Abstract
Background
Type 2 diabetes is prevalent in cardiovascular disease and contributes to excess morbidity and mortality. We sought to investigate the effect of glycemia on functional cardiac improvement, morbidity, and mortality in durable left ventricular assist device (LVAD) recipients.
Methods and Results
Consecutive patients with an LVAD were prospectively evaluated (n=531). After excluding patients missing pre‐LVAD glycated hemoglobin (HbA1c) measurements or having inadequate post‐LVAD follow‐up, 375 patients were studied. To assess functional cardiac improvement, we used absolute left ventricular ejection fraction change (ΔLVEF: LVEF post‐LVAD−LVEF pre‐LVAD). We quantified the association of pre‐LVAD HbA1c with ΔLVEF as the primary outcome, and all‐cause mortality and LVAD‐related adverse event rates (ischemic stroke/transient ischemic attack, intracerebral hemorrhage, gastrointestinal bleeding, LVAD‐related infection, device thrombosis) as secondary outcomes. Last, we assessed HbA1c differences pre‐ and post‐LVAD. Patients with type 2 diabetes were older, more likely men suffering ischemic cardiomyopathy, and had longer heart failure duration. Pre‐LVAD HbA1c was inversely associated with ΔLVEF in patients with nonischemic cardiomyopathy but not in those with ischemic cardiomyopathy, after adjusting for age, sex, heart failure duration, and left ventricular end‐diastolic diameter. Pre‐LVAD HbA1c was not associated with all‐cause mortality, but higher pre‐LVAD HbA1c was shown to increase the risk of intracerebral hemorrhage, LVAD‐related infection, and device thrombosis by 3 years on LVAD support (P<0.05 for all). HbA1c decreased from 6.68±1.52% pre‐LVAD to 6.11±1.33% post‐LVAD (P<0.001).
Conclusions
Type 2 diabetes and pre‐LVAD glycemia modify the potential for functional cardiac improvement and the risk for adverse events on LVAD support. The degree and duration of pre‐LVAD glycemic control optimization to favorably affect these outcomes warrants further investigation.
Keywords: diabetes, heart assist device, heart failure, left ventricular assist device, myocardial recovery, reverse remodeling
Subject Categories: Heart Failure, Remodeling, Cardiomyopathy, Complications, Quality and Outcomes
Nonstandard Abbreviations and Acronyms
- ICM
ischemic cardiomyopathy
- INTERMACS
Interagency Registry for Mechanically Assisted Circulatory Support
- MCS
mechanical circulatory support
- NICM
nonischemic cardiomyopathy
- T2D
type 2 diabetes
Clinical Perspective.
What Is New?
Type 2 diabetes and pre‐left ventricular assist device glycemia might affect the potential for functional cardiac improvement and the risk for adverse events on left ventricular assist device support.
What Are the Clinical Implications?
Optimization of pre‐left ventricular assist device glycemic control could improve outcomes in these patients.
Findings from patients with advanced heart failure receiving mechanical circulatory support could inform the care of patients with earlier stage heart failure and concomitant type 2 diabetes or prediabetes.
Type 2 diabetes (T2D) affects 34.1 million adults in the United States, accounting for 13% of the total adult population, and its prevalence is projected to increase. 1 The association of T2D with cardiovascular disease is well established, with people with diabetes having a substantially increased risk for developing heart failure (HF). 2 , 3 , 4 , 5 It has been shown that a 1% increase in serum glycated hemoglobin (HbA1c) increases the risk for developing HF by 16%. 6 At the same time, patients with HF exhibit marked insulin resistance, 7 which increases their risk of developing T2D. The above suggest a bidirectional relationship between T2D and HF, with each disease increasing the risk of each other and adversely affecting prognosis and outcomes. 2 , 8
HF has long been deemed unidirectional and progressive, inevitably leading to advanced disease. This notion has been challenged by the occurrence of cardiac improvement in different clinical settings, from spontaneous improvement in acute myocarditis and stress‐induced cardiomyopathy, to facilitated improvement by electrical or pharmacological therapies, and even advanced HF treated with left ventricular (LV) assist devices (LVADs). 9 Mechanical circulatory support (MCS) with LVADs is an established treatment modality for patients with refractory HF symptoms despite guideline‐directed medical therapy. Through volume and pressure unloading of the ailing left ventricle, it can facilitate structural and functional cardiac improvement in varying degrees. 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17
In this study, we sought to investigate how T2D and glycemia affect the potential for functional cardiac improvement, as well as morbidity and mortality in patients with advanced HF on durable MCS. Findings from the advanced HF/MCS investigational setting could have prognostic and therapeutic implications for the greater population of patients with earlier stage HF and concomitant T2D or prediabetes.
METHODS
Data Sharing
The data and analytic methods of the study will be made available from the corresponding author upon reasonable request.
Study Population
Patients with advanced HF receiving a continuous‐flow LVAD between May 2008 and November 2020 at 1 of the institutions comprising the Utah Cardiac Recovery Program (University of Utah Health and School of Medicine, Intermountain Medical Center, and George E. Wahlen Department of Veterans Affairs Medical Center) were prospectively evaluated. Patients were followed until LVAD explantation due to heart transplantation or cardiac recovery, loss to follow‐up, death, or study conclusion in February 2023. The study was approved by the institutional review boards of the participating institutions, and written informed consent was obtained from all patients.
Patients with hypertrophic or infiltrative cardiomyopathy, baseline LV ejection fraction (LVEF) ≥40%, consent withdrawal, or inadequate (<3 months) post‐LVAD follow‐up (early heart transplantation, death, or unavailable echocardiographic follow‐up) were excluded. To investigate the effect of T2D and glycemic control on LVAD‐mediated cardiac recovery, we also excluded patients with missing HbA1c measurements before LVAD implantation or diagnosed with type 1 diabetes.
Clinical Management and Definitions
Data collection included demographics, comorbidities, medications, laboratory values, and hemodynamic data obtained via right heart catheterization before and closest to LVAD implantation. Cardiac imaging data were obtained before and during LVAD support to assess the structural and functional effects of mechanical unloading on the failing heart. The duration of HF was defined as the time from HF symptom onset to LVAD implantation as ascertained through chart review. The estimated glomerular filtration rate was calculated using the Chronic Kidney Disease Epidemiology collaboration 2021 creatinine‐based equation. 18 Patients were considered diabetic if previously diagnosed with T2D or if they had an HbA1c ≥6.5% in the 12‐month period preceding LVAD implantation. Pre‐LVAD HbA1c was recorded as the average of all available measurements in the 12‐month period preceding LVAD implantation, whereas post‐LVAD HbA1c was recorded as the average of all available measurements in the 12‐month period following LVAD implantation.
The effect of LVAD unloading on cardiac size, shape, and function was assessed by echocardiography and invasive hemodynamic measurements following LVAD implantation and before discharge. LVAD speed was adjusted to optimize flows and left ventricle decompression with positioning of the interventricular and interatrial septa in the midline, minimal mitral valve regurgitation, and intermittent aortic valve opening, in order of decreasing priority. Subsequent speed adjustments were made as indicated by patient symptoms and/or clinical events. Patients were medically managed at the discretion of the treating physicians within the participating institutions per established standard HF and T2D therapy guidelines.
Functional Cardiac Improvement Assessment
Functional cardiac changes on LVAD support were prospectively assessed using a protocol developed and tested at the Utah Cardiac Recovery Program. 11 Transthoracic echocardiograms were performed within the 2 weeks preceding and serially at 1, 3, 6, 9, and 12 months following LVAD implantation. The standard of care clinical protocol entailed 2 sets of echocardiographic measurements: (1) at full LVAD support and (2) after 30 minutes of limited support, at the lowest setting recommended by the device manufacturer (turndown study). The absence of prior stroke, transient ischemic attack, LVAD thrombosis, or hemolysis, along with a therapeutic international normalized ratio, were prerequisites for a turndown study. Complete echocardiographic assessment, including 2‐dimensional, M‐mode, and Doppler modalities, was performed according to the 2015 American Society of Echocardiography and European Association of Cardiovascular Imaging guidelines. 19
To quantitatively estimate functional cardiac improvement on LVAD support, we used the following formula: absolute LVEF change (ΔLVEF)=LVEF post‐LVAD−LVEF pre‐LVAD. For LVEF pre‐LVAD we used the measurement before and closest to LVAD implantation, whereas for LVEF post‐LVAD we used the maximum LVEF achieved within the 12‐month period following LVAD implantation. Pre‐turndown LVEF measurements were used, because a turndown study might not have been performed for the reasons mentioned above. We have previously shown that LVEF measurements do not significantly differ between pre‐ and post‐turndown studies. 11 , 20
Study Outcomes
The primary outcome was ΔLVEF by 12 months, and the secondary outcomes were all‐cause mortality and LVAD‐related adverse event rates by 3 years on LVAD support. The following LVAD‐related adverse events were prospectively captured using the INTERMACS (Interagency Registry for Mechanically Assisted Circulatory Support) definitions and were adjudicated via chart review (C.P.K., I.T.) 21 : ischemic stroke, intracerebral hemorrhage, transient ischemic attack, gastrointestinal bleeding, LVAD‐related infection (percutaneous site infection, infection of external or blood‐containing surfaces of an implantable component), and device thrombus. Additionally, we assessed glycemia before and following LVAD support, in the subset of patients with available pre‐ and post‐LVAD HbA1c measurements.
Statistical Analysis
Patient baseline characteristics were summarized using standard summary statistics including frequencies, percentages, and means. Measures of variation were presented as mean and SD or median and interquartile range, as appropriate. Differences between patient groups for categorical variables were evaluated using the χ2 test or Fisher exact test, as appropriate, and continuous variables were evaluated using the 2‐group Student t test or Mann‐Whitney 2‐sample test, as appropriate.
Linear regression was used to assess the primary outcome (association of ΔLVEF with pre‐LVAD HbA1c). Multivariable linear regression was used to test this association while adjusting for variables that have been suggested to significantly affect functional cardiac improvement, and by extension ΔLVEF in previous studies. 12 , 13 , 14 , 15 , 16 , 17 , 22 Additionally, we used statistical interaction terms to determine potential modification of HbA1c impact by variables previously shown to affect functional cardiac improvement. 12 , 13 , 14 , 15 , 16 , 17 , 22 To properly interpret potential interaction effects, data were mean‐centered (raw value mean). 23 Linear regression is robust with regard to the assumptions of homogeneity of variance and normality of residual errors, 24 , 25 and graphical evaluation of the data allowed us to conclude these assumptions were not violated. Robust estimates of variance were reported. We used a paired‐sample t test to assess the difference in glycemia before and after LVAD support in the subset of patients with available pre‐ and post‐LVAD HbA1c measurements. A 2‐sided P value <0.05 was considered significant.
Cox proportional hazards regression modeling was used to examine the association between pre‐LVAD HbA1c and all‐cause mortality or LVAD‐related adverse events by 3 years on LVAD support.
RESULTS
Overall, 531 patients receiving durable continuous‐flow LVAD were prospectively enrolled within the study period. After excluding patients with hypertrophic or infiltrative cardiomyopathies (n=4), a baseline LVEF ≥40% (n=6), consent withdrawal (n=8), inadequate (<3 months) post‐LVAD follow‐up due to death or heart transplantation (n=53), unavailable echocardiographic data (n=43), or absent HbA1c measurements before LVAD implantation (n=42), 375 patients comprised our study cohort (Figure 1). After applying the above exclusion criteria, patients with type 1 diabetes were not included in our study.
Figure 1. Flow diagram for inclusion and exclusion of patients.
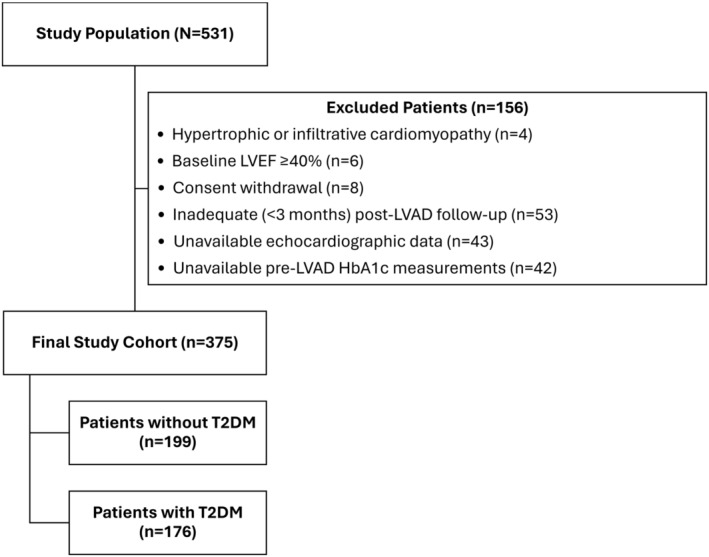
HbA1c indicates glycated hemoglobin; LVAD, left ventricular assist device; LVEF, left ventricular ejection fraction; and T2DM, type 2 diabetes mellitus.
Baseline demographic and clinical characteristics after stratifying patients into diabetic and nondiabetic are presented in Table 1. Patients with T2D were more likely to be older men with a history of systemic hypertension and a higher body mass index. They had a longer duration of HF and more commonly suffered ischemic cardiomyopathy (ICM). No differences were observed in terms of disease severity as evidenced by New York Heart Association classification and INTERMACS profile or preoperative use of vasoactive agents or temporary MCS. Last, the proportion of patients treated with guideline‐directed HF medical therapy pre‐LVAD was comparable between the 2 groups, whereas patients with T2D were less commonly on an aldosterone antagonist and more commonly on a diuretic 3 months post‐LVAD (Table S1).
Table 1.
Baseline Demographic and Clinical Characteristics in the Total Cohort and Patients Without and With T2D
| Variables | Total cohort (N=375) | Patients without T2D (n=199) | Patients with T2D (n=176) | P value |
|---|---|---|---|---|
| Male sex, n (%) | 319 (85.1%) | 155 (77.9%) | 164 (93.2%) | <0.001 |
| Age, y | 59 [49–66] | 58 [39–66] | 61 [55–66] | <0.001 |
| Race, n (%) | 0.85 | |||
| White | 287 (81.8%) | 145 (79.7%) | 142 (84.0%) | |
| Black | 31 (8.8%) | 18 (9.9%) | 13 (7.7%) | |
| American Indian or Alaska Native | 14 (4.0%) | 7 (3.8%) | 7 (4.2%) | |
| Native Hawaiian or Other Pacific Islander | 4 (1.1%) | 2 (1.1%) | 2 (1.2%) | |
| Asian | 3 (0.9%) | 2 (1.1%) | 1 (0.6%) | |
| Not reported or multiple races | 12 (3.4%) | 8 (4.4%) | 4 (2.3%) | |
| Ethnicity, n (%) | ||||
| Hispanic or Latino | 25 (7.1%) | 13 (7.1%) | 12 (7.1%) | 0.99 |
| Body mass index, kg/m2 | 28.3±5.8 | 27.5±5.8 | 29.3±5.7 | 0.003 |
| Medical history, n (%) | ||||
| Smoking | 188 (50.0%) | 103 (51.8%) | 85 (48.3%) | 0.50 |
| Hypertension | 190 (50.7%) | 73 (36.7%) | 117 (66.5%) | <0.001 |
| Ethanol use | 169 (45.1%) | 96 (48.2%) | 73 (41.5%) | 0.19 |
| Atrial fibrillation | 161 (43.1%) | 76 (38.2%) | 85 (48.6%) | 0.04 |
| Previous thoracotomy | 98 (26.3%) | 39 (19.9%) | 59 (33.5%) | 0.003 |
| Electrical therapies, n (%) | 0.34 | |||
| None | 58 (15.6%) | 35 (17.7%) | 23 (13.2%) | |
| CRT‐D | 177 (47.6%) | 88 (44.4%) | 89 (51.2%) | |
| ICD | 137 (36.8%) | 75 (37.9%) | 62 (35.6%) | |
| Preoperative supportive therapies, n (%) | ||||
| Inotrope dependency | 260 (69.3%) | 134 (67.3%) | 126 (71.6%) | 0.37 |
| Intra‐aortic balloon pump | 27 (7.2%) | 11 (5.6%) | 16 (9.1%) | 0.19 |
| Percutaneous VAD/VA‐ECMO | 22 (6.0%) | 16 (8.0%) | 6 (3.4%) | 0.06 |
| New York Heart Association class IV, n (%) | 271 (72.3%) | 143 (71.9%) | 128 (72.7%) | 0.85 |
| Heart failure duration, mo | 88.6±84.1 | 77.1±80.4 | 101.5±86.5 | 0.005 |
| Heart failure cause, n (%) | ||||
| Ischemic cardiomyopathy | 172 (45.9%) | 69 (34.7%) | 103 (58.5%) | <0.001 |
| INTERMACS profile, n (%) | 0.08 | |||
| 1 | 26 (7.1%) | 20 (10.2%) | 6 (3.5%) | |
| 2 | 66 (17.9%) | 32 (16.3%) | 34 (19.6%) | |
| 3 | 159 (43.1%) | 81 (41.3%) | 78 (45.1%) | |
| ≥4 | 118 (32.0%) | 63 (32.1%) | 55 (31.8%) | |
| VAD indication, n (%) | 0.05 | |||
| BTT | 212 (56.5%) | 117 (58.8%) | 95 (54.0%) | |
| DT | 139 (37.1%) | 64 (32.2%) | 75 (42.6%) | |
| BTD | 13 (3.5%) | 10 (5.0%) | 3 (1.7%) | |
| BTR | 11 (2.9%) | 8 (4.0%) | 3 (1.7%) | |
| VAD type, n (%) | 0.29 | |||
| HeartMate 2 | 139 (37.1%) | 66 (33.2%) | 73 (41.5%) | |
| HeartMate 3 | 44 (11.7%) | 22 (11.1%) | 22 (12.5%) | |
| HeartWare | 170 (45.3%) | 99 (49.8%) | 71 (40.3%) | |
| Other | 22 (5.9%) | 12 (6.0%) | 10 (5.7%) | |
| VAD configuration, n (%) | ||||
| Centrifugal | 199 (56.4%) | 110 (59.8%) | 89 (52.7%) | 0.18 |
| Pre‐VAD heart failure medications, n (%) | ||||
| β‐Blocker | 241 (64.3%) | 128 (64.3%) | 113 (64.2%) | 0.98 |
| ARNI/ARB/ACE inhibitor | 242 (64.5%) | 124 (62.3%) | 118 (67.1%) | 0.34 |
| Aldosterone antagonist | 227 (60.5%) | 127 (63.8%) | 100 (56.8%) | 0.17 |
| Diuretic | 354 (94.4%) | 185 (93.0%) | 169 (96.0%) | 0.20 |
| Pre‐VAD T2D medications, n (%) | ||||
| Insulin | … | … | 75 (42.9%) | … |
| Metformin | … | … | 42 (23.9%) | … |
| DPP‐4 inhibitors | … | … | 7 (4.0%) | … |
| GLP‐1 agonists | … | … | 10 (5.7%) | … |
| SGLT‐2 inhibitors | … | … | 2 (1.2%) | … |
| α‐Glucosidase inhibitors | … | … | 1 (0.6%) | … |
Continuous variables are presented as mean±SD or median [interquartile range]. ACE indicates angiotensin‐converting enzyme; ARB, angiotensin receptor blocker; ARNI, angiotensin receptor‐neprilysin inhibitor; BTD, bridge‐to‐decision; BTR, bridge‐to‐recovery; BTT, bridge‐to‐transplant; CRT‐D, cardiac resynchronization therapy‐defibrillator; DPP‐4, dipeptidyl peptidase‐4; DT, destination therapy; GLP‐1; glucagon‐like peptide‐1; ICD, implantable cardioverter‐defibrillator; INTERMACS, Interagency Registry of Mechanically Assisted Circulatory Support; SGLT‐2, sodium‐glucose cotransporter‐2; T2D, type 2 diabetes; VAD, ventricular assist device; and VA‐ECMO, veno‐arterial extracorporeal membrane oxygenation.
Hemodynamic, echocardiographic, and laboratory measurements before LVAD implantation are presented in Table 2. Patients in both the T2D and non‐T2D groups had elevated cardiac filling pressures, severely impaired cardiac function, and abnormal cardiac structure. No differences were identified between the 2 groups in baseline hemodynamic and echocardiographic parameters, a higher LVEF, and a thicker interventricular septum in patients with diabetes. Laboratory assessment revealed higher creatinine, blood urea nitrogen, as well as lower estimated glomerular filtration rate, albumin, and alanine aminotransferase values in patients with versus without T2D. As expected, patients with diabetes had higher blood glucose and HbA1c values compared with those without.
Table 2.
Baseline Hemodynamic, Echocardiographic, and Laboratory Characteristics in the Total Cohort and Patients Without and With T2D
| Variables | Total cohort (N=375) | Patients without T2D (n=199) | Patients with T2D (n=176) | P value |
|---|---|---|---|---|
| Hemodynamic measurements | ||||
| Systolic blood pressure, mm Hg | 104.6±15.4 | 103.0±15.2 | 106.8±15.4 | 0.06 |
| Diastolic blood pressure, mm Hg | 68.3±11.4 | 68.2±11.1 | 68.3±11.8 | 0.94 |
| Mean blood pressure, mm Hg | 79.4±12.4 | 80.0±12.3 | 78.6±12.5 | 0.42 |
| Heart rate, bpm | 86.6±19.8 | 88.1±21.3 | 84.9±17.9 | 0.15 |
| Mean right atrial pressure, mm Hg | 11.7±6.2 | 11.6±6.5 | 11.9±5.8 | 0.66 |
| Systolic right ventricular pressure, mm Hg | 51.5±13.7 | 50.1±13.6 | 52.9±13.7 | 0.07 |
| Diastolic right ventricular pressure, mm Hg | 7.6±6.7 | 7.9±7.7 | 7.2±5.3 | 0.38 |
| Mean right ventricular pressure, mm Hg | 16.1±8.5 | 15.2±8.1 | 17.1±8.9 | 0.19 |
| Systolic pulmonary artery pressure, mm Hg | 52.5±14.6 | 51.5±14.4 | 53.7±14.8 | 0.16 |
| Diastolic pulmonary artery pressure, mm Hg | 26.4±8.7 | 26.3±8.6 | 26.5±8.8 | 0.84 |
| Mean pulmonary artery pressure, mm Hg | 36.9±9.9 | 36.6±9.6 | 37.2±10.2 | 0.55 |
| Pulmonary capillary wedge pressure, mm Hg | 24.6±8.2 | 24.7±8.0 | 24.5±8.5 | 0.79 |
| Systemic vascular resistance, dynes×s×cm−5 | 1458 [1121–1837] | 1496 [1162–1853] | 1414 [1073–1683] | 0.41 |
| Pulmonary vascular resistance, Wood units | 3.64±2.35 | 3.56±2.23 | 3.73±2.47 | 0.51 |
| Cardiac output, L/min | 3.77±1.29 | 3.74±1.48 | 3.81±1.05 | 0.62 |
| Cardiac index, L/min per m2 | 1.86±0.63 | 1.88±0.72 | 1.85±0.53 | 0.72 |
| Echocardiographic measurements | ||||
| Left ventricular ejection fraction, % | 18.1±6.9 | 17.2±6.5 | 19.0±7.2 | 0.01 |
| Left ventricular end‐diastolic diameter, cm | 6.77±1.04 | 6.82±1.01 | 6.71±1.07 | 0.33 |
| Interventricular septum thickness end‐diastole, cm | 0.98±0.42 | 0.93±0.24 | 1.04±0.56 | 0.02 |
| Posterior wall thickness end‐diastole, cm | 0.95±0.25 | 0.94±0.24 | 0.97±0.25 | 0.31 |
| Laboratory measurements | ||||
| Hemoglobin, g/dL | 12.3±2.3 | 12.5±2.4 | 12.2±2.2 | 0.20 |
| White blood cell count, ×103/μL | 8.3±3.5 | 8.3±3.3 | 8.3±3.7 | 0.95 |
| Neutrophil/lymphocyte count ratio | 5.2±4.2 | 5.0±4.1 | 5.5±4.3 | 0.21 |
| C‐reactive protein, mg/dL | 3.2±4.0 | 3.1±4.3 | 3.3±3.7 | 0.73 |
| Platelet count, ×103/μL | 205 [161–249] | 207 [159–244] | 202 [164–259] | 0.84 |
| International normalized ratio | 1.40±1.16 | 1.40±1.15 | 1.40±1.18 | 0.93 |
| Sodium, mEq/L | 134.3±5.3 | 134.5±5.1 | 134.0±5.5 | 0.40 |
| Potassium, mEq/L | 4.09±0.51 | 4.08±0.55 | 4.11±0.45 | 0.50 |
| Creatinine, mg/dL | 1.38±0.52 | 1.30±0.50 | 1.47±0.52 | 0.002 |
| Blood urea nitrogen, mg/dL | 27 [20–39] | 25 [18–34] | 29 [22–44] | <0.001 |
| Estimated glomerular filtration rate, mL/min | 67.6±27.6 | 72.9±29.1 | 61.6±24.4 | <0.001 |
| Aspartate aminotransferase, mg/dL | 30 [22–45] | 32 [23–47] | 27 [21–40] | 0.017 |
| Alanine aminotransferase, mg/dL | 27 [19–51] | 30 [20–61] | 25 [17–40] | 0.015 |
| Alkaline phosphatase, mg/dL | 94 [72–124] | 90 [71–115] | 99 [74–129] | 0.044 |
| Total bilirubin, mg/dL | 1.1 [0.7–1.6] | 1.2 [0.7–1.8] | 1.0 [0.8–1.6] | 0.25 |
| Uric acid, mg/dL | 8.6±3.1 | 8.5±3.2 | 8.8±3.1 | 0.48 |
| Total serum protein, g/dL | 6.97±0.77 | 7.02±0.75 | 6.92±0.79 | 0.24 |
| Albumin, g/dL | 3.72±0.47 | 3.78±0.49 | 3.66±0.45 | 0.02 |
| Blood glucose, g/dL | 129.0±53.5 | 114.1±40.9 | 145.6±60.6 | <0.001 |
| Hemoglobin A1c, % | 6.4±1.2 | 5.7±0.4 | 7.2±1.2 | <0.001 |
| B‐type natriuretic peptide, pg/mL | 1013 [455–1873] | 1100 [506–2099] | 844 [428–1546] | 0.078 |
Continuous variables are presented as mean±SD or median [interquartile range]. T2D indicates type 2 diabetes.
Glycemia and Functional Cardiac Improvement on LVAD Support
Pre‐LVAD HbA1c was inversely associated with ΔLVEF at a univariable level (linear regression coefficient: −1.02; P value: 0.05), as shown in Figure 2. The impact of pre‐LVAD HbA1c on ΔLVEF was subsequently assessed after adjusting for clinical factors previously shown to associate with functional cardiac improvement on LVAD support, including age, sex, duration of HF symptoms, HF cause, and LV end‐diastolic diameter. 12 , 14 , 15 , 22 Statistical interaction terms were created between pre‐LVAD HbA1c and the same set of clinical factors to assess for potential effect modification. A significant interaction was identified between pre‐LVAD HbA1c and ICM (P=0.039). Based on the above, patients were stratified into ICM and non‐ICM (NICM). Although pre‐LVAD HbA1c was found to be significantly associated with ΔLVEF in patients with NICM after adjusting for age, sex, HF duration, and LV end‐diastolic diameter, this was not evident in patients with ICM (Figure 3). The multivariable linear regression models in ICM and NICM are shown in Figure 3.
Figure 2. Association of pre‐LVAD HbA1c with left ventricular functional improvement (absolute LVEF change: LVEF post‐LVAD−LVEF pre‐LVAD) at a univariable level.
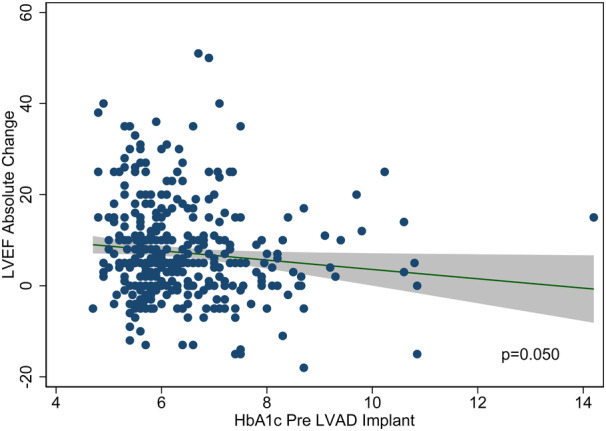
HbA1c indicates glycated hemoglobin; LVAD: left ventricular assist device; and LVEF, left ventricular ejection fraction.
Figure 3. Main effects between pre‐LVAD HbA1c and left ventricular functional improvement (absolute LVEF change: LVEF post‐LVAD−LVEF pre‐LVAD) in nonischemic and ischemic patients with HF.
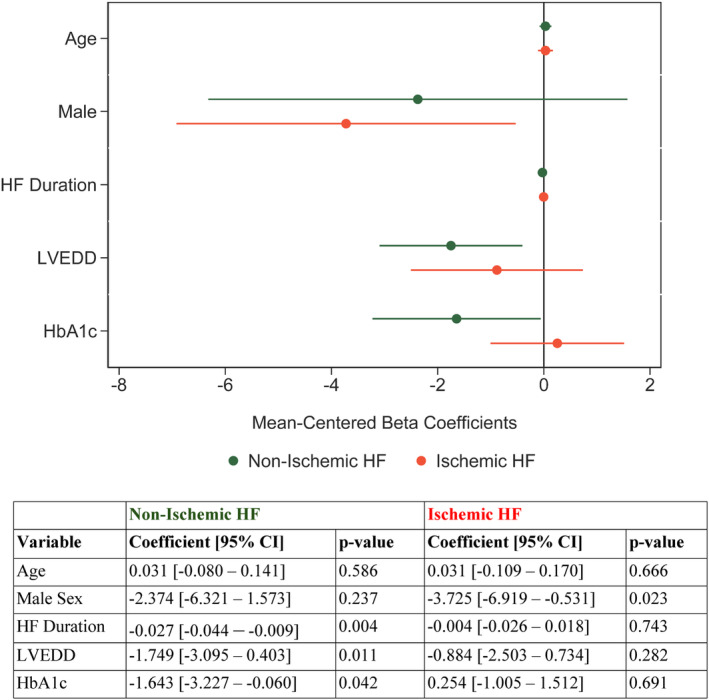
HbA1c indicates glycated hemoglobin; HF, heart failure; LVEDD, left ventricular end‐diastolic diameter; and LVEF, left ventricular ejection fraction.
Glycemia and All‐Cause Mortality on LVAD Support
Of 375 patients, 74 (19.7%) were deceased by 3 years on LVAD support (Figure S1). Pre‐LVAD HbA1c was not associated with the risk of all‐cause mortality by 3 years on LVAD support at a univariable level (hazard ratio [HR], 1.12 [95% CI, 0.95–1.31]; P=0.177), or after adjusting for age, sex, duration of HF symptoms, HF cause, and LV end‐diastolic diameter (HR, 1.13 [95% CI, 0.96–1.34]; P=0.152) (Table S2).
Glycemia and LVAD‐Related Adverse Events
Pre‐LVAD HbA1c was shown to increase the risk of intracerebral hemorrhage (HR, 1.31 [95% CI, 1.13–1.52]; P<0.001), device thrombosis (HR, 1.28 [95% CI, 1.07–1.54]; P=0.008), and LVAD‐related infection (HR, 1.31 [95% CI, 1.17–1.47]; P<0.001), but not ischemic stroke/transient ischemic attack (HR, 1.03 [95% CI, 0.79–1.35]; P=0.811), or gastrointestinal bleeding (HR, 1.14 [95% CI, 1.00–1.31]; P=0.052) by 3 years on LVAD support. The associations above remained significant after adjusting for age, sex, and LVAD type for all outcomes (Figure S2).
Glycemia Pre‐ and Post‐LVAD Support
In the subset of patients with available HbA1c measurements before and following LVAD support (n=127), HbA1c decreased from 6.68±1.52% pre‐LVAD to 6.11±1.33% post‐LVAD support (P<0.001) (Figure 4).
Figure 4. HbA1c values pre‐ and post‐LVAD support.
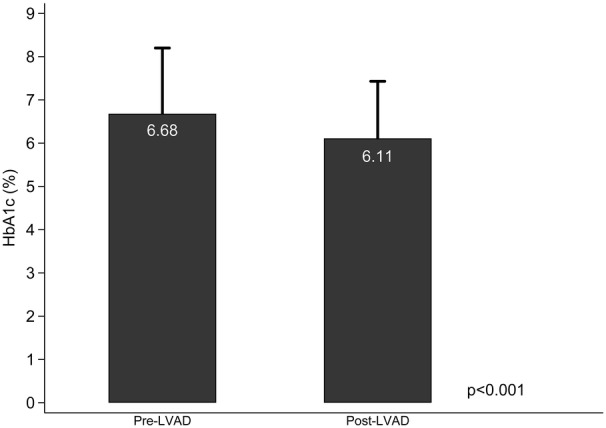
The bars represent mean values and the caps the SD. HbA1c indicates glycated hemoglobin; and LVAD, left ventricular assist device.
DISCUSSION
The findings of the present study suggest that T2D and glycemia affect the potential for cardiac functional improvement on LVAD support. Decreased pre‐LVAD HbA1c was found to be independently associated with greater ΔLVEF, after adjusting for clinical factors known to associate with LVAD‐mediated cardiac recovery, including age, sex, duration of HF symptoms, and LV end‐diastolic diameter. 12 , 14 , 15 , 22 This was not evident, however, after stratifying patients based on their underlying HF cause. Decreased pre‐LVAD HbA1c was shown to independently associate with increased ΔLVEF in patients with NICM but not in those with ICM, after adjusting for the above‐mentioned clinical variables.
T2D and HF have a bidirectional relationship and are often coexistent. 2 , 8 , 26 , 27 , 28 Risk factors for HF, such as hypertension, coronary artery disease, valvular heart disease, chronic kidney disease, and obesity, are often coexistent with T2D and can accelerate LV adverse remodeling and dysfunction; however, HF might develop in their absence. 2 , 29 Several preclinical and clinical studies have yielded results strongly suggesting that diabetic cardiomyopathy is a unique clinical entity, with hyperglycemia leading to abnormal cardiac structure and function independent of traditional cardiac risk factors. 30 , 31 Among the potentially implicated pathophysiologic derangements are insulin resistance and impaired insulin signaling, glucotoxicity and lipotoxicity, upregulation of inflammatory pathways, oxidative stress, mitochondrial dysfunction, formation of advanced glycation end‐products, and cardiac fibrosis. 27 , 30 , 31 , 32
By unloading the failing heart, LVADs create a favorable environment for the reversal of adverse structural and functional cardiac changes, 10 , 11 , 12 , 13 , 14 , 15 with functional cardiac improvement recently shown to associate with improved outcomes on LVAD support. 33 , 34 Clinical factors previously shown to associate with LVAD‐mediated cardiac recovery include a younger age, an underlying NICM, a shorter duration of HF, and smaller left ventricle dimensions. 12 , 14 , 15 , 22 At the cellular and molecular level, it has been suggested that glucose metabolism, mitochondrial function, and myocardial and systemic inflammation might influence cardiac recovery upon mechanical unloading. 35 , 36 , 37 , 38 These derangements have been implicated in the pathophysiology of diabetes, 27 , 30 , 31 , 32 and might explain why T2D could play a role in LVAD‐mediated cardiac recovery.
Insulin resistance is a key component of the metabolic syndrome, a cluster of systemic metabolic abnormalities implicated in the pathogenesis of cardiovascular disease. 39 Neurohormonal activation and chronic inflammation appear to be the common final pathway leading to changes in cardiac metabolism and signaling pathways that might contribute to myocardial dysfunction. 28 , 39 We have previously studied the effect of tissue and serum inflammatory markers on LVAD‐mediated myocardial recovery. 38 Circulating levels of cytokines were lower, whereas the signal transducer and activator of transcription‐3, an inflammatory response regulator, was less activated in the cardiac tissue of patients significantly improving the function and structure of their heart on an LVAD (responders) compared with nonresponders. As such, pre‐LVAD metabolic dysfunction, including both insulin resistance/hyperglycemia and inflammation, seem to affect the potential for myocardial recovery.
It has been suggested that strategies to correct the systemic metabolic derangements associated with insulin resistance could impact the prognosis and outcomes of patients with HF. 28 In a study comparing intensive blood glucose control (target HbA1c ≤6.5%) versus standard of care treatment, combined micro‐ and macrovascular events risk were reduced by 10%, an effect largely driven by a reduction in the risk of microvascular events, especially nephropathy. 40 In another large study investigating blood glucose reduction, an overall benefit was shown during the period in which the HbA1c curves were separated, 41 whereas in a separate analysis, patients with low coronary artery calcium had the greatest benefit. 42 These findings might be suggestive of a differential effect of improved glycemic control on cardiac reverse remodeling based on the extent of already established macro‐ and microvascular coronary artery disease. This agrees with our finding that glycemia might play a more pronounced role in affecting functional cardiac improvement in patients with NICM compared with patients with ICM. Furthermore, the relationships between changes in cardiac and systemic metabolism and myocardial recovery are currently under intensive investigation by several groups, including our group, 35 , 36 , 37 , 43 and the emerging findings might shed additional light into the mechanisms driving the findings of the current study.
T2D is associated with worse outcomes in patients suffering from HF. 8 It has been suggested that even insulin resistance in the absence of overt T2D is independently associated with a worse prognosis. 7 Specifically in patients with advanced HF supported with LVADs, prior reports studying the impact of T2D and glycemic control on mortality and LVAD‐related adverse events have not been conclusive. 44 , 45 , 46 , 47 In a meta‐analysis, T2D was not shown to significantly affect all‐cause mortality or LVAD‐related adverse events. 48 In a recently published observational study of 154 patients with continuous‐flow LVADs, patients with and without diabetes had comparable 30‐day, 1‐year, and 3‐year mortality rates; however, T2D was an independent predictor of 5‐year mortality (HR, 2.09; P=0.004). 49 Additionally, patients with T2D had higher rates of major infection on LVAD support (59% versus 47%, P=0.044). 49 In our study, we found that pre‐LVAD HbA1c was not associated with the risk of all‐cause mortality by 3 years on LVAD support. Patients with a higher HbA1c before LVAD support, however, had a higher chance of developing intracerebral hemorrhage, LVAD‐related infection, or device thrombosis by 3 years on LVAD support.
Last, we found that in patients with available HbA1c measurements before and following LVAD implantation, HbA1c decreased from 6.69±1.54% pre‐LVAD to 6.10±1.35% post‐LVAD support (P<0.001). It should be acknowledged, however, that only a small proportion of patients were included in the analysis (127 out of 375), with the small sample size potentially affecting this finding. It has been reported that glycemic control improves following LVAD implantation with decreased fasting blood glucose and HbA1c levels, as well as antidiabetic medical therapy requirements. 44 , 47 , 50 , 51 , 52 Multiple physiologic factors might be driving these findings, stemming from improved hemodynamics, cardiac output, and tissue perfusion. Low cardiac output in advanced HF leads to upregulation of the renin‐angiotensin‐aldosterone system, and increases cortisol and catecholamine levels, which in turn lead to insulin resistance. 53 MCS helps correct these metabolic disturbances by increasing cardiac output and enhancing blood flow to peripheral tissues. It has also been shown that LVAD support reduces inflammation, which might also contribute to ameliorate insulin resistance. 54 , 55 Improved physical activity, more frequent follow‐up, and better care coordination and medication optimization could also play a role. 51 Last, it should be noted that red blood cell lifespan and turnover rate are affecting HbA1c levels and might be contributing to decreased levels on LVAD support. MCS devices can lead to mechanical damage and destruction of red blood cells, leading to reduced hemoglobin and increased reticulocyte counts, 56 , 57 potentially overestimating a favorable glycemic control.
Limitations of the current study include the potential selection bias introduced by the inclusion of patients with available HbA1c measurements before LVAD implantation and at least 3 months echocardiographic follow‐up on LVAD support, excluding patients who died or underwent heart transplantation before this time point. Additional limitations to be mentioned are the relatively small sample size and the inclusion of data from patients treated across a consortium of collaborating sites (ie, Utah Cardiac Recovery Program). Although the collaborating environment and research infrastructure allows for the rigorous, prospective follow‐up of patients, it poses limitations on the generalizability of our findings. Additional limitations include the use of only 1 modality to assess cardiac functional improvement and the absence of information on micro‐ and macrovascular complications of T2D that could affect the study outcomes, as well as the limited number of patients treated with sodium‐glucose cotransporter‐2 inhibitors, which did not allow us to examine the impact of these medications on our results. Moreover, HF and T2D pharmacotherapy is presented for the 3‐month post‐LVAD time point. We acknowledge that pharmacologic regimen might have changed in subsequent time points. Last, the inclusion of available HbA1c values within the 1‐year period preceding LVAD implantation does not allow for the assessment of longer‐term glycemia and its potential effect on study outcomes.
The findings of our study suggest that T2D and pre‐LVAD glycemia might affect the potential for functional cardiac improvement in patients with advanced HF supported with a durable LVAD. Moreover, it seems that glycemia before LVAD support does not affect all‐cause mortality rates, but it affects the risk for the development of LVAD‐related adverse events by 3 years on MCS. The degree and duration of glycemic control optimization before LVAD implantation to potentially promote and sustain cardiac functional improvement and improve outcomes warrants further investigation. Findings from the advanced HF/MCS investigational setting could inform the care of patients with earlier‐stage HF and concomitant T2D or prediabetes, and follow‐up studies in this patient population are warranted.
Sources of Funding
This work was supported by the American Heart Association Heart Failure Strategically Focused Research Network 16SFRN29020000 (Drs Drakos, Stehlik, and Selzman), National Heart, Lung, and Blood Institute (NHLBI) R01 HL135121‐01 (Dr Drakos), NHLBI R01 HL132067‐01A1 (Dr Drakos), Nora Eccles Treadwell Foundation (Dr Drakos), U.S. Department of Veterans Affairs Merit Review Award I01 CX002291 (Dr Drakos), NHLBI 2T32HL007576‐36 (Dr Kyriakopoulos), NHLBI 5T32HL007576‐33 (Dr Taleb), and NHLBI K23 HL150322‐01A1 (Dr Wever‐Pinzon).
Disclosures
Dr Fang serves on the steering committee of the Dapagliflozin Evaluation to Improve the Lives of Patients with Preserved Ejection Fraction Heart Failure (DELIVER) trial and on the data and safety monitoring board of the Dapagliflozin in Preserved Ejection Fraction Heart Failure (PRESERVED‐HF) trial for AstraZeneca. Dr Drakos serves as a consultant for Abbott Laboratories and Pfizer and has received research support from Novartis and Merck. The remaining authors have nothing to disclose.
Supporting information
Tables S1–S2
Figures S1–S2
Acknowledgments
C.P.K.: Study conception and design, data acquisition, data analysis, interpretation of results, original draft article preparation. I.T.: Study conception and design, data acquisition, interpretation of results, article review and editing. E.T.: Data acquisition, interpretation of results, article review and editing. K.S.: Data acquisition, interpretation of results, article review and editing. R.H.: Interpretation of results, article review and editing. E.M.: Data acquisition, interpretation of results, article review and editing. M.N.: Interpretation of results, article review and editing. E.K.: Interpretation of results, article review and editing. S.S.: Interpretation of results, article review and editing. J.R.V.: Interpretation of results, article review and editing. E.D.: Study conception and design, data analysis, interpretation of results, article review and editing. M.L.G.: Interpretation of results, article review and editing. R.A.: Interpretation of results, article review and editing. O.W.‐P.: Interpretation of results, article review and editing. J.C.F.: Interpretation of results, article review and editing. J.S.: Interpretation of results, article review and editing. C.H.S.: Interpretation of results, article review and editing. T.C.H.: Interpretation of results, article review and editing. S.G.D.: Study conception and design, funding acquisition, data acquisition, interpretation of results, article review and editing. All authors have approved the content of the article.
This article was sent to Sula Mazimba, MD, MPH, Associate Editor, for review by expert referees, editorial decision, and final disposition.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.123.032936
For Sources of Funding and Disclosures, see page 10.
References
- 1. Centers for Disease Control and Prevention . National Diabetes Statistics Report, 2020. Centers for Disease Control and Prevention, U.S. Dept of Health and Human Services; 2020. https://www.cdc.gov/diabetes/data/statistics‐report/index.html [Google Scholar]
- 2. Pop‐Busui R, Januzzi JL, Bruemmer D, Butalia S, Green JB, Horton WB, Knight C, Levi M, Rasouli N, Richardson CR. Heart failure: an underappreciated complication of diabetes. A consensus report of the American Diabetes Association. Diabetes Care. 2022;45:1670–1690. doi: 10.2337/dci22-0014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chahal H, Bluemke DA, Wu CO, McClelland R, Liu K, Shea SJ, Burke G, Balfour P, Herrington D, Shi P, et al. Heart failure risk prediction in the multi‐ethnic study of atherosclerosis. Heart. 2015;101:58–64. doi: 10.1136/heartjnl-2014-305697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ingelsson E, Sundstrom J, Arnlov J, Zethelius B, Lind L. Insulin resistance and risk of congestive heart failure. JAMA. 2005;294:334–341. doi: 10.1001/jama.294.3.334 [DOI] [PubMed] [Google Scholar]
- 5. Kannel WB, McGee DL. Diabetes and cardiovascular disease. The Framingham study. JAMA. 1979;241:2035–2038. doi: 10.1001/jama.241.19.2035 [DOI] [PubMed] [Google Scholar]
- 6. Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, Hadden D, Turner RC, Holman RR. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321:405–412. doi: 10.1136/bmj.321.7258.405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Doehner W, Rauchhaus M, Ponikowski P, Godsland IF, von Haehling S, Okonko DO, Leyva F, Proudler AJ, Coats AJ, Anker SD. Impaired insulin sensitivity as an independent risk factor for mortality in patients with stable chronic heart failure. J Am Coll Cardiol. 2005;46:1019–1026. doi: 10.1016/j.jacc.2005.02.093 [DOI] [PubMed] [Google Scholar]
- 8. Dei Cas A, Khan SS, Butler J, Mentz RJ, Bonow RO, Avogaro A, Tschoepe D, Doehner W, Greene SJ, Senni M, et al. Impact of diabetes on epidemiology, treatment, and outcomes of patients with heart failure. JACC Heart Fail. 2015;3:136–145. doi: 10.1016/j.jchf.2014.08.004 [DOI] [PubMed] [Google Scholar]
- 9. Mann DL, Barger PM, Burkhoff D. Myocardial recovery and the failing heart: myth, magic, or molecular target? J Am Coll Cardiol. 2012;60:2465–2472. doi: 10.1016/j.jacc.2012.06.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Birks EJ, Tansley PD, Hardy J, George RS, Bowles CT, Burke M, Banner NR, Khaghani A, Yacoub MH. Left ventricular assist device and drug therapy for the reversal of heart failure. N Engl J Med. 2006;355:1873–1884. doi: 10.1056/NEJMoa053063 [DOI] [PubMed] [Google Scholar]
- 11. Drakos SG, Wever‐Pinzon O, Selzman CH, Gilbert EM, Alharethi R, Reid BB, Saidi A, Diakos NA, Stoker S, Davis ES, et al. Magnitude and time course of changes induced by continuous‐flow left ventricular assist device unloading in chronic heart failure: insights into cardiac recovery. J Am Coll Cardiol. 2013;61:1985–1994. doi: 10.1016/j.jacc.2013.01.072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Topkara VK, Garan AR, Fine B, Godier‐Furnemont AF, Breskin A, Cagliostro B, Yuzefpolskaya M, Takeda K, Takayama H, Mancini DM, et al. Myocardial recovery in patients receiving contemporary left ventricular assist devices: results from the interagency registry for mechanically assisted circulatory support (INTERMACS). Circ Heart Fail. 2016;9. doi: 10.1161/CIRCHEARTFAILURE.116.003157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Birks EJ, Drakos SG, Patel SR, Lowes BD, Selzman CH, Starling RC, Trivedi J, Slaughter MS, Alturi P, Goldstein D, et al. Prospective multicenter study of myocardial recovery using left ventricular assist devices (RESTAGE‐HF [remission from stage D heart failure]): medium‐term and primary end point results. Circulation. 2020;142:2016–2028. doi: 10.1161/CIRCULATIONAHA.120.046415 [DOI] [PubMed] [Google Scholar]
- 14. Shah P, Psotka M, Taleb I, Alharethi R, Shams MA, Wever‐Pinzon O, Yin M, Latta F, Stehlik J, Fang JC, et al. Framework to classify reverse cardiac remodeling with mechanical circulatory support: the Utah‐Inova stages. Circ Heart Fail. 2021;14:e007991. doi: 10.1161/CIRCHEARTFAILURE.120.007991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wever‐Pinzon O, Drakos SG, McKellar SH, Horne BD, Caine WT, Kfoury AG, Li DY, Fang JC, Stehlik J, Selzman CH. Cardiac recovery during long‐term left ventricular assist device support. J Am Coll Cardiol. 2016;68:1540–1553. doi: 10.1016/j.jacc.2016.07.743 [DOI] [PubMed] [Google Scholar]
- 16. Kanwar MK, Selzman CH, Ton VK, Miera O, Cornwell WK III, Antaki J, Drakos S, Shah P. Clinical myocardial recovery in advanced heart failure with long term left ventricular assist device support. J Heart Lung Transplant. 2022;41:1324–1334. doi: 10.1016/j.healun.2022.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kyriakopoulos CP, Kapelios CJ, Stauder EL, Taleb I, Hamouche R, Sideris K, Koliopoulou AG, Bonios MJ, Drakos SG. LVAD as a bridge to remission from advanced heart failure: current data and opportunities for improvement. J Clin Med. 2022;11:3542. doi: 10.3390/jcm11123542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Inker LA, Eneanya ND, Coresh J, Tighiouart H, Wang D, Sang Y, Crews DC, Doria A, Estrella MM, Froissart M, et al. New creatinine‐ and cystatin C‐based equations to estimate GFR without race. N Engl J Med. 2021;385:1737–1749. doi: 10.1056/NEJMoa2102953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lang RM, Badano LP, Mor‐Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39. doi: 10.1016/j.echo.2014.10.003 [DOI] [PubMed] [Google Scholar]
- 20. Wever‐Pinzon J, Selzman CH, Stoddard G, Wever‐Pinzon O, Catino A, Kfoury AG, Diakos NA, Reid BB, McKellar S, Bonios M, et al. Impact of ischemic heart failure etiology on cardiac recovery during mechanical unloading. J Am Coll Cardiol. 2016;68:1741–1752. doi: 10.1016/j.jacc.2016.07.756 [DOI] [PubMed] [Google Scholar]
- 21. STS Intermacs Adverse Events Definitions, Appendix A, Version Date: 10/11/2021. https://www.uab.edu/medicine/intermacs/intermacs‐documents.
- 22. Antonides CFJ, Schoenrath F, de By T, Muslem R, Veen K, Yalcin YC, Netuka I, Gummert J, Potapov EV, Meyns B, et al. Outcomes of patients after successful left ventricular assist device explantation: a EUROMACS study. ESC Heart Fail. 2020;7:1085–1094. doi: 10.1002/ehf2.12629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Aiken LS, West SG, Reno RR. Multiple Regression: Testing and Interpreting Interactions. SAGE; 1991. [Google Scholar]
- 24. Box GEP. Some theorems on quadratic forms applied in the study of analysis of variance problems, I. Effect of inequality of variance in the one‐way classification. Ann Math Stat. 1954;25:290–302, 213. doi: 10.1214/aoms/1177728786 [DOI] [Google Scholar]
- 25. Fleiss JL. Design and Analysis of Clinical Experiments. John Wiley & Sons; 2011. [Google Scholar]
- 26. Aroor AR, Mandavia CH, Sowers JR. Insulin resistance and heart failure: molecular mechanisms. Heart Fail Clin. 2012;8:609–617. doi: 10.1016/j.hfc.2012.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Boudina S, Abel ED. Diabetic cardiomyopathy revisited. Circulation. 2007;115:3213–3223. doi: 10.1161/CIRCULATIONAHA.106.679597 [DOI] [PubMed] [Google Scholar]
- 28. Riehle C, Abel ED. Insulin signaling and heart failure. Circ Res. 2016;118:1151–1169. doi: 10.1161/CIRCRESAHA.116.306206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Segar MW, Vaduganathan M, Patel KV, McGuire DK, Butler J, Fonarow GC, Basit M, Kannan V, Grodin JL, Everett B, et al. Machine learning to predict the risk of incident heart failure hospitalization among patients with diabetes: the WATCH‐DM risk score. Diabetes Care. 2019;42:2298–2306. doi: 10.2337/dc19-0587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Marwick TH, Ritchie R, Shaw JE, Kaye D. Implications of underlying mechanisms for the recognition and Management of Diabetic Cardiomyopathy. J Am Coll Cardiol. 2018;71:339–351. doi: 10.1016/j.jacc.2017.11.019 [DOI] [PubMed] [Google Scholar]
- 31. Tan Y, Zhang Z, Zheng C, Wintergerst KA, Keller BB, Cai L. Mechanisms of diabetic cardiomyopathy and potential therapeutic strategies: preclinical and clinical evidence. Nat Rev Cardiol. 2020;17:585–607. doi: 10.1038/s41569-020-0339-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jia G, Hill MA, Sowers JR. Diabetic cardiomyopathy: an update of mechanisms contributing to this clinical entity. Circ Res. 2018;122:624–638. doi: 10.1161/CIRCRESAHA.117.311586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kyriakopoulos CP, Horne BD, Sideris K, Taleb I, Griffin RJ, Sheffield E, Alharethi R, Hanff TC, Stehlik J, Selzman CH, et al. Left ventricular functional improvement appears to contribute to lower rates of device thrombosis in patients on durable mechanical circulatory support. J Heart Lung Transplant. 2023;42:853–858. doi: 10.1016/j.healun.2023.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Olsen C, Mandawat A, Sun JL, Triana T, Chiswell K, Karra R. Recovery of left ventricular function is associated with improved outcomes in LVAD recipients. J Heart Lung Transplant. 2022;41:1055–1062. doi: 10.1016/j.healun.2022.03.008 [DOI] [PubMed] [Google Scholar]
- 35. Badolia R, Ramadurai DKA, Abel ED, Ferrin P, Taleb I, Shankar TS, Krokidi AT, Navankasattusas S, McKellar SH, Yin M, et al. The role of nonglycolytic glucose metabolism in myocardial recovery upon mechanical unloading and circulatory support in chronic heart failure. Circulation. 2020;142:259–274. doi: 10.1161/CIRCULATIONAHA.119.044452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cluntun AA, Badolia R, Lettlova S, Parnell KM, Shankar TS, Diakos NA, Olson KA, Taleb I, Tatum SM, Berg JA, et al. The pyruvate‐lactate axis modulates cardiac hypertrophy and heart failure. Cell Metab. 2021;33:629–648. doi: 10.1016/j.cmet.2020.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Diakos NA, Navankasattusas S, Abel ED, Rutter J, McCreath L, Ferrin P, McKellar SH, Miller DV, Park SY, Richardson RS, et al. Evidence of glycolysis up‐regulation and pyruvate mitochondrial oxidation mismatch during mechanical unloading of the failing human heart: implications for cardiac reloading and conditioning. JACC Basic Transl Sci. 2016;1:432–444. doi: 10.1016/j.jacbts.2016.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Diakos NA, Taleb I, Kyriakopoulos CP, Shah KS, Javan H, Richins TJ, Yin MY, Yen CG, Dranow E, Bonios MJ, et al. Circulating and myocardial cytokines predict cardiac structural and functional improvement in patients with heart failure undergoing mechanical circulatory support. J Am Heart Assoc. 2021;10:e020238. doi: 10.1161/JAHA.120.020238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rochlani Y, Pothineni NV, Kovelamudi S, Mehta JL. Metabolic syndrome: pathophysiology, management, and modulation by natural compounds. Ther Adv Cardiovasc Dis. 2017;11:215–225. doi: 10.1177/1753944717711379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Group AC , Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, Marre M, Cooper M, Glasziou P, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560–2572. doi: 10.1056/NEJMoa0802987 [DOI] [PubMed] [Google Scholar]
- 41. Reaven PD, Emanuele NV, Wiitala WL, Bahn GD, Reda DJ, McCarren M, Duckworth WC, Hayward RA, Investigators V. Intensive glucose control in patients with type 2 diabetes ‐ 15‐year follow‐up. N Engl J Med. 2019;380:2215–2224. doi: 10.1056/NEJMoa1806802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Koska J, Saremi A, Howell S, Bahn G, De Courten B, Ginsberg H, Beisswenger PJ, Reaven PD, Investigators V. Advanced glycation end products, oxidation products, and incident cardiovascular events in patients with type 2 diabetes. Diabetes Care. 2018;41:570–576. doi: 10.2337/dc17-1740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tseliou E, Lavine KJ, Wever‐Pinzon O, Topkara VK, Meyns B, Adachi I, Zimpfer D, Birks EJ, Burkhoff D, Drakos SG. Biology of myocardial recovery in advanced heart failure with long‐term mechanical support. J Heart Lung Transplant. 2022;41:1309–1323. doi: 10.1016/j.healun.2022.07.007 [DOI] [PubMed] [Google Scholar]
- 44. Asleh R, Briasoulis A, Schettle SD, Tchantchaleishvili V, Pereira NL, Edwards BS, Clavell AL, Maltais S, Joyce DL, Joyce LD, et al. Impact of diabetes mellitus on outcomes in patients supported with left ventricular assist devices: a single institutional 9‐year experience. Circ Heart Fail. 2017;10:e004213. doi: 10.1161/CIRCHEARTFAILURE.117.004213 [DOI] [PubMed] [Google Scholar]
- 45. Mohamedali B, Yost G, Bhat G. Is diabetes mellitus a risk factor for poor outcomes after left ventricular assist device placement? Tex Heart Inst J. 2017;44:115–119. doi: 10.14503/THIJ-15-5698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Usoh CO, Sherazi S, Szepietowska B, Kutyifa V, McNitt S, Papernov A, Wang M, Alexis JD. Influence of diabetes mellitus on outcomes in patients after left ventricular assist device implantation. Ann Thorac Surg. 2018;106:555–560. doi: 10.1016/j.athoracsur.2018.02.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Vest AR, Mistak SM, Hachamovitch R, Mountis MM, Moazami N, Young JB. Outcomes for patients with diabetes after continuous‐flow left ventricular assist device implantation. J Card Fail. 2016;22:789–796. doi: 10.1016/j.cardfail.2016.02.010 [DOI] [PubMed] [Google Scholar]
- 48. Zhou P, Xiao Z, Zhu P, Nie Z, Pavan D, Zheng S. Diabetes mellitus is not a risk factor for patients supported with left ventricular assist device. Ann Thorac Surg. 2020;109:1614–1622. doi: 10.1016/j.athoracsur.2019.09.021 [DOI] [PubMed] [Google Scholar]
- 49. Kogan A, Frogel J, Ram E, Jamal T, Peled‐Potashnik Y, Maor E, Grupper A, Morgan A, Segev A, Raanani E, et al. The impact of diabetes on short‐, intermediate‐ and long‐term mortality following left ventricular assist device implantation. Eur J Cardiothorac Surg. 2022;61:1432–1437. doi: 10.1093/ejcts/ezab575 [DOI] [PubMed] [Google Scholar]
- 50. Goetz ME, Charnigo R, Guglin M. Implantation of left ventricular assist device results in immediate improvement of glucose metabolism in patients with and without diabetes mellitus. Heart Lung Circ. 2020;29:931–935. doi: 10.1016/j.hlc.2019.05.181 [DOI] [PubMed] [Google Scholar]
- 51. Patel N, Gluck JA, Radojevic J, Coleman CI, Baker WL. Left ventricular assist device implantation improves glycaemic control: a systematic review and meta‐analysis. ESC Heart Fail. 2018;5:1141–1149. doi: 10.1002/ehf2.12337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Uriel N, Naka Y, Colombo PC, Farr M, Pak SW, Cotarlan V, Albu JB, Gallagher D, Mancini D, Ginsberg HN, et al. Improved diabetic control in advanced heart failure patients treated with left ventricular assist devices. Eur J Heart Fail. 2011;13:195–199. doi: 10.1093/eurjhf/hfq204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Heck PM, Dutka DP. Insulin resistance and heart failure. Curr Heart Fail Rep. 2009;6:89–94. doi: 10.1007/s11897-009-0014-8 [DOI] [PubMed] [Google Scholar]
- 54. Goldstein DJ, Moazami N, Seldomridge JA, Laio H, Ashton RC Jr, Naka Y, Pinsky DJ, Oz MC. Circulatory resuscitation with left ventricular assist device support reduces interleukins 6 and 8 levels. Ann Thorac Surg. 1997;63:971–974. doi: 10.1016/s0003-4975(96)01117-4 [DOI] [PubMed] [Google Scholar]
- 55. Torre‐Amione G, Bozkurt B, Deswal A, Mann DL. An overview of tumor necrosis factor alpha and the failing human heart. Curr Opin Cardiol. 1999;14:206–210. doi: 10.1097/00001573-199905000-00003 [DOI] [PubMed] [Google Scholar]
- 56. Olia SE, Maul TM, Antaki JF, Kameneva MV. Mechanical blood trauma in assisted circulation: sublethal RBC damage preceding hemolysis. Int J Artif Organs. 2016;39:150–159. doi: 10.5301/ijao.5000478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Pierce CN, Larson DF. Inflammatory cytokine inhibition of erythropoiesis in patients implanted with a mechanical circulatory assist device. Perfusion. 2005;20:83–90. doi: 10.1191/0267659105pf793oa [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S2
Figures S1–S2


