Abstract
Protein post-translational modification (PTM) is a covalent process that occurs in proteins during or after translation through the addition or removal of one or more functional groups, and has a profound effect on protein function. Glycosylation is one of the most common PTMs, in which polysaccharides are transferred to specific amino acid residues in proteins by glycosyltransferases. A growing body of evidence suggests that glycosylation is essential for the unfolding of various functional activities in organisms, such as playing a key role in the regulation of protein function, cell adhesion and immune escape. Aberrant glycosylation is also closely associated with the development of various diseases. Abnormal glycosylation patterns are closely linked to the emergence of various health conditions, including cancer, inflammation, autoimmune disorders, and several other diseases. However, the underlying composition and structure of the glycosylated residues have not been determined. It is imperative to fully understand the internal structure and differential expression of glycosylation, and to incorporate advanced detection technologies to keep the knowledge advancing. Investigations on the clinical applications of glycosylation focused on sensitive and promising biomarkers, development of more effective small molecule targeted drugs and emerging vaccines. These studies provide a new area for novel therapeutic strategies based on glycosylation.
Subject terms: Haematological cancer, Epigenetics, Tumour biomarkers, Drug development
Introduction
Protein post-translational modification (PTM) is a covalent alteration occurring during or after protein synthesis, often by adding or removing functional groups, profoundly impacting protein function.1 More than 300 different PTMs have been identified, including methylation, ubiquitination, acetylation, phosphorylation and glycosylation. Among them, glycosylation is one of the richest and most diverse PTMs, and there are many different types of PTMs in human body, mainly including N-glycosylation with asparagine (Asn) linkage, O-glycosylation with serine (Ser) and threonine (Thr) linkage, C-glycosylation with tryptophan (Trp) linkage, and glycosylphosphatidylinositol (GPI)-anchored attachment, which shows great structural changes.2 The glycosylation process begins with the initial transfer of glycosyl in the cell’s endoplasmic reticulum (ER), followed by its entry into the Golgi apparatus, where a range of glycans are added to facilitate glycan maturation, involving numerous glycosyltransferases (GTs) and glycosidases in the process.3
The glycosylation pathway produces different protein forms of modified proteins that play a role in many biological functions. Diverse cell adhesion molecules exhibit significant alterations in glycosylation is exhibited in cell adhesion molecules, which are vital for cancer progression, tumor metastasis, and immune evasion.4 The significance of glycosylation in signaling pathways, including the complex modulation of signal transduction within the transforming growth factor β (TGF-β) pathway, cannot be understated.5 Abnormal glycosylation leads to protein malfunction and disruption of biological processes that can lead to serious diseases. Up-regulation of the glycosyltransferase β-1,3-N-acetylglucosaminyl transferase leads to abnormal levels of programmed cell death molecule ligand 1 (PD-L1) glycosylation, which has been implicated in the development of triple-negative breast cancer (TNBC).6 Blockade of N-glycosylation of sterol regulatory element-binding protein cleavage-activating protein improves epidermal growth factor receptor (EGFR) VIII-driven glioblastoma growth.7 The importance of glycosylation in normal physiological functions and diseases in the human body has revealed the need to explore its mechanisms in depth and investigate more effective and tolerable strategies to stop disease production and improve patient prognosis.
In recent years, glycosylation has been widely studied as a biomarker for the diagnosis and prognosis of diseases, and great progress has been made (Fig. 1). Glycans participate in various stages of tumor progression, and glycosylation provides new targets for cancer therapy through their involvement in biosynthetic pathways. Various assays to determine the structure and mechanism of action of glycosylation are also in full swing from research to clinical and scientific studies. The current focus is to utilize this knowledge and technological tools of glycosylation to develop more powerful and safer drugs that are truly applicable to patients in the clinic.
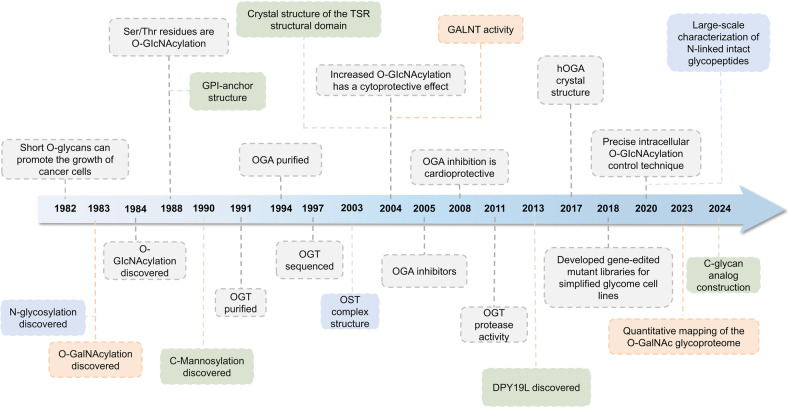
Fig. 1.
Timeline of the four major glycosylations development and further investigation. In the early 1980s, there was a preliminary understanding of glycosylation. As the research progressed, the corresponding initiating enzymes and chemical structures were gradually deciphered and recognized. For N-glycosylation, the determination of the Asn-X-Thr/Ser consensus sequence and the structure of the OST complex subunit and some accessory subunits around 2000 led to the in-depth study. Years later, N-linked intact glycopeptides in human serum were identified on a large scale using HILIC enrichment and spectral library searches, and the relationship between N-glycosylation levels and glucose metabolic stress, iron apoptosis, and so on were found. O-glycosylation is mainly classified into two types: O-GlcNAcylation and O-GalNAcylation. Since the 2010s, the close connection between O-glycosylation and physiological processes such as inflammatory response, immune escape, viral infection, cell adhesion, metastasis, apoptosis, etc. has been continuously discovered. Technological breakthroughs around 2020 have also led to the emergence of quantitative determination of maps of the O-glycosylated proteome and the development of gene editing libraries. The understanding of C-glycosylation began in the 1990s with the ongoing discovery of the structure of tryptophan residues in human Rnase linking mannose via C-C bonds. By the 2000s it was understood that C-glycosylation may be a necessary step for ER export in mucin biosynthesis. Recently it has been possible to construct C-glycosylation-modified glycopeptide drugs or glycan analogs in vitro, which greatly improve biological functions. GPI-anchoring has been well understood since the complete structural elucidation in 1988 and the discovery of the initiator enzyme, Transamidase, in 1992. In 2023, the phenomenon and molecular mechanism of feedback regulation of cellular maintenance of GPI-anchored proteins were first elucidated. DPY19L, dpy-19 like C-Man transferase; Gal, d-galactose; GalNAc, N-acetyl-d-galactosamine; GALNT, polypeptide GalNAc transferase; Glc, d-glucose; GlcNAc, N-acetyl-d-glucosamine; GPI, glycosylphosphatidylinositol; HILIC, hydrophilic interaction liquid chromatography; OGA, O-GlcNAcase; OGT, O-GlcNAc transferase; OST, oligosaccharyltransferase; TSR, thrombospondin type 1 repeat
In this review, we briefly summarized the mechanisms of glycosylation, and their biological functions, and discussed the impact of glycosylation in various human diseases. We further presented the potential value of glycosylation that can be used as biomarkers for disease diagnosis and prognosis, and summarized the current assays for glycosylation, briefly discussing the advantages and limitations of each. In addition, the targeted therapies related to glycosylation, a highly promising approach for the treatment of cancer, and challenges encountered in clinical development were discussed.
Mechanism of glycosylation
Protein is essential for life and involved in all cellular processes. Proteins undergo dynamic changes and various PTMs such as phosphorylation, methylation, acetylation, and notably glycosylation.8 Glycosylation, being one of the known PTMs, is essential in the unfolding of various functional activities in living organisms.9 Glycosylation of proteins is the process of covalently binding oligosaccharides in the form of glycosides to certain amino acid residues on proteins.10 The amino acids and glycans are classified into four categories according to their linkage: O-glycosylation, N-glycosylation, C-glycosylation and GPI-anchored attachment10,11 (Fig. 2). Among them, N-glycosylation and O-glycosylation are the most common types and they contain most glycosylation machinery associated with the pathogenesis and progression of disease.
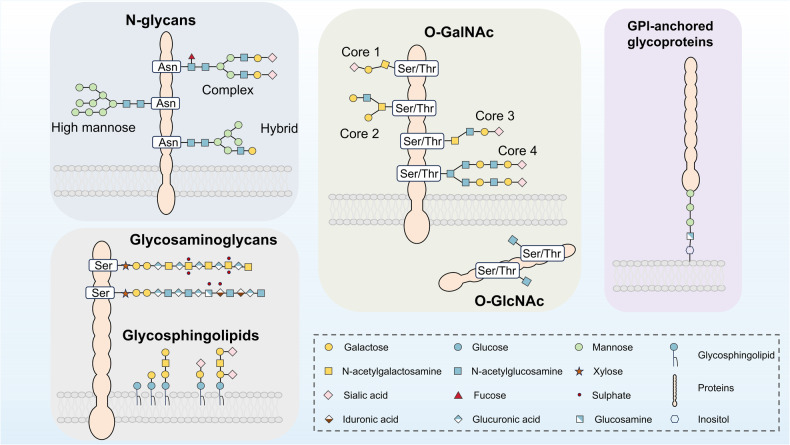
Fig. 2.
Classification of major manifestations of glycosylation on proteins. Glycosylation of proteins is capable of occurring when a saccharide is covalently attached to the polypeptide backbone via N-linkage to Asn or O-linkage to Ser/Thr. N-glycans that have complex, hybrid, or high mannose forms, are linked to Asn via GlcNAc. GalNAc links O-glycans to Ser/Thr with a variety of core structures and extensions, most of which are sialylated and fucosylated. Single GlcNAc molecules are linked to the Ser/Thr residue of intracellular proteins in the cytoplasm, mitochondria, and nucleus through the process of O-GlcNAc glycosylation. Certain glycoproteins known as glycosylphosphatidylinositol (GPI)-anchored proteins are also present in the plasma membrane’s outer leaflet and are connected to a phosphatidylinositol. A significant portion of the cell plasma membrane’s outer leaflet is made up of glycosphingolipids. Terminal sialic acids can be used to further modify the various series of structures that make up these ceramide-linked glycans. GalNAc, N-acetyl-d-galactosamine; GlcNAc, N-acetyl-d-glucosamine; GPI, glycosylphosphatidylinositol
N-glycosylation
N-glycosylation is a common glycosylation process facilitated by glycosyltransferases. It involves the binding of oligosaccharide N-acetylglucosamine (GlcNAc) to protein aspartate residues on the ER. This process results in three primary N-glycan subtypes, differentiated by their side chain branching into complex, mixed, and high mannose polysaccharides.12 N-glycans are conserved in the early synthesis of the ER, and their heterogeneity is seen in their subsequent processing. There is a common core structure for all N-glycans (asn-GlcNAc2Man3), which is even further extended by terminal glycan residues.13 These glycan residues, which mainly include GlcNAc, mannose, galactose, fucose and sialic acid (N-acetylneuraminic acid/Neu5Ac), could significantly affect the structure of N-glycan.
In the ER lumen, Asn-linked glycosylation (ALG) gene products assemble lipid-linked oligosaccharide precursors (Glc3Man9-GlcNAc2) onto dolpolypterpene phosphate (Dol-P) carriers. Key enzymes include GlcNAc-1-P transferase Alg7p (or GPT) with Alg13p/Alg14p uridine 5’-diphosphate-N-acetylglucosamine (UDP-GlcNAc) transferase, forming a linkage between N-glycan and the polypeptide chain.14 This assembly is followed by α-glucosidase I (GluI) and α-glucosidase II (GluII), which remove glucose residues, aiding in protein folding.15
The glycan is subsequently trimmed by ER mannosidase I, which removes terminal mannose residues from the branches of N-linked oligosaccharides.16 This structure is then recognized by lectin mannose-binding 1 (LMAN1). Matrix metalloproteinase-9 (MMP-9) is a secreted glycoprotein protease that further trims glycosylation and secretes correctly folded proteins during the passage from the ER to the Golgi apparatus. LMAN1 can act as a lectin carrier protein to mediate the efficient secretion of MMP-9, which facilitates the loading of glycosylated proteins into the coat protein complex II (COPII). COPII then transports these proteins from ER to Golgi via the ER-Golgi intermediate compartment.17,18 In the Golgi, various glycosidases and GTs further process the majority of N-glycans into complex and/or hybrid form.19
It was reported that aberrant expression of high mannose-type N-glycan structures is found in various malignancies, contributing to tumor proliferation, invasion, and migration through diverse mechanisms.20 However, the precise mechanism underlying the role of high mannose-based N-glycans in malignant tumor proliferation, immunosurveillance and tumor immunotherapy is still poorly understood and requires further investigation.
O-glycosylation
O-glycosylation is generally an oxygen linkage of glycans to the Ser or Thr residues, followed by a gradual addition of monosaccharides. Unlike N-glycosylation, which generally occurs in the ER and Golgi apparatus, O-glycosylation occurs mainly in the nucleus and cytoplasm.21 The two most common types of O-linked glycosylation are O-acetylgalactosamine (O-GalNAc) and O-linked-β-D-N-acetylglucosamine (O-GlcNAc).22 O-GalNAc glycosylation is initiated by polypeptide N-acetylgalactosaminyl-transferase, GalNAc monosaccharides are attached to Ser or Thr residues of proteins by O-glycosidic bonds.23,24 This dense O-GalNAc glycosylation, also known as O-glycosylation mucins, is expressed in a variety of tumor types and plays a role in cell-cell interaction, and cell-matrix interactions.25 On the other hand, the process of O-GlcNAc glycosylation is primarily regulated by two enzymes, O-GlcNAc transferase (OGT) and O-GlcNAcase (OGA). Glucose synthesizes UDP-GlcNAc is synthesized via the hexosamine biosynthesis pathway (HBP). Subsequently, UDP-GlcNAc is attached to Ser or Thr residues via β-O bonding, thereby enhancing the extent of protein O-GlcNAc glycosylation in the presence of OGT.26 OGA hydrolyzes and removes GlcNAc from proteins and reduces protein O-GlcNAc glycosylation.
Dysregulated expression levels of O-glycan enzymes are associated with tumor development, progression, invasion, and metastasis. It is shown that mucin-type O-glycan biosynthesis is an important pathway of colon carcinogenesis through single-cell transcriptomic analysis.27 Additionally, it has been reported that N-acetylgalactosaminyltransferase 7 (GALNT7) is upregulated in prostate cancer and can enhance its proliferation by regulating the O-glycosylation of prostate cancer cells.28
C-glycosylation and GPI anchor
C-glycosylation, a rare phenomenon in living organisms, involves the attachment of a mannose molecule to the tryptophan indole ring at position C via a C-C bond.29 The process of GPI-anchored attachment entails the binding of a GPI anchor, comprising the glycan core, to the C-terminus of a protein, thereby attaching it to the cell membrane. The general structure of the GPI anchor is ethanolamine, glycan core, and inositoll.30 GPI anchoring occurs in the ER and is a reversible modification where phospholipases can detach the anchored proteins from the cell membrane.
Biological functions of glycosylation
Regulation of protein functions
Glycosylation has multiple biological functions and can affect protein folding, quality control, stability, and transportation (Fig. 3).
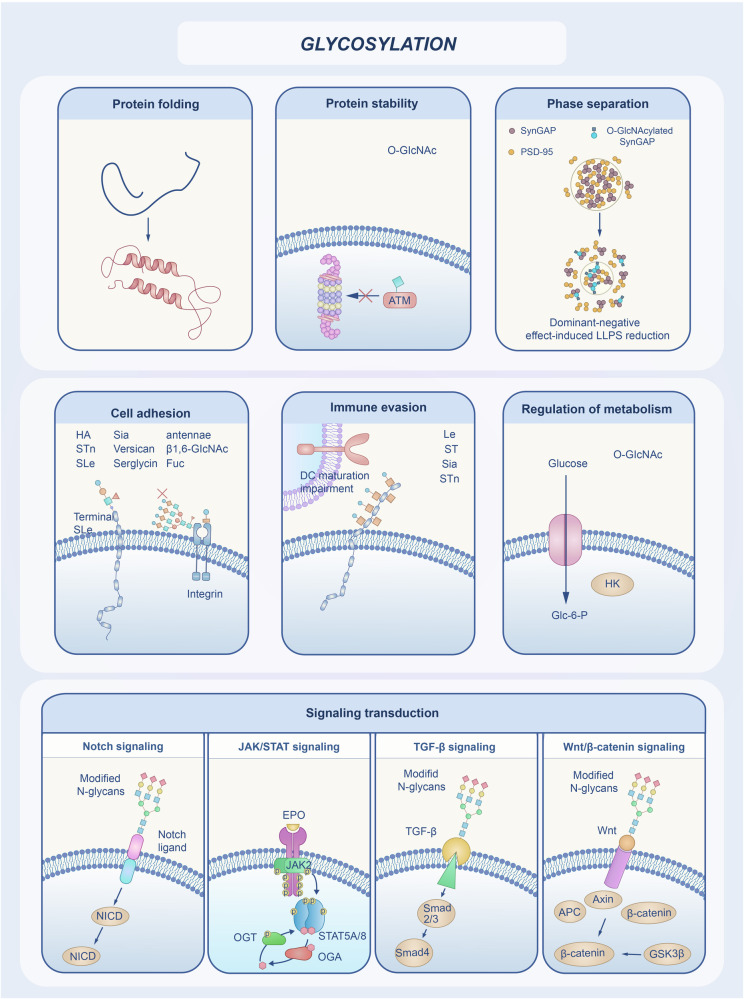
Fig. 3.
Biological functions of glycosylation. Glycosylation has a variety of biological functions that can affect protein folding and stability, for example, O-GlcNAcylation accelerates protein degradation in cells and decreases protein stability, it also regulates activities such as protein aggregation and phase separation. Glycosylation modulates cell-matrix interactions and promotes integrin-dependent signaling, which regulates adhesion activity. An effective immune response depends on the successful activation and maturation of dendritic cells, whereas abnormally glycosylated protein antigens impair the function of dendritic cells, allowing the cells to evade the host’s immune response. O-GlcNAc modifications have been found to directly regulate a variety of important biological processes within cells, such as cellular metabolism. Glycosylation can regulate enzyme activities or interact with other proteins and participate in cell signaling processes. These include Notch signaling, JAK-STAT signaling, TGF-β signaling, Wnt/β-catenin signaling pathways. GlcNAc, N-acetyl-d-glucosamine; LLPS, liquid-liquid phase separation; PSD, postsynaptic density; Glc-6-P, Glucose-6-phosphate; JAK-STAT, Janus kinase (JAK)-signal transducer and activator of transcription; OGT, O-GlcNAc transferase; OGA, O-GlcNAcase; TGF, transforming growth factor
Protein folding
Protein function depends on its high-level structure, and correct protein folding plays an important role. Protein folding is the process by which a polypeptide chain folds into its natural three-dimensional structure of a biologically active protein, aligning functional groups for specificity and minimum energy. Protein folding is crucial for modulating biological activity, regulating cell growth and differentiation, and facilitating molecular transport. Misfolded proteins tend to denature and lose their structure and function, even leading to diseases such as Alzheimer’s disease, which is caused by incorrect folding of the secondary beta-structure of fibrillar beta-amyloid proteins found in the brain.31 Protein folding is affected by a variety of factors, including electric and magnetic fields, temperature, pH, spatial confinement and molecular crowding, and it has been found that glycosylation also plays a role in protein folding. Glycosylation is also one of the factors affecting protein folding, which on the one hand helps directly by stabilizing polypeptide structures and on the other hand can help indirectly through interactions with lectins, glycosidases, and GTs as recognition “tags”.32
In the ER, N-glycosylation directs the initial steps of protein folding and its quality control.32,33 N-glycosylation allows the newly synthesized glycoprotein to interact with the lectin-based chaperone system in the ER.34 In mammalian cells, calnexin, calreticulin, and related factors play a crucial role in facilitating the proper folding and oligomerization of numerous glycoproteins. They offer specialized quality control and chaperone functions tailored specifically for glycoproteins in the ER.34 Lectins could act as chaperones in glycoprotein folding.34 O-glycosylation stabilizes the folded protein domain and promotes protein secretion.2 The O-linked mannose structure is simpler and has recently been found to have a unique function in protein quality control that does not depend on the intricate structure of N-linked glycan.35
Protein stability
Glycosylation is crucial in regulating protein stability. Glycosylation can enhance the stability and solubility of proteins, thereby improving their half-life and drug properties in vivo. A recent report indicated that the O-GlcNAcylation of hepatocyte growth factor-regulated tyrosine kinase substrate (HGS), a critical protein in the EGFR trafficking pathway, could hasten the degradation of HGS protein in cells, thereby diminishing its stability.36 O-GlcNAcylation could stabilize PPM1K and promote dephosphorylation of BCKDHA, thereby promoting catabolism of branched-chain amino acid in hepatocellular carcinoma (HCC).37 CD98 (SLC3A2) is a type II glycoprotein, which is a multifunctional protein, and mutations in its N-glycosylation site can severely affect stability and influence migration to the plasma membrane and various cellular processes.38
Phase separation
Phase separation (or liquid-liquid phase separation, LLPS) refers to the phenomenon that biological macromolecules (such as proteins, RNA, etc.) form a temporary liquid-liquid phase separation state in the cell.39 Phase separation is a widespread liquid state in physical chemistry. In cells, phase separation plays a crucial role in maintaining the cellular complex structure, function and signal transmission.40 Recent studies have shown that O-glycosylation plays a crucial and multifaceted role in regulating amyloid protein aggregation and LLPS under physiological conditions.41 Lv et al. synthesized site-specific O-GlcNAc -modified SynGAP using a protein semi-synthesis strategy, and found that O-GlcNAcylation could modulate LLPS of SynGAP/PSD-95 complex.42 Furthermore, Chen et al. found that O-GlcNAcylation could attenuate the translation-promoting effect of YTHDF1 and YTHDF3 by inhibiting their associations with proteins involved in mRNA translation. The abundant O-GlcNAc modification of YTHDF1/3 enhanced its dynamic characteristics within the phase separation components and accelerated the depolymerization phase separation, low abundance O-GlcNAc modification of YTHDF1/3 maintained its stability in phase separation components.43 These results suggest that O-GlcNAc modification may act as a universal regulatory mechanism to regulate a variety of LLPS processes.
Regulation of cell adhesion
Proper cell-cell and cell-extracellular matrix proteins (ECM) communications and interactions are essential to maintain healthy tissues, both structurally and functionally. These interactions are largely carried out by adhesion molecules, peripheral cell membrane proteins. Most of the proteins involved in cell adhesion are glycosylated. There are several groups of adhesions molecules on cell, including cadherin, selectin, sialic acid-binding immunoglobulin-like lectins (Siglecs), and integrin family.44
Different adhesion molecules apparently play different, although sometimes overlapped, roles. Cadherins, a group of transmembrane glycoproteins, are a class of Ca2+-dependent homophilic cell adhesion molecules responsible for facilitating cell-cell adhesion. There are multiple members, E-cadherin, N-cadherin, and P-cadherin in the cadherin family with E-cadherin being studied the most. Evidence suggests that glycosylation can affect the adhesion function of the family E-cadherins. E-cadherins contain a single transmembrane structural domain, a cytoplasmic structural domain (C-terminus), and an extracellular domain, and the said extracellular domain consists of five repetitive structural domains (EC 1 to EC 5).45,46 O-glycosylation of newly synthesized E-cadherin has been reported to impede cell-surface translocation, leading to decreased intercellular adhesion, which was suggested due to the impaired binding of E-cadherin to p120-catenin. In addition to phosphorylation, cytoplasmic O-glycosylation of E-cadherin becomes an alternative mechanism to regulate cell adhesion.47 The N-glycosylation sites of E-cadherin are located in the extracellular structural domains, with four sites clustered in the EC 4 to EC 5 region. It has been emphasized that reduced N-glycosylation of E-calmodulin promotes the establishment of a stable adherens-junction. However, excessive N-glycosylation of E-calmodulin weakens the adherens-junction.48,49 E-calmodulin N-glycosylation is mainly controlled by the GnT-III, GnT-V, and fucosyltransferase 8 (FUT8), which are involved in the regulation of cell-cell adhesion.50 Significant alterations in glycosylation have been observed for several cell adhesion molecules in the tumor microenvironment, predominantly cadherin, and selectins, becoming a common feature of cancer progression.
Selectins, a class of Ca2+-dependent heterophilic cell adhesion molecules, comprise three cell adhesion molecules: L-selectin expressed on leukocytes, E-selectin expressed on activated endothelial cells, and P-selectin expressed on activated platelets and endothelial cells.4,51,52 They could specifically recognize and adhere to specific glycosyl groups in oligosaccharide chains on the surface of other cells, and mediate the recognition and adhesion of leukocytes primarily to vascular endothelial cells or platelets, specializing in the capture of leukocytes from the bloodstream to the vessel wall.53 Selectins are single-transmembrane-penetrating glycoproteins with an intracellular region that binds to microfilaments via anchoring proteins. The extracellular region consists of three major structural domains: an N-terminal C lectin-like structural domain, an epidermal growth factor (EGF)-like structural domain, and a structural domain that is homologous to complement regulatory protein.54 Among them, the C lectin-like structural domain is the active site that recognizes specific glycosyl groups and engages in selective adhesion.55
Leukocytes move in a rolling fashion, mediated by selectins, by adhesion-detachment and re-adhesion-detachment with vascular endothelial cells in the vasculature at the site of inflammation.56 Selectins act as receptors that bind to glycoligands with structural modifications of sialyl-Lewisx(sLeX) or sialyl-Lewisa(sLea) at the end to regulate cell migration, and cell metastasis in cancer. The expression and adhesion function of sLeX on trophoblast cells and L-selectin on uterine epithelial cells mediate adhesion at the fetal-maternal interface. The key enzyme for the synthesis of sLeX is fucosyltransferase VII (FUT7), and when the expression of FUT7 was up-regulated, the synthesis of sLeX was increased, and the rate of adhesion of trophoblast cells to human uterine epithelial cells was significantly increased.57 There are at least 14 different Siglec receptors in human, which are predominantly found in immune cells.58 By binding to its ligands including sialoglycans, Siglec receptors modulate immune cell activity through recruiting SHP1 and SHP2 phosphatases.58 The Siglec-sialoglycan axis plays a strong role in regulating both the innate and adaptive immune responses and has been found to be strongly associated with tumor evasion which involves altered glycosylation.59,60 Integrins are a large family of heterodimeric alpha/beta receptors, bind to ECM so they are the molecules that “sense” the cellular environment. This binding is specific and highly regulated. The binding of integrin to specific ECM component could trigger the activation of intracellular signaling pathways, release growth factors and cytokines embedded in the ECM, and sensing the mechanic changes in the cellular environment, all of which can contribute to cell proliferation, survival, differentiation, and migration.61–63 Under diseased conditions, such as cancer and immunological disorders, integrins have become attractive drug targets. In fact, certain integrins are clinically validated drug targets. Changes in glycosylation of integrins observed in tumors impact the intracellular signaling and cell adhesion activity.64 For example, cancer patients, such as breast cancer patients, with increased GlcNAc branching on the N-glycans showed poor prognosis.65 Core 1 β1,3-galactosyltransferase (C1GALT1) modifies the O-glycan on integrin β1, regulates its activity, and enhances the invasiveness of HCC.66 β-galactoside α2,6-sialyltransferase 1 (ST6Gal-I) was identified as poor prognosis marker as hypersialylation on integrins in multiple cancers. It was found to link to increase of tumor migration in colon and ovarian cancer cells.67,68 Clearly glycosylation on adhesion molecules, including integrins, alters cell proliferation and function.
Immune evasion
Immune evasion refers to the development of many mechanisms by infectious organisms (including bacteria, viruses, parasites, etc.) and cancer cells that prevent the host’s immune system to respond and trigger their elimination from the host. Glycosylation plays a critical role in immune regulation under normal and pathogenic conditions, including immune evasion by pathogenic organisms and cancer cells,69 which is not surprising, since most of the key molecules that are involved in immune response, both innate and adaptive, are glycosylated.70 The products of protein glycosylation, glycans, and the glycosylated proteins, are all well-documented in immune modulation and immune evasions by pathogens. Specifically, three major glycan-binding proteins, galectins, Siglecs, C-type lectin family (CTLF) are known to play a role in immune evasion.71,72 Pathogenic organisms utilize host glycans or secret their own molecules as glycan mimicry as shield to avoid immune surveillance.73 Cancer cells could produce abnormal amount of glycans, glycan-binding proteins, and cell surface glycosylated proteins with structural changes. The quantity and qualitative changes in glycosylation and interaction of glycosylated proteins favor tumor cells to escape the host immune response.74 Among the best examples, sialic acids are rich in tumor environments and have been shown to be extensively involved in a variety of cancer cells for immune suppression.75 Removal of sialic acids was found to be associated with tumor inhibition in glioblastoma.76 The changes in glycosylation, quantitively or/and qualitatively, in cell surface glycans alter the interactions between cancer cells with host glycan binding proteins such as galectins, Siglecs, and CTLF. Cell-surface glycans usually end in sialic acid, a structure that binds to a variety of endogenous receptors, among which Siglecs have been associated with immune evasion in tumor cells.77 Siglecs are a series of sialic acid-binding immunoglobulin-like lectins that modulate cellular functions in the innate and adaptive immune systems through glycan recognition.58 The extracellular portion of Siglecs contains an N-terminal carbohydrate-recognizing domain (CRD) and a C2 structural domain, and the intracellular portion contains immunoreceptor tyrosine-based inhibition motif (ITIM) or ITIM-like structures that mediate immunosuppression.78 Several studies have found that increased sialic acid density leads to increased involvement of inhibitory Siglecs receptors on immune cells and modulates the immune response to cancer, which has been observed in lung cancer and melanoma samples.79 Many cancers show an increase in sialylated glycans which may be associated with sialyltransferases, and sialic acid synthesis genes. The hypersalivation Siglecs axis may be another way for tumor cells to escape immune surveillance.60 Siglec-7 is involved in remodeling the sialylation status of cancer cells and thereby affecting NK cell immunoevasion.80 Galectins binding to LacNAc-containing glycans has an important role in regulation T cell functions.81 The amount and the nature of the modification of galectins all affect immune cell activity. For example, high level of Galectin-1 expression is strongly associated with low T cell infiltration to tumor tissues in head-and-neck cancers.82 Similarly, Gal-3 and Gal-9 were found to play a role in the downregulation of immune response in other solid tumors.83,84
Modulation of signaling transduction
Glycosylation can regulate enzyme activity or its interaction with other proteins to participate in cell signaling and recognition processes.
Notch signaling
As a highly conserved signaling pathway, Notch signaling plays an important role in a variety of biological processes such as cell growth, development, and tissue repair.85 In mammals, Notch is activated by the binding of its extracellular domain to ligands (Delta and Jagged/Serrate) on the surface of apposed cells.86 Accumulating studies have confirmed that extracellular PTMs in the form of glycosylation are essential for the Notch signaling pathway.87–89 Abnormal O-glycosylation of Notch receptors and their ligands leading to acquired functional mutations or signaling dysregulation of genes in the Notch signaling pathway is associated with several diseases.90 The extracellular domain of Notch is comprised of a series of EGF repeats, which can range up to 36 in number. Studies have shown that aberrant expression of Notch-modified glycosyltransferase, including protein O-glucosyltransferase 1 (POGLUT1) and protein O-fucosyltransferase 1 (POFUT1), can be found increased in several diseases, such as oral squamous cell carcinoma.91 POGLUT1 functions as a protein-O-glucosyltransferase, facilitating the transfer of glucose to the EGF-like domains found in Notch receptors and other signaling receptors. POGLUT1 O-glucosylation is mainly carried out by three POGLUTs (POGLUT1, 2, and 3) that use uridine diphosphate-Glc as a substrate for modification of the EGF repetitive sequences for their O-glucosylation activities.92,93 In a family with autosomal recessive limb-girdle muscular dystrophy, a missense mutation was identified in the POGLUT1 gene. This mutation is known to induce decreased O-glucosyltransferase activity on Notch receptors, leading to impaired muscle development.94 POFUT1 is localized in the region of 20q11.21, which is heavily amplified in acute myeloid leukemia (AML).95 Recent findings indicate that POFUT1, the enzyme responsible for adding O-fucose to Notch receptors, may also play a role in quality control within the ER. Several studies have demonstrated that Fringe, a glycosyltransferase, operates within the Golgi complex to modify the glycosylation patterns of Notch receptors.96,97 The modulation of Jagged1-induced Notch signaling by Fringe requires the involvement of beta 4galactosyltransferase-1, which adds a galactose residue to GlcNAc beta 3Fuc. This suggests that beta 4GalT-1 may serve as a novel regulator of Notch signaling.98
O-GlcNAcylation is a common PTM, which can be divided into OGT-catalyzed intracellular form and EGF-domain specific O-GlcNAc transferase (EOGT)-catalyzed extracellular form. Several studies have found that O-GlcNAcylation is regulated by the Notch signaling pathway and is involved in disease development.99 OGT directly interacts with Notch1 to catalyze the O-GlcNAcylation of the Notch TM/ICD fragment, promoting its binding to the E3 ubiquitin ligase Itch and reducing degradation, which plays an important role in regulating mammalian neurogenesis and cognition.100 In addition, O-GlcNAcylation can also be catalyzed by EOGT, which augments Delta-like ligand-mediated Notch signaling and promotes human Adams-Oliver syndrome.101
Investigations conducted by multiple groups have demonstrated the crucial role of O-fucosylation in ligand-mediated Notch signaling. The modification of the O-fucose saccharide structure, induced by Fringe, influences the response of Notch to its ligands. Zhou et al. reported that Notch-dependent signaling could control myelopoiesis, and fucosylation of Notch could regulate the ligand binding activity and efficiency of Notch signaling in myeloid progenitors.102 O-fucose site mutations have been found in anaplastic large cell lymphoma as two mutations in NOTCH1 (p.T311P and p.T349P) result in deletion of the O-fucose site in the eighth and ninth EGF repetitive sequences in the NOTCH1 ECD.103 Furthermore, certain O-fucose molecules are elongated through the activity of β-1,3-N-acetylglucosaminyltransferases (β3GNTs) from the Fringe family. GCNT1, a glycosyltransferase, could mediate O-Glycosylation of the Sialomucin CD43, thereby act as an indicator of Notch signaling in T cells.104
JAK-STAT signaling
The Janus kinase (JAK)-signal transducer and activator of transcription (STAT) pathway is one of the central pathways for cellular function. A variety of cytokines and growth factors, such as hormones, interferons, and interleukins, have been identified to initiate this pathway. JAK-STAT pathway is involved in multiple processes such as immune adaptation, apoptosis, and inflammation. Deletion or mutation of JAK-STAT components is associated with a variety of diseases.105 The binding of cytokine to the receptor causes dimerization of the receptor, simultaneous tyrosine phosphorylation of the intracellular terminus of the receptor, and the phosphorylation of the two JAK kinase molecules that are recruited to the receptor. The phosphorylated receptor binds to the STAT protein, activating STAT phosphorylation and triggering downstream pathways.106
The STAT protein family includes seven members, among which STAT5 plays an important function in the regulation of growth and development and tumor immunity.107 O-GlcNAcylation promotes oncogenic transcription by enhancing STAT5 tyrosine phosphorylation and oligomerization to drive myeloid transformation.108 It was found that O-GlcNAcylation, a modification necessary for STAT5-induced transcription, occurs at Thr at the N-terminal site 92 of the STAT5 protein.109 Defective or hyperactive STAT5 variants of O-GlcNAcylation differ greatly in oncogenic potential. In Burkitt lymphoma and acquired immunodeficiency syndrome (AID)-related lymphoma, this region of the T58 locus of the c-Myc protein is a hotspot for mutation. The study could observe that O-GlcNAcylation of Thr at the T58 site occurs with phosphorylation modification, which also serves as evidence that O-GlcNAcylation regulates gene transcription signaling in tumor cells.110 In addition, it was identified that the JAK/STAT1 pathway could act as a regulator of glycolysis, enhanced glucose turnover was associated with abundant STAT1-O-GlcNAcylation in mesenchymal stem cells.111 The above studies demonstrate the critical role of glycosylation in the JAK-STAT signaling pathway and reveal the possible functions involved in glycosylation.
TGF-β signaling
TGF-β signaling plays crucial roles in the preservation of organismal integrity. TGF-β signaling governs the regulation of cell proliferation, phenotypic plasticity, metabolic adaptation, and immune surveillance in diverse cell types. Malfunctions in TGF-β signaling have the potential to disturb immune tolerance, induce inflammation, and contribute to the development of fibrosis and cancer.112
Both core fucosylation and sialylation primarily act as stimulatory factors in TGF-β signaling during various biological processes, including fibrosis, migration, differentiation, and immune evasion.5 ALG3, one of ALG members, has been to promote radiation resistance in breast cancer by regulating glycosylation of TGF-β receptor II.113 Kim et al. demonstrated that the use of glycosylation inhibitors, such as tunicamycin and kifunenine, or mutations in the N-linked glycosylation site of TβRII attenuated ALG3 overexpression to varying degrees, could prevente the effective transport of TβRII protein to the cell surface, thereby reducing cell sensitivity to TGF-β.114 Core fucosylation catalyzed by FUT8 is important for signaling receptors. Upregulated expression of FUT8 induced high levels of core fucosylation of TGF-β type I and type II receptors, facilitating TGF-β binding and downstream targets, thereby promoting cell invasiveness of breast cancer cells.115
Sialylation-mediated regulations on TGF-β signaling are linked with epithelial-mesenchymal transition (EMT).116 ST3Gal1, a key sialyltransferase, could mediate sialylation of vasorin to facilitate TGF-β1-mediated angiogenesis and progression of tumor.117 In addition, GalNAc-type O-glycosylation is initiated by polypeptide N-acetylgalactosaminyltransferases, is involved in TGF-β signaling regulation. Inhibition of breast cancer cell migration and invasion, specifically through the EMT process, could be achieved by targeting ppGalNAc-T4-catalyzed TGF-β receptor O-GalNAcylation. This suggests that focusing on ppGalNAc-T4 may hold promise as a therapeutic strategy for breast cancer.118 Together, these studies suggested the regulatory role of glycosylation in TGF-β signaling, more investigations are needed to better understand the process and its implications.
Wnt/β-catenin signaling
The Wnt signaling pathway plays a pivotal role in regulating cell-fate determination, cell migration, cell polarity, neural patterning, and organogenesis during embryonic development.119 Abnormal Wnt signaling can lead to developmental diseases or cancer. Wnt is a class of secreted protein molecules with glycosylation and fatty acidification modification. To date, 19 Wnt proteins have been identified in humans. Wnt pathway is commonly divided into β-catenin-dependent (canonical) and independent (non-canonical) signaling.120 Among the mechanisms that have been documented to affect Wnt/β-catenin activity, modification of N-glycans by L-fucose is the recently understood. Hong et al. revealed that increased α (1-3)-fucosylation of GlcNAc in the Galβ (1-4)-GlcNAc sequences of complex N-glycans could suppress the activation of Wnt/β-catenin signaling through elevates the endocytosis of lipid-raft-localized low-density lipoprotein receptor-related protein 6 (LRP6).121 Conversely, Wnt suppression induced by cell-surface α (1-3)-fucosylation can be rescued by addition of free fucose.121 B3GnT2, a member of the β1,3-N-acetylglucosaminyltransferase (B3GnT) family, could promote the extension of polylactosamine chains on multiple N-glycans on LRP6, thereby enhancing LRP6 transport to the plasma membrane and promoting Wnt/β-catenin signaling.122
In addition, β1,4-Galactosyltransferase V is engaged in embryogenesis and plays a crucial role in catalyzing the attachment of galactose to GlcNAcβ1-4Man residues located on N-glycans. This enzymatic process holds significance in regulating cell stemness through glycosylation mechanisms, leading to the stabilization of Frizzled-1 and activation of the Wnt/β-catenin signaling pathway specifically in breast cancer.123 Overexpression of the polypeptide N-acetylgalactosamine-transferase 1 could activate the Wnt/β-catenin signaling through modulating CD44 O-glycosylation in gastric cancer.124 Further validation of the regulatory mechanism of glycosylation will contribute to a better understanding of the function of Wnt/β-catenin signaling.
Regulation of metabolism
Recently, accumulating studies have demonstrated the importance of protein glycosylation in regulating lipid metabolism.125 N-glycosylation plays a regulatory role in proteins involved in lipid synthesis, packaging, and the clearance of lipoproteins. N-glycosylation in humans reduces low-density lipoprotein by enhancing low density lipoprotein (LDL) receptor expression, suggesting that N-glycosylation could act as a vital regulator of LDL metabolism.126 In addition, the specific glycan patterns of high-density lipoprotein (HDL)-associated ApoE are intricately involved in the activity and functionality of HDL. The enzyme galNAc-T2, encoded by GALNT2, plays a crucial role in catalyzing the attachment of GalNAc in O-glycosylation processes. Genetic studies have demonstrated that the variants in GALNT2 could impact HDL and triglyceride levels in humans.127
In terms of glutamine metabolism, a study on pancreatic ductal adenocarcinoma revealed that the cancer cells are highly dependent on a specific glutamine catabolic pathway, and malate dehydrogenase (MDH1) is a key enzyme in this pathway.128 Zhu et al. found that Ser189 was the glycosylation site of MDH1, and its O-GlcNAc glycosylation could promote Gln catabolism. Moreover, Ser189 O-GlcNAc could act as a “molecular glue” to stabilize the substrate binding pocket on the MDH1 monomer, improve substrate binding and stability, and ultimately promote the enzymatic activity of MDH1.129 Further determination of the mechanism by which protein glycosylation modifies metabolic reprogramming will open up novel insights for therapeutic intervention in metabolic diseases and tumors.
Abnormal glycosylation in human diseases
Glycans, together with proteins, nucleic acids and lipids, are important biomolecules in the human body. Protein glycosylation is widespread in living organisms and involves various processes such as cellular immunity, protein translation regulation, and signaling pathways. Abnormal glycosylation is associated with the onset and progression of various diseases130 (Fig. 4).
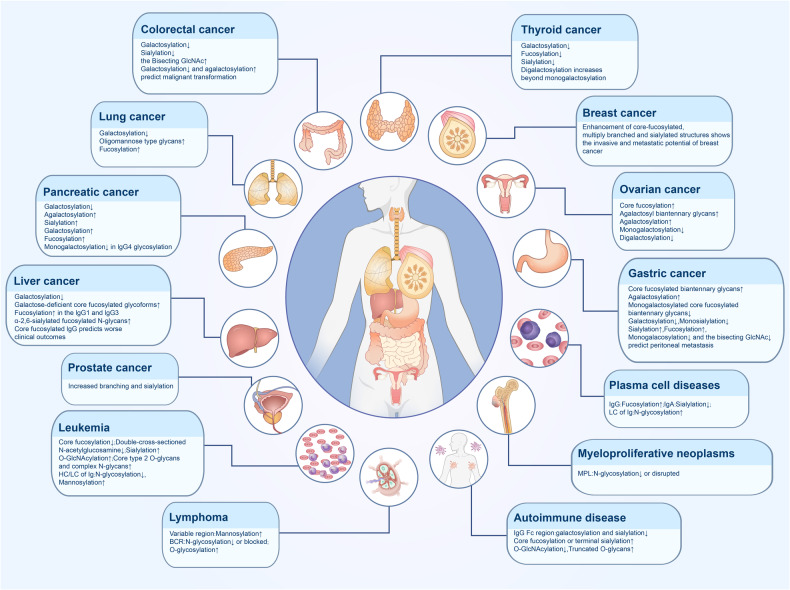
Fig. 4.
Altered glycosylation in human disease. Glycosylation is widely present in organisms, and aberrant glycosylation is closely associated with the development of a variety of diseases. Altered glycosylation present in solid tumors, hematological malignancies, and autoimmune diseases are summarized in the figure
Glycosylation in hematologic malignancies
Hematological malignancies are a group of diseases with high heterogeneity in molecular and phenotypical characteristics. Increasing evidence suggests that protein glycosylation is involved in the pathogenesis and development of hematological malignancies, including lymphoma, leukemia, and plasma diseases.
Leukemia
Acute myeloid leukemia
Acute myeloid leukemia (AML) is a highly diverse malignancy originating from hematopoietic stem and progenitor cells.131 Fms-like tyrosine kinase 3 (FLT3), a receptor tyrosine kinase of type III, plays a critical role in regulating the growth and maturation of hematopoietic cells, is one of the commonly mutated genes in AML.132 Mutations in the FLT3 gene are detected in about 30% of adult AML patients, and the mutations are mainly in the FLT3-internal tandem duplication (FLT3-ITD) and FLT3-tyrosine kinase domain (FLT3-TKD), of which FLT3-ITD mutation is more common and suggests a poorer prognosis.133 FLT3 is synthesized in the ER as a 130 KD precursor of glycosylation-deficient or mannose-rich material, is transferred to the Golgi apparatus and then continues glycosylation to complete the complex 150 KD structure before transferring to the cell surface.134,135 If the progression of FLT3 in the Golgi is disrupted, FLT3 protein carrying immature glycosylation is then accumulated in the ER. This promotes apoptosis or initiates signaling pathways that are distinct from normal glycosylation.136 It has been illustrated that knockdown of FUT8 leads to the deletion of core fucosylation of FLT3, which results in sustained ligand-independent and autonomous activation of its downstream pathway and unlimited proliferation of tumor cells.137 2-Deoxy-D-glucose (2DG) could induce cell death by inhibiting the N-glycosylation of FLT3 in FLT3-ITD-mutated AML, decreasing the cell surface expression of FTL3-ITD. 2DG is also effective in c-KIT-mutated AML cells. In the latter, O-glycosylation of Sp1 was modulated and c-KIT expression was decreased, triggering ER stress and activation of the unfolded protein response, which induced apoptosis.138–140 Tyrosine kinase inhibitors (TKIs) increase the surface level of FLT3 as well as FLT3-ITD and FLT3-D835Y mutants by upregulating their glycosylation.141 Statins such as lovastatin can reduce mutant FLT3 kinase activity by blocking complicated glycosylation at the receptor. This in turn contributes to changes in positioning and signaling, leading to cell apoptosis.136,142 Therefore, TKI combined with FLT3 immunotherapy, which attenuates the level of glycosylation, may benefit AML patients.
In addition, the interaction between AML and the bone marrow microenvironment is crucial in disease progression.143 In MDS/AML patients, a significant down-regulation of double-cross-sectioned GlcNAc structures was observed in bone marrow stromal cells, whereas enhanced expression of MCAM, a marker of bone marrow niche, further activates the ERK signaling pathway and promotes bone marrow cell growth.144 The membrane scaffold CD82 in the microenvironment undergoes N-glycosylation, and inhibition of its glycosylation increases the molecular accumulation of N-cadherin, promotes bone marrow homing of AML cells, and affects AML cell migration in vivo.145
Studies have revealed that leukemia cells expressing CD43 are enriched with sialic acid glycosyl groups on their surface, while CD43 is a heavily O-glycosylated protein, and the glycosylation status of the CD43 protein was associated with cytotoxic T cell (CTL)-mediated cytolysis.146 Sialic acid residue epitopes on CD43 have been studied as targets for bispecific T cell-engaging antibodies, which have been shown to specifically induce CTL cells and mediate cytotoxicity in both in vivo and in vitro studies, providing evidence for the therapeutic potential of targeting the sialic acid residue epitope of CD43.147 The role of sialic acid modification on CD43 demonstrates the relevance of glycosylation in AML pathophysiology. At present, there are more emerging studies that have taken a deeper dive at glycan characterization. It can be concluded that glycosylation plays a major part in the development and treatment of leukemia. However, more comprehensive studies on their mechanisms are needed in order to provide a stronger rational for the development of new therapies for AML and new perspectives for targeted therapy.
Acute lymphoblastic leukemia
Acute lymphoblastic leukemia (ALL) is characterized by the uncontrolled growth and infiltration of immature lymphocytes in the bone marrow, peripheral blood, and other organs including B-ALL and T-ALL. High levels of C-Myc expression promote leukemia cells to enhance self-renewal and be more insensitive to chemotherapy-induced differentiation.148 O-GlcNAcylation, plays a pivotal role in modulating the activity of nuclear proteins involved in gene expression, signal transduction, and cell growth.149 High expression of O-GlcNAcylation of c-Myc promotes rapid proliferation of pre-B cells. Evidence of decreased pre-B cell number was also observed in mice administered O-GlcNAc inhibitor.150 Oliveira et al. found that B-cell precursor ALL (BCP-ALL) cells with mixed lineage leukemia gene rearrangement exhibited increased core type 2 O-glycans and complex N-glycans, alongside significant changes in sialic acid and fucose glycosylation.151
The role of glycosylation is also captured in T-ALL. CD95 (Fas/Apo-1), a type I transmembrane protein encoded by the Fas gene that binds to CD95L and induces apoptosis, through which virus-infected cells, damaged cells and cancer cells are eliminated.152 In a study treating cells with interferon-gamma, it was found that the high molecular weight form CD95, most of which is caused by N-linked glycosylation.153 SHIP-1 (SH2 (Src homology 2)-containing inositol 5’-phosphatase-1) is a negative regulator of the immune response, partially located in the ER, and promotes the glycosylation of CD95, resulting in its inability to oligomerize, leading to the formation of the death-inducing signaling complex and impaired downstream apoptotic cascades.154
Chronic lymphocytic leukemia
Chronic lymphocytic leukemia (CLL) is a mature B-lymphocyte clonal proliferative neoplasm, characterized by lymphocyte aggregation in the peripheral blood, bone marrow, spleen and lymph nodes.155 The B cell receptor (BCR) is an immunoglobulin expressed on the membrane surface of B lymphocytes during B lymphocyte development, and is a specific receptor for B lymphocytes to recognize and bind antigens. The structure of the immunoglobulin heavy chain (IGH) is critical for the function of BCR.156 After the development and maturation of B lymphocytes, the IGH is stimulated by antigen and undergoes somatic high-frequency mutation (SHM), which enhances the affinity of the BCR for the antigen. In CLL cells, the IGH of the BCR can be characteristically altered.157
CD79 is a heterodimer molecule involved in signal transduction as a component of BCR. CD79 has two subunits, CD79a and CD79b (Igα and Igβ, respectively), and is highly expressed in CLL cells.158 A study showed that low levels of BCR expression in CLL cells was due to impaired glycosylation and folding of mu and CD79a.159 SHM frequently leads to the creation and/or disruption of N-glycosylation sites within the variable structural domains of the Ig H and L chains, which appears to be related to the natural history of CLL.160 In addition, the heavy chain constant region of the BCR in CLL cells carries a high level of mannosylation modification, which could relate to a more aggressive CLL.161
Lymphoma
Follicular lymphoma
Lymphoma is one of the first hematologic malignancies to be identified.162 Non-Hodgkin lymphoma (NHL) is the most prevalent hematologic malignancy globally, responsible for approximately 3% of cancer cases and fatalities. Follicular lymphoma (FL) is a class of NHL originating from follicle-centered B-cells, with a typical immunophenotype of CD5-CD10 + CD19+ with t(14;18) (q32; q21), and whose BCR undergoes SHM to produce a high degree of tumor heterogeneity.163,164 FL cells undergo persistent somatic hypermutation in the variable region gene of their immunoglobulins. DNA sequences encoding Asn-X-Ser/Thr (N-glycosylation site) were introduced by sustained somatic hypermutation in the variable region.165,166 Following contact with calcium-dependent lectins exposed to tissue macrophages, the unique additional oligosaccharide activates the BCR signaling pathway. That route seemed to be crucial for tumor growth and proliferation.167
N-glycosylation sites are introduced into the variable region of the BCR in FL and exhibit a high mannose type. Binding of these glycosylated groups to mannose-specific lectins triggers sustained BCR signaling activation, which is critical for tumor cell survival and proliferation.161 The introduction of N-glycosylation sites by SHM can occur in a variety of B-cell diseases, but has the highest prevalence in FL. Most of the neo-glycosylation sites are located in the complementary decision region in the variable region and less frequently in non-functional V(H) sequences.166 High throughput sequencing analysis revealed that sustained SHM could result in subclones with different amino acid compositions in different disease events, but a large majority of the resulting DNA sequences still encode an N-glycosylation site.167 In addition, a recurrent type of N-glycosylation, NX(S/T), was identified in mutations in the variable structural domain in FL.168 This study suggested that the opportunistic interactions occurred at BCR and cells containing mannose-binding lectins may contribute to the pathogenesis of FL.169
Studies addressing differences in N-glycosylation patterns between FL subgroups with and without t(14;18) found a significant difference in the frequency of newly acquired N-glycosylation sites in t(14;18)-positive and t(14;18)-negative FL stage III/IV patients, which was not observed in FL stage I/II patients.170 In contrast, newly acquired N-glycosylation sites were significantly reduced in t(14;18)-negative advanced (stage III/IV) FL, suggesting that signaling in addition to the current BCR pathway. It has been found that N-glycosylation site-negative FL cells can also expand and may be more dependent on the energy metabolism pathway than the BCR signaling.171
Diffuse large B-cell lymphoma
Diffuse large B-cell lymphoma (DLBCL) is the most prevalent type of NHL, which derived from mature B cells.172 Glycosylation in different proteins can regulate the development of DLBCL from multiple pathways. BCR signaling pathway plays an important role in the development of DLBCL. Inhibition of BCR glycosylation by oligosaccharyltransferase-B reduces BCR clustering and internalization, thereby attenuating PI3K and NF-κB activation.173 This finding suggests that agents against the oligosaccharyltransferase-B glycosylation pathway may be useful for the treatment of DLBCL.
CD45 is classified as a type 1 transmembrane protein tyrosine phosphatase, a glycoprotein of 180-220kDa.174 CD45 consists of two cytoplasmic domains, a transmembrane domain and an extracellular structural domain, and mature CD45 is highly O- and N-glycosylated.175 Gal-3 is a soluble beta-galactoside-binding protein that is widely distributed in various tissues and organs.176 Gal-3 binds to a variety of ligands on the cell surface and in the extracellular matrix, and plays an important role in apoptosis, adhesion, proliferation, migration, inflammatory response, and immune response. Gal-3 is overexpressed in DLBCL and binds mainly to CD45, which is regulated by C2GnT-1 glycosyltransferase.177 The activity of tyrosine phosphatase is regulated by the binding of gal-3 to CD45. Removal of gal-3 from CD45 on the cell surface with the multivalent glycan inhibitor GCS-100 increased the sensitivity of DLBCL cells to chemotherapeutic agents.178 This sheds light on the importance of specific glycosylation of CD45 in regulating gal-3 pathway signaling and implication in DLBCL chemotherapy treatment.
Tumor immune escape mediated by the programmed cell death molecule 1 (PD-1)/PD-L1 signaling pathway is one of the important mechanisms of tumorigenesis and progression.179 PD-1 is mainly expressed on the surface of activated T cells, and its ligand PD-L1 is mainly exposed on the surface of tumor cells. The binding of PD-1 to its ligand PD-L1 affects the downstream signaling pathway through dephosphorylation of key molecules and inhibits T-cell receptor/CD28 signaling. Thus, it acts as a negative immune response regulator, leading to immune escapement of neoplastic cells.180,181 Immune checkpoint inhibitor drugs, anti-PD-1 or anti-PD-L1 antibodies, have been developed and found to be efficacious in treating a variety of human cancers.182 Glycosyltransferase 1 domain-containing 1 (GLT1D1) was found to be highly up-regulated in early relapsed DLBCL. GLT1D1 transfers N-linked glycans to PD-L1, which was found to promote the immunosuppressive function of glycosylated PD-L1 and attenuates the cytotoxic function of T cells against lymphoma cells.183 Thus, inhibition of GLT1D1 activity may be a new therapeutic direction for DLBCL.
Plasma cell diseases
Multiple myeloma
Plasma cell disease is a heterogeneous group of diseases of plasma cell origin caused by the over-proliferation of clonal plasma cells or that produce immunoglobulins.184 Monoclonal immunoglobulins or their fragments (called M-proteins) are emitted in most cases in serum or urine, with some end-organ damage. The synthesis and secretion of excess, structurally homogeneous M-protein is a common feature of plasma cell disease.185 IgG is the most abundant in serum immunoglobulins. The decrease or deficiency of IgG makes individuals susceptible to repetitive infections, demonstrating its role in immune defense.186 Glycosylation is important for the functional realization of IgG. It was previously reported that IgG from selected myeloma patients had a high degree of fucosylation in the Fab fraction.187 Multiple myeloma (MM) patients with bone disease showed less galactose on IgG than patients without bone disease. Moreover, less sialic acid on IgG was found in MM patients, compared to healthy control.188 Fucosylation glycosylation-modified IgG could play a role in myeloma development. Compared to IgG, IgA subtypes in MM are relatively rare. The level of IgA could have value, but more studies are needed.189 A study that collected sera from 35 patients with monoclonal IgA, isolated and purified IgA, and found that patients with monoclonal gammopathy of undetermined significance (MGUS) had less sialylated IgA than healthy volunteers.190
In addition to exploring the mechanisms of plasma cell disease development, glycosylation can also be potentially valuable in its diagnosis and staging. For example, serum N-glycoprotein changes were significant in the pathologic findings of IgD-MM. Different N-glycans levels correspond to different peaks and can be used in the differential diagnosis of IgD-MM and light chain (LC)-MM.191
Light-chain amyloidosis
Light-chain amyloidosis (AL) is caused by the misfolding of immunoglobulin LCs. A study using cryo-electron microscopy to demonstrate the structure of λ1-AL amyloid fibrils showed that N-glycosylation acts as a key regulator in fibrin misfolding.192 A study using MASS-FIX, an immunoenrichment-based matrix-assisted laser desorption ionization time-of-flight mass spectrometry, confirmed the LC N-glycosylation in 5% of MASS-FIX-positive patients with clinically significant rare monoclonal diseases including AL amyloidosis and cold agglutinin disease.193 In addition, Nevone et al. revealed the specific sequence and spatial pattern of N-glycosylation in amyloidogenic κ LCs, where the majority of N-glycosylation sites are located in framework region 3, specifically inside the E chain.194 Monoclonal LC N-glycosylation is a detrimental element for progression to AL amyloidosis, myeloma, and other plasma cell diseases, and it is hoped that these findings will yield an earlier diagnosis and minimize morbidity and mortality.195 Single-cell RNA sequencing presented the upregulation of transcriptional program of glycosylation-modified, tumor-associated plasma cells in AL, which may provide a new direction for understanding plasma cell development and transcriptional reorganization.196 Although important links between glycosylation and plasma cell disease have been identified, the precise functions and molecular mechanisms involved still require more detailed studies to be fully elucidated.
Myeloproliferative neoplasms
Typical myeloproliferative neoplasms (MPN) are classified as chronic myeloid leukemia, polycythaemia vera, primary thrombocytosis, and primary myelofibrosis.197 JAK2(V617F) point mutation is strongly associated with MPN. Cell surface type I cytokine receptors such as erythropoietin receptor, granulocyte colony-stimulating factor receptor (G-CSFR), and thrombopoietin receptor are homodimers. When cytokines bind to their receptors, JAK2 forms a dimer and phosphorylates each other, which in turn activates STAT5 to exert transcription factor activity and ultimately causes the generation, proliferation and differentiation of the corresponding hematopoietic progenitor cells.198 JAK2, myeloproliferative leukemia protein (MPL), and CALR were found in close correlation with the development, progression and regression of MPN, and all of them were involved in glycosylation, and the activation of JAK-STAT may be the central link in the pathogenesis of MPN.199 Mutations in the CALR gene encoding the ER-resident chaperone protein calreticulin are associated with most MPN, and deletion and insertion of its exon 9 leads to the 21/12 transition.200 The mutated calreticulin activates MPL by binding to the N-glycosylation site of MPL through its new C-terminus, particularly the two Asn residues of N-117 and N-178, which in turn induces ligand-independent activation of the JAK2-STAT/ PI3K and MAPK pathways.201 In mutant CALR-transformed cells, genes that have undergone N-glycosylation are depleted to varying degrees. Chemical inhibition of N-glycosylation expression on the surface of MPL cells impairs the growth of mutant CALR-transformed cells.202
Granulocyte colony-stimulating factor (G-CSF) is a polypeptide chain of granulocyte growth factor that mobilizes a variety of bone marrow precursor cells by binding to the G-CSFR to promote the proliferation, differentiation and migration of granulocytes and enhance the function of mature granulocytes.203 CSF3R is the gene encoding G-CSFR, and acquired mutations in CSF3R have been associated with malignant events.204 Mutations in CSF3R are common in patients with chronic neutrophilic leukemia (CNL) or atypical CML.205 CSF3R T618I mutation is a point mutation in the proximal extra-membrane structural region, as T618I is located at the O-glycosylation site, and glycosylation changes at this site are associated with tumorigenesis.206 There is also a rarer point mutation at N610 of CSF3R, as the Asn residue at N610 is N-linked.207 The mutation disrupts the N-glycan as part of the consensus glycosylation motif, losing its role in folding, transport and thermodynamic stabilization of the protein.208 These N610 mutants allow CSF3R to activate independently of its ligand G-CSF, promoting tumor cell expansion through the JAK-STAT signaling, with potent oncogenicity. Kinase inhibitors can prevent the growth of cells possessing this mutation.203,208 Point mutations in CSF3R T618I and CSF3R N610 hyperactivate CSF3R, resulting in cytokine-independent growth of leukemia cells.208 The above studies revealed the critical function of N-glycosylation in maintaining the normal function of CSF3R, and provide a basis for conducting studies on the regulation of glycans in receptors.
Glycosylation in solid malignancies
Glycosylation changes associated with cancer were first identified more than 50 years ago.209 Today, aberrant alterations in glycosylation are widely recognized in various cancers. These alterations include truncated O-glycans, altered N-glycan branching, and the incorporation of aberrant glycan chains.210,211 Tumor cells may experience aberrant glycosylation due to several factors, firstly, including the aberrant expression of GTs and hydrolases, changes to the peptide backbone or glycan chain backbone, and variability in substrates and donor availability.212–215 Recognizing abnormal glycosylation as a hallmark of cancer has been pivotal. In-depth investigation into the mechanisms of glycosylation in cancer development is now a critical aspect of tumor biology research.67,216
Respiratory neoplasms
Lung cancer is a prevalent malignant tumor that is associated with high morbidity and mortality rates worldwide.217 The role of glycosylation in lung cancer has been an area of significant research interest, and several important findings have been made in recent years. Studies have shown that lung cancer cells often exhibit altered glycosylation patterns compared to normal lung cells.218,219 These changes include variations in N-linked and O-linked glycosylation, which can affect the behavior of cancer cells, including their growth, invasion, and metastasis.220 For instance, changes in the glycosylation of adhesion molecules can influence the ability of cancer cells to detach from the primary tumor and spread to other parts of the body.221 Importantly, aberrant glycosylation in lung cancer can help the tumor evade immune detection and induce resistance to immunotherapy including ICIs. Glycosylation of PD-L1 is a major mechanism that induces its stabilization, reduces the detection by PD-L1 antibody and consequently results in immune escape.222 Removing the N-linked glycosylation significantly enhanced anti-PD-1/PD-L1 therapy efficacy.223 In addition, changes in glycosylation patterns in lung cancer cells also have potential as biomarkers for early diagnosis and prognosis. Certain glycan structures have been identified that are uniquely present or altered in lung cancer, which could aid in detecting the disease early and predicting patient outcomes.224,225 Ongoing studies continue to uncover more about how glycosylation influences lung cancer at the molecular level, which may shed light on new therapeutical targets and strategies.
Gastrointestinal tumor
As reported previously, the digestive system cancers often exhibit altered glycosylation patterns, which affect cell signaling, adhesion, and invasion, playing a crucial role in cancer development and progression.226–228 For instance, increased sialylation and fucosylation of glycoproteins and glycolipids on cancer cells can enhance cell ability to detach, invade, and form metastases in colorectal cancer.229,230 Modified glycan structures on the surface of cancer cells can impair the immune system’s ability to recognize and target them, facilitating tumor growth and survival.228,231 Glycosylation in digestive system cancers also affects the tumor microenvironment, affecting tumor growth and response to therapy.232 The aberrant glycosylation pathways in digestive system cancers present new targets for therapeutic intervention.
Moreover, sialylation is mediated by sialyltransferases, and increased overall sialylation, particularly ST6Gal-I-mediated α2,6-linked sialylation is strongly associated with gastrointestinal tract cancers, as well as being a marker of poor prognosis.233,234 Further cellular and animal experiments have also shown that ST6Gal-I is associated with invasiveness and metastasis in gastrointestinal tumor.68,235 It was found that most hemagglutinins showed diminished binding to β-galactosides ending in α 2,6-sialic acid.236 ST6Gal-I adds an α2,6-sialic acid to the terminal galactose of the N-glycan, a modification that prevents binding of hemagglutinin to β-galactoside and resists galectin-mediated apoptosis.237
sLeX is a ligand for selectin, a vascular cell adhesion molecule belonging to the family of C-type lectins. The production of the selectin-recognized sLeX and sLea tetrasaccharides induced by increased sialylation of the outer chains of the N-glycan branches may also be one of the mechanisms that enhance the progression of the cancer process. The high expression of sLeX and sLea in tumor cells correlates with a poor prognosis in patients with gastrointestinal cancers.238 sLeX interacts with selectin to induce cancer cell adhesion to platelets and arrest on endothelial cells, regulating tumor cell metastasis in colorectal carcinoma.239 sLea, or carbohydrate antigen19-9 (CA19-9), is the commonly used biomarker for pancreatic cancer, and high expression is also detected in gastrointestinal tumors.240 The CA19-9 locus has now become one of the research targets for cancer therapy, and therapeutic strategies such as antibodies and vaccines, and CA19-9-guided nanoparticles are emerging.241
Reproductive cancers
The reproductive cancers, including cervical, ovarian, and endometrial cancers in women, and prostate cancer in men, exhibit varied incidence rates. The role of glycosylation in cancers of the reproductive system has been the subject of significant research, revealing critical insights into how these complex biochemical modifications influence the development, and treatment of these cancers.242,243
Endometrial cancer
Altered glycosylation patterns in endometrial cancer have been identified, with changes in N-linked and O-linked glycosylation affecting tumor growth and the potential to metastasize.244,245 These alterations in glycosylation patterns can also impact the immune system’s ability to recognize and respond to the tumor.246 Changes in glycosylation patterns in reproductive system cancers offer potential as biomarkers. For instance, certain glycosylation patterns in ovarian and endometrial cancers may aid in early diagnosis, monitor disease progression, and predict response to therapy.247–249 Targeting specific glycosylation pathways and enzymes involved in glycosylation has shown promise in preclinical models, suggesting potential therapeutic strategies. It is also worth noticing that the altered glycosylation in these cancers not only contributes to tumor progression but also impacts the fertility and overall quality of life of patients, making it a critical area of research for patient-centric therapies.250
Ovarian cancer
Aberrant glycosylation has been observed in ovarian cancer, particularly in the form of altered sialylation and fucosylation patterns. These changes in glycosylation affect cell adhesion, migration, and invasion, which are critical for tumor metastasis.251,252
Prostate cancer
In prostate cancer, changes in glycosylation, particularly in the forms of complex N-glycans, have been linked to tumor progression and metastasis. Specific glycan structures, such as increased branching and sialylation, have been associated with more aggressive diseases.28,253,254
Breast cancer
Abnormal glycosylation in breast cancer impacts cell signaling, adhesion, and immune recognition, contributing to the cancer’s aggressiveness.6,255 Moreover, aberrant glycosylation enables breast cancer cells to evade immune detection, facilitating tumor progression.181,256 Current biomarkers for breast cancer prognosis and treatment still lack applicability and precision. Glycosylation patterns in breast cancer are potential biomarkers for early diagnosis, prognosis, and treatment monitoring, with unique glycan structures identified in breast cancer cells.257 Targeted therapies aiming at these altered glycosylation pathways, such as inhibiting specific glycosylation enzymes, show promise in reducing tumor growth and enhancing treatment efficacy. Furthermore, research suggests glycosylation characteristics might vary among breast cancer subtypes, like TNBC indicating the possibility of subtype-specific treatment approaches.258,259
Basement membrane degradation and myoepithelial cell reduction are key features of breast cancer progression, and glycosylation plays a role in disrupting cell-cell adhesion and cell polarity, driving breast cancer progression.260,261 E-calmodulin is a cell surface glycoprotein that is essential in mammary cell development and epithelial cell dissemination. When E-calmodulin undergoes down-regulation of O-mannosylation and an increase in N-branched complex glycans, its signaling is aberrant and cell cycle progression is dysregulated.262 Breast cancer cells present upregulation of glycolysis, prompting some glucose to enter the HBP, increasing the level of O-GlcNAc and enhancing the expression of OGT, regulating cell metabolism, promoting tumor cell growth and accelerating cancer progression.263 Studies have shown that OGT can be inhibited by targeting the oncogenic transcription factor FoxM1, which is a potential new therapeutic target for breast cancer.264 These insights affirm glycosylation’s pivotal role in breast cancer pathophysiology.
The figure of glycosylation is very common in breast cancer cell metastasis and invasion. Matteo Rossi et al. found that deletion of phosphoglycerate dehydrogenase (PHGDH) in breast cancer patients promotes metastatic spread of tumor cells. Low expression of PHGDH loses its interaction with phosphofructokinase, which activates the hexosamine-sialic acid pathway, and increases sialylation of integrin αvβ3, thus facilitating cancer cell migration and invasion.265 Paweł et al. found that podoplanin (PDPN) from a subpopulation of breast cancer tumor-associated macrophages (TAMs) promotes cancer lymphangiogenesis and distant metastasis through attachment to lymphatic endothelial cells (LECs).266 And this process is predicated on the presence of a large amount of O-glycosylation in the extracellular structural domain of PDPN. Klára et al. found metastatic site specificity of N-glycosylation in breast cancer metastasis. While high-mannose glycans were most frequently elevated in breast cancer metastases, it was the increase in core fucosylation that was more pronounced in bone metastases.267 This suggests the importance of glycosylation as a diagnostic marker for metastatic breast cancer.
Inhibition of glycosylation has potential in tumor immunosuppression.258 Li et al. found that targeting glycosylated PD-L1 and blocking PD-L1/PD-1 interactions promoted PD-L1 internalization and degradation, and was able to eradicate TNBC cells.181 Monoclonal antibodies targeting glycosylation have become another direction of immune checkpoint therapy. Targeting glycosylation may be a strategy to combat breast cancer drug resistance. Researchers have found that upregulation of dolichyl-phosphate N-acetylglucosaminyltransferase (DPAGT1) maintains high levels of HER2 shedding, which produces trastuzumab resistance in breast cancer. And this process relies on DPAGT1 to induce N-glycosylation levels of the shedding enzyme, ADAM metallopeptidase domain 10 (ADAM10).268 Blocking the glycosylation level may be a new direction to combat trastuzumab resistance. Naoomi et al. found that by inhibiting the expression of ribophorin II (RPN2), the level of N-glycosylation of the tetraspanin CD63 could be reduced, leading to the localization of multidrug resistance protein 1 (MDR1) to be moved out of the cell surface, and decreasing drug resistance in malignant breast cancer cells.269 Glycosylation plays a variety of roles in the process of breast cancer occurrence, progression and migration, and also provides a variety of new target directions for tumor therapy, which still requires in-depth study of its mechanism to explore its endless clinical potential in the future.
Central nervous system tumors
Aberrant glycosylation in central nervous system (CNS) tumors has been linked to tumor growth, invasion, and metastasis.270 For instance, alterations in the glycosylation of cell surface proteins can influence the way cancer cells interact with their environment, facilitating invasion into surrounding brain tissue.271 Glycosylation can significantly impact the tumor microenvironment in the CNS. This includes altering interactions with immune cells, impacting the blood-brain barrier, and influencing the response to therapies.272 Glycosylation in CNS tumors also affects how these tumors interact with normal neural cells. For instance, altered glycosylation patterns in CNS tumors have potential as biomarkers for diagnosis and prognosis, and also offer potential therapeutic targets.273 However, it’s worth noting that there is considerable heterogeneity in glycosylation patterns among different types of CNS tumors. This variability reflects the diverse origins and characteristics of these tumors and has implications for treatment and prognosis. The study of glycosylation in CNS tumors is complex due to the intricate nature of the brain and its environment.
Neuroendocrine neoplasms
Research on glycosylation in neuroendocrine neoplasms (NENs) highlights its critical role in tumor behavior and patient outcomes. NENs display altered glycosylation patterns, including changes in N-linked and O-linked glycosylation, which influence tumor proliferation, differentiation, and metastasis.274 These glycosylation changes also present potential as diagnostic and prognostic biomarkers, and offer novel therapeutic targets, particularly in disrupting tumor growth and spread. Additionally, glycosylation in NENs intersects with hormonal pathways and regulating the immune response, impacting the efficacy of hormonal and immunotherapies.275 The heterogeneity in glycosylation patterns among different NENs further underscores its significance in the pathogenesis and treatment of these tumors.
Sarcoma
Investigation of the roles of glycosylation in sarcoma, including osteosarcoma, is an emerging field that has already provided key insights. Sarcoma, like other cancer types, often displays altered glycosylation patterns. These changes can significantly impact tumor growth, invasion, and metastasis. Specifically, modifications in the glycosylation of cell adhesion molecules may enhance sarcoma cells’ ability to metastasize by altering their interaction with the surrounding environment.265,276 Glycosylation also affects sarcoma cells’ interactions within the tumor microenvironment, influencing growth and therapeutic response.277,278 In osteosarcoma, glycosylation is believed to contribute to disease pathogenesis and progression, but the detailed mechanisms and effects are less clear compared to other cancers. The field is still evolving, and further research may provide novel diagnostic and treatment approaches for this disease.
Glycosylation in autoimmune diseases
Autoimmune diseases are caused by the immune response to autoantibodies, which resulting in damage to tissues.279 Immunoglobulin (Ig), the most abundant glycoprotein in serum, plays a crucial role in humoral immunity, and glycosylation can regulate the function of Ig-mediated cellular and humoral responses. These glycosylation changes can modify immunoglobulin G (IgG)’s binding affinity to Fcγ fragment receptors (FcγR) or C1q complement, thus influencing IgG’s role in regulating immune cell activities towards pro- or anti-inflammatory responses.280 Specifically, the IgG1 Fc segment at Asn-297, composed of GlcNAc and mannose, can undergo further glycan modifications. Such alterations in the Fc segment’s glycosylation can impact its affinity for FcγR, with reduced sialylation, for instance, enhancing pro-inflammatory FcγR activation.281–283
Rheumatoid arthritis
Rheumatoid arthritis (RA), a long-term autoimmune disorder that affects the joints, shows significant glycosylation alterations in IgG molecules, particularly in the Fc region, marked by reduced galactose and sialic acid. These changes enhance pro-inflammatory responses and are linked to RA’s severity.283 Additionally, these glycosylation variations, influenced by genetic and environmental factors, open avenues for personalized medicine in RA management. Compared with healthy controls, RA patients showed specific glycosylation patterns, such as lower galactosylation, lower sialylation, and higher IgG fucosylation.284,285 The first report on the abnormal N-glycosylation of serum IgG in patients with RA was published in 1985.286
For diagnosing RA, rheumatoid factor (RF) and anti-cyclic citrullinated polypeptide antibodies are the most reliable autoantibodies. Despite being clinically manageable, RA lacks a cure, underscoring the need to investigate its pathogenesis. Low expression of N-glycosylated sialylation is a pathogenetic feature of immunoglobulin to in RA patients.282,287 A study comparing IgG glycosylation in RA patients and healthy individuals revealed that IgG RF with decreased sialylation correlates with heightened RF activity. Additionally, elevated serum C-reactive protein levels intensify arthritic symptoms.288 In contrast, anti-citrullinated protein antibodies exhibit abnormally low sialylated Fc glycans, promoting osteoclastogenesis and susceptibility to inflammatory bone loss.289,290 However, studies found no significant link between IgA glycosylation and RA activity.291
The synovium, a specialized mesenchymal tissue in the joint cavity, is maintained by synovial fibroblasts (SFs), which preserve a homeostatic environment in healthy joints but become pro-inflammatory in RA.292 This transformation is linked to a decrease in the cytokine TNF-dependent glycosyltransferase ST6Gal-I and α2-6 sialylation, altering glycosylation and turning the synovial membrane pro-inflammatory.293 The shift may be due to healthy SFs having high levels of core fucosylation or terminal sialylation, regulated by TNF. This results in reduced sialylation of CD90+ SFs in the sub synovial layer, contributing to the pathological changes observed in RA.294
Systemic lupus erythematosus
Systemic lupus erythematosus (SLE) is classified as an autoimmune disease involving innate and adaptive immunity, characterized by symptoms like rashes, nephritis, and arthritis, largely due to excessive autoantibodies and immune complexes, notably anti-double-stranded DNA (anti-dsDNA) antibodies.295,296 Despite its high mortality and relapse rates, specific preventive or therapeutic measures for SLE are lacking. Research into altered glycosylation in SLE seeks to understand its mechanisms. A study utilizing quantitative mass spectrometry for glycan chain analysis, along with cluster analysis and machine learning, assessed the link between glycosylation of anti-dsDNA IgG and SLE activity. A significant positive correlation was observed between the level of fucosylation in anti-dsDNA IgG1 and SLE disease activity.297
Defects in N-glycosylation is associated with immune system dysregulation in SLE patients. The enzyme alpha-Mannosidase II, crucial for complex N-glycosylation through mannose residue removal, plays a key role. Mice deficient in alpha-Mannosidase II exhibit N-glycosylation anomalies, leading to an autoimmune disease resembling human SLE.298 Additionally, a study on lupus nephritis revealed that renal tissues from lupus nephritis patients are rich in mannosylation, a feature absent in other renal diseases. This is thought to result from inadequate complex N-glycosylation combined with mature O-glycosylation.299 Additional research suggests that complex N-glycosylation plays a protective role against autoimmune diseases like SLE by preventing endogenous non-autoimmune signals and reducing sterile inflammatory reactions.300
Inflammatory bowel disease
Inflammatory bowel disease (IBD) is a group of chronic inflammatory bowel diseases characterized by a chronic and prolonged course, mediated by a combination of genetic, immune, and environmental factors, including ulcerative colitis (UC) and Crohn’s disease (CD).301 The development of IBD is associated with an elevated expression of truncated O-glycans and changes in the expression of terminal glycan structures.302 A study compared plasma N-glycosylation levels between IBD patients and healthy individuals. Plasma specimens from patients with IBD were found to be characterized by more macroglycans, reduced relative abundance of heterodimeric and high-mannose structures, lower fucosylation, lower galactosylation, and higher levels of sialic acidification.303 Elevated IgG agalactosylation (G0), decreased IgG monogalactosylation (G1) and digalactosylation (G2) were found in IBD patients.304,305 In a retrospective analysis of 3441 plasma samples from patients with IBD, it was found that CD patients showed an increase in core fucosylation of IgG1 and IgG2/3, while UC patients exhibited a decrease in core fucosylation of IgG2/3.305 The severity of colitis in chemical and T cell-transfer-induced IBD mouse models was found to be less pronounced in Fut8 knockout mice. This finding provides further evidence supporting the involvement of Ig fucosylation in the development of IBD.306 Sialylation is another possible Ig glycosylation pattern involved in IBD development. Decreased IgG sialylation levels were observed in both CD patients and UC patients.305
In addition, there was a significant correlation between antibody glycosylation and disease activity in patients with IBD. For example, increased IgG2/3 agalactosylation, decreased IgG2/3 digalactosylation, or sialylation was associated with disease worsening in CD patients. Furthermore, elevated IgG1 agalactosylation or increased agalactosylated and fucosylated IgG to digalactosylation and fucosylated IgG ratio (G0F/G2F) associated with more extensive in CD patients.305,307 CD patients who received infliximab treatment exhibited a significant reduction in the agalactosylation of IgG.308
Despite advances in understanding IBD, the role of T cells’ glycosylation-modified structures remains largely unexplored.309 In IBD, memory CD4+ T cells produce colitis-associated glycoprotein, featuring immature O-glycosylation. CAG aids in forming CD4+ T cell immune synapses, thus promoting memory CD4+ T cell expansion in the inflamed gut and intensifying intestinal inflammation.310 Additionally, a study in IBD mice found increased core fucosylation on T-cells, essential for signaling, inflammatory factor production, and intestinal inflammation induction.306 Disruption of glycosylation promotes inflammation by perturbing intestinal barrier function, gut microbiota, glycan-lectin interactions, as well as mucosal immunity in IBD.302 These findings suggest that both N- and O-glycosylation are crucial in IBD’s pathogenesis, highlighting potential therapeutic targets in glycosylation inhibition.
Autoimmune liver disease
Autoimmune hepatitis (AIH) is a parenchymal inflammation of the liver mediated by an autoimmune response against hepatocytes, including primary biliary cholangitis (PBC) and primary sclerosing cholangitis (PSC).311 AIH is characterized by serum autoantibody positivity, hyperimmunoglobulin G and/or gamma-globulinemia, and the presence of hepatic histology of interfacial hepatitis, with a variety of clinical manifestations.312,313 A site-specific glycomic analysis of Ig in patients with autoimmune cholestatic liver diseases demonstrated elevated levels of IgG1 agalactosylation and reduced levels of IgG1 galactosylation in both PBC and PSC patients.314 PSC patients exhibited higher levels of IgG2 fucosylation compared to PBC patients. Conversely, decreased glycosylation of IgG3/4 was observed in PBC patients. In addition, elevated IgA 1/2 bisecting glycoforms, declined IgA diantennary glycoforms, and increased IgA 1/2 asialylation levels were observed in PBC patients.314 The presence of IgG2 agalactosylation showed a positive correlation with various stages of PBC,314 indicating the potential significance of Ig glycoforms as biomarkers and for monitoring disease progression in autoimmune cholestatic liver diseases.
Another study revealed the regulatory role of glycosylation in the pathogenesis of autoimmune hepatitis by establishing an O-GlcNAc glycosylation-deficient rat model.
The severity of AIH is linked to the activity of CD4+ and CD8+ T lymphocytes.315 While the exact role of glycosylation in AIH is unclear, recent studies have shed light on its significance. Research using an O-GlcNAc glycosylation-deficient rat model revealed that a lack of this glycosylation intensifies liver damage and prolongs recovery in AIH. This glycosylation deficit increases CD4+ T cell infiltration and impacts Treg differentiation, thus disrupting the Notch signaling in CD4+ T cells and accelerating AIH progression.316 Further, O-glycosylation inhibitors have been shown to worsen AIH, potentially due to altered cytokine levels and T-cell proliferation.317
Myasthenia gravis
Myasthenia gravis (MG) is an autoimmune disease that affects neuromuscular transmission. The most common subtype of MG is the production of autoantibodies directed against the nicotinic acetylcholine receptor (AChR) and is controlled primarily by the IgG1 and IgG3 subclasses.318,319 In addition to this, autoantibodies against muscle-specific tyrosine kinase (MuSK) are also present in many patients.320 Unlike AChR MG, MuSK MG isoforms are most often associated with IgG4 subclass autoantibodies.321,322 The level of Fab glycosylation of IgG4 antibodies was significantly elevated in MuSK MG, but this change was not found in the IgG subclass of AChR MG. One study explains that it may be related to the fact that the MuSK antigen is positively charged and the AChR antigen has a negative charge.323–325
MG patients have an increased frequency of N-linked glycosylation of IgG V regions (IgG-VN-Glyc) which may be associated with biased use of V gene fragments and somatic hypermutation. However, it has been experimentally confirmed that the binding of MG autoantigen-specific monoclonal antibodies (mAbs) to autoantigens is not altered by glycosylation.326 One study evaluated for the first time the changes in IgG Fc N-glycosylation in MG, observing low levels of IgG2 galactosylation.327
Nephropathy
IgG glycosylation plays an important role in the occurrence of kidney injury. Galactosylation, sialylation, and level of bisecting GlcNAc of the IgG glycans significantly correlate with renal function.328 One of the causative mechanisms of IgA nephropathy may be abnormal O-glycosylation of IgA1. The IgA1 O-glycosylation chain is truncated, resulting in increased terminal GalNAc and insufficient galactosylation.329 This overproduction and increased systemic presence of low O-glycosylated galactose-deficient IgA1 is recognized by anti-glycan autoantibodies, which form macromolecular IgA1 immune complexes that deposit in the glomerular mesangium, leading to renal inflammation and scarring.330
Glycosylation in other diseases
In addition to the above alterations in glycosylation in tumors and autoimmune diseases, this PTM is increasingly being studied in metabolic and cardiovascular diseases. For example, it has been found that patients with type 1 diabetes mellitus have a relatively low abundance of simple dual antennae N-glycan (NA2), while complex multibranched, galactosylated, and sialylated modifications are characterized by a higher relative abundance of N-glycans.331 In chronic thromboembolic pulmonary hypertension, the distribution of IgG galactosylation is closely associated with N-terminal pro-B-type natriuretic peptide, demonstrating the pro-inflammatory properties of IgG N-glycosylation.332 In summary, glycosylation is involved in the occurrence and development of a variety of diseases. Further exploration of the value of glycosylation in disease diagnosis and prognosis is expected to provide a new choice for treatment strategy.333
Cardiovascular diseases
Glycosylation plays a role in transcriptional regulation, signal transduction, and energy metabolism, and is closely related to the development of cardiovascular disease (CVD). Among them, the role of O-GlcNAcylation in CVD is very important, but the mechanism is still not completely clear.334 Several studies have suggested a potential role for O-GlcNAcylation in cardiomyopathy through regulation of mitochondrial energy, autophagy, or gene expression.335,336 Decreased activity and expression levels of peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α) when modified by O-GlcNAcylation and down-regulation of its expression in the myocardium may be involved in the formation of cardiac hypertrophy.337 Chen et al. also confirmed that the increase of O-GlcNAcylation in cardiomyocytes is related to myocardial hypertrophy, and hyperglycemia and hyperlipidemia can lead to the enhancement of protein O-GlcNAcylation, thus suggesting the importance of dietary control in the treatment of myocardial hypertrophy.338 In addition to cardiomyopathy, O-GlcNAcylation has also been shown to be associated with coronary microvascular disease.339 Reduced degradation of P53 protein modified by O-GlcNAcylation induces coronary microvascular disease and impairment of myocardial contractility.340 O-GlcNAcylation-modified synaptosome-associated protein 29 interferes with autophagic responses and impairs myocardial diastolic function.341
Changes in protein glycosylation could affect the interactions between blood cells and basement membranes or soluble plasma proteins, modulating blood viscosity, promoting local cell adhesion and inducing pro-inflammatory responses, leading to plasma cell extravasation, which is an important determinant of atherosclerosis and myocardial infarction.342–344 For example, the shear stress of sialidase affects cellular desialylation, leading to cell membrane stiffness and increased susceptibility to lysis and inflammation.345
Glycosylation of IgG is also associated with the pathophysiology of myocardial infarction. Some analyses have shown decreased serum galactosylation and increased levels of sialylation, fucosylation, and N-acetylglucosamine in patients with myocardial infarction.346 Three IgG N-glycosylation types associated with CVD were identified in a prospective study, with gender-specific associations.347 In conclusion, N-glycosylation of IgG may affect the risk of CVD, which may be an additional therapeutic target.
The key to clinical intervention in CVDs, such as myocardial infarction and ischemic stroke, is the application of thrombolytic enzymes in the acute phase to dissolve clots. Although drugs such as alteplase and urokinase are widely used today, they still suffer from insufficient clot dissolution rate and high risk of side effects.348 Glycosylation engineering could play a role in improving thrombolytic enzyme performance and resolving glycan covalent linkages.349 For example, tenecteplase, the most successful glycosylated engineered variant of alteplase, carries a total of six point-mutations in three different regions of the original base: Thr103Asn, Asn117Gln, and tetra-alanine substitution Lys296Ala+His297Ala+Arg298Ala+Arg299Ala.350 These glycosylation sites lead to a longer half-life of tenecteplase and significantly improved fibrin selectivity.351,352
Metabolic diseases
The incidence of metabolic diseases is increasing as a result of rapid economic development, population ageing and dietary adjustments. Metabolic diseases have a long course, affect the functioning of systemic systems, which means the prevention and control of these diseases will be of great benefit. Glycosylation, as one of the common types of PTMs, plays an important role in the development of metabolic diseases.
The EPIC-Potsdam study established a weighted score consisting of five IgG N-glycosylation characteristic glycans of type 2 diabetes and validated the association between the model and the risk of developing type 2 diabetes.347 IgG N-glycosylation is strongly associated with the incidence of diabetes. O-GlcNAcylation regulates pancreatic β-cell survival and function and affects insulin secretion. Decreased total insulin content and impaired glucose tolerance were found in transgenic mice overexpressing OGA in pancreatic islet β-cells in young month-old mice. With increasing months of age, O-GlcNAcylation in pancreatic islets increased and β-cell function was gradually restored.353 This suggests an important effect of O-GlcNAcylation on pancreatic islet function. O-GlcNAcylation is also a nutrient-sensing response, and studies have shown that induced removal of OGT decreases O-GlcNAcylation levels of lipid droplet-associated perilipin 1, leading to enhanced visceral lipolysis.354 Targeting OGT emerges as a potential target for the treatment of obesity.
Glycosylation also plays a role in lipid metabolism. It has been found that apolipoproteins, one of the key HDL-associated proteins, contain seven mucin-type O-glycosylation sites that correlate with HDL functional capacity and are not affected by short-term diet.355 The GALNT2 gene has been identified as a plasma lipid-related locus that encodes GalNAc-T2, which catalyzes GalNAc linkage in O-glycosylation and is a regulator of high-density lipoprotein cholesterol (HDL-C) metabolism.356 GALNT2 deficiency decreases the levels of HDL-C and phospholipid transfer protein (PLTP).357 Perhaps this suggests that GALNT2 affects HDL-C levels by regulating PLTP, which provides new therapeutic directions for dyslipidemia.
Neurodegenerative diseases
Normal brain function depends on protein homeostasis and high levels of glucose. The nutrient-sensitive O-GlcNAcylation pathway senses changes in glucose levels and utilizes glucose to convert and synthesize it into UDP-GlcNAc, which is added to proteins and completes PTM of proteins.358 This has been linked to the deposition of protein aggregates in neurodegenerative diseases such as Alzheimer’s disease(AD), Parkinson’s disease(PD), and Huntington’s diseases.359
One of the characteristic changes in the brains of AD patients is that the microtubule-associated protein tau is abnormally hyperphosphorylated and aggregates into neurofibrillary tangles, and it has been found that this may be based on reduced levels of O-GlcNAcylation.360 A low glucose uptake-induced decrease in O-GlcNAcylation, which negatively regulates tau phosphorylation in a site-specific manner in vitro and in vivo, occurs in a mouse model mimicking AD, leading to excessively elevated levels of its phosphorylation.361 The OGA inhibitor Thiamet-G was also shown to block tau protein hyperphosphorylation in vivo.362 Another characteristic change in the brain of AD patients is the deposition of β-amyloid (Aβ) as neuritic plaques, and it was found that O-GlcNAcylation enzyme inhibitors could also inhibit this process. Kim et al. applied the O-GlcNAcylation enzyme inhibitor, NButGT, to AD mice, and analyzed that NButGT acted on the S708 residue of nicotinic acid proteins to reduce the activity of γ-secretase and decrease the production of Aβ.363 The above results suggest that O-GlcNAcylation may be a potential target for the treatment of AD.
However, data from different models of neurodegenerative diseases suggest that increased levels of O-GlcNAcylation can also have a negative effect on disease. For example, in a model of Caenorhabditis elegans models, the absence of OGT enzyme activity associated with increased O-GlcNAc levels attenuates proteotoxicity.364 The characteristic manifestation of PD is Lewy bodies, the formation of cytoplasmic perinuclear inclusion bodies in neurons, which are predominantly α-synuclein.365 O-GlcNAcylation may affect the degradation of α-synuclein. By inhibiting OGA enzyme activity associated with reduced O-GlcNAc levels, total α-synuclein levels are increased.366 In conclusion, O-GlcNAcylation has been associated with the progression of a variety of neurodegenerative diseases, and it is crucial to study its regulation in depth and to grasp the pathophysiological process of diseases caused by O-GlcNAcylation dysregulation.
Diagnostic and prognostic value of glycosylation in diseases
Potential diagnostic value of glycosylation as a biomarker
As mentioned above, abnormalities in glycosylation are crucial in several human diseases. Accumulating studies indicated the value of glycosylation in disease diagnosis and prognosis.367 A variety of glycoproteins have been used as tumor markers in clinical diagnoses and predictor activities, such as alpha-fetoprotein in liver cancer, prostate-specific antigen (PSA) in prostate cancer, or glycol-residue-related markers, such as CA19-9 in pancreatic cancer.241,368
Combining serum IgG galactosylation with quantitative changes in CA125 may provide a more effective differential diagnosis for ovarian cancer, with the diagnostic specificity improved from 65.2% (CA125 test alone) to 84.6%.369 The large-sample-based analysis further confirmed that the distribution of IgG galactosylation could serve as a non-invasive pan-cancer biomarker for early cancer detection and cancer screening.370 The diagnostic and prognostic value of glycosyltransferase-related genes were evaluated in several tumors, which may also provide good biomarkers for individualized medicine.244,371
In addition, several studies have indicated the prognostic value and clinical relevance of ST6Gal-I. ST6Gal-I was demonstrated to enhance HIF-1α signaling, thereby protecting ovarian and pancreatic tumor cells against hypoxic stress.372 The glycosyltransferase ST6Gal-I is upregulated in ovarian and pancreatic cancers and expressed at high levels in metastatic tumors.373 In addition, the glycosylation patterns of glycoproteins associated with circulating extracellular vesicles, as well as mucins, present potential targets for the development of detection methods. Detection of glycosylation cancer-associated mucin 1, mucin 16 and CD63 may contribute to the accurate and effective differentiation of primary breast cancer.374 Inadequate glycosylation of MUC1 leads to exposure to novel epitopes that can be used as specific targets for treatment and diagnosis.375 The subcellular localization of tumor-associated MUC1 is significantly different from that of normal cells. Alirezapour et al. synthesized 64Cu-DOTA-PR81, a tracer by combining 64Cu labeling with PR81(a high-affinity mAb to MUC1), and showed high sensitivity and specificity in tumor diagnosis.375,376
In addition to the detection of markers in serum, the specific glycoproteins contained in urine and saliva, which are non-invasive specimens, would simplify the screening and monitoring of pathologies.377,378 The combination of glycosylation detection and current diagnostic tools can better manage diseases and promote accurate diagnosis and treatment of diseases and efficacy monitoring.
Potential prognostic value of glycosylation
A variety of glycosylation-modifying proteins have been used for cancer-specific diagnosis, meanwhile, several studies have explored the prognostic value of GTs expression levels in cancer. Recently, a prognostic signature in HCC based on six GTs was established, which showed a good performance in predicting OS.379 Seventeen glycosyltransferases, which play a role in the initial stages of N- or O-glycosylation, as well as glycolipid biosynthesis and sialylation, were unequivocally identified as being associated with a poor prognosis in malignancy. Among them, ALG3, GALNT2, B4GALNT1, POFUT1, B4GALT5, B3GNT5 and ST3GAL2 as the most consistently malignancy-associated enzymes, compared to the published experimental data points.380
In addition to glycosyltransferases, characteristic glycosylation of IgG can serve as a prognostic biomarker. Theodoratou et al. demonstrated that IgG with reduced galactosylation, reduced levels of sialylation, and increased bifurcated GlcNAc was strongly correlated with all-cause and colorectal cancer mortality, with a good predictive effect.381 FUTs are also considered as an essential biomarker for the detection and prediction of several types of cancer, because altered protein fucosylation is a characteristic of cancer. Overexpression of FUT3 is linked to reduced overall survival in TNBC patients.382 FUT8 was significantly associated with poor survival in non-small cell lung cancer.383
In addition, the expression of glycosylation genes may be associated with cancer prognosis.384 Zhao et al. identified three subtypes of glycosylation-regulated genes that are highly compatible with the immunophenotypes of HCC and further constructed glycosylation-related model scores.385 Another study, also for HCC, used bioinformatics analysis to construct a five-gene signature that predicted the prognosis of patients with HCC and delineated risk stratification.386 In lung adenocarcinoma, a risk model based on differentially expressed GTs-related genes was conducted, which could predict the survival rate of patients.387 Both the use of GTs as effective prognostic markers for tumors and the construction of a genetic prognostic model for glycosylation exemplify the potential of glycosylation in assessing cancer prognosis. It is important to explore the potential of glycosylated proteins as biomarkers for human diseases (Table 1).
Table 1.
Diagnostic and prognostic biomarkers related to glycosylation
| Biomarker | Cancer type | Glycosylation | References |
|---|---|---|---|
| AFP | liver cancer | Core fucosylated biantennary or hybrid glycans or bisecting glycans↑ | 486 |
| CA125(MUC16) | ovarian cancer | Core-fucosylated biantennary monosialylated glycans↑ | 487,488 |
| CA15-3(MUC1) | breast cancer, ovarian cancer | Truncated O-glycans↑ | 489–491 |
| CA19-9 | gastric cancer, pancreatic cancer, colorectal cancer, ovarian cancer | Sialylation↑ | 492–495 |
| CEA | breast cancer, colorectal cancer, pancreatic cancer | Highly heterogeneous in sialylation and fucosylation | 496–499 |
| CP | liver cancer, pancreatic cancer | Fucosylation↑ | 500,501 |
| hAGP | pancreatic cancer | Core-fucosylation↑ | 502 |
| Hp | liver cancer, ovarian cancer | Fucosylation↑ | 252,503,504 |
| IgG | liver cancer, gastric cancer, lung cancer, colorectal cancer |
Galactosylation ↓ , sialic acids and more complex highly branched N-glycan ↑ ; α-2,6-sialylated fucosylated N-glycans↑ |
505–508 |
| PSA | prostatic cancer | Core 2 O-sLe(x)↑, Neu5Ac α2-6 sialylated glycans ↑ , galactosylated glycans ↑ , N-acetylgalactosaminylated glycans↑ | 449,509 |
| RNase 1 | pancreatic cancer | Core-fucosylation↑ | 510 |
| PON1 | liver cancer, head and neck cancer | Fucosylation↑ | 511,512 |
| sLeX | lung cancer, breast cancer | Sialylation ↑ , Core-fucosylation↓ | 513–515 |
| STn | gastric cancer, ovarian cancer | Truncated O-glycans↑ | 491,516 |
| A1AT | liver cancer, lung cancer | Core-fucosylation↓ | 517,518 |
AFP alpha-fetoprotein, CA125 carbohydrate antigen 125, MUC16 mucin 16, CA15-3 carbohydrate antigen 153, CA19-9 carbohydrate antigen19-9, CEA carcinoembryonic antigen, PSA prostate-specific antigen, RNase 1 ribonuclease 1, CP ceruloplasmin, hAGP human α1-acid-glycoprotein, HP haptoglobin, IgG immunoglobulin G, PON1 serum paraoxonase 1, sLeX sialyl-Lewisx, STn sialyl-Tn, A1AT α1-Antitrypsin
Targeting glycosylation for the treatment of diseases
Glycan-based therapies are approaches with great potential in the treatment of cancer, including protein glycosylation inhibitors, small molecule inhibitors of glycosyltransferases, glycosylation-modified mAbs, glycosylation-mediated targeted drug delivery, and vaccine design (Table 2). The choice between glycosylation-related targeted treatments and other targeted treatments depends on the specific molecular characteristics of the tumor, the general health status of patients, and the potential benefits and risks.
Table 2.
Strategies targeting glycosylation in clinical trials
| Approach | Target | Drug candidate | Cancers | Phase | Identifier |
|---|---|---|---|---|---|
| Inhibitors of glycosylation | Galectin-1 | OTX008 | solid tumors | I | NCT01724320 |
| Galectin-3 | GB1211, GCS-100 | metastatic melanoma, head and neck squamous cell carcinoma, non-small cell lung cancer, diffuse large B-cell lymphoma, chronic lymphocytic leukemia | I/II | ||
| Galectin-1 and -3 | GR-MD-02, GM-CT-01 | melanoma, non-small cell lung cancer, squamous cell carcinoma of the head and neck, colorectal cancer, cancer of the bile duct, gallbladder cancer | I/II | ||
| Selectins | GMl-1271 | acute myeloid leukemia, multiple myeloma | I/II/III | ||
| Fucosylation | SGN-2FF | non-small cell lung cancer, renal cell carcinoma, breast neoplasms, urinary bladder neoplasm | I | NCT02952989 | |
| Glycan-based antibodies | Gangliosides (e.g., GD2, GD3, GM2, GM3 and fucosyl- GM1) | hu3F8, ch14.18, hu14.18- IL2, PF-06688992, KW- 2871, BMS-986012 | neuroblastoma, lung cancer, osteosarcoma, melanoma, brain and central nervous system tumors, ovarian cancer, retinoblastoma, sarcoma, small intestine cancer, melanomas, other solid tumors | I/II/III | |
| Globo H | OBI-888, OBI-999 | solid tumors | I/II | NCT03573544, NCT04084366 | |
| Truncated O-glycan (e.g., Tn, and STn) | PankoMab-GEX™ | ovarian epithelial cancer, fallopian tube cancer, primary peritoneal cancer, breast cancer | I/II/III | ||
| Lewis antigens (e.g., LeA, and LeY) |
MVT-5873 hu3S193 |
pancreatic cancer, ovarian cancer, small cell lung cancer | I/II | ||
| MUC-1 |
TAB004, SAR566658, MUC1 + CD3 BsAb |
breast cancer | I/II | ||
| Glycan-based vaccines | Glycolipids (e.g., GD2, GD3, GM2, GM3, and α-galactose containing) |
bivalent vaccine, GM2-KLH vaccine, BEC2 Vaccine, BCG vaccine |
sarcoma, neuroblastoma, small cell lung cancer, breast cancer, fallopian tubes, ovarian cancer, peritoneal cancer, metastatic melanoma | I/II/III | |
| Globo H | OBI-822 | breast cancer | II/III | NCT03562637 | |
| Truncated O-glycan (e.g., T, Tn and STn) |
MAG-Tn3 + AS15, THERATOPE STn-KLH vaccine |
ovarian cancer, peritoneal cancer, breast cancer, prostate cancer | I/III | ||
| Lewis antigens |
sialyl Lewis A-KLH + QS21 |
breast cancer | Not Applicable | NCT00470574 | |
| Polysialic acid | Polysialic acid-KLH + QS21 | small cell lung cancer | II | NCT00004249 | |
| MUC-1 | YB-01 | breast cancer, other solid tumors | I/II | ||
| Polyvalent vaccines |
(Bivalent vaccine+ OPT-821), (Trivalent vaccine- KLH conjugated + OPT-821), (Globo-H-GM2-STn-TF-Tn-KLH-QS21), (GD2L, GD3L, Globo H, fucosyl- GM1, and N- propionylated polysialic acid -KLH + OPT-821) |
neuroblastoma, sarcoma, ovarian cancer, breast cancer, small cell lung cancer | I/II | ||
| CAR-T cells against aberrant glycosylation | Gangliosides (e.g., GD2) |
GD2Bi-aATC, 4SCAR-GD2, 1RG-CART, GD2-CART01, GINAKIT |
neuroblastoma, sarcoma | I/II | |
| Lewis antigen (e.g., LeY) | LeY-CAR-T | lung cancer, fallopian tube cancer, other solid tumors | I/II | NCT03198052, NCT03851146 | |
| MUC-1/MUC1-Tn |
MUC-1 CART huMNC2-CAR44 |
intrahepatic cholangiocarcinoma, breast cancer, lung cancer, ovarian cancer, fallopian tube cancer, multiple myeloma, pancreatic ductal adenocarcinoma | I/II |
MUC-1 mucin 1, CAR chimeric antigen receptor, STn sialyl-Tn
Inhibitors of protein glycosylation
Inhibitors of the glycosylation mechanism have been proved to be efficacy in cancer treatment. Among them, most are small-molecule proteins that are readily taken up by cells, thus providing an opportunity for researchers to design drugs.388 A variety of protein glycosylation inhibitors are currently under investigation or have been put into clinical use as effective strategies to block cancer progression. Per-O-acetylated GlcNAcbeta1,3Galbeta-O-naphthalenemethanol inhibits sLeX in tumor cells and inhibits hematogenous dissemination of mouse lung cancer cells.389
Increased aerobic glycolysis characterizes cancer cells, and 2DG, as a glycolysis inhibitor, became an effective strategy to cause cancer cell death, yet not through direct inhibition of glycolytic processes. 2DG treatment of lymphocytic choriomeningitis virus resulted in a dramatic reduction of pathogenic particles, which was further demonstrated to be done by inhibiting the N-glycosylation of viral glycoproteins.390 2DG impairs the N-glycosylation of the key oncogenic receptors Axl and Met in SCC15 cells, an oral squamous cell carcinoma cell line, decreasing cell viability and colony-forming ability.391 Thus, 2DG could be used with other drugs to synergistically treat the disease. In addition, 2DG in combination with metformin inhibits protein N-glycosylation and induces an unfolded protein response, which combined with adequate energy stress results in mitochondrial enlargement and improved anti-tumor immunity.392
Overexpression of mucin glycans is an important mechanism for cancer development, and inhibitors targeting mucin glycans would be very promising therapeutic directions.393 As an example, MUC1, which is currently the most well-characterized, expresses mainly O-glycosylation and N-glycosylation.394 Knockdown of O-glycosylation of MUC1 significantly restored chemosensitivity in wild-type WT breast cancer cells, whereas insertion of the O-glycosylation site increased drug resistance.395 MUC1-based antibodies, radiopharmaceuticals, vaccines and chimeric antigen receptor (CAR)-T cell therapy are continuously being developed. A mAb (TAB004) specifically targeting human MUC1 was developed, which binds to MUC1 to induce ER stress and anaerobic responses in PAAD cells and inhibit cell proliferation.396 Salouti et al. generated 99mTc-HYNIC-PR81 by radiolabeling PR81, an antibody specific for MUC1, with 99mTc, which improved the stability and immunoreactivity of PR81.397 DNA vaccine (pcDNA3.1-VNTR) controls tumor growth and extends survival time in mice by enhancing MUC1-induced CTL activity.398 Novel CAR-T cells targeting MUC1-Tn inhibit the growth of MUC1-Tn-positive cancer cells in leukemia and PAAD mouse models.399 In conclusion, targeted therapies based on inhibition of MUC1 glycosylation are promising, but further in-depth studies are needed to obtain more robust therapeutic responses.
Small molecule inhibitors of enzymes
Glycosidases and GTs are key enzymes in the glycosylation process, and their expression levels regulate the function, properties, stability, and localization of glycosylated proteins. Several studies of small molecule inhibitors targeting these enzymes have been conducted, which provide novel strategies for cancer treatment.400 Shi’s team found that knocking down the MAN2A1 gene, which encodes the N-glycan maturation enzyme, resulted in the deletion of MAN2a1 in cancer cells, but increased the infiltration of tumor cytotoxic T-cells under anti-PD-L1 treatment.401 It further demonstrated the utility of N-glycosylation in combination therapy with immune checkpoint inhibitors. Pancreatic cancer ductal cells, referred to as Panc1 cells, express core 2 N-acetylglucosaminyltransferase-M. When Panc1 cells are subjected to heat shock, the non-muscle myosin IIA-C2GnT-M complex is increased, as is the polyubiquitination and proteasomal degradation of C2GnT-M, which induces Golgi disruption.402
Targeting the enzymes that play a role in angiogenesis and regulating the glycosylation process to achieve anti-angiogenesis, is also a new direction of glycosylation in cancer therapy. The α-glucosidase I inhibitor castanospermine converts high-mannose-type N-glycosylation to complex oligosaccharides, alters endothelial cell proliferation and attachment capacity, prevents angiogenesis, and inhibits tumor growth.403 The enzymatic activity of GnTs catalyzes the N-glycosylation pattern in proteins, thereby regulating the glycosylation status of key angiogenic factors such as VEGFR2 and Notch.404
Glycosylation of mAbs
mAbs are biological agents that are widely used in various diseases and are highly regarded for their specificity, sensitivity and relatively low cross-reactivity. The structure and stability of glycosylation are crucial for the function of mAbs. Altering the Fc glycosylation structure enhances the antibody’s ability to attract innate immune responses.405 N-glycosylation is the most common form of glycosylation in mAb therapeutics.406 The CH2 domain of deglycosylated antibodies is less thermally stable, more susceptible to loss of higher structures in space and more susceptible to papain.407 Kayser’s team found that glycosylated mAbs are less stable and appear to improve solubility and stability, which may be important for long-term storage.408
The addition of 2-fluoroperacetylated fucose, an inhibitor of FUT, to cell cultures or transfection of EG2-hFc-producing cells with the prokaryotic GDP- 6-deoxy-D-lyxo-4-hexanone glucose reductase (RMD) gene resulted in a reduction in the level of fucosylation of the antibody glycan in CHO cells in both methods.409 This alteration enhances antibody-dependent cytotoxicity, Fcγ receptor binding, and affects antibody effector function. Glycosylation in mAbs and common antigens require further research to control these components in vivo and to selectively utilize glycosylation targeting. Moreover, deletion of the intramembrane protease signaling peptide peptidase-like 3, which resides within the Golgi, leads to hyperglycosylation of CD19, an alteration that directly inhibits effector function and suppresses antitumor cytotoxicity in CAR-T cells.410 Dysregulation of this PTM of glycosylation of the leukocyte differentiation antigen CD19 might be one of the mechanisms of antigen escape from CAR T cell therapy.
Glycosylation-mediated targeted drug delivery
Glycosylated structures are an important part of the tumor cell membrane and selectively bind to the cell. Molecules such as mannose, galactose, and glucose are generally used to bind to the delivery system and are non-toxic, non-immunogenic, and have good histocompatibility.411 Therefore, targeted drug delivery systems based on glycosylated structures could control drug distribution, maximize therapeutic efficacy, and minimize unfavorable drug behavior.412 The lectin receptor is one of the targets of glycans, which is important in the immune system. For example, it establishes the first line of defense against microorganisms, recognizes self and non-self, and regulates inflammatory response processes.413 The lectin receptor and glucose transporter subtype I (GLUT1) are widely studied. Drug delivery systems and glycosidic ligands can form conjugates that selectively concentrate drugs at lesion sites. Lectin receptors exert their targeting properties through covalent binding to mannose and galactose.414 GLUT1-specific glycans bind to drug carriers to cross the blood-brain barrier for tumor-targeted drug delivery.415
Nanoparticles receiving galactosylation or mannosylation modifications loaded with the tumor-specific antigen SIINFEKL peptide (OVA) rapidly enhanced antigen uptake and dendritic cell maturation, and were able to promote the toxic effects of CD8+ tumor-infiltrating lymphocytes on lymphoma cells.416 A tobacco mosaic virus-based drug delivery system with a glycan structure (mannose or lactosylation modification) as the targeting ligand, loaded with the anticancer drug cisplatin, exhibited robust endocytosis and apoptosis in cell lines.417 The galactose-installed photo-crosslinked pH-sensitive degradable micelles system loaded with paclitaxel indexed significant tumor cell death with less damage to the liver and kidney.418 Targeting up-regulated glycan transporters in glioblastoma using glucose, mannose or galactose molecules as ligands conjugated to hydroxyl-terminated polyamidoamine dendrimers significantly improves targeting, specificity and aggregation kinetics of tumor macrophages in glioblastoma.419 Glycosylation-modified polymers are effective nanocarriers for loading anticancer drugs and have important cancer intervention implications. However glycosylated modified proteins may have multiple problems at the same time. For example, multiple recognition problems affect targeting efficiency. Conjugation strategies based on glycosylation may alter the characteristics of the delivery vehicle, such as surface charge or size. It may also produce off-target effects affecting other organs, etc., all of which require further in-depth studies.420,421
Novel vaccines based on glycosylation
Vaccines prevent various diseases by inducing humoral and/or cellular immunity, with carbohydrates on cell surfaces playing a key role in both innate and adaptive immunity, making them attractive antigens for vaccine development. Covalent linking of carbohydrates to immunogenic proteins enhances their immunogenicity.422 A study integrated the prostate cancer cell antigen carbohydrate molecule globo H hexasaccharide with keyhole grouper blood blue protein, together with the immune adjuvant QS-21, to patients who have recurred from radiation or surgery.423 Synthesis of two- or three-component antitumor vaccines with glycopeptides of the tumor-associated mucin MUC1, T-cell epitope peptides, and Pam(3)CSK(4) lipopeptides also produced a strong immune response that induced the killing of tumor cells.424 Despite numerous glycosylation-based vaccines reaching clinical trials, they predominantly remain in vitro, and none have yet met clinical application criteria.425 Identifying optimal immune components and assembling effective vaccines to prevent or halt disease progression remains a significant challenge
Glycoconjugate drugs
Despite the remarkable efficacy of most first-line anticancer drugs, the prevalence of “off-target” drug effects has led to the occurrence of serious adverse events such as alopecia, nausea, and vomiting, which have a significant influence on the patient’s quality of life and disease prognosis. To achieve better clinical efficacy of chemotherapeutic drugs, glycan conjugation methods of drugs have been figured out. Covalent conjugation of glycan structures to drugs improves drug compatibility, reduces toxicity, and minimizes adverse effects. In 1995, the first glycoconjugate drug, glufosfamide, was reported to be better tolerated, less toxic, and more efficacious, and entered clinical trials.426 Chloramphenicol with the addition of glycosylated structures such as N-alkoxyamine- and N-acylhydrazine- exhibits a broader tumor specificity, as well as greater potency.427 Mikuni et al. found that the in vivo potency of galactose-conjugated docetaxel in a mouse leukemia tumor model was superior to paclitaxel in terms of antitumor activity.428 The addition of polysaccharides to common drugs also enhances the drug’s ability for action. The antacid-alginate combination (under the trade name Gaviscon) is a drug that effectively inhibits gastroesophageal reflux disease by forming a gel that floats on top of the stomach contents. Conjugation with a glycan base greatly reduces the adhesion of Helicobacter pylori to the gastric epithelial cells and improves drug action.429 Glycoconjugated drugs often improve the water solubility and stability of the drug and may enable selective targeting of tumor cells.430
How to design specificity for targeting glycosylated drugs
To deliver therapeutic drugs to cancer cells more effectively while minimizing toxicity and off-target effects of the drugs on normal cells, maximizing the eradication of cancer cells, and improving the specificity of targeted drugs, dual or multiple targeting strategies can be adopted. Systemic administration using dual-targeted heterodimers significantly improves DNA sensing, DC cross-presentation, and anti-tumor T-cell responses, and tumor cell-specific dual-targeting enables better tumor control.431 Dual/multi-targeted drugs are favorable to solve the problems of limited efficacy, poor safety and drug resistance that a single target has. Based on a full understanding of the structural biology of glycosylation, rational use of bioinformatics tools and proteomic information provides the possibility of designing highly effective and safe multi-targeted anticancer drugs.432
Novel detection methods for glycosylation
Glycosylation has emerged as a variety of assays over the course of several years of research, which are briefly summarized below.
Liquid chromatography and mass spectrometry
As mentioned earlier, biomarkers of glycosylation are of great value in tumor diagnosis and prognosis, and thus glycosylation analysis is a critical step. However, this is challenging because of the need to identify both glycosylation sites and glycan structures.433 Liquid chromatography (LC)is the most widely used technique in the separation of glycopeptides and released glycans.434,435 A study has revealed the high performance of three hydrophilic interaction liquid chromatography (HILIC) columns for the separation of human IgG glycopeptides, as well as the successful separation of the isomeric A2G1F1 glycopeptide of IgG and the structural potential of the isomeric glycopeptide.436 Hernandez-Hernandez’s group used HILIC with reversed-phase liquid chromatography to isolate the O-glycopeptide structure of bovine casein myopeptide.437 Although LC is the most commonly used separation technique for glycoproteomics analysis, it has its limitations. For example, the use of multiple solvents as mobile phases results in higher analytical costs, complicated operation and environmental pollution.
Mass spectrometry (MS) is a method of ionizing molecules in a sample and separating the ions in a magnetic or electric field. It has become a powerful tool in the analysis of saccharides and glycomacroproteomics.438 A high-throughput matrix-assisted laser desorption ionization mass spectrometry (MALDI-MS) method based on microarray technology for the successful detection of N-glycosylated peptides of IgG1 produced during in vitro cell culture.439 Some studies have used MS to detect glycosylation of serum glycoprotein transferrin and used N-glycosylation levels to determine subtypes of congenital disorders of glycosylation.440 Kotsias’ research group detected multiple O-glycosylation compositions of in vitro cancer cell lines using ultra-high resolution MALDI-FT-ICR MS, enabling semi-automated, high-throughput glycan identification and quantitative analysis.441 Some studies have used porous graphitized carbon chromatography coupled to tandem mass spectrometry to analyze the epitopes of N- and O-glycosylation in AML and to identify glycan profiles.90
MS ionization is inefficient and MS alone does not always provide complete structural and quantitative information, and needs to be combined with other techniques. For example, the separation step prior to MS, which mainly consists of LC and capillary electrophoresis methods, can more reliably identify and quantify glycans and glycopeptides. In a study using LC-MS/MS for glycosylation analysis, increased expression levels of high mannose and α2,6-linked glycosylated complex N-glycans were observed in colorectal cancer cell lines and tissues.442 Determination of glycosylation in glycan-conjugated vaccines using solid-phase extraction coupled with LC was performed, and tandem mass spectrometry detected 14 and 12 glycosylation sites in the CRM197 vaccine and tetanus toxin-based vaccine, respectively, and was achieved with high sensitivity and a wide linear dynamic range.443 Naumann’s group performed high-throughput glycosylation analysis of intact mAbs based on LC and MS in combination with capillary electrophoresis, and efficiently determined the five major glycosylation patterns of the tested antibodies.444 In addition to this, MS is capable of combining various techniques, such as fluorimetry, electrospray ionization, and isotope-specific labeling, for qualitative and quantitative studies of glycosylation and in-depth characterization.445–447
Lectin-based techniques
In addition to the traditional approach of using LC to isolate and purify glycoproteins and then MS to identify glycans, a variety of lectin-based assays based on the interaction of glycans and glycan-binding proteins have been developed. For example, lectin microarrays, lectin chromatography and lectin immunosorbent assay are used to detect glycosylation patterns.448,449 The development of high-density lectin microarrays is critical for identifying lectins specific to disease-related protein glycosylation.450,451 Lectin microarrays enable rapid and simple analysis of glycosylation to determine the most appropriate lectin to enrich for glycoproteins.452 Although lectin microarrays provide data on lectin-glycoprotein interactions, they are unable to account for changes in glycosylation patterns of individual proteins or of glycoproteins as a whole and need to be combined with other techniques.453 Patwa et al. combined lectin microarrays with all-liquid-phase enrichment and pre-fractionation methods to discover differences in glycosylation between pancreatic cancer and normative human sera, particularly with respect to mannosylation and fucosylation.454 Early lectin detection techniques, such as tandem lectin affinity chromatography, did not meet the criteria for glycosylation analysis. Lectin microarrays and lectin immunosorbent assay, which analyze the structure of glycans in a comprehensive, high-throughput manner, are not only simple to perform, but also offer significant advantages in terms of sensitivity and reproducibility.455
Electrophoresis
Conventional sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was unable to meet the criteria for separating glycosylation patterns, and more advanced techniques were developed. Previous studies have confirmed that two-dimensional gel electrophoresis. Which could analyze peptide mixtures, can be used for the analysis of glycosylated peptides.456 Polyacrylamide Gel Electrophoresis is a new technique introduced based on gel electrophoresis method for detecting the levels and dynamics of O-GlcNAc. Polyacrylamide Gel Electrophoresis can separate native O-GlcNAc proteins from their unmodified counterparts, does not require complex sample handling and advanced equipment beyond the electrophoresis apparatus, and has a high degree of specificity and affinity, making it particularly suitable for O-GlcNAc analysis.457 In addition, Liu et al. developed a new technique of supported molecular matrix electrophoresis to detect glycosylation patterns of Fut8+/+ and Fut8-/- mouse serum proteins, and further analyzed the N-glycosylation and O-glycosylation patterns in the isolated γ-bands by mass spectrometry. Supported molecular matrix electrophoresis is more economical in the amount of protein samples compared to SDS-PAGE, and the serum fractionation is simpler and faster, and the compatibility of glycoproteins with the separated bands.458
Capillary gel electrophoresis (CGE) has been used in the past in conjunction with LC and MS as a method for the structural separation of oligosaccharides because of its high performance in separation, resolution, and sample handling.459 Combined with laser-induced fluorescence decolorization, the CGE-based method has become a highly efficient analytical technique for the characterization of glycosylation patterns, with high performance in terms of low cost of separation, robustness, speed of analysis, and sensitivity.460 Multiplexed CGE using laser-induced fluorescence detection was able to monitor the structural properties of N-glycosylated glycans.461 It is of great value in the ability to identify small differences in glycosylation composition and structure.462
Radiolabelling
While MS is able to identify the glycopeptide composition and glycan structure, it cannot obtain the accurate stereo-structure of glycans, and at the same time, it cannot distinguish the types of glycan residues with the same molecular weight.463 Schubert’s team proposed nuclear magnetic resonance (NMR) spectroscopy experiments to analyze glycosylation patterns.464 Structural changes in the O-glycosylation of calcitonin derivatives have been reported using NMR analysis, which revealed localized residue lengthening at the O-glycosylation site, thus disturbing the original α-helical structure of calcitonin.465 NMR allows the detection of glycan stereochemistry, composition and type of linkage bonds and quality control of the produced proteins, regardless of molecular size and without isotopic labeling.466 It has the disadvantage of not being able to identify modification sites in the protein sequence.
On the other hand, Raman imaging was conducted to monitor the glycosylation metabolism in brain and breast cancers. The researchers identified Raman markers based on the vibrational characteristics of glycogen, glycans, etc., and combined them with chemometrics to distinguish protein glycosylation patterns in different tissues.467 The structure of the secreted polymeric gel MUC5B was analyzed by Raman imaging and found to be rich in O-glycosylation.468 Raman imaging is a powerful visualization method that is sensitive and specific, does not require staining, and has the spatial and spectral resolution to provide important information to help analyze the multiple components present in a cell and map the distribution of glycans in tissues. The disadvantage of Raman imaging is the inability to quantify glycosylation levels.
Molecular fluorescence
Fluorescence detection has been widely used in the identification of glycosylation structures because of its advantages of high sensitivity and direct observation. A series of fluorescent boron-dipyrromethene dyes can be incorporated and labeled with glycan groups during glycosylation structure synthesis, while relying on their chemical stability not to react with the glycan molecules. Meanwhile boron-dipyrromethene labeled glycans have high fluorescence efficiency in water.469 Zaro’s group has visualized the mucin-type O-glycosylation using in-gel fluorescence detection.470 However, there is also the problem that some fluorescent dyes are not sensitive to glycosylation patterns. Recently, imaging in combination with new techniques has been continuously applied. Haga et al. used azide-tagged GFP-labeled proteins to image cell-surface glycoproteins by Förster resonance energy transfer fluorescence microscopy.471 However, limited by the GFP protein, this method is unable to image endogenous glycoproteins. Belardi’s group has built on this foundation and achieved imaging of glycosylated forms of endogenous proteins using azidoglycan labeling and two-photon fluorescence lifetime imaging microscopy.472
Since most protein molecules are similar in size to nanomaterials, nanomaterials can be used as a backbone for the assembly of glycosylated probes, and glycosylation-modified fluorescent probes have been created.473 Zhang’s team designed nano-based metal-organic framework fluorescent probes with Zr (IV) and boric acid as active centers.474 Specific glycosylation sites were identified and in situ fluorescence imaging was performed by structural modification of metal-organic framework and alizarin red modulated fluorescence. Together, fluorescent labeling is hopefully to be widely promoted in glycosylation pattern identification due to its unique advantages of direct visualization and simplified operation procedures.
Nanotechnology and other detection technologies
In addition to the common assays, there is a proliferation of novel techniques for the detection of glycosylation (Table 3). Maglia’s research group devised the nanopore method, which takes advantage of high ionic strength, and low pH environments and uses nanopores containing phenylalanine for identifying hydrophilic peptides and distinguishing between mono- and di-glycosylated peptides. The method enables qualitative detection and quantitative quantification of protein glycosylation.475
Table 3.
Novel detection methods for glycosylation
| Methods | Principle | Advantages | Limitations | Reference | |
|---|---|---|---|---|---|
| Separation enrichment affinity technique | LAC | Isolation and enrichment of intact glycopeptides is achieved by selective recognition of monosaccharides or glycans with specific structures by lectins immobilized on agarose or magnetic beads. |
Widely used for the enrichment of specific N- and O-glycoproteins and glycopeptides. Multilectin affinity chromatography allows for the simultaneous enrichment of more diverse protein glycosylation by mixing multiple lectins. |
Lectin specificity needs to be determined and multiple binding produces inertia. | 519–523 |
| Hydrazine chemical enrichment |
Trapping of glycopeptides by formation of covalent hydrazone bonds between the aldehyde group generated by oxidation of the glycan chain and the amino group on the hydrazide resin. Removal of the N-glycosyl chain by the PNGase F enzyme or chemical methods. |
Simplified protein glycosylation and effective localization of N-glycosylation sites at the peptide level. | Complicated experimental procedures, especially the loss of glycan chain information. | 524 | |
| HILIC | A broad-spectrum enrichment method for the separation and enrichment of intact glycopeptides by the difference in polarity between glycosylated and non-glycosylated peptides. |
Preserves the structure of intact glycopeptides. Enriches all types of intact glycopeptides without bias. Suitable for analyzing compounds in complex systems. Does not require expensive ion-pairing reagents and can be easily coupled with MS. |
Co-expression of glycopeptides and non-glycopeptides leads to lower enrichment specificity. | 525–527 | |
| MAC | Glycosylated peptides are negatively charged and hydrophilic, which are selectively enriched by electrostatic interactions with metals. |
High stability and low non-specific binding. Wide range of pH values. Multiple metal ions can be tested on the same resin to determine which metal ion is best suited for purifying the target protein. |
Cannot be used on proteins that do not have a metal affinity. Some amino acids are susceptible to metal-catalyzed oxidation reactions leading to protein damage. |
528 | |
| Boric acid chemical method | Enrichment of intact glycopeptides using boric acid to form reversible covalent bonds with monosaccharides. | Captures and releases intact glycopeptides without affecting the structure of the glycan chain. | Different affinities between boric acid and different glycan chains. | 529–531 | |
| Methods for glycoprotein identification/glycosylation site determination | Enzymatic method for PNGase F | Catalyze the non-lysosomal hydrolysis of an N(4)-(acetyl-β-d-glucosaminyl) asparagine residue into N-acetyl-β-d-glucosaminyl-amine and a peptide containing an aspartate residue. |
Simplifies analysis of N-glycosylation. Good reusability and thermal stability. |
Incomplete deglycosylation may lead to biased results. | 532,533 |
| NMR | Caused by the spin motion of the nucleus. |
Binding affinity and epitope information can be obtained. Analyze the structure, conformation and dynamics of carbohydrates and their interactions with biomolecular receptors such as proteins. Regardless of molecular size and without isotopic labeling. |
Severe overlap of signal peaks, difficult resolution and low sensitivity. Multidimensional NMR requires milligram samples. |
466,534,535 | |
| Raman imaging | Utilizes spectral and spatial information to generate images. |
A powerful visualization method with high sensitivity and specificity, without staining, with spatial and spectral resolution. Ability to record vibrational spectra in multiple regions to map the spatial distribution of glycans in human tissues. |
Unable to quantify glycosylation levels. Detection of low abundance molecules requires next generation vibrating labels with higher sensitivity |
467,536 | |
| Other detection technologies | Glycosylation-related gene testing | Using molecular biology and informatics techniques, the gene sequence of a glycan residue or glycosyltransferase is analyzed to derive a differential expression profile. | Suitable for the study of the specific structure of the lack of glycan, simple operation. | Technical and instrumental requirements. | 537 |
LAC lectin affinity chromatography, HILIC hydrophilic interaction liquid chromatography, MS mass spectrometry, MAC metal affinity chromatography, NMR nuclear magnetic resonance
In addition to the methods mentioned above, there are also several new platforms being established. Some researchers have developed a software tool called GlypID 2.0, which incorporates CID and HCD information to achieve accurate characterization of N-glycosylation sites.476 A simplified GlycoSense method utilizes carbohydrate sensing microspheres assembled into a multi-suspension array to achieve the goal of simultaneous detection of multiple orthogonal glycosylation profiles simultaneously using common instrumentation.477 Blöchl et al. integrated glycomics with transcriptomics from public databases to unearth distinct glycochemical profiles based on FAB system classification.478 An anti-Tn antibody microarray platform was recently established for the detection of Tn+ glycoproteins in detecting sub-nanogram levels, which are almost universally present in cancer patients.479
Conclusions and future perspectives
Glycosylation is an extremely complex and the most abundant type of PTM, which not only greatly contributes to the expansion of protein diversity, but also has far-reaching effects on protein functions. Glycosylation in the form of multiple glycan residues brings considerable changes to the proteome, and the myriad forms of polysaccharides or oligosaccharides linked together are a source of stereostructural diversity in proteins. Glycosylation is closely related to protein solubility, stability, and activity. For example, in the ER, the composition of glycans in the chaperone proteins calnexin and calreticulin tracks the folding state of the nascent polypeptide. N-glycosylation is localized on the side of the conserved structural domains of hepcidin, which regulates calcineurin secretion and promotes hepcidin maturation. If N-glycosylation occurs elsewhere in hepcidin, it misfolds and accumulates with calnexin over time.480
Abnormal or disturbed glycosylation cause human diseases. Congenital disorders of glycosylation are genetic disorders marked by hypoglycosylation of proteins and lipids, were first described in 1980 and are characterized by mutations in the gene encoding phosphomannanase.481,482 In addition, altered protein glycosylation is considered a hallmark of cancer. Glycosyltransferase GALNT7 alters O-glycosylation in prostate cancer cells and facilitates tumor growth.28 Unraveling glycan-based mechanisms in cancer can help decipher the molecular mechanisms of cancer biology.
New approaches are needed for early diagnosis, risk prediction and treatment of cancer, and glycans can be used to develop new non-invasive biomarkers. Some of the most commonly used serum biomarkers in clinical practice for cancer diagnosis and monitoring prognosis are glycoproteins. These include PSA, a major biomarker widely used in prostate cancer patients, CA125 in ovarian cancer patients, CEA in colon cancer patients, and CA199 in pancreatic cancer patients.368,483,484 Unfortunately, these glycoproteins are not exclusively found in one cancer type or not exclusively in one stage of the tumor. Finding the most specific and sensitive assay for detecting cancer then becomes a topic for research. For example, serum PSA testing for prostate disease has been around for a long time, but has led to problems of overdiagnosis and treatment. Researchers have studied the glycosylation patterns of PSA and found that a significant increase in core fucosylation with sialylation is more representative of prostate cancer.485
Combined protein binding and glycosylation pattern detection may provide better diagnostic and prognostic performance, but how one can accurately detects alterations in glycosylation sites and expression levels in clinic settings has become a major challenge. Advancement of detection methods for glycosylation patterns has been constantly pursued, from the initial MS and electrophoretic analysis to visualization imaging such as fluorescence and NMR, to the current gene detection technology and sophisticated data analysis platform. Increasingly, the latest advances in glycomics, proteomics, genomics, transcriptomics, and metabolomics data gathering and analysis are being used to better, understand how glycosylation regulates protein biological functions, and how new therapies can be developed by targeting glycosylation. Whether they are small molecule inhibitors targeting enzymes in the glycosylation process, or glycosylation-modified mAbs and novel vaccines, these modalities show the great potential to target glycosylation for therapy development. In conclusion, glycosylation serves as molecular “switches” to provide proteins or lipids with more diverse functions and play crucial roles in various aspects of life. Targeted therapies at glycosylation, either alone or in combination with more conventional therapeutics, have the potential to provide cancer patients and patients with diseases with better treatment options.
Acknowledgements
This study was supported by National Natural Science Foundation (No.82270200, No.82170189, No.82070203, No.81800194, No.81770210); Key Research and Development Program of Shandong Province (No.2018CXGC1213); Taishan Scholars Program of Shandong Province (No. tspd20230610); China Postdoctoral Science Foundation (No. 2021T1404223); Translational Research Grant of NCRCH (No.2021WWB02, No.2020ZKMB01); Shandong Provincial Natural Science Foundation (ZR2021YQ51); Shandong Provincial Engineering Research Center of Lymphoma; Academic Promotion Program of Shandong First Medical University (No. 2019QL018).
Author contributions
X.W. and X.X.Z. designed the review and revised the manuscript. M.Y.H. and X.X.Z. did literature search and wrote the manuscript and drafted figures and tables. X.W. and X.X.Z. revised the tables. All authors have read and approved the review article.
Competing interests
The authors declare no competing interests.
Footnotes
Contributor Information Mengyuan He, Email: 202235893@mail.sdu.edu.cn. Xiangxiang Zhou, Email: xiangxiangzhou@sdu.edu.cn. Xin Wang, Email: xinw007@126.com.
Contributor Information
Xiangxiang Zhou, Email: xiangxiangzhou@sdu.edu.cn.
Xin Wang, Email: xinw007@126.com.
References
- 1.Walsh, C. T., Garneau-Tsodikova, S. & Gatto, G. J. Jr Protein posttranslational modifications: the chemistry of proteome diversifications. Angew. Chem. Int. Ed. Engl.44, 7342–7372 (2005). 10.1002/anie.200501023 [DOI] [PubMed] [Google Scholar]
- 2.Schjoldager, K. T., Narimatsu, Y., Joshi, H. J. & Clausen, H. Global view of human protein glycosylation pathways and functions. Nat. Rev. Mol. Cell Biol.21, 729–749 (2020). 10.1038/s41580-020-00294-x [DOI] [PubMed] [Google Scholar]
- 3.Stanley, P. Golgi glycosylation. Cold Spring Harb Perspect Biol3, a005199 (2011). [DOI] [PMC free article] [PubMed]
- 4.Laubli, H. & Borsig, L. Altered cell adhesion and glycosylation promote cancer immune suppression and metastasis. Front Immunol.10, 2120 (2019). 10.3389/fimmu.2019.02120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen, Z. et al. Mutual regulation between glycosylation and transforming growth factor-beta isoforms signaling pathway. Int. J. Biol. Macromol.236, 123818 (2023). 10.1016/j.ijbiomac.2023.123818 [DOI] [PubMed] [Google Scholar]
- 6.Caval, T., Alisson-Silva, F. & Schwarz, F. Roles of glycosylation at the cancer cell surface: opportunities for large scale glycoproteomics. Theranostics13, 2605–2615 (2023). 10.7150/thno.81760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng, C. et al. Glucose-mediated N-glycosylation of SCAP is essential for SREBP-1 activation and tumor growth. Cancer Cell28, 569–581 (2015). 10.1016/j.ccell.2015.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuehnel, K. Glycosylation: gluing on glycans. Nat. Chem. Biol.14, 199 (2018). 10.1038/nchembio.2579 [DOI] [PubMed] [Google Scholar]
- 9.Oliveira-Ferrer, L., Legler, K. & Milde-Langosch, K. Role of protein glycosylation in cancer metastasis. Semin Cancer Biol.44, 141–152 (2017). 10.1016/j.semcancer.2017.03.002 [DOI] [PubMed] [Google Scholar]
- 10.Hirabayashi, J., Arata, Y. & Kasai, K. Glycome project: concept, strategy and preliminary application to Caenorhabditis elegans. Proteomics1, 295–303 (2001). [DOI] [PubMed] [Google Scholar]
- 11.Wiederschain, G. Y. Wiederschain, G. Y. Glycobiology: progress, problems, and perspectives. Biochemistry78, 679–696 (2013). [DOI] [PubMed]
- 12.Schwarz, F. & Aebi, M. Mechanisms and principles of N-linked protein glycosylation. Curr. Opin. Struct. Biol.21, 576–582 (2011). 10.1016/j.sbi.2011.08.005 [DOI] [PubMed] [Google Scholar]
- 13.Medzihradszky, K. F., Kaasik, K. & Chalkley, R. J. Tissue-specific glycosylation at the glycopeptide level. Mol. Cell Proteom.14, 2103–2110 (2015). 10.1074/mcp.M115.050393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kelleher, D. J., Banerjee, S., Cura, A. J., Samuelson, J. & Gilmore, R. Dolichol-linked oligosaccharide selection by the oligosaccharyltransferase in protist and fungal organisms. J. Cell Biol.177, 29–37 (2007). 10.1083/jcb.200611079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bickel, T., Lehle, L., Schwarz, M., Aebi, M. & Jakob, C. A. Biosynthesis of lipid-linked oligosaccharides in Saccharomyces cerevisiae: Alg13p and Alg14p form a complex required for the formation of GlcNAc(2)-PP-dolichol. J. Biol. Chem.280, 34500–34506 (2005). 10.1074/jbc.M506358200 [DOI] [PubMed] [Google Scholar]
- 16.Tu, H. C. et al. Up-regulation of Golgi alpha-mannosidase IA and down-regulation of Golgi alpha-mannosidase IC activates unfolded protein response during hepatocarcinogenesis. Hepatol. Commun.1, 230–247 (2017). 10.1002/hep4.1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duellman, T., Burnett, J., Shin, A. & Yang, J. LMAN1 (ERGIC-53) is a potential carrier protein for matrix metalloproteinase-9 glycoprotein secretion. Biochem Biophys. Res Commun.464, 685–691 (2015). 10.1016/j.bbrc.2015.06.164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zeyen, L., Doring, T. & Prange, R. Hepatitis B virus exploits ERGIC-53 in conjunction with COPII to exit cells. Cells9, 9081889 (2020). [DOI] [PMC free article] [PubMed]
- 19.Aebi, M. N-linked protein glycosylation in the ER. Biochim Biophys. Acta1833, 2430–2437 (2013). 10.1016/j.bbamcr.2013.04.001 [DOI] [PubMed] [Google Scholar]
- 20.Mendoza, L., Olaso, E., Anasagasti, M. J., Fuentes, A. M. & Vidal-Vanaclocha, F. Mannose receptor-mediated endothelial cell activation contributes to B16 melanoma cell adhesion and metastasis in liver. J. Cell Physiol.174, 322–330 (1998). [DOI] [PubMed] [Google Scholar]
- 21.Steentoft, C. et al. Mining the O-glycoproteome using zinc-finger nuclease-glycoengineered SimpleCell lines. Nat. Methods8, 977–982 (2011). 10.1038/nmeth.1731 [DOI] [PubMed] [Google Scholar]
- 22.Brockhausen, I., Wandall, H. H., Hagen, K. G. T. & Stanley, P. in Essentials of Glycobiology (eds A. Varki et al.) 117–128 (2022).
- 23.Ten Hagen, K. G., Fritz, T. A. & Tabak, L. A. All in the family: the UDP-GalNAc:polypeptide N-acetylgalactosaminyltransferases. Glycobiology13, 1R–16R (2003). 10.1093/glycob/cwg007 [DOI] [PubMed] [Google Scholar]
- 24.Bennett, E. P. et al. Control of mucin-type O-glycosylation: a classification of the polypeptide GalNAc-transferase gene family. Glycobiology22, 736–756 (2012). 10.1093/glycob/cwr182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brockhausen, I. Biosynthesis and functions of O-glycans and regulation of mucin antigen expression in cancer. Biochem Soc. Trans.25, 871–874 (1997). 10.1042/bst0250871 [DOI] [PubMed] [Google Scholar]
- 26.Akella, N. M., Ciraku, L. & Reginato, M. J. Fueling the fire: emerging role of the hexosamine biosynthetic pathway in cancer. BMC Biol.17, 52 (2019). 10.1186/s12915-019-0671-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun, R. et al. Single-cell transcriptomic analysis of normal and pathological tissues from the same patient uncovers colon cancer progression. Cell Biosci.13, 62 (2023). 10.1186/s13578-023-01002-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scott, E. et al. Upregulation of GALNT7 in prostate cancer modifies O-glycosylation and promotes tumour growth. Oncogene42, 926–937 (2023). 10.1038/s41388-023-02604-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li, J. & Richards, J. C. Functional glycomics and glycobiology: an overview. Methods Mol. Biol.600, 1–8 (2010). 10.1007/978-1-60761-454-8_1 [DOI] [PubMed] [Google Scholar]
- 30.Jing, W. et al. Clostridium septicum alpha-toxin activates the NLRP3 inflammasome by engaging GPI-anchored proteins. Sci. Immunol.7, eabm1803 (2022). 10.1126/sciimmunol.abm1803 [DOI] [PubMed] [Google Scholar]
- 31.DeToma, A. S., Salamekh, S., Ramamoorthy, A. & Lim, M. H. Misfolded proteins in Alzheimer’s disease and type II diabetes. Chem. Soc. Rev.41, 608–621 (2012). 10.1039/C1CS15112F [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Helenius, A. & Aebi, M. Roles of N-linked glycans in the endoplasmic reticulum. Annu Rev. Biochem73, 1019–1049 (2004). 10.1146/annurev.biochem.73.011303.073752 [DOI] [PubMed] [Google Scholar]
- 33.Jayaprakash, N. G. & Surolia, A. Role of glycosylation in nucleating protein folding and stability. Biochem J.474, 2333–2347 (2017). 10.1042/BCJ20170111 [DOI] [PubMed] [Google Scholar]
- 34.Trombetta, E. S. & Helenius, A. Lectins as chaperones in glycoprotein folding. Curr. Opin. Struct. Biol.8, 587–592 (1998). 10.1016/S0959-440X(98)80148-6 [DOI] [PubMed] [Google Scholar]
- 35.Xu, C. & Ng, D. T. Glycosylation-directed quality control of protein folding. Nat. Rev. Mol. Cell Biol.16, 742–752 (2015). 10.1038/nrm4073 [DOI] [PubMed] [Google Scholar]
- 36.Wu, L. et al. O-GlcNAcylation regulates epidermal growth factor receptor intracellular trafficking and signaling. Proc. Natl. Acad. Sci. USA119, e2107453119 (2022). 10.1073/pnas.2107453119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang, D. et al. Branched-chain amino acid catabolism breaks glutamine addiction to sustain hepatocellular carcinoma progression. Cell Rep.41, 111691 (2022). 10.1016/j.celrep.2022.111691 [DOI] [PubMed] [Google Scholar]
- 38.Console, L. et al. N-glycosylation is crucial for trafficking and stability of SLC3A2 (CD98). Sci. Rep.12, 14570 (2022). 10.1038/s41598-022-18779-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tsang, B., Pritisanac, I., Scherer, S. W., Moses, A. M. & Forman-Kay, J. D. Phase Separation as a Missing Mechanism for Interpretation of Disease Mutations. Cell183, 1742–1756 (2020). 10.1016/j.cell.2020.11.050 [DOI] [PubMed] [Google Scholar]
- 40.Gu, X. et al. Phase separation drives tumor pathogenesis and evolution: all roads lead to Rome. Oncogene41, 1527–1535 (2022). 10.1038/s41388-022-02195-z [DOI] [PubMed] [Google Scholar]
- 41.Li, X., Pinou, L., Du, Y., Chen, X. & Liu, C. Emerging roles of O-glycosylation in regulating protein aggregation, phase separation, and functions. Curr. Opin. Chem. Biol.75, 102314 (2023). 10.1016/j.cbpa.2023.102314 [DOI] [PubMed] [Google Scholar]
- 42.Lv, P. et al. O-GlcNAcylation modulates liquid-liquid phase separation of SynGAP/PSD-95. Nat. Chem.14, 831–840 (2022). 10.1038/s41557-022-00946-9 [DOI] [PubMed] [Google Scholar]
- 43.Chen, Y. et al. O-GlcNAcylation determines the translational regulation and phase separation of YTHDF proteins. Nat. Cell Biol.25, 1676–1690 (2023). 10.1038/s41556-023-01258-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mui, K. L., Chen, C. S. & Assoian, R. K. The mechanical regulation of integrin-cadherin crosstalk organizes cells, signaling and forces. J. Cell Sci.129, 1093–1100 (2016). 10.1242/jcs.183699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moh, M. C. & Shen, S. The roles of cell adhesion molecules in tumor suppression and cell migration: a new paradox. Cell Adh Migr.3, 334–336 (2009). 10.4161/cam.3.4.9246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Makrilia, N., Kollias, A., Manolopoulos, L. & Syrigos, K. Cell adhesion molecules: role and clinical significance in cancer. Cancer Invest27, 1023–1037 (2009). 10.3109/07357900902769749 [DOI] [PubMed] [Google Scholar]
- 47.Zhu, W., Leber, B. & Andrews, D. W. Cytoplasmic O-glycosylation prevents cell surface transport of E-cadherin during apoptosis. EMBO J.20, 5999–6007 (2001). 10.1093/emboj/20.21.5999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhao, H. et al. N-glycosylation affects the adhesive function of E-Cadherin through modifying the composition of adherens junctions (AJs) in human breast carcinoma cell line MDA-MB-435. J. Cell Biochem104, 162–175 (2008). 10.1002/jcb.21608 [DOI] [PubMed] [Google Scholar]
- 49.Liwosz, A., Lei, T. & Kukuruzinska, M. A. N-glycosylation affects the molecular organization and stability of E-cadherin junctions. J. Biol. Chem.281, 23138–23149 (2006). 10.1074/jbc.M512621200 [DOI] [PubMed] [Google Scholar]
- 50.Pinho, S. S. et al. Modulation of E-cadherin function and dysfunction by N-glycosylation. Cell Mol. Life Sci.68, 1011–1020 (2011). 10.1007/s00018-010-0595-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vestweber, D. & Blanks, J. E. Mechanisms that regulate the function of the selectins and their ligands. Physiol. Rev.79, 181–213 (1999). 10.1152/physrev.1999.79.1.181 [DOI] [PubMed] [Google Scholar]
- 52.McEver, R. P. Selectins: lectins that initiate cell adhesion under flow. Curr. Opin. Cell Biol.14, 581–586 (2002). 10.1016/S0955-0674(02)00367-8 [DOI] [PubMed] [Google Scholar]
- 53.Ley, K. The role of selectins in inflammation and disease. Trends Mol. Med9, 263–268 (2003). 10.1016/S1471-4914(03)00071-6 [DOI] [PubMed] [Google Scholar]
- 54.Sladek, V., Smak, P. & Tvaroska, I. How E-, L-, and P-Selectins Bind to sLe(x) and PSGL-1: a quantification of critical residue interactions. J. Chem. Inf. Model63, 5604–5618 (2023). 10.1021/acs.jcim.3c00704 [DOI] [PubMed] [Google Scholar]
- 55.Weis, W. I., Taylor, M. E. & Drickamer, K. The C-type lectin superfamily in the immune system. Immunol. Rev.163, 19–34 (1998). 10.1111/j.1600-065X.1998.tb01185.x [DOI] [PubMed] [Google Scholar]
- 56.Cappenberg, A., Kardell, M. & Zarbock, A. Selectin-mediated signaling-shedding light on the regulation of integrin activity in neutrophils. Cells11, 11081310 (2022). [DOI] [PMC free article] [PubMed]
- 57.Liu, S., Yang, X., Liu, Y., Wang, X. & Yan, Q. sLeX/L-selectin mediates adhesion in vitro implantation model. Mol. Cell Biochem350, 185–192 (2011). 10.1007/s11010-010-0697-x [DOI] [PubMed] [Google Scholar]
- 58.Crocker, P. R., Paulson, J. C. & Varki, A. Siglecs and their roles in the immune system. Nat. Rev. Immunol.7, 255–266 (2007). 10.1038/nri2056 [DOI] [PubMed] [Google Scholar]
- 59.Stanczak, M. A. et al. Self-associated molecular patterns mediate cancer immune evasion by engaging Siglecs on T cells. J. Clin. Invest128, 4912–4923 (2018). 10.1172/JCI120612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Beatson, R. et al. The mucin MUC1 modulates the tumor immunological microenvironment through engagement of the lectin Siglec-9. Nat. Immunol.17, 1273–1281 (2016). 10.1038/ni.3552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Seguin, L., Desgrosellier, J. S., Weis, S. M. & Cheresh, D. A. Integrins and cancer: regulators of cancer stemness, metastasis, and drug resistance. Trends Cell Biol.25, 234–240 (2015). 10.1016/j.tcb.2014.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Desgrosellier, J. S. & Cheresh, D. A. Integrins in cancer: biological implications and therapeutic opportunities. Nat. Rev. Cancer10, 9–22 (2010). 10.1038/nrc2748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Aoudjit, F. & Vuori, K. Integrin signaling inhibits paclitaxel-induced apoptosis in breast cancer cells. Oncogene20, 4995–5004 (2001). 10.1038/sj.onc.1204554 [DOI] [PubMed] [Google Scholar]
- 64.Marsico, G., Russo, L., Quondamatteo, F. & Pandit, A. Glycosylation and integrin regulation in cancer. Trends Cancer4, 537–552 (2018). 10.1016/j.trecan.2018.05.009 [DOI] [PubMed] [Google Scholar]
- 65.de-Souza-Ferreira, M., Ferreira, E. E. & de-Freitas-Junior, J. C. M. Aberrant N-glycosylation in cancer: MGAT5 and beta1,6-GlcNAc branched N-glycans as critical regulators of tumor development and progression. Cell Oncol. (Dordr.)46, 481–501 (2023). 10.1007/s13402-023-00770-4 [DOI] [PubMed] [Google Scholar]
- 66.Liu, C. H. et al. C1GALT1 promotes invasive phenotypes of hepatocellular carcinoma cells by modulating integrin beta1 glycosylation and activity. PLoS One9, e94995 (2014). 10.1371/journal.pone.0094995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pinho, S. S. & Reis, C. A. Glycosylation in cancer: mechanisms and clinical implications. Nat. Rev. Cancer15, 540–555 (2015). 10.1038/nrc3982 [DOI] [PubMed] [Google Scholar]
- 68.Seales, E. C. et al. Hypersialylation of beta1 integrins, observed in colon adenocarcinoma, may contribute to cancer progression by up-regulating cell motility. Cancer Res65, 4645–4652 (2005). 10.1158/0008-5472.CAN-04-3117 [DOI] [PubMed] [Google Scholar]
- 69.Smith, B. A. H. & Bertozzi, C. R. The clinical impact of glycobiology: targeting selectins, Siglecs and mammalian glycans. Nat. Rev. Drug Discov.20, 217–243 (2021). 10.1038/s41573-020-00093-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rudd, P. M., Elliott, T., Cresswell, P., Wilson, I. A. & Dwek, R. A. Glycosylation and the immune system. Science291, 2370–2376 (2001). 10.1126/science.291.5512.2370 [DOI] [PubMed] [Google Scholar]
- 71.Macauley, M. S., Crocker, P. R. & Paulson, J. C. Siglec-mediated regulation of immune cell function in disease. Nat. Rev. Immunol.14, 653–666 (2014). 10.1038/nri3737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rabinovich, G. A. & Croci, D. O. Regulatory circuits mediated by lectin-glycan interactions in autoimmunity and cancer. Immunity36, 322–335 (2012). 10.1016/j.immuni.2012.03.004 [DOI] [PubMed] [Google Scholar]
- 73.van Kooyk, Y. & Rabinovich, G. A. Protein-glycan interactions in the control of innate and adaptive immune responses. Nat. Immunol.9, 593–601 (2008). 10.1038/ni.f.203 [DOI] [PubMed] [Google Scholar]
- 74.Stanczak, M. A. et al. Targeting cancer glycosylation repolarizes tumor-associated macrophages allowing effective immune checkpoint blockade. Sci. Transl. Med14, eabj1270 (2022). 10.1126/scitranslmed.abj1270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.van de Wall, S., Santegoets, K. C. M., van Houtum, E. J. H., Bull, C. & Adema, G. J. Sialoglycans and siglecs can shape the tumor immune microenvironment. Trends Immunol.41, 274–285 (2020). 10.1016/j.it.2020.02.001 [DOI] [PubMed] [Google Scholar]
- 76.Kuliesiute, U. et al. Sialic acid metabolism orchestrates transcellular connectivity and signaling in glioblastoma. Neuro Oncol.25, 1963–1975 (2023). 10.1093/neuonc/noad101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rodriguez, E. et al. Sialic acids in pancreatic cancer cells drive tumour-associated macrophage differentiation via the Siglec receptors Siglec-7 and Siglec-9. Nat. Commun.12, 1270 (2021). 10.1038/s41467-021-21550-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bochner, B. S. & Zimmermann, N. Role of siglecs and related glycan-binding proteins in immune responses and immunoregulation. J. Allergy Clin. Immunol.135, 598–608 (2015). 10.1016/j.jaci.2014.11.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Moreira, R. S. et al. Siglec 15 as a biomarker or a druggable molecule for non-small cell lung cancer. J. Cancer Res Clin. Oncol.149, 17651–17661 (2023). 10.1007/s00432-023-05437-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hudak, J. E., Canham, S. M. & Bertozzi, C. R. Glycocalyx engineering reveals a Siglec-based mechanism for NK cell immunoevasion. Nat. Chem. Biol.10, 69–75 (2014). 10.1038/nchembio.1388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Antonopoulos, A. et al. Loss of effector function of human cytolytic T lymphocytes is accompanied by major alterations in N- and O-glycosylation. J. Biol. Chem.287, 11240–11251 (2012). 10.1074/jbc.M111.320820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nambiar, D. K. et al. Galectin-1-driven T cell exclusion in the tumor endothelium promotes immunotherapy resistance. J. Clin. Invest129, 5553–5567 (2019). 10.1172/JCI129025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Caputo, S. et al. Galectin-3 in prostate cancer stem-like cells is immunosuppressive and drives early metastasis. Front Immunol.11, 1820 (2020). 10.3389/fimmu.2020.01820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wiersma, V. R., de Bruyn, M., Helfrich, W. & Bremer, E. Therapeutic potential of Galectin-9 in human disease. Med Res Rev.33(Suppl 1), E102–E126 (2013). [DOI] [PubMed] [Google Scholar]
- 85.Zhou, B. et al. Notch signaling pathway: architecture, disease, and therapeutics. Signal Transduct. Target Ther.7, 95 (2022). 10.1038/s41392-022-00934-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kopan, R. & Ilagan, M. X. The canonical Notch signaling pathway: unfolding the activation mechanism. Cell137, 216–233 (2009). 10.1016/j.cell.2009.03.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Haines, N. & Irvine, K. D. Glycosylation regulates Notch signalling. Nat. Rev. Mol. Cell Biol.4, 786–797 (2003). 10.1038/nrm1228 [DOI] [PubMed] [Google Scholar]
- 88.Rampal, R., Luther, K. B. & Haltiwanger, R. S. Notch signaling in normal and disease states: possible therapies related to glycosylation. Curr. Mol. Med7, 427–445 (2007). 10.2174/156652407780831593 [DOI] [PubMed] [Google Scholar]
- 89.Takeuchi, H. & Haltiwanger, R. S. Role of glycosylation of Notch in development. Semin Cell Dev. Biol.21, 638–645 (2010). 10.1016/j.semcdb.2010.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Blochl, C. et al. Integrated N- and O-Glycomics of Acute Myeloid Leukemia (AML) Cell Lines. Cells10, 10113058 (2021). [DOI] [PMC free article] [PubMed]
- 91.Yokota, S. et al. Protein O-fucosyltransferase 1: a potential diagnostic marker and therapeutic target for human oral cancer. Int J. Oncol.43, 1864–1870 (2013). 10.3892/ijo.2013.2110 [DOI] [PubMed] [Google Scholar]
- 92.Takeuchi, H. et al. Two novel protein O-glucosyltransferases that modify sites distinct from POGLUT1 and affect Notch trafficking and signaling. Proc. Natl Acad. Sci. USA115, E8395–E8402 (2018). 10.1073/pnas.1804005115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shao, L., Luo, Y., Moloney, D. J. & Haltiwanger, R. O-glycosylation of EGF repeats: identification and initial characterization of a UDP-glucose: protein O-glucosyltransferase. Glycobiology12, 763–770 (2002). 10.1093/glycob/cwf085 [DOI] [PubMed] [Google Scholar]
- 94.Servian-Morilla, E. et al. A POGLUT1 mutation causes a muscular dystrophy with reduced Notch signaling and satellite cell loss. EMBO Mol. Med8, 1289–1309 (2016). 10.15252/emmm.201505815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mackinnon, R. N. et al. The paradox of 20q11.21 amplification in a subset of cases of myeloid malignancy with chromosome 20 deletion. Genes Chromosomes Cancer49, 998–1013 (2010). 10.1002/gcc.20806 [DOI] [PubMed] [Google Scholar]
- 96.Blair, S. S. Notch signaling: Fringe really is a glycosyltransferase. Curr. Biol.10, R608–R612 (2000). 10.1016/S0960-9822(00)00633-3 [DOI] [PubMed] [Google Scholar]
- 97.Moloney, D. J. et al. Fringe is a glycosyltransferase that modifies Notch. Nature406, 369–375 (2000). 10.1038/35019000 [DOI] [PubMed] [Google Scholar]
- 98.Chen, J., Moloney, D. J. & Stanley, P. Fringe modulation of Jagged1-induced Notch signaling requires the action of beta 4galactosyltransferase-1. Proc. Natl. Acad. Sci. USA98, 13716–13721 (2001). 10.1073/pnas.241398098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ogawa, M., Sawaguchi, S., Kamemura, K. & Okajima, T. Intracellular and extracellular O-linked N-acetylglucosamine in the nervous system. Exp. Neurol.274, 166–174 (2015). 10.1016/j.expneurol.2015.08.009 [DOI] [PubMed] [Google Scholar]
- 100.Chen, J. et al. Ogt controls neural stem/progenitor cell pool and adult neurogenesis through modulating Notch signaling. Cell Rep.34, 108905 (2021). 10.1016/j.celrep.2021.108905 [DOI] [PubMed] [Google Scholar]
- 101.Ogawa, M. & Okajima, T. Structure and function of extracellular O-GlcNAc. Curr. Opin. Struct. Biol.56, 72–77 (2019). 10.1016/j.sbi.2018.12.002 [DOI] [PubMed] [Google Scholar]
- 102.Zhou, L. et al. Notch-dependent control of myelopoiesis is regulated by fucosylation. Blood112, 308–319 (2008). 10.1182/blood-2007-11-115204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Larose, H. et al. Whole Exome Sequencing reveals NOTCH1 mutations in anaplastic large cell lymphoma and points to Notch both as a key pathway and a potential therapeutic target. Haematologica106, 1693–1704 (2021). 10.3324/haematol.2019.238766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Perkey, E. et al. GCNT1-Mediated O-Glycosylation of the Sialomucin CD43 Is a Sensitive Indicator of Notch Signaling in Activated T Cells. J. Immunol.204, 1674–1688 (2020). 10.4049/jimmunol.1901194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hu, X., Li, J., Fu, M., Zhao, X. & Wang, W. The JAK/STAT signaling pathway: from bench to clinic. Signal Transduct. Target Ther.6, 402 (2021). 10.1038/s41392-021-00791-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Liu, K. D., Gaffen, S. L. & Goldsmith, M. A. JAK/STAT signaling by cytokine receptors. Curr. Opin. Immunol.10, 271–278 (1998). 10.1016/S0952-7915(98)80165-9 [DOI] [PubMed] [Google Scholar]
- 107.Gatzka, M., Piekorz, R., Moriggl, R., Rawlings, J. & Ihle, J. N. A role for STAT5A/B in protection of peripheral T-lymphocytes from postactivation apoptosis: insights from gene expression profiling. Cytokine34, 143–154 (2006). 10.1016/j.cyto.2006.04.003 [DOI] [PubMed] [Google Scholar]
- 108.Freund, P. et al. O-GlcNAcylation of STAT5 controls tyrosine phosphorylation and oncogenic transcription in STAT5-dependent malignancies. Leukemia31, 2132–2142 (2017). 10.1038/leu.2017.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gewinner, C. et al. The coactivator of transcription CREB-binding protein interacts preferentially with the glycosylated form of Stat5. J. Biol. Chem.279, 3563–3572 (2004). 10.1074/jbc.M306449200 [DOI] [PubMed] [Google Scholar]
- 110.Jozwiak, P., Forma, E., Brys, M. & Krzeslak, A. O-GlcNAcylation and Metabolic Reprograming in Cancer. Front Endocrinol. (Lausanne)5, 145 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jitschin, R. et al. Inflammation-induced glycolytic switch controls suppressivity of mesenchymal stem cells via STAT1 glycosylation. Leukemia33, 1783–1796 (2019). 10.1038/s41375-018-0376-6 [DOI] [PubMed] [Google Scholar]
- 112.Massague, J. & Sheppard, D. TGF-beta signaling in health and disease. Cell186, 4007–4037 (2023). 10.1016/j.cell.2023.07.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sun, X. et al. ALG3 contributes to stemness and radioresistance through regulating glycosylation of TGF-beta receptor II in breast cancer. J. Exp. Clin. Cancer Res40, 149 (2021). 10.1186/s13046-021-01932-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kim, Y. W., Park, J., Lee, H. J., Lee, S. Y. & Kim, S. J. TGF-beta sensitivity is determined by N-linked glycosylation of the type II TGF-beta receptor. Biochem J.445, 403–411 (2012). 10.1042/BJ20111923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tu, C. F., Wu, M. Y., Lin, Y. C., Kannagi, R. & Yang, R. B. FUT8 promotes breast cancer cell invasiveness by remodeling TGF-beta receptor core fucosylation. Breast Cancer Res19, 111 (2017). 10.1186/s13058-017-0904-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lu, J. et al. beta-Galactoside alpha2,6-sialyltranferase 1 promotes transforming growth factor-beta-mediated epithelial-mesenchymal transition. J. Biol. Chem.289, 34627–34641 (2014). 10.1074/jbc.M114.593392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yeo, H. L. et al. Sialylation of vasorin by ST3Gal1 facilitates TGF-beta1-mediated tumor angiogenesis and progression. Int J. Cancer144, 1996–2007 (2019). 10.1002/ijc.31891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wu, Q. et al. ppGalNAc-T4-catalyzed O-Glycosylation of TGF-beta type II receptor regulates breast cancer cells metastasis potential. J. Biol. Chem.296, 100119 (2021). 10.1074/jbc.RA120.016345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nusse, R. & Clevers, H. Wnt/beta-Catenin signaling, disease, and emerging therapeutic modalities. Cell169, 985–999 (2017). 10.1016/j.cell.2017.05.016 [DOI] [PubMed] [Google Scholar]
- 120.Flanagan, D. J., Vincan, E. & Phesse, T. J. Wnt signaling in cancer: not a binary ON:OFF Switch. Cancer Res79, 5901–5906 (2019). 10.1158/0008-5472.CAN-19-1362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hong, S. et al. In Situ Fucosylation of the Wnt Co-receptor LRP6 increases its endocytosis and reduces Wnt/beta-Catenin signaling. Cell Chem. Biol.27, 1140–1150.e1144 (2020). 10.1016/j.chembiol.2020.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Xu, R. et al. N-Glycosylation of LRP6 by B3GnT2 promotes Wnt/beta-Catenin signalling. Cells12, 12060863 (2023). [DOI] [PMC free article] [PubMed]
- 123.Tang, W., Li, M., Qi, X. & Li, J. beta1,4-Galactosyltransferase V modulates breast cancer stem cells through Wnt/beta-catenin signaling pathway. Cancer Res. Treat.52, 1084–1102 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhang, J. et al. GALNT1 enhances malignant phenotype of gastric cancer via modulating CD44 glycosylation to activate the Wnt/beta-catenin signaling pathway. Int. J. Biol. Sci.18, 6068–6083 (2022). 10.7150/ijbs.73431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wu, X. et al. Targeting protein modifications in metabolic diseases: molecular mechanisms and targeted therapies. Signal Transduct. Target Ther.8, 220 (2023). 10.1038/s41392-023-01439-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.van den Boogert, M. A. W. et al. N-Glycosylation defects in humans lower low-density lipoprotein cholesterol through increased low-density lipoprotein receptor expression. Circulation140, 280–292 (2019). 10.1161/CIRCULATIONAHA.118.036484 [DOI] [PubMed] [Google Scholar]
- 127.Kathiresan, S. et al. Common variants at 30 loci contribute to polygenic dyslipidemia. Nat. Genet41, 56–65 (2009). 10.1038/ng.291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wang, Y. P. et al. Arginine methylation of MDH1 by CARM1 inhibits glutamine metabolism and suppresses pancreatic cancer. Mol. Cell64, 673–687 (2016). 10.1016/j.molcel.2016.09.028 [DOI] [PubMed] [Google Scholar]
- 129.Zhu, Q. et al. O-GlcNAcylation promotes pancreatic tumor growth by regulating malate dehydrogenase 1. Nat. Chem. Biol.18, 1087–1095 (2022). 10.1038/s41589-022-01085-5 [DOI] [PubMed] [Google Scholar]
- 130.Pang, X., Li, H., Guan, F. & Li, X. Multiple roles of glycans in hematological malignancies. Front Oncol.8, 364 (2018). 10.3389/fonc.2018.00364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Shimony, S., Stahl, M. & Stone, R. M. Acute myeloid leukemia: 2023 update on diagnosis, risk-stratification, and management. Am. J. Hematol.98, 502–526 (2023). 10.1002/ajh.26822 [DOI] [PubMed] [Google Scholar]
- 132.Daver, N., Schlenk, R. F., Russell, N. H. & Levis, M. J. Targeting FLT3 mutations in AML: review of current knowledge and evidence. Leukemia33, 299–312 (2019). 10.1038/s41375-018-0357-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Choudhary, C. et al. AML-associated Flt3 kinase domain mutations show signal transduction differences compared with Flt3 ITD mutations. Blood106, 265–273 (2005). 10.1182/blood-2004-07-2942 [DOI] [PubMed] [Google Scholar]
- 134.Schmidt-Arras, D. E. et al. Tyrosine phosphorylation regulates maturation of receptor tyrosine kinases. Mol. Cell Biol.25, 3690–3703 (2005). 10.1128/MCB.25.9.3690-3703.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Schmidt-Arras, D. et al. Anchoring of FLT3 in the endoplasmic reticulum alters signaling quality. Blood113, 3568–3576 (2009). 10.1182/blood-2007-10-121426 [DOI] [PubMed] [Google Scholar]
- 136.Williams, A. B. et al. Fluvastatin inhibits FLT3 glycosylation in human and murine cells and prolongs survival of mice with FLT3/ITD leukemia. Blood120, 3069–3079 (2012). 10.1182/blood-2012-01-403493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Radomska, H. S. et al. Targeting CDK1 promotes FLT3-activated acute myeloid leukemia differentiation through C/EBPalpha. J. Clin. Invest122, 2955–2966 (2012). 10.1172/JCI43354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Larrue, C. et al. Antileukemic activity of 2-Deoxy-d-Glucose through Inhibition of N-linked glycosylation in acute myeloid leukemia with FLT3-ITD or c-KIT mutations. Mol. Cancer Ther.14, 2364–2373 (2015). 10.1158/1535-7163.MCT-15-0163 [DOI] [PubMed] [Google Scholar]
- 139.Parodi, A. J. Protein glucosylation and its role in protein folding. Annu Rev. Biochem69, 69–93 (2000). 10.1146/annurev.biochem.69.1.69 [DOI] [PubMed] [Google Scholar]
- 140.Andresen, L. et al. 2-deoxy D-glucose prevents cell surface expression of NKG2D ligands through inhibition of N-linked glycosylation. J. Immunol.188, 1847–1855 (2012). 10.4049/jimmunol.1004085 [DOI] [PubMed] [Google Scholar]
- 141.Reiter, K. et al. Tyrosine kinase inhibition increases the cell surface localization of FLT3-ITD and enhances FLT3-directed immunotherapy of acute myeloid leukemia. Leukemia32, 313–322 (2018). 10.1038/leu.2017.257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hamadmad, S. N. & Hohl, R. J. Lovastatin suppresses erythropoietin receptor surface expression through dual inhibition of glycosylation and geranylgeranylation. Biochem Pharm.74, 590–600 (2007). 10.1016/j.bcp.2007.04.028 [DOI] [PubMed] [Google Scholar]
- 143.Kumar, B. et al. Acute myeloid leukemia transforms the bone marrow niche into a leukemia-permissive microenvironment through exosome secretion. Leukemia32, 575–587 (2018). 10.1038/leu.2017.259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Feng, J. et al. Loss of bisecting GlcNAcylation on MCAM of bone marrow stoma determined pro-tumoral niche in MDS/AML. Leukemia37, 113–121 (2023). 10.1038/s41375-022-01748-1 [DOI] [PubMed] [Google Scholar]
- 145.Marjon, K. D. et al. Tetraspanin CD82 regulates bone marrow homing of acute myeloid leukemia by modulating the molecular organization of N-cadherin. Oncogene35, 4132–4140 (2016). 10.1038/onc.2015.449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hasegawa, K. et al. Glycosylation status of CD43 protein is associated with resistance of leukemia cells to CTL-mediated cytolysis. PLoS One11, e0152326 (2016). 10.1371/journal.pone.0152326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Bartels, L. et al. A chemo-enzymatically linked bispecific antibody retargets T cells to a sialylated epitope on CD43 in acute myeloid leukemia. Cancer Res.79, 3372–3382 (2019). 10.1158/0008-5472.CAN-18-0189 [DOI] [PubMed] [Google Scholar]
- 148.Tomita, N. BCL2 and MYC dual-hit lymphoma/leukemia. J. Clin. Exp. Hematop51, 7–12 (2011). 10.3960/jslrt.51.7 [DOI] [PubMed] [Google Scholar]
- 149.Bond, M. R. & Hanover, J. A. O-GlcNAc cycling: a link between metabolism and chronic disease. Annu Rev. Nutr.33, 205–229 (2013). 10.1146/annurev-nutr-071812-161240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lee, D. H. et al. Increased O-GlcNAcylation of c-Myc promotes pre-B cell proliferation. Cells9, 9010158 (2020). [DOI] [PMC free article] [PubMed]
- 151.Oliveira, T. et al. Glycoproteome remodeling in MLL-rearranged B-cell precursor acute lymphoblastic leukemia. Theranostics11, 9519–9537 (2021). 10.7150/thno.65398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Haymour, L., Jean, M., Smulski, C. & Legembre, P. CD95 (Fas) and CD95L (FasL)-mediated non-canonical signaling pathways. Biochim Biophys. Acta Rev. Cancer1878, 189004 (2023). 10.1016/j.bbcan.2023.189004 [DOI] [PubMed] [Google Scholar]
- 153.Dorrie, J., Sapala, K. & Zunino, S. J. Interferon-gamma increases the expression of glycosylated CD95 in B-leukemic cells: an inducible model to study the role of glycosylation in CD95-signalling and trafficking. Cytokine18, 98–107 (2002). 10.1006/cyto.2002.1030 [DOI] [PubMed] [Google Scholar]
- 154.Charlier, E. et al. SHIP-1 inhibits CD95/APO-1/Fas-induced apoptosis in primary T lymphocytes and T leukemic cells by promoting CD95 glycosylation independently of its phosphatase activity. Leukemia24, 821–832 (2010). 10.1038/leu.2010.9 [DOI] [PubMed] [Google Scholar]
- 155.Rai, K. R. & Jain, P. Chronic lymphocytic leukemia (CLL)-Then and now. Am. J. Hematol.91, 330–340 (2016). 10.1002/ajh.24282 [DOI] [PubMed] [Google Scholar]
- 156.Burger, J. A. & Wiestner, A. Targeting B cell receptor signalling in cancer: preclinical and clinical advances. Nat. Rev. Cancer18, 148–167 (2018). 10.1038/nrc.2017.121 [DOI] [PubMed] [Google Scholar]
- 157.Kostareli, E., Gounari, M., Agathangelidis, A. & Stamatopoulos, K. Immunoglobulin gene repertoire in chronic lymphocytic leukemia: insight into antigen selection and microenvironmental interactions. Mediterr. J. Hematol. Infect. Dis.4, e2012052 (2012). 10.4084/mjhid.2012.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Wienands, J. & Engels, N. Multitasking of Ig-alpha and Ig-beta to regulate B cell antigen receptor function. Int Rev. Immunol.20, 679–696 (2001). 10.3109/08830180109045585 [DOI] [PubMed] [Google Scholar]
- 159.Vuillier, F. et al. Lower levels of surface B-cell-receptor expression in chronic lymphocytic leukemia are associated with glycosylation and folding defects of the mu and CD79a chains. Blood105, 2933–2940 (2005). 10.1182/blood-2004-09-3643 [DOI] [PubMed] [Google Scholar]
- 160.Iatrou, A. et al. N-Glycosylation of the Ig receptors shapes the antigen reactivity in chronic lymphocytic leukemia subset #201. J. Immunol.211, 743–754 (2023). 10.4049/jimmunol.2300330 [DOI] [PubMed] [Google Scholar]
- 161.Hollander, N. & Haimovich, J. Altered N-linked glycosylation in follicular lymphoma and chronic lymphocytic leukemia: involvement in pathogenesis and potential therapeutic targeting. Front Immunol.8, 912 (2017). 10.3389/fimmu.2017.00912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Roman, E. & Smith, A. G. Epidemiology of lymphomas. Histopathology58, 4–14 (2011). 10.1111/j.1365-2559.2010.03696.x [DOI] [PubMed] [Google Scholar]
- 163.Tomonaga, M. Outline and direction of revised WHO classification of Tumors of Haematopoietic and Lymphoid Tissues. Rinsho Ketsueki50, 1401–1406 (2009). [PubMed] [Google Scholar]
- 164.Vitolo, U., Ferreri, A. J. & Montoto, S. Follicular lymphomas. Crit. Rev. Oncol. Hematol.66, 248–261 (2008). 10.1016/j.critrevonc.2008.01.014 [DOI] [PubMed] [Google Scholar]
- 165.Carlotti, E. et al. High throughput sequencing analysis of the immunoglobulin heavy chain gene from flow-sorted B cell sub-populations define the dynamics of follicular lymphoma clonal evolution. PLoS One10, e0134833 (2015). 10.1371/journal.pone.0134833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Zhu, D. et al. Acquisition of potential N-glycosylation sites in the immunoglobulin variable region by somatic mutation is a distinctive feature of follicular lymphoma. Blood99, 2562–2568 (2002). 10.1182/blood.V99.7.2562 [DOI] [PubMed] [Google Scholar]
- 167.Odabashian, M. et al. IGHV sequencing reveals acquired N-glycosylation sites as a clonal and stable event during follicular lymphoma evolution. Blood135, 834–844 (2020). 10.1182/blood.2019002279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Mamessier, E. et al. Contiguous follicular lymphoma and follicular lymphoma in situ harboring N-glycosylated sites. Haematologica100, e155–e157 (2015). 10.3324/haematol.2014.115782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Radcliffe, C. M. et al. Human follicular lymphoma cells contain oligomannose glycans in the antigen-binding site of the B-cell receptor. J. Biol. Chem.282, 7405–7415 (2007). 10.1074/jbc.M602690200 [DOI] [PubMed] [Google Scholar]
- 170.Leich, E. et al. Follicular lymphoma subgroups with and without t(14;18) differ in their N-glycosylation pattern and IGHV usage. Blood Adv.5, 4890–4900 (2021). 10.1182/bloodadvances.2021005081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Haebe, S. et al. Follicular lymphoma evolves with a surmountable dependency on acquired glycosylation motifs in the B-cell receptor. Blood142, 2296–2304 (2023). 10.1182/blood.2023020360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.A clinical evaluation of the International Lymphoma Study Group classification of non-Hodgkin’s lymphoma. The Non-Hodgkin’s lymphoma classification project. Blood89, 3909–3918 (1997). [PubMed] [Google Scholar]
- 173.Scheich, S. et al. Targeting N-linked glycosylation for the therapy of aggressive lymphomas. Cancer Discov.13, 1862–1883 (2023). 10.1158/2159-8290.CD-22-1401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Chen, I. J., Chen, H. L. & Demetriou, M. Lateral compartmentalization of T cell receptor versus CD45 by galectin-N-glycan binding and microfilaments coordinate basal and activation signaling. J. Biol. Chem.282, 35361–35372 (2007). 10.1074/jbc.M706923200 [DOI] [PubMed] [Google Scholar]
- 175.Pulido, R. & Sanchez-Madrid, F. Glycosylation of CD45: carbohydrate composition and its role in acquisition of CD45R0 and CD45RB T cell maturation-related antigen specificities during biosynthesis. Eur. J. Immunol.20, 2667–2671 (1990). 10.1002/eji.1830201221 [DOI] [PubMed] [Google Scholar]
- 176.Dumic, J., Dabelic, S. & Flogel, M. Galectin-3: an open-ended story. Biochim Biophys. Acta1760, 616–635 (2006). 10.1016/j.bbagen.2005.12.020 [DOI] [PubMed] [Google Scholar]
- 177.Hoyer, K. K. et al. An anti-apoptotic role for galectin-3 in diffuse large B-cell lymphomas. Am. J. Pathol.164, 893–902 (2004). 10.1016/S0002-9440(10)63177-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Clark, M. C. et al. Galectin-3 binds to CD45 on diffuse large B-cell lymphoma cells to regulate susceptibility to cell death. Blood120, 4635–4644 (2012). 10.1182/blood-2012-06-438234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Wang, M. et al. Identification of a monoclonal antibody that targets PD-1 in a manner requiring PD-1 Asn58 glycosylation. Commun. Biol.2, 392 (2019). 10.1038/s42003-019-0642-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Okazaki, T. & Honjo, T. PD-1 and PD-1 ligands: from discovery to clinical application. Int Immunol.19, 813–824 (2007). 10.1093/intimm/dxm057 [DOI] [PubMed] [Google Scholar]
- 181.Li, C. W. et al. Eradication of triple-negative breast cancer cells by targeting glycosylated PD-L1. Cancer Cell33, 187–201.e110 (2018). 10.1016/j.ccell.2018.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.De Sousa Linhares, A. et al. Therapeutic PD-L1 antibodies are more effective than PD-1 antibodies in blocking PD-1/PD-L1 signaling. Sci. Rep.9, 11472 (2019). 10.1038/s41598-019-47910-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Liu, X. et al. Overexpression of GLT1D1 induces immunosuppression through glycosylation of PD-L1 and predicts poor prognosis in B-cell lymphoma. Mol. Oncol.14, 1028–1044 (2020). 10.1002/1878-0261.12664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Gupta, N., Sharma, A. & Sharma, A. Emerging biomarkers in multiple myeloma: a review. Clin. Chim. Acta503, 45–53 (2020). 10.1016/j.cca.2019.12.026 [DOI] [PubMed] [Google Scholar]
- 185.Bergon, E. & Miravalles, E. Retrospective study of monoclonal gammopathies detected in the clinical laboratory of a Spanish healthcare district: 14-year series. Clin. Chem. Lab Med45, 190–196 (2007). 10.1515/CCLM.2007.029 [DOI] [PubMed] [Google Scholar]
- 186.Nimmerjahn, F. & Ravetch, J. V. Four keys to unlock IgG. J. Exp. Med.218, 20201753 (2021). [DOI] [PMC free article] [PubMed]
- 187.Kinoshita, N. et al. Glycosylation at the Fab portion of myeloma immunoglobulin G and increased fucosylated biantennary sugar chains: structural analysis by high-performance liquid chromatography and antibody-lectin enzyme immunoassay using Lens culinaris agglutinin. Cancer Res51, 5888–5892 (1991). [PubMed] [Google Scholar]
- 188.Westhrin, M. et al. Monoclonal immunoglobulins promote bone loss in multiple myeloma. Blood136, 2656–2666 (2020). 10.1182/blood.2020006045 [DOI] [PubMed] [Google Scholar]
- 189.Visram, A. et al. Disease monitoring with quantitative serum IgA levels provides a more reliable response assessment in multiple myeloma patients. Leukemia35, 1428–1437 (2021). 10.1038/s41375-021-01180-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Bosseboeuf, A. et al. Analysis of the targets and glycosylation of monoclonal IgAs From MGUS and myeloma patients. Front Immunol.11, 854 (2020). 10.3389/fimmu.2020.00854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Chen, J. et al. N-glycosylation of serum proteins for the assessment of patients with IgD multiple myeloma. BMC Cancer17, 881 (2017). 10.1186/s12885-017-3891-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Radamaker, L. et al. Role of mutations and post-translational modifications in systemic AL amyloidosis studied by cryo-EM. Nat. Commun.12, 6434 (2021). 10.1038/s41467-021-26553-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Mellors, P. W. et al. MASS-FIX for the detection of monoclonal proteins and light chain N-glycosylation in routine clinical practice: a cross-sectional study of 6315 patients. Blood Cancer J.11, 50 (2021). 10.1038/s41408-021-00444-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Nevone, A. et al. An N-glycosylation hotspot in immunoglobulin kappa light chains is associated with AL amyloidosis. Leukemia36, 2076–2085 (2022). 10.1038/s41375-022-01599-w [DOI] [PubMed] [Google Scholar]
- 195.Dispenzieri, A. et al. N-glycosylation of monoclonal light chains on routine MASS-FIX testing is a risk factor for MGUS progression. Leukemia34, 2749–2753 (2020). 10.1038/s41375-020-0940-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Alameda, D. et al. Tumor cells in light-chain amyloidosis and myeloma show distinct transcriptional rewiring of normal plasma cell development. Blood138, 1583–1589 (2021). 10.1182/blood.2020009754 [DOI] [PubMed] [Google Scholar]
- 197.Greenfield, G., McMullin, M. F. & Mills, K. Molecular pathogenesis of the myeloproliferative neoplasms. J. Hematol. Oncol.14, 103 (2021). 10.1186/s13045-021-01116-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Venugopal, S. & Mascarenhas, J. Novel therapeutics in myeloproliferative neoplasms. J. Hematol. Oncol.13, 162 (2020). 10.1186/s13045-020-00995-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Vainchenker, W. & Constantinescu, S. N. JAK/STAT signaling in hematological malignancies. Oncogene32, 2601–2613 (2013). 10.1038/onc.2012.347 [DOI] [PubMed] [Google Scholar]
- 200.How, J., Hobbs, G. S. & Mullally, A. Mutant calreticulin in myeloproliferative neoplasms. Blood134, 2242–2248 (2019). 10.1182/blood.2019000622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Araki, M. et al. Activation of the thrombopoietin receptor by mutant calreticulin in CALR-mutant myeloproliferative neoplasms. Blood127, 1307–1316 (2016). 10.1182/blood-2015-09-671172 [DOI] [PubMed] [Google Scholar]
- 202.Jutzi, J. S. et al. Whole-genome CRISPR screening identifies N-glycosylation as a genetic and therapeutic vulnerability in CALR-mutant MPNs. Blood140, 1291–1304 (2022). 10.1182/blood.2022015629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.de Figueiredo, L. L., de Abreu e Lima, R. S. & Rego, E. M. Granulocyte colony-stimulating factor and leukemogenesis. Mediators Inflamm.13, 145–150 (2004). 10.1080/09511920410001713574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Skokowa, J., Dale, D. C., Touw, I. P., Zeidler, C. & Welte, K. Severe congenital neutropenias. Nat. Rev. Dis. Prim.3, 17032 (2017). 10.1038/nrdp.2017.32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Maxson, J. E. et al. Oncogenic CSF3R mutations in chronic neutrophilic leukemia and atypical CML. N. Engl. J. Med368, 1781–1790 (2013). 10.1056/NEJMoa1214514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Maxson, J. E. et al. Ligand independence of the T618I mutation in the colony-stimulating factor 3 receptor (CSF3R) protein results from loss of O-linked glycosylation and increased receptor dimerization. J. Biol. Chem.289, 5820–5827 (2014). 10.1074/jbc.M113.508440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Price, A. et al. T618I CSF3R mutations in chronic neutrophilic leukemia induce oncogenic signals through aberrant trafficking and constitutive phosphorylation of the O-glycosylated receptor form. Biochem Biophys. Res. Commun.523, 208–213 (2020). 10.1016/j.bbrc.2019.12.030 [DOI] [PubMed] [Google Scholar]
- 208.Spiciarich, D. R. et al. A novel germline variant in CSF3R reduces N-Glycosylation and exerts potent oncogenic effects in leukemia. Cancer Res.78, 6762–6770 (2018). 10.1158/0008-5472.CAN-18-1638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Hakomori, S. I. & Murakami, W. T. Glycolipids of hamster fibroblasts and derived malignant-transformed cell lines. Proc. Natl Acad. Sci. USA59, 254–261 (1968). 10.1073/pnas.59.1.254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Gill, D. J. et al. Initiation of GalNAc-type O-glycosylation in the endoplasmic reticulum promotes cancer cell invasiveness. Proc. Natl Acad. Sci. USA110, E3152–E3161 (2013). 10.1073/pnas.1305269110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Powers, T. W. et al. MALDI imaging mass spectrometry profiling of N-glycans in formalin-fixed paraffin embedded clinical tissue blocks and tissue microarrays. PLoS One9, e106255 (2014). 10.1371/journal.pone.0106255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Kannagi, R., Yin, J., Miyazaki, K. & Izawa, M. Current relevance of incomplete synthesis and neo-synthesis for cancer-associated alteration of carbohydrate determinants-Hakomori’s concepts revisited. Biochim Biophys. Acta1780, 525–531 (2008). 10.1016/j.bbagen.2007.10.007 [DOI] [PubMed] [Google Scholar]
- 213.Kakugawa, Y. et al. Up-regulation of plasma membrane-associated ganglioside sialidase (Neu3) in human colon cancer and its involvement in apoptosis suppression. Proc. Natl Acad. Sci. USA99, 10718–10723 (2002). 10.1073/pnas.152597199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Kumamoto, K. et al. Increased expression of UDP-galactose transporter messenger RNA in human colon cancer tissues and its implication in synthesis of Thomsen-Friedenreich antigen and sialyl Lewis A/X determinants. Cancer Res61, 4620–4627 (2001). [PubMed] [Google Scholar]
- 215.Schietinger, A. et al. A mutant chaperone converts a wild-type protein into a tumor-specific antigen. Science314, 304–308 (2006). 10.1126/science.1129200 [DOI] [PubMed] [Google Scholar]
- 216.Hakomori, S. Glycosylation defining cancer malignancy: new wine in an old bottle. Proc. Natl. Acad. Sci. USA99, 10231–10233 (2002). 10.1073/pnas.172380699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Zhang, Y. et al. Global variations in lung cancer incidence by histological subtype in 2020: a population-based study. Lancet Oncol.24, 1206–1218 (2023). 10.1016/S1470-2045(23)00444-8 [DOI] [PubMed] [Google Scholar]
- 218.Lastwika, K. J. et al. Posttranslational modifications induce autoantibodies with risk prediction capability in patients with small cell lung cancer. Sci. Transl. Med15, eadd8469 (2023). 10.1126/scitranslmed.add8469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Kondo, K. et al. Identification of distinct N-glycosylation patterns on extracellular vesicles from small-cell and non-small-cell lung cancer cells. J. Biol. Chem.298, 101950 (2022). 10.1016/j.jbc.2022.101950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Saitou, A. et al. N-glycosylation regulates MET processing and signaling. Cancer Sci.113, 1292–1304 (2022). 10.1111/cas.15278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Reticker-Flynn, N. E. & Bhatia, S. N. Aberrant glycosylation promotes lung cancer metastasis through adhesion to galectins in the metastatic niche. Cancer Discov.5, 168–181 (2015). 10.1158/2159-8290.CD-13-0760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Cui, Y. et al. B4GALT1 promotes immune escape by regulating the expression of PD-L1 at multiple levels in lung adenocarcinoma. J. Exp. Clin. Cancer Res.42, 146 (2023). 10.1186/s13046-023-02711-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Lee, H. H. et al. Removal of N-linked glycosylation enhances PD-L1 detection and predicts anti-PD-1/PD-L1 therapeutic efficacy. Cancer Cell36, 168–178.e164 (2019). 10.1016/j.ccell.2019.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Wang, Y. et al. Comprehensive serum N-glycan profiling identifies a biomarker panel for early diagnosis of non-small-cell lung cancer. Proteomics23, e2300140 (2023). 10.1002/pmic.202300140 [DOI] [PubMed] [Google Scholar]
- 225.Alvarez, M. R. et al. Glycomic, glycoproteomic, and proteomic profiling of philippine lung cancer and peritumoral tissues: case series study of patients Stages I-III. Cancers (Basel)15, 15051559 (2023). [DOI] [PMC free article] [PubMed]
- 226.Mun, D. G. et al. Proteogenomic characterization of human early-onset gastric cancer. Cancer Cell35, 111–124.e110 (2019). 10.1016/j.ccell.2018.12.003 [DOI] [PubMed] [Google Scholar]
- 227.Pinho, S. S. et al. Gastric cancer: adding glycosylation to the equation. Trends Mol. Med19, 664–676 (2013). 10.1016/j.molmed.2013.07.003 [DOI] [PubMed] [Google Scholar]
- 228.Krug, J. et al. N-glycosylation regulates intrinsic IFN-gamma resistance in colorectal cancer: implications for immunotherapy. Gastroenterology164, 392–406.e395 (2023). 10.1053/j.gastro.2022.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.He, L., Guo, Z., Wang, W., Tian, S. & Lin, R. FUT2 inhibits the EMT and metastasis of colorectal cancer by increasing LRP1 fucosylation. Cell Commun. Signal21, 63 (2023). 10.1186/s12964-023-01060-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Irimura, T. et al. Colorectal cancer metastasis determined by carbohydrate-mediated cell adhesion: role of sialyl-LeX antigens. Semin Cancer Biol.4, 319–324 (1993). [PubMed] [Google Scholar]
- 231.Li, J. et al. The ligation between ERMAP, galectin-9 and dectin-2 promotes Kupffer cell phagocytosis and antitumor immunity. Nat. Immunol.24, 1813–1824 (2023). 10.1038/s41590-023-01634-7 [DOI] [PubMed] [Google Scholar]
- 232.Liang, Y. et al. Systemic delivery of glycosylated-PEG-masked oncolytic virus enhances targeting of antitumor immuno-virotherapy and modulates T and NK cell infiltration. Theranostics13, 5452–5468 (2023). 10.7150/thno.87498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Dall’Olio, F. & Chiricolo, M. Sialyltransferases in cancer. Glycoconj. J.18, 841–850 (2001). 10.1023/A:1022288022969 [DOI] [PubMed] [Google Scholar]
- 234.Lise, M. et al. Clinical correlations of alpha2,6-sialyltransferase expression in colorectal cancer patients. Hybridoma19, 281–286 (2000). 10.1089/027245700429828 [DOI] [PubMed] [Google Scholar]
- 235.Bresalier, R. S., Rockwell, R. W., Dahiya, R., Duh, Q. Y. & Kim, Y. S. Cell surface sialoprotein alterations in metastatic murine colon cancer cell lines selected in an animal model for colon cancer metastasis. Cancer Res.50, 1299–1307 (1990). [PubMed] [Google Scholar]
- 236.Leppanen, A., Stowell, S., Blixt, O. & Cummings, R. D. Dimeric galectin-1 binds with high affinity to alpha2,3-sialylated and non-sialylated terminal N-acetyllactosamine units on surface-bound extended glycans. J. Biol. Chem.280, 5549–5562 (2005). 10.1074/jbc.M412019200 [DOI] [PubMed] [Google Scholar]
- 237.Zhuo, Y. & Bellis, S. L. Emerging role of alpha2,6-sialic acid as a negative regulator of galectin binding and function. J. Biol. Chem.286, 5935–5941 (2011). 10.1074/jbc.R110.191429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Trinchera, M., Aronica, A. & Dall’Olio, F. Selectin ligands Sialyl-Lewis a and Sialyl-Lewis x in gastrointestinal cancers. Biology (Basel)6, 6010016 (2017). [DOI] [PMC free article] [PubMed]
- 239.Nakamori, S. et al. Increased expression of sialyl Lewisx antigen correlates with poor survival in patients with colorectal carcinoma: clinicopathological and immunohistochemical study. Cancer Res.53, 3632–3637 (1993). [PubMed] [Google Scholar]
- 240.Marrelli, D. et al. Preoperative positivity of serum tumor markers is a strong predictor of hematogenous recurrence of gastric cancer. J. Surg. Oncol.78, 253–258 (2001). 10.1002/jso.1163 [DOI] [PubMed] [Google Scholar]
- 241.Luo, G. et al. Roles of CA19-9 in pancreatic cancer: biomarker, predictor and promoter. Biochim Biophys. Acta Rev. Cancer1875, 188409 (2021). 10.1016/j.bbcan.2020.188409 [DOI] [PubMed] [Google Scholar]
- 242.Xu, Z., Zhang, Y., Ocansey, D. K. W., Wang, B. & Mao, F. Glycosylation in cervical cancer: new insights and clinical implications. Front Oncol.11, 706862 (2021). 10.3389/fonc.2021.706862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Shen, H., Lee, C. Y. & Chen, C. H. Protein glycosylation as biomarkers in gynecologic cancers. Diagnostics (Basel)12, 12123177 (2022). [DOI] [PMC free article] [PubMed]
- 244.Wu, J. et al. Glycosyltransferase-related prognostic and diagnostic biomarkers of uterine corpus endometrial carcinoma. Comput Biol. Med.163, 107164 (2023). 10.1016/j.compbiomed.2023.107164 [DOI] [PubMed] [Google Scholar]
- 245.Mittal, P. et al. Altered N-linked glycosylation in endometrial cancer. Anal. Bioanal. Chem.413, 2721–2733 (2021). 10.1007/s00216-020-03039-z [DOI] [PubMed] [Google Scholar]
- 246.Yang, Y., Zhu, G., Yang, L. & Yang, Y. Targeting CD24 as a novel immunotherapy for solid cancers. Cell Commun. Signal21, 312 (2023). 10.1186/s12964-023-01315-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Payne, K. K. et al. BTN3A1 governs antitumor responses by coordinating alphabeta and gammadelta T cells. Science369, 942–949 (2020). 10.1126/science.aay2767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Gautam, S. K. et al. Mucins as potential biomarkers for early detection of cancer. Cancers (Basel)15, 15061640 (2023). [DOI] [PMC free article] [PubMed]
- 249.Memarzadeh, S. et al. Urokinase plasminogen activator receptor: prognostic biomarker for endometrial cancer. Proc. Natl. Acad. Sci. USA99, 10647–10652 (2002). 10.1073/pnas.152127499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Kacperczyk, M., Kmieciak, A. & Kratz, E. M. The role of ApoE expression and variability of its glycosylation in human reproductive health in the light of current information. Int. J. Mol. Sci.22, 22137197 (2021). [DOI] [PMC free article] [PubMed]
- 251.Cao, K. et al. Attenuation of sialylation augments antitumor immunity and improves response to immunotherapy in ovarian cancer. Cancer Res.83, 2171–2186 (2023). 10.1158/0008-5472.CAN-22-3260 [DOI] [PubMed] [Google Scholar]
- 252.Thompson, S., Dargan, E. & Turner, G. A. Increased fucosylation and other carbohydrate changes in haptoglobin in ovarian cancer. Cancer Lett.66, 43–48 (1992). 10.1016/0304-3835(92)90278-4 [DOI] [PubMed] [Google Scholar]
- 253.Scott, E. et al. ST6GAL1-mediated aberrant sialylation promotes prostate cancer progression. J. Pathol.261, 71–84 (2023). 10.1002/path.6152 [DOI] [PubMed] [Google Scholar]
- 254.Hatano, K. et al. Simultaneous analysis of serum alpha2,3-linked sialylation and core-type fucosylation of prostate-specific antigen for the detection of high-grade prostate cancer. Br. J. Cancer126, 764–770 (2022). 10.1038/s41416-021-01637-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Liang, D. et al. Glycosylation in breast cancer progression and mammary development: molecular connections and malignant transformations. Life Sci.326, 121781 (2023). 10.1016/j.lfs.2023.121781 [DOI] [PubMed] [Google Scholar]
- 256.Gupta, R., Ponangi, R. & Indresh, K. G. Role of glycosylation in breast cancer progression and metastasis: implications for miRNA, EMT and multidrug resistance. Glycobiology33, 545–555 (2023). 10.1093/glycob/cwad046 [DOI] [PubMed] [Google Scholar]
- 257.Chang, X. et al. Glycosylated proteins with abnormal glycosylation changes are potential biomarkers for early diagnosis of breast cancer. Int J. Biol. Macromol.236, 123855 (2023). 10.1016/j.ijbiomac.2023.123855 [DOI] [PubMed] [Google Scholar]
- 258.Huang, Y. et al. FUT8-mediated aberrant N-glycosylation of B7H3 suppresses the immune response in triple-negative breast cancer. Nat. Commun.12, 2672 (2021). 10.1038/s41467-021-22618-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Taifour, T. et al. The tumor-derived cytokine Chi3l1 induces neutrophil extracellular traps that promote T cell exclusion in triple-negative breast cancer. Immunity56, 2755–2772.e2758 (2023). 10.1016/j.immuni.2023.11.002 [DOI] [PubMed] [Google Scholar]
- 260.Polyak, K. Breast cancer: origins and evolution. J. Clin. Invest117, 3155–3163 (2007). 10.1172/JCI33295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Berx, G. & van Roy, F. Involvement of members of the cadherin superfamily in cancer. Cold Spring Harb. Perspect. Biol.1, a003129 (2009). 10.1101/cshperspect.a003129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Valla, S. et al. Syndecan-1 depletion has a differential impact on hyaluronic acid metabolism and tumor cell behavior in luminal and triple-negative breast cancer cells. Int. J. Mol. Sci.22, 22115874 (2021). [DOI] [PMC free article] [PubMed]
- 263.Ma, Z. & Vosseller, K. Cancer metabolism and elevated O-GlcNAc in oncogenic signaling. J. Biol. Chem.289, 34457–34465 (2014). 10.1074/jbc.R114.577718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Caldwell, S. A. et al. Nutrient sensor O-GlcNAc transferase regulates breast cancer tumorigenesis through targeting of the oncogenic transcription factor FoxM1. Oncogene29, 2831–2842 (2010). 10.1038/onc.2010.41 [DOI] [PubMed] [Google Scholar]
- 265.Rossi, M. et al. PHGDH heterogeneity potentiates cancer cell dissemination and metastasis. Nature605, 747–753 (2022). 10.1038/s41586-022-04758-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Bieniasz-Krzywiec, P. et al. Podoplanin-expressing macrophages promote lymphangiogenesis and lymphoinvasion in breast cancer. Cell Metab.30, 917–936.e910 (2019). 10.1016/j.cmet.2019.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Scupakova, K. et al. Clinical importance of high-mannose, fucosylated, and complex N-glycans in breast cancer metastasis. JCI Insight6, 146945 (2021). [DOI] [PMC free article] [PubMed]
- 268.Yang, M. et al. Inhibition of DPAGT1 suppresses HER2 shedding and trastuzumab resistance in human breast cancer. J. Clin. Invest.133, 164428 (2023). [DOI] [PMC free article] [PubMed]
- 269.Tominaga, N. et al. RPN2-mediated glycosylation of tetraspanin CD63 regulates breast cancer cell malignancy. Mol. Cancer13, 134 (2014). 10.1186/1476-4598-13-134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Ho, W. L., Hsu, W. M., Huang, M. C., Kadomatsu, K. & Nakagawara, A. Protein glycosylation in cancers and its potential therapeutic applications in neuroblastoma. J. Hematol. Oncol.9, 100 (2016). 10.1186/s13045-016-0334-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Hall, M. K. et al. Limited N-glycan processing impacts chaperone expression patterns, cell growth and cell invasiveness in neuroblastoma. Biology (Basel)12, 12020293 (2023). [DOI] [PMC free article] [PubMed]
- 272.Stip, M. C. et al. IgA antibody immunotherapy targeting GD2 is effective in preclinical neuroblastoma models. J. Immunother Cancer11, 006948 (2023). [DOI] [PMC free article] [PubMed]
- 273.Qin, W. et al. Serum protein N-Glycosylation signatures of neuroblastoma. Front Oncol.11, 603417 (2021). 10.3389/fonc.2021.603417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Xiao, Z. et al. EGFR is a potential therapeutic target for highly glycosylated and aggressive pancreatic neuroendocrine neoplasms. Int. J. Cancer153, 164–172 (2023). 10.1002/ijc.34499 [DOI] [PubMed] [Google Scholar]
- 275.Liu, Z. et al. Detecting tumor antigen-specific T cells via interaction-dependent fucosyl-biotinylation. Cell183, 1117–1133.e1119 (2020). 10.1016/j.cell.2020.09.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Dela Cruz, C. S. et al. N-linked glycosylation is required for optimal function of Kaposi’s sarcoma herpesvirus-encoded, but not cellular, interleukin 6. J. Exp. Med199, 503–514 (2004). 10.1084/jem.20031205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Huijbers, E. J. M. et al. Targeting tumor vascular CD99 inhibits tumor growth. Front Immunol.10, 651 (2019). 10.3389/fimmu.2019.00651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Darzi, M., Gorgin, S., Majidzadeh, A. K. & Esmaeili, R. Gene co-expression network analysis reveals immune cell infiltration as a favorable prognostic marker in non-uterine leiomyosarcoma. Sci. Rep.11, 2339 (2021). 10.1038/s41598-021-81952-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Wahren-Herlenius, M. & Dorner, T. Immunopathogenic mechanisms of systemic autoimmune disease. Lancet382, 819–831 (2013). 10.1016/S0140-6736(13)60954-X [DOI] [PubMed] [Google Scholar]
- 280.Aoyama, M. et al. Effects of terminal galactose residues in mannose alpha1-6 arm of Fc-glycan on the effector functions of therapeutic monoclonal antibodies. MAbs11, 826–836 (2019). 10.1080/19420862.2019.1608143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Scallon, B. J., Tam, S. H., McCarthy, S. G., Cai, A. N. & Raju, T. S. Higher levels of sialylated Fc glycans in immunoglobulin G molecules can adversely impact functionality. Mol. Immunol.44, 1524–1534 (2007). 10.1016/j.molimm.2006.09.005 [DOI] [PubMed] [Google Scholar]
- 282.Anthony, R. M. et al. Recapitulation of IVIG anti-inflammatory activity with a recombinant IgG Fc. Science320, 373–376 (2008). 10.1126/science.1154315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Zhou, X. et al. Antibody glycosylation in autoimmune diseases. Autoimmun. Rev.20, 102804 (2021). 10.1016/j.autrev.2021.102804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Su, Z., Xie, Q., Wang, Y. & Li, Y. Abberant Immunoglobulin G Glycosylation in Rheumatoid Arthritis by LTQ-ESI-MS. Int. J. Mol. Sci.21, 21062045 (2020). [DOI] [PMC free article] [PubMed]
- 285.Huang, C., Liu, Y., Wu, H., Sun, D. & Li, Y. Characterization of IgG glycosylation in rheumatoid arthritis patients by MALDI-TOF-MS(n) and capillary electrophoresis. Anal. Bioanal. Chem.409, 3731–3739 (2017). 10.1007/s00216-017-0302-1 [DOI] [PubMed] [Google Scholar]
- 286.Parekh, R. B. et al. Association of rheumatoid arthritis and primary osteoarthritis with changes in the glycosylation pattern of total serum IgG. Nature316, 452–457 (1985). 10.1038/316452a0 [DOI] [PubMed] [Google Scholar]
- 287.Kaneko, Y., Nimmerjahn, F. & Ravetch, J. V. Anti-inflammatory activity of immunoglobulin G resulting from Fc sialylation. Science313, 670–673 (2006). 10.1126/science.1129594 [DOI] [PubMed] [Google Scholar]
- 288.Matsumoto, A., Shikata, K., Takeuchi, F., Kojima, N. & Mizuochi, T. Autoantibody activity of IgG rheumatoid factor increases with decreasing levels of galactosylation and sialylation. J. Biochem128, 621–628 (2000). 10.1093/oxfordjournals.jbchem.a022794 [DOI] [PubMed] [Google Scholar]
- 289.Harre, U. et al. Glycosylation of immunoglobulin G determines osteoclast differentiation and bone loss. Nat. Commun.6, 6651 (2015). 10.1038/ncomms7651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Bernard, N. J. Rheumatoid arthritis: changes in ACPA Fc glycosylation patterns prior to RA onset. Nat. Rev. Rheumatol.9, 697 (2013). 10.1038/nrrheum.2013.162 [DOI] [PubMed] [Google Scholar]
- 291.Bondt, A. et al. IgA N- and O-glycosylation profiling reveals no association with the pregnancy-related improvement in rheumatoid arthritis. Arthritis Res. Ther.19, 160 (2017). 10.1186/s13075-017-1367-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Orr, C. et al. Synovial tissue research: a state-of-the-art review. Nat. Rev. Rheumatol.13, 463–475 (2017). 10.1038/nrrheum.2017.115 [DOI] [PubMed] [Google Scholar]
- 293.Xu, Z. et al. Integrative proteomics and N-glycoproteomics analyses of rheumatoid arthritis synovium reveal immune-associated glycopeptides. Mol. Cell Proteom.22, 100540 (2023). 10.1016/j.mcpro.2023.100540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Wang, Y. et al. Loss of alpha2-6 sialylation promotes the transformation of synovial fibroblasts into a pro-inflammatory phenotype in arthritis. Nat. Commun.12, 2343 (2021). 10.1038/s41467-021-22365-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Kiriakidou, M. & Ching, C. L. Systemic lupus erythematosus. Ann. Intern Med172, ITC81–ITC96 (2020). 10.7326/AITC202006020 [DOI] [PubMed] [Google Scholar]
- 296.Wang, X. & Xia, Y. Anti-double stranded DNA antibodies: origin, pathogenicity, and targeted therapies. Front Immunol.10, 1667 (2019). 10.3389/fimmu.2019.01667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Han, J. et al. Fucosylation of anti-dsDNA IgG1 correlates with disease activity of treatment-naive systemic lupus erythematosus patients. EBioMedicine77, 103883 (2022). 10.1016/j.ebiom.2022.103883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Chui, D. et al. Genetic remodeling of protein glycosylation in vivo induces autoimmune disease. Proc. Natl Acad. Sci. USA98, 1142–1147 (2001). 10.1073/pnas.98.3.1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Alves, I. et al. Protein mannosylation as a diagnostic and prognostic biomarker of lupus nephritis: an unusual glycan neoepitope in systemic lupus erythematosus. Arthritis Rheumatol.73, 2069–2077 (2021). 10.1002/art.41768 [DOI] [PubMed] [Google Scholar]
- 300.Green, R. S. et al. Mammalian N-glycan branching protects against innate immune self-recognition and inflammation in autoimmune disease pathogenesis. Immunity27, 308–320 (2007). 10.1016/j.immuni.2007.06.008 [DOI] [PubMed] [Google Scholar]
- 301.Bruner, L. P., White, A. M. & Proksell, S. Inflammatory bowel disease. Prim. Care50, 411–427 (2023). 10.1016/j.pop.2023.03.009 [DOI] [PubMed] [Google Scholar]
- 302.Kudelka, M. R., Stowell, S. R., Cummings, R. D. & Neish, A. S. Intestinal epithelial glycosylation in homeostasis and gut microbiota interactions in IBD. Nat. Rev. Gastroenterol. Hepatol.17, 597–617 (2020). 10.1038/s41575-020-0331-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Clerc, F. et al. Plasma N-Glycan signatures are associated with features of inflammatory bowel diseases. Gastroenterology155, 829–843 (2018). 10.1053/j.gastro.2018.05.030 [DOI] [PubMed] [Google Scholar]
- 304.Trbojevic Akmacic, I. et al. Inflammatory bowel disease associates with proinflammatory potential of the immunoglobulin G glycome. Inflamm. Bowel Dis.21, 1237–1247 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Simurina, M. et al. Glycosylation of immunoglobulin G associates with clinical features of inflammatory bowel diseases. Gastroenterology154, 1320–1333.e1310 (2018). 10.1053/j.gastro.2018.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Fujii, H. et al. Core fucosylation on T cells, required for activation of T-cell receptor signaling and induction of colitis in mice, is increased in patients with inflammatory bowel disease. Gastroenterology150, 1620–1632 (2016). 10.1053/j.gastro.2016.03.002 [DOI] [PubMed] [Google Scholar]
- 307.Shinzaki, S. et al. IgG oligosaccharide alterations are a novel diagnostic marker for disease activity and the clinical course of inflammatory bowel disease. Am. J. Gastroenterol.103, 1173–1181 (2008). 10.1111/j.1572-0241.2007.01699.x [DOI] [PubMed] [Google Scholar]
- 308.Shinzaki, S. et al. Lectin-based immunoassay for aberrant IgG glycosylation as the biomarker for Crohn’s disease. Inflamm. Bowel Dis.19, 321–331 (2013). 10.1097/MIB.0b013e318280eade [DOI] [PubMed] [Google Scholar]
- 309.Muller, S. et al. Galectin-3 modulates T cell activity and is reduced in the inflamed intestinal epithelium in IBD. Inflamm. Bowel Dis.12, 588–597 (2006). 10.1097/01.MIB.0000225341.37226.7c [DOI] [PubMed] [Google Scholar]
- 310.Nishida, A. et al. Inducible colitis-associated glycome capable of stimulating the proliferation of memory CD4+ T cells. J. Exp. Med209, 2383–2394 (2012). 10.1084/jem.20112631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Li, Y., Tang, R., Leung, P. S. C., Gershwin, M. E. & Ma, X. Bile acids and intestinal microbiota in autoimmune cholestatic liver diseases. Autoimmun. Rev.16, 885–896 (2017). 10.1016/j.autrev.2017.07.002 [DOI] [PubMed] [Google Scholar]
- 312.Lohse, A. W. & Mieli-Vergani, G. Autoimmune hepatitis. J. Hepatol.55, 171–182 (2011). 10.1016/j.jhep.2010.12.012 [DOI] [PubMed] [Google Scholar]
- 313.Muratori, L., Lohse, A. W. & Lenzi, M. Diagnosis and management of autoimmune hepatitis. BMJ380, e070201 (2023). 10.1136/bmj-2022-070201 [DOI] [PubMed] [Google Scholar]
- 314.Zhou, X. et al. Glycomic analysis of antibody indicates distinctive glycosylation profile in patients with autoimmune cholangitis. J. Autoimmun.113, 102503 (2020). 10.1016/j.jaut.2020.102503 [DOI] [PubMed] [Google Scholar]
- 315.Hardtke-Wolenski, M. et al. Genetic predisposition and environmental danger signals initiate chronic autoimmune hepatitis driven by CD4+ T cells. Hepatology58, 718–728 (2013). 10.1002/hep.26380 [DOI] [PubMed] [Google Scholar]
- 316.Hao, X. et al. Deficient O-GlcNAc glycosylation impairs regulatory T cell differentiation and notch signaling in autoimmune hepatitis. Front Immunol.9, 2089 (2018). 10.3389/fimmu.2018.02089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Hao, X. et al. Deficiency of O-linked-glycosylation regulates activation of T cells and aggravates Concanavalin A-induced liver injury. Toxicology433-434, 152411 (2020). 10.1016/j.tox.2020.152411 [DOI] [PubMed] [Google Scholar]
- 318.Vincent, A. Unravelling the pathogenesis of myasthenia gravis. Nat. Rev. Immunol.2, 797–804 (2002). 10.1038/nri916 [DOI] [PubMed] [Google Scholar]
- 319.Rodgaard, A., Nielsen, F. C., Djurup, R., Somnier, F. & Gammeltoft, S. Acetylcholine receptor antibody in myasthenia gravis: predominance of IgG subclasses 1 and 3. Clin. Exp. Immunol.67, 82–88 (1987). [PMC free article] [PubMed] [Google Scholar]
- 320.Hoch, W. et al. Auto-antibodies to the receptor tyrosine kinase MuSK in patients with myasthenia gravis without acetylcholine receptor antibodies. Nat. Med7, 365–368 (2001). 10.1038/85520 [DOI] [PubMed] [Google Scholar]
- 321.Koneczny, I., Cossins, J., Waters, P., Beeson, D. & Vincent, A. MuSK myasthenia gravis IgG4 disrupts the interaction of LRP4 with MuSK but both IgG4 and IgG1-3 can disperse preformed agrin-independent AChR clusters. PLoS One8, e80695 (2013). 10.1371/journal.pone.0080695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Huijbers, M. G. et al. MuSK myasthenia gravis monoclonal antibodies: Valency dictates pathogenicity. Neurol. Neuroimmunol. Neuroinflamm6, e547 (2019). 10.1212/NXI.0000000000000547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Koers, J. et al. Differences in IgG autoantibody Fab glycosylation across autoimmune diseases. J. Allergy Clin. Immunol.151, 1646–1654 (2023). 10.1016/j.jaci.2022.10.035 [DOI] [PubMed] [Google Scholar]
- 324.Meltzer, R. H. et al. Electrostatic steering at acetylcholine binding sites. Biophys. J.91, 1302–1314 (2006). 10.1529/biophysj.106.081463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Fichtner, M. L. et al. Affinity maturation is required for pathogenic monovalent IgG4 autoantibody development in myasthenia gravis. J. Exp. Med.217, 20200513 (2020). [DOI] [PMC free article] [PubMed]
- 326.Mandel-Brehm, C. et al. Elevated N-linked glycosylation of IgG V regions in myasthenia gravis disease subtypes. J. Immunol.207, 2005–2014 (2021). 10.4049/jimmunol.2100225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Selman, M. H. et al. IgG fc N-glycosylation changes in Lambert-Eaton myasthenic syndrome and myasthenia gravis. J. Proteome Res10, 143–152 (2011). 10.1021/pr1004373 [DOI] [PubMed] [Google Scholar]
- 328.Barrios, C. et al. Glycosylation profile of IgG in moderate kidney dysfunction. J. Am. Soc. Nephrol.27, 933–941 (2016). 10.1681/ASN.2015010109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Allen, A. C., Bailey, E. M., Barratt, J., Buck, K. S. & Feehally, J. Analysis of IgA1 O-glycans in IgA nephropathy by fluorophore-assisted carbohydrate electrophoresis. J. Am. Soc. Nephrol.10, 1763–1771 (1999). 10.1681/ASN.V1081763 [DOI] [PubMed] [Google Scholar]
- 330.Stamellou, E. et al. IgA nephropathy. Nat. Rev. Dis. Prim.9, 67 (2023). 10.1038/s41572-023-00476-9 [DOI] [PubMed] [Google Scholar]
- 331.Nemcic, M. et al. N-glycosylation of serum proteins in adult type 1 diabetes mellitus exposes further changes compared to children at the disease onset. Clin. Chim. Acta543, 117298 (2023). 10.1016/j.cca.2023.117298 [DOI] [PubMed] [Google Scholar]
- 332.Zhang, Z. J. et al. Human plasma IgG N-Glycome profiles reveal a proinflammatory phenotype in chronic thromboembolic pulmonary hypertension. Hypertension80, 1929–1939 (2023). 10.1161/HYPERTENSIONAHA.123.21408 [DOI] [PubMed] [Google Scholar]
- 333.Cvetko, A. et al. Plasma N-glycome shows continuous deterioration as the diagnosis of insulin resistance approaches. BMJ Open Diabetes Res. Care9, 002263 (2021). [DOI] [PMC free article] [PubMed]
- 334.Wang, H. F. et al. Protein O-GlcNAcylation in cardiovascular diseases. Acta Pharm. Sin.44, 8–18 (2023). 10.1038/s41401-022-00934-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Arany, Z. et al. Transcriptional coactivator PGC-1 alpha controls the energy state and contractile function of cardiac muscle. Cell Metab.1, 259–271 (2005). 10.1016/j.cmet.2005.03.002 [DOI] [PubMed] [Google Scholar]
- 336.Yu, H., Wen, L. & Mu, Y. O-GlcNAcylation is essential for autophagy in cardiomyocytes. Oxid. Med Cell Longev.2020, 5602396 (2020). 10.1155/2020/5602396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Brainard, R. E. & Facundo, H. T. Cardiac hypertrophy drives PGC-1alpha suppression associated with enhanced O-glycosylation. Biochim Biophys. Acta Mol. Basis Dis.1867, 166080 (2021). 10.1016/j.bbadis.2021.166080 [DOI] [PubMed] [Google Scholar]
- 338.Chen, X. et al. Increased O-GlcNAcylation induces myocardial hypertrophy. Vitr. Cell Dev. Biol. Anim.56, 735–743 (2020). 10.1007/s11626-020-00503-z [DOI] [PubMed] [Google Scholar]
- 339.Si, R. et al. Overexpression of p53 due to excess protein O-GlcNAcylation is associated with coronary microvascular disease in type 2 diabetes. Cardiovasc Res116, 1186–1198 (2020). 10.1093/cvr/cvz216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Yang, W. H. et al. Modification of p53 with O-linked N-acetylglucosamine regulates p53 activity and stability. Nat. Cell Biol.8, 1074–1083 (2006). 10.1038/ncb1470 [DOI] [PubMed] [Google Scholar]
- 341.Huang, L. et al. O-GlcNAc-modified SNAP29 inhibits autophagy-mediated degradation via the disturbed SNAP29-STX17-VAMP8 complex and exacerbates myocardial injury in type I diabetic rats. Int J. Mol. Med42, 3278–3290 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Ferro, F. et al. Glycosylation of blood cells during the onset and progression of atherosclerosis and myocardial infarction. Trends Mol. Med30, 178–196 (2024). 10.1016/j.molmed.2023.11.013 [DOI] [PubMed] [Google Scholar]
- 343.Bottcher, M. et al. Extracorporeal blood circulation in the unanesthetized free-moving rabbit-an animal model for biocompatibility studies. Z. Exp. Chir. Transpl. Kunstliche Organe21, 139–144 (1988). [PubMed] [Google Scholar]
- 344.Eckardt, V., Weber, C. & von Hundelshausen, P. Glycans and Glycan-Binding Proteins in Atherosclerosis. Thromb. Haemost.119, 1265–1273 (2019). 10.1055/s-0039-1692720 [DOI] [PubMed] [Google Scholar]
- 345.Mironov, A. A. & Beznoussenko, G. V. Opinion: On the way towards the new paradigm of atherosclerosis. Int. J. Mol. Sci.23, 23042152 (2022). [DOI] [PMC free article] [PubMed]
- 346.Lim, S. Y. et al. N-glycan profiles of acute myocardial infarction patients reveal potential biomarkers for diagnosis, severity assessment and treatment monitoring. Glycobiology32, 469–482 (2022). 10.1093/glycob/cwab129 [DOI] [PubMed] [Google Scholar]
- 347.Birukov, A. et al. Immunoglobulin G N-glycosylation signatures in incident type 2 diabetes and cardiovascular disease. Diabetes Care45, 2729–2736 (2022). 10.2337/dc22-0833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Mican, J., Toul, M., Bednar, D. & Damborsky, J. Structural biology and protein engineering of thrombolytics. Comput Struct. Biotechnol. J.17, 917–938 (2019). 10.1016/j.csbj.2019.06.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Toul, M. et al. Identification, characterization, and engineering of glycosylation in thrombolytics. Biotechnol. Adv.66, 108174 (2023). 10.1016/j.biotechadv.2023.108174 [DOI] [PubMed] [Google Scholar]
- 350.Keyt, B. A. et al. A faster-acting and more potent form of tissue plasminogen activator. Proc. Natl Acad. Sci. USA91, 3670–3674 (1994). 10.1073/pnas.91.9.3670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Paoni, N. F. et al. Involvement of residues 296-299 in the enzymatic activity of tissue-type plasminogen activator. Protein Eng.5, 259–266 (1992). 10.1093/protein/5.3.259 [DOI] [PubMed] [Google Scholar]
- 352.Refino, C. J. et al. A variant of t-PA (T103N, KHRR 296-299 AAAA) that, by bolus, has increased potency and decreased systemic activation of plasminogen. Thromb. Haemost.70, 313–319 (1993). 10.1055/s-0038-1649572 [DOI] [PubMed] [Google Scholar]
- 353.Soesanto, Y. et al. Pleiotropic and age-dependent effects of decreased protein modification by O-linked N-acetylglucosamine on pancreatic beta-cell function and vascularization. J. Biol. Chem.286, 26118–26126 (2011). 10.1074/jbc.M111.249508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Yang, Y. et al. O-GlcNAc transferase inhibits visceral fat lipolysis and promotes diet-induced obesity. Nat. Commun.11, 181 (2020). 10.1038/s41467-019-13914-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Zhu, C. et al. Site-specific glycoprofiles of HDL-associated apoe are correlated with HDL functional capacity and unaffected by short-term diet. J. Proteome Res18, 3977–3984 (2019). 10.1021/acs.jproteome.9b00450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Teslovich, T. M. et al. Biological, clinical and population relevance of 95 loci for blood lipids. Nature466, 707–713 (2010). 10.1038/nature09270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Khetarpal, S. A. et al. Loss of function of GALNT2 lowers high-density lipoproteins in humans, nonhuman primates, and rodents. Cell Metab.24, 234–245 (2016). 10.1016/j.cmet.2016.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Bouche, C., Serdy, S., Kahn, C. R. & Goldfine, A. B. The cellular fate of glucose and its relevance in type 2 diabetes. Endocr. Rev.25, 807–830 (2004). 10.1210/er.2003-0026 [DOI] [PubMed] [Google Scholar]
- 359.Chatham, J. C., Zhang, J. & Wende, A. R. Role of O-linked N-acetylglucosamine protein modification in cellular (Patho)Physiology. Physiol. Rev.101, 427–493 (2021). 10.1152/physrev.00043.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Grundke-Iqbal, I. et al. Microtubule-associated protein tau. A component of Alzheimer paired helical filaments. J. Biol. Chem.261, 6084–6089 (1986). 10.1016/S0021-9258(17)38495-8 [DOI] [PubMed] [Google Scholar]
- 361.Liu, F., Iqbal, K., Grundke-Iqbal, I., Hart, G. W. & Gong, C. X. O-GlcNAcylation regulates phosphorylation of tau: a mechanism involved in Alzheimer’s disease. Proc. Natl Acad. Sci. USA101, 10804–10809 (2004). 10.1073/pnas.0400348101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Yuzwa, S. A. et al. A potent mechanism-inspired O-GlcNAcase inhibitor that blocks phosphorylation of tau in vivo. Nat. Chem. Biol.4, 483–490 (2008). 10.1038/nchembio.96 [DOI] [PubMed] [Google Scholar]
- 363.Kim, C. et al. O-linked beta-N-acetylglucosaminidase inhibitor attenuates beta-amyloid plaque and rescues memory impairment. Neurobiol. Aging34, 275–285 (2013). 10.1016/j.neurobiolaging.2012.03.001 [DOI] [PubMed] [Google Scholar]
- 364.Wang, P. et al. O-GlcNAc cycling mutants modulate proteotoxicity in Caenorhabditis elegans models of human neurodegenerative diseases. Proc. Natl Acad. Sci. USA109, 17669–17674 (2012). 10.1073/pnas.1205748109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Ross, C. A. & Poirier, M. A. Opinion: What is the role of protein aggregation in neurodegeneration? Nat. Rev. Mol. Cell Biol.6, 891–898 (2005). 10.1038/nrm1742 [DOI] [PubMed] [Google Scholar]
- 366.Wani, W. Y. et al. O-GlcNAc regulation of autophagy and alpha-synuclein homeostasis; implications for Parkinson’s disease. Mol. Brain10, 32 (2017). 10.1186/s13041-017-0311-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Reily, C., Stewart, T. J., Renfrow, M. B. & Novak, J. Glycosylation in health and disease. Nat. Rev. Nephrol.15, 346–366 (2019). 10.1038/s41581-019-0129-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Gilgunn, S., Conroy, P. J., Saldova, R., Rudd, P. M. & O’Kennedy, R. J. Aberrant PSA glycosylation-a sweet predictor of prostate cancer. Nat. Rev. Urol.10, 99–107 (2013). 10.1038/nrurol.2012.258 [DOI] [PubMed] [Google Scholar]
- 369.Qian, Y. et al. Quantitative analysis of serum IgG galactosylation assists differential diagnosis of ovarian cancer. J. Proteome Res12, 4046–4055 (2013). 10.1021/pr4003992 [DOI] [PubMed] [Google Scholar]
- 370.Ren, S. et al. Distribution of IgG galactosylation as a promising biomarker for cancer screening in multiple cancer types. Cell Res26, 963–966 (2016). 10.1038/cr.2016.83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Xu, X. et al. A signature based on glycosyltransferase genes provides a promising tool for the prediction of prognosis and immunotherapy responsiveness in ovarian cancer. J. Ovarian Res16, 5 (2023). 10.1186/s13048-022-01088-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Jones, R. B., Dorsett, K. A., Hjelmeland, A. B. & Bellis, S. L. The ST6Gal-I sialyltransferase protects tumor cells against hypoxia by enhancing HIF-1alpha signaling. J. Biol. Chem.293, 5659–5667 (2018). 10.1074/jbc.RA117.001194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Schultz, M. J. et al. The tumor-associated glycosyltransferase ST6Gal-I regulates stem cell transcription factors and confers a cancer stem cell phenotype. Cancer Res.76, 3978–3988 (2016). 10.1158/0008-5472.CAN-15-2834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Terava, J. et al. Primary breast cancer biomarkers based on glycosylation and extracellular vesicles detected from human serum. Cancer Rep. (Hoboken)5, e1540 (2022). 10.1002/cnr2.1540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Qing, L., Li, Q. & Dong, Z. MUC1: an emerging target in cancer treatment and diagnosis. Bull. Cancer109, 1202–1216 (2022). 10.1016/j.bulcan.2022.08.001 [DOI] [PubMed] [Google Scholar]
- 376.Alirezapour, B. et al. Development of [(6)(4)Cu]-DOTA-PR81 radioimmunoconjugate for MUC-1 positive PET imaging. Nucl. Med Biol.43, 73–80 (2016). 10.1016/j.nucmedbio.2015.07.012 [DOI] [PubMed] [Google Scholar]
- 377.Xu, M. et al. Protein glycosylation in urine as a biomarker of diseases. Transl. Res253, 95–107 (2023). 10.1016/j.trsl.2022.08.001 [DOI] [PubMed] [Google Scholar]
- 378.Ragusa, A. et al. Differential glycosylation levels in saliva from patients with lung or breast cancer: a preliminary assessment for early diagnostic purposes. Metabolites11, 11090566 (2021). [DOI] [PMC free article] [PubMed]
- 379.Zhou, Z. et al. Identification of a novel glycosyltransferase prognostic signature in hepatocellular carcinoma based on LASSO algorithm. Front Genet13, 823728 (2022). 10.3389/fgene.2022.823728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Pucci, M., Duca, M., Malagolini, N. & Dall’Olio, F. Glycosyltransferases in cancer: prognostic biomarkers of survival in patient cohorts and impact on malignancy in experimental models. Cancers (Basel)14, 14092128 (2022). [DOI] [PMC free article] [PubMed]
- 381.Theodoratou, E. et al. Glycosylation of plasma IgG in colorectal cancer prognosis. Sci. Rep.6, 28098 (2016). 10.1038/srep28098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.do Nascimento, J. C. F., Beltrao, E. I. C. & Rocha, C. R. C. High FUT3 expression is a marker of lower overall survival of breast cancer patients. Glycoconj. J.37, 263–275 (2020). 10.1007/s10719-020-09914-2 [DOI] [PubMed] [Google Scholar]
- 383.Honma, R. et al. Expression of fucosyltransferase 8 is associated with an unfavorable clinical outcome in non-small cell lung cancers. Oncology88, 298–308 (2015). 10.1159/000369495 [DOI] [PubMed] [Google Scholar]
- 384.Peric, L. et al. Glycosylation alterations in cancer cells, prognostic value of glycan biomarkers and their potential as novel therapeutic targets in breast cancer. Biomedicines10, 10123265 (2022). [DOI] [PMC free article] [PubMed]
- 385.Zhao, L. et al. Characterization of glycosylation regulator-mediated glycosylation modification patterns and tumor microenvironment infiltration in hepatocellular carcinoma. Front Genet13, 1001901 (2022). 10.3389/fgene.2022.1001901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Shi, Y. et al. Glycosylation-related molecular subtypes and risk score of hepatocellular carcinoma: Novel insights to clinical decision-making. Front Endocrinol. (Lausanne)13, 1090324 (2022). 10.3389/fendo.2022.1090324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Liang, J. X. et al. A novel glycosylation-related gene signature predicts survival in patients with lung adenocarcinoma. BMC Bioinforma.23, 562 (2022). 10.1186/s12859-022-05109-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Thomas, D., Rathinavel, A. K. & Radhakrishnan, P. Altered glycosylation in cancer: a promising target for biomarkers and therapeutics. Biochim Biophys. Acta Rev. Cancer1875, 188464 (2021). 10.1016/j.bbcan.2020.188464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Brown, J. R. et al. A disaccharide-based inhibitor of glycosylation attenuates metastatic tumor cell dissemination. Clin. Cancer Res12, 2894–2901 (2006). 10.1158/1078-0432.CCR-05-2745 [DOI] [PubMed] [Google Scholar]
- 390.Badurova, L. et al. 2-Deoxy-D-glucose inhibits lymphocytic choriomeningitis virus propagation by targeting glycoprotein N-glycosylation. Virol. J.20, 108 (2023). 10.1186/s12985-023-02082-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Lee, N., Jang, W. J., Seo, J. H., Lee, S. & Jeong, C. H. 2-Deoxy-d-Glucose-Induced metabolic alteration in human oral squamous SCC15 cells: involvement of N-Glycosylation of Axl and Met. Metabolites9, 9090188 (2019). [DOI] [PMC free article] [PubMed]
- 392.Repas, J. et al. Dual effect of combined metformin and 2-Deoxy-D-Glucose treatment on mitochondrial biogenesis and PD-L1 expression in triple-negative breast cancer cells. Cancers (Basel)14, 14051343 (2022). [DOI] [PMC free article] [PubMed]
- 393.Julien, S. et al. Sialyl-Tn vaccine induces antibody-mediated tumour protection in a relevant murine model. Br. J. Cancer100, 1746–1754 (2009). 10.1038/sj.bjc.6605083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Chugh, S. et al. Pathobiological implications of mucin glycans in cancer: Sweet poison and novel targets. Biochim Biophys. Acta1856, 211–225 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Xi, X. et al. Glycosylated modification of MUC1 maybe a new target to promote drug sensitivity and efficacy for breast cancer chemotherapy. Cell Death Dis.13, 708 (2022). 10.1038/s41419-022-05110-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Bose, M. et al. Targeting tumor-associated MUC1 overcomes anoikis-resistance in pancreatic cancer. Transl. Res.253, 41–56 (2023). 10.1016/j.trsl.2022.08.010 [DOI] [PubMed] [Google Scholar]
- 397.Salouti, M., Rajabi, H., Babaei, M. H. & Rasaee, M. J. Breast tumor targeting with (99m)Tc-HYNIC-PR81 complex as a new biologic radiopharmaceutical. Nucl. Med Biol.35, 763–768 (2008). 10.1016/j.nucmedbio.2008.07.002 [DOI] [PubMed] [Google Scholar]
- 398.Rong, Y. et al. Induction of protective and therapeutic anti-pancreatic cancer immunity using a reconstructed MUC1 DNA vaccine. BMC Cancer9, 191 (2009). 10.1186/1471-2407-9-191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Posey, A. D. Jr. et al. Engineered CAR T cells targeting the cancer-associated Tn-glycoform of the membrane mucin MUC1 control adenocarcinoma. Immunity44, 1444–1454 (2016). 10.1016/j.immuni.2016.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Gerber-Lemaire, S. & Juillerat-Jeanneret, L. Glycosylation pathways as drug targets for cancer: glycosidase inhibitors. Mini Rev. Med Chem.6, 1043–1052 (2006). 10.2174/138955706778195162 [DOI] [PubMed] [Google Scholar]
- 401.Shi, S. et al. Inhibition of MAN2A1 enhances the immune response to Anti-PD-L1 in human tumors. Clin. Cancer Res.26, 5990–6002 (2020). 10.1158/1078-0432.CCR-20-0778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Petrosyan, A. & Cheng, P. W. Golgi fragmentation induced by heat shock or inhibition of heat shock proteins is mediated by non-muscle myosin IIA via its interaction with glycosyltransferases. Cell Stress Chaperones19, 241–254 (2014). 10.1007/s12192-013-0450-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Pili, R. et al. The alpha-glucosidase I inhibitor castanospermine alters endothelial cell glycosylation, prevents angiogenesis, and inhibits tumor growth. Cancer Res.55, 2920–2926 (1995). [PubMed] [Google Scholar]
- 404.Bousseau, S. et al. Glycosylation as new pharmacological strategies for diseases associated with excessive angiogenesis. Pharm. Ther.191, 92–122 (2018). 10.1016/j.pharmthera.2018.06.003 [DOI] [PubMed] [Google Scholar]
- 405.Goede, V. et al. Obinutuzumab plus chlorambucil in patients with CLL and coexisting conditions. N. Engl. J. Med370, 1101–1110 (2014). 10.1056/NEJMoa1313984 [DOI] [PubMed] [Google Scholar]
- 406.Zhang, Z., Pan, H. & Chen, X. Mass spectrometry for structural characterization of therapeutic antibodies. Mass Spectrom. Rev.28, 147–176 (2009). 10.1002/mas.20190 [DOI] [PubMed] [Google Scholar]
- 407.Zheng, K., Bantog, C. & Bayer, R. The impact of glycosylation on monoclonal antibody conformation and stability. MAbs3, 568–576 (2011). 10.4161/mabs.3.6.17922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Kayser, V. et al. Glycosylation influences on the aggregation propensity of therapeutic monoclonal antibodies. Biotechnol. J.6, 38–44 (2011). 10.1002/biot.201000091 [DOI] [PubMed] [Google Scholar]
- 409.Mishra, N., Spearman, M., Donald, L., Perreault, H. & Butler, M. Comparison of two glycoengineering strategies to control the fucosylation of a monoclonal antibody. J. Biotechnol.324S, 100015 (2020). 10.1016/j.btecx.2020.100015 [DOI] [PubMed] [Google Scholar]
- 410.Heard, A. et al. Antigen glycosylation regulates efficacy of CAR T cells targeting CD19. Nat. Commun.13, 3367 (2022). 10.1038/s41467-022-31035-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Chen, F., Huang, G. & Huang, H. Sugar ligand-mediated drug delivery. Future Med Chem.12, 161–171 (2020). 10.4155/fmc-2019-0114 [DOI] [PubMed] [Google Scholar]
- 412.Nahar, M. et al. Functional polymeric nanoparticles: an efficient and promising tool for active delivery of bioactives. Crit. Rev. Ther. Drug Carr. Syst.23, 259–318 (2006). 10.1615/CritRevTherDrugCarrierSyst.v23.i4.10 [DOI] [PubMed] [Google Scholar]
- 413.Peterslund, N. A., Koch, C., Jensenius, J. C. & Thiel, S. Association between deficiency of mannose-binding lectin and severe infections after chemotherapy. Lancet358, 637–638 (2001). 10.1016/S0140-6736(01)05785-3 [DOI] [PubMed] [Google Scholar]
- 414.Sun, Y. et al. Galactose-containing polymer-DOX conjugates for targeting drug delivery. AAPS PharmSciTech18, 749–758 (2017). 10.1208/s12249-016-0557-4 [DOI] [PubMed] [Google Scholar]
- 415.Qu, B. et al. Design, synthesis and biological evaluation of multivalent glucosides with high affinity as ligands for brain targeting liposomes. Eur. J. Med Chem.72, 110–118 (2014). 10.1016/j.ejmech.2013.10.007 [DOI] [PubMed] [Google Scholar]
- 416.Chou, P. Y. et al. Glycosylation of OVA antigen-loaded PLGA nanoparticles enhances DC-targeting for cancer vaccination. J. Control Release351, 970–988 (2022). 10.1016/j.jconrel.2022.10.002 [DOI] [PubMed] [Google Scholar]
- 417.Liu, X. et al. Glyco-decorated tobacco mosaic virus as a vector for cisplatin delivery. J. Mater. Chem. B5, 2078–2085 (2017). 10.1039/C7TB00100B [DOI] [PubMed] [Google Scholar]
- 418.Zou, Y., Yang, W., Meng, F. & Zhong, Z. Targeted hepatoma chemotherapy in vivo using galactose-decorated crosslinked pH-sensitive degradable micelles. J. Control Release213, e125–e126 (2015). 10.1016/j.jconrel.2015.05.212 [DOI] [PubMed] [Google Scholar]
- 419.Sharma, R. et al. Glycosylation of PAMAM dendrimers significantly improves tumor macrophage targeting and specificity in glioblastoma. J. Control Release337, 179–192 (2021). 10.1016/j.jconrel.2021.07.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Auriti, C. et al. Mannose-binding lectin: biologic characteristics and role in the susceptibility to infections and ischemia-reperfusion related injury in critically Ill neonates. J. Immunol. Res.2017, 7045630 (2017). 10.1155/2017/7045630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421.Yu, W., Zhang, N. & Li, C. Saccharide modified pharmaceutical nanocarriers for targeted drug and gene delivery. Curr. Pharm. Des.15, 3826–3836 (2009). 10.2174/138161209789649547 [DOI] [PubMed] [Google Scholar]
- 422.Buskas, T., Li, Y. & Boons, G. J. The immunogenicity of the tumor-associated antigen Lewis(y) may be suppressed by a bifunctional cross-linker required for coupling to a carrier protein. Chemistry10, 3517–3524 (2004). 10.1002/chem.200400074 [DOI] [PubMed] [Google Scholar]
- 423.Slovin, S. F. et al. Carbohydrate vaccines in cancer: immunogenicity of a fully synthetic globo H hexasaccharide conjugate in man. Proc. Natl. Acad. Sci. USA96, 5710–5715 (1999). 10.1073/pnas.96.10.5710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.Cai, H. et al. Fully synthetic self-adjuvanting thioether-conjugated glycopeptide-lipopeptide antitumor vaccines for the induction of complement-dependent cytotoxicity against tumor cells. Chemistry19, 1962–1970 (2013). 10.1002/chem.201203709 [DOI] [PubMed] [Google Scholar]
- 425.Mettu, R., Chen, C. Y. & Wu, C. Y. Synthetic carbohydrate-based vaccines: challenges and opportunities. J. Biomed. Sci.27, 9 (2020). 10.1186/s12929-019-0591-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Pohl, J. et al. D-19575-a sugar-linked isophosphoramide mustard derivative exploiting transmembrane glucose transport. Cancer Chemother. Pharm.35, 364–370 (1995). 10.1007/s002800050248 [DOI] [PubMed] [Google Scholar]
- 427.Goff, R. D. & Thorson, J. S. Assessment of chemoselective neoglycosylation methods using chlorambucil as a model. J. Med Chem.53, 8129–8139 (2010). 10.1021/jm101024j [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428.Mikuni, K. et al. In vivo antitumor activity of novel water-soluble taxoids. Biol. Pharm. Bull.31, 1155–1158 (2008). 10.1248/bpb.31.1155 [DOI] [PubMed] [Google Scholar]
- 429.Moayedi, S. et al. Sugar codes conjugated alginate: an innovative platform to make a strategic breakthrough in simultaneous prophylaxis of GERD and helicobacter pylori infection. Drug Des. Devel Ther.14, 2405–2412 (2020). 10.2147/DDDT.S255611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430.Calvaresi, E. C. & Hergenrother, P. J. Glucose conjugation for the specific targeting and treatment of cancer. Chem. Sci.4, 2319–2333 (2013). 10.1039/c3sc22205e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431.Liu, X. et al. Dual targeting of innate and adaptive checkpoints on tumor cells limits immune evasion. Cell Rep.24, 2101–2111 (2018). 10.1016/j.celrep.2018.07.062 [DOI] [PubMed] [Google Scholar]
- 432.Raghavendra, N. M., Pingili, D., Kadasi, S., Mettu, A. & Prasad, S. Dual or multi-targeting inhibitors: the next generation anticancer agents. Eur. J. Med Chem.143, 1277–1300 (2018). 10.1016/j.ejmech.2017.10.021 [DOI] [PubMed] [Google Scholar]
- 433.Liu, Z. et al. Recent development in hydrophilic interaction liquid chromatography stationary materials for glycopeptide analysis. Anal. Methods14, 4437–4448 (2022). 10.1039/D2AY01369J [DOI] [PubMed] [Google Scholar]
- 434.Kozlik, P., Goldman, R. & Sanda, M. Hydrophilic interaction liquid chromatography in the separation of glycopeptides and their isomers. Anal. Bioanal. Chem.410, 5001–5008 (2018). 10.1007/s00216-018-1150-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435.van der Burgt, Y. E. M., Siliakus, K. M., Cobbaert, C. M. & Ruhaak, L. R. HILIC-MRM-MS for linkage-specific separation of sialylated glycopeptides to quantify prostate-specific antigen proteoforms. J. Proteome Res19, 2708–2716 (2020). 10.1021/acs.jproteome.0c00050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436.Molnarova, K., Duris, A., Jecmen, T. & Kozlik, P. Comparison of human IgG glycopeptides separation using mixed-mode hydrophilic interaction/ion-exchange liquid chromatography and reversed-phase mode. Anal. Bioanal. Chem.413, 4321–4328 (2021). 10.1007/s00216-021-03388-3 [DOI] [PubMed] [Google Scholar]
- 437.Hernandez-Hernandez, O., Quintanilla-Lopez, J. E., Lebron-Aguilar, R., Sanz, M. L. & Moreno, F. J. Characterization of post-translationally modified peptides by hydrophilic interaction and reverse phase liquid chromatography coupled to quadrupole-time-of-flight mass spectrometry. J. Chromatogr. A1428, 202–211 (2016). 10.1016/j.chroma.2015.07.096 [DOI] [PubMed] [Google Scholar]
- 438.Illiano, A. et al. Protein glycosylation investigated by mass spectrometry: an overview. Cells9, 9091986 (2020). [DOI] [PMC free article] [PubMed]
- 439.Hajduk, J. et al. Monitoring of antibody glycosylation pattern based on microarray MALDI-TOF mass spectrometry. J. Biotechnol.302, 77–84 (2019). 10.1016/j.jbiotec.2019.06.306 [DOI] [PubMed] [Google Scholar]
- 440.O’Brien, J. F., Lacey, J. M. & Bergen, H. R., 3rd. Detection of hypo-N-glycosylation using mass spectrometry of transferrin. Curr Protoc. Hum. Genet Chapter 17, Unit 17 14 (2007). [DOI] [PubMed]
- 441.Kotsias, M. et al. A semi-automated, high throughput approach for O-glycosylation profiling of in vitro established cancer cell lines by MALDI-FT-ICR MS. Glycoconj. J.38, 747–756 (2021). 10.1007/s10719-021-10003-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442.Sethi, M. K., Hancock, W. S. & Fanayan, S. Identifying N-Glycan biomarkers in colorectal cancer by mass spectrometry. Acc. Chem. Res.49, 2099–2106 (2016). 10.1021/acs.accounts.6b00193 [DOI] [PubMed] [Google Scholar]
- 443.Long, Z. et al. Determination of glycosylation degree for glycoconjugate vaccines using a solid-phase extraction combined with liquid chromatography and tandem mass spectrometry method. J. Sep Sci.43, 2880–2888 (2020). 10.1002/jssc.202000075 [DOI] [PubMed] [Google Scholar]
- 444.Naumann, L. et al. High-throughput glycosylation analysis of intact monoclonal antibodies by mass spectrometry coupled with capillary electrophoresis and liquid chromatography. J. Sep Sci.45, 2034–2044 (2022). 10.1002/jssc.202100865 [DOI] [PubMed] [Google Scholar]
- 445.Moran, A. B. et al. Profiling the proteoforms of urinary prostate-specific antigen by capillary electrophoresis—mass spectrometry. J. Proteom.238, 104148 (2021). 10.1016/j.jprot.2021.104148 [DOI] [PubMed] [Google Scholar]
- 446.Gardner, R. A., Urbanowicz, P. A. & Spencer, D. I. R. N-glycan characterization by liquid chromatography coupled with fluorimetry and mass spectrometry. Methods Mol. Biol.2370, 267–280 (2022). 10.1007/978-1-0716-1685-7_13 [DOI] [PubMed] [Google Scholar]
- 447.Wu, C. C. et al. Identification of fucosylated SERPINA1 as a novel plasma marker for pancreatic cancer using lectin affinity capture coupled with iTRAQ-based quantitative glycoproteomics. Int. J. Mol. Sci.22, 22116079 (2021). [DOI] [PMC free article] [PubMed]
- 448.Li, Y., Tao, S. C., Zhu, H. & Schneck, J. P. High-throughput lectin microarray-based analysis of live cell surface glycosylation. Curr. Protoc. Protein Sci.12, 12 19 11–12 19 17 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449.Li, Y. et al. Detection and verification of glycosylation patterns of glycoproteins from clinical specimens using lectin microarrays and lectin-based immunosorbent assays. Anal. Chem.83, 8509–8516 (2011). 10.1021/ac201452f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 450.Tao, S. C. et al. Lectin microarrays identify cell-specific and functionally significant cell surface glycan markers. Glycobiology18, 761–769 (2008). 10.1093/glycob/cwn063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 451.Kuno, A. et al. Focused differential glycan analysis with the platform antibody-assisted lectin profiling for glycan-related biomarker verification. Mol. Cell Proteom.8, 99–108 (2009). 10.1074/mcp.M800308-MCP200 [DOI] [PubMed] [Google Scholar]
- 452.Hsu, K. L. & Mahal, L. K. A lectin microarray approach for the rapid analysis of bacterial glycans. Nat. Protoc.1, 543–549 (2006). 10.1038/nprot.2006.76 [DOI] [PubMed] [Google Scholar]
- 453.Zhao, J., Patwa, T. H., Lubman, D. M. & Simeone, D. M. Protein biomarkers in cancer: natural glycoprotein microarray approaches. Curr. Opin. Mol. Ther.10, 602–610 (2008). [PMC free article] [PubMed] [Google Scholar]
- 454.Patwa, T. H., Zhao, J., Anderson, M. A., Simeone, D. M. & Lubman, D. M. Screening of glycosylation patterns in serum using natural glycoprotein microarrays and multi-lectin fluorescence detection. Anal. Chem.78, 6411–6421 (2006). 10.1021/ac060726z [DOI] [PubMed] [Google Scholar]
- 455.Hirabayashi, J. Lectin-based glycomics: how and when was the technology born? Methods Mol. Biol.1200, 225–242 (2014). 10.1007/978-1-4939-1292-6_20 [DOI] [PubMed] [Google Scholar]
- 456.Loster, K. & Kannicht, C. 2-dimensional electrophoresis: detection of glycosylation and influence on spot pattern. Methods Mol. Biol.446, 199–214 (2008). 10.1007/978-1-60327-084-7_14 [DOI] [PubMed] [Google Scholar]
- 457.Fu, C. & van Aalten, D. M. F. Native detection of protein O-GlcNAcylation by gel electrophoresis. Analyst145, 6826–6830 (2020). 10.1039/C9AN02506E [DOI] [PMC free article] [PubMed] [Google Scholar]
- 458.Liu, D. et al. Rapid glycosylation analysis of mouse serum glycoproteins separated by supported molecular matrix electrophoresis. J. Proteom.234, 104098 (2021). 10.1016/j.jprot.2020.104098 [DOI] [PubMed] [Google Scholar]
- 459.Khatri, K. et al. Microfluidic capillary electrophoresis-mass spectrometry for analysis of monosaccharides, oligosaccharides, and glycopeptides. Anal. Chem.89, 6645–6655 (2017). 10.1021/acs.analchem.7b00875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 460.Guttman, A. & Pritchett, T. Capillary gel electrophoresis separation of high-mannose type oligosaccharides derivatized by 1-aminopyrene-3,6,8-trisulfonic acid. Electrophoresis16, 1906–1911 (1995). 10.1002/elps.11501601314 [DOI] [PubMed] [Google Scholar]
- 461.Yamane-Ohnuki, N. et al. Establishment of FUT8 knockout Chinese hamster ovary cells: an ideal host cell line for producing completely defucosylated antibodies with enhanced antibody-dependent cellular cytotoxicity. Biotechnol. Bioeng.87, 614–622 (2004). 10.1002/bit.20151 [DOI] [PubMed] [Google Scholar]
- 462.Cajic, S., Hennig, R., Burock, R. & Rapp, E. Capillary (Gel) electrophoresis-based methods for immunoglobulin (G) glycosylation analysis. Exp. Suppl.112, 137–172 (2021). [DOI] [PubMed] [Google Scholar]
- 463.Silva, A. M. N., Vitorino, R., Domingues, M. R. M., Spickett, C. M. & Domingues, P. Post-translational modifications and mass spectrometry detection. Free Radic. Biol. Med65, 925–941 (2013). 10.1016/j.freeradbiomed.2013.08.184 [DOI] [PubMed] [Google Scholar]
- 464.Schubert, M., Walczak, M. J., Aebi, M. & Wider, G. Posttranslational modifications of intact proteins detected by NMR spectroscopy: application to glycosylation. Angew. Chem. Int Ed. Engl.54, 7096–7100 (2015). 10.1002/anie.201502093 [DOI] [PubMed] [Google Scholar]
- 465.Tagashira, M., Iijima, H. & Toma, K. An NMR study of O-glycosylation induced structural changes in the alpha-helix of calcitonin. Glycoconj. J.19, 43–52 (2002). 10.1023/A:1022532930708 [DOI] [PubMed] [Google Scholar]
- 466.Fan, T. W. & Lane, A. N. Applications of NMR spectroscopy to systems biochemistry. Prog. Nucl. Magn. Reson Spectrosc.92-93, 18–53 (2016). 10.1016/j.pnmrs.2016.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 467.Kopec, M., Imiela, A. & Abramczyk, H. Monitoring glycosylation metabolism in brain and breast cancer by Raman imaging. Sci. Rep.9, 166 (2019). 10.1038/s41598-018-36622-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 468.Davies, H. S. et al. Secondary structure and glycosylation of mucus glycoproteins by Raman spectroscopies. Anal. Chem.88, 11609–11615 (2016). 10.1021/acs.analchem.6b03095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 469.Uriel, C. et al. BODIPYs as chemically stable fluorescent tags for synthetic glycosylation strategies towards fluorescently labeled saccharides. Chemistry26, 5388–5399 (2020). 10.1002/chem.201905780 [DOI] [PubMed] [Google Scholar]
- 470.Zaro, B. W., Bateman, L. A. & Pratt, M. R. Robust in-gel fluorescence detection of mucin-type O-linked glycosylation. Bioorg. Med Chem. Lett.21, 5062–5066 (2011). 10.1016/j.bmcl.2011.04.038 [DOI] [PubMed] [Google Scholar]
- 471.Haga, Y. et al. Visualizing specific protein glycoforms by transmembrane fluorescence resonance energy transfer. Nat. Commun.3, 907 (2012). 10.1038/ncomms1906 [DOI] [PubMed] [Google Scholar]
- 472.Belardi, B. et al. Imaging the glycosylation state of cell surface glycoproteins by two-photon fluorescence lifetime imaging microscopy. Angew. Chem. Int. Ed. Engl.52, 14045–14049 (2013). 10.1002/anie.201307512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 473.de la Fuente, J. M. & Penades, S. Glyconanoparticles: types, synthesis and applications in glycoscience, biomedicine and material science. Biochim Biophys. Acta1760, 636–651 (2006). 10.1016/j.bbagen.2005.12.001 [DOI] [PubMed] [Google Scholar]
- 474.Zhang, W. et al. In situ fluorescence imaging of the levels of glycosylation and phosphorylation by a MOF-based nanoprobe in depressed mice. Anal. Chem.92, 3716–3721 (2020). 10.1021/acs.analchem.9b04878 [DOI] [PubMed] [Google Scholar]
- 475.Versloot, R. C. A. et al. Quantification of protein glycosylation using nanopores. Nano Lett.22, 5357–5364 (2022). 10.1021/acs.nanolett.2c01338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 476.Mayampurath, A. M., Wu, Y., Segu, Z. M., Mechref, Y. & Tang, H. Improving confidence in detection and characterization of protein N-glycosylation sites and microheterogeneity. Rapid Commun. Mass Spectrom.25, 2007–2019 (2011). 10.1002/rcm.5059 [DOI] [PubMed] [Google Scholar]
- 477.Saunders, M. J., Woods, R. J. & Yang, L. Simplifying the detection and monitoring of protein glycosylation during in vitro glycoengineering. Sci. Rep.13, 567 (2023). 10.1038/s41598-023-27634-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 478.Blochl, C., Wang, D., Mayboroda, O. A., Lageveen-Kammeijer, G. S. M. & Wuhrer, M. Transcriptionally imprinted glycomic signatures of acute myeloid leukemia. Cell Biosci.13, 31 (2023). 10.1186/s13578-023-00981-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 479.Kudelka, M. R. et al. Targeting altered glycosylation in secreted tumor glycoproteins for broad cancer detection. Glycobiology33, 567–578 (2023). 10.1093/glycob/cwad035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 480.Bergeron, J. J. M. & Thomas, D. Y. N-glycosylation mediated folding and quality control in serine proteases of the hepsin family. FEBS J.290, 3963–3965 (2023). 10.1111/febs.16779 [DOI] [PubMed] [Google Scholar]
- 481.Ondruskova, N., Cechova, A., Hansikova, H., Honzik, T. & Jaeken, J. Congenital disorders of glycosylation: Still “hot” in 2020. Biochim Biophys. Acta Gen. Subj.1865, 129751 (2021). 10.1016/j.bbagen.2020.129751 [DOI] [PubMed] [Google Scholar]
- 482.Matthijs, G. et al. Mutations in PMM2, a phosphomannomutase gene on chromosome 16p13, in carbohydrate-deficient glycoprotein type I syndrome (Jaeken syndrome). Nat. Genet16, 88–92 (1997). 10.1038/ng0597-88 [DOI] [PubMed] [Google Scholar]
- 483.Zurawski, V. R. Jr., Orjaseter, H., Andersen, A. & Jellum, E. Elevated serum CA 125 levels prior to diagnosis of ovarian neoplasia: relevance for early detection of ovarian cancer. Int J. Cancer42, 677–680 (1988). 10.1002/ijc.2910420507 [DOI] [PubMed] [Google Scholar]
- 484.Goldstein, M. J. & Mitchell, E. P. Carcinoembryonic antigen in the staging and follow-up of patients with colorectal cancer. Cancer Invest.23, 338–351 (2005). 10.1081/CNV-58878 [DOI] [PubMed] [Google Scholar]
- 485.Saldova, R., Fan, Y., Fitzpatrick, J. M., Watson, R. W. & Rudd, P. M. Core fucosylation and alpha2-3 sialylation in serum N-glycome is significantly increased in prostate cancer comparing to benign prostate hyperplasia. Glycobiology21, 195–205 (2011). 10.1093/glycob/cwq147 [DOI] [PubMed] [Google Scholar]
- 486.Zhao, T. et al. Heterogeneities of site-specific N-glycosylation in HCC tumors with low and high AFP concentrations. Front Oncol.10, 496 (2020). 10.3389/fonc.2020.00496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 487.Bast, R. C. Jr. et al. A radioimmunoassay using a monoclonal antibody to monitor the course of epithelial ovarian cancer. N. Engl. J. Med309, 883–887 (1983). 10.1056/NEJM198310133091503 [DOI] [PubMed] [Google Scholar]
- 488.Saldova, R. et al. Exploring the glycosylation of serum CA125. Int J. Mol. Sci.14, 15636–15654 (2013). 10.3390/ijms140815636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 489.Kandylis, K. et al. Diagnostic significance of the tumour markers CEA, CA 15-3 and CA 125 in malignant effusions in breast cancer. Ann. Oncol.1, 435–438 (1990). 10.1093/oxfordjournals.annonc.a057798 [DOI] [PubMed] [Google Scholar]
- 490.Choi, J. W. et al. Use of CA15‑3 for screening breast cancer: an antibody‑lectin sandwich assay for detecting glycosylation of CA15‑3 in sera. Oncol. Rep.40, 145–154 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 491.Coelho, R. et al. Mucins and truncated O-glycans unveil phenotypic discrepancies between serous ovarian cancer cell lines and primary tumours. Int. J. Mol. Sci.19, 19072045 (2018). [DOI] [PMC free article] [PubMed]
- 492.Engle, D. D. et al. The glycan CA19-9 promotes pancreatitis and pancreatic cancer in mice. Science364, 1156–1162 (2019). 10.1126/science.aaw3145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 493.Han, C. et al. A novel multiple biomarker panel for the early detection of high-grade serous ovarian carcinoma. Gynecol. Oncol.149, 585–591 (2018). 10.1016/j.ygyno.2018.03.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 494.Thomsen, M. et al. Prognostic role of carcinoembryonic antigen and carbohydrate antigen 19-9 in metastatic colorectal cancer: a BRAF-mutant subset with high CA 19-9 level and poor outcome. Br. J. Cancer118, 1609–1616 (2018). 10.1038/s41416-018-0115-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 495.Kambara, Y. et al. CA19-9 is a significant prognostic marker of patients with stage III gastric cancer. Eur. J. Surg. Oncol.46, 1918–1924 (2020). 10.1016/j.ejso.2020.05.003 [DOI] [PubMed] [Google Scholar]
- 496.Ito, H., Hoshi, K., Honda, T. & Hashimoto, Y. Lectin-based assay for glycoform-specific detection of alpha2,6-sialylated transferrin and carcinoembryonic antigen in tissue and body fluid. Molecules23, 23061314 (2018). [DOI] [PMC free article] [PubMed]
- 497.Tang, S. et al. CEA in breast ductal secretions as a promising biomarker for the diagnosis of breast cancer: a systematic review and meta-analysis. Breast Cancer23, 813–819 (2016). 10.1007/s12282-016-0680-9 [DOI] [PubMed] [Google Scholar]
- 498.van Manen, L. et al. Elevated CEA and CA19-9 serum levels independently predict advanced pancreatic cancer at diagnosis. Biomarkers25, 186–193 (2020). 10.1080/1354750X.2020.1725786 [DOI] [PubMed] [Google Scholar]
- 499.Pont, L. et al. Site-specific N-linked glycosylation analysis of human carcinoembryonic antigen by sheathless capillary electrophoresis-tandem mass spectrometry. J. Proteome Res.20, 1666–1675 (2021). 10.1021/acs.jproteome.0c00875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 500.Cramer, D. A. T., Franc, V., Caval, T. & Heck, A. J. R. Charting the proteoform landscape of serum proteins in individual donors by high-resolution native mass spectrometry. Anal. Chem.94, 12732–12741 (2022). 10.1021/acs.analchem.2c02215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 501.Lin, Y. et al. A fucosylated glycopeptide as a candidate biomarker for early diagnosis of NASH hepatocellular carcinoma using a stepped HCD method and PRM evaluation. Front Oncol.12, 818001 (2022). 10.3389/fonc.2022.818001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 502.Gimenez, E. et al. Quantitative analysis of N-glycans from human alfa-acid-glycoprotein using stable isotope labeling and zwitterionic hydrophilic interaction capillary liquid chromatography electrospray mass spectrometry as tool for pancreatic disease diagnosis. Anal. Chim. Acta866, 59–68 (2015). 10.1016/j.aca.2015.02.008 [DOI] [PubMed] [Google Scholar]
- 503.Zhu, J. et al. Analysis of serum haptoglobin fucosylation in hepatocellular carcinoma and liver cirrhosis of different etiologies. J. Proteome Res.13, 2986–2997 (2014). 10.1021/pr500128t [DOI] [PMC free article] [PubMed] [Google Scholar]
- 504.Zhang, Y. et al. ESI-LC-MS method for haptoglobin fucosylation analysis in hepatocellular carcinoma and liver cirrhosis. J. Proteome Res.14, 5388–5395 (2015). 10.1021/acs.jproteome.5b00792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 505.Jin, W. et al. Glycoqueuing: Isomer-Specific Quantification for Sialylation-Focused Glycomics. Anal. Chem.91, 10492–10500 (2019). 10.1021/acs.analchem.9b01393 [DOI] [PubMed] [Google Scholar]
- 506.Ruhaak, L. R. et al. The Serum Immunoglobulin G Glycosylation Signature of Gastric Cancer. EuPA Open Proteom.6, 1–9 (2015). 10.1016/j.euprot.2014.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 507.Komaromy, A., Reider, B., Jarvas, G. & Guttman, A. Glycoprotein biomarkers and analysis in chronic obstructive pulmonary disease and lung cancer with special focus on serum immunoglobulin G. Clin. Chim. Acta506, 204–213 (2020). 10.1016/j.cca.2020.03.041 [DOI] [PubMed] [Google Scholar]
- 508.Gu, Y. et al. Serum IgG N-glycans enable early detection and early relapse prediction of colorectal cancer. Int J. Cancer152, 536–547 (2023). 10.1002/ijc.34298 [DOI] [PubMed] [Google Scholar]
- 509.Chen, Z., Gulzar, Z. G., St Hill, C. A., Walcheck, B. & Brooks, J. D. Increased expression of GCNT1 is associated with altered O-glycosylation of PSA, PAP, and MUC1 in human prostate cancers. Prostate74, 1059–1067 (2014). 10.1002/pros.22826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 510.Barrabes, S. et al. Glycosylation of serum ribonuclease 1 indicates a major endothelial origin and reveals an increase in core fucosylation in pancreatic cancer. Glycobiology17, 388–400 (2007). 10.1093/glycob/cwm002 [DOI] [PubMed] [Google Scholar]
- 511.Zhang, S., Jiang, K., Zhang, Q., Guo, K. & Liu, Y. Serum fucosylated paraoxonase 1 as a potential glycobiomarker for clinical diagnosis of early hepatocellular carcinoma using ELISA Index. Glycoconj. J.32, 119–125 (2015). 10.1007/s10719-015-9576-8 [DOI] [PubMed] [Google Scholar]
- 512.Rodriguez-Tomas, E. et al. Serum paraoxonase-1-related variables and lipoprotein profile in patients with lung or head and neck cancer: effect of radiotherapy. Antioxidants (Basel)8, 8070213 (2019). [DOI] [PMC free article] [PubMed]
- 513.Arnold, J. N. et al. Novel glycan biomarkers for the detection of lung cancer. J. Proteome Res.10, 1755–1764 (2011). 10.1021/pr101034t [DOI] [PubMed] [Google Scholar]
- 514.Cohen, E. N. et al. Elevated serum levels of sialyl Lewis X (sLe(X)) and inflammatory mediators in patients with breast cancer. Breast Cancer Res. Treat.176, 545–556 (2019). 10.1007/s10549-019-05258-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 515.Mizuguchi, S. et al. Serum Sialyl Lewis x and cytokeratin 19 fragment as predictive factors for recurrence in patients with stage I non-small cell lung cancer. Lung Cancer58, 369–375 (2007). 10.1016/j.lungcan.2007.07.002 [DOI] [PubMed] [Google Scholar]
- 516.Freitas, D. et al. O-glycans truncation modulates gastric cancer cell signaling and transcription leading to a more aggressive phenotype. EBioMedicine40, 349–362 (2019). 10.1016/j.ebiom.2019.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 517.Blanchard, V. et al. N-glycosylation and biological activity of recombinant human alpha1-antitrypsin expressed in a novel human neuronal cell line. Biotechnol. Bioeng.108, 2118–2128 (2011). 10.1002/bit.23158 [DOI] [PubMed] [Google Scholar]
- 518.Yang, P. et al. Alpha1-antitrypsin and neutrophil elastase imbalance and lung cancer risk. Chest128, 445–452 (2005). 10.1378/chest.128.1.445 [DOI] [PubMed] [Google Scholar]
- 519.Liu, L., Qin, H. & Ye, M. Recent advances in glycopeptide enrichment and mass spectrometry data interpretation approaches for glycoproteomics analyses. Se Pu39, 1045–1054 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 520.Trinidad, J. C., Schoepfer, R., Burlingame, A. L. & Medzihradszky, K. F. N- and O-glycosylation in the murine synaptosome. Mol. Cell Proteom.12, 3474–3488 (2013). 10.1074/mcp.M113.030007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 521.Tsai, I. J., Su, E. C., Tsai, I. L. & Lin, C. Y. Clinical assay for the early detection of colorectal cancer using mass spectrometric wheat germ agglutinin multiple reaction monitoring. Cancers (Basel)13, 13092190 (2021). [DOI] [PMC free article] [PubMed]
- 522.Gbormittah, F. O. et al. Characterization of glycoproteins in pancreatic cyst fluid using a high-performance multiple lectin affinity chromatography platform. J. Proteome Res.13, 289–299 (2014). 10.1021/pr400813u [DOI] [PMC free article] [PubMed] [Google Scholar]
- 523.Gao, C. et al. Unique binding specificities of proteins toward isomeric asparagine-linked glycans. Cell Chem. Biol.26, 535–547.e534 (2019). 10.1016/j.chembiol.2019.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 524.Tian, Y., Zhou, Y., Elliott, S., Aebersold, R. & Zhang, H. Solid-phase extraction of N-linked glycopeptides. Nat. Protoc.2, 334–339 (2007). 10.1038/nprot.2007.42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 525.McCalley, D. V. Understanding and manipulating the separation in hydrophilic interaction liquid chromatography. J. Chromatogr. A1523, 49–71 (2017). 10.1016/j.chroma.2017.06.026 [DOI] [PubMed] [Google Scholar]
- 526.Zhang, Q., Yang, F. Q., Ge, L., Hu, Y. J. & Xia, Z. N. Recent applications of hydrophilic interaction liquid chromatography in pharmaceutical analysis. J. Sep Sci.40, 49–80 (2017). 10.1002/jssc.201600843 [DOI] [PubMed] [Google Scholar]
- 527.Buszewski, B. & Noga, S. Hydrophilic interaction liquid chromatography (HILIC)-a powerful separation technique. Anal. Bioanal. Chem.402, 231–247 (2012). 10.1007/s00216-011-5308-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 528.Cheung, R. C., Wong, J. H. & Ng, T. B. Immobilized metal ion affinity chromatography: a review on its applications. Appl Microbiol Biotechnol.96, 1411–1420 (2012). 10.1007/s00253-012-4507-0 [DOI] [PubMed] [Google Scholar]
- 529.Zhang, L. et al. Boronic acid functionalized core-satellite composite nanoparticles for advanced enrichment of glycopeptides and glycoproteins. Chemistry15, 10158–10166 (2009). 10.1002/chem.200901347 [DOI] [PubMed] [Google Scholar]
- 530.Xie, Y., Liu, Q., Li, Y. & Deng, C. Core-shell structured magnetic metal-organic framework composites for highly selective detection of N-glycopeptides based on boronic acid affinity chromatography. J. Chromatogr. A1540, 87–93 (2018). 10.1016/j.chroma.2018.02.013 [DOI] [PubMed] [Google Scholar]
- 531.Liu, L., Jin, S., Mei, P. & Zhou, P. Preparation of cotton wool modified with boric acid functionalized titania for selective enrichment of glycopeptides. Talanta203, 58–64 (2019). 10.1016/j.talanta.2019.05.050 [DOI] [PubMed] [Google Scholar]
- 532.Zhang, L. et al. Site-specific, covalent immobilization of PNGase F on magnetic particles mediated by microbial transglutaminase. Anal. Chim. Acta1250, 340972 (2023). 10.1016/j.aca.2023.340972 [DOI] [PubMed] [Google Scholar]
- 533.Zhang, W. et al. PNGase F-mediated incorporation of (18)O into glycans for relative glycan quantitation. Analyst140, 1082–1089 (2015). 10.1039/C4AN02073A [DOI] [PubMed] [Google Scholar]
- 534.Marchetti, R. et al. Rules of engagement” of protein-glycoconjugate interactions: a molecular view achievable by using NMR spectroscopy and molecular modeling. ChemistryOpen5, 274–296 (2016). 10.1002/open.201600024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 535.Arda, A. & Jimenez-Barbero, J. The recognition of glycans by protein receptors. Insights from NMR spectroscopy. Chem. Commun. (Camb.)54, 4761–4769 (2018). 10.1039/C8CC01444B [DOI] [PubMed] [Google Scholar]
- 536.Shen, Y., Hu, F. & Min, W. Raman imaging of small biomolecules. Annu Rev. Biophys.48, 347–369 (2019). 10.1146/annurev-biophys-052118-115500 [DOI] [PubMed] [Google Scholar]
- 537.Mohamed Abd-El-Halim, Y. et al. A glycosyltransferase gene signature to detect pancreatic ductal adenocarcinoma patients with poor prognosis. EBioMedicine71, 103541 (2021). 10.1016/j.ebiom.2021.103541 [DOI] [PMC free article] [PubMed] [Google Scholar]