Abstract
Background
With the introduction of transcatheter aortic valve implantation, the role of surgical aortic valve replacement (SAVR) in elderly patients has been called into question. We investigated the short-term outcomes of SAVR in the elderly population.
Methods
All patients aged ≥ 70 years who underwent isolated SAVR in our centre between 2008 and 2017 were included in the study. Survival at 30 days and 1 year were compared for patients aged 70–79 years (n = 809) versus patients aged ≥ 80 years (n = 322). Factors associated with poorer survival outcomes were identified using multivariable Cox regression analysis.
Results
Patients aged 70–79 years and patients aged ≥ 80 years had similar survival rates at 30 days (98.1% vs. 98.4%, p = 0.732) and 1 year (96.0% vs. 94.1%, p = 0.162) post-SAVR. This remained true after multivariable adjustment. Risk factors for 30 day all-cause mortality included insulin dependent diabetes (HR 6.17, 95% CI 1.32–28.92, p = 0.021) and increasing cardiopulmonary bypass time (HR 2.72, 95% CI 1.89–3.91, p < 0.0001). Significant risk factors for 1 year all-cause mortality were New York Heart Association (NYHA) class IV (HR 6.25, 95% CI 1.55–25.24, p = 0.010) and longer cardiopulmonary bypass time (HR 1.94, 95% CI 1.40–2.69, p < 0.0001). Similar results were obtained for cardiac-specific mortality.
Conclusions
Short-term outcomes of SAVR are excellent in elderly patients and age alone is not a predictor of poorer outcomes. However, the increased risk of mortality in patients with insulin-dependent diabetes and those with severe functional impairment (NYHA class IV) should be carefully considered when selecting patients for SAVR in this elderly population.
Keywords: Surgical aortic valve replacement (SAVR), Transcatheter aortic valve implantation (TAVI), Elderly patients, Octogenarians, Surgical outcomes
Introduction
The prevalence of aortic stenosis (AS) increases with age and is estimated to be around 10–13% in people over the age of 80 years [1, 2]. This is expected to increase further as the population continues to age [3]. Elderly patients with AS pose a therapeutic challenge as they often have complex comorbidity, frailty and rehabilitation issues. Surgical aortic valve replacement (SAVR) can offer a considerable improvement in survival and quality of life but comes with significant potential risks, particularly in this elderly population. The introduction of transcatheter aortic valve implantation (TAVI), a less invasive procedure with comparable results to SAVR [4], has called into question the role of SAVR in elderly patients.
In the UK, TAVI is recommended for patients who are unsuitable for surgery due to high operative risk [5, 6]. This practice is based on current guidelines, usually without consideration of the frailty status of the patient. Indeed, the most recent American College of Cardiology (ACC) and American Heart Association (AHA) guidelines make a Class 1 A recommendation for transfemoral TAVI in preference to SAVR for patients above the age of 80 years who have severe, symptomatic AS and suitable anatomy [7].
The aim of this study was to compare 30-day and 1-year survival after SAVR for patients aged 70–79 years versus those aged 80 years and over, as well as to identify the risk factors which impact outcomes after SAVR in this elderly population.
Methods
This was a single-centre analysis of all consecutive patients undergoing isolated SAVR in the Golden Jubilee National Hospital, Glasgow between 1 April 2008 and 31 January 2017 with follow-up until 31 January 2018. After excluding patients younger than 70 years old and those with missing data, the final study population included 1131 patients (Fig. 1). All patients had a minimum of 1-year follow-up. Patient demographics, medical history, operative details, discharge destination and mortality data were extracted from the prospectively maintained hospital electronic record system. The impact of cardiac disease on patient functional status was recorded as per the New York Heart Association (NYHA) classification system [8].
Fig. 1.
Flowchart showing selection of study population
Clinical outcomes were compared for patients aged 70–79 versus patients aged 80 years and over. The primary outcome was survival at 30 days and at 1 year. Secondary outcomes were risk factors predictive of mortality after SAVR at 30 days and 1 year. Data on cause of mortality was collected from Information Services Division (ISD) Scotland and General Register Office Scotland (GROS) databases. All-cause mortality and cardiac-specific mortality were analysed separately. Categorical data were presented as number and percentage and compared with chi-squared tests. Non-parametric continuous data were presented as median and inter-quartile range and compared with Mann-Whitney U tests. Survival was calculated using the Kaplan Meier method with log-rank test. Multivariable analysis was undertaken using Cox proportional hazards regression to identify factors associated with poorer survival. Individual models were created for 30-day and 1-year survival as well as for all-cause and cardiac mortality. A decision was made a priori to include the variables age group, year of procedure and sex in all models. Other variables with p < 0.1 on univariable analysis were tested for inclusion in multivariable models and those leading to a significant (p < 0.05) change in log likelihood were retained using a manual backward elimination method. Potential interactions between all variables were tested and none were found to be significant. All data were analysed using SAS 9.4 (SAS Institute, Cary, NC, USA).
Results
A total of 1131 patients were included in the study, comprising 809 patients aged 70–79 years and 322 patients aged 80 years and over. The two groups of patients had similar baseline characteristics, except for a higher proportion of females in the 80 years and over group (Table 1). In both groups the majority of operations were elective, used biological valves and had a median cardiopulmonary bypass (CPB) time of approximately 1.5 h. The distribution of NYHA category and left ventricular ejection fraction was similar between the two groups, as was the proportion of patients with pre-operative diabetes, hypertension, peripheral vascular disease and neurological dysfunction.
Table 1.
Baseline characteristics of patients included in the study
| Variable | Age 70 - 79 years (n = 809) |
Age ≥ 80 years (n = 322) |
p-value |
|---|---|---|---|
| Gender (n, %) | 0.021 | ||
| Male | 383 (47.3%) | 128 (39.8%) | |
| Female | 426 (52.7%) | 194 (60.3%) | |
| Urgency (n, %) | 0.260 | ||
| Elective | 711 (87.9%) | 275 (85.4%) | |
| Emergency | 98 (12.1%) | 47 (14.6%) | |
| Redo operation (n, %) | 2 (0.25%) | 0 (0.00%) | 0.372 |
| Implant valve (n, %) | 0.178 | ||
| Mechanical | 17 (2.1%) | 3 (0.9%) | |
| Biological | 792 (97.9%) | 319 (99.1%) | |
| Cardiopulmonary bypass time in minutes (median, IQR) | 93 (80–109) | 91 (76–106) | 0.672 |
| Aortic cross clamp time in minutes (median, IQR) | 68 (59–81) | 67 (58–79) | 0.111 |
| NYHA category (n, %) | 0.423 | ||
| I | 94 (11.6%) | 27 (8.4%) | |
| II | 375 (46.4%) | 151 (46.9%) | |
| III | 313 (38.7%) | 134 (41.6%) | |
| IV | 27 (3.3%) | 10 (3.1%) | |
| Left ventricular ejection fraction (n, %) | 0.599 | ||
| Good (> 50%) | 689 (85.2%) | 275 (85.4%) | |
| Moderate (31–50%) | 100 (12.4%) | 35 (10.9%) | |
| Poor (21–30%) | 19 (2.4%) | 11 (3.4%) | |
| Very poor (< 21%) | 1 (0.1%) | 1 (0.3%) | |
| Diabetes (n, %) | 0.123 | ||
| Yes – dietary control | 39 (4.8%) | 18 (5.6%) | |
| Yes – oral medicine | 103 (12.7%) | 26 (8.1%) | |
| Yes – insulin | 21 (2.6%) | 6 (1.9%) | |
| Hypertension history (n, %) | 568 (70.2%) | 215 (66.8%) | 0.258 |
| Peripheral vascular disease (n, %) | 97 (12.0%) | 28 (8.7%) | 0.111 |
| Neurological dysfunction (n, %) | 22 (2.7%) | 4 (1.2%) | 0.135 |
NYHA, New York Heart Association
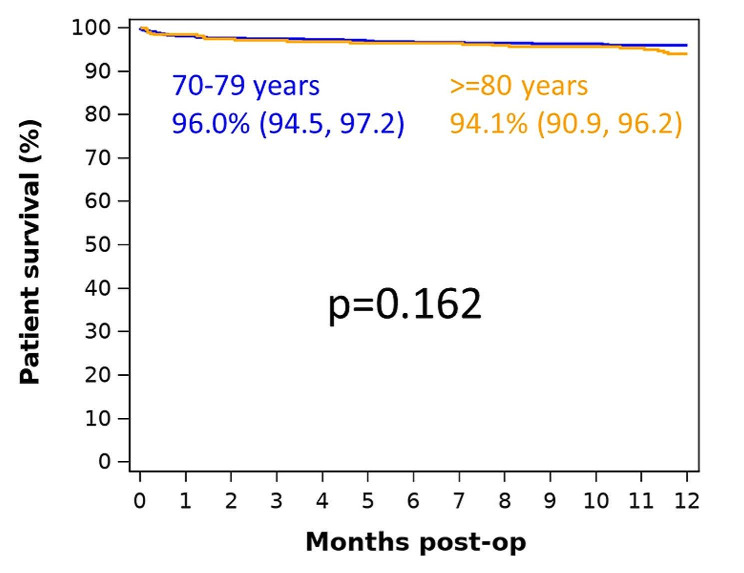
There were no significant differences in discharge destination between the two groups, with the large majority being discharged home (Fig. 2). There was also no significant difference in the proportion of inpatient deaths between the two groups (2.9% in the 70-79-year-old group versus 3.8% in the 80 years and over group, p = 0.672).
Fig. 2.
Discharge destination by age group
30-day survival
There was no significant difference in 30-day survival for all-cause mortality after SAVR between patients aged 70–79 years (98.1%, 95% confidence interval [CI] 96.9–98.9) versus those aged 80 years and over (98.4%, 95% CI 96.3–99.4) (Fig. 3). This remained the case after adjusting for confounding factors in the multivariable analysis (hazard ratio [HR] 0.84, 95% CI 0.30–2.33, p = 0.741 for 80 years and over versus 70–79 years), as shown in Table 2. The multivariable analysis also identified diabetes requiring insulin (HR 6.17, 95% CI 1.32–28.92, p = 0.021) and increasing CPB time (HR 2.72, 95% CI 1.89–3.91, p < 0.0001 for each additional hour of bypass time) as significant risk factors for 30-day all-cause mortality after SAVR (Table 2).
Fig. 3.

30-day survival (all-cause mortality)
Table 2.
Multivariable analysis of factors affecting 30-day all-cause mortality
| Variable | Hazard ratio | 95% CI | p-value | |
|---|---|---|---|---|
| LCL | UCL | |||
| Age group | ||||
| 70–79 | 1 (ref) | |||
| ≥ 80 | 0.84 | 0.30 | 2.33 | 0.741 |
| Year of procedure (per year) | 0.91 | 0.75 | 1.11 | 0.358 |
| Sex | ||||
| Male | 1 (ref) | |||
| Female | 1.47 | 0.59 | 3.64 | 0.401 |
| Diabetes | ||||
| No | 1 (ref) | |||
| Yes (dietary control) | 1.42 | 0.19 | 10.90 | 0.734 |
| Yes (oral medicine) | 0.90 | 0.20 | 4.03 | 0.893 |
| Yes (insulin) | 6.17 | 1.32 | 28.92 | 0.021 |
| Cardiopulmonary bypass time (per hour) | 2.72 | 1.89 | 3.91 | < 0.0001 |
CI, confidence interval; LCL, lower confidence limit; UCL, upper confidence limit.
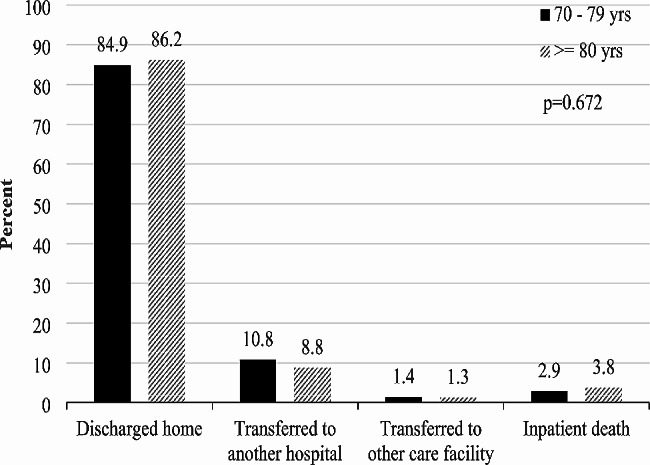
Similarly, there was no significant difference in 30-day cardiac-specific mortality after SAVR between the two groups (98.5% survival, 95% CI 97.4–99.2 for 70–79 years versus 98.4% survival, 95% CI 96.3–99.4 for 80 years and over, p = 0.931) (Fig. 4).
Fig. 4.
30-day survival (cardiac mortality)
In multivariable analysis, there continued to be no difference in 30-day cardiac mortality between the two age groups (HR 1.01, 95% CI 0.35–2.89, p = 0.984 for 80 years and over versus 70–79 years) (Table 3). Significant risk factors for 30-day cardiac mortality after SAVR included diabetes requiring insulin (HR 7.31, 95% CI 1.51–35.47, p = 0.014) and CPB time (HR 3.01, 95% CI 2.09–4.32, p < 0.0001 for each additional hour of CPB time) (Table 3).
Table 3.
Multivariable analysis of factors affecting 30-day cardiac mortality
| Variable | Hazard ratio | 95% CI | p-value | |
|---|---|---|---|---|
| LCL | UCL | |||
| Age group | ||||
| 70–79 | 1 (ref) | |||
| ≥ 80 | 1.01 | 0.35 | 2.89 | 0.984 |
| Year of procedure (per year) | 0.92 | 0.74 | 1.14 | 0.442 |
| Sex | ||||
| Male | 1 (ref) | |||
| Female | 1.78 | 0.65 | 4.89 | 0.263 |
| Diabetes | ||||
| No | 1 (ref) | |||
| Yes (dietary control) | 1.77 | 0.23 | 13.75 | 0.587 |
| Yes (oral medicine) | 0.54 | 0.07 | 4.24 | 0.559 |
| Yes (insulin) | 7.31 | 1.51 | 35.47 | 0.014 |
| Cardiopulmonary bypass time (per hour) | 3.01 | 2.09 | 4.32 | < 0.0001 |
CI, confidence interval; LCL, lower confidence limit; UCL, upper confidence limit
1-year survival
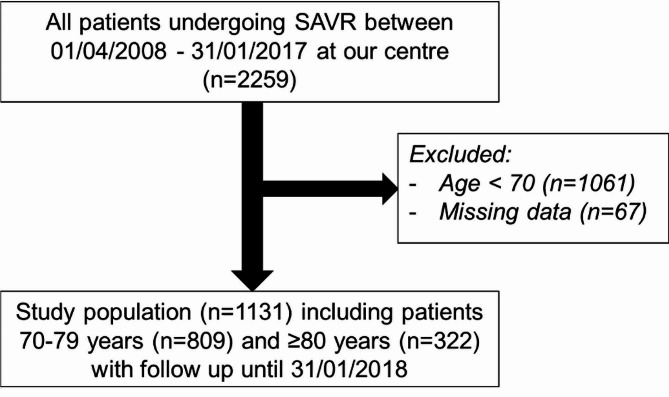
At 1 year, there was no significant difference in survival (all-cause mortality) after SAVR between patients aged 70–79 (96.0%, 95% CI 94.5–97.2) and 80 years and over (94.1%, 95% CI 90.9–96.2) (Fig. 5). This remained true after adjustment using multivariable analysis (HR 1.46, 95% CI 0.81–2.61, p = 0.206 for 80 years and over versus 70–79 years), Table 4. The multivariable analysis showed that higher NYHA category and increased CPB time were significant risk factors for 1-year all-cause mortality after SAVR (Table 4).
Fig. 5.
1-year survival (all-cause mortality)
Table 4.
Multivariable analysis of factors affecting 1-year all-cause mortality
| Variable | Hazard ratio | 95% CI | p-value | |
|---|---|---|---|---|
| LCL | UCL | |||
| Age group | ||||
| 70–79 | 1 (ref) | |||
| ≥ 80 | 1.46 | 0.81 | 2.61 | 0.206 |
| Year of procedure (per year) | 1.02 | 0.91 | 1.15 | 0.702 |
| Sex | ||||
| Male | 1 (ref) | |||
| Female | 1.10 | 0.62 | 1.95 | 0.757 |
| NYHA category | ||||
| I | 1 (ref) | |||
| II | 1.57 | 0.47 | 5.28 | 0.467 |
| III | 1.61 | 0.47 | 5.48 | 0.444 |
| IV | 6.25 | 1.55 | 25.24 | 0.010 |
| Cardiopulmonary bypass time (per hour) | 1.94 | 1.40 | 2.69 | < 0.0001 |
CI, confidence interval; LCL, lower confidence limit; UCL, upper confidence limit; NYHA, New York Heart Association
In terms of cardiac mortality, there was no significant difference in survival after SAVR between the two groups (97.1% survival, 95% CI 95.7–98.1 for 70–79 years versus 95.6% survival, 95% CI 92.7–97.4 for 80 years and over, p = 0.204) (Fig. 6). In multivariable analysis, there continued to be no difference in 1-year cardiac mortality between the two groups (HR 1.51, 95% CI 0.77–2.94, p = 0.227 for 80 years and over versus 70–79 years) (Table 5). Significant risk factors for 1-year cardiac mortality after SAVR included higher NYHA category and longer CPB time (Table 5).
Fig. 6.
1-year survival (cardiac mortality)
Table 5.
Multivariable analysis of factors affecting 1-year cardiac mortality
| Variable | Hazard ratio | 95% CI | p-value | |
|---|---|---|---|---|
| LCL | UCL | |||
| Age group | ||||
| 70–79 | 1 (ref) | |||
| ≥ 80 | 1.51 | 0.77 | 2.94 | 0.227 |
| Year of procedure (per year) | 1.02 | 0.89 | 1.17 | 0.766 |
| Sex | ||||
| Male | 1 (ref) | |||
| Female | 1.09 | 0.56 | 2.11 | 0.805 |
| NYHA category | ||||
| I | 1 (ref) | |||
| II | 3.53 | 0.57 | 26.75 | 0.221 |
| III | 3.99 | 0.53 | 30.27 | 0.181 |
| IV | 12.26 | 1.36 | 11.61 | 0.026 |
| Cardiopulmonary bypass time (per hour) | 2.08 | 1.48 | 2.93 | < 0.0001 |
CI, confidence interval; LCL, lower confidence limit; UCL, upper confidence limit; NYHA, New York Heart Association
Of note, aortic cross clamp time was significantly associated with 30-day and 1-year survival on univariate analysis in all models, however it became non-significant on multivariable analysis. This is likely because it is a confounder for overall CPB time and when both variables are included in the multivariable model, overall CPB time has a stronger association with the outcomes and hence was preferentially retained in the model.
Discussion
The standard of care for definitive treatment of severe, symptomatic AS has been SAVR, as recommended by international guidelines [5]. The longevity of SAVR has been demonstrated by numerous studies. Bouhout et al. [9] showed excellent outcomes in a cohort of 450 patients under the age of 65 years who underwent mechanical SAVR with actuarial survival at 1, 5, and 10 years of 98 ± 1%, 95 ± 1%, and 87 ± 1%, respectively. Actuarial freedom from prosthetic valve dysfunction was 99 ± 0.4%, 95 ± 1%, and 91 ± 1% at 1, 5, and 10 years, respectively. Actuarial freedom from valve reintervention in this study, was 98 ± 1%, 96 ± 1%, and 94 ± 1% at 1, 5 and 10 years, respectively. Similarly, Bourguignon et al. conducted a 20-year follow-up of the Carpentier-Edwards Perimount pericardial aortic valve bioprosthesis in 2659 patients aged 50–65 years and showed excellent outcomes, with expected valve durability of 19.7 years for the entire cohort [10]. Yoshikawa et al. [11] studied long-term outcomes of 1202 patients, with a median age of 76 years, who underwent SAVR with the Mosaic aortic porcine prosthesis. The authors noted that 12-year actuarial survival rate was 59.9 ± 7.5%, and freedom from valve-related death was 81.1 ± 7.9%. Freedom from reoperation was 86.4 ± 2.6% at 12 years. Freedom from structural valve deterioration at 12 years was 93.5 ± 2.9% for patients aged above 65 years and 98.2 ± 1.8% for those aged below 65 years. Cappabianca et al. [12] reported 12-year outcomes in octogenarians who had undergone SAVR. The authors noted that there was no statistically significant difference in long-term survival of the cohort when compared to an age- and gender-matched general population living in the same area. Mechanical and tissue SAVR have therefore demonstrated long-term durability across a wide age spectrum.
In 2002, Cribier performed the first TAVI, on a patient with calcific AS in cardiogenic shock, with multiple co-morbidities and who was deemed inoperable. Excellent valvular function and haemodynamic results were obtained but, unfortunately, the patient died 17 weeks later due to non-cardiac causes, including worsening of pre-procedural limb ischaemia [13]. TAVI has since evolved to become the intervention of choice in those unsuitable for surgery, as demonstrated in the first of the PARTNER trials [14]. Subsequent trials have shown that TAVI was a non-inferior alternative to surgery in patients with severe AS and intermediate surgical risk [15, 16] and, more recently, in low-risk patients [17, 18]. The NOTION trial demonstrated equivalent composite outcomes of all-cause mortality, stroke or myocardial infarction (MI) for TAVI versus SAVR at 5 years in a cohort of low-risk patients aged above 70 years [19]. However, the same authors noted that in those patients who had undergone TAVI, the incidence of moderate/severe total aortic regurgitation (8.2% versus 0.0%, P < 0.001) and need for a new permanent pacemaker (PPM) (43.7% versus 8.7%, P < 0.001) was significantly higher than in the SAVR group. Concerns have been raised by numerous authors about the long-term durability of the TAVI prostheses [20], rates of paravalvular leak (PVL) [21] and the need for a new PPM [21]. A Cochrane review from 2019 analysed the pooled results of four randomised controlled trials (RCTs) of TAVI versus SAVR in 2818 low-risk patients, most of whom were older than 70 years. The authors concluded that there was probably little or no difference in the short-term outcomes of all-cause and cardiac-specific mortality, stroke or MI. TAVI was associated with reduced risk of atrial fibrillation (AF), acute kidney injury (AKI), bleeding and, possibly, short-term rehospitalisation but at the expense of increased PPM implantation [22].
The impact of short-term complications of TAVI upon quality of life and mortality were assessed by Arnold et al. following the Partner 2 trial of intermediate and high-risk patients. They found that major stroke and stage 3 AKI were associated with markedly increased risk for 1-year mortality, as well as poorer quality of life amongst survivors. Additionally, moderate or severe PVL and life-threatening or major bleeding were associated with a modest increase in mortality and decrease in quality of life [23]. Furthermore, reintervention, whether surgical or TAVI valve-in-valve, would increase overall costs and potentially offset initial savings. Therefore, whether a healthcare system is funded by insurance, taxation or individual payment, there are ethical issues in proceeding with a treatment of unknown long-term cost-effectiveness and durability.
One fundamental omission in previous TAVI trials is that frailty of patients has not been analysed separately. Advanced age has been associated with frailty in a biased manner. Although 23% of the total global burden of disease is attributable to disorders in people aged 60 years and older [24–26], there is no simple correlation between age and frailty. Current ESC/EACTS guidelines [5] suggest considering TAVI in patients over the age of 75 years and AHA/ACC guidelines [7] in those older than 80 years. These guidelines are likely to heavily influence multi-disciplinary meetings and decision-making regarding intervention route in these patients.
Age per se is not a risk factor for excess mortality after SAVR [18]. Our study further supports this. Modality of treatment of aortic valve disease should not be decided on the patient’s age alone. As physicians treating elderly patients, we need to embrace the concept of frailty, which is a recognised clinical entity independent of age, co-morbidity and disability. It is defined as a state of reduced physiological reserve and is associated with an increased susceptibility to poor healthcare outcomes [27]. Frailty has been shown to be associated with poorer outcomes in all surgical specialties in the short- and long-term [28–30]. Although frailty now features in the most recent international guidelines [5, 7], we feel that it has not become widely incorporated into routine clinical practice. There are various methods and scoring systems to assess and quantify frailty, such as the 5-metre walking time test [31, 32] and Edmonton frailty scale [27], which are simple, reproducible and objective. These assessment tools could be used to assess frailty in elderly patients awaiting aortic valve intervention.
A male aged 65 years in the UK can now expect to live, on average, to 83.2 and a female of the same age to 85.7 [33]. At present, there is insufficient evidence to advocate the use of TAVI as definitive treatment for severe, symptomatic AS, to the exclusion of SAVR. Frailty, and not chronological age, should be one of the major determinants of intervention modality. We recommend incorporating a measure of frailty in the decision-making process for elderly patients awaiting aortic valve intervention.
In this single-institution study, we have demonstrated no statistically significant difference in 30-day and 1-year all-cause and cardiac-specific mortality after SAVR between two groups of elderly patients (70–79 year olds versus those older than 80 years). The factors that contributed to increased 30-day all-cause and cardiac-specific mortality in these patients were increased CPB time and the presence of diabetes requiring insulin. The factors that contributed to increased 1-year all-cause and cardiac mortality were preoperative NYHA class IV symptoms, which reflects the degree of preoperative heart failure, and increased CPB time. Apart from these risk factors, age group per se did not influence the 30-day and 1-year all-cause or cardiac mortality of this elderly cohort undergoing SAVR.
In a comparable study, Hussain et al. [34] analysed morbidity and mortality after SAVR in 351 patients categorised into age below 70 years, 70–79 and above 80. Median 1-year survival was 97%, 95% and 94%, respectively. In contrast to our study, they found a statistically significant difference between each of the age groups. Additionally, despite an increased rate of post-operative delirium and infection in the over 80 group, length of stay, rehospitalisation rate and risks of PPM, stroke, MI and heart failure were similar between the study groups.
Limitations of the study
This study has a number of limitations. Firstly, the data are from a single centre and, therefore, applicability to a wider patient cohort will be affected by local patient demographics, clinical practices and institutional protocols. Secondly, as this was not a randomised study, the effect of selection bias on the study population is unknown. A number of elderly patients may have been referred for TAVI or medical therapy and, therefore, not selected for surgery due to co-morbidities, frailty, patient choice and referring physician preference. Thirdly, although the two age groups were fairly well-matched with regard to baseline characteristics, there was a higher proportion of women in the group aged 80 years and above. This could also represent a degree of selection bias, for instance, if there are biological differences between age-matched men and women causing the latter to be more likely to be referred for SAVR. Since there is conflicting evidence regarding gender-specific differences in outcomes following SAVR [35], the effect of this discrepancy is unknown.
Further study should involve longer-term follow-up and, moreover, more detailed analysis of pre-operative and post-operative characteristics. Data on frailty and co-morbidities, such as coronary, pulmonary and chronic renal disease, and the presence of AF and pulmonary hypertension should be included. An extension to the study would be to compare rates of major post-operative morbidity between the two age groups, as well as quality of life and recovery time.
Author contributions
D.W., P.L., D.V. Wrote the main manuscript text. D.W. Performed the statistical analyses and prepared the figures and tables. N.A.A., K.S., V.Z., S.N. Revised the manuscript. All authors reviewed the manuscript.
Funding
None.
Data availability
Available upon request to the corresponding author and after authorisation from our Clinical Governance and Audit Department.
Declarations
Competing interests
V.Z. Is editor of the Journal of Cardiothoracic Surgery.
Ethical approval
Not applicable.
Footnotes
Abstract presented at the 14th Panhellenic Congress of The Hellenic Society of Thoracic and Cardiovascular Surgeons, November 24–26 2022, Thessaloniki, Greece.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Diana A. Wu and Peter Lang Contributed equally as first authors.
References
- 1.Osnabrugge RL, Mylotte D, Head SJ, Van Mieghem NM, Nkomo VT, LeReun CM, et al. Aortic stenosis in the elderly: disease prevalence and number of candidates for transcatheter aortic valve replacement: a meta-analysis and modeling study. J Am Coll Cardiol. 2013;62(11):1002–12. 10.1016/j.jacc.2013.05.015 [DOI] [PubMed] [Google Scholar]
- 2.Eveborn GW, Schirmer H, Heggelund G, Lunde P, Rasmussen K. The evolving epidemiology of valvular aortic stenosis. The Tromsø study. Heart. 2013;99(6):396–400. 10.1136/heartjnl-2012-302265 [DOI] [PubMed] [Google Scholar]
- 3.Danielsen R, Aspelund T, Harris TB, Gudnason V. The prevalence of aortic stenosis in the elderly in Iceland and predictions for the coming decades: the AGES-Reykjavík study. Int J Cardiol. 2014;176(3):916–22. 10.1016/j.ijcard.2014.08.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nagaraja V, Raval J, Eslick GD, Ong ATL. Transcatheter versus surgical aortic valve replacement: a systematic review and meta-analysis of randomised and non-randomised trials. Open Heart. 2014;1(1):e000013. 10.1136/openhrt-2013-000013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vahanian A, Beyersdorf F, Praz F, et al. 2021 ESC/EACTS guidelines for the management of valvular heart disease: developed by the Task Force for the management of valvular heart disease of the European Society of Cardiology (ESC) and the European Association for Cardio-thoracic surgery (EACTS). Euro Heart J. 2022;43(7):561–632. 10.1093/eurheartj/ehab395 [DOI] [Google Scholar]
- 6.National Institute for Health and Care Excellence (NICE). Transcatheter aortic valve implantation for aortic stenosis. Interventional procedures guidance [IPG586]; 2017.
- 7.Otto CM, Nishimura RA, Bonow RO, et al. 2020 ACC/AHA guidelines for the management of patients with Valvular Heart Disease. A report of the American College of Cardiologists/American Heart Association Joint Committee on Clinical Practice guidelines. Circulation. 2021;143:e72–227. [DOI] [PubMed] [Google Scholar]
- 8.Criteria Committee of the New York Heart Association. Nomenclature and criteria or diagnosis of diseases of the heart and blood vessels. Boston: Little Brown; 1964. [Google Scholar]
- 9.Bouhout I, et al. Long-term outcomes after elective isolated mechanical aortic valve replacement in young adults. J Thorac Cardiovasc Surg. 2014;148(4):1341–6. e1. 10.1016/j.jtcvs.2013.10.064 [DOI] [PubMed] [Google Scholar]
- 10.Bourguignon T, et al. Very long-term outcomes of the Carpentier-Edwards Perimount valve in aortic position. Ann Thorac Surg. 2015;99(3):831–7. 10.1016/j.athoracsur.2014.09.030 [DOI] [PubMed] [Google Scholar]
- 11.Yoshikawa Y, et al. Long-term outcomes of the Mosaic Aortic Porcine Bioprosthesis in Japan- results from the Japan Mosaic Valve Long-Term Multicenter Study. Circ J. 2020;84(8):1261–70. 10.1253/circj.CJ-19-1113 [DOI] [PubMed] [Google Scholar]
- 12.Cappabianca G, et al. Predictive factors of long-term survival in the octogenarian undergoing surgical aortic valve replacement: 12-year single-centre follow-up. Heart Vessels. 2016;31(11):1798–805. 10.1007/s00380-016-0804-3 [DOI] [PubMed] [Google Scholar]
- 13.Cribier A, Eltchaninoff H, Bash A, Borenstein N, Tron C, Bauer F, Derumeaux G, Anselme F, Laborde F, Leon MB. Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: first human case description. Circulation. 2002;106(24):3006–8. 10.1161/01.CIR.0000047200.36165.B8 [DOI] [PubMed] [Google Scholar]
- 14.Leon MB, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363(17):1597–607. 10.1056/NEJMoa1008232 [DOI] [PubMed] [Google Scholar]
- 15.Reardon MJ, et al. Surgical or transcatheter aortic-valve replacement in intermediate-risk patients. N Engl J Med. 2017;376(14):1321–31. 10.1056/NEJMoa1700456 [DOI] [PubMed] [Google Scholar]
- 16.Leon MB, et al. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N Engl J Med. 2016;374(17):1609–20. 10.1056/NEJMoa1514616 [DOI] [PubMed] [Google Scholar]
- 17.Mack MJ, et al. Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. N Engl J Med. 2019;380(18):1695–705. 10.1056/NEJMoa1814052 [DOI] [PubMed] [Google Scholar]
- 18.Popma JJ, et al. Transcatheter aortic-valve replacement with a self-expanding valve in low-risk patients. N Engl J Med. 2019;380(18):1706–15. 10.1056/NEJMoa1816885 [DOI] [PubMed] [Google Scholar]
- 19.Hans Gustav Hørsted Thyregod NI, Troels Højsgaard Jørgensen H, Nissen BJ, Kjeldsen P, Petursson Y, Chang OW, Franzen T, Engstrøm P, Clemmensen. Peter Bo Hansen, Lars Willy Andersen, Daniel Andreas Steinbruüchel, Peter Skov Olsen, Lars Søndergaard, five-year clinical and echocardiographic outcomes from the NOTION Randomized Clinical Trial in patients at Lower Surgical Risk. Circulation. 2019;139:2714–23. 10.1161/CIRCULATIONAHA.118.036606 [DOI] [PubMed] [Google Scholar]
- 20.Ferguson C, Blum P. Experts warn expanded heart valve use risks patient safety in a world full of dizzying innovation, marketing blitzes and conflicts of interest, doctors and patients must decide: how much risk is too much? Intern Consort Invest Journ 2018. https://www.icij.org/investigations/implant-files/experts-warn-expanded-heart-valve-use-risks-patient-safety/
- 21.Polimeni A et al. Transcatheter Versus Surgical aortic valve replacement in low-risk patients for the treatment of severe aortic stenosis. J Clin Med. 2020; 9(2). [DOI] [PMC free article] [PubMed]
- 22.Kolkailah AA, Doukky R, Pelletier MP, et al. Transcatheter aortic valve implantation versus surgical aortic valve replacement for severe aortic stenosis in people with low surgical risk (review). Cochrane Database Syst Reviews. 2019;12:CD013319. 10.1002/14651858.CD013319. 10.1002/14651858.CD013319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arnold SV, Zhang Y, Baron SJ, et al. Impact of short-term complications on mortality and quality of life after transcatheter aortic valve replacement. JACC: Cardiovasc Interv. 2019;12(4):362–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gilbert GH. ‘Ageism’ in dental care delivery. J Am Dent Assoc. 1989;118(5):545–8. 10.14219/jada.archive.1989.0076 [DOI] [PubMed] [Google Scholar]
- 25.Levy BR, et al. Ageism amplifies cost and prevalence of Health conditions. Gerontologist. 2020;60(1):174–81. 10.1093/geront/gny131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prince MJ, et al. The burden of disease in older people and implications for health policy and practice. Lancet. 2015;385(9967):549–62. 10.1016/S0140-6736(14)61347-7 [DOI] [PubMed] [Google Scholar]
- 27.Koizia L, et al. Use of the reported Edmonton frail scale in the assessment of patients for transcatheter aortic valve replacement: a possible selection tool in very high-risk patients? J Geriatr Cardiol. 2018;15(6):463–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robinson TN, et al. Simple frailty score predicts postoperative complications across surgical specialties. Am J Surg. 2013;206(4):544–50. 10.1016/j.amjsurg.2013.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McIsaac DI, et al. The impact of frailty on outcomes and healthcare resource usage after total joint arthroplasty: a population-based cohort study. Bone Joint J. 2016;98–B(6):799–805. 10.1302/0301-620X.98B6.37124 [DOI] [PubMed] [Google Scholar]
- 30.McIsaac DI, Bryson GL, van Walraven C. Association of frailty and 1-year postoperative mortality following major elective noncardiac surgery: a population-based cohort study. JAMA Surg. 2016;151(6):538–45. 10.1001/jamasurg.2015.5085 [DOI] [PubMed] [Google Scholar]
- 31.Wilson CM, Kostsuca SR, Boura JA. Utilization of a 5-meter walk test in evaluating self-selected gait speed during preoperative screening of patients scheduled for cardiac surgery. Cardiopulm Phys Ther Journ. 2013;24(3):36–43. 10.1097/01823246-201324030-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chaker Z, Badhwar V, Alqatani F, et al. Sex differences in the utilization and outcomes of surgical aortic valve replacement for severe aortic stenosis. J Am Heart Assoc. 2017;6:e006370. 10.1161/JAHA.117.006370. 10.1161/JAHA.117.006370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.National life tables: Great Britain 2020-2022. Office for National Statistics.
- 34.Hussain AI, Auensen A, Brunborg C, et al. Age-dependent morbidity and mortality outcomes after surgical aortic valve replacement. Interact CardioVasc Thor Surg. 2018;27:650–6. 10.1093/icvts/ivy154 [DOI] [PubMed] [Google Scholar]
- 35.Society of Thoracic Surgeons (STS). Adult Cardiac Surgery Database. Data Collection Form Version 4.20.2.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Available upon request to the corresponding author and after authorisation from our Clinical Governance and Audit Department.