Abstract
Background
Available evidence on the effects of vitamin D on mortality has been inconclusive. In a recent systematic review, we found evidence that vitamin D3 may decrease mortality in mostly elderly women. The present systematic review updates and reassesses the benefits and harms of vitamin D supplementation used in primary and secondary prophylaxis of mortality.
Objectives
To assess the beneficial and harmful effects of vitamin D supplementation for prevention of mortality in healthy adults and adults in a stable phase of disease.
Search methods
We searched The Cochrane Library, MEDLINE, EMBASE, LILACS, the Science Citation Index–Expanded and Conference Proceedings Citation Index–Science (all up to February 2012). We checked references of included trials and pharmaceutical companies for unidentified relevant trials.
Selection criteria
Randomised trials that compared any type of vitamin D in any dose with any duration and route of administration versus placebo or no intervention in adult participants. Participants could have been recruited from the general population or from patients diagnosed with a disease in a stable phase. Vitamin D could have been administered as supplemental vitamin D (vitamin D3 (cholecalciferol) or vitamin D2 (ergocalciferol)) or as an active form of vitamin D (1α‐hydroxyvitamin D (alfacalcidol) or 1,25‐dihydroxyvitamin D (calcitriol)).
Data collection and analysis
Six review authors extracted data independently. Random‐effects and fixed‐effect meta‐analyses were conducted. For dichotomous outcomes, we calculated the risk ratios (RRs). To account for trials with zero events, we performed meta‐analyses of dichotomous data using risk differences (RDs) and empirical continuity corrections. We used published data and data obtained by contacting trial authors.
To minimise the risk of systematic error, we assessed the risk of bias of the included trials. Trial sequential analyses controlled the risk of random errors possibly caused by cumulative meta‐analyses.
Main results
We identified 159 randomised clinical trials. Ninety‐four trials reported no mortality, and nine trials reported mortality but did not report in which intervention group the mortality occurred. Accordingly, 56 randomised trials with 95,286 participants provided usable data on mortality. The age of participants ranged from 18 to 107 years. Most trials included women older than 70 years. The mean proportion of women was 77%. Forty‐eight of the trials randomly assigned 94,491 healthy participants. Of these, four trials included healthy volunteers, nine trials included postmenopausal women and 35 trials included older people living on their own or in institutional care. The remaining eight trials randomly assigned 795 participants with neurological, cardiovascular, respiratory or rheumatoid diseases. Vitamin D was administered for a weighted mean of 4.4 years. More than half of the trials had a low risk of bias. All trials were conducted in high‐income countries. Forty‐five trials (80%) reported the baseline vitamin D status of participants based on serum 25‐hydroxyvitamin D levels. Participants in 19 trials had vitamin D adequacy (at or above 20 ng/mL). Participants in the remaining 26 trials had vitamin D insufficiency (less than 20 ng/mL). Vitamin D decreased mortality in all 56 trials analysed together (5,920/47,472 (12.5%) vs 6,077/47,814 (12.7%); RR 0.97 (95% confidence interval (CI) 0.94 to 0.99); P = 0.02; I2 = 0%). More than 8% of participants dropped out. 'Worst‐best case' and 'best‐worst case' scenario analyses demonstrated that vitamin D could be associated with a dramatic increase or decrease in mortality. When different forms of vitamin D were assessed in separate analyses, only vitamin D3 decreased mortality (4,153/37,817 (11.0%) vs 4,340/38,110 (11.4%); RR 0.94 (95% CI 0.91 to 0.98); P = 0.002; I2 = 0%; 75,927 participants; 38 trials). Vitamin D2, alfacalcidol and calcitriol did not significantly affect mortality. A subgroup analysis of trials at high risk of bias suggested that vitamin D2 may even increase mortality, but this finding could be due to random errors. Trial sequential analysis supported our finding regarding vitamin D3, with the cumulative Z‐score breaking the trial sequential monitoring boundary for benefit, corresponding to 150 people treated over five years to prevent one additional death. We did not observe any statistically significant differences in the effect of vitamin D on mortality in subgroup analyses of trials at low risk of bias compared with trials at high risk of bias; of trials using placebo compared with trials using no intervention in the control group; of trials with no risk of industry bias compared with trials with risk of industry bias; of trials assessing primary prevention compared with trials assessing secondary prevention; of trials including participants with vitamin D level below 20 ng/mL at entry compared with trials including participants with vitamin D levels equal to or greater than 20 ng/mL at entry; of trials including ambulatory participants compared with trials including institutionalised participants; of trials using concomitant calcium supplementation compared with trials without calcium; of trials using a dose below 800 IU per day compared with trials using doses above 800 IU per day; and of trials including only women compared with trials including both sexes or only men. Vitamin D3 statistically significantly decreased cancer mortality (RR 0.88 (95% CI 0.78 to 0.98); P = 0.02; I2 = 0%; 44,492 participants; 4 trials). Vitamin D3 combined with calcium increased the risk of nephrolithiasis (RR 1.17 (95% CI 1.02 to 1.34); P = 0.02; I2 = 0%; 42,876 participants; 4 trials). Alfacalcidol and calcitriol increased the risk of hypercalcaemia (RR 3.18 (95% CI 1.17 to 8.68); P = 0.02; I2 = 17%; 710 participants; 3 trials).
Authors' conclusions
Vitamin D3 seemed to decrease mortality in elderly people living independently or in institutional care. Vitamin D2, alfacalcidol and calcitriol had no statistically significant beneficial effects on mortality. Vitamin D3 combined with calcium increased nephrolithiasis. Both alfacalcidol and calcitriol increased hypercalcaemia. Because of risks of attrition bias originating from substantial dropout of participants and of outcome reporting bias due to a number of trials not reporting on mortality, as well as a number of other weaknesses in our evidence, further placebo‐controlled randomised trials seem warranted.
Keywords: Adolescent; Adult; Aged; Aged, 80 and over; Female; Humans; Male; Middle Aged; Young Adult; Mortality; Calcitriol; Calcitriol/therapeutic use; Cause of Death; Cholecalciferol; Cholecalciferol/therapeutic use; Dietary Supplements; Ergocalciferols; Ergocalciferols/therapeutic use; Hydroxycholecalciferols; Hydroxycholecalciferols/therapeutic use; Randomized Controlled Trials as Topic; Vitamins; Vitamins/therapeutic use
Plain language summary
Vitamin D supplementation for prevention of mortality in adults
Review question
To assess the beneficial and harmful effects of vitamin D for prevention of mortality in healthy adults and adults in a stable phase of disease.
Background
Numerous observational studies suggest that optimal vitamin D status may be associated with fewer occurrences of cancer and cardiovascular disease (such as heart attack or stroke). Vitamin D is synthesised in the skin as vitamin D3 (cholecalciferol) or is obtained from dietary sources or supplements as vitamin D3 or vitamin D2 (ergocalciferol). Our Cochrane systematic review from 2011, which analysed the influence of different forms of vitamin D on mortality, showed that vitamin D3 (cholecalciferol) decreased mortality. This systematic review is now updated, and all included trials have been reassessed in accordance with improved Cochrane methodology, developed to enhance the validity of the conclusions.
Study characteristics
In the 56 trials that provided data for the analyses, a total of 95,286 participants were randomly assigned to vitamin D versus no treatment or placebo. More than half of the trials were considered to have low risk of bias. All trials were conducted in high‐income countries. The age of participants ranged from 18 to 107 years. The mean proportion of women was 77%. Vitamin D was administered for an average of 4.4 years.
This plain language summary is as current as of February 2012.
Key results
This review suggests that vitamin D3 may reduce mortality, showing that about 150 participants need to be treated over five years for one additional life to be saved. We found comparable effects of vitamin D3 in studies that included only women compared with studies including both women and men. Vitamin D3 also seemed to decrease cancer mortality, showing a reduction in mortality of 4 per 1000 persons treated for five to seven years. We also observed adverse effects to vitamin D such as renal stone formation (seen for vitamin D3 combined with calcium) and elevated blood levels of calcium (seen for both alfacalcidol and calcitriol). In conclusion, we found some evidence that vitamin D3 seems to decrease mortality in elderly people not dependent on help or living in institutional care.
Quality of the evidence
A large number of study participants left the trial before completion, and this raises concerns regarding the validity of the results. More randomised clinical trials are needed on the effects of vitamin D3 on mortality in younger, healthy persons, as well as in elderly community‐dwelling and institutionalised persons without apparent vitamin D deficiency.
Summary of findings
Summary of findings for the main comparison. Vitamin D supplementation for prevention of mortality in adults.
| Vitamin D supplementation for prevention of mortality in adults | ||||||
| Population: adults Settings: any Intervention: vitamin D Comparison: placebo or no intervention | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo or no intervention | Vitamin D | |||||
|
All‐cause mortality in trials using vitamin D3
(cholecalciferol) (Follow‐up: 0.08 to 7 years) |
Study population | RR 0.94 (0.91 to 0.98) | 75,927 (38) | ⊕⊕⊕⊝ moderatea |
Trial sequential analysis of all trials irrespective of bias risks showed that the required information size had not yet been reached and that the cumulative Z‐curve crossed the trial sequential monitoring boundary for benefit. If this is correct, the intervention effect corresponds to a number needed to treat for a beneficial outcome (NNTB) of 150 participants over five years to save one additional life | |
| 114 per 1000 | 107 per 1000 (104 to 112) | |||||
| Moderate risk | ||||||
| 46 per 1000 | 43 per 1000 (42 to 45) | |||||
|
Cardiovascular mortality in trials using vitamin D3 (cholecalciferol) (Follow‐up: 0.31 to 6.2 years) |
Study population | RR 0.98 (0.90 to 1.07) | 47,267 (10) | ⊕⊕⊝⊝ lowb |
Trial sequential analysis showed that the cumulative Z‐curve did not cross the conventional monitoring boundary for benefit. The required information size was 2,539,845 participants | |
| 42 per 1000 | 41 per 1000 (38 to 45) | |||||
| Moderate risk | ||||||
| 13 per 1000 | 11 per 1000 (12 to 15) | |||||
|
Cancer mortality in trials using vitamin D3 (cholecalciferol) (Follow‐up: 5 to 7 years) |
Study population | RR 0.88 (0.78 to 0.98) | 44,492 (4) | ⊕⊕⊕⊝ moderatea |
Trial sequential analysis showed that the cumulative Z‐curve did not cross the conventional monitoring boundary for benefit. The required information size was 66,724 participants | |
| 29 per 1000 | 25 per 1000 (22 to 31) | |||||
| Moderate risk | ||||||
| 21 per 1000 | 19 per 1000 (16 to 21) | |||||
|
Adverse events: nephrolithiasis in trials using vitamin D3 combined with calcium (Follow‐up: 1.25 to 7 years) |
Study population | RR 1.17 (1.02 to 1.34) | 42,876 (4) | ⊕⊕⊕⊝ moderatea | ||
| 18 per 1000 | 21 per 1000 (18 to 24) | |||||
| Moderate risk | ||||||
| 9 per 1000 | 11 per 1000 (9 to 12) | |||||
|
Adverse events: hypercalcaemia in trials using the active forms of vitamin D (alfacalcidol and calcitriol) (Follow‐up: 0.75 to 3 years) |
Study population | RR 3.18 (1.17 to 8.68) | 710 (3) | ⊕⊕⊝⊝ lowb | ||
| 23 per 1000 | 72 per 1000 (27 to 197) | |||||
| Moderate risk | ||||||
| 11 per 1000 | 15 per 1000 (4 to 23) | |||||
|
Health‐related quality of life (Follow‐up: 0.38 years) |
See comment | See comment | Not estimable | 105 (1) |
See comment | Insufficient information: significant worsening in disease‐specific quality of life in the vitamin D2 group compared with the placebo group was reported. The between‐group difference at 20 weeks was 5.3 (0.5 to 10.2), and the minimally important difference (MID) is estimated to be 5 points in either direction |
|
Health economics (Follow‐up: 4 years) |
See comment | See comment | Not estimable | 3270 (1) |
See comment | Insufficient information: authors reported that vitamin D3 and calcium supplementation prevented 46 hip fractures in every 1000 women treated |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio; RRR: relative risk reduction | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
aDowngraded by one level because of risk of attrition bias bDowngraded by two levels because of risk of attrition bias and imprecision
Background
Description of the condition
Vitamin D is synthesised in the skin as vitamin D3 (cholecalciferol) or is obtained from dietary sources or supplements as vitamin D3 or vitamin D2 (ergocalciferol). Vitamins D3 and D2 are metabolised in the liver to 25‐hydroxyvitamin D and in the kidneys to the biologically active 1,25‐dihydroxyvitamin D (calcitriol), which functions as a steroid‐like hormone (Horst 2005; Lips 2006). The effects of vitamin D are mediated by its binding to vitamin D receptors in the cells (Wesley Pike 2005). Renal production of 1,25‐dihydroxyvitamin D is regulated by parathyroid hormone levels, by serum calcium and phosphorus levels and by the phosphaturic hormone fibroblast growth factor‐23 (Kovesdy 2013).
Under conditions of hypocalcaemia, synthesis of the biologically active form of vitamin D (1,25‐dihydroxyvitamin D or calcitriol) is stimulated. This, in turn, stimulates the transport of calcium out of the intestine, kidneys and bones into the blood (Lips 2006). Therefore, homeostasis of vitamin D and calcium levels is essential for bone health (Holick 2007a; Horst 2005; Lips 2006). Current interest in vitamin D has been provoked by the discovery that most cells and tissues in our body contain vitamin D receptors (Holick 2006). During past decades, observational studies have suggested that vitamin D is effective for prevention of malignant, cardiovascular, autoimmune and infectious diseases (Holick 2007a; Nnoaham 2008; Rosen 2011; Souberbielle 2010).
Vitamin D status
Vitamin D status is determined by measurement of the serum 25‐hydroxyvitamin D level, which is a functional indicator of 'vitamin D status' (Bischoff‐Ferrari 2009c; Dawson‐Hughes 2005; Lips 2004). The US Institute of Medicine recently recommended a target serum 25‐hydroxyvitamin D level of 20 ng/mL (50 nmol/L) (IOM 2011). The worldwide prevalence of suboptimal vitamin D status is estimated to be high (Holick 2007a; Mithal 2009). Major causes of vitamin D deficiency include insufficient exposure to sunlight, decreased dietary intake, skin pigmentation, obesity and advanced age (Lips 2006). Vitamin D deficiency in adults precipitates or exacerbates osteopenia and osteoporosis and induces osteomalacia (Holick 2007a). Vitamin D insufficiency is linked to increased risk of malignant, cardiovascular, autoimmune and infectious diseases (Holick 2007a; Rosen 2011; Souberbielle 2010). An opposing hypothesis that vitamin D insufficiency is a consequence of disease but not its cause has been postulated by Marshall et al (Marshall 2008).
How the intervention might work
Vitamin D supplementation (vitamin D3 (cholecalciferol), vitamin D2 (ergocalciferol), 1α‐hydroxyvitamin D (alfacalcidol) or 1,25‐dihydroxyvitamin D (calcitriol)) seems to prevent osteoporosis, osteomalacia and fractures (Holick 2007a; Lamberg‐Allardt 2006). It has been speculated that vitamin D may confer benefits beyond the skeletal system (Davis 2007). Evidence on whether vitamin D may prevent cancer, cardiovascular disease and mortality is contradictory (Bjelakovic 2011; Davis 2007; Giovannucci 2005; Michos 2008; Pittas 2010; Wang 2010; Zittermann 2006).
Adverse effects of the intervention
Excessive vitamin D intake over a prolonged time may lead to vitamin D toxicity. However, evidence that ingestion of high quantities of vitamin D is harmful is sparse. Most trials have reported hypercalcaemia, hypercalciuria or nephrocalcinosis when vitamin D was administered to participants with renal failure (Cranney 2007). Excessive exposure to sunlight does not seem to lead to vitamin D intoxication (Holick 2007b).
Why it is important to do this review
Available evidence on vitamin D and mortality is intriguing and for the most inconclusive. Most observational studies have associated low vitamin D status with increased risk of death (Johansson 2012; Zittermann 2012). Several systematic reviews and meta‐analyses found beneficial effects of vitamin D in elderly people with vitamin deficiency or in people who received vitamin D as monotherapy or in combination with calcium for osteoporosis, fractures and falls (Bischoff‐Ferrari 2005; Bischoff‐Ferrari 2009a; Jackson 2007; Latham 2003b; Richy 2005; Tang 2007). Vitamin D supplementation revealed positive effects in maintaining glucose homeostasis (Pittas 2007a) and in preventing tuberculosis (Nnoaham 2008). However, Izaks et al (Izaks 2007) and Boonen et al (Boonen 2006) found no statistically significant effects of vitamin D supplementation on these outcomes in the general population. A meta‐analysis by Autier and Gandini (Autier 2007) of 18 randomised clinical trials found significantly lower mortality among vitamin D–supplemented participants (Autier 2007). A Cochrane systematic review of 16 randomised trials on prevention of fractures found only a non‐significant tendency of vitamin D to reduce mortality (Avenell 2009). In our published Cochrane review in 2011, data from 50 randomised clinical trials with 94,148 participants suggested a beneficial effect of vitamin D3 on mortality (Bjelakovic 2011). Since the time of that review (Bjelakovic 2011), the results of several new randomised trials conducted to test the influence of vitamin D supplementation on mortality have become available. Also, we wanted to analyse further the influence of participants' sex on the effects of vitamin D3 and to implement the improved Cochrane methodology in performing data assessment. The present review is an update of the former review (Bjelakovic 2011).
Objectives
To assess the beneficial and harmful effects of vitamin D supplementation for prevention of mortality in healthy adults and adults in a stable phase of disease.
Methods
Criteria for considering studies for this review
Types of studies
Randomised clinical trials, irrespective of blinding, publication status or language, that have assessed supplemental vitamin D (vitamin D3 (cholecalciferol) or vitamin D2 (ergocalciferol)) or an active form of vitamin D (1α‐hydroxyvitamin D (alfacalcidol) or 1,25‐dihydroxyvitamin D (calcitriol)). We included primary prevention trials (defined as trials that seek to prevent disease before it occurs) and secondary prevention trials (defined as trials undertaken to prevent recurrences or exacerbations of a disease that has already been diagnosed) (Starfield 2008).
Types of participants
We included adult participants (18 years of age or older) who were.
Healthy or were recruited from the general population (primary prevention), irrespective of vitamin D status in the blood.
Diagnosed with a specific disease and in a stable phase (secondary prevention), irrespective of vitamin D status in the blood.
Diagnosed with vitamin D deficiency (secondary prevention).
We excluded trials that included:
Patients with secondary induced osteoporosis (e.g. glucocorticoid‐induced osteoporosis, thyroidectomy, primary hyperparathyroidism, chronic kidney disease, liver cirrhosis, Crohn's disease, gastrointestinal bypass surgery).
Pregnant or lactating women (as they usually are in need of vitamin D).
Patients with cancer.
Types of interventions
Intervention
Vitamin D at any dose and for any duration, administered as monotherapy or in combination with calcium. The route of administration could have been enteral or parenteral.
Vitamin D could have been administered as supplemental vitamin D (vitamin D3 (cholecalciferol) or vitamin D2 (ergocalciferol)) or as an active form of vitamin D (1α‐hydroxyvitamin D (alfacalcidol) or 1,25‐dihydroxyvitamin D (calcitriol)).
Control
Identical placebo or no intervention. Calcium in the control group was allowed if used equally in the vitamin D groups of the trial.
Types of outcome measures
Primary outcomes
All‐cause mortality.
Adverse events: depending on the availability of data, we attempted to classify adverse events as serious and non‐serious. A serious adverse event was defined as any untoward medical occurrence that was life threatening; resulted in death, or in persistent or significant disability or incapacity; or was a congenital anomaly/birth defect; or any medical event that might have jeopardised the participant or required intervention to prevent it (ICH‐GCP 1997). All other adverse events (i.e. medical occurrences not necessarily having a causal relationship to the treatment but causing a dose reduction or discontinuation of treatment) were considered as non‐serious.
Secondary outcomes
Cancer‐related mortality.
Cardiovascular mortality.
Fracture‐related mortality.
Other causes of mortality.
Health‐related quality of life.
Health economics.
Co‐variates, effect modifiers and confounders
We recorded any possible co‐variates, effect modifiers and confounders such as dosage and form of vitamin D, dosing schedule, duration of supplementation, duration of follow‐up, mean age, risk of bias, calcium co‐administration, other medications, compliance and attrition.
Timing of outcome measurement
We applied no restrictions regarding duration of the intervention or length of follow‐up. We assessed outcome data at the end of the trial follow‐up period.
Search methods for identification of studies
Electronic searches
We searched the following sources from inception to the specified date to identify trials that met our criteria.
The Cochrane Library (Issue 2, February 2012).
MEDLINE (until February 2012).
EMBASE (until February 2012).
LILACS (until February 2012).
Science Citation Index–Expanded (until February 2012).
Conference Proceedings Citation Index–Science (until February 2012).
We also searched Clinicaltrials.gov (http://clinicaltrials.gov/) and the World Health Organization International Clinical Trials Registry Platform (ICTRP 2011) to look for ongoing trials.
The search strategies for the databases we have searched are given in Appendix 1.
Searching other resources
We identified additional trials by searching reference lists of included trials and systematic reviews, meta‐analyses and health technology assessment reports. We also contacted experts and main manufacturers of vitamin D to ask about unpublished randomised trials.
Data collection and analysis
The present updated review expands on the previously published review in 2011 (Bjelakovic 2011) and the protocol published in 2008 (Bjelakovic 2008a).
Selection of studies
One review author (GB) performed the electronic searches. Six review authors (GB, LLG, DN, KW, RGS and MB) participated in the manual searches, identified trials eligible for inclusion from the search results and extracted data from the included trials. GB listed the excluded studies along with the reasons for exclusion. When a discrepancy occurred in trial selection or data extraction, the review author CG was consulted so consensus could be reached. We contacted authors of the trials to ask for missing information. Interrater agreement for trial selection was measured using the Kappa statistic (Cohen 1960). Agreement between the review authors was very good (Kappa = 0.85). An adapted PRISMA flow diagram of study selection is included in the review (Moher 2009).
Data extraction and management
Six review authors (GB, LLG, DN, KW, RGS and MB) independently extracted data on the relevant population and intervention characteristics, as well as on the risk of bias components, from trials that fulfilled the inclusion criteria of our review protocol. We used standard templates for data extraction. We searched for duplicate publications. Disagreements were resolved by discussion or, when needed, by the review author CG.
Assessment of risk of bias in included studies
Because of the risk of overestimation of beneficial intervention effects in randomised clinical trials with unclear or inadequate methodological quality (Kjaergard 2001; Lundh 2012; Moher 1998; Savovic 2012; Schulz 1995; Wood 2008), we assessed the influence of the risk of bias on our results. We used the following domains: allocation sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting, industry bias and other apparent biases (Higgins 2011). The following definitions were used.
Allocation sequence generation
Low risk of bias: sequence generation was achieved using computer random number generation or a random number table. Drawing lots, tossing a coin, shuffling cards and throwing dice are adequate if performed by an independent person not otherwise involved in the trial.
Uncertain risk of bias: the method of sequence generation was not specified.
High risk of bias: the sequence generation method was not random.
Allocation concealment
Low risk of bias: the participant allocations could not have been foreseen in advance of, or during, enrolment. Allocation was controlled by a central and independent randomisation unit. The allocation sequence was unknown to the investigators (e.g. if the allocation sequence was hidden in sequentially numbered, opaque and sealed envelopes).
Uncertain risk of bias: the method used to conceal the allocation was not described so that intervention allocations may have been foreseen in advance of, or during, enrolment.
High risk of bias: the allocation sequence was likely to be known to the investigators who assigned the participants.
Blinding of participants, personnel and outcome assessors
Low risk of bias: blinding was performed adequately, or the assessment of outcomes was not likely to be influenced by lack of blinding.
Uncertain risk of bias: information was insufficient to allow assessment of whether blinding was likely to induce bias on the results.
High risk of bias: no blinding or incomplete blinding was provided, and assessment of outcomes was likely to be influenced by lack of blinding.
Incomplete outcome data
Low risk of bias: missing data were unlikely to make treatment effects depart from plausible values. Sufficient methods, such as multiple imputation, have been employed to handle missing data.
Uncertain risk of bias: information was insufficient to allow assessment of whether missing data in combination with the method used to handle missing data were likely to induce bias on the results.
High risk of bias: the results were likely to be biased because of missing data.
Selective outcome reporting
Low risk of bias: all outcomes were predefined and reported, or all clinically relevant and reasonably expected outcomes were reported.
Uncertain risk of bias: it is unclear whether all predefined and clinically relevant and reasonably expected outcomes were reported.
High risk of bias: one or more clinically relevant and reasonably expected outcomes were not reported, and data on these outcomes were likely to have been recorded.
To be assessed with low risk of bias in the selective outcome reporting domain, the trial should have been registered on the www.clinicaltrials.gov website or a similar register, or a protocol should exist (e.g. published in a paper journal). In cases where the trial was run and published during the years when trial registration was not required, we tried to carefully scrutinise the publication reporting on the trial to identify the trial objectives and outcomes. If usable data on all outcomes specified in the trial objectives were provided in the publication's results section, the trial was considered to have low risk of bias in the 'Selective outcome reporting' domain.
Industry bias
Low risk of bias: the trial is not funded by a manufacturer of vitamin D.
Uncertain risk of bias: the source of funding is not clear.
High risk of bias: the trial is funded by a manufacturer of vitamin D.
Other bias
Low risk of bias: the trial appears to be free of other components that could put it at risk of bias.
Uncertain risk of bias: the trial may or may not be free of other components that could put it at risk of bias.
High risk of bias: other factors in the trial could put it at risk of bias (e.g. authors have conducted trials on the same topic, etc).
Trials assessed as having 'low risk of bias' in all of the individual domains specified above were considered 'trials with low risk of bias'. Trials assessed as having 'uncertain risk of bias' or 'high risk of bias' in one or more of the specified individual domains were considered trials with 'high risk of bias' (Gluud 2011).
Dealing with missing data
We tried to obtain relevant missing data from authors of the included trials. We performed an evaluation of important numerical data such as screened, eligible and randomly assigned participants, as well as intention‐to‐treat (ITT) and per‐protocol (PP) populations. We investigated attrition (i.e. dropouts, losses to follow‐up, and withdrawals).
Dealing with duplicate publications
In the case of duplicate publications and companion papers of a primary trial, we tried to maximise the yield of information by simultaneously evaluating all available data. When doubts arose, the publication that reported the longest follow‐up (usually the most recent publication) was given priority.
Assessment of heterogeneity
We identified heterogeneity through visual inspection of the forest plots by using a standard Chi2 test and a significance level of α = 0.1. In view of the low power of such tests, we also examined heterogeneity by using the I2 statistic (Higgins 2002); I2 values of 50% or more indicate a substantial level of heterogeneity (Higgins 2003). When heterogeneity was found, we attempted to determine potential reasons for it by examining individual trial characteristics and subgroups of the main body of evidence. For heterogeneity adjustment of the required information size, we used diversity, the D2 statistic (Wetterslev 2009).
Assessment of reporting biases
Funnel plots were used to assess the potential existence of bias (Lau 2006). Several explanations can be offered for the asymmetry of a funnel plot, including true heterogeneity of effect with respect to trial size, poor methodological design (and hence bias of small trials) and publication bias. We performed adjusted rank correlation (Begg 1994) and a regression asymmetry test for detection of bias (Egger 1997).
Data synthesis
We performed this review and meta‐analyses in accordance with the recommendations of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
For the statistical analyses, we used Review Manager 5.2 (RevMan 2012), Trial Sequential Analysis version 0.9 beta (TSA 2011), STATA 8.2 (STATA Corp, College Station, Texas) and Sigma Stat 3.0 (SPSS Inc, Chicago, Illinois). For dichotomous outcomes, we calculated the Mantel‐Haenszel risk ratios (RRs) (Gluud 2008). For all association measures, 95% confidence intervals (CIs) were used. We analysed the data with both fixed‐effect (DeMets 1987) and random‐effects (DerSimonian 1986) model meta‐analyses. In cases where no difference in statistical significance was observed between the results obtained with the two models, we presented the result of the random‐effects model analysis. Otherwise, we presented the results of both analyses.
We calculated weighted averages for factors related to the trials such as duration of the intervention and length of the follow‐up period.
Analyses were performed using the intention‐to‐treat (ITT) principle, including all randomly assigned participants, irrespective of completeness of data. Participants with missing data were included in the analyses using a carry forward of the last observed response. Accordingly, participants who had been lost to follow‐up were counted as being alive.
Review Manager 5.2 does not include trials with zero events in both intervention groups when calculating RR (RevMan 2012). To account for trials with zero events, meta‐analyses of dichotomous data were repeated using risk differences (RDs) (Friedrich 2007; Keus 2009). The influence of trials with zero events in the treatment, control or both groups was also assessed by recalculating the random‐effects model meta‐analyses with 0.5, 0.01 and 0.001 as empirical continuity corrections (Bradburn 2007; Sweeting 2004) using Trial Sequential Analysis version 0.9 beta (TSA 2011; www.ctu.dk/tsa).
For trials using a factorial design that tested vitamin D parallel to any other intervention (i.e. hormone replacement therapy, other vitamins, etc), we used 'inside the table' analysis in which we compared only the vitamin D intervention group versus the placebo or no intervention group. Otherwise, we used 'at margins' analysis (McAlister 2003). In trials with parallel‐group design with more than two intervention groups and additional therapy, we compared the vitamin D singly administered group versus the placebo or no intervention group.
We included in the analyses individually randomised trials as well as cluster‐randomised trials. Data from cluster‐randomised trials were incorporated using the generic inverse variance method. We explored the association between intervention effects of vitamin D and the subgrouping of individually randomised and cluster‐randomised trials. The influence of cluster‐randomised trials on our results was also explored in sensitivity analyses, which either included or excluded them.
We compared the intervention effects in subgroups of trials using the method described by Bornstein et al (Borenstein 2009) and implemented in RevMan 5.2 for all types of meta‐analyses.
Trial sequential analysis
A cumulative meta‐analysis runs the risk of random errors due to analysis of sparse data and repetitive testing of data (Thorlund 2009; Thorlund 2011a; Thorlund 2011b; Wetterslev 2008). We conducted trial sequential analyses to control the risk of random errors and to prevent premature statements of superiority of the experimental or control intervention or probably falsely declarations of absence of effect in cases for which we have too few data (Thorlund 2011a; Thorlund 2011b; Wetterslev 2008). We performed trial sequential analyses with a type I error of 5%, a type II error of 20% (80% power) and a diversity‐adjusted required information size (Brok 2008; Brok 2009; Thorlund 2009; Wetterslev 2008; Wetterslev 2009). We assumed an event proportion of 10% of deaths in the control group (Autier 2007) and an anticipated intervention effect of 5% relative risk reduction or otherwise as stated. Trials were entered into trial sequential analyses according to year of publication, and in cases where more than one trial was published in a year, trial entrance followed alphabetically the family name of the first author.
Subgroup analysis and investigation of heterogeneity
We performed subgroup analyses in cases where one of the primary outcome measures showed statistically significant differences between intervention groups.
We performed the following subgroup analyses.
Trials at low risk of bias compared with trials at high risk of bias.
Placebo‐controlled trials compared with trials with no intervention in the control group.
Individually randomised trials compared with cluster‐randomised trials.
Primary prevention trials compared with secondary prevention trials.
Vitamin D3 compared with placebo or no intervention.
Trials that administered vitamin D3 singly compared with trials that administered vitamin D3 combined with calcium.
Trials that administered low‐dose vitamin D3 compared with trials that administered high‐dose vitamin D3.
Trials that administered vitamin D3 daily compared with trials that administered vitamin D3 intermittently.
Trials that administered vitamin D3 to vitamin D–sufficient participants compared with trials that administered vitamin D3 to vitamin D–insufficient participants.
Vitamin D2 compared with placebo or no intervention.
Trials that administered vitamin D2 singly compared with trials that administered vitamin D2 combined with calcium.
Trials that administered low‐dose vitamin D2 compared with trials that administered high‐dose vitamin D2.
Trials that administered vitamin D2 daily compared with trials that administered vitamin D2 intermittently.
Trials that administered vitamin D2 to vitamin D–sufficient participants compared with trials that administered vitamin D2 to vitamin D–insufficient participants.
Alfacalcidol compared with placebo or no intervention.
Trials that administered alfacalcidol to vitamin D–sufficient participants compared with trials that administered alfacalcidol to vitamin D–insufficient participants.
Calcitriol compared with placebo or no intervention.
Trials that administered calcitriol to vitamin D–sufficient participants compared with trials that administered calcitriol to vitamin D–insufficient participants.
Sensitivity analysis
We performed the following sensitivity analyses to explore the influence of these factors on the intervention effect size.
Repeating the analysis while excluding cluster‐randomised trials.
Repeating the analysis while including trials with zero mortality in both intervention groups.
Repeating the analysis while taking attrition bias into consideration.
Results
Description of studies
Results of the search
We identified a total of 5995 references of possible interest by searching The Cochrane Library (n = 1118), MEDLINE (n = 1263), EMBASE (n = 1836), LILACS (n = 505), Science Citation Index–Expanded (n = 1205), Conference Proceedings Citation Index–Science (n = 28) and reference lists (n = 40). We excluded 4802 duplicates and 842 clearly irrelevant references by reading the abstracts. Accordingly, 351 references were retrieved for further assessment. Of these, we excluded 95 references describing 82 studies because they were not randomised clinical trials or did not fulfil our review protocol inclusion criteria. Reasons for exclusion are listed in the table Characteristics of excluded studies.
In total, 159 randomised trials described in 256 publications fulfilled our inclusion criteria (Figure 1). They included a total of 105,992 participants. In total, 94 trials described in 114 publications reported no deaths (Abu‐Mouch 2011; Aloia 1988; Aloia 1990; Aloia 2008; Aloia 2010; Andersen 2009; Angeles‐Agdeppa 2010; Armas 2004b; Arvold 2009; Bang 2011; Barnes 2006; Barnes 2011; Biancuzzo 2010; Braam 2004; Bunout 2006; Burton 2010; Caniggia 1984; Cashman 2008; Chen 1997; Christiansen 1980; Christiansen 1981; Dawson‐Hughes 1991; Deroisy 2002; Dhesi 2004; Di 2004; Domrongkitchaiporn 2000; Ebeling 2001; Fliser 1997; Forsythe 2012; Gallagher 1982; Gorai 1999; Green 2010; Harris 1999; Harris 2002; Himeno 2009; Himmelstein 1990; Holick 2008c; Hulshof 2000; Hunter 2000; Ishida 2004; Islam 2010; Jensen 1982a; Jensen 1982b; Jensen 1985; Johnson 1980; Jorde 2008; Jorde 2009; Jorde 2010a; Jorde 2010b; Jorde 2010c; Jorde 2010d; Jorde 2010e; Kenny 2003; Khaw 1994; Kimball 2011; Kruger 2010; Kuwabara 2009; Laaksi 2010; Lambrinoudaki 2000; Lappe 2008; Li‐Ng 2009; Lind 1989; Lind 1992; Lips 1988; Ljunghall 1987; Major 2007; Major 2009; Maki 2011; Malhotra 2009; Martin‐Bautista 2010; Menczel 1994; Mitri 2011; Nagpal 2009; Nelson 2009; Nordin 1985; Ongphiphadhanakul 2000; Orimo 1994; Orwoll 1988; Orwoll 1994; Patel 2001; Pfeifer 2000; Pfeifer 2001; Pfeifer 2009; Pignotti 2010; Pilz 2011; Schaafsma 2000; Scragg 1995a; Scragg 1995b; Shiomi 1999a; Shiomi 1999b; Shiraki 1985; Shiraki 1996; Shiraki 2004; Sneve 2008; Son 2001; Songpatanasilp 2009; Sorva 1991; Sugden 2008; Urbain 2011; Ushiroyama 1995; Ushiroyama 2001; Ushiroyama 2002; Van Der Klis 1996; Viljakainen 2006; Viljakainen 2009; von Hurst 2008; von Hurst 2009; von Hurst 2010a; von Hurst 2010b; Weisman 1986; Wicherts 2010; Yusupov 2010; Zittermann 2009b; Zubillaga 2006). We contacted the authors, and the authors of 62 trials confirmed that mortality was indeed zero. For 32 trials, we did not obtain such confirmation. Nine trials reported on deaths (n ≈ 50), but they did not report the trial intervention group in which the deaths occurred (Cashman 2009; Chapuy 1987; Doetsch 2004; Fedirko 2010; Gallagher 1989; Keane 1998; Moreira‐Pfrimer 2009; Orwoll 1990; Peacock 2000). The study authors did not reply to our request for additional information.
1.
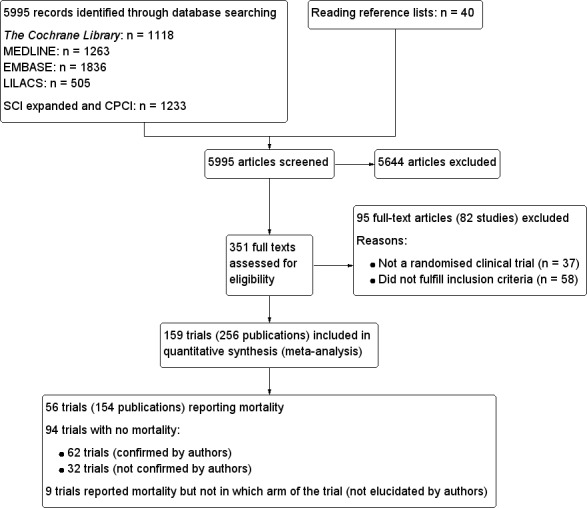
Study flow diagram.
In total, 56 trials described in 154 publications, with 95,286 participants, provided data for our analyses of mortality. A further 62 trials with zero mortality in both experimental and control groups were included in our sensitivity analyses.
We contacted 139 study authors to ask for the missing information and received answers from authors of 91 randomised clinical trials (65%).
We identified an additional 11 ongoing randomised clinical trials by searching databases of ongoing trials. Data from these trials will be included in future updates of this review.
Included studies
The included trials are described in detail in the tables Characteristics of included studies; Table 2; Table 3; Table 4; Table 5; Appendix 2; Appendix 3; Appendix 4; Appendix 5; and Appendix 6.
1. Characteristics of included trials (I).
|
Characteristic Study ID |
Design | Arms | Bias risk | Blinding | Participants [N] | Women [%] | Mean age [years] |
| Aloia 2005 | Parallel | 2 | Low | PL | 208 | 100 | 60 |
| Avenell 2004 | 2 × 2 | 4 | High | NI | 134 | 83 | 77 |
| Avenell 2012 | 2 × 2 | 4 | Low | PL | 5292 | 85 | 77 |
| Baeksgaard 1998 | Parallel | 3 | High | PL | 240 | 100 | 62.5 |
| Bischoff 2003 | Parallel | 2 | High | PL | 122 | 100 | 85.3 |
| Bjorkman 2007 | Parallel | 3 | Low | PL | 218 | 82 | 84.5 |
| Bolton‐Smith 2007 | 2 × 2 | 4 | Low | PL | 244 | 100 | 68 |
| Brazier 2005 | Parallel | 2 | High | PL | 192 | 100 | 74.6 |
| Broe 2007 | Parallel | 5 | Low | PL | 124 | 73 | 89 |
| Brohult 1973 | Parallel | 2 | High | PL | 50 | 68 | 52 |
| Burleigh 2007 | Parallel | 2 | Low | PL | 205 | 59 | 83 |
| Campbell 2005 | 2 × 2 | 4 | High | NI | 391 | 68 | 83.6 |
| Chapuy 1992 | Parallel | 2 | High | PL | 3270 | 100 | 84 |
| Chapuy 2002 | Parallel | 3 | High | PL | 610 | 100 | 85 |
| Chel 2008 | Parallel | 6 | High | PL | 338 | 77 | 84 |
| Cherniack 2011 | Parallel | 2 | High | PL | 46 | 2 | 80 |
| Cooper 2003 | Parallel | 2 | Low | PL | 187 | 100 | 56 |
| Corless 1985 | Parallel | 2 | High | PL | 65 | 78 | 82.4 |
| Daly 2008 | Parallel | 2 | High | NI | 167 | 0 | 61.9 |
| Dawson‐Hughes 1997 | Parallel | 2 | Low | PL | 389 | 55 | 71 |
| Dukas 2004 | Parallel | 2 | Low | PL | 378 | 51 | 71 |
| Flicker 2005 | Parallel | 2 | Low | PL | 625 | 95 | 83.4 |
| Gallagher 2001 | 2 × 2 | 4 | Low | PL | 489 | 100 | 71.5 |
| Glendenning 2012 | Parallel | 2 | Low | PL | 686 | 100 | 76.7 |
| Grady 1991 | Parallel | 2 | High | PL | 98 | 54 | 79.1 |
| Grimnes 2011 | Parallel | 2 | Low | PL | 104 | 49 | 52 |
| Harwood 2004 | Parallel | 4 | High | NI | 150 | 100 | 81.2 |
| Jackson 2006 | Parallel | 2 | Low | PL | 36,282 | 100 | 62.4 |
| Janssen 2010 | Parallel | 2 | Low | PL | 70 | 100 | 80.8 |
| Komulainen 1999 | 2 × 2 | 4 | Low | PL | 464 | 100 | 52.7 |
| Krieg 1999 | Parallel | 2 | High | NI | 248 | 100 | 84.5 |
| Kärkkäinen 2010 | Parallel | 2 | High | NI | 3139 | 100 | 67 |
| Lappe 2007 | Parallel | 3 | High | PL | 1179 | 100 | 66.7 |
| Larsen 2004 | 2 × 2 | 4 | High | NI | 9605 | 60 | 75 |
| Latham 2003 | 2 × 2 | 4 | Low | PL | 243 | 53 | 79.5 |
| Law 2006 | Parallel | 2 | High | NI | 3717 | 76 | 85 |
| Lehouck 2012 | Parallel | 2 | Low | PL | 181 | 20 | 68 |
| Lips 1996 | Parallel | 2 | Low | PL | 2578 | 74 | 80 |
| Lips 2010 | Parallel | 2 | Low | PL | 226 | NR | 78 |
| Lyons 2007 | Parallel | 2 | Low | PL | 3440 | 76 | 84 |
| Meier 2004 | Parallel | 2 | High | NI | 55 | 65 | 56.5 |
| Mochonis 2006 | Parallel | 3 | High | NI | 112 | 100 | 60.3 |
| Ooms 1995 | Parallel | 2 | Low | PL | 348 | 100 | 80.3 |
| Ott 1989 | Parallel | 2 | High | PL | 86 | 100 | 67.5 |
| Porthouse 2005 | Parallel | 2 | High | NI | 3314 | 100 | 76.8 |
| Prince 2008 | Parallel | 2 | Low | PL | 302 | 100 | 77.2 |
| Sanders 2010 | Parallel | 2 | Low | PL | 2258 | 100 | 76.0 |
| Sato 1997 | Parallel | 2 | High | PL | 64 | 45 | 68.5 |
| Sato 1999a | Parallel | 2 | High | PL | 86 | 78 | 70.6 |
| Sato 1999b | Parallel | 3 | High | NI | 103 | 56 | 70.7 |
| Sato 2005a | Parallel | 2 | Low | PL | 96 | 100 | 74.1 |
| Schleithoff 2006 | Parallel | 2 | Low | PL | 123 | 17 | 51 |
| Smith 2007 | Parallel | 2 | Low | PL | 9440 | 54 | 79.1 |
| Trivedi 2003 | Parallel | 2 | Low | PL | 2686 | 24 | 74.7 |
| Witham 2010 | Parallel | 2 | Low | PL | 105 | 34 | 79.7 |
| Zhu 2008 | Parallel | 3 | Low | PL | 120 | 100 | 75 |
NI: no intervention; NR: not reported; PL: placebo
2. Characteristics of included trials (II).
|
Characteristic Study ID |
Participants | Outcome Measures | Country | Sponsor |
| Aloia 2005 | Black postmenopausal African‐American women | Bone mineral density | USA | No |
| Avenell 2004 | Elderly people with an osteoporotic fracture within the past 10 years | Recruitment, compliance and retention within a randomised trial | UK | Yes |
| Avenell 2012 | Elderly people with low‐trauma osteoporotic fracture in the previous 10 years | Fractures | UK | Yes |
| Baeksgaard 1998 | Postmenopausal women | Bone mineral density | Denmark | Yes |
| Bischoff 2003 | Elderly women living in institutional care | Falls | Switzerland | Yes |
| Bjorkman 2007 | Chronically bedridden patients | Parathyroid function and bone mineral density | Finland | Yes |
| Bolton‐Smith 2007 | Elderly non‐osteoporotic women | Bone mineral density | UK | Yes |
| Brazier 2005 | Elderly vitamin D–insufficient women | Bone mineral density | France | Yes |
| Broe 2007 | Nursing home residents | Falls | USA | Yes |
| Brohult 1973 | Patients with rheumatoid arthritis | Objective and subjective improvement | Sweden | Yes |
| Burleigh 2007 | Older geriatric inpatients | Falls | UK | Yes |
| Campbell 2005 | Elderly people with visual impairment | Numbers of falls and injuries resulting from falls | New Zealand | No |
| Chapuy 1992 | Healthy ambulatory women | Fractures | France | Yes |
| Chapuy 2002 | Elderly people living in institutional care | Biochemical variables of calcium homeostasis, femoral neck bone mineral density and hip fracture risk | France | Yes |
| Chel 2008 | Nursing home residents | Vitamin D status | Netherlands | Yes |
| Cherniack 2011 | Elderly people | Vitamin D status | USA | Yes |
| Cooper 2003 | Postmenopausal women | Bone mineral density | Australia | Yes |
| Corless 1985 | Elderly patients from the geriatric wards | Abilities to carry out basic activities of daily life | UK | Yes |
| Daly 2008 | Healthy ambulatory men | Bone mineral density | Australia | Yes |
| Dawson‐Hughes 1997 | Healthy ambulatory participants | Bone mineral density | USA | Yes |
| Dukas 2004 | Elderly people | Falls | Switzerland | Yes |
| Flicker 2005 | Elderly people living in institutional care | Falls and fractures | Australia | No |
| Gallagher 2001 | Elderly women | Bone mineral density | USA | No |
| Glendenning 2012 | Elderly community‐dwelling ambulatory women | Falls, muscular strength and mobility | Australia | No |
| Grady 1991 | Elderly people | Muscle strength | USA | Yes |
| Grimnes 2011 | Healthy people with a low vitamin D status | Insulin sensitivity and secretion | Norway | No |
| Harwood 2004 | Elderly women following surgery for hip fracture | Bone mineral density, falls and fractures | UK | Yes |
| Jackson 2006 | Postmenopausal women | Fractures | USA | Yes |
| Janssen 2010 | Elderly vitamin D–insufficient women | Muscle strength, power and functional mobility | Netherlands | Yes |
| Komulainen 1999 | Postmenopausal women | Bone mineral density | Finland | Yes |
| Krieg 1999 | Elderly institutionalised women | Bone mineral density | Switzerland | Yes |
| Kärkkäinen 2010 | Postmenopausal women | Falls | Finland | Yes |
| Lappe 2007 | Healthy postmenopausal white women | Fractures | USA | Yes |
| Larsen 2004 | Older community‐dwelling residents | Falls | Denmark | Yes |
| Latham 2003 | Frail elderly people | Self‐rated physical health and falls | New Zealand | No |
| Law 2006 | Nursing home residents | Falls and fractures | UK | No |
| Lehouck 2012 | Patients with chronic obstructive pulmonary disease | Time to first exacerbation | Belgium | Yes |
| Lips 1996 | Elderly people | Fractures | Netherlands | Yes |
| Lips 2010 | Elderly people with vitamin D insufficiency | Postural stability, muscle strength and safety | Netherlands | No |
| Lyons 2007 | Older people living in institutional care | Fractures | UK | No |
| Meier 2004 | Healthy volunteers | Bone mineral density | Germany | No |
| Mochonis 2006 | Postmenopausal women | Bone mineral density | Greece | Yes |
| Ooms 1995 | Elderly people | Bone mineral density | Netherlands | Yes |
| Ott 1989 | Postmenopausal women | Bone mass | USA | Yes |
| Porthouse 2005 | Elderly women with one or more risk factors for hip fracture | Fractures | UK | Yes |
| Prince 2008 | Elderly women with a history of falling and vitamin D insufficiency | Falls | Australia | Yes |
| Sanders 2010 | Elderly women at high risk of fracture | Falls and fractures | Australia | Yes |
| Sato 1997 | Outpatients with hemiplegia after stroke | Bone mineral density and fractures | Japan | No |
| Sato 1999a | Elderly patients with Parkinson's disease | Fractures | Japan | No |
| Sato 1999b | Outpatients with hemiplegia after stroke | Bone mineral density | Japan | Yes |
| Sato 2005a | Hospitalised elderly women with post‐stroke hemiplegia | Falls | Japan | No |
| Schleithoff 2006 | Patients with congestive heart failure | Mortality | Germany | Yes |
| Smith 2007 | Elderly people | Fractures | UK | No |
| Trivedi 2003 | Elderly people | Mortality, fractures | UK | No |
| Witham 2010 | Patients with systolic heart failure | Exercise capacity | UK | No |
| Zhu 2008 | Elderly women | Bone mineral density | Australia | No |
3. Characteristics of included trials (III).
|
Characteristic Study ID |
D3 [IU] | D2 [IU] | 1α(OH)D [µg] | 1,25(OH)2D [µg] | Ca [mg] | Regimen | Route | Treatment [years] | Follow‐up [years] |
| Aloia 2005 | 800 2000 | 1200‐1500a | Daily | Oral | 3 | 3 | |||
| Avenell 2004 | 800 | 1000b | Daily | Oral | 1 | 1 | |||
| Avenell 2012 | 800 | 500b | Daily | Oral | 3.75 | 6.2 | |||
| Baeksgaard 1998 | 560 | 1000 | Daily | Oral | 2 | 2 | |||
| Bischoff 2003 | 800 | 1200a | Daily | Oral | 0.25 | 0.25 | |||
| Bjorkman 2007 | 400 1200 | 500a | Daily | Oral | 0.5 | 0.5 | |||
| Bolton‐Smith 2007 | 400 | 1000 | Daily | Oral | 2 | 2 | |||
| Brazier 2005 | 800 | 1000 | Daily | Oral | 1 | 1 | |||
| Broe 2007 | 200 400 600 800 | Daily | Oral | 0.42 | 0.42 | ||||
| Brohult 1973 | 100,000 | Daily | Oral | 1 | 1 | ||||
| Burleigh 2007 | 800 | 1200a | Daily | Oral | 0.08 | 0.08 | |||
| Campbell 2005 | 50,000 100,000 |
Monthly | Oral | 1 | 1 | ||||
| Chapuy 1992 | 800 | 1200 | Daily | Oral | 1.5 | 4 | |||
| Chapuy 2002 | 800 | 1200 | Daily | Oral | 2 | 2 | |||
| Chel 2008 | 600 4200 18.000 | 800 1600 | Daily Weekly Monthly | Oral | 0.33 | 0.33 | |||
| Cherniack 2011 | 2000 | 1200a | Daily | Oral | 0.5 | 0.5 | |||
| Cooper 2003 | 10,000 | 1000a | Weekly | Oral | 2 | 2 | |||
| Corless 1985 | 9000 | Daily | Oral | 0.75 | 0.75 | ||||
| Daly 2008 | 800 | 1000 | Daily | Oral | 2 | 3.5 | |||
| Dawson‐Hughes 1997 | 700 | 500 | Daily | Oral | 3 | 3 | |||
| Dukas 2004 | 1 | Daily | Oral | 0.75 | 0.75 | ||||
| Flicker 2005 | 1000 10,000 | 600a | Daily Weekly | Oral | 2 | 2 | |||
| Gallagher 2001 | 0.5 | Daily | Oral | 3 | 5 | ||||
| Glendenning 2012 | 150,000 | Three‐monthly | Oral | 0.5 | 0.75 | ||||
| Grady 1991 | 0.5 | Daily | Oral | 0.5 | 0.5 | ||||
| Grimnes 2011 | 20,000 | Twice weekly | Oral | 0.5 | 0.5 | ||||
| Harwood 2004 | 800 | 300,000 | 1000 | Single dose daily | Intramuscular Oral |
1 | 1 | ||
| Jackson 2006 | 400 | 1000 | Daily | Oral | 7 | 7 | |||
| Janssen 2010 | 400 | 500a | Daily | Oral | 0.5 | 0.5 | |||
| Komulainen 1999 | 300 | 500 | Daily | Oral | 5 | 5 | |||
| Krieg 1999 | 880 | 1000 | Daily | Oral | 2 | 2 | |||
| Kärkkäinen 2010 | 800 | 1000 | Daily | Oral | 3 | 3 | |||
| Lappe 2007 | 1000 | 1400‐1500b | Daily | Oral | 4 | 4 | |||
| Larsen 2004 | 400 | 1000 | Daily | Oral | 3.5 | 3.5 | |||
| Latham 2003 | 300,000 | Single dose | Oral | 0.003 | 0.5 | ||||
| Law 2006 | 100,000 | Four‐monthly | Oral | 0.83 | 0.83 | ||||
| Lehouck 2012 | 100,000 | Monthly | Oral | 1 | 1 | ||||
| Lips 1996 | 400 | Daily | Oral | 3.5 | 3.5 | ||||
| Lips 2010 | 8400 | 500a | weekly | Oral | 0.31 | 0.31 | |||
| Lyons 2007 | 100,000 | Four‐monthly | Oral | 3 | 3 | ||||
| Meier 2004 | 500 | 500 | Daily | Oral | 0.5 | 1 | |||
| Mochonis 2006 | 300 | 1200b | Daily | Oral | 1 | 1 | |||
| Ooms 1995 | 400 | Daily | Oral | 2 | 2 | ||||
| Ott 1989 | 0.5 2 | 1000a | Daily | Oral | 2 | 2 | |||
| Porthouse 2005 | 800 | 1000 | Daily | Oral | 2 | 2 | |||
| Prince 2008 | 1000 | 1000a | Daily | Oral | 1 | 1 | |||
| Sanders 2010 | 500,000 | Yearly | Oral | 2.96 | 2.96 | ||||
| Sato 1997 | 1 | 300a | Daily | Oral | 0.5 | 0.5 | |||
| Sato 1999a | 1 | Daily | Oral | 1.5 | 1.5 | ||||
| Sato 1999b | 1 | Daily | Oral | 1 | 1 | ||||
| Sato 2005a | 1000 | Daily | Oral | 2 | 2 | ||||
| Schleithoff 2006 | 2000 | 500a | Daily | Oral | 0.75 | 1.25 | |||
| Smith 2007 | 300,000 | Yearly | Intramuscular | 3 | 3 | ||||
| Trivedi 2003 | 100,000 | Four‐monthly | Oral | 5 | 5 | ||||
| Witham 2010 | 100,000 | 10‐weekly | Oral | 0.38 | 0.38 | ||||
| Zhu 2008 | 1000 | 1200b | Daily | Oral | 5 | 5 |
aEqual dose of calcium was administered to a control group bCalcium was tested singly in one arm of the trial as well as combined with vitamin D; placebo or no intervention group of the trial was not supplemented with calcium 1α(OH)D: alfacalcidol; 1,25(OH)2D: calcitriol; IU: international units; µg: microgram
4. Overview of study populations.
|
Characteristic Study ID |
Intervention(s) and control(s) | [N] screened / eligible | [N] randomised | [N] ITT | [N] finishing study | [%] of randomised participants finishing study |
| 1. Aloia 2005 | I: vitamin D3 plus calcium | 322 | 104 | 104 | 74 | 71 |
| C: placebo | 104 | 104 | 74 | 71 | ||
| total: | 208 | 208 | 148 | 71 | ||
| 2. Avenell 2004 | I: vitamin D3 | 180 | 70 | 70 | ‐ | ‐ |
| C: no intervention | 64 | 64 | ‐ | ‐ | ||
| total: | 134 | 134 | ‐ | ‐ | ||
| 3. Avenell 2012 | I: vitamin D3 | 15,024 | 2649 | 2649 | 1813 | 68 |
| C: matched placebo tablets | 2643 | 2643 | 1762 | 67 | ||
| total: | 5292 | 5292 | 3575 | 68 | ||
| 4. Baeksgaard 1998 | I: vitamin D3 plus calcium | ‐ | 80 | 80 | 65 | 81 |
| C: matched placebo tablets | 80 | 80 | 64 | 80 | ||
| total: | 160 | 160 | 129 | 80 | ||
| 5. Bischoff 2003 | I: vitamin D3 plus calcium | 130 | 62 | 62 | ‐ | ‐ |
| C: calcium | 60 | 60 | ‐ | ‐ | ||
| total: | 122 | 122 | 89 | 73 | ||
| 6. Bjorkman 2007 | I: vitamin D3 plus calcium | 1215 | 150 | 150 | 123 | 82 |
| C: calcium | 68 | 68 | 59 | 87 | ||
| total: | 218 | 218 | 182 | 83 | ||
| 7. Bolton‐Smith 2007 | I: vitamin D3 plus calcium | ‐ | 62 | 62 | 50 | 81 |
| C: matched placebo | 61 | 61 | 56 | 92 | ||
| total: | 123 | 123 | 106 | 86 | ||
| 8. Brazier 2005 | I: vitamin D3 plus calcium | 360 | 95 | 95 | 74 | 78 |
| C: matched placebo tablets | 97 | 97 | 68 | 70 | ||
| total: | 192 | 192 | 142 | 74 | ||
| 9. Broe 2007 | I: vitamin D2 | 126 | 99 | 99 | 96 | 97 |
| C: matched placebo tablets | 25 | 25 | 25 | 100 | ||
| total: | 124 | 124 | 121 | 98 | ||
| 10. Brohult 1973 | I: vitamin D3 | ‐ | 25 | 25 | 24 | 96 |
| C: placebo | 25 | 25 | 25 | 100 | ||
| total: | 50 | 50 | 49 | 98 | ||
| 11. Burleigh 2007 | I: vitamin D3 plus calcium | 515 | 101 | 101 | 98 | 97 |
| C: placebo | 104 | 104 | 101 | 97 | ||
| total: | 205 | 205 | 199 | 97 | ||
| 12. Campbell 2005 | I: home safety assessment and modification programme | 391 | 195 | 195 | 177 | 91 |
| C: social visits | 196 | 196 | 184 | 94 | ||
| total: | 391 | 391 | 361 | 92 | ||
| 13. Chapuy 1992 | I: vitamin D3 plus calcium | ‐ | 1634 | 1634 | 1590 | 97 |
| C: double placebo | 1636 | 1636 | 1573 | 96 | ||
| total: | 3270 | 3270 | 3163 | 96 | ||
| 14. Chapuy 2002 | I: vitamin D3 plus calcium | 639 | 393 | 393 | ‐ | ‐ |
| C: double placebo | 190 | 190 | ‐ | ‐ | ||
| total: | 583 | 583 | ‐ | ‐ | ||
| 15. Chel 2008 | I: vitamin D3 | 1006 | 166 | 166 | 139 | 84 |
| C: matched placebo tablets | 172 | 172 | 137 | 80 | ||
| total: | 338 | 338 | 276 | 82 | ||
| 16. Cherniack 2011 | I: vitamin D3 plus calcium | 52 | 23 | 23 | 17 | 74 |
| C: matched placebo plus calcium | 23 | 23 | 17 | 74 | ||
| total: | 46 | 46 | 34 | 74 | ||
| 17. Cooper 2003 | I: vitamin D2 plus calcium | ‐ | 93 | 93 | 73 | 78 |
| C: calcium | 94 | 94 | 80 | 85 | ||
| total: | 187 | 187 | 153 | 82 | ||
| 18. Coreless 1985 | I: vitamin D2 | 320 | 32 | 32 | 8 | 25 |
| C: placebo | 33 | 33 | 17 | 51 | ||
| total: | 65 | 65 | 25 | 38 | ||
| 19. Daly 2006 | I: calcium‐vitamin D3–fortified milk plus calcium | 422 | 85 | 85 | 76 | 89 |
| C: no intervention | 82 | 82 | 73 | 89 | ||
| total: | 167 | 167 | 149 | 89 | ||
| 20. Dawson‐Hughes 1997 | I: vitamin D3 plus calcium | 545 | 187 | 187 | 148 | 79 |
| C: placebo | 202 | 202 | 170 | 84 | ||
| total: | 389 | 389 | 318 | 82 | ||
| 21. Dukas 2004 | I: alfacalcidol | 410 | 192 | 192 | ‐ | ‐ |
| C: placebo | 186 | 186 | ‐ | ‐ | ||
| total: | 378 | 378 | ‐ | ‐ | ||
| 22. Flicker 2005 | I: vitamin D3 plus calcium | 1767 | 313 | 313 | 269 | 86 |
| C: calcium | 312 | 312 | 271 | 87 | ||
| total: | 625 | 625 | 540 | 86 | ||
| 23. Gallagher 2001 | I: calcitriol | 1905 | 123 | 123 | 101 | 82 |
| C: matched placebo | 123 | 123 | 112 | 91 | ||
| total: | 246 | 246 | 213 | 87 | ||
| 24. Glendenning 2012 | I: cholecalciferol 150,000 three‐monthly | 2110 | 353 | 353 | 331 | 94 |
| C: placebo vitamin D | 333 | 333 | 307 | 92 | ||
| total: | 686 | 686 | 638 | 93 | ||
| 25. Grady 1991 | I: calcitriol | 98 | 50 | 50 | 49 | 98 |
| C: placebo vitamin D | 48 | 48 | 47 | 98 | ||
| total: | 98 | 98 | 96 | 98 | ||
| 26. Grimnes 2011 | I: vitamin D3 | 108 | 51 | 51 | 49 | 96 |
| C: placebo | 53 | 53 | 45 | 85 | ||
| total: | 104 | 104 | 94 | 90 | ||
| 27. Harwood 004 | I: vitamin D plus calcium | 208 | 113 | 113 | ‐ | ‐ |
| C: no intervention | 37 | 37 | ‐ | ‐ | ||
| total: | 150 | 150 | ‐ | ‐ | ||
| 28. Jackson 2006 | I: vitamin D3 plus calcium | 68,132 | 18,176 | 18,176 | 16,936 | 93 |
| C: matched placebo | 18,106 | 18,106 | 16,815 | 93 | ||
| total: | 36,282 | 36,282 | 33,751 | 93 | ||
| 29. Janssen 2010 | I: vitamin D3 plus calcium | 91 | 36 | 36 | 18 | 50 |
| C: matched placebo vitamin D3 plus calcium | 34 | 34 | 31 | 91 | ||
| total: | 70 | 70 | 49 | 70 | ||
| 30. Komulainen 1999 | I: oestradiol valerate and cyproterone acetate | 13,100 | 116 | 116 | ‐ | ‐ |
| C: placebo | 116 | 116 | ‐ | ‐ | ||
| total: | 232 | 232 | ‐ | ‐ | ||
| 31. Krieg 1999 | I: vitamin D3 plus calcium | ‐ | 124 | 124 | 50 | 40 |
| C: no treatment | 124 | 124 | 53 | 43 | ||
| total: | 248 | 248 | 103 | 41 | ||
| 32. Kärkkäinen 2010 | I: vitamin D3 plus calcium | 5407 | 1718 | 1718 | 1566 | 91 |
| C: no treatment | 1714 | 1714 | 1573 | 92 | ||
| total: | 3432 | 3432 | 3139 | 91 | ||
| 33. Lappe 2007 | I: vitamin D3 plus calcium | 1180 | 446 | 446 | ‐ | ‐ |
| C: calcium plus placebo tablets | 733 | 733 | ‐ | ‐ | ||
| total: | 1179 | 1179 | ‐ | ‐ | ||
| 34. Larsen 2004 | I: home safety inspection, vitamin D3 plus calcium | 62,000 | 4957 | 4957 | ‐ | ‐ |
| C: no intervention | 4648 | 4648 | ‐ | ‐ | ||
| total: | 9605 | 9605 | ‐ | ‐ | ||
| 35. Latham 2003 | I: vitamin D3 | 3,028 | 121 | 121 | 108 | 89 |
| C: matched placebo tablets | 122 | 122 | 114 | 93 | ||
| total: | 243 | 243 | 222 | 91 | ||
| 36. Law 2006 | I: vitamin D2 | ‐ | 1762 | 1762 | 1366 | 77 |
| C: no intervention | 1955 | 1955 | 1569 | 80 | ||
| total: | 3717 | 3717 | 2935 | 79 | ||
| 37. Lehouck 2012 | I: vitamin D3 | 419 | 91 | 91 | 72 | 79 |
| C: matched placebo | 91 | 91 | 78 | 86 | ||
| total: | 182 | 182 | 150 | 82 | ||
| 38. Lips 1996 | I: vitamin D3 | ‐ | 1291 | 1291 | 1061 | 82 |
| C: matched placebo | 1287 | 1287 | 1029 | 80 | ||
| total: | 2578 | 2578 | 2090 | 81 | ||
| 39. Lips 2010 | I: vitamin D3 | 593 | 114 | 114 | 105 | 92 |
| C: matched placebo | 112 | 112 | 97 | 87 | ||
| total: | 226 | 226 | 202 | 89 | ||
| 40. Lyons 2007 | I: vitamin D2 | 5745 | 1725 | 1725 | 778 | 45 |
| C: matched placebo tablets | 1715 | 1715 | 762 | 44 | ||
| total: | 3440 | 3440 | 1540 | 44 | ||
| 41. Meier 2004 | I: vitamin D3 | ‐ | 30 | 30 | 27 | 90 |
| C: no intervention | 25 | 25 | 16 | 64 | ||
| total: | 55 | 55 | 43 | 78 | ||
| 42. Mochonis 2006 | I: vitamin D3 plus calcium | ‐ | 72 | 72 | 65 | 90 |
| C: no intervention | 40 | 40 | 36 | 90 | ||
| total: | 112 | 112 | 101 | 90 | ||
| 43. Ooms 1995 | I: vitamin D3 | ‐ | 177 | 177 | 126 | 71 |
| C: matched placebo | 171 | 171 | 118 | 69 | ||
| total: | 348 | 348 | 244 | 70 | ||
| 44. Ott 1989 | I: vitamin D3 plus calcium | ‐ | 43 | 43 | 39 | 91 |
| C: matched placebo vitamin D plus calcium | 43 | 43 | 37 | 86 | ||
| total: | 86 | 86 | 76 | 88 | ||
| 45. Porthouse 2005 | I: vitamin D3 plus calcium | 11,022 | 1321 | 1321 | 1212 | 92 |
| C: no intervention | 1993 | 1993 | 1862 | 93 | ||
| total: | 3454 | 3454 | 3074 | 92 | ||
| 46. Prince 2008 | I: vitamin D2 plus calcium | 827 | 151 | 151 | 144 | 95 |
| C: matched placebo tablets of vitamin D plus calcium | 151 | 151 | 145 | 96 | ||
| total: | 302 | 302 | 289 | 95 | ||
| 47. Sanders 2010 | I: vitamin D3 | 7204 | 1131 | 1131 | 1015 | 90 |
| C: matched placebo tablets | 1127 | 1127 | 1017 | 90 | ||
| total: | 2258 | 2258 | 1032 | 90 | ||
| 48. Sato 1997 | I: vitamin D (alfacalcidol) plus calcium | ‐ | 45 | 45 | 30 | 67 |
| C: matched placebo tablets of vitamin D and calcium | 39 | 39 | 34 | 87 | ||
| total: | 84 | 84 | 64 | 76 | ||
| 49. Sato 1999a | I: vitamin D (alfacalcidol) | ‐ | 43 | 43 | 40 | 93 |
| C: matched placebo tablets of vitamin D | 43 | 43 | 40 | 93 | ||
| total: | 86 | 86 | 80 | 93 | ||
| 50. Sato 1999b | I: vitamin D (alfacalcidol) | ‐ | 34 | 34 | 32 | 94 |
| C: matched placebo tablet of vitamin D | 35 | 35 | 32 | 91 | ||
| total: | 69 | 69 | 64 | 93 | ||
| 51. Sato 2005a | I: vitamin D2 | ‐ | 48 | 48 | 43 | 90 |
| C: matched placebo tablets of vitamin D | 48 | 48 | 42 | 87 | ||
| total: | 96 | 96 | 85 | 88 | ||
| 52. Schleithoff 2006 | I: vitamin D3 plus calcium | ‐ | 61 | 61 | 42 | 69 |
| C: matched placebo vitamin D plus calcium | 62 | 62 | 51 | 82 | ||
| total: | 103 | 103 | 93 | 90 | ||
| 53. Smith 2007 | I: vitamin D2 | 13,487 | 4727 | 4727 | 2304 | 49 |
| C: matched placebo intramuscular injection | 4713 | 4713 | 2266 | 48 | ||
| total: | 9440 | 9440 | 4570 | 48 | ||
| 54. Trivedi 2003 | I: vitamin D3 | ‐ | 1345 | 1345 | 1262 | 94 |
| C: matched placebo vitamin D | 1341 | 1341 | 1264 | 94 | ||
| total: | 2696 | 2696 | 2526 | 94 | ||
| 55. Witham 2010 | I: vitamin D2 | 173 | 53 | 53 | 48 | 91 |
| C: matched placebo tablets | 52 | 52 | 48 | 91 | ||
| total: | 105 | 105 | 96 | 91 | ||
| 56. Zhu 2008 | I: vitamin D2 plus calcium | ‐ | 39 | 39 | 33 | 85 |
| C: matched placebo vitamin D and calcium | 81 | 81 | 74 | 91 | ||
| total: | 120 | 120 | 107 | 89 | ||
| Grand total | All interventions | 47,472 | 45,351 | |||
| All controls | 47,814 | 45,278 | ||||
| All interventions and controls | 95,286 | 90,629a | ||||
"‐" denotes not reported
aNumbers not available for all studies
C: control; I: intervention; ITT: intention‐to‐treat
Trial characteristics
Of the 56 trials reporting mortality, 54 trials randomly assigned participants individually and two trials as clusters (Larsen 2004; Law 2006). Forty‐eight trials used a parallel‐group design, and eight trials (Avenell 2004; Avenell 2012; Bolton‐Smith 2007; Campbell 2005; Gallagher 2001; Komulainen 1999; Larsen 2004; Latham 2003) used the 2 × 2 factorial design (Pocock 2004). The 56 trials were published from 1973 to 2012.
The trials were conducted in Europe (n = 34), North America (n = 9), Oceania (n = 9) and Asia (n = 4). All 56 trials came from high‐income countries.
In 38 trials (69%), vitamin D was provided free of charge by pharmaceutical companies. In the other 18 trials, funding was not reported.
The 62 trials reporting no mortality included a total of 10,723 participants. These trials were mostly phase I or phase II short‐term clinical trials assessing the pharmacokinetic or pharmacodynamic properties of vitamin D. These trials had typical outcome measures that are non‐validated potential surrogates for participant‐relevant outcomes (Gluud 2006).
Participants
A total of 95,286 participants were randomly assigned in the 56 trials reporting mortality (Table 5). The number of participants in each trial ranged from 46 to 36,282 participants (median 226). The age range of participants was from 18 to 107 years. The mean proportion of women was 77% (Table 2).
Forty‐eight trials were primary prevention trials that included 94,491 apparently healthy participants. Of these 48 trials, four trials included healthy volunteers, nine trials postmenopausal women and 35 trials older people living independently or in institutional care.
Eight trials with 795 participants were secondary prevention trials that included participants with neurological (Sato 1997; Sato 1999a; Sato 1999b; Sato 2005a), cardiovascular (Schleithoff 2006; Witham 2010), respiratory (Lehouck 2012) or rheumatoid disease (Brohult 1973) (Table 3).
Of the 56 trials reporting mortality, 45 trials (80%) reported the baseline vitamin D status of participants based on serum 25‐hydroxyvitamin D levels. Participants in 19 trials (Bjorkman 2007; Bolton‐Smith 2007; Broe 2007; Burleigh 2007; Chel 2008; Cooper 2003; Daly 2008; Dawson‐Hughes 1997; Dukas 2004; Flicker 2005; Gallagher 2001; Glendenning 2012; Grady 1991; Meier 2004; Moschonis 2006; Ott 1989; Smith 2007; Trivedi 2003; Zhu 2008) had baseline 25‐hydroxyvitamin D levels at or above vitamin D adequacy (20 ng/mL). Participants in the remaining 26 trials had baseline 25‐hydroxyvitamin D levels within a range of vitamin D insufficiency (less than 20 ng/mL). Eleven trials did not report the baseline vitamin D status of participants (Avenell 2004; Baeksgaard 1998; Brohult 1973; Campbell 2005; Komulainen 1999; Lappe 2007; Larsen 2004; Law 2006; Lyons 2007; Porthouse 2005; Sato 1997).
The main outcomes in the trials were bone mineral density, numbers of falls and fractures and mortality (Table 3).
Experimental interventions
Vitamin D3 (cholecalciferol)
Vitamin D was administered as vitamin D3 (cholecalciferol) in 38 trials (75,927 participants; 76.8% women; age range 51 to 85 years). Vitamin D3 was tested singly in 11 trials and combined with calcium in 25 trials. An additional two trials tested vitamin D3 both singly and combined with calcium (Avenell 2004; Avenell 2012). Vitamin D3 was tested orally in all trials. Vitamin D3 was administered daily in 30 trials and intermittently in eight trials (daily, weekly or monthly (Chel 2008); twice weekly (Grimnes 2011); weekly (Lips 2010); monthly (Campbell 2005; Lehouck 2012); three‐monthly (Glendenning 2012); four‐monthly (Trivedi 2003); or yearly (Sanders 2010)). The dose of vitamin D3 was 300 IU to 500,000 IU (mean daily dose 3650 IU; median daily dose 800 IU). The duration of supplementation in trials using vitamin D3 was one day to seven years (weighted mean 4.9 years), and the length of the follow‐up period was one month to seven years (weighted mean 5.2 years) (Table 4).
Vitamin D2 (ergocalciferol)
Vitamin D was administered as vitamin D2 (ergocalciferol) in 12 trials (18,349 participants; 82% women; age range 56 to 89 years). Vitamin D2 was tested singly in seven trials and combined with calcium in four trials. An additional one trial tested vitamin D2 both singly and combined with calcium (Harwood 2004). Vitamin D2 was administered orally in 10 trials. One trial administered vitamin D2 orally and parenterally (single intramuscular injection) (Harwood 2004), and one trial administered vitamin D2 parenterally (single intramuscular injection yearly) (Smith 2007). The dosing schedule for vitamin D2 was daily in five trials (Broe 2007; Corless 1985; Prince 2008; Sato 2005a; Zhu 2008) and intermittently in five trials (weekly (Cooper 2003), 10‐weekly (Witham 2010), three‐monthly (Law 2006), four‐monthly (Lyons 2007) or yearly (Smith 2007)). One trial tested vitamin D2 first weekly and then daily (Flicker 2005). The dose of vitamin D2 was 200 IU to 300,000 IU (mean daily dose 1661 IU; median daily dose 1000 IU). The duration of supplementation and follow‐up in trials using vitamin D2 was one day to seven years (weighted mean 2.4 years) (Table 4).
Alfacalcidol (1α‐hydroxyvitamin D)
Vitamin D was administered as alfacalcidol in four trials (617 participants; 57% women; age range 68 to 71 years). Alfacalcidol was tested singly in three trials and combined with calcium in one trial (Sato 1997). Alfacalcidol was administered orally and daily in all trials. The dose of alfacalcidol was 1 μg in all four trials. The duration of supplementation and follow‐up in trials using alfacalcidol was six months to one year (weighted mean 0.9 years) (Table 4).
Calcitriol (1,25‐dihydroxyvitamin D)
Vitamin D was administered as calcitriol in three trials (430 participants; 85% women; age range 67 to 79 years). Calcitriol was tested singly in two trials and combined with calcium in one trial (Ott 1989). Calcitriol was administered orally and daily in all trials. The dose of calcitriol was 0.5 μg in two trials (Gallagher 2001; Grady 1991), and one trial tested two doses of calcitriol 0.5 μg and 2 μg (Ott 1989). The duration of supplementation in trials using calcitriol was two to five years (weighted mean 2.2 years) and the follow‐up period lasted two to five years (weighted mean four years) (Table 4).
Control interventions
A total of 44 trials used placebo vitamin D and 12 trials used no intervention in the control group (Table 2).
Co‐interventions
Thirty‐four trials used vitamin D in combination with calcium in the experimental intervention groups. Calcium was administered orally and daily in all 34 trials. The dose of calcium was 300 mg to 1600 mg (mean 920 mg; median 1000 mg) (Table 4).
Thirteen trials used calcium combined with vitamin D placebo in the control group. The dose of calcium was 300 mg to 1500 mg (mean 835 mg; median 1000 mg). These trials used an equal dose of calcium in the experimental intervention groups (Table 4).
One trial with a 2 × 2 factorial design tested a combination of vitamin D3, vitamin K1 and calcium in one of the intervention groups (Bolton‐Smith 2007). The factorial design of this trial allowed us to compare only the vitamin D3 plus calcium group versus the placebo group of this trial. Another two trials with parallel‐group designs and three intervention groups tested in one of the groups the combination of calcium and multivitamins (Baeksgaard 1998) or ipriflavone (Sato 1999b). The parallel‐group design of these trials allowed us to compare the vitamin D group versus the placebo group. Two trials with a 2 × 2 factorial design tested vitamin D and hormone replacement (Gallagher 2001; Komulainen 1999). We have compared only the vitamin D group with the placebo group of these trials.
Risk of bias in included studies
Thirty trials reporting mortality (54% of the trials; 71% of the participants) were considered as having low risk of bias. The remaining 26 trials had unclear bias control in one or more of the components assessed (Table 2; Figure 2; Figure 3). Inspection of the funnel plot does not suggest potential bias (asymmetry) (Figure w7, http://ctu.dk/publications/supplementary‐material.aspx). The adjusted‐rank correlation test (P = 0.44) and the regression asymmetry test (P = 0.08) found no statistically significant evidence of bias.
2.
Risk of bias according to bias domains in the 56 randomised clinical trials on vitamin D and mortality.
3.
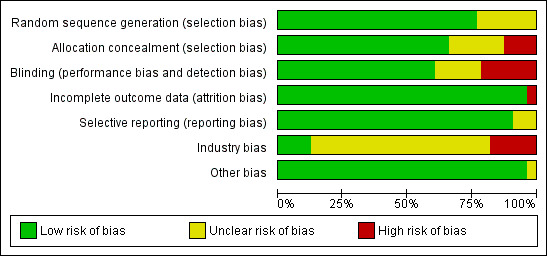
Risk of bias in the included 56 randomised clinical trials on vitamin D and mortality.
Allocation
The generation of the allocation sequence was adequately described in 43 trials. The remaining 13 trials were described as randomised, but the method used for sequence generation was not described (Baeksgaard 1998; Bischoff 2003; Brohult 1973; Chapuy 1992; Chapuy 2002; Chel 2008; Grady 1991; Krieg 1999; Larsen 2004; Meier 2004; Ott 1989; Sato 1997; Sato 1999b).
The method used to conceal allocation was adequately described in 37 trials. The method used for allocation concealment was judged as unclear in 12 trials (Baeksgaard 1998; Bischoff 2003; Brohult 1973; Chapuy 1992; Chapuy 2002; Chel 2008; Corless 1985; Grady 1991; Meier 2004; Ott 1989; Sato 1997; Sato 1999a) and inadequate in seven trials (Avenell 2004; Daly 2008; Krieg 1999; Moschonis 2006; Larsen 2004; Law 2006; Sato 1999b).
Blinding
The method of blinding was adequately described in 34 trials. The method of blinding was unclear in 10 trials (Brazier 2005; Brohult 1973; Chapuy 1992; Chapuy 2002; Chel 2008; Corless 1985; Grady 1991; Ott 1989; Sato 1997; Sato 1999a). Twelve trials were not blinded (Avenell 2004; Campbell 2005; Daly 2008; Harwood 2004; Krieg 1999; Kärkkäinen 2010; Larsen 2004; Law 2006; Meier 2004; Moschonis 2006; Porthouse 2005; Sato 1999b).
Incomplete outcome data
Incomplete data were addressed adequately in 54 trials. In two trials, information is insufficient to allow assessment of whether the missing data mechanism in combination with the method used to handle missing data is likely to induce bias on the estimate of effect (Lappe 2007; Larsen 2004).
Selective reporting
Predefined primary and secondary outcomes were reported in 51 trials. Five trials did not report all predefined or clinically relevant and reasonably expected outcomes (Baeksgaard 1998; Brohult 1973; Larsen 2004; Porthouse 2005; Sato 1997). The 103 randomised clinical trials that could not provide data for mortality analyses represent an unknown reservoir of outcome reporting bias.
Industry bias
Seven trials were not funded by industry (Campbell 2005; Flicker 2005; Janssen 2010; Lyons 2007; Meier 2004; Trivedi 2003; Witham 2010). Ten trials were funded by industry (Bischoff 2003; Brazier 2005; Brohult 1973; Chapuy 2002; Harwood 2004; Komulainen 1999; Lips 2010; Moschonis 2006; Porthouse 2005; Smith 2007) and 32 trials reported that trial medications were funded by industry (Aloia 2005; Avenell 2004; Avenell 2012; Baeksgaard 1998; Bjorkman 2007; Bolton‐Smith 2007; Broe 2007; Burleigh 2007; Chapuy 1992; Chel 2008; Cherniack 2011; Cooper 2003; Daly 2008; Dawson‐Hughes 1997; Dukas 2004; Gallagher 2001; Grady 1991; Grimnes 2011; Jackson 2006; Kärkkäinen 2010; Krieg 1999; Lappe 2007; Larsen 2004; Latham 2003; Lehouck 2012; Lips 1996; Ooms 1995; Ott 1989; Prince 2008; Sanders 2010; Schleithoff 2006; Zhu 2008). The source of funding is not clear for seven trials (Corless 1985; Glendenning 2012; Law 2006; Sato 1997; Sato 1999a; Sato 1999b; Sato 2005a).
Other potential sources of bias
Two trials had other factors that could put the trials at risk of bias, such as recruitment bias (Larsen 2004; Law 2006). The remaining 54 trials appeared to be free of other components that could put them at risk of bias.
Effects of interventions
See: Table 1
All‐cause mortality in all trials
Overall, vitamin D significantly decreased all‐cause mortality (RR 0.97 (95% CI 0.94 to 0.99); P = 0.02; I2 = 0%; 95,286 participants; 56 trials; Analysis 1.1). A total of 5920 of 47,472 participants (12.5%) randomly assigned to the vitamin D group versus 6077 of 47,814 participants (12.7%) randomly assigned to the placebo or no intervention group died. A sensitivity analysis excluding the cluster‐randomised trials had no noticeable effect on the result (RR 0.96 (95% CI 0.93 to 0.99); P = 0.01; I2 = 0%; 81,964 participants; 54 trials; Analysis 1.2). The difference between the estimate of the effect of vitamin D on mortality in individually randomised and cluster‐randomised trials was not statistically significant by the test of interaction (Chi2 = 0.48; P = 0.49; Analysis 1.2).
1.1. Analysis.
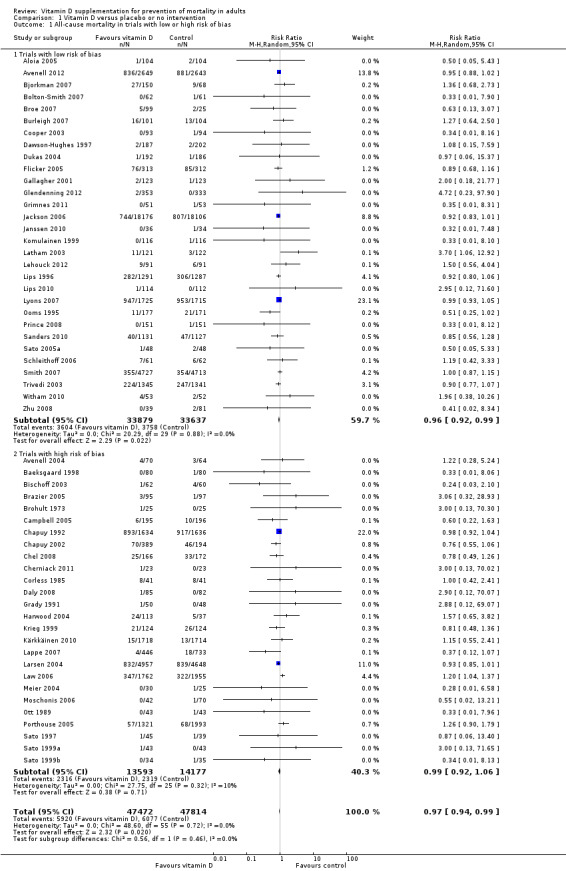
Comparison 1 Vitamin D versus placebo or no intervention, Outcome 1 All‐cause mortality in trials with low or high risk of bias.
1.2. Analysis.
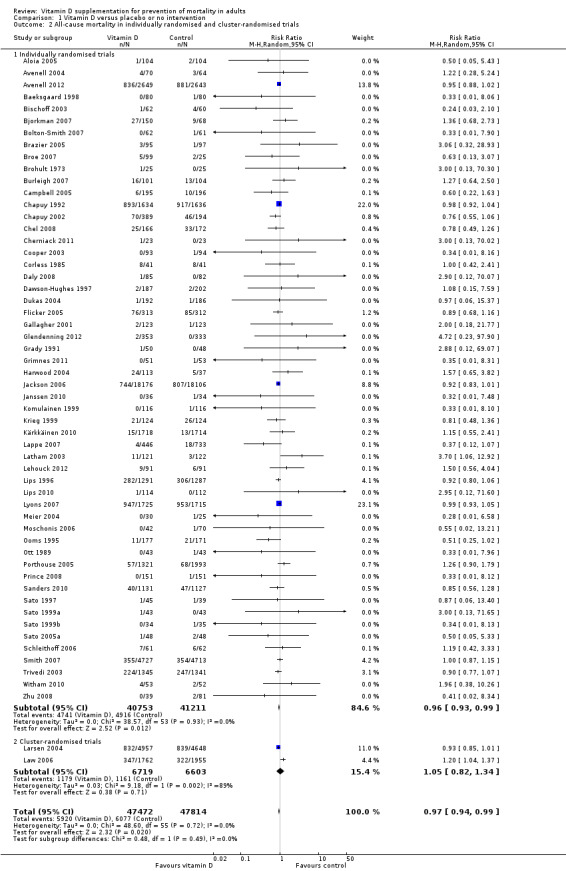
Comparison 1 Vitamin D versus placebo or no intervention, Outcome 2 All‐cause mortality in individually randomised and cluster‐randomised trials.
Intervention effects according to bias risk of trials
In the trials with low risk of bias, mortality was significantly decreased in the vitamin D group (RR 0.96 (95% CI 0.92 to 0.99); P = 0.02; I2 = 0%; 67,516 participants; 30 trials; Analysis 1.1). In the trials with high risk of bias, vitamin D did not significantly affect all‐cause mortality (RR 0.99 (95% CI 0.92 to 1.06); P = 0.71; I2 = 10%; 27,770 participants; 26 trials; Analysis 1.1). The difference between the estimate of the effect of vitamin D on mortality in low‐ and high‐bias risk trials was not statistically significant by the test of interaction (Chi2 = 0.56; P = 0.46; Analysis 1.1).
Placebo‐controlled trials compared with trials with no intervention in the control group
Vitamin D significantly decreased mortality in the placebo‐controlled trials (RR 0.96 (95% CI 0.93 to 0.99); P = 0.009; I2 = 0%; 73,892 participants; 44 trials; Analysis 1.3). Vitamin D had no statistically significant effect on mortality in the trials with no intervention in the control group (RR 1.05 (95% CI 0.91 to 1.21); P = 0.51; I2 = 29%; 21,394 participants; 12 trials; Analysis 1.3.2). The difference between the estimate of the effect of vitamin D on mortality in the placebo‐controlled trials and in trials with no intervention in the control group was not statistically significant by the test of interaction (Chi2 = 1.50; P = 0.22; Analysis 1.3).
1.3. Analysis.
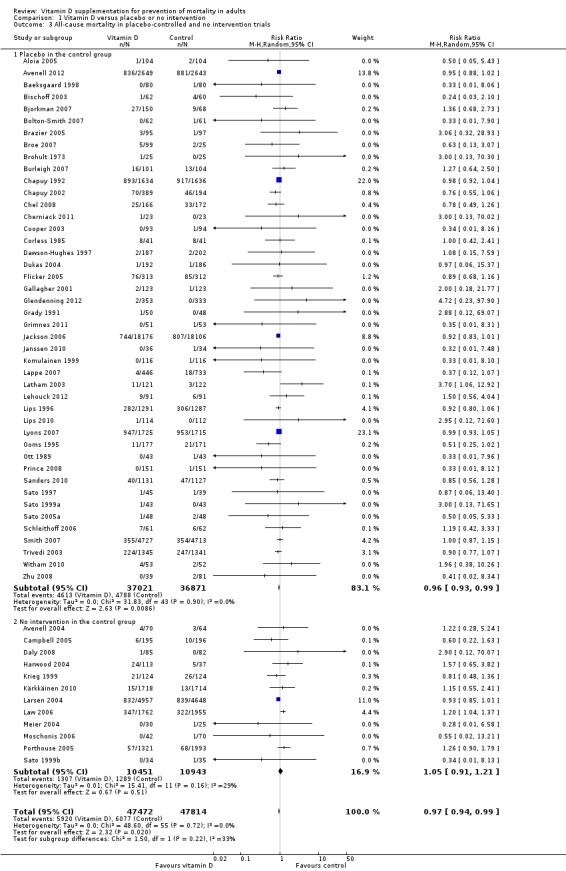
Comparison 1 Vitamin D versus placebo or no intervention, Outcome 3 All‐cause mortality in placebo‐controlled and no intervention trials.
Trials without risk of industry bias compared to trials with risk of industry bias
Vitamin D had no significant effect on mortality in the trials without risk of industry bias (RR 0.97, 95% CI 0.92 to 1.03; P = 0.32; I2 = 0%; 7,372 participants; 7 trials; Analysis 1.4). Vitamin D significantly decreased mortality in the trials with risk of industry bias (RR 0.96 (95% CI 0.93 to 1.00); P = 0.003; I2 = 0%; 87,914 participants; 49 trials; Analysis 1.4). The difference between the estimate of the effect of vitamin D on mortality in the trials without risk of industry bias and the trials with risk of industry bias was not statistically significant by the test of interaction (Chi2 = 0.07; P = 0.80; Analysis 1.4).
1.4. Analysis.
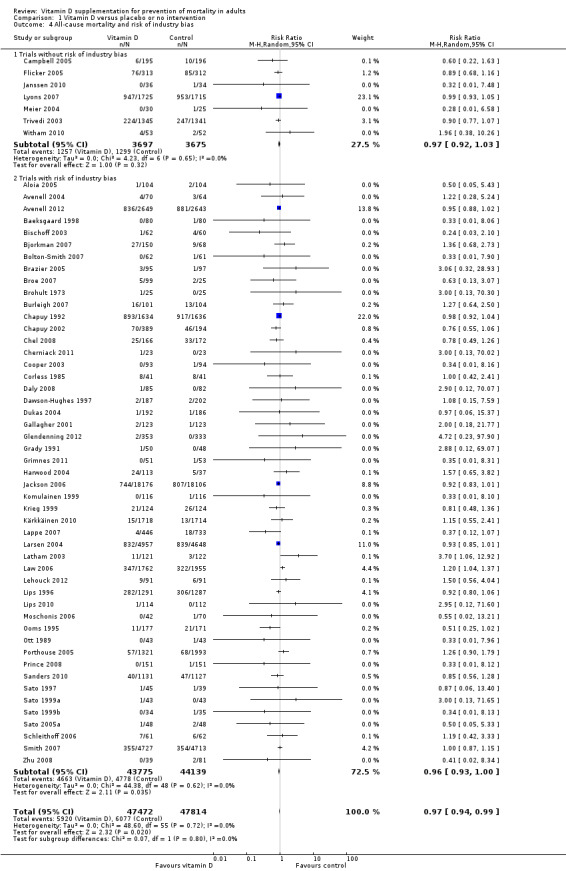
Comparison 1 Vitamin D versus placebo or no intervention, Outcome 4 All‐cause mortality and risk of industry bias.
Primary prevention compared with secondary prevention
Vitamin D significantly decreased mortality in the primary prevention trials (RR 0.97 (95% CI 0.94 to 0.99); P = 0.02; I2 = 0%; 94,491 participants; 48 trials; Analysis 1.5). Vitamin D had no statistically significant effect on mortality in the secondary prevention trials (RR 1.31 (95% CI 0.73 to 2.35); P = 0.37; I2 = 0%; 795 participants; 8 trials; Analysis 1.5). The difference between the estimates of the effect of vitamin D on mortality in the primary prevention and the secondary prevention trials was not statistically significant by the test of interaction (Chi2 = 1.04; P = 0.31; Analysis 1.5).
1.5. Analysis.
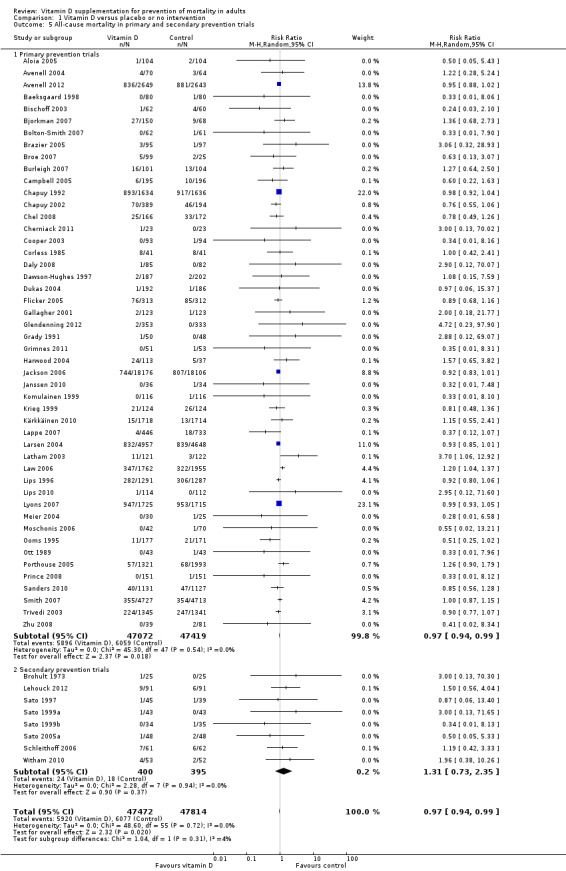
Comparison 1 Vitamin D versus placebo or no intervention, Outcome 5 All‐cause mortality in primary and secondary prevention trials.
Intervention effects according to vitamin D status at entry
Vitamin D significantly decreased mortality in participants with vitamin D insufficiency at entry (RR 0.95 (95% CI 0.91 to 0.99); P = 0.01; I2 = 0%; 56,697 participants; 26 trials; Analysis 1.6). Vitamin D had no statistically significant effect on mortality in the trials including participants with vitamin D adequacy (RR 0.95 (95% CI 0.87 to 1.05); P = 0.30; I2 = 0%; 16,283 participants; 19 trials; Analysis 1.6). A similar finding was obtained in the trials including participants with unknown vitamin D status (Analysis 1.6). The difference between the estimates of the effect of vitamin D on mortality in the trials including participants with vitamin D insufficiency and the trials including participants with vitamin D adequacy was not statistically significant by the test of interaction (Chi2 = 1.59; P = 0.45; Analysis 1.6).
1.6. Analysis.
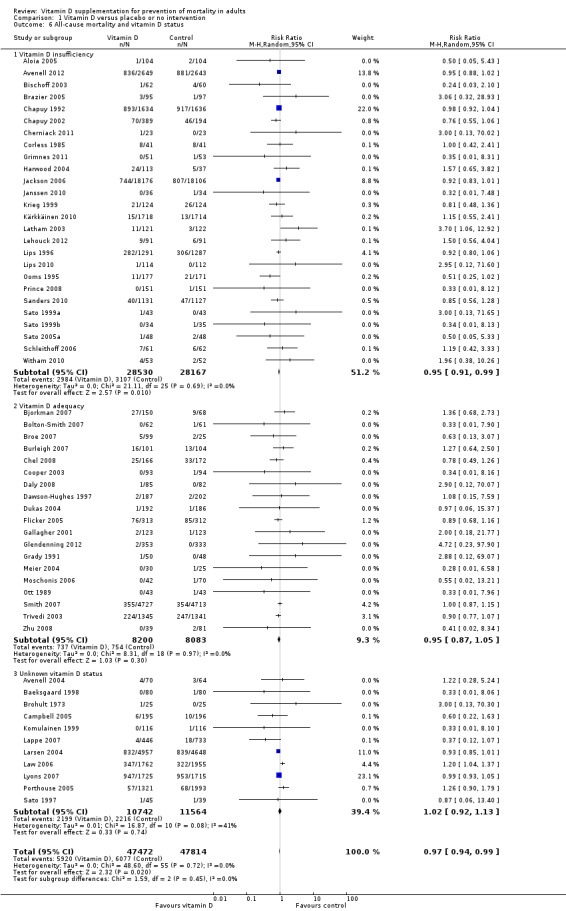
Comparison 1 Vitamin D versus placebo or no intervention, Outcome 6 All‐cause mortality and vitamin D status.
Trials including participants living independently compared with trials including participants living in care institutions
Vitamin D significantly decreased mortality in ambulatory participants (RR 0.95 (95% CI 0.92 to 0.98); P = 0.0003; I2 = 0%; 86,071 participants; 45 trials; Analysis 1.7). Vitamin D had no statistically significant effect on mortality in the trials including institutionalised participants (RR 1.02 (95% CI 0.92 to 1.13); P = 0.74; I2 = 21%; 9215 participants; 11 trials; Analysis 1.7). The difference between the estimates of the effect of vitamin D on mortality in the trials including ambulatory participants and the trials including institutionalised participants was not statistically significant by the test of interaction (Chi2 = 1.60; P = 0.21; Analysis 1.7).
1.7. Analysis.
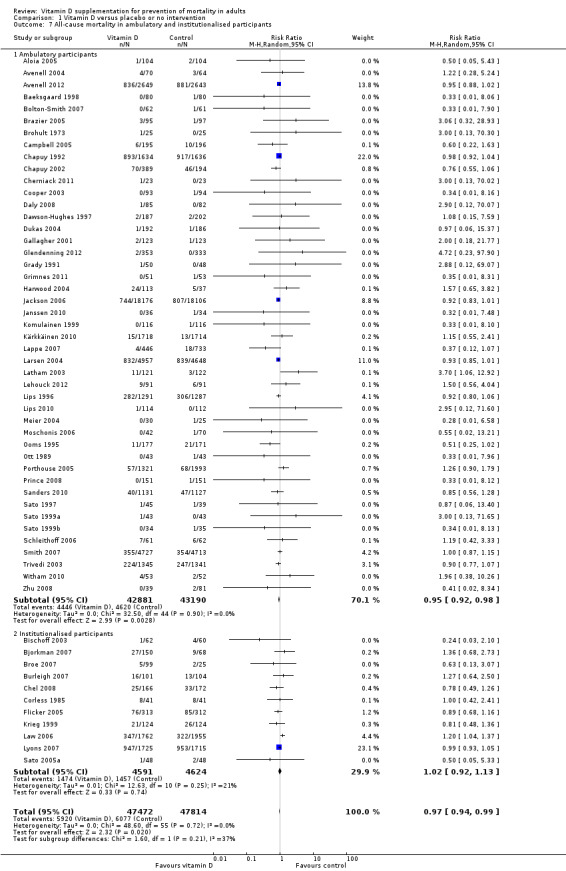
Comparison 1 Vitamin D versus placebo or no intervention, Outcome 7 All‐cause mortality in ambulatory and institutionalised participants.
Sensitivity analyses taking attrition into consideration
Of the 56 trials reporting mortality, 53 trials reported the exact numbers of participants with missing outcomes in the intervention and control groups. Two trials did not report losses to follow‐up (Larsen 2004; Sato 1997), and one trial did not report losses to follow‐up for the intervention groups separately (Lappe 2007). A total of 3634 of 42,024 participants (8.6%) had missing outcomes in the vitamin D group versus 3523 of 42,394 participants (8.3%) in the control group.
'Best‐worst case' scenario
If we assume that all participants lost to follow‐up in the experimental intervention group survived and all those with missing outcomes in the control intervention group died, vitamin D significantly decreased mortality (RR 0.40 (95% CI 0.32 to 0.51); P < 0.00001; I2 = 96%; 84,418 participants; 53 trials; Analysis 1.8).
1.8. Analysis.
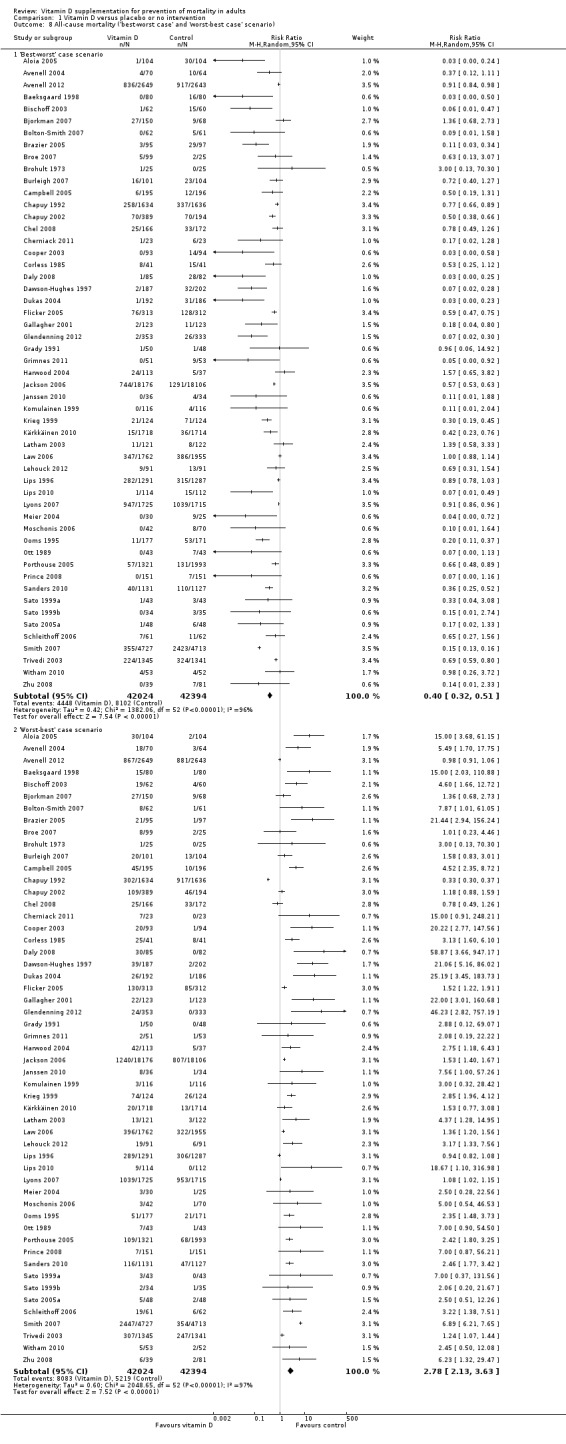
Comparison 1 Vitamin D versus placebo or no intervention, Outcome 8 All‐cause mortality ('best‐worst case' and 'worst‐best case' scenario).
'Worst‐best case' scenario
If we assume that all participants lost to follow‐up in the experimental intervention group died and all those lost to follow‐up in the control intervention group survived, vitamin D significantly increased mortality (RR 2.78 (95% CI 2.13 to 3.63); P < 0.00001; I2 = 97%; 84,418 participants; 53 trials; Analysis 1.8).
Sensitivity analyses taking zero event trials into account
In addition to the 56 trials reporting mortality, 62 trials with 10,804 participants had zero mortality in both experimental and control groups. We assessed the influence of these trials by recalculating the RR with 0.5, 0.01 and 0.001 as empirical continuity corrections. The random‐effects model RR for the three continuity corrections was not noticeably influenced (RR 0.97 (95% CI 0.94 to 0.99); P = 0.020; RR 0.97 (95% CI 0.94 to 1.00); P = 0.022; RR 0.97 (95% CI 0.94 to 1.00); P = 0.023; respectively). We also tested the influence of zero event trials using risk difference as the measure of association. Vitamin D significantly decreased all‐cause mortality using the fixed‐effect model meta‐analysis (RD ‐0.004 (95% CI ‐0.016 to ‐0.008); P = 0.015). Heterogeneity was substantial (I2 = 64%). The random‐effects model revealed no statistically significant effect of vitamin D on all‐cause mortality (RD ‐0.002 (95% CI ‐0.005 to 0.002); P = 0.30).
Vitamin D3 (cholecalciferol)
Vitamin D3 was tested in 38 trials (75,927 participants). Inspection of the funnel plot did not suggest potential bias (asymmetry) (Figure w8, http://ctu.dk/publications/supplementary‐material.aspx). The adjusted‐rank correlation test (P = 0.79) and the regression asymmetry test (P = 0.97) found no statistically significant evidence of bias. Overall, vitamin D3 significantly decreased mortality (RR 0.94 (95% CI 0.91 to 0.98); P = 0.002; I2 = 0; 75,927 participants; 38 trials; Analysis 1.9). Vitamin D3 significantly decreased mortality in the trials with low risk of bias (RR 0.93 (95% CI 0.89 to 0.98); P = 0.009; I2 = 0%; 52,645 participants; 20 trials; Analysis 1.9). Vitamin D3 had no statistically significant effect on mortality in the trials with high risk of bias (RR 0.95 (95% CI 0.91 to 1.00); P = 0.06; I2 = 0%; 23,282 participants; 18 trials; Analysis 1.7.2). The difference between estimates of the effect of vitamin D3 on mortality in the trials with low risk of bias and the trials with high risk of bias was not statistically significant by the test of interaction (Chi2 = 0.39; P = 0.53; Analysis 1.9).
1.9. Analysis.
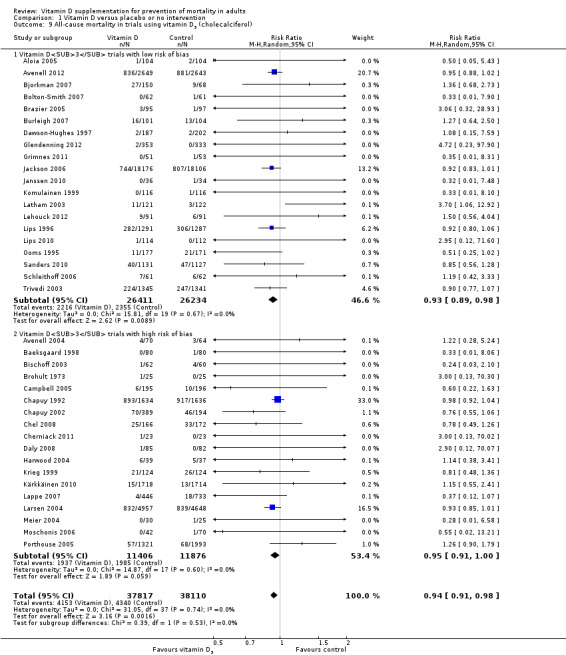
Comparison 1 Vitamin D versus placebo or no intervention, Outcome 9 All‐cause mortality in trials using vitamin D3 (cholecalciferol).
Trial sequential analysis of all 38 vitamin D3 trials was constructed on the basis of diversity‐adjusted required information size calculated using mortality of 10% in the control group, a relative risk reduction of 5% with vitamin D3, a type I error of 5% and a type II error of 20% (80% power). No diversity was noted. The trial sequential analysis showed that the required information size had not yet been reached and that the cumulative Z‐curve crossed the trial sequential monitoring boundary for benefit in 2006 during the 22nd trial. The trial sequential analysis excludes risk of random errors (Figure 4). The intervention effect corresponds to the number needed to treat for an additional beneficial outcome (NNTB) of 150 participants treated over five years to save one additional life.
4.
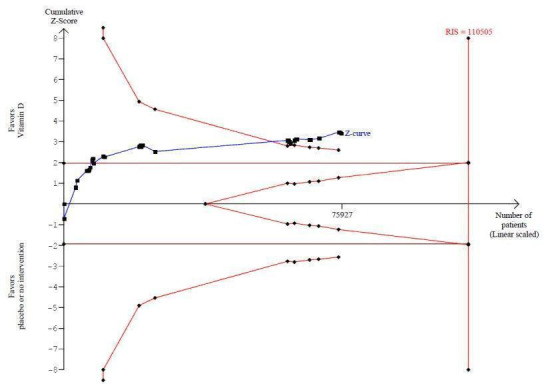
Trial sequential analysis on mortality in 38 vitamin D3 trials The diversity‐adjusted required information size (RIS) was calculated based on mortality in the control group of 10%; relative risk reduction of 5% in the experimental group; type I error of 5%; and type II error of 20% (80% power). No diversity was noted. The required information size was 110,505 participants. The cumulative Z‐curve (blue line) crossed the trial sequential monitoring boundaries for benefit (red inward sloping line) after the 22nd trial. Accordingly, the risk of random error in the finding seems acceptable according to the O'Brien Fleming stopping rule for an individual trial interim analysis. Subsequently, 16 trials have been published.
Vitamin D3 and calcium
Vitamin D3 administered singly versus placebo or no intervention had no statistically significant effect on mortality (RR 0.92 (95% CI 0.85 to 1.00); P = 0.06; I2 = 5%; 12,609 participants; 13 trials; Analysis 1.10). Vitamin D3 combined with calcium versus placebo or no intervention significantly decreased mortality (RR 0.96 (95% CI 0.92 to 0.99); P = 0.03; I2 = 0%; 63,051 participants; 27 trials; Analysis 1.10). The difference between the estimate of the effect of vitamin D3 on mortality in the trials using vitamin D3 singly and the trials using vitamin D3 combined with calcium was not statistically significant by the test of interaction (Chi2 = 0.49; P = 0.49; Analysis 1.10).
1.10. Analysis.
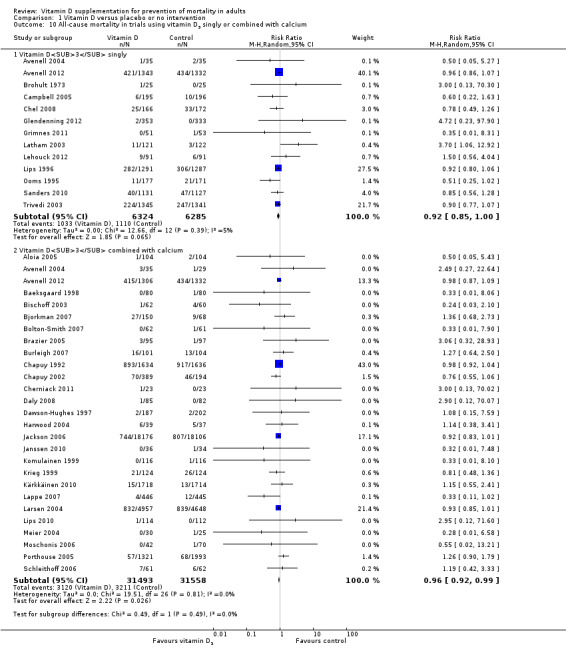
Comparison 1 Vitamin D versus placebo or no intervention, Outcome 10 All‐cause mortality in trials using vitamin D3 singly or combined with calcium.
The trial sequential analysis on mortality in the 27 trials that administered vitamin D3 combined with calcium showed that the cumulative Z‐curve did not cross the trial sequential monitoring boundary for benefit (Figure w9, http://ctu.dk/publications/supplementary‐material.aspx).
Dose of vitamin D3
A dose of vitamin D3 less than 800 IU a day significantly decreased mortality (RR 0.92 (95% CI 0.87 to 0.97); P = 0.005; I2 = 0%; 50,437 participants; 13 trials; Analysis 1.11). A dose of vitamin D3 equal to or greater than 800 IU a day had no statistically significant effect on mortality (RR 0.96 (95% CI 0.92 to 1.00); P = 0.07; I2 = 0%; 25,558 participants; 26 trials; Analysis 1.11). The difference between the estimate of the effect of vitamin D3 on mortality in the trials using a low dose of vitamin D3 and the trials using a high dose of vitamin D3 was not statistically significant by the test of interaction (Chi2 = 1.37; P = 0.24; Analysis 1.11).
1.11. Analysis.
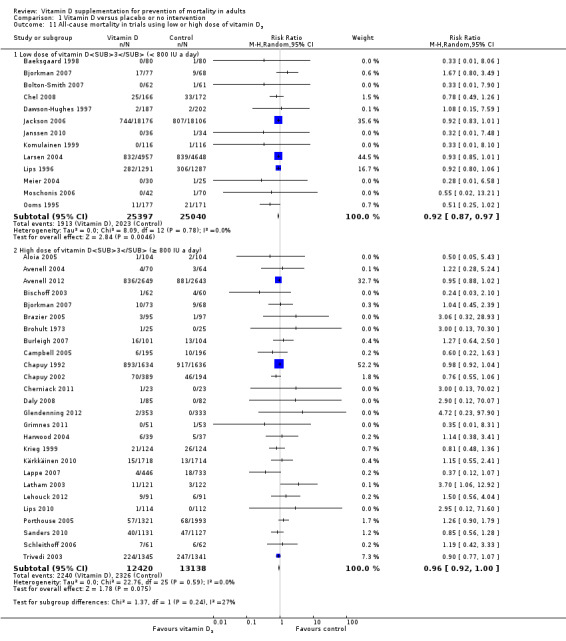
Comparison 1 Vitamin D versus placebo or no intervention, Outcome 11 All‐cause mortality in trials using low or high dose of vitamin D3.
The trial sequential analysis on mortality in the 13 trials that administered a low dose of vitamin D3 showed that the cumulative Z‐curve did not cross the trial sequential monitoring boundary for benefit (Figure 5).
5.
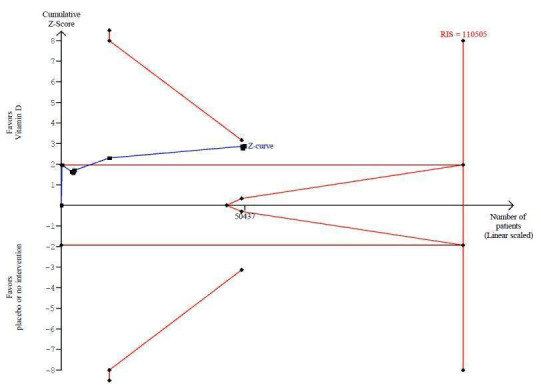
Trial sequential analysis on mortality in the 13 trials that administered low dose of vitamin D3 (i.e. a dose less than 800 IU per day) The diversity‐adjusted required information size (RIS) was calculated based on mortality in the control group of 10%; relative risk reduction of 5% in the experimental group; type I error of 5%; and type II error of 20% (80% power). No diversity was noted. The required information size was 110,505 participants. The cumulative Z‐curve (blue line) did not cross the trial sequential monitoring boundaries for benefit (red line) at any time. Accordingly, the crossing of the conventional statistical 5% boundary (the horizontal brown line) may be due to random errors.
Dosing schedule of vitamin D3
Vitamin D3 administered daily significantly decreased mortality (RR 0.95 (95% CI 0.91 to 0.98); P = 0.004; I2 = 0%; 69,168 participants; 31 trials; Analysis 1.12). Vitamin D3 administered intermittently had no statistically significant effect on mortality (RR 0.89 (95% CI 0.77 to 1.03); P = 0.11; I2 = 0%; 6871 participants; 8 trials; Analysis 1.12). The difference between the estimate of the effect of vitamin D3 on mortality in the trials that administered vitamin D3 daily and the trials that administered vitamin D3 intermittently was not statistically significant by the test of interaction (Chi2 = 0.66; P = 0.41; Analysis 1.12).
1.12. Analysis.
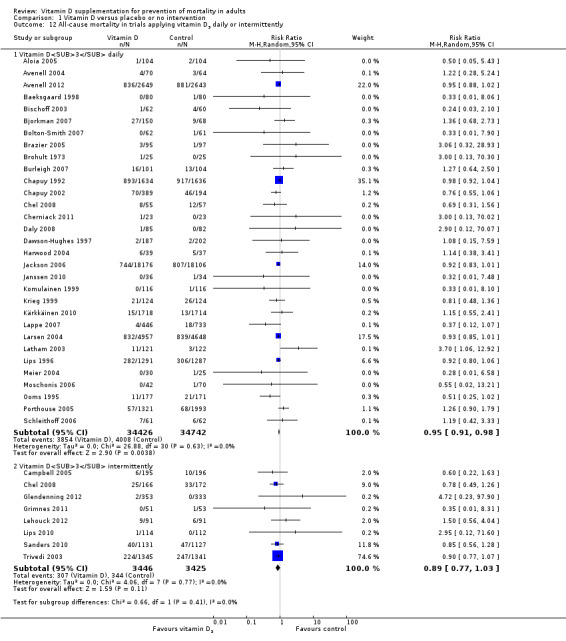
Comparison 1 Vitamin D versus placebo or no intervention, Outcome 12 All‐cause mortality in trials applying vitamin D3 daily or intermittently.
Intervention effect of vitamin D3 according to vitamin D status at entry
Vitamin D3 significantly decreased mortality in the trials including participants with vitamin D insufficiency (RR 0.95 (95% CI 0.91 to 0.99); P = 0.009; I2 = 0%; 55,883 participants; 20 trials; Analysis 1.13). Vitamin D3 had no statistically significant effect on mortality in the trials including participants with vitamin D adequacy (RR 0.92 (95% CI 0.80 to 1.07); P = 0.29; I2 = 0%; 4979 participants; 10 trials; Analysis 1.13). The difference between the estimate of the effect of vitamin D3 on mortality in the trials including participants with vitamin D insufficiency and the trials including participants with vitamin D adequacy was not statistically significant by the test of interaction (Chi2= 0.1; P = 0.75; Analysis 1.13).
1.13. Analysis.
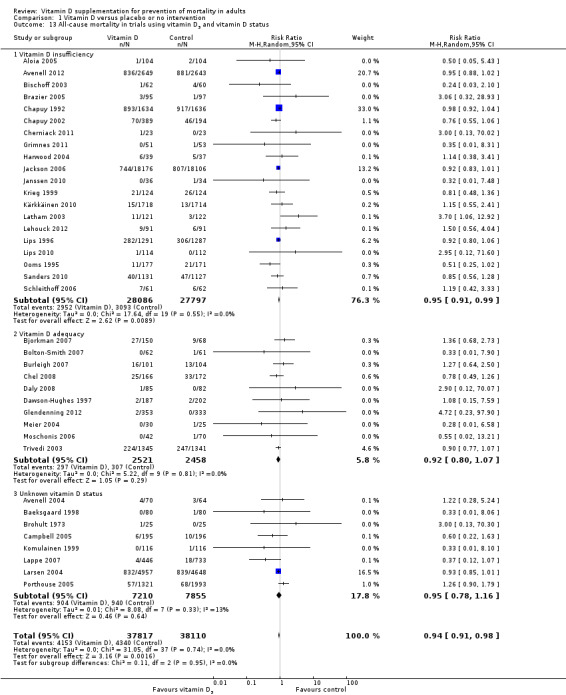
Comparison 1 Vitamin D versus placebo or no intervention, Outcome 13 All‐cause mortality in trials using vitamin D3 and vitamin D status.
Intervention effect of vitamin D3 according to the sex of the trial participants
Vitamin D3 had no statistically significant effect on mortality in the trials that exclusively included women (RR 0.93 (95% CI 0.84 to 1.03); P = 0.16; I2 = 22%; 53,062 participants; 19 trials; Analysis 1.14). Vitamin D3 significantly decreased mortality in the trials including both men and women, or including only men (one trial by Daly 2008) (RR 0.94 (95% CI 0.89 to 0.99); P = 0.01; I2 = 0%; 22,865 participants; 19 trials; Analysis 1.14). The difference between the estimate of the effect of vitamin D3 on mortality in the trials including only women and the trials including both men and women or only men was not statistically significant by the test of interaction (Chi2 = 0.03; P = 0.87; Analysis 1.14).
1.14. Analysis.
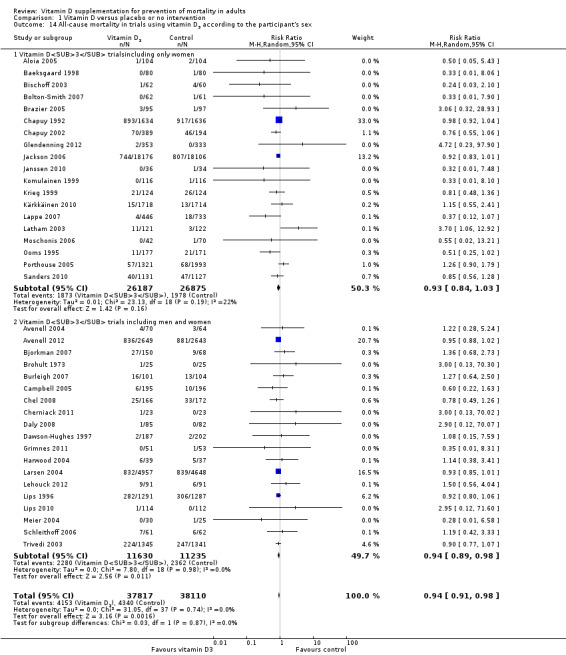
Comparison 1 Vitamin D versus placebo or no intervention, Outcome 14 All‐cause mortality in trials using vitamin D3 according to the participant's sex.
Vitamin D2 (ergocalciferol)
Vitamin D2 was tested in 12 trials (18,349 participants). Inspection of the funnel plot did not suggest potential bias (asymmetry) (Figure w10, http://ctu.dk/publications/supplementary‐material.aspx). The adjusted‐rank correlation test (P = 0.60) and the regression asymmetry test (P = 0.55) found no statistically significant evidence of bias. Overall, vitamin D2 had no statistically significant effect on mortality (RR 1.02 (95% CI 0.96 to 1.08); P = 0.54; I2 = 4%; Analysis 1.15). Vitamin D2 had no statistically significant effect on mortality in the trials with low risk of bias (RR 0.98 (95% CI 0.93 to 1.04); P = 0.57; I2 = 0%; 14,439 participants; 9 trials; Analysis 1.15). Vitamin D2 significantly increased mortality in the trials with high risk of bias (RR 1.20 (95% CI 1.05 to 1.37); P = 0.007; I2 = 0%; 3910 participants; 3 trials; Analysis 1.15). The difference between the estimate of effect of vitamin D2 on mortality in the trials with low risk of bias and the trials with high risk of bias was statistically significant by the test of interaction (Chi2 = 7.28; P = 0.007; Analysis 1.15).
1.15. Analysis.
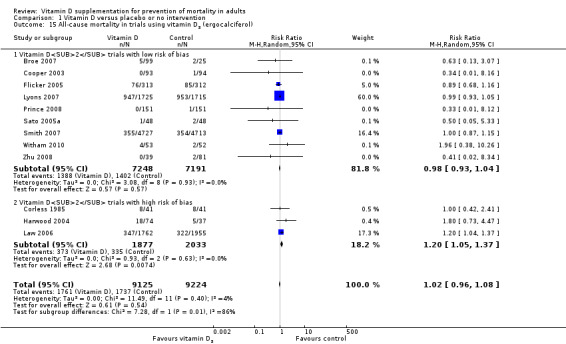
Comparison 1 Vitamin D versus placebo or no intervention, Outcome 15 All‐cause mortality in trials using vitamin D2 (ergocalciferol).
The trial sequential analysis of all vitamin D2 trials suggests that we reached the futility area after the eighth trial, allowing us to conclude that any possible intervention effect, if present, is lower than a 5% relative risk reduction, or that the number needed to treat for an additional beneficial outcome (NNTB) is greater than 150 (Figure 6).
6.
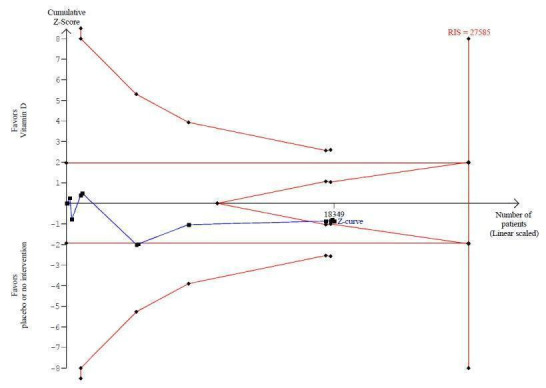
Trial sequential analysis of mortality in 12 vitamin D2 trials The diversity‐adjusted required information size (RIS) was conducted based on 10% mortality in the control group; relative risk reduction of 10% in the experimental group; type I error of 5%; and type II error of 20% (80% power). No diversity was noted. The required information size was 27,585 participants. The cumulative Z‐curve (blue line) crossed the trial sequential monitoring boundaries for futility (red outward sloping line) after the eighth trial.
Vitamin D2 and calcium
Vitamin D2 administered singly had no statistically significant effect on mortality (RR 1.03 (95% CI 0.96 to 1.12); P = 0.37; I2 = 14%; 17,079 participants; 8 trials; Analysis 1.16). Vitamin D2 combined with calcium had no statistically significant effect on mortality (RR 1.00 (95% CI 0.64 to 1.57); P = 1.00; I2 = 11%; 1307 participants; 5 trials; Analysis 1.16). The difference between the estimates of effect of vitamin D2 on mortality in the trials using vitamin D2 singly and the trials using vitamin D2 combined with calcium was not statistically significant by the test of interaction (Chi2 = 0.02; P = 0.88; Analysis 1.16).
1.16. Analysis.
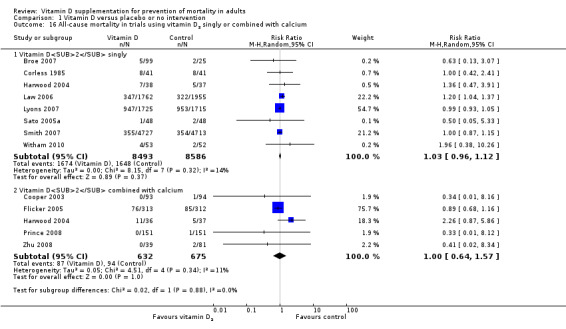
Comparison 1 Vitamin D versus placebo or no intervention, Outcome 16 All‐cause mortality in trials using vitamin D2 singly or combined with calcium.
Dose of vitamin D2
A dose of vitamin D2 less than 800 IU a day, tested in one trial, had no statistically significant effect on mortality (RR 0.82 (95% CI 0.17 to 3.98); P = 0.81; 101 participants; Analysis 1.17). A dose of vitamin D2 equal to or greater than 800 IU a day had no statistically significant effect on mortality (RR 1.02 (95% CI 0.95 to 1.10); P = 0.51; I2 = 9%; 18,273 participants; 12 trials; Analysis 1.17). The difference between the estimate of effect of vitamin D2 on mortality in the trials using a high dose of vitamin D2 and the trial using low‐dose vitamin D2 was not statistically significant by the test of interaction (Chi2 = 0.07; P = 0.79; Analysis 1.17).
1.17. Analysis.
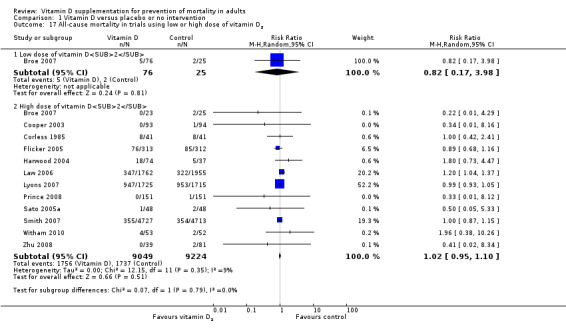
Comparison 1 Vitamin D versus placebo or no intervention, Outcome 17 All‐cause mortality in trials using low or high dose of vitamin D2.
Dosing schedule of vitamin D2
Vitamin D2 administered daily had no statistically significant effect on mortality (RR 0.88 (95% CI 0.68 to 1.12); P = 0.30; I2 = 0%; 1349 participants; 6 trials; Analysis 1.18). Vitamin D2 administered intermittently had no statistically significant effect on mortality (RR 1.06 (95% CI 0.95 to 1.18); P = 0.33; I2 = 46%; 17,000 participants; 6 trials; Analysis 1.18). The difference between the estimates of effect of vitamin D2 on mortality in the trials that administered vitamin D2 daily and the trials that administered vitamin D2 intermittently was not statistically significant by the test of interaction (Chi2 = 1.81; P = 0.18; Analysis 1.18).
1.18. Analysis.
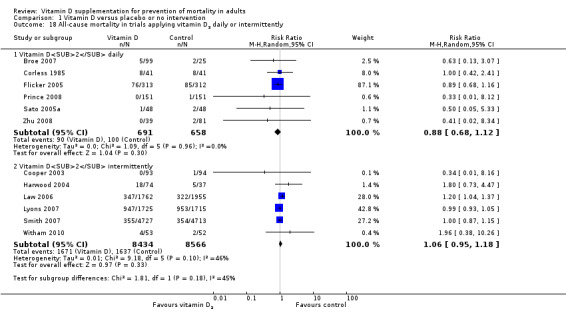
Comparison 1 Vitamin D versus placebo or no intervention, Outcome 18 All‐cause mortality in trials applying vitamin D2 daily or intermittently.
Intervention effect of vitamin D2 according to vitamin D status
Vitamin D2 significantly increased mortality in the trials including participants with vitamin D insufficiency (RR 1.20 (95% CI 1.05 to 1.37); P = 0.008; I2 = 0%; 4413 participants; 6 trials; Analysis 1.19). Vitamin D2 had no statistically significant effect on mortality in the trials including participants with vitamin D adequacy (RR 0.97 (95% CI 0.86 to 1.10); P = 0.62; I2 = 0%; 10,496 participants; 5 trials; Analysis 1.19). The difference between the estimates of effect of vitamin D2 on mortality in the trials including participants with vitamin D insufficiency and the trials including participants with vitamin D adequacy was statistically significant by the test of interaction (Chi2 = 5.23; P = 0.02; Analysis 1.19).
1.19. Analysis.
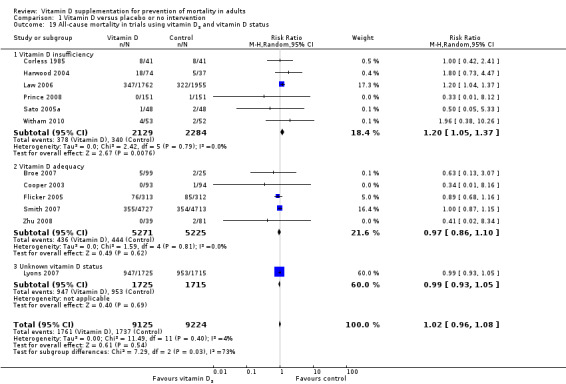
Comparison 1 Vitamin D versus placebo or no intervention, Outcome 19 All‐cause mortality in trials using vitamin D2 and vitamin D status.
Alfacalcidol (1α‐hydroxyvitamin D)
Alfacalcidol was tested in four trials (617 participants). Inspection of the funnel plot did not suggest potential bias (asymmetry) (Figure w11, http://ctu.dk/publications/supplementary‐material.aspx). The adjusted‐rank correlation test (P = 1.00) found no significant evidence of bias. Alfacalcidol had no statistically significant effect on mortality (RR 0.96 (95% CI 0.22 to 4.15); P = 0.95; I2 = 0%; Analysis 1.20). The effect of alfacalcidol on mortality was not dependent on vitamin D status (Analysis 1.21).
1.20. Analysis.
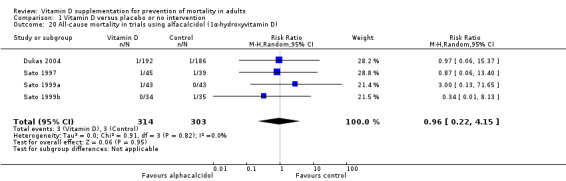
Comparison 1 Vitamin D versus placebo or no intervention, Outcome 20 All‐cause mortality in trials using alfacalcidol (1α‐hydroxyvitamin D).
1.21. Analysis.
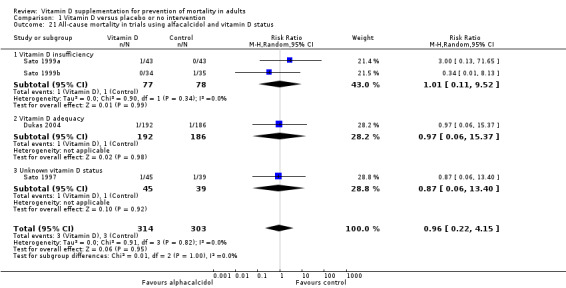
Comparison 1 Vitamin D versus placebo or no intervention, Outcome 21 All‐cause mortality in trials using alfacalcidol and vitamin D status.
Calcitriol (1,25‐dihydroxyvitamin D)
Calcitriol was tested in three trials (430 participants). Inspection of the funnel plot did not suggest potential bias (asymmetry) (Figure w12, http://ctu.dk/publications/supplementary‐material.aspx). Calcitriol had no statistically significant effect on mortality (RR 1.37 (95% CI 0.27 to 7.03); P = 0.71; I2 = 0%; Analysis 1.22). The effect of calcitriol on mortality was not dependent on vitamin D status (Analysis 1.23).
1.22. Analysis.
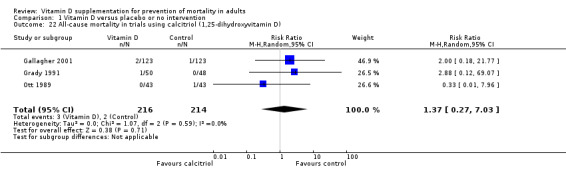
Comparison 1 Vitamin D versus placebo or no intervention, Outcome 22 All‐cause mortality in trials using calcitriol (1,25‐dihydroxyvitamin D).
1.23. Analysis.
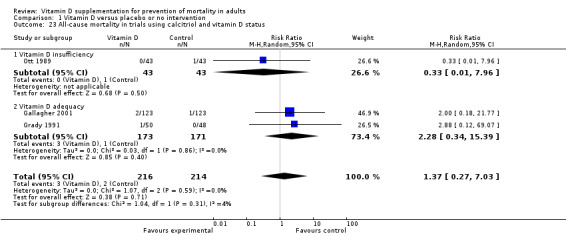
Comparison 1 Vitamin D versus placebo or no intervention, Outcome 23 All‐cause mortality in trials using calcitriol and vitamin D status.
Cause‐specific mortality
Vitamin D3 statistically significantly decreased cancer mortality (RR 0.88 (95% CI 0.78 to 0.98); P = 0.02; I2 = 0%; 44,492 participants; 4 trials; Analysis 1.24).
1.24. Analysis.
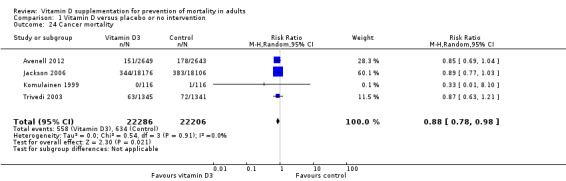
Comparison 1 Vitamin D versus placebo or no intervention, Outcome 24 Cancer mortality.
Trial sequential analysis on cancer mortality in the four trials that administered vitamin D3 was performed on the basis of mortality in the control group of 2.85%; relative risk reduction (based on trials with low risk of bias) of 12.28% in the experimental group; type I error of 5%; and type II error of 20% (80% power). No diversity was noted. The required information size was 66,724 participants. The cumulative Z‐curve (blue line) did not cross the trial sequential monitoring boundary for benefit (red line) (Figure w13, http://ctu.dk/publications/supplementary‐material.aspx).
Vitamin D3 had no significant effect on cardiovascular mortality (RR 0.98 (95% CI 0.90 to 1.07); P = 0.68; I2 = 0%; 47,267 participants; 10 trials; Analysis 1.25).
1.25. Analysis.
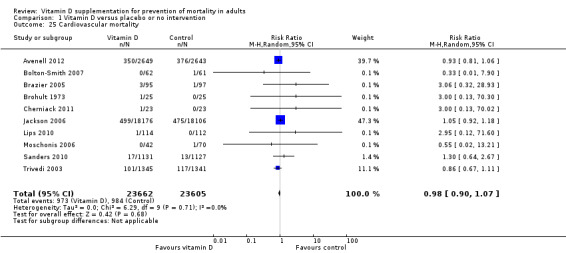
Comparison 1 Vitamin D versus placebo or no intervention, Outcome 25 Cardiovascular mortality.
The trial sequential analysis on cardiovascular mortality in the 10 trials that administered vitamin D3 was performed on the basis of mortality in the control group of 4.17%; relative risk reduction (based on trials with low risk of bias) of 1.68% in the experimental group; type I error of 5%; and type II error of 20% (80% power). No diversity was noted. The required information size was 2,539,845 participants. The cumulative Z‐curve (blue line) did not cross the conventional monitoring boundary for benefit (red line) (Figure w14, http://ctu.dk/publications/supplementary‐material.aspx).
We were not able to extract from the included trials relevant data on fracture‐related mortality and other causes of mortality.
Adverse events
Several adverse events were reported (e.g. hypercalcaemia, nephrolithiasis, hypercalciuria, renal insufficiency, gastrointestinal disorders, cardiovascular disorders, psychiatric disorders, skin disorders, cancer).
The supplemental forms of vitamin D (D3 and D2) had no statistically significant effect on the risk of hypercalcaemia (RR 1.36 (95% CI 0.85 to 2.18); P = 0.21; I2 = 0%; 11,323 participants; 15 trials; Analysis 1.26).
1.26. Analysis.
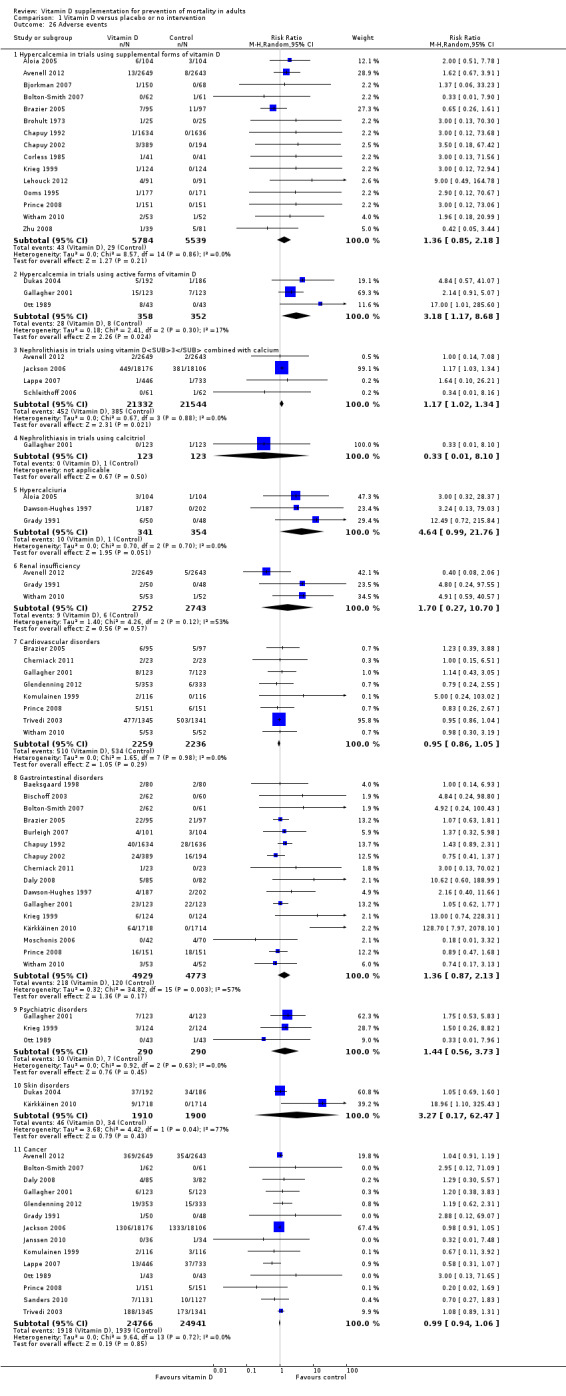
Comparison 1 Vitamin D versus placebo or no intervention, Outcome 26 Adverse events.
The active forms of vitamin D (alfacalcidol and calcitriol) statistically significantly increased the risk of hypercalcaemia (RR 3.18 (95% CI 1.17 to 8.68); P = 0.02; I2 = 17%; 710 participants; 3 trials; Analysis 1.26). The difference between the estimate of effect of vitamin D on hypercalcaemia in the trials that administered supplemental forms of vitamin D (D3 and D2) and the trials that administered active forms of vitamin D (alfacalcidol or calcitriol) was not statistically significant by the test of interaction (Chi2 = 2.27; P = 0.13; Analysis 1.26).
Vitamin D3 combined with calcium significantly increased nephrolithiasis (RR 1.17 (95% CI 1.02 to 1.34); P = 0.02; I2 = 0%; 42,876 participants; 4 trials; Analysis 1.26).
The effect of vitamin D on the other adverse events was not statistically significant (hypercalciuria: RR 4.64 (95% CI 0.99 to 21.76; P = 0.05; I2 = 0%; 695 participants; 3 trials; Analysis 1.26 renal insufficiency: RR 1.70 (95% CI 0.27 to 10.70); P = 0.57; I2 = 53%; 5495 participants; 3 trials; Analysis 1.26; cardiovascular disorders: RR 0.95 (95% CI 0.86 to 1.05); P = 0.29; I2 = 0%; 4495 participants; 8 trials; Analysis 1.26; gastrointestinal disorders: RR 1.36 (95% CI 0.87 to 2.13); P = 0.17; I2 = 57%; 9702 participants; 16 trials; Analysis 1.26; psychiatric disorders: RR 1.44 (95% CI 0.56 to 3.73); P = 0.45; I2 = 0%; 580 participants; 3 trials; Analysis 1.26; skin disorders: RR 3.27 (95% CI 0.17 to 62.47); P = 0.43; I2 = 77%; 3810 participants; 2 trials; Analysis 1.26; cancer: RR 0.99 (95% CI 0.94 to 1.06); P = 0.85; I2 = 0%; 49,707 participants; 14 trials; Analysis 1.26).
Health‐related quality of life
Only one trial published data on health‐related quality of life (Witham 2010). Authors reported significant worsening in disease‐specific quality of life (MLWHF, Minnesota Living With Heart Failure score) in the vitamin D2 group compared with the placebo group (Witham 2010). The between‐group difference at 20 weeks was 5.3 (0.5 to 10.2), and the minimally important difference (MID) was estimated to be 5 points in either direction.
Health economics
We found only one randomised clinical trial (Chapuy 1992) that reported a cost‐effectiveness analysis (Lilliu 2003). The authors found that vitamin D3 and calcium supplementation prevented 46 hip fractures in every 1000 women treated and concluded that vitamin D3 with calcium supplementation is cost‐effective (Lilliu 2003). Mortality was not addressed.
Discussion
Summary of main results
Our systematic review contains a number of important findings. We found evidence suggesting that vitamin D3 may significantly benefit survival of elderly ambulatory participants living in institutional care who were likely to be vitamin D deficient with significant risk of falls and fractures, when we disregard the risks of attrition bias and outcome reporting bias. However, if these bias risks are considered, we do not yet know whether vitamin D3 affects mortality. Vitamin D2, alfacalcidol and calcitriol had no statistically significant effect on mortality, but these estimates are at risk of type II errors because of the fact that much smaller groups of participants were examined compared with the trials assessing vitamin D3.
A subgroup analysis of trials with high risk of bias suggests that vitamin D2 may increase mortality, but a trial sequential analysis opens the possibility that this could be a random error. Alfacalcidol and calcitriol significantly increased the risk of hypercalcaemia, and vitamin D3 combined with calcium significantly increased nephrolithiasis. Vitamin D had no clear effect on other adverse events, including cancer.
Compared with our previous version of this systematic review (Bjelakovic 2011), the number of included trials in the present review has increased, with six new trials (12%) adding another 1,138 participants (1.2%). In addition, we have obtained updated results of a longer follow‐up from one large‐scale randomised trial (Avenell 2012). In spite of these additional amounts of information, our results remain largely the same, but our assessment of the robustness of our findings has weakened.
Overall completeness and applicability of evidence
Our published protocol described our plan to analyse the effect of vitamin D on mortality in primary and secondary prevention randomised clinical trials in adults. All eligible randomised clinical trials up to February 2012 were included. All trials were conducted in high‐income countries. Both sexes were included. Most of the participants were elderly persons, They were living alone or were living in institutions. A vast majority of the participants came from primary prevention trials, and we assume that they were apparently healthy when included in the trials. Few trials with very few participants were included in the secondary prevention trials, so our ability to say anything about such patients is week to absent. We included randomised trials with both vitamin D–deficient participants and persons who seemed to have adequate vitamin D levels at entry. We were unable to detect significant differences regarding these variables on the estimated intervention effect on mortality. Surprisingly little heterogeneity was found in all of our analyses. Most trials assessed vitamin D3, and our major conclusions are related to this intervention. Although more than half of the trials were considered of low risk of bias, our analyses revealed that outcome reporting on more than 8% of participants was lacking. This number is too high when mortality is about 12% to 13% in the placebo or no intervention group. Accordingly, our 'best‐worst case' and 'worst‐best case' analyses revealed that our results were compatible with both a very large beneficial effect and a very large detrimental effect of vitamin D3 on mortality. Although these extreme sensitivity analyses are unlikely, they reveal how few unaccounted for patients should have died to substantially change our findings of modest benefit into nil effect or maybe even harm. Therefore, we warn against uncritical application of our findings.
Quality of the evidence
Our review follows the overall plan of a published, peer‐reviewed Cochrane protocol (Bjelakovic 2008a). It represents a comprehensive review of the topic, including 159 randomised trials with more than 105,000 participants. A total of 56 trials including more than 94,000 participants reported on mortality. This increases the precision and power of our analyses (Higgins 2011). Previous meta‐analyses of preventive trials of vitamin D supplements have included substantially less information and have not examined the separate influence of different forms of vitamin D on mortality. We conducted a thorough review in accordance with The Cochrane Collaboration methodology (Higgins 2011) while implementing findings of methodological studies (Kjaergard 2001; Lundh 2012; Moher 1998; Savovic 2012; Schulz 1995; Wood 2008). Between‐trial heterogeneity is almost absent in our meta‐analyses. This may emphasise the consistency of our findings but should also raise concern (Ioannidis 2006). Furthermore, all‐cause mortality should generally be connected with unbiased estimates (Savovic 2012; Wood 2008). We also performed trial sequential analyses to control the risk of random errors in a cumulative meta‐analysis and to prevent premature statements of superiority of vitamin D based on estimation of the diversity‐adjusted required information size (Brok 2008; Brok 2009; Thorlund 2009; Thorlund 2011a; Thorlund 2011b; Wetterslev 2008; Wetterslev 2009).
A major drawback in most of the included trials is the relatively large proportion of more than 8% of participants who dropped out. This opens up for attrition bias, and our 'best‐worst' and 'worst‐best' intention‐to‐treat analyses demonstrate that the intervention effect of vitamin D may be either beneficial or harmful. Although both of the two extreme scenarios are unlikely, they demonstrate that we cannot depend fully on the estimates we arrive at. The percentage of participants lost to follow‐up in both experimental and control groups was about 8.5%. Our 'best‐worst case' and 'worst‐best case' scenario analyses revealed much more extreme confidence limits (95% CI 0.32 to 3.63) compared with our 'complete‐case' scenario analysis (95% CI 0.93 to 0.99), and they convey a message of a noticeable degree of uncertainty regarding our results. This observation calls for more comprehensive meta‐analyses of individual participant data plus further large randomised clinical trials. We have abstained from conducting 'uncertainty' analyses (Gamble 2005). The latter analyses accept the point estimate from the complete‐participant analysis, assuming that the distribution of deaths among the participants lost to follow‐up is equal to the distribution of deaths among all participants. But the distribution of dead participants among the lost to follow‐up participants may indeed be different from the distribution of dead participants among participants actually followed through the whole observation period, making the 'uncertainty' analyses themselves uncertain.
We conducted a number of subgroup analyses. We observed no statistically significant different effects of the intervention effect of vitamin D on mortality in subgroup analyses of trials with low risk of bias compared with trials with high risk of bias; of trials using placebo compared with trials using no intervention in the control group; of trials with no risk of industry bias compared with trials with risk of industry bias; of trials assessing primary prevention compared with trials assessing secondary prevention; of trials including participants with vitamin D level below 20 mg/mL at entry compared with trials including participants with normal vitamin D levels at entry; of trials including ambulatory participants compared with trials including institutionalised participants; of vitamin D3 trials using concomitant calcium supplementation compared with vitamin D3 trials without calcium; of trials using a dose of vitamin D3 less than 800 IU per day compared with trials using doses greater than 800 IU per day; of vitamin D3 trials including only women compared with vitamin D3 trials including both sexes or only men.
In addition to the 56 trials reporting mortality, 62 trials with 10,804 participants had zero mortality in both the experimental and control groups. These trials were mostly phase I and phase II randomised clinical trials assessing the effects of short‐term vitamin D administration on surrogate outcomes. These trials were excluded from the meta‐analyses by using RR as the association measure. We assessed the influence of these trials by recalculating the RR with 0.5, 0.01 and 0.001 as empirical continuity corrections. The random‐effects model RR for the three continuity corrections was not noticeably influenced. We also tested the influence of zero event trials using a risk difference as the measure of association. Vitamin D significantly decreased all‐cause mortality using the fixed‐effect model meta‐analysis. Heterogeneity was substantial. The random‐effects model revealed no statistically significant effect of vitamin D on all‐cause mortality. Accordingly, the decreased mortality could be an artefact created by exclusion of trials with zero events in both intervention groups (Bradburn 2007; Sweeting 2004).
Two trials had other factors that could put them at risk of bias (i.e. recruitment bias) (Larsen 2004; Law 2006). These trials were cluster‐randomised. We explored the association between intervention effects of vitamin D and the subgrouping of individually randomised and cluster‐randomised trials. The influence of cluster‐randomised trials on our results was also explored in sensitivity analyses, which included or excluded them. The difference between the estimate of the effect of vitamin D on mortality in individually randomised compared with cluster‐randomised trials was not statistically significant. Our sensitivity analyses by including or excluding cluster‐randomised trials revealed no noticeable effect on our results.
We conducted trial sequential analyses to control the risk of random errors and to prevent premature statements of superiority of the experimental or control intervention or probably false declarations of absence of effect in the cases for which we had too few data (Thorlund 2011a; Thorlund 2011b; Wetterslev 2008). The finding of significantly decreased mortality with vitamin D3 (cholecalciferol) did not seem to be due to a random error. The cumulative Z‐curve crossed the trial sequential monitoring boundary for benefit after the 22nd trial. However, such an analysis cannot remove risks of bias‐detected or undetected. The trial sequential analysis for vitamin D2 (ergocalciferol) suggests that we reached the futility area after the eighth trial, allowing us to conclude that any possible intervention effect, if present, is lower than a 5% relative risk reduction. One should discuss, however, how much evidence one would require when dealing with potential benefit or harm. On the one hand, beneficial or harmful effects can occur as the result of random errors; therefore, sufficient information needs to be assessed to demonstrate benefit or harm beyond reasonable doubt.
Potential biases in the review process
We repeatedly searched several databases and contacted authors of trials and industry producing vitamin D supplements. Therefore, we believe that we have not overlooked important randomised clinical trials. On the other hand, only about every second trial is reported (Gluud 2008), so we cannot exclude reporting biases, although our funnel plots did not suggest publication bias. On the positive side, we managed to obtain much more information on a number of trials from this update. However, this does not detract from the fact that we did not have access to individual participant data. Accordingly, we have no chance of analysing the effect of vitamin D in only women or in only men. When we separate trials with only women from trials with men and women combined, we see no significant difference in the intervention effect of vitamin D.
We selected all trials and extracted all data in duplicate, and we reached a high level of agreement. We did not conduct the quality assessments or data extractions blinded for authors and bias risks.
In this review update, we have now presented a more conservative and, we believe, a more correct interpretation of our findings compared with interpretations in the first version of this review.
Agreements and disagreements with other studies or reviews
In our present systematic review, we found no significant effects of bias on our estimates of intervention of vitamin D in general or of vitamin D3 specifically.
On the other hand, most of the trials were conducted with some type of support from the industry, and in general, the risk of potential industry bias was poorly described or accounted for. However, the difference in the estimates of vitamin D effect on mortality in the trials sponsored by industry compared with trials that were not sponsored by industry was not statistically significant. Accordingly, we could not confirm results from a recently published Cochrane review (Lundh 2012), which found that sponsorship of a trial by the manufacturing company leads to more favourable results and conclusions compared with trials having no sponsors.
No difference in the estimates of vitamin D effect on mortality was evident in the primary and secondary prevention trials. The number of trials with secondary prevention was low, and these trials included very few participants. Our findings may seem to contrast with earlier claims in the literature that vitamin D might be beneficial for patients with cardiovascular, malignant, infectious or autoimmune diseases (Holick 2007a; Rosen 2011; Souberbielle 2010). Assessment of vitamin D supplementation for participant groups with active disease was outside the scope of the present systematic review.
We found no statistically significant difference regarding the effect of vitamin D on mortality in trials including participants with vitamin D insufficiency (25‐hydroxyvitamin D level less than 20 ng/mL) compared with trials including participants with optimal vitamin D status. The optimal vitamin D status, reached by using the blood level of 25‐hydroxyvitamin D that maximally suppresses serum parathyroid hormone, varies widely (8 ng/mL to 44 ng/mL) (Dawson‐Hughes 2005; Lips 2004; Vieth 2006). The level of 25‐hydroxyvitamin D in the blood also depends much on the laboratory methods used for assessment of vitamin D concentration (Binkley 2009; Holick 2009; Lips 1999). Many external factors (latitude, season, time of the day, air pollution) and internal factors (skin colour, age, clothing, use of sunscreen) influence the cutaneous synthesis of vitamin D, and consequently the 25‐hydroxyvitamin D levels (Webb 2006). According to a recent report of the Institute of Medicine (IOM 2011), a serum 25‐hydroxyvitamin D level of 20 ng/mL (50 nmol/L) meets the vitamin D requirements of at least 97.5% of the population. Our results do not support earlier claims that participants with insufficient vitamin D status may benefit from vitamin D supplementation (Bischoff‐Ferrari 2009c; Holick 2008a; Zittermann 2009a).
No difference was noted in the estimates of vitamin D effect on mortality in trials including ambulatory participants compared with trials including institutionalised participants. This could be due to random error associated with the fact that a much smaller number of institutionalised participants were analysed.
Our review identified a possible difference between the two forms of supplemental vitamin D, that is, vitamin D3 and vitamin D2. Vitamin D3 seemed to significantly decrease mortality, while the effect of vitamin D2 may be neutral or even detrimental. The World Health Organization officially regards these two forms as equivalent, based on the results of quite old studies on rickets prevention (World Health Organization 1950). Biological differences between vitamins D3 and D2 are found in some species such as birds and monkeys (Hoy 1988; Marx 1989). Evidence on biological differences between the two vitamins in humans has been sparse and contradictory. A number of recently published clinical trials found evidence that vitamin D3 increases serum 25‐hydroxyvitamin D more efficiently than vitamin D2 (Armas 2004; Heaney 2011; Leventis 2009; Romagnoli 2008; Trang 1998). However, a randomised clinical trial found that vitamin D3 and vitamin D2 were comparable in maintaining serum 25‐hydroxyvitamin D levels (Holick 2008b). A recently published systematic review and meta‐analysis indicated that vitamin D3 is more efficacious than vitamin D2 in raising serum 25‐hydroxyvitamin D concentrations (Tripkovic 2012). An emerging body of evidence suggests several plausible explanations for this observation. The plasma half‐life of vitamin D3 is longer, and it has higher affinity to the vitamin D binding protein, hepatic vitamin D hydroxylase, and the vitamin D receptor (Holmberg 1986; Houghton 2006; Mistretta 2008). Vitamin D3 is the only naturally occurring form of vitamin D produced endogenously in our body, while vitamin D2 can be obtained only through the diet (Norman 2008). Vitamin D2 seems to upregulate several enzymes that degrade administered vitamin D2 and endogenous D3 (Heaney 2008). Our result could be of interest to health policy makers in different countries. The predominant supplemental form of vitamin D in the United States is vitamin D2 (Houghton 2006). In Europe, Japan and Canada, vitamin D supplements principally contain vitamin D3 (Holick 2008a), although in some European countries, like France and Great Britain, vitamin D2 is also available on the market.
Furthermore, we found no statistically significant difference between the intervention effects of vitamin D3 on mortality in trials using vitamin D3 singly and trials using vitamin D3 combined with calcium. Vitamin D3 was tested in combination with calcium in 27 trials and alone in 13 trials. Because of the small number of included trials assessing vitamin D3 alone, the findings could be due to a type II error. Our finding seems consistent with the result obtained by Autier et al, who found that calcium supplements did not affect mortality (Autier 2007), but opposite to the results of recent meta‐analyses examining the influence of vitamin D on mortality (Rejnmark 2012) or bone health (DIPART 2010). These meta‐analyses concluded that vitamin D is effective in preventing mortality (Rejnmark 2012) and hip fractures (DIPART 2010) only when combined with calcium. The complex interactions between vitamin D and calcium make it difficult to separate their effects. More research seems needed.
The current recommendation for adequate intake of calcium for adults is in the range of 1000 mg to 1200 mg. The tolerable upper limit is 2,000 mg (IOM 2011). The dosages used in the trials included in our meta‐analysis are in accordance with recommended intakes. In most of the included trials, the primary outcome measure was bone health. Vitamin D and calcium are well‐recognised nutritional factors related to bone health. Fractures, especially in elderly people, are associated with increased mortality risk (Haentjens 2010). We speculate that by preventing fractures, especially in elderly people, vitamin D combined with calcium can indirectly decrease mortality. Our results concur with the results of a recently published Cochrane review, which found that vitamin D singly could not prevent hip fracture but combined with calcium had a significant beneficial effect (Avenell 2009). However, Avenell et al found no statistically significant effect of vitamin D on mortality (Avenell 2009), although the review authors assessed a much more limited number of trials. A number of meta‐analyses of randomised trials found that vitamin D combined with calcium could prevent falls and fractures (Bischoff‐Ferrari 2005; Bischoff‐Ferrari 2009a; Bischoff‐Ferrari 2009b; Tang 2007). A recent meta‐analysis observed that calcium supplementation (with or without co‐administration of vitamin D) is associated with increased risk of cardiovascular events, especially myocardial infarction (Bolland 2010; Bolland 2011). Another review of prospective studies and randomised clinical trials found neutral effects of calcium (Patel 2012). A US Preventive Services Task Force recently recommended against daily supplementation with 400 IU or less of vitamin D3 and 1000 mg or less of calcium for the primary prevention of fractures in noninstitutionalised postmenopausal women (Moyer 2013).
A further important outcome of our review is that we found no significant differences in the effect of vitamin D3 on mortality in trials assessing doses less than 800 IU a day compared with trials assessing doses equal to or greater than 800 IU a day. The cutoff value for dividing trials was the median daily dose of vitamin D3 in the included trials (800 IU). The trial sequential analysis revealed that we may need more randomised trials assessing the influence of low doses of vitamin D3 (less than 800 IU) on mortality if we are to obtain the required information size. Controversy persists about the optimal dosage of vitamin D. Recommended daily intakes of vitamin D proposed by the Institute of Medicine are 600 IU per day for adults up to 70 years of age and 800 IU per day for those 70 years of age and older (IOM 2011). Recent randomised trials and meta‐analyses of randomised trials that have falls and fractures as the primary outcome have concluded that the reduction in risk for falls and hip and non‐vertebral fractures is dose dependent (Bischoff‐Ferrari 2009a; Bischoff‐Ferrari 2009b; Bischoff‐Ferrari 2009c; Bischoff‐Ferrari 2012). Conversely, two recent randomised clinical trials (Sanders 2010; Smith 2007) identified a potential harm associated with high doses of vitamin D. Furthermore, recent studies undertaken to examine how vitamin D status in the blood relates to all‐cause mortality found a U‐ or J‐shaped association between vitamin D status and all‐cause mortality (Durup 2012; Michaëlsson 2010), as well as cancer mortality (Michaëlsson 2010). Both high and low concentrations of plasma 25‐hydroxyvitamin D were associated with elevated risks of mortality (Durup 2012; Michaëlsson 2010). Amer et al evaluated the association of 25‐hydroxyvitamin D with all‐cause and cardiovascular mortality using National Health and Nutrition Examination Survey data (2001 to 2004) (Amer 2013). They found an inverse association between 25‐hydroxyvitamin D and all‐cause mortality in healthy adults with serum 25‐hydroxyvitamin D levels equal to or less than 21 ng/mL (Amer 2013). These results should warn us to be very cautious about the changes in recommended daily intakes of vitamin D (Bischoff‐Ferrari 2010b; Holick 2011; Sanders 2013).
It still is not known which dosing schedules are optimal for vitamin D3 supplementation. We found no significant differences in the effects of vitamin D3 on mortality in trials that administered vitamin D3 orally and daily compared with trials that applied vitamin D3 orally and intermittently. This could be due to type II errors. The randomised trial by Chel et al comparing daily, weekly and monthly dosing of vitamin D3 found that daily dosing was more effective than weekly and monthly dosing for preventing fractures (Chel 2008). A recently completed randomised clinical trial that assessed annual high‐dose vitamin D3 reported an increase in the primary outcome of fractures compared with placebo (Sanders 2010).
Most of the trial participants were women. However, when we compared the effect of vitamin D3 on all‐cause mortality in trials including participants of both sexes or only men versus the effect of vitamin D3 on all‐cause mortality in trials including only women, no statistically significant difference was noted. Therefore, our results are compatible with vitamin D3 having similar effects in men and women. Obviously, further randomised trials stratifying for sex and reporting effects according to the sex of the participants are needed.
We observed that vitamin D2 may increase mortality in trials with high risk of bias, as well as in vitamin D–insufficient participants. These subgroup findings may be due to random errors, and our trial sequential analysis supports this assessment. Until more data become available, regulatory authorities need to consider how this information should be handled.
We lack evidence for drawing any firm conclusions about the influence of the active forms of vitamin D (alfacalcidol and calcitriol) on mortality. Available evidence suggests that alfacalcidol and calcitriol have no statistically significant effect on mortality risk. However, only a few trials were conducted, and the risk of type II errors is high. We were not able to identify other meta‐analyses or systematic reviews assessing the influence of alfacalcidol and calcitriol on mortality. A recent systematic review that examined the influence of alfacalcidol and calcitriol on falls and fractures found no significant effect on vertebral fractures, a beneficial effect on non‐vertebral fractures and falls and increased risk of hypercalcaemia (O'Donnell 2008). Occurrences of hypercalcaemia due to the active forms of vitamin D were increased significantly in our review.
Vitamin D had no significant effect on cardiovascular mortality. Much debate in the literature has surrounded the possible beneficial effect of vitamin D on cardiovascular disease (Holick 2004; Scragg 2010; Zittermann 2006; Zittermann 2010). Results of recently published population‐based cohort studies are inconsistent (Schottker 2013; Skaaby 2012). Four recently published systematic reviews summarised the role of vitamin D in cardiovascular disease (Elamin 2011; Myung 2013; Pittas 2010; Wang 2010). These review authors found no evidence to support the use of vitamin D for prevention or treatment of cardiovascular disease (Elamin 2011; Myung 2013; Pittas 2010; Wang 2010).
Vitamin D seems to decrease cancer mortality. However, data were sparse, and selective outcome reporting bias is likely. Furthermore, the cumulative Z‐curve did not cross the trial sequential monitoring boundary in our analysis of cancer mortality, and additional evidence seems needed. Pilz and coworkers recently reviewed the evidence on vitamin D status and cancer mortality (Pilz 2009b). They concluded that epidemiological data were inconsistent in favour of the hypothesis that optimal vitamin D status was related to decreased cancer mortality. However, they lacked evidence from randomised clinical trials on intervention with vitamin D to strengthen their conclusion (Pilz 2009b). Although our present data are encouraging, we need more trials to exclude risks of systematic errors and risks of random errors.
We found that vitamin D had no significant effect on cancer occurrence (Bjelakovic 2008b). A large number of observational studies have provided evidence suggesting that vitamin D may have a role in cancer prevention (Garland 2007; Gorham 2007; Schwartz 2007). The first evidence came from ecological studies that found an inverse relationship between exposure to sunlight and cancer risk (Apperly 1941; Garland 1980). Several mechanisms have been proposed to explain how vitamin D may modify cancer risk. Experimental studies revealed that vitamin D inhibits cellular proliferation and stimulates apoptosis (Artaza 2010; Pan 2010). However, some observational studies found that high vitamin D status was connected with increased oesophageal (Chen 2007), pancreatic (Stolzenberg 2006), breast (Goodwin 2009) and prostate cancer risks (Ahn 2008). One should consider the possibility of a U‐shaped relation between vitamin D status and cancer risk (Toner 2010). Our results are in accordance with the conclusions of the recently published International Agency for Research on Cancer and Institute of Medicine reports stating that vitamin D status is not correlated with cancer occurrence (IARC 2008; IOM 2011). Recently, an updated meta‐analysis prepared for the US Preventive Services Task Force found inconclusive evidence regarding vitamin D supplementation for the prevention of cancer (Chung 2011). We still lack evidence; therefore, we need additional randomised clinical trials if we are to better understand the potential effect of vitamin D on cancer.
Vitamin D3 combined with calcium significantly increased nephrolithiasis. Active forms of vitamin D significantly increased hypercalcaemia. Other adverse events such as elevated urinary calcium excretion, renal insufficiency, cancer and cardiovascular, gastrointestinal, psychiatric or skin disorders were not statistically significantly influenced by vitamin D supplementation.
We lack sufficient evidence on the effect of vitamin D supplementation on health‐related quality of life and on the cost‐effectiveness of vitamin D supplementation. However, vitamin D3 products and calcium are relatively cheap, so these interventions are likely to be cost‐effective if they work sufficiently well.
In conclusion, we see a potentially positive effect of vitamin D3 on mortality, but we caution against thinking that now we know what to do in clinical practice because of the following. Our collection of trials showed a large dropout rate, which could seriously influence our results. The 'worst‐best case' scenario analysis does not exclude a risk of increased mortality associated with vitamin D. We found no significant difference in mortality between vitamin D3 given singly compared with combined with calcium, or vitamin D3 given in doses greater than compared with less than 800 IU/d. Vitamin D3 in doses less than 800 IU did not cross the trial sequential monitoring boundary for benefit, so random errors cannot be excluded. The effect of vitamin D3 on participants with adequate vitamin D status is unknown. Furthermore, we do not know the harm‐to‐benefit ratio when the intervention is used over a longer time. Moreover, we lack information on the effect in men and in younger persons of both sexes. All these reservations lead us to conclude that more research is urgently needed.
A great debate has been documented in the literature about the possible beneficial health effects of vitamin D supplementation. A lot of evidence indicates that vitamin D has beneficial effects, in addition to its effects on bones (Cavalier 2009; Stechschulte 2009; Wang 2009). It has been speculated that optimal vitamin D status is related to prevention of a spectrum of chronic diseases, including malignant and cardiovascular diseases (Fleet 2008; Ingraham 2008; Judd 2009; Zittermann 2010). Vitamin D insufficiency has been associated with increased mortality (Hutchinson 2010; Melamed 2008; Pilz 2009a; Pilz 2012; Zittermann 2009a). Two recently published evidence reports prepared for The Agency for Healthcare Research and Quality have assessed the influence of vitamin D and calcium on different health outcomes (Chung 2009; Cranney 2007). Most of the findings on bone health and different health outcomes were inconsistent (Chung 2009; Cranney 2007). The Institute of Medicine recently reported that available evidence supports a role of vitamin D and calcium in skeletal health (IOM 2011). However, the evidence was considered insufficient and inconclusive for extraskeletal outcomes, including mortality (IOM 2011). A recent meta‐analysis on the effects of vitamin D supplements on bone mineral density concluded that vitamin D supplementation for osteoporosis prevention in community‐dwelling adults without specific risk factors for vitamin D deficiency seems inappropriate (Reid 2013; Rosen 2013).
Authors' conclusions
Implications for practice.
We found some evidence that vitamin D3 may decrease all‐cause mortality and cancer mortality in predominantly elderly participants living independently or in institutional care. Vitamin D3 combined with calcium increased nephrolithiasis. Vitamin D2, alfacalcidol and calcitriol had no statistically significant effect on mortality. Alfacalcidol and calcitriol increased hypercalcaemia. Elevated urinary calcium excretion, renal insufficiency, cancer and cardiovascular, gastrointestinal, psychiatric or skin disorders were not statistically significantly influenced by vitamin D supplementation. However, because of risks of attrition bias, of outcome reporting bias and other biases, we cannot yet recommend or refute the use of vitamin D for preventing all‐cause mortality or cancer mortality.
Implications for research.
More randomised clinical trials are needed on the effects of vitamin D3 on mortality in younger, healthy persons and in elderly community‐dwelling and institutionalised persons without apparent vitamin D deficiency. Before drawing conclusions, we need more evidence on the effect of vitamin D on cancer and cardiovascular disease, especially when we consider the different forms of vitamin D used for supplementation. More randomised clinical trials are needed to test the efficacy of vitamin D3 applied singly or in combination with calcium and to compare different doses of vitamin D3. The effects of vitamin D on health‐related quality of life and cost‐effectiveness deserve further investigation. A number of issues are still insufficiently addressed. We do not know the importance of daily doses of vitamin D3, the influence of vitamin D insufficiency, the influence of dietary habits, the influence of sun exposure, the influence of latitude on the globe, the influence of sex of the participants and the influence of age. Future randomised clinical trials ought to be conducted without influence of industry on the design and reporting and ought to stratify participants for age and sex. Future trials ought to be designed according to the SPIRIT guidelines (Chan 2013) and reported according to the CONSORT guidelines (www.consort‐statement.org). Future trials ought to report individual participant data, so that proper individual participant data meta‐analyses of the effects of vitamin D in subgroups can be conducted.
What's new
| Date | Event | Description |
|---|---|---|
| 5 April 2012 | New search has been performed | The present review version is an update of the review published in 2011 (Bjelakovic 2011). |
| 4 April 2012 | New citation required but conclusions have not changed | New searches were performed in February 2012. We found and included six new randomised clinical trials with 1138 participants. |
Acknowledgements
We extend our gratitude to all participants and investigators in the randomised clinical trials. We are grateful to the many authors of publications who kindly responded to our requests for further information on the trials in which they were involved.
Appendices
Appendix 1. Search strategies
| Search terms for various databases |
| Unless otherwise stated, search terms are free‐text terms. Abbreviations: '$': stands for any character; '?': substitutes one or no character; adj: adjacent (i.e. number of words within range of search term); exp: exploded MeSH; MeSH: medical subject heading (MEDLINE medical index term); pt: publication type; sh: MeSH; tw: text word. |
| The Cochrane Library |
| 1. MeSH descriptor Vitamin D explode all trees 2. MeSH descriptor Cholecalciferol explode all trees 3. MeSH descriptor Ergocalciferols explode all trees 4. MeSH descriptor Dihydrotachysterol explode all trees 5. MeSH descriptor 25‐hydroxyvitamin D 2 explode all trees 6. MeSH descriptor Hydroxycholecalciferols explode all trees 7. ( (vitamin* in All Text and d in All Text and 2 in All Text) or (vitamin* in All Text and d2 in All Text) ) 8. (cholecalciferol* in All Text or calciferol* in All Text or calcitriol* in All Text or dihydrotachysterol* in All Text or (hydroxyvitamin* in All Text and d* in All Text) ) 9. (alfacalcidol* in All Text or alphacalcidol* in All Text or cholecalciferol* in All Text) 10. (#1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9) 11. MeSH descriptor Mortality explode all trees 12. (mortality in All Text or mortaliti* in All Text) 13. (#11 or #12) 14. MeSH descriptor Primary Prevention explode all trees 15. prevent* in All Text 16. MeSH descriptor Neoplasms explode all trees 17. (cancer* in All Text or neoplasm* in All Text or tumo?r* in All Text) 18. (#14 or #15 or #16 or #17) 19. (#10 and #13) 20. (#10 and #18) 21. (#19 or #20) |
| MEDLINE |
| 1. exp Vitamin D/ 2. exp Cholecalciferol/ 3. exp ergocalciferols/ or exp dihydrotachysterol/ or exp 25‐hydroxyvitamin d 2/ 4. exp Hydroxycholecalciferols/ 5. vitamin D?.tw,ot. 6. (cholecalciferol$ or calcifediol$ or calcitriol$ or dihydrotachysterol$ or hydroxyvitamin$ d?).tw,ot. 7. (alfacalcidol$ or alphacalcidol$ or colecalciferol$).tw,ot. 8. or/1‐7 9. exp Mortality/ 10. mortality.tw,ot. 11. mortaliti$.tw,ot. 12. or/9‐11 13. exp Primary Prevention/ 14. (prevention$ or prevent$).tw,ot. 15. exp Neoplasm/ 16. (cancer$ or neoplasm$ or tumo?r$).tw,ot. 17. or/13‐16 18. exp Randomized Controlled Trials as topic/ 19. Randomized Controlled Trial.pt. 20. exp Controlled Clinical Trials as topic/ 21. Controlled Clinical Trial.pt. 22. exp Random Allocation/ 23. exp Double‐Blind Method/ 24. exp Single‐Blind Method/ 25. or/18‐24 26. exp "Review Literature as topic"/ 27. exp Technology Assessment, Biomedical/ 28. exp Meta‐analysis as topic/ 29. Meta‐analysis.pt. 30. hta.tw,ot. 31. (health technology adj6 assessment$).tw,ot. 32. (meta analy$ or metaanaly$ or meta?analy$).tw,ot. 33. ((review$ or search$) adj10 (literature$ or medical database$ or medline or pubmed or embase or cochrane or cinahl or psycinfo or psyclit or healthstar or biosis or current content$ or systemat$)).tw,ot. 34. or/26‐33 35. 25 or 34 36. 8 and 17 and 35 37. 8 and 12 and 35 38 36 or 37 39. limit 38 to animals 40. limit 38 to humans 41. 39 not 40 42 38 not 41 |
| EMBASE |
| 1. exp ergocalciferol/ or exp vitamin D/ 2. exp colecalciferol/ 3. exp dihydrotachysterol/ 4. exp 25 hydroxyvitamin D/ 5. exp hydroxycolecalciferol/ 6. (vitamin* D? or vitamin*D?).tw,ot. 7. (cholecalciferol* or colecalciferol* or calcifediol* or calcitriol* or dihydrotachysterol* or hydroxyvitamin* d?).tw,ot. 8. exp alfacalcidol/ 9. (alfacalcidol* or alphacalcidol*).tw,ot. 10. or/1‐9 11. exp mortality/ 12. (mortality or mortaliti*).tw,ot. 13. 11 or 12 14. exp prevention/ 15. prevent*.tw,ot. 16. exp neoplasm/ 17. or/14‐16 18. randomized controlled trial/ 19. double blind procedure/ 20. single blind procedure/ 21. exp randomization/ 22. exp controlled clinical trial/ 23. or/18‐22 24. exp meta analysis/ 25. (metaanaly$ or meta analy$ or meta?analy$).ab,ti,ot. 26. ((review$ or search$) adj10 (literature$ or medical database$ or medline or pubmed or embase or cochrane or cinahl or psycinfo or psyclit or healthstar or biosis or current content$ or systematic$)).ab,ti,ot. 27. exp Literature/ 28. exp Biomedical Technology Assessment/ 29. hta.tw,ot. 30. (health technology adj6 assessment$).tw,ot. 31. or/24‐30 32. (comment or editorial or historical‐article).pt. 33. 31 not 32 34. 23 or 33 35. 10 and 13 and 34 36. 10 and 17 and 34 37. 35 or 36 38. limit 37 to human |
| LILACS |
| 1. Vitamin D 2. Cholecalciferol 3. Ergocalciferol 4. Alfacalcidol 5. Calcitriol |
| ISI Web of Science |
| 1. TS=(vitamin d2 OR vitamin d 2 OR hydroxyvitamin* OR cholecalciferol* OR calciferol* OR calcitriol* OR calcifediol* OR dihydrotachysterol* OR alfacalcidol* OR alphacalcidol* OR colecalciferol*) 2. TS=(mortalit* OR prevent* OR cancer* OR neoplasm* OR tumor* OR tumour*) 3. #2 AND #1 4. TS=(random* OR blind* OR placebo* OR meta‐analys*) 5. #4 AND #3 |
Appendix 2. Description of interventions
|
Characteristic Study ID |
Intervention(s) [route, frequency, total dose/day] | Control(s) [route, frequency, total dose/day] |
| Aloia 2005 | Vitamin D3 (800 IU) plus calcium (1200 to 1500 mg) orally, daily | Matched placebo tablets plus calcium (1200 to 1500 mg) orally, daily |
| Avenell 2004 | Vitamin D3 (800 IU) orally, daily | No intervention |
| Calcium (1000 mg) orally, daily | ||
| Vitamin D3 (800 IU) plus calcium (1000 mg) orally, daily | ||
| Avenell 2012 | Vitamin D3 (800 IU) orally, daily | Matched placebo tablets orally, daily |
| Calcium (1000 mg) orally, daily | ||
| Vitamin D3 (800 IU) plus calcium (1000 mg) orally, daily | ||
| Baeksgaard 1998 | Vitamin D3 (560 IU) plus calcium (1000 mg) orally, daily | Matched placebo tablets orally, daily |
| Vitamin D3 (560 IU) plus calcium (1000 mg) plus multivitamin orally, daily | ||
| Bischoff 2003 | Vitamin D3 (800 IU) plus calcium 1200 mg orally, daily | Calcium 1200 mg orally, daily |
| Bjorkman 2007 | Vitamin D3 (1200 IU) plus calcium (500 mg) orally, daily | Calcium (500 mg) orally, daily |
| Vitamin D3 (400 IU) plus calcium (500 mg) orally, daily | ||
| Bolton‐Smith 2007 | Vitamin D3 (400 IU) plus calcium 1000 mg orally, daily | Matched placebo orally, daily |
| Vitamin D3 (400 IU) plus calcium 1000 mg plus vitamin K1 200 μg orally, daily | ||
| Vitamin K1 200 μg orally, daily | ||
| Brazier 2005 | Vitamin D3 (800 IU) plus calcium (1000 mg) orally, daily | Matched placebo tablets orally, daily |
| Broe 2007 | Vitamin D2 (800 IU) orally, daily | Matched placebo tablets orally, daily |
| Vitamin D2 (600 IU) orally, daily | ||
| Vitamin D2 (400 IU) orally, daily | ||
| Vitamin D2 (200 IU) orally, daily | ||
| Brohult 1973 | Vitamin D3 (100,000 IU) daily | Placebo daily |
| Burleigh 2007 | Vitamin D3 (800 IU) plus calcium (1200 mg) orally, daily | Calcium (1200 mg) orally, daily |
| Campbell 2005 | Home safety assessment and modification programme | Social visits |
| Exercise programme plus vitamin D3 100,000 IU initially and then 50,000 IU orally, monthly | ||
| Both interventions | ||
| Chapuy 1992 | Vitamin D3 (800 IU) plus calcium (1200 mg) orally, daily | Double placebo orally, daily |
| Chapuy 2002 | Vitamin D3 (800 IU) plus calcium (1200 mg) (fixed combination) orally, daily | Double placebo orally, daily |
| Vitamin D3 (800 IU) plus calcium (1200 mg), (separate combination) orally, daily | ||
| Chel 2008 | Vitamin D3 (600 IU) orally, daily | Matched placebo tablets orally, daily |
| Vitamin D3 (4200 IU) orally, weekly | Matched placebo tablets orally, weekly | |
| Vitamin D3 (18,000 IU) orally, monthly | Matched placebo powder orally, monthly | |
| Cherniack 2011 | Vitamin D3 2000 IU plus calcium 1200 mg orally, daily | Matched placebo plus calcium 1200 mg orally, daily |
| Cooper 2003 | Vitamin D2 (10,000 IU) orally, weekly plus calcium (1000 mg) orally, daily | Calcium (1000 mg) orally, daily |
| Coreless 1985 | Vitamin D2 (9000 IU) orally, daily | Placebo orally, daily |
| Daly 2006 | Calcium‐vitamin D3–fortified milk containing vitamin D3 (800 IU) plus calcium (1000 mg) daily | No intervention |
| Dawson‐Hughes 1997 | Vitamin D3 (700 IU) plus calcium (500 mg) orally, daily | Double placebo orally, daily |
| Dukas 2004 | Alfacalcidol (1 μg) orally, daily | Placebo orally, daily |
| Flicker 2005 | Vitamin D3 (10,000 IU) weekly and thereafter vitamin D31000 IU daily plus calcium (600 mg) orally, daily | Calcium (600 mg) orally, daily |
| Gallagher 2001 | Calcitriol (0.5 μg) daily | Matched placebo pills orally, daily |
| Conjugated oestrogens 0.625 mg/daily plus medroxyprogesterone acetate 2.5 mg orally, daily | ||
| Calcitriol (0.5 μg daily) plus conjugated oestrogens 0.625 mg/daily plus medroxyprogesterone acetate 2.5 mg orally, daily | ||
| Glendenning 2012 | Cholecalciferol 150,000 three‐monthly | Placebo vitamin D three‐monthly |
| Grady 1991 | Calcitriol (0.5 μg) orally, daily | Placebo vitamin D orally, daily |
| Grimnes 2011 | Vitamin D3 (20,000 IU) orally, twice weekly | Placebo orally, twice weekly. |
| Harwood 2004 | Single injection of 300,000 IU of vitamin D2 | No intervention |
| Single injection of 300,000 IU of vitamin D2 plus oral calcium (1000 mg) daily | ||
| Oral vitamin D3 (800 IU) plus calcium (1000 mg) daily | ||
| Jackson 2006 | Vitamin D3 (400 IU) plus calcium (1000 mg) orally, daily | Matched placebo orally, daily |
| Janssen 2010 | Vitamin D3 (400 IU) plus calcium (500 mg) orally, daily | Matched placebo vitamin D3 plus calcium (500 mg) orally, daily |
| Komulainen 1999 | Sequential combination of 2 mg oestradiol valerate (days 1 to 21) and 1 mg cyproterone acetate (days 12 to 21) and a treatment‐free interval (days 22 to 28) | Placebo |
| Vitamin D3 (300 IU) plus calcium (500 mg) dailya | ||
| Sequential combination of 2 mg oestradiol valerate (days 1 to 21) and 1 mg cyproterone acetate (days 12 to 21) and a treatment‐free interval (days 22 to 28) plus vitamin D3 (300 IU) and calcium (500 mg) orally, daily | ||
| Krieg 1999 | Vitamin D3 (880 IU) plus calcium (1000 mg) orally, daily | No treatment |
| Kärkkäinen 2010 | Vitamin D3 800 IU plus calcium 1000 mg orally, daily | No intervention |
| Lappe 2007 | Vitamin D3 (1000 IU) plus calcium (1400 to 1500 mg) orally, daily | Double placebo tablets, orally, daily |
| Calcium (1400 to 1500 mg) plus a vitamin D placebo orally, daily | ||
| Larsen 2004 | Home safety inspection | No intervention |
| Vitamin D3 (400 IU) plus calcium (1000 mg) orally, daily | ||
| Both interventions | ||
| Latham 2003 | Resistance exercise | Matched placebo tablets, orally |
| Attention control | ||
| Vitamin D3 (300,000 IU) single dose, orally | ||
| Law 2006 | Vitamin D2 100,000 IU every three months, orally | No intervention |
| Lehouck 2012 | Vitamin D3 100,000 IU monthly, orally | Matched placebo orally, monthly |
| Lips 1996 | Vitamin D3 400 IU orally, daily | Matched placebo orally, daily |
| Lips 2010 | Vitamin D3 8400 IU orally, weekly | Matched placebo orally, weekly. |
| Lyons 2007 | Vitamin D2 100,000 IU four‐monthly, orally | Matched placebo tablets four‐monthly, orally |
| Meier 2004 | Vitamin D3 (500 IU) orally, daily | No intervention |
| Mochonis 2006 | Vitamin D3 300 IU plus calcium 1200 mg orally, daily | No intervention |
| Calcium 1200 mg, orally, daily | ||
| Ooms 1995 | Vitamin D3 400 IU orally, daily | Matched placebo orally, daily |
| Ott 1989 | Vitamin D3 17.2 IU plus calcium 1000 mg | Matched placebo vitamin D plus calcium 1000 mg orally, daily |
| Porthouse 2005 | Vitamin D3 (800 IU) plus calcium (1000 mg), orally, daily | No interventionb |
| Prince 2008 | Vitamin D2 1000 IU plus calcium 1000 mg orally, daily | Matched placebo tablets of vitamin D plus calcium 1000 mg orally, daily |
| Sanders 2010 | Vitamin D3 500,000 IU orally, yearly | Matched placebo tablets of vitamin D orally, yearly |
| Sato 1997 | Vitamin D (alfacalcidol) (1 μg) plus calcium 300 mg orally, daily | Matched placebo tablets of vitamin D and calcium orally, daily |
| Sato 1999a | Vitamin D (alfacalcidol) (1 μg) orally, daily | Matched placebo tablets of vitamin D orally, daily |
| Sato 1999b | Vitamin D (alfacalcidol) (1 μg) orally, daily | No treatment |
| Ipriflavone 600 mg orally, daily | ||
| Sato 2005a | Vitamin D2 (1000 IU) orally, daily | Matched placebo tablets of vitamin D daily |
| Schleithoff 2006 | Vitamin D3 2000 IU plus calcium 500 mg orally, daily | Matched placebo vitamin D plus calcium 500 mg orally, daily |
| Smith 2007 | Vitamin D2 300,000 IU intramuscular injection, yearly | Matched placebo intramuscular injection yearly |
| Trivedi 2003 | Vitamin D3 100,000 IU every four months orally | Matched placebo vitamin D every four months orally |
| Witham 2010 | Vitamin D2 (10,000 IU) orally, daily | Matched placebo tablets orally, daily |
| Zhu 2008 | Vitamin D2 (1000 IU) plus calcium (1200 mg) orally, daily | Matched placebo vitamin D and placebo calcium orally, daily |
| Calcium 1200 mg plus placebo vitamin D orally, daily | ||
|
Footnotes aNo intake during June to August; the vitamin D3 dosage was lowered to 100 IU/d after four years of treatment bInformation leaflet on dietary calcium intake and prevention of falls | ||
Appendix 3. Baseline characteristics (I)
|
Characteristic Study ID |
Duration of interv ention [years] |
Duration of follow‐up [years] | Participating population | Country | Setting | Ethnic groups |
| Aloia 2005 | 3 | 3 | Postmenopausal African‐American women | USA | Outpatients | All black |
| Avenell 2004 | 1 | 1 | Elderly people with an osteoporotic fracture within the past 10 years | UK | Outpatients | ‐ |
| Avenell 2012 | 3.75 | 6.2 | Elderly people with low‐trauma, osteoporotic fracture in the previous 10 years | UK | Outpatients | ‐ |
| Baeksgaard 1998 | 2 | 2 | Postmenopausal women | Demark | Outpatients | ‐ |
| Bischoff 2003 | 0.25 | 0.25 | Elderly women living in institutional care | Switzerland | Inpatients | ‐ |
| Bjorkman 2007 | 0.5 | 0.5 | Chronically bedridden patients | Finland | ‐ | ‐ |
| Bolton‐Smith 2007 | 2 | 2 | Elderly non‐osteoporotic women | UK | Outpatients | ‐ |
| Brazier 2005 | 1 | 1 | Elderly vitamin D–insufficient women | France | Outpatients | ‐ |
| Broe 2007 | 0.42 | 0.42 | Nursing home residents | USA | Inpatients | ‐ |
| Brohult 1973 | 1 | 1 | Patients with rheumatoid arthritis | Sweden | Outpatients | ‐ |
| Burleigh 2007 | 0.08 | 0.08 | Elderly people | UK | Inpatients | ‐ |
| Campbell 2005 | 1 | 1 | Elderly people with visual impairment | New Zealand | Outpatients | ‐ |
| Chapuy 1992 | 1.5 | 4 | Healthy ambulatory women | France | Outpatients | ‐ |
| Chapuy 2002 | 2 | 2 | Elderly people living in institutional care | France | Outpatients | ‐ |
| Chel 2008 | 0.33 | 0.33 | Nursing home residents | Netherlands | Inpatients | ‐ |
| Cherniack 2011 | 0.5 | 0.5 | Elderly people | USA | Outpatients | ‐ |
| Cooper 2003 | 2 | 2 | Postmenopausal women | Australia | Outpatients | All white |
| Coreless 1985 | 0.75 | 0.75 | Elderly patients from geriatric wards | UK | Inpatients | ‐ |
| Daly 2006 | 2 | 3.5 | Healthy ambulatory men | Australia | Outpatients | ‐ |
| Dawson‐Hughes 1997 | 3 | 3 | Healthy ambulatory participants | USA | Outpatients | ‐ |
| Dukas 2004 | 0.75 | 0.75 | Elderly people | Switzerland | Outpatients | ‐ |
| Flicker 2005 | 2 | 2 | Elderly people living in institutional care | Australia | Inpatients | ‐ |
| Gallagher 2001 | 3 | 5 | Elderly women | USA | Outpatients | ‐ |
| Grady 1991 | 0.5 | 0.5 | Elderly people | USA | Outpatients | ‐ |
| Glendenning 2012 | 0.5 | 0.75 | Elderly women | Australia | Outpatients | ‐ |
| Grimnes 2011 | 0.5 | 0.5 | Healthy people with low vitamin D status | Norway | Outpatients | ‐ |
| Harwood 004 | 1 | 1 | Elderly women | UK | Outpatients | ‐ |
| Jackson 2006 | 7 | 7 | Postmenopausal women | USA | Outpatients | ‐ |
| Janssen 2010 | 1 | 1 | Elderly vitamin D–insufficient women | Netherlands | Outpatients | ‐ |
| Komulainen 1999 | 5 | 5 | Postmenopausal women | Finland | Outpatients | ‐ |
| Krieg 1999 | 2 | 2 | Elderly institutionalised women | Switzerland | Outpatients | ‐ |
| Kärkkäinen 2010 | 3 | 3 | Postmenopausal women | Finland | Outpatients | ‐ |
| Lappe 2007 | 4 | 4 | Postmenopausal women | USA | Outpatients | All white |
| Larsen 2004 | 3.5 | 3.5 | Elderly people | Denmark | Outpatients | ‐ |
| Latham 2003 | 0.003 | 0.5 | Elderly people | New Zealand | Outpatients | ‐ |
| Law 2006 | 0.83 | 0.83 | Elderly people | UK | Inpatients | ‐ |
| Lehouck 2012 | 1 | 1 | Patients with chronic obstructive pulmonary disease | Belgium | Outpatients | ‐ |
| Lips 1996 | 3.5 | 3.5 | Elderly people | Netherlands | Outpatients | ‐ |
| Lips 2010 | 0.31 | 0.31 | Elderly people with vitamin D insufficiency | Netherlands | Outpatients | ‐ |
| Lyons 2007 | 3 | 3 | Elderly people living in institutional care | UK | Inpatients | ‐ |
| Meier 2004 | 0.5 | 1 | Healthy volunteers | Germany | Outpatients | ‐ |
| Mochonis 2006 | 1 | 1 | Postmenopausal women | Greece | Outpatients | ‐ |
| Ooms 1995 | 2 | 2 | Elderly people | Netherlands | Outpatients | ‐ |
| Ott 1989 | 2 | 2 | Postmenopausal women | USA | Outpatients | ‐ |
| Porthouse 2005 | 2 | 2 | Elderly women | UK | Outpatients | ‐ |
| Prince 2008 | 1 | 1 | Elderly women with vitamin D insufficiency | Australia | Outpatients | ‐ |
| Sanders 2010 | 2.96 | 2.96 | Elderly women | Australia | Outpatients | ‐ |
| Sato 1997 | 0.5 | 0.5 | Patients with hemiplegia after stroke | Japan | Outpatients | ‐ |
| Sato 1999a | 1.5 | 1.5 | Elderly patients with Parkinson's disease | Japan | Outpatients | ‐ |
| Sato 1999b | 1 | 1 | Patients with hemiplegia after stroke | Japan | Outpatients | ‐ |
| Sato 2005a | 2 | 2 | Elderly women with hemiplegia after stroke | Japan | Outpatients | ‐ |
| Schleithoff 2006 | 0.75 | 1.25 | Patients with congestive heart failure | Germany | Inpatients | ‐ |
| Smith 2007 | 3 | 3 | Elderly people | UK | Outpatients | ‐ |
| Trivedi 2003 | 5 | 5 | Elderly people | UK | Outpatients | ‐ |
| Witham 2010 | 0.38 | 0.38 | Patients with systolic heart failure | UK | Outpatients | ‐ |
| Zhu 2008 | 5 | 5 | Elderly women | Australia | Outpatients | ‐ |
|
Footnotes "‐" denotes not reported. | ||||||
Appendix 4. Baseline characteristics (II)
|
Characteristic Study ID |
Duration of disease [mean years (SD) / range] | Sex [female %] | Age [mean years (SD) / range] | Co‐medications/ Co‐interventions | Co‐morbidities |
| Aloia 2005 | ‐ | 100 | 60 (50 to 75) | ‐ | ‐ |
| Avenell 2004 | ‐ | 83 | 77 | ‐ | ‐ |
| Avenell 2012 | ‐ | 85 | 77 | ‐ | Low‐trauma osteoporotic fracture in the previous 10 years |
| Baeksgaard 1998 | ‐ | 100 | 62.5 (58 to 67) | ‐ | ‐ |
| Bischoff 2003 | ‐ | 100 | 85.3 | ‐ | ‐ |
| Bjorkman 2007 | ‐ | 82 | 84.5 (65 to 104) | ‐ | ‐ |
| Bolton‐Smith 2007 | ‐ | 100 | 68 | ||
| Brazier 2005 | ‐ | 100 | 74.6 | ‐ | ‐ |
| Broe 2007 | 73 | 89 | |||
| Brohult 1973 | 2 (2 to 7) | 68 | 52 (18 to 69) | ‐ | Rheumatoid arthritis |
| Burleigh 2007 | ‐ | 59 | 83 | ‐ | ‐ |
| Campbell 2005 | ‐ | 68 | 83.6 (75 to 96) | ‐ | ‐ |
| Chapuy 1992 | ‐ | 100 | 84 (69 to 106) | ‐ | ‐ |
| Chapuy 2002 | ‐ | 100 | 85 (64 to 99) | ‐ | ‐ |
| Chel 2008 | ‐ | 77 | 84 | ‐ | ‐ |
| Cherniack 2011 | ‐ | 2 | 80 | ‐ | ‐ |
| Cooper 2003 | ‐ | 100 | 56 | ‐ | ‐ |
| Coreless 1985 | ‐ | 78 | 82.4 | ‐ | ‐ |
| Daly 2006 | ‐ | 0 | 61.9 | ‐ | ‐ |
| Dawson‐Hughes 1997 | 55 | 71 | ‐ | ‐ | |
| Dukas 2004 | ‐ | 51 | 71 | ‐ | ‐ |
| Flicker 2005 | ‐ | 95 | 83.4 | ‐ | ‐ |
| Gallagher 2001 | ‐ | 100 | 71.5 | ‐ | ‐ |
| Glendenning 2012 | ‐ | 100 | 76.7 | ‐ | ‐ |
| Grady 1991 | ‐ | 54 | 79.1 (70 to 97) | ‐ | ‐ |
| Grimnes 2011 | ‐ | 49 | 52 | ‐ | ‐ |
| Harwood 2004 | ‐ | 100 | 81.2 (67 to 91) | ‐ | ‐ |
| Jackson 2006 | ‐ | 100 | 62.4 (50 to 79) | ||
| Janssen 2010 | ‐ | 100 | 80.8 | ‐ | ‐ |
| Komulainen 1999 | ‐ | 100 | 52.7 (47 to 56) | ‐ | ‐ |
| Krieg 1999 | ‐ | 100 | 84.5 (62 to 98) | ‐ | ‐ |
| Kärkkäinen 2010 | ‐ | 100 | 67 (65 to 71) | ‐ | ‐ |
| Lappe 2007 | ‐ | 100 | 66.7 | ‐ | ‐ |
| Larsen 2004 | ‐ | 60 | 75 (66 to 103) | ‐ | ‐ |
| Latham 2003 | ‐ | 53 | 85 (64 to 99) | ‐ | ‐ |
| Law 2006 | ‐ | 76 | 85 | ‐ | ‐ |
| Lehouck 2012 | ‐ | 20 | 68 | ‐ | Chronic obstructive pulmonary disease |
| Lips 1996 | ‐ | 74 | 80 (70 to 97) | ‐ | ‐ |
| Lips 2010 | ‐ | ‐ | 78 | ‐ | ‐ |
| Lyons 2007 | ‐ | 76 | 84 (62 to 107) | ‐ | ‐ |
| Meier 2004 | ‐ | 65 | 56.5 (33 to 78) | ‐ | ‐ |
| Mochonis 2006 | ‐ | 100 | 60.3 (55 to 65) | ‐ | ‐ |
| Ooms 1995 | ‐ | 100 | 80.3 | ‐ | ‐ |
| Ott 1989 | ‐ | 100 | 67.5 | ‐ | ‐ |
| Porthouse 2005 | ‐ | 100 | 76.8 | ‐ | ‐ |
| Prince 2008 | ‐ | 100 | 77.2 (70 to 90) | ‐ | ‐ |
| Sanders 2010 | ‐ | 100 | 76.0 | ‐ | ‐ |
| Sato 1997 | ‐ | 45 | 68.5 | ‐ | Hemiplegia after stroke |
| Sato 1999a | ‐ | 78 | 70.6 (65 to 88) | ‐ | Parkinson's disease |
| Sato 1999b | ‐ | 56 | 70.7 | Ipriflavone | Hemiplegia after stroke |
| Sato 2005a | ‐ | 100 | 74.1 | ‐ | Hemiplegia after stroke |
| Schleithoff 2006 | ‐ | 17 | 51 (50 to 63) | ‐ | Congestive heart failure |
| Smith 2007 | ‐ | 54 | 79.1 | ‐ | ‐ |
| Trivedi 2003 | ‐ | 24 | 74.7 (65 to 85) | ‐ | ‐ |
| Witham 2010 | ‐ | 34 | 79.7 | ‐ | Systolic heart failure |
| Zhu 2008 | ‐ | 100 | 75 (70 to 80) | ‐ | ‐ |
|
Footnotes "‐" denotes not reported SD: standard deviation | |||||
Appendix 5. Matrix of study endpoints
|
Characteristic Study ID |
Primary endpoint(s) [time of measurement] | Secondary endpoint(s) [time of measurement] | Other endpoint(s) [time of measurement] |
| Aloia 2005 | Bone mineral density (6, 12, 18, 24 mo) | ‐ | Overall mortality (24 mo) |
| Avenell 2004 | Recruitment, compliance and retention within a randomised trial (12 mo) | ‐ | Overall mortality (12 mo) |
| Avenell 2012 | Fractures | Overall mortality, vascular disease mortality, cancer mortality and cancer occurrence (3.75, 6.2 yr) | ‐ |
| Baeksgaard 1998 | Bone mineral density (0, 12, 24 mo | Serum calcium, serum phosphate and serum intact parathyroid hormone (0, 24 mo) | Overall mortality (24 mo) |
| Bischoff 2003 | Falls | Musculoskeletal function and bone remodeling (3 mo) | Overall mortality (3 mo) |
| Bjorkman 2007 | Parathyroid function and bone mineral density (0, 6 mo) | ‐ | Overall mortality (6 mo) |
| Bolton‐Smith 2007 | Bone mineral density (6, 12, 18, 24 mo) | Markers of bone turnover and vitamin status (0, 24 mo) | Overall mortality (24 mo) |
| Brazier 2005 | Bone mineral density (0, 12 mo) | Clinical and laboratory safety of treatment (0, 12 mo) | Overall mortality (12 mo) |
| Broe 2007 | Falls (5 mo) | ‐ | Overall mortality (5 mo) |
| Brohult 1973 | Objective and subjective improvement (12 mo) | ‐ | Overall mortality (12 mo) |
| Burleigh 2007 | Falls (1 mo) | ‐ | Overall mortality (1 mo) |
| Campbell 2005 | Numbers of falls, injuries resulting from falls (3, 6, 12 mo) | Costs of implementing the home safety programme | Overall mortality (3, 6, 12 mo) |
| Chapuy 1992 | Fractures (6, 12, 18 mo) | Adverse events (6, 12, 18 mo) | Overall mortality (18 mo) |
| Chapuy 2002 | Biochemical variables of calcium homeostasis, femoral neck bone mineral density and hip fracture risk (3, 6, 9, 12, 15, 18, 21, 24 mo) | ‐ | Overall mortality (24 mo) |
| Chel 2008 | Efficacy of different doses and intervals of oral vitamin D3 supplementation with the same total dose (4 mo) | Effect of calcium supplementation following vitamin D supplementation on serum PTH and markers of bone turnover (4 mo) | Overall mortality (4 mo) |
| Cherniack 2011 | Serum calcium, 25‐hydroxyvitamin D, parathyroid hormone and 24‐hour urinary calcium (6, 12 mo) |
‐ | Overall mortality (18 mo) |
| Cooper 2003 | Bone mineral density (0, 6, 12, 18, 24 mo) | ‐ | Overall mortality (24 mo) |
| Coreless 1985 | Abilities to carry out basic activities of daily life (2, 9 mo) | ‐ | Overall mortality (9 mo) |
| Daly 2006 | Bone mineral density | ‐ | Overall mortality (24 mo) |
| Dawson‐Hughes 1997 | Bone mineral density, biochemical measures of bone metabolism and the incidence of nonvertebral fractures (1, 6, 18, 24, 30, 36 mo) | ‐ | Overall mortality (36 mo) |
| Dukas 2004 | Falls (9 mo) | Serum concentrations of 25‐hydroxyvitamin D, 1,25 dihydroxyvitamin D and intact parathormone (0, 3, 6 mo) | Overall mortality (9 mo) |
| Flicker 2005 | Falls and fractures (24 mo) | ‐ | Overall mortality (24 mo) |
| Gallagher 2001 | Bone mineral density (1.5, 3, 6, 12, 18, 24, 30, 36 mo) | ‐ | Overall mortality (24 mo) |
| Glendenning 2012 | Falls, muscle strength and mobility (0, 3, 6, 9 mo) | Serum 25‐hydroxyvitamin D levels and adverse events (0, 3, 6, 9 mo) | Overall mortality (9 mo) |
| Grady 1991 | Muscle strength (1, 2, 4, 8, 12, 18, 24 wk) | ‐ | Overall mortality (24 mo) |
| Grimnes 2011 | Insulin sensitivity and secretion (6 mo) | Blood lipid levels (6 mo) | Adverse events (6 mo), overall mortality (6 mo) |
| Harwood 2004 | Bone biochemical markers, bone mineral density and rate of falls (3, 6, 12 mo) | ‐ | Overall mortality (12 mo) |
| Jackson 2006 | Fractures, cancer occurrence, mortality (3, 7 yr) | ‐ | ‐ |
| Janssen 2010 | Muscle strength, power and functional mobility (0, 6 mo) | ‐ | Overall mortality (6 mo) |
| Komulainen 1999 | Bone mineral density (0, 1, 2, 3, 4, 5 yr) | ‐ | Adverse events (5 yr), overall mortality (5 yr) |
| Krieg 1999 | Quantitative ultrasound parameters of bones and metabolic disturbances (0, 12, 24 mo) | ‐ | Overall mortality (24 mo) |
| Kärkkäinen 2010 | Bone mineral density (0, 3 yr) | Vitamin D status (0, 3 yr) | Overall mortality (0, 3 yr) |
| Lappe 2007 | Fractures | Cancer occurrence (0, 6, 12, 18, 24, 30, 36, 42, 48 mo), vitamin D status (0, 12 mo) | Overall mortality (48 mo) |
| Larsen 2004 | Falls (3.5 yr) | ‐ | Overall mortality (3.5 yr) |
| Latham 2003 | Physical health (3 mo), falls (6 mo) | Physical performance (6 mo), self‐rated function (6 mo) | Overall mortality (6 mo) |
| Law 2006 | Non‐vertebral fractures (10 mo), falls (10 mo) | ‐ | Overall mortality (10 mo) |
| Lehouck 2012 | Time to first exacerbation | Exacerbation rate, time to first hospitalisation, time to second exacerbation, quality of life, overall mortality |
‐ |
| Lips 1996 | Fractures (1, 2, 3.5 yr) | Overall mortality (3.5 yr) | ‐ |
| Lips 2010 | Mediolateral body sway with eyes open (4 mo) | Short physical performance battery (4 mo), vitamin D status (4 mo), calcium concentration (4 mo), phosphate concentration (4 mo), adverse events (4 mo) |
‐ |
| Lyons 2007 | Incidence of first fracture | Hip fractures, fractures at common osteoporotic sites (hip, wrist, forearm, vertebrae) (3 yr), overall mortality (3 yr) | ‐ |
| Meier 2004 | Circannual changes in bone turnover and bone mass (1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12 mo) |
‐ | Overall mortality (12 mo) |
| Mochonis 2006 | Bone mineral density (0, 6, 12 mo) | ‐ | Overall mortality (12 mo) |
| Ooms 1995 | Bone mineral density (0, 6, 12, 18, 24 mo), biochemical markers of bone turnover (0, 6, 12, 18, 24 mo) | ‐ | Overall mortality (24 mo) |
| Ott 1989 | Bone mass (0, 6, 12, 18, 24 mo) | Adverse events (24 mo) | Overall mortality (24 mo) |
| Porthouse 2005 | Fractures (excluding those of the digits, rib, face and skull) (0, 6, 12, 18, 25 mo) | Hip fracture; quality of life, visits to the doctor, hospital admissions, falls, fear of falling (0, 6, 12, 18, 25 mo), overall mortality (25 mo) | ‐ |
| Prince 2008 | Falls (12 mo) | Adverse events (12 mo) | Overall mortality (12 mo) |
| Sanders 2010 | Falls and fractures (3, 9, 15, 24, 27, 36 mo) | Adverse events (36 mo) | Overall mortality (36 mo) |
| Sato 1997 | Bone mineral density, hip fractures (6 mo) | ‐ | Overall mortality (6 mo) |
| Sato 1999a | Non‐vertebral fractures (18 mo) | Progression of osteopenia (0, 18 mo) | Overall mortality (0, 18 mo) |
| Sato 1999b | Bone mineral density (0, 12 mo) | ‐ | Overall mortality (12 mo) |
| Sato 2005a | Falls (24 mo) | Muscular strength, morphological changes of muscle (0, 24 mo) | Overall mortality (24 mo) |
| Schleithoff 2006 | Overall mortality (15 mo], biochemical variables (15 mo) | Left ventricular ejection fraction (15 mo], left ventricular end‐diastolic diameter (15 mo), the cardiothoracic ratio (15 mo), maximal oxygen intake (15 mo) and blood pressure (15 mo) | Vitamin D status (15 mo) |
| Smith 2007 | Non‐vertebral fractures (0, 6, 12, 18, 24, 30, 36 mo) | Fractures of the hip or wrist, falls (0, 6, 12, 18, 24, 30, 36 mo) | Overall mortality (36 mo) |
| Trivedi 2003 | Fractures (5 yr], cause‐specific mortality (5 yr) | Cancer occurrence (5 yr), cardiovascular disease (5 yr) | Overall mortality (5 yr) |
| Witham 2010 | Six‐minute walk test (0, 10, 20 wk) | Timed get‐up‐and‐go test (0, 10, 20 wk), daily physical activity levels (0, 10, 20 wk), health status (0, 10, 20 wk), cardiovascular and inflammatory markers (0, 10, 20 wk), adverse events (20 wk) | Overall mortality (20 wk) |
| Zhu 2008 | Bone mineral density (0, 1, 3, 5 yr], plasma 25‐hydroxyvitamin D (0, 1, 3, 5 yr), biomarkers of bone turnover (0, 1, 3, 5 yr), parathyroid hormone (0, 1, 3, 5 yr), intestinal calcium absorption (0, 1, 3, 5 yr) | Adverse events (5 yr) | Overall mortality (5 yr) |
|
Footnotes Primary or secondary endpoint(s) refer to verbatim statements in the publication; other endpoints relate to outcomes that were not specified as 'primary' or 'secondary' outcomes in the publication "‐" denotes not reporte mo: month; wk: weeks; yr: year | |||
Appendix 6. Adverse events
|
Characteristic Study ID |
Intervention(s) and control(s) | Deaths [n/N] | All adverse events [n/N (%)] | Severe/serious adverse events [n/N (%)] | Left study because of adverse events [n/N (%)] |
| Aloia 2005 | I: vitamin D3, calcium | 1/104 | ‐ | 8/104 (7.7) | ‐ |
| C: placebo | 2/104 | ‐ | 7/104 (6.7) | ‐ | |
| total: | 222 | ||||
| Avenell 2004 | I: vitamin D3 | 4/70 | ‐ | ‐ | ‐ |
| C: no intervention | 3/64 | ‐ | ‐ | ‐ | |
| total: | 8 (6) | ‐ | |||
| Avenell 2012 | I: vitamin D3 | 836/2649 | 363/2649 (13.7) | ‐ | ‐ |
| C: matched placebo | 881/2643 | 386/2643 (14.6) | ‐ | ‐ | |
| total: | 33 (0.6) | ‐ | |||
| Baeksgaard 1998 | I: vitamin D3 plus calcium | 0/80 | 2/80 (2.5) | ‐ | ‐ |
| C: matched placebo | 1/80 | 2/80 (2.5) | ‐ | ‐ | |
| Bischoff 2003 | I: vitamin D3 plus calcium | 1/62 | 2/62 (3.2) | ‐ | ‐ |
| C: matched placebo plus calcium | 4/60 | 0/60 (0.0) | ‐ | ‐ | |
| Bjorkman 2007 | I: vitamin D3 plus calcium | 27/150 | ‐ | ‐ | ‐ |
| C: calcium | 9/68 | ‐ | ‐ | ‐ | |
| Bolton‐Smith 2007 | I: vitamin D3 plus calcium | 0/62 | ‐ | ‐ | ‐ |
| C: matched placebo | 1/60 | ‐ | ‐ | ‐ | |
| Brazier 2005 | I: vitamin D3 plus calcium | 3/95 | 15/95 (15.8) | 8/95 (8.4) | 8/95 (8.4) |
| C: matched placebo | 1/97 | 17/97 (17.5) | 10/97 (10.3) | 10/97 (10.3) | |
| Broe 2007 | I: vitamin D2 | 5/99 | ‐ | ‐ | ‐ |
| C: matched placebo | 2/25 | ‐ | ‐ | ‐ | |
| Brohult 1973 | I: vitamin D3 | 1/25 | 3/25 (12.0) | 1/25 (4.0) | 0/25 (0.0) |
| C: placebo | 0/25 | 0/25 (0) | 0/25 (0) | 0/25 (0.0) | |
| Burleigh 2007 | I: vitamin D3 plus calcium | 16/104 | 4/101 (4.0) | 0/101 (0.0) | 1/101 (1.0) |
| C: matched placebo vitamin D3 plus calcium | 13/104 | 3/104 (2.9) | 0/104 (0.0) | 0/104 (0.0) | |
| Campbell 2005 | I: home safety programme | 6/195 | ‐ | ‐ | ‐ |
| C: social visits | 10/196 | ‐ | ‐ | ‐ | |
| Chapuy 1992 | I: vitamin D3 plus calcium | 893/1634 | 40/1634 (2.4) | 40/1634 (2.4) | 40/1634 (2.4) |
| C: double placebo | 917/1636 | 28/1636 (1.7) | 28/1636 (1.7) | 28/1636 (1.7) | |
| Chapuy 2002 | I1: vitamin D3 plus calcium | 70/389 | 27/389 (6.9) | 3/389 (0.8) | ‐ |
| I2: vitamin D3 plus calcium | 46/194 | 16/194 (8.2) | 0/194 (8.2) | ‐ | |
| C: double placebo | 6/583 (1.0) | 6/583 (1.0) | |||
| Chel 2008 | I: vitamin D3 | ‐ | ‐ | ‐ | ‐ |
| C: matched placebo | ‐ | ‐ | ‐ | ‐ | |
| Cherniack 2011 | I: vitamin D3 plus calcium | 1/23 | 4/23 (17.4) | 3/23 (13.0) | 3/23 (13.0) |
| C: matched placebo plus calcium | 0/23 | 4/23 (17.4) | 4/23 (17.4) | 4/23 (17.4) | |
| Cooper 2003 | I: vitamin D2 plus calcium | 0/93 | 8/93 (8.6) | ‐ | 8/93 (8.6) |
| C: calcium | 1/94 | 1/94 (1.1) | ‐ | 1/94 (1.1) | |
| total: | 6/187 (3.2) | ||||
| Coreless 1985 | I: vitamin D2 | 8/41 | 3/41 (7.3) | 1/41 (2.4) | 1/41 (2.4) |
| C: matched placebo | 8/41 | 0/41 (0.0) | 0/41 (0.0) | 0/41 (0.0) | |
| Daly 2006 | I: calcium‐vitamin D3–fortified milk plus calcium | 1/85 | 9/85 (10.6) | 9/85 (10.6) | 9/85 (10.6) |
| C: no intervention | 0/82 | 2/82 (2.4) | 2/82 (2.4) | 2/82 (2.4) | |
| Dawson‐Hughes 1997 | I: vitamin D3 plus calcium | 2/187 | 6/187 (3.2) | 6/187 (3.2) | 6/187 (3.2) |
| C: placebo | 2/202 | 3/202 (1.5) | 3/202 (1.5) | 3/202 (1.5) | |
| Dukas 2004 | I: alfacalcidol | 1/192 | 75/192 (39.0) | 0/192 (0.0) | 0/192 (0.0) |
| C: placebo | 1/186 | 82/186 (44.1) | 0/186 (0.0) | 0/186 (0.0) | |
| Flicker 2005 | I: vitamin D3 plus calcium | ‐ | ‐ | ‐ | ‐ |
| C: calcium | ‐ | ‐ | ‐ | ‐ | |
| Gallagher 2001 | I: calcitriol | 2/123 | 87/123 (71.0) | 55/123 (45.0) | ‐ |
| C: matched placebo | 1/123 | 56/123 (45.5) | 46/123 (37.0) | ‐ | |
| Glendenning 2012 | I: cholecalciferol 150,000 three‐monthly | 2/353 | 24/353 (6.8) | 19/353 (5.4) | ‐ |
| C: placebo vitamin D three‐monthly | 0/333 | 21/333 (6.3) | 15/333 (4.5) | ‐ | |
| Grady 1991 | I: calcitriol | 1/50 | 7/50 (14.0) | 7/50 (14.0) | ‐ |
| C: placebo vitamin D | 0/48 | 2/48 (4.2) | 2/48 (4.2) | ‐ | |
| Grimnes 2011 | I: vitamin D3 | 0/51 | 45/51 (88.0) | ‐ | ‐ |
| C: placebo | 1/53 | 46/53 (87.0) | ‐ | ‐ | |
| Harwood 2004 | I: vitamin D2 plus calcium | 24/113 | ‐ | ‐ | 0/113 (0.0) |
| C: no intervention | 5/37 | ‐ | ‐ | 0/37 (0.0) | |
| Jackson 2006 | I: vitamin D3 plus calcium | 744/18176 | 449/18,176 (2.5) | 449/18,176 (2.5) | ‐ |
| C: matched placebo | 807/18106 | 381/18,106 (2.1) | 381/18,106 (2.1) | ‐ | |
| Janssen 2010 | I: vitamin D3 plus calcium | 0/36 | ‐ | ‐ | ‐ |
| C: matched placebo vitamin D3 plus calcium | 0/34 | ‐ | ‐ | ‐ | |
| Komulainen 1999 | I: vitamin D3 plus calcium | 0/116 | ‐ | 5/116 (4.3) | ‐ |
| C: placebo | 1/116 | ‐ | 4/116 (3.4) | ‐ | |
| Krieg 1999 | I: vitamin D3 plus calcium | 21/124 | 21/124 | 10/124 (8.1) | ‐ |
| C: no treatment | 26/124 | 26/124 | 2/124 (1.6) | ‐ | |
| Kärkkäinen 2010 | I: vitamin D3 plus calcium | 15/1718 | 17/1718 (0.99) | ‐ | ‐ |
| C: no intervention | 13/1714 | 0/1714 (0.0) | ‐ | ‐ | |
| Lappe 2007 | I: vitamin D3 plus calcium | 4/446 | 1/446 (0.2) | 13/446 (2.9) | ‐ |
| C: calcium plus vitamin D placebo | 18/733 | 4/733 (0.5) | 20/733 (2.7) | ‐ | |
| Larsen 2004 | I: home safety inspection | 832/4957 | ‐ | ‐ | ‐ |
| C: vitamin D3 plus calcium | 839/4648 | ‐ | ‐ | ‐ | |
| Latham 2003 | I: vitamin D3 | 11/121 | ‐ | ‐ | ‐ |
| C: matched placebo | 3/122 | ‐ | ‐ | ‐ | |
| Law 2006 | I: vitamin D2 | 347/1762 | ‐ | 28/1762 (1.6) | 28/1762 (1.6) |
| C: no intervention | 322/1955 | ‐ | 1955 (0.0) | 1955 (0.0) | |
| Lehouck 2012 | I: vitamin D3 | 9/91 | 4/91 (4.4) | ‐ | ‐ |
| C: matched placebo | 6/91 | 0/91 (0.0) | ‐ | ‐ | |
| Lips 1996 | I: vitamin D3 | 282/1291 | ‐ | ‐ | ‐ |
| C: matched placebo | 306/1287 | ‐ | ‐ | ‐ | |
| Lips 2010 | I: vitamin D3 | 1/114 | 24/114 (21.0) | 3/114 (2.6) | 3/114 (2.6) |
| C: matched placebo | 0/112 | 26/112 (23.2) | 2/112 (2.7) | 2/112 (2.7) | |
| Lyons 2007 | I: vitamin D2 | 947/1725 | ‐ | ‐ | ‐ |
| C: matched placebo | 953/1715 | ‐ | ‐ | ‐ | |
| Meier 2004 | I: vitamin D3 (500 IU) orally, daily | 0/30 | ‐ | ‐ | ‐ |
| C: no intervention | 1/25 | ‐ | ‐ | ‐ | |
| Mochonis 2006 | I: vitamin D3 plus calcium | 0/42 | 0/42 (0.0) | ‐ | 0/42 (0.0) |
| C: no intervention | 1/70 | 4/70 (5.7) | ‐ | 4/70 (5.7) | |
| Ooms 1995 | I: vitamin D3 | 11/177 | 2/177 (1.1) | ‐ | ‐ |
| C: matched placebo | 21/171 | 0/171 (0.0) | ‐ | ‐ | |
| Ott 1989 | I: vitamin D3 plus calcium | 0/43 | 11/43 (25.6) | ‐ | ‐ |
| C: matched placebo vitamin D plus calcium | 1/43 | 1/43 (2.3) | ‐ | ‐ | |
| Porthouse 2005 | I: vitamin D3 plus calcium | 57/1321 | ‐ | ‐ | ‐ |
| C: no intervention | 68/1993 | ‐ | ‐ | ‐ | |
| Prince 2008 | I: vitamin D2 plus calcium | 0/151 | ‐ | ‐ | ‐ |
| C: matched placebo tablet of vitamin D plus calcium | 1/151 | ‐ | ‐ | ‐ | |
| Sanders 2010 | I: vitamin D3 | 40/1131 | 223/1131 (19.7) | 244/1131 (19.7) | ‐ |
| C: matched placebo tablet | 47/1127 | 201/1127 (17.8) | 207/1127 (17.8) | ‐ | |
| Sato 1997 | I: vitamin D (alfacalcidol) plus calcium | 1/45 | ‐ | ‐ | ‐ |
| C: matched placebo tablets of vitamin D and calcium | 1/39 | ‐ | ‐ | ‐ | |
| Sato 1999a | I: vitamin D (alfacalcidol) | 1/43 | ‐ | ‐ | ‐ |
| C: matched placebo tablet of vitamin D | 0/43 | ‐ | ‐ | ‐ | |
| Sato 1999b | I: vitamin D (alfacalcidol) | 0/34 | ‐ | ‐ | ‐ |
| C: matched placebo tablet of vitamin D | 1/35 | ‐ | ‐ | ‐ | |
| Sato 2005a | I: vitamin D2 | 1/48 | ‐ | ‐ | ‐ |
| C: matched placebo tablet of vitamin D | 2/48 | ‐ | ‐ | ‐ | |
| Schleithoff 2006 | I: vitamin D3 2000 IU plus calcium 500 mg orally, daily | 7/61 | ‐ | ‐ | ‐ |
| C: matched placebo vitamin D plus calcium | 6/62 | ‐ | ‐ | ‐ | |
| Smith 2007 | I: vitamin D2 | 355/4727 | ‐ | ‐ | ‐ |
| C: matched placebo intramuscular injection | 354/4713 | ‐ | ‐ | ‐ | |
| Trivedi 2003 | I: vitamin D3 | 224/1345 | ‐ | ‐ | ‐ |
| C: matched placebo vitamin D | 247/1341 | ‐ | ‐ | ‐ | |
| Witham 2010 | I: vitamin D2 (10,000 IU) orally, daily | 4/53 | 20/53 (37.7) | ‐ | ‐ |
| C: matched placebo tablet | 2/52 | 25/52 (48.1) | ‐ | ‐ | |
| Zhu 2008 | I: vitamin D2 plus calcium | 0/39 | ‐ | ‐ | ‐ |
| C: calcium plus placebo vitamin D | 2/81 | ‐ | ‐ | ‐ | |
|
Footnotes "‐" denotes not reported C: control; I: intervention | |||||
Data and analyses
Comparison 1. Vitamin D versus placebo or no intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All‐cause mortality in trials with low or high risk of bias | 56 | 95286 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.94, 0.99] |
| 1.1 Trials with low risk of bias | 30 | 67516 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.92, 0.99] |
| 1.2 Trials with high risk of bias | 26 | 27770 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.92, 1.06] |
| 2 All‐cause mortality in individually randomised and cluster‐randomised trials | 56 | 95286 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.94, 0.99] |
| 2.1 Individually randomised trials | 54 | 81964 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.93, 0.99] |
| 2.2 Cluster‐randomised trials | 2 | 13322 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.82, 1.34] |
| 3 All‐cause mortality in placebo‐controlled and no intervention trials | 56 | 95286 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.94, 0.99] |
| 3.1 Placebo in the control group | 44 | 73892 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.93, 0.99] |
| 3.2 No intervention in the control group | 12 | 21394 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.91, 1.21] |
| 4 All‐cause mortality and risk of industry bias | 56 | 95286 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.94, 0.99] |
| 4.1 Trials without risk of industry bias | 7 | 7372 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.92, 1.03] |
| 4.2 Trials with risk of industry bias | 49 | 87914 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.93, 1.00] |
| 5 All‐cause mortality in primary and secondary prevention trials | 56 | 95286 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.94, 0.99] |
| 5.1 Primary prevention trials | 48 | 94491 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.94, 0.99] |
| 5.2 Secondary prevention trials | 8 | 795 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [0.73, 2.35] |
| 6 All‐cause mortality and vitamin D status | 56 | 95286 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.94, 0.99] |
| 6.1 Vitamin D insufficiency | 26 | 56697 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.91, 0.99] |
| 6.2 Vitamin D adequacy | 19 | 16283 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.87, 1.05] |
| 6.3 Unknown vitamin D status | 11 | 22306 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.92, 1.13] |
| 7 All‐cause mortality in ambulatory and institutionalised participants | 56 | 95286 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.94, 0.99] |
| 7.1 Ambulatory participants | 45 | 86071 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.92, 0.98] |
| 7.2 Institutionalised participants | 11 | 9215 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.92, 1.13] |
| 8 All‐cause mortality ('best‐worst case' and 'worst‐best case' scenario) | 53 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 8.1 'Best‐worst' case scenario | 53 | 84418 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.32, 0.51] |
| 8.2 'Worst‐best' case scenario | 53 | 84418 | Risk Ratio (M‐H, Random, 95% CI) | 2.78 [2.13, 3.63] |
| 9 All‐cause mortality in trials using vitamin D3 (cholecalciferol) | 38 | 75927 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.91, 0.98] |
| 9.1 Vitamin D3 trials with low risk of bias | 20 | 52645 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.89, 0.98] |
| 9.2 Vitamin D3 trials with high risk of bias | 18 | 23282 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.91, 1.00] |
| 10 All‐cause mortality in trials using vitamin D3 singly or combined with calcium | 38 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 10.1 Vitamin D3 singly | 13 | 12609 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.85, 1.00] |
| 10.2 Vitamin D3 combined with calcium | 27 | 63051 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.92, 0.99] |
| 11 All‐cause mortality in trials using low or high dose of vitamin D3 | 38 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 11.1 Low dose of vitamin D3 (< 800 IU a day) | 13 | 50437 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.87, 0.97] |
| 11.2 High dose of vitamin D3 (≥ 800 IU a day) | 26 | 25558 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.92, 1.00] |
| 12 All‐cause mortality in trials applying vitamin D3 daily or intermittently | 38 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 12.1 Vitamin D3 daily | 31 | 69168 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.91, 0.98] |
| 12.2 Vitamin D3 intermittently | 8 | 6871 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.77, 1.03] |
| 13 All‐cause mortality in trials using vitamin D3 and vitamin D status | 38 | 75927 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.91, 0.98] |
| 13.1 Vitamin D insufficiency | 20 | 55883 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.91, 0.99] |
| 13.2 Vitamin D adequacy | 10 | 4979 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.80, 1.07] |
| 13.3 Unknown vitamin D status | 8 | 15065 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.78, 1.16] |
| 14 All‐cause mortality in trials using vitamin D3 according to the participant's sex | 38 | 75927 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.91, 0.98] |
| 14.1 Vitamin D3 trialsincluding only women | 19 | 53062 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.84, 1.03] |
| 14.2 Vitamin D3 trials including men and women | 19 | 22865 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.89, 0.98] |
| 15 All‐cause mortality in trials using vitamin D2 (ergocalciferol) | 12 | 18349 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.96, 1.08] |
| 15.1 Vitamin D2 trials with low risk of bias | 9 | 14439 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.93, 1.04] |
| 15.2 Vitamin D2 trials with high risk of bias | 3 | 3910 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [1.05, 1.37] |
| 16 All‐cause mortality in trials using vitamin D2 singly or combined with calcium | 12 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 16.1 Vitamin D2 singly | 8 | 17079 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.96, 1.12] |
| 16.2 Vitamin D2 combined with calcium | 5 | 1307 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.64, 1.57] |
| 17 All‐cause mortality in trials using low or high dose of vitamin D2 | 12 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 17.1 Low dose of vitamin D2 | 1 | 101 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.17, 3.98] |
| 17.2 High dose of vitamin D2 | 12 | 18273 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.95, 1.10] |
| 18 All‐cause mortality in trials applying vitamin D2 daily or intermittently | 12 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 18.1 Vitamin D2 daily | 6 | 1349 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.68, 1.12] |
| 18.2 Vitamin D2 intermittently | 6 | 17000 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.95, 1.18] |
| 19 All‐cause mortality in trials using vitamin D2 and vitamin D status | 12 | 18349 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.96, 1.08] |
| 19.1 Vitamin D insufficiency | 6 | 4413 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [1.05, 1.37] |
| 19.2 Vitamin D adequacy | 5 | 10496 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.86, 1.10] |
| 19.3 Unknown vitamin D status | 1 | 3440 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.93, 1.05] |
| 20 All‐cause mortality in trials using alfacalcidol (1α‐hydroxyvitamin D) | 4 | 617 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.22, 4.15] |
| 21 All‐cause mortality in trials using alfacalcidol and vitamin D status | 4 | 617 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.22, 4.15] |
| 21.1 Vitamin D insufficiency | 2 | 155 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.11, 9.52] |
| 21.2 Vitamin D adequacy | 1 | 378 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.06, 15.37] |
| 21.3 Unknown vitamin D status | 1 | 84 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.06, 13.40] |
| 22 All‐cause mortality in trials using calcitriol (1,25‐dihydroxyvitamin D) | 3 | 430 | Risk Ratio (M‐H, Random, 95% CI) | 1.37 [0.27, 7.03] |
| 23 All‐cause mortality in trials using calcitriol and vitamin D status | 3 | 430 | Risk Ratio (M‐H, Random, 95% CI) | 1.37 [0.27, 7.03] |
| 23.1 Vitamin D insufficiency | 1 | 86 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 7.96] |
| 23.2 Vitamin D adequacy | 2 | 344 | Risk Ratio (M‐H, Random, 95% CI) | 2.28 [0.34, 15.39] |
| 24 Cancer mortality | 4 | 44492 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.78, 0.98] |
| 25 Cardiovascular mortality | 10 | 47267 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.90, 1.07] |
| 26 Adverse events | 35 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 26.1 Hypercalcemia in trials using supplemental forms of vitamin D | 15 | 11323 | Risk Ratio (M‐H, Random, 95% CI) | 1.36 [0.85, 2.18] |
| 26.2 Hypercalcemia in trials using active forms of vitamin D | 3 | 710 | Risk Ratio (M‐H, Random, 95% CI) | 3.18 [1.17, 8.68] |
| 26.3 Nephrolithiasis in trials using vitamin D3 combined with calcium | 4 | 42876 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [1.02, 1.34] |
| 26.4 Nephrolithiasis in trials using calcitriol | 1 | 246 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 8.10] |
| 26.5 Hypercalciuria | 3 | 695 | Risk Ratio (M‐H, Random, 95% CI) | 4.64 [0.99, 21.76] |
| 26.6 Renal insufficiency | 3 | 5495 | Risk Ratio (M‐H, Random, 95% CI) | 1.70 [0.27, 10.70] |
| 26.7 Cardiovascular disorders | 8 | 4495 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.86, 1.05] |
| 26.8 Gastrointestinal disorders | 16 | 9702 | Risk Ratio (M‐H, Random, 95% CI) | 1.36 [0.87, 2.13] |
| 26.9 Psychiatric disorders | 3 | 580 | Risk Ratio (M‐H, Random, 95% CI) | 1.44 [0.56, 3.73] |
| 26.10 Skin disorders | 2 | 3810 | Risk Ratio (M‐H, Random, 95% CI) | 3.27 [0.17, 62.47] |
| 26.11 Cancer | 14 | 49707 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.94, 1.06] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Aloia 2005.
| Methods | Randomised, double‐blind, placebo‐controlled trial using parallel group design (two intervention groups). | |
| Participants | Country: United States. Number of participants randomised: 208 healthy calcium‐replete, black postmenopausal African American women, 50 to 75 (mean 60) years of age. African American ancestry of the participants was assessed by self‐declaration that both parents and at least three of four grandparents were African American. Inclusion criteria: ambulatory postmenopausal African American women not receiving hormone therapy. Exclusion criteria: previous treatment with bone active agents and any medication or illness that affects skeletal metabolism. |
|
| Interventions | Participants were randomly assigned to receive: Intervention group: vitamin D3 (800 IU) plus calcium (1200 to 1500 mg) daily, (n = 104); Control group: matched placebo plus calcium (1200 to 1500 mg) daily, (n = 104); for a two‐year period. After two years, the vitamin D3 dose was increased to 2000 IU daily in the intervention group, and the trial continued for an additional year. The calcium supplements were provided as calcium carbonate. |
|
| Outcomes | The primary outcome measure was the bone mineral density of the total hip. | |
| Stated aim of study | "To examine the effect of vitamin D3 supplementation on bone loss in African American women." | |
| Notes | "81 participants from the intervention group and 78 participants form the control group completed two years in the trial. 81 participants from the intervention group switched to vitamin D3 2000 IU daily plus 1200 to 1500 mg of calcium daily after two years. 78 participants from the control group switched to matched placebo plus 1200 to 1500 mg of calcium daily after two years. 74 participants from the intervention group completed 36 months of trial. 74 participants from the control group completed 36 months of the trial. A total of 222 adverse events were reported in the trial over three years. There were 15 serious adverse events, eight in the intervention group and seven in the control group. Mean pill count compliance was 87% ± 8% of vitamin D3 pills consumed after the randomisation visit." Vitamin D3 capsules and matched placebo capsules were custom manufactured for the trial (Tishcon Corp, Westbury, NY). Vitamin D3 content was also analysed in an independent laboratory (Vitamin D, Skin, and Bone Research Laboratory, Department of Medicine, Boston University School of Medicine, Boston, Mass). The calcium supplements were provided as calcium carbonate." Additional information on the risk of bias domains was received through personal communication with Dr John F Aloia (30.01.2009; 03.02.2009). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequence generation was achieved using computer random number generation. |
| Allocation concealment (selection bias) | Low risk | Allocation was controlled by a central and independent randomisation unit so that intervention allocations could not have been foreseen in advance of, or during, enrolment. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was described as blinded, the parties that were blinded, and the method of blinding was described, so that knowledge of allocation was adequately prevented during the trial. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The numbers and reasons for dropouts and withdrawals in all intervention groups were described. |
| Selective reporting (reporting bias) | Low risk | Pre‐defined, or clinically relevant and reasonably expected outcomes are reported on. |
| Industry bias | Unclear risk | Vitamin D3 capsules and matched placebo capsules were custom manufactured for the trial (Tishcon Corp, Westbury, NY). |
| Other bias | Low risk | The trial appears to be free of other components that could put it at risk of bias. |
Avenell 2004.
| Methods | Randomised clinical trial using 2 x 2 factorial design. | |
| Participants | Country: United Kingdom. Number of participants randomised: 134, aged 70 years or over (mean age 77), 83% women. Inclusion criteria: people aged 70 years or over with an osteoporotic fracture within the last 10 years. Exclusion criteria: daily oral treatment with more than 200 IU (5 µg) vitamin D or more than 500 mg calcium or other bone active medications. |
|
| Interventions | Participants were randomly assigned to receive: Intervention group 1: vitamin D3 (800 IU) daily (n = 35); Intervention group 2: calcium (1000 mg) daily (n = 29); Intervention group 3: vitamin D3 (800 IU) plus calcium (1000 mg) daily (n = 35); Intervention group 4 (Control group): no tablets daily (n = 35); for a one‐year period. The calcium supplements were provided as calcium carbonate. |
|
| Outcomes | Primary outcomes were recruitment, compliance, and retention within a randomised trial. | |
| Stated aim of study | "To assess the effects of an open trial design (without placebo and participants knowing what tablets they were given) when compared with a blinded, placebo‐controlled design on recruitment, compliance, and retention within a randomised trial of secondary osteoporotic fracture prevention." | |
| Notes | "All participants were asked to return unconsumed tablets for a tablet count compliance. Compliance amongst those who returned their tablet containers was similar (overall 85% versus 84.5% of tablet takers took their tablets on more than 80% of days). The same pattern was observed for self‐reported tablet consumption at four, eight or 12 months during the trial." "Shire Pharmaceuticals funded the capsules, which were co‐funded and manufactured by Nycomed." Additional information on mortality was received through personal communication with Dr Alison Avenell (28.01.2009). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequence generation was achieved using computer random number generation. |
| Allocation concealment (selection bias) | High risk | Participants were told to which compound they had been allocated. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Trial was not blinded, so that the allocation was known during the trial. Participants were told to which compound they had been allocated. Placebo was not used. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The numbers and reasons for dropouts and withdrawals in all intervention groups were described. |
| Selective reporting (reporting bias) | Low risk | Pre‐defined, or clinically relevant and reasonably expected outcomes are reported on. |
| Industry bias | Unclear risk | "Shire Pharmaceuticals funded the capsules, which were co‐funded and manufactured by Nycomed." |
| Other bias | Low risk | The trial appears to be free of other components that could put it at risk of bias. |
Avenell 2012.
| Methods | Randomised Evaluation of Calcium Or vitamin D (RECORD). Multicentre, randomised, double‐blind, placebo‐controlled trial using 2 x 2 factorial design. |
|
| Participants | Country: United Kingdom. Number of participants randomised: 5292 people (85% women) aged 70 and over (mean 77 years) with low‐trauma, osteoporotic fracture in the previous 10 years. Inclusion criteria: elderly people aged 70 years or older, who were mobile before developing a low‐trauma fracture. Exclusion criteria: bed or chair bound before fracture; cognitive impairment indicated by an abbreviated mental test score of less than seven; cancer in the past 10 years that was likely to metastasise to bone; fracture associated with pre‐existing local bone abnormality; those known to have hypercalcaemia; renal stone in the past 10 years; life expectancy of less than 6 months; individuals known to be leaving the United Kingdom; daily intake of more than 200 IU vitamin D or more than 500 mg calcium supplements; intake in the past 5 years of fluoride, bisphosphonates, calcitonin, tibolone, hormone‐replacement therapy, selective oestrogen‐receptor modulators, or any vitamin D metabolite (e.g., calcitriol); and vitamin D by injection in the past year. |
|
| Interventions | Participants were randomly assigned to receive: Intervention group 1: vitamin D3 (800 IU) daily (n = 1343); Intervention group 2: calcium (500 mg) daily (n = 1311); Intervention group 3: vitamin D3 (800 IU) plus calcium (500 mg) daily (n = 1306); Intervention group 4 (Control group): matched placebo tablets (n = 1332); for a 45 month period. Participants were followed for a period of 6.2 years. Tablets varied in size and taste, and thus each had matching placebos. |
|
| Outcomes | The primary outcome measure was all‐new low‐energy fractures including clinical, radiologically confirmed vertebral fractures, but not those of the face or skull. | |
| Stated aim of study | "To assess whether vitamin D3 and calcium, either alone or in combination, were effective in prevention of secondary fractures." | |
| Notes | "Compliance was measured by a postal questionnaire sent every four months, in which participants were asked how many days of the past seven days they had taken tablets. A randomly selected 10% sample was asked to return unused tablets for pill counting. Based on questionnaire responses at 24 months, 2886 (54,5%) of 5292 were still taking tablets. Throughout the trial about 80% of those taking tablets did so on more than 80% of days, which is consistent with pill counts in the subsample (data not shown). However, the number who were taking any tablets fell over time. At 24 months, 2268 of 4841 (46,8%), who returned questionnaires, had taken pills on more than 80% of days." Shire Pharmaceuticals co‐funded the drugs, with Nycomed, who also manufactured the drugs. Additional information received through personal communication with Dr Alison Avenell (02.02.2009). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequence generation was achieved using computer random number generation. |
| Allocation concealment (selection bias) | Low risk | Allocation was controlled by a central and independent randomisation unit so that intervention allocations could not have been foreseen in advance of, or during, enrolment. "Allocation was controlled by a central and independent randomisation unit. The allocation programme was written by the trial programmer and the allocation remained concealed until the final analyses (other than for confidential reports to the data monitoring committee)." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was described as blinded, the parties that were blinded, and the method of blinding was described, so that knowledge of allocation was adequately prevented during the trial. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The numbers and reasons for dropouts and withdrawals in all intervention groups were described. |
| Selective reporting (reporting bias) | Low risk | Pre‐defined, or clinically relevant and reasonably expected outcomes are reported on. |
| Industry bias | Unclear risk | Shire Pharmaceuticals co‐funded the drugs, with Nycomed, who also manufactured the drugs. |
| Other bias | Low risk | The trial appears to be free of other components that could put it at risk of bias. |
Baeksgaard 1998.
| Methods | Randomised, double‐blind, placebo‐controlled trial using parallel group design (three intervention groups). | |
| Participants | Country: Denmark. Number of participants randomised: 240 healthy postmenopausal women, 58 to 67 (mean 62.5) years of age. Inclusion criteria: Caucasian background, age 58 to 67 years, good general health and postmenopausal status defined as cessation of menstrual bleeding for at least six months. Exclusion criteria: treatment with oestrogen or calcitonin during the previous 12 months or with bisphosphonates in the previous 24 months, presence of diseases known to affect bone metabolism, renal disease with serum creatinine above 120 mmol/L, and hepatic disease with increased alanine aminotransferase and/or decreased extrinsic coagulation factors II, VII and X. |
|
| Interventions | Participants were randomly assigned to receive: Intervention group 1: vitamin D3 (560 IU) plus calcium 1000 mg daily, (n = 80); Intervention group 2: vitamin D3 (560 IU) plus calcium (1000 mg) plus multivitamin containing retinol 800 μg; thiamine 1.4 mg; riboflavine 1.6 mg; pyridoxine 2 mg; cyanocobalamin 1 μg; folic acid 100 μg; niacin 18 mg; pantothenic acid 6 mg; biotin 150 μg; ascorbic acid 60 mg; D‐alpha tocopherol 10 mg; and phylloquinone 70 μg; daily, (n = 80); Intervention group 3 (Control group): matched placebo in a similar combination daily (n = 80); for a two‐year period. Participants were asked to take no calcium or vitamin D supplement other than the supplement supplied for the trial. Calcium was in the form of calcium carbonate. |
|
| Outcomes | The primary outcome was changes from baseline in the bone mineral density (BMD) in the lumbar spine (L2–4). Secondary outcome measures were hip BMD, forearm BMD, serum calcium, serum phosphate and serum intact parathyroid hormone. | |
| Stated aim of study | "To evaluate the effect of vitamin D supplement and a calcium supplement plus or minus multivitamins on bone loss at the hip, spine, and forearm." | |
| Notes | "For all variables measured, authors observed no significant differences between the two experimental intervention groups. In presenting the results, authors, therefore, considered the two groups as one group. During the trial, 41 of the 240 women dropped out. No significant difference in drop‐out rate was found between the groups. One hundred and ninety‐nine women completed all visits. In the analysis, an additional two women were excluded due to development of radiologically verified vertebral fractures in the lumbar spine. No formal assessment of compliance, such as tablet counting, was made. At each visit, the participants were questioned about their compliance with the trial medication and encouraged to comply." All placebo and active treatment tablets were provided by Lube Ltd. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The trial is described as randomised but the method of sequence generation was not specified. |
| Allocation concealment (selection bias) | Unclear risk | The trial was described as randomised but the method used to conceal the allocation was not described, so that intervention allocations may have been foreseen in advance of, or during, enrolment. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was described as blinded, the parties that were blinded, and the method of blinding was described, so that knowledge of allocation was adequately prevented during the trial. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The numbers and reasons for dropouts and withdrawals in all intervention groups were described |
| Selective reporting (reporting bias) | Unclear risk | Not all pre‐defined or clinically relevant and reasonably expected outcomes are reported on. |
| Industry bias | Unclear risk | All placebo and active treatment tablets were provided by Lube Ltd. |
| Other bias | Low risk | The trial appears to be free of other components that could put it at risk of bias. |
Bischoff 2003.
| Methods | Randomised, double‐blind, controlled trial using parallel group design (two intervention groups). | |
| Participants | Country: Switzerland. Number of participants randomised: 122 elderly women in long‐stay geriatric care, aged 60 years or older (mean age 85.3 years). Inclusion criteria: age 60 or older and the ability to walk three meters with or without a walking aid. Exclusion criteria: primary hyperparathyroidism, hypocalcaemia, hypercalciuria, renal insufficiency, and fracture or stroke within the last three months, any treatment with hormone replacement therapy, calcitonin, fluoride, or bisphosphonates during the previous 24 months. |
|
| Interventions | Participants were randomly assigned to receive: Intervention group 1: vitamin D3 (800 IU) plus calcium 1200 mg daily (n = 62); Intervention group 2 (Control group): calcium 1200 mg daily (n = 60); for a three‐month period. |
|
| Outcomes | The primary outcome measure was number of falls per person. Secondary outcome measures were musculoskeletal function and bone remodeling. | |
| Stated aim of study | "To evaluate hypothesis that higher vitamin D serum levels may increase muscle strength and reduce the number of falls." | |
| Notes | "Tablets containing vitamin D and calcium or calcium alone were taken in the presence of the trial nurse to ensure compliance." The trial was supported by Strathmann AG, Germany. Authors reported deaths but not according to intervention group of the trial. All‐cause mortality data was taken from a Cochrane systematic review prepared by Avenell et al (Avenell 2009) who obtained mortality data by personal communication with Bischoff trial authors. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The trial is described as randomised but the method of sequence generation was not specified. |
| Allocation concealment (selection bias) | Low risk | Allocation was controlled by sealed envelopes so that intervention allocations could not have been foreseen in advance of, or during, enrolment. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was described as blinded, the parties that were blinded, and the method of blinding was described, so that knowledge of allocation was adequately prevented during the trial. "Tablets in both groups had an identical appearance. Participants, nurses, and all investigators were blinded to the intervention assignment throughout the trial." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The numbers and reasons for dropouts and withdrawals in all intervention groups were described. |
| Selective reporting (reporting bias) | Low risk | Pre‐defined, or clinically relevant and reasonably expected outcomes are reported on. |
| Industry bias | High risk | The trial was supported by Strathmann AG, Germany. |
| Other bias | Low risk | The trial appears to be free of other components that could put it at risk of bias. |
Bjorkman 2007.
| Methods | Randomised, double‐blind, placebo‐controlled trial using parallel group design (three intervention groups). | |
| Participants | Country: Finland. Number of participants randomised: 218 chronically bedridden patients (81.7 % women), 65 to 104 (mean 84.5) years of age. Inclusion criteria: age over 65 years, chronically impaired mobility, stable general condition, and no known present disease (except osteoporosis) or medication (vitamin D supplements, glucocorticoids, antiepileptics, etc.) affecting calcium or bone metabolism. Exclusion criteria: markedly elevated creatinine levels (> 125 µmol/L) hypercalcaemia (ionised calcium > 1.32 mmol/L), hypothyroidism (thyrotropin > 5.3 mU/L) or hyperthyroidism (thyrotropin < 0.2 mU/L). |
|
| Interventions | Participants were randomly assigned to receive: Intervention group 1: vitamin D3 (1200 IU) daily, (n = 73); 17 participants from this group received calcium 500 mg daily; Intervention group 2: vitamin D3 (400 IU) daily, (n = 77); 11 participants from this group received calcium 500 mg daily; Intervention group 3 (Control group): matched placebo vitamin D3 (0 IU) daily (n = 68), 15 participants from this group received calcium 500 mg daily; for a six‐month period. "Participants received vitamin D3 (Vigantol, Merck KGaA, Darmstadt, Germany 20,000 IU/ml in Migliol oil) in doses of 0 µg, 140 µg, or 420 µg (groups 1, 2, 3) every 2 weeks, equivalent with average daily intakes of 0 IU, 400 IU, or 1200 IU. To ensure that all three groups received identical volumes (26 drops = 0.84 ml), medication oil was diluted three‐fold with Migliol oil in group 2, and group 1 received plain Migliol oil. Furthermore, the oil was swallowed entirely in the presence of the nurse and given with a small amount of food or drink, if necessary." "Before the start of the intervention, the use of dairy products was roughly evaluated to be insufficient among 40 patients, who received a daily calcium carbonate substitution of 500 mg during the intervention. Three other patients also received a previous daily medication of 500 mg calcium carbonate at entry, which they continued to receive through the intervention." |
|
| Outcomes | The primary outcome measures were parathyroid function and bone turnover. | |
| Stated aim of study | "To evaluate the effects of vitamin D supplementation on parathyroid function and bone turnover in aged, chronically immobile patients." | |
| Notes | "Vitamin D supplementation was well tolerated. One patient, however, developed a mild hypercalcaemia (ionised calcium from 1.24 to 1.40 mmol/L) in group 3." Treatment agents were produced by Vigantol, Merck KGaA, Darmstadt, Germany. Authors did not provide data about compliance. Additional information on the risk of bias domains was received through personal communication with Dr Mikko Björkman (31.01.2009). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequence generation was achieved using computer random number generation. |
| Allocation concealment (selection bias) | Low risk | "Allocation was controlled by coded bottles. Each bottle was individually coded to blind the participants and the ward nurses of not only the content of the bottles but also of the group labels (1, 2, 3)." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was described as blinded, the parties that were blinded, and the method of blinding was described, so that knowledge of allocation was adequately prevented during the trial. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The numbers and reasons for dropouts and withdrawals in all intervention groups were described. |
| Selective reporting (reporting bias) | Low risk | Pre‐defined, or clinically relevant and reasonably expected outcomes are reported on. |
| Industry bias | Unclear risk | Treatment agents were produced by Vigantol, Merck KGaA, Darmstadt, Germany. |
| Other bias | Low risk | The trial appears to be free of other components that could put it at risk of bias. |
Bolton‐Smith 2007.
| Methods | Randomised, double‐blind, placebo controlled trial using 2 x 2 factorial design. | |
| Participants | Country: United Kingdom. Number of participants randomised: 244 healthy, non‐osteoporotic women, aged 60 years or over (mean 68). Inclusion criteria: healthy, non‐osteoporotic women, aged 60 years or over. Exclusion criteria: clinical osteoporosis or chronic disease (e.g., diabetes mellitus, cardiovascular disease, cancer, fat malabsorption syndromes), routine medication that interferes with vitamin K, vitamin D, or bone metabolism (notably warfarin and steroids), and consumption of nutrient supplements that provided in excess of 30 µg vitamin K, 400 IU vitamin D, or 500 mg calcium daily. |
|
| Interventions | Participants were randomly assigned to receive: Intervention group 1: vitamin D3 (400 IU) plus calcium 1000 mg daily, (n = 62); Intervention group 2: vitamin D3 (400 IU) plus calcium 1000 mg plus vitamin K1 200 μg daily, (n = 61); Intervention group 3: vitamin K1 200 μg daily (n = 60); Intervention group 4 (Control group): matched placebo daily (n = 61); for a two‐year period. |
|
| Outcomes | The primary outcome measure was bone mineral density. Secondary outcome measure was possible interaction with vitamin K, of vitamin D and calcium. | |
| Stated aim of study | "The putative beneficial role of high dietary vitamin K1 (phylloquinone) on bone mineral density and the possibility of interactive benefits with vitamin D were studied." | |
| Notes | "Of the 244 eligible women randomised in the trial, 209 (85.6%) completed the two‐year trial. Compliance with the trial intervention was good based on pill count (median, 99; interquartile range, 97.3 to 99.8%)." Hoffmann‐La Roche (Basel, Switzerland) provided the supplementation tablets. Additional information on mortality, adverse events, and risk of bias domains was received through personal communication with Dr Martin J Shearer (03.02.2009; 05.02.2010). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequence generation was achieved using computer random number generation. |
| Allocation concealment (selection bias) | Low risk | Allocation was controlled by a central and independent randomisation unit so that intervention allocations could not have been foreseen in advance of, or during, enrolment. "An independent statistician at Hoffmann‐La Roche, who had no other connection to the trial, provided a randomisation list to the researchers." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was described as blinded, the parties that were blinded, and the method of blinding was described, so that knowledge of allocation was adequately prevented during the trial. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The numbers and reasons for dropouts and withdrawals in all intervention groups were described. |
| Selective reporting (reporting bias) | Low risk | Pre‐defined, or clinically relevant and reasonably expected outcomes are reported on. |
| Industry bias | Unclear risk | Hoffmann‐La Roche (Basel, Switzerland) provided the supplementation tablets. |
| Other bias | Low risk | The trial appears to be free of other components that could put it at risk of bias. |
Brazier 2005.
| Methods | Multicentre, randomised, double‐blind, placebo‐controlled trial using parallel group design (two intervention groups). | |
| Participants | Country: France. Number of participants randomised: 192 women with a 25‐hydroxyvitamin D level ≤ 12 ng/mL, mean age 74.6 years. Inclusion criteria: community‐dwelling ambulatory women aged > 65 years who spontaneously consulted a practitioner and presented with vitamin D insufficiency (i.e., serum 25‐hydroxy vitamin D ≤ 12 ng/mL). Exclusion criteria: hypercalcaemia (serum calcium > 2.62 mmol/L), primary hyperparathyroidism, renal insufficiency (serum creatinine >130 pmol/L), hepatic insufficiency, treatment with a bisphosphonate, calcitonin, vitamin D or its metabolites, oestrogen, raloxifene, fluoride, anticonvulsives, or any other drug acting on bone metabolism (e.g., glucocorticoids) in the past six months. |
|
| Interventions | Participants were randomly assigned to receive: Intervention group 1: vitamin D3 (800 IU) plus calcium (1000 mg) daily (n = 95); Intervention group 2 (Control group): matched placebo tablets (n = 97); for a one‐year period. |
|
| Outcomes | The primary outcome was to assess the effects of vitamin D3 plus calcium on bone mineral density and biochemical markers of bone formation and resorption. Secondary outcome was to evaluate the clinical and laboratory safety of treatment. | |
| Stated aim of study | "An evaluation of the clinical and laboratory safety of a one‐year course of treatment with a combination vitamin D and calcium tablets in ambulatory women aged > 65 years with vitamin D insufficiency." | |
| Notes | Fifty women (21/95 vitamin D plus calcium, 29/97 placebo) were prematurely withdrawn from the trial for various reasons. Treatment‐related adverse events were reported in 21 and 23 women in the respective intervention groups. These events consisted mainly of metabolic disorders (9 and 10), particularly hypercalcaemia (6 and 8) and gastrointestinal disorders (9 and 8). "Treatment compliance was assessed at each visit based on counts of the number of tablets taken compared with the number that was to be taken. Compliance at each visit ranged from a median of 93% to 94% in the vitamin D plus calcium group and from 93% to 96.5% in the placebo group. Global compliance was 92% in the vitamin D plus calcium group and 92.5% in the placebo group. No significant difference in compliance was observed between the two groups at any visit." This trial was supported by Innothera Laboratories, Arcueil, France. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequence generation was achieved using computer random number generation. |
| Allocation concealment (selection bias) | Unclear risk | The trial was described as randomised but the method used to conceal the allocation was not described, so that intervention allocations may have been foreseen in advance of, or during, enrolment. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | The trial was described as double blind, but the method of blinding was not described, so that knowledge of allocation was possible during the trial. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The numbers and reasons for dropouts and withdrawals in all intervention groups were described. |
| Selective reporting (reporting bias) | Low risk | Pre‐defined, or clinically relevant and reasonably expected outcomes are reported on. |
| Industry bias | High risk | This trial was supported by Innothera Laboratories, Arcueil, France. |
| Other bias | Low risk | The trial appears to be free of other components that could put it at risk of bias. |
Broe 2007.
| Methods | Randomised, double‐blind, placebo‐controlled trial using parallel group design (five intervention groups). | |
| Participants | Country: United States. Number of participants randomised: 124 nursing home residents (73% women), mean 89 years of age. Inclusion criteria: a life expectancy of at least six months, the ability to swallow medication, and three months residency at Hebrew Rehabilitation Center for the Aged. Exclusion criteria: use of glucocorticoids, anti‐seizure medication, or pharmacological doses of vitamin D; calcium metabolism disorders; severe mobility limitations; or fracture within the previous six months. |
|
| Interventions | Participants were randomly assigned to receive: Intervention group 1: vitamin D2 (800 IU) daily (n = 23); Intervention group 2: vitamin D2 (600 IU) daily (n = 25); Intervention group 3: vitamin D2 (400 IU) daily (n = 25); Intervention group 4: vitamin D2 (200 IU) daily (n = 26); Intervention group 5 (Control group): matched placebo tablets daily (n = 25); for a five‐month period. |
|
| Outcomes | The primary outcome measure was effect of the vitamin D doses on falls over the trial period. | |
| Stated aim of study | "To determine the effect of four vitamin D supplement doses on the risk of falls in elderly nursing home residents." | |
| Notes | "Over the 5‐month trial period, 114 completed the trial. Of the 10 participants who did not complete the trial, seven died and three withdrew. There were no significant differences between the intervention groups in the number who did not complete the 5‐month trial period with a loss of one to three participants from each intervention group." "Compliance was calculated as the number of pills taken, as determined according to blister pack counts after the completion of the trial divided by the total days a participant was actively participating (alive, living at Hebrew Rehabilitation Center for Aged, not withdrawn from the trial)." "Average compliance was 97.6%, with only two participants having a compliance level of less than 50%. Compliance did not differ between the intervention groups." The vitamin D2 tablets were purchased from Tishcon Corporation (Westbury, NY). Vitamin D content of the supplements was verified at the BU Vitamin D Laboratory. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequence generation was achieved using computer random number generation. |
| Allocation concealment (selection bias) | Low risk | Allocation was controlled by a central and independent randomisation unit so that intervention allocations could not have been foreseen in advance of, or during, enrolment. "The pharmacy of The Hebrew Rehabilitation Center for the Aged randomised participants in blocks of 15 to one of the five intervention groups." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was described as blinded, the parties that were blinded, and the method of blinding was described, so that knowledge of allocation was adequately prevented during the trial. "The pharmacy labelled pill blister packs with names and patient identification numbers only. Blister packs and tablets from all five groups were identical in appearance and taste, so nursing staff, participants, and the trial team were unaware of the group assignment." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The numbers and reasons for dropouts and withdrawals in all intervention groups were described. |
| Selective reporting (reporting bias) | Low risk | Pre‐defined, or clinically relevant and reasonably expected outcomes are reported on. |
| Industry bias | Unclear risk | The vitamin D2 tablets were purchased from Tishcon Corporation (Westbury, NY). |
| Other bias | Low risk | The trial appears to be free of other components that could put it at risk of bias. |
Brohult 1973.
| Methods | Randomised, double‐blind, controlled trial using parallel group design (two intervention groups). | |
| Participants | Country: Sweden. Number of participants randomised: 100 (68 % women), aged 18 to 69 years (mean age 52). Inclusion criteria: ambulatory patients with rheumatoid arthritis of at least two years duration. Exclusion criteria: patients with steroid, gold, or antimalaria therapy. |
|
| Interventions | Participants were randomly assigned to receive: Intervention group 1: vitamin D3 (100,000 IU) daily (n = 25); Intervention group 2 (Control group): placebo daily (n = 25); for a one year period. |
|
| Outcomes | The primary outcomes were subjective an objective improvement. | |
| Stated aim of study | To determine the effect of vitamin D supplementation on objective and subjective improvement of patients with rheumatoid arthritis. | |
| Notes | The trial was supported financially by a grant from Ekhagastiftelsen. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The trial is described as randomised, but the method of sequence generation was not specified. |
| Allocation concealment (selection bias) | Unclear risk | The trial was described as randomised, but the method used to conceal the allocation was not described so that intervention allocations may have been foreseen in advance of, or during, enrolment. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | There is insufficient information to assess whether the type of blinding used is likely to induce bias on the estimate of effect. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The underlying reasons for missing data are unlikely to make treatment effects depart from plausible values. |
| Selective reporting (reporting bias) | Unclear risk | Pre‐defined, or clinically relevant and reasonably expected outcomes are reported on. |
| Industry bias | High risk | The trial was supported financially by a grant from Ekhagastiftelsen. |
| Other bias | Low risk | The trial appears to be free of other components that could put it at risk of bias. |
Burleigh 2007.
| Methods | Randomised, double‐blind, controlled trial using parallel group design (two intervention groups). | |
| Participants | Country: United Kingdom. Number of participants randomised: 205 (59 % women), aged 65 years or over (mean age 83), acute admissions to a geriatric medical unit. Inclusion criteria: patients newly transferred or admitted into the general assessment and rehabilitation wards in an acute geriatric unit aged 65 years or over. Exclusion criteria: known hypercalcaemia, urolithiasis or renal dialysis therapy, terminal or bed‐bound patients with a reduced Glasgow Coma Scale, those already prescribed vitamin D supplements and calcium, and those who were deemed 'nil by mouth'. |
|
| Interventions | Participants were randomly assigned to receive: Intervention group 1: vitamin D3 (800 IU) plus calcium (1200 mg) daily (n = 101); Intervention group 2 (Control group): calcium (1200 mg) daily (n = 104); for a 30‐day period. |
|
| Outcomes | The primary outcomes were numbers of fallers and falls. | |
| Stated aim of study | "To determine whether routine supplementation with vitamin D plus calcium reduces numbers of fallers and falls in a cohort of hospital admissions while they are inpatients." | |
| Notes | "Vitamin D and calcium were well tolerated in the total trial cohort with a median compliance level of 88%." Strakan Pharmaceuticals supplied all trial drugs free of charge. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequence generation was achieved using a random number table. |
| Allocation concealment (selection bias) | Low risk | Allocation was controlled by a central and independent randomisation unit so that intervention allocations could not have been foreseen in advance of, or during, enrolment. Randomisation was known only to the statistician and pharmacist. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was described as blinded, the parties that were blinded, and the method of blinding was described, so that knowledge of allocation was adequately prevented during the trial. "Statistician and pharmacist subsequently issued an appropriate uniquely numbered drug blister pack to each patient's ward. Thereafter, trained staff nurses administered trial drugs as part of routine drug rounds. The researchers, therapists, and patients remained blinded to trial drug allocation." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The numbers and reasons for dropouts and withdrawals in all intervention groups were described. |
| Selective reporting (reporting bias) | Low risk | Pre‐defined, or clinically relevant and reasonably expected outcomes are reported on. |
| Industry bias | Unclear risk | Strakan Pharmaceuticals supplied all trial drugs free of charge. |
| Other bias | Low risk | The trial appears to be free of other components that could put it at risk of bias. |
Campbell 2005.
| Methods | Randomised controlled trial using 2 x 2 factorial design. The VIP (visual impairment) trial. |
|
| Participants | Country: New Zealand. Number of participants randomised: 391 elderly people (68 % women) aged 75 to 96 (mean 83.6) years, with visual acuity of 6/24 or worse, who were living in the community. Inclusion criteria: elderly people aged 75 years or over with visual acuity of 6/24 or worse who were living in the community. Exclusion criteria: those who could not walk around their own residence, who were receiving physiotherapy at the time of recruitment, or could not understand the trial requirements. |
|
| Interventions | Participants were randomly assigned to receive: Intervention group 1: home safety assessment and modification programme delivered by an occupational therapist (n = 100); Intervention group 2: an exercise programme prescribed at home by a physiotherapist plus vitamin D3 100,000 IU initially and then 50,000 IU monthly (n = 97); Intervention group 3: both interventions (intervention 1 plus intervention 2) (n = 98); Intervention group 4 (Control group): social visits (n = 96); for a one‐year period. The one‐year exercise intervention consisted of the specific muscle strengthening and balance retraining exercises that progress in difficulty and a walking plan, modified for those with severe visual acuity loss, with vitamin D supplementation. The home safety assessment and modification programme was specifically designed for people with severe visual impairments. The occupational therapist visited the person at home and used a home safety assessment checklist to identify hazards and to initiate discussion with the participant about any items, behaviour, or lack of equipment that could lead to falls. Research staff made two home visits lasting an hour each during the first six months of the trial to participants in intervention group four. |
|
| Outcomes | The primary outcome measures were number of falls and number of injuries resulting from falls. Secondary outcome measure was costs of implementing the home safety programme. | |
| Stated aim of study | "To assess the efficacy and cost effectiveness of a home safety programme and a home exercise programme to reduce falls and injuries in older people with poor vision." | |
| Notes | Additional information received through personal communication with Professor John Campbell (19.02.2010). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequence generation was achieved using a random number table. |
| Allocation concealment (selection bias) | Low risk | Allocation was controlled by a central and independent randomisation unit so that intervention allocations could not have been foreseen in advance of, or during, enrolment. "The schedule was held by an independent person at a separate site and was accessed by a research administrator for the trial, who telephoned after each baseline assessment was completed. The administrator then informed the occupational therapist, physiotherapist, or social visitor, who delivered the assigned intervention to that participant where possible within the next two weeks." |
| Blinding (performance bias and detection bias) All outcomes | High risk | Trial was not blinded, so that the allocation was known during the trial. Placebo was not used. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The numbers and reasons for dropouts and withdrawals in all intervention groups were described. |
| Selective reporting (reporting bias) | Low risk | Pre‐defined, or clinically relevant and reasonably expected outcomes are reported on. |
| Industry bias | Low risk | The trial is not funded by a manufacturer of vitamin D. |
| Other bias | Low risk | The trial appears to be free of other components that could put it at risk of bias. |
Chapuy 1992.
| Methods | Vitamin D, Calcium, Lyon Study I (DECALYOS I). Randomised, double‐blind, placebo controlled trial using parallel group design (two intervention groups). |
|
| Participants | Country: France. Number of participants randomised: 3270, 69 to 106 (mean 84) years of age, healthy ambulatory women. Inclusion criteria: ambulatory woman (with activity levels ranging from going outdoors easily to walk indoors with a cane or a walker), with no serious medical conditions, and with a life expectancy of at least 18 months. Exclusion criteria: receiving drugs known to alter bone metabolism, such as corticosteroids, thyroxine, or anticonvulsant drugs within the past year, women who had been treated with fluoride salts for more than three months, or with vitamin D or calcium during the previous six months or for more than one year within the past five years. |
|
| Interventions | Participants were randomly assigned to receive: Intervention group 1: vitamin D3 (800 IU) plus calcium (1200 mg) daily (n = 1634); Intervention group 2 (Control group): double placebo daily (n = 1636); for a 18 month period. Participants were followed for four years. Calcium was in a form of tricalcium phosphate powder in an aqueous suspension. Placebo pills contained lactose and suspension of lactose, kaolin, and starch. The supplements were taken in the presence of a nurse to ensure compliance. |
|
| Outcomes | The primary outcome was frequency of hip fractures and other nonvertebral fractures, identified radiologically. | |
| Stated aim of study | "To evaluate if vitamin D and calcium supplements reduce the risk of hip fractures and other nonvertebral fractures identified radiologically." | |
| Notes | Duphar and Company Laboratories provided the vitamin D3 (Devaron), and Merck‐Clevenot Laboratories provided the tricalcium phosphate (Ostram). Additional information on mortality was received through personal communication with Professor Pierre Meunier (27.02.2010). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The trial is described as randomised but the method of sequence generation was not specified. |
| Allocation concealment (selection bias) | Unclear risk | The trial was described as randomised but the method used to conceal the allocation was not described, so that intervention allocations may have been foreseen in advance of, or during, enrolment. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | The trial was described as double blind, but the method of blinding was not described, so that knowledge of allocation was possible during the trial. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The numbers and reasons for dropouts and withdrawals in all intervention groups were described. |
| Selective reporting (reporting bias) | Low risk | Pre‐defined, or clinically relevant and reasonably expected outcomes are reported on. |
| Industry bias | Unclear risk | Duphar and Company Laboratories provided the vitamin D3 (Devaron), and Merck‐Clevenot Laboratories provided the tricalcium phosphate (Ostram). |
| Other bias | Low risk | The trial appears to be free of other components that could put it at risk of bias. |
Chapuy 2002.
| Methods | Vitamin D, Calcium, Lyon Study II (DECALYOS II). Multicenter, randomised, double‐blind, placebo controlled trial using parallel group design (three intervention groups). |
|
| Participants | Country: France. Number of participants randomised: 610, 64 to 99 (mean 85) years of age, healthy ambulatory women. Inclusion criteria: ambulatory woman (able to walk indoors with a cane or a walker) and life expectancy of at least 24 months. Exclusion criteria: intestinal malabsorption, hypercalcaemia (serum calcium 42.63 mmol/L) or chronic renal failure (serum creatinine 4150 mmol/L), receiving drugs known to alter bone metabolism, such as corticosteroids, anticonvulsants, or a high dose of thyroxine within the past year, treatments with fluoride salts (43 months), bisphosphonates, calcitonin (41 month), calcium (4500 mg/day), and vitamin D (4100 IU/day) during the last 12 months. |
|
| Interventions | Participants were randomly assigned to receive: Intervention group 1: vitamin D3 (800 IU) plus calcium (1200 mg) daily (fixed combination) (n = 199); Intervention group 2: vitamin D3 (800 IU) plus calcium (1200 mg) daily (separate combination) (n = 194); Intervention group 3 (Control group): double placebo daily (n = 190); for a two‐year period. "The sachet of the calcium–vitamin D3 fixed combination (Ostram–vitamin D3, Merck KGaA) contains a fixed combination of 1200 mg elemental calcium in the form of tricalcium phosphate and 800 IU of vitamin D3. The calcium (Ostram, Merck KGaA) contains 1200 mg of elemental calcium in the form of tricalcium phosphate. Vitamin D3 (Devaron, i.e., cholecalciferol, Duphar Solvay) was given in two pills of 400 IU each. Each day women in intervention groups one and two received 1200 mg of elemental calcium and 800 IU of vitamin D3 given either by a sachet of calcium–vitamin D3 fixed combination (Ca–D3 group) or as a sachet of calcium and two tablets of vitamin D3 (Ca+D3 group). The other women received a placebo of vitamin D3 and calcium (one sachet containing lactose, microcrystalline cellulose and the same excipient as the active treatment and two tablets of vitamin D3 placebo)." |
|
| Outcomes | The primary outcomes were biochemical variables of calcium homeostasis, femoral neck bone mineral density, and hip fracture risk. | |
| Stated aim of study | "To confirm the effects of combined vitamin D supplementation and calcium on biochemical variables of calcium homeostasis, femoral neck bone mineral density, and hip fracture risk." | |
| Notes | "The supplements were taken in the presence of a nurse to ensure compliance. The mean compliance was more than 95% for both sachets and tablets in each treatment group." The trial was sponsored by MERCK KGaA, Darmstadt, Germany. Additional information on mortality was received through personal communication with Professor Pierre Meunier (27.02.2010). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The trial is described as randomised but the method of sequence generation was not specified. |
| Allocation concealment (selection bias) | Unclear risk | The trial was described as randomised but the method used to conceal the allocation was not described, so that intervention allocations may have been foreseen in advance of, or during, enrolment. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | The trial was described as double blind, but the method of blinding was not described, so that knowledge of allocation was possible during the trial. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The numbers and reasons for dropouts and withdrawals in all intervention groups were described |
| Selective reporting (reporting bias) | Low risk | Pre‐defined, or clinically relevant and reasonably expected outcomes are reported on. |
| Industry bias | High risk | The trial was sponsored by MERCK KGaA, Darmstadt, Germany. |
| Other bias | Low risk | The trial appears to be free of other components that could put it at risk of bias. |
Chel 2008.
| Methods | Randomised, double‐blind, placebo‐controlled trial using parallel group design (six intervention groups). | |
| Participants | Country: the Netherlands. Number of participants randomised: 338 (77% women), aged 70 years or over (mean age 84), nursing home residents. Inclusion criteria: nursing home residents aged 70 years or over. Exclusion criteria: going outside in the sunshine more than once a week, the use of vitamin D or calcium supplementation, the use of more than one vitamin D fortified food or drink per day, complete immobilisation and a very poor life expectancy. |
|
| Interventions | Participants were randomly assigned to receive: Intervention group 1: vitamin D3 (600 IU) daily (n = 55); Intervention group 2 (control group): matched placebo tablet daily (n = 57); Intervention group 3: vitamin D3 (4200 IU) weekly (n = 54); Intervention group 4 (Control group): matched placebo tablets weekly (n = 58); Intervention group 5: vitamin D3 (18,000 IU) powder monthly (n = 57); Intervention group 6 (Control group): matched placebo powder monthly (n = 57); for a four and a half month period. The treatment period of four and a half months was completed by 276 out of 338 participants. The 276 participants who completed the vitamin D intervention trial were randomly assigned to receive: Intervention group: calcium 800 mg or 1600 mg daily (n = 138); Control group: matched placebo tablet daily (n = 138); for the period of 4 months. The treatment was completed by 269 participants. The first 156 randomised participants received 800 mg calcium carbonate or placebo; the subsequent 120 participants received 1600 mg calcium carbonate or placebo. |
|
| Outcomes | The primary outcome was to assess efficacy of different doses and intervals of oral vitamin D3 supplementation with the same total dose. Secondary outcome measure was to assess the additional effect of calcium supplementation following vitamin D supplementation on serum parathyroid hormone and markers of bone turnover. |
|
| Stated aim of study | "To investigate, in a Dutch nursing home population, whether there is a difference in efficacy of different doses and intervals of oral vitamin D3 supplementation with the same total dose compared with placebo. A second aim was to assess the additional effect of calcium supplementation following vitamin D supplementation on serum parathyroid hormone and markers of bone turnover." | |
| Notes | "The trial medication was centrally distributed to ensure compliance. Random samples of the returned medication were counted in order to verify compliance." "The compliance assessed within 96 random samples of the returned medication was good. In the daily administration group, all 33 participants were compliant, used at least 80% of the tablets. For weekly administration, 80% of the 35 participants were compliant, used at least 80% of the tablets. For monthly administration, 93% of the 28 participants were compliant, used at least four out of five powders." Solvay Pharmaceuticals supplied the research medication. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The trial is described as randomised but the method of sequence generation was not specified. |
| Allocation concealment (selection bias) | Unclear risk | The trial was described as randomised but the method used to conceal the allocation was not described, so that intervention allocations may have been foreseen in advance of, or during, enrolment. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | The trial was described as double blind, but the method of blinding was not described so that knowledge of allocation was possible during the trial. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The numbers and reasons for dropouts and withdrawals in all intervention groups were described. |
| Selective reporting (reporting bias) | Low risk | Pre‐defined, or clinically relevant and reasonably expected outcomes are reported on. |
| Industry bias | Unclear risk | Solvay Pharmaceuticals supplied the research medication. |
| Other bias | Low risk | The trial appears to be free of other components that could put it at risk of bias. |
Cherniack 2011.
| Methods | Randomised, double‐blind, controlled trial using parallel group design (two intervention groups). | |
| Participants | Country: United States. Number of participants randomised: 46 (2% women), aged 70 years an older (mean age 80). Inclusion criteria: community‐dwelling elderly veterans living in south Florida who were aged 70 and older. Exclusion criteria: current users of vitamin D or corticosteroids; or had hypo‐ or hypercalcaemia, hypercalciuria, hyperparathyroidism, chronic serum creatinine greater than 2.0 mg/dL, or cholestatic liver disease; or were unable to take medication daily. |
|
| Interventions | Participants were randomly assigned to receive: Intervention group 1: vitamin D3 (2000 IU) daily (n = 23); Intervention group 2 (Control group): placebo daily (n = 23); for a one year period. The 41 participants found to have inadequate calcium intake (< 1200 mg/d) according to dietary questionnaire were also dispensed a calcium supplement to provide adequate daily intake. |
|
| Outcomes | The primary outcomes were serum calcium, 25‐hydroxyvitamin D, parathyroid hormone, and 24‐hour urinary calcium. | |
| Stated aim of study | "To determine the prevalence of hypovitaminosis D (serum 25‐hydroxyvitamin D < 32 ng/mL; HVD) in a population of elderly veterans and conduct a preliminary assessment of the efficacy of supplementation with cholecalciferol in correcting HVD". | |
| Notes | Carlson Laboratories donated the cholecalciferol and placebo capsules. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequence generation was achieved using computer random number generation |
| Allocation concealment (selection bias) | Low risk | Allocation was controlled by a central and independent randomisation unit so that intervention allocations could not have been foreseen in advance of, or during, enrolment. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The outcome measurement is not likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There is insufficient information to assess whether the missing data mechanism in combination with the method used to handle missing data are likely to induce bias on the estimate of effect. |
| Selective reporting (reporting bias) | Low risk | Pre‐defined, or clinically relevant and reasonably expected outcomes are reported on. |
| Industry bias | Unclear risk | Carlson Laboratories donated the cholecalciferol and placebo capsules. |
| Other bias | Low risk | The trial appears to be free of other components that could put it at risk of bias. |
Cooper 2003.
| Methods | Randomised, double‐blind, placebo controlled trial using parallel group design (two intervention groups). | |
| Participants | Country: Australia. Number of participants randomised: 187 healthy, white, postmenopausal women, mean age 56 years. Inclusion criteria: healthy, white women who were postmenopausal for one to ten years, and who were not receiving hormone replacement therapy. Exclusion criteria: malignant disease, renal, hepatic, endocrine, or gastrointestinal disorder associated with abnormal calcium metabolism, use of oestrogen, progesterone, glucocorticoids, anticonvulsants, thiazide diuretics, vitamin D supplements, or other medications known to affect calcium or bone metabolism in the previous 12 months. Participants with laboratory evidence of renal, hepatic, or endocrine disorder; a serum follicle‐stimulating hormone concentration < 40 mIU/mL, or bone mineral density at any site ± two standard deviation from the mean for potential participant matched for age were also excluded. |
|
| Interventions | Participants were randomly assigned to receive: Intervention group 1: vitamin D2 (10,000 IU) weekly plus calcium (1000 mg) daily (n = 93); Intervention group 2 (Control group): calcium (1000 mg) daily (n = 94); for a two‐year period. Calcium was in a form of tricalcium phosphate powder in an aqueous suspension. |
|
| Outcomes | The primary outcome was bone mineral density. | |
| Stated aim of study | "To examine the effects of vitamin D2 supplementation on changes in bone mineral density in younger (age: 56 years) postmenopausal women who were also given 1000 mg calcium daily and to compare those changes with the changes in bone mineral density in women given 1000 mg calcium daily only." | |
| Notes | "Compliance was assessed by tablet counts and diary review. Compliance with treatment was 98.2 ± 6.1% for the calcium plus vitamin D group and 97.7 ± 5.4% for the calcium group." Vitamin D2 was provided by Ostelin; Boots Healthcare Pharmaceuticals, Sydney, Australia. Calcium carbonate was provided by Cal‐Sup; 3M Pharmaceutical, Sydney, Australia. Additional information on mortality and risk of bias domains was received through personal communication with Professor Philip Clifton‐Bligh (12.11.2007; 08.02.2010). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequence generation was achieved using computer random number generation. |
| Allocation concealment (selection bias) | Low risk | The trial was described as randomised but the method used to conceal the allocation was not described, so that intervention allocations may have been foreseen in advance of, or during, enrolment. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Allocation was controlled by a central and independent randomisation unit so that intervention allocations could not have been foreseen in advance of, or during, enrolment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The numbers and reasons for dropouts and withdrawals in all intervention groups were described. |
| Selective reporting (reporting bias) | Low risk | Pre‐defined, or clinically relevant and reasonably expected outcomes are reported on. |
| Industry bias | Unclear risk | Vitamin D2 was provided by Ostelin; Boots Healthcare Pharmaceuticals, Sydney, Australia. Calcium carbonate was provided by Cal‐Sup; 3M Pharmaceutical, Sydney, Australia. |
| Other bias | Low risk | The trial appears to be free of other components that could put it at risk of bias. |
Corless 1985.
| Methods | Randomised double‐blind placebo controlled trial using parallel group design (two intervention groups). | |
| Participants | Country: United Kingdom. Number of participants randomised: 82, elderly hospital patients (78% women), mean age 82.4 years. Inclusion criteria: elderly hospital patients. Exclusion criteria: overt clinical osteomalacia, either plasma calcium less than 1.95 mmol/L or Looser's zones, or on calciferol therapy; a judgement that he or she was unlikely to be able to co‐operate in the trial; plasma creatinine more than 150/mmol/L, potassium less than 3.3 mmol/L; plasma 25(OH)D more than 40nmol/L (16ng/ml); refused consent or unable to give informed consent. |
|
| Interventions | Participants were randomly assigned to receive: Intervention group 1: vitamin D2 (9000 IU) daily (n = 32); Intervention group 2 (Control group): matching placebo tablets daily (n = 33); for a nine‐month period. Placebo tablets were identical in appearance to the vitamin D2 tablets containing lactose. |
|
| Outcomes | The primary outcome measure was abilities of elderly hospital patients to carry out basic activities of daily life. | |
| Stated aim of study | "To evaluate the effect of oral vitamin D supplements on the ability of elderly hospital patients with low or low normal plasma 25(OH)D to perform basic activities of daily living." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequence generation was achieved using computer random number generation. |
| Allocation concealment (selection bias) | Unclear risk | The trial was described as randomised but the method used to conceal the allocation was not described, so that intervention allocations may have been foreseen in advance of, or during, enrolment. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | The trial was described as double blind, but the method of blinding was not described, so that knowledge of allocation was possible during the trial. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The numbers and reasons for dropouts and withdrawals in all intervention groups were described. |
| Selective reporting (reporting bias) | Low risk | Pre‐defined, or clinically relevant and reasonably expected outcomes are reported on. |
| Industry bias | Unclear risk | The source of funding is not clear. |
| Other bias | Low risk | The trial appears to be free of other components that could put it at risk of bias. |
Daly 2008.
| Methods | Randomised controlled trial using parallel group design (two intervention groups). | |
| Participants | Country: Australia. Number of participants randomised: 167 ambulatory community living men 50 to 87 (mean 61.9) years of age. Inclusion criteria: ambulatory community living men aged 50 years or over. Exclusion criteria: taking calcium and/or vitamin D supplements in the preceding 12 months, participating in regular high‐intensity resistance training in the previous six months or more, then 150 minutes a week of moderate‐ to high‐impact weight‐bearing exercise, had a body mass index > 35 kg/m2, lactose intolerance, consuming more than four alcoholic beverages per day, a history of osteoporotic fracture or medical disease, or medication use that is known to affect metabolism of bones. |
|
| Interventions | Participants were randomly assigned to receive: Intervention group 1: calcium‐vitamin D3‐fortified milk containing vitamin D3 (800 IU) plus calcium (1000 mg) daily (n = 85); Intervention group 2 (Control group): usual diet (n = 82); for a two‐year period. Participants were followed for additional a year and a half. |
|
| Outcomes | The primary outcome measure was bone mineral density. | |
| Stated aim of study | "To assess the effects of calcium and vitamin D3 fortified milk on bone mineral density in community living men > 50 years of age." | |
| Notes | "To monitor milk compliance, participants were asked to record the number of tetra packs consumed per day on a compliance calendar, which was collected and checked every three months. Compliance proportion (expressed as a percentage) was calculated as the actual number of tetra packs consumed, divided by the expected consumption each month. The overall mean reported milk compliance, calculated as the percentage of the tetra packs consumed and based on daily diaries was 85.1%. Milk was specifically formulated by Murray Goulburn Cooperative Co. (Brunswick, Australia). The added milk calcium salt (Natra‐Cal) was prepared by Murray Goulburn Cooperative Co. The vitamin D (Vitamin D3) used to fortify the milk was obtained from DSM Nutritional Products Pty (NSW, Australia)." Additional information on mortality was received through personal communication with Professor Robin Daly (04.02.2009). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequence generation was achieved using a random number table. |
| Allocation concealment (selection bias) | High risk | The allocation sequence was known to the investigators who assigned participants. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Trial was not blinded, so that the allocation was known during the trial. Placebo was not used. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The numbers and reasons for dropouts and withdrawals in all intervention groups were described. |
| Selective reporting (reporting bias) | Low risk | Pre‐defined, or clinically relevant and reasonably expected outcomes are reported on. |
| Industry bias | Unclear risk | The vitamin D (Vitamin D3) used to fortify the milk was obtained from DSM Nutritional Products Pty (NSW, Australia). |
| Other bias | Low risk | The trial appears to be free of other components that could put it at risk of bias. |
Dawson‐Hughes 1997.
| Methods | Boston STOP IT (Sites Testing Osteoporosis Prevention Intervention Treatment). Randomised, double‐blind, placebo controlled trial using parallel group design (two intervention groups). |
|
| Participants | Country: United States. Number of participants randomised: 389, healthy, ambulatory participants (55% women), aged 65 years or older (mean 71). Inclusion criteria: healthy, ambulatory men and women 65 years of age or older. Exclusion criteria: current cancer or hyperparathyroidism; a kidney stone in the past five years; renal disease; bilateral hip surgery; therapy with a bisphosphonate, calcitonin, oestrogen, tamoxifen, or testosterone in the past six months or fluoride in the past two years; femoral‐neck bone mineral density more than 2 SD below the mean for participants of the same age and sex; dietary calcium intake exceeding 1500 mg per day; and laboratory evidence of kidney or liver disease. |
|
| Interventions | Participants were randomly assigned to receive: Intervention group 1: vitamin D3 (700 IU) plus calcium (500 mg) daily (n = 187); Intervention group 2 (Control group): matched placebo tablets daily (n = 202); for a three‐year period. Calcium was in the form of calcium citrate malate. Placebo pills contained microcrystalline cellulose. |
|
| Outcomes | The primary outcome measures were bone mineral density, biochemical measures of bone metabolism, and the incidence of nonvertebral fractures. | |
| Stated aim of study | "To examine the effects of combined vitamin D supplementation and calcium on bone loss, biochemical measures of bone metabolism, and the incidence of nonvertebral fractures in men and women 65 years of age or older who were living in the community." | |
| Notes | Procter & Gamble, Cincinnati manufactured calcium tablets. Additional information on mortality was received through personal communication with Professor Bess Dawson‐Hughes (04.02.2009). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequence generation was achieved using computer random number generation. |
| Allocation concealment (selection bias) | Low risk | Allocation was controlled by a central and independent randomisation unit so that intervention allocations could not have been foreseen in advance of, or during, enrolment. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was described as blinded, the parties that were blinded, and the method of blinding was described, so that knowledge of allocation was adequately prevented during the trial. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The numbers and reasons for dropouts and withdrawals in all intervention groups were described. |
| Selective reporting (reporting bias) | Low risk | Pre‐defined or clinically relevant and reasonably expected outcomes are reported on. |
| Industry bias | Unclear risk | Procter & Gamble, Cincinnati manufactured calcium tablets. |
| Other bias | Low risk | The trial appears to be free of other components that could put it at risk of bias. |
Dukas 2004.
| Methods | Randomised, double‐blind, placebo‐controlled trial using parallel group design (two intervention groups). | |
| Participants | Country: Switzerland. Number of participants randomised: 378 (51% women), mean age 71 years, community‐dwelling elderly people. Inclusion criteria: community‐dwelling elderly people who are mobile and have an independent life style. Exclusion criteria: primary hyperparathyroidism, polyarthritis or inability to walk, calcium intake by supplement of more than 500 mg daily, vitamin D intake of more than 200 IU daily, active kidney stone disease, history of hypercalcuria or cancer or other incurable diseases, dementia, elective surgery within the next three months, severe renal insufficiency (creatinine clearance < 20 mL/min, and fracture or stroke within the last 3 months. Calcium supplementation of 500 mg/d or less was accepted. |
|
| Interventions | Participants were randomly assigned to receive: Intervention group 1: 1 α(OH)D3 (alfacalcidol), (1 μg) daily (n = 192); Intervention group 2 (Control group): placebo (n = 186); for a nine‐month period. |
|
| Outcomes | The primary outcome measure was number of fallers. Secondary outcome measures were muscle strength, balance, blood pressure, and bone quality. | |
| Stated aim of study | "To evaluate whether treatment with alfacalcidol, a precursor of the D hormone calcitriol, reduces the number of fallers and falls in community‐dwelling men and women." | |
| Notes | Trial medication was provided by TEVA Pharmaceuticals Industries Ltd, Israel. Additional information on the risk of bias domains was received through personal communication with Dr Laurent C Dukas (28.01.2010). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequence generation was achieved using computer random number generation. |
| Allocation concealment (selection bias) | Low risk | Allocation was controlled by a central and independent randomisation unit so that intervention allocations could not have been foreseen in advance of, or during, enrolment. "An independent statistical group performed the blinding and randomisation. All investigators and staff conducting the trial remained blinded throughout the intervention period." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was described as blinded, the parties that were blinded, and the method of blinding was described, so that knowledge of allocation was adequately prevented during the trial. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The numbers and reasons for dropouts and withdrawals in all intervention groups were described. |
| Selective reporting (reporting bias) | Low risk | Pre‐defined, or clinically relevant and reasonably expected outcomes are reported on. |
| Industry bias | Unclear risk | Trial medication was provided by TEVA Pharmaceuticals Industries Ltd, Israel. |
| Other bias | Low risk | The trial appears to be free of other components that could put it at risk of bias. |
Flicker 2005.
| Methods | Randomised, double‐blind, placebo‐controlled trial using parallel group design (two intervention groups). | |
| Participants | Country: Australia. Number of participants randomised: 625, older residents (mean age 83.4), 95% females, with serum 25‐hydroxyvitamin D levels between 25 and 90 nmol/L. Inclusion criteria: older people resident in hostels and nursing homes with serum 25‐hydroxyvitamin D levels between 25 and 90 nmol/L. Exclusion criteria: use of agents that could affect bone and mineral metabolism, such as warfarin, chronic heparin therapy, vitamin D therapy within the previous three months, glucocorticoids at an average daily dose of greater than 5 mg prednisolone (or equivalent) for more than one month within the preceding year, current use of bisphosphonates, and hormone replacement therapy, thyrotoxicosis within the previous three years, primary hyperparathyroidism treated within the previous three years, multiple myeloma, Paget’s disease of bone, history of malabsorption, intercurrent active malignancy, and other disorders affecting bone and mineral metabolism. |
|
| Interventions | Participants were randomly assigned to receive: Intervention group: vitamin D3 (10000 IU) weekly until November 1998 and thereafter vitamin D31000 IU daily plus calcium (600 mg) daily (n = 313); Control group: calcium (600 mg) (n = 312); for a two‐year period. |
|
| Outcomes | The primary outcomes were falls and fractures. | |
| Stated aim of study | "To test whether administration of vitamin D could reduce the incidence of falls and fractures in nursing home residents." | |
| Notes | "Supplements and placebos were purchased commercially, and the suppliers played no role in the trial design or in the collection, analysis, or interpretation of data." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequence generation was achieved using computer random number generation. |
| Allocation concealment (selection bias) | Low risk | Allocation was controlled by a central and independent randomisation unit so that intervention allocations could not have been foreseen in advance of, or during, enrolment. An individual who was not involved in contact with the participants or the residential care institutions performed randomisation. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was described as blinded, the parties that were blinded, and the method of blinding was described, so that knowledge of allocation was adequately prevented during the trial. "Participants were randomised to receive sequentially numbered bottles containing vitamin D or placebo. Both interventions had matching placebo preparations given in identical fashion, and residents, institutional staff, and trial staff were blinded to treatment allocation." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The numbers and reasons for dropouts and withdrawals in all intervention groups were described. |
| Selective reporting (reporting bias) | Low risk | Pre‐defined, or clinically relevant and reasonably expected outcomes are reported on. |
| Industry bias | Low risk | The trial is not funded by a manufacturer of vitamin D. |
| Other bias | Low risk | The trial appears to be free of other components that could put it at risk of bias. |
Gallagher 2001.
| Methods | Sites Testing Osteoporosis Prevention / Intervention Treatment (STOP IT). Randomised, double‐blind, placebo‐controlled trial using 2 x 2 factorial design. |
|
| Participants | Country: United States. Number of participants randomised: 489 healthy elderly women 65 to 77 (mean 71.5) years of age. Inclusion criteria: healthy elderly women 65 to 77 years of age and femoral neck density within the normal range for their age. Exclusion criteria: severe chronic illness, primary hyperparathyroidism or active renal stone disease, and were on certain medications, such as bisphosphonates, anticonvulsants, oestrogen, fluoride, or thiazide diuretics in the previous 6 months. |
|
| Interventions | Participants were randomly assigned to receive: Intervention group 1: calcitriol (0.5 μg) daily (n = 123); Intervention group 2: conjugated oestrogens (Premarin) 0.625 mg/daily plus medroxyprogesterone acetate (Provera) 2.5 mg daily (n = 121); Intervention group 3: calcitriol (0.5 μg) plus conjugated oestrogens daily; (Premarin) 0.625 mg/daily plus medroxyprogesterone acetate (Provera) 2.5 mg daily (n = 122); Intervention group 4 (Control group): matched placebo daily (n = 123); for a three‐year period. |
|
| Outcomes | The primary outcome measure was the change in bone mineral density of the femoral neck and spine. Secondary outcome measure was incidence of nonvertebral fractures. | |
| Stated aim of study | "To examine the effect of oestrogen and 1,25‐dihydroxyvitamin D therapy given individually or in combination on bone loss in elderly women." | |
| Notes | "Compliance to trial medication was evaluated by pill counts. At 36 months, treatment group differences in adherence to assigned therapy were evident, with 78% of those assigned to placebo, 70% of those assigned to calcitriol, 65% of those assigned to HRT/ERT and 62% of those assigned to HRT/ERT calcitriol still adherent to their assigned medication. Among those still on medication the compliance for the groups calculated at six months and compared with 36 months, respectively, was: conjugated estrogens, 86% and 92%; medroxyprogesterone acetate, 91% and 94%; calcitriol, 87% and 93%; placebos, 94% and 92%." The active trial drug and placebo were supplied by Wyeth‐Ayerst Laboratories, Inc Pharm, Hoffman‐LaRoche Inc and Pharmacia & Upjohn, Inc. Additional information on mortality and risk of bias domains was received through personal communication with Dr John Gallagher (09.02.2009; 11.03.2010). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequence generation was achieved using computer random number generation. |
| Allocation concealment (selection bias) | Low risk | Allocation was controlled by a central and independent randomisation unit so that intervention allocations could not have been foreseen in advance of, or during, enrolment. An independent statistical group performed the blinding and randomisation. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was described as blinded, the parties that were blinded, and the method of blinding was described, so that knowledge of allocation was adequately prevented during the trial. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The numbers and reasons for dropouts and withdrawals in all intervention groups were described. |
| Selective reporting (reporting bias) | Low risk | Pre‐defined, or clinically relevant and reasonably expected outcomes are reported on. |
| Industry bias | Unclear risk | The active trial drug and placebo were supplied by Wyeth‐Ayerst Laboratories, Inc Pharm, Hoffman‐LaRoche Inc and Pharmacia & Upjohn, Inc. |
| Other bias | Low risk | The trial appears to be free of other components that could put it at risk of bias. |
Glendenning 2012.
| Methods | Randomised, double‐blind, placebo‐controlled trial using parallel group design (two intervention groups). | |
| Participants | Country: Australia. Number of participants randomised: 686 community‐dwelling ambulant women aged over 70 years (mean 76.7). Inclusion criteria: age over 70 years, registration with a general practitioner, and likelihood, in the investigators’ opinion, of attending four study visits over 9 months. Exclusion criteria: consumption of vitamin D supplementation either in isolation or as part of a combination treatment; e.g., Actonel combi +D or Fosamax plus, cognitive impairment (Mini Mental State Score < 24), and individuals who in the investigators’ opinion would not be suitable for the study. |
|
| Interventions | Participants were randomly assigned to receive: Intervention group 1: cholecalciferol 150,000 three‐monthly (n = 353); Intervention group 2 (Control group): placebo vitamin D three‐monthly (n = 333); for a nine‐month period. |
|
| Outcomes | The primary outcome measures were falls, muscle strength, and mobility. Secondary outcome measures were serum 25‐hidrohyvitamin D levels, and adverse events. | |
| Stated aim of study | "to evaluate the effects of cholecalciferol treatment and lifestyle advice compared to lifestyle advice alone on falls, serum 25OHD levels, physical function, and adverse events in 686 women aged over 70 years" | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequence generation was achieved using computer random number generation |
| Allocation concealment (selection bias) | Low risk | Allocation was controlled by a central and independent randomisation unit so that intervention allocations could not have been foreseen in advance of, or during, enrolment. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The outcome measurement is not likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The underlying reasons for missing data are unlikely to make treatment effects depart from plausible values. |
| Selective reporting (reporting bias) | Low risk | Pre‐defined, or clinically relevant and reasonably expected outcomes are reported on. |
| Industry bias | Unclear risk | The source of funding is not clear. |
| Other bias | Low risk | The trial appears to be free of other components that could put it at risk of bias. |
Grady 1991.
| Methods | Randomised, double‐blind, placebo‐controlled trial using parallel group design (two intervention groups). | |
| Participants | Country: United States. Number of participants randomised: 98 elderly ambulatory men and women (54%) women, aged 70 to 97 (mean 79.1) years of age. Inclusion criteria: elderly ambulatory men and women. Exclusion criteria: serum calcium levels of 2.57 mmol/L or more, urinary calcium levels of 7.28 mmol/day or more, creatinine clearance less than 0.42 mmol/s, history of hypercalcaemia, nephrolithiasis, seizure disorder, hyperparathyroidism, treatment with calcium, vitamin D or thiazide diuretics, and average calcium intake greater than 1000 mg/day. |
|
| Interventions | Participants were randomly assigned to receive: Intervention group 1: calcitriol (0.5 μg) daily (n = 50); Intervention group 2 (Control group): placebo vitamin D (n = 48); for a six‐month period. |
|
| Outcomes | The primary outcome measure was muscle strength. | |
| Stated aim of study | "To test the hypothesis that the weakness associated with aging is in part due to inadequate serum concentrations of 1,25‐(OH2)D3." | |
| Notes | "Participants were evaluated at 1, 2, 4, 8, 12, 18, and 24 weeks of intervention regimen to maintain compliance. Participants in both groups took more than 95% of the assigned medication." Calcitriol and placebo capsules were provided by Hoffman‐LaRoche (Nutley, NJ). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The trial is described as randomised but the method of sequence generation was not specified. |
| Allocation concealment (selection bias) | Unclear risk | The trial was described as randomised but the method used to conceal the allocation was not described, so that intervention allocations may have been foreseen in advance of, or during, enrolment. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | The trial was described as double blind, but the method of blinding was not described, so that knowledge of allocation was possible during the trial. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The numbers and reasons for dropouts and withdrawals in all intervention groups were described. |
| Selective reporting (reporting bias) | Low risk | Pre‐defined, or clinically relevant and reasonably expected outcomes are reported on. |
| Industry bias | Unclear risk | Calcitriol and placebo capsules were provided by Hoffman‐LaRoche (Nutley, NJ). |
| Other bias | Low risk | The trial appears to be free of other components that could put it at risk of bias. |
Grimnes 2011.
| Methods | Randomised, double‐blind, placebo‐controlled trial using parallel group design (two intervention groups). | |
| Participants | Country: Norway. Number of participants randomised: 104 (45% women), mean age 51.5 years. Inclusion criteria: participants with low serum 25‐hydroxyvitamin D levels. Exclusion criteria: diabetes, acute myocardial infarction or stroke during the past 12 months, cancer during the past 5 years, steroid use, serum creatinine ≥130 μmol/L (males) ≥ 110 μmol/L (females), possible primary hyperparathyroidism (plasma parathyroid hormone [PTH] > 5.0 pmol/L combined with serum calcium > 2.50 mmol/L), sarcoidosis, systolic blood pressure > 175 mmHg or diastolic blood pressure > 105 mmHg, and specifically for women, pregnancy, lactation, or fertile age and no contraception use. |
|
| Interventions | Participants were randomly assigned to receive: Intervention group 1: vitamin D3 (20,000 IU) twice weekly (n = 51); Intervention group 2 (Control group): placebo twice weekly (n = 53); for a six months period. |
|
| Outcomes | The primary outcomes were insulin sensitivity and secretion. Secondary outcome measure was blood lipid level. | |
| Stated aim of study | "to compare insulin sensitivity (the primary end point) and secretion and lipids in subjects with low and high serum 25(OH)D (25‐hydroxyvitamin D) levels and to assess the effect of vitamin D supplementation on the same outcomes among the participants with low serum 25(OH)D levels." | |
| Notes | Vitamin D3 was manufactured by Dekristol; Mibe, Brehna, Germany. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequence generation was achieved using computer random number generation |
| Allocation concealment (selection bias) | Low risk | Allocation was controlled by a central and independent randomisation unit so that intervention allocations could not have been foreseen in advance of, or during, enrolment. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The outcome measurement is not likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The underlying reasons for missing data are unlikely to make treatment effects depart from plausible values. |
| Selective reporting (reporting bias) | Low risk | Pre‐defined, or clinically relevant and reasonably expected outcomes are reported on. |
| Industry bias | Unclear risk | Vitamin D3 was manufactured by Dekristol; Mibe, Brehna, Germany. |
| Other bias | Low risk | The trial appears to be free of other components that could put it at risk of bias. |
Harwood 2004.
| Methods | The Nottingham Neck of Femur Study (NONOF). Randomised controlled trial, using parallel group design (four intervention groups). |
|
| Participants | Country: United Kingdom. Number of participants randomised: 150 previously independent elderly women, 67 to 92 (mean 81.2) years of age, recruited following surgery for hip fracture. Inclusion criteria: elderly women post‐hip fracture, previous community residence, independence in activities of daily living. Exclusion criteria: institutionalised patients, diseases or medication known to affect bone metabolism, and those with a 10‐point abbreviated mental test score less than seven at the time of recruitment. |
|
| Interventions | Participants were randomly assigned to receive: Intervention group 1: single injection of 300,000 IU of vitamin D2 (n = 38); Intervention group 2: single injection of 300,000 IU of vitamin D2 plus oral calcium (1000 mg) daily (n = 36); Intervention group 3: oral vitamin D3 (800 IU) plus calcium (1000 mg) daily (n = 39); Intervention group 4 (Control group): no treatment (n = 37); for a one‐year period. |
|
| Outcomes | The primary outcomes were bone biochemical markers, bone mineral density, and rate of falls and new fractures. | |
| Stated aim of study | "To compare the effects of different calcium and vitamin D supplementation regimens on bone biochemical markers, bone mineral density, and rate of falls in elderly women post‐hip fracture." | |
| Notes | "There were no cases of hypercalcaemia, and no participants were withdrawn because of adverse effects of trial medication." The trial was supported by Provalis Healthcare Ltd. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequence generation was achieved using computer random number generation. |
| Allocation concealment (selection bias) | Low risk | Allocation was controlled by a opaque and sealed envelopes. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Trial was not blinded, so that the allocation was known during the trial. Placebo was not used. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The numbers and reasons for dropouts and withdrawals in all intervention groups were described. |
| Selective reporting (reporting bias) | Low risk | Pre‐defined, or clinically relevant and reasonably expected outcomes are reported on. |
| Industry bias | High risk | The trial was supported by Provalis Healthcare Ltd. |
| Other bias | Low risk | The trial appears to be free of other components that could put it at risk of bias. |
Jackson 2006.
| Methods | Women’s Health Initiative (WHI). Multicentre, randomised, double‐blind, placebo controlled trial using parallel group design (two intervention groups). |
|
| Participants | Country: United States. Number of participants randomised: 36,282 50 to 79 (mean 62) years of age, healthy postmenopausal women. Inclusion criteria: postmenopausal women 50 to 79 years of age at the initial screening without evidence of a medical condition associated with a predicted survival of less than three years and no safety, adherence, or retention risks. Exclusion criteria: hypercalcaemia, renal calculi, corticosteroid use, and calcitriol use. Personal supplemental calcium (up to 1000 mg per day) and vitamin D (up to 600 IU per day) were allowed. In 1999, the upper limit of personal vitamin D intake was raised to 1000 IU. The calcium with vitamin D trial permitted the use of bisphosphonates and calcitonin. Use of oestrogen (with or without a progestin) was according to randomisation among women in the Hormone Therapy trial. Independent use of hormone therapy or selective oestrogen‐receptor modulators was permitted for women in the Dietary Modification trial. |
|
| Interventions | Participants were randomly assigned to receive: Intervention group 1: vitamin D3 (400 IU) plus calcium (1000 mg) daily (n = 18176); Intervention group 2 (Control group): matched placebo daily (n = 18106); for a seven‐year period. |
|
| Outcomes | The primary outcome measure was hip fracture. The secondary outcomes were other fractures and colorectal cancer. | |
| Stated aim of study | "To test the primary hypothesis that postmenopausal women randomly assigned to vitamin D supplementation plus calcium would have a lower risk of hip fracture, and, secondarily, of all fractures than women assigned to placebo. Another secondary hypothesis was that women receiving calcium with vitamin D supplementation would have a lower rate of colorectal cancer than those receiving placebo." | |
| Notes | "The Women’s Health Initiative was clinical investigation of strategies for the prevention of some of the most common causes of morbidity and mortality among postmenopausal women. It consisted of two components, the randomised controlled clinical trial and observational study. Randomised controlled trial tested two interventions (hormone therapy and dietary modification. Women who were ineligible or unwilling to enrol in randomised trial were invited to participate in the observational study. One year later participants enrolled in the dietary modification trial, hormone therapy trials, or both were invited to join the Women Health Initiative calcium‐vitamin D trial." "Adherence to the trial medication was established by weighing returned pill bottles during clinic visits. The rate of adherence (defined as use of 80% or more of the assigned trial medication) ranged from 60% to 63% during the first three years of follow‐up, with an additional 13% to 21% of the participants taking at least half of their trial pills. At the end of the trial, 76% were still taking the trial medication, and 59% were taking 80% or more of it." The active trial drug and placebo were supplied by GlaxoSmithKline Consumer Healthcare (Pittsburgh). We extracted data about cancer occurrence and cancer mortality from the article: Brunner RL, Wactawski‐Wende J, Caan BJ, Cochrane BB, Chlebowski RT, Gass ML, et al. The effect of calcium plus vitamin D on risk for invasive cancer: results of the Women's Health Initiative (WHI) calcium plus vitamin D randomised clinical trial. Nutrition an Cancer 2011;63(6):827‐41. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequence generation was achieved using computer random number generation. |
| Allocation concealment (selection bias) | Low risk | Allocation was controlled by a central and independent randomisation unit so that intervention allocations could not have been foreseen in advance of, or during, enrolment. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was described as blinded, the parties that were blinded, and the method of blinding was described, so that knowledge of allocation was adequately prevented during the trial. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The numbers and reasons for dropouts and withdrawals in all intervention groups were described. |
| Selective reporting (reporting bias) | Low risk | Pre‐defined, or clinically relevant and reasonably expected outcomes are reported on. |
| Industry bias | Unclear risk | The active trial drug and placebo were supplied by GlaxoSmithKline Consumer Healthcare (Pittsburgh). |
| Other bias | Low risk | The trial appears to be free of other components that could put it at risk of bias. |
Janssen 2010.
| Methods | Randomised, double‐blind, placebo‐controlled trial using parallel group design (two intervention groups). | |
| Participants | Country: Netherlands. Number of participants randomised: 70 female geriatric patients older than 65 years with serum 25 hydroxyvitamin D concentrations between 20 and 50 nmol/L. Inclusion criteria: vitamin D insufficient geriatric patients able to walk and follow simple instructions. Exclusion criteria: treatment with vitamin D or steroids in the previous six months, a history of hypercalcaemia or renal stones, liver cirrhosis, serum creatinine > 200 μmol/L, malabsorptive bowel syndrome, primary hyperparathyroidism, uncontrolled thyroid disease, anticonvulsant drug therapy, and/or presence of any other condition that would probably interfere with the patients compliance (i.e., surgery planned). |
|
| Interventions | Participants were randomly assigned to receive: Intervention group 1: vitamin D3 (400 IU) plus calcium (500 mg) daily (n = 36); Intervention group 2 (Control group): placebo vitamin D3 plus calcium (500 mg) daily (n = 34); for a six months period. |
|
| Outcomes | The primary outcomes were muscle strength, power and functional mobility. | |
| Stated aim of study | "To test the hypothesis that vitamin D plus calcium supplementation improves muscle strength and mobility, compared with calcium monotherapy in vitamin D insufficient female geriatric patients." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequence generation was achieved using computer random number generation |
| Allocation concealment (selection bias) | Low risk | Allocation was controlled by a central and independent randomisation unit. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The outcome measurement is not likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The underlying reasons for missing data are unlikely to make treatment effects depart from plausible values. |
| Selective reporting (reporting bias) | Low risk | Pre‐defined, or clinically relevant and reasonably expected outcomes are reported on. |
| Industry bias | Low risk | The trial is not funded by a manufacturer of vitamin D. |
| Other bias | Low risk | The trial appears to be free of other components that could put it at risk of bias. |
Komulainen 1999.
| Methods | Randomised, double‐blind, placebo‐controlled trial using 2 x 2 factorial design. | |
| Participants | Country: Finland. Number of participants randomised: 464, recently postmenopausal women without contraindications to hormone replacement therapy 47 to 56 (mean 52.7) years of age. Inclusion criteria: nonosteoporotic, early postmenopausal women (6 to 24 months had elapsed since their last menstruation). Exclusion criteria: history of breast or endometrial cancer, thromboembolic diseases, and medication‐resistant hypertension. |
|
| Interventions | Participants were randomly assigned to receive: Intervention group 1: sequential combination of 2 mg estradiol valerate (E2Val; days 1 to 21) and 1 mg cyproterone acetate (days 12 to 21) and a treatment‐free interval (days 22 to 28) (n = 116); Intervention group 2: vitamin D3 (300 IU) plus calcium (500 mg) daily, intervention‐free interval June‐August, the Vit D3 dosage was lowered to 100 IU/day after 4 years of treatment because of adverse lipid changes noticed during the first years of the trial (N = 116); Intervention group 3: sequential combination of 2 mg estradiol valerate (E2Val; days 1 to 21) and 1 mg cyproterone acetate (days 12 to 21) and a intervention‐free interval (days 22 to 28) plus vitamin D3 (300 IU) and calcium (500 mg) daily (n = 116); Intervention group 4 (Control group): placebo daily (n = 116); for a five‐year period. |
|
| Outcomes | The primary outcome was bone mineral density. | |
| Stated aim of study | "To examine the long term effects of a sequential oestrogen‐progestin combination therapy (estradiol valerate and cyproterone acetate) and low dose vitamin D3 supplementation on bone mineral density in nonosteoporotic, early postmenopausal women and to determine whether vitamin D3 supplementation can give additional benefit to hormone replacement therapy." | |
| Notes | "Of the 464 women enrolled in the trial, 435 (94%) eligible women completed it. Among the 29 drop‐outs were 20 women who could not be contacted in the end of the trial and 3 who died from unrelated causes during the trial period. In addition, 6 osteoporotic women were withdrawn from the trial after enrolment when participant eligibility data were available (baseline lumbar or femoral BMD above ‐2 SD of the mean of the whole trial population)." The trial was supported by Leiras Oy, Finland and Schering AG, Germany. Hormone replacement therapy provided by Climen, Schering AG, Germany; Vitamin D3 by D‐Calsor, Orion Ltd, Finland, and calcium by Rohto Ltd, Tampere, Finland. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequence generation was achieved using computer random number generation. |
| Allocation concealment (selection bias) | Low risk | Allocation was controlled by a central and independent randomisation unit, so that intervention allocations could not have been foreseen in advance of, or during, enrolment. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was described as blinded, the parties that were blinded, and the method of blinding was described, so that knowledge of allocation was adequately prevented during the trial. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The numbers and reasons for dropouts and withdrawals in all intervention groups were described. |
| Selective reporting (reporting bias) | Low risk | Pre‐defined, or clinically relevant and reasonably expected outcomes are reported on. |
| Industry bias | High risk | The trial was supported by Leiras Oy, Finland and Schering AG, Germany. Hormone replacement therapy provided by Climen, Schering AG, Germany; Vitamin D3 by D‐Calsor, Orion Ltd, Finland, and calcium by Rohto Ltd, Tampere, Finland. |
| Other bias | Low risk | The trial appears to be free of other components that could put it at risk of bias. |
Krieg 1999.
| Methods | Randomised clinical trial using parallel group design (two intervention groups). | |
| Participants | Country: Switzerland. Number of participants randomised: 248 elderly institutionalised women 62 to 98 (mean 84.5) years of age. Inclusion criteria: elderly institutionalised women. Exclusion criteria: not reported. |
|
| Interventions | Participants were randomly assigned to receive: Intervention group 1: vitamin D3 (880 IU) plus calcium (1000 mg) daily (n = 124); Intervention group 2 (Control group): no treatment (n = 124); for a two‐year period. |
|
| Outcomes | The primary outcomes were quantitative ultrasound parameters of bones and metabolic disturbances. | |
| Stated aim of study | "To assess the effect of supplementation with vitamin D and calcium on quantitative ultrasound parameters and metabolic disturbances in elderly institutionalised women." | |
| Notes | "The drugs were given by the nursing staff to avoid lack of compliance." Trial agents were provided by Novartis Pharma, Basle, Switzerland. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The trial is described as randomised but the method of sequence generation was not specified. |
| Allocation concealment (selection bias) | High risk | The allocation sequence was known to the investigators who assigned participants. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Trial was not blinded, so that the allocation was known during the trial. Placebo was not used. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The numbers and reasons for dropouts and withdrawals in all intervention groups were described. |
| Selective reporting (reporting bias) | Low risk | Pre‐defined or clinically relevant and reasonably expected outcomes are reported on. |
| Industry bias | Unclear risk | Trial agents were provided by Novartis Pharma, Basle, Switzerland. |
| Other bias | Low risk | The trial appears to be free of other components that could put it at risk of bias. |
Kärkkäinen 2010.
| Methods | Osteoporosis Risk Factor and Prevention Study‐Fracture Prevention Study (OSTPRE‐FPS). Randomised controlled trial using parallel group design (two intervention groups). |
|
| Participants | Country: Finland. Number of participants randomised: 3139 ambulatory postmenopausal women, aged 65 to 71 (mean 67) years. Inclusion criteria: ambulatory women aged 65 years or more at the end of November 2002, living in Kuopio province area at the onset of the trial, and not belonging to the former OSTPRE bone densitometry sample. Exclusion criteria: none stated. |
|
| Interventions | Participants were randomly assigned to receive: Intervention group 1: vitamin D3 800 IU plus calcium (calcium carbonate) 1000 mg daily (n = 1718); Intervention group 2 (Control group): no intervention (n = 1714); for a three‐year period. |
|
| Outcomes | The primary outcome measure was the occurrence of falls. | |
| Stated aim of study | "To test the hypothesis that the calcium and vitamin D supplementation prevents falls at the population level." | |
| Notes | This trial was based on the OSTPRE‐FPS (Osteoporosis Risk Factor and Prevention Study‐Fracture Prevention Study) which began in 2003 in Kuopio, Finland. "The compliance was calculated as the dispensed tablets on prescriptions and not on exact number of tablets consumed. The mean compliance in the entire trial population was 78%. The values for 70%, 80% and 90% compliance were 77.4%, 74.2% and 69.1% of the intervention group (entire trial population), respectively." Supported by Leiras‐Nycomed Ltd with calcium and vitamin D supplementation. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequence generation was achieved using computer random number generation. |
| Allocation concealment (selection bias) | Low risk | Allocation was controlled by a central and independent randomisation unit so that intervention allocations could not have been foreseen in advance of, or during, enrolment. |
| Blinding (performance bias and detection bias) All outcomes | High risk | The trial was not blinded, so that the allocation was known during the trial. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The numbers and reasons for dropouts and withdrawals in all intervention groups were described. |
| Selective reporting (reporting bias) | Low risk | Pre‐defined, or clinically relevant and reasonably expected outcomes are reported on. |
| Industry bias | Unclear risk | The trial was supported by Leiras‐Nycomed Ltd with calcium and vitamin D supplementation. |
| Other bias | Low risk | The trial appears to be free of other components that could put it at risk of bias. |
Lappe 2007.
| Methods | Randomised, double‐blind, placebo‐controlled trial using parallel group design (three intervention groups). | |
| Participants | Country: United States. Number of participants randomised: 1179 healthy postmenopausal white women, 55 years of age and older (mean 66.7). Inclusion criteria: age > 55 years, at least four years past last menses; in generally good health, living independently in the community, and weighing less than 300 pounds. Exclusion criteria: a medical diagnosis of any chronic kidney disease, Paget's or other metabolic bone disease, and history of cancer except for superficial basal or squamous cell carcinoma of the skin and other malignancies treated curatively more than 10 years prior to entry into the trial. |
|
| Interventions | Participants were randomly assigned to receive: Intervention group 1: vitamin D3 (1000 IU) plus calcium (1400 to 1500 mg) daily (n = 446); Intervention group 2: vitamin D3 placebo plus calcium (1400 to 1500 mg) daily (n = 445); Intervention group 3 (Control group): placebo, consisting of both vitamin D3 placebo and a brand‐specific calcium placebo daily (n = 288); for a four‐year period. |
|
| Outcomes | The primary outcome was fracture incidence, and the principal secondary outcome was cancer occurrence. | |
| Stated aim of study | "To determine the efficacy of calcium alone and calcium plus vitamin D in reducing incident cancer risk of all types." | |
| Notes | "Compliance with trial medication was assessed at six months intervals by bottle weight. Mean adherence (defined as taking 80% of assigned doses) was 85.7% for the vitamin D component of the combined regimen and 74.4% for the calcium component." The calcium supplements were provided by Mission Pharmacal (San Antonio, TX) and GlaxoSmithKline (Parsippany, NJ). The vitamin D3 was obtained from Tishcon Corporation (Westbury, NY). Additional information on mortality was received through personal communication with Professor Joan M Lappe (21.11.2007). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequence generation was achieved using computer random number generation. |
| Allocation concealment (selection bias) | Low risk | Allocation was controlled by a central and independent randomisation unit so that intervention allocations could not have been foreseen in advance of, or during, enrolment. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was described as blinded, the parties that were blinded, and the method of blinding was described, so that knowledge of allocation was adequately prevented during the trial. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The numbers and reasons for dropouts and withdrawals in all intervention groups were not described. |
| Selective reporting (reporting bias) | Low risk | Pre‐defined, or clinically relevant and reasonably expected outcomes are reported on. |
| Industry bias | Unclear risk | The calcium supplements were provided by Mission Pharmacal (San Antonio, TX) and GlaxoSmithKline (Parsippany, NJ). The vitamin D3 was obtained from Tishcon Corporation (Westbury, NY). |
| Other bias | Low risk | The trial appears to be free of other components that could put it at risk of bias. |
Larsen 2004.
| Methods | Cluster‐randomised clinical trial using 2 x 2 factorial design. | |
| Participants | Country: Denmark. Number of participants randomised: 9605, (60 % women), 66 to 103 (mean 75) years or over community‐dwelling residents. Inclusion criteria: community‐dwelling residents, aged 66 years or over. Exclusion criteria: elderly, who were living in nursing homes, severely impaired persons living in sheltered homes for the elderly, as well as elderly with mental retardation who were unable to give informed consent. |
|
| Interventions | Municipality of Randers, Denmark was divided into four comparable blocks. The four blocks were allocated at random to three different fracture prevention programs or no intervention. Intervention group 1: home safety inspection by a community nurse to identify and remedy possible hazards and identify and correct potential health or dietary problems. The nurse evaluated the resident’s prescribed medication to identify possible errors or necessary dose adjustments. Those who accepted a home visit in this area were given leaflets with information of different ways to avoid falling (n = 2532); Intervention group 2: vitamin D3 (400 IU) plus calcium (1000 mg) daily. Furthermore, these participants were offered an evaluation of their prescribed medication. This revision also ensured that the elderly took no other types of vitamin D products and calcium. If the participants used cardiovascular medicine (digoxin or calcium antagonists) that may interact with calcium, they were referred to their general practitioner. Those who accepted a home visit were given leaflets with information of different ways to avoid osteoporosis (n = 2426); Intervention group 3: a combination of the intervention 1 and intervention 2 (n = 2531); Intervention group 4 (Control group): no intervention (n = 2116); for a three and a half year period. |
|
| Outcomes | The primary outcome was osteoporotic fractures leading to acute hospital admission. | |
| Stated aim of study | "To evaluate the effect of two programmes for the prevention of fractures leading to acute hospital admission in a population of elderly community‐dwelling Danish residents. One programme included the provision of vitamin D and calcium, whereas the other programme offered an evaluation of and suggestions for the improvement of the domestic environment. Both programmes included revision of the resident’s current pharmaceutical treatment." | |
| Notes | The trial was supported by Nycomed DAK. Nycomed DAK supplied the free vitamin D tablets and calcium (Calcichew). Additional information on mortality was received through personal communication with Dr Leif Mosekilde and Dr Lars Rejnmark (06.02.2009). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The trial is described as randomised, but the method of sequence generation was not specified. |
| Allocation concealment (selection bias) | High risk | The allocation sequence was known to the investigators who assigned participants. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Trial was not blinded, so that the allocation was known during the trial. Placebo was not used. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The number or reasons for dropouts and withdrawals were not described. |
| Selective reporting (reporting bias) | Unclear risk | Not all pre‐defined, or clinically relevant and reasonably expected outcomes are reported on or are not reported fully, or it is unclear whether data on these outcomes were recorded or not. |
| Industry bias | Unclear risk | The trial was supported by Nycomed DAK. Nycomed DAK supplied the free vitamin D tablets and calcium (Calcichew). |
| Other bias | Unclear risk | There are other factors in the trial that could put it at risk of bias. Recruitment bias was judged as probably adequate. |
Latham 2003.
| Methods | The Frailty Interventions Trial in Elderly Subjects (FITNESS). Multicentre, randomised, placebo controlled trial using 2 x 2 factorial design. |
|
| Participants | Country: New Zealand. Number of participants randomised: 243, 64 to 99 (mean 85) years of age, healthy ambulatory women. Inclusion criteria: aged 65 and older, considered frail according to simple clinical measures of frailty and no clear indication or contraindication to either of the trial interventions (i.e., the clinician had substantial uncertainty about the benefits or harms of either interventions for a specific patient). Exclusion criteria: if patients were considered not frail (i.e., fit and independent or fully dependent in activity of daily living) or if, in the opinion of the responsible clinician, that treatment was considered to be potentially hazardous or definitely indicated for a patient; had a poor prognosis and were unlikely to survive six months; severe cognitive impairment that would compromise adherence to the exercise programme (generally people with scores < 20 on a 30‐point Mini‐Mental State Examination); physical limitations that could limit adherence to the exercise programme (e.g., poor upper limb function that limited application of the weights); unstable cardiac status, or large ulcers about the ankles that would preclude safe application of the ankle weights. In addition, because of difficulties that would arise with their follow‐up assessments, people who lived outside the hospitals' normal geographical zones and patients who were not fluent in English were excluded. |
|
| Interventions | Participants were randomly assigned to receive: Intervention group 1: resistance exercise to the quadriceps muscles with frequency‐matched social home visits (ten week programme) (n = 120); Intervention group 2: vitamin D3 (300,000 IU) (n = 121); Intervention group 3: attention control (n = 123); Intervention group 4 (Control group): placebo vitamin D3 (n = 122); for a six‐month period. The vitamin D intervention was given in a single oral dose. Patients received either six vitamin D3 (300,000 IU) or matching placebo tablets. A trial nurse administered the tablets. Overall, vitamin D received 121 participant and placebo 122 participants. |
|
| Outcomes | The primary outcomes were self‐rated physical health at three months and falls over the sixth‐month period. Secondary outcomes were physical performance and self‐rated function. | |
| Stated aim of study | "To determine whether a simple home‐based programme of resistance exercise to the quadriceps muscles or a single high dose of vitamin D could improve self‐reported physical health and reduce the risk of falls in frail older people who had recently been discharged from hospital." | |
| Notes | "Compliance was monitored using a participants diary. Compliance with the single high dose of calciferol or placebo was 100%. No participants were lost to follow‐up." Additional information on mortality and form of vitamin D used in the trial was received through personal communication with Professor Nancy K Latham (01.02.2009) and Professor Ian Cameron (24.02.2010). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The trial biostatistician generated the randomisation sequence using a computerised central randomisation scheme. |
| Allocation concealment (selection bias) | Low risk | Allocation was controlled by a central and independent randomisation unit so that intervention allocations could not have been foreseen in advance of, or during, enrolment. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was described as blinded, the parties that were blinded, and the method of blinding was described, so that knowledge of allocation was adequately prevented during the trial. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | It was specified that there were no dropouts or withdrawals. |
| Selective reporting (reporting bias) | Low risk | Pre‐defined, or clinically relevant and reasonably expected outcomes are reported on. |
| Industry bias | Unclear risk | The trial is supported by grants from the Health Research Council of New Zealand, the Auckland University of Technology Research Fund, and a bequest from the Lenore Wilson Estate. |
| Other bias | Low risk | The trial appears to be free of other components that could put it at risk of bias. |
Law 2006.
| Methods | Cluster‐randomised clinical trial using parallel group design (two intervention groups). | |
| Participants | Country: United Kingdom. Number of participants randomised: 3717 participating residents (76% women), average age 85 years. Inclusion criteria: elderly people aged 60 years or over. Exclusion criteria: temporary residents admitted for respite care, residents who were already taking calcium/vitamin D or drugs that increase bone density (such as bisphosphonates), and residents who had sarcoidosis or malignancy, or other life‐threatening illness. |
|
| Interventions | Participants (30‐bedded units) were randomly assigned to receive: Intervention group 1: vitamin D2 (1100 IU) daily (n = 1762); Intervention group 2 (Control group): no intervention (n = 1955); for a ten‐month period. Vitamin D was given as tablets containing vitamin D2 (ergocalciferol) 100,000 IU (Norton Healthcare (now Ivax Pharmaceuticals)) every three months; Residents in the control group took no vitamin D (there was no placebo). |
|
| Outcomes | The primary outcomes were non‐vertebral fractures and falls. | |
| Stated aim of study | "To determine whether vitamin D supplementation reduces the risk of fracture or falls in elderly people in care home accommodation." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequence generation was achieved using cluster randomisation by computer. |
| Allocation concealment (selection bias) | High risk | The allocation sequence was known to the investigators who assigned participants. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Trial was not blinded, so that the allocation was known during the trial. Placebo was not used. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The numbers and reasons for dropouts and withdrawals in all intervention groups were described. |
| Selective reporting (reporting bias) | Low risk | Pre‐defined, or clinically relevant and reasonably expected outcomes are reported on. |
| Industry bias | Unclear risk | The source of funding is not clear. |
| Other bias | Unclear risk | The trial may or may not be free of other components that could put it at risk of bias. There was potential selection bias as no data given on non‐participants. Recruitment bias judged as unknown. |
Lehouck 2012.
| Methods | Randomised, double‐blind, placebo‐controlled trial using parallel group design (two intervention groups). | |
| Participants | Country: Belgium. Number of participants randomised: 182 patients with chronic obstructive pulmonary disease (COPD), (20% women), mean age 68 years. Inclusion criteria: current or former smokers, older than 50 years, diagnosis of COPD according to the Global Initiative for Chronic Obstructive Lung Disease definition (postbronchodilator FEV1– FVC ratio < 0.7), and had an FEV1 less than 80% predicted. Exclusion criteria: a history of hypercalcaemia, sarcoidosis, or active cancer. |
|
| Interventions | Participants were randomly assigned to receive: Intervention group 1: vitamin D3 100,000 IU monthly (n = 91); Intervention group 2 (Control group): matched placebo monthly (n = 91); for one year. |
|
| Outcomes | The primary outcome was time to first exacerbation. Secondary outcomes were exacerbation rate, time to first hospitalisation, time to second exacerbation, FEV1, quality of life, and death. | |
| Stated aim of study | "To explore the effect of adequate vitamin D supplementation on exacerbations in patients with moderate to very severe COPD." | |
| Notes | Laboratoires SMB Brussels, Belgium, provided the study medication free of charge. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequence generation was achieved using computer random number generation |
| Allocation concealment (selection bias) | Low risk | Allocation was controlled by a central and independent randomisation unit so that intervention allocations could not have been foreseen in advance of, or during, enrolment. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The outcome measurement is not likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The underlying reasons for missing data are unlikely to make treatment effects depart from plausible values. |
| Selective reporting (reporting bias) | Low risk | Pre‐defined, or clinically relevant and reasonably expected outcomes are reported on. |
| Industry bias | Unclear risk | Laboratoires SMB Brussels, Belgium, provided the study medication free of charge. |
| Other bias | Low risk | The trial appears to be free of other components that could put it at risk of bias. |
Lips 1996.
| Methods | Randomised, double‐blind, placebo‐controlled trial using parallel group design (two intervention groups). | |
| Participants | Country: the Netherlands. Number of participants randomised: 2578 independently living elderly persons (74% women), 70 to 97 (mean 80) years of age. Inclusion criteria: elderly people, aged 70 years or over, reasonable healthy and able to give informed consent. Exclusion criteria: history of hip fracture or total hip arthroplasty, known hypercalcaemia, sarcoidosis, or recent urolithiasis (< 5 years earlier), diseases or medications that influence bone metabolism (such as thyroid disease or glucocorticoid medication). |
|
| Interventions | Participants were randomly assigned to receive: Intervention group 1: vitamin D3 400 IU daily (n = 1291); Intervention group 2 (Control group): matched placebo daily (n = 1287); for a three and a half year period. |
|
| Outcomes | The primary outcomes were hip fractures and other peripheral bone fractures. | |
| Stated aim of study | "To determine whether vitamin D supplementation decreases the incidence of hip fractures and other peripheral bone fractures." | |
| Notes | "Compliance was checked when the tablet containers were replaced (every 6 months), by questionnaire (every year), and by measurement of the serum 25(OH)D concentration. Compliance was considered to be adequate if the participants reported on the questionnaire that they took the tablets five or more days per week. This occurred in 85% of the participants and was similar in both groups." Vitamin D and placebo tablets were provided by Solvay‐Duphar, Inc, Weesp, the Netherlands. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequence generation was achieved using computer random number generation or a random number table. |
| Allocation concealment (selection bias) | Low risk | Allocation was controlled by a central and independent randomisation unit so that intervention allocations could not have been foreseen in advance of, or during, enrolment. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was described as blinded, the parties that were blinded, and the method of blinding was described, so that knowledge of allocation was adequately prevented during the trial. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The numbers and reasons for dropouts and withdrawals in all intervention groups were described. |
| Selective reporting (reporting bias) | Low risk | Pre‐defined, or clinically relevant and reasonably expected outcomes are reported on. |
| Industry bias | Unclear risk | Vitamin D and placebo tablets were provided by Solvay‐Duphar, Inc, Weesp, the Netherlands. |
| Other bias | Low risk | The trial appears to be free of other components that could put it at risk of bias. |
Lips 2010.
| Methods | Randomised, double‐blind, placebo‐controlled multicenter trial using parallel group design (two intervention groups). | |
| Participants | Country: the Netherlands. Number of participants randomised: 226 men and women aged ≥ 70 (mean 78) years who were vitamin D insufficient (serum 25‐hydroxyvitamin D concentrations ≤ 20 but ≥ 6 ng/mL). Inclusion criteria: ambulatory elderly people who were vitamin D insufficient, aged 70 years or over, able to walk 10 feet without a walking aid) and mentally competent. If patients had serum 25‐hydroxyvitamin D concentrations ≥ 6 but ≤ 9 ng/mL, they needed to have 24‐h urine calcium concentrations ≥ 50 mg/d and bone‐specific alkaline phosphatase concentrations not higher than the upper limit of normal. Exclusion criteria: primary hyperparathyroidism, active thyroid disease, impaired renal function, osteomalacia, neurologic impairment, peripheral neuropathy, myocardial infarction within 6 months of screening, uncontrolled hypertension, postural hypotension, malabsorption syndrome, alcohol abuse (i.e., > 2 drinks/day), cancer, treatment with oral glucocorticoids, anabolic steroids, or a growth hormone within 12 months of screening; treatment with > 800 IU vitamin D a day or with active metabolites of vitamin D within 6 months of screening; or treatment with any drug that might affect vitamin D metabolism or interfere with postural stability at screening. |
|
| Interventions | Participants were randomly assigned to receive: Intervention group 1: vitamin D3 8400 IU weekly (n = 114); Intervention group 2 (Control group): matched placebo weekly (n = 112); for a 16 weeks period. "For participants with a daily dietary calcium intake <1000 mg (as assessed by a questionnaire at screening), daily calcium carbonate containing 500 mg elemental calcium was also prescribed." |
|
| Outcomes | The primary outcome measure was mediolateral sway with eyes open. Secondary outcome measures were change in functional status assessed with the short physical performance battery, mean serum 25‐hydroxyvitamin D, calcium, and phosphate concentrations, and adverse events. | |
| Stated aim of study | "To assess whether a once‐weekly treatment with 8400 IU vitamin D3 would improve body postural stability and lower‐extremity function in elderly people with low vitamin D status (serum 25‐hydroxyvitamin D concentrations ≤ 20 ng/mL)." | |
| Notes | "All patients who completed the trial were adherent to treatment, which was defined as taking ≥ 13 of the 16 total doses prescribed." Supported by Merck & Co Inc. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequence generation was achieved using computer random number generation. |
| Allocation concealment (selection bias) | Low risk | Allocation was controlled by a central and independent randomisation unit. Participants were stratified (2:1) at randomisation according to baseline serum 25‐hydroxyvitamin D concentration. Patients were assigned a unique allocation number according to their appropriate stratification block. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was described as blinded, the parties that were blinded, and the method of blinding was described, so that knowledge of allocation was adequately prevented during the trial. Investigators were blinded to serum 25‐hydroxyvitamin D concentrations and to stratum definitions. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The numbers and reasons for dropouts and withdrawals in all intervention groups were described or if it was specified that there were no dropouts or withdrawals. |
| Selective reporting (reporting bias) | Low risk | Pre‐defined, or clinically relevant and reasonably expected outcomes are reported on. |
| Industry bias | High risk | The trial is funded by a manufacturer of vitamin D. |
| Other bias | Low risk | The trial appears to be free of other components that could put it at risk of bias. |
Lyons 2007.
| Methods | Randomised, double‐blind, placebo‐controlled trial using parallel group design (two intervention groups). | |
| Participants | Country: United Kingdom. Number of participants randomised: 3440 older people living in institutional care (76% women), 62 to 107 (mean 84) years of age. Inclusion criteria: elderly people, including those with mobility, cognitive, visual, hearing or communication impairments living in nursing homes, residential homes, and sheltered housing. Exclusion criteria: people already receiving ≥ 400 IU of vitamin D/day and those already known to have contraindications to vitamin D supplementation. |
|
| Interventions | Participants were randomly assigned to receive: Intervention group 1: vitamin D2 100,000 IU three times a year (four‐monthly) (n = 1725); Intervention group 2 (Control group): matched placebo tablet three times a year (four‐monthly) (n = 1715); for a three‐year period. |
|
| Outcomes | The primary outcome measure was the incidence of first fracture. Secondary outcome measures were the incidence of hip fractures, fractures at common osteoporotic sites (hip/wrist/forearm/vertebrae), and mortality rates. | |
| Stated aim of study | "To examine the effect of vitamin D supplementation on fracture rate in people living in sheltered accommodation." | |
| Notes | "Dosing was supervised by the research nurse to ensure adherence, but nurse, participant, and analysts were blinded to the allocation. Adherence among participants in the trial was 80% overall (percentage of occasions observed to take tablets whilst in the trial)." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequence generation was achieved using computer random number generation. |
| Allocation concealment (selection bias) | Low risk | Allocation was controlled by a central and independent randomisation unit so that intervention allocations could not have been foreseen in advance of, or during, enrolment. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was described as blinded, the parties that were blinded, and the method of blinding was described, so that knowledge of allocation was adequately prevented during the trial. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The numbers and reasons for dropouts and withdrawals in all intervention groups were described |
| Selective reporting (reporting bias) | Low risk | Pre‐defined, or clinically relevant and reasonably expected outcomes are reported on. |
| Industry bias | Low risk | The trial is not funded by a manufacturer of vitamin D. |
| Other bias | Low risk | The trial appears to be free of other components that could put it at risk of bias. |
Meier 2004.
| Methods | Randomised controlled trial using parallel group design (two intervention groups). | |
| Participants | Country: Germany. Number of participants randomised: 55 healthy volunteers (65% postmenopausal women), 33 to 78 (mean 55,8) years of age. Inclusion criteria: healthy volunteers. Exclusion criteria: history or clinical evidence of significant skeletal or nonskeletal disease, taking any medication known to affect bone metabolism, including vitamin D and mineral supplements. |
|
| Interventions | Participants were randomly assigned to receive: Intervention group 1: vitamin D3 500 IU daily plus calcium 500 mg daily (n = 30); Intervention group 2 (Control group): no intervention (n = 25); for a six‐month period. Participants were followed an additional six‐month period. The first year of the trial after randomisation was designed as an observation period only, during which the participants followed their usual daily routine with no intervention per protocol. During the winter of the second year, from October to March, the participants assigned to the intervention group received a daily supplement of oral vitamin D3 (500 IU) and calcium (500 mg), whereas the participants in the control group received no supplements and were asked to remain off such agents. The trial medication was open label. |
|
| Outcomes | The primary outcomes were circannual changes in bone turnover, and bone mineral density and rates of bone turnover and bone loss during the winter months. | |
| Stated aim of study | "To evaluate the circannual changes in bone turnover, and bone mineral density and to determine the effect of oral calcium and vitamin D3 supplementation on rates of bone turnover and bone loss during the winter months." | |
| Notes | "Adherence to intervention was checked in monthly intervals through personal interviews." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The trial is described as randomised but the method of sequence generation was not specified. |
| Allocation concealment (selection bias) | Unclear risk | The trial was described as randomised but the method used to conceal the allocation was not described, so that intervention allocations may have been foreseen in advance of, or during, enrolment. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Trial was not blinded, so that the allocation was known during the trial. Placebo was not used. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The numbers and reasons for dropouts and withdrawals in all intervention groups were described. |
| Selective reporting (reporting bias) | Low risk | Pre‐defined, or clinically relevant and reasonably expected outcomes are reported on. |
| Industry bias | Low risk | The trial is not funded by a manufacturer of vitamin D. |
| Other bias | Low risk | The trial appears to be free of other components that could put it at risk of bias. |
Moschonis 2006.
| Methods | Postmenopausal Health Study (PMHS). Randomised controlled trial using parallel group design (two intervention groups). |
|
| Participants | Country: Greece. Number of participants randomised: 112 postmenopausal women, aged 55 to 65 (mean 60.3) years. Inclusion criteria: postmenopausal non‐osteoporotic women. Exclusion criteria: a T‐score lower than 22.5, taking medications (i.e., thiazide diuretics, glucocorticoids) and/or dietary supplements (calcium, magnesium, phosphate or vitamin D) that affect bone metabolism, having any kind of degenerative chronic disease (i.e., diabetes, nephrolithiasis, heart disease, cancer, hyper‐ and hypothyroidism, hyperparathyroidism, impaired renal and liver function), smoking and being postmenopausal for less than 1 year |
|
| Interventions | Participants were randomly assigned to receive: Intervention group 1: vitamin D3 300 IU plus calcium 1200 mg daily (n = 42); Intervention group 2: calcium 1200 mg (n = 30); Intervention group 3 (Control group): no intervention (n = 40); for a one‐year period. |
|
| Outcomes | The primary outcome measure was bone mineral density. | |
| Stated aim of study | "To examine whether the use of calcium supplementation could prevent bone loss in healthy postmenopausal women or more favourable outcomes could be obtained using a holistic approach combining dietary intervention and consumption of dairy products fortified with calcium and vitamin D3." | |
| Notes | "To ensure compliance with the intervention scheme, ‘Health and Nutrition Education’ sessions were held biweekly within the settings of the university and the required quantities of fortified dairy products for the next two weeks were provided at the end of the sessions. Adherence of the participants in the calcium group was assessed by checking for remaining calcium tablets in the returned packages but also via weekly phone calls. Compliance to the intervention scheme was reaching a rate of 93% (range 89 to 100 %). Compliance rate in calcium group was approximately 95% (range 91 to 100 %)." The trial was supported by a research grant from Friesland Foods Hellas. Additional information on mortality was received through personal communication with Dr George Moschonis (23.02.2010). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequence generation was achieved using a random number table. |
| Allocation concealment (selection bias) | High risk | The allocation sequence was known to the investigators who assigned participants. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Trial was not blinded, so that the allocation was known during the trial. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The numbers and reasons for dropouts and withdrawals in all intervention groups were described. |
| Selective reporting (reporting bias) | Low risk | Pre‐defined, or clinically relevant and reasonably expected outcomes are reported on. |
| Industry bias | High risk | The trial was supported by a research grant from Friesland Foods Hellas. |
| Other bias | Low risk | The trial appears to be free of other components that could put it at risk of bias. |
Ooms 1995.
| Methods | Randomised, double‐blind, placebo‐controlled trial using parallel group design (two intervention groups). | |
| Participants | Country: The Netherlands. Number of participants randomised: 348 women, aged 70 years or older, who were reasonably mobile. Inclusion criteria: elderly mobile women aged 70 years or older. Exclusion criteria: hip fracture in the past, total hip prosthesis, and recent history of urolithiasis, hypercalcaemia, or sarcoidosis. |
|
| Interventions | Participants were randomly assigned to receive: Intervention group 1: vitamin D3 400 IU daily (n = 177); Intervention group 2 (Control group): matched placebo daily (n = 171); for a two‐year period. |
|
| Outcomes | The primary outcome measures were bone mineral density of both hips (femoral neck and trochanter) and the distal radius, as well as biochemical markers of bone turnover. | |
| Stated aim of study | "To determine the effect of vitamin D supplementation on bone turnover and bone loss in elderly women." | |
| Notes | "Compliance was established by questionnaire, by pill counting, and by measuring serum 250HD levels in blood. If participants were suspected of poor compliance resulting from memory problems, the nursing staff were asked to supervise the taking of the trial intervention or to administer it." "The compliance was good in both groups. According to the yearly questionnaire, 85% used one tablet daily, and 14% used between three and six tablets weekly. The analysis of the remaining tablets showed a slightly better compliance in the second trial year. In the first year, 63% had used between six and seven tablets weekly, and 4% had used less than three weekly; in the second year, these compliance rates were 78% and 1%, respectively. Of the women receiving the vitamin D supplement, only 5 participants (3%) did not achieve a serum 25 hydroxyvitamin D level higher than 30 nmol/L, whereas 68.4% of the participants in the placebo group had serum levels below 30 nmol/L." The trial medication was provided by Duphar Nederland BV, Amsterdam, the Netherlands. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequence generation was achieved using computer random number generation. |
| Allocation concealment (selection bias) | Low risk | Allocation was controlled by a central and independent randomisation unit so that intervention allocations could not have been foreseen in advance of, or during, enrolment. Randomisation was performed by the hospital pharmacy, and double‐blinding was assured. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was described as blinded, the parties that were blinded, and the method of blinding was described, so that knowledge of allocation was adequately prevented during the trial. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The numbers and reasons for dropouts and withdrawals in all intervention groups were described. |
| Selective reporting (reporting bias) | Low risk | Pre‐defined, or clinically relevant and reasonably expected outcomes are reported on. |
| Industry bias | Unclear risk | The trial medication was provided by Duphar Nederland BV, Amsterdam, the Netherlands. |
| Other bias | Low risk | The trial appears to be free of other components that could put it at risk of bias. |
Ott 1989.
| Methods | Randomised, double‐blind, placebo‐controlled trial using parallel group design (two intervention groups). | |
| Participants | Country: United States. Number of participants randomised: 86 postmenopausal women, 50 to 80 (mean 67.5) years of age. Inclusion criteria: postmenopausal women with at least two compression fractures (> 15% reduction in anterior height) without history of serious trauma. Exclusion criteria: history of corticosteroid use, malnutrition, sarcoidosis, liver disease, rheumatoid arthritis, nephrolithiasis, renal disease, or recent malignancy. |
|
| Interventions | Participants were randomly assigned to receive: Intervention group 1: calcitriol 0.25 to 2 μg plus calcium 1000 mg (n = 43); Intervention group 2 (Control group): placebo vitamin D plus calcium 1000 mg daily (n = 43); for a two‐year period. |
|
| Outcomes | The primary outcome measure was bone mass. Secondary outcome measure was adverse effects of calcitriol. | |
| Stated aim of study | "To determine if calcitriol is an effective treatment in postmenopausal osteoporosis." | |
| Notes | Hoffman‐La Roche (Nutley, New Jersey) supplied the vitamin D supplements. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The trial is described as randomised but the method of sequence generation was not specified. |
| Allocation concealment (selection bias) | Unclear risk | The trial was described as randomised but the method used to conceal the allocation was not described, so that intervention allocations may have been foreseen in advance of, or during, enrolment. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | The trial was described as double blind, but the method of blinding was not described, so that knowledge of allocation was possible during the trial. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The numbers and reasons for dropouts and withdrawals in all intervention groups were described. |
| Selective reporting (reporting bias) | Low risk | Pre‐defined, or clinically relevant and reasonably expected outcomes are reported on. |
| Industry bias | Unclear risk | Hoffman‐La Roche (Nutley, New Jersey) supplied the vitamin D supplements. |
| Other bias | Low risk | The trial appears to be free of other components that could put it at risk of bias. |
Porthouse 2005.
| Methods | Randomised controlled trial using parallel group design (two intervention groups). | |
| Participants | Country: United Kingdom. Number of participants randomised: 3314 women, aged 70 and over (mean 76.8) years, with one or more risk factors for hip fracture. Inclusion criteria: elderly women, aged 70 years or older, who had at least one self reported risk factor for hip fracture: low bodyweight (< 58 kg), any previous fracture, maternal history of hip fracture, smoker, and poor or fair health. Exclusion criteria: unable to give written consent, receiving of any calcium supplementation of more than 500 mg a day, a history of kidney or bladder stones, renal failure, or hypercalcaemia. |
|
| Interventions | Participants were randomly assigned to receive: Intervention group 1: vitamin D3 800 IU plus calcium 1000 mg daily (n = 1321); Intervention group 2 (Control group): information leaflet on dietary calcium intake and prevention of falls, or leaflet only (n = 1993); for a 25‐month period. |
|
| Outcomes | The primary outcome measure was fracture, excluding those of the digits, rib, face, and skull. Secondary outcomes included hip fracture; quality of life as measured by the 12 item short‐form health survey questionnaire, and the European quality of life instrument, death, visits to the doctor and hospital admissions, falls and fear of falling. | |
| Stated aim of study | "To assess whether supplementation with calcium and vitamin D3 reduces the risk of fracture in women with one or more risk factors for fracture of the hip." | |
| Notes | "Adherence was measured through self report every six months. Rates for adherence at 12 months were about 63%." The trial was supported by Shire and Nycomed. Shire supplied the vitamin D supplements and calcium. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequence generation was achieved using computer random number generation. |
| Allocation concealment (selection bias) | Low risk | Allocation was controlled by a central and independent randomisation unit so that intervention allocations could not have been foreseen in advance of, or during, enrolment. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Trial was not blinded, so that the allocation was known during the trial. Placebo was not used. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The numbers and reasons for dropouts and withdrawals in all intervention groups were described. |
| Selective reporting (reporting bias) | Unclear risk | Not all clinically relevant and reasonably expected outcomes are reported on. Adverse events were not reported. |
| Industry bias | High risk | The trial was supported by Shire and Nycomed. Shire supplied the vitamin D supplements and calcium. |
| Other bias | Low risk | The trial appears to be free of other components that could put it at risk of bias. |
Prince 2008.
| Methods | Randomised, double‐blind, placebo‐controlled trial using parallel group design (two intervention groups). | |
| Participants | Country: Australia. Number of participants randomised: 302 community‐dwelling ambulant older women aged 70 to 90 (mean 77.2) years with a history of falling and vitamin D insufficiency. Inclusion criteria: community‐dwelling ambulant older women with a history of falling in the past 12 months and a plasma 25 hydroxyvitamin D concentration of less than 24.0 ng/mL. Exclusion criteria: current vitamin D consumption; current consumption of bone or mineral active agents apart from calcium; a bone mineral density z score at the total hip site of less than ‐2.0; medical conditions or disorders that influence bone mineral metabolism, including laboratory evidence of renal insufficiency (a creatinine level more than two‐fold above the reference range); a fracture in the past 6 months; a Mini‐Mental State Examination score of less than 24; or the presence of marked neurological conditions likely to substantially impair balance or physical activity, such as stroke and Parkinson's disease. |
|
| Interventions | Participants were randomly assigned to receive: Intervention group 1: vitamin D2 1000 IU plus calcium 1000 mg daily (n = 151); Intervention group 2 (Control group): matched placebo tablet of vitamin D plus calcium 1000 mg daily (n = 151); for a one‐year period. |
|
| Outcomes | The primary outcome measure was risk of falls in older women at high risk of falling. | |
| Stated aim of study | "To evaluate the effect of vitamin D2 and calcium supplementation compared with calcium alone on the risk of falls in older women at high risk of falling." | |
| Notes | "Adherence to the trial medications was established by counting tablets returned at the clinic visits at 6 and 12 months. The rate of compliance with trial medication in participants who continued to receive the medication, as determined from tablet counting, was 86% in both groups." Vitamin D2 (ergocalciferol) or identical placebo was provided by Ostelin; Boots Healthcare, North Ryde, Australia. Calcium as calcium citrate was provided by Citracal; Mission Pharmacal, Key Pharmaceutical Pty Ltd, Rhodes, Australia. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequence generation was achieved using computer random number generation. |
| Allocation concealment (selection bias) | Low risk | Allocation was controlled by a central and independent randomisation unit so that intervention allocations could not have been foreseen in advance of, or during, enrolment. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was described as blinded, the parties that were blinded, and the method of blinding was described, so that knowledge of allocation was adequately prevented during the trial. Randomisation schedule was kept in the pharmacy department, where the bottles were labelled and dispensed to the participants.The trial participants and the trial staff remained blinded to the treatment code until all the data had been entered, evaluated for accuracy, and the a priori hypotheses reviewed. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The numbers and reasons for dropouts and withdrawals in all intervention groups were described. |
| Selective reporting (reporting bias) | Low risk | Pre‐defined, or clinically relevant and reasonably expected outcomes are reported on. |
| Industry bias | Unclear risk | Vitamin D2 (ergocalciferol) or identical placebo was provided by Ostelin; Boots Healthcare, North Ryde, Australia. Calcium as calcium citrate was provided by Citracal; Mission Pharmacal, Key Pharmaceutical Pty Ltd, Rhodes, Australia. |
| Other bias | Low risk | The trial appears to be free of other components that could put it at risk of bias. |
Sanders 2010.
| Methods | Single centre, randomised, double‐blind, placebo‐controlled trial using parallel group design (two intervention groups). The Vital D study. |
|
| Participants | Country: Australia. Number of participants randomised: 2258 community‐dwelling women, 70 years or older (mean age 76 years) considered to be at high risk of fracture. Inclusion criteria: community‐dwelling women at higher risk of hip fracture, defined by criteria such as maternal hip fracture, past fracture, or self‐reported faller. Exclusion criteria: unable to provide informed consent or information about falls or fractures; permanently resided at a high‐level care facility; had an albumin‐corrected calcium level higher than 2.65 mmol/L; or had a creatinine level higher than 150 μmol/L, or currently took vitamin D doses of 400 IU or more, calcitriol, or antifracture therapy. |
|
| Interventions | Participants were randomly assigned to receive: Intervention group 1: vitamin D3 500,000 IU yearly (n = 1131); Intervention group 2 (Control group): matched placebo tablet of vitamin D yearly (n = 1127); for a three to five years (in autumn or winter), median 2.96 years. "Ten tablets were mailed to participants annually (March‐August, determined by recruitment date) with instructions to take all tablets on a single day. Study staff confirmed by telephone the ingestion of study medication within 2 weeks. Subsequent dosing occurred within 2 weeks of the anniversary of the first dose." |
|
| Outcomes | The primary outcome measures were falls and fractures. Secondary outcome measures were serum 25‐hydroxycholecalciferol and intact parathyroid hormone levels. | |
| Stated aim of study | "To determine whether a single annual dose of 500 000 IU of cholecalciferol administered orally to older women in autumn or winter would improve adherence and reduce the risk of falls and fracture." | |
| Notes | "Study staff confirmed by telephone the ingestion of study medication." Study medication was supplied by PSM Healthcare, Auckland, New Zealand. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequence generation was achieved using computer random number generation. |
| Allocation concealment (selection bias) | Low risk | "Allocation was performed by an independent statistician. Treatment allocation status was e‐mailed directly to the hospital clinical trials pharmacist responsible for dispensing study medication." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was described as blinded, the parties that were blinded, and the method of blinding was described, so that knowledge of allocation was adequately prevented during the trial. The participants and study staff were blinded to intervention group. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The numbers and reasons for dropouts and withdrawals in all intervention groups were described. |
| Selective reporting (reporting bias) | Low risk | Pre‐defined, or clinically relevant and reasonably expected outcomes are reported on. |
| Industry bias | Unclear risk | Study medication was supplied by PSM Healthcare, Auckland, New Zealand. |
| Other bias | Low risk | The trial appears to be free of other components that could put it at risk of bias. |
Sato 1997.
| Methods | Randomised, double‐blind, placebo‐controlled trial using parallel group design (two intervention groups). | |
| Participants | Country: Japan. Number of participants randomised: 64 (45% women) mean age 68.5 years) outpatients with hemiplegia after stroke. Inclusion criteria: patients with hemiplegia after stroke. Exclusion criteria: shoulder‐hand syndrome, multiple strokes, history of hip fracture, a stroke duration of less than 1 month, or the use of medication known to affect bone metabolism, including oestrogen, calcium, vitamin D, corticosteroids, thyroxine, or anticonvulsants. |
|
| Interventions | Participants were randomly assigned to receive: Intervention group 1: vitamin D in the form of 1(OH)D3 (alfacalcidol) 1 μg plus calcium 300 mg daily (n = 45); Intervention group 2 (Control group): matched placebo tablet of vitamin D plus calcium 300 mg daily (n = 39); for a six‐month period. |
|
| Outcomes | The primary outcome measures were bone mineral density and hip fractures. | |
| Stated aim of study | "To evaluate the efficacy of 1(OH)D3and supplemental elemental calcium in reducing the severity of osteopenia in the second metacarpals and decreasing the risk of hip fractures in chronically ill stroke patients with hemiplegia." | |
| Notes | Additional information on mortality was received through personal communication with Dr Yoshiro Sato (05.02.2009). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The trial is described as randomised but the method of sequence generation was not specified. |
| Allocation concealment (selection bias) | Unclear risk | The trial was described as randomised but the method used to conceal the allocation was not described, so that intervention allocations may have been foreseen in advance of, or during, enrolment. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | The method of blinding was not described, so that knowledge of allocation was possible during the trial. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The numbers and reasons for dropouts and withdrawals in all intervention groups were described. |
| Selective reporting (reporting bias) | Unclear risk | Not all pre‐defined, or clinically relevant and reasonably expected outcomes are reported on. Adverse events were not reported. |
| Industry bias | Unclear risk | The source of funding is not clear. |
| Other bias | Low risk | The trial appears to be free of other components that could put it at risk of bias. |
Sato 1999a.
| Methods | Randomised, double‐blind, placebo‐controlled trial using parallel group design (two intervention groups). | |
| Participants | Country: Japan. Number of participants randomised: 86 elderly patients (78% women) aged 65 to 88 (mean 70.6) with Parkinson's disease. Inclusion criteria: elderly patients with Parkinson's disease and low serum 1,25‐dihydroxyvitamin D concentrations. Exclusion criteria: other known causes of osteoporosis, such as hyperparathyroidism or renal osteodystrophy; impairment of renal, cardiac, or thyroid function; a history of therapy with corticosteroids, estrogens, calcitonin, etidronate, calcium, or vitamin D for three months or longer during the 18 months preceding the trial; or even brief treatment of this nature during the two months immediately preceding the trial. Patients at Hoehn and Yahr stage 5 were excluded because their poor ambulation status largely precluded any chance of fracture. Patients with a history of non‐vertebral fracture were also excluded. |
|
| Interventions | Participants were randomly assigned to receive: Intervention group 1: vitamin D in a form of 1‐α hydroxyvitamin D3 (alfacalcidol) (1 μg) daily (n = 43); Intervention group 2 (Control group): matched placebo tablet daily (n = 43); for a 18‐month period. |
|
| Outcomes | The primary outcome measure was non‐vertebral fractures. Secondary outcome was progression of osteopenia in the second metacarpal bone. | |
| Stated aim of study | "To evaluate the efficacy of 1‐α hydroxyvitamin D3 (alfacalcidol) in reducing progression of osteopenia in the second metacarpal and in decreasing non‐vertebral fractures in elderly patients with Parkinson's disease with low serum 1,25‐dihydroxyvitamin D concentrations." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequence generation was achieved using computer random number generation. |
| Allocation concealment (selection bias) | Unclear risk | The trial was described as randomised but the method used to conceal the allocation was not described, so that intervention allocations may have been foreseen in advance of, or during, enrolment. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | The trial was described as double blind, but the method of blinding was not described, so that knowledge of allocation was possible during the trial. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The numbers and reasons for dropouts and withdrawals in all intervention groups were described |
| Selective reporting (reporting bias) | Low risk | Pre‐defined, or clinically relevant and reasonably expected outcomes are reported on. |
| Industry bias | Unclear risk | The source of funding is not clear. |
| Other bias | Low risk | The trial appears to be free of other components that could put it at risk of bias. |
Sato 1999b.
| Methods | Randomised controlled trial using parallel group design (three intervention groups). | |
| Participants | Country: Japan. Number of participants randomised: 103 patients (56% women), mean age 70.7 with hemiplegia after stroke. Inclusion criteria: outpatients with post‐stroke hemiplegia of more than one year duration. Exclusion criteria: congestive heart failure or obstructive pulmonary disease, other known causes of osteoporosis, such as hyperparathyroidism or renal osteodystrophy; impairment of renal, cardiac, or thyroid function; a history of therapy with corticosteroids, estrogens, calcitonin, etidronate, calcium, or vitamin D for 3 months or longer during the 12 months preceding the trial; or even brief treatment of this nature during the 2 months immediately preceding the trial. |
|
| Interventions | Participants were randomly assigned to receive: Intervention group 1: vitamin D in a form of 1‐α hydroxyvitamin D3 (alfacalcidol) (1 μg) daily (n = 34); Intervention group 2: ipriflavone 600 mg daily (n = 34); Intervention group 2 (Control group): no treatment (n = 35); for a one‐year period. |
|
| Outcomes | The primary outcome measures was bone mineral density. | |
| Stated aim of study | "To evaluate the effect of ipriflavone and 1 alpha‐hydroxyvitamin D3 administration on bone mineral density preservation." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The trial is described as randomised but the method of sequence generation was not specified. |
| Allocation concealment (selection bias) | High risk | The allocation sequence was known to the investigators who assigned participants. |
| Blinding (performance bias and detection bias) All outcomes | High risk | The trial was not blinded, so that the allocation was known during the trial. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The numbers and reasons for dropouts and withdrawals in all intervention groups were described. |
| Selective reporting (reporting bias) | Low risk | Pre‐defined, or clinically relevant and reasonably expected outcomes are reported on. |
| Industry bias | Unclear risk | The source of funding is not clear. |
| Other bias | Low risk | The trial appears to be free of other components that could put it at risk of bias. |
Sato 2005a.
| Methods | Randomised, double‐blind, placebo‐controlled trial using parallel group design (two intervention groups). | |
| Participants | Country: Japan. Number of participants randomised: 96 hospitalised elderly women with post stroke hemiplegia mean age 74.1 years. Inclusion criteria: hospitalised elderly women with post stroke hemiplegia who had first‐ever cerebral infarction or haemorrhage more than two years before and were in a convalescent stage with post‐stroke hemiplegia. Exclusion criteria: dementia, total disability, or hospitalisation of less than two years' duration, receiving any drugs known to alter vitamin D metabolism, such as anticonvulsants, calcium, or vitamin D, during the 12 months preceding the trial. |
|
| Interventions | Participants were randomly assigned to receive: Intervention group 1: vitamin D2 (1000 IU) daily (n = 48); Intervention group 2 (Control group): matched placebo tablet daily (n = 48); for a two‐year period. |
|
| Outcomes | The primary outcome measure was number of falls. Secondary outcome measures were muscular strength and morphological changes of muscle. | |
| Stated aim of study | "To evaluate the efficacy of vitamin D2 therapy in reducing the risk of falls in elderly women with stroke. Histochemical examination of skeletal muscles was performed to assess the effect of the therapy." | |
| Notes | Additional information on mortality was received through personal communication with Dr Yoshiro Sato (05.02.2009). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequence generation was achieved using computer random number generation or a random number table. |
| Allocation concealment (selection bias) | Low risk | Allocation was controlled by a central and independent randomisation unit so that intervention allocations could not have been foreseen in advance of, or during, enrolment. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was described as blinded, the parties that were blinded, and the method of blinding was described, so that knowledge of allocation was adequately prevented during the trial. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The numbers and reasons for dropouts and withdrawals in all intervention groups were described. |
| Selective reporting (reporting bias) | Low risk | Pre‐defined, or clinically relevant and reasonably expected outcomes are reported on. |
| Industry bias | Unclear risk | The source of funding is not clear. |
| Other bias | Low risk | The trial appears to be free of other components that could put it at risk of bias. |
Schleithoff 2006.
| Methods | Randomised, double‐blind, placebo‐controlled trial using parallel group design (two intervention groups). | |
| Participants | Country: Germany. Number of participants randomised: 123 patients (17% women) aged 50 to 63 (mean 51) years with congestive heart failure. Inclusion criteria: patients with congestive heart failure and New York Heart Association functional class II. Exclusion criteria: hypercalcaemia, serum creatinine concentration > 2 mg/dL, nephrolithiasis, sarcoidosis, use of a biventricular pacemaker, acute heart insufficiency, and an actual intake of supplements containing vitamin D and calcium. |
|
| Interventions | Participants were randomly assigned to receive: Intervention group 1: vitamin D3 (2000 IU) plus calcium (500 mg) daily (n = 61); Intervention group 2 (Control group): matched placebo tablet of vitamin D plus calcium 500 mg daily (n = 62); for a nine‐month period. Participants were followed‐up for a 15‐month period. |
|
| Outcomes | The primary outcome measures were survival rates, and biochemical variables such as natriuretic peptides and cytokines. Secondary outcomes were those haemodynamic variables, which were assessed routinely during the ambulatory visits, such as left ventricular ejection fraction, left ventricular end‐diastolic diameter, the cardiothoracic ratio, maximal oxygen intake (spiroergometry; O2max), and blood pressure. | |
| Stated aim of study | "To evaluate the effect of vitamin D supplementation on the survival rate and different biochemical variables in patients with congestive heart failure." | |
| Notes | "Compliance was measured by controlling the trial medication at each visit (bottle counts) and by the analysis of serum 25 hydroxyvitamin D concentrations." Vitamin D3 was provided by Vigantol Oel; Merck, Darmstadt, Germany, and placebo by Migliol‐Oel; Merck, Darmstadt, Germany. Additional information received thorough personal communication with Professor Armin Zittermann (10.02.2010). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequence generation was achieved using computer random number generation. |
| Allocation concealment (selection bias) | Low risk | Allocation was controlled by a central and independent randomisation unit so that intervention allocations could not have been foreseen in advance of, or during, enrolment. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was described as blinded, the parties that were blinded, and the method of blinding was described, so that knowledge of allocation was adequately prevented during the trial. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The numbers and reasons for dropouts and withdrawals in all intervention groups were described |
| Selective reporting (reporting bias) | Low risk | Pre‐defined, or clinically relevant and reasonably expected outcomes are reported on. |
| Industry bias | Unclear risk | Vitamin D3 was provided by Vigantol Oel; Merck, Darmstadt, Germany, and placebo by Migliol‐Oel; Merck, Darmstadt, Germany. |
| Other bias | Low risk | The trial appears to be free of other components that could put it at risk of bias. |
Smith 2007.
| Methods | Wessex Fracture Prevention Trial (WFPT). Randomised, double‐blind, placebo‐controlled trial using parallel group design (two intervention groups). | |
| Participants | Country: United Kingdom. Number of participants randomised: 9440 elderly people (54% women) aged 75 years and over. Inclusion criteria: elderly people aged 75 years and over. Exclusion criteria: current cancer or any history of treated osteoporosis, taking 400 IU or more vitamin D daily, bilateral total hip replacement, renal failure, renal stones, hypercalcaemia or sarcoidosis. |
|
| Interventions | Participants were randomly assigned to receive: Intervention group 1: vitamin D2 (300,000 IU) intramuscular injection yearly (n = 4727); Intervention group 2 (Control group): matched placebo intramuscular injection of vitamin D yearly (n = 4713); for a three‐year period. Active or placebo injections were administered every autumn at annual intervals and concealed in the same way as the first injection. |
|
| Outcomes | The primary outcome measure was all non‐vertebral fracture. Secondary outcome measures were hip and wrist fractures, and all falls. | |
| Stated aim of study | "To evaluate if vitamin D2 is effective in preventing non‐vertebral fractures among elderly men and women resident in the general population." | |
| Notes | The trial was supported by Celltech UK plc. Additional information on mortality was received through personal communication with Professor Cyrus Cooper and Dr Sarah Crozier (16.11.2007). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequence generation was achieved using computer random number generation. |
| Allocation concealment (selection bias) | Low risk | Allocation was controlled by a central and independent randomisation unit so that intervention allocations could not have been foreseen in advance of, or during, enrolment. Packing and labelling were carried out by an external contractor; allocation was concealed from investigators, practice nurses, and participants. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was described as blinded, the parties that were blinded, and the method of blinding was described, so that knowledge of allocation was adequately prevented during the trial. Each participating practice was sent mixed boxes containing previously randomised, numbered ampoules of either vitamin D or placebo, which were identical in visual appearance and consistency. As each participant consented to participate in the trial, they were allocated consecutive ampoules. The number of the ampoule was then linked to the participant's name and phoned to a central location. This trial number remained with the participant for the duration of the trial. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The numbers and reasons for dropouts and withdrawals in all intervention groups were described. |
| Selective reporting (reporting bias) | Low risk | Pre‐defined, or clinically relevant and reasonably expected outcomes are reported on. |
| Industry bias | High risk | The trial was supported by Celltech UK plc. |
| Other bias | Low risk | The trial appears to be free of other components that could put it at risk of bias. |
Trivedi 2003.
| Methods | Randomised double‐blind placebo‐controlled trial with parallel group design (two intervention groups). | |
| Participants | Country: United Kingdom. Number of participants randomised: 2686 elderly people (24% women) aged 65 to 85 (mean 74.7) years. Inclusion criteria: elderly people living in the general community. Exclusion criteria: already taking vitamin D supplements and conditions that were contraindications to vitamin D supplementation (a history of renal stones, sarcoidosis, or malignancy). |
|
| Interventions | Participants were randomly assigned to receive: Intervention group 1: vitamin D3 (100,000 IU) every four months orally (n = 1345); Intervention group 2 (Control group): matched placebo every four months orally (n = 1341); for a five‐year period. |
|
| Outcomes | The primary outcome measures were fracture incidence and total mortality by cause. | |
| Stated aim of study | "To determine the effect of four monthly vitamin D supplementation on the rate of fractures in men and women aged 65 years and over living in the community." | |
| Notes | "Seventy six percent of participants had at least 80% compliance (12/15 doses). Compliance for the final dose was 66%; excluding participants who had died, compliance was estimated to be 80%. The 100,000 IU vitamin D supplement or placebo used in this trial was specially prepared by the Ipswich Hospital Pharmacy." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequence generation was achieved using computer random number generation. |
| Allocation concealment (selection bias) | Low risk | Allocation was controlled by a central and independent randomisation unit so that intervention allocations could not have been foreseen in advance of, or during, enrolment. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was described as blinded, the parties that were blinded, and the method of blinding was described, so that knowledge of allocation was adequately prevented during the trial. Participants and investigators were blinded to the treatment until the trial ended, when Ipswich Pharmacy revealed the coding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The numbers and reasons for dropouts and withdrawals in all intervention groups were described. |
| Selective reporting (reporting bias) | Low risk | Pre‐defined, or clinically relevant and reasonably expected outcomes are reported on. |
| Industry bias | Low risk | The trial is not funded by a manufacturer of vitamin D. |
| Other bias | Low risk | The trial appears to be free of other components that could put it at risk of bias. |
Witham 2010.
| Methods | Randomised, double‐blind, placebo‐controlled trial using parallel group design (two intervention groups). | |
| Participants | Country: United Kingdom. Number of participants randomised: 105 patients with systolic heart failure aged 70 or over (mean 79.7) years, 34% females with 25‐hydroxyvitamin D levels < 50nmol/L (20 ng/ml). Inclusion criteria: aged 70 years or over with a previously recorded clinical diagnosis of chronic heart failure, previously documented left ventricular systolic dysfunction by echocardiography, radionuclide ventriculography or angiography as part of their usual clinical care, a New York Heart Association class II or III symptoms, and a 25‐hydroxyvitamin D level of < 50nmol/L (20 ng/ml). Exclusion criteria: a clinical diagnosis of osteomalacia, under investigation for recurrent falls, already taking vitamin D supplements, moderate to severe cognitive impairment, defined as a Folstein mini‐mental state examination < 15/30), serum creatinine > 200umol/L, liver function tests (bilirubin, alanine aminotransferase, alkaline phosphatase) > 3 times the upper limit of the local reference range, systolic blood pressure < 90mmHg, albumin adjusted calcium > 2.55 mmol/L or < 2.20 mmol/L), metastatic malignancy, and wheelchair bound patients unable to perform the primary outcome, and excluded patients unwilling or unable to give informed consent. |
|
| Interventions | Participants were randomly assigned to receive: Intervention group 1: vitamin D2 (10,000 IU) tablet at baseline and 10 weeks (n = 53); Intervention group 2 (Control group): matched placebo tablet at baseline and 10 weeks (n = 52). Participants were followed for 20 weeks. |
|
| Outcomes | The primary outcome measure was the six‐minute walk test, a measure of submaximal exercise capacity. Secondary outcomes were muscle function, daily physical activity levels, health status/health‐related quality of life, cardiovascular and inflammatory markers. | |
| Stated aim of study | "To examine whether vitamin D supplementation could improve parameters that are directly relevant to older people with heart failure – i.e., exercise capacity, physical function and quality of life." | |
| Notes | "Administration of vitamin D2 was supervised in the participant’s own home by the research nurse to ensure 100% adherence." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed using computer generated random number tables by DHP Pharmaceuticals (Gwent, UK). |
| Allocation concealment (selection bias) | Low risk | Allocation was controlled by a central and independent randomisation unit. Code allocation was concealed from the research nurse and investigators until after data analysis was complete. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was described as blinded, the parties that were blinded, and the method of blinding was described, so that knowledge of allocation was adequately prevented during the trial. DHP Pharmaceuticals (Gwent, UK) encapsulated the trial medication to render it identical to placebo. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The numbers and reasons for dropouts and withdrawals in all intervention groups were described. |
| Selective reporting (reporting bias) | Low risk | Pre‐defined, or clinically relevant and reasonably expected outcomes are reported on. |
| Industry bias | Low risk | The trial is not funded by a manufacturer of vitamin D. |
| Other bias | Low risk | The trial appears to be free of other components that could put it at risk of bias. |
Zhu 2008.
| Methods | Randomised, double‐blind, placebo‐controlled trial using parallel group design (three intervention groups). | |
| Participants | Country: Australia. Number of participants randomised: 120 community‐dwelling women aged 70 to 80 (mean 75) years. Inclusion criteria: aged over 70 year old, likely to survive a five year trial, and not receiving bone active agent. Exclusion criteria: none stated. |
|
| Interventions | Participants were randomly assigned to receive: Intervention group 1: vitamin D2 (1000 IU) plus calcium (1200 mg) daily (n = 39); Intervention group 2: calcium 1200 mg plus placebo vitamin D daily (n = 40); Intervention group 3 (Control group): matched placebo vitamin D and placebo calcium daily (n = 41); for a five year period. |
|
| Outcomes | The primary outcome measures were bone mineral density, plasma 25‐hydroxyvitamin D, biomarkers of bone turnover, parathyroid hormone, and intestinal calcium absorption. | |
| Stated aim of study | "To evaluate the relative benefits of 5 year of calcium supplementation of 1200 mg with or without 1000 IU vitamin D2, compared with placebo, on hip BMD and bone‐related biochemistry in ambulant elderly women aged 70 to 80 year living in a sunny climate." | |
| Notes | "This trial was nested within the larger Calcium Intake Fracture Outcome Study, a five year double‐blinded, randomised, controlled calcium supplementation trial, in which 1500 community‐living ambulant women over the age of 70 years old were randomised to received either 1200 mg calcium per day or identical placebo. The first 120 sequential participants presenting in September 1998 (end of winter in Western Australia) enrolled in this substudy and were randomised." "Adherence to the trial interventions was established by counting tablets returned every 12 months. There were no significant differences among the three groups in the compliance rates determined by tablet counting for calcium or placebo in the intervention groups 1, 2, and 3 (80.7, 80.9, and 86.9%, respectively) or for vitamin D or placebo (84.2, 86.9, and 89.8%, respectively)." Vitamin D2 (ergocalciferol) or identical placebo was provided by Ostelin; Boots Healthcare, North Ryde, New South Wales, Australia. Calcium as calcium citrate was provided by Caltrate; Wyeth Consumer Healthcare, Baulkham Hills, New South Wales, Australia. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequence generation was achieved using computer random number generation. |
| Allocation concealment (selection bias) | Low risk | Allocation was controlled by a central and independent randomisation unit so that intervention allocations could not have been foreseen in advance of, or during, enrolment. "Randomisation was undertaken by an independent research fellow and was kept in the Pharmacy Department of the Sir Charles Gairdner Hospital, in which the bottles were labelled and dispensed to participants. The trial participants and trial staff remained blinded to the treatment code until all the data had been entered, evaluated for accuracy, and the a priori hypotheses reviewed." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was described as blinded, the parties that were blinded, and the method of blinding was described, so that knowledge of allocation was adequately prevented during the trial. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The numbers and reasons for dropouts and withdrawals in all intervention groups were described. |
| Selective reporting (reporting bias) | Low risk | Pre‐defined, or clinically relevant and reasonably expected outcomes are reported on. |
| Industry bias | Unclear risk | Vitamin D2 (ergocalciferol) or identical placebo was provided by Ostelin; Boots Healthcare, North Ryde, New South Wales, Australia. Calcium as calcium citrate was provided by Caltrate; Wyeth Consumer Healthcare, Baulkham Hills, New South Wales, Australia. |
| Other bias | Low risk | The trial appears to be free of other components that could put it at risk of bias. |
Abbreviations: BMD: bone mineral density; HRT: hormone replacement therapy; ERT: oestrogen replacement therapy; FEV: Forced expiratory volume; FEV: forced vital capacity
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Adachi 1996 | Randomised controlled trial. This trial included patients receiving corticosteroids (diagnosed with polymyalgia rheumatica, temporal arteritis, asthma, vasculitis, or systemic lupus erythematosus). |
| Andersen 2008 | Randomised controlled trial. This trial included participants younger than 18 years (adolescent girls median age 12.2 years). |
| Arthur 1990 | Randomised controlled trial. All participants received vitamin D. |
| Bacon 2008 | Randomised controlled trial. All participants received vitamin D. |
| Bernstein 1996 | Randomised controlled trial. This trial included patients receiving corticosteroids (diagnosed with inflammatory bowel disease). |
| Berry 2010 | This is not a randomised controlled trial. |
| Binkley 2011 | Randomised controlled trial. All participants received vitamin D. |
| Bischoff‐Ferrari 2010a | Randomised controlled trial. All participants received vitamin D. |
| Bizzarri 2010 | Randomised controlled trial. This trial included participants younger than 18 years. |
| Buckley 1996 | Randomised controlled trial. This trial included patients receiving corticosteroids (diagnosed with rheumatoid arthritis). |
| Caniggia 1992 | This is not a randomised controlled trial. |
| Chapuy 1996 | This is not a randomised controlled trial. |
| Chen 2001 | Randomised controlled trial. All women received hormone replacement therapy. |
| Dawson‐Hughes 1995 | Randomised controlled trial. All participants received vitamin D. |
| den Uyl 2010 | Randomised controlled trial. All participants received vitamin D. |
| Diamond 2005 | This is not a randomised controlled trial. |
| Dykman 1984 | Randomised controlled trial in patients with glucocorticoid‐induced osteopenia. |
| Falch 1987 | Randomised controlled trial. All participants received vitamin D. |
| Francis 1996 | Randomised controlled trial. All participants received vitamin D. |
| Gallagher 1990 | Randomised controlled trial. All participants received 400 IU of vitamin D2. |
| Gannage‐Yared 2003 | This is not a randomised controlled trial. |
| Geusens 1986 | Randomised controlled trial comparing the effect of nandrolone decanoate, 1‐alphahydroxyvitamin D3 and intermittent calcium infusions. Vitamin D group was not supplemented with calcium. |
| Giusti 2010 | Randomised controlled trial. All participants received vitamin D. |
| Glendenning 2009 | Randomised controlled trial. All participants received vitamin D. |
| Goswami 2008a | This is not a randomised controlled trial. |
| Goussous 2005 | Randomised controlled trial. All participants received vitamin D. |
| Gupta 2010 | This is not a randomised controlled trial. |
| Heaney 2011 | Randomised controlled trial. All participants received vitamin D. |
| Hedström 2002 | Randomised controlled trial. Vitamin D group also received anabolic steroids. |
| Heikinheimo 1992 | This is not a randomised controlled trial. Participants were divided into treatment groups according to month of birth. |
| Hill 2010 | Randomised controlled trial. All participants received vitamin D. |
| Holecki 2008 | This is not a randomised controlled trial. |
| Holick 2008b | Randomised controlled trial. This trial did not fulfil our inclusion criteria. |
| Holvik 2007 | Randomised controlled trial. All participants received vitamin D. |
| Inkovaara 1983 | Quasi‐randomised trial. Participants randomised by date of birth. |
| Inomata 1986 | This is not a randomised controlled trial. |
| Ish‐Shalom 2008 | Randomised controlled trial. All participants received vitamin D. |
| Iwamoto 2000 | Randomised controlled trial. Participants in the control group supplemented with calcium. Participants in the vitamin D group were not supplemented with calcium. |
| Javanbakht 2011 | Randomised controlled trial. This trial included participants younger than 18 years. |
| Kamel 1996 | This is not a randomised controlled trial. |
| Keane 1992 | Randomised controlled trial. Participants in a control group supplemented with small dose of vitamin D. |
| Kenny 2004 | Randomised controlled trial. All participants received vitamin D. |
| Kilpinen‐Loisa 2009 | This is not a randomised controlled trial. |
| Lakatos 2000 | Randomised controlled trial. This trial included patients receiving corticosteroids (diagnosed with systemic lupus erythematodes, multiple sclerosis, rheumatoid arthritis or asthma bronchiale). |
| Leventis 2009 | This is not a randomised controlled trial. |
| Lind 1988 | Randomised controlled trial. This trial included participants with primary hyperparathyroidism. |
| Lind 1989c | This is not a randomised controlled trial. |
| Matsumoto 2010 | Randomised controlled trial. All participants received vitamin D or vitamin D analogs. |
| Meyer 2002 | Quasi‐randomised trial. Before the trial started, the days of the month (1–31 days) were divided randomly into group A and group B, and based on the day of birth, a participant was placed automatically in group A or group B when registered in the trial database. |
| Nugent 2009 | This is not a randomised controlled trial. |
| Nuti 2006 | Randomised controlled trial. All participants received vitamin D. |
| Orwoll 1989 | Randomised controlled trial. Participants received 25‐hydroxyvitamin D3. |
| Pekkarinen 2010 | Randomised controlled trial. All participants received vitamin D. |
| Prestwood 1996 | This is not a randomised controlled trial. |
| Reginster 1999 | Randomised controlled trial. This trial included patients receiving high doses of corticosteroids (cardiac transplant, severe inflammatory syndrome, etc). |
| Reginster 2001 | Randomised controlled trial. All participants received vitamin D. |
| Romagnoli 2008 | Randomised controlled trial. All participants received vitamin D. |
| Rosenblum 2012 | Randomised controlled trial. Participants received a combination of vitamin D, vitamin C, vitamin B1, vitamin B12, and folate. |
| Russo 2011 | This is not a randomised controlled trial. |
| Sambrook 1993 | Randomised controlled trial. This trial included patients on a long‐term corticosteroid therapy. |
| Sambrook 2000 | Randomised controlled trial in patients after cardiac or lung transplantation. |
| Sambrook 2003 | Randomised controlled trial. All participants received vitamin D2 plus calcium, vitamin D3 or alendronate plus calcium. There is no control group of the trial. |
| Sato 2005b | Randomised controlled trial. All participants received vitamin D. |
| Sato 2005c | Randomised controlled trial. Participants received a combination of menatrenone, vitamin D2, and calcium. |
| Sato 2006 | Randomised controlled trial. Participants were randomised to a combination of alendronate and vitamin D2. |
| Sebert 1995 | Randomised controlled trial. All participants received vitamin D. |
| Serhan 2005 | Randomised controlled trial. All participants received vitamin D. |
| Shipowick 2009 | This is not a randomised controlled trial. |
| Shiraki 1991 | This is not a randomised controlled trial. |
| Sidbury 2008 | Randomised controlled trial in children. |
| Slatkovska 2011 | Randomised controlled trial. All participants received vitamin D. |
| Smith 2009 | Randomised controlled trial. All participants received vitamin D. |
| Stein 2011 | Randomised controlled trial. All participants received vitamin D. |
| Stephens 1981 | Randomised controlled trial. All participants received vitamin D. Participants younger than 18 years were included. |
| Tfelt‐Hansen 2004 | Randomised controlled trial. All participants received vitamin D. |
| Tilyard 1992 | Randomised controlled trial. Participants in active treatment group treated with vitamin D and participants in the control group treated with calcium. |
| Trang 1998 | Randomised controlled trial. All participants received vitamin D. |
| Verschueren 2010 | Randomised controlled trial. All participants received vitamin D. |
| Vieth 2004 | Randomised controlled trial. All participants received vitamin D. |
| Viljakainen 2006b | Randomised controlled trial in adolescent girls. |
| von Restorff 2009 | This is not a randomised controlled trial. |
| Wejse 2009 | Randomised controlled trial in patients with tuberculosis starting antituberculosis treatment. |
Characteristics of ongoing studies [ordered by study ID]
Aloia 2008b.
| Trial name or title | The interaction between calcium and vitamin D Intake |
| Methods | Randomised, double‐blind, placebo‐controlled trial using 2 × 2 factorial design |
| Participants | Country: United States Estimated number of participants: 120 Inclusion criteria: healthy women aged 45 and above who have been menopausal for at least one year (absence of menstrual period for a period of 12 months or longer) Exclusion criteria: any chronic medical illness including uncontrolled diabetes mellitus, recent history of myocardial infarction or heart failure, malignancy, uncontrolled hypertension, obesity (BMI > 35 kg/m2), history of anaemia, leukaemia or other hematological abnormalities, lupus, rheumatoid arthritis, or other rheumatological disease, or kidney disease of any kind as determined by history and physical examination; participants with osteoporosis of the hip (total hip T‐score equal to or less than ‐2.5) or taking medications for osteoporosis such as bisphosphonate, pregnancy, use of medication that influences bone metabolism (i.e. anticonvulsant medications, long‐term use of steroids and high‐dose diuretics), significant deviation from normal in medical history, physical examination or laboratory tests as evaluated by the primary investigator, history of hypercalciuria, hypercalcaemia, nephrolithiasis and active sarcoidosis, participation in another investigational trial in the past 30 days before the screening evaluation, unexplained weight loss of > 15% during the previous year or history of anorexia nervosa, medications that interfere with vitamin D metabolism; patients with a habitual dietary calcium intake that exceeds 800 mg/day; smokers greater than one pack per day, patients reporting alcohol intake greater than two drinks daily and serum 25‐hydroxyvitamin D level > 75 nmol/L |
| Interventions | Participants will be randomly assigned to receive: Intervention 1: vitamin D3 (4000 IU) daily; Intervention 2: calcium (1200 mg) daily; Intervention 3: vitamin D3 (4000 IU) plus calcium (1200 mg) daily; or Intervention group 4 (control group): placebo daily for a period of six months |
| Outcomes | Primary outcome measures will be the influence of calcium supplementation alone on serum parathyroid hormone levels and bone markers in healthy adult women. Secondary outcome measures will be the interaction between calcium and vitamin D supplementation and their combined effect on serum parathyroid hormone levels and bone markers in healthy adult women |
| Starting date | November 2008. Expected completion: 2009 |
| Contact information | John F Aloia, MD; jaloia@winthrop.org |
| Notes |
Baron 2004.
| Trial name or title | Vitamin D/calcium polyp prevention study |
| Methods | Randomised, double‐blind, placebo‐controlled trial using 2 × 2 factorial design |
| Participants | Country: United States Estimated number of participants: 2200 Inclusion criteria: aged 45 to 75 years; one or more histologically verified neoplastic polyp (adenoma) that measures at least 2 mm removed from the large bowel, with the entire large bowel examined by colonoscopy and documented to be free of further polyps or areas suspicious for neoplasia within 120 days of trial entry; anticipated colonoscopic follow‐up three years or five years after the qualifying colonoscopy; agreement to avoid pregnancy (i.e. use of standard contraception); willingness to forego calcium supplementation (including multivitamins containing calcium) or, for women only, option of taking calcium supplementation of 1200 mg daily (contained in the trial pills); willingness to forego vitamin D supplementation (including multivitamins containing vitamin D); agreement to daily dietary intake of the equivalent of not more than 1200 mg calcium; agreement to daily dietary intake of the equivalent of not more than 400 IU vitamin D; blood calcium level within normal range; blood creatinine level not to exceed 20% above upper limit of normal; serum 25‐hydroxyvitamin D within lower limit of normal to 70 ng/mL; ability and willingness to follow the trial protocol, as indicated by provision of informed consent to participate; good general health, with no severely debilitating diseases or active malignancy that might compromise the participant's ability to complete the trial Exclusion criteria: participation in another colorectal (bowel) trial in the past five years; current participation in any other clinical trial (intervention trial); pregnancy or lactation; a diagnosis of narcotic or alcohol dependence in the past five years; a diagnosis of dementia (e.g. Alzheimer's) in the past five years; a diagnosis of a significant psychiatric disability (e.g. schizophrenia, refractory bipolar disorder, current severe depression) in the past five years; any diagnosis of kidney stones; a diagnosis of granulomatous diseases (e.g. sarcoidosis), active chronic fungal or mycobacterial infection (tuberculosis, histoplasmosis, coccidioidomycosis, blastomycosis), berylliosis, Wegener's granulomatosis in the past five years; hyperparathyroidism or other serious disturbance of calcium metabolism in the past five years; a diagnosis of severe kidney disease (e.g. chronic renal failure) in the past five years; unexplained hypercalcaemia in the past five years; osteoporosis with physician recommendation for treatment of low bone mass; two or more low trauma fractures in the past five years; medical condition requiring treatment with vitamin D (e.g. osteomalacia) in the past five years; invasive carcinoma of the large bowel (even if confined to a polyp); familial colorectal cancer syndromes (e.g. familial adenomatous polyposis (FAP), including Gardner syndrome, Turcot's syndrome), hereditary nonpolyposis colorectal cancer (HNPCC), hamartomatous polyposis syndromes (including Peutz‐Jeghers or familial juvenile polyposis); inflammatory bowel disease (e.g. Crohn's disease, ulcerative colitis); a diagnosis of chronic intestinal malabsorption syndromes (e.g. celiac sprue, bacterial overgrowth, chronic pancreatitis, pancreatic insufficiency) in the past five years; large bowel resection; a diagnosis of malignancy other than non‐melanoma skin cancer in the past five years; severe lung disease class three or four (e.g. COPD or emphysema requiring oxygen) in the past five years; severe heart disease: cardiovascular disease functional class three or four in the past five years; severe liver disease (e.g. cirrhosis); any HIV‐positive diagnosis; active hepatitis B, defined as Hep B surface antigen positive; active hepatitis C, defined as measurable HCV RNA; use of long‐term oral corticosteroid therapy in the past five years; use of lithium in the past five years; use of phenytoin in the past five years; use of quinidine in the past five years; use of therapeutic vitamin D in the past five years |
| Interventions | Participants will be randomly assigned to receive: Intervention group 1: vitamin D3 (1000 IU) daily; Intervention group 2: calcium (1200 mg) daily; Intervention group 3: vitamin D3 (1000 IU) plus calcium (1200 mg) daily; or Intervention group 4 (control group): placebo daily for a period of five years. Women who decline to forego calcium supplementation will be randomly assigned only to calcium alone or to calcium plus vitamin D intervention |
| Outcomes | Primary outcome measure will be new adenomas detected on follow‐up colonoscopy |
| Starting date | July 2004. Expected completion: December 2017 |
| Contact information | John A Baron, MD, Principal Investigator, Dartmouth‐Hitchcock Medical Center |
| Notes |
Gallagher 2007.
| Trial name or title | Vitamin D supplementation in younger women |
| Methods | Randomised, double‐blind, placebo‐controlled trial using parallel‐group design (five intervention groups) |
| Participants | Country: United States Estimated number of participants: 200 Inclusion criteria: premenopausal Caucasian or African American women, aged 25 to 45 years (women with hysterectomy and/or oophorectomy must have a premenopausal follicle‐stimulating hormone level); serum 25‐hydroxyvitamin D level 5 to 20 ng/mL; BMI < 45 kg/m2; willing to discontinue vitamin D supplements after entering the trial; negative pregnancy test before BMD and calcium absorption tests; willing to give signed informed consent form Exclusion criteria: cancer (exceptions: basal cell carcinoma or cancer that occurred more than 10 years ago) or terminal illness; previous hip fracture; hemiplegia; uncontrolled type I diabetes ± significant proteinuria or fasting blood sugar > 140 mg in type II diabetes; kidney stones more than two in a lifetime; chronic renal failure (serum creatinine > 1.4 mg/dL); evidence of chronic liver disease, including alcoholism; physical conditions such as severe osteoarthritis, rheumatoid arthritis, heart failure severe enough to prevent reasonable physical activity; previous treatment with bisphosphonates (longer than three months), parathyroid hormone (PTH) or PTH derivatives (e.g. teriparatide or fluoride) in the past six months; previous treatment within the past six months with calcitonin or oestrogen (except birth control pills); long‐term high‐dose corticosteroid therapy (> 10 mg/day) for over six months and not within the past six months; anticonvulsant therapy (Dilantin, phenobarbital); high‐dose thiazide therapy (> 37.5 mg); 24‐hour urine calcium > 290 mg on two baseline tests; serum calcium exceeding upper normal limit on two baseline tests; bone mineral density; T‐score less than ‐3.0 for spine or hip |
| Interventions | Participants will be randomly assigned to receive: Intervention group 1: vitamin D3 (400 IU) daily; Intervention group 2: vitamin D3 (800 IU) daily; Intervention group 3: vitamin D3 (1600 IU) daily; Intervention group 4: vitamin D3 (2400 IU) daily; or Intervention group 5 (control group): placebo daily for a period of one year |
| Outcomes | Primary outcome measures will be serum 25‐hydroxyvitamin D and parathyroid hormone. Secondary outcome measures will be serum and urine calcium levels |
| Starting date | October 2007; Expected completion: January 2012 |
| Contact information | JC Gallagher, MD; tel: 402‐280‐4518; bones@creighton.edu |
| Notes |
Giovannucci 2007.
| Trial name or title | Vitamin D for chemoprevention |
| Methods | Randomised, double‐blind, placebo‐controlled trial using parallel‐group design (four intervention groups) |
| Participants | Country: United States Estimated number of participants: 320 Inclusion criteria: healthy black participants 30 to 80 years of age; comfortable communicating in English; currently with a primary care physician; willing to discontinue vitamin D or calcium supplements; willing to have all protocol specific tests run Exclusion criteria: plans on taking a vacation or travelling to a sunny region within three months of vitamin supplementation period except for a short period (i.e. one weekend); pregnant or breast feeding or planning on becoming pregnant in the following year; pre‐existing calcium (including hypercalcaemia), parathyroid conditions (including hyperparathyroidism), sarcoidosis; no concurrent active malignancies (other than non‐melanoma skin cancer) or previous diagnosis of prostate cancer; cognitively impaired; active thyroid disease (e.g. Graves', Hashimoto's or thyroiditis); history of nephrolithiasis, chronic liver disease, chronic renal disease or renal dialysis |
| Interventions | Participants will be randomly assigned to receive: Intervention group 1: vitamin D3 (1000 IU) daily; Intervention group 2: vitamin D3 (2000 IU) daily; Intervention group 3: vitamin D3 (4000 IU) daily; or Intervention group 4 (control group): placebo daily for a period of three months. Participants will be followed six months |
| Outcomes | Primary outcome measures will include to identify among blacks a dose of oral vitamin D supplementation that will result in levels of plasma 25‐hydroxyvitamin D that would be predicted to reduce colorectal cancer occurrence. Secondary outcome measures will be to determine the influence of oral vitamin D supplementation on inflammatory markers and to compare germline polymorphic variation in vitamin D pathway genes between blacks and a cohort of whites |
| Starting date | October 2007; Expected completion: October 2009 |
| Contact information | Charles Fuchs, MD; tel: (617) 632‐5840; Charles_Fuchs@dfci.harvard.edu |
| Notes |
Harris 2008.
| Trial name or title | Vitamin D, glucose control and insulin sensitivity in African‐Americans |
| Methods | Randomised, double‐blind, placebo‐controlled trial using parallel‐group design (two intervention groups) |
| Participants | Country: United States Estimated number of participants: 96 Inclusion criteria: African‐American by self designation aged 40 and older; glucose intolerance; body mass index 25.0 to 39.9 Exclusion criteria: diabetes potentially requiring pharmacotherapy, defined as A1c > 7%; uncontrolled thyroid disease; current parathyroid, liver or kidney disease; renal stone within five years; sarcoidosis, current pancreatitis, active tuberculosis, hemiplegia, gout; inflammatory bowel disease, colostomy, malabsorption; cancer other than basal cell skin cancer within five years; uncontrolled arrhythmia in past year; albinism or other condition associated with reduced skin pigmentation; pregnancy over the past year; intent to become pregnant; menopause onset within one year; any other unstable medical condition laboratory tests; fasting plasma glucose < 100; haemoglobin A1c > 7%; laboratory evidence of liver disease (e.g. AST > 70 U/L or ALT > 72 IU/L); laboratory evidence of kidney disease (e.g. estimated glomerular filtration rate < 60 mL/min/1.73 m2); elevated spot urine calcium‐to‐creatinine ratio > 0.38 mg/dL; abnormal serum calcium (serum calcium > 10.5 mg/dL); anaemia (hematocrit < 36% in men, < 33% in women); medications (use in past three months; oestrogen or testosterone); prescription vitamin D, lithium; oral corticosteroids; antiseizure medications; unstable doses of psychotropics or phenothiazines; cholestyramine supplements (current use may discontinue after screening); vitamin D supplements, cod liver oil, calcium supplements; body mass index < 25 or > 39.9; consumption of more than 14 alcoholic drinks per week; inability to attend all three trial visits as scheduled; inability to provide written informed consent; age < 40 years; not African‐American (by self designation); participation in another research intervention trial; corresponds to a 24‐hour urinary calcium excretion > 400 mg |
| Interventions | Participants will be randomly assigned to receive: Intervention group 1: vitamin D3 (4000 IU) daily; or Intervention group 2 (control group): placebo daily for a period of 12 weeks |
| Outcomes | Primary outcome measure will be insulin secretion, insulin sensitivity and glucose control |
| Starting date | July 2008; Expected completion: February 2011 |
| Contact information | Nancy Palermo, BS; tel: 617‐556‐3073; nancy.palermo@tufts.edu |
| Notes |
Manson 2009.
| Trial name or title | Vitamin D and omega‐3 trial (VITAL) |
| Methods | Randomised, double‐blind, placebo‐controlled trial using 2 × 2 factorial design |
| Participants | Country: United States Estimated number of participants: 20,000 Inclusion criteria: men aged 50 or older or women aged 55 or older who have at least a high school education Exclusion criteria: history of cancer (except non‐melanoma skin cancer), heart attack, stroke, transient ischaemic attack, angina pectoris, coronary artery bypass graft or percutaneous coronary intervention; history of renal failure or dialysis, hypercalcaemia, hypoparathyroidism or hyperparathyroidism, severe liver disease (cirrhosis) or sarcoidosis or other granulomatous diseases such as active chronic tuberculosis or Wegener's granulomatosis; allergy to fish or soy; other serious illness that would preclude participation; consuming no more than 800 IU of vitamin D from all supplemental sources combined (individual vitamin D supplements, calcium + vitamin D supplements, medications with vitamin D [e.g. Fosamax Plus D] and multivitamins), or, if taking, willing to decrease or forego such use during the trial; consuming no more than 1200 mg/d of calcium from all supplemental sources combined, or, if taking, willing to decrease or forego such use during the trial; taking fish oil supplements, or, if taking, willing to forego their use during the trial |
| Interventions | Intervention group 1: vitamin D3 and omega‐3; Intervention group 2: vitamin D3 and omega‐3 placebo; Intervention group 3: vitamin D placebo and omega‐3; or Intervention group 4 (control group): vitamin D placebo and omega‐3 placebo orally, daily for a two‐year period |
| Outcomes | Cancer and cardiovascular disease |
| Starting date | July 2010 |
| Contact information | Project manager; 1‐800‐388‐3963; vitalstudy@rics.bwh.harvard.edu www.vitalstudy.org |
| Notes |
Pande 2006.
| Trial name or title | A trial to study the effect of vitamin D supplementation on glucose and insulin metabolism in centrally obese men |
| Methods | Randomised, double‐blind, placebo‐controlled trial using parallel‐group design (two intervention groups) |
| Participants | Country: India Estimated number of participants: 100 Inclusion criteria: male, aged 35 years or older, waist circumference ≥ 78 cm Exclusion criteria: diabetic (fasting blood sugar > 126 mg/dL or on anti‐diabetic medication; blood pressure > 140/90 mmHg or on antihypertensive medication; receiving Vitamin D or calcium supplementation; chronic disease renal/hepatic/malignancy/gastrointestinal; on any medication within the last month that could potentially influence insulin secretion, insulin sensitivity, vitamin D or calcium metabolism; febrile illness or infective morbidity in the past 10 days; past history of nephrolithiasis |
| Interventions | Paraticipants will be randomly assigned to receive: Intervention group 1: vitamin D weekly; or Intervention group 2 (control group): placebo weekly for a period of six weeks |
| Outcomes | Primary outcome measure will be oral glucose insulin sensitivity (OGIS). Secondary outcome measures will be lipid profile, CRP, ApoA1, ApoB and blood pressure |
| Starting date | July 2006; Expected completion: September 2006 |
| Contact information | Jitendra N Pande, MD; Sitaram Bhartia Institute of Science and Research, New Delhi 110016 India |
| Notes |
Schwartz 2008.
| Trial name or title | Effects of vitamin D on lipids |
| Methods | Randomised, double‐blind, placebo‐controlled trial using parallel‐group design (three intervention groups) |
| Participants | Country: United States Estimated number of participants: 90 Inclusion criteria: any medically stable person with hypercholesterolaemia able to swallow pills Exclusion criteria: clinical instability of underlying disease process (e.g. recent hospitalisation, change of dosages of medications within the prior two weeks, or new medications within one month); recent transfusion; severe renal failure or dialysis; hypercalcaemia; malignancy under active treatment; feeding tube; intestinal bypass surgery; inability to swallow tablets |
| Interventions | Paraticipants will be randomly assigned to receive: Intervention group 1: vitamin D2 (1000 IU) daily; Intervention group 2: vitamin D3 (1000 IU) daily; or Intervention group 3 (control group): placebo daily for a period of 12 weeks |
| Outcomes | Primary outcome measure will be low‐density lipoprotein‐cholesterol. Secondary outcome measures will be vitamin D and metabolite concentrations with supplementation and time course of repletion in deficient or insufficient participants, measures of inflammatory markers |
| Starting date | July 2008; Expected completion: April 2010 |
| Contact information | Janice B Schwartz, MD; Jewish Home, University of California, San Francisco |
| Notes |
Scragg 2011.
| Trial name or title | ViDA (vitamin D assessment) trial |
| Methods | Randomised, double‐blind, placebo‐controlled trial using parallel‐group design (two intervention groups) |
| Participants | Country: New Zealand Estimated number of participants: 5100 Inclusion criteria: age 50 to 84 years; ability to give informed consent; resident in Auckland at recruitment; anticipated residence in New Zealand for the four‐year study period Exclusion criteria: current use of vitamin D supplements (> 600 IU per day if aged 50 to 70 years; > 800 IU per day if aged 71 to 84 years); diagnosis of psychiatric disorders that would limit ability to comply with study protocol (i.e. history of regular exacerbation of major psychosis (schizophrenia, bipolar disorder) in past two years); history of hypercalcaemia, nephrolithiasis, sarcoidosis, parathyroid disease or gastric bypass surgery; enrolled in another study, which could affect participation in the vitamin D study; serum calcium from baseline blood sample > 2.50 mmol/L |
| Interventions | Intervention group 1: vitamin D3 200,000 IU oral capsule at baseline, then 100,000 IU oral capsule monthly (aside from 200,000 IU oral capsule in each June); or Intervention group 2 (control group): placebo (sunflower lecithin) for four years |
| Outcomes | Incidence rate of fatal and non‐fatal cardiovascular disease, as assessed by mortality, hospital discharges and family doctors |
| Starting date | 7/04/2011 |
| Contact information | |
| Notes |
Shapses 2007.
| Trial name or title | The effect of vitamin D supplementation during caloric restriction on intestinal calcium absorption |
| Methods | Randomised, double‐blind, placebo‐controlled trial using parallel‐group design (two intervention groups) |
| Participants | Country: United Kingdom Estimated number of participants: 60 Inclusion criteria: postmenopausal women aged 50 to 70 years who are more than two years since last menses; obese or overweight; living in the geographic vicinity of Rutgers University Exclusion criteria: currently taking any medication known to influence calcium or bone metabolism, including hormone replacement therapy, or with evidence of diseases known to influence calcium metabolism (i.e. metabolic bone disease), hyperparathyroidism, untreated thyroid disease, significant immune, hepatic, or renal disease, significant cardiac disease (i.e. heart attack or stroke in the past six months, abnormal electrocardiogram), active malignancy or cancer therapy within the past year; history of kidney stones; weight gain or weight loss (5% of body weight) within three months before recruitment; participation in other investigational studies during the 12‐month trial period; travel for longer than two consecutive weeks during the trial period; usually have a very high or low intake of calcium (more than 1500 mg or less than 500 mg per day) |
| Interventions | Paraticipants will be randomly assigned to receive: Intervention group 1: vitamin D3 (1200 IU) daily plus weight loss; Intervention group 2: (control group): placebo daily plus weight loss; Intervention group 3: vitamin D3 (1200 IU) daily plus weight maintenance; or Intervention group 4 (control group): vitamin D3 (1200 IU) daily plus weight maintenance for a period of five weeks |
| Outcomes | Primary outcome measure will be changes in calcium absorption. Secondary outcome measures will be changes in serum and urine bone markers, hormones, proteins and genes |
| Starting date | March 2007; Expected completion: May 2011 |
| Contact information | Sue Shapses, PhD, RD; shapses@aesop.rutgers.edu |
| Notes |
Witte 2009.
| Trial name or title | The impact of vitamin D supplementation in chronic heart failure |
| Methods | Randomised, double‐blind, placebo‐controlled trial using parallel‐group design (two intervention groups) |
| Participants | Country: United Kingdom Estimated number of participants: 100 Inclusion criteria: patients aged 18 years or over with class II and III heart failure due to left ventricular systolic dysfunction (left ventricular ejection fraction less than or equal to 40%); stable symptoms for three months on maximally tolerated medical therapy with no recent change in medication; able to give informed written consent Exclusion criteria: currently taking (or have taken in the previous three months) calcium or other vitamin supplements; currently prescribed amlodipine or other calcium channel antagonists (intake of spironolactone will be recorded); chronic heart failure due to untreated valvular heart disease; history of primary hyperparathyroidism, sarcoidosis, tuberculosis or lymphoma; vitamin D levels greater than 50 nmol/L |
| Interventions | Patients will be randomly assigned to receive: Intervention group 1: vitamin D3 (4000 IU) daily; or Intervention group 2 (control group): placebo daily for a period of one year |
| Outcomes | Primary outcome measures will be left ventricular function assessed at baseline and 12 months, measured by cardiac magnetic resonance. Secondary outcome measures will be symptom status (New York Heart Association status), measured at baseline, one month, four months, eight months, 12 months; exercise tolerance, measured at baseline and 12 months; quality of life (Minnesota Living With Heart Failure questionnaire, European Quality of Life instrument and a 19‐item Likert scale index), measured at baseline, one month, four months, eight months, 12 months; flow‐mediated dilatation, measured at baseline and 12 months; immune status, measured at baseline and 12 months; insulin resistance, measured at baseline and 12 months; autonomic activation (measured by heart rate variability), measured at baseline and 12 months; renal function, measured at baseline and 1, 4, 8 and 12 months; B‐type natriuretic peptide, measured at baseline and 1, 4, 8 and 12 months |
| Starting date | 01.01.2009; Expected completion: 31.12.2012 |
| Contact information | Klaus Witte Division of Cardiovascular and Diabetes Research LIGHT building University of Leeds, Leeds, United Kingdom, LS2 9JT; klauswitte@hotmail.com |
| Notes |
AST: aspartate aminotransferase; ALT: alanine aminotransferase; BMI: body mass index; CRP: C‐reactive protein; DMSO: dimethyl sulphoxide; DNA: deoxyribonucleic acid; MSM: methylsulfonylmethane
Differences between protocol and review
Difference between the last published review version and the present review version
We interpreted our results much more conservatively as the result of extensive discussion of the validity of our results among the review authors.
Contributions of authors
Goran Bjelakovic (GB): performed the literature search, data extraction and statistical analyses and drafted the review. Lise Lotte Gluud (LLG): performed data extraction and revised the review. Dimitrinka Nikolova (DN): performed data extraction and revised the review. Kate Whitfield (KW): developed the search strategy, performed data extraction and revised the review. Jørn Wetterslev (JW): performed data extraction and revised the review. Rosa G Simonetti (RGS): performed data extraction and revised the review. Marija Bjelakovic (MB) performed data extraction and revised the review. Christian Gluud (CG): initiated the systematic review, acted as arbiter for disagreements and revised the review.
Sources of support
Internal sources
The Copenhagen Trial Unit, Centre for Clinical Intervention Research, Rigshospitalet, Denmark.
External sources
Ministry of Science Republic of Serbia, Serbia.
Declarations of interest
None known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Aloia 2005 {published data only}
- Aloia JF, Talwar SA, Pollack S, Feuerman M, Yeh JK. Optimal vitamin D status and serum parathyroid hormone concentrations in African American women. American Journal of Clinical Nutrition 2006;84(3):602‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aloia JF, Talwar SA, Pollack S, Yeh J. A randomized controlled trial of vitamin D3 supplementation in African American women. Archives of Internal Medicine 2005;165(14):1618‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talwar SA, Aloia JF, Pollack S, Yeh JK. Dose response to vitamin D supplementation among postmenopausal African American women. American Journal of Clinical Nutrition 2008;86(6):1657‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Avenell 2004 {published data only}
- Avenell A, Grant AM, McGee M, McPherson G, Campbell MK, McGee MA. The effects of an open design on trial participant recruitment, compliance and retention—a randomized controlled trial comparison with a blinded, placebo‐controlled design. Clinical Trials 2004;1(6):490‐8. [DOI] [PubMed] [Google Scholar]
Avenell 2012 {published data only}
- Alexander C. Prevention of low‐trauma fractures in older people. Lancet 2005;366(9485):544. [DOI] [PubMed] [Google Scholar]
- Avenell A, Cook JA, Maclennan GS, Macpherson GC. Vitamin D supplementation to prevent infections: a sub‐study of a randomised placebo‐controlled trial in older people (RECORD trial, ISRCTN 51647438). Age and Ageing 2007;36(5):574‐7. [DOI] [PubMed] [Google Scholar]
- Avenell A, MacLennan GS, Jenkinson DJ, McPherson GC, McDonald AM, Pant PR, et al. Long‐term follow‐up for mortality and cancer in a randomized placebo‐controlled trial of vitamin D(3) and/or calcium (RECORD trial). Journal of Clinical Endocrinology and Metabolism 2012;97(2):614‐22. [DOI] [PubMed] [Google Scholar]
- Cameron ID, Kurrle SE. Prevention of low‐trauma fractures in older people. Lancet 2005;366(9485):543. [DOI] [PubMed] [Google Scholar]
- Grant AM, Avenell A, Campbell MK, McDonald AM, MacLennan GS, McPherson GC, et al. Oral vitamin D3 and calcium for secondary prevention of low‐trauma fractures in elderly people (Randomised Evaluation of Calcium Or vitamin D, RECORD): a randomised placebo‐controlled trial. Lancet 2005;365(9471):1621‐8. [DOI] [PubMed] [Google Scholar]
- McDonald A, Campbell MK, Ross S, for the RECORD Study Group. Delivering clinical trial supplies by post to elderly trial participants: a feasibility study. Applied Clinical Trials 2004;Feb:58‐9. [Google Scholar]
- Sambrook P. Vitamin D and fractures: quo vadis. Lancet 2005;365(9471):1599‐600. [DOI] [PubMed] [Google Scholar]
- Scharla S. Prevention of low‐trauma fractures in older people. Lancet 2005;366(9485):543. [DOI] [PubMed] [Google Scholar]
Baeksgaard 1998 {published data only}
- Baeksgaard L, Andersen KP, Hyldstrup L. Calcium and vitamin D supplementation increases spinal BMD in healthy, postmenopausal women. Osteoporosis International 1998;8(3):255‐60. [DOI] [PubMed] [Google Scholar]
Bischoff 2003 {published data only}
- Bischoff HA, Stähelin HB, Dick W, Akos R, Knecht M, Salis C, et al. Effects of vitamin D and calcium supplementation on falls: a randomized controlled trial. Journal of Bone and Mineral Research 2003;18(2):343‐51. [DOI] [PubMed] [Google Scholar]
Bjorkman 2007 {published data only}
- Aspray TJ, Francis RM. Vitamin D deficiency—can old age learn from childhood?. Age and Ageing 2008;37(1):6‐7. [DOI] [PubMed] [Google Scholar]
- Björkman M, Sorva A, Risteli J, Tilvis R. Vitamin D supplementation has minor effects on parathyroid hormone and bone turnover markers in vitamin D‐deficient bedridden older patients. Age and Ageing 2008;37(1):25‐31. [DOI] [PubMed] [Google Scholar]
- Björkman M, Sorva A, Tilvis R. Vitamin D supplementation has no major effect on pain or pain behavior in bedridden geriatric patients with advanced dementia. Aging Clinical and Experimental Research 2008;20(4):316‐21. [DOI] [PubMed] [Google Scholar]
Bolton‐Smith 2007 {published data only}
- Bolton‐Smith C, McMurdo ME, Paterson CR, Mole PA, Harvey JM, Fenton ST, et al. Two‐year randomized controlled trial of vitamin K1 (phylloquinone) and vitamin D3 plus calcium on the bone health of older women. Journal of Bone and Mineral Research 2007;22(4):509‐19. [DOI] [PubMed] [Google Scholar]
Brazier 2005 {published data only}
- Brazier M, Grados F, Kamel S, Mathieu M, Morel A, Maamer M, et al. Clinical and laboratory safety of one year's use of a combination calcium + vitamin D tablet in ambulatory elderly women with vitamin D insufficiency: results of a multicenter, randomized, double‐blind, placebo‐controlled study. Clinical Therapeutics 2005;27(12):1885‐93. [DOI] [PubMed] [Google Scholar]
- Grados F, Brazier M, Kamel S, Duver S, Heurtebize N, Maamer M, et al. Effects on bone mineral density of calcium and vitamin D supplementation in elderly women with vitamin D deficiency. Joint Bone Spine 2003;70(3):203‐8. [DOI] [PubMed] [Google Scholar]
- Grados F, Brazier M, Kamel S, Mathieu M, Hurtebize N, Maamer M, et al. Prediction of bone mass density variation by bone remodeling markers in postmenopausal women with vitamin D insufficiency treated with calcium and vitamin D supplementation. Journal of Clinical Endocrinology and Metabolism 2003;88(11):5175‐9. [DOI] [PubMed] [Google Scholar]
Broe 2007 {published data only}
- Broe KE, Chen TC, Weinberg J, Bischoff‐Ferrari HA, Holick MF, Kiel DP. A higher dose of vitamin d reduces the risk of falls in nursing home residents: a randomized, multiple‐dose study. Journal of the American Geriatrics Society 2007;55(2):234‐9. [DOI] [PubMed] [Google Scholar]
Brohult 1973 {published data only}
- Brohult J, Jonson B. Effects of large doses of calciferol on patients with rheumatoid arthritis. A double‐blind clinical trial. Scandinavian Journal of Rheumatology 1973;2(4):173‐6. [DOI] [PubMed] [Google Scholar]
Burleigh 2007 {published data only}
- Burleigh E, McColl J, Potter J. Does vitamin D stop inpatients falling? A randomised controlled trial. Age and Ageing 2007;36(5):507‐13. [DOI] [PubMed] [Google Scholar]
Campbell 2005 {published data only}
- Campbell AJ, Robertson MC, Grow SJ, Kerse NM, Sanderson GF, Jacobs RJ, et al. Randomised controlled trial of prevention of falls in people aged > or =75 with severe visual impairment: the VIP trial. BMJ 2005;331(7520):817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grow SJ, Robertson MC, Campbell AJ, Clarke GA, Kerse NM. Reducing hazard related falls in people 75 years and older with significant visual impairment: how did a successful program work. Injury Prevention 2006;12(5):296‐301. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chapuy 1992 {published data only}
- Chapuy MC, Arlot ME, Delmas PD, Meunier PJ. Effect of calcium and cholecalciferol treatment for three years on hip fractures in elderly women. BMJ 1994;308(6936):1081‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapuy MC, Arlot ME, Duboeuf F, Brun J, Crouzet B, Arnaud S, et al. Vitamin D3 and calcium to prevent hip fractures in the elderly women. New England Journal of Medicine 1992;327(23):1637‐42. [DOI] [PubMed] [Google Scholar]
- Torgerson D, Campbell M. Calcium, vitamin D, and hip fractures. Vitamin D alone may be helpful. BMJ 1994;309(6948):193. [PMC free article] [PubMed] [Google Scholar]
Chapuy 2002 {published data only}
- Chapuy MC, Pamphile R, Paris E, Kempf C, Schlichting M, Arnaud S, et al. Combined calcium and vitamin D3 supplementation in elderly women: confirmation of reversal of secondary hyperparathyroidism and hip fracture risk: the Decalyos II study. Osteoporosis International 2002;13(3):257‐64. [DOI] [PubMed] [Google Scholar]
Chel 2008 {published data only}
- Chel V, Wijnhoven HA, Smit JH, Ooms M, Lips P. Efficacy of different doses and time intervals of oral vitamin D supplementation with or without calcium in elderly nursing home residents. Osteoporosis International 2008;19(5):663‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holick MF. Does vitamin D3 dosing schedule influence treatment efficacy in nursing home residents with vitamin D deficiency. Nature Clinical Practice. Endocrinology & Metabolism 2008;4(12):656‐7. [DOI] [PubMed] [Google Scholar]
- Vieth R. Comment on Chel et al.: efficacy of different doses and time intervals of oral vitamin D supplementation with or without calcium in elderly nursing home residents. Osteoporosis International 2008;19(5):721‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cherniack 2011 {published data only}
- Cherniack EP, Florez HJ, Hollis BW, Roos BA, Troen BR, Levis S. The response of elderly veterans to daily vitamin D3 supplementation of 2,000 IU: a pilot efficacy study. Journal of the American Geriatrics Society 2011;59(2):286‐90. [DOI] [PubMed] [Google Scholar]
Cooper 2003 {published data only}
- Cooper L, Clifton‐Bligh PB, Nery ML, Figtree G, Twigg S, Hibbert E, et al. Vitamin D supplementation and bone mineral density in early postmenopausal women. American Journal of Clinical Nutrition 2003;77(5):1324‐9. [DOI] [PubMed] [Google Scholar]
Corless 1985 {published data only}
- Corless D, Dawson E, Fraser F, Ellis M, Evans SJ, Perry JD, et al. Do vitamin D supplements improve the physical capabilities of elderly hospital patients. Age and Ageing 1985;14(2):76‐84. [DOI] [PubMed] [Google Scholar]
Daly 2008 {published data only}
- Daly RM, Bass S, Nowson C. Long‐term effects of calcium‐vitamin‐D3‐fortified milk on bone geometry and strength in older men. Bone 2006;39(4):946‐53. [DOI] [PubMed] [Google Scholar]
- Daly RM, Brown M, Bass S, Kukuljan S, Nowson C. Calcium‐ and vitamin D3‐fortified milk reduces bone loss at clinically relevant skeletal sites in older men: a 2‐year randomized controlled trial. Journal of Bone and Mineral Research 2006;21(3):397‐405. [DOI] [PubMed] [Google Scholar]
- Daly RM, Petrass N, Bass S, Nowson CA. The skeletal benefits of calcium‐ and vitamin D3‐fortified milk are sustained in older men after withdrawal of supplementation: an 18‐mo follow‐up study. American Journal of Clinical Nutrition 2008;87(3):771‐7. [DOI] [PubMed] [Google Scholar]
Dawson‐Hughes 1997 {published data only}
- Bischoff‐Ferrari HA, Orav EJ, Dawson‐Hughes B. Additive benefit of higher testosterone levels and vitamin D plus calcium supplementation in regard to fall risk reduction among older men and women. Osteoporosis International 2008;19(9):1307‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff‐Ferrari HA, Orav EJ, Dawson‐Hughes B. Effect of cholecalciferol plus calcium on falling in ambulatory older men and women: a 3‐year randomized controlled trial. Archives of Internal Medicine 2006;166(4):424‐30. [DOI] [PubMed] [Google Scholar]
- Blum M, Dallal GE, Dawson‐Hughes B. Body size and serum 25 hydroxy vitamin D response to oral supplements in healthy older adults. Journal of the American College of Nutrition 2008;27(2):274‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson‐Hughes B, Harris SS, Krall EA, Dallal GE. Effect of calcium and vitamin D supplementation on bone density in men and women 65 years of age or older. New England Journal of Medicine 1997;337(10):670‐6. [DOI] [PubMed] [Google Scholar]
- Dawson‐Hughes B, Harris SS, Krall EA, Dallal GE. Effect of withdrawal of calcium and vitamin D supplements on bone mass in elderly men and women. American Journal of Clinical Nutrition 2000;72(3):745‐50. [DOI] [PubMed] [Google Scholar]
- Krall EA, Wehler C, Garcia RI, Harris SS, Dawson‐Hughes B. Calcium and vitamin D supplements reduce tooth loss in the elderly. American Journal of Medicine 2001;111(6):452‐6. [DOI] [PubMed] [Google Scholar]
- Niramitmahapanya S, Harris SS, Dawson‐Hughes B. Type of dietary fat is associated with the 25‐hydroxyvitamin D3 increment in response to vitamin D supplementation. Journal of Clinical Endocrinology and Metabolism 2011;96(10):3170‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittas AG, Harris SS, Stark PC, Dawson‐Hughes B. The effects of calcium and vitamin D supplementation on blood glucose and markers of inflammation in nondiabetic adults. Diabetes Care 2007;30(4):980‐6. [DOI] [PubMed] [Google Scholar]
Dukas 2004 {published data only}
- Dukas L, Bischoff HA, Lindpaintner LS, Schacht E, Birkner‐Binder D, Damm TN, et al. Alfacalcidol reduces the number of fallers in a community‐dwelling elderly population with a minimum calcium intake of more than 500 mg daily. Journal of the American Geriatrics Society 2004;52(2):230‐6. [DOI] [PubMed] [Google Scholar]
Flicker 2005 {published data only}
- Flicker L, MacInnis RJ, Stein MS, Scherer SC, Mead KE, Nowson CA, et al. Should older people in residential care receive vitamin D to prevent falls? Results of a randomized trial. Journal of the American Geriatrics Society 2005;53(11):1881‐8. [DOI] [PubMed] [Google Scholar]
- Flicker L, Mead K, MacInnis RJ, Nowson C, Scherer S, Stein MS, et al. Serum vitamin D and falls in older women in residential care in Australia. Journal of the American Geriatrics Society 2003;51(11):1533‐8. [DOI] [PubMed] [Google Scholar]
- Gau JT, Barcikowski RS. Falls and supplementation of vitamin D and calcium. Journal of the American Geriatrics Society 2006;54(6):1020‐2. [DOI] [PubMed] [Google Scholar]
Gallagher 2001 {published data only}
- Gallagher JC. The effects of calcitriol on falls and fractures and physical performance tests. Journal of Steroid Biochemistry and Molecular Biology 2004;89‐90(1‐5):497‐501. [DOI] [PubMed] [Google Scholar]
- Gallagher JC, Fowler SE, Detter JR, Sherman SS. Combination treatment with estrogen and calcitriol in the prevention of age‐related bone loss. Journal of Clinical Endocrinology and Metabolism 2001;86(8):3618‐28. [DOI] [PubMed] [Google Scholar]
- Gallagher JC, Rapuri PB, Haynatzki G, Detter JR. Effect of discontinuation of estrogen, calcitriol, and the combination of both on bone density and bone markers. Journal of Clinical Endocrinology and Metabolism 2002;87(11):4914‐23. [DOI] [PubMed] [Google Scholar]
- Rapuri PB, Gallagher JC, Haynatzki G. Effect of vitamins D2 and D3 supplement use on serum 25OHD concentration in elderly women in summer and winter. Calcified Tissue International 2004;74(2):150‐6. [DOI] [PubMed] [Google Scholar]
- Sai AJ, Gallagher JC, Fang X. Effect of hormone therapy and calcitriol on serum lipid profile in postmenopausal older women: association with estrogen receptor‐α genotypes. Menopause 2011;18(10):1101‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Glendenning 2012 {published data only}
- Glendenning P, Zhu K, Inderjeeth C, Howat P, Lewis JR, Prince RL. Effects of three monthly oral 150,000 IU cholecalciferol supplementation on falls, mobility and muscle strength in older postmenopausal women: a randomised controlled trial. Journal of Bone and Mineral Research 2012;27(1):170‐6. [DOI] [PubMed] [Google Scholar]
Grady 1991 {published data only}
- Grady D, Halloran B, Cummings S, Leveille S, Wells L, Black D, et al. 1,25‐Dihydroxyvitamin D3 and muscle strength in the elderly: a randomized controlled trial. Journal of Endocrinology and Metabolism 1991;73(5):1111‐7. [DOI] [PubMed] [Google Scholar]
Grimnes 2011 {published data only}
- Grimnes G, Figenschau Y, Almås B, Jorde R. Vitamin D, insulin secretion, sensitivity, and lipids: results from a case‐control study and a randomized controlled trial using hyperglycemic clamp technique. Diabetes 2011;60(11):2748‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
Harwood 2004 {published data only}
- Harwood RH, Sahota O, Gaynor K, Masud T, Hosking DJ, The Nottingham Neck of Femur (NONOF) Study. A randomised, controlled comparison of different calcium and vitamin D supplementation regimens in elderly women after hip fracture: The Nottingham Neck of Femur (NONOF) Study. Age and Ageing 2004;33(1):45‐51. [DOI] [PubMed] [Google Scholar]
- Sahota O, Gaynor K, Harwood RH, Hosking DJ. Hypovitaminosis D and "functional hypoparathyroidism"—the NoNoF (Nottingham Neck of Femur Study). Age and Ageing 2003;32(4):465‐6. [DOI] [PubMed] [Google Scholar]
Jackson 2006 {published data only}
- Brunner RL, Cochrane B, Jackson RD, Larson J, Lewis C, Limacher M, et al. Calcium, vitamin D supplementation, and physical function in the Women's Health Initiative. Journal of the American Dietetic Association 2008;108(9):1472‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner RL, Wactawski‐Wende J, Caan BJ, Cochrane BB, Chlebowski RT, Gass ML, et al. The effect of calcium plus vitamin D on risk for invasive cancer: results of the Women's Health Initiative (WHI) calcium plus vitamin D randomized clinical trial. Nutrition and Cancer 2011;63(6):827‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caan B, Neuhouser M, Aragaki A, Lewis CB, Jackson R, LeBoff MS. Calcium plus vitamin D supplementation and the risk of postmenopausal weight gain. Archives of Internal Medicine 2007;167(9):893‐902. [DOI] [PubMed] [Google Scholar]
- Chlebowski RT, Johnson KC, Kooperberg C, Pettinger M, Wactawski‐Wende J, Rohan T, et al. Calcium plus vitamin D supplementation and the risk of breast cancer. Journal of the National Cancer Institute 2008;100(22):1581‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding EL, Mehta S, Fawzi WW, Giovannucci EL. Interaction of estrogen therapy with calcium and vitamin D supplementation on colorectal cancer risk: reanalysis of Women's Health Initiative randomized trial. International Journal of Cancer 2008;122(8):1690‐4. [DOI] [PubMed] [Google Scholar]
- Hsia J, Heiss G, Ren H, Allison M, Dolan NC, Greenland P, et al. Calcium/vitamin D supplementation and cardiovascular events. Circulation 2007;115(7):846‐54. [DOI] [PubMed] [Google Scholar]
- Jackson RD, LaCroix AZ, Cauley JA, McGowan J. The Women's Health Initiative calcium‐vitamin D trial: overview and baseline characteristics of participants. Annals of Epidemiology 2003;13 Suppl(9):98‐106. [DOI] [PubMed] [Google Scholar]
- Jackson RD, LaCroix AZ, Gass M, Wallace RB, Robbins J, Lewis CE, et al. Calcium plus vitamin D supplementation and the risk of fractures. New England Journal of Medicine 2006;354(7):669‐83. [DOI] [PubMed] [Google Scholar]
- Jackson RD, Shidham S. The role of hormone therapy and calcium plus vitamin D for reduction of bone loss and risk for fractures: lessons learned from the Women's Health Initiative. Current Osteoporosis Reports 2007;5(4):153‐9. [DOI] [PubMed] [Google Scholar]
- Jackson RD, Wright NC, Beck TJ, Sherrill D, Cauley JA, Lewis CE, et al. Calcium plus vitamin D supplementation has limited effects on femoral geometric strength in older postmenopausal women: the Women's Health Initiative. Calcified Tissue International 2011;88(3):198‐208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manson JE, Allison MA, Carr JJ, Langer RD, Cochrane BB, Hendrix SL, et al. Calcium/vitamin D supplementation and coronary artery calcification in the Women's Health Initiative. Menopause 2010;17(4):683‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis KL, Ray RM, Horn L, Manson JE, Allison MA, Black HR, et al. Effect of calcium and vitamin D supplementation on blood pressure: the Women's Health Initiative Randomized Trial. Hypertension 2008;52(5):847‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prentice RL, Anderson GL. The women's health initiative: lessons learned. Annual Review of Public Health 2008;29:131‐50. [DOI] [PubMed] [Google Scholar]
- Rajpathak SN, Xue X, Wassertheil‐Smoller S, Horn L, Robinson JG, Liu S, et al. Effect of 5 y of calcium plus vitamin D supplementation on change in circulating lipids: results from the Women's Health Initiative. American Journal of Clinical Nutrition 2010;91(4):894‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohan TE, Negassa A, Chlebowski RT, Ceria‐Ulep CD, Cochrane BB, Lane DS, et al. A randomized controlled trial of calcium plus vitamin D supplementation and risk of benign proliferative breast disease. Breast Cancer Research and Treatment 2009;116(2):339‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang JY, Fu T, Leblanc E, Manson JE, Feldman D, Linos E, et al. Calcium plus vitamin D supplementation and the risk of nonmelanoma and melanoma skin cancer: post hoc analyses of the women's health initiative randomized controlled trial. Journal of Clinical Oncology 2011;29(22):3078‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Women’s Health Initiative Study Group. Design of the Women's Health Initiative clinical trial and observational study. The Women's Health Initiative Study Group. Controlled Clinical Trials 1998;19(1):61‐109. [DOI] [PubMed] [Google Scholar]
- Twombly R. Negative Women's Health Initiative findings stir consternation, debate among researchers. Journal of the National Cancer Institute 2006;98(8):508‐10. [DOI] [PubMed] [Google Scholar]
- Wallace RB, Wactawski‐Wende J, O'Sullivan MJ, Larson JC, Cochrane B, Gass M, et al. Urinary tract stone occurrence in the Women's Health Initiative (WHI) randomized clinical trial of calcium and vitamin D supplements. American Journal of Clinical Nutrition 2011;94(1):270‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittes J, Barrett‐Connor E, Braunwald E, Chesney M, Cohen HJ, Demets D, et al. Monitoring the randomized trials of the Women's Health Initiative: the experience of the Data and Safety Monitoring Board. Clinical Trials 2007;4(3):218‐34. [DOI] [PubMed] [Google Scholar]
- Boer IH, Tinker LF, Connelly S, Curb JD, Howard BV, Kestenbaum B, et al. Calcium plus vitamin D supplementation and the risk of incident diabetes in the Women's Health Initiative. Diabetes Care 2008;31(4):701‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Janssen 2010 {published data only}
- Janssen HC, Samson MM, Verhaar HJ. Muscle strength and mobility in vitamin D‐insufficient female geriatric patients: a randomized controlled trial on vitamin D and calcium supplementation. Aging Clinical an Experimental Research 2010;22(1):78‐84. [DOI] [PubMed] [Google Scholar]
Kärkkäinen 2010 {published data only}
- Kärkkäinen M, Tuppurainen M, Salovaara K, Sandini L, Rikkonen T, Sirola J, et al. Effect of calcium and vitamin D supplementation on bone mineral density in women aged 65‐71 years: a 3‐year randomized population‐based trial (OSTPRE‐FPS). Osteoporosis International 2010;21(12):2047‐55. [DOI] [PubMed] [Google Scholar]
- Kärkkäinen MK, Tuppurainen M, Salovaara K, Sandini L, Rikkonen T, Sirola J, et al. Does daily vitamin D 800IU and calcium 1000mg supplementation decrease the risk of falling in ambulatory women aged 65‐71 years? A 3‐year randomized population‐based trial (OSTPRE‐FPS). Maturitas 2010;65(4):359‐65. [DOI] [PubMed] [Google Scholar]
- Salovaara K, Tuppurainen M, Kärkkäinen M, Rikkonen T, Sandini L, Sirola J, et al. Effect of vitamin D3 and calcium on fracture risk in 65‐ to 71‐year old women—a population‐based 3‐year randomized controlled trial: OSTPRE‐FPS study. Journal of Bone and Mineral Research 2010;25(7):1487‐95. [DOI] [PubMed] [Google Scholar]
Komulainen 1999 {published data only}
- Heikkinen AM, Niskanen L, Ylä‐Herttuala S, Luoma J, Tuppurainen MT, Komulainen M, et al. Postmenopausal hormone replacement therapy and autoantibodies against oxidized LDL. Maturitas 1998;29(2):155‐61. [DOI] [PubMed] [Google Scholar]
- Heikkinen AM, Parviainen M, Niskanen L, Komulainen M, Tuppurainen MT, Kröger H, et al. Biochemical bone markers and bone mineral density during postmenopausal hormone replacement therapy with and without vitamin D3: a prospective, controlled, randomized study. Journal of Clinical Endocrinology and Metabolism 1997;82(8):2476‐82. [DOI] [PubMed] [Google Scholar]
- Heikkinen AM, Tuppurainen MT, Niskanen L, Komulainen M, Penttilä I, Saarikoski S, et al. Long‐term vitamin D3 supplementation may have adverse effects on serum lipids during postmenopausal hormone replacement therapy. European Journal of Endocrinology/European Federation of Endocrine Societies 1997;137(5):495‐502. [DOI] [PubMed] [Google Scholar]
- Komulainen M, Kröger H, Tuppurainen MT, Heikkinen AM, Alhava E, Honkanen R, et al. Prevention of femoral and lumbar bone loss with hormone replacement therapy and vitamin D3 in early postmenopausal women: a population‐based 5‐year randomized trial. Journal of Clinical Endocrinology and Metabolism 1999;84(2):546‐52. [DOI] [PubMed] [Google Scholar]
- Komulainen M, Kröger H, Tuppurainen MT, Heikkinen AM, Honkanen R, Saarikoski S. Identification of early postmenopausal women with no bone response to HRT: results of a five‐year clinical trial. Osteoporosis International 2000;11(3):211‐8. [DOI] [PubMed] [Google Scholar]
- Komulainen M, Tuppurainen MT, Kröger H, Heikkinen AM, Puntila E, Alhava E, et al. Vitamin D and HRT: no benefit additional to that of HRT alone in prevention of bone loss in early postmenopausal women. A 2.5‐year randomized placebo‐controlled study. Osteoporosis International 1997;7(2):126‐32. [DOI] [PubMed] [Google Scholar]
- Komulainen MH, Kröger H, Tuppurainen MT, Heikkinen AM, Alhava E, Honkanen R, et al. HRT and Vit D in prevention of non‐vertebral fractures in postmenopausal women; a 5 year randomized trial. Maturitas 1998;31(1):45‐54. [DOI] [PubMed] [Google Scholar]
- Salmen T, Heikkinen AM, Mahonen A, Kröger H, Komulainen M, Pallonen H, et al. Relation of aromatase gene polymorphism and hormone replacement therapy to serum estradiol levels, bone mineral density, and fracture risk in early postmenopausal women. Annals of Medicine 2003;35(4):282‐8. [DOI] [PubMed] [Google Scholar]
- Salmén T, Heikkinen AM, Mahonen A, Kröger H, Komulainen M, Pallonen H, et al. Relation of androgen receptor gene polymorphism to bone mineral density and fracture risk in early postmenopausal women during a 5‐year randomized hormone replacement therapy trial. Journal of Bone and Mineral Research 2003;18(2):319‐24. [DOI] [PubMed] [Google Scholar]
- Salmén T, Heikkinen AM, Mahonen A, Kröger H, Komulainen M, Saarikoski S, et al. Early postmenopausal bone loss is associated with PvuII estrogen receptor gene polymorphism in Finnish women: effect of hormone replacement therapy. Journal of Bone and Mineral Research 2000;15(2):315‐21. [DOI] [PubMed] [Google Scholar]
- Salmén T, Heikkinen AM, Mahonen A, Kröger H, Komulainen M, Saarikoski S, et al. Relation of estrogen receptor‐alpha gene polymorphism and hormone replacement therapy to fall risk and muscle strength in early postmenopausal women. Annals of Medicine 2002;34(1):64‐72. [DOI] [PubMed] [Google Scholar]
- Salmén T, Heikkinen AM, Mahonen A, Kröger H, Komulainen M, Saarikoski S, et al. The protective effect of hormone‐replacement therapy on fracture risk is modulated by estrogen receptor alpha genotype in early postmenopausal women. Journal of Bone and Mineral Research 2000;15(12):2479‐86. [DOI] [PubMed] [Google Scholar]
- Tuppurainen M, Heikkinen AM, Penttilä I, Saarikoski S. Does vitamin D3 have negative effects on serum levels of lipids? A follow‐up study with a sequential combination of estradiol valerate and cyproterone acetate and/or vitamin D3. Maturitas 1995;22(1):55‐61. [DOI] [PubMed] [Google Scholar]
- Tuppurainen MT, Komulainen M, Kröger H, Honkanen R, Jurvelin J, Puntila E, et al. Does vitamin D strengthen the increase in femoral neck BMD in osteoporotic women treated with estrogen. Osteoporosis International 1998;8(1):32‐8. [DOI] [PubMed] [Google Scholar]
Krieg 1999 {published data only}
- Krieg MA, Jacquet AF, Bremgartner M, Cuttelod S, Thiébaud D, Burckhardt P. Effect of supplementation with vitamin D3 and calcium on quantitative ultrasound of bone in elderly institutionalized women: a longitudinal study. Osteoporosis International 1999;9(6):483‐8. [DOI] [PubMed] [Google Scholar]
Lappe 2007 {published data only}
- Bolland MJ, Reid IR. Calcium supplementation and cancer incidence. American Journal of Clinical Nutrition 2008;87(3):792‐3. [DOI] [PubMed] [Google Scholar]
- Lappe JM, Davies KM, Travers‐Gustafson D, Heaney RP. Vitamin D status in a rural postmenopausal female population. Journal of the American College of Nutrition 2006;25(5):395‐402. [DOI] [PubMed] [Google Scholar]
- Lappe JM, Travers‐Gustafson D, Davies KM, Recker RR, Heaney RP. Vitamin D and calcium supplementation reduces cancer risk: results of a randomized trial. American Journal of Clinical Nutrition 2007;85(6):1586‐91. [DOI] [PubMed] [Google Scholar]
- Ojha RP, Felini MJ, Fischbach LA. Vitamin D for cancer prevention: valid assertion or premature anointment. American Journal of Clinical Nutrition 2007;86(6):1804‐5. [DOI] [PubMed] [Google Scholar]
- Schabas R. Artifact in the control group undermines the conclusions of a vitamin D and cancer study. American Journal of Clinical Nutrition 2008;87(3):792. [DOI] [PubMed] [Google Scholar]
- Sood MM, Sood AR. Dietary vitamin D and decreases in cancer rates: Canada as the national experiment. American Journal of Clinical Nutrition 2007;86(5):1549. [DOI] [PubMed] [Google Scholar]
Larsen 2004 {published data only}
- Larsen ER, Mosekilde L, Foldspang A. Determinants of acceptance of a community‐based program for the prevention of falls and fractures among the elderly. Preventive Medicine 2001;33(2 Pt 1):115‐9. [DOI] [PubMed] [Google Scholar]
- Larsen ER, Mosekilde L, Foldspang A. Vitamin D and calcium supplementation prevents osteoporotic fractures in elderly community dwelling residents: a pragmatic population‐based 3‐year intervention study. Journal of Bone and Mineral Research 2004;19(3):370‐8. [DOI] [PubMed] [Google Scholar]
- Larsen ER, Mosekilde L, Foldspang A. Vitamin D and calcium supplementation prevents severe falls in elderly community‐dwelling women: a pragmatic population‐based 3‐year intervention study. Aging Clinical and Experimental Research 2005;17(2):125‐32. [DOI] [PubMed] [Google Scholar]
Latham 2003 {published data only}
- Latham NK, Anderson CS, Lee A, Bennett DA, Moseley A, Cameron ID, et al. A randomized, controlled trial of quadriceps resistance exercise and vitamin D in frail older people: the Frailty Interventions Trial in Elderly Subjects (FITNESS). Journal of the American Geriatrics Society 2003;51(3):291‐9. [DOI] [PubMed] [Google Scholar]
Law 2006 {published data only}
- Grant WB. Cholecalciferol, not ergocalciferol, should be used for vitamin D supplementation. Age and Ageing 2006;35(6):645. [DOI] [PubMed] [Google Scholar]
- Law M, Withers H, Morris J, Anderson F. Vitamin D supplementation and the prevention of fractures and falls: results of a randomised trial in elderly people in residential accommodation. Age and Ageing 2006;35(5):482‐6. [DOI] [PubMed] [Google Scholar]
- Zeimer H. Vitamin D supplementation and the prevention of fractures and falls. Age and Ageing 2006;36(2):232‐3. [DOI] [PubMed] [Google Scholar]
Lehouck 2012 {published data only}
- Lehouck A, Mathieu C, Carremans C, Baeke F, Verhaegen J, Eldere J, et al. High doses of vitamin D to reduce exacerbations in chronic obstructive pulmonary disease: a randomized trial. Annals of Internal Medicine 2012;156(2):105‐14. [DOI] [PubMed] [Google Scholar]
Lips 1996 {published data only}
- Graafmans WC, Lips P, Ooms ME, Leeuwen JP, Pols HA, Uitterlinden AG. The effect of vitamin D supplementation on the bone mineral density of the femoral neck is associated with vitamin D receptor genotype. Journal of Bone and Mineral Research 1997;12(8):1241‐5. [DOI] [PubMed] [Google Scholar]
- Lips P, Graafmans WC, Ooms ME, Bezemer PD, Bouter LM. Vitamin D supplementation and fracture incidence in elderly persons. A randomized, placebo‐controlled clinical trial. Annals of Internal Medicine 1996;124(4):400‐6. [DOI] [PubMed] [Google Scholar]
Lips 2010 {published data only}
- Lips P, Binkley N, Pfeifer M, Recker R, Samanta S, Cohn DA, et al. Once‐weekly dose of 8400 IU vitamin D(3) compared with placebo: effects on neuromuscular function and tolerability in older adults with vitamin D insufficiency. American Journal of Clinical Nutrition 2010;91(4):985‐91. [DOI] [PubMed] [Google Scholar]
Lyons 2007 {published data only}
- Lyons RA, Johansen A, Brophy S, Newcombe RG, Phillips CJ, Lervy B, et al. Preventing fractures among older people living in institutional care: a pragmatic randomised double blind placebo controlled trial of vitamin D supplementation. Osteoporosis International 2007;18(6):811‐8. [DOI] [PubMed] [Google Scholar]
Meier 2004 {published data only}
- Meier C, Woitge HW, Witte K, Lemmer B, Seibel MJ. Supplementation with oral vitamin D3 and calcium during winter prevents seasonal bone loss: a randomized controlled open‐label prospective trial. Journal of Bone and Mineral Research 2004;19(8):1221‐30. [DOI] [PubMed] [Google Scholar]
Moschonis 2006 {published data only}
- Manios Y, Moschonis G, Koutsikas K, Papoutsou S, Petraki I, Bellou E, et al. Changes in body composition following a dietary and lifestyle intervention trial: the postmenopausal health study. Maturitas 2009;62(1):58‐65. [DOI] [PubMed] [Google Scholar]
- Manios Y, Moschonis G, Lyritis GP. Seasonal variations of vitamin D status in Greek postmenopausal women receiving enriched dairy products for 30 months: the Postmenopausal Health Study. European Journal of Clinical Nutrition 2011;65(3):412‐4. [DOI] [PubMed] [Google Scholar]
- Manios Y, Moschonis G, Panagiotakos DB, Farajian P, Trovas G, Lyritis GP. Changes in biochemical indices of bone metabolism in post‐menopausal women following a dietary intervention with fortified dairy products. Journal of Human Nutrition and Dietetics 2009;22(2):156‐65. [DOI] [PubMed] [Google Scholar]
- Manios Y, Moschonis G, Trovas G, Lyritis GP. Changes in biochemical indexes of bone metabolism and bone mineral density after a 12‐mo dietary intervention program: the Postmenopausal Health Study. American Journal of Clinical Nutrition 2007;86(3):781‐9. [DOI] [PubMed] [Google Scholar]
- Moschonis G, Katsaroli I, Lyritis GP, Manios Y. The effects of a 30‐month dietary intervention on bone mineral density: the Postmenopausal Health Study. British Journal of Nutrition 2010;104(1):100‐7. [DOI] [PubMed] [Google Scholar]
- Moschonis G, Manios Y. Skeletal site‐dependent response of bone mineral density and quantitative ultrasound parameters following a 12‐month dietary intervention using dairy products fortified with calcium and vitamin D: the Postmenopausal Health Study. British Journal of Nutrition 2006;96(6):1140‐8. [DOI] [PubMed] [Google Scholar]
- Moschonis G, Tanagra S, Koutsikas K, Nikolaidou A, Androutsos O, Manios Y. Association between serum 25‐hydroxyvitamin D levels and body composition in postmenopausal women: the postmenopausal Health Study. Menopause 2009;16(4):701‐7. [DOI] [PubMed] [Google Scholar]
- Tenta R, Moschonis G, Koutsilieris M, Manios Y. Calcium and vitamin D supplementation through fortified dairy products counterbalances seasonal variations of bone metabolism indices: the Postmenopausal Health Study. European Journal of Nutrition 2011;50(5):341‐9. [DOI] [PubMed] [Google Scholar]
Ooms 1995 {published data only}
- Graafmans WC, Ooms ME, Hofstee HM, Bezemer PD, Bouter LM, Lips P. Falls in the elderly: a prospective study of risk factors and risk profiles. American Journal of Epidemiology 1996;143(11):1129‐36. [DOI] [PubMed] [Google Scholar]
- Lips P, Ooms ME, Ter Schegget RM. Prevention of hip fractures in the elderly by vitamin D supplementation. In: Christiansen C, Overgaard K editor(s). Osteoporosis. Copenhagen, Denmark: Osteopress Aps, 1990:604‐6. [Google Scholar]
- Ooms ME, Roos JC, Bezemer PD, Vijgh WJ, Bouter LM, Lips P. Prevention of bone loss by vitamin D supplementation in elderly women: a randomized double‐blind trial. Journal of Clinical Endocrinology and Metabolism 1995;80(4):1052‐8. [DOI] [PubMed] [Google Scholar]
Ott 1989 {published data only}
- Ott SM, Chesnut CH 3rd. Calcitriol treatment is not effective in postmenopausal osteoporosis. Annals of Internal Medicine 1989;110(4):267‐74. [DOI] [PubMed] [Google Scholar]
Porthouse 2005 {published data only}
- Allain TJ. Vitamin D and fracture prevention‐‐treatment still indicated but clarification needed. Age and Ageing 2005;34(6):542‐4. [DOI] [PubMed] [Google Scholar]
- Dumville JC, Miles JN, Porthouse J, Cockayne S, Saxon L, King C. Can vitamin D supplementation prevent winter‐time blues? A randomised trial among older women. Journal of Nutrition, Health and Aging 2006;10(2):151‐3. [PubMed] [Google Scholar]
- Porthouse J, Cockayne S, King C, Saxon L, Steele E, Aspray T, et al. Randomised controlled trial of calcium and supplementation with cholecalciferol (vitamin D3) for prevention of fractures in primary care. BMJ 2005;330(7498):1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radecki TE. Calcium and vitamin D in preventing fractures: vitamin K supplementation has powerful effect. BMJ 2005;331(7508):108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasquez A, Cannell J. Calcium and vitamin D in preventing fractures: data are not sufficient to show inefficacy. BMJ 2005;331(7508):108‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Prince 2008 {published data only}
- Prince RL, Austin N, Devine A, Dick IM, Bruce D, Zhu K. Effects of ergocalciferol added to calcium on the risk of falls in elderly high‐risk women. Archives of Internal Medicine 2008;168(1):103‐8. [DOI] [PubMed] [Google Scholar]
- Zhu K, Bruce D, Austin N, Devine A, Ebeling PR, Prince RL. Randomized controlled trial of the effects of calcium with or without vitamin D on bone structure and bone‐related chemistry in elderly women with vitamin D insufficiency. Journal of Bone and Mineral Research 2008;23(8):1343‐8. [DOI] [PubMed] [Google Scholar]
Sanders 2010 {published data only}
- Sanders KM, Stuart AL, Merriman EN, Read ML, Kotowicz MA, Young D, et al. Trials and tribulations of recruiting 2,000 older women onto a clinical trial investigating falls and fractures: Vital D study. BMC Medical Research Methodology 2009;9:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders KM, Stuart AL, Williamson EJ, Jacka FN, Dodd S, Nicholson G, et al. Annual high‐dose vitamin D3 and mental well‐being: randomised controlled trial. British Journal of Psychiatry 2011;198(5):357‐64. [DOI] [PubMed] [Google Scholar]
- Sanders KM, Stuart AL, Williamson EJ, Simpson JA, Kotowicz MA, Young D, et al. Annual high‐dose oral vitamin D and falls and fractures in older women: a randomized controlled trial. JAMA 2010;303(18):1815‐22. [DOI] [PubMed] [Google Scholar]
Sato 1997 {published data only}
- Sato Y, Maruoka H, Oizumi K. Amelioration of hemiplegia‐associated osteopenia more than 4 years after stroke by 1 alpha‐hydroxyvitamin D3 and calcium supplementation. Stroke 1997;28(4):736‐9. [DOI] [PubMed] [Google Scholar]
Sato 1999a {published data only}
- Sato Y, Manabe S, Kuno H, Oizumi K. Amelioration of osteopenia and hypovitaminosis D by 1alpha‐hydroxyvitamin D3 in elderly patients with Parkinson's disease. Journal of Neurology, Neurosurgery and Psychiatry 1999;66(1):64‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sato 1999b {published data only}
- Sato Y, Kuno H, Kaji M, Saruwatari N, Oizumi K. Effect of ipriflavone on bone in elderly hemiplegic stroke patients with hypovitaminosis D. American Journal of Physical Medicine and Rehabilitation 1999;78(5):457‐63. [DOI] [PubMed] [Google Scholar]
Sato 2005a {published data only}
- Sato Y, Iwamoto J, Kanoko T, Satoh K. Low‐dose vitamin D prevents muscular atrophy and reduces falls and hip fractures in women after stroke: a randomized controlled trial. Cerebrovascular Diseases 2005;20(3):187‐92. [DOI] [PubMed] [Google Scholar]
Schleithoff 2006 {published data only}
- Schleithoff SS, Zittermann A, Tenderich G, Berthold HK, Stehle P, Koerfer R. Combined calcium and vitamin D supplementation is not superior to calcium supplementation alone in improving disturbed bone metabolism in patients with congestive heart failure. European Journal of Clinical Nutrition 2008;62(12):1388‐94. [DOI] [PubMed] [Google Scholar]
- Schleithoff SS, Zittermann A, Tenderich G, Berthold HK, Stehle P, Koerfer R. Vitamin D supplementation improves cytokine profiles in patients with congestive heart failure: a double‐blind, randomized, placebo‐controlled trial. American Journal of Clinical Nutrition 2006;83(4):754‐9. [DOI] [PubMed] [Google Scholar]
- Vieth R, Kimball S. Vitamin D in congestive heart failure. American Journal of Clinical Nutrition 2006;83(4):731‐2. [DOI] [PubMed] [Google Scholar]
Smith 2007 {published data only}
- Francis RM. The vitamin D paradox. Rheumatology (Oxford) 2007;46(12):1749‐50. [DOI] [PubMed] [Google Scholar]
- Smith H, Anderson F, Raphael H, Maslin P, Crozier S, Cooper C. Effect of annual intramuscular vitamin D on fracture risk in elderly men and women—a population‐based, randomized, double‐blind, placebo‐controlled trial. Rheumatology (Oxford) 2007;46(12):1852‐7. [DOI] [PubMed] [Google Scholar]
Trivedi 2003 {published data only}
- Trivedi DP, Doll R, Khaw KT. Effect of four monthly oral vitamin D3 (cholecalciferol) supplementation on fractures and mortality in men and women living in the community: randomised double blind controlled trial. BMJ 2003;326(7387):469. [DOI] [PMC free article] [PubMed] [Google Scholar]
Witham 2010 {published data only}
- Witham MD, Crighton LJ, Gillespie ND, Struthers AD, McMurdo ME. The effects of vitamin D supplementation on physical function and quality of life in older heart failure patients: a randomised controlled trial. Circulation Heart Failure 2010;3(2):195‐201. [DOI] [PubMed] [Google Scholar]
Zhu 2008 {published data only}
- Prince RL, Devine A, Dhaliwal SS, Dick IM. Effects of calcium supplementation on clinical fracture and bone structure: results of a 5‐year, double‐blind, placebo‐controlled trial in elderly women. Archives of Internal Medicine 2006;166(8):869‐75. [DOI] [PubMed] [Google Scholar]
- Zhu K, Devine A, Dick IM, Wilson SG, Prince RL. Effects of calcium and vitamin D supplementation on hip bone mineral density and calcium‐related analytes in elderly ambulatory Australian women: a five‐year randomized controlled trial. The Journal of Clinical Endocrinology and Metabolism 2008;93(3):743‐9. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Adachi 1996 {published data only}
- Adachi JD, Bensen WG, Bianchi F, Cividino A, Pillersdorf S, Sebaldt RJ, et al. Vitamin D and calcium in the prevention of corticosteroid induced osteoporosis: a 3 year follow‐up. Journal of Rheumatology 1996;23(6):995‐1000. [PubMed] [Google Scholar]
Andersen 2008 {published data only}
- Andersen R, Mølgaard C, Skovgaard LT, Brot C, Cashman KD, Jakobsen J, et al. Effect of vitamin D supplementation on bone and vitamin D status among Pakistani immigrants in Denmark: a randomised double‐blinded placebo‐controlled intervention study. British Journal of Nutrition 2008;100(1):197‐207. [DOI] [PubMed] [Google Scholar]
Arthur 1990 {published data only}
- Arthur RS, Piraino B, Candib D, Cooperstein L, Chen T, West C, et al. Effect of low‐dose calcitriol and calcium therapy on bone histomorphometry and urinary calcium excretion in osteopenic women. Mineral and Electrolyte Metabolism 1990;16(6):385‐90. [PubMed] [Google Scholar]
Bacon 2008 {published data only}
- Bacon CJ, Gamble GD, Horne AM, Scott MA, Reid IR. High‐dose oral vitamin D(3) supplementation in the elderly. Osteoporosis International 2008;20(8):1407‐15. [DOI] [PubMed] [Google Scholar]
Bernstein 1996 {published data only}
- Bernstein CN, Seeger LL, Anton PA, Artinian L, Geffrey S, Goodman W, et al. A randomized, placebo‐controlled trial of calcium supplementation for decreased bone density in corticosteroid‐using patients with inflammatory bowel disease: a pilot study. Alimentary Pharmacology and Therapeutics 1996;10(5):777‐86. [DOI] [PubMed] [Google Scholar]
Berry 2010 {published data only}
- Berry SD, Misra D, Hannan MT, Kiel DP. Low acceptance of treatment in the elderly for the secondary prevention of osteoporotic fracture in the acute rehabilitation setting. Aging Clinical and Experimental Research 2010;22(3):231‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Binkley 2011 {published data only}
- Binkley N, Gemar D, Engelke J, Gangnon R, Ramamurthy R, Krueger D, et al. Evaluation of ergocalciferol or cholecalciferol dosing, 1,600 IU daily or 50,000 IU monthly in older adults. Journal of Clinical Endocrinology and Metabolism 2011;96(4):981‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bischoff‐Ferrari 2010a {published data only}
- Bischoff‐Ferrari HA, Dawson‐Hughes B, Platz A, Orav EJ, Stähelin HB, Willett WC, et al. Effect of high‐dosage cholecalciferol and extended physiotherapy on complications after hip fracture: a randomized controlled trial. Archives of Internal Medicine 2010;170(9):813‐20. [DOI] [PubMed] [Google Scholar]
Bizzarri 2010 {published data only}
- Bizzarri C, Pitocco D, Napoli N, Stasio E, Maggi D, Manfrini S, et al. No protective effect of calcitriol on beta‐cell function in recent‐onset type 1 diabetes: the IMDIAB XIII trial. Diabetes Care 2010;33(9):1962‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Buckley 1996 {published data only}
- Buckley LM, Leib ES, Cartularo KS, Vacek PM, Cooper SM. Calcium and vitamin D3 supplementation prevents bone loss in the spine secondary to low‐dose corticosteroids in patients with rheumatoid arthritis. A randomized, double‐blind, placebo‐controlled trial. Annals of Internal Medicine 1996;125(12):961‐8. [DOI] [PubMed] [Google Scholar]
Caniggia 1992 {published data only}
- Caniggia A, Loré F, Nuti R, Martini G, Frediani B, Cairano G. Role of the active vitamin D metabolite and 1 alpha‐hydroxylated analogs in the treatment of postmenopausal osteoporosis. Journal of Nutritional Science and Vitaminology 1992;Spec No:232‐5. [DOI] [PubMed] [Google Scholar]
Chapuy 1996 {published data only}
- Chapuy MC, Chapuy P, Thomas JL, Hazard MC, Meunier PJ. Biochemical effects of calcium and vitamin D supplementation in elderly, institutionalized, vitamin D‐deficient patients. Revue du Rhumatisme (English ed.) 1996;63(2):135‐40. [PubMed] [Google Scholar]
Chen 2001 {published data only}
- Chen M, Chow SN. Additive effect of alfacalcidol on bone mineral density of the lumbar spine in Taiwanese postmenopausal women treated with hormone replacement therapy and calcium supplementation: a randomized 2‐year study. Clinical Endocrinology 2001;55(2):253‐8. [DOI] [PubMed] [Google Scholar]
Dawson‐Hughes 1995 {published data only}
- Dawson‐Hughes B, Harris SS, Krall EA, Dallal GE, Falconer G, Green CL. Rates of bone loss in postmenopausal women randomly assigned to one of two dosages of vitamin D. American Journal of Clinical Nutrition 1995;61(5):1140‐5. [DOI] [PubMed] [Google Scholar]
den Uyl 2010 {published data only}
- Uyl D, Geusens PP, Berkum FN, Houben HH, Jebbink MC, Lems WF. Patient preference and acceptability of calcium plus vitamin D3 supplementation: a randomised, open, cross‐over trial. Clinical Rheumatology 2010;29(5):465‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Diamond 2005 {published data only}
- Diamond TH, Ho KW, Rohl PG, Meerkin M. Annual intramuscular injection of a megadose of cholecalciferol for treatment of vitamin D deficiency: efficacy and safety data. The Medical Journal of Australia 2005;183(1):10‐2. [DOI] [PubMed] [Google Scholar]
Dykman 1984 {published data only}
- Dykman TR, Haralson KM, Gluck OS, Murphy WA, Teitelbaum SL, Hahn TJ, et al. Effect of oral 1,25‐dihydroxyvitamin D and calcium on glucocorticoid‐induced osteopenia in patients with rheumatic diseases. Arthritis and Rheumatism 1984;27(12):1336‐43. [DOI] [PubMed] [Google Scholar]
Falch 1987 {published data only}
- Falch JA, Odegaard OR, Finnanger AM, Matheson I. Postmenopausal osteoporosis: no effect of three years treatment with 1,25‐dihydroxycholecalciferol. Acta Medica Scandinavica 1987;221(2):199‐204. [DOI] [PubMed] [Google Scholar]
Francis 1996 {published data only}
- Francis RM, Boyle IT, Moniz C, Sutcliffe AM, Davis BS, Beastall GH, et al. A comparison of the effects of alfacalcidol treatment and vitamin D2 supplementation on calcium absorption in elderly women with vertebral fractures. Osteoporosis International 1996;6(4):284‐90. [DOI] [PubMed] [Google Scholar]
Gallagher 1990 {published data only}
- Gallagher JC. Metabolic effects of synthetic calcitriol (Rocaltrol) in the treatment of postmenopausal osteoporosis. Metabolism 1990;39 Suppl 1(4):27‐9. [DOI] [PubMed] [Google Scholar]
- Gallagher JC, Goldgar D. Treatment of postmenopausal osteoporosis with high doses of synthetic calcitriol. A randomized controlled study. Annals of Internal Medicine 1990;113(9):649‐55. [DOI] [PubMed] [Google Scholar]
Gannage‐Yared 2003 {published data only}
- Gannagé‐Yared MH, Azoury M, Mansour I, Baddoura R, Halaby G, Naaman R. Effects of a short‐term calcium and vitamin D treatment on serum cytokines, bone markers, insulin and lipid concentrations in healthy post‐menopausal women. Journal of Endocrinological Investigation 2003;26(9):748‐53. [DOI] [PubMed] [Google Scholar]
Geusens 1986 {published data only}
- Geusens P, Dequeker J. Long‐term effect of nandrolone decanoate, 1 alpha‐hydroxyvitamin D3 or intermittent calcium infusion therapy on bone mineral content, bone remodeling and fracture rate in symptomatic osteoporosis: a double‐blind controlled study. Bone and MIneral 1986;1(4):347‐57. [PubMed] [Google Scholar]
Giusti 2010 {published data only}
- Giusti A, Barone A, Pioli G, Girasole G, Razzano M, Pizzonia M, et al. Heterogeneity in serum 25‐hydroxy‐vitamin D response to cholecalciferol in elderly women with secondary hyperparathyroidism and vitamin D deficiency. Journal of the American Geriatrics Society 2010;58(8):1489‐95. [DOI] [PubMed] [Google Scholar]
Glendenning 2009 {published data only}
- Glendenning P, Chew GT, Seymour HM, Gillett MJ, Goldswain PR, Inderjeeth CA, et al. Serum 25‐hydroxyvitamin D levels in vitamin D‐insufficient hip fracture patients after supplementation with ergocalciferol and cholecalciferol. Bone 2009;45(5):870‐5. [DOI] [PubMed] [Google Scholar]
Goswami 2008a {published data only}
- Goswami R, Gupta N, Ray D, Singh N, Tomar N. Pattern of 25‐hydroxy vitamin D response at short (2 month) and long (1 year) interval after 8 weeks of oral supplementation with cholecalciferol in Asian Indians with chronic hypovitaminosis D. British Journal of Nutrition 2008;100(3):526‐9. [DOI] [PubMed] [Google Scholar]
Goussous 2005 {published data only}
- Goussous R, Song L, Dallal GE, Dawson‐Hughes B. Lack of effect of calcium intake on the 25‐hydroxyvitamin D response to oral vitamin D3. Journal of Clinical Endocrinology and Metabolism 2005;90(2):707‐11. [DOI] [PubMed] [Google Scholar]
Gupta 2010 {published data only}
- Gupta A, Gupta N, Singh N, Goswami R. Presence of impaired intestinal calcium absorption in chronic hypovitaminosis D and its change after cholecalciferol supplementation: assessment by the calcium load test. Journal of Human Nutrition and Dietetics 2010;23(1):54‐60. [DOI] [PubMed] [Google Scholar]
Heaney 2011 {published data only}
- Heaney RP, Recker RR, Grote J, Horst RL, Armas LA. Vitamin D(3) is more potent than vitamin D(2) in humans. Journal of Clinical Endocrinology and Metabolism 2011;96(3):E447‐52. [DOI] [PubMed] [Google Scholar]
Hedström 2002 {published data only}
- Hedström M, Sjöberg K, Brosjö E, Aström K, Sjöberg H, Dalén N. Positive effects of anabolic steroids, vitamin D and calcium on muscle mass, bone mineral density and clinical function after a hip fracture. A randomised study of 63 women. Journal of Bone and Joint Surgery. British Volume 2002;84(4):497‐503. [DOI] [PubMed] [Google Scholar]
Heikinheimo 1992 {published data only}
- Heikinheimo RJ, Inkovaara JA, Harju EJ, Haavisto MV, Kaarela RH, Kataja JM, et al. Annual injection of vitamin D and fractures of aged bones. Calcified Tissue International 1992;51(2):105‐10. [DOI] [PubMed] [Google Scholar]
Hill 2010 {published data only}
- Hill DA, Cacciatore M, Lamvu GM. Electronic prescribing influence on calcium supplementation: a randomized controlled trial. American Journal of Obstetrics and Gynecology 2010;202(3):236. [DOI] [PubMed] [Google Scholar]
Holecki 2008 {published data only}
- Holecki M, Zahorska‐Markiewicz B, Wiecek A, Mizia‐Stec K, Nieszporek T, Zak‐Golab A. Influence of calcium and vitamin D supplementation on weight and fat loss in obese women. Obesity Facts 2008;1(5):274‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Holick 2008b {published data only}
- Holick MF, Biancuzzo RM, Chen TC, Klein EK, Young A, Bibuld D, et al. Vitamin D2 is as effective as vitamin D3 in maintaining circulating concentrations of 25‐hydroxyvitamin D. Journal of Clinical Endocrinology and Metabolism 2008;93(3):677‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Holvik 2007 {published data only}
- Holvik K, Madar AA, Meyer HE, Lofthus CM, Stene LC. A randomised comparison of increase in serum 25‐hydroxyvitamin D concentration after 4 weeks of daily oral intake of 10 microg cholecalciferol from multivitamin tablets or fish oil capsules in healthy young adults. British Journal of Nutrition 2007;98(3):620‐5. [DOI] [PubMed] [Google Scholar]
Inkovaara 1983 {published data only}
- Inkovaara J, Gothoni G, Halttula R, Heikinheimo R, Tokola O. Calcium, vitamin D and anabolic steroid in treatment of aged bones: double‐blind placebo‐controlled long‐term clinical trial. Age and Ageing 1983;12(2):124‐30. [DOI] [PubMed] [Google Scholar]
Inomata 1986 {published data only}
- Inomata S, Kadowaki S, Yamatani T, Fukase M, Fujita T. Effect of 1 alpha (OH)‐vitamin D3 on insulin secretion in diabetes mellitus. Bone and Mineral 1986;1(3):187‐92. [PubMed] [Google Scholar]
Ish‐Shalom 2008 {published data only}
- Ish‐Shalom S, Segal E, Salganik T, Raz B, Bromberg IL, Vieth R. Comparison of daily, weekly, and monthly vitamin D3 in ethanol dosing protocols for two months in elderly hip fracture patients. Journal of Endocrinology and Metabolism 2008;93(9):3430‐5. [DOI] [PubMed] [Google Scholar]
Iwamoto 2000 {published data only}
- Iwamoto J, Takeda T, Ichimura S. Effect of combined administration of vitamin D3 and vitamin K2 on bone mineral density of the lumbar spine in postmenopausal women with osteoporosis. Journal of Orthopaedic Science 2000;5(6):546‐51. [DOI] [PubMed] [Google Scholar]
Javanbakht 2011 {published data only}
- Javanbakht MH, Keshavarz SA, Djalali M, Siassi F, Eshraghian MR, Firooz A, et al. Randomized controlled trial using vitamins E and D supplementation in atopic dermatitis. Journal of Dermatological Treatment 2011;22(3):144‐50. [DOI] [PubMed] [Google Scholar]
Kamel 1996 {published data only}
- Kamel S, Brazier M, Rogez JC, Vincent O, Maamer M, Desmet G, et al. Different responses of free and peptide‐bound cross‐links to vitamin D and calcium supplementation in elderly women with vitamin D insufficiency. Journal of Endocrinology and Metabolism 1996;81(10):3717‐21. [DOI] [PubMed] [Google Scholar]
Keane 1992 {published data only}
- Keane EM, Rochfort A, Cox J, McGovern D, Coakley D, Walsh JB. Vitamin‐D‐fortified liquid milk—a highly effective method of vitamin D administration for house‐bound and institutionalised elderly. Gerontology 1992;38(5):280‐4. [DOI] [PubMed] [Google Scholar]
Kenny 2004 {published data only}
- Kenny AM, Prestwood KM, Biskup B, Robbins B, Zayas E, Kleppinger A, et al. Comparison of the effects of calcium loading with calcium citrate or calcium carbonate on bone turnover in postmenopausal women. Osteoporosis International 2004;15(4):290‐4. [DOI] [PubMed] [Google Scholar]
Kilpinen‐Loisa 2009 {published data only}
- Kilpinen‐Loisa P, Arvio M, Ilvesmäki V, Mäkitie O. Vitamin D status and optimal supplementation in institutionalized adults with intellectual disability. Journal of Intellectual Disability Research 2009;53(12):1014‐23. [DOI] [PubMed] [Google Scholar]
Lakatos 2000 {published data only}
- Lakatos P, Nagy Z, Kiss L, Horvath C, Takacs I, Foldes J, et al. Prevention of corticosteroid‐induced osteoporosis by alfacalcidol [Praevention der kortikosteroidinduzierten osteoporose durch alfacalcidol]. Zeitschrift für Rheumatologie 2000;59 Suppl 1:48‐52. [DOI] [PubMed] [Google Scholar]
Leventis 2009 {published data only}
- Leventis P, Kiely PD. The tolerability and biochemical effects of high‐dose bolus vitamin D2 and D3 supplementation in patients with vitamin D insufficiency. Scandinavian Journal of Rheumatology 2009;38(2):149‐53. [DOI] [PubMed] [Google Scholar]
Lind 1988 {published data only}
- Lind L, Wengle B, Lithell H, Ljunghall S. Plasma ionized calcium and cardiovascular risk factors in mild primary hyperparathyroidism: effects of long‐term treatment with active vitamin D (alphacalcidol). Journal of Internal Medicine 1992;231(4):427‐32. [DOI] [PubMed] [Google Scholar]
- Lind L, Wengle B, Lithell H, Ljunghall S. Reduction in serum alkaline phosphatase levels by treatment with active vitamin D (alphacalcidol) in primary and secondary hyperparathyroidism and in euparathyroid individuals. Scandinavian Journal of Urology and Nephrology 1991;25(3):233‐6. [DOI] [PubMed] [Google Scholar]
- Lind L, Wengle B, Ljunghall S. Blood pressure is lowered by vitamin D (alphacalcidol) during long‐term treatment of patients with intermittent hypercalcaemia. A double‐blind, placebo‐controlled study. Acta Medica Scandinavica 1987;222(5):423‐7. [DOI] [PubMed] [Google Scholar]
- Lind L, Wengle B, Sorensen OH, Wide L, Akerström G, Ljunghall S. Treatment with active vitamin D (alphacalcidol) in patients with mild primary hyperparathyroidism. Acta Endocrinologica 1989;120(2):250‐6. [DOI] [PubMed] [Google Scholar]
- Lind L, Wengle B, Wide L, Sörensen OH, Ljunghall S. Hypertension in primary hyperparathyroidism—reduction of blood pressure by long‐term treatment with vitamin D (alphacalcidol). A double‐blind, placebo‐controlled study. American Journal of Hypertension 1988;1(4 Pt 1):397‐402. [DOI] [PubMed] [Google Scholar]
Lind 1989c {published data only}
- Lind L, Pollare T, Hvarfner A, Lithell H, Sørensen OH, Ljunghall S. Long‐term treatment with active vitamin D (alphacalcidol) in middle‐aged men with impaired glucose tolerance. Effects on insulin secretion and sensitivity, glucose tolerance and blood pressure. Diabetes Research 1989;11(3):141‐7. [PubMed] [Google Scholar]
Matsumoto 2010 {published data only}
- Matsumoto T, Takano T, Yamakido S, Takahashi F, Tsuji N. Comparison of the effects of eldecalcitol and alfacalcidol on bone and calcium metabolism. Journal of Steroid Biochemistry and Molecular Biology 2010;121(1‐2):261‐4. [DOI] [PubMed] [Google Scholar]
Meyer 2002 {published data only}
- Kvaavik E, Meyer HE, Smedshaug GB, Falch JA, Tverdal A, Pedersen JI. The intervention study “Prevention of Hip Fractures.” Method and implementation [Intervensjonsstudien ”Forebyggelse av lårhalsbrudd.” Metode og praktisk gjennomføring]. Norwegian Journal of Epidemiology 2001;10(1):78–85. [Google Scholar]
- Meyer HE, Smedshaug GB, Kvaavik E, Falch JA, Tverdal A, Pedersen JI. Can vitamin D supplementation reduce the risk of fracture in the elderly? A randomized controlled trial. Journal of Bone and Mineral Research 2002;17(4):709‐15. [DOI] [PubMed] [Google Scholar]
Nugent 2009 {published data only}
- Nugent C, Roche K, Wilson S, Fitzgibbon M, Griffin D, Nichaidhin N. The effect of intramuscular vitamin D (cholecalciferol) on serum 25OH vitamin D levels in older female acute hospital admissions. Irish Journal of Medical Science 2010;179(1):57‐61. [DOI] [PubMed] [Google Scholar]
Nuti 2006 {published data only}
- Nuti R, Bianchi G, Brandi ML, Caudarella R, D'Erasmo E, Fiore C, et al. Superiority of alfacalcidol compared to vitamin D plus calcium in lumbar bone mineral density in postmenopausal osteoporosis. Rheumatology International 2006;26(5):445‐53. [DOI] [PubMed] [Google Scholar]
Orwoll 1989 {published data only}
- Orwoll ES, McClung MR, Oviatt SK, Recker RR, Weigel RM. Histomorphometric effects of calcium or calcium plus 25‐hydroxyvitamin D3 therapy in senile osteoporosis. Journal of Bone and Mineral Research 1989;4(1):81‐8. [DOI] [PubMed] [Google Scholar]
Pekkarinen 2010 {published data only}
- Pekkarinen T, Välimäki VV, Aarum S, Turpeinen U, Hämäläinen E, Löyttyniemi E, et al. The same annual dose of 292000 IU of vitamin D (cholecalciferol) on either daily or four monthly basis for elderly women: 1‐year comparative study of the effects on serum 25(OH)D concentrations and renal function. Clinical Endocrinology 2010;72(4):455‐61. [DOI] [PubMed] [Google Scholar]
Prestwood 1996 {published data only}
- Prestwood KM, Pannullo AM, Kenny AM, Pilbeam CC, Raisz LG. The effect of a short course of calcium and vitamin D on bone turnover in older women. Osteoporosis International 1996;6(4):314‐9. [DOI] [PubMed] [Google Scholar]
Reginster 1999 {published data only}
- Reginster JY, Kuntz D, Verdickt W, Wouters M, Guillevin L, Menkès CJ, et al. Prophylactic use of alfacalcidol in corticosteroid‐induced osteoporosis. Osteoporosis International 1999;9:75‐81. [DOI] [PubMed] [Google Scholar]
Reginster 2001 {published data only}
- Reginster JY, Zegels B, Lejeune E, Micheletti MC, Kvsaz A, Seidel L, et al. Influence of daily calcium and vitamin D supplementation on parathyroid hormone secretion. Gynecological Endocrinology 2001;15(1):56‐62. [PubMed] [Google Scholar]
- Reginster JY, Zegels B, Lejeune E, Micheletti MC, Kvsaz A, Seidel L, et al. Influence of daily regimen calcium and vitamin D supplementation on parathyroid hormone secretion. Calcified Tissue International 2002;70(2):78‐82. [DOI] [PubMed] [Google Scholar]
Romagnoli 2008 {published data only}
- Romagnoli E, Mascia ML, Cipriani C, Fassino V, Mazzei F, D'Erasmo E, et al. Short and long‐term variations in serum calciotropic hormones after a single very large dose of ergocalciferol (vitamin D2) or cholecalciferol (vitamin D3) in the elderly. Journal of Clinical Endocrinology and Metabolism 2008;93(8):3015‐20. [DOI] [PubMed] [Google Scholar]
Rosenblum 2012 {published data only}
- Rosenblum JL, Castro VM, Moore CE, Kaplan LM. Calcium and vitamin D supplementation is associated with decreased abdominal visceral adipose tissue in overweight and obese adults. American Journal of Clinical Nutrition 2012;95(1):101‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Russo 2011 {published data only}
- Russo S, Carlucci L, Cipriani C, Ragno A, Piemonte S, Fiacco RD, et al. Metabolic changes following 500 μg monthly administration of calcidiol: a study in normal females. Calcified Tissue International 2011;89(3):252‐7. [DOI] [PubMed] [Google Scholar]
Sambrook 1993 {published data only}
- Sambrook P, Birmingham J, Kelly P, Kempler S, Nguyen T, Pocock N, et al. Prevention of corticosteroid osteoporosis. A comparison of calcium, calcitriol, and calcitonin. New England Journal of Medicine 1993;328(24):1747‐52. [DOI] [PubMed] [Google Scholar]
Sambrook 2000 {published data only}
- Sambrook P, Henderson NK, Keogh A, MacDonald P, Glanville A, Spratt P, et al. Effect of calcitriol on bone loss after cardiac or lung transplantation. Journal of Bone and Mineral Research 2000;15(9):1818‐24. [DOI] [PubMed] [Google Scholar]
Sambrook 2003 {published data only}
- Sambrook PN, Kotowicz M, Nash P, Styles CB, Naganathan V, Henderson‐Briffa KN, et al. Prevention and treatment of glucocorticoid‐induced osteoporosis: a comparison of calcitriol, vitamin D plus calcium, and alendronate plus calcium. Journal of Bone and Mineral Research 2003;18(5):919‐24. [DOI] [PubMed] [Google Scholar]
Sato 2005b {published data only}
- Sato Y, Kanoko T, Satoh K, Iwamoto J. The prevention of hip fracture with risedronate and ergocalciferol plus calcium supplementation in elderly women with Alzheimer disease: a randomized controlled trial. Annals of Internal Medicine 2005;165(15):1737‐42. [DOI] [PubMed] [Google Scholar]
Sato 2005c {published data only}
- Sato Y, Kanoko T, Satoh K, Iwamoto J. Menatetrenone and vitamin D2 with calcium supplements prevent nonvertebral fracture in elderly women with Alzheimer's disease. Bone 2005;36(1):61‐8. [DOI] [PubMed] [Google Scholar]
Sato 2006 {published data only}
- Sato Y, Iwamoto J, Kanoko T, Satoh K. Alendronate and vitamin D2 for prevention of hip fracture in Parkinson's disease: a randomized controlled trial. Movement Disorders 2006;21(7):924‐9. [DOI] [PubMed] [Google Scholar]
Sebert 1995 {published data only}
- Sebert JL, Garabedian M, Chauvenet M, Maamer M, Agbomson F, Brazier M. Evaluation of a new solid formulation of calcium and vitamin D in institutionalized elderly subjects. A randomized comparative trial versus separate administration of both constituents. Revue du Rhumatisme (English ed.) 1995;62(4):288‐94. [PubMed] [Google Scholar]
Serhan 2005 {published data only}
- Serhan E, Holland MR. Calcium and vitamin D supplementation failed to improve bone mineral density in Indo‐Asians suffering from hypovitaminosis D and secondary hyperparathyroidism. Rheumatology International 2005;25(4):276‐9. [DOI] [PubMed] [Google Scholar]
Shipowick 2009 {published data only}
- Shipowick CD, Moore CB, Corbett C, Bindler R. Vitamin D and depressive symptoms in women during the winter: a pilot study. Applied Nursing Research 2009;22(3):221‐5. [DOI] [PubMed] [Google Scholar]
Shiraki 1991 {published data only}
- Shiraki M, Orimo H. The effect of estrogen and, sex‐steroids and thyroid hormone preparation on bone mineral density in senile osteoporosis—a comparative study of the effect of 1 alpha‐hydroxycholecalciferol (1 alpha‐OHD3) on senile osteoporosis. Nippon Naibunpi Gakkai Zasshi. Folia Endocrinologica Japonica 1991;67(2):84‐95. [DOI] [PubMed] [Google Scholar]
Sidbury 2008 {published data only}
- Sidbury R, Sullivan AF, Thadhani RI, Camargo CA Jr. Randomized controlled trial of vitamin D supplementation for winter‐related atopic dermatitis in Boston: a pilot study. British Journal of Dermatology 2008;159(1):245‐7. [DOI] [PubMed] [Google Scholar]
Slatkovska 2011 {published data only}
- Slatkovska L, Alibhai SM, Beyene J, Hu H, Demaras A, Cheung AM. Effect of 12 months of whole‐body vibration therapy on bone density and structure in postmenopausal women: a randomized trial. Annals of Internal Medicine 2011;155(10):668‐79. [DOI] [PubMed] [Google Scholar]
Smith 2009 {published data only}
- Smith SM, Gardner KK, Locke J, Zwart SR. Vitamin D supplementation during Antarctic winter. American Journal of Clinical Nutrition 2009;89(4):1092‐8. [DOI] [PubMed] [Google Scholar]
Stein 2011 {published data only}
- Stein MS, Liu Y, Gray OM, Baker JE, Kolbe SC, Ditchfield MR, et al. A randomized trial of high‐dose vitamin D2 in relapsing‐remitting multiple sclerosis. Neurology 2011;77(17):1611‐8. [DOI] [PubMed] [Google Scholar]
Stephens 1981 {published data only}
- Stephens WP, Klimiuk PS, Berry JL, Mawer EB. Annual high‐dose vitamin D prophylaxis in Asian immigrants. Lancet 1981;2(8257):1199‐202. [DOI] [PubMed] [Google Scholar]
Tfelt‐Hansen 2004 {published data only}
- Tfelt‐Hansen J, Tørring O. Calcium and vitamin D3 supplements in calcium and vitamin D3 sufficient early postmenopausal healthy women. European Journal of Clinical Nutrition 2004;58(10):1420‐4. [DOI] [PubMed] [Google Scholar]
Tilyard 1992 {published data only}
- Tilyard MW, Spears GF, Thomson J, Dovey S. Treatment of postmenopausal osteoporosis with calcitriol or calcium. New England Journal of Medicine 1992;326(6):357‐62. [DOI] [PubMed] [Google Scholar]
Trang 1998 {published data only}
- Trang HM, Cole DE, Rubin LA, Pierratos A, Siu S, Vieth R. Evidence that vitamin D3 increases serum 25‐hydroxyvitamin D more efficiently than does vitamin D2. American Journal of Clinical Nutrition 1998;68(4):854‐8. [DOI] [PubMed] [Google Scholar]
Verschueren 2010 {published data only}
- Bogaerts A, Delecluse C, Boonen S, Claessens AL, Milisen K, Verschueren SM. Changes in balance, functional performance and fall risk following whole body vibration training and vitamin D supplementation in institutionalized elderly women. A 6 month randomized controlled trial. Gait & Posture 2011;33(4):448‐54. [DOI] [PubMed] [Google Scholar]
- Verschueren SM, Bogaerts A, Delecluse C, Claessens AL, Haentjens P, Vanderschueren D, et al. The effects of whole‐body vibration training and vitamin D supplementation on muscle strength, muscle mass, and bone density in institutionalized elderly women: a 6‐month randomized, controlled trial. Journal of Bone and Mineral Research 2010;26(1):42‐9. [DOI] [PubMed] [Google Scholar]
Vieth 2004 {published data only}
- Vieth R, Kimball S, Hu A, Walfish PG. Randomized comparison of the effects of the vitamin D3 adequate intake versus 100 mcg (4000 IU) per day on biochemical responses and the wellbeing of patients. Nutrition Journal 2004;3:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Viljakainen 2006b {published data only}
- Viljakainen HT, Natri AM, Kärkkäinen M, Huttunen MM, Palssa A, Jakobsen J, et al. A positive dose‐response effect of vitamin D supplementation on site‐specific bone mineral augmentation in adolescent girls: a double‐blinded randomized placebo‐controlled 1‐year intervention. Journal of Bone and Mineral Research 2006;21(6):836‐44. [DOI] [PubMed] [Google Scholar]
von Restorff 2009 {published data only}
- Restorff C, Bischoff‐Ferrari HA, Theiler R. High‐dose oral vitamin D3 supplementation in rheumatology patients with severe vitamin D3 deficiency. Bone 2009;45(4):747‐9. [DOI] [PubMed] [Google Scholar]
Wejse 2009 {published data only}
- Wejse C, Gomes VF, Rabna P, Gustafson P, Aaby P, Lisse IM, et al. Vitamin D as supplementary treatment for tuberculosis: a double‐blind, randomized, placebo‐controlled trial. American Journal of Respiratory and Critical Care Medicine 2009;179(9):843‐50. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
Aloia 2008b {published data only}
- The interaction between calcium and vitamin D Intake. Ongoing study November 2008. Expected completion: 2009.
Baron 2004 {published data only}
- Vitamin D/calcium polyp prevention study. Ongoing study July 2004. Expected completion: December 2017.
Gallagher 2007 {published data only}
- Vitamin D supplementation in younger women. Ongoing study October 2007; Expected completion: January 2012.
Giovannucci 2007 {published data only}
- Vitamin D for chemoprevention. Ongoing study October 2007; Expected completion: October 2009.
Harris 2008 {published data only}
- Vitamin D, glucose control and insulin sensitivity in African‐Americans. Ongoing study July 2008; Expected completion: February 2011.
Manson 2009 {published data only}
- Manson JE, Bassuk SS, Lee IM, Cook NR, Albert MA, Gordon D, et al. The VITamin D and OmegA‐3 TriaL (VITAL): rationale and design of a large randomized controlled trial of vitamin D and marine omega‐3 fatty acid supplements for the primary prevention of cancer and cardiovascular disease. Contemporary Clinical Trials 2012;33(1):159‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pande 2006 {published data only}
- A trial to study the effect of vitamin D supplementation on glucose and insulin metabolism in centrally obese men. Ongoing study July 2006; Expected completion: September 2006.
Schwartz 2008 {published data only}
- Effects of vitamin D on lipids. Ongoing study July 2008; Expected completion: April 2010.
Scragg 2011 {published data only}
- ViDA (vitamin D assessment) trial. Ongoing study 7/04/2011.
Shapses 2007 {published data only}
- The effect of vitamin D supplementation during caloric restriction on intestinal calcium absorption. Ongoing study March 2007; Expected completion: May 2011.
Witte 2009 {published data only}
- The impact of vitamin D supplementation in chronic heart failure. Ongoing study 01.01.2009; Expected completion: 31.12.2012.
Additional references
Abu‐Mouch 2011
- Abu‐Mouch S, Fireman Z, Jarchovsky J, Zeina AR, Assy N. Vitamin D supplementation improves sustained virologic response in chronic hepatitis C (genotype 1)‐naîve patients. World Journal of Gastroenterology 2011;17(47):5184‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ahn 2008
- Ahn J, Peters U, Albanes D, Purdue MP, Abnet CC, Chatterjee N, et al. Serum vitamin D concentration and prostate cancer risk: a nested case‐control study. Journal of the National Cancer Institute 2008;100(11):796‐804. [DOI] [PMC free article] [PubMed] [Google Scholar]
Aloia 1988
- Aloia JF, Vaswani A, Yeh JK, Ellis K, Yasumura S, Cohn SH. Calcitriol in the treatment of postmenopausal osteoporosis. American Journal of Medicine 1988;84(3 Pt 1):401‐8. [DOI] [PubMed] [Google Scholar]
Aloia 1990
- Aloia JF. Role of calcitriol in the treatment of postmenopausal osteoporosis. Metabolism 1990;39(4 Suppl 1):35‐8. [DOI] [PubMed] [Google Scholar]
Aloia 2008
- Aloia JF, Patel M, Dimaano R, Li‐Ng M, Talwar SA, Mikhail M, et al. Vitamin D intake to attain a desired serum 25‐hydroxyvitamin D concentration. American Journal of Clinical Nutrition 2008;87(6):1952‐8. [DOI] [PubMed] [Google Scholar]
Aloia 2010
- Aloia J, Bojadzievski T, Yusupov E, Shahzad G, Pollack S, Mikhail M. The relative influence of calcium intake and vitamin D status on serum parathyroid hormone and bone turnover biomarkers in a double‐blind, placebo‐controlled parallel group, longitudinal factorial design. Journal of Clinical Endocrinology and Metabolism 2010;95(7):3216‐24. [DOI] [PubMed] [Google Scholar]
Amer 2013
- Amer Muhammad, Qayyum Rehan. Relationship between 25‐hydroxyvitamin D and all‐cause and cardiovascular disease mortality. The American Journal of Medicine 2013;x(Epub ahead of print):x. [DOI] [PubMed] [Google Scholar]
Andersen 2009
- Andersen R, Brot C, Mejborn H, Mølgaard C, Skovgaard LT, Trolle E, et al. Vitamin D supplementation does not affect serum lipids and lipoproteins in Pakistani immigrants. European Journal of Clinical Nutrition 2009;63(9):1150‐3. [DOI] [PubMed] [Google Scholar]
Angeles‐Agdeppa 2010
- Angeles‐Agdeppa I, Capanzana MV, Li‐Yu J, Schollum LM, Kruger MC. High‐calcium milk prevents overweight and obesity among postmenopausal women. Food and Nutrition Bulletin 2010;31(3):381‐90. [DOI] [PubMed] [Google Scholar]
Apperly 1941
- Apperly FL. The relation of solar radiation to cancer mortality in North American. Cancer Research 1941;1:191‐5. [DOI] [PubMed] [Google Scholar]
Armas 2004
- Armas LA, Hollis BW, Heaney RP. Vitamin D2 is much less effective than vitamin D3 in humans. Journal of Clinical Endocrinology and Metabolism 2004;89(11):5387‐91. [DOI] [PubMed] [Google Scholar]
Artaza 2010
- Artaza JN, Sirad F, Ferrini MG, Norris KC. 1,25(OH)(2)vitamin D(3) inhibits cell proliferation by promoting cell cycle arrest without inducing apoptosis and modifies cell morphology of mesenchymal multipotent cells. Journal of Steroid Biochemistry and Molecular Biology 2010;119(1‐2):73‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Arvold 2009
- Arvold DS, Odean MJ, Dornfeld MP, Regal RR, Arvold JG, Karwoski GC, et al. Correlation of symptoms with vitamin D deficiency and symptom response to cholecalciferol treatment: a randomized controlled trial. Endocrine Practice 2009;15(3):203‐12. [DOI] [PubMed] [Google Scholar]
Autier 2007
- Autier P, Gandini S. Vitamin D supplementation and total mortality: a meta‐analysis of randomized controlled trials. Archives of Internal Medicine 2007;167(16):1730‐7. [DOI] [PubMed] [Google Scholar]
Avenell 2009
- Avenell A, Gillespie WJ, Gillespie LD, O’Connell DL. Vitamin D and vitamin D analogues for preventing fractures associated with involutional and post‐menopausal osteoporosis. The Cochrane Database of Systematic Reviews 2009, Issue 2. Art. No.: CD000227.DOI: 10.1002/14651858.CD000227.pub3. [DOI] [PubMed]
Bang 2011
- Bang UC, Matzen P, Benfield T, Beck Jensen JE. Oral cholecalciferol versus ultraviolet radiation B: effect on vitamin D metabolites in patients with chronic pancreatitis and fat malabsorption—a randomized clinical trial. Pancreatology 2011;11(4):376‐82. [DOI] [PubMed] [Google Scholar]
Barnes 2006
- Barnes MS, Robson PJ, Bonham MP, Strain JJ, Wallace JM. Effect of vitamin D supplementation on vitamin D status and bone turnover markers in young adults. European Journal of Clinical Nutrition 2006;60(6):727‐33. [DOI] [PubMed] [Google Scholar]
Barnes 2011
- Barnes MS, Horigan G, Cashman KD, Hill TR, Forsythe LK, Lucey AJ, et al. Maintenance of wintertime vitamin D status with cholecalciferol supplementation is not associated with alterations in serum cytokine concentrations among apparently healthy younger or older adults. Journal of Nutrition 2011;141(3):476‐81. [DOI] [PubMed] [Google Scholar]
Begg 1994
- Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50(4):1088‐101. [PubMed] [Google Scholar]
Biancuzzo 2010
- Biancuzzo RM, Young A, Bibuld D, Cai MH, Winter MR, Klein EK, et al. Fortification of orange juice with vitamin D(2) or vitamin D(3) is as effective as an oral supplement in maintaining vitamin D status in adults. American Journal of Clinical Nutrition 2010;91(6):1621‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Binkley 2009
- Binkley N, Krueger D, Lensmeyer G. 25‐Hydroxyvitamin D measurement, 2009: a review for clinicians. Journal of Clinical Densitometry 2009;12(4):417‐27. [DOI] [PubMed] [Google Scholar]
Bischoff‐Ferrari 2005
- Bischoff‐Ferrari HA, Willett WC, Wong JB, Giovannucci E, Dietrich T, Dawson‐Hughes B. Fracture prevention with vitamin D supplementation: a meta‐analysis of randomized controlled trials. JAMA 2005;293:2257‐64. [DOI] [PubMed] [Google Scholar]
Bischoff‐Ferrari 2009a
- Bischoff‐Ferrari HA, Dawson‐Hughes B, Staehelin HB, Orav JE, Stuck AE, Theiler R, et al. Fall prevention with supplemental and active forms of vitamin D: a meta‐analysis of randomised controlled trials. BMJ 2009;339:843‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bischoff‐Ferrari 2009b
- Bischoff‐Ferrari HA, Willett WC, Wong JB, Stuck AE, Staehelin HB, Orav EJ, et al. Prevention of nonvertebral fractures with oral vitamin D and dose dependency: a meta‐analysis of randomized controlled trials. Archives of Internal Medicine 2009;169(6):551‐61. [DOI] [PubMed] [Google Scholar]
Bischoff‐Ferrari 2009c
- Bischoff‐Ferrari H. Vitamin D: what is an adequate vitamin D level and how much supplementation is necessary. Best Practice & Research. Clinical Rheumatology 2009;23(6):789‐95. [DOI] [PubMed] [Google Scholar]
Bischoff‐Ferrari 2010b
- Bischoff‐Ferrari HA, Shao A, Dawson‐Hughes B, Hathcock J, Giovannucci E, Willett WC. Benefit‐risk assessment of vitamin D supplementation. Osteoporosis International 2010;21(7):1121‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bischoff‐Ferrari 2012
- Bischoff‐Ferrari Heike A, Willett Walter C, Orav Endel J, Lips Paul, Meunier Pierre J, Lyons Ronan A, et al. A pooled analysis of vitamin D dose requirements for fracture prevention. New England Journal of Medicine 2012;367(1):40‐9. [DOI] [PubMed] [Google Scholar]
Bjelakovic 2008a
- Bjelakovic G, Gluud LL, Nikolova D, Whitfield K, Wetterslev J, Gluud C. Vitamin D supplementation for prevention of mortality in adults. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD007470] [DOI] [Google Scholar]
Bjelakovic 2008b
- Bjelakovic G, Gluud LL, Nikolova D, Whitfield K, Wetterslev J, Gluud C. Vitamin D supplementation for prevention of cancer in adults. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD007469] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bolland 2010
- Bolland MJ, Avenell A, Baron JA, Grey A, MacLennan GS, Gamble GD, et al. Effect of calcium supplements on risk of myocardial infarction and cardiovascular events: meta‐analysis. BMJ 2010;341:c3691. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bolland 2011
- Bolland MJ, Grey A, Avenell A, Gamble GD, Reid IR. Calcium supplements with or without vitamin D and risk of cardiovascular events: reanalysis of the Women's Health Initiative limited access dataset and meta‐analysis. BMJ 2011;342:d2040. [DOI: 10.1136/bmj.d2040] [DOI] [PMC free article] [PubMed] [Google Scholar]
Boonen 2006
- Boonen S, Bischoff‐Ferrari HA, Cooper C, Lips P, Ljunggren O, Meunier PJ, et al. Addressing the musculoskeletal components of fracture risk with calcium and vitamin D: a review of the evidence. Calcified Tissue International 2006;78:257‐70. [DOI] [PubMed] [Google Scholar]
Borenstein 2009
- Bornstein M, Hedges LV, Higgins T, Rothstein HR. Introduction to Meta‐Analysis. Chichester: John Wiley & Sons, 2009. [Google Scholar]
Braam 2004
- Braam LA, Hoeks AP, Brouns F, Hamulyak K, Gerichhausen MJ, Vermeer C. Beneficial effects of vitamins D and K on the elastic properties of the vessel wall in postmenopausal women: a follow‐up study. Thrombosis and Haemostasis 2004;91(2):373‐80. [DOI] [PubMed] [Google Scholar]
Bradburn 2007
- Bradburn MJ, Deeks JJ, Berlin JA, Russell Localio A. Much ado about nothing: a comparison of the performance of meta‐analytical methods with rare events. Statistics in Medicine 2007;26:53‐77. [DOI] [PubMed] [Google Scholar]
Brok 2008
- Brok J, Thorlund K, Gluud C, Wetterslev J. Trial sequential analysis reveals insufficient information size and potentially false positive results in many meta‐analyses. Journal of Clinical Epidemiology 2008;61(8):763‐9. [DOI] [PubMed] [Google Scholar]
Brok 2009
- Brok J, Thorlund K, Wetterslev J, Gluud C. Apparently conclusive meta‐analyses may be inconclusive—Trial sequential analysis adjustment of random error risk due to repetitive testing of accumulating data in apparently conclusive neonatal meta‐analyses. International Journal of Epidemiology 2009;38(1):287‐98. [DOI] [PubMed] [Google Scholar]
Bunout 2006
- Bunout D, Barrera G, Leiva L, Gattas V, Maza MP, Avendano M, et al. Effects of vitamin D supplementation and exercise training on physical performance in Chilean vitamin D deficient elderly subjects. Experimental Gerontology 2006;41(8):746‐52. [DOI] [PubMed] [Google Scholar]
Burton 2010
- Burton JM, Kimball S, Vieth R, Bar‐Or A, Dosch HM, Cheung R, et al. A phase I/II dose‐escalation trial of vitamin D3 and calcium in multiple sclerosis. Neurology 2010;74(23):1852‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Caniggia 1984
- Caniggia A, Delling G, Nuti R, Lore F, Vattimo A. Clinical, biochemical and histological results of a double‐blind trial with 1,25‐dihydroxyvitamin D3, estradiol and placebo in post‐menopausal osteoporosis. Acta Vitaminologica et Enzymologica 1984;6(2):117‐28. [PubMed] [Google Scholar]
Cashman 2008
- Cashman KD, Hill TR, Lucey AJ, Taylor N, Seamans KM, Muldowney S, et al. Estimation of the dietary requirement for vitamin D in healthy adults. American Journal of Clinical Nutrition 2008;88(6):1535‐42. [DOI] [PubMed] [Google Scholar]
Cashman 2009
- Cashman KD, Wallace JM, Horigan G, Hill TR, Barnes MS, Lucey AJ, et al. Estimation of the dietary requirement for vitamin D in free‐living adults >=64 y of age. American Journal of Clinical Nutrition 2009;89(5):1366‐74. [DOI] [PubMed] [Google Scholar]
Cavalier 2009
- Cavalier E, Delanaye P, Chapelle JP, Souberbielle JC. Vitamin D: current status and perspectives. Clinical Chemistry and Laboratory Medicine 2009;47(2):120‐7. [DOI] [PubMed] [Google Scholar]
Chan 2013
- Chan A‐W, Tetzlaff JM, Altman DG, Laupacis A, Gøtzsche PC, Krleža‐Jerić K, et al. SPIRIT 2013 Statement: Defining standard protocol items for clinical trials. Annals of Internal Medicine 2013;158:200‐207. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chapuy 1987
- Chapuy MC, Chapuy P, Meunier PJ. Calcium and vitamin D supplements: effects on calcium metabolism in elderly people. American Journal of Clinical Nutrition 1987;46(2):324‐8. [DOI] [PubMed] [Google Scholar]
Chen 1997
- Chen JT, Shiraki M, Hasumi K, Tanaka N, Katase K, Kato T, et al. 1‐Alpha‐hydroxyvitamin D3 treatment decreases bone turnover and modulates calcium‐regulating hormones in early postmenopausal women. Endocrine Journal 1997;20(6):557‐62. [DOI] [PubMed] [Google Scholar]
Chen 2007
- Chen W, Dawsey SM, Qiao YL, Mark SD, Dong ZW, Taylor PR, et al. Prospective study of serum 25(OH)‐vitamin D concentration and risk of oesophageal and gastric cancers. British Journal of Cancer 2007;97(1):123‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Christiansen 1980
- Christiansen C, Christensen MS, McNair P, Hagen C, Stocklund KE, Transb›l I. Prevention of early postmenopausal bone loss: controlled 2‐year study in 315 normal females. European Journal of Clinical Investigation 1980;10(4):273‐9. [DOI] [PubMed] [Google Scholar]
Christiansen 1981
- Christiansen C, Christensen MS, R›odbro P, Hagen C, Transb›l I. Effect of 1,25‐dihydroxy‐vitamin D3 in itself or combined with hormone treatment in preventing postmenopausal osteoporosis. European Journal of Clinical Investigation 1981;11(4):305‐9. [DOI] [PubMed] [Google Scholar]
Chung 2009
- Chung M, Balk EM, Brendel M, Ip S, Lau J, Lee J, et al. Vitamin D and calcium: a systematic review of health outcomes. Evidence Report No. 183. AHRQ publication No. 09‐E015 Rockville, MD: Agency for Healthcare Research and Quality, August 2009.
Chung 2011
- Chung M, Lee J, Terasawa T, Lau J, Trikalinos TA. Vitamin D with or without calcium supplementation for prevention of cancer and fractures: an updated meta‐analysis for the U.S. Preventive Services Task Force. Annals of Internal Medicine 2011;155(12):827‐38. [DOI] [PubMed] [Google Scholar]
Cohen 1960
- Cohen J. A coefficient of agreement for nominal scales. Educational and Psychological Measurement 1960;20:37‐46. [Google Scholar]
Cranney 2007
- Cranney A, Horsley T, O'Donnell S, Weiler H, Puil L, Ooi D, et al. Effectiveness and safety of vitamin D in relation to bone health. Evidence Report/Technology Assessment No. 158. AHRQ publication No. 07‐E013 Rockville, MD: Agency for Healthcare Research and Quality, August 2007. [PMC free article] [PubMed]
Davis 2007
- Davis CD, Dwyer JT. The "sunshine vitamin": benefits beyond bone?. Journal of the National Cancer Institute 2007;99(21):1563‐5. [DOI] [PubMed] [Google Scholar]
Dawson‐Hughes 1991
- Dawson‐Hughes B, Dallal GE, Krall EA, Harris S, Sokoll LJ, Falconer G. Effect of vitamin D supplementation on wintertime and overall bone loss in healthy postmenopausal women. Annals of Internal Medicine 1991;115(7):505‐12. [DOI] [PubMed] [Google Scholar]
Dawson‐Hughes 2005
- Dawson‐Hughes B, Heaney RP, Holick MF, Lips P, Meunier PJ, Vieth R. Estimates of optimal vitamin D status. Osteoporosis International 2005;16(7):713‐6. [DOI] [PubMed] [Google Scholar]
DeMets 1987
- DeMets DL. Methods for combining randomized clinical trials: strengths and limitations. Statistics in Medicine 1987;6(3):341‐50. [DOI] [PubMed] [Google Scholar]
Deroisy 2002
- Deroisy R, Collette J, Albert A, Jupsin I, Reginster JY. Administration of a supplement containing both calcium and vitamin D is more effective than calcium alone to reduce secondary hyperparathyroidism in postmenopausal women with low 25(OH)vitamin D circulating levels. Aging Clinical and Experimental Research 2002;14(1):13‐7. [DOI] [PubMed] [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. [DOI] [PubMed] [Google Scholar]
Dhesi 2004
- Dhesi JK, Jackson SH, Bearne LM, Moniz C, Hurley MV, Swift CG, et al. Vitamin D supplementation improves neuromuscular function in older people who fall. Age and Ageing 2004;33(6):589‐95. [DOI] [PubMed] [Google Scholar]
Di 2004
- Daniele N, Carbonelli MG, Candeloro N, Iacopino L, Lorenzo A, Andreoli A. Effect of supplementation of calcium and vitamin D on bone mineral density and bone mineral content in peri‐ and post‐menopause women; a double‐blind, randomized, controlled trial. Pharmacological Research 2004;50(6):637‐41. [DOI] [PubMed] [Google Scholar]
DIPART 2010
- DIPART (Vitamin D Individual Patient Analysis of Randomized Trials) Group. Patient level pooled analysis of 68 500 patients from seven major vitamin D fracture trials in US and Europe. BMJ 2010;340:b5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
Doetsch 2004
- Doetsch AM, Faber J, Lynnerup N, Wätjen I, Bliddal H, Danneskiold‐Samsøe B. The effect of calcium and vitamin D3 supplementation on the healing of the proximal humerus fracture: a randomized placebo‐controlled study. Calcified Tissue International 2004;75(3):183‐8. [DOI] [PubMed] [Google Scholar]
Domrongkitchaiporn 2000
- Domrongkitchaiporn S, Ongphiphadhanakul B, Stitchantrakul W, Piaseu N, Chansirikam S, Puavilai G, et al. Risk of calcium oxalate nephrolithiasis after calcium or combined calcium and calcitriol supplementation in postmenopausal women. Osteoporosis International 2000;11(6):486‐92. [DOI] [PubMed] [Google Scholar]
Durup 2012
- Durup D, Jørgensen HL, Christensen J, Schwarz P, Heegaard AM, Lind B. A reverse J‐shaped association of all‐cause mortality with serum 25‐hydroxyvitamin D in general practice, the CopD study. Journal of Clinical Endocrinology and Metabolism 2012;May 9:[Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
Ebeling 2001
- Ebeling PR, Wark JD, Yeung S, Poon C, Salehi N, Nicholson GC, et al. Effects of calcitriol or calcium on bone mineral density, bone turnover, and fractures in men with primary osteoporosis: a two‐year randomized, double blind, double placebo study. Journal of Clinical Endocrinology and Metabolism 2001;86(9):4098‐103. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey SG, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ (Clinical Research Ed) 1997;315(7109):629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Elamin 2011
- Elamin MB, Abu Elnour NO, Elamin KB, Fatourechi MM, Alkatib AA, Almandoz JP, et al. Vitamin D and cardiovascular outcomes: a systematic review and meta‐analysis. Journal of Clinical Endocrinology and Metabolism 2011;96(7):1931‐42. [DOI] [PubMed] [Google Scholar]
Fedirko 2010
- Fedirko V, Bostick RM, Long Q, Flanders WD, McCullough ML, Sidelnikov E, et al. Effects of supplemental vitamin D and calcium on oxidative DNA damage marker in normal colorectal mucosa: a randomized clinical trial. Cancer Epidemiology, Biomarkers and Prevention 2010;19(1):280‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fleet 2008
- Fleet JC. Molecular actions of vitamin D contributing to cancer prevention. Molecular Aspects of Medicine 2008;29(6):388‐96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fliser 1997
- Fliser D, Stefanski A, Franek E, Fode P, Gudarzi A, Ritz E. No effect of calcitriol on insulin‐mediated glucose uptake in healthy subjects. European Journal of Clinical Investigation 1997;27(7):629‐33. [DOI] [PubMed] [Google Scholar]
Forsythe 2012
- Forsythe LK, Livingstone MB, Barnes MS, Horigan G, McSorley EM, Bonham MP, et al. Effect of adiposity on vitamin D status and the 25‐hydroxycholecalciferol response to supplementation in healthy young and older Irish adults. British Journal of Nutrition 2012;107(1):126‐34. [DOI] [PubMed] [Google Scholar]
Friedrich 2007
- Friedrich JO, Adhikari NK, Beyene J. Inclusion of zero total event trials in meta‐analyses maintains analytic consistency and incorporates all available data. BMC Medical Research Methodology 2007; Vol. 7, issue 5. [DOI] [PMC free article] [PubMed]
Gallagher 1982
- Gallagher JC, Jerpbak CM, Jee WS, Johnson KA, DeLuca HF, Riggs BL. 1,25‐Dihydroxyvitamin D3: short‐ and long‐term effects on bone and calcium metabolism in patients with postmenopausal osteoporosis. Proceedings of the National Academy of Sciences of the United States of America 1982;79(10):3325‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gallagher 1989
- Gallagher JC, Riggs BL, Recker RR, Goldgar D. The effect of calcitriol on patients with postmenopausal osteoporosis with special reference to fracture frequency. Proceedings of the Society for Experimental Biology and Medicine 1989;191(3):287‐92. [DOI] [PubMed] [Google Scholar]
Gamble 2005
- Gamble C, Hollis S. Uncertainty method improved on best‐worst case analysis in a binary meta‐analysis. Journal of Clinical Epidemiology 2005;58(6):579‐88. [DOI] [PubMed] [Google Scholar]
Garland 1980
- Garland CF, Garland FC. Do sunlight and vitamin D reduce the likelihood of colon cancer?. International Journal of Epidemiology 1980;9:227‐31. [DOI] [PubMed] [Google Scholar]
Garland 2007
- Garland CF, Gorham ED, Mohr SB, Grant WB, Giovannucci EL, Lipkin M, et al. Vitamin D and prevention of breast cancer: pooled analysis. The Journal of Steroid Biochemistry and Molecular Biology 2007;103(3‐5):708‐11. [DOI] [PubMed] [Google Scholar]
Giovannucci 2005
- Giovannucci E. The epidemiology of vitamin D and cancer incidence and mortality: a review (United States). Cancer Causes & Control 2005;16(2):83‐95. [DOI] [PubMed] [Google Scholar]
Gluud 2006
- Gluud C. The culture of designing hepato‐biliary randomised trials. Journal of Hepatology 2006;44(3):607‐15. [DOI] [PubMed] [Google Scholar]
Gluud 2008
Gluud 2011
- Gluud C, Nikolova D, Klingenberg SL, Alexakis N, Als‐Nielsen B, Colli A, et al. Cochrane Hepato‐Biliary Group. About The Cochrane Collaboration (Cochrane Review Groups (CRGs)). 2011, Issue 10. Art. No.: LIVER.
Goodwin 2009
- Goodwin PJ, Ennis M, Pritchard KI, Koo J, Hood N. Prognostic effects of 25‐hydroxyvitamin D levels in early breast cancer. Journal of Clinical Oncology 2009;27(23):3757‐63. [DOI] [PubMed] [Google Scholar]
Gorai 1999
- Gorai I, Chaki O, Taguchi Y, Nakayama M, Osada H, Suzuki N, et al. Early postmenopausal bone loss is prevented by estrogen and partially by 1alpha‐OH‐vitamin D3: therapeutic effects of estrogen and/or 1alpha‐OH‐vitamin D3. Calcified Tissue International 1999;65(1):16‐22. [DOI] [PubMed] [Google Scholar]
Gorham 2007
- Gorham ED, Garland CF, Garland FC, Grant WB, Mohr SB, Lipkin M, et al. Optimal vitamin D status for colorectal cancer prevention: a quantitative meta analysis. American Journal of Preventive Medicine 2007;32(3):210‐6. [DOI] [PubMed] [Google Scholar]
Green 2010
- Green TJ, Skeaff CM, Rockell JE. Milk fortified with the current adequate intake for vitamin D (5 microg) increases serum 25‐hydroxyvitamin D compared to control milk but is not sufficient to prevent a seasonal decline in young women. Asian Journal of Clinical Nutrition 2010;19(2):195‐9. [PubMed] [Google Scholar]
Haentjens 2010
- Haentjens P, Magaziner J, Colon‐Emeric CS, Vanderschueren D, Milisen K, Velkeniers B, et al. Meta‐analysis: excess mortality after hip fracture among older women and men. Annals of Internal Medicine 2010;152(6):380‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Harris 1999
- Harris SS, Dawson‐Hughes B, Perrone GA. Plasma 25‐hydroxyvitamin D responses of younger and older men to three weeks of supplementation with 1800 IU/day of vitamin D. Journal of the American College of Nutrition 1999;18(5):470‐4. [DOI] [PubMed] [Google Scholar]
Harris 2002
- Harris SS, Dawson‐Hughes B. Plasma vitamin D and 25OHD responses of young and old men to supplementation with vitamin D3. Journal of the American College of Nutrition 2002;21(4):357‐62. [DOI] [PubMed] [Google Scholar]
Heaney 2008
- Heaney RP. Vitamin D in health and disease. Clinical Journal of the American Society of Nephrology 2008;3(5):1535‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analysis. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1. [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Himeno 2009
- Himeno M, Tsugawa N, Kuwabara A, Fujii M, Kawai N, Kato Y, et al. Effect of vitamin D supplementation in the institutionalized elderly. Journal of Bone and Mineral Research 2009;27(6):733‐7. [DOI] [PubMed] [Google Scholar]
Himmelstein 1990
- Himmelstein S, Clemens TL, Rubin A, Lindsay R. Vitamin D supplementation in elderly nursing home residents increases 25(OH)D but not 1,25(OH)2D. American Journal of Clinical Nutrition 1990;52(4):701‐6. [DOI] [PubMed] [Google Scholar]
Holick 2004
- Holick MF. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. American Journal of Clinical Nutrition 2004;80 Suppl(6):1678–88. [DOI] [PubMed] [Google Scholar]
Holick 2006
- Holick MF. Vitamin D: Its role in cancer prevention and treatment. Progress in Biophysics and Molecular Biology 2006;92:49‐59. [DOI] [PubMed] [Google Scholar]
Holick 2007a
- Holick MF. Vitamin D deficiency. New England Journal of Medicine 2007;357(3):266‐81. [DOI] [PubMed] [Google Scholar]
Holick 2007b
- Holick MF, Chen TC, Lu Z, Sauter E. Vitamin D and skin physiology: a D‐lightful story. Journal of Bone and Mineral Research 2007;22 Suppl 2:V28‐33. [DOI] [PubMed] [Google Scholar]
Holick 2008a
- Holick MF, Chen TC. Vitamin D deficiency: a worldwide problem with health consequences. American Journal of Clinical Nutrition 2008;87(4):1080S‐6S. [DOI] [PubMed] [Google Scholar]
Holick 2008c
- Holick MF, Biancuzzo RM, Chen TC, Klein EK, Young A, Bibuld D, et al. Vitamin D2 is as effective as vitamin D3 in maintaining circulating concentrations of 25‐hydroxyvitamin D. Journal of Clinical Endocrinology and Metabolism 2008;93(3):677‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Holick 2009
- Holick MF. Vitamin D status: measurement, interpretation, and clinical application. Annals of Epidemiology 2009;19(2):73‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Holick 2011
- Holick Michael F, Binkley Neil C, Bischoff‐Ferrari Heike A, Gordon Catherine M, Hanley David A, Heaney Robert P, et al. Evaluation, treatment, and prevention of vitamin D Deficiency: an Endocrine Society clinical practice guideline. Journal of Clinical Endocrinology & Metabolism 2011;96(7):1911‐30. [DOI] [PubMed] [Google Scholar]
Holmberg 1986
- Holmberg I, Berlin T, Ewerth S, Björkhem I. 25‐Hydroxylase activity in subcellular fractions from human liver. Evidence for different rates of mitochondrial hydroxylation of vitamin D2 and D3. Scandinavian Journal of Clinical and Laboratory Investigation 1986;46(8):785‐90. [DOI] [PubMed] [Google Scholar]
Horst 2005
- Horst RL, Reinhardt TA, Satyanarayana Reddy G. Vitamin D metabolism. In: Feldman D, Wesley Pike J, Glorieux FH editor(s). Vitamin D. 2nd Edition. Amsterdam, Boston, Heidelberg, London, New York, Oxford, Paris, San Diego, San Francisco, Singapore, Sydney, Tokyo: Elsevier Academic Press, 2005:15‐36. [Google Scholar]
Houghton 2006
- Houghton LA, Vieth R. The case against ergocalciferol (vitamin D2) as a vitamin supplement. American Journal of Clinical Nutrition 2006;84(4):694‐7. [DOI] [PubMed] [Google Scholar]
Hoy 1988
- Hoy DA, Ramberg CF, Horst RL. Evidence that discrimination against ergocalciferol by the chick is the result of enhanced metabolic clearance rates for its mono‐ and dihydroxylated metabolites. Journal of Nutrition 1988;118(5):633‐8. [DOI] [PubMed] [Google Scholar]
Hulshof 2000
- Hulshof MM, Bouwes Bavinck JN, Bergman W, Masclee AA, Heickendorff L, et al. Double‐blind, placebo‐controlled study of oral calcitriol for the treatment of localized and systemic scleroderma. Journal of the American Academy of Dermatology 2000;43(6):1017‐23. [DOI] [PubMed] [Google Scholar]
Hunter 2000
- Hunter D, Major P, Arden N, Swaminathan R, Andrew T, MacGregor AJ, et al. A randomized controlled trial of vitamin D supplementation on preventing postmenopausal bone loss and modifying bone metabolism using identical twin pairs. Journal of Bone and Mineral Research 2000;15(11):2276‐83. [DOI] [PubMed] [Google Scholar]
Hutchinson 2010
- Hutchinson MS, Grimnes G, Joakimsen RM, Figenschau Y, Jorde R. Low serum 25‐hydroxyvitamin D levels are associated with increased all‐cause mortality risk in a general population: the Tromsø study. European Journal of Endocrinology 2010;162(5):935‐42. [DOI] [PubMed] [Google Scholar]
IARC 2008
- IARC. Vitamin D and Cancer. IARC Working Group Reports. Lyon, France: International Agency for Research on Cancer, 2008; Vol. 5.
ICH‐GCP 1997
- International Committee on Harmonization. Code of Federal Regulations & Guidelines. Vol. 1, Philadelphia, US: Barnett International/PAREXEL, 1997.
ICTRP 2011
- World Health Organization. International Clinical Trials Registry Platform (ICTRP), 2011. http://www.who.int/ictrp/en/.
Ingraham 2008
- Ingraham BA, Bragdon B, Nohe A. Molecular basis of the potential of vitamin D to prevent cancer. Current Medical Research and Opinion 2008;24(1):139‐49. [DOI] [PubMed] [Google Scholar]
Ioannidis 2006
- Ioannidis JP, Trikalinos TA, Zintzaras E. Extreme between‐study homogeneity in meta‐analyses could offer useful insights. Journal of Clinical Epidemiology 2006;59(10):1023‐32. [DOI] [PubMed] [Google Scholar]
IOM 2011
- Institute of Medicine. Dietary reference intakes for calcium and vitamin D. Washington, DC: The National Academies Press, 2011. [PubMed] [Google Scholar]
Ishida 2004
- Ishida Y, Kawai S. Comparative efficacy of hormone replacement therapy, etidronate, calcitonin, alfacalcidol, and vitamin K in postmenopausal women with osteoporosis: The Yamaguchi Osteoporosis Prevention Study. American Journal of Medicine 2004;117(8):549‐55. [DOI] [PubMed] [Google Scholar]
Islam 2010
- Islam MZ, Shamim AA, Viljakainen HT, Akhtaruzzaman M, Jehan AH, Khan HU. Effect of vitamin D, calcium and multiple micronutrient supplementation on vitamin D and bone status in Bangladeshi premenopausal garment factory workers with hypovitaminosis D: a double‐blinded, randomised, placebo‐controlled 1‐year intervention. British Journal of Nutrition 2010;104(2):241‐7. [DOI] [PubMed] [Google Scholar]
Izaks 2007
- Izaks GJ. Fracture prevention with vitamin D supplementation: considering the inconsistent results. BMC Musculoskeletal Disorders 2007; Vol. 8, issue 26. [DOI] [PMC free article] [PubMed]
Jackson 2007
- Jackson C, Gaugris S, Sen SS, Hosking D. The effect of cholecalciferol (vitamin D3) on the risk of fall and fracture: a meta‐analysis. The Quarterly Journal of Medicine 2007;100:185‐92. [DOI] [PubMed] [Google Scholar]
Jensen 1982a
- Jensen GF, Christiansen C, Transb›l. 1,25(OH)2D3 and renal function. A controlled clinical study in normal elderly women. Acta Medica Scandinavica 1982;211(1‐2):51‐4. [PubMed] [Google Scholar]
Jensen 1982b
- Jensen GF, Christiansen C, Transb›l I. Treatment of post menopausal osteoporosis. A controlled therapeutic trial comparing oestrogen/gestagen, 1,25‐dihydroxy‐vitamin D3 and calcium. Clinical Endocrinology 1982;16(5):515‐24. [DOI] [PubMed] [Google Scholar]
Jensen 1985
- Jensen GF, Meinecke B, Boesen J, TransbÇ÷l I. Does 1,25(OH)2D3 accelerate spinal bone loss? A controlled therapeutic trial in 70‐year‐old women. Clinical Orthopaedics and Related Research 1985;192:215‐21. [PubMed] [Google Scholar]
Johansson 2012
- Johansson H, Odén A, Kanis J, McCloskey E, Lorentzon M, Ljunggren Ö, et al. Low serum vitamin D is associated with increased mortality in elderly men: MrOS Sweden. Osteoporosis International 2012;23(3):991‐9. [DOI] [PubMed] [Google Scholar]
Johnson 1980
- Johnson KR, Jobber J, Stonawski BJ. Prophylactic vitamin D in the elderly. Age and Ageing 1980;9:121‐7. [DOI] [PubMed] [Google Scholar]
Jorde 2008
- Jorde R, Sneve M, Figenschau Y, Svartberg J, Waterloo K. Effects of vitamin D supplementation on symptoms of depression in overweight and obese subjects: randomized double blind trial. Journal of Internal Medicine 2008;264(6):599‐609. [DOI] [PubMed] [Google Scholar]
Jorde 2009
- Jorde R, Figenschau Y. Supplementation with cholecalciferol does not improve glycaemic control in diabetic subjects with normal serum 25‐hydroxyvitamin D levels. European Journal of Nutrition 2009;48(6):349‐54. [DOI] [PubMed] [Google Scholar]
Jorde 2010a
- Jorde R, Sneve M, Torjesen P, Figenschau Y, Hansen JB. Parameters of the thrombogram are associated with serum 25‐hydroxyvitamin D levels at baseline, but not affected during supplementation with vitamin D. Thrombosis Research 2010;125(5):e210‐3. [DOI] [PubMed] [Google Scholar]
Jorde 2010b
- Jorde R, Sneve M, Torjesen P, Figenschau Y. No improvement in cardiovascular risk factors in overweight and obese subjects after supplementation with vitamin D3 for 1 year. Journal of Internal Medicine 2010;267(5):462‐72. [DOI] [PubMed] [Google Scholar]
Jorde 2010c
- Jorde R, Sneve M, Emaus N, Figenschau Y, Grimnes G. Cross‐sectional and longitudinal relation between serum 25‐hydroxyvitamin D and body mass index: the Troms study. European Journal of Nutrition 2010;49(7):401‐7. [DOI] [PubMed] [Google Scholar]
Jorde 2010d
- Jorde R, Sneve M, Torjesen PA, Figenschau Y, Goransson LG, Omdal R. No effect of supplementation with cholecalciferol on cytokines and markers of inflammation in overweight and obese subjects. Cytokines 2010;50(2):175‐80. [DOI] [PubMed] [Google Scholar]
Jorde 2010e
- Jorde R, Sneve M, Torjesen PA, Figenschau Y, Hansen JB, Grimnes G. No significant effect on bone mineral density by high doses of vitamin D3 given to overweight subjects for one year. Nutrition Journal 2010;9(1):1‐1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Judd 2009
- Judd SE, Tangpricha V. Vitamin D deficiency and risk for cardiovascular disease. American Journal of Medical Sciences 2009;338(1):40‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Keane 1998
- Keane EM, Healy M, O'Moore R, Coakley D, Walsh JB. Vitamin D‐fortified liquid milk: benefits for the elderly community‐based population. Calcified Tissue International 1998;62(4):300‐2. [DOI] [PubMed] [Google Scholar]
Kenny 2003
- Kenny AM, Biskup B, Robbins B, Marcella G, Burleson JA. Effects of vitamin D supplementation on strength, physical function, and health perception in older, community‐dwelling men. Journal of the American Geriatrics Society 2003;51(12):1762‐7. [DOI] [PubMed] [Google Scholar]
Keus 2009
- Keus F, Wetterslev J, Gluud C, Gooszen HG, Laarhoven CJ. Robustness assessments are needed to reduce bias in meta‐analyses that include zero‐event randomized trials. American Journal of Gastroenterology 2009;104(3):546‐51. [DOI] [PubMed] [Google Scholar]
Khaw 1994
- Khaw KT, Scragg R, Murphy S. Single‐dose cholecalciferol suppresses the winter increase in parathyroid hormone concentrations in healthy older men and women: a randomized trial. American Journal of Clinical Nutrition 1994;59(5):1040‐4. [DOI] [PubMed] [Google Scholar]
Kimball 2011
- Kimball S, Vieth R, Dosch HM, Bar‐Or A, Cheung R, Gagne D, et al. Cholecalciferol plus calcium suppresses abnormal PBMC reactivity in patients with multiple sclerosis. Journal of Clinical Endocrinology and Metabolism 2011;96(9):2826‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kjaergard 2001
- Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta‐analyses. Annals of Internal Medicine 2001;135(11):982‐9. [DOI] [PubMed] [Google Scholar]
Kovesdy 2013
- Kovesdy Csaba P, Quarles Leigh Darryl. Fibroblast growth factor‐23: what we know, what we don't know, and what we need to know. Nephrology Dialysis Transplantation 2013;Apr 25:Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kruger 2010
- Kruger MC, Schollum LM, Kuhn‐Sherlock B, Hestiantoro A, Wijanto P, Li‐Yu J, et al. The effect of a fortified milk drink on vitamin D status and bone turnover in post‐menopausal women from South East Asia. Bone 2010;46(3):759‐67. [DOI] [PubMed] [Google Scholar]
Kuwabara 2009
- Kuwabara A, Tsugawa N, Tanaka K, Fujii M, Kawai N, Mukae S, et al. Improvement of vitamin D status in Japanese institutionalized elderly by supplementation with 800 IU of vitamin D(3). Journal of Nutritional Science and Vitaminology 2009;55(6):453‐8. [DOI] [PubMed] [Google Scholar]
Laaksi 2010
- Laaksi I, Ruohola JP, Mattila V, Auvinen A, Ylikomi T, Pihlajamaki H. Vitamin D supplementation for the prevention of acute respiratory tract infection: a randomized, double‐blinded trial among young Finnish men. Journal of Infectious Diseases 2010;202(5):809‐14. [DOI] [PubMed] [Google Scholar]
Lamberg‐Allardt 2006
- Lamberg‐Allardt C. Vitamin D in foods and as supplements. Progress in Biophysics and Molecular Biology 2006;92:33‐8. [DOI] [PubMed] [Google Scholar]
Lambrinoudaki 2000
- Lambrinoudaki I, Chan DT, Lau CS, Wong RW, Yeung SS, Kung AW. Effect of calcitriol on bone mineral density in premenopausal Chinese women taking chronic steroid therapy. A randomized, double blind, placebo controlled study. Journal of Rheumatology 2000;27(7):1759‐65. [PubMed] [Google Scholar]
Lappe 2008
- Lappe J, Cullen D, Haynatzki G, Recker R, Ahlf R, Thompson K. Calcium and vitamin d supplementation decreases incidence of stress fractures in female navy recruits. Journal of Bone and Mineral Research 2008;23(5):741‐9. [DOI] [PubMed] [Google Scholar]
Latham 2003b
- Latham NK, Anderson CS, Reid IR. Effects of vitamin D supplementation on strength, physical performance, and falls in older persons: a systematic review. Journal of the American Geriatrics Society 2003;51:1219‐26. [DOI] [PubMed] [Google Scholar]
Lau 2006
- Lau J, Ioannidis JPA, Terrin N, Schmid CH, Olkin I. The case of the misleading funnel plot. BMJ 2006;333:597‐600. [DOI] [PMC free article] [PubMed] [Google Scholar]
Li‐Ng 2009
- Li‐Ng M, Aloia JF, Pollack S, Cunha BA, Mikhail M, Yeh J, et al. A randomized controlled trial of vitamin D3 supplementation for the prevention of symptomatic upper respiratory tract infections. Epidemiology and Infection 2009;137(10):1396‐404. [DOI] [PubMed] [Google Scholar]
Lilliu 2003
- Lilliu H, Pamphile R, Chapuy MC, Schulten J, Arlot M, Meunier PJ. Calcium‐vitamin D3 supplementation is cost‐effective in hip fractures prevention. Maturitas 2003;44(4):299‐305. [DOI] [PubMed] [Google Scholar]
Lind 1989
- Lind L, Wengle B, Wide L, Ljunghall S. Reduction of blood pressure during long‐term treatment with active vitamin D (alphacalcidol) is dependent on plasma renin activity and calcium status. A double‐blind, placebo‐controlled study. American Journal of Hypertension 1989;2(1):20‐5. [DOI] [PubMed] [Google Scholar]
Lind 1992
- Lind L, Wengle B, Sorensen OH, Ljunghall S. Serum levels of 1,25‐(OH)2‐vitamin D are not altered by long‐term supplementation with alphacalcidol (1‐OH‐vitamin D3). A double‐blind, placebo‐controlled study. Experimental and Clinical Endocrinology 1992;99(3):151‐3. [DOI] [PubMed] [Google Scholar]
Lips 1988
- Lips P, Wiersinga A, Ginkel FC, Jongen MJ, Netelenbos JC, Hackeng WH, et al. The effect of vitamin D supplementation on vitamin D status and parathyroid function in elderly subjects. Journal of Clinical Endocrinology and Metabolism 1988;67(4):644‐50. [DOI] [PubMed] [Google Scholar]
Lips 1999
- Lips P, Chapuy MC, Dawson‐Hughes B, Pols HA, Holick MF. An international comparison of serum 25‐hydroxyvitamin D measurements. Osteoporosis International 1999;9(5):394‐7. [DOI] [PubMed] [Google Scholar]
Lips 2004
- Lips P. Which circulating level of 25‐hydroxyvitamin D is appropriate. Journal of Steroid Biochemistry and Molecular Biology 2004;89‐90(1‐5):611‐4. [DOI] [PubMed] [Google Scholar]
Lips 2006
- Lips P. Vitamin D physiology. Progress in Biophysics and Molecular Biology 2006;92(1):4‐8. [DOI] [PubMed] [Google Scholar]
Ljunghall 1987
- Ljunghall S, Lind L, Lithell H, Skarfors E, Selinus I, Sorensen OH, et al. Treatment with one‐alpha‐hydroxycholecalciferol in middle‐aged men with impaired glucose tolerance—a prospective randomized double‐blind study. Acta Medica Scandinavica 1987;222(4):361‐7. [DOI] [PubMed] [Google Scholar]
Lundh 2012
- Lundh A, Sismondo S, Lexchin J, Busuioc OA, Bero L. Industry sponsorship and research outcome. Cochrane Database of Systematic Reviews 2012, Issue 12. [DOI: 10.1002/14651858.MR000033.pub2] [DOI] [PubMed] [Google Scholar]
Major 2007
- Major GC, Alarie F, Dore J, Phouttama S, Tremblay A. Supplementation with calcium + vitamin D enhances the beneficial effect of weight loss on plasma lipid and lipoprotein concentrations. American Journal of Clinical Nutrition 2007;85(1):54‐9. [DOI] [PubMed] [Google Scholar]
Major 2009
- Major GC, Alarie FP, Dore J, Tremblay A. Calcium plus vitamin D supplementation and fat mass loss in female very low‐calcium consumers: potential link with a calcium‐specific appetite control. British Journal of Nutrition 2009;101(5):659‐63. [DOI] [PubMed] [Google Scholar]
Maki 2011
- Maki KC, Rubin MR, Wong LG, McManus JF, Jensen CD, Lawless A. Effects of vitamin D supplementation on 25‐hydroxyvitamin D, high‐density lipoprotein cholesterol, and other cardiovascular disease risk markers in subjects with elevated waist circumference. International Journal of Food Sciences and Nutrition 2011;62(4):318‐27. [DOI] [PubMed] [Google Scholar]
Malhotra 2009
- Malhotra N, Mithal A, Gupta S, Shukla M, Godbole M. Effect of vitamin D supplementation on bone health parameters of healthy young Indian women. Archives of Osteoporosis 2009;4(1‐2):47‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Marshall 2008
- Marshall TG. Vitamin D discovery outpaces FDA decision making. Bioessays 2008;30(2):173‐82. [DOI] [PubMed] [Google Scholar]
Martin‐Bautista 2010
- Martin‐Bautista E, Munoz‐Torres M, Fonolla J, Quesada M, Poyatos A, Lopez‐Huertas E. Improvement of bone formation biomarkers after 1‐year consumption with milk fortified with eicosapentaenoic acid, docosahexaenoic acid, oleic acid, and selected vitamins. Nutrition Research 2010;30(5):320‐6. [DOI] [PubMed] [Google Scholar]
Marx 1989
- Marx SJ, Jones G, Weinstein RS, Chrousos GP, Renquist DM. Differences in mineral metabolism among nonhuman primates receiving diets with only vitamin D3 or only vitamin D2. Journal of Clinical Endocrinology and Metabolism 1989;69(6):1282‐90. [DOI] [PubMed] [Google Scholar]
McAlister 2003
- McAlister FA, Straus SE, Sackett DL, Altman DG. Analysis and reporting of factorial trials—A systematic review. JAMA 2003;289:2545‐53. [DOI] [PubMed] [Google Scholar]
Melamed 2008
- Melamed ML, Michos ED, Post W, Astor B. 25‐Hydroxyvitamin D levels and the risk of mortality in the general population. Archives of Internal Medicine 2008;168(15):1629‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Menczel 1994
- Menczel J, Foldes J, Steinberg R, Leichter I, Shalita B, Bdolah‐Abram T, et al. Alfacalcidol (alpha D3) and calcium in osteoporosis. Clinical Orthopaedics and Related Research 1994;300:241‐7. [PubMed] [Google Scholar]
Michaëlsson 2010
- Michaëlsson K, Baron JA, Snellman G, Gedeborg R, Byberg L, Sundström J, et al. Plasma vitamin D and mortality in older men: a community‐based prospective cohort study. American Journal of Clinical Nutrition 2010;92(4):841‐8. [DOI] [PubMed] [Google Scholar]
Michos 2008
- Michos ED, Melamed ML. Vitamin D and cardiovascular disease risk. Current Opinion in Clinical Nutrition and Metabolic Care 2008;11(1):7‐12. [DOI] [PubMed] [Google Scholar]
Mistretta 2008
- Mistretta VI, Delanaye P, Chapelle JP, Souberbielle JC, Cavalier E. Vitamin D2 or vitamin D3 [Vitamine D2 ou vitamine D3]. La Revue de Medicine Interne 2008;29(10):815‐20. [DOI] [PubMed] [Google Scholar]
Mithal 2009
- Mithal A, Wahl DA, Bonjour JP, Burckhardt P, Dawson‐Hughes B, Eisman JA, et al. Global vitamin D status and determinants of hypovitaminosis D. Osteoporosis International 2009;20(11):1807‐20. [DOI] [PubMed] [Google Scholar]
Mitri 2011
- Mitri J, Dawson‐Hughes B, Hu FB, Pittas AG. Effects of vitamin D and calcium supplementation on pancreatic ×ý cell function, insulin sensitivity, and glycemia in adults at high risk of diabetes: the Calcium and Vitamin D for Diabetes Mellitus (CaDDM) randomized controlled trial. American Journal of Clinical Nutrition 2011;94(2):486‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moher 1998
- Moher D, Pham B, Jones A, Cook DJ, Jadad A, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analysis. Lancet 1998;352(9128):609‐13. [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. Preferred reporting items for systematic reviews and meta‐analyses: The PRISMA Statement. PLoS Medicine 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moreira‐Pfrimer 2009
- Moreira‐Pfrimer LD, Pedrosa MA, Teixeira L, Lazaretti‐Castro M. Treatment of vitamin D deficiency increases lower limb muscle strength in institutionalized older people independently of regular physical activity: a randomized double‐blind controlled trial. Annals of Nutrition and Metabolism 2009;54(4):291‐300. [DOI] [PubMed] [Google Scholar]
Moyer 2013
- Moyer Virginia A. Vitamin D and calcium supplementation to prevent fractures in adults: U.S. Preventive Services Task Force recommendation statement. Annals of Internal Medicine 2013;158(9):691‐6. [DOI] [PubMed] [Google Scholar]
Myung 2013
- Myung SK, Ju W, Cho B, Oh SW, Park SM, Koo BK, et al. Efficacy of vitamin and antioxidant supplements in prevention of cardiovascular disease: systematic review and meta‐analysis of randomised controlled trials. BMJ 2013;346:f10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nagpal 2009
- Nagpal J, Pande JN, Bhartia A. A double‐blind, randomized, placebo‐controlled trial of the short‐term effect of vitamin D3 supplementation on insulin sensitivity in apparently healthy, middle‐aged, centrally obese men. Diabetic Medicine 2009;26(1):19‐27. [DOI] [PubMed] [Google Scholar]
Nelson 2009
- Nelson ML, Blum JM, Hollis BW, Rosen C, Sullivan SS. Supplements of 20 microg/d cholecalciferol optimized serum 25‐hydroxyvitamin D concentrations in 80% of premenopausal women in winter. Journal of Nutrition 2009;139(3):540‐6. [DOI] [PubMed] [Google Scholar]
Nnoaham 2008
- Nnoaham KE, Clarke A. Low serum vitamin D levels and tuberculosis: a systematic review and meta‐analysis. International Journal of Epidemiology 2008;37:113‐9. [DOI] [PubMed] [Google Scholar]
Nordin 1985
- Nordin BE, Baker MR, Horsman A, Peacock M. A prospective trial of the effect of vitamin D supplementation on metacarpal bone loss in elderly women. American Journal of Clinical Nutrition 1985;42(3):470‐4. [DOI] [PubMed] [Google Scholar]
Norman 2008
- Norman AW. From vitamin D to hormone D: fundamentals of the vitamin D endocrine system essential for good health. American Journal of Clinical Nutrition 2008;88(2):491S‐9S. [DOI] [PubMed] [Google Scholar]
O'Donnell 2008
- O'Donnell S, Moher D, Thomas K, Hanley DA, Cranney A. Systematic review of the benefits and harms of calcitriol and alfacalcidol for fractures and falls. Journal of Bone and Mineral Metabolism 2008;26(6):531‐42. [DOI] [PubMed] [Google Scholar]
Ongphiphadhanakul 2000
- Ongphiphadhanakul B, Piaseu N, Tung SS, Chailurkit L, Rajatanavin R. Prevention of postmenopausal bone loss by low and conventional doses of calcitriol or conjugated equine estrogen. Maturitas 2000;34(2):179‐84. [DOI] [PubMed] [Google Scholar]
Orimo 1994
- Orimo H, Shiraki M, Hayashi Y, Hoshino T, Onaya T, Miyazaki S, et al. Effects of 1 alpha‐hydroxyvitamin D3 on lumbar bone mineral density and vertebral fractures in patients with postmenopausal osteoporosis. Calcified Tissue International 1994;54(5):370‐6. [DOI] [PubMed] [Google Scholar]
Orwoll 1988
- Orwoll ES, Weigel RM, Oviatt SK, McClung MR, Deftos LJ. Calcium and cholecalciferol: effects of small supplements in normal men. American Journal of Clinical Nutrition 1988;48(1):127‐30. [DOI] [PubMed] [Google Scholar]
Orwoll 1990
- Orwoll ES, Oviatt S. Relationship of mineral metabolism and long‐term calcium and cholecalciferol supplementation to blood pressure in normotensive men. American Journal of Clinical Nutrition 1990;52(4):717‐21. [DOI] [PubMed] [Google Scholar]
Orwoll 1994
- Orwoll E, Riddle M, Prince M. Effects of vitamin D on insulin and glucagon secretion in non‐insulin‐dependent diabetes mellitus. American Journal of Clinical Nutrition 1994;59(5):1083‐7. [DOI] [PubMed] [Google Scholar]
Pan 2010
- Pan L, Matloob AF, Du J, Pan H, Dong Z, Zhao J, et al. Vitamin D stimulates apoptosis in gastric cancer cells in synergy with trichostatin A /sodium butyrate‐induced and 5‐aza‐2'‐deoxycytidine‐induced PTEN upregulation. FEBS J 2010;277(4):989‐99. [DOI] [PubMed] [Google Scholar]
Patel 2001
- Patel R, Collins D, Bullock S, Swaminathan R, Blake GM, Fogelman I. The effect of season and vitamin D supplementation on bone mineral density in healthy women: a double‐masked crossover study. Osteoporosis International 2001;12(4):319‐25. [DOI] [PubMed] [Google Scholar]
Patel 2012
- Patel VB, Vacek JL, Graves L, Bhattacharya RK. Calcium effects on vascular endpoints. Nutrition and Metabolism 2012;9(1):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Peacock 2000
- Peacock M, Liu G, Carey M, McClintock R, Ambrosius W, Hui S, et al. Effect of calcium or 25OH vitamin D3 dietary supplementation on bone loss at the hip in men and women over the age of 60. Journal of Clinical Endocrinology and Metabolism 2000;85(9):3011‐9. [DOI] [PubMed] [Google Scholar]
Pfeifer 2000
- Pfeifer M, Begerow B, Minne HW, Abrams C, Nachtigall D, Hansen C. Effects of a short‐term vitamin D and calcium supplementation on body sway and secondary hyperparathyroidism in elderly women. Journal of Bone and Mineral Research 2000;15(6):1113‐8. [DOI] [PubMed] [Google Scholar]
Pfeifer 2001
- Pfeifer M, Begerow B, Minne HW, Nachtigall D, Hansen C. Effects of a short‐term vitamin D(3) and calcium supplementation on blood pressure and parathyroid hormone levels in elderly women. Journal of Clinical Endocrinology and Metabolism 2001;86(4):1633‐7. [DOI] [PubMed] [Google Scholar]
Pfeifer 2009
- Pfeifer M, Begerow B, Minne HW, Suppan K, Fahrleitner‐Pammer A, Dobnig H. Effects of a long‐term vitamin D and calcium supplementation on falls and parameters of muscle function in community‐dwelling older individuals. Osteoporosis International 2009;20(2):315‐22. [DOI] [PubMed] [Google Scholar]
Pignotti 2010
- Pignotti GA, Genaro PS, Pinheiro MM, Szejnfeld VL, Martini LA. Is a lower dose of vitamin D supplementation enough to increase 25(OH)D status in a sunny country. European Journal of Nutrition 2010;49(5):277‐83. [DOI] [PubMed] [Google Scholar]
Pilz 2009a
- Pilz S, Dobnig H, Nijpels G, Heine RJ, Stehouwer CD, Snijder MB, et al. Vitamin D and mortality in older men and women. Clinical Endocrinology 2009;71(5):666‐72. [DOI] [PubMed] [Google Scholar]
Pilz 2009b
- Pilz S, Tomaschitz A, Obermayer‐Pietsch B, Dobnig H, Pieber TR. Epidemiology of vitamin D insufficiency and cancer mortality. Anticancer Research 2009;29(9):3699‐704. [PubMed] [Google Scholar]
Pilz 2011
- Pilz S, Frisch S, Koertke H, Kuhn J, Dreier J, Obermayer‐Pietsch B, et al. Effect of vitamin D supplementation on testosterone levels in men. Hormone and Metabolic Research 2011;43(3):223‐5. [DOI] [PubMed] [Google Scholar]
Pilz 2012
- Pilz S, Dobnig H, Tomaschitz A, Kienreich K, Meinitzer A, Friedl C, et al. Low 25‐hydroxyvitamin D is associated with increased mortality in female nursing home residents. Journal of Clinical Endocrinology and Metabolism 2012;97(4):E653‐7. [DOI] [PubMed] [Google Scholar]
Pittas 2007a
- Pittas AG, Lau J, Hu FB, Dawson‐Hughes B. The role of vitamin D and calcium in type 2 diabetes. A systematic review and meta‐analysis. The Journal of Clinical Endocrinology and Metabolism 2007;92(6):2017‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pittas 2010
- Pittas AG, Chung M, Trikalinos T, Mitri J, Brendel M, Patel K, et al. Systematic review: vitamin D and cardiometabolic outcomes. Annals of Internal Medicine 2010;152(5):307‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pocock 2004
- Pocock SJ. Clinical Trials: A Practical Approach. New York: John Wiley & Sons, 2004. [Google Scholar]
Reid 2013
- Ried IR, Bolland MJ, Grey A. Effects of vitamin D supplements on bone mineral density: a systematic review and meta‐analysis. The Lancet 2013;(in press):doi:10.1016/S0140‐6736(13)61647‐5. [DOI] [PubMed] [Google Scholar]
Rejnmark 2012
- Rejnmark L, Avenell A, Masud T, Anderson F, Meyer HE, Sanders KM, et al. Vitamin D with calcium reduces mortality: patient level pooled analysis of 70,528 patients from eight major vitamin D trials. Journal of Clinical Endocrinology and Metabolism 2012;May 17:[Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2012 [Computer program]
- Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Richy 2005
- Richy F, E.Schacht E, Bruyere O, Ethgen O, Gourlay M, Reginster JY. Vitamin D analogs versus native vitamin D in preventing bone loss and osteoporosis‐related fractures: a comparative meta‐analysis. Calcified Tissue International 2005;76:176‐86. [DOI] [PubMed] [Google Scholar]
Rosen 2011
- Rosen CJ. Clinical practice. Vitamin D insufficiency. New England Journal of Medicine 2011;364(3):248‐54. [DOI] [PubMed] [Google Scholar]
Rosen 2013
- Rosen CJ. Vitamin D supplementation: bones of contention. The Lancet 2013;(in press):doi:10.1016/S0140‐6736(13)61721‐3. [DOI] [PubMed] [Google Scholar]
Sanders 2013
- Sanders KM, Nicholson GC, Ebeling PR. Is high dose vitamin D harmful?. Calcified Tissue International 2013;92(2):191‐206. [DOI] [PubMed] [Google Scholar]
Savovic 2012
- Savović J, Jones H, Altman D, Harris R, Jűni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomised controlled trials: combined analysis of meta‐epidemiological studies. Health Technology Assessment 2012;16(35):1‐82. [DOI] [PubMed] [Google Scholar]
Schaafsma 2000
- Schaafsma A, Muskiet FA, Storm H, Hofstede GJ, Pakan I, Veer E. Vitamin D(3) and vitamin K(1) supplementation of Dutch postmenopausal women with normal and low bone mineral densities: effects on serum 25‐hydroxyvitamin D and carboxylated osteocalcin. European Journal of Clinical Nutrition 2000;54(8):626‐31. [DOI] [PubMed] [Google Scholar]
Schottker 2013
- Schottker B, Haug U, Schomburg L, Kohrle J, Perna L, Muller H, et al. Strong associations of 25‐hydroxyvitamin D concentrations with all‐cause, cardiovascular, cancer, and respiratory disease mortality in a large cohort study. The American Journal of Clinical Nutrition 2013;97(4):782‐93. [DOI] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408‐12. [DOI] [PubMed] [Google Scholar]
Schwartz 2007
- Schwartz GG, Skinner HG. Vitamin D status and cancer: new insights. Current Opinion in Clinical Nutrition and Metabolic Care 2007;10(1):6‐11. [DOI] [PubMed] [Google Scholar]
Scragg 1995a
- Scragg R, Khaw KT, Murphy S. Life‐style factors associated with winter serum 25‐hydroxyvitamin D levels in elderly adults. Age and Ageing 1995;24(4):271‐5. [DOI] [PubMed] [Google Scholar]
Scragg 1995b
- Scragg R, Khaw KT, Murphy S. Effect of winter oral vitamin D3 supplementation on cardiovascular risk factors in elderly adults. European Journal of Clinical Nutrition 1995;49(9):640‐6. [PubMed] [Google Scholar]
Scragg 2010
- Scragg RK, Camargo CA Jr, Simpson RU. Relation of serum 25‐hydroxyvitamin D to heart rate and cardiac work (from the National Health and Nutrition Examination Surveys). American Journal of Cardiology 2010;105(1):122‐8. [DOI] [PubMed] [Google Scholar]
Shiomi 1999a
- Shiomi S, Masaki K, Habu D, Takeda T, Nishiguchi S, Kuroki T, et al. Calcitriol for bone loss in patients with primary biliary cirrhosis. Journal of Gastroenterology 1999;34(2):241‐5. [DOI] [PubMed] [Google Scholar]
Shiomi 1999b
- Shiomi S, Masaki K, Habu D, Takeda T, Nishiguchi S, Kuroki T, et al. Calcitriol for bone disease in patients with cirrhosis of the liver. Journal of Gastroenterology and Hepatology 1999;14(6):547‐52. [DOI] [PubMed] [Google Scholar]
Shiraki 1985
- Shiraki M, Orimo H, Ito H, Akiguchi I, Nakao J, Takahashi R, et al. Long‐term treatment of postmenopausal osteoporosis with active vitamin D3, 1‐alpha‐hydroxycholecalciferol (1 alpha‐OHD3) and 1, 24‐dihydroxycholecalciferol (1, 24(OH)2D3). Endocrinologia Japonica 1985;32(2):305‐15. [DOI] [PubMed] [Google Scholar]
Shiraki 1996
- Shiraki M, Kushida K, Yamazaki K, Nagai T, Inoue T, Orimo H. Effects of 2 years treatment of osteoporosis with 1 alpha‐hydroxy vitamin D3 on bone mineral density and incidence of fracture: a placebo‐controlled, double‐blind prospective study. Endocrine Journal 1996;43(2):211‐20. [DOI] [PubMed] [Google Scholar]
Shiraki 2004
- Shiraki M, Fukuchi M, Kiriyama T, Okamoto S, Ueno T, Sakamoto H, et al. Alfacalcidol reduces accelerated bone turnover in elderly women with osteoporosis. Journal of Bone and Mineral Metabolism 2004;22(4):352‐9. [DOI] [PubMed] [Google Scholar]
Skaaby 2012
- Skaaby T, Lise Lotte Nystrup H, Charlotta P, Torben J, Thuensen BH, Mogens F, et al. Vitamin D status and cause‐specific mortality: a general population study. PLoS ONE 2012;7(12):e52423. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sneve 2008
- Sneve M, Figenschau Y, Jorde R. Supplementation with cholecalciferol does not result in weight reduction in overweight and obese subjects. European Journal of Endocrinology 2008;159(6):675‐84. [DOI] [PubMed] [Google Scholar]
Son 2001
- Son SM, Chun YM. Effect of oral therapy with alphacalcidol or calcium in Korean elderly women with osteopenia and low dietary calcium. Nutrition Research 2001;21:1347‐55. [Google Scholar]
Songpatanasilp 2009
- Songpatanasilp T, Chailurkit LO, Nichachotsalid A, Chantarasorn M. Combination of alfacalcidol with calcium can improve quadriceps muscle strength in elderly ambulatory Thai women who have hypovitaminosis D: a randomized controlled trial. Journal of the Medical Association of Thailand 2009;92(Suppl 5):S30‐41. [PubMed] [Google Scholar]
Sorva 1991
- Sorva A, Risteli J, Risteli L, Valimaki M, Tilvis R. Effects of vitamin D and calcium on markers of bone metabolism in geriatric patients with low serum 25‐hydroxyvitamin D levels. Calcified Tissue International 1991;49(Supp 1):S88‐9. [DOI] [PubMed] [Google Scholar]
Souberbielle 2010
- Souberbielle JC, Body JJ, Lappe JM, Plebani M, Shoenfeld Y, Wang TJ, et al. Vitamin D and musculoskeletal health, cardiovascular disease, autoimmunity and cancer: recommendations for clinical practice. Autoimmunity Reviews 2010;9(11):709‐15. [DOI] [PubMed] [Google Scholar]
Starfield 2008
- Starfield B, Hyde J, Gérvas J, Heath I. The concept of prevention: a good idea gone astray. Journal of Epidemiology and Community Health 2008;62(7):580‐3. [DOI] [PubMed] [Google Scholar]
Stechschulte 2009
- Stechschulte SA, Kirsner RS, Federman DG. Vitamin D: bone and beyond, rationale and recommendations for supplementation. American Journal of Medicine 2009;122(9):793‐802. [DOI] [PubMed] [Google Scholar]
Stolzenberg 2006
- Stolzenberg‐Solomon RZ, Vieth R, Azad A, Pietinen P, Taylor PR, Virtamo J, et al. A prospective nested case‐control study of vitamin D status and pancreatic cancer risk in male smokers. Cancer Research 2006;66(20):10213‐9. [DOI] [PubMed] [Google Scholar]
Sugden 2008
- Sugden JA, Davies JI, Witham MD, Morris AD, Struthers AD. Vitamin D improves endothelial function in patients with Type 2 diabetes mellitus and low vitamin D levels. Diabetic Medicine 2008;25(3):320‐5. [DOI] [PubMed] [Google Scholar]
Sweeting 2004
- Sweeting MJ, Sutton AJ, Lambert PC. What to add to nothing? Use and avoidance of continuity corrections in meta‐analyses of sparse data. Statistics in Medicine 2004;23:1351‐75. [DOI] [PubMed] [Google Scholar]
Tang 2007
- Tang BMP, Eslick GD, Nowson C, Smith C, Bensoussan A. Use of calcium or calcium in combination with vitamin D supplementation to prevent fractures and bone loss in people aged 50 years and older: a meta‐analysis. Lancet 2007;370:657‐66. [DOI] [PubMed] [Google Scholar]
Thorlund 2009
- Thorlund K, Devereaux PJ, Wetterslev J, Guyatt G, Ioannidis JP, Thabane L, et al. Can trial sequential monitoring boundaries reduce spurious inferences from meta‐analyses. International Journal of Epidemiology 2009;38(1):276‐86. [DOI] [PubMed] [Google Scholar]
Thorlund 2011a
- Thorlund K, Imberger G, Walsh M, Chu R, Gluud C, Wetterslev J, et al. The number of patients and events required to limit the risk of overestimation of intervention effects in meta‐analysis—a simulation study. PloS One 2011;6(10):e25491. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thorlund 2011b
- Thorlund K, Engstrøm J, Wetterslev J, Brok J, Imberger G, Gluud C. User Manual for Trial Sequential Analysis (TSA). Copenhagen Trial Unit, Centre for Clinical Intervention Research, Copenhagen, Denmark, 2011. www.ctu.dk/tsa.
Toner 2010
- Toner CD, Davis CD, Milner JA. The vitamin D and cancer conundrum: aiming at a moving target. Journal of the American Dietetic Association 2010;110(10):1492‐500. [DOI] [PubMed] [Google Scholar]
Tripkovic 2012
- Tripkovic L, Lambert H, Hart K, Smith CP, Bucca G, Penson S, et al. Comparison of vitamin D2 and vitamin D3 supplementation in raising serum 25‐hydroxyvitamin D status: a systematic review and meta‐analysis. American Journal of Clinical Nutrition 2012;95(6):1357‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
TSA 2011 [Computer program]
- Copenahgen Trial Unit, 2011. Trial Sequential Analysis, version 0.9 beta.
Urbain 2011
- Urbain P, Singler F, Ihorst G, Biesalski HK, Bertz H. Bioavailability of vitamin Dƒ'' from UV‐B‐irradiated button mushrooms in healthy adults deficient in serum 25‐hydroxyvitamin D: a randomized controlled trial. European Journal of Clinical Nutrition 2011;65(8):965‐71. [DOI] [PubMed] [Google Scholar]
Ushiroyama 1995
- Ushiroyama T, Okamura S, Ikeda A, Ueki M. Efficacy of ipriflavone and 1 alpha vitamin D therapy for the cessation of vertebral bone loss. International Journal of Gynaecology and Obstetrics 1995;48(3):283‐8. [DOI] [PubMed] [Google Scholar]
Ushiroyama 2001
- Ushiroyama T, Ikeda A, Sakai M, Higashiyama T, Ueki M. Effects of the combined use of calcitonin and 1 alpha‐hydroxycholecalciferol on vertebral bone loss and bone turnover in women with postmenopausal osteopenia and osteoporosis: a prospective study of long‐term and continuous administration with low dose calcitonin. Maturitas 2001;40(3):229‐38. [DOI] [PubMed] [Google Scholar]
Ushiroyama 2002
- Ushiroyama T, Ikeda A, Ueki M. Effect of continuous combined therapy with vitamin K(2) and vitamin D(3) on bone mineral density and coagulofibrinolysis function in postmenopausal women. Maturitas 2002;41(3):211‐21. [DOI] [PubMed] [Google Scholar]
Van Der Klis 1996
- Klis FR, Jonxis JH, Doormaal JJ, Sikkens P, Saleh AE, Muskiet FA. Changes in vitamin‐D metabolites and parathyroid hormone in plasma following cholecalciferol administration to pre‐ and postmenopausal women in the Netherlands in early spring and to postmenopausal women in Curacao. British Journal of Nutrition 1996;75(4):637‐46. [DOI] [PubMed] [Google Scholar]
Vieth 2006
- Vieth R. What is the optimal vitamin D status. Progress in Biophysics and Molecular Biology 2006;92(1):26‐32. [DOI] [PubMed] [Google Scholar]
Viljakainen 2006
- Viljakainen HT, Palssa A, Karkkainen M, Jakobsen J, Lamberg‐Allardt C. How much vitamin D3 do the elderly need. Journal of the American College of Nutrition 2006;25(5):429‐35. [DOI] [PubMed] [Google Scholar]
Viljakainen 2009
- Viljakainen HT, Vaisanen M, Kemi V, Rikkonen T, Kroger H, Laitinen EK, et al. Wintertime vitamin D supplementation inhibits seasonal variation of calcitropic hormones and maintains bone turnover in healthy men. Journal of Bone and Mineral Research 2009;24(2):346‐52. [DOI] [PubMed] [Google Scholar]
von Hurst 2008
- Hurst PR, Stonehouse W, Matthys C, Conlon C, Kruger MC, Coad J. Study protocol—metabolic syndrome, vitamin D and bone status in South Asian women living in Auckland, New Zealand: a randomised, placebo‐controlled, double‐blind vitamin D intervention. BMC Public Health 2008;8:267. [DOI] [PMC free article] [PubMed] [Google Scholar]
von Hurst 2009
- Hurst PR, Stonehouse W, Coad J. Vitamin D status and attitudes towards sun exposure in South Asian women living in Auckland, New Zealand. Public Health Nutrition 2009;13(4):531‐6. [DOI] [PubMed] [Google Scholar]
von Hurst 2010a
- Hurst PR, Stonehouse W, Kruger MC, Coad J. Vitamin D supplementation suppresses age‐induced bone turnover in older women who are vitamin D deficient. Journal of Steroid Biochemistry and Molecular Biology 2010;121:293‐6. [DOI] [PubMed] [Google Scholar]
von Hurst 2010b
- Hurst PR, Stonehouse W, Coad J. Vitamin D supplementation reduces insulin resistance in South Asian women living in New Zealand who are insulin resistant and vitamin D deficient—a randomised, placebo‐controlled trial. British Journal of Nutrition 2010;103(4):549‐55. [DOI] [PubMed] [Google Scholar]
Wang 2009
- Wang S. Epidemiology of vitamin D in health and disease. Nutrition Research Reviews 2009;22(2):188‐203. [DOI] [PubMed] [Google Scholar]
Wang 2010
- Wang L, Manson JE, Song Y, Sesso HD. Systematic review: vitamin D and calcium supplementation in prevention of cardiovascular events. Annals of Internal Medicine 2010;152(5):315‐23. [DOI] [PubMed] [Google Scholar]
Webb 2006
- Webb AR. Who, what, where and when‐influences on cutaneous vitamin D synthesis. Progress in Biophysics and Molecular Biology 2006;92(1):17‐25. [DOI] [PubMed] [Google Scholar]
Weisman 1986
- Weisman Y, Schen RJ, Eisenberg Z, Amarilio N, Graff E, Edelstein‐Singer M, et al. Single oral high‐dose vitamin D3 prophylaxis in the elderly. Journal of the American Geriatrics Society 1986;34(7):515‐8. [DOI] [PubMed] [Google Scholar]
Wesley Pike 2005
- Wesley Pike J, Shevde NK. The vitamin D receptor. In: Feldman D, Wesley Pike J, Glorieux FH editor(s). Vitamin D. 2nd Edition. Amsterdam, Boston, Heidelberg, London, New York, Oxford, Paris, San Diego, San Francisco, Singapore, Sydney, Tokyo: Elsevier Academic Press, 2005:167‐91. [Google Scholar]
Wetterslev 2008
- Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta‐analysis. Journal of Clinical Epidemiology 2008;61(1):64‐75. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wetterslev 2009
- Wetterslev J, Thorlund K, Brok J, Gluud C. Estimating required information size by quantifying diversity in random‐effects model meta‐analyses. BMC Medical Research Methodology 2009;9:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wicherts 2010
- Wicherts IS, Boeke AJ, Meer IM, Schoor NM, Knol DL, Lips P. Sunlight exposure or vitamin D supplementation for vitamin D‐deficient non‐western immigrants: a randomized clinical trial. Osteoporosis International 2011;22(3):873‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wood 2008
- Wood L, Egger M, Gluud LL, Schulz KF, Jüni P, Altman DG, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta‐epidemiological study. BMJ 2008;336(7644):601‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
World Health Organization 1950
- World Health Organization. Expert committee on biological standardization, report of the subcommittee on fat‐soluble vitamins. World Health Organization Technical Report Series 1950; Vol. 3:1‐9.
Yusupov 2010
- Yusupov E, Li‐Ng M, Pollack S, Yeh JK, Mikhail M, Aloia JF. Vitamin D and serum cytokines in a randomized clinical trial. International Journal of Endocrinology 2010;2010:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zittermann 2006
- Zittermann A. Vitamin D and disease prevention with special reference to cardiovascular disease. Progress in Biophysics and Molecular Biology 2006;92(1):39‐48. [DOI] [PubMed] [Google Scholar]
Zittermann 2009a
- Zittermann A, Gummert JF, Börgermann J. Vitamin D deficiency and mortality. Current Opinion in Clinical Nutrition and Metabolic Care 2009;12(6):634‐9. [DOI] [PubMed] [Google Scholar]
Zittermann 2009b
- Zittermann A, Frisch S, Berthold HK, Gotting C, Kuhn J, Kleesiek K, et al. Vitamin D supplementation enhances the beneficial effects of weight loss on cardiovascular disease risk markers. American Journal of Clinical Nutrition 2009;89(5):1321‐7. [DOI] [PubMed] [Google Scholar]
Zittermann 2010
- Zittermann A, Gummert JF. Sun, vitamin D, and cardiovascular disease. Journal of Photochemistry and Photobiology. B, Biology 2010;101(2):124‐9. [DOI] [PubMed] [Google Scholar]
Zittermann 2012
- Zittermann A, Iodice S, Pilz S, Grant WB, Bagnardi V, Gandini S. Vitamin D deficiency and mortality risk in the general population: a meta‐analysis of prospective cohort studies. American Journal of Clinical Nutrition 2012;95(1):91‐100. [DOI] [PubMed] [Google Scholar]
Zubillaga 2006
- Zubillaga P, Garrido A, Mugica I, Ansa J, Zabalza R, Emparanza JI. Effect of vitamin D and calcium supplementation on bone turnover in institutionalized adults with Down's Syndrome. European Journal of Clinical Nutrition 2006;60(5):605‐9. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Bjelakovic 2011
- Bjelakovic G, Gluud LL, Nikolova D, Whitfield K, Wetterslev J, Simonetti RG, et al. Vitamin D supplementation for prevention of mortality in adults. Cochrane Database of Systematic Reviews 2011, Issue 7. [DOI: 10.1002/14651858.CD007470.pub2] [DOI] [PubMed] [Google Scholar]


