Abstract
Background and Aims
East African countries have high rates of maternal and child mortality and morbidity. Studies have shown that the involvement of male partners in reproductive health can benefit maternal and child health (MCH). This scoping review aims to provide an overview of the evidence across East Africa that describes male partner involvement and its effect on maternal, reproductive, and child well‐being.
Methods
Ten databases were searched to identify quantitative data on male's involvement in East Africa. Studies reporting qualitative data, “intention to use” data or only reporting on male partner's education or economic status were excluded. Studies were organized into five a priori categories: antenatal care (ANC), human immunodeficiency virus, breastfeeding, family planning, and intimate partner violence with further categories developed based on studies included.
Results
A total of 2787 records were identified; 644 full texts were reviewed, and 96 studies were included in this review. Data were reported on 118,967 mothers/pregnant women and 15,361 male partners. Most of the studies (n = 83) were reported from four countries Ethiopia (n = 49), Kenya (n = 14), Tanzania (n = 12) and Uganda (n = 10). The evidence indicates that male partner involvement and support is associated with improved reproductive, MCH across a wide range of outcomes. However, the studies were heterogeneous, using diverse exposure and outcome measures. Also, male partners' lack of practical and emotional support, and engagement in violent behaviors towards partners, were associated with profound negative impacts on MCH and well‐being.
Conclusions
The body of evidence, although heterogeneous, provides compelling support for male involvement in reproductive health programs designed to support MCH. To advance research in this field, an agreement is needed on a measure of male partner “involvement.” To optimize benefits of male partners' involvement, developing core outcome sets and regional coordination are recommended.
Keywords: child health, East Africa, male partner involvement, maternal health, reproductive health care
1. INTRODUCTION
In 2017, sub‐Saharan African countries alone accounted for two‐thirds of the estimated global maternal deaths 1 and a child born in a sub‐Saharan African country is 10 times more likely to die in their first month of life, compared to a child born in a high‐income country. 2 In sub‐Saharan countries, high rates of health‐related problems and unmet health care needs frequently result in elevated rates of maternal morbidities such as haemorrhage, infection, hypertensive disorders, and uterine ruptures. 3 High human immunodeficiency virus (HIV) infection rates and mother‐to‐child‐transmissions (MTCT), 4 inadequate nutrition/breastfeeding 5 and intimate partner violence (IPV) 6 affect both the mother's and child's health. 7 , 8 The Sustainable Development Goal (SDG) to “Ensure healthy lives and promote well‐being for all at all ages” aims to end preventable deaths of newborns and children under 5 years of age and reduce the global maternal mortality ratio to less than 70 per 100,000 live births. 9 Although maternal mortality in sub‐Saharan countries has decreased over recent decades rates remain at over 500 deaths per 100,000 live births. 10
Maternal and newborn health (MNH) in East Africa has generally been viewed as exclusively a woman's responsibility within a women's health framework, which limits male participation due to cultural norms that see the involvement of mothers‐in‐law and other family members as essential. 11 As well, various cultural traditions limit women's involvement in decision making, and position male partners as decision‐makers for all aspects of family life while removing them from knowledge of and involvement with maternal and newborn care. 11 Involving male partners in reproductive health can have significant benefits for MNH, particularly in low‐ and middle‐income country settings. 11 The World Health Organization (WHO) has recommended involving fathers, stating that next to improving health care, “The inclusion of fathers is important as they can play a role as caregivers to the newborn and as a source of support for the mother.” 12
Over the last decades, studies from numerous African regions have confirmed that male partner involvement has the potential to influence health outcomes directly, and can also indirectly improve MNH by increasing couple communication and joint decision making. 13 A partner providing significant emotional, 14 practical 15 and financial 16 support has been found to reduce barriers for women to access health services and can normalize women's care‐seeking behaviors. 17 For example, women are more likely to use antenatal (ANC) and postnatal care (PNC) facilities, accept medical tests and counseling and seek skilled birth attendants (SBAs) when their partners are supportive. 17 , 18 , 19 Other areas in which male partner emotional and material support are beneficial are family planning, HIV care, breastfeeding practices and child nutrition and maternal health. 19 , 20 , 21
Systematic reviews examining male partner involvement in reproductive health care include numerous studies reporting a positive association between men's active engagement in the pregnancy, birth and postnatal period in low and middle income countries settings. 22 , 23 , 24 However, there are three features of this body of evidence that limit its usefulness for designing interventions and policies to address maternal and infant health in East African countries: the body of evidence is fragmented in geography, focused on specific health conditions or behaviors, and male partner involvement is poorly defined. This scoping review aims to support a more integrated approach to increasing partner support for mothers and infants in East African countries by identifying the geographic spread, health outcome, and male partner involvement measures among studies demonstrating a link between male partner involvement and maternal and child well‐being.
2. METHODS
This scoping review was informed by the Joanna Briggs Institute Reviewers' Manual 2015. 25 A scoping review aims to identify literature relating to a broad research topic and map the findings to inform key concepts, theories, sources of evidence, and research gaps. 26 , 27
2.1. Eligibility criteria
We included all research articles written in English, including pilot studies, randomized and non‐randomized controlled trials and pre‐ and post‐test designs. Literature reviews, qualitative studies, case studies, study protocols, dissertations, studies with participants (as parents) younger than 12 years or with data restricted to male partners' level of education, income, or their absence from home, and conference abstracts were ineligible. Only studies reporting at least one outcome measure related to maternal and/or infant outcomes from conception to infant age 2 years were included, however “intention to use” outcomes were excluded. Studies involving “ever‐pregnant” women and reported on male partners' beliefs, knowledge, attitudes, or behaviors about their partner's and/or infant's health were included. The United Nations “Standard Country or Area Codes for Statistical Use” 28 was used to determine countries or territories considered “East African.” Additionally, systematic reviews were retrieved from the search and screened for eligible studies. Those studies reporting male partner involvement and mother, or infant outcomes were identified and screened full text by two reviewers using the eligibility criteria.
2.2. Search strategy
Ten databases were used to search for literature (Medline, Scopus, EMBASE, Emcare, POPLINE, EBSCO, PsychINFO, Maternity and Infant Care, CINAHL and African Index Medicus) by multiple authors on September 24, 2018 and updated on July 15, 2021 July 15, 2020. The following is an example of the search terms used for Scopus: (TITLE‐ABS‐KEY ((pregnan* OR maternal OR antenatal OR prenatal OR newborn OR infant OR breastfeed* OR “breastfeed*” OR “family health” OR “womens health” OR perinatal OR infant* OR child* OR baby* OR babies* OR mother*)) AND TITLE‐ABS‐KEY (((dad OR dads OR paternal OR father* OR husband* OR “male partner*” Or spouse*) AND (belief* OR attitude* OR knowledge OR behavior* OR behavior* OR engag* OR involve*))) AND TITLE‐ABS‐KEY ((“east* africa*” OR “british Indian ocean” OR “french southern territor* OR “south sudan” OR Burundi* OR comoros* OR djibout* OR Eritrea* OR Ethiopia* OR Kenya* OR madagasca* OR Malawi* OR mauriti* OR mayotte* OR mozambiqu* OR reunion* OR Rwanda* OR seychell* OR Somalia* OR Uganda* OR Tanzania* OR Zambia* OR Zimbabwe*))). We imported all references retrieved from the searches into Covidence, an online software for managing systematic reviews. 29
2.3. Study selection process and outcome definition
In Covidence, all records were screened for relevance by two authors using the eligibility criteria. All listed authors independently screened the abstracts, with conflicts resolved by a third reviewer. Two reviewers (R. F., B. S., R. L., C. R., or N. V.) independently screened the full text of studies with conflicts resolved by a third reviewer (Figure 1). The perinatal outcomes and conclusions reported in this scoping review are framed by recommendations or guidelines specifying treatment regimens or behaviors set by authoritative national or international bodies (Table 1).
Figure 1.
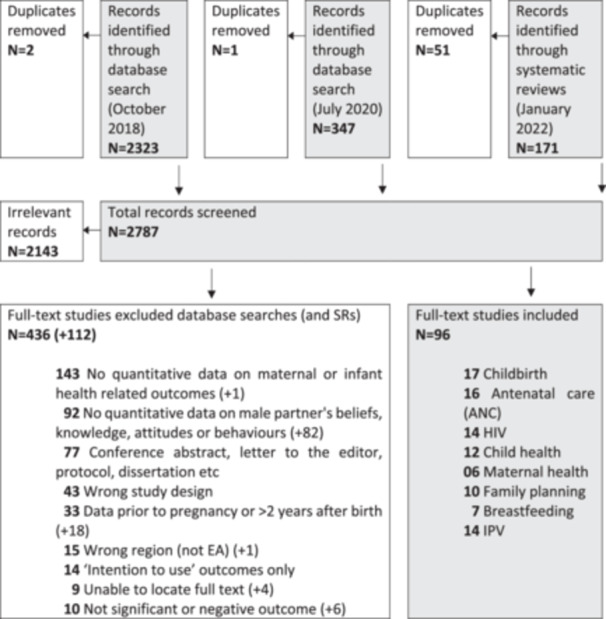
Flowchart of studies through review of male partner involvement and its impacts on maternal and child well‐being.
Table 1.
Guidelines for maternal and infant health outcomes.
| Outcomes | Guidelines |
|---|---|
| ANC | The WHO recommends a minimum of eight ANC contacts for women throughout their pregnancy, 30 some countries have adopted the 4‐visit model. 31 |
| Childbirth | A skilled birth attendant is a skilled health professional present during labor and birth. 32 |
| HIV | Outcomes related to the human immunodeficiency virus (HIV) include a husband's attitude towards a mother's positive HIV status, the likelihood of the father and/or mother being tested, mothers using and adhering to HIV medication and mother‐to‐child transmission (MTCT) of HIV. The WHO has recommended “Option B+” for the prevention of MTCT and sexual transmissions since 2012, which is a program offering medication to women from first identification as HIV positive and continuing the rest of their lives. 33 |
| Breastfeeding | Exclusive breastfeeding (EBF) is globally recommended for an infant's first 6 months of life 34 and also for HIV positive mothers. The WHO states that mothers living with HIV should breastfeed at least 6 months while being treated with antiretroviral medication. 35 While there are risks of HIV transmission through breastfeeding, inappropriate or inadequate foods and drinks risks malnutrition, diarrhoea and pneumonia. 36 |
| Family planning | Preventing unplanned pregnancies is a vital component of the WHO's strategy in preventing MTCT. 37 Modern contraceptive methods are recommended to avoid short birth‐spacing (i.e. under 18 months) which is associated with higher risks of maternal and infant mortality and complications. 38 |
| Child health | A child's health is commonly measured using height and weight for age (i.e. Weight for Height in children [WHZ] and Height for Age in children [HAZ]). Additionally, a diverse diet that includes all food groups is considered a good indication of a child's nutritional status and health. 39 “Child mortality” refers to mortality of children under the age of five is indicated. 40 |
| Maternal health | Outcomes in maternal health include mortality, 10 morbidities, 41 smoking and alcohol consumption, 42 low birthweight, preterm birth and stillbirth. 43 , 44 , 45 |
| IPV | Many incidents of violence against women involve their male intimate partners, or ex‐partners. 46 Intimate partner violence (IPV) includes physical abuse, and/or sexual abuse and/or emotional/psychological abuse. |
Abbreviations: ANC, antenatal care; WHO, World Health Organization.
2.4. Data extraction and synthesis of results
The data were extracted into an Excel spreadsheet by R. F., B. S., C. R., and N. V. Recorded data included author, year of publication, country, participant information, study aims, design and data collection, outcomes and results. Several outcomes were expected and proposed before commencing and others emerged following discussion during data extractions. The final list of outcomes is as follows: (1) ANC, (2) Childbirth, (3) HIV care, (4) Breastfeeding, (5) Child health, (6) Family planning (7) Maternal health and (8) IPV. Data for each outcome was collated and evidence tables were developed to present each topic separately.
3. RESULTS
A database search in September 2018 identified 2323 articles; a further database search in July 2020 identified another 347. Forty‐four systematic reviews identified 171 articles. After the exclusion of duplicates and screening on title and abstract, full‐text screening was undertaken on 644 papers. (Figure 1). Thirteen papers identified in the searches were excluded as they were unable to be accessed for screening. Continued screening during extraction resulted in 96 papers identified as eligible for inclusion. A full list of included studies, by primary topic and country, is provided in Table 2.
Table 2.
Number of studies per topic, participant status, and countries.
| Topic | Number of studies | Participants | Countries | |
|---|---|---|---|---|
| Mothers/pregnant women | Fathers/male partner | |||
| Antenatal care | 16 | 19,812 | 8264 | Ethiopia, Kenya, Mozambique, Tanzania, |
| Childbirth | 17 | 17,008 | 1556 | Ethiopia, Kenya, Malawi, Tanzania, Uganda, Zambia |
| Human immunodeficiency virus | 14 | 23,816 | ‐ | Ethiopia, Kenya, Malawi, Tanzania, Uganda, Zimbabwe |
| Breastfeeding | 7 | 2856 | ‐ | Ethiopia, Somaliland, Uganda |
| Child Health | 12 | 9260 | 3936 | Ethiopia, Kenya, Rwanda |
| Family Planning | 10 | 9316 | 1000 | Ethiopia, Kenya, Malawi, Rwanda, Tanzania, Uganda |
| Maternal Health | 16 | 4161 | ‐ | Ethiopia, Uganda |
| Intimate Partner Violence | 14 | 32,738 | 605 | Burundi, Ethiopia, Kenya, Rwanda, Tanzania, Uganda, Zambia |
| Total | 96 | 118,967 | 15,361 | |
3.1. Study characteristics
Many of the studies originated from Ethiopia (n = 49), Kenya (n = 14), Tanzania (n = 12) and Uganda (n = 10). The remaining studies were from Malawi (n = 4), Zambia (n = 2), Mozambique (n = 2), Rwanda (n = 3), Zimbabwe (n = 1), and Somaliland (n = 1) although one study (118) included data from Burundi, Rwanda, Tanzania, Uganda and Kenya. Most of the studies (n = 93/96) used surveys administered by an interviewer to collect quantitative data. Mothers were the primary participants of the included studies and data were collected from a total of 118,967 mothers/pregnant women and 15,361 fathers/male partners (Table 2). Data on fathers and male partners were typically collected by the mothers/female partners reporting on the father's/male partner's involvement.
Six categories were identified by grouping specific factors of male partner behaviors, knowledge or attitudes within the outcome categories across the 96 studies included in the scoping review: presence; attitudes; partner communication (i.e., joint decision making or discussion); health behaviors and knowledge; sexual and reproductive intentions; and IPV (Table 1 Supporting Information). Below we describe findings for each topic, mapped against these categories of male partner behaviors, knowledge, and attitudes. Supporting Information S1: Table 2‐5 Supporting Information details the country, participants, design, aim, and results for each study.
3.2. Impact of male partner involvement on ANC and childbirth
Male partner involvement was measured as financial support, attitudes (or acceptance/approval) toward ANC, presence (including accompaniment to ANC visits and involvement in hard labor tasks, and joint decision making). Several studies showed a strong association between male partner involvement and ANC service uptake. 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 (Supporting Information S1: Table 2 Supporting Information) For example, women receiving financial support from their male partner were more likely to use ANC than those who did not. 47 Women whose male partners expressed positive attitudes or acceptance/approval towards ANC were 3.5−9 times more likely to utilize ANC 49 , 50 , 51 and more likely to complete four ANC visits 52 , 53 , 62 compared to women whose partners perceived ANC negatively. Male partner accompaniment of women to ANC visits was associated with a higher uptake of ANC visits. 54 Conversely, women who attended ANC without their male partner were nearly seven times more likely to delay the commencement of ANC. 57 Women were more likely to utilize ANC services if they decided together with their husbands on ANC services or household purchases. 60 Increased male partner involvement assessed across multiple supportive behaviors related to maternal health services was associated with a greater likelihood of timely initiation of ANC. 61
Sixteen studies explored how male partner involvement can positively or negatively influence SBA and/or childbirth at a health facility. 18 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 (Supporting Information S1: Table 2) Male partner involvement was measured as an accompaniment to ANC visits, attitudes (or acceptance/approval) toward childbirth health care, and involvement in decision making and discussion. Women who were accompanied by their partners to ANC visits were more likely to have skilled birth attendance 18 , 63 and deliver at a health facility 64 compared to women who were not accompanied by their partners. Women whose male partner preferred home childbirth were more likely to deliver at home, 65 and women whose male partner expressed positive attitudes towards ANC were more likely to give birth in health facilities. 66 Women with a male partner who was involved in decision making regarding childbirth place were 1.9−6.8 times more likely to deliver at a health facility 69 , 70 , 71 , 72 , 73 compared to women making decisions on their own.
3.3. Impact of male partner involvement on breastfeeding and HIV care
Male partner involvement in breastfeeding primarily consists of support, which is linked to exclusive breastfeeding and timely initiation of complementary feeding. Several studies report a positive impact of partner support on child nutrition indicators. 79 , 80 , 81 , 82 , 83 , 84 , 85 (Supporting Information S1: Table 3) For example, mothers with husbands who were supportive of breastfeeding were 2.3−4.9 more likely to exclusively breastfeed compared to those who did not get their father's support. 79 , 80 , 81 , 82 Mothers with husband support during child feeding were more likely to initiate complementary feeding at an appropriate time compared to those without husband support. 83 Conversely, women who did not get support from their husbands to exclusively breastfeed were 68%−74% less likely to have good practices in exclusive breastfeeding. 84 , 85
Male partner impact on HIV care included presence/involvement (attendance in couple HIV testing, counseling and PMTCT activities), attitudes (or acceptance/approval) toward family planning and IPV, communication (joint decision making and spousal discussion), and health behaviors (alcohol consumption). Several studies reported that such factors were found to impact nevirapine uptake, ART treatment adherence, PMTCT continuum adherence, condom use, facility‐based childbirth, receive HIV test results, HIV self‐testing, postpartum HIV status, infant HIV acquisition through MTCT, MTCT knowledge and uptake of family planning. 16 , 86 , 87 , 88 , 89 , 90 , 91 , 92 , 93 , 94 , 95 , 96 , 97 (Supporting Information S1: Table 3) For example, women with partners who were involved in HIV testing, counseling or other PMTCT activities were more likely to: return for follow‐up to receive nevirapine 86 ; take maternal or infant dose of nevirapine 86 ; adhere to Option B plus ART treatment 87 ; successfully complete all steps in the PMTCT continuum 88 ; and, show improved retention in HIV care. 89 Postpartum women with a partner who ever refused the use of a condom were more likely to have a positive HIV status (Adjusted Odds Ratio = 1.88; 95% confidence interval 1.20, 2.90) compared to women with a partner who never refused. 94
3.4. Impact of male partner involvement on family planning, maternal and child health (MCH)
Male partner involvement in family planning included ANC attendance, approval or support toward contraceptive use, spousal discussion and joint decision making, and reproductive intentions. Such factors were found to have an impact on family planning service uptake and maternal intention for more children. 98 , 99 , 100 , 101 , 102 , 103 , 104 , 105 , 106 , 107 For example, women with partners involved in a discussion about contraceptive use were more likely to initiate postpartum contraceptive utilization on time, 104 utilize family planning services, 105 use contraceptives since childbirth, 105 and were less likely to discontinue contraceptive use 102 compared to those who have never discussed contraception with their partners. Furthermore, HIV positive women with a partner who desired more children were also more likely to intend to have more children. 107
Male partner involvement in the child's health is demonstrated by his: presence (including childcare activities, infant feeding, and ANC attendance); positive attitudes and support; communication (joint decision making); and health knowledge was shown to impact the child's dietary diversity and complementary feeding, development, HIV infection, and vaccination status. Several studies reported better health outcomes for children in families having supportive partner. 108 , 109 , 110 , 111 , 112 , 113 , 114 , 115 , 116 , 117 , 118 (Supporting Information S1: Table 4) For example, infants born to women with poor partner ANC attendance had an increased risk of death or infection due to HIV compared to those born to women with partner attendance. 113 One study that assessed urban and rural families separately found that among both types, children whose fathers had good knowledge of keeping the child healthy, food groups, and childcare were 2.9−8.4 times more likely to meet the minimum dietary diversity compared to those with poor knowledge. 108
Male partner involvement in maternal health was measured as involvement and support during pregnancy, which was found to have an impact on birth preparedness and antenatal depression. 119 , 120 , 121 , 122 (Supporting Information S1: Table 4) For example, women with husbands who accompanied them to the place of childbirth were more likely to report good birth preparedness 119 compared to women with husbands who did not accompany them. Also, women whose partners were unsupportive and uninvolved during pregnancy were more likely to demonstrate clinically significant symptoms of antenatal depression 120 compared to women with involved and supportive partners during pregnancy.
3.5. Impact of IPV on maternal and infant outcomes
IPV was found to be associated with several maternal and infant outcomes including depression, exclusive breastfeeding, health facility utilization (including ANC), HIV testing, maternal alcohol consumption, pregnancy loss and infant mortality. 6 , 123 , 124 , 125 , 126 , 127 , 128 , 129 , 130 , 131 , 132 , 133 , 134 , 135 , 136 , 137 (Supporting Information S1: Table 5) For example, women who were physically abused by their partner were less likely to (i) deliver in a health facility; (ii) get HIV testing; (iii) use a skilled delivery attendant; (iv) attend four or more ANC visits 130 ; (v) to seek treatment for child's diarrhoea and ARI 133 ; (vi) to have unplanned pregnancy, 135 (vii) repeat‐induced abortion, 136 and (viii) pregnancy loss 137 and infant mortality. 6
4. DISCUSSION
As part of a global effort to reduce maternal and infant mortality and morbidity, there has been a focus on interventions in the perinatal period. In East Africa, where poor maternal and infant health outcomes are prevalent, gender inequality is widely recognized as a major contributing factor. 138 Consequently, there has been increasing interest in the role of male partners in supporting maternal physical and mental health and improving infant well‐being. This review shows that a partner's attitudes, knowledge, and behaviors are crucial for a woman's well‐being and that of her infant throughout her journey from conception, through pregnancy and birth, to infant feeding and care. Although mothers are often the focus of health services and public health interventions, a partner's involvement can enhance or impair her access and engagement which will, in turn, affect the health and well‐being of their children. The partner's support can include his attitudes, to HIV testing for example, or his behaviors such as providing transport or accompanying the mother to the clinic. Partner engagement, in couple counseling or discussing family planning, can reduce health risks while violence to the mother can directly impair her well‐being as well as prevent her from accessing health services.
A limitation in the evidence is that studies are typically confined to a particular aspect of maternal or infant health thereby hindering the development of family‐based interventions which address the range of conditions and causal factors impinging on health outcomes. This scoping review is a critical first step in conceptualizing an approach to maternal and infant health in the perinatal period that is inclusive of male partners across health conditions. While it is accepted that the evidence presented in this review is variable in the design and method of data collection, the demonstrated link between male partner's involvement and improved reproductive, mother and infant outcomes support efforts to include male partners to meet the SDG for maternal and infant health. Studies in each topic area demonstrate the potential for improved family health by engaging male partners.
Researchers authoring many of the studies included in this review add their voices to the call from international and national bodies for more efforts to involve male partners in every aspect of reproductive health. 12 , 139 However, achieving significant change in this area has proved to be elusive. Several reviews have documented successful strategies for increasing male partner involvement, some reported decades ago 16 , 23 , 140 yet initiatives remain small‐scale and reliant on research or NGO funding. While assessing strategies for improving male partner involvement was not the purpose of this review, several points can be drawn from the data which may assist in developing more comprehensive and sustainable change.
Many counties within the East African region currently lack data on male partner involvement to guide their approach to improving maternal and infant health—despite an abundance of such studies being conducted. In this review Ethiopia, Kenya, Tanzania, and Uganda were the most frequently studied populations. While 11 countries were represented in the evidence base there were no studies from half of the 22 East African countries. However, all countries in this region face similar serious situations where rates of maternal and infant mortality and morbidity are unacceptably high. As well as improving co‐operation between stakeholders within countries, 141 a regional approach to sharing research evidence and intervention planning would avoid the potentially long time delay while studies from those countries without evidence replicate what is already well established in Ethiopian, Ugandan and Tanzanian studies. Such an approach would build on existing regional co‐ordination mechanisms, such as the East Africa Community linking countries across the region undertaking joint action towards the prevention and control of communicable and noncommunicable diseases, 142 as well as previous initiatives trialing interventions to include male partners in reproductive health involving several East African countries. 143
There are also lessons to be drawn from this review for designing future research to support male partner involvement in the perinatal period. Developing interventions that take a continuum of care approach to support men's engagement may be key to moving forward. For example, investigations of male partner involvement in addressing health conditions such as HIV have targeted male partner behaviors and attitudes that might also impact other important conditions. A male partner who encourages his partner to attend antenatal clinics with her and willingly undertakes couple counseling and HIV testing is also likely to be supportive of early ANC and may be more receptive to information describing the possible benefits of breastfeeding or dietary diversity for their infant. Theoretical frameworks for broadening health promotion and service perspectives may offer a template for supporting inclusive approaches to male partner involvement. 144 , 145
Developing more rigorous methods for assessing the impact of male partners' beliefs and behaviors may also improve future research and interventions. The outcome measures for MCH across the wide range of conditions reported in this review were diverse and within topic areas data collection methods varied considerably. Male partners' positive engagement with pregnancy, birth and PNC was captured under the rubric of “male partner involvement.” For some studies a male partner accompanying the mother to a clinic qualified as involvement. For others, a male partner needed to provide emotional and practical support and be willing to share decision making on key health choices such as birth location, contraception, HIV assessment and treatment to be judged as involved.
Developing an agreed‐upon tool for measuring male partner involvement may go some way to address this need. The “male involvement index” 146 has shown to be useful for specifically assessing male involvement in HIV interventions, involving two factors, communication‐based involvement and action‐based involvement. More recently a global framework was developed by Galle et al. 147 consisting of five categories for assessing male involvement comprehensively: involvement in communication, involvement in decision making, practical involvement, physical involvement and emotional involvement. The latter instrument will need further refinement depending on the context but may well be useful for comparing studies and interventions across the maternal and infant health field. Improving the measurement of male partner behaviors, however, may not address the concerns of those who see increasing women's autonomy and share in decision making as critical to improving maternal and infant health outcomes. 148 , 149 Reports linking male partner involvement in perinatal health services and reduced women's autonomy have prompted calls for interventions to explicitly seek to reduce inequalities between men and women and to examine men's and women's subjective experiences of partner relationships following male partner involvement interventions. 150 , 151 , 152 , 153 In addition, the role of others, such as mothers‐in‐law, who may influence male partners' participation in caring roles, and the health system itself should be examined. 154 , 155 And for some conditions, such as birth complications, a husband's knowledge may be critical. 156
4.1. Limitations
This review presents an overview of the literature from East African countries about male partner involvement and maternal and infant outcomes. However, we could not access the full text of a small number of articles, studies not in English were excluded and we did not include studies that reported qualitative data that may have been relevant to our findings. Furthermore, following the scoping review methodology, 26 quality assessment of the included studies was not included. Most reviewed articles featured mothers as participants, with fathers' data often reported by their partners. Thus, the findings predominantly reflect mothers' perspectives. Men's views on their role in reproductive and MCH may differ, seeing themselves primarily as financial providers, in line with societal expectations.
5. CONCLUSION
Finding avenues to lower the rates of mortality and morbidity of mothers and infants in East Africa is an international imperative. However, the evidence base for interventions must be grounded in research from the region. This scoping review presents an overview of health conditions where male partner involvement can make a significant improvement. The level of evidence, although uneven, provides a compelling case for male partner involvement interventions across a continuum of reproductive health care with attention to measurement of “involvement” and the possibility of regional coordination to maximize impact. To enhance MCH outcomes in East African countries, it is crucial to actively promote male involvement in health strategies. Policymakers and health care providers should develop and implement initiatives that engage men not only as supporters but also as active participants in MCH. This includes creating culturally sensitive educational programs that redefine gender roles and emphasize the importance of shared responsibility in reproductive health. Additionally, community outreach and communication campaigns should be tailored to overcome traditional views that limit male participation, thereby fostering a more inclusive approach to MCH.
AUTHOR CONTRIBUTIONS
Richard Fletcher: Conceptualization; methodology; data curation; writing—review and editing; writing—original draft; project administration. Faye Forbes: Conceptualization; methodology; data curation; writing—review and editing; writing—original draft; project administration. Abel Fekadu Dadi: Methodology; data curation; writing—review and editing and corresponded the study. Getachew Mullu Kassa: Methodology; data curation; writing—review and editing. Casey Regan: Conceptualization; methodology; data curation; writing—review and editing; writing—original draft; project administration. Anna Galle: Conceptualization; methodology; data curation; writing—review and editing; writing—original draft; project administration. Addisu Beyene: Methodology; data curation; writing—review and editing. Rebecca Liackman: Conceptualization; methodology; data curation; writing—review and editing; writing—original draft; project administration. Marleen Temmerman: Conceptualization; methodology; data curation; writing—review and editing; writing—original draft; project administration. All authors have read and approved the final version of the manuscript. Richard Fletcher had full access to all the data in this study and takes complete responsibility for the integrity of the data and the accuracy of the data analysis.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
TRANSPARENCY STATEMENT
The lead author Abel Fekadu Dadi affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Supporting information
Supporting information.
ACKNOWLEDGMENTS
Jordan Tait assisted in the development of the extraction table and screened papers. Open access publishing facilitated by Charles Darwin University, as part of the Wiley ‐ Charles Darwin University agreement via the Council of Australian University Librarians.
Fletcher R, Forbes F, Dadi AF, et al. Effect of male partners' involvement and support on reproductive, maternal and child health and well‐being in East Africa: a scoping review. Health Sci Rep. 2024;7:e2269. 10.1002/hsr2.2269
DATA AVAILABILITY STATEMENT
This is a scoping review where all included studies with their data can be accessed online or are included in the main manuscript or supplementary information. However, any other required documents and summary materials can be accessed from the first author upon reasonable request.
REFERENCES
- 1. World Health Organisation . Maternal mortality. 2020.
- 2. World Health Organisation . Newborns: improving survival and wellbeing. 2020.
- 3. Geller SE, Koch AR, Garland CE, MacDonald EJ, Storey F, Lawton B. A global view of severe maternal morbidity: moving beyond maternal mortality. Reprod Health. 2018;15(1):98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. UNAIDS . UNAIDS DATA 2021. 2021.
- 5. Abrahams Z, Mchiza Z, Steyn NP. Diet and mortality rates in Sub‐Saharan Africa: stages in the nutrition transition. BMC Pub Health. 2011;11(1):801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Memiah P, Bond T, Opanga Y, et al. Neonatal, infant, and child mortality among women exposed to intimate partner violence in East Africa: a multi‐country analysis. BMC Womens Health. 2020;20(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. World Health Organisation . Trends in Maternal Mortality: 1990 to 2015. Estimates by WHO, UNICEF, UNFPA, World Bank Group and the United Nations Population Division. 2015.
- 8. World Health Organisation . Strategies toward ending preventable maternal mortality (EPMM). 2015.
- 9. World Health Organisation . World health statistics 2018: monitoring health for the SDGs, sustainable development goals. 2018.
- 10. World Health Organisation . Trends in maternal mortality 2000 to 2017: estimates by WHO, UNICEF, UNFPA, World Bank Group and the United Nations Population Division. 2019.
- 11. Kinanee JB, Ezekiel‐Hart J. Men as partners in maternal health: implications for reproductive health counselling in Rivers State Nigeria. Int J Psychol Counse. 2009;1(3):039‐044. [Google Scholar]
- 12. World Health Organisation . WHO recommendation on male involvement interventions for maternal and neonatal health. 2015.
- 13. Hartmann M, Gilles K, Shattuck D, Kerner B, Guest G. Changes in couples' communication as a result of a male‐involvement family planning intervention. J Health Commun. 2012;17(7):802‐819. [DOI] [PubMed] [Google Scholar]
- 14. Mukuria AG, Martin SL, Egondi T, Bingham A, Thuita FM. Role of social support in improving infant feeding practices in Western Kenya: a quasi‐experimental study. Global Heal Sci Pract. 2016;4(1):55‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nguyen PH, Frongillo EA, Sanghvi T, et al. Engagement of husbands in a maternal nutrition program substantially contributed to greater intake of micronutrient supplements and dietary diversity during pregnancy: results of a cluster‐randomized program evaluation in Bangladesh. J Nutr. 2018;148(8):1352‐1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kalembo FW, Zgambo M, Mulaga AN, Yukai D, Ahmed NI. Association between male partner involvement and the uptake of prevention of mother‐to‐child transmission of HIV (PMTCT) interventions in Mwanza district, Malawi: a retrospective cohort study. PLoS One. 2013;8(6):e66517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tancred T, Marchant T, Hanson C, Schellenberg J, Manzi F. Birth preparedness and place of birth in Tandahimba district, Tanzania: what women prepare for birth, where they go to deliver, and why. BMC Pregn Childbir. 2016;16(1):165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Teklesilasie W, Deressa W. Husbands' involvement in antenatal care and its association with women's utilization of skilled birth attendants in Sidama zone, Ethiopia: a prospective cohort study. BMC Pregn Childbir. 2018;18(1):315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Manjate Cuco RM, Munguambe K, Bique Osman N, Degomme O, Temmerman M, Sidat MM. Male partners' involvement in prevention of mother‐to‐child HIV transmission in sub‐Saharan Africa: a systematic review. SAHARA‐J J Soc Asp HIV/AIDS. 2015;12(1):87‐105. [DOI] [PubMed] [Google Scholar]
- 20. Victora CG, Bahl R, Barros AJD, et al. Breastfeeding in the 21st century: epidemiology, mechanisms, and lifelong effect. Lancet. 2016;387(10017):475‐490. [DOI] [PubMed] [Google Scholar]
- 21. Matare CR, Craig HC, Martin SL, et al. Barriers and opportunities for improved exclusive breast‐feeding practices in Tanzania: household trials with mothers and fathers. Food Nutr Bull. 2019;40(3):308‐325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ayebare E, Mwebaza E, Mwizerwa J, Namutebi E, Kinengyere AA, Smyth R. Interventions for male involvement in pregnancy and labour: a systematic review. African J Midwif Wom Health. 2015;9(1):23‐28. [Google Scholar]
- 23. Yargawa J, Leonardi‐Bee J. Male involvement and maternal health outcomes: systematic review and meta‐analysis. J Epidemiol Commu Health. 2015;69(6):604‐612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Boltena MT, Kebede AS, El‐Khatib Z, et al. Male partners' participation in birth preparedness and complication readiness in low‐and middle‐income countries: a systematic review and meta‐analysis. BMC Pregn Childbirth. 2021;21(1):556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Peters M, Godfrey C, McInerney P, Soares C, Khalil H, Parker D. The Joanna Briggs Institute reviewers' manual 2015: methodology for JBI scoping reviews, 2015. [Google Scholar]
- 26. Levac D, Colquhoun H, O'Brien KK. Scoping studies: advancing the methodology. Implement Sci. 2010;5(1):69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Peters MD, Godfrey CM, Khalil H, McInerney P, Parker D, Soares CB. Guidance for conducting systematic scoping reviews. Int J Evid Health. 2015;13(3):141‐146. [DOI] [PubMed] [Google Scholar]
- 28. United Nations . Standard country or area codes for statistical use (M49). 2019.
- 29. Veritas Health Innovation . Covidence systematic review software. Melbourne, Australia. 2020.
- 30. World Health Organisation . WHO recommendations on antenatal care for a positive pregnancy experience. 2016. [PubMed]
- 31. Getachew T, Abajobir AA, Aychiluhim M. Focused antenatal care service utilization and associated factors in Dejen and Aneded districts, Northwest Ethiopia. Prim Heal Care Open Access. 2014;4(4):1‐8. [Google Scholar]
- 32. World Health Organisation . Making pregnancy safer: the critical role of the skilled attendant. 2004.
- 33. O'Brien L, Shaffer N, Sangrujee N, Abimbola TO. The incremental cost of switching from option B to option B+ for the prevention of mother‐to‐child transmission of HIV. Bull World Health Organ. 2014;92:162‐170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. World Health Organisation . The world health organization's infant‐feeding recommendation. WHO Weekly Epidemiol Record. 1995:17. [Google Scholar]
- 35. World Health Organisation . Infant and young child feeding. 2021.
- 36. Coutsoudis A, Pillay K, Spooner E, Coovadia H, Pembrey L, Newell ML. Morbidity in children born to women infected with human immunodeficiency virus in South Africa: does mode of feeding matter? Acta Paediatr (Stockholm). 2003;92(8):890‐895. [PubMed] [Google Scholar]
- 37. Collins LD. Andrew. Preventing HIV and unintended pregnancies: strategic framework, 2012:2011‐2015. [Google Scholar]
- 38. World Health Organisation . Report of a WHO technical consultation on birth spacing, 2007. [Google Scholar]
- 39. Kennedy GL. Evaluation of dietary diversity scores for assessment of micronutrient intake and food security in developing countries. Wageningen University and Research; 2009. [Google Scholar]
- 40. UNICEF . Child Mortality. 2021.
- 41. Alder J, Fink N, Bitzer J, Hösli I, Holzgreve W. Depression and anxiety during pregnancy: a risk factor for obstetric, fetal and neonatal outcome? A critical review of the literature. J Mater Fetal Neona Med. 2007;20(3):189‐209. [DOI] [PubMed] [Google Scholar]
- 42. Lee AM, Lam SK, Sze Mun Lau SM, Chong CS, Chui HW, Fong DY. Prevalence, course, and risk factors for antenatal anxiety and depression. Obstet Gynecol. 2007;110(5):1102‐1112. [DOI] [PubMed] [Google Scholar]
- 43. Nasreen HE, Kabir ZN, Forsell Y, Edhborg M. Low birth weight in offspring of women with depressive and anxiety symptoms during pregnancy: results from a population based study in Bangladesh. BMC Pub Health. 2010;10(1):515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rao D, Kumar S, Mohanraj R, Frey S, Manhart LE, L. Kaysen D. The impact of domestic violence and depressive symptoms on preterm birth in South India. Soc Psychiatry Psychiatr Epidemiol. 2016;51(2):225‐232. [DOI] [PubMed] [Google Scholar]
- 45. Saeed A, Raana T, Saeed AM, Humayun A. Effect of antenatal depression on maternal dietary intake and neonatal outcome: a prospective cohort. Nutr J. 2015;15(1):64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. World Health Organisation . Understanding and addressing violence against women: Intimate partner violence. 2012.
- 47. Umer A, Zinsstag J, Schelling E, et al. Antenatal care and skilled delivery service utilisation in Somali pastoral communities of eastern Ethiopia. Trop Med Int Health. 2020;25(3):328‐337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gross K, Alba S, Glass TR, Schellenberg JA, Obrist B. Timing of antenatal care for adolescent and adult pregnant women in south‐eastern Tanzania. BMC Pregn Childbir. 2012;12(1):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Abosse Z, Woldie M, Ololo S. Factors influencing antenatal care service utilization in hadiya zone. Ethiop J Health Sci. 2011;20(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Birmeta K, Dibaba Y, Woldeyohannes D. Determinants of maternal health care utilization in Holeta town, central Ethiopia. BMC Health Serv Res. 2013;13(1):256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Tewodros B, Dibaba Y. Factors affecting antenatal care utilization in Yem special woreda, southwestern Ethiopia. Ethiop J Health Sci. 2009;19(1). [Google Scholar]
- 52. Ftwi M, Gebretsadik GG, Berhe H, Haftu M, Gebremariam G, Tesfau YB. Coverage of completion of four ANC visits based on recommended time schedule in Northern Ethiopia: a community‐based cross‐sectional study design. PLoS One. 2020;15(8):e0236965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Tizazu MA, Asefa EY, Muluneh MA, Haile AB. Utilizing a minimum of four antenatal care visits and associated factors in debre berhan town, north shewa, amhara, Ethiopia, 2020. Risk Manag Healthc Policy. 2020;13:2783‐2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Audet CM, Blevins M, Chire YM, et al. Engagement of men in antenatal care services: increased HIV testing and treatment uptake in a community participatory action program in Mozambique. AIDS Behav. 2016;20(9):2090‐2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Odeny B, McGrath CJ, Langat A, et al. Male partner antenatal clinic attendance is associated with increased uptake of maternal health services and infant BCG immunization: a national survey in Kenya. BMC Pregn Childbirth. 2019;19(1):284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Roney E, Morgan C, Gatungu D, et al. Men's and women's knowledge of danger signs relevant to postnatal and neonatal care‐seeking: A cross sectional study from Bungoma County, Kenya. PLoS One. 2021;16(5):e0251543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Weldemariam S, Damte A, Endris K, et al. Late antenatal care initiation: the case of public health centers in Ethiopia. BMC Res Notes. 2018;11(1):562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Niedfeldt HJ, Sever TE, Smith R, et al. The role of men during pregnancy: a cross‐sectional study of perceptions and beliefs of primary caregivers in Tanzania. J Fam Issues. 2022;43(1):3‐19. [Google Scholar]
- 59. Galle A, De Melo M, Griffin S, Osman N, Roelens K, Degomme O. A cross‐sectional study of the role of men and the knowledge of danger signs during pregnancy in Southern Mozambique. BMC Pregn Childbir. 2020;20(1):572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Nebeb G, Salgedo W, Alemayehu Y. Antenatal care utilization in Debre Tabor, North West Ethiopia. Gynecol Obstet. 2015;5(339):2161‐0932.10003. [Google Scholar]
- 61. Mohammed BH, Johnston JM, Vackova D, Hassen SM, Yi H. The role of male partner in utilization of maternal health care services in Ethiopia: a community‐based couple study. BMC Pregn Childbirth. 2019;19(1):28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Forbes F, Wynter K, Wade C, Zeleke BM, Fisher J. Male partner attendance at antenatal care and adherence to antenatal care guidelines: secondary analysis of 2011 Ethiopian demographic and health survey data. BMC Pregn Childbirth. 2018;18(1):145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Mangeni JN, Mwangi A, Mbugua S, Mukthar VK. Male involvement in maternal healthcare as a determinant of utilisation of skilled birth attendants in Kenya. East Afr Med J. 2012;89(11):372‐383. [PubMed] [Google Scholar]
- 64. Kashitala J, Nyambe N, Mwalo S, et al. Is male involvement in ANC and PMTCT associated with increased facility‐based obstetric delivery in pregnant women? Afr J Reprod Health. 2015;19(2):116‐123. [PubMed] [Google Scholar]
- 65. Mekonnen Y, Ayichiluhm M, Dejenu G. Prevalence and determinants of home birth after antenatal care attendance in Gozamin District, Northwest Ethiopia. Health Sci J. 2015;9(6):1. [Google Scholar]
- 66. Dadi LS, Berhane M, Ahmed Y, et al. Maternal and newborn health services utilization in Jimma Zone, Southwest Ethiopia: a community based cross‐sectional study. BMC Pregn Childbirth. 2019;19(1):178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Danforth E, Kruk M, Rockers P, Mbaruku G, Galea S. Household decision making about delivery in health facilities: evidence from Tanzania. J Health Popul Nutr. 2009;27(5):696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Habte F, Demissie M. Magnitude and factors associated with institutional delivery service utilization among childbearing mothers in Cheha district, Gurage zone, SNNPR, Ethiopia: a community based cross sectional study. BMC Pregn Childbirth. 2015;15(1):299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Assefa L, Alemayehu M, Debie A. Magnitude of institutional delivery service utilization and associated factors among women in pastoral community of Awash Fentale district Afar Regional State, Ethiopia. BMC Res Notes. 2018;11(1):162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Wayessa ZJ, Dukale UG. Factors associated with institutional delivery among women in Bule Hora Town, Southern Ethiopia. Midwifery. 2021;97:102968. [DOI] [PubMed] [Google Scholar]
- 71. Rao N, Esber A, Turner A, Chilewani J, Banda V, Norris A. The impact of joint partner decision making on obstetric choices and outcomes among Malawian women. Int J Gynaecol Obst. 2016;135(1):61‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Kabakyenga JK, Östergren P‐O, Turyakira E, Pettersson KO. Influence of birth preparedness, decision making on location of birth and assistance by skilled birth attendants among women in South‐western Uganda. PLoS One. 2012;7(4):e35747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Atnafu H, Belete Z, Kinfu H, Tadesse M, Amin M, Ballard KD. Can a community‐based maternal care package in rural Ethiopia increase the use of health facilities for childbirth and reduce the stillbirth rate? Int J Women's Health. 2016;8:421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Mpembeni RN, Killewo JZ, Leshabari MT, et al. Use pattern of maternal health services and determinants of skilled care during delivery in Southern Tanzania: implications for achievement of MDG‐5 targets. BMC Pregn Childbirth. 2007;7(1):29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Debelie TZ, Abdo AA, Anteneh KT, et al. Birth preparedness and complication readiness practice and associated factors among pregnant women in Northwest Ethiopia: 2018. PLoS One. 2021;16(4):e0249083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Berhe R, Nigusie A. Magnitude of home delivery and associated factors among child bearing age mothers in Sherkole District, Benishangul Gumuz regional state‐Western‐Ethiopia. BMC Public Health. 2020;20(1):796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Wondimu MS, Woldesemayat EM. Factors associated with institutional delivery among women in Bule Hora Town, Southern Ethiopia. Risk Manag Healthc Policy. 2020;13:2159‐2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Ssebunya R, Matovu JKB. Factors associated with utilization of motorcycle ambulances by pregnant women in rural eastern Uganda: a cross‐sectional study. BMC Pregn Childbirth. 2016;16(1):46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Matovu S, Kirunda B, Rugamba‐Kabagambe G, Tumwesigye N, Nuwaha F. Factors influencing adherence to exclusive breast feeding among HIV positive mothers in Kabarole district, Uganda. East Afr Med J. 2008;85(4):162‐170. [DOI] [PubMed] [Google Scholar]
- 80. Odongkara B, Kiguli S, Edison M. Factors influencing replacement feeding practices among HIV‐positive mothers attending the PMTCT program at Uganda. J Ped Infect Dise. 2015;08(01):007‐017. [Google Scholar]
- 81. Tewabe T, Mandesh A, Gualu T, Alem G, Mekuria G, Zeleke H. Exclusive breastfeeding practice and associated factors among mothers in Motta town, East Gojjam zone, Amhara Regional State, Ethiopia, 2015: a cross‐sectional study. Int Breastfeed J. 2016;12(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Tewabe T. Prelacteal feeding practices among mothers in Motta town, Northwest Ethiopia: a cross‐sectional study. Ethiop J Health Sci. 2019;28(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Reda EB, Teferra AS, Gebregziabher MG. Time to initiate complementary feeding and associated factors among mothers with children aged 6–24 months in Tahtay Maichew district, Northern Ethiopia. BMC Res Notes. 2019;12(1):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Jama A, Gebreyesus H, Wubayehu T, et al. Exclusive breastfeeding for the first six months of life and its associated factors among children age 6‐24 months in Burao district, Somaliland. Int Breastfeed J. 2020;15(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Mazengia AL, Demissie H. Knowledge and practice of employed mothers towards exclusive breastfeeding and its associated factors in mecha district, northwest Ethiopia. J Nutr Metab. 2020;2020:1‐9. [Google Scholar]
- 86. Farquhar C, Kiarie JN, Richardson BA, et al. Antenatal couple counseling increases uptake of interventions to prevent HIV‐1 transmission. JAIDS J Acqu Imm Defic Synd. 2004;37(5):1620‐1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Gebretsadik GG, Gebretnsae H, Ftwi M, Tesfahunegn A. Alarm Clock‐Based reminder for improving low adherence on option B plus antiretroviral therapy among HIV positive pregnant and lactating mothers in Northern Ethiopia. HIV AIDS. 2020;12:687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Hampanda K, Helova A, Odwar T, et al. Male partner involvement and successful completion of the prevention of mother‐to‐child transmission continuum of care in Kenya. Int J Gynaecol Obst. 2021;152(3):409‐415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Wesevich A, Mtande T, Saidi F, et al. Role of male partner involvement in ART retention and adherence in Malawi's option B+ program. AIDS Care. 2017;29(11):1417‐1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Msuya SE, Mbizvo E, Uriyo J, Stray‐Pedersen B, Sam NE, Hussain A. Predictors of failure to return for HIV test results among pregnant women in Moshi, Tanzania. JAIDS J Acquir Immune Defic Syndrom. 2006;43(1):85‐90. [DOI] [PubMed] [Google Scholar]
- 91. Nyandat J, Van Rensburg G. Are male partners the missing link to eliminating mother‐to‐child transmission of HIV in sub‐Saharan Africa? Evidence from a retrospective case‐control study. J Assoc Nurses AIDS Care. 2020;31(4):439‐447. [DOI] [PubMed] [Google Scholar]
- 92. Aluisio A, Richardson BA, Bosire R, John‐Stewart G, Mbori‐Ngacha D, Farquhar C. Male antenatal attendance and HIV testing are associated with decreased infant HIV infection and increased HIV free survival. JAIDS J Acquir Imm Defic Synd. 2011;56(1):76‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Beyene GA, Dadi LS, Mogas SB. Determinants of HIV infection among children born to mothers on prevention of mother to child transmission program of HIV in Addis Ababa, Ethiopia: a case control study. BMC Infect Dis. 2018;18(1):327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Rwafa T, Shamu S, Christofides N. Relationship power and HIV sero‐status: an analysis of their relationship among low‐income urban Zimbabwean postpartum women. BMC Pub Health. 2019;19(1):792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Liyeh TM. Determinant factor of married women's knowledge on vertical transmission of HIV in Mecha district, Ethiopia; a community‐based study. PLoS One. 2020;15(12):e0242659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Mbonye AK, Hansen KS, Wamono F, Magnussen P. Barriers to prevention of mother‐to‐child transmission of HIV services in Uganda. J Biosoc Sci. 2010;42(2):271‐283. [DOI] [PubMed] [Google Scholar]
- 97. Fanta W, Worku A. Determinants for refusal of HIV testing among women attending for antenatal care in Gambella Region, Ethiopia. Reprod Health. 2012;9(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Achwoka D, Pintye J, McGrath CJ, et al. Uptake and correlates of contraception among postpartum women in Kenya: results from a national cross‐sectional survey. Contraception. 2018;97(3):227‐235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Garrison‐Desany HM, Wilson E, Munos M, et al. The role of gender power relations on women's health outcomes: evidence from a maternal health coverage survey in Simiyu region, Tanzania. BMC Pub Health. 2021;21(1):909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Kanakuze CA, Kaye DK, Musabirema P, Nkubito P, Mbalinda SN. Factors associated with the uptake of immediate postpartum intrauterine contraceptive devices (PPIUCD) in Rwanda: a mixed methods study. BMC Pregn Childbirth. 2020;20(1):650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Berta M, Feleke A, Abate T, Worku T, Gebrecherkos T. Utilization and associated factors of modern contraceptives during extended postpartum period among women who gave birth in the last 12 months in gondar town, northwest Ethiopia. Ethiop J Health Sci. 2018;28(2):207‐216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Sarnak DO, Wood SN, Zimmerman LA, et al. The role of partner influence in contraceptive adoption, discontinuation, and switching in a nationally representative cohort of Ugandan women. PLoS One. 2021;16(1):e0238662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Bwazi C, Maluwa A, Chimwaza A, Pindani M. Utilization of postpartum family planning services between six and twelve months of delivery at Ntchisi District Hospital, Malawi. Health. 2014;06:1724‐1737. [Google Scholar]
- 104. Dona A, Abera M, Alemu T, Hawaria D. Timely initiation of postpartum contraceptive utilization and associated factors among women of child bearing age in Aroressa District, Southern Ethiopia: a community based cross‐sectional study. BMC Pub Health. 2018;18(1):1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Sileo KM, Wanyenze RK, Lule H, Kiene SM. Determinants of family planning service uptake and use of contraceptives among postpartum women in rural Uganda. Int J Pub Health. 2015;60(8):987‐997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Williams P, Santos N, Azman‐Firdaus H, et al. Predictors of postpartum family planning in Rwanda: the influence of male involvement and healthcare experience. BMC Womens Health. 2021;21(1):112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Atukunda EC, Mugyenyi GR, Atuhumuza EB, et al. Factors associated with pregnancy intentions amongst postpartum women living with HIV in rural Southwestern Uganda. AIDS Behav. 2019;23(6):1552‐1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Bilal SM, Dinant G, Blanco R, Crutzen R, Mulugeta A, Spigt M. The influence of father's child feeding knowledge and practices on children's dietary diversity: a study in urban and rural districts of Northern Ethiopia, 2013. Matern Child Nutr. 2016;12(3):473‐483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Kuche D, Moss C, Eshetu S, et al. Factors associated with dietary diversity and length‐for‐age z‐score in rural Ethiopian children aged 6–23 months: A novel approach to the analysis of baseline data from the sustainable undernutrition reduction in Ethiopia evaluation. Matern Child Nutr. 2020;16(1):12852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Oryono A, Iraguha B, Musabende A, et al. Father involvement in the care of children born small and sick in Rwanda: association with children's nutrition and development. Child Care Health Dev. 2021;47(4):451‐464. [DOI] [PubMed] [Google Scholar]
- 111. Dangura D, Gebremedhin S. Dietary diversity and associated factors among children 6‐23 months of age in gorche district, Southern Ethiopia: cross‐sectional study. BMC Pediatr. 2017;17(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Gebremedhin S, Baye K, Bekele T, et al. Predictors of dietary diversity in children ages 6 to 23 mo in largely food‐insecure area of south wollo, Ethiopia. Nutrition. 2017;33:163‐168. [DOI] [PubMed] [Google Scholar]
- 113. Aluisio AR, Bosire R, Bourke B, et al. Male partner participation in antenatal clinic services is associated with improved HIV‐free survival among infants in Nairobi, Kenya: a prospective cohort study. JAIDS J Acquir Immune Defic Synd. 2016;73(2):169‐176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Bayih WA, Mekonen DK, Kebede SD. Prevalence and associated factors of prelacteal feeding among neonates admitted to neonatal intensive care units, North central Ethiopia, 2019. BMC Public Health. 2020;20(1):1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Beyene EZ, Worku A, Bisrat F, Fantahun M. Factors associated with immunization coverage among children age 12‐23 months: the case of Zone 3, Afar Regional State, Ethiopia. Ethiop Med J. 2013;51 Suppl 1:41‐50. [PubMed] [Google Scholar]
- 116. Semahegn A, Tesfaye G, Bogale A. Complementary feeding practice of mothers and associated factors in Hiwot Fana Specialized Hospital, Eastern Ethiopia. Pan African Medical Journal. 2014;18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Berhanu Z, Alemu T, Argaw D. Predictors of inappropriate complementary feeding practice among children aged 6 to 23 months in Wonago District, South Ethiopia, 2017; case control study. BMC Pediatr. 2019;19(1):146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Young MR, Odoyo‐June E, Nordstrom SK, et al. Factors associated with uptake of infant male circumcision for HIV prevention in Western Kenya. Pediatrics. 2012;130(1):e175‐e182. [DOI] [PubMed] [Google Scholar]
- 119. Timša L, Marrone G, Ekirapa E, Waiswa P. Strategies for helping families prepare for birth: experiences from eastern central Uganda. Glob Health Action. 2015;8(1):23969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Duko B, Ayano G, Bedaso A. Depression among pregnant women and associated factors in Hawassa city, Ethiopia: an institution‐based cross‐sectional study. Reprod Health. 2019;16(1):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Biratu A, Haile D. Prevalence of antenatal depression and associated factors among pregnant women in Addis Ababa, Ethiopia: a cross‐sectional study. Reprod Health. 2015;12(1):99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Godefay H, Byass P, Graham WJ, Kinsman J, Mulugeta A. Risk factors for maternal mortality in rural Tigray, northern Ethiopia: a case‐control study. PLoS One. 2015;10(12):e0144975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Dibaba Y, Fantahun M, Hindin MJ. The association of unwanted pregnancy and social support with depressive symptoms in pregnancy: evidence from rural Southwestern Ethiopia. BMC Pregn Childbirth. 2013;13(1):135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Necho M, Belete A, Zenebe Y. The association of intimate partner violence with postpartum depression in women during their first month period of giving delivery in health centers at Dessie town, 2019. Ann Gen Psychiatry. 2020;19(1):59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Manongi R, Rogathi J, Sigalla G, et al. The association between intimate partner violence and signs of depression during pregnancy in Kilimanjaro region, Northern Tanzania. J Interpers Violence. 2020;35(23‐24):5797‐5811. [DOI] [PubMed] [Google Scholar]
- 126. Belay S, Astatkie A, Emmelin M, Hinderaker SG. Intimate partner violence and maternal depression during pregnancy: a community‐based cross‐sectional study in Ethiopia. PLoS One. 2019;14(7):e0220003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Kjerulff Madsen F, Holm‐Larsen CE, Wu C, et al. Intimate partner violence and subsequent premature termination of exclusive breastfeeding: a cohort study. PLoS One. 2019;14(6):e0217479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Hampanda K. Intimate partner violence against HIV‐positive women is associated with sub‐optimal infant feeding practices in Lusaka, Zambia. Matern Child Health J. 2016;20(12):2599‐2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Jambola ET, Gelagay AA, Belew AK, Abajobir AA. Early resumption of sexual intercourse and its associated factors among postpartum women in western ethiopia: a cross‐sectional study. Int J Women's Health. 2020;12:381‐391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Mohammed BH, Johnston JM, Harwell JI, Yi H. Tsang KW‐k, haidar JA. intimate partner violence and utilization of maternal health care services in Addis Ababa, Ethiopia. BMC Health Serv Res. 2017;17(1):1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Goo L, Harlow SD. Intimate partner violence affects skilled attendance at most recent delivery among women in Kenya. Matern Child Health J. 2012;16(5):1131‐1137. [DOI] [PubMed] [Google Scholar]
- 132. Stöckl H, Watts C, Kilonzo Mbwambo JK. Physical violence by a partner during pregnancy in Tanzania: prevalence and risk factors. Reprod Health Matt. 2010;18(36):171‐180. [DOI] [PubMed] [Google Scholar]
- 133. Geda NR, Feng CX, Whiting SJ, Lepnurm R, Henry CJ, Janzen B. Disparities in mothers' healthcare seeking behavior for common childhood morbidities in Ethiopia: based on nationally representative data. BMC Health Serv Res. 2021;21(1):670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Vrana‐Diaz CJ, Korte JE, Gebregziabher M, et al. Relationship gender equality and couples' uptake of oral human immunodeficiency virus self‐testing kits delivered by pregnant women in Kenya. Sex Transm Dis. 2019;46(9):588‐593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Musa A, Chojenta C, Loxton D. High rate of partner violence during pregnancy in eastern Ethiopia: findings from a facility‐based study. PLoS One. 2020;15(6):e0233907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Waktola MI, Mekonen DG, Nigussie TS, Cherkose EA, Abate AT. Repeat induced abortion and associated factors among women seeking abortion care services at Debre Markos town health institutions, Amhara regional state, Ethiopia, 2017. BMC Res Notes. 2020;13(1):44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Stöckl H, Filippi V, Watts C, Mbwambo JK. Induced abortion, pregnancy loss and intimate partner violence in Tanzania: a population based study. BMC Pregn Childbirth. 2012;12(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Graham W, Woodd S, Byass P, et al. Diversity and divergence: the dynamic burden of poor maternal health. Lancet. 2016;388(10056):2164‐2175. [DOI] [PubMed] [Google Scholar]
- 139. Ministry of Health and Social Welfare URoT . The National Road Map Strategic Plan To Accelerate Reduction of Maternal, Newborn and Child Deaths in Tanzania. 2008.
- 140. Dunlap J, Foderingham N, Bussell S, Wester CW, Audet CM, Aliyu MH. Male involvement for the prevention of mother‐to‐child HIV transmission: a brief review of initiatives in East, West, and Central Africa. Curr HIV/AIDS Rep. 2014;11(2):109‐118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Galle A, Cossa H, Griffin S, Osman N, Roelens K, Degomme O. Policymaker, health provider and community perspectives on male involvement during pregnancy in Southern Mozambique: a qualitative study. BMC Pregnancy Childbirth. 2019;19(1):384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Community EA East African Community. 1999. https://www.eac.int/
- 143. Besada D, Rohde S, Goga A, et al. Strategies to improve male involvement in PMTCT option B+ in four African countries: a qualitative rapid appraisal. Glob Health Action. 2016;9(1):33507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Ergo A, Eichler R, Koblinsky M, Shah N. Strengthening health systems to improve maternal, neonatal and child health outcomes: a framework. MCHIP, USAID; 2011. [Google Scholar]
- 145. Orlando S, Palla I, Ciccacci F, et al. Improving treatment adherence and retention of HIV‐positive women through behavioral change interventions aimed at their male partners: protocol for a prospective, controlled before‐and‐after study. JMIR Res Protoc. 2021;10(1):e19384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Rodriguez VJ, Parrish MS, Jones DL, Peltzer K. Factor structure of a male involvement index to increase the effectiveness of prevention of mother‐to‐child HIV transmission (PMTCT) programs: revised male involvement index. AIDS Care. 2020;32(10):1304‐1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Galle A, Griffin S, Osman N, Roelens K, Degomme O. Towards a global framework for assessing male involvement in maternal health: results of an international Delphi study. BMJ Open. 2021;11(9):e051361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Tokhi M, Comrie‐Thomson L, Davis J, Portela A, Chersich M, Luchters S. Involving men to improve maternal and newborn health: a systematic review of the effectiveness of interventions. PLoS One. 2018;13(1):e0191620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Jennings L, Na M, Cherewick M, Hindin M, Mullany B, Ahmed S. Women's empowerment and male involvement in antenatal care: analyses of demographic and health surveys (DHS) in selected African countries. BMC Pregnancy Childbirth. 2014;14(1):297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Comrie‐Thomson L, Tokhi M, Ampt F, et al. Challenging gender inequity through male involvement in maternal and newborn health: critical assessment of an emerging evidence base. Cult Health Sex. 2015;17 Suppl 2(sup2):177‐189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Comrie‐Thomson L, Mavhu W, Makungu C, et al. Male involvement interventions and improved couples' emotional relationships in Tanzania and Zimbabwe:‘When we are walking together, I feel happy'. Cult Health Sex. 2020;22(6):722‐739. [DOI] [PubMed] [Google Scholar]
- 152. Peneza AK, Maluka SO. Unless you come with your partner you will be sent back home': strategies used to promote male involvement in antenatal care in Southern Tanzania. Glob Health Action. 2018;11(1):1449724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Galle A, Plaieser G, Van Steenstraeten T, et al. Systematic review of the concept ‘male involvement in maternal health’ by natural language processing and descriptive analysis. BMJ Glob Health. 2021;6(4):e004909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Hay K, McDougal L, Percival V, et al. Disrupting gender norms in health systems: making the case for change. Lancet. 2019;393(10190):2535‐2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Onyango MA, Owoko S, Oguttu M. Factors that influence male involvement in sexual and reproductive health in Western Kenyaa qualitative study. Afr J Reprod Health. 2010;14(4). [PubMed] [Google Scholar]
- 156. Forbes F, Wynter K, Zeleke BM, Fisher J. Male partner involvement in birth preparedness, complication readiness and obstetric emergencies in Sub‐Saharan Africa: a scoping review. BMC Pregn Childbirth. 2021;21(1):128. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Data Availability Statement
This is a scoping review where all included studies with their data can be accessed online or are included in the main manuscript or supplementary information. However, any other required documents and summary materials can be accessed from the first author upon reasonable request.


