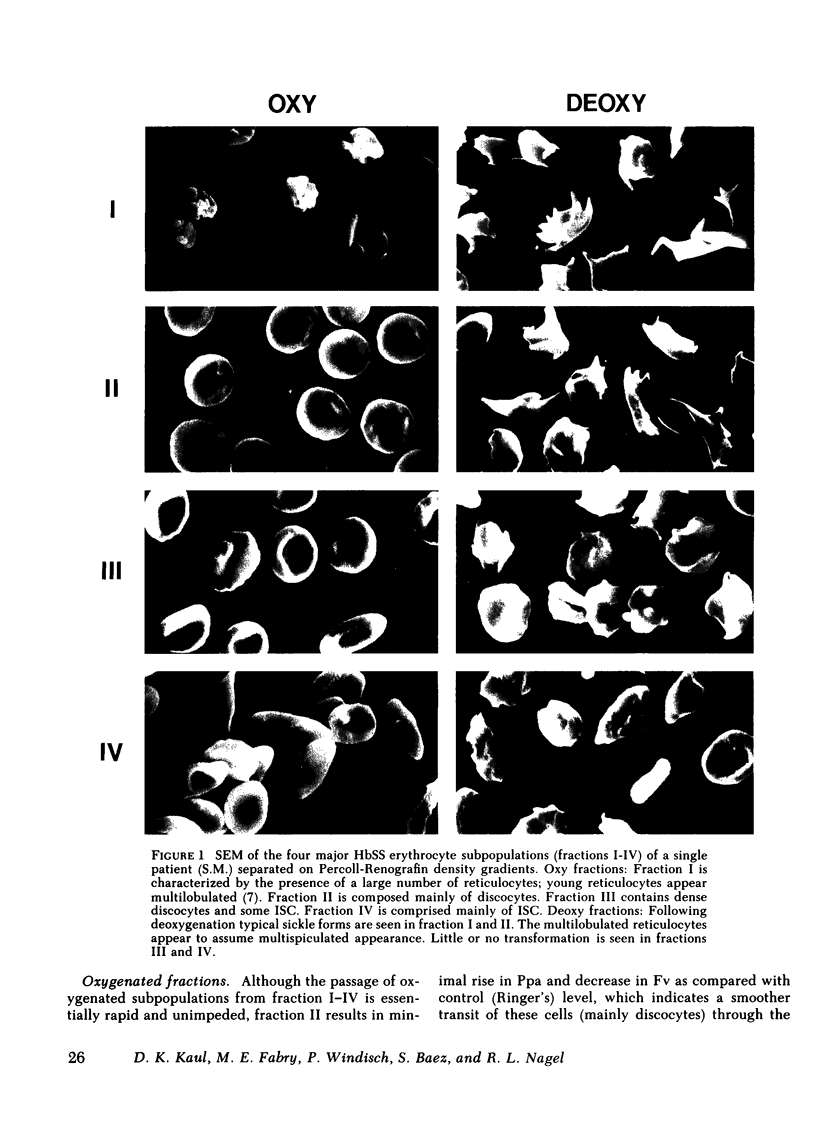
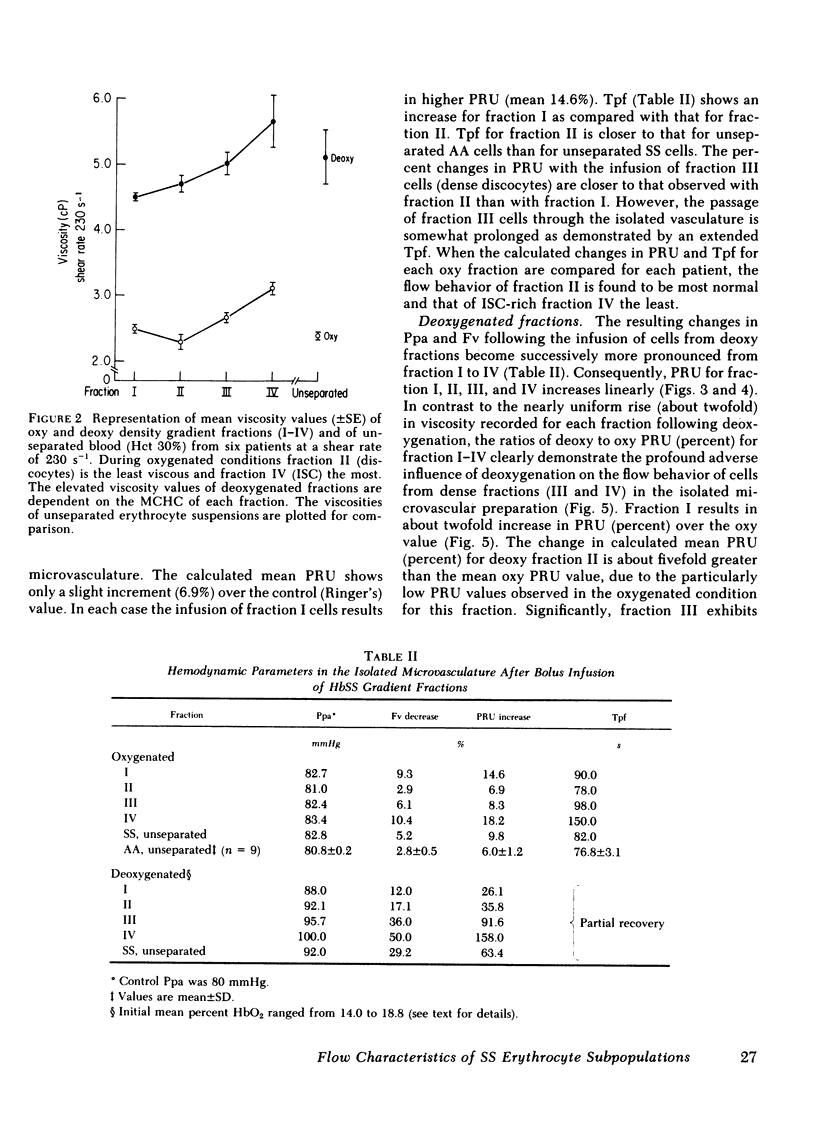
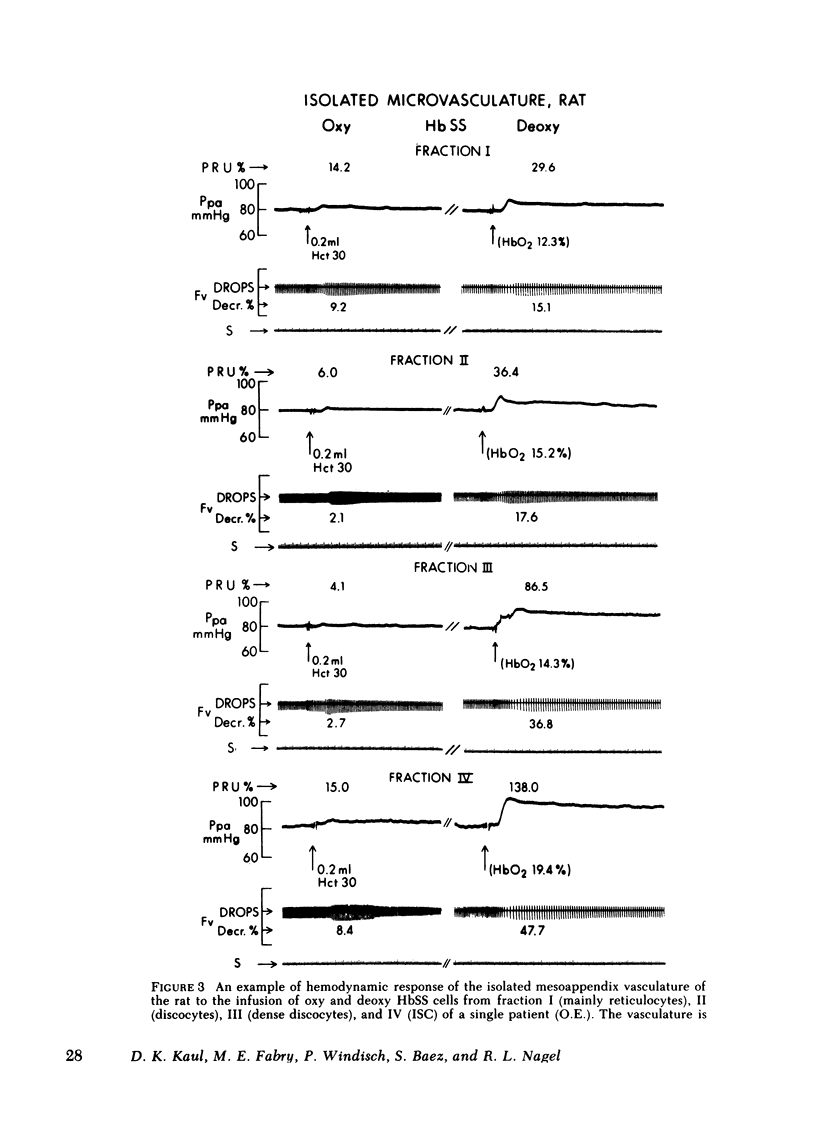
Abstract
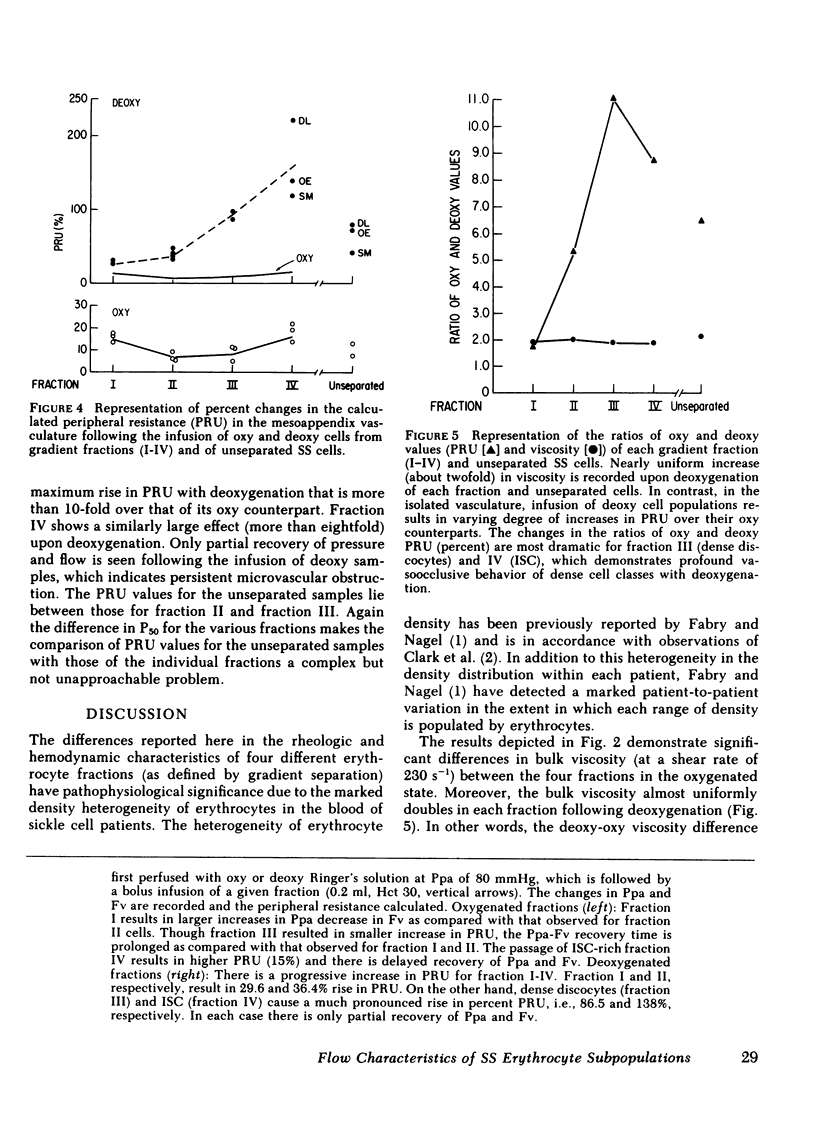
To understand the contribution to the pathophysiology of sickle cell anemia of the different erythrocyte density types present in the blood of these patients, we have studied the viscosimetric and hemodynamic characteristics of four major classes of hemoglobin SS erythrocytes. We have isolated reticulocytes, discocytes, dense discocytes, and irreversibly sickled cells (fractions I-IV) on Percoll-Renografin density gradients. Bulk viscosity was studied in a coneplate viscosimeter and the hemodynamic studies were performed on the isolated, artificially perfused mesoappendix vasculature of the rat (Baez preparation). Bulk viscosity measurements at shear rates of 230 S-1 demonstrate that when the cells are oxygenated, fraction I (reticulocyte rich) has a higher viscosity than expected from its low intracellular hemoglobin concentration. The rest of the fractions exhibit moderate increases in bulk viscosity pari-passu with the corresponding increases in density (mean corpuscular hemoglobin concentration). When deoxygenated, all cell fractions nearly doubled their bulk viscosity and the deoxy-oxy differences remained constant. The Baez preparation renders a different picture: oxygenated fractions behave as predicted by the viscosimetric data, but, when deoxygenated, cell fractions exhibit dramatically increased peripheral resistance and the deoxy-oxy difference are directly proportional to cell density, thus, the largest increases were observed for fractions III and IV. The differences between the rheological and the hemodynamic measurements are most probably due to the different sensitivity of the two methods to the extent of intracellular polymerization. These results also demonstrate that the hitherto unrecognized fraction III cells (very dense discocytes that change shape very little on deoxygenation) are as detrimental to the microcirculation as the irreversibly sickled cell-rich fraction IV. They may, however, induce obstruction by a different mechanism. As the extent to which these fractions are populated by erythrocytes varies considerably from patient to patient, the distribution function of cell densities in each sickle cell anemia patient might have consequences for the type of pathophysiological events occurring in their microcirculation.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baez S., Kaul D. K., Nagel R. L. Microvascular determinants of blood flow behavior and HbSS erythrocyte plugging in microcirculation. Blood Cells. 1982;8(1):127–137. [PubMed] [Google Scholar]
- Bertles J. F., Milner P. F. Irreversibly sickled erythrocytes: a consequence of the heterogeneous distribution of hemoglobin types in sickle-cell anemia. J Clin Invest. 1968 Aug;47(8):1731–1741. doi: 10.1172/JCI105863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien S., Usami S., Bertles J. F. Abnormal rheology of oxygenated blood in sickle cell anemia. J Clin Invest. 1970 Apr;49(4):623–634. doi: 10.1172/JCI106273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark M. R., Guatelli J. C., Mohandas N., Shohet S. B. Influence of red cell water content on the morphology of sickling. Blood. 1980 May;55(5):823–830. [PubMed] [Google Scholar]
- Clark M. R., Mohandas N., Shohet S. B. Deformability of oxygenated irreversibly sickled cells. J Clin Invest. 1980 Jan;65(1):189–196. doi: 10.1172/JCI109650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulombel L., Tchernia G., Mohandas N. Human reticulocyte maturation and its relevance to erythropoietic stress. J Lab Clin Med. 1979 Sep;94(3):467–474. [PubMed] [Google Scholar]
- Fabry M. E., Nagel R. L. Heterogeneity of red cells in the sickler: a characteristic with practical clinical and pathophysiological implications. Blood Cells. 1982;8(1):9–15. [PubMed] [Google Scholar]
- Havell T. C., Hillman D., Lessin L. S. Deformability characteristics of sickle cells by microelastimetry. Am J Hematol. 1978;4(1):9–16. doi: 10.1002/ajh.2830040103. [DOI] [PubMed] [Google Scholar]
- Hofrichter J., Ross P. D., Eaton W. A. Supersaturation in sickle cell hemoglobin solutions. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3035–3039. doi: 10.1073/pnas.73.9.3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leblond P. F., Coulombe L., Lyonnais J. Erythrocyte populations in pyruvate kinase deficiency anaemia following splenectomy. II. Cell deformability. Br J Haematol. 1978 May;39(1):63–70. doi: 10.1111/j.1365-2141.1978.tb07128.x. [DOI] [PubMed] [Google Scholar]
- Leblond P. F., Coulombe L. The measurement of erythrocyte deformability using micropore membranes. A sensitive technique with clinical applications. J Lab Clin Med. 1979 Jul;94(1):133–143. [PubMed] [Google Scholar]
- Lipowsky H. H., Usami S., Chien S. Human SS red cell rheological behavior in the microcirculation of cremaster muscle. Blood Cells. 1982;8(1):113–126. [PubMed] [Google Scholar]
- Murphy J. R., Wengard M., Brereton W. Rheological studies of Hb SS blood: influence of hematocrit, hypertonicity, separation of cells, deoxygenation, and mixture with normal cells. J Lab Clin Med. 1976 Mar;87(3):475–486. [PubMed] [Google Scholar]
- Pfafferott C., Wenby R., Meiselman H. J. Morphologic and internal viscosity aspects of RBC rheologic behavior. Blood Cells. 1982;8(1):65–78. [PubMed] [Google Scholar]
- Steinberg M. H., Dreiling B. J., Lovell W. J. Sickle cell anemia: erythrokinetics, blood volumes, and a study of possible determinants of severity. Am J Hematol. 1977;2(1):17–23. doi: 10.1002/ajh.2830020103. [DOI] [PubMed] [Google Scholar]
- Vettore L., De Matteis M. C., Zampini P. A new density gradient system for the separation of human red blood cells. Am J Hematol. 1980;8(3):291–297. doi: 10.1002/ajh.2830080307. [DOI] [PubMed] [Google Scholar]