Abstract
We have recently noted marked differences between the in vitro responses of human B lymphocytes to stimulation with soluble antigens vs. stimulation with mitogens. In the present study, these differences were analyzed in terms of the precursor frequencies for the T cells and B cells involved and in terms of the radiation sensitivity of the T cells providing help in the two systems. Marked differences were found between antigen-induced and mitogen-induced systems with regard to T cell precursor frequencies and radiation sensitivity. In contrast, the precursor frequencies for the B cells involved in the two systems were approximately the same. In addition, having developed a system for the study of human antigen-specific B cell responses, we were interested in delineating the nature of the allogeneic effects that might be operative in this system. Marked allogeneic effects, both positive and negative, were noted in this system and will need to be taken into account in any studies that try to address the question of the genetic restriction, if any, that exists in human antigen-specific T cell-B cell collaboration. Appreciation of the marked differences between the antigen-specific and mitogen-induced activation and immunoregulation of human B cell responses will be of importance in understanding the relationship between specificity and nonspecificity of antibody production in normal and disease states.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
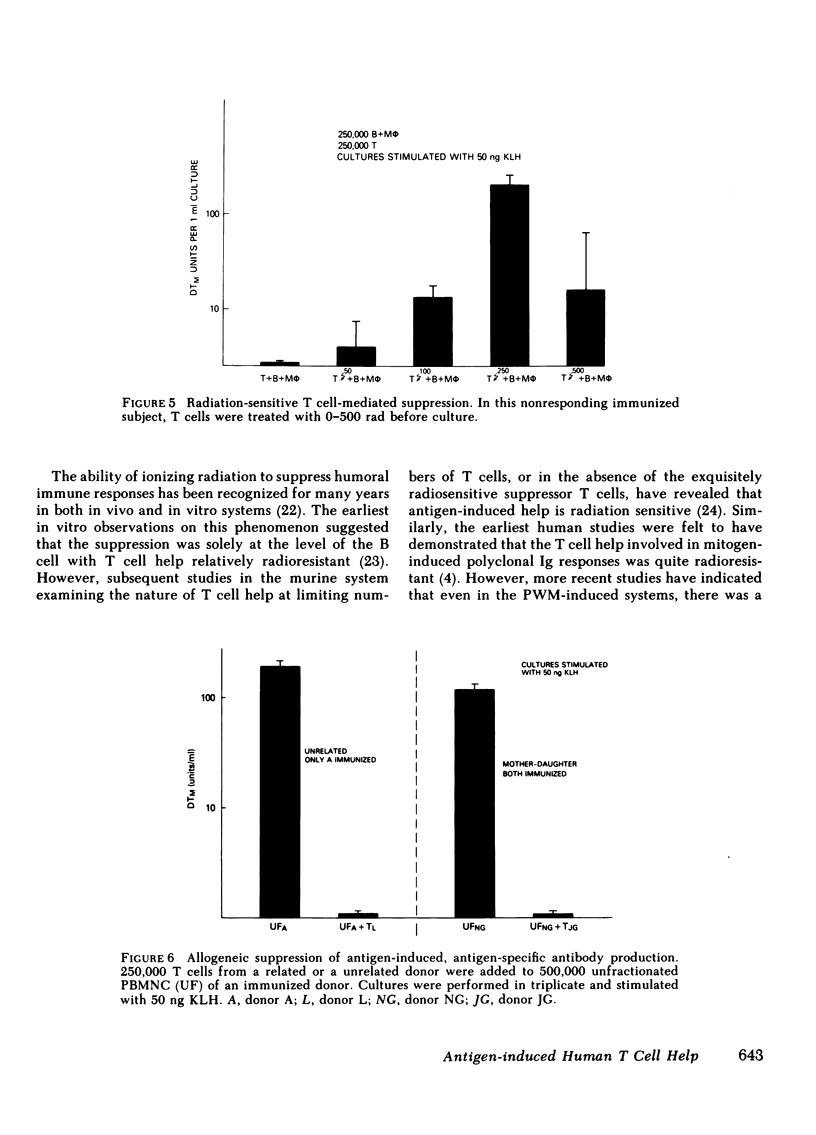
- Agarossi G., Pozzi L., Mancini C., Doria G. Radiosensitivity of the helper cell function. J Immunol. 1978 Nov;121(5):2118–2121. [PubMed] [Google Scholar]
- Anderson R. E., Warner N. L. Ionizing radiation and the immune response. Adv Immunol. 1976;24:215–335. doi: 10.1016/s0065-2776(08)60331-4. [DOI] [PubMed] [Google Scholar]
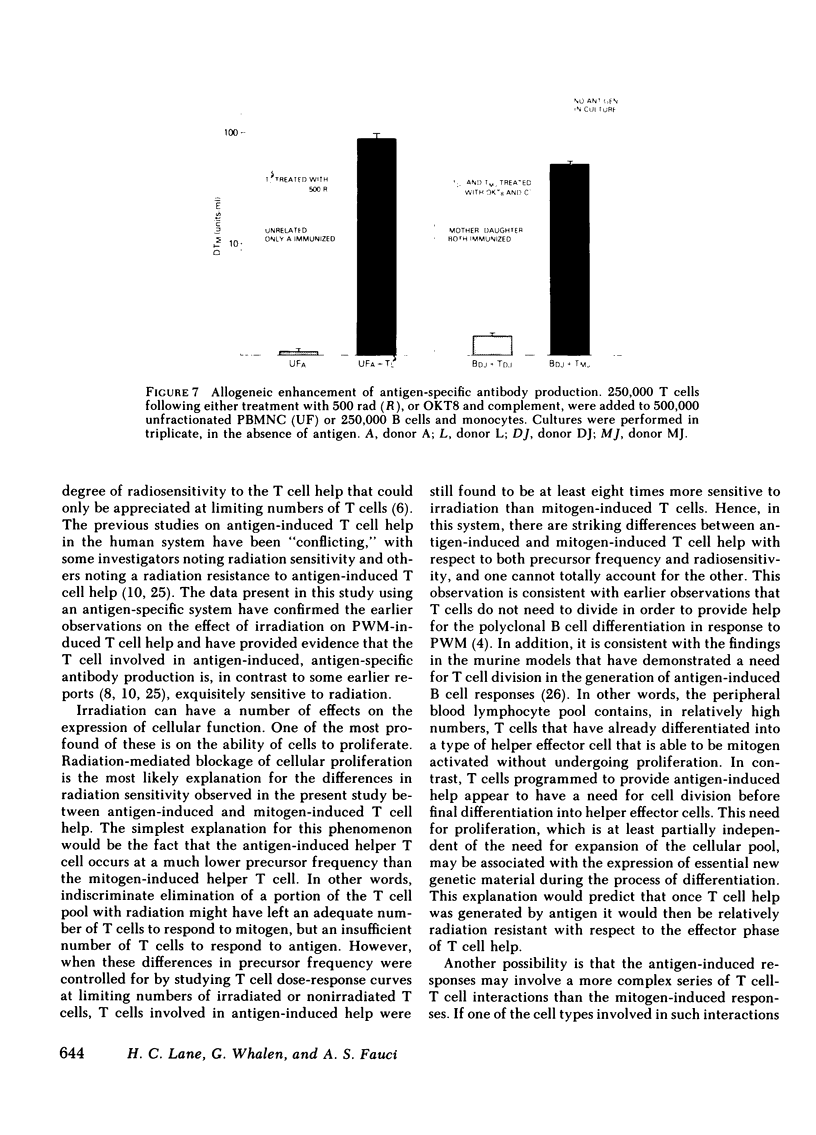
- Brenner M. K., Munro A. J. T-cell help in human in vitro antibody-producing systems: role of inhibitory T cells in masking allogeneic help. Cell Immunol. 1981 Jan 1;57(1):201–208. doi: 10.1016/0008-8749(81)90132-5. [DOI] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Callard R. E., McCaughan G. W., Babbage J., Souhami R. L. Specific in vitro antibody responses by human blood lymphocytes: apparent nonresponsiveness of PBL is due to a lack of recirculating memory B cells. J Immunol. 1982 Jul;129(1):153–156. [PubMed] [Google Scholar]
- Callard R. E., Smith C. M. Histocompatibility requirements for T cell help in specific in vitro antibody responses to influenza virus by human blood lymphocytes. Eur J Immunol. 1981 Mar;11(3):206–212. doi: 10.1002/eji.1830110309. [DOI] [PubMed] [Google Scholar]
- Callard R. E. Specific in vitro antibody response to influenza virus by human blood lymphocytes. Nature. 1979 Dec 13;282(5740):734–736. doi: 10.1038/282734a0. [DOI] [PubMed] [Google Scholar]
- Chiorazzi N., Fu S. M., Kunkel H. G. Induction of polyclonal antibody synthesis by human allogeneic and autologous helper factors. J Exp Med. 1979 Jun 1;149(6):1543–1548. doi: 10.1084/jem.149.6.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosch H. M., Shore A., Gelfand E. W. Regulation of the specific plaque-forming cell response in man: restraint of B cell responsiveness. Eur J Immunol. 1979 Sep;9(9):702–707. doi: 10.1002/eji.1830090908. [DOI] [PubMed] [Google Scholar]
- Engvall E., Perlmann P. Enzyme-linked immunosorbent assay, Elisa. 3. Quantitation of specific antibodies by enzyme-labeled anti-immunoglobulin in antigen-coated tubes. J Immunol. 1972 Jul;109(1):129–135. [PubMed] [Google Scholar]
- Gebel H. M., Scott J. R., Parvin C. A., Rodey G. E. In vitro immunization to KLH. II. Limiting dilution analysis of antigen-reactive cells in primary and secondary culture. J Immunol. 1983 Jan;130(1):29–32. [PubMed] [Google Scholar]
- Heijnen C. J., UytdeHaag F., Gmelig-Meyling F. H., Ballieux R. E. Localization of human antigen-specific helper and suppressor function in distinct T-cell subpopulations. Cell Immunol. 1979 Mar 15;43(2):282–292. doi: 10.1016/0008-8749(79)90173-4. [DOI] [PubMed] [Google Scholar]
- Janossy G., Gomez de la Concha E., Luquetti A., Snajdr M. J., Waxdal M. J., Platts-Mills T. A. T-cell regulation of immunoglobulin synthesis and proliferation in pokeweed (Pa-1)-stimulated human lymphocyte cultures. Scand J Immunol. 1977;6(1-2):109–123. doi: 10.1111/j.1365-3083.1977.tb00326.x. [DOI] [PubMed] [Google Scholar]
- Katz D. H., Osborne D. P., Jr The allogeneic effect in inbred mice. II. Establishment of the cellular interactions required for enhancement of antibody production by the graft-versus-host reaction. J Exp Med. 1972 Sep 1;136(3):455–465. doi: 10.1084/jem.136.3.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keightley R. G., Cooper M. D., Lawton A. R. The T cell dependence of B cell differentiation induced by pokeweed mitogen. J Immunol. 1976 Nov;117(5 Pt 1):1538–1544. [PubMed] [Google Scholar]
- Kettman J., Dutton R. W. Radioresistance of the enhancing effect of cells from carrier-immunized mice in an in vitro primary immune response. Proc Natl Acad Sci U S A. 1971 Apr;68(4):699–703. doi: 10.1073/pnas.68.4.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
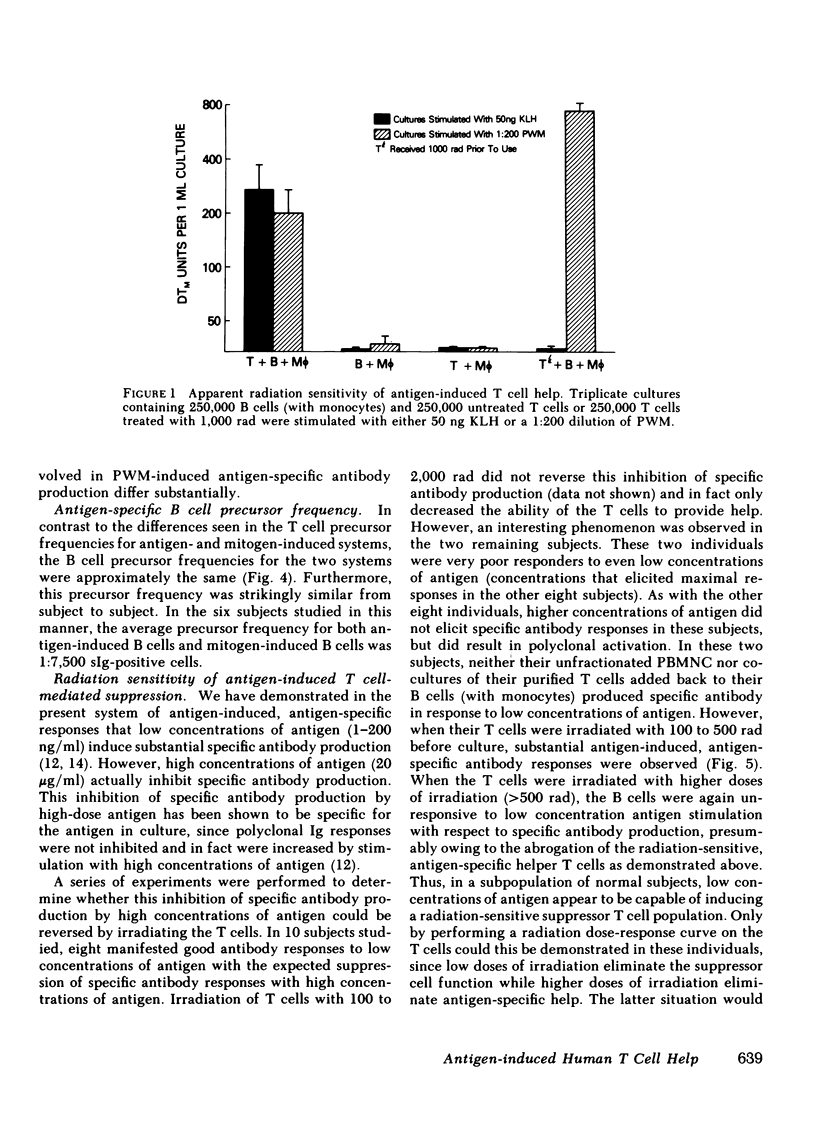
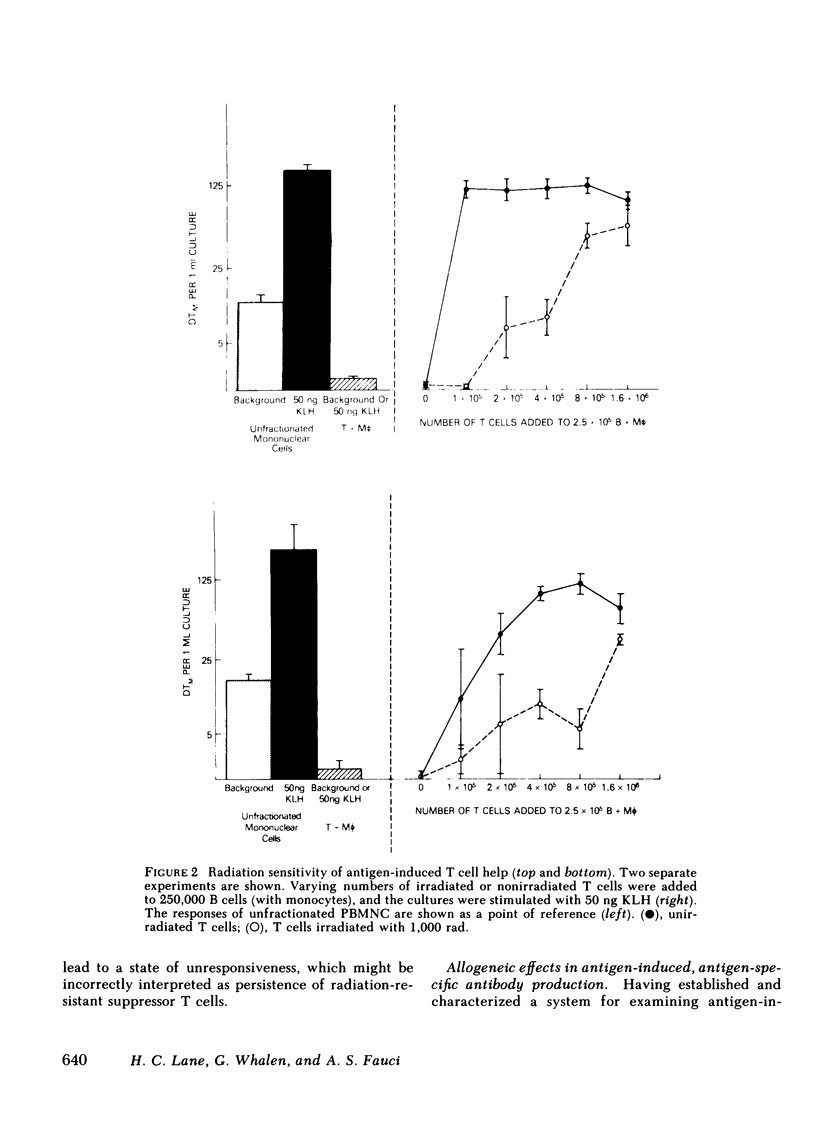
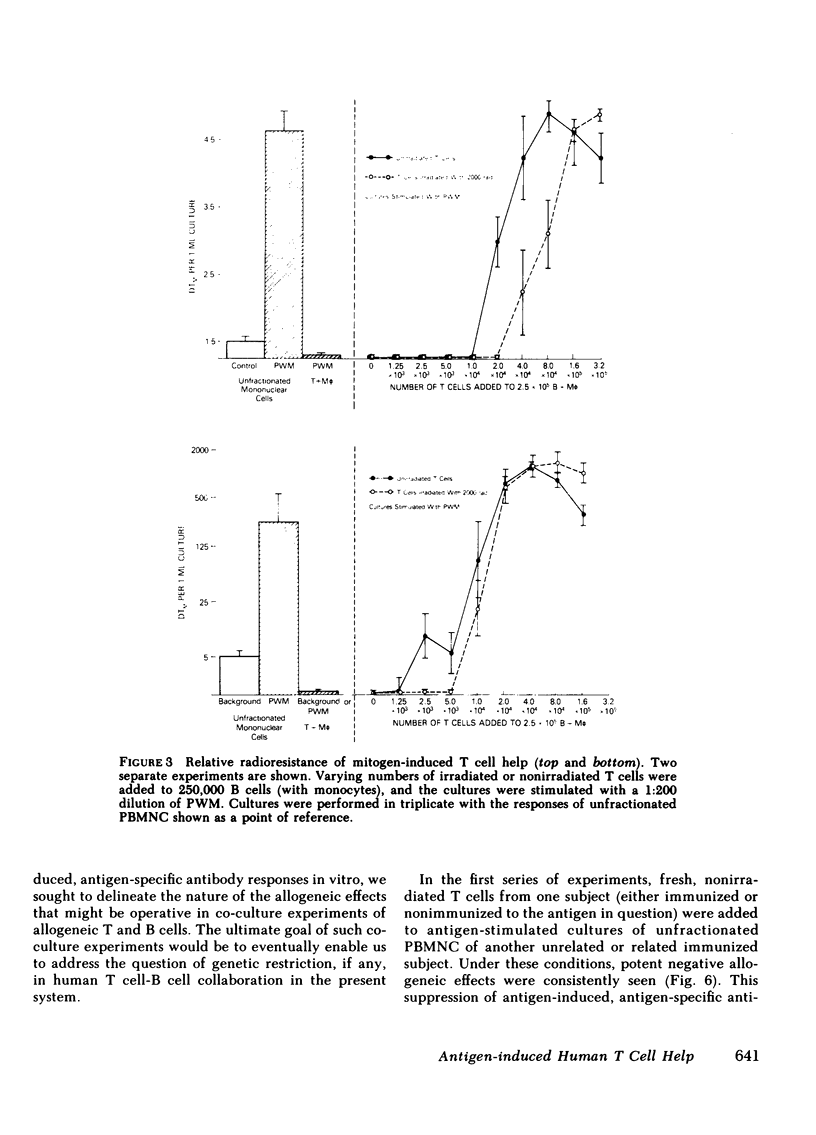
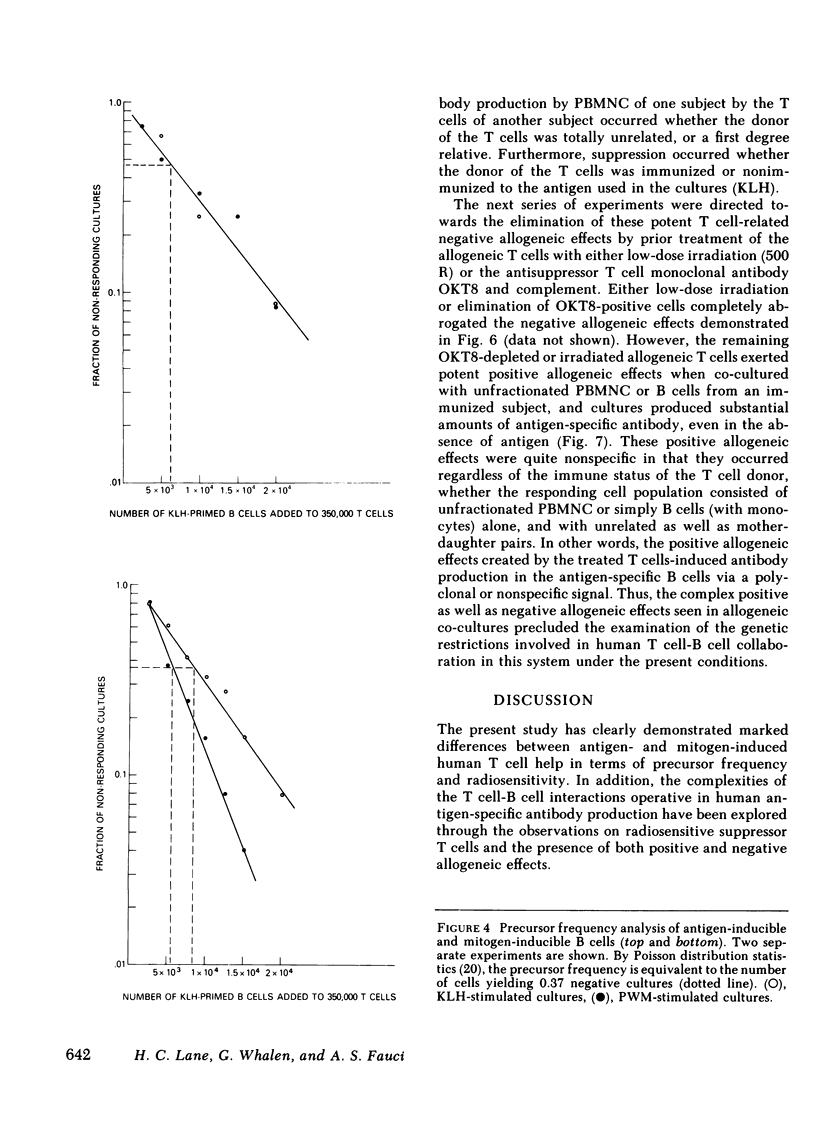
- Lane H. C., Volkman D. J., Whalen G., Fauci A. S. In vitro antigen-induced, antigen-specific antibody production in man. Specific and polyclonal components, kinetics, and cellular requirements. J Exp Med. 1981 Oct 1;154(4):1043–1057. doi: 10.1084/jem.154.4.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MITCHISON N. A. INDUCTION OF IMMUNOLOGICAL PARALYSIS IN TWO ZONES OF DOSAGE. Proc R Soc Lond B Biol Sci. 1964 Dec 15;161:275–292. doi: 10.1098/rspb.1964.0093. [DOI] [PubMed] [Google Scholar]
- Misiti J., Waldmann T. A. In vitro generation of antigen-specific hemolytic plaque-forming cells from human peripheral blood mononuclear cells. J Exp Med. 1981 Oct 1;154(4):1069–1084. doi: 10.1084/jem.154.4.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretta L., Webb S. R., Grossi C. E., Lydyard P. M., Cooper M. D. Functional analysis of two human T-cell subpopulations: help and suppression of B-cell responses by T cells bearing receptors for IgM or IgG. J Exp Med. 1977 Jul 1;146(1):184–200. doi: 10.1084/jem.146.1.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrino M. A., Ferrone S., Dierich M. P., Reisfeld R. A. Enhancement of sheep red blood cell human lymphocyte rosette formation by the sulfhydryl compound 2-amino ethylisothiouronium bromide. Clin Immunol Immunopathol. 1975 Jan;3(3):324–333. doi: 10.1016/0090-1229(75)90019-7. [DOI] [PubMed] [Google Scholar]
- Quintáns J., Lefkovits I. Precursor cells specific to sheep red cells in nude mice. Estimation of frequency in the microculture system. Eur J Immunol. 1973 Jul;3(7):392–397. doi: 10.1002/eji.1830030704. [DOI] [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Goldstein G., Schlossman S. F. Further characterization of the human inducer T cell subset defined by monoclonal antibody. J Immunol. 1979 Dec;123(6):2894–2896. [PubMed] [Google Scholar]
- Siegal F. P., Siegal M. Enhancement by irradiated T cells of human plasma cell production: dissection of helper and suppressor functions in vitro. J Immunol. 1977 Feb;118(2):642–647. [PubMed] [Google Scholar]
- Sredni B., Volkman D., Schwartz R. H., Fauci A. S. Antigen-specific human T-cell clones: development of clones requiring HLA-DR-compatible presenting cells for stimulation in presence of antigen. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1858–1862. doi: 10.1073/pnas.78.3.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain S. L., Dutton R. W. Negative allogeneic effects in vitro. I. Allogeneic T cells markedly suppress the secondary antibody-forming cell response. J Immunol. 1977 Jun;118(6):2262–2268. [PubMed] [Google Scholar]
- Swain S. L., Trefts P. E., Tse H. Y., Dutton R. W. The significance of T-B collaboration across haplotype barriers. Cold Spring Harb Symp Quant Biol. 1977;41(Pt 2):597–609. doi: 10.1101/sqb.1977.041.01.069. [DOI] [PubMed] [Google Scholar]
- Thomas Y., Sosman J., Irigoyen O., Friedman S. M., Kung P. C., Goldstein G., Chess L. Functional analysis of human T cell subsets defined by monoclonal antibodies. I. Collaborative T-T interactions in the immunoregulation of B cell differentiation. J Immunol. 1980 Dec;125(6):2402–2408. [PubMed] [Google Scholar]
- UytdeHaag F., Heynen C. J., Ballieux R. E. Induction of antigen-specific human suppressor T lymphocytes in vitro. Nature. 1978 Feb 9;271(5645):556–557. doi: 10.1038/271556a0. [DOI] [PubMed] [Google Scholar]
- Volkman D. J., Lane H. C., Fauci A. S. Antigen-induced in vitro antibody production in humans: a model for B cell activation and immunoregulation. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2528–2531. doi: 10.1073/pnas.78.4.2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner C., Klouda P. T., Corréa M. C., Vassalli P., Jeannet M. Isolation of B and T lymphocytes by nylon fiber columns. Tissue Antigens. 1977 Apr;9(4):227–229. doi: 10.1111/j.1399-0039.1977.tb01112.x. [DOI] [PubMed] [Google Scholar]
- Winchester R. J., Fu S. M. Lymphocyte surface membrane immunoglobulin. Scand J Immunol. 1976 Jun;Suppl 5:77–82. doi: 10.1111/j.1365-3083.1976.tb03858.x. [DOI] [PubMed] [Google Scholar]
- Yarchoan R., Murphy B. R., Strober W., Schneider H. S., Nelson D. L. Specific anti-influenza virus antibody production in vitro by human peripheral blood mononuclear cells. J Immunol. 1981 Dec;127(6):2588–2594. [PubMed] [Google Scholar]





