Abstract
Objective
This study aimed to assess differences in various scalp parameters between patients with androgenetic alopecia (AGA) and healthy volunteers using 22 MHz ultrasound.
Methods
Thirty patients with AGA (AGA group) and 30 healthy volunteers (control group) who visited the Department of Dermatology at the Second Affiliated Hospital of Soochow University from September 2021 to June 2022 were randomly selected. The patients with AGA met the diagnostic criteria outlined in the Chinese Guidelines for the Diagnosis and Treatment of Androgenetic Alopecia. The severity of alopecia was assessed for males between grades 2 and 4 on the Norwood–Hamilton scale, and for females between stages 2 and 3 on the Ludwig scale. No artificial interventions were conducted at the vertex, and all examination conditions remained consistent. Ultrasound examinations at 22 MHz were performed on the scalp at the vertex in both the AGA and control groups. Seven parameters were measured, namely, epidermis + dermis thickness, entire scalp thickness, subcutaneous tissue thickness, average follicle width, average follicle length, follicle count, and the presence of color flow signals in the subcutaneous tissue. The differences in these parameters were then compared.
Results
The AGA group showed reduced thickness of the entire scalp and subcutaneous tissue, narrower average follicle width, shorter average follicle length, lower hair follicle count, and fewer instances of color flow signals in the subcutaneous tissue at the vertex area (p < 0.05).
Conclusion
High‐frequency (22 MHz) ultrasonography can be employed to visualize the entrance echo, dermis, subcutaneous tissue, and hair follicles of the scalp, thereby providing imaging for the clinical assessment of hair loss.
Keywords: androgenetic alopecia, follicle, scalp, ultrasound
1. INTRODUCTION
Androgenetic alopecia (AGA) is a non‐scarring, inherited condition characterized by the progressive worsening of alopecia symptoms. 1 The distribution of alopecia differs between the sexes: men commonly experience an upward shift of the frontal hairline and a decrease in hair density on the top of the head, while women typically experience a decrease in hair density on the top of the head. 2 Coronavirus disease 2019 (COVID‐19) can exacerbate hair loss. 3 Trichoscopy is a rapid, non‐invasive tool that enables dermatologists to identify morphologic hair structures not visible to the naked eye. It aids in diagnosing the disease, assessing disease severity and activity, and conducting follow‐up assessments. 4 However, trichoscopy cannot visualize deeper skin structures such as hair follicles. 5 , 6 Ultrasonography has been widely used in dermatology for diagnosis and efficacy assessment of conditions, such as melanocytic nevi, 7 hidradenitis suppurativa, 8 basal cell carcinoma, 9 and many others. High‐frequency ultrasonography can also visualize the structure of hair follicles within the skin layer, 10 , 11 allowing observation of parameters such as the number, width, and depth of hair follicles. 12 In this study, we aim to investigate whether differences exist in the ultrasound parameters of hair follicle‐related structures in the scalp area between patients with AGA and healthy volunteers. The goal is to identify imaging changes in hair follicles, thus providing an imaging basis for the clinical assessment of hair loss.
2. METHODS
2.1. Study population and grouping
Thirty patients with AGA who attended the Department of Dermatology at the Second Affiliated Hospital of Soochow University from September 2021 to June 2022 were randomly selected as the AGA group. Additionally, 30 healthy volunteers were selected as the control group.
2.2. Inclusion and exclusion criteria
The inclusion criteria were as follows:.(1) hair loss diagnostic criteria for patients with AGA as per the Chinese Guidelines for the Diagnosis and Treatment of Androgenetic Alopecia 13 ; (2) severity of hair loss graded for males according to the Norwood–Hamilton scale, with grades 2−4 selected 14 (Table 1); and for females according to the Ludwig scale, with stages 2−3 selected 15 (Table 2). Healthy volunteers were free of hair disorders and seborrheic dermatitis and had no history of radiation or chemotherapy. The exclusion criteria were as follows: breakout, scar formation, or alopecia areata at the vertex. 16
TABLE 1.
Norwood‐Hamilton Scale.
| Type | Clinical definition |
|---|---|
| I | Minimal recession of the hairline along the anterior border in the frontotemporal (FT) region The anterior border of the hair in the FT region has triangular areas of recession that tend to be symmetrical |
| II | These areas extend no further posterior than approximately 2 cm anterior to a line drawn in a coronal plane between the external auditory meatus on both sides. Hari is either lost or sparse along the mid‐frontal border of the scape |
| III | Characterized by deep FT hair recession, usually symmetrical and either bald or sparsely covered with hair. These areas of hair recession extend further posterior than a point that lies approximately 2 cm anterior to a line drawn in a coronal plane between the external auditory meatus on either side |
| IV | The frontal and FT recession is more severe than type III. There is also sparseness or absence of hair in the vertex area. These bald areas are extensive, but separated from each other by a band of moderately dense hair that joins the fully haired fringe on each side of the head |
| V | The hair loss over the vertex and FT areas is larger than in type IV and the band of hair between them is narrower and sparser |
| VI | The hair loss over the FT and vertex regions is confluent and the bridge of hair that crosses the crown is absent |
| VII | There is only a narrow horseshoe‐shaped band of hair that begins laterally just anterior to the ear and extends posteriorly on the sides and fairly low on the occipital area |
TABLE 2.
Ludwig Scale.
| Type | Clinical definition |
|---|---|
| Stage 1 | Thinning of hair is seen mainly over the anterior part of the crown with minimal widening of the parting width |
| Stage 2 | Thinning of the crown becomes more evident because of an increase in the number of thin and short hairs |
| Stage 3 | The crown becomes almost total bald. There is signify cant widening of the parting width, but the frontal hairline is still maintained |
3. METHODS
The MyLab Twice ultrasound diagnostic system (Italy) was used. The system was equipped with an SL3116 linear array probe operating at a frequency of 22 MHz, with an image depth of 15 mm and a width of 12.7 mm.
The examination was performed in a quiet, separate room with the temperature controlled at 25°C−28°C.
Ultrasound was used to observe the scalp of patients with AGA at the vertex (2 cm to the left of the midline of the body's vertex 16 ) in a seated position. A 7 mm thick sound guide pad (Foshan Team En Technology) was placed at the hair seam after the application of a coupling agent. The ultrasound probe was gently placed on the acoustic coupler pad, and the image focus was adjusted to the dermis layer for optimal imaging. Grayscale ultrasound was used to observe the scalp entrance echo, dermis, subcutaneous tissue, and hair follicles (Figure 1). The color doppler mode was then activated (blood flow velocity scale 0.08 m/s), and the color gain was adjusted to the critical point where noise was about to appear, to observe the subcutaneous tissue for color blood flow signals. The entire scanning process was stored in video file format on the machine's hard disk for later analysis, and the examination conditions were kept consistent throughout.
FIGURE 1.
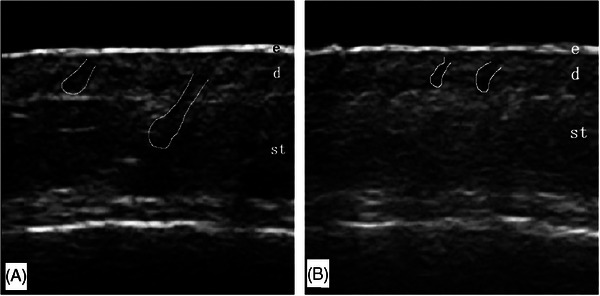
Scalp ultrasonography image. (A) Scalp ultrasound image of a healthy volunteers. The ultrasound image depicts the scalp of a 48‐year‐old male healthy volunteers. (B) Scalp ultrasound image of a patient with AGA. The ultrasound image depicts the scalp of a 52‐year‐old male patient with AGA. d, dermis; e, epidermis; st, subcutaneous tissue. White outlined circles indicate follicles located in the dermis or subcutaneous tissue layer.
This research protocol was approved by the ethics committee of the hospital under approval number 2021–089, and all participants provided informed consent.
3.1. Ultrasound instrumentation and method
The video files were played back, and images of the scalp skin layer and hair follicles were captured. Measurements and counts were taken and repeated three times to obtain the mean value. The parameters used in the study were as follows: (1) Epidermis + dermis thickness: The vertical distance from the entrance echo to the boundary of the deep dermis 6 ; (2) Entire scalp thickness: The vertical distance from the entrance echo to the cranial surface 17 ; (3) Subcutaneous tissue thickness: The vertical distance from the deep dermal boundary to the cranial surface, calculated as the difference between the entire scalp thickness and the epidermis + dermis thickness 17 ; (4) Follicle count: The number of hair follicles visible in the image; (5) Average follicle width: The widest diameter along the axis of the follicle, 6 with the average width calculated from all follicles in the image; (6) Average follicle length: The distance from the lower border of the entrance echo to the far end of the follicle along the axis of the follicle, 6 with the average length calculated from all follicles in the image; and (7) Color blood flow signal count: The number of color blood flow signals detected in the subcutaneous tissue.
3.2. Statistical analysis
Statistical analysis was performed using SPSS version 26.0. Normality was assessed using the Shapiro–Wilk test. Normally distributed data were expressed as mean ± standard deviation, and between‐group comparisons were made using the independent‐samples t test. Skewed data were presented as median (quartiles), and between‐group comparisons were made using the nonparametric Mann–Whitney U test. Categorical variables were presented as counts, and comparisons between different frequency arrays were made using the chi‐square test or Fisher's exact probability test. A p value < 0.05 indicated a statistically significant difference.
The intraclass correlation coefficient (ICC) was used to assess the agreement between the two sonographers in measuring and counting the ultrasound parameters.
4. RESULTS
4.1. General information and results
The AGA group comprised 21 males and 9 females, aged 23−56 years, with a mean age of 39.40 ± 8.99 years, a mean body mass index of 22.99 ± 1.74, and a disease duration of 1−20 years. Among the males, 21 had Norwood–Hamilton scale scores of II in 3 cases, III in 8 cases, and IV in 10 cases. Among the females, nine had Ludwig scale ratings of stage 2 in six cases and stage 3 in three cases. The control group comprised 16 males and 14 females, aged 22−57 years, with a mean age of 36.73 ± 9.46 years, and a mean body mass index of 23.32 ± 1.79. Both groups of subjects tested negative for COVID‐19 infection antigens during the observation period. No statistically significant differences were found in terms of gender, age, and body mass index between the patients with AGA and the healthy volunteers (p > 0.05).
4.2. Intraclass correlation coefficient test
Two associate physicians with 11 years and 11 years and 3 months of working experience and whose daily work focuses on diagnosing skin diseases analyzed the ultrasound images of the scalps of 30 cases from the AGA group. The best images were selected from the video files during playback in a double‐blind manner to measure and count the parameters. The two ultrasonographers counted the same number of colored blood flow signals in the subcutaneous tissue, eliminating the need for a consistency test for this parameter. The results of the ICC test for the remaining parameters are presented in Table 3. The lowest ICC was 0.822 (95% CI 0.625−0.915), indicating a high level of agreement between different observers for both image recognition and parameter measurement.
TABLE 3.
Intraclass correlation coefficient test.
| ICC(95% CI) | |
|---|---|
| Epidermis + dermis thickness | 0.978(0.954–0.990) |
| Entire scalp thickness | 0.989(0.977–0.995) |
| Follicle count | 0.822(0.625–0.915) |
| Average follicle width | 0.837(0.658–0.923) |
| Average follicle length | 0.864(0.714–0.935) |
Abbreviations: CI, confidence interval; ICC, intraclass correlation coefficient.
4.3. Ultrasound examination results
No statistically significant differences were found in each ultrasound parameter between genders in the AGA group (p > 0.05, Table 4).
TABLE 4.
Comparison of ultrasound parameters between males and females in the AGA group.
| Ultrasound parameters | Male (n = 21) | Female (n = 9) | Statistical value | p |
|---|---|---|---|---|
| Epidermis + dermis thickness (mm) | 1.56 ± 0.27 | 1.59 ± 0.17 | −0.306 | 0.763 |
| Entire scalp thickness (mm) | 4.64 ± 0.97 | 4.94 ± 0.60 | −1.011 | 0.322 |
| Subcutaneous tissue thickness (mm) | 3.08 ± 0.81 | 3.35 ± 0.44 | −1.178 | 0.250 |
| Follicle count (roots) | 13.00(12.00, 13.00) | 13.00(12.00, 13.50) | −0.143 | 0.886 |
| Average follicle width (mm) | 0.50 ± 0.07 | 0.54 ± 0.07 | −1.325 | 0.204 |
| Average follicle length (mm) | 1.21 ± 0.19 | 1.32 ± 0.15 | −1.796 | 0.088 |
| Subcutaneous tissue with colored blood flow signals (cases) | 9 | 2 | 0.419 |
Abbreviation: AGA, androgenetic alopecia.
A comparison of the ultrasound parameters of the AGA group with those of the control group revealed that the subjects in the AGA group had thinner entire scalp and subcutaneous tissue thickness, fewer hair follicles, narrower average follicle width, shorter average follicle length, and fewer instances of colorful blood flow signals in the subcutaneous tissue (p < 0.05, Table 5).
TABLE 5.
Comparison of ultrasound parameters between AGA and control group.
| Ultrasound parameters | Control group (n = 30) | AGA group (n = 30) | Statistical value | p |
|---|---|---|---|---|
| Epidermis + dermis thickness (mm) | 1.61 ± 0.23 | 1.57 ± 0.24 | −0.718 | 0.476 |
| Entire scalp thickness (mm) | 5.76 ± 1.14 | 4.73 ± 0.88 | −3.912 | <0.001 |
| Subcutaneous tissue thickness (mm) | 4.14 ± 1.10 | 3.16 ± 0.72 | −4.104 | <0.001 |
| Follicle count (roots) | 16 (15, 17) | 13 (12, 13) | −6.425 | <0.001 |
| Average follicle width (mm) | 0.70 ± 0.06 | 0.51 ± 0.07 | −11.020 | <0.001 |
| Average follicle length (mm) | 1.97 ± 0.22 | 1.24 ± 0.18 | −13.769 | <0.001 |
| Subcutaneous tissue with colored blood flow signals (cases) | 21 | 11 | 6.696 | <0.05 |
Abbreviation: AGA, androgenetic alopecia.
5. CONCLUSION
The hair follicle growth cycle comprises three phases: anagen, telogen, and alopecia areata. 18 The pathogenesis of AGA is primarily associated with dihydrotestosterone (DHT) and transforming growth factor β−1. 19 Excessive DHT binding to androgen receptors leads to progressive thinning and shortening of hair follicles during the anagen phase. 20 Moreover, androgen‐induced overexpression of transforming growth factor β−1 in dermal papilla cells accelerates the abscission phase, ultimately resulting in hair loss. 21 The reason for exacerbate hair loss caused by COVID‐19 is thought to be related to the psychological state of individuals, as well as to the increased levels of inflammatory cytokines [such as IL‐1, IL‐6, IL‐2, IL‐17, interferon (IFN‐), monocyte chemoattractant protein 1 (MCP‐1), IP10, and many others] that suppress the growth cycle of hair follicles and accelerate hair shedding. It is also associated with hypoxemia due to lung damage secondary to COVID‐19 infection, which causes skin ischemia and affects hair follicle growth. 22
Currently, only oral finasteride and topical minoxidil are approved by the Food and Drug Administration for treating AGA. 23 Finasteride, a 5α‐reductase inhibitor, is particularly effective in stimulating hair growth on the scalp's crown and is primarily prescribed for adult males with AGA. However, its side effects predominantly include sexual dysfunction and an elevated risk of prostate cancer in males, along with a potential teratogenic risk to fetal genital development in females. 24 Minoxidil, acting as a potassium channel blocker, can be used in women to enhance blood flow to terminal vessels. This facilitates the influx of oxygen and nutrient‐rich blood into hair follicles, thereby promoting hair growth. Minoxidil is administered topically, and its primary adverse effect is itching or peeling caused by skin irritation. 25 Other treatment methods for AGA include topical laser irradiation, 26 topical injections of botulinum toxin type A, 27 or platelet‐rich plasma, 28 and the topical application of derivatives, such as tea seed oil 29 and pumpkin seed oil. 30
Currently, the invasive method for clinically assessing alopecia is tissue biopsy, which is considered the best method for diagnosing the disease. However, owing to its invasive nature, this method cannot be routinely employed. 31 Non‐invasive assessment methods primarily include questionnaire surveys, 60‐s hair loss counts, before‐ and after‐treatment photo comparisons, and trichoscopy. 32 Questionnaires are utilized to evaluate treatment responses and changes in appearance, but they are simple and highly subjective. 28 The 60‐s hair count, involving a monthly tally of hair loss after 60 s of combing before shampooing over 6 months, is time‐consuming and prone to bias in the results. 33 Comparing photographs taken before and after treatment necessitates consistency in hair length, color, shape, and machine settings each time a photograph is captured to minimize interference. 34 Trichoscopy can identify morphological structures of hairs not visible to the naked eye but cannot observe hair follicle structures. Ultrasonography can observe changes in hair follicles, compensating for the limitation of trichoscopy.
In this study, the use of sound guide pads can prevent artifactual interference and enhance the clarity of images of superficial tissues. The study demonstrates that the use of sound guide pads does not affect parameter measurements and may enhance their utility in the differential diagnosis of superficial lesions. 35
In their comparative observation between patients with AGA and healthy volunteers, Ten et al. 17 utilized 14 MHz ultrasound to examine the middle part of the hairline in both groups. The results indicated that the entire scalp was thinner in patients with AGA. Similarly, Soga et al. 36 employed 3T magnetic resonance imaging to observe the top of the head area in patients with AGA and healthy volunteers. Their findings revealed that the thickness of both the entire scalp and subcutaneous tissue (measured as the vertical distance from the superficial dermis to the surface of the skull) was thinner in patients with AGA. In the present study, 22 MHz ultrasound was utilized for observation, and the results similarly indicated thinner entire scalp and subcutaneous tissue in patients with AGA. This phenomenon is believed to be associated with the acceleration of skin aging owing to hair loss. 17 Skin aging encompasses both intrinsic and extrinsic factors. Intrinsic aging primarily involves a reduction in the number of fibroblasts and thinning of subcutaneous fat, leading to skin atrophy. 37 Extrinsic aging is predominantly caused by ultraviolet light induced damage to collagen and elastin fibers, a process that is particularly pronounced in the scalp region. 38 Choy et al. 39 employed a micrometer to measure the thickness of the scalp skin layer in both patients with AGA and healthy volunteers after lifting, and their results indicated a greater thickness of the entire scalp in patients with AGA. This contrasts with the results of the present study. The discrepancy is attributable to differences in measurement methods, as the external force exerted during the lifting procedure may influence skin tension and consequently affect thickness measurements.
In a previous study, AGA hair follicles exhibited a progressive reduction in size, 1 with the ratio of initial to final growth changing from 12:1 to 5:0. 20 The findings of the present study corroborated the decrease in the number of hair follicles in patients with AGA. A study by Mikiel et al. 6 similarly demonstrated a reduction in the number of hair follicles in patients with AGA.
The positioning of hair follicle ends within the skin layer varies depending on the growth phase, with the anagen phase predominantly situated in the subcutaneous tissue, a smaller proportion in the superficial or deep dermis, and the resting phase in the superficial dermis. 12 AGA alters the growth phase of the hair follicle, resulting in a shift in the location of the follicle ends. 19 Wortsman et al. 14 utilized 15−18 MHz ultrasonography to investigate hair follicle width (using the same methodology as in the present study) and depth (the distance from the entrance echo to the visible distal‐most portion of the hair follicle along the hair shaft direction). The mean depth of the hair follicle was shallower in patients with AGA than in healthy volunteers, while the difference in the mean width of the follicle was not statistically significant. Mikiel et al. 6 employed 20 MHz ultrasonography to examine follicle width and length (the distance from the entrance echo to the bottom portion of the follicle in the hair shaft direction), and the length was defined similarly to Wortsman et al.’s 14 hair follicle depth. The findings indicated that the mean width of hair follicles was narrower in patients with AGA than in healthy volunteers, although the length of hair follicles was not compared with that of healthy volunteers. However, the measurement may not be accurate because the authors speculated that the hair follicles were located deeper in the skin layer in healthy volunteers. In the present study, we followed the methodology outlined by Mikiel et al. 6 for measuring hair follicle width and length. The results indicated that the mean width of hair follicles was narrower, and the mean length was shorter in subjects with AGA.
Scalp blood supply originates from the distal peripheral vasculature, 40 and the microvessels associated with the distal vessels and hair papillae are affected by the lack of microcirculation, which reduces or even prevents blood supply, resulting in vascular occlusion. 41 The results of the present study showed even fewer instances of colored blood flow signals in the subcutaneous tissues of patients with AGA, confirming the reduced blood supply to the scalp at the sites of hair loss.
This study has several limitations. First, there is a lack of histological basis for the observed changes in hair follicles detected via ultrasonography. Second, the research was a single‐center study. Data from larger samples and multicenter studies are required in the future to enhance generalizability. Additionally, this study solely utilized ultrasonography to observe hair follicle structure, without employing trichoscopy on the hairs, which may have resulted in some inadequacies in the results. During the observation period of this study, none of the cases contracted COVID‐19, therefore we are unable to provide comparative observations of hair loss exacerbated by COVID‐19 infection. This is an interesting research direction that could be further explored in subsequent studies.
In conclusion, ultrasonography can be employed to visualize the entrance echo, dermis, subcutaneous tissue, and hair follicles of the scalp. The quantitative data obtained regarding hair follicles can be applied to clinical hair loss assessment to provide a clinical imaging basis.
CONFLICT OF INTEREST STATEMENT
The authors have no conflict of interest to declare.
ACKNOWLEDGMENTS
The author would like to express his gratitude to all those who participated in this study. He is even more grateful to my supervisor, Mr. Qi Ma, who provided me with assistance in research design, case collection, and paper revision. I sincerely appreciate all the volunteers who participated in this study. I also want to extend special thanks to my supervisor, Mr. Qi Ma, for his invaluable guidance and support throughout the research design, data collection, and paper writing process.
Li L, Ma Q, Luo W, Ji J, Zhang X, Hong D. High‐frequency ultrasonography of the scalp: A comparison between androgenetic alopecia and healthy volunteers. Skin Res Technol. 2024;e13863. 10.1111/srt.13863
DATA AVAILABILITY STATEMENT
The data supporting the findings of this study are available from the corresponding author, Qi Ma, upon reasonable request.
REFERENCES
- 1. Alessandrini A, Bruni F, Piraccini BM, Starace M. Common causes of hair loss—clinical manifestations, trichoscopy and therapy. J Eur Acad Dermatol Venereol. 2021;35(3):629‐640. [DOI] [PubMed] [Google Scholar]
- 2. Suchonwanit P, Iamsumang W, Leerunyakul K. Topical finasteride for the treatment of male androgenetic alopecia and female pattern hair loss: a review of the current literature. J Dermatolog Treat. 2022;33(2):643‐648. [DOI] [PubMed] [Google Scholar]
- 3. Gentile P. Hair loss and telogen effluvium related to COVID‐19: the potential implication of adipose‐derived mesenchymal stem cells and platelet‐rich plasma as regenerative strategies. Int J Mol Sci. 2022;23(16):9116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Panchaprateep R, Tanus A, Tosti A. Clinical, dermoscopic, and histopathologic features of body hair disorders. J Am Acad Dermatol. 2015;72(5):890‐900. [DOI] [PubMed] [Google Scholar]
- 5. Olszewska M, Rudnicka L, Rakowska A, Kowalska‐Oledzka E, Slowinska M. Trichoscopy. Arch Dermatol. 2008;144(8):1007. [DOI] [PubMed] [Google Scholar]
- 6. Mikiel D, Polanska A, Zaba R, Adamski Z, Danczak‐Pazdrowska A. Usefulness of high‐frequency ultrasonography in the assessment of alopecia areata—comparison of ultrasound images with trichoscopic images. Postepy Dermatol Alergol. 2022;39(1):132‐140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang YK, Gao YJ, Liu J, et al. A comparative study of melanocytic nevi classification with dermoscopy and high‐frequency ultrasound. Skin Res Technol. 2022;28(2):265‐273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Oranges T, Vitali S, Benincasa B, et al. Advanced evaluation of hidradenitis suppurativa with ultra‐high frequency ultrasound: a promising tool for the diagnosis and monitoring of disease progression. Skin Res Technol. 2020;26(4):513‐519. [DOI] [PubMed] [Google Scholar]
- 9. Han X, Li J, Zeng F, Liu H, He Y. Differential diagnosis of basal cell carcinoma by high‐resolution ultrasound elastography. Skin Res Technol. 2022;28(2):350‐354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wortsman X, Carreño L, Ferreira‐Wortsman C, et al. Ultrasound characteristics of the hair follicles and tracts, sebaceous glands, montgomery glands, apocrine glands, and arrector pili muscles. J Ultrasound Med. 2019;38:1995‐2004. [DOI] [PubMed] [Google Scholar]
- 11. Porrino‐Bustamante ML, Fernandez‐Pugnaire MA, Castellote‐Caballero L, Arias‐Santiago S. Colour doppler ultrasound study in patients with frontal fibrosing alopecia. Skin Res Technol. 2021;27(5):709‐714. [DOI] [PubMed] [Google Scholar]
- 12. Wortsman X, Wortsman J, Matsuoka L, et al. Sonography in pathologies of scalp and hair. Br J Radiol. 2012;85(1013):647‐655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hu ZQ, Miao Y. Guidelines for the diagnosis and treatment of androgenetic alopecia in Chinese. Chinese J Aesthetic Plastic Surg. 2019;30(01):8‐12. (in Chinese). [Google Scholar]
- 14. Wortsman X, Guerrero R, Wortsman J. Hair morphology in androgenetic alopecia: sonographic and electron microscopic studies. J Ultrasound Med. 2014;33(7):1265‐1272. [DOI] [PubMed] [Google Scholar]
- 15. Ludwig E. Classification of the types of androgenetic alopecia (common baldness) occurring in the female sex. Br J Dermatol. 1977;97(3):247–254. [DOI] [PubMed] [Google Scholar]
- 16. Mikiel D, Polańska A, Żaba R, Adamski Z, Dańczak‐Pazdrowska A. Suitability of high‐frequency ultrasonography (20 MHz) in evaluation of various forms of primary cicatricial alopecia in relation to trichoscopy—pilot study. Skin Res Technol. 2021;27(5):774–784. [DOI] [PubMed] [Google Scholar]
- 17. Ten B, Kaya TI, Balci Y, et al. The place of B‐mode ultrasonography, shear‐wave elastography, and superb microvascular imaging in the pre‐diagnosis of androgenetic alopecia. J Cosmet Dermatol. 2022;21(7):2962–2970. [DOI] [PubMed] [Google Scholar]
- 18. Wall D, Meah N, Fagan N, York K, Sinclair R. Advances in hair growth. Fac Rev. 2022;11(2022):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. English RS Jr, Ruiz S. Use of botulinum toxin for androgenic alopecia: a systematic review. Skin Appendage Disord. 2022;8(2):93‐100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bienenfeld A, Azarchi S, Lo Sicco K, Marchbein S, Shapiro J, Nagler AR. Androgens in women: androgen‐mediated skin disease and patient evaluation. J Am Acad Dermatol. 2019;80(6):1497–1506. [DOI] [PubMed] [Google Scholar]
- 21. Tellez‐Segura R. Involvement of mechanical stress in androgenetic alopecia. Int J Trichology. 2015;7(3):95–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gentile P. Preliminary investigation on micro‐needling with low‐level LED therapy and growth factors in hair loss related to COVID‐19. J Clin Med. 2022;11(19):5760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. James JF, Jamerson TA, Aguh C. Efficacy and safety profile of oral spironolactone use for androgenic alopecia: a systematic review. J Am Acad Dermatol. 2022;86(2):425–429. [DOI] [PubMed] [Google Scholar]
- 24. Ali AK, Heran BS, Etminan M. Persistent sexual dysfunction and suicidal ideation in young men treated with low‐dose finasteride: a pharmacovigilance study. Pharmacotherapy. 2015;35(7):687–695. [DOI] [PubMed] [Google Scholar]
- 25. Randolph M, Tosti A. Oral minoxidil treatment for hair loss: a review of efficacy and safety. J Am Acad Dermatol. 2021;84(3):737‐746. [DOI] [PubMed] [Google Scholar]
- 26. Suchonwanit P, Chalermroj N, Khunkhet S. Low‐level laser therapy for the treatment of androgenetic alopecia in Thai men and women: a 24‐week, randomized, double‐blind, sham device‐controlled trial. Lasers Med Sci. 2018;34(6):1107–1114. [DOI] [PubMed] [Google Scholar]
- 27. Singh S, Neema S, Vasudevan B. A pilot study to evaluate effectiveness of botulinum toxin in treatment of androgenetic alopecia in males. J Cutan Aesthet Surg. 2017;10(3):163–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Verma K, Tegta GR, Verma G, Gupta M, Negi A, Sharma R. A study to compare the efficacy of platelet‐rich plasma and minoxidil therapy for the treatment of androgenetic alopecia. Int J Trichology. 2019;11(2):68–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Riangjanapatee P, Khongkow M, Treetong A, et al. Development of tea seed oil nanostructured lipid carriers and in vitro studies on their applications in inducing human hair growth. Pharmaceutics. 2022;14(5):984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ibrahim IM, Hasan MS, Elsabaa KI, Elsaie ML. Pumpkin seed oil vs. minoxidil 5% topical foam for the treatment of female pattern hair loss: a randomized comparative trial. J Cosmet Dermatol. 2021;20(9):2867–2873. [DOI] [PubMed] [Google Scholar]
- 31. Hillmann K, Blume‐Peytavi U. Diagnosis of hair disorders. Semin Cutan Med Surg. 2009; 28(1): 33–38. [DOI] [PubMed] [Google Scholar]
- 32. Kurtti A, Jagdeo J, Eisinger A, Sukhdeo K. New diagnostic tools to evaluate hair loss. Dermatol Clin. 2021;39(3):375–381. [DOI] [PubMed] [Google Scholar]
- 33. Wasko CA, Mackley CL, Sperling LC, Mauger D, Miller JJ. Standardizing the 60‐second hair count. Arch Dermatol. 2008;144(6):759–762. [DOI] [PubMed] [Google Scholar]
- 34. Dhurat R, Saraogi P. Hair evaluation methods: merits and demerits. Int J Trichology. 2009;1(2):108–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Corvino A, Sandomenico F, Corvino F, et al. Utility of a gel stand‐off pad in the detection of doppler signal on focal nodular lesions of the skin. J Ultrasound. 2020;23(1):45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Soga S, Koyama T, Mikoshi A, et al. Quantitative analysis of the anatomical changes in the scalp and hair follicles in androgenetic alopecia using magnetic resonance imaging. Skin Res Technol. 2021;27(1):56–61. [DOI] [PubMed] [Google Scholar]
- 37. Khavkin J, Ellis DA. Aging skin: histology, physiology, and pathology. Facial Plast Surg Clin North Am. 2011;19(2):229–234. [DOI] [PubMed] [Google Scholar]
- 38. Kim EJ, Kim YK, Kim JE, e al. UV modulation of subcutaneous fat metabolism. J Invest Dermatol. 2011;131(8):1720–1726. [DOI] [PubMed] [Google Scholar]
- 39. Choy H. Detumescence therapy of human scalp for natural hair regrowth. J Clin Exp Dermatol Res. 2014;3(1):138. [Google Scholar]
- 40. Zhang L, Yu Q, Wang Y, Ma Y, Shi Y, Li X. A small dose of botulinum toxin A is effective for treating androgenetic alopecia in Chinese patients. Dermatol Ther. 2019;32(4):e12785. [DOI] [PubMed] [Google Scholar]
- 41. Yang K, Fu C, Ling JQ, Huo R. Mechanism and research progress of botulinum toxin type A in the treatment of androgenetic alopecia. Chinese J Aesthetic Plastic Surg. 2022;33(01):23–25. (in Chinese). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data supporting the findings of this study are available from the corresponding author, Qi Ma, upon reasonable request.


