Abstract
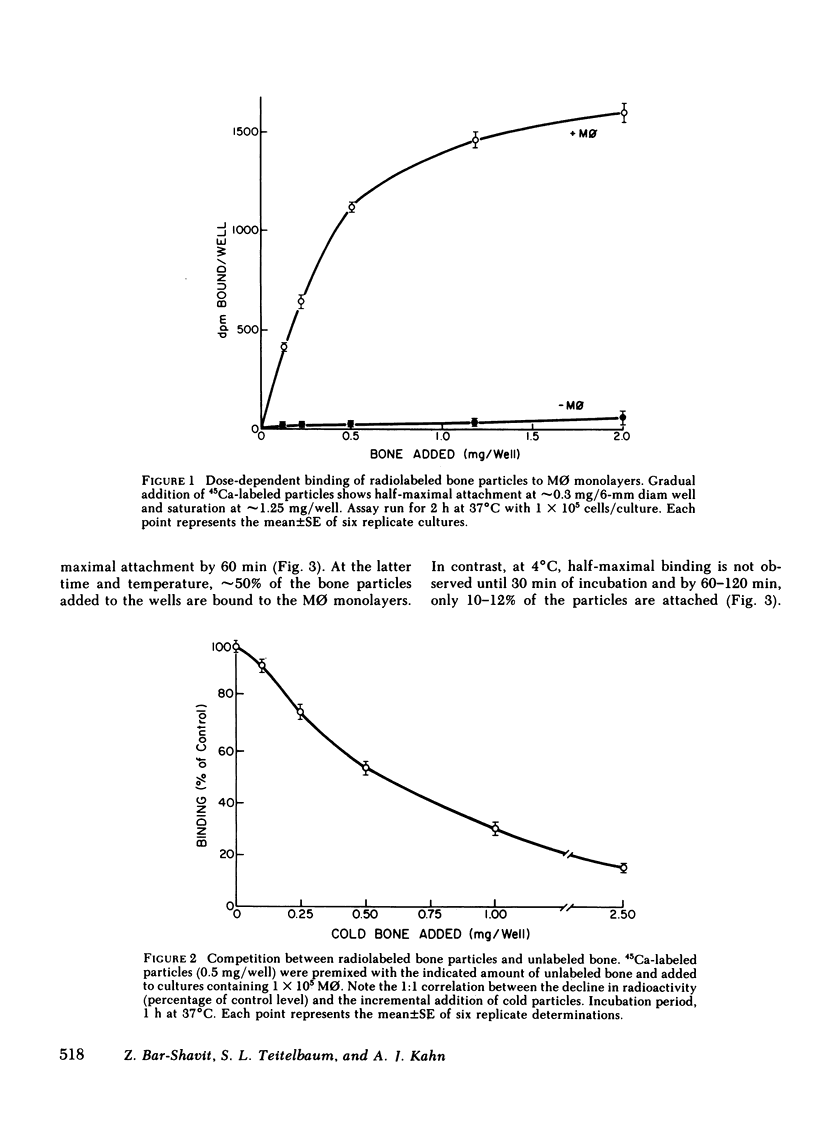
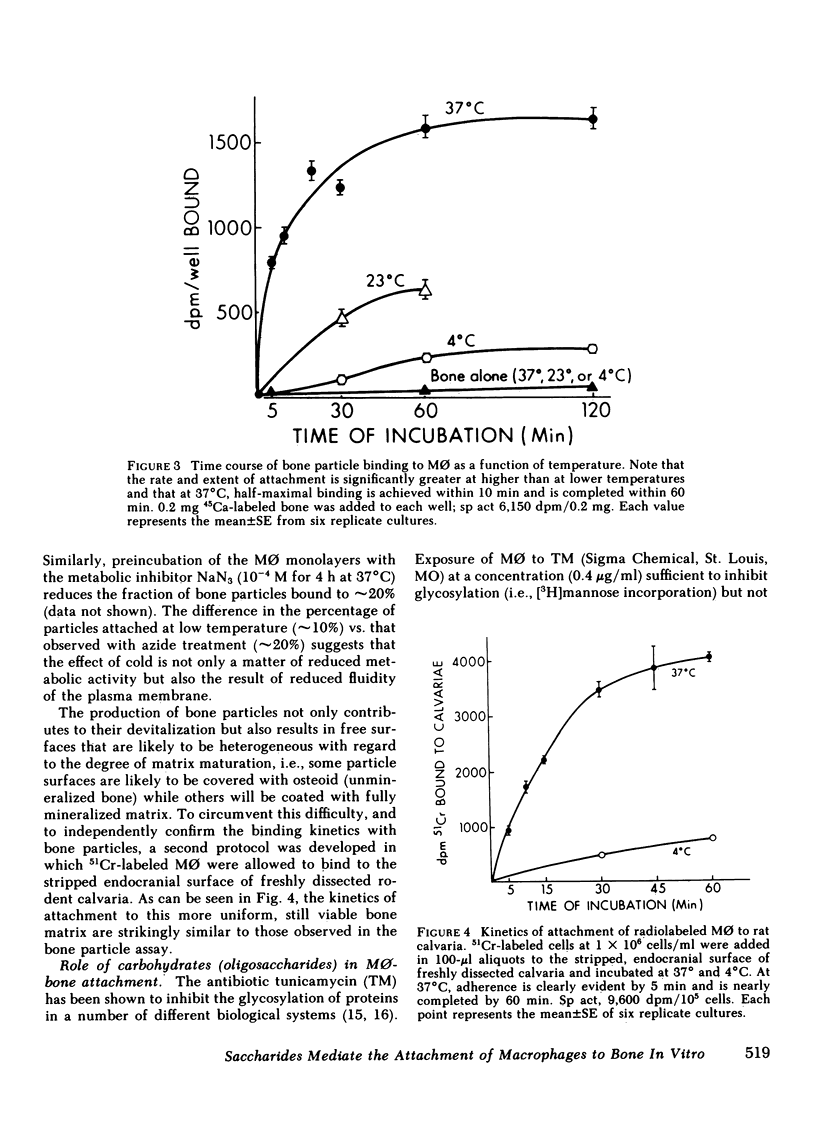
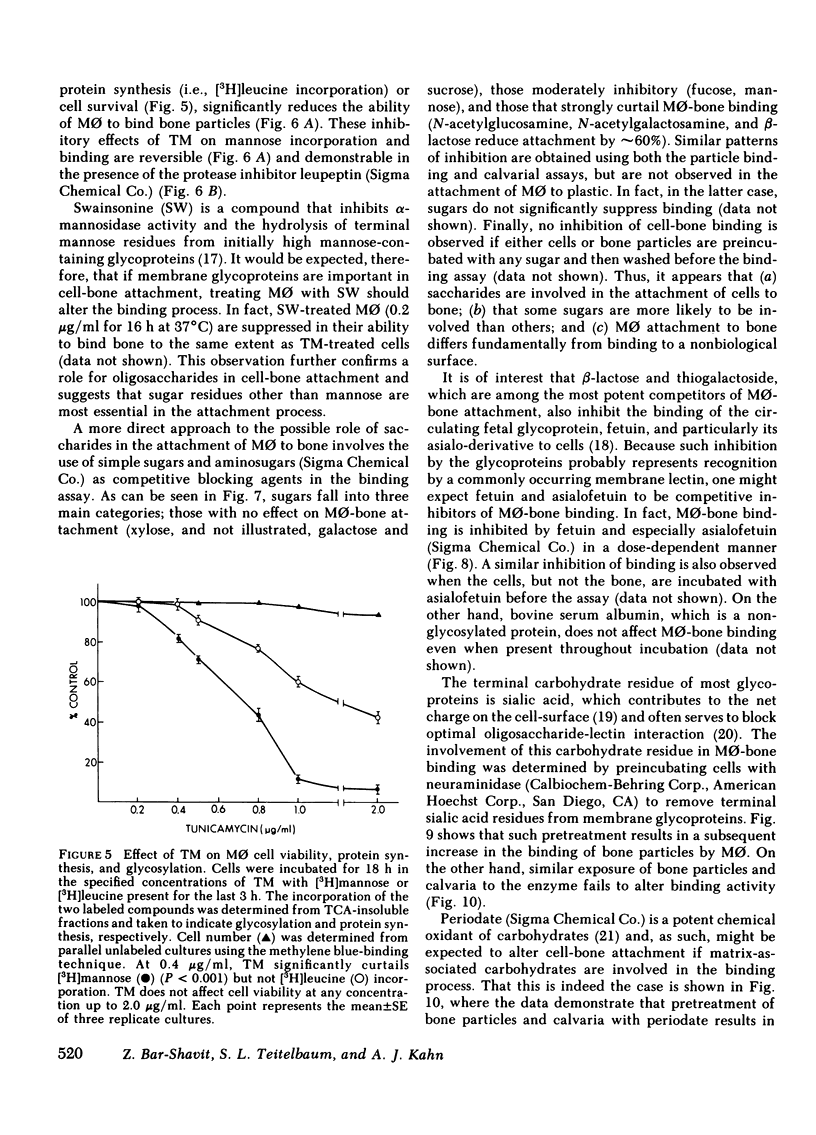
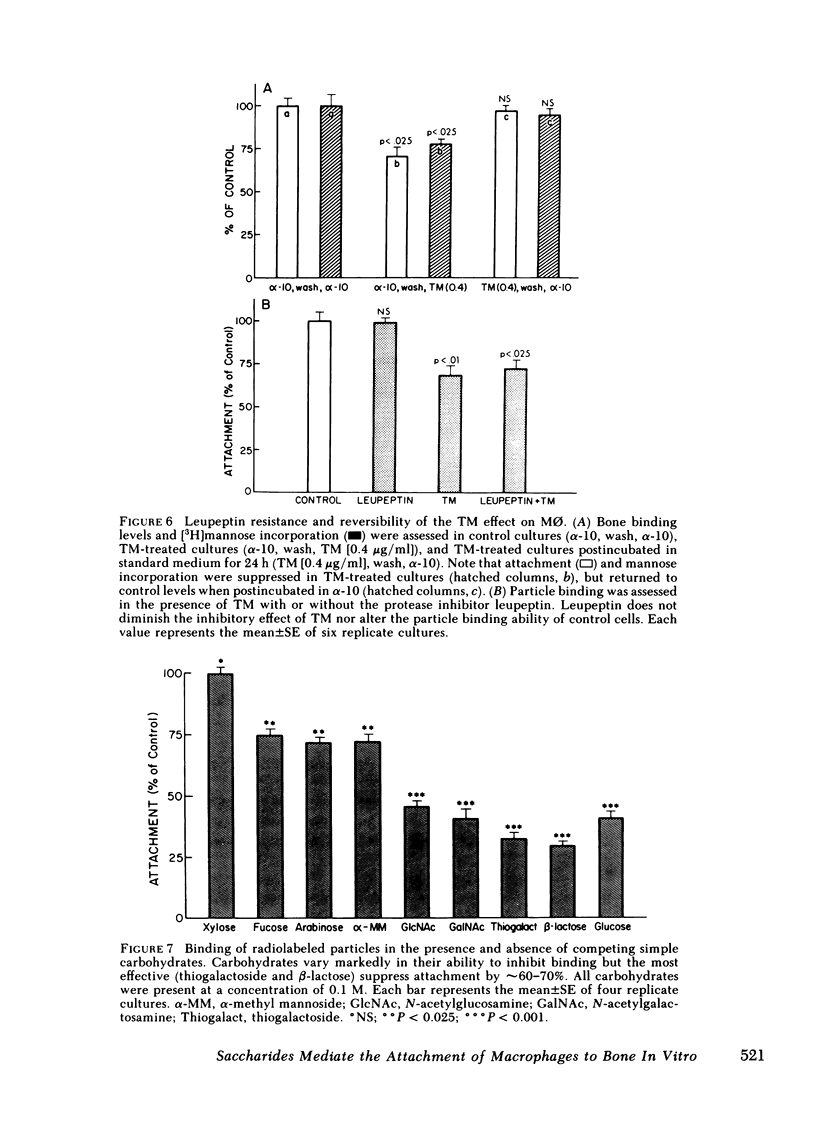
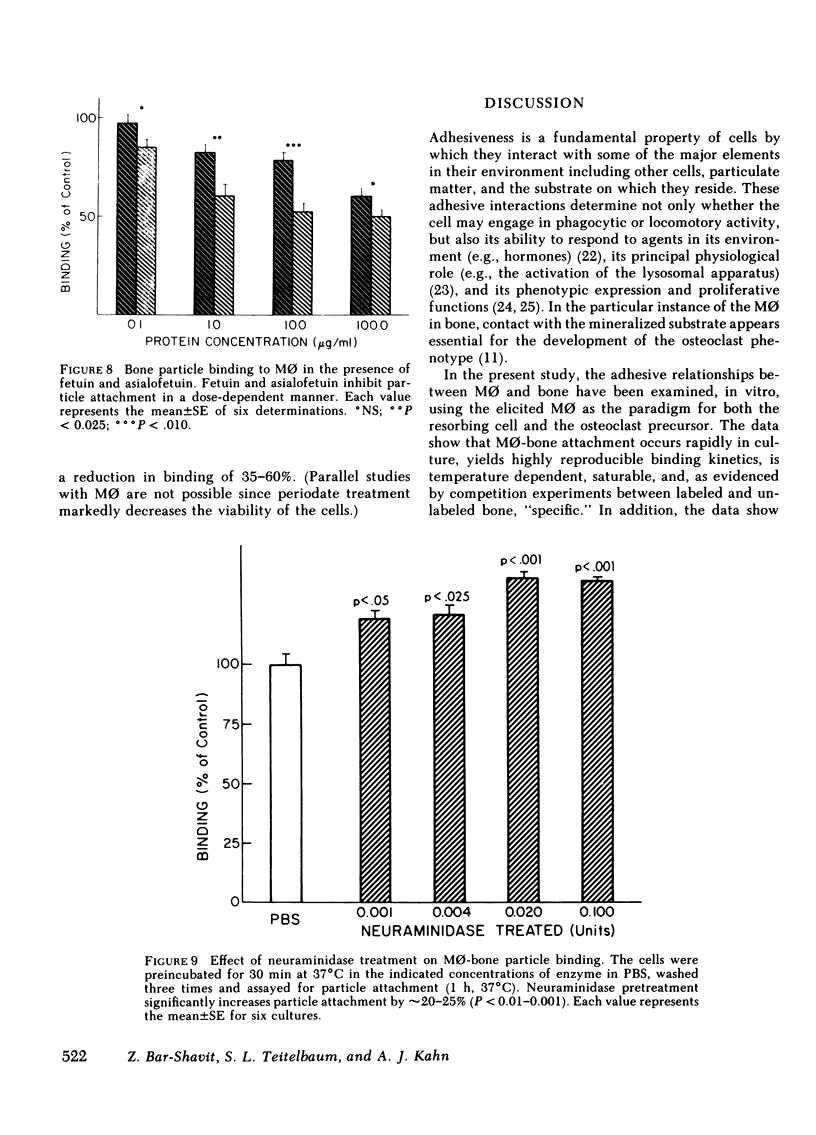
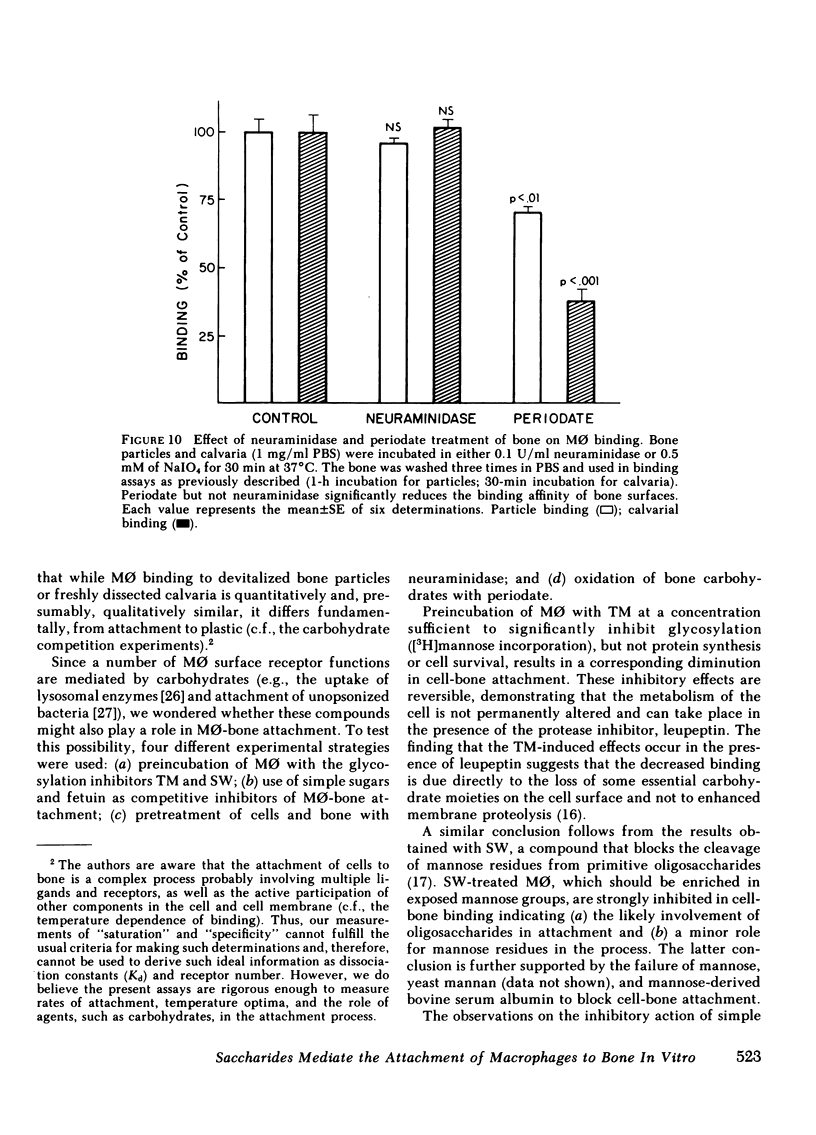
Macrophages (M phi) are multipotential cells capable of giving rise to osteoclasts and of resorbing bone. Since both of these processes are ultimately dependent upon the attachment of cells to a mineralized bone surface, we have examined in this study the mechanism by which such attachment is achieved. The data show that elicited rat peritoneal M phi bind to bone in a temperature-dependent and -saturable manner with half-maximal attachment occurring within 10 min at 37 degrees C and reaching a plateau by approximately 60 min. The kinetics of binding are essentially the same whether devitalized bone particles or viable calvaria are used as a substrate. The attachment of M phi to bone is inhibited by some sugars (e.g., N-acetyl-galactosamine, thiogalactoside, beta-lactose), fetuin and asialofetuin, and by pretreating the bone with periodate. Binding is also significantly reduced when M phi are preincubated with tunicamycin and swainsonine at nontoxic concentrations sufficient to inhibit or alter glycosylation. On the other hand, exposing the cells to neuraminidase increases the capacity of M phi to bind to bone. Collectively, our observations indicate that the attachment of M phi to bone is a highly regulated process and is mediated, at least in part, by saccharides located on both the cell and the bone surface.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bar-Shavit Z., Goldman R., Ofek I., Sharon N., Mirelman D. Mannose-binding activity of Escherichia coli: a determinant of attachment and ingestion of the bacteria by macrophages. Infect Immun. 1980 Aug;29(2):417–424. doi: 10.1128/iai.29.2.417-424.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Shavit Z., Kahn A. J., Teitelbaum S. L. Defective binding of macrophages to bone in rodent osteomalacia and vitamin D deficiency. In vitro evidence for a cellular defect and altered saccharides in the bone matrix. J Clin Invest. 1983 Aug;72(2):526–534. doi: 10.1172/JCI111000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair H., Kahn A. J., Sadow J., Malone J. D., Teitelbaum S. L. Disassociation of lysosomal enzyme secretion and macrophage-mediated bone resorption. Biochem Biophys Res Commun. 1981 Jun 16;100(3):959–964. doi: 10.1016/0006-291x(81)91916-1. [DOI] [PubMed] [Google Scholar]
- Burger E. H., Van der Meer J. W., van de Gevel J. S., Gribnau J. C., Thesingh G. W., van Furth R. In vitro formation of osteoclasts from long-term cultures of bone marrow mononuclear phagocytes. J Exp Med. 1982 Dec 1;156(6):1604–1614. doi: 10.1084/jem.156.6.1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czop J. K., Fearon D. T., Austen K. F. Membrane sialic acid on target particles modulates their phagocytosis by a trypsin-sensitive mechanism on human monocytes. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3831–3835. doi: 10.1073/pnas.75.8.3831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorling P. R., Huxtable C. R., Colegate S. M. Inhibition of lysosomal alpha-mannosidase by swainsonine, an indolizidine alkaloid isolated from Swainsona canescens. Biochem J. 1980 Nov 1;191(2):649–651. doi: 10.1042/bj1910649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkman J., Moscona A. Role of cell shape in growth control. Nature. 1978 Jun 1;273(5661):345–349. doi: 10.1038/273345a0. [DOI] [PubMed] [Google Scholar]
- Goldman R., Bar-Shavit Z. Dual effect of normal and stimulated macrophages and their conditioned media on target cell proliferation. J Natl Cancer Inst. 1979 Oct;63(4):1009–1016. [PubMed] [Google Scholar]
- Kahn A. J., Stewart C. C., Teitelbaum S. L. Contact-mediated bone resorption by human monocytes in vitro. Science. 1978 Mar 3;199(4332):988–990. doi: 10.1126/science.622581. [DOI] [PubMed] [Google Scholar]
- Keefer L. M., De Meyts P. Glycosylation of cell surface receptors: tunicamycin treatment decreases insulin and growth hormone binding to different levels in cultured lymphocytes. Biochem Biophys Res Commun. 1981 Jul 16;101(1):22–29. doi: 10.1016/s0006-291x(81)80005-8. [DOI] [PubMed] [Google Scholar]
- Kimmel D. B., Jee W. S. Bone cell kinetics during longitudinal bone growth in the rat. Calcif Tissue Int. 1980;32(2):123–133. doi: 10.1007/BF02408531. [DOI] [PubMed] [Google Scholar]
- Ko J. S., Bernard G. W. Osteoclast formation in vitro from bone marrow mononuclear cells in osteoclast-free bone. Am J Anat. 1981 Aug;161(4):415–425. doi: 10.1002/aja.1001610407. [DOI] [PubMed] [Google Scholar]
- Krukowski M., Kahn A. J. Inductive specificity of mineralized bone matrix in ectopic osteoclast differentiation. Calcif Tissue Int. 1982 Sep;34(5):474–479. doi: 10.1007/BF02411288. [DOI] [PubMed] [Google Scholar]
- Marikovsky Y., Lotan R., Lis H., Sharon N., Danon D. Agglutination and labeling density of soybean agglutinin on young and old human red blood cells. Exp Cell Res. 1976 May;99(2):453–456. doi: 10.1016/0014-4827(76)90607-8. [DOI] [PubMed] [Google Scholar]
- Mørland B. Studies on selective induction of lysosomal enzyme activities in mouse peritoneal macrophages. J Reticuloendothel Soc. 1979 Dec;26(6):749–762. [PubMed] [Google Scholar]
- Olden K., Pratt R. M., Yamada K. M. Role of carbohydrate in biological function of the adhesive glycoprotein fibronectin. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3343–3347. doi: 10.1073/pnas.76.7.3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz A., Lotan R. Lectin-like activities associated with human and murine neoplastic cells. Cancer Res. 1981 Sep;41(9 Pt 1):3642–3647. [PubMed] [Google Scholar]
- Schenk R. K., Spiro D., Wiener J. Cartilage resorption in the tibial epiphyseal plate of growing rats. J Cell Biol. 1967 Jul;34(1):275–291. doi: 10.1083/jcb.34.1.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiro R. G., Bhoyroo V. D. Structure of the O-glycosidically linked carbohydrate units of fetuin. J Biol Chem. 1974 Sep 25;249(18):5704–5717. [PubMed] [Google Scholar]
- Teitelbaum S. L., Stewart C. C., Kahn A. J. Rodent peritoneal macrophages as bone resorbing cells. Calcif Tissue Int. 1979 Jul 3;27(3):255–261. doi: 10.1007/BF02441194. [DOI] [PubMed] [Google Scholar]
- Weisbrode S. E., Capen C. C., Nagode L. A. Fine structural and enzymatic evaluation of bone in thyroparathyroidectomized rats receiving various levels of vitamin D. Lab Invest. 1973 Jan;28(1):29–37. [PubMed] [Google Scholar]
- van Furth R. Current view on the mononuclear phagocyte system. Immunobiology. 1982 Apr;161(3-4):178–185. doi: 10.1016/S0171-2985(82)80072-7. [DOI] [PubMed] [Google Scholar]


