Abstract
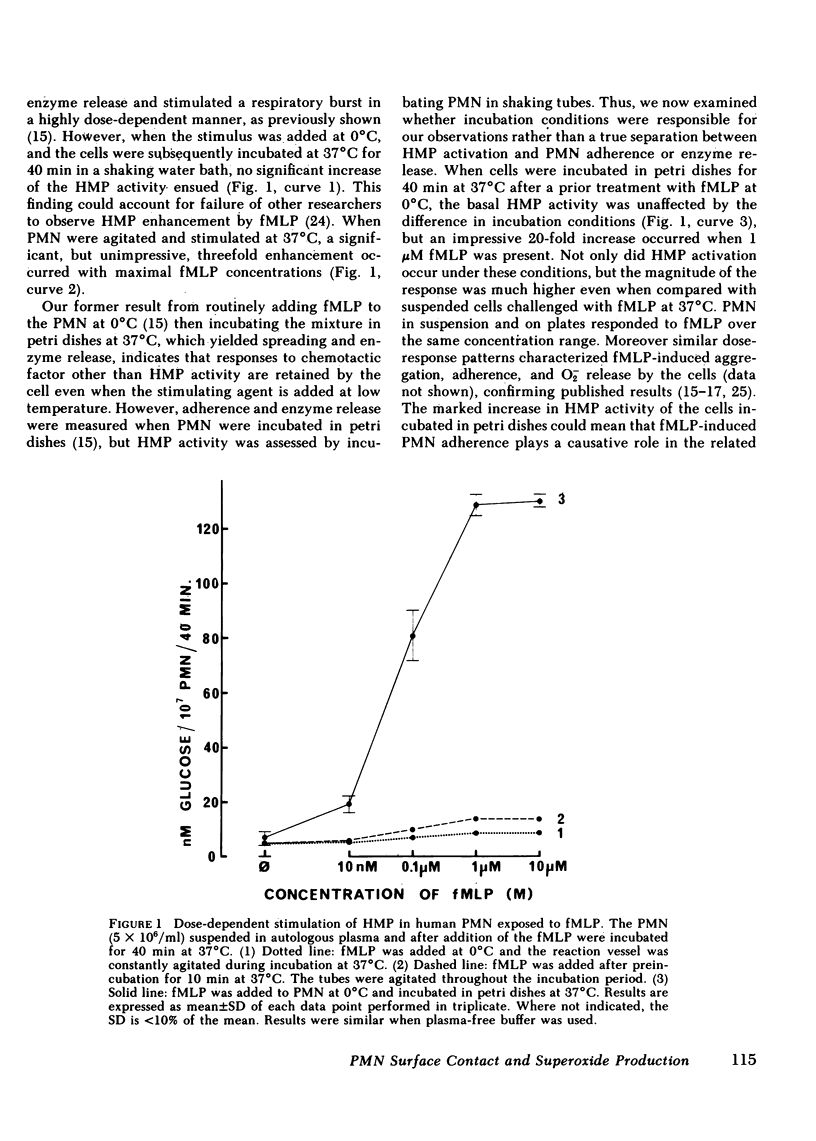
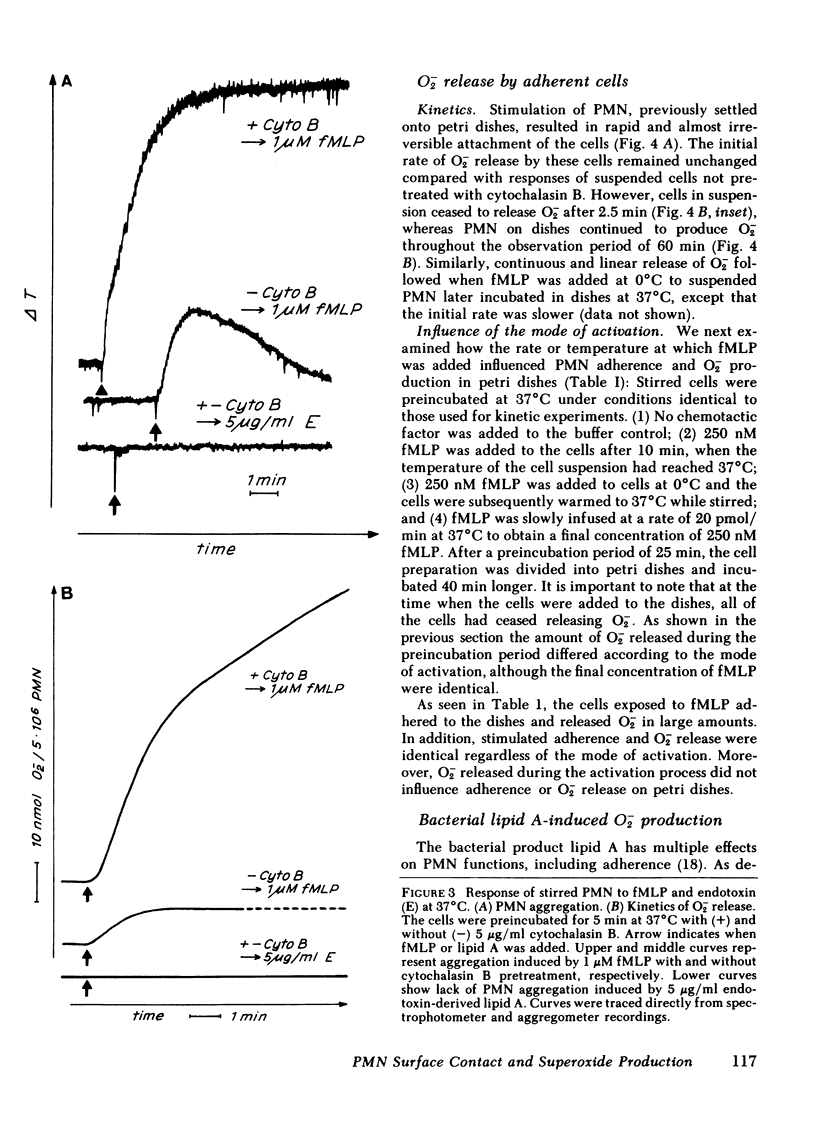
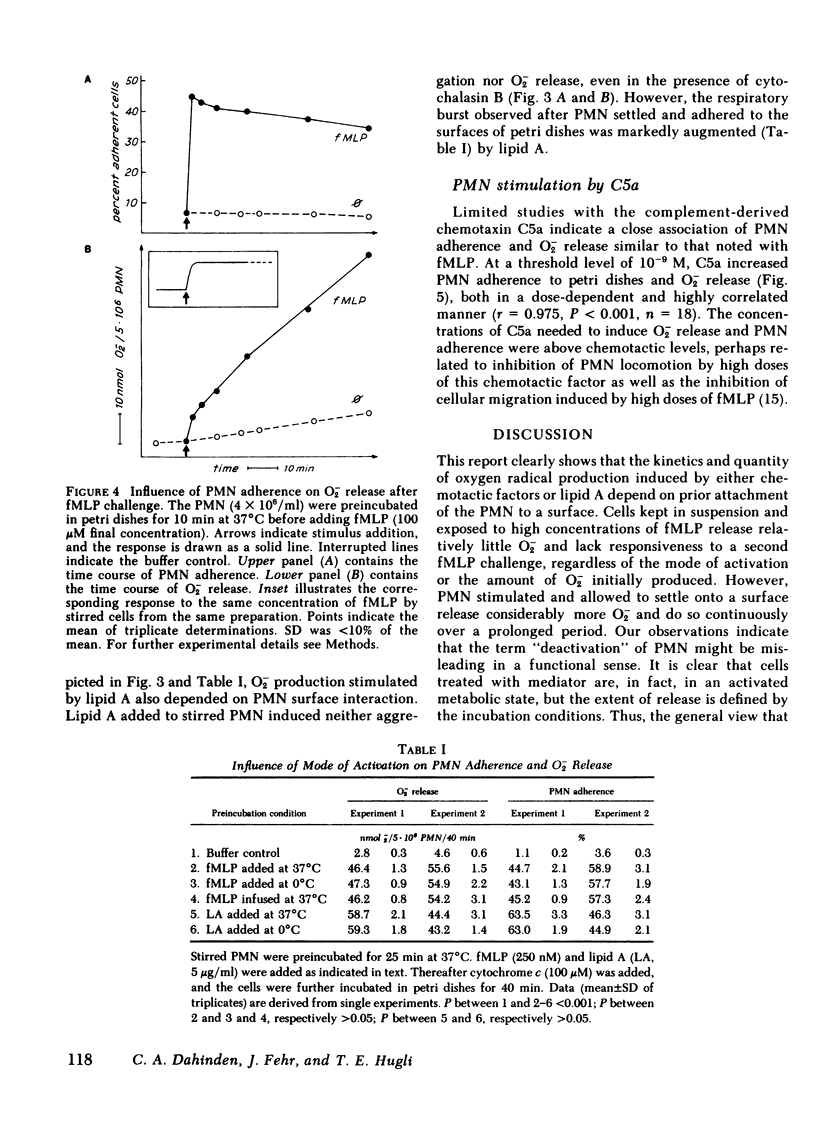
The complement-derived anaphylatoxin C5a and a putative analogue of bacterial chemotactic factor (N-formyl-methionyl-leucyl-phenylalanyl [fMLP]), as well as bacterial lipid A, all stimulate human granulocyte (PMN) adhesiveness and superoxide (O-2) production in a concentration-dependent manner. Since attachment of particulate matter to the PMN membrane is an early event in the triggering of respiratory burst of these cells, we further examined how adherence might modulate the release of O-2 induced by soluble mediators of inflammation. We found that both the quantity and kinetics of O-2 production depend on prior attachment of the cells to a surface. In stirred suspensions of PMN, fMLP induces only a short burst (2.5 min) of O-2 release associated with reversible PMN aggregation. The magnitude, but not the time course, of both these responses depend on the fMLP concentration. Unlike the short respiratory response of cells in suspension, PMN allowed to settle onto stationary petri dishes, then overlaid with fMLP, rapidly spread and attach to the surface where they remain and release O-2 throughout the 60-min test period. Prolonged O-2 release also follows fMLP stimulation in suspensions of PMN pretreated with cytochalasin B, in which case aggregation becomes irreversible during the 20-min burst. If fMLP is slowly infused into a suspension of cells at 37 degrees C or if PMN are challenged at 0 degrees C, and then warmed to 37 degrees C, O-2 release greatly decreases or becomes undetectable. Suspended PMN do not respond to a second challenge by the same stimulus regardless of the rate or temperature at which the first stimulus was added, a phenomenon formerly described as desensitization. However, if PMN challenged with fMLP in suspension undergo the short respiratory response and then are later placed in petri dishes, they adhere and resume production of O-2 without further stimulation. Chemotactic factor-induced adherence and O-2 release of PMN on a surface is entirely independent of either the mode of activation or prior O-2 release during preincubation in suspension. Human C5a also promotes PMN adherence and prolonged O-2 release in petri dishes. Furthermore, lipid A increases O-2 release and adherence of settled PMN, but fails to elicit either response from suspended PMN. These results indicate that cell surface contact plays an essential role in triggering the respiratory burst of PMN activated by soluble stimuli. This long-lasting O-2 release by chemotactic factor-stimulated PMN may play a significant role in inflammatory reactions when PMN become adherent in vivo.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babior B. M., Curnutte J. T., McMurrich B. J. The particulate superoxide-forming system from human neutrophils. Properties of the system and further evidence supporting its participation in the respiratory burst. J Clin Invest. 1976 Oct;58(4):989–996. doi: 10.1172/JCI108553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babior B. M., Kipnes R. S., Curnutte J. T. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J Clin Invest. 1973 Mar;52(3):741–744. doi: 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass D. A., Dechatelet L. R., McCall C. E. Independent stimulation of motility and the oxidative metabolic burst of human polymorphonuclear leukocytes. J Immunol. 1978 Jul;121(1):172–178. [PubMed] [Google Scholar]
- Cohen H. J., Newburger P. E., Chovaniec M. E., Whitin J. C., Simons E. R. Opsonized zymosan-stimulated granulocytes-activation and activity of the superoxide-generating system and membrane potential changes. Blood. 1981 Nov;58(5):975–982. [PubMed] [Google Scholar]
- Craddock P. R., White J. G., Jacob H. S. Potentiation of complement (C5a)-induced granulocyte aggregation by cytochalasin B. J Lab Clin Med. 1978 Mar;91(3):490–499. [PubMed] [Google Scholar]
- Curnutte J. T., Babior B. M., Karnovsky M. L. Fluoride-mediated activation of the respiratory burst in human neutrophils. A reversible process. J Clin Invest. 1979 Apr;63(4):637–647. doi: 10.1172/JCI109346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahinden C., Fehr J. Receptor-directed inhibition of chemotactic factor-induced neutrophil hyperactivity by pyrazolon derivatives. Definition of a chemotactic peptide antagonist. J Clin Invest. 1980 Nov;66(5):884–891. doi: 10.1172/JCI109955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahinden C., Galanos C., Fehr J. Granulocyte activation by endotoxin. I. Correlation between adherence and other granulocyte functions, and role of endotoxin structure on biologic activity. J Immunol. 1983 Feb;130(2):857–862. [PubMed] [Google Scholar]
- Dewald B., Baggiolini M., Curnutte J. T., Babior B. M. Subcellular localization of the superoxide-forming enzyme in human neutrophils. J Clin Invest. 1979 Jan;63(1):21–29. doi: 10.1172/JCI109273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- English D., Roloff J. S., Lukens J. N. Regulation of human polymorphonuclear leukocyte superoxide release by cellular responses to chemotactic peptides. J Immunol. 1981 Jan;126(1):165–171. [PubMed] [Google Scholar]
- Fehr J., Dahinden C. Modulating influence of chemotactic factor-induced cell adhesiveness on granulocyte function. J Clin Invest. 1979 Jul;64(1):8–16. doi: 10.1172/JCI109466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez H. N., Hugli T. E. Primary structural analysis of the polypeptide portion of human C5a anaphylatoxin. Polypeptide sequence determination and assignment of the oligosaccharide attachment site in C5a. J Biol Chem. 1978 Oct 10;253(19):6955–6964. [PubMed] [Google Scholar]
- Galanos C., Lüderitz O. Electrodialysis of lipopolysaccharides and their conversion to uniform salt forms. Eur J Biochem. 1975 Jun;54(2):603–610. doi: 10.1111/j.1432-1033.1975.tb04172.x. [DOI] [PubMed] [Google Scholar]
- Gallin J. I. Degranulating stimuli decrease the neagative surface charge and increase the adhesiveness of human neutrophils. J Clin Invest. 1980 Feb;65(2):298–306. doi: 10.1172/JCI109672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein I. M., Roos D., Kaplan H. B., Weissmann G. Complement and immunoglobulins stimulate superoxide production by human leukocytes independently of phagocytosis. J Clin Invest. 1975 Nov;56(5):1155–1163. doi: 10.1172/JCI108191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafeman D. G., McConnell H. M., Gray J. W., Dean P. N. Neutrophil activation monitored by flow cytometry: stimulation by phorbol diester is an all-or-none event. Science. 1982 Feb 5;215(4533):673–675. doi: 10.1126/science.6800035. [DOI] [PubMed] [Google Scholar]
- Hugli T. E., Gerard C., Kawahara M., Scheetz M. E., 2nd, Barton R., Briggs S., Koppel G., Russell S. Isolation of three separate anaphylatoxins from complement-activated human serum. Mol Cell Biochem. 1981 Dec 4;41:59–66. doi: 10.1007/BF00225297. [DOI] [PubMed] [Google Scholar]
- Jandl R. C., André-Schwartz J., Borges-DuBois L., Kipnes R. S., McMurrich B. J., Babior B. M. Termination of the respiratory burst in human neutrophils. J Clin Invest. 1978 May;61(5):1176–1185. doi: 10.1172/JCI109033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston R. B., Jr, Lehmeyer J. E. Elaboration of toxic oxygen by-products by neutrophils in a model of immune complex disease. J Clin Invest. 1976 Apr;57(4):836–841. doi: 10.1172/JCI108359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebanoff S. J., Rosen H. Ethylene formation by polymorphonuclear leukocytes. Role of myeloperoxidase. J Exp Med. 1978 Aug 1;148(2):490–506. doi: 10.1084/jem.148.2.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korchak H. M., Weissmann G. Changes in membrane potential of human granulocytes antecede the metabolic responses to surface stimulation. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3818–3822. doi: 10.1073/pnas.75.8.3818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmeyer J. E., Snyderman R., Johnston R. B., Jr Stimulation of neutrophil oxidative metabolism by chemotactic peptides: influence of calcium ion concentration and cytochalasin B and comparison with stimulation by phorbol myristate acetate. Blood. 1979 Jul;54(1):35–45. [PubMed] [Google Scholar]
- Lehrer R. I., Cohen L. Receptor-mediated regulation of superoxide production in human neutrophils stimulated by phorbol myristate acetate. J Clin Invest. 1981 Nov;68(5):1314–1320. doi: 10.1172/JCI110378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPhail L. C., DeChatelet L. R., Johnston R. B., Jr Generation of chemiluminescence by a particulate fraction isolated from human neutrophils. Analysis of molecular events. J Clin Invest. 1979 Apr;63(4):648–655. doi: 10.1172/JCI109347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newburger P. E., Chovaniec M. E., Cohen H. J. Activity and activation of the granulocyte superoxide-generating system. Blood. 1980 Jan;55(1):85–92. [PubMed] [Google Scholar]
- O'Flaherty J. T., Kreutzer D. L., Showell H. J., Vitkauskas G., Becker E. L., Ward P. A. Selective neutrophil desensitization to chemotactic factors. J Cell Biol. 1979 Mar;80(3):564–572. doi: 10.1083/jcb.80.3.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Flaherty J. T., Kreutzer D. L., Showell H. J., Ward P. A. Influence of inhibitors of cellular function on chemotactic factor-induced neutrophil aggregation. J Immunol. 1977 Nov;119(5):1751–1756. [PubMed] [Google Scholar]
- O'Flaherty J. T., Showell H. J., Becker E. L., Ward P. A. Substances which aggregate neutrophils. Mechanism of action. Am J Pathol. 1978 Jul;92(1):155–166. [PMC free article] [PubMed] [Google Scholar]
- Repine J. E., Eaton J. W., Anders M. W., Hoidal J. R., Fox R. B. Generation of hydroxyl radical by enzymes, chemicals, and human phagocytes in vitro. Detection with the anti-inflammatory agent, dimethyl sulfoxide. J Clin Invest. 1979 Dec;64(6):1642–1651. doi: 10.1172/JCI109626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeo D., Zabucchi G., Berton G., Schneider C. Metabolic stimulation of polymorphonuclear leucocytes: effects of tetravalent and divalent concanavalin A. J Membr Biol. 1978 Dec 29;44(3-4):321–330. doi: 10.1007/BF01944227. [DOI] [PubMed] [Google Scholar]
- Root R. K., Metcalf J. A. H2O2 release from human granulocytes during phagocytosis. Relationship to superoxide anion formation and cellular catabolism of H2O2: studies with normal and cytochalasin B-treated cells. J Clin Invest. 1977 Dec;60(6):1266–1279. doi: 10.1172/JCI108886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen H., Klebanoff S. J. Hydroxyl radical generation by polymorphonuclear leukocytes measured by electron spin resonance spectroscopy. J Clin Invest. 1979 Dec;64(6):1725–1729. doi: 10.1172/JCI109637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacks T., Moldow C. F., Craddock P. R., Bowers T. K., Jacob H. S. Oxygen radicals mediate endothelial cell damage by complement-stimulated granulocytes. An in vitro model of immune vascular damage. J Clin Invest. 1978 May;61(5):1161–1167. doi: 10.1172/JCI109031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simchowitz L., Atkinson J. P., Spilberg I. Stimulus-specific deactivation of chemotactic factor-induced cyclic AMP response and superoxide generation by human neutrophils. J Clin Invest. 1980 Oct;66(4):736–747. doi: 10.1172/JCI109911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sklar L. A., Jesaitis A. J., Painter R. G., Cochrane C. G. The kinetics of neutrophil activation. The response to chemotactic peptides depends upon whether ligand-receptor interaction is rate-limiting. J Biol Chem. 1981 Oct 10;256(19):9909–9914. [PubMed] [Google Scholar]
- Till G. O., Johnson K. J., Kunkel R., Ward P. A. Intravascular activation of complement and acute lung injury. Dependency on neutrophils and toxic oxygen metabolites. J Clin Invest. 1982 May;69(5):1126–1135. doi: 10.1172/JCI110548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams A. J., Cole P. J. Polymorphonuclear leucocyte membrane-stimulated oxidative metabolic activity---the effect of divalent cations and cytochalasins. Immunology. 1981 Dec;44(4):847–858. [PMC free article] [PubMed] [Google Scholar]


