Abstract
Diabetes mellitus (DM) and Alzheimer's disease (AD) are two major health concerns that have seen a rising prevalence worldwide. Recent studies have indicated a possible link between DM and an increased risk of developing AD. Insulin, while primarily known for its role in regulating blood sugar, also plays a vital role in protecting brain functions. Insulin resistance (IR), especially prevalent in type 2 diabetes, is believed to play a significant role in AD's development. When insulin signalling becomes dysfunctional, it can negatively affect various brain functions, making individuals more susceptible to AD's defining features, such as the buildup of beta-amyloid plaques and tau protein tangles. Emerging research suggests that addressing insulin-related issues might help reduce or even reverse the brain changes linked to AD. This review aims to explore the rela-tionship between DM and AD, with a focus on the role of IR. It also explores the molecular mechanisms by which IR might lead to brain changes and assesses current treatments that target IR. Understanding IR's role in the connection between DM and AD offers new possibilities for treatments and highlights the importance of continued research in this interdisciplinary field.
Keywords: Alzheimer's disease, Insulin resistance, Obesity, Dementia, Diabetes, Metabolic syndrome
Core Tip: Insulin resistance (IR), commonly associated with type 2 diabetes, is a crucial factor linking diabetes mellitus to Alzheimer's disease (AD). While insulin is primarily known for regulating blood sugar, it also plays a significant role in brain health. Dysfunctional insulin signaling, characteristic of IR, adversely impacts brain functions and is implicated in the development of AD's defining features, such as beta-amyloid plaques and tau protein tangles. Understanding and addressing IR early could offer new treatment strategies for AD, highlighting the importance of continued research in this interdisciplinary field.
INTRODUCTION
The interplay between metabolic and neurodegenerative disorders has emerged as a focal point of scientific inquiry, highlighting the complex relationship between insulin resistance (IR) and Alzheimer's disease (AD)[1-3]. While Type 2 diabetes mellitus (T2DM) stands as a global concern predominantly characterized by IR, the scope of research extends beyond T2DM to elucidate IR's broader impact on neurological health[4]. Notably, AD, the foremost cause of dementia, exhibits a profound connection with IR, suggesting a complex metabolic-neurodegenerative link[5,6].
Globally, the prevalence of both T2DM and AD is on an upward trajectory, presenting significant public health challenges. In 2022, France reported nearly 1.65 million individuals with mild cognitive impairment due to AD, with over 925000 cases evolving into clinical AD dementia[7]. Similarly, the United States has demonstrated significant racial and ethnic disparities in AD prevalence, with notably higher rates observed among non-Hispanic Blacks and Hispanics[8]. Regions such as Maryland, New York, and Mississippi noted particularly high AD prevalence[9]. Simultaneously, the global diabetic population, driven by factors such as obesity, reached approximately 529 million in 2021, with projections indicating a rise to over 1.31 billion by 2050[10]. Importantly, the prevalence of dementia, including AD, among individuals with T2DM in Spain increased significantly over a decade, highlighting a notable intersection between these diseases[11]. This convergence accentuates the imperative for early detection and management of IR to possibly attenuate AD progression and highlights the necessity for multidisciplinary research aimed at unveiling novel therapeutic strategies and deciphering the intricate metabolic-neurodegenerative interplay.
AD is classified into two types: Early-onset, which has a genetic basis and typically manifests in the forties or fifties, and late-onset, appearing after the age of 65[12]. The traditional understanding of AD, focused on the accumulation of beta-amyloid (Aβ) plaques and hyperphosphorylated tau protein tangles, is evolving[13-16]. Current insights reveal insulin's broader neuroprotective role, suggesting that its dysregulation contributes significantly to neuronal damage and cognitive decline, key features of AD[17-23]. This revelation has led to the conceptualization of AD as "type 3 diabetes", a term that highlights impaired insulin signaling within the brain as a pivotal aspect of AD's pathology, drawing parallels with the systemic IR observed in T2DM[24-26].
Epidemiological, clinical, neuroimaging, and post-mortem studies collectively affirm the correlation between IR and an elevated risk of AD, highlighting shared pathophysiological pathways that contribute to neuronal dysfunction and cognitive impairment[6,27-29]. This body of evidence, demonstrating reduced insulin receptor density, altered phos-phorylation of insulin receptor substrates, and decreased insulin activity in brain regions critical for memory and cognition, supports the notion that brain IR might actively contribute to AD's development rather than merely result from it[30-33].
Despite progress in understanding the mechanisms linking IR and AD, including the role of IR in disrupting glucose metabolism and exacerbating oxidative stress and neuroinflammation within the central nervous system (CNS), significant gaps remain[27-29]. The exacerbation of neuroinflammation and oxidative stress by peripheral IR—affecting both metabolic dysfunction and neural health[34-39], highlights the need for targeted therapies that can modify AD's progression by addressing IR specifically.
This bi-directional influence mandates a comprehensive treatment approach, addressing both systemic IR and its neurological manifestations. Such dual-pathway exploration not only enriches our understanding of the disease mechanism but also unveils new therapeutic targets for AD, fostering optimism in a field that has faced considerable challenges in achieving treatment breakthroughs.
INSULIN'S ROLE IN BRAIN HEALTH AND FUNCTION
Insulin, a peptide hormone synthesized by pancreatic β-cells, plays a crucial role in regulating blood glucose levels by facilitating glucose uptake in muscle and adipose tissues and inhibiting hepatic glucose production, thereby maintaining euglycemia[40]. This hormonal regulation is critical for energy balance and metabolic stability, with dysregulation leading to diabetes mellitus, characterised by hyperglycemia and associated metabolic complications[41]. The rising global incidence of T2DM, exacerbated by increasing obesity rates and sedentary lifestyles, underscores the pressing challenge of IR[41,42].
Beyond its well-known role in peripheral glucose metabolism, insulin also exerts significant effects within the CNS, influencing neuronal growth, synaptic plasticity, and neurotransmitter regulation, key processes for cognitive functions[6,43-46]. These actions are mediated via insulin receptors distributed across the brain, particularly in regions integral to memory and cognition such as the hippocampus and cerebral cortex[47,48]. Disruption in insulin signaling is linked to neurodegenerative diseases like AD, contributing to pathological outcomes including impaired glucose metabolism, oxidative stress, and altered lipid metabolism[38,49,50].
At the molecular level, insulin activates pathways such as phosphoinositide 3-kinase (PI3K)/Akt and glycogen synthase kinase-3β (GSK-3β) within the brain, influencing cell survival, tau phosphorylation, and amyloid-beta production, central elements in AD pathology[6,51-53]. Furthermore, insulin regulates neurotransmitters’ release, including acetylcholine, dopamine, and serotonin[54,55], with IR in the brain leading to cognitive decline and mood disorders[56]. Additionally, insulin supports neurogenesis and brain plasticity, which are compromised under IR conditions, raising the risk of neurodegenerative diseases[23,38,45].
Insulin's source in the brain involves transport from peripheral circulation across the blood-brain barrier (BBB) and potentially local production within the brain itself[57,58], This transport occurs via the choroid plexus into the cerebrospinal fluid (CSF)[59] and directly from plasma into the brain's endothelial cells[60], with insulin concentrations in the CSF being notably lower than in plasma[61,62], a difference exacerbated in obesity[63]. This mechanism may involve insulin receptors or megalin, a transporter associated with insulin and leptin transport[64]. Intranasal insulin delivery has emerged as a promising method for bypassing slower transport mechanisms, directly enhancing CSF insulin levels without affecting plasma concentrations and showing potential for cognitive enhancement in AD[65].
Local insulin synthesis within the brain has been a contentious topic. Early studies proposed high brain insulin levels compared to plasma[48], but later research challenged these findings[66], leading to debates about the brain's insulin production capabilities. Despite challenges in differentiating between pancreatic and brain-sourced insulin due to identical epitopes recognized by anti-insulin antibodies[67], evidence supports brain insulin synthesis, as indicated by the localization of C-peptide and proinsulin-like immunoreactivity in the CNS and gene expression analyses[68] which further validate local brain insulin production, especially in areas like the hippocampus, suggesting a link between neuronal insulin production and local metabolic demands[69,70].
Furthermore, insulin's role within the CNS extends to modulating peripheral metabolic functions, including indirect regulation of hepatic glucose production via neuronal pathways, a phenomenon documented in rodent models but debated in human studies[71-75]. Intranasal insulin administration in humans provides evidence of the brain's capability to sense insulin and influence hepatic glucose production, although the physiological relevance of this interaction requires further clarification[76,77].
Furthermore, insulin exerts effects on lipolysis and lipogenesis, influencing the body's lipid storage and utilization processes[78]. Additionally, insulin within the CNS is instrumental in managing reproductive health, overseeing the hormonal regulation critical for fertility in both sexes[79]. A significant aspect of insulin's central function includes mediating the counterregulatory response to hypoglycemia, whereby insulin enhances the brain's capacity to detect low glucose levels and trigger necessary physiological responses to restore euglycemia[80].
Insulin's central effects on lipid metabolism, reproductive health, and the counterregulatory response to hypoglycemia further illuminate its comprehensive roles beyond glucose regulation, emphasizing its critical contribution to metabolic homeostasis and underscoring the necessity for continued research into insulin's multifaceted roles in health and disease.
Recent research challenges the traditional belief that the brain's glucose uptake is independent of insulin, suggesting instead that insulin may influence glucose transporters and metabolism within the CNS, particularly under conditions of IR. This significant revelation implies that peripheral IR could detrimentally affect brain functionality and health, potentially accelerating the progression of neurodegenerative diseases[81,82]. However, the impact of peripheral insulin on brain insulin levels and activity remains a subject of debate, due to the selective permeability of the BBB and unique insulin sensitivity regulation mechanisms within the brain. This ongoing discussion highlights the necessity for further research into the complex role of insulin in the CNS and its links to neurodegenerative disorders[83-85].
IR AND AD
The complex relationship between IR and AD is increasingly recognized through a blend of epidemiological, molecular, and clinical research. These studies collectively suggest that IR significantly contributes to cognitive impairments and AD's hallmark pathologies. Impaired insulin signaling, evident even in the early stages of AD absent of diabetes, may serve as a potential biomarker for AD, underscoring IR's pivotal role in the disease's development[27,30,32,33,53].
Dysfunctional insulin signaling in the brain is detrimental, exacerbating oxidative stress and inflammation, fostering an environment conducive to the production of neurotoxic species. This cascade of metabolic dysfunction further precipitates neuronal damage and cognitive decline. One of the most compelling connections between IR and AD is the impact of insulin dysregulation on the accumulation of Aβ plaques and tau protein tangles, the defining pathological features of AD. Insulin has been shown to regulate enzymes which are involved in the production and clearance of Aβ[32,86]. Dysfunctional insulin signaling can lead to an imbalance in these enzyme activities, resulting in increased production or decreased clearance of Aβ, contributing to plaque formation[87,88]. Concurrently, altered insulin pathways contribute to the hyperphosphorylation of tau proteins, a process that results in the formation of neurofibrillary tangles, another hallmark of AD. This hyperphosphorylation impairs tau's ability to stabilize microtubules, essential for neuron structure and function, further contributing to neurodegeneration[89-91].
MOLECULAR MECHANISMS LINKING IR TO AD
The intricate relationship between IR and AD can be elucidated through an in-depth examination of the molecular pathways affected by IR and their contribution to AD pathogenesis. This section explores the underlying molecular mechanisms that bridge IR to AD, highlighting the critical pathways disrupted by IR and analyzing how these disruptions contribute to the development of AD.
IR, fundamentally, is characterized by the impaired signaling of insulin through its primary pathways. The canonical insulin signaling pathway involves the binding of insulin to its receptor, activating the receptor's tyrosine kinase activity. This activation leads to the phosphorylation of insulin receptor substrates, which in turn triggers downstream signaling cascades involving PI3K and Akt (protein kinase B). These cascades play critical roles in glucose uptake, glycogen synthesis, lipid metabolism, and protein synthesis. In the context of IR, there is a disruption in these signaling pathways, leading to reduced glucose uptake, altered lipid metabolism, and impaired cell survival and growth mechanisms.
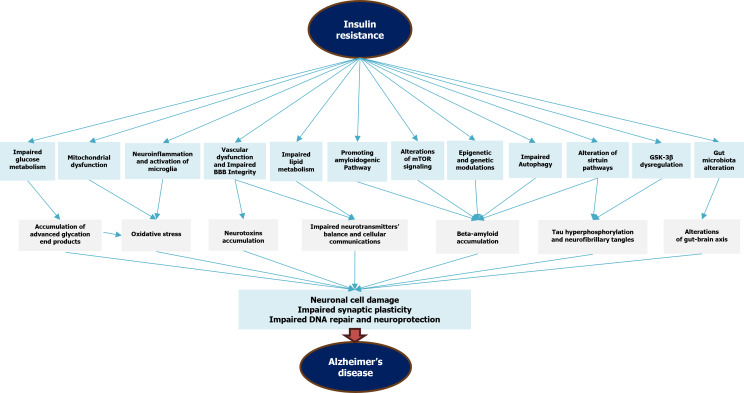
In the brain, these pathways are crucial for neuronal health, synaptic plasticity, and cognitive function. IR-induced impairments in these pathways have been associated with reduced neuronal survival, altered synaptic transmission, and impaired cognitive function, all of which are characteristic of AD. Figure 1 graphically represents the intricate molecular mechanisms linking IR and AD.
Figure 1.
Simplified mechanisms of insulin resistance-induced Alzheimer’s disease. BBB: Blood-brain barrier; GSK-3B: Glycogen synthase kinase-3β; mTOR: Mechanistic target of rapamycin pathway.
Glucose metabolism disruption
One of the key aspects of IR's contribution to AD is through the dysregulation of glucose metabolism in the brain. The brain relies heavily on glucose as its primary energy source, and IR impairs the brain's ability to utilize glucose efficiently, leading to an energy deficit in neurons[92]. This scenario is nuanced by findings from euglycemic hyperinsulinemic conditions, where enhanced brain glucose uptake is observed in insulin-resistant individuals, suggesting an acute compensatory response aimed at maintaining energy supply despite peripheral IR[93]. However, this increased brain glucose uptake does not mitigate the chronic metabolic disturbances caused by IR, including the significant energy deficit that culminates in reduced ATP production. The consequent escalation in reactive oxygen species (ROS) production induces oxidative stress, a condition that damages DNA, proteins, and lipids, and may trigger apoptotic pathways. Such oxidative stress and neuronal damage are critical factors in the pathogenesis of AD[94-97].
Aβ accumulation and tau hyperphosphorylation
IR influences the metabolism of the amyloid precursor protein (APP), promoting the amyloidogenic pathway[98]. This pathway involves the sequential cleavage of APP by beta-secretase (BACE1) and gamma-secretase, resulting in the production of Aβ peptides. In IR, the activity of BACE1 is increased, leading to an accumulation of Aβ peptides which aggregate to form amyloid plaques[98,99]. Furthermore, insulin regulates enzymes like GSK-3β, a key kinase involved in tau phosphorylation. In the setting of IR, the activity of GSK-3β is dysregulated, leading to the hyperphosphorylation of tau proteins and subsequent formation of neurofibrillary tangles[23,30,53].
Neuroinflammation and microglial activation
The chronic inflammation characteristic of IR significantly influences AD development[100]. Elevated pro-inflammatory cytokine levels, such as Tumour necrosis factor alpha and Interleukin 6, exacerbate neuronal damage[101,102]. Activation of microglia, the brain's innate immune cells, is observed in IR. These activated microglia can release pro-inflammatory cytokines, which may promote neurodegeneration[103-105]. Chronic inflammation driven by IR may facilitate the progression of AD by sustaining a harmful cycle of neuronal damage and inflammatory response. This process can induce the expression of cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase, leading to further neuronal damage and contributing to the pathogenesis of AD[106-110].
Research into AD and IR has unveiled a myriad of interconnected pathways that extend beyond the core mechanisms of glucose metabolism disruption, mitochondrial dysfunction, and inflammation. These additional mechanisms not only deepen our understanding of the intricate relationship between metabolic dysfunctions and neurodegeneration but also open new avenues for therapeutic interventions.
Autophagy impairment and protein aggregation
Insulin signaling is involved in the regulation of autophagy, a cellular process essential for clearing misfolded proteins and damaged organelles. IR can impair autophagy in neurons, leading to the accumulation of toxic proteins, including Aβ and phosphorylated tau. This impairment contributes to the aggregation of these proteins and the formation of the characteristic plaques and tangles in AD[111-113].
Mitochondrial dysfunction and endoplasmic reticulum stress
IR precipitates a cascade of cellular stress responses in the brain, critically undermining neuronal health and exacerbating AD pathology through mitochondrial dysfunction and endoplasmic reticulum (ER) stress. Mitochondrial dysfunction manifests as diminished energy production and escalated oxidative stress, leading to a depletion of neuronal ATP and an increase in ROS. This mitochondrial impairment results in energy shortages, cellular damage, and ultimately, neuronal death, contributing to the neurodegenerative processes observed in AD[114]. Concurrently, IR triggers ER stress within neurons, activating the unfolded protein response (UPR) pathways, including IRE1, ATF6, and PERK[115,116]. Persistent ER stress and UPR activation exacerbate the accumulation of misfolded proteins, a hallmark of AD, driving neuronal degeneration[117]. The interplay between mitochondrial dysfunction and ER stress, fueled by IR, underscores a multifaceted mechanism contributing to the neuronal vulnerability and cognitive decline characteristic of AD, high-lighting the intricate relationship between metabolic dysfunction and neurodegeneration.
Lipid metabolism alterations
IR is associated with dysregulated lipid metabolism, leading to altered lipid profiles in the brain. These changes can affect the composition and fluidity of neuronal membranes, impacting neurotransmitter receptor function and synaptic plasticity. Altered lipid profiles can also influence the processing of APP, potentially increasing the production of Aβ[118-120].
Neurovascular dysfunction and impaired BBB integrity
IR contributes to neurovascular dysfunction, compromising the BBB integrity and cerebral blood flow regulation. This dysfunction can facilitate the entry of neurotoxic substances into the brain parenchyma and impair the clearance of Aβ, further contributing to AD pathology. The vascular impairment can contribute to the cerebral hypoperfusion and hypoxia observed in AD, exacerbating neuronal damage and cognitive decline[121-125]. Additionally, IR is associated with the degradation of tight junction proteins, such as claudins and occludins, essential for maintaining the BBB's selective permeability. Consequently, the BBB becomes more permeable, facilitating the entry of peripheral immune cells into the brain and the buildup of neurotoxic elements, including Aβ. These changes contribute significantly to AD's pathology by promoting inflammation, neuronal damage, and further accumulation of pathological proteins[126-128]. Addressing the vascular components and enhancing BBB integrity could play a pivotal role in slowing or preventing the progression of AD, highlighting the importance of targeting metabolic and vascular dysfunctions in comprehensive AD management strategies.
Neurotransmitter imbalance and alterations in neurotrophic factor signaling
IR significantly impacts neurotransmitter balance in the brain, contributing to the cognitive deficits observed in AD. IR affects key neurotransmitters like acetylcholine, dopamine, and serotonin, essential for memory, mood, and cognitive function[129-131]. This imbalance leads to a disruption in the equilibrium between excitatory and inhibitory neurotransmissions, particularly impacting the cholinergic system and exacerbating memory loss[132,133]. Furthermore, IR-induced chronic inflammation and alterations in pathways of neurotrophic factors, notably brain-derived neurotrophic factor (BDNF), further destabilize neurotransmitter systems. BDNF plays a pivotal role in neuronal survival, growth, and synaptic plasticity. IR disrupts BDNF signaling, notably through the TrkB receptor pathway, precipitating synaptic dysfunction, and neuronal loss[134-135]. The reduction in BDNF activity correlates with the cognitive decline observed in AD, emphasizing the intertwined roles of metabolic dysfunction, neurotransmitter imbalance, and neurotrophic factor signaling disruption in the disease's neuropathology. Addressing both metabolic dysfunction and neurotransmitter imbalance through therapeutic strategies could therefore be crucial in mitigating AD's progression, highlighting the role of IR in the disease's neuropathology[136].
Epigenetic and genetic modulations
Emerging evidence suggests a strong link between IR and epigenetic alterations that may contribute to AD's progression. Epigenetic modifications, including DNA methylation and histone modification, are key regulators of gene expression without altering the DNA sequence itself. These changes can profoundly affect neuronal function and are implicated in the regulation of genes associated with AD pathology[137].
DNA methylation, an epigenetic mechanism involving the addition of a methyl group to the DNA molecule, can influence the expression of genes critical for neural function and the pathological processes underlying AD[137,138]. Studies have shown that abnormal DNA methylation patterns are associated with the dysregulation of APP and tau protein genes, leading to increased Aβ production and tau hyperphosphorylation, hallmark features of AD[138-140]. For instance, hypermethylation of the promoter region of the APP gene has been linked to its increased expression and subsequent amyloid-beta accumulation[141,142].
Histone modifications, another form of epigenetic regulation, involve the chemical alteration of histone proteins around which DNA is wrapped, influencing chromatin structure and gene accessibility. Alterations in histone acetylation and methylation have been observed in AD, affecting genes involved in synaptic plasticity, neuronal survival, and inflammatory responses[143,144]. These modifications can exacerbate or mitigate AD pathology by regulating the transcriptional activity of genes implicated in Aβ deposition and tau pathology. Furthermore, genetic factors, such as polymorphisms in the apolipoprotein E (APOE) gene, have been well-documented to affect the risk of developing AD[145]. The APOE E4 allele is the strongest genetic risk factor for sporadic AD, influencing Aβ aggregation and clearance. Research suggests that individuals with IR carrying the APOE E4 allele have an increased risk of cognitive decline and AD, potentially due to synergistic effects on lipid metabolism, Aβ accumulation, and brain insulin signaling[146-148].
Insulin-derived amyloidosis
Recent studies have highlighted a novel aspect of insulin's behavior, showing that it can aggregate into amyloid-like fibrils under specific conditions, such as changes in pH, temperature, and ionic strength, which are reminiscent of the environment found in IR[149,150]. This phenomenon, primarily observed in vitro, suggests a potential link between elevated insulin levels associated with IR and the formation of amyloid fibrils in the brain, akin to those seen in AD[151]. The structural similarities between insulin-derived amyloids and Aβ plaques highlight a possible mechanistic connection between metabolic dysfunctions, such as IR, and neurodegenerative processes. This emerging insight not only enriches the understanding of the relationship between IR and AD but also proposes new therapeutic targets for AD, focusing on preventing or disrupting the amyloidogenic potential of insulin as a means to address neurodegeneration in patients with metabolic disorders[152-154].
Hormonal dysregulation
IR significantly affects the neuroprotective hormonal axis, particularly leptin and ghrelin, potentially accelerating AD's pathogenesis[155]. Leptin, beyond its critical role in energy regulation and appetite suppression, exerts significant neuroprotective effects. It fosters neuronal growth, synaptic plasticity, and protects neurons from apoptotic triggers. Leptin resistance, induced by IR, impairs neuroprotection, affecting memory and learning, further contributing to the pathophysiology of AD[156-160]. Similarly, ghrelin, often known as the "hunger hormone," has been recognized for its roles beyond appetite stimulation, including the promotion of neuronal survival, enhancement of neurogenesis, and facilitation of synaptic plasticity. Ghrelin's neuroprotective effects are particularly pronounced in the hippocampus, a brain region pivotal for memory formation and one of the first regions to suffer damage in the course of AD[161-163]. IR-associated alterations in ghrelin levels and signaling can disrupt these beneficial processes, leading to impaired cognitive functions and increased risk of neurodegeneration. This hormonal imbalance due to IR contributes to cognitive decline and AD pathology, highlighting the need for therapeutic strategies targeting these hormonal pathways to mitigate AD progression in individuals with metabolic disorders[162,164,165].
Impaired cellular communication
IR disrupts critical brain signaling pathways, particularly the Wnt/β-catenin pathway, which is essential for neurodevelopment and synaptic plasticity. This disruption affects the communication between neurons and glial cells, including astrocytes and oligodendrocytes, pivotal for supporting neuronal function and integrity. The consequences of disrupted Wnt/β-catenin signaling extend beyond synaptic plasticity, affecting various aspects of brain function and health by altering gene expression, neuronal connectivity, and the brain's ability to respond to neural damage, potentially contributing to cognitive dysfunction and the pathogenesis of neurodegenerative diseases such as AD[166-168]. The debate continues on how directly IR-induced signaling disruptions contribute to neurodegeneration and whether these effects are reversible through interventions that improve insulin sensitivity[169-170]. Ongoing research aims to unravel the complexities of cellular signaling affected by IR and explore therapeutic strategies to restore brain function and mitigate neurodegenerative processes[169,170,171]. Understanding the role of IR in impaired cellular communication within the brain is crucial for developing targeted treatments for AD and related conditions[172].
Altered ion homeostasis and neuronal excitability
Ion channels and transporters play critical roles in maintaining the electrochemical gradients essential for neuron firing, signal transduction, and synaptic activity. IR, possibly disrupts the regulatory mechanisms governing these ion channels and transporters, leading to altered neuronal excitability and impaired signaling[173,174].
One of the most significant impacts of IR on ion homeostasis is observed in the regulation of calcium ions (Ca2+). Calcium plays a pivotal role in numerous neuronal processes, including neurotransmitter release, synaptic plasticity, and activation of intracellular signaling pathways. Disruption in calcium homeostasis due to IR can lead to an imbalance in intracellular Ca2+ levels, potentially triggering synaptic dysfunction and promoting neuronal death. These disturbances in calcium signaling are critical contributors to the pathophysiology of AD, as they can exacerbate the neurodegenerative processes characteristic of the disease[175].
Furthermore, altered ion homeostasis in the context of IR can influence the activity of other essential ions, such as sodium (Na+) and potassium (K+), further complicating neuronal excitability and signaling. The dysregulation of these ion channels and transporters contributes to a cascade of neural dysfunctions, laying the groundwork for synaptic loss, neuronal death, and cognitive decline observed in AD[176,177].
Advanced glycation end products and receptor for advanced glycation end products activation
The interplay between IR and the accumulation of advanced glycation end products (AGEs) presents a significant potential pathway contributing to the pathogenesis of AD. AGEs are complex molecules formed through the non-enzymatic glycation of proteins, lipids, and nucleic acids. IR exacerbates the formation of AGEs due to persistent hyperglycemia and altered metabolic states, leading to an accumulation of these harmful compounds in various tissues, including the brain[178-180].
AGEs exert their detrimental effects primarily through interaction with the receptor for AGEs (RAGE) expressed on neuronal cells and other brain cells, such as microglia and astrocytes[181,182]. The binding of AGEs to RAGE triggers a cascade of downstream signaling pathways that promote oxidative stress and inflammation, two critical processes implicated in the neurodegenerative mechanisms of AD. The oxidative stress induced by this interaction contributes to neuronal damage and death, while the inflammatory response exacerbates the pathological environment within the AD brain[183,184].
Alteration of sirtuin pathways
Sirtuins, a family of NAD+-dependent deacetylases, play a pivotal role in cellular metabolism, stress resistance, and longevity. Among them, sirtuin 1 (SIRT1) is of particular interest due to its extensive involvement in metabolic regulation, DNA repair, and neuroprotection. Research has demonstrated that SIRT1 exerts a protective effect against neurodegeneration, promoting neuronal survival, enhancing DNA repair mechanisms, and modulating inflammatory responses in the brain[185,186].
In the context of IR, the activity of SIRT1 and other sirtuins can be significantly impacted. IR, characterized by a diminished response to insulin signaling, leads to metabolic disturbances not only in peripheral tissues but also within the CNS. These disturbances can alter the NAD+/NADH ratio, a critical cofactor for sirtuin activity, thereby affecting the functional capacity of SIRT1 and its neuroprotective effects[187,188].
The potential role of sirtuins, especially SIRT1, in AD stems from their ability to modulate several pathways implicated in the disease's pathogenesis. SIRT1 can influence amyloid-beta metabolism, tau protein phosphorylation, and cellular stress responses, all of which are key factors in AD development. By deacetylating transcription factors and other proteins, SIRT1 can suppress the expression of genes involved in amyloid-beta production and promote pathways that enhance neuronal survival and plasticity[187].
However, the exact mechanisms through which IR affects SIRT1 activity in the brain and its implications for AD remain areas of active research. Some studies suggest that enhancing SIRT1 activity could offer a therapeutic strategy to mitigate the effects of IR on neuronal health and slow AD progression[186,187]. Conversely, the multifaceted roles of SIRT1 in different cellular contexts highlight the complexity of targeting this pathway for disease intervention. The challenge lies in elucidating the specific conditions under which SIRT1 activation or inhibition could be beneficial in the context of AD and IR.
Alterations of mechanistic target of rapamycin signaling
The mechanistic target of rapamycin (mTOR) pathway, critical for cellular metabolism and growth, intersects significantly with IR and AD. IR disrupts insulin signaling, leading to mTOR dysregulation, which is implicated in AD pathogenesis through the accumulation of amyloid-beta and hyperphosphorylated tau proteins[189,190]. This dysregulation hampers autophagy, essential for clearing these neurotoxic proteins[191,192]. Conversely, targeted mTOR inhibition has shown potential in reducing AD markers in experimental models, suggesting modulation of this pathway as a therapeutic strategy[193,194]. However, the complexity of mTOR's role in integrating various cellular signals necessitates nuanced approaches to leverage its therapeutic potential without disrupting essential cellular functions. Understanding the intricate relationship between mTOR signaling, metabolic dysfunction, and neurodegeneration highlights a promising avenue for AD research and treatment development.
Gut-brain axis
The gut-brain axis plays a crucial role in linking IR with AD through complex interactions involving the gut microbiome and neuroinflammatory processes[195,196]. Alterations in the gut microbiota due to IR can increase intestinal per-meability, leading to systemic inflammation and exacerbating neuroinflammation, which contributes to AD pathology[197,198]. The gut microbiota also influences the production of neuroactive substances like short-chain fatty acids, which have anti-inflammatory properties and support the BBB's integrity[199,200]. Moreover, gut-derived metabolites can affect neurotransmitter production in the brain, impacting mood and cognitive functions. This evidence suggests the gut-brain axis as a potential target for AD therapeutic strategies, focusing on dietary interventions and probiotics to restore gut health, reduce inflammation, and slow AD progression[201,202]. Further research is needed to fully understand these interactions and their implications for AD treatment.
Lipid rafts and cell signaling
IR may disrupt the composition of lipid rafts in neuronal membranes, impacting the organization and function of essential signaling molecules. This disruption affects various cellular pathways crucial for neuronal health, synaptic function, and the processing of APP, potentially leading to increased Aβ accumulation, a key feature of AD. Furthermore, altered lipid raft integrity may impair receptor-mediated signaling, including insulin and neurotransmitter receptors, contributing to synaptic dysfunction and cognitive decline observed in AD. Addressing the changes in lipid raft composition due to IR could offer new therapeutic avenues for mitigating AD progression[203-207].
It's essential to note that while these mechanisms provide a deeper understanding of the potential links between IR and AD, the exact contribution and interplay of each mechanism in the pathophysiology of AD remain areas of active research. Further studies are needed to elucidate these relationships fully and to develop effective therapeutic strategies targeting these mechanisms.
CURRENT THERAPEUTIC APPROACHES TARGETING IR
Effective management of IR is crucial in addressing not only metabolic disorders but also neurodegenerative diseases like AD. This section synthesizes current treatment modalities for IR, examines their potential impact on AD progression, and assesses their effectiveness in mitigating AD symptoms, integrating recent data and research findings.
Current therapeutic landscape for IR
The management of IR employs a multidimensional strategy focused on enhancing insulin sensitivity and addressing metabolic imbalances. Fundamental to this approach are lifestyle modifications, where dietary optimization aimed at reducing refined sugars and increasing fiber intake, combined with regular physical activity, has been proven to significantly improve insulin sensitivity. Such modifications are pivotal in IR management, highlighting their importance in promoting metabolic health[208-210]. Pharmacologically, metformin stands as a cornerstone in treating type 2 diabetes by improving insulin sensitivity and reducing hepatic glucose production, underlining its key role in diabetes care[211,212]. Additionally, thiazolidinediones (TZDs), such as pioglitazone, act as PPAR-γ agonists to substantially increase tissue responsiveness to insulin, demonstrating their effectiveness in enhancing metabolic functions[213,214]. Glucagon-like peptide-1 (GLP-1) receptor agonists, like liraglutide, not only augment insulin secretion but also offer neuroprotective benefits, indicating their dual benefit in IR treatment[215,216]. Furthermore, SGLT2 inhibitors, represented by empag-liflozin, contribute to lowering glucose reabsorption in the kidneys, thus indirectly boosting insulin sensitivity and presenting an innovative tactic in IR management[217,218].
Impact of IR treatments on AD’s progression
Emerging research highlights the potential impact of IR therapies on the progression of AD. Metformin, known for its glycemic control properties, has been observed to reduce the risk of cognitive decline and dementia in diabetic patients, potentially through the activation of AMP-activated protein kinase and reduction in neuroinflammation[219,220]. TZDs, with pioglitazone in particular, have demonstrated the potential to decrease AD risk, likely attributed to their anti-inflammatory effects and improvements in cerebral glucose metabolism[221,222]. GLP-1 receptor agonists, such as liraglutide, are being explored for their ability to reduce amyloid plaque formation and enhance cognitive function, showing promising results in early trials[223]. SGLT2 inhibitors, originally utilized for diabetes management, are under research for their possible neuroprotective effects and impact on glucose metabolism in AD[224,225].
The effectiveness of these IR treatments in the context of AD is an area of active research. Observational studies of metformin suggest neuroprotective benefits, yet randomized controlled trials are needed to confirm these effects[211,220]. Clinical trials involving TZDs have yielded mixed results, with some indicating cognitive benefits in the early stages of AD, while others report minimal impact[222]. Initial trials with GLP-1 receptor agonists hint at cognitive improvements in patients with mild AD, but further, more extensive research is required to solidify these findings[223]. The potential neuroprotective role of SGLT2 inhibitors in AD remains an exciting field of study, indicating the necessity for continued investigation into the efficacy of IR treatments in mitigating AD progression[225].
Emerging and adjunctive therapies
Emerging and adjunctive therapies are broadening the horizon of IR management, encompassing a range of innovative and supplementary strategies. Novel insulin sensitizers are being developed to enhance insulin sensitivity through new mechanisms, aiming to minimize the side effects associated with current drugs like TZDs[226]. Nutraceuticals, including omega-3 fatty acids, curcumin, and resveratrol, are gaining attention for their potential to improve insulin sensitivity, attributed to their anti-inflammatory and antioxidant properties[227,228]. Dietary approaches such as intermittent fasting and caloric restriction are linked to both improved insulin sensitivity and neuroprotection, suggesting a beneficial effect on metabolic and neuronal pathways[229-231]. Cognitive training, though not directly addressing IR, is proposed to boost brain plasticity and possibly curb cognitive decline related to IR and AD[232,233]. The modulation of the gut microbiome through probiotics, prebiotics, and dietary changes is another area of interest, reflecting the growing recognition of the gut-brain axis in influencing insulin sensitivity and cognitive function[234,235]. Gene and cell therapies represent cutting-edge interventions in the early research stages, with the potential to directly target metabolic pathways and restore insulin sensitivity[236,237]. Anti-inflammatory therapies are being explored for their capacity to specifically target inflammatory pathways common to both IR and AD, highlighting the intertwined role of inflammation in these conditions[238,239]. Lastly, a holistic approach that integrates medication, lifestyle modifications, and cognitive training offers a comprehensive strategy for managing IR and its potential repercussions on AD, underlining the necessity of a multifaceted treatment paradigm to address the complex interplay between metabolic dysfunction and neurodegeneration.
In summary, the spectrum of therapeutic approaches targeting IR, from lifestyle modifications to emerging pharmacological interventions, offers promising avenues not only in managing metabolic symptoms but also in potentially influencing AD progression. While preliminary studies and trials indicate beneficial roles for these treatments in AD, extensive and long-term research is essential to fully ascertain their efficacy and mechanisms of action. This evolving field underscores the importance of a multifaceted approach in treating conditions with overlapping metabolic and neurological implications, like IR and AD.
FUTURE PERSPECTIVES AND RESEARCH DIRECTIONS
The exploration of the complex relationship between IR and AD presents promising avenues for advancements in early detection, therapeutic interventions, and a deeper understanding of both conditions. The emphasis on early identification and management of IR as a strategy to potentially alter AD's progression is gaining momentum. Research suggests that metabolic disturbances, including IR, often precede the onset of neurodegenerative changes, offering a critical window for early intervention[227]. Developing biomarkers for IR associated with cognitive decline and exploring non-invasive detection methods for IR-related metabolic dysfunctions in at-risk individuals are key research directions[240].
Moreover, emerging therapeutic strategies are increasingly addressing both the metabolic and neurological facets of IR and AD. Novel pharmacological agents targeting molecular pathways common to both conditions, drugs enhancing insulin sensitivity or mimicking insulin's neuroprotective effects without worsening peripheral IR, and the modulation of neurotrophic factors to mitigate IR's adverse effects in the brain are under investigation. The exploration of anti-inflammatory agents also reflects the recognized role of chronic inflammation in the pathogenesis of both IR and AD[100,220,221]. This ongoing research highlights the need for continued interdisciplinary efforts to uncover effective treatments and preventive measures, highlighting the critical intersection of metabolic dysfunction and neurodegeneration in advancing the approach to managing IR and AD.
CONCLUSION
The intricate link between IR and AD underscores a critical intersection in metabolic and neurodegenerative disorders. Research reveals that IR, a hallmark of DM, significantly contributes to the pathogenesis of AD through shared pathways such as impaired insulin signaling, inflammation, and disrupted glucose metabolism. This connection not only highlights the increased AD risk among diabetic patients but also opens new avenues for treatment, including metabolic health interventions and repurposing diabetic medications for AD. Ongoing interdisciplinary research in this domain is vital, promising transformative advances in understanding and managing these prevalent conditions.
Footnotes
Conflict-of-interest statement: The author has nothing to disclose.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country of origin: Malaysia
Peer-review report’s classification
Scientific Quality: Grade A, Grade A, Grade C, Grade C
Novelty: Grade A, Grade A, Grade B
Creativity or Innovation: Grade A, Grade A, Grade B
Scientific Significance: Grade A, Grade A, Grade B
P-Reviewer: Moriyama K, Japan; Qureshi W, India; Soreq L, United Kingdom S-Editor: Liu H L-Editor: A P-Editor: Chen YX
References
- 1.Mitra S, Banik A, Saurabh S, Maulik M, Khatri SN. Neuroimmunometabolism: A New Pathological Nexus Underlying Neurodegenerative Disorders. J Neurosci. 2022;42:1888–1907. doi: 10.1523/JNEUROSCI.0998-21.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Motamedi S, Karimi I, Jafari F. The interrelationship of metabolic syndrome and neurodegenerative diseases with focus on brain-derived neurotrophic factor (BDNF): Kill two birds with one stone. Metab Brain Dis. 2017;32:651–665. doi: 10.1007/s11011-017-9997-0. [DOI] [PubMed] [Google Scholar]
- 3.Neto A, Fernandes A, Barateiro A. The complex relationship between obesity and neurodegenerative diseases: an updated review. Front Cell Neurosci. 2023;17:1294420. doi: 10.3389/fncel.2023.1294420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.James BD, Bennett DA. Causes and Patterns of Dementia: An Update in the Era of Redefining Alzheimer's Disease. Annu Rev Public Health. 2019;40:65–84. doi: 10.1146/annurev-publhealth-040218-043758. [DOI] [PubMed] [Google Scholar]
- 5.Zhao WQ, Townsend M. Insulin resistance and amyloidogenesis as common molecular foundation for type 2 diabetes and Alzheimer's disease. Biochim Biophys Acta. 2009;1792:482–496. doi: 10.1016/j.bbadis.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 6.Ramasubbu K, Devi Rajeswari V. Impairment of insulin signaling pathway PI3K/Akt/mTOR and insulin resistance induced AGEs on diabetes mellitus and neurodegenerative diseases: a perspective review. Mol Cell Biochem. 2023;478:1307–1324. doi: 10.1007/s11010-022-04587-x. [DOI] [PubMed] [Google Scholar]
- 7.Gabelle A, Guéry M, Doutriaux A, Bettayeb K. Forecasting the Prevalence of Alzheimer's Disease at Mild Cognitive Impairment and Mild Dementia Stages in France in 2022. J Prev Alzheimers Dis. 2023;10:259–266. doi: 10.14283/jpad.2023.22. [DOI] [PubMed] [Google Scholar]
- 8.Gillis C, Montenigro P, Nejati M, Maserejian N. Estimating prevalence of early Alzheimer's disease in the United States, accounting for racial and ethnic diversity. Alzheimers Dement. 2023;19:1841–1848. doi: 10.1002/alz.12822. [DOI] [PubMed] [Google Scholar]
- 9.Dhana K, Beck T, Desai P, Wilson RS, Evans DA, Rajan KB. Prevalence of Alzheimer's disease dementia in the 50 US states and 3142 counties: A population estimate using the 2020 bridged-race postcensal from the National Center for Health Statistics. Alzheimers Dement. 2023;19:4388–4395. doi: 10.1002/alz.13081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.GBD 2021 Diabetes Collaborators. Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: a systematic analysis for the Global Burden of Disease Study 2021. Lancet. 2023;402:203–234. doi: 10.1016/S0140-6736(23)01301-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lopez-de-Andres A, Jimenez-Garcia R, Zamorano-Leon JJ, Omaña-Palanco R, Carabantes-Alarcon D, Hernández-Barrera V, De Miguel-Diez J, Cuadrado-Corrales N. Prevalence of Dementia among Patients Hospitalized with Type 2 Diabetes Mellitus in Spain, 2011-2020: Sex-Related Disparities and Impact of the COVID-19 Pandemic. Int J Environ Res Public Health. 2023;20 doi: 10.3390/ijerph20064923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Strooper B. Loss-of-function presenilin mutations in Alzheimer disease. Talking Point on the role of presenilin mutations in Alzheimer disease. EMBO Rep. 2007;8:141–146. doi: 10.1038/sj.embor.7400897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bayer TA, Wirths O. Intracellular accumulation of amyloid-Beta - a predictor for synaptic dysfunction and neuron loss in Alzheimer's disease. Front Aging Neurosci. 2010;2:8. doi: 10.3389/fnagi.2010.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rao CV, Asch AS, Carr DJJ, Yamada HY. "Amyloid-beta accumulation cycle" as a prevention and/or therapy target for Alzheimer's disease. Aging Cell. 2020;19:e13109. doi: 10.1111/acel.13109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang HC, Jiang ZF. Accumulated amyloid-beta peptide and hyperphosphorylated tau protein: relationship and links in Alzheimer's disease. J Alzheimers Dis. 2009;16:15–27. doi: 10.3233/JAD-2009-0960. [DOI] [PubMed] [Google Scholar]
- 16.Bancher C, Brunner C, Lassmann H, Budka H, Jellinger K, Wiche G, Seitelberger F, Grundke-Iqbal I, Iqbal K, Wisniewski HM. Accumulation of abnormally phosphorylated tau precedes the formation of neurofibrillary tangles in Alzheimer's disease. Brain Res. 1989;477:90–99. doi: 10.1016/0006-8993(89)91396-6. [DOI] [PubMed] [Google Scholar]
- 17.Hölscher C. First clinical data of the neuroprotective effects of nasal insulin application in patients with Alzheimer's disease. Alzheimers Dement. 2014;10:S33–S37. doi: 10.1016/j.jalz.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 18.Ramalingam M, Kim SJ. Insulin exerts neuroprotective effects via Akt/Bcl-2 signaling pathways in differentiated SH-SY5Y cells. J Recept Signal Transduct Res. 2015;35:1–7. doi: 10.3109/10799893.2014.922576. [DOI] [PubMed] [Google Scholar]
- 19.Zakharova I, Bayunova L, Avrova D, Avrova N. Neuroprotective Effect of Insulin on Rat Cortical Neurons in Oxidative Stress Is Mediated by Autophagy and Apoptosis Inhibition in vitro. J Evol Biochem Physiol. 2023;59:1536–1550. [Google Scholar]
- 20.Zhang HY, Jiang YC, Li JR, Yan JN, Wang XJ, Shen JB, Ke KF, Gu XS. Neuroprotective effects of insulin-like growth factor-2 in 6-hydroxydopamine-induced cellular and mouse models of Parkinson's disease. Neural Regen Res. 2023;18:1099–1106. doi: 10.4103/1673-5374.355815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Andrade LJO, de Oliveira LM, Bittencourt AMV, Lourenço LGC, de Oliveira GCM. Brain insulin resistance and Alzheimer's disease: a systematic review. Dement Neuropsychol. 2024;18:e20230032. doi: 10.1590/1980-5764-DN-2023-0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang JJ. Brain insulin resistance and the therapeutic value of insulin and insulin-sensitizing drugs in Alzheimer's disease neuropathology. Acta Neurol Belg. 2022;122:1135–1142. doi: 10.1007/s13760-022-01907-2. [DOI] [PubMed] [Google Scholar]
- 23.Yoon JH, Hwang J, Son SU, Choi J, You SW, Park H, Cha SY, Maeng S. How Can Insulin Resistance Cause Alzheimer's Disease? Int J Mol Sci. 2023;24 doi: 10.3390/ijms24043506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pilcher H. Alzheimer's disease could be "type 3 diabetes". Lancet Neurol. 2006;5:388–389. doi: 10.1016/s1474-4422(06)70434-3. [DOI] [PubMed] [Google Scholar]
- 25.Nguyen TT, Ta QTH, Nguyen TKO, Nguyen TTD, Giau VV. Type 3 Diabetes and Its Role Implications in Alzheimer's Disease. Int J Mol Sci. 2020;21 doi: 10.3390/ijms21093165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de la Monte SM, Tong M, Lester-Coll N, Plater M Jr, Wands JR. Therapeutic rescue of neurodegeneration in experimental type 3 diabetes: relevance to Alzheimer's disease. J Alzheimers Dis. 2006;10:89–109. doi: 10.3233/jad-2006-10113. [DOI] [PubMed] [Google Scholar]
- 27.Zhang J, Chen C, Hua S, Liao H, Wang M, Xiong Y, Cao F. An updated meta-analysis of cohort studies: Diabetes and risk of Alzheimer's disease. Diabetes Res Clin Pract. 2017;124:41–47. doi: 10.1016/j.diabres.2016.10.024. [DOI] [PubMed] [Google Scholar]
- 28.Ferreira ST, Clarke JR, Bomfim TR, De Felice FG. Inflammation, defective insulin signaling, and neuronal dysfunction in Alzheimer's disease. Alzheimers Dement. 2014;10:S76–S83. doi: 10.1016/j.jalz.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 29.Akhtar A, Sah SP. Insulin signaling pathway and related molecules: Role in neurodegeneration and Alzheimer's disease. Neurochem Int. 2020;135:104707. doi: 10.1016/j.neuint.2020.104707. [DOI] [PubMed] [Google Scholar]
- 30.Chatterjee S, Mudher A. Alzheimer's Disease and Type 2 Diabetes: A Critical Assessment of the Shared Pathological Traits. Front Neurosci. 2018;12:383. doi: 10.3389/fnins.2018.00383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Valente T, Gella A, Fernàndez-Busquets X, Unzeta M, Durany N. Immunohistochemical analysis of human brain suggests pathological synergism of Alzheimer's disease and diabetes mellitus. Neurobiol Dis. 2010;37:67–76. doi: 10.1016/j.nbd.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 32.Alafuzoff I, Aho L, Helisalmi S, Mannermaa A, Soininen H. Beta-amyloid deposition in brains of subjects with diabetes. Neuropathol Appl Neurobiol. 2009;35:60–68. doi: 10.1111/j.1365-2990.2008.00948.x. [DOI] [PubMed] [Google Scholar]
- 33.Chornenkyy Y, Wang WX, Wei A, Nelson PT. Alzheimer's disease and type 2 diabetes mellitus are distinct diseases with potential overlapping metabolic dysfunction upstream of observed cognitive decline. Brain Pathol. 2019;29:3–17. doi: 10.1111/bpa.12655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kinney JW, Bemiller SM, Murtishaw AS, Leisgang AM, Salazar AM, Lamb BT. Inflammation as a central mechanism in Alzheimer's disease. Alzheimers Dement (N Y) 2018;4:575–590. doi: 10.1016/j.trci.2018.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rosales-Corral SA, Acuña-Castroviejo D, Coto-Montes A, Boga JA, Manchester LC, Fuentes-Broto L, Korkmaz A, Ma S, Tan DX, Reiter RJ. Alzheimer's disease: pathological mechanisms and the beneficial role of melatonin. J Pineal Res. 2012;52:167–202. doi: 10.1111/j.1600-079X.2011.00937.x. [DOI] [PubMed] [Google Scholar]
- 36.Ajoolabady A, Lindholm D, Ren J, Pratico D. ER stress and UPR in Alzheimer's disease: mechanisms, pathogenesis, treatments. Cell Death Dis. 2022;13:706. doi: 10.1038/s41419-022-05153-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ganguly U, Kaur U, Chakrabarti SS, Sharma P, Agrawal BK, Saso L, Chakrabarti S. Oxidative Stress, Neuroinflammation, and NADPH Oxidase: Implications in the Pathogenesis and Treatment of Alzheimer's Disease. Oxid Med Cell Longev. 2021;2021:7086512. doi: 10.1155/2021/7086512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Perluigi M, Di Domenico F, Butterfield DA. Oxidative damage in neurodegeneration: roles in the pathogenesis and progression of Alzheimer disease. Physiol Rev. 2024;104:103–197. doi: 10.1152/physrev.00030.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rajkumar M, Kannan S, Thangaraj R. Voglibose attenuates cognitive impairment, Aβ aggregation, oxidative stress, and neuroinflammation in streptozotocin-induced Alzheimer's disease rat model. Inflammopharmacology. 2023;31:2751–2771. doi: 10.1007/s10787-023-01313-x. [DOI] [PubMed] [Google Scholar]
- 40.Norton L, Shannon C, Gastaldelli A, DeFronzo RA. Insulin: The master regulator of glucose metabolism. Metabolism. 2022;129:155142. doi: 10.1016/j.metabol.2022.155142. [DOI] [PubMed] [Google Scholar]
- 41.Frank N, Tadros EM. Insulin dysregulation. Equine Vet J. 2014;46:103–112. doi: 10.1111/evj.12169. [DOI] [PubMed] [Google Scholar]
- 42.Petersen MC, Shulman GI. Mechanisms of Insulin Action and Insulin Resistance. Physiol Rev. 2018;98:2133–2223. doi: 10.1152/physrev.00063.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nguyen V, Thomas P, Pemberton S, Babin A, Noonan C, Weaver R, Banks WA, Rhea EM. Central nervous system insulin signaling can influence the rate of insulin influx into brain. Fluids Barriers CNS. 2023;20:28. doi: 10.1186/s12987-023-00431-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhao F, Siu JJ, Huang W, Askwith C, Cao L. Insulin Modulates Excitatory Synaptic Transmission and Synaptic Plasticity in the Mouse Hippocampus. Neuroscience. 2019;411:237–254. doi: 10.1016/j.neuroscience.2019.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grillo CA, Piroli GG, Lawrence RC, Wrighten SA, Green AJ, Wilson SP, Sakai RR, Kelly SJ, Wilson MA, Mott DD, Reagan LP. Hippocampal Insulin Resistance Impairs Spatial Learning and Synaptic Plasticity. Diabetes. 2015;64:3927–3936. doi: 10.2337/db15-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.de Bartolomeis A, De Simone G, De Prisco M, Barone A, Napoli R, Beguinot F, Billeci M, Fornaro M. Insulin effects on core neurotransmitter pathways involved in schizophrenia neurobiology: a meta-analysis of preclinical studies. Implications for the treatment. Mol Psychiatry. 2023;28:2811–2825. doi: 10.1038/s41380-023-02065-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Werner H, LeRoith D. Insulin and insulin-like growth factor receptors in the brain: physiological and pathological aspects. Eur Neuropsychopharmacol. 2014;24:1947–1953. doi: 10.1016/j.euroneuro.2014.01.020. [DOI] [PubMed] [Google Scholar]
- 48.Havrankova J, Roth J, Brownstein M. Insulin receptors are widely distributed in the central nervous system of the rat. Nature. 1978;272:827–829. doi: 10.1038/272827a0. [DOI] [PubMed] [Google Scholar]
- 49.Velazquez R, Tran A, Ishimwe E, Denner L, Dave N, Oddo S, Dineley KT. Central insulin dysregulation and energy dyshomeostasis in two mouse models of Alzheimer's disease. Neurobiol Aging. 2017;58:1–13. doi: 10.1016/j.neurobiolaging.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Griffith CM, Eid T, Rose GM, Patrylo PR. Evidence for altered insulin receptor signaling in Alzheimer's disease. Neuropharmacology. 2018;136:202–215. doi: 10.1016/j.neuropharm.2018.01.008. [DOI] [PubMed] [Google Scholar]
- 51.Gabbouj S, Ryhänen S, Marttinen M, Wittrahm R, Takalo M, Kemppainen S, Martiskainen H, Tanila H, Haapasalo A, Hiltunen M, Natunen T. Altered Insulin Signaling in Alzheimer's Disease Brain - Special Emphasis on PI3K-Akt Pathway. Front Neurosci. 2019;13:629. doi: 10.3389/fnins.2019.00629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zheng M, Wang P. Role of insulin receptor substance-1 modulating PI3K/Akt insulin signaling pathway in Alzheimer's disease. 3 Biotech. 2021;11:179. doi: 10.1007/s13205-021-02738-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang Y, Huang NQ, Yan F, Jin H, Zhou SY, Shi JS, Jin F. Diabetes mellitus and Alzheimer's disease: GSK-3β as a potential link. Behav Brain Res. 2018;339:57–65. doi: 10.1016/j.bbr.2017.11.015. [DOI] [PubMed] [Google Scholar]
- 54.Cools R, Arnsten AFT. Neuromodulation of prefrontal cortex cognitive function in primates: the powerful roles of monoamines and acetylcholine. Neuropsychopharmacology. 2022;47:309–328. doi: 10.1038/s41386-021-01100-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Patel JC, Carr KD, Rice ME. Actions and Consequences of Insulin in the Striatum. Biomolecules. 2023;13 doi: 10.3390/biom13030518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Spinelli M, Fusco S, Grassi C. Brain Insulin Resistance and Hippocampal Plasticity: Mechanisms and Biomarkers of Cognitive Decline. Front Neurosci. 2019;13:788. doi: 10.3389/fnins.2019.00788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rhea EM, Rask-Madsen C, Banks WA. Insulin transport across the blood-brain barrier can occur independently of the insulin receptor. J Physiol. 2018;596:4753–4765. doi: 10.1113/JP276149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Banks WA. The source of cerebral insulin. Eur J Pharmacol. 2004;490:5–12. doi: 10.1016/j.ejphar.2004.02.040. [DOI] [PubMed] [Google Scholar]
- 59.Wallum BJ, Taborsky GJ Jr, Porte D Jr, Figlewicz DP, Jacobson L, Beard JC, Ward WK, Dorsa D. Cerebrospinal fluid insulin levels increase during intravenous insulin infusions in man. J Clin Endocrinol Metab. 1987;64:190–194. doi: 10.1210/jcem-64-1-190. [DOI] [PubMed] [Google Scholar]
- 60.Genders AJ, Frison V, Abramson SR, Barrett EJ. Endothelial cells actively concentrate insulin during its transendothelial transport. Microcirculation. 2013;20:434–439. doi: 10.1111/micc.12044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Banks WA, Jaspan JB, Kastin AJ. Selective, physiological transport of insulin across the blood-brain barrier: novel demonstration by species-specific radioimmunoassays. Peptides. 1997;18:1257–1262. doi: 10.1016/s0196-9781(97)00198-8. [DOI] [PubMed] [Google Scholar]
- 62.Schwartz MW, Sipols A, Kahn SE, Lattemann DF, Taborsky GJ Jr, Bergman RN, Woods SC, Porte D Jr. Kinetics and specificity of insulin uptake from plasma into cerebrospinal fluid. Am J Physiol. 1990;259:E378–E383. doi: 10.1152/ajpendo.1990.259.3.E378. [DOI] [PubMed] [Google Scholar]
- 63.Kern W, Benedict C, Schultes B, Plohr F, Moser A, Born J, Fehm HL, Hallschmid M. Low cerebrospinal fluid insulin levels in obese humans. Diabetologia. 2006;49:2790–2792. doi: 10.1007/s00125-006-0409-y. [DOI] [PubMed] [Google Scholar]
- 64.Dietrich MO, Spuch C, Antequera D, Rodal I, de Yébenes JG, Molina JA, Bermejo F, Carro E. Megalin mediates the transport of leptin across the blood-CSF barrier. Neurobiol Aging. 2008;29:902–912. doi: 10.1016/j.neurobiolaging.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 65.Born J, Lange T, Kern W, McGregor GP, Bickel U, Fehm HL. Sniffing neuropeptides: a transnasal approach to the human brain. Nat Neurosci. 2002;5:514–516. doi: 10.1038/nn849. [DOI] [PubMed] [Google Scholar]
- 66.Baskin DG, Stein LJ, Ikeda H, Woods SC, Figlewicz DP, Porte D Jr, Greenwood MR, Dorsa DM. Genetically obese Zucker rats have abnormally low brain insulin content. Life Sci. 1985;36:627–633. doi: 10.1016/0024-3205(85)90166-3. [DOI] [PubMed] [Google Scholar]
- 67.Di Guglielmo GM, Drake PG, Baass PC, Authier F, Posner BI, Bergeron JJ. Insulin receptor internalization and signalling. Mol Cell Biochem. 1998;182:59–63. [PubMed] [Google Scholar]
- 68.Kuwabara T, Kagalwala MN, Onuma Y, Ito Y, Warashina M, Terashima K, Sanosaka T, Nakashima K, Gage FH, Asashima M. Insulin biosynthesis in neuronal progenitors derived from adult hippocampus and the olfactory bulb. EMBO Mol Med. 2011;3:742–754. doi: 10.1002/emmm.201100177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Devaskar SU, Singh BS, Carnaghi LR, Rajakumar PA, Giddings SJ. Insulin II gene expression in rat central nervous system. Regul Pept. 1993;48:55–63. doi: 10.1016/0167-0115(93)90335-6. [DOI] [PubMed] [Google Scholar]
- 70.Molnár G, Faragó N, Kocsis ÁK, Rózsa M, Lovas S, Boldog E, Báldi R, Csajbók É, Gardi J, Puskás LG, Tamás G. GABAergic neurogliaform cells represent local sources of insulin in the cerebral cortex. J Neurosci. 2014;34:1133–1137. doi: 10.1523/JNEUROSCI.4082-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Obici S, Zhang BB, Karkanias G, Rossetti L. Hypothalamic insulin signaling is required for inhibition of glucose production. Nat Med. 2002;8:1376–1382. doi: 10.1038/nm1202-798. [DOI] [PubMed] [Google Scholar]
- 72.Pocai A, Lam TK, Gutierrez-Juarez R, Obici S, Schwartz GJ, Bryan J, Aguilar-Bryan L, Rossetti L. Hypothalamic K(ATP) channels control hepatic glucose production. Nature. 2005;434:1026–1031. doi: 10.1038/nature03439. [DOI] [PubMed] [Google Scholar]
- 73.Ramnanan CJ, Saraswathi V, Smith MS, Donahue EP, Farmer B, Farmer TD, Neal D, Williams PE, Lautz M, Mari A, Cherrington AD, Edgerton DS. Brain insulin action augments hepatic glycogen synthesis without suppressing glucose production or gluconeogenesis in dogs. J Clin Invest. 2011;121:3713–3723. doi: 10.1172/JCI45472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Edgerton DS, Kraft G, Smith M, Farmer B, Williams PE, Coate KC, Printz RL, O'Brien RM, Cherrington AD. Insulin's direct hepatic effect explains the inhibition of glucose production caused by insulin secretion. JCI Insight. 2017;2:e91863. doi: 10.1172/jci.insight.91863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Edgerton DS, Lautz M, Scott M, Everett CA, Stettler KM, Neal DW, Chu CA, Cherrington AD. Insulin's direct effects on the liver dominate the control of hepatic glucose production. J Clin Invest. 2006;116:521–527. doi: 10.1172/JCI27073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dash S, Xiao C, Morgantini C, Koulajian K, Lewis GF. Intranasal insulin suppresses endogenous glucose production in humans compared with placebo in the presence of similar venous insulin concentrations. Diabetes. 2015;64:766–774. doi: 10.2337/db14-0685. [DOI] [PubMed] [Google Scholar]
- 77.Heni M, Wagner R, Kullmann S, Gancheva S, Roden M, Peter A, Stefan N, Preissl H, Häring HU, Fritsche A. Hypothalamic and Striatal Insulin Action Suppresses Endogenous Glucose Production and May Stimulate Glucose Uptake During Hyperinsulinemia in Lean but Not in Overweight Men. Diabetes. 2017;66:1797–1806. doi: 10.2337/db16-1380. [DOI] [PubMed] [Google Scholar]
- 78.Koch L, Wunderlich FT, Seibler J, Könner AC, Hampel B, Irlenbusch S, Brabant G, Kahn CR, Schwenk F, Brüning JC. Central insulin action regulates peripheral glucose and fat metabolism in mice. J Clin Invest. 2008;118:2132–2147. doi: 10.1172/JCI31073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Brüning JC, Gautam D, Burks DJ, Gillette J, Schubert M, Orban PC, Klein R, Krone W, Müller-Wieland D, Kahn CR. Role of brain insulin receptor in control of body weight and reproduction. Science. 2000;289:2122–2125. doi: 10.1126/science.289.5487.2122. [DOI] [PubMed] [Google Scholar]
- 80.Fisher SJ, Brüning JC, Lannon S, Kahn CR. Insulin signaling in the central nervous system is critical for the normal sympathoadrenal response to hypoglycemia. Diabetes. 2005;54:1447–1451. doi: 10.2337/diabetes.54.5.1447. [DOI] [PubMed] [Google Scholar]
- 81.Seaquist ER, Damberg GS, Tkac I, Gruetter R. The effect of insulin on in vivo cerebral glucose concentrations and rates of glucose transport/metabolism in humans. Diabetes. 2001;50:2203–2209. doi: 10.2337/diabetes.50.10.2203. [DOI] [PubMed] [Google Scholar]
- 82.Fujikawa T. Central regulation of glucose metabolism in an insulin-dependent and -independent manner. J Neuroendocrinol. 2021;33:e12941. doi: 10.1111/jne.12941. [DOI] [PubMed] [Google Scholar]
- 83.Havrankova J, Roth J, Brownstein MJ. Concentrations of insulin and insulin receptors in the brain are independent of peripheral insulin levels. Studies of obese and streptozotocin-treated rodents. J Clin Invest. 1979;64:636–642. doi: 10.1172/JCI109504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sartorius T, Peter A, Heni M, Maetzler W, Fritsche A, Häring HU, Hennige AM. The brain response to peripheral insulin declines with age: a contribution of the blood-brain barrier? PLoS One. 2015;10:e0126804. doi: 10.1371/journal.pone.0126804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Agrawal R, Reno CM, Sharma S, Christensen C, Huang Y, Fisher SJ. Insulin action in the brain regulates both central and peripheral functions. Am J Physiol Endocrinol Metab. 2021;321:E156–E163. doi: 10.1152/ajpendo.00642.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Aulston BD, Schapansky J, Huang Y, Odero GL, Glazner GW. Secreted amyloid precursor protein alpha activates neuronal insulin receptors and prevents diabetes-induced encephalopathy. Exp Neurol. 2018;303:29–37. doi: 10.1016/j.expneurol.2018.01.013. [DOI] [PubMed] [Google Scholar]
- 87.Krishnaswamy S, Verdile G, Groth D, Kanyenda L, Martins RN. The structure and function of Alzheimer's gamma secretase enzyme complex. Crit Rev Clin Lab Sci. 2009;46:282–301. doi: 10.3109/10408360903335821. [DOI] [PubMed] [Google Scholar]
- 88.Meakin PJ, Mezzapesa A, Benabou E, Haas ME, Bonardo B, Grino M, Brunel JM, Desbois-Mouthon C, Biddinger SB, Govers R, Ashford MLJ, Peiretti F. The beta secretase BACE1 regulates the expression of insulin receptor in the liver. Nat Commun. 2018;9:1306. doi: 10.1038/s41467-018-03755-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Špolcová A, Mikulášková B, Kršková K, Gajdošechová L, Zórad Š, Olszanecki R, Suski M, Bujak-Giżycka B, Železná B, Maletínská L. Deficient hippocampal insulin signaling and augmented Tau phosphorylation is related to obesity- and age-induced peripheral insulin resistance: a study in Zucker rats. BMC Neurosci. 2014;15:111. doi: 10.1186/1471-2202-15-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rodriguez-Rodriguez P, Sandebring-Matton A, Merino-Serrais P, Parrado-Fernandez C, Rabano A, Winblad B, Ávila J, Ferrer I, Cedazo-Minguez A. Tau hyperphosphorylation induces oligomeric insulin accumulation and insulin resistance in neurons. Brain. 2017;140:3269–3285. doi: 10.1093/brain/awx256. [DOI] [PubMed] [Google Scholar]
- 91.Peng D, Pan X, Cui J, Ren Y, Zhang J. Hyperphosphorylation of tau protein in hippocampus of central insulin-resistant rats is associated with cognitive impairment. Cell Physiol Biochem. 2013;32:1417–1425. doi: 10.1159/000356579. [DOI] [PubMed] [Google Scholar]
- 92.Willette AA, Bendlin BB, Starks EJ, Birdsill AC, Johnson SC, Christian BT, Okonkwo OC, La Rue A, Hermann BP, Koscik RL, Jonaitis EM, Sager MA, Asthana S. Association of Insulin Resistance With Cerebral Glucose Uptake in Late Middle-Aged Adults at Risk for Alzheimer Disease. JAMA Neurol. 2015;72:1013–1020. doi: 10.1001/jamaneurol.2015.0613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rebelos E, Bucci M, Karjalainen T, Oikonen V, Bertoldo A, Hannukainen JC, Virtanen KA, Latva-Rasku A, Hirvonen J, Heinonen I, Parkkola R, Laakso M, Ferrannini E, Iozzo P, Nummenmaa L, Nuutila P. Insulin Resistance Is Associated With Enhanced Brain Glucose Uptake During Euglycemic Hyperinsulinemia: A Large-Scale PET Cohort. Diabetes Care. 2021;44:788–794. doi: 10.2337/dc20-1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dienel GA. Brain Glucose Metabolism: Integration of Energetics with Function. Physiol Rev. 2019;99:949–1045. doi: 10.1152/physrev.00062.2017. [DOI] [PubMed] [Google Scholar]
- 95.Mosconi L, Pupi A, De Leon MJ. Brain glucose hypometabolism and oxidative stress in preclinical Alzheimer's disease. Ann N Y Acad Sci. 2008;1147:180–195. doi: 10.1196/annals.1427.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Blázquez E, Hurtado-Carneiro V, LeBaut-Ayuso Y, Velázquez E, García-García L, Gómez-Oliver F, Ruiz-Albusac JM, Ávila J, Pozo MÁ. Significance of Brain Glucose Hypometabolism, Altered Insulin Signal Transduction, and Insulin Resistance in Several Neurological Diseases. Front Endocrinol (Lausanne) 2022;13:873301. doi: 10.3389/fendo.2022.873301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hirvonen J, Virtanen KA, Nummenmaa L, Hannukainen JC, Honka MJ, Bucci M, Nesterov SV, Parkkola R, Rinne J, Iozzo P, Nuutila P. Effects of insulin on brain glucose metabolism in impaired glucose tolerance. Diabetes. 2011;60:443–447. doi: 10.2337/db10-0940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kim B, Elzinga SE, Henn RE, McGinley LM, Feldman EL. The effects of insulin and insulin-like growth factor I on amyloid precursor protein phosphorylation in in vitro and in vivo models of Alzheimer's disease. Neurobiol Dis. 2019;132:104541. doi: 10.1016/j.nbd.2019.104541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Koelsch G. BACE1 Function and Inhibition: Implications of Intervention in the Amyloid Pathway of Alzheimer's Disease Pathology. Molecules. 2017;22 doi: 10.3390/molecules22101723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Vinuesa A, Pomilio C, Gregosa A, Bentivegna M, Presa J, Bellotto M, Saravia F, Beauquis J. Inflammation and Insulin Resistance as Risk Factors and Potential Therapeutic Targets for Alzheimer's Disease. Front Neurosci. 2021;15:653651. doi: 10.3389/fnins.2021.653651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lee S, Tong M, Hang S, Deochand C, de la Monte S. CSF and Brain Indices of Insulin Resistance, Oxidative Stress and Neuro-Inflammation in Early versus Late Alzheimer's Disease. J Alzheimers Dis Parkinsonism. 2013;3:128. doi: 10.4172/2161-0460.1000128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Morimoto K, Horio J, Satoh H, Sue L, Beach T, Arita S, Tooyama I, Konishi Y. Expression profiles of cytokines in the brains of Alzheimer's disease (AD) patients compared to the brains of non-demented patients with and without increasing AD pathology. J Alzheimers Dis. 2011;25:59–76. doi: 10.3233/JAD-2011-101815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Haas CB, de Carvalho AK, Muller AP, Eggen BJL, Portela LV. Insulin activates microglia and increases COX-2/IL-1β expression in young but not in aged hippocampus. Brain Res. 2020;1741:146884. doi: 10.1016/j.brainres.2020.146884. [DOI] [PubMed] [Google Scholar]
- 104.Brabazon F, Bermudez S, Shaughness M, Khayrullina G, Byrnes KR. The effects of insulin on the inflammatory activity of BV2 microglia. PLoS One. 2018;13:e0201878. doi: 10.1371/journal.pone.0201878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Edison P. Neuroinflammation, microglial activation, and glucose metabolism in neurodegenerative diseases. Int Rev Neurobiol. 2020;154:325–344. doi: 10.1016/bs.irn.2020.03.017. [DOI] [PubMed] [Google Scholar]
- 106.Giovannini MG, Scali C, Prosperi C, Bellucci A, Pepeu G, Casamenti F. Experimental brain inflammation and neurodegeneration as model of Alzheimer's disease: protective effects of selective COX-2 inhibitors. Int J Immunopathol Pharmacol. 2003;16:31–40. [PubMed] [Google Scholar]
- 107.Guan PP, Wang P. Integrated communications between cyclooxygenase-2 and Alzheimer's disease. FASEB J. 2019;33:13–33. doi: 10.1096/fj.201800355RRRR. [DOI] [PubMed] [Google Scholar]
- 108.Dubey H, Gulati K, Ray A. Alzheimer's Disease: A Contextual Link with Nitric Oxide Synthase. Curr Mol Med. 2020;20:505–515. doi: 10.2174/1566524019666191129103117. [DOI] [PubMed] [Google Scholar]
- 109.Katakam PV, Snipes JA, Steed MM, Busija DW. Insulin-induced generation of reactive oxygen species and uncoupling of nitric oxide synthase underlie the cerebrovascular insulin resistance in obese rats. J Cereb Blood Flow Metab. 2012;32:792–804. doi: 10.1038/jcbfm.2011.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Onyango AN. Cellular Stresses and Stress Responses in the Pathogenesis of Insulin Resistance. Oxid Med Cell Longev. 2018;2018:4321714. doi: 10.1155/2018/4321714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Barlow AD, Thomas DC. Autophagy in diabetes: β-cell dysfunction, insulin resistance, and complications. DNA Cell Biol. 2015;34:252–260. doi: 10.1089/dna.2014.2755. [DOI] [PubMed] [Google Scholar]
- 112.Sadeghi A, Niknam M, Momeni-Moghaddam MA, Shabani M, Aria H, Bastin A, Teimouri M, Meshkani R, Akbari H. Crosstalk between autophagy and insulin resistance: evidence from different tissues. Eur J Med Res. 2023;28:456. doi: 10.1186/s40001-023-01424-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pickford F, Masliah E, Britschgi M, Lucin K, Narasimhan R, Jaeger PA, Small S, Spencer B, Rockenstein E, Levine B, Wyss-Coray T. The autophagy-related protein beclin 1 shows reduced expression in early Alzheimer disease and regulates amyloid beta accumulation in mice. J Clin Invest. 2008;118:2190–2199. doi: 10.1172/JCI33585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.De Felice FG, Ferreira ST. Inflammation, defective insulin signaling, and mitochondrial dysfunction as common molecular denominators connecting type 2 diabetes to Alzheimer disease. Diabetes. 2014;63:2262–2272. doi: 10.2337/db13-1954. [DOI] [PubMed] [Google Scholar]
- 115.Hughes D, Mallucci GR. The unfolded protein response in neurodegenerative disorders - therapeutic modulation of the PERK pathway. FEBS J. 2019;286:342–355. doi: 10.1111/febs.14422. [DOI] [PubMed] [Google Scholar]
- 116.Pandey VK, Mathur A, Kakkar P. Emerging role of Unfolded Protein Response (UPR) mediated proteotoxic apoptosis in diabetes. Life Sci. 2019;216:246–258. doi: 10.1016/j.lfs.2018.11.041. [DOI] [PubMed] [Google Scholar]
- 117.Ghemrawi R, Khair M. Endoplasmic Reticulum Stress and Unfolded Protein Response in Neurodegenerative Diseases. Int J Mol Sci. 2020;21 doi: 10.3390/ijms21176127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Nizari S, Carare RO, Hawkes CA. Increased Aβ pathology in aged Tg2576 mice born to mothers fed a high fat diet. Sci Rep. 2016;6:21981. doi: 10.1038/srep21981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Martín MG, Dotti CG. Plasma membrane and brain dysfunction of the old: Do we age from our membranes? Front Cell Dev Biol. 2022;10:1031007. doi: 10.3389/fcell.2022.1031007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.van Deijk AF, Camargo N, Timmerman J, Heistek T, Brouwers JF, Mogavero F, Mansvelder HD, Smit AB, Verheijen MH. Astrocyte lipid metabolism is critical for synapse development and function in vivo. Glia. 2017;65:670–682. doi: 10.1002/glia.23120. [DOI] [PubMed] [Google Scholar]
- 121.Kisler K, Nelson AR, Montagne A, Zlokovic BV. Cerebral blood flow regulation and neurovascular dysfunction in Alzheimer disease. Nat Rev Neurosci. 2017;18:419–434. doi: 10.1038/nrn.2017.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Nwafor DC, Brichacek AL, Mohammad AS, Griffith J, Lucke-Wold BP, Benkovic SA, Geldenhuys WJ, Lockman PR, Brown CM. Targeting the Blood-Brain Barrier to Prevent Sepsis-Associated Cognitive Impairment. J Cent Nerv Syst Dis. 2019;11:1179573519840652. doi: 10.1177/1179573519840652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Cockerill I, Oliver JA, Xu H, Fu BM, Zhu D. Blood-Brain Barrier Integrity and Clearance of Amyloid-β from the BBB. Adv Exp Med Biol. 2018;1097:261–278. doi: 10.1007/978-3-319-96445-4_14. [DOI] [PubMed] [Google Scholar]
- 124.Daulatzai MA. Cerebral hypoperfusion and glucose hypometabolism: Key pathophysiological modulators promote neurodegeneration, cognitive impairment, and Alzheimer's disease. J Neurosci Res. 2017;95:943–972. doi: 10.1002/jnr.23777. [DOI] [PubMed] [Google Scholar]
- 125.Kwon KJ, Lee EJ, Kim MK, Kim SY, Kim JN, Kim JO, Kim HJ, Kim HY, Han JS, Shin CY, Han SH. Diabetes augments cognitive dysfunction in chronic cerebral hypoperfusion by increasing neuronal cell death: implication of cilostazol for diabetes mellitus-induced dementia. Neurobiol Dis. 2015;73:12–23. doi: 10.1016/j.nbd.2014.08.034. [DOI] [PubMed] [Google Scholar]
- 126.Hawkins BT, Lundeen TF, Norwood KM, Brooks HL, Egleton RD. Increased blood-brain barrier permeability and altered tight junctions in experimental diabetes in the rat: contribution of hyperglycaemia and matrix metalloproteinases. Diabetologia. 2007;50:202–211. doi: 10.1007/s00125-006-0485-z. [DOI] [PubMed] [Google Scholar]
- 127.Yoo DY, Yim HS, Jung HY, Nam SM, Kim JW, Choi JH, Seong JK, Yoon YS, Kim DW, Hwang IK. Chronic type 2 diabetes reduces the integrity of the blood-brain barrier by reducing tight junction proteins in the hippocampus. J Vet Med Sci. 2016;78:957–962. doi: 10.1292/jvms.15-0589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Robles-Osorio ML, Sabath E. Tight junction disruption and the pathogenesis of the chronic complications of diabetes mellitus: A narrative review. World J Diabetes. 2023;14:1013–1026. doi: 10.4239/wjd.v14.i7.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ruggiero RN, Rossignoli MT, Marques DB, de Sousa BM, Romcy-Pereira RN, Lopes-Aguiar C, Leite JP. Neuromodulation of Hippocampal-Prefrontal Cortical Synaptic Plasticity and Functional Connectivity: Implications for Neuropsychiatric Disorders. Front Cell Neurosci. 2021;15:732360. doi: 10.3389/fncel.2021.732360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Chen ZR, Huang JB, Yang SL, Hong FF. Role of Cholinergic Signaling in Alzheimer's Disease. Molecules. 2022;27 doi: 10.3390/molecules27061816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Peeyush KT, Savitha B, Sherin A, Anju TR, Jes P, Paulose CS. Cholinergic, dopaminergic and insulin receptors gene expression in the cerebellum of streptozotocin-induced diabetic rats: functional regulation with Vitamin D3 supplementation. Pharmacol Biochem Behav. 2010;95:216–222. doi: 10.1016/j.pbb.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 132.Meneses A. Neurotransmitters and memory: cholinergic, glutamatergic, gabaergic, dopaminergic, serotonergic, signaling, and memory. Identification Neural Markers Accompanying Mem. 2014:5–45. [Google Scholar]
- 133.Bekdash RA. The Cholinergic System, the Adrenergic System and the Neuropathology of Alzheimer's Disease. Int J Mol Sci. 2021;22 doi: 10.3390/ijms22031273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Tanila H. The role of BDNF in Alzheimer's disease. Neurobiol Dis. 2017;97:114–118. doi: 10.1016/j.nbd.2016.05.008. [DOI] [PubMed] [Google Scholar]
- 135.Son Y, Yang M, Wang H, Moon C. Hippocampal dysfunctions caused by cranial irradiation: a review of the experimental evidence. Brain Behav Immun. 2015;45:287–296. doi: 10.1016/j.bbi.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 136.Ghosh I, Liu CS, Swardfager W, Lanctôt KL, Anderson ND. The potential roles of excitatory-inhibitory imbalances and the repressor element-1 silencing transcription factor in aging and aging-associated diseases. Mol Cell Neurosci. 2021;117:103683. doi: 10.1016/j.mcn.2021.103683. [DOI] [PubMed] [Google Scholar]
- 137.Esposito M, Sherr GL. Epigenetic Modifications in Alzheimer's Neuropathology and Therapeutics. Front Neurosci. 2019;13:476. doi: 10.3389/fnins.2019.00476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Miller CA, Sweatt JD. Covalent modification of DNA regulates memory formation. Neuron. 2007;53:857–869. doi: 10.1016/j.neuron.2007.02.022. [DOI] [PubMed] [Google Scholar]
- 139.Liu X, Jiao B, Shen L. The Epigenetics of Alzheimer's Disease: Factors and Therapeutic Implications. Front Genet. 2018;9:579. doi: 10.3389/fgene.2018.00579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Bufill E, Ribosa-Nogué R, Blesa R. The Therapeutic Potential of Epigenetic Modifications in Alzheimer’s Disease. In: Alzheimer’s Disease: Drug Discovery [Internet]. Brisbane (AU): Exon Publications; 2020-Dec-18. [PubMed] [Google Scholar]
- 141.Iwata A, Nagata K, Hatsuta H, Takuma H, Bundo M, Iwamoto K, Tamaoka A, Murayama S, Saido T, Tsuji S. Altered CpG methylation in sporadic Alzheimer's disease is associated with APP and MAPT dysregulation. Hum Mol Genet. 2014;23:648–656. doi: 10.1093/hmg/ddt451. [DOI] [PubMed] [Google Scholar]
- 142.Lunnon K, Smith R, Hannon E, De Jager PL, Srivastava G, Volta M, Troakes C, Al-Sarraj S, Burrage J, Macdonald R, Condliffe D, Harries LW, Katsel P, Haroutunian V, Kaminsky Z, Joachim C, Powell J, Lovestone S, Bennett DA, Schalkwyk LC, Mill J. Methylomic profiling implicates cortical deregulation of ANK1 in Alzheimer's disease. Nat Neurosci. 2014;17:1164–1170. doi: 10.1038/nn.3782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Gräff J, Kim D, Dobbin MM, Tsai LH. Epigenetic regulation of gene expression in physiological and pathological brain processes. Physiol Rev. 2011;91:603–649. doi: 10.1152/physrev.00012.2010. [DOI] [PubMed] [Google Scholar]
- 144.Rodrigues DA, Pinheiro PSM, Sagrillo FS, Bolognesi ML, Fraga CAM. Histone deacetylases as targets for the treatment of neurodegenerative disorders: Challenges and future opportunities. Med Res Rev. 2020;40:2177–2211. doi: 10.1002/med.21701. [DOI] [PubMed] [Google Scholar]
- 145.Xiao H, Gao Y, Liu L, Li Y. Association between polymorphisms in the promoter region of the apolipoprotein E (APOE) gene and Alzheimer's disease: A meta-analysis. EXCLI J. 2017;16:921–938. doi: 10.17179/excli2017-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Michaelson DM. APOE ε4: the most prevalent yet understudied risk factor for Alzheimer's disease. Alzheimers Dement. 2014;10:861–868. doi: 10.1016/j.jalz.2014.06.015. [DOI] [PubMed] [Google Scholar]
- 147.Uddin MS, Kabir MT, Al Mamun A, Abdel-Daim MM, Barreto GE, Ashraf GM. APOE and Alzheimer's Disease: Evidence Mounts that Targeting APOE4 may Combat Alzheimer's Pathogenesis. Mol Neurobiol. 2019;56:2450–2465. doi: 10.1007/s12035-018-1237-z. [DOI] [PubMed] [Google Scholar]
- 148.Gomez-Isla T, West HL, Rebeck GW, Harr SD, Growdon JH, Locascio JJ, Perls TT, Lipsitz LA, Hyman BT. Clinical and pathological correlates of apolipoprotein E epsilon 4 in Alzheimer's disease. Ann Neurol. 1996;39:62–70. doi: 10.1002/ana.410390110. [DOI] [PubMed] [Google Scholar]
- 149.Iannuzzi C, Borriello M, Portaccio M, Irace G, Sirangelo I. Insights into Insulin Fibril Assembly at Physiological and Acidic pH and Related Amyloid Intrinsic Fluorescence. Int J Mol Sci. 2017;18 doi: 10.3390/ijms18122551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Manno M, Craparo EF, Podestà A, Bulone D, Carrotta R, Martorana V, Tiana G, San Biagio PL. Kinetics of different processes in human insulin amyloid formation. J Mol Biol. 2007;366:258–274. doi: 10.1016/j.jmb.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 151.Matveyenka M, Zhaliazka K, Kurouski D. Concentration of Phosphatidylserine Influence Rates of Insulin Aggregation and Toxicity of Amyloid Aggregates In Vitro. ACS Chem Neurosci. 2023;14:2396–2404. doi: 10.1021/acschemneuro.3c00277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Alam P, Beg AZ, Siddiqi MK, Chaturvedi SK, Rajpoot RK, Ajmal MR, Zaman M, Abdelhameed AS, Khan RH. Ascorbic acid inhibits human insulin aggregation and protects against amyloid induced cytotoxicity. Arch Biochem Biophys. 2017;621:54–62. doi: 10.1016/j.abb.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 153.Matveyenka M, Rizevsky S, Pellois JP, Kurouski D. Lipids uniquely alter rates of insulin aggregation and lower toxicity of amyloid aggregates. Biochim Biophys Acta Mol Cell Biol Lipids. 2023;1868:159247. doi: 10.1016/j.bbalip.2022.159247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.de Tullio MB, Castelletto V, Hamley IW, Martino Adami PV, Morelli L, Castaño EM. Proteolytically inactive insulin-degrading enzyme inhibits amyloid formation yielding non-neurotoxic aβ peptide aggregates. PLoS One. 2013;8:e59113. doi: 10.1371/journal.pone.0059113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kumar R, Mal K, Razaq MK, Magsi M, Memon MK, Memon S, Afroz MN, Siddiqui HF, Rizwan A. Association of Leptin With Obesity and Insulin Resistance. Cureus. 2020;12:e12178. doi: 10.7759/cureus.12178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Bonda DJ, Stone JG, Torres SL, Siedlak SL, Perry G, Kryscio R, Jicha G, Casadesus G, Smith MA, Zhu X, Lee HG. Dysregulation of leptin signaling in Alzheimer disease: evidence for neuronal leptin resistance. J Neurochem. 2014;128:162–172. doi: 10.1111/jnc.12380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Flores-Cordero JA, Pérez-Pérez A, Jiménez-Cortegana C, Alba G, Flores-Barragán A, Sánchez-Margalet V. Obesity as a Risk Factor for Dementia and Alzheimer's Disease: The Role of Leptin. Int J Mol Sci. 2022;23 doi: 10.3390/ijms23095202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Signore AP, Zhang F, Weng Z, Gao Y, Chen J. Leptin neuroprotection in the CNS: mechanisms and therapeutic potentials. J Neurochem. 2008;106:1977–1990. doi: 10.1111/j.1471-4159.2008.05457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Doherty GH, Beccano-Kelly D, Yan SD, Gunn-Moore FJ, Harvey J. Leptin prevents hippocampal synaptic disruption and neuronal cell death induced by amyloid β. Neurobiol Aging. 2013;34:226–237. doi: 10.1016/j.neurobiolaging.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 160.Ma J, Hou YH, Liao ZY, Ma Z, Zhang XX, Wang JL, Zhu YB, Shan HL, Wang PY, Li CB, Lv YL, Wei YL, Dou JZ. Neuroprotective Effects of Leptin on the APP/PS1 Alzheimer's Disease Mouse Model: Role of Microglial and Neuroinflammation. Degener Neurol Neuromuscul Dis. 2023;13:69–79. doi: 10.2147/DNND.S427781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Davies JS. Ghrelin mediated hippocampal neurogenesis. Vitam Horm. 2022;118:337–367. doi: 10.1016/bs.vh.2021.12.003. [DOI] [PubMed] [Google Scholar]
- 162.Eslami M, Sadeghi B, Goshadrou F. Chronic ghrelin administration restores hippocampal long-term potentiation and ameliorates memory impairment in rat model of Alzheimer's disease. Hippocampus. 2018;28:724–734. doi: 10.1002/hipo.23002. [DOI] [PubMed] [Google Scholar]
- 163.Stoyanova I, Lutz D. Ghrelin-Mediated Regeneration and Plasticity After Nervous System Injury. Front Cell Dev Biol. 2021;9:595914. doi: 10.3389/fcell.2021.595914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.McLaughlin T, Abbasi F, Lamendola C, Frayo RS, Cummings DE. Plasma ghrelin concentrations are decreased in insulin-resistant obese adults relative to equally obese insulin-sensitive controls. J Clin Endocrinol Metab. 2004;89:1630–1635. doi: 10.1210/jc.2003-031572. [DOI] [PubMed] [Google Scholar]
- 165.Gahete MD, Rubio A, Córdoba-Chacón J, Gracia-Navarro F, Kineman RD, Avila J, Luque RM, Castaño JP. Expression of the ghrelin and neurotensin systems is altered in the temporal lobe of Alzheimer's disease patients. J Alzheimers Dis. 2010;22:819–828. doi: 10.3233/JAD-2010-100873. [DOI] [PubMed] [Google Scholar]
- 166.Wang Q, Huang X, Su Y, Yin G, Wang S, Yu B, Li H, Qi J, Chen H, Zeng W, Zhang K, Verkhratsky A, Niu J, Yi C. Activation of Wnt/β-catenin pathway mitigates blood-brain barrier dysfunction in Alzheimer's disease. Brain. 2022;145:4474–4488. doi: 10.1093/brain/awac236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Wang J, Zhao J, Zhang J, Luo X, Gao K, Zhang M, Li L, Wang C, Hu D. Association of Canonical Wnt/β-Catenin Pathway and Type 2 Diabetes: Genetic Epidemiological Study in Han Chinese. Nutrients. 2015;7:4763–4777. doi: 10.3390/nu7064763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Ali A, Ali A, Ahmad W, Ahmad N, Khan S, Nuruddin SM, Husain I. Deciphering the Role of WNT Signaling in Metabolic Syndrome-Linked Alzheimer's Disease. Mol Neurobiol. 2020;57:302–314. doi: 10.1007/s12035-019-01700-y. [DOI] [PubMed] [Google Scholar]
- 169.Jia L, Piña-Crespo J, Li Y. Restoring Wnt/β-catenin signaling is a promising therapeutic strategy for Alzheimer's disease. Mol Brain. 2019;12:104. doi: 10.1186/s13041-019-0525-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Vallée A, Vallée JN, Lecarpentier Y. WNT/β-catenin Pathway: a Possible Link Between Hypertension and Alzheimer's Disease. Curr Hypertens Rep. 2022;24:465–475. doi: 10.1007/s11906-022-01209-1. [DOI] [PubMed] [Google Scholar]
- 171.Kim DY, Jung SY, Kim K, Kim CJ. Treadmill exercise ameliorates Alzheimer disease-associated memory loss through the Wnt signaling pathway in the streptozotocin-induced diabetic rats. J Exerc Rehabil. 2016;12:276–283. doi: 10.12965/jer.1632678.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Assaf N, El-Shamarka ME, Salem NA, Khadrawy YA, El Sayed NS. Neuroprotective effect of PPAR alpha and gamma agonists in a mouse model of amyloidogenesis through modulation of the Wnt/beta catenin pathway via targeting alpha- and beta-secretases. Prog Neuropsychopharmacol Biol Psychiatry. 2020;97:109793. doi: 10.1016/j.pnpbp.2019.109793. [DOI] [PubMed] [Google Scholar]
- 173.Alberti P, Semperboni S, Cavaletti G, Scuteri A. Neurons: The Interplay between Cytoskeleton, Ion Channels/Transporters and Mitochondria. Cells. 2022;11 doi: 10.3390/cells11162499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Dar TA, Sheikh IA, Ganie SA, Ali R, Singh LR, Gan SH, Kamal MA, Zargar MA. Molecular linkages between diabetes and Alzheimer's disease: current scenario and future prospects. CNS Neurol Disord Drug Targets. 2014;13:290–298. doi: 10.2174/18715273113126660135. [DOI] [PubMed] [Google Scholar]
- 175.Zündorf G, Reiser G. Calcium dysregulation and homeostasis of neural calcium in the molecular mechanisms of neurodegenerative diseases provide multiple targets for neuroprotection. Antioxid Redox Signal. 2011;14:1275–1288. doi: 10.1089/ars.2010.3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Albaik M, Sheikh Saleh D, Kauther D, Mohammed H, Alfarra S, Alghamdi A, Ghaboura N, Sindi IA. Bridging the gap: glucose transporters, Alzheimer's, and future therapeutic prospects. Front Cell Dev Biol. 2024;12:1344039. doi: 10.3389/fcell.2024.1344039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Kshatri AS, Gonzalez-Hernandez A, Giraldez T. Physiological Roles and Therapeutic Potential of Ca(2+) Activated Potassium Channels in the Nervous System. Front Mol Neurosci. 2018;11:258. doi: 10.3389/fnmol.2018.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Srikanth V, Maczurek A, Phan T, Steele M, Westcott B, Juskiw D, Münch G. Advanced glycation endproducts and their receptor RAGE in Alzheimer's disease. Neurobiol Aging. 2011;32:763–777. doi: 10.1016/j.neurobiolaging.2009.04.016. [DOI] [PubMed] [Google Scholar]
- 179.Ko SY, Ko HA, Chu KH, Shieh TM, Chi TC, Chen HI, Chang WC, Chang SS. The Possible Mechanism of Advanced Glycation End Products (AGEs) for Alzheimer's Disease. PLoS One. 2015;10:e0143345. doi: 10.1371/journal.pone.0143345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Ottum MS, Mistry AM. Advanced glycation end-products: modifiable environmental factors profoundly mediate insulin resistance. J Clin Biochem Nutr. 2015;57:1–12. doi: 10.3164/jcbn.15-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Gonzalez-Reyes RE, Rubiano MG. Astrocyte´s RAGE: More Than Just a Question of Mood. Cent Nerv Syst Agents Med Chem. 2018;18:39–48. doi: 10.2174/1871524916999160505105121. [DOI] [PubMed] [Google Scholar]
- 182.Choi BR, Cho WH, Kim J, Lee HJ, Chung C, Jeon WK, Han JS. Increased expression of the receptor for advanced glycation end products in neurons and astrocytes in a triple transgenic mouse model of Alzheimer's disease. Exp Mol Med. 2014;46:e75. doi: 10.1038/emm.2013.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Gasparotto J, Ribeiro CT, da Rosa-Silva HT, Bortolin RC, Rabelo TK, Peixoto DO, Moreira JCF, Gelain DP. Systemic Inflammation Changes the Site of RAGE Expression from Endothelial Cells to Neurons in Different Brain Areas. Mol Neurobiol. 2019;56:3079–3089. doi: 10.1007/s12035-018-1291-6. [DOI] [PubMed] [Google Scholar]
- 184.Fang F, Lue LF, Yan S, Xu H, Luddy JS, Chen D, Walker DG, Stern DM, Schmidt AM, Chen JX, Yan SS. RAGE-dependent signaling in microglia contributes to neuroinflammation, Abeta accumulation, and impaired learning/memory in a mouse model of Alzheimer's disease. FASEB J. 2010;24:1043–1055. doi: 10.1096/fj.09-139634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Jiao F, Gong Z. The Beneficial Roles of SIRT1 in Neuroinflammation-Related Diseases. Oxid Med Cell Longev. 2020;2020:6782872. doi: 10.1155/2020/6782872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Song Y, Wu Z, Zhao P. The protective effects of activating Sirt1/NF-κB pathway for neurological disorders. Rev Neurosci. 2022;33:427–438. doi: 10.1515/revneuro-2021-0118. [DOI] [PubMed] [Google Scholar]
- 187.Ma X, Sun Z, Han X, Li S, Jiang X, Chen S, Zhang J, Lu H. Neuroprotective Effect of Resveratrol via Activation of Sirt1 Signaling in a Rat Model of Combined Diabetes and Alzheimer's Disease. Front Neurosci. 2019;13:1400. doi: 10.3389/fnins.2019.01400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Yang H, Tang L, Qu Z, Lei SH, Li W, Wang YH. Hippocampal insulin resistance and the Sirtuin 1 signaling pathway in diabetes-induced cognitive dysfunction. Neural Regen Res. 2021;16:2465–2474. doi: 10.4103/1673-5374.313051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Sun Q, Wei LL, Zhang M, Li TX, Yang C, Deng SP, Zeng QC. Rapamycin inhibits activation of AMPK-mTOR signaling pathway-induced Alzheimer's disease lesion in hippocampus of rats with type 2 diabetes mellitus. Int J Neurosci. 2019;129:179–188. doi: 10.1080/00207454.2018.1491571. [DOI] [PubMed] [Google Scholar]
- 190.de la Monte SM. Malignant Brain Aging: The Formidable Link Between Dysregulated Signaling Through Mechanistic Target of Rapamycin Pathways and Alzheimer's Disease (Type 3 Diabetes) J Alzheimers Dis. 2023;95:1301–1337. doi: 10.3233/JAD-230555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Maiese K. Dysregulation of metabolic flexibility: The impact of mTOR on autophagy in neurodegenerative disease. Int Rev Neurobiol. 2020;155:1–35. doi: 10.1016/bs.irn.2020.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Rapaka D, Bitra VR, Challa SR, Adiukwu PC. mTOR signaling as a molecular target for the alleviation of Alzheimer's disease pathogenesis. Neurochem Int. 2022;155:105311. doi: 10.1016/j.neuint.2022.105311. [DOI] [PubMed] [Google Scholar]
- 193.Van Skike CE, Jahrling JB, Olson AB, Sayre NL, Hussong SA, Ungvari Z, Lechleiter JD, Galvan V. Inhibition of mTOR protects the blood-brain barrier in models of Alzheimer's disease and vascular cognitive impairment. Am J Physiol Heart Circ Physiol. 2018;314:H693–H703. doi: 10.1152/ajpheart.00570.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Spilman P, Podlutskaya N, Hart MJ, Debnath J, Gorostiza O, Bredesen D, Richardson A, Strong R, Galvan V. Inhibition of mTOR by rapamycin abolishes cognitive deficits and reduces amyloid-beta levels in a mouse model of Alzheimer's disease. PLoS One. 2010;5:e9979. doi: 10.1371/journal.pone.0009979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Longo S, Rizza S, Federici M. Microbiota-gut-brain axis: relationships among the vagus nerve, gut microbiota, obesity, and diabetes. Acta Diabetol. 2023;60:1007–1017. doi: 10.1007/s00592-023-02088-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Solas M, Milagro FI, Ramírez MJ, Martínez JA. Inflammation and gut-brain axis link obesity to cognitive dysfunction: plausible pharmacological interventions. Curr Opin Pharmacol. 2017;37:87–92. doi: 10.1016/j.coph.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 197.Luca M, Di Mauro M, Luca A. Gut Microbiota in Alzheimer's Disease, Depression, and Type 2 Diabetes Mellitus: The Role of Oxidative Stress. Oxid Med Cell Longev. 2019;2019:4730539. doi: 10.1155/2019/4730539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Köhler CA, Maes M, Slyepchenko A, Berk M, Solmi M, Lanctôt KL, Carvalho AF. The Gut-Brain Axis, Including the Microbiome, Leaky Gut and Bacterial Translocation: Mechanisms and Pathophysiological Role in Alzheimer's Disease. Curr Pharm Des. 2016;22:6152–6166. doi: 10.2174/1381612822666160907093807. [DOI] [PubMed] [Google Scholar]
- 199.Guo B, Zhang J, Zhang W, Chen F, Liu B. Gut microbiota-derived short chain fatty acids act as mediators of the gut-brain axis targeting age-related neurodegenerative disorders: a narrative review. Crit Rev Food Sci Nutr. 2023:1–22. doi: 10.1080/10408398.2023.2272769. [DOI] [PubMed] [Google Scholar]
- 200.Dalile B, Van Oudenhove L, Vervliet B, Verbeke K. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat Rev Gastroenterol Hepatol. 2019;16:461–478. doi: 10.1038/s41575-019-0157-3. [DOI] [PubMed] [Google Scholar]
- 201.Chen Y, Xu J, Chen Y. Regulation of Neurotransmitters by the Gut Microbiota and Effects on Cognition in Neurological Disorders. Nutrients. 2021;13 doi: 10.3390/nu13062099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Swer NM, Venkidesh BS, Murali TS, Mumbrekar KD. Gut microbiota-derived metabolites and their importance in neurological disorders. Mol Biol Rep. 2023;50:1663–1675. doi: 10.1007/s11033-022-08038-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Mesa-Herrera F, Taoro-González L, Valdés-Baizabal C, Diaz M, Marín R. Lipid and Lipid Raft Alteration in Aging and Neurodegenerative Diseases: A Window for the Development of New Biomarkers. Int J Mol Sci. 2019;20 doi: 10.3390/ijms20153810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Rushworth JV, Hooper NM. Lipid Rafts: Linking Alzheimer's Amyloid-β Production, Aggregation, and Toxicity at Neuronal Membranes. Int J Alzheimers Dis. 2010;2011:603052. doi: 10.4061/2011/603052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Marrano N, Biondi G, Borrelli A, Rella M, Zambetta T, Di Gioia L, Caporusso M, Logroscino G, Perrini S, Giorgino F, Natalicchio A. Type 2 Diabetes and Alzheimer's Disease: The Emerging Role of Cellular Lipotoxicity. Biomolecules. 2023;13 doi: 10.3390/biom13010183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Lee HJ, Ryu JM, Jung YH, Lee SJ, Kim JY, Lee SH, Hwang IK, Seong JK, Han HJ. High glucose upregulates BACE1-mediated Aβ production through ROS-dependent HIF-1α and LXRα/ABCA1-regulated lipid raft reorganization in SK-N-MC cells. Sci Rep. 2016;6:36746. doi: 10.1038/srep36746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Messiha BAS, Ali MRA, Khattab MM, Abo-Youssef AM. Perindopril ameliorates experimental Alzheimer's disease progression: role of amyloid β degradation, central estrogen receptor and hyperlipidemic-lipid raft signaling. Inflammopharmacology. 2020;28:1343–1364. doi: 10.1007/s10787-020-00724-4. [DOI] [PubMed] [Google Scholar]
- 208.Abolfotoh MM, Khloussy HA, Mustafa H, Eshra MA. Effects of Life Style Modifications (Dietary and Exercise) on Insulin Resistance in Egyptian Men with Impaired Glucose Tolerance. J Adv Zool. 2023;44 [Google Scholar]
- 209.Bruckner F, Gruber JR, Ruf A, Edwin Thanarajah S, Reif A, Matura S. Exploring the Link between Lifestyle, Inflammation, and Insulin Resistance through an Improved Healthy Living Index. Nutrients. 2024;16 doi: 10.3390/nu16030388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.McAuley KA, Williams SM, Mann JI, Goulding A, Chisholm A, Wilson N, Story G, McLay RT, Harper MJ, Jones IE. Intensive lifestyle changes are necessary to improve insulin sensitivity: a randomized controlled trial. Diabetes Care. 2002;25:445–452. doi: 10.2337/diacare.25.3.445. [DOI] [PubMed] [Google Scholar]
- 211.Smiley D, Umpierrez G. Metformin/rosiglitazone combination pill (Avandamet) for the treatment of patients with Type 2 diabetes. Expert Opin Pharmacother. 2007;8:1353–1364. doi: 10.1517/14656566.8.9.1353. [DOI] [PubMed] [Google Scholar]
- 212.Moreno-Cabañas A, Morales-Palomo F, Alvarez-Jimenez L, Mora-Gonzalez D, Ortega JF, Mora-Rodriguez R. Metformin and exercise effects on postprandial insulin sensitivity and glucose kinetics in pre-diabetic and diabetic adults. Am J Physiol Endocrinol Metab. 2023;325:E310–E324. doi: 10.1152/ajpendo.00118.2023. [DOI] [PubMed] [Google Scholar]
- 213.Al Neyadi SS, Adem A, Amir N, Ghattas MA, Abdou IM, Salem AA. Novel Thiazolidinedione and Rhodanine Derivatives Regulate Glucose Metabolism, Improve Insulin Sensitivity, and Activate the Peroxisome Proliferator-Activated γ Receptor. ACS Omega. 2024;9:5463–5484. doi: 10.1021/acsomega.3c07149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Reginato MJ, Lazar MA. Mechanisms by which Thiazolidinediones Enhance Insulin Action. Trends Endocrinol Metab. 1999;10:9–13. doi: 10.1016/s1043-2760(98)00110-6. [DOI] [PubMed] [Google Scholar]
- 215.Cimmaruta D, Maiorino MI, Scavone C, Sportiello L, Rossi F, Giugliano D, Esposito K, Capuano A. Efficacy and safety of insulin-GLP-1 receptor agonists combination in type 2 diabetes mellitus: a systematic review. Expert Opin Drug Saf. 2016;15:77–83. doi: 10.1080/14740338.2016.1221402. [DOI] [PubMed] [Google Scholar]
- 216.Madsbad S. Exenatide and liraglutide: different approaches to develop GLP-1 receptor agonists (incretin mimetics)--preclinical and clinical results. Best Pract Res Clin Endocrinol Metab. 2009;23:463–477. doi: 10.1016/j.beem.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 217.Andrianesis V, Doupis J. The role of kidney in glucose homeostasis--SGLT2 inhibitors, a new approach in diabetes treatment. Expert Rev Clin Pharmacol. 2013;6:519–539. doi: 10.1586/17512433.2013.827399. [DOI] [PubMed] [Google Scholar]
- 218.Brown E, Wilding JPH, Alam U, Barber TM, Karalliedde J, Cuthbertson DJ. The expanding role of SGLT2 inhibitors beyond glucose-lowering to cardiorenal protection. Ann Med. 2021;53:2072–2089. doi: 10.1080/07853890.2020.1841281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Zhang JH, Zhang XY, Sun YQ, Lv RH, Chen M, Li M. Metformin use is associated with a reduced risk of cognitive impairment in adults with diabetes mellitus: A systematic review and meta-analysis. Front Neurosci. 2022;16:984559. doi: 10.3389/fnins.2022.984559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Samaras K, Makkar S, Crawford JD, Kochan NA, Wen W, Draper B, Trollor JN, Brodaty H, Sachdev PS. Metformin Use Is Associated With Slowed Cognitive Decline and Reduced Incident Dementia in Older Adults With Type 2 Diabetes: The Sydney Memory and Ageing Study. Diabetes Care. 2020;43:2691–2701. doi: 10.2337/dc20-0892. [DOI] [PubMed] [Google Scholar]
- 221.Alhowail A, Alsikhan R, Alsaud M, Aldubayan M, Rabbani SI. Protective Effects of Pioglitazone on Cognitive Impairment and the Underlying Mechanisms: A Review of Literature. Drug Des Devel Ther. 2022;16:2919–2931. doi: 10.2147/DDDT.S367229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Saunders AM, Burns DK, Gottschalk WK. Reassessment of Pioglitazone for Alzheimer's Disease. Front Neurosci. 2021;15:666958. doi: 10.3389/fnins.2021.666958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Kong F, Wu T, Dai J, Zhai Z, Cai J, Zhu Z, Xu Y, Sun T. Glucagon-like peptide 1 (GLP-1) receptor agonists in experimental Alzheimer's disease models: a systematic review and meta-analysis of preclinical studies. Front Pharmacol. 2023;14:1205207. doi: 10.3389/fphar.2023.1205207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Mancinetti F, Xenos D, De Fano M, Mazzieri A, Porcellati F, Boccardi V, Mecocci P. Diabetes-Alzheimer's connection in older age: SGLT2 inhibitors as promising modulators of disease pathways. Ageing Res Rev. 2023;90:102018. doi: 10.1016/j.arr.2023.102018. [DOI] [PubMed] [Google Scholar]
- 225.Ali L. The Neuroprotective Effects of SGLT2 or Nox1/Nox4 Selective Inhibitors on Alzheimer’s-Like Symptoms Development in Diabetic Mice. 2020. Available from: http://hdl.handle.net/10938/22129 .
- 226.Chen Y, Ma H, Zhu D, Zhao G, Wang L, Fu X, Chen W. Discovery of Novel Insulin Sensitizers: Promising Approaches and Targets. PPAR Res. 2017;2017:8360919. doi: 10.1155/2017/8360919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Dama A, Shpati K, Daliu P, Dumur S, Gorica E, Santini A. Targeting Metabolic Diseases: The Role of Nutraceuticals in Modulating Oxidative Stress and Inflammation. Nutrients. 2024;16 doi: 10.3390/nu16040507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Méndez L, Medina I. Polyphenols and Fish Oils for Improving Metabolic Health: A Revision of the Recent Evidence for Their Combined Nutraceutical Effects. Molecules. 2021;26 doi: 10.3390/molecules26092438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Martin B, Mattson MP, Maudsley S. Caloric restriction and intermittent fasting: two potential diets for successful brain aging. Ageing Res Rev. 2006;5:332–353. doi: 10.1016/j.arr.2006.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Pani G. Neuroprotective effects of dietary restriction: Evidence and mechanisms. Semin Cell Dev Biol. 2015;40:106–114. doi: 10.1016/j.semcdb.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 231.Anson RM, Guo Z, de Cabo R, Iyun T, Rios M, Hagepanos A, Ingram DK, Lane MA, Mattson MP. Intermittent fasting dissociates beneficial effects of dietary restriction on glucose metabolism and neuronal resistance to injury from calorie intake. Proc Natl Acad Sci U S A. 2003;100:6216–6220. doi: 10.1073/pnas.1035720100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Sabbagh M, Sadowsky C, Tousi B, Agronin ME, Alva G, Armon C, Bernick C, Keegan AP, Karantzoulis S, Baror E, Ploznik M, Pascual-Leone A. Effects of a combined transcranial magnetic stimulation (TMS) and cognitive training intervention in patients with Alzheimer's disease. Alzheimers Dement. 2020;16:641–650. doi: 10.1016/j.jalz.2019.08.197. [DOI] [PubMed] [Google Scholar]
- 233.Li BY, Tang HD, Qiao Y, Chen SD. Mental Training for Cognitive Improvement in Elderly People: What have We Learned from Clinical and Neurophysiologic Studies? Curr Alzheimer Res. 2015;12:543–552. doi: 10.2174/156720501206150716112918. [DOI] [PubMed] [Google Scholar]
- 234.Baldi S, Mundula T, Nannini G, Amedei A. Microbiota shaping - the effects of probiotics, prebiotics, and fecal microbiota transplant on cognitive functions: A systematic review. World J Gastroenterol. 2021;27:6715–6732. doi: 10.3748/wjg.v27.i39.6715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Coutts L, Ibrahim K, Tan QY, Lim SER, Cox NJ, Roberts HC. Can probiotics, prebiotics and synbiotics improve functional outcomes for older people: a systematic review. Eur Geriatr Med. 2020;11:975–993. doi: 10.1007/s41999-020-00396-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Banerjee A, Sharma D, Trivedi R, Singh J. Treatment of insulin resistance in obesity-associated type 2 diabetes mellitus through adiponectin gene therapy. Int J Pharm. 2020;583:119357. doi: 10.1016/j.ijpharm.2020.119357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Bi S, Nie Q, Wang WQ, Zhu YL, Ma XM, Wang CM, Zhang BC, Li HY, Zhang Q, Chen G. Human Umbilical Cord Mesenchymal Stem Cells Therapy for Insulin Resistance: A Novel Strategy in Clinical Implication. Curr Stem Cell Res Ther. 2018;13:658–664. doi: 10.2174/1574888X13666180810154048. [DOI] [PubMed] [Google Scholar]
- 238.Ozben T, Ozben S. Neuro-inflammation and anti-inflammatory treatment options for Alzheimer's disease. Clin Biochem. 2019;72:87–89. doi: 10.1016/j.clinbiochem.2019.04.001. [DOI] [PubMed] [Google Scholar]
- 239.Moore AH, O'Banion MK. Neuroinflammation and anti-inflammatory therapy for Alzheimer's disease. Adv Drug Deliv Rev. 2002;54:1627–1656. doi: 10.1016/s0169-409x(02)00162-x. [DOI] [PubMed] [Google Scholar]
- 240.Zhao X, Han Q, Lv Y, Sun L, Gang X, Wang G. Biomarkers for cognitive decline in patients with diabetes mellitus: evidence from clinical studies. Oncotarget. 2018;9:7710–7726. doi: 10.18632/oncotarget.23284. [DOI] [PMC free article] [PubMed] [Google Scholar]