Interleukin (IL)-22, which was discovered in 2000 as a T-cell-derived cytokine, is probably the only cytokine produced by immune cells (e.g., T helper [Th]17 cells, Th22 cells, activated natural killer [NK] cells, and NKT cells) that does not target immune cells.1–3 Instead, IL-22 mainly targets epithelial cells, including hepatocytes in the liver, by binding to IL-22R1 and IL-10R2.1,2 In 2004, it was demonstrated, for the first time, that IL-22 is a hepatoprotective cytokine that ameliorates hepatocellular damage.4 Subsequently, the epithelial protective effects of IL-22 were also demonstrated in many other organs, including the pancreas, lung, intestine, and kidneys (see a review5 and references therein). The hepatoprotective function of IL-22 is mediated by binding to IL-22R1 and IL-10R2, followed by activation of signal transducer and activator of transcription 3 (STAT3) and subsequent induction of several antiapoptotic proteins, including B-cell lymphoma 2 (Bcl-2), B-cell lymphoma-extra large (Bcl-xL), and myeloid cell leukemia 1 (Mcl-1; Fig. 1).4 It was generally accepted that IL-22R1 expression is restricted to cells of epithelial origin, including hepatocytes and liver progenitor cells in the liver, but to our surprise, we found that hepatic stellate cells (HSCs), which are not of epithelial origin, also expressed high levels of IL-22R1. In vitro and in vivo IL-22 treatment induced HSC senescence by induction of suppressor of cytokine signaling 3 (SOCS3) and subsequent stabilization of p53, thereby inhibiting liver fibrosis in a mouse model of CCl4-induced liver fibrosis (Fig. 1).6 The antifibrotic function of IL-22 was also confirmed in another mouse model of bile duct ligation–induced liver fibrosis.7 Although the hepatoprotective and antifibrotic effects of IL-22 are well documented in animal models, the functions of IL-22 in the pathogenesis of human liver diseases have not been carefully examined.
Fig. 1.
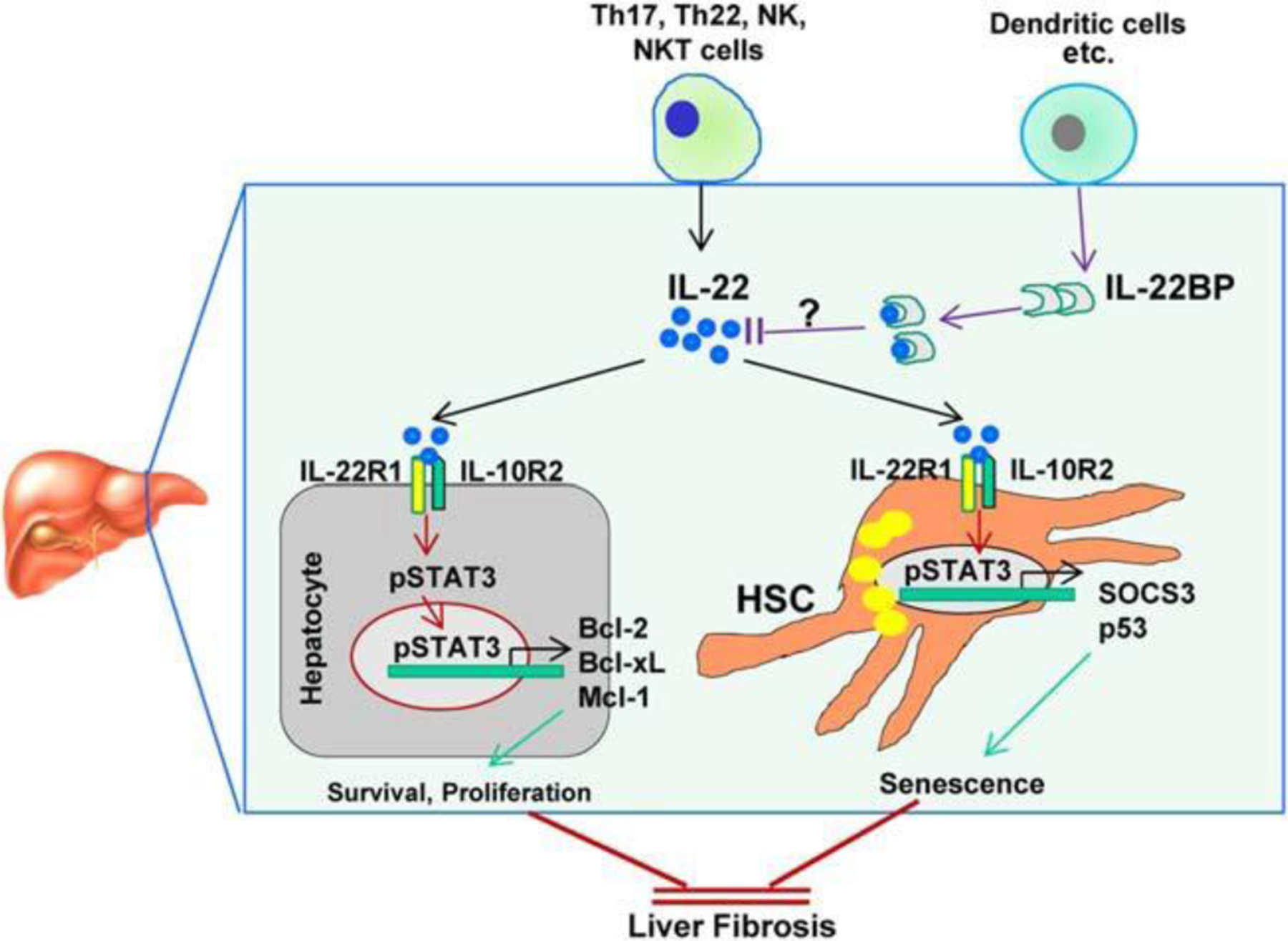
Interplay between IL-22 and IL-22BP in regulating liver fibrosis. IL-22, which is produced by immune cells, targets hepatocytes and HSCs by binding to IL-22R1 and IL-10R2. The well-documented hepatoprotective and antifibrotic effects of IL-22 are mediated by the promotion of hepatocyte survival and induction of HSC senescence, respectively. IL-22BP, which lacks both transmembrane and intracellular domains, binds to IL-22 with a 20- to 1,000-fold higher binding affinity than IL-22R1 and subsequently blocks IL-22 activity, thereby exacerbating liver fibrogenesis.
Several previous studies have reported that serum or hepatic levels of IL-22 were elevated and correlated positively with severity of liver disease in patients with cirrhosis8 or chronic hepatitis B virus (HBV) infection.9,10 In addition, elevated hepatic IL-22 expression was reported to be positively correlated with the number of liver progenitor cells in patients with chronic HBV or hepatitis C virus (HCV) infection, and IL-22 treatment promoted liver progenitor cell proliferation.11 Collectively, these findings suggest that elevated IL-22 contributes to disease progression or plays a compensatory role in promoting liver repair and regeneration. In this issue of HEPATOLOGY, Sertorio et al.12 examined IL-22 production by peripheral blood mononuclear cells (PBMCs) in 66 Chinese fishermen infected with Schistosoma japonicum and found that PBMCs from these patients had a greater ability to produce IL-22 with or without schistosome egg stimulation, compared with PBMCs from normal healthy controls. Moreover, IL-22 production by resting and egg-stimulated PBMCs from patients was inversely correlated with degree of liver fibrosis and portal vein diameter, suggesting that IL-22 plays a role in protecting against liver fibrosis during S. japonicum infection. The protective function of IL-22 against schistosome-induced liver fibrosis is believed to be mediated by promoting liver repair and inducing HSC senescence (Fig. 1). In addition, IL-22 has been shown to contribute to the host defense against intracellular parasite infection through stimulation of antimicrobial effectors and matrix metalloproteinase 9,13 which may also be involved in the protective action of IL-22 in schistosome-induced liver fibrosis. Furthermore, Sertorio et al.12 reported that expression of IL-22, IL-22 binding protein (IL-22BP), and IL-22R1 in the intestine and liver was altered in mice infected with S. mansoni; however, the functions of these molecules were not examined in this mouse model.
Another important finding in the study by Sertorio et al.12 was the demonstration of the association between IL-22BP polymorphisms and the degree of liver fibrosis in patients with S. japonicum or chronic HCV infection. IL-22BP, also called IL-22RA2, is a soluble IL-22 receptor that lacks both transmembrane and intracellular domains and thereby acts as an inhibitor of IL-22 by preventing the binding of IL-22 to membrane-bound IL-22R1. Because it has a 20- to 1,000-fold higher binding affinity to IL-22 than IL-22R1,14 IL-22BP likely plays a significant role in regulating liver disease severity by inhibiting IL-22 activity (Fig. 1). However, the role of IL-22BP in the pathogenesis of liver diseases has not been investigated in animal models or in patients to date. The study by Sertorio et al.12 is probably the first article investigating the potential implications of IL-22BP in the pathogenesis of liver disease. They demonstrated that variants of IL-22BP that are associated with a high abundance of IL-22BP transcripts positively correlated with severe hepatic fibrosis in Chinese patients infected with S. japonicum and in Sudanese and Brazilian patients infected with S. mansoni as well as in Brazilian patients with chronic HCV infection. The profibrotic effect of IL-22BP is likely mediated by blocking IL-22 protein activity. Although the researchers reported that several variants of IL-22BP are associated with a high abundance of IL-22BP transcripts in healing skin tissues, hepatic and serum IL-22BP levels were not examined in patients with liver disease. It has been reported that IL-22BP is constitutively expressed in secondary lymphoid organs, breast, and epithelial tissues (e.g., intestine, lung, and skin).15 In addition, IL-22BP is constitutively expressed by a subset of conventional dendritic cells (DCs), and its expression in these cells is highly induced by retinoic acid.16 As we know, HSCs store 75% of the body’s vitamin A and produce large amounts of retinoic acid when activated in fibrotic livers. Production of retinoic acid by HSCs has been shown to regulate various types of immune cells.17 Thus, it is likely that HSCs produce retinoic acid during fibrosis, which then stimulates DCs to produce IL-22BP and subsequently reduces IL-22 activity. Further studies are needed to confirm this hypothesis. Because of its many beneficial effects and few side effects (owing to the restricted expression of IL-22R1), the functions and therapeutic applications of IL-22 in the treatment of various acute diseases that damage epithelial tissues, such as acute liver failure (ALF), have been actively investigated, and IL-22 protein is currently under consideration for a phase II clinical trial for ALF.5,18 Hepatic and serum IL-22 levels have been examined in patients with various types of liver diseases. For example, hepatic IL-22 levels are highly elevated in patients with chronic viral hepatitis infection,9–11 but are undetectable in patients with alcoholic hepatitis (AH).19 These results suggest that AH patients may be sensitive to IL-22 therapy owing to low levels of endogenous IL-22, whereas viral hepatitis patients may not benefit because of high basal IL-22 levels. However, few studies have examined hepatic and serum levels of IL-22BP and the pathophysiological functions of IL-22BP in liver diseases. This is an important question that needs to be addressed in the future because IL-22BP is a potent inhibitor for IL-22, and high levels of IL-22BP could diminish the therapeutic effects of IL-22.
Acknowledgments
This work was supported by the intramural program of the National Institute on Alcohol Abuse and Alcoholism, National Institutes of Health.
Abbreviations:
- AH
alcoholic hepatitis
- ALF
acute liver failure
- Bcl-2
B-cell lymphoma 2
- Bcl-xL
B-cell lymphoma-extra large
- DCs
dendritic cells
- HBV
hepatitis B virus
- HCV
hepatitis C virus
- HSCs
hepatic stellate cells
- IL
interleukin
- IL-22BP
IL-22 binding protein
- Mcl-1
myeloid cell leukemia 1
- NK
natural killer
- PBMCs
peripheral blood mononuclear cells
- SOCS3
suppressor of cytokine signaling 3
- STAT3
signal transducer and activator of transcription 3
- Th
T helper
Footnotes
Potential conflict of interest: Nothing to report.
References
- 1.Sonnenberg GF, Fouser LA, Artis D. Border patrol: regulation of immunity, inflammation and tissue homeostasis at barrier surfaces by IL-22. Nat Immunol 2011;12:383–390. [DOI] [PubMed] [Google Scholar]
- 2.Wolk K, Witte E, Witte K, Warszawska K, Sabat R. Biology of interleukin-22. Semin Immunopathol 2010;32:17–31. [DOI] [PubMed] [Google Scholar]
- 3.Rutz S, Eidenschenk C, Ouyang W. IL-22, not simply a Th17 cytokine. Immunol Rev 2013;252:116–132. [DOI] [PubMed] [Google Scholar]
- 4.Radaeva S, Sun R, Pan HN, Hong F, Gao B. Interleukin 22 (IL-22) plays a protective role in T cell-mediated murine hepatitis: IL-22 is a survival factor for hepatocytes via STAT3 activation. HEPATOLOGY 2004; 39:1332–1342. [DOI] [PubMed] [Google Scholar]
- 5.Muhl H, Scheiermann P, Bachmann M, Härdle L, Heinrichs A, Pfeilschifter J IL-22 in tissue-protective therapy. Br J Pharmacol 2013; 169:761–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kong X, Feng D, Wang H, Hong F, Bertola A, Wang FS, Gao B. Interleukin-22 induces hepatic stellate cell senescence and restricts liver fibrosis in mice. HEPATOLOGY 2012;56:1150–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meng F, Wang K, Aoyama T, Grivennikov SI, Paik Y, Scholten D, et al. Interleukin-17 signaling in inflammatory, Kupffer cells, and hepatic stellate cells exacerbates liver fibrosis in mice. Gastroenterology 2012;143:765–776.e1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kronenberger B, Rudloff I, Bachmann M, Brunner F, Kapper L, Filmann N, et al. Interleukin-22 predicts severity and death in advanced liver cirrhosis: a prospective cohort study. BMC Med 2012;10:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao J, Zhang Z, Luan Y, Zou Z, Sun Y, Li Y, et al. Pathological functions of interleukin-22 in chronic liver inflammation and fibrosis with hepatitis B virus infection by promoting T helper 17 cell recruitment. HEPATOLOGY 2014;59:1331–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Y, Cobleigh MA, Lian JQ, Huang CX, Booth CJ, Bai XF, Robek MD. A proinflammatory role for interleukin-22 in the immune response to hepatitis B virus. Gastroenterology 2011;141:1897–1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feng D, Kong X, Weng H, Park O, Wang H, Dooley S, et al. Interleukin-22 promotes proliferation of liver stem/progenitor cells in mice and patients with chronic hepatitis B virus infection. Gastroenterology 2012;143:188–198.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sertorio M, Hou X, Carmo RF, Dessein H, Cabantous S, Abdelwahed M, et al. Interleukin-22 and IL-22 binding protein (IL-22BP) regulate fibrosis and cirrhosis in hepatitis C virus and schistosome infections. HEPATOLOGY 2015;61:1321–1331. [DOI] [PubMed] [Google Scholar]
- 13.Stange J, Hepworth MR, Rausch S, Zajic L, Kühl AA, Uyttenhove C, et al. IL-22 mediates host defense against an intestinal intracellular parasite in the absence of IFN-gamma at the cost of Th17-driven immunopathology. J Immunol 2012;188:2410–2418. [DOI] [PubMed] [Google Scholar]
- 14.Jones BC, Logsdon NJ, Walter MR. Structure of IL-22 bound to its high-affinity IL-22R1 chain. Structure 2008;16:1333–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dumoutier L, Lejeune D, Colau D, Renauld JC. Cloning and characterization of IL-22 binding protein, a natural antagonist of IL-10-related T cell-derived inducible factor/IL-22. J Immunol 2001;166: 7090–7095. [DOI] [PubMed] [Google Scholar]
- 16.Martin JC, Bériou G, Heslan M, Chauvin C, Utriainen L, Aumeunier A, et al. Interleukin-22 binding protein (IL-22BP) is constitutively expressed by a subset of conventional dendritic cells and is strongly induced by retinoic acid. Mucosal Immunol 2014;7:101–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee YS, Jeong WI. Retinoic acids and hepatic stellate cells in liver disease. J Gastroenterol Hepatol 2012;27(Suppl 2):75–79. [DOI] [PubMed] [Google Scholar]
- 18.Sabat R, Ouyang W, Wolk K. Therapeutic opportunities of the IL-22-IL-22R1 system. Nat Rev Drug Discov 2014;13:21–38. [DOI] [PubMed] [Google Scholar]
- 19.Ki SH, Park O, Zheng M, Morales-Ibanez O, Kolls JK, Bataller R, Gao B. Interleukin-22 treatment ameliorates alcoholic liver injury in a murine model of chronic-binge ethanol feeding: role of signal transducer and activator of transcription 3. HEPATOLOGY 2010;52:1291–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]


