Abstract
STUDY QUESTION
What is the impact of the EuroNet-PHL-C2 treatment protocol for children with classical Hodgkin lymphoma (cHL) on gonadal function in girls, based on assessment of serum anti-Müllerian hormone (AMH)?
SUMMARY ANSWER
Serum AMH levels decreased after induction chemotherapy and increased during subsequent treatment and 2 years of follow-up, with lowest levels in patients treated for advanced stage cHL.
WHAT IS KNOWN ALREADY
Treatment for cHL, particularly alkylating agents and pelvic irradiation, can be gonadotoxic and result in premature reduction of primordial follicles in females. The current EuroNet-PHL-C2 trial aims to reduce the use of radiotherapy in standard childhood cHL treatment, by intensifying chemotherapy. This study aims to assess the gonadotoxic effect of the EuroNet-PHL-C2 protocol.
STUDY DESIGN, SIZE, DURATION
This international, prospective, multicenter cohort study is embedded in the EuroNet-PHL-C2 trial, an European phase-3 treatment study evaluating the efficacy of standard cHL treatment with OEPA-COPDAC-28 (OEPA: vincristine, etoposide, prednisone, and doxorubicin; COPDAC-28: cyclophosphamide, vincristine, prednisone, and dacarbazine) versus intensified OEPA-DECOPDAC-21 (DECOPDAC-21: COPDAC with additional doxorubicin and etoposide and 25% more cyclophosphamide) in a randomized setting. Participants were recruited between January 2017 and September 2021.
PARTICIPANTS/MATERIALS, SETTING, METHODS
Female patients aged ≤18 years, treated according to the EuroNet-PHL-C2 protocol for cHL were recruited across 18 sites in the Netherlands, Belgium, Germany, Austria, and Czech Republic. All parents and patients (aged ≥12 years old) provided written informed consent. Serum AMH levels and menstrual cycle characteristics were evaluated over time (at diagnosis, one to three times during treatment and 2 up to 5 years post-diagnosis) and compared between treatment-levels (TL1, TL2, and TL3) and treatment-arms (OEPA-COPDAC-28 and OEPA-DECOPDAC-21). Serum samples obtained from patients after receiving pelvic radiotherapy were excluded from the main analyses.
MAIN RESULTS AND THE ROLE OF CHANCE
A total of 104 females, with median age at diagnosis of 15.6 years (IQR 13.7; 17.0), were included in the analysis. Ninety-nine were (post)pubertal. Eighteen girls were diagnosed with an early stage of cHL (TL1) and 86 with intermediate or advanced stage disease (50 TL2 and 36 TL3, 66% received COPDAC-28 and 34% DECOPDAC-21). Five patients received pelvic radiotherapy. Median AMH level at diagnosis was 1.7 µg/l (IQR 0.9; 2.7). After two courses of OEPA chemotherapy, AMH levels decreased substantially in all patients (98% <0.5 µg/l), followed by a significant increase during the consolidation treatment and follow-up. After 2 years, 68% of patients reached their baseline AMH value, with overall median recovery of 129% (IQR 75.0; 208.9) compared to baseline measurement. Five patients (7%) had AMH <0.5 µg/l. In patients treated for advanced stage disease, AMH levels remained significantly lower compared to early- or intermediate stage disease, with median serum AMH of 1.3 µg/l (IQR 0.8; 2.1) after 2 years. Patients who received DECOPDAC-21 consolidation had lower AMH levels during treatment than patients receiving COPDAC-28, but the difference was no longer statistically significant at 2 years post-diagnosis. Of the 35 postmenarchal girls who did not receive hormonal co-treatment, 19 (54%) experienced treatment-induced amenorrhea, two girls had persisting amenorrhea after 2 years.
LIMITATIONS, REASONS FOR CAUTION
The studied population comprises young girls with diagnosis of cHL often concurring with pubertal transition, during which AMH levels naturally rise. There was no control population, while the interpretation of AMH as a biomarker during childhood is complex. The state of cHL disease may affect AMH levels at diagnosis, potentially complicating assessment of AMH recovery as a comparison with baseline AMH. The current analysis included data up to 2–5 years post-diagnosis.
WIDER IMPLICATIONS OF THE FINDINGS
The current PANCARE guideline advises to use the cyclophosphamide-equivalent dose score (CED-score, as an estimation of cumulative alkylating agent exposure) with a cut-off of 6000 mg/m2 to identify females aged <25 years at high risk of infertility. All treatment-arms of the EuroNet-PHL-C2 protocol remain below this cut-off, and based on this guideline, girls treated for cHL should therefore be considered low-risk of infertility. However, although we observed an increase in AMH after chemotherapy, it should be noted that not all girls recovered to pre-treatment AMH levels, particularly those treated for advanced stages of cHL. It remains unclear how our measurements relate to age-specific expected AMH levels and patterns. Additional (long-term) data are needed to explore clinical reproductive outcomes of survivors treated according to the EuroNet-PHL-C2 protocol.
STUDY FUNDING/COMPETING INTEREST(S)
The fertility add-on study was funded by the Dutch charity foundation KiKa (project 257) that funds research on all forms of childhood cancer. C.M-K., D.K., W.H.W., D.H., M.C., A.U., and A.B. were involved in the development of the EuroNet-PHL-C2 regimen. The other authors indicated no potential conflicts of interest.
TRIAL REGISTRATION NUMBER
N/A.
Keywords: childhood Hodgkin lymphoma, gonadotoxicity, anti-Müllerian hormone, treatment-induced amenorrhea, late effects, chemotherapy
Introduction
Current treatment for childhood classical Hodgkin lymphoma (cHL) is highly effective with survival rates exceeding 90% (Mauz-Körholz et al., 2022). Treatment protocols are constantly adapted in effort to reduce late effects such as second malignancies and a reduced reproductive function, that substantially affect quality of life during survivorship (Absolom et al., 2008; Schaapveld et al., 2015). In the European EuroNet-PHL-C1 randomized protocol, the use of radiotherapy was reduced for early stage disease (to lower the risk of secondary malignancies) and the gonadotoxic agent procarbazine was successfully omitted in intermediate and advanced stage disease by the introduction of cHL treatment with OEPA-COPDAC-28 (OEPA: vincristine, etoposide, prednisone and doxorubicin; COPDAC-28: cyclophosphamide, vincristine, prednisone and dacarbazine) as an alternative to OEPA-COPP (COPP: cyclophosphamide, vincristine, prednisone, and procarbazine) (Mauz-Körholz et al., 2022). The current EuroNet-PHL-C2 protocol aims to further reduce the use of radiotherapy by intensifying chemotherapy (European Network-Paediatric Hodgkin Lymphoma Study Group (EuroNet-PHL), 2015). The cumulative dose of cyclophosphamide is increased by 25% in the intensified treatment-arm, in which patients receive DECOPDAC-21 consolidation chemotherapy (DECOPDAC-21: COPDAC with a higher dose of cyclophosphamide and additional doxorubicin and etoposide) instead of COPDAC-28. Since cyclophosphamide is an alkylating gonadotoxic agent, an adverse effect of treatment on gonadal function could be more pronounced in patients receiving the intensified chemotherapy-arm of the EuroNet-PHL-C2 protocol, as this contains higher doses of this drug. Knowledge, however, of the impact of the different treatment arms of the underlying treatment protocol for cHL on gonadal function is lacking, while this is utmost importance for adequate counseling on future fertility and decisions on potential fertility preservation.
As girls are born with a finite pool of primordial follicles in the ovaries, any gonadotoxic insult may result in a reduction of the number of primordial follicles by inducing apoptosis and atrophy during and directly after treatment (Meirow, 2000; Wallace and Kelsey, 2010; Sonigo et al., 2019). This premature reduction in ovarian reserve can potentially reduce the fertile lifespan and result in premature ovarian insufficiency (POI) during survivorship (Webber et al., 2016; Spears et al., 2019). Anti-Müllerian hormone (AMH) is generally considered a marker of ovarian reserve and is produced by granulosa cells of early developing follicles (Broer et al., 2014; Anderson and Su, 2020). Generally, an increase of serum AMH is seen during infancy and puberty. After the age of ∼25 years old, AMH progressively declines to undetectable levels before menopausal transition (Kelsey et al., 2011; Lie Fong et al., 2012; Broer et al., 2014). Previous studies have shown that AMH levels tend to drop considerably in females during chemotherapy due to the acute cytotoxic effect of treatment (Anderson et al., 2022). In the months thereafter, AMH commonly increases as the follicular recruitment resumes. However, recovery patterns are highly variable and in most female adult cancer survivors AMH levels do not reach pre-treatment levels. There appears to be a regimen- and dose-related effect, with higher gonadotoxicity correlating with lower AMH levels post-treatment (Krawczuk-Rybak et al., 2013; Anderson et al., 2018, 2022; Oktem et al., 2018; Decanter et al., 2021).
The objective of the present study is to assess the gonadotoxicity of the EuroNet-PHL-C2 treatment protocol for female childhood cHL patients ≤18 years of age, by evaluating serum AMH and menstrual cycle characteristics in newly diagnosed cHL patients up to 5 years post-treatment.
Materials and methods
Study design and study population
This international, prospective, multicenter cohort study is embedded in the EuroNet-PHL-C2 study, an European phase-3 treatment study evaluating the efficacy of cHL treatment with OEPA-COPDAC-28 versus OEPA-DECOPDAC-21 in a randomized setting (Clinicaltrials NCT02684708; EudraCT number 2012-004053-88). Boys and girls with a confirmed diagnosis of cHL before or at the age of 18 years, treated according to the EuroNet-PHL-C2 regimen between January 2017 and September 2021 in one of the 18 participating study sites across five countries (The Netherlands, Belgium, Germany, Austria, and Czech Republic) were eligible for the fertility add-on study. The present report comprises the results of the included girls in the fertility add-on study. Reproductive outcomes among the participating boys will be described separately.
cHL treatment regimen
All patients were treated according to the EuroNet-PHL-C2 protocol (European Network-Paediatric Hodgkin Lymphoma Study Group (EuroNet-PHL), 2015). The treatment flow is schematically depicted in Supplementary Fig. S1. In brief, assigned treatment depended on treatment level (i.e. TL-stage based on Ann Arbor classification and the presence of risk factors) and treatment response (determined by PET-CT, AR = adequate response, IR = inadequate response). Patients in all treatment arms initially received two cycles of OEPA induction treatment. TL1 patients (early stage) subsequently received either one cycle of COPDAC-28 (if in AR) or 20 Gy involved-field radiotherapy (if in IR) as consolidation treatment. TL2 and TL3 patients (intermediate and advanced stages of cHL) were randomized to receive either two (TL2) or four (TL3) additional COPDAC-28 or DECOPDAC-21 courses. Patients who refused randomization, received COPDAC-28 consolidation chemotherapy. IR patients underwent a second (late) response assessment (LRA). TL2 and TL3 COPDAC-28 patients with IR received radiotherapy to all initially involved sites (20 Gy + 10 Gy boost to LRA PET+ sites), whereas IR patients of the DECOPDAC-21-group only received radiotherapy to LRA PET-positive sites (30 Gy) or no radiotherapy if in AR at LRA. On 31 December 2020 enrolment onto the EuroNet-PHL-C2 trial ended, while the fertility add-on study continued to enroll patients until 30 September 2021. After the end of accrual of the EuroNet-PHL-C2 trial, newly diagnosed cHL patients were treated with the standard OEPA-COPDAC-28 regimen. On the discretion of the treating physician and in special situations, if potential large radiotherapy fields were assumed, also DECOPDAC-21 consolidation could be given (e.g. to advanced stage patients). Received treatment was analyzed per protocol in the present observational study.
Data collection and measurements
Data were collected at diagnosis (T0) and during regular checkup visits after 2× OEPA (T1), after 1× COPDAC-28 (T1b, only for TL1 patients), after 2× (DE)COPDAC (T2, for TL2/TL3 patients), after 4× (DE)COPDAC (T3, only for TL3 patients), 2 years post-diagnosis (T4) and 5 years post-diagnosis or as soon as the patient turned 18 years old (T5). If the patient had reached the age of 18 years earlier than 2 years post-diagnosis, data collection was considered complete after the T4 follow-up. Study participation and data collection ended per direct in case of disease progression or recurrence, death or lost to follow-up.
The primary outcome of the present study was gonadal function assessed by serum AMH, compared between the TL-stages and COPDAC-28/DECOPDAC-21 consolidation schemes. Timing of menarche, the occurrence of treatment-induced amenorrhea, and regularity of the menstrual cycle during follow-up were included as secondary outcomes. In order to adjust for relevant confounders, data on age at time of sampling, the use of oral contraceptives and prescription of GnRH-analogues were recorded in the dataset.
Blood sampling
Blood samples were drawn at diagnosis and at each follow-up and stored at local sites at (minimal) −20°C. In 2023 (January–April), all available samples were collected and shipped on dry-ice to the Netherlands. AMH levels were assessed at the laboratory of Amsterdam UMC, the Netherlands. Lower limit of quantitation was 0.03 µg/l, with intra-assay variation <1.3% and inter-assay variation of <3.5% over the whole concentration range (Cobas, Roche Diagnostics).
Menstrual cycle characteristics and hormonal co-treatment
Data on Tanner stage M: mammae, menarchal status (including age at time of menarche), menstrual cycle characteristics and hormonal cotreatment were obtained using a predesigned case-report form (CRF). Pubertal stage was considered prepubertal in case of Tanner-M = 1 and (post)pubertal if Tanner-M > 1. Post-menarchal girls who were not receiving hormonal contraceptives or GnRH-analogues (gonadotropin releasing hormone analogues) during treatment and experienced at least 6 months without menses were considered to have ‘treatment-induced amenorrhea’. Regularity of the menstrual cycle after 2 years was self-reported.
cHL staging and received treatment
Treatment data (TL, randomization result, planned and administered treatments including radiotherapy sites and doses) were retrieved from the central EuroNet-PHL-C2 study database, as well as data regarding date of diagnosis, age, Ann arbor stage of disease, presence of B symptoms (i.e. drenching night sweats, unexplained fever >38.5°C and weight loss ≥10% in 6 months), elevated ESR (erythrocyte sedimentation rate ≥30mm in the first hour), bulk tumour volume (>200cc), E-lesions, tumour site and regions involved (infradiaphragmatic region includes tumour sites in the porta hepatis, splenic hilum, mesenteric, upper para-aortic, lower para-aortic, iliac and inguinal area) and potential recurrence or secondary malignancies. Data of the patients who were included in the fertility add-on study after December 2020 (and were not included in the main EuroNet-PHL-C2 study) were collected from local medical files. All available data up to October 2023 were included in the present analysis.
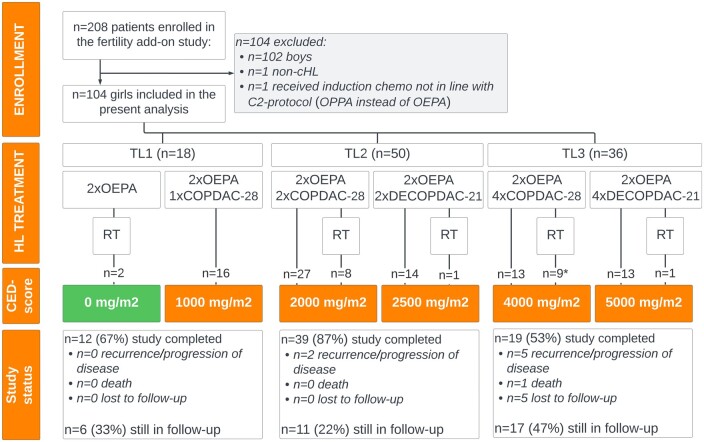
The cumulative alkylating agent exposure per TL and received scheme was estimated by calculating the cyclophosphamide-equivalent dose (CED-score), see Fig. 1 (Green et al., 2014).
Figure 1.
Study flowchart. Flow diagram of the fertility add-on study, depicting enrollment, assigned treatment according to the EuroNet-PHL-C2 protocol with corresponding cyclophosphamide equivalent dose (CED) score, and follow-up/end of study for the present study. cHL, classical Hodgkin lymphoma; COPDAC-28, cyclophosphamide, vincristine, prednisone and dacarbazine; DECOPDAC-21, doxorubicin, etoposide, cyclophosphamide, vincristine, prednisone and dacarbazine; OEPA, vincristine, etoposide, prednisone, doxorubicin; OPPA, vincristine, procarbazine, prednisone, doxorubicin; TL, treatment level; RT, radiotherapy; n, number. *a total of 5 patients assigned to TL3 (receiving OEPA induction and COPDAC-28 consolidation chemotherapy) received pelvic radiotherapy.
Statistical analysis
To evaluate ‘very low’ AMH levels, measurements were dichotomized into <0.5 µg/l and ≥0.5 µg/l (Revelli et al., 2016). Recovery of AMH was calculated using the following equation; AMH level at 2 years post-diagnosis (T4)/AMH level at diagnosis (T0) * 100%. In cases where AMH recovery was ≥100%, patients were considered to have reached their baseline value. A total of 46 laboratory measurements that were reported as ‘AMH <0.03 µg/l’ were recoded into 0.03 µg/l to allow statistical analyses.
Baseline information, serum AMH, and data on the menstrual cycle characteristics were compared between treatment-schemes using Chi-square, Fishers exact or Mann–Whitney U tests. Linear mixed models were used to estimate the effect of treatment on log-transformed AMH levels over time (T0–T5) with a random intercept at patient level to correct for correlated observations within the same patient. Potential differences in outcomes between treatment levels (TL1, TL2, TL3) and treatment-schemes (TL2/TL3 DECOPDAC-21 and COPDAC-28) were assessed in the analyses by adding these variables of interest. Moreover, linear regression analyses were used to study the impact of B-symptoms and/or elevated ESR (as signs of an elevated inflammatory state) on log-transformed baseline AMH levels.
All analyses were adjusted for age and use of oral contraceptives or GnRH-analogues at time of sampling. Outcomes were re-transformed into the original scale and were presented as geometric-mean ratio (GMR) with their corresponding 95% confidence interval (95% CI) and P-value. Samples obtained from patients after receiving radiotherapy were excluded from the main analyses and separately assessed in unadjusted comparative analyses (n = 4 T4 samples and n = 1 T5 sample). Statistical analyses were performed using IBM SPSS Statistics version 28.0 (IBM Corp., 2021) and P-values below 0.05 were considered statistically significant.
Ethical approval
All parents and patients (aged ≥12 years old) provided written informed consent for study participation. The study was approved by local ethical boards in each participating country. This study was performed in accordance with Good Clinical Practice and the Declaration of Helsinki.
Results
Included girls
A total of 106 girls were included in the fertility-add on study of whom 104 girls were included in the present analysis. Two girls were excluded, one because of a final non-cHL diagnosis and another because she received procarbazine-based induction treatment instead of the OEPA chemotherapy of the C2-protocol.
Baseline data are presented in Table 1. Median age at diagnosis was 15.6 years (IQR 13.7–17.0, range 7.3–18.8). Ninety-nine (95%) girls were (post)pubertal. Eighteen girls were diagnosed with an early stage cHL (TL1) and 86 with intermediate (TL2, n = 50) and advanced stages of disease (TL3, n = 36). Fifty-seven TL2/3 girls (66%) received COPDAC-28- and 29 (34%) received DECOPDAC-21 consolidation chemotherapy. Overall, CED-score of received treatment ranged between 0 and 5000 mg/2. Twenty-one (20%) patients received radiotherapy, of whom five patients (5%) were irradiated in the pelvic area (19.8 Gy), all of these patients were assigned to TL3 and treated with COPDAC-28 consolidation.
Table 1.
Baseline characteristics of all included girls.
| All included girls(n = 104) | Treatment arms |
||||
|---|---|---|---|---|---|
| TL1 (n = 18) |
TL2/3 COPDAC-28 arm (n = 57) | TL2/3 DECOPDAC-21 arm (n = 29) | COPDAC-28 vs DECOPDAC-21 (P-value) |
||
| Age at diagnosis (years), median (IQR) | 15.6[13.7; 17.0] | 15.8 [13.9; 17.1] | 15.8 [14.4; 16.8] | 15.0 [13.2; 17.0] | 0.40 |
| (Post)pubertal at diagnosisa | 99 (95%) | 17 (94%) | 56 (98%) | 26 (90%) | 0.11 |
| Hormonal contraceptives during treatmentb | 38/87 (44%) | 7/15(47%) | 25/49 (45%) | 6/23 (26%) | 0.06 |
| GnRH-analogues during treatmentb | 17/87 (20%) | 3/15 (20%) | 9/49 (18%) | 5/22 (23%) | 1.000 |
| Ann Arbor stage of disease | |||||
| 1 | 0 (0%) | 0 (0%) | — | — | 0.10 |
| 2 | 62 (60%) | 18 (100%) | 33 (58%) | 11 (38%) | |
| 3 | 19 (18%) | 0 (0%) | 9 (16%) | 10 (35%) | |
| 4 | 23 (22%) | 0 (0%) | 15 (26%) | 8 (28%) | |
| B-symptomsc | 33 (32%) | 1 (6%) | 18 (32%) | 14 (48%) | 0.20 |
| ESR ≥ 30 mm/h | 75 (72%) | 0 (0%) | 51 (90%) | 24 (83%) | 0.50 |
| Bulky diseased | 53 (52%) | 0 (0%) | 36 (64%) | 17 (59%) | 0.78 |
| Involved tumor sites in infradiaphragmatic regione | 32 (31%) | 1 (6%) | 12 (21%) | 15 (52%) | 0.008 |
| Treatment level (TL) and ERA response | |||||
| TL1, adequate response | 16 (15%) | 16 (89%) | — | — | 0.24 |
| TL1, inadequate response | 2 (2%) | 2 (11%) | — | — | |
| TL2, adequate response | 37 (36%) | — | 24 (42%) | 13 (45%) | |
| TL2, inadequate response | 13 (13%) | — | 11 (19%) | 2 (7%) | |
| TL3, adequate response | 18 (17%) | — | 9 (16%) | 9 (31%) | |
| TL3, inadequate response | 18 (17%) | — | 13 (23%) | 5 (17%) | |
| Radiotherapy | |||||
| Received radiotherapy | 21 (20%) | 2 (11%) | 17 (30%) | 2 (7%) | 0.03 |
| Radiotherapy assigned but not given | 3 (3%) | 0 (0%) | 3 (5%) | 0 (0%) | |
| Ended study participation before end of treatment | 5 (5%) | 0 (0%) | 3 (5%) | 2 (7%) | |
| Pelvic radiotherapyf | 5 (5%) | 0 (0%) | 5 (9%) | 0 (0%) | 0.16 |
| Median follow-up, months (IQR) | 24.0 [23.0; 28.0] | 25.0 [23.0; 26.0] | 24.0 [22.0; 28.0] | 25.0 [23.0; 29.0] | 0.32 |
| End of study | |||||
| Recurrence/progression of disease | 7 (7%) | 0 (0%) | 5 (9%) | 2 (7%) | 0.58 |
| Secondary malignancy | 1 (1%) | 0 (0%) | 1 (2%) | 0 (0%) | |
| Death | 1 (1%) | 0 (0%) | 1 (2%) | 0 (0%) | |
| Lost to follow-up | 5 (5%) | 0 (0%) | 4 (7%) | 1 (3%) | |
P values were calculated by Mann–Whitney U test (continuous) or Chi-square/Fishers exact (categorical). Assigned treatment was according to the EuroNET-PHL-C2 protocol (see treatment-flow in Supplementary Fig. S1). TL1 patients receive 2× OEPA induction followed by either 1× COPDAC-28 or involved node radiotherapy. TL2/TL3 patients are randomized between the COPDAC-28 and DECOPDAC-21 treatment-arm and receive 2× OEPA induction followed by 2× (TL2) or 4× (TL3) (DE)COPDAC consolidation. Indication for radiotherapy depends on treatment response and treatment-group.
OEPA, vincristine, etoposide, prednisone, doxorubicin; COPDAC-28, cyclophosphamide, vincristine, prednisone, and dacarbazine; DECOPDAC-21, doxorubicin, etoposide, cyclophosphamide, vincristine, prednisone and dacarbazine; ESR, erythrocyte sedimentation rate; ERA, early response rate; IQR, interquartile range; TL, treatment level.
(Post)pubertal is defined as tanner stage (mammae) >1.
Reported percentages of girls using hormonal contraceptives or GNRH-analogues were calculated in post-menarchal girls.
B symptoms, i.e. unexplained fever >38.5°C, weight loss of 10% during the past 6 months and drenching night sweats.
Bulky disease is defined as contiguous tumour volume of at least 200 ml.
Including tumour sites in the porta hepatis, splenic hilum, mesenteric, upper para-aortic, lower para-aortic, iliac and inguinal area.
All patients received 19.8 Gy.
Median duration of follow-up was 24.0 months (IQR 23.0–28.0). At present, 70 out of the 104 patients (67%) have completed follow-up. A total of 53 patients reached the age of 18 years, four patients were 5 years post-treatment and study participation ended prematurely in 13 patients (n = 3 progression of disease, n = 4 recurrence, n = 1 died during follow-up because of a secondary malignancy, n = 5 lost to follow-up). The remaining 34 patients are still in follow-up. An overview of the inclusion, assigned treatment and follow-up is included in the flowchart in Fig. 1.
Anti-Müllerian hormone
At diagnosis
Blood samples at diagnosis were available for 97 (93%) girls, with median age at time of blood sampling 15.6 years (IQR 13.7–17.2). Median AMH levels were 1.7 µg/l (IQR 0.9; 2.7), 12 (12%) girls had AMH levels <0.5 µg/l (Table 2). Baseline AMH levels were not statistically significantly different between the TL stages, and there were also no significant differences between the AMH levels of 81 patients who presented with signs of an elevated inflammatory state (i.e. B symptoms and/or ESR ≥30 mm/h), compared to 23 patients without B symptoms and ESR <30 mm/h (adjusted GMR 0.76 (95% 0.5; 1.3) P = 0.31).
Table 2.
Anti-Müllerian hormone (AMH) at diagnosis, and 2 years post-diagnosis.
| At diagnosis (n = 97) | 2 years post-diagnosis (n = 73)** | |
|---|---|---|
| Age at sampling in years, median (IQR) | 15.6 [13.7; 17.2] | 17.8 [15.8; 19.1] |
| Used HC at time of blood sampling | 25 (25.8%) | 25 (34.2%) |
| AMH, median (IQR) | 1.7 [0.9; 2.7] | 2.1 [1.1; 3.8] |
| AMH <0.5 µg/l | 12 (12%) | 5 (7%) |
| Reached baseline AMH | 46 (68%) | |
| AMH recovery*, median (IQR) | — | 129 [75.0; 208.9] |
HC, hormonal contraceptives; IQR, interquartile range.
Reached percentage of baseline AMH, calculated as AMH T4/AMH T0 *100%.
Samples drawn in patients after receiving pelvic radiotherapy (i.e. n = 4 blood samples) were excluded from the analyses.
During treatment
A total of 220 samples were drawn during treatment (T1: 100 samples, T1b: 15 samples, T2: 76 samples and T3: 29 samples). Of these, 33 samples were obtained from TL1 patients, 121 from TL2/TL3 COPDAC-28 patients and 66 from TL2/TL3 DECOPDAC-21 patients. There was a significant decrease in serum AMH in all patients after two courses of OEPA, with a median AMH of 0.06 µg/l (IQR 0.03; 0.1) and 98% of measurements below 0.5 µg/l. In all treatment-arms, the serum AMH level significantly increased during consolidation treatment. However, adjusted serum AMH levels remained significantly lower at T2 and T3 checkups in the DECOPDAC-21-group when compared to the COPDAC-28-group (T2: estimated effect for DECOPDAC-21 GMR 0.17 (95% CI 0.10; 0.29), P =< 0.001; T3 GMR 0.30 (95% CI 0.07; 0.31), P =< 0.001). AMH values over time are depicted in boxplots in Fig. 2 (split for TL-stage) and Fig. 3 (split for COPDAC-28/DECOPDAC-21 scheme). Median values and prevalence of AMH < 0.5 µg/l per checkup are included in Supplementary Table S1 and results of the linear mixed models are reported in Table 3.
Figure 2.
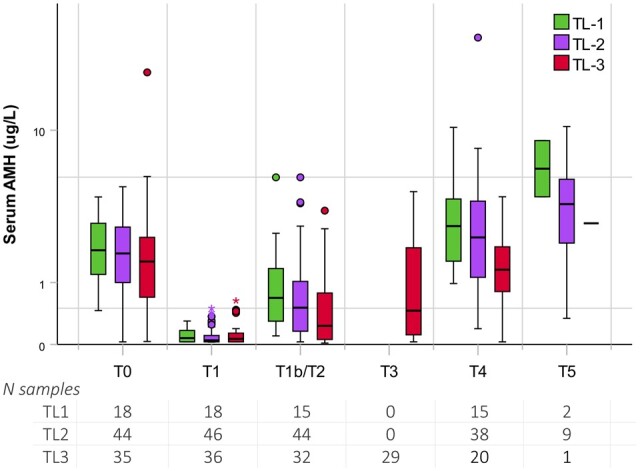
Serum anti-Müllerian hormone (AMH) during treatment for childhood classical Hodgkin lymphoma and follow-up, compared between treatment levels. Boxplots depicting the distribution of uncorrected serum AMH levels from diagnosis up to 5 years post-diagnosis, including the median (centerline), interquartile range (end of the box) and range (end of the whiskers). Separate dots are outliers. Patients within TL1 are shown in green, TL2 in purple and TL3 in red. TL, treatment level; N, number. Timing sampling: T0, at diagnosis; T1, after 2× OEPA; T1b, after 1× COPDAC-28; T2, after 2× (DE)COPDAC; T3, after 4× (DE)COPDAC; T4, 2 years post-diagnosis; T5, 5 years post-diagnosis or as soon as the patient turned 18 years old. Assigned treatment was according to the EuroNet-PHL-C2 protocol (see treatment-flow in Supplementary Fig. S1). TL1 patients received 2 × OEPA ± 1× COPDAC, TL2 patients received 2 × OEPA + 2 × (DE)COPDAC, TL3 patients received 2 × OEPA + 4× (DE)COPDAC. OEPA, vincristine, etoposide, prednisone, doxorubicin; COPDAC-28, cyclophosphamide, vincristine, prednisone and dacarbazine; DECOPDAC-21, doxorubicin, etoposide, cyclophosphamide, vincristine, prednisone and dacarbazine. A total of five patients in the TL3 group received pelvic radiotherapy. Samples drawn in these patients after receiving radiotherapy (n = 4 T4 samples and n = 1 T5 sample) were excluded from the analyses.
Figure 3.
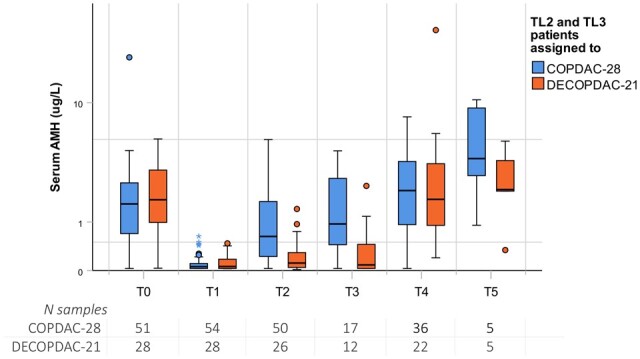
Serum anti-Müllerian hormone (AMH) during treatment for childhood classical Hodgkin lymphoma and follow-up, compared between COPDAC-28 and DECOPDAC-21 treatment schemes. Boxplots depicting the distribution of uncorrected serum AMH levels from diagnosis up to 5 years post-diagnosis, including the median (centerline), interquartile range (end of the box) and range (end of the whiskers). Separate dots are outliers. TL2/TL3 Patients who received COPDAC-28 consolidation are shown in blue and DECOPDAC-21 patients in orange. TL, treatment level; N, number. Timing sampling: T0, at diagnosis; T1, after 2 × OEPA; T2, after 2 × (DE)COPDAC; T3, after 4 × (DE)COPDAC; T4, 2 years post-diagnosis; T5, 5 years post-diagnosis or as soon as the patient turned 18 years old. Assigned treatment was according to the EuroNet-PHL-C2 protocol (see treatment-flow in Supplementary Fig. S1). TL2 patients received 2 × OEPA + 2× (DE)COPDAC, TL3 received 2× OEPA + 4× (DE)COPDAC. OEPA, vincristine, etoposide, prednisone, doxorubicin; COPDAC-28, cyclophosphamide, vincristine, prednisone and dacarbazine; DECOPDAC-21, doxorubicin, etoposide, cyclophosphamide, vincristine, prednisone and dacarbazine. A total of five patients in the TL3 group received pelvic radiotherapy. Samples drawn in these patients after receiving radiotherapy (n = 4 T4 samples and n = 1 T5 sample) were excluded from the analyses.
Table 3.
Results of the mixed models performed on serum anti-Müllerian hormone (AMH).
| Including all available samples* | Treatment level |
Treatment scheme |
||||||
|---|---|---|---|---|---|---|---|---|
| TL1 | TL2 | TL3 | TL2 versus TL3 (P-value) | TL2/3 COPDAC-28 arm | TL2/3 DECOPDAC-21 arm | COPDAC-28 vs DECOPDAC-21 (P-value) | ||
| AMH | ||||||||
| Intercept: T0: at diagnosis | 0.63 (0.19; 2.05) | 0.71 (0.20; 2.57) | 0.82 (0.24; 2.85) | 0.65 (0.20; 2.09) | 0.139 | 0.45(0.12; 1.66) | 0.54 (0.15; 1.94) | 0.504 |
| T1: after 2× OEPA | 0.05 (0.04; 0.07), P < 0.001 | 0.05 (0.03; 0.09), P < 0.001 | 0.04 (0.03; 0.06), P < 0.001 | 0.07 (0.05; 0.10), P < 0.001 | 0.717 | 0.05 (0.04; 0.07), P < 0.001 | 0.05 (0.03; 0.08), P < 0.001 | 0.629 |
| T1b/T2: after 1× COPDAC/2× (DE)COPDAC | 0.25 (0.20; 0.32), P < 0.001 | 0.36 (0.20; 0.65), P = 0.001 | 0.25 (0.17; 0.36), P < 0.001 | 0.21 (0.14; 0.32), P < 0.001 | 0.042 | 0.43 (0.31; 0.59), P < 0.001 | 0.07 (0.05; 0.11), P < 0.001 | <0.001 |
| T3: after 4× (DE)COPDAC | 0.32 (0.22; 0.47), P < 0.001 | — | — | 0.34 (0.22; 0.52), P < 0.001 | NA | 0.67 (0.42; 1.07), P = 0.091 | 0.10 (0.06; 0.18), P < 0.001 | <0.001 |
| T4: 2 years PD | 1.06 (0.78; 1.46), P = 0.699 | 1.35 (0.73; 2.51), P = 0.333 | 1.17 (0.77; 1.79), P = 0.458 | 0.76 (0.46; 1.26), P = 0.283 | 0.003 | 0.95 (0.63; 1.43), P = 0.808 | 0.86 (0.53; 1.41), P = 0.555 | 0.654 |
| T5: 2–5 years PD | 1.22 (0.65; 2.28), P = 0.539 | 1.65 (0.41; 6.67), P = 0.480 | 1.18 (0.57; 2.46), P = 0.648 | 0.76 (0.12; 4.69), P = 0.767 | 0.030 | 1.26 (0.52; 3.03), P = 0.609 | 0.73 (0.30; 1.78), P = 0.493 | 0.994 |
ACOPDAC-28, cyclophosphamide, vincristine, prednisone and dacarbazine; DECOPDAC-21, doxorubicin, etoposide, cyclophosphamide, vincristine, prednisone and dacarbazine; TL, treatment level; yPD, years post diagnosis.
Linear mixed models on log-transformed serum AMH. Analyses were adjusted for age and hormonal co-treatment (either hormonal contraceptives or GnRH-analogues) at time of sampling. Results were retransformed into the original scale and presented as geometric-mean ratio (GMR) with their corresponding 95% confidence interval (95%CI) and p value. Reported GMRs of all treatment-level and treatment scheme subgroups were calculated using the AMH at diagnosis (T0) as intercept in the model. P values represent the estimated differences between subgroups, using the respective T follow-up as intercept in the model.
Samples drawn in patients after receiving pelvic radiotherapy (n = 4 T4 and n = 1 T5 samples) were excluded from the analyses.
Up to 5 years post-diagnosis
Overall, median serum AMH after 2 years was 2.1 µg/l (IQR 1.1; 3.8), measured in 73 samples with age at time of sampling 17.8 years (IQR 15.8; 19.1) (Table 2). In total, 5 (7%) patients had AMH levels <0.5 µg/l at 2 years post diagnosis (i.e. n = 2 TL2-COPDAC-28, n = 1 TL3-COPDAC-28, n = 2 TL3-DECOPDAC-21). Median AMH was 1.3 µg/l (IQR 0.8; 2.1) in TL3 patients and there was a significant association with lower AMH levels in higher treatment levels (intercept T4: estimated effect for TL3 versus TL2 GMR 0.40 (95% CI 0.22; 0.73), P = 0.003, estimated effect for TL3 versus TL1 GMR 0.36 (95% CI 0.16; 0.77), P = 0.009).
When considering AMH recovery, 68% of all patients reached their baseline AMH (n = 46/68), with an overall median observed recovery of 129% (IQR 75.0; 208.9). More specifically, in the advanced stage TL3 group, 10 out of 20 patients (50%) achieved their baseline AMH value and median recovery was 94% (IQR 34.0; 168.6), compared to 24 out of 33 patients (73%) and a median recovery of 139% (IQR 99.5; 225.0) in the TL2 group (P = 0.17 reached baseline, P = 0.06 median recovery, respectively) (Supplementary Table S1). Furthermore, after 2 years, there were no significant differences in median AMH levels and the observed recovery rates between the COPDAC-28 and DECOPDAC-21 schemes (AMH: T4 estimated effect for DECOPDAC-21 GMR 1.14 (95% CI 0.65; 1.99), P = 0.654, median recovery COPDAC-28 136% (IQR 99.9; 209.3) versus DECOPDAC-21 117% (IQR 63.5; 207.4), P = 0.16 respectively). Unadjusted median AMH after 2 years was lower among the patients who had received pelvic radiotherapy compared to those who were not irradiated (in the pelvic area) (median AMH 0.5 µg/l, IQR 0.3; 0.8, n = 4 versus 2.1 µg/l (1.1; 3.8) n = 73, P = 0.011). However, the irradiated patients also had remarkably low AMH levels before treatment (AMH 0.3 µg/l, IQR 0.2; 0.5, versus 1.6 µg/l, IQR 1.0; 2.8, P = 0.003). The patients who received pelvic radiotherapy all had advanced stage cHL (all TL3 with inadequate treatment response).
A total of 12 T5 samples were included in the analyses, with a median time since diagnosis of 3.0 years (range 2.3–5.2 years). The observed median AMH T5 level appeared lower in patients of the DECOPDAC-21 scheme, when compared to those treated with COPDAC-28 (2.2 µg/l (1.2; 4.6) in five patients receiving DECOPDAC-21 and 4.0 µg/l (1.9; 9.8) in five patients receiving COPDAC-28, respectively). However, the sample size was fairly limited and no statistically significant differences were observed in the linear mixed models (intercept T5: estimated effect for DECOPDAC-21 versus COPDAC-28 GMR 1.00 (95% CI 0.33; 3.01), P = 0.994).
Menstrual cycle characteristics and hormonal co-treatment
Data on the menstrual cycle characteristics at diagnosis and during follow-up are presented in Supplementary Table S2. Contraceptives and GnRH-analogues were prescribed to 38 (44%) and 17 (20%) post-menarchal girls during treatment, respectively. Out of the 35 post-menarchal girls who were not taking any contraceptives or GnRH-analogues, 19 (54%) experienced treatment-induced amenorrhea. Self-reported regularity of the menstrual cycle at 2 years post-diagnosis was known in 89 girls. The menstrual cycle was regular in 33 girls, irregular in 14 girls and absent in 2 girls (i.e. n = 1 TL3-COPDAC-28 and n = 1 TL3-DECOPDAC-21). Thirty-four girls used hormonal contraceptives, and the remaining six had not yet experienced their menarche. There were no statistically significant differences in the occurrence of treatment-induced amenorrhea and regularity of the menstrual cycle during follow-up between the COPDAC-28 and DECOPDAC-21 treatment schemes.
Discussion
This study prospectively assessed markers of gonadal function among girls treated for newly diagnosed cHL according to the current EuroNet-PHL-C2 protocol. In all patients, we observed a rapid and steep decrease in serum AMH after the administration of two OEPA induction chemotherapy courses. Serum AMH increased significantly during consolidation chemotherapy and 2 years of follow-up, although not all girls recovered to pre-treatment AMH levels. There was a clear association between higher TL-stage, i.e. a higher chemotherapy treatment burden, and lower AMH during treatment and after 2 years, after adjustment for age and hormonal co-treatment. There were no statistically significant differences in observed serum AMH between the standard and intensified chemotherapy arms after 2 years.
The observed AMH levels at diagnosis seemed relatively low for the age group of the studied cohort of girls, with an observed group median of 1.7 µg/l and 12 girls with AMH levels <0.5 µg/l. Normative models often report AMH reference values between 3–4 µg/l for girls aged between 14 and 18 years old (Kelsey et al., 2011; Li et al., 2012; Lie Fong et al., 2012; Yates et al., 2019). Previous studies have reported reduced baseline AMH levels in newly diagnosed cancer patients, which were hypothesized to be related to an overall impaired health status (Dorp et al., 2014; Drechsel et al., 2023a). Most of our patients were diagnosed with a more advanced stage of cHL, yet no significant association between serum AMH and the treatment-level nor the presence of B symptoms or elevated ESR was established in the analyses. It is unknown if there is a negative effect of the cHL disease on AMH levels and if such an effect resolves after treating the disease. However, if such an effect were to exist, we should be aware that this could introduce bias when discussing AMH recovery relative to baseline values.
Based on previous studies, recovery of AMH is to be expected up to 1 or 2 years after completion of treatment (Brougham et al., 2012; Anderson et al., 2018, 2022; Anderson and Su, 2020; Berjeb et al., 2020; Irene Su et al., 2020; Decanter et al., 2021). In our population, AMH levels started to recover after induction chemotherapy, i.e. during subsequent ongoing consolidation treatment and 2 years of follow-up, and appeared to further increase between the T4 and T5-checkups (2–5 years post-diagnosis). However, it remains uncertain how the observed values relate to expected age-specific AMH (reference) values. Ideally, laboratory results should have been compared to an age-matched control cohort, which was unfortunately not available. The performance of additional statistical analyses based on AMH reference values was considered unreliable because of the substantial impact of the AMH assay variability and variance between the limited number of available reference measurements in young and healthy children. Nevertheless, observed median AMH was 1.3 µg/l among girls receiving the highest number of chemotherapy cycles for advanced stage disease and five girls had AMH <0.5 µg/l, which will generally be considered low for women in the reproductive phase in daily clinical practice. AMH may fluctuate during childhood and adolescence, and could potentially fall at the onset of puberty (Hagen et al., 2012). However, most of the included girls were aged between 16 and 19 years old at their 2 year post-diagnosis check-up and it seems unlikely that lower AMH levels during follow-up could be fully attributed to a peripubertal state. Besides, an additional natural age-expected increase in AMH is to be expected in the studied cohort, and returning to baseline AMH alone may not be sufficient. The observed AMH levels in these girls may still be relatively lower than expected, which, despite the recovery, could still suggest gonadotoxicity.
Adjusted AMH levels remained significantly lower during treatment with DECOPDAC-21 chemotherapy when compared to the standard COPDAC-28 consolidation treatment. In addition to intensified chemotherapy, the DECOPDAC-21 courses are given every 3 weeks instead of every 4 weeks in the COPDAC-28 arm, which potentially allows less time for gonadal recovery during treatment. At 2 years post-diagnosis, we no longer observed statistically significant differences in AMH levels between the two treatment schemes. However, given that the T5 analysis was based only 10 samples collected from Tl2/TL3 patients between 2 and 5 years post-diagnosis, there is still too little data available to rule out potential long-term differences in recovery.
The overall objective of the introduction of DECOPDAC-21 chemotherapy is to reduce radiotherapy. Data on efficacy of the EuroNet-PHL-C2 protocol are still pending. In our study, none of the DECOPDAC-21 patients received pelvic radiotherapy, while actually more of these patients had active tumour sites within the infradiaphragmatic region at time of diagnosis, thus were potentially at risk of receiving pelvic radiotherapy. The sample size was too limited to perform adjusted sensitivity analyses on the subgroup of patients that received pelvic radiotherapy, but unadjusted AMH levels were considerably low after treatment and based on previous research, the risk of impaired fertility is especially high among these patients (Wallace et al., 2003, 2005; Fong et al., 2009; Elchuri et al., 2016; Van Den Berg et al., 2018; van de Loo et al., 2019; van Dijk et al., 2020; Drechsel et al., 2023a). Samples obtained from patients after receiving pelvic radiotherapy were excluded from the primary analysis to more accurately evaluate the impact of chemotherapy on serum AMH. Nevertheless, any successful reduction of radiotherapy within the DECOPDAC-21 arm should also be considered when comparing overall gonadotoxicity (and risk of other late effects) of both treatment schemes.
In line with previous evidence, treatment-induced amenorrhea was commonly reported (54%) among those girls not receiving hormonal co-treatment, with subsequent recovery in the vast majority of girls (Behringer et al., 2013; Boltežar et al., 2016; Jacobson et al., 2016). However, although the resumption of menses confirms remaining gonadal function, it does not predict how long the gonadal function will remain and it cannot offer reassurance of future reproductive ability (Letourneau et al., 2012; Jacobson et al., 2016). The menstrual cycle was absent in two girls at 2 years from diagnosis. Additional testing on the cause of secondary amenorrhea has not been performed within this study, thus it was not possible to differentiate between POI or other causes of amenorrhea.
Strengths and limitations
The fertility add-on study to the EuroNet-PHL-C2 protocol is the first study to prospectively evaluate reproductive markers in children treated for cHL, for this report focusing on females. The longitudinal design of the study increases knowledge on the effect of cancer treatment on serum AMH in a larger context as a surrogate marker for gonadal function. Nevertheless, several study limitations should be addressed. The studied population comprises young girls with diagnosis of cHL often concurring with pubertal transition, during which AMH levels naturally rise. There was no control population available, while the interpretation of AMH as a biomarker during childhood is complex. The current analysis included data up to 2–5 years post-diagnosis and potential long-term recovery and clinical reproductive outcomes remain unknown. Serum follicle stimulating hormone (FSH) was not included as a reproductive marker in this study. FSH measurement would have required timing of blood sampling based on the menstrual cycle, which was not feasible nor desirable during intensive treatment for cHL. In addition, most girls receive oral contraceptives during chemotherapy to prevent excess bleeding related to thrombocytopenia during menstruation, which would have severely affected serum FSH levels. Still, the measured circulating AMH could also have been affected by the hormonal contraceptive use, although most girls used the contraceptives for a brief period. Contraceptive use was comparable between both treatment schemes (COPDAC-28/DECOPDAC-21) and results were adjusted for this potential confounder. Furthermore, data on the regularity of the menstrual cycle after treatment was self-reported, thus potentially not completely reliable.
The sample size of the DECOPDAC-21 treatment-arm was lower than the COPDAC-28 treatment-arm and overall power to study the effect of pelvic radiotherapy or potential differences between pubertal stage groups was too limited. Similarly, our planned analysis of the effect of GnRH-analogues on serum AMH were impossible, as the sample size was too low to perform analysis adjusted for confounding factors such as age and intensity of received treatment.
Clinical implications and future research
In principle, cHL patients treated according to the EuroNet-PHL-C2 protocol will receive chemotherapy with a CED-score <6000 mg/m2, which is considered low-risk of infertility in females aged <25 years according to the current PANCARE guidelines (Mulder et al., 2021b). However, it should be acknowledged that the CED-score calculator provides a conversion of only 10 alkylating drugs, based on evidence derived from a fairly limited number of studies (n = 17) (Green et al., 2014). It is important to remain critical about the potential gonadotoxicity of treatment, since not all alkylating agents used to treat cancer are part of the calculator (e.g. dacarbazine) and risks could well be underestimated. Although we observed an increase in AMH after chemotherapy, it should be noted that not all girls recovered to pre-treatment AMH levels, particularly those treated for advanced stages of cHL. Additional data are needed to interpret our observations in relation to expected age-specific AMH in healthy adolescents. Present study results are still insufficient to provide full reassurance regarding the gonadotoxic risk of both standard and intensified treatment arms of the EuroNet-PHL-C2 protocol, mostly due to limited sample size and duration of follow-up.
Nevertheless, previous studies suggested that the chance to become pregnant as a childhood cancer survivor is relatively reassuring even in a setting where AMH levels are low, especially when attempting pregnancy at a young age (van Dijk et al., 2020; Drechsel et al., 2023a,b). The latter however would require careful and appropriate counseling. Therefore, it remains valuable to conduct research on gonadotoxicity of treatment and long-term reproductive outcomes in cancer survivors.
Summarizing conclusions
A clear direct adverse effect of cHL treatment according to the EuroNet-PHL-C2 protocol on AMH levels was observed in girls aged ≤18 years at time of treatment. Although we observed an increase in AMH within 2 years following diagnosis, not all girls recovered to pre-treatment AMH levels. Additional (long-term) data are needed to explore clinical reproductive outcomes of survivors treated according to the EuroNet-PHL-C2 protocol. These data are also needed to determine whether gonadotoxicity of the DECOPDAC-21 treatment-arm is comparable to the standard COPDAC-28 treatment in the long term.
Supplementary Material
Acknowledgements
The authors gratefully acknowledge Salena Meivis, Lisanne Raasen and all other involved research nurses and personnel who assisted data collection of this study.
Contributor Information
K C E Drechsel, Pediatric Oncology, Cancer Center Amsterdam, Emma Children’s Hospital, Amsterdam UMC, Vrije Universiteit Amsterdam, Amsterdam, The Netherlands; Department of Paediatric Haemato-Oncology, Princess Máxima Center for Pediatric Oncology, Utrecht, The Netherlands; Cancer Center Amsterdam, Treament and quality of life, Amsterdam UMC, Vrije Universiteit Amsterdam, The Netherlands.
S L Broer, Department of Reproductive Medicine & Gynecology, University Medical Center Utrecht, Utrecht, The Netherlands.
F S Stoutjesdijk, Pediatric Oncology, Cancer Center Amsterdam, Emma Children’s Hospital, Amsterdam UMC, Vrije Universiteit Amsterdam, Amsterdam, The Netherlands.
E van Dulmen-den Broeder, Pediatric Oncology, Cancer Center Amsterdam, Emma Children’s Hospital, Amsterdam UMC, Vrije Universiteit Amsterdam, Amsterdam, The Netherlands.
A Beishuizen, Department of Paediatric Haemato-Oncology, Princess Máxima Center for Pediatric Oncology, Utrecht, The Netherlands; Department of Haematology/Oncology, Erasmus MC—Sophia Children’s Hospital, Rotterdam, The Netherlands.
W H Wallace, Department of Haematology/Oncology, Royal Hospital for Sick Children, Edinburgh, UK.
D Körholz, Department of Pediatric Hematology and Oncology, Universitätsklinikum Giessen und Marburg GmbH, Standort Giessen—Zentrum für Kinderheilkunde und Jugendmedizin, Giessen, Germany.
C Mauz-Körholz, Department of Pediatric Hematology and Oncology, Universitätsklinikum Giessen und Marburg GmbH, Standort Giessen—Zentrum für Kinderheilkunde und Jugendmedizin, Giessen, Germany; Clinic for Paediatric and Adolescent Medicine, Medical Faculty of the Martin, Luther University of Halle, Halle, Germany.
D Hasenclever, Institut für Medizinische Informatik, Statistik und Epidemiologie, Universität Leipzig, Leipzig, Germany.
M Cepelova, Department of Pediatric Hematology and Oncology, Faculty Hospital Motol and 2nd Medical Faculty, Charles University, Prague, Czech Republic.
A Uyttebroeck, Department of Paediatric Haematology and Oncology, KU Leuven, UZ Leuven, Leuven, Belgium.
L Ronceray, Pediatric Hematology and Oncology, St Anna Children's Hospital, Medical University of Vienna, Wien, Austria.
J W R Twisk, Department of Epidemiology and Data Science, Amsterdam UMC, Vrije Universiteit Amsterdam, Amsterdam, The Netherlands.
G J L Kaspers, Pediatric Oncology, Cancer Center Amsterdam, Emma Children’s Hospital, Amsterdam UMC, Vrije Universiteit Amsterdam, Amsterdam, The Netherlands; Department of Paediatric Haemato-Oncology, Princess Máxima Center for Pediatric Oncology, Utrecht, The Netherlands.
M A Veening, Pediatric Oncology, Cancer Center Amsterdam, Emma Children’s Hospital, Amsterdam UMC, Vrije Universiteit Amsterdam, Amsterdam, The Netherlands; Department of Paediatric Haemato-Oncology, Princess Máxima Center for Pediatric Oncology, Utrecht, The Netherlands.
Data availability
Individual participant data that underlie this article cannot be shared publicly because of privacy. Pseudonymized data will be shared on reasonable request for an ethically approved study protocol, after compiling a data-sharing agreement. Data sharing requests can be directed to ma.veening@prinsesmaximacentrum.nl.
Authors’ roles
M.A.V., A.B., G.J.L.K., W.H.W., E.v.D-d.B., D.K., C.M.-K., M.C., A.U., L.R., and F.S.S. were responsible for trial design and study setup. D.H. and K.C.E.D. were also involved during the data collection. K.C.E.D. cleaned the data and performed the statistical analysis, supported by J.W.R.T., S.L.B., and M.A.V. K.C.E.D. wrote the first version of the manuscript. All authors critically reviewed the manuscript and approved the submitted final version.
Funding
The Dutch charity foundation KiKa (project 257) that funds research on all forms of childhood cancer.
Conflict of interest
C.M.-K., D.K., W.H.W., D.H., M.C., A.U., and A.B. were involved in the development of the EuroNet-PHL-C2 regimen. The other authors indicated no potential conflicts of interest.
References
- Absolom K, Eiser C, Turner L, Ledger W, Ross R, Davies H, Coleman R, Hancock B, Snowden J, Greenfield D; Late Effects Group Sheffield. Ovarian failure following cancer treatment: current management and quality of life. Hum Reprod 2008;23:2506–2512. [DOI] [PubMed] [Google Scholar]
- Anderson RA, Cameron D, Clatot F, Demeestere I, Lambertini M, Nelson SM, Peccatori F. Anti-Müllerian hormone as a marker of ovarian reserve and premature ovarian insufficiency in children and women with cancer: a systematic review. Hum Reprod Update 2022;28:417–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RA, Remedios R, Kirkwood AA, Patrick P, Stevens L, Clifton-Hadley L, Roberts T, Hatton C, Kalakonda N, Milligan DW et al. Determinants of ovarian function after response-adapted therapy in patients with advanced Hodgkin’s lymphoma (RATHL): a secondary analysis of a randomised phase 3 trial. Lancet Oncol 2018;19:1328–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RA, Su HI. The clinical value and interpretation of anti-Müllerian Hormone in women with cancer. Front Endocrinol (Lausanne) 2020;11:574263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behringer K, Mueller H, Goergen H, Thielen I, Eibl AD, Stumpf V, Wessels C, Wiehlpütz M, Rosenbrock J, Halbsguth T, et al. Gonadal function and fertility in survivors after Hodgkin lymphoma treatment within the German Hodgkin study group HD13 to HD15 Trials. J Clin Oncol 2013;31:231–239. [DOI] [PubMed] [Google Scholar]
- Berjeb KK, Debbabi L, Braham M, Zemni Z, Chtourou S, Hannachi H, Hamdoun M, Ayadi M, Kacem K, Zhioua F, et al. Evaluation of ovarian reserve before and after chemotherapy. J Gynecol Obstet Hum Reprod 2020;50:102035. [DOI] [PubMed] [Google Scholar]
- Boltežar L, Pintarić K, Jezeršek Novaković B. Fertility in young patients following treatment for Hodgkin’s lymphoma: a single center survey. J Assist Reprod Genet 2016;33:325–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broer SL, Broekmans FJM, Laven JSE, Fauser BCJM. Anti-Müllerian hormone: ovarian reserve testing and its potential clinical implications. Hum Reprod Update 2014;20:688–701. [DOI] [PubMed] [Google Scholar]
- Brougham MFH, Crofton PM, Johnson EJ, Evans N, Anderson RA, Wallace WHB. Anti-Müllerian hormone is a marker of gonadotoxicity in pre- and postpubertal girls treated for cancer: A prospective study. J Clin Endocrinol Metab 2012;97:2059–2067. [DOI] [PubMed] [Google Scholar]
- Decanter C, Delepine J, Behal H, Manier S, Bruno B, Barbatti M, Robin C, Labreuche J, Morschhauser F, Pigny P. Longitudinal study of AMH variations in 122 adolescents and young Adults (AYA) and non-AYA lymphoma patients to evaluate the chemo-induced ovarian toxicity to further personalise fertility preservation counselling. Hum Reprod 2021;36:2743–2752. [DOI] [PubMed] [Google Scholar]
- Dorp W, VanVries ACH, DePluijm SMF, Visser JA, Pieters R, Laven JSE, Dorp W, vanHeuvel-Eibrink MM, van denVries AC, dePluijm SMF, et al. Decreased serum anti-Müllerian hormone levels in girls with newly diagnosed cancer. Hum Reprod 2014;29:337–342. [DOI] [PubMed] [Google Scholar]
- Drechsel K, Pilon M, Stoutjesdijk F, Meivis S, Schoonmade LJ, Wallace W, Dulmen-den Broeder E, vanBeishuizen A, Kaspers G, Broer S, et al. Reproductive ability in survivors of childhood, adolescent and young adult Hodgkin lymphoma: a review. Hum Reprod Update 2023a;29:486–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drechsel KCE, Broer SL, Stoutjesdijk FS, Twisk JWR, Berg MH, van denLambalk CB, Leeuwen FE, vanOverbeek A, Heuvel-Eibrink MM, van denDorp W, et al. ; LATER-VEVO Study Group . Clinical and self-reported markers of reproductive function in female survivors of childhood Hodgkin lymphoma. J Cancer Res Clin Oncol 2023b;149:13677–13695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elchuri SV, Patterson BC, Brown M, Bedient C, Record E, Wasilewski-Masker K, Mertens AC, Meacham LR. Low anti-Müllerian hormone in pediatric cancer survivors in the early years after gonadotoxic therapy. J Pediatr Adolesc Gynecol 2016;29:393–399. [DOI] [PubMed] [Google Scholar]
- European Network-Paediatric Hodgkin Lymphoma Study Group (EuroNet-PHL). Second international inter-group study for classical Hodgkin lymphoma in children and adolescent: EuroNet-PHL-C2, 2015. https://classic.clinicaltrials.gov/ct2/show/NCT02684708 (24 February 2024, date last accessed).
- Fong SL, Laven JSE, Hakvoort-Cammel FGAJ, Schipper I, Visser JA, Themmen APN, De Jong FH, Van Den Heuvel-Eibrink MM. Assessment of ovarian reserve in adult childhood cancer survivors using anti-Müllerian hormone. Hum Reprod 2009;24:982–990. [DOI] [PubMed] [Google Scholar]
- Green DM, Nolan VG, Goodman PJ, Whitton JA, Srivastava DK, Leisenring WM, Neglia JP, Sklar CA, Kaste SC, Hudson MM, et al. The cyclophosphamide equivalent dose as an approach for quantifying alkylating agent exposure: A report from the childhood cancer survivor study. Pediatr Blood Cancer 2014;61:53–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagen CP, Aksglaede L, Sørensen K, Mouritsen A, Andersson AM, Petersen JH, Main KM, Juul A. Individual serum levels of anti-Müllerian hormone in healthy girls persist through childhood and adolescence: a longitudinal cohort study. Hum Reprod 2012;27:861–866. [DOI] [PubMed] [Google Scholar]
- IBM Corp. IBM SPSS Statistics for Windows, Version 28.0. Armonk, NY: IBM Corp, 2021. https://www.ibm.com/support/pages/how-cite-ibm-spss-statistics-or-earlier-versions-spss.
- Irene Su H, Kwan B, Whitcomb BW, Shliakhsitsava K, Dietz AC, Stark SS, Martinez E, Sluss PM, Sammel MD, Natarajan L. Modeling variation in the reproductive lifespan of female adolescent and young adult cancer survivors using AMH. J Clin Endocrinol Metab 2020;105:2740–2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson MH, Mertens AC, Spencer JB, Manatunga AK, Howards PP. Menses resumption after cancer treatment-induced amenorrhea occurs early or not at all. Fertil Steril 2016;105:765–772e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelsey TW, Wright P, Nelson SM, Anderson RA, Wallace WHB. A validated model of serum anti-Müllerian hormone from conception to menopause. PLoS One 2011;6:e22024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krawczuk-Rybak M, Leszczynska E, Poznanska M, Zelazowska-Rutkowska B, Wysocka J. Anti-Müllerian hormone as a sensitive marker of ovarian function in young cancer survivors. Int J Endocrinol 2013;2013:125080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letourneau JM, Ebbel EE, Katz PP, Oktay KH, McCulloch CE, Ai WZ, Chien AJ, Melisko ME, Cedars MI, Rosen MP. Acute ovarian failure underestimates age-specific reproductive impairment for young women undergoing chemotherapy for cancer. Cancer 2012;118:1933–1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li HWR, Ng EHY, Wong BPC, Anderson RA, Ho PC, Yeung WSB. Correlation between three assay systems for anti-Müllerian hormone (AMH) determination. J Assist Reprod Genet 2012;29:1443–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lie Fong S, Visser JA, Welt CK, Rijke YBD, Eijkemans MJC, Broekmans FJ, Roes EM, Peters WHM, Hokken-Koelega ACS, Fauser BCJM, et al. Serum anti-Müllerian hormone levels in healthy females: a nomogram ranging from infancy to adulthood. J Clin Endocrinol Metab 2012;97:4650–4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauz-Körholz C, Landman-Parker J, Balwierz W, Ammann RA, Anderson RA, Attarbaschi A, Bartelt JM, Beishuizen A, Boudjemaa S, Cepelova M et al. Response-adapted omission of radiotherapy and comparison of consolidation chemotherapy in children and adolescents with intermediate-stage and advanced-stage classical Hodgkin lymphoma (EuroNet-PHL-C1): a titration study with an open-label, embedded, multinational, non-inferiority, randomised controlled trial. Lancet Oncol 2022;23:125–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meirow D. Reproduction post-chemotherapy in young cancer patients. Mol Cell Endocrinol 2000;169:123–131. [DOI] [PubMed] [Google Scholar]
- Mulder RL, Font-Gonzalez A, Green DM, Loeffen EAH, Hudson MM, Loonen J, Yu R, Ginsberg JP, Mitchell RT, Byrne J, et al. ; PanCareLIFE Consortium. Fertility preservation for male patients with childhood, adolescent, and young adult cancer: recommendations from the PanCareLIFE Consortium and the International Late Effects of Childhood Cancer Guideline Harmonization Group. Lancet Oncol 2021a;22:e57–e67. [DOI] [PubMed] [Google Scholar]
- Mulder RL, Font-Gonzalez A, Hudson MM, Santen HM, vanLoeffen EAH, Burns KC, Quinn GP, Dulmen-den Broeder E, vanByrne J, Haupt R, et al. ; PanCareLIFE Consortium. Fertility preservation for female patients with childhood, adolescent, and young adult cancer: recommendations from the PanCareLIFE Consortium and the International Late Effects of Childhood Cancer Guideline Harmonization Group. Lancet Oncol 2021b;22:e45–e56. [DOI] [PubMed] [Google Scholar]
- Oktem O, Kim SS, Selek U, Schatmann G, Urman B. Ovarian and uterine functions in female survivors of childhood cancers. Oncologist 2018;23:214–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revelli A, Biasoni V, Gennarelli G, Canosa S, Dalmasso P, Benedetto C. IVF results in patients with very low serum AMH are significantly affected by chronological age. J Assist Reprod Genet 2016;33:603–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaapveld M, Aleman BMP, Eggermond AM, VanJanus CPM, Krol ADG, Maazen RWM, Van DerRoesink J, Raemaekers JMM, Boer JP, DeZijlstra JM, et al. Second cancer risk up to 40 years after treatment for Hodgkin’s lymphoma. N Engl J Med 2015;373:2499–2511. [DOI] [PubMed] [Google Scholar]
- Sonigo C, Beau I, Binart N, Grynberg M. The impact of chemotherapy on the ovaries: molecular aspects and the prevention of ovarian damage. Int J Mol Sci 2019;20:5342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spears N, Lopes F, Stefansdottir A, Rossi V, Felici M, DeAnderson RA, Klinger FG. Ovarian damage from chemotherapy and current approaches to its protection. Hum Reprod Update 2019;25:673–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Loo LEXM, van den Berg MH, Overbeek A, van Dijk M, Damen L, Lambalk CB, Ronckers CM, van den Heuvel-Eibrink MM, Kremer LCM, van der Pal HJ, et al. ; DCOG LATER-VEVO Study Group. Uterine function, pregnancy complications, and pregnancy outcomes among female childhood cancer survivors. Fertil Steril 2019;111:372–380. [DOI] [PubMed] [Google Scholar]
- Van Den Berg MH, Overbeek A, Lambalk CB, Kaspers GJL, Bresters D, Van Den Heuvel-Eibrink MM, Kremer LC, Loonen JJ, Van Der Pal HJ, Ronckers CM, et al. ; DCOG LATER-VEVO Study Group.Long-term effects of childhood cancer treatment on hormonal and ultrasound markers of ovarian reserve. Human Reproduction 2018;33:1474–1488. [DOI] [PubMed] [Google Scholar]
- van Dijk M, van Leeuwen FE, Overbeek A, Lambalk CB, van den Heuvel-Eibrink MM, van Dorp W, Tissing WJ, Kremer LC, Loonen JJ, Versluys B, et al. Pregnancy, time to pregnancy and obstetric outcomes among female childhood cancer survivors: results of the DCOG LATER-VEVO study. J Res Clin Oncol 2020;146:1451–1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace WHB, Kelsey TW. Human ovarian reserve from conception to the menopause. PLoS One 2010;5:e8772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace WHB, Thomson AB, Kelsey TW. The radiosensitivity of the human oocyte. Hum Reprod 2003;18:117–121. [DOI] [PubMed] [Google Scholar]
- Wallace WHB, Thomson AB, Saran F, Kelsey TW. Predicting age of ovarian failure after radiation to a field that includes the ovaries. Int J Radiat Oncol Biol Phys 2005;62:738–744. [DOI] [PubMed] [Google Scholar]
- Webber L, Davies M, Anderson R, Bartlett J, Braat D, Cartwright B, Cifkova R, Keizer-Schrama M, De S, Hogervorst E, et al. ESHRE guideline: management of women with premature ovarian insufficiency. Hum Reprod 2016;31:926–937. [DOI] [PubMed] [Google Scholar]
- Yates AP, Jopling HM, Burgoyne NJ, Hayden K, Chaloner CM, Tetlow L. Paediatric reference intervals for plasma anti-Müllerian hormone: comparison of data from the Roche Elecsys assay and the Beckman Coulter Access assay using the same cohort of samples. Ann Clin Biochem 2019;56:536–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Individual participant data that underlie this article cannot be shared publicly because of privacy. Pseudonymized data will be shared on reasonable request for an ethically approved study protocol, after compiling a data-sharing agreement. Data sharing requests can be directed to ma.veening@prinsesmaximacentrum.nl.