Abstract
Amino acid metabolism plays a pivotal role in tumor microenvironment, influencing various aspects of cancer progression. The metabolic reprogramming of amino acids in tumor cells is intricately linked to protein synthesis, nucleotide synthesis, modulation of signaling pathways, regulation of tumor cell metabolism, maintenance of oxidative stress homeostasis, and epigenetic modifications. Furthermore, the dysregulation of amino acid metabolism also impacts tumor microenvironment and tumor immunity. Amino acids can act as signaling molecules that modulate immune cell function and immune tolerance within the tumor microenvironment, reshaping the anti-tumor immune response and promoting immune evasion by cancer cells. Moreover, amino acid metabolism can influence the behavior of stromal cells, such as cancer-associated fibroblasts, regulate ECM remodeling and promote angiogenesis, thereby facilitating tumor growth and metastasis. Understanding the intricate interplay between amino acid metabolism and the tumor microenvironment is of crucial significance. Expanding our knowledge of the multifaceted roles of amino acid metabolism in tumor microenvironment holds significant promise for the development of more effective cancer therapies aimed at disrupting the metabolic dependencies of cancer cells and modulating the tumor microenvironment to enhance anti-tumor immune responses and inhibit tumor progression.
Keywords: Amino acid metabolism, Tumor microenvironment, Redox, Epigenetic regulation, Immune tolerance, Angiogenesis
Introduction
The role of amino acid metabolism in the context of cancer
Metabolic reprogramming has emerged as a hallmark of cancer, playing a crucial role in sustaining the aberrant proliferation and providing survival advantage for tumor cells [1, 2]. Among the various metabolic alterations in cancer, the dysregulation of amino acid metabolism has gained increasing attention due to its profound impact on tumor development and progression [3, 4]. Amino acids, as the building blocks of proteins and vital signaling molecules, are intricately involved in various cellular processes, including energy production, redox homeostasis, nucleotide synthesis and epigenetic regulation [5–8]. In recent years, extensive research has elucidated the complex interplay between amino acid metabolic reprogramming and the tumor microenvironment, revealing its multifaceted effects on immune evasion, angiogenesis, and metastasis [9–12]. Furthermore, the dysregulated amino acid metabolism in cancer cells has been linked to therapeutic resistance, highlighting its clinical significance [13].
Importance of the tumor microenvironment in cancer progression and treatment
The tumor microenvironment (TME) is a complex and dynamic milieu consisting of various cell types, extracellular matrix components, and signaling molecules, which collectively shape the behavior of tumor cells [14, 15]. The reciprocal interactions between tumor cells and the TME play a critical role in cancer progression, metastasis, and therapeutic response [16–18]. Tumor cells can actively remodel the TME to create a supportive niche for their survival and growth, while TME also exerts profound influences on tumor cell behavior, including immune evasion and drug resistance [19–22]. Stromal cells within the TME, such as cancer-associated fibroblasts (CAFs), can promote tumor growth and invasion through the secretion of growth factors, cytokines, and extracellular matrix remodeling [23, 24]. TME also plays a critical role in angiogenesis, providing the necessary vasculature for tumor growth and metastasis [25]. Additionally, the immune components of the TME, such as T cells and myeloid-derived suppressor cells (MDSCs), can exert pro-tumorigenic and anti-tumorigenic effects, contributing to the subtle balance between immune surveillance and immune evasion in cancer. The TME is also recognized as a key determinant of the heterogeneous nature of tumors, contributing to the spatial and functional diversity within tumor. Moreover, emerging evidence suggests that the TME plays a significant part in modulating the response of tumors to various therapeutic interventions [22, 26]. Understanding the intricate interactions within the TME is crucial for unraveling the complexities of cancer biology and devising effective therapeutic strategies.
This review aims to provide a comprehensive overview of the important effects of amino acid metabolic reprogramming on the tumor microenvironment, shedding light on its implications for cancer biology and therapy.
The role of amino acids in tumor cells
Metabolic reprogramming of amino acids in tumor cells is a pivotal aspect of cancer biology. Tumor cells undergo alterations in amino acid metabolism to meet their increased demands for energy, nucleic acid synthesis, signaling pathway regulation, redox balance maintenance, and epigenetic modifications.
Amino acids as building blocks for protein synthesis
The equilibrium between protein synthesis and degradation within the organism is dynamically regulated under physiological conditions. The standard genetic code incorporates 22 natural amino acids into the process of protein synthesis, facilitating the accurate transmission of genetic information from DNA to mRNA and ultimately to protein, thereby enabling the fulfillment of biological functions [27, 28]. Protein synthesis necessitates both anabolic stimuli and amino acid constituents [29]. Amino acids and their corresponding mRNA codons selectively bind through chemical affinity and synthesize proteins. Leucine, a branched-chain amino acid, has the capacity to generate metabolic cues and effectively facilitate growth, with supplementary leucine intake being capable of stimulating protein synthesis in skeletal muscle [30, 31]. Furthermore, leucine collaborates with other anabolic signals, such as insulin, to augment protein synthesis in vivo [32, 33]. Free amino acids in the body can be incorporated into proteins as part of the proteome, or they can be oxidized into amino acid pools to produce carbon dioxide and nitrogen [33].
Amino acid metabolism is involved in nucleic acid synthesis
Amino acid metabolism is important for the formation of nucleotides, the building blocks of nucleic acids [34]. Amino acids serve as precursors for the synthesis of purine and pyrimidine nucleotides, which are essential for DNA and RNA formation. Specific amino acids contribute carbon, nitrogen, and functional groups necessary for the de novo synthesis and salvage pathways of nucleotide biosynthesis [35]. Furthermore, amino acids are involved in the regulation of nucleotide pools and the maintenance of nucleotide balance. Glutamate produces pyruvate by transaminase action, which can provide 5-phosphoribose for nucleotide synthesis by entering the pentose phosphate pathway. Alanine can act as a precursor to purine and pyrimidine nucleotides by producing pyruvate through combined deaminatio. Aspartic acid can be converted to aspartic acid diphosphate, which is an important precursor for adenosine and uracil synthesis, and glutamic acid can also participate in nucleic acid synthesis by converting to aspartic acid. Moreover, serine, glycine, histidine, etc. can be used as a carbon unit donor and participate in nucleotide synthesis [36]. Folate cycling based on serine, glycine and other alternative one carbon donors such as choline and histidine is strongly associated with metastasis and poor prognosis of malignant tumors such as breast cancer and colorectal cancer [37–39]. Downstream conversion of serine to carbon units and formate contributes to enhanced cell migration induced by purine depletion [40]. In addition, branched chain amino acids provide nitrogen atoms for nucleotides synthesis [41]. The sophisticated interplay between amino acid metabolism and nucleotide formation is fundamental for the accurate transmission of genetic information and the sustenance of cellular processes.
Interaction of amino acids with signaling molecules
Amino acids exert remarkable function as signaling molecules in cancer. The reprogrammed metabolism of amino acids in cancer cells can influence signaling pathways that promote tumor growth, invasion, and metastasis. Here are some hallmarks in which amino acids act as signaling molecules in cancer:
mTOR Signaling: The mammalian target of rapamycin (mTOR) is a central regulator for cell growth, proliferation, and metabolism [42]. Active mTOR promotes cellular anabolism and blocks catabolic processes. Activated mTORC1 drives tumor cell growth and drug resistance by regulating ribosome biogenesis, nucleotide, and protein synthesis. The metabolism of arginine and the methionine metabolite SAM can activate the mTORC1 signaling pathway by destabilizing the GATOR2 complex and GATOR1 complex [43, 44]. Asparagine can maintain the growth of tumor cells even when the respiratory electron transport chain (ETC) is inhibited by regulating the feedback loop of ATF4 and enhancing mTORC1 activity [45]. Amino acids, particularly leucine, isoleucine, and valine, can activate the mTOR signaling pathway [46–48]. Leucine can induce the phosphorylation of p70α through oxidative carboxylation as a mitochondrial fuel and as a mutational antagonist of glutamate dehydrogenase, suggesting that leucine regulates mTOR function by regulating mitochondrial function and AMPK [49]. Dysregulated mTOR signaling is commonly observed in cancer and is associated with increased protein synthesis and tumor progression [50–52]. However, under conditions of amino acid limitation, aberrant mTORC1 activation significantly impacts protein synthesis, leading to the induction of endoplasmic reticulum stress and cellular apoptosis [53].
AMPK Signaling: Adenosine monophosphate-activated protein kinase (AMPK) plays a pivotal role in regulating cellular energy balance. AMPK modulates metabolism by directly phosphorylating enzymes, or by influencing metabolic transcription through the phosphorylation of transcription factors and co-regulators [54, 55]. AMPK activation can be triggered by amino acids like alanine, aspartate, and cysteine [55, 56]. Phosphorylation of branched-chain ketoacid dehydrogenase kinase regulates EMT genes, contributing to colorectal cancer metastasis [57]. Furthermore, phosphorylation of GLS promotes tumorigenesis, while histone phosphorylation controls gene transcription activation and apoptosis in tumor cells [58, 59].
MYC Signaling: The oncogene MYC regulates the expression of multiple key metabolic enzymes. Glutamine serves as a source of energy and nitrogen for nucleotide and amino acid biosynthesis [60]. Glutamine metabolism influences signaling pathways such c-MYC, which are implicated in cancer cell growth and survival [61, 62]. Arginine metabolism and the production of polyamines, such as spermine and spermidine, are dysregulated in cancer [63]. Polyamines are important for cell proliferation, and altered arginine metabolism can affect signaling pathways involved in cancer cell growth and survival [64]. Many carcinogenic signals, including MYC, JUN, FOS, KRAS and BRAF, are crosstalk with polyamine metabolism, which maintains the continuous proliferation of tumor cells [65, 66]. Cancer cells often exhibit increased serine and glycine metabolism, which can contribute to nucleotide synthesis and maintenance of redox balance [67]. The serine-glycine one-carbon metabolism pathway can interact with oncogenic signaling and supports cancer cell proliferation. c-MYC plays a role in regulating serine biosynthesis, and P53 enables cancer cells to overcome cellular stress by surmounting serine deprivation [68, 69].
AHR Pathway: Aryl hydrocarbon receptor (AHR) is a ligand-activated transcription factor that regulates numerous critical cellular functions. Tryptophan metabolism through the kynurenine pathway can generate metabolites that modulate immune responses and promote tumor immune evasion [70–72]. Indoleamine 2,3-dioxygenase (IDO) and tryptophan 2,3-dioxygenase (TDO) are key enzymes involved in this pathway, whose activity can impact tumor progression [73]. Kynurenine promotes nuclear translocation of AHR in colorectal cancer, and blocking the interplay between AHR and Kynurenine with CH223191 reduces the proliferation of colon cancer cells [74].
Other signaling pathways are also included in the signaling alteration of amino acid metabolic reprogramming. For example, tumor cells can enhance tumor cell proliferation and suppress the intratumoral infiltration of CD8+ T cells by converting glutamine into γ-aminobutyric acid (GABA), thereby promoting Wnt/β-catenin signaling [75]. NOTCH1 has been found to promote glutaminolysis in acute lymphoblastic leukemia (T-ALL), whereas the inhibition of NOTCH in T-ALL cells significantly suppresses glutaminolysis and induces autophagy [76]. Additionally, the glutaminase C (GAC) inhibitor C968 has demonstrated efficacy in inhibiting the growth of erlotinib-resistant non-small cell lung cancer (NSCLC) cells, suggesting a potential influence of glutaminase (GLS) on the epidermal growth factor receptor (EGFR) signaling pathway [77, 78].
Overall, the dysregulated metabolism of amino acids in cancer cells can influence multiple signaling pathways that contribute to tumor development and progression (Table 1). Understanding the role of amino acids as signaling molecules in cancer can shed light for the development of novel targeted therapies.
Table 1.
Interaction of amino acids with signaling molecules
| Signaling Molecules | Description | Amino Acids and Metabolites | Reference |
|---|---|---|---|
| MTOR | mTOR is a central regulator for cell growth, proliferation, and metabolism. | Arginine | [43] |
| Methionine | [44] | ||
| Asparagine | [45] | ||
| BCAAs | [46–49] | ||
| Glutamine | [49] | ||
| AMPK | AMPK plays a pivotal role in regulating cellular energy balance. | Alanine | [55] |
| Aspartate | [56] | ||
| Cysteine | [56] | ||
| BCAAs | [57] | ||
| Glutamine | [58] | ||
| MYC | The oncogene MYC regulates the expression of multiple key metabolic enzymes. | Glutamine | [60–62] |
| Arginine | [63] | ||
| Serine | [67] | ||
| Glycine | [67] | ||
| Polyamine | [64–66] | ||
| AHR | AHR is a ligand-activated transcription factor that regulates numerous critical cellular functions. | Tryptophan | [70–72] |
| Kynurenine | [73, 74] | ||
| KRAS | KRAS is the best-known oncogene with the highest mutation rate among all cancers. | polyamine | [65, 66] |
| P53 | The tumor suppressor p53 halts the cell cycle and cellular repair or induces apoptosis. | Serine | [68, 69] |
| Wnt/β-Catenin | The Wnt signaling pathway is a highly conserved pathway throughout species evolution, playing a critical role in physiological processes such as organ formation and tissue regeneration. | GABA | [75] |
| NORTCH | The Notch signaling pathway influences cell differentiation, apoptosis, and proliferation. | Glutamine | [76] |
| EGFR | EGFR is a receptor for epidermal growth factor, linked to tumor cell proliferation, angiogenesis, tumor invasion, metastasis, and inhibition of apoptosis. | Glutamine | [77, 78] |
BCAA Branched chain amino acid, GABA γ-aminobutyric acid
Amino acids as regulators of cell metabolism
Amino acids can serve as precursors for the tricarboxylic acid (TCA) cycle via the gluconeogenesis pathway to fuel tumor cells during nutrient scarcity. Moreover, amino acids also play a regulatory role in tumor cell metabolism [79]. Branched-chain amino acids can impact systemic glucose metabolism independently of mTOR signaling by regulating insulin secretion and the sensitivity of peripheral tissues to insulin, thereby coordinating amino acid and carbohydrate metabolism in the organism [80]. Serine exerts regulatory control over the glycolytic rate of tumor cells through allosteric modulation of PKM2 [81]. Within the glycolytic pathway, pyruvate kinase (PK) facilitates the conversion of phosphoenolpyruvate (PEP) to pyruvate [82]. The accumulation of glycolytic intermediates serves as a reservoir for metabolic pathways such as the pentose phosphate pathway (PPP) and serine biosynthesis [83]. PKM2 activity diminishes with serine deprivation, leading to a shift of pyruvate towards the oxidative phosphorylation pathway in mitochondria to fuel cellular metabolism, while glycolytic metabolites redirect towards serine synthesis to sustain cellular proliferation [84]. Consequently, serine sustains aerobic glycolysis and lactic acid production, critical for the growth and survival of cancer cells.
Amino acid metabolism regulates redox homeostasis
The proliferation of cancer cells leads to the accumulation of reactive oxygen species (ROS), which can damage biological macromolecules and ultimately result in cell death. Throughout the stages of tumor development, the ability to counteract oxidative stress is essential for the survival of cancer cells. In this context, cancer cells depend on the synthesis of glutathione (GSH), a crucial antioxidant, which is achieved through the utilization of glutamate, glycine, and cysteine to regulate the cellular redox balance [85, 86]. Glutamine is catalytically converted to glutamate by glutaminase (GLS) upon transport into the cell by transporters, which further participating in the biosynthesis of GSH. Cancer cells inhibit cytochrome C-mediated apoptosis through regulation dependent on the production of intracellular glutathione [87]. Xc- cystine transporter is implicated in pancreatic cancer growth by enhancing glutathione biosynthesis [88]. Moreover, the induction of autophagy by PNO1 can upregulate the cystine/glutamate antiporter SLC7A11, leading to increased intracellular levels of cysteine and glutamate, thereby sustaining glutathione synthesis and protecting HCC cells from ferroptosis [89]. In the presence of oxidative stress, cysteine undergoes conversion to cystine, and tumor cells demonstrate vulnerability in cystine metabolism [90]. SLC7A11 overexpression at moderate levels has been observed to shield cancer cells from oxidative stress induced by H2O2 and prevent cell death [91]. Conversely, heightened SLC7A11 expression leads to excessive NADPH consumption and triggers disulfide stress, ultimately promoting cell death under H2O2 treatment. Furthermore, elevated SLC7A11 expression fosters primary tumor growth while impeding tumor metastasis [92]. Thus, the role of SLC7A11 is contingent on environmental factors [93].
In addition, Thioredoxin (Trx) and thioredoxin reductase (TrxR) also play crucial roles in maintaining the intracellular redox balance. Thioredoxin, characterized by the presence of two adjacent cysteine residues in the CXXC motif, can reversibly reduce disulfide bonds and improve the antioxidant capacity of tumor cells [94]. Trxs form disulfides upon oxidation, which are subsequently reduced in a reaction catalyzed by TrxR. This process involves the transfer of electrons from NADPH to restore the reduced dithiol form of Trxs. Reduction of Trx enables electron transfer to ribonucleotide reductase for DNA synthesis, or to peroxiredoxins for the elimination of peroxides [95].
NADPH is an essential reducing coenzyme that participates in various biochemical reactions within the cell, including the maintenance of the reduced states of GSH and thioredoxin. The cytoplasmic NADPH serves as a crucial substrate for the synthesis of fatty acids and reduced GSH. In proliferating cells, cytosolic NADPH is primarily derived from the oxidative pentose phosphate pathway. Glutamate is catalyzed by glutamate dehydrogenase GDH to convert to α-KG, which participates in the TCA cycle and further produces NADPH. Additionally, in serine-driven one-carbon metabolism, 5,10-methylenetetrahydrofolate can be oxidized to 10-formyltetrahydrofolate and coupled with NADP+ to generate NADPH, suggesting an alternative function of serine-driven one-carbon metabolism in promoting tumor cell proliferation beyond nucleotide synthesis [96]. Moreover, aspartate and proline biosynthesis play an important role in the maintenance of NAD+ consumption and regeneration [97, 98]. The transportation of glutamine-derived aspartate from mitochondria to the cytoplasm is essential for generating the metabolic precursor NADPH [99]. Proline is synthesized from glutamine by PYCR1 to support the oxidation of NADH [97, 99].
Amino acid metabolism can also modulate the intracellular redox equilibrium by furnishing intermediates for the TCA cycle. Amino acids can be catabolized into TCA cycle intermediates, such as citric acid and alpha-ketoglutaric acid, which can enter the cycle and partake in energy generation and biomolecular synthesis. Simultaneously, these intermediates can serve as substrates for redox reactions, influencing cellular redox balance [7]. Additionally, active oxygen species and active nitrogen directly affect redox sensitive amino acids (mainly cysteine, tryptophan, tyrosine, etc.) in proteins, thus affecting signal transduction [100, 101]. Under oxidative stress, proteins are vulnerable to oxidative damage, and tumor cells can use amino acid metabolism to provide amino acids required for protein repair or synthesis, thereby maintaining the normal structure and function of cellular proteins and reducing oxidative stress [102] (Fig. 1).
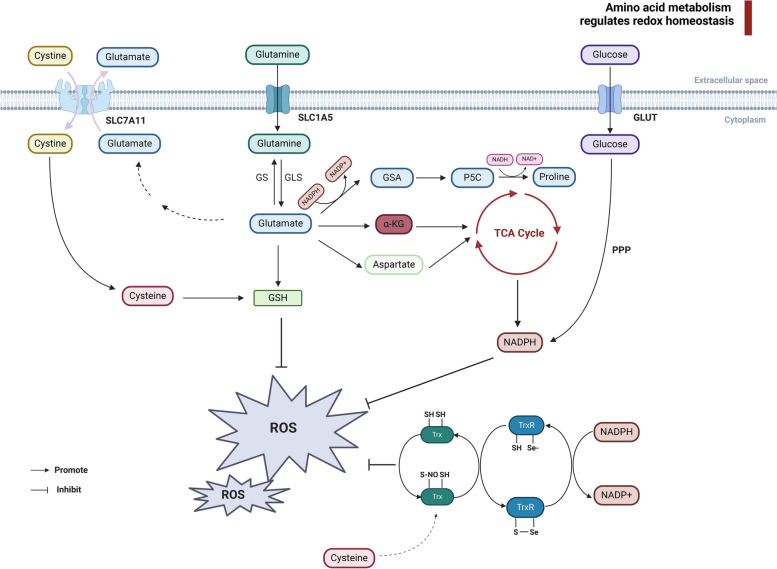
Fig. 1.
Amino acid metabolism maintains the redox homeostasis in tumor cells. The intracellular maintenance of redox balance primarily relies on glutathione, Thioredoxin and NADPH. Cysteine and glutamate are transported into and out of tumor cells via the bidirectional transporter SLC7A11. Cysteine is intracellularly converted to cystine, while glutamine enters cells through the transporter SLC1A5 and is converted to glutamate by GLS. Furthermore, glutamate can also be converted to glutamine through GS. Both glutamate and cysteine serve as precursors for GSH synthesis. Glutamate can further be metabolized into α-KG and aspartate, generating NADPH through the TCA cycle. Moreover, the PPP pathway of glucose metabolism also contributes to NADPH production. Additionally, glutamate can be transformed into GSA and P5C, eventually leading to proline synthesis, a process that involves the interconversion of NADPH and NADP+. Within thioredoxin, cysteine participates in antioxidant activities by serving as a substrate for protein reduction, facilitated by sulfhydryl-disulfide exchange reactions with TrxR and the NADPH system. SLC7A11 Solute carrier family 7 member 11, SLC1A5 Solute carrier family 1 member 5, NADPH nicotinamide adenine dinucleotide phosphate diaphorase, GSH glutathione, GLS glutaminase, GS glutamine synthetase, GSA glutamic semialdehyde, P5C pyrroline-5-carboxylate, TCA tricarboxylic acid cycle, PPP pentose phosphate pathway. Image created with BioRender.com
Amino acid metabolism regulates epigenetic modifications
Amino acid metabolism can support cancer cell survival and malignant behaviors by modulating epigenetic modifications (Fig. 2). Amino acid metabolism is intricately involved in the modulation of epigenetic processes, encompassing methylation, acetylation, succinylation, citrullination and B-Hydroxybutyrylation.
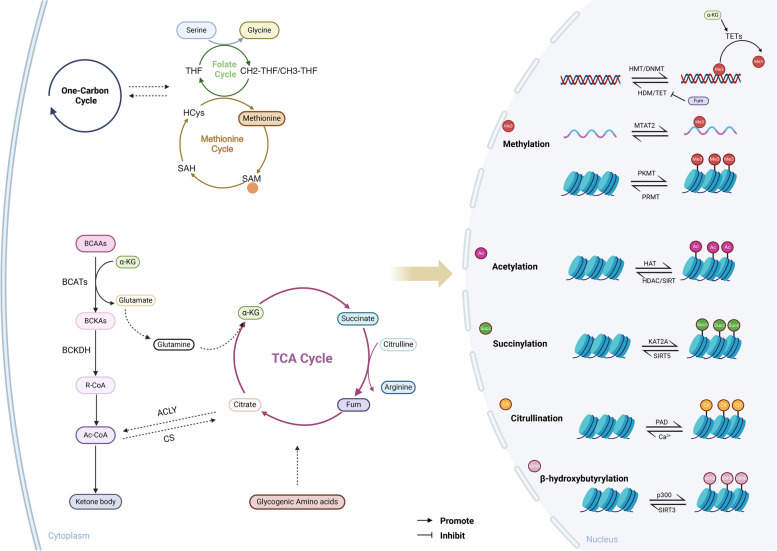
Fig. 2.
Amino acid metabolism regulates the epigenetic modification in tumor cells. Amino acid metabolism participates in epigenetic regulation by providing methyl groups, acetyl coenzyme A, succinyl-CoA, citrulline, β-hydroxybutyrylation and serving as modification sites. In the intricate processes of the folate and methionine cycles involving one-carbon metabolism, the conversions of serine and glycine play pivotal roles. Within the methionine cycle, homocysteine and methionine yield SAM, functioning as a methyl donor for DNA, RNA, and histone methylation. Branch-chain amino acids undergo transamination to form BCKAs, eventually leading to the conversion of α-ketoglutarate to glutamate. Glutamate further transforms into glutamine, participating in TCA cycle. The BCKAs are catalyzed by BCKDH to yield R-CoA, subsequently metabolized into acetyl-CoA. Acetyl-CoA can either be converted into citrate by CS or via ACLY to replenish the acetyl-CoA pool. Additionally, gluconeogenic amino acids can fuel the TCA cycle. Succinyl-CoA, an intermediate metabolite from branch-chain amino acid breakdown and TCA cycle, serves as a substrate for histone succinylation. Moreover, acetyl-CoA participates in ketone body-mediated modifications and the conversion of glutamate to arginine within the TCA cycle supports histone citrullination. SAM S-adenosylmethionine, BCKAs branched-chain keto acids, ATP-citrate lyase, BCKDH branched-chain α-ketoacid dehydrogenase complex, CS citrate synthase. Image created with BioRender.com
DNA methylation is facilitated by DNA methyltransferase, targeting 5-carbocytosine residues within CpG dinucleotides [103]. Methionine is a sulfur-containing amino acid necessary for epigenetic modification. Methionine can be converted to S-adenylyl methionine (SAM) by methionine adenylytransferase (MAT), which is a universal methyl donor for DNA and histone methylation [104]. SAM can not only provide methylation donors for DNA and histone methylation, but also help maintain RNA methylation modification and promote tumorigenesis and metastasis [105]. mTORC1 can also increase the expression of methionine adenosine transferase 2A (MAT2A) and further stimulate m6A RNA modification, thus promoting tumor proliferation [106]. Histone methylation is catalyzed by histone methyltransferase, predominantly occurs at the N-terminal of arginine or lysine, thereby influencing gene expression activation and inhibition [107]. Arginine methylation is associated with DNA damage repair, transcription, splicing and cell cycle [108]. Protein arginine methyltransferases (PRMTs) catalyze the methylation of guanidine in arginine of histone or other proteins [109]. Arginine methylation has a dichotomous function in the target protein depending on the type of methyltransferase [8, 110]. Moreover, Histone lysine methylation involves the transfer of one to three methyl groups from S-adenosylmethionine to the ε-nitrogen group of lysine residues [111]. The methylation of lysine is catalyzed by histone lysine methyltransferases (HKMTs) [112]. Methionine cycle and folate cycle are central components of one-carbon metabolism. Folate cycle supplies methyl groups for the conversion of homocysteine to methionine. The interconnectedness of the methionine cycle with the folate cycle facilitates the metabolism of one-carbon units (methyl groups) [113]. In addition to serving as methyl donors, amino acid metabolism can also regulate the activity of demethylases. The catalytic process of branched-chain amino acid transaminase (BCAT1/2) on branched-chain amino acids can facilitate the conversion of α-KG to glutamine, leading to a decrease in intracellular α-KG levels [114]. Given that α-KG serves as a co-factor for various DNA demethylases and histone demethylases, the metabolism of branched-chain amino acids can thereby indirectly regulate methylation modifications [115].
Histone acetylation is regulated by histone acetyltransferases (HATs) and histone deacetylases (HDACs), governing the electrostatic interaction between histones and DNA to enhance gene expression, with the acetyl group primarily derived from acetyl-CoA [116]. Branched-chain amino acids (BCAAs) are converted to branched-chain alpha-ketoacids (BCKAs) catalyzed by branched-chain aminotransferases (BCATs), which are further metabolized to acetyl-CoA and succinyl-CoA for the TCA cycle [117]. Some gluconeogenic and ketogenic amino acids can also generate acetyl-CoA through pathways such as gluconeogenesis and glycolysis, providing substrates for histone acetylation. Under specific stress, acetate can also serve as a carbon source to specifically promote the acetylation of histone H4K16 (H4K16ac) near telomeres, disrupting telomeric heterochromatin structure and accelerating cellular aging [118].
The intermediate product of branched-chain amino acid metabolism, succinyl-CoA, serves as a substrate for histone succinylation, catalyzed by succinyltransferase OXCT1 adding onto lysine residues [119]. Succinylation of histones, akin to acetylation, activates gene expression. Accumulation of succinyl-CoA in the process of branched-chain amino acid metabolism can promote cancer initiation and progression [120]. Succinylation modification promoting the activation of the anticancer protein LACTB, inhibiting LACTB's protease activity, can enhance hepatocellular carcinoma development [119]. In addition, succinylation of GLS increases enzyme activity, promoting glutaminolysis and tumor growth [121].
Histone citrullination is catalyzed by peptidylarginine deiminases (PADs) as a post-translational modification. PAD4-mediated histone citrullination can promote tumor development through NETs (Neutrophil extracellular traps) [122]. PAD4 can inhibit the expression of p53 target gene OKL38, thus regulating apoptosis [123]. Moreover, PAD4 can interact with HDAC2, participating in the regulation of gene expression during DNA damage [124]. Furthermore, Citrullinated histone H3 (H3Cit) can predict venous thromboembolism (VTE) in cancer patients [125].
β-hydroxybutyrylation emerges as a novel histone modification, where acetyltransferase p300 catalyzes the addition of β-hydroxybutyrate to lysine (Kbhb), while histone deacetylases 1 (HDAC1) and HDAC2 enzymatically remove Kbhb [126]. Acetyl-CoA interconverts with ketone bodies and serves as a precursor to β-hydroxybutyrate. Research by Liu et al. has demonstrated that p53 Kbhb leads to reduced acetylation levels of p53, resulting in decreased expression of downstream gene p21, reduced apoptosis, and promotion of tumorigenesis and progression [127].
In conclusion, amino acid metabolism can directly and indirectly participate in tumor epigenetic regulation by providing methyl groups, acetyl-CoA, succinyl-CoA, citrulline, β-hydroxybutyrylation and serving as modification sites.
The role of amino acid metabolism in tumor microenvironment
Amino acid metabolism plays a pivotal part in sculpting the tumor microenvironment, with significant impact on diverse facets of tumorigenesis such as regulation of immune cells function (Table 2), immune evasion, remodeling extracellular matrix and angiogenesis. Additionally, the elaborate interplay between tumor cells and the surrounding stromal and immune cells highlights the substantial role of amino acid metabolism in orchestrating the tumor immune landscape and fostering tumor growth.
Table 2.
Influence of amino acid metabolism on immune cells
| Type | Immune Cells | Amino Acids and Metabolites | Influence | Reference |
|---|---|---|---|---|
| Lymphoid cells | T cells | Methionine | T cells enhance the expression of the methionine transporter following antigen stimulation. Methionine promotes T cells infiltration and function. | [106, 129–132] |
| Cystine | Cysteine enables T cells to exert anti-tumor immune responses, recruitment and infiltration. | [133–136] | ||
| Glutamine | Glutamine modulates the activation and differentiation of T cells, polarization of Th1 and Th17. | [137–141] | ||
| Tryptophan | Tryptophan promotes T cell proliferation and anti-tumor immunity. | [142, 143, 149] | ||
| Kynurenine | Kynurenine inhibits T cell response and proliferation. | [144, 147] | ||
| Glutaric acid | Glutaric acid influences T cell metabolism and differentiation. | [145, 146] | ||
| I3A | I3A stimulates the production of interferon-γ by T cells. | [148] | ||
| Arginine | Arginine promotes the function and proliferation of T cells. | [150–154] | ||
| Polyamines | Polyamines promote the proliferation, cell cycle and histone acetylation modifications of T cells, and induce cell lysis of T lymphocytes after TCR stimulation. | [155–159] | ||
| Serine | Serine provides single carbon metabolites to support T cell proliferation and adaptive immunity. | [160] | ||
| Glycine | Glycine promotes T cell proliferation and adaptive immunity. | [160] | ||
| Branched-chain amino acids | BCAAs provide nutrients for T cell survival and enhance anti-tumor immune function. | [161–163] | ||
| Asparagine | Asn enhances the activity and proliferation of T cells. | [43, 164, 165] | ||
| Tregs | Methionine | Methionine is important for Treg survival. | [168] | |
| Cystine | Cystine promotes the proliferation and function of Tregs. | [169] | ||
| Glutamate | Glutamate is essential for the proliferation and function of Tregs. | [169] | ||
| Serine | Serine inhibits the function of Tregs. | [170] | ||
| Tryptophan | Tryptophan promotes the function of Tregs. | [171, 172] | ||
| Polyamines | Polyamines promote Tregs differentiation. | [173, 174] | ||
| Natural killer cells | Glutamine | Glutamine enhances the anti-tumor activity of NK cells. | [177–179] | |
| Tryptophan | L-kynurenine induce NK cell apoptosis in an AHR-independent manner. | [180, 181] | ||
| Selenium-containing amino acids | Se-AAs enhance the activity of NK cells. | [182, 183] | ||
| B cells | Leucine | Leucine promotes B cell differentiation and support the production of IgG and cytokine. | [184] | |
| Glutamine | Glutamine affects IgM production in B cells. | [185] | ||
| Tryptophan | Tryptophan affects B cell development. | [186] | ||
| Methionine | Methionine regulates epigenome remodeling in B cells. | [187] | ||
| Arginine | Polyamines promote B cell activation and tumor antigen presentation. | [188] | ||
| GABA | B cells secrete GABA to promote the proliferation of Tregs. | [189] | ||
| Myeloid cells | Macrophages | Methionine | Methionine induces M1/classical macrophage activation. | [194–196] |
| Tryptophan | NAD+ enhances macrophage phagocytosis; PA activates the pro-inflammatory function of macrophages. TAMs exert immunosuppressive function through tryptophan metabolism. | [197–200] | ||
| Glutamine | Glutamine facilitates the polarization towards M2 macrophages. | [201–203] | ||
| Arginine | Activated macrophages uptake arginine; Creatine promotes the polarization of M2-like macrophages. | [204–207] | ||
| Branched-chain amino acids | Accumulated BCAAs activates the TLR4/NF-κB signaling pathway. | [208] | ||
| Serine | Serine metabolism is intricately linked to M1 macrophage polarization. | [209–212] | ||
| Neutrophils | Leucine | Leucine regulates the antigen presentation mechanism of neutrophils. | [214] | |
| Glutamine | Glutamine inhibits inflammatory response, migration and Redox balance of Neutrophils. | [215–217] | ||
| Branched-chain amino acids | Neutrophils release BCAAs when they contact with fibronectin. | [218] | ||
| Aromatic amino acids | Aromatic amino acids were released by neutrophils. | [218] | ||
| Dendritic cells | Glutamine | Glutamine enhances CD8+ T cell immunity mediated by DC1s; tumor cells and DC1s compete for glutamine uptake. | [224] | |
| Tryptophan | IDO inhibits the tryptophan-sensitive differentiation in DCs. | [225–227] | ||
| Arginine | Arginine metabolism transform DCs into an immunosuppressive phenotype. | [228] | ||
| Myeloid-derived suppressor cells | Arginine | MDSCs outcompete tumor immune cells for arginine, arginase-1 have been found to effectively suppress T cell responses. | [230, 231] | |
| Tryptophan | Tryptophan metabolism helps MDSCs to exert immunosuppressive effects. | [232, 233] | ||
| Glutamate | Glutamate promotes the infiltration of MDSCs. | [234] | ||
| Glutamine | Glutamine promotes generation and mobilization of MDSCs, but other studies indicates that glutamine deprivation promotes the generation of MDSCs. | [235–237] | ||
| Asparagine | Asparagine promotes immunosuppressive function of MDSCs by ERK/ETS2 signaling. | [234] | ||
| Cystine | MDSCs express the cystine transporter xc-, diminishing the cysteine supply for T cells. | [134] |
BCAA Branched chain amino acid, GABA γ-aminobutyric acid, MDSCs Myeloid-derived suppressor cells
Influence of amino acid metabolism on immune cells
T cells
T cells are instrumental to cellular immunity, being capable of recognizing tumor-associated antigens and selectively eliminating tumor cells. T cells can be classified into CD4+ T cell subsets, encompassing follicular helper T cells (Tfh), helper T cells (Th), and regulatory T cells (Treg) based on their membrane markers, and CD8+ T clusters comprising of cytotoxic T cells and memory T cells [128].
Methionine and cystine are of significance to the immune infiltration and function of T cells. T cells enhance the expression of the methionine transporter SLC7A5 following antigen stimulation [129]. Conversely, tumor cells competitively acquire methionine via the methionine transporters SLC7A5 and SLC43A2 [130]. Restriction of methionine results in diminished histone H3K4 methylation (H3K4me3), leading to a reduction in T-cell mediated neuroinflammation and disease, while impelling immune function in tumors [131]. However, methionine and its downstream catabolic product S-adenosylmethionine (SAM) modulate the DNA methylation and chromatin accessibility of CD8+ T cells, thereby attenuating the anti-tumor capabilities of T cells [132]. Methionine can also act as a methyl-donor to assist RNA m6A methylation of T cells, while methionine deprivation can enhance infiltrating levels of CD8+ T cells and prevent tumor growth [106]. Amino acid metabolism can affect the function of T cells, and T cells can also affect the amino acid metabolism of tumor cells. Cystine plays a crucial role as an intermediate metabolite in both methionine metabolism and the sulfur transfer pathway. Cysteine is significant in enabling T cells to exert anti-tumor immune responses. However, T cells lack cystathionase and the xc- transporter, necessitating reliance on exogenous cysteine [133]. In instances where extracellular cysteine is constrained, tumor-specific T cells fail to activate, resulting in the suppression of anti-tumor immunity [134]. Effector T cells have the capacity to release interferon (IFN) upon activation, which in turn down-regulates the expression of SLC7A11 and SLC3A2 in tumor cells. This downregulation effectively inhibits cystine uptake, ultimately culminating in ferroptosis and subsequent cell death [135]. Additionally, H2S-mediated sulfhydration of cysteine increases the expression of adaptor-associated protein kinase 1 (AAK1), leading to the inhibition of recruitment and infiltration of CD8+ T cells [136].
Glutamine is central to modulate the activation and functionality of T cells. It serves as a precursor for the acylation of protein O-GlcNAc and is involved in the regulation of T cell self-renewal [137]. Furthermore, glutamine catabolism promotes the de novo synthesis of glutathione (GSH), thereby impacting T cell differentiation [138]. The overexpression of the glutamine transporter SLC38A1 significantly enhances the mitochondrial function of CD4+ T cells [139]. Additionally, another glutamine transporter, SLC38A2 regulates T cell production and memory function by modulating mTORC1 activity to some extent [140]. SLC1A5 deficiency can hinder Glutamine internal flow and damage the polarization of Th1 and Th17 [141].
Tryptophan depletion can lead to T cell energy deficiency and proliferation arrest, while tryptophan metabolites can act as immunosuppressive factors of T cells. During stem cell transplantation, the up-regulation of extracellular kynurenine can effectively inhibit T cell response and proliferation [142, 143]. The ratio of tryptophan to kynuridine can be used as a predictor of CD4+ T cell populations [144]. Glutaric acid in the form of Coenzyme A (CoA) is an important intermediate in the catabolism of tryptophan and lysine. Glutaric acid and its metabolites succinate and fumarate are structural analogues of α-ketoglutarate (α-KG), all of which can act as competitive inhibitors of α-KG dependent dioxygenase (α-KGDDs), strongly influencing cell metabolism and T cell differentiation [145, 146]. Metformin increases the availability of tryptophan in CD8+ T cells, and promotes anti-tumor immunity by inhibiting tryptophan metabolism in colorectal cancer cells [147]. Nevertheless, the dietary tryptophan metabolite I3A has been shown to stimulate the production of interferon-γ by CD8+ T cells, thereby augmenting the therapeutic efficacy of immune checkpoint inhibitors (ICIs) and demonstrating anti-tumor immune activity [148]. Indoleamine 2,3-dioxygenase 1 (IDO1) can participate in the anti-tumor effects mediated by IFNγ through the depletion of tryptophan. However, IDO1 inhibition not only reduces the production of tryptophan metabolites but also exerts unfavorable protective effects, rendering melanoma cells less susceptible to the influence of IFNγ [149].
Arginine can promote the function and proliferation of T cells [150]. Arginine transporter cationic amino acid transporter-1 (CAT1) maintains the proliferation and activation of naive and memory CD4+ T cells and CD8+ T cells [151]. The arginine transported by SLC7A1 regulates the production and persistence of memory T cells and the T cell cycle through mTORC1 [140]. Extracellular L-arginine concentration regulates antigen recognition function of T cells [152]. Methylglyoxal, a derivative metabolite of glycine, can enter CD8+ T cells and bind to arginine, depleting free L-arginine, thereby inhibiting the activation and function of CD8+ T cells [153]. Arginase 1 drives the immunosuppressive microenvironment of pancreatic cancer by consuming arginine and inhibiting T cell activation [154]. Polyamines, a metabolite of arginine, have a dual potential to influence inflammation and tumor immunogenicity. Polyamines can promote the proliferation of T cells and induce cell lysis of T lymphocytes after TCR stimulation [155, 156]. In addition, polyamines regulate the cell cycle and modulate differentiation of helper CD4+ T cells into their functional subsets [157, 158]. Polyamines can also regulate the histone acetylation modifications of the T cell epigenome through the synthesis of hypusine [157]. Deletion of the histone acetyltransferase (HAT) can restore differentiation of polyamine-deficient Th cells [159].
Serine synthesis is increased in activated T cells to provide intracellular glycine and single carbon metabolites to support T cell proliferation and promote adaptive immunity [160].
Branched-chain amino acids (BCAAs) serve as essential nutrients for T cell survival. In PP2Cm deficient mice with impaired BCAA degradation, heightened activity of CD8+ T cells and enhanced anti-tumor immune function have been observed. Exogenous supplementation of BCAAs exhibits a synergistic effect with anti-PD-1 treatment, positioning BCAAs as a supplementary component to augment the clinical efficacy of anti-PD-1 immunotherapy in cancer treatment [161]. The metabolite of BCAAs, β-hydroxy-β-methylbutyrate (HMB), is conducive to the conversion of Th1 cells to Th2 cells [162]. Antigen signaling mediated by the interaction of T cell receptor (TCR) and leucine/glutamine bidirectional transporter LAT1 increases leucine uptake [163]. In addition, Leucine can also regulate T cell proliferation and differentiation by controlling mTOR activity [163].
Asparagine (Asn) has been shown to enhance the activity of CD8+ T cells by upregulating the LCK signaling pathway. In vitro experiments have demonstrated that Asn promotes the activation of CD8+ T cells through uptake by T cells rather than acting as an exchange factor, thus enhancing the function of immune cells to suppress tumor growth, challenging previous notions of Asn's pro-cancer properties [45, 164]. Interestingly, Asparagine deprivation inhibits the early activation of CD8+ T cells, yet reshapes the metabolic program by triggering the nuclear factor erythroid 2 related factor 2 (NRF2) dependent stress response during differentiation. This leads to a reduction in overall glucose and glutamine consumption while increasing intracellular nucleotides to promote proliferation, thereby endowing CD8+ T cells with robust proliferative and effector functions [165].
Tregs
Tregs, characterized as CD4+ CD25+ T cells, exert a significant regulatory role in immune surveillance and anti-tumor immune responses by suppressing the activity of CD4+ helper and CD8+ cytotoxic T cells [166]. The upregulation of immunosuppressive molecules, including CTLA-4, PD-1, and PD-L1, is also essential for immune evasion during tumorigenesis [167].
Methionine transporter SLC43A2 is indispensable for Treg survival, and its downregulation reduces methionine uptake, thereby promoting Treg apoptosis [168]. Cystine/glutamate reverse transporter SLC7A11 is essential for the proliferation and function of Tregs. This transporter may facilitate the capture of free cystine in the tumor microenvironment by tumor cells or immunosuppressive cells, thereby promoting tumor growth and undermining the function of anti-tumor immunity [169]. Hydrogen sulfide (H2S) promotes Treg activation through sulfhydrating enolase 1 (ENO1) on cysteine residues via ELK4 [136]. However, Serine can activate the mTOR signaling pathway to inhibit the function of Tregs, but the conversion of serine to glutathione via glutamate-cysteine ligase (Gclc) helps maintain the immunosuppressant function of Tregs [170]. Tryptophan can be converted into N-formylkynurenine through indoleamine 2,3-dioxygenase enzyme (IDO), which is upregulated in DCs and M2-like macrophages to promote the function of Tregs, leading to the suppression of immune response [171, 172]. Polyamines and Spermidine can promote T cell differentiation to regulatory phenotype by inducing FOXP3 [173, 174].
Natural killer cells
NK cells can mediate direct cytotoxicity to alloantigens and tumor-associated antigen [175]. Human NK cells can be distinguished by two main subgroups including CD56dim cells and CD56bright cells based on cell marker expression [176].
Glutamine has been shown to enhance the anti-tumor activity of NK cells. SLC7A5 functions as a principal systemic L-amino acid transporter in activated NK cells and activates mTORC1 signaling [177]. Furthermore, cytokines such as IL-2, IL-12, and IL-18 have the ability to stimulate the expression of SLC7A5/SLC3A2, consequently promoting the proliferation of NK cells through the mTORC1 pathway [178]. The membrane protein metabolic glutamate receptor 5 (mGluR5) augments the cytotoxicity of NK cells following glutamate activation [179]. Moreover, L-kynurenine generated through IDO-catalyzed tryptophan metabolism can induce NK cell apoptosis in an AHR-independent manner, contributing to immunosuppression in gastric cancer [180]. This is different from previous reports that kyn/AhR signals enhance the cytotoxicity of NK cells, reflecting the complexity of kyn's effect on NK cells [181]. Additionally, Selenium-containing amino acids (Se-AAs) have the ability to modulate crosstalk between immune and tumor cells, thereby reshaping the Tumor Microenvironment (TME) [182]. By activating a variety of enzyme systems within lymphocytes, Se-AAs not only enhance the activity of immune cells such as NK cells but also further stimulate the secretion of lymphocytic factors [183].
B cells
B cells play a central part in humoral immunity. B cells defend against foreign antigens through antibody secretion, antigen presentation and direct killing through antibody-dependent cellular cytotoxicity (ADCC).
Leucine is transported into B cells via SLC7A5, targeting mTORC1 to promote B cell differentiation and support the production of IgG and cytokine [184]. Glutamine transporter SLC1A5 or key enzyme of glutamine metabolism can affect IgM production in B cells [185]. In addition, tryptophan can affect B-cell development in bone marrow [186]. Methionine metabolism may regulate epigenome remodeling in B cells infected with Epstein-Barr virus by influencing methylation potential [187]. The increase of polyamines, a metabolite of arginine, promotes B cell activation and tumor antigen presentation [188]. Moreover, B cells can also secrete GABA, thus inhibiting the function of CD4+ T cells and cytotoxic CD8+ T cells, while promoting the proliferation of regulatory T cells [189].
Macrophages
Macrophages are essential myeloid cells and represent a major cell type within the mononuclear phagocyte system (MPS) [190]. They polarize into M1/M2 macrophages in response to various internal and external stimuli. M1 macrophages exert anti-tumor effects by recognizing and clearing tumor cells, whereas M2 macrophages promote tumor growth, invasion, and metastasis, exhibiting pro-tumorigenic effects [191].
Cell competition represents a mechanism for cellular malignant potential and acquisition of adaptive advantages [192]. In the context of breast cancer, overexpression of MYC in cancer cells mediates the mTORC1 signaling pathway, leading to adaption and evolution of dominant cancer cells through cell competition. These dominant cancer cells engulf and eliminate fragments of other cancer cells to acquire nutrients. However, tumor-associated macrophages (TAMs) can modulate their own mTORC1 signaling activity through regulation of protein intake and genetic reprogramming, which allows TAMs to engage in competition with cancer cells, exerting a suppressive effect on tumor growth [193].
Methionine induces M1/classical macrophage activation, correlated with pro-inflammatory responses characterized by TNF-α release [194]. Methionine also modulates extracellular nucleotide metabolism, facilitating an increase in ATPase/ADPase activity within macrophages [194]. Moreover, activated macrophages fine-tune their metabolic pathways to drive a pro-inflammatory phenotype [195]. The one-carbon units provided by serine metabolism can synergistically integrate into the methionine cycle, promoting SAM production during LPS-induced inflammatory processes. SAM is an essential metabolite for inflammatory macrophages, and disruption of the metabolic pathways results in anti-inflammatory outcomes [196].
Tryptophan undergoes decomposition into small molecules, including NAD+, pyridinic acid (PA), ATP, and CO2 via the kynuridine pathway [197]. NAD+ has been shown to enhance macrophage phagocytosis [198], while PA is capable of activating the pro-inflammatory function of macrophage [199]. Tumor-associated macrophages can exert their immunosuppressive function through tryptophan metabolism or the conversion of dietary tryptophan into indole [200].
Glutamine has been found to facilitate the polarization towards M2 macrophages, while glutamine deprivation inhibits M2 polarization and the production of the chemokine CCL22 [201]. The cleavage of glutamine to produce α-KG has been shown to promote the activation of M2-like macrophages, and a reduction in α-KG enhances the pro-inflammatory function of M1-like macrophage [202]. Furthermore, glutamine metabolism has been demonstrated to enhance macrophage phagocytosis for the elimination of apoptotic cells [203].
Arginine can be transported into activated macrophages via SLC7A2 and undergo distinct metabolic pathways [204]. In M1-like macrophages, arginine is metabolized to produce NO, which inhibits tumor development [205]. Conversely, M2-like macrophages consume arginine through arginase-1, thereby limiting the uptake of arginine by other anti-tumor immune cells [206]. Moreover, the metabolism of arginine can generate creatine through glycine aminotransferase and methyltransferase guanidinoacetate, which can promote the polarization of M2-like macrophages [207].
The impaired BCAAs catabolism in human monocytes and mouse macrophages has been associated with atherosclerosis (AS), while promoting BCAAs catabolism in macrophages may improve the progression of AS. The accumulated BCAAs activates the mitochondrial H2O2 (mtH2O2) signaling pathway and increases the formation of disulfide HMGB1, thus activating the TLR4/NF-κB signaling pathway and its downstream cascade [208]. However, the regulation of branched-chain amino acids in macrophages within tumors is currently underreported.
Serine metabolism is intricately linked to the function of macrophages, where exogenous serine supports M1 macrophage polarization through mTOR signaling or synthesis of SAM and GSH [209, 210]. However, inhibiting PHGDH to suppress endogenous serine synthesis can also promote M1 macrophage polarization by increasing IGF1 expression through SAM [211]. These conflicting results suggest that serine metabolism, encompassing both exogenous acquisition and de novo serine synthesis, may regulate macrophage function under different conditions [212].
Neutrophils
Neutrophils play a crucial role in innate immune responses as effectors, regulating various processes such as injury and repair, cancer, immunity, and inflammatory processes [213].
Neutrophils expressing HLA-DR+CD74+ can serve as alternative antigen-presenting cells in various cancers. The leucine metabolism-dependent axis of acetyl-CoA/H3K27ac/MHC-II can regulate the antigen presentation mechanism of neutrophils [214]. Leucine-rich diet may enhance the efficacy of PD-1 immunotherapy for cancer. The utilization rate of glutamine in neutrophils is very high. Glutamine can inhibit the generation of TNF-a in LPS-treated neutrophils, thereby inhibiting their inflammatory response. Glutamine can regulate the expression of ICAM-1 and VCAM-1 in cells, thereby regulating neutrophil migration [215]. Castell et al. [216] demonstrated that glutamine increases the respiratory burst and superoxide anion generation in human neutrophils, affecting the oxidative-reductive balance of neutrophils. Moreover, tumor cells prepare for tumor invasion and migration by regulating the remodeling of the extracellular matrix, a process accompanied by the infiltration, migration, and adhesion of neutrophils into tissues [217]. When neutrophils come into contact with fibronectin, they release branched amino acids, aromatic amino acids, and positively charged free amino acids. However, when neutrophils encounter cell adhesion inhibitors such as cell-relaxing factor D, the release of hydroxylysine sharply decreases while phenylalanine increases [218]. Therefore, amino acid metabolism can regulate the immune function of neutrophils, and neutrophils can also serve as a source of some amino acids in the processes of migration and functional activation.
Dendritic cells
Dendritic cells (DCs) serve as the primary antigen presenting cells (APCs) and are pivotal in regulating adaptive immunity and immune tolerance [219]. DCs can be categorized into DC1 and DC2 subgroups, each of which plays a distinct role in promoting the cytotoxicity of CD8+ T cells and the activation of CD4+ T cells. These cells are involved in the cross-presentation of tumor-associated antigens to cytotoxic CD8+ T cells and blockade of immune checkpoints, thereby facilitating anti-tumor immune responses [220, 221]. However, the immunosuppressive TME can impair the functional activity of DCs by their recognition and binding of immune checkpoints, leading to immune evasion [204]. Specifically, DCs expressing CD80/CD86 engage with T cells expressing CTLA-4, transmitting co-stimulatory signals and dampening T cell response [222]. Additionally, DCs with elevated PD-L1 expression interact with the PD-1 receptor of T cells, enabling tumor cells to evade destruction by cytotoxic T cells [223].
Glutamine enhances CD8+ T cell immunity mediated by DC1s to inhibit tumor growth, while tumor cells and DC1s compete for glutamine uptake through the transporter SLC38A2 and form a metabolic crosstalk to regulate anti-tumor immunity [224]. A positive feedback loop exists between DCs and Tregs, where the expression of CTLA-4 in Tregs promotes the secretion of indoleamine 2,3-dioxygenase (IDO) by DCs. Inhibition of CTLA-4 effectively reduces the production of kynurenine by DCs [225]. Bruton's tyrosine kinase (BTK) serves as a key regulatory factor in the immunosuppressive function of bone marrow-derived suppressor cells and DCs [226]. The BTK-IDO axis inhibits the tryptophan-sensitive differentiation pathway in DCs [227]. Furthermore, Arginase can promote arginine metabolism to produce polyamines and transform DCs into an immunosuppressive phenotype [228].
Myeloid-derived suppressor cells
Myeloid-derived suppressor cells (MDSCs) originate from myeloid cells and share phenotypic and morphological similarities with neutrophils and monocytes, while exerting immunosuppressive functions [229].
MDSCs have been shown to outcompete tumor immune cells for arginine, a crucial amino acid for T cell function. Furthermore, MDSCs expressing arginase-1 have been found to effectively suppress T cell responses [230]. MDSCs can also competitively inhibit cytotoxic T cell proliferation and function by increasing the consumption of L-arginine and downregulating the generation of polyamine [231]. Within MDSCs, IDO can exert immunosuppressive effects in tumors by modulating tryptophan metabolism [232, 233]. Glutamate transporter SLC25A22 promotes the transcription of CXCL1, driving the infiltration of MDSCs, and creating an immunosuppressive microenvironment by enhancing asparagine and downstream ERK/ETS2 signaling [234]. Moreover, restricted glutamine metabolism significantly inhibits the generation and mobilization of MDSCs, further promoting anti-tumor immunity [235]. However, there are also studies indicating that glutamine deprivation promotes the generation of MDSCs [236], suggesting that the function of MDSCs may be microenvironment-dependent. There may be crosstalk of glutamine with arginine metabolism and tryptophan metabolism, as in glutamine-restricted condition, the activity of iNOS is upregulated in MDSC [237], and inhibiting glutamine metabolism also disrupts IDO expression in MDSCs [235]. Additionally, MDSCs have the ability to express the cystine transporter xc-, while lacking expression of the neutral amino acid transporter, allowing them to acquire cystine from the environment and convert it into cysteine. However, they are unable to export cystine, thereby diminishing the cysteine supply for T cells. This limitation can restrict the activation and functionality of T cells [134] (Fig. 3).
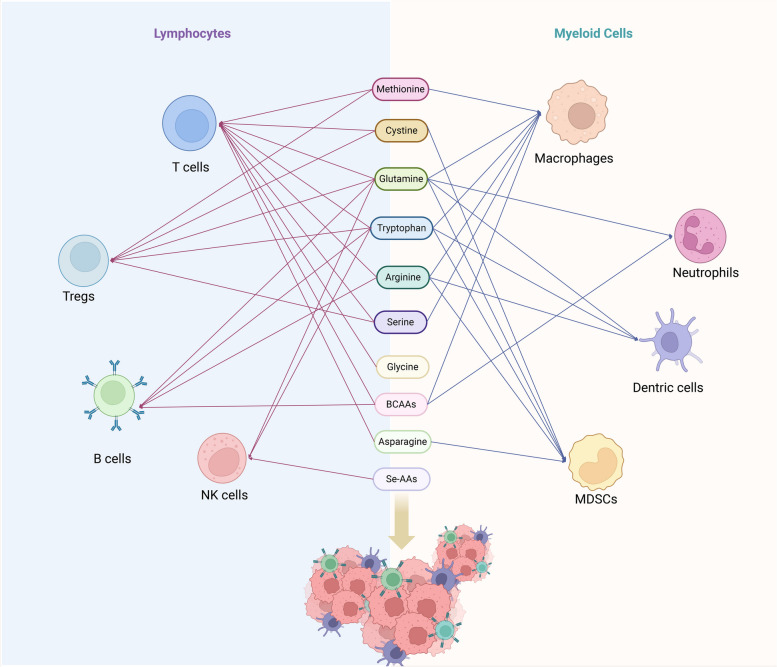
Fig. 3.
The influence of amino acids in regulating the activity and function of immune cells in microenvironment. Lymphoid immune cells encompass T cells, regulatory T cells, NK cells, and B cells, while myeloid immune cells consist of macrophages, neutrophils, dendritic cells, and MDSCs. Various studies have indicated that amino acids such as arginine, cysteine, glycine, glutamine, tryptophan, arginine, serine, BCAAs, aspartate, and selenium-containing amino acids play regulatory roles in the activation, proliferation, and function of immune cells within the tumor microenvironment. The red line represents the effect of amino acids on lymphocytes and the blue line represents the effect on myeloid cells. BCAAs branched-chain amino acids, MDSCs myeloid-derived suppressor cells. Image created with BioRender.com
Influence of amino acid metabolism on immune tolerance by mediating ferroptosis
Ferroptosis is a programmed cell death induced by iron-dependent lipid peroxidation, which can be modulated by amnio acid metabolism, other cellular metabolic and signaling pathways and plays a dual role in immune tolerance [238].
Ferroptosis can influence immune tolerance through co-stimulatory or co-inhibitory signals, immune checkpoints of T cells and the susceptibility of immune cell. The ferroptosis score correlates positively with CTLA4 expression, although the role of ferroptosis in upregulating CTLA4 mediated immune tolerance varies across different tumors and is environment-dependent [239]. Ferroptosis may induce the death of GPX4-deficient Tregs via the CD28 co-stimulatory pathway, thereby impeding immune tolerance [240]. Additionally, ferroptosis may inhibit immune tolerance through the ATP-P2X7-CD40/CD86 axis [241, 242]. Heterogeneous nuclear ribonucleoprotein L upregulates PD-L1 to suppress T cell-mediated ferroptosis in castration-resistant prostate cancer cells [243]. Activated T cells release IFN-γ, leading to inactivation of cysteine uptake, thereby promoting lipid peroxidation and ferroptosis in tumor cells [135]. A system-level crosstalk exists between ferroptosis and immune activation to restrain tumor progression. Ferroptosis vulnerability exhibits in CD8+ T cells, with GPX4-deficient T cells showing heightened sensitivity to ferroptosis and loss of anti-tumor effects [240]. Overexpression of GPX4 can inhibit ferroptosis in CD8+ T cells in vitro, restoring the production of cytotoxic cytokines. Furthermore, GPX4 overexpression increases the infiltration of CD8+ T cells in tumors, exerting anti-tumor immune functions. In addition, TAM2 and DCs are also vulnerable to ferroptosis, while MDSCs and TAM1 are more resistant to ferroptosis, leading to a diversity of immune tolerance regulation [244–246].
In the regulation of ferroptosis, amino acid metabolism plays a crucial role, with GPX4 and system-xc emerging as two key molecular players. GPX4, a member of the GPX family, contains a catalytic center with selenocysteine. It catalyzes the conversion of reduced glutathione (GSH) to its oxidized form (GSSG) and reduces cytotoxic lipid hydroperoxides (L-OOH) [247]. Inhibition of GPX4 activity and limited availability of glutathione can lead to the accumulation of lipid hydroperoxides, thereby triggering ferroptosis [248]. System-xc functions as an amino acid antiporter, facilitating cysteine uptake and promoting GSH synthesis. Reduced activity of system xc- can result in decreased GPX4 activity, diminished cellular antioxidant capacity, and the onset of ferroptosis and oxidative stress [249]. Moreover, the enzyme cysteine dioxygenase 1 (CDO1) competitively catalyzes the conversion of cysteine to taurine, in opposition to the synthesis of glutathione catalyzed by glutamate-cysteine ligase (GCL). This process reduces glutathione production, thereby mediating oxidative stress and ferroptosis [250]. Therefore, the intricate regulation of immune tolerance through the control of ferroptosis by amino acid metabolism is complex.
Amino acid metabolism reprogramming in fibroblasts
Metabolic reprogramming in non-cancer cells, such as stromal fibroblasts and bone marrow adipocytes, as well as metabolic interactions among various cell types within the tumor microenvironment, represent emerging characteristics of cancer metabolism [1]. Overexpression of serotonin N-acetyltransferase-5 (SNAT5) in tumor cells or stromal cells in glutamine-dystrophic tumors may support metabolic exchange in the tumor microenvironment and play an important role in promoting cancer [251]. Cancer-associated fibroblasts (CAFs) serve as primary contributors to the extracellular matrix (ECM) components, fostering tumor progression. There is a metabolic crosstalk between CAFs and cancer cells. Moreover, tumors can promote metabolic reprogramming of various non-cancer cells by secreting exosomal miRNAs to support tumor growth. Thus, reprogrammed fibroblasts enhance glycoglutamine hydrolysis, providing fuel for neighboring cancer cells while converting metabolic waste ammonium into energy-rich metabolite [252]. The extracellular vesicles secreted by breast cancer cells and their cargo of miRNA can comprehensively reduce the translation of mRNA in fibroblasts by inhibiting mTOR signaling, reprogramming fibroblast metabolism to provide metabolic flux to cancer cells, and dynamically regulating extracellular matrix proteins in response to nutrient fluctuations [253]. Additionally, Proline is an amino acid abundant in collagen proteins, with PYCR1 being a crucial enzyme in proline synthesis. Targeting PYCR1 in CAFs may hold the potential to impede the production of tumor-promoting extracellular matrix [254].
Effects of amino acid metabolism on angiogenesis
The vascular system consists of a branching network of endothelial cells (ECs) that deliver oxygen and nutrients to tissues in the body, and angiogenesis is important for tumor growth, spread, and distant metastasis [255]. Metabolic reprogramming of endothelial cells is vital in controlling angiogenesis and vasculature in tumors. Apart from glucose and lipid metabolism, amino acid metabolism can also affect the proliferation and migration of endothelial cells [11, 256]. Amino acid metabolism also plays an important role in the regulation and maintenance of vascular functions such as vascular tone, coagulation and fibrinolysis, redox homeostasis, and immune response [257] (Fig. 4).
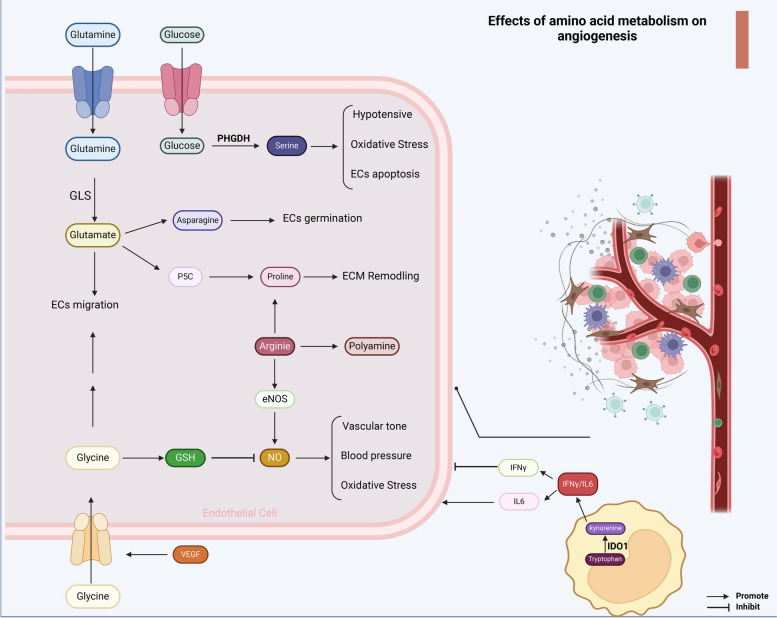
Fig. 4.
Amino acid metabolism regulates tumor angiogenesis. The metabolism of amino acids can impact not only the function of endothelial cells but also the formation and function of blood vessels. Glutamine is catalyzed by GLS to form glutamate, which can be converted to aspartate to promote endothelial cell germination. Glutamate can also be converted to P5C and proline, further facilitating ECM remodeling. Following VEGF signaling stimulation, endothelial cells exhibit increased glycine uptake, which can enhance endothelial cell migration. Glycine is also involved in GSH synthesis, inhibiting vascular tone, blood pressure, and oxidative stress mediated by NO. Arginine, through the synthesis of proline, polyamines, and NO, plays a regulatory role in angiogenesis. The serine synthesis pathway dependent on PHGDH can promote antihypertensive effects, regulate oxidative stress, and resist endothelial cell apoptosis. Additionally, tryptophan metabolism in MDSCs generates kynurenine via IDO-1, regulating the balance between IFNγ and IL6. While IL6 promotes angiogenesis, IFNγ exerts the opposite effect. GSH glutathione, GLS glutaminase, ECM extracellular matrix, VEGF vascular Endothelial Growth Factor, PHGDH phosphoglycerate dehydrogenase. Image created with BioRender.com
Glutamine uptake by ECs is highly regulated by the sodium-dependent system ASC [258]. Restriction of glutamine in endothelial cells or inhibition of glutaminase 1 (GLS1) leads to impaired endothelial cell migration and defective vascular germination [259]. Glutamine can provide nitrogen for asparagine synthesis to maintain cellular homeostasis. Although endothelial cells can absorb asparagine, restriction of asparagine synthetase activity can still hinder EC germination [260].
Serine serves as a primary one-carbon unit donor in folate cycle, synthesizing precursors for glycine and cysteine, while also promoting the generation of glutathione. The serine synthesis pathway (SSP) is mainly catalyzed by phosphoglycerate dehydrogenase (PHGDH) within the glycolytic branch. Studies have demonstrated that neonatal mice lacking PHGDH in ECs exhibit severe vascular defects [261]. Furthermore, disruption of the SSP due to PHGDH knockout induces EC apoptosis even in the absence of serine deprivation. Silencing PHGDH also impacts the levels of ROS in tumor endothelial cells [262]. Additionally, serine exerts a significant hypotensive effect under conditions of nitric oxide damage [263]. Thus, endothelial cells rely on the serine synthesis pathway for serine generation and utilization, while serine also modulates endothelial cell redox balance and maintains vascular function.
Glycine acts as a precursor to glutathione, maintaining the redox homeostasis of endothelial cells. Furthermore, studies have indicated that exogenous glycine can impede angiogenesis and tumor growth by reducing iNOS expression [264]. Interestingly, other research has suggested that glycine can promote angiogenesis, as stimulation with VEGF activates the glycine transporter protein 1 in endothelial cells, increasing glycine uptake [265].
Arginine shows regulatory influence in tumor angiogenesis through its involvement in protein synthesis and the generation of nitric oxide (NO) via endothelial nitric oxide synthase (eNOS) [266]. NO is involved in the regulation of diverse vascular functions, including vascular tone, blood pressure, neurotransmission, immune response, and oxidative stress [267]. Furthermore, the effects of NO on angiogenesis can be either inhibitory or stimulatory, whose function is context-dependent [268]. Additionally, arginine promotes angiogenesis by generating polyamine precursors, facilitating protein synthesis and gene expression [158, 269]. Arginine in endothelial cells can also be converted into proline, which is used to synthesize collagen and generate extracellular matrix, thus playing an important role in vascular remodeling [270]. Proline analogs can also facilitate endothelial cell migration and vascular formation by providing an anchoring matrix through continuous collagen synthesis [271].
In addition, tryptamine catabolic enzyme indoleamine 2,3-dioxygenase (IDO1) in MDSCs can modulate inflammatory neovascularization by orchestrating the equilibrium between IFNγ and IL6 [272, 273]. IFN-γ exerts an antiangiogenic influence, while IL6 can stimulate angiogenesis. IFN-γ is capable of upregulating the expression of IDO1 [274]. However, IDO1 can exert either a positive or negative impact on the induction of IL6 [275–277].
The influence of mechanical cues on amino acid metabolism in the TME
Mechanical cues and forces can impact cellular function and metabolic processes, while metabolic feedback also influences the mechanical and physical properties of cells and tissues within the TME. Cancer cells directly regulate ECM components and collagen crosslinking, or indirectly influence the activity of stromal cells such as CAFs and macrophages, contributing to the maintenance of mechanical properties within the TME [278]. Within the TME, mechanical stimulation of the ECM activates integrins in cell-ECM adhesions, sensed through calponin and interacting with the cell nucleus via the LINC protein complex [279]. Numerous studies suggest that mechanical cues, both intra- and extracellular, activate key oncogenic pathways such as YAP, RAS, and mTOR in cancer cells, regulating processes including survival, proliferation, metastasis, and drug resistance [280–282]. Furthermore, cancer cells proliferate excessively, invade surrounding tissues, alter ECM composition and stiffness to promote physical changes within the TME. High ECM stiffness in CAFs and cancer cells promotes the upregulation of glutamate transporters SLC1A3 and GLS1, leading to increased glutamate uptake and production [283]. Glutamate is utilized to produce glutathione and aspartate, supporting redox homeostasis, nucleotide synthesis, and eventual ECM remodeling. ECM stiffening-induced glutaminolysis depletion results in heightened microtubule stability, promoting cancer cell proliferation and invasive migration [284]. Reduced glutaminolysis levels also facilitate the reversal of the TCA cycle and increased acetyl-CoA levels, enhancing H3K27 acetylation on the PYCR1 gene promoter for proline biosynthesis essential for collagen production [254]. In macrophages, elevated ECM hardness leads to arginine/proline metabolic reprogramming, secreting arginase, thereby impairing CD8+ T cell anti-tumor activity [285].
Amino acid metabolic crosstalk between tumor cells and TME
Amino acid metabolic reprogramming is a hallmark in tumor microenvironment, where lies dynamic crosstalk between immune cells, stromal cells, and tumor cells. Activated T cells and macrophages enhance glutamine metabolism to sustain cell proliferation and immune responses [202]. Tumor cells also rely on glutamine for energy to support their growth and proliferation. Therefore, a competitive relationship exists between tumor cells and immune cells, as tumor cells competitively consume glutamine to prevent T cell proliferation, activation, and cytokine secretion, thereby creating an immunosuppressive microenvironment [286]. In addition, fibroblasts interact with tumor cells and transform into CAFs during tumorigenesis, which promote extracellular matrix remodeling and the formation of the tumor niche [287]. The metabolic crosstalk between tumor cells and stromal CAFs highlights the complexity of tumor biology. The acquisition of aspartate represents a metabolic constraint that tumors encounter within their native environment, and overcoming this limitation favors tumor growth [288]. SLC1A3 can function as Asp/Glu co-transporter, enabling CAFs and cancer cells to share metabolites. The production of aspartate by CAFs is crucial for maintaining cancer cell proliferation [283]. Moreover, Glutamine derived from CAFs can be transported to support cancer cell proliferation, whereas glutamate derived from cancer cells balances the redox state of CAFs and promotes extracellular matrix remodeling [289]. In nutrient-deprived regions where glutamine is depleted, CAFs can be guided by the polarized protein kinase B (AKT2) to migrate towards glutamine-rich areas. This glutamine-dependent process accelerates the migration and invasion of CAFs, thereby facilitating the migration of cancer cells towards nutrient-rich regions [290]. Moreover, there is also crosstalk in proline metabolism between tumor cells and CAFs. Under hypoxic conditions, PYCR1 regenerates NAD+ in an oxygen-independent manner, supporting the activity of the TCA cycle. Loss of PYCR1 in tumors leads to increased hypoxia, decreased cell viability, and necrosis [97]. Tumor cells promote overall proline catabolic metabolism, utilizing the energy derived from proline degradation to support invasive processes [291]. Stimulation by transforming growth factor β induces CAFs to enhance proline synthesis [292].
Amino acid-based strategies for cancer therapy
The heterogeneity of tumor metabolism exerts a profound influence on the spatial distribution of nutrients within the tumor microenvironment, thereby impacting the functionality of both tumor cells and immune cells. Moreover, intricate competitive metabolic interplays and crosstalk occur between tumor cells, immunosuppressive cells, and anti-tumor immune cells, thereby significantly modulating the efficacy of cancer therapies. Simultaneously, the targeting of amino acids [293–296], amino acid transporters [297–301], metabolic sensors [200, 302–304], enzymes [227, 305–309], downstream signal pathways [310, 311], and metabolites [72, 312–322] holds promise in offering potential therapeutic avenues for addressing tumors.
Given that other therapeutic approaches pertaining to the targeting of amino acid metabolism have been comprehensively reviewed and documented [204, 323, 324], our focus lies in exploring the potential of amino acid as a novel supplementary strategy for tumor treatment.
CAR T cells
Chimeric antigen receptor (CAR) T cells and tumor cells compete for limited amino acids in the environment. Amino acid metabolic alterations also influence the function of CAR T cells. CAR T cells can be re-engineered to up-regulate the expression of SLC7A5/SLC7A11 and arginase 1/arginase 2 enzymes, thus enhancing its proliferation and anti-tumor activity [325]. Moreover, CD229 CAR-T cells have demonstrated efficacy in eliminating multiple myeloma cells. However, the inadvertent targeting and damage of healthy lymphocytes expressing CD229 by CD229 CAR-T cells have been observed. To address this issue, the use of single amino acid substitution within the CAR binding domain has shown promise in enhancing the selectivity of CD229 CAR-T cells towards MM cells [326]. Yang et al [327]. engineered a novel BCKDK-modified CAR-T cell based on genotype modifications, reprogramming the BCAA metabolism in the tumor microenvironment. This intervention significantly increased the proportion of CAR-T cells in the peripheral circulation, enhancing the efficiency of cancer cell lysis and prolonging the survival of mice. Additionally, accumulation of Kynurenine promotes an immune-suppressive tumor microenvironment. KYNU-modified CAR-T cells exhibit superior cytotoxicity towards cancer cells by metabolizing Kyn in the immune-suppressive TME [328].
Nanomedicines
Competition for amino acid metabolism by tumor cells can indirectly lead to amino acid deprivation within the tumor, affecting the availability of amino acids for anti-tumor immune cells, thereby hindering the activation and differentiation of immune cells [329]. Furthermore, the metabolic reshaping of the tumor microenvironment due to metabolic dysregulation is characterized by nutrient deficiency, hypoxia, acidity, and the accumulation of immunosuppressive metabolites, further impeding the anti-tumor function of tumor-infiltrating immune cells (TIICs) and reducing the effectiveness of immunotherapy. Nanomedicines offer various advantages such as co-delivery of multiple drugs, cell and organelle-specific targeting, controlled drug release, and multimodal therapy [330]. Nanomedicines for tumor metabolism intervention could serve as a potential therapeutic approach to restore anti-tumor immunity.
Nanoparticle drug carriers offer numerous advantages over traditional chemotherapy, including the ability to protect drug activity and prolong circulation in the body [331]. However, they still need to overcome biological barriers within the body. Poly (amino acid)s, characterized by their multifunctional amphiphilic macromolecular structure and excellent biocompatibility [332], have emerged as a promising material. They have the potential to enhance tumor targeting, effectively control tumor growth, and mitigate the side effects of Redox on normal tissues and organs during treatment [333]. Cationic nanomaterials of polyaspartic acid can inhibit NET-DNA-mediated cancer cell migration by disrupting the interaction between NET-DNA and CCDC25. Due to its excellent liver retention properties, this material shows promising prospects for the treatment of liver metastatic tumors [334]. In addition to influencing the properties of nanomaterials through the polar side chains of amino acid molecules, they can also serve as targets for the carried drugs in therapy. Tang et al. [335] engineered a two-dimensional nanomedical drug delivery system targeting tumor cells, incorporating the glutamine metabolic inhibitor (V9302) and anti-PD-L1 (MoS2-apdl1-V9302). This formulation effectively suppressed the uptake of glutamine by tumor cells, resulting in elevated levels of glutamine in TME and subsequently enhancing tumor invasion and activation of CD8+ Teff cells. Xie et al. [336] developed a cancer cell membrane-coated nanosystem (CTTPA-G) loaded with a Type I aggregation-induced emission (AIE) photosensitizer (PS) and a glutamine antagonist to facilitate synergistic photodynamic therapy (PDT) and tumor metabolic reprogramming, thereby providing Teff cells with sufficient glutamine to restore their anti-tumor function. Furthermore, Mai et al. [337] devised a carrier-free immunotherapy nanoenhancer (C9SN) that functions by modulating glutamine metabolism to polarize M2-like TAMs into an M1-like phenotype and recruit Teff cells. A pH-responsive arginine nanoassembly (ArgNP) has also been developed to enhance tumor immunotherapy through tumor arginine intervention. When combined with anti-PD-L1 treatment, ArgNP has been shown to promote the infiltration levels of Teff cells in tumor and significantly increase the proportion of CD8+/CD4+ T cells [338].
AAT-PET
There are two primary classes of amino acid transporters, namely large amino acid transporters (LAT) and Na+-independent transporters (alanine-serine-cysteine transporters, ASCT). The intracellular uptake and imaging of radiolabeled amino acid tracers (AAT) via LAT and ASCT form the foundation of AAT-PET. This technique plays a crucial role in the initial diagnosis and treatment planning for glioma, metastatic brain cancer, and other neurological tumors, as well as in the assessment of therapeutic efficacy [339, 340].
Conclusion
In summary, amino acid metabolism reprogramming stands as a crucial hallmark in the development and progression of cancer. Within tumor cells, reshaping amino acid metabolism can engage in protein and nucleic acid biosynthesis, serve as signaling molecules, regulate tumor metabolism, maintain oxidative stress balance, and modulate epigenetic modifications. Within the tumor microenvironment, amino acid metabolism can influence the functions of lymphoid and myeloid immune cells, regulate CAFs and the formation of extracellular matrix, modulate immune tolerance, tumor vascularization as well as mechanical cues. Furthermore, the crosstalk of amino acid metabolism exerts intricate interactions within both tumor cells and the tumor microenvironment. A comprehensive understanding of the significant role of amino acid metabolism in cancer is crucial for the development of novel therapeutic strategies.
Acknowledgements
We acknowledge the BioRender website (https://app.biorender.com/) for providing the platform in drawing the illustrations in this review.
Authors' contributions
L. Y. and Y. Z. designed and revised the manuscript. X. L. wrote the main manuscript text. B. R. and J. R. elaborated the Figs. 1, 2, 3, 4. M. G. polished the manuscript and gave useful suggestions. All authors reviewed the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (grant number 81972321 and 82273455 L. Y., 82303504 to B.R.), the CAMS Innovation Fund for Medical Sciences (CIFMS) (grant number 2021-I2M-1-002 to Y. Z.), Non-proft Central Research Institute Fund of Chinese Academy of Medical Sciences (No. 2018PT32014), National High Level Hospital Clinical Research Funding (No. 2022-PUMCH-D-001) and National Multidisciplinary Cooperative Diagnosis and Treatment Capacity Building Project for Major Diseases (Z190022).
Availability of data and materials
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xiaohong Liu and Bo Ren contributed equally to this work.
Contributor Information
Lei You, Email: florayo@163.com.
Yupei Zhao, Email: zhao8028@263.net.
References
- 1.Pavlova NN, Thompson CB. The Emerging Hallmarks of Cancer Metabolism. Cell metabolism. 2016;23(1):27–47. 10.1016/j.cmet.2015.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Faubert B, Solmonson A, DeBerardinis RJ. Metabolic reprogramming and cancer progression. Science (New York, NY). 2020;368(6487):eaaw5473. 10.1126/science.aaw5473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vettore L, Westbrook RL, Tennant DA. New aspects of amino acid metabolism in cancer. Brit J Cancer. 2020;122(2):150–6. 10.1038/s41416-019-0620-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Safrhansova L, Hlozkova K, Starkova J. Targeting amino acid metabolism in cancer. Int Rev Cell Mol Biol. 2022;373:37–79. 10.1016/bs.ircmb.2022.08.001 [DOI] [PubMed] [Google Scholar]
- 5.DeBerardinis RJ, Cheng T. Q’s next: the diverse functions of glutamine in metabolism, cell biology and cancer. Oncogene. 2010;29(3):313–24. 10.1038/onc.2009.358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang Y, Morar M, Ealick SE. Structural biology of the purine biosynthetic pathway. Cell Mol Life Sci. 2008;65(23):3699–724. 10.1007/s00018-008-8295-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ryan DG, Yang M, Prag HA, et al. Disruption of the TCA cycle reveals an ATF4-dependent integration of redox and amino acid metabolism. eLife. 2021;10:e72593. 10.7554/eLife.72593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li X, Zhang HS. Amino acid metabolism, redox balance and epigenetic regulation in cancer. FEBS J. 2023;291(3):412–29. 10.1111/febs.16803 [DOI] [PubMed] [Google Scholar]
- 9.Zhao H, Yang L, Baddour J, et al. Tumor microenvironment derived exosomes pleiotropically modulate cancer cell metabolism. eLife. 2016;5:e10250. 10.7554/eLife.10250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Z, Li B, Li S, et al. Metabolic control of CD47 expression through LAT2-mediated amino acid uptake promotes tumor immune evasion. Nat Comm. 2022;13(1):6308. 10.1038/s41467-022-34064-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oberkersch RE, Santoro MM. Role of amino acid metabolism in angiogenesis. Vasc Pharmacol. 2019;112:17–23. 10.1016/j.vph.2018.11.001 [DOI] [PubMed] [Google Scholar]
- 12.Karno B, Edwards DN, Chen J. Metabolic control of cancer metastasis: role of amino acids at secondary organ sites. Oncogene. 2023;42(47):3447–56. 10.1038/s41388-023-02868-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yoo HC, Han JM. Amino Acid Metabolism in Cancer Drug Resistance. Cells. 2022;11(1):140. 10.3390/cells11010140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arneth B. Tumor microenvironment. Medicina (Kaunas, Lithuania). 2019;56(1):15. [DOI] [PMC free article] [PubMed]
- 15.Del Prete A, Schioppa T, Tiberio L, Stabile H, Sozzani S. Leukocyte trafficking in tumor microenvironment. Curr Opin Pharmacol. 2017;35:40–7. 10.1016/j.coph.2017.05.004 [DOI] [PubMed] [Google Scholar]
- 16.Naleskina LA, Kunska LM, Chekhun VF. Modern views on the role of main components of stroma and tumor microinvironment in invasion, migration and metastasis. Exp Oncol. 2020;42(4):252–62. 10.32471/exp-oncology.2312-8852.vol-42-no-4.15401 [DOI] [PubMed] [Google Scholar]
- 17.Yuan Y, Jiang YC, Sun CK, Chen QM. Role of the tumor microenvironment in tumor progression and the clinical applications (Review). Oncol Rep. 2016;35(5):2499–515. 10.3892/or.2016.4660 [DOI] [PubMed] [Google Scholar]
- 18.Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19(11):1423–37. 10.1038/nm.3394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen C, Wang Z, Ding Y, Qin Y. Tumor microenvironment-mediated immune evasion in hepatocellular carcinoma. Front Immunol. 2023;14:1133308. 10.3389/fimmu.2023.1133308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang A, Miao K, Sun H, Deng CX. Tumor heterogeneity reshapes the tumor microenvironment to influence drug resistance. Int J Biol Sci. 2022;18(7):3019–33. 10.7150/ijbs.72534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang X, Wang J, Deng X, et al. The role of microenvironment in tumor angiogenesis. J Exp Clin Cancer Res. 2020;39(1):204. 10.1186/s13046-020-01709-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsai CH, Chuang YM, Li X, et al. Immunoediting instructs tumor metabolic reprogramming to support immune evasion. Cell Metabol. 2023;35(1):118-33.e7. 10.1016/j.cmet.2022.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lavie D, Ben-Shmuel A, Erez N, Scherz-Shouval R. Cancer-associated fibroblasts in the single-cell era. Nat Cancer. 2022;3(7):793–807. 10.1038/s43018-022-00411-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DeNardo DG, Ruffell B. Macrophages as regulators of tumour immunity and immunotherapy. Nat Rev Immunol. 2019;19(6):369–82. 10.1038/s41577-019-0127-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fu LQ, Du WL, Cai MH, Yao JY, Zhao YY, Mou XZ. The roles of tumor-associated macrophages in tumor angiogenesis and metastasis. Cell Immunol. 2020;353:104119. 10.1016/j.cellimm.2020.104119 [DOI] [PubMed] [Google Scholar]
- 26.Bejarano L, Jordāo MJC, Joyce JA. Therapeutic Targeting of the Tumor Microenvironment. Cancer Disc. 2021;11(4):933–59. 10.1158/2159-8290.CD-20-1808 [DOI] [PubMed] [Google Scholar]
- 27.Fan Y, Evans CR, Ling J. Rewiring protein synthesis: From natural to synthetic amino acids. Biochimica et biophysica acta General subjects. 2017;1861(11 Pt B):3024–9. 10.1016/j.bbagen.2017.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brosnan J, Rooyackers O. The importance of amino acids as independent metabolites, signalling molecules and as building blocks for protein. Curr Opin Clin Nutr Metab Care. 2012;15(1):47–8. 10.1097/MCO.0b013e32834de431 [DOI] [PubMed] [Google Scholar]
- 29.Neinast M, Murashige D, Arany Z. Branched Chain Amino Acids. Ann Rev Physiol. 2019;81:139–64. 10.1146/annurev-physiol-020518-114455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.May ME, Buse MG. Effects of branched-chain amino acids on protein turnover. Diabetes Metabol Rev. 1989;5(3):227–45. 10.1002/dmr.5610050303 [DOI] [PubMed] [Google Scholar]
- 31.Anthony JC, Yoshizawa F, Anthony TG, Vary TC, Jefferson LS, Kimball SR. Leucine stimulates translation initiation in skeletal muscle of postabsorptive rats via a rapamycin-sensitive pathway. J Nutr. 2000;130(10):2413–9. 10.1093/jn/130.10.2413 [DOI] [PubMed] [Google Scholar]
- 32.Anthony JC, Lang CH, Crozier SJ, et al. Contribution of insulin to the translational control of protein synthesis in skeletal muscle by leucine. Am J Physiol Endocrinol Metabol. 2002;282(5):E1092-101. 10.1152/ajpendo.00208.2001 [DOI] [PubMed] [Google Scholar]
- 33.James HA, O’Neill BT, Nair KS. Insulin Regulation of Proteostasis and Clinical Implications. Cell Metabol. 2017;26(2):310–23. 10.1016/j.cmet.2017.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nelsestuen GL. Amino acid-directed nucleic acid synthesis. A possible mechanism in the origin of life. J Mol Evol. 1978;11(2):109–20. 10.1007/BF01733887 [DOI] [PubMed] [Google Scholar]
- 35.Zhu J, Thompson CB. Metabolic regulation of cell growth and proliferation. Nat Rev Mol Cell Biol. 2019;20(7):436–50. 10.1038/s41580-019-0123-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang M, Vousden KH. Serine and one-carbon metabolism in cancer. Nat Rev Cancer. 2016;16(10):650–62. 10.1038/nrc.2016.81 [DOI] [PubMed] [Google Scholar]
- 37.Lee Y, Vousden KH, Hennequart M. Cycling back to folate metabolism in cancer. Nat Cancer. 2024;5(5):701–15. 10.1038/s43018-024-00739-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kiweler N, Delbrouck C, Pozdeev VI, et al. Mitochondria preserve an autarkic one-carbon cycle to confer growth-independent cancer cell migration and metastasis. Nat Comm. 2022;13(1):2699. 10.1038/s41467-022-30363-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ternes D, Tsenkova M, Pozdeev VI, et al. The gut microbial metabolite formate exacerbates colorectal cancer progression. Nat Metabol. 2022;4(4):458–75. 10.1038/s42255-022-00558-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Soflaee MH, Kesavan R, Sahu U, et al. Purine nucleotide depletion prompts cell migration by stimulating the serine synthesis pathway. Nat Comm. 2022;13(1):2698. 10.1038/s41467-022-30362-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Biswas D, Duffley L, Pulinilkunnil T. Role of branched-chain amino acid-catabolizing enzymes in intertissue signaling, metabolic remodeling, and energy homeostasis. FASEB J. 2019;33(8):8711–31. 10.1096/fj.201802842RR [DOI] [PubMed] [Google Scholar]
- 42.Tokunaga C, Yoshino K, Yonezawa K. mTOR integrates amino acid- and energy-sensing pathways. Biochem Biophys Res Commun. 2004;313(2):443–6. 10.1016/j.bbrc.2003.07.019 [DOI] [PubMed] [Google Scholar]
- 43.Durán RV, Oppliger W, Robitaille AM, et al. Glutaminolysis activates Rag-mTORC1 signaling. Mol Cell. 2012;47(3):349–58. 10.1016/j.molcel.2012.05.043 [DOI] [PubMed] [Google Scholar]
- 44.Gu X, Orozco JM, Saxton RA, et al. SAMTOR is an S-adenosylmethionine sensor for the mTORC1 pathway. Sci (New York, NY). 2017;358(6364):813–8. 10.1126/science.aao3265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Krall AS, Mullen PJ, Surjono F, et al. Asparagine couples mitochondrial respiration to ATF4 activity and tumor growth. Cell Metabol. 2021;33(5):1013-26.e6. 10.1016/j.cmet.2021.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jiang H, Zhang L, Yang M, et al. Branched-chain amino acids promote thrombocytopoiesis by activating mTOR signaling. J Thromb Haemost. 2023;21(11):3224–35. 10.1016/j.jtha.2023.06.039 [DOI] [PubMed] [Google Scholar]
- 47.Zhang YK, Qu YY, Lin Y, et al. Enoyl-CoA hydratase-1 regulates mTOR signaling and apoptosis by sensing nutrients. Nat Comm. 2017;8(1):464. 10.1038/s41467-017-00489-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen J, Ou Y, Luo R, et al. SAR1B senses leucine levels to regulate mTORC1 signalling. Nature. 2021;596(7871):281–4. 10.1038/s41586-021-03768-w [DOI] [PubMed] [Google Scholar]
- 49.Xu G, Kwon G, Cruz WS, Marshall CA, McDaniel ML. Metabolic regulation by leucine of translation initiation through the mTOR-signaling pathway by pancreatic beta-cells. Diabetes. 2001;50(2):353–60. 10.2337/diabetes.50.2.353 [DOI] [PubMed] [Google Scholar]
- 50.Saxton RA, Sabatini DM. mTOR Signaling in Growth, Metabolism, and Disease. Cell. 2017;168(6):960–76. 10.1016/j.cell.2017.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhou H, Huang S. Role of mTOR signaling in tumor cell motility, invasion and metastasis. Curr Protein Pept Sci. 2011;12(1):30–42. 10.2174/138920311795659407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Murugan AK. mTOR: Role in cancer, metastasis and drug resistance. Semin Cancer Biol. 2019;59:92–111. 10.1016/j.semcancer.2019.07.003 [DOI] [PubMed] [Google Scholar]
- 53.Solanki S, Sanchez K, Ponnusamy V, et al. Dysregulated Amino Acid Sensing Drives Colorectal Cancer Growth and Metabolic Reprogramming Leading to Chemoresistance. Gastroenterol. 2023;164(3):376-91.e13. 10.1053/j.gastro.2022.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Herzig S, Shaw RJ. AMPK: guardian of metabolism and mitochondrial homeostasis. Nat Rev Mol Cell Biol. 2018;19(2):121–35. 10.1038/nrm.2017.95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Deng L, Yao P, Li L, et al. p53-mediated control of aspartate-asparagine homeostasis dictates LKB1 activity and modulates cell survival. Nat Comm. 2020;11(1):1755. 10.1038/s41467-020-15573-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Adachi Y, De Sousa-Coelho AL, Harata I, et al. l-Alanine activates hepatic AMP-activated protein kinase and modulates systemic glucose metabolism. Mol Metabol. 2018;17:61–70. 10.1016/j.molmet.2018.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tian Q, Yuan P, Quan C, et al. Phosphorylation of BCKDK of BCAA catabolism at Y246 by Src promotes metastasis of colorectal cancer. Oncogene. 2020;39(20):3980–96. 10.1038/s41388-020-1262-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Han T, Zhan W, Gan M, et al. Phosphorylation of glutaminase by PKCε is essential for its enzymatic activity and critically contributes to tumorigenesis. Cell Res. 2018;28(6):655–69. 10.1038/s41422-018-0021-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Castro-Muñoz LJ, Ulloa EV, Sahlgren C, Lizano M, De La Cruz-Hernández E, Contreras-Paredes A. Modulating epigenetic modifications for cancer therapy (Review). Oncol Rep. 2023;49(3):59. 10.3892/or.2023.8496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yoo HC, Yu YC, Sung Y, Han JM. Glutamine reliance in cell metabolism. Exp Mol Med. 2020;52(9):1496–516. 10.1038/s12276-020-00504-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yuneva M, Zamboni N, Oefner P, Sachidanandam R, Lazebnik Y. Deficiency in glutamine but not glucose induces MYC-dependent apoptosis in human cells. J Cell Biol. 2007;178(1):93–105. 10.1083/jcb.200703099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bodineau C, Tomé M, Murdoch PDS, Durán RV. Glutamine, MTOR and autophagy: a multiconnection relationship. Autophagy. 2022;18(11):2749–50. 10.1080/15548627.2022.2062875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen CL, Hsu SC, Ann DK, Yen Y, Kung HJ. Arginine Signaling and Cancer Metabolism. Cancers. 2021;13(14):3541. 10.3390/cancers13143541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Casero RA Jr, Murray Stewart T, Pegg AE. Polyamine metabolism and cancer: treatments, challenges and opportunities. Nat Rev Cancer. 2018;18(11):681–95. 10.1038/s41568-018-0050-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tomasi ML, Ryoo M, Skay A, et al. Polyamine and methionine adenosyltransferase 2A crosstalk in human colon and liver cancer. Exp Cell Res. 2013;319(12):1902–11. 10.1016/j.yexcr.2013.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Arruabarrena-Aristorena A, Zabala-Letona A, Carracedo A. Oil for the cancer engine: The cross-talk between oncogenic signaling and polyamine metabolism. Science advances. 2018;4(1):eaar2606. 10.1126/sciadv.aar2606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sun W, Zhao E, Cui H. Target enzymes in serine-glycine-one-carbon metabolic pathway for cancer therapy. Int J Cancer. 2023;152(12):2446–63. 10.1002/ijc.34353 [DOI] [PubMed] [Google Scholar]
- 68.Nilsson LM, Forshell TZ, Rimpi S, et al. Mouse genetics suggests cell-context dependency for Myc-regulated metabolic enzymes during tumorigenesis. PLoS Genet. 2012;8(3):e1002573. 10.1371/journal.pgen.1002573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Amelio I, Cutruzzolá F, Antonov A, Agostini M, Melino G. Serine and glycine metabolism in cancer. Trends Biochem Sci. 2014;39(4):191–8. 10.1016/j.tibs.2014.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Salminen A. Role of indoleamine 2,3-dioxygenase 1 (IDO1) and kynurenine pathway in the regulation of the aging process. Ageing Res Rev. 2022;75:101573. 10.1016/j.arr.2022.101573 [DOI] [PubMed] [Google Scholar]
- 71.Gong X, Chang R, Zou J, Tan S, Huang Z. The role and mechanism of tryptophan - kynurenine metabolic pathway in depression. Rev Neurosci. 2023;34(3):313–24. 10.1515/revneuro-2022-0047 [DOI] [PubMed] [Google Scholar]
- 72.Basson C, Serem JC, Hlophe YN, Bipath P. The tryptophan-kynurenine pathway in immunomodulation and cancer metastasis. Cancer Med. 2023;12(18):18691–701. 10.1002/cam4.6484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Badawy AA. Tryptophan metabolism and disposition in cancer biology and immunotherapy. Biosci Rep. 2022;42(11):BSR20221682. 10.1042/BSR20221682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Venkateswaran N, Lafita-Navarro MC, Hao YH, et al. MYC promotes tryptophan uptake and metabolism by the kynurenine pathway in colon cancer. Genes Dev. 2019;33(17–18):1236–51. 10.1101/gad.327056.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Huang D, Wang Y, Thompson JW, et al. Cancer-cell-derived GABA promotes β-catenin-mediated tumour growth and immunosuppression. Nat Cell Biol. 2022;24(2):230–41. 10.1038/s41556-021-00820-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Herranz D, Ambesi-Impiombato A, Sudderth J, et al. Metabolic reprogramming induces resistance to anti-NOTCH1 therapies in T cell acute lymphoblastic leukemia. Nat Med. 2015;21(10):1182–9. 10.1038/nm.3955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xie C, Jin J, Bao X, et al. Inhibition of mitochondrial glutaminase activity reverses acquired erlotinib resistance in non-small cell lung cancer. Oncotarget. 2016;7(1):610–21. 10.18632/oncotarget.6311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Altman BJ, Stine ZE, Dang CV. From Krebs to clinic: glutamine metabolism to cancer therapy. Nat Rev Cancer. 2016;16(10):619–34. 10.1038/nrc.2016.71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Grasmann G, Smolle E, Olschewski H, Leithner K. Gluconeogenesis in cancer cells - Repurposing of a starvation-induced metabolic pathway? Biochim Biophys Acta Rev Cancer. 2019;1872(1):24–36. 10.1016/j.bbcan.2019.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Muoio DM. Metabolic inflexibility: when mitochondrial indecision leads to metabolic gridlock. Cell. 2014;159(6):1253–62. 10.1016/j.cell.2014.11.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ye J, Mancuso A, Tong X, et al. Pyruvate kinase M2 promotes de novo serine synthesis to sustain mTORC1 activity and cell proliferation. Proc Natl Acad Sci U S A. 2012;109(18):6904–9. 10.1073/pnas.1204176109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Noguchi T, Yamada K, Inoue H, Matsuda T, Tanaka T. The L- and R-type isozymes of rat pyruvate kinase are produced from a single gene by use of different promoters. J Biol Chem. 1987;262(29):14366–71. 10.1016/S0021-9258(18)47947-1 [DOI] [PubMed] [Google Scholar]
- 83.Mazurek S, Zwerschke W, Jansen-Dürr P, Eigenbrodt E. Metabolic cooperation between different oncogenes during cell transformation: interaction between activated ras and HPV-16 E7. Oncogene. 2001;20(47):6891–8. 10.1038/sj.onc.1204792 [DOI] [PubMed] [Google Scholar]
- 84.Chaneton B, Hillmann P, Zheng L, et al. Serine is a natural ligand and allosteric activator of pyruvate kinase M2. Nature. 2012;491(7424):458–62. 10.1038/nature11540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lapenna D. Glutathione and glutathione-dependent enzymes: From biochemistry to gerontology and successful aging. Ageing Res Rev. 2023;92:102066. 10.1016/j.arr.2023.102066 [DOI] [PubMed] [Google Scholar]
- 86.Wu G, Fang YZ, Yang S, Lupton JR, Turner ND. Glutathione metabolism and its implications for health. J Nutr. 2004;134(3):489–92. 10.1093/jn/134.3.489 [DOI] [PubMed] [Google Scholar]
- 87.Vaughn AE, Deshmukh M. Glucose metabolism inhibits apoptosis in neurons and cancer cells by redox inactivation of cytochrome c. Nat Cell Biol. 2008;10(12):1477–83. 10.1038/ncb1807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lo M, Ling V, Wang YZ, Gout PW. The xc- cystine/glutamate antiporter: a mediator of pancreatic cancer growth with a role in drug resistance. Brit J Cancer. 2008;99(3):464–72. 10.1038/sj.bjc.6604485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hu X, He Y, Han Z, et al. PNO1 inhibits autophagy-mediated ferroptosis by GSH metabolic reprogramming in hepatocellular carcinoma. Cell Death Dis. 2022;13(11):1010. 10.1038/s41419-022-05448-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Liu X, Zhang Y, Zhuang L, Olszewski K, Gan B. NADPH debt drives redox bankruptcy: SLC7A11/xCT-mediated cystine uptake as a double-edged sword in cellular redox regulation. Genes Dis. 2021;8(6):731–45. 10.1016/j.gendis.2020.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Liu X, Nie L, Zhang Y, et al. Actin cytoskeleton vulnerability to disulfide stress mediates disulfidptosis. Nat Cell Biol. 2023;25(3):404–14. 10.1038/s41556-023-01091-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Liu X, Olszewski K, Zhang Y, et al. Cystine transporter regulation of pentose phosphate pathway dependency and disulfide stress exposes a targetable metabolic vulnerability in cancer. Nat Cell Biol. 2020;22(4):476–86. 10.1038/s41556-020-0496-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yan Y, Teng H, Hang Q, et al. SLC7A11 expression level dictates differential responses to oxidative stress in cancer cells. Nat Comm. 2023;14(1):3673. 10.1038/s41467-023-39401-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Du Y, Zhang H, Lu J, Holmgren A. Glutathione and glutaredoxin act as a backup of human thioredoxin reductase 1 to reduce thioredoxin 1 preventing cell death by aurothioglucose. The J Biol Chem. 2012;287(45):38210–9. 10.1074/jbc.M112.392225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Arnér ES, Holmgren A. The thioredoxin system in cancer. Sem Cancer Biol. 2006;16(6):420–6. 10.1016/j.semcancer.2006.10.009 [DOI] [PubMed] [Google Scholar]
- 96.Fan J, Ye J, Kamphorst JJ, Shlomi T, Thompson CB, Rabinowitz JD. Quantitative flux analysis reveals folate-dependent NADPH production. Nature. 2014;510(7504):298–302. 10.1038/nature13236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Westbrook RL, Bridges E, Roberts J, et al. Proline synthesis through PYCR1 is required to support cancer cell proliferation and survival in oxygen-limiting conditions. Cell Rep. 2022;38(5):110320. 10.1016/j.celrep.2022.110320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Helenius IT, Madala HR, Yeh JJ. An Asp to Strike Out Cancer? Therapeutic Possibilities Arising from Aspartate’s Emerging Roles in Cell Proliferation and Survival. Biomolecules. 2021;11(11):1666. 10.3390/biom11111666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Raho S, Capobianco L, Malivindi R, et al. KRAS-regulated glutamine metabolism requires UCP2-mediated aspartate transport to support pancreatic cancer growth. Nat Metabol. 2020;2(12):1373–81. 10.1038/s42255-020-00315-1 [DOI] [PubMed] [Google Scholar]
- 100.Radi R. Oxygen radicals, nitric oxide, and peroxynitrite: Redox pathways in molecular medicine. Proc Natl Acad Sci U S A. 2018;115(23):5839–48. 10.1073/pnas.1804932115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chang CF, Diers AR, Hogg N. Cancer cell metabolism and the modulating effects of nitric oxide. Free Radic Biol Med. 2015;79:324–36. 10.1016/j.freeradbiomed.2014.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lai KY, Galan SRG, Zeng Y, et al. LanCLs add glutathione to dehydroamino acids generated at phosphorylated sites in the proteome. Cell. 2021;184(10):2680-95.e26. 10.1016/j.cell.2021.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fuks F. DNA methylation and histone modifications: teaming up to silence genes. Curr Opin Genet Dev. 2005;15(5):490–5. 10.1016/j.gde.2005.08.002 [DOI] [PubMed] [Google Scholar]
- 104.Coppedè F. One-carbon epigenetics and redox biology of neurodegeneration. Free Radic Biol Med. 2021;170:19–33. 10.1016/j.freeradbiomed.2020.12.002 [DOI] [PubMed] [Google Scholar]
- 105.Villa E, Sahu U, O’Hara BP, et al. mTORC1 stimulates cell growth through SAM synthesis and m(6)A mRNA-dependent control of protein synthesis. Mol Cell. 2021;81(10):2076-93.e9. 10.1016/j.molcel.2021.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Li T, Tan YT, Chen YX, et al. Methionine deficiency facilitates antitumour immunity by altering m(6)A methylation of immune checkpoint transcripts. Gut. 2023;72(3):501–11. 10.1136/gutjnl-2022-326928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Black JC, Van Rechem C, Whetstine JR. Histone lysine methylation dynamics: establishment, regulation, and biological impact. Mol Cell. 2012;48(4):491–507. 10.1016/j.molcel.2012.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Plotnikov A, Kozer N, Cohen G, et al. PRMT1 inhibition induces differentiation of colon cancer cells. Sci Reports. 2020;10(1):20030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hwang JW, Cho Y, Bae GU, Kim SN, Kim YK. Protein arginine methyltransferases: promising targets for cancer therapy. Exp Mol Med. 2021;53(5):788–808. 10.1038/s12276-021-00613-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bryant JP, Heiss J, Banasavadi-Siddegowda YK. Arginine Methylation in Brain Tumors: Tumor Biology and Therapeutic Strategies. Cells. 2021;10(1):124. 10.3390/cells10010124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Toubhans B, Alkafri N, Quintela M, et al. Selenium nanoparticles modulate histone methylation via lysine methyltransferase activity and S-adenosylhomocysteine depletion. Redox Biol. 2023;61:102641. 10.1016/j.redox.2023.102641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Li Y, Ge K, Li T, Cai R, Chen Y. The engagement of histone lysine methyltransferases with nucleosomes: structural basis, regulatory mechanisms, and therapeutic implications. Prot Cell. 2023;14(3):165–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Li JT, Yang H, Lei MZ, et al. Dietary folate drives methionine metabolism to promote cancer development by stabilizing MAT IIA. Signal Transduct Target Ther. 2022;7(1):192. 10.1038/s41392-022-01017-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lu C, Thompson CB. Metabolic regulation of epigenetics. Cell Metabol. 2012;16(1):9–17. 10.1016/j.cmet.2012.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sivanand S, Vander Heiden MG. Emerging Roles for Branched-Chain Amino Acid Metabolism in Cancer. Cancer Cell. 2020;37(2):147–56. 10.1016/j.ccell.2019.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lieu EL, Nguyen T, Rhyne S, Kim J. Amino acids in cancer. Exp Mol Med. 2020;52(1):15–30. 10.1038/s12276-020-0375-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Dimou A, Tsimihodimos V, Bairaktari E. The Critical Role of the Branched Chain Amino Acids (BCAAs) Catabolism-Regulating Enzymes, Branched-Chain Aminotransferase (BCAT) and Branched-Chain α-Keto Acid Dehydrogenase (BCKD), in Human Pathophysiology. Int J Mol Sci. 2022;23(7):192. 10.3390/ijms23074022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chen W, Yu X, Wu Y, et al. The SESAME complex regulates cell senescence through the generation of acetyl-CoA. Nat Metabol. 2021;3(7):983–1000. 10.1038/s42255-021-00412-9 [DOI] [PubMed] [Google Scholar]
- 119.Ma W, Sun Y, Yan R, et al. OXCT1 functions as a succinyltransferase, contributing to hepatocellular carcinoma via succinylating LACTB. Mol Cell. 2024;84(3):538-51.e7. 10.1016/j.molcel.2023.11.042 [DOI] [PubMed] [Google Scholar]
- 120.Smestad J, Erber L, Chen Y, Maher LJ 3rd. Chromatin Succinylation Correlates with Active Gene Expression and Is Perturbed by Defective TCA Cycle Metabolism. iScience. 2018;2:63–75. 10.1016/j.isci.2018.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tong Y, Guo D, Lin SH, et al. SUCLA2-coupled regulation of GLS succinylation and activity counteracts oxidative stress in tumor cells. Mol Cell. 2021;81(11):2303-16.e8. 10.1016/j.molcel.2021.04.002 [DOI] [PubMed] [Google Scholar]
- 122.Shi L, Yao H, Liu Z, Xu M, Tsung A, Wang Y. Endogenous PAD4 in Breast Cancer Cells Mediates Cancer Extracellular Chromatin Network Formation and Promotes Lung Metastasis. Mol Cancer Res. 2020;18(5):735–47. 10.1158/1541-7786.MCR-19-0018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tanikawa C, Espinosa M, Suzuki A, et al. Regulation of histone modification and chromatin structure by the p53-PADI4 pathway. Nat Comm. 2012;3:676. 10.1038/ncomms1676 [DOI] [PubMed] [Google Scholar]
- 124.Li P, Wang D, Yao H, et al. Coordination of PAD4 and HDAC2 in the regulation of p53-target gene expression. Oncogene. 2010;29(21):3153–62. 10.1038/onc.2010.51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Mauracher LM, Posch F, Martinod K, et al. Citrullinated histone H3, a biomarker of neutrophil extracellular trap formation, predicts the risk of venous thromboembolism in cancer patients. J Thrombosis Haemostasis. 2018;16(3):508–18. 10.1111/jth.13951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Huang H, Zhang D, Weng Y, et al. The regulatory enzymes and protein substrates for the lysine β-hydroxybutyrylation pathway. Science Adv. 2021;7(9):eabe2771. 10.1126/sciadv.abe2771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Liu K, Li F, Sun Q, et al. p53 β-hydroxybutyrylation attenuates p53 activity. Cell Death Dis. 2019;10(3):243. 10.1038/s41419-019-1463-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kaech SM, Wherry EJ, Ahmed R. Effector and memory T-cell differentiation: implications for vaccine development. Nat Rev Immunol. 2002;2(4):251–62. 10.1038/nri778 [DOI] [PubMed] [Google Scholar]
- 129.Sinclair LV, Howden AJ, Brenes A, et al. Antigen receptor control of methionine metabolism in T cells. Life. 2019;8:e44210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bian Y, Li W, Kremer DM, et al. Cancer SLC43A2 alters T cell methionine metabolism and histone methylation. Nature. 2020;585(7824):277–82. 10.1038/s41586-020-2682-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Roy DG, Chen J, Mamane V, et al. Methionine Metabolism Shapes T Helper Cell Responses through Regulation of Epigenetic Reprogramming. Cell Metabol. 2020;31(2):250-66.e9. 10.1016/j.cmet.2020.01.006 [DOI] [PubMed] [Google Scholar]
- 132.Hung MH, Lee JS, Ma C, et al. Tumor methionine metabolism drives T-cell exhaustion in hepatocellular carcinoma. Nat Comm. 2021;12(1):1455. 10.1038/s41467-021-21804-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gmünder H, Eck HP, Benninghoff B, Roth S, Dröge W. Macrophages regulate intracellular glutathione levels of lymphocytes. Evidence for an immunoregulatory role of cysteine. Cell Immuno. 1990;129(1):32–46. 10.1016/0008-8749(90)90184-S [DOI] [PubMed] [Google Scholar]
- 134.Srivastava MK, Sinha P, Clements VK, Rodriguez P, Ostrand-Rosenberg S. Myeloid-derived suppressor cells inhibit T-cell activation by depleting cystine and cysteine. Cancer Res. 2010;70(1):68–77. 10.1158/0008-5472.CAN-09-2587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wang W, Green M, Choi JE, et al. CD8(+) T cells regulate tumour ferroptosis during cancer immunotherapy. Nature. 2019;569(7755):270–4. 10.1038/s41586-019-1170-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yue T, Li J, Zhu J, et al. Hydrogen Sulfide Creates a Favorable Immune Microenvironment for Colon Cancer. Cancer Res. 2023;83(4):595–612. 10.1158/0008-5472.CAN-22-1837 [DOI] [PubMed] [Google Scholar]
- 137.Swamy M, Pathak S, Grzes KM, et al. Glucose and glutamine fuel protein O-GlcNAcylation to control T cell self-renewal and malignancy. Nat Immunol. 2016;17(6):712–20. 10.1038/ni.3439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lian G, Gnanaprakasam JR, Wang T, et al. Glutathione de novo synthesis but not recycling process coordinates with glutamine catabolism to control redox homeostasis and directs murine T cell differentiation. eLife. 2018;7:e36158. 10.7554/eLife.36158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Song M, Sandoval TA, Chae CS, et al. IRE1α-XBP1 controls T cell function in ovarian cancer by regulating mitochondrial activity. Nature. 2018;562(7727):423–8. 10.1038/s41586-018-0597-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Huang H, Zhou P, Wei J, et al. In vivo CRISPR screening reveals nutrient signaling processes underpinning CD8(+) T cell fate decisions. Cell. 2021;184(5):1245-61.e21. 10.1016/j.cell.2021.02.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Nakaya M, Xiao Y, Zhou X, et al. Inflammatory T cell responses rely on amino acid transporter ASCT2 facilitation of glutamine uptake and mTORC1 kinase activation. Immunity. 2014;40(5):692–705. 10.1016/j.immuni.2014.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hainz U, Obexer P, Winkler C, et al. Monocyte-mediated T-cell suppression and augmented monocyte tryptophan catabolism after human hematopoietic stem-cell transplantation. Blood. 2005;105(10):4127–34. 10.1182/blood-2004-05-1726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Uyttenhove C, Pilotte L, Théate I, et al. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat Med. 2003;9(10):1269–74. 10.1038/nm934 [DOI] [PubMed] [Google Scholar]
- 144.Byakwaga H, Boum Y 2nd, Huang Y, et al. The kynurenine pathway of tryptophan catabolism, CD4+ T-cell recovery, and mortality among HIV-infected Ugandans initiating antiretroviral therapy. J Infect Dis. 2014;210(3):383–91. 10.1093/infdis/jiu115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Tyrakis PA, Palazon A, Macias D, et al. S-2-hydroxyglutarate regulates CD8(+) T-lymphocyte fate. Nature. 2016;540(7632):236–41. 10.1038/nature20165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Baksh SC, Finley LWS. Metabolic Coordination of Cell Fate by α-Ketoglutarate-Dependent Dioxygenases. Trends Cell Biol. 2021;31(1):24–36. 10.1016/j.tcb.2020.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Huang X, Sun T, Wang J, et al. Metformin Reprograms Tryptophan Metabolism to Stimulate CD8+ T-cell Function in Colorectal Cancer. Cancer Res. 2023;83(14):2358–71. 10.1158/0008-5472.CAN-22-3042 [DOI] [PubMed] [Google Scholar]
- 148.Bender MJ, McPherson AC, Phelps CM, et al. Dietary tryptophan metabolite released by intratumoral Lactobacillus reuteri facilitates immune checkpoint inhibitor treatment. Cell. 2023;186(9):1846-62.e26. 10.1016/j.cell.2023.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kenski JCN, Huang X, Vredevoogd DW, et al. An adverse tumor-protective effect of IDO1 inhibition. Cell Rep Med. 2023;4(2):100941. 10.1016/j.xcrm.2023.100941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zhu X, Pribis JP, Rodriguez PC, et al. The central role of arginine catabolism in T-cell dysfunction and increased susceptibility to infection after physical injury. Ann Surg. 2014;259(1):171–8. 10.1097/SLA.0b013e31828611f8 [DOI] [PubMed] [Google Scholar]
- 151.Werner A, Amann E, Schnitzius V, et al. Induced arginine transport via cationic amino acid transporter-1 is necessary for human T-cell proliferation. Eur J Immunol. 2016;46(1):92–103. 10.1002/eji.201546047 [DOI] [PubMed] [Google Scholar]
- 152.Rodriguez PC, Zea AH, Culotta KS, Zabaleta J, Ochoa JB, Ochoa AC. Regulation of T cell receptor CD3zeta chain expression by L-arginine. J Biol Chem. 2002;277(24):21123–9. 10.1074/jbc.M110675200 [DOI] [PubMed] [Google Scholar]
- 153.Baumann T, Dunkel A, Schmid C, et al. Regulatory myeloid cells paralyze T cells through cell-cell transfer of the metabolite methylglyoxal. Nat Immunol. 2020;21(5):555–66. 10.1038/s41590-020-0666-9 [DOI] [PubMed] [Google Scholar]
- 154.Menjivar RE, Nwosu ZC, Du W, et al. Arginase 1 is a key driver of immune suppression in pancreatic cancer. eLife. 2023;12:e80721. 10.7554/eLife.80721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Gnanaprakasam JN, Wang R. MYC in Regulating Immunity: Metabolism and Beyond. Genes. 2017;8(3):88. 10.3390/genes8030088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Bowlin TL, McKown BJ, Sunkara PS. Increased ornithine decarboxylase activity and polyamine biosynthesis are required for optimal cytolytic T lymphocyte induction. Cell Immunol. 1987;105(1):110–7. 10.1016/0008-8749(87)90060-8 [DOI] [PubMed] [Google Scholar]
- 157.Puleston DJ, Baixauli F, Sanin DE, et al. Polyamine metabolism is a central determinant of helper T cell lineage fidelity. Cell. 2021;184(16):4186-202.e20. 10.1016/j.cell.2021.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Holbert CE, Cullen MT, Casero RA Jr, Stewart TM. Polyamines in cancer: integrating organismal metabolism and antitumour immunity. Nat Rev Cancer. 2022;22(8):467–80. 10.1038/s41568-022-00473-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Wilson CB, Rowell E, Sekimata M. Epigenetic control of T-helper-cell differentiation. Nat Rev Immunol. 2009;9(2):91–105. 10.1038/nri2487 [DOI] [PubMed] [Google Scholar]
- 160.Ma EH, Bantug G, Griss T, et al. Serine Is an Essential Metabolite for Effector T Cell Expansion. Cell Metabol. 2017;25(2):345–57. 10.1016/j.cmet.2016.12.011 [DOI] [PubMed] [Google Scholar]
- 161.Yao CC, Sun RM, Yang Y, et al. Accumulation of branched-chain amino acids reprograms glucose metabolism in CD8(+) T cells with enhanced effector function and anti-tumor response. Cell Rep. 2023;42(3):112186. 10.1016/j.celrep.2023.112186 [DOI] [PubMed] [Google Scholar]
- 162.Nunes EA, Lomax AR, Noakes PS, Miles EA, Fernandes LC, Calder PC. β-Hydroxy-β-methylbutyrate modifies human peripheral blood mononuclear cell proliferation and cytokine production in vitro. Nutrition. 2011;27(1):92–9. 10.1016/j.nut.2009.12.008 [DOI] [PubMed] [Google Scholar]
- 163.Sinclair LV, Rolf J, Emslie E, Shi YB, Taylor PM, Cantrell DA. Control of amino-acid transport by antigen receptors coordinates the metabolic reprogramming essential for T cell differentiation. Nat Immunol. 2013;14(5):500–8. 10.1038/ni.2556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Wu J, Li G, Li L, Li D, Dong Z, Jiang P. Asparagine enhances LCK signalling to potentiate CD8(+) T-cell activation and anti-tumour responses. Nat Cell Biol. 2021;23(1):75–86. 10.1038/s41556-020-00615-4 [DOI] [PubMed] [Google Scholar]
- 165.Gnanaprakasam JNR, Kushwaha B, Liu L, et al. Asparagine restriction enhances CD8(+) T cell metabolic fitness and antitumoral functionality through an NRF2-dependent stress response. Nat Metabol. 2023;5(8):1423–39. 10.1038/s42255-023-00856-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Baecher-Allan C, Viglietta V, Hafler DA. Human CD4+CD25+ regulatory T cells. Sem Immunol. 2004;16(2):89–98. 10.1016/j.smim.2003.12.005 [DOI] [PubMed] [Google Scholar]
- 167.Togashi Y, Shitara K, Nishikawa H. Regulatory T cells in cancer immunosuppression - implications for anticancer therapy. Nat Rev Clin Oncol. 2019;16(6):356–71. 10.1038/s41571-019-0175-7 [DOI] [PubMed] [Google Scholar]
- 168.Saini N, Naaz A, Metur SP, et al. Methionine uptake via the SLC43A2 transporter is essential for regulatory T-cell survival. Life Sci Alliance. 2022;5(12):e202201663. 10.26508/lsa.202201663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Koppula P, Zhuang L, Gan B. Cystine transporter SLC7A11/xCT in cancer: ferroptosis, nutrient dependency, and cancer therapy. Protein Cell. 2021;12(8):599–620. 10.1007/s13238-020-00789-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kurniawan H, Franchina DG, Guerra L, et al. Glutathione Restricts Serine Metabolism to Preserve Regulatory T Cell Function. Cell Metabol. 2020;31(5):920-36.e7. 10.1016/j.cmet.2020.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Toulmonde M, Penel N, Adam J, et al. Use of PD-1 Targeting, Macrophage Infiltration, and IDO Pathway Activation in Sarcomas: A Phase 2 Clinical Trial. JAMA Oncol. 2018;4(1):93–7. 10.1001/jamaoncol.2017.1617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Sharma MD, Baban B, Chandler P, et al. Plasmacytoid dendritic cells from mouse tumor-draining lymph nodes directly activate mature Tregs via indoleamine 2,3-dioxygenase. J Clin Invest. 2007;117(9):2570–82. 10.1172/JCI31911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Carriche GM, Almeida L, Stüve P, et al. Regulating T-cell differentiation through the polyamine spermidine. J Allergy Clin Immunol. 2021;147(1):335-48.e11. 10.1016/j.jaci.2020.04.037 [DOI] [PubMed] [Google Scholar]
- 174.Wagner A, Wang C, Fessler J, et al. Metabolic modeling of single Th17 cells reveals regulators of autoimmunity. Cell. 2021;184(16):4168-85.e21. 10.1016/j.cell.2021.05.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Kiessling R, Klein E, Wigzell H. “Natural” killer cells in the mouse. I. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Specificity and distribution according to genotype. Eur J Immunol. 1975;5(2):112–7. 10.1002/eji.1830050208 [DOI] [PubMed] [Google Scholar]
- 176.Crinier A, Milpied P, Escalière B, et al. High-Dimensional Single-Cell Analysis Identifies Organ-Specific Signatures and Conserved NK Cell Subsets in Humans and Mice. Immunity. 2018;49(5):971-86.e5. 10.1016/j.immuni.2018.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Loftus RM, Assmann N, Kedia-Mehta N, et al. Amino acid-dependent cMyc expression is essential for NK cell metabolic and functional responses in mice. Nat Comm. 2018;9(1):2341. 10.1038/s41467-018-04719-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Almutairi SM, Ali AK, He W, et al. Interleukin-18 up-regulates amino acid transporters and facilitates amino acid-induced mTORC1 activation in natural killer cells. J Biol Chem. 2019;294(12):4644–55. 10.1074/jbc.RA118.005892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Choi WM, Ryu T, Lee JH, et al. Metabotropic Glutamate Receptor 5 in Natural Killer Cells Attenuates Liver Fibrosis by Exerting Cytotoxicity to Activated Stellate Cells. Hepatol (Baltimore, Md). 2021;74(4):2170–85. 10.1002/hep.31875 [DOI] [PubMed] [Google Scholar]
- 180.Cui JX, Xu XH, He T, et al. L-kynurenine induces NK cell loss in gastric cancer microenvironment via promoting ferroptosis. J Exp Clin Cancer Res. 2023;42(1):52. 10.1186/s13046-023-02629-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Yang SL, Tan HX, Niu TT, Li DJ, Wang HY, Li MQ. Kynurenine promotes the cytotoxicity of NK cells through aryl hydrocarbon receptor in early pregnancy. J Reprod Immunol. 2021;143:103270. 10.1016/j.jri.2020.103270 [DOI] [PubMed] [Google Scholar]
- 182.Liang R, Cheng A, Lu S, et al. Seleno-amino Acid Metabolism Reshapes the Tumor Microenvironment: from Cytotoxicity to Immunotherapy. Int J Biol Sci. 2024;20(7):2779–89. 10.7150/ijbs.95484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Pan S, Guan J, Xianyu B, Tan Y, Li T, Xu H. A Nanotherapeutic Strategy to Reverse NK Cell Exhaustion. Adv Mater. 2023;35(23):e2211370. 10.1002/adma.202211370 [DOI] [PubMed] [Google Scholar]
- 184.Torigoe M, Maeshima K, Ozaki T, et al. l-Leucine influx through Slc7a5 regulates inflammatory responses of human B cells via mammalian target of rapamycin complex 1 signaling. Mod Rheumatol. 2019;29(5):885–91. 10.1080/14397595.2018.1510822 [DOI] [PubMed] [Google Scholar]
- 185.Jiang S, Yan W, Wang SE, Baltimore D. Let-7 Suppresses B Cell Activation through Restricting the Availability of Necessary Nutrients. Cell Metabol. 2018;27(2):393-403.e4. 10.1016/j.cmet.2017.12.007 [DOI] [PubMed] [Google Scholar]
- 186.van Beek AA, Hugenholtz F, Meijer B, et al. Frontline Science: Tryptophan restriction arrests B cell development and enhances microbial diversity in WT and prematurely aging Ercc1(-/Δ7) mice. J Leukocyte Biol. 2017;101(4):811–21. 10.1189/jlb.1HI0216-062RR [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Guo R, Liang JH, Zhang Y, et al. Methionine metabolism controls the B cell EBV epigenome and viral latency. Cell Metabol. 2022;34(9):1280-97.e9. 10.1016/j.cmet.2022.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Nitta T, Igarashi K, Yamashita A, Yamamoto M, Yamamoto N. Involvement of polyamines in B cell receptor-mediated apoptosis: spermine functions as a negative modulator. Exp Cell Res. 2001;265(1):174–83. 10.1006/excr.2001.5177 [DOI] [PubMed] [Google Scholar]
- 189.Zhang B, Vogelzang A, Miyajima M, et al. B cell-derived GABA elicits IL-10(+) macrophages to limit anti-tumour immunity. Nature. 2021;599(7885):471–6. 10.1038/s41586-021-04082-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Hume DA. The mononuclear phagocyte system. Curr Opinion Immunol. 2006;18(1):49–53. 10.1016/j.coi.2005.11.008 [DOI] [PubMed] [Google Scholar]
- 191.Locati M, Curtale G, Mantovani A. Diversity, Mechanisms, and Significance of Macrophage Plasticity. Ann Rev Pathol. 2020;15:123–47. 10.1146/annurev-pathmechdis-012418-012718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Baker NE. Emerging mechanisms of cell competition. Nat Rev Genet. 2020;21(11):683–97. 10.1038/s41576-020-0262-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Zhang X, Li S, Malik I, et al. Reprogramming tumour-associated macrophages to outcompete cancer cells. Nature. 2023;619(7970):616–23. 10.1038/s41586-023-06256-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Dos Santos LM, da Silva TM, Azambuja JH, et al. Methionine and methionine sulfoxide treatment induces M1/classical macrophage polarization and modulates oxidative stress and purinergic signaling parameters. Mol Cell Biochem. 2017;424(1–2):69–78. 10.1007/s11010-016-2843-6 [DOI] [PubMed] [Google Scholar]
- 195.Wen D, Wang S, Yu J, Yu T, Liu Z, Li Y. Analysis of clinical significance and molecular characteristics of methionine metabolism and macrophage-related patterns in hepatocellular carcinoma based on machine learning. Cancer Biomarkers. 2024;39(1):37–48. 10.3233/CBM-220421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Yu W, Wang Z, Zhang K, et al. One-Carbon Metabolism Supports S-Adenosylmethionine and Histone Methylation to Drive Inflammatory Macrophages. Mol Cell. 2019;75(6):1147-60.e5. 10.1016/j.molcel.2019.06.039 [DOI] [PubMed] [Google Scholar]
- 197.Platten M, Nollen EAA, Röhrig UF, Fallarino F, Opitz CA. Tryptophan metabolism as a common therapeutic target in cancer, neurodegeneration and beyond. Nat Rev Drug Discov. 2019;18(5):379–401. 10.1038/s41573-019-0016-5 [DOI] [PubMed] [Google Scholar]
- 198.Minhas PS, Liu L, Moon PK, et al. Macrophage de novo NAD(+) synthesis specifies immune function in aging and inflammation. Nat Immunol. 2019;20(1):50–63. 10.1038/s41590-018-0255-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Bosco MC, Rapisarda A, Massazza S, Melillo G, Young H, Varesio L. The tryptophan catabolite picolinic acid selectively induces the chemokines macrophage inflammatory protein-1 alpha and -1 beta in macrophages. J Immunol. 2000;164(6):3283–91. 10.4049/jimmunol.164.6.3283 [DOI] [PubMed] [Google Scholar]
- 200.Hezaveh K, Shinde RS, Klötgen A, et al. Tryptophan-derived microbial metabolites activate the aryl hydrocarbon receptor in tumor-associated macrophages to suppress anti-tumor immunity. Immunity. 2022;55(2):324-40.e8. 10.1016/j.immuni.2022.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Jha AK, Huang SC, Sergushichev A, et al. Network integration of parallel metabolic and transcriptional data reveals metabolic modules that regulate macrophage polarization. Immunity. 2015;42(3):419–30. 10.1016/j.immuni.2015.02.005 [DOI] [PubMed] [Google Scholar]
- 202.Liu PS, Wang H, Li X, et al. α-ketoglutarate orchestrates macrophage activation through metabolic and epigenetic reprogramming. Nat Immunol. 2017;18(9):985–94. 10.1038/ni.3796 [DOI] [PubMed] [Google Scholar]
- 203.Merlin J, Ivanov S, Dumont A, et al. Non-canonical glutamine transamination sustains efferocytosis by coupling redox buffering to oxidative phosphorylation. Nat Metabol. 2021;3(10):1313–26. 10.1038/s42255-021-00471-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Yang L, Chu Z, Liu M, et al. Amino acid metabolism in immune cells: essential regulators of the effector functions, and promising opportunities to enhance cancer immunotherapy. J Hematol Oncol. 2023;16(1):59. 10.1186/s13045-023-01453-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.MacMicking J, Xie QW, Nathan C. Nitric oxide and macrophage function. Ann Rev Immunol. 1997;15:323–50. 10.1146/annurev.immunol.15.1.323 [DOI] [PubMed] [Google Scholar]
- 206.Munder M, Eichmann K, Modolell M. Alternative metabolic states in murine macrophages reflected by the nitric oxide synthase/arginase balance: competitive regulation by CD4+ T cells correlates with Th1/Th2 phenotype. J Immunol. 1998;160(11):5347–54. 10.4049/jimmunol.160.11.5347 [DOI] [PubMed] [Google Scholar]
- 207.Ji L, Zhao X, Zhang B, et al. Slc6a8-Mediated Creatine Uptake and Accumulation Reprogram Macrophage Polarization via Regulating Cytokine Responses. Immunity. 2019;51(2):272-84.e7. 10.1016/j.immuni.2019.06.007 [DOI] [PubMed] [Google Scholar]
- 208.Zhao S, Zhou L, Wang Q, et al. Elevated branched-chain amino acid promotes atherosclerosis progression by enhancing mitochondrial-to-nuclear H(2)O(2)-disulfide HMGB1 in macrophages. Redox Biol. 2023;62:102696. 10.1016/j.redox.2023.102696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Chen S, Xia Y, He F, et al. Serine Supports IL-1β Production in Macrophages Through mTOR Signaling. Front Immunol. 2020;11:1866. 10.3389/fimmu.2020.01866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Rodriguez AE, Ducker GS, Billingham LK, et al. Serine Metabolism Supports Macrophage IL-1β Production. Cell Metabol. 2019;29(4):1003-11.e4. 10.1016/j.cmet.2019.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Shan X, Hu P, Ni L, et al. Serine metabolism orchestrates macrophage polarization by regulating the IGF1-p38 axis. Cell Mol Immunol. 2022;19(11):1263–78. 10.1038/s41423-022-00925-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Wang C, Chen Q, Chen S, et al. Serine synthesis sustains macrophage IL-1β production via NAD(+)-dependent protein acetylation. Mol Cell. 2024;84(4):744-59.e6. 10.1016/j.molcel.2024.01.002 [DOI] [PubMed] [Google Scholar]
- 213.Liew PX, Kubes P. The Neutrophil’s Role During Health and Disease. Physiol Rev. 2019;99(2):1223–48. 10.1152/physrev.00012.2018 [DOI] [PubMed] [Google Scholar]
- 214.Wu Y, Ma J, Yang X, et al. Neutrophil profiling illuminates anti-tumor antigen-presenting potency. Cell. 2024;187(6):1422-39.e24. 10.1016/j.cell.2024.02.005 [DOI] [PubMed] [Google Scholar]
- 215.Lagranha CJ, Levada-Pires AC, Sellitti DF, Procopio J, Curi R, Pithon-Curi TC. The effect of glutamine supplementation and physical exercise on neutrophil function. Amino Acids. 2008;34(3):337–46. 10.1007/s00726-007-0560-x [DOI] [PubMed] [Google Scholar]
- 216.Castell LM, Newsholme EA. The relation between glutamine and the immunodepression observed in exercise. Amino Acids. 2001;20(1):49–61. 10.1007/s007260170065 [DOI] [PubMed] [Google Scholar]
- 217.Galkina SI, Fedorova NV, Ksenofontov AL, et al. Neutrophil Adhesion and the Release of the Free Amino Acid Hydroxylysine. Cells. 2021;10(3):563. 10.3390/cells10030563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Galkina SI, Fedorova NV, Ksenofontov AL, Stadnichuk VI, Baratova LA, Sud’Ina GF. Neutrophils as a source of branched-chain, aromatic and positively charged free amino acids. Cell Adh Migr. 2019;13(1):98–105. 10.1080/19336918.2018.1540903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Steinman RM, Cohn ZA. Identification of a novel cell type in peripheral lymphoid organs of mice. I. Morphology, quantitation, tissue distribution. J Exp Med. 1973;137(5):1142–62. 10.1084/jem.137.5.1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Cabeza-Cabrerizo M, Cardoso A, Minutti CM, da Pereira Costa M, Reis e Sousa C. Dendritic Cells Revisited. Ann Rev Immunol. 2021;39:131–66. 10.1146/annurev-immunol-061020-053707 [DOI] [PubMed] [Google Scholar]
- 221.Wculek SK, Cueto FJ, Mujal AM, Melero I, Krummel MF, Sancho D. Dendritic cells in cancer immunology and immunotherapy. Nat Rev Immunol. 2020;20(1):7–24. 10.1038/s41577-019-0210-z [DOI] [PubMed] [Google Scholar]
- 222.Stamper CC, Zhang Y, Tobin JF, et al. Crystal structure of the B7–1/CTLA-4 complex that inhibits human immune responses. Nature. 2001;410(6828):608–11. 10.1038/35069118 [DOI] [PubMed] [Google Scholar]
- 223.Peng Q, Qiu X, Zhang Z, et al. PD-L1 on dendritic cells attenuates T cell activation and regulates response to immune checkpoint blockade. Nat Comm. 2020;11(1):4835. 10.1038/s41467-020-18570-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Guo C, You Z, Shi H, et al. SLC38A2 and glutamine signalling in cDC1s dictate anti-tumour immunity. Nature. 2023;620(7972):200–8. 10.1038/s41586-023-06299-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Fallarino F, Grohmann U, Hwang KW, et al. Modulation of tryptophan catabolism by regulatory T cells. Nat Immunol. 2003;4(12):1206–12. 10.1038/ni1003 [DOI] [PubMed] [Google Scholar]
- 226.Stiff A, Trikha P, Wesolowski R, et al. Myeloid-Derived Suppressor Cells Express Bruton’s Tyrosine Kinase and Can Be Depleted in Tumor-Bearing Hosts by Ibrutinib Treatment. Cancer Res. 2016;76(8):2125–36. 10.1158/0008-5472.CAN-15-1490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Sharma MD, Pacholczyk R, Shi H, et al. Inhibition of the BTK-IDO-mTOR axis promotes differentiation of monocyte-lineage dendritic cells and enhances anti-tumor T cell immunity. Immunity. 2021;54(10):2354-71.e8. 10.1016/j.immuni.2021.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Mondanelli G, Bianchi R, Pallotta MT, et al. A Relay Pathway between Arginine and Tryptophan Metabolism Confers Immunosuppressive Properties on Dendritic Cells. Immunity. 2017;46(2):233–44. 10.1016/j.immuni.2017.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Gabrilovich DI, Bronte V, Chen SH, et al. The terminology issue for myeloid-derived suppressor cells. Cancer Res. 2007;67(1):425 author reply 6. 10.1158/0008-5472.CAN-06-3037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Pallett LJ, Gill US, Quaglia A, et al. Metabolic regulation of hepatitis B immunopathology by myeloid-derived suppressor cells. Nat Med. 2015;21(6):591–600. 10.1038/nm.3856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Bronte V, Zanovello P. Regulation of immune responses by L-arginine metabolism. Nat Rev Immunol. 2005;5(8):641–54. 10.1038/nri1668 [DOI] [PubMed] [Google Scholar]
- 232.Yu J, Du W, Yan F, et al. Myeloid-derived suppressor cells suppress antitumor immune responses through IDO expression and correlate with lymph node metastasis in patients with breast cancer. J Immunol. 2013;190(7):3783–97. 10.4049/jimmunol.1201449 [DOI] [PubMed] [Google Scholar]
- 233.Li A, Barsoumian HB, Schoenhals JE, et al. Indoleamine 2,3-dioxygenase 1 inhibition targets anti-PD1-resistant lung tumors by blocking myeloid-derived suppressor cells. Cancer Lett. 2018;431:54–63. 10.1016/j.canlet.2018.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Zhou Q, Peng Y, Ji F, et al. Targeting of SLC25A22 boosts the immunotherapeutic response in KRAS-mutant colorectal cancer. Nat Comm. 2023;14(1):4677. 10.1038/s41467-023-39571-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Oh MH, Sun IH, Zhao L, et al. Targeting glutamine metabolism enhances tumor-specific immunity by modulating suppressive myeloid cells. J Clin Invest. 2020;130(7):3865–84. 10.1172/JCI131859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Sun HW, Wu WC, Chen HT, et al. Glutamine Deprivation Promotes the Generation and Mobilization of MDSCs by Enhancing Expression of G-CSF and GM-CSF. Front Immunol. 2020;11:616367. 10.3389/fimmu.2020.616367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Hammami I, Chen J, Bronte V, DeCrescenzo G, Jolicoeur M. L-glutamine is a key parameter in the immunosuppression phenomenon. Biochem Biophys Res Commun. 2012;425(4):724–9. 10.1016/j.bbrc.2012.07.139 [DOI] [PubMed] [Google Scholar]
- 238.Dang Q, Sun Z, Wang Y, Wang L, Liu Z, Han X. Ferroptosis: a double-edged sword mediating immune tolerance of cancer. Cell Death Dis. 2022;13(11):925. 10.1038/s41419-022-05384-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Marangoni F, Zhakyp A, Corsini M, et al. Expansion of tumor-associated Treg cells upon disruption of a CTLA-4-dependent feedback loop. Cell. 2021;184(15):3998-4015.e19. 10.1016/j.cell.2021.05.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Xu C, Sun S, Johnson T, et al. The glutathione peroxidase Gpx4 prevents lipid peroxidation and ferroptosis to sustain Treg cell activation and suppression of antitumor immunity. Cell Rep. 2021;35(11):109235. 10.1016/j.celrep.2021.109235 [DOI] [PubMed] [Google Scholar]
- 241.Friedmann Angeli JP, Krysko DV, Conrad M. Ferroptosis at the crossroads of cancer-acquired drug resistance and immune evasion. Nat Rev Cancer. 2019;19(7):405–14. 10.1038/s41568-019-0149-1 [DOI] [PubMed] [Google Scholar]
- 242.Tanaka S, Ise W, Inoue T, et al. Tet2 and Tet3 in B cells are required to repress CD86 and prevent autoimmunity. Nat Immunol. 2020;21(8):950–61. 10.1038/s41590-020-0700-y [DOI] [PubMed] [Google Scholar]
- 243.Zhou X, Zou L, Liao H, et al. Abrogation of HnRNP L enhances anti-PD-1 therapy efficacy via diminishing PD-L1 and promoting CD8(+) T cell-mediated ferroptosis in castration-resistant prostate cancer. Acta Pharmaceutica Sinica B. 2022;12(2):692–707. 10.1016/j.apsb.2021.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Tesi RJ. MDSC; the Most Important Cell You Have Never Heard Of. Trends Pharmacol Sci. 2019;40(1):4–7. 10.1016/j.tips.2018.10.008 [DOI] [PubMed] [Google Scholar]
- 245.Kapralov AA, Yang Q, Dar HH, et al. Redox lipid reprogramming commands susceptibility of macrophages and microglia to ferroptotic death. Nat Chem Biol. 2020;16(3):278–90. 10.1038/s41589-019-0462-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Giuliani C. The Flavonoid Quercetin Induces AP-1 Activation in FRTL-5 Thyroid Cells. Antioxidants (Basel). 2019;8(5):112. 10.3390/antiox8050112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Li J, Cao F, Yin HL, et al. Ferroptosis: past, present and future. Cell Death Disease. 2020;11(2):88. 10.1038/s41419-020-2298-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Yang WS, SriRamaratnam R, Welsch ME, et al. Regulation of ferroptotic cancer cell death by GPX4. Cell. 2014;156(1–2):317–31. 10.1016/j.cell.2013.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Hayano M, Yang WS, Corn CK, Pagano NC, Stockwell BR. Loss of cysteinyl-tRNA synthetase (CARS) induces the transsulfuration pathway and inhibits ferroptosis induced by cystine deprivation. Cell Death Differ. 2016;23(2):270–8. 10.1038/cdd.2015.93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Kang YP, Torrente L, Falzone A, et al. Cysteine dioxygenase 1 is a metabolic liability for non-small cell lung cancer. Life. 2019;8:e45572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Taurino G, Chiu M, Bianchi MG, Griffini E, Bussolati O. The SLC38A5/SNAT5 amino acid transporter: from pathophysiology to pro-cancer roles in the tumor microenvironment. Am J Physiol Cell Physiol. 2023;325(2):C550-c62. 10.1152/ajpcell.00169.2023 [DOI] [PubMed] [Google Scholar]
- 252.Yan W, Wu X, Zhou W, et al. Cancer-cell-secreted exosomal miR-105 promotes tumour growth through the MYC-dependent metabolic reprogramming of stromal cells. Nat Cell Biol. 2018;20(5):597–609. 10.1038/s41556-018-0083-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Fong MY, Yan W, Ghassemian M, et al. Cancer-secreted miRNAs regulate amino-acid-induced mTORC1 signaling and fibroblast protein synthesis. EMBO Rep. 2021;22(2):e51239. 10.15252/embr.202051239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Kay EJ, Paterson K, Riera-Domingo C, et al. Cancer-associated fibroblasts require proline synthesis by PYCR1 for the deposition of pro-tumorigenic extracellular matrix. Nat Metabol. 2022;4(6):693–710. 10.1038/s42255-022-00582-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Risau W. Mechanisms of angiogenesis. Nature. 1997;386(6626):671–4. 10.1038/386671a0 [DOI] [PubMed] [Google Scholar]
- 256.Longchamp A, Mirabella T, Arduini A, et al. Amino Acid Restriction Triggers Angiogenesis via GCN2/ATF4 Regulation of VEGF and H(2)S Production. Cell. 2018;173(1):117-29.e14. 10.1016/j.cell.2018.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Li X, Sun X, Carmeliet P. Hallmarks of Endothelial Cell Metabolism in Health and Disease. Cell Metabol. 2019;30(3):414–33. 10.1016/j.cmet.2019.08.011 [DOI] [PubMed] [Google Scholar]
- 258.Pan M, Wasa M, Ryan U, Souba W. Inhibition of pulmonary microvascular endothelial glutamine transport by glucocorticoids and endotoxin. JPEN J Parenter Enteral Nutr. 1995;19(6):477–81. 10.1177/0148607195019006477 [DOI] [PubMed] [Google Scholar]
- 259.Kim B, Li J, Jang C, Arany Z. Glutamine fuels proliferation but not migration of endothelial cells. EMBO J. 2017;36(16):2321–33. 10.15252/embj.201796436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Huang H, Vandekeere S, Kalucka J, et al. Role of glutamine and interlinked asparagine metabolism in vessel formation. EMBO J. 2017;36(16):2334–52. 10.15252/embj.201695518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Vandekeere S, Dubois C, Kalucka J, et al. Serine Synthesis via PHGDH Is Essential for Heme Production in Endothelial Cells. Cell Metabol. 2018;28(4):573-87.e13. 10.1016/j.cmet.2018.06.009 [DOI] [PubMed] [Google Scholar]
- 262.Cantelmo AR, Conradi LC, Brajic A, et al. Inhibition of the Glycolytic Activator PFKFB3 in Endothelium Induces Tumor Vessel Normalization, Impairs Metastasis, and Improves Chemotherapy. Cancer Cell. 2016;30(6):968–85. 10.1016/j.ccell.2016.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Mishra RC, Tripathy S, Quest D, et al. L-Serine lowers while glycine increases blood pressure in chronic L-NAME-treated and spontaneously hypertensive rats. J Hypertens. 2008;26(12):2339–48. 10.1097/HJH.0b013e328312c8a3 [DOI] [PubMed] [Google Scholar]
- 264.Rose ML, Madren J, Bunzendahl H, Thurman RG. Dietary glycine inhibits the growth of B16 melanoma tumors in mice. Carcinogenesis. 1999;20(5):793–8. 10.1093/carcin/20.5.793 [DOI] [PubMed] [Google Scholar]
- 265.Guo D, Murdoch CE, Xu H, et al. Vascular endothelial growth factor signaling requires glycine to promote angiogenesis. Sci Rep. 2017;7(1):14749. 10.1038/s41598-017-15246-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Morris SM Jr. Recent advances in arginine metabolism: roles and regulation of the arginases. Brit J Pharmacol. 2009;157(6):922–30. 10.1111/j.1476-5381.2009.00278.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Tousoulis D, Kampoli AM, Tentolouris C, Papageorgiou N, Stefanadis C. The role of nitric oxide on endothelial function. Curr Vasc Pharmacol. 2012;10(1):4–18. 10.2174/157016112798829760 [DOI] [PubMed] [Google Scholar]
- 268.Cooke JP. NO and angiogenesis. Atherosclerosis Supplements. 2003;4(4):53–60. 10.1016/S1567-5688(03)00034-5 [DOI] [PubMed] [Google Scholar]
- 269.Soda K. The mechanisms by which polyamines accelerate tumor spread. J Exp Clin Cancer Res. 2011;30(1):95. 10.1186/1756-9966-30-95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Li H, Meininger CJ, Hawker JR Jr, et al. Regulatory role of arginase I and II in nitric oxide, polyamine, and proline syntheses in endothelial cells. Am J Physiol Endocrinol Metab. 2001;280(1):E75-82. 10.1152/ajpendo.2001.280.1.E75 [DOI] [PubMed] [Google Scholar]
- 271.McAuslan BR, Reilly W, Hannan GN, Schindhelm K, Milthorpe B, Saur BA. Induction of endothelial cell migration by proline analogs and its relevance to angiogenesis. Exp Cell Res. 1988;176(2):248–57. 10.1016/0014-4827(88)90328-X [DOI] [PubMed] [Google Scholar]
- 272.Nilsson MB, Langley RR, Fidler IJ. Interleukin-6, secreted by human ovarian carcinoma cells, is a potent proangiogenic cytokine. Cancer Res. 2005;65(23):10794–800. 10.1158/0008-5472.CAN-05-0623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Mondal A, Smith C, DuHadaway JB, et al. IDO1 is an Integral Mediator of Inflammatory Neovascularization. EBioMed. 2016;14:74–82. 10.1016/j.ebiom.2016.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Taylor MW, Feng GS. Relationship between interferon-gamma, indoleamine 2,3-dioxygenase, and tryptophan catabolism. FASEB J. 1991;5(11):2516–22. 10.1096/fasebj.5.11.1907934 [DOI] [PubMed] [Google Scholar]
- 275.Dey S, Mondal A, DuHadaway JB, et al. IDO1 Signaling through GCN2 in a Subpopulation of Gr-1(+) Cells Shifts the IFNγ/IL6 Balance to Promote Neovascularization. Cancer Immunol Res. 2021;9(5):514–28. 10.1158/2326-6066.CIR-20-0226 [DOI] [PubMed] [Google Scholar]
- 276.Smith C, Chang MY, Parker KH, et al. IDO is a nodal pathogenic driver of lung cancer and metastasis development. Cancer Discov. 2012;2(8):722–35. 10.1158/2159-8290.CD-12-0014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Liu H, Huang L, Bradley J, et al. GCN2-dependent metabolic stress is essential for endotoxemic cytokine induction and pathology. Mol Cell Biol. 2014;34(3):428–38. 10.1128/MCB.00946-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Hayward MK, Muncie JM, Weaver VM. Tissue mechanics in stem cell fate, development, and cancer. Dev Cell. 2021;56(13):1833–47. 10.1016/j.devcel.2021.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Phuyal S, Romani P, Dupont S, Farhan H. Mechanobiology of organelles: illuminating their roles in mechanosensing and mechanotransduction. Trends Cell Biol. 2023;33(12):1049–61. 10.1016/j.tcb.2023.05.001 [DOI] [PubMed] [Google Scholar]
- 280.Nyga A, Ganguli S, Matthews HK, Baum B. The role of RAS oncogenes in controlling epithelial mechanics. Trends Cell Biol. 2023;33(1):60–9. 10.1016/j.tcb.2022.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Piccolo S, Panciera T, Contessotto P, Cordenonsi M. YAP/TAZ as master regulators in cancer: modulation, function and therapeutic approaches. Nat Cancer. 2023;4(1):9–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Capaci V, Mantovani F, Del Sal G. Amplifying Tumor-Stroma Communication: An Emerging Oncogenic Function of Mutant p53. Front Oncol. 2020;10:614230. 10.3389/fonc.2020.614230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Bertero T, Oldham WM, Grasset EM, et al. Tumor-Stroma Mechanics Coordinate Amino Acid Availability to Sustain Tumor Growth and Malignancy. Cell Metabol. 2019;29(1):124-40.e10. 10.1016/j.cmet.2018.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Torrino S, Grasset EM, Audebert S, et al. Mechano-induced cell metabolism promotes microtubule glutamylation to force metastasis. Cell Metabol. 2021;33(7):1342-57.e10. 10.1016/j.cmet.2021.05.009 [DOI] [PubMed] [Google Scholar]
- 285.Bertolio R, Napoletano F, Del Sal G. Dynamic links between mechanical forces and metabolism shape the tumor milieu. Curr Opin Cell Biol. 2023;84:102218. 10.1016/j.ceb.2023.102218 [DOI] [PubMed] [Google Scholar]
- 286.Nabe S, Yamada T, Suzuki J, et al. Reinforce the antitumor activity of CD8(+) T cells via glutamine restriction. Cancer Sci. 2018;109(12):3737–50. 10.1111/cas.13827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Pickup MW, Mouw JK, Weaver VM. The extracellular matrix modulates the hallmarks of cancer. EMBO Rep. 2014;15(12):1243–53. 10.15252/embr.201439246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Garcia-Bermudez J, Baudrier L, La K, et al. Aspartate is a limiting metabolite for cancer cell proliferation under hypoxia and in tumours. Nat Cell Biol. 2018;20(7):775–81. 10.1038/s41556-018-0118-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Yang L, Achreja A, Yeung TL, et al. Targeting Stromal Glutamine Synthetase in Tumors Disrupts Tumor Microenvironment-Regulated Cancer Cell Growth. Cell Metabol. 2016;24(5):685–700. 10.1016/j.cmet.2016.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Zhu Y, Li X, Wang L, Hong X, Yang J. Metabolic reprogramming and crosstalk of cancer-related fibroblasts and immune cells in the tumor microenvironment. Front Endocrinol. 2022;13:988295. 10.3389/fendo.2022.988295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Scott GK, Yau C, Becker BC, et al. Targeting Mitochondrial Proline Dehydrogenase with a Suicide Inhibitor to Exploit Synthetic Lethal Interactions with p53 Upregulation and Glutaminase Inhibition. Mol Cancer Ther. 2019;18(8):1374–85. 10.1158/1535-7163.MCT-18-1323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Schwörer S, Berisa M, Violante S, et al. Proline biosynthesis is a vent for TGFβ-induced mitochondrial redox stress. EMBO J. 2020;39(8):e103334. 10.15252/embj.2019103334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Chitapanarux I, Traisathit P, Chitapanarux T, et al. Arginine, glutamine, and fish oil supplementation in cancer patients treated with concurrent chemoradiotherapy: A randomized control study. Curr Prob Cancer. 2020;44(1):100482. 10.1016/j.currproblcancer.2019.05.005 [DOI] [PubMed] [Google Scholar]
- 294.Cao Y, Wang Q, Du Y, et al. l-arginine and docetaxel synergistically enhance anti-tumor immunity by modifying the immune status of tumor-bearing mice. Int Immunopharmacol. 2016;35:7–14. 10.1016/j.intimp.2016.03.002 [DOI] [PubMed] [Google Scholar]
- 295.Sittitrai P, Ruenmarkkaew D, Booyaprapa S, Kasempitakpong B. Effect of a perioperative immune-enhancing diet in clean-contaminated head and neck cancer surgery: A randomized controlled trial. Int J Surg (London, England). 2021;93:106051. 10.1016/j.ijsu.2021.106051 [DOI] [PubMed] [Google Scholar]
- 296.Szefel J, Ślebioda T, Walczak J, et al. The effect of l-arginine supplementation and surgical trauma on the frequency of myeloid-derived suppressor cells and T lymphocytes in tumour and blood of colorectal cancer patients. Adv Med Sci. 2022;67(1):66–78. 10.1016/j.advms.2021.12.005 [DOI] [PubMed] [Google Scholar]
- 297.Wang R, Dillon CP, Shi LZ, et al. The transcription factor Myc controls metabolic reprogramming upon T lymphocyte activation. Immunity. 2011;35(6):871–82. 10.1016/j.immuni.2011.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Li S, Wu Q, Jiang Z, et al. miR-31-5p Regulates Type I Interferon by Targeting SLC15A4 in Plasmacytoid Dendritic Cells of Systemic Lupus Erythematosus. J Inflamm Res. 2022;15:6607–16. 10.2147/JIR.S383623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Morotti M, Bridges E, Valli A, et al. Hypoxia-induced switch in SNAT2/SLC38A2 regulation generates endocrine resistance in breast cancer. Proc Natl Acad Sci U S A. 2019;116(25):12452–61. 10.1073/pnas.1818521116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Elorza A, Soro-Arnáiz I, Meléndez-Rodríguez F, et al. HIF2α acts as an mTORC1 activator through the amino acid carrier SLC7A5. Mol Cell. 2012;48(5):681–91. 10.1016/j.molcel.2012.09.017 [DOI] [PubMed] [Google Scholar]
- 301.Jensen H, Potempa M, Gotthardt D, Lanier LL. Cutting Edge: IL-2-Induced Expression of the Amino Acid Transporters SLC1A5 and CD98 Is a Prerequisite for NKG2D-Mediated Activation of Human NK Cells. J Immunol. 2017;199(6):1967–72. 10.4049/jimmunol.1700497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Shi J, Chen C, Ju R, et al. Carboxyamidotriazole combined with IDO1-Kyn-AhR pathway inhibitors profoundly enhances cancer immunotherapy. J Immunother Cancer. 2019;7(1):246. 10.1186/s40425-019-0725-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Schmidt S, Gay D, Uthe FW, et al. A MYC-GCN2-eIF2α negative feedback loop limits protein synthesis to prevent MYC-dependent apoptosis in colorectal cancer. Nat Cell Biol. 2019;21(11):1413–24. 10.1038/s41556-019-0408-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Morikawa N, Tachibana M, Ago Y, Goda H, Sakurai F, Mizuguchi H. LY341495, an mGluR2/3 Antagonist, Regulates the Immunosuppressive Function of Myeloid-Derived Suppressor Cells and Inhibits Melanoma Tumor Growth. Biol Pharm Bull. 2018;41(12):1866–9. 10.1248/bpb.b18-00055 [DOI] [PubMed] [Google Scholar]
- 305.Dolšak A, Gobec S, Sova M. Indoleamine and tryptophan 2,3-dioxygenases as important future therapeutic targets. Pharmacol Ther. 2021;221:107746. 10.1016/j.pharmthera.2020.107746 [DOI] [PubMed] [Google Scholar]
- 306.Majd N, Waguespack SG, Janku F, et al. Efficacy of pembrolizumab in patients with pituitary carcinoma: report of four cases from a phase II study. J Immunother Cancer. 2020;8(2):e001532. 10.1136/jitc-2020-001532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Schramme F, Crosignani S, Frederix K, et al. Inhibition of Tryptophan-Dioxygenase Activity Increases the Antitumor Efficacy of Immune Checkpoint Inhibitors. Cancer Immunol Res. 2020;8(1):32–45. 10.1158/2326-6066.CIR-19-0041 [DOI] [PubMed] [Google Scholar]
- 308.Qin H, Yang L, Chukinas JA, et al. Systematic preclinical evaluation of CD33-directed chimeric antigen receptor T cell immunotherapy for acute myeloid leukemia defines optimized construct design. J Immunother Cancer. 2021;9(9):e003149. 10.1136/jitc-2021-003149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Cronin SJF, Seehus C, Weidinger A, et al. The metabolite BH4 controls T cell proliferation in autoimmunity and cancer. Nature. 2018;563(7732):564–8. 10.1038/s41586-018-0701-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Tabata S, Kojima Y, Sakamoto T, et al. L-2hydroxyglutaric acid rewires amino acid metabolism in colorectal cancer via the mTOR-ATF4 axis. Oncogene. 2023;42(16):1294–307. 10.1038/s41388-023-02632-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Huynh C, Ryu J, Lee J, Inoki A, Inoki K. Nutrient-sensing mTORC1 and AMPK pathways in chronic kidney diseases. Nat Rev Nephrol. 2023;19(2):102–22. 10.1038/s41581-022-00648-y [DOI] [PubMed] [Google Scholar]
- 312.Sun C, Ye Y, Tan Z, et al. Tumor-associated nonmyelinating Schwann cell-expressed PVT1 promotes pancreatic cancer kynurenine pathway and tumor immune exclusion. Sci Adv. 2023;9(5):eadd6995. 10.1126/sciadv.add6995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.León-Letelier RA, Dou R, Vykoukal J, et al. The kynurenine pathway presents multi-faceted metabolic vulnerabilities in cancer. Front Oncol. 2023;13:1256769. 10.3389/fonc.2023.1256769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Shadboorestan A, Koual M, Dairou J, Coumoul X. The Role of the Kynurenine/AhR Pathway in Diseases Related to Metabolism and Cancer. Int J Tryptophan Res. 2023;16:11786469231185102. 10.1177/11786469231185102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Lee MS, Dennis C, Naqvi I, et al. Ornithine aminotransferase supports polyamine synthesis in pancreatic cancer. Nature. 2023;616(7956):339–47. 10.1038/s41586-023-05891-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Chen Y, León-Letelier RA, Abdel Sater AH, et al. c-MYC-Driven Polyamine Metabolism in Ovarian Cancer: From Pathogenesis to Early Detection and Therapy. Cancers. 2023;15(3):623. 10.3390/cancers15030623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Coni S, Bordone R, Ivy DM, et al. Combined inhibition of polyamine metabolism and eIF5A hypusination suppresses colorectal cancer growth through a converging effect on MYC translation. Cancer Lett. 2023;559:216120. 10.1016/j.canlet.2023.216120 [DOI] [PubMed] [Google Scholar]
- 318.Zimmermann A, Hofer SJ, Madeo F. Molecular targets of spermidine: implications for cancer suppression. Cell Stress. 2023;7(7):50–8. 10.15698/cst2023.07.281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Chamoto K, Zhang B, Tajima M, Honjo T, Fagarasan S. Spermidine - an old molecule with a new age-defying immune function. Trends Cell Biol. 2023;34(5):363–70. 10.1016/j.tcb.2023.08.002 [DOI] [PubMed] [Google Scholar]
- 320.Lores S, Gámez-Chiachio M, Cascallar M, et al. Effectiveness of a novel gene nanotherapy based on putrescine for cancer treatment. Biomaterials Sci. 2023;11(12):4210–25. 10.1039/D2BM01456D [DOI] [PubMed] [Google Scholar]
- 321.Lores S, Gámez-Chiachio M, Cascallar M, et al. Correction: Effectiveness of a novel gene nanotherapy based on putrescine for cancer treatment. Biomaterials Sci. 2023;11(12):4397. 10.1039/D3BM90029K [DOI] [PubMed] [Google Scholar]
- 322.Ding N, Cheng Y, Liu H, et al. Fusobacterium nucleatum Infection Induces Malignant Proliferation of Esophageal Squamous Cell Carcinoma Cell by Putrescine Production. Microbiol Spectrum. 2023;11(2):e0275922. 10.1128/spectrum.02759-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Pandey P, Khan F, Upadhyay TK, Maqsood R. Review to Understand the Crosstalk between Immunotherapy and Tumor Metabolism. Molecules (Basel, Switzerland). 2023;28(2):862. 10.3390/molecules28020862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Zheng Y, Yao Y, Ge T, et al. Amino acid metabolism reprogramming: shedding new light on T cell anti-tumor immunity. J Exp Clin Cancer Res. 2023;42(1):291. 10.1186/s13046-023-02845-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Panetti S, McJannett N, Fultang L, et al. Engineering amino acid uptake or catabolism promotes CAR T-cell adaption to the tumor environment. Blood Adv. 2023;7(9):1754–61. 10.1182/bloodadvances.2022008272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Vander Mause ER, Baker JM, Dietze KA, et al. Systematic single amino acid affinity tuning of CD229 CAR T cells retains efficacy against multiple myeloma and eliminates on-target off-tumor toxicity. Sci Transl Med. 2023;15(705):eadd7900. 10.1126/scitranslmed.add7900 [DOI] [PubMed] [Google Scholar]
- 327.Yang Q, Zhu X, Huang P, et al. BCKDK modification enhances the anticancer efficacy of CAR-T cells by reprogramming branched chain amino acid metabolism. Mol Ther. 2024;S1525–0016(24):00319–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Yang Q, Hao J, Chi M, et al. Superior antitumor immunotherapy efficacy of kynureninase modified CAR-T cells through targeting kynurenine metabolism. Oncoimmunol. 2022;11(1):2055703. 10.1080/2162402X.2022.2055703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Kelly B, Pearce EL. Amino Assets: How Amino Acids Support Immunity. Cell Metabol. 2020;32(2):154–75. 10.1016/j.cmet.2020.06.010 [DOI] [PubMed] [Google Scholar]
- 330.Dong X, Xia S, Du S, et al. Tumor Metabolism-Rewriting Nanomedicines for Cancer Immunotherapy. ACS Cent Sci. 2023;9(10):1864–93. 10.1021/acscentsci.3c00702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Hu Z, Wang G, Zhang R, et al. Sustained-release behavior and the antitumor effect of charge-convertible poly(amino acid)s drug-loaded nanoparticles. Drug Deliv Transl Res. 2023;13(9):2394–406. 10.1007/s13346-023-01323-w [DOI] [PubMed] [Google Scholar]
- 332.Geng C, Wang S, Wang H. Recent Advances in Thermoresponsive OEGylated Poly(amino acid)s. Polymers. 2021;13(11):1813. 10.3390/polym13111813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Hu Z, Wang G, Zhang R, et al. Construction of poly(amino acid)s nano-delivery system and sustained release with redox-responsive. Colloids Surf B Biointerfaces. 2023;224:113232. 10.1016/j.colsurfb.2023.113232 [DOI] [PubMed] [Google Scholar]
- 334.Liang H, Du Y, Zhu C, et al. Nanoparticulate Cationic Poly(amino acid)s Block Cancer Metastases by Destructing Neutrophil Extracellular Traps. ACS Nano. 2023;17(3):2868–80. 10.1021/acsnano.2c11280 [DOI] [PubMed] [Google Scholar]
- 335.Tang Y, Wang S, Li Y, et al. Simultaneous glutamine metabolism and PD-L1 inhibition to enhance suppression of triple-negative breast cancer. J Nanobiotechnol. 2022;20(1):216. 10.1186/s12951-022-01424-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Xie W, Chen B, Wen H, et al. Biomimetic Nanoplatform Loading Type I Aggregation-Induced Emission Photosensitizer and Glutamine Blockade to Regulate Nutrient Partitioning for Enhancing Antitumor Immunotherapy. ACS Nano. 2022;16(7):10742–53. 10.1021/acsnano.2c02605 [DOI] [PubMed] [Google Scholar]
- 337.Mai Z, Zhong J, Zhang J, et al. Carrier-Free Immunotherapeutic Nano-Booster with Dual Synergistic Effects Based on Glutaminase Inhibition Combined with Photodynamic Therapy. ACS Nano. 2023;17(2):1583–96. 10.1021/acsnano.2c11037 [DOI] [PubMed] [Google Scholar]
- 338.Zang J, Yang Y, Zheng X, et al. Dynamic tagging to drive arginine nano-assembly to metabolically potentiate immune checkpoint blockade therapy. Biomaterials. 2023;292:121938. 10.1016/j.biomaterials.2022.121938 [DOI] [PubMed] [Google Scholar]
- 339.Soni N, Ora M, Jena A, et al. Amino Acid Tracer PET MRI in Glioma Management: What a Neuroradiologist Needs to Know. AJNR Am J Neuroradiol. 2023;44(3):236–46. 10.3174/ajnr.A7762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Galldiks N, Lohmann P, Fink GR, Langen KJ. Amino Acid PET in Neurooncology. J Nucl Med. 2023;64(5):693–700. 10.2967/jnumed.122.264859 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.