Abstract
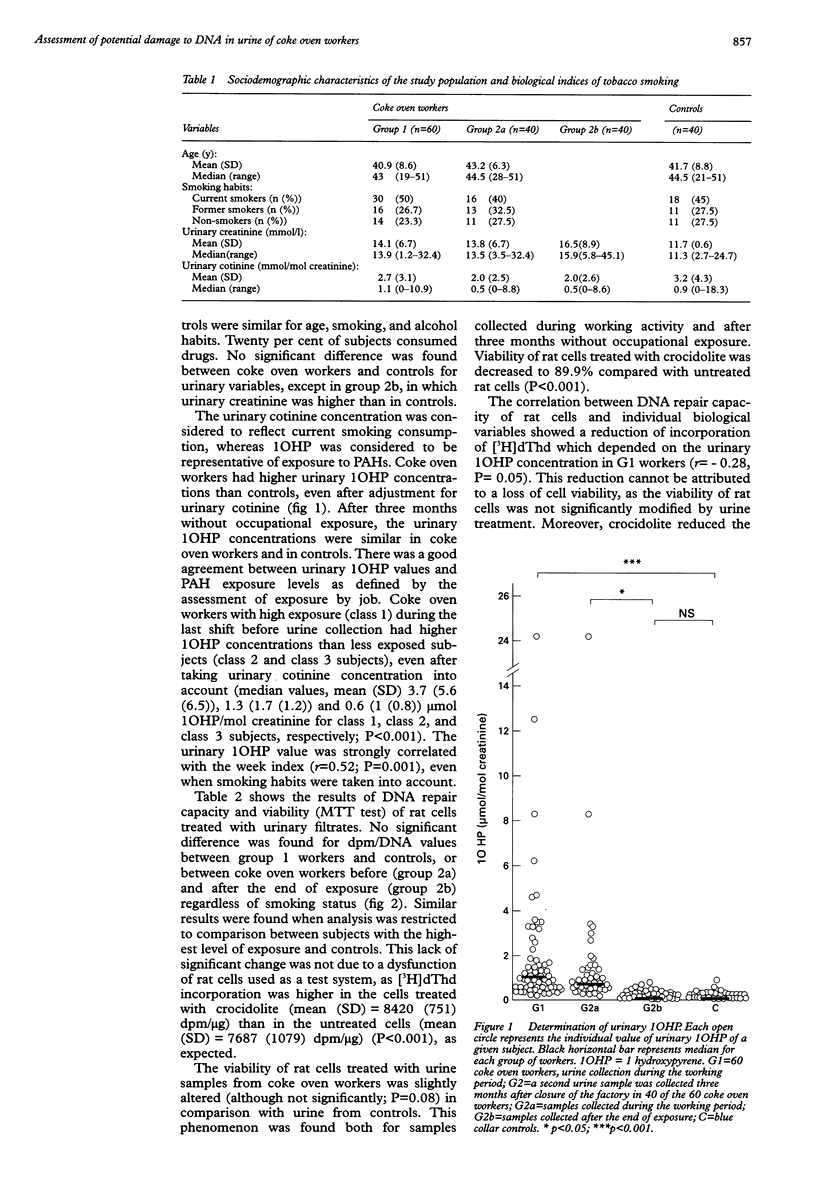
OBJECTIVES: A study was conducted in coke oven workers to evaluate the biological consequences of the exposure of these workers, particularly production of potential genotoxic factors. METHODS: 60 coke oven workers and 40 controls were recruited in the same iron and steel works. Exposure to polycyclic aromatic hydrocarbons (PAHs) was assessed by job and measurement of 1-hydroxypyrene (1OHP) in urine samples. An unscheduled DNA synthesis assay was performed on rat pleural mesothelial cells used as a test system to evaluate the effect of the workers' filtered urine on the DNA repair capacity of rat cells to determine whether DNA damaging agents are present in the urine of these workers. RESULTS: Urinary concentrations of 1OHP ranged from 0.06 to 24.2 (mean (SD) 2.1 (3.6)) mumol/mol creatinine in exposed coke oven workers, and from 0.01 to 0.9 in controls (0.12 (0.15)). These high concentrations in coke oven workers reflected recent exposure to PAHs and were in agreement with the assessment of exposure by job. No significant difference was found between coke oven workers and controls in the DNA repair level of rat cells treated with urine samples. However, the rat cell repair capacity decreased with increasing 1OHP concentrations in the exposed population (r = -0.28, P < 0.05). CONCLUSIONS: As high concentrations of 1OHP were found in the urine of some workers, a more stringent control of exposures to PAHs in the workplace is required. Exposure to PAHs was not associated with a clear cut modification of the urinary excretion of DNA damaging factors in this test, as shown by the absence of increased unscheduled DNA synthesis in rat cells. However, impairment of some repair mechanisms by urinary constituents is suspected.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buard A., Beaune P. H., Renier A., Jaurand M. C., Bignon J., Laurent P. Expression of cytochrome P450 in rat pleural mesothelial cells in secondary cultures. J Cell Physiol. 1994 Jul;160(1):176–183. doi: 10.1002/jcp.1041600120. [DOI] [PubMed] [Google Scholar]
- Buchet J. P., Ferreira M., Jr, Burrion J. B., Leroy T., Kirsch-Volders M., Van Hummelen P., Jacques J., Cupers L., Delavignette J. P., Lauwerys R. Tumor markers in serum, polyamines and modified nucleosides in urine, and cytogenetic aberrations in lymphocytes of workers exposed to polycyclic aromatic hydrocarbons. Am J Ind Med. 1995 Apr;27(4):523–543. doi: 10.1002/ajim.4700270406. [DOI] [PubMed] [Google Scholar]
- Buchet J. P., Gennart J. P., Mercado-Calderon F., Delavignette J. P., Cupers L., Lauwerys R. Evaluation of exposure to polycyclic aromatic hydrocarbons in a coke production and a graphite electrode manufacturing plant: assessment of urinary excretion of 1-hydroxypyrene as a biological indicator of exposure. Br J Ind Med. 1992 Nov;49(11):761–768. doi: 10.1136/oem.49.11.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley T. J., Lioy P. J. An examination of the time course from human dietary exposure to polycyclic aromatic hydrocarbons to urinary elimination of 1-hydroxypyrene. Br J Ind Med. 1992 Feb;49(2):113–124. doi: 10.1136/oem.49.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clonfero E., Granella M., Marchioro M., Barra E. L., Nardini B., Ferri G., Foà V. Urinary excretion of mutagens in coke oven workers. Carcinogenesis. 1995 Mar;16(3):547–554. doi: 10.1093/carcin/16.3.547. [DOI] [PubMed] [Google Scholar]
- Costantino J. P., Redmond C. K., Bearden A. Occupationally related cancer risk among coke oven workers: 30 years of follow-up. J Occup Environ Med. 1995 May;37(5):597–604. doi: 10.1097/00043764-199505000-00009. [DOI] [PubMed] [Google Scholar]
- Emerit I., Levy A., Pagano G., Pinto L., Calzone R., Zatterale A. Transferable clastogenic activity in plasma from patients with Fanconi anemia. Hum Genet. 1995 Jul;96(1):14–20. doi: 10.1007/BF00214180. [DOI] [PubMed] [Google Scholar]
- Emerit I. Reactive oxygen species, chromosome mutation, and cancer: possible role of clastogenic factors in carcinogenesis. Free Radic Biol Med. 1994 Jan;16(1):99–109. doi: 10.1016/0891-5849(94)90246-1. [DOI] [PubMed] [Google Scholar]
- Ferreira Júnior M. F., Tas S., dell'Omo M., Goormans G., Buchet J. P., Lauwerys R. Determinants of benzo(a)pyrenediol epoxide adducts to haemoglobin in workers exposed to polycyclic aromatic hydrocarbons. Occup Environ Med. 1994 Jul;51(7):451–455. doi: 10.1136/oem.51.7.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira M., Jr, Buchet J. P., Burrion J. B., Moro J., Cupers L., Delavignette J. P., Jacques J., Lauwerys R. Determinants of urinary thioethers, D-glucaric acid and mutagenicity after exposure to polycyclic aromatic hydrocarbons assessed by air monitoring and measurement of 1-hydroxypyrene in urine: a cross-sectional study in workers of coke and graphite-electrode-producing plants. Int Arch Occup Environ Health. 1994;65(5):329–338. doi: 10.1007/BF00405698. [DOI] [PubMed] [Google Scholar]
- Granella M., Clonfero E. Urinary excretion of 1-pyrenol in automotive repair workers. Int Arch Occup Environ Health. 1993;65(4):241–245. doi: 10.1007/BF00381197. [DOI] [PubMed] [Google Scholar]
- Hansen A. M., Omland O., Poulsen O. M., Sherson D., Sigsgaard T., Christensen J. M., Overgaard E. Correlation between work process-related exposure to polycyclic aromatic hydrocarbons and urinary levels of alpha-naphthol, beta-naphthylamine and 1-hydroxypyrene in iron foundry workers. Int Arch Occup Environ Health. 1994;65(6):385–394. doi: 10.1007/BF00383249. [DOI] [PubMed] [Google Scholar]
- Hatjian B. A., Edwards J. W., Harrison J., Williams F. M., Blain P. G. Ambient, biological, and biological effect monitoring of exposure to polycyclic aromatic hydrocarbons (PAHs). Toxicol Lett. 1995 May;77(1-3):271–279. doi: 10.1016/0378-4274(95)03307-6. [DOI] [PubMed] [Google Scholar]
- Jongeneelen F. J., Anzion R. B., Henderson P. T. Determination of hydroxylated metabolites of polycyclic aromatic hydrocarbons in urine. J Chromatogr. 1987 Jan 23;413:227–232. doi: 10.1016/0378-4347(87)80230-x. [DOI] [PubMed] [Google Scholar]
- Jongeneelen F. J., van Leeuwen F. E., Oosterink S., Anzion R. B., van der Loop F., Bos R. P., van Veen H. G. Ambient and biological monitoring of cokeoven workers: determinants of the internal dose of polycyclic aromatic hydrocarbons. Br J Ind Med. 1990 Jul;47(7):454–461. doi: 10.1136/oem.47.7.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen L. E., Boisen T., Christensen J. M., Jelnes J. E., Jensen G. E., Jensen J. C., Lundgren K., Lundsteen C., Pedersen B., Wassermann K. Biomonitoring of genotoxic exposure among stainless steel welders. Mutat Res. 1992 May 16;279(2):129–143. doi: 10.1016/0165-1218(92)90255-x. [DOI] [PubMed] [Google Scholar]
- Knudsen L. E., Sorsa M. Human biological monitoring of occupational genotoxic exposures. Pharmacol Toxicol. 1993;72 (Suppl 1):86–92. doi: 10.1111/j.1600-0773.1993.tb01675.x. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983 Dec 16;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Omland O., Sherson D., Hansen A. M., Sigsgaard T., Autrup H., Overgaard E. Exposure of iron foundry workers to polycyclic aromatic hydrocarbons: benzo(a)pyrene-albumin adducts and 1-hydroxypyrene as biomarkers for exposure. Occup Environ Med. 1994 Aug;51(8):513–518. doi: 10.1136/oem.51.8.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovrebø S., Haugen A., Farmer P. B., Anderson D. Evaluation of biomarkers in plasma, blood, and urine samples from coke oven workers: significance of exposure to polycyclic aromatic hydrocarbons. Occup Environ Med. 1995 Nov;52(11):750–756. doi: 10.1136/oem.52.11.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peach H., Ellard G. A., Jenner P. J., Morris R. W. A simple, inexpensive urine test of smoking. Thorax. 1985 May;40(5):351–357. doi: 10.1136/thx.40.5.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera F. P., Dickey C., Santella R., O'Neill J. P., Albertini R. J., Ottman R., Tsai W. Y., Mooney L. A., Savela K., Hemminki K. Carcinogen-DNA adducts and gene mutation in foundry workers with low-level exposure to polycyclic aromatic hydrocarbons. Carcinogenesis. 1994 Dec;15(12):2905–2910. doi: 10.1093/carcin/15.12.2905. [DOI] [PubMed] [Google Scholar]
- Pilliere F., Levy F., Renier A., Brochard P., Jaurand M. C. Induction of DNA-repair synthesis (UDS) in rat pleural mesothelial cells by urine of subjects exposed to genotoxic agents. J Toxicol Clin Toxicol. 1992;30(2):223–238. doi: 10.3109/15563659209038634. [DOI] [PubMed] [Google Scholar]
- Popp W., Vahrenholz C., Schfll C., Kraus R., von Bülow J., Müller G., Norpoth K. Risk estimation in coke-oven workers by determining some biomarkers of carcinogen exposure. Exp Toxicol Pathol. 1995 Dec;47(6):440–442. doi: 10.1016/s0940-2993(11)80323-4. [DOI] [PubMed] [Google Scholar]
- Quinlan R., Kowalczyk G., Gardiner K., Calvert I. Exposure to polycyclic aromatic hydrocarbons in coal liquefaction workers: impact of a workwear policy on excretion of urinary 1-hydroxypyrene. Occup Environ Med. 1995 Sep;52(9):600–605. doi: 10.1136/oem.52.9.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rago R., Mitchen J., Wilding G. DNA fluorometric assay in 96-well tissue culture plates using Hoechst 33258 after cell lysis by freezing in distilled water. Anal Biochem. 1990 Nov 15;191(1):31–34. doi: 10.1016/0003-2697(90)90382-j. [DOI] [PubMed] [Google Scholar]
- Reid I. R., Veale A. G., France J. T. Glucocorticoid osteoporosis. J Asthma. 1994;31(1):7–18. doi: 10.3109/02770909409056764. [DOI] [PubMed] [Google Scholar]
- Renier A., Lévy F., Pillière F., Jaurand M. C. Unscheduled DNA synthesis in rat pleural mesothelial cells treated with mineral fibres. Mutat Res. 1990 Aug;241(4):361–367. doi: 10.1016/0165-1218(90)90066-b. [DOI] [PubMed] [Google Scholar]
- Rojas M., Alexandrov K., Auburtin G., Wastiaux-Denamur A., Mayer L., Mahieu B., Sebastien P., Bartsch H. Anti-benzo[a]pyrene diolepoxide--DNA adduct levels in peripheral mononuclear cells from coke oven workers and the enhancing effect of smoking. Carcinogenesis. 1995 Jun;16(6):1373–1376. doi: 10.1093/carcin/16.6.1373. [DOI] [PubMed] [Google Scholar]
- Van Hummelen P., Gennart J. P., Buchet J. P., Lauwerys R., Kirsch-Volders M. Biological markers in PAH exposed workers and controls. Mutat Res. 1993 Aug;300(3-4):231–239. doi: 10.1016/0165-1218(93)90055-i. [DOI] [PubMed] [Google Scholar]
- Van Rooij J. G., Van Lieshout E. M., Bodelier-Bade M. M., Jongeneelen F. J. Effect of the reduction of skin contamination on the internal dose of creosote workers exposed to polycyclic aromatic hydrocarbons. Scand J Work Environ Health. 1993 Jun;19(3):200–207. doi: 10.5271/sjweh.1322. [DOI] [PubMed] [Google Scholar]
- Van Rooij J. G., Veeger M. M., Bodelier-Bade M. M., Scheepers P. T., Jongeneelen F. J. Smoking and dietary intake of polycyclic aromatic hydrocarbons as sources of interindividual variability in the baseline excretion of 1-hydroxypyrene in urine. Int Arch Occup Environ Health. 1994;66(1):55–65. doi: 10.1007/BF00386580. [DOI] [PubMed] [Google Scholar]
- van Schooten F. J., Jongeneelen F. J., Hillebrand M. J., van Leeuwen F. E., de Looff A. J., Dijkmans A. P., van Rooij J. G., den Engelse L., Kriek E. Polycyclic aromatic hydrocarbon-DNA adducts in white blood cell DNA and 1-hydroxypyrene in the urine from aluminum workers: relation with job category and synergistic effect of smoking. Cancer Epidemiol Biomarkers Prev. 1995 Jan-Feb;4(1):69–77. [PubMed] [Google Scholar]


