Abstract
Background
Major depressive disorder (MDD), or depression, is a syndrome characterised by a number of behavioural, cognitive and emotional features. It is most commonly associated with a sad or depressed mood, a reduced capacity to feel pleasure, feelings of hopelessness, loss of energy, altered sleep patterns, weight fluctuations, difficulty in concentrating and suicidal ideation. There is a need for more effective and better tolerated antidepressants to combat this condition. Agomelatine was recently added to the list of available antidepressant drugs; it is a novel antidepressant that works on melatonergic (MT1 and MT2), 5‐HT 2B and 5‐HT2C receptors. Because the mechanism of action is claimed to be novel, it may provide a useful, alternative pharmacological strategy to existing antidepressant drugs.
Objectives
The objective of this review was 1) to determine the efficacy of agomelatine in alleviating acute symptoms of major depressive disorder in comparison with other antidepressants, 2) to review the acceptability of agomelatine in comparison with other antidepressant drugs, and, 3) to investigate the adverse effects of agomelatine, including the general prevalence of side effects in adults.
Search methods
We searched the Cochrane Collaboration's Depression, Anxiety and Neurosis Review Group's Specialised Register (CCDANCTR) to 31 July 2013. The CCDANCTR includes relevant randomised controlled trials from the following bibliographic databases: CENTRAL (the Cochrane Central Register of Controlled Trials) (all years), EMBASE (1974 onwards), MEDLINE (1950 onwards) and PsycINFO (1967 onwards). We checked reference lists of relevant studies together with reviews and regulatory agency reports. No restrictions on date, language or publication status were applied to the search. Servier Laboratories (developers of agomelatine) and other experts in the field were contacted for supplemental data.
Selection criteria
Randomised controlled trials allocating adult participants with major depression to agomelatine versus any other antidepressive agent.
Data collection and analysis
Two review authors independently extracted data and a double‐entry procedure was employed. Information extracted included study characteristics, participant characteristics, intervention details and outcome measures in terms of efficacy, acceptability and tolerability.
Main results
A total of 13 studies (4495 participants) were included in this review. Agomelatine was compared to selective serotonin reuptake inhibitors (SSRIs), namely paroxetine, fluoxetine, sertraline, escitalopram, and to the serotonin–norepinephrine reuptake inhibitor (SNRI), venlafaxine. Participants were followed up for six to 12 weeks. Agomelatine did not show any advantage or disadvantage over the other antidepressants for our primary outcome, response to treatment (risk ratio (RR) 1.01, 95% confidence interval (CI) 0.95 to 1.08, P value 0.75 compared to SSRIs, and RR 1.06; 95% CI 0.98 to 1.16, P value 0.16 compared to venlafaxine). Also, agomelatine showed no advantage or disadvantage over other antidepressants for remission (RR 0.83; 95% CI 0.68 to 1.01, P value 0.07 compared to SSRIs, and RR 1.08; 95% CI 0.94 to 1.24, P value 0.73 compared to venlafaxine). Overall, agomelatine appeared to be better tolerated than venlafaxine in terms of lower rates of drop outs (RR 0.40; 95% CI 0.24 to 0.67, P value 0.0005), and showed the same level of tolerability as SSRIs (RR 0.95; 95% CI 0.83 to 1.09, P value 0.44). Agomelatine induced a lower rate of dizziness than venlafaxine (RR 0.19, 95% CI 0.06 to 0.64, P value 0.007).
With regard to the quality of the body of evidence, there was a moderate risk of bias for all outcomes, due to the number of included unpublished studies. There was some heterogeneity, particularly between published and unpublished studies. The included studies were conducted in inpatient and outpatient settings, thus limiting the generalisability of the results to primary care settings. With regard to precision, the efficacy outcomes were precise, but the tolerability outcomes were mostly imprecise. Publication bias was variable and depended on the outcome of the trial. Our review included unpublished studies, and we think that this reduced the impact of publication bias. The overall methodological quality of the studies was not very good. Almost all of the studies were sponsored by the pharmaceutical company that manufactures agomelatine (Servier), and some of these were unpublished. Attempts to contact the pharmaceutical company Servier for additional information on all unpublished studies were unsuccessful.
Authors' conclusions
Agomelatine did not seem to provide a significant advantage in efficacy over other antidepressive agents for the acute‐phase treatment of major depression. Agomelatine was better tolerated than paroxetine and venlafaxine in terms of overall side effects, and fewer participants treated with agomelatine dropped out of the trials due to side effects compared to sertraline and venlafaxine, but data were limited because the number of included studies was small. We found evidence that compared agomelatine with only a small number of other active antidepressive agents, and there were only a few trials for each comparison, which limits the generalisability of the results. Moreover, the overall methodological quality of the studies was low, and, therefore, no firm conclusions can be drawn concerning the efficacy and tolerability of agomelatine.
Keywords: Adult; Humans; Acetamides; Acetamides/adverse effects; Acetamides/therapeutic use; Antidepressive Agents; Antidepressive Agents/adverse effects; Antidepressive Agents/therapeutic use; Depressive Disorder, Major; Depressive Disorder, Major/drug therapy; Melatonin; Melatonin/agonists; Randomized Controlled Trials as Topic; Selective Serotonin Reuptake Inhibitors; Selective Serotonin Reuptake Inhibitors/adverse effects; Selective Serotonin Reuptake Inhibitors/therapeutic use
Plain language summary
Agomelatine versus other antidepressant medication for depression
Why is this review important?
Major depression is a serious illness that can cause significant distress both to sufferers and their families. Major depression affects people's work, relationships and self‐esteem. It also affects people physically, changing their sleep patterns, concentration and appetite. The symptoms of major depression can lead people to feel hopeless and even suicidal. Antidepressant medications are an effective treatment option for major depression, but many have unpleasant side‐effects.
This review is important because it compares a new antidepressant, called agomelatine, with some other antidepressants used to treat major depression. Agomelatine works in a different way to existing antidepressants, it affects the hormone melatonin in the brain, and stimulates release of the brain chemicals dopamine and norepinephrine.
Who may be interested in this review?
People affected by major depression.
General Practitioners (GPs), psychiatrists and pharmacists.
Professionals working in adult mental health services.
Families and friends of people who suffer from major depression.
What questions does this review aim to answer?
Does agomelatine work better than other antidepressant medications?
Do patients tolerate agomelatine better than other antidepressants?
How do the side‐effects of agomelatine compare with other antidepressants?
Which studies were included in the review?
In July 2013, we used electronic medical databases to find all published and unpublished medical trials that compared agomelatine with any other antidepressant. We also contacted Servier Laboratories (the developers of agomelatine) for additional information. To be included in the review, medical trials had to have a randomised design (i.e. be randomised controlled trials), and have adult participants (aged over 18) with a diagnosis of major depression.
We identified 13 medical trials, involving a total of 4495 participants, that could be included in the review. The reviewers rated the overall quality of the trials as 'moderate'. Almost all of the trials included were sponsored by the pharmaceutical company that developed agomelatine (Servier), which could introduce bias (research shows that funding strongly affects the outcomes of research studies).
What does the evidence from the review tell us?
The review included trials comparing agomelatine with a group of antidepressants called selective serotonin reuptake inhibitors (SSRIs), and one antidepressant from the serotonin–norepinephrine reuptake inhibitor group, called venlafaxine. Participants in the studies were followed up for between six to 12 weeks.
‐ Agomelatine was no more or less effective in reducing symptoms of depression than any of the other antidepressants.
‐ Agomelatine was no more or less effective in preventing relapse of depression than any of the other antidepressants.
‐ Agomelatine was tolerated better than venlafaxine (fewer people discontinued treatment), but the same as the SSRIs.
‐ Agomelatine caused a lower rate of dizziness than venlafaxine.
‐ Agomelatine caused a lower rate of vomiting, nausea and sexual side‐effects than SSRIs.
What should happen next?
The reviewers conclude that agomelatine is not more effective than other antidepressants currently on the market. It did seem to be more tolerable to patients in terms of lower rates of some side‐effects, however, the quality of trials was low and there were only a few trials that compared agomelatine with each medication. No firm conclusion on agomelatine can be made because of problems with reporting of data in the trials included. The authors recommend that further trials of agomelatine versus placebo (dummy pill), particularly in primary care settings (where the majority of patient/practitioner contact take place, e.g. GP surgeries), should be carried out to improve the quality of evidence.
Summary of findings
Summary of findings for the main comparison. Agomelatine compared to SSRI for major depression.
| Agomelatine compared to SSRI for major depression | ||||||
| Patient or population: patients with major depression Settings: inpatients and outpatients Intervention: agomelatine Comparison: SSRI | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| SSRI | Agomelatine | |||||
| Response rates Number of participants showing a reduction of at least 50% on the Hamilton Depression Rating Scale for Depression, the Montgomery‐Asberg Depression Rating Scale or any other depression scale Follow‐up: 6 to 12 weeks | Study population | RR 1.01 (0.95 to 1.08) | 3826 (10 studies) | ⊕⊝⊝⊝ very low1,2 | ||
| 610 per 1000 | 616 per 1000 (579 to 658) | |||||
| Moderate | ||||||
| 557 per 1000 | 563 per 1000 (529 to 602) | |||||
| Remission rates The number of participants who achieved remission as defined by: a score of 7 or less on the 17‐item Hamilton Depression Rating Scale; 10 or less on the Montgomery‐Asberg Depression Rating Scale; 'not ill or borderline mentally ill' on the Clinical Global Impression ‐ Severity; or any other equivalent value on a depression scale defined by the authors Follow‐up: 6 to 12 weeks | Study population | RR 0.83 (0.68 to 1.01) | 3826 (10 studies) | ⊕⊝⊝⊝ very low1,2,3 | Most of the difference in heterogeneity existed between published and unpublished studies | |
| 363 per 1000 | 302 per 1000 (247 to 367) | |||||
| Moderate | ||||||
| 264 per 1000 | 219 per 1000 (180 to 267) | |||||
| Total drop outs Total number of participants who dropped out during the trial as a proportion of the total number of randomised participants Follow‐up: 6 to 12 weeks | Study population | RR 0.95 (0.83 to 1.09) | 3826 (10 studies) | ⊕⊝⊝⊝ very low1,2 | ||
| 189 per 1000 | 180 per 1000 (157 to 206) | |||||
| Moderate | ||||||
| 188 per 1000 | 179 per 1000 (156 to 205) | |||||
| Drop out due to inefficacy Number of participants who dropped out due to inefficacy during the trial, as a proportion of the total number of randomised participants Follow‐up: 6 to 12 weeks | Study population | RR 0.99 (0.71 to 1.37) | 3377 (9 studies) | ⊕⊝⊝⊝ very low1,2,3 | ||
| 44 per 1000 | 43 per 1000 (31 to 60) | |||||
| Moderate | ||||||
| 49 per 1000 | 49 per 1000 (35 to 67) | |||||
| Drop outs due to side effects Number of participants who dropped out due to side effects during the trial, as a proportion of the total number of randomised participants Follow‐up: 6 to 12 weeks | Study population | RR 0.68 (0.51 to 0.91) | 3377 (9 studies) | ⊕⊝⊝⊝ very low1,2 | ||
| 70 per 1000 | 47 per 1000 (35 to 63) | |||||
| Moderate | ||||||
| 65 per 1000 | 44 per 1000 (33 to 59) | |||||
| Total number of participants with side effects Total number of participants experiencing at least one side effect Follow‐up: 6 to 12 weeks | Study population | RR 0.91 (0.84 to 0.98) | 2490 (6 studies) | ⊕⊝⊝⊝ very low1,2 | ||
| 594 per 1000 | 540 per 1000 (499 to 582) | |||||
| Moderate | ||||||
| 611 per 1000 | 556 per 1000 (513 to 599) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low quality: we are very uncertain about the estimate | ||||||
1 Most studies were funded by the pharmaceutical company that manufactures agomelatine (Servier). Four out of ten studies were unpublished 2 The studies included in our review were conducted in inpatient and outpatient settings. Results may not be generalisable for a primary care setting 3 Heterogeneity is very high
Summary of findings 2. Agomelatine compared to SNRI for major depression.
| Agomelatine compared to SNRI for major depression | ||||||
| Patient or population: patients with major depression Settings: inpatients and outpatients Intervention: agomelatine Comparison: SNRI | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| SNRI | Agomelatine | |||||
| Response rates Number of participants showing a reduction of at least 50% on the Hamilton Depression Rating Scale for Depression, the Montgomery‐Asberg Depression Rating Scale or any other depression scale Follow‐up: 6 to 12 weeks | Study population | RR 1.06 (0.98 to 1.16) | 669 (3 studies) | ⊕⊝⊝⊝ very low1,2 | ||
| 727 per 1000 | 771 per 1000 (712 to 843) | |||||
| Moderate | ||||||
| 707 per 1000 | 749 per 1000 (693 to 820) | |||||
| Remission rates The number of participants who achieved remission as defined by: a score of 7 or less on the 17‐item Hamilton Depression Rating Scale; 10 or less on the Montgomery‐Asberg Depression Rating Scale; 'not ill or borderline mentally ill' on the Clinical Global Impression ‐ Severity; or any other equivalent value on a depression scale defined by the authors Follow‐up: 6 to 12 weeks | Study population | RR 1.08 (0.94 to 1.24) | 669 (3 studies) | ⊕⊝⊝⊝ very low1,2 | ||
| 454 per 1000 | 490 per 1000 (427 to 563) | |||||
| Moderate | ||||||
| 333 per 1000 | 360 per 1000 (313 to 413) | |||||
| Total drop outs Total number of participants who dropped out during the trial as a proportion of the total number of randomised participants Follow‐up: 6 to 8 weeks | Study population | RR 0.4 (0.24 to 0.67) | 392 (2 studies) | ⊕⊝⊝⊝ very low1,2 | ||
| 228 per 1000 | 91 per 1000 (55 to 153) | |||||
| Moderate | ||||||
| 258 per 1000 | 103 per 1000 (62 to 173) | |||||
| Drop out due to inefficacy Number of participants who dropped out due to inefficacy during the trial, as a proportion of the total number of randomised participants Follow‐up: 6 weeks | Study population | RR 1.01 (0.21 to 4.94) | 332 (1 study) | ⊕⊝⊝⊝ very low1,2 | ||
| 18 per 1000 | 18 per 1000 (4 to 89) | |||||
| Moderate | ||||||
| 18 per 1000 | 18 per 1000 (4 to 89) | |||||
| Drop outs due to side effects Number of participants who dropped out due to side effects during the trial, as a proportion of the total number of randomised participants Follow‐up: 6 to 12 weeks | Study population | RR 0.3 (0.15 to 0.59) | 608 (2 studies) | ⊕⊝⊝⊝ very low1,2 | ||
| 111 per 1000 | 33 per 1000 (17 to 66) | |||||
| Moderate | ||||||
| 109 per 1000 | 33 per 1000 (16 to 64) | |||||
| Total number of patients with side effects Total number of participants experiencing at least one side effect | Study population | RR 0.72 (0.44 to 1.18) | 611 (2 studies) | ⊕⊝⊝⊝ very low1,2,3 | ||
| 481 per 1000 | 346 per 1000 (211 to 567) | |||||
| Moderate | ||||||
| 471 per 1000 | 339 per 1000 (207 to 556) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low quality: we are very uncertain about the estimate | ||||||
1 The studies included in our review were conducted in inpatient and outpatient settings. Results may not be generalisable for a primary care setting 2 Most studies were funded by the pharmaceutical company that manufactures agomelatine (Servier) 3 There is high heterogeneity
Background
Description of the condition
Major depressive disorder (MDD), or depression, is a syndrome characterised by a number of behavioural, cognitive and emotional features. It is most commonly associated with a sad or depressed mood, a reduced capacity to feel pleasure, hopelessness, loss of energy, altered sleep patterns, weight fluctuations, difficulty in concentrating and suicidal ideation (APA 2000). MDD is among the most common medical illnesses. The National Co‐morbidity Survey Replication estimated the lifetime prevalence of MDD to be 16.2% and the 12‐month prevalence to be 6.6%, with a mean depressive episode duration of 16 weeks (Kessler 2003). Women were 1.7 times more likely than men to have MDD (Kessler 2003). MDD is associated with marked personal, social and economic morbidity; loss of functioning and productivity; and creates significant demands on service providers in terms of workload (NICE 2004). It is the third leading cause of burden from disease and accounts for 4.5% of all human disability (WHO 2008). Additionally, MDD is expected to show a rising trend in the next 20 years (WHO 2008).
Description of the intervention
The treatment of depression includes both psychological (most commonly cognitive behavioural and interpersonal therapies) and pharmacological therapies. With the arrival of selective serotonin reuptake inhibitors (SSRIs) in the 1980s, and subsequent new antidepressant drugs, the use of antidepressants to treat depression has increased greatly (Middleton 2001). SSRIs have been recommended for first‐line drug use in depression by most of the treatment guidelines (APA 2000a; Lam 2009; NICE 2004; Taylor 2009), largely because of having a better safety profile, rather than superior efficacy. SSRIs are not without problems. Their effect size has been found to be small in comparison to placebo, in mild to moderate depression (Fournier 2010). Moreover, in clinical trials, SSRIs are associated with sexual dysfunction in 30% to 70% of participants (Clayton 2006). SSRIs also cause difficulties in sleep and agitation (Dording 2002). Therefore, the search is on for better antidepressant drugs. The ideal medication for depression would be a drug with a high level of effectiveness and a favourable side‐effect profile. Until now there has been little evidence to support one antidepressant over another. A number of previous meta‐analyses have concluded that there are no significant differences in either efficacy or acceptability between the various second‐generation antidepressants currently on the market (AHRQ 2007; Hansen 2005), although a recent multiple treatment meta‐analysis of 12 new‐generation antidepressant drugs, which used both direct and indirect data, reported the possible superiority of sertraline and escitalopram in terms of efficacy and acceptability (Cipriani 2009).
Agomelatine was recently added to the list of available antidepressant drugs; it works on melatonergic (MT1 and MT2), 5‐HT2B and 5‐HT2C receptors. Since its mechanism of action is claimed to be novel, it may provide a useful alternative pharmacological strategy to existing antidepressant drugs. Its positive effect on the sleep‐wake cycle and lack of serious side effects, including sexual side effects, may provide added advantages (Dolder 2008). Agomelatine has been licensed in the EU for the treatment of depression since 2009 (EMEA 2009). Novartis discontinued the development of agomelatine for the US market in 2011 (Novartis 2012).
How the intervention might work
Agomelatine is a new antidepressant drug with a unique mechanism of action. It acts as an agonist on melatonin MT1 and MT2 receptors, and an antagonist on 5‐HT2B and 5‐HT2C receptors. The MT1 receptor directly inhibits firing of the neurons in the suprachiasmatic areas in the hypothalamus, regulating the amplitude of circadian rhythmicity, whilst the MT2 receptor is responsible for the entrainment of circadian rhythms (San 2008). Antagonism at 5‐HT2C receptor causes a postulated increase in frontal dopamine transmission in animal models (Millan 2003). Agomelatine has shown some antidepressant‐like activity in animal models of depression (Popoli 2009). It has been studied for major depression in adults at doses of 25 mg to 50 mg/day given in the evenings (Popoli 2009).
Why it is important to do this review
Agomelatine is a unique antidepressant drug that has been found to be effective when compared with placebo and other antidepressant drugs (Kasper 2008). Its efficacy and tolerability compared to other antidepressants have been assessed in a recent meta‐analyses of published trials (Singh 2011), and a pooled analysis of selected trials (Kasper 2013). Another systematic review of placebo controlled trials (Koesters 2013), questioned whether there is a clinically important difference between agomelatine and placebo. However, as shown by Howland 2011 and Koesters 2013, published evidence for agomelatine may be influenced by publication bias. Therefore, there is good reason to conduct a systematic quantitative review using both published and unpublished evidence for its comparative efficacy, and adverse effects, against other antidepressant drugs.
Objectives
The objective of this review was 1) to determine the efficacy of agomelatine in alleviating acute symptoms of major depressive disorder in comparison with other antidepressants, 2) to review the acceptability of agomelatine in comparison with other antidepressant drugs, and, 3) to investigate the adverse effects of agomelatine, including the general prevalence of side effects in adults.
Methods
Criteria for considering studies for this review
Types of studies
We included all randomised controlled trials using a parallel group design that compared agomelatine with other antidepressant agents as monotherapies. For cross‐over trials we included only the results from the first randomised period.
Types of participants
Participants of both sexes, aged 18 years or older, with a primary diagnosis of major depression.
We included studies that used any standardised criteria to define unipolar major depression. We expected that most studies would use DSM‐IV (APA 1994), DSM‐ IV‐TR (APA 2000), or ICD‐10 (WHO 1992). Older studies might have used ICD‐9 (WHO 1978), DSM‐III (APA 1980), DSM‐ III‐R (APA 1987), or other diagnostic systems. We excluded studies that used ICD‐9 because it does not have operationalised criteria, but only disease names and no diagnostic criteria. However, we included studies that used Feighner criteria (Feighner 1972), or Research Diagnostic Criteria (Spitzer 1978).
We included studies in which less than 20% of participants were suffering from bipolar depression, but we examined the validity of the decision in a sensitivity analysis.
We did not consider a concurrent secondary diagnosis of another psychiatric disorder as a criterion for exclusion, though we did consider a concurrent primary diagnosis of Axis I or II disorders as an exclusion criterion. We excluded participants with a concurrent DSM‐IV diagnosis of schizophrenia, delusional disorder, or a psychosis not otherwise specified. We excluded antidepressant trials in depressive participants with a serious concomitant medical illness.
Types of interventions
Experimental intervention
Agomelatine
Comparator interventions
Selective serotonin reuptake inhibitors (SSRIs; fluoxetine, fluvoxamine, citalopram, paroxetine, escitalopram)
Serotonin–norepinephrine reuptake inhibitors (SNRIs; venlafaxine, duloxetine, milnacipran)
Other antidepressive agents (tricyclic or heterocyclic antidepressants; monoamine oxidase inhibitors (MAOIs); newer agents (mirtazapine, bupropion, reboxetine); atypical antipsychotics in monotherapy (risperidone, paliperidone, olanzapine, quetiapine, aripiprazole, amisulpride, ziprasidone); non‐conventional (herbal products such as Hypericum).
In future updates, if studies become available, we will group the other antidepressants according to classes in further comparisons.
We applied no restrictions regarding dose, frequency, intensity or duration.
We excluded trials in which agomelatine was used as an augmentation strategy.
Types of outcome measures
Primary outcomes
Our primary outcome measure for efficacy was:
the number of participants who responded to treatment, showing a reduction of at least 50% on the Hamilton Rating Scale for Depression (HAM‐D) (Hamilton 1960), the Montgomery Asberg Depression Rating Scale (MADRS) (Montgomery 1979), or any other depression scale (e.g. the Beck Depression Inventory (Beck 1987), or the CES‐D scale (Radloff 1977)); or were 'much or very much improved' (score 1 or 2) on the Clinical Global Impression‐Improvement (CGI‐I) (Guy 1976).
Secondary outcomes
Our secondary outcome measures included:
the number of participants who achieved remission as defined by: a score of 7 or less on the 17‐item HAM‐D, or 8 or less on the longer version of HAM‐D; 10 or less on the MADRS; 'not ill or borderline mentally ill' on the CGI‐S (Guy 1976); or any other equivalent value on a depression scale defined by the authors. We preferred remission rates defined by the HAM‐D or MADRS scores.
group mean scores at endpoint on HAM‐D, MADRS, CGI‐S or any other depression rating scale score.
Acceptability was evaluated using the following outcome measures:
total drop‐out rate: i.e. total number of participants who dropped out during the trial as a proportion of the total number of randomised participants.
drop‐out rates due to inefficacy: i.e. number of participants who dropped out due to inefficacy during the trial as a proportion of the total number of randomised participants.
drop‐out rates due to side effects: i.e. number of participants who dropped out due to side effects during the trial as a proportion of the total number of randomised participants.
Tolerability was evaluated using the following outcome measures:
total number of participants experiencing at least some side effects.
-
total number of participants experiencing the following specific side effects:
sleepiness or drowsiness;
insomnia;
dry mouth;
constipation;
dizziness;
hypotension;
agitation or anxiety;
suicide wishes, gestures or attempts;
completed suicide;
vomiting or nausea;
diarrhoea;
sexual dysfunction;
abnormal liver function tests;
weight gain;
hypertension.
In order not to miss any relatively rare or unexpected yet important side effects, in the data extraction phase we collected all side‐effect data reported in the literature and discussed ways to summarize them later.
Search methods for identification of studies
The Cochrane Depression, Anxiety and Neurosis Review Group's Specialised Register (CCDANCTR)
The Cochrane Depression, Anxiety and Neurosis Group (CCDAN) maintains two clinical trials registers at the editorial base in Bristol, UK: a references register and a studies‐based register. The CCDANCTR‐References Register contains over 31,500 reports of RCTs in depression, anxiety and neurosis. Approximately 65% of these references have been tagged to individual, coded trials. The coded trials are held in the CCDANCTR‐Studies Register and records are linked between the two registers through the use of unique Study ID tags. Coding of trials is based on the EU‐Psi coding manual, using a controlled vocabulary (please contact the CCDAN Trials Search Co‐ordinator for further details). Reports of trials for inclusion in the Group's registers are collated from routine (weekly), generic searches of MEDLINE (1950 onwards), EMBASE (1974 onwards) and PsycINFO (1967 onwards); quarterly searches of the Cochrane Central Register of Controlled Trials (CENTRAL) and review‐specific searches of additional databases. Reports of trials are also sourced from international trials registers c/o the World Health Organization's (WHO) trials portal (the International Clinical Trials Registry Platform (ICTRP)), pharmaceutical companies, the handsearching of key journals, conference proceedings and other (non‐Cochrane) systematic reviews and meta‐analyses.
Details of CCDAN's generic search strategies (used to identify RCTs) can be found on the Group's website.
Electronic searches
The CCDANCTR‐Studies Register was searched using the following terms: Diagnosis = depress* and Intervention = agomelatine and Age group = (adult or aged or unclear or "not stated")
The CCDANCTR‐References Register was searched using free‐text terms to identify additional untagged/uncoded reports of RCTs: (depress* and agomelatine)
International trial registers were searched via the WHO International Clinical Trials Registry Platform (ICTRP), which includes access to Controlled‐Trials.com where Servier Laboratories (developers of agomelatine) register their trial protocols. The following search terms were used: (agomelatine or valdoxan or thymanax)
The literature search was last updated on 31 July 2013.
Searching other resources
Personal communication
We contacted experts in the field for information on unpublished or ongoing studies, or to request additional trial data. We contacted Servier Laboratories directly in Slough (UK) and Paris (France), but they failed to respond to our request for additional data.
Reference checking
We checked the reference lists of all included studies, relevant reviews and regulatory agency reports to identify additional studies missed from the original electronic searches, and also conducted a cited reference search on the Web of Science.
Data collection and analysis
Selection of studies
The selection of trials for inclusion in this systematic review was done by two of the review authors.
Both review authors inspected the search hits by reading the titles and the abstracts to see if they met the inclusion criteria. Possible doubts were resolved by consultation with each other. We obtained each potentially relevant study located in the search as a full article, then two review authors independently assessed each for inclusion, and, if there was disagreement sought resolution through discussion between review authors. The discordance in the selection of studies was calculated using Cohen’s Kappa (k) (Cohen 1960), a more robust measure than a simple per cent agreement calculation since it takes into account the agreement between review authors that occurs by chance. Where it was not possible to evaluate the study because of language problems or missing information, we have classified the study as a 'study awaiting classification' until we can obtain either a translation or further information. We have reported the reasons for exclusion of trials in the 'Characteristics of included studies' table.
Data extraction and management
Two review authors independently used a data extraction form to extract the data from included studies concerning participant characteristics (age, sex, severity of depression, study setting), intervention details (dosage, duration of study, sponsorship), study characteristics (blinding, allocation etc.) and outcome measures of interest. Again, any disagreement was resolved by consensus or by the third member of the review team. If necessary, we contacted authors of studies to obtain clarification.
Main comparisons
Agomelatine versus SSRIs
Agomelatine versus SNRIs
Agomelatine versus other antidepressive agents (see Types of interventions)
Where sufficient data were available, we presented the results grouped by substance as well.
Assessment of risk of bias in included studies
Again working independently, two authors assessed risk of bias using the tool described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). This tool encourages consideration of how the sequence was generated, how allocation was concealed, the integrity of blinding at outcome assessment, the completeness of outcome data, selective reporting and other biases. We also considered sponsorship bias.
The risk of bias, in each domain and overall, was assessed and categorised as either:
low risk of bias, i.e. plausible bias unlikely to seriously alter the results; or
high risk of bias, i.e. plausible bias that seriously weakens confidence in the results; or
unclear risk of bias, i.e. plausible bias that raises some doubt about the results.
If the assessors disagreed, the final rating was made by consensus or with the involvement of another member of the review group. Where insufficient details of randomisation and other characteristics of trials were provided, we contacted authors of the studies in order to obtain further information. We agreed to report non‐concurrence in quality assessment.
Measures of treatment effect
The main outcome result was response to treatment. The improvement is usually presented as either a change in a depression scale(s) (mean and standard deviation), or as a dichotomous outcome (responder or non‐responder, remitted or not‐remitted), or both.
(1) Binary or dichotomous data
For binary outcomes we calculated a standard estimation of the random‐effects model risk ratio (RR) and its 95% confidence interval (CI). It has been shown that RR is more intuitive than odds ratio (Boissel 1999), and odds ratios tend to be interpreted as RR by clinicians (Deeks 2000). This misinterpretation then leads to an overestimation of the impression of the effect. For statistically significant results we calculated the number needed to treat to benefit or harm statistic (NNTB or NNTH) and its 95% CI using Visual Rx (http://www.nntonline.net/), taking account of the event rate in the control group.
(2) Continuous data
(a) Summary statistics
It was likely that different studies would use a variety of depression rating scales; therefore we used standardised mean difference (SMD). If all included studies had used the same instrument, we would have used mean differences (MD).
(b) Endpoint versus change data
We preferred to use scale endpoint data, which typically cannot have negative values and are easier to interpret from a clinical point of view. When endpoint data were unavailable, we used the change data. If we used MD, we pooled results based on change data and endpoint data in the same analysis.
Unit of analysis issues
(1) Cross‐over trials
Cross‐over trials are trials in which all participants receive both the control and intervention treatment but not in the same order. The major problem with this design of trial is a carry‐over effect from the first phase to the second phase of the study, especially if the condition of interest is unstable (Elbourne 2002). As this is the case with depression, randomised cross‐over studies were eligible, but we would only use data up to the point of first cross‐over.
(2) Studies with multiple treatment groups
Where a study involved more than two agomelatine arms, especially two appropriate dose groups of an antidepressant drug, the different dose arms were pooled and considered to be one. For dichotomous outcomes sample sizes and the event numbers were summed across groups. For continuous outcomes, means and standard deviations were grouped using the methods described in Chapter 7 (Section 7.7.3.8) of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). In three‐armed trials with placebo groups, we only considered data from active treatments.
Dealing with missing data
We tried to contact the authors for all relevant missing data.
(1) Dichotomous outcomes
Response, or remission on treatment, was calculated using an intention‐to‐treat analysis (ITT). We followed the principle 'once randomised always analyse'. In trials where participants left the study before the intended endpoint, it was be assumed that they would have experienced a negative outcome. The validity of the above assumption was tested by sensitivity analysis, applying worst‐case and best‐case scenarios. When dichotomous outcomes were not reported, but the baseline mean and standard deviation on a depression scale were reported, we calculated the number of responding or remitted participants according to a validated imputation method (Furukawa 2005). We analysed the validity of the above approach by sensitivity analysis.
(2) Continuous outcomes
The Cochrane Handbook for Systematic Reviews of Interventions recommends avoiding imputations of continuous data and suggests that the data must be used in the form they were presented by the original authors. We preferred ITT data, when available, to 'per‐protocol analysis'.
(3) Skewed or qualitative data
We presented skewed and qualitative data descriptively.
We considered several strategies for skewed data. If papers reported a mean and standard deviation, and there was also an absolute minimum possible value for the outcome, we divided the mean by the standard deviation. If this was less than two, then we concluded that there was some indication of skewness. If it was less than one (that is the standard deviation was bigger than the mean) then skewness was almost certainly present. If papers had not reported the skewness and simply reported means, standard deviations and sample sizes, these numbers were used. We did this because there was a possibility that these data may not have been properly analysed, and can also be misleading; we conducted analyses with and without these studies. If the data had been log‐transformed for analysis, and the geometric means were reported, skewness was reduced. This is the recommended method of analysis of skewed data. If papers used non‐parametric tests and described averages using medians, they could not be pooled formally in the analysis. We followed the recommendation made by the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) that results of these studies are reported in a table in our review, along with all other papers. This meant that the data were not lost from the review and these results could be considered when drawing conclusions, even if they could not be pooled formally in the analyses.
(4) Missing statistics
When only P or standard error (SE) values were reported, we calculated standard deviations (SDs) (Altman 1996). In the absence of supplementary data after requests to the authors, we calculated the SDs according to a validated imputation method (Furukawa 2006). We examined the validity of these imputations in the sensitivity analyses. We applied ITT analyses, in which all the drop outs not included in the analyses were considered to be non‐responders. We examined the validity of this decision in the sensitivity analyses by applying worst‐case and best‐case scenarios. We presented symptom levels as either continuous (mean ± SD) or dichotomous outcomes (improved or not improved).
Assessment of heterogeneity
We followed the Cochrane Handbook for Systematic Reviews of Interventions' recommendations (I2 statistic values 0% to 40%: might be important; 30% to 60%: may represent moderate heterogeneity; 50% to 90%: may represent substantial heterogeneity; 75% to 100%: represent considerable heterogeneity). We also used Chi2 and its P value to determine the direction and magnitude of the treatment effects. In a meta‐analysis of few trials, Chi2 will be underpowered to detect heterogeneity, if it exists. Therefore, a P value of less than 0.10 was used as the threshold for statistical significance (Higgins 2011).
Assessment of reporting biases
Reporting biases arise when the dissemination of research findings is influenced by the nature and direction of results. These are described in section 10.1 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). A funnel plot is usually used to investigate publication bias. However, it has a limited role when there are only a few studies of similar size. Secondly, asymmetry of a funnel plot does not always reflect publication bias. We did not use funnel plots for outcomes if there were 10 or fewer studies, or if all studies were of similar size.
Data synthesis
We used a random‐effects model to calculate the treatment effects. We preferred the random‐effects model as it takes in to account differences between studies even when there is no evidence of statistical heterogeneity. It gives a more conservative estimate than the fixed‐effect model. We note that the random‐effects model gives added weight to small studies, which can either increase or decrease the effect size. We also did the analysis using a fixed‐effect model to see whether it changed the effect size markedly.
Subgroup analysis and investigation of heterogeneity
We note that subgroup analyses are often exploratory in nature and should be interpreted very cautiously: firstly, because they often involve multiple analyses and this can lead to false positive results; and secondly, because these analyses lack power and are more likely to result in false positive results. Bearing the above reservations in mind, we performed the following subgroup analyses.
Agomelatine dosing (fixed low dosage: 25 mg/day; fixed high dosage: 50 mg and above; flexible high dose and flexible low dose). The standard dose of agomelatine is 25 mg to 50 mg per day. There is evidence to suggest that low dose antidepressants may be associated with better outcomes both in terms of effectiveness and tolerability (Bollini 1999). Similarly, fixed versus flexible dose can also affect the estimate of treatment effectiveness (Khan 2003).
Severity of depression (mild, moderate, severe depression). This analysis might have been useful in assessing the efficacy of agomelatine in different subpopulations of participants divided by severity.
Treatment settings (primary care, psychiatric inpatients, or psychiatric outpatients). This analysis might have been useful in identifying whether agomelatine could have been more or less effective in different treatment settings.
Older participants (participants aged 65 years or more) separately from other adult participants. This analysis might have been useful for finding out whether agomelatine could have more or less efficacy among older participants.
Examination of 'wish bias' by comparing agomelatine as the investigational drug versus agomelatine as the comparator, as there is evidence to suspect that a new antidepressant might perform worse when used as a comparator than when used as an experimental agent (Barbui 2004).
Examination of publication bias by assessing response to agomelatine in unpublished studies versus response to agomelatine in published studies. This analysis might be useful in determining whether there is a significant difference in reported outcome in published studies versus unpublished studies, in order to be able to assess publication bias.
If groups within any of the subgroups were found to be significantly different from one another, we planned to run meta‐regressions for exploratory analyses of additive or multiplicative influences of the variables in question.
Sensitivity analysis
The following sensitivity analyses were planned a priori. By limiting the included studies to those of higher quality, we examined whether the results changed, and checked for the robustness of the observed findings.
Excluding trials with unclear concealment of random allocation or unclear double blinding, or both; and trials with inadequate concealment of random allocation.
Excluding trials with drop‐out rates greater than 20%.
Performing the worst‐case scenario ITT (i.e. all the participants in the experimental group experienced the negative outcome and all those allocated to the comparison group experienced the positive outcome) and the best‐case scenario ITT (i.e. all the participants in the experimental group experienced the positive outcome and all those allocated to the comparison group experienced the negative outcome).
Excluding trials for which the response rates had to be calculated via the imputation method (Furukawa 2005), and those for which the SD had to be borrowed from other trials (Furukawa 2006).
Excluding all cross‐over trials.
Excluding studies funded by the pharmaceutical company marketing agomelatine (Servier). This sensitivity analysis is particularly important in view of the repeated findings that funding strongly affects the outcomes of research studies (Als‐Nielsen 2003; Bhandari 2004; Lexchin 2003), and because industry sponsorship and authorship of clinical trial reports has increased over the last 20 years (Buchkowsky 2004).
Excluding studies in which there were any participants diagnosed with bipolar depression.
Our routine application of random‐effects and fixed‐effect models, as well as our secondary outcomes of remission rates and continuous severity measures, might be considered to be additional forms of sensitivity analyses.
'Summary of findings' table
A 'Summary of findings' (SoF) table was made for each comparison (agomelatine versus SSRI and agomelatine versus SNRI). Six outcomes were chosen to build the two tables, based on their importance (response rate, remission rates, total drop outs, drop out due to inefficacy, drop outs due to side effects, total number of participants experiencing at least one side effect). The SoF table is based on the GRADE (Grading of Recommendations Assessment Development and Evaluation) system (Rosenbaum 2010). The GRADE system is a formal way of evaluating the quality of evidence, using several parameters, in order to achieve transparency and simplicity (Guyatt 2008). Initially, evidence for each RCT is considered as high, and then downgraded for a variety of reasons, including study limitations, inconsistency of results, indirectness of evidence, imprecision and reporting bias. On the basis of these criteria, the evidence can be downgraded to moderate, low or very low (Guyatt 2008). The inclusion of SoF tables in Cochrane reviews improves understanding and rapid retrieval of key findings (Rosenbaum 2010).
Results
Description of studies
See Characteristics of included studies; Characteristics of excluded studies; Characteristics of studies awaiting classification; Characteristics of ongoing studies.
Results of the search
Initially, we identified 120 references, 88 through database searching and 32 through other sources. These included 28 duplicates that were excluded, and a further 44 records were excluded after screening of abstracts. We retrieved full‐text copies of the remaining 48 references for more detailed evaluation. Thirty‐five studies were excluded for a variety of reasons. In the end, 13 studies were included in the qualitative and quantitative synthesis. The literature search was last updated in July 2013.
See Figure 1 for more details on the result of the search.
1.

Study flow diagram
Included studies
A total of 13 studies was included in this systematic review. Four of these were unpublished trials carried out by a pharmaceutical company (Servier; CAGO2303; CL3‐022; CL3‐023; CL3‐024). Attempts to contact the pharmaceutical company (Servier) for additional information on all unpublished studies were unsuccessful. With the exception of one study (Martinotti 2012), requests to authors concerning missing data were also unsuccessful.
Design
All the included studies were randomised trials. Eleven were reported to be double blind, and one study used an open label parallel group design (Martinotti 2012). Five studies were three‐armed with agomelatine, an active comparator and placebo (CAGO2303; CL3‐022; CL3‐023; CL3‐024; Loo 2002a). Seven studies were two‐armed with agomelatine versus another antidepressant (Corruble 2013; Hale 2010; Kasper 2010; Kennedy 2008; Lemoine 2007; Martinotti 2012; Quera‐Salva 2011).
Sample sizes
Overall, the studies included 4495 participants in active treatment arms. Of these, 2457 were randomised to agomelatine. Of the remaining 2048 participants, 1701 were randomised to SSRIs: 862 to fluoxetine, 453 to paroxetine,159 to sertraline and 227 to escitalopram. The remaining 337 participants were randomised to an SNRI, venlafaxine. The mean sample size per arm was 156 participants (range 30 to 314). Only one study recruited fewer than 100 participants overall (Martinotti 2012).
Setting
All studies were multicentre trials. Three studies were conducted in a single nation, namely, the USA (CAGO2303), France (CL3‐022), and Italy (Martinotti 2012). One study was conducted in France and Spain (Lemoine 2007). One multinational study recruited Asian participants only (Shu 2013). The other studies were multinational across continents.
Three studies enrolled both inpatients and outpatients (CL3‐022; CL3‐023; CL3‐024). Eight studies recruited outpatients (Corruble 2013; Hale 2010; Kasper 2010; Kennedy 2008; Lemoine 2007; Martinotti 2012; Quera‐Salva 2011; Shu 2013). Two studies did not report the setting (CAGO2303; Loo 2002a).
Participants
All studies included participants with a diagnosis of major depressive disorder (MDD). One study also included participants with bipolar II depression (Loo 2002a), although the proportion suffering from this condition was no more than 3%. As per our protocol for the review, Loo 2002a was included in the present review as less than 20% of the participants in the study had bipolar disorder.
All studies randomised participants from 18 years of age, but the age ranges recruited varied: two studies recruited participants up to 70 years of age (CAGO2303; Corruble 2013); three studies recruited between the ages of 18 to 59 years (CL3‐022; CL3‐023; CL3‐024); four studies recruited between the ages of 18 to 60 (Kasper 2010; Kennedy 2008; Martinotti 2012; Quera‐Salva 2011); and the final four studies recruited between the ages of 18 to 65 (Hale 2010; Lemoine 2007; Loo 2002a; Shu 2013).
Interventions and comparators
Most studies compared agomelatine to SSRIs: three compared agomelatine to paroxetine (CAGO2303; CL3‐023; Loo 2002a); four compared agomelatine to fluoxetine (CL3‐022; CL3‐024; Hale 2010; Shu 2013); two compared agomelatine to escitalopram (Corruble 2013; Quera‐Salva 2011); and one compared agomelatine to sertraline (Kasper 2010). Three studies compared agomelatine to an SNRI, venlafaxine (Kennedy 2008; Lemoine 2007; Martinotti 2012).
Outcomes
Efficacy data (either as dichotomous or as continuous outcome) and tolerability/acceptability data were available for all 13 included studies and could be entered into a meta‐analysis.
All but one study used HAM‐D as rating scale for primary or secondary outcome measures; Kennedy 2008 used MADRS instead. Ten studies reported response rates: data for one study was provided by the authors upon request (Martinotti 2012), and we imputed response rates for the remaining two studies from continuous data (CL3‐023; CL3‐024). Seven studies reported remission rates: data for one study was provided by the authors upon request (Martinotti 2012), and we imputed remission rates for the remaining five studies from continuous data (CL3‐023; CL3‐024; Lemoine 2007; Quera‐Salva 2011; Shu 2013).
All but one study reported drop outs due to any reason (Kennedy 2008). Nine studies reported drop outs due to side effects (CAGO2303; CL3‐022; CL3‐023; Corruble 2013; Hale 2010; Kasper 2010; Loo 2002a; Quera‐Salva 2011; Shu 2013), and the same nine also reported drop outs due to inefficacy. Six studies reported the total number of participants who experienced side effects (CAGO2303; Hale 2010; Kasper 2010; Loo 2002a; Quera‐Salva 2011; Shu 2013).
Excluded studies
Overall, we excluded 20 studies from the systematic review because of the inclusion criteria. Nineteen of these did not have an active control group and one trial was withdrawn prior to enrolment (see Characteristics of excluded studies and Figure 1).
Ongoing studies
We identified six ongoing studies (see Characteristics of ongoing studies) (CL3‐060; CL3‐074; CL3‐083; GENRAS; Lundbeck 2011; NCT01483053).
Studies awaiting classification
We classified nine studies as awaiting classification (CL3‐027; CL3‐048; CL3‐062; CL3‐073; CRSC11003; CTRI/2011/04/001659; Karaiskos 2013; Montgomery 2004; Vasile 2011. Those trials were either published (Karaiskos 2013; Montgomery 2004), included in EMEA documents (CL3‐027; CL3‐048; CL3‐062; CL3‐073), retreived from Clinical Trial Registry India (CRSC11003; CTRI/2011/04/001659) or retreived from conference proceedings (Vasile 2011). One of the published studies was designed to examine withdrawal symptoms (Montgomery 2004); data for the first 12 weeks of the study, comparing agomelatine (25 mg/day) and paroxetine (20 mg/day) were not adequately reported to be included (see: Characteristics of studies awaiting classification). The other published study was rated as awaiting classification because it was unclear whether the trial was randomised (Karaiskos 2013); a request to the authors remains unanswered.
Risk of bias in included studies
See: Characteristics of included studies, Figure 2, Figure 3.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study
The overall methodological quality of the studies was not very good; every study judged as having high or unclear risk of bias in at least one domain (see Figure 2 and Figure 3 for summary graphs). Almost all of the studies were sponsored by the pharmaceutical company that manufactures agomelatine (Servier), and some of them were unpublished. This last source of bias should be taken into account when interpreting the results.
Allocation
Ten studies reported no details about the randomisation procedure and so were judged as having unclear risk of bias (CAGO2303; CL3‐022; CL3‐023; CL3‐024; Hale 2010; Kasper 2010; Kennedy 2008; Lemoine 2007; Loo 2002a; Shu 2013). One study reported that randomisation was non‐adaptive, balanced, and stratified on the centre and that an interactive computer‐based system allocated a therapeutic unit number (Martinotti 2012). One study reported that randomisation was balanced but, did not provide any more details on the sequence generation (Quera‐Salva 2011).
Blinding
All RCTs apart from Martinotti 2012 were reported as double blind and so were deemed to have low risk of bias. Martinotti 2012, which was an open‐label parallel group trial, was judged as having a high risk of bias in this domain. All but two double‐blind studies reported details of measures employed to ensure successful blinding (CAGO2303; Loo 2002a).
Incomplete outcome data
Overall, the drop out rate was around 18% for agomelatine and 19% for the control medication; this ranged from 10% in Quera‐Salva 2011 to 22% in CAGO2303. Three studies had a drop‐out rate of more than 20% (CAGO2303; CL3‐024; Loo 2002a).
Selective reporting
Study protocols were not available for all the studies. Data from CL3‐022; CL3‐023; CL3‐024 were limited, as we could not gain access to the full dataset because the studies were unpublished; attempts to obtain data from the pharmaceutical company manufacturing agomelatine (Servier) were unsuccessful. Loo 2002a, CL3‐022, CL3‐023 and CL3‐024 were not registered, thus increasing risk of selective reporting, and Martinotti 2012 did not report full data on adverse events. CL3‐022, CL3‐023, CL3‐024, Loo 2002a, Martinotti 2012 and Corruble 2013 were all judged as having a high risk of bias in this domain. The risk of bias in Shu 2013 was assessed as unclear, and the remaining studies were deemed as having low risk of bias.
Other potential sources of bias
All but two of the included RCTs were sponsored by the company that manufactures agomelatine (Servier) and so were assessed as having high risk of bias. Martinotti 2012 was funded by a research department without industry support and it was unclear whether Loo 2002a was sponsored or not, so these two studies were assessed as having unclear risk of bias in this domain.
Effects of interventions
We have reported the results of the present systematic review by grouping the comparators into three classes: SSRIs, SNRIs and other antidepressive agents. Where possible, each comparator is presented in subgroups.
Comparison one: agomelatine versus other SSRIs
Primary outcome
1.1 Efficacy ‐ number of participants who responded to treatment
There was no evidence that agomelatine was less or more effective than SSRIs as a whole (Mantel‐Haenszel risk ratio (RR) 1.01, 95% confidence interval (CI) 0.95 to 1.08, P value 0.75; 10 trials, 3826 participants), statistical heterogeneity was moderate (I² = 31%). Considering each comparator, there was no evidence that agomelatine was less or more effective than: paroxetine (RR 0.92, 95% CI 0.77 to 1.09, P value 0.33, I² = 56%; three trials, 1189 participants); fluoxetine (RR 1.01, 95% CI 0.92 to 1.11, P value 0.80, I² = 31%; four trials, 1862 participants); sertraline (RR 1.11, 95% CI 0.94 to 1.30, P value 0.23, one trial, 313 participants); or escitalopram (RR 1.05, 95% CI 0.95 to 1.16, P value 0.33, I² = 0%; two trials, 462 participants) (see Analysis 1.1 and Figure 4).
1.1. Analysis.
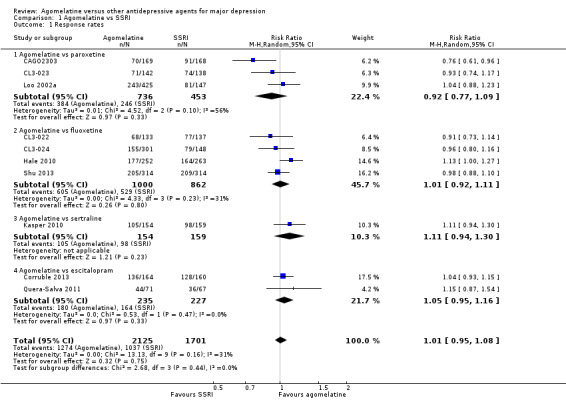
Comparison 1 Agomelatine vs SSRI, Outcome 1 Response rates.
4.

Forest plot of comparison: 1 Agomelatine vs SSRI, outcome: 1.1 Response rates
Secondary outcomes
1.2 Efficacy ‐ number of participants who achieve remission
There was no significant difference in the number of participants who achieved remission between agomelatine and SSRIs as a whole (RR 0.83, 95% CI 0.68 to 1.01, P value 0.07; ten trials, 3826 participants). Heterogeneity was substantial between studies (I² = 78%). There was no significant difference in the number of participants who achieved remission between: agomelatine and paroxetine (RR 0.61, 95% CI 0.32 to 1.18, P value 0.14, I² = 84%; three trials, 1189 participants); fluoxetine (RR 0.76, 95% CI 0.55 to 1.05, P value 0.10, I² = 84%; four trials, 1862 participants); sertraline (RR 1.12, 95% CI 0.80 to 1.58, P value 0.50; one trial, 313 participants); and escitalopram (RR 1.13, 95% CI 0.94 to 1.35, P value 0.20, I² = 0%; two trials, 462 participants) (see Analysis 1.2 and Figure 5).
1.2. Analysis.

Comparison 1 Agomelatine vs SSRI, Outcome 2 Remission rates.
5.

Forest plot of comparison: 1 Agomelatine vs SSRI, outcome: 1.2 Remission rates
1.3 Acceptability ‐ total drop‐out rate
There was no evidence that agomelatine was associated with a lower or higher total drop‐out rate than SSRIs as a whole (RR 0.95, 95% CI 0.83 to 1.09, P value 0.44, I² = 0%; ten trials, 3826 participants). There was no evidence that agomelatine was associated with a higher total drop‐out rate than: paroxetine (RR 1.01, 95% CI 0.80 to 1.28, P value 0.94; three trials, 1189 participants); fluoxetine (RR 0.96, 95% CI 0.74 to 1.26, P value 0.78, I² = 43%; four trials, 1862 participants); sertraline (RR 0.72, 95% CI 0.43 to 1.21, P value 0.21; one trial, 313 participants); and escitalopram (RR 0.81, 95% CI 0.50 to 1.32, P value 0.76, I² = 0%; two trials, 462 participants) (see Analysis 1.3 and Figure 6).
1.3. Analysis.

Comparison 1 Agomelatine vs SSRI, Outcome 3 Total drop outs.
6.
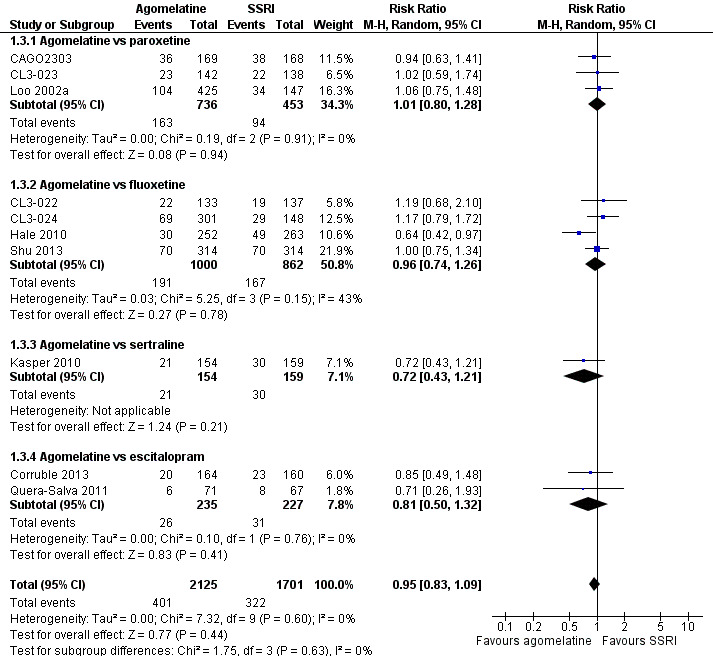
Forest plot of comparison: 1 Agomelatine vs SSRI, outcome: 1.3 Total drop outs
1.4 Acceptability ‐ drop‐out rates due to inefficacy
There was no evidence that agomelatine was associated with a higher drop‐out rate due to inefficacy than SSRIs as a whole (RR 0.99, 95% CI 0.71 to 1.37, P value 0.95, I² = 0%; nine trials, 3377 participants). There was no evidence that agomelatine was associated with a lower or higher drop‐out rate due to inefficacy than: paroxetine (RR 1.07, 95% CI 0.64 to 1.80, P value 0.80, I² = 0%; three trials, 1189 participants); fluoxetine (RR 0.97, 95% CI 0.57 to 1.65, P value 0.91, I² = 17%; three trials, 1413 participants); sertraline (RR 0.52, 95% CI 0.16 to 1.68, P value 0.27; one trial, 313 participants); or escitalopram (RR 1.34, 95% CI 0.43 to 4.21, P value 0.61, I² = 0%; two trials, 462 participants) (see Analysis 1.4).
1.4. Analysis.

Comparison 1 Agomelatine vs SSRI, Outcome 4 Drop out due to inefficacy.
1.5 Acceptability ‐ drop‐out rates due to side effects
There was evidence that fewer participants dropped out due to side effects when treated with agomelatine compared to other SSRIs as a whole (RR 0.68, 95% CI 0.51 to 0.91, P value 0.009, I² = 0%; nine studies, 3377 participants, NNTH = 47).
There was no evidence that fewer or more people dropped out due to side effects when treated with agomelatine compared to: paroxetine (RR 0.83, 95% CI 0.49 to 1.41, P value 0.49, I² = 0%; three studies, 1189 participants); fluoxetine (RR 0.74, 95% CI 0.50 to 1.09, P value 0.13, I² = 0%; three studies, 1413 participants); and escitalopram (RR 0.40, 95% CI 0.15 to 1.06, P value 0.07; two studies, 462 participants). There was some evidence (albeit with a small effect size) that agomelatine was associated with a lower drop‐out rate due to side effects compared to sertraline (RR 0.37, 95% CI 0.14 to 1.00, P value 0.05; one study, 313 participants, NNTH = 18) (see Analysis 1.5).
1.5. Analysis.

Comparison 1 Agomelatine vs SSRI, Outcome 5 Drop outs due to side effects.
1.6 Tolerability ‐ total number of participants experiencing at least some side effects
There was evidence (albeit with a small effect size) that fewer participants experienced side effects when treated with agomelatine than when treated with other SSRIs as a whole (RR 0.91, 95% CI 0.84 to 0.98, P value 0.01, I² = 28%; six studies, 2490 participants, NNTH = 23).
There was evidence that fewer participants experienced at least some side effects when treated with agomelatine compared to paroxetine (RR 0.86, 95% CI 0.78 to 0.94, P value 0.0010, I² = 0%; two studies, 905 participants, NNTH = 7). There was no evidence that fewer or more participants experienced at least some side effects when treated with agomelatine compared to either fluoxetine (RR 1.00, 95% CI 0.89 to 1.11, P value 0.95, I² = 0%; two studies, 1141 participants) or sertraline (RR 0.98, 95% CI 0.78 to 1.23, P value 0.86; one study, 307 participants). There was some evidence (albeit with a small effect size) that agomelatine was associated with a lower rate of participants experiencing side effects compared to escitalopram (RR 0.81, 95% CI 0.66 to 0.99, P value 0.04; one study, 137 participants, NNTH = 6). See Analysis 1.6.
1.6. Analysis.

Comparison 1 Agomelatine vs SSRI, Outcome 6 Total number of patients with side effects.
We also analysed the six outcomes above using a fixed‐effect model; this produced no change in significance levels and only negligible changes in effect sizes.
1.7 Tolerability ‐ total number of participants experiencing specific side effects
(a) Sleepiness or drowsiness
There was no evidence that agomelatine was associated with a lower or higher rate of participants experiencing sleepiness or drowsiness compared to all other SSRIs as a group (RR 0.96, 95% CI 0.43 to 2.15, P value 0.92, I² = 68%; five studies, 1868 participants).
There was no evidence that agomelatine was associated with a lower or higher rate of participants experiencing sleepiness or drowsiness than: paroxetine (RR 0.63, 95% CI 0.32 to 1.21, P value 0.16; two studies, 905 participants); fluoxetine (RR 1.75, 95% CI 0.78 to 3.93, P value 0.17; one study, 513 participants); or escitalopram (RR 0.19, 95% CI 0.02 to 1.55, P value 0.12; one study, 137 participants). There was evidence that agomelatine was associated with a higher rate of participants experiencing sleepiness or drowsiness compared to sertraline (RR 4.65, 95% CI 1.02 to 21.16, P value 0.05; one study, 313 participants, NNTH = 22) (see Analysis 1.7).
1.7. Analysis.

Comparison 1 Agomelatine vs SSRI, Outcome 7 Sleepiness or drowsiness.
(b) Insomnia
There was no evidence that agomelatine was associated with a lower or higher rate of participants experiencing Insomnia compared to other SSRIs as a group (RR 0.78, 95% CI 0.38 to 1.59, P value 0.49, I² = 14%; two studies, 1192 participants).
There was no evidence that agomelatine was associated with a lower or higher rate of participants experiencing insomnia than paroxetine (RR 0.54, 95% CI 0.21 to 1.38, P value 0.20; one study, 572 participants) or fluoxetine (RR 1.13, 95% CI 0.44 to 2.88, P value 0.81; one study, 620 participants) (see Analysis 1.8). No data were available for sertraline or escitalopram for this outcome.
1.8. Analysis.

Comparison 1 Agomelatine vs SSRI, Outcome 8 Insomnia.
(c) Dry mouth
There was no evidence that agomelatine was associated with a lower or higher rate of participants experiencing dry mouth compared to all other SSRIs as a group (RR 0.94, 95% CI 0.64 to 1.40, P value 0.77, I² = 0%; five studies, 2349 participants).
There was no evidence that agomelatine was associated with a higher rate of participants experiencing dry mouth than: paroxetine (RR 0.90, 95% CI 0.51 to 1.57, P value 0.71; two studies, 905 participants); fluoxetine (RR 0.96, 95% CI 0.49 to 1.89, P value 0.92, I² = 0%; two studies, 1133 participants); or sertraline (RR 1.05, 95% CI 0.40 to 2.72, P value 0.93; one study, 311 participants) (see Analysis 1.9). No data were available for escitalopram for this outcome.
1.9. Analysis.

Comparison 1 Agomelatine vs SSRI, Outcome 9 Dry mouth.
(d) Constipation
There was no evidence that agomelatine was associated with a higher rate of participants experiencing constipation than fluoxetine (RR 2.81, 95% CI 0.75 to 10.46, P value 0.12; one study, 513 participants). See Analysis 1.10. No data were available for paroxetine, sertraline and escitalopram for this outcome.
1.10. Analysis.

Comparison 1 Agomelatine vs SSRI, Outcome 10 Constipation.
(e) Dizziness
There was no evidence that agomelatine was associated with a lower or higher rate of participants experiencing dizziness compared to all other SSRIs as a group (RR 1.00, 95% CI 0.64 to 1.55; P value 0.99, I² = 3%; four studies, 1603 participants).
There was no evidence that agomelatine was associated with a lower or higher rate of participants experiencing dizziness than: paroxetine (RR 0.80, 95% CI 0.32 to 1.96, P value 0.62; one study, 333 participants); fluoxetine (RR 1.17, 95% CI 0.71 to 1.94, P value 0.54; I² = 0%; two studies, 1133 participants); or escitalopram (RR 0.23, 95% CI 0.03 to 2.03, P value 0.19; one study, 137 participants) (see Analysis 1.11). No data were available for sertraline for this outcome.
1.11. Analysis.

Comparison 1 Agomelatine vs SSRI, Outcome 11 Dizziness.
(f) Hypotension
None of the included studies reported data for this outcome.
(g) Agitation or anxiety
There was no evidence that agomelatine was associated with a lower or higher rate of participants experiencing agitation or anxiety compared to other SSRIs as a group (RR 1.02, 95% CI 0.46 to 2.27, P value 0.97, I² = 0%; two studies, 1192 participants).
There was no evidence that agomelatine was associated with a higher rate of participants experiencing agitation and anxiety than paroxetine (RR 1.21, 95% CI 0.40 to 3.62, P value 0.73; one study, 572 participants) or fluoxetine (RR 0.83; 95% CI 0.26 to 2.70, P value 0.76; one study, 620 participants) (see Analysis 1.12). No data were available for sertraline and escitalopram for this outcome.
1.12. Analysis.

Comparison 1 Agomelatine vs SSRI, Outcome 12 Agitation or anxiety.
(h) Suicide wishes, gestures or attempts
There was no evidence that agomelatine was associated with a higher rate of participants experiencing suicide wishes, gestures or attempts than paroxetine (RR 0.86, 95% CI 0.17 to 4.41, P value 0.86; one study, 572 participants) (see Analysis 1.13). No data were available for fluoxetine, sertraline and escitalopram for this outcome.
1.13. Analysis.

Comparison 1 Agomelatine vs SSRI, Outcome 13 Suicide wishes, gestures or attempts.
(i) Completed suicide
There was no evidence that agomelatine was associated with a higher rate of participants completing suicide than paroxetine (RR 0.35 , 95% CI 0.02 to 5.49, P value 0.45; one study, 572 participants) (see Analysis 1.14). No data were available for fluoxetine, sertraline and escitalopram for this outcome.
1.14. Analysis.

Comparison 1 Agomelatine vs SSRI, Outcome 14 Completed suicide.
(j) Vomiting or nausea
There was no evidence that agomelatine was associated with a lower rate of participants experiencing vomiting or nausea compared to all other SSRIs as a group (RR 0.70, 95% CI 0.33 to 1.45, P value 0.34, I² = 83%; five studies, 2175 participants).
There was evidence that agomelatine was associated with a lower rate of participants experiencing vomiting or nausea than paroxetine (RR 0.34, 95% CI 0.23 to 0.52, P value < 0.00001, I² = 0%; two studies, 905 participants, NNTH = 9) There was no evidence that agomelatine was associated with a lower rate of participants experiencing vomiting or nausea compared to fluoxetine (RR 1.54, 95% CI 0.30 to 7.90, P value 0.60, I² = 90%; two studies, 1133 participants) or escitalopram (RR 0.65, 95% CI 0.26 to 1.61, P value 0.35; one study, 137 participants) (see Analysis 1.15). No data were available for sertraline for this outcome.
1.15. Analysis.

Comparison 1 Agomelatine vs SSRI, Outcome 15 Vomiting or nausea.
(k) Diarrhoea
There was no evidence that agomelatine was associated with a lower or higher rate of participants experiencing diarrhoea compared to all other SSRIs as a group (RR 0.80, 95% CI 0.46 to 1.40, P value 0.43, I² = 0%; four studies, 1533 participants).
There was no evidence that agomelatine was associated with a lower or higher rate of participants experiencing diarrhoea than: paroxetine (RR 0.52, 95% CI 0.19 to 1.43, P value 0.21; one study, 572 participants); fluoxetine (RR 1.05, 95% CI 0.37 to 2.96, P value 0.92; one study, 513 participants); sertraline (RR 0.70, 95% CI 0.25 to 1.91, P value 0.48; one study, 311 participants); or escitalopram (RR 1.86, 95% CI 0.35 to 9.82, P value 0.47; one study, 137 participants) (see Analysis 1.16).
1.16. Analysis.

Comparison 1 Agomelatine vs SSRI, Outcome 16 Diarrhoea.
(l) Sexual dysfunction
There was evidence that agomelatine was associated with a lower rate of participants experiencing sexual dysfunction than paroxetine (RR 0.14, 95% CI 0.04 to 0.47, P value 0.001; one study, 333 participants, NNTH = 9) (see Analysis 1.17). No data were available for fluoxetine, sertraline and escitalopram for this outcome.
1.17. Analysis.
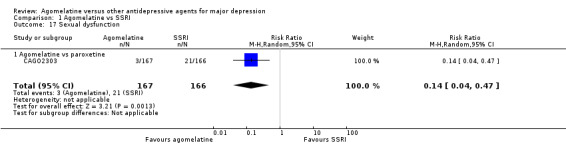
Comparison 1 Agomelatine vs SSRI, Outcome 17 Sexual dysfunction.
(m) Abnormal liver function tests
There was no statistically significant higher rate of abnormal liver function tests in participants treated with agomelatine compared to all other SSRIs as a group (RR 3.04, 95% CI 0.90 to 10.22, P value 0.07, I² = 0%; four studies, 1755 participants).
There was no statistically significant higher rate of abnormal liver function tests in participants treated with agomelatine compared to: paroxetine (RR 3.04, 95% CI 0.32 to 28.89, P value 0.33; one study, 318 participants); fluoxetine (RR 3.02, 95% CI 0.60 to 15.17, P value 0.18; two studies, 1124 participants); or sertraline (RR 3.10, 95% CI 0.13 to 75.44, P value 0.49; one study, 313 participants). See Analysis 1.18. No data were available for escitalopram for this outcome.
1.18. Analysis.

Comparison 1 Agomelatine vs SSRI, Outcome 18 Abnormal liver function tests.
(n) Weight gain
No data reported for this outcome.
(o) Hypertension
No data reported for this outcome.
1.8 Continuous outcome ‐ depression scale endpoint score
There was no evidence that agomelatine was associated with a statistically significant difference compared to the other SSRIs as a group (SMD 0.00; 95% CI ‐0.11 to 0.12, P value 0.94, I² = 66%; ten studies, 3457 participants). There was no evidence that agomelatine was associated with a lower or higher depression endpoint score compared to: paroxetine (SMD 0.16; 95% CI ‐0.11 to 0.43, P value 0.24, I² = 75%; three studies, 882 participants); fluoxetine (SMD ‐0.01; 95% CI ‐0.15 to 0.13, P value 0.87, I² = 54%; four studies, 1816 participants); or escitalopram (SMD ‐0.08; 95% CI ‐0.26 to 0.11, P value 0.42, I² = 0%; two studies, 453 participants).There was a small but statistically significant advantage of agomelatine compared to sertraline (SMD ‐0.23; 95% CI ‐0.46 to ‐0.01, P value 0.04; one study, 306 participants) (see Analysis 1.19).
1.19. Analysis.

Comparison 1 Agomelatine vs SSRI, Outcome 19 Depression scales endpoint score.
Comparison two: agomelatine versus other SNRIs
Primary outcome
2.1 Efficacy ‐ number of participants who responded to treatment
There was no evidence that agomelatine was less or more effective than venlafaxine (RR 1.06, 95% CI 0.98 to 1.16, P value 0.16, I² = 0%; three trials, 669 participants) (see Analysis 2.1 and Figure 7).
2.1. Analysis.

Comparison 2 Agomelatine vs SNRI, Outcome 1 Response rates.
7.

Forest plot of comparison: 2 Agomelatine vs SNRI, outcome: 2.1 Response rates
Secondary outcomes
2.2 Efficacy ‐ number of participants who achieved remission
There was no significant difference in the number of participants who achieved remission between agomelatine and venlafaxine (RR 1.08, 95% CI 0.94 to 1.24, P value 0.26, I² = 0%; three trials, 669 participants) (see Analysis 2.2 and Figure 8).
2.2. Analysis.

Comparison 2 Agomelatine vs SNRI, Outcome 2 Remission rates.
8.
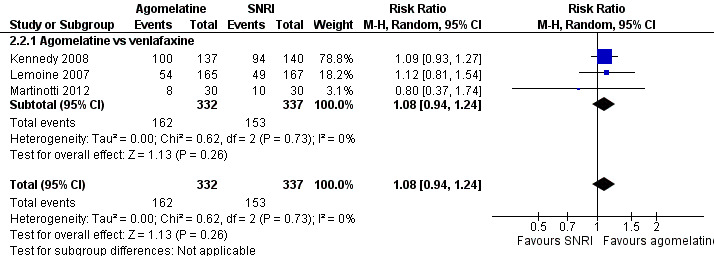
Forest plot of comparison: 2 Agomelatine vs SNRI, outcome: 2.2 Remission rates
2.3 Acceptability ‐ total drop‐out rate
There was evidence that agomelatine was associated with a lower total drop‐out rate than venlafaxine (RR 0.40, 95% CI 0.24 to 0.67, P value 0.0005, I² = 0%; two trials, 392 participants, NNTB = 2) (See Analysis 2.3 and Figure 9).
2.3. Analysis.
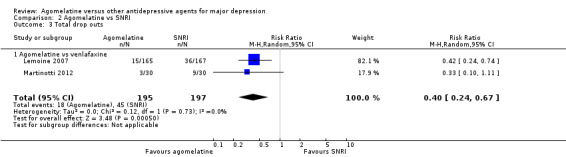
Comparison 2 Agomelatine vs SNRI, Outcome 3 Total drop outs.
9.

Forest plot of comparison: 2 Agomelatine vs SNRI, outcome: 2.3 Total drop outs
2.4 Acceptability ‐ drop‐out rates due to inefficacy
There was no evidence that agomelatine was associated with a lower or higher drop‐out rate due to inefficacy compared to venlafaxine (RR 1.01, 95% CI 0.21 to 4.94, P value 0.99; one trial, 332 participants) (see Analysis 2.4).
2.4. Analysis.

Comparison 2 Agomelatine vs SNRI, Outcome 4 Drop out due to inefficacy.
2.5 Acceptability ‐ drop‐out rates due to side effects
There was evidence that agomelatine was associated with a lower drop‐out rate due to side effects compared to venlafaxine (RR 0.30, 95% CI 0.15 to 0.59, P value 0.0006, I² = 0%; two trials, 608 participants, NNTH = 13) (see Analysis 2.5).
2.5. Analysis.

Comparison 2 Agomelatine vs SNRI, Outcome 5 Drop outs due to side effects.
2.6 Tolerability ‐ total number of participants experiencing at least some side effects
There was no evidence that fewer or more participants experienced at least some side effects compared to venlafaxine (RR 0.72, 95% CI 0.44 to 1.18, P value 0.19, I² = 80%; two trials, 611 participants) (see Analysis 2.6).
2.6. Analysis.

Comparison 2 Agomelatine vs SNRI, Outcome 6 Total number of patients with side effects.
We also analysed the six outcomes above using a fixed‐effect model and noted no change in significance levels and only negligible changes in effect sizes.
2.7 Tolerability ‐ total number of participants experiencing specific side effects
(a) Sleepiness or drowsiness
There was no evidence that agomelatine was associated with a higher rate of participants experiencing sleepiness or drowsiness than venlafaxine (RR 0.76, 95% CI 0.27 to 2.14, P value 0.60; one study, 334 participants) (see Analysis 2.7).
2.7. Analysis.

Comparison 2 Agomelatine vs SNRI, Outcome 7 Sleepiness or drowsiness.
(b) Insomnia
There was no evidence that agomelatine was associated with a higher rate of participants experiencing insomnia than venlafaxine (RR 0.25, 95% CI 0.03 to 2.24, P value 0.22; one study, 332 participants) (see Analysis 2.8).
2.8. Analysis.

Comparison 2 Agomelatine vs SNRI, Outcome 8 Insomnia.
(c) Dry mouth
There was no evidence that agomelatine was associated with a higher rate of participants experiencing dry mouth than venlafaxine (RR 0.51, 95% CI 0.13 to 1.99, P value 0.33; one study, 332 participants) (see Analysis 2.9).
2.9. Analysis.

Comparison 2 Agomelatine vs SNRI, Outcome 9 Dry mouth.
(d) Constipation
There was no evidence that agomelatine was associated with a higher rate of participants experiencing constipation than venlafaxine (RR 0.87, 95% CI 0.30 to 2.53, P value 0.79; one study, 332 participants) (see Analysis 2.10).
2.10. Analysis.

Comparison 2 Agomelatine vs SNRI, Outcome 10 Constipation.
(e) Dizziness
There was evidence that agomelatine was associated with a lower rate of participants experiencing dizziness than venlafaxine (RR 0.19, 95% CI 0.06 to 0.64, P value 0.007, one study, 332 participants, NNTH = 13) (see Analysis 2.11).
2.11. Analysis.

Comparison 2 Agomelatine vs SNRI, Outcome 11 Dizziness.
(f) Hypotension
No data were available for the comparison of agomelatine and venlafaxine for this outcome.
(g) Agitation or anxiety
No data were available for the comparison of agomelatine and venlafaxine for this outcome.
(h) Suicide wishes, gestures or attempts
No data were available for the comparison of agomelatine and venlafaxine for this outcome.
(i) Completed suicide
No data were available for the comparison of agomelatine and venlafaxine for this outcome.
(j) Vomiting or nausea
There was no evidence that agomelatine was associated with a higher rate of participants experiencing vomiting and nausea than venlafaxine (RR 0.42, 95% CI 0.17 to 1.08, P value 0.07, I² = 80%; two studies, 609 participants) (see Analysis 2.12).
2.12. Analysis.

Comparison 2 Agomelatine vs SNRI, Outcome 12 Vomiting or nausea.
(k) Diarrhoea
There was no evidence that agomelatine was associated with a higher rate of participants experiencing diarrhoea than venlafaxine (RR 2.70, 95% CI 0.73 to 10.00, P value 0.14; one study, 332 participants) (see Analysis 2.13).
2.13. Analysis.

Comparison 2 Agomelatine vs SNRI, Outcome 13 Diarrhoea.
(l) Sexual dysfunction
No data were available for the comparison of agomelatine and venlafaxine for this outcome.
(m) Abnormal liver function tests
No data were available for the comparison of agomelatine and venlafaxine for this outcome.
(n) Weight gain
No data were available for the comparison of agomelatine and venlafaxine for this outcome.
(o) Hypertension
No data were available for the comparison of agomelatine and venlafaxine for this outcome.
2.8 Continuous outcome ‐ depression scale endpoint score
There was no evidence that agomelatine was associated with a statistically significant difference compared to venlafaxine (SMD ‐0.07; 95% CI ‐0.22 to 0.08, P value 0.37, I² = 0%; three studies, 668 participants) (see Analysis 2.14).
2.14. Analysis.

Comparison 2 Agomelatine vs SNRI, Outcome 14 Depression scales endpoint score.
Comparison three: agomelatine versus other antidepressive agents
No studies were found comparing agomelatine with tricyclic or heterocyclic antidepressants, MAOIs, newer agents (mirtazapine, bupropion, reboxetine), atypical antipsychotics in monotherapy (risperidone, paliperidone, olanzapine, quetiapine, aripiprazole, amisulpride, ziprasidone) or non‐conventional antidepressive agents (herbal products such as Hypericum).
Subgroup analyses
Where possible, the pre‐planned subgroup analyses were conducted for the primary outcome. Results were interpreted with caution, because of the small number of studies included. Furthermore, multiple comparisons could lead to false positive conclusions (Oxman 1992).
(a) Agomelatine dosing (fixed low dosage: 25 mg/day; fixed high dosage: 50 mg and above; flexible high and flexible low)
Subgroup analyses of fixed versus flexible dosages were performed for the comparisons of agomelatine with SSRIs (Analysis 1.20), and agomelatine with venlafaxine (Analysis 2.15). There was no evidence for a difference between flexible or fixed dosage schemes for the former comparison (P value 0.42) or the latter (P value 0.74).
1.20. Analysis.

Comparison 1 Agomelatine vs SSRI, Outcome 20 Subgroup analysis: dosing ‐ response rates.
2.15. Analysis.

Comparison 2 Agomelatine vs SNRI, Outcome 15 Subgroup analysis: dosing ‐ response rates.
(b) Severity of depression (mild, moderate, severe depression)
Mean HAM‐D scores of the agomelatine groups ranged from 25.7 (CL3‐023), to 28.7 (Martinotti 2012). Kennedy 2008 reported a mean MADRS score of 17.9 at baseline. We defined 'severe depression' at a threshold of 25 or more for HAM‐D and 31 or more on MADRS (Müller 2003); this meant that all the trials, except for one (Kennedy 2008), were rated as trials in severe depression. The subgroup analysis was conducted, therefore, for agomelatine versus SNRIs only. There was no evidence (P value 0.59) of a difference in response rates of agomelatine compared to venlafaxine for severely depressed (RR 1.09, 95% CI 0.96 to 1.23, P value 0.18; two studies, 392 participants) or moderate depressed participants (RR 1.04, 95% CI 0.93 to 1.17, P value 0.50; one study, 277 participants) (see Analysis 2.16).
2.16. Analysis.

Comparison 2 Agomelatine vs SNRI, Outcome 16 Subgroup analysis: severity ‐ response rates.
(c) Treatment settings (primary care, psychiatric inpatients, or psychiatric outpatients)
No study was conducted in a primary care or inpatient setting only. One study did not report detailed setting information (CAGO2303). All other studies included outpatients only or in‐ and outpatients without separating the two groups. Therefore, it was not meaningful to carry out this subgroup‐analysis.
(d) Older participants (participants aged 65 years or more) versus other adult participants
None of the included studies specifically recruited older participants. Only one study recruited participants up to 70 years of age (CAGO2303), all others included participants under 65 years of age. Therefore, this subgroup analysis could not be performed.
(e) Examination of 'wish bias' by comparing agomelatine as the investigational drug versus agomelatine as the comparator
Only one study that used agomelatine as the investigational drug was not conducted by the manufacturer of agomelatine (Martinotti 2012). Accordingly, it was not considered meaningful to perform this subgroup analyses.
(f) Examination of publication bias by assessing response to agomelatine in unpublished studies versus response to agomelatine in published studies
Four unpublished studies were included in the review, all comparing agomelatine to SSRIs (see CAGO2303, CL3‐022, CL3‐023, CL3‐024 and Analysis 1.30). This analysis showed a significant difference between published and unpublished trials (P value 0.009), with a better outcome for participants in the published trials who were treated with agomelatine.
1.30. Analysis.

Comparison 1 Agomelatine vs SSRI, Outcome 30 Additional subgroup analysis: unpublished vs published trials ‐ response rates.
Sensitivity analyses
(a) Excluding trials with unclear concealment of random allocation or unclear double blinding, or both; and trials with inadequate concealment of random allocation
We rated all but two studies as having an unclear risk of bias for allocation concealment (Martinotti 2012; Quera‐Salva 2011). Martinotti 2012 compared agomelatine to venlafaxine (an SNRI), and Quera‐Salva 2011 compared agomelatine to escitalopram (an SSRI). Consequently, it was not considered meaningful to conduct this sensitivity analysis.
(b) Excluding trials with drop‐out rates greater than 20%
Four studies reported overall drop‐out rates of more than 20% and were excluded (CAGO2303; CL3‐024; Loo 2002a; Shu 2013). Kennedy 2008 did not report drop‐out rates and was also excluded. Martinotti 2012 reported drop‐out rates of exactly 20%. Excluding the studies with drop‐out rates over 20%, results from the sensitivity analysis changed the findings only marginally (see Analysis 1.21 and Analysis 2.17).
1.21. Analysis.

Comparison 1 Agomelatine vs SSRI, Outcome 21 Sensitivity analysis: excluding trials with > 20% drop outs ‐ response rates.
2.17. Analysis.

Comparison 2 Agomelatine vs SNRI, Outcome 17 Sensitivity analysis: excluding trials with > 20% drop outs.
(c) Worst‐case scenario and best‐case scenario ITT analyses
All studies calculated response and remission rates based on ITT population as defined by the authors. Therefore, participants who terminated their participation in the study early by dropping out could also be responders or remitters. Accordingly, we calculated the best‐ and worst‐case scenarios only for participants who were not included in the ITT analysis. Two studies did not report difference between ITT samples and randomised participants and were left unchanged (Lemoine 2007; Martinotti 2012). One study did not report the number of participants included in the ITT analysis and was also left unchanged (Corruble 2013). Although the analyses did not differ significantly from the main analyses (see Analysis 1.25; Analysis 1.26; Analysis 1.27; Analysis 1.28; Analysis 2.19; Analysis 2.20; Analysis 2.21; Analysis 2.22), the worst‐case scenario for remission rates showed a small but significant effect in favour of SSRIs (RR 0.73, 95% CI 0.57 to 0.94, P value 0.01, I² = 76%; ten studies, 3502 participants, see Analysis 1.28).
1.25. Analysis.

Comparison 1 Agomelatine vs SSRI, Outcome 25 Sensitivity analysis: response rates ‐ best case.
1.26. Analysis.

Comparison 1 Agomelatine vs SSRI, Outcome 26 Sensitivity analysis: response rates ‐ worst case.
1.27. Analysis.

Comparison 1 Agomelatine vs SSRI, Outcome 27 Sensitivity analysis: remission rates ‐ best case.
1.28. Analysis.

Comparison 1 Agomelatine vs SSRI, Outcome 28 Sensitivity analysis:remission rates ‐ worst case.
2.19. Analysis.

Comparison 2 Agomelatine vs SNRI, Outcome 19 Sensitivity analysis: response rates ‐ best case.
2.20. Analysis.

Comparison 2 Agomelatine vs SNRI, Outcome 20 Sensitivity analysis: response rates ‐ worst case.
2.21. Analysis.

Comparison 2 Agomelatine vs SNRI, Outcome 21 Sensitivity analysis: remission rates ‐ best case.
2.22. Analysis.

Comparison 2 Agomelatine vs SNRI, Outcome 22 Sensitivity analysis: remission rates ‐ worst case.
(d) Excluding trials for which the response or remission rates had to be calculated based on the imputation method and those for which the SD had to be borrowed from other trials
Response rates were imputed for two SSRI studies (CL3‐023; CL3‐024). The exclusion of these trials changed the results only marginally (Analysis 1.22). No response rates were imputed in studies that compared agomelatine to SNRIs.
1.22. Analysis.

Comparison 1 Agomelatine vs SSRI, Outcome 22 Sensitivity analysis: excluding imputed response rates.
Remission rates were imputed for four studies that compared agomelatine to SSRIs (CL3‐023; CL3‐024; Quera‐Salva 2011; Shu 2013), and one study that compared agomelatine to an SNRI (Lemoine 2007). The exclusion of these studies did not change the results of the overall estimates comparing agomelatine with SSRIs as a group or SNRIs substantially (Analysis 1.23 and Analysis 2.18). However, exclusion of one study, CL3‐023, led to a significant difference in the number of participants who achieved remission between agomelatine and paroxetine, with a lower number of remitters when treated with agomelatine (RR 0.90, 95% CI 0.67 to 1.21, P value 0.02; I² = 78%; two trials, 909 participants).
1.23. Analysis.

Comparison 1 Agomelatine vs SSRI, Outcome 23 Sensitivity analysis: excluding imputed remission rates.
2.18. Analysis.
Comparison 2 Agomelatine vs SNRI, Outcome 18 Sensitivity analysis: excluding imputed remission rates.
Post test SDs were imputed for one study (Corruble 2013). Exclusion of this study did not change the results significantly (see Analysis 1.24).
1.24. Analysis.

Comparison 1 Agomelatine vs SSRI, Outcome 24 Sensitivity analysis: excluding trials with imputed SDs.
(e) Excluding all cross‐over trials
This review did not include any cross‐over trials.
(f) Excluding studies funded by the pharmaceutical company that manufactures agomelatine
Only one of the included studies was not conducted by Servier, the manufacturer of agomelatine (Martinotti 2012), so it was not considered meaningful to conduct this sensitivity analysis.
(g) Excluding studies in which there were any participants diagnosed with bipolar depression
Only one study allowed the inclusion of bipolar participants (Loo 2002a). Exclusion of this study showed no difference between agomelatine and paroxetine (RR 0.84, 95% CI 0.70 to 1.03, P value 0.09, I² = 32%; two studies, 617 participants) (see Analysis 1.29).
1.29. Analysis.

Comparison 1 Agomelatine vs SSRI, Outcome 29 Sensitivity anal': excluding studies with bipolar participants ‐ response rates.
Reporting bias
We did not use funnel plots to investigate publication bias, because none of the comparisons contained more than 10 studies. The review included four unpublished trials (CAGO2303; CL3‐022; CL3‐023; CL3‐024), but the Studies awaiting classification section lists further unpublished trials. We added an additional subgroup analyses to compare published and unpublished trials (Analysis 1.30).
Discussion
Summary of main results
A total of 13 studies (4495 participants) were included in this review. Agomelatine does not seem to have any clinically significant advantage over other antidepressants, in terms of efficacy, for response or remission, for the acute phase treatment of major depression. It may even have some disadvantages compared to other antidepressants, in terms of remission. Overall, agomelatine appeared to be slightly better tolerated than venlafaxine, and showed the same level of tolerability as the other SSRIs examined (fluoxetine, sertraline, paroxetine and escitalopram). Agomelatine was associated with a lower rate of dizziness than venlafaxine, and a lower rate of vomiting and nausea than paroxetine. Agomelatine also appeared to be associated with less sexual dysfunction than paroxetine, although only one study was included in that analysis. With regard to liver function test abnormalities, the evidence for agomelatine compared to paroxetine, fluoxetine or sertraline, is inconclusive. Although our analysis did not reach statistical significance, all comparisons showed a risk ratio greater than 3, indicating an increased risk of liver function abnormalities in people treated with agomelatine, but only four studies could be included in these analyses. In the light of the recently updated contraindication for people with increased transaminase levels due to reports of liver injuries and hepatic failures (Servier 2013), it seems likely that these elevations are clinically relevant and masked in our review due to a reporting bias. Unfortunately, agomelatine was compared to a limited number of antidepressants, only SSRIs and venlafaxine, which limits the generalisability of the results. We found no clear evidence that agomelatine has advantages over other antidepressants. There is limited evidence that agomelatine could be more tolerable than venlafaxine, but this is based only on two studies with a limited number of events.
Overall completeness and applicability of evidence
The present review focused on the comparison between agomelatine and other antidepressants. This may limit the applicability of the findings as, ideally, an agent should be assessed against placebo. Data from seven unpublished studies were included in our review, but, given the number of completed studies awaiting assessment, we can not be sure that all unpublished material has been included. Furthermore, there was a high chance of selective reporting bias, especially for analysis of side effects. For example, data from placebo‐controlled studies submitted to the European Medicines Agency showed that agomelatine was consistently associated with a significantly higher incidence of abnormal liver function tests than placebo. Moreover, a recent review based on surveillance data from the French national monitoring system, EU periodic safety update reports (PSURs), and the European pharmacovigilance database concluded that the harms associated with agomelatine may outweigh its benefits (Prescrire 2013). Thus, the evidence is insufficient to guide treatment decisions based on considerations of side effects. Furthermore, the number of studies for each comparator was quite small, limiting the applicability of the findings. Indeed, only one trial included participants over 70 years of age, and no study was conducted in a primary care setting, which further limits the generalisability of the evidence. It may also be possible that the population selected, given the stringent exclusion criteria employed in the studies, may not have been representative of the 'real world' population. Often, 'real' patients with depressive disorder have several co‐morbidities (physical or psychiatric), and this may not be accurately reflected in the severity of the condition evident in participants in the RCTs.
Quality of the evidence
We chose six outcomes for assessing the quality of evidence and to construct the 'Summary of findings' tables (the chosen outcomes being response rates; remission rates; total drop outs; drop outs due to inefficacy; drop outs due to side effects; total number of participants with side effects). We constructed two separate 'Summary of findings' tables: one for agomelatine compared to SSRIs (Table 1), and one for agomelatine compared to SNRIs (Table 2). The quality of the evidence was low and outcomes may have been selectively reported, particularly in the unpublished material, which limits the generalisability of our findings. Ten out of 11 studies were sponsored by the pharmaceutical company that manufactures agomelatine (Servier). Evidence shows that sponsored trials may be associated with a more favourable outcome (Lundh 2012), so the results in this review should be considered with caution.
Study limitations (risk of bias)
We judged that the studies have a moderate risk of bias for all outcomes. There were several unpublished studies in our review, which may not have reported all the outcomes. Also, unpublished studies may not have been subject to peer review. We decided to downgrade the quality of evidence by one level accordingly.
Consistency of effect
Studies showed some heterogeneity, and our additional analysis revealed a significant difference between published and unpublished studies in the 'response rates' outcome; we decided to downgrade the quality of evidence by one level for this outcome. The other outcomes did not show any significant evidence of heterogeneity and, therefore, we did not downgrade the level of evidence.
Indirectness
The studies included in our review were conducted in inpatient and outpatient settings, so results may not be generalisable to the primary care setting. Therefore we downgraded the quality of evidence for all the outcomes, by one level.
Imprecision
The results on efficacy showed a variable level of precision depending on the outcome. With regard to the efficacy outcomes, the 'response rates' and 'remission rates' showed sufficient precision. The tolerability outcomes, on the other hand, were mostly imprecise. The 'total drop outs' and 'drop outs due to inefficacy' outcomes had large CIs. The 'drop outs due to side effects' outcome had a narrow confidence interval but the event rate was low. We decided to the downgrade for imprecision, based on the tolerability outcomes. The 'total number of participants with side effects' outcome, showed a narrow confidence interval in addition to a high event rate, so we did not downgrade it for imprecision.
Publication bias
In accordance with the protocol, publication bias was not formally assessed by funnel plot analyses, because all analyses included fewer than 10 studies. Our review included unpublished studies and we think that this reduced the impact of publication bias. Our additional subgroup analysis showed a clear difference between published and unpublished trials, and we are aware of a number of unpublished studies that we were not able to include. Therefore, our results may still be biased to some extent. Furthermore, these studies may raise further doubts with regard to the generalisabilty of the results. For example, one unpublished study compared agomelatine to paroxetine in older people (CL3‐048), and according to data from this study included in a pooled analysis (Kasper 2013), agomelatine was not more effective than paroxetine in this study.
Overall the quality of the evidence was quite low.
Potential biases in the review process
Some biases and limitations can be noted.
We tried to perform a thorough article search, however, it is possible that we missed some relevant studies. Some of the data from this review come from unpublished material, and it may be possible that more unpublished studies have not been identified.
Some of the included studies were not designed to test efficacy of agomelatine as a primary outcome (Lemoine 2007; Martinotti 2012; Quera‐Salva 2011), but focused on sleep (Lemoine 2007; Quera‐Salva 2011), and anhedonia (Martinotti 2012), though they did include data for efficacy and tolerability. This means that the applicability of the results may be limited, because the studies may not have been sufficiently powered to detect differences in depressive symptoms.
Agreements and disagreements with other studies or reviews
A recent pooled analysis on agomelatine compared to SSRIs and SNRIs has been published (Kasper 2013). This review is very similar in scope to our review, but the authors did not conduct a systematic literature review and only included trials without placebo control groups. The authors concluded that agomelatine can be used as first‐line treatment for depression, however, the study included only six of the studies that are analysed in the present review. Moreover, only one unpublished study was included, which could mean that Kasper 2013 is at greater risk of having publication bias than this systematic review. Another systematic review of published trials comparing agomelatine to placebo and SSRIs and SNRIs concluded that agomelatine had a marginal superior effect compared to other antidepressants (Singh 2011). Although the review included published trials only, the authors judged the effect to be 'fragile' and not clinically relevant. The review included only five of the studies included in our review and combined SSRIs and SNRIs as comparators.
Authors' conclusions
Implications for practice.
The results of this review suggest that agomelatine does not offer any significant advantage over other new antidepressant agents in the acute‐phase treatment of major depression (with the exception of venlafaxine in terms of tolerability and acceptability). In terms of tolerability, agomelatine did not show a statistically significant higher rate of liver function test abnormalities compared to paroxetine, fluoxetine, or sertraline for up to 12 weeks of initial treatment, but risk ratios for all comparisons were high. There is weak evidence that agomelatine may be tolerated better overall than venlafaxine and paroxetine. We only found evidence comparing agomelatine with a small number of other active antidepressive agents and found only a few trials for each comparison. This limits the generalisability of the results. It is important to point out that no firm conclusion regarding the efficacy and tolerability of agomelatine can be drawn, due the low quality of the studies included.
Implications for research.
Given the limited number of studies comparing agomelatine with other antidepressants, it is essential that more high quality randomised controlled trials (RCTs) are designed to compare agomelatine with other antidepressants. These RCTs should be independently funded. The studies included in the present review were relatively short‐term (no more than 12 weeks). No data from RCTs are available currently for agomelatine compared to other active treatments in the medium‐ to long‐term. There is a need to explore the use of agomelatine beyond 12 weeks, so future trials should be designed with longer follow‐up times. Also, the population included in trials in this review may not be representative of the population routinely seen in primary care clinics or mental health services. RCTs using less restrictive criteria would be useful for elucidating the role of agomelatine in a wider patient population.
Acknowledgements
CRG Funding Acknowledgement
The National Institute for Health Research (NIHR) is the largest single funder of the Cochrane Depression, Anxiety and Neurosis Group.
Disclaimer: the views and opinions expressed herein are those of the authors and do not necessarily reflect those of the NIHR, NHS or the Department of Health.
Data and analyses
Comparison 1. Agomelatine vs SSRI.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Response rates | 10 | 3826 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.95, 1.08] |
| 1.1 Agomelatine vs paroxetine | 3 | 1189 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.77, 1.09] |
| 1.2 Agomelatine vs fluoxetine | 4 | 1862 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.92, 1.11] |
| 1.3 Agomelatine vs sertraline | 1 | 313 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.94, 1.30] |
| 1.4 Agomelatine vs escitalopram | 2 | 462 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.95, 1.16] |
| 2 Remission rates | 10 | 3826 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.68, 1.01] |
| 2.1 Agomelatine vs paroxetine | 3 | 1189 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.32, 1.18] |
| 2.2 Agomelatine vs fluoxetine | 4 | 1862 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.55, 1.05] |
| 2.3 Agomelatine vs sertraline | 1 | 313 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.80, 1.58] |
| 2.4 Agomelatine vs escitalopram | 2 | 462 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.94, 1.35] |
| 3 Total drop outs | 10 | 3826 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.83, 1.09] |
| 3.1 Agomelatine vs paroxetine | 3 | 1189 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.80, 1.28] |
| 3.2 Agomelatine vs fluoxetine | 4 | 1862 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.74, 1.26] |
| 3.3 Agomelatine vs sertraline | 1 | 313 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.43, 1.21] |
| 3.4 Agomelatine vs escitalopram | 2 | 462 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.50, 1.32] |
| 4 Drop out due to inefficacy | 9 | 3377 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.71, 1.37] |
| 4.1 Agomelatine vs paroxetine | 3 | 1189 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.64, 1.80] |
| 4.2 Agomelatine vs fluoxetine | 3 | 1413 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.57, 1.65] |
| 4.3 Agomelatine vs sertraline | 1 | 313 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.16, 1.68] |
| 4.4 Agomelatine vs escitalopram | 2 | 462 | Risk Ratio (M‐H, Random, 95% CI) | 1.34 [0.43, 4.21] |
| 5 Drop outs due to side effects | 9 | 3377 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.51, 0.91] |
| 5.1 Agomelatine vs paroxetine | 3 | 1189 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.49, 1.41] |
| 5.2 Agomelatine vs fluoxetine | 3 | 1413 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.50, 1.09] |
| 5.3 Agomelatine vs sertraline | 1 | 313 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.14, 1.00] |
| 5.4 Agomelatine vs escitalopram | 2 | 462 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.15, 1.06] |
| 6 Total number of patients with side effects | 6 | 2490 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.84, 0.98] |
| 6.1 Agomelatine vs paroxetine | 2 | 905 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.78, 0.94] |
| 6.2 Agomelatine vs fluoxetine | 2 | 1141 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.89, 1.11] |
| 6.3 Agomelatine vs sertraline | 1 | 307 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.78, 1.23] |
| 6.4 Agomelatine vs escitalopram | 1 | 137 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.66, 0.99] |
| 7 Sleepiness or drowsiness | 5 | 1868 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.43, 2.15] |
| 7.1 Agomelatine vs paroxetine | 2 | 905 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.32, 1.21] |
| 7.2 Agomelatine vs fluoxetine | 1 | 513 | Risk Ratio (M‐H, Random, 95% CI) | 1.75 [0.78, 3.93] |
| 7.3 Agomelatine vs sertraline | 1 | 313 | Risk Ratio (M‐H, Random, 95% CI) | 4.65 [1.02, 21.16] |
| 7.4 Agomelatine vs escitalopram | 1 | 137 | Risk Ratio (M‐H, Random, 95% CI) | 0.19 [0.02, 1.55] |
| 8 Insomnia | 2 | 1192 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.38, 1.59] |
| 8.1 Agomelatine vs paroxetine | 1 | 572 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.21, 1.38] |
| 8.2 Agomelatine vs fluoxetine | 1 | 620 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.44, 2.88] |
| 9 Dry mouth | 5 | 2349 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.64, 1.40] |
| 9.1 Agomelatine vs paroxetine | 2 | 905 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.51, 1.57] |
| 9.2 Agomelatine vs fluoxetine | 2 | 1133 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.49, 1.89] |
| 9.3 Agomelatine vs sertraline | 1 | 311 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.40, 2.72] |
| 10 Constipation | 1 | 513 | Risk Ratio (M‐H, Random, 95% CI) | 2.81 [0.75, 10.46] |
| 10.1 Agomelatine vs fluoxetine | 1 | 513 | Risk Ratio (M‐H, Random, 95% CI) | 2.81 [0.75, 10.46] |
| 11 Dizziness | 4 | 1603 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.64, 1.55] |
| 11.1 Agomelatine vs paroxetine | 1 | 333 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.32, 1.96] |
| 11.2 Agomelatine vs fluoxetine | 2 | 1133 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.71, 1.94] |
| 11.3 Agomelatine vs escitalopram | 1 | 137 | Risk Ratio (M‐H, Random, 95% CI) | 0.23 [0.03, 2.03] |
| 12 Agitation or anxiety | 2 | 1192 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.46, 2.27] |
| 12.1 Agomelatine vs paroxetine | 1 | 572 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.40, 3.62] |
| 12.2 Agomelatine vs fluoxetine | 1 | 620 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.26, 2.70] |
| 13 Suicide wishes, gestures or attempts | 1 | 572 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.17, 4.41] |
| 13.1 Agomelatine vs paroxetine | 1 | 572 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.17, 4.41] |
| 14 Completed suicide | 1 | 572 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.02, 5.49] |
| 14.1 Agomelatine vs paroxetine | 1 | 572 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.02, 5.49] |
| 15 Vomiting or nausea | 5 | 2175 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.33, 1.45] |
| 15.1 Agomelatine vs paroxetine | 2 | 905 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.23, 0.52] |
| 15.2 Agomelatine vs fluoxetine | 2 | 1133 | Risk Ratio (M‐H, Random, 95% CI) | 1.54 [0.30, 7.90] |
| 15.3 Agomelatine vs escitalopram | 1 | 137 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.26, 1.61] |
| 16 Diarrhoea | 4 | 1533 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.46, 1.40] |
| 16.1 Agomelatine vs paroxetine | 1 | 572 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.19, 1.43] |
| 16.2 Agomelatine vs fluoxetine | 1 | 513 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.37, 2.96] |
| 16.3 Agomelatine vs sertraline | 1 | 311 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.25, 1.91] |
| 16.4 Agomelatine vs escitalopram | 1 | 137 | Risk Ratio (M‐H, Random, 95% CI) | 1.86 [0.35, 9.82] |
| 17 Sexual dysfunction | 1 | 333 | Risk Ratio (M‐H, Random, 95% CI) | 0.14 [0.04, 0.47] |
| 17.1 Agomelatine vs paroxetine | 1 | 333 | Risk Ratio (M‐H, Random, 95% CI) | 0.14 [0.04, 0.47] |
| 18 Abnormal liver function tests | 4 | 1755 | Risk Ratio (M‐H, Random, 95% CI) | 3.04 [0.90, 10.22] |
| 18.1 Agomelatine vs paroxetine | 1 | 318 | Risk Ratio (M‐H, Random, 95% CI) | 3.04 [0.32, 28.89] |
| 18.2 Agomelatine vs fluoxetine | 2 | 1124 | Risk Ratio (M‐H, Random, 95% CI) | 3.02 [0.60, 15.17] |
| 18.3 Agomelatine vs sertraline | 1 | 313 | Risk Ratio (M‐H, Random, 95% CI) | 3.10 [0.13, 75.44] |
| 19 Depression scales endpoint score | 10 | 3457 | Std. Mean Difference (IV, Random, 95% CI) | 0.00 [‐0.11, 0.12] |
| 19.1 Agomelatine vs paroxetine | 3 | 882 | Std. Mean Difference (IV, Random, 95% CI) | 0.16 [‐0.11, 0.43] |
| 19.2 Agomelatine vs fluoxetine | 4 | 1816 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.15, 0.13] |
| 19.3 Agomelatine vs sertraline | 1 | 306 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.23 [‐0.46, ‐0.01] |
| 19.4 Agomelatine vs escitalopram | 2 | 453 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.26, 0.11] |
| 20 Subgroup analysis: dosing ‐ response rates | 10 | 3826 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.95, 1.08] |
| 20.1 Flexible dosing | 6 | 2255 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.94, 1.12] |
| 20.2 Fixed dosing | 4 | 1571 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.88, 1.07] |
| 21 Sensitivity analysis: excluding trials with > 20% drop outs ‐ response rates | 5 | 1516 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.97, 1.16] |
| 21.1 Agomelatine vs paroxetine | 1 | 280 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.74, 1.17] |
| 21.2 Agomelatine vs fluoxetine | 2 | 785 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.84, 1.27] |
| 21.3 Agomelatine vs sertraline | 1 | 313 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.94, 1.30] |
| 21.4 Agomelatine vs escitalopram | 1 | 138 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.87, 1.54] |
| 22 Sensitivity analysis: excluding imputed response rates | 8 | 3097 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.95, 1.10] |
| 22.1 Agomelatine vs paroxetine | 2 | 909 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.67, 1.21] |
| 22.2 Agomelatine vs fluoxetine | 3 | 1413 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.91, 1.15] |
| 22.3 Agomelatine vs sertraline | 1 | 313 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.94, 1.30] |
| 22.4 Agomelatine vs escitalopram | 2 | 462 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.95, 1.16] |
| 23 Sensitivity analysis: excluding imputed remission rates | 6 | 2331 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.83, 1.14] |
| 23.1 Agomelatine vs paroxetine | 2 | 909 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.67, 0.97] |
| 23.2 Agomelatine vs fluoxetine | 2 | 785 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.67, 1.45] |
| 23.3 Agomelatine vs sertraline | 1 | 313 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.80, 1.58] |
| 23.4 Agomelatine vs escitalopram | 1 | 324 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.93, 1.35] |
| 24 Sensitivity analysis: excluding trials with imputed SDs | 8 | 2524 | Std. Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.15, 0.16] |
| 24.1 Agomelatine vs paroxetine | 3 | 882 | Std. Mean Difference (IV, Random, 95% CI) | 0.16 [‐0.11, 0.43] |
| 24.2 Agomelatine vs fluoxetine | 3 | 1207 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.23, 0.18] |
| 24.3 Agomelatine vs sertraline | 1 | 306 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.23 [‐0.46, ‐0.01] |
| 24.4 Agomelatine vs escitalopram | 1 | 129 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.21 [‐0.55, 0.14] |
| 25 Sensitivity analysis: response rates ‐ best case | 10 | 3826 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.99, 1.11] |
| 25.1 Agomelatine vs paroxetine | 3 | 1189 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.83, 1.11] |
| 25.2 Agomelatine vs fluoxetine | 4 | 1862 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.99, 1.14] |
| 25.3 Agomelatine vs sertraline | 1 | 313 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.98, 1.35] |
| 25.4 Agomelatine vs escitalopram | 2 | 462 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.93, 1.26] |
| 26 Sensitivity analysis: response rates ‐ worst case | 10 | 3826 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.92, 1.04] |
| 26.1 Agomelatine vs paroxetine | 3 | 1189 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.73, 1.07] |
| 26.2 Agomelatine vs fluoxetine | 4 | 1862 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.90, 1.07] |
| 26.3 Agomelatine vs sertraline | 1 | 313 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.92, 1.27] |
| 26.4 Agomelatine vs escitalopram | 2 | 462 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.94, 1.13] |
| 27 Sensitivity analysis: remission rates ‐ best case | 9 | 3502 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.72, 1.15] |
| 27.1 Agomelatine vs paroxetine | 3 | 1189 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.48, 1.17] |
| 27.2 Agomelatine vs fluoxetine | 4 | 1862 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.61, 1.30] |
| 27.3 Agomelatine vs sertraline | 1 | 313 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.87, 1.69] |
| 27.4 Agomelatine vs escitalopram | 1 | 138 | Risk Ratio (M‐H, Random, 95% CI) | 1.42 [0.74, 2.71] |
| 28 Sensitivity analysis:remission rates ‐ worst case | 9 | 3502 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.57, 0.94] |
| 28.1 Agomelatine vs paroxetine | 3 | 1189 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.29, 1.12] |
| 28.2 Agomelatine vs fluoxetine | 4 | 1862 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.52, 1.05] |
| 28.3 Agomelatine vs sertraline | 1 | 313 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.77, 1.50] |
| 28.4 Agomelatine vs escitalopram | 1 | 138 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.43, 1.43] |
| 29 Sensitivity anal': excluding studies with bipolar participants ‐ response rates | 2 | 617 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.70, 1.03] |
| 29.1 Agomelatine vs paroxetine | 2 | 617 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.70, 1.03] |
| 30 Additional subgroup analysis: unpublished vs published trials ‐ response rates | 10 | 3826 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.95, 1.08] |
| 30.1 Unpublished | 4 | 1336 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.81, 1.00] |
| 30.2 Published | 6 | 2490 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [1.00, 1.11] |
Comparison 2. Agomelatine vs SNRI.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Response rates | 3 | 669 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.98, 1.16] |
| 1.1 Agomelatine vs venlafaxine | 3 | 669 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.98, 1.16] |
| 2 Remission rates | 3 | 669 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.94, 1.24] |
| 2.1 Agomelatine vs venlafaxine | 3 | 669 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.94, 1.24] |
| 3 Total drop outs | 2 | 392 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.24, 0.67] |
| 3.1 Agomelatine vs venlafaxine | 2 | 392 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.24, 0.67] |
| 4 Drop out due to inefficacy | 1 | 332 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.21, 4.94] |
| 4.1 Agomelatine vs venlafaxine | 1 | 332 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.21, 4.94] |
| 5 Drop outs due to side effects | 2 | 608 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.15, 0.59] |
| 5.1 Agomelatine vs venlafaxine | 2 | 608 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.15, 0.59] |
| 6 Total number of patients with side effects | 2 | 611 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.44, 1.18] |
| 6.1 Agomelatine vs venlafaxine | 2 | 611 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.44, 1.18] |
| 7 Sleepiness or drowsiness | 1 | 334 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.27, 2.14] |
| 7.1 Agomelatine vs venlafaxine | 1 | 334 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.27, 2.14] |
| 8 Insomnia | 1 | 332 | Risk Ratio (M‐H, Random, 95% CI) | 0.25 [0.03, 2.24] |
| 8.1 Agomelatine vs venlafaxine | 1 | 332 | Risk Ratio (M‐H, Random, 95% CI) | 0.25 [0.03, 2.24] |
| 9 Dry mouth | 1 | 332 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.13, 1.99] |
| 9.1 Agomelatine vs venlafaxine | 1 | 332 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.13, 1.99] |
| 10 Constipation | 1 | 332 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.30, 2.53] |
| 10.1 Agomelatine vs venlafaxine | 1 | 332 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.30, 2.53] |
| 11 Dizziness | 1 | 332 | Risk Ratio (M‐H, Random, 95% CI) | 0.19 [0.06, 0.64] |
| 11.1 Agomelatine vs venlafaxine | 1 | 332 | Risk Ratio (M‐H, Random, 95% CI) | 0.19 [0.06, 0.64] |
| 12 Vomiting or nausea | 2 | 609 | Risk Ratio (M‐H, Random, 95% CI) | 0.42 [0.17, 1.08] |
| 12.1 Agomelatine vs venlafaxine | 2 | 609 | Risk Ratio (M‐H, Random, 95% CI) | 0.42 [0.17, 1.08] |
| 13 Diarrhoea | 1 | 332 | Risk Ratio (M‐H, Random, 95% CI) | 2.70 [0.73, 10.00] |
| 13.1 Agomelatine vs venlafaxine | 1 | 332 | Risk Ratio (M‐H, Random, 95% CI) | 2.70 [0.73, 10.00] |
| 14 Depression scales endpoint score | 3 | 668 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.22, 0.08] |
| 14.1 Agomelatine vs venlafaxine | 3 | 668 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.22, 0.08] |
| 15 Subgroup analysis: dosing ‐ response rates | 3 | 669 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.98, 1.16] |
| 15.1 Flexible dosing | 1 | 332 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.95, 1.23] |
| 15.2 Fixed dosing | 2 | 337 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.94, 1.17] |
| 16 Subgroup analysis: severity ‐ response rates | 3 | 669 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.98, 1.16] |
| 16.1 Agomelatine vs venlafaxine (moderate depression) | 1 | 277 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.93, 1.17] |
| 16.2 Agomelatine vs venlafaxine (severe depression) | 2 | 392 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.96, 1.23] |
| 17 Sensitivity analysis: excluding trials with > 20% drop outs | 2 | 392 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.96, 1.23] |
| 17.1 Agomelatine vs venlafaxine | 2 | 392 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.96, 1.23] |
| 18 Sensitivity analysis: excluding imputed remission rates | 2 | 337 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.93, 1.26] |
| 18.1 Agomelatine vs venlafaxine | 2 | 337 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.93, 1.26] |
| 19 Sensitivity analysis: response rates ‐ best case | 3 | 669 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.98, 1.16] |
| 19.1 Agomelatine vs venlafaxine | 3 | 669 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.98, 1.16] |
| 20 Sensitivity analysis: response rates ‐ worst case | 3 | 669 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.97, 1.15] |
| 20.1 Agomelatine vs venlafaxine | 3 | 669 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.97, 1.15] |
| 21 Sensitivity analysis: remission rates ‐ best case | 3 | 669 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.95, 1.25] |
| 21.1 Agomelatine vs venlafaxine | 3 | 669 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.95, 1.25] |
| 22 Sensitivity analysis: remission rates ‐ worst case | 3 | 669 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.94, 1.24] |
| 22.1 Agomelatine vs venlafaxine | 3 | 669 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.94, 1.24] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
CAGO2303.
| Methods | Multicentre (51 centres), double‐blind controlled trial, randomised, parallel group design Study conducted from 28 March 2007‐20 June 2008 Duration: 8 weeks Participants were randomised to 25 mg/day of agomelatine, 20 mg/day of paroxetine and placebo, in a 1:1:1 ratio |
|
| Participants | Men and women aged 18‐70 (inclusive) with MDD according to DSM‐IV criteria Eligibility criteria: HAMD‐17 score of 22 and over Age: 18‐70 years Sample size: agomelatine = 169; paroxetine = 168; placebo = 166 Setting: unclear Country: USA |
|
| Interventions | Agomelatine (25 mg/day) Paroxetine (20 mg/day) Placebo (identically coated tablets) Participants who had not improved by Week 4 received an increase to dose level 2 (agomelatine 50 mg/day or paroxetine 40 mg/day or matching placebo) The core study comprised a pre‐randomisation phase and a 8‐week double‐blind phase, followed by a 1‐week double‐blind taper phase. Participants who did not enrol into the open‐label extension phase and participants who prematurely discontinued were required to attend a follow‐up interview |
|
| Outcomes | Primary efficacy variables: ‐ Change from baseline to Week 8 on HAMD‐17 total score Secondary efficacy variables: ‐ Change from baseline to Week 8 (LOCF) on the total score of the Arizona Sexual Experience Scale ‐ Proportion of participants with clinical improvement as defined by a score of 1 or 2 in CGI at Week 8 (LOCF) ‐ Proportion of participants who achieved clinical remission as defined by a total score of ≤ 7 on HAMD‐17 (LOCF) ‐ Symptoms of anxiety and depression as measured by the change from baseline to Week 8 (LOCF) on the total score of the HADS Safety variables: ‐ Frequency of adverse events and serious adverse events during the 8‐week treatment period ‐ Changes in laboratory values during the 8‐week treatment period ‐ Changes in ECGs during the 8‐week treatment period ‐ Changes in vitals signs during the 8‐week treatment period |
|
| Notes | Unpublished study. No details on study authors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: no information provided |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: " . . . Matching placebo film‐coated oral tablets and paroxetine 20 mg", " . . . double‐blind trial". Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: " . . . double blind" Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: " . . . the primary efficacy analysis was performed on the ITT population" Attrition was balanced between groups |
| Selective reporting (reporting bias) | Low risk | The trial was registered, all mentioned outcomes listed in the report. However, no protocol was available. Adverse effects were reported only if the incidence was at least 2% per group, but serious adverse effects were reported |
| Other bias | High risk | Study funded by Servier, the drug company manufacturing agomelatine |
CL3‐022.
| Methods | Multicenter (74 centres), randomised, parallel group, double‐blind controlled trial Study conducted from September 1999‐August 2001 Duration: 6 weeks The randomisation of treatments (agomelatine, placebo, fluoxetine) was non‐adaptive, non‐centralised, and balanced with a 1:1:1 ratio. There was no stratification and permutation blocks were of fixed size = 6 |
|
| Participants | Men or women suffering from a single or recurrent episode of MDD according to DSM‐IV criteria, with or without melancholic features, without atypical features, and without psychotic features Eligibility criteria: HAM‐D total score of 22 or over. The decrease in HAM‐D total score should not be more than 20% between start of run‐in and inclusion visits, and a severity of illness ≥ 4 on the CGI Age: 18‐59 years (inclusive) Sample size: agomelatine = 133; fluoxetine = 137; placebo = 149 Setting: inpatients and outpatients Country: France |
|
| Interventions | Agomelatine (25 mg/day) Fluoxetine (25 mg/day) Placebo The study comprised a single‐blind run‐in placebo period with a duration ranging from 7‐14 days, an active double‐blind placebo‐controlled treatment period of 6 weeks (W0‐W6), an optional double‐blind placebo‐controlled extension treatment period of 18 weeks (W6‐W24), and a follow‐up period of 1 week without treatment (W7 or W25) |
|
| Outcomes | Primary efficacy variable: ‐ last post‐baseline value of HAM‐D total score assessed by the investigator Secondary efficacy variables: ‐ CGI ‐ MADRS ‐ HAM‐A ‐ LSEQ All but the LSEQ were assessed by the investigator |
|
| Notes | Unpublished study. No details on study authors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: " . . . The randomisation of treatments [ . . . ] was non‐adaptive, non‐centralised, and balanced with a 1:1:1 ratio. There was no stratification and permutation blocks were of fixed size = 6". Comment: probably done |
| Allocation concealment (selection bias) | Unclear risk | Quote: " . . . non centralised" Comment: not enough information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: " . . . agomelatine, placebo and fluoxetine [ . . . ] were disguised in tablets or capsules (or tablets) of identical appearance and taste" Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: " . . . double blind" Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The attrition was balanced between groups. However, no more information was provided |
| Selective reporting (reporting bias) | High risk | The study was a trial of a study conducted by the pharmaceutical company (Servier) to obtain approval of agomelatine in Europe, from EMA. Data are from EMA report only, and no protocol is available |
| Other bias | High risk | Trial sponsored by drug manufacturer The pharmaceutical company (Servier) was contacted, but they were not able to provide us with any additional data |
CL3‐023.
| Methods | Multicentre, randomised, double‐blind, parallel group, controlled trial Study conducted from December 1999‐September 2001 Duration: 6 weeks The randomisation of treatments (agomelatine, placebo, paroxetine) was non‐adaptive, non‐centralised, and balanced with a 1:1:1 ratio. There was no stratification and permutation blocks were of fixed size = 6 |
|
| Participants | Men and women suffering from a single or recurrent episode of MDD according to DSM‐IV criteria, with or without melancholic features, without atypical features, and without psychotic features Eligibility criteria: HAM‐D Total score of ≥ 22 Age: 18‐59 years (inclusive) Sample size: agomelatine = 142; paroxetine = 138; placebo = 137 Setting: inpatients and outpatients Country: international (countries not stated) |
|
| Interventions | Agomelatine (25 mg/day) Paroxetine (20 mg/day) Placebo The study comprised a single blind run‐in placebo period with duration ranging from 7‐14 days, an active double‐blind placebo‐controlled treatment period of 6 weeks (W0‐W6), an optional double‐blind placebo‐controlled extension treatment period of 18 weeks (W6‐W24), and a follow‐up period of 1 week without treatment (W7 or W25) |
|
| Outcomes | Primary efficacy variable: ‐ Last post‐baseline value of HAM‐D total score assessed by the investigator Secondary efficacy variables: ‐ CGI ‐ MADRS ‐ HAM‐A ‐ LSEQ All but the LSEQ were assessed by the investigator |
|
| Notes | Unpublished study. No details on study authors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: " . . . The randomisation of treatments [. . . ] was non‐adaptive, non‐centralised, and balanced with a 1:1:1 ratio. There was no stratification and permutation blocks were of fixed size = 6" Comment: probably done |
| Allocation concealment (selection bias) | Unclear risk | Quote: ". . . non centralised" Comment: not enough information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: ". . . Agomelatine, placebo and [. . . ] paroxetine were disguised in tablets or capsules (or tablets) of identical appearance and taste" Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: ". . . double blind". Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Attrition was balanced between groups, however, no further information was provided |
| Selective reporting (reporting bias) | High risk | The study was a trial of a study conducted by the pharmaceutical company (Servier) to obtain approval for agomelatine in Europe, from EMA. Data are from EMA report only, and no protocol is available |
| Other bias | High risk | The pharmaceutical company (Servier) was contacted, but they were not able to provide us with any additional data |
CL3‐024.
| Methods | Multicentre, randomised, parallel group, double‐blind controlled trial Study conducted from June 2000‐May 2002 Duration: 6 weeks The randomisation of treatments (agomelatine, placebo, fluoxetine) was non‐adaptive, non‐centralised, and balanced with a 1:1:1 ratio. There was no stratification and permutation blocks were of fixed size = 6 |
|
| Participants | Men or women suffering from a single or recurrent episode of MDD according to DSM‐IV criteria, with or without melancholic features, without atypical features, and without psychotic features Eligibility criteria: HAM‐D Total score of ≥ 22 Age: 18‐59 years (inclusive) Sample size: agomelatine 25 mg/d = 150; agomelatine 50 mg/d = 151; fluoxetine = 148; placebo = 158 Setting: inpatients and outpatients Country: multinational (countries not stated) |
|
| Interventions | Agomelatine (25 mg/day) Agomelatine (50 mg/day) Fluoxetine (20 mg/day) Placebo The study comprised a single blind run‐in placebo period with a duration ranging from 7‐14 days, an active double‐blind placebo‐controlled treatment period of 6 weeks (W0‐W6), an optional double‐blind placebo‐controlled extension treatment period of 18 weeks (W6‐W24), and a follow‐up period of 1 week without treatment (W7 or W25) |
|
| Outcomes | Primary efficacy variable: ‐ Last post‐baseline value of HAM‐D total score assessed by the investigator Secondary efficacy variables: ‐ CGI ‐ MADRS ‐ HAM‐A ‐ LSEQ All but the LSEQ were assessed by the investigator |
|
| Notes | Unpublished study. No details on study authors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: " . . . The randomisation of treatments [. . . ] was non‐adaptive, non‐centralised, and balanced with a 1:1:1 ratio. There was no stratification and permutation blocks were of fixed size = 6" Comment: probably done |
| Allocation concealment (selection bias) | Unclear risk | Quote: ". . . non centralised" Comment: not enough information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: ". . . agomelatine, placebo and [. . .] paroxetine were disguised in tablets or capsules (or tablets) of identical appearance and taste" Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: ". . . double blind". Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Attrition was balanced between groups. However, no more information was provided |
| Selective reporting (reporting bias) | High risk | The study was a trial of a study conducted by the pharmaceutical company Servier to obtain approval of agomelatine in Europe, from EMA. Data are from EMA report only, and no protocol is available |
| Other bias | High risk | The pharmaceutical company (Servier) was contacted, but they were not able to provide us with any additional data |
Corruble 2013.
| Methods | Multicentre (51 centres), randomised, parallel group, double‐blind controlled trial Duration: 12 weeks (with 12‐week extension) |
|
| Participants | Men or women suffering from a moderate to severe MDD according to DSM‐IV‐TR criteria, single or recurrent episode (at least 4 weeks), with or without melancholic features, without seasonal pattern, without psychotic features and without catatonic features Eligibility criteria: HAM‐D 17 total score of ≥ 22; CGI‐S score of ≥ 4; HADS score of ≥ 11. HAM‐D 17 total score had to be stable between election and inclusion (decrease < 20%) Age: 18‐70 years (inclusive) Sample size: agomelatine = 164; escitalopram = 160 Setting: outpatients Country: multinational (Australia, Brasil, Canada, France, Russia, South Africa, UK) |
|
| Interventions | Agomelatine (25‐50 mg/day) Escitalopram (10‐20 mg/day) A double‐blind treatment period of 24 weeks (a 12‐week mandatory double‐blind treatment period followed by a 12‐week extension period (for participants having a CGI‐I score 42 at week 12)) preceded by a 3–7‐day run‐in selection period between selection and inclusion visits (week 0). At week 0, participants were randomised to 1 of 2 treatment groups: agomelatine or escitalopram. From week 0, participants received 25 mg/d agomelatine or 10 mg/d escitalopram. In case of insufficient improvement (criteria blind for the investigator and participant), the dosage of agomelatine was increased to 50 mg/d and that of escitalopram to 20 mg/d from 2 weeks onwards |
|
| Outcomes | Primary efficacy variables: ‐ HAMD‐17 ‐ CGI ‐ Sleep satisfaction Visual Analogue Scale ‐ Global Assessment of Functioning Secondary efficacy variables: ‐ Oxford Questionnaire on Emotional Side‐Effects of Antidepressants (OQUESA) Safety variables: ‐ spontaneous report of adverse events by participants at each visit ‐ 12‐lead ECG abnormalities at week 0, 12 and 24 or at time of withdrawal ‐ biological samplings at week 0, 12 and 24 or at time of withdrawal ‐ physical examination at selection, inclusion and 24 weeks |
|
| Notes | 12 weeks of mandatory treatment, 12 week extension period for participants with CGI‐I ≤ 2 (after the first 12 weeks of mandatory treatment) Data were extracted for the first 12 week of mandatory treatment only. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Eligible patients were assigned to agomelatine or escitalopram treatment according to a balanced (nonadaptive) randomization with stratification on the clinical centre [ . . . ] in a blinded condition manner for patients and investigators" Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote: ". . . The treatment allocation and the dose increase were done centrally using an Interactive Response System . . . " Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Treatments were identically labelled" Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: ". . . double blind". Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Attrition was balanced between groups. However, no more information was provided |
| Selective reporting (reporting bias) | High risk | The FAS analysis is not fully reported, so it is unclear if all patients were included. Also, safety data were reported at 24 weeks only, in spite of the fact that they were collected at week 12 |
| Other bias | High risk | Trial sponsored by drug manufacturer |
Hale 2010.
| Methods | Multicentre (41 centres), double‐blind, randomised, parallel‐group comparative study Study conducted from 2005‐2008 Duration: 8 weeks |
|
| Participants | Men and women (outpatients), with a diagnosis of MDD of severe intensity according to DSM‐IV‐TR, confirmed by the MINI Elgibility criteria: HAMD‐17 ≥ 25, CGI ≥ 4, at least 7 symptoms among symptoms A1–A9 of the diagnostic criteria for major depressive episode (marked interference with occupational functioning, usual social activities, or relationships with others) with a single or recurrent episode that had already lasted at least 4 weeks. The HAMD‐17 total score did not decrease between selection and inclusion by more than 20%. At inclusion, the sum of items 1 (depressed mood), 2 (feelings of guilt), 5 (insomnia, middle of the night), 6 (insomnia, early hours of the morning), 7 (work and activities), 8 (retardation), 10 (psychic anxiety), and 13 (general somatic symptoms) were at least 55% of the HAMD‐17 total score, to ensure the expected weight of the depression symptoms the HAMD‐17 total score was still at least 25, and the CGI‐S score was still at least 4. Exclusion criteria: criteria related to the depressive episode included seasonal pattern, psychotic features, and postpartum onset. Other exclusion criteria included: marked suicidal intent and/or known suicidal tendencies for the current episode (score of 4 on HAMD‐17 item 3 or the investigator’s opinion); bipolar disorder; anxiety symptoms such as panic attacks; obsessive compulsive disorder; post‐traumatic stress disorder; drug abuse or dependency; resistance to fluoxetine for current episode; treatment with ECT or formal psychotherapy within the last 3 months, or light‐therapy started within the last 2 weeks; previous failure to respond to the administration of an appropriate dose of 2 different antidepressant treatments (including fluoxetine) for at least 4 weeks each, for the current and previous episodes; neurologic disorders (dementia, seizure, and stroke); and severe or uncontrolled organic disorders (neoplastic, cardiovascular, pulmonary, or digestive disorders, unstabilised type 1 or 2 diabetes) Age: 18‐65 years Sample size: agomelatine = 252; fluoxetine = 263 Setting: outpatients Countries: international (Argentina, Brazil, Italy, Spain, UK) |
|
| Interventions | Agomelatine (25‐50 mg/day) Fluoxetine (20‐40 mg/day) After a 3‐7‐day drug‐free period (according to the half‐life of any earlier treatment), participants were randomly assigned to a treatment group, with visits every 2 weeks. When there was insufficient improvement according to predefined criteria, the dose of agomelatine was increased to 50 mg/day after 2 weeks, and fluoxetine to 40 mg/day after 4 weeks, in line with current prescribing guidelines for the 2 agents. Other antidepressants, hypnotics, anxiolytics, and neuroleptic agents were prohibited during the study and for a variable period before inclusion, depending on the half‐life. Notably, treatment with hypnotics or anxiolytics had to be stopped at selection visit at the latest, though zolpidem was allowed until week 2 (maximal dose 10 mg/day), if required by the patient’s condition |
|
| Outcomes | Primary efficacy variables:
‐ HAMD‐17 Secondary efficacy variables: ‐ CGI‐S ‐ CGI‐I ‐ sleep (using HAM‐D sleep items) ‐ anxiety (using HAM‐A total score and psychic and somatic scores). ‐ responders (defined by a decrease of at least 50% in total score of HAMD‐17 from baseline or a CGI‐I score of 1 or 2) ‐ remitters (participants with HAM‐D17 total score of 6 or less or a CGI‐I score of 1) ‐ HAM‐A scale Safety variables: ‐ adverse events reported by the participants ‐ somatic complaints expressed spontaneously or upon enquiry ‐ body mass index (weeks 0 and 8) ‐ sitting blood pressure (weeks 0 and 8) ‐ heart rate (weeks 0 and 8) ‐ ECG (weeks 0 and 8) ‐ biochemistry and hematology (weeks 0 and 8) Compliance assessed by counting returned capsules at each visit from week 2 onwards |
|
| Notes | Study funded by Servier, the drug company manufacturing agomelatine. The authors have received honoraria, research grants, or both, from Servier | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote:" . . . patients were randomly assigned to receive agomelatine (25 mg/day) or fluoxetine (20 mg/day)" Comment: not enough information provided |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote:" . . . During the entire duration of the study, all patients took two capsules orally in the morning and one capsule in the evening, irrespective [of] the treatment and daily dosage allocated. Agomelatine was administered in the evening, whereas fluoxetine was administered in the morning according to current recommendations. The appearance and taste of the study treatment were the same from inclusion to the end of the study for all patients. The packaging and labelling were identical". Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote:" . . . Both investigators and patients were blind to the criteria determining insufficient improvement, as well as the decision to up‐titrate, which were applied centrally using an interactive voice‐response system" Quote: " . . . double‐blind" Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Balanced attrition between groups (30/252 for agomelatine and 49/263 for fluoxetine) Quote: " . . . The full analysis set (FAS) (intention to treat principle) consisted of patients in the randomised set who took at least one dose of the study treatment and with a baseline (week 0) evaluation and with at least one post‐baseline evaluation of the primary criterion over the period from week 0 to week 8. The safety set was defined as all included patients who took at least one dose of study treatment". Comment: probably done |
| Selective reporting (reporting bias) | Low risk | All outcomes and safety variables stated were reported |
| Other bias | High risk | Study was funded by Servier, the pharmaceutical company that manufactures agomelatine |
Kasper 2010.
| Methods | Multicentre (37 centres), double‐blind, randomised exploratory study with parallel‐group design Study conducted from 2005‐2006 Duration: 6 weeks |
|
| Participants | Outpatients, men and women with a primary diagnosis of MDD, single or recurrent, of moderate or severe intensity according to DSM‐IV‐TR criteria, confirmed by the MINI Eligibility criteria: an HDRS score ≥ 22, and a sum of 3 on HAM‐D items 5 ('insomnia: middle of the night') and 6 ('insomnia: early hours of the morning'). Exclusion criteria: seasonal pattern; psychotic features, or catatonic symptoms; postpartum depression; current episode had already lasted at least 4 weeks; high risk of suicide or a previous suicide attempt within 6 months (score 2 on HDRS item 3); bipolar disorder; anxiety symptoms such as current panic disorder, obsessive‐compulsive disorder, post‐traumatic stress disorder, or acute stress disorder; drug abuse or dependency within the past 12 months; previous depression resistance to antidepressants; treatment with ECT or formal psychotherapy within 3 months, or light‐therapy started within last 2 weeks; screening positive on clinical screening evaluation for sleep disorders, including obstructive sleep apnoea and restless legs syndrome; neurologic disorders (dementia, seizure, and stroke); obesity with functional impairment; serious or not stabilised organic disease (neoplasia, cardiovascular or pulmonary disease, or uncontrolled type 1 or type 2 diabetes). Other antidepressants, hypnotics, anxiolytics, and neuroleptic agents were prohibited during the study and for a variable period before inclusion, depending on half‐life. When the washout period of hypnotics or anxiolytics was applicable for the study, the treatment had to be stopped at selection visit at the latest appointment. Age: 18 to 60 years Sample size: agomelatine = 154; sertaline = 159 Setting: outpatients Countries: multinational (France, Germany, Austria, Spain, Italy, and Poland) |
|
| Interventions | Agomelatine (25‐50 mg/day) Sertraline (50‐100 mg/day) After a washout period according to previous drugs’ half‐lives of at least 1 week, increasing to 2 weeks if the participant had previously received monoamine oxidase inhibitors, and to a maximum of 5 weeks if the participant had previously been treated with fluoxetine or trazodone, eligible participants were randomly assigned to a treatment group. 4 visits were scheduled: at week 0 (baseline) and then every 2 weeks at weeks 2, 4, and 6. After 2 weeks, a dose increase to agomelatine 50 mg/d or sertraline 100 mg/d was possible if there was insufficient improvement according to predefined criteria |
|
| Outcomes | Efficacy variables: ‐ actigraphy ‐ Non‐Parametric Circadian Rhythms Analysis (NPCRA) ‐ LSEQ ‐ HAMD‐17 ‐ HAM‐A ‐ CGI‐S ‐ CGI‐I Safety variables: ‐ adverse events reported by the participants ‐ somatic complaints expressed spontaneously or upon enquiry ‐ body mass index (weeks 0 and 6) ‐ blood pressure (weeks 0 and 6) ‐ heart rate (weeks 0 and 6) ‐ ECG (week 0 and in case of withdrawal from the study) ‐ biochemistry and haematology (weeks 0 and 6) Compliance was assessed by counting returned capsules at each visit from week 2 |
|
| Notes | Study sponsored by Servier, the pharmaceutical company that manufactures agomelatine Conflicts of interest declaration: "Dr Kasper has received grant/research support from Eli Lilly, Lundbeck, Bristol‐Myers Squibb, GlaxoSmithKline, Organon, Sepracor, and Servier; has served as a consultant or on advisory boards for AstraZeneca, Bristol‐Myers Squibb, GlaxoSmithKline, Eli Lilly, Lundbeck, Pfizer, Organon, Schwabe, Sepracor, Servier, Janssen, and Novartis; and has served on speakers’ bureaus for AstraZeneca, Eli Lilly, Lundbeck, Schwabe, Sepracor, Servier, Pierre Fabre, and Janssen. Dr Hajak has served on the speakers boards of AstraZeneca, Bayer Vital, Bristol‐Myers Squibb, Boehringer Ingelheim, Cephalon, EuMeCom, GlaxoSmithKline, Janssen‐Cilag, Eli Lilly, Lundbeck, Merz, Neurim, Novartis, Organon, Pfizer, Sanofi‐Aventis, Servier, Takeda, and Wyeth; has been a consultant or member of advisory board of Actelion, AstraZeneca, Bristol‐Myers Squibb, Euro RSCG Life Worldwide, Gerson Lerman Group, Janssen‐Cilag, Eli Lilly, Lundbeck, McKinsey, Merck, Network of Advisors, Neurim, Neurocrine, Novartis, Organon, Pfizer, Proctor & Gamble, Purdue, Sanofi‐Aventis, Schering Plough, Sepracor, Servier, Takeda, and Wyeth; and has received research funding from Actelion, AstraZeneca, Boehringer Ingelheim, BrainLab, GlaxoSmithKline, Lundbeck, Neurim, Novartis, Organon, Orphan, Sanofi‐Aventis, Sepracor, Servier, and Takeda. Dr Wulff has received honoraria for data analysis and consulting from Servier. Dr Hoogendijk has lectured and been a member of advisory boards for Lundbeck, Servier, Eli Lilly, and Organon/Schering Plough, for which the Foundation for Depression Research GGZBA has received grants and salary. Dr Montejo has received honoraria from Servier, Eli Lilly, Bristol‐ Myers Squibb, GlaxoSmithKline, Sanofi, AstraZeneca, Boehringer, and Wyeth and has served on advisory boards for Eli Lilly, Boehringer, GlaxoSmithKline, Servier, and AstraZeneca. Dr Smeraldi has served as consultant or speaker for Janssen‐Cilag, Innova‐Pharma, and Wyeth. Dr Rybakowski has served as consultant or speaker for Adamed‐Poland, AstraZeneca, Bristol‐Myers Squibb, Eli Lilly, Janssen‐Cilag, Lundbeck, Organon, Pfizer, Sanofi‐Aventis, and Servier. Dr Quera‐Salva has served as a consultant or advisory board member for Servier. Dr Wirz‐Justice has been a consultant and speaker for Servier. Dr Picarel‐Blanchot is an employee of Servier. Dr Baylé has received honoraria from Bristol‐Myers Squibb, Janssen‐Cilag, Servier, Sanofi‐Aventis, UCB Pharma Research Support Bioprojet, Eli Lilly, Janssen‐Cilag, and Servier." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: " . . . Eligible patients were randomly assigned" Comment: not enough information provided |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: " . . . During the entire duration of the study, all patients took orally 2 tablets once a day in the evening, irrespective of the treatment and daily dosage allocated. The appearance and the taste of the study treatment were the same from inclusion to the end of the treatment period for all patients. The packaging and the labelling were identical" Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: " . . . the dose increase were applied centrally using an Interactive Voice Response System, and the investigator and the patient were blind to them" Quote: " . . . double‐blind" Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Balanced attrition between groups (21/154 for agomelatine and 30/159 for sertraline) No missing outcome data Comment: probably done |
| Selective reporting (reporting bias) | Low risk | All outcomes and safety variables stated are reported |
| Other bias | High risk | Study was funded by Servier, the pharmaceutical company manufacturing agomelatine |
Kennedy 2008.
| Methods | Multicentre (43 centres), double‐blind double‐dummy, randomised, parallel‐ group, placebo‐controlled study Study conducted from October 2002‐May 2004 Duration: 12 weeks Participants in the agomelatine group received 2 placebo capsules in the morning and 2 agomelatine 25 mg capsules in the evening. Participants in the venlafaxine group were prescribed 2 placebo capsules in the evening throughout the study and 2 venlafaxine 37.5 mg capsules in the morning for the first 2 weeks, followed by 2 x 75 mg capsules in the morning for the remaining 10 weeks. Outcome measures were administered at baseline and after 2, 6, 10, and 12 weeks |
|
| Participants | Men and women with DSM‐IV criteria for MDD Eligibility criteria: before treatment required 1) to score ≥ 20 on MADRS and to be free of antidepressant medication for a minimum of 7 days (3 weeks in the case of fluoxetine or nonselective monoamine oxidase inhibitors); and 2) to have engaged in sexual activity (intercourse and/or self‐stimulation) in the past 2 weeks Exclusion criteria: concurrent anxiety disorders; substance abuse or dependence disorders; bipolar disorder; MDD with psychotic or catatonic features; postpartum depression; pregnancy, lactation, or inadequate contraception; evidence of active suicidal ideation or suicide attempt in the past 6 months; previous failure to respond to either of the protocol treatments; and neurological or unstable medical conditions. Age: 18 to 60 years Sample size: agomelatine = 137; venlafaxine = 140 Setting: outpatients Countries: multinational (Canada, France, UK) |
|
| Interventions | Agomelatine (50 mg/day) Venlafaxine (75‐150 mg/day) |
|
| Outcomes | Primary variables: ‐ Sex function scale Secondary variables: ‐ MADRS ‐ CGI‐S ‐ CGI‐I Safety variables: ‐ spontaneously reported adverse events |
|
| Notes | Primary outcome was the change on Sex FX and not efficacy Study sponsored by Servier, the pharmaceutical company manufacturing agomelatine Conflict of interest declaration: "Dr Kennedy has received research funding from Astra‐ Zeneca, Eli Lilly, GlaxoSmithKline, Janssen Ortho, Lundbeck, and Merck Frosst. At the time of the article publication he was a consultant to ANS, Biovail, Boehringer Ingelheim, Eli Lilly, GlaxoSmithKline, Institut de Recherches Internationales Servier, Janssen‐Ortho, Lundbeck, Organon, Pfizer, and Wyeth. Dr Rasmussen is a consultant to Institut de Recherches Internationales Servier" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: " . . . 277 were randomised to receive either agomelatine (n = 137) or venlafaxine XR (n = 140)" Comment: not enough information provided |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: " . . . A double‐dummy design was applied" Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: ". . . double‐blind" Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: " . . . Of 320 outpatients aged 18 to 60 years who were enrolled, 277 were randomised to receive either agomelatine (n = 137) or venlafaxine XR (n = 140). Nonrandomisation occurred mainly because of abnormal laboratory values or unacceptable concomitant medications" Comment: no data on exclusion criteria divided by medication |
| Selective reporting (reporting bias) | Low risk | All outcomes and safety variables stated were reported |
| Other bias | High risk | Study was sponsored by the drug company that manufactures agomelatine (Servier) |
Lemoine 2007.
| Methods | Multicentre (56 centres), double‐blind, randomised, parallel‐group study Study conducted from November 2002‐June 2004 Duration: 6 weeks |
|
| Participants | Men and women with DSM‐IV criteria for MDD Eligibility criteria: HAM‐D score of ≥ 20 Exclusion criteria: psychotic features or catatonic symptoms; postpartum depression; high risk of suicide or previous suicide attempt within 6 months; bipolar disorder; anxiety symptoms such as panic attacks; obsessive‐compulsive disorders; post‐traumatic stress disorders; drug abuse or dependency; previous depression resistant to antidepressants; treatment with ECT 3 months or formal psychotherapy within 1 month; screening positive on clinical screening evaluation for sleep disorders, including obstructive sleep apnoea and restless legs syndrome; recent or planned transmeridian air travel (time change of ≥ 3 hours) or phototherapy within 2 weeks; neurologic disorders (dementia, seizure, stroke); obesity with functional impairment; serious or not stabilised organic disorders (neoplasia, cardiovascular, pulmonary, uncontrolled type 1 or type 2 diabetes) Age: 18‐65 years Sample size: agomelatine = 165; venlafaxine = 167 Setting: outpatients Countries: multinational (France and Spain) |
|
| Interventions | Agomelatine (25‐50 mg/day) Venlafaxine (75‐150 mg/day) After a brief (< 7 days) washout period without study treatment, participants were randomly assigned to receive agomelatine 25 mg/day or venlafaxine 75 mg/day for a 6‐week treatment period Participants took 2 capsules daily, 1 in the morning and 1 in the evening. Agomelatine‐treated participants took the placebo capsule in the morning and the agomelatine 25 mg capsule in the evening; venlafaxine‐treated participants took a capsule of venlafaxine 37.5 mg in the morning and evening. In participants with insufficient response at week 2, based on a predetermined cut‐off on the 17‐item HAM‐D and global improvement score of the CGI the doses were increased to 1 capsule of agomelatine 50 mg in the evening and 1 placebo capsule in the morning and to venlafaxine 150 mg (75 mg twice daily) |
|
| Outcomes | Efficacy variables: ‐ LSEQ ‐ visual Analogue Scale for 'daytime sleepiness' and 'feeling well' ‐ sleep diary ‐ HAM‐D ‐ CGI‐I Safety variables ‐ adverse events presented or reported by the participants ‐ any abnormal value judged to be clinically relevant by the investigator |
|
| Notes | Primary outcome was the 'getting to sleep score' on LSEQ Study sponsored by Servier, the pharmaceutical company that manufactures agomelatine The authors received honoraria from Servier in conjunction with this study |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: " . . . patients were randomly assigned.." Comment: not enough information provided |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: " . . . All capsules were identical in appearance" Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: " . . . double‐blind." Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Slightly higher attrition rare with venlafaxine (15/165) than agomelatine(36/167) but reasons reported. No missing outcome data Comment: probably done |
| Selective reporting (reporting bias) | Low risk | All outcomes and safety variables stated were reported |
| Other bias | High risk | Study was sponsored by the drug company that manufactures agomelatine (Servier) |
Loo 2002a.
| Methods | Multicentre (102 centres), randomised, double‐blind, placebo‐controlled trial Duration: 8 weeks In order to exclude rapid placebo responders, participants received placebo for 1 week before inclusion in the study; those with a reduction of 20% in the HAM‐D total score were not included |
|
| Participants | Men and women with a DSM‐IV diagnosis of MDD and bipolar II depression Eligibility criteria: HAM‐D 17 score of ≥ 22 Exclusion criteria: severe unstable disease or disease that could interfere with study evaluations or circadian rhythms Age: 18‐65 years Sample size: agomelatine 1 mg = 141; agomelatine 5 mg = 147; agomelatine 25 mg = 137; paroxetine = 147; placebo = 139 Setting: inpatients and outpatients Countries: multinational (Belgium, France, UK) |
|
| Interventions | Agomelatine (1 mg, 5 mg and 25 mg/day) Paroxetine (40 mg/day) Placebo Concomitant treatment with psychotropic drugs was not allowed with the exception of benzodiazepines at restricted doses. High potency benzodiazepines, such as alprazolam and triazolam,were not permitted. Drugs that were thought to be able to influence study evaluations by acting on participant’s mood or circadian rhythms, such as beta‐blockers, central alpha‐blockers, nonsteroidal anti‐inflammatory drugs and exogenous melatonin, were not allowed. In participants receiving prior psychotropic medication, a washout period of 1–4 weeks was required depending on the drugs' half‐life |
|
| Outcomes | Primary outcomes: ‐ HAM‐D final score ‐ response (defined as 50% reduction of HAMD‐17) ‐ remission (defined as HAMD‐17 of < 7) Secondary outcomes: ‐ MADRS ‐ HAM‐A ‐ CGI Safety variables: ‐ number of participants experiencing adverse events ‐ physical examination ‐ standard biological tests centrally assessed ‐ ECG |
|
| Notes | The primary goal of the study was to compare 3 different doses of agomelatine with both placebo and the active comparator paroxetine No information about conflict of interest reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: no information provided |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: " . . . patients were randomised in double‐blind conditions . . . " Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: " . . . double blind" Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Attrition was balanced between groups. However, data are available only for included participants. No data on number of people randomised |
| Selective reporting (reporting bias) | High risk | All outcomes and safety variables stated were reported. However, the trial was not registered and there is no protocol available |
| Other bias | Unclear risk | It was not clear whether the study was sponsored or not. The authors did not report any information on potential conflict of interest |
Martinotti 2012.
| Methods | Multisite (2 sites), open‐label, randomised, parallel group pilot study Study conducted from January‐November 2010 Duration: 8 weeks |
|
| Participants | Men and women with DSM‐IV‐TR diagnosis of MDD Exclusion criteria: presence of a medical condition that could either interfere with the assessment of the drug treatment or be unsafe for the participant (i.e. cirrhosis, renal impairment, unstable hypertension, hypotension, diabetes mellitus, convulsions); history of bipolar disorder; schizophrenia; schizoaffective disorder; eating disorder; obsessive‐compulsive disorder; substance dependence; concomitant use of other antidepressant drugs (in this case, a washout period of 7 days was required); and pregnancy and breastfeeding or non‐effective contraception. Age: 18‐60 years Sample size: agomelatine = 30; venlafaxine = 30 Setting:outpatients Countries: Italy |
|
| Interventions | Agomelatine (25 mg/day) Venlafaxine (75 mg/day) After screening to assess eligibility, participants were randomly started on agomelatine at a dose of 25 mg/d (n = 30) or on venlafaxine 75 mg/d (n = 30). A single dose of 25 mg/d agomelatine was administered at 8:00 pm. If after 2 weeks there was no clinical response, in the clinician’s judgment, the dosage could be increased to a single dose of 50 mg/d. Venlafaxine was administered as a single dose of 75 mg/d, at 8:00 am. If after 2 weeks there was no clinical response, in the clinician’s judgment, the dosage could be increased to a single dose of 150 mg/d |
|
| Outcomes | Primary outcomes: ‐ SHAPS (anhedonia) Secondary outcomes: ‐ HAM‐D ‐ HAM‐A ‐ CGI Safety parameters: ‐ ECG at the start and end of the study ‐ urinalysis at the start and end of the study ‐ haematological and clinical chemical analyses of blood samples (including liver enzymes) at the start and end of the study ‐ self‐reported adverse events |
|
| Notes | Primary aim of the study was the comparison of the effects of agomelatine and venlafaxine on anhedonia in people with major depression The authors declared no conflicts of interest. Study was not sponsored by any pharmaceutical organisation |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomised". "Randomization was nonadaptive, balanced, and stratified on the center. After recruitment of a patient, an interactive computer‐based system allocated a therapeutic unit number . . . " Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote: " . . . randomisation was nonadaptive, balanced, and stratified on the center. After recruitment of a patient, an interactive computer‐based system allocated a therapeutic unit number" Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "open‐label" Comment: study was open‐label |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "open‐label" Comment: study was open‐label |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: " . . . A total of 92 patients were screened, of whom 32 were excluded from the study" Comment: no data on the reasons for exclusion |
| Selective reporting (reporting bias) | High risk | No information provided about type(s) of adverse event |
| Other bias | Unclear risk | No information available |
Quera‐Salva 2011.
| Methods | Multicentre (24 centres), randomised, parallel‐group, double‐blind controlled trial Study conducted from May 2007‐October 2008 Duration: 6 weeks |
|
| Participants | Men and women with DSM‐IV‐TR diagnosis of MDD, confirmed by the MINI Eligibility criteria: MDD (single or recurrent episode) of moderate or severe intensity, HAM‐D total score of at least 22. Exclusion criteria: seasonal pattern; postpartum onset; psychotic; or catatonic features; marked suicidal risk (HAM‐D item 3 score > 2); resistant depression (i.e. people who have not responded previously to 2 different antidepressant treatments of at least 4 weeks at an appropriate dose); ECT for the current episode; sleep deprivation or light therapy during the 2 weeks before selection; bipolar disorder and drug abuse or dependency; shift workers or people with recent transmeridian travel; uncontrolled organic disease; women without effective contraception; and after the first Polysomnographic recording (PSG), people with > 10 apnoeas– hypopnoeas per hour of sleep or > 10 periodic leg movements per hour of sleep with arousal Age: 18‐60 years Sample size: agomelatine = 71; escitalopram = 67 Setting: outpatients Countries: international (Australia, Austria, Brazil, Finland, France, Germany, Spain, Taiwan) |
|
| Interventions | Agomelatine (25‐50 mg/day) Escitalopram (10‐20 mg/day) After a maximum run‐in period of 10 days without treatment, participants were randomly assigned to receive agomelatine 25 mg/day or escitalopram 10 mg/day for 2 weeks, after which the dose could be increased either to agomelatine 50 mg/day or to escitalopram 20 mg/day if there was insufficient improvement of depressive symptoms. Participants scoring > 4 on the CGI‐I after 6 weeks of treatment were not allowed to continue in the extension period |
|
| Outcomes | Efficacy variables: ‐ PSG recordings ‐ HAM‐D ‐ CGI‐S ‐ CGI‐I Safety variables: ‐ adverse events reported by the participant or upon enquiry, assessed and recorded at each visit (the investigator had to evaluate the event in terms of severity, relationship to study medication, and seriousness) ‐ heart rate at selection and at weeks 6 and 24 ‐ blood pressure at selection and at weeks 6 and 24 ‐ body mass index at selection and at weeks 6 and 24 ‐ biochemistry and haematology at selection, week 6, and week 24 ‐ ECG at selection and at the last visit |
|
| Notes | The main aim of the study was to characterise the effects of agomelatine on sleep and wake parameters by comparing its effect with that of the SSRI escitalopram The authors have received honoraria, research grants, or both, from Servier. The authors declared they have no other relevant affiliations or financial involvement with any organisation or entity in conflict with the subject matter or materials discussed in the manuscript apart from those disclosed below Conflict of interest from other pharmaceutical companies: Dr MA Quera Salva: advisory board member of Ferrer international (Barcelona, Spain) Professor Goeran Hajak: speakers board, consultant, advisory board member author fees or research funding by Actelion, Affectis, Astra‐Zeneca, Bayerische Motorenwerke, Bayer Vital, Brain Lab, Bristol‐Myers, Cephalon, Daimler Benz, Elsevier, EuMeCom, Essex, Georg Thieme, Gerson Lerman Group Council Healthcare Advisors, GlaxoSmithKline, Janssen‐Cilag, Lilly, Lundbeck, McKinsey, Merck, Merz, Network of Cilag, Lilly, Lundbeck, Mc Kinsey, Merck, Merz, Network of Advisors, Neurim, Neurocrine, Novartis, Organon, Orphan, Pfizern Pharmacia, Proctor and Gamble, Purdue, Sanofi‐Aventis, Schering‐Ploungh, Sepracor, Springer, Takeda, Transcept, Urban Fischer, Volkswagen, and Wyeth |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: " . . . patients were randomly assigned" Comment: not enough information provided |
| Allocation concealment (selection bias) | Low risk | Quote: " . . . The centralized, balanced randomisation was stratified by center and age (≤ 40 vs >40 years) using an interactive voice response system" Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: " . . . The patients and the investigators were blinded to the dose increase, which was determined centrally by interactive voice response system according to the predefined criteria", "The appearance and taste of the study treatments were the same from inclusion to the end of the treatment period for all patients. The packaging and the labeling were identical" Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "double‐blind" Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition rate was balanced between groups |
| Selective reporting (reporting bias) | Low risk | All outcomes and safety variables stated were reported |
| Other bias | High risk | The authors have received honoraria, research grants, or both, from Servier |
Shu 2013.
| Methods | Multicentre (39 centres), randomised, parallel‐group, double‐blind controlled trial Duration: 8 weeks |
|
| Participants | Men and women with DSM‐IV‐TR diagnosis of MDD Eligibility criteria: MDD (single or recurrent episode that had already lasted at least 4 weeks) of moderate or severe intensity; HAM‐D total score of at least 22 and CGI‐S score of at least 4; with or without melancholic features; without seasonal pattern; without psychotic features and without postpartum onset for the current episode Exclusion criteria: all other psychiatric disorders comorbid with MDD; physical problems (including γGT or transaminases values > 3 times the upper normal limit (> 2 times the upper normal limit in mainland China) or/and creatinemia >150 μmol/l (1.7 mg/dL), and/or alpha‐fetoprotein abnormal value (in mainland China); any of the following disorders from DSM IV‐TR, identified with the MINI: 1) chronic depression (> 2 years of a depressive episode); bipolar disorder I and II; MDD superimposed on dysthymic disorder according to DSM IV‐TR (double depression); current panic disorder; obsessive compulsive disorder; post‐traumatic stress disorder; acute stress disorder; schizoaffective disorder of depressive type; or any other psychotic disorder, including major depression with psychotic features; 2) alcohol or drug abuse or dependence within the past 12 months; any personality disorder that might compromise the study. Patients were also excluded if they were at risk of suicide (according to the investigator) or had a rating of 4 points on item 3 of HAMD‐17; had not responded to previous administration of 2 different antidepressant treatments at an appropriate dose of for at least 4 weeks (including fluoxetine) for the current episode; had neurologic disorders (dementia, seizure, and stroke); severe or uncontrolled organic disorders (neoplastic, cardiovascular, pulmonary, or digestive disorders, unstabilised type 1 or 2 diabetes). People were also excluded if they had received any of the following recent/concomitant therapies: insight‐oriented and structured psychotherapy (interpersonal therapy, psychoanalysis, cognitive behavioural therapy) started within 3 months of inclusion; light therapy started within 2 weeks; oral antipsychotic drugs within 4 weeks; depot neuroleptics within 6 months; ECT within the last 3 months. Age: 18‐65 years Sample size: agomelatine = 314; fluoxetine = 314 Setting: outpatients Countries: international Asia (Hong Kong, China, Singapore, Malaysia, Taiwan, South Korea) |
|
| Interventions | Agomelatine (25‐50 mg/day) Fluoxetine (20‐40 mg/day) Washout time required for antidepressants was usually 1 week (2 weeks for non selective MAOIs and tricyclics). Hypnotics, anxiolytics, and neuroleptic agents were prohibited during the study and for a variable period before inclusion, depending on the half‐life. Notably, treatment with hypnotics or anxiolytics had to be stopped at selection visit at the latest, though zolpidem was allowed until week 2 (maximal dose 10 mg/day), if required by the patient’s condition A double‐blind treatment period of 8 weeks (from W0‐W8) was preceded by a selection period of 4‐10 days before inclusion (W0) visit and followed by a 1 week untreated period until last study visit (follow‐up visit). At W0, participants were randomised to 1 of 2 treatment groups: agomelatine or fluoxetine. From W0, participants received agomelatine 25 mg/day or fluoxetine 20 mg/day. If there was insufficient improvement ‐ determined using blinded criteria defined by the sponsor prior to the study start ‐ dosage of agomelatine was increased to 50 mg/day after 2 weeks, and dosage of fluoxetine to 40 mg/day after 4 weeks |
|
| Outcomes | Primary outcomes: ‐ HAM‐D final score Secondary outcomes: ‐ Response (defined as 50% reduction of HAMD‐17) ‐ HAM‐A ‐ CGI‐S ‐ CGI‐I ‐ LSEQ ‐ TESS Safety variables: ‐ emergent adverse events spontaneously reported by the participant at each visit ‐ physical examination ‐ vital signs (SBP and DBP, heart rate, and weight) at selection and week 8 ‐ standard biological tests ‐ liver B ultrasound (for China only) ‐ ECG |
|
| Notes | Completed trial still in press; unregistered trial, no further data available | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: " . . . patients were randomised in one of the two treatment groups" Comment: not enough information provided |
| Allocation concealment (selection bias) | Low risk | Quote: "Eligible patients were assigned to agomelatine or fluoxetine treatment according to a balanced (non‐adaptive) randomisation with stratification on the clinical centre. The computer‐generated randomisation list was drawn up in blind by I.R.I.S. Biometry Department." Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The dosage schedule (three capsules once a day), the study treatment appearance (2 red capsules in the morning and 1 yellow capsule in the evening) and the taste were the same from inclusion to the end of the treatment period for all patients. Capsules were packaged in identical blisters with identical labelling.", " "All study personnel and participants were blinded to treatment assignment and dose for the duration of the study." Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "double‐blind", "All study personnel and participants were blinded to treatment assignment and dose for the duration of the study." Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Attrition rate was balanced between groups |
| Selective reporting (reporting bias) | Unclear risk | Some outcomes and safety variables stated were reported. More safety data and different exclusion criteria were reported for data from China, but these were not reported in the paper |
| Other bias | High risk | It was not clear whether the study was sponsored by a drug company or not. Trial number not reported |
Abbreviations
> = greater/more than < = less than ≥ = greater than or equal to ≤ = less than or equal to CGI = Clinical Global Impression CGI‐I = Clinical Global Impression ‐ Improvement CGI‐S = Clinical Global Impression ‐ Severity DBP = diastolic blood pressure DSM‐IV = Diagnostic and Statistical Manual of Mental Disorders, 4th Edition DSM‐IV‐TR = Diagnostic and Statistical Manual of Mental Disorders, 4th Edition, Text Revision ECG = electrocardiogram ECT = electroconvulsive therapy FAS = full analysis set EMA = European Medicines Agency HADS = Hospital Anxiety and Depression Scale HAM‐A = Hamilton Anxiety rating scale HAM‐D = Hamilton Rating Scale for Depression HAMD‐17 = original 17‐point version of HAM‐D/HDRS HDRS = Hamilton Rating Scale for Depression LOCF = last observation carried forward LSEQ = Leeds Sleep Evaluation Questionnaire MADRS = Montgomery and Asberg Depression Rating Scale MDD = Major Depressive Disorder MINI = Mini‐International Neuropsychiatric Interview OQUESA = Oxford Questionnaire on Emotional Side effect of Antidepressants SBP = systolic blood pressure SHAPS = Snaith Hamilton Rating Scale SexFX = the sex effects scale TESS = Traumatic Exposure Severity Scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| CAGO2301 | Wrong comparison (no active control group) |
| CAGO2302 | Wrong comparison (no active control group) |
| CAGO2304 | Wrong comparison (no active control group) |
| CL2‐009 | Wrong comparison (no active control group) |
| CL3‐021 | Wrong comparison (no active control group) |
| CL3‐025 | Wrong comparison (no active control group) |
| CL3‐026 | Wrong comparison (no active control group) |
| Corral 2009 | Wrong comparison (no active control group) |
| Goodwin 2009 | Wrong comparison (no active control group) |
| Heun 2013 | Wrong comparison (no active control group) |
| Kennedy 2006 | Wrong comparison (no active control group) |
| Loo 2002b | Wrong comparison (comparisons of 2 agomelatine doses) |
| Olie 2007 | Wrong comparison (no active control group) |
| Rouillon 2008 | Wrong comparison (no active control group) |
| Saletu 2011 | Wrong comparison (no active control group) |
| Save 2011 | Wrong comparison (no active control group) |
| Serfaty 2010 | Wrong comparison (no active control group) |
| Stahl 2010 | Wrong comparison (no active control group) |
| Tseng 2007 | Withdrawn prior to enrolment |
| Zajecka 2010 | Wrong comparison (no active control group) |
Characteristics of studies awaiting assessment [ordered by study ID]
CL3‐027.
| Methods | Unclear |
| Participants | " . . . largely resistant hospitalised depressed patients" |
| Interventions | Unclear, probably only placebo controlled |
| Outcomes | Unclear |
| Notes | This unpublished study is mentioned in the EMA report, no further information available |
CL3‐048.
| Methods | 12‐week multicentre, randomised, double‐blind controlled trial |
| Participants | Older, depressed outpatients with major depression according to DSM‐IV |
| Interventions | Agomelatine (25‐50 mg/day), paroxetine (20‐30 mg/d) |
| Outcomes | LSEQ, HAM‐D, CGI, MADRS, HAM‐A |
| Notes | Completed but unpublished trial, some data were reported in Kasper 2013, but were insufficient for inclusion |
CL3‐062.
| Methods | 6‐month randomised double‐blind, parallel group international multicentre trial |
| Participants | Outpatients with major depression according to DSM‐IV |
| Interventions | Agomelatine (25‐50 mg/day) vs "SNRI" |
| Outcomes | HAM‐D, CGI, Pittsburgh Sleep Quality Index, LESQ, SDS |
| Notes | Completed but unpublished trial, no further data available |
CL3‐073.
| Methods | 3‐week, randomised, double then single‐blind, controlled, parallel groups, international, multicentre safety trial with a 5‐week open extension period |
| Participants | Outpatients with major depression according to DSM‐IV |
| Interventions | Agomelatine (25 mg/day), paroxetine, venlafaxine |
| Outcomes | Number of discontinuation emergent symptoms according to the DESS check‐list |
| Notes | Completed but unpublished trial. Primary objective: "Compare three different ways to initiate agomelatine after antidepressant treatment by SSRI or SNRI" |
CRSC11003.
| Methods | 6‐week multicentre, randomised, double‐blind controlled trial |
| Participants | Older depressed outpatients with major depression according to DSM‐IV |
| Interventions | Agomelatine (25‐50 mg/day), paroxetine (20‐30 mg) |
| Outcomes | HAM‐D, CGI |
| Notes | Completed but unpublished trial, no further data available |
CTRI/2011/04/001659.
| Methods | Randomised, open‐label multicentre trial |
| Participants | People with major depression according to DSM‐IV |
| Interventions | Agomelatine (25‐50 mg/day), venlafaxine (37.5‐150 mg/day) |
| Outcomes | HAM‐D, CGI |
| Notes | Completed but unpublished trial, no further data available |
Karaiskos 2013.
| Methods | 4‐month, observational, open‐label study |
| Participants | People with major depression according to DSM‐IV‐TR and comorbid non‐optimally controlled type 2 diabetes mellitus |
| Interventions | Agomelatine (25‐50 mg/day), sertraline (50‐100 mg/day) |
| Outcomes | HAM‐D, HAM‐A |
| Notes | Unclear randomisation |
Montgomery 2004.
| Methods | 14‐week multicentre, randomised, double‐blind controlled trial, (2 weeks placebo controlled discontinuation phase) |
| Participants | People with major depression according to DSM‐IV, sustained remitters (MADRS ≤12 at week 8,10,12) in the placebo trial |
| Interventions | Agomelatine (25 mg/day), paroxetine (20 mg/day), placebo |
| Outcomes | DESS symptoms during first week of withdrawal, MADRS, HAM‐A, CGI, partial relapse |
| Notes | Data for the first trial period (agomelatine vs paroxetine for 12 weeks) were not reported |
Vasile 2011.
| Methods | 6 month single‐blind trial |
| Participants | People with MDD and diabetes mellitus |
| Interventions | Agomelatine, fluoxetine, sertraline |
| Outcomes | HAM‐D, CGI, GAF |
| Notes | Conference abstract, no further information available, randomisation unclear |
Abbreviations
≤ = less than or equal to CGI = Clinical Global Impression DESS = Discontinuation Emergent Signs and Symptoms DSM‐IV = Diagnostic and Statistical Manual of Mental Disorders, 4th Edition DSM‐IV‐TR = Diagnostic and Statistical Manual of Mental Disorders, 4th Edition, Text Revision EMA = European Medicines Agency GAF = Global Assessment of Functioning HAM‐A = Hamilton Anxiety rating scale HAM‐D = Hamilton Rating Scale for Depression LSEQ = Leeds Sleep Evaluation Questionnaire MADRS = Montgomery and Asberg Depression Rating Scale MDD = Major Depressive Disorder SDS = Sheehan Disability Scale
Characteristics of ongoing studies [ordered by study ID]
CL3‐060.
| Trial name or title | CL3‐20098‐060 |
| Methods | 6‐month randomised, double‐blind, parallel‐group multicentre trial |
| Participants | Adult outpatients with major depression according to DSM‐IV |
| Interventions | Agomelatine (25‐50 mg/day) versus escitalopram (10‐20 mg/day) |
| Outcomes | "emotional experiences", "antidepressant efficacy" |
| Starting date | June 2012 |
| Contact information | Institut de Recherches Internationales Servier; clinicaltrials@servier.com |
| Notes |
CL3‐074.
| Trial name or title | CL3‐20098‐074 |
| Methods | 8‐week randomised, double‐blind, parallel‐group, multicentre trial |
| Participants | Adult Indian outpatients with MDD according to DSM‐IV |
| Interventions | Agomelatine (25‐50 mg/day), sertraline (50‐100 mg/day) |
| Outcomes | HAM‐D, CGI, HADS, SDS, LESQ |
| Starting date | January 2011 |
| Contact information | Dr Vihang Vahia (vvahia@hotmail.com); Dr Ashutosh Shah (ashutosh.shah@in.netgrs.com) |
| Notes |
CL3‐083.
| Trial name or title | CL3‐20098‐083 |
| Methods | 12‐week, randomised, double‐blind, parallel‐group, multicentre trial |
| Participants | Outpatients with major depression according to DSM‐IV |
| Interventions | "Therapeutic oral doses of agomelatine and therapeutic oral doses of selective serotonin reuptake inhibitors" |
| Outcomes | Unclear: "early effect of agomelatine" |
| Starting date | May 2011 |
| Contact information | Prof Tudor Udristoiu, Spitalul Clinic de Neuropsihiatrie Craiova, Clinica 1 Psihiatrie, Aleea Potelu No 24, Craiova, 200317, Romania |
| Notes |
GENRAS.
| Trial name or title | GENRAS |
| Methods | Randomised, single‐blind trial |
| Participants | In‐ and outpatients with major depression according to DSM‐IV |
| Interventions | Agomelatine (25‐50 mg/day), escitalopram (20 mg/day) |
| Outcomes | The genetics of antidepressant response to agomelatine and escitalopram |
| Starting date | October 2010 |
| Contact information | Medizinische Universität Wien,Univ Klinik f Psychiatrie und Psychotherapie |
| Notes |
Lundbeck 2011.
| Trial name or title | |
| Methods | 12 week, randomised, double‐blind, parallel‐group, multicentre trial |
| Participants | Adult outpatients treated with inadequate response to an SRI antidepressant (monotherapy) prescribed to treat major depression according to DSM‐IV |
| Interventions | Lu AA21004 (vortioxetine, 10‐20 mg/day) versus agomelatine (25‐50 mg/day) |
| Outcomes | MADRS |
| Starting date | January 2012 |
| Contact information | H Lundbeck A/S; LundbeckClinicalTrials@lundbeck.com |
| Notes |
NCT01483053.
| Trial name or title | NCT01483053 "Cardiovascular effects of agomelatine and escitalopram in patients with major depressive disorder" |
| Methods | Randomised, open label parallel‐group, multicentre trial |
| Participants | People with MDD or MDD with melancholia according to DSM‐IV |
| Interventions | Agomelatine (25‐50 mg/day), escitalopram (10‐20 mg/day) |
| Outcomes | Sympathetic nervous activity, blood pressure regulation |
| Starting date | Not yet open for participant recruitment (August 2012) |
| Contact information | Markus Schlaich, Baker IDI Heart and Diabetes Institute |
| Notes |
Abbreviations
CGI = Clinical Global Impression DSM‐IV = Diagnostic and Statistical Manual of Mental Disorders, 4th Edition HAM‐D = Hamilton Rating Scale for Depression LSEQ = Leeds Sleep Evaluation Questionnaire MADRS = Montgomery and Asberg Depression Rating Scale MDD = Major Depressive Disorder SDS = Sheehan Disability Scale SRI = serotonin reuptake inhibitor
Differences between protocol and review
Since we judged that it would have not made much difference in data analysis and interpretation, the analysis for the fixed‐effect model was calculated only for the main outcomes that were included in the 'Summary of findings' tables.
The protocol did not list continuous data as an outcome. We amended the analysis and we included continuous data as a secondary outcome.
We did not run any meta‐regression analyses, as the low number of studies available made these analyses obsolete.
In the protocol phase, we thought of grouping all antidepressants together. At the review stage, we decided to group the antidepressants by classes (SSRI, SNRI and others).
In order to assess publication bias better, we added a subgroup analysis (Analysis 1.30), that examined differences in outcomes for agomelatine in published versus unpublished studies.
Contributions of authors
GG: conceived the idea, supervised protocol writing, and wrote part of the review SG: wrote the protocol and extracted data from studies DC: read the protocol and the review, and provided methodological input SJCD: wrote the descriptions of the condition and of the intervention, and provided content supervision KH: helped with data extraction and with revision of the review MK: extracted data from studies, wrote part of the review and also analysed the data
Sources of support
Internal sources
None, Not specified.
External sources
None, Not specified.
Declarations of interest
Giuseppe Guaiana: none known Sumeet Gupta: none known Debbie Chiodo: none known Simon JC Davies: none known Katja Haederle: none known Markus Koesters: none known
New
References
References to studies included in this review
CAGO2303 {unpublished data only}
- Novartis Pharmaceuticals. A placebo‐ and paroxetine‐controlled study of the efficacy, safety and tolerability of agomelatine (25 or 50 mg) in the treatment of major depressive disorder (MDD) [NCT00463242]. ClinicalTrials.gov [www.clinicaltrials.gov] [accessed 10 November 2012] 2009. [Google Scholar]
- Novartis Pharmaceuticals. An 8‐week, multicenter, randomized, double‐blind, placebo‐and paroxetine‐controlled study of the efficacy, safety and tolerability of agomelatine 25 or 50 mg given once daily in the treatment of Major Depressive Disorder (MDD). http://www.novctrd.com/ctrdWebApp/clinicaltrialrepository/displayFile.do?trialResult=2659 [accessed 10 November 2012] 2009. [Google Scholar]
CL3‐022 {unpublished data only}
- European Medicines Agency. CHMP Assessment report for Thymanax [Procedure No. EMEA/H/C/000916, Doc. Ref. EMEA/97539/2009].http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_‐_Public_assessment_report/human/000915/WC500046226.pdf. [Accessed 22 October 2012] 2008.
- European Medicines Agency. CHMP Assessment report for Thymanax [Procedure No. EMEA/H/C/000916, Doc. Ref. EMEA/97539/2009].http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_‐_Public_assessment_report/human/000916/WC500038315.pdf. [Accessed 25 October 2012] 2009.
- European Medicines Agency. CHMP Assessment report for Valdoxan [Procedure No. EMEA/H/C/656, Doc. Ref. EMEA/CHMP/87018/2006].http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_‐_Public_assessment_report/human/000656/WC500070527.pdf. [Accessed 22 January 2013] 2006.
CL3‐023 {unpublished data only}
- European Medicines Agency. CHMP Assessment report for Thymanax [Procedure No. EMEA/H/C/000916, Doc. Ref. EMEA/97539/2009].http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_‐_Public_assessment_report/human/000915/WC500046226.pdf. [Accessed 22 October 2012] 2008.
- European Medicines Agency. CHMP Assessment report for Thymanax [Procedure No. EMEA/H/C/000916, Doc. Ref. EMEA/97539/2009].http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_‐_Public_assessment_report/human/000916/WC500038315.pdf. [Accessed 25 October 2012] 2009.
- European Medicines Agency. CHMP Assessment report for Valdoxan [Procedure No. EMEA/H/C/656, Doc. Ref. EMEA/CHMP/87018/2006].http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_‐_Public_assessment_report/human/000656/WC500070527.pdf. [Accessed 22 January 2013] 2006.
CL3‐024 {unpublished data only}
- European Medicines Agency. CHMP Assessment report for Thymanax [Procedure No. EMEA/H/C/000916, Doc. Ref. EMEA/97539/2009].http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_‐_Public_assessment_report/human/000915/WC500046226.pdf. [Accessed 22 October 2012] 2008.
- European Medicines Agency. CHMP Assessment report for Thymanax [Procedure No. EMEA/H/C/000916, Doc. Ref. EMEA/97539/2009].http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_‐_Public_assessment_report/human/000916/WC500038315.pdf. [Accessed 25 October 2012] 2009.
- European Medicines Agency. CHMP Assessment report for Valdoxan [Procedure No. EMEA/H/C/656, Doc. Ref. EMEA/CHMP/87018/2006].http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_‐_Public_assessment_report/human/000656/WC500070527.pdf. [Accessed 22 January 2013] 2006.
Corruble 2013 {published data only}
- Corruble E, Belaidi C, Goodwin GM. Agomelatine versus escitalopram in major depressive disorders. European Psychiatry [abstracts from the 19th European Congress of Psychiatry, EPA 2011 Mar 12‐15; Vienna, Austria] 2011;26(S1):619. [Google Scholar]
- Corruble E, Bodinat C, Belaidi C, Goodwin GM. Efficacy of agomelatine and escitalopram on depression, subjective sleep and emotional experiences in patients with major depressive disorder: a 24‐wk randomized, controlled, double‐blind trial. International Journal of Neuropsychopharmacology 2013:1‐16 [epub ahead of print]. [DOI] [PubMed] [Google Scholar]
Hale 2010 {published data only (unpublished sought but not used)}
- Hale A. Efficacy and safety of agomelatine (25 mg/day with potential adjustment to 50 mg) given orally for eight weeks in out‐patients with severe major depressive disorder: a randomised double‐blind, parallel groups, international study versus selective serotonin reuptake inhibitor (SSRI) with a double‐blind extension period of 16 weeks. Controlled‐Trials.com 2008 (http://www.controlled‐trials.com/ISRCTN19313268/servier).
- Hale A, Corral RM, Mencacci C, Ruiz JS, Severo CA, Gentil V. Superior antidepressant efficacy results of agomelatine versus fluoxetine in severe MDD patients: a randomized, double‐blind study. International Clinical Psychopharmacology 2010;25(6):305‐14. [DOI] [PubMed] [Google Scholar]
Kasper 2010 {published data only (unpublished sought but not used)}
- Kasper S, Hajak G, Wulff K, Hoogendijk WJG, Montejo AL, Smeraldi E, et al. Efficacy of the novel antidepressant agomelatine on the circadian rest‐activity cycle and depressive and anxiety symptoms in patients with major depressive disorder: A randomized, double‐blind comparison with sertraline [ISRCTN49376288]. Journal of Clinical Psychiatry 2010;71(2):109‐20. [DOI] [PubMed] [Google Scholar]
Kennedy 2008 {published data only (unpublished sought but not used)}
- Kennedy SH, Rizvi S, Fulton K, Rasmussen J. A double‐blind comparison of sexual functioning, antidepressant efficacy, and tolerability between agomelatine and venlafaxine XR. Journal of Clinical Psychopharmacology 2008;28(3):329‐33. [DOI] [PubMed] [Google Scholar]
Lemoine 2007 {published data only (unpublished sought but not used)}
- Lemoine P, Guilleminault C, Alvarez E. Improvement in subjective sleep in major depressive disorder with a novel antidepressant, agomelatine: randomized, double‐blind comparison with venlafaxine. Journal of Clinical Psychiatry 2007;68(11):1723‐32. [DOI] [PubMed] [Google Scholar]
Loo 2002a {published data only}
- Loo H, Hale A, D'haenen H. Determination of the dose of agomelatine, a melatoninergic agonist and selective 5‐HT(2C) antagonist, in the treatment of major depressive disorder: a placebo‐controlled dose range study. International Clinical Psychopharmacology 2002;17(5):239‐47. [DOI] [PubMed] [Google Scholar]
Martinotti 2012 {published data only}
- Martinotti G, Sepede G, Nicola M, Iorio G, Gambi F, Risio L, et al. Agomelatine versus venlafaxine in the treatment of anhedonia in major depressive subjects: A pilot study [oral presentation]. European Psychiatry [abstracts of the 20th European Congress of Psychiatry, EPA. 2012 Mar 3‐6; Prague Czech Republic] 2012;27(Suppl 1):O‐35. [Google Scholar]
- Martinotti G, Sepede G, Gambi F, Iorio G, Berardis D, Nicola M, et al. Agomelatine versus venlafaxine XR in the treatment of anhedonia in major depressive disorder: A pilot study. Journal of Clinical Psychopharmacology 2012;32(4):487‐91. [DOI] [PubMed] [Google Scholar]
Quera‐Salva 2011 {published data only (unpublished sought but not used)}
- Quera‐Salva M‐A, Hajak G, Philip P, Montplaisir J, Keufer‐Le Gall S, Laredo J, et al. Comparison of agomelatine and escitalopram on nighttime sleep and daytime condition and efficacy in major depressive disorder patients. International Clinical Psychopharmacology 2011;26(5):252‐62. [DOI] [PubMed] [Google Scholar]
- Quera‐Salva M‐A, Servier Laboratories. Effects of agomelatine on sleep electroencephalogram parameters compared to selective serotonin reuptake inhibitors in patients with major depressive disorder: a six‐week randomised, double‐blind parallel group study versus comparator, followed by a double‐blind optional treatment extension period up to six months [ISRCTN44737909; CL3‐20098‐056; EUCTR2006‐004716‐48]. Controlled‐Trials.com [www.controlled‐trials.com] 2007. [Google Scholar]
Shu 2013 {published data only}
- Shu L, Sulaiman AH, Huang YS, et al. Comparable efficacy and safety of 8 weeks treatment with agomelatine 25‐50mg or fluoxetine 20‐40mg in Asian out‐patients with major depressive disorder. Asian Journal of Psychiatry 2013;(in press). [DOI: 10.1016/j.ajp.2013.09.009] [DOI] [PubMed] [Google Scholar]
- Shu L, Zhang Y, Servier Laboratories. Efficacy and safety of agomelatine with flexible dose (25 mg/day with potential adjustment at 50 mg) given orally for 8 weeks in out‐patients with Major Depressive Disorder. A randomised flexible dose double‐blind international multicentric study with parallel groups, versus fluoxetine (20 mg/day with potential adjustment at 40 mg) [ChiCTR‐TRC‐11001668]. Chinese Clinical Trial Registry [http://www.chictr.org/en] 2011. [Google Scholar]
References to studies excluded from this review
CAGO2301 {published data only}
- Novartis Pharmaceuticals. An 8‐week, randomized, double‐blind, placebo‐controlled, parallel‐group, multi‐center study of the efficacy and safety of agomelatine 0.5 mg and 1 mg sublingual tablets administered once daily in patients with major depressive disorder (MDD) [NCT01110889; CAGO178C2301]. ClinicalTrials.gov [www.clinicaltrials.gov] 2010.
CAGO2302 {published data only}
- Novartis Pharmaceuticals. A 8‐week, randomized, double‐blind, placebo‐controlled, parallel‐group, multi‐center study of the efficacy and safety of agomelatine 0.5 mg and 1 mg sublingual tablets administered once daily in patients with major depressive disorder (MDD) [NCT01110902; CAGO178C2302]. ClinicalTrials.gov [www.clinicaltrials.gov] 2010.
CAGO2304 {published data only}
- Novartis Pharmaceuticals. A 52‐week, randomized, double‐blind, placebo‐controlled, multi‐center, parallel‐group study of the long‐term efficacy, tolerability and safety of agomelatine 25 and 50 mg in the prevention of relapse of major depressive disorder (MDD) following open‐label treatment of 16‐24 weeks [NCT00467402; CAGO178A2304]. ClinicalTrials.gov [www.clinicaltrials.gov] 2007.
CL2‐009 {published data only}
- Servier Laboratories. Efficacy and safety of 3 doses (0.25, 0.5 and 1mg/day) of agomelatine sublingual administration over an 8‐week treatment period, in out‐patients with Major Depressive Disorder. An 8‐week randomised, double‐blind, fixed dose, international multicentre, placebo‐controlled study with parallel groups, followed by an extension double‐blind treatment period of 16 weeks [CL2‐90098‐009; EUCTR2009‐014045‐92]. EU Clinical Trials Register [.www.clinicaltrialsregister.eu] 2009.
CL3‐021 {published data only}
- European Medicines Agency. CHMP Assessment report for Thymanax [Procedure No. EMEA/H/C/000916, Doc. Ref. EMEA/97539/2009].http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_‐_Public_assessment_report/human/000915/WC500046226.pdf [Accessed 22 October 2012] 2008.
- European Medicines Agency. CHMP Assessment report for Thymanax [Procedure No. EMEA/H/C/000916, Doc. Ref. EMEA/97539/2009].http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_‐_Public_assessment_report/human/000916/WC500038315.pdf. [Accessed 25 October 2012] 2009.
CL3‐025 {published data only}
- European Medicines Agency. CHMP Assessment report for Thymanax [Procedure No. EMEA/H/C/000916, Doc. Ref. EMEA/97539/2009].http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_‐_Public_assessment_report/human/000915/WC500046226.pdf. [Accessed 22 October 2012] 2008.
- European Medicines Agency. CHMP Assessment report for Thymanax [Procedure No. EMEA/H/C/000916, Doc. Ref. EMEA/97539/2009].http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_‐_Public_assessment_report/human/000916/WC500038315.pdf. [Accessed 25 October 2012] 2009.
CL3‐026 {published data only}
- European Medicines Agency. CHMP Assessment report for Thymanax [Procedure No. EMEA/H/C/000916, Doc. Ref. EMEA/97539/2009].http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_‐_Public_assessment_report/human/000915/WC500046226.pdf. [Accessed 22 October 2012] 2008.
- European Medicines Agency. CHMP Assessment report for Thymanax [Procedure No. EMEA/H/C/000916, Doc. Ref. EMEA/97539/2009].http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_‐_Public_assessment_report/human/000916/WC500038315.pdf. [Accessed 25 October 2012] 2009.
Corral 2009 {published data only}
- Corral RM, Servier Laboratories. Efficacy and safety of three dose regimens of agomelatine (10, 25, 25 ‐ 50 mg) versus placebo given once a day for 6 weeks in out‐patients suffering from moderate to severe major depressive disorder: a 6‐week randomised, double‐blind, placebo‐controlled, parallel groups study followed by a double‐blind optional 18‐week extension period [ISRCTN10845256; CL3‐20098‐069; EUCTR2009‐011238‐84]. Controlled‐Trials.com [www.controlled‐trials.com] 2009.
Goodwin 2009 {published data only}
- Goodwin GM, Emsley R, Rembry S, Rouillon F, Agomelatine Study Group. Agomelatine prevents relapse in patients with major depressive disorder without evidence of a discontinuation syndrome: a 24‐week randomized, double‐blind, placebo‐controlled trial [ISRCTN53193024]. Journal of Clinical Psychiatry 2009;70(8):1128‐37. [DOI] [PubMed] [Google Scholar]
Heun 2013 {published data only}
- Heun R, Ahokas A, Boyer P, Gimenez‐Montesinos N, Pontes‐Soares F, Olivier V. The efficacy of agomelatine in elderly patients with recurrent major depressive disorder: a placebo‐controlled study. Journal of Clinical Psychiatry 2013;74(6):587‐94. [DOI] [PubMed] [Google Scholar]
- Heun R, Servier Laboratories. Efficacy and safety of agomelatine oral administration (25 to 50 mg/day) in elderly patients suffering from major depressive disorder: an 8‐week, randomised, double‐blind, flexible‐dose, parallel groups, placebo‐controlled, international, multicentre study followed by an extension double‐blind treatment period of 16 weeks [ISRCTN57507360; CL3‐20098‐070; EUCTR2009‐011795‐29]. Controlled‐Trials.com [www.controlled‐trials.com] 2010.
Kennedy 2006 {published data only}
- Kennedy SH, Emsley R. Placebo‐controlled trial of agomelatine in the treatment of major depressive disorder. European Neuropsychopharmacology 2006;16(2):93‐100. [DOI] [PubMed] [Google Scholar]
Loo 2002b {published data only}
- Loo H, Dalery J, Macher JP, Payen A. Pilot study comparing in blind the therapeutic effect of two doses of agomelatine, melatoninergic agonist and selective 5ht2c receptors antagonist, in the treatment of major depressive disorders. L'Encephale 2002;28(4):356‐62. [PubMed] [Google Scholar]
Olie 2007 {published data only}
- Olie JP, Kasper S. Efficacy of agomelatine, a MT1/MT2 receptor agonist with 5‐HT2C antagonistic properties, in major depressive disorder. International Journal of Neuropsychopharmacology 2007;10(5):661‐73. [DOI] [PubMed] [Google Scholar]
Rouillon 2008 {published data only}
- Rouillon F, Servier Laboratories. Efficacy and safety of two doses of S 90098 (1 and 2 mg/day), sublingual formulation for 8 weeks in out‐patients with major depressive disorder: An 8‐week randomised, double‐blind, fixed dose, international, multicentre, placebo‐controlled study with parallel groups, followed by an extension double‐blind treatment period for 16 weeks [ISRCTN38378163; CL2‐90098‐005]. Controlled‐Trials.com [www.controlled‐trials.com] 2008.
Saletu 2011 {published data only}
- Saletu M, Saletu‐Zyhlarz GM, Anderer P, Rosales‐Rodriguez S, Saletu B. On the acute effect of agomelatine on sleep and awakening in major depression: controlled polysomnographic and psychometric studies [abstract]. Somnologie [abstracts of the 19th Jahrestagung der DGSM; 2011 Nov 10‐12; Mannheim Germany] 2011. [Google Scholar]
Save 2011 {published data only}
- Save D, Precise Chemipharma Pvt Ltd. A multicentric, open‐label, randomized, comparative, parallel‐group, active‐controlled Phase III clinical trial of the efficacy and safety of agomelatine oral tablets in patients with major depressive disorder [CTRI/2011/08/001946]. Clinical Trials Registry ‐ India [http://ctri.nic.in] 2011.
Serfaty 2010 {published data only}
- Serfaty MA, Osborne D, Buszewicz MJ, Blizard R, Raven PW. A randomized double‐blind placebo‐controlled trial of treatment as usual plus exogenous slow‐release melatonin (6 mg) or placebo for sleep disturbance and depressed mood. International Clinical Psychopharmacology 2010;25(3):132‐42. [DOI] [PubMed] [Google Scholar]
Stahl 2010 {published data only}
- Stahl SM, Fava M, Trivedi MH, Caputo A, Shah A, Post A. Agomelatine in the treatment of major depressive disorder: an 8‐week, multicenter, randomized, placebo‐controlled trial [NCT00411242]. Journal of Clinical Psychiatry 2010;71(5):616‐26. [DOI] [PubMed] [Google Scholar]
Tseng 2007 {published data only}
- Tseng M‐C. The effect of agomelatine or fluoxentine on heart rate variability in patients with major depressive disorder [NCT00451490]. ClinicalTrials.gov [www.clinicaltrials.gov] 2007. [Google Scholar]
Zajecka 2010 {published data only}
- Zajecka J, Schatzberg A, Stahl S, Shah A, Caputo A, Post A. Efficacy and safety of agomelatine in the treatment of major depressive disorder: a multicenter, randomized, double‐blind, placebo‐controlled trial. Journal of Clinical Psychopharmacology 2010;30(2):135‐44. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
CL3‐027 {published data only}
- European Medicines Agency. CHMP Assessment report for Valdoxan [Procedure No. EMEA/H/C/656, Doc. Ref. EMEA/CHMP/87018/2006].http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_‐_Public_assessment_report/human/000656/WC500070527.pdf. [Accessed 22 January 2013] 2006.
CL3‐048 {published data only}
- Bougerol T, Servier Laboratories. Efficacy of agomelatine given orally on the quality of remission in elderly depressed patients, after a 12‐week treatment period. A randomised, double‐blind, flexible‐dose international multicentre study with parallel groups versus SSRI drug. Twelve‐week treatment plus optional continuation for 12 weeks [ISRCTN68222771; CL3‐20098‐048; EUCTR2005‐002388‐95]. Controlled‐Trials.com [www.controlled‐trials.com] 2006.
CL3‐062 {published data only}
- Marey C, Servier Laboratories. Evaluation of efficacy and clinical benefit of agomelatine in patients with major depressive disorder compared to serotonin‐norepinephrine reuptake inhibitor (SNRI) [ISRCTN96725312; CL3‐20098‐062; EUCTR2008‐004642‐92]. Controlled‐Trials.com [www.controlled‐trials.com] 2009.
CL3‐073 {published data only}
- Lejoyeux M, Servier Laboratories. Initiation of agomelatine after antidepressant treatment in outpatients suffering major depressive disorder [ISRCTN97599615; CL3‐20098‐073; EUCTR2010‐019556‐44]. Controlled‐Trials.com [www.controlled‐trials.com] 2010.
CRSC11003 {published data only}
- Cadila Pharmaceuticals Limited. A randomized, multi‐center, double blind, controlled, comparative study of the efficacy and safety of agomelatine 25 mg (or 50 mg) and paroxetine 20mg (or 30 mg) in patients with major depressive disorder (MDD) [CTRI/2012/03/002480; CRSC11003]. Clinical Trials Registry ‐ India [http://ctri.nic.in] 2012.
CTRI/2011/04/001659 {published data only}
- Borgharkar S, Sun Pharmaceutical Industries Ltd. Evaluation of efficacy and safety of agomelatine versus venlafaxine ER in the treatment of major depressive disorder. Clinical Trials Registry ‐ India [http://ctri.nic.in] 2011. [Google Scholar]
Karaiskos 2013 {published data only}
- Karaiskos D, Tzavellas E, Ilias I, Liappas I, Paparrigopoulos T. Agomelatine and sertraline for the treatment of depression in type 2 diabetes mellitus. International Journal of Clinical Practice 2013;67(3):257‐60. [DOI] [PubMed] [Google Scholar]
Montgomery 2004 {published data only}
- Montgomery SA, Kennedy SH, Burrows GD, Lejoyeux M, Hindmarch I. Absence of discontinuation symptoms with agomelatine and occurrence of discontinuation symptoms with paroxetine: a randomized, double‐blind, placebo‐controlled discontinuation. International Clinical Psychopharmacology 2004;19(5):271‐80. [DOI] [PubMed] [Google Scholar]
Vasile 2011 {published data only}
- Vasile D, Vasiliu O, Vasile ML, Terpan M, Ojog DG. Agomelatine versus selective serotoninergic reuptake inhibitors in major depressive disorder and comorbid diabetes mellitus [conference abstract]. European Neuropsychopharmacology [abstracts from the 24th Congress of the European College of Neuropsychopharmacology, ECNP 2011 Paris France, 3‐7 Sept] 2011;21(Suppl 3):S383‐4. [Google Scholar]
References to ongoing studies
CL3‐060 {published data only}
- Servier Laboratories. Effects of agomelatine versus escitalopram on emotional experiences in outpatients suffering from major depressive disorder. An exploratory, randomised, double‐blind, international, multicentre study with parallel groups: agomelatine (25 to 50 mg/day) versus escitalopram (10 to 20 mg/day) over a 6‐month period [EUCTR2011‐005320‐17‐GB; CL3‐20098‐060]. EU Clinical Trials Register [www.clinicaltrialsregister.eu] [Accessed 20 September 2013] 2012.
CL3‐074 {published data only}
- Shah A, Rajarshi M. Efficacy and safety of agomelatine with flexible dose (25 mg/day with blinded adjustment at 50 mg) given orally for 8 weeks in Indian outpatients with major depressive disorder. A randomised double‐blind national multicentric study with parallel groups, versus sertraline (50 mg/day with blinded potential adjustment at 100 mg) [CTRI/2010/091/006081; CL3‐20098‐074]. Clinical Trials Registry ‐ India [http://ctri.nic.in] [Accessed 20 September 2013] 2012.
CL3‐083 {published data only}
- Udristoiu T, Servier Laboratories. Early effect of agomelatine on general interest in outpatients suffering major depressive disorder: a parallel group, randomised, double‐blind, multicentre study [ISRCTN28327843; CL3‐20098‐083]. Controlled‐Trials.com [www.controlled‐trials.com] [Accessed 20 September 2013] 2011.
GENRAS {published data only}
- Medizinische Universität Wien Univ Klinik f Psychiatrie und Psychotherapie. GENRAS (GENetics of Response to Agomelatine vs. EScitalopram) [EUCTR2010‐019423‐61‐AT]. EU Clinical Trials Register [www.clinicaltrialsregister.eu] [Accessed 20 September 2013] 2010.
Lundbeck 2011 {published data only}
- H Lundbeck A/S. A randomised, double‐blind, parallel‐group, active‐controlled, flexible dose study evaluating the effects of Lu AA21004 versus agomelatine in adult patients suffering from major depressive disorder with inadequate response to antidepressant treatment [NCT01488071; EUCTR2011‐002362‐21]. ClinicalTrials.gov [www.clinicaltrials.gov] [Accessed 20 September 2013] 2011.
NCT01483053 {published data only}
- Baker IDI Heart and Diabetes Institute. A randomised trial investigating the cardiovascular effects of agomelatine and escitalopram in patients with major depressive disorder [NCT01483053]. ClinicalTrials.gov [www.clinicaltrials.gov] [Accessed 20 September 2013] 2011.
Additional references
AHRQ 2007
- Agency for Healthcare Research and Quality. Comparative effectiveness of second‐generation antidepressants in the pharmacologic treatment of adult depression. http://www.effectivehealthcare.ahrq.gov/ehc/products/7/61/Antidepressants_Executive_Summary.pdf (accessed 9 April 2010). [PubMed]
Als‐Nielsen 2003
- Als‐Nielsen B, Chen W, Gluud C, Kjaegard LL. Association of funding and conclusions in randomized drug trials. JAMA 2003;290:921‐8. [DOI] [PubMed] [Google Scholar]
Altman 1996
- Altman DG, Bland MJ. Detecting skewness for summary information. BMJ 1996;313:1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
APA 1980
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorder (DSM‐III), 3rd edition. American Psychiatric Press, 1980. [Google Scholar]
APA 1987
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM‐III‐R), 3rd edition revised. American Psychiatric Publishing, 1987. [Google Scholar]
APA 1994
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM‐IV), 4th edition. American Psychiatric Publishing, 2004. [Google Scholar]
APA 2000
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM‐IV‐TR) 4th Edition ‐ revised. American Psychiatric Publishing, 2000. [Google Scholar]
APA 2000a
- American Psychiatric Association. Practice guideline for the treatment of patients with major depressive disorder (revision). American Journal of Psychiatry 2000;157:1‐45. [PubMed] [Google Scholar]
Barbui 2004
- Barbui C, Cipriani A, Brambilla P, Hotopf M. "Wish bias" in antidepressant drug trials?. Journal of Clinical Psychopharmacology 2004;2:126‐30. [DOI] [PubMed] [Google Scholar]
Beck 1987
- Beck AT, Steer R. Beck Depression Inventory. Psychological Corporation, 1987. [Google Scholar]
Bhandari 2004
- Bhandari M, Busse JW, Jackowski D, Montori VM, Schunemann H, Sprague S, et al. Association between industry funding and statistically significant pro‐industry findings in medical and surgical randomized trials. CMAJ 2004;170:477‐80. [PMC free article] [PubMed] [Google Scholar]
Boissel 1999
- Boissel JP, Cucherat M, Li W, Chatellier G, Gueyffier F, Buyse M, et al. The problem of therapeutic efficacy indices. Comparison of the indices and their use. Therapie 1999;54:405‐11. [PubMed] [Google Scholar]
Bollini 1999
- Bollini P, Pampallona S, Tibaldi G, Kupelnick B, Munizza C. Effectiveness of antidepressants. Meta‐analysis of dose‐effect relationships in randomised clinical trials. British Journal of Psychiatry 1999;174:297‐303. [DOI] [PubMed] [Google Scholar]
Buchkowsky 2004
- Buchkowsky SS, Jewesson PJ. Industry sponsorship and authorship of clinical trials over 20 years. The Annals of Pharmacotherapy 2004;38:579‐85. [DOI] [PubMed] [Google Scholar]
Cipriani 2009
- Cipriani A, Furukawa TA, Salanti G, Geddes JR, Higgins JPT, Churchill R, et al. Comparative efficacy and acceptability of 12 new‐generation antidepressants: a multiple‐treatments meta‐analysis. Lancet 2009;373:746‐58. [DOI] [PubMed] [Google Scholar]
Clayton 2006
- Clayton AH, Montejo AL. Major depressive disorder, antidepressants,and sexual dysfunction. Journal of Clinical Psychiatry 2006;67(Suppl 6):33‐7. [PubMed] [Google Scholar]
Cohen 1960
- Cohen J. A coefficient of agreement for nominal scales. Educational and Psychological Measurement 1960;20:37‐46. [Google Scholar]
Deeks 2000
- Deeks J. Issues in the selection for meta‐analyses of binary data. Proceedings of the 8th International Cochrane Colloquium; Cape Town, South Africa. 2000 Oct 25‐28th.
Dolder 2008
- Dolder CR, Nelson M, Snider M. Agomelatine treatment of major depressive disorder. The Annals of Pharmacotherapy 2008;421:822‐31. [DOI] [PubMed] [Google Scholar]
Dording 2002
- Dording CM, Mischoulon D, Petersen TJ, Kornbluh R, Gordon J, Nierenberg AA, et al. The pharmacologic management of SSRI‐induced side effects: a survey of psychiatrists. Annals of Clinical Psychiatry 2002;14:143‐7. [DOI] [PubMed] [Google Scholar]
Elbourne 2002
- Elbourne DR, Altman DG, Higgins JP, Curtin F, Worthington HV, Vail A. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31:140‐9. [DOI] [PubMed] [Google Scholar]
EMEA 2009
- European Medicines Agency. CHMP Assessment report for Thymanax [Procedure No. EMEA/H/C/000916, Doc. Ref. EMEA/97539/2009].http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_‐_Public_assessment_report/human/000916/WC500038315.pdf. (Accessed 25 October 2012) 2009.
Feighner 1972
- Feighner JP, Robins E, Guze SB, Woodruff RA, Winokur G, Munoz R. Diagnostic criteria for use in psychiatric research. Archives of General Psychiatry 1972;26:57‐63. [DOI] [PubMed] [Google Scholar]
Fournier 2010
- Fournier JC, DeRubeis RJ, Hollon SD, Dimidjian S, Amsterdam JD, Shelton RC, et al. Antidepressant drug effects and depression severity: a patient‐level meta‐analysis. JAMA 2010;303:47‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Furukawa 2005
- Furukawa TA, Cipriani A, Barbui C, Brambilla P, Watanabe N. Imputing response rates from means and standard deviations in meta‐analysis. International Clinical Psychopharmacology 2005;20:49‐52. [DOI] [PubMed] [Google Scholar]
Furukawa 2006
- Furukawa TA, Barbui C, Cipriani A, Brambilla P, Watanabe N. Imputing missing standard deviations in meta‐analyses can provide accurate results. Journal of Clinical Epidemiology 2000;59:7‐10. [DOI] [PubMed] [Google Scholar]
Guy 1976
- Guy W (editor). ECDEU Assessment Manual for Psychopharmacology. US Department of Health, Education and Welfare, 1976. [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Junz R, Falck‐Vitter Y, Alonso‐Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hamilton 1960
- Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery and Psychiatry 1960;23:56‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hansen 2005
- Hansen RA, Gartlhner G, Lohr KN, Gaynes BN, Carey TS. Efficacy and safety of second‐generation antidepressants in the treatment of major depressive disorder. Annals of Internal Medicine 2005;143:415‐26. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. Chichester: John Wiley & Sons.
Howland 2011
- Howland RH. Publication bias and outcome reporting bias: agomelatine as a case example. Journal of Psychosocial Nursing and Mental Health Services 2011;49:11‐4. [DOI] [PubMed] [Google Scholar]
Kasper 2008
- Kasper S, Laigle L, Baylè F. Superior antidepressant efficacy of agomelatine vs sertraline: A randomized, double‐blind study. Poster presentation 21st ECNP Congress, Barcelona, Spain. 30 August ‐ 3 September 2008.
Kasper 2013
- Kasper S, Corruble E, Hale A, Lemoine P, Montgomery SA, Quera‐Salva MA. Antidepressant efficacy of agomelatine versus SSRI/SNRI: results from a pooled analysis of head‐to‐head studies without a placebo control. International Clinical Psychopharmacology 2013;28:12‐9. [DOI] [PubMed] [Google Scholar]
Kessler 2003
- Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, et al. National Comorbidity Survey Replication. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS‐R). JAMA 2003;289:3095‐105. [DOI] [PubMed] [Google Scholar]
Khan 2003
- Khan A, Khan SR, Walens G, Kolts R, Giller EL. Frequency of positive studies among fixed and flexible dose antidepressant clinical trials: an analysis of the food and drug administration summary basis of approval reports. Neuropsychopharmacology 2003;28:552‐7. [DOI] [PubMed] [Google Scholar]
Koesters 2013
- Koesters M, Guaiana G, Cipriani A, Becker T, Barbui C. Agomelatine efficacy and acceptability revisited: systematic review and meta‐analysis of published and unpublished randomised trials. The British Journal of Psychiatry 2013;203(3):179‐87. [DOI] [PubMed] [Google Scholar]
Lam 2009
- Lam RW, Kennedy SH, Grigoriadis S, McIntyre RS, Milev R, Ramasubbu R, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT). Canadian Network for Mood and Anxiety Treatments (CANMAT) clinical guidelines for the management of major depressive disorder in adults. III Pharmacotherapy. Journal of Affective Disorder 2009;117 Suppl 1:26‐43. [DOI] [PubMed] [Google Scholar]
Lexchin 2003
- Lexchin J, Bero LA, Djulbegovic B, Clark O. Pharmaceutical industry sponsorship and research outcome and quality: systematic review. BMJ 2003;326:1167‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lundh 2012
- Lundh A, Sismondo S, Lexchin J, Busuioc OA, Bero L. Industry sponsorship and research outcome. Cochrane Database of Systematic Reviews 2012, Issue 12. [DOI: 10.1002/14651858.MR000033.pub2] [DOI] [PubMed] [Google Scholar]
Middleton 2001
- Middleton N, Gunnell D, Whitley E, Dorling D, Frankel S. Secular trends in antidepressants prescribing in the UK, 1975‐98. Journal of Public Health Medicine 2001;23:262‐7. [DOI] [PubMed] [Google Scholar]
Millan 2003
- Millan MG, Gobert A, Lejeune F, Dekeyne A, Newman‐Tancredi A, Pasteau V, et al. The novel melatonin agonist agomelatine (S20098) is an antagonist at 5‐hydroxytryptamine2C receptors, blockade of which enhances the activity of frontocortical dopaminergic and adrenergic pathways. Journal of Pharmacology and Experimental Therapeutics 2003;306:954‐64. [DOI] [PubMed] [Google Scholar]
Montgomery 1979
- Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. British Journal of Psychiatry 1979;134:382‐9. [DOI] [PubMed] [Google Scholar]
Müller 2003
- Müller MJ, Himmerich H, Kienzle B, Szegedi A. Differentiating moderate and severe depression using the Montgomery Asberg depression rating scale (MADRS). Journal of Affective Disorders 2003;77(3):255‐60. [DOI] [PubMed] [Google Scholar]
NICE 2004
- National Institute of Clinical Excellence. Depression: management of depression in primary and secondary care. National Institute of Clinical Excellence, 2004. [Google Scholar]
Novartis 2012
- Novartis. Annual report 2011. http://www.novartis.com/downloads/investors/reports/novartis‐annual‐report‐2011‐en.pdf [Accessed 20 June 2013] 2012.
Oxman 1992
- Oxman AD, Guyatt GH. A consumer's guide to subgroup analyses. Annals of Internal Medicine 1992;116(1):78‐84. [DOI] [PubMed] [Google Scholar]
Popoli 2009
- Popoli M. Agomelatine: innovative pharmacological approach in depression. CNS Drugs 2009;23 Suppl 2:27‐34. [DOI] [PubMed] [Google Scholar]
Prescrire 2013
- Agomelatine: a review of adverse effects. Prescrire International 2013;136:70‐1. [PubMed] [Google Scholar]
Radloff 1977
- Radloff LS. The CES‐D scale: a self‐report depression scale for research in the general population. Applied Psychological Measurement 1977;1:385‐401. [Google Scholar]
Rosenbaum 2010
- Rosenbaum SE, Glenton C, Oxman AD. Summary‐of‐findings tables in Cochrane reviews improved understanding and rapid retrieval of key information. Journal of Clinical Epidemiology 2010;63:620‐6. [DOI] [PubMed] [Google Scholar]
San 2008
- San L, Arranz B. Agomelatine: a novel mechanism of antidepressant action involving the melatonergic and the serotonergic system. European Psychiatry 2008;23:396‐402. [DOI] [PubMed] [Google Scholar]
Servier 2013
- Servier Laboratories Ltd. Agomelatine (Valdoxan): monitor liver function and do not use in people with high transaminase levels (>3 x ULN) or ≥ 75 years. http://www.servier.co.uk/pdfs/direct‐healthcare‐professional‐communication.pdf [Accessed 20 September 2013] 2013. [Google Scholar]
Singh 2011
- Singh SP, Singh V, Kar N. Efficacy of agomelatine in major depressive disorder: meta‐analysis and appraisal. International Journal of Neuropsychopharmacology 2011;23:1‐12. [DOI] [PubMed] [Google Scholar]
Spitzer 1978
- Spitzer RL, Robins E. Research diagnostic criteria: rationale and reliability. Archives of General Psychiatry 1978;35:773‐82. [DOI] [PubMed] [Google Scholar]
Taylor 2009
- Taylor D, Paton C, Kapur S. The Maudesley Prescribing Guidelines. The Maudsley Prescribing Guidelines. Informa Healthcare, 2009. [Google Scholar]
WHO 1978
- World Health Organization. The Ninth Revision of the International Classification of Diseases and Related Health Problems (ICD‐9). World Health Organization, 1978. [Google Scholar]
WHO 1992
- World Health Organization. The Tenth Revision of the International Classification of Diseases and Related Health Problems (ICD‐10). World Health Organization, 1992. [Google Scholar]
WHO 2008
- World Health Organization. The Global Burden of Disease. 2004 update. World Health Organization, 2008. [Google Scholar]


