Abstract
With emerging Asian-derived diet quality indices and data-driven dietary patterns available, we aimed to synthesize the various dietary patterns and quantify its association with cardiovascular diseases (CVDs) among Asian populations. We systematically searched PubMed, Embase, Scopus, and Web of Science for observational studies in South, Southeast, and East Asia. Dietary patterns were grouped “high-quality,” which included high intakes of three or more of the following food groups: 1) fruits and vegetables, 2) whole grains, 3) healthy protein sources (legumes and nuts, fish and seafood, low-fat dairy, and lean meat and poultry), and 4) liquid plant oils. High-quality patterns were further subcategorized based on their derivation methods: non-Asian indices, Asian indices, data-driven patterns, and plant-based indices. Dietary patterns were grouped “low-quality,” which included high intakes of two or more of the following: 5) ultraprocessed food, 6) beverages and foods with added sugars, 7) foods high in salt, and 8) alcoholic beverages. Data-driven dietary patterns characterized by animal food sources were labeled “animal-based,” and studies using dietary diversity scores were labeled “diet diversity indices.” Dietary patterns that could not be meaningfully categorized were summarized narratively. Study-specific effect estimates were pooled using a random-effects model. Forty-one studies were included in this review. Higher adherence to high-quality dietary patterns in the top compared with bottom tertile defined by non-Asian indices (RR: 0.78; 95% CI: 0.69, 0.88; GRADE: moderate), Asian indices (RR: 0.84; 95% CI: 0.79, 0.90; GRADE: low), and data-driven patterns (RR: 0.81; 95% CI: 0.74, 0.89; GRADE: moderate) were associated with lower CVD risk. Plant-based, low-quality, animal-based, and diet diversity indices dietary patterns were not associated with CVD. Associations of Asian diet quality indices and CVD risk were weaker than those with non-Asian indices, highlighting the need for current Asian diet quality criteria to be updated to better capture the impact of diet on CVD.
The systematic review and meta-analysis was registered at PROSPERO as CRD42021244318.
Keywords: diet, dietary pattern, diet quality, cardiovascular disease, Asia, meta-analysis
Statements of significance.
To our knowledge, this is the first meta-analysis to comprehensively describe the associations between non-Asian diet quality indices, Asian-derived diet quality indices, and data-driven dietary patterns and CVD risk among South, Southeast, and East Asian populations. It demonstrates that current Asian-derived diet quality indices predict a weaker association with CVD than non-Asian diet quality indices, highlighting the need for current Asian-derived diet quality indices to be reviewed to reflect up-to-date dietary recommendations for cardiovascular health.
Introduction
Cardiovascular diseases (CVDs) are among the major leading causes of mortality in the world [1], with a high proportion of premature CVD deaths occurring in Asia [2]. A diet high in salt, sugars, saturated and trans fats and low in fiber and healthy plant foods is a well-recognized modifiable risk factor for CVD [1,3].
The shift toward dietary patterns analysis has emerged as a more holistic research approach considering an individual’s overall diet, capturing the interrelationship between foods, food groups, and nutrients [4]. Dietary patterns are often evaluated by the following: 1) an index-based or a priori method, which measures adherence to dietary recommendations using predefined dietary indices from epidemiologic research findings or 2) a data-driven or a posteriori method, which uses population-based dietary intake data to derive possible consumption patterns via statistical methods [4].
Traditional Asian diet is rich in fruits and vegetables and in plant foods such as soy or lentils, with use of unsweetened tea, fermented foods, and herbs and spices [5,6]. Taking into consideration the marked differences in dietary habits from non-Asian populations, a priori dietary scores to measure adherence to country-specific dietary recommendations within Asia has been increasingly developed, such as the Japanese Food Guide Spinning Top and Chinese Food Pagoda (CHFP) scores which capture information on intake by food groups and specific foods that are distinctive to the population [7,8].
Previous reviews and meta-analyses conducted on dietary patterns and CVD were focused on either diet quality indices developed based on data from non-Asian populations (e.g., Alternative Healthy Eating Index [AHEI], Dietary Approaches to Stop Hypertension [DASH] score) [[9], [10], [11], [12], [13], [14], [15], [16]] or data-driven methods [[17], [18], [19]]. Although an inverse association between a higher quality diet and lower CVD risk has been established consistently, existing research on a heart-healthy dietary pattern are mostly conducted in the non-Asian populations. Furthermore, a previous review that conducted a subgroup analysis on Asian-derived data-driven prudent dietary patterns (n = 5 studies) found a weaker inverse association with CVD mortality when compared with those in non-Asian populations [18]. However, only publications available up to 2014 were considered, suggesting the need for up-to-date evidence to examine whether Asian-derived dietary patterns are associated with lower CVD risk.
Given the emerging published literature on dietary patterns in South, Southeast, and East Asia, we aimed to conduct a systematic review and meta-analysis on the associations between dietary patterns and CVD among these Asian populations.
Methods
This systematic review and meta-analysis was performed in accordance with the Meta-analyses of Observation Studies in Epidemiology (MOOSE) reporting guidelines [20]. The review protocol was registered in PROSPERO International Prospective Register of Systematic Reviews (CRD42021244318).
Search strategy
A systematic search was conducted in PubMed, Embase, Scopus, and Web of Science for articles published up to July 2023, with no language or time restriction. The search strategy combined key words “diet,” “dietary pattern,” “diet quality,” “cardiovascular disease,” “prospective,” and “Asia.” Details of the full search strategy can be found in Supplemental Table 1. In addition, reference list of the selected articles and previous systematic reviews was also hand-searched for relevant studies.
Selection criteria
We used the PECOS (Population, Exposure, Comparator, Outcomes, Study design) criteria to identify eligible studies as summarized in Table 1. Studies were included if they met the following criteria: 1) prospective studies conducted in South (e.g., Bangladesh and India), Southeast (e.g., Indonesia and Singapore), and East Asian (e.g., China and Japan) populations; 2) generally healthy adults aged 18 years and older; 3) studies that evaluated the associations between index-based or data-driven dietary patterns as exposure of interest; and 4) ultimate end points of CVD incidence or mortality as outcome of interest. Studies were excluded if they 1) were conducted in West (e.g., Iraq and Saudi Arabia) and Central Asian (e.g., Kazakhstan and Uzbekistan) populations owing to differences in culinary culture and ethnicity, and migrated Asians due to dietary acculturation; 2) were performed in populations with underlying medical conditions such as diabetes and renal disease or examined intermediate CVD outcomes such as blood pressure, blood glucose concentrations or blood lipids, and 3) examined a single food or nutrient, food group, or dietary component (e.g., low carbohydrate diet and low glycemic index diet), dietary patterns derived by intermediate methodologies such as reduced rank regression or reflected only a few foods that are a minor part of the diet, nutrient-based scores (e.g., DASH nutrient score), or combined diet and exercise interventions as the exposure of interest.
TABLE 1.
Inclusion and exclusion criteria of articles using the PECOS criteria
| Parameter | Inclusion criteria | Exclusion criteria |
|---|---|---|
| Population | South, Southeast, East Asian populations | Migrated Asians, West and Central Asian populations, population with underlying medical conditions (e.g., diabetes and renal disease) |
| Exposure | Dietary patterns (data-driven1 or index-based2) | Studies examining single food or nutrient, food group, or dietary component (e.g., low glycemic index diet), dietary patterns derived by intermediate methodologies such as reduced rank regression, dietary patterns that reflect only a few foods that are a minor part of the diet, nutrient-based scores (e.g., DASH nutrient score), combined diet and exercise interventions |
| Comparator | Highest vs. lowest category of dietary patterns3 | Not applicable |
| Outcomes | CVD incidence and mortality | Intermediate CVD outcomes (e.g., blood pressure, blood glucose concentrations, and blood lipids), other health outcomes (e.g., type 2 diabetes mellitus, metabolic syndrome, cancers, and renal or liver or neurogenerative diseases), risk prediction scores for CVD |
| Study design | Prospective cohort studies, nested case–control studies, case–cohort studies | Literature/narrative reviews, cross-sectional studies, case–control studies, ecological studies, nonhuman studies |
Abbreviations: CVD, cardiovascular disease; DASH, Dietary Approaches to Stop Hypertension; PECOS, Population, Exposure, Comparator, Outcomes, Study design.
Derived by data-driven approach such as principal component analysis or factor analysis.
Combination of food-based and nutrient-based diet quality indices constructed to measure adherence to national dietary guidelines or recommendations.
Highest and lowest category of dietary patterns (e.g., highest and lowest quantile or score categories defined by cutoff points) reported in the original study.
Two authors independently screened the titles and abstracts of articles, and full texts of the potential articles were retrieved and evaluated for eligibility. The initial search for articles published through March 2022 was conducted by NN and YQL. The search was updated by GHL and AC for articles published through July 2023. Disagreements were resolved by discussion with two investigators (RMvD and MF-FC).
Data extraction
A standardized data extraction form was designed to extract the following data from each study: 1) study characteristics (geographic region, study design, sample size, and follow-up duration); 2) population characteristics (age, sex, BMI, medical history, and inclusion and exclusion criteria); 3) dietary patterns (a priori and a posteriori) and its components, level of intake (score categories, tertile, and quartile or quintile), and method of dietary assessment (food frequency questionnaire, 24-hour dietary recall, and food record); 4) types of study outcome, number of cases, and ascertainment method; 5) statistical analysis method, effect estimates with its corresponding 95% confidence intervals (CIs) for the most adjusted model alongside the covariates included. For this study, the outcome of interest was CVD incidence and CVD-related mortality, which encompasses major CVD subtypes including ischemic heart disease (IHD), acute myocardial infarction (AMI), cerebrovascular diseases, and heart failure, according to the International Classification of Diseases (ICD) [21,22]. When necessary, we contacted the respective authors to obtain additional information that would be relevant. Data extraction was carried out by two pairs of independent authors (NN and SHP; GHL and ZHK). Any disagreements were resolved by discussion with two investigators (RMvD and AC).
Risk of bias assessment
Independent critical appraisal of the included studies was conducted by three authors (NN, GHL, and ZHK) using risk of bias in nonrandomized studies of interventions (ROBINS-I) tool [23]. The ROBINS-I tool was adapted for use to appraise observational cohort studies by substituting the two domains on interventions to exposure instead. The tool comprises seven domains: 1) bias due to confounding, 2) bias in selection of participants, 3) bias in classification of exposure measurement, 4) bias in misclassification of exposure during follow-up, 5) bias due to missing data, 6) bias in measurement of outcomes, and 7) bias in selection of reported result. A predefined set of confounders were selected a priori to assess the first domain on bias due to confounding: a history of CVD, diabetes, or hypertension; age; sex; BMI; physical activity; smoking; alcohol consumption; and energy intake. Studies were assessed for each domain and classified as “low” (comparable with a well-performed randomized trial), “moderate” (sound for a nonrandomized study), “serious” (important methodological problems), or “critical” (too problematic to provide any useful evidence) risk of bias. To reach an overall judgment, the study was appraised “low” when the response option for all domains were low; “moderate” when there were both low and moderate responses across the domains; “serious” when ≥1 domain was assessed a serious risk of bias; and “critical” when ≥1 domain was assessed a critical risk of bias (Supplemental Table 2). Differences in the overall judgment between authors were resolved by discussion with two investigators to arrive at a consensus (RMvD and AC).
Certainty of evidence assessment
The GRADE (Grading of Recommendations, Assessment, Development and Evaluation) tool was used to assess the certainty of synthesized findings for each pooled effect estimate [24]. Certainty of evidence was rated as follows: “very low” indicating that the true effect is likely to be substantially different from the effect estimate; “low” indicating that the true effect may be substantially different from the effect estimate; “moderate” indicating that the true effect is likely to be close to the effect estimate but with the possibility of substantial difference; and “high” indicating that we are very confident that the true effect is similar to the effect estimate. According to the framework described by Schünemann et al. [25], observational studies that were assessed for risk of bias using ROBINS-I may start with a high certainty of evidence and subsequently rated down if there is a presence of moderate or serious risk of bias, inconsistency, indirectness, imprecision, and/or publication bias (Supplemental Table 3).
Grouping of dietary patterns derived from studies
Using the 2021 American Heart Association (AHA) scientific statement on Dietary Guidance to Improve Cardiovascular Health [3] as a guide, index-based and data-driven dietary patterns were broadly categorized as high-quality if they were characterized by high intakes of three or more of the following food groups: 1) fruits and vegetables, 2) whole grains, 3) healthy protein sources (legumes and nuts, fish and seafood, low-fat dairy, and lean meat and poultry), and 4) liquid plant oils. High-quality dietary patterns were further subdivided into groups based on their derivation methods (i.e., data-driven or indices, which were further subcategorized into Asian or non-Asian indices and plant-based indices) to examine their performance when related to CVD: 1) non-Asian indices, comprising studies adopting diet quality indices or pattern scores derived from non-Asian populations, such as the Alternative Healthy Eating Index-2010 (AHEI-2010) and Mediterranean diet score; 2) Asian indices, comprising studies using indices and scores derived from an Asian country or study cohort, such as the CHFP score, 3) data-driven patterns, comprising studies using exploratory patterns that were statistically derived, and 4) plant-based indices, comprising studies that adopted a scoring algorithm to measure consumption of healthy plant foods.
Dietary patterns were categorized as low-quality if they were characterized by high intakes of two or more of the following food/beverage groups described in the 2021 AHA Dietary Guidance: 5) ultraprocessed food, 6) beverages and foods with added sugars, 7) foods high in salt, and 8) alcoholic beverages. Next, dietary patterns that were data-driven and had high factor loadings of similar animal food sources such as meat and poultry, processed meat, and fish were categorized as animal-based dietary pattern. Finally, studies using dietary diversity scores as an indicator of diet adequacy and quality were categorized as diet diversity indices.
Common dietary patterns, regardless of the naming of the dietary pattern, were grouped and meta-analyzed if they shared similar constituent foods. A detailed description of the dietary patterns included in meta-analysis is presented in Supplemental Table 4.
If similar dietary patterns within the same cohort were identified, results from the study with a longer follow-up and/or larger number of cases were included in the main analysis, while the remaining patterns were considered in sensitivity analysis by including one pattern at a time to determine the robustness of result. Dietary patterns that could not be meaningfully categorized into the aforementioned categories (e.g., dairy product pattern, vegetarian pattern, and absence of factor loadings for data-driven patterns) [[26], [27], [28], [29], [30], [31]]; a study with effect estimates reported in odds ratio [32], which may not be an accurate approximation of the effect size when expressed as relative risk (RR) in the meta-analysis, recommended by the Cochrane Handbook for Systematic Reviews of Interventions [33]; and studies with effect estimates referenced to other dietary patterns within the study cohort [34,35] were summarized narratively (Supplemental Table 5).
Statistical methods
To examine the overall association between dietary patterns and CVD expressed in RR, we combined study-specific effect estimates using the random-effects model, which was chosen to account for between-study variations [33,36]. As studies included in the meta-analysis reported a mixture of equal-sized groups (i.e., tertile/quartile/quintile) [7,8,[26], [27], [28],30,[37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51]] and unequal-sized groups (i.e., score categories defined by cutoff points) [37,44,[52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [65], [66]], we transformed the effect estimates from each study to a standard scale of effect, facilitating comparison between the RR at top and bottom tertiles, according to the methods described previously [[67], [68], [69]]. Under a log-normal distribution, the difference in mean between the top and bottom groups of tertile, quartile, and quintile can be expressed by 2.18, 2.54, and 2.80 standard deviation (SD, respectively. Therefore, the log RR and corresponding standard error were multiplied by the following: 1) 2.18/2.54, for conversion of top and bottom quartile to tertile; 2) 2.18/2.80, for conversion of top and bottom quintile to tertile; and 3) 2.18/x for conversion of unequal-sized groups (i.e., score categories) to tertile, where x is the difference in mean scores expressed in SD between the top and bottom categories.
Similar to previous meta-analyses [9,17], we combined outcomes on CVD incidence and CVD mortality to examine its association with dietary patterns. When the study reported effect estimates on mortality for multiple CVD subtypes separately, for example, stroke and IHD, we pooled the estimates for each subtype using a fixed-effects model to obtain an overall estimate for total CVD mortality to be used in the meta-analysis. If multiple outcome measurements were reported within the same cohort, for instance, CVD incidence and CVD mortality, results from the study with a longer follow-up and/or larger number of cases were included in the main analysis while the others were considered in sensitivity analysis by including one study at a time.
Publication bias was assessed by visual inspection of funnel plots and quantified by Egger’s test for funnel plot asymmetry when there were ≥5 studies included in the meta-analysis [33]. We evaluated heterogeneity using Cochran’s Q test (P-heterogeneity) and I2 statistics. Significant heterogeneity was considered if I2 value was >50% [33,70]. When there were 10 or more studies, potential sources of heterogeneity were further explored by performing meta-regression analyses [33]. We used the sequential exclusion strategy described by Patsopoulos et al. [70] to examine whether overall estimates were influenced by the substantial heterogeneity observed. Studies that accounted for the largest proportion of heterogeneity were sequentially and cumulatively excluded until the I2 value was <50%. We then examined the overall estimates before and after exclusion for consistency. Further sensitivity analyses were also conducted by excluding one study at a time and recalculating the pooled effect estimate for the remaining studies, excluding studies considered to have serious risk of bias, and calculating the pooled effect estimates using unconverted data to examine the robustness of our findings.
Results of the meta-analysis were presented using forest plots, illustrating the pooled and individual study effect estimates with its corresponding 95% CI, weightage of each study, and I2 statistic. All statistical analyses were performed using Stata 18 (StataCorp), and two-tailed P values of <0.05 were considered statistically significant.
Results
Study selection
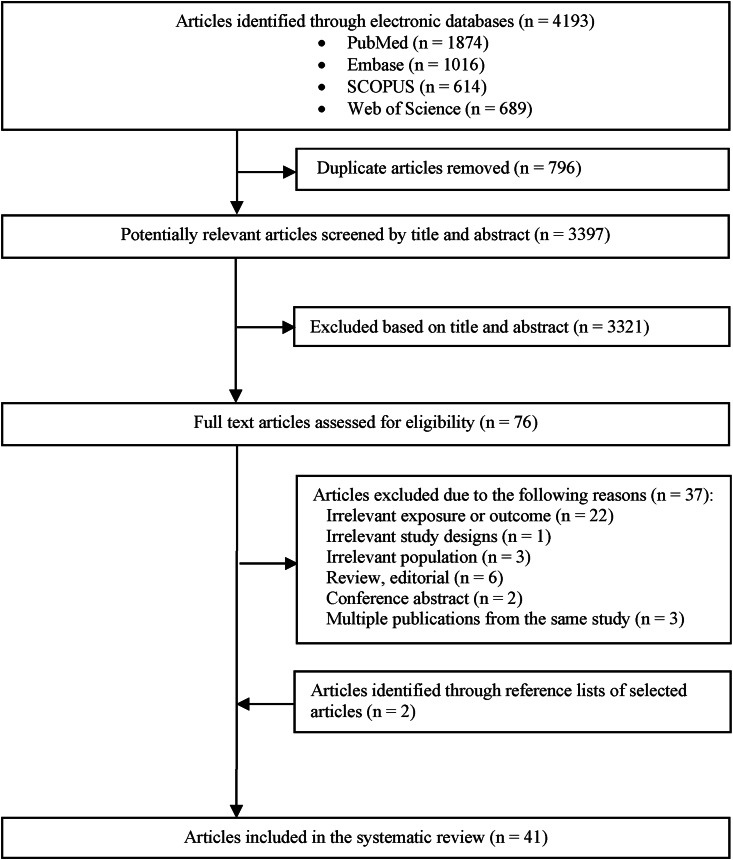
A flow chart of the study selection process is shown in Figure 1. In brief, 3397 unique articles were identified in the initial search. After screening by title and abstract, full texts of 76 articles were evaluated and 37 articles were excluded. Two additional studies were identified from hand-searching of reference lists. Finally, 41 studies were included in this systematic review.
FIGURE 1.
Flowchart of the study selection process.
Study characteristics
All studies were conducted in Asia. There were 14 studies conducted in China [7,29,30,43,50,51,53,[57], [58], [59], [60], [61],63,65], 12 in Japan [8,26,27,31,34,40,41,45,48,49,56,62], 5 in Taiwan [35,39,52,55,66], 3 in Singapore [32,38,42], 3 in Hong Kong [37,44,47], 2 in South Korea [28,54], and 2 in South Asian countries (Bangladesh, India, and Pakistan) [46,64]. All were prospective cohort studies, except for one study which had a nested case–control study design [32]. The number of participants in these studies ranged from 799 to 636,000 and the follow-up duration from 2.4 to 19.4 years.
Dietary intake was mostly assessed by food frequency questionnaire; one study used a three-day 24-hour dietary recall [43], and another study used a three-day weighed food record [49]. Twenty-four studies reported on CVD mortality [7,8,[26], [27], [28],34,37,38,[40], [41], [42],[45], [46], [47], [48], [49],51,[54], [55], [56],59,62,63,66], eight studies on CVD (or CVD subtypes) incidence [29,30,35,43,50,53,60,65], and nine studies on both [31,32,39,44,52,57,58,61,64]. The measures of association were commonly adjusted for age, sex, BMI, physical activity, education level, and a history of diabetes and hypertension. Characteristics of the studies are summarized in Table 2, and covariates adjusted for in the multivariable models are illustrated in Supplemental Table 6.
TABLE 2.
Characteristics of studies included in systematic review and meta-analysis of dietary patterns and CVD in Asia
| Cohort | Lead author | Sample size1,2 | Age (y)3 | Baseline survey years | Follow-up (y) | Dietary pattern | Outcome type | |
|---|---|---|---|---|---|---|---|---|
| China | ||||||||
| 4C Study | Li et al. [57] | 130,780 | 57.0 | 2010–2011 | 3.8 | Healthy diet score | CVD incidence and mortality | |
| China-PAR4 | Han et al. [61] | 93,987 | 51.6 | China MUCA: 1998 | 15 | Healthy diet score | CVD incidence and mortality | |
| InterASIA: 2000–2001 | 13 | |||||||
| CIMIC: 2007–2008 | 6 | |||||||
| China PEACE MPP | Zhang et al. [63] | 636,000 | 54.1 | 2015–2019 | 2.4 | Healthy diet score | CVD mortality | |
| CHNS | Shi and Ganji [43] | 13,055 | 44 | 1991–2011 | 9 | Traditional Modern |
CVD incidence | |
| CKB | Han et al. [29] | 459,606 | 51.25 | 2004–2008 | 11.2 | Unhealthy diet6 | CVD incidence | |
| Qin et al. [30] | 477,465 | 51.1 | 2004–2008 | 10.5 | Traditional northern6 Modern |
CVD incidence | ||
| Zhu et al. [59] | 487,198 | 51.5 | 2004–2008 | 10.2 | Healthy diet score | CVD mortality | ||
| Su et al. [60] (Suzhou subcohort) | 31,727 | 48.1-50.1 | 2004–2008 | 10.1 | Healthy diet score | CVD incidence | ||
| CMEC | Chen et al. [53] | 22,160 | 51.4 | 2018–2019 | 3.8 | Modified DASH | Stroke incidence | |
| CVD-China | Dong et al. [65] | 8754 | 35-64 | 2009–2010 | 6.3 | Ideal diet score | CVD incidence | |
| GPHCS | Wang et al. [50] | 7841 | 44.2 | 2010–2012 | 6.6 | Higher bound score | Ischemic stroke incidence | |
| PURE—China | Li et al. [58] | 47,262 | 51.1 | 2005–2009 | 11.9 | PURE diet score | CVD incidence and mortality | |
| SMHS | Yu et al. [7] | 61,239, men | 54.8-55.9 | 2002–2006 | 6.5 | CHFP score Modified DASH Modified AHEI-2010 |
CVD mortality | |
| SWHS | 73,216, women | 52.2-53.0 | 1996–2000 | 12 | ||||
| Cai et al. [51] | 52,698, women | 52.0-60.55 | 1996–2000 | 5.7 | Vegetable-rich6 Fruit-rich6 Meat-rich |
CVD mortality | ||
| Japan | ||||||||
| JACC | Maruyama et al. [27] | 26,598, men | 53.7-58.2 | 1988–1990 | 12.6 | Vegetable Animal food Dairy product6 |
CVD mortality | |
| 37,439, women | 54.6-58.9 | |||||||
| Okada et al. [56] | 23,162, men | 54.2-57.2 | 1988–1990 | 18.9 | Japanese food score | CVD mortality | ||
| 35,605, women | 55.9-56.7 | 19.4 | ||||||
| JPHC | Kobayashi et al. [48] | 37,240, men | 55.7-57.2 | 1995–1999 | 14.9 | DDS | CVD mortality | |
| 42,664, women | 56.5-57.5 | |||||||
| Kurotani et al. [8] | 79,594 | 50.4-51.5 | 1995–1998 | 14.9 | Japanese food guide spinning top | CVD mortality | ||
| Matsuyama et al. [62] | 92,969 | 56.6 | 1995–1998 | 18.9 | JDI8 | CVD mortality | ||
| Nanri et al. [45] | 81,720 | 53.9-60.1 | 1995–1998 | 14.8 | Prudent Westernized Traditional Japanese |
CVD mortality | ||
| NILS-LSA | Otsuka et al. [49] | 799 | 67.9-68.4 | 1997–2000 | 15.7 | DDS | CVD mortality | |
| NIPPONDATA80 | Nakamura et al. [41] | 9086 | 49.1-51.7 | 1980 | 19 | Reduced-salt Japanese diet score | CVD mortality | |
| Ohsaki | Shimazu et al. [26] | 40,547 | 57.0-63.8 | 1994 | 7 | Japanese Animal food High-dairy, high-fruit-and-vegetable,and low-alcohol6 |
CVD mortality | |
| SAKUCESS | Li et al. [34] | 12,883 | 53.3 | 1980–1990 | 10 | Healthy6 Traditional6 Western6 |
CVD mortality | |
| Shibata study | Seino et al. [31] | 2283 | Not reported | 1977 | 15.5 | Westernized6 | Stroke incidence and mortality | |
| Takayama study | Oba et al. [40] | 13,355, men | 53.1-55.7 | 1992 | NR | Japanese food guide spinning top | CVD mortality | |
| 15,724, women | 54.5-56.1 | |||||||
| Taiwan | ||||||||
| 2002 TwSHHH | Tsai et al. [52] | 6048 | 44.9 | 2002 | 12.5 | Alternative MDS | CVD incidence and mortality | |
| CVDFACTS | Lin et al. [39] | 2061 | 45.5 | 1990–1993 | 12 | DASH | Stroke incidence and mortality | |
| NAHSIT 1999–2000 | Lee et al. [66] | 1743 | 72.1-74.4 | 1999–2000 | NR | ODI-R DDS |
CVD mortality | |
| NAHSIT 1993–1996 | Chuang et al. [55] | 2475 | 43.5 | 1993–1996 | 17.8 | Taiwanese Eating Approach Score | CVD mortality | |
| Cohort 1: TCHS | Chiu et al. [35] | 5050 | 51.7-53.8 | 2007–2009 | 6.1 | Vegetarian6 Nonvegetarian6 |
Stroke incidence | |
| Cohort 2: TCVS | 8302 | 49.2-50.1 | 2005 | 9.2 | ||||
| Singapore | ||||||||
| SCHS | Neelakantan et al. [38] | 57,078 | 56 | 1993–1998 | 17 | aMED AHEI-2010 DASH |
CVD mortality | |
| Odegaard et al. [42] | 52,584 | 53.6-58.3 | 1993–1998 | 15.1 | Vegetable, fruit, and soy rich Dim sum and meat-rich |
CVD mortality | ||
| Neelakantan et al. [32] | 2194 | Cases: 59.5,Controls: 59.3 | 1993–1998 | NR | AHEI-20106 | MI incidence and mortality | ||
| Hong Kong | ||||||||
| Mr. and Ms. Os | Chan et al. [37] | 932, men | 73 | 2001–2003 | 12 | Vegetables and fruits Snacks, drinks, and milk products Meat and fish DQI-I MDS DASH MIND Okinawan diet score |
CVD mortality | |
| 1127, women | 12.7 | |||||||
| Chan et al. [44] | 1338, men | 72.2 | 2001–2003 | 5.7 | Vegetables and fruits Snacks, drinks, and milk products Meat and fish MDS DASH |
Stroke incidence and mortality | ||
| 1397, women | 72.4 | |||||||
| Lo et al. [47] | 3991 | 72.4-72.6 | 2001–2003 | 11.1 | Portfolio diet score | CVD mortality | ||
| Korea | ||||||||
| KoGES_HEXA | Kim et al. [28] | 118,577 | 52.7 | 2004–2013 | 10 | Overall plant-based dietindex (PDI)6 Healthful PDI Unhealthful PDI |
CVD mortality | |
| Seoul Male | Kim et al. [54] | 12,538, men | 47.3-50 | 1993 | 19 | Healthy diet score | CVD mortality | |
| South Asian countries (Bangladesh, India, and Pakistan) | ||||||||
| HEALS | Chen et al. [46] | 11,116 | 35.7-39.4 | 2000–2002 | 6.6 | Balanced diet Animal protein Gourd and root vegetable6 |
CVD mortality | |
| PURE—South Asia | Joseph et al. [64] | 33,583 | 48.4 | 2003–2009 | 11 | PURE diet score | CVD incidence and mortality | |
Abbreviations: AHEI, Alternative Healthy Eating Index; aMED, alternate Mediterranean diet; CHFP, Chinese Food Guide Pagoda; CHNS, China Health and Nutrition Survey; CKB, China Kadoorie Biobank; CMEC, China Multi-Ethnic Cohort; CVD, cardiovascular disease; CVDFACTS, CardioVascular Disease risk FACtor Two-township Study; DASH, Dietary Approaches to Stop Hypertension; DDS, Dietary Diversity Score; DQI-I, Diet Quality Index-International; FFQ, food frequency questionnaire; GPHCS, Guizhou Population Health Cohort Study; HEALS, Health Effects of Arsenic Longitudinal Study; IHD, ischemic heart disease; JACC, Japan Collaborative Cohort; JDI8, 8-item Japanese Diet Index; JPHC, Japan Public Health Center-based Prospective Study; KoGES_HEXA, Korean Genome and Epidemiology Study Health Examinees; MDS, Mediterranean diet score; MI, myocardial infarction; MIND, Mediterranean-DASH diet Intervention for Neurodegenerative Delay; NAHSIT, Nutrition and Health Survey in Taiwan; NILS-LSA, National Institute for Longevity Sciences-Longitudinal Study of Aging; NIPPONDATA80, National Integrated Project for Prospective Observation of Non-Communicable Diseases and its Trends in the Aged, 1980; NR, not reported; ODI-R, Overall Dietary Index-Revised; PEACE MPP, Patient-centered Evaluative Assessment of Cardiac Events Million Persons Project; PURE, Prospective Urban Rural Epidemiology; SAKUCESS, Saku Cancer Etiology Surveillance Study; SCHS, Singapore Chinese Health Study; SMHS, Shanghai Men’s Health Study; SWHS, Shanghai Women’s Health Study; TCHS, Tzu Chi Health Study; TCVS, Tzu Chi Vegetarian Study; TwSHHH, Taiwan Survey of Hypertensive, Hyperglycemia, Hyperlipidemia Survey; 4C, China Cardiometabolic Disease and Cancer Cohort.
Number of participants included in current CVD analysis.
Participants consist of both men and women, unless otherwise specified.
Presented as mean or range.
The China-PAR project consists of 3 cohorts: 1) China MUCA, China Multi-Center Collaborative Study of Cardiovascular Epidemiology; 2) InterASIA, International Collaborative Study of CVD in Asia; 3) CIMIC, Community Intervention of Metabolic Syndrome in China and Chinese Family Health Study.
Data for specific population included in CVD analysis were not available; mean age based on entire study cohort reported in original study was taken.
Dietary patterns not included in meta-analysis.
Risk of bias assessment
All studies were assessed to have a moderate risk of bias, except for 11 studies that were considered to have serious risk of bias due to potential confounding by general health status or medical history [31,39,46,48,57,59,63,65,66]; a lack of validated instrument to assess dietary intakes [41,63]; or the use of self-reported outcomes that were not validated by medical records [43] (Supplemental Table 2).
Association between dietary patterns and CVD
High-quality dietary patterns.
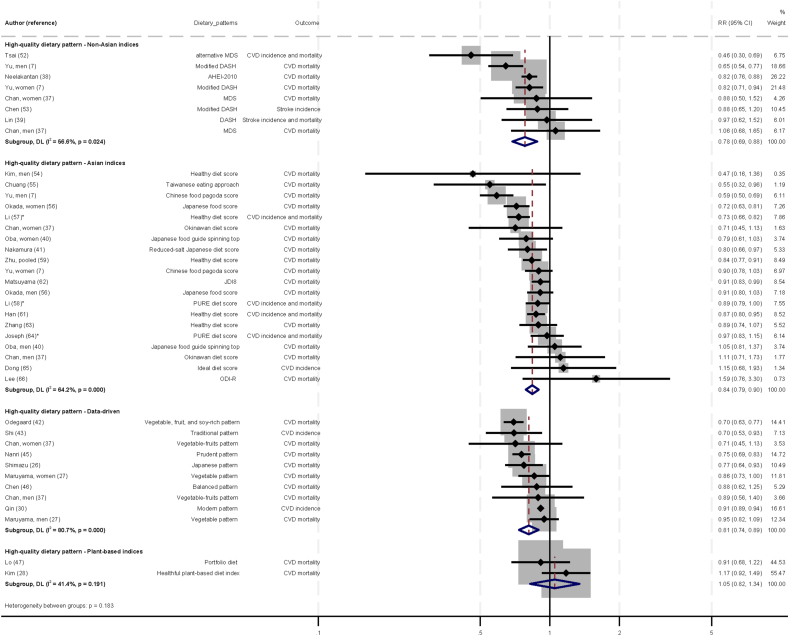
The associations between a high-quality dietary pattern (defined by non-Asian indices, Asian indices, data-driven patterns, and plant-based indices) and CVD are illustrated in Figure 2 [71]. Six studies were included in the analysis of non-Asian indices [7,[37], [38], [39],52,53], 16 studies in the analysis of Asian indices [7,37,40,41,[54], [55], [56], [57], [58], [59],[61], [62], [63], [64], [65], [66]], and 8 studies in the analysis of data-driven patterns [26,27,30,37,42,43,45,46]. When data were pooled within each group, the direction of associations with CVD were similar, suggesting that higher adherence to a high-quality dietary pattern, whether defined by non-Asian indices, Asian indices, or data-driven patterns, was associated with lower CVD risks when comparing between the top and bottom tertiles (non-Asian indices—RR: 0.78; 95% CI: 0.69, 0.88; I2 = 56.6%; P-heterogeneity = 0.024; Asian indices—RR: 0.84; 95% CI: 0.79, 0.90; I2 = 64.2; P-heterogeneity < 0.001; data-driven patterns—RR: 0.81; 95% CI: 0.74, 0.89; I2 = 80.7; P-heterogeneity < 0.001). In contrast, higher scores for plant-based indices [28,47] were not associated with CVD (RR: 1.05; 95% CI: 0.82, 1.34; I2 = 41.4; P-heterogeneity = 0.191; n = 2 studies). The GRADE certainty of evidence was moderate for non-Asian indices and data-driven patterns, and low for Asian indices and plant-based indices.
FIGURE 2.
Associations between high-quality dietary pattern (defined by non-Asian indices, Asian indices, data-driven patterns, and plant-based indices) and cardiovascular diseases in Asia, comparing top and bottom tertile of dietary patterns. Individual effect estimates and corresponding 95% CI for each study are a black dot and horizontal line. Weight of each study is represented by size of gray square. Pooled RR from a random-effects model are represented by diamonds. ∗To obtain the difference in mean dietary pattern scores expressed in SD units between top and bottom categories: 1) distribution of healthy diet scores based on original study population was used for calculation in the publication by Li et al. [57]; 2) distribution of PURE diet scores were obtained from Mente et al. [71] as this information were not available in the original publications [58,64]. CI, confidence interval; CVD, cardiovascular disease; RR, relative risk.
Low-quality dietary pattern.
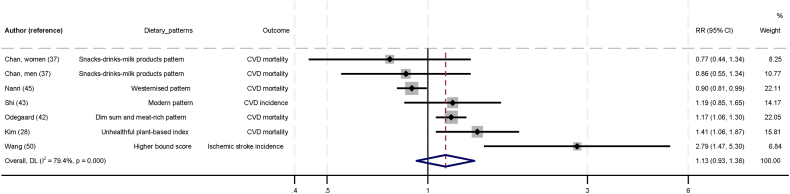
Figure 3 shows the association between low-quality dietary pattern and CVD. In the six studies that evaluated low-quality dietary patterns [28,37,42,43,45,50], no statistically significant association with CVD was found (RR: 1.13; 95% CI: 0.93, 1.38; I2 = 79.4; P-heterogeneity < 0.001). The GRADE certainty of evidence was low.
FIGURE 3.
Association between low-quality dietary pattern and cardiovascular diseases in Asia, comparing top and bottom tertile of dietary patterns. Individual effect estimates and corresponding 95% CI for each study are a black dot and horizontal line. Weight of each study is represented by size of gray square. Pooled RR from a random-effects model are represented by diamonds. CI, confidence interval; CVD, cardiovascular disease; RR, relative risk.
Animal-based dietary pattern.
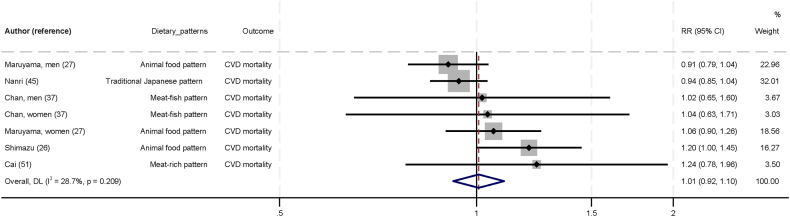
Figure 4 shows the association between animal-based dietary pattern and CVD. No statistically significant association was found in the five studies on animal-based dietary patterns [26,27,37,45,51] and CVD (RR: 1.01; 95% CI: 0.92, 1.10; I2 = 28.7; P-heterogeneity = 0.209). The GRADE certainty of evidence was low.
FIGURE 4.
Association between animal-based dietary pattern and cardiovascular diseases in Asia, comparing top and bottom tertile of dietary patterns. Individual effect estimates and corresponding 95% CI for each study are a black dot and horizontal line, respectively. Weight of each study is represented by size of gray square. Pooled RR from a random-effects model are represented by diamonds. CI, confidence interval; CVD, cardiovascular disease; RR, relative risk.
Diet diversity indices dietary pattern.
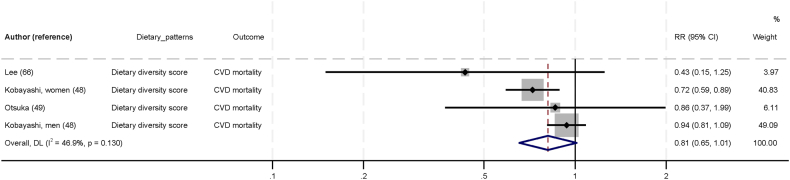
Figure 5 shows the association between diet diversity indices dietary pattern and CVD. In the three studies that evaluated dietary diversity indices [48,49,66], higher scores of diversity showed a trend toward lower CVD risk when comparing between the top and bottom tertiles (RR: 0.81; 95% CI: 0.65, 1.01; I2 = 46.9; P-heterogeneity = 0.130). The GRADE certainty of evidence was very low.
FIGURE 5.
Association between diet diversity indices dietary pattern and cardiovascular diseases in Asia, comparing top and bottom tertile of dietary patterns. Individual effect estimates and corresponding 95% CI for each study are a black dot and horizontal line, respectively. Weight of each study is represented by size of gray square. Pooled RR from a random-effects model are represented by diamonds. CI, confidence interval; CVD, cardiovascular disease; RR, relative risk.
Publication bias
No evidence of publication bias was detected by visual inspection of the funnel plot and Egger’s test: non-Asian indices (Supplemental Figure 1A): Egger’s test P = 0.821; Asian indices (Supplemental Figure 1B): Egger’s test P = 0.979; data-driven patterns (Supplemental Figure 1C): Egger’s test P = 0.100; low-quality dietary pattern (Supplemental Figure 1D): Egger's test P = 0.476; and animal-based dietary pattern (Supplemental Figure 1E): Egger’s test P = 0.245.
Identifying sources of heterogeneity
For dietary patterns with 10 or more studies, we performed meta-regression analyses on studies of Asian indices of high-quality dietary pattern to examine the potential sources of heterogeneity. We did not detect statistically significant differences in pooled effect estimates between any of the subgroups (Table 3).
TABLE 3.
Meta-regression analyses for association between high-quality dietary pattern defined by Asian indices and CVD
| Characteristics | Asian indices |
||||
|---|---|---|---|---|---|
| n | RR (95% CI) | I2 (%) | P-heterogeneity | P-difference | |
| Overall | 20 | 0.84 (0.79, 0.90) | 64.2 | <0.001 | — |
| Region | |||||
| China | 10 | 0.82 (0.75, 0.90) | 69.4 | 0.001 | Ref |
| Japan | 6 | 0.85 (0.77, 0.94) | 62.2 | 0.021 | 0.38 |
| Others1 | 4 | 0.85 (0.55, 1.30) | 59.1 | 0.062 | 0.62 |
| Sample size | |||||
| <25,000 | 10 | 0.88 (0.78, 1.00)2 | 30.5 | 0.165 | Ref |
| ≥25000 | 10 | 0.83 (0.76, 0.89) | 76.8 | <0.001 | 0.44 |
| Sex | |||||
| Mixed | 11 | 0.86 (0.81, 0.92) | 49.5 | 0.031 | Ref |
| Male | 5 | 0.84 (0.63, 1.11) | 83.4 | <0.001 | 0.63 |
| Female | 4 | 0.79 (0.69, 0.91) | 47.2 | 0.128 | 0.38 |
| Age (y) | |||||
| <55 | 12 | 0.87 (0.83, 0.91) | 0.8 | 0.436 | Ref |
| ≥55 | 8 | 0.80 (0.70, 0.92) | 80.8 | <0.001 | 0.18 |
| Follow-up duration (y) | |||||
| <10 | 6 | 0.81 (0.68, 0.96) | 75.8 | 0.001 | Ref |
| ≥103 | 14 | 0.86 (0.82, 0.91) | 41.4 | 0.053 | 0.26 |
| Baseline survey years | |||||
| <2000 | 10 | 0.85 (0.78, 0.94) | 55.0 | 0.018 | Ref |
| ≥20003 | 10 | 0.83 (0.76, 0.91) | 71.7 | <0.001 | 0.76 |
| Mode of dietary intake assessment | |||||
| Self-administered | 7 | 0.85 (0.76, 0.94) | 58.5 | 0.025 | Ref |
| Interviewer-administered | 13 | 0.84 (0.77, 0.91) | 68.5 | <0.001 | 0.92 |
| Outcomes | |||||
| Incidence and mortality | 4 | 0.86 (0.77, 0.95) | 71.4 | 0.015 | Ref |
| Incidence | 1 | 1.15 (0.68, 1.93) | — | — | 0.375 |
| Mortality | 15 | 0.83 (0.76, 0.90) | 66.0 | <0.001 | 0.691 |
| Adjustment for confounders | |||||
| Age | |||||
| Yes | 19 | 0.84 (0.78, 0.90) | 66.1 | <0.001 | 0.97 |
| No | 1 | 0.84 (0.77, 0.91) | — | — | Ref |
| Education | |||||
| Yes | 18 | 0.84 (0.78, 0.90) | 65.5 | <0.001 | 0.78 |
| No | 2 | 0.88 (0.79, 0.98) | 30.0 | 0.232 | Ref |
| Income | |||||
| Yes | 5 | 0.80 (0.66, 0.96) | 81.7 | <0.001 | 0.23 |
| No | 15 | 0.86 (0.82, 0.91) | 38.9 | 0.062 | Ref |
| BMI | |||||
| Yes | 14 | 0.82 (0.75, 0.90) | 71.0 | <0.001 | 0.39 |
| No | 6 | 0.88 (0.81, 0.95) | 22.1 | 0.268 | Ref |
| Energy intake | |||||
| Yes | 7 | 0.81 (0.71, 0.93) | 80.8 | <0.001 | 0.45 |
| No | 13 | 0.86 (0.80, 0.92) | 44.5 | 0.042 | Ref |
| Physical activity | |||||
| Yes | 15 | 0.85 (0.78, 0.92) | 68.3 | <0.001 | 0.76 |
| No | 5 | 0.81 (0.75, 0.89) | 37.8 | 0.169 | Ref |
| Alcohol | |||||
| Yes | 12 | 0.83 (0.76, 0.91) | 71.6 | <0.001 | 0.71 |
| No | 8 | 0.84 (0.77, 0.93) | 47.0 | 0.067 | Ref |
| Smoking | |||||
| Yes | 16 | 0.84 (0.78, 0.91) | 66.4 | <0.001 | 0.88 |
| No | 4 | 0.82 (0.73, 0.92) | 53.3 | 0.093 | Ref |
| History of diabetes and hypertension | |||||
| Yes | 15 | 0.83 (0.77, 0.91) | 64.6 | <0.001 | 0.72 |
| No | 5 | 0.85 (0.76, 0.97) | 70.5 | 0.009 | Ref |
| Family history of CVD | |||||
| Yes | 4 | 0.81 (0.74, 0.89) | 58.1 | 0.067 | 0.54 |
| No | 16 | 0.85 (0.78, 0.93) | 66.4 | <0.001 | Ref |
Abbreviations: CVD, cardiovascular disease; IHD, ischemic heart disease; RR, relative risk.
Other regions included were South Korea, Taiwan, and South Asia (Bangladesh, India, and Pakistan).
P = 0.045.
Categorization for study by Han et al. [61] with overlapping years was based on majority of the study duration falling within the category of follow-up years of ≥10 years and baseline survey years ≥2000.
When studies that contributed to the largest proportion of heterogeneity were sequentially excluded until I2 value was <50%, pooled estimates remained similar to the original estimates—non-Asian indices (RR: 0.83; 95% CI: 0.78, 0.88; I2 = 0.0%): exclusion of men cohort in the study by Yu et al. [7] and Tsai et al. [52]; Asian indices (RR: 0.86; 95% CI: 0.81, 0.91; I2 = 47.5): exclusion of men cohort in the study by Yu et al. [7]; and data-driven patterns (RR: 0.79; 95% CI: 0.73, 0.86; I2 = 47.0): exclusion of the study by Qin et al. [30]. Substantial heterogeneity was still observed in low-quality dietary pattern, where the lowest I2 value remained >50% regardless of studies excluded (RR: 1.21; 95% CI: 0.97, 1.49; I2 = 60.3%: exclusion of the study by Nanri et al. [45]).
Sensitivity analyses
We conducted sensitivity analyses by including similar dietary patterns from the same study cohort alternately and omitting one study at a time (Supplemental Table 7). None of these changes substantially influenced the pooled effect estimates, with the only exception observed in studies under diet diversity indices where a statistically significant inverse association was observed (RR: 0.72; 95% CI: 0.59, 0.87) on omitting the men cohort in the study by Kobayashi et al. [48]. Similar estimates were also observed when we excluded studies considered to have serious risk of bias and using unconverted data (Supplemental Table 7).
Results of other dietary patterns
Results of dietary patterns that could not be evaluated in the meta-analysis are summarized in Supplemental Table 5. Higher adherence to the AHEI-2010 in Singapore was associated with a lower odds of incident AMI [32]. The traditional Northern pattern (wheat, other staples and egg, moderate intake of dairy products, and low intakes of rice and preserved vegetables) in China was associated with lower risk of incident CVD [30]. Following a vegetarian pattern in Taiwan was associated with lower risk of incident stroke in one of the two cohorts included in the study [35]. Higher adherence to a dairy product pattern (high intakes of milk and dairy products, butter, margarine, fruits, coffee, and tea) in Japan was associated with lower risk of CVD mortality in women, but not in men [27].
Conversely, both traditional (miso, pickles, and rice, less frequent consumption of vegetables, milk, and meat) and Western (high intake of fat and meat) dietary patterns in Japan were associated with higher risk of incident CVD than that with a healthy dietary pattern [34]. An unhealthy dietary pattern (nondaily eating of vegetables, fruits, and eggs and eating red meat daily) in China was associated with incident ischemic stroke, whereas no association was observed with incident IHD and hemorrhagic stroke [29]. No significant associations with CVD were detected for overall plant-based diet index pattern [28], westernized [31], high-dairy, high–fruit-and-vegetable, low-alcohol [26], gourd and root vegetable [46], vegetable-rich, and fruit-rich patterns [51].
Discussion
This systematic review and meta-analysis of observational studies evaluated the association between dietary patterns and CVD in South, Southeast, and East Asian populations. We found that greater adherence to high-quality dietary patterns were inversely associated with CVD risk, regardless of whether they had been derived from indices from Asian or non-Asian populations or data-driven methods. These high-quality dietary patterns reflect higher intakes from fruits and vegetables; whole grains; healthy protein sources such as legumes and nuts, fish, and seafood; low-fat dairy and lean meat and poultry; and/or liquid plant oils. The evidence from non-Asian indices and data-driven patterns were supported by moderate certainty, although Asian indices were of lower certainty. We did not find significant associations for high-quality dietary pattern defined by plant-based indices, low-quality, animal-based, and diet diversity indices dietary patterns. To our knowledge, this is the first study to quantify and examine the associations between diet quality indices established in non-Asian countries with those derived in Asia, as well as the association between Asian data-driven dietary patterns and CVD risk.
Comparison with existing systematic reviews and meta-analyses
A previous meta-analysis of cohort studies worldwide published in 2020 on diet quality indices (Healthy Eating Index, AHEI, and DASH) found 20% lower risk in CVD [9]. This finding is consistent with the association between high-quality dietary pattern defined by non-Asian indices and 22% lower CVD risk in our meta-analysis of studies in Asian populations. In the analysis of Asian indices, greater adherence in the top tertile than that in the bottom tertile was associated with 16% lower CVD risk, revealing a weaker association when compared with non-Asian indices.
With data-driven high-quality dietary pattern, we found that greater adherence in the top tertile was associated with 19% lower risk in CVD than that in the bottom tertile. Although a previous meta-analysis that examined data-driven prudent dietary pattern observed greater decreased risk of 31% in CVD, there were only five cohort studies included and none of the studies were conducted in Asia [17]. Another meta-analysis that performed a subgroup analysis on five studies within Asia found 14% lower risk specifically in CVD mortality with data-driven prudent dietary pattern [18].
Similar to previous systematic reviews and meta-analyses comprising predominantly studies conducted in non-Asian countries [17,18,72], our analysis of data-driven high-quality dietary pattern was characterized by higher intakes of fruits, vegetables, and fish, suggesting that these are consistent key foods that constitutes a high-quality dietary pattern favoring cardiovascular health [3,72]. Conversely, distinct differences were highlighted in the type of protein consumed among Asians, where higher intakes of fish, soy, and soy products and lower intakes of red meat and dairy were observed, aligned with the findings from a comprehensive systematic review, which demonstrated that dietary patterns that are also characterized by higher intakes of legumes, fish/seafood, and lower intakes of red and processed meat are associated with decreased risk of CVD [72].
We did not find a significant association between plant-based indices and CVD. In contrast, a previous meta-analysis comprising studies conducted in non-Asian countries demonstrated 13% lower CVD risk with greater adherence to healthful plant-based dietary pattern [73]. However, further investigation of plant-based indices in Asian populations is needed considering the small number of studies (n = 2) available in our meta-analysis.
Results from our meta-analysis also add to existing evidence demonstrating the lack of association between low-quality dietary pattern and CVD [[17], [18], [19]]. In the low-quality dietary pattern characterized by fast food, processed meat, and added sugars in sweets and beverages, not all the individual studies in the pooled analysis found a statistically significant association. The inconsistency in results across studies may be explained by the wide-ranging food items in each pattern, with only one or two items correlated with CVD [74]. For instance, dairy products and red and processed meat were represented in the modern dietary pattern by Shi et al. [75] and westernized dietary pattern by Nanri et al. [45]. However, red and processed meat is a recognized risk factor for CVD [3,76], but not dairy products [3,77]. Likewise, the lack of association between an animal-based dietary pattern and CVD in our study may be explained by the presence of other animal protein sources, including fish and seafood, in the evaluated dietary patterns. For example, long chain ω-3 (n–3) polyunsaturated fatty acids in seafood may reduce endothelial inflammation and dysfunction [78]. As the animal-based dietary pattern comprises data-driven patterns, non-animal food sources that were captured in the dietary pattern analysis could have also attenuated the result although these were foods of a lower factor loading. Our findings suggest that additional studies are warranted to establish the role of low-quality and animal-based dietary patterns on CVD.
To our knowledge, the association between dietary diversity scores and CVD has not been previously quantified in a meta-analysis. Most diversity scores do not distinguish between the effects of healthy and unhealthy food [79]; therefore, its applicability in predicting chronic diseases risk may not be entirely suitable.
Investigation of heterogeneity
Heterogeneity in our meta-analysis was mostly significant. Because of insufficient number of studies in the other dietary patterns, subgroup analyses and meta-regression were performed only in the high-quality dietary pattern defined by Asian indices. However, we were unable to explain the observed heterogeneity. This is likely attributable to variations in the scoring standards and dietary sources to define a high-quality diet, as recommended serving sizes or population-specific median intake for each dietary component may differ between indices. For high-quality dietary pattern defined by non-Asian indices, heterogeneity was reduced to below an I2 value of 50% when we sequentially and cumulatively excluded studies conducted by Yu et al. [7] and Tsai et al. [52], suggesting that substantial variation in the original estimate could be due to the removal of dietary sources [7] or modification of scoring components [52] in the non-Asian diet quality indices. Likewise, heterogeneity in data-driven high-quality dietary pattern reduced markedly after the removal of study by Qin et al. [30]. Protein sources (i.e., meat, poultry, fish, and dairy products) in the modern pattern identified in the study by Qin et al. [30] were characterized by higher factor loadings, and vegetables had a relatively lower factor loading than the dietary patterns in other studies. For low-quality dietary pattern, heterogeneity reduced slightly after excluding the Japanese study by Nanri et al. [45], although overall I2 value was still >50%. Nanri et al. [45] suggested that the inverse association between the westernized dietary pattern and CVD in their study was due to a low meat consumption in the Japanese population, and foods such as coffee, tea, and dairy products included in the dietary pattern may be associated with lower risk of CVD mortality.
Public health implications
Given the wide range of diet quality indices available to define a high-quality diet, our study demonstrated that both non-Asian indices and Asian indices were consistently associated with lower CVD risk in South, Southeast, and East Asian populations. Although non-Asian diet quality indices such as AHEI and DASH reflect recommended intake levels that correspond to the Dietary Guidelines for Americans to evaluate eating patterns and predict chronic disease risk [72,[80], [81], [82]], its construct based on broad food groups and nutrients allow these indices to be adapted to include Asian-specific food sources when used in Asian populations. For example, nuts and legumes component in the AHEI-2010 can be specified as soy and fermented soy products in East and Southeast Asian countries and lentils in South Asian countries. On the contrary, possible reasons for the weaker association between Asian indices and CVD risk may be alluded to the fewer food groups in its construct, such as the lack of, or less emphasis on, components such as added sugars, distinction between types of meat and fat (e.g., saturated compared with unsaturated fat), suggesting an Asian diet quality index that has yet to be optimally defined.
We also observed in the study by Yu et al. [7] that total scoring for AHEI-2010 and DASH were modified by removing the whole grains component due to low consumption in the Chinese population in Shanghai. Similarly, the data-driven high-quality dietary pattern did not reflect a high intake of whole grains, suggesting that refined grains may still be largely embedded in a typical Asian diet [83], explaining the difference in pooled effect estimates when compared with non-Asian indices. Given that current national dietary guidelines in Asian countries do not uniformly distinguish between protein sources and refined and whole grains [[84], [85], [86], [87]], a lack of clear distinction may give the impression that health benefits from, for example, brown rice against white rice, or white meat against red meat, are equivalent when consumed in the same amount [88,89]. Higher consumption of whole grains has been consistently associated with lower CVD risk, but this has not been observed for refined grains [3,90,91]. Protein sources, further classified into animal or plant-based, have been recognized to exert differential risks on CVD: high consumption of unprocessed red meat was shown to be associated with higher CVD risk [76]; instead, substitution with fish lowered CVD risk [89], while higher consumption of plant protein sources such as soy, nuts, beans, and lentils has been associated with lower risk of CVD mortality [92,93] with favorable reductions in serum total and LDL cholesterol levels [94]. In turn, it is pertinent to define the type of protein and encourage whole grains consumption in these Asian dietary guidelines to guide recommendations and public health messaging. Additionally, the development of Asian diet quality indices could also move toward a scoring metric that measures consumption of a larger number of food groups, as it was observed that Asian indices [54,61] based on only two or three food groups may not reflect the overall quality of an individual’s dietary intake.
The emergence of notable dietary features that are distinct to Asian populations in our data-driven high-quality dietary pattern also has important public health implications. A recent randomized controlled trial has successfully demonstrated the blood pressure-lowering effect in Chinese adults with a Chinese heart-healthy diet made up of four different versions of Chinese cuisines that represents typical dietary styles of the Chinese population [95]. Although well-established heart-healthy diets such as traditional Mediterranean diet in the West may be reputable for its cardiovascular benefits, certain practices such as olive oil as primary source of fat and moderate consumption of alcohol, specifically red wine, may not be feasible or well accepted in the Asian populations as dietary choices are not only based on availability and staple foods but also deeply rooted in culture and traditions [5,95]. Taken together, variations in the makeup of healthful dietary patterns between non-Asian countries and Asia may exist; therefore, dietary recommendations and interventions based on a high-quality dietary pattern that aligns with the consumption habits of Asian populations are more likely to improve adherence to dietary recommendations.
Strengths and limitations
Our study has marked a significant step forward in presenting the first and an up-to-date comprehensive review on current dietary patterns and CVD in Asia. Although dietary influences by geographical location, ethnicity, or culture may inevitably give rise to possible variations between-study-specific dietary patterns in each grouping, we sought the method of considering index-based dietary patterns with multiple similar dietary components and, similarly, for data-driven patterns by considering similar dietary components of high factor loadings to minimize diversity. Results of our sensitivity analyses also suggested the robustness of our findings on the dietary patterns derived and its association with CVD. Furthermore, our meta-analysis considered the transformation of each study-specific effect estimate to a standard scale (i.e., tertile) to facilitate a fair comparison, which has not been performed in previous meta-analyses [9,17,18].
Our review is not without limitations. First, we were unable to perform subgroup and meta-regression analyses on most of the dietary patterns identified with significant heterogeneity due to the small number of studies available. However, overall estimates remained consistent after sequential exclusion of studies that contributed to substantial heterogeneity in the dietary patterns, suggesting that the pooled effect estimates were not influenced by the high levels of heterogeneity. Second, our study is based on observational studies; therefore, we are unable to establish a causal association. Third, although majority of the studies adjusted for potential confounding factors rather extensively, residual confounding cannot be excluded. Fourth, we excluded studies conducted in West and Central Asia owing to dietary variations and ethnicity; hence, our results may not be generalizable to these populations. Finally, most research has been predominantly conducted on East Asian populations, and further studies in South Asian and Southeast Asian countries are urgently needed. An improved understanding of the variation in dietary patterns across different ethnic groups and its association with CVD risks within the diverse Asian regions is also warranted.
Conclusion
We presented our findings that adherence to a high-quality dietary pattern, whether defined by non-Asian indices, Asian indices, or data-driven patterns, is consistently associated with lower CVD risks in South, Southeast, and East Asian populations. Given the evidence that associations between current Asian-derived diet quality indices and CVD risk were weaker than those with established non-Asian diet quality indices such as AHEI and DASH, there may be a need for current dietary guidelines in Asian countries and Asian diet quality indices to be optimized to reflect up-to-date dietary recommendations for cardiovascular health.
Author contributions
The authors’ responsibilities were as follows – AC, MF-FC, NN, RMvD: contributed to study conception and design; AC, GHL, NN: conducted the literature search, data extraction and risk of bias assessment, with assistance from YQL, SHP, and ZHK; AC, GHL: performed statistical analysis and data interpretation; GHL: drafted the manuscript; and all authors: read and approved the final manuscript.
Conflict of interest
The authors report no conflicts of interest.
Funding
This work was supported by Population Health Metrics and Analytics Programme, National University of Singapore and National University Health System, Singapore.
Acknowledgments
We thank Ratnala Sukanya Naidu and Wong Suei Nee, senior medical librarians from National University of Singapore, for their assistance with development of search strategy and implementation. We also thank Zhang Xingyi and Li Xi (National Clinical Research Center for Cardiovascular Diseases, Fuwai Hospital, Beijing, China) for clarification of the data from the China PEACE Million Persons Project.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.advnut.2024.100249.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.World Health Organization . World Health Organization; 2021. Cardiovascular diseases (CVDs)https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) [Internet] [updated 11 June 2021; cited 3 November 2023 ]. Available from: [Google Scholar]
- 2.Zhao D. Epidemiological features of cardiovascular disease in Asia. JACC Asia. 2021;1(1):1–13. doi: 10.1016/j.jacasi.2021.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lichtenstein A.H., Appel L.J., Vadiveloo M., Hu F.B., Kris-Etherton P.M., Rebholz C.M., et al. Dietary guidance to improve cardiovascular health: a scientific statement from the American Heart Association. Circulation. 2021;144(23):e472–e487. doi: 10.1161/CIR.0000000000001031. 2021. [DOI] [PubMed] [Google Scholar]
- 4.Hu F.B. Dietary pattern analysis: a new direction in nutritional epidemiology. Curr. Opin. Lipidol. 2002;13(1):3–9. doi: 10.1097/00041433-200202000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Kwan T.W., Wong S.S., Hong Y., Kanaya A.M., Khan S.S., Hayman L.L., et al. Epidemiology of diabetes and atherosclerotic cardiovascular disease among Asian American adults: implications, management, and future directions: a scientific statement from the American Heart Association. Circulation. 2023;148(1):74–94. doi: 10.1161/CIR.0000000000001145. [DOI] [PubMed] [Google Scholar]
- 6.Hu F.B. Diet strategies for promoting healthy aging and longevity: an epidemiological perspective. J. Intern. Med. 2024;295(4):508–531. doi: 10.1111/joim.13728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu D., Zhang X., Xiang Y.B., Yang G., Li H., Gao Y.T., et al. Adherence to dietary guidelines and mortality: a report from prospective cohort studies of 134,000 Chinese adults in urban Shanghai. Am. J. Clin. Nutr. 2014;100(2):693–700. doi: 10.3945/ajcn.113.079194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kurotani K., Akter S., Kashino I., Goto A., Mizoue T., Noda M., et al. Quality of diet and mortality among Japanese men and women: Japan Public Health Center based prospective study. BMJ. 2016;352:i1209. doi: 10.1136/bmj.i1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morze J., Danielewicz A., Hoffmann G., Schwingshackl L. Diet quality as assessed by the healthy eating index, alternate healthy eating index, dietary approaches to stop hypertension score, and health outcomes: a second update of a systematic review and meta-analysis of cohort studies. J. Acad. Nutr. Diet. 2020;120(12):1998–2031.e15. doi: 10.1016/j.jand.2020.08.076. [DOI] [PubMed] [Google Scholar]
- 10.Schwingshackl L., Bogensberger B., Hoffmann G. Diet quality as assessed by the healthy eating index, alternate healthy eating index, dietary approaches to stop hypertension score, and health outcomes: an updated systematic review and meta-analysis of cohort studies. J. Acad. Nutr. Diet. 2018;118(1):74–100.e11. doi: 10.1016/j.jand.2017.08.024. [DOI] [PubMed] [Google Scholar]
- 11.Schwingshackl L., Hoffmann G. Diet quality as assessed by the Healthy Eating Index, the Alternate Healthy Eating Index, the Dietary Approaches to Stop Hypertension score, and health outcomes: a systematic review and meta-analysis of cohort studies. J. Acad. Nutr. Diet. 2015;115(5):780–800.e5. doi: 10.1016/j.jand.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 12.Soltani S., Arablou T., Jayedi A., Salehi-Abargouei A. Adherence to the dietary approaches to stop hypertension (DASH) diet in relation to all-cause and cause-specific mortality: a systematic review and dose-response meta-analysis of prospective cohort studies. Nutr. J. 2020;19(1):37. doi: 10.1186/s12937-020-00554-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chiavaroli L., Viguiliouk E., Nishi S.K., Blanco Mejia S., Rahelić D., Kahleová H., et al. DASH dietary pattern and cardiometabolic outcomes: an umbrella review of systematic reviews and meta-analyses. Nutrients. 2019;11(2):338. doi: 10.3390/nu11020338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salehi-Abargouei A., Maghsoudi Z., Shirani F., Azadbakht L. Effects of Dietary Approaches to Stop Hypertension (DASH)-style diet on fatal or nonfatal cardiovascular diseases—incidence: a systematic review and meta-analysis on observational prospective studies. Nutrition. 2013;29(4):611–618. doi: 10.1016/j.nut.2012.12.018. [DOI] [PubMed] [Google Scholar]
- 15.Chen G.C., Neelakantan N., Martín-Calvo N., Koh W.P., Yuan J.M., Bonaccio M., et al. Adherence to the Mediterranean diet and risk of stroke and stroke subtypes. Eur. J. Epidemiol. 2019;34(4):337–349. doi: 10.1007/s10654-019-00504-7. [DOI] [PubMed] [Google Scholar]
- 16.Rosato V., Temple N.J., La Vecchia C, Castellan G., Tavani A., Guercio V. Mediterranean diet and cardiovascular disease: a systematic review and meta-analysis of observational studies. Eur. J. Nutr. 2019;58(1):173–191. doi: 10.1007/s00394-017-1582-0. [DOI] [PubMed] [Google Scholar]
- 17.Rodríguez-Monforte M., Flores-Mateo G., Sánchez E. Dietary patterns and CVD: a systematic review and meta-analysis of observational studies. Br. J. Nutr. 2015;114(9):1341–1359. doi: 10.1017/S0007114515003177. [DOI] [PubMed] [Google Scholar]
- 18.Li F., Hou L.N., Chen W., Chen P.L., Lei C.Y., Wei Q., et al. Associations of dietary patterns with the risk of all-cause, CVD and stroke mortality: a meta-analysis of prospective cohort studies. Br. J. Nutr. 2015;113(1):16–24. doi: 10.1017/S000711451400289X. [DOI] [PubMed] [Google Scholar]
- 19.Hou L., Li F., Wang Y., Ou Z., Xu D., Tan W., et al. Association between dietary patterns and coronary heart disease: a meta-analysis of prospective cohort studies. Int. J. Clin. Exp. Med. 2015;8(1):781–790. [PMC free article] [PubMed] [Google Scholar]
- 20.Stroup D.F., Berlin J.A., Morton S.C., Olkin I., Williamson G.D., Rennie D., et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA. 2000;283(15):2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 21.World Health Organization . 2nd ed. World Health Organization; Geneva: 2004. ICD-10: international statistical classification of diseases and related health problems: 10th revision. [Google Scholar]
- 22.World Health Organization . World Health Organization; Geneva: 1978. International classification of diseases: 9th revision, basic tabulation list with alphabetic index. [Google Scholar]
- 23.Sterne J.A., Hernán M.A., Reeves B.C., Savović J., Berkman N.D., Viswanathan M., et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schünemann H., Brożek J, Guyatt G., Oxman A. Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach. October 2013. https://gdt.gradepro.org/app/handbook/handbook.html [Internet], Available from.
- 25.Schünemann H.J., Cuello C., Akl E.A., Mustafa R.A., Meerpohl J.J., Thayer K., et al. GRADE guidelines: 18. How ROBINS-I and other tools to assess risk of bias in nonrandomized studies should be used to rate the certainty of a body of evidence. J. Clin. Epidemiol. 2019;111:105–114. doi: 10.1016/j.jclinepi.2018.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shimazu T., Kuriyama S., Hozawa A., Ohmori K., Sato Y., Nakaya N., et al. Dietary patterns and cardiovascular disease mortality in Japan: a prospective cohort study. Int. J. Epidemiol. 2007;36(3):600–609. doi: 10.1093/ije/dym005. [DOI] [PubMed] [Google Scholar]
- 27.Maruyama K., Iso H., Date C., Kikuchi S., Watanabe Y., Wada Y., et al. Dietary patterns and risk of cardiovascular deaths among middle-aged Japanese: JACC study. Nutr. Metab. Cardiovasc. Dis. 2013;23(6):519–527. doi: 10.1016/j.numecd.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 28.Kim J., Kim H., Giovannucci E.L. Plant-based diet quality and the risk of total and disease-specific mortality: a population-based prospective study. Clin. Nutr. 2021;40(12):5718–5725. doi: 10.1016/j.clnu.2021.10.013. [DOI] [PubMed] [Google Scholar]
- 29.Han Y., Hu Y., Yu C., Guo Y., Pei P., Yang L., et al. Lifestyle, cardiometabolic disease, and multimorbidity in a prospective Chinese study. Eur. Heart J. 2021;42(34):3374–3384. doi: 10.1093/eurheartj/ehab413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qin C., Lv J., Yu C., Guo Y., Bian Z., Gao M., et al. Dietary patterns and cardiometabolic diseases in 0.5 million Chinese adults: a 10-year cohort study. Nutr. J. 2021;20(1):74. doi: 10.1186/s12937-021-00730-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seino F., Date C., Nakayama T., Yoshiike N., Yokoyama T., Yamaguchi M., et al. Dietary lipids and incidence of cerebral infarction in a Japanese rural community. J. Nutr. Sci. Vitaminol (Tokyo). 1997;43(1):83–99. doi: 10.3177/jnsv.43.83. [DOI] [PubMed] [Google Scholar]
- 32.Neelakantan N., Naidoo N., Koh W.P., Yuan J.M., van Dam R.M. The alternative healthy eating index is associated with a lower risk of fatal and nonfatal acute myocardial infarction in a Chinese adult population. J. Nutr. 2016;146(7):1379–1386. doi: 10.3945/jn.116.231605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Higgins J.P.T., Thomas J., Chandler J., Cumpston M., Li T., Page M.J., Welch V.A., editors. Cochrane handbook for systematic reviews of interventions. Cochrane; 2023. https://training.cochrane.org/handbook [Internet]. (updated August 2023). Available from: [Google Scholar]
- 34.Li Y., Sato Y., Yamaguchi N. Lifestyle factors as predictors of general cardiovascular disease: use for early self-screening. Asia Pac. J. Public Health. 2014;26(4):414–424. doi: 10.1177/1010539511423067. [DOI] [PubMed] [Google Scholar]
- 35.Chiu T.H.T., Chang H.R., Wang L.Y., Chang C.C., Lin M.N., Lin C.L. Vegetarian diet and incidence of total, ischemic, and hemorrhagic stroke in 2 cohorts in Taiwan. Neurology. 2020;94(11):e1112–e1121. doi: 10.1212/WNL.0000000000009093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Borenstein M., Hedges L.V., Higgins J.P., Rothstein H.R. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res. Synth. Methods. 2010;1(2):97–111. doi: 10.1002/jrsm.12. [DOI] [PubMed] [Google Scholar]
- 37.Chan R.S.M., Yu B.W.M., Leung J., Lee J.S.W., Auyeung T.W., Kwok T., et al. How dietary patterns are related to inflammaging and mortality in community-dwelling older Chinese adults in Hong Kong—a prospective analysis. J. Nutr. Health Aging. 2019;23(2):181–194. doi: 10.1007/s12603-018-1143-0. [DOI] [PubMed] [Google Scholar]
- 38.Neelakantan N., Koh W.P., Yuan J.M., Van Dam R.M. Diet-quality indexes are associated with a lower risk of cardiovascular, respiratory, and all-cause mortality among Chinese adults. J. Nutr. 2018;148(8):1323–1332. doi: 10.1093/jn/nxy094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lin P.H., Yeh W.T., Svetkey L.P., Chuang S.Y., Chang Y.C., Wang C., et al. Dietary intakes consistent with the DASH dietary pattern reduce blood pressure increase with age and risk for stroke in a Chinese population. Asia Pac. J. Clin. Nutr. 2013;22(3):482–491. [PubMed] [Google Scholar]
- 40.Oba S., Nagata C., Nakamura K., Fujii K., Kawachi T., Takatsuka N., et al. Diet based on the Japanese food guide spinning top and subsequent mortality among men and women in a general Japanese population. J. Am. Diet. Assoc. 2009;109(9):1540–1547. doi: 10.1016/j.jada.2009.06.367. [DOI] [PubMed] [Google Scholar]
- 41.Nakamura Y., Ueshima H., Okamura T., Kadowaki T., Hayakawa T., Kita Y., et al. A Japanese diet and 19-year mortality: national integrated project for prospective observation of non-communicable diseases and its trends in the aged, 1980. Br. J. Nutr. 2009;101(11):1696–1705. doi: 10.1017/S0007114508111503. [DOI] [PubMed] [Google Scholar]
- 42.Odegaard A.O., Koh W.P., Yuan J.M., Gross M.D., Pereira M.A. Dietary patterns and mortality in a Chinese population. Am. J. Clin. Nutr. 2014;100(3):877–883. doi: 10.3945/ajcn.114.086124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shi Z., Ganji V. Dietary patterns and cardiovascular disease risk among Chinese adults: a prospective cohort study. Eur. J. Clin. Nutr. 2020;74(12):1725–1735. doi: 10.1038/s41430-020-0668-6. [DOI] [PubMed] [Google Scholar]
- 44.Chan R., Chan D., Woo J. The association of a priori and a posterior dietary patterns with the risk of incident stroke in Chinese older people in Hong Kong. J. Nutr. Health Aging. 2013;17(10):866–874. doi: 10.1007/s12603-013-0334-y. [DOI] [PubMed] [Google Scholar]
- 45.Nanri A., Mizoue T., Shimazu T., Ishihara J., Takachi R., Noda M., et al. Dietary patterns and all-cause, cancer, and cardiovascular disease mortality in Japanese men and women: the Japan public health center-based prospective study. PLoS One. 2017;12(4) doi: 10.1371/journal.pone.0174848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen Y., McClintock T.R., Segers S., Parvez F., Islam T., Ahmed A., et al. Prospective investigation of major dietary patterns and risk of cardiovascular mortality in Bangladesh. Int. J. Cardiol. 2013;167(4):1495–1501. doi: 10.1016/j.ijcard.2012.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lo K., Glenn A.J., Yeung S., Kendall C.W.C., Sievenpiper J.L., Jenkins D.J.A., et al. Prospective association of the portfolio diet with all-cause and cause-specific mortality risk in the Mr. OS and Ms. OS study, Nutrients. 2021;13(12):4360. doi: 10.3390/nu13124360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kobayashi M., Sasazuki S., Shimazu T., Sawada N., Yamaji T., Iwasaki M., et al. Association of dietary diversity with total mortality and major causes of mortality in the Japanese population: JPHC study. Eur. J. Clin. Nutr. 2020;74(1):54–66. doi: 10.1038/s41430-019-0416-y. [DOI] [PubMed] [Google Scholar]
- 49.Otsuka R., Tange C., Nishita Y., Kato Y., Tomida M., Imai T., et al. Dietary diversity and all-cause and cause-specific mortality in Japanese community-dwelling older adults. Nutrients. 2020;12(4):1052. doi: 10.3390/nu12041052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang Y., Su X., Chen Y., Wang Y., Zhou J., Liu T., et al. Unfavorable dietary quality contributes to elevated risk of ischemic stroke among residents in Southwest China: based on the Chinese Diet Balance Index 2016 (DBI-16) Nutrients. 2022;14(3):694. doi: 10.3390/nu14030694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cai H., Shu X.O., Gao Y.T., Li H., Yang G., Zheng W. A prospective study of dietary patterns and mortality in Chinese women. Epidemiology. 2007;18(3):393–401. doi: 10.1097/01.ede.0000259967.21114.45. [DOI] [PubMed] [Google Scholar]
- 52.Tsai M.C., Yeh T.L., Hsu H.Y., Hsu L.Y., Lee C.C., Tseng P.J., et al. Comparison of four healthy lifestyle scores for predicting cardiovascular events in a national cohort study. Sci. Rep. 2021;11(1) doi: 10.1038/s41598-021-01213-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen L., Tang W., Wu X., Zhang R., Ding R., Liu X., et al. Eating spicy food, Dietary Approaches to Stop Hypertension (DASH) score, and their interaction on incident stroke in southwestern Chinese aged 30–79: a prospective cohort study. Nutrients. 2023;15(5):1222. doi: 10.3390/nu15051222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim J.Y., Ko Y.J., Rhee C.W., Park B.J., Kim D.H., Bae J.M., et al. Cardiovascular health metrics and all-cause and cardiovascular disease mortality among middle-aged men in Korea: the Seoul male cohort study. J. Prev. Med. Public Health. 2013;46(6):319–328. doi: 10.3961/jpmph.2013.46.6.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chuang S.Y., Chang H.Y., Fang H.L., Lee S.C., Hsu Y.Y., Yeh W.T., et al. The Healthy Taiwanese Eating Approach is inversely associated with all-cause and cause-specific mortality: a prospective study on the Nutrition and Health Survey in Taiwan, 1993–1996. PLoS One. 2021;16(5) doi: 10.1371/journal.pone.0251189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Okada E., Nakamura K., Ukawa S., Wakai K., Date C., Iso H., et al. The Japanese food score and risk of all-cause, CVD and cancer mortality: the Japan Collaborative Cohort Study. Br. J. Nutr. 2018;120(4):464–471. doi: 10.1017/S000711451800154X. [DOI] [PubMed] [Google Scholar]
- 57.Li M., Xu Y., Wan Q., Shen F., Xu M., Zhao Z., et al. Individual and combined associations of modifiable lifestyle and metabolic health status with new-onset diabetes and major cardiovascular events: the China cardiometabolic disease and cancer cohort (4C) study. Diabetes Care. 2020;43(8):1929–1936. doi: 10.2337/dc20-0256. [DOI] [PubMed] [Google Scholar]
- 58.Li S., Liu Z., Joseph P., Hu B., Yin L., Tse L.A., et al. Modifiable risk factors associated with cardiovascular disease and mortality in China: a PURE substudy. Eur. Heart J. 2022;43(30):2852–2863. doi: 10.1093/eurheartj/ehac268. [DOI] [PubMed] [Google Scholar]
- 59.Zhu N., Yu C., Guo Y., Bian Z., Han Y., Yang L., et al. Adherence to a healthy lifestyle and all-cause and cause-specific mortality in Chinese adults: a 10-year prospective study of 0.5 million people. Int. J. Behav. Nutr. Phys. Act. 2019;16(1):98. doi: 10.1186/s12966-019-0860-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Su J., Geng H., Chen L., Fan X., Zhou J., Wu M., et al. Association of healthy lifestyle with incident cardiovascular diseases among hypertensive and normotensive Chinese adults. Front. Cardiovasc. Med. 2023;10 doi: 10.3389/fcvm.2023.1046943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Han C., Liu F., Yang X., Chen J., Li J., Cao J., et al. Ideal cardiovascular health and incidence of atherosclerotic cardiovascular disease among Chinese adults: the China-PAR project. Sci. China Life Sci. 2018;61(5):504–514. doi: 10.1007/s11427-018-9281-6. [DOI] [PubMed] [Google Scholar]
- 62.Matsuyama S., Sawada N., Tomata Y., Zhang S., Goto A., Yamaji T., et al. Association between adherence to the Japanese diet and all-cause and cause-specific mortality: the Japan Public Health Center-based Prospective Study. Eur. J. Nutr. 2021;60(3):1327–1336. doi: 10.1007/s00394-020-02330-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang X., Lu J., Wu C., Cui J., Wu Y., Hu A., et al. Healthy lifestyle behaviours and all-cause and cardiovascular mortality among 0.9 million Chinese adults. Int. J. Behav. Nutr. Phys. Act. 2021;18(1):162. doi: 10.1186/s12966-021-01234-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Joseph P., Kutty V.R., Mohan V., Kumar R., Mony P., Vijayakumar K., et al. Cardiovascular disease, mortality, and their associations with modifiable risk factors in a multi-national South Asia cohort: a PURE substudy. Eur. Heart J. 2022;43(30):2831–2840. doi: 10.1093/eurheartj/ehac249. [DOI] [PubMed] [Google Scholar]
- 65.Dong Y., Hao G., Wang Z., Wang X., Chen Z., Zhang L. Ideal cardiovascular health status and risk of cardiovascular disease or all-cause mortality in Chinese middle-aged population. Angiology. 2019;70(6):523–529. doi: 10.1177/0003319718813448. [DOI] [PubMed] [Google Scholar]
- 66.Lee M.S., Huang Y.C., Su H.H., Lee M.Z., Wahlqvist M.L. A simple food quality index predicts mortality in elderly Taiwanese. J. Nutr. Health Aging. 2011;15(10):815–821. doi: 10.1007/s12603-011-0081-x. [DOI] [PubMed] [Google Scholar]
- 67.Danesh J., Collins R., Appleby P., Peto R. Association of fibrinogen, C-reactive protein, albumin, or leukocyte count with coronary heart disease: meta-analyses of prospective studies. JAMA. 1998;279(18):1477–1482. doi: 10.1001/jama.279.18.1477. [DOI] [PubMed] [Google Scholar]
- 68.Araujo A.B., Dixon J.M., Suarez E.A., Murad M.H., Guey L.T., Wittert G.A. Clinical review: endogenous testosterone and mortality in men: a systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 2011;96(10):3007–3019. doi: 10.1210/jc.2011-1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chia A.R., Chen L.W., Lai J.S., Wong C.H., Neelakantan N., van Dam R.M., et al. Maternal dietary patterns and birth outcomes: a systematic review and meta-analysis. Adv. Nutr. 2019;10(4):685–695. doi: 10.1093/advances/nmy123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Patsopoulos N.A., Evangelou E., Ioannidis J.P. Sensitivity of between-study heterogeneity in meta-analysis: proposed metrics and empirical evaluation. Int. J. Epidemiol. 2008;37(5):1148–1157. doi: 10.1093/ije/dyn065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mente A., Dehghan M., Rangarajan S., O’Donnell M., Hu W., Dagenais G., et al. Diet, cardiovascular disease, and mortality in 80 countries. Eur. Heart J. 2023;44(28):2560–2579. doi: 10.1093/eurheartj/ehad269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Boushey C., Ard J., Bazzano L., Heymsfield S., Mayer-Davis E., Sabaté J., et al. Dietary patterns and risk of cardiovascular disease: a systematic review. July 2020. [Internet], Available from. [DOI] [PubMed]
- 73.Quek J., Lim G., Lim W.H., Ng C.H., So W.Z., Toh J., et al. The association of plant-based diet with cardiovascular disease and mortality: a meta-analysis and systematic review of prospect cohort studies. Front. Cardiovasc. Med. 2021;8 doi: 10.3389/fcvm.2021.756810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schulze M.B., Hoffmann K. Methodological approaches to study dietary patterns in relation to risk of coronary heart disease and stroke. Br. J. Nutr. 2006;95(5):860–869. doi: 10.1079/BJN20061731. [DOI] [PubMed] [Google Scholar]
- 75.Shi Z., Zhen S., Zimmet P.Z., Zhou Y., Zhou Y., Magliano D.J., et al. Association of impaired fasting glucose, diabetes and dietary patterns with mortality: a 10-year follow-up cohort in Eastern China. Acta Diabetol. 2016;53(5):799–806. doi: 10.1007/s00592-016-0875-8. [DOI] [PubMed] [Google Scholar]
- 76.Shi W., Huang X., Schooling C.M., Zhao J.V. Red meat consumption, cardiovascular diseases, and diabetes: a systematic review and meta-analysis. Eur. Heart J. 2023;44(28):2626–2635. doi: 10.1093/eurheartj/ehad336. [DOI] [PubMed] [Google Scholar]
- 77.Dehghan M., Mente A., Rangarajan S., Sheridan P., Mohan V., Iqbal R., et al. Association of dairy intake with cardiovascular disease and mortality in 21 countries from five continents (PURE): a prospective cohort study. Lancet. 2018;392(10161):2288–2297. doi: 10.1016/S0140-6736(18)31812-9. [DOI] [PubMed] [Google Scholar]
- 78.Jayedi A., Shab-Bidar S. Fish consumption and the risk of chronic disease: an umbrella review of meta-analyses of prospective cohort studies. Adv. Nutr. 2020;11(5):1123–1133. doi: 10.1093/advances/nmaa029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hu F.B., Rimm E.B., Stampfer M.J., Ascherio A., Spiegelman D., Willett W.C. Prospective study of major dietary patterns and risk of coronary heart disease in men. Am. J. Clin. Nutr. 2000;72(4):912–921. doi: 10.1093/ajcn/72.4.912. [DOI] [PubMed] [Google Scholar]
- 80.Fung T.T., Chiuve S.E., McCullough M.L., Rexrode K.M., Logroscino G., Hu F.B. Adherence to a DASH-style diet and risk of coronary heart disease and stroke in women. Arch. Intern. Med. 2008;168(7):713–720. doi: 10.1001/archinte.168.7.713. [DOI] [PubMed] [Google Scholar]
- 81.Chiuve S.E., Fung T.T., Rimm E.B., Hu F.B., McCullough M.L., Wang M., et al. Alternative dietary indices both strongly predict risk of chronic disease. J. Nutr. 2012;142(6):1009–1018. doi: 10.3945/jn.111.157222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.US Department of Agriculture and US Department of Health and Human Services, Dietary Guidelines for Americans, 2020-2025, [Internet] 9th Edition. December 2020. Available from: DietaryGuidelines.gov. [Google Scholar]
- 83.Hu F.B. Globalization of diabetes: the role of diet, lifestyle, and genes. Diabetes Care. 2011;34(6):1249–1257. doi: 10.2337/dc11-0442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Food and Agriculture Organization (FAO) Food-based dietary guidelines, Asia and the Pacific [Internet] Food and Agriculture Organization of the United Nations, Rome. 2023 https://www.fao.org/nutrition/education/food-dietary-guidelines/regions/asia-pacific/en/ [cited 9 November 2023]. Available from: [Google Scholar]
- 85.Centre for Health Protection . Department of Health, The Government of the Hong Kong Special Administrative Region; 2017. The food pyramid—a guide to a balanced diet [Internet]https://www.chp.gov.hk/en/static/90017.html [updated 16 August 2023; cited 9 November 2023]. Available from: [Google Scholar]
- 86.HealthHub. My Healthy Plate. Ministry of Health; Singapore: 2023. https://www.healthhub.sg/programmes/nutrition-hub/eat-more [Internet]. [cited 9 November 2023]. Available from: [Google Scholar]
- 87.Health Promotion Administration . Ministry of Health and Welfare; Taiwan: 2019. 1. What is “My Plate”? Do you take in a diverse diet?https://www.hpa.gov.tw/EngPages/Detail.aspx?nodeid=4106&pid=11729 [Internet] [updated 31 October 2019; cited 9 November 2023]. Available from: [Google Scholar]
- 88.Aljuraiban G.S., Gibson R., Oude Griep L.M., Okuda N., Steffen L.M., Van Horn L., et al. Perspective: the application of a priori diet quality scores to cardiovascular disease risk-a critical evaluation of current scoring systems. Adv. Nutr. 2020;11(1):10–24. doi: 10.1093/advances/nmz059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhubi-Bakija F., Bajraktari G., Bytyçi I., Mikhailidis D.P., Henein M.Y., Latkovskis G., et al. The impact of type of dietary protein, animal versus vegetable, in modifying cardiometabolic risk factors: a position paper from the International Lipid Expert Panel (ILEP) Clin. Nutr. 2021;40(1):255–276. doi: 10.1016/j.clnu.2020.05.017. [DOI] [PubMed] [Google Scholar]
- 90.Aune D., Keum N., Giovannucci E., Fadnes L.T., Boffetta P., Greenwood D.C., et al. Whole grain consumption and risk of cardiovascular disease, cancer, and all cause and cause specific mortality: systematic review and dose-response meta-analysis of prospective studies. BMJ. 2016;353:i2716. doi: 10.1136/bmj.i2716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hu H., Zhao Y., Feng Y., Yang X., Li Y., Wu Y., et al. Consumption of whole grains and refined grains and associated risk of cardiovascular disease events and all-cause mortality: a systematic review and dose-response meta-analysis of prospective cohort studies. Am. J. Clin. Nutr. 2023;117(1):149–159. doi: 10.1016/j.ajcnut.2022.10.010. [DOI] [PubMed] [Google Scholar]
- 92.Qi X.X., Shen P. Associations of dietary protein intake with all-cause, cardiovascular disease, and cancer mortality: a systematic review and meta-analysis of cohort studies. Nutr. Metab. Cardiovasc. Dis. 2020;30(7):1094–1105. doi: 10.1016/j.numecd.2020.03.008. [DOI] [PubMed] [Google Scholar]
- 93.Naghshi S., Sadeghi O., Willett W.C., Esmaillzadeh A. Dietary intake of total, animal, and plant proteins and risk of all cause, cardiovascular, and cancer mortality: systematic review and dose-response meta-analysis of prospective cohort studies. BMJ. 2020;370:m2412. doi: 10.1136/bmj.m2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Guasch-Ferré M., Satija A., Blondin S.A., Janiszewski M., Emlen E., O’Connor L.E., et al. Meta-analysis of randomized controlled trials of red meat consumption in comparison with various comparison diets on cardiovascular risk factors. Circulation. 2019;139(15):1828–1845. doi: 10.1161/CIRCULATIONAHA.118.035225. [DOI] [PubMed] [Google Scholar]
- 95.Wang Y., Feng L., Zeng G., Zhu H., Sun J., Gao P., et al. Effects of cuisine-based Chinese heart-healthy diet in lowering blood pressure among adults in China: multicenter, single-blind, randomized, parallel controlled feeding trial. Circulation. 2022;146(4):303–315. doi: 10.1161/CIRCULATIONAHA.122.059045. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.