Abstract
Background
Sarcoidosis is a heterogeneous granulomatous disease with no accurate biomarkers of disease progression. Therefore, we profiled and integrated the DNA methylome, mRNAs, and microRNAs to identify molecular changes associated with sarcoidosis and disease progression that might illuminate underlying mechanisms of disease and potential biomarkers.
Methods
Bronchoalveolar lavage cells from 64 sarcoidosis subjects and 16 healthy controls were used. DNA methylation was profiled on Illumina HumanMethylationEPIC arrays, mRNA by RNA-sequencing, and miRNAs by small RNA-sequencing. Linear models were fit to test for effect of sarcoidosis diagnosis and progression phenotype, adjusting for age, sex, smoking, and principal components of the data. We built a supervised multi-omics model using a subset of features from each dataset.
Results
We identified 1,459 CpGs, 64 mRNAs, and five miRNAs associated with sarcoidosis versus controls and four mRNAs associated with disease progression. Our integrated model emphasized the prominence of the PI3K/AKT1 pathway, which is important in T cell and mTOR function. Novel immune related genes and miRNAs including LYST, RGS14, SLFN12L, and hsa-miR-199b-5p, distinguished sarcoidosis from controls. Our integrated model also demonstrated differential expression/methylation of IL20RB, ABCC11, SFSWAP, AGBL4, miR-146a-3p, and miR-378b between non-progressive and progressive sarcoidosis.
Conclusions
Leveraging the DNA methylome, transcriptome, and miRNA-sequencing in sarcoidosis BAL cells, we detected widespread molecular changes associated with disease, many which are involved in immune response. These molecules may serve as diagnostic/prognostic biomarkers and/or drug targets, although future testing is required for confirmation.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12931-024-02919-7.
Keywords: Sarcoidosis, Epigenetics, Multi-omics, DNA methylation, Gene expression, microRNA
Introduction
Sarcoidosis is a heterogeneous disease characterized by non-caseating granulomatous inflammation that differentially impacts Black individuals and women [1]. The lungs are involved in over 90% of individuals [2]. Those with pulmonary sarcoidosis can be asymptomatic or demonstrate remission / resolution; however, progression can result in impairment and/or pulmonary fibrosis, the main cause of mortality [3]. The course of pulmonary sarcoidosis is unpredictable, with at least 25% of patients developing chronic or progressive disease requiring treatment [4]. Driven by a combination of genetic, environmental, and host immunologic factors, the underlying cause(s) of sarcoidosis are currently unknown. Multiple lines of evidence point towards an antigenic stimulus including HLA allele associations, environmental, seasonal, and regional patterns, and a Th1 predominate immune response in which CD4 + T cells secrete IFN-γ and TNF-α [5]. In addition, aberrant and dysfunctional immune responses are associated with sarcoidosis and supported by genome wide transcriptome studies [6]. While previous studies have elucidated many contributors to disease development and progression, many knowledge gaps remain.
Epigenetic mechanisms such as DNA methylation (DNAm) and microRNAs (miRNAs) mediate gene expression, are modified by exposures, and are dynamic and reversible, making them candidates for gene regulation in sarcoidosis as well as promising biomarkers and therapeutic targets. Epigenetic dysregulation has been identified in many lung diseases and likely drives sarcoidosis progression and manifestations. We have previously demonstrated changes in DNAm and mRNA gene expression in bronchoalveolar lavage (BAL) cells from chronic beryllium disease (a granulomatous lung disease caused by beryllium exposure) patients and a small sample of sarcoidosis patients [7, 8]. No other studies have evaluated epigenome-wide DNAm in sarcoidosis although others have identified miRNAs associated with disease [9, 10]. Additionally, BALF miRNAs such as miR-27b, miR-192, and miR-221 have been associated with pulmonary sarcoidosis progression [11]. These studies were limited by small sample sizes, targeted as opposed to genome-wide approaches, and a lack of integration of different omics modalities.
With a larger integrated approach utilizing several genomic data types, we hypothesize that epigenomic studies could link risk factors to disease pathobiology to better understand disease course, and subclassify patients based on molecular profiling; ultimately this would direct focused research on disease manifestations and treatment. In a first step, we conducted this study to profile genome-wide DNAm, mRNA and miRNA expression in sarcoidosis BAL cells, stratified by disease progression. Analyzing each dataset separately, we identified many molecular features associated with disease overall. By next constructing a sparse multi-omic model incorporating DNAm, mRNAs, and miRNAs, we identified features associated with sarcoidosis and pulmonary progression.
Methods
Study population
Sarcoidosis BAL cell samples were obtained from the Genomic Research in Alpha-1 Antitrypsin Deficiency and Sarcoidosis (GRADS) consortium [12] including National Jewish Health (NJH, n = 17) and non-NJH GRADS cases (n = 39) and cases from the Granuloma Biorepository at NJH (n = eight). Controls with no history of lung disease were obtained from the NJH Donor Lung Core (n = 16). All sarcoidosis subjects met the ATS/ERS criteria [13] for tissue biopsy confirmation of diagnosis of sarcoidosis. For all NJH cases, medical records were reviewed for clinical features including acuity of presentation (acute/non-acute), organ involvement, pulmonary function tests (PFT), chest imaging and immunosuppressive treatment at time of BAL and up to two years after BAL. For the non-NJH GRADS cases, acuity of presentation, PFTs, and immunosuppressive treatment were available at and up to six months after BAL. Cases with fibrotic/Scadding stage 4 chest radiographic disease and/or on immunosuppressive treatment at time of BAL were excluded.
Sarcoidosis cases were categorized as non-progressive or progressive pulmonary phenotypes. The non-progressive phenotype was defined as having either acute (i.e. consistent with Lofgren’s syndrome) or non-acute disease presentation, no new organ involvement, lung function testing with < 10% decline in FVC or FEV1, < 15% decline in DLCO, and stable chest imaging within two years after BAL. The progressive phenotype had a non-acute disease presentation; lung function testing with ≥ 10% decline in FVC or FEV1; or ≥ 15% decline in DLCO; worsening chest imaging; and/or required initiation of systemic immunosuppressive treatment any time up to two years after BAL. Non-NJH GRADS cases were phenotyped based on disease acuity, PFT, and treatment status only.
Bronchoscopy and nucleic acid processing
Bronchoscopy with BAL was performed as previously described [12, 14]. Cells were isolated and frozen at -80 C in RLT buffer. DNA and RNA were extracted using the Qiagen AllPrep DNA/RNA extraction mini kit. Purified genomic DNA was bisulfite-converted with the Zymo EZ-96 DNA Methylation bisulfite conversion kit, followed by whole-genome amplification and enzymatic fragmentation. DNA was denatured and hybridized to Illumina Infinium HumanMethylationEPIC BeadChips, followed by single base extension. Hybridized BeadChips were stained, washed, and scanned using Illumina’s iScan System. mRNA libraries were prepared from 500 ng total RNA with TruSeq stranded mRNA library preparation kits (Illumina) and miRNA libraries were constructed using Lexogen Small RNA-Seq library preparation kits. RNA libraries were sequenced at an average depth of 80M 150 bp paired-end reads on the Illumina NovaSeq 6000.
Data analysis
RNA-Sequencing data was previously produced on 28 samples by the GRADS consortium [6]. RNA paired-end reads from both GRADS and NJH samples were aligned at the gene level to Ensembl GrCh38 using STAR [15]. The forward read of miRNA FASTQ files were aligned to sequences from miRBase v22.1 [16–21] using miR-MaGiC [22]. miRNAs present in at least 50% of samples were retained (n = 818). Illumina idat signal intensity files were processed using SeSAMe; within-sample normalization with out-of-band probes and dye bias correction were performed [23]. Probes with non-unique mapping and off-target hybridization were removed. Additionally, probes with an average detection p-value ≥ 0.05 within samples (indicating an inability to statistically discern signal from background noise) and sex chromosome probes were removed prior to analysis. Methylation levels were analyzed as M values, and are presented in results as β values [24].
For each dataset, linear models were fit to each feature testing for an effect of sarcoidosis diagnosis or progressive vs. non-progressive disease while adjusting for age, sex, and smoking status (using the R package limma [25] for methylation data and DESeq2 [26] for RNA datasets). We further adjusted for three principal components derived from the data in methylation and mRNA case-control comparisons. Shrunken effect sizes from RNA comparisons (expressed as log2(fold change)) were calculated using Approximate Posterior Estimation for the GLM (apeglm) [27]. Null test statistic distributions were derived for each analysis using bacon to reduce bias and inflation [28]. P-values were adjusted to a 5% false discovery rate (FDR) to account for multiple testing using the Benjamini-Hochberg procedure [29]. Enrichment of significant results in the Gene Ontology (GO) resource [30] and the Kyoto Encyclopedia of Genes and Genomes (KEGG) [31] was performed using GOmeth [32] for DNAm data and clusterProfiler [33] for mRNA data. CpGs were annotated to CpG islands, shelves, and shores, and gene elements using the annotatr R package [34]. Experimentally confirmed miRNA target genes were obtained from MirTarBase [35]. For each miRNA, we retained target genes that had at least 2 independent sources of experimental evidence of association with the relevant miRNA, including at least one source using assays considered strong evidence by MirTarBase (reporter assay, Western blot, or qPCR).
Data integration analysis for biomarker discovery using latent cOmponents (DIABLO)
Methylation M values and normalized mRNA and miRNA gene expression values were used as input for a DIABLO [36] model implemented in the mixOmics R package [37]. Gene expression values were normalized to library size using DESeq2 [26] and transformed to account for heteroscedasticity with a variance stabilizing transformation (VST) [38]. A subset of features from each dataset for two model components were selected for model input using LASSO regression and five-fold cross-validation was repeated ten times.
Results
Demographics of study population
We recruited 64 sarcoidosis cases, including 26 progressive and 38 non-progressive, and 16 healthy controls. Demographic information at time of BAL is displayed in Table 1. No significant differences were observed in race, ethnicity, or age. Non-progressive cases were more likely female than progressive cases. Progressive cases presented with significantly reduced FEV1 and FVC and more Stage 2 disease.
Table 1.
Demographic information of control, progressive sarcoidosis, and non-progressive sarcoidosis cases at time of bronchoscopy with lavage
| Control | Non-Progressive | Progressive | p | |
|---|---|---|---|---|
| n | 16 | 38 | 26 | |
| Age (mean (SD)) | 55.62 (9.74) | 51.68 (9.88) | 51.19 (9.61) | 0.316* |
| Sex = Male (%) | 11 (68.8) | 11 (28.9) | 16 (61.5) | 0.006** |
| Race (%) | 0.242** | |||
| Asian | 1 (6.2) | 1 (2.6) | 0 (0.0) | |
| Black | 0 (0.0) | 6 (15.8) | 5 (19.2) | |
| White | 15 (93.8) | 31 (81.6) | 21 (80.8) | |
| Ethnicity = Non-Hispanic (%) | 15 (93.8) | 36 (94.7) | 25 (100.0) | 0.441** |
| Smoking Status = Former (%) | 0 (0.0) | 12 (31.6) | 6 (23.1) | 0.0275** |
| FVC (mean (SD)) | 97.42 (11.25) | 85.92 (14.21) | 0.001# | |
| FEV1 (mean (SD)) | 101.03 (12.19) | 86.40 (15.04) | < 0.001* | |
| DLCO (mean (SD)) | 87.71 (21.81) | 84.42 (12.64) | 0.491* | |
| Scadding Stage (%) | < 0.001** | |||
| 0 | 13 (34.2) | 1 (3.8) | ||
| 1 | 20 (52.6) | 1 (3.8) | ||
| 2 | 4 (10.5) | 22 (84.6) | ||
| 3 | 1 (2.6) | 2 (7.7) | ||
*One-way ANOVA, **Fisher’s exact test, #Mann-Whitney U test. FVC: forced vital capacity. FEV1: forced expiratory volume over 1 s. DLCO: diffusing capacity of the lung for carbon monoxide
Immune gene expression differs by disease status and to a lesser degree by disease progression
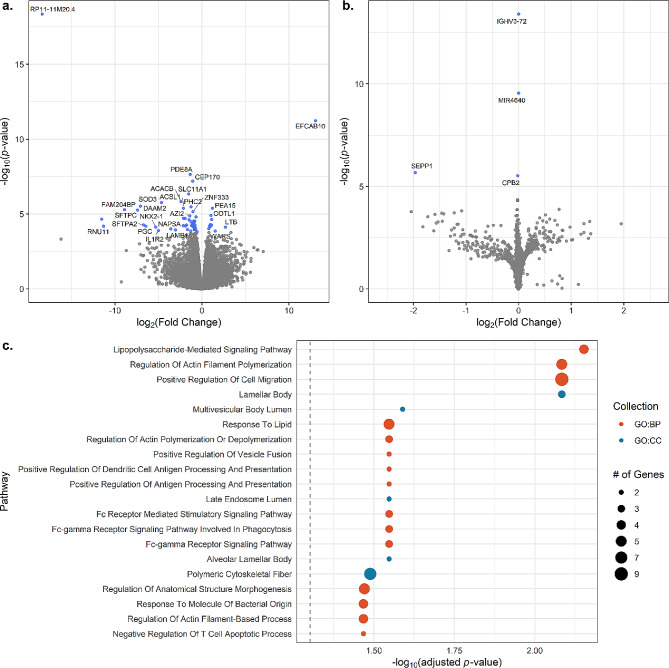
We analyzed gene expression data to assess differences between both cases and controls and progressive and non-progressive disease. In the sarcoidosis-control comparison, we detected 1,842 differentially expressed genes (DEGs), of which 379 (20.6%) were significantly increased in sarcoidosis (Supplementary Figure S1; Supplementary Table S1A). Due to p-value inflation (λ = 1.558) and a skewed test-statistic distribution (Supplementary Figure S1), we ran an additional model adjusting for three principal components to improve the model fit; this resulted in 64 DEGs that meet the FDR-adjusted p < 0.05 and an additional 61 at FDR-adjusted p < 0.1 (Fig. 1A; Supplementary Table S1B). Many of the significant genes from this model are also included in the 1,842 DEGs (20/64; 31.3%). Top significant upregulated genes involved many genes relating to immunity, including cytokine and chemokine signaling (chemokine CCL5/RANTES, chemokine receptor CXCR3, IL16), T lymphocytes (CD6, IL16, LTB), and HLA class II. Most significant downregulated DEGs include surfactant genes SFTPA2 and SFTPC, and transcription factors important in lung cell specification such as CEBPD (CCAAT Enhancer Binding Protein Delta) and NKX2-1 (NK2 homeobox 1). Four DEGs (IGHV3-72, MIR4640, SEPP1, & CPB2) were found in progressive vs. non-progressive cases, although all except SEPP1 displayed small effect sizes (|log2(fold change)| < 0.01) driven by low expression (Fig. 1B).
Fig. 1.
Differentially expressed genes in sarcoidosis. A) Differentially expressed genes in sarcoidosis vs. controls. Significant genes at an FDR-adjusted p-value threshold of 5% are colored in blue. Unadjusted p-values are plotted on the y-axis. B) Differentially expressed genes in progressive vs. non-progressive sarcoidosis. Significant genes at an FDR-adjusted p-value threshold of 5% are colored in blue. Unadjusted p-values are plotted on the y-axis. C) Pathway enrichment of mRNAs. The gray dashed line indicates an FDR-adjusted p-value threshold of 5%. GO: BP: Gene Ontology Biological Process, GO: CC: Gene Ontology Cellular Component
We next tested whether 125 sarcoidosis DEGs (FDR-adjusted p-value < = 0.1) were overrepresented in GO [30] and KEGG pathways using clusterProfiler [33] (Fig. 1C; Supplementary Table S2). Significant genes were enriched for 29 GO and one KEGG pathways. The most significantly enriched GO terms include lipopolysaccharide-mediated signaling pathway, regulation of actin filament polymerization, and positive regulation of cell migration. The significant KEGG pathway was epithelial cell signaling in Helicobacter pylori infection.
DNA methylation differs between cases and controls and overlaps with gene expression
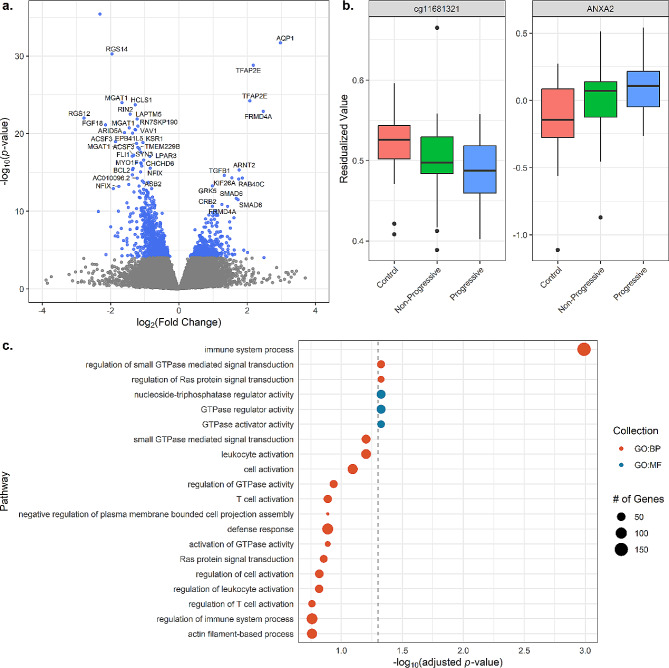
We next tested for differential methylation (DM) and identified 46,812 CpG sites associated with sarcoidosis, of which 38,504 map to 15,208 unique genes (Supplementary Table S3A). As with mRNA, due to poor model fit (Supplementary Figure S2), we ran an additional model adjusting for three principal components to improve the model fit; this resulted in 1,459 CpGs that meet the FDR-adjusted p < 0.05 (Fig. 2A; Supplementary Table 3B). 897 (61.4%) of CpGs are shared with the model without PCs. We did not detect any DNAm sites significantly associated with disease progression. The majority of significant CpGs were hypomethylated in sarcoidosis (948; 64.9%). Significant probes were enriched for intronic regions (66.7% vs. 60.6%; Fisher’s exact test p-value = 2.0 × 10− 6) and FANTOM5 enhancers (15.7% vs. 4.10%; Fisher’s exact test p-value = 1.5 × 10− 66) relative to all tested DNAm sites. Hypomethylated DM sites were significantly enriched for six GO terms, with top hits including immune system process, GTPase activator activity, and GTPase regulator activity (Fig. 2C; Supplementary Table S4). The most significant hypomethylated DM sites include CpGs that map to an intergenic region 17 kb upstream of CCR7 and a promoter of RGS14 as well as an alternative promoter of MGAT1. Top hypermethylated DM sites mapped to the first exon of AQP1 and an exon of TFAP2E.
Fig. 2.
Differentially methylated sites in sarcoidosis. A) Differentially methylated sites in sarcoidosis vs. controls. Significant sites at an FDR-adjusted p-value threshold of 5% are colored in blue. Unadjusted p-values are plotted on the y-axis. Sites are labeled by associated gene. B) Boxplots of ANXA2 cg11681321 methylation and expression by sample group. Omics values were residualized by age, sex, and three principal components from the relevant data type. C) Pathway enrichment of hypomethylated DNAm sites. The gray dashed line indicates an FDR-adjusted p-value threshold of 5%. GO: BP: Gene Ontology Biological Process, GO: MF: Gene Ontology Molecular Function
We next overlapped DEGs and differentially methylated probes (DMPs) in sarcoidosis vs. controls based on gene ID. A single gene, ANXA2, showed evidence of both differential methylation and expression (Fig. 2B). After regressing out covariates, ANXA2 displays increased expression in cases as well as hypomethylation of cg11681321, a CpG within an intron of ANXA2.
miRNA expression differs between cases and controls and targets DEGs
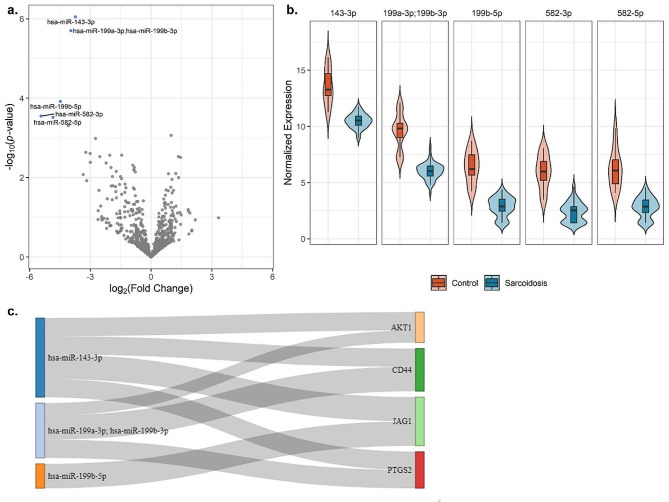
We next compared miRNA expression in sarcoidosis vs. controls and identified five miRNAs (hsa-miR-143-3p, hsa-miR-199a-3p/hsa-miR-199b-3p, hsa-miR-199b-5p, hsa-miR-582-3p & hsa-miR-582-5p) downregulated in sarcoidosis (Fig. 3A-B; Supplementary Table S5). No miRNAs were associated with progression. We derived experimentally validated target genes for each significant miRNA using MirTarBase [35]. We identified 67 target genes, of which four (AKT1, CD44, JAG1, PTGS2) are targeted by two DE miRNAs (Fig. 3C).
Fig. 3.
Differentially expressed microRNAs in sarcoidosis. A) Differentially expressed miRNAs in sarcoidosis vs. controls. Significant genes at an FDR-adjusted p-value threshold of 5% are colored in blue. Unadjusted p-values are plotted on the y-axis. B) Distribution of significant miRNAs’ expression in cases and controls. C) Sankey plot connecting DE miRNAs to target genes targeted by > 1 DE miRNA. Connection width represents number of sources confirming relationship
Integrated model reveals disease and progression associations not found in individual analyses
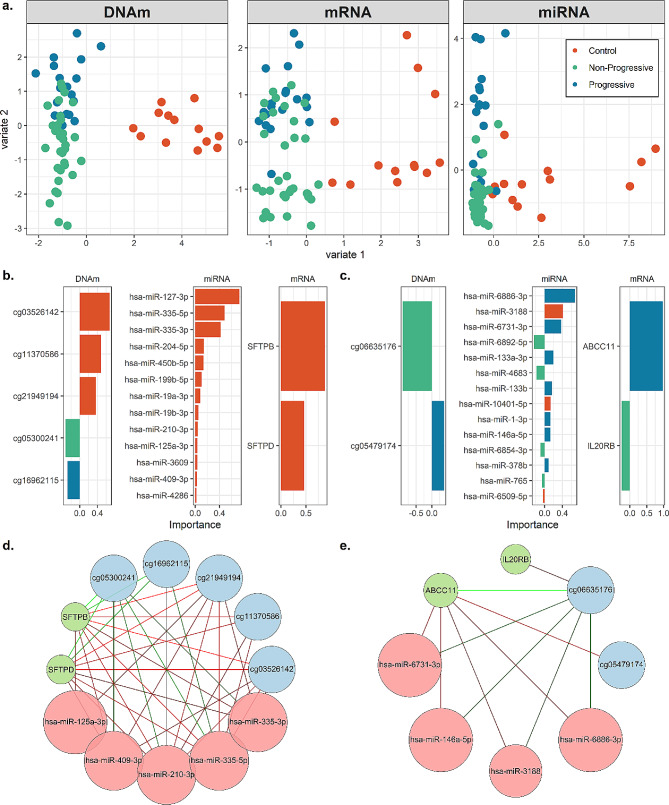
We used DIABLO to integrate the three datasets, including individuals present in all datasets after QC (n = 65; 13 controls, 19 progressive, 33 non-progressive sarcoidosis). With a goal to determine molecular features separating three groups (controls, progressive, and non-progressive sarcoidosis), we constructed a multi-omic model with two latent variables, which are linear combinations of input features. Using 5-fold cross validation repeated ten times, we determined that selecting five DNAm sites, two mRNAs, and 13 miRNAs for latent variable 1 and two DNAm sites, two mRNAs, and 14 miRNAs for latent variable 2 maximized the prediction accuracy of the model and resulted in clustering based on diagnosis (Fig. 4A; Supplementary Table S6). Latent variable 1 separates controls from sarcoidosis samples and latent variable 2 separates progressive from non-progressive sarcoidosis. Features included in each latent variable are shown in Fig. 4B; Supplementary Table S7. We further constructed networks based on correlations of feature weights for each latent variable (Fig. 4C). The two mRNAs contributing to latent variable 1 were SFTPB and SFTPD. DNAm sites contributing to latent variable 1 include DNAm sites hypermethylated in cases cg16962115, within an intron of LYST, and cg05300241 within an intron of PIK3CD. The remaining three DNAm sites were hypomethylated and included cg21949194 within an enhancer of SOS1 (SOS Ras/Rac guanine nucleotide exchange factor 1), cg11370586 within the predicted promoter of RGS14, and cg03526142 within an exon of SLFN12L. All DNAm sites contributing to latent variable 1 were DMPs in our previous methylation analysis. In addition to the DE miR hsa-miR-199b-5p, miRNA features on latent variable 1 include hsa-miR-204-5p. Features contributing to latent variable 2 include the DNAm sites cg05479174, within an exon of SFSWAP, and cg06635176 within an intron of AGBL4. IL20RB (interleukin 20 receptor subunit beta) mRNA was upregulated in non-progressive sarcoidosis, while ABCC11 (ATP binding cassette subfamily C member 11) expression was higher in progressive sarcoidosis. miRNAs contributing to latent variable 1 include hsa-miR-146a-3p and hsa-miR-378b.
Fig. 4.
A sparse multi-omic model of sarcoidosis and progression. A) Projection of samples based on selected feature weights. Points represent samples, colored by disease group, and are plotted for each data type on the top latent variables from the DIABLO multi-omic model. B) Feature importance of selected features for latent variable (1) Bars are colored by strongest sample group loading. C) Feature importance of selected features for latent variable (2) Bars are colored by strongest sample group loading. D) Network constructed from feature correlations on latent variable (1) Features are colored by dataset. Edges are colored by feature correlation, with green representing positive correlation. Features in the network share at least 90% correlation. E) Network constructed from feature correlations on latent variable (2) Features are colored by dataset. Edges are colored by feature correlation, with green representing positive correlation. Features in the network share at least 50% correlation
Discussion
In this study, we present the first application of multi-omic integration in sarcoidosis, leveraging coding and miRNA expression with DNA methylation data to construct a multiomic network. Through initial genome-wide profiling of DNA methylation, mRNA expression, and miRNA expression in sarcoidosis BAL, we identified 64 DEGs, 1,459 DMPs, and five DE miRNAs in sarcoidosis relative to healthy controls, as well as four DEGs associated with sarcoidosis progression. By integrating omic datasets, we define pathogenic molecules/genes in sarcoidosis not identified using conventional modeling methods in single-omics datasets. While our single-omics approach only demonstrated four DEGs for progression, the multiomic model identified several progression-associated methylation, mRNA, and miRNA features. We identify previously reported molecules and pathways associated with disease as well as implicate novel molecular features as potential drivers and modifiers of sarcoidosis, thus demonstrating the potential of integrative approaches.
Our single-omic analyses implicate multiple genes in the pathogenesis of sarcoidosis, with many of these genes involved in processes relating to inflammation and immunity (potentially associated with those involved in viral/atypical bacterial infections and autoimmunity) and extracellular matrix signaling. While we detected many associations with case status, we detected few omic associations with progression. We identified four mRNAs associated with progression, including SEPP1. Results from the GRADS study demonstrated that SEPP1 levels were inversely correlated with DLCO (%) and FVC (% pred) in sarcoidosis individuals [6]. These finding support a progressive phenotype, including lung function changes that we and others have used in the definition of progressive pulmonary disease.
DNAm and mRNA association analyses both identified differential regulation of the gene ANXA2, which displays hypomethylation and increased gene expression in sarcoidosis samples. ANXA2 protein levels have previously been reported to be increased in BALF from sarcoidosis cases [39]. In addition, ANXA2 is found on macrophages, as a cytoplasmic and cell surface molecule that is involved in endocytosis, a pathway frequently found associated with sarcoidosis; however the exact role of this gene in sarcoidosis and/or progression is unclear at this time and will require future study.
Our study also demonstrated five DE miRNAs downregulated in sarcoidosis cases in the first study of genome-wide miRNAs in sarcoidosis. While these associations are novel, multiple target genes of these miRNAs have been implicated in sarcoidosis previously, including the genes AKT1, CD44, JAG1, and PTGS2. For example, CD44 has been found in areas of granuloma formation and fibrosis [40] and is differentially expressed between Lofgren syndrome versus non-Lofgren syndrome subjects [41].
Our integrated model revealed several novel genes and miRNAs between sarcoidosis and controls, including LYST, RGS14, SLFN12L, and hsa-miR-199b-5p. The first three genes appear to have important roles in immune function. Specifically, LYST, a regulator of endosome/lysosome trafficking, can regulate TLR3 and TLR4 mediated pathways [42], genes involved in the innate immune system which have been implicated in sarcoidosis [6, 43, 44]. RGS14 is expressed in lymphocytes and regulates chemokine receptors to control immune responses to exogenous agents [45]. Finally, SLFN21L regulates thymocyte development and is downregulated in T-cell activation, suggesting a role as an immune response regulator [46].
Our integrated analyses demonstrated miRNAs DE between progressive/non-progressive sarcoidosis including those previously described. Specifically, miR-146a-3p upregulated in progressive sarcoidosis is an indicator of inflammation and oxidative stress that may target TLR4 and was previously found elevated in sarcoidosis BALF, as well as serum [47, 48]. miR-378b, which was upregulated in progressive cases, was previously found associated with sarcoidosis [49]. We also identified novel genes including IL20RB, ABCC11, SFSWAP and AGBL4. Interestingly, IL20RB expression was increased in non-progressive versus progressive sarcoidosis in our study, suggesting that the activation of this inflammatory pathway is increased in the non-progressive phenotype. ABCC11 is a gene that influences macrophage differentiation and induces TNF-α and IL17 through TLR4 signaling [50]; these results as well as the novel findings above support the importance of innate immune response genes in sarcoidosis.
Both our individual and integrative analyses identify molecules involved in PI3K/AK1 signaling, a pathway already recognized in sarcoidosis pathogenesis. For example, we observe hypermethylation of PI3KCD (phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit delta), which encodes a component of PI3K, and complexes with AKT1 to impact T cell differentiation and function [51]. Our miRNA results demonstrate AKT1 as a target of downregulated miRNAs (miR-143-3p and miR-199a/miR-199b) in sarcoidosis. Both downregulation and upregulation of the PI3K/AKT signaling pathways have been associated with sarcoidosis [39, 51]. Previous studies demonstrated both reduced proliferative response and exhaustion of T cells in progressive sarcoidosis is thought to be driven in part by inhibition of PI3K/AKT1 signaling [51]. The PI3K/AKT1 pathway has also been implicated in activation of mTOR [39], which has been associated with granuloma formation [52]. Interestingly, in the recent GRADS study, PI3K activation was associated with an endotype of sarcoidosis characterized by hilar lymphadenopathy, pulmonary reticulation, less multiorgan involvement, and more environmental associations [6].
While our sample size is larger than most previous sarcoidosis omic studies, power in our analyses was limited, especially in our phenotype analyses. In future studies, it will be important to both increase sample sizes and investigate associations with better-powered quantitative measures related to disease such as pulmonary function testing variables. We were unable to correct for cell proportions in sarcoidosis subjects relative to controls as controls lacked cell differentials. Despite this, final case-control models (adjusting for principal components in the mRNA and DNAm comparisons) display minimal test-statistic inflation (λ = 1.09–1.31). Altered cell proportions may explain the downregulated epithelial genes in sarcoidosis BAL (e.g. decreased surfactant proteins in sarcoidosis) and some of the DNAm sites and DEGs detected in sarcoidosis versus control comparisons, although in general epithelial cells are not a large proportion of BAL. Finally, our multi-omic models did not take into account demographic information. Regressing out demographic information such as age and sex may lead to more predictive networks. Despite these shortcomings, we detected widespread molecular changes and constructed multi-omic networks associated with sarcoidosis as well as progression.
Conclusions
Using integrative methods, we identified DNA methylation, mRNA, and microRNA associations with sarcoidosis and disease progression in bronchoalveolar lavage cells. Some of these molecular markers have been identified previously in the literature, while others are novel. Molecules discovered in these analyses shed light on disease pathogenesis and may also be leveraged therapeutically or as biomarkers after replication/validation in additional populations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank the study participants and staff for their invaluable contributions.
Author contributions
I.R.K., N.W.L., S.Y.L., C.L., K.M., M.M.M, E.D., C.I.R., S. S., L. L., L.A.M., and I.V.Y. acquired, analyzed and/or interpreted the data. L.A.M, I.V.Y. designed the study. I.R.K., N.W.L., L.A.M, and I.V.Y wrote the initial manuscript. All authors critically revised and approved the final manuscript.
Funding
This work was supported by NIH awards R01HL140357, R01ES023826, TL1TR0025331 and UL1TR001082, and FSR grant 22-505-RFP.
Data availability
Data generated as a part of this study (DNAm, miRNA, and mRNA datasets, as well as relevant deidentified phenotypic data) have been deposited in the National Center for Biotechnology Information Gene Expression Omnibus. IDAT files and a processed matrix of DNAm data are available through accession GSE263432, a counts table of mRNA data is available through GSE263448, and a counts table of miRNA data is available through GSE263451.
Declarations
Ethics approval and consent to participate
All subjects provided written informed consent to participate in this study. The study was approved by the NJH Institutional Review Board (HS-3159-528).
Consent for publication
Not applicable.
Competing interests
L.A.M. has received grant support from the Foundation for Sarcoidosis and Mallinckrodt Pharmaceuticals and serves on the advisory board for the Foundation for Sarcoidosis and the sarcoidosis advisory board for Boehringer Ingelheim. I.V.Y. has received financial support to attend the 2023 World Association for Sarcoidosis and Other Granulomatous Disorders conference and consulting fees from ElevenP15 unrelated to this work. The remaining authors do not have conflicts of interest relating to this work to disclose.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Iain R. Konigsberg, Nancy W. Lin, Lisa A. Maier, Ivana V. Yang contributed equally to this work.
Contributor Information
Iain R. Konigsberg, Email: iain.konigsberg@cuanschutz.edu
Nancy W. Lin, Email: nlin15@jh.edu
References
- 1.Baughman RP, Field S, Costabel U, Crystal RG, Culver DA, Drent M, et al. Sarcoidosis in America. Analysis based on Health Care Use. Annals Am Thorac Soc. 2016;13(8):1244–52. 10.1513/AnnalsATS.201511-760OC [DOI] [PubMed] [Google Scholar]
- 2.Baughman RP, Teirstein AS, Judson MA, Rossman MD, Yeager H, Bresnitz EA, et al. Clinical characteristics of patients in a Case Control Study of Sarcoidosis. Am J Respir Crit Care Med. 2001;164(10):1885–9. 10.1164/ajrccm.164.10.2104046 [DOI] [PubMed] [Google Scholar]
- 3.Swigris JJ, Olson AL, Huie TJ, Fernandez-Perez ER, Solomon J, Sprunger D, et al. Sarcoidosis-related mortality in the United States from 1988 to 2007. Am J Respir Crit Care Med. 2011;183(11):1524–30. 10.1164/rccm.201010-1679OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gerke AK, Judson MA, Cozier YC, Culver DA, Koth LL. Disease Burden and Variability in Sarcoidosis. Annals Am Thorac Soc. 2017;14(Supplement6):S421–8. 10.1513/AnnalsATS.201707-564OT [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Asano M, Minagawa T, Ohmichi M, Hiraga Y. Detection of endogenous cytokines in sera or in lymph nodes obtained from patients with sarcoidosis. Clin Exp Immunol. 1991;84(1):92–6. 10.1111/j.1365-2249.1991.tb08129.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vukmirovic M, Yan X, Gibson KF, Gulati M, Schupp JC, DeIuliis G et al. Transcriptomics of bronchoalveolar lavage cells identifies new molecular endotypes of sarcoidosis. Eur Respir J. 2021;58(6). [DOI] [PMC free article] [PubMed]
- 7.Yang IV, Konigsberg I, MacPhail K, Li L, Davidson EJ, Mroz PM, et al. DNA methylation changes in Lung Immune cells are Associated with Granulomatous Lung Disease. Am J Respir Cell Mol Biol. 2019;60(1):96–105. 10.1165/rcmb.2018-0177OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li L, Konigsberg IR, Bhargava M, Liu S, MacPhail K, Mayer A, et al. Multiomic signatures of Chronic Beryllium Disease Bronchoalveolar Lavage cells relate to T-Cell function and innate immunity. Am J Respir Cell Mol Biol. 2022;67(6):632–40. 10.1165/rcmb.2022-0077OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ascoli C, Huang Y, Schott C, Turturice BA, Metwally A, Perkins DL, et al. A circulating MicroRNA signature serves as a Diagnostic and Prognostic Indicator in Sarcoidosis. Am J Respir Cell Mol Biol. 2018;58(1):40–54. 10.1165/rcmb.2017-0207OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou T, Casanova N, Pouladi N, Wang T, Lussier Y, Knox KS, et al. Identification of Jak-STAT signaling involvement in sarcoidosis severity via a novel microRNA-regulated peripheral blood mononuclear cell gene signature. Sci Rep. 2017;7(1):4237. 10.1038/s41598-017-04109-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kiszalkiewicz J, Piotrowski WJ, Pastuszak-Lewandoska D, Gorski P, Antczak A, Gorski W, et al. Altered miRNA expression in pulmonary sarcoidosis. BMC Med Genet. 2016;17:2. 10.1186/s12881-016-0266-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moller DR, Koth LL, Maier LA, Morris A, Drake W, Rossman M, et al. Rationale and design of the Genomic Research in Alpha-1 Antitrypsin Deficiency and Sarcoidosis (GRADS) study. Sarcoidosis Protocol. Ann Am Thorac Soc. 2015;12(10):1561–71. 10.1513/AnnalsATS.201503-172OT [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crouser ED, Maier LA, Wilson KC, Bonham CA, Morgenthau AS, Patterson KC, et al. Diagnosis and detection of Sarcoidosis. An official American thoracic Society Clinical Practice Guideline. Am J Respir Crit Care Med. 2020;201(8):e26–51. 10.1164/rccm.202002-0251ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maier LA, Sawyer RT, Tinkle SS, Kittle LA, Barker EA, Balkissoon R, et al. IL-4 fails to regulate in vitro beryllium-induced cytokines in berylliosis. Eur Respir J. 2001;17(3):403–15. 10.1183/09031936.01.17304030 [DOI] [PubMed] [Google Scholar]
- 15.Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29(1):15–21. 10.1093/bioinformatics/bts635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kozomara A, Birgaoanu M, Griffiths-Jones S. miRBase: from microRNA sequences to function. Nucleic Acids Res. 2019;47(D1):D155–62. 10.1093/nar/gky1141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kozomara A, Griffiths-Jones S. miRBase: annotating high confidence microRNAs using deep sequencing data. Nucleic Acids Res. 2014;42(Database issue):D68–73. 10.1093/nar/gkt1181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kozomara A, Griffiths-Jones S. miRBase: integrating microRNA annotation and deep-sequencing data. Nucleic Acids Res. 2011;39(Database issue):D152–7. 10.1093/nar/gkq1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Griffiths-Jones S, Saini HK, van Dongen S, Enright AJ. miRBase: tools for microRNA genomics. Nucleic Acids Res. 2008;36(Database issue):D154–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Griffiths-Jones S, Grocock RJ, van Dongen S, Bateman A, Enright AJ. miRBase: microRNA sequences, targets and gene nomenclature. Nucleic Acids Res. 2006;34(Database issue):D140–4. 10.1093/nar/gkj112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Griffiths-Jones S. The microRNA Registry. Nucleic Acids Res. 2004;32(Database issue):D109–11. 10.1093/nar/gkh023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Russell PH, Vestal B, Shi W, Rudra PD, Dowell R, Radcliffe R et al. miR-MaGiC improves quantification accuracy for small RNA-seq. BMC Research Notes. 2018;11(1). [DOI] [PMC free article] [PubMed]
- 23.Zhou W, Triche TJ Jr., Laird PW, Shen H. SeSAMe: reducing artifactual detection of DNA methylation by Infinium BeadChips in genomic deletions. Nucleic Acids Res. 2018;46(20):e123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Du P, Zhang X, Huang C-C, Jafari N, Kibbe WA, Hou L et al. Comparison of Beta-value and M-value methods for quantifying methylation levels by microarray analysis. BMC Bioinformatics. 2010;11(1). [DOI] [PMC free article] [PubMed]
- 25.Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, et al. Limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43(7):e47. 10.1093/nar/gkv007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Love MI, Huber W, Anders S. Moderated estimation of Fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15(12):550. 10.1186/s13059-014-0550-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu A, Ibrahim JG, Love MI, Stegle O. Heavy-tailed prior distributions for sequence count data: removing the noise and preserving large differences. Bioinformatics. 2019;35(12):2084–92. 10.1093/bioinformatics/bty895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Iterson M, van Zwet EW, Heijmans BT. Controlling bias and inflation in epigenome- and transcriptome-wide association studies using the empirical null distribution. Genome Biol. 2017;18(1). [DOI] [PMC free article] [PubMed]
- 29.Benjamini Y, Hochberg Y. Controlling the false Discovery rate: a practical and powerful Approach to multiple testing. J Royal Stat Soc Ser B (Methodological). 1995;57(1):289–300. 10.1111/j.2517-6161.1995.tb02031.x [DOI] [Google Scholar]
- 30.Carbon S, Douglass E, Good BM, Unni DR, Harris NL, Mungall CJ, et al. The Gene Ontology resource: enriching a GOld mine. Nucleic Acids Res. 2021;49(D1):D325–34. 10.1093/nar/gkaa1113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kanehisa M, Furumichi M, Sato Y, Ishiguro-Watanabe M, Tanabe M. KEGG: integrating viruses and cellular organisms. Nucleic Acids Res. 2021;49(D1):D545–51. 10.1093/nar/gkaa970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maksimovic J, Oshlack A, Phipson B. Gene set enrichment analysis for genome-wide DNA methylation data. Genome Biol. 2021;22(1). [DOI] [PMC free article] [PubMed]
- 33.Wu T, Hu E, Xu S, Chen M, Guo P, Dai Z et al. clusterProfiler 4.0: a universal enrichment tool for interpreting omics data. Innov. 2021;2(3). [DOI] [PMC free article] [PubMed]
- 34.Cavalcante RG, Sartor MA. Annotatr: genomic regions in context. Bioinformatics. 2017;33(15):2381–3. 10.1093/bioinformatics/btx183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang HY, Lin YC, Cui S, Huang Y, Tang Y, Xu J et al. miRTarBase update 2022: an informative resource for experimentally validated miRNA-target interactions. Nucleic Acids Res. 2021. [DOI] [PMC free article] [PubMed]
- 36.Singh A, Shannon CP, Gautier B, Rohart F, Vacher M, Tebbutt SJ, et al. DIABLO: an integrative approach for identifying key molecular drivers from multi-omics assays. Bioinformatics. 2019;35(17):3055–62. 10.1093/bioinformatics/bty1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schneidman D, Rohart F, Gautier B, Singh A, Lê Cao K-A, mixOmics. An R package for ‘omics feature selection and multiple data integration. PLoS Comput Biol. 2017;13(11). [DOI] [PMC free article] [PubMed]
- 38.Anders S, Huber W. Differential expression analysis for sequence count data. Genome Biol. 2010;11(10):R106. 10.1186/gb-2010-11-10-r106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bhargava M, Viken KJ, Barkes B, Griffin TJ, Gillespie M, Jagtap PD, et al. Novel protein pathways in development and progression of pulmonary sarcoidosis. Sci Rep. 2020;10(1):13282. 10.1038/s41598-020-69281-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aleksoniene R, Besusparis J, Gruslys V, Jurgauskiene L, Laurinaviciene A, Laurinavicius A, et al. CD31(+), CD38(+), CD44(+), and CD103(+) lymphocytes in peripheral blood, bronchoalveolar lavage fluid and lung biopsy tissue in sarcoid patients and controls. J Thorac Dis. 2021;13(4):2300–18. 10.21037/jtd-20-2396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kaiser Y, Lakshmikanth T, Chen Y, Mikes J, Eklund A, Brodin P, et al. Mass Cytometry identifies distinct lung CD4(+) T cell patterns in Lofgren’s syndrome and Non-lofgren’s syndrome sarcoidosis. Front Immunol. 2017;8:1130. 10.3389/fimmu.2017.01130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Westphal A, Cheng W, Yu J, Grassl G, Krautkramer M, Holst O, et al. Lysosomal trafficking regulator Lyst links membrane trafficking to toll-like receptor-mediated inflammatory responses. J Exp Med. 2017;214(1):227–44. 10.1084/jem.20141461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wiken M, Grunewald J, Eklund A, Wahlstrom J. Higher monocyte expression of TLR2 and TLR4, and enhanced pro-inflammatory synergy of TLR2 with NOD2 stimulation in sarcoidosis. J Clin Immunol. 2009;29(1):78–89. 10.1007/s10875-008-9225-0 [DOI] [PubMed] [Google Scholar]
- 44.Schurmann M, Kwiatkowski R, Albrecht M, Fischer A, Hampe J, Muller-Quernheim J, et al. Study of toll-like receptor gene loci in sarcoidosis. Clin Exp Immunol. 2008;152(3):423–31. 10.1111/j.1365-2249.2008.03621.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moratz C, Harrison K, Kehrl JH. Regulation of chemokine-induced lymphocyte migration by RGS proteins. Methods Enzymol. 2004;389:15–32. 10.1016/S0076-6879(04)89002-5 [DOI] [PubMed] [Google Scholar]
- 46.Puck A, Aigner R, Modak M, Cejka P, Blaas D, Stockl J. Expression and regulation of Schlafen (SLFN) family members in primary human monocytes, monocyte-derived dendritic cells and T cells. Results Immunol. 2015;5:23–32. 10.1016/j.rinim.2015.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dyskova T, Fillerova R, Novosad T, Kudelka M, Zurkova M, Gajdos P, et al. Correlation Network Analysis reveals relationships between MicroRNAs, transcription factor T-bet, and deregulated Cytokine/Chemokine-Receptor network in Pulmonary Sarcoidosis. Mediators Inflamm. 2015;2015:121378. 10.1155/2015/121378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Novosadova E, Chabronova A, Kolek V, Petrek M, Navratilova Z. The serum expression of selected miRNAs in Pulmonary Sarcoidosis with/without Lofgren’s syndrome. Mediators Inflamm. 2016;2016:1246129. 10.1155/2016/1246129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jazwa A, Kasper L, Bak M, Sobczak M, Szade K, Jozkowicz A, et al. Differential inflammatory microRNA and cytokine expression in pulmonary sarcoidosis. Arch Immunol Ther Exp (Warsz). 2015;63(2):139–46. 10.1007/s00005-014-0315-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vogl T, Tenbrock K, Ludwig S, Leukert N, Ehrhardt C, van Zoelen MA, et al. Mrp8 and Mrp14 are endogenous activators of toll-like receptor 4, promoting lethal, endotoxin-induced shock. Nat Med. 2007;13(9):1042–9. 10.1038/nm1638 [DOI] [PubMed] [Google Scholar]
- 51.Celada LJ, Rotsinger JE, Young A, Shaginurova G, Shelton D, Hawkins C, et al. Programmed Death-1 inhibition of phosphatidylinositol 3-Kinase/AKT/Mechanistic target of Rapamycin Signaling impairs sarcoidosis CD4(+) T cell proliferation. Am J Respir Cell Mol Biol. 2017;56(1):74–82. 10.1165/rcmb.2016-0037OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Linke M, Pham HT, Katholnig K, Schnoller T, Miller A, Demel F, et al. Chronic signaling via the metabolic checkpoint kinase mTORC1 induces macrophage granuloma formation and marks sarcoidosis progression. Nat Immunol. 2017;18(3):293–302. 10.1038/ni.3655 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data generated as a part of this study (DNAm, miRNA, and mRNA datasets, as well as relevant deidentified phenotypic data) have been deposited in the National Center for Biotechnology Information Gene Expression Omnibus. IDAT files and a processed matrix of DNAm data are available through accession GSE263432, a counts table of mRNA data is available through GSE263448, and a counts table of miRNA data is available through GSE263451.