Abstract
The genus Prevotella includes more than 50 characterized species that occur in varied natural habitats, although most Prevotella spp. are associated with humans. In the human microbiome, Prevotella spp. are highly abundant in various body sites, where they are key players in the balance between health and disease. Host factors related to diet, lifestyle and geography are fundamental in affecting the diversity and prevalence of Prevotella species and strains in the human microbiome. These factors, along with the ecological relationship of Prevotella with other members of the microbiome, likely determine the extent of the contribution of Prevotella to human metabolism and health. Here we review the diversity, prevalence and potential connection of Prevotella spp. in the human host, highlighting how genomic methods and analysis have improved and should further help in framing their ecological role. We also provide suggestions for future research to improve understanding of the possible functions of Prevotella spp. and the effects of the Western lifestyle and diet on the host–Prevotella symbiotic relationship in the context of maintaining human health.
Prevotella is a diverse genus of Gram-negative anaerobic bacteria that was first described in 1990 by Shar and Collins to include some oral species with specific phenotypic traits (moderately saccharolytic capabilities and bile salt sensitivity) that were formerly placed in the genus Bacteroides. Prevotella was named after the pioneering anaerobic microbiologist A. R. Prévot1. The type strain for the genus is from Prevotella melaninogenica, which was isolated by Oliver and Wherry in 1921 from various human body sites and was named for the black pigmentation of its colonies1,2. Prevotella spp. are non-spore-forming, non-motile short rods that are saccharolytic or moderately saccharolytic3,4. The genus Prevotella is part of the family Prevotellaceae, which also contains the three closely related genera Paraprevotella, Alloprevotella and Hallella.
Prevotella spp. have been isolated from various animal hosts and even occur free living in the environment. In comparison with other genera, which contain members with clear associations with disease or that are easily cultured, Prevotella has received less attention, maybe due to limitations in culturing these bacteria and confusion in phenotype-based taxonomic classification of isolates5,6. Nevertheless, the advent of cultivation-free microbial profiling showed that Prevotella spp. are common, abundant, consistent features of mammal-associated microbial communities (microbiomes) and in humans have been found to inhabit multiple body sites, including the skin, oral cavity, vagina and gastrointestinal tract. Prevotella spp. are not rare in human microbiomes, and they are members of one of the most dominant genera in the oral cavity7. In rural populations that follow a more pre-industrial, ‘traditional’ lifestyle and diet — so-called ‘non-Westernized populations’ — Prevotella spp. tend to dominate the gut microbiome8–15. Westernization is the consequence of industrialization over the past 200 years. While separating populations into Westernized and non-Westernized categories demarcates what is best seen as a continuum and is not without its difficulties (as discussed elsewhere14–16), multiple reports have consistently highlighted a decreased prevalence of Prevotella spp. in Westernized populations, which is generally compensated by the domination of Bacteroides species8–15. Our understanding of the human microbiome is skewed towards Western populations, and therefore Bacteroides spp. have received more attention than Prevotella spp., although as the number of non-Westernized populations sampled expands, the diversity, prevalence, abundance and importance of Prevotella is becoming increasingly evident. This is particularly true for intestinal Prevotella spp., which have been shown to be long-standing key members of our co-evolved microbiome, supporting the hypothesis that the modern Western lifestyle is causal in the loss of Prevotella diversity and further raising the question of what are the consequences of this loss from a health perspective16.
Despite the abundance and prevalence of Prevotella spp. associated with humans, their involvement in health and disease, their role within the microbiome and their contribution to host–microbiome crosstalk are unclear. Prevotella contains no known obligate pathogenic species, yet members have been implicated in multiple diseases, including inflammatory autoimmune diseases17, opportunistic infections18–21, bacterial vaginosis22 and oral biofilm formation and diseases23,24, although direct causation of disease is uncertain. Similarly, in the intestine, there are conflicting reports regarding whether Prevotella spp. are beneficial or detrimental to health, particularly in glucose homeostasis25–27. Establishing general associations and specific causal links between Prevotella and disease is likely further confounded by the recent discovery that this genus may be more diverse than previously appreciated4,16. In the view of the human microbiome as an integral part of human biology, Prevotella is an exemplar case of a genus that is likely involved in intricate ecological interactions and crosstalk with the host, with indirect but potentially substantial effects on human health.
In light of the open questions regarding these enigmatic bacteria, it is timely to discuss the genus Prevotella and its association and distribution on and within the human host, across ages, lifestyles and diseases. Using available genomes from cultivated strains and those recovered directly from thousands of metagenomic samples (BOX 1), we explore and summarize the overall diversity and current knowledge of the genus Prevotella, as well as the role of Prevotella spp. in human health and disease.
BOX 1 |. Metagenomic approaches to uncover the diversity of Prevotella spp.
The study of human-associated Prevotella spp. has been hampered by the intrinsic difficulty in cultivating in vitro some taxa, especially anaerobic strains in the gut. By surveying the genetic content of complex microbial samples by high-throughput sequencing (that is, metagenomics60), it is possible not only to identify and quantify known species but also to discover previously uncharacterized ‘novel’ species. This approach is becoming increasingly effective by metagenomic assembly, which first reconstructs relatively long fragments of the genomes (called ‘contigs’)181,182 and then groups contigs into draft genomes termed ‘metagenome-assembled genomes’ (MAGs) by exploiting read coverage and intrinsic genetic features (such as tetranucleotide frequency distribution) across contigs183. After quality control of the resulting MAGs184, high-quality sequences can be phylogenetically and taxonomically contextualized185, revealing in many cases that MAGs do not belong to any previously named or catalogued species. As metagenomic assembly reaches maturity, large collections of MAGs are being built and analysed15,49,50. Currently, more than 200,000 MAGs have been deposited in catalogues that are based on many large human metagenomic studies; thousands of these MAGs have expanded the strain-level genetic diversity of known species, while many others define new species-level genome bins, which are interpreted as yet-to-be cultivated new candidate species. Applied to the genus Prevotella, such approaches confirmed the existence of at least four genetically and functionally distinct clades of Prevotella copri that form a species complex4,16, and a total of 4,886 human-associated MAGs that are assigned to the family Prevotellaceae but do not belong to any known Prevotella spp.15. (FIG. 3). While in vitro cultivation remains indispensable to understand the physiology of bacterial species and their possible involvement in host diseases, metagenomic assembly is emerging as a key tool to characterize previously overlooked bacterial diversity, drive targeted cultivation efforts and formulate hypotheses on the biomedical relevance of some taxa.
Phylogenetic and ecological diversity
Since the initial isolation of Prevotella spp. from the human oral cavity and respiratory tract, they have been shown to occupy ecologically diverse habitats. Currently, 57 species of Prevotella for which isolates are publicly available (FIG. 1; TABLE 1; Supplementary Table 1) have been characterized. By far the largest number of isolates and known species are associated with human hosts, but Prevotella spp. can also be found associated with other mammalian hosts. For example, Prevotella spp. are a common and predominant feature of the gut microbiota of ruminants; in particular, Prevotella bryantii, Prevotella ruminicola, Prevotella brevis and Prevotella albensis are well-known members28,29. Prevotella spp. are integral in carbohydrate utilization in the rumen30 and are a particularly common feature of the swine gut microbiome31,32. Genomes of other members of the Prevotellaceae have been recovered from non-human primates, dogs and mice33,34 (Supplementary Table 1) and non-mammalian hosts, including from the fermentative crop of the tropical bird Opisthocomus hoazin (the hoatzin) and have been found free living in natural habitats, including soil35.
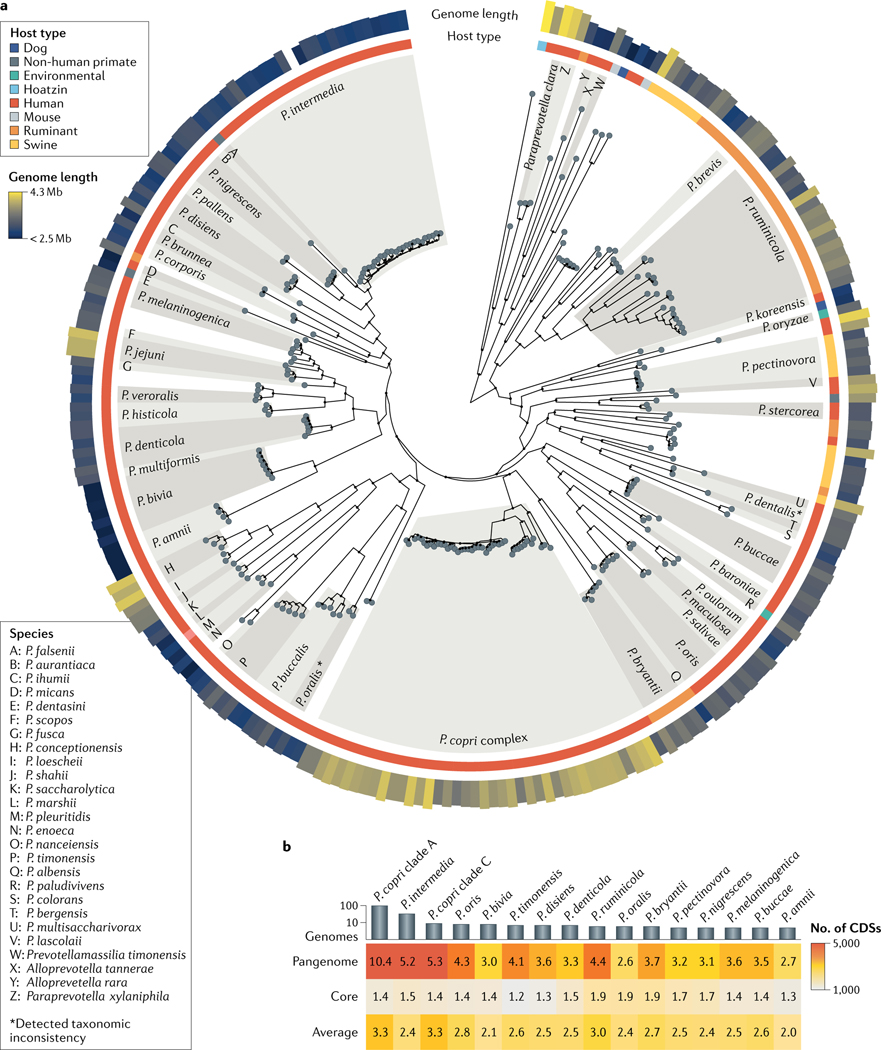
Fig. 1 |. Genomic overview of the genus Prevotella.
a | Phylogenetic tree of reference genomes of strains or species of Prevotella and other Prevotellaceae species, with an indication of their ecological niches. The largest number of genomes are associated with human hosts, although Prevotella genomes are also identified in several other host types. Prevotella spp. exhibit variable genome length, ranging from 4.26 Mb for Prevotella copri to 2.37 Mb for Prevotella amnii. Only named species are reported in the tree, which was built using a maximum likelihood approach applied on marker genes. b | Genome characteristics for Prevotella spp. with at least five available genomes. The average number of coding sequences (CDSs) ranges between 2,000 and 3,300. The core size (that is, the number of CDSs that are present in almost all genomes) ranges from 1,200 to 1,900 genes. The pangenome size ranges between 2,600 and 10,400 genes; however, this is partially affected by the number of available genomes (Spearman correlation between genome number and pangenome size of 0.64). Data were generated by whole-genome analysis of currently available Prevotella genomes in public repositories. TABLE 1 and Supplementary Table 1 contain the genomic characteristics of Prevotella spp. considered in this figure and the list of available reference genomes. Numbers in the heat map are in thousands.
Table 1 |.
Genomic characteristics of Prevotella species
| Prevotella speciesa | Number of sequenced genomes | Genome lengthb (Mb) | G+C contentb (%) | Host | Host sites |
|---|---|---|---|---|---|
| P copri complex | 106 | 3.65 ± 0.21 | 44.93 ± 0.23 | Human | Gut |
| P. intermedia | 33 | 2.79 ± 0.13 | 43.45 ± 0.15 | Human | Oral cavity, empyema |
| P. ruminicola | 9 | 3.53 ± 0.30 | 47.69 ± 0.71 | Ruminant | Rumen |
| P. bivia | 8 | 2.49 ± 0.08 | 39.79 ± 0.17 | Human | Vagina |
| P. denticola | 7 | 3.06 ± 0.11 | 50.04 ± 0.18 | Human | Vagina, oral cavity |
| P. disiens | 7 | 2.86 ± 0.13 | 39.93 ± 0.21 | Human | Vagina, gut, Bartholin abscess |
| P. timonensis | 7 | 3.09 ± 0.16 | 42.41 ± 0.16 | Human | Vagina, breast abscess |
| P. bryantii | 6 | 3.41 ± 0.14 | 38.8 ± 0.19 | Ruminant | Rumen |
| P. amnii | 5 | 2.4 ± 0.03 | 36.52 ± 0.08 | Human | Vagina, amniotic fluid |
| P. melaninogenica | 5 | 3.14 ± 0.12 | 41.02 ± 0.23 | Human | Vagina, oral cavity, sputum |
| P. nigrescens | 5 | 2.85 ± 0.16 | 42.64 ± 0.11 | Human | Oral cavity |
| P. oralis | 5 | 2.87 ± 0.07 | 44.54 ± 0.09 | Human | Vagina, gut, oral cavity |
| P. oris | 5 | 3.18 ± 0.12 | 43.86 ± 0.09 | Human | Oral cavity, airways |
| P. pectinovora | 5 | 3.17 ± 0.12 | 47.74 ± 0.25 | Swine | Gut |
| P. buccae | 4 | 3.22 ± 0.12 | 51.15 ± 0.19 | Human | Oral cavity |
| P. buccalis | 4 | 2.99 ± 0.20 | 45.5 ± 0.18 | Human | Vagina |
| P. baroniae | 3 | 3.09 ± 0.06 | 53.1 ± 0.17 | Human | Oral cavity |
| P. corporis | 3 | 2.81 ± 0.04 | 44.1 ± 0.10 | Human | Vagina |
| P. histicola | 3 | 3.0 ± 0.06 | 41.2 ± 0.01 | Human | Vagina, oral cavity |
| P. loescheii | 3 | 3.49 ± 0.02 | 46.6 ± 0.01 | Human | Oral cavity |
| P. maculosa | 3 | 3.25 ± 0.09 | 47.5 ± 0.17 | Human | Oral cavity |
| P. micans | 3 | 2.45 ± 0.03 | 45.5 ± 0.01 | Human | Oral cavity |
| P. oulorum | 3 | 2.82 ± 0.02 | 46.8 ± 0.01 | Human | Oral cavity |
| P. pallens | 3 | 3.1 ± 0.04 | 37.47 ± 0.06 | Human | Oral cavity |
| P. salivae | 3 | 3.22 ± 0.10 | 41.5 ± 0.17 | Human | Gut, oral cavity |
| P. scopos | 3 | 3.23 ± 0.06 | 40.7 ± 0.01 | Human | Oral cavity |
| P. stercorea | 3 | 3.11 ± 0.01 | 48.93 ± 0.06 | Human | Gut |
| P. veroralis | 3 | 2.89 ± 0.09 | 41.83 ± 0.06 | Human | Oral cavity |
Supplementary Table 1 contains the complete species and genome lists.
Only species with at least three sequenced genomes are included.
Values are reported as averages and the standard deviations.
The distinguishing feature between human and non-human isolates of Prevotella is their species-level evolutionary history, as inferred from whole-genome phylogenetics (FIG. 1; Supplementary BOX 1). There is clustering of large subtrees, in which human-associated species are mostly separated from those of non-human origin (FIG. 1), and a large multispecies clade is present, which comprises P. ruminicola and P. brevis and numerous unnamed species that are specific to swine and ruminants. The genome size of the isolates differs: the largest genome (4.26 Mb) is from a member of the Prevotella copri complex, and Prevotella amnii has the smallest genome (2.37 Mb) (FIG. 1; TABLE 1). In comparison, the average genome size for Bacteroides spp. (5.46 Mb from 1,116 isolate genomes) is larger. There is also a considerable spread in G+C content across the genus, ranging from 36.4% in P. amnii to 56.1% in Prevotella dentalis (TABLE 1), which is further evidence of the remarkable diversity of Prevotella. A comparison of the core genome for species for which there are multiple independent isolates reveals that Prevotella spp. have a core of ~1,200–1,900 genes (accounting for ~50–75% of the genome), which is consistent for species from different host types (FIG. 1). Prevotella is a diverse microbial genus, yet like most isolate collections, it likely suffers from sampling bias; increased isolation efforts from animal species and undersampled environments will surely expand the number of hosts and diversity of Prevotella spp.
The ecological importance of bacteriophages (phages) in shaping the complex host-associated microbiome has been established; nevertheless, investigation of these phage communities is still in its infancy36. Consequently, little is known about Prevotella-specific phages, with only a few reports describing phages associated with Prevotella spp. in ruminants37,38. Recently, metagenomics-based discovery has identified large ‘megaphages’ termed ‘Lak phages,’ which are associated with Prevotella spp. in humans, non-human primates, other mammalian hosts and reptiles39,40. These phages use an alternative genetic code and, with a genome size of up to ~660 kb, they are the largest intestinal phages discovered to date40. The identification of these diverse phages raises intriguing questions about their ability to modulate Prevotella populations in the intestine and the implications of this modulation on the whole microbiome and ultimately on its influence on the host.
Prevotella in humans across body sites
Even within a specific host, different Prevotella spp. are present in multiple different body locations. In humans, and similarly to other bacterial genera41, distinct Prevotella spp. have been identified and isolated from the oral cavity, respiratory tract, vagina, skin and intestine. Surveying the prevalence and abundance of the known characterized Prevotella spp. in more than 9,500 individuals from multiple integrated datasets15 reveals that, with the exception of vaginal Prevotella spp., most species are body-site-specific (FIG. 2a,b; Supplementary BOX 2). A genome-based analysis of human Prevotella isolates identified differing functional potential at different body sites, suggesting the existence of niche-specific differentiation and specialization42. Further stratification is also observed across lifestyles; non-Westernized populations, which follow a more traditional lifestyle and diet, tend to have a higher prevalence of several distinct Prevotella spp.8–13 in both the gut and the oral cavity (FIG. 2c). The distribution of Prevotella spp. is also influenced by age; in the gut, both P. copri and Prevotella stercorea show an age-dependent increase in prevalence regardless of Westernization, although their relative abundance drops from adulthood to old age (more than 65 years) (FIG. 2a,b). However, some species (Prevotella bivia, Prevotella timonensis, Prevotella buccalis, Prevotella disiens and Prevotella corporis) seem to have the highest prevalence in the gut of elderly individuals in Westernized populations and, intriguingly, they have a correspondingly similar prevalence pattern in the vagina (FIG. 2a,b). Sex effects have also been observed, with Prevotella being more prevalent in men than women43,44. However, female hormone metabolism seems to be linked to the regulation of Prevotella spp. in the human body. Steroidal hormones can favour the growth of Prevotella intermedia and P. melaninogenica in the oral cavity45,46 and affect oral microbial ecology and the occurrence of gingivitis in hormone-driven diseases47. It is evident that the distribution and prevalence of Prevotella spp. in the human host are driven by multiple factors, including body site, lifestyle, sex and age, and this is an area of continued research.
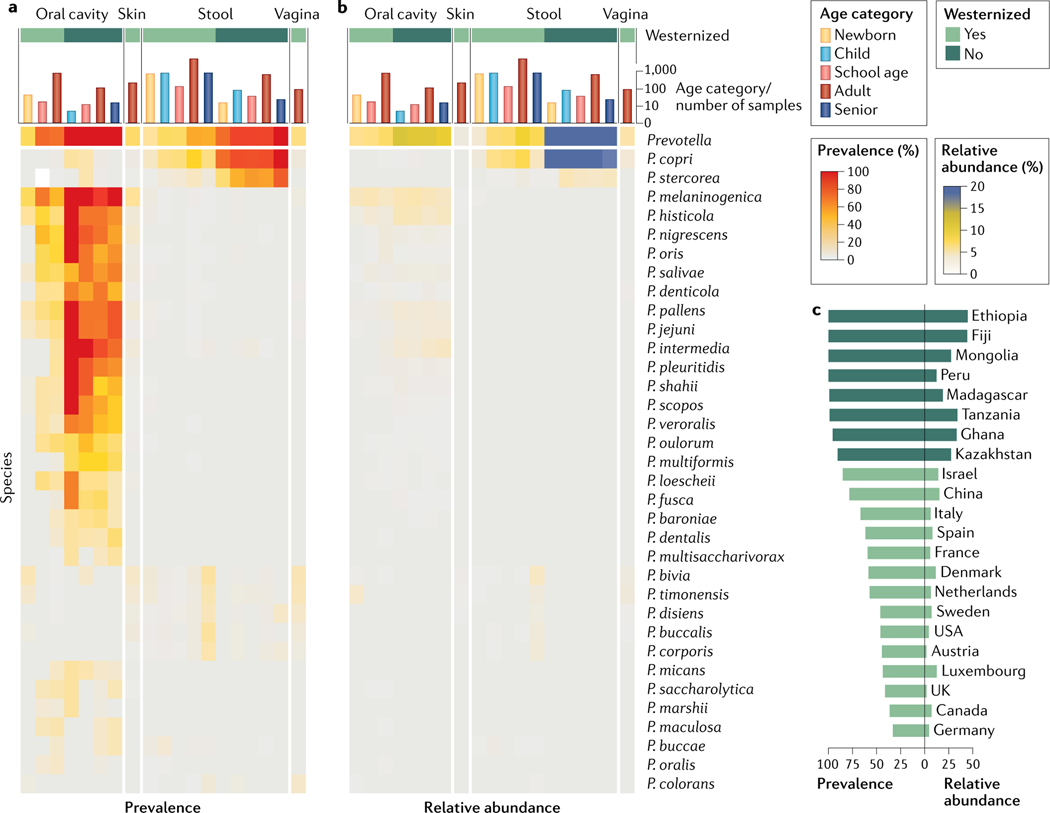
Fig. 2 |. Distribution and stratification of Prevotella spp. across human populations and body sites.
Prevalence (part a) and relative abundance (part b) of known Prevotella spp. across four main body sites (that is, the oral cavity (saliva or oral swabs), the skin (cutaneous swabs), the gut (stool samples) and the vagina (vaginal swabs)), five age categories (that is, newborn, child, school-age child, adult and senior) and two main lifestyles (that is, Westernized and non-Westernized). These data were obtained from public repositories of more than 9,500 profiled human metagenomes (curated metadata information is available elsewhere109). Quantitative taxonomic profiles were generated with MetaPhlAn 3.0 (REF.180). Only named species with a prevalence greater than 0.1% in at least one category are reported. Most species are body-site-specific, and further differences are observed across age categories. The prevalence and relative abundance of Prevotella in the gut (part c) are largely influenced by lifestyle. Non-Westernized populations, which follow a more traditional diet and lifestyle, are enriched in Prevotella spp., and this is consistently observed across countries. Extended heat maps are available in Supplementary Fig. 1.
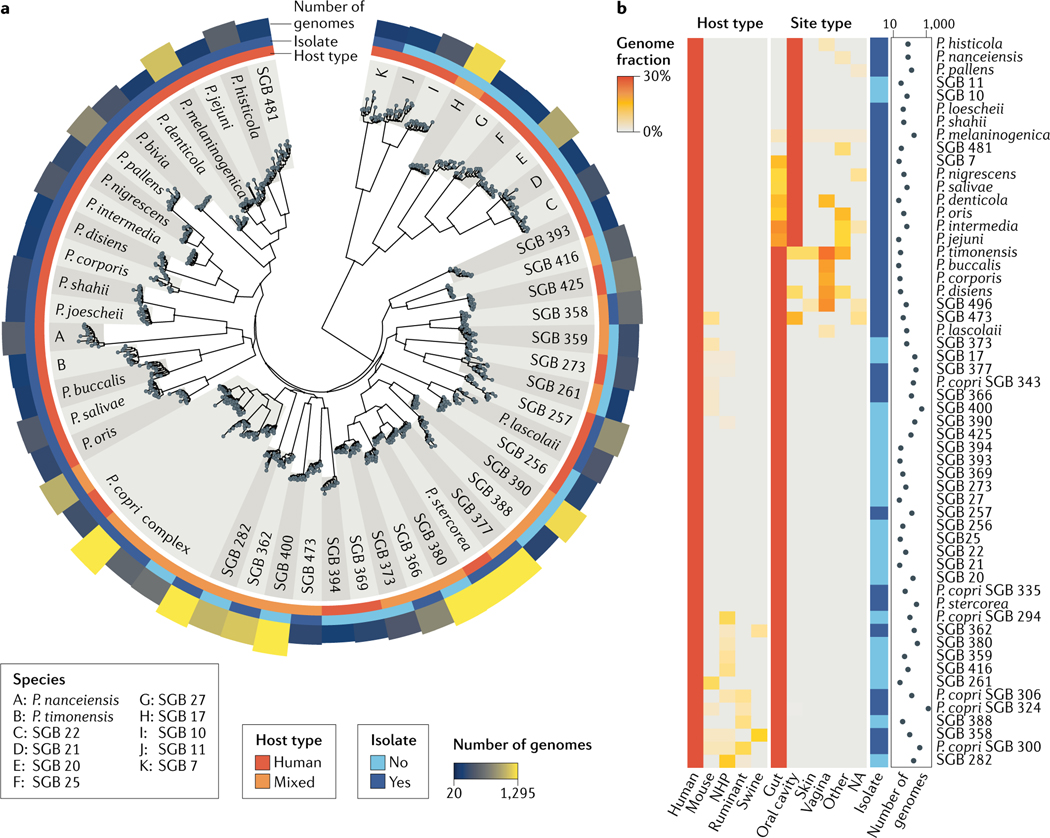
While strain isolation has been, and continues to be, the predominant approach in microbiological research, culture-independent methods offer a new complementary approach in microbial discovery. For example, recovering and identifying genomes directly from mixed environmental samples (metagenomes) has begun to transform our understanding of the true microbial diversity in natural systems4,15,16,48–51 (BOX 1) and gives an unprecedented opportunity to characterize the role and ecology of Prevotella by exploiting the increasingly available large metagenomic datasets. To assess Prevotella diversity in the human host, we examined 7,480 Prevotella-assigned metagenome-assembled genomes (MAGs) from more than 9,500 human-associated metagenomes covering multiple geographic populations, lifestyles and body sites in combination with the isolate genomes from multiple sources in public repositories15. Fifty-six Prevotella species-level genome bins (SGBs; using a 5% nucleotide whole-genome distance threshold to define the bins) were identified with at least 20 genomes reconstructed from human sources. Of these, only 32 SGBs were populated with at least one isolate genome with assigned taxonomy (FIG. 3a, Supplementary BOX 3), demonstrating that human-associated Prevotella spp. are far more diverse than appreciable from the current catalogue of Prevotella isolates. These unnamed and uncharacterized Prevotella SGBs are not all rare metagenomic occurrences, as in some cases hundreds of genomes were recovered from independent metagenomic samples (FIG. 3a,b). Of note, nine uncharacterized SGBs are at the boundary of the Prevotella genus (FIG. 3a; subtree at the root) and contain only two isolate genomes, labelled as ‘Prevotellaceae bacterium’.
Fig. 3 |. Current diversity of Prevotella spp. in the human microbiome.
a | A large phylogenetic tree spanning the 56 most prevalent Prevotella species (with at least 20 genomes retrieved from human microbiomes). Isolate sequences were integrated with metagenome-assembled genomes (MAGs) retrieved from more than 10,000 metagenomes and more than 50 human populations. For each species, 15 randomly selected genomes are reported along with the information on the host type (that is, human or mixed sources), the public availability of isolate sequences and the number of retrieved genomes. The tree highlights the gap in strain isolation, with 24 human-associated species that completely lack isolate genomes, the within-species strain diversity and the remarkably well-defined taxonomic species based on the interspecies versus the intraspecies diversity. b | The fraction of Prevotella genomes retrieved from human and non-human hosts (both from isolation and assignment to MAGs) and different human site types suggest multiple ecological patterns. In addition to human-specific species, other species are present in two or more host types. Of note, a few species are ecologically adapted to multiple human body sites, which might also be due to the influx of oral species into the lower gastrointestinal tract71. Supplementary Table 1 provides a list of available reference genomes and reconstructed MAGs with their genomic characteristics for the species considered in this figure. NA, not assigned; NHP, non-human primate; SGB, species-level genome bin.
Prevotella in the oral cavity.
The oral cavity is the body site that hosts by far the largest diversity of known human Prevotella spp. (FIG. 2; FIG. 3). While the human gut may still harbour many undiscovered Prevotella spp. that are particularly recalcitrant to cultivation (FIG. 3b), the oral Prevotella taxa were studied long before the advent of cultivation-free surveys52 owing to their less complex cultivation conditions and their potential (sometimes confirmed) role in oral diseases53 and systemic infections54. According to the first comprehensive 16S rRNA gene sequencing investigation, the total abundance of the genus Prevotella in the oral cavity of healthy individuals exceeds, on average, 10% of the whole microbiome of the saliva (13.0%), tongue (10.3%), tonsils (11.4%) and throat (11.6%)7. Recent microbiome studies confirmed even higher average abundances in the saliva (from 12% to 17%) in multiple socio-economic and age categories55,56, making Prevotella the most abundant oral genus after Streptococcus. Slightly lower Prevotella abundance is estimated in oral locations that have more divergent structural and biochemical conditions, such as the dental plaque, the gingiva and the buccal mucosa7. Our analysis (FIG. 2) gives an overall Prevotella prevalence of 85% in Westernized populations when all oral sites are combined and 100% in non-Westernized populations, with an average abundance of 7.4% and 11.5% respectively. Our oral metagenomic data for non-Westernized populations are based on a single cohort from Fiji57, but reanalysis of another dataset of Filipino individuals58 confirmed the intriguing observation of higher prevalence and abundance of Prevotella in the oral cavity of individuals from non-Westernized populations. Interestingly, the levels of P. intermedia in the saliva microbiome decrease when a Mediterranean diet nutritional intervention is initiated in individuals with obesity59, suggesting a dependence of oral Prevotella on lifestyle factors that may include those connected with the Westernization process.
Genus-level quantification clearly conceals extensive species-level and interindividual diversity that is now readily detectable by shotgun metagenomics60. Available metagenomic analyses61–65 point to the presence of at least 15 known Prevotella spp. in the oral cavity (and a couple of taxa that are still to be characterized; FIG. 3); however, the oral microbiome in individuals is usually dominated by P. melaninogenica and, to a slightly lesser extent, Prevotella histicola and P. intermedia in adult non-Westernized populations (FIG. 2). Recent analyses for other oral genera in the context of the healthy oral microbiome, such as Neisseria41, Streptococcus66 and other genera67,68, revealed extensive strain-level variation hidden at the species level, and that strains within a species are individual-specific except for related individuals such as mother–infant couples in which direct transmission can occur63,69,70. Currently, such strain-level analyses are not available for oral Prevotella spp., and while the extent of within-species genetic and genomic variability is species-specific, it is likely that similar strain-level variation and individual specificity will also be observed for Prevotella spp.
As for other members of the oral microbiome, oral Prevotella spp. have the potential to colonize the lower gastrointestinal tract. Indeed, a systematic metagenomic investigation of oral to gut microbial transmission71 found that all oral Prevotella spp. can occasionally be found in the stool microbiome (FIG. 2b) and, in most cases, the oral and gut strains were the same within a host, suggesting a direct oral–faecal route in healthy individuals. Interestingly, high enrichment of oral species in the gut has been observed in patients with newly diagnosed colorectal cancer72,73, although only P. intermedia and, to a lesser extent, Prevotella nigrescens are biomarkers of this cancer. Oral Prevotella seeding of the gut environment seems to be a physiological mechanism, despite oral Prevotella species occasionally being implicated in systemic infections74.
In the oral cavity, Prevotella is also involved in the complex process of biofilm formation, which is potentially connected with poor oral hygiene24 and involved in the cause of oral diseases, such as prevalent gingivitis and periodontitis23. In particular, P. nigrescens and P. intermedia are associated with the plaque microbiome in periodontitis, an association that was already established in the presequencing era75, and these two species may promote tissue inflammation by driving T helper 17 cell (TH17 cell)-mediated immune responses23,76. Intriguingly, complementary fluorescence in situ hybridization-based investigations of dental plaque77,78 identified Prevotella spp. as one of the early colonizers that are necessary for stable biofilm formation. Several Prevotella spp., including Prevotella loescheii, P. intermedia and Prevotella denticola, may have a role in the early and middle stages of biofilm formation by promoting cell–cell adhesion and creating the physical and biochemical conditions that favour later colonizers79,80. The microbial consortia in oral biofilms can be very diverse; for example, in biofilm models in the absence of Streptococcus species, the abundance of P. intermedia increases and filamented chains that resemble those of streptococci are formed81. Prevotella spp. can also synergistically support Aggregatibacter actinomycetemcomitans, a species that is strongly associated with aggressive periodontitis82, by molecular interspecies regulation83. Although an area of continuous research for many years, the complexity of oral biofilm formation and the intricate interactions of the biofilm community with host tissues and immunity remain only partially understood. Furthermore, the hypothetical role of low-abundance taxa and of synergistic bacterial interactions84 further underlines the relevance of biofilm formation as a model for microbial ecology and as a potential therapeutic target. Prevotella is likely one of the most relevant genera in this context, as physical proximity analysis revealed that, together with Actinomyces, this genus had the highest intergenus interactions77.
Despite their abundance and role in oral biofilm formation, oral Prevotella spp. are still understudied compared with other oral bacteria. In particular, comparative analysis of oral Prevotella spp. is limited85, and clearly more studies are required to better understand their characteristics and ecological role in the oral microbiome86,87.
Vaginal Prevotella spp.
The human vaginal microbiome has been comprehensively surveyed, and Prevotella stands out as one of the dominant genera88. The most prevalent vaginal species are P. bivia, P. timonensis, P. buccalis, P. disiens and P. corporis. Interestingly, these species belong to separate phylogenetic clusters and none occurs uniquely in the urogenital tract, as they are also found in the oral and/or gut microbiome (FIG. 2). However, it is possible that for these species, genomic adaptation to the different body sites may occur at the subspecies level and is therefore undetectable at the species level. Prevotella spp. have a recognized role in bacterial vaginosis, which occurs when the protective guilds of the normally dominant Lactobacillus species are lost and strictly anaerobic microorganisms become established22. Prevotella spp. are the most heritable vaginal bacteria and are also associated with increased body mass index22,89. P. bivia is an important source of ammonia, lipopolysaccharide and sialidase activity in vaginal mucus and is linked to epithelial cytokine production, which occurs in infected uterine and placental tissues and amniotic fluid during pregnancy complications90–92. P. bivia often co-occurs with Gardnerella vaginalis, and this is recognized as a clear example of how microbial species can interact synergistically in certain environments. These two bacteria are co-abundant in the vagina and both are increased in abundance in cases of bacterial vaginosis93, although neither induces a robust inflammatory response94. Their co-inoculation has no major effect on their growth in mouse models of bacterial vaginosis, although co-colonization seemed to increase the chances of P. bivia ascension to the uterus90, which is relevant for the recognized association of Prevotella spp. with increased risk of preterm birth95–97. Interestingly, a network of other Prevotella spp., including P. amnii, P. buccalis and P. timonensis, can co-occur in other infections, such as those caused by Chlamydia trachomatis98, and have a role in bacterial vaginosis and increased predisposition to viral infections such as HIV infection99,100. P. timonensis interacts with vaginal dendritic cells, which are involved in mucosal inflammation101, and has also recently been associated with persistence and slower regression of cervical intraepithelial neoplasia102.
In the absence of further data to clarify the role of Prevotella spp. and strains in the development of bacterial vaginosis and related diseases, Prevotella simply appears as an inhabitant of the female urogenital tract that opportunistically increases in abundance when the abundance of protective vaginal lactobacilli is reduced.
Gut Prevotella spp. and P. copri.
The human gut is home to many microbial species, belonging predominantly to the phylum Firmicutes or Bacteroidetes61,103. Of the Bacteroidetes, two genera tend to dominate, Bacteroides and Prevotella, while the dominant Firmicutes genera include Ruminococcus, Blautia, Eubacterium and Faecalibacterium. The observation that these groups of taxa drive a subject-specific microbiome type led to the concept of human gut enterotypes104. Since its introduction almost 10 years ago, this concept has fed further studies and fuelled debate within the field105, which resulted in a revisited formulation of the enterotypes as non-discrete microbiome types106. It is clear that Prevotella spp. (particularly members of the P. copri complex) seem to be a discrete and well-defined feature of the gut microbiome, and their relative abundance is inversely correlated with that of the Bacteroides; that is, the presence of one seems to result in exclusion of the other61,104,107. Nevertheless, quantitative profiling based on absolute abundance suggests that this inverse correlation may simply be an artefact of microbiome profiling approaches based on relative abundance108. In the human gut, the average prevalence of Prevotella spp. ranges from 20% to 100% depending on the population surveyed109. Of the characterized Prevotella spp., P. stercorea and the P. copri complex are the two most common. Of note, despite both species being present in the gut, they are more phylogenetically distant from each other compared with other Prevotella spp. that occupy other body sites (FIGS 1,2). However, analysis of MAGs suggests that the number of Prevotella spp. in the gut is underestimated, with 22 additional SGBs (containing at least 20 MAGs) being identified that are not represented by a genome from an isolate (FIG. 3).
Of the human intestinal Prevotella spp., the most recognized and relevant member is P. copri. Although P. copri is not universally present, when identified it tends to be highly abundant15. P. copri has increasingly come under the spotlight owing to conflicting reports about whether its effect on human health is positive or detrimental25–27. Detrimental effects include inflammatory conditions, such as rheumatoid arthritis17,110,111, increased mucosal and systemic immune activation in patients with HIV infection112 or ankylosing spondylitis113, and exacerbating Listeria monocytogenes intestinal infection114. The association of Prevotella and P. copri with rheumatoid arthritis115 was reported on the basis of their abundance in the faecal microbiome17,116,117 and presence in the synovial fluid110,118 (Supplementary Table 2). An increased Prevotella abundance has been associated not only with patients with symptomatic rheumatoid arthritis but also with individuals at high risk of developing the disease, including those with rheumatoid arthritis-predisposing genotypes111,119. In preclinical models, faecal transplantation from patients with early-stage rheumatoid arthritis who have increased abundance of P. copri induced a proinflammatory TH17 cell response and rheumatoid arthritis phenotype in mice prone to rheumatoid arthritis120. Proinflammatory TH17 and TH1 cell immune responses mediated by P. copri-specific antibodies have also been reported in patients with rheumatoid arthritis17,110,111. Beyond associations with disease, studies have also suggested contrasting diet-dependent effects of P. copri on insulin resistance (discussed later). Of note, studies of Prevotella and particularly P. copri associations with diet or disease have relied mostly on the type strain DSM18205 (REF.121), despite P. copri displaying considerable subspecies strain-level variation67,122,123. Therefore, future mechanistic and intervention studies should better factor in such strain-level diversity, as some of the observed host phenotypes could be subspecies-dependent.
Two studies in 2019 expanded the concept of P. copri genetic and functional diversity, combining a large-scale meta-analysis of publicly available metagenomes (more than 6,800) with a targeted isolation and sequencing of human P. copri isolates4,16. These studies revealed that, in contrast to what had been previously assumed, P. copri is not monotypic (that is, it is not a single species-level lineage) but is a complex comprising four genetically distinct clades designated A–D16 (FIG. 1). The average nucleotide identity (13–21.4%) shared between clades is greater than would be expected within a single species lineage (less than 5–6%)124–126. Prevotella spp. have long been associated with non-Westernized populations, where they tend to be the dominant bacteria in the gut8–13. Analysis of the prevalence of the P. copri complex in these populations confirmed that it is very common (95.4% in non-Westernized populations versus 29.6% in Westernized populations)16 and is a long-standing constituent of the human microbiome, having been identified in ancient faecal and intestinal contents (BOX 2), but is rapidly being lost, most likely owing to the process of Westernization. Another revelation was the presence of multiple cohabiting P. copri clades within an individual. The presence of multiple P. copri clades was more likely than the presence of a single clade in non-Westernized individuals (more than two clades present in 93.5% of non-Westernized individuals versus 32.1% in Westernized individuals)16. This result suggests that the members of the P. copri complex are niche separated and potentially complementary, as the same result has also been obtained in mice studies127.
BOX 2 |. Ancient and evolutionary Prevotella genomic fossils.
The human microbiome is the result of a continuous interplay and co-evolution of microbial communities with the human host. Understandably, our knowledge of the microbiome relating to human health has been gleaned almost exclusively from biological samples collected from modern contemporary populations; more specifically, populations that have undergone Westernization, a process driven by industrialization and culminating in dietary and lifestyle changes. Recently, comparison of the microbiome of Westernized populations with that of contemporary non-Westernized populations (that is, those that follow a more traditional lifestyle and diet) revealed stark differences8–15. This raises interesting questions: how similar is the microbiome in Westernized populations to the long established, co-evolved ‘ancestral’ human microbiome, and are non-Westernized populations a better representation of this ancestral microbiome? Ancient samples have started to give a historical snapshot of the microorganisms that were present in our ancestors and how they have evolved, with the help of accurate dating of samples using molecular clocks186. These studies have focused primarily on the evolution of human pathogens and were made possible from samples where microbial DNA has been preserved, such as dental calculus or pulp and other skeletal remains. Examples include the unravelling of the evolutionary and adaptive trajectories of Yersinia pestis, the cause of the Black Death187, and Mycobacterium tuberculosis188 (reviewed elsewhere189).
Although small in number, ancient microbiomes from stool or intestinal samples have become available, including those from coprolites (fossilized faeces) and the Iceman (Ötzi)16, a natural ice mummy190. These studies have provided an initial awareness of how the ancestral human microbiome compares with that of contemporary populations. For example, analysis of the Iceman offered an insight into the historical global spread and evolutionary history of the stomach parasite Helicobacter pylori191. Ancient samples also revealed that the divergence of Prevotella copri into four distinct clades may possibly predate modern humans, and P. copri was almost certainly a core species in the microbiome of populations before the waves of human migration out of Africa16. As a consequence, the decreased prevalence of P. copri in the microbiome of Westernized populations compared with non-Westernized populations suggests that the non-Westernized microbiome is more representative of the co-evolved microbiome. However, to definitively answer this question, efforts are needed to increase the number of ancient samples from different human populations. Nevertheless, the increasing evidence that Westernized microbiomes are not accurate representations of our ancestral microbiome raises the question of whether this perturbation is ultimately detrimental to overall health, and warrants further investigation.
Gut Prevotella, diet and health.
Intestinal Prevotella spp. and the P. copri complex in particular are commonly associated with non-Western diets and nutritional patterns rich in carbohydrates, resistant starch and fibre8,128–132. In Western-style diets, members of the class Clostridia (Ruminococcaceae and Lachnospiraceae) often contribute as degraders of dietary fibre133, although nutritional interventions with fibre-rich foods usually result in an increase in Prevotella abundance131,134–137. In addition, a loss of members of the order Bacteroidales (including Prevotella) was observed in humanized mice fed a diet with low amounts of microbiota-accessible carbohydrates from dietary fibre138. The first attempts to develop a microbiome-oriented personalized nutrition demonstrated that stratifying individuals on the basis of microbiome composition may be a way to maximize clinical responses to dietary treatments. Indeed, having a Prevotella-rich gut microbiome potentiates weight loss139–142, decreases cholesterol levels143 and limits the bifidogenic effect144 in individuals consuming a fibre-rich diet. Improved glucose metabolism from fibre consumption has also been attributed to a higher Prevotella-to-Bacteroides ratio and succinate metabolism145,146. A study examining the association between gut bacteria and postprandial responses in more than 1,000 individuals identified P. copri as potentially beneficial in glucose homeostasis and host metabolism147. By contrast, another study found that P. copri was associated with insulin resistance148, and lower baseline levels of P. copri were linked to a reduction in insulin resistance in overweight individuals following a Mediterranean diet intervention149.
These effects might be explained by the potential of Prevotella spp. to degrade the complex polysaccharides in individuals with a mixed, high-fibre diet. This is relevant to human metabolism because the human genome encodes enzymes for the degradation of only a limited number of carbohydrates, including sucrose, lactose and starch150. Consequently, the ability of gut microorganisms to ferment polysaccharides is crucial in human nutrition. Prevotella spp. are proficient producers of the short-chain fatty acid propionate from arabinoxylans and fructo-oligosaccharides in vitro151, and the efficiency of specific strains in degrading these polysaccharides is linked with dominance patterns of Prevotella in mice127. In addition, the P. copri complex is capable of breaking down plant polysaccharides and host-derived mucin but not dietary-derived animal polysaccharides4,152. This fact might explain the decreased gut Prevotella diversity in industrialized, Westernized populations153, in which a high intake of diverse plant-based foods is rarely maintained. A highly specialized enzymatic potential, with remarkable strain-level variation, has been demonstrated in vitro and is responsible for complex polysaccharide degradation by different strains of the P. copri complex4. The diversity and mechanism of complex polysaccharide digestion is driven by the starch utilization system (Sus) comprising different transmembrane proteins (such as SusC) that transport polysaccharides into the periplasm. The genes encoding these transport proteins are usually located in polysaccharide utilization loci (together with degradation enzyme genes) and are selectively overexpressed on exposure of P. copri strains to different plant polysaccharides, with such exposure influencing growth capability4. Consequently, it might be plausible that the higher the P. copri diversity in the gut, the greater the number of types of complex polysaccharides that can be potentially utilized. Indeed, strain-level variation in P. copri genomes seems to be dependent on different dietary habits123,154, and such diversity may simply be driven by selective exposure of individuals to different types and amounts of plant polysaccharides. Remarkably, diet-driven selection can have unexpected health implications, as P. copri strains associated with an omnivorous diet have a higher prevalence of the leuB gene, which is involved in branched-chain amino acid biosynthesis, a risk factor for glucose intolerance and type 2 diabetes mellitus123.
Overall, the literature supporting a key role for Prevotella, and especially the P. copri complex, in driving individual clinical and metabolic responses to diet variation and to health status is substantial. However, the mechanisms of microbial contributions to these responses are far from clear, and this uncertainty is likely the cause of debate about whether Prevotella and P. copri are beneficial or detrimental in human health26,27,155. Factors that prevent clarity on the role of Prevotella spp. in gut health include the species-level and strain-level variability of gut Prevotella, in particular how many clades of the P. copri complex are present, which can hugely affect the response to different diets; and the variability of the dietary patterns studied, which all have a different supply of fibre and/or fibre-rich foods. The Prevotella arsenal of enzymes for digestion of polysaccharides represents an invaluable resource for optimal digestion dynamics and gut homeostasis. Thus, the current most likely interpretation is that the higher the diversity of Prevotella spp. (and other fibre degraders), the more advantageous the fermenting ability of the microbiome will be for the benefit of the human gut.
Prevotella spp. as potential pathogens
Prevotella spp. have been implicated in local and systemic infections, so to determine the extent of their involvement, we analysed the available literature by using a systematic approach (see Supplementary BOX 4). Studies linking Prevotella spp. with human infections are mostly observational (43%), but there are also case reports and retrospective and prospective studies (Supplementary Table 2; Supplementary Fig. 2a). Prevotella spp. are most commonly associated with infections in the oral cavity, with periodontal infections (n = 31) and endodontic infections (n = 35) being the most frequently reported. Of the non-oral infections, musculoskeletal, head and neck, lower respiratory tract and skin infections have been reported (Supplementary Fig. 2b). Interestingly, oral infections may cause secondary systemic or organ-related infections156. For example, cases of Prevotella-related bacteraemia after dental extraction157 or periodontal probing158 have been documented, as well as a case of liver pyogenic abscess related to dental disease159. Furthermore, a relationship between oral infections and cardiac diseases has also been proposed160. In other cases, Prevotella-related infections have occurred as a result of major diseases, such as heart failure161, in individuals with a compromised immune system162 or as a complication of surgery162–166.
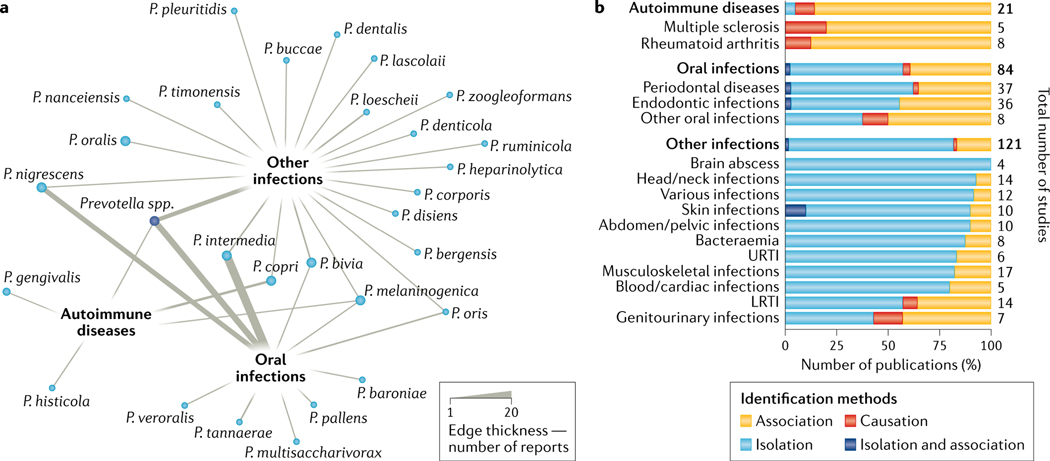
P. intermedia, P. bivia, P. nigrescens and P. melaninogenica are the species most frequently associated with infections in humans (FIG. 4a; Supplementary Fig. 2d). However, the methods that are routinely used to identify Prevotella taxa have ranged from standard clinical microbiology methods to low-throughput or high-throughput molecular approaches, and species-level identification was reliably achieved in only a fraction of these clinical cases. Bacterial isolation and cultivation were the most prevalent methods (FIG. 4b; Supplementary Fig. 2c), especially in clinical case reports. Particular attention must be paid to the ‘causation’ link in clinical cases, as the mere presence of Prevotella spp. does not indicate causality, whereas isolation of Prevotella spp. from blood or from infection fluids provides some evidence that they are the cause of infection, especially if the infection involves a single bacterial species. However, for oral infections, Prevotella can be present in the infection site as a consequence of its presence in the (healthy) oral microbiome. A causative role for Prevotella spp. in infections has rarely been confirmed by reproducing the human disease phenotype in animal models. In a bacterial vaginosis mouse model, co-inoculation of P. bivia with Gardnerella vaginalis reproduced some features of human bacterial vaginosis, including uterus ascension, epithelial exfoliation and sialidase activity90. Overall, only a small percentage of studies have addressed the causative role of Prevotella spp. (FIG. 4b), and for only a few infections (for example, genitourinary infections) or in autoimmunity (for example, multiple sclerosis and rheumatoid arthritis) has the pathogenic potential of Prevotella strains been further questioned. Furthermore, in most cases (60%; Supplementary Fig. 2b) Prevotella spp. tend to co-infect humans with other microorganisms167,168, suggesting that, while not obligate pathogens, Prevotella spp. may be opportunistic pathogens, alone or synergistically with other microorganisms, when the ecological conditions allow.
Fig. 4 |. Evidence for a role of Prevotella spp. in human infections and autoimmunity.
a | Network analysis showing the association of each Prevotella species with one or more diseases (grouped into three broad categories: autoimmune diseases, oral infections or other infections) based on a total of 226 studies (see Supplementary BOX 4 for the search and inclusion criteria for the systematic literature review; Supplementary Table 2 includes all the data extracted from the articles included in this work). The thickness of the edges is proportional to the number of articles reporting the involvement of Prevotella spp. in diseases. While some species were specifically associated with one disease group, a few were shared among the three categories. Cases for which species identification was not available are labelled as ‘Prevotella spp.’. The number of the four most abundant Prevotella spp. associated with each detailed disease is shown in Supplementary Fig. 4d. b | Proposed role of Prevotella spp. in human diseases. The percentage of studies for each disease (with at least four studies linking them with Prevotella spp.) are categorized on the basis of the identification method, namely ‘isolation’ (standard microbiology approaches of isolation and cultivation without mechanistic or causative investigation), ‘association’ (low-input or high-input molecular approaches, such as species-specific PCR or 16S rRNA gene sequencing that surveys the presence of bacteria in a sample) and ‘causation’ (studies in which the causative role of Prevotella spp. in the infection was demonstrated in animal models). Most of the studies providing evidence of a link between Prevotella and disease are isolation-based or merely associative. Diseases are ordered on the basis of a descending ‘isolation’ order, and the total number of studies considered per disease is indicated. Supplementary Fig. 2c provides further details of the method used to identify the Prevotella spp. in each disease and extends the results presented here. LRTI, lower respiratory tract infection; URTI, upper respiratory tract infection.
Anaerobic bacterial infections, such those involving Prevotella spp., are usually treated with broad-spectrum antibiotics. However, the increased incidence of antibiotic resistance may constitute a major limitation to resolving infections and poses a major issue for global health care. Despite this, the available information on antibiotic resistance in Prevotella spp. is scarce. Indeed, of the 226 studies we considered here, only 80 (35.4%) investigated the antibiotic resistance of Prevotella spp. through either biochemical or genetic methods. Resistance to β-lactam antibiotics, in particular penicillin and ampicillin, was reported in Prevotella isolates from head and neck infections169. Metronidazole resistance is also possible and has been reported in the clinic170, more specifically for Prevotella buccae171 and P. bivia172. While Prevotella does not seem to be one of the major resistant infectious threats, antibiotic resistance should be tested whenever possible, especially in patients with critical conditions, such as cystic fibrosis173–177.
Conclusions and outlook
Prevotella is the second most abundant genus in the human oral cavity, and when present, it is frequently the most abundant in the gut microbiome. Therefore, it is surprising how little is known about this genus and it is puzzling why other oral and intestinal genera have received much more attention. This discrepancy can be explained only partially by the substantially non-pathogenic nature of Prevotella spp. and the difficulty in cultivating intestinal Prevotella taxa. However, two main aspects are stimulating the study of this genus: the increased availability of (untargeted) metagenomic datasets (BOX 1) of the human microbiome that can reveal the hidden Prevotella diversity across worldwide populations, and the recognition that intestinal Prevotella spp. are key players in host–microbiome interactions, especially in relation to nutrition and metabolism.
A number of important questions and research directions could have a considerable impact on the current understanding of Prevotella genomics and ecology. First, there is now overwhelming evidence that the abundance of Prevotella spp. in oral and intestinal microbiomes is higher in non-Westernized populations than in Westernized populations8–13,15,57,58. Analysis of ancient microbiome samples supports the theory, at least for the P. copri complex, that the loss of Prevotella diversity is associated with, and is likely driven by, the process of Westernization and is not just a common feature of modern non-industrialized communities16. Other gut bacteria may be subject to the same forces, but the link with a Western lifestyle is stronger for Prevotella on the basis of the observed differential prevalence and within-subject genus-level diversity. Because Prevotella spp. in the intestine also tend to be highly abundant, some of the differences observed for other bacterial species across populations with different lifestyles could partially be a statistical artefact owing to the use of microbiome profiling methods based on relative abundance as well as a consequence of Prevotella-induced ecological effects. Further investigation to understand the genetic and ecological features driving the distinct host–Prevotella equilibrium is thus urgently needed. Unravelling such features will be particularly crucial in combination with a good study design when assessing which of the multiple lifestyle aspects (including diet, availability of antimicrobial drugs and hygiene practices) that differentiate Westernized and non-Westernized populations are most clearly related to differences in Prevotella prevalence and diversity. Ultimately, the major outstanding question is whether the decreased Prevotella prevalence and diversity observed at the population level in modern industrialized society is a reversible process and whether reversing this trend is indeed desirable from a health perspective. If it is desirable, then studies will be necessary to understand whether individual dietary-induced and lifestyle-induced microbiome alterations are sufficient to restore gut homeostasis or whether direct supplementation of Prevotella diversity would be required.
Second, it is crucial that future research be directed at unravelling the biomedical relevance of Prevotella spp. Indeed, while their pathogenic potential has proved to be limited and mostly connected with opportunistic infections, the role of Prevotella spp. in the onset and development of immune-mediated diseases remains unclear. Initial reports of a link with diseases such as rheumatoid arthritis17 were only partially confirmed by follow-up studies110,111,116. The complexity of Prevotella spp. interactions with mucosal immunity23,178 could explain the involvement of this genus in both autoimmunity and increased protection against pathobionts, and strain-level differences are likely responsible for the variability in immunological phenotypes between individuals, especially in light of the expanded genomic diversity of the P. copri complex16. Therefore, studies involving larger cohorts, with longitudinal sampling and accounting for host population differences, immunological aspects and strain-level Prevotella diversity, are needed to clarify this link, which could have a broad translational impact.
Third, the relationship between intestinal Prevotella spp. and nutrition should receive more research focus. Multiple convincing studies have demonstrated an association between Prevotella spp. and dietary patterns and cardiometabolic health, but such studies often report conflicting results128,130,139–143,145,146,148,149. Again, subspecies and strain diversity may obscure the consistency of experimental outcomes, which argues for improved study designs and larger clinical and preclinical cohorts. Because of the potential to manipulate the gut microbiome, a clear understanding of the role of Prevotella in human metabolism and nutrition might have tremendous clinical applications, such as the development of advanced probiotics and prebiotics, similar to those that are starting to be implemented for other bacteria, such as Akkermansia muciniphila179. For this application, efforts to isolate Prevotella strains will be pivotal in the coming years. The possibility to characterize strains, particularly from individuals with different diets and lifestyles, and observe their behaviour in vitro or in animal models will be of utmost importance to clarify the role of Prevotella spp. in health and how effective lifestyle features are in selecting strains with defined attributes.
Intriguingly, these three open questions could be interconnected in one hypothetical scenario. Considering the clear dietary implications of the Westernization process, the link between Prevotella and diet might be driving its decreasing presence in Westernized populations with their fat-rich and fibre-poor diet. Such a changing equilibrium potentially affects the host–Prevotella symbiotic relationship that was established over hundreds of thousands of years of co-evolution, leading in turn to impaired host–microorganism interactions that could make the host more prone to disease. This scenario remains for now an unproven hypothesis, but Prevotella seems to be the most promising genus to test this hypothesis.
Supplementary Material
Acknowledgements
The authors thank F. Cumbo, A. Blanco-Miguez, P. Manghi and F. Asnicar for support in retrieving and organizing the metagenome-assembled genomes. The work was supported by the European Research Council (ERC-STG project MetaPG), MIUR ‘Futuro in Ricerca’ (grant no. RBFR13EWWI_001), the National Cancer Institute of the US National Institutes of Health (1U01CA230551), the Premio Internazionale Lombardia e Ricerca 2019 and the European Union Horizon 2020 project ONCOBIOME-825410 to N.S, by the MASTER-818368 project to D.E. and N.S., and by the JPI HDHL-INTIMIC - Knowledge Platform of Food, Diet, Intestinal Microbiomics and Human Health (ID 790) and PRIN2017 (20174FHBWR_005) granted by the Italian Ministry of University and Research to D.E.
Footnotes
Competing interests
The authors declare no competing interests.
Supplementary information
The online version contains supplementary material available at https://doi.org/10.1038/s41579-021-00559-y.
Peer review information Nature Reviews Microbiology thanks T. Strowig, H. Flint, G. Belibasakis, who co-reviewed with D. Manoil, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
References
- 1. Shah HN & Collins DM Prevotella, a new genus to include Bacteroides melaninogenicus and related species formerly classified in the genus Bacteroides. Int. J. Syst. Bacteriol 40, 205–208 (1990). This work describes the initial identification, naming and description of the genus Prevotella.
- 2.Oliver WW & Wherry WB Notes on some bacterial parasites of the human mucous membranes. J. Infect. Dis 28, 341–344 (1921). [Google Scholar]
- 3.Shah HN, Chattaway MA, Rajakurana L. & Gharbia SE Prevotella. Bergey’s Manual of Systematics of Archaea and Bacteria 1–25 (Springer, 2015). [Google Scholar]
- 4. Fehlner-Peach H. et al. distinct polysaccharide utilization profiles of human intestinal Prevotella copri isolates. Cell Host Microbe 26, 680–690.e5 (2019). This study highlights how different strains in the P. copri complex have different abilities to target different types of polysaccharides.
- 5.Gmür R. & Thurnheer T. Direct quantitative differentiation between Prevotella intermedia and Prevotella nigrescens in clinical specimens. Microbiology 148, 1379–1387 (2002). [DOI] [PubMed] [Google Scholar]
- 6.Zambon JJ, Reynolds HS & Slots J. Black-pigmented Bacteroides spp. in the human oral cavity. Infect. Immun 32, 198–203 (1981). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Segata N. et al. Composition of the adult digestive tract bacterial microbiome based on seven mouth surfaces, tonsils, throat and stool samples.Genome Biol. 13, R42 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yatsunenko T. et al. Human gut microbiome viewed across age and geography. Nature 486, 222–227 (2012). This is one of the first and most comprehensive reports on the higher abundance and prevalence of Prevotella spp. in non-Westernized populations by 16S rRNA gene sequencing.
- 9.Smits SA et al. Seasonal cycling in the gut microbiome of the Hadza hunter-gatherers of Tanzania. Science 357, 802–806 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schnorr SL et al. Gut microbiome of the Hadza hunter-gatherers. Nat. Commun 5, 3654 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Obregon-Tito AJ et al. Subsistence strategies in traditional societies distinguish gut microbiomes. Nat. Commun 6, 6505 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hansen MEB et al. Population structure of human gut bacteria in a diverse cohort from rural Tanzania and Botswana. Genome Biol. 20, 16 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. De Filippo C. et al. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc. Natl Acad. Sci. USA 107, 14691–14696 (2010). This work reports one of the first pieces of evidence that Prevotella spp. dominate the gut microbiome in ‘non-Westernized’ populations.
- 14.Brewster R. et al. Surveying gut microbiome research in Africans: toward improved diversity and representation. Trends Microbiol. 27, 824–835 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pasolli E. et al. Extensive unexplored human microbiome diversity revealed by over 150,000 genomes from metagenomes spanning age, geography, and lifestyle. Cell 176, 649–662.e20 (2019). This study shows how microbial genomes can be reconstructed from metagenomic sequencing on a large scale, which is crucial to better understand the genetic basis and variability of human-associated Prevotella spp.
- 16. Tett A. et al. The Prevotella copri complex comprises four distinct clades underrepresented in westernized populations. Cell Host Microbe 26, 666–679.e7 (2019). This work reports the discovery that P. copri is not monotypic but comprises genetically distinct clades, and that this diversity should be considered in future studies.
- 17. Scher JU et al. Expansion of intestinal Prevotella copri correlates with enhanced susceptibility to arthritis. eLife 2, e01202 (2013). This is the first report of a link between P. copri and rheumatoid arthritis, which has since been expanded to other cohorts and related diseases.
- 18.Zhao-Fleming HH. et al. Traditional culture methods fail to detect principle pathogens in necrotising soft tissue infection: a case report. J. Wound Care 27, S24–S28 (2018). [DOI] [PubMed] [Google Scholar]
- 19.Bein T, Brem J. & Schüsselbauer T. Bacteremia and sepsis due to Prevotella oris from dentoalveolar abscesses. Intensive Care Med. 29, 856 (2003). [DOI] [PubMed] [Google Scholar]
- 20.Teanpaisan R, Douglas CW, Eley AR & Walsh TF Clonality of Porphyromonas gingivalis, Prevotella intermedia and Prevotella nigrescens isolated from periodontally diseased and healthy sites. J. Periodontal Res 31, 423–432 (1996). [DOI] [PubMed] [Google Scholar]
- 21.Baumgartner JC, Watkins BJ, Bae KS & Xia T. Association of black-pigmented bacteria with endodontic infections. J. Endod 25, 413–415 (1999). [DOI] [PubMed] [Google Scholar]
- 22.Si J, You HJ, Yu J, Sung J. & Ko G. Prevotella as a hub for vaginal microbiota under the influence of host genetics and their association with obesity. Cell Host Microbe 21, 97–105 (2017). [DOI] [PubMed] [Google Scholar]
- 23.Larsen JM The immune response to Prevotella bacteria in chronic inflammatory disease. Immunology 151, 363–374 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Teles FR et al. Early microbial succession in redeveloping dental biofilms in periodontal health and disease. J. Periodontal Res 47, 95–104 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cani PD Human gut microbiome: hopes, threats and promises. Gut 67, 1716–1725 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ley RE Gut microbiota in 2015: Prevotella in the gut: choose carefully. Nat. Rev. Gastroenterol. Hepatol 13, 69–70 (2016). [DOI] [PubMed] [Google Scholar]
- 27. Claus SP The strange case of Prevotella copri: Dr. Jekyll or Mr. Hyde? Cell Host Microbe 26, 577–578 (2019). This commentary summarizes some of the conflicting evidence for a favourable or detrimental role of P. copri.
- 28.Henderson G. et al. Rumen microbial community composition varies with diet and host, but a core microbiome is found across a wide geographical range. Sci. Rep 5, 14567 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deusch S. et al. A structural and functional elucidation of the rumen microbiome influenced by various diets and microenvironments. Front. Microbiol 8, 1605 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Accetto T. & Avguštin G. The diverse and extensive plant polysaccharide degradative apparatuses of the rumen and hindgut Prevotella species: a factor in their ubiquity? Syst. Appl. Microbiol 42, 107–116 (2019). [DOI] [PubMed] [Google Scholar]
- 31.Guevarra RB et al. Piglet gut microbial shifts early in life: causes and effects. J. Anim. Sci. Biotechnol 10, 1 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang X. et al. Longitudinal investigation of the swine gut microbiome from birth to market reveals stage and growth performance associated bacteria. Microbiome 7, 109 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Coil DA et al. Genomes from bacteria associated with the canine oral cavity: A test case for automated genome-based taxonomic assignment. PLoS ONE 14, e0214354 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kogawa M, Hosokawa M, Nishikawa Y, Mori K. & Takeyama H. Obtaining high-quality draft genomes from uncultured microbes by cleaning and co-assembly of single-cell amplified genomes. Sci. Rep 8, 2059 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ueki A, Akasaka H, Satoh A, Suzuki D. & Ueki K. Prevotella paludivivens sp. nov., a novel strictly anaerobic, Gram-negative, hemicellulose-decomposing bacterium isolated from plant residue and rice roots in irrigated rice-field soil. Int. J. Syst. Evol. Microbiol 57, 1803–1809 (2007). [DOI] [PubMed] [Google Scholar]
- 36.Sutton TDS & Hill C. Gut bacteriophage: current understanding and challenges. Front. Endocrinol 10, 784 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gregg K, Kennedy BG & Klieve AV Cloning and DNA sequence analysis of the region containing attP of the temperate phage ΦAR29 of Prevotella ruminicola AR29. Microbiology 140, 2109–2114 (1994). [DOI] [PubMed] [Google Scholar]
- 38.Ambrozic J, Ferme D, Grabnar M, Ravnikar M. & Avgustin G. The bacteriophages of ruminal prevotellas. Folia Microbiol. 46, 37–39 (2001). [DOI] [PubMed] [Google Scholar]
- 39. Devoto AE et al. Megaphages infect Prevotella and variants are widespread in gut microbiomes. Nat. Microbiol 4, 693–700 (2019). This study reports the discovery of large intestine megaphages associated with Prevotella and some initial characterization of their genetic features, such as the use of an alternative genetic code.
- 40.Crisci MA, Chen LX, Devoto AE, Borges AL & Bordin N. Wide distribution of alternatively coded Lak megaphages in animal microbiomes. bioRxiv 10.1101/2021.01.08.425732 (2021). [DOI] [Google Scholar]
- 41.Donati C. et al. Uncovering oral Neisseria tropism and persistence using metagenomic sequencing. Nat. Microbiol 1, 16070 (2016). [DOI] [PubMed] [Google Scholar]
- 42.Gupta VK, Chaudhari NM, Iskepalli S. & Dutta C. Divergences in gene repertoire among the reference Prevotella genomes derived from distinct body sites of human. BMC Genomics 16, 153 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mueller S. et al. Differences in fecal microbiota in different European study populations in relation to age, gender, and country: a cross-sectional study. Appl. Environ. Microbiol 72, 1027–1033 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Santos-Marcos JA et al. Sex differences in the gut microbiota as potential determinants of gender predisposition to disease. Mol. Nutr. Food Res 63, 1800870 (2019). [DOI] [PubMed] [Google Scholar]
- 45.Kornman KS & Loesche WJ The subgingival microbial flora during pregnancy. J. Periodontal Res 15, 111–122 (1980). [DOI] [PubMed] [Google Scholar]
- 46.Kornman KS & Loesche WJ Effects of estradiol and progesterone on Bacteroides melaninogenicus and Bacteroides gingivalis. Infect. Immun 35, 256–263 (1982). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Akcalı A. et al. Association between polycystic ovary syndrome, oral microbiota and systemic antibody responses. PLoS ONE 9, e108074 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Karcher N. et al. Analysis of 1321 Eubacterium rectale genomes from metagenomes uncovers complex phylogeographic population structure and subspecies functional adaptations. Genome Biol. 21, 138 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Almeida A. et al. A new genomic blueprint of the human gut microbiota. Nature 568, 499–504 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Almeida A. et al. A unified catalog of 204,938 reference genomes from the human gut microbiome. Nat. Biotechnol 39, 105–114 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nayfach S, Shi ZJ, Seshadri R, Pollard KS & Kyrpides NC New insights from uncultivated genomes of the global human gut microbiome. Nature 568, 505–510 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Könönen E. Pigmented Prevotella species in the periodontally healthy oral cavity. FEMS Immunol. Med. Microbiol 6, 201–205 (1993). [DOI] [PubMed] [Google Scholar]
- 53.Mättö J. et al. Role of Porphyromonas gingivalis, Prevotella intermedia, and Prevotella nigrescens in extraoral and some odontogenic infections. Clin. Infect. Dis 25, S194–S198 (1997). [DOI] [PubMed] [Google Scholar]
- 54.Brook I. Prevotella and Porphyromonas infections in children. J. Med. Microbiol 42, 340–347 (1995). [DOI] [PubMed] [Google Scholar]
- 55.Renson A. et al. Sociodemographic variation in the oral microbiome. Ann. Epidemiol 35, 73–80.e2 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Willis JR et al. Citizen science charts two major ‘stomatotypes’ in the oral microbiome of adolescents and reveals links with habits and drinking water composition. Microbiome 6, 218 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brito IL et al. Mobile genes in the human microbiome are structured from global to individual scales. Nature 535, 435–439 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lassalle F. et al. Oral microbiomes from hunter-gatherers and traditional farmers reveal shifts in commensal balance and pathogen load linked to diet. Mol. Ecol 27, 182–195 (2018). [DOI] [PubMed] [Google Scholar]
- 59.Laiola M, De Filippis F, Vitaglione P. & Ercolini D. A Mediterranean diet intervention reduces the levels of salivary periodontopathogenic bacteria in overweight and obese subjects. Appl. Environ. Microbiol 86, e00777–20 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Quince C, Walker AW, Simpson JT, Loman NJ & Segata N. Shotgun metagenomics, from sampling to analysis. Nat. Biotechnol 35, 833–844 (2017). [DOI] [PubMed] [Google Scholar]
- 61.Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature 486, 207–214 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Castro-Nallar E. et al. Composition, taxonomy and functional diversity of the oropharynx microbiome in individuals with schizophrenia and controls. PeerJ 3, e1140 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ferretti P. et al. Mother-to-infant microbial transmission from different body sites shapes the developing infant gut microbiome. Cell Host Microbe 24, 133–145.e5 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Olm MR et al. Identical bacterial populations colonize premature infant gut, skin, and oral microbiomes and exhibit different in situ growth rates. Genome Res. 27, 601–612 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ghensi P. et al. Strong oral plaque microbiome signatures for dental implant diseases identified by strain-resolution metagenomics. NPJ Biofilms Microbiomes 6, 47 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Eren AM, Borisy GG, Huse SM & Mark Welch JL Oligotyping analysis of the human oral microbiome. Proc. Natl Acad. Sci. USA 111, E2875–E2884 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Truong DT, Tett A, Pasolli E, Huttenhower C. & Segata N. Microbial strain-level population structure and genetic diversity from metagenomes. Genome Res. 27, 626–638 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Van Rossum T, Ferretti P, Maistrenko OM & Bork P. Diversity within species: interpreting strains in microbiomes. Nat. Rev. Microbiol 18, 491–506 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yassour M. et al. Strain-Level Analysis of Mother-to-Child Bacterial Transmission during the First Few Months of Life. Cell Host Microbe 24, 146–154.e4 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Korpela K. et al. Selective maternal seeding and environment shape the human gut microbiome. Genome Res. 28, 561–568 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Schmidt TS et al. Extensive transmission of microbes along the gastrointestinal tract. eLife 8, e42693 (2019). This work provides evidence that transmission to the large intestine by oral microorganisms is common and is particularly relevant for Prevotella spp.
- 72.Thomas AM et al. Metagenomic analysis of colorectal cancer datasets identifies cross-cohort microbial diagnostic signatures and a link with choline degradation. Nat. Med 25, 667–678 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wirbel J. et al. Meta-analysis of fecal metagenomes reveals global microbial signatures that are specific for colorectal cancer. Nat. Med 25, 679–689 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nagy E. Anaerobic infections: update on treatment considerations. Drugs 70, 841–858 (2010). [DOI] [PubMed] [Google Scholar]
- 75. Socransky SS, Haffajee AD, Cugini MA, Smith C. & Kent RL Jr. Microbial complexes in subgingival plaque. J. Clin. Periodontol 25, 134–144 (1998). This is the seminal work in the presequencing era associating species in the dental plaque biofilm, including Prevotella spp., with oral diseases.
- 76.Schincaglia GP et al. Clinical, immune, and microbiome traits of gingivitis and peri-implant mucositis. J. Dent. Res 96, 47–55 (2017). [DOI] [PubMed] [Google Scholar]
- 77.Valm AM et al. Systems-level analysis of microbial community organization through combinatorial labeling and spectral imaging. Proc. Natl Acad. Sci. USA 108, 4152–4157 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mark Welch JL, Rossetti BJ, Rieken CW, Dewhirst FE & Borisy GG Biogeography of a human oral microbiome at the micron scale. Proc. Natl Acad. Sci. USA 113, E791–E800 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kolenbrander PE, Palmer RJ, Periasamy S. & Jakubovics NS Oral multispecies biofilm development and the key role of cell–cell distance. Nat. Rev. Microbiol 8, 471–480 (2010). [DOI] [PubMed] [Google Scholar]
- 80.Kolenbrander PE Oral microbial communities: biofilms, interactions, and genetic systems. Annu. Rev. Microbiol 54, 413–437 (2000). [DOI] [PubMed] [Google Scholar]
- 81.Ammann TW, Belibasakis GN & Thurnheer T. Impact of early colonizers on in vitro subgingival biofilm formation. PLoS ONE 8, e83090 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fine DH et al. Aggregatibacter actinomycetemcomitans and its relationship to initiation of localized aggressive periodontitis: longitudinal cohort study of initially healthy adolescents. J. Clin. Microbiol 45, 3859–3869 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bao K, Bostanci N, Selevsek N, Thurnheer T. & Belibasakis GN Quantitative proteomics reveal distinct protein regulations caused by Aggregatibacter actinomycetemcomitans within subgingival biofilms. PLoS ONE 10, e0119222 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hajishengallis G, Darveau RP & Curtis MA The keystone-pathogen hypothesis. Nat. Rev. Microbiol 10, 717–725 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ibrahim M, Subramanian A. & Anishetty S. Comparative pan genome analysis of oral Prevotella species implicated in periodontitis. Funct. Integr. Genomics 17, 513–536 (2017). [DOI] [PubMed] [Google Scholar]
- 86.Könönen E, Nyfors S, Máttö J, Asikainen S. & Somer HJ β-lactamase production by oral pigmented Prevotella species isolated from young children. Clin. Infect. Dis 25, S272–S274 (1997). [DOI] [PubMed] [Google Scholar]
- 87.Falagas ME & Siakavellas E. Bacteroides, Prevotella, and Porphyromonas species: a review of antibiotic resistance and therapeutic options. Int. J. Antimicrob. Agents 15, 1–9 (2000). [DOI] [PubMed] [Google Scholar]
- 88.Diop K, Dufour J-C, Levasseur A. & Fenollar F. Exhaustive repertoire of human vaginal microbiota. Hum. Microbiome J 11, 100051 (2019). [Google Scholar]
- 89.Ravel J. et al. Vaginal microbiome of reproductive-age women. Proc. Natl Acad. Sci. USA 108, 4680–4687 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gilbert NM. et al. Gardnerella vaginalis and Prevotella bivia trigger distinct and overlapping phenotypes in a mouse model of bacterial vaginosis. J. Infect. Dis 220, 1099–1108 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Randis TM & Ratner AJ Gardnerella and Prevotella: co-conspirators in the pathogenesis of bacterial vaginosis. J. Infect. Dis 220, 1085–1088 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Aroutcheva A, Ling Z. & Faro S. Prevotella bivia as a source of lipopolysaccharide in the vagina. Anaerobe 14, 256–260 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Muzny CA et al. Identification of key bacteria involved in the induction of incident bacterial vaginosis: a prospective study. J. Infect. Dis 218, 966–978 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Muzny CA, Łaniewski P, Schwebke JR & Herbst-Kralovetz MM Host-vaginal microbiota interactions in the pathogenesis of bacterial vaginosis. Curr. Opin. Infect. Dis 33, 59–65 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fettweis JM et al. The vaginal microbiome and preterm birth. Nat. Med 25, 1012–1021 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Brown RG et al. Establishment of vaginal microbiota composition in early pregnancy and its association with subsequent preterm prelabor rupture of the fetal membranes. Transl. Res 207, 30–43 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Callahan BJ et al. Replication and refinement of a vaginal microbial signature of preterm birth in two racially distinct cohorts of US women. Proc. Natl Acad. Sci. USA 114, 9966–9971 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Filardo S. et al. Selected immunological mediators and cervical microbial signatures in women with Chlamydia trachomatis infection. mSystems 4, e00094–19 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Abdool Karim SS, Baxter C, Passmore J-AS, McKinnon LR & Williams BL The genital tract and rectal microbiomes: their role in HIV susceptibility and prevention in women. J. Int. Aids Soc 22, e25300 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cohen J. Vaginal microbiome affects HIV risk. Science 353, 331–331 (2016). [DOI] [PubMed] [Google Scholar]
- 101.van Teijlingen NH et al. Vaginal dysbiosis associated-bacteria Megasphaera elsdenii and Prevotella timonensis induce immune activation via dendritic cells. J. Reprod. Immunol 138, 103085 (2020). [DOI] [PubMed] [Google Scholar]
- 102.Mitra A. et al. The vaginal microbiota associates with the regression of untreated cervical intraepithelial neoplasia 2 lesions. Nat. Commun 11, 1999 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Qin J. et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 464, 59–65 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Arumugam M. et al. Enterotypes of the human gut microbiome. Nature 473, 174–180 (2011). This study introduces the concept of enterotypes, including the Prevotella enterotype, which is one of the enterotypes with the strongest evidence in recent refinements of the concept.
- 105.Cheng M. & Ning K. Stereotypes about enterotype: the old and new ideas. Genomics Proteom. Bioinforma 17, 4–12 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Costea PI et al. Enterotypes in the landscape of gut microbial community composition. Nat. Microbiol 3, 8–16 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Faust K. et al. Microbial co-occurrence relationships in the human microbiome. PLoS Comput. Biol 8, e1002606 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Vandeputte D. et al. Quantitative microbiome profiling links gut community variation to microbial load. Nature 551, 507–511 (2017). [DOI] [PubMed] [Google Scholar]
- 109.Pasolli E. et al. Accessible, curated metagenomic data through ExperimentHub. Nat. Methods 14, 1023–1024 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pianta A. et al. Evidence of the immune relevance of Prevotella copri, a gut microbe, in patients with rheumatoid arthritis. Arthritis Rheumatol. 69, 964–975 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Alpizar-Rodriguez D. et al. Prevotella copri in individuals at risk for rheumatoid arthritis. Ann. Rheum. Dis 78, 590–593 (2019). [DOI] [PubMed] [Google Scholar]
- 112.Dillon SM et al. An altered intestinal mucosal microbiome in HIV-1 infection is associated with mucosal and systemic immune activation and endotoxemia. Mucosal Immunol. 7, 983–994 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wen C. et al. Quantitative metagenomics reveals unique gut microbiome biomarkers in ankylosing spondylitis. Genome Biol. 18, 142 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rolhion N. et al. A Listeria monocytogenes bacteriocin can target the commensal Prevotella copri and modulate intestinal infection. Cell Host Microbe 26, 691–701.e5 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Iljazovic A, Amend L, Galvez EJC, de Oliveira R. & Strowig T. Modulation of inflammatory responses by gastrointestinal Prevotella spp. – from associations to functional studies. Int. J. Med. Microbiol 311, 151472 (2021). [DOI] [PubMed] [Google Scholar]
- 116.Kishikawa T. et al. Metagenome-wide association study of gut microbiome revealed novel aetiology of rheumatoid arthritis in the Japanese population. Ann. Rheum. Dis 79, 103–111 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lee J-Y et al. Comparative analysis of fecal microbiota composition between rheumatoid arthritis and osteoarthritis patients. Genes 10, 748 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhao Y. et al. Detection and characterization of bacterial nucleic acids in culture-negative synovial tissue and fluid samples from rheumatoid arthritis or osteoarthritis patients. Sci. Rep 8, 14305 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wells PM et al. Associations between gut microbiota and genetic risk for rheumatoid arthritis in the absence of disease: a cross-sectional study. Lancet Rheumatol. 2, e418–e427 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Maeda Y. et al. Dysbiosis contributes to arthritis development via activation of autoreactive T cells in the intestine. Arthritis Rheumatol. 68, 2646–2661 (2016). [DOI] [PubMed] [Google Scholar]
- 121.Hayashi H, Shibata K, Sakamoto M, Tomita S. & Benno Y. Prevotella copri sp. nov. and Prevotella stercorea sp. nov., isolated from human faeces. Int. J. Syst. Evol. Microbiol 57, 941–946 (2007). [DOI] [PubMed] [Google Scholar]
- 122.Vangay P. et al. US Immigration westernizes the human gut microbiome. Cell 175, 962–972.e10 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. De Filippis F. et al. Distinct genetic and functional traits of human intestinal Prevotella copri strains are associated with different habitual diets. Cell Host Microbe 25, 444–453.e3 (2019). This work reports important recent evidence of the effect of diet in shaping the subspecies pangenomic diversity of P. copri.
- 124.Goris J. et al. DNA-DNA hybridization values and their relationship to whole-genome sequence similarities. Int. J. Syst. Evol. Microbiol 57, 81–91 (2007). [DOI] [PubMed] [Google Scholar]
- 125.Jain C, Rodriguez-R LM, Phillippy AM, Konstantinidis KT & Aluru S. High throughput ANI analysis of 90K prokaryotic genomes reveals clear species boundaries. Nat. Commun 9, 5114 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Konstantinidis KT & Tiedje JM Genomic insights that advance the species definition for prokaryotes. Proc. Natl Acad. Sci. USA 102, 2567–2572 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Gálvez EJC et al. Distinct polysaccharide utilization determines interspecies competition between intestinal Prevotella spp. Cell Host Microbe 28, 838–852.e6 (2020). This work describes how distinct Prevotella spp. compete in vivo for similar plant-derived polysaccharides.
- 128.Wu GD et al. Linking long-term dietary patterns with gut microbial enterotypes. Science 334, 105–108 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ou J. et al. Diet, microbiota, and microbial metabolites in colon cancer risk in rural Africans and African Americans. Am. J. Clin. Nutr 98, 111–120 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.De Filippis F. et al. High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut 65, 1812–1821 (2016). [DOI] [PubMed] [Google Scholar]
- 131.Haro C. et al. Consumption of two healthy dietary patterns restored microbiota dysbiosis in obese patients with metabolic dysfunction. Mol. Nutr. Food Res 61, 1700300 (2017). [DOI] [PubMed] [Google Scholar]
- 132.Precup G. & Vodnar D-C Gut Prevotella as a possible biomarker of diet and its eubiotic versus dysbiotic roles: a comprehensive literature review. Br. J. Nutr 122, 131–140 (2019). [DOI] [PubMed] [Google Scholar]
- 133.Gomez A. et al. Gut microbiome of coexisting BaAka pygmies and bantu reflects gradients of traditional subsistence patterns. Cell Rep. 14, 2142–2153 (2016). [DOI] [PubMed] [Google Scholar]
- 134.Benítez-Páez A. et al. A multi-omics approach to unraveling the microbiome-mediated effects of arabinoxylan oligosaccharides in overweight humans. mSystems 4, e00209–19 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Roager HM et al. Whole grain-rich diet reduces body weight and systemic low-grade inflammation without inducing major changes of the gut microbiome: a randomised cross-over trial. Gut 68, 83–93 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Marungruang N, Tovar J, Björck I. & Hållenius FF Improvement in cardiometabolic risk markers following a multifunctional diet is associated with gut microbial taxa in healthy overweight and obese subjects. Eur. J. Nutr 57, 2927–2936 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ghosh TS et al. Mediterranean diet intervention alters the gut microbiome in older people reducing frailty and improving health status: the NU-AGE 1-year dietary intervention across five European countries. Gut 69, 1218–1228 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sonnenburg ED et al. Diet-induced extinctions in the gut microbiota compound over generations. Nature 529, 212–215 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Christensen L, Roager HM, Astrup A. & Hjorth MF Microbial enterotypes in personalized nutrition and obesity management. Am. J. Clin. Nutr 108, 645–651 (2018). [DOI] [PubMed] [Google Scholar]
- 140.Hjorth MF et al. Pre-treatment microbial Prevotella-to-Bacteroides ratio, determines body fat loss success during a 6-month randomized controlled diet intervention. Int. J. Obes 42, 284 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hjorth MF et al. Prevotella-to-Bacteroides ratio predicts body weight and fat loss success on 24-week diets varying in macronutrient composition and dietary fiber: results from a post-hoc analysis. Int. J. Obes 43, 149–157 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ortega-Santos CP & Whisner CM The key to successful weight loss on a high-fiber diet may be in gut microbiome Prevotella abundance. J. Nutr 149, 2083–2084 (2019). [DOI] [PubMed] [Google Scholar]
- 143.Eriksen AK et al. Effects of whole-grain wheat, rye, and lignan supplementation on cardiometabolic risk factors in men with metabolic syndrome: a randomized crossover trial. Am. J. Clin. Nutr 111, 864–876 (2020). [DOI] [PubMed] [Google Scholar]
- 144.Chung WSF et al. Relative abundance of the Prevotella genus within the human gut microbiota of elderly volunteers determines the inter-individual responses to dietary supplementation with wheat bran arabinoxylan-oligosaccharides. BMC Microbiol. 20, 283 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kovatcheva-Datchary P. et al. Dietary fiber-induced improvement in glucose metabolism is associated with increased abundance of Prevotella. Cell Metab. 22, 971–982 (2015). [DOI] [PubMed] [Google Scholar]
- 146.De Vadder F. et al. Microbiota-produced succinate improves glucose homeostasis via intestinal gluconeogenesis. Cell Metab. 24, 151–157 (2016). [DOI] [PubMed] [Google Scholar]
- 147.Asnicar F. et al. Microbiome connections with host metabolism and habitual diet from 1,098 deeply phenotyped individuals. Nat. Med 27, 321–332 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Pedersen HK et al. Human gut microbes impact host serum metabolome and insulin sensitivity. Nature 535, 376–381 (2016). [DOI] [PubMed] [Google Scholar]
- 149.Meslier V. et al. Mediterranean diet intervention in overweight and obese subjects lowers plasma cholesterol and causes changes in the gut microbiome and metabolome independently of energy intake. Gut 69, 1258–1268 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kaoutari AE et al. The abundance and variety of carbohydrate-active enzymes in the human gut microbiota. Nat. Rev. Microbiol 11, 497–504 (2013). [DOI] [PubMed] [Google Scholar]
- 151.Chen T. et al. Fiber-utilizing capacity varies in Prevotella- versus Bacteroides-dominated gut microbiota. Sci. Rep 7, 2594 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wright DP, Rosendale DI & Robertson AM Prevotella enzymes involved in mucin oligosaccharide degradation and evidence for a small operon of genes expressed during growth on mucin. FEMS Microbiol. Lett 190, 73–79 (2000). [DOI] [PubMed] [Google Scholar]
- 153.Shanahan F, Ghosh TS & O’Toole PW The healthy microbiome — what is the definition of a healthy gut microbiome? Gastroenterology 160, 483–494 (2021). [DOI] [PubMed] [Google Scholar]
- 154.De Filippis F, Pellegrini N, Laghi L, Gobbetti M. & Ercolini D. Unusual sub-genus associations of faecal Prevotella and Bacteroides with specific dietary patterns. Microbiome 4, 57 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Metwaly A. & Haller D. Strain-level diversity in the gut: the P. copri case. Cell Host Microbe 25, 349–350 (2019). [DOI] [PubMed] [Google Scholar]
- 156.Li X, Kolltveit KM, Tronstad L. & Olsen I. Systemic diseases caused by oral infection. Clin. Microbiol. Rev 13, 547–558 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Rajasuo A, Perkki K, Nyfors S, Jousimies-Somer H. & Meurman JH Bacteremia following surgical dental extraction with an emphasis on anaerobic strains. J. Dent. Res 83, 170–174 (2004). [DOI] [PubMed] [Google Scholar]
- 158.Daly C, Mitchell D, Grossberg D, Highfield J. & Stewart D. Bacteraemia caused by periodontal probing. Aust. Dent. J 42, 77–80 (1997). [DOI] [PubMed] [Google Scholar]
- 159.Lei W-Y, Chang W-H, Shih S-C, Liu C-J & Shih C-H Pyogenic liver abscess with Prevotella species and Fusobacterium necrophorum as causative pathogens in an immunocompetent patient. J. Formos. Med. Assoc 108, 253–257 (2009). [DOI] [PubMed] [Google Scholar]
- 160.Kholy KE, Genco RJ & Van Dyke TE Oral infections and cardiovascular disease. Trends Endocrinol. Metab 26, 315–321 (2015). [DOI] [PubMed] [Google Scholar]
- 161.Posteraro P. et al. First bloodstream infection caused by Prevotella copri in a heart failure elderly patient with Prevotella-dominated gut microbiota: a case report. Gut Pathog. 11, 44 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Teanpaisan R, Douglas CW & Nittayananta W. Isolation and genotyping of black-pigmented anaerobes from periodontal sites of HIV-positive and non-infected subjects in Thailand. J. Clin. Periodontol 28, 311–318 (2001). [DOI] [PubMed] [Google Scholar]
- 163.Steingruber I, Bach CM, Czermak B, Nogler M. & Wimmer C. Infection of a total hip arthroplasty with Prevotella loeschii. Clin. Orthop. Relat. Res 418, 222–224 (2004). [DOI] [PubMed] [Google Scholar]
- 164.Myers C. et al. Postoperative gram-negative anaerobic bacterial endocarditis. Pediatr. Infect. Dis. J 26, 369 (2007). [DOI] [PubMed] [Google Scholar]
- 165.Mehmood M, Jaffar NA, Nazim M. & Khasawneh FA Bacteremic skin and soft tissue infection caused by Prevotella loescheii. BMC Infect. Dis 14, 162 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Thomaidis PC et al. Sonication assisted microbiological diagnosis of implant-related infection caused by Prevotella disiens and Staphylococcus epidermidis in a patient with cranioplasty. BMC Res. Notes 8, 307 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Krüger W, Vielreicher S, Kapitan M, Jacobsen ID & Niemiec MJ Fungal-bacterial interactions in health and disease. Pathogens 8, 70 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Contreras A. & Slots J. Herpesviruses in human periodontal disease. J. Periodontal Res 35, 3–16 (2000). [DOI] [PubMed] [Google Scholar]
- 169.Bancescu G, Didilescu A, Bancescu A. & Bari M. Antibiotic susceptibility of 33 Prevotella strains isolated from Romanian patients with abscesses in head and neck spaces. Anaerobe 35, 41–44 (2015). [DOI] [PubMed] [Google Scholar]
- 170.Mory F. et al. Bacteremia caused by a metronidazole-resistant Prevotella sp. strain. J. Clin. Microbiol 43, 5380 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Cobo F, Rodríguez-Granger J, Sampedro A. & Navarro-Marí JM Infected breast cyst due to Prevotella buccae resistant to metronidazole. Anaerobe 48, 177–178 (2017). [DOI] [PubMed] [Google Scholar]
- 172.Veloo ACM, Chlebowicz M, Winter HLJ, Bathoorn D. & Rossen JWA Three metronidazole-resistant Prevotella bivia strains harbour a mobile element, encoding a novel nim gene, inimK, and an efflux small MDR transporter. J. Antimicrob. Chemother 73, 2687–2690 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Sherrard LJ et al. Antibiotic resistance in Prevotella species isolated from patients with cystic fibrosis. J. Antimicrob. Chemother 68, 2369–2374 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Sherrard LJ et al. Mechanisms of reduced susceptibility and genotypic prediction of antibiotic resistance in Prevotella isolated from cystic fibrosis (CF) and non-CF patients. J. Antimicrob. Chemother 69, 2690–2698 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Sherrard LJ et al. Production of extended-spectrum β-lactamases and the potential indirect pathogenic role of Prevotella isolates from the cystic fibrosis respiratory microbiota. Int. J. Antimicrob. Agents 47, 140–145 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Tunney MM et al. Use of culture and molecular analysis to determine the effect of antibiotic treatment on microbial community diversity and abundance during exacerbation in patients with cystic fibrosis. Thorax 66, 579–584 (2011). [DOI] [PubMed] [Google Scholar]
- 177.Zhao J. et al. Decade-long bacterial community dynamics in cystic fibrosis airways. Proc. Natl Acad. Sci. USA 109, 5809–5814 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Iljazovic A. et al. Perturbation of the gut microbiome by Prevotella spp. enhances host susceptibility to mucosal inflammation. Mucosal. Immunol 14, 113–124 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Depommier C. et al. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: a proof-of-concept exploratory study. Nat. Med 25, 1096–1103 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Truong DT et al. MetaPhlAn2 for enhanced metagenomic taxonomic profiling. Nat. Methods 12, 902–903 (2015). [DOI] [PubMed] [Google Scholar]
- 181.Li D, Liu C-M, Luo R, Sadakane K. & Lam T-W MEGAHIT: an ultra-fast single-node solution for large and complex metagenomics assembly via succinct de Bruijn graph. Bioinformatics 31, 1674–1676 (2015). [DOI] [PubMed] [Google Scholar]
- 182.Nurk S, Meleshko D, Korobeynikov A. & Pevzner PA metaSPAdes: a new versatile metagenomic assembler. Genome Res. 27, 824–834 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Kang DD et al. MetaBAT 2: an adaptive binning algorithm for robust and efficient genome reconstruction from metagenome assemblies. PeerJ 7, e7359 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Parks DH, Imelfort M, Skennerton CT, Hugenholtz P. & Tyson GW CheckM: assessing the quality of microbial genomes recovered from isolates, single cells, and metagenomes. Genome Res. 25, 1043–1055 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Asnicar F. et al. Precise phylogenetic analysis of microbial isolates and genomes from metagenomes using PhyloPhlAn 3.0. Nat. Commun 11, 2500 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Bromham L. & Penny D. The modern molecular clock. Nat. Rev. Genet 4, 216–224 (2003). [DOI] [PubMed] [Google Scholar]
- 187.Bos KI et al. A draft genome of Yersinia pestis from victims of the Black Death. Nature 478, 506–510 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Comas I. et al. Out-of-Africa migration and Neolithic coexpansion of Mycobacterium tuberculosis with modern humans. Nat. Genet 45, 1176–1182 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Spyrou MA, Bos KI, Herbig A. & Krause J. Ancient pathogen genomics as an emerging tool for infectious disease research. Nat. Rev. Genet 20, 323–340 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Spindler K. The Man in the Ice (Weidenfeld and Nicolson, 1994). [Google Scholar]
- 191. Maixner F. et al. The 5300-year-old Helicobacter pylori genome of the Iceman. Science 351, 162–165 (2016). This is one of the first studies showing that reconstruction of genomes from ancient samples is possible, which is particularly relevant to study the evolutionary history of Prevotella spp.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.