Abstract
ABSTRACT
Objective
There is limited information regarding the incidence of treatment-related adverse events (AE) following antiretroviral therapy (ART) in women. So, this review aimed to describe the incidence of AE of ART in women living with HIV/AIDS.
Design
Systematic review and meta-analysis.
Data sources
Medline, Embase, Cochrane Library, Epistemonikos, Lilacs and Who Index, from inception to 9 April 2023.
Eligibility criteria
We included randomised controlled trials with at least 12 weeks of follow-up and evaluated AE of ART in women at any age living with HIV/AIDS, without restrictions on status, year or language of publication. We excluded post hoc or secondary analyses and open-label extensions without comparator, and trials involving pregnant or breastfeeding women or with a focus on coinfection with tuberculosis, hepatitis B or C. The primary outcomes were the incidence rate of participants with any clinical and/or laboratory AE related or not to ART and treatment discontinuation.
Data extraction and synthesis
Two independent reviewers extracted data and assessed the risk of bias using Cochrane’s risk of bias tool 2. We used Bayesian random-effects meta-analysis to summarise event rates. Results were presented as event rates per 1000 person-years (95% credibility intervals, 95% CrI). The pooled incidence rate per 1000 person-years adjusted for duration and loss to follow-up was estimated. We assessed the certainty of the evidence using Grading of Recommendations, Assessment, Development and Evaluation.
Results
A total of 24 339 studies were identified for screening, of which 10 studies (2871 women) met the eligibility criteria, with 11 different antiretrovirals (ARVs) regimens. Seven studies included exclusively women, while in the remaining three, the proportion of women ranged from 11% to 46%. Nine studies received industry funding. The pooled analysis showed a mean incidence rate of ART-related clinical and laboratory AE of 341.60 events per 1000 person-years (95% CrI 133.60–862.70), treatment discontinuation of 20.78 events per 1000 person-years (95% CrI 5.58–57.31) and ART-related discontinuation of 4.31 per 1000 person-years (95% CrI 0.13–54.72). Summary estimates were subject to significant uncertainty due to the limited number of studies and sparse data. The certainty of the evidence was graded as very low for all outcomes assessed.
Conclusion
Existing randomised trials do not provide sufficient evidence on the incidence rates of safety outcomes from antiretroviral treatment in women living with HIV/AIDS. Large comparative studies in well-characterised populations are needed to provide a more comprehensive landscape of the safety profile of these ARV therapies in women with HIV/AIDS.
PROSPERO registration number
CRD42021251051.
Keywords: HIV & AIDS, Systematic Review, Health policy
Strengths and limitations of this study.
This study is probably the first to assess the occurrence of therapy adverse events (AE) in women with HIV/AIDS for all antiretrovirals regimens in use in current clinical practice.
Women are under-represented in clinical trials on antiretroviral therapy, which makes knowledge about AE in this population essential for managing adherence and ensuring treatment goals.
AE are usually reported as secondary outcomes, which may reflect a lack of uniformity in their reporting by primary studies, limiting the findings of this review.
Introduction
It is estimated that 84.2 million people worldwide have already been infected with HIV. In 2021, 38.4 million people were still living with the virus, 54% of which were women or girls, with 650 000 deaths from diseases related to AIDS. At the end of that year, 28.7 million people worldwide were using antiretroviral therapy (ART). Of women aged 15 years or older, 80% had access to treatment.1
Currently, HIV/AIDS standard treatment guidelines from several countries and the WHO recommend the treatment of all people living with HIV/AIDS, regardless of whether they have the disease.2,5 All these documents reinforce the importance of treatment adherence to obtain an undetectable viral load and reduce morbidity and mortality from the disease and the risk of transmission of the virus.
Maintaining adherence to therapy, especially considering the chronic nature of HIV/AIDS, implies monitoring and managing possible adverse effects of ART. Although women represent just over half of the people living with the virus in the world, a systematic review identified that their median participation in clinical trials of the therapy is only 19%.6 Therefore, treatment recommendations are extrapolated from studies in which men predominate. In 2005, an article drew attention to possible differences in the toxicity of antiretrovirals (ARVs) between genders, which could be greater in women, due to the higher concentration of drugs that they demonstrated in pharmacokinetic studies.7
Observational studies suggest possible consequences of the use of ARV on female sex hormones, which may negatively impact women’s reproductive life and bone health. A cross-sectional study suggested increased menstrual abnormalities,8 which was not confirmed by another cohort study.9 A meta-analysis10 of six observational studies showed an increase in amenorrhoea, which may be related to lower bone mineral density (BMD). Research is contradictory on the effect of ART or HIV infection itself on bone. A systematic review11 with one clinical trial and four cross-sectional studies showed that women using protease inhibitors, a class of ARV drugs, showed a difference of more than 3% in BMD loss at the femoral neck. Another review,12 of mainly cross-sectional studies, associated a reduction in BMD in women with HIV infection, concluding that further research is needed to establish the effect of therapy on this issue.
In addition to being scarce, systematic reviews so far do not include all ART currently used in clinical practice. Understanding the differences in the occurrence of AE between genders, especially in women, and the effect of ARV on female hormones is essential for management and adherence to treatment. This review aimed to describe the incidence of AE in women with HIV/AIDS using ART in different age groups.
Methods
Study design
This is a systematic review and meta-analysis, whose objective was to describe the incidence of AE of ART in women living with HIV/AIDS. We previously provided an outline of our methods, and the current review pertains to objective 2 of our protocol.13 Our review was registered on PROSPERO, and we followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines14 when reporting this systematic review and meta-analysis (online supplemental file 1).
Search strategy
We conducted searches in MEDLINE via PubMed, Embase via Elsevier, Cochrane Central Register of Controlled Trials, Latin American and Caribbean Health Sciences Literature (Lilacs) via Virtual Health Library, Epistemonikos and Global Index Medicus from inception to 9 April 2023. The search was systematised and structured by an experienced librarian and will be reviewed by another professional librarian. Search strategies are described in online supplemental table S1 (online supplemental file 2).
Eligibility criteria
Type of studies
We included only controlled and randomised clinical trials (RCTs) that compared ART with a placebo or a different ARV regimen. The study should describe AE of interest in women living with HIV/AIDS receiving ART. The minimum follow-up duration was 12 weeks after randomisation. This cut-off was chosen because the studies usually have at least this follow-up duration and to identify treatment AE that may only become apparent or clinically detectable after prolonged use.
We excluded post hoc or secondary analyses and open-label extensions without a comparator. We have not imposed restrictions on status, year or language of publication. A professional translation service would be accessed if required.
Participants
The population of interest included women receiving ART for HIV/AIDS, regardless of age group. We excluded trials involving pregnant or breastfeeding women or with a focus on coinfection with tuberculosis, hepatitis B or C.
Trials that only reported results for both sexes combined or investigations that concentrated solely on males were excluded.
Interventions
We included all study arms involving ART for HIV/AIDS management currently being used in the clinical practice as described in online supplemental table S2 (online supplemental file 3). We excluded placebo groups from all analyses.
We also excluded studies on pre-exposure prophylaxis or postexposure prophylaxis, and arms of studies that allowed the use of multiple ARV regimens, since it would be difficult to attribute any reported AE to a specific ART regimen.
Outcomes
The outcomes used in this review, considering the number of women affected in each arm of the studies, are described in online supplemental table S3 (online supplemental file 4). We prespecified the primary outcomes to capture the most commonly observed AE in clinical practice among women taking ART. These provide a comprehensive assessment of the safety and tolerability profile of ARV drugs. We anticipated that these outcomes could have variable definitions across studies. However, our approach mitigates the risk of overlooking any key AE that is typically observed in this patient population.
The protocol predicted the assessment of the frequency of AE in the female reproductive and bone systems. These outcomes were not addressed or were rarely reported in the studies and we chose to include the predicted outcomes for the other objective described in our protocol. We also adopted the description of clinical (signs and symptoms), laboratory and total (clinical and laboratory) AE, since studies used to present results in this way. We separated AE into grades 3 or 4, grade 3, grade 4, and serious, according to the definition used by the authors of the studies.
We chose not to use death related to any AE, as this definition was not uniform across studies, and could include adverse effects of therapy, complications of the disease, and even deaths with no defined cause. Therefore, we used the number of all-cause deaths and those attributed by the study to be ART-related.
Study selection and data extraction
The selection and extraction of data went through an initial calibration by the researchers involved. Then they were carried out in pairs independently, with disagreements resolved by consensus or by a third researcher, if necessary. We selected titles and abstracts on the Covidence15 and Rayyan16 websites, and full text by an Excel spreadsheet (https://1drv.ms/x/s!AkzxfYpR4grGgoh2zxgXfN_wiYZ6rw?e=Kj474f). Eligible studies that referred to the same participants were grouped under a single identification number, including registration protocols and posted data, when available. Supplementary study materials were also consulted. For data extraction, we used an Excel spreadsheet (https://1drv.ms/x/s!AkzxfYpR4grGgoh9sGFm6ohljhL01g?e=ERjhK4).
Risk of bias
Two investigators independently assessed the risk of bias in the included studies using Cochrane’s risk of bias tool 2 (RoB2).17 Disagreements were resolved by consensus and, if necessary, by a third reviewer. Publication bias does not apply to the type of analysis performed in our review, which describes the incidence rate of AE in the study arms and not measures of effect that compare different ART. In any case, more details about this are presented in online supplemental file.
Statistical analysis
We changed the prespecified measure of outcome frequency from incidence risk to incidence rate. This adjustment was necessary due to considerable differences between trials regarding the length of follow-up and the number of participants lost to follow-up. Using the incidence rate, we could account for variations in duration and loss to follow-up across studies.
Data that were not normally distributed were reported as median (interquartile range, IQR). Categorical variables were presented as numbers (percentages). We approximated the number of events when required based on reported proportions and incidence rates. We imputed the mean follow-up (in person-years) using the available summary statistics.
When the actual total time at risk was not reported, we approximated the total person-years at risk using the mean follow-up duration, accounting for the all-cause losses to follow-up or withdrawals. We multiplied the reported mean follow-up by the number of participants analysed, subtracting from it the product of half the mean follow-up and the number of participants not included in the analyses (all-cause dropouts/withdrawals).
In our systematic review, the unit of analysis was the treatment group. Thus, the same trial could contribute to one, two or more independent groups. We used Poisson 95% confidence intervals (95% CIs) to represent the uncertainty around point estimates derived for individual groups. We summarised estimates across groups using Bayesian random-effects meta-analysis models. We employed a Poisson likelihood with a log link for incidence rates. Non-informative prior distributions were used in all models. We quantified the between-study heterogeneity using 95% predictive intervals, which describe the expected variation in true incidence rates over different settings and populations.18
Summary estimates were presented as events per 1000 person-years to facilitate interpretation. These estimates were obtained via posterior medians and 2.5th and 97.5th percentiles. Models were implemented in OpenBUGS (V.2.0, Cambridge, UK), and estimates were obtained using Markov chain Monte Carlo simulation via 500 000 iterations (with a burn-in of 50 000 simulations).
We describe the approaches to model diagnostics in online supplemental file 5. We used a multilevel frequentist random-effects meta-analysis model to conduct a sensitivity analysis. Specifically, we employed a mixed-effects Poisson regression model, as this approach outperforms inverse-variance methods in meta-analyses with sparse data.
Patient and public involvement
Patients and the public were not involved in this study.
Results
Study selection
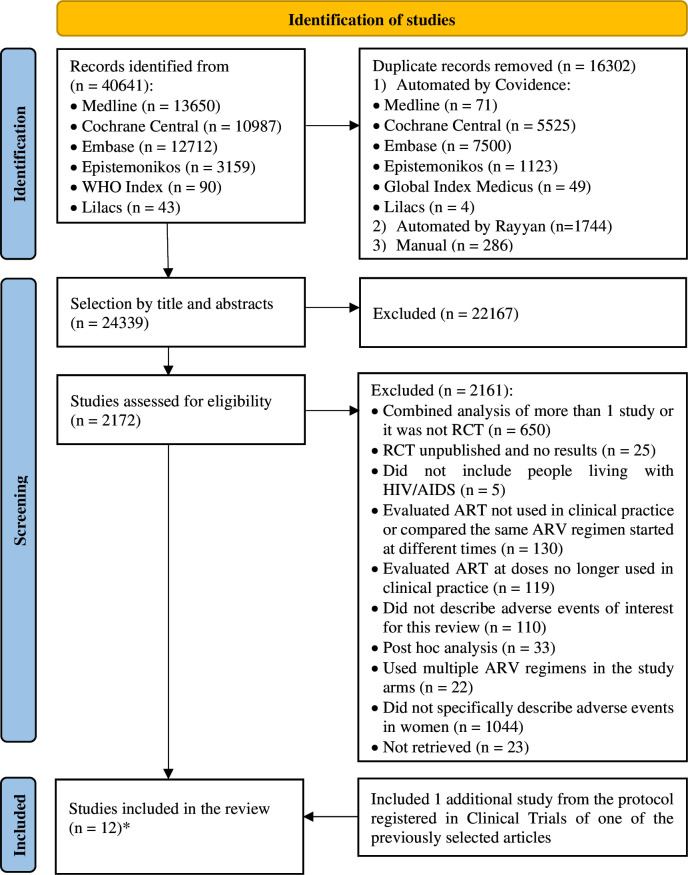
The selection process and the reasons for excluding the studies are shown in figure 1, prepared according to PRISMA,19 and the list of excluded studies is in online supplemental table S4 (online supplemental file 6). We included 12 publications. The studies by Campbell et al and Firnhaber et al20 21 contained the same participants and were linked to the same protocol, being grouped under a single identification number. This was similar to the studies by Eron et al and Rashbaum et al.22 23 Two other studies,24 25 despite having a common protocol, assessed different participants and were considered separately. Therefore, we had 10 studies included for analysis of results.
Figure 1. Study selection flowchart according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses. *Although 12 articles were individually included, they were evaluated as 10 studies, as two pairs belonged to the same protocol, contributing data on the same participants. ART, antiretroviral therapy; RCT, randomised controlled trial.
Study characteristics
The general characteristics of the included studies are described in table 1 and the arms of each study used in this review are in table 2. In general, they were multicentric. Only two took place in a single country, one in South Africa26 and one in England.27
Table 1. General characteristics of the studies.
| Author, year, protocol | Financing | Study locations | Total number of participants | Number who received at least one dose of treatment | Duration of treatment (weeks) | Prior exposure to ART | Interventions other than ART and contraceptive measures | Blinding | Allocation concealment |
| Squires et al, 2016, NCT0170557430 | Industry | 80 hospitals, outpatient and university services in 12 countries | 583 | 575 | 48 | No | None | Double-blind | Not described |
| Firnhaber et al, 2015, NCT0008413621 | Mixed* | 46 clinics, universities, research centres and hospitals in 9 countries | 1045 | 1042 | 184 | No | ART was changed in case of virological failure or serious AE | Open | There is not |
| Orrell et al, 2017, NCT0191040229 | Industry | 86 hospitals, universities and clinics in 13 countries | 499 | 495 | 48 | No | None | Open | There is not |
| Lockman et al, 2010, NCT0008950524 | Mixed* | Hospitals, universities and research centres in 7 African countries | 243 | 241 | 153 | No | ART was changed in case of intolerance or lack of clinical response | Open | There is not |
| Lockman et al, 2012, NCT0008950525 | Mixed* | Hospitals, universities and research centres in 7 African countries | 502 | 500 | 168 | No | ART was changed in case of intolerance or lack of clinical response | Open | There is not |
| Rashbaum et al, 2019, NCT0243124723 | Industry | 121 locations in 10 countries | 725 | 725 | 48 | No | None | Double-blind | Not described |
| Kityo et al, 2019, NCT0265262428 | Industry | 58 clinics in 6 countries | 235 | 234 | 48 | Yes | Not reported | Open | There is not |
| Naicker et al, 2017, NCT0138702226 | Governmental agencies | Two cities from a province in South Africa | 59 | 59 | 156 | No | Another NRTI was used when there was a contraindication to zidovudine | Open | There is not |
| Huhn et al, 2019, NCT0226991731 | Industry | 106 hospitals and clinics in 9 countries | 766 | 763 | 48 | Yes | None | Open | There is not |
| Ibrahim et al, 2020, EudraCT 2015-005297-3727 | Industry | Nine HIV clinics in the UK | 59 | 59 | 96 | Yes | Not reported | Open | There is not |
Mixed: pharmaceutical industry and governmental agencies.
ART, antiretroviral therapy; ARV, antiretroviral; NRTI, nucleoside and nucleotide reverse transcriptase inhibitors
Table 2. Characteristics of the study arms.
| Author, year | Intervention | Total number of participants | Number who received at least one dose of treatment (no of losses) | Number of women (%) | Age range (years) | CD4 level(cells/μL) | Comorbidities |
| Squires et al, 201630 | E/C/F/TDF 150/150/200/300 mg/day | 293 | 289 (29) | 289 (100) | Median (IQR)34 (28–43) | Median (IQR)344 (246–466) mean (SD)376 (199.6) | HBsAg positive 9 (4%); positive anti-HCV 22 (8%); history of STI 76 (26%); history of anxiety/depression 51 (18%) |
| ATV 300 mg/day+RTV 100 mg/day+F/TDF 200/300 mg/day | 290 | 285 (45) | 285 (100) | Median (IQR)35 (29–42) | Median (IQR)370 (244–489) mean (SD)385 (210.2) | HBsAg positive 7 (3%); positive anti-HCV 25 (9%); history of STI 80 (28%); history of anxiety/depression 60 (21%) | |
| Firnhaber et al, 201521 | EFZ 600 mg/day+3TC/AZT 150/300 mg 12/12 hours | 519 | 517 (NR) | 241 (46) | Mean (SD)35 (9) | Mean (SD)163 (85) | Pulmonary tuberculosis 28, extrapulmonary tuberculosis 10, chronic hepatitis C 12, chronic hepatitis B 3, any infection 153 |
| EFZ 600 mg/day+F/TDF 200/300 mg/day | 526 | 525 (NR) | 242 (46) | Mean (SD)35 (9) | Mean (SD)155 (81) | Pulmonary tuberculosis 25, extrapulmonary tuberculosis 7, chronic hepatitis C 7, chronic hepatitis B 4, any infection 144 | |
| Orrell et al, 201729 | DTG/ABC/3TC 50/600/300 mg/day | 250 | 248 (42) | 248 (100) | Mean (SD)38.1 (11.15) | Median*340(197–497.5) | Hepatitis C 16 (6%) |
| ATV 300 mg/day+RTV 100 mg/day+F/TDF 200/300 mg/day | 249 | 247 (55) | 247 (100) | Mean (SD)37.8 (10.14) | Median*350(241–487) | Hepatitis C 21 (9%) | |
| Lockman et al, 201024 | NVP 200 mg 12/12 hours+F/TDF 200/300 mg/day | 123 | 121 (9) | 121 (100) | Mean (SD)30 (5) | Mean (SD)136 (60) | HBsAg positive 9 (8%) |
| LPV/RTV 400/100 mg 12/12 hours+F/TDF 200/300 mg/day | 120 | 120 (2) | 120 (100) | Mean (SD)31 (5) | Mean (SD)135 (61) | HBsAg positive 18 (7%) | |
| Lockman et al, 201225 | NVP 200 mg 12/12 hours+F/TDF 200/300 mg/day | 251 | 249 (21) | 249 (100) | Mean (SD)35 (7) | Mean (SD)126 (68) | HBsAg positive 18 (7%) |
| LPV/RTV 400/100 mg 12/12 hours+F/TDF 200/300 mg/day | 251 | 251 (21) | 251 (100) | Mean (SD)35 (8) | Mean (SD)125 (71) | HBsAg positive 17 (7%) | |
| Rashbaum et al, 201923 | DRV/C/F/TAF 800/150/200/10 mg/day | 362 | 362 (NR) | 44 (12) | Median (IQR)34 (27–42) | Median (IQR) 461,5 (342–617) | Not reported |
| DRV/C 800/150 mg/day+F/TDF 200/300 mg/day | 363 | 363 (NR) | 41 (11) | Median (IQR)34 (27–42) | Median (IQR) 440 (325–594) | Not reported | |
| Kityo et al, 201928 | BIC/F/TAF 50/200/25 mg/day | 235 | 234 (3) | 234 (100) | Median (min–max)39 (21–63) | Median (IQR)667 (532–852) | Hepatitis B/hepatitis C coinfection 2 (5%) |
| Naicker et al, 201726 | EFZ 600 mg/day+TDF 300 mg/day+3TC 300 mg/day | 29 | 29 (1) | 29 (100) | Median (IQR)28 (25–33) | Median (IQR)345 (289–431) | Not reported |
| EFZ 600 mg/day+AZT 12/12 hour+3TC 300 mg/day | 30 | 30 (4) | 30 (100) | Median (IQR)28 (26–30) | Median (IQR)335 (280–411) | Not reported | |
| Huhn et al, 201931 | DRV/C/F/TAF 800/150/200/10 mg/day | 766 | 763 (NR) | 140 (18) | Median (min–max)46 (19–75) | Median 630IQR 468–806min–max 111–1921 | Not reported |
| Ibrahim et al, 202027 | DTG/ABC/3TC 50/600/300 mg/day | 59 | 59 (8) | 59 (100) | Mean (SD)50.97 | Median (IQR)612 (454–807) | Diabetes 3 (5.1%), hypertension 13 (22%), smoking 4 (9.8%) |
ABCabacavirATVatazanavirAZTzidovudineBICbictegravirCcobicistatDRVdarunavirDTGdolutegravirEelvitegravirEFZefavirenzFemtricitabineHBsAghepatitis B surface antigenHCVhepatitis C virusLPVlopinavirNPVnevirapineNRnot reportedRTVritonavirSTIsexually transmitted infectionTAFtenofovir alafenamideTClamivudineTDFtenofovir disoproxil fumarate
The 10 studies had 4716 people randomised, of whom 4693 received at least one dose of the treatment. Seven studies included only women.24,30 In the other three,21 23 31 their percentage of participation ranged from 11% to 46%. This review included a total of 2871 women who had taken at least one dose of ART.
Three27 28 31 of the 10 studies contributed information on AE in women in only one arm, totalling 17 arms, with 11 different ARV regimens (table 2). The study by Naicker et al26 described that zidovudine was used every 12 hours, without specifying the dose, neither in the published study nor in the protocol. As the usual dose of this drug, when used in conjunction with efavirenz and lamivudine, is usually 300 mg every 12 hours, we assume that this should have been adopted.
Seven studies2123,26 29 31 classified the gravity (grade) of AE using the DAIDS Table for Grading the Severity of Adult and Pediatric Adverse Events (from the Division of AIDS of the US Department of Health and Human Services, National Institutes of Health, National Institute of Allergy and Infectious Diseases). The others did not describe which source they used for this classification.27 28 30
In one of the three mixed-funded (governmental agencies and pharmaceutical industry) studies, the industry only contributed to the supply of drugs.21 In two others, participation went further: in one of them, the industry participated in the study group, but not in the data analysis,24 and in another, the industry contributed to the protocol and with suggestions about the manuscript (at the discretion of the authors), but not with its writing, data analysis and decision to publish.25
Risk of bias
We performed the per-protocol quality assessment because most studies presented data and results for the population that received at least one dose of treatment and because the assessment of AE usually includes participants who were exposed to the intervention (those who took any drug dose).
We preferentially considered the primary outcome of ART discontinuation due to any AE, which was evaluated by nine of the studies,23,31 rather than discontinuation due to AE specifically caused by ART, reported by only half.23 25 26 28 31 We considered the impact of the lack of blinding of most studies on our other primary outcomes: the total number of AE and the total number of treatment-related AE. Only in the study by Firnhaber et al21 the reference for the analysis was the total number of laboratory AE since it only contributed to this review with information about this outcome.
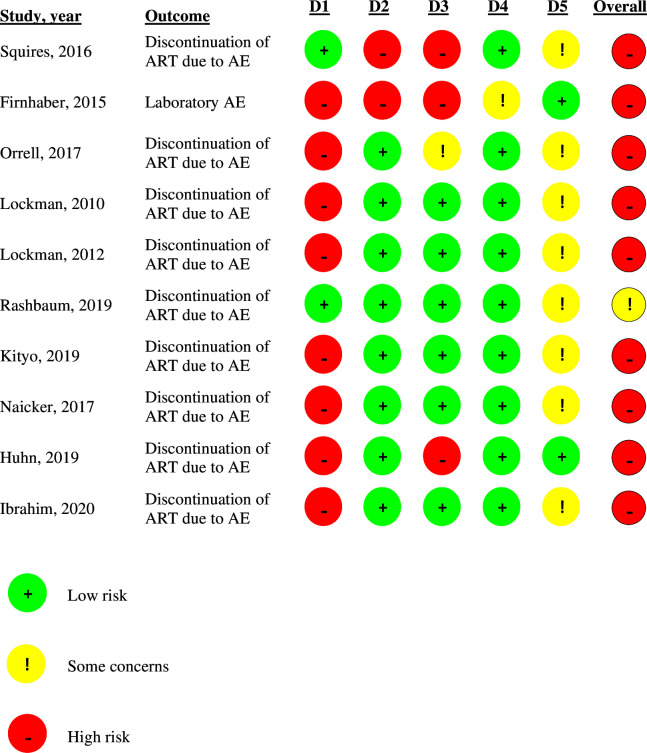
Most studies (80%) were not blinded.2124,29 31 Only two were double-blinded,23 30 but although they performed the randomisation properly, they did not describe how the allocation concealment took place. Four studies had follow-up losses that could interfere with their results, three21 30 31 were classified as at high risk of bias in this domain and one29 with some concern about bias.
Nine studies2124,31 were at high risk of bias by the Cochrane RoB2 tool,17 while only one23 had some concern about the risk of bias, as can be seen in figure 2.
Figure 2. Assessment of the risk of bias by the Cochrane RoB2 tool. D1, randomisation process; D2, deviations from the intended interventions; D3, missing outcome data; D4, measurement of the outcome; D5, selection of the reported result. AE, adverse events; ART, antiretroviral therapy.
Publication bias normally applies to comparative studies that use measures of association, which is not the case in this review. Despite this, we present funnel plots for the outcomes in which there were at least 10 arms evaluated with the occurrence of at least one event, using the incidence rate as a summary estimate, although the arms of the studies represent different ARV regimens (online supplemental figures S1 and S2; online supplemental file 7).
Study outcomes
The incidence rate of AE for pooled ART arms is shown in table 3. Detailed results for each ART grouped by outcome and by ARV regimen are shown in online supplemental tables S5 and S6, respectively (online supplemental file 8). Events whose incidence was zero in all studies that evaluated them were not included in the tables but were described in the text.
Table 3. Incidence rate of adverse events for pooled ART arms.
| Adverse event | Number of different ART | Number of study arms | Number of women | Incidence rate (number of events/1000 person-years) | Credibility interval (95%) |
| Osteoporosis from adverse events | 1 | 1 | 59 | 6.15 | 0.01–4037.00 |
| Discontinuation or dropouts/withdrawals due to adverse events | 10 | 15 | 2388 | 20.78 | 5.58–57.31 |
| Discontinuation or dropouts/withdrawals due to ART-related adverse events | 6 | 7 | 988 | 4.31 | 0.13–54.72 |
| Clinical and/or laboratory adverse events | 5 | 6 | 954 | 888.20 | 759.90–1045.00 |
| Clinical and/or laboratory adverse events related to ART | 5 | 6 | 954 | 341.60 | 133.60–862.70 |
| Clinical adverse events related to ART | 2 | 2 | 575 | 431.50 | 13.84–13630.00 |
| Grade 3 and/or 4 clinical and/or laboratory adverse events | 4 | 5 | 284 | 96.34 | 55.04–158.90 |
| Grade 3 and/or 4 clinical adverse events | 4 | 6 | 1316 | 59.93 | 33.74–104.60 |
| Grade 3 and/or 4 laboratory adverse events | 5 | 7 | 1550 | 145.10 | 57.71–359.90 |
| Grade 3 clinical and/or laboratory adverse events | 2 | 2 | 495 | 125.50 | 3.51–4295.00 |
| Grade 4 clinical and/or laboratory adverse events | 2 | 2 | 495 | 25.05 | 0.50–1017.00 |
| Grade 4 clinical adverse events | 2 | 2 | 500 | 9.31 | 0.39–216.30 |
| Serious clinical and/or laboratory adverse events | 9 | 13 | 1813 | 49.34 | 31.60–77.10 |
| Grade 3 and/or 4 clinical and/or laboratory adverse events related to ART | 2 | 3 | 225 | 27.75 | 2.56–272.90 |
| Serious clinical and/or laboratory adverse events related to ART | 5 | 6 | 954 | 1.09 | 0.01–21.47 |
| Serious clinical adverse events related to ART | 5 | 5 | 1197 | 1.53 | 0.01–21.29 |
| Death from all causes | 10 | 15 | 2388 | 4.47 | 1.42–7.91 |
| Death from adverse events related to ART | 10 | 15 | 2388 | 0.18 | 0.00–1.56 |
ART, antiretroviral therapy
Total AE
The outcome of any clinical and/or laboratory AE had a mean incidence rate of 888.20 events per 1000 person-years (95% credibility interval (95% CrI) 759.90–1045.00) for all pooled treatments (online supplemental figure S3; online supplemental file 9). In the analyses for each ARV regimen separately, the result ranged from 718.40 with bictegravir/emtricitabine/tenofovir alafenamide 50/200/25 mg/day to 972.10 with atazanavir 300/day+ritonavir 100 mg/day+emtricitabine/fumarate of tenofovir disoproxil 200/300 mg/day, but the CrIs were generally very wide, as shown in online supplemental table S5 (online supplemental file 8). The occurrence of any laboratory AE was evaluated by a single study.21 No articles reported the incidence of any clinical AE.
The pooled study arms showed a mean incidence rate of ART-related clinical and/or laboratory AE of 341.60 events per 1000 person-years (95% CrI 133.60–862.70). Individually, the ARV regimens had a variation in this outcome from 91.31 to 594.80, but also with very wide CrIs (onlinesupplemental tables S3 S5). Clinical AE related to ART were reported only in the study by Squires et al30 and none described the laboratory AE related to ART separately.
Discontinuation or dropouts/withdrawals due to AE
The incidence rate of treatment discontinuation due to any AE ranged from 0 to 126.40 events per 1000 person-years among the different ART. Bictegravir/emtricitabine/tenofovir alafenamide 50/200/25 mg/day and efavirenz 600 mg/day+tenofovir disoproxil fumarate 300 mg/day+lamivudine 300 mg/day were not discontinued, but both were evaluated in single studies26 28 and the second regimen was used by only 29 women.26 Darunavir/cobicistat 800/150 mg/day+emtricitabine/tenofovir disoproxil fumarate 200/300 mg/day, in one study,23 n=41, and efavirenz 600 mg/day+lamivudine/zidovudine 150/300 mg 12/12 hours, in one study,26 n=30, showed, respectively, 121.30 (95% CrI 0.21–67 219.50) and 126.40 (95% CrI 0.23–69 389.00) discontinuations per 1000 person-years. However, both were used by a few women and the CrI was very wide. Pooled analysis of all ART showed a mean treatment discontinuation incidence rate of 20.78 events per 1000 person-years (95% CrI 5.58–57.31; online supplemental figure S4; online supplemental file 9).
Discontinuation due to ART-related AE was reported by half of the studies23 25 26 28 31 and the incidence rate ranged from 0 to 121.30 events per 1000 person-years. All treatments pooled had an average ART-related discontinuation of 4.31 per 1000 person-years (95% CrI 0.13–54.72; online supplemental figure S5; online supplemental file 9).
Grade 3 and/or 4 and serious AE
Grade 3 and/or 4 clinical and/or laboratory AE were evaluated in only 284 women, who used four different ARV regimens containing efavirenz or darunavir/cobicistat associated with two nucleoside and nucleotide reverse transcriptase inhibitors (NRTIs). The incidence rate for this outcome ranged from 77.39 to 137.80 events per 1000 person-years, but with wide CrIs (online supplemental table S5; online supplemental file 8). The pooled analysis of these ART showed 96.34 grade 3 and/or 4 clinical and/or laboratory AE per 1000 person-years (95% CrI 55.04–158.90).
The incidence rate of grade 3 and/or 4 clinical AE was analysed for four different ARV regimens, ranging from 45.40 to 117.60 events per 1000 person-years, and the mean pooled result for these ART was 59.93 (95% CrI 33.74–104.60). Grade 3 and/or 4 laboratory AE ranged from 71.04 to 703.70 in five different ART, with a pooled analysis of 145.10 events per 1000 person-years (95% CrI 57.71–359.90).
Grade 3 clinical and/or laboratory AE and grade 4 clinical and/or laboratory AE were evaluated by only one study,29 with a mean incidence rate in the pooled analysis of the two arms of, respectively, 125.50 (95% CrI 3.51–4295.00) and 25.05 (95% CrI 0.50–1017.00). The same occurred with grade 4 clinical AE, assessed by the study by Lockman et al,25 with a pooled result of the two evaluated ARV regimens of 9.31 (95% CrI 0.39–216.30).
The occurrence of serious clinical and/or laboratory AE was reported for nine of the 11 ARV regimens included in this review. The pooled mean incidence rate of all treatments was 49.34 events per 1000 person-years (95% CrI 31.60–77.10). When looking at ART separately, the event incidence rate ranged from 20.74 (95% CrI 0.78–491.70) with lopinavir/ritonavir 400/100 mg two times a day+emtricitabine/tenofovir disoproxil fumarate 200/300 mg/day (n=371) to 126.40 per 1000 person-years (95% CrI 0.23–69 389.00) with efavirenz 600 mg/day+lamivudine/zidovudine 150/300 mg 12/12 hours (n=30).
The other grade 3 and/or 4 and serious AE described in the methods had no data reported in any of the included studies. For events of this nature described by the studies as related to ART, four outcomes were contemplated: (1) grade 3 and/or 4 clinical and/or laboratory AE; (2) serious clinical and/or laboratory AE; (3) serious clinical AE; (4) serious laboratory AE.
Grade 3 and/or 4 clinical and/or laboratory AE related to ART were evaluated in two studies, with 225 women, who used two ARV regimens containing darunavir/cobicistat+emtricitabine associated with tenofovir alafenamide or tenofovir disoproxil fumarate. The pooled mean incidence rate for these treatments was 22.75 events per 1000 person-years (95% CrI 2.56–272.90).
The incidence rate of serious clinical and/or laboratory AE related to ART ranged from no events with three treatment regimens to 68.36 per 1000 person-years. However, for the two regimens in which an event was reported, the CrI was quite wide, as shown in online supplemental table S5 (online supplemental file 8).
Serious clinical AE related to ART were absent with three regimens, while two others had 10.21 and 18.85 per 1000 person-years, also with wide CrIs (online supplemental table S5; online supplemental file 8). Three ARV regimens (dolutegravir/abacavir/lamivudine 50/600/300 mg/day; darunavir/cobicistat/emtricitabine/tenofovir alafenamide 800/150/200/10 mg/day; bictegravir/emtricitabine/tenofovir alafenamide 50/200/25 mg/day) were evaluated for serious laboratory AE related to ART, with no events recorded.
AE of the female reproductive system
None of the studies evaluated AE in the female reproductive system. The total or ART-related occurrence of delayed puberty, amenorrhoea, menstrual irregularity, early menopause, full hot flushes, and severe, moderate and mild hot flashes could not be extracted from randomised controlled trials.
Osteopenia, osteoporosis and osteoporotic fractures
Only one study27 analysed the occurrence of osteoporosis, whose incidence rate was 6.15 events per 1000 person-years (CrI 0.01–4037.00). There were no data on osteopenia and only two studies23 31 reported that there were no fractures due to osteoporosis.
Death
All-cause death was assessed in 2388 women for ten different ARV regimens. The pooled mean incidence rate for all these treatments was 4.47 events per 1000 person-years (95% CrI 1.42–7.91; online supplemental figure S6; online supplemental file 9). Four of the ten ARTs had no deaths in their treatment groups, as shown in online supplemental table S5 (online supplemental file 8).
Eight of these ten ARV regimens had no deaths from ART-related AE, with the pooled mean incidence rate being 0.2 events per 1000 person-years (95% CrI 0.00–1.60; online supplemental figure S7; online supplemental file 9). The two treatments with the occurrence of events were nevirapine 200 mg 12/12 hours+emtricitabine/tenofovir disoproxil fumarate 200/300 mg/day (one death) and lopinavir/ritonavir 400/100 mg 12/12 hours+emtricitabine/tenofovir disoproxil fumarate 200/300 mg/day (three deaths), both in the Lockman et al study.25 In the other study24 in which these two regimens were evaluated, there were no ART-related deaths. The mean incidence rate was, respectively, 0.29 (95% CrI 0.00–20.77) and 0.94 (95% CrI 0.00–60.39).
Heterogeneity, subgroup analysis and assessment of evidence certainty
These analyses are available in onlinesupplemental tables S7 S8 (online supplemental file 10).
Discussion
Summary of main results
The pooled analysis showed a mean incidence rate of ART-related clinical and laboratory AE of 341.60 events per 1000 person-years (95% CrI 133.60–862.70), treatment discontinuation of 20.78 events per 1000 person-years (95% CrI 5.58–57.31) and ART-related discontinuation of 4.31 per 1000 person-years (95% CrI 0.13–54.72). The certainty of the evidence was graded as very low for all outcomes assessed. Despite the extensive literature search performed, few studies specifically analysed the occurrence of AE in women with HIV/AIDS. Most of the studies included in this review were published between 2015 and 2020. Despite being recent studies, we still noticed a lack of standardisation between studies in the collection and analysis of AE, as already reported by Pitrou et al.32 We could not establish recommendations on a preferential ARV regimen for use in women with HIV/AIDS, aiming to minimise the risk of AE and improve treatment adherence. Less than half of the ARV drugs used in current clinical practice comprised the evaluated treatment regimens. We found few data on elderly women and none of the studies included children.
Comparison with other reviews
A systematic review33 that evaluated the bone safety of ART, not specifically in women, and included 11 clinical trials with the efavirenz+emtricitabine+tenofovir disoproxil fumarate regimen, did not identify treatment-related bone fractures. The only study in our review that evaluated this ARV regimen21 did not address bone health outcomes. Another systematic review11 analysed osteopenia, osteoporosis and fractures due to osteoporosis in women with HIV/AIDS using ARVs and found only cross-sectional studies. It demonstrated 40% osteopenia in the proximal femur (one study) and 22% in the proximal femur and lumbar spine (one study). Osteoporosis was described in the lumbar spine in 42.8% (one study of postmenopausal women), in the proximal femur in 5% (one study) and at both sites in 7.3% (one study). There was only one case of vertebral fracture in a postmenopausal woman. Despite the concern about the effect of ART on the risk of osteoporosis and bone fractures, especially in women, these outcomes are still little discussed in the literature, especially in RCTs. In our review, only one trial reported the occurrence of osteoporosis and two studies evaluated fractures related to this condition, but without any events. However, the follow-up duration for both was only 48 weeks, which is insufficient for an adequate assessment of this outcome.
Discontinuation of ARVs due to AE compromises the current goal of treating all people with HIV/AIDS. A systematic review,34 which included 64 clinical trials and 15 cohorts with both sexes, found that the most common cause of treatment interruption was the occurrence of AE (9%). In our review, discontinuation varied greatly between different ARV regimens. The result found for the two regimens with the highest risk of discontinuation due to AE should be considered with caution, as the number of women evaluated was very small. A meta-analysis35 sought to assess the safety of regimens containing lopinavir/ritonavir in women with HIV/AIDS. Discontinuation due to AE occurred in 6.8% of women. In our review, lopinavir/ritonavir was evaluated in two studies,24 25 linked to the same protocol, associated with emtricitabine/tenofovir disoproxil fumarate, with similar results. The mean incidence rate of discontinuation due to AE with this regimen was 0.31 per 1000 person-years, corresponding to 6.2% of women evaluated for this outcome.
Deaths from AE related to ART occurred with only two treatment regimens out of ten evaluated for this outcome. One with nevirapine+emtricitabine/tenofovir disoproxil fumarate (0.27% of women receiving this regimen) and three with lopinavir/ritonavir+emtricitabine/tenofovir disoproxil fumarate (0.81%). This last finding was similar to ART discontinuation due to death in the meta-analysis by Hermes et al,35 who evaluated regimens containing lopinavir/ritonavir in women with HIV/AIDS, in which five women died (0.8%).
Strengths and weaknesses
As far as we know, our review was the first that sought to gather the existing scientific knowledge about AE in women with HIV/AIDS of all ART in use in current clinical practice. Previously, published reviews on women have focused on specific AE, such as bone health, or the comparative evaluation of no more than two regimens containing certain ARVs. We highlight the broad search we carried out in the literature, with a large number of studies screened in the selection stage, without limitation of publication date or language.
Summary estimates were subject to significant uncertainty due to the limited number of studies and sparse data. We assessed whether we could carry out a network meta-analysis with studies that included 100% women, but this was not possible.
The level of treatment adherence may influence the incidence of AE reported by studies as they may be less prominent if participants take fewer doses of the medication than recommended. Three of the studies did not describe treatment adherence.21 27 29 The others considered adherence by counting the difference between the total number of pills supplied and the number of pills not taken (returned), divided by the total number of pills delivered. A high level of adherence was usually considered when at least 95% of expected doses were taken. One of the studies reported adherence by region in which the study was conducted, ranging from 61% to 92%.30 In the others, adherence varied from 81% to 99.7%. However, none of the studies presented the results of AE according to percentage or level of treatment adherence.
Since HIV/AIDS has become a chronic condition that requires lifelong treatment, it is important to recognise the AE that therapy can cause in the long term. In our review, the study with the longest follow-up lasted 184 weeks and contributed data for only one of the outcomes we sought to evaluate. Half of the studies lasted just under 1 year (48 weeks). This follow-up duration is insufficient to analyse the incidence of long-term AE of ART in women.
We chose to select only randomised controlled clinical trials, mainly due to the other objective foreseen in our protocol, which intends to evaluate the difference between genders in the occurrence of AE, comparing the different ART. However, for the description of the frequency of AE specifically in women, we used data from only one arm of some studies, since the comparator had multiple ARV regimens. Thus, results from single-arm experimental studies reporting AE in women could have been included. Future research may expand the search for this type of study to update the findings of our review.
Implications for research
Our review demonstrates the need for more research to assess the AE of ART in women, especially with ARV who did not appear in any of the studies. Research should compare isolated ARV regimens instead of allowing multiple ARV regimens in any of the arms of the study, or describe AE separately for each of the regimens used, to enable the assessment of safety for each specific regimen. As knowledge and management of the adverse effects of ART have a direct implication on adherence to treatment, it is necessary to consider carrying out pragmatic clinical trials, which allow the incorporation of the individual needs of the participants, evaluating the results in conditions that are close to everyday clinical practice.36
Clinical implications
The scarcity of information on AE of ART specifically in women, combined with the high risk of bias in almost all existing studies, do not allow the establishment of recommendations for clinical practice guidelines or for approaches aimed at minimising the risk of AE and improving the adherence to ARVs in this population.
The Brazilian and WHO clinical practice guidelines2 5 recommend the use of tenofovir disoproxil fumarate in the ARV regimen for the initial treatment of people with HIV/AIDS. One of the adverse effects of this drug is the possibility of reducing BMD, with a consequent increase in the risk of pathological fractures.2 An American guideline4 recommends that this drug be avoided in those with osteoporosis. However, tenofovir disoproxil fumarate was evaluated for the risk of fractures due to osteoporosis in only one study.23
Conclusion
Existing randomised trials do not provide sufficient evidence on the incidence rates of safety outcomes from ART in women living with HIV/AIDS. Large comparative studies in well-characterised populations are needed to provide a more comprehensive landscape of the safety profile of these ARV therapies in women with HIV/AIDS. In this sense, pragmatic clinical trials can contribute to the evaluation of the effectiveness of currently available therapies, by generating results that are closer to the reality faced in daily clinical practice.
supplementary material
Acknowledgements
The authors would like to thank Genevieve Gore and Mabel Figueiro, experienced librarians, for their collaboration with the review and definition of the final search strategy for the present study. The authors would like to thank Filipe Perini as well, for his contributions to the search questions, choice of medicines to be included and the functioning of the current Brazilian public policies for HIV. Our great thanks also go to Tiago Veiga Pereira, from the Clinical Trial Service Unit and Epidemiological Studies Unit, at the University of Oxford, who reviewed the study proposal, described and performed the statistical analyses, and contributed to the final version of the article.
Footnotes
Funding: This research received funding from the São Paulo Research Foundation (FAPESP), grant no. 2018/16893- 8.
Prepublication history and additional supplemental material for this paper are available online. To view these files, please visit the journal online (https://doi.org/10.1136/bmjopen-2023-079292).
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Not applicable.
Ethics approval: Not applicable.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Contributor Information
Jardel Corrêa de Oliveira, Email: jardel_coli@yahoo.com.br.
Maíra Ramos Alves, Email: mairaramosalves@gmail.com.
Luis Phillipe Nagem Lopes, Email: luis.pharma20161@gmail.com.
Fabiane Raquel Motter, Email: fabianemotter@gmail.com.
Rodrigo Suguimoto Iwami, Email: rodrigo.iwami@gmail.com.
Cristiane de Cássia Bergamaschi, Email: cristiane.bergamaschi@prof.uniso.br.
Marcus Tolentino Silva, Email: marcusts@gmail.com.
Diogo Luis Scalco, Email: dscalco@gmail.com.
Donavan de Souza Lucio, Email: donavanlucio@gmail.com.
Lauren Giustti Mazzei, Email: lauren.mazzei@prof.uniso.br.
Rodrigo D’Agostini Derech, Email: dagostiniderech@gmail.com.
Alexander Itria, Email: alexitria@ufscar.br.
Jorge Otávio Maia Barreto, Email: jorgeomaia@hotmail.com.
Luciane Cruz Lopes, Email: luslopesbr@gmail.com.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
References
- 1.UNAIDS Global HIV & AIDS statistics — fact sheet. 2022. https://www.unaids.org/en/resources/fact-sheet Available.
- 2.Secretaria de Vigilância em Saúde, Departamento de Vigilância, Prevenção e Controle das Infecções Sexualmente Transmissíveis, do HIV/Aids e das Hepatites Virais . Protocolo clínico e diretrizes terapêuticas para manejo da infecção pelo HIV em adultos. Brasília: Ministério da Saúde; 2018. p. 412. [Google Scholar]
- 3.European AIDS Clinical Society Guidelines [Internet] 2021. https://www.eacsociety.org/media/final2021eacsguidelinesv11.0_oct2021.pdf Available.
- 4.National Institute of Health Guidelines for the use of antiretroviral agents in adults and adolescents living with HIV. 2019. https://clinicalinfo.hiv.gov/en/guidelines/hiv-clinical-guidelines-adult-and-adolescent-arv/initiation-antiretroviral-therapy Available.
- 5.World Health Organization . Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. 2o. 2016. p. 480. edn. [PubMed] [Google Scholar]
- 6.Curno MJ, Rossi S, Hodges-Mameletzis I, et al. A systematic review of the inclusion (or exclusion) of women in HIV research: from clinical studies of antiretrovirals and vaccines to cure strategies. J Acquir Immune Defic Syndr . 2016;71:181–8. doi: 10.1097/QAI.0000000000000842. [DOI] [PubMed] [Google Scholar]
- 7.Clark RA, Squires KE. Gender-specific considerations in the antiretroviral management of HIV-infected women. Expert Rev Anti Infect Ther . 2005;3:213–27. doi: 10.1586/14787210.3.2.213. [DOI] [PubMed] [Google Scholar]
- 8.Valiaveettil C, Loutfy M, Kennedy VL, et al. High prevalence of abnormal menstruation among women living with HIV in Canada. PLoS One. 2019;14:e0226992. doi: 10.1371/journal.pone.0226992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Massad LS, Evans CT, Minkoff H, et al. Effects of HIV infection and its treatment on self-reported menstrual abnormalities in women. J Womens Health (Larchmt) . 2006;15:591–8. doi: 10.1089/jwh.2006.15.591. [DOI] [PubMed] [Google Scholar]
- 10.King EM, Albert AY, Murray MCM. HIV and amenorrhea: a meta-analysis. AIDS. 2019;33:483–91. doi: 10.1097/QAD.0000000000002084. [DOI] [PubMed] [Google Scholar]
- 11.Carvalho ÉH de, Gelenske T, Bandeira F, et al. Bone mineral density in HIV-infected women taking antiretroviral therapy: a systematic review. Arq Bras Endocrinol Metab . 2010;54:133–42. doi: 10.1590/S0004-27302010000200008. [DOI] [PubMed] [Google Scholar]
- 12.Cezarino PYA, Simões R dos S, Baracat EC, et al. Are women living with HIV prone to osteoporosis in postmenopause? A systematic review. Rev Assoc Med Bras. 1992;64:469–73. doi: 10.1590/1806-9282.64.05.469. [DOI] [PubMed] [Google Scholar]
- 13.de Oliveira JC, Alves MR, Lopes LPN, et al. Sex differences and adverse events of antiretrovirals in people living with HIV/AIDS: a systematic review and meta-analysis protocol. BMJ Open . 2022;12:e057094. doi: 10.1136/bmjopen-2021-057094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Covidence [Internet] Covidence - better systematic review management. 2022. https://www.covidence.org/ Available.
- 16.Rayyan [internet] https://rayyan.ai/reviews/640676 n.d. Available.
- 17.Cochrane Revised cochrane risk-of-bias tool for randomized trials (Rob 2) 2019.
- 18.IntHout J, Ioannidis JPA, Rovers MM, et al. Plea for routinely presenting prediction intervals in meta-analysis. BMJ Open. 2016;6:e010247. doi: 10.1136/bmjopen-2015-010247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haddaway NR, McGuinness LA, Pritchard CC. PRISMA2020: R package and shinyapp for producing PRISMA 2020 compliant flow diagrams. Zenodo. 2021 doi: 10.1002/cl2.1230. https://zenodo.org/record/4287834 Available. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Campbell TB, Smeaton LM, Kumarasamy N, et al. Efficacy and safety of three antiretroviral regimens for initial treatment of HIV-1: a randomized clinical trial in diverse multinational settings. PLoS Med. 2012;9:e1001290. doi: 10.1371/journal.pmed.1001290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Firnhaber C, Smeaton LM, Grinsztejn B, et al. Differences in antiretroviral safety and efficacy by sex in a multinational randomized clinical trial. HIV Clin Trials. 2015;16:89–99. doi: 10.1179/1528433614Z.0000000013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eron JJ, Orkin C, Gallant J, et al. A week-48 randomized phase-3 trial of darunavir/cobicistat/emtricitabine/tenofovir alafenamide in treatment-naive HIV-1 patients. AIDS. 2018;32:1431–42. doi: 10.1097/QAD.0000000000001817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rashbaum B, Spinner CD, McDonald C, et al. Darunavir/cobicistat/emtricitabine/tenofovir alafenamide in treatment-naïve patients with HIV-1: subgroup analyses of the phase 3 AMBER study. HIV Res Clin Pract. 2019;20:24–33. doi: 10.1080/15284336.2019.1608714. [DOI] [PubMed] [Google Scholar]
- 24.Lockman S, Hughes MD, McIntyre J, et al. Antiretroviral therapies in women after single-dose nevirapine exposure. N Engl J Med. 2010;363:1499–509. doi: 10.1056/NEJMoa0906626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lockman S, Hughes M, Sawe F, et al. Nevirapine- versus lopinavir/ritonavir-based initial therapy for HIV-1 infection among women in africa: a randomized trial. PLoS Med. 2012;9:e1001236. doi: 10.1371/journal.pmed.1001236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Naicker N, Naidoo A, Werner L, et al. Efficacy and safety of tenofovir-containing antiretroviral therapy in women who acquired HIV while enrolled in tenofovir gel prophylaxis trials. Antivir Ther . 2017;22:287–93. doi: 10.3851/IMP3106. [DOI] [PubMed] [Google Scholar]
- 27.Ibrahim F, Samarawickrama A, Hamzah L, et al. Bone mineral density, kidney function, weight gain and insulin resistance in women who switch from TDF/FTC/NNRTI to ABC/3TC/DTG. HIV Med. 2021;22:83–91. doi: 10.1111/hiv.12961. [DOI] [PubMed] [Google Scholar]
- 28.Kityo C, Hagins D, Koenig E, et al. Switching to fixed-dose bictegravir, emtricitabine, and tenofovir alafenamide (B/F/TAF) in virologically suppressed HIV-1 infected women: a randomized, open-label, multicenter, active-controlled, phase 3, noninferiority trial. JAIDS J Acquir Immune Defic Syndr. 2019;82:321–8. doi: 10.1097/QAI.0000000000002137. [DOI] [PubMed] [Google Scholar]
- 29.Orrell C, Hagins DP, Belonosova E, et al. Fixed-dose combination dolutegravir, abacavir, and lamivudine versus ritonavir-boosted atazanavir plus tenofovir disoproxil fumarate and emtricitabine in previously untreated women with HIV-1 infection (ARIA): week 48 results from a randomised, open-label, non-inferiority, phase 3b study. Lancet HIV. 2017;4:e536–46. doi: 10.1016/S2352-3018(17)30095-4. [DOI] [PubMed] [Google Scholar]
- 30.Squires K, Kityo C, Hodder S, et al. Integrase inhibitor versus protease inhibitor based regimen for HIV-1 infected women (WAVES): a randomised, controlled, double-blind, phase 3 study. Lancet HIV. 2016;3:e410–20. doi: 10.1016/S2352-3018(16)30016-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huhn GD, Eron JJ, Girard P-M, et al. Darunavir/cobicistat/emtricitabine/tenofovir alafenamide in treatment-experienced, virologically suppressed patients with HIV-1: subgroup analyses of the phase 3 EMERALD study. AIDS Res Ther. 2019;16:23. doi: 10.1186/s12981-019-0235-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pitrou I, Boutron I, Ahmad N, et al. Reporting of safety results in published reports of randomized controlled trials. Arch Intern Med . 2009;169:1756–61. doi: 10.1001/archinternmed.2009.306. [DOI] [PubMed] [Google Scholar]
- 33.Bedimo R, Rosenblatt L, Myers J. Systematic review of renal and bone safety of the antiretroviral regimen efavirenz, emtricitabine, and tenofovir disoproxil fumarate in patients with HIV infection. HIV Clin Trials . 2016;17:246–66. doi: 10.1080/15284336.2016.1243363. [DOI] [PubMed] [Google Scholar]
- 34.Carr A, Amin J. Efficacy and tolerability of initial antiretroviral therapy: a systematic review. AIDS. 2009;23:343–53. doi: 10.1097/QAD.0b013e32831db232. [DOI] [PubMed] [Google Scholar]
- 35.Hermes A, Squires K, Fredrick L, et al. Meta-analysis of the safety, tolerability, and efficacy of lopinavir/ritonavir-containing antiretroviral therapy in HIV-1-infected women. HIV Clin Trials . 2012;13:308–23. doi: 10.1310/hct1306-308. [DOI] [PubMed] [Google Scholar]
- 36.Coutinho E da SF, Huf G, Bloch KV. Ensaios clínicos pragmáticos: uma opção na construção de evidências em saúde. Cad Saúde Pública. 2003;19:1189–93. doi: 10.1590/S0102-311X2003000400039. [DOI] [PubMed] [Google Scholar]