Abstract
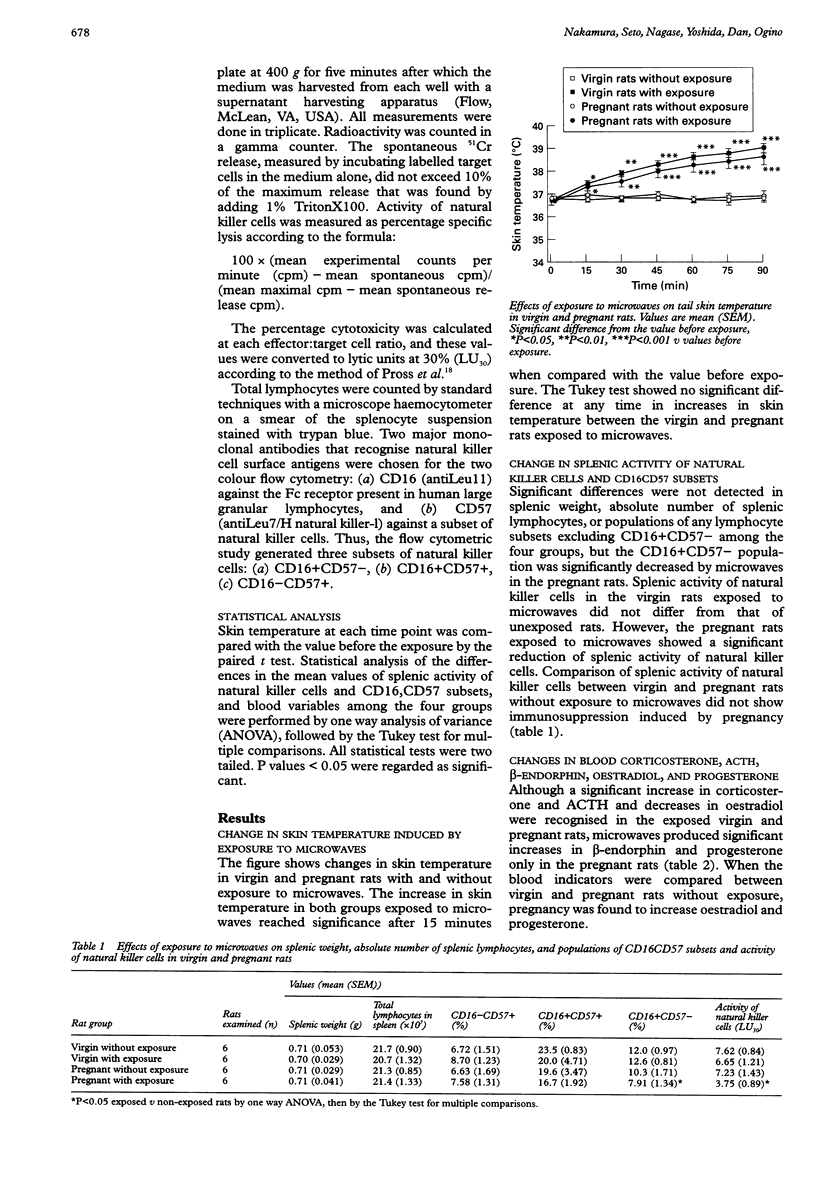
OBJECTIVES: Microwaves produce various detrimental changes based on actions of heat or non-specific stress, although the effects of microwaves on pregnant organisms has not been uniform. This study was designed to clarify the effect of exposure to microwaves during pregnancy on endocrine and immune functions. METHODS: Natural killer cell activity and natural killer cell subsets in the spleen were measured, as well as some endocrine indicators in blood--corticosterone and adrenocorticotrophic hormone (ACTH) as indices of the hypothalamic-pituitary-adrenal axis--beta-endorphin, oestradiol, and progesterone in six female virgin rats and six pregnant rats (nine to 11 days gestation) exposed to microwaves at 10 mW/cm2 incident power density at 2450 MHz for 90 minutes. The same measurements were performed in control rats (six virgin and six pregnant rats). RESULTS: Skin temperature in virgin and pregnant rats increased immediately after exposure to microwaves. Although splenic activity of natural killer cells and any of the subset populations identified by the monoclonal antibodies CD16 and CD57 did not differ in virgin rats with or without exposure to microwaves, pregnant rats exposed to microwaves showed a significant reduction of splenic activity of natural killer cells and CD16+CD57-. Although corticosterone and ACTH increased, and oestradiol decreased in exposed virgin and pregnant rats, microwaves produced significant increases in beta-endorphin and progesterone only in pregnant rats. CONCLUSIONS: Microwaves at the power of 10 mW/cm2 produced activation of the hypothalamic-pituitary-adrenal axis and increased oestradiol in both virgin and pregnant rats, suggesting that microwaves greatly stress pregnant organisms. These findings in pregnant rats suggest that--with exposure to microwaves--pregnancy induces immunosuppression, which could result in successful maintainance of pregnancy. This enhancement of adaptability to heat stress with pregnancy may be mediated by activation of placental progesterone and placental or pituitary beta-endorphin.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Branch D. W. Physiologic adaptations of pregnancy. Am J Reprod Immunol. 1992 Oct-Dec;28(3-4):120–122. doi: 10.1111/j.1600-0897.1992.tb00771.x. [DOI] [PubMed] [Google Scholar]
- Brent R. L. The effect of embryonic and fetal exposure to x-ray, microwaves, and ultrasound: counseling the pregnant and nonpregnant patient about these risks. Semin Oncol. 1989 Oct;16(5):347–368. [PubMed] [Google Scholar]
- Giglio T., Imro M. A., Filaci G., Scudeletti M., Puppo F., De Cecco L., Indiveri F., Costantini S. Immune cell circulating subsets are affected by gonadal function. Life Sci. 1994;54(18):1305–1312. doi: 10.1016/0024-3205(94)00508-7. [DOI] [PubMed] [Google Scholar]
- Gregory C. D., Shah L. P., Lee H., Scott I. V., Golding P. R. Cytotoxic reactivity of human natural killer (NK) cells during normal pregnancy: a longitudinal study. J Clin Lab Immunol. 1985 Dec;18(4):175–181. [PubMed] [Google Scholar]
- Grossman C. J. Interactions between the gonadal steroids and the immune system. Science. 1985 Jan 18;227(4684):257–261. doi: 10.1126/science.3871252. [DOI] [PubMed] [Google Scholar]
- Inaba R., Shishido K., Okada A., Moroji T. Effects of whole body microwave exposure on the rat brain contents of biogenic amines. Eur J Appl Physiol Occup Physiol. 1992;65(2):124–128. doi: 10.1007/BF00705068. [DOI] [PubMed] [Google Scholar]
- Inman R. D. Immunologic sex differences and the female predominance in systemic lupus erythematosus. Arthritis Rheum. 1978 Sep-Oct;21(7):849–852. doi: 10.1002/art.1780210718. [DOI] [PubMed] [Google Scholar]
- Jacoby D. R., Olding L. B., Oldstone M. B. Immunologic regulation of fetal-maternal balance. Adv Immunol. 1984;35:157–208. doi: 10.1016/s0065-2776(08)60576-3. [DOI] [PubMed] [Google Scholar]
- Lanier L. L., Le A. M., Phillips J. H., Warner N. L., Babcock G. F. Subpopulations of human natural killer cells defined by expression of the Leu-7 (HNK-1) and Leu-11 (NK-15) antigens. J Immunol. 1983 Oct;131(4):1789–1796. [PubMed] [Google Scholar]
- Lipman R. M., Tripathi B. J., Tripathi R. C. Cataracts induced by microwave and ionizing radiation. Surv Ophthalmol. 1988 Nov-Dec;33(3):200–210. doi: 10.1016/0039-6257(88)90088-4. [DOI] [PubMed] [Google Scholar]
- Léonard A., Berteaud A. J., Bruyère A. An evaluation of the mutagenic, carcinogenic and teratogenic potential of microwaves. Mutat Res. 1983 Sep;123(1):31–46. doi: 10.1016/0165-1110(83)90045-3. [DOI] [PubMed] [Google Scholar]
- Michaelson S. M. Health implications of exposure to radiofrequency/microwave energies. Br J Ind Med. 1982 May;39(2):105–119. doi: 10.1136/oem.39.2.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelson S. M., Houk W. M., Lebda N. J., Lu S. T., Magin R. L. Biochemical and neuroendocrine aspects of exposure to microwaves. Ann N Y Acad Sci. 1975 Feb 28;247:21–45. doi: 10.1111/j.1749-6632.1975.tb35981.x. [DOI] [PubMed] [Google Scholar]
- Nakai Y., Nakao K., Oli S., Imura H. Presence of immunoreactive beta-lipotropin and beta-endorphin in human placenta. Life Sci. 1978 Nov 13;23(20):2013–2018. doi: 10.1016/0024-3205(78)90233-3. [DOI] [PubMed] [Google Scholar]
- Newnham J. P., Tomlin S., Ratter S. J., Bourne G. L., Rees L. H. Endogenous opioid peptides in pregnancy. Br J Obstet Gynaecol. 1983 Jun;90(6):535–538. doi: 10.1111/j.1471-0528.1983.tb08963.x. [DOI] [PubMed] [Google Scholar]
- Petraglia F., Baraldi M., Giarrè G., Facchinetti F., Santi M., Volpe A., Genazzani A. R. Opioid peptides of the pituitary and hypothalamus: changes in pregnant and lactating rats. J Endocrinol. 1985 May;105(2):239–245. doi: 10.1677/joe.0.1050239. [DOI] [PubMed] [Google Scholar]
- Pross H. F., Baines M. G., Rubin P., Shragge P., Patterson M. S. Spontaneous human lymphocyte-mediated cytotoxicity against tumor target cells. IX. The quantitation of natural killer cell activity. J Clin Immunol. 1981 Jan;1(1):51–63. doi: 10.1007/BF00915477. [DOI] [PubMed] [Google Scholar]
- RUBIN A., ERDMAN W. J., 2nd Microwave exposure of the human female pelvis during early pregnancy and prior to conception: case reports. Am J Phys Med. 1959 Dec;38:219–220. [PubMed] [Google Scholar]
- Ratcliffe W. A., Carter G. D., Dowsett M., Hillier S. G., Middle J. G., Reed M. J. Oestradiol assays: applications and guidelines for the provision of a clinical biochemistry service. Ann Clin Biochem. 1988 Sep;25(Pt 5):466–483. doi: 10.1177/000456328802500502. [DOI] [PubMed] [Google Scholar]
- SILBER R. H., BUSCH R. D., OSLAPAS R. Practical procedure for estimation of corticosterone or hydrocortisone. Clin Chem. 1958 Aug;4(4):278–285. [PubMed] [Google Scholar]
- Salméron O. J., Vaquer S., Salmerón I., Moltó L., Lapeña P., Manzano L., de las Heros J. I., Alvarez-Mon M. Pregnancy is associated with a reduction in the pattern of the cytotoxic activity displayed by lymphokine-activated killer cells. Am J Reprod Immunol. 1991 Dec;26(4):150–155. doi: 10.1111/j.1600-0897.1991.tb00716.x. [DOI] [PubMed] [Google Scholar]
- Shavit Y., Terman G. W., Martin F. C., Lewis J. W., Liebeskind J. C., Gale R. P. Stress, opioid peptides, the immune system, and cancer. J Immunol. 1985 Aug;135(2 Suppl):834s–837s. [PubMed] [Google Scholar]
- Thwaites C. J. Embryo mortality in the heat stressed ewe. I. The influence of breed. J Reprod Fertil. 1967 Aug;14(1):5–14. doi: 10.1530/jrf.0.0140005. [DOI] [PubMed] [Google Scholar]
- Trinchieri G. Biology of natural killer cells. Adv Immunol. 1989;47:187–376. doi: 10.1016/S0065-2776(08)60664-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber R. J., Pert A. The periaqueductal gray matter mediates opiate-induced immunosuppression. Science. 1989 Jul 14;245(4914):188–190. doi: 10.1126/science.2749256. [DOI] [PubMed] [Google Scholar]
- Wiebold J. L., Stanfield P. H., Becker W. C., Hillers J. K. The effect of restraint stress in early pregnancy in mice. J Reprod Fertil. 1986 Sep;78(1):185–192. doi: 10.1530/jrf.0.0780185. [DOI] [PubMed] [Google Scholar]
- Yoshimi H., Matsukura S., Sueoka S., Fukase M., Yokota M., Hirata Y., Imura H. Radioimmunoassay for beta-endorphin: presence of immunoreactive "big-big" beta-endorphin ("big" beta-lipotropin) in human and rat pituitaries. Life Sci. 1978 Jun 26;22(24):2189–2195. doi: 10.1016/0024-3205(78)90570-2. [DOI] [PubMed] [Google Scholar]


