Abstract
Background.
Managing psychological distress is an objective of palliative care. No meta-analysis has evaluated whether palliative care reduces psychological distress.
Objectives.
Examine the effects of palliative care on depression, anxiety, and general psychological distress for adults with life-limiting illnesses and their caregivers.
Design.
We searched PubMed, PsycInfo, Embase, and CINAHL for randomized clinical trials (RCTs) of palliative care interventions. RCTs were included if they enrolled adults with life-limiting illnesses or their caregivers, reported data on psychological distress at 3 months after study intake, and if authors had described the intervention as “palliative care.”
Results.
We identified 38 RCTs meeting our inclusion criteria. Many (14/38) included studies excluded participants with common mental health conditions. There were no statistically significant improvements in patient or caregiver anxiety (patient SMD: −0.008, P = 0.96; caregiver SMD: −0.21, P = 0.79), depression (patient SMD: −0.13, P = 0.25; caregiver SMD −0.27, P = 0.08), or psychological distress (patient SMD: 0.26, P = 0.59; caregiver SMD: 0.04, P = 0.78).
Conclusions.
Psychological distress is not likely to be reduced in the context of a typical palliative care intervention. The systemic exclusion of patients with common mental health conditions in more than 1/3 of the studies raises ethical questions about the goals of palliative care RCTS and could perpetuate inequalities.
Keywords: Meta-analysis, palliative care interventions, anxiety, depression, patient, caregiver, psychological distress
Introduction
Palliative care clinicians encounter psychological distress regularly in their practices.1 Psychological distress can include depression, sadness, anxiety, negative affect, and fear.2 Approximately 30%–40% of patients with cancer experience a mood disorder,3 and depression is similarly common among patients with chronic heart failure and chronic obstructive pulmonary disease.4 In addition, patients with cancers, heart failure, and lung disease frequently experience increased depression symptoms as they approach the end of life.5 Goals of palliative care include identifying, assessing, and managing pain and physical, psychological, social, and spiritual concerns among patients experiencing serious illnesses and their families.6,7 Some studies have shown that palliative care may improve mental health symptoms8,9 and may even be associated with reduced likelihood of death by suicide among people with serious illnesses.10–12
Prior systematic reviews and meta-analyses have evaluated the effects of palliative care on improving quality of life with mixed findings.13–17 Among patients with cancer, outpatient palliative care interventions had a positive impact on quality of life.17 Three systematic reviews found some evidence for palliative care improving quality of life among mixed-disease samples (including cancer and noncancer patients).13,14,16 The single review specifically investigating palliative care interventions among noncancer patients found no effect of palliative care on quality of life.15 One review of 23 trials on patient mood outcomes13 found mixed evidence, but four of the five trials at low risk of bias reported statistically significantly improved mood. However, the patient mood was not a primary outcome analyzed in this review, so the authors did not perform a meta-analysis.
We performed a systematic review and meta-analysis of palliative care randomized controlled trials (RCTs) and estimated the association between palliative care and psychological distress symptoms in adult patients with life-limiting illnesses and their caregivers. We also conducted several moderator analyses to examine differences in effect sizes between studies focusing on patients with cancer vs. noncancer illnesses, examining psychological distress as a primary vs. secondary outcome, using a manualized therapeutic psychosocial intervention, including a specialty mental health clinician on the intervention team, and specifying a theoretical basis for the psychological component of the intervention. We also examined the moderating role of the study’s risk of bias.
We hypothesized that studies focusing on cancer populations would have more improvement in psychological distress than those focused on patients with non-cancer illnesses because cancer care is better integrated within palliative care18–20 and trajectories of decline are better established for cancer.21–23 Consequently, palliative care may be better positioned to address psychological distress symptoms at the right times in cancer settings. We also hypothesized that studies using a manualized therapeutic intervention to target psychological distress would have improved outcomes relative to those that did not as the psychological component of the intervention would be standardized, leading to less heterogeneity. It was hypothesized that the inclusion of a specialty mental health clinician on the intervention team would be associated with improved outcomes because participants would be receiving care from a clinician trained to manage psychological distress. We hypothesized that studies with theoretical bases for the psychological component of the intervention would have improved outcomes relative to those without because the mechanism through which the intervention would work is specified. We hypothesized that studies with psychological distress identified as a primary outcome would have stronger outcomes because the intervention would be tailored for psychological distress. And finally, we expected that studies with lower risk of bias would have more precise estimates of the intervention effect because studies with lower risk of bias are less likely to over-estimate effects.24
Methods
Protocol and Registration
This study is a protocol-based systematic review and meta-analysis (PROSPERO ID: CRD42021255958) conducted according to the Cochrane Handbook for Systematic Review of Interventions and the Preferred Reporting Items for Systematic Review and Meta-Analysis statement 27-item checklist.25
Search Strategy
PubMed, PsycInfo, Embase, and CINAHL were searched for articles published anytime from inception to June 11, 2021. The primary author (M. A. N.) conducted the searches with assistance from a research librarian (see appendix for search examples). The primary author also screened other resources, including searching the NIH Clinical Trials registration webpage26 and bibliographic references from prior systematic reviews on similar topics and from retrieved papers of interest, for additional studies and data not identified in the original search strategy. An example of the search strategy can be found in the appendix.
Study Selection
Two of the reviewers (M.A.N. and either S. K. or an undergraduate research assistant) screened and independently evaluated all records for eligibility criteria. Disagreements between the two primary reviewers were adjudicated by the third. Studies were included for full review if they had a RCT or cluster RCT study design, the patient population was adults with a life-limiting illness, and the study evaluated a palliative care intervention (as defined by the authors of the included study), and assessed psychological distress symptoms three months postintervention. Life-limiting illnesses were defined as an incurable condition that would likely limit lifespan.27 Studies of palliative care interventions selected for full review were subsequently included if the intervention was delivered by at least one person who had received palliative care training. This criterion included interventions using palliative care-boarded and/or experienced physicians, nurses, or other clinicians to deliver the intervention. It also included studies in which the individuals delivering the intervention received study-specific palliative care training, even if they did not have prior palliative care experience. Studies reporting at least one of the following outcomes for either the patient or the patient’s caregiver were included: depression symptoms, anxiety symptoms, mood symptoms, or psychological distress symptoms. Studies using an overall symptom or quality of life scale, such as the Edmonton Symptom Assessment Scale, were included only if they reported results from a psychological symptoms subscale. Studies reporting a diagnosis of depression or anxiety were included only if they also reported a dimensional assessment of symptoms. There were no limitations placed on types of comparison groups; we allowed both active and nonactive controls. Studies were excluded if psychological distress symptoms were not assessed at three months (plus or minus one month) after study intake. Studies involving pediatric patients were excluded due to differences in pediatric and adult palliative care.28 Studies not written in English were also excluded, as the investigative team did not have multiple speakers of other languages. Two authors (M. A. N. and S. K.) extracted all the data.
Risk of Bias Assessment
The risk of bias was assessed using the Joanna Briggs Institute Critical Appraisal Checklist for RCTs.29 This checklist includes 12 characteristics: true randomization, concealment of allocation, treatment groups similar at baseline, participants blind to assignment, assessors blind to assignment, groups treated identically except for intervention, follow-up complete, intention-to-treat analysis, outcome measurement same for treatment groups, reliable assessment of outcomes, appropriate statistical analysis, and appropriate trial design. Of these 12 characteristics, 11 were applicable to the studies included in this meta-analysis, as it is not reasonable to blind participants to their treatment assignment in a behavioral RCT. Each study was assessed (yes, no, unclear) on all 11 characteristics. The total number of “yes” categorizations within each study was summed, and studies with seven or more characteristics were considered lower risk of bias, while those with six or fewer were considered higher risk of bias. This approach has been used previously.30
Synthesis of Results
A narrative synthesis was performed to describe the populations, diseases studied, nature of the palliative care interventions, and psychological distress outcomes for the included studies. For the meta-analysis, psychological distress symptoms were selected as the focal outcome, even if they were not the primary outcome of the included palliative care intervention.
Summary Measures
Given that several instruments were used to measure psychological distress, standardized mean differences (SMDs) were calculated using Hedge’s adjusted g estimator to correct for small sample bias.31 Among studies that did not report SMDs, means and standard deviations or other summary statistics of outcome measures were collected and SMDs calculated according to the Cochrane training handbook.32 When studies included multiple psychological distress measures and multiple time points within the 2–4 months after study intake, estimates were pooled,33 based on the imputation of an estimate of intercorrelation among the outcome measures. Based on the prior literature,34,35 we used an estimate of r = 0.8 and conducted sensitivity analyses for r = 0.5. When necessary, individual studies were directionally corrected such that lower scores on distress measures represented lower levels of psychological distress. For studies that did not report numeric data on the psychological distress outcomes in the paper (e.g., only reported results were “not significant”), authors were contacted and asked to share their data. If authors did not respond or did not agree to share their data, the study was not included in the meta-analysis. Because we used SMDs as the outcome, studies that did not report change scores or psychological distress measures at study intake were excluded.
Statistical Analysis
Six separate meta-analyses were performed for patient anxiety, patient depression, general patient psychological distress, caregiver anxiety, caregiver depression, and general caregiver psychological distress. Six potential moderators of patient anxiety and depression outcomes were explored: risk of bias (lower vs. higher risk of bias), illness type (cancer or noncancer), whether a manualized therapeutic intervention was included, whether a specialty mental health clinician was part of the study team, whether the authors specified a theoretical basis for the psychological component of the intervention, and whether psychological distress was indicated as a primary outcome. Moderators of caregiver outcomes and general distress outcomes were not explored because there were few studies.36 Heterogeneity was examined using the I2 statistic value and chi-square tests P-values from each meta-analysis. Heterogeneity is considered high at values over 75%. Outcomes were pooled using a random-effects model including a random study effect to account for heterogeneity among studies.31 Forest plots and funnel plots were created for each of the main analyses. The analysis was carried out using R (version 4.0.1),37 the metafor package (version 3.0.2),38 and the dmetar package.39 All analyses used a two-sided P-value of 0.05.
Results
There were 2806 unique records identified from the literature search, of which 224 were deemed eligible for full review (see Fig. 1 for more detail). A total of 38 studies with 6336 patients and 1667 caregivers were included. Study characteristics can be found in Table 1 and in Appendix Table 1. Twenty-one (55.3%) were conducted in the US,8,9,40–59 three (7.9%) in Denmark,60–62 two (5.3%) in each of Canada,63,64 Italy,65,66 Hong Kong,67,68 and Belgium.69,70 More than two-thirds of the included studies (N = 26, 68.4%) enrolled patients with cancer.8,9,42,43,45–49,52–54,56,58–65,70–75 Six (15.8%) studies enrolled heart failure patients.40,44,51,55,57,68 The remaining six studies were evenly divided among neurological conditions,66,76 pulmonary conditions, 50,69 and other conditions.41,67 Most (23) studies had a higher or unclear risk of bias8,9,42,43,46–48,50,53–55,57–61,65,67,69,71–75 and 15 had a lower risk of bias.40,41,44,45,49,51,52,56,62–64,66,68,70,76
Fig. 1.
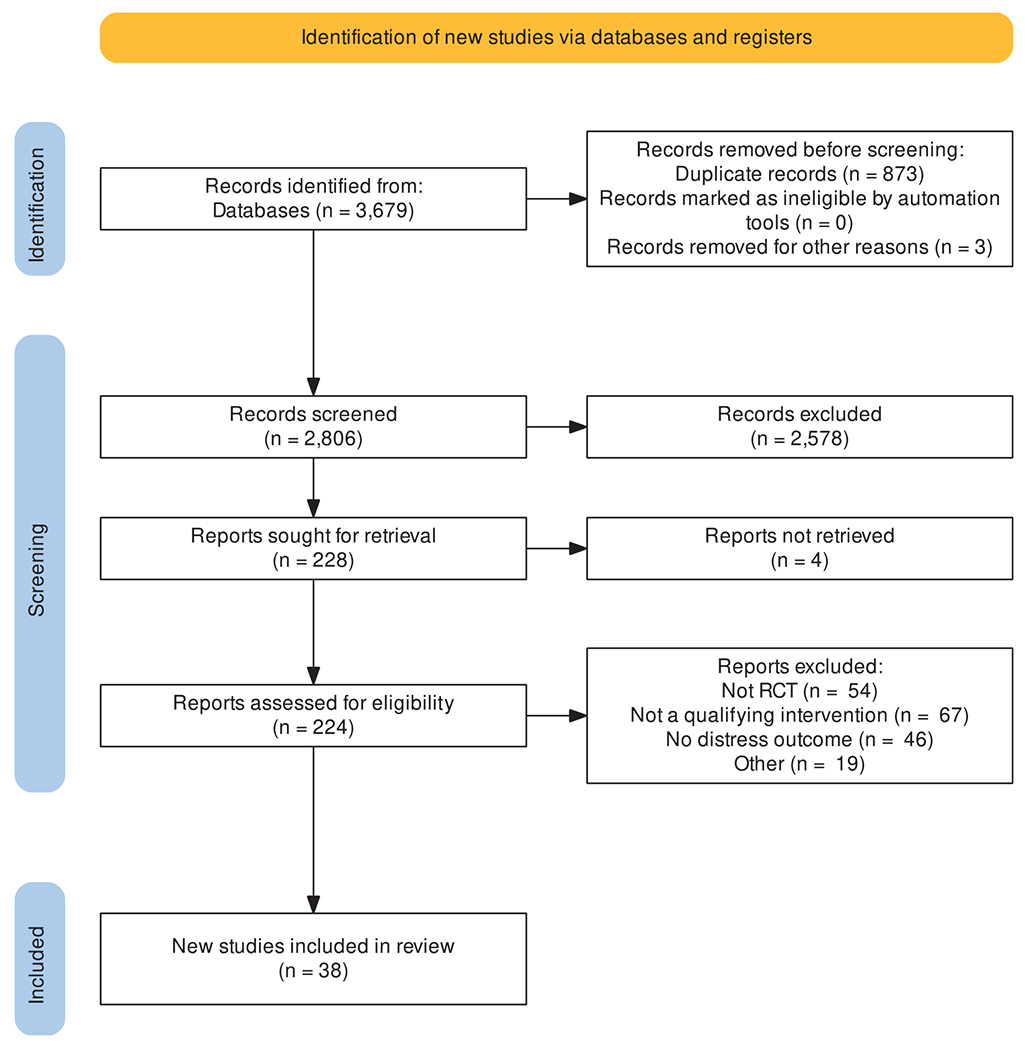
PRISMA flow diagram of included studies. For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.
Table 1.
Characteristics of Interventions and Their Samples
| Study | # Subjects | Patient Illness(es) | Patient or Caregiver Outcomes | Specialty MH Clinician? | Primary Outcome Distress? | Manualized Psychological Intervention | Theoretical or Conceptual Background | Care Delivery Team | Content of Intervention | Excluded Elevated Distress? | Baseline Distress Measures |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ammari et al.60 | 57 | Advanced solid cancer patients | Patient | no | no | – | Lazarus’s stress and coping theory | Nurses | appraise and cope with problems, support | No | Intervention HADS-D: 6.89 (2.86) Intervention HADS-A:6.36 (4.56); Control D:6.86 (2.72), A: 4.66 (3.68) |
| Bakitas et al.9 | 279 | Advanced cancer patients | Patient | no | no | problem solving therapy | chronic care model | APN with specialty in PC | problem solving, communication and social support, symptom management, advance care planning, unfinished business | excluded axis I diagnoses and people with substance use | not reported alone, only change reported |
| Bakitas et al.52 | 207 | Advanced cancer | Patient | no | no | problem solving therapy | – | Board-certified PC clinician, APN coach | problem solving, self-care, local resources, communication, decision making, ACP; life review and creating legacy | excluded if active axis I diagnosis or active substance use disorder | Intervention CESD: 14.99(10.64); Control: 13.42(9.47) |
| Bakitas et al.51 | 415 | Advanced heart failure | Patient | no | yes | problem solving therapy | chronic care model, National Consensus Project Guidelines for Quality Palliative Care, Outlook intervention | PC clinician, nurse coach | COPE (creativity, optimism, planning, expert information); self-care (healthy eating, exercise, meditation, spirituality and partnering); physical and emotional symptom management; decision making; life review and creating legacy | excluded if untreated axis I diagnosis or active substance use disorder | Intervention: HADS-A:6.6 (3.5), HADS-D:5.7(4.3); Control: HADS-A:6.8(3.7), HADS-D:5.8(4.2) |
| Bekelman et al.40 | 314 | Chronic heart failure patients | Patient | yes | no | – | interpersonal and behavioral activation psychotherapies | Team: nurse, primary care clinician, social worker, PC specialist, cardiologist | symptom management, grief and loss, change in role, behavioral activation, pacing, medical review | excluded those with substance use and SMI | phq-9 at baseline: median IQR: 9 (5-14) |
| Brims et al.75 | 174 | Mesothelioma | Caregiver | no | no | – | – | PC physician | assessment of patient physical, psychological, social, and spiritual needs | excluded those with a psych-related hospitalization in last 12 mo | didn’t report baseline |
| Carson et al.41 | 365 | Chronic critical illness that required mechanical ventilation | Caregivera | no | yes | – | – | PC physician and NP; team could also include social workers, chaplains, and other disciplines as needed | No | Intervention HADS total: 16.0 (8.1) Control: 16.4 (8.4) |
|
| Chan et al.67 | 29 | Kidney failure | Caregivera | no | yes | – | – | PC nurse, social worker, PC physician | enhanced psychosocial support (including education), symptom advice and prevention, psychosocial-spiritual support, social support and advice about financial issues | No | Intervention HADS-A:9.9 (3.3), HADS-D: 5.4 (4.5); Control: HADS-A: 9.1 (2.3), HADS-D:6.4 (2.9) |
| Dionne-Odom et al.42, Dionne-Odom et al.43 | 122 | Advanced cancer | Caregivera | no | yes | problem solving therapy | – | nurse coaches | COPE (creativity, optimism, planning, expert information); self-care (healthy eating, exercise, meditation, spirituality and partnering); communication; decision making; decision support; ACP | excluded if untreated axis I diagnosis or active substance use disorder | Early group: CESD: 13.4 (SE 1.3), Delayed group: 15.9 (1.4) |
| Dionne-Odom et al.44 | 158 | Advanced heart failure | Caregivera | no | yes | problem solving therapy | Wagner’s chronic care model; National Consensus Project Guidelines for Quality Palliative Care | Nurse coaches with specific training for this PC intervention | COPE (creativity, optimism, planning, expert information); self-care (healthy eating, exercise, meditation, spirituality and partnering); communication; decision making; decision support; ACP | excluded documented active axis I disorder | Intervention: HADS-A: 3.9(3.1), HADS-D: 4.7 (3.1); Control: HADS-A: 3.7(2.9), HADS-D: 4.8 (3.3) |
| do Carmo et al.74 | 63 | Advanced cancer | Patient | yes | yes | CBT | session structure method | PC Physician | psychoeducation; discuss functioning of anxiety and techniques to manage symptoms, discuss depressive symptoms | excluded if receiving psychological treatment for any psych disorder or any pharmacological antidepressants for depression and/or anxiety | Arm A: HADS-A:5.79 (3.92), HADS-D:5.11 (4.51), PHQ-9:9.7 (6.5); Arm B: HADS-A: 7.14 (3.41), HADS-D: 7.14 (4.12), PHQ-9:10.3 (6.0); Arm C: HADS-A:6.27 (4.84), HADS-D: 7.18 (4.92), PHQ-9: 9.8 (6.5) |
| El-Jawahri et al.45 | 137 | Incurable lung or non-colorectal GI cancer | Caregiver | no | no | – | – | Boarded PC physician or APN | addressing symptoms, enhancing coping efforts, establishing rapport, illness and prognostic understanding, treatment decisions, ACP, discussing disposition | none for CG | not reported alone, only change reported |
| El-Jawahri et al.46 | 160 | Hematologic cancer | Patient | no | no | – | – | Inpatient PC physician or APN | managing physical and psychological symptoms | those with existing psych condition the oncologist thought may interfere were exclude | Control: PHQ-9 5.4, HADS-D: 4.9 (4.1), HADS-A:5.4 (3.8); Intervention PHQ-9: 4.8 (4.4), HADS-D: 4.0 (3.2), HADS-A:4.6(3.6) |
| El-Jawahri et al.47 | 160 | cancer | Patient | no | no | – | – | PC physician, inpatient palliative care physician, advance practice nurse, or physician assistant | address symptoms, assess understanding, goals and expectations, decision-making | those with existing psych condition the oncologist thought may interfere were excluded | Intervention: HADS-A:(4.6), HADS-D:(3.9), PHQ-9:6.3 (5.0); Control: HADS-A:6.2 (4.1), HADS-D:5.6 (3.3), PHQ-9:6.9 (4.9) |
| Ferrell et al.48 | 479 | Cancer | Patient | no | yes | – | – | nurses, chaplain, SW, oncologist | teaching sessions addressing symptoms and QOL | not clear | did not report distress at study intake |
| Gao et al.76 | 350 | long-term neurological conditions (any advanced stage MS, motor neuron disease, idiopathic Parkinson disease multiple system atrophy, or progressive supranuclear palsy) | Patient | no | no | – | Medical Research Council framework for evaluating complex interventions | Existing multidisciplinary PC teams | assessment, personalized care planning, case management/care coordination, advising existing care providers | no | Intervention: HADS-A: 7.78(6.78,8.77), HADS-D: 8.13(7.29, 8.97); Control: HADS-A: 7.51 (6.52, 8.50), HADS-D: 8.31 (7.47, 9.16) |
| Grudzen et al.49 | 136 | cancer | Patient | no | yes | – | – | physician, NP, social worker, chaplain | symptom assessment/treatment, goals of care and ACP, and transition planning | no | Intervention: 35% had MDD, Control: 29% with MDD |
| Hoek et al.73 | 74 | Advanced cancer | Patient | no | yes | yes | – | mostly delivered by nurse, but GP was involved when possible | no | Intervention: HADS-A:7.24 (4.7), HADS-D: 7.66(3.87); Control: HADS-A:6.22 (3.91), HADS-D:6.49(4.57) | |
| Janssen et al.50 | 22 | Idiopathic pulmonary fibrosis | Patient | no | no | – | – | physicians, nurses, SWs | intro to PC, symptom and QOL assessment, support network assessment, prognostic understanding, planning decisions, care goals | no | HADS-A: 5.3 (4.3), HADS-D:4.0(3.2), PHQ-9: 5.4(5.3) |
| Johnsen et al.61 | 297 | Stage IV cancer or stage III/IV CNS cancer | Patient | yes | no | – | European Association for Palliative Care and WHO guidelines | at least 4 different disciplines, always including nurses, physicians, all had psychologist | no | Intervention HADS-A: 6.9 (4.1), HADS-D: 6.3 (3.9); Control: HADS-A: 6.8 (3.9), HADS-D: 6.3 (3.7) | |
| Jordhoy et al.72 | 434 | Advanced cancer | Patient | no | no | – | – | PC Program includes GPs, home care nurses, nursing homes, Pall medicine unit physician & nurse | no | Intervention: IES-Avoidance:17(11), IES-Intrusion:14 (10); Control: IES-Avoidance:18(11), IES-Intrusion: 15 (10) | |
| Maltoni et al.65 | 186 | Advanced/metastatic pancreatic cancer | Patient | no | no | – | – | PC specialist | no | Intervention elevated HADS-A:42.3%, HADS-D:39.4%; Control HADS-A:45.3%, HADS-D:28% | |
| McCorkle et al.53 | 146 | Late stage gynecological and lung cancer | Patient | no | no | – | – | APNs, Pas, MSWs | symptom management, teaching patients and caregivers, enhancing QOL, goals of care; enhance patient problem solving, decision making, and self-efficacy | no | PHQ-9:5.10(4.33) |
| McDonald et al.63 | 182 | Stage IV cancer or stage III cancer with poor prognosis | Caregiver | no | no | – | – | PC physician and nurse | symptom management, goals of care, ACP, social/emotional/spiritual needs | no | Intervention: 43.5 (40.7 46.3); Control: 42.7(40.1, 45.2) |
| Ng & Wong68 | 84 | End-stage heart failure | Patient | no | no | – | Omaha system | PC nurse case managers and trained volunteer nursing students | physical and psychological symptoms management, social support, spiritual support, goals of care, treatment preference and EOL issues | excluded those with psych disorder requiring active treatment | Median and IQR, Intervention: ESAS-A: 2 (0,6), ESAS-D: 2 (0,6); Control: ESAS-A: 3 (0,6), ESAS-D: 3, (0,6) |
| Nordly et al.54 | 322 | Cancer | Patient | yes | no | existential-phenomenological therapy | – | nurse, GP, psychologist, in addition to specialized PC team | no | Intervention HADS-A:5.8 (3.8), HADS-D: 4.5(3.6); Control HADS-A: 5.0(4.0), HADS-D: 4.4(3.6) | |
| O’Riordan et al.55 | 30 | Heart failure | Patient | no | yes | – | – | Interdisciplinary PC team including NP, physician, social worker, chaplain | meds for symptoms, ACP, psychosocial and spiritual support | no, just excluded active illicit drug use | Intervention: HADS-A: 5.9(3.5-8.2), HADS-D: 5.4(3.4-7.4); Control: HADS-A:7.4(4.9-9.9) HADS-D:6.5(4.-8.6); |
| Rodin et al.64 | 42 | Acute leukemia | Patient | yes | no | supportive psychotherapy and CBT, based on Anxiety Reduction Treatment for Acute Trauma intervention | – | MSW as psychosocial clinician, hematology physicians, nurses, allied health; consultation with PC nurses and PC physicians in 1st week and as needed | problem solving, education, modulate emotions | excluded those already receiving psychological or psychiatric care | Intervention BDI:10.25 (SE 1.73); Control: 13.20(SE 1.83) |
| Scheerens et al.69 | 39 | COPD | Patient | yes | no | – | – | HCNs, PHC physicians, and psychologists | disease insight and coping, symptom management, care planning, caregiver support, psychosocial/existential/spiritual support | no | Intervention: HADS-A: 8.5(5.4, 11.6), HADS-D: 7.9 (5.8,10.0); Control: HADS-A: 8.3 (5.2,11.4), HADS-D: 10.2 (8.1, 12.3) |
| Schenker et al.56 | 672 | cancer | Patient | no | no | – | chronic care model | nurse delivered, shared plans with oncologists | establish rapport, addressing symptom needs, choosing surrogate decision maker, treatment preferences, completion of AD | no | HADS-A: 5.78 (3.90), HADS-D: 5.41(3.75) |
| Sidebottom et al.57 | 232 | Acute heart failure | Patient | no | no | – | – | PC team: 4 HPM boarded physicians, 2 AP PC nurses, social worker, chaplain | symptom burdens, emotional/spiritual/psychosocial aspects of care, coordination of care, recommendations in treatment, referrals, care planning | no | PHQ-9: 8.3(5.2) |
| Slama et al.71 | 126 | cancer | Patient | yes | no | – | – | referred out from PC physician to SW, psychologist etc as needed | pain and symptom management, coping strategies, need for psychosocial support | no | Intervention: elevated anxiety:35.7%, elevated depression: 28.3%; Control: elevated anxiety: 34.8%, elevated depression: 28.8% |
| Solari et al.66 | 78 | Severe MS | Patient | yes | no | – | – | Home-based PC team including a physician (neurology, physiatrist), nurse with specialty training in PC, psychologist, social worker | no | Intervention: HADS-A: 6.4 (3.9), HADS-D: 6.9 (4.4); Control: HADS-A: 6.6 (3.9), HADS-D: 7.1(3.6) | |
| Temel et al.58 | 350 | Newly diagnosed incurable lung or non-colorectal GI cancer | Patient | no | no | – | National Consensus Project for Quality Palliative Care | physicians and APNs | illness understanding/education, symptom management, decision-making, coping with life-threatening illness, referrals/prescription | excluded if significant psychiatric condition prohibiting participation | Intervention: PHQ-9:6.39 (5.49), HADS-D: 4.72(4.28), HADS-A: 5.05(3.95); Control: PHQ-9: 6.50(5.19), HADS-D: 4.58(3.73), HADS-A: 5.57(3.88) |
| Temel et al.8 | 151 | Metastatic non-small cell lung cancer | Patient | no | no | – | National Consensus Project for Quality Palliative Care | PC physicians and APNs | physical and psychosocial symptoms, goals of care, decision-making, and coordinating care | no | Intervention HADS-A:36% elevated, HADS-D: 22%, PHQ-9: 12%; Control: HADS-A:33%, HADS-D: 25%, PHQ-9: 17% |
| Temel et al.59 | 405 | cancer | Patient | no | no | – | – | PC physicians and APNs | symptom assessment/treatment, support of coping with advanced cancer, prognostic awareness, decision-making, planning for EOL care | no | Intervention HADS-A: 7.2(3.3), HADS-D: 5.4 (4.2); Control: HADS-A:7.2 (3.7), HADS-D: 5.8 (4.2) |
| Vanbutsele et al.70 | 186 | Advanced cancer | Patient | no | no | – | – | PC nurse with ability to refer to PC physician as needed | illness understanding, symptom burden, psychological coping, spiritual coping, decision making | no | not reported alone, only change reported |
| von Heymann-Horan et al.62 | 340 | Incurable cancer | Caregiver | yes | no | existential-phenomenological therapy | – | Specialized PC teams which included physicians, and at least 2 other professions (e.g., psychologists, social workers) | psych intervention aiming to decrease distress in patients and caregivers | no | Intervention: Anxiety elevated: 28%, depression elevated: 24%; Control: anxiety elevated: 27%, depression elevated: 23% |
Abbreviations: CNS = centra nervous system; PC = palliative care; ACP = advance care planning; APN = advanced practice nurse; NP = nurse practitioner; MSW = masters in social work; SW = social worker; GI = gastro-intestinal; PA = physician assistant; MS = multiple sclerosis; GP = general practitioner; EOL = end of life; CBT = cognitive behavioral therapy.
Denotes that caregivers were primary target of intervention.
Intervention Characteristics.
Twelve studies (31.6%) described a theoretical background for the psychological component of the intervention such as the chronic care model or Lazarus’s stress and coping theory,8,9,40,44,51,56,58,60,61,68,74,76 while the other 26 (68.4%) did not. Only nine studies (23.7%) included a specialty mental health clinician (inclusive of psychologists, social workers providing structured psychosocial care, and psychiatric nurses) on the intervention team.40,54,61,62,64,66,69,71,74 Ten studies (26.3%) included a manualized educational, behavioral, or therapeutic intervention such as problem-solving therapy or cognitive behavioral therapy (CBT).9,42–44,51,52,54,62,64,73,74 Most interventions (N = 26, 68.4%) included interdisciplinary teams of at least two types of clinicians;8,40,41,47–59,61–64,66,67,69,72,76 while seven (17.9%) were performed only by nurses9,42–44,60,68,70,73; four (10.3%) by palliative care physicians,65,71,74,75 and two had either a physician or a nurse.45,46 Fifteen studies (39.5%) were conducted in a mix of settings (e.g., inpatient and outpatient),40,44,52,53,55,58,61,62,64,68,70,72,67 four (10.5%) were conducted in an outpatient setting,8,50,56,71 six (15.8%) in an inpatient setting,51,57,64,72 three (7.9%) were performed in-home,54,66,69 two (5.3%) by telemedicine,42,43,73 and eight (21.1%) did not specify a setting.9,41,45,60,65,74,75 Most interventions (N = 21, 55.3%) explicitly included content aimed at improving psychological distress.8,9,40,42–44,46,51,52,55,57,60,62,64,68–71,74,75,67
Outcome Assessments.
Most included studies (N = 32, 84.2%) had been pre-registered on ClinicalTrials.gov or another similar trial registration webpage,8,9,40–49,51,52,54–56,58–66,68,70,73–76, 67 10 of which included a psychological distress measure as a primary outcome.41–44,48,49,51,55,73,74,67 Five (50%) studies that prespecified a primary psychological outcome included a manualized therapeutic intervention.42–44,51,73,74 The majority of studies considered quality of life as a primary outcome and psychological distress as a secondary outcome. Of the 38 included studies, six (15.8%) investigated only depression symptoms,9,42,43,49,52,57,64 27 (71.5%) evaluated both depression and anxiety symptoms,8,40,40,44–47,50,51,53,55,56,58–62,65,66,68–71,73,74,76,67 and five (13.2%) assessed general psychological distress.41,54,63,72,77 No study had an inclusion criterion that required an elevated score on a psychological distress outcome, while 14 (36.8%) excluded potential subjects with certain diagnosed mental or behavioral health diagnoses, including depression, anxiety, and substance use disorders.
Most included studies (N = 24, 63.2%) did not find a significant difference in psychological distress symptoms among the intervention and control group (Table 2).41,44,49,51–56,58,60,61,63–66,68–72,74–76 Eight studies (21.1%) found a positive effect of the intervention on the corresponding psychological distress outcome, meaning distress was lowered in the intervention group relative to the control.8,9,40,42,43,47,48,57,62 Six studies (15.7%) found a mix of effects,45,46,50,59,73,67 with four studies having both a finding that distress decreased on one measure and didn’t change on another.45,46,59,67 The other two studies with mixed results found distress increased on one measure and didn’t change on another.50,73
Table 2.
Outcomes for Each Included Study
| Study | Study | Psych Distress Outcome Measure(s) | Time Period(s) for Outcome | Symptoms Decreased | Symptoms increased | No change in symptoms |
|---|---|---|---|---|---|---|
| Ammari et al.60 | Ammari et al. 2018 | HADS-A, HADS-D | 16 wk | No differences | ||
| Bakitas et al.9 | Bakitas et al., 2009 | CESD | 4 mo | Mood improved both in longitudinal analyses and in one mo look-back analyses from time of death | ||
| Bakitas et al.52 | Bakitas et al., 2015 | CESD | 3 mo | No difference | ||
| Bakitas et al.51 | Bakitas et al., 2020 | HADS-A, HADS-D | 8 wk, 16 wk (endpoints combined as an average of the two) | No differences at combined endpoint | ||
| Bekelman et al.40 | Bekelman et al., 2018 | PHQ-9, GAD-7 | 3 mo | PHQ-9, GAD-7 | ||
| Brims et al.75 | Brims et al., 2019 | GHQ-12 | 12 wk | No difference | ||
| Carson et al.41 | Carson et al., 2016 | HADS | 3 mo | No difference | ||
| Chan et al., 201667 | Chan et al., 2016 | HADS-A; HADS-D | 3 mo | HADS-A | No difference in HADS-D | |
| Dionne-Odom et al.42; Dionne-Odom et al.43 | Dionne-Odom et al., 2015; Dionne-Odom et al., 2016 | CESD | 3 mo | CESD | ||
| Dionne-Odom et al.44 | Dionne-Odom et al. 2020 | HADS-A, HADS-D | 8 wk and 16 wk | No differences at either time point | ||
| do Carmo et al.74 | do Carmo et al., 2017 | PHQ-9, HADS-D, HADS-A | 90 and 120 d after baseline | No differences | ||
| El-Jawahri et al.45 | El-Jawahri et al., 2017 | HADS-A, HADS-D | 3 mo | HADS-D | No difference in HADS-A | |
| El-Jawahri et al.46 | El-Jawahri et al., 2016 | HADS-A, HADS-D, PHQ-9 | 12 wk after baseline | HADS-D, PHQ-9 | No difference in HADS-A | |
| El-Jawahri et al.47 | El-Jawahri et al., 2021 | HADS A, HADS-D | 12 wk | HADS-D, HADS-A | ||
| Ferrell et al.48 | Ferrell et al., 2021 | Distress thermometer | 12 wk | Distress thermometer | ||
| Gao et al.76 | Gao et al., 2020 | HADS-D, HADS-A | 12 wk | No differences | ||
| Grudzen et al.49 | Grudzen et al., 2016 | PHQ-9 | 12 wk | No differences | ||
| Hoek et al.73 | Hoek et al., 2017 | HADS-A, HADS-D | 12 wk | HADS-A | HADS-D | |
| Janssen et al.50 | Janssen et al., 2020 | HADS-A, HADS-D, PHQ-9 | 3 mo | PHQ-9 | HADS-A, HADS-D | |
| Johnsen et al.61 | Johnsen et al., 2020 | HADS-A and HADS-D | 8 wk | No differences | ||
| Jordhoy et al. 72 | Jordhoy et al., 2001 | IES | 4 mo | No difference | ||
| Maltoni et al. 65 | Maltoni et al., 2016 | HADS-A, HADS-D | 12 wk | No differences | ||
| McCorkle et al.53 | McCorkle et al., 2015 | HADS-A, PHQ-9 | 3 mo | No differences | ||
| McDonald et al.63 | McDonald et al, 2017 | SF-36 MCS | 3 and 4 mo | No differences at either time point | ||
| Ng & Wong68 | Ng & Wong, 2018 | ESAS-A, ESAS-D | 12 wk | ESAS-A, ESAS-D | ||
| Nordly et al.54 | Nordly et al, 2019 | HADS QOL | 8 wk | No difference | ||
| O’Riordan et al.55 | O’Riordan et al., 2019 | HADS-A, HADS-D | 3 mo | No differences | ||
| Rodin et al.64 | Rodin et al., 2020 | BDI-II | 8, 12 wk | No differences | ||
| Scheerens et al.69 | Scheerens et al., 2020 | HADS-A, HADS-D | 12 wk | No differences | ||
| Schenker et al.56 | Schencker et al., 2021 | HADS-A, HADS-D | 3 mo | No differences | ||
| Sidebottom et al.57 | Sidebottom et al., 2015 | PHQ-9 | 3 mo | PHQ-9 | ||
| Slama et al.71 | Slama et al., 2020 | HADS-A, HADS-D | 3 mo | No differences | ||
| Solari et al.66 | Solari et al., 2018 | HADS-A, HADS-D | 3 mo | No differences | ||
| Temel et al.58 | Temel et al., 2010 | HADS-total, PHQ-9 | 12 wk | PHQ-9 and HAD (dichotomized) | ||
| Temel et al.8 | Temel et al., 2017 | HADS-A, HADS-D, PHQ-9 | 12 wk | No differences | ||
| Temel et al.59 | Temel et al., 2020 | HADS A, HADS D | 12 wk | >HADS-A significantly decreased at 12 wk | HADS-D, PHQ-9 | |
| Vanbutsele et al.70 | Vanbutsele et al., 2018 | HADS, PHQ-9 | 12 wk | No differences | ||
| von Heymann-Horan et al. 62 | von Heymann-Horan et al. 2018 | Anxiety and depression subscales of Symptom Checklist-92 (SCL-92) | 8 wk | Anxiety and depression |
Statistical Results: Intervention Effects
Meta-analytic pooling of effects of a palliative care intervention on psychological distress symptoms can be found in Table 3. None of the SMDs for any effects were statistically significant. However, the anxiety and depression SMDs were all negative, that is, in the predicted direction. The strongest effect was observed in the five studies that examined caregiver depression, with an SMD of −0.27 (P = 0.08). In addition, five of the six primary analyses had statistically significant heterogeneity, and four had heterogeneity statistics higher than 75%, indicating possible substantial to considerable heterogeneity.32 Figs. 1–7 show the forest plot results for each of the main outcomes.
Table 3.
Pooled Effect Sizes and Precision for Each Outcome Type, With P-Value for Significantly Different From Null
| Outcome | Standardized Mean Difference | 95% CI | P-Value | I2 statistic, P-Value |
|---|---|---|---|---|
| Patient anxiety (N = 17) | −0.008 | (−0.37, 0.36) | 0.96 | 95%, P < 0.001 |
| Caregiver anxiety (N = 4) | −0.21 | (−1.79, 1.36) | 0.79 | 98%, P < 0.001 |
| Patient depression (N = 22) | −0.13 | (−0.34, 0.09) | 0.25 | 88%, P < 0.001 |
| Caregiver depression (N = 5) | −0.27 | (−0.57, 0.03) | 0.08 | 64%, P =0.02 |
| Patient psychological distress (N = 2) | 0.26 | (−0.70, 1.23) | 0.59 | 94%, P < 0.001 |
| Caregiver psychological distress (N = 3) | 0.04 | (−0.25, 0.33) | 0.78 | 62%, P = 0.07 |
Fig. 7.
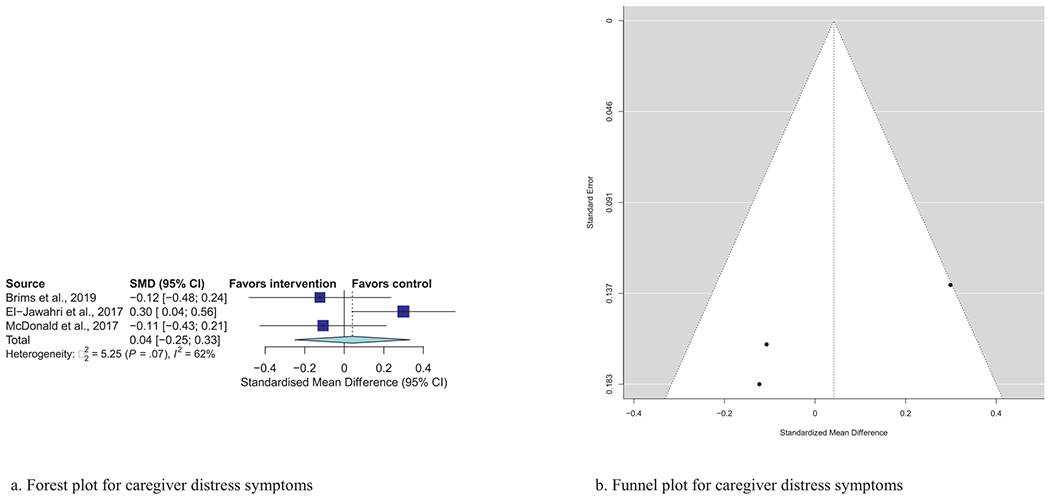
a) Forest plot for caregiver distress symptoms. b) Funnel plot for caregiver distress symptoms. For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.
Statistical Results: Moderator Analyses
None of the moderator analyses resulted in significant findings, and as such, none aligned with our hypotheses. However, for both patient anxiety and depression, studies enrolling only cancer patients had negative (i.e., favorable) SMDs with small effect sizes, while noncancer studies had positive SMDs. Despite not being statistically significant, these findings are in the direction we expected. In addition, studies with lower risk of bias had larger (though not statistically significant) effect sizes for both patient anxiety (SMD of −0.47 for lower risk of bias vs. SMD of 0.12 for higher risk of bias) and patient depression (SMD of −0.28 for lower risk of bias vs. SMD of −0.05 for higher risk of bias). The direction of these nonsignificant findings is consistent with our predictions. A similar pattern was seen for patients with depression, with more favorable findings emerging studies with a lower risk of bias than those with a higher or unclear risk of bias. We also conducted moderator analyses to compare studies that indicated psychological distress was a primary outcome vs. those with psychological distress as a secondary outcome for both patient anxiety (SMD of 0.01 for primary outcome vs. SMD of −0.03 for secondary outcome) and patient depression (SMD of 0.52 for primary outcome vs. −0.23 for secondary outcome). This finding did not align with our predicted direction of effect. Moderator analyses comparing interventions with and without a specialty mental health clinician for patient anxiety (SMD of −0.07 for a specialty mental health clinician vs −0.007 for no specialty mental health clinician) did not support our hypothesis. When depression was the outcome (SMD of 0.08 for specialty mental health clinician vs. −0.22 for no specialty mental health clinician), the direction of effect was inconsistent with predictions. Finally, identifying a theoretical basis for the psychological component of the intervention was not statistically significant for patient anxiety (SMD −0.09 for theory vs. SMD .02 for no theory) or patient depression (SMD of −0.27 for theory vs. SMD of −0.03 for no theory). However, the direction of these effects was as expected. Nearly all moderator analyses had heterogeneity statistics indicating substantial to considerable heterogeneity. However, four subgroups in the patient anxiety moderator analyses saw less heterogeneity: distress as primary outcome (I2 = 32%), those with a theoretical basis (I2 = 13%), those without a manualized intervention (I2 = 42%), and those with a specialty mental health clinician (I2 = 20%). Results for moderator analyses can be seen in Table 4.
Table 4.
Moderator Analyses Results for Patient Outcomes, With SMDs and 95% CI for Subgroup Effects and P-values for Between Subgroup Differences
| Outcome | Subgroup | Standardized Mean Difference | 95% CI | P-Value | I2 Statistic |
|---|---|---|---|---|---|
| Patient anxiety | Cancer (N = 7) | −0.28 | (−0.87, 0.31) | 0.14 | 96% |
| Non-cancer (N = 7) | 0.23 | (−0.09, 0.54) | 80% | ||
| Higher risk of bias (N = 12) | 0.12 | (−0.13, 0.37) | .18 | 72% | |
| Lower risk of bias (N = 5) | −0.47 | (−1.30, 0.36) | 98% | ||
| Distress primary outcome (N = 4) | 0.01 | (−0.35, 0.38) | .87 | 32% | |
| Distress not primary outcome (N = 13) | −0.03 | (−0.47, 0.40) | 96% | ||
| Theoretical basis (N = 6) | −0.09 | (−0.22, 0.04) | .74 | 13% | |
| No theoretical basis (N = 11) | 0.02 | (−0.58, 0.62) | 96% | ||
| Manualized therapy (N = 5) | −0.003 | (−1.09, 1.08) | .88 | 98% | |
| No manualized therapy (N = 12) | −0.09 | (−0.23, 0.06) | 42% | ||
| Specialty MH clinician on team (N = 6) | −0.07 | (−0.24, 0.11) | .82 | 20% | |
| No specialty MH clinician (N = 11) | −0.007 | (−0.54, 0.52) | 97% | ||
| Patient depression | Cancer (N = 13) | −0.23 | (−0.53, 0.07) | .25 | 91% |
| Non-cancer (N = 9) | 0.003 | (−0.26, 0.26) | 75% | ||
| Higher risk of bias (N = 16) | −0.04 | (−0.33, 24) | .32 | 88% | |
| Lower risk of bias (N = 6) | −0.28 | (−0.64, 0.09) | 91% | ||
| Distress primary outcome (N = 4) | 0.52 | (−0.27, 1.30) | .07 | 86% | |
| Distress not primary outcome (N = 18) | −0.23 | (−0.46, −0.01) | 88% | ||
| Theoretical basis (N = 9) | −0.27 | (−0.62, 0.08) | .25 | 93% | |
| No theoretical basis (N = 13) | −0.03 | (−0.25, 0.20) | 71% | ||
| Manualized therapy (N = 6) | −0.02 | (−0.32, 0.28) | .47 | 75% | |
| No manualized therapy (N = 16) | −0.17 | (−0.43, 0.10) | 90% | ||
| Specialty MH clinician on team (N = 7) | 0.08 | (−0.24, 0.41) | .16 | 74% | |
| No specialty MH clinician (N = 15) | −0.22 | (−0.48, 0.04) | 90% |
Discussion
Our analyses spanned 38 RCTs, with more than 6000 patients and 1500 caregivers. Only one-quarter of included studies found a significant improvement in psychological distress symptoms, and the current meta-analysis suggests that, on average, palliative care interventions do not lead to reductions in psychological distress. While not statistically significant, studies focusing on caregiver anxiety and depression had moderate effect sizes in favor of the intervention. In addition, four of the six primary analyses had heterogeneity statistics higher than 75%, which is likely attributable to the variety of diseases, treatment settings, and interventions included in the meta-analysis studied. In our moderator analyses, we did observe some findings favoring intervention, though none rose to the level of statistical significance. Standardized mean differences in patients with cancer and among studies with lower risk of bias trended toward favoring intervention, with small to moderate effect sizes (from −0.21 to −0.47).
The overall null pattern of findings can be partially explained by the fact that none of the studies required elevated psychological distress scores for study entry. Moreover, some studies systematically excluded individuals with common anxiety and depression diagnoses. This could lead to a floor effect with no improvements in symptoms to detect.
The exclusion of patients with mental health conditions not only decreases variability on the outcome variable, but it also raises troubling ethical concerns. One of the goals of palliative care is to address psychological symptoms.6,7 RCTs, such as those included in the current study, often influence medical care and inform evidence based practice. When studies exclude patients with psychological disorders, as over 1/3 of the studies in this review did, then the very individuals who may be most in need of palliative care’s integrative approach to suffering are not represented in the clinical trial. The systematic exclusion of individuals with psychopathology is not uncommon,78 even in trials of treatments for mental health conditions,79 and likely contributes to and perpetuates health inequities.
In addition, the included studies also had a wide variety of intervention approaches to address psychological distress among enrolled subjects, which constitutes a challenge for conducting a systematic review and meta-analysis.80 This issue is underscored by the large heterogeneity statistics for most of the analyses. This may have added more difficulty to determining an effect of interventions on psychological distress outcomes because the interventions themselves varied greatly, possibly diffusing effects.
Our findings from specialty mental health clinician moderator analyses were nonsignificant. This may be due to the lack of specificity of training background of clinicians (inclusive of psychologists, social workers, and psychiatric nurses). Furthermore, some mental health clinicians may offer general support while others may offer medication management or evidence-based psychotherapies. Just over one quarter of studies included a manualized therapeutic or behavioral intervention such as CBT. In palliative care, general psychosocial support is often offered,1 but less effective than CBT in managing anxiety symptoms.81 Future studies should consider the type of psychological intervention as a potential moderator of the effect of palliative care on psychological distress symptoms, and its specificity in addressing different distress outcomes (e.g., symptoms of anxiety, depression, PTSD, etc.).
The current findings seem to contrast with preliminary evidence from observational studies that have demonstrated that palliative care service use may reduce suicidal self-violence among veterans with11,12 and without10 cancer diagnoses. If palliative care does indeed decrease suicide risk, but does not reliably decrease psychological distress, it will be important to examine alternative mechanisms by which palliative care mitigates suicide risk (e.g., reducing pain; increasing social connection).
The current study is consistent with and extends prior work on mental health services in palliative care. A 2018 review of psychological interventions in palliative care found that many studies failed to describe how psychological symptoms were identified and treated, which team member within the palliative care team delivered psychological treatment, and whether symptoms improved as a result.82 In the current systematic review, we similarly observed that descriptions of the palliative care interventions often lack enough detail to determine who is delivering particular components of the intervention and whether there is a specific psychological treatment component of the intervention, let alone if the psychological treatment is manualized or evidence-based. However, there seems to be improvement over the last few years, with several of the more recent RCTs providing more detail than earlier studies. Our findings add to the literature by including more studies and a meta-analysis to quantify the effect of the interventions.
Our study demonstrated a need to carefully consider whether improvement in psychological distress symptoms is a realistic outcome of general palliative care interventions or if perhaps nonworsening psychological symptoms may be a more realistic goal for patients nearing end of life. If study participants are enrolled late in their illness trajectory, as would be expected in many palliative care studies, it may be more difficult to reduce psychological distress at that point in the disease trajectory given that distress tends to increase as end of life approaches, especially in illnesses with high symptom burden.5,83 Furthermore, researchers should consider how they expect palliative care to impact psychological symptoms, whether directly through psychosocial treatment or indirectly through other symptom management, and design RCTs accordingly. We recommend that researchers include the mechanism through which they expect to impact psychological symptoms. More research is needed in efficacy trials to identify effective and appropriate psychological interventions for use in palliative care settings.
Limitations.
This study is subject to some limitations. First, only RCTs published in English were included due to language limitations of the study team. Future work should include studies written in many languages. Next, studies were included only if they reported outcomes between two and four months after study intake. The longer-term effects of palliative care interventions on psychological distress are not yet estimated in a meta-analysis. Next, this meta-analysis investigated the effects of heterogeneous interventions on heterogeneous populations seen in heterogeneous settings by heterogeneous palliative care teams. While several moderator analyses were performed to investigate the effects of some dimensions of variation among both interventions and populations, future work could focus on specific aspects of palliative care interventions or on specific populations.
Implications for Policy, Practice, and Future Research
Palliative care is a relatively new field which has demonstrated efficacy for managing burdensome symptoms and improving quality of life for people with serious illness. While psychological science and psychiatry have made strides in improving psychological distress symptoms, these advances have not been fully integrated into palliative care. Future RCTs may benefit from including theoretically-grounded psychological interventions that are adapted for and integrated into palliative care settings. The field will also benefit from increasing transparency and accountability through trial preregistration, providing sample size estimates to detect an effect in the psychological distress variable, and specifying a basis for the assessment time points. We also believe that research teams must include patients with existing mental health conditions in their studies to improve quality of care for this group that is often under-represented in clinical trials.
In conclusion, this systematic review and meta-analysis uncovered no evidence to support the idea that palliative care interventions reduce psychological distress, but we did identify conceptual and methodological problems in the literature that could be remedied. More work is needed to adapt and integrate theoretically-grounded, evidence-based psychological interventions into studies of palliative care and rigorously evaluate outcomes in seriously ill populations.
Fig. 2.
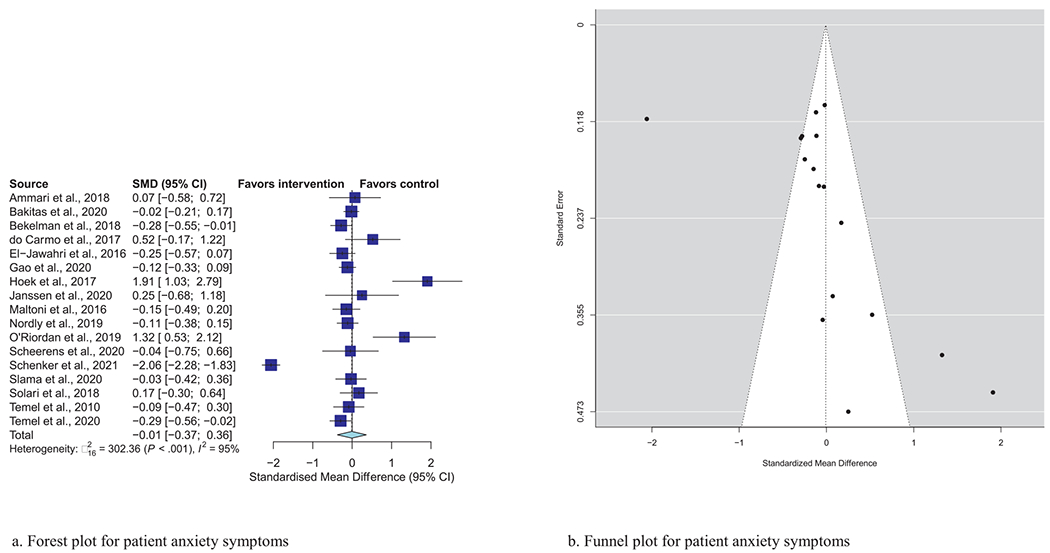
a) Forest plot for patient anxiety symptoms. b) Funnel plot for patient anxiety symptoms. For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.
Fig. 3.
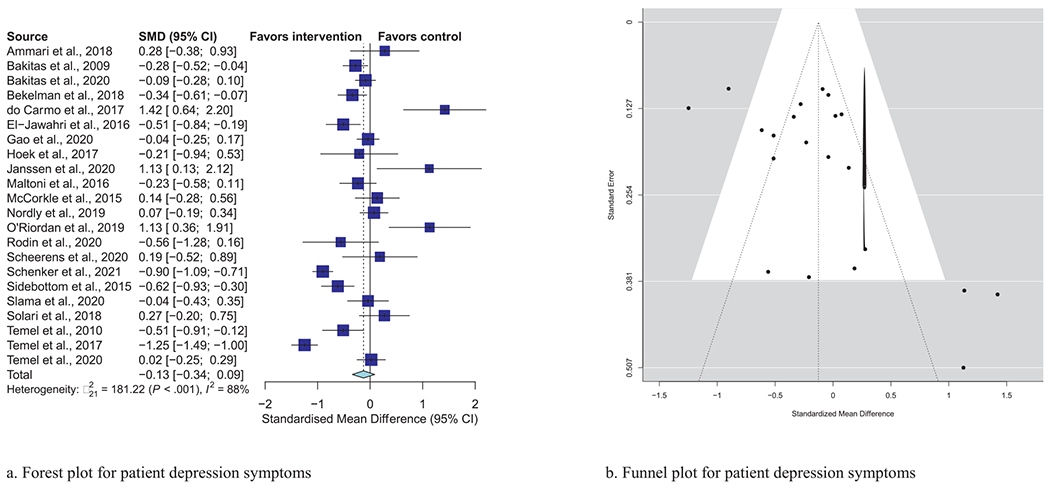
a) Forest plot for patient depression symptoms. b) Funnel plot for patient depression symptoms. For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.
Fig. 4.
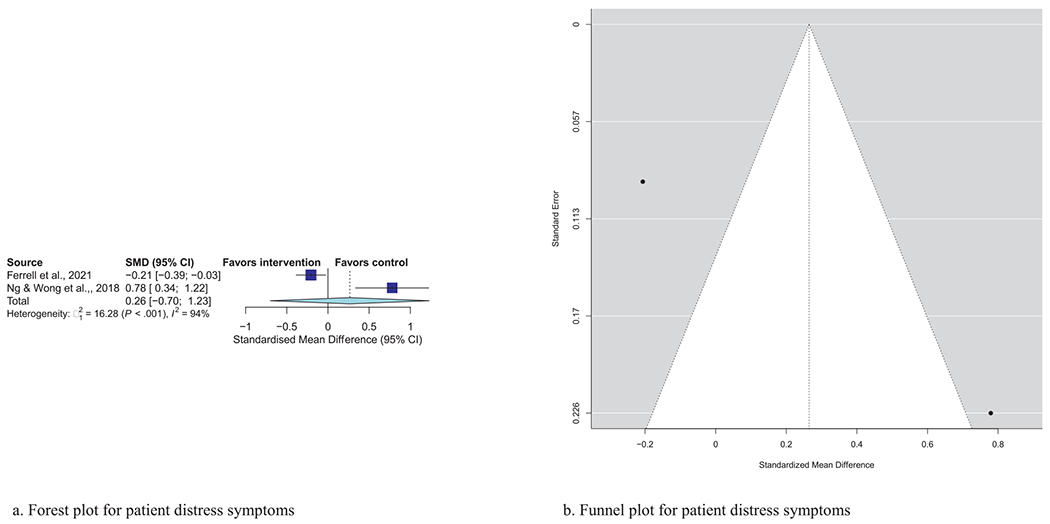
a) Forest plot for patient distress symptoms. b) Funnel plot for patient distress symptoms. For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.
Fig. 5.
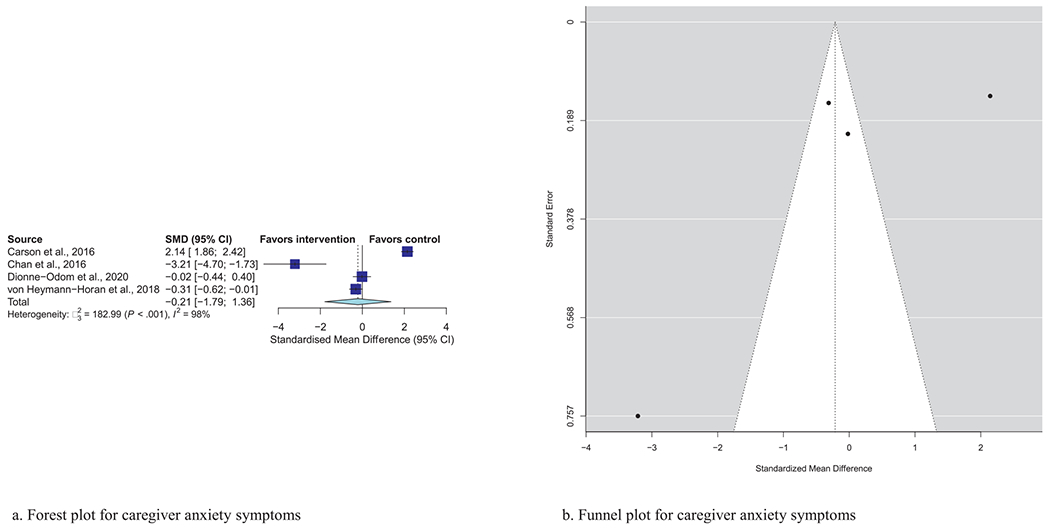
a) Forest plot for caregiver anxiety symptoms. b) Funnel plot for caregiver anxiety symptoms. For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.
Fig. 6.
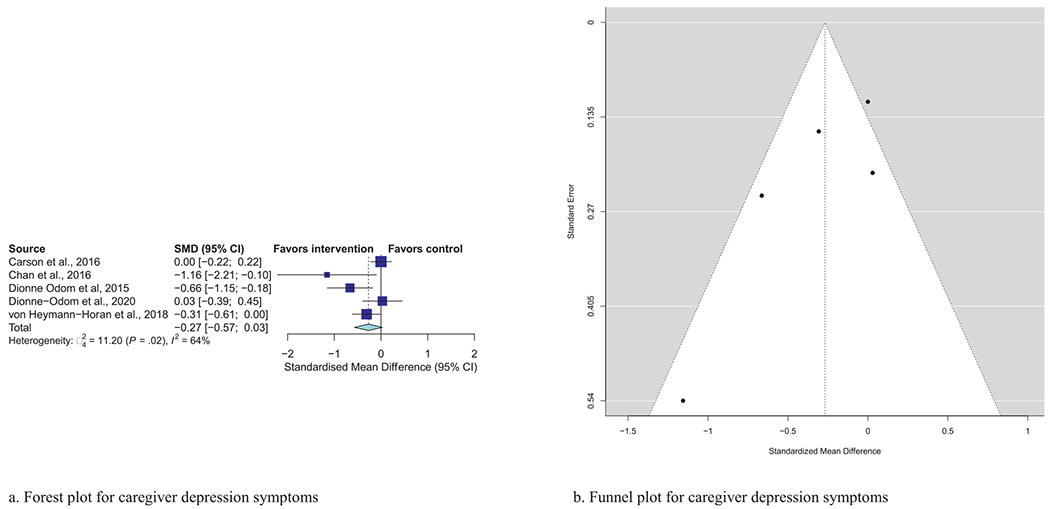
a) Forest plot for caregiver depression symptoms. b) Funnel plot for caregiver depression symptoms. For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.
Disclosures and Acknowledgments
The authors would like to thank Yingting Zhang for her assistance with developing the literature search strategy, and Shoshanna Tokar for her assistance with screening and reviewing studies. This research received no specific funding/grant from any funding agency in the public, commercial, or not-for-profit sectors. The authors declare no conflicts of interest.
Appendix
Appendix Table 1.
Appendix Items
PubMed:
(palliative care [MeSH] OR palliative [TiAb]) AND ((depression) OR (anxiety) OR (distress) OR (quality of life) OR (mood))
Randomized Controlled Trial filter ON
−896 results
| Study | Country | Mean Age | Baseline Psychological Distress, Mean (SD) | Racial Group | Ethnicity | Gender Distribution | Marital Status | Educational Attainment | SES |
|---|---|---|---|---|---|---|---|---|---|
| Ammari et al.60 | Denmark | 67.4 | NA | NA | 68% men | NA | NA | NA | |
| Bakitas et al.9 | USA | Intervention: 65.4 Control: 65.2 |
Intervention: 98.6% White Control: 98.5% White |
No Hispanic participants | Intervention: 62.1% men Control: 58.2% men |
Intervention: 73.1% married Control: 67.2% married |
Intervention: 57.2% HS+ Control: 55.2% HS+ |
NA | |
| Bekelman et al.40 | USA | Intervention: 64.5 Control: 66.5 |
NA | NA | intervention: 81.5% men Control: 75.8% men |
NA | Intervention: 33.1% HS graduate or less Control: 27.4% HS graduate or less |
Intervention: 41.6% less than $20k Control: 44.0% less than $20k |
|
| Brims et al.75 | UK and Australia | Intervention: 72.1 Control: 72.8 |
NA | NA | Intervention: 77% male Control: 82.8% male |
NA | NA | NA | |
| Carson et al.41 | USA | 51 | NA | int: 15% Hispanic, control: 13% | Intervention: 70% female Control: 72.7% female |
Intervention: 59% married Control: 66% married |
NA | Intervention: 57% employed Control: 51% employed |
|
| Chan et al.67 | Hong Kong | 45% 40-59 years, 38% 60-79 years | NA | NA | 76% female | 79% married | 80% HS education or lower | NA | |
| Dionne-Odom et al.42; Dionne-Odom et al.43 | USA | Intervention: 61 Control: 57.9 |
Intervention: 90.2% white Control: 95.1% white |
NA | Intervention: 77% women Control: 80.3% women |
Intervention: 88.5% married Control 95.1% married/cohabitating |
Intervention: 60.7% some collge or less Control: 54.1% some college or less |
Intervention: 37.7% employed Control: 60.7% employed |
|
| Dionne-Odom et al.44 | USA | Intervention: 58.2 Control: 57.6 |
Intervention: 39% white, 56.1% BlackControl; 50% white, 47.4% Black | NA | Intervention: 89% women Control: 81.6% women |
Intervention: 69.5% married/cohabitating Control: 72.4% married/cohabitating |
Intervention: 36.5% HS or less Control: 36.3% HS or less |
Intervention: 30.5% employed Control 39.5% employed |
|
| do Carmo et al.74 | Brazil | Intervention group1: 49.1 Intervention group 2: 52.7 Control: 57 |
Intervention group 1:63.2% white, 15.8% Black Intervention group 2: 45.5% white, 36.4% Black Control: 68.2% White, 9.1% Black |
recorded with race | Intervention group 1: 68.4% women Intervention group 2: 62.6% women Control: 63.6% women |
Intervention group 1:68.5% married/stable union Intervention group 2: 59.1% married/stable union Control: 72.7% married/stable union |
Intervention 1: 57.9% “low educational level” Intevention 2: 45.5% “low educational level” Control: 54.5% “low educational level” |
Intervention 1:42.1% employed Intervention 2: 36.4% employed Control: 54.5% employed |
|
| El-Jawahri et al.45 | USA | Intervention: 57.5 Control: 57.2 |
Intervention: 92.7% white Control: 92.8% white |
1.5% (PC) Hispanic; 2.2% (UC) Hispanic | Intervention: 68.6% women Control: 69.6% women |
NA | Intervention: 19% HS, 54.7% college Control: 34.1% HS, 45.7% college |
Intervention: 54.0% working Control: 50% working |
|
| El-Jawahri et al.46 | USA | Intervention: 57.2 Control: 56.9 |
Intervention: 85.2% white Control: 88.6% white |
NA | Intervention: 59.3% women Control: 54.4% women |
Intervention: 77.8% married Control: 69.6% married |
Intervention: 28.4% HS, 43.2% college Control: 30.4% HS, 53.2% college |
Intervention: 27.4% less than $51k Control: 40.3% less than $51K |
|
| El-Jawahri et al.47 | USA | Median (IQR): 64.4 (19.7-80.1) | 86.2% white | NA | 40.0% women | NA | NA | NA | |
| Ferrell et al.48 | USA | Median (IQR): 62 (53–69) | 7.1% African American, 9.6% Asian, 69.3% Caucasian, 9.0% Hispanic/Latino, 1.3% Native Hawaiian, 2.5% Mixed race, 1.3% Other | Hispanic Latino 43 (9.0%) | 56.8% women | 74.9% married/living with partner | 22.8% HS grad, 53.9% college grad, 20.0% graduate school | 60.3% greater than $50k | |
| Gao et al.76 | UK | Intervention: 67.3 Control: 66.4 |
Intervention: 94.3% NH white Control: 86.2% NH white |
combined with race | PC: 48.9% M, UC: 53.5% M | Intervention: 64.8% married/civil partner Control: 67.2% marrried/civil partner |
Intervention: 38.1% no formal education Control: 41.4% no formal education |
Intervention: 98.3% not employed Control: 96.0% not employed |
|
| Grudzen et al.49 | USA | Intervention: 55.1 Control: 57.8 |
Intervention: 34% white, 27% Black, 6% Asian, 1% AI/AN, 1% multi-racial, 30% other Control: 30% white, 23% Black, 3% Asian, 3% AI/AN, 1% multi-racial, 38% other |
Hispanic or Latino control 29 (43%) Intervention 20 (29%) |
Intervention: 57% women Control: 55% women |
NA | Intervention: 46% HS or less Control: 54% HS or less |
Intervention: 62% <$50k Control: 61% <$50k |
|
| Hoek et al.73 | Netherlands | Intervention: 62.3 Control: 61.9 |
NA | NA | Intervention: 29% women Control: 39% women |
Intervention: 71% married/permanent relationship Control: 81% married/permanent relationship |
Intervention: 24% college+ Control: 25% college+ |
NA | |
| Janssen et al.50 | USA | 71.1 | NA | NA | 90% Male | NA | NA | NA | |
| Johnsen et al.61 | Denmark | Modal range: 60-69 | NA | NA | Intervention: 57% women Control: 59% women |
NA | Intervention: 18% didn’t go beyond mandatory education Control: 12% |
NA | |
| Jordhoy et al.72 | Norway | Median Intervention: 70 Control: 69 |
NA | NA | Intervention: 44% women Control: 51% women |
NA | Intervention: 13% 13 years or more Control: 17% |
NA | |
| Bakitas et al.51 | USA | Intervention: 63.5 Control: 64.1 |
Intervention: 44.2% white, 54.3% Black Control: 44.4% white, 54.6% Black |
NA | Intervention: 53.4% male Control: 53.1% male |
Intervention: 50.5% married/living with partner Control: 46.4% married/living with partner |
Intervention: 53.3% HS or less Control: 38.2% |
Intervention: 2.9% unemployed 48.6% disabled Control: 2.4% unemployed, 46.9% disabled |
|
| Bakitas et al.52 | USA | Intervention: 64.03 Control: 64.6 |
Intervention: 90.08% white Control: 95.15% white |
NA | Intervention: 53.85% male Control: 51.46% male |
Intervention: 66.35% married/living with partner Control: 64.08% |
Intervention: 66.34% HS or less Control: 51.45% |
Intervention: 24.04% employed, 47.12% retired Control: 23.3% employed, 48.54% retired |
|
| Maltoni et al.65 | Italy | Intervention: 67 Control: 66 |
NA | NA | Intervention: 61.5% male Control: 52.8% male |
Intervention: 76.9% married Control: 78.6% married |
NA | NA | |
| McCorkle et al.53 | USA | 62.3% younger than 65, 37.7% 65+ | NH white: 85.9%, other: 15.1% | combined with race | 56.2% Female | 56.2% married, 43.8% single/widowed/divorced | 28.8% HS or less, 71.2% college or more | 34.2% working, 31.6% retired, 34.2% other | |
| McDonald et al.63 | Canada | Median Intervention: 58 Control: 57 |
NA | int: 80.9% “European ethnicity”, 88.2% control | Intervention: 61.7% women Control: 69.3% women |
Intervention: 94.5% married Control: 96.6% married |
Intervention: 73.1% greater than HS Control: 56.5% greater than HS |
Intervention: 39.4% retired, 52.1% employed Control: 31.8% retired, 47.7% employed |
|
| Ng & Wong68 | Hog Kong | Intervention: 78.3 Control: 78.4 |
NA | NA | Intervention: 43.9% male Control: 61.90% male |
Interventoin: 62.8% married, 30.2% widowed Control: 68.3% married, 22.1% widoed |
Intervention: 44.2% no schooling, 37.2% primary, 16.3% secondary Control: 29.3% no schooling, 48.8% primary, 17.1% scondary |
Intervention: 25.6% makemore than enough money Control: 24.4% makemore than enough money |
|
| Nordly et al.54 | USA | Intervention: 66.3 Control: 65.2 |
NA | NA | Intervention: Male 48.7 % Control: Male 48.8% |
Intervention: 69.8% married Control: 66% married |
Intervention: 58.3% with at least some higher education Control: 51.9% |
NA | |
| O’Riordan et al.55 | USA, California | Intervention: 71 Control: 59 |
Intervention: 44% white Control: 43% white |
NA | Intervention: 69% female Control: 28% female |
NA | Intervention: 81% 4 year degree Control: 64% 4-year degree |
NA | |
| Rodin et al.64 | Canada, Toronto | 52.86 | 76.2% white | combined with race | 38.1% female | 71.4% married/common law | 66.7% post-secondary education | 54.8% employed, 43.3% with income less than 60k | |
| Scheerens et al.69 | Belgium | Intervention: 67.5 Control: 67 |
NA | NA | Intervention: 55% male Control: 57.9% male |
Intervention: 65% married Control 73.7% married |
Intervention: 45.0% Lower secondary, primary education, or less Control: 73.7% lower secondary, primary education, or less |
NA | |
| Schencker et al.56 | USA | 69.3 | 4.9% Black, 0.7% Asian, 94% white | Latino 9 (1.3%) others - non latino 663 (98.7%) | 53.6% women | 56.8% married | High school/GED or less (49.8%), Some college or college degree 289 (43.0%), Graduate or professional degree 41 (6.1%) | 6.8% Cannot make ends meet, 33.6%, Just manage to get by | |
| Sidebottom et al.57 | USA, Minnesota | 73.4 | 93.9% white, 4.3% Black, 1.3% American Indian | 0.9% Hispanic | 47.4% female | 53% married/partnered | NA | NA | |
| Slama et al.71 | Czech Republic | NA | NA | NA | NA | NA | |||
| Intervention: 61.1 Control: 63.5 |
InterventionL 61.7% male Control: 57.6% male |
||||||||
| Solari et al.66 | Italy | Intervention: 60.5 Control: 56.8 |
NA | NA | Intervention: 62% female Control: 46% female |
NA | Intervention: 20% university Control: 24% university |
Intervention: 78% retired due to disability Control: 85% retired due to disabilty |
|
| Temel et al.58 | USA, Massachusetts | Intervention: 65.64 Control: 64.03 |
Intervention: 89.1% white, 3.4% Black, 4% Hispanic, 2.9% Asian, 2.3% American Indian Control: 95.4% White, 1.7% Asian, 2.3% Black, 1.1% Hispanic |
combined with race | Intervention: 52% male Control: 56% male |
Intervention: 69.1% married Control: 70.9% married |
Intervention: 33.1% HS or less, 43.3% some or completed college Control: 41.7% HS or less, 39.4% some or completed college |
NA | |
| Temel et al.8 | USA, Massachusetts | Intervention: 64.98 Control: 64.87 |
Intervention: 100% white Control: 95% white, 4% Black, 1% Asian |
both groups 1% Hispanic | Intervention: 55% female Control: 49% female |
Intervention: 62% married Control: 61% married |
NA | NA | |
| Temel et al.59 | USA | Intervention: 65.5 Control: 65 |
Intervention: 75.9% white, 12.3% Black, 6.2% unknown, 4.6% Asian, 1% AI/AN Control: 79.1% white, 11.2% Black, 4.6% unknown, 3.1% AI/AN, 1% Native Hawaiian |
Hispanic intervention 8 (4.1%) Control 6 (3.1%) | Intervention: 58.5% male Control: 54.6% male |
Intervention: 61.6% married/partner Control: 59.2% married/partner |
Intervention: HS or less 37.4% Control: HS or less 41.4% |
NA | |
| Vanbutsele et al.70 | Belgium | Intervention: 64.5 Control: 65.0 |
NA | NA | NA | NA | Intervention: 26% college or higher Control: 32% college or higher |
NA | |
| von Heymann-Horan et al.62 | Denmark | Intervention: 61 Control: 62 |
NA | NA | Intervention: 63% female Control: 65% female |
Intervention: 92% married/cohabitating Control: 90% married/cohabitating |
Intervention; 38% HS or less Control: 41% HS or less |
NA |
Appendix Table 2.
Risk of Bias for Each Included Study
| Authors | True randomization? | Allocation to treatment groups concealed? | Treatment groups similar at baseline? | Outcomes assessors blind to treatment assignment? | Treatment groups treated identically other than intervention? | Follow up complete, if not were differences btwn groups in terms of their follow up adequately described and analyzed? | Participatns analyzed in the groups to which they wewre randomized? | Were outcomes measured in the same way for treatment groups? | Were outcomes measured in a reliable way? | Was appropriate statistical analysis used? | Was trial design appropriate, and any deviations from standard RCT design accounted for in conduct and analysis of the trial? |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ammari et al.11 | yes | unclear | yes | no | yes | unclear | yes | yes | unclear | no | yes |
| Bakitas et al.12 | yes | unclear | yes | no | unclear | no | yes | yes | yes | no | yes |
| Bekelman et al.13 | yes | yes | yes | no | unclear | unclear | yes | yes | unclear | yes | yes |
| Brims et al.16 | yes | unclear | yes | no | unclear | unclear | yes | yes | unclear | yes | yes |
| Carson et al.17 | yes | yes | yes | no | unclear | no | yes | yes | unclear | yes | yes |
| Chan et al.18 | yes | unclear | yes | no | unclear | no | unclear | yes | unclear | unclear | yes |
| Dionne-Odom et al.19; Dionne-Odom et al.20 | unclear | unclear | yes | no | unclear | no | yes | yes | unclear | yes | yes |
| Dionne-Odom et al.21 | yes | yes | yes | no | unclear | unclear | yes | yes | unclear | yes | yes |
| do Carmo et al.22 | yes | unclear | no | no | unclear | unclear | yes | yes | yes | yes | yes |
| El-Jawahri et al.23 | yes | yes | yes | no | yes | no | unclear | yes | unclear | yes | yes |
| El-Jawahri et al.46 | yes | unclear | yes | no | unclear | no | unclear | yes | unclear | yes | yes |
| El-Jawahri et al.47 | yes | unclear | yes | no | unclear | no | unclear | yes | unclear | yes | yes |
| Ferrell et al.48 | yes | unclear | yes | unclear | unclear | no | yes | yes | unclear | yes | yes |
| Gao et al.25; patient | yes | unclear | yes | yes | unclear | no | yes | yes | unclear | yes | yes |
| Grudzen et al.49 | yes | yes | yes | yes | yes | no | yes | yes | unclear | yes | yes |
| Hoek et al.26 | yes | yes | no | unclear | yes | no | unclear | yes | unclear | yes | yes |
| Janssen et al.27 | unclear | unclear | no | unclear | unclear | no | yes | yes | unclear | yes | yes |
| Johnsen et al.29 | yes | unclear | yes | No | yes | no | no | yes | unclear | unclear | yes |
| Jordhoy et al.30 | unclear | no | yes | unclear | unclear | no | unclear | yes | unclear | unclear | yes |
| Bakitas et al.32 | yes | yes | yes | yes | unclear | no | yes | yes | unclear | yes | yes |
| Bakitas et al.33 | yes | unclear | yes | yes | yes | no | yes | yes | unclear | yes | yes |
| Maltoni et al.34 | yes | no | yes | No | unclear | no | yes | yes | unclear | yes | yes |
| McCorkle et al.35 | yes | no | no | unclear | yes | no | unclear | yes | yes | maybe | unclear |
| McDonald et al, 20136 | yes | unclear | yes | No | yes | no | yes | yes | unclear | yes | yes |
| Ng & Wong, 201837 | yes | yes | yes | No | yes | no | yes | yes | unclear | yes | yes |
| Nordly et al.54 | yes | yes | yes | No | unclear | no | no | yes | unclear | yes | yes |
| O’Riordan et al.39 | yes | unclear | no | yes | unclear | no | unclear | yes | unclear | yes | yes |
| Rodin et al.42 | yes | yes | yes | No | yes | no | yes | yes | unclear | no | yes |
| Scheerens et al.69 | yes | yes | no | No | yes | no | unclear | yes | unclear | yes | yes |
| Schencker et al.56 | yes | yes | no | yes | unclear | no | yes | yes | unclear | yes | yes |
| Sidebottom et al.44 | unclear | yes | no | unclear | yes | no | yes | yes | un | yes | yes |
| Slama et al.71 | yes | unclear | yes | No | unclear | no | unclear | yes | unclear | non | yes |
| Solari et al.45 | yes | yes | no | yes | unclear | no | yes | yes | unclear | yes | yes |
| Temel et al.46 | yes | yes | no | unclear | yes | no | unclear | yes | unclear | yes | yes |
| Temel et al.47 | yes | unclear | yes | No | unclear | No | yes | yes | un | yes | yes |
| Temel et al.59 | yes | unclear | no | No | unclear | No | unclear | yes | unclear | yes | yes |
| Vanbutsele et al.48 | yes | yes | no | No | yes | No | yes | yes | un | yes | yes |
| von Heymann-Horan et al.49 | yes | yes | yes | No | yes | No | yes | yes | unclear | yes | yes |
References
- 1.Kozlov E, Eghan C, Moran S, Herr K, Reid MC. Palliative care providers’ practices surrounding psychological distress screening and treatment: a National Survey. Am J Hosp Palliat Med 2018;35:938–944. 10.1177/1049909117743960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ridner SH. Psychological distress: concept analysis. J Adv Nurs 2004;45:536–545. 10.1046/j.1365-2648.2003.02938.x. [DOI] [PubMed] [Google Scholar]
- 3.Mitchell AJ, Chan M, Bhatti H, et al. Prevalence of depression, anxiety, and adjustment disorder in oncological, haematological, and palliative-care settings: a meta-analysis of 94 interview-based studies. Lancet Oncol 2011;12:160–174. 10.1016/S1470-2045(11)70002-X. [DOI] [PubMed] [Google Scholar]
- 4.Yohannes AM, Willgoss TG, Baldwin RC, Connolly MJ. Depression and anxiety in chronic heart failure and chronic obstructive pulmonary disease: prevalence, relevance, clinical implications and management principles. Int J Geriatr Psychiatry 2010;25:1209–1221. 10.1002/gps.2463. [DOI] [PubMed] [Google Scholar]
- 5.Kozlov E, Dong X, Kelley AS, Ankuda C The epidemiology of depressive symptoms in the last year of life. J Am Geriatr Soc 2020;68:321–328. 10.1111/jgs.16197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.National Consensus Project for Quality Palliative Care. Clinical Practice Guidelines for Quality Palliative Care. 4th Edition National Coalition for Hospice and Palliative Care.; 2018. https://www.nationalcoalitionhpc.org/ncp. [DOI] [PubMed]
- 7.World Health Organization. Palliative Care. 2020. Accessed November 20, 2021. Available at: https://www.who.int/news-room/fact-sheets/detail/palliative-care
- 8.Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non–small-cell lung cancer. N Engl J Med 2010;363:733–742. 10.1056/NEJMoa1000678. [DOI] [PubMed] [Google Scholar]
- 9.Bakitas M, Lyons KD, Hegel MT, et al. Effects of a palliative care intervention on clinical outcomes in patients with advanced cancer: the Project ENABLE II randomized controlled trial. JAMA 2009;302:741–749. 10.1001/jama.2009.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kutney-Lee A, Khazanov GK, Carpenter JG, et al. Palliative care and documented suicide: association among veterans with high mortality risk. J Pain Symptom Manage 2022;64:e63–e69. 10.1016/j.jpainsymman.2022.04.179 [DOI] [PubMed] [Google Scholar]
- 11.Sullivan DR, Forsberg CW, Golden SE, Ganzini L, Dobscha SK, Slatore CG. Incidence of suicide and association with palliative care among patients with advanced lung cancer. Ann Am Thorac Soc 2018;15:1357–1359. 10.1513/AnnalsATS.201805-299RL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nugent SM, Morasco BJ, Handley R, et al. Risk of suicidal self-directed violence among US veteran survivors of head and neck cancer. JAMA Otolaryngol Neck Surg 2021;147:981–989. 10.1001/jamaoto.2021.2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kavalieratos D, Corbelli J, Zhang D, et al. Association between palliative care and patient and caregiver outcomes: a systematic review and meta-analysis. JAMA 2016;316:2104. 10.1001/jama.2016.16840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gaertner J, Siemens W, et al. Effect of specialist palliative care services on quality of life in adults with advanced incurable illness in hospital, hospice, or community settings: systematic review and meta-analysis. BMJ 2017;357:j2925. 10.1136/bmj.j2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Quinn KL, Shurrab M, Gitau K, et al. Association of receipt of palliative care interventions with health care use, quality of life, and symptom burden among adults with chronic noncancer illness: a systematic review and meta-analysis. JAMA 2020;324:1439–1450. 10.1001/jama.2020.14205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singer AE, Goebel JR, Kim YS, et al. Populations and interventions for palliative and end-of-life care: a systematic review. J Palliat Med 2016;19:995–1008. 10.1089/jpm.2015.0367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoerger M, Wayser G, Schwing G, Suzuki A, Perry L. Impact of Interdisciplinary outpatient specialty palliative care on survival and quality of life in adults with advanced cancer: a meta-analysis of randomized controlled trials. Ann Behav Med 2019;53:674–685. 10.1093/abm/kay077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hui D, Bruera E. Models of integration of oncology and palliative care. Ann Palliat Med 2015;4:898. [DOI] [PubMed] [Google Scholar]
- 19.Kaasa S, Loge J, Aapro M, et al. Integration of oncology and palliative care: a Lancet Oncology Commission. LANCET Oncol 2018;19:E588–E653. 10.1016/S1470-2045(18)30415-7. [DOI] [PubMed] [Google Scholar]
- 20.Hui D, Kim Y, Park J, et al. Integration of oncology and palliative care: a systematic review. Oncologist 2015;20:77–83. 10.1634/theoncologist.2014-0312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lunney JR, Lynn J, Foley DJ, Lipson S, Guralnik JM. Patterns of functional decline at the end of life. JAMA 2003;289:2387–2392. 10.1001/jama.289.18.2387. [DOI] [PubMed] [Google Scholar]
- 22.Cagle JG, Bunting M, Kelemen A, Lee J, Terry D, Harris R. Psychosocial needs and interventions for heart failure patients and families receiving palliative care support: a systematic review. Heart Fail Rev 2017;22:565–580. 10.1007/s10741-017-9596-5. [DOI] [PubMed] [Google Scholar]
- 23.Garland EL, Bruce A, Stajduhar K. Exposing barriers to end-of-life communication in heart failure: an integrative review. Can J Cardiovasc Nurs J Can En Soins Infirm Cardio-Vasc 2013;23:12–18. [PubMed] [Google Scholar]
- 24.Savović J, Turner RM, Mawdsley D, et al. Association between risk-of-bias assessments and results of randomized trials in cochrane reviews: the ROBES Meta-Epidemiologic Study. Am J Epidemiol 2018;187:1113–1122. 10.1093/aje/kwx344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.PRISMA. Accessed September 19, 2021. Available at: http://prisma-statement.org/prismastatement/Checklist.aspx
- 26.NIH U.S. National Library of Medicine. Home - ClinicalTrials.gov. Accessed April 5, 2022. https://clinicaltrials.gov/
- 27.Zwakman M, Jabbarian L, van Delden J, et al. Advance care planning: a systematic review about experiences of patients with a life-threatening or life-limiting illness. Palliat Med 2018;32:1305–1321. 10.1177/0269216318784474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mellor C, Hain R. Paediatric palliative care: not so different from adult palliative care? Br J Hosp Med 2010;71:36–39. 10.12968/hmed.2010.71.L45971. [DOI] [PubMed] [Google Scholar]
- 29.Aromataris E, Munn Z (Editors). JBI Manual for Evidence Synthesis. JBI, 2020. Available at: https://synthesismanual.jbi.global. Accessed July 17, 2022. [Google Scholar]
- 30.Xiang B, Wong HM, Perfecto AP, McGrath CPJ. The effectiveness of behavioral interventions to improve oral health in adolescents at different periods of follow-up: a systematic review and meta-analysis. Patient Educ Couns 2020;103:725–733. 10.1016/j.pec.2019.11.030. [DOI] [PubMed] [Google Scholar]
- 31.Borenstein M Introduction to meta-analysis. West Sussex, United Kingdom: John Wiley & Sons; 2009. [Google Scholar]
- 32.Higgins J, Thomas J. Cochrane handbook for systematic reviews of interventions. Available at: https://training.cochrane.org/handbook/current. Accessed December 18, 2022.
- 33.Borenstein M, Hedges L, Higgins J, Rothstein H. Multiple outcomes or time-points within a study. Introduction to meta-analysis. John Wiley & Sons, Ltd; 2009. p. 225–238. [Google Scholar]
- 34.Terrill AL, Hartoonian N, Beier M, Salem R, Alschuler K. The 7-item generalized anxiety disorder scale as a tool for measuring generalized anxiety in multiple sclerosis. Int J MS Care 2015;17:49–56. 10.7224/1537-2073.2014-008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cameron IM, Crawford JR, Lawton K, Reid IC. Psychometric comparison of PHQ-9 and HADS for measuring depression severity in primary care. Br J Gen Pract 2008;58:32–36. 10.3399/bjgp08X263794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fu R, Gartlehner G, Grant M, et al. Conducting quantitative synthesis when comparing medical interventions: AHRQ and the Effective Health Care Program. J Clin Epidemiol 2011;64:1187–1197. [DOI] [PubMed] [Google Scholar]
- 37.R Core Team. R: a language and environment for statistical computing. Available at: https://www.R-project.org/.Accessed June 15, 2021.
- 38.Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw 2010;36:1–48. 10.18637/jss.v036.i03. [DOI] [Google Scholar]
- 39.Harrer M, Cuijpers P, Furukawa T, Ebert DD. dmetar: companion R package for the guide “Doing Meta-Analysis in R.” 2019. Available at: http://dmetar.protectlab.org/. Accessed August 21, 2021. [Google Scholar]
- 40.. Bekelman DB, Allen LA, McBryde CF, et al. Effect of a collaborative care intervention vs usual care on health status of patients with chronic heart failure: the CASA randomized clinical trial. JAMA Intern Med 2018;178:511–519. 10.1001/jamainternmed.2017.8667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carson SS, Cox CE, Wallenstein S, et al. Effect of palliative care-led meetings for families of patients with chronic critical illness: a randomized clinical trial. JAMA 2016;316:51–62. 10.1001/jama.2016.8474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dionne-Odom JN, Azuero A, Lyons KD, et al. Benefits of early versus delayed palliative care to informal family care-givers of patients with advanced cancer: outcomes from the ENABLE III randomized controlled trial. J Clin Oncol Off J Am Soc Clin Oncol 2015;33:1446–1452. 10.1200/JCO.2014.58.7824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dionne-Odom JN, Azuero A, Lyons KD, et al. Family caregiver depressive symptom and grief outcomes from the ENABLE III randomized controlled trial. J Pain Symptom Manage 2016;52:378–385. 10.1016/j.jpain-symman.2016.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dionne-Odom JN, Ejem DB, Wells R, et al. Effects of a telehealth early palliative care intervention for family caregivers of persons with advanced heart failure: the ENABLE CHF-PC randomized clinical trial. JAMA Netw Open 2020;3:e202583. 10.1001/jamanetworkopen.2020.2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.El-Jawahri A, Greer JA, Pirl WF, et al. Effects of Early integrated palliative care on caregivers of patients with lung and gastrointestinal cancer: a randomized clinical trial. The oncologist. 2017;22:1528–1534. 10.1634/the-oncologist.2017-0227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.El-Jawahri A, LeBlanc T, VanDusen H, et al. Effect of inpatient palliative care on quality of life 2 weeks after hematopoietic stem cell transplantation: a randomized clinical trial. JAMA 2016;316:2094–2103. 10.1001/jama.2016.16786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.El-Jawahri A, LeBlanc TW, Kavanaugh A, et al. effective ness of integrated palliative and oncology care for patients with acute myeloid leukemia: a randomized clinical trial. JAMA Oncol 2021;7:238–245. 10.1001/jamaoncol.2020.6343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ferrell B, Chung V, Hughes MT, et al. A palliative care intervention for patients on phase 1 studies. J Palliat Med 2021;24:846–856. 10.1089/jpm.2020.0597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Grudzen CR, Richardson LD, Johnson PN, et al. Emergency department–initiated palliative care in advanced cancer: a randomized clinical trial. JAMA Oncol 2016;2:591–598. 10.1001/jamaoncol.2015.5252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Janssen K, Rosielle D, Wang Q, Kim HJ. The impact of palliative care on quality of life, anxiety, and depression in idiopathic pulmonary fibrosis: a randomized controlled pilot study. Respir Res 2020;21:2. 10.1186/s12931-019-1266-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bakitas MA, Dionne-Odom JN, Ejem DB, et al. Effect of an early palliative care telehealth intervention vs usual care on patients with heart failure: the ENABLE CHF-PC randomized clinical trial. JAMA Intern Med 2020;180:1203–1213. 10.1001/jamainternmed.2020.2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bakitas MA, Tosteson TD, Li Z, et al. Early versus delayed initiation of concurrent palliative oncology care: patient out-comes in the ENABLE III randomized controlled trial. J Clin Oncol 2015;33:1438–1445. 10.1200/JCO.2014.58.6362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McCorkle R, Jeon S, Ercolano E, et al. An advanced practice nurse coordinated multidisciplinary intervention for patients with late-stage cancer: a cluster randomized trial. J Palliat Med 2015;18:962–969. 10.1089/jpm.2015.0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nordly M, Skov Benthien K, Vadstrup ES, et al. Systematic fast-track transition from oncological treatment to dyadic specialized palliative home care: DOMUS - a randomized clinical trial. Palliat Med 2019;33:135–149. 10.1177/0269216318811269. [DOI] [PubMed] [Google Scholar]
- 55.O’Riordan DL, Rathfon MA, Joseph DM, et al. Feasibility of implementing a palliative care intervention for people with heart failure: learnings from a pilot randomized clinical trial. J Palliat Med 2019;22:1583–1588. 10.1089/jpm.2018.0633 [DOI] [PubMed] [Google Scholar]
- 56.Schenker Y, Althouse AD, Rosenzweig M, et al. Effect of an oncology nurse—led primary palliative care intervention on patients with advanced cancer: the CONNECT cluster randomized clinical trial. JAMA Intern Med 2021;181:1451. 10.1001/jamainternmed.2021.5185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sidebottom AC, Jorgenson A, Richards H, Kirven J, Sillah A. Inpatient palliative care for patients with acute heart failure: outcomes from a randomized trial. J Palliat Med 2015;18:134–142. 10.1089/jpm.2014.0192. [DOI] [PubMed] [Google Scholar]
- 58.Temel JS, Greer JA, El-Jawahri A, et al. Effects of early integrated palliative care in patients with lung and GI cancer: a randomized clinical trial. J Clin Oncol Off J Am Soc Clin Oncol 2017;35:834–841. 10.1200/JCO.2016.70.5046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Temel JS, Sloan J, Zemla T, et al. Multisite, randomized trial of early integrated palliative and oncology care in patients with advanced lung and gastrointestinal cancer: alliance A221303. J Palliat Med 2020;23:922–929. 10.1089/jpm.2019.0377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ammari ABH, Hendriksen C, Rydahl-Hansen S. Results from the family and coping oriented palliative homecare intervention study (FamCope)-a randomized controlled trial. J Psychosoc Oncol 2018;36:557–581. 10.1080/07347332.2018.1460003. [DOI] [PubMed] [Google Scholar]
- 61.Johnsen AT, Petersen MA, Sjøgren P, et al. Exploratory analyses of the Danish Palliative Care Trial (DanPaCT): a randomized trial of early specialized palliative care plus standard care versus standard care in advanced cancer patients. Support Care Cancer Off J Multinatl Assoc Support Care Cancer 2020;28:2145–2155. 10.1007/s00520-019-05021-7. [DOI] [PubMed] [Google Scholar]
- 62.von Heymann-Horan A, Bidstrup P, Guldin MB, et al. Effect of home-based specialised palliative care and dyadic psychological intervention on caregiver anxiety and depression:a randomised controlled trial. Br J Cancer 2018;119:1307–1315. 10.1038/s41416-018-0193-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McDonald J, Swami N, Hannon B, et al. Impact of early palliative care on caregivers of patients with advanced cancer: cluster randomised trial. Ann Oncol Off J Eur Soc Med Oncol 2017;28:163–168. 10.1093/annonc/mdw438. [DOI] [PubMed] [Google Scholar]
- 64.Rodin G, Malfitano C, Rydall A, et al. Emotion And Symptom-focused Engagement (EASE): a randomized phase II trial of an integrated psychological and palliative care intervention for patients with acute leukemia. Support Care Cancer Off J Multinatl Assoc Support Care Cancer 2020;28:163–176. 10.1007/s00520-019-04723-2. [DOI] [PubMed] [Google Scholar]
- 65.Maltoni M, Scarpi E, Dall’Agata M, et al. Systematic versus on-demand early palliative care: results from a multicentre, randomised clinical trial. Eur J Cancer Oxf Engl 1990 2016;65:61–68. 10.1016/j.ejca.2016.06.007. [DOI] [PubMed] [Google Scholar]
- 66.Solari A, Giordano A, Patti F, et al. Randomized con trolled trial of a home-based palliative approach for people with severe multiple sclerosis. Mult Scler Houndmills Basingstoke Engl 2018;24:663–674. 10.1177/1352458517704078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chan KY, Yip T, Yap DYH, et al. Enhanced psychosocial support for caregiver burden for patients with chronic kidney failure choosing not to be treated by dialysis or transplantation: a pilot randomized controlled trial. Am J Kidney Dis Off J Natl Kidney Found 2016;67:585–592. 10.1053/j.ajkd.2015.09.021. [DOI] [PubMed] [Google Scholar]
- 68.Ng AYM, Wong FKY. Effects of a home-based palliative heart failure program on quality of life, symptom burden, satisfaction and caregiver burden: a randomized controlled trial. J Pain Symptom Manage 2018;55:1–11. 10.1016/jjpainsymman.2017.07.047. [DOI] [PubMed] [Google Scholar]
- 69.Scheerens C, Pype P, Van Cauwenberg J, et al. Early integrated palliative home care and standard care for end-stage COPD (EPIC): a phase II pilot RCT testing feasibility, acceptability, and effectiveness. J Pain Symptom Manage 2020;59:206. 10.1016/jjpainsymman.2019.09.012.-+ -+. [DOI] [PubMed] [Google Scholar]
- 70.Vanbutsele G, Pardon K, Van Belle S, et al. Effect of early and systematic integration of palliative care in patients with advanced cancer: a randomised controlled trial. Lancet Oncol 2018;19:394–404. 10.1016/S1470-2045(18)30060-3. [DOI] [PubMed] [Google Scholar]
- 71.Slama O, Pochop L, Sedo J, et al. Effects of early and systematic integration of specialist palliative care in patients with advanced cancer: randomized controlled trial PALINT. J Palliat Med 2020;23:1586–1593. 10.1089/jpm.2019.0697. [DOI] [PubMed] [Google Scholar]
- 72.Jordhøy MS, Fayers P, Loge JH, Ahlner-Elmqvist M, Kaasa S. Quality of life in palliative cancer care: results from a cluster randomized trial. J Clin Oncol Off J Am Soc Clin Oncol 2001;19:3884–3894. 10.1200/JCO.2001.19.18.3884. [DOI] [PubMed] [Google Scholar]
- 73.Hoek PD, Schers HJ, Bronkhorst EM, Vissers KCP, Hasselaar JGJ. The effect of weekly specialist palliative care telecon-sultations in patients with advanced cancer -a randomized clinical trial. BMC Med 2017;15:119. 10.1186/s12916-017-0866-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.do Carmo TM, Paiva BSR, de Oliveira CZ, Nascimento MS de A, Paiva CE. The feasibility and benefit of a brief psychosocial intervention in addition to early palliative care in patients with advanced cancer to reduce depressive symptoms: a pilot randomized controlled clinical trial. BMC Cancer 2017;17:564. 10.1186/s12885-017-3560-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Brims F, Gunatilake S, Lawrie I, et al. Early specialist palliative care on quality of life for malignant pleural mesothelioma: a randomised controlled trial. Thorax 2019;74:354–361. 10.1136/thoraxjnl-2018-212380. [DOI] [PubMed] [Google Scholar]
- 76.Gao W, Wilson R, Hepgul N, et al. Effect of short-term integrated palliative care on patient-reported outcomes among patients severely affected with long-term neurological conditions: a randomized clinical trial. JAMA Netw Open 2020;3:e2015061. 10.1001/jamanetwor-kopen.2020.15061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ferrell BR, Paterson CL, Hughes MT, Chung V, Koczywas M, Smith TJ. Characteristics of participants enrolled onto a randomized controlled trial of palliative care for patients on phase I studies. J Palliat Med 2017;20:1338–1344. 10.1089/jpm.2017.0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Humphreys K, Blodgett JC, Roberts LW. The exclusion of people with psychiatric disorders from medical research. J Psychiatr Res 2015;70:28–32. 10.1016/jjpsy-chires.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 79.Iltis AS, McCall WV, Deria R. Suicidality, depression, and the FDA: health inequities and the ethical conduct of research. J Clin Psychiatry 2020;81:20306. 10.4088/JCP.19m13050. [DOI] [PubMed] [Google Scholar]
- 80.Fletcher J. What is heterogeneity and is it important? BMJ 2007;334:94–96. 10.1136/bmj.39057.406644.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ayers CR, Sorrell JT, Thorp SR, Wetherell JL. Evidence-based psychological treatments for late-life anxiety. Psychol Aging 2007;22:8–17. 10.1037/0882-7974.22.1.8. [DOI] [PubMed] [Google Scholar]
- 82.Kozlov E, Niknejad B, Reid MC. Palliative care gaps in providing psychological treatment: a review of the current state of research in multidisciplinary palliative care. Am J Hosp Palliat Med 2018;35:505–510. 10.1177/1049909117723860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kozlov E, Phongtankuel V, Prigerson H, et al. Prevalence, severity, and correlates of symptoms of anxiety and depression at the very end of life. J Pain Symptom Manage 2019;58:80–85. 10.1016/jjpainsymman.2019.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]


