Abstract
Background
In especially, low and middle-income nations (LMICs), where healthcare access may be restricted, high blood pressure (BP) is a major risk factor for cardiovascular disease and stroke, both of which can even lead to death. Altering one's lifestyle, in conjunction with medical therapy, has been demonstrated to be effective in lowering BP. Recent research has shown that physical activity (PA), in a variety of guises and to varying degrees, can be an effective means of lowering BP.
Objective
The purpose of this meta-analysis and systematic review was to evaluate the impact that PA plays in the development of hypertension in LMICs nations.
Methods
An exhaustive search of the available research was carried out in order to locate studies that were pertinent. We searched a number of online databases, such as SCOPUS, Medline, and Web of Science, looking for clinical trials that were published before March of 2023. Studies were only considered for inclusion if they were randomized controlled trials (RCTs), reported on the association between PA and BP, and were carried out in LMICs countries.
Results
This meta-analysis incorporated a comprehensive collection of 60 studies, encompassing a total of 11,002 people, consisting of 5,630 cases and 5372 controls. The findings indicate that engaging in PA had a notable impact on decreasing systolic blood pressure (SBP), as seen by a weighted mean difference (WMD) of -7.70 mmHg, with a 95% confidence interval (CI) ranging from -9.50 to -5.91 (p < 0.001). Additionally, PA was found to have a significant influence on reducing diastolic blood pressure (DBP), as indicated by a WMD of -3.60 mmHg, with a 95% CI ranging from -4.48to -2.73(p < 0.001). The findings from subgroup analysis indicate that the observed results remained statistically significant when considering individuals with baseline SBP of 120 mmHg or lower and DBP of 80 mmHg or lower.
Conclusion
The incorporation of PA can significantly contribute to the mitigation of high BP within LMICs nations. Additional investigation is required to ascertain the most effective form and amount of PA in order to mitigate BP levels within these specific individuals.
Graphical Abstract

Supplementary Information
The online version contains supplementary material available at 10.1186/s40885-024-00281-w.
Keywords: Blood pressure, Physical activity, Middle- and low-income nations
Introduction
Hypertension is diagnosed when an individual's systolic blood pressure (SBP) is equal to or greater than 140 mmHg, and/or their diastolic blood pressure (DBP) is equal to or greater than 90 mmHg [1]. It is a major contributor to global mortality, affecting 40% of adults and is linked to an increased risk of strokes and heart disease [2–7]. Up to 10 million deaths worldwide can be attributed to arterial hypertension [8]. In fact, a reduction in SBP, at least 10 mmHg, can decrease the risk of CVD by about 20–30 percent [2]. Various studies have examined symptoms of hypertension such as headaches, hot flushes, and mood disorders. Hypertension is often seen as asymptomatic, but these symptoms may indicate secondary hypertension caused by another medical condition [1, 9–11]. The prevalence of hypertension among adults is higher in low and middle-income countries (LMICs) in comparison to high-income nations [12]. Records from 2019 shows the highest normalized death rate caused by cardiovascular disease and SBP with regards to age in LMICs [13]. The World Health Organization (WHO) presented a table from The World Bank that categorizes countries as LMICs based on Gross National Income (GNI). A nation that possesses GNI per capita of $1,135 or lower is classified as a low-income country, whereas a nation with a GNI per capita ranging from $1,136 to $13,846 falls into the category of a middle-income country [14, 15]. According to the data presented in the table, there are a total of 26 countries classified as low-income and 108 countries classified as middle-income [14]. Patients with hypertension require a combination of lifestyle modifications and medication for effective medical care and self-management [16, 17]. WHO has just published a comprehensive inventory of indispensable pharmaceuticals for the management of hypertension. This list encompasses a range of medications, including angiotensin-converting enzyme (ACE) inhibitors, calcium channel blockers (CCBs), angiotensin receptor blockers (ARBs), and diuretics. In addition to the aforementioned considerations, it is imperative to address the management of other cardiovascular risk factors, such as smoking, diabetes, and lipid abnormalities [18–22]. Engaging in healthy lifestyle practices, including the maintenance of a normal BMI and waist circumference, regular physical activity, abstaining from smoking, moderate alcohol consumption, adherence to the DASH diet, and utilization of dietary supplements such as garlic, cocoa, vitamin C, coenzyme Q10, omega‐3 fatty acids, calcium, potassium, and magnesium, has been associated with favorable health outcomes among individuals diagnosed with hypertension [17, 23–28]. Moreover, lifestyle modifications may hold greater significance than pharmacological interventions for those with moderate hypertension. Thus, exercise training and other non-pharmacologic treatments should be recommended as the primary treatment for stage 1 hypertension in conjunction with medication [17]. Due to the significant health issues caused by hypertension and the lack of comprehensive studies. Therefore, a comprehensive systematic review and meta-analysis of clinical trial studies was undertaken to aggregate existing evidence pertaining to the correlation between physical activity intervention and blood pressure.
Materials and methods
The PRISMA procedure, which is used for reporting systematic reviews and meta-analyses, served as the basis for the planning, execution, and reporting of this work [29].
Information sources and search strategy
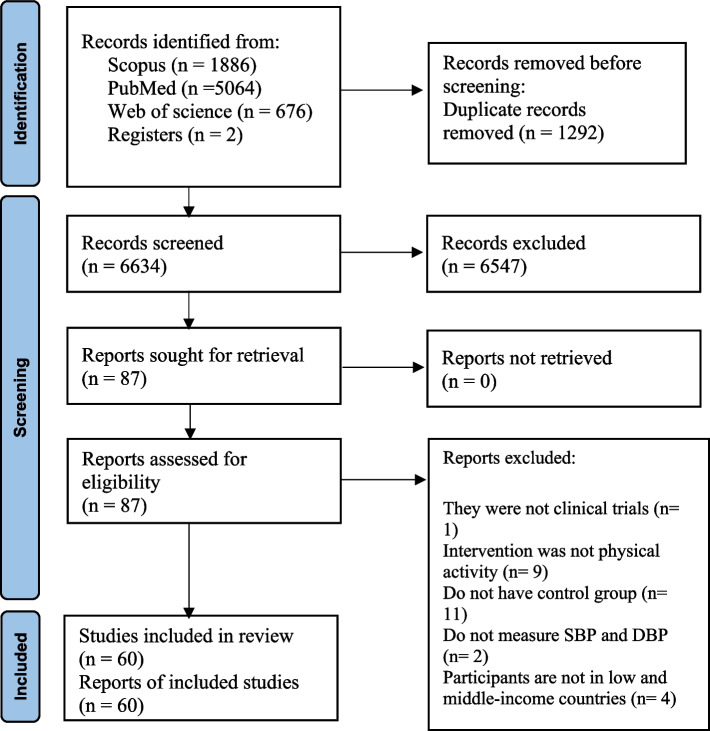
Online databases including SCOPUS (http://www.scopus.com), Medline (http://www.ncbi.nlm.nih.gov/PubMed), and Web of Science (https://clarivate.com › scientific-and-academic-research) were searched to find all the relevant clinical trials up to March 2023. We used the following search terms in our search in the mentioned databases: (Exercise OR "Exercise therapy" OR "Exercise test" OR "Exercise Movement Techniques" OR kinesiotherapy OR "Physical Endurance" OR Anaerobic OR aerobic OR "Resistance Training" OR "Motor activity" OR "Physical Activity" OR "Locomotor Activity" OR "relaxation therapy" OR tai-ji OR yoga) AND ("Blood pressure" OR Hypertension OR "Systolic Pressure" OR "Diastolic Pressure" OR "Pulse Pressure") AND ("Developing Country" OR "Under Developed country" OR "Underdeveloped country" OR "less Developed country" OR "Developing nation" OR "Under Developed nation" OR "Underdeveloped nation" OR "less Developed nation" OR "Third World" OR "low resource country" OR "low resource nation" OR Africa OR Africa OR "South America" OR "South America" OR "Latin America" OR "central America" OR Asia). All of the studies that were looked up were incorporated into the Endnote software (version X9, for Windows, Thomson Reuters, Philadelphia, PA, USA) and screened. After that, duplicate citations were removed from the articles. The present meta-analysis did not take into account information from unpublished sources or those considered as grey literature, such as conference abstracts, theses, and patents. In addition to this, we also conducted a hand search of the reference lists of the papers that were obtained as well as the reviews that were done previously in order to include any other studies that might be suitable. There were no limitations on language and date. The detailed steps of the literature search are depicted in (Fig. 1).
Fig. 1.
Flow diagram of the selection of the included studies
Inclusion criteria
Human trials were included in the meta-analysis if they fulfilled the following inclusion criteria: (A) Were RCTs with either parallel or crossover designs; (B) Reported blood pressure indices before and after intervention in each group; (C) Compared intervention group with the placebo group; (D) Reported data as mean and SD or can be converted to it; (E) Were listed in LMICs according to the World Bank incoming group list used by WHO (Supplementary file).
Exclusion criteria
Studies were excluded if they: (A) Were non clinical trials; (B) Duplicate studies (C) Animal, in vitro, and review studies; (D) Pregnant women; (E) Did not provide effects sizes on blood pressure before and after the trial in placebo and intervention groups; (F) They were conducted solely in developed (high-income) countries according to the World Bank incoming group list used by WHO.
Data extraction
The following data were extracted with a standardized data collection form by three researchers working independently (Hashemi M, Javid R, Kiani F). After reviewing the titles and abstracts of the studies, relevant research was incorporated into the present study. The disagreements that arose amongst the reviewers were settled through discussion and by coming to an agreement with the assistance of a fourth reviewer (Monfared V). The following information was extracted: first author, publication year, mean age, gender, study design, sample size, intervention type, duration of intervention, mean and SD in BMI, health status, and mean and SD changes in SBP and DBP. The corrected mean changes and standard deviations of blood pressure measurements were calculated during the study for both the intervention group and the control group. If the data on the blood pressure were provided in a different unit, we converted them to the unit that was used the most frequently.
Risk of bias assessment
We applied the Cochrane quality assessment tool for assessing the risk of bias for each study included in the current meta-analysis [30]. This instrument comprised seven components, which included random sequence generation, allocation concealment, reporting bias, performance bias, detection bias, attrition bias, and additional sources of bias. A score of "high risk" was assigned to each domain if the study contained methodological concerns that could have affected its findings. A score of "low risk" was assigned if there was no imperfection for that domain, and a score of "unclear risk" was assigned if the information was insufficient to determine the impact of the study. If the trial met the criteria for "low risk" in all dimensions, then the study was regarded as being of excellent quality and carrying an extremely low risk of being biased overall. Both of the reviewers worked independently on the assessment of the potential for bias (Hashemi M and Yousefi M) (Table 2).
Table 2.
Risk of bias assessment
| Study | Random sequence generation | Allocation concealment | Selective reporting | Other sources of bias | Blinding (participants and personnel) | Blinding (outcome assessment) | Incomplete outcome data | General risk of bias |
|---|---|---|---|---|---|---|---|---|
| Mandal et al., 2021 [78] | L | L | L | H | U | H | L | Moderate risk |
| Yu et al., 2021 [66] | U | L | L | H | U | H | L | Moderate risk |
| Gu et al., 2020 [35] | U | L | L | L | U | U | L | Low risk |
| Hooshmand-moghadam et al., 2020 [36] | L | L | L | L | U | U | L | Low risk |
| Ranasinghe et al., 2020 [46] | U | U | L | H | L | L | L | Low risk |
| Rosa Santos et al., 2020 [67] | U | L | L | H | U | H | L | Moderate risk |
| Ac et al., 2019 [68] | L | L | L | H | H | H | L | High risk |
| Leung et al., 2019 [51] | L | U | L | H | H | H | L | High risk |
| Mouodi et al., 2019 [76] | L | L | L | L | U | H | L | Low risk |
| Chhugani et al., 2018 [83] | U | U | L | H | U | U | L | Low risk |
| Gradidg et al., 2018 [34] | L | L | H | H | L | L | L | Moderate risk |
| Ma et al., 2018 [47] | U | U | L | H | U | U | L | Low risk |
| Cavalcante et al., 2017 [81] | L | H | L | L | U | L | L | Low risk |
| Frih et al., 2017 [33] | L | L | L | L | H | H | L | Moderate risk |
| Kanegusuku et al., 2017 [39] | U | H | H | H | H | H | H | High risk |
| Chang et al., 2016 [82] | L | L | L | H | U | U | L | Low risk |
| Farinatti et al., 2016 [86] | L | L | L | H | U | U | L | Low risk |
| Gallo-Villegas et al., 2016 [69] | L | L | L | L | H | H | L | Moderate risk |
| Rubinstein et al., 2016 [42] | L | L | L | L | H | U | L | Low risk |
| Lau et al., 2015 [44] | H | U | L | H | H | H | L | High risk |
| Ezema et al., 2014 [85] | L | U | L | H | U | U | L | Low risk |
| Sikiru et al., 2014 [53] | L | L | L | H | L | L | L | Low risk |
| Sujatha et al., 2014 [59] | L | L | L | H | U | H | L | Moderate risk |
| Wu et al., 2014 [63] | L | L | L | L | L | H | L | Low risk |
| Yan et al., 2014 [64] | U | L | L | H | U | H | L | Moderate risk |
| Yu et al., 2014 [65] | H | L | L | L | U | H | L | Moderate risk |
| Hou et al., 2014 [37] | L | U | L | L | U | U | L | Low risk |
| Ajiboye et al., 2013 [41] | L | U | L | H | U | U | L | Low risk |
| Brito et al., 2013 [56] | L | U | L | H | U | U | L | Low risk |
| Lamina et al., 2013 [75] | U | L | L | H | L | U | L | Low risk |
| Pal et al., 2013 [43] | L | L | L | H | U | U | L | Low risk |
| Sikiru et al., 2013 [52] | U | L | L | H | L | L | L | Low risk |
| Tsai et al., 2013 [61] | H | U | L | H | U | H | L | High risk |
| Lamina et al., 2012 [72] | L | L | L | H | U | H | L | Moderate risk |
| Lo et al., 2012 [48] | H | H | L | H | U | U | L | High risk |
| M. Cunha et al., 2012 [55] | U | H | L | H | H | H | L | High risk |
| Mizuno et al., 2012 [50] | H | U | L | H | U | U | L | Moderate risk |
| Vianna et al., 2012 [94] | L | U | L | L | U | U | L | Low risk |
| Kanegusuku et al., 2011 [91] | L | U | L | L | U | L | L | Low risk |
| Lamina et al., 2011 [73] | U | L | L | H | L | U | L | Low risk |
| Lamina et al., 2011 [74] | U | L | L | H | L | U | L | Low risk |
| Luk et al., 2011[79] | L | L | L | H | H | H | L | High risk |
| Mortimer et al., 2011 [49] | U | U | L | H | U | U | L | Low risk |
| Ansari et al., 2010 [84] | L | L | L | H | U | U | L | Low risk |
| Monteiro et al., 2010 [92] | L | U | L | H | U | U | L | Low risk |
| Mujica et al., 2010 [80] | L | U | L | H | L | H | L | Moderate risk |
| Arora et al., 2009 [90] | L | U | L | L | U | U | L | Low risk |
| Bündchen et al., 2009 [88] | L | U | L | H | U | U | L | Low risk |
| Jafar et al., 2009 [54] | L | L | L | L | L | L | L | Low risk |
| Barroso et al., 2008 [87] | L | U | H | L | U | U | L | Low risk |
| Meirelles et al., 2008 [89] | U | U | L | H | U | U | L | Low risk |
| Jiang et al., 2007 [38] | L | L | L | H | H | H | L | High risk |
| Lee et al., 2007 [40] | L | L | L | H | H | H | L | High risk |
| Pazoki et al., 2007 [70] | U | U | L | H | U | H | L | Moderate risk |
| Wu et al., 2007 [93] | U | U | L | H | U | U | L | Low risk |
| Mendivil et al., 2006 [45] | L | U | L | L | U | U | L | Low risk |
| McCaffrey et al., 2005 [71] | U | U | L | H | U | H | L | Moderate risk |
| Thomas et al., 2005 [60] | L | L | L | H | U | H | L | Moderate risk |
| Tsai et al., 2003 [62] | L | L | L | L | L | H | L | Low risk |
| Tsai et al., 2002 [58] | U | L | L | H | U | H | L | Moderate risk |
General Low risk < 2 high risk
General moderate risk = 2 high risk
General high risk > 2 high risk
Statistical analysis
The overall effect sizes were calculated using the mean changes in blood pressure measurements and their respective standard deviations for both the intervention group and the control group. We were able to determine mean changes when they were not reported by considering the changes in blood pressure that occurred during the intervention. The standard deviation (SD) was determined in research projects that reported the standard error of means (SEM) by multiplying the SEM by the square root of the sample size, as follows: SD = SEM × √n. We used a random-effects model that considering differences from one study to the next in order to get an accurate picture of the overall effect sizes. The I2 statistic and the Cochrane's Q test were used to determine the presence of heterogeneity. It was determined that there was significant heterogeneity between studies if the I2 value was > 50% or P < 0.05 [31]. To find probable sources of heterogeneity, subgroup analyses were performed according to the predefined variables including Type of physical activities (Resistant training, Aerobic training, Combined exercise), Gender (Male, Female, Both), Baseline SBP (> 120 mmHg, ≤ 120 mmHg), Baseline DBP (> 80 mmHg, ≤ 80 mmHg), Age (> 50 years, ≤ 50 years), Health status (Healthy, Disease),Baseline BMI (Normal (18.5–24.9 kg/m2), Overweight (25–29.9 kg/m2), Obese (> 30 kg/m2)), Ethinc (Asia, Africa, America), and Trial duration (> 24 weeks, < 24 weeks) (Table 3). A sensitivity analysis was performed in order to determine whether or not the overall effect size was dependent on a specific study. The formal test developed by Begg looked into the possibility of publication bias. Stata, version 17/0 was utilized in the process of doing the meta-analysis, and P value < 0.05 was considered as significant level.
Table 3.
Subgroup analyses of physical activity on blood pressure in low and middle income countries
| NO | WMD (95%CI) | P-value | heterogeneity | |||
|---|---|---|---|---|---|---|
| P heterogeneity | I2 | P between sub-groups | ||||
| Subgroup analyses of physical activity on SBP | ||||||
| Overall effect | 84 | -7.70 (-9.49, -5.91) | < 0.001 | < 0.001 | 97.3 | |
| Baseline SBP (mmHg) | ||||||
| > 120 | 73 | -8.15 (-10.43, -5.86) | < 0.001 | < 0.001 | 97.5 | < 0.001 |
| ≤ 120 | 9 | -4.73 (-6.26, -3.20) | < 0.001 | 0.001 | 70.9 | |
| Trial duration (week) | ||||||
| ≤ 12 | 50 | -9.64 (-12.50, -6.79) | < 0.001 | < 0.001 | 97.8 | < 0.001 |
| > 12 | 31 | -4.72 (-6.37, -3.06) | < 0.001 | < 0.001 | 91 | |
| Age (year) | ||||||
| > 50 | 54 | -7.19 (-9.48, -4.90) | < 0.001 | < 0.001 | 97.6 | < 0.001 |
| ≤ 50 | 26 | -8.76 (-12.34, -5.17) | < 0.001 | < 0.001 | 96.8 | |
| Health status | ||||||
| Disease | 57 | -8.94 (-11.33, 6.55) | < 0.001 | < 0.001 | 98.1 | < 0.001 |
| Healthy | 26 | -4.95 (-6.30, -3.60) | < 0.001 | < 0.001 | 64.1 | |
| Both | 1 | -2.24 (-5.63, 1.15) | 0.196 | 0 | 0 | |
| Gender | ||||||
| Both | 60 | -6.84 (-8.80, -4.88) | < 0.001 | < 0.001 | 97 | < 0.001 |
| Male | 13 | -14.26 (-16.24, -12.29) | < 0.001 | < 0.001 | 84.9 | |
| Female | 11 | -4.90 (-11.79, 1.98) | 0.163 | < 0.001 | 97 | |
| Baselin BMI (kg/m2) | ||||||
| Normal (18.5–24.9) | 18 | -11.75 (-14.95, -8.55) | < 0.001 | < 0.001 | 96 | < 0.001 |
| Overweight (25–29.9) | 50 | -7.04 ( -9.88, -4.20) | < 0.001 | < 0.001 | 96.5 | |
| Obese (> 30) | 5 | -3.48 (-6.02, -0.95) | 0.007 | < 0.001 | 87.9 | |
| Ethinc | ||||||
| Asia | 41 | -6.22 (-8.52, -3.91) | < 0.001 | < 0.001 | 96.2 | < 0.001 |
| Africa | 15 | -14.26 (-16.84, -11.68) | < 0.001 | < 0.001 | 90.4 | |
| America | 27 | -6.62 (-9.97, -3.28) | < 0.001 | < 0.001 | 97.4 | |
| Types of physical activities | 84 | |||||
| Resistant training | 1 | -3.93 (-9.32, 1.45) | 0.158 | < 0.001 | 97.1 | < 0.001 |
| Aerobic training | 38 | -9.17 (-12.46, -5.87) | < 0.001 | < 0.001 | 97.9 | |
| Combined exercise | 32 | -7.47 (-9.85, -5.09) | < 0.001 | < 0.001 | 96.2 | |
| Subgroup analyses of physical activity on DBP | ||||||
| Overall effect | 78 | -3.60 (-4.48, -2.72) | < 0.001 | < 0.001 | 94.5 | |
| Baseline DBP (mmHg) | ||||||
| > 80 | 42 | -4.96 (-6.24, -3.68) | < 0.001 | < 0.001 | 96.5 | < 0.001 |
| ≤ 80 | 36 | -1.91 (-2.62, -1.19) | < 0.001 | < 0.001 | 63.8 | |
| Trial duration (week) | ||||||
| > 12 | 29 | -2.36 (-3.69, -1.04) | < 0.001 | < 0.001 | 94.1 | < 0.001 |
| ≤ 12 | 46 | -4.40 (-5.52, -3.27) | < 0.001 | < 0.001 | 93.8 | |
| Age (year) | ||||||
| > 50 | 49 | -3.52 (-4.56, -2.48) | < 0.001 | < 0.001 | 94.4 | < 0.001 |
| ≤ 50 | 26 | -3.70 (-5.58, -1.82) | < 0.001 | < 0.001 | 94.5 | |
| Health status | ||||||
| Disease | 54 | -4.36 (-5.46, -3.25) | < 0.001 | < 0.001 | 95.8 | < 0.001 |
| Healthy | 23 | -2.05 (-2.96, -1.15) | < 0.001 | < 0.001 | 64.3 | |
| Both | 1 | 0.30 (-1.85, 2.45) | 0.785 | 0 | 0 | |
| Gender | ||||||
| Both | 55 | -3.34 (-4.52, -2.17) | < 0.001 | < 0.001 | 94.8 | < 0.001 |
| Male | 13 | -5.01 (-6.43, -3.59) | < 0.001 | < 0.001 | 93.7 | |
| Female | 10 | -3.23 (-6.76, 0.29) | 0.073 | < 0.001 | 91.9 | |
| Baselin BMI (kg/m2) | ||||||
| Normal (18.5–24.9) | 18 | -4.18 (-5.37, -2.99) | < 0.001 | < 0.001 | 90.8 | < 0.001 |
| Overweight (25–29.9) | 45 | -3.89 (-5.34, -2.43) | < 0.001 | < 0.001 | 94.4 | |
| Obese (> 30) | 4 | -1.95 (-3.67, -0.24) | 0.025 | 0.001 | 81.3 | |
| Ethinc | ||||||
| Asia | 41 | -3.08 (-4.45, -1.71) | < 0.001 | < 0.001 | 95 | 0.038 |
| Africa | 14 | -4.50 (-5.70, -3.30) | < 0.001 | < 0.001 | 88.4 | |
| America | 23 | -4.00 (-6.07, -1.93) | < 0.001 | < 0.001 | 95.4 | |
| Types of physical activities | 78 | < 0.001 | ||||
| Resistant training | 10 | -3.03 (-6.17, -0.10) | 0.058 | < 0.001 | 95 | |
| Aerobic training | 36 | -4.21 (-5.59, -2.83) | < 0.001 | < 0.001 | 95 | |
| Combined exercise | 32 | -3.00 (-4.01, -1.98) | < 0.001 | < 0.001 | 89.9 | |
Abbreviations: CI Confidence interval, WMD Weighted mean differences, SBP Systolic blood pressure, DBP Diastolic blood pressure
Certainty assessment
The overall certainty of evidence across the studies was graded according to the GRADE guidelines Working Group [32]. According to the relevant assessment standards, the standard of the evidence can be divided into the following four categories: high, moderate, low, and extremely low. Monfared V and Hasani M, two pairs of authors, independently utilized the GRADE assessment and then consensus to reach a single result (Table 4).
Table 4.
GRADE profile of physical activity for SBP and DBP
| Quality assessment | Summary of findings | Quality of evidence | ||||||
|---|---|---|---|---|---|---|---|---|
| Outcomes | Risk of bias | Inconsistency | Indirectness | Imprecision | Publication Bias | Number of intervention/control | WMD (95%CI) | |
| SBP | No serious limitation | Very serious limitationa | No serious limitation | No serious limitation | No serious limitation | 5630/5372 | -7.70 (-9.50, -5.91) |
⊕ ⊕ ⊕ ◯ Moderate |
| DBP | No serious limitation | Very serious limitationb | No serious limitation | No serious limitation | No serious limitation | 5495/5226 | -3.60 (-4.48, -2.73) |
⊕ ⊕ ⊕ ◯ Moderate |
aThe test for heterogeneity is significant, and the I2 is high, 97.3%
bThe test for heterogeneity is significant, and the I2 is high, 94.5%
Results
Study selection
Out of a total of 7,626 articles obtained from three databases (Scopus, PubMed, and Web of Science), the primary search yielded 1,886 items from Scopus, 5,064 articles from PubMed, and 676 articles from Web of Science. After excluding 1,292 duplicate studies, a total of 6,634 unique articles remained for further analysis. Following the examination of titles and abstracts, a total of 6,547 studies that did not meet our inclusion criteria were excluded. These exclusions were based on the following reasons: 5,779 studies had titles and abstracts that were irrelevant to our research topic, 465 studies had animal subjects, and 222 studies were review articles. As a result, a total of 87 studies were retained for analysis. Following a thorough examination of the full-text articles, one study was excluded due to its lack of a clinical trial design. Additionally, in nine studies, the intervention did not involve physical activity. Furthermore, 11 studies lacked an appropriate control group. Two study failed to measure SBP and DBP, while four studies did not include participants from LMICs countries. Ultimately, a total of 60 studies were identified that satisfied all the specified inclusion criteria and were then included in the meta-analysis. Figure 1 illustrates the PRISMA flow diagram, which outlines the search process.
Study characteristics
Overall, 60 studies, with 11,002participants (5,630cases and 5,372controls), were included [33–94]. Included studies were published between 2002 [58] and 2021 [66]. The duration period ranged from 2 [55] days to 96 [54] weeks, and the sample size of the included studies ranged from 20 to [56] to 674 [54] participants. The mean age ranged from 13 [67] to 71 [40], and BMI ranged from 20 [67] to 32 [34] All of the studies were parallel RCTs, except for three studies that were cross-over designs [52, 53, 55]. Selected studies enrolled subjects with hypertension [36, 40, 47, 48, 50, 52–56, 58, 59, 62, 71–75, 86–89],metabolic syndrome [44, 51, 69, 80, 82], healthy persons [34, 35, 37, 49, 60, 61, 63, 65–67, 70, 76, 78, 83, 84, 91, 94], type 2 diabetes mellitus [46, 64, 90, 92], peripheral artery disease [68], symptomatic peripheral artery disease [81], chronic hemodialysis patient [33], Parkinson disease [39], Prehypertension [42], HIV/AIDS [85], chronic heart failure [41], coronary artery disease [43], Stable coronary artery disease [79], coronary heart disease [38], cardiovascular risk [45, 93]. The investigations were conducted in various countries, including the India [43, 59, 78, 83, 90], China [35, 37, 38, 44, 47, 51, 60, 63, 65, 66, 79], Iran [36, 70, 76], Sri Lanka [46], Brazil [39, 50, 55, 56, 67, 68, 81, 86–88, 91, 92, 94, 95], Taiwan [40, 48, 61, 62, 82, 93, 95], East Africa [34, 49], South Africa [64], Tunisia [33], Colombia [45, 69], Thailand [71], Nigeria [41, 52, 53, 72–75, 85], one multinational study in (Argentina،Guatemala، Peru) [42], Egypt [84], Chile [80], and Pakistan [54]. Characteristics of the included studies are abstracted in Table 1.
Table 1.
Characteristic of included studies in meta-analysis
| Studies | Year | Country | Study Design | Participant | Sample size and Sex | Sample size | Trial Duration (Week) |
Means Age | Means BMI | Health Status | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IG | CG | IG | CG | IG | CG | IG | CG | |||||||
| Mandal et al | 2021 | India | RCT | Yoga program (asana, pranayama, and deep relaxation technique) | No intervention | 110, Both | 58 | 52 | 12 | 35 ± 7.9 | 32.5 ± 6.8 | 25.9 ± 4.2 | 24.4 ± 4.4 | Healthy |
| Yu et al | 2021 | China | CT | Brisk walking level 1 | Daily walking | 277, Both | 92 | 185 | 12 | 37.0 ± 9.9 | 37.0 ± 9.9 | 23.9 ± 3.9 | 23.9 ± 3.9 | Healthy and sedentary lifestyle |
| Yu et al | 2021 | China | CT | Brisk walking level 2 | Daily walking | 359, Both | 174 | 185 | 12 | 37.0 ± 9.9 | 37.0 ± 9.9 | 23.9 ± 3.9 | 23.9 ± 3.9 | Healthy and sedentary lifestyle |
| Yu et al | 2021 | China | CT | Brisk walking level 3 | Daily walking | 422, Both | 237 | 185 | 12 | 37.0 ± 9.9 | 37.0 ± 9.9 | 23.9 ± 3.9 | 23.9 ± 3.9 | Healthy and sedentary lifestyle |
| Gu et al | 2020 | China | Prospective self- CT | Walking and self-monitoring by a pedometer | No Intervention | 262, Both | 150 | 112 | 100 DAY | NR | NR | 23.7 ± 3.0 | 22.7 ± 3.1 | Healthy |
| Hooshmand-moghadam et al | 2020 | Iran | RCT | Resistance training (RT) | No Intervention | 24, Male | 12 | 12 | 18 | 63.2 ± 8.09 | 62.5 ± 10.25 | 28.2 ± 2.25 | 28.4 ± 2.25 | Hypertension |
| Hooshmand-moghadam et al | 2020 | Iran | RCT | RT and Saffron (RTS) | No Intervention | 24, Male | 12 | 12 | 18 | 63.5 ± 8.09 | 62.5 ± 10.25 | 28.4 ± 2.25 | 28.4 ± 2.25 | Hypertension |
| Ranasinghe et al | 2020 | Sri Lanka | RCT | Aerobic Exercise Training | Standard Care | 58, Both | 28 | 30 | 12 | 52 ± 9.8 | 49.3 ± 7.0 | 26.77 ± 4.23 | 25.85 ± 4.38 | Type 2 Diabetes Mellitus |
| Ranasinghe et al | 2020 | Sri Lanka | RCT | Resistance Exercise Training | Standard Care | 58, Both | 28 | 30 | 12 | 49.0 ± 9.2 | 49.3 ± 7.0 | 26.86 ± 4.23 | 25.85 ± 4.39 | Type 2 Diabetes Mellitus |
| Rosa Santos et al | 2020 | Brazil | RCT | Resistance training | No intervention | 122, Both | 61 | 61 | 12 | 13.39 ± 0.97 | 13 ± 1.04 | 20.30 ± 2.99 | 18.39 ± 2.76 | Healthy |
| Ac et al | 2019 | Brazil | RCT | Isometric handgrip training (IHT) | Placebo | 79, Both | 29 | 50 | 8 | 66 ± 12 | 67 ± 11 | 27 ± 5 | 26 ± 7 | Peripheral artery disease |
| Ac et al | 2019 | Brazil | RCT | Isometric handgrip training (IHT) | Placebo | 73, Both | 27 | 46 | 8 | 66 ± 12 | 67 ± 11 | 27 ± 5 | 26 ± 7 | Peripheral artery disease |
| Ac et al | 2019 | Brazil | RCT | Isometric handgrip training (IHT) | Placebo | 102, Both | 50 | 52 | 8 | 66 ± 12 | 67 ± 11 | 27 ± 5 | 26 ± 7 | Peripheral artery disease |
| Ac et al | 2019 | Brazil | RCT | Isometric handgrip training (IHT) | Placebo | 98, Both | 47 | 51 | 8 | 66 ± 12 | 67 ± 11 | 27 ± 5 | 26 ± 7 | Peripheral artery disease |
| Leung et al | 2019 | China | RCT | Tai Chi Exercise Program | Usual Daily Activities | 35, Both | 18 | 17 | 12 | 62.19 ± 5.93 | 65.52 ± 9.34 | 27.40 ± 4.82 | 27.25 ± 4.34 | Metabolic Syndrome |
| Mouodi et al | 2019 | Iran | Population-based RCT | Nutrition and physical activity training classes (aerobic), individual nutrition consultation, educational package and weekly aerobic exercises | Educational package | 203, Both | 106 | 97 | 16 | 49.8 ± 5.2 | 49.5 ± 5.7 | 28.3 ± 4.3 | 28.2 ± 4.3 | Healthy |
| Mouodi et al | 2019 | Iran | Population-based RCT | Nutrition training classes, individual nutrition consultation and educational package | Educational package | 195, Both | 98 | 97 | 16 | 49.8 ± 5.4 | 49.5 ± 5.7 | 28.2 ± 4.6 | 28.2 ± 4.3 | Healthy |
| Chhugani et al | 2018 | India | A Controlled Pilot Study | Integrated Yoga (IY) | No Intervention | 30, Female | 17 | 13 | 4 | 34 ± 8.4 | 34 ± 8.4 | NR | NR | Healthy |
| Gradidg et al | 2018 | South Africa | RCT | Physical activity (walking) | No Intervention | 115, Female | 49 | 66 | 12 | NR | NR | 32.1 ± 6.61 | 31 ± 6.57 | Healthy |
| Ma et al | 2018 | China | CT | Tai Chi Training | Usual Care | 158, Both | 79 | 79 | 24 | 70.24 ± 10.25 | 69.71 ± 10.84 | 26.01 ± 3.38 | 25.96 ± 2.91 | Hypertension |
| Cavalcante et al | 2017 | Brazil | Prospective, controlled, crossover | Arm crank Exercise | Control session | 11, Male | 4 | 7 | NR | 64 ± 8 | 64 ± 8 | 25.05 | 25.05 | Symptomatic peripheral artery disease |
| Frih et al | 2017 | Tunisia | RCT, SB | Resistance and endurance training exercises | No Intervention | 41, Male | 21 | 20 | 16 | 64.2 ± 3.4 | 65.2 ± 3.1 | 25.4 ± 2.8 | 24.3 ± 3.2 | Chronic hemodialysis patient |
| Kanegusuku et al | 2017 | Brazil | RCT | Resistance Training | Usual Lifestyle | 27, Both | 15 | 12 | 12 | 67 ± 8 | 63 ± 8 | 26.3 ± 3.5 | 25.9 ± 4.3 | Parkinson disease |
| Kanegusuku et al | 2017 | Brazil | RCT | Resistance Training | Usual Lifestyle | 31, Both | 15 | 16 | 12 | 67 ± 8 | 68 ± 10 | 26.3 ± 3.5 | 24.8 ± 3.0 | Parkinson disease |
| Chang et al | 2016 | Taiwan | RCT | Community-based program (provided exercise environments, exercise skills and volunteers’ reminding) | No Intervention | 131, Both | 65 | 66 | 24 | 55.41 ± 14.05 | 55.08 ± 14.40 | 29.95 ± 3.11 | 30.42 ± 2.59 | Obese individuals (BMI ≥ 27 kg/m2) with Metabolic syndrome |
| Farinatti et al | 2016 | Brazil | CT | Home-based exercise programs (light- to moderate-intensity walking and complementary stretching exercises three times per week) | No Intervention | 43, Both | 29 | 14 | 64 | 53 ± 11 | 48 ± 5 | 30.5 ± 4.6 | 30.4 ± 4.5 | Hypertension |
| Gallo-Villegas et al | 2016 | Colombia | RCT | Aerobic exercise at 3 times a week, muscle strength training twice a week, and the nutrition education program 2-h workshops each week | General recommendations about exercise and healthy eating | 59, Both | 30 | 29 | 12 | 49.2 ± 8.7 | 52. ± 6.8 | 29 ± 3 | 28.3 ± 4 | Metabolic syndrome |
| Rubinstein et al | 2016 | Argentina،Guatemala، Peru | RCT | Mobile phone-based health intervention (physical activity and diet) | Usual Care | 553, Both | 266 | 287 | 48 | 43.6 ± 8.4 | 43.2 ± 8.4 | 30.2 ± 5.2 | 30.8 ± 5.3 | Prehypertension |
| Lau et al | 2015 | China | RCT | Hatha Yoga Intervention | Routine Activities and not to Begin any Exercise | 87, Both | 44 | 43 | 12 | 52.44 ± 7.15 | 51.52 ± 7.78 | 24.44 ± 3.84 | 25.90 ± 3.90 | with Metabolic Syndrome |
| Lau et al | 2015 | China | RCT | Hatha Yoga Intervention | Routine Activities and not to Begin any Exercise | 86, Both | 43 | 43 | 12 | 52.44 ± 7.15 | 51.52 ± 7.78 | 24.44 ± 3.84 | 25.90 ± 3.90 | without Metabolic Syndrome |
| Lau et al | 2015 | China | RCT | Hatha Yoga Intervention | Routine Activities and not to Begin any Exercise | 173, Both | 87 | 86 | 12 | 52.44 ± 7.15 | 51.52 ± 7.78 | 24.44 ± 3.84 | 25.90 ± 3.90 | with/without Metabolic Syndrome |
| Ezema et al | 2014 | Nigeria | RCT | Moderate intensity continuous exercise training + Conventional therapy | Conventional therapy | 30, Both | 15 | 15 | 8 | 40.07 ± 9.72 | 32.47 ± 10.41 | 28.65 ± 7.11 | 29.01 ± 4.42 | HIV/AIDS |
| Sikiru et al | 2014 | Nigeria | Cross over, DB, RCT | Bicycle ergometer at a low intensity, 8 weeks of continuous training, 3 times per week | Remain Sedentary | 217, Male | 112 | 105 | 8 | 58.63 ± 7.22 | 58.27 ± 6.24 | 22.48 ± 2.89 | 24.16 ± 4.91 | Hypertension |
| Sujatha et al | 2014 | India | RCT | Yoga program | No intervention | 238, Both | 118 | 120 | 12 | NR | NR | 27.61 ± 4.96 | 27.29 ± 5.28 | Hypertension |
| Wu et al | 2014 | China | RCT | Lifestyle: diet and moderate-intensity aerobic or endurance-type activities, such as pulse-controlled brisk walking, jogging, dancing | Usual eating patterns and activities | 100, Female | 53 | 47 | 48 | 50.62 ± 3.92 | 49.06 ± 4.30 | 24.14 ± 2.70 | 24.33 ± 3.24 | Healthy |
| Yan et al | 2014 | East Africa | RCT | low intensity exercise (LEX), vigorous-intensity exercise (VEX) | No intervention | 41, Male | 31 | 10 | 48 | 53 ± 2 | 55 ± 3 | 27.2 ± 0.7 | 27 ± 1.3 | Type 2 diabetes mellitus |
| Yu et al | 2014 | China | Community-based CT | Lifestyle: improving physical activity (activity between 3 and 6 METs and lasting at least 6 min) and dietary patterns | Usual standard health care | 273, Both | 175 | 98 | 24 | 60.9 ± 10.2 | 60.1 ± 10 | NR | NR | Older adults with diabetes and hypertension |
| Hou et al | 2014 | China | RCT | Chinese traditional healthcare exercises (CTHE) | No Intervention | 136, Both | 68 | 68 | 7 | 13.9 | 13.7 | NR | NR | Healthy |
| Ajiboye et al | 2013 | Nigeria | RCT | Exercise Training (12 weeks of aerobic and resistance training 3 session per week) | Usual Drug Therapy | 32, Both | 17 | 15 | 12 | 54.1 ± 9.07 | 53.7 ± 11.6 | 30.4 ± 5.36 | 27 ± 7.35 | Chronic Heart Failure |
| Brito et al | 2013 | Brazil | RCT | High-intensity resistance exercise (50%) | Control session | 20, Female | 10 | 10 | NR | 65.5 ± 3 | 65.5 ± 3 | 28.7 ± 3 | 28.7 ± 3 | Hypertensive elderly |
| Brito et al | 2013 | Brazil | RCT | High-intensity resistance exercise (80%) | Control session | 20, Female | 10 | 10 | NR | 65.5 ± 3 | 65.5 ± 3 | 28.7 ± 3 | 28.7 ± 3 | Hypertensive elderly |
| Lamina et al | 2013 | Nigeria | RCT | Aerobic exercise training program | Sedentary | 245, Male | 140 | 105 | 8 | 58.40 ± 6.91 | 58.27 ± 6.24 | 24.96 ± 3.88 | 24.16 ± 4.91 | Essential hypertension |
| Pal et al | 2013 | India | RCT | Medication + Yoga | Medication Only | 208, Both | 105 | 103 | 72 | 59.1 ± 9.9 | 56.4 ± 10.9 | 24.8 ± 3.8 | 25.1 ± 4.8 | Coronary artery disease |
| Sikiru et al | 2013 | Nigeria | Cross over, DB, RCT | Bicycle ergometer at a low intensity, 8 weeks of continuous training, 3 times per week | Remain Sedentary | 245, Male | 140 | 105 | 8 | 58.40 ± 6.91 | 58.27 ± 6.24 | 24.96 ± 3.88 | 24.16 ± 4.91 | Mild to Moderate Hypertension |
| Tsai et al | 2013 | Taiwan | Non-randomized quasi-experimental design | Low intensity exercise | No intervention | 59, Both | 30 | 29 | 12 | 34.8 ± 7.0 | 33.3 ± 9.4 | NR | NR | Healthy |
| Tsai et al | 2013 | Taiwan | Non-randomized quasi-experimental design | High intensity exercise | No intervention | 59, Both | 30 | 29 | 12 | 41.0 ± 7.2 | 33.3 ± 9.4 | NR | NR | Healthy |
| Lamina et al | 2012 | Nigeria | RCT | Aerobic exercise training (bicycle ergometer) | No intervention | 217, Male | 112 | 105 | 8 | 58.63 ± 7.22 | 58.27 ± 6.24 | 22.92 ± 2.2 | 23.37 ± 3.87 | Essential hypertension |
| Lo et al | 2012 | Taiwan | RCT | Yang-Style Tai Chi Exercise Program | Routine Care | 58, Both | 27 | 31 | 8 | 58.47 ± 7.46 | 58.47 ± 7.46 | NR | NR | Hypertension |
| M. Cunha et al | 2012 | Brazil | Cross over،RCT | Aerobic and Water Exercise | Did not enter the pool and did not exercise | 32, Female | 16 | 16 | 2 DAYS | 66 ± 2.94 | 66 ± 2.94 | 27.32 ± 4.30 | 27.32 ± 4.30 | Hypertensive elderly women |
| Mizuno et al | 2012 | Brazil | RCT | Yoga Exercises | Gave Up Yoga Classes | 33, Both | 17 | 16 | 16 | 67 ± 7 | 62 ± 12 | 27.4 ± 4.4 | 26.4 ± 5.3 | Arterial Hypertension |
| Vianna et al | 2012 | Brazil | CT | Aerobics exercises (walking, hydrogymnastics, weight-training exercises, and stretching exercises) | Was advised to keep their daily routines | 70, Both | 35 | 35 | 16 | 68.66 ± 5.93 | 69.8 ± 8.05 | 26.74 ± 3.81 | 26.09 ± 4.58 | Healthy |
| Kanegusuku et al | 2011 | Brazil | RCT | Strength training | Normotensive older adults who did not take part in any training program | 24, Both | 13 | 11 | 16 | 63 ± 3.60 | 63 ± 3.31 | 27.4 ± 5.76 | 27.3 ± 4.97 | Healthy |
| Kanegusuku et al | 2011 | Brazil | RCT | Strength training | Normotensive older adults who did not take part in any training program | 24, Both | 13 | 11 | 16 | 63 ± 3.60 | 63 ± 3.31 | 27.4 ± 5.76 | 27.3 ± 4.97 | Healthy |
| Kanegusuku et al | 2011 | Brazil | RCT | Power training | Normotensive older adults who did not take part in any training program | 26, Both | 15 | 11 | 16 | 65 ± 3.87 | 63 ± 3.31 | 26.5 ± 4.64 | 27.3 ± 4.97 | Healthy |
| Kanegusuku et al | 2011 | Brazil | RCT | Power training | Normotensive older adults who did not take part in any training program | 26, Both | 15 | 11 | 16 | 65 ± 3.87 | 63 ± 3.31 | 26.5 ± 4.64 | 27.3 ± 4.97 | Healthy |
| Lamina et al | 2011 | Nigeria | RCT, DB | Exercise training (interval) | Sedentary | 245, Male | 140 | 105 | 8 | 58.40 ± 6.91 | 58.27 ± 6.24 | 24.96 ± 3.88 | 24.16 ± 4.91 | Essential mild to moderate hypertension |
| Lamina et al | 2011 | Nigeria | RCT, DB | Exercise on a bicycle ergometer | No intervention | 245, Male | 140 | 105 | 8 | 58.63 ± 7.22 | 58.27 ± 6.24 | 22.48 ± 2.89 | 24.16 ± 4.91 | Essential hypertension |
| Lamina et al | 2011 | Nigeria | RCT, DB | Exercise on a bicycle ergometer | No intervention | 217, Male | 112 | 105 | 8 | 58.40 ± 6.91 | 58.27 ± 6.24 | 24.96 ± 3.88 | 24.16 ± 4.91 | Essential hypertension |
| Luk et al | 2011 | China | RCT | 12 Each session would last for an hour with combined endurance and resistance exercise, beginning with warm-up stretching exercise | Non-exercise | 64, Both | 32 | 32 | 8 | 67.7 ± 9.0 | 66.6 ± 7.9 | 24.7 ± 2.4 | 25.1 ± 2.6 | Stable coronary artery disease |
| Mortimer et al | 2011 | South Africa | RCT | Handgrip Training | Without Exercising | 18, Female | 9 | 9 | 5 DAYS | 47.88 ± 5.4 | 49.88 ± 4.2 | 24.92 ± 3.9 | 27.26 ± 2.4 | Healthy |
| Ansari et al | 2010 | Egypt | Quantitative study | One hour of moderate exercise three times a week for three months | No Intervention | 90, Female | 45 | 45 | 12 | 15.7 ± 1.8 | 15.4 ± 1.6 | 21.6 ± 4.5 | 21.4 ± 3.8 | Healthy |
| Ansari et al | 2010 | Egypt | Quantitative study | One hour of moderate exercise three times a week for three months | No Intervention | 70, Male | 35 | 35 | 12 | 15.7 ± 1.8 | 15.4 ± 1.6 | 20.9 ± 4.1 | 21.2 ± 3.6 | Healthy |
| Monteiro et al | 2010 | Brazil | CT | Aerobic training program | Attended educational lectures once a week | 22, Female | 11 | 11 | 13 | 61 ± 1.18 | 60.2 ± 0.4 | 27.5 ± 4 | 28.1 ± 6.61 | Elderly women with type-2 diabetes mellitus |
| Mujica et al | 2010 | Chile | RCT | Exercise program (walking) | No intervention | 51, Both | 27 | 24 | 18 | 49.4 ± 6.2 | 51.1 ± 5.3 | 31.8 ± 4.0 | 29.6 ± 3.8 | Metabolic syndrome |
| Arora et al | 2009 | India | RCT | Aerobic exercise | Went under no training | 20, Both | 10 | 10 | 8 | 52.2 ± 9.3 | 58.4 ± 1.8 | 26.23 ± 3.2 | 24.98 ± 3 | With type 2 diabetes |
| Arora et al | 2009 | India | RCT | Progressive resistance training (PRT) | Went under no training | 19, Both | 9 | 10 | 8 | 49.6 ± 5.2 | 58.4 ± 1.8 | 26.99 ± 4.1 | 24.98 ± 3 | With type 2 diabetes |
| Bündchen et al | 2009 | Brazil | RCT | Aerobic and resistance exercises 30 to 60 min 3 times a week | Did not participate in the exercise programs | 111, Both | 57 | 54 | 12 | 58 ± 8.9 | 60 ± 7.7 | 30.1 ± 5 | 32.3 ± 7 | Hypertensive patients with overweight or obesity |
| Jafar et al | 2009 | Pakistan | Cluster RCT | General Practitioner (GP) AND Home Health Education (HHE) | No Intervention | 658, Both | 332 | 326 | 96 | 54 ± 11.5 | 53.3 ± 11.5 | NR | NR | Hypertension |
| Jafar et al | 2009 | Pakistan | Cluster RCT | General Practitioner (GP) Education | No Intervention | 661, Both | 335 | 326 | 96 | 55.3 ± 11.5 | 53.3 ± 11.5 | NR | NR | Hypertension |
| Jafar et al | 2009 | Pakistan | Cluster RCT | Home Health Education (HHE) | No Intervention | 674, Both | 348 | 326 | 96 | 52.7 ± 11.4 | 53.3 ± 11.5 | NR | NR | Hypertension |
| Barroso et al | 2008 | Brazil | RCT | Non-pharmacological treatment and physical activity consisting of 1-h sessions, 3 times a week | Non-pharmacological treatment (NPT) | 35, Both | 22 | 13 | 24 | 66.5 ± 4 | 70.8 ± 6.3 | NR | NR | With stage I hypertension who were not using antihypertensive medication |
| Meirelles et al | 2008 | Brazil | CT | Aerobic, three times a week for 12 weeks | Sedentary hypertensive patients on stage 1 not doing the exercise | 19, Both | 13 | 6 | 12 | 49 ± 1 | 50 ± 4 | 30 ± 1 | 32 ± 2 | Sedentary hypertensive patients on stage 1 |
| Meirelles et al | 2008 | Brazil | CT | Aerobic, three times a week for 12 weeks | Sedentary hypertensive patients on stage 1 not doing the exercise | 19, Both | 13 | 6 | 12 | 49 ± 1 | 50 ± 4 | 30 ± 1 | 32 ± 2 | Sedentary hypertensive patients on stage 1 |
| Jiang et al | 2007 | China | RCT | Walking Performance, Step II diet | Routine care | 167, Both | 83 | 84 | 24 | 62.11 ± 7.44 | 61.37 ± 7.61 | NR | NR | Coronary Heart Disease Patients |
| Lee et al | 2007 | Taiwan | RCT | Walking intervention | Usual Primary Health Care | 184, Both | 91 | 93 | 24 | 71.3 ± 6.4 | 71.3 ± 5.7 | 25.4 ± 3.8 | 25.31 ± 3.5 | Mild to Moderate Hypertension |
| Pazoki et al | 2007 | Iran | RCT | 10 min per day of moderate-intensity physical activity and encouraged to do 30 min of physical activity daily and healthy eating | No intervention | 335, Female | 170 | 165 | 8 | NR | NR | 28.02 ± 4.74 | 27.82 ± 5.39 | Healthy |
| Wu et al | 2007 | Taiwan | CT | Treadmill training program for 30 min each time, 3 times a week | Maintained their previous lifestyles | 36, Female | 18 | 18 | 8 | 49.7 ± 6.1 | 51.8 ± 6.4 | 28.4 ± 3.1 | 27.3 ± 3.7 | Cardiovascular risk |
| Mendivil et al | 2006 | Colombia | RCT | Nutritional Intervention Program plus physical activity (Aerobic dancing, soccer, basketball, recreational kickboxing, and a few resistance activities to strengthen localized muscular groups) | Nutritional Intervention Program | 49, Both | 28 | 21 | 16 | 50.03 ± 8.09 | 53.04 ± 8.18 | 26.3 ± 4.72 | 27.5 ± 4.09 | Cardiovascular risk |
| McCaffrey et al | 2005 | Thailand | CT | Yoga program | Typical outpatient teaching about hypertension | 54, Both | 27 | 27 | 8 | 56.7 | 56.2 | 25.74 ± 2.87 | 25.32 ± 3.19 | Hypertensive persons |
| Thomas et al | 2005 | China | Longitudinal, randomized, controlled intervention | Tai Chi | Usual level of physical activity | 142, Both | 64 | 78 | 48 | 68.9 ± 2.8 | 69 ± 3 | 23.8 ± 3.9 | 24.2 ± 3 | Healthy elderly subjects |
| Thomas et al | 2005 | China | Longitudinal, randomized, controlled intervention | Resistance training | Usual level of physical activity | 143, Both | 65 | 78 | 48 | 69.1 ± 3.2 | 69 ± 3 | 24.2 ± 3.8 | 24.2 ± 3 | Healthy elderly subjects |
| Tsai et al | 2003 | Taiwan | RCT | Tai Chi Chuan exercise training program | Sedentary life | 76, Both | 37 | 39 | 12 | 51.6 ± 16.3 | 50.5 ± 9.8 | 23.8 ± 2.4 | 24.1 ± 1.8 | Stage I hypertension |
| Tsai et al | 2003 | Taiwan | RCT | Tai Chi Chuan exercise training program 3 times per week | Maintained their usual lifestyle behaviors | 76, Both | 37 | 39 | 12 | 51.6 ± 16.3 | 50.5 ± 9.8 | 23.8 ± 2.4 | 24.1 ± 1.8 | Stage I hypertension |
| Tsai et al | 2002 | Taiwan | RCT | Moderate-intensity exercise | No exercise | 23, Both | 12 | 11 | 12 | 49.6 ± 9.3 | 46.2 ± 5.6 | 26.1 ± 4.5 | 25 ± 1.8 | Mild hypertensive patients |
Abbreviations: IG Intervention group, CG Control group, DB Double-blinded, CT Control trial, SB Single-blinded, PC Placebo-controlled, CO Controlled, RA Randomized, NR Not reported, F Female, M Male, NR Not reported
Quality assessment
The Cochrane scoring system was used to assess the quality of the included studies (Table 2). This system consists of seven criteria to evaluate the risk of bias, which are as follows: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other biases. Bias was assessed (high, low, or unclear) for individual elements, interpreted as high risk, low risk, and unknown risk, respectively. We classified 34 studies as low risk [35–37, 41–43, 45–47, 49, 52–54, 56, 62, 63, 72–76, 79, 81–88, 90–95], 16 moderate risk [33, 34, 50, 58–60, 64–67, 69–72, 78, 80], and 10 high-risk studies [38–40, 44, 48, 51, 55, 61, 68, 79].
Effect of physical activity on SBP
A total of sixty studies, encompassing 84 effect sizes, were analyzed in this research. The studies involved a combined sample size of 11,002 participants, with 5,630 individuals classified as cases and 5,372 as controls. The primary focus of these studies was to investigate the impact of physical exercise on SBP. The results indicated that physical activity had a statistically significant impact on SBP (weighted mean difference [WMD]: -7.70 mmHg; 95% confidence interval [CI]: -9.50, -5.91; p < 0.001) (Fig. 2A). Nevertheless, a notable level of variability was observed (I2 = 97.3%). The subgroup analysis revealed that several factors, including type of physical activities, gender, the health status of the research participants, baseline SBP, BMI, trial duration, ethnicity, and age, accounted for the observed variability. The subgroup analysis revealed that the observed outcomes remained statistically significant when the baseline SBP was equal to or less than 120 mmHg, chose walking as a physical activity as well as in groups characterized as healthy. Healthy individuals had the greatest reduction in heterogeneity among the groups, also in people with African ethnic and male gender, they had the greatest reduction in SBP, but the heterogeneity in this group was very high.(Table 3).
Fig. 2.
A Forest plot of randomized controlled trials to investigate the effect of physical activity on systolic blood pressure. B Forest plot of randomized controlled trials to investigate the effect of physical activity on diastolic blood pressure
Effect of physical activity on DBP
A total of fifty-six studies, comprising 78 effect sizes, were included in the analysis. These investigations involved a combined sample size of 10,721 participants, with 5,495 cases and 5,226controls. The primary focus of these research was to investigate the impact of physical activity on DBP. The results indicate that physical activity had a substantial impact on DBP (weighted mean difference [WMD]: -3.60 mmHg; 95% confidence interval [CI]: -4.48, -2.73; p < 0.001) as shown in Fig. 2B. Nevertheless, a notable level of diversity was observed, as indicated by the I2 statistic of 94.5%. The subgroup analysis revealed that many factors, including type of physical activities, gender, the health status of the research participants, baseline DBP, baseline BMI, trial duration, ethnicity, and age, accounted for the observed variability. The subgroup analysis revealed that the observed outcomes continued to be statistically significant when the baseline DBP was equal to or less than 80 mmHg, chose walking as a physical activity as well as in populations without any pre-existing health conditions. People who had lower DBP equal to 80 had the greatest reduction in heterogeneity among the groups, also in people with male gender, they had the greatest reduction in DBP, but the heterogeneity in this group was very high. (Table 3).
Sensitivity analysis and publication bias
The results of the sensitivity analysis revealed that the magnitude of the overall effect regarding the association between physical activity on SBP and DBP did not depend on a single study. Also, Visual inspection of the funnel plot revealed no evidence of publication bias in the studies that evaluated the effect of physical activity on SBP (Begg: 0.454) and DBP (Begg: 0.360) (Fig. 3A, B).
Fig. 3.
A Funnel plot detailing publication bias in the studies reporting the impact of physical activity on systolic blood pressure. B the sensitivity analysis of included studies reporting the impact of physical activity on diastolic blood pressure
Meta-regression analysis
Meta-regression was used to investigate the potential linear association between duration of intervention, age, BMI, baseline SBP, and DBP with changes in SBP and DBP. Accordingly, meta-regression analysis did reveal a significant association between the duration of intervention and changes in SBP (P linearity = < 0.001) and DBP (P linearity = 0.004). The findings indicate that the longer the intervention period, the less impact it has on both SBP and DBP levels. In other words, the effectiveness of the intervention decreases as its duration increases.(Fig. 4A, B). Also, there was a significant association between baseline SBP and changes in SBP (P linearity = < 0.001) and between baseline DBP and changes in DBP (Plinearity = 0.001) (Figs. 4C, D). Based on the graphs, it can be observed that as the baseline BP level increases, there is a corresponding increase in the blood pressure level during physical activity. In other words, the higher the baseline BP, the greater decrease in the level of blood pressure was observed. However, meta-regression analysis did not show any significant association between age and changes in SBP (Plinearity = 0.282), DBP (Plinearity = 0.631), and BMI with changes in SBP (Plinearity = 0.871) and DBP (Plinearity = 0.208) (Figs. 4E–H).
Fig. 4.
Result of meta-regression analysis for exploring the potential linear association between duration of intervention (A, B), baseline SBP and DBP (C, D), age (E, F) and BMI (G, H) with changes in SBP and DBP
Grading of evidence
The GRADE protocol was used to assess the certainty of the evidence (Table 4). Accordingly, studies investigating the effect of physical activity on SBP and DBP were regarded as moderate quality due to the high heterogeneity between studies.
Discussion
We conducted a meta-analysis that indicates a relation between blood pressure (SBP and DBP) and physical activity (PA). Our findings also, suggest that PA is a good lifestyle intervention for reducing BP. This study represents the first meta-analysis conducted to examine the effects of PA on blood pressure across low and middle-income countries (LMICs), as far as our current understanding allows us to ascertain. The rigorous evaluation of the outcomes of this investigation is crucial owing to the significant heterogeneity seen throughout the analyses. To account for this heterogeneity, subgroup analyses were performed based on type of physical activities (Resistant training, Aerobic training,Combined exercise), trial duration (≤ 12 vs. > 12 weeks), age (≤ 50 vs. > 50 years), health status (healthy vs. disease vs. both), gender (male, female, both), ethnicity (Asian vs. African vs. American), baseline BMI (normal (18.5–24.9) vs. overweight (25–29.9) vs. obese (≥ 30)), baseline SBP (≤ 120 vs. > 120 mmHg), and baseline DBP (≤ 80 vs. > 80 mmHg). Subgroup analysis showed that the results remained significant when baseline SBP ≤ 120 mmHg and in healthy populations for SBP and the results remained significant when baseline DBP ≤ 80 mmHg and in healthy populations in DBP, respectively. The findings of our study align with other research indicating that engaging in leisure-time PA has the potential to decrease both SBP and DBP levels [96]. Nevertheless, it is important to acknowledge a constraint of this study, namely the limited number of studies that were encompassed, potentially impeding the extent to which the findings can be applied to a broader population [96]. Moreover, another recent study supports the perspective that a higher intensity of PA is associated with larger reductions in DBP [97]. The primary focus of our analysis was on LMICs, and it encompassed a substantial number of RCTs. In contrast, previous investigations have examined this correlation in smaller cohorts and did not specifically target this particular demographic. Different kinds of medication are used in the treatment of hypertension and they can lower the BP in different ways. The ACE enzyme facilitates the transformation of angiotensin I to angiotensin II, which results in heightened blood pressure. ACEIs impede this process, whereas ARBs obstruct the receptors. CCBs curtail vascular resistance by hindering calcium channels. Diuretics decrease blood pressure by impeding sodium reabsorption at different segments of the nephron. Thiazides target the distal convoluted tubule to check the sodium-chloride cotransporter [98]. However, the role of PA in reducing BP can be as important as pharmacological treatment [16]. The precise mechanisms via which PA mitigates the development of hypertension are still uncertain and subject to debate. This is mostly attributed to the complex and multifaceted character of hypertension, as well as the ambiguous ways in which several contributing factors interact with each other. According to the research conducted by Millar and colleagues (Millar PJ et al.), it is proposed that the decrease in resting blood pressure (BP) resulting from isometric exercise training necessitates modifications in either one or both of the factors that determine mean arterial pressure, namely cardiac output and total peripheral resistance [99]. Following mechanisms can be mentioned: PA induces a vasodilatory response that widens blood vessels and diminishes their constriction [100, 101]. This response includes decreasing cardiac output, sympathetic nervous system activity, levels of plasma norepinephrine, and total peripheral resistance [102, 103]. One of the other mechanisms is improving endothelial function which can be compromised in individuals with hypertension [102, 104–106]. Based on a comprehensive study of existing research studies and a synthesis of their findings, it has been determined that aerobic endurance training has the capacity to lower BP by diminishing vascular resistance. This effect is believed to be mediated via the involvement of the sympathetic nervous system and the renin-angiotensin system [107]. Furthermore, hyperinsulinemia and insulin resistance can lead to hypertension. This happens because insulin causes the body to hold onto sodium, makes the nervous system more active, and causes the proliferation of muscles inside blood vessels [102, 108]. Exercise improves insulin sensitivity, providing another possible mechanism for its antihypertensive effect [102, 109]. Additionally, PA may decrease plasma viscosity, leading to less resistance in blood vessels and improving blood flow [102]. Apart from what was previously mentioned, doing exercise can lower the chance of getting high BP by helping maintain a healthy weight and reducing fat in the body which is the main reason for high BP [102, 110–113]. Besides affecting the constriction and relaxation of blood vessels, exercise also helps to reduce BP by decreasing the levels of oxidative stress and inflammation [100]. According to a recent systematic review and meta-analysis of randomized clinical trials, Strength training can help reduce BP by improving cardiovascular function, increasing muscle strength and endurance, promoting weight loss, and reducing peripheral vascular resistance [114]. It can also improve insulin sensitivity and glucose metabolism, which can contribute to a reduction in BP [114]. One possible explanation for this finding could be that strength training makes the body produce more nitric oxide, which in turn leads to the vasodilation of blood vessels [114].
Strengths and limitations
There are some noteworthy strengths inherent in this study that warrant acknowledgment. This study encompasses a thorough investigation and examination of the existing research about the influence of physical activity on blood pressure. The search technique employed in this study was comprehensive and encompassed a variety of databases, hence reducing the likelihood of inadvertently omitting relevant studies. Furthermore, we have incorporated data from 19 LMICs, so enhancing the generalizability of our findings to all nations of similar economic status. Furthermore, our investigations did not identify any potential sources for conducting sensitivity analysis. Nevertheless, it is important to acknowledge the presence of several limitations in our study that may have been mitigated. The RCTs that were included in our investigation exhibited variability in terms of both sample size and length of the intervention. Moreover, it is worth noting that the individuals in each study exhibited varying health statuses, as well as diverse dietary and lifestyle patterns, which may have influenced the outcomes observed.
Conclusion
Recent research consistently validates the connection between PA and BP. Given the rise in high BP cases in LMICs, notable emphasis has been placed on the fact that our comprehensive analysis also verifies the substantial impact of PA in lowering BP within these countries.
Supplementary Information
Acknowledgements
We are very grateful to all the colleagues who helped us in carrying out this project.
Declaration of generative AI in scientific writing
The authors state that during the writing or even the search for this study, they did not use artificial intelligence or artificial intelligence-assisted technologies.
Authors’ contributions
Conceptualization, V.M, and M.H.; methodology, M.Y. and F.K; validation, V.M., and M.V; investigation and data curation. V.M, and M.H and R.J; writing—original draft preparation, V.M and M.H; writing—review and editing, M.H and M.V; visualization, V.M., and M.H; supervision, M.H.; project administration, M.H.; All authors have read and agreed to the published version of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
The present research was approved and supported by the Golestan University of Medical Sciences, under ethical code IR.GOUMS.REC.1402.393 and fund number [10-113804].
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Unger T, Borghi C, Charchar F, Khan NA, Poulter NR, Prabhakaran D, et al. 2020 International Society of Hypertension global hypertension practice guidelines. Hypertension. 2020;75(6):1334–57. 10.1161/HYPERTENSIONAHA.120.15026 [DOI] [PubMed] [Google Scholar]
- 2.Carey RM, Moran AE, Whelton PK. Treatment of hypertension: a review. JAMA. 2022;328(18):1849–61. 10.1001/jama.2022.19590 [DOI] [PubMed] [Google Scholar]
- 3.Keating SE, Coombes JS, Stowasser M, Bailey TG. The role of exercise in patients with obesity and hypertension. Curr Hypertens Rep. 2020;22(10):77. 10.1007/s11906-020-01087-5 [DOI] [PubMed] [Google Scholar]
- 4.Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, et al. Heart disease and stroke statistics-2020 update: a report from the American Heart Association. Circulation. 2020;141(9):e139–596. 10.1161/CIR.0000000000000757 [DOI] [PubMed] [Google Scholar]
- 5.Wajngarten M, Silva GS. Hypertension and stroke: update on treatment. Eur Cardiology. 2019;14(2):111–5. 10.15420/ecr.2019.11.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yusuf S, Joseph P, Rangarajan S, Islam S, Mente A, Hystad P, et al. Modifiable risk factors, cardiovascular disease, and mortality in 155 722 individuals from 21 high-income, middle-income, and low-income countries (PURE): a prospective cohort study. Lancet. 2020;395(10226):795–808. 10.1016/S0140-6736(19)32008-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Z, Zhao L, Zhou X, Meng X, Zhou X. Role of inflammation, immunity, and oxidative stress in hypertension: New insights and potential therapeutic targets. Front Immunol. 2022;13:1098725. 10.3389/fimmu.2022.1098725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hengel FE, Sommer C, Wenzel U. Arterial hypertension. Dtsch Med Wochenschr. 2022;147(7):414–28. [DOI] [PubMed] [Google Scholar]
- 9.Goodhart AK. Hypertension from the patient’s perspective. Br J Gen Pract. 2016;66(652):570. 10.3399/bjgp16X687757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Granados-Gámez G, Roales-Nieto JG, Gil-Luciano A, Moreno-San Pedro E, Márquez-Hernández VV. A longitudinal study of symptoms beliefs in hypertension. Int J Clin Health Psychol. 2015;15(3):200–7. 10.1016/j.ijchp.2015.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Puar TH, Mok Y, Debajyoti R, Khoo J, How CH, Ng AK. Secondary hypertension in adults. Singapore Med J. 2016;57(5):228–32. 10.11622/smedj.2016087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mills KT, Stefanescu A, He J. The global epidemiology of hypertension. Nat Rev Nephrol. 2020;16(4):223–37. 10.1038/s41581-019-0244-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schutte AE, Srinivasapura Venkateshmurthy N, Mohan S, Prabhakaran D. Hypertension in low- and middle-income countries. Circ Res. 2021;128(7):808–26. 10.1161/CIRCRESAHA.120.318729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bank TW. World Bank Country and Lending Groups. Country Classification. 2022. https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups.
- 15.World Health Organization. Life tables by World Bank income group Lower-middle-income countries. Last updated 2020. https://apps.who.int/gho/data/view.searo.LIFEWBLMI?lang=en.
- 16.Lu Q, Zhang Y, Geng T, Yang K, Guo K, Min X, et al. Association of lifestyle factors and antihypertensive medication use with risk of all-cause and cause-specific mortality among adults with hypertension in China. JAMA Netw Open. 2022;5(2):e2146118. 10.1001/jamanetworkopen.2021.46118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ozemek C, Tiwari S, Sabbahi A, Carbone S, Lavie CJ. Impact of therapeutic lifestyle changes in resistant hypertension. Prog Cardiovasc Dis. 2020;63(1):4–9. 10.1016/j.pcad.2019.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Al-Makki A, DiPette D, Whelton PK, Murad MH, Mustafa RA, Acharya S, et al. Hypertension pharmacological treatment in adults: a World Health Organization guideline executive summary. Hypertension. 2022;79(1):293–301. 10.1161/HYPERTENSIONAHA.121.18192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Husain MJ, Datta BK, Kostova D, Joseph KT, Asma S, Richter P, et al. Access to cardiovascular disease and hypertension medicines in developing countries: an analysis of essential medicine lists, price, availability, and affordability. J Am Heart Assoc. 2020;9(9):e015302. 10.1161/JAHA.119.015302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salam A, Huffman MD, Kanukula R, Hari Prasad E, Sharma A, Heller DJ, et al. Two-drug fixed-dose combinations of blood pressure-lowering drugs as WHO essential medicines: an overview of efficacy, safety, and cost. J Clin Hypertens (Greenwich). 2020;22(10):1769–79. 10.1111/jch.14009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Petrie JR, Guzik TJ, Touyz RM. Diabetes, hypertension, and cardiovascular disease: clinical insights and vascular mechanisms. Can J Cardiol. 2018;34(5):575–84. 10.1016/j.cjca.2017.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosendorff C, Lackland DT, Allison M, Aronow WS, Black HR, Blumenthal RS, et al. Treatment of hypertension in patients with coronary artery disease: a scientific statement from the American Heart Association, American College of Cardiology, and American Society of Hypertension. J Am Soc Hypertens. 2015;9(6):453–98. 10.1016/j.jash.2015.03.002 [DOI] [PubMed] [Google Scholar]
- 23.Filippini T, Naska A, Kasdagli MI, Torres D, Lopes C, Carvalho C, et al. Potassium intake and blood pressure: a dose-response meta-analysis of randomized controlled trials. J Am Heart Assoc. 2020;9(12):e015719. 10.1161/JAHA.119.015719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Samadian F, Dalili N, Jamalian A. Lifestyle modifications to prevent and control hypertension. Iran J Kidney Dis. 2016;10(5):237–63. [PubMed] [Google Scholar]
- 25.Zhang X, Ritonja JA, Zhou N, Chen BE, Li X. Omega-3 polyunsaturated fatty acids intake and blood pressure: a dose-response meta-analysis of randomized controlled trials. J Am Heart Assoc. 2022;11(11):e025071. 10.1161/JAHA.121.025071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matsutomo T. Potential benefits of garlic and other dietary supplements for the management of hypertension. Exp Ther Med. 2020;19(2):1479–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dominguez L, Veronese N, Barbagallo M. Magnesium and hypertension in old age. Nutrients. 2020;13(1):139. 10.3390/nu13010139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Verma N, Rastogi S, Chia YC, Siddique S, Turana Y, Cheng HM, et al. Non-pharmacological management of hypertension. J Clin Hypertens (Greenwich). 2021;23(7):1275–83. 10.1111/jch.14236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2020;2021:372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–58. 10.1002/sim.1186 [DOI] [PubMed] [Google Scholar]
- 32.Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–6. 10.1136/bmj.39489.470347.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Frih B, Jaafar H, Mkacher W, Ben Salah Z, Hammami M, Frih A. The effect of interdialytic combined resistance and aerobic exercise training on health related outcomes in chronic hemodialysis patients: the Tunisian randomized controlled study. Front Physiol. 2017;8:288. 10.3389/fphys.2017.00288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gradidge PJ, Golele PN. Walking as a feasible means of effecting positive changes in BMI, waist, and blood pressure in black South African women. Afr Health Sci. 2018;18(4):917–21. 10.4314/ahs.v18i4.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gu M, Wang Y, Shi Y, Yu J, Xu J, Jia Y, et al. Impact of a group-based intervention program on physical activity and health-related outcomes in worksite settings. BMC Public Health. 2020;20(1):935. 10.1186/s12889-020-09036-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hooshmand-Moghadam B, Eskandari M, Shabkhiz F, Mojtahedi S, Mahmoudi N. Saffron (Crocus sativus L.) in combination with resistance training reduced blood pressure in the elderly hypertensive men: a randomized controlled trial. Br J Clin Pharmacol. 2021;87(8):3255–67. 10.1111/bcp.14746 [DOI] [PubMed] [Google Scholar]
- 37.Hou ZW, Li YL, Zhang LH, Yu H, Miao YH, Gu XH. Effect of Chinese traditional healthcare exercises on 136 junior school students. Chin J Integr Med. 2014;20(3):232–4. 10.1007/s11655-014-1689-6 [DOI] [PubMed] [Google Scholar]
- 38.Jiang X, Sit JW, Wong TK. A nurse-led cardiac rehabilitation programme improves health behaviours and cardiac physiological risk parameters: evidence from Chengdu, China. J Clin nursing. 2007;16(10):1886–97. 10.1111/j.1365-2702.2007.01838.x [DOI] [PubMed] [Google Scholar]
- 39.Kanegusuku H, Silva-Batista C, Peçanha T, Nieuwboer A, Silva ND Jr, Costa LA, et al. Effects of progressive resistance training on cardiovascular autonomic regulation in patients with Parkinson disease: a randomized controlled trial. Arch Phys Med Rehabil. 2017;98(11):2134–41. 10.1016/j.apmr.2017.06.009 [DOI] [PubMed] [Google Scholar]
- 40.Lee L-L, Arthur A, Avis M. Evaluating a community-based walking intervention for hypertensive older people in Taiwan: a randomized controlled trial. Prev Med. 2007;44(2):160–6. 10.1016/j.ypmed.2006.09.001 [DOI] [PubMed] [Google Scholar]
- 41.Ajiboye OA, Anigbogu CN, Ajuluchukwu JN, Jaja SI. Therapeutic effects of exercise training on selected cardio-pulmonary parameters and body composition of Nigerians with chronic heart failure (a preliminary study). Nig Q J Hosp Med. 2013;23(4):295–301. [PubMed] [Google Scholar]
- 42.Rubinstein A, Miranda JJ, Beratarrechea A, Diez-Canseco F, Kanter R, Gutierrez L, et al. Effectiveness of an mHealth intervention to improve the cardiometabolic profile of people with prehypertension in low-resource urban settings in Latin America: a randomised controlled trial. Lancet Diabetes Endocrinol. 2016;4(1):52–63. 10.1016/S2213-8587(15)00381-2 [DOI] [PubMed] [Google Scholar]
- 43.Pal A, Srivastava N, Narain VS, Agrawal GG, Rani M. Effect of yogic intervention on the autonomic nervous system in the patients with coronary artery disease: a randomized controlled trial. Eastern Mediterranean health journal. 2013;19(5):452–8. 10.26719/2013.19.5.452 [DOI] [PubMed] [Google Scholar]
- 44.Lau C, Yu R, Woo J. Effects of a 12-week hatha yoga intervention on metabolic risk and quality of life in Hong Kong Chinese adults with and without metabolic syndrome. PLoS One. 2015;10(6):e0130731. 10.1371/journal.pone.0130731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mendivil CO, Cortés E, Sierra ID, Ramírez A, Molano LM, Tovar LE, et al. Reduction of global cardiovascular risk with nutritional versus nutritional plus physical activity intervention in Colombian adults. European journal of cardiovascular prevention and rehabilitation : official journal of the European Society of Cardiology, Working Groups on Epidemiology & Prevention and Cardiac Rehabilitation and Exercise Physiology. 2006;13(6):947–55. 10.1097/01.hjr.0000219114.48285.7a [DOI] [PubMed] [Google Scholar]
- 46.Ranasinghe C, Devage S, Constantine GR, Katulanda P, Hills AP, King NA. Glycemic and cardiometabolic effects of exercise in South Asian Sri Lankans with type 2 diabetes mellitus: a randomized controlled trial Sri Lanka diabetes aerobic and resistance training study (SL-DARTS). Diabetes & metabolic syndrome. 2021;15(1):77–85. 10.1016/j.dsx.2020.12.011 [DOI] [PubMed] [Google Scholar]
- 47.Ma C, Zhou W, Tang Q, Huang S. The impact of group-based Tai chi on health-status outcomes among community-dwelling older adults with hypertension. Heart & lung : the journal of critical care. 2018;47(4):337–44. 10.1016/j.hrtlng.2018.04.007 [DOI] [PubMed] [Google Scholar]
- 48.Lo HM, Yeh CY, Chang SC, Sung HC, Smith GD. A Tai Chi exercise programme improved exercise behaviour and reduced blood pressure in outpatients with hypertension. Int J Nurs Pract. 2012;18(6):545–51. 10.1111/ijn.12006 [DOI] [PubMed] [Google Scholar]
- 49.Mortimer J, McKune AJ. Effect of short-term isometric handgrip training on blood pressure in middle-aged females. Cardiovasc J Afr. 2011;22(5):257–60. 10.5830/CVJA-2010-090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mizuno J, Monteiro HL. An assessment of a sequence of yoga exercises to patients with arterial hypertension. J Bodyw Mov Ther. 2013;17(1):35–41. 10.1016/j.jbmt.2012.10.007 [DOI] [PubMed] [Google Scholar]
- 51.Leung LYL, Chan AWK, Sit JWH, Liu T, Taylor-Piliae RE. Tai Chi in Chinese adults with metabolic syndrome: a pilot randomized controlled trial. Complementary therapies in medicine. 2019;46:54–61. 10.1016/j.ctim.2019.07.008 [DOI] [PubMed] [Google Scholar]
- 52.Sikiru L, Okoye G. Effect of interval training programme on pulse pressure in the management of hypertension: a randomized controlled trial. Afr Health Sci. 2013;13(3):571–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sikiru L, Okoye G. Therapeutic effect of continuous exercise training program on serum creatinine concentration in men with hypertension: a randomized controlled trial. Ghana Med J. 2014;48(3):135–42. 10.4314/gmj.v48i3.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jafar TH, Hatcher J, Poulter N, Islam M, Hashmi S, Qadri Z, et al. Community-based interventions to promote blood pressure control in a developing country: a cluster randomized trial. Ann Intern Med. 2009;151(9):593–601. 10.7326/0003-4819-151-9-200911030-00004 [DOI] [PubMed] [Google Scholar]
- 55.Cunha RM, Macedo CB, Araújo SF, Santos JC, Borges VS, Soares AA, et al. Subacute blood pressure response in elderly hypertensive women after a water exercise session: a controlled clinical trial. High Blood Pressure & Cardiovascular Prevention. 2012;19:223–7. 10.1007/BF03297634 [DOI] [PubMed] [Google Scholar]
- 56.Brito ADF, de Oliveira CVC, Santos MDSB, Santos ADC. High-intensity exercise promotes postexercise hypotension greater than moderate intensity in elderly hypertensive individuals. Clin Physiol Funct Imaging. 2014;34(2):126–32. 10.1111/cpf.12074 [DOI] [PubMed] [Google Scholar]
- 57.Ha M, Lee J, Jeong W, Kim H, Son W. Combined intervention of aqua exercise and burdock extract synergistically improved arterial stiffness: a randomized, double-blind, controlled trial. Metabolites. 2022;12:970. s Note: MDPI stays neu-tral with regard to jurisdictional claims in …; 2022. 10.3390/metabo12100970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tsai J-C, Chang W-Y, Kao C-C, Lu M-S, Chen Y-J, Chan P. Beneficial effect on blood pressure and lipid profile by programmed exercise training in Taiwanese patients with mild hypertension. Clin Exp Hypertens. 2002;24(4):315–24. 10.1081/CEH-120004234 [DOI] [PubMed] [Google Scholar]
- 59.Sujatha T, Judie A. Effectiveness of a 12-week yoga program on physiopsychological parameters in patients with hypertension. International Journal of Pharmaceutical and Clinical Research. 2014;6(4):329–35. [Google Scholar]
- 60.Thomas GN, Hong AW, Tomlinson B, Lau E, Lam CW, Sanderson JE, et al. Effects of Tai Chi and resistance training on cardiovascular risk factors in elderly Chinese subjects: a 12-month longitudinal, randomized, controlled intervention study. Clin Endocrinol. 2005;63(6):663–9. 10.1111/j.1365-2265.2005.02398.x [DOI] [PubMed] [Google Scholar]
- 61.Tsai HH, Yeh CY, Su CT, Chen CJ, Peng SM, Chen RY. The effects of exercise program on burnout and metabolic syndrome components in banking and insurance workers. Ind Health. 2013;51(3):336–46. 10.2486/indhealth.2012-0188 [DOI] [PubMed] [Google Scholar]
- 62.Tsai JC, Wang WH, Chan P, Lin LJ, Wang CH, Tomlinson B, et al. The beneficial effects of Tai Chi Chuan on blood pressure and lipid profile and anxiety status in a randomized controlled trial. Journal of alternative and complementary medicine (New York, NY). 2003;9(5):747–54. 10.1089/107555303322524599 [DOI] [PubMed] [Google Scholar]
- 63.Wu L, Chen R, Ma D, Zhang S, Walton-Moss B, He Z. Effects of lifestyle intervention improve cardiovascular disease risk factors in community-based menopausal transition and early postmenopausal women in China. Menopause (New York, NY). 2014;21(12):1263–8. 10.1097/GME.0000000000000248 [DOI] [PubMed] [Google Scholar]
- 64.Yan H, Prista A, Ranadive SM, Damasceno A, Caupers P, Kanaley JA, et al. Effect of aerobic training on glucose control and blood pressure in T2DDM East African males. ISRN Endocrinology. 2014;2014:864897. 10.1155/2014/864897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yu R, Yan LL, Wang H, Ke L, Yang Z, Gong E, et al. Effectiveness of a community-based individualized lifestyle intervention among older adults with diabetes and hypertension, Tianjin, China, 2008–2009. Prev Chronic Dis. 2014;11:E84. 10.5888/pcd11.120333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yu Y, Chang C, Wu Y, Guo C, Xie L. Dose-effect relationship between brisk walking and blood pressure in Chinese occupational population with sedentary lifestyles: exercise and blood pressure. J Clin Hypertens (Greenwich). 2021;23(9):1734–43. 10.1111/jch.14340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rosa Santos LD, Araujo SS, Vieira E, Estevam CDS, Santos JLD, Wichi RB, et al. Effects of 12 weeks of resistance training on cardiovascular risk factors in school adolescents. Medicina (Kaunas, Lithuania). 2020;56(5):220. 10.3390/medicina56050220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ac M, Oliveira PL, Farah BQ, Vianna LC, Wolosker N, Puech-Leao P, et al. Effects of isometric handgrip training in patients with peripheral artery disease: a randomized controlled trial. Journal of the American Heart Association. 2020;9(4):e013596. 10.1161/JAHA.119.013596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pérez-Idárraga A, Valencia Gómez K, Gallo Villegas J, Arenas Sosa M, Quintero Velásquez MA. [Intervention with rumba and nutrition education to modify cardiovascular risk factors in adults with metabolic syndrome]. Revista panamericana de salud publica = Pan American journal of public health. 2015;37(1):29–37. [PubMed] [Google Scholar]
- 70.Pazoki R, Nabipour I, Seyednezami N, Imami SR. Effects of a community-based healthy heart program on increasing healthy women’s physical activity: a randomized controlled trial guided by Community-based Participatory Research (CBPR). BMC Public Health. 2007;7:216. 10.1186/1471-2458-7-216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.McCaffrey R, Ruknui P, Hatthakit U, Kasetsomboon P. The effects of yoga on hypertensive persons in Thailand. Holist Nurs Pract. 2005;19(4):173–80. 10.1097/00004650-200507000-00009 [DOI] [PubMed] [Google Scholar]
- 72.Lamina S, Okoye G. Effects of aerobic exercise training on psychosocial status and serum uric Acid in men with essential hypertension: a randomized controlled trial. Ann Med Health Sci Res. 2012;2(2):161–8. 10.4103/2141-9248.105665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lamina S. Comparative effect of interval and continuous training programs on serum uric acid in management of hypertension: a randomized controlled trial. J Strength Cond Res. 2011;25(3):719–26. 10.1519/JSC.0b013e3181d09edf [DOI] [PubMed] [Google Scholar]
- 74.Lamina S, Okoye CG. Uricaemia as a cardiovascular events risk factor in hypertension: the role of interval training programme in its downregulation. J Assoc Physicians India. 2011;59:23–8. [PubMed] [Google Scholar]
- 75.Lamina S, Okoye CG, Hanif SM. Randomised controlled trial: effects of aerobic exercise training programme on indices of adiposity and metabolic markers in hypertension. JPMA The Journal of the Pakistan Medical Association. 2013;63(6):680–7. [PubMed] [Google Scholar]
- 76.Mouodi S, Hosseini SR, Ghadimi R, Cumming RG, Bijani A, Mouodi M, et al. Lifestyle interventions to promote healthy nutrition and physical activity in middle-age (40–60 Years) adults: a randomized controlled trial in the north of Iran. Journal of research in health sciences. 2019;19(1):e00434. [PMC free article] [PubMed] [Google Scholar]
- 77.Onagbiye SO, Moss SJ, Cameron M. Managing noncommunicable diseases in an African community: effects, compliance, and barriers to participation in a 4-week exercise intervention. Int Q Community Health Educ. 2016;36(3):165–76. 10.1177/0272684X16647357 [DOI] [PubMed] [Google Scholar]
- 78.Mandal S, Misra P, Sharma G, Sagar R, Kant S, Dwivedi SN, et al. Effect of structured yoga program on stress and professional quality of life among nursing staff in a tertiary care hospital of Delhi-a small scale phase-II trial. Journal of evidence-based integrative medicine. 2021;26:2515690x21991998. 10.1177/2515690X21991998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Luk TH, Dai YL, Siu CW, Yiu KH, Chan HT, Lee SW, et al. Effect of exercise training on vascular endothelial function in patients with stable coronary artery disease: a randomized controlled trial. Eur J Prev Cardiol. 2012;19(4):830–9. 10.1177/1741826711415679 [DOI] [PubMed] [Google Scholar]
- 80.Mujica V, Urzúa A, Leiva E, Díaz N, Moore-Carrasco R, Vásquez M, et al. Intervention with education and exercise reverses the metabolic syndrome in adults. Journal of the American Society of Hypertension : JASH. 2010;4(3):148–53. 10.1016/j.jash.2010.02.008 [DOI] [PubMed] [Google Scholar]
- 81.Cavalcante BR, Ritti-Dias RM, Soares AH, Lima AH, Correia MA, De Matos LD, et al. A single bout of Arm-crank exercise promotes positive emotions and post-exercise hypotension in patients with symptomatic peripheral artery disease. European journal of vascular and endovascular surgery : the official journal of the European Society for Vascular Surgery. 2017;53(2):223–8. 10.1016/j.ejvs.2016.11.021 [DOI] [PubMed] [Google Scholar]
- 82.Chang SH, Chen MC, Chien NH, Lin HF. Effectiveness of community-based exercise intervention programme in obese adults with metabolic syndrome. J Clin Nurs. 2016;25(17–18):2579–89. 10.1111/jocn.13301 [DOI] [PubMed] [Google Scholar]
- 83.Chhugani KJ, Metri K, Babu N, Nagendra HR. Effects of integrated yoga intervention on psychopathologies and sleep quality among professional caregivers of older adults with Alzheimer’s disease: a controlled pilot study. Adv Mind Body Med. 2018;32(3):18–22. [PubMed] [Google Scholar]
- 84.El Ansari W, El Ashker S, Moseley L. Associations between physical activity and health parameters in adolescent pupils in Egypt. Int J Environ Res Public Health. 2010;7(4):1649–69. 10.3390/ijerph7041649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ezema CI, Onwunali AA, Lamina S, Ezugwu UA, Amaeze AA, Nwankwo MJ. Effect of aerobic exercise training on cardiovascular parameters and CD4 cell count of people living with human immunodeficiency virus/acquired immune deficiency syndrome: a randomized controlled trial. Niger J Clin Pract. 2014;17(5):543–8. 10.4103/1119-3077.141414 [DOI] [PubMed] [Google Scholar]
- 86.Farinatti P, Monteiro WD, Oliveira RB. Long term home-based exercise is effective to reduce blood pressure in low income Brazilian hypertensive patients: a controlled trial. High blood pressure & cardiovascular prevention : the official journal of the Italian Society of Hypertension. 2016;23(4):395–404. 10.1007/s40292-016-0169-9 [DOI] [PubMed] [Google Scholar]
- 87.Barroso WKS, Jardim PCBV, Vitorino PV, Bittencourt A, Miquetichuc F. Influência da atividade física programada na pressão arterial de idosos hipertensos sob tratamento não-farmacológico. Rev Assoc Med Bras. 2008;54:328–33. 10.1590/S0104-42302008000400018 [DOI] [PubMed] [Google Scholar]
- 88.Bündchen D, Panigas C, Dipp T, Panigas T, Richter C, Belli K, et al. Ausência de influência da massa corporal na redução da pressão arterial após exercício físico. Arquivos Brasileiros De Cardiologia - ARQ BRAS CARDIOL. 2010;94:678. 10.1590/S0066-782X2010005000030 [DOI] [PubMed] [Google Scholar]
- 89.de Meirelles LR, Mendes-Ribeiro AC, Mendes MA, da Silva MN, Ellory JC, Mann GE, et al. Chronic exercise reduces platelet activation in hypertension: upregulation of the L-arginine-nitric oxide pathway. Scand J Med Sci Sports. 2009;19(1):67–74. 10.1111/j.1600-0838.2007.00755.x [DOI] [PubMed] [Google Scholar]
- 90.Arora E, Shenoy S, Sandhu JS. Effects of resistance training on metabolic profile of adults with type 2 diabetes. Indian J Med Res. 2009;129(5):515–9. 10.4103/0971-5916.171275 [DOI] [PubMed] [Google Scholar]
- 91.Kanegusuku H, Queiroz ACC, Chehuen MdR, Costa LAR, Wallerstein LF, Mello MTd, et al. Strength and power training did not modify cardiovascular responses to aerobic exercise in elderly subjects. Brazilian Journal of Medical and Biological Research. 2011;44:864–70. 10.1590/S0100-879X2011007500100 [DOI] [PubMed] [Google Scholar]
- 92.Monteiro LZ, Fiani CRV, Freitas MCFd, Zanetti ML, Foss MC. Decrease in blood pressure, body mass index and glycemia after aerobic training in elderly women with type 2 diabetes. Arq Brasi Cardiologia. 2010;95:563–70. 10.1590/S0066-782X2010005000135 [DOI] [PubMed] [Google Scholar]
- 93.Wu T, Yeh H, Chan P, Chiou Y, Tsai J. The effects of simple eight-week regular exercise on cardiovascular disease risk factors in middle-aged women at risk in Taiwan. Acta Cardiologica Sinica. 2007;23(3):169. [Google Scholar]
- 94.Vianna MVA, Cáder SA, Gomes ALM, Guimarães AC, Seixas-da-Silva IA, do Rêgo ARDO, et al. Aerobic conditioning, blood pressure (BP) and body mass index (BMI) of older participants of the Brazilian Family Health Program (FHP) after 16 weeks of guided physical activity. Archives of gerontology and geriatrics. 2012;54(1):210–3. 10.1016/j.archger.2011.02.013 [DOI] [PubMed] [Google Scholar]
- 95.Bueno N, Fletcher BJ, Fletcher GF, Serra S, Cruz P, Kelly D, et al. Coronary risk factors in adult children of parents with coronary heart disease: a comparison survey of southeastern Brazil and southeastern United States. Prev Cardiol. 2005;8(3):149–54. 10.1111/j.1520-037X.2005.2248.x [DOI] [PubMed] [Google Scholar]
- 96.Shariful Islam M, Fardousi A, Sizear MI, Rabbani MG, Islam R, Saif-Ur-Rahman KM. Effect of leisure-time physical activity on blood pressure in people with hypertension: a systematic review and meta-analysis. Sci Rep. 2023;13(1):10639. 10.1038/s41598-023-37149-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Schneider VM, Domingues LB, Umpierre D, Tanaka H, Ferrari R. Exercise characteristics and blood pressure reduction after combined aerobic and resistance training: a systematic review with meta-analysis and meta-regression. J Hypertens. 2023;41(7):1068–76. 10.1097/HJH.0000000000003455 [DOI] [PubMed] [Google Scholar]
- 98.Ojha U, Ruddaraju S, Sabapathy N, Ravindran V, Worapongsatitaya P, Haq J, et al. Current and emerging classes of pharmacological agents for the management of hypertension. American journal of cardiovascular drugs : drugs, devices, and other interventions. 2022;22(3):271–85. 10.1007/s40256-021-00510-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Millar PJ, McGowan CL, Cornelissen VA, Araujo CG, Swaine IL. Evidence for the role of isometric exercise training in reducing blood pressure: potential mechanisms and future directions. Sports medicine (Auckland, NZ). 2014;44(3):345–56. 10.1007/s40279-013-0118-x [DOI] [PubMed] [Google Scholar]
- 100.Tian D, Meng J. Exercise for prevention and relief of cardiovascular disease: prognoses, mechanisms, and approaches. Oxid Med Cell Longev. 2019;2019:3756750. 10.1155/2019/3756750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tsukiyama Y, Ito T, Nagaoka K, Eguchi E, Ogino K. Effects of exercise training on nitric oxide, blood pressure and antioxidant enzymes. Journal of clinical biochemistry and nutrition. 2017;60(3):180–6. 10.3164/jcbn.16-108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Liu X, Zhang D, Liu Y, Sun X, Han C, Wang B, et al. Dose-response association between physical activity and incident hypertension: a systematic review and meta-analysis of cohort studies. Hypertension. 2017;69(5):813–20. 10.1161/HYPERTENSIONAHA.116.08994 [DOI] [PubMed] [Google Scholar]
- 103.Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Böhm M, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J. 2013;34(28):2159–219. 10.1093/eurheartj/eht151 [DOI] [PubMed] [Google Scholar]
- 104.DeSouza CA, Shapiro LF, Clevenger CM, Dinenno FA, Monahan KD, Tanaka H, et al. Regular aerobic exercise prevents and restores age-related declines in endothelium-dependent vasodilation in healthy men. Circulation. 2000;102(12):1351–7. 10.1161/01.CIR.102.12.1351 [DOI] [PubMed] [Google Scholar]
- 105.Linder L, Kiowski W, Bühler FR, Lüscher TF. Indirect evidence for release of endothelium-derived relaxing factor in human forearm circulation in vivo. Blunted response in essential hypertension. Circulation. 1990;81(6):1762–7. 10.1161/01.CIR.81.6.1762 [DOI] [PubMed] [Google Scholar]
- 106.Panza JA, Quyyumi AA, Brush JE Jr, Epstein SE. Abnormal endothelium-dependent vascular relaxation in patients with essential hypertension. N Engl J Med. 1990;323(1):22–7. 10.1056/NEJM199007053230105 [DOI] [PubMed] [Google Scholar]
- 107.de Barcelos GT, Heberle I, Coneglian JC, Vieira BA, Delevatti RS, Gerage AM. Effects of aerobic training progression on blood pressure in individuals with hypertension: a systematic review with meta-analysis and meta-regression. Frontiers in sports and active living. 2022;4:719063. 10.3389/fspor.2022.719063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rahmouni K, Correia ML, Haynes WG, Mark AL. Obesity-associated hypertension: new insights into mechanisms. Hypertension. 2005;45(1):9–14. 10.1161/01.HYP.0000151325.83008.b4 [DOI] [PubMed] [Google Scholar]
- 109.Ryan BJ, Schleh MW, Ahn C, Ludzki AC, Gillen JB, Varshney P, et al. Moderate-intensity exercise and high-intensity interval training affect insulin sensitivity similarly in obese adults. J Clin Endocrinol Metab. 2020;105(8):e2941–59. 10.1210/clinem/dgaa345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Brandt C, Pedersen BK. Physical activity, obesity and weight loss maintenance. Handb Exp Pharmacol. 2022;274:349–69. 10.1007/164_2021_575 [DOI] [PubMed] [Google Scholar]
- 111.Carrasquilla GD, García-Ureña M, Fall T, Sørensen TIA, Kilpeläinen TO. Mendelian randomization suggests a bidirectional, causal relationship between physical inactivity and adiposity. eLife. 2022;11:e70386. 10.7554/eLife.70386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Haslam DW, James WP. Obesity. Lancet. 2005;366(9492):1197–209. 10.1016/S0140-6736(05)67483-1 [DOI] [PubMed] [Google Scholar]
- 113.Shariq OA, McKenzie TJ. Obesity-related hypertension: a review of pathophysiology, management, and the role of metabolic surgery. Gland Surg. 2020;9(1):80–93. 10.21037/gs.2019.12.03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Correia RR, Veras ASC, Tebar WR, Rufino JC, Batista VRG, Teixeira GR. Strength training for arterial hypertension treatment: a systematic review and meta-analysis of randomized clinical trials. Sci Rep. 2023;13(1):201. 10.1038/s41598-022-26583-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.