Abstract
Obesity condition causes morphological and functional alterations involving the cardiovascular system. These can represent the substrates for different cardiovascular diseases, such as atrial fibrillation, coronary artery disease, sudden cardiac death, and heart failure (HF) with both preserved ejection fraction (EF) and reduced EF. Different pathogenetic mechanisms may help to explain the association between obesity and HF including left ventricular remodelling and epicardial fat accumulation, endothelial dysfunction, and coronary microvascular dysfunction. Multi‐imaging modalities are required for appropriate recognition of subclinical systolic dysfunction typically associated with obesity, with echocardiography being the most cost‐effective technique. Therapeutic approach in patients with obesity and HF is challenging, particularly regarding patients with preserved EF in which few strategies with high level of evidence are available. Weight loss is of extreme importance in patients with obesity and HF, being a primary therapeutic intervention. Sodium–glucose co‐transporter‐2 inhibitors have been recently introduced as a novel tool in the management of HF patients. The present review aims at analysing the most recent studies supporting pathogenesis, diagnosis, and management in patients with obesity and HF.
Keywords: Heart failure, Obesity, Natriuretic peptides, Left ventricular remodelling, Atrial fibrillation, Sudden cardiac death, Sacubitril/valsartan, Sodium–glucose co‐transporter‐2 inhibitors, Implantable cardioverter–defibrillators, Direct‐acting oral anticoagulants
Introduction
Heart failure (HF) is a complex and multifaceted syndrome with various aetiologies and relevant impact on public health. In fact, it has been estimated that HF affects 1–2% of adult population in Western countries. 1 In European countries, the prevalence of HF ranges from 1.2% (Spain) to 3.9% (Germany). 2 Based on epidemiological data, it is reasonable to estimate an increase of HF incidence in the next few years. It was calculated that HF will affect 8.5 million individuals in 2030 in the United States. 3
HF is characterized by a severe prognosis. Despite the efforts made over the last decades to improve its treatment, 5 year mortality of HF patients is ≈20%, and symptomatic HF has a 1 year mortality ranging from 53% to 67%. 4 , 5 HF has a considerable impact on the healthcare systems, because its management requires elevated costs for frequent hospitalizations, rehabilitation programmes, pharmacological therapies, and interventional and surgical procedures. On this regard, the European annual costs for care of a single HF patient range from €3.150 (Germany, 2012) 6 to €11.864 (Italy, 2014). 7
For many years, HF has been exclusively identified with an impairment of left ventricular (LV) systolic function, and the reduction of LV ejection fraction (LVEF) was considered its typical feature. In the last two decades, the definition of HF has completely changed, and upon the values of LVEF, it is possible to categorize HF in (i) HF with preserved ejection fraction (HFpEF, LVEF ≥ 50%), (ii) HF with reduced ejection fraction (HFrEF, LVEF ≤ 40%), and (iii) HF with mildly reduced ejection fraction (HFmrEF, LVEF > 40% and <50%). 1 , 8 The global prevalence of HFpEF is 2%. 9 HFpEF and HFrEF are the consequences of different pathogenic mechanisms. In general, HFpEF is triggered by a cluster of systemic inflammatory and/or metabolic disorders. 10 , 11 Although, in patients with HFpEF, high morbidity and mortality rates have been reported, the risk of death is lower compared with patients with HFrEF and HFmrEF. 12 , 13 In contrast, they have similar rate of hospitalization (21% vs. 22%). 14
To note, due to the lack of solid clinical evidence, the current guidelines for the management of HF do not recommend the use of all therapeutic strategies demonstrated to improve survival in HFpEF patients, such as treatment with sacubitril/valsartan (SAC/VAL) or the use of implantable cardioverter–defibrillator (ICD) and resynchronization therapy. 8 , 10 Only recently, an update of the European Society of Cardiology guidelines for the treatment of HF recommends the use of the sodium–glucose co‐transporter‐2 (SGLT2) inhibitors for the reduction of the cardiovascular (CV) risk and death in HFpEF patients. 15
Obesity is characterized by an increase of fat accumulation 11 and is classified upon the degree of body mass index (BMI) increase. A BMI of 25–30 kg/m2 is defined as overweight, while a BMI of 30–35, 35–40, and above 40 kg/m2 is categorized as obesity of I, II, and III degrees, respectively. 16
The global prevalence of obesity significantly raised over the last decades, and it has been documented that the prevalence of obesity in adult men and women increased from 3.2% to 10.8% and from 6.4% to 14.9%, respectively, between 1975 and 2014. 17 Nowadays, it has been estimated that 39% and 13% of the adult world population are affected by overweight and obesity, respectively. 18 Thus, it is reasonable to assert that obesity currently represents a worldwide health issue. 17 , 19
Obesity is frequently associated with additional CV risk factors, such as arterial hypertension, diabetes, and dyslipidaemia, which exert their noxious impact on cardiac function in a cumulative way. 20 , 21 , 22 Obesity increases CV mortality and predisposes progression towards HF. 23 As a matter of fact, obesity can be considered the perfect storm for CV disease, representing a leading cause of HF. As the result of the ageing involving the general population and the increased rate of obesity worldwide, the number of HF patients is expected to rise soon. In particular, HF prevalence is expected to achieve 50% by 2035 in ageing populations. 24 However, it is reasonable to speculate that this projection possibly underestimates the real incidence of HF in the next future, because it does not consider the remarkable prevalence of overweight and obesity among children and adolescents that has been recorded over the last years. Indeed, almost 40 million children under 5 years are estimated to be affected by obesity or overweight all over the world, and 340 million children and adolescents ageing between 5 and 19 years are overweight or obese. 25 More alarmingly, the increased incidence of obesity in young age has been recorded also in the developing countries, in which it had increased from 8.1% to 12.9% among boys and from 8.4% to 13.4% among girls between 1980 and 2013. 26 , 27
Epidemiological studies indicate that obesity is more frequently associated with HFpEF, rather than HFrEF. 28 , 29 The relationship between obesity and HF is an intricate phenomenon that is mediated not only by the excess of adipose tissue but also by comorbidities associated with obesity. These conditions share with obesity the same pathogenic mechanisms, such as insulin resistance (IR), low‐grade systemic inflammation, 30 oxidative stress, 31 and similar behavioural habits such as sedentary lifestyle, hypercaloric food consumption, and sugar‐sweetened beverage usage. 32 , 33
Obesity‐related HFpEF is often associated with atrial fibrillation (AF), chronic kidney disease (CKD), different forms of anaemia, chronic obstructive pulmonary disease (COPD), and obstructive sleep apnoea (OSAS). These conditions determine atrial and LV remodelling that account for increased LV filling pressure and for diastolic dysfunction. The combination of these haemodynamic abnormalities with metabolic alterations and with chronic proinflammatory state is responsible for fatigue, exertional dyspnoea, and reduced exercise tolerance. 34 The combination of metabolic abnormalities with cardiac structural and functional impairment is currently identified with the term cardiometabolic‐based chronic disease (CMBCD). 35
The purpose of this review is (i) to outline the principal pathogenic mechanisms that are involved in the development of HF in obese people, (ii) to summarize the recent progresses in CV imaging for diagnosis of HF in obese individuals, and (iii) to consider pathogenetic mechanism‐based therapeutic strategies to improve prognosis in obese patients with HF.
Pathogenetic mechanisms
The pathogenesis of HF in obesity is a complex and multifactorial process that involves metabolic, functional, structural, and neurohormonal determinants (Figure 1 ).
Figure 1.
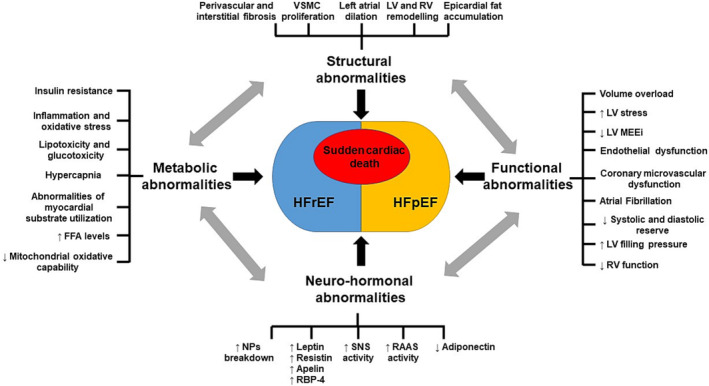
Schematic representation of the principal mechanisms involved in the pathogenesis of HF in obesity. FFA, free fatty acid; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; LV, left ventricular; MEEi, mechano‐energetic efficiency; NPs, natriuretic peptides; RAAS, renin–angiotensin–aldosterone system; RBP‐4, retinol‐binding protein‐4; RV, right ventricular; SNS, sympathetic nervous system; VSMC, vascular smooth muscle cell.
Left ventricular remodelling and epicardial fat accumulation
Presence of obesity is associated with exaggerated LV response to increased haemodynamic load mainly through non‐haemodynamic effects, mediated by the visceral adiposity. In obesity, LV remodelling can be related to the excess of fat mass, which explains the main geometric abnormalities found in obese patients. In fact, obesity represents the most relevant risk factor for LV remodelling even in patients with hypertension. 36
Increased LV mass in obesity could be explained not only by the increased muscle component but also by the infiltration of non‐muscular component (adipocyte infiltration and fibrosis) in myocardial tissue. 37 The impact of body composition on CV abnormalities in obesity also deserves attention. Obese patients with low fat‐free mass and excess of adipose mass (so‐called sarcopenic obese patients) are considered at higher CV risk. 38 In this subset of patients, metabolic and inflammatory abnormalities are more prevalent. 39 Thus, assessment of body composition might help in the management and risk stratification of obese patients.
Epicardial fat accumulation is the result of combined stimuli derived from obesity and associated low‐grade inflammation. It is characterized by lipolysis with release of fatty acid and reactive inflammation. 40 Because of the intimacy of the epicardium–myocardium interface, the deranged biological activity of epicardial adipose tissue shifts from a source of nourishment to a nidus for dysfunction, inflammation, and fibrosis, 41 ultimately leading to an impairment of LV functional reserve. 42
The accumulation of fat in the epicardium can act as a stimulus for the impairment of myocardial circulation leading to the development of increased myocardial stiffness, diastolic dysfunction, and left atrial dilatation. 43 , 44 These alterations are the hallmarks of HFpEF, particularly prevalent in obese patients, suggesting that epicardial fat might be a therapeutic target in HFpEF. Aggressive weight reduction, mainly related to bariatric surgery, can reduce epicardial fat and the associated systemic inflammation, reducing the risk of HF. Other drugs including statins, metformin, SGLT2 inhibitors, and mineralocorticoid antagonists reduce the accumulation and inflammation of epicardial adipose tissue and may, thereby, reduce the development of HFpEF. 45 , 46 , 47
Obstructive sleep apnoea syndrome
Obesity or overweight is associated with poor sleep quantity and quality. 48 , 49 On the other hand, inadequate sleep during the night and daytime sleepness predisposes patients to weight gain. 50
OSAS is a sleep‐related upper airway respiratory condition in which the partial or complete collapse of the upper aerodigestive tract causes intermittent temporary cessation of airflow during sleep with hypoxaemia/apnoea episodes.
Obesity represents a risk factor for OSAS; indeed, the prevalence of OSAS in the general population is 3–7% for men and 2–5% for women, whereas the rates are higher in obese patients reaching 77% in those who undergo bariatric surgery. 51
OSAS is an independent risk factor for CV and cerebrovascular diseases, including hypertension, stroke, HF, coronary artery disease (CAD), and AF. 52 , 53 Hypoxia related to OSAS increases the oxidative stress due to an overproduction of reactive oxygen species. The oxidative stress is a proinflammatory trigger leading to an increase of inflammatory markers such as C‐reactive protein (CRP), tumour necrosis factor‐α (TNF‐α), and interleukin‐6 (IL‐6) possibly causing endothelial dysfunction and favouring atherosclerosis development. Furthermore, the mechanical effects of obesity and sleep apnoea can lead to increased afterload, LV hypertrophy (LVH), and left atrial fibrosis and remodelling. These changes can result in an increased risk of AF development, 54 as well as of cardiac dysfunction. 55 , 56
Atrial fibrillation
Obesity directly contributes to incident arrhythmias, especially to the development of AF. 57 There is now compelling evidence supporting the importance of excess adiposity in increasing the risk of incident AF: each 5‐unit increment in BMI confers a ≈29% greater risk of incident AF, 57 and about one out of five cases of AF can be attributable to overweight or obesity. 58 Of note, obesity severity increases the likelihood progression from a paroxysmal to a permanent form of AF; indeed, patients with Class 2 obesity carry an increased risk of 87%. 59 Obesity is associated with several conditions that increase the risk of AF. However, obesity could represent itself a risk factor for the onset of AF and/or persistence of AF, regardless of other risk factors. Obesity is a chronic disorder associated with low‐grade levels of inflammation with elevated CRP level, 60 enhanced neurohormonal activation, dysregulation of the renin–angiotensin–aldosterone system, and increase in transforming growth factor (TGF)‐β. These factors are associated with signalling pathways involved in myocardial hypertrophy and fibrosis. Thus, obesity may lead to AF by inducing structural, functional, and electrical changes of the left atrium. 61 Increased atrial fibrosis and left atrial pressure, left atrial enlargement, and increased atrial conduction heterogeneity have all been described in an obese ovine model, 62 and these changes are reversible with weight loss.
In addition, the link between obesity and AF can be explained by the paracrine and endocrine properties of the epicardial adipose tissue, which is of larger dimensions in obese individuals. 63 This fatty infiltration predominates in the posterior wall of the left atria, at the junction of the pulmonary veins, and produces several hormonal factors, cytokines, chemokines, adipokines, and growth factors with proinflammatory and profibrotic effects on myocardial tissue (as activin A, a member of the TGF family). 64
As demonstrated by Mahajan et al. in an ovine model of obesity, the epicardial fat infiltrated the posterior left atrium with consequent reduced posterior endocardial voltage, conduction abnormalities, reduced effective atrial refractory period, fractionated electrograms, increased profibrotic TGF‐β1 expression, interstitial atrial fibrosis, and an increased burden of AF. 65
Of interest, it has been recently demonstrated that a large left atrial epicardial fat mass may reflect an early stage of AF, and as the disease progresses, there is an evolution towards an enlargement of the left atrium. The latter is associated with a high prevalence of persistent/permanent AF. Thus, increased left atrial epicardial fat mass without large left atrial volume may reflect the early AF disease process. 66
Catheter ablation (CA) is increasingly used for drug‐resistant symptomatic AF patients. In obese patients, CA improves quality of life, assessed by the Short Form 36 (SF‐36) score, across all BMI categories. 67 However, evidence demonstrated that a BMI above 35 kg/m2 severely impacts on ablation outcomes and increases minor complications. 68 Sivasambu et al. demonstrated that AF recurrence after ablation is already higher in overweight patients (with BMI ≥ 25 to <30 kg/m2). 69 BMI, thus, seems to be an independent predictor of AF relapse. 70 Lifestyle changes in this setting have demonstrated promising results. Indeed, a strict programme for weight reduction was demonstrated to decrease the symptoms burden and their severity and to be beneficial on cardiac remodelling. 71 Furthermore, the SORT‐AF randomized trial demonstrated that weight loss together with AF ablation, although not reducing AF burden as assessed by loop recorder, is able to improve exercise activity in patients with persistent AF. 72
Coronary microcirculation and coronary artery disease
Recent progresses in invasive and non‐invasive CV imaging techniques have allowed to identify further mechanisms that are involved in the pathogenesis of obesity‐induced HF. Coronary microvascular disease (CMVD) plays a key role in the pathogenesis of HFpEF. 9 , 73 CMVD affects coronary arterioles and capillary endothelium, resulting in an impairment of myocardial perfusion regulation. CMVD is responsible for reduced coronary flow reserve (CFR) and is diagnosed by the concomitant presence of the following criteria: (i) symptoms or documentation of ischaemic heart disease (IHD), (ii) evidence of non‐significant epicardial CAD, and (iii) evidence of reduced coronary blood flow (CBF). 74 The clinical relevance of CMVD has dramatically grown over the last years and is currently considered a specific disease, also called ‘coronary microvascular disease’, rather than as a merely pathogenic mechanism. 75 Moreover, CMVD is the typical feature of cardiometabolic disease. For instance, CMVD is responsible for severe CV prognosis 76 and accounts for the residual CV risk after a successful myocardial revascularization. 73 , 77
The pathogenic mechanisms that are involved in the development of CMVD can be classified as follows: (i) microvascular remodelling that includes the structural abnormalities of coronary microvasculature, such as intimal thickening, vascular smooth muscle cell proliferation, reduced capillary density, and perivascular fibrosis; (ii) dynamic abnormalities of coronary circulation such as reduction of the diastolic time, increase of interstitial fibrosis and LV mass, enhanced myocardial metabolic demand, and increase of end‐diastolic pressure; and (iii) functional abnormalities of coronary circulation such as blunted vasodilation and microvascular constriction. 78
Endothelial dysfunction is the result of the imbalance between the synthesis/release and degradation of vasodilator (i.e. nitric oxide, endothelium‐derived hyperpolarizing factor, and prostacyclin) and vasoconstrictor agents (i.e. reactive oxygen species, endothelin‐1, prostanoids, and thromboxane), resulting in a vasodilation impairment. Endothelial dysfunction is one of the mechanisms that play a key role in the pathogenesis of CMVD. In the specific, IR, low‐grade chronic inflammation mediated mainly by adipocytokines, and excess of oxidative stress are the principal determinants of endothelial dysfunction in obesity.
CFR is defined by the rate of CBF during hyperaemia compared with baseline. It is a feasible method to measure coronary microvascular function. A value of CFR < 2 or 2.5 is diagnostic for CMVD. An inverse relationship between BMI and CFR was found in obese patients undergoing positron emission tomography (PET) myocardial perfusion imaging for the assessment of suspected IHD. More interestingly, during a median follow‐up of 5.6 years, the adjusted incidence of composite endpoints also including HF was significantly associated with both increased BMI and decreased CFR. 79 These results were consistent with previous observations documenting that increased BMI was independently associated with abnormalities in coronary vasomotion and that the blunted endothelium‐dependent coronary vasodilation of overweight individuals was replaced by the abolition of the vasodilator capacity in obese individuals. 80
These data support the key role of CMVD as a pathogenic determinant of HF in obese patients.
Excess adiposity could also affect epicardial coronary arteries, and obesity was demonstrated to be associated with higher risk of incident CAD. 81 , 82 The pathogenetic mechanisms involved in this process include enhanced inflammation, increased reactive oxygen species, alterations in adipokine secretion, insulin resistance, being responsible of the advancement in the atherosclerotic process, and development and progression of CAD in obese patients. 83 , 84 In addition, the proinflammatory modifications induced by the obesity condition, and intensified by the presence of multiple CV risk factors, could also promote vascular thrombosis that might be involved in the acute coronary syndrome pathophysiological process. 85 Lifestyle modifications with associated weight loss determine an improvement in systemic inflammation, endothelial dysfunction, and metabolic syndrome. 86 Interventional trials aiming at medical weight loss failed in demonstrating a prominent reduction in CAD rates. 87 On the contrary, reduced CAD risk was showed in obese patients undergoing bariatric surgery when compared with nonsurgical obese patients. 88 The contrasting results of medical and surgical weight loss studies are likely due to the different degree of weight loss achieved (5–10 kg with medical weight loss vs. 10–40 kg with surgery) and the risk factor reduction obtained with bariatric surgery. 89 Nonetheless, lifestyle modifications and physical activity accompanied by weight loss in obese patients with CAD seem to improve prognosis 90 and thus are highly recommended.
The role of natriuretic peptides
Cardiomyocytes produce two hormones, atrial natriuretic peptide (ANP) and brain natriuretic peptide (BNP), belonging to the natriuretic peptides (NPs) family, 91 as pre‐pro‐peptides. This family of hormones contributes to the control of CV haemodynamic and remodelling by playing both endocrine functions (natriuresis, diuresis, and vasodilation) and several autocrine/paracrine effects in the heart and vasculature (Figure 2 ). The latter include anti‐inflammatory, anti‐fibrotic, anti‐hypertrophic, anti‐apoptotic, proliferative, and angiogenic properties, 92 regulation of myocardial perfusion, 93 and stimulation of the autophagy process to protect cardiomyocytes from the ischaemic insult. 94 All effects are mediated by guanylyl cyclase (GC) type A NP receptor (NPRA), whereas the type C NP receptor mediates the ANP and BNP clearance through internalization and lysosomal degradation. 91 Both hormones stimulate lipolysis, being similar in potency to catecholamines, promote lipid oxidation, favour adiponectin synthesis, inhibit leptin secretion, and antagonize IR. 95 Moreover, NPs promote ‘browning’ of white adipocytes to increase energy expenditure and reduce white fat mass. 95 A third component of the NP family, C‐type NP, being produced mainly by the endothelium, 91 controls the processes of adipogenesis and thermogenesis by both type B NP receptor and type C NP receptor. 96 Of note, a state of reduced NP level has been found in obese patients along with increased sodium retention and volume expansion. 97 , 98 Consistently, a significantly reduced NP level, inversely correlated with BMI, is reported in obese patients with HF. 99 Both reduced synthesis and increased clearance can explain the condition of reduced NP concentration. In this regard, adipose cells show an increased expression of type C NP receptor. 95 In addition, higher level of neprilysin (NEP), the neutral endopeptidase highly involved in NP degradation, has been reported in obesity. 100 Therefore, increased NP clearance mechanisms can explain a state of reduced NP level in obese individuals. On the other hand, a reduced cardiac synthesis or secretion of NP cannot be excluded in obesity. 98 Plasma NP‐level assessment is relevant in clinical diagnosis and prognosis of HF as a reasonable surrogate for intracardiac volumes and filling pressures. In the acute HF setting, BNP level >100 pg/mL, N‐terminal pro‐brain NP (NT‐proBNP) >300 pg/mL, and midregional N‐terminal pro‐ANP (NT‐proANP) >120 pg/mL support the diagnosis. 1 In non‐acute HF condition, the upper normal limits are 35 and 125 pg/mL for BNP and NT‐proBNP, respectively. 1 Because of lower NP levels detected in obese HF patients, a 50% reduction of the upper limits has been proposed by the European Society of Cardiology to assess the HF condition in this setting. 101
Figure 2.
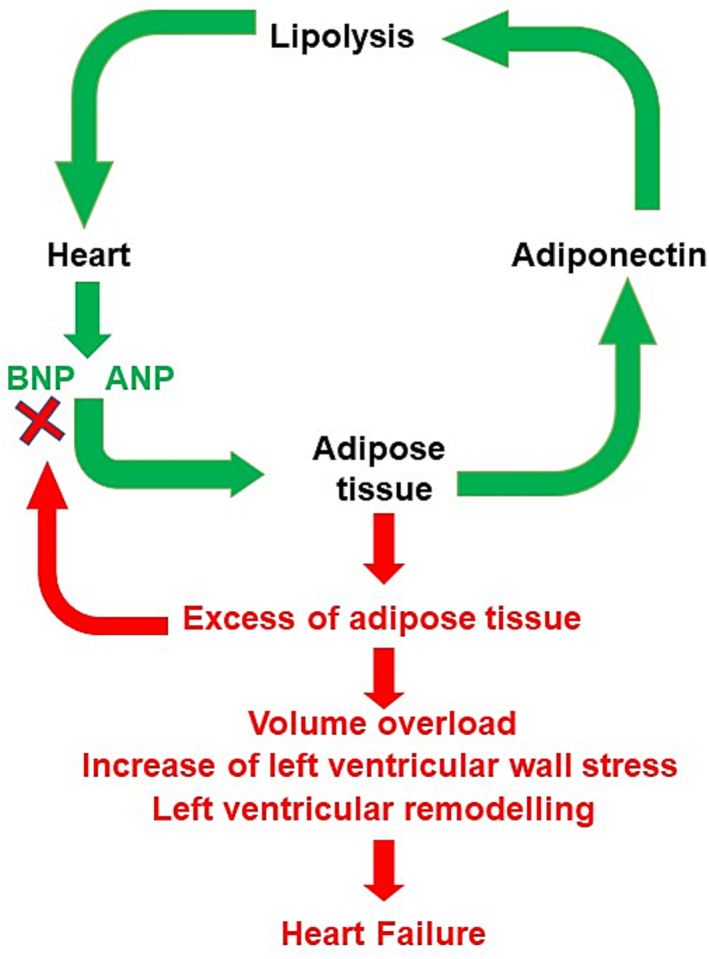
Role of natriuretic peptides (NPs) in the regulation of homeostasis of the adipose tissue. In normal conditions, NPs [i.e. atrial NP (ANP) and BNP], released by the cardiomyocytes, act on the adipose tissue stimulating the release of adiponectin, which, in turn, promotes the lipolysis. This mechanism contributes to maintain the homeostasis of adipose tissue (green arrows). A combination of increased degradation and decreased cardiac release may contribute to relative deficiency of NPs in obesity, leading to volume overload, increased ventricular wall stress, and cardiac remodelling (red arrows). Altogether, these abnormalities favour the development of HF.
Based on the limitation inherent to the lower diagnostic ability of NP level in obesity associated with HF, additional biomarkers in this condition are worth of investigation.
New insights in cardiovascular imaging techniques
CV imaging allows the investigation of multiple aspects related to the obesity condition using different imaging techniques, able to detect cardiac morphological and functional abnormalities. 102 , 103 , 104
Echocardiography represents the most widely available technique for the evaluation of LV remodelling and function but is limited by both operator and acoustic window dependency. 21 Obesity leads to an increased cardiac output and workload, and augmentation in LV mass, resulting in LVH. 105 , 106 , 107 LV mass indexation for height powered to 2.7 has advantages over indexing to body surface area (BSA), to avoid the underestimation of the rate of LVH in overweight/obese subjects. 108
Several studies showed an early impairment of LV performance involving both systolic function and diastolic function in obese patients. 109 , 110 , 111 Early LV systolic dysfunction was detectable by an impairment in LV myocardial mechano‐energetic efficiency (MEEi) indexed for LV mass, representing a sensitive index of LV systolic function. 112 , 113 The alteration of this parameter anticipated a drop in LVEF in patients with IR, a feature commonly present in obese patients. 114 , 115 , 116
Regarding advanced echocardiography, a subclinical LV systolic dysfunction was demonstrated by an impairment in longitudinal strain by speckle tracking echocardiography, even in very young subjects (Figure 3 ). 109 , 117 Longitudinal strain mainly reflects the deformation of myocardial subendocardial fibres, the ones damaged in early stages of multiple conditions. 118 , 119 , 120 In obese patients, alterations in LV geometry and LV strain deformations were also reported using three‐dimensional echocardiography, in particular involving LV longitudinal, radial, and area strains. 121 , 122 , 123 The obesity condition could modify myocardial morphology inducing LVH, metabolic dysregulation, favouring IR and a proinflammatory condition leading to myocardial fibrosis. 124 The combination of LVH and myocardial fibrosis could underlie the impairment of strain deformations and the early systolic impairment.
Figure 3.
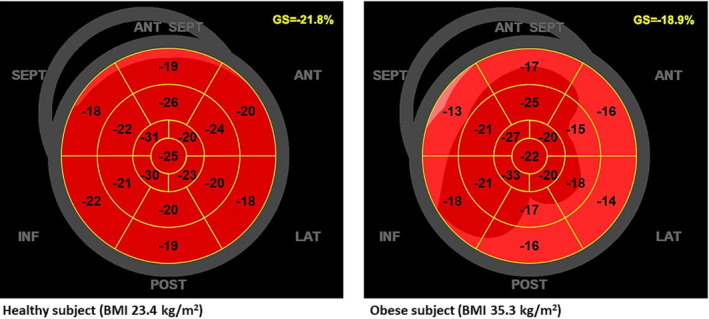
Two‐dimensional global longitudinal strain by speckle tracking echocardiography in a healthy subject with body mass index (BMI) of 23.4 kg/m2 (left panel) and in an obese subject with BMI of 35.3 kg/m2 (right panel). GS, global longitudinal strain.
Among strain‐derived myocardial work components, obesity correlated to a reduction of global constructive work and work index, without a significant affection of global wasted work, independently of systolic blood pressure in the Characteristics and Course of Heart Failure STAges A/B and Determinants of Progression (STAAB) cohort study. 125 In addition, after adjusting for systolic blood pressure, myocardial work indices appeared more severely affected by obesity in women than in men, suggesting a different sex‐related impact of this condition. 125 , 126 , 127
Obesity also showed a deleterious effect on diastolic function, predisposing to the development of HFpEF. Indeed, a significant correlation was shown between BMI and E/e′ ratio, a surrogate of LV filling pressures. 128 In obese patients, left atrial dilation is also correlated to diastolic impairment. It was demonstrated that BMI was related to both left atrial dimension and progressive dilation, independently of blood pressure. 129 Furthermore, in obese patients, left atrial dysfunction was identified in terms of impairment of left atrial strain, with a reduction of both reservoir function and conduit function. 130 Thus, left atrial dilation and dysfunction related to the obese condition represented an independent risk factor for the occurrence of AF. 131
Furthermore, echocardiography could reveal morpho‐functional alterations related to obesity involving the right ventricle. 132 , 133 Right ventricular hypertrophy and dysfunction were indeed described in obese patients, also associated with increased pulmonary arterial pressure. 134 In addition, impairment of right ventricular longitudinal strain was associated with obesity and overweight independently of sleep apnoea. 135
Moreover, the evaluation of epicardial adipose tissue, whose assessment can be done by echocardiography, and by cardiac magnetic resonance imaging (MRI) and computed tomography (CT), is relevant in this setting. Epicardial fat was suggested to have metabolic activity, producing several bioactive molecules potentially impacting negatively on myocardial morphology and function and thus defining an unfavourable CV risk profile. 136 Indeed, higher epicardial fat thickness in morbidly obese subjects was demonstrated to be significantly related to the left atrial dilation and diastolic dysfunction. 43
Besides echocardiography, cardiac MRI is gaining importance not only as gold standard technique for the assessment of LV mass and function and for the evaluation of epicardial fat but also for its ability to define tissue characterization, to identify regional fibrosis by late gadolinium enhancement and diffuse myocardial fibrosis by T1 mapping and evaluation of extracellular volume fraction. 137 The synergic negative effect of obesity and diabetes mellitus in HFpEF patients was demonstrated to be associated with diffuse myocardial fibrosis and deterioration in longitudinal strain by cardiac MRI in comparison with patients presenting only one of the two risk factors. 138 Furthermore, cardiac MRI has emerged as a feasible tool for the evaluation of the underlying substrate in AF patients, a condition often present in obese patients. Indeed, cardiac MRI allows localization and quantification of atrial structural remodelling. 139
In addition, obesity represents an independent risk factor for the development of IHD, both accelerating its progression and predisposing to IR and type 2 diabetes mellitus, which could hasten its evolution and worsen the prognosis. 140 Cardiac MRI performed after pharmacological stress with adenosine and injection of paramagnetic contrast can give accurate information about myocardial perfusion and IHD. Elevated BMI did not negatively impact the diagnostic quality and the risk stratification of patients undergoing stress cardiac MRI. 141 Indeed, stress cardiac MRI has been demonstrated to be useful in defining the prognosis of obese patients, because a very low rate of CV events was identified in obese patients without ischaemia/infarction. 142
CAD could also be investigated in an anatomical way in obese patients by contrast CT, despite limited in routine assessment because of the use of ionizing radiation and iodine contrast agent. Tube current modulation system allows adequate image quality using limited radiation dose, improving the diagnostic capability even in obese patients. 143 In severely obese patients, the evidence of a normal coronary CT before bariatric surgery was shown to be prognostically relevant to exclude major cardiac events in the postoperative period. 144
Myocardial perfusion can be also studied by single‐photon emission CT (SPECT) and PET/CT imaging. Because obese patients could have poor exercise capability, the use of pharmacological stressors is usually preferred. In obese patients, soft tissue attenuation of radioactive tracers could lead to artefacts and poor signal‐to‐noise ratios. 145 Nonetheless, SPECT sensitivity has improved, thanks to high‐efficiency cameras with optimal capability in identifying CAD in obese patients. 146 Furthermore, PET/CT provided higher diagnostic accuracy and specificity in the identification of obstructive CAD in comparison with SPECT imaging even in patients affected by severe obesity. 147 In addition, PET/CT represents the gold standard for the evaluation of CFR, the latter expressing the myocardial blood supply, related to the ability of coronary arteries to increase blood flow under stress. In the absence of flow‐limiting CAD, an impaired CFR reflects a coronary microvascular dysfunction, a phenomenon strongly associated with the development of HFpEF. 148 Indeed, an impaired CFR by PET/CT was demonstrated to be independently associated with elevated BMI and adverse outcomes. 79 CFR could be also investigated by dipyridamole stress echocardiography. 149 Even with this approach, a reduction of this parameter was reported in patients affected by metabolic syndrome, a disorder sharing common metabolic alterations with the obesity condition. 150
Table 1 describes the advantages and disadvantages of different imaging tools in obese condition.
Table 1.
Advantages and disadvantages of different imaging tools in obese setting
| Advantages | Disadvantages | |
|---|---|---|
| Echocardiography |
|
|
| Cardiac MRI |
|
|
| CT |
|
|
| SPECT/PET |
|
|
CFR, coronary flow reserve; CT, computed tomography; LV, left ventricular; MRI, magnetic resonance imaging; PET, positron emission tomography; SPECT, single‐photon emission computed tomography.
In summary, a multi‐imaging approach could be useful in providing multiple information about structural and functional modifications involving the CV system possibly present at the same time in obese patients, allowing the definition of better risk stratification and prognostic profile in this setting. The choice of the imaging technique to employ should be performed considering the information to achieve, the feasibility of the imaging techniques, and a cost/benefit basis (Figure 4 ).
Figure 4.
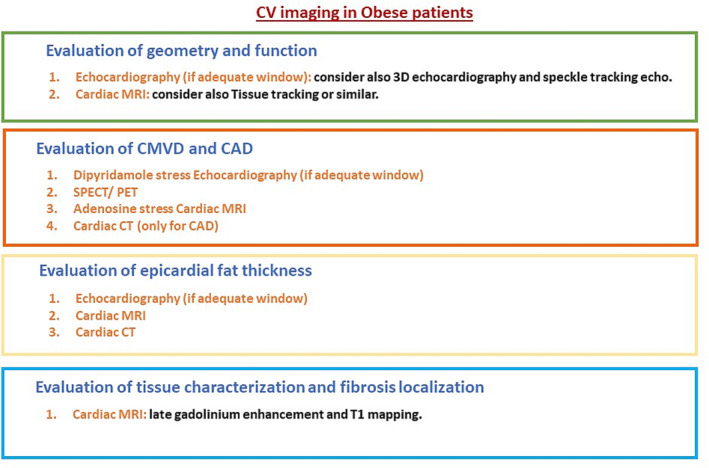
Cardiovascular (CV) imaging in obese patients for the evaluation of cardiac geometry and function, epicardial fat thickness, coronary microvascular disease (CMVD) and coronary artery disease (CAD), and tissue characterization and fibrosis localization with a proposal of the imaging techniques in the order of priority on a cost/benefit basis. CT, computed tomography; MRI, magnetic resonance imaging; PET, positron emission tomography; SPECT, single‐photon emission computed tomography.
Therapeutic perspectives
The therapy of obese HF patients should include the following: (i) weight loss, (ii) prescription of drugs that interfere specifically with the mechanisms underlying the pathogenesis of HF, and (iii) interventions aimed at preventing the arrhythmogenic sudden cardiac death (SCD) and cardioembolic stroke. Weight loss is the first therapeutical goal that should be achieved in obese HF patients.
Weight loss and the obesity paradox
Weight loss and weight maintenance should be the principal goals to prevent the development and the worsening of HF in obese individuals. However, in obesity, the complex relationship between body weight and HF outcome is also enriched by the ‘obesity paradox’. This phenomenon was described for the first time in 2001 by Horwich et al. that found, in a large cohort of patients with end‐stage HF screened for heart transplant, that obesity was not associated with an increased mortality rate but, paradoxically, with an improved survival. 151 This tendency was subsequently confirmed by randomized controlled trials (RCTs), observational studies, and meta‐analyses. A post hoc analysis of the CHARM programme showed that patients with HF and BMI > 30 kg/m2 had a lower rate of mortality compared with those having a lower BMI, independently of confounding variables, during a median follow‐up of 37.7 months. 152 These data were consistent with the results of the Val‐HeFT 153 and I‐PRESERVE 154 studies. The ADHERE study, an observational registry of hospital data on patients admitted for acute HF, showed that patients in the highest quartile of BMI had a lower intrahospital mortality. 155 Finally, several meta‐analyses documented favourable effect of obesity on HF prognosis. 156 , 157 Thus, obesity although being an established risk factor for HF, paradoxically, seems to ameliorate the prognosis of HF, rather than to worsen it. According to this view, weight reduction should have a detrimental effect on outcome. However, several doubts are present about the real existence of this phenomenon and its clinical implications. In particular, (i) the better clinical outcome detected in obese individuals is not peculiar for HF, but it is present in several chronic diseases; (ii) the supposed protective effect of obesity is not detectable in patients with severe obesity (BMI > 40 kg/m2); (iii) it is clearly documented that in HF patients, the correlation between major CV events (MACEs) and BMI does not have a linear relationship, but rather a U‐shaped profile; (iv) because cachexia is associated with poor outcome, the observed effect of obesity, rather than to be really protective, is related to the detrimental effect of the extreme weight loss; (v) there is existing evidence that the ‘obesity paradox’ is the result of statistical artefact; and (vi) many studies used BMI to identify obesity, but this parameter does not allow to differentiate between fat, muscle, skeletal, and water weight; thus, the diagnosis of obesity could be biased. Because the notion of ‘obesity paradox’ contains many pitfalls, nowadays, the clinical relevance of this phenomenon has been markedly reduced. 158 , 159 Thus, body weight reduction in patients with HF is a desirable goal, because it ameliorates symptoms and LV function. 160 , 161 At this regard, the Heart Failure Society of America Scientific Statements Committee recommends a 5–10% weight loss in patients with HF and BMI ≥ 35 kg/m2. 162
Lifestyle changes, including hypocaloric diet and physical training, induce a weight reduction of ~5%. 32 Pharmacological treatment of obesity with glucagon‐like peptide 1 (GLP‐1) receptor agonists, such as liraglutide and semaglutide, decreased weight of 10–15%. 163 , 164 Recently, the STEP‐HFpEF trial showed that 52 weeks of treatment with semaglutide reduced body weight and improved several functional parameters in obese patients with HFpEF. Interestingly, the extent of favourable effects was proportional to the magnitude of weight loss. 165 Moreover, a dual agonist for glucose‐dependent insulinotropic polypeptide (GIP) and GLP‐1, tirzepatide, was tested in obese patients in a placebo‐controlled trial. After 72 weeks of treatment, the decrease in body weight ranged from 15% to 21% based on the drug dose used, highlighting the benefit of targeting multiple endogenous nutrient‐stimulated hormone pathways for effective weight reduction. 166 Retatrutide is an agonist of the GIP, GLP‐1, and glucagon receptors. The use of a triple agonist significantly reduced body weight in patients with or without diabetes. 167 Notably, recent evidence from the SELECT trial has demonstrated the important protective effect of semaglutide in terms of CV morbidity and mortality in overweight/obese patients. 168
The lowering of body weight obtained by bariatric surgery ranges between 15% and 17%. 169 In addition, the beneficial effect on body weight obtained with these strategies is only temporary and is followed by a rapid weight regain. This phenomenon is known as ‘weight cycling’ or ‘yo‐yo effect’. Certainly, weight loss over time in obese individuals remains inadequate in most cases. Thus, obesity should be considered a chronic disease coexisting with HF rather than simply a risk factor for HF. This has important therapeutic implications. Considering obesity as a chronic condition, therapy should prevent the worsening of signs and symptoms of HF and SCD. Paradoxically, due to the deficiency of evidence‐based proven therapies, current guidelines for the treatment of obesity and HF do not adequately attempt this specific demand. 170 Moreover, the perception by the cardiologists that overweight and obesity are ‘minor’ CV risk factors further worsens this scenario. 171 Therefore, nowadays, the treatment of HF in obese subjects can be considered an unmet need, requiring specific therapeutic interventions.
Potential role of angiotensin receptor–neprilysin inhibitor
Angiotensin receptor–NEP inhibitor (ARNI)‐based treatment has been demonstrated to reduce the incidence of both hospitalizations for HF and death from CV causes by 20% in patients with systolic HF. 172 For this reason, current guidelines highly recommend the use of ARNI for the treatment of HFrEF. 10 Conversely, the favourable actions of ARNI have not being confirmed so far in HFpEF. 173 At this regard, in the PARALLAX study, SAC/VAL compared with standard renin–angiotensin system inhibitor treatment or placebo resulted in a significant decrease in plasma NT‐proBNP levels at 12 weeks, but did not significantly improve 6 min walking distance at 24 weeks. 174
Because HFpEF represents the most common form of HF in obesity, SAC/VAL is not currently prescribed in obese individuals with HF. Interestingly, this drug has induced a substantially lower decrease in NT‐proBNP levels in obese patients with HFpEF than those with reduced LVEF. 175 As previously reported, the lower‐than‐expected level of NPs in obese patients may be related to an attenuated cardiac wall stress because cardiac or pericardial fibrosis or microcirculatory rarefaction limits the capacity of the left ventricle to enlarge in response to plasma volume expansion. 176 , 177 However, the administration of ARNI in obese individuals may have its own pathophysiological rationale. In fact, mature adipocytes express increased levels of NEP on their cell surface, and soluble levels of NEP are increased in states of adiposity. 178 The increased activity of renal sympathetic nerves, a common feature of obesity, can also lead to an increased renal NEP expression. 179 In addition, obesity is accompanied by NEP activation. Increased NEP activity reduces NPs, bradykinin, apelin, substance P, and adrenomedullin levels that lead to inhibition of myocardial fibrosis. 180 Moreover, obesity is strongly associated with aldosterone overproduction. 181 Adipokine leptin can directly enhance aldosterone secretion from the adrenal gland, 182 and the adipocytes directly synthesize aldosterone. 183 The increased NEP activity in obesity minimizes the influence of NPs that can inhibit aldosterone secretion. 184 Hyperaldosteronism not only causes sodium retention but also promotes the accumulation and inflammation of epicardial adipose tissue and the development of microvascular rarefaction and fibrosis in the underlying cardiac muscle. 185 The transformation of perivisceral fat into a maladaptive proinflammatory phenotype may depend on mineralocorticoid receptor signalling. 186 It has been demonstrated that mineralocorticoid receptor antagonism may attenuate biomarkers of fibrosis and normalize estimated elevated cardiac filling pressures in obese patients not having yet received a diagnosis of HFpEF. 187 Intriguingly, patients were more likely to receive benefits from spironolactone if they had circulating NP levels lower than the median value. 188 Similarly, SAC/VAL was shown to be an effective drug combination to blunt progression of asymptomatic diastolic dysfunction and vascular stiffness to HFpEF development in a preclinical model of obesity‐associated prediabetes. 189 More recently, SAC/VAL treatment was associated with a significant reduction of CV death and MACEs in patients with pre‐HFpEF. 190 Surprisingly, these results were observed despite a greater increase in left atrial and LV volumes compared with valsartan alone. The contemporary reduction in filling pressures, vascular stiffness, and cardiac chamber stiffness could explain a change in the pressure–volume relationship and partly justify the apparent benefit of left atrial and LV enlargement in this clinical setting. However, future studies are needed to understand the long‐term effects of SAC/VAL in patients with pre‐HFpEF.
Potential role of sodium–glucose co‐transporter‐2 inhibitors
Although SGLT2 inhibitors are glucose‐lowering agents, they represent the new frontier in the treatment of HF. In fact, when added to the standard therapy in HFrEF patients, they reduce the risk of CV death by 25% and 27% in diabetic and non‐diabetic individuals, respectively. 191 They are recommended with level of evidence 1A to reduce the rate of hospitalization for HF and cardiac death, independently of diabetes. Recently, on the basis of the results of the EMPEROR‐Preserved and DELIVER studies 192 , 193 that documented the beneficial effects of SGLT2 inhibitors even in patients with HFpEF and HFmrEF, the European Society of Cardiology updated the class and the level of recommendation to 1A. 1 , 15
The SGLT2 inhibitors interfere with many of the pathophysiological derangements in obesity‐related HFpEF.
These drugs block sodium‐retentive mechanisms in the renal tubules that are activated in obesity 194 ; as a result, they cause a marked reduction in plasma volume and haemoconcentration. 195 Furthermore, SGLT2 inhibitors reduce epicardial fat accumulation and dysfunction and inhibit proinflammatory and profibrotic processes in the myocardium, thus ameliorating the development of HFpEF phenotype. 196 As a result of these actions, SGLT2 inhibitors could antagonize the deleterious biological effects of many adipocytokines, such as leptin, contributing to sodium retention and systemic inflammation. 197 SGLT2 inhibitors may also ameliorate the effects of systemic inflammation on the vasculature and visceral organs. 198 These favourable effects are corroborated by the results of large‐scale trials in type 2 diabetes where the use of empagliflozin and canagliflozin decreased the risk of new‐onset HF. 199 , 200 More recently, dapagliflozin was demonstrated to improve CV outcomes across the spectrum of BMI, leading to greater symptom improvement in patients with obesity, compared with those without, and having the additional benefit of causing modest weight loss. 201
Interestingly, SGLT2 inhibitors seem to be working through overlapping mechanisms (such as interference with sodium retention and cardiac inflammation, microvascular rarefaction, and fibrosis) with ARNI and mineralocorticoid receptor antagonists. Therefore, the benefits of adding an SGLT2 inhibitor to either a mineralocorticoid receptor antagonist or an ARNI in patients with HFpEF may be additive. In fact, a sub‐analysis of the DELIVER trial has demonstrated that the efficacy (in terms of worsening HF or CV death reduction) and safety of dapagliflozin were similar, regardless of background treatment with an aldosterone receptor antagonist or ARNI. In particular, the benefit of the effect of dapagliflozin compared with placebo on the primary outcome was slightly superior, even if it did not reach the statistical significance for aldosterone receptor antagonists and ARNI users compared with non‐users. 202
Thus, hopefully the effects of SLGT‐2 inhibitors addition to other treatments, recommended in recent guidelines, will be more extensively tested in obese people affected by HFpEF.
Potential role of implantable cardioverter–defibrillator
HFpEF is a feature of obesity and is a risk factor for SCD. SCD accounts for 25% of death in HFpEF. 203 However, obesity increases SCD risk independently of HF. 57 In this regard, it has been documented that for each 5‐unit increase of BMI, the risk for SCD enhances by 16%. 204 The pathophysiological basis of this association can be resumed in (i) increased sympathetic nervous activity, (ii) electrical and structural LV remodelling, (iii) abnormalities of electrolyte homeostasis and excitation–contraction coupling, (iv) LV infiltration of epicardial adipose tissue, and (v) increased oxidative stress. These abnormalities increase the susceptibility to lethal ventricular arrhythmias (i.e. ventricular tachycardia/ventricular fibrillation), by the development of reentrant circuits or delayed ventricular repolarization. Obesity was detected in 20% of unexpected SCD in young individuals (age ≤35 years). 205 Moreover, obesity was significantly associated with increased risk of SCD in the cohort of the Atherosclerosis Risk in Communities (ARIC) study. 206 The key role of obesity as a risk factor for ventricular tachyarrhythmias has been also documented in a sub‐analysis of the MADIT II study, showing that individuals with BMI ≥ 30 kg/m2 had a significantly higher risk of ventricular tachyarrhythmias in post‐infarction patients with impaired LVEF. 207
Therapeutic strategies aimed at minimizing the occurrence of SCD in obese HF patients should be considered. The use of ICD is the unique therapeutic option that has been demonstrated to reduce arrhythmogenic SCD. Up to date, this therapeutic option is recommended, in primary prevention, only in HFrEF. 10 This represents an important weakness of the evidence‐based medicine, because the recommendation for ICD in primary prevention of SCD in HF is based on trials performed more than 20 years ago. Nowadays, the epidemiological scenario is completely changed, and preventive medicine requires updated solutions for the ongoing problems. Based on the available data, it is reasonable to estimate that ICD use in obese patients with HFpEF may reduce the occurrence of SCD by 20–25%. Thus, controlled studies aimed at clarifying this issue should be performed.
Potential role of direct‐acting oral anticoagulants
Obesity increases the risk of stroke 208 even in the absence of concomitant CV risk factors. 209 This association has been ascribed to the strong association between obesity and AF. 210 However, it should be underlined that obese people are at increased risk of thrombo‐embolic stroke independently from AF. 211 Low‐grade systemic inflammation plays a mechanistic role in this association. In fact, the chronic state of inflammation per se activates the thrombotic process and, at the same time, plays a role in the pathogenesis of ‘atrial myopathy’, responsible of both HFpEF and AF. 212 The combination of AF and HFpEF has a synergistic effect on thrombo‐embolic events. 213 Unfortunately, thrombo‐embolic risk in obese patients with HFpEF is often undertreated. In fact, current guidelines recommend prescription of direct‐acting oral anticoagulants (DOACs) in primary prevention of stroke only in the presence of AF with CHA2DS2‐VASc score ≥2 and ≥3 in men and women, respectively. 214 In addition, obesity is not included in the calculation of CHA2DS2‐VASc score. Thus, obese people with AF with or without HF, but with CHA2DS2‐VASc scores that do not achieve the threshold values, are not eligible for treatment with DOACs. These two circumstances seriously limit the strategies of primary prevention of thrombo‐embolic stroke in obese HF patients, especially in young people. Thus, there is the need to define new scores for risk stratification of thrombo‐embolic stroke and consequently to modify the recommendations for the prescription of DOACs in obese people with or without HF.
An additional potential limitation in primary prevention of thrombo‐embolic stroke in obese patients with AF is represented by the therapeutic efficacy of DOACs. In fact, it is possible that the changes of metabolism in obese people may affect pharmacokinetics, pharmacodynamics, and efficacy and safety of DOACs. In this regard, no RCTs have investigated the efficacy and safety of DOACs in the obese population. For this reason, the International Society on Thrombosis and Haemostasis and the European Society of Cardiology Working Group on Thrombosis have raised perplexity about the administration of DOACs in extreme obesity (i.e. BMI ≥ 40 kg/m2). 215 However, based on few available clinical data, it has been suggested that apixaban and rivaroxaban have a higher safety and efficacy profiles compared with edoxaban and dabigatran in III‐degree obesity. 216 This scenario highlights the need of RCTs to clarify which DOACs are safe and efficacious in obese patients and at which doses.
The therapeutic strategies that can ameliorate the prognosis of obese people with HF are summarized in Figure 5 .
Figure 5.

Future directions for the treatment of obesity with heart failure. AF, atrial fibrillation; DOACs, direct‐acting oral anticoagulants; HFpEF, heart failure with preserved ejection fraction.
Conclusions
HF is a complex and multifactorial syndrome characterized by poor prognosis and elevated management costs. Therefore, the prevention and optimization of HF treatment in this setting represent a challenge of public health.
In the industrialized and developing countries, the prevalence of obesity has achieved pandemic proportions. This phenomenon has reached alarming dimensions because it involves also young individuals.
Obesity is a leading cause of HF, especially HFpEF. It is reasonable to estimate that, in the next few years, the incidence of obesity‐induced HF will dramatically increase.
Unfortunately, nowadays, there are no clearly defined strategies for the diagnosis and treatment of obesity‐induced HF. This issue is an unmet clinical need that should be promptly and adequately addressed.
Conflict of interest
None declared.
Funding
None.
Acknowledgements
The authors are grateful to Federica De Luise for her assistance with manuscript preparation.
Lembo, M. , Strisciuglio, T. , Fonderico, C. , Mancusi, C. , Izzo, R. , Trimarco, V. , Bellis, A. , Barbato, E. , Esposito, G. , Morisco, C. , and Rubattu, S. (2024) Obesity: the perfect storm for heart failure. ESC Heart Failure, 11: 1841–1860. 10.1002/ehf2.14641.
References
- 1. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Bohm M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure of the European Society of Cardiology (ESC). With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2021;42:3599‐3726. doi: 10.1093/eurheartj/ehab368 [DOI] [PubMed] [Google Scholar]
- 2. Seferovic PM, Vardas P, Jankowska EA, Maggioni AP, Timmis A, Milinkovic I, et al. The Heart Failure Association atlas: Heart failure epidemiology and management statistics 2019. Eur J Heart Fail 2021;23:906‐914. doi: 10.1002/ejhf.2143 [DOI] [PubMed] [Google Scholar]
- 3. Heidenreich PA, Albert NM, Allen LA, Bluemke DA, Butler J, Fonarow GC, et al. Forecasting the impact of heart failure in the United States: A policy statement from the American Heart Association. Circ Heart Fail 2013;6:606‐619. doi: 10.1161/HHF.0b013e318291329a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gerber Y, Weston SA, Redfield MM, Chamberlain AM, Manemann SM, Jiang R, et al. A contemporary appraisal of the heart failure epidemic in Olmsted County, Minnesota, 2000 to 2010. JAMA Intern Med 2015;175:996‐1004. doi: 10.1001/jamainternmed.2015.0924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tsao CW, Lyass A, Enserro D, Larson MG, Ho JE, Kizer JR, et al. Temporal trends in the incidence of and mortality associated with heart failure with preserved and reduced ejection fraction. JACC Heart Fail 2018;6:678‐685. doi: 10.1016/j.jchf.2018.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Biermann J, Neumann T, Angermann CE, Erbel R, Maisch B, Pittrow D, et al. Economic burden of patients with various etiologies of chronic systolic heart failure analyzed by resource use and costs. Int J Cardiol 2012;156:323‐325. doi: 10.1016/j.ijcard.2012.01.099 [DOI] [PubMed] [Google Scholar]
- 7. Maggioni AP, Orso F, Calabria S, Rossi E, Cinconze E, Baldasseroni S, et al. The real‐world evidence of heart failure: Findings from 41 413 patients of the ARNO database. Eur J Heart Fail 2016;18:402‐410. doi: 10.1002/ejhf.471 [DOI] [PubMed] [Google Scholar]
- 8. Authors/Task Force Members , McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure of the European Society of Cardiology (ESC). With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2022;24:4‐131. doi: 10.1002/ejhf.2333 [DOI] [PubMed] [Google Scholar]
- 9. Paulus WJ, Tschope C. A novel paradigm for heart failure with preserved ejection fraction: Comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol 2013;62:263‐271. doi: 10.1016/j.jacc.2013.02.092 [DOI] [PubMed] [Google Scholar]
- 10. Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: A report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022;145:e895‐e1032. doi: 10.1161/CIR.0000000000001063 [DOI] [PubMed] [Google Scholar]
- 11. World Health Organization . Obesity: Preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser 2000;894:1‐253. [PubMed] [Google Scholar]
- 12. Meta‐analysis Global Group in Chronic Heart Failure . The survival of patients with heart failure with preserved or reduced left ventricular ejection fraction: An individual patient data meta‐analysis. Eur Heart J 2012;33:1750‐1757. doi: 10.1093/eurheartj/ehr254 [DOI] [PubMed] [Google Scholar]
- 13. Lam CSP, Gamble GD, Ling LH, Sim D, Leong KTG, Yeo PSD, et al. Mortality associated with heart failure with preserved vs. reduced ejection fraction in a prospective international multi‐ethnic cohort study. Eur Heart J 2018;39:1770‐1780. doi: 10.1093/eurheartj/ehy005 [DOI] [PubMed] [Google Scholar]
- 14. Lenzen MJ, Scholte op Reimer WJ, Boersma E, Vantrimpont PJ, Follath F, Swedberg K, et al. Differences between patients with a preserved and a depressed left ventricular function: A report from the EuroHeart Failure Survey. Eur Heart J 2004;25:1214‐1220. doi: 10.1016/j.ehj.2004.06.006 [DOI] [PubMed] [Google Scholar]
- 15. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Bohm M, et al. 2023 focused update of the 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure of the European Society of Cardiology (ESC). With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2023;44:3627‐3639. doi: 10.1093/eurheartj/ehad195 [DOI] [PubMed] [Google Scholar]
- 16. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults—The evidence report. National Institutes of Health. Obes Res 1998;6:51S‐209S. [PubMed] [Google Scholar]
- 17. Bluher M. Obesity: Global epidemiology and pathogenesis. Nat Rev Endocrinol 2019;15:288‐298. doi: 10.1038/s41574-019-0176-8 [DOI] [PubMed] [Google Scholar]
- 18. Chooi YC, Ding C, Magkos F. The epidemiology of obesity. Metabolism 2019;92:6‐10. doi: 10.1016/j.metabol.2018.09.005 [DOI] [PubMed] [Google Scholar]
- 19. Abdelaal M, le Roux CW, Docherty NG. Morbidity and mortality associated with obesity. Ann Transl Med 2017;5:161. doi: 10.21037/atm.2017.03.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Van Gaal LF, Maggioni AP. Overweight, obesity, and outcomes: Fat mass and beyond. Lancet 2014;383:935‐936. doi: 10.1016/S0140-6736(13)62076-0 [DOI] [PubMed] [Google Scholar]
- 21. Cameli M, Lembo M, Sciaccaluga C, Bandera F, Ciccone MM, D'Andrea A, et al. Identification of cardiac organ damage in arterial hypertension: Insights by echocardiography for a comprehensive assessment. J Hypertens 2020;38:588‐598. doi: 10.1097/HJH.0000000000002323 [DOI] [PubMed] [Google Scholar]
- 22. Kisialiou A, Grella R, Carrizzo A, Pelone G, Bartolo M, Zucchella C, et al. Risk factors and acute ischemic stroke subtypes. J Neurol Sci 2014;339:41‐46. doi: 10.1016/j.jns.2014.01.014 [DOI] [PubMed] [Google Scholar]
- 23. Ebong IA, Goff DC Jr, Rodriguez CJ, Chen H, Bertoni AG. Mechanisms of heart failure in obesity. Obes Res Clin Pract 2014;8:e540‐e548. doi: 10.1016/j.orcp.2013.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bui AL, Horwich TB, Fonarow GC. Epidemiology and risk profile of heart failure. Nat Rev Cardiol 2011;8:30‐41. doi: 10.1038/nrcardio.2010.165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. NCD Risk Factor Collaboration (NCD‐RisC) . Worldwide trends in body‐mass index, underweight, overweight, and obesity from 1975 to 2016: A pooled analysis of 2416 population‐based measurement studies in 128.9 million children, adolescents, and adults. Lancet 2017;390:2627‐2642. doi: 10.1016/S0140-6736(17)32129-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Andersson C, Vasan RS. Epidemiology of cardiovascular disease in young individuals. Nat Rev Cardiol 2018;15:230‐240. doi: 10.1038/nrcardio.2017.154 [DOI] [PubMed] [Google Scholar]
- 27. Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014;384:766‐781. doi: 10.1016/S0140-6736(14)60460-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Savji N, Meijers WC, Bartz TM, Bhambhani V, Cushman M, Nayor M, et al. The association of obesity and cardiometabolic traits with incident HFpEF and HFrEF. JACC Heart Fail 2018;6:701‐709. doi: 10.1016/j.jchf.2018.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ho JE, Lyass A, Lee DS, Vasan RS, Kannel WB, Larson MG, et al. Predictors of new‐onset heart failure: Differences in preserved versus reduced ejection fraction. Circ Heart Fail 2013;6:279‐286. doi: 10.1161/CIRCHEARTFAILURE.112.972828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pugliese NR, Pellicori P, Filidei F, De Biase N, Maffia P, Guzik TJ, et al. Inflammatory pathways in heart failure with preserved left ventricular ejection fraction: Implications for future interventions. Cardiovasc Res 2023;118:3536‐3555. doi: 10.1093/cvr/cvac133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mancusi C, Izzo R, di Gioia G, Losi MA, Barbato E, Morisco C. Insulin resistance the hinge between hypertension and type 2 diabetes. High Blood Press Cardiovasc Prev 2020;27:515‐526. doi: 10.1007/s40292-020-00408-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Iaccarino G, Franco D, Sorriento D, Strisciuglio T, Barbato E, Morisco C. Modulation of insulin sensitivity by exercise training: Implications for cardiovascular prevention. J Cardiovasc Transl Res 2021;14:256‐270. doi: 10.1007/s12265-020-10057-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Giallauria F, Strisciuglio T, Cuomo G, Di Lorenzo A, D'Angelo A, Volpicelli M, et al. Exercise training: The holistic approach in cardiovascular prevention. High Blood Press Cardiovasc Prev 2021;28:561‐577. doi: 10.1007/s40292-021-00482-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mancusi C, Lembo M, Manzi MV, Basile C, Fucile I, Morisco C. From structural to functional hypertension mediated target organ damage—A long way to heart failure with preserved ejection fraction. J Clin Med 2022;11:5377. doi: 10.3390/jcm11185377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mechanick JI, Farkouh ME, Newman JD, Garvey WT. Cardiometabolic‐based chronic disease, adiposity and dysglycemia drivers: JACC state‐of‐the‐art review. J Am Coll Cardiol 2020;75:525‐538. doi: 10.1016/j.jacc.2019.11.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Buono F, Crispo S, Pagano G, Rengo G, Petitto M, Grieco F, et al. Determinants of left ventricular hypertrophy in patients with recent diagnosis of essential hypertension. J Hypertens 2014;32:166‐173. doi: 10.1097/HJH.0b013e328365c87d [DOI] [PubMed] [Google Scholar]
- 37. de Simone G, Mancusi C, Izzo R, Losi MA, Aldo Ferrara L. Obesity and hypertensive heart disease: Focus on body composition and sex differences. Diabetol Metab Syndr 2016;8:79. doi: 10.1186/s13098-016-0193-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ferrara LA, Capaldo B, Mancusi C, Lee ET, Howard BV, Devereux RB, et al. Cardiometabolic risk in overweight subjects with or without relative fat‐free mass deficiency: The Strong Heart Study. Nutr Metab Cardiovasc Dis 2014;24:271‐276. doi: 10.1016/j.numecd.2013.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Stenholm S, Harris TB, Rantanen T, Visser M, Kritchevsky SB, Ferrucci L. Sarcopenic obesity: Definition, cause and consequences. Curr Opin Clin Nutr Metab Care 2008;11:693‐700. doi: 10.1097/MCO.0b013e328312c37d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. van Dam AD, Boon MR, Berbee JFP, Rensen PCN, van Harmelen V. Targeting white, brown and perivascular adipose tissue in atherosclerosis development. Eur J Pharmacol 2017;816:82‐92. doi: 10.1016/j.ejphar.2017.03.051 [DOI] [PubMed] [Google Scholar]
- 41. Packer M. Epicardial adipose tissue may mediate deleterious effects of obesity and inflammation on the myocardium. J Am Coll Cardiol 2018;71:2360‐2372. doi: 10.1016/j.jacc.2018.03.509 [DOI] [PubMed] [Google Scholar]
- 42. Nesti L, Pugliese NR, Chiriaco M, Trico D, Baldi S, Natali A. Epicardial adipose tissue thickness is associated with reduced peak oxygen consumption and systolic reserve in patients with type 2 diabetes and normal heart function. Diabetes Obes Metab 2023;25:177‐188. doi: 10.1111/dom.14861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Iacobellis G, Leonetti F, Singh N. Relationship of epicardial adipose tissue with atrial dimensions and diastolic function in morbidly obese subjects. Int J Cardiol 2007;115:272‐273. doi: 10.1016/j.ijcard.2006.04.016 [DOI] [PubMed] [Google Scholar]
- 44. Pugliese NR, Paneni F, Mazzola M, De Biase N, Del Punta L, Gargani L, et al. Impact of epicardial adipose tissue on cardiovascular haemodynamics, metabolic profile, and prognosis in heart failure. Eur J Heart Fail 2021;23:1858‐1871. doi: 10.1002/ejhf.2337 [DOI] [PubMed] [Google Scholar]
- 45. Soucek F, Covassin N, Singh P, Ruzek L, Kara T, Suleiman M, et al. Effects of atorvastatin (80 mg) therapy on quantity of epicardial adipose tissue in patients undergoing pulmonary vein isolation for atrial fibrillation. Am J Cardiol 2015;116:1443‐1446. doi: 10.1016/j.amjcard.2015.07.067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cameron AR, Morrison VL, Levin D, Mohan M, Forteath C, Beall C, et al. Anti‐inflammatory effects of metformin irrespective of diabetes status. Circ Res 2016;119:652‐665. doi: 10.1161/CIRCRESAHA.116.308445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bouchi R, Terashima M, Sasahara Y, Asakawa M, Fukuda T, Takeuchi T, et al. Luseogliflozin reduces epicardial fat accumulation in patients with type 2 diabetes: A pilot study. Cardiovasc Diabetol 2017;16:32. doi: 10.1007/s13300-017-0279-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Vorona RD, Winn MP, Babineau TW, Eng BP, Feldman HR, Ware JC. Overweight and obese patients in a primary care population report less sleep than patients with a normal body mass index. Arch Intern Med 2005;165:25‐30. doi: 10.1001/archinte.165.1.25 [DOI] [PubMed] [Google Scholar]
- 49. Gangwisch JE, Malaspina D, Boden‐Albala B, Heymsfield SB. Inadequate sleep as a risk factor for obesity: Analyses of the NHANES I. Sleep 2005;28:1289‐1296. doi: 10.1093/sleep/28.10.1289 [DOI] [PubMed] [Google Scholar]
- 50. Jehan S, Zizi F, Pandi‐Perumal SR, Wall S, Auguste E, Myers AK, et al. Obstructive sleep apnea and obesity: Implications for public health. Sleep Med Disord 2017;1. [PMC free article] [PubMed] [Google Scholar]
- 51. Sareli AE, Cantor CR, Williams NN, Korus G, Raper SE, Pien G, et al. Obstructive sleep apnea in patients undergoing bariatric surgery—A tertiary center experience. Obes Surg 2011;21:316‐327. doi: 10.1007/s11695-009-9928-1 [DOI] [PubMed] [Google Scholar]
- 52. Tietjens JR, Claman D, Kezirian EJ, De Marco T, Mirzayan A, Sadroonri B, et al. Obstructive sleep apnea in cardiovascular disease: A review of the literature and proposed multidisciplinary clinical management strategy. J Am Heart Assoc 2019;8:e010440. doi: 10.1161/JAHA.118.010440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Cowie MR, Linz D, Redline S, Somers VK, Simonds AK. Sleep disordered breathing and cardiovascular disease: JACC state‐of‐the‐art review. J Am Coll Cardiol 2021;78:608‐624. doi: 10.1016/j.jacc.2021.05.048 [DOI] [PubMed] [Google Scholar]
- 54. Marulanda‐Londono E, Chaturvedi S. The interplay between obstructive sleep apnea and atrial fibrillation. Front Neurol 2017;8:668. doi: 10.3389/fneur.2017.00668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Lanfranchi PA, Somers VK, Braghiroli A, Corra U, Eleuteri E, Giannuzzi P. Central sleep apnea in left ventricular dysfunction: Prevalence and implications for arrhythmic risk. Circulation 2003;107:727‐732. doi: 10.1161/01.CIR.0000049641.11675.EE [DOI] [PubMed] [Google Scholar]
- 56. Buonauro A, Galderisi M, Santoro C, Canora A, Bocchino ML, Lo Iudice F, et al. Obstructive sleep apnoea and right ventricular function: A combined assessment by speckle tracking and three‐dimensional echocardiography. Int J Cardiol 2017;243:544‐549. doi: 10.1016/j.ijcard.2017.05.002 [DOI] [PubMed] [Google Scholar]
- 57. Powell‐Wiley TM, Poirier P, Burke LE, Despres JP, Gordon‐Larsen P, Lavie CJ, et al. Obesity and cardiovascular disease: A scientific statement from the American Heart Association. Circulation 2021;143:e984‐e1010. doi: 10.1161/CIR.0000000000000973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Huxley RR, Lopez FL, Folsom AR, Agarwal SK, Loehr LR, Soliman EZ, et al. Absolute and attributable risks of atrial fibrillation in relation to optimal and borderline risk factors: The Atherosclerosis Risk in Communities (ARIC) study. Circulation 2011;123:1501‐1508. doi: 10.1161/CIRCULATIONAHA.110.009035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Tsang TS, Barnes ME, Miyasaka Y, Cha SS, Bailey KR, Verzosa GC, et al. Obesity as a risk factor for the progression of paroxysmal to permanent atrial fibrillation: A longitudinal cohort study of 21 years. Eur Heart J 2008;29:2227‐2233. doi: 10.1093/eurheartj/ehn324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Anderson JL, Allen Maycock CA, Lappe DL, Crandall BG, Horne BD, Bair TL, et al. Frequency of elevation of C‐reactive protein in atrial fibrillation. Am J Cardiol 2004;94:1255‐1259. doi: 10.1016/j.amjcard.2004.07.108 [DOI] [PubMed] [Google Scholar]
- 61. Tadic M, Ivanovic B, Cuspidi C. What do we currently know about metabolic syndrome and atrial fibrillation? Clin Cardiol 2013;36:654‐662. doi: 10.1002/clc.22163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Mahajan R, Lau DH, Brooks AG, Shipp NJ, Wood JPM, Manavis J, et al. Atrial fibrillation and obesity: Reverse remodeling of atrial substrate with weight reduction. JACC Clin Electrophysiol 2021;7:630‐641. doi: 10.1016/j.jacep.2020.11.015 [DOI] [PubMed] [Google Scholar]
- 63. Alpert MA, Omran J, Bostick BP. Effects of obesity on cardiovascular hemodynamics, cardiac morphology, and ventricular function. Curr Obes Rep 2016;5:424‐434. doi: 10.1007/s13679-016-0235-6 [DOI] [PubMed] [Google Scholar]
- 64. Venteclef N, Guglielmi V, Balse E, Gaborit B, Cotillard A, Atassi F, et al. Human epicardial adipose tissue induces fibrosis of the atrial myocardium through the secretion of adipo‐fibrokines. Eur Heart J 2015;36:795‐805a. doi: 10.1093/eurheartj/eht099 [DOI] [PubMed] [Google Scholar]
- 65. Mahajan R, Lau DH, Brooks AG, Shipp NJ, Manavis J, Wood JP, et al. Electrophysiological, electroanatomical, and structural remodeling of the atria as consequences of sustained obesity. J Am Coll Cardiol 2015;66:1‐11. doi: 10.1016/j.jacc.2015.04.058 [DOI] [PubMed] [Google Scholar]
- 66. van Rosendael AR, Smit JM, El'Mahdiui M, van Rosendael PJ, Leung M, Delgado V, et al. Association between left atrial epicardial fat, left atrial volume, and the severity of atrial fibrillation. Europace 2022;24:1223‐1228. doi: 10.1093/europace/euac031 [DOI] [PubMed] [Google Scholar]
- 67. Cha YM, Friedman PA, Asirvatham SJ, Shen WK, Munger TM, Rea RF, et al. Catheter ablation for atrial fibrillation in patients with obesity. Circulation 2008;117:2583‐2590. doi: 10.1161/CIRCULATIONAHA.107.716712 [DOI] [PubMed] [Google Scholar]
- 68. Winkle RA, Mead RH, Engel G, Kong MH, Fleming W, Salcedo J, et al. Impact of obesity on atrial fibrillation ablation: Patient characteristics, long‐term outcomes, and complications. Heart Rhythm 2017;14:819‐827. doi: 10.1016/j.hrthm.2017.02.023 [DOI] [PubMed] [Google Scholar]
- 69. Sivasambu B, Balouch MA, Zghaib T, Bajwa RJ, Chrispin J, Berger RD, et al. Increased rates of atrial fibrillation recurrence following pulmonary vein isolation in overweight and obese patients. J Cardiovasc Electrophysiol 2018;29:239‐245. doi: 10.1111/jce.13388 [DOI] [PubMed] [Google Scholar]
- 70. Providencia R, Adragao P, de Asmundis C, Chun J, Chierchia G, Defaye P, et al. Impact of body mass index on the outcomes of catheter ablation of atrial fibrillation: A European observational multicenter study. J Am Heart Assoc 2019;8:e012253. doi: 10.1161/JAHA.119.012253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Abed HS, Wittert GA, Leong DP, Shirazi MG, Bahrami B, Middeldorp ME, et al. Effect of weight reduction and cardiometabolic risk factor management on symptom burden and severity in patients with atrial fibrillation: A randomized clinical trial. JAMA 2013;310:2050‐2060. doi: 10.1001/jama.2013.280521 [DOI] [PubMed] [Google Scholar]
- 72. Kalarus Z, Mairesse GH, Sokal A, Boriani G, Sredniawa B, Casado‐Arroyo R, et al. Searching for atrial fibrillation: Looking harder, looking longer, and in increasingly sophisticated ways. An EHRA position paper. EP Europace 2023;25:185‐198. doi: 10.1093/europace/euac144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Niccoli G, Scalone G, Lerman A, Crea F. Coronary microvascular obstruction in acute myocardial infarction. Eur Heart J 2016;37:1024‐1033. doi: 10.1093/eurheartj/ehv484 [DOI] [PubMed] [Google Scholar]
- 74. Thakker RA, Rodriguez Lozano J, Rodriguez Lozano P, Motiwala A, Rangasetty U, Khalife W, et al. Coronary microvascular disease. Cardiol Ther 2022;11:23‐31. doi: 10.1007/s40119-021-00250-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Taqueti VR, Di Carli MF. Coronary microvascular disease pathogenic mechanisms and therapeutic options: JACC state‐of‐the‐art review. J Am Coll Cardiol 2018;72:2625‐2641. doi: 10.1016/j.jacc.2018.09.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Chen C, Wei J, AlBadri A, Zarrini P, Bairey Merz CN. Coronary microvascular dysfunction—Epidemiology, pathogenesis, prognosis, diagnosis, risk factors and therapy. Risk Fact Ther Circ J 2016;81:3‐11. doi: 10.1253/circj.CJ-16-1002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Mangiacapra F, Bressi E, Di Gioia G, Pellicano M, Di Serafino L, Peace AJ, et al. Coronary microcirculation and peri‐procedural myocardial injury during elective percutaneous coronary intervention. Int J Cardiol 2020;306:42‐46. doi: 10.1016/j.ijcard.2019.12.042 [DOI] [PubMed] [Google Scholar]
- 78. Konst RE, Guzik TJ, Kaski JC, Maas A, Elias‐Smale SE. The pathogenic role of coronary microvascular dysfunction in the setting of other cardiac or systemic conditions. Cardiovasc Res 2020;116:817‐828. doi: 10.1093/cvr/cvaa009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Bajaj NS, Osborne MT, Gupta A, Tavakkoli A, Bravo PE, Vita T, et al. Coronary microvascular dysfunction and cardiovascular risk in obese patients. J Am Coll Cardiol 2018;72:707‐717. doi: 10.1016/j.jacc.2018.05.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Schindler TH, Cardenas J, Prior JO, Facta AD, Kreissl MC, Zhang XL, et al. Relationship between increasing body weight, insulin resistance, inflammation, adipocytokine leptin, and coronary circulatory function. J Am Coll Cardiol 2006;47:1188‐1195. doi: 10.1016/j.jacc.2005.10.062 [DOI] [PubMed] [Google Scholar]
- 81. Bogers RP, Bemelmans WJ, Hoogenveen RT, Boshuizen HC, Woodward M, Knekt P, et al. Association of overweight with increased risk of coronary heart disease partly independent of blood pressure and cholesterol levels: A meta‐analysis of 21 cohort studies including more than 300 000 persons. Arch Intern Med 2007;167:1720‐1728. doi: 10.1001/archinte.167.16.1720 [DOI] [PubMed] [Google Scholar]
- 82. Folsom AR, Stevens J, Schreiner PJ, McGovern PG, Atherosclerosis Risk in Communities Study Investigators . Body mass index, waist/hip ratio, and coronary heart disease incidence in African Americans and whites. Am J Epidemiol 1998;148:1187‐1194. doi: 10.1093/oxfordjournals.aje.a009608 [DOI] [PubMed] [Google Scholar]
- 83. McGill HC Jr, McMahan CA, Herderick EE, Zieske AW, Malcom GT, Tracy RE, et al. Obesity accelerates the progression of coronary atherosclerosis in young men. Circulation 2002;105:2712‐2718. doi: 10.1161/01.CIR.0000018121.67607.CE [DOI] [PubMed] [Google Scholar]
- 84. Rocha VZ, Libby P. Obesity, inflammation, and atherosclerosis. Nat Rev Cardiol 2009;6:399‐409. doi: 10.1038/nrcardio.2009.55 [DOI] [PubMed] [Google Scholar]
- 85. Ross R. Atherosclerosis is an inflammatory disease. Am Heart J 1999;138:S419‐S420. doi: 10.1016/s0002-8703(99)70266-8 [DOI] [PubMed] [Google Scholar]
- 86. Bassi N, Karagodin I, Wang S, Vassallo P, Priyanath A, Massaro E, et al. Lifestyle modification for metabolic syndrome: A systematic review. Am J Med 2014;127:1242 e1‐e10. doi: 10.1016/j.amjmed.2014.06.035 [DOI] [PubMed] [Google Scholar]
- 87. Look ARG, Wing RR, Bolin P, Brancati FL, Bray GA, Clark JM, et al. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med 2013;369:145‐154. doi: 10.1056/NEJMoa1212914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Sjostrom L, Peltonen M, Jacobson P, Sjostrom CD, Karason K, Wedel H, et al. Bariatric surgery and long‐term cardiovascular events. JAMA 2012;307:56‐65. doi: 10.1001/jama.2011.1914 [DOI] [PubMed] [Google Scholar]
- 89. Batsis JA, Sarr MG, Collazo‐Clavell ML, Thomas RJ, Romero‐Corral A, Somers VK, et al. Cardiovascular risk after bariatric surgery for obesity. Am J Cardiol 2008;102:930‐937. doi: 10.1016/j.amjcard.2008.05.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Poirier P, Giles TD, Bray GA, Hong Y, Stern JS, Pi‐Sunyer FX, et al. Obesity and cardiovascular disease: Pathophysiology, evaluation, and effect of weight loss: An update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation 2006;113:898‐918. doi: 10.1161/CIRCULATIONAHA.106.171016 [DOI] [PubMed] [Google Scholar]
- 91. Goetze JP, Bruneau BG, Ramos HR, Ogawa T, de Bold MK, de Bold AJ. Cardiac natriuretic peptides. Nat Rev Cardiol 2020;17:698‐717. doi: 10.1038/s41569-020-0381-0 [DOI] [PubMed] [Google Scholar]
- 92. Volpe M, Gallo G, Rubattu S. Endocrine functions of the heart: From bench to bedside. Eur Heart J 2023;44:643‐655. doi: 10.1093/eurheartj/ehac759 [DOI] [PubMed] [Google Scholar]
- 93. Woodard GE, Rosado JA. Natriuretic peptides in vascular physiology and pathology. Int Rev Cell Mol Biol 2008;268:59‐93. doi: 10.1016/S1937-6448(08)00803-4 [DOI] [PubMed] [Google Scholar]
- 94. Forte M, Marchitti S, Di Nonno F, Stanzione R, Schirone L, Cotugno M, et al. NPPA/atrial natriuretic peptide is an extracellular modulator of autophagy in the heart. Autophagy 2023;19:1087‐1099. doi: 10.1080/15548627.2022.2115675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Rukavina Mikusic NL, Kouyoumdzian NM, Puyo AM, Fernandez BE, Choi MR. Role of natriuretic peptides in the cardiovascular‐adipose communication: A tale of two organs. Pflugers Arch 2022;474:5‐19. doi: 10.1007/s00424-021-02596-1 [DOI] [PubMed] [Google Scholar]
- 96. Perez‐Ternero C, Aubdool AA, Makwana R, Sanger GJ, Stimson RH, Chan LF, et al. C‐type natriuretic peptide is a pivotal regulator of metabolic homeostasis. Proc Natl Acad Sci U S A 2022;119:e2116470119. doi: 10.1073/pnas.2116470119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Jordan J, Birkenfeld AL, Melander O, Moro C. Natriuretic peptides in cardiovascular and metabolic crosstalk: Implications for hypertension management. Hypertension 2018;72:270‐276. doi: 10.1161/HYPERTENSIONAHA.118.11081 [DOI] [PubMed] [Google Scholar]
- 98. Wang TJ, Larson MG, Levy D, Benjamin EJ, Leip EP, Wilson PW, et al. Impact of obesity on plasma natriuretic peptide levels. Circulation 2004;109:594‐600. doi: 10.1161/01.CIR.0000112582.16683.EA [DOI] [PubMed] [Google Scholar]
- 99. Mehra MR, Uber PA, Park MH, Scott RL, Ventura HO, Harris BC, et al. Obesity and suppressed B‐type natriuretic peptide levels in heart failure. J Am Coll Cardiol 2004;43:1590‐1595. doi: 10.1016/j.jacc.2003.10.066 [DOI] [PubMed] [Google Scholar]
- 100. Standeven KF, Hess K, Carter AM, Rice GI, Cordell PA, Balmforth AJ, et al. Neprilysin, obesity and the metabolic syndrome. Int J Obes (Lond) 2011;35:1031‐1040. doi: 10.1038/ijo.2010.227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Castiglione V, Aimo A, Vergaro G, Saccaro L, Passino C, Emdin M. Biomarkers for the diagnosis and management of heart failure. Heart Fail Rev 2022;27:625‐643. doi: 10.1007/s10741-021-10105-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Mavrogeni SI, Bacopoulou F, Markousis‐Mavrogenis G, Chrousos G, Charmandari E. Cardiovascular imaging in obesity. Nutrients 2021;13:3. doi: 10.3390/nu13030744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Lembo M, Manzi MV, Mancusi C, Morisco C, Rao MAE, Cuocolo A, et al. Advanced imaging tools for evaluating cardiac morphological and functional impairment in hypertensive disease. J Hypertens 2022;40:4‐14. doi: 10.1097/HJH.0000000000002967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Tumuklu MM, Etikan I, Kisacik B, Kayikcioglu M. Effect of obesity on left ventricular structure and myocardial systolic function: Assessment by tissue Doppler imaging and strain/strain rate imaging. Echocardiography 2007;24:802‐809. doi: 10.1111/j.1540-8175.2007.00484.x [DOI] [PubMed] [Google Scholar]
- 105. Bella JN, Devereux RB, Roman MJ, O'Grady MJ, Welty TK, Lee ET, et al. Relations of left ventricular mass to fat‐free and adipose body mass: The Strong Heart Study. Strong Heart Stud Invest Circ 1998;98:2538‐2544. doi: 10.1161/01.CIR.98.23.2538 [DOI] [PubMed] [Google Scholar]
- 106. Aurigemma GP, de Simone G, Fitzgibbons TP. Cardiac remodeling in obesity. Circ Cardiovasc Imaging 2013;6:142‐152. doi: 10.1161/CIRCIMAGING.111.964627 [DOI] [PubMed] [Google Scholar]
- 107. Mancusi C, Gerdts E, Losi MA, D'Amato A, Manzi MV, Canciello G, et al. Differential effect of obesity on prevalence of cardiac and carotid target organ damage in hypertension (the Campania Salute Network). Int J Cardiol 2017;244:260‐264. doi: 10.1016/j.ijcard.2017.06.045 [DOI] [PubMed] [Google Scholar]
- 108. Cuspidi C, Meani S, Negri F, Giudici V, Valerio C, Sala C, et al. Indexation of left ventricular mass to body surface area and height to allometric power of 2.7: Is the difference limited to obese hypertensives? J Hum Hypertens 2009;23:728‐734. doi: 10.1038/jhh.2009.16 [DOI] [PubMed] [Google Scholar]
- 109. Blomstrand P, Sjoblom P, Nilsson M, Wijkman M, Engvall M, Lanne T, et al. Overweight and obesity impair left ventricular systolic function as measured by left ventricular ejection fraction and global longitudinal strain. Cardiovasc Diabetol 2018;17:113. doi: 10.1186/s12933-018-0756-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Di Bello V, Fabiani I, Conte L, Barletta V, Delle Donne MG, Cuono C, et al. New echocardiographic techniques in the evaluation of left ventricular function in obesity. Obesity (Silver Spring) 2013;21:881‐892. doi: 10.1002/oby.20071 [DOI] [PubMed] [Google Scholar]
- 111. Dini FL, Fabiani I, Miccoli M, Galeotti GG, Pugliese NR, D'Agostino A, et al. Prevalence and determinants of left ventricular diastolic dysfunction in obese subjects and the role of left ventricular global longitudinal strain and mass normalized to height. Echocardiography 2018;35:1124‐1131. doi: 10.1111/echo.13890 [DOI] [PubMed] [Google Scholar]
- 112. Mancusi C, Losi MA, Izzo R, Canciello G, Manzi MV, Sforza A, et al. Effect of diabetes and metabolic syndrome on myocardial mechano‐energetic efficiency in hypertensive patients. The Campania Salute Network. J Hum Hypertens 2017;31:424. doi: 10.1038/jhh.2017.3 [DOI] [PubMed] [Google Scholar]
- 113. Lembo M, Acampa W, Rao MAE, Manzi MV, Morisco C, Esposito G, et al. Left ventricular mechano‐energetic efficiency identifies an early impairment of myocardial blood flow in arterial hypertension. Hypertension 2023;80:1534‐1543. doi: 10.1161/HYPERTENSIONAHA.123.21071 [DOI] [PubMed] [Google Scholar]
- 114. Mancusi C, de Simone G, Best LG, Wang W, Zhang Y, Roman MJ, et al. Myocardial mechano‐energetic efficiency and insulin resistance in non‐diabetic members of the Strong Heart Study cohort. Cardiovasc Diabetol 2019;18:56. doi: 10.1186/s12933-019-0862-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Manzi MV, Mancusi C, Lembo M, Esposito G, Rao MAE, de Simone G, et al. Low mechano‐energetic efficiency is associated with future left ventricular systolic dysfunction in hypertensives. ESC Heart Fail 2022;9:2291‐2300. doi: 10.1002/ehf2.13908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Lembo M, Trimarco V, Manzi MV, Mancusi C, Esposito G, Esposito S, et al. Determinants of improvement of left ventricular mechano‐energetic efficiency in hypertensive patients. Front Cardiovasc Med 2022;9:977657. doi: 10.3389/fcvm.2022.977657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Kibar AE, Pac FA, Ece I, Oflaz MB, Balli S, Bas VN, et al. Effect of obesity on left ventricular longitudinal myocardial strain by speckle tracking echocardiography in children and adolescents. Balkan Med J 2015;32:56‐63. doi: 10.5152/balkanmedj.2015.15136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Lembo M, Santoro C, Sorrentino R, Fazio V, Canonico ME, Chiariello L, et al. Prominent basal and middle strain longitudinal involvement in newly‐diagnosed and never treated hypertensive patients without clear‐cut hypertrophy. Int J Cardiol 2020;304:179‐184. doi: 10.1016/j.ijcard.2020.01.038 [DOI] [PubMed] [Google Scholar]
- 119. Lembo M, Santoro C, Sorrentino R, Canonico ME, Fazio V, Trimarco B, et al. Interrelation between midwall mechanics and longitudinal strain in newly diagnosed and never‐treated hypertensive patients without clinically defined hypertrophy. J Hypertens 2020;38:295‐302. doi: 10.1097/HJH.0000000000002257 [DOI] [PubMed] [Google Scholar]
- 120. Santoro C, Esposito R, Lembo M, Sorrentino R, De Santo I, Luciano F, et al. Strain‐oriented strategy for guiding cardioprotection initiation of breast cancer patients experiencing cardiac dysfunction. Eur Heart J Cardiovasc Imaging 2019;20:1345‐1352. doi: 10.1093/ehjci/jez194 [DOI] [PubMed] [Google Scholar]
- 121. Monte IP, Mangiafico S, Buccheri S, Arcidiacono AA, Lavanco V, Privitera F, et al. Early changes of left ventricular geometry and deformational analysis in obese subjects without cardiovascular risk factors: A three‐dimensional and speckle tracking echocardiographic study. Int J Cardiovasc Imaging 2014;30:1037‐1047. doi: 10.1007/s10554-014-0429-5 [DOI] [PubMed] [Google Scholar]
- 122. Lembo M, Santoro C, Sorrentino R, Trimarco B, Galderisi M, Esposito R. Impact of left ventricular mass/end‐diastolic volume ratio by three‐dimensional echocardiography on two‐dimensional global longitudinal strain and diastolic function in native hypertensive patients. J Hypertens 2019;37:2041‐2047. doi: 10.1097/HJH.0000000000002147 [DOI] [PubMed] [Google Scholar]
- 123. Lembo M, Esposito R, Santoro C, Lo Iudice F, Schiano‐Lomoriello V, Fazio V, et al. Three‐dimensional echocardiographic ventricular mass/end‐diastolic volume ratio in native hypertensive patients: Relation between stroke volume and geometry. J Hypertens 2018;36:1697‐1704. doi: 10.1097/HJH.0000000000001717 [DOI] [PubMed] [Google Scholar]
- 124. Mureddu GF, Greco R, Rosato GF, Cella A, Vaccaro O, Contaldo F, et al. Relation of insulin resistance to left ventricular hypertrophy and diastolic dysfunction in obesity. Int J Obes Relat Metab Disord 1998;22:363‐368. doi: 10.1038/sj.ijo.0800596 [DOI] [PubMed] [Google Scholar]
- 125. Sahiti F, Morbach C, Cejka V, Tiffe T, Wagner M, Eichner FA, et al. Impact of cardiovascular risk factors on myocardial work—Insights from the STAAB cohort study. J Hum Hypertens 2022;36:235‐245. doi: 10.1038/s41371-021-00509-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Park CS, Park JB, Park JJ, Park JH, Cho GY. Impact of sex and myocardial function on association of obesity with mortality in Asian patients with acute heart failure: A retrospective analysis from the STRATS‐AHF registry. BMJ Open 2020;10:e031608. doi: 10.1136/bmjopen-2019-031608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Petitto M, Esposito R, Sorrentino R, Lembo M, Luciano F, De Roberto AM, et al. Sex‐specific echocardiographic reference values: The women's point of view. J Cardiovasc Med (Hagerstown) 2018;19:527‐535. doi: 10.2459/JCM.0000000000000696 [DOI] [PubMed] [Google Scholar]
- 128. Miyoshi H, Oishi Y, Mizuguchi Y, Iuchi A, Nagase N, Ara N, et al. Contribution of obesity to left atrial and left ventricular dysfunction in asymptomatic patients with hypertension: A two‐dimensional speckle‐tracking echocardiographic study. J Am Soc Hypertens 2014;8:54‐63. doi: 10.1016/j.jash.2013.08.005 [DOI] [PubMed] [Google Scholar]
- 129. McManus DD, Xanthakis V, Sullivan LM, Zachariah J, Aragam J, Larson MG, et al. Longitudinal tracking of left atrial diameter over the adult life course: Clinical correlates in the community. Circulation 2010;121:667‐674. doi: 10.1161/CIRCULATIONAHA.109.885806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Chirinos JA, Sardana M, Satija V, Gillebert TC, De Buyzere ML, Chahwala J, et al. Effect of obesity on left atrial strain in persons aged 35–55 years (the Asklepios study). Am J Cardiol 2019;123:854‐861. doi: 10.1016/j.amjcard.2018.11.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Vyas V, Lambiase P. Obesity and atrial fibrillation: Epidemiology, pathophysiology and novel therapeutic opportunities. Arrhythmia Electrophysiol Rev 2019;8:28‐36. doi: 10.15420/aer.2018.76.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Liu J, Li J, Yu J, Xia C, Pu H, He W, et al. Regional fat distributions are associated with subclinical right ventricular dysfunction in adults with uncomplicated obesity. Front Cardiovasc Med 2022;9:814505. doi: 10.3389/fcvm.2022.999845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Santoro C, Sorrentino R, Esposito R, Lembo M, Capone V, Rozza F, et al. Cardiopulmonary exercise testing and echocardiographic exam: An useful interaction. Cardiovasc Ultrasound 2019;17:29. doi: 10.1186/s12947-019-0180-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Chahal H, McClelland RL, Tandri H, Jain A, Turkbey EB, Hundley WG, et al. Obesity and right ventricular structure and function: The MESA‐Right Ventricle Study. Chest 2012;141:388‐395. doi: 10.1378/chest.11-0172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Wong CY, O'Moore‐Sullivan T, Leano R, Hukins C, Jenkins C, Marwick TH. Association of subclinical right ventricular dysfunction with obesity. J Am Coll Cardiol 2006;47:611‐616. doi: 10.1016/j.jacc.2005.11.015 [DOI] [PubMed] [Google Scholar]
- 136. Iacobellis G, Corradi D, Sharma AM. Epicardial adipose tissue: Anatomic, biomolecular and clinical relationships with the heart. Nat Clin Pract Cardiovasc Med 2005;2:536‐543. doi: 10.1038/ncpcardio0319 [DOI] [PubMed] [Google Scholar]
- 137. Messroghli DR, Moon JC, Ferreira VM, Grosse‐Wortmann L, He T, Kellman P, et al. Clinical recommendations for cardiovascular magnetic resonance mapping of T1, T2, T2* and extracellular volume: A consensus statement by the Society for Cardiovascular Magnetic Resonance (SCMR) endorsed by the European Association for Cardiovascular Imaging (EACVI). J Cardiovasc Magn Reson 2017;19:75. doi: 10.1186/s12968-017-0389-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Kucukseymen S, Neisius U, Rodriguez J, Tsao CW, Nezafat R. Negative synergism of diabetes mellitus and obesity in patients with heart failure with preserved ejection fraction: A cardiovascular magnetic resonance study. Int J Cardiovasc Imaging 2020;36:2027‐2038. doi: 10.1007/s10554-020-01915-4 [DOI] [PubMed] [Google Scholar]
- 139. Zhao Y, Dagher L, Huang C, Miller P, Marrouche NF. Cardiac MRI to manage atrial fibrillation. Arrhythmia Electrophysiol Rev 2020;9:189‐194. doi: 10.15420/aer.2020.21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Wilson PW, D'Agostino RB, Sullivan L, Parise H, Kannel WB. Overweight and obesity as determinants of cardiovascular risk: The Framingham experience. Arch Intern Med 2002;162:1867‐1872. doi: 10.1001/archinte.162.16.1867 [DOI] [PubMed] [Google Scholar]
- 141. Ge Y, Steel K, Antiochos P, Bingham S, Abdullah S, Mikolich JR, et al. Stress CMR in patients with obesity: Insights from the Stress CMR Perfusion Imaging in the United States (SPINS) registry. Eur Heart J Cardiovasc Imaging 2021;22:518‐527. doi: 10.1093/ehjci/jeaa281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Shah RV, Heydari B, Coelho‐Filho O, Abbasi SA, Feng JH, Neilan TG, et al. Vasodilator stress perfusion CMR imaging is feasible and prognostic in obese patients. JACC Cardiovasc Imaging 2014;7:462‐472. doi: 10.1016/j.jcmg.2013.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Law WY, Huang GL, Yang CC. Effect of body mass index in coronary CT angiography performed on a 256‐slice multi‐detector CT scanner. Diagnostics (Basel) 2022;12:319. doi: 10.3390/diagnostics12020319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Messerli M, Maywald C, Walti S, Warschkow R, Wildermuth S, Alkadhi H, et al. Prognostic value of negative coronary CT angiography in severely obese patients prior to bariatric surgery: A follow‐up after 6 years. Obes Surg 2017;27:2044‐2049. doi: 10.1007/s11695-017-2592-y [DOI] [PubMed] [Google Scholar]
- 145. Lim SP, Arasaratnam P, Chow BJ, Beanlands RS, Hessian RC. Obesity and the challenges of noninvasive imaging for the detection of coronary artery disease. Can J Cardiol 2015;31:223‐226. doi: 10.1016/j.cjca.2014.12.003 [DOI] [PubMed] [Google Scholar]
- 146. Nakazato R, Tamarappoo BK, Kang X, Wolak A, Kite F, Hayes SW, et al. Quantitative upright‐supine high‐speed SPECT myocardial perfusion imaging for detection of coronary artery disease: Correlation with invasive coronary angiography. J Nucl Med 2010;51:1724‐1731. doi: 10.2967/jnumed.110.078782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Harnett DT, Hazra S, Maze R, Mc Ardle BA, Alenazy A, Simard T, et al. Clinical performance of Rb‐82 myocardial perfusion PET and Tc‐99m‐based SPECT in patients with extreme obesity. J Nucl Cardiol 2019;26:275‐283. doi: 10.1007/s12350-017-0855-6 [DOI] [PubMed] [Google Scholar]
- 148. Marinescu MA, Loffler AI, Ouellette M, Smith L, Kramer CM, Bourque JM. Coronary microvascular dysfunction, microvascular angina, and treatment strategies. JACC Cardiovasc Imaging 2015;8:210‐220. doi: 10.1016/j.jcmg.2014.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Lembo M, Sicari R, Esposito R, Rigo F, Cortigiani L, Lo Iudice F, et al. Association between elevated pulse pressure and high resting coronary blood flow velocity in patients with angiographically normal epicardial coronary arteries. J Am Heart Assoc 2017;6: doi: 10.1161/JAHA.117.005710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Pirat B, Bozbas H, Simsek V, Yildirir A, Sade LE, Gursoy Y, et al. Impaired coronary flow reserve in patients with metabolic syndrome. Atherosclerosis 2008;201:112‐116. doi: 10.1016/j.atherosclerosis.2008.02.016 [DOI] [PubMed] [Google Scholar]
- 151. Horwich TB, Fonarow GC, Hamilton MA, MacLellan WR, Woo MA, Tillisch JH. The relationship between obesity and mortality in patients with heart failure. J Am Coll Cardiol 2001;38:789‐795. doi: 10.1016/S0735-1097(01)01448-6 [DOI] [PubMed] [Google Scholar]
- 152. Kenchaiah S, Pocock SJ, Wang D, Finn PV, Zornoff LA, Skali H, et al. Body mass index and prognosis in patients with chronic heart failure: Insights from the Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity (CHARM) program. Circulation 2007;116:627‐636. doi: 10.1161/CIRCULATIONAHA.106.679779 [DOI] [PubMed] [Google Scholar]
- 153. Cicoira M, Maggioni AP, Latini R, Barlera S, Carretta E, Janosi A, et al. Body mass index, prognosis and mode of death in chronic heart failure: Results from the Valsartan Heart Failure Trial. Eur J Heart Fail 2007;9:397‐402. doi: 10.1016/j.ejheart.2006.10.016 [DOI] [PubMed] [Google Scholar]
- 154. Haass M, Kitzman DW, Anand IS, Miller A, Zile MR, Massie BM, et al. Body mass index and adverse cardiovascular outcomes in heart failure patients with preserved ejection fraction: Results from the Irbesartan in Heart Failure with Preserved Ejection Fraction (I‐PRESERVE) trial. Circ Heart Fail 2011;4:324‐331. doi: 10.1161/CIRCHEARTFAILURE.110.959890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Fonarow GC, Srikanthan P, Costanzo MR, Cintron GB, Lopatin M, ADHERE Scientific Advisory Committee and Investigators . An obesity paradox in acute heart failure: Analysis of body mass index and inhospital mortality for 108 927 patients in the Acute Decompensated Heart Failure National Registry. Am Heart J 2007;153:74‐81. doi: 10.1016/j.ahj.2006.09.007 [DOI] [PubMed] [Google Scholar]
- 156. Sharma A, Lavie CJ, Borer JS, Vallakati A, Goel S, Lopez‐Jimenez F, et al. Meta‐analysis of the relation of body mass index to all‐cause and cardiovascular mortality and hospitalization in patients with chronic heart failure. Am J Cardiol 2015;115:1428‐1434. doi: 10.1016/j.amjcard.2015.02.024 [DOI] [PubMed] [Google Scholar]
- 157. Oreopoulos A, Padwal R, Kalantar‐Zadeh K, Fonarow GC, Norris CM, McAlister FA. Body mass index and mortality in heart failure: A meta‐analysis. Am Heart J 2008;156:13‐22. doi: 10.1016/j.ahj.2008.02.014 [DOI] [PubMed] [Google Scholar]
- 158. Vest AR, Wu Y, Hachamovitch R, Young JB, Cho L. The heart failure overweight/obesity survival paradox: The missing sex link. JACC Heart Fail 2015;3:917‐926. doi: 10.1016/j.jchf.2015.06.009 [DOI] [PubMed] [Google Scholar]
- 159. Nagarajan V, Kohan L, Holland E, Keeley EC, Mazimba S. Obesity paradox in heart failure: A heavy matter. ESC Heart Fail 2016;3:227‐234. doi: 10.1002/ehf2.12120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Alpert MA, Terry BE, Mulekar M, Cohen MV, Massey CV, Fan TM, et al. Cardiac morphology and left ventricular function in normotensive morbidly obese patients with and without congestive heart failure, and effect of weight loss. Am J Cardiol 1997;80:736‐740. doi: 10.1016/S0002-9149(97)00505-5 [DOI] [PubMed] [Google Scholar]
- 161. Frohlich H, Frey N, Frankenstein L, Tager T. The obesity paradox in heart failure: Is it still valid in light of new therapies? Cardiology 2022;147:529‐538. doi: 10.1159/000527332 [DOI] [PubMed] [Google Scholar]
- 162. Vest AR, Chan M, Deswal A, Givertz MM, Lekavich C, Lennie T, et al. Nutrition, obesity, and cachexia in patients with heart failure: A consensus statement from the Heart Failure Society of America Scientific Statements Committee. J Card Fail 2019;25:380‐400. doi: 10.1016/j.cardfail.2019.03.007 [DOI] [PubMed] [Google Scholar]
- 163. Wilding JPH, Batterham RL, Calanna S, Davies M, Van Gaal LF, Lingvay I, et al. Once‐weekly semaglutide in adults with overweight or obesity. N Engl J Med 2021;384:989‐1002. doi: 10.1056/NEJMoa2032183 [DOI] [PubMed] [Google Scholar]
- 164. Pi‐Sunyer X, Astrup A, Fujioka K, Greenway F, Halpern A, Krempf M, et al. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med 2015;373:11‐22. doi: 10.1056/NEJMoa1411892 [DOI] [PubMed] [Google Scholar]
- 165. Borlaug BA, Kitzman DW, Davies MJ, Rasmussen S, Barros E, Butler J, et al. Semaglutide in HFpEF across obesity class and by body weight reduction: A prespecified analysis of the STEP‐HFpEF trial. Nat Med 2023;29:2358‐2365. doi: 10.1038/s41591-023-02526-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Jastreboff AM, Aronne LJ, Ahmad NN, Wharton S, Connery L, Alves B, et al. Tirzepatide once weekly for the treatment of obesity. N Engl J Med 2022;387:205‐216. doi: 10.1056/NEJMoa2206038 [DOI] [PubMed] [Google Scholar]
- 167. Jastreboff AM, Kaplan LM, Frias JP, Wu Q, Du Y, Gurbuz S, et al. Triple–hormone‐receptor agonist retatrutide for obesity—A phase 2 trial. N Engl J Med 2023;389:514‐526. doi: 10.1056/NEJMoa2301972 [DOI] [PubMed] [Google Scholar]
- 168. Lincoff AM, Brown‐Frandsen K, Colhoun HM, Deanfield J, Emerson SS, Esbjerg S, et al. Semaglutide and cardiovascular outcomes in obesity without diabetes. N Engl J Med 2023; doi: 10.1056/NEJMoa2307563 [DOI] [PubMed] [Google Scholar]
- 169. Carlsson LMS, Sjoholm K, Jacobson P, Andersson‐Assarsson JC, Svensson PA, Taube M, et al. Life expectancy after bariatric surgery in the Swedish obese subjects study. N Engl J Med 2020;383:1535‐1543. doi: 10.1056/NEJMoa2002449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Yumuk V, Tsigos C, Fried M, Schindler K, Busetto L, Micic D, et al. European guidelines for obesity management in adults. Obes Facts 2015;8:402‐424. doi: 10.1159/000442721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Ferrera A, Battistoni A, Lanza O, Rossi C, Tocci G, Volpe M. Low perception of obesity as a pathological condition among Italian cardiologists. High Blood Press Cardiovasc Prev 2023;30:351‐356. doi: 10.1007/s40292-023-00588-z [DOI] [PubMed] [Google Scholar]
- 172. McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, et al. Angiotensin–neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014;371:993‐1004. doi: 10.1056/NEJMoa1409077 [DOI] [PubMed] [Google Scholar]
- 173. Solomon SD, McMurray JJV, Anand IS, Ge J, Lam CSP, Maggioni AP, et al. Angiotensin–neprilysin inhibition in heart failure with preserved ejection fraction. N Engl J Med 2019;381:1609‐1620. doi: 10.1056/NEJMoa1908655 [DOI] [PubMed] [Google Scholar]
- 174. Pieske B, Wachter R, Shah SJ, Baldridge A, Szeczoedy P, Ibram G, et al. Effect of sacubitril/valsartan vs standard medical therapies on plasma NT‐proBNP concentration and submaximal exercise capacity in patients with heart failure and preserved ejection fraction: The PARALLAX randomized clinical trial. JAMA 2021;326:1919‐1929. doi: 10.1001/jama.2021.18463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Khalid U, Wruck LM, Quibrera PM, Bozkurt B, Nambi V, Virani SS, et al. BNP and obesity in acute decompensated heart failure with preserved vs. reduced ejection fraction: The Atherosclerosis Risk in Communities Surveillance Study. Int J Cardiol 2017;233:61‐66. doi: 10.1016/j.ijcard.2017.01.130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Obokata M, Reddy YNV, Pislaru SV, Melenovsky V, Borlaug BA. Evidence supporting the existence of a distinct obese phenotype of heart failure with preserved ejection fraction. Circulation 2017;136:6‐19. doi: 10.1161/CIRCULATIONAHA.116.026807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Schelbert EB, Fridman Y, Wong TC, Abu Daya H, Piehler KM, Kadakkal A, et al. Temporal relation between myocardial fibrosis and heart failure with preserved ejection fraction: Association with baseline disease severity and subsequent outcome. JAMA Cardiol 2017;2:995‐1006. doi: 10.1001/jamacardio.2017.2511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Schling P, Schafer T. Human adipose tissue cells keep tight control on the angiotensin II levels in their vicinity. J Biol Chem 2002;277:48066‐48075. doi: 10.1074/jbc.M204058200 [DOI] [PubMed] [Google Scholar]
- 179. Polhemus DJ, Trivedi RK, Gao J, Li Z, Scarborough AL, Goodchild TT, et al. Renal sympathetic denervation protects the failing heart via inhibition of neprilysin activity in the kidney. J Am Coll Cardiol 2017;70:2139‐2153. doi: 10.1016/j.jacc.2017.08.056 [DOI] [PubMed] [Google Scholar]
- 180. Cunningham JW, Claggett BL, O'Meara E, Prescott MF, Pfeffer MA, Shah SJ, et al. Effect of sacubitril/valsartan on biomarkers of extracellular matrix regulation in patients with HFpEF. J Am Coll Cardiol 2020;76:503‐514. doi: 10.1016/j.jacc.2020.05.072 [DOI] [PubMed] [Google Scholar]
- 181. Bentley‐Lewis R, Adler GK, Perlstein T, Seely EW, Hopkins PN, Williams GH, et al. Body mass index predicts aldosterone production in normotensive adults on a high‐salt diet. J Clin Endocrinol Metab 2007;92:4472‐4475. doi: 10.1210/jc.2007-1088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Huby AC, Antonova G, Groenendyk J, Gomez‐Sanchez CE, Bollag WB, Filosa JA, et al. Adipocyte‐derived hormone leptin is a direct regulator of aldosterone secretion, which promotes endothelial dysfunction and cardiac fibrosis. Circulation 2015;132:2134‐2145. doi: 10.1161/CIRCULATIONAHA.115.018226 [DOI] [PubMed] [Google Scholar]
- 183. Briones AM, Nguyen Dinh Cat A, Callera GE, Yogi A, Burger D, He Y, et al. Adipocytes produce aldosterone through calcineurin‐dependent signaling pathways: Implications in diabetes mellitus‐associated obesity and vascular dysfunction. Hypertension 2012;59:1069‐1078. doi: 10.1161/HYPERTENSIONAHA.111.190223 [DOI] [PubMed] [Google Scholar]
- 184. Miura S, Nakayama A, Tomita S, Matsuo Y, Suematsu Y, Saku K. Comparison of aldosterone synthesis in adrenal cells, effect of various AT1 receptor blockers with or without atrial natriuretic peptide. Clin Exp Hypertens 2015;37:353‐357. doi: 10.3109/10641963.2014.987391 [DOI] [PubMed] [Google Scholar]
- 185. Brown NJ. Contribution of aldosterone to cardiovascular and renal inflammation and fibrosis. Nat Rev Nephrol 2013;9:459‐469. doi: 10.1038/nrneph.2013.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Hirata A, Maeda N, Nakatsuji H, Hiuge‐Shimizu A, Okada T, Funahashi T, et al. Contribution of glucocorticoid–mineralocorticoid receptor pathway on the obesity‐related adipocyte dysfunction. Biochem Biophys Res Commun 2012;419:182‐187. doi: 10.1016/j.bbrc.2012.01.139 [DOI] [PubMed] [Google Scholar]
- 187. Kosmala W, Przewlocka‐Kosmala M, Szczepanik‐Osadnik H, Mysiak A, Marwick TH. Fibrosis and cardiac function in obesity: A randomised controlled trial of aldosterone blockade. Heart 2013;99:320‐326. doi: 10.1136/heartjnl-2012-303329 [DOI] [PubMed] [Google Scholar]
- 188. Anand IS, Claggett B, Liu J, Shah AM, Rector TS, Shah SJ, et al. Interaction between spironolactone and natriuretic peptides in patients with heart failure and preserved ejection fraction: From the TOPCAT trial. JACC Heart Fail 2017;5:241‐252. doi: 10.1016/j.jchf.2016.11.015 [DOI] [PubMed] [Google Scholar]
- 189. Aroor AR, Mummidi S, Lopez‐Alvarenga JC, Das N, Habibi J, Jia G, et al. Sacubitril/valsartan inhibits obesity‐associated diastolic dysfunction through suppression of ventricular‐vascular stiffness. Cardiovasc Diabetol 2021;20:80. doi: 10.1186/s12933-021-01270-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Ledwidge M, Dodd JD, Ryan F, Sweeney C, McDonald K, Fox R, et al. Effect of sacubitril/valsartan vs valsartan on left atrial volume in patients with pre‐heart failure with preserved ejection fraction: The PARABLE randomized clinical trial. JAMA Cardiol 2023;8:366‐375. doi: 10.1001/jamacardio.2023.0065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Petrie MC, Verma S, Docherty KF, Inzucchi SE, Anand I, Belohlavek J, et al. Effect of dapagliflozin on worsening heart failure and cardiovascular death in patients with heart failure with and without diabetes. JAMA 2020;323:1353‐1368. doi: 10.1001/jama.2020.1906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Bohm M, et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med 2021;385:1451‐1461. doi: 10.1056/NEJMoa2107038 [DOI] [PubMed] [Google Scholar]
- 193. Solomon SD, McMurray JJV, Claggett B, de Boer RA, DeMets D, Hernandez AF, et al. Dapagliflozin in heart failure with mildly reduced or preserved ejection fraction. N Engl J Med 2022;387:1089‐1098. doi: 10.1056/NEJMoa2206286 [DOI] [PubMed] [Google Scholar]
- 194. Packer M. Activation and inhibition of sodium‐hydrogen exchanger is a mechanism that links the pathophysiology and treatment of diabetes mellitus with that of heart failure. Circulation 2017;136:1548‐1559. doi: 10.1161/CIRCULATIONAHA.117.030418 [DOI] [PubMed] [Google Scholar]
- 195. Hallow KM, Helmlinger G, Greasley PJ, McMurray JJV, Boulton DW. Why do SGLT2 inhibitors reduce heart failure hospitalization? A differential volume regulation hypothesis. Diabetes Obes Metab 2018;20:479‐487. doi: 10.1111/dom.13126 [DOI] [PubMed] [Google Scholar]
- 196. Kolijn D, Pabel S, Tian Y, Lodi M, Herwig M, Carrizzo A, et al. Empagliflozin improves endothelial and cardiomyocyte function in human heart failure with preserved ejection fraction via reduced pro‐inflammatory‐oxidative pathways and protein kinase Gα oxidation. Cardiovasc Res 2021;117:495‐507. doi: 10.1093/cvr/cvaa123 [DOI] [PubMed] [Google Scholar]
- 197. Packer M. Leptin‐aldosterone‐neprilysin axis: Identification of its distinctive role in the pathogenesis of the three phenotypes of heart failure in people with obesity. Circulation 2018;137:1614‐1631. doi: 10.1161/CIRCULATIONAHA.117.032474 [DOI] [PubMed] [Google Scholar]
- 198. Youssef ME, Yahya G, Popoviciu MS, Cavalu S, Abd‐Eldayem MA, Saber S. Unlocking the full potential of SGLT2 inhibitors: Expanding applications beyond glycemic control. Int J Mol Sci 2023;24:6039. doi: 10.3390/ijms24076039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Ott C, Jumar A, Striepe K, Friedrich S, Karg MV, Bramlage P, et al. A randomised study of the impact of the SGLT2 inhibitor dapagliflozin on microvascular and macrovascular circulation. Cardiovasc Diabetol 2017;16:26. doi: 10.1186/s12933-017-0510-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Neal B, Perkovic V, Matthews DR. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017;377:2099. doi: 10.1056/NEJMc1712572 [DOI] [PubMed] [Google Scholar]
- 201. Adamson C, Kondo T, Jhund PS, de Boer RA, Cabrera Honorio JW, Claggett B, et al. Dapagliflozin for heart failure according to body mass index: The DELIVER trial. Eur Heart J 2022;43:4406‐4417. doi: 10.1093/eurheartj/ehac481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Yang M, Butt JH, Kondo T, Jering KS, Docherty KF, Jhund PS, et al. Dapagliflozin in patients with heart failure with mildly reduced and preserved ejection fraction treated with a mineralocorticoid receptor antagonist or sacubitril/valsartan. Eur J Heart Fail 2022;24:2307‐2319. doi: 10.1002/ejhf.2722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Cho JH. Sudden death and ventricular arrhythmias in heart failure with preserved ejection fraction. Korean Circ J 2022;52:251‐264. doi: 10.4070/kcj.2021.0420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Aune D, Schlesinger S, Norat T, Riboli E. Body mass index, abdominal fatness, and the risk of sudden cardiac death: A systematic review and dose–response meta‐analysis of prospective studies. Eur J Epidemiol 2018;33:711‐722. doi: 10.1007/s10654-017-0353-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Finocchiaro G, Papadakis M, Dhutia H, Cole D, Behr ER, Tome M, et al. Obesity and sudden cardiac death in the young: Clinical and pathological insights from a large national registry. Eur J Prev Cardiol 2018;25:395‐401. doi: 10.1177/2047487317751291 [DOI] [PubMed] [Google Scholar]
- 206. Adabag S, Huxley RR, Lopez FL, Chen LY, Sotoodehnia N, Siscovick D, et al. Obesity related risk of sudden cardiac death in the atherosclerosis risk in communities study. Heart 2015;101:215‐221. doi: 10.1136/heartjnl-2014-306238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Pietrasik G, Goldenberg I, McNitt S, Moss AJ, Zareba W. Obesity as a risk factor for sustained ventricular tachyarrhythmias in MADIT II patients. J Cardiovasc Electrophysiol 2007;18:181‐184. doi: 10.1111/j.1540-8167.2006.00680.x [DOI] [PubMed] [Google Scholar]
- 208. Liu X, Zhang D, Liu Y, Sun X, Hou Y, Wang B, et al. A J‐shaped relation of BMI and stroke: Systematic review and dose–response meta‐analysis of 4.43 million participants. Nutr Metab Cardiovasc Dis 2018;28:1092‐1099. doi: 10.1016/j.numecd.2018.07.004 [DOI] [PubMed] [Google Scholar]
- 209. Caleyachetty R, Thomas GN, Toulis KA, Mohammed N, Gokhale KM, Balachandran K, et al. Metabolically healthy obese and incident cardiovascular disease events among 3.5 million men and women. J Am Coll Cardiol 2017;70:1429‐1437. doi: 10.1016/j.jacc.2017.07.763 [DOI] [PubMed] [Google Scholar]
- 210. Schnabel RB, Yin X, Gona P, Larson MG, Beiser AS, McManus DD, et al. 50 year trends in atrial fibrillation prevalence, incidence, risk factors, and mortality in the Framingham Heart Study: A cohort study. Lancet 2015;386:154‐162. doi: 10.1016/S0140-6736(14)61774-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Bodenant M, Kuulasmaa K, Wagner A, Kee F, Palmieri L, Ferrario MM, et al. Measures of abdominal adiposity and the risk of stroke: The MOnica Risk, Genetics, Archiving and Monograph (MORGAM) study. Stroke 2011;42:2872‐2877. doi: 10.1161/STROKEAHA.111.614099 [DOI] [PubMed] [Google Scholar]
- 212. Packer M. HFpEF is the substrate for stroke in obesity and diabetes independent of atrial fibrillation. JACC Heart Fail 2020;8:35‐42. doi: 10.1016/j.jchf.2019.09.002 [DOI] [PubMed] [Google Scholar]
- 213. Oluleye OW, Rector TS, Win S, McMurray JJ, Zile MR, Komajda M, et al. History of atrial fibrillation as a risk factor in patients with heart failure and preserved ejection fraction. Circ Heart Fail 2014;7:960‐966. doi: 10.1161/CIRCHEARTFAILURE.114.001523 [DOI] [PubMed] [Google Scholar]
- 214. Wolfes J, Ellermann C, Frommeyer G, Eckardt L. Evidence‐based treatment of atrial fibrillation around the globe: Comparison of the latest ESC, AHA/ACC/HRS, and CCS guidelines on the management of atrial fibrillation. Rev Cardiovasc Med 2022;23:56. doi: 10.31083/j.rcm2302056 [DOI] [PubMed] [Google Scholar]
- 215. Martin K, Beyer‐Westendorf J, Davidson BL, Huisman MV, Sandset PM, Moll S. Use of the direct oral anticoagulants in obese patients: Guidance from the SSC of the ISTH. J Thromb Haemost 2016;14:1308‐1313. doi: 10.1111/jth.13323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Covert K, Branam DL. Direct‐acting oral anticoagulant use at extremes of body weight: Literature review and recommendations. Am J Health Syst Pharm 2020;77:865‐876. doi: 10.1093/ajhp/zxaa059 [DOI] [PubMed] [Google Scholar]


