Abstract
Prior research has identified associations between immune cells and aplastic anaemia (AA); however, the causal relationships between them have not been conclusively established. A two-sample Mendelian randomisation analysis was conducted to investigate the causal link between 731 immune cell signatures and AA risk using publicly available genetic data. Four types of immune signatures, including relative cell, absolute cell (AC), median fluorescence intensities and morphological parameters, were considered sensitivity analyses were also performed to verify the robustness of the results and assess potential issues such as heterogeneity and horizontal pleiotropy. Following multiple test adjustments using the False Discovery Rate (FDR) method, no statistically significant impact of any immunophenotype on AA was observed. However, twelve immunophenotypes exhibited a significant correlation with AA without FDR correction (p of IVW < 0.01), of which eight were harmful to AA: CD127- CD8br %T cell (Treg panel), CD25 on IgD + CD38dim (B cell panel), CD38 on naive-mature B cell (B cell panel), CD39 + resting Treg % CD4 Treg (Treg panel), CD39 + secreting Treg AC (Treg panel), CD8 on CD28 + CD45RA- CD8br (Treg panel), HLA DR + NK AC (TBNK panel), Naive DN (CD4−CD8−) AC (Maturation stages of T cell panel); and four were protective to AA: CD86 on CD62L + myeloid DC (cDC panel), DC AC (cDC panel), DN (CD4−CD8−) NKT %T cell (TBNK panel), and TD CD4 + AC (Maturation stages of T cell panel). The results of this study demonstrate a close link between immune cells and AA by genetic means, thereby improving the current understanding of the interaction between immune cells and AA risk and providing guidance for future clinical research.
Keywords: Aplastic anaemia, Immunity, Causal inference, Mendelian randomisation study
Subject terms: Medical research, Immunology
Introduction
Aplastic anaemia (AA), characterised by bone marrow failure resulting in hypocellular marrow and pancytopenia, exhibits common symptoms such as fatigue, easy bruising or bleeding, and susceptibility to infections1,2. This condition is rare but potentially life-threatening, with a rising incidence in recent years. Current data indicates 6–9 million cases of aplastic anaemia annually in East Asia, which is 2–3 times higher than in Western countries3,4. Fortunately, advances in understanding its pathophysiology and improved treatment approaches, including hematopoietic stem cell transplantation and thrombopoietin receptor agonists, have significantly increased the survival rates of AA patients5,6. Nevertheless, aplastic anaemia remains a substantial burden for patients, their families, and the healthcare system due to its often-prolonged disease course, associated high morbidity, and the uncertainty surrounding clinical outcomes7.
The exact cause and pathogenesis of AA have not been fully elucidated. Numerous clinical and preclinical investigations have indicated that AA patients exhibit immune dysfunction, involving abnormalities in both cellular and humoral immunity. Notably, the effectiveness of immunosuppressive therapies (ISTs) such as antithymocyte globulin (ATG) and/or cyclosporine A (CSA) suggests the involvement of one or more immune system components in the disease's pathogenesis2. It appears that an elevated myeloid dendritic cell/plasmacytoid dendritic cell ratio leads to an imbalance in the T helper (Th)1/Th2 ratio in favour of Th1, ultimately resulting in abnormal activation of cytotoxic T lymphocytes (CTLs)8. However, the specific antigens triggering T cell responses in AA are still unknown. Further, regulatory T cells (Treg), Th17 cells, natural killer (NK) cells, memory T cells, and negative hematopoietic regulatory factors also play roles in this process9–14. Nonetheless, the precise contribution of immune cells to the development of AA remains elusive, possibly due to the intricate nature of the immune system, flaws in study design, limited sample sizes, and the influence of confounding factors not fully addressed in existing research.
Based on Mendelian independent distribution law, Mendelian randomisation (MR) is an analytical method mainly used in epidemiological aetiology inference. Recent advances in large-scale genome-wide association studies (GWAS) and MR approaches have facilitated the assessment of causal relationships between immune traits and diseases15–17. Multiple studies have substantiated the utility of MR investigations in elucidating causal relationships in blood-related disorders18–20. MR studies are adept at mitigating confounding variables and deciphering reverse causal associations within the realm of causal inference. An extensive two-sample MR analysis was used in this study to determine the causal relationship between immune cell characteristics and AA.
Methods
Study design
A two-sample MR design was established to explore the causal relationships between 731 immune cell signatures (7 groups) and AA. To ensure the reliability of the finding, each MR analysis had to fulfil the following three key assumptions21: (1) the instrumental variable was directly related to exposure; (2) the instrumental variable was are not related to any confounders affecting both exposure and outcome; (3) the instrumental variable had an effect on the outcome only through its effect on the exposure, without involvement in any other causal pathways. The studies included in this analysis was approved by the review boards of relevant institutional, and participants provided informed consent.
Immunity-wide Genome-wide association study (GWAS) data sources
GWAS summary statistics for each immunophenotype are available from the GWAS Catalog, accession number from GCST90001391 to GCST9000212117. A total of 731 immunophenotypes were covered, including of median fluorescence intensities (MFI) reflecting surface antigen levels (n = 389), absolute cell (AC) counts (n = 118), relative cell (RC) counts (n = 192) and morphological parameters (MP) (n = 32). It is important to note that the MFI, AC, and RC features encompassed various immune cell types, including B cells, CTLs, mature stages of T cells, monocytes, myeloid cells, and TBNK (T cells, B cells, natural killer cells). The MP feature, on the other hand, included panels related to CTL and TBNK cell types. The original GWAS on immune signature was performed using data from 3757 European individuals without overlapping cohorts. Approximately 22 million SNPs were genotyped using high-density arrays, and imputation was performed using a Sardinian sequence-based reference panel. Associations were measured while taking into account covariates, including age and gender22.
GWAS data sources for AA
GWAS summary statistics for AA were got from FinnGen and UK Biobank. A total of 473, 500 European individuals (Ncase = 4128, Ncontrol = 469,372) were included in the GWAS analysis of AA, and approximately 25 million variants were analysed after quality control and imputation23. Based on the source information of the participants, there is no sample overlap between the immunophenotypes and AA GWAS datasets.
Selection of instrumental variables (IVs)
The significance level of IVs for each immunophenotype was set at 1 × 10−5 based on a recent Mendelian randomisation study on immune traits15. The 1000 Genomes Project linkage disequilibrium structure (r2 < 0.1 with any other associated SNP within 10 Mb) was performed among the initially selected SNPs to ensure that the selected IVs were able to independently predict exposure. In addition, the proportion of phenotypic variation explained (PVE) and F statistic were calculated for each IV, so as to assess the strength of IV and avoid weak instrumental bias. SNPs with an F-statistic below 10 were determined to be weak instruments and subsequently ruled out from the IVs24. F-statistic was estimated using the formula: F = R2 (N2)/(1 − R2), where R2 was the proportion of phenotypic variation explained by the SNP and N was the sample size of the GWAS of SNPs with the trait. The R2 values were estimated using the formula: R2 = 2 × EAF × (1 − EAF) × β2, where EAF was the effect allele frequency (EAF) of the SNP and β was the estimated effect of SNP on trait25. We also conducted a search using the PhenoScanner database to clarify whether the selected SNPs were associated with AA potential confounders (e.g., benzene exposure, ionizing radiation, organic solvents, viral infections, etc.)20.
Statistical analysis
All analyses were conducted using R software (version 4.3.1, https://cran.r-project.org/src/base/R-4/R-4.3.1.tar.gz). To assess the causal link between 731 immunophenotypes and AA, statistical methods were used such as Inverse Variance Weighting (IVW), Weighted Median (WM), and Mendelian Randomisation–Egger (MR-Egger) from the 'Mendelian Randomisation' package (version 0.4.3)26. Heterogeneity among instrumental variables was checked using corresponding p values and Cochran's Q statistic, visualising it with a random effect model27. To account for potential horizontal pleiotropy, MR-Egger was applied. The MR-PRESSO method was used to identify and exclude possible pleiotropic outliers28. Scatter and funnel plots confirmed the robustness of the results and the absence of heterogeneity29. In addition, the statistical power for the MR results was calculated to clarify the probability of committing a type II statistical error for a negative result30.
Ethics approval and consent to participate
The study was approved by the Sardinian Regional Ethics Committee (protocol no. 2171/CE). All participants provided written, informed consent. Informed consent was obtained from all participants and/or their LAR. This study was conducted in accordance to relevant guidelines and regulations.
Results
Development of the IVs used to genetically predict each immunophenotype
A total of 3–724 independent, non-palindromic and significant SNPs were selected as the IVs for 731 immunophenotypes. These IVs accounted for variance ranging from 0.005 to 5.199% in their respective immunophenotypes. Notably, all the genetic instruments had F statistics exceeding 10, signifying their robust strength (See Supplementary Table 1). In addition, as shown in Supplementary Table 2, after searching in the PhenoScanner database, no selected SNPs were found to be associated with the AA potential confounders (e.g., benzene exposure, ionizing radiation, organic solvents, viral infections, etc.).
Exploration of the causal effect of immunophenotypes on AA
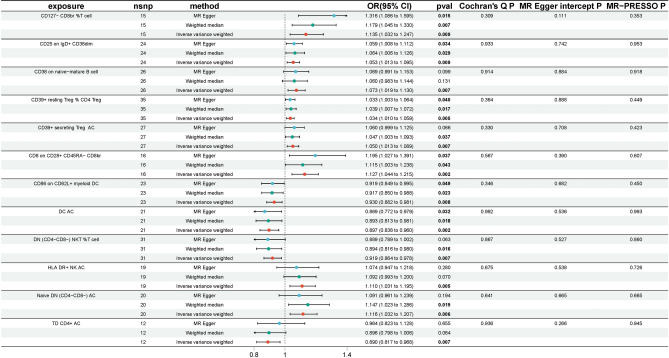
To explore the causal effects of immunophenotypes on AA, we performed a two-sample MR analysis and used the IVW method as the primary analysis. Following multiple test adjustments using the False Discovery Rate (FDR) method, no statistically significant effect of any immunophenotype on AA was observed (Supplementary Table 2). However, twelve immunophenotypes exhibited a significant correlation with AA without FDR correction (p of IVW < 0.01), of which eight were harmful to AA: CD127- CD8br %T cell (Treg panel), CD25 on IgD + CD38dim (B cell panel), CD38 on naive-mature B cell (B cell panel), CD39 + resting Treg % CD4 Treg (Treg panel), CD39 + secreting Treg AC (Treg panel), CD8 on CD28 + CD45RA- CD8br (Treg panel), HLA DR + NK AC (TBNK panel), Naive DN (CD4−CD8−) AC (Maturation stages of T cell panel); and four were protective to AA: CD86 on CD62L + myeloid DC (cDC panel), DC AC (cDC panel), DN (CD4−CD8−) NKT %T cell (TBNK panel), and TD CD4 + AC (Maturation stages of T cell panel).
Specifically, by using the IVW method, the odds ratio (OR) of CD127- CD8br %T cell on AA risk was estimated to be 1.135 (95% CI 1.032–1.247, P = 0.009), the OR of CD25 on IgD + CD38dim on AA risk was estimated to be 1.053 (95% CI 1.013–1.095, P = 0.009), the OR of CD38 on naive-mature B cell on AA risk was estimated to be 1.073 (95% CI 1.019–1.130, P = 0.007), the OR of CD39 + resting Treg % CD4 Treg on AA risk was estimated to be 1.034 (95% CI 1.010–1.059, P = 0.005), the OR of CD39 + secreting Treg AC on AA risk was estimated to be 1.050 (95% CI 1.013–1.089, P = 0.007), the OR of CD8 on CD28 + CD45RA- CD8br on AA risk was estimated to be 1.127 (95% CI 1.044–1.215, P = 0.002), the OR of HLA DR + NK AC on AA risk was estimated to be 1.110 (95% CI 1.031–1.195, P = 0.005), the OR of Naive DN (CD4−CD8−) AC on AA risk was estimated to be 1.116 (95% CI 1.032–1.207, P = 0.006), the OR of CD86 on CD62L + myeloid DC on AA risk was estimated to be 0.930 (95% CI 0.882–0.981, P = 0.008), the OR of DC AC on AA risk was estimated to be 0.897 (95% CI 0.838–0.960, P = 0.002), the OR of DN (CD4−CD8−) NKT %T cell on AA risk was estimated to be 0.919 (95% CI 0.864–0.978, P = 0.007), and the OR of TD CD4 + AC on AA risk was estimated to be 0.890 (95% CI = 0.817–0.968, P = 0.007). Both the WM and MR-Egger methods, employed as complementary tests, yielded results consistent with the IVW analysis (Fig. 1), which reinforced the confidence in the findings. In the investigation of causality, the p value of Cochran's Q exceeded 0.05, indicating the absence of heterogeneity in the results. Additionally, MR-Egger did not detect any evidence of pleiotropy, as the p value for its intercept was greater than 0.05. Neither MR-PRESSO nor leave-one-out plots identified any outliers (Supplementary Fig. 1).
Figure 1.
The significant positive MR results of 731 immunophenotypes on AA without FDR correction (p < 0.01).
Additionally, the MR results for all immunophenotypes are shown in Supplementary Table 3, the scatter plots, forest plots and funnel plots are presented in Supplementary Figs. 2, 3 and 4, respectively.
Notably, even taking the immunophenotypes related to Treg cells to primary analyses and the remaining immunophenotypes to secondary analyses, it was found that positive results were still not detected after FDR correction, as shown in Supplementary Tables 4 and 5, respectively.
Discussion
To the present knowledge, our study is the first MR study to explore the causal relationship between multiple immunophenotypes and AA. The results of this study demonstrated that no statistically significant effect of any immunophenotype on AA was found following multiple test adjustment according to the FDR method. However, twelve immunophenotypes exhibited a significant correlation with AA without FDR correction (p of IVW < 0.01).
Dendritic cells (DCs) play a crucial role in the immune system by processing antigens for presentation to T cells and regulating their differentiation and function31. Previous research suggested that the activation of DCs induced by various factors can lead to abnormal activation of downstream T cells32, resulting in a pathological immune response, imbalance, apoptosis of bone marrow hematopoietic cells, and subsequent hematopoietic dysfunction and pancytopenia in AA33. It has also been observed that the number of myeloid dendritic cells (MDCs) and the expression of costimulatory molecules on DCs, including CD40, CD80, and CD86, increased in patients with severe AA from Asia9,34. MDCs exhibited strong phagocytic activity in patients with severe AA, leading to an increase in the number of CTLs35. Inconsistent with previous findings, our study based on European databases demonstrated that the decreased levels of DC AC and CD86 on CD62L + myeloid DC appeard to be significantly associated with the risk of AA. The inconsistency of the above results may be influenced by ethnicity, disease severity, sample size and other factors, so further research is necessary.
In the present study, associations were observed between an increased risk of AA and specific T cell-related factors. These factors included Naive DN (CD4−CD8−) AC of maturation stages of T cell and four types of Treg cells (CD127- CD8br %T cell, CD39 + resting Treg % CD4 Treg, CD39 + secreting Treg AC, CD8 on CD28 + CD45RA- CD8br). Numerous studies have emphasised the critical role of T cells in AA, with findings indicating the importance of T cells in the disease8. AA patients often exhibit an elevated number of CTLs and a shift in the CD4+ to CD8+T cell ratio. These activated CTLs tend to produce pro-inflammatory cytokines such as INF-γ and TNF-α, leading to apoptosis through the Fas/FasL pathway. This apoptosis can inhibit bone marrow's hematopoietic functions and result in hematopoietic cell destruction2,7,36. Furthermore, abnormalities in the number and/or function of CD4+ cells, including interferon (IFN)-γ-producing CD4+T cells (Th1 cells), interleukin (IL)-4-producing CD4+T cells (Th2 cells), interleukin-17 (IL-17)-producing CD4+T cells (Th17 cells) and Tregs, have been reported in patients with AA, suggesting their potential roles in the disease's pathogenesis8,37,38. It has been reported that significant increases in the number of Th1 cells and the Th1/Th2 cell ratio have been observed in AA, leading to elevated production of IFN-γ, a potent stimulator of CD8+T cells37,39. The number of Th17 cells is also increased, which induces the terminal differentiation of CTLs into effector memory CD8+T cells via IL-17 and IL-22 stimulation, and reduces the number and function of Treg cells in AA38,40,41. Our study demonstrated Naive DN (CD4−CD8−) AC of maturation stages of T cell was related to the risk of AA. However, due to the lack of relevant data on several subsets of CD4+T cell in the 731 immune cell signatures, we were unable to separately analyse the causal link between Th1, Th2, and Th17 cells and risk of AA. Additionally, close correlation has been observed between the severity of AA and significant decreases in the number and functions of Treg cells, which improved with successful IST37,42. However, the presenting study demonstrated that CD39 + Treg cells (CD39 + resting Treg % CD4 Treg and CD39 + secreting Treg AC), which were highly active and suppressive, increased the risk of AA43. The role of T cells in the onset and progression of AA remains elusive. AA is a highly heterogeneous disease, and the triggering events and underlying pathogenesis may vary among patients. At the same time, immune signatures differ between early-stage or non-severe AA and late-stage or severe AA, as well as among different treatment approaches. Whether the changes in T cell profiles are the cause or the consequence of AA remains a subject of inquiry. Therefore, further research is necessary to elucidate the intricate relationship between T cells and the risk of AA40,44.
Two types of B cells (that is, CD25 on IgD + CD38dim and CD38 on naive-mature B cell) were found to be associated with increased risk of AA. Unlike T cells, the role of B cells in AA remains unclear. Recent reports have suggested that the numbers of circulating B regulatory cells decreased at the time of diagnosis and subsequently recoverd following IST26. Additionally, patients with acquired AA have been found to exhibit various autoantibodies in their serum, including anti-moesin, diazepam-binding inhibitor-related protein 1 (DRS1), and kinectin antibodies, which are associated with proteins found in hematopoietic cells40.
In the present study, HLA DR + NK AC was found to be associated with an increased risk of AA, and the percent of DN (CD4−CD8−) NKT %T cell was associated with a reduced risk of AA. Natural killer (NK) cells are innate immune system lymphocytes with effector functions. The role of NK cells in AA has shown conflicting results. In severe AA patients, both the numbers and functions of NK cells in peripheral blood have been observed to be significantly reduced, with subsequent recovery following successful IST45. However, a separate study focused on paediatric-acquired AA did not report a correlation between NK cell frequency and disease severity or treatment response46. Additionally, in non-severe AA patients, there was an increase in the percentage of CD56bright NK cells and heightened expression of the activating receptor NKG2D, while the expression of the inhibitory receptor CD158a was low. These findings suggested that the increased and activated CD56bright NK cells might have a protective role in the development of non-severe aplastic anaemia47.
In the present study, a two-sample MR analysis was employed based on data from large GWAS cohorts, involving approximately 48,000 individuals, thereby ensuring high statistical power. The findings were drawn from genetic instrumental variables, and causal inference was performed by various MR analysis methods. The results were robust and not influenced by horizontal pleiotropy and other potential confounders. However, several limitations need to be acknowledged. Firstly, despite conducting multiple sensitivity analyses, there remains a partial limitation in assessing horizontal pleiotropy comprehensively. Secondly, the lack of detailed information about individuals prevents further stratification within the population. Thirdly, the generalisability of the findings is limited as the study utilised European databases exclusively, making it challenging to extend the conclusions to other ethnic groups. Fourthly, the potential risk factors for AA highlighted in this study were all significant correlations exhibited without FDR correction, and thus the false positives need to be taken into account and validated by more in-depth studies in the future. Lastly, a more lenient threshold was employed to assess the results, which might increase the likelihood of false positives but also provide a more comprehensive picture of the close association between immunophenotypes and AA.
Conclusions
In conclusion, causal associations between various immunophenotypes and AA were established using a comprehensive MR analysis. Given the intricate nature of AA's pathogenesis and the evident clinical heterogeneity of immune cell types implicated in AA, the present research sheds light on the complex interplay between the immune system and AA. Moreover, the present findings mitigate the influence of confounding factors, reverse causality, and other potential biases. The present study not only offers new insights into the biological mechanisms of AA but also suggests directions for the development of novel therapeutic interventions.
Supplementary Information
Acknowledgements
The authors thank the contributors to the ieu open gwas project (https://gwas.mrcieu.ac.uk/) for sharing data.
Author contributions
Y.L. and S.F. designed the experiments. S.F. and Y.D. carried out the experiments. F.M. and T.P. analyzed the data. S.F. and T.P. wrote the manuscript. H.H. contributed to statistical analysis. Y.L. supervised the study and revised the paper. All authors have read and agreed to the published version of the manuscript. All authors agree to publish.
Funding
This study was supported by grants from the Health Special Project of Jilin Province (No. JLSWSRCZX2020-0065) and Natural Science Foundation of Jilin Province (No. 20210101259JC).
Data availability
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article. Further inquiries can be directed to the corresponding authors.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-69104-0.
References
- 1.DeZern, A. E. & Churpek, J. E. Approach to the diagnosis of aplastic anemia. Blood Adv.5, 2660–2671. 10.1182/bloodadvances.2021004345 (2021). 10.1182/bloodadvances.2021004345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Young, N. S. Aplastic anemia. N. Engl. J. Med.379, 1643–1656. 10.1056/NEJMra1413485 (2018). 10.1056/NEJMra1413485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vaht, K. et al. Incidence and outcome of acquired aplastic anemia: Real-world data from patients diagnosed in Sweden from 2000–2011. Haematologica102, 1683–1690. 10.3324/haematol.2017.169862 (2017). 10.3324/haematol.2017.169862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Norasetthada, L. et al. Adult aplastic anemia in Thailand: Incidence and treatment outcome from a prospective nationwide population-based study. Ann. Hematol.100, 2443–2452. 10.1007/s00277-021-04566-0 (2021). 10.1007/s00277-021-04566-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scheinberg, P. Acquired severe aplastic anaemia: How medical therapy evolved in the 20th and 21st centuries. Br. J. Haematol.194, 954–969. 10.1111/bjh.17403 (2021). 10.1111/bjh.17403 [DOI] [PubMed] [Google Scholar]
- 6.Drexler, B. & Passweg, J. Current evidence and the emerging role of eltrombopag in severe aplastic anemia. Ther. Adv. Hematol.12, 2040620721998126. 10.1177/2040620721998126 (2021). 10.1177/2040620721998126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Furlong, E. & Carter, T. Aplastic anaemia: Current concepts in diagnosis and management. J. Paediatr. Child Health56, 1023–1028. 10.1111/jpc.14996 (2020). 10.1111/jpc.14996 [DOI] [PubMed] [Google Scholar]
- 8.Liu, C., Sun, Y. & Shao, Z. Current concepts of the pathogenesis of aplastic anemia. Curr. Pharm. Des.25, 236–241. 10.2174/1381612825666190313113601 (2019). 10.2174/1381612825666190313113601 [DOI] [PubMed] [Google Scholar]
- 9.Liu, C. et al. Differential expression of the proteome of myeloid dendritic cells in severe aplastic anemia. Cell Immunol.285, 141–148. 10.1016/j.cellimm.2013.09.007 (2013). 10.1016/j.cellimm.2013.09.007 [DOI] [PubMed] [Google Scholar]
- 10.Zheng, M. et al. Abnormal immunomodulatory ability on memory T cells in humans with severe aplastic anemia. Int. J. Clin. Exp. Pathol.8, 3659–3669 (2015). [PMC free article] [PubMed] [Google Scholar]
- 11.Hosokawa, K. et al. Memory stem T cells in autoimmune disease: High frequency of circulating CD8+ memory stem cells in acquired aplastic anemia. J. Immunol.196, 1568–1578. 10.4049/jimmunol.1501739 (2016). 10.4049/jimmunol.1501739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang, J. et al. Single-cell analysis highlights a population of Th17-polarized CD4+ naive T cells showing IL6/JAK3/STAT3 activation in pediatric severe aplastic anemia. J. Autoimmun.136, 103026. 10.1016/j.jaut.2023.103026 (2023). 10.1016/j.jaut.2023.103026 [DOI] [PubMed] [Google Scholar]
- 13.Lu, T. et al. Decreased circulating Th22 and Th17 cells in patients with aplastic anemia. Clin. Chim. Acta450, 90–96. 10.1016/j.cca.2015.07.031 (2015). 10.1016/j.cca.2015.07.031 [DOI] [PubMed] [Google Scholar]
- 14.Lim, S. P. et al. Treg sensitivity to FasL and relative IL-2 deprivation drive idiopathic aplastic anemia immune dysfunction. Blood136, 885–897. 10.1182/blood.2019001347 (2020). 10.1182/blood.2019001347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang, C. et al. Causal role of immune cells in schizophrenia: Mendelian randomization (MR) study. BMC Psychiatry23, 590. 10.1186/s12888-023-05081-4 (2023). 10.1186/s12888-023-05081-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gu, J. et al. Assessing the causal relationship between immune traits and systemic lupus erythematosus by bi-directional Mendelian randomization analysis. Mol. Genet. Genomics MGG298, 1493–1503. 10.1007/s00438-023-02071-9 (2023). 10.1007/s00438-023-02071-9 [DOI] [PubMed] [Google Scholar]
- 17.Orrù, V. et al. Complex genetic signatures in immune cells underlie autoimmunity and inform therapy. Nat. Genet.52, 1036–1045. 10.1038/s41588-020-0684-4 (2020). 10.1038/s41588-020-0684-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li, H. et al. Mendelian randomization analysis reveals causality of inflammatory bowel disease on risks of Henoch-Schönlein purpura and immune thrombocytopenia. Dig. Liver Dis.56, 92–97. 10.1016/j.dld.2023.08.044 (2024). 10.1016/j.dld.2023.08.044 [DOI] [PubMed] [Google Scholar]
- 19.Xu, P., Han, S., Hou, M., Zhao, Y. & Xu, M. The serum lipid profiles in immune thrombocytopenia: Mendelian randomization analysis and a retrospective study. Thromb. J.21, 107. 10.1186/s12959-023-00551-x (2023). 10.1186/s12959-023-00551-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kjaergaard, A. D. et al. Thyroid function, pernicious anemia and erythropoiesis: A two-sample Mendelian randomization study. Hum. Mol. Genet.31, 2548–2559. 10.1093/hmg/ddac052 (2022). 10.1093/hmg/ddac052 [DOI] [PubMed] [Google Scholar]
- 21.Davey Smith, G., Holmes, M. V., Davies, N. M. & Ebrahim, S. Mendel’s laws, Mendelian randomization and causal inference in observational data: Substantive and nomenclatural issues. Eur. J. Epidemiol.35, 99–111. 10.1007/s10654-020-00622-7 (2020). 10.1007/s10654-020-00622-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sidore, C. et al. Genome sequencing elucidates Sardinian genetic architecture and augments association analyses for lipid and blood inflammatory markers. Nat. Genet.47, 1272–1281. 10.1038/ng.3368 (2015). 10.1038/ng.3368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sakaue, S. et al. A cross-population atlas of genetic associations for 220 human phenotypes. Nat. Genet.53, 1415–1424. 10.1038/s41588-021-00931-x (2021). 10.1038/s41588-021-00931-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fu, S. et al. Effects of selenium on chronic kidney disease: A Mendelian randomization study. Nutrients10.3390/nu14214458 (2022). 10.3390/nu14214458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang, Q., Shi, Q., Lu, J., Wang, Z. & Hou, J. Causal relationships between inflammatory factors and multiple myeloma: A bidirectional Mendelian randomization study. Int. J. Cancer151, 1750–1759. 10.1002/ijc.34214 (2022). 10.1002/ijc.34214 [DOI] [PubMed] [Google Scholar]
- 26.Yavorska, O. O. & Burgess, S. MendelianRandomization: An R package for performing Mendelian randomization analyses using summarized data. Int. J. Epidemiol.46, 1734–1739. 10.1093/ije/dyx034 (2017). 10.1093/ije/dyx034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yan, D. et al. A Mendelian randomization study revealed a causal link between napping and deep vein thrombosis (DVT). Sleep Breath.10.1007/s11325-023-02940-y (2023). 10.1007/s11325-023-02940-y [DOI] [PubMed] [Google Scholar]
- 28.Lyu, B., Ma, J., Bai, Y. & Feng, Z. Casual effects of gut microbiota on risk of infections: A two-sample Mendelian randomization study. Front. Microbiol.14, 1284723. 10.3389/fmicb.2023.1284723 (2023). 10.3389/fmicb.2023.1284723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Richardson, T. G., Leyden, G. M. & Davey Smith, G. Time-varying and tissue-dependent effects of adiposity on leptin levels: A Mendelian randomization study. eLife10.7554/eLife.84646 (2023). 10.7554/eLife.84646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burgess, S. Sample size and power calculations in Mendelian randomization with a single instrumental variable and a binary outcome. Int. J. Epidemiol.43, 922–929. 10.1093/ije/dyu005 (2014). 10.1093/ije/dyu005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mellman, I. Dendritic cells: Master regulators of the immune response. Cancer Immunol. Res.1, 145–149. 10.1158/2326-6066.CIR-13-0102 (2013). 10.1158/2326-6066.CIR-13-0102 [DOI] [PubMed] [Google Scholar]
- 32.Ochyl, L. J. & Moon, J. J. Dendritic cell membrane vesicles for activation and maintenance of antigen-specific T cells. Adv. Healthc. Mater.8, e1801091. 10.1002/adhm.201801091 (2019). 10.1002/adhm.201801091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gao, M., Zhang, D. & Xu, R. Advances in understanding the role of dendritic cells in aplastic anaemia. Scand. J. Immunol.97, e13265. 10.1111/sji.13265 (2023). 10.1111/sji.13265 [DOI] [PubMed] [Google Scholar]
- 34.Zonghong, S. et al. Circulating myeloid dendritic cells are increased in individuals with severe aplastic anemia. Int. J. Hematol.93, 156–162. 10.1007/s12185-010-0761-z (2011). 10.1007/s12185-010-0761-z [DOI] [PubMed] [Google Scholar]
- 35.Sun, Y. et al. Myeloid dendritic cells in severe aplastic anemia patients exhibit stronger phagocytosis. J. Clin. Lab. Anal.35, e24063. 10.1002/jcla.24063 (2021). 10.1002/jcla.24063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu, C. Y. et al. Fas/FasL in the immune pathogenesis of severe aplastic anemia. Genet. Mol. Res.13, 4083–4088. 10.4238/2014.May.30.3 (2014). 10.4238/2014.May.30.3 [DOI] [PubMed] [Google Scholar]
- 37.Kordasti, S. et al. Functional characterization of CD4+T cells in aplastic anemia. Blood119, 2033–2043. 10.1182/blood-2011-08-368308 (2012). 10.1182/blood-2011-08-368308 [DOI] [PubMed] [Google Scholar]
- 38.de Latour, R. P. et al. Th17 immune responses contribute to the pathophysiology of aplastic anemia. Blood116, 4175–4184. 10.1182/blood-2010-01-266098 (2010). 10.1182/blood-2010-01-266098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Giannakoulas, N. C. et al. Clinical relevance of balance between type 1 and type 2 immune responses of lymphocyte subpopulations in aplastic anaemia patients. Br. J. Haematol.124, 97–105. 10.1046/j.1365-2141.2003.04729.x (2004). 10.1046/j.1365-2141.2003.04729.x [DOI] [PubMed] [Google Scholar]
- 40.Patel, B. A., Giudice, V. & Young, N. S. Immunologic effects on the haematopoietic stem cell in marrow failure. Best Pract. Res. Clin. Haematol.34, 101276. 10.1016/j.beha.2021.101276 (2021). 10.1016/j.beha.2021.101276 [DOI] [PubMed] [Google Scholar]
- 41.Giudice, V., Cardamone, C., Triggiani, M. & Selleri, C. Bone marrow failure syndromes, overlapping diseases with a common cytokine signature. Int. J. Mol. Sci.22, 200. 10.3390/ijms22020705 (2021). 10.3390/ijms22020705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yan, L. et al. Abnormal quantity and function of regulatory T cells in peripheral blood of patients with severe aplastic anemia. Cell Immunol.296, 95–105. 10.1016/j.cellimm.2015.04.001 (2015). 10.1016/j.cellimm.2015.04.001 [DOI] [PubMed] [Google Scholar]
- 43.Álvarez-Sánchez, N. et al. Peripheral CD39-expressing T regulatory cells are increased and associated with relapsing-remitting multiple sclerosis in relapsing patients. Sci. Rep.9, 2302. 10.1038/s41598-019-38897-w (2019). 10.1038/s41598-019-38897-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Giudice, V. & Selleri, C. Aplastic anemia: Pathophysiology. Semin. Hematol.59, 13–20. 10.1053/j.seminhematol.2021.12.002 (2022). 10.1053/j.seminhematol.2021.12.002 [DOI] [PubMed] [Google Scholar]
- 45.Liu, C. et al. Abnormalities of quantities and functions of natural killer cells in severe aplastic anemia. Immunol. Invest.43, 491–503. 10.3109/08820139.2014.888448 (2014). 10.3109/08820139.2014.888448 [DOI] [PubMed] [Google Scholar]
- 46.Sutton, K. S., Shereck, E. B., Nemecek, E. R. & Kurre, P. Immune markers of disease severity and treatment response in pediatric acquired aplastic anemia. Pediatr. Blood Cancer60, 455–460. 10.1002/pbc.24247 (2013). 10.1002/pbc.24247 [DOI] [PubMed] [Google Scholar]
- 47.Li, Y. et al. Abnormalities of quantities and functions of CD56bright natural killer cells in non-severe aplastic Anemia. Hematology24, 405–412. 10.1080/16078454.2019.1590963 (2019). 10.1080/16078454.2019.1590963 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article. Further inquiries can be directed to the corresponding authors.