Abstract
Efficient local expression from recombinant adeno-associated virus (rAAV)-cystic fibrosis (CF) transmembrane conductance regulator (CFTR) vectors has been observed in the airways of rabbits and monkeys for up to 6 months following a single bronchoscopic delivery. However, it is likely that repeated administrations of rAAV vectors will be necessary for sustained correction of the CF defect in the airways. The current study was designed to test the feasibility of repeated airway delivery of rAAV vectors in the rabbit lung. After two doses of rAAV-CFTR to the airways, rabbits generated high titers of serum anti-AAV neutralizing antibodies. Rabbits then received a third dose of a rAAV vector containing the green fluorescent protein (GFP) reporter gene packaged in either AAV serotype 2 (AAV2) or serotype 3 (AAV3) capsids. Each dose consisted of 1 ml containing 5 × 109 DNase-resistant particles of rAAV vector, having no detectable replication-competent AAV or adenovirus. Three weeks later, GFP expression was observed in airway epithelial cells despite high anti-AAV neutralizing titers at the time of delivery. There was no significant difference in the efficiency of DNA transfer or expression between the rAAV3 and rAAV2 groups. No significant inflammatory responses to either repeated airway exposure to rAAV2-CFTR vectors or to GFP expression were observed. These experiments demonstrate that serum anti-AAV neutralizing antibody titers do not predict airway neutralization in vivo and that repeated airway delivery rAAV allows for safe and effective gene transfer.
The ultimate goal of cystic fibrosis (CF) transmembrane conductance regulator (CFTR) gene transfer to treat cystic fibrosis (CF) lung disease is to achieve persistent expression of CFTR protein in the airways such that the pathophysiologic sequelae of CF lung disease are ameliorated or prevented. Recombinant adeno-associated viral (rAAV) vectors are very promising agents for use to achieve this goal. rAAV vectors efficiently transduce a number of different cell types, including nondividing cells in vivo, as demonstrated in rabbit and monkey lung (13, 18, 25), mouse and guinea pig retina (6, 55), cochlea (35), rat and monkey brain (5, 14, 29, 52), skeletal muscle (11, 16, 31, 48, 49, 53), and liver (32). With these vectors, local transduction and long-term expression of transgene have been demonstrated in immunocompetent animals after a single dose (11, 18, 25, 29, 31, 48, 53).
rAAV-CFTR vectors were first developed to transfer a copy of the normal human CFTR (hCFTR) cDNA to mammalian cells (18, 19) and were shown to correct the chloride channel defect (15). The rAAV-CFTR vectors were tested in two animal models, the New Zealand White (NZW) rabbit and the rhesus macaque. In each case, expression of hCFTR was observed for up to 6 months following a single dose of rAAV-CFTR to the endobronchial surface of the lower lobe of the lung (13, 18). A phase I trial of rAAV-CFTR delivery to the maxillary sinuses of CF patients demonstrated efficient gene transfer which persisted for up to 10 weeks after a single administration (50). Endobronchial delivery of rAAV-CFTR vectors is also being evaluated in a phase I clinical trial in adult CF patients with mild lung disease (17).
Because rAAV vectors currently in use, including the rAAV-CFTR vectors, are deleted for the genes encoding the AAV nonstructural Rep proteins, vector integration or long-term persistence may occur by a different mechanism. Rep proteins are required for the establishment of the typical pattern of wild-type AAV latency, with site-specific integration into a region of human chromosome 19 (24, 33, 34, 37, 45). Rep-deleted rAAV vectors persist through a distinct mechanism that may involve a combination of episomal persistence and random-site integration (1, 20, 30, 42). Although it is unknown whether this altered pattern of persistence will eventually lead to loss of vector genomes, in vivo data from muscle, retina, spinal cord, brain, liver, and lung all indicate that rAAV transduction is quite persistent. Thus, prolonged expression within a given individual more likely will be limited by the life span of the cells that are transduced. Most of the cells transduced by rAAV-CFTR in the NZW rabbit and rhesus macaque following endobronchial delivery are surface epithelial cells. The life span of these cells in humans is estimated to be 120 days in normal individuals (2) and much shorter in individuals with CF (36). It is likely that maintenance of rAAV-mediated hCFTR expression in the airways of a given individual will require multiple administrations to transduce a sufficient number of cells to achieve the estimated 5 to 10% global correction thought to be required to overcome the electrophysiologic defect (27) in CF airways.
With respect to repeated delivery of a viral vector such as rAAV, the immune response to repeated capsid antigen exposure must be considered. The immune response to natural or wild-type AAV is not completely characterized. It is well established that AAV, uniquely among all the DNA viruses, is defective for replication, such that in the absence of a helper virus such as adenovirus (Ad), AAV remains latent in the host and integrates site specifically. In the presence of helper virus, AAV undergoes a productive infection replicating to yield a burst of progeny virus (reviewed in reference 3). Antibodies to AAV, as measured by complement fixation, were found in the serum of at least 30% of all people studied regardless of their exposure to Ad (10). However, detectable AAV infection or seroconversion to AAV usually occurs only in the setting of symptomatic Ad infection (7, 10). Preexisting neutralizing antibody to wild-type AAV did not prevent infection with AAV even during a concurrent symptomatic Ad type 1 (Ad 1) infection but did seem to prevent prolonged AAV shedding from the gastrointestinal and respiratory tracts (7). It is unclear whether direct airway inoculation with nonreplicating rAAV vectors will generate a humoral immune response capable of preventing reinfection of the airway.
Of the five well-characterized AAV serotypes, AAV1 to AAV5, 50 to 80% of adults and children over the age of 10 are seropositive to AAV by complement fixation or neutralization assays, indicating that natural AAV infection is common in humans (7, 9, 38, 41). AAV2 and AAV3 were first isolated from anal and throat specimens of children during an outbreak of Ad gastrointestinal illness (7, 8), while AAV1 and AAV4 have never been isolated from humans. Although antibodies to AAV5 were detected in 50% of adults in the former West Germany, AAV5 has been isolated only from a single human penile chondylomatous lesion (4, 23). The majority of individuals with anti-AAV antibodies are positive to more than one type of AAV, typically AAV1, AAV2, and/or AAV3, indicating that infection with a single type produces a heterotypic antibody response or that multiple infections are common (7, 9).
Since it has been shown that AAV2 and AAV3 commonly infect humans without causing disease and that preexisting neutralizing antibodies do not prevent wild-type infection, we have chosen to study repeated delivery with these two serotypes. In this study, we used bronchoscopic delivery to determine the feasibility of repeated delivery of rAAV vectors to the rabbit airway. We examined rAAV-mediated transgene expression following repeated delivery and show that high serum neutralizing titers to AAV capsid protein were developed. However, anti-AAV capsid host immune responses did not interfere with successful redelivery.
MATERIALS AND METHODS
Study design and animals.
The study was designed to assess the efficiency of rAAV-mediated gene transfer after the development of high serum neutralizing AAV antibody titers and the extent of airway inflammation associated with repeated bronchoscopic delivery of the rAAV2-CFTR vector. The study protocol is shown in Fig. 1. Fifty Pasteurella-free NZW male rabbits were obtained (Covance, Philadelphia, Pa.) and housed according to the Johns Hopkins University Animal Care and Use Committee guidelines. After acclimatization and physical examination, the rabbits were entered into the study. All rabbits in the experimental groups (n = 31) received rAAV2-CFTR vector at week 0 and again at week 3 instilled directly to three sites of the respiratory tract: (i) to the right lower lobe (RLL) bronchus (at the point of segmental branching), (ii) along the middle portion of the posterior tracheal wall, and (iii) in the right nostril. All three sites were inoculated with the rAAV2-encapsidated vector. At week 17, the rabbits were randomly assigned to three different groups and received a final dose of either rAAV2-green fluorescent protein (GFP) (n = 11), rAAV3-GFP (n = 10), or rAAV2-CFTR (n = 10) vector to both the RLL and naive left lower lobe (LLL) bronchi. At each time point, each dose to each portion of the respiratory tract (i.e., nose, trachea, RLL, or LLL) contained 5 × 109 DNase-resistant particles (DRP) in a 1-ml volume given through the suction channel of a 3.5-mm flexible fiberoptic bronchoscope (Olympus, Melville, N.Y.) (for the bronchus and trachea) or through a syringe (for the nose). Animals that received the vectors with the heterologous transgene, GFP, as the final dose were sacrificed at week 20, 3 weeks after the final dose of vector. Those that received three instillations of rAAV2-CFTR were sacrificed 4 weeks after the final dose (week 21). Additional groups of naive rabbits served as controls as follows. Single-dose vector-treated animals received a single dose of either rAAV2-GFP or rAAV3-GFP (n = 5) and were sacrificed at week 3 or received rAAV2-CFTR (n = 2) and were sacrificed at week 4. We have previously demonstrated in the rabbit bronchoscopy model that in vivo GFP expression from TR-UF5 (in the absence of wild-type AAV and Ad) peaks at week 3 and begins to decrease by week 4 (22). A vehicle-instilled control group of rabbits received either one (n = 5) or two (separated by 3 weeks; n = 3) bronchoscopic instillations of sterile lactated Ringer’s balanced salt solution (pH 7) (LR) to the RLL and were sacrificed 3 to 4 weeks after the final instillation. Finally, an uninstrumented control group (n = 4) was analyzed.
FIG. 1.
Study design. rAAV2-CFTR vector was instilled into the RLL, tracheas, and noses of NZW rabbits (n = 31) at week 0, and a second dose was given at week 3. The rabbits were then divided into three groups and received either rAAV3-GFP (n = 10), rAAV2-GFP (n = 11), or rAAV2-CFTR (n = 10) to both the RLL and LLL at week 17. Animals that received the heterologous transgene (GFP) vectors were sacrificed at week 20; rabbits that received the homologous transgene (CFTR) as the third dose were sacrificed at week 21. X indicates the study week during which the indicated intervention was performed. *, the GFP groups underwent cytologic brushings of the bronchial mucosa prior to sacrifice. BAL fluid (for total cell counts and neutralizing antibody) and serum (for neutralizing antibody) were obtained immediately prior to each delivery and at sacrifice. Additional serum was obtained at week 6 for neutralizing antibody assay. n indicates number of rabbits in each group.
BAL, brushing, and necropsy.
The rabbits were sedated with ketamine and acepromazine delivered intramuscularly prior to bronchoscopy, venipuncture, or sacrifice. Low-flow supplemental oxygen was provided during bronchoscopy. Prior to each vector delivery, and prior to sacrifice, bronchoalveolar lavage (BAL) was performed with sterile LR (6 ml) instilled into the lobar bronchus of either the RLL or LLL. A portion of the BAL fluid was kept on ice for determination of cell counts, while the remainder was stored at −80°C for further analyses. All rAAV-GFP-treated animals (n = 21) underwent cytologic brushings of the RLL and LLL just prior to sacrifice (week 20). Cytologic brushings were performed by inserting a sterile 1.1- by 2.0-mm cytology brush (Mill-Rose Laboratories, Inc., Mentor, Ohio) through the suction port of the bronchoscope and vigorously brushing the lower airway mucosa in the approximate area to which the vector had been instilled at earlier time points. The brushes were held on ice (2 to 4°C) and processed for fluorescence-activated cell sorting (FACS) analysis of GFP expression. In all cases, BAL and venipuncture were performed prior to vector administration or brushing. Serum was collected by venipuncture of ear veins at the times shown in Fig. 1. Animals were sacrificed by pentobarbital overdose. The lungs were removed from the thoracic cavity en bloc. The trachea was cannulated and perfused with sterile LR to physiologic inflation. The RLL and LLL of each animal were isolated and sectioned transversely into proximal, middle, and distal sections. The proximal and distal sections were preserved in 10% formalin for thin (5-μm) sectioning. The middle third was placed in a sterile container, immediately frozen in liquid nitrogen and stored at −80°C for molecular analyses. Separate sterile instruments for each individual lobe were used to minimize cross-contamination, and aseptic technique was used for all procedures.
Vectors.
The rAAV2-CFTR vector used in this study was the same as that previously used in preclinical studies in rhesus macaques (13), contains the full-length hCFTR cDNA inserted between the ITRs of AAV2, and is packaged in AAV2 capsids using a rescuable vector-containing cell line as previously described (12). Figure 2 depicts the vector constructs. The biological titer was 1.4 × 108 replication units per ml (equivalent to 109 infectious units [IU]/ml), and the physical titer was to 5 × 1011 DRP per ml. Thus, the particle-to-infectivity ratio was 500:1. The vectors were tested for replication competent AAV (rc-AAV) and Ad by a modified replication center assay and found to have less than 3 IU of either rc-AAV or rc-Ad for each 1010 DRP of vector. One hundred-fold dilutions in sterile LR were made for doses of 5 × 109 DRP per ml.
FIG. 2.
Map of vector and packaging constructs. The DNA contained in the rAAV-CFTR vector contains full-length hCFTR cDNA inserted between the ITRs of AAV2 (ITR-2). This vector was packaged in AAV2 capsids by using a cell line that expresses Cap protein from AAV2 (Cap-2) and is referred to as rAAV2-CFTR in this study. The DNA contained in the rAAV-GFP vectors, TR-UF5, contains the cytomegalovirus (CMV)-driven Neor gene and humanized GFP cDNA reporter gene cassette inserted between the ITRs of AAV2 (ITR-2). rAAV-GFP vector DNA was packaged in AAV2 capsids by using the packaging construct pRS5 and is referred to as rAAV2-GFP vector. rAAV-GFP vector DNA was also packaged in AAV3 capsids by using the construct pSB-Cap3.6, which contains the Cap gene from AAV3 (Cap-3) in place of Cap from AAV2 (Cap-2) in the packaging construct (see text for explanation), and is referred to as rAAV3-GFP vector. TK, thymidine kinase; LTR, long terminal repeat.
The rAAV-GFP vector is the previously described TR-UF5 (55). It contains the humanized GFP reporter gene expressed from the cytomegalovirus immediate-early promoter and a herpes simplex virus thymidine kinase promoter-driven neomycin resistance (Neor) gene cassette inserted between the ITRs of AAV2. rAAV2-GFP vector was packaged in AAV2 capsids by using the packaging construct pRS5 (21). rAAV3-GFP vector was packaged in AAV3 capsids, using a packaging construct, pSB-Cap3.6, in which the 2.3-kb KpnI fragment of the AAV3 genome (containing 89% of the Cap gene) was substituted for the homologous fragment of AAV2. Sequencing of pSB-Cap3.6 confirmed homology with the KpnI Cap fragment of wild-type AAV3. rAAV-GFP vectors were packaged by calcium phosphate precipitation in Ad-infected 293 cells, purified by CsCl ultracentrifugation as previously described (21), and found to be free of detectable rc-Ad and rc-AAV as determined by a quantitative competitive PCR. The biological titers of the rAAV3-GFP and the rAAV2-GFP vectors were each 109 IU/ml as determined by replication center assay and or fluorescence assay and quantitative competitive PCR. The particle to-infectivity ratios of the two GFP vectors and the rAAV-CFTR vector were equivalent (500:1) (Fig. 3).
FIG. 3.
GFP expression in 293 cells following infection with 104 DRP of rAAV2-GFP vector (B) or rAAV3-GFP vector (C) per cell. (A) Uninfected 293 cells.
Serum and BAL anti-AAV neutralizing antibody titer.
To measure neutralizing antibody titers to AAV in sera taken from animals at each previously mentioned time point, serial twofold dilutions of serum were preincubated with 8,000 IU of wild-type AAV2 (for a multiplicity of 1 IU per cell) and added to 293 cells preinfected with Ad5 (multiplicity of 5 IU per cell) in a 96-well dish. After 72 h of incubation at 37°C, the lysate was probed for AAV replication by using a 32P-labeled AAV2 Rep fragment probe and analyzed on a PhosphorImager (Molecular Dynamics, Sunnyvale, Calif.). Assays were performed in quadruplicate for all serum samples. The inverse of the most dilute serum that completely inhibited AAV replication in all four wells is reported as the neutralizing titer. Statistical significance of the titers and log2 of the titers was determined by analysis of variance using the SPSS program; the n for each group is listed in the study design; P values are given in Fig. 4. In the text, titers are reported as the mean titer ± standard error. Neutralizing antibody to AAV was similarly measured in BAL fluid (diluted to 1:10) in quadruplicate at all time points.
FIG. 4.
Serum neutralizing AAV antibody response. The left-hand side shows the baseline titer at week 0, the response 3 weeks after the first dose (week 3), and the responses 3 and 14 weeks after the second dose (week 6 and week 17, respectively). The right-hand side shows the responses 3 weeks after administration of the third dose of either rAAV3-GFP (A) or rAAV2-GFP (B) and 4 weeks after readministration of a dose of rAAV2-CFTR (C). Bars represent median values, shaded boxes represent 2 quartiles of values, whiskers represent ranges of values, ∗ indicates significance at P < 0.001, and n/s (not significant) indicates significance at P > 0.05. Note that the titer is plotted on a log2 scale. n values are as indicated in Fig. 1.
Anti-GFP antibody detection.
Anti-GFP antibodies were measured in the sera of rAAV-GFP-treated rabbits at week 21 (n = 21) and in a selected group of pretreated rabbits at week 0 (n = 6) and week 17 (n = 6) in an enzyme-linked immunosorbent assay (ELISA). Immulon 4 (Dynatech, Chantilly, Va.) flat-bottomed microtiter plates were coated with 100 ng of purified GFP protein, blocked with 0.5% bovine serum albumin, and then incubated with a 1:100 dilution of each test rabbit serum. Anti-GFP antibody levels in each test serum were quantified by an o-phenylenediamine dihydrochloride detection reaction (Sigma, St. Louis, Mo.) after binding with a goat anti-rabbit immunoglobulin G (IgG)-peroxidase-conjugated secondary antibody (1:5,000 dilution). The assay was linear down to 1:800 dilution of positive control polyclonal rabbit serum.
DNA PCR.
The RLL and LLL of all rabbits were tested for the presence of rAAV-GFP or rAAV-CFTR vector-specific DNA, or both, by nested DNA PCR. Individual RLL or LLL frozen lung was homogenized with an individual sterile frozen mortar and pestle and divided for DNA or RNA extraction. Thus, RNA and DNA were extracted from the same homogenate. DNA was extracted from frozen lung homogenates either by phenol chloroform extraction or according to specifications in the Qiagen (Valencia, Calif.) Midi-DNA extraction kit. The DNA concentration was determined by spectrophotometry. Two hundred nanograms of DNA was used in the first round of PCR, and 4 μl of the first-round product was used in the second round. Primer pairs used for rAAV-CFTR or rAAV-GFP vector-specific DNA and assay sensitivities were the same as those used for reverse transcription (RT)-PCR described below. The presence of rabbit DNA in the samples was confirmed by performing a nested PCR on 4 μl of the first-round PCR, using primers specific for the ubiquitous rabbit major histocompatibility complex (MHC) class I gene as described below. Samples were reported as positive if they showed the appropriate size band after nested vector-specific DNA PCR and showed the presence of rabbit DNA in the sample after nested MHC PCR. To eliminate false negative reporting, a sample was reported as negative only if vector-specific DNA was absent and rabbit MHC DNA was present. Statistical significance of the number of rabbits positive for detectable vector DNA was determined by independent t test using SPSS; values are reported as mean ± standard error, and differences are reported at a level of significance of P < 0.05.
RT-PCR.
The RLL and LLL of all rabbits that received the GFP vectors were tested for the presence of TR-UF5 vector-specific RNA by RT-PCR. Only the rabbits that were positive for hCFTR by DNA PCR were tested for the presence of rAAV-CFTR vector-specific RNA by RT-PCR. RNA was isolated from fresh frozen rabbit lung homogenates as instructed for the Trizol RNA extraction kit (Sigma); concentration determined by optical density reading at 260 nm. Four μg of RNA was treated with DNase (Sigma) prior to the RT-PCR assay. The DNase-treated product was divided into two tubes (with [RT+] and without [RT−] reverse transcriptase). Reverse transcriptase was then inactivated in the negative tubes by heating to 94°C for 10 min. Products then underwent amplification by RT-PCR according to kit instructions (Amersham Life Science Inc., Arlington Heights, Ill.). For detection of rAAV-GFP vector-specific cDNA, a nested PCR was performed with the outer primer pair 5′-TATGGGATCGGCCATTGAAC–3′-GGACTACGAGAAGCAGGTCT and the inner pair 5′-GG TGGAGAGGCTATTCGGCTATG–3′-AGGACTAGCTGTTCTGGCCGA AGG after the RT reaction. The expected size of the nested PCR product was 355 bp. The lower limit of detection, 10−11 μg of rAAV-GFP DNA or one copy of rAAV-GFP mRNA (or DNA) per 1,000 cells, was measured by performing nested PCR on serial 10-fold dilutions of a known amount pTR-UF5 DNA in a background of 200 ng of rabbit DNA. For amplification of rAAV-CFTR vector-specific cDNA, the outer primer pair 5′-CTGTGAGCCGAGTCTTTAAG–3′-GTTGGTTTTTTGTGTGTACG and the inner pair 5′-CCTACCAAGTCAACCAAACC–3′-GAGCTCAGATGCATCGATCAG, corresponding to hCFTR vector sequences, were used. The sensitivity of this assay was one copy of hCFTR mRNA (or DNA) per 1,000 cells.
Two methods were used to ensure that amplification of genomic or vector DNA did not contaminate the results of the RT-PCR assay. (i) Each sample underwent amplification in the absence of reverse transcriptase in a parallel nested PCR to check for amplification of vector DNA. (ii) Using primers specific for the ubiquitous rabbit MHC RLA region class I 19-1 gene complex (accession no. K02819), the presence of genomic versus cDNA in the RT-PCR products was determined by nested PCR by using primer pairs in adjacent exons with 4 μl from the original RT reactions. Thus, amplification of genomic DNA yielded a larger product (355 bp with the intervening intron sequence) than amplification of cDNA (242 bp). The MHC outer primers were 5′-CTCACTGACCTGGCAGCGGGATG and 5′-CTCCAAGAACTCCAGCAAC, corresponding to nucleotides 2186 to 2208 and 2523 to 2541, and the inner primers were 5′-GAGCTCGTGGAGACCAGGCCTG and 5′-CAATTCCCACTATGAGCGCGGTG, corresponding to nucleotides 2232 to 2253 and 2501 to 2524 in adjacent exons 4 and 5 of the rabbit MHC gene. MHC PCR confirmed the presence of cDNA in the samples negative for vector sequences. Samples were reported as positive for presence of vector RNA if the all of the following criteria were met: (i) presence of a product of the expected size from the nested vector-specific RT-PCR, (ii) presence of amplified MHC cDNA in the RT+ reactions, and (iii) absence of amplified genomic MHC DNA in the RT− and RT+ reactions. To eliminate false negative reporting, samples were reported as negative for presence of vector RNA if the two following criteria were met: (i) absence of a product of the expected size from the nested vector-specific RT-PCR and (ii) presence of amplified MHC cDNA in the RT+ reactions.
Detection of GFP expression by FACS.
Cells brushed from the approximate site of vector instillation were analyzed for GFP expression by FACS using a Becton Dickinson (Philadelphia, Pa.) flow cytometer. Bronchial brushings were taken from the RLL and LLL of all the rabbits that received the GFP vectors 3 weeks after the final dose of vector as described above. Negative controls were provided by rabbits in which only LR (n = 3) or only rAAV2-CFTR (n = 3) was instilled. A separate group of rabbits analyzed 3 weeks after a single dose of either rAAV2-GFP or rAAV3-GFP vector served as a positive control group (n = 5). Brushes were incubated in 1 ml of trypsin at 37°C for 5 min. Cells, collected by centrifugation at 1,500 rpm for 5 min, were resuspended in 1 ml of phosphate-buffered saline and promptly analyzed. Cells with a light-scatter profile typical of epithelial cells were included in the analysis, whereas erythrocytes were excluded from the analysis (Fig. 6B). Negative controls always contained less than 0.5% of the epithelial cell population showing fluorescence, whereas single-dose GFP-treated animals always had at least 2% of the epithelial cell population showing distinct fluorescence. Thus, samples were scored as positive for GFP expression if at least 2% of the epithelial cells showed distinct fluorescence and at least 1,000 epithelial cells were counted. Samples with an inadequate amount of epithelial cells (i.e., fewer than 1,000 epithelial cells) were excluded in the analysis. For each group, the number of animals from which a sufficient number of cells were obtained for analysis is shown in Table 1. Statistical significance of the number of positive rabbits per group and the number of positive cells per sample was determined by independent t tests using SPSS, values are reported as mean ± standard error, differences are reported at a level of significance of P < 0.05.
FIG. 6.
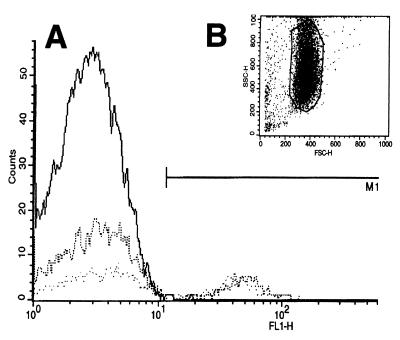
FACS analysis of brushed bronchial epithelial cells taken 3 weeks after the third dose of AAV vector. (A) Representative histogram of brushed bronchial epithelial cells from the RLL and LLL (dotted lines) of an AAV3-GFP-treated rabbit showing 45 and 25% of cells with characteristic GFP fluorescence compared to an untreated control animal (solid line) showing less than 0.5% fluorescing cells. (B) Forward/side scatter profile of epithelial cells gated for analysis.
TABLE 1.
Summary of vector DNA transfer as detected by PCR and mRNA as detected by RT-PCR
| Vector/means of detection | No. of rabbits positive for vector detection/no. with available DNA for assaya
|
|||||
|---|---|---|---|---|---|---|
| AAV3- GFP
|
AAV2- GFP
|
AAV2-CFTR
|
||||
| LLL | RLL | LLL | RLL | LLL | RLL | |
| GFP by DNA PCR | 4/8 | 9/10 | 6/7 | 7/8 | NA | NA |
| GFP mRNA by RT-PCR | 1/6 | 0/6 | 0/9 | 1/9 | NA | NA |
| hCFTR by DNA PCR | NA | 1/9 | NA | 1/9 | 1/10 | 4/10 |
After third dose of vector at indicated site of delivery. NA, not applicable.
Detection of GFP by fluorescence microscopy.
Transverse 5-μm sections were made through the RLL or LLL bronchus at the point of segmental branching. Unstained sections were examined for GFP expression, using a Zeiss (Thornwood, N.Y.) Axioskop upright epifluorescence microscope equipped with a GFP-specific filter set (excitation, HQ470/40x; emitter, HQ515/30M). Images were processed using MetaMorph software (Crane Digital Media, Inc., Santa Fe, N. Mex.).
BAL fluid cell counts and cytokine assays.
To assess the inflammatory response to repeated airway delivery of rAAV vectors, total cell counts in BAL fluid were measured. Total cell counts were performed on 10 μl of fresh BAL fluid in a cell counting chamber slide. Differential cell counts were performed on glutaraldehyde-fixed, Wright-Giemsa-stained 100-μl BAL fluid samples deposited onto glass microscope slides, using a Shandon Cytospin-3 cytocentrifuge (Shandon Scientific, Cheshire, United Kingdom). BAL fluid levels of interleukin-8 (IL-8) were measured by using an ELISA (R&D Systems, Minneapolis, Minn.) that detects a human IL-8 and was normalized to the extracellular lining fluid (ELF) by comparison of blood and BAL urea nitrogen levels by the formula [IL-8]ELF = [IL-8]BAL × [urea nitrogen]serum/[urea nitrogen]serum (43). Statistical significance was determined by analysis of variance using SPSS. Differences are presented as the mean ± standard error at a level of significance of P < 0.05. For the IL-8 assay, BAL fluid samples from two additional rabbits infected with an Ad vector (107 PFU) were used as positive controls to show that the assay was capable of capturing an IL-8-like rabbit homologue.
Histopathology.
One or two 5-μm slides from the proximal RLL, distal RLL, proximal LLL, and distal LLL of each animal in the study were stained with hematoxylin and eosin and reviewed for evidence of inflammation without knowledge of prior treatment. The extent of bronchus-associated lymphoid tissue (BALT) was counted in terms of number of airways with and without BALT, the number of lymphoid aggregates per airway, and the percentage of airway circumference that the lymphoid aggregates occupied. Neutrophilic, eosinophilic, or lymphocytic infiltration (other than BALT) around vessels, in and around airways, in the interstitium and in the alveolar space was assessed and quantified according to location, severity, and distribution. Structural changes in vessels, airways, interstitium, and air spaces were assessed and classified as mild, moderate, or severe.
RESULTS
Vector packaging.
To study the serotype specificity of anti-AAV humoral immune responses, we constructed complementing packaging plasmids capable of providing trans functions for encapsidating rAAV2 vector genomes into either AAV2 or AAV3 capsids (Fig. 2). The feasibility of packaging recombinant AAV2 genomes into AAV3 capsids was confirmed by cotransfection of the rAAV-GFP construct with either the rAAV2 complementing construct, pRS5, or the rAAV3 complementing construct, pSB-Cap3.6, into Ad5-infected 293 cells. After purification by CsCl ultracentrifugation, rAAV3-GFP was found to be as infectious as rAAV2-GFP, as evidenced by its ability to efficiently transduce 293 cells (Fig. 3) as well as the CF bronchial epithelial cell line IB3-1 (data not shown). The particle-to-infectivity ratios of the rAAV3-GFP and rAAV2-GFP vectors were equivalent (500:1).
Clinical status of experimental animals.
All rabbits that received repeated delivery of rAAV vectors via bronchoscopy remained healthy throughout the study period, with vigorous appetites, normal behavior, and no signs of respiratory difficulties. Each animal tolerated three bronchoscopic instillations of rAAV vector. One rabbit contracted a self-limited, unilateral, culture-negative conjunctivitis that was most likely due to drying of the conjunctival mucosa during sedation. We subsequently modified our procedure to include artificial tears and taping the eyes closed during sedation. Two rabbits became apneic and stopped breathing during sedation prior to instillation of the second or third dose and did not recover. The cause of death was determined as pulmonary edema secondary to asphyxiation from oversedation without evidence of infection or structural lung changes. These two rabbits were excluded from the study.
Neutralizing AAV antibody titer rises.
To determine whether two doses of rAAV2-CFTR to the upper and lower respiratory tract elicited a significant humoral immune response, sera from treated animals were analyzed for anti-AAV neutralizing antibodies at predetermined intervals described in the study design. There was a statistically significant rise in titer after the first dose, but seroconversion, defined as a fourfold or greater rise in titer, was not observed in all animals until after the second dose (Fig. 4). The high titer was sustained at week 17. Thus, all rabbits had significantly elevated anti-AAV2 titers at the time of third dose instillation. After the third dose, titers showed a typical boost. Interestingly after the third dose, there was no difference in the magnitude of the anti-AAV2 titer between the AAV2-GFP and AAV3-GFP groups, likely reflecting the cross-reactivity between the AAV3 and AAV2 serotypes. The neutralizing response to three doses of rAAV2-CFTR was the most robust but cannot be directly compared to the AAV-GFP groups since the serum from the AAV-CFTR group was drawn 1 week later. Humoral immune responses against GFP itself were also assessed by ELISA. Anti-GFP antibodies were not detectable in these animals either before or after rAAV-GFP administration, because AAV-mediated GFP expression just begins to peak at week 3.
Anti-AAV2 neutralization in diluted BAL fluid was undetectable before and after delivery at all time points outlined in the study design (data not shown). These negative results may reflect the low overall quantity of immunoglobulin in diluted BAL fluid or the presence of nonspecific inhibitors of the assay.
DNA transfer and mRNA expression.
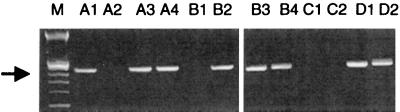
In spite of the elevated levels of serum anti-AAV neutralizing antibodies at the time of delivery, DNA transfer to the lungs of treated rabbits was observed in the RLL and LLL of both the AAV3-GFP and AAV2-GFP groups (Table 1). Agarose gel electrophoresis of amplified TR-UF5 (GFP) vector DNA sequences demonstrated an amplification product of the expected size (Fig. 5). The observation of gene transfer was fairly consistent in that vector DNA was detected in 9 of 10 RLLs and 4 of 8 LLLs in the AAV3-GFP group and in 7 of 8 RLLs and 6 of 7 LLLs in the AAV2-GFP group. There was no statistical difference in the number of rabbits positive for vector DNA detection between the RLL and LLL of either group (P = 0.089 and P = 0.926, respectively) or between the AAV3- and AAV2-GFP-treated groups (P = 0.316). mRNA detection was less readily observed in that GFP vector mRNA was detected in one of six LLLs and in none of the RLLs of the rAAV3-GFP and one of nine RLLs and none of the LLLs in the AAV2-GFP group. hCFTR cDNA was detected in one of nine RLLs in the AAV3-GFP group and in one of nine RLLs in the rAAV2-GFP-treated rabbits (Table 1). hCFTR vector mRNA was not detected as measured by RT-PCR in these lobes. hCFTR cDNA was not detected in the LLL of these groups, as they did not receive rAAV-CFTR vector in this area.
FIG. 5.
Ethidium bromide-stained 1.5% agarose gel electrophoresis analysis of vector-specific DNA amplified from the RLL (even numbers) or LLL (odd numbers) of rAAV3-GFP-treated (A1 to A4), rAAV2-GFP-treated (B1 to B4), and vehicle-treated (C1 to C2) animals. pTR-UF5 plasmid dilutions D1 (10−14 g) and D2 (10−17 g) in a background of 200 ng of untreated rabbit lung DNA show the sensitivity of the PCR. M is the 100-bp marker; the arrow marks expected 355-bp PCR product.
In the group that was challenged with three doses of rAAV2-CFTR only, vector hCFTR was amplified in 4 of 10 RLLs, while only 1 of 10 LLLs of the same group had detectable hCFTR DNA by PCR (Table 1). Thus, hCFTR vector DNA was detected more readily in the thrice-challenged RLL than in the once-challenged LLL; however the differences between the two groups did not reach statistical significance (P = 0.138). Since the relative percentage of target epithelial cells in a given sample can vary, the hCFTR DNA PCR assay was repeated in the thrice-dosed group in a different lab using freshly prepared lung homogenates and different primer pairs for hCFTR; the results were identical, indicating that the amount of vector hCFTR DNA in the positive samples was consistently above the threshold of detection. This suggests that local immune factors may not play a role in blocking vector uptake and that DNA transfer may be cumulative after the three doses. hCFTR vector mRNA was detected by RT-PCR in the RLL of one animal in this group.
The presence or absence of vector DNA or mRNA in an individual rabbit did not correlate with the magnitude of the anti-AAV neutralizing titer. For example, the mean titer of all rabbits that had no detectable third dose vector DNA, 792 ± 206, was not significantly higher than the mean titer of all rabbits that had detectable third dose vector, 457 ± 55 (P = 0.132), indicating that serum neutralizing anti-AAV antibody was not a significant variable in limiting vector uptake.
Expression after repeated dosing as detected by FACS and fluorescence microscopy.
In accordance with the DNA transfer data described above, 50 to 80% of rAAV2-GFP- or rAAV3-GFP-treated rabbits showed GFP expression by FACS analysis (Fig. 6). The proportion of fluorescent cells in each sample ranged from 2.6 to 46% of total cells in the AAV3-GFP group and from 2.8 to 28% of total cells in the AAV2-GFP group. There was a significant trend toward a higher percentage of fluorescent cells in the AAV3-GFP group compared to the AAV2-GFP group (P = 0.028) (Table 2). However, there was no statistically significant difference between the number of rabbits demonstrating expression in the AAV3-GFP versus AAV2-GFP groups (P = 0.736) or between the right and left lobes within the AAV3-GFP or AAV2-GFP group (P = 0.336 and P = 0.430, respectively) (Table 2). The wide range of the number of positive cells per sample may reflect the imprecise sampling technique of brushing at the expected site of delivery. The magnitude of the serum neutralizing titer did not correlate with the presence or absence of GFP expression in an individual rabbit. For example, the mean serum neutralizing AAV titer in all rabbits negative for GFP expression was 340 ± 70, versus 580 ± 76 in the rabbits positive for GFP expression (P = 0.095), indicating the titer of serum neutralizing anti-AAV antibody did not negatively influence uptake and consequently expression.
TABLE 2.
Summary of FACS analysis
| Group | Site of delivery | Total no. of rabbits with FACS data (>1,000 cells counted) | Range of % fluorescent cells over background | % of rabbits positive for GFP [(no. positive/no. with data)] ×100) |
|---|---|---|---|---|
| 3rd dose of vector: | ||||
| AAV3-GFP | LLL | 6 | 14.4–45.3 | 50 |
| RLL | 5 | 2.6–28.5 | 80 | |
| AAV2-GFP | LLL | 9 | 2.8–27.6 | 78 |
| RLL | 10 | 2.8–27.1 | 60 | |
| Controls | ||||
| Positive | RLL | 6 | 2.1–48 | 100 |
| Negative | RLL or LLL | 6 | 0.15–0.51 | 0 |
GFP expression after repeated delivery was confirmed in the bronchial epithelium by epifluorescence microscopic examination of tissue sections (Fig. 7). GFP expression was limited to the epithelium of large cartilaginous airways, and in particular appeared to be localized to the cytoplasm of ciliated columnar epithelial cells. Resolution did not allow further differentiation of cells to determine if basal cells were transduced. Since rabbits do not normally have submucosal glands the transduction efficiency at that site could not be assessed in this model. No expression was seen in alveolar cells. Minimal autofluorescence in the muscle layer and cartilage was further minimized when a GFP-specific filter set was used in place of a fluorescein isothiocyanate filter set.
FIG. 7.
GFP expression in rabbit bronchial epithelium after the third dose of rAAV vector. Proximal RLLs were sectioned at the level of segmental branching, fixed in 10% formalin, and analyzed by GFP-specific fluorescent microscopy. (A) GFP in the cytoplasm of ciliated columnar epithelial cells in the RLL of an AAV3-GFP-treated rabbit; (B) vehicle-treated rabbit airway showing minimal background autofluorescence. Original magnification, ×400. Sections were examined under differential interference contrast, captured, colored blue, and overlaid onto the fluorescent image to show underlying airway architecture.
Minimal inflammation as judged by BAL fluid cell counts, cytokines, and histopathology.
Host immune responses may play a role in triggering inflammatory cell infiltration in the lung. In rabbits that received repeated doses of rAAV vectors, there was no rise in BAL fluid cell counts after the first or second dose of rAAV-CFTR vector. After the third dose, cell counts were elevated in the RLL of 3 of 10 rabbits in the rAAV3-GFP-treated group, giving a statistically significant rise in the mean compared to predose controls in this group (Fig. 8B). Otherwise, the counts following the third dose were not statistically different from baseline or single-dose controls. Differential cell counts in all groups after three doses of rAAV vectors were normal compared to predose values, including the RLL of the AAV3-GFP group (Fig. 8C to E). In all cases, the predominant cell type in each group was the alveolar macrophage (>92%) and the percentage of lymphocytes was less than 7.5%, indicating absence of chronic inflammation to vector proteins. Given the normal distribution of cell types in the RLL of the AAV3-GFP group, the increased total cell counts likely represent a concentration artifact in those samples and not a pathologic process. The percentage of eosinophils was significantly higher statistically but not clinically in the RLL of the AAV2-GFP group versus the control group. All groups had less than 2.5% eosinophils and less than 1.9% neutrophils, indicating absence of an acute inflammatory response to vector protein expression.
FIG. 8.
Inflammatory profile of BAL fluid before and after treatment with rAAV vectors. (A) IL-8 in rabbit BAL fluid after three doses of rAAV vector and in BAL fluid from Ad vector-infected rabbits (striped bar at the bottom; n = 4); (B) total BAL fluid cell counts; (C) percentages of macrophages in BAL fluid; (D) percentages of lymphocytes in BAL fluid; (E) percentages of eosinophils (Eos) in BAL fluid. Designations for repeatedly dosed groups (diagonally hatched bars; n = 10 to 11 in each group), single-dose controls (stippled bars; n = 2 in each group), and pre-first dose (open bar at top; n = 31) apply to all panels. Bars represent means, whiskers represent standard errors of the means, and ∗ indicates significance at P < 0.05.
The concentration of an IL-8-like cytokine, as judged by an anti-human IL-8 antigen capture ELISA, was measured in rabbit BAL fluid and normalized to the ELF. The ability of this antigen-capture ELISA to detect a rabbit homologue of IL-8 was demonstrated in an assay using BAL fluid from Ad-infected rabbits (Fig. 8A). In this assay, very low levels of IL-8-like molecules were detected in the BAL fluids of all groups; however, there was no difference in the concentration after three doses of rAAV vector compared to predose controls.
An extensive review of hematoxylin-and-eosin-stained proximal and distal lung sections revealed no identifiable differences among the groups. There was no evidence of destructive acute or chronic inflammation in the lungs of rabbits after three doses of rAAV vector compared to single-dose, twice-vehicle-instilled, or uninstrumented rabbits. All groups showed bronchus-associated lymphoid aggregates around the cartilaginous and bronchiolar airways, features common in the rabbit airway (18). A few rabbits in every group, including single-dose, vehicle-instilled, and uninstrumented rabbits, had mild to moderate perivascular or peribronchial/bronchiolar lymphocytic or eosinophilic accumulation in small focal areas, rarely in a diffuse pattern (Fig. 9). There was no evidence of any destructive inflammatory changes in or around the airways, alveoli, vasculature, or interstitium in the rAAV-treated or control rabbits.
FIG. 9.
Histopathology of rabbit lung. Five-micrometer sections of formalin-fixed lung, stained with hematoxylin and eosin, from each animal in the study were examined as described in Materials and Methods. (A) LLL of the thrice-dosed AAV2-CFTR group (received only one local dose of rAAV2-CFTR). Note BALT in the submucosa of this cartilaginous airway and the intact alveolar and airway architecture. This finding was typical in all treated and untreated rabbits in the study. Original magnification, ×40. (B) RLL after three instillations of rAAV2-CFTR. Note minimal lymphocytic infiltration in the submucosa of a large airway with preservation of airway architecture. This degree of lymphocytic infiltration was common in treated and untreated rabbits. Original magnification, ×100. (C) RLL of a single-dose vehicle-treated control rabbit showing intact epithelium, normal alveolar architecture, and BALT (upper left-hand corner), typical of all rAAV-treated vehicle-treated, and untreated rabbits in the study. Original magnification, ×40. (D) For comparison, a rabbit airway 3 days after instillation of 107 PFU of an Ad vector, showing inflammatory cell disruption of the epithelium and submucosa of a large airway. This was not seen in any rAAV- or vehicle-treated rabbit in the study. Original magnification, ×40. (E) Same animal as in panel A, showing small focal aggregate of lymphocytes and eosinophils around a bronchiole and structural integrity of alveolar air spaces, epithelium, and submucosa. This finding was typical of all treated and untreated rabbits in the study. Original magnification, ×100. (F) Same animal as in panel B, showing structural integrity of epithelium and basement membrane, with occasional lymphocytes and eosinophils, and lack of neutrophils in submucosa. This finding was typical of all treated and untreated rabbits in the study. Original magnification, ×200. (G) Same as animal as in panel C, showing integrity of epithelium and basement membrane, typical of all rAAV- and vehicle-treated rabbits in the study. Original magnification, ×200. (H) Same animal as panel D, showing inflammatory cell infiltration. Note neutrophils and eosinophils invading the columnar epithelial cells, which were not seen in any rAAV- or vehicle-treated rabbit in the study. Original magnification, ×100.
DISCUSSION
Host immune responses to vector capsid proteins have created a significant barrier to repeated delivery of Ad vectors in a number of animal models (40, 51, 54). The data presented here indicate that host immune responses to rAAV vectors delivered to the rabbit airway are sufficiently compartmentalized to allow for effective repeated gene transfer. More specifically, our results show that expression may occur from repeated airway doses of rAAV vectors despite the development of neutralizing antibodies in the serum.
There are several possible explanations for the finding that serum neutralizing antibodies do not prevent reinfection with rAAV vectors. Our neutralization assay measured a total serum antibody response of which IgG is the predominant immunoglobulin. In the absence of inflammation and capillary leakage of serum proteins, IgG is generally not available on the large airway surface to neutralize foreign antigen. The predominant surface immunoglobulin is secretory IgA (39). Although we did not measure secretory IgA directly, our ability to reinfect the animals strongly suggests the absence of such a response.
Early studies of AAV seroepidemiology showed that antibody to AAV was common in the serum of children and young adults independently of the individual’s exposure to Ad and that wild-type AAV infection was not prevented by preexisting serum neutralizing antibodies (10). This is consistent with our findings which show that preexisting serum neutralizing antibodies to AAV2 did not prevent direct airway reinfection with either rAAV2- or rAAV3-encapsidated vectors. Our findings that vector DNA or protein expression was detected in a given rabbit sample despite elevated levels of serum neutralizing antibody at the time of delivery indicates that serum neutralizing anti-AAV antibody does not play a significant role in neutralization of rAAV vectors in the normal airway.
The data in this study are consistent with previous reports (13, 18) that a single dose of rAAV-CFTR vector to the airway does not elicit an inflammatory response and is not associated with lymphocytic inflammation. We have also shown here that repeated airway delivery of rAAV-CFTR does not instigate inflammatory changes or induce clearance of rAAV-mediated transgene expression. Our findings are also similar to those in muscle where rAAV-mediated transgene expression has been particularly long term, with inflammation being notably absent (31, 53). The mechanism by which AAV evades immunologic responses following injection into muscle has been studied by Jooss et al., who showed that rAAV was unable to efficiently transduce antigen presenting cells (dendritic cells) in muscle, thus accounting for the lack of CTL against rAAV vector transgene and the long-term persistence (28). In our study, endobronchial delivery targeted vector primarily to the segmental branches of the airway where pulmonary dendritic cells lace the submucosal area of the epithelium and function as antigen-presenting cells (46, 47). Since AAV is inefficient at transducing dendritic cells, perhaps the lack of immune response in the airways affords AAV the same protection as the muscle. This possibility is supported by our observations that readministration of highly purified AAV vector yields substantial expression when delivered to an immunologically compartmentalized site, such as the lung.
Host immune responses may be more readily mounted against vectors that can replicate. If AAV replication took place, the immune response would likely be enhanced due to the processing of synthesized capsid proteins through the MHC class I, CD8+ cell-mediated pathway, leading to CTL destruction of cells expressing vector-derived proteins, as well as boosting the humoral arm. The rAAV vectors used in our experiments did not contain the Cap gene and were free of detectable replication-competent forms of both Ad and AAV, thus making it unlikely for replication and augmentation to take place.
In contrast, a study by Halbert et al. reported failure to transduce rabbit lung upon readministration of homologous serotype rAAV vector in association with the development of neutralizing antibodies to AAV (25). First, it is possible that greater airway trauma was produced in their study since an intralumenal balloon catheter rather than a fiberoptic bronchoscope was used to deliver vector. This procedure might have allowed for additional protein leakage across the airway surface or more efficient antigen presentation at the site of delivery. Second, in the same study, vector preparations were reported to be contaminated with wild-type AAV, which correlated with decreased transduction efficiency following the first dose compared to less contaminated preparations. Low-level Rep and Cap expression from wild-type AAV contamination might have augmented the development of neutralizing antibodies and adversely influenced uptake from a second dose of same serotype vector. Alternatively, expression of Rep proteins from wild-type AAV might have down-regulated expression from rAAV. In a second study reporting failure to transduce mouse lung following repeated aspiration of homologous serotype rAAV vectors (26), the alveolar region was transduced following the first but not second administration of rAAV vector in association with the development of serum neutralizing anti-AAV antibodies. In that study (26), the second dose may have been neutralized by plasma derived IgG, since IgG is predominantly distributed in the alveolar region of the lung (44).
We examined the strategy of switching vector capsid by using AAV2 and AAV3 and were able to develop a wild-type-free packaging system for rAAV3 vectors that produced infectious pseudotyped rAAV3 vectors with physical and biological titers equivalent to those of rAAV2 vectors. The similar rise in titer after the third dose of either rAAV3 or rAAV2 in this study reflects the cross-reactivity between the two serotypes (compare Fig. 4A to Fig. 4B). Our findings are consistent with those of earlier investigators who found that antibody to AAV2 does not prevent reinfection with either AAV2 or AAV3 (9). Following the third dose, the rAAV3-GFP-treated group tended to have a higher percentage of GFP-expressing cells per sample than the rAAV2-GFP-treated group, but there was no difference in the number of rabbits expressing GFP between the groups. The difference in the range of positive cells between the groups likely reflects the sampling variability in this model rather than a true difference between the groups. The yield of vector mRNA detection was low despite the presence of GFP expression as detected by FACS. The FACS analysis was performed on a specific population of targeted epithelial cells and accounts for the higher level of GFP detection. In contrast, for the RT-PCR, RNA was isolated from whole lung homogenates; thus, the percentage of isolated mRNA specifically from targeted epithelial cells is small relative to the total amount of RNA in each sample and accounts for the lower level of detection. Following repeated delivery of rAAV2-CFTR vector to the airway, both rAAV2- and rAAV3-encapsidated vectors yielded substantial expression of transgene, and switching from serotype 2 to serotype 3 did not provide a further advantage in this setting.
The presence of hCFTR vector DNA may be a cumulative phenomenon, as demonstrated by the comparison of DNA transfer in the thrice-dosed RLL (40%) as opposed to the once-dosed LLL (10%). The increased detection of hCFTR following three doses may reflect incremental increase in the number of transduced cells or proliferation of transduced progenitor cells. Alternatively, the number of hCFTR genomes per cell may have increased over time due to concatemer formation as noted in other studies (1, 13, 16, 48, 49). Most of the animals in this group were not brushed, and so selective epithelial trauma from the brushing technique does not explain the discrepancy between the right and left lobes. Although the study design did not allow us to determine from which dose(s) the hCFTR vector DNA resulted and the sampling methods did not allow for precise quantification of vector transfer, the consistent results upon repeated testing suggest there may be an incremental increase in vector hCFTR DNA after three doses. In any case, there was no evidence of inflammation or lymphocytic destruction of epithelial cells in the lobes positive for hCFTR vector DNA. We have shown persistence of rAAV-CFTR genomes for at least 17 weeks following repeated bronchoscopic delivery, indicating that repeated airway delivery of homologous rAAV vectors is a feasible approach to maintain rAAV-mediated expression in the airway of an individual.
In summary, we have demonstrated that the repeated bronchoscopic administration of highly purified rAAV vectors to the rabbit airway results in incremental expression of transgene without detectable toxicity. Furthermore, we detected no toxicity from either GFP expression or repeated exposure to rAAV-CFTR. Repeated bronchoscopic administration is one way to maintain persistent rAAV-hCFTR in the airways. As rAAV-CFTR clinical applications are developed, future studies should consider that mucosal immunity might be influenced by the purity of vector, the targeted area of delivery, and the condition of the delivery surface. Pseudotyping of rAAV vectors with serologically distinct capsid proteins may then be necessary to circumvent host responses. However, in the setting of a normal airway, repeated bronchoscopic delivery of highly purified rAAV vectors is successful.
ACKNOWLEDGMENTS
This research was partially funded by the Cystic Fibrosis Foundation (S.E.B.) and by National Institutes of Health grants NIH PO1 HL51811-06 (W.B.G.) and NHLBI P01, HL51811, and NIDDK R01 DK51809 (T.R.F.). T.R.F. is an inventor of two patented technologies regarding the use of AAV in CF patients. W.B.G. is an inventor of one patented technology regarding the use of AAV in CF patients. These technologies have been licensed to Targeted Genetics Corporation. T.R.F. and W.B.G., the National Institutes of Health and The Johns Hopkins University (the holders of the two patents), conceivably could benefit monetarily from royalties paid by Targeted Genetics Corporation if this gene therapy treatment proves beneficial in human patients suffering from CF.
The technical expertise of Holly Bowers, Amy Poirier, and Lawrence Evans is greatly appreciated. Special thanks go to Carol J. Blaisdell for critically reviewing the manuscript.
REFERENCES
- 1.Afione S A, Conrad C K, Kearns W G, Chunduru S, Adams R, Reynolds T C, Guggino W B, Cutting G R, Carter B J, Flotte T R. In vivo model of adeno-associated virus vector persistence and rescue. J Virol. 1996;70:3235–3241. doi: 10.1128/jvi.70.5.3235-3241.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ayers M M, Jeffery P K. Proliferation and differentiation in mammalian airway epithelium. Eur Respir J. 1988;1:58–80. [PubMed] [Google Scholar]
- 3.Balcklow N R. Adeno-associated viruses of humans. In: Pattison J R, editor. Parvoviruses and human disease. Boca Raton, Fla: CRC Press, Inc.; 1988. pp. 165–174. [Google Scholar]
- 4.Bantel-Schaal U, zur Hausen H H. Characterization of the DNA of a defective human parvovirus isolated from a genital site. Virology. 1984;134:52–63. doi: 10.1016/0042-6822(84)90271-x. [DOI] [PubMed] [Google Scholar]
- 5.Bartlett J S, Samulski R J, McCown T J. Selective and rapid uptake of adeno-associated virus type 2 in brain. Hum Gene Ther. 1998;9:1181–1186. doi: 10.1089/hum.1998.9.8-1181. [DOI] [PubMed] [Google Scholar]
- 6.Bennett J, Duan D, Engelhardt J F, Maguire A M. Real-time, noninvasive in vivo assessment of adeno-associated virus-mediated retinal transduction. Investig Ophthalmol Visual Sci. 1997;38:2857–2863. [PubMed] [Google Scholar]
- 7.Blacklow N R, Hoggan M D, Kapikian A Z, Austin J B, Rowe W P. Epidemiology of adenovirus-associated virus infection in a nursery population. Am J Epidemiol. 1968;88:368–378. doi: 10.1093/oxfordjournals.aje.a120897. [DOI] [PubMed] [Google Scholar]
- 8.Blacklow N R, Hoggan M D, Rowe W P. Isolation of adenovirus-associated viruses from man. Proc Natl Acad Sci USA. 1967;58:1410–1415. doi: 10.1073/pnas.58.4.1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blacklow N R, Hoggan M D, Rowe W P. Serologic evidence for human infection with adenovirus-associated viruses. J Natl Cancer Inst. 1968;40:319–327. [PubMed] [Google Scholar]
- 10.Blacklow N R, Hoggan M D, Sereno M S, Brandt C D, Kim H W, Parrott R H, Chanock R M. A seroepidemiologic study of adenovirus-associated virus infection in infants and children. Am J Epidemiol. 1971;94:359–366. doi: 10.1093/oxfordjournals.aje.a121331. [DOI] [PubMed] [Google Scholar]
- 11.Clark K R, Sferra T J, Johnson P R. Recombinant adeno-associated viral vectors mediate long-term transgene expression in muscle. Hum Gene Ther. 1997;8:659–669. doi: 10.1089/hum.1997.8.6-659. [DOI] [PubMed] [Google Scholar]
- 12.Clark K R, Voulgaropoulou F, Fraley D M, Johnson P R. Cell lines for the production of recombinant adeno-associated virus. Hum Gene Ther. 1995;6:1329–1341. doi: 10.1089/hum.1995.6.10-1329. [DOI] [PubMed] [Google Scholar]
- 13.Conrad C K, Allen S S, Afione S A, Reynolds T C, Beck S E, Fee-Maki M, Barrazza-Ortiz X, Adams R, Askin F B, Carter B J, Guggino W B, Flotte T R. Safety of single-dose administration of an adeno-associated virus (AAV)-CFTR vector in the primate lung. Gene Ther. 1996;3:658–668. [PubMed] [Google Scholar]
- 14.During M J, Samulski R J, Elsworth J D, Kaplitt M G, Leone P, Xiao X, Li J, Freese A, Taylor J R, Roth R H, Sladek J R J, O’Malley K L, Redmond D E J. in vivo expression of therapeutic human genes for dopamine production in the caudates of MPTP-treated monkeys using an AAV vector. Gene Ther. 1998;5:820–827. doi: 10.1038/sj.gt.3300650. [DOI] [PubMed] [Google Scholar]
- 15.Egan M, Flotte T, Afione S, Solow R, Zeitlin P L, Carter B J, Guggino W B. Defective regulation of outwardly rectifying Cl-channels by protein kinase A corrected by insertion of CFTR. Nature. 1992;358:581–584. doi: 10.1038/358581a0. [DOI] [PubMed] [Google Scholar]
- 16.Fisher K J, Jooss K, Alston J, Yang Y, Haecker S E, High K, Pathak R, Raper S E, Wilson J M. Recombinant adeno-associated virus for muscle directed gene therapy. Nat Med. 1997;3:306–312. doi: 10.1038/nm0397-306. [DOI] [PubMed] [Google Scholar]
- 17.Flotte T, Carter B, Conrad C, Guggino W, Reynolds T, Rosenstein B, Taylor G, Walden S, Wetzel R. A phase I study of an adeno-associated virus-CFTR gene vector in adult CF patients with mild lung disease. Hum Gene Ther. 1996;7:1145–1159. doi: 10.1089/hum.1996.7.9-1145. [DOI] [PubMed] [Google Scholar]
- 18.Flotte T R, Afione S A, Conrad C, McGrath S A, Solow R, Oka H, Zeitlin P L, Guggino W B, Carter B J. Stable in vivo expression of the cystic fibrosis transmembrane conductance regulator with an adeno-associated virus vector. Proc Natl Acad Sci USA. 1993;90:10613–10617. doi: 10.1073/pnas.90.22.10613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flotte T R, Afione S A, Solow R, Drumm M L, Markakis D, Guggino W B, Zeitlin P L, Carter B J. Expression of the cystic fibrosis transmembrane conductance regulator from a novel adeno-associated virus promoter. J Biol Chem. 1993;268:3781–3790. [PubMed] [Google Scholar]
- 20.Flotte T R, Afione S A, Zeitlin P L. Adeno-associated virus vector gene expression occurs in nondividing cells in the absence of vector DNA integration. Am J Respir Cell Mol Biol. 1994;11:517–521. doi: 10.1165/ajrcmb.11.5.7946381. [DOI] [PubMed] [Google Scholar]
- 21.Flotte T R, Barraza-Ortiz X, Solow R, Afione S A, Carter B J, Guggino W B. An improved system for packaging recombinant adeno-associated virus vectors capable of in vivo transduction. Gene Ther. 1995;2:29–37. [PubMed] [Google Scholar]
- 22.Flotte T R, Beck S E, Chesnut K, Potter M, Poirier A, Zolotukhin S. A fluorescence video-endoscopy technique for detection of gene transfer and expression. Gene Ther. 1998;5:166–173. doi: 10.1038/sj.gt.3300579. [DOI] [PubMed] [Google Scholar]
- 23.Georg-Fries B, Biederlack S, Wolf J, zur Hausen H H. Analysis of proteins, helper dependence, and seroepidemiology of a new human parvovirus. Virology. 1984;134:64–71. doi: 10.1016/0042-6822(84)90272-1. [DOI] [PubMed] [Google Scholar]
- 24.Giraud C, Winocour E, Berns K I. Site-specific integration by adeno-associated virus is directed by a cellular DNA sequence. Proc Natl Acad Sci USA. 1994;91:10039–10043. doi: 10.1073/pnas.91.21.10039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Halbert C L, Standaert T A, Aitken M L, Alexander I E, Russell D W, Miller A D. Transduction by adeno-associated virus vectors in the rabbit airway: efficiency, persistence, and readministration. J Virol. 1997;71:5932–5941. doi: 10.1128/jvi.71.8.5932-5941.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Halbert C L, Standaert T A, Wilson C B, Miller A D. Successful readministration of adeno-associated virus vectors to the mouse lung requires transient immunosuppression during the initial exposure. J Virol. 1998;72:9795–9805. doi: 10.1128/jvi.72.12.9795-9805.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnson L G, Olsen J C, Sarkadi B, Moore K L, Swanstrom R, Boucher R C. Efficiency of gene transfer for restoration of normal airway epithelial function in cystic fibrosis. Nat Genet. 1992;2:21–25. doi: 10.1038/ng0992-21. [DOI] [PubMed] [Google Scholar]
- 28.Jooss K, Yang Y, Fisher K J, Wilson J M. Transduction of dendritic cells by DNA viral vectors directs the immune response to transgene products in muscle fibers. J Virol. 1998;72:4212–4223. doi: 10.1128/jvi.72.5.4212-4223.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaplitt M G, Leone P, Samulski R J, Xiao X, Pfaff D W, O’Malley K L, During M J. Long-term gene expression and phenotypic correction using adeno-associated virus vectors in the mammalian brain. Nat Genet. 1994;8:148–154. doi: 10.1038/ng1094-148. [DOI] [PubMed] [Google Scholar]
- 30.Kearns W G, Afione S A, Fulmer S B, Pang M C, Erikson D, Egan M, Landrum M J, Flotte T R, Cutting G R. Recombinant adeno-associated virus (AAV-CFTR) vectors do not integrate in a site-specific fashion in an immortalized epithelial cell line. Gene Ther. 1996;3:748–755. [PubMed] [Google Scholar]
- 31.Kessler P D, Podsakoff G M, Chen X, McQuiston S A, Colosi P C, Matelis L A, Kurtzman G J, Byrne B J. Gene delivery to skeletal muscle results in sustained expression and systemic delivery of a therapeutic protein. Proc Natl Acad Sci USA. 1996;93:14082–14087. doi: 10.1073/pnas.93.24.14082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koeberl D D, Alexander I E, Halbert C L, Russell D W, Miller A D. Persistent expression of human clotting factor IX from mouse liver after intravenous injection of adeno-associated virus vectors. Proc Natl Acad Sci USA. 1997;94:1426–1431. doi: 10.1073/pnas.94.4.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kotin R M, Linden R M, Berns K I. Characterization of a preferred site on human chromosome 19q for integration of adeno-associated virus DNA by non-homologous recombination. EMBO J. 1992;11:5071–5078. doi: 10.1002/j.1460-2075.1992.tb05614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kotin R M, Siniscalco M, Samulski R J, Zhu X D, Hunter L, Laughlin C A, McLaughlin S, Muzyczka N, Rocchi M, Berns K I. Site-specific integration by adeno-associated virus. Proc Natl Acad Sci USA. 1990;87:2211–2215. doi: 10.1073/pnas.87.6.2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lalwani A, Walsh B, Reilly P, Carvalho G, Zolotukhin S, Muzyezka N, Mhatre A. Long-term in vivo cochlear transgene expression mediated by recombinant adeno-associated virus. Gene Ther. 1998;5:277–281. doi: 10.1038/sj.gt.3300573. [DOI] [PubMed] [Google Scholar]
- 36.Leigh M W, Kylander J E, Yankaskas J R, Boucher R C. Cell proliferation in bronchial epithelium and submucosal glands of cystic fibrosis patients. Am J Respir Cell Mol Biol. 1995;12:605–612. doi: 10.1165/ajrcmb.12.6.7766425. [DOI] [PubMed] [Google Scholar]
- 37.Linden R M, Ward P, Giraud C, Winocour E, Berns K I. Site-specific integration by adeno-associated virus. Proc Natl Acad Sci USA. 1996;93:11288–11294. doi: 10.1073/pnas.93.21.11288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mayor H D, Carrier S, Jordan L. Complementation of adeno-associated satellite virus (AAV) by temperature-sensitive mutants of adenovirus type 31. J Gen Virol. 1977;35:545–553. doi: 10.1099/0022-1317-35-3-545. [DOI] [PubMed] [Google Scholar]
- 39.Merrill W W, Naegel G P, Olchowski J J, Reynolds H Y. Immunoglobulin G subclass proteins in serum and lavage fluid of normal subjects. Quantitation and comparison with immunoglobulins A and E. Am Rev Respir Dis. 1985;131:584–587. doi: 10.1164/arrd.1985.131.4.584. [DOI] [PubMed] [Google Scholar]
- 40.Otake K, Ennist D L, Harrod K, Trapnell B C. Nonspecific inflammation inhibits adenovirus-mediated pulmonary gene transfer and expression independent of specific acquired immune responses. Hum Gene Ther. 1998;9:2207–2222. doi: 10.1089/hum.1998.9.15-2207. [DOI] [PubMed] [Google Scholar]
- 41.Parks W P, Melnick J L, Taber L H, Yow M D. Seroepidemiological and ecological studies of the adeno-associated satellite viruses. Infect Immun. 1970;2:716–722. doi: 10.1128/iai.2.6.716-722.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ponnazhagan S, Erikson D, Kearns W G, Zhou S Z, Nahreini P, Wang X S, Srivastava A. Lack of site-specific integration of the recombinant adeno-associated virus 2 genomes in human cells. Hum Gene Ther. 1997;8:275–284. doi: 10.1089/hum.1997.8.3-275. [DOI] [PubMed] [Google Scholar]
- 43.Rennard S I, Basset G, Lecossier D, O’Donnell K M, Pinkston P, Martin P G, Crystal R G. Estimation of volume of epithelial lining fluid recovered by lavage using urea as marker of dilution. J Appl Physiol. 1986;60:532–538. doi: 10.1152/jappl.1986.60.2.532. [DOI] [PubMed] [Google Scholar]
- 44.Reynolds H Y, Huck J L. Immunologic responses in the lung. Respiration. 1990;57:221–228. doi: 10.1159/000195845. [DOI] [PubMed] [Google Scholar]
- 45.Samulski R J, Zhu X, Xiao X, Brook J D, Housman D E, Epstein N, Hunter L A. Targeted integration of adeno-associated virus (AAV) into human chromosome 19. EMBO J. 1991;10:3941–3950. doi: 10.1002/j.1460-2075.1991.tb04964.x. . (Erratum, 11:1228, 1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schon-Hegrad M A, Oliver J, McMenamin P G, Holt P G. Studies on the density, distribution, and surface phenotype of intraepithelial class II major histocompatibility complex antigen (Ia)-bearing dendritic cells (DC) in the conducting airways. J Exp Med. 1991;173:1345–1356. doi: 10.1084/jem.173.6.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sertl K, Takemura T, Tschachler E, Ferrans V J, Kaliner M A, Shevach E M. Dendritic cells with antigen-presenting capability reside in airway epithelium, lung parenchyma, and visceral pleura. J Exp Med. 1986;163:436–451. doi: 10.1084/jem.163.2.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Snyder R O, Spratt S K, Lagarde C, Bohl D, Kaspar B, Sloan B, Cohen L K, Danos O. Efficient and stable adeno-associated virus-mediated transduction in the skeletal muscle of adult immunocompetent mice. Hum Gene Ther. 1997;8:1891–1900. doi: 10.1089/hum.1997.8.16-1891. [DOI] [PubMed] [Google Scholar]
- 49.Song S, Morgan M, Ellis T, Poirier A, Chesnut K, Wang J, Brantly M, Muzyczka N, Byrne B J, Atkinson M, Flotte T R. Sustained secretion of human alpha-1-antitrypsin from murine muscle transduced with adeno-associated virus vectors. Proc Natl Acad Sci USA. 1998;95:14384–14388. doi: 10.1073/pnas.95.24.14384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wagner J A, Reynolds T, Moran M L, Moss R B, Wine J J, Flotte T R, Gardner P. Efficient and persistent gene transfer of AAV-CFTR in maxillary sinus. Lancet. 1998;351:1702–1703. doi: 10.1016/S0140-6736(05)77740-0. . (Letter.) [DOI] [PubMed] [Google Scholar]
- 51.Wilmott R W, Amin R S, Perez C R, Wert S E, Keller G, Boivin G P, Hirsch R, De I J, Lu P, Reising S F, Yei S, Whitsett J A, Trapnell B C. Safety of adenovirus-mediated transfer of the human cystic fibrosis transmembrane conductance regulator cDNA to the lungs of nonhuman primates. Hum Gene Ther. 1996;7:301–318. doi: 10.1089/hum.1996.7.3-301. [DOI] [PubMed] [Google Scholar]
- 52.Xiao X, Li J, McCown T J, Samulski R J. Gene transfer by adeno-associated virus vectors into the central nervous system. Exp Neurol. 1997;144:113–124. doi: 10.1006/exnr.1996.6396. [DOI] [PubMed] [Google Scholar]
- 53.Xiao X, Li J, Samulski R J. Efficient long-term gene transfer into muscle tissue of immunocompetent mice by adeno-associated virus vector. J Virol. 1996;70:8098–8108. doi: 10.1128/jvi.70.11.8098-8108.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yang Y, Su Q, Wilson J M. Role of viral antigens in destructive cellular immune responses to adenovirus vector-transduced cells in mouse lungs. J Virol. 1996;70:7209–7212. doi: 10.1128/jvi.70.10.7209-7212.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zolotukhin S, Potter M, Hauswirth W W, Guy J, Muzyczka N. A “humanized” green fluorescent protein cDNA adapted for high-level expression in mammalian cells. J Virol. 1996;70:4646–4654. doi: 10.1128/jvi.70.7.4646-4654.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]