Abstract
OBJECTIVES:
The American Academy of Pediatrics National Registry for the Surveillance and Epidemiology of Perinatal coronavirus disease 2019 (COVID-19) (NPC-19) was developed to provide information on the effects of perinatal severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection.
METHODS:
National Registry for the Surveillance and Epidemiology of Perinatal COVID-19 participating centers entered maternal and newborn data for pregnant persons who tested positive for SARS-CoV-2 infection between 14 days before and 10 days after delivery. Incidence of and morbidities associated with maternal and newborn SARS-CoV-2 infection were assessed.
RESULTS:
From April 6, 2020 to March 19, 2021, 242 centers in the United States centers reported data for 7524 pregnant persons; at the time of delivery, 78.1% of these persons were asymptomatic, 18.2% were symptomatic but not hospitalized specifically for COVID-19, 3.4% were hospitalized for COVID-19 treatment, and 18 (0.2%) died in the hospital of COVID-related complications. Among 7648 newborns, 6486 (84.8%) were tested for SARS-CoV-2, and 144 (2.2%) were positive; the highest rate of newborn infection was observed when mothers first tested positive in the immediate postpartum period (17 of 125, 13.6%). No newborn deaths were attributable to SARS-CoV-2 infection. Overall, 15.6% of newborns were preterm: among tested newborns, 30.1% of polymerase chain reaction-positive and 16.2% of polymerase chain reaction-negative were born preterm (P < .001). Need for mechanical ventilation did not differ by newborn SARS-CoV-2 test result, but those with positive tests were more likely to be admitted to a NICU.
CONCLUSIONS:
Early in the pandemic, SARS-CoV-2 infection was acquired by newborns at variable rates and without apparent short-term effects. During a period that preceded widespread availability of vaccines, we observed higher than expected numbers of preterm births and maternal in-hospital deaths.
At the onset of the coronavirus disease 2019 (COVID-19) pandemic, clinicians had limited data on which to base perinatal care decisions when pregnant persons developed severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. Prior experience with perinatal respiratory viral disease provided reason for concern. Influenza infections among pregnant persons are associated with higher rates of maternal death and preterm birth, and newborns acutely infected with influenza, respiratory syncytial virus, and varicella can develop severe illness if infected in the immediate postnatal period.1–6 Initial reports of infected pregnant persons in China described immediate maternal and newborn separation at birth to prevent postnatal newborn infection.7–9 Interim guidance from organizations such as the American Academy of Pediatrics (AAP), the American College of Obstetricians and Gynecologists, the United States Centers for Disease Control and Prevention (CDC), and the World Health Organization variably recommended a panel of infection prevention measures to protect newborns from acquiring SARS-CoV-2 from infected mothers while preserving dyad contact and breast milk feeding.10
Perinatal disease surveillance in the United States is frequently challenged by healthcare structures that provide obstetric and pediatric care in separate health systems. To address these challenges and to characterize the risks of perinatal COVID-19, the AAP Section on Neonatal-Perinatal Medicine launched the National Registry for the Surveillance and Epidemiology of Perinatal COVID-19 (NPC-19) in March 2020. The goals of the registry were to quantify and characterize SARS-CoV-2 infections by selected maternal and newborn characteristics and care practices and to describe the short-term health outcomes of pregnant persons and their newborns affected by perinatal COVID-19. This report summarizes NPC-19 information on 7524 pregnant persons and their newborns reported between April 6, 2020 and March 19, 2021.
METHODS
NPC-19 Database and Regulatory Processes
The NPC-19 database was developed by select authors (M.L.H., D.D.F., S.M., K.M.P.) and finalized with input from members of the Section on Neonatal-Perinatal Medicine Executive Committee and representatives of the Vermont-Oxford Network and the Mednax medical group. The University of Florida (UFL) Institutional Review Board approved NPC-19 as a data repository of deidentified data with a waiver of consent as a minimal risk study. Two hundred forty-two medical centers voluntarily participated with center-specific IRB approval. Centers and investigators contributing data to this report are listed in Supplemental Table 4; geographic distribution is shown in Supplemental Fig 3. Data were abstracted by center data specialists and entered into Research Electronic Data Capture (REDCap), a web-based, password-protected database hosted at UFL.11,12 Case-subject linkage to medical record numbers was held at the submitting center; the UFL coordinating team queried centers regarding missing data. This activity was reviewed by CDC and was conducted consistent with applicable federal law and CDC policy.
Study Population
The manuscript uses “pregnant persons” to be inclusive of all pregnant persons, regardless of gender. The words “maternal” and “mother” are also used to refer to the person who gave birth to infants included in the study. However, the authors are aware that pregnancy is not equated with the decision to parent nor do all parents who give birth identify as mothers. The manuscript accounts for maternal race and ethnicity because of prepandemic evidence that these social constructs are associated with preterm birth and maternal mortality. NPC-19 collected information about pregnant persons with positive nasopharyngeal polymerase chain reaction (PCR) testing for SARS-CoV-2 during pregnancy or the immediate postpartum period and their newborns. This report is limited to data reported from April 6, 2020 to March 19, 2021 and included pregnant persons who first tested positive from 14 days before delivery through 10 days after delivery. With the goal to assess newborn SARS-CoV-2 infections and hospital outcomes, we excluded pregnancies resulting in stillbirth or neonatal death in the delivery room (Fig 1). NPC-19 data elements collected via medical record abstraction are provided in Supplemental Table 5. Gestational age was determined by the abstractor based on review of the medical record; preterm delivery was defined as birth at <37 0/7 weeks’ gestation. Maternal or newborn separation was defined as any care structure where mother and newborn did not share the birth hospital room. For infants cared for in the NICU, and for those born in centers that did not practice mother or newborn rooming-in, separation was defined by practices that deviated from routine prepandemic mother or newborn interaction. Determination of whether the maternal or newborn death was related to SARS-CoV-2 infection was based on the judgment of local providers.
FIGURE 1.
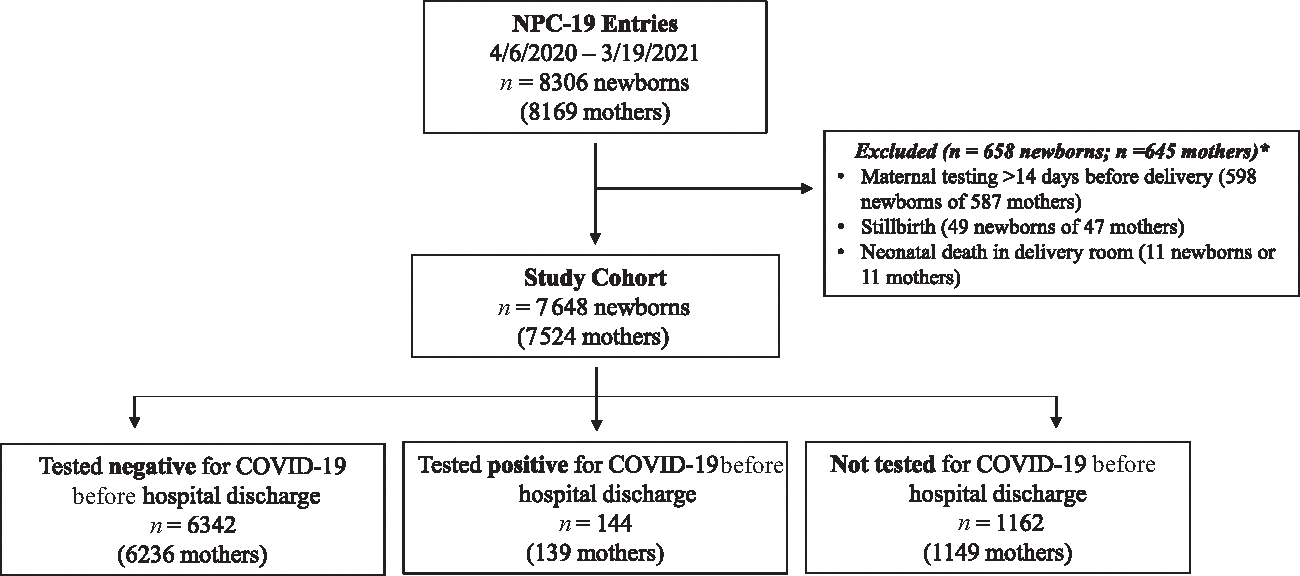
STROBE Diagram, American Academy of Pediatrics Perinatal COVID 19 Registry, United States, April 6, 2020 to March 19, 2021. Because of 11 twin gestations, there were 587 mothers and 598 newborns excluded because of maternal positive test for SARS-CoV-2 occurring >14 days before delivery. Because of 2 twin gestations, there were 47 mothers and 49 newborns excluded because of stillbirth.
Statistical Analysis
Descriptive statistics including medians and interquartile ranges (interquartile range [IQR], 25th and 75th percentiles) or frequencies and percentages were generated, and bivariate analyses were performed to examine associations by comparing groups, using Wilcoxon rank sum tests for continuous outcome variables, and Pearson’s χ2 tests or Fisher’s exact tests for categorical outcome variables. P value less than .05 was considered statistically significant. All analyses were performed using SAS v9.4 (SAS Institute Inc, Cary, North Carolina).
RESULTS
Study Population
During the study period, 242 of 296 registered sites entered data pertaining to 8306 maternal/newborn dyads (Fig 1). A total of 587 pregnant persons (598 newborns) were excluded for having positive test results >14 days before or >10 days after delivery. We excluded 49 stillbirths (47 mothers) and 11 newborns (11 mothers) who died in the delivery room; the final study cohort consisted of 7524 pregnant persons and 7648 newborns. Data were reported from 41 states and the District of Columbia (Supplemental Fig 3); in 2019, these medical centers accounted for 18.1% of all US births (data not shown).
Maternal Characteristics and Outcomes
Persons of Hispanic ethnicity comprised 44.4% of the cohort (Table 1). One-third (35.3%) of newborns were born by caesarean delivery. The majority (5584 of 7524, 74.2%) of pregnant persons were tested for SARS-CoV-2 infection because of obstetric hospital admission policy; 1947 (25.9%) were tested because of clinical symptoms and 588 (7.8%) were tested because of travel or exposure to another infected person. Most (78.1%) reported no symptoms of COVID-19 at the time of admission for delivery. Among the 254 pregnant persons hospitalized before delivery for COVID-19 treatment, the highest level of respiratory support administered was supplemental oxygen in 112 (44.1%), continuous positive airway pressure in 6 (2.4%); mechanical ventilation in 70 (27.6%), and extracorporeal membrane oxygenation (ECMO) in 10 (3.9%) cases. One pregnant person undergoing ECMO received a lung transplant after delivery and another required tracheostomy. Death before hospital discharge occurred in 18 (0.2%) mothers; maternal outcome was unknown for 6 persons who were transferred from the birth hospital to an outside facility. Among those who died, 8 mothers were non-Hispanic Black, 7 were Hispanic, 1 non-Hispanic white, and 2 were other race and ethnicity. Mothers who died trended older than those who survived (median 30.5 years (IQR 27–37) versus 28 years (IQR 24–33), P = .08) and a higher proportion were non-Hispanic Black (8 of 18, 44.4%) compared with those who survived (1449 of 7506, 19.3%) (P = .013). Of those who died, 9 of 18 had been hospitalized because of COVID-19 during the 14 days before delivery, and 8 of 18 presented with symptoms at the time of admission for delivery. The clinical presentation for 1 person who died was not reported.
TABLE 1.
Characteristics of Pregnant Persons in the American Academy of Pediatrics Perinatal COVID-19 Registry, United States, April 6, 2020 to March 19, 2021
| All (n = 7524) | Newborn PCR Positive (n = 139) | Newborn PCR Negative (n = 6236) | P | |
|---|---|---|---|---|
|
| ||||
| Age at delivery admission, y, median (IQR) | 28 (24–33) | 28 (23–33) | 28 (24–33) | .988 |
| Gravidity, n, median (IQR) | 2 (1–4) | 2 (1–4) | 2 (1–4) | .917 |
| Race and ethnicity, n (%) | .187 | |||
| Hispanic | 3337 (44.4) | 67 (48.2) | 2898 (46.5) | |
| Non-Hispanic Black | 1457 (19.4) | 17 (12.2) | 1249 (20.0) | |
| Non-Hispanic white | 1846 (24.5) | 38 (27.3) | 1366 (21.9) | |
| Asian | 290 (3.9) | 8 (5.8) | 230 (3.7) | |
| American Indian or Alaska Native | 63 (0.8) | 1 (0.7) | 50 (0.8) | |
| Native Hawaiian or other Pacific Islander | 62 (0.8) | 0 (0) | 49 (0.8) | |
| Other or unknowna | 469 (6.2) | 8 (5.8) | 394 (6.3) | |
| Multiple gestation, n (%)b | 145 (1.9) | 10 (7.2) | 123 (2.0) | .005 |
| Betamethasone before delivery, n (%) | 571/7381 (7.7) | 16/135 (11.9) | 505/6117 (8.3) | .135 |
| Labor, n (%) | .074 | |||
| Spontaneous | 3443 (45.8) | 73 (52.5) | 2809 (45.0) | |
| Induced | 2845 (37.8) | 40 (28.8) | 2376 (38.1) | |
| No labor | 1200 (15.9) | 26 (18.7) | 1022 (16.4) | |
| Unknown | 36 (0.5) | 0 | 29 (0.5) | |
| Duration of ROM, hours, median (IQR) | 2.5 (0–8) | 2.0 (0–8) | 2.1 (0–9) | .832 |
| Delivery mode, n (%)c | .655 | |||
| Vaginal | 4919/7648 (64.3) | 87/144 (60.4) | 3996/6342 (63.0) | |
| Cesarean | 2696/7648 (35.3) | 55/144 (38.2) | 2319/6342 (36.6) | |
| Unknown | 33/7648 (0.4) | 2/144 (1.4) | 27/6342 (0.4) | |
| Gestation at delivery, weeks, median (IQR) | 39 (37–39) | 38 (36–39) | 39 (37–39) | .001 |
| Maternal COVID-19 status at admission, n (%) | .002 | |||
| Asymptomatic | 5875 (78.1) | 91 (65.5) | 4857 (77.9) | |
| Symptomatic but not hospitalized specifically for COVID-19 treatment | 1369 (18.2) | 41 (29.5) | 1133 (18.2) | |
| Hospitalized for COVID-19 treatment ≤14 d before delivery | 254 (3.4) | 5 (3.6) | 228 (3.7) | |
| Unknown | 26 (0.4) | 2 (1.4) | 18 (0.3) | |
| Duration of hospitalization, d, median (IQR) | 3 (2–4) | 3 (2–5) | 3 (2–4) | .027 |
| Maternal status at discharge, n (%) | >.99 | |||
| Discharged from the hospital | 7483 (99.5) | 138 (99.3) | 6197 (99.4) | |
| Transferred | 21 (0.3) | 0 (0) | 21 (0.3) | |
| Died of COVID-related complication | 18 (0.2) | 0 (0) | 17 (0.3) | |
| Unknown | 2 (0) | 1 (0.7) | 1 (0) | |
Newborn PCR test results were not available or not reported for 1149 persons. IQR, interquartile range (25th and 75th percentiles); ROM, rupture of membranes.
Other or unknown category includes 171 persons with other race or ethnicity, 250 persons with unknown ethnicity, and 48 persons of non-Hispanic ethnicity with unknown race.
Among multiple gestation PCR-positive multiple pairs, registry includes both twins in only 5 of 10 instances.
Denominator is for total infants: 144 for PCR positive, 6342 for PCR negative.
Newborn Characteristics and Outcomes
SARS-CoV-2 testing data were available for 6486 of 7648 (84.8%) newborns. Most (6337 of 6486, 97.7%) newborns were tested by the third day after birth and 3035 of 6486 (46.8%) were tested more than once. Preterm newborns were more frequently tested compared with those born at term (1069 of 1190, 89.8% vs 5417 of 6458, 83.9%, P < .001). Differences were observed between tested and untested newborns (Supplemental Tables 6 and 7): those untested were more frequently born to asymptomatic and non-Hispanic white mothers and were less likely to be separated from their mother after birth.
Overall, 15.6% of newborns were born preterm (Table 2). Among PCR-positive newborns, 30.1% were born preterm compared with 16.2% among PCR-negative newborns (P < .001). Maternal race and ethnicity and COVID-19 status were associated with preterm birth (Table 3). Among the 1104 pregnant persons who delivered preterm, a greater proportion were symptomatic, received COVID-specific therapies, and/or were hospitalized for COVID-19 ≤14 days before delivery, compared with pregnant persons delivering at term (Table 3).
TABLE 2.
Characteristics of Newborns in the American Academy of Pediatrics Perinatal COVID-19 Registry, United States, April 6, 2020 to March 19, 2021
| All Newborns (n = 7648) | PCR Positive (n = 144) | PCR Negative (n = 6342) | P | |
|---|---|---|---|---|
|
| ||||
| Birth wt, g, median (IQR) | 3210 (2830–3540) | 3040 (2518–3493) | 3200 (2820–3530) | .007 |
| Sex, n (%) | .797 | |||
| Female | 3678 (48.1) | 70 (48.3) | 3011 (47.5) | |
| Male | 3966 (51.9) | 74 (51.4) | 3327 (52.5) | |
| Unreported | 4 (0.1) | 0 (0) | 4 (0.1) | |
| Multiple gestation, n (%)a | 269/7632 (3.5) | 15/143 (10.5) | 229/6327 (3.6) | .008 |
| Small for gestational age, n (%)b | 748/7641 (9.8) | 14/144 (9.7) | 632/6337 (10.0) | .907 |
| Preterm, n (%) | <.001 | |||
| Born <37 wk’ | 1190 (15.6) | 43 (29.9) | 1026 (16.2) | |
| Born ≥37 wk’ gestation | 6458 (84.4) | 101 (70.1) | 5316 (83.8) | |
| Infants born ≥37 wk’ gestation (n = 6458) | ||||
| Apgar <7 at 5 min, n (%)c | 75/6406 (1.2) | 3/101 (3.0) | 62/5268 (1.2) | .125 |
| Delivery room resuscitation, n (%)d | 484/6458 (7.5) | 7/101 (6.9) | 427/5316 (8.0) | .469 |
| NICU admission, n (%)e | 901/6392 (14.1) | 24/100 (24.0) | 831/5264 (15.8) | .036 |
| Mechanical ventilation, n (%)f | 63/6394 (1.0) | 3/100 (3.0) | 55/5259 (1.0) | .083 |
| Infants born <37 wk’ gestation (n = 1190) | ||||
| Gestational age, weeks, n (%) | .254 | |||
| <28 | 42/1190 (3.5) | 3/43 (7.0) | 32/1026 (3.1) | |
| 28–31 | 149/1190 (12.5) | 4/43 (9.3) | 136/1026 (13.3) | |
| 32–33 | 163/1190 (13.7) | 10/43 (23.3) | 142/1026 (13.8) | |
| 34–36 | 836/1190 (70.3) | 26/43 (60.5) | 716/1026 (69.8) | |
| Apgar <7 at 5 min, n (%)g | 121/1190 (10.2) | 5/43 (11.6) | 104/1011 (10.3) | .782 |
| Delivery room resuscitation, n (%)d | 511/1190 (42.9) | 23/43 (53.5) | 458/1026 (44.6) | .274 |
| NICU admission, n (%)h | 699/1177 (59.4) | 32/41 (78.0) | 627/1016 (61.7) | .044 |
| Mechanical ventilation, n (%)i | 184/1182 (15.6) | 11/43 (25.6) | 162/1019 (15.9) | .103 |
| Newborn isolation procedures, n (%)j | .039 | |||
| None | 725 (9.5) | 10 (6.9) | 397 (6.3) | |
| Enhanced respiratory precautions | 2053 (26.8) | 44 (30.6) | 1731 (27.3) | |
| Airborne precautions | 2886 (37.7) | 45 (31.3) | 2492 (39.3) | |
| Negative pressure isolation | 1693 (22.1) | 41 (28.5) | 1488 (23.5) | |
| Other or not reported | 291 (3.8) | 4 (2.8) | 234 (3.7) | |
| Maternal-infant separation at birth, n (%)k | 2414/7610 (31.7) | 55/144 (38.2) | 2247/6316 (35.6) | .541 |
| Any breast milk feeding, n (%) | 5401 (70.6) | 105 (72.9) | 4352 (68.6) | .284 |
| Duration of hospitalization, days, median (IQR) | 2 (2–3) | 3 (2–8.5) | 3 (2–4) | .001 |
| Infant disposition, n (%) | .041 | |||
| Discharge home | 7541 (98.6) | 139 (96.5) | 6268 (98.8) | |
| Transferred | 78 (1.0) | 3 (2.1) | 59 (0.9) | |
| Died | 29 (0.4) | 2 (1.4) | 15 (0.2) | |
Table includes all live births that survived beyond delivery room and with available postnatal testing data. IQR, interquartile range (25th and 75th percentiles).
In 5 sets of twins, both twins tested positive. In 3 sets of twins, 1 twin tested positive and 1 tested negative. In 2 additional cases, 1 infant of a set of twins was positive, but no data were recorded for the other twin.
Data missing for 18 infants.
Data missing for 52 infants.
Delivery room resuscitation defined as requiring continuous positive airway pressure, positive pressure ventilation, or intubation.
Data missing for 66 infants.
Data missing for 64 infants. Extracorporeal membrane oxygenation (ECMO) support provided to 2 infants.
Data missing for 15 infants.
Data missing for 13 infants.
Data missing for 8 infants. No infants born <37 wk’ gestation were treated with ECMO.
Newborn isolation measures were defined by strictest level of precaution used: enhanced respiratory precautions defined as use of gown, gloves, surgical mask, and eye protection; airborne precautions defined by use of N95 mask or equivalent; negative pressure isolation defined as use of negative pressure room for newborn care.
Data missing for 38 infants.
TABLE 3.
Maternal Factors Associated With Preterm Delivery in the American Academy of Pediatrics Perinatal COVID-19 Registry, United States, April 6, 2020 to March 19, 2021
| All (n = 7524) | Delivery ≥37 wk (n = 6420) | Delivery <37 wk (n = 1104) | P | |
|---|---|---|---|---|
|
| ||||
| Age, y, median (IQR) | 28 (24–33) | 28 (24–33) | 29 (24–34) | .644 |
| Gravidity, n, median (IQR) | 2 (1–4) | 2 (1–4) | 3 (1–4) | .464 |
| Race and ethnicity, n (%) | .001 | |||
| Hispanic | 3337 (44.4) | 2869 (44.7) | 468 (42.4) | |
| Non-Hispanic Black | 1457 (19.4) | 1199 (18.7) | 258 (23.4) | |
| Non-Hispanic white | 1846 (24.5) | 1608 (25.0) | 238 (21.6) | |
| Asian | 290 (3.9) | 244 (3.8) | 46 (4.2) | |
| American Indian or Alaska Native | 63 (0.8) | 46 (0.7) | 17 (1.5) | |
| Native Hawaiian or other Pacific Islander | 62 (0.8) | 50 (0.8) | 12 (1.1) | |
| Othera | 469 (6.2) | 404 (6.3) | 65 (5.9) | |
| COVID-19 status, n (%) | <.001 | |||
| Asymptomatic | 5875 (78.1) | 5184 (80.7) | 691 (62.6) | |
| Symptomatic but did not require hospitalization | 1369 (18.2) | 1127 (17.6) | 242 (21.9) | |
| Hospitalized for COVID-19 ≤14 d before delivery | 254 (3.4) | 90 (1.4) | 164 (14.9) | |
| Unknown | 26 (0.4) | 19 (0.3) | 7 (0.6) | |
| Maternal COVID-19 medical therapy administered, n (%)b | 517/7418 (7.0) | 297/6339 (4.7) | 220/1079 (20.4) | <.001 |
IQR, interquartile range (25th and 75th percentiles).
Other or unknown category includes 171 persons with other race and ethnicity; 250 persons with unknown ethnicity; 48 persons non-Hispanic ethnicity with unknown race.
One hundred six mothers had unknown or unreported data for maternal COVID-19 medical therapy: 81 unknown or unreported among the 6420 mothers with term deliveries; and 25 unknown or unreported among the 1104 mothers with preterm delivery.
Overall, 31.7% of dyads were separated at birth (Table 2); the frequency of separation was 54.2% for dyads during April to June 2020 and 22.0% during January to March 2021 (data not shown). Only 9.5% of newborns were cared for without any infection control precautions. Among 7537 newborns with available breastfeeding data, 70.6% were fed maternal or donor human milk, ranging from 59.1% of newborns during April to June 2020 to 77.1% during January to March 2021 (data not shown). Dyad separation at birth, newborn isolation precautions, and human milk feeding did not differ by newborn SARS-CoV-2 test result. Stratified by term or preterm birth, the rates of low 5-minute Apgar score and need for resuscitation at birth were similar among newborns testing positive or negative for SARS-CoV-2, but rates of NICU admission were significantly higher among those who tested PCR-positive (Table 2). Overall, 29 of 7648 (0.4%) newborns died, but no deaths were attributed to SARS-CoV-2 infection; 78 of 7648 (1.0%) were transferred from the birth hospital for higher level of care.
Maternal COVID-19 Clinical Status on Admission for Delivery and Newborn SARS-CoV-2 Infection
There were no differences in infant SARS CoV-2 infection by maternal race and ethnicity (Table 1). The proportion of deliveries with at least 1 newborn testing positive was higher for pregnant persons who were symptomatic versus asymptomatic at the time of delivery (Fig 2 and Supplemental Table 8; 41 of 1174, 3.5% vs 91 of 4948, 1.8%, P = .001). The proportion of deliveries with a newborn testing positive for SARS-CoV-2 was highest (17 of 125, 13.6%) in cases where pregnant persons were asymptomatic on admission for delivery but tested positive in the postpartum period (Fig 2 and Supplemental Table 8). Among these cases, maternal and newborn separation occurred in 47 of 125. The rate at which at least 1 newborn tested positive was higher among unseparated dyads (15 of 78, 19.2%) compared with those separated (2 of 47, 4.3%) (P = .029).
FIGURE 2.
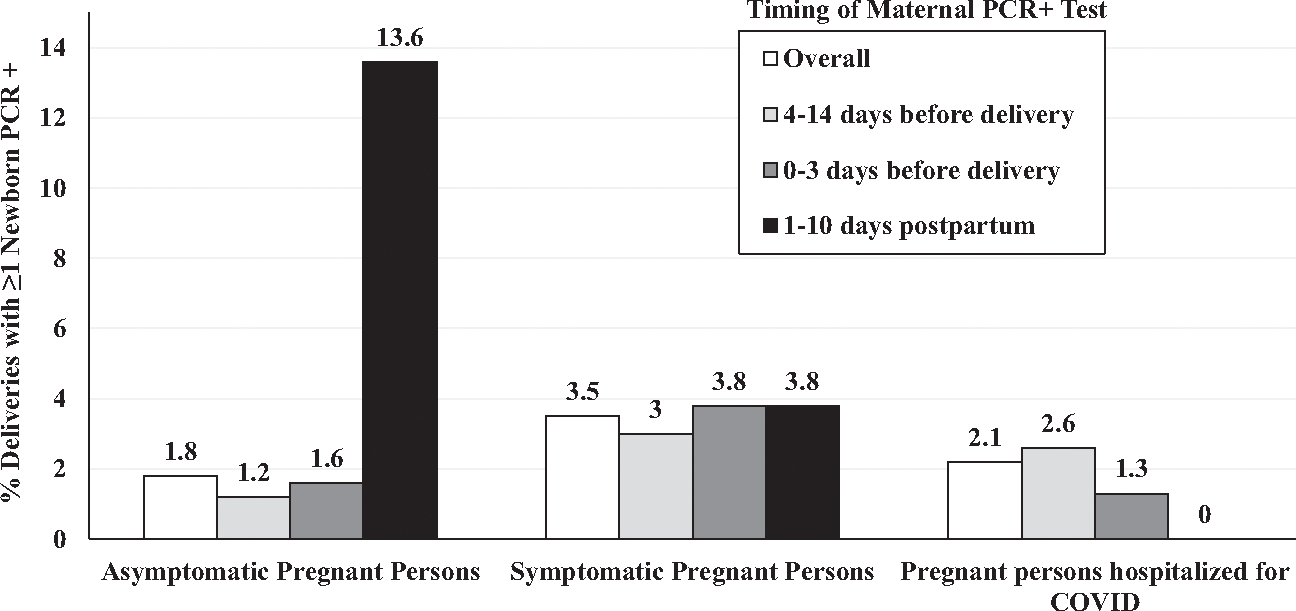
PCR+ newborns in relation to maternal COVID status at delivery and timing of maternal infection, American Academy of Pediatrics Perinatal COVID-19 Registry, United States, April 6, 2020 to March 19, 2021. Graph shows proportions of deliveries with at least 1 newborn who tested positive for SARS-CoV-2 by nasopharyngeal PCR testing in relation to maternal clinical COVID status on admission to the hospital, by category (asymptomatic, symptomatic, or hospitalized for COVID-19 treatment), overall and by timing of maternal positive PCR testing relative to childbirth within each category.
DISCUSSION
The NPC-19 registry data reported here are the result of an extraordinary and unprecedented effort supported by AAP, in collaboration with the CDC, to harvest volunteer assistance from “clinicians on the ground” to submit data on early pandemic perinatal care and outcomes. We report in-hospital outcomes for pregnant persons and their newborns across the United States diagnosed with SARS-CoV-2 infection near the time of childbirth from April 2020 to March 2021. This period was marked by uncertainty regarding the effects of SARS-CoV-2 infection on pregnant persons and newborns and preceded widespread availability of COVID vaccines. By focusing on this period, we aimed to assess the outcomes of perinatal infection without consideration of the potential effects of immunization. Our analysis has 3 key findings. First, newborns were at variable risk of acquiring SARS-CoV-2 from infected mothers during birth hospitalization; second, preterm birth was associated with symptomatic maternal infection around the time of birth; and third, maternal in-hospital death occurred more frequently than expected based on national prepandemic statistics.11 Diagnostic testing, clinical approaches and therapeutics for COVID-19 care, infection control measures, vaccine availability, and circulating virus variants have all rapidly evolved since the study period, but each of these findings remains relevant to perinatal counsel and care.
Among the 6486 newborns tested for SARS-CoV-2 infection in our cohort, we found that 2.2% tested positive after birth, with preterm (4.0%) newborns more likely to test positive compared with term newborns (1.9%). These results align with a report of 3816 newborns of persons infected with SARS-CoV-2 during January to December 2020.13 The proportion of newborns testing positive in this study ranged from 2.5% when mothers were diagnosed 7 to 14 days before delivery, to 4.6% when mothers were diagnosed 0 to 2 days before delivery, with higher rates of infection among preterm (5.7%) compared with term (3.4%) newborns.14 International single and multicenter studies report newborn SARS-CoV-2 positive test rates of 0% to 12.9% in cohorts ranging from 67 to 416 newborns with variable definitions of pregnancy-associated infection.15–19 Overall, we found no difference in newborns testing positive by maternal and newborn separation status. This finding should be interpreted with caution. Universal testing for persons presenting for hospital care was adopted in the United States early in the pandemic, and three-quarters of pregnant persons in the NPC-19 registry were identified by such protocols. With the recognition that PCR tests can detect viral nucleic acid days to weeks after an infected person is no longer shedding intact virus,20 it is likely that many asymptomatic pregnant persons who tested positive at obstetric admission were not contagious. Further, among persons potentially infected earlier in pregnancy, the development and transplacental transfer of spike protein-specific antibody may lower the risk to the newborn if exposed to intact virus.21 We observed higher rates (≥3.0%) of newborn infections among cases where mothers were symptomatic, and the highest rate of transmission was observed among infants of mothers first PCR positive in the days after delivery (13.6%). These findings suggest that when mothers are contagious around the time of birth, the risk of newborn infection is substantial.
We did not find significant differences in newborn in-hospital morbidity between newborns with PCR positive and negative testing, although NICU admission occurred more frequently among PCR-positive infants. We did observe that 15.6% of newborns were born at <37 weeks’ gestation. The overall US preterm birth rate from 2017 to 2019 was 10.1%, with variation by race and ethnicity (Hispanic 9.8%, non-Hispanic Black 14.2%, non-Hispanic white 9.1%).22–24 Accounting for race and ethnicity distribution, we would estimate an expected preterm birth rate of 10.3% in our cohort. Preterm birth was associated with maternal illness severity, with a higher proportion of symptomatic persons and persons hospitalized for COVID-19 delivering preterm. Factors known to increase the risk of preterm birth - including maternal age, body-mass index, and comorbidities including hypertension and diabetes mellitus – can also affect the severity of SARS-CoV-2 infection.25 Therefore, we could not determine if SARS-CoV-2 infection itself increased the risk of preterm birth among all pregnant persons, or if the infection results in more severe illness among those at higher baseline risk of preterm birth. Although initial studies of the overall birth population have reported lower or unchanged rates of preterm birth comparing prepandemic and pandemic periods,26,27 other studies focused on pregnant persons with SARS-CoV-2 report increased risks of both spontaneous and medically indicated preterm birth.28–32
We stratified by gestational age when assessing newborn hospital outcomes. Among term newborns, rates of low Apgar scores and delivery room resuscitation fell within expected values and did not differ by SARS-CoV-2 test result.33,34 Cohort rates of mechanical ventilation (1% overall and 3% among PCR-positive term newborns) were higher than national data for mechanical ventilation among term newborns (0.5% to 0.6%).34,35 Rates of NICU admission (14% overall and 23% for those PCR-positive) were higher compared with national rates of NICU admission (~5%) among term newborns.34 We did not collect admission indication for each newborn, but some centers may have used NICU isolation rooms to manage newborns who otherwise would not require NICU care. Among preterm newborns, the cohort rates of low Apgar score and delivery room resuscitation were within expected ranges.34 The national rate of NICU admission among preterm newborns in 2019 was 50%32 and all cohort measures (overall preterm, tested preterm, and PCR-positive preterm) were higher. Overall, rates of mechanical ventilation (15.5%) and the rate among PCR-positive preterm newborns (25.6%) were higher than reported in national data for preterm infants (10.8%).34 Among infants born <37 weeks’ gestation, our cohort had the expected proportion of births at 34 to 36 weeks’ gestation (~70%) but a smaller proportion of births at <28 weeks’ gestation (3.5% of the preterm cohort, compared with 6% to 7% in national data),34 so the findings among preterm infants cannot be attributed to gestational age distribution. Further detailed studies will be needed to determine the underlying etiology of both excess preterm births and potentially increased neonatal morbidity.
SARS-CoV-2 infection caused a mild illness in the majority of pregnant persons reported to the NPC-19, but others in this prevaccine era cohort experienced severe illness and death. Approximately 1 of every 30 infected pregnant persons required hospital admission for respiratory support, and more than 1 of every 100 required mechanical ventilation and/or ECMO support. In contrast, 2019 US national vital statistics report that roughly 1 in every 500 to 600 pregnant persons required admission to an ICU for any cause in the peripartum period.34 Most concerning is that 18 maternal deaths were recorded before final hospital discharge, an in-hospital mortality rate of 240 per 100000 live births in this study. For comparison, in 2015, in-hospital mortality rate ranged from 3.7 per 100000 delivery hospitalizations among white women to 10.9 per 100000 delivery hospitalizations among Black women.35 Additionally, in 2020 there were 23.8 maternal deaths (defined as death while pregnant or within 42 days of end of pregnancy from any cause aggravated by the pregnancy or its management) per 100000 livebirths.13 We were unable to determine an outcome for 6 pregnant persons transferred from birth hospital for higher level of care, meaning our data may underestimate COVID-19-related maternal deaths in the cohort. Severe outcomes among infected pregnant persons in the prevaccine era remain of concern: SARS-CoV-2 vaccine coverage among pregnant persons in the United States has plateaued, with approximately 30% unvaccinated between January 1, 2022 to September 24, 2022.36
There are limitations to the information collected in NPC-19. Participant centers voluntarily shared data, and it is possible that individual case ascertainment was incomplete and varied by center. Multiple data elements, including gestational age and cause of death, were ascertained by judgment of local investigators, and SARS-CoV-2 test results were not available for 15.2% of reported newborns. Not all potential confounders for adverse outcomes were collected (eg, maternal BMI). The registry did not collect information on maternal COVID-19 vaccination history; however, as of March 6, 2021, only 6% to 7% of pregnant persons in the United States were estimated to have received at least 1 dose of a COVID-19 vaccine.36 NPC-19 did not collect data for contemporaneous uninfected control dyads. The exclusion of pregnant persons with infection >14 days before delivery, stillbirths, and deaths at time of delivery may have introduced a bias that underestimates the effects of perinatal COVID-19. Strengths of this study include the large number of cases reported from geographically diverse US centers, and the linkage of maternal to newborn information that provides a granularity of clinical data about perinatal SARS-CoV-2 not typically associated with retrospective administrative datasets. Data were collected by clinical teams who cared for the SARS-CoV-2-affected dyad and who provided corrections in response to queries from the coordinating center.
CONCLUSIONS
Among a large cohort of pregnant persons with perinatal SARS-CoV-2 infection and their newborns, we observed variable rates of newborn infection and preterm births and in-hospital maternal deaths, which were higher than expected based on national prepandemic statistics. Our findings provide strong rationale for mitigation measures, including maternal vaccination against SARS-CoV-2 infection, particularly with evidence of vaccine effectiveness to prevent severe disease among both pregnant persons and young infants.37,38 Although most term newborns suffered neither viral infection nor COVID-19 related morbidity, these findings are balanced by multiple case reports of rare severe outcomes of newborn SARS-CoV-2 infections, including multisystem inflammatory-like illness and neurologic injury.39–43 It remains to be determined if newborn SARS-CoV-2 infection with past or future variants will have long-term consequences.
Supplementary Material
WHAT’S KNOWN ON THIS SUBJECT:
A lack of large-scale data on outcomes of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections among pregnant persons and their newborns impacted perinatal care decisions during the coronavirus disease 2019 pandemic.
WHAT THIS STUDY ADDS:
Among 7524 pregnant persons with SARS-CoV-2 near the time of delivery, 2.2% of newborns tested were positive for SARS-CoV-2 before hospital discharge. Increased rates of preterm delivery and in-hospital maternal death were observed in this prevaccine cohort.
ACKNOWLEDGMENTS
We thank all the investigators who contributed data to the American Academy of Pediatrics National Registry for the Surveillance and Epidemiology of Perinatal COVID-19 Registry.
FUNDING:
This study was partially supported by Centers for Disease Control and Prevention BAA 75D301-20-R-67897.
ABBREVIATIONS
- AAP
American Academy of Pediatrics
- CDC
Centers for Disease Control and Prevention
- COVID-19
coronavirus disease 2019
- ECMO
extracorporeal membrane oxygenation
- IQR
interquartile range
- PCR
polymerase chain reaction
- SARS-CoV-2
severe acute respiratory syndrome coronavirus 2
- SONPM
Section on Neonatal-Perinatal Medicine
- US
United States
Footnotes
CONFLICT OF INTEREST DISCLOSURES: The authors have indicated they have no conflicts of interest to disclose. The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention (CDC). Investigators from CDC participated in data interpretation and manuscript review.
REFERENCES
- 1.Wen T, Arditi B, Riley LE, et al. Influenza complicating delivery hospitalization and its association with severe maternal morbidity in the United States, 2000–2018. Obstet Gynecol. 2021;138(2):218–227 [DOI] [PubMed] [Google Scholar]
- 2.Izurieta HS, Thompson WW, Kramarz P, et al. Influenza and the rates of hospitalization for respiratory disease among infants and young children. N Engl J Med. 2000;342(4):232–239 [DOI] [PubMed] [Google Scholar]
- 3.Munoz FM. Influenza virus infection in infancy and early childhood. Paediatr Respir Rev. 2003;4(2):99–104 [PubMed] [Google Scholar]
- 4.Rha B, Curns AT, Lively JY, et al. Respiratory syncytial virus-associated hospitalizations among young children: 2015–2016. Pediatrics. 2020;146(1):e20193611. [DOI] [PubMed] [Google Scholar]
- 5.Tam J, Papenburg J, Fanella S, et al. Pediatric investigators collaborative network on infections in Canada study of respiratory syncytial virus-associated deaths in pediatric patients in Canada, 2003–2013. Clin Infect Dis. 2019;68(1):113–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Petersen R, Miller AS. Varicella zoster virus infection in neonates. NeoReviews. 2016;17(9):e507–e514 [Google Scholar]
- 7.Chen H, Guo J, Wang C, et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395(10226):809–815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zeng L, Xia S, Yuan W, et al. Neonatal early-onset infection with SARS-CoV-2 in 33 neonates born to mothers with COVID-19 in Wuhan, China. JAMA Pediatr. 2020;174(7):722–725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Office Editorial. Fighting the novel coronavirus: the publication of the Chinese expert consensus on the perinatal and neonatal management for the prevention and control of the 2019 novel coronavirus infection (First edition). Ann Palliat Med. 2020;9(2):524–525 [DOI] [PubMed] [Google Scholar]
- 10.Flannery DD, Puopolo KM. Perinatal COVID-19: guideline development, implementation, and challenges. Curr Opin Pediatr. 2021;33(2):188–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harris PA, Taylor R, Minor BL, et al. ; REDCap Consortium. The REDCap consortium: Building an international community of software platform partners. J Biomed Inform. 2019;95:103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoyert DL. Maternal Mortality Rates in the United States, 2020. Hyattsville, MD: NCHS Health E-Stats; 2022 [Google Scholar]
- 14.Olsen EO, Roth NM, Aveni K, et al. SARS-CoV-2 infections among neonates born to pregnant people with SARS-CoV-2 infection: maternal, pregnancy and birth characteristics. Paediatr Perinat Epidemiol. 2022;36(4):476–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Angelidou A, Sullivan K, Melvin PR, et al. Association of maternal perinatal SARS-CoV-2 infection with neonatal outcomes during the COVID-19 pandemic in Massachusetts. JAMA Netw Open. 2021;4(4):e217523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Villar J, Ariff S, Gunier RB, et al. Maternal and neonatal morbidity and mortality among pregnant women with and without COVID-19 infection: the INTERCOVID multinational cohort study. JAMA Pediatr. 2021;175(8):817–826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dumitriu D, Emeruwa UN, Hanft E, et al. Outcomes of neonates born to nothers with severe acute respiratory syndrome coronavirus 2 infection at a large medical center in New York City. JAMA Pediatr. 2021;175(2):157–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Remaeus K, Savchenko J, Brismar Wendel S, et al. Characteristics and short-term obstetric outcomes in a case series of 67 women test-positive for SARS-CoV-2 in Stockholm, Sweden. Acta Obstet Gynecol Scand. 2020;99(12):1626–1631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hcini N, Maamri F, Picone O, et al. Maternal, fetal and neonatal outcomes of large series of SARS-CoV-2 positive pregnancies in peripartum period: a single-center prospective comparative study. Eur J Obstet Gynecol Reprod Biol. 2021;257:11–18) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Owusu D, Pomeroy MA, Lewis NM, et al. ; Household Transmission Study Team. Persistent SARS-CoV-2 RNA shedding without evidence of infectiousness: a cohort study of individuals with COVID-19. J Infect Dis. 2021;224(8):1362–1371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Flannery DD, Gouma S, Dhudasia MB, et al. Assessment of maternal and neonatal cord blood SARS-CoV-2 antibodies and placental transfer ratios. JAMA Pediatr. 2021;175(6):594–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martin JA, Hamilton BE, Osterman MJK, Driscoll AK, Drake P. Births: final data for 2017. Natl Vital Stat Rep. 2018;67(8):1–50 [PubMed] [Google Scholar]
- 23.Martin JA, Hamilton BE, Osterman MJK, Driscoll AK. Births: final data for 2018. Natl Vital Stat Rep. 2019;68(13):1–47 [PubMed] [Google Scholar]
- 24.Martin JA, Hamilton BE, Osterman MJK, Driscoll AK. Births: final data for 2019. Natl Vital Stat Rep. 2021;70(2):1–51 [PubMed] [Google Scholar]
- 25.Galang RR, Newton SM, Woodworth KR, et al. Centers for Disease Control and Prevention COVID-19 Response Pregnancy and Infant Linked Outcomes Team. Risk factors for illness severity among pregnant women with confirmed severe acute respiratory syndrome coronavirus 2 infection-surveillance for emerging threats to mothers and babies network, 22 state, local, and territorial health departments, March 29, 2020-March 5, 2021. Clin Infect Dis. 2021;73(Suppl 1):S17–S23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goldenberg RL, McClure EM. Have coronavirus disease 2019 (COVID-19) community lockdowns reduced preterm birth rates? Obstet Gynecol. 2021;137(3):399–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Handley SC, Mullin AM, Elovitz MA, et al. Changes in preterm birth phenotypes and stillbirth at 2 Philadelphia hospitals during the SARS-CoV-2 pandemic, March-June 2020. JAMA. 2021;325(1):87–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Villar J, Ariff S, Gunier RB, et al. Maternal and neonatal morbidity and mortality among pregnant women with and without COVID-19 infection: the INTERCOVID multinational cohort study. JAMA Pediatr. 2021;175(8):817–826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mullins E, Hudak ML, Banerjee J, et al. ; PAN-COVID investigators and the National Perinatal COVID-19 Registry Study Group. Pregnancy and neonatal outcomes of COVID-19: coreporting of common outcomes from PAN-COVID and AAP-SONPM registries. Ultrasound Obstet Gynecol. 2021;57(4):573–581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ko JY, DeSisto CL, Simeone RM, et al. Adverse pregnancy outcomes, maternal complications, and severe illness among US delivery hospitalizations with and without a coronavirus disease 2019 (COVID-19) diagnosis. Clin Infect Dis. 2021;73(Suppl 1):S24–S31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jamieson DJ, Rasmussen SA. An update on COVID-19 and pregnancy. Am J Obstet Gynecol. 2022;226(2):177–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Woodworth KR, Olsen EO, Neelam V, et al. ; CDC COVID-19 Response Pregnancy and Infant Linked Outcomes Team; COVID-19 Pregnancy and Infant Linked Outcomes Team (PILOT). Birth and infant outcomes following laboratory-confirmed SARS-CoV-2 infection in pregnancy - SET-NET, 16 jurisdictions, March 29-October 14, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(44):1635–1640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weiner GM, Zaichkin J, Kattwinkel J, eds. Textbook of Neonatal Resuscitation, 8th ed. Itasca, Illinois: American Academy of Pediatrics; 2021 [Google Scholar]
- 34.United States Department of Health and Human Services (US DHHS). Centers for Disease Control and Prevention (CDC), National Center for Health Statistics (NCHS), Division of Vital Statistics, Natality public-use data 2016–2020, on CDC WONDER Online Database. Available at: http://wonder.cdc.gov/natality-expanded-current.html. Accessed Dec 15, 2021
- 35.Fingar KF, Hambrick MM, Heslin KC, Moore JE. Trends and Disparities in Delivery Hospitalizations Involving Severe Maternal Morbidity, 2006–2015. HCUP Statistical Brief #243. Rockville, MD: Agency for Healthcare Research and Quality; 2018 [PubMed] [Google Scholar]
- 36.COVID Data Tracker. COVID-19 Vaccination Among Pregnant People Aged 18–49 Years Overall, by Race/Ethnicity, and Date Reported to CDC - Vaccine Safety Datalink, United States. Atlanta, GA: US Department of Health and Human Services, CDC; 2022 [Google Scholar]
- 37.Butt AA, Chemaitelly H, Al Khal A, et al. SARS-CoV-2 vaccine effectiveness in preventing confirmed infection in pregnant women. J Clin Invest. 2021;131(23):e153662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Halasa NB, Olson SM, Staat MA, et al. ; Overcoming COVID-19 Investigators; Overcoming COVID-19 Network. Effectiveness of maternal vaccination with mRNA COVID-19 vaccine during pregnancy against COVID-19-associated hospitalization in infants aged <6 months - 17 states, July 2021-January 2022. MMWR Morb Mortal Wkly Rep. 2022;71(7):264–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yildiz H, Yarci E, Bozdemir SE, et al. COVID-19-associated cerebral white matter injury in a newborn infant with afebrile seizure. Pediatr Infect Dis J. 2021;40(7):e268–e269 [DOI] [PubMed] [Google Scholar]
- 40.Fragoso DC, Marx C, Dutra BG, et al. COVID-19 as a cause of acute neonatal encephalitis and cerebral cytotoxic edema. Pediatr Infect Dis J. 2021;40(7):e270–e271 [DOI] [PubMed] [Google Scholar]
- 41.Kimberlin DW, Puopolo KM. Neonatal brain injury from SARS-CoV-2: fact or fiction? Pediatr Infect Dis J. 2021;40(7):e266–e267 [DOI] [PubMed] [Google Scholar]
- 42.Godfred-Cato S, Tsang CA, Giovanni J, et al. Multisystem inflammatory syndrome in infants <12 months of age, United States, May 2020-January 2021. Pediatr Infect Dis J. 2021;40(7):601–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ryan L, Plötz FB, van den Hoogen A, et al. Neonates and COVID-19: state of the art : neonatal sepsis series. Pediatr Res. 2022;91(2):432–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


