO4), potassium hydroxide (KOH), potassium carbonate (K2CO3), sodium carbonate (Na2CO3), aluminum chloride (AlCl3), sodium nitrite (NaNO2), sodium hydroxide (NaOH), sodium acetate (CH3COONa), formic acid, ethanol, hexane, acetone, acetonitrile, and methanol were all purchased from Merck & Co. Inc. (Rahway, New Jersey, USA).
Sampling of Salvia species
A total of 102 Salvia samples were handpicked between April and July 2020 from various regions in West Azerbaijan and identified by Dr. Shahram Bahadori of the Department of Biology, University of Tehran, Tehran (Iran). West Azerbaijan Province is recognized as one of the primary centers of genetic diversity of the Lamiaceae family in Iran. The 102 collected samples represent 20 different Salvia species (Fig. 1, Table 1), making them a comprehensive representation of the entire Salvia family. Moreover, even within the same Salvia species, the samples exhibit variations in collection location and altitude. The samples were collected in different cities within the Western Azerbaijan Province that can be categorized into three distinct collection areas (Fig. 2): the northern region extending from Maku to Salmas (highlighted in green), the central area from Urmia to Sardasht (in red), and the southern area from Miandoab to Takab (in purple). Furthermore, the samples were gathered at different altitudes, some at sea level, while others were collected in the mountains or near lakes. Consequently, even samples from the same species were exposed to different meteorological conditions. Table S1 provides details of the specific areas where the various samples of each of the 20 Salvia species were collected.
Preparation of methanolic extracts
The aerial parts of the selected plants were separated and then washed thoroughly with running water to remove surface dust. The collected samples were then air dried for few days, shrunken into powder, and stored in plastic bags for use. The plant powders (0.1 g) were placed in test tubes, and methanol (10 mL) was added to each tube to soak the plant powder, which was then shaken well. The solutions were then filtered by filter paper, and the resulting filtrates were collected separately and used for phytochemical analysis. Methanol was chosen as an extraction solvent, based on the results of Bajkacz et al., who demonstrated that methanol outperformed other solvents in extracting polyphenols100. Indeed, their comparative analysis revealed that methanol was the most effective solvent, followed by ethanol and its combinations.
Total phenolic content (TPC)
The Folin-Ciocalteu colorimetric method was employed to determine the total phenolic content in the methanolic extracts from the 102 Salvia samples, according to Ul-Haq et al.101. The concentration of the extracts was 20 µg/mL and their absorbances were measured at 765 nm using an HALO DB-20 UV–Vis spectrophotometer (Dynamica Scientific LTD, Livingston, UK). A standard curve was built using different concentrations of gallic acid (10–50 µg/mL). The total phenolic content for each sample was expressed in terms of gallic acid equivalents per gram of dry weight sample (mg of GAE/g of dry weight sample).
Total flavonoid content (TFC)
The total flavonoid content of each sample was determined using the aluminum chloride colorimetric assay102. Briefly, 0.15 mL of each diluted extract was separately mixed with 1.5 mL of methanol, 0.1 mL of potassium acetate (1.0 M), 0.1 mL of aluminum chloride (10%, w/v), and 2.8 mL of distilled water, and the mixture was incubated at room temperature for 35 min. Then, the absorbance of each sample was measured at 415 nm. A calibration curve using quercetin as standard (10–50 µg/mL) was drawn. The results of the TFC were expressed as mg of quercetin equivalent per gram of dry weight sample (mg QUE/g DW).
Total tannin content (TTC)
The amount of total tannin content for each extract was evaluated according to the Vanillin reagent method as reported by Bharath et al.103. The reaction mixture consisted of 0.1 mL of extract, 2 mL of vanillin (4% w/v in methanol), and 1 mL of HCl (concentrated grade). The reaction mixture was shaken well and incubated for 30 min at 30 °C. The absorbance of each sample was measured at 500 nm. The total tannin content was evaluated using the linear regression equation obtained from the standard tannic acid and expressed in mg tannic acid equivalent per g of extract (mg TAE/g extract).
Ascorbic acid content (AAC)
The total concentration of ascorbic acid was determined using to the method of Klein and Perry104. Briefly, 0.1 g of sample powder was extracted using 3 mL of a 1% metaphosphoric acid solution leaving the mixture at − 4 °C for 35 min, followed by filtration at room temperature. The filtrate was mixed with 2,6-dichloroindophenol (2 mL) and the absorbance was recorded within 30 min at 520 nm against a blank. The content of ascorbic acid was assessed based on a standard calibration curve built using authentic l-ascorbic acid (10–50 mg/mL). Results were expressed as mg of ascorbic acid per gram dry weight (mg AA/g DW).
Antioxidant activity by DPPH assay
The free radical scavenging activity was measured using the elimination of DPPH radicals according to the method of Shimada et al.105 with some modifications. Different concentrations of each extract were separately added to 4 mL of DPPH methanol solution (0.004%). The mixture was shaken and kept for 30 min at room temperature in the dark. The absorbance was measured at 517 nm. All determinations were performed in triplicate. The antioxidant activity was determined as the percent inhibition caused by the hydrogen donor activity of each sample according to the following equation: Inhibition (%) = (1 − absorbance of the sample/absorbance of the blank) × 100. The results are reported as micrograms of ascorbic acid equivalent per milliliter (µg AAE/mL). The percentage obtained for each sample was placed in the formula of the standard curve (y = 1.5112 x + 32.644) of ascorbic acid and thus the amount of DPPH was obtained based on AAE.
Antioxidant activity by FRAP assay
FRAP activity assay was carried out according to the protocol of Miao et al.106 with minor modifications. Briefly, the FRAP reagent consisted of 300 mmol/L acetate buffer (pH 3.6), 20 mmol/L ferric chloride (FeCl3) solution, and 10 mmol/L 2,4,6-tripyridyls-triazine (TPTZ) solution, mixed in a 10:1:1 (v/v) ratio, respectively. The FRAP reagent was freshly prepared and warmed at 37 °C in a water bath before use. 100 μL of each plant extract was separately added to 3 mL of the FRAP reagent. The mixture was vortexed, and absorbance of the solution was evaluated at 593 nm after incubating at 37 °C for 35 min. Different concentrations (from 50 to 600 μmol/L) of Fe2+ solution were used to build the standard curve. Results were expressed in terms of micromoles of Fe2+ per gram of dry weight (μmol Fe2+/g DW).
Photosynthetic pigments content
Chlorophylls (Ca and Cb), total carotenoids (Cx+c), and β-carotene were measured spectrophotometrically using plant extracts prepared in 80% acetone/distilled water (v/v), following the methods outlined in the studies by Lichtenthaler107 and Nagata et al.108. The contents of these compounds were calculated for each extract using the following formulas:
where A662, A645, and A470 are the absorbance values determined at the wavelengths of 662, 645, and 470 nm, respectively.
Identification of polyphenolic compounds by UHPLC-HRMS
Polyphenolic compounds’ profiling was performed by using an UHPLC system (Dionex UltiMate 3000, Thermo Fisher Scientific, Waltham, MA, USA) coupled to a Q-Exactive Orbitrap mass spectrometer (UHPLC, Thermo Fisher Scientific, Waltham, MA, USA). A Luna Omega PS 1.6 µm column (50 × 2.1 mm, Phenomenex, Torrance, CA, USA) was employed for the chromatographic separation setting the temperature at 25 °C. The mobile phase was composed of solvent A (water containing 0.1% of formic acid), and solvent B (acetonitrile containing 0.1% of formic acid). Polyphenolic compounds were eluted using the following gradient program: 0–1 min, 0% B; 1–2 min, 0–95% B; 2–2.5 min, 95% B; 2.5–5 min, 95–75% B; 5–6 min, 75–60% B. Subsequently, the gradient returned to 0% B in 0.5 min and held for 2.5 min for column re-equilibration. The flow rate was 0.4 mL/min and the injection volume was 5 µL. The autosampler temperature was set at 10 °C.
The mass spectrometer was operated in negative ion mode (ESI−) due to the acidic nature of the phenolic hydroxyl groups. In this mode, the molecules (M) lose a proton (H), forming anions (M–H)−. This results in higher sensitivity and better signal stability for polyphenols, as they tend to form stable negative ions. Two scan events (full ion MS and all ion fragmentation, AIF) were set up for all compounds of interest. Full scan data were acquired setting a resolving power of 35,000 FWHM (full width at half maximum) at m/z 200, whereas AIF scan events were acquired by setting a resolving power of 17,500 FWHM and collision energy values of 10, 20, and 45 eV. In both cases, the mass parameters were the following: Spray Voltage, − 3.5 kV; Sheath Gas Flow Rate, 45 arbitrary units; Auxiliary Gas Flow Rate, 10 arbitrary units; Capillary Temperature, 275 °C; Auxiliary Gas Heater Temperature, 350 °C; S-lens RF level, 50; Scan Range m/z, 80–1200. Data acquisition and processing were performed with Quan/Qual Browser Xcalibur software, v. 3.1.66.10 (Xcalibur, Thermo Fisher Scientific, (Thermo Fisher Scientific, Waltham, MA, USA). Analyses were performed in triplicate.
Statistical analysis
Data were analyzed by one-way ANOVA using SAS software version 9.4 (SAS Institute Inc., Cary, NC, USA). Means of three replicates were compared using the Duncan’s Multiple Range Test (DMRT). For multivariate data analysis, raw data obtained from UHPLC-HRMS analysis were aligned based on the m/z value and retention time of the ion signals, then collected in a data matrix consisted of 102 rows (samples) and 40 columns (polyphenols data from UHPLC-HRMS analysis). Such data matrix was then imported into Matlab R2015b (The Mathworks Inc., Natick, MA, USA) to compute multivariate analysis. A principal component analysis (PCA) was performed using the PLS Toolbox 8.6.1 (Eigenvector Research Inc., Wenatchee, WA, USA) under Matlab environment. Before PCA, data was scaled to unit variance (autoscaling). Autoscaling employs both the standard deviation as a scaling factor, thus giving all metabolites the same chance to affect the model, and the mean-centering, which is needed to compute PCA.
Supplementary Information
Acknowledgements
The present research was realized in the frame of the international agreement between the Department of Pharmacy of the University of Naples Federico II and the Department of Horticultural Science of the Urmia University (n. 2022/0059068).
Author contributions
Conceptualization: A.A. and J.A.; Methodology and investigation: A.M.N., N.I., F.R., G.G, and H.A.; Supervision: N.I., A.A., and J.A.; Manuscript writing: A.M.N., F.R., A.A., and J.A. All authors have read and agreed to the published version of the manuscript.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding authors on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Atefeh Moshari-Nasirkandi and Nunzia Iaccarino.
Contributor Information
Abolfazl Alirezalu, Email: a.alirezalu@urmia.ac.ir.
Jussara Amato, Email: jussara.amato@unina.it.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-68421-8.
References
- 1.Walker, J. B., Sytsma, K. J., Treutlein, J. & Wink, M. Salvia (Lamiaceae) is notmonophyletic: Implications for the systematics, radiation, and ecological specializations of Salvia and tribe Mentheae. Am. J. Bot.91, 1115–1125 (2004). 10.3732/ajb.91.7.1115 [DOI] [PubMed] [Google Scholar]
- 2.Zare, S. et al. Antileishmanial and pharmacophore modeling of abietane-type diterpenoids extracted from the roots of Salvia hydrangea. J. Mol. Struct.1228, 129447 (2021). 10.1016/j.molstruc.2020.129447 [DOI] [Google Scholar]
- 3.Zhumaliyeva, G. et al. Natural compounds of Salvia L. genus and molecular mechanism of their biological activity. Biomedicines11, 3151 (2023). 10.3390/biomedicines11123151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roby, M. H. H., Sarhan, M. A., Selim, K.A.-H. & Khalel, K. I. Evaluation of antioxidant activity, total phenols and phenolic compounds in thyme (Thymus vulgaris L.), sage (Salvia officinalis L.), and marjoram (Origanum majorana L.) extracts. Ind. Crops Prod.43, 827–831 (2013). 10.1016/j.indcrop.2012.08.029 [DOI] [Google Scholar]
- 5.Kahnamoei, M. B. et al. Chemical constituents from the ethyl acetate extract of Salvia hydrangea. Nat. Prod. Commun.14, 1934578–1984885 (2019). [Google Scholar]
- 6.Kan, Y., Gökbulut, A., Kartal, M., Konuklugil, B. & Yılmaz, G. Development and validation of a LC method for the analysis of phenolic acids in turkish Salvia species. Chromatographia66, 147–152 (2007). 10.1365/s10337-007-0278-7 [DOI] [Google Scholar]
- 7.Zhou, Y. et al. Qualitative and quantitative analysis of diterpenoids in Salvia species by liquid chromatography coupled with electrospray ionization quadrupole time-of-flight tandem mass spectrometry. J. Chromatogr. A1216, 4847–4858 (2009). 10.1016/j.chroma.2009.04.017 [DOI] [PubMed] [Google Scholar]
- 8.Wu, Y.-B. et al. Constituents from Salvia species and their biological activities. Chem. Rev.112, 5967–6026 (2012). 10.1021/cr200058f [DOI] [PubMed] [Google Scholar]
- 9.Lu, Y. & Yeap Foo, L. Polyphenolics of Salvia—A review. Phytochemistry59, 117–140 (2002). 10.1016/S0031-9422(01)00415-0 [DOI] [PubMed] [Google Scholar]
- 10.Bonesi, M. et al. Anti-inflammatory and antioxidant agents from Salvia genus (Lamiaceae): An assessment of the current state of knowledge. Antiinflamm. Antiallergy Agents Med. Chem.16, 70 (2017). 10.2174/1871523016666170502121419 [DOI] [PubMed] [Google Scholar]
- 11.Lopresti, A. L. Salvia (sage): A review of its potential cognitive-enhancing and protective effects. Drugs R. D.17, 53–64 (2017). 10.1007/s40268-016-0157-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kamatou, G. P. P., Viljoen, A. M. & Steenkamp, P. Antioxidant, antiinflammatory activities and HPLC analysis of South African Salvia species. Food Chem.119, 684–688 (2010). 10.1016/j.foodchem.2009.07.010 [DOI] [Google Scholar]
- 13.Adzet, T., Caiñigueral, S. & Iglesias, J. A chromatographic survey of polyphenols from Salvia species. Biochem. Syst. Ecol.16, 29–32 (1988). 10.1016/0305-1978(88)90113-5 [DOI] [Google Scholar]
- 14.Farhat, M. B., Landoulsi, A., Chaouch-Hamada, R., Sotomayor, J. A. & Jordán, M. J. Profiling of essential oils and polyphenolics of Salvia argentea and evaluation of its by-products antioxidant activity. Ind. Crops Prod.47, 106–112 (2013). 10.1016/j.indcrop.2013.02.007 [DOI] [Google Scholar]
- 15.Tepe, B., Eminagaoglu, O., Akpulat, H. A. & Aydin, E. Antioxidant potentials and rosmarinic acid levels of the methanolic extracts of Salvia verticillata (L.) subsp. verticillata and S. verticillata (L.) subsp. amasiaca (Freyn & Bornm.) Bornm. Food Chem.100, 985–989 (2007). 10.1016/j.foodchem.2005.10.062 [DOI] [Google Scholar]
- 16.Ghorbani, A. & Esmaeilizadeh, M. Pharmacological properties of Salvia officinalis and its components. J. Tradit. Complement. Med.7, 433–440 (2017). 10.1016/j.jtcme.2016.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jassbi, A. R., Zare, S., Firuzi, O. & Xiao, J. Bioactive phytochemicals from shoots and roots of Salvia species. Phytochem. Rev.15, 829–867 (2016). 10.1007/s11101-015-9427-z [DOI] [Google Scholar]
- 18.Sharifi-Rad, M. et al.Salvia spp. plants-from farm to food applications and phytopharmacotherapy. Trends Food Sci. Technol.80, 242–263 (2018). 10.1016/j.tifs.2018.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Topçu, G. Bioactive triterpenoids from Salvia species. J. Nat. Prod.69, 482–487 (2006). 10.1021/np0600402 [DOI] [PubMed] [Google Scholar]
- 20.Fierascu, I. et al. Phytochemical profile and biological activities of Satureja hortensis L.: A review of the last decade. Molecules23, 2458 (2018). 10.3390/molecules23102458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cardile, V. et al. Essential oils of Salvia bracteata and Salvia rubifolia from Lebanon: Chemical composition, antimicrobial activity and inhibitory effect on human melanoma cells. J. Ethnopharmacol.126, 265–272 (2009). 10.1016/j.jep.2009.08.034 [DOI] [PubMed] [Google Scholar]
- 22.Mancini, E. et al. Chemical composition and phytotoxic effects of essential oils of Salvia hierosolymitana Boiss. and Salvia multicaulis Vahl. var. simplicifolia Boiss. Growing wild in lebanon. Molecules14, 4725–4736 (2009). 10.3390/molecules14114725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Canzoneri, M. et al. Chemical composition and biological activity of Salvia verbenaca essential oil. Nat. Prod. Commun.6, 1023–1026 (2011). [PubMed] [Google Scholar]
- 24.Tenore, G. C. et al. Antimicrobial and antioxidant properties of the essential oil of Salvia lanigera from Cyprus. Food Chem. Toxicol.49, 238–243 (2011). 10.1016/j.fct.2010.10.022 [DOI] [PubMed] [Google Scholar]
- 25.El Sheikha, A. F. Nutritional profile and health benefits of ganoderma lucidum “Lingzhi, Reishi, or Mannentake” as functional foods: Current scenario and future perspectives. Foods11, 1030 (2022). 10.3390/foods11071030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ahmed, M. et al. Comprehensive phytochemical profiling, biological activities, and molecular docking studies of Pleurospermum candollei: An insight into potential for natural products development. Molecules27, 4113 (2022). 10.3390/molecules27134113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rudrapal, M. et al. Dietary polyphenols and their role in oxidative stress-induced human diseases: Insights into protective effects, antioxidant potentials and mechanism(s) of action. Front. Pharmacol.13, 806470 (2022). 10.3389/fphar.2022.806470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alizadeh, A. & Shaabani, M. Essential oil composition, phenolic content, antioxidant and antimicrobial activity in Salvia officinalis L. cultivated in Iran. Adv. Environ. Biol.6, 221–226 (2012). [Google Scholar]
- 29.Dastan, D., Salehi, P. & Maroofi, H. Chemical composition, antioxidant, and antimicrobial activities on Laserpitium carduchorum Hedge & Lamond essential oil and extracts during various growing stages. Chem. Biodivers.13, 1397–1403 (2016). 10.1002/cbdv.201600087 [DOI] [PubMed] [Google Scholar]
- 30.Nantitanon, W., Yotsawimonwat, S. & Okonogi, S. Factors influencing antioxidant activities and total phenolic content of guava leaf extract. LWT Food Sci. Technol.43, 1095–1103 (2010). 10.1016/j.lwt.2010.02.015 [DOI] [Google Scholar]
- 31.Atmani, D. et al. Antioxidant capacity and phenol content of selected Algerian medicinal plants. Food Chem.112, 303–309 (2009). 10.1016/j.foodchem.2008.05.077 [DOI] [Google Scholar]
- 32.Al-Jaber, H. I., Shakya, A. K. & Elagbar, Z. A. HPLC profiling of selected phenolic acids and flavonoids in Salvia eigii, Salvia hierosolymitana and Salvia viridis growing wild in jordan and their in vitro antioxidant activity. PeerJ8, e9769 (2020). 10.7717/peerj.9769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khiya, Z. et al. Correlation of total polyphenolic content with antioxidant activity of hydromethanolic extract and their fractions of the Salvia officinalis leaves from different regions of Morocco. J. Chem.2021, 1–11 (2021). 10.1155/2021/8585313 [DOI] [Google Scholar]
- 34.Krimat, S., Dob, T., Toumi, M., Kesouri, A. & Noasri, A. Assessment of phytochemicals, antioxidant, antimicrobial and cytotoxic properties of Salvia chudaei Batt. et Trab. endemic medicinal plant from Algeria. J. Mater. Environ. Sci.6, 70–78 (2015). [Google Scholar]
- 35.Moshari-Nasirkandi, A., Alirezalu, A., Alipour, H. & Amato, J. Screening of 20 species from Lamiaceae family based on phytochemical analysis, antioxidant activity and HPLC profiling. Sci. Rep.13, 16987 (2023). 10.1038/s41598-023-44337-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Makkar, H. P. Effects and fate of tannins in ruminant animals, adaptation to tannins, and strategies to overcome detrimental effects of feeding tannin-rich feeds. Small Rumin. Res.49, 241–256 (2003). 10.1016/S0921-4488(03)00142-1 [DOI] [Google Scholar]
- 37.Cheynier, V. Polyphenols in foods are more complex than often thought. Am. J. Clin. Nutr.81, 223–229 (2005). 10.1093/ajcn/81.1.223S [DOI] [PubMed] [Google Scholar]
- 38.Barbehenn, R. V. & Peter Constabel, C. Tannins in plant–herbivore interactions. Phytochemistry72, 1551–1565 (2011). 10.1016/j.phytochem.2011.01.040 [DOI] [PubMed] [Google Scholar]
- 39.Huang, Q., Liu, X., Zhao, G., Hu, T. & Wang, Y. Potential and challenges of tannins as an alternative to in-feed antibiotics for farm animal production. Anim. Nutr.4, 137–150 (2018). 10.1016/j.aninu.2017.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lila, M. A. From beans to berries and beyond. Ann. N. Y. Acad. Sci.1114, 372–380 (2007). 10.1196/annals.1396.047 [DOI] [PubMed] [Google Scholar]
- 41.de Sousa Leal, A., de Carvalho Leal, L. H., da Silva, D., Cunha Nunes, L. C. & Dantas Lopes, J. A. Incorporation of tannic acid in formulations for topical use in wound healing: A technological prospecting. Afr. J. Pharm. Pharmacol.9, 662–674 (2015). 10.5897/AJPP2015.4361 [DOI] [Google Scholar]
- 42.Luna-Guevara, M. L., Luna-Guevara, J. J., Hernández-Carranza, P., Ruíz-Espinosa, H. & Ochoa-Velasco, C. E. Phenolic compounds: A good choice against chronic degenerative diseases. Stud. Nat. Prod. Chem.59, 79–108 (2018). 10.1016/B978-0-444-64179-3.00003-7 [DOI] [Google Scholar]
- 43.Moores, J. Vitamin C: A wound healing perspective. Br. J. Community Nurs.18, S6–S11 (2013). 10.12968/bjcn.2013.18.Sup12.S6 [DOI] [PubMed] [Google Scholar]
- 44.Lee, J., Koo, N. & Min, D. B. Reactive oxygen species, aging, and antioxidative nutraceuticals. Compr. Rev. Food Sci. Food Saf.3, 21–33 (2004). 10.1111/j.1541-4337.2004.tb00058.x [DOI] [PubMed] [Google Scholar]
- 45.Shen, J. et al. Ascorbate oxidation by iron, copper and reactive oxygen species: Review, model development, and derivation of key rate constants. Sci. Rep.11, 7417 (2021). 10.1038/s41598-021-86477-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pizzino, G. et al. Oxidative stress: Harms and benefits for human health. Oxid. Med. Cell. Longev.2017, 1–13 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Njus, D., Kelley, P. M., Tu, Y.-J. & Schlegel, H. B. Ascorbic acid: The chemistry underlying its antioxidant properties. Free Radic. Biol. Med.159, 37–43 (2020). 10.1016/j.freeradbiomed.2020.07.013 [DOI] [PubMed] [Google Scholar]
- 48.Dobrikova, A. G. et al. Cadmium toxicity in Salvia sclarea L.: An integrative response of element uptake, oxidative stress markers, leaf structure and photosynthesis. Ecotoxicol. Environ. Saf.209, 111851 (2021). 10.1016/j.ecoenv.2020.111851 [DOI] [PubMed] [Google Scholar]
- 49.Sharafzadeh, S. H. & Alizadeh, O. Chlorophyll a, chlorophyll b and carotenoids of garden thyme (Thymus vulgaris L.) as affected by nutrients. Adv. Environ. Biol.5, 3725–3728 (2011). [Google Scholar]
- 50.Sledz, M. & Witrowa-Rajchert, D. Influence of microwave-convective drying of chlorophyll content and colour of herbs. Acta Geophys.19, 4 (2012). [Google Scholar]
- 51.Tounekti, T., Abreu, M. E., Khemira, H. & Munné-Bosch, S. Canopy position determines the photoprotective demand and antioxidant protection of leaves in salt-stressed Salvia officinalis L. plants. Environ. Exp. Bot.78, 146–156 (2012). 10.1016/j.envexpbot.2011.12.037 [DOI] [Google Scholar]
- 52.Pellegrini, E., Francini, A., Lorenzini, G. & Nali, C. Ecophysiological and antioxidant traits of Salvia officinalis under ozone stress. Environ. Sci. Pollut. Res.22, 13083–13093 (2015). 10.1007/s11356-015-4569-5 [DOI] [PubMed] [Google Scholar]
- 53.Mapes, C. & Xu, Y. Photosynthesis, vegetative habit and culinary properties of sage (Salvia officinalis) in response to low-light conditions. Can. J. Plant Sci.94, 881–889 (2014). 10.4141/cjps-2014-010 [DOI] [Google Scholar]
- 54.Eghbaliferiz, S. & Iranshahi, M. Prooxidant activity of polyphenols, flavonoids, anthocyanins and carotenoids: Updated review of mechanisms and catalyzing metals. Phyther. Res.30, 1379–1391 (2016). 10.1002/ptr.5643 [DOI] [PubMed] [Google Scholar]
- 55.Divya, P., Puthusseri, B. & Neelwarne, B. Carotenoid content, its stability during drying and the antioxidant activity of commercial coriander (Coriandrum sativum L.) varieties. Food Res. Int.45, 342–350 (2012). 10.1016/j.foodres.2011.09.021 [DOI] [Google Scholar]
- 56.Rezai, S., Etemadi, N., Nikbakht, A., Yousefi, M. & Majidi, M. M. Effect of light intensity on leaf morphology, photosynthetic capacity, and chlorophyll content in sage (Salvia officinalis L.). Hortic. Sci. Technol.36, 46–57 (2018). [Google Scholar]
- 57.Zhang, Y., Li, X. & Wang, Z. Antioxidant activities of leaf extract of Salvia miltiorrhiza Bunge and related phenolic constituents. Food Chem. Toxicol.48, 2656–2662 (2010). 10.1016/j.fct.2010.06.036 [DOI] [PubMed] [Google Scholar]
- 58.Grzegorczyk, I., Matkowski, A. & Wysokińska, H. Antioxidant activity of extracts from in vitro cultures of Salvia officinalis L.. Food Chem.104, 536–541 (2007). 10.1016/j.foodchem.2006.12.003 [DOI] [Google Scholar]
- 59.López, C. C. et al. Assessment of antioxidant and antibacterial potential of borojo fruit (Borojoa patinoi Cuatrecasas) from the rainforests of South America. Ind. Crops Prod.63, 79–86 (2015). 10.1016/j.indcrop.2014.10.047 [DOI] [Google Scholar]
- 60.Vámos-Vigyázó, L. & Haard, N. F. Polyphenol oxidases and peroxidases in fruits and vegetables. C R C Crit. Rev. Food Sci. Nutr.15, 49–127 (1981). 10.1080/10408398109527312 [DOI] [PubMed] [Google Scholar]
- 61.Moshari-Nasirkandi, A., Alirezalu, A. & Hachesu, M. A. Effect of lemon verbena bio-extract on phytochemical and antioxidant capacity of strawberry (Fragaria×ananassa Duch. cv. Sabrina) fruit during cold storage. Biocatal. Agric. Biotechnol.25, 101613 (2020). 10.1016/j.bcab.2020.101613 [DOI] [Google Scholar]
- 62.Mamache, W., Amira, S., Ben Souici, C., Laouer, H. & Benchikh, F. In vitro antioxidant, anticholinesterases, anti-α-amylase, and anti-α-glucosidase effects of Algerian Salvia aegyptiaca and Salvia verbenaca. J. Food Biochem.44, e13472 (2020). 10.1111/jfbc.13472 [DOI] [PubMed] [Google Scholar]
- 63.Wojdyło, A., Oszmiański, J. & Czemerys, R. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem.105, 940–949 (2007). 10.1016/j.foodchem.2007.04.038 [DOI] [Google Scholar]
- 64.Fındık, E., Ceylan, M. & Elmastaş, M. Isoeugenol-based novel potent antioxidants: Synthesis and reactivity. Eur. J. Med. Chem.46, 4618–4624 (2011). 10.1016/j.ejmech.2011.07.041 [DOI] [PubMed] [Google Scholar]
- 65.Erdemoglu, N., Turan, N. N., Cakõcõ, I., Sener, B. & Aydõn, A. Antioxidant activities of some Lamiaceae plant extracts. Phyther. Res.20, 9–13 (2006). 10.1002/ptr.1816 [DOI] [PubMed] [Google Scholar]
- 66.Grzegorczyk-Karolak, I. & Kiss, A. Determination of the phenolic profile and antioxidant properties of Salvia viridis L. shoots: A comparison of aqueous and hydroethanolic extracts. Molecules23, 1468 (2018). 10.3390/molecules23061468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Luca, S. V. et al. Chemical profile and bioactivity evaluation of Salvia species from Eastern Europe. Antioxidants12, 1514 (2023). 10.3390/antiox12081514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jiang, R.-W. et al. Chemistry and biological activities of caffeic acid derivatives from Salvia miltiorrhiza. Curr. Med. Chem.12, 237–246 (2005). 10.2174/0929867053363397 [DOI] [PubMed] [Google Scholar]
- 69.Nickavar, B., Kamalinejad, M. & Izadpanah, H. In vitro free radical scavenging activity of five Salvia species. Pak. J. Pharm. Sci.20, 291–294 (2007). [PubMed] [Google Scholar]
- 70.Aleksovski, S. A. & Sovová, H. Supercritical CO2 extraction of Salvia officinalis L.. J. Supercrit. Fluids40, 239–245 (2007). 10.1016/j.supflu.2006.07.006 [DOI] [Google Scholar]
- 71.Cuvelier, M., Richard, H. & Berset, C. Antioxidative activity and phenolic composition of pilot-plant and commercial extracts of sage and rosemary. J. Am. Oil Chem. Soc.73, 645–652 (1996). 10.1007/BF02518121 [DOI] [Google Scholar]
- 72.European Medicines Agency. European Union herbal monograph on Salvia officinalis L. folium. J. Sci. Med. Health1, 1–8 (2016). [Google Scholar]
- 73.Afonso, A. F. et al. Phytochemical composition and bioactive effects of Salvia africana, Salvia officinalis ‘Icterina’ and Salvia mexicana aqueous extracts. Molecules24, 4327 (2019). 10.3390/molecules24234327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kamatou, G. P. P., Van Vuuren, S. F., Van Heerden, F. R., Seaman, T. & Viljoen, A. M. Antibacterial and antimycobacterial activities of South African Salvia species and isolated compounds from S. chamelaeagnea. S. Afr. J. Bot.73, 552–557 (2007). 10.1016/j.sajb.2007.05.001 [DOI] [Google Scholar]
- 75.Skendi, A., Irakli, M., Chatzopoulou, P. & Papageorgiou, M. Aromatic plants of Lamiaceae family in a traditional bread recipe: Effects on quality and phytochemical content. J. Food Biochem.43, e13020 (2019). 10.1111/jfbc.13020 [DOI] [PubMed] [Google Scholar]
- 76.Moshari Nasirkandi, A., Alirezalu, A. & Bahadori, S. Phenolic compounds and antioxidant activity of Nepeta fissa—First report from Iran. Nat. Prod. Res.35, 4596–4599 (2021). 10.1080/14786419.2019.1693566 [DOI] [PubMed] [Google Scholar]
- 77.Saccenti, E., Hoefsloot, H. C. J., Smilde, A. K., Westerhuis, J. A. & Hendriks, M. M. W. B. Reflections on univariate and multivariate analysis of metabolomics data. Metabolomics10, 361–374 (2014). 10.1007/s11306-013-0598-6 [DOI] [Google Scholar]
- 78.Iaccarino, N. et al. Impact of phytosterols on liver and distal colon metabolome in experimental murine colitis model: An explorative study. J. Enzyme Inhib. Med. Chem.34, 1041 (2019). 10.1080/14756366.2019.1611802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Granato, D., Santos, J. S., Escher, G. B., Ferreira, B. L. & Maggio, R. M. Use of principal component analysis (PCA) and hierarchical cluster analysis (HCA) for multivariate association between bioactive compounds and functional properties in foods: A critical perspective. Trends Food Sci. Technol.72, 83–90 (2018). 10.1016/j.tifs.2017.12.006 [DOI] [Google Scholar]
- 80.Laincer, F. et al. Characterization of monovarietal extra virgin olive oils from the province of Béjaïa (Algeria). Food Res. Int.89, 1123–1133 (2016). 10.1016/j.foodres.2016.04.024 [DOI] [Google Scholar]
- 81.Ford, C. W. & Hartley, R. D. Cyclodimers of p-coumaric and ferulic acids in the cell walls of tropical grasses. J. Sci. Food Agric.50, 29–43 (1990). 10.1002/jsfa.2740500105 [DOI] [Google Scholar]
- 82.Wang, J. et al. Biosynthesis, chemistry, and pharmacology of polyphenols from Chinese Salvia species: A review. Molecules24, 155 (2019). 10.3390/molecules24010155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Srinivasulu, C., Ramgopal, M., Ramanjaneyulu, G., Anuradha, C. M. & Suresh Kumar, C. Syringic acid (SA)—A review of its occurrence, biosynthesis, pharmacological and industrial importance. Biomed. Pharmacother.108, 547–557 (2018). 10.1016/j.biopha.2018.09.069 [DOI] [PubMed] [Google Scholar]
- 84.Ecevit, K., Barros, A. A., Silva, J. M. & Reis, R. L. Preventing microbial infections with natural phenolic compounds. Futur. Pharmacol.2, 460–498 (2022). 10.3390/futurepharmacol2040030 [DOI] [Google Scholar]
- 85.Abaza, M.-S. et al. Syringic acid from Tamarix aucheriana possesses antimitogenic and chemo-sensitizing activities in human colorectal cancer cells. Pharm. Biol.51, 1110–1124 (2013). 10.3109/13880209.2013.781194 [DOI] [PubMed] [Google Scholar]
- 86.Ramachandran, V. & Raja, B. Protective effects of syringic acid against acetaminophen-induced hepatic damage in albino rats. J. Basic Clin. Physiol. Pharmacol.21, 369–386 (2010). 10.1515/JBCPP.2010.21.4.369 [DOI] [PubMed] [Google Scholar]
- 87.Wu, Y., Xu, S. & Tian, X. Y. The effect of salvianolic acid on vascular protection and possible mechanisms. Oxid. Med. Cell. Longev.2020, 1–9 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Paudel, P. et al. Rosmarinic acid derivatives’ inhibition of glycogen synthase kinase-3β Is the pharmacological basis of kangen-karyu in Alzheimer’s disease. Molecules23, 2919 (2018). 10.3390/molecules23112919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zengin, G. et al. Novel perceptions on chemical profile and biopharmaceutical properties of Mentha spicata extracts: Adding missing pieces to the scientific puzzle. Plants11, 233 (2022). 10.3390/plants11020233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Li, D. et al. Ferulic acid: A review of its pharmacology, pharmacokinetics and derivatives. Life Sci.284, 119921 (2021). 10.1016/j.lfs.2021.119921 [DOI] [PubMed] [Google Scholar]
- 91.Chowdhury, S., Ghosh, S., Rashid, K. & Sil, P. C. Deciphering the role of ferulic acid against streptozotocin-induced cellular stress in the cardiac tissue of diabetic rats. Food Chem. Toxicol.97, 187–198 (2016). 10.1016/j.fct.2016.09.011 [DOI] [PubMed] [Google Scholar]
- 92.Xiao, J. Dietary flavonoid aglycones and their glycosides: which show better biological significance? Crit. Rev. Food Sci. Nutr.57, 1874–1905 (2017). [DOI] [PubMed] [Google Scholar]
- 93.Cai, Y.-Z., Sun, M., Xing, J., Luo, Q. & Corke, H. Structure–radical scavenging activity relationships of phenolic compounds from traditional Chinese medicinal plants. Life Sci.78, 2872–2888 (2006). 10.1016/j.lfs.2005.11.004 [DOI] [PubMed] [Google Scholar]
- 94.Ye, X.-P., Song, C.-Q., Yuan, P. & Mao, R.-G. α-Glucosidase and α-amylase inhibitory activity of common constituents from traditional Chinese medicine used for diabetes mellitus. Chin. J. Nat. Med.8, 349–352 (2010). 10.3724/SP.J.1009.2010.00349 [DOI] [Google Scholar]
- 95.Mok, S.-Y. & Lee, S. Identification of flavonoids and flavonoid rhamnosides from Rhododendron mucronulatum for albiflorum and their inhibitory activities against aldose reductase. Food Chem.136, 969–974 (2013). 10.1016/j.foodchem.2012.08.091 [DOI] [PubMed] [Google Scholar]
- 96.Nurul Islam, M., Jung, H. A., Sohn, H. S., Kim, H. M. & Choi, J. S. Potent α-glucosidase and protein tyrosine phosphatase 1B inhibitors from Artemisia capillaris. Arch. Pharm. Res.36, 542–552 (2013). 10.1007/s12272-013-0069-7 [DOI] [PubMed] [Google Scholar]
- 97.Brownlee, M. Biochemistry and molecular cell biology of diabetic complications. Nature414, 813–820 (2001). 10.1038/414813a [DOI] [PubMed] [Google Scholar]
- 98.Fan, P., Hay, A.-E., Marston, A. & Hostettmann, K. Acetylcholinesterase-inhibitory activity of linarin from Buddleja davidii, structure–activity relationships of related flavonoids, and chemical investigation of buddleja nitida. Pharm. Biol.46, 596–601 (2008). 10.1080/13880200802179592 [DOI] [Google Scholar]
- 99.Pick, A. et al. Structure–activity relationships of flavonoids as inhibitors of breast cancer resistance protein (BCRP). Bioorg. Med. Chem.19, 2090–2102 (2011). 10.1016/j.bmc.2010.12.043 [DOI] [PubMed] [Google Scholar]
- 100.Bajkacz, S., Baranowska, I., Buszewski, B., Kowalsi, B. & Ligor, M. Determination of flavonoids and phenolic acids in plant materials using SLE-SPE-UHPLC-MS/MS method. Food Anal. Methods11, 3563–3575 (2018). 10.1007/s12161-018-1332-9 [DOI] [Google Scholar]
- 101.Ul-Haq, I. et al. Antioxidant and cytotoxic activities and phytochemical analysis of Euphorbia wallichii root extract and its fractions. Iran. J. Pharm. Res.11, 241–249 (2012). [PMC free article] [PubMed] [Google Scholar]
- 102.Chang, C.-C., Yang, M.-H., Wen, H.-M. & Chern, J.-C. Estimation of total flavonoid content in propolis by two complementary colometric methods. J. Food Drug Anal.10, 3 (2020). [Google Scholar]
- 103.Bharath, B., Pavithra, A. N., Divya, A. & Perinbam, K. Chemical composition of ethanolic extracts from some seaweed species of the south indian coastal zone, their antibacterial and membrane-stabilizing activity. Russ. J. Mar. Biol.46, 370–378 (2020). 10.1134/S1063074020050041 [DOI] [Google Scholar]
- 104.Klein, B. P. & Perry, A. K. Ascorbic acid and vitamin A activity in selected vegetables from different geographical areas of the united states. J. Food Sci.47, 941–945 (1982). 10.1111/j.1365-2621.1982.tb12750.x [DOI] [Google Scholar]
- 105.Shimada, K., Fujikawa, K., Yahara, K. & Nakamura, T. Antioxidative properties of xanthan on the autoxidation of soybean oil in cyclodextrin emulsion. J. Agric. Food Chem.40, 945–948 (1992). 10.1021/jf00018a005 [DOI] [Google Scholar]
- 106.Miao, J. et al. Chemical composition and bioactivities of two common chaenomeles fruits in china: Chaenomeles speciosa and Chaenomeles sinensis. J. Food Sci.81, 2049 (2016). 10.1111/1750-3841.13377 [DOI] [PubMed] [Google Scholar]
- 107.Lichtenthaler, H. K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol.148, 350–382 (1987). 10.1016/0076-6879(87)48036-1 [DOI] [Google Scholar]
- 108.Nagata, M., Noguchi, Y., Imanishi, S. & Sugiyama, K. A simple method for the estimation of alpha- and beta-carotene in carrots. Acta Hortic.768, 565–569 (2008). 10.17660/ActaHortic.2008.768.76 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding authors on reasonable request.