This cohort study investigates the association of sociodemographic and lifestyle factors with biological age as measured by epigenetic clocks among younger adults.
Key Points
Question
Are epigenetic clocks, measures of biological aging developed mainly on older adult samples, meaningful for younger adults, and are sociodemographic and lifestyle characteristics associated with clock measures in expected patterns found in prior aging research?
Findings
This cohort study of 4237 younger adults found that sociodemographic and lifestyle factors were associated with biological aging in clocks trained to estimate morbidity and mortality, showing accelerated aging among individuals with lower levels of education and income and those with severe obesity, no weekly exercise, and tobacco use.
Meaning
These findings suggest that age-related molecular processes may be identified in younger adults before disease manifests and may represent potential end points for interventions targeting social inequalities in heathy aging and longevity.
Abstract
Importance
Epigenetic clocks represent molecular evidence of disease risk and aging processes and have been used to identify how social and lifestyle characteristics are associated with accelerated biological aging. However, most research is based on samples of older adults who already have measurable chronic disease.
Objective
To investigate whether and how sociodemographic and lifestyle characteristics are associated with biological aging in a younger adult sample across a wide array of epigenetic clock measures.
Design, Setting, and Participants
This cohort study was conducted using data from the National Longitudinal Study of Adolescent to Adult Health, a US representative cohort of adolescents in grades 7 to 12 in 1994 followed up for 25 years to 2018 over 5 interview waves. Participants who provided blood samples at wave V (2016-2018) were analyzed, with samples tested for DNA methylation (DNAm) in 2021 to 2024. Data were analyzed from February 2023 to May 2024.
Exposure
Sociodemographic (sex, race and ethnicity, immigrant status, socioeconomic status, and geographic location) and lifestyle (obesity status by body mass index [BMI] in categories of reference range or underweight [<25], overweight [25 to <30], obesity [30 to <40], and severe obesity [≥40]; exercise level; tobacco use; and alcohol use) characteristics were assessed.
Main Outcome and Measure
Biological aging assessed from banked blood DNAm using 16 epigenetic clocks.
Results
Data were analyzed from 4237 participants (mean [SD] age, 38.4 [2.0] years; percentage [SE], 51.3% [0.01] female and 48.7% [0.01] male; percentage [SE], 2.7% [<0.01] Asian or Pacific Islander, 16.7% [0.02] Black, 8.7% [0.01] Hispanic, and 71.0% [0.03] White). Sociodemographic and lifestyle factors were more often associated with biological aging in clocks trained to estimate morbidity and mortality (eg, PhenoAge, GrimAge, and DunedinPACE) than clocks trained to estimate chronological age (eg, Horvath). For example, the β for an annual income less than $25 000 vs $100 000 or more was 1.99 years (95% CI, 0.45 to 3.52 years) for PhenoAgeAA, 1.70 years (95% CI, 0.68 to 2.72 years) for GrimAgeAA, 0.33 SD (95% CI, 0.17 to 0.48 SD) for DunedinPACE, and −0.17 years (95% CI, −1.08 to 0.74 years) for Horvath1AA. Lower education, lower income, higher obesity levels, no exercise, and tobacco use were associated with faster biological aging across several clocks; associations with GrimAge were particularly robust (no college vs college or higher: β = 2.63 years; 95% CI, 1.67-3.58 years; lower vs higher annual income: <$25 000 vs ≥$100 000: β = 1.70 years; 95% CI, 0.68-2.72 years; severe obesity vs no obesity: β = 1.57 years; 95% CI, 0.51-2.63 years; no weekly exercise vs ≥5 bouts/week: β = 1.33 years; 95% CI, 0.67-1.99 years; current vs no smoking: β = 7.16 years; 95% CI, 6.25-8.07 years).
Conclusions and Relevance
This study found that important social and lifestyle factors were associated with biological aging in a nationally representative cohort of younger adults. These findings suggest that molecular processes underlying disease risk may be identified in adults entering midlife before disease is manifest and inform interventions aimed at reducing social inequalities in heathy aging and longevity.
Introduction
Research on aging has long documented social and demographic differentials in morbidity and mortality risk but has only recently been able to explore underlying molecular and cellular changes that accompany aging processes and shorten life. New developments in geroscience have identified biological hallmarks of aging,1,2,3 about which studies now collect data to examine social determinants of biological aging.4,5,6,7,8 In particular, epigenomic profiling has greatly increased the availability of novel indicators of biological aging in the form of epigenetic clocks,9,10,11 composite measures of DNA methylation (DNAm) that represent molecular evidence of disease risk and aging processes.12,13 Epigenetic clocks estimate epigenetic age, and the relative comparison of epigenetic age with chronological age represents epigenetic age acceleration (hereafter, biological aging), providing a measurement of differences in biological aging among individuals of the same calendar age.14 Epigenetic clock measures have been associated with many age-related diseases and mortality.15,16,17,18,19,20,21,22,23,24,25,26 Thus, they potentially represent useful measures for interventions intended to reduce social inequalities in healthy aging and longevity,26 particularly if the clocks can detect biological aging in young individuals without apparent disease.
Considerable progress has been made in measuring biological age using numerous sources of data to create epigenetic clocks.7,27,28 First-generation clocks were constructed as molecular estimators of chronological age.29,30,31 However, attention soon shifted to estimate aging outcomes beyond chronological age. Thus, second-generation epigenetic clocks were calibrated on differences in biological aging reflected by disease and mortality risks.20,32 Recent advances exploit longitudinal measurement of physical and cognitive function and disease risk over time to construct clocks that capture the pace of change in biological aging over time,33,34 representing a third generation of epigenetic clocks.
There is an increasing body of research on social and lifestyle factors associated with measured biological aging,7,28 providing insights for differential exposures that are associated with aging.35,36,37,38,39 To the extent that certain social and lifestyle factors are known to be associated with increased age-related health risks, we would expect these factors to be associated with more rapid biological aging, affirming the research value of epigenetic clocks as markers of aging, particularly in younger adults. Indeed, lower socioeconomic status in education, income, and wealth has been associated with more rapid epigenetic aging.8,40 Variability in biological aging by sex generally shows that males experience more rapid epigenetic aging,28,39,41 whereas variability by race and ethnicity is inconsistent, with positive, negative, and null differences observed in previous research comparing Black or Hispanic with White individuals.5,9 Among lifestyle factors, existing research shows strong and consistently positive associations of tobacco use and body mass index (BMI; calculated as weight in kilograms divided by height in meters squared) with biological aging in second- and third-generation clocks but more mixed results for alcohol use and exercise.7,8,9,28 Overall, results suggest that more recent, second- and third-generation clocks are more sensitive to social and environmental exposures, although more work is needed to better understand whether and how clocks capture shared or distinct aspects of aging.
Existing research examining sociodemographic factors and biological aging has notable gaps. First, many studies use a single or a few clocks, making it difficult to ascertain whether results are consistent across clocks. Second, most epigenetic research relies on small, often local, and nondiverse samples, limiting generalizability.7,13,14,40 Some recent studies have used large representative samples that examined a range of clocks but focused on older adults.10,41 In fact, most research on epigenetic aging has been based on samples of older adults10,14,15,20,22,23,24,38,42,43,44,45 (exceptions include studies by Aanes et al46 and Raffington et al47). Given that older adults are more likely to have chronic comorbidities, it is difficult to disentangle outcomes associated with underlying disease from those associated with sociodemographic exposures. Furthermore, as age increases, biological age may become a less reliable estimator of health outcomes due to mortality selection and increased biological heterogeneity in older age.
Our research addresses existing gaps by investigating the association of sociodemographic and lifestyle factors with biological age according to 16 DNAm measures in a diverse population of adults aged 33 to 44 years from the US representative National Longitudinal Study of Adolescent to Adult Health (Add Health). Using new methylation data with national representation of racial and ethnic, socioeconomic, and geographic groups, we contribute to the limited research on epigenetic aging in younger adults. To our knowledge, our study is one of few to examine the emergence of sociodemographic inequalities in aging before adults enter midlife across established epigenetic clock measures.
Methods
Data in this cohort study come from Add Health, a nationally representative cohort study of US adolescents in grades 7 to 12 in 1994 who were followed up for 25 years across 5 interview waves.44 We use data from wave I (WI; 1994-1995) and wave V (WV; 2016-18), when the cohort was aged 33 to 44 years. During the WV survey, 5381 of 12 300 participants consented to and completed a follow-up in-person home exam, when venous blood was drawn (93.1% consent rate) for DNAm assay. After removal of samples that did not pass quality control and elimination of replicates, the DNAm sample included 4582 participants. The sample size was further reduced to 4237 participants due to missing values on sociodemographic factors. Population representation was maintained across waves and samples (eTable 1 in Supplement 1).
Methylation analysis was conducted using the Illumina Infinium chemistry.48 DNAm levels across approximately 850 000 CpG sites were measured using the Infinium Methylation EPIC BeadChip (Illumina, Inc). We measured β values for CpG sites across the genome according to kit protocols and filtered to remove polymorphic positions. Remaining CpG sites were restricted to a set of 30 484 CpG sites used with DNA methylation calculator49 and Methylcipher50 and Dunedin33,34 calculators; principal component (PC) clocks were based on 78 464 CpG sites and used code publicly available on GitHub (eAppendix 1 in Supplement 1).
Adolescent participants and their parents or caregivers provided written consent at WI; young adult participants provided consent at WV for the survey administration and epigenetic data collection. Consent obtained at WV extends to the current study. This study was approved by the institutional review board of the University of North Carolina at Chapel Hill. We followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Epigenetic Clocks
We constructed 16 epigenetic clocks when the mean (SD) cohort age was 38.4 (2.0) years at the time of venous blood draw (Table 1; eAppendix 2 in Supplement 1). Clocks are shown in order of their generation, beginning with 5 first-generation clocks: Horvath1, Horvath2, Hannum, VidalBralo, and Zhang2019. The 2 Horvath clocks were constructed across multiple tissues as a pantissue clock of chronological age and differ by the number of CpG sites on which the algorithm was based.30,31 The Hannum29 and VidalBralo45 clocks were trained on blood samples, and Zhang201951 was trained on blood and saliva samples to estimate chronological age. We examined 3 second-generation clocks trained on disease phenotypes and mortality in estimation of epigenetic age, including Lin,43 PhenoAge,20 and GrimAge,21 which also incorporated smoking-associated methylation changes.
Table 1. Descriptive Statistics for Epigenetic Clocks and Pearson Correlation With Age (N = 4237)a.
| Type of measure | Weighted mean (SD) [range]a | Pearson r | Pearson probability |
|---|---|---|---|
| Chronological age, y | 38.4 (2.0) [33.1 to 44.8] | >.99 | <0.01 |
| Clock-measured epigenetic age, y | |||
| Horvath1 | 39.1 (4.3) [15.5 to 62.3] | 0.42 | <0.01 |
| Horvath2 | 35.4 (3.5) [17.7 to 57.4] | 0.58 | <0.01 |
| Hannum | 31.6 (3.9) [13.2 to 47.5] | 0.40 | <0.01 |
| PhenoAge | 30.1 (5.7) [12.8 to 50.9] | 0.32 | <0.01 |
| GrimAge | 52.5 (4.6) [39.1 to 71.9] | 0.39 | <0.01 |
| Lin | 23.1 (5.2) [4.0 to 44.0] | 0.32 | <0.01 |
| VidalBralo | 54.9 (3.5) [38.2 to 77.6] | 0.27 | <0.01 |
| Zhang2019 | 32.0 (4.1) [19.9 to 58.3] | 0.62 | <0.01 |
| PC clock–measured epigenetic age, y | |||
| PCHorvath1 | 45.3 (3.7) [31.6 to 66.0] | 0.36 | <0.01 |
| PCHorvath2 | 41.2 (4.1) [26.6 to 62.5] | 0.32 | <0.01 |
| PCHannum | 46.1 (3.8) [31.5 to 64.8] | 0.38 | <0.01 |
| PCPhenoAge | 41.0 (5.3) [21.7 to 66.4] | 0.34 | <0.01 |
| PCGrimAge | 54.6 (3.9) [43.6 to 71.3] | 0.43 | <0.01 |
| Clock-measured age acceleration, y | |||
| Horvath1AA | 0.1 (5.5) [−25.1 to 26.0] | <0.01 | 0.78 |
| Horvath2AA | <0.1 (3.9) [−15.9 to 21.8] | −0.01 | 0.52 |
| HannumAA | 0.1 (5.0) [−25.5 to 25.0] | <0.01 | 0.93 |
| PhenoAgeAA | 0.1 (7.8) [−32.4 to 29.9] | 0.01 | 0.66 |
| GrimAgeAA | 0.3 (6.2) [−15.7 to 26.6] | 0.01 | 0.54 |
| LinAA | 0.1 (7.2) [−30.1 to 31.8] | −0.01 | 0.65 |
| VidalBraloAA | −0.1 (4.8) [−23.0 to 24.0] | <0.01 | 0.94 |
| Zhang2019AA | <0.1 (4.4) [−18.2 to 26.2] | −0.02 | 0.28 |
| PC clock–measured age acceleration, y | |||
| PCHorvath1AA | 0.1 (5.0) [−17.9 to 31.8] | <0.01 | 0.97 |
| PCHorvath2AA | <0.1 (5.5) [−20.7 to 26.9] | <0.01 | 0.90 |
| PCHannumAA | 0.1 (5.0) [−24.4 to 29.8] | −0.01 | 0.67 |
| PCPhenoAgeAA | 0.1 (7.2) [−29.1 to 32.8] | <0.01 | 0.89 |
| PCGrimAgeAA | 0.3 (5.2) [−14.5 to 25.4] | <0.01 | 0.78 |
| Clock-measured rate of aging, SD | |||
| Dunedin PoAm | <0.1 (1.0) [−3.9 to 4.9] | −0.01 | 0.52 |
| Dunedin PACE | <0.1 (1.0) [−4.5 to 4.6] | 0.08 | <0.01 |
| Zhang2017-measured risk of mortality, RR | −1.3 (0.4) [−2.6 to 0.4] | 0.17 | <0.01 |
Abbreviations: PC, principal component; RR risk ratio.
Chronological age was assessed at the wave V blood draw. Clock measures are in units of years of epigenetic (ie, biological) age. Age acceleration measures are in units of years that one’s epigenetic age was higher (positive number) or lower (negative number) compared with the epigenetic age of others with the same chronological age. Rate of aging measures are in SD units representing a faster (positive) or a slower (negative) rate of epigenetic aging (ie, biological aging) relative to the rate of chronological age over time. Risk of mortality is in units of mortality RR, with negative values indicating a lower risk and positive values indicating a higher risk.
To reduce technical variation in CpG β values on which epigenetic clocks were based, Higgins-Chen et al52 retrained Hannum, Horvath1, Horvath2, GrimAge, and PhenoAge clocks on PCs of CpG methylation values rather than individual CpGs, with the goal of reducing the effects of technical noise at any given individual CpG. We refer to these as PC clocks (Table 1). For each of these 13 first- and second-generation clocks, we calculated biological age by taking residuals of the clock values regressed on chronological age.
We refer to the final set of 3 clocks as third generation, which use a different unit of measurement: DunedinPoAm, Dunedin PACE, and Zhang2017. DunedinPoAm33 and Dunedin PACE34 estimate the pace of biological aging in SD units based on changes in biomarkers of organ system dysfunction, and Zhang2017 estimates a continuous risk score of all-cause mortality.53
Sociodemographic and Lifestyle Characteristics
Age was a continuous measure of chronological age at WV blood draw. Participants reported sex assigned at birth at WI (female or male). We assessed differences in epigenetic aging by race and ethnicity because prior evidence reported mixed results.5,9 Race or ethnicity were self-identified at WV based on 1 question that asked participants, “What is your race or ethnic origin?” Response categories included American Indian or Alaska Native, Asian, Black or African American, Hispanic, Pacific Islander, White, and some other race or origin. For participants with missing data at WV, we used WI self-reports of race and ethnicity. Small sample sizes required us to combine Pacific Islander with the Asian category and American Indian or Alaska Native with the other category, although we show the full distribution in eTable 1 in Supplement 1. We measured immigrant generation at WI (first, second, or ≥third) and education (≥college, some college, or no college), annual household income (>$100 000, $50 000-$100 000, $25 000-$50 000, and <$25 000), region of residence (Northeast, West, Midwest, and South), and rural or urban residence (metropolitan, micropolitan, and small town or rural) at WV. Lifestyle measures at WV included obesity status by BMI (reference range or underweight [<25], overweight [25 to <30], obesity [30 to <40], and severe obesity [≥40]), bouts of moderate to rigorous exercise per week (≥5, 1-4, and 0), tobacco use (never, former, and current), and alcohol use (none, light [<daily and no binge drinking], and heavy [daily] or binge). See eAppendix 3 in Supplement 1 for details on variable construction.
Statistical Analysis
We conducted descriptive statistical analysis on the 16 clocks and sample characteristics and examined correlations among epigenetic clocks, chronological age, and measures of accelerated and pace of aging. We performed weighted linear regression of accelerated biological aging on sociodemographic and lifestyle factors in bivariate and multivariable models adjusted for chronological age54 with and without controls for cell composition (see eMethods in Supplement 1 for model assumptions and eTable 2 in Supplement 1 for distributional attributes of clock measures). We used sampling weights in all analyses to produce national estimates44,55,56 and the R statistical library Survey.57,58 Data were analyzed using R statistical software version 4.2.1 (R Project for Statistical Computing) from February 2023 to May 2024. We conducted 2-sided tests of significance for P values ranging from <.001 to <.05.
Results
We analyzed 4237 participants (mean [SD] age 38.4 [2.0] years; percentage [SE], 51.3% [0.01] female and 48.7% [0.01] male; percentage [SE], 2.7% [<0.01] Asian or Pacific Islander, 16.7% [0.02] Black, 8.7% [.01] Hispanic, and 71.0% [0.03] White). Sociodemographic and lifestyle characteristics of the sample (Table 2) reflect national representation of sex, racial and ethnic groups, and immigrant status (percentage [SE], 4.4% [0.02] first-generation and 10.0% [.01] second-generation immigrants). Most participants had at least some college education (percentage [SE], 42.7% [0.02] ≥college and 40.0% [0.01] some college), and one-third had annual household incomes greater than $100 000 (percentage [SE], 32.5% [0.02]). Reflecting current health trends, the percentage (SE) of participants with obesity (32.7% [.01]) and severe obesity (9.9% [<.01]) was high, while the percentage (SE) was 22.6% (0.01) for getting no moderate to rigorous exercise per week, 25.9% (0.01) for current smokers, and 46.8% (0.01) for heavy or binge drinking.
Table 2. Participant Characteristics.
| Characteristic | Participants, unweighted No. (weighted % [SE]) (N = 4237) |
|---|---|
| Sex | |
| Female | 2569 (51.30 [0.01]) |
| Male | 1668 (48.70 [0.01]) |
| Race or ethnicity | |
| Asian or Pacific Islander | 218 (2.70 [0.006]) |
| Black | 811 (16.70 [0.023]) |
| Hispanic | 435 (8.70 [0.014]) |
| White | 2746 (71.00 [0.029]) |
| Othera | 27 (0.80 [0.002]) |
| Immigrant generation | |
| First | 200 (4.40 [0.008]) |
| Second | 551 (10.00 [0.01]) |
| ≥Third | 3486 (85.70 [0.016]) |
| Education | |
| ≥College | 2005 (42.70 [0.02]) |
| Some college | 1622 (40.00 [0.014]) |
| No college | 610 (17.30 [0.014]) |
| Annual income, $ | |
| >100 000 | 1491 (32.50 [0.015]) |
| 50 000-100 000 | 1403 (33.60 [0.012]) |
| 25 000-50 000 | 743 (18.40 [0.009]) |
| <25 000 | 600 (15.50 [0.012]) |
| Region of residence | |
| Northeast | 438 (9.60 [0.01]) |
| West | 991 (17.70 [0.017]) |
| Midwest | 1162 (31.20 [0.027]) |
| South | 1646 (41.40 [0.021]) |
| Rural or urban residence | |
| Metropolitan | 3711 (85.90 [0.017]) |
| Micropolitan | 286 (7.90 [0.013]) |
| Small town or rural | 240 (6.10 [0.011]) |
| Obesityb | |
| Reference range or underweight | 1173 (26.00 [0.012]) |
| Overweight | 1304 (31.50 [0.011]) |
| Obesity | 1334 (32.70 [0.011]) |
| Severe obesity | 426 (9.90 [0.007]) |
| Moderate to rigorous exercise, bouts/wk | |
| ≥5 | 1612 (38.10 [0.012]) |
| 1-4 | 1656 (39.30 [0.01]) |
| 0 | 969 (22.60 [0.013]) |
| Tobacco | |
| Never | 2429 (52.30 [0.016]) |
| Former | 817 (21.90.[0.012]) |
| Current | 991 (25.90 [0.013]) |
| Alcoholc | |
| None | 231 (6.08 [0.007]) |
| Light | 2035 (47.11 [0.012]) |
| Heavy or binge | 1971 (46.81 [0.013]) |
The other category for race or ethnicity includes participants who identified as American Indian or Alaska Native or who checked some other race or origin.
Body mass index (BMI; calculated as weight in kilograms divided by height in meters squared) ranges for obesity categories are reference range or underweight (<25), overweight (25 to <30), obesity (30 to <40), and severe obesity (≥40).
Alcohol use is categorized as none if participants indicated they never drank alcohol, light if participants reported drinking alcohol less than daily and did not binge drink, and heavy or binge if participants reported engaging in binge drinking in the last year or drinking daily in the last month or last year.
The weighted mean and range of epigenetic ages for the various clocks (Table 1) showed considerable variation, from a mean (SD) of 23.1 (5.2) years for Lin to 54.9 (3.5) for VidalBralo for the cohort. Estimates of epigenetic age were positively correlated with chronological age. In general, first-generation clocks had higher Pearson r values for correlation with age except for VidalBralo (0.27), ranging from 0.40 for Hannum to 0.62 for Zhang2019. Second-generation clocks and PC clocks had moderate r values for correlations with chronological age, ranging from 0.32 for PhenoAge, Lin, and PCHorvath 2 to 0.43 for PCGrimAge. While DunedinPoAm was not correlated with age, DunedinPACE (Pearson r = 0.08) and the Zhang2017 (Pearson r = 0.17) risk of mortality measure were correlated with age.
All clocks were positively correlated with each other (Figure 1A), with the higher Pearson r values for correlation between first-generation clocks (except for VidalBralo) and especially among PC versions of these clocks. Among second-generation clocks, PhenoAge had a more consistent positive correlation with other clocks. Pearson r values for correlations among age acceleration measures were somewhat lower and less likely to reach statistical significance (Figure 1B). In general, Pearson r values for correlations between age acceleration measures were higher within generations of clocks compared with across generations, with the exception of PhenoAgeAA.
Figure 1. Correlations Between Epigenetic Clocks and Epigenetic Age Acceleration.
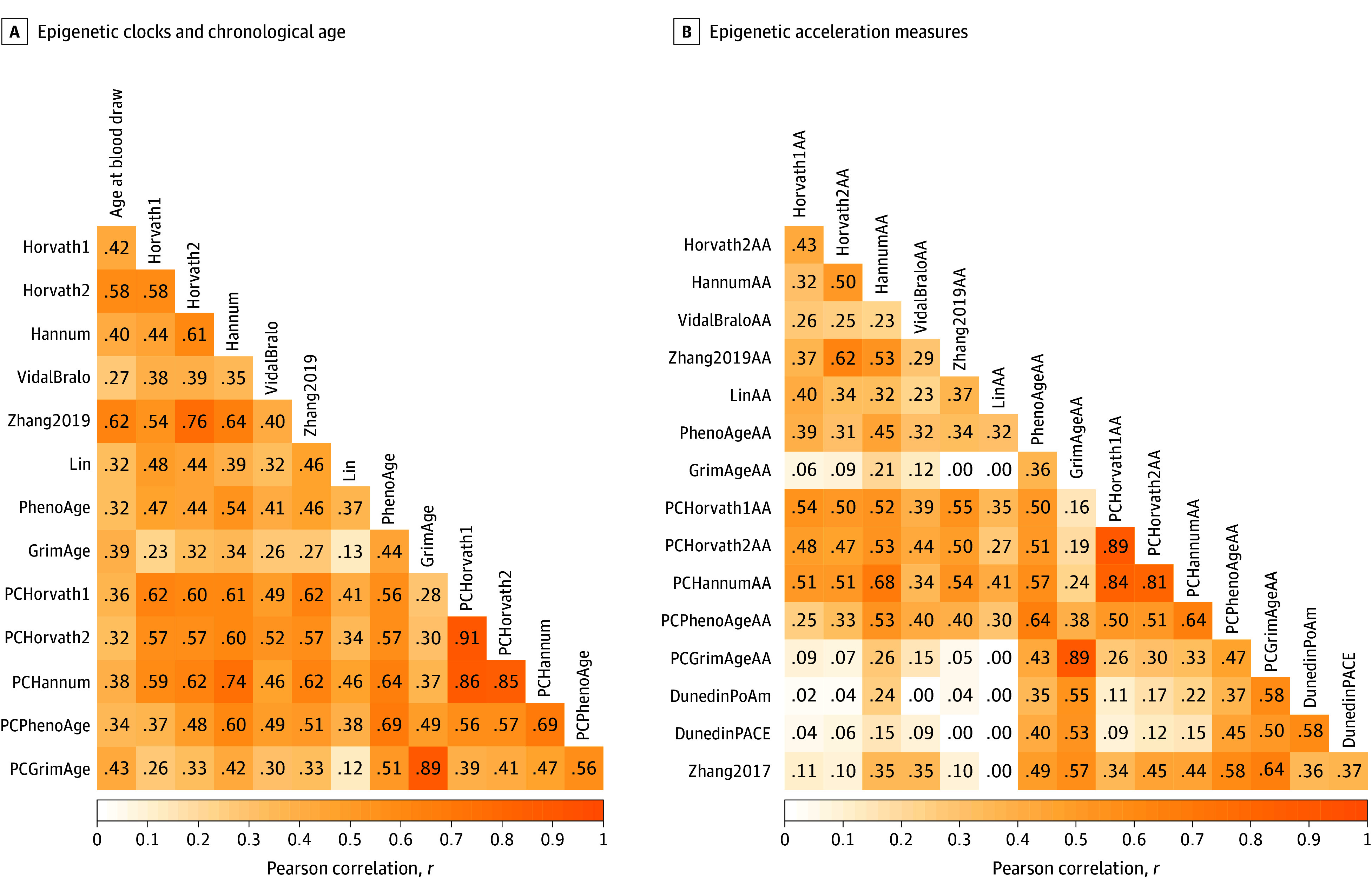
Correlations between epigenetic clocks and epigenetic age acceleration measures are depicted as a heat map. A, Pearson correlations among all epigenetic clocks for chronological age are presented. B, Pearson correlations among all epigenetic age acceleration measures, including third-generation rates of aging, are presented. Darker hues indicated a higher Pearson r correlation value.
We tested bivariate associations of sociodemographic and lifestyle factors and with biological aging in which β values represent the increase (positive coefficient) or decrease (negative coefficient) in years of biological age associated with each sociodemographic category compared with the referent category. In this analysis, β values were higher among second-generation clocks PhenoAgeAA and GrimAgeAA and third-generation DunedinPACE than first-generation Horvath1AA; for example, the β for no college education vs college or more was 3.12 years (95% CI, 1.98-4.26 years) for PhenoAgeAA, 6.55 years (95% CI, 5.44-7.66 years) for GrimAgeAA, 0.90 SD (95% CI, 0.79-1.02 SD) for DunedinPACE, and 0.80 years (95% CI, −.09 to 1.69 years) for Horvath1AA (Figure 2; eTable 3 in Supplement 2). Among remaining clocks (eFigure in Supplement 1 and eTable 3 in Supplement 2), LinAA had the fewest associations among second-generation clocks, and social and lifestyle factors had high β values for associations with third-generation clocks DunedinPoAm and Zhang2017.
Figure 2. Bivariate Associations Between Sociodemographics and Epigenetic Age in Selected Clocks.

Weighted bivariate associations between sociodemographic characteristics and epigenetic age acceleration for selected first-, second-, and third-generation clocks are presented in a forest plot. Error bars indicate 95% CIs. Age acceleration units are expressed in years for Horvath1AA, PhenoAgeAA, and GrimAgeAA, while DunedinPACE is measured as a rate of biological aging in SD. Horvath1AA is a first-generation clock, while PhenoAgeAA and GrimAgeAA are second-generation clocks and DunedinPACE is a third-generation clock. The other category for race or ethnicity includes participants who identified as American Indian or Alaska Native or who checked some other race or origin. Alcohol use categories were none, light drinking (<daily and no binge drinking), and heavy (daily drinking) or binge drinking.
Overall, males had more rapid biological aging than females across most clocks. Consistent with the literature, there were mixed results for race and ethnicity; 7 first-generation clocks showed slower biological aging and 6 second- and third-generation clocks showed faster epigenetic aging among Black adults compared with White adults. More consistent results were found for Asian and Pacific Islander adults, with slower biological aging in 10 clocks, and among Hispanic adults, with slower biological aging in 5 clocks compared with White adults. Foreign-born, first-generation immigrants also had slower biological aging compared with US-born adults (eTable 3 in Supplement 2).
For the most part, second- and third-generation clocks showed expected associations, in which lower levels of education and income were associated with accelerated biological aging. A few clocks showed associations between geographic location and accelerated biological aging in the South and Midwest compared with the Northeast and in more rural locales compared with urban areas (Figure 2; eFigure 1 in Supplement 1 and eTable 3 in Supplement 2).
The lifestyle factor with the most consistent bivariate association with biological aging and highest β values was obesity status. Across 15 clocks, individuals with severe obesity (eg, BMI ≥40) experienced faster biological aging compared with those with reference range or underweight BMI status (β values ranged from 1.02 years; 95% CI, 0.22-1.83 years for Horvath2AA to 6.06 years; 95% CI, 4.42-7.69 years for PhenoAgeAA) (eTable 3 in Supplement 2); 9 clocks found faster biological aging among individuals with obesity compared with those with reference range or underweight status. Weekly exercise was also consistently and frequently associated with biological aging, showing that individuals who got less exercise had accelerated biological aging for 9 clocks. Alcohol and tobacco use results were mixed, with some clocks estimating slower aging and some faster aging with greater use. However, among second-generation clocks (except for Lin), current or former smokers had accelerated biological aging (Figure 2; eFigure 1 in Supplement 1 and eTable 3 in Supplement 2).
We tested for independent associations in a multivariable model adjusting for all sociodemographic and lifestyle characteristics (Table 3; eTable 4 in Supplement 2). Associations with the highest β values were found for second- and third-generation measures of biological aging. For example, the β for an annual income less than $25 000 vs $100 000 or more was 1.99 years (95% CI, 0.45 to 3.52 years) for PhenoAgeAA, 1.70 years (95% CI, 0.68 to 2.72 years) for GrimAgeAA, 0.33 SD (95% CI, 0.17 to 0.48 SD) for DunedinPACE, and −0.17 years (95% CI, −1.08 to 0.74 years) for Horvath1AA (Table 3). Focusing on these clocks and highlighting GrimAgeAA as an example, consistent independent associations with faster biological aging were also found for the following characteristics: lower levels of education (no college: β = 2.63 years; 95% CI, 1.67 to 3.58 years; some college: β = 0.93 years; 95% CI, 0.45 to 1.40 years) compared with college or higher, severe obesity status (β = 1.57 years; 95% CI, 0.51 to 2.63 years) compared with reference range or underweight status, lack of exercise (no bouts/wk: β = 1.33 years; 95% CI, 0.67 to 1.99 years) compared with 5 or more bouts per week, and tobacco use (current smoker: β = 7.16 years; 95% CI, 6.25 to 8.07 years) compared with never a smoker, which was a particularly high β value given that the GrimAge algorithm includes smoking pack-years. Most clocks (except PCPhenoAgeAA and DunedinPACE) showed males with faster biological aging than females (eg, GrimAgeAA: β = 1.78 years; 95% CI, 1.26 to 2.30 years). Adjusted results for race and ethnicity remained mixed as Black, Asian or Pacific Islander, and Hispanic adults had slower rates of biological aging compared with White adults across most clocks. The slower biological aging among foreign-born immigrants was independently significant only for GrimAge clocks, while HannumAA showed slower aging among US-born individuals in the third generation. Models that controlled for cell composition (eTable 5 in Supplement 2) showed similar results.
Table 3. Multivariate Models of Social and Demographic Characteristics and Selected Clock Estimates (N = 4237).
| Variable | Horvath1AA | PhenoAgeAA | GrimAgeAA | DunedinPACE |
|---|---|---|---|---|
| β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) | |
| Age per 1 y | −0.09 (−0.25 to 0.06) | 0.01 (−0.20 to 0.22) | −0.1 (−0.22 to 0.03) | 0.02 (0.00 to 0.04) |
| Sex | ||||
| Female | [Reference] | [Reference] | [Reference] | [Reference] |
| Male | 2.40 (1.80 to 2.99) | −0.47 (−1.27 to 0.34) | 1.78 (1.26 to 2.30) | −0.23 (−0.29 to −0.16) |
| Race or ethnicity | ||||
| Asian or Pacific Islander | −0.18 (−2.06 to 1.71) | −3.09 (−5.90 to −0.29) | −0.98 (−2.65 to 0.68) | 0.14 (-0.13 to 0.41) |
| Black | −0.36 (−1.17 to 0.45) | −1.93 (−3.19 to −0.68) | 1.16 (0.51-1.82) | 0.33 (0.22 to 0.44) |
| Hispanic | −0.99 (−2.51 to 0.52) | −0.84 (−2.72 to 1.04) | −1.45 (−2.43 to −0.47) | 0.20 (0.04 to 0.36) |
| White | [Reference] | [Reference] | [Reference] | [Reference] |
| Othera | 0.58 (−1.68 to 2.84) | 1.31 (−2.22 to 4.83) | 0.10 (−1.94 to 2.14) | 0.60 (0.35 to 0.86) |
| Immigrant generation | ||||
| First | [Reference] | [Reference] | [Reference] | [Reference] |
| Second | 0.35 (−1.88 to 2.57) | −0.03 (−2.73 to 2.66) | 1.66 (0.36 to 2.96) | −0.07 (−0.29 to 0.15) |
| ≥Third | 0.38 (−2.01 to 2.77) | −0.84 (−3.78 to 2.11) | 1.72 (0.38 to 3.07) | −0.06 (−0.28 to 0.16) |
| Education | ||||
| ≥College | [Reference] | [Reference] | [Reference] | [Reference] |
| Some college | −0.12 (−0.76 to 0.51) | −0.05 (−0.77 to 0.67) | 0.93 (0.45 to 1.40) | 0.11 (0.02 to 0.19) |
| No college | 0.44 (−0.50 to 1.39) | 0.83 (−0.47 to 2.13) | 2.63 (1.67 to 3.58) | 0.30 (0.18 to 0.42) |
| Annual income, $ | ||||
| >100 000 | [Reference] | [Reference] | [Reference] | [Reference] |
| 50 00-100 000 | −0.78 (−1.40 to −0.15) | 0.60 (−0.21 to 1.41) | 0.28 (−0.30 to 0.86) | 0.07 (−0.01 to 0.15) |
| 25 000-50 000 | −0.21 (−1.07 to 0.65) | 1.13 (−0.16 to 2.41) | 1.14 (0.18 to 2.10) | 0.17 (0.06 to 0.28) |
| <25 000 | −0.17 (−1.08 to 0.74) | 1.99 (0.45 to 3.52) | 1.70 (0.68 to 2.72) | 0.33 (0.17 to 0.48) |
| Region | ||||
| Northeast | [Reference] | [Reference] | [Reference] | [Reference] |
| West | 0.63 (−0.27 to 1.53) | 0.90 (−0.34 to 2.14) | 0.22 (−0.56 to 0.99) | 0.06 (−0.04 to 0.16) |
| Midwest | 0.80 (−0.07 to 1.68) | 2.08 (0.88 to 3.29) | 0.95 (0.23 to 1.67) | 0.13 (0.05 to 0.22) |
| South | 0.18 (−0.66 to 1.02) | 0.66 (−0.48 to 1.79) | 0 (−0.69 to 0.70) | 0.10 (0.01 to 0.19) |
| Rural or urban | ||||
| Metropolitan | [Reference] | [Reference] | [Reference] | [Reference] |
| Micropolitan | 0.37 (−0.67 to 1.41) | 0.29 (−1.05 to 1.62) | 0.46 (−0.43 to 1.36) | 0.03 (−0.07 to 0.13) |
| Town or rural | −1.07 (−1.97 to −0.17) | −0.59 (−2.08 to 0.91) | 0.26 (−0.60 to 1.12) | 0.07 (−0.09 to 0.23) |
| Obesityb | ||||
| Reference range | [Reference] | [Reference] | [Reference] | [Reference] |
| Overweight | −0.08 (−0.85 to 0.70) | 0.81 (−0.13 to 1.75) | 0.11 (-0.53 to 0.75) | 0.21 (0.12 to 0.30) |
| Obesity | 0.65 (−0.11 to 1.42) | 2.13 (0.98 to 3.28) | 0.05 (−1.01 to 1.10) | 0.58 (0.48 to 0.68) |
| Severe obesity | 2.93 (1.69 to 4.18) | 5.24 (3.55 to 6.92) | 1.57 (0.51 to 2.63) | 1.16 (1.04 to 1.28) |
| Exercise bouts/wk | ||||
| ≥5 | [Reference] | [Reference] | [Reference] | [Reference] |
| 1-4 | −0.05 (−0.66 to 0.56) | 0.55 (-0.22 to 1.31) | 0.52 (0.03 to 1.02) | 0.11 (0.03 to 0.18) |
| 0 | 0.29 (−0.35 to 0.92) | 1.84 (0.65 to 3.04) | 1.33 (0.67 to 1.99) | 0.28 (0.18 to 0.38) |
| Tobacco | ||||
| Never | [Reference] | [Reference] | [Reference] | [Reference] |
| Former | −0.46 (−1.27 to 0.35) | −0.15 (−0.92 to 0.61) | 1.57 (1.13 to 2.01) | 0.13 (0.05 to 0.20) |
| Current | −0.54 (−1.33 to 0.25) | 1.38 (0.32 to 2.44) | 7.16 (6.25 to 8.07) | 0.66 (0.55 to 0.77) |
| Alcoholc | ||||
| None | [Reference] | [Reference] | [Reference] | [Reference] |
| Light | 0.61 (−1.46 to 2.68) | −0.81 (−3.43 to 1.82) | −0.70 (−2.01 to 0.60) | −0.12 (−0.32 to 0.09) |
| Heavy or binge | 0.51 (−1.47 to 2.50) | −0.79 (−3.34 to 1.76) | −0.80 (−2.12 to 0.52) | −0.19 (−0.40 to 0.02) |
The other category for race or ethnicity includes participants who identified as American Indian or Alaska Native or who checked some other race or origin.
Body mass index (BMI, calculated as weight in kilograms divided by height in meters squared) ranges for the obesity categories are: reference range or underweight, BMI<25; overweight, 25 ≤ BMI < 30; obesity, 30 ≤ BMI < 40; severe obesity, BMI ≥ 40.
Alcohol use is categorized as: “none” if participants indicated they never drank alcohol; “light” if participants reported drinking alcohol less than daily and did not binge drink; and “heavy or binge” if participants reported engaging in binge drinking in the last year or drinking daily in the last month or last year.
Discussion
Based on the current literature, this cohort study estimated 16 epigenetic clocks primarily developed for older cohorts to assess their distribution, correlations with chronological age and with each other, and variability across sociodemographic and lifestyle characteristics known to estimate morbidity and mortality in prior research using the younger adult Add Health cohort. While it makes sense that most epigenetic research focuses on older adults, there is increasing recognition that molecular processes underlying disease risk begin long before overt disease is evident in chronologically older adults.59,60
We found that clock measures displayed a range of estimated epigenetic ages for younger adults that had moderate Pearson r values for correlation with chronological age and with each other. This result is consistent with prior epigenetic clock research on older populations.9,11 These wide-ranging results suggest that different clocks may reflect distinct aspects of aging given that they are based on the assessment of methylation at highly disparate numbers of CpG sites, trained on different populations that vary by age and race and ethnicity, and developed in different tissues.5
Nevertheless, many social and lifestyle factors were associated with biological aging as shown by second- and third-generation clocks in the expected direction according to prior research on inequalities in health and mortality risks26,61,62 even in this sample of adults about to enter midlife. In particular, GrimAge, PCGrimAge, and DunedinPACE showed accelerated aging among individuals with no or some college education compared with those with a college degree and for those at near or below poverty-level incomes compared with those with incomes greater than $100 000. Importantly and consistent with other research, severe obesity and lack of weekly exercise were also associated with faster biological aging in second- and third-generation clocks. Given that second- and third-generation clocks were trained to estimate disease and mortality risks while first-generation clocks were trained on chronological age, our findings support conclusions that the biological aging process may be underway prior to midlife and later life and that these second- and third-generation clocks are sensitive measures of this process before age-related disease comorbidities are present. Thus, our findings suggest that epigenetic clocks may represent surrogate end points in interventions designed to address social determinants of healthy aging.
We found interesting new results for immigrant status. Despite the often stressful and discriminatory contexts in which immigrants live, those with US-born status even if they had foreign-born parents in the second generation experienced faster aging, suggesting that the immigrant advantage found in much prior research remains biologically embedded.63,64 However, this result may be driven by the large and heterogenous Hispanic population that comprised immigrant groups given that Hispanic individuals tend to have lower biological aging for second- and third-generation clocks.
Limitations
This study has several limitations. One potential limitation of our research is the small age range of adults in Add Health. This limitation could be related to inconsistent results we found for race and ethnicity and sex, although these findings were also consistent with prior research.5,9,11,35,65,66 There are differing views on whether epigenetic markers can be analyzed in pooled racial and ethnic samples, especially because many epigenetic clocks have been developed in predominantly White samples.7,13,20,67 It remains to be investigated whether there are varying responses to social and environmental exposures in different racial and ethnic groups.67 Prior research identified changes in biological aging by sex that is chronological age related (eg, females experience more rapid aging during menopause38), which should not affect our young adult sample. However, females also have variability in immunity over time, especially during and after pregnancy, and experience variation in autoimmunity and hormones.68 This variation could be related to some outcomes on which clocks are or are not trained. In addition, other social factors we did not include may explain some of the mixed sex and race and ethnicity findings.67
Conclusions
There remains great promise in research using epigenetic clocks to understand underlying molecular and cellular changes that accompany aging processes and the development of chronic disease. Future epigenetic research should prioritize representative and diverse samples, such as Add Health, to evaluate whether cellular and molecular changes vary by sex and race and ethnicity so we can improve the measurement of biological aging. This cohort study further showed that cellular change in underlying disease processes was already evident in younger adults, which may inform prevention efforts. Future research should include a broader range of ages to investigate potential moderation by age or life stage to better understand when and how social inequalities in biological aging emerge across the life course.
eAppendix 1. Data Quality and Curation of Epigenetic Data in Add Health
eAppendix 2. Description of Epigenetic Clock Measures
eAppendix 3. Sociodemographic and Lifestyle Measures
eMethods.
eFigure. Bivariate Associations Between Sociodemographic Factors and Epigenetic Age According to Clocks
eTable 1. Add Health Sample Distribution of Demographic Characteristics for Wave I, Wave V Full Sample, and Wave V Epigenetic Sample
eTable 2. Univariate Statistics for DNA Methylation Epigenetic Clocks (N = 4237)
eTable 3. Bivariate Models of Social and Demographic Characteristics and Clock Estimates (N = 4237)
eTable 4. Multivariate Models of Social and Demographic Characteristics and Clock Estimates (N = 4237)
eTable 5. Multivariate Models of Social and Demographic Characteristics and Clock Estimates With Cell Compositions (N = 4237)
Data Sharing Statement
References
- 1.Kennedy BK, Berger SL, Brunet A, et al. Geroscience: linking aging to chronic disease. Cell. 2014;159(4):709-713. doi: 10.1016/j.cell.2014.10.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. Hallmarks of aging: an expanding universe. Cell. 2023;186(2):243-278. doi: 10.1016/j.cell.2022.11.001 [DOI] [PubMed] [Google Scholar]
- 3.Sierra F, Kohanski R. Geroscience and the trans-NIH Geroscience Interest Group, GSIG. Geroscience. 2017;39(1):1-5. doi: 10.1007/s11357-016-9954-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crimmins EM. Social hallmarks of aging: suggestions for geroscience research. Ageing Res Rev. 2020;63:101136. doi: 10.1016/j.arr.2020.101136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu Z, Chen BH, Assimes TL, Ferrucci L, Horvath S, Levine ME. The role of epigenetic aging in education and racial/ethnic mortality disparities among older U.S. women. Psychoneuroendocrinology. 2019;104:18-24. doi: 10.1016/j.psyneuen.2019.01.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martin EM, Fry RC. Exposure- associated DNA methylation in human populations. Annu Rev Public Health. 2018;39:309-333. doi: 10.1146/annurev-publhealth-040617-014629 [DOI] [PubMed] [Google Scholar]
- 7.Ryan J, Wrigglesworth J, Loong J, Fransquet PD, Woods RL. A systematic review and meta-analysis of environmental, lifestyle, and health factors associated with DNA methylation age. J Gerontol A Biol Sci Med Sci. 2020;75(3):481-494. doi: 10.1093/gerona/glz099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fiorito G, McCrory C, Robinson O, et al. ; BIOS Consortium; Lifepath consortium . Socioeconomic position, lifestyle habits and biomarkers of epigenetic aging: a multi-cohort analysis. Aging (Albany NY). 2019;11(7):2045-2070. doi: 10.18632/aging.101900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crimmins EM, Thyagarajan B, Levine ME, Weir DR, Faul J. Associations of age, sex, race/ethnicity, and education with 13 epigenetic clocks in a nationally representative U.S. sample: the Health and Retirement Study. J Gerontol A Biol Sci Med Sci. 2021;76(6):1117-1123. doi: 10.1093/gerona/glab016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Non AL. Social epigenomics: are we at an impasse? Epigenomics. 2021;13(21):1747-1759. doi: 10.2217/epi-2020-0136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schmitz LL, Zhao W, Ratliff SM, et al. The socioeconomic gradient in epigenetic ageing clocks: evidence from the Multi-Ethnic Study of Atherosclerosis and the Health and Retirement Study. Epigenetics. 2022;17(6):589-611. doi: 10.1080/15592294.2021.1939479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones MJ, Goodman SJ, Kobor MS. DNA methylation and healthy human aging. Aging Cell. 2015;14(6):924-932. doi: 10.1111/acel.12349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levine ME. Assessment of epigenetic clocks as biomarkers of aging in basic and population research. J Gerontol A Biol Sci Med Sci. 2020;75(3):463-465. doi: 10.1093/gerona/glaa021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hillary RF, Stevenson AJ, McCartney DL, et al. Epigenetic measures of ageing predict the prevalence and incidence of leading causes of death and disease burden. Clin Epigenetics. 2020;12(1):115. doi: 10.1186/s13148-020-00905-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen BH, Marioni RE, Colicino E, et al. DNA methylation-based measures of biological age: meta-analysis predicting time to death. Aging (Albany NY). 2016;8(9):1844-1865. doi: 10.18632/aging.101020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Christiansen L, Lenart A, Tan Q, et al. DNA methylation age is associated with mortality in a longitudinal Danish twin study. Aging Cell. 2016;15(1):149-154. doi: 10.1111/acel.12421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fransquet PD, Wrigglesworth J, Woods RL, Ernst ME, Ryan J. The epigenetic clock as a predictor of disease and mortality risk: a systematic review and meta-analysis. Clin Epigenetics. 2019;11(1):62. doi: 10.1186/s13148-019-0656-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marioni RE, Shah S, McRae AF, et al. The epigenetic clock is correlated with physical and cognitive fitness in the Lothian Birth Cohort 1936. Int J Epidemiol. 2015;44(4):1388-1396. doi: 10.1093/ije/dyu277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levine ME, Hosgood HD, Chen B, Absher D, Assimes T, Horvath S. DNA methylation age of blood predicts future onset of lung cancer in the women’s health initiative. Aging (Albany NY). 2015;7(9):690-700. doi: 10.18632/aging.100809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levine ME, Lu AT, Quach A, et al. An epigenetic biomarker of aging for lifespan and healthspan. Aging (Albany NY). 2018;10(4):573-591. doi: 10.18632/aging.101414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu AT, Quach A, Wilson JG, et al. DNA methylation GrimAge strongly predicts lifespan and healthspan. Aging (Albany NY). 2019;11(2):303-327. doi: 10.18632/aging.101684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maddock J, Castillo-Fernandez J, Wong A, et al. DNA methylation age and physical and cognitive aging. J Gerontol A Biol Sci Med Sci. 2020;75(3):504-511. doi: 10.1093/gerona/glz246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCartney DL, Stevenson AJ, Walker RM, et al. Investigating the relationship between DNA methylation age acceleration and risk factors for Alzheimer’s disease. Alzheimers Dement (Amst). 2018;10:429-437. doi: 10.1016/j.dadm.2018.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perna L, Zhang Y, Mons U, Holleczek B, Saum KU, Brenner H. Epigenetic age acceleration predicts cancer, cardiovascular, and all-cause mortality in a German case cohort. Clin Epigenetics. 2016;8:64. doi: 10.1186/s13148-016-0228-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marioni RE, Shah S, McRae AF, et al. DNA methylation age of blood predicts all-cause mortality in later life. Genome Biol. 2015;16(1):25. doi: 10.1186/s13059-015-0584-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martin CL, Ghastine L, Lodge EK, Dhingra R, Ward-Caviness CK. Understanding health inequalities through the lens of social epigenetics. Annu Rev Public Health. 2022;43:235-254. doi: 10.1146/annurev-publhealth-052020-105613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bell CG, Lowe R, Adams PD, et al. DNA methylation aging clocks: challenges and recommendations. Genome Biol. 2019;20(1):249. doi: 10.1186/s13059-019-1824-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oblak L, van der Zaag J, Higgins-Chen AT, Levine ME, Boks MP. A systematic review of biological, social and environmental factors associated with epigenetic clock acceleration. Ageing Res Rev. 2021;69:101348. doi: 10.1016/j.arr.2021.101348 [DOI] [PubMed] [Google Scholar]
- 29.Hannum G, Guinney J, Zhao L, et al. Genome-wide methylation profiles reveal quantitative views of human aging rates. Mol Cell. 2013;49(2):359-367. doi: 10.1016/j.molcel.2012.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Horvath S. DNA methylation age of human tissues and cell types. Genome Biol. 2013;14(10):R115. doi: 10.1186/gb-2013-14-10-r115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Horvath S, Raj K. DNA methylation-based biomarkers and the epigenetic clock theory of ageing. Nat Rev Genet. 2018;19(6):371-384. doi: 10.1038/s41576-018-0004-3 [DOI] [PubMed] [Google Scholar]
- 32.Lu AT, Seeboth A, Tsai PC, et al. DNA methylation-based estimator of telomere length. Aging (Albany NY). 2019;11(16):5895-5923. doi: 10.18632/aging.102173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Belsky DW, Caspi A, Arseneault L, et al. Quantification of the pace of biological aging in humans through a blood test, the DunedinPoAm DNA methylation algorithm. Elife. 2020;9:e54870. doi: 10.7554/eLife.54870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Belsky DW, Caspi A, Corcoran DL, et al. DunedinPACE, a DNA methylation biomarker of the pace of aging. Elife. 2022;11:e73420. doi: 10.7554/eLife.73420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Horvath S, Gurven M, Levine ME, et al. An epigenetic clock analysis of race/ethnicity, sex, and coronary heart disease. Genome Biol. 2016;17(1):171. doi: 10.1186/s13059-016-1030-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li S, Nguyen TL, Wong EM, et al. Genetic and environmental causes of variation in epigenetic aging across the lifespan. Clin Epigenetics. 2020;12(1):158. doi: 10.1186/s13148-020-00950-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu Z, Chen X, Gill TM, Ma C, Crimmins EM, Levine ME. Associations of genetics, behaviors, and life course circumstances with a novel aging and healthspan measure: evidence from the Health and Retirement Study. PLoS Med. 2019;16(6):e1002827. doi: 10.1371/journal.pmed.1002827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Levine ME, Lu AT, Chen BH, et al. Menopause accelerates biological aging. Proc Natl Acad Sci U S A. 2016;113(33):9327-9332. doi: 10.1073/pnas.1604558113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McCrory C, Fiorito G, McLoughlin S, et al. Epigenetic clocks and allostatic load reveal potential sex-specific drivers of biological aging. J Gerontol A Biol Sci Med Sci. 2020;75(3):495-503. doi: 10.1093/gerona/glz241 [DOI] [PubMed] [Google Scholar]
- 40.Schmitz LL, Duque V. In utero exposure to the Great Depression is reflected in late-life epigenetic aging signatures. Proc Natl Acad Sci U S A. 2022;119(46):e2208530119. doi: 10.1073/pnas.2208530119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao M, Qin J, Yin H, et al. Distinct epigenomes in CD4+ T cells of newborns, middle-ages and centenarians. Sci Rep. 2016;6:38411. doi: 10.1038/srep38411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang S, Wu Z, Xie J, Yang Y, Wang L, Qiu H. DNA methylation exploration for ARDS: a multi-omics and multi-microarray interrelated analysis. J Transl Med. 2019;17(1):345. doi: 10.1186/s12967-019-2090-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lin Q, Weidner CI, Costa IG, et al. DNA methylation levels at individual age-associated CpG sites can be indicative for life expectancy. Aging (Albany NY). 2016;8(2):394-401. doi: 10.18632/aging.100908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harris KM, Halpern CT, Whitsel EA, et al. Cohort profile: the national longitudinal study of adolescent to adult health (add health). Int J Epidemiol. 2019;48(5):1415-1415k. doi: 10.1093/ije/dyz115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vidal-Bralo L, Lopez-Golan Y, Gonzalez A. Simplified assay for epigenetic age estimation in whole blood of adults. Front Genet. 2016;7:126. doi: 10.3389/fgene.2016.00126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aanes H, Bleka Ø, Dahlberg PS, et al. A new blood based epigenetic age predictor for adolescents and young adults. Sci Rep. 2023;13(1):2303. doi: 10.1038/s41598-023-29381-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Raffington L, Belsky DW, Kothari M, Malanchini M, Tucker-Drob EM, Harden KP. Socioeconomic disadvantage and the pace of biological aging in children. Pediatrics. 2021;147(6):e2020024406. doi: 10.1542/peds.2020-024406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pidsley R, Zotenko E, Peters TJ, et al. Critical evaluation of the Illumina MethylationEPIC BeadChip microarray for whole-genome DNA methylation profiling. Genome Biol. 2016;17(1):208. doi: 10.1186/s13059-016-1066-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Horvath S; Clock Foundation Team . DNA methylation age calculator. University of California, Los Angeles. Accessed June 25, 2024. https://dnamage.genetics.ucla.edu
- 50.Thrush KL, Higgins-Chen AT, Liu Z, Levine ME. R methylCIPHER: a methylation clock investigational package for hypothesis-driven evaluation & research. bioRxiv. Preprint posted online July 16, 2022. doi: 10.1101/2022.07.13.499978 [DOI]
- 51.Zhang Q, Vallerga CL, Walker RM, et al. Improved precision of epigenetic clock estimates across tissues and its implication for biological ageing. Genome Med. 2019;11(1):54. doi: 10.1186/s13073-019-0667-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Higgins-Chen AT, Thrush KL, Wang Y, et al. A computational solution for bolstering reliability of epigenetic clocks: implications for clinical trials and longitudinal tracking. Nat Aging. 2022;2(7):644-661. doi: 10.1038/s43587-022-00248-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang Y, Wilson R, Heiss J, et al. DNA methylation signatures in peripheral blood strongly predict all-cause mortality. Nat Commun. 2017;8:14617. doi: 10.1038/ncomms14617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Krieger N, Chen JT, Testa C, et al. Use of correct and incorrect methods of accounting for age in studies of epigenetic accelerated aging: implications and recommendations for best practices. Am J Epidemiol. 2023;192(5):800-811. doi: 10.1093/aje/kwad025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Biemer PP, Harris KM, Burke BJ, Liao D, Halpern CT. Transitioning a panel survey from in-person to predominantly web data collection: results and lessons learned. J R Stat Soc Ser A Stat Soc. 2022;185(3):798-821. doi: 10.1111/rssa.12750 [DOI] [Google Scholar]
- 56.Biemer P, Harris KM, Liao D, Dean S, Halpern C. Sampling and Mixed-Mode Survey Design. Carolina Digital Repository. Accessed June 25, 2024. https://cdr.lib.unc.edu/concern/scholarly_works/h415pf93q?locale=en
- 57.Campbell KA, Colacino JA, Park SK, Bakulski KM. Cell types in environmental epigenetic studies: biological and epidemiological frameworks. Curr Environ Health Rep. 2020;7(3):185-197. doi: 10.1007/s40572-020-00287-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lumley T. Survey analysis in R. Accessed December 22, 2023. https://r-survey.r-forge.r-project.org/survey/
- 59.Cole SW, Shanahan MJ, Gaydosh L, Harris KM. Population-based RNA profiling in Add Health finds social disparities in inflammatory and antiviral gene regulation to emerge by young adulthood. Proc Natl Acad Sci U S A. 2020;117(9):4601-4608. doi: 10.1073/pnas.1821367117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Harris KM, McDade TW. The biosocial approach to human development, behavior, and health across the life course. RSF. 2018;4(4):2-26. doi: 10.7758/RSF.2018.4.4.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gutin I, Hummer RA. Social inequality and the future of U.S. life expectancy. Annu Rev Sociol. 2021;47(1):501-520. doi: 10.1146/annurev-soc-072320-100249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Crimmins EM, Zhang YS. Aging populations, mortality, and life expectancy. Annu Rev Sociol. 2019;45:69-89. doi: 10.1146/annurev-soc-073117-041351 [DOI] [Google Scholar]
- 63.Lu Y, Denier N, Wang JSH, Kaushal N. Unhealthy assimilation or persistent health advantage: a longitudinal analysis of immigrant health in the United States. Soc Sci Med. 2017;195:105-114. doi: 10.1016/j.socscimed.2017.11.019 [DOI] [PubMed] [Google Scholar]
- 64.Riosmena F, Kuhn R, Jochem WC. Explaining the immigrant health advantage: self-selection and protection in health-related factors among five major national-origin immigrant groups in the United States. Demography. 2017;54(1):175-200. doi: 10.1007/s13524-016-0542-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhao W, Ammous F, Ratliff S, et al. Education and lifestyle factors are associated with DNA methylation clocks in older African Americans. Int J Environ Res Public Health. 2019;16(17):3141. doi: 10.3390/ijerph16173141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McCrory C, Fiorito G, Ni Cheallaigh C, et al. How does socio-economic position (SEP) get biologically embedded: a comparison of allostatic load and the epigenetic clock(s). Psychoneuroendocrinology. 2019;104:64-73. doi: 10.1016/j.psyneuen.2019.02.018 [DOI] [PubMed] [Google Scholar]
- 67.Watkins SH, Testa C, Chen JT, et al. Epigenetic clocks and research implications of the lack of data on whom they have been developed: a review of reported and missing sociodemographic characteristics. Environ Epigenet. 2023;9(1):dvad005. doi: 10.1093/eep/dvad005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol. 2016;16(10):626-638. doi: 10.1038/nri.2016.90 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix 1. Data Quality and Curation of Epigenetic Data in Add Health
eAppendix 2. Description of Epigenetic Clock Measures
eAppendix 3. Sociodemographic and Lifestyle Measures
eMethods.
eFigure. Bivariate Associations Between Sociodemographic Factors and Epigenetic Age According to Clocks
eTable 1. Add Health Sample Distribution of Demographic Characteristics for Wave I, Wave V Full Sample, and Wave V Epigenetic Sample
eTable 2. Univariate Statistics for DNA Methylation Epigenetic Clocks (N = 4237)
eTable 3. Bivariate Models of Social and Demographic Characteristics and Clock Estimates (N = 4237)
eTable 4. Multivariate Models of Social and Demographic Characteristics and Clock Estimates (N = 4237)
eTable 5. Multivariate Models of Social and Demographic Characteristics and Clock Estimates With Cell Compositions (N = 4237)
Data Sharing Statement


