Abstract
The organ-specific toxicity resulting from microplastic (MP) exposure has been extensively explored, particularly concerning the gut, liver, testis, and lung. However, under natural conditions, these effects are not restricted to specific organs or tissues. Investigating whether MP exposure presents a systemic threat to an entire organism, impacting factors such as lifespan, sleep, and fecundity, is essential. In this study, we investigated the effects of dietary exposure to two different doses of MPs (1–5 μm) using the terrestrial model organism Drosophila melanogaster. Results indicated that the particles caused gut damage and remained within the digestive system. Continuous MP exposure significantly shortened the lifespan of adult flies. Even short-term exposure disrupted sleep patterns, increasing the length of daytime sleep episodes. Additionally, one week of MP exposure reduced ovary size, with a trend towards decreased egg-laying in mated females. Although MPs did not penetrate the brain or ovaries, transcriptome analysis revealed altered gene expression in these tissues. In the ovary, Gene Ontology (GO) analysis indicated genotoxic effects impacting inflammation, circadian regulation, and metabolic processes, with significant impacts on extracellular structure-related pathways. In the brain, GO analysis identified changes in pathways associated with proteolysis and carbohydrate metabolism. Overall, this study provides compelling evidence of the systemic negative effects of MP exposure, highlighting the urgent need to address and mitigate environmental MP pollution.
Keywords: Microplastics, Sleep, Lifespan, Reproduction, Risk assessment, Drosophila melanogaster
INTRODUCTION
An estimated 5 billion tons of plastics exist on Earth, with an additional 400 million tons produced annually (Lim, 2021). Recently, intensive attention has been directed towards microplastic (MP) pollutants, which result from the degradation of disposed plastics into particle fragments smaller than 5 mm in diameter (Hartmann et al., 2019). These MPs are ubiquitously distributed in the environment (Lim, 2021), with more than 5 trillion plastic pieces, containing at least 14.9 trillion MP particles and weighing more than 25×104 tons (Eriksen et al., 2014), floating in the ocean (Van Sebille et al., 2015). MP deposition occurs both indoors and outdoors, with central London samples showing deposition rates between 575 and 1 008 microplastics/m2/day (Wright et al., 2020). In eastern China, indoor air MP concentrations (1 583±1 180 n/m3) exceed outdoor air concentrations (189±85 n/m3), and urban air concentrations (224±70 n/m3) are higher than those in rural areas (101±47 n/m3) (Liao et al., 2021).
Humans are not exempt from MP exposure. Studies have demonstrated the transfer of MPs from the environment to organisms (Mallik et al., 2021; Van Raamsdonk et al., 2020). Previous research has suggested that children and adults may ingest more than 105 MPs daily (Nor et al., 2021), and MPs have been detected in human fecal samples (Schwabl et al., 2019; Zhang et al., 2021; Ho et al., 2022). Although concerns about the potential public health risks of MPs are increasing, direct evidence from the general population remains scarce. Animal studies have shown that MP exposure can pose health risks to organisms, including the gut (Jin et al., 2019; Lu et al., 2018; Zhang et al., 2020b), liver (Lu et al., 2016, 2018), testes (Deng et al., 2021; Hou et al., 2021; Jin et al., 2021; Wang et al., 2019a; Lin et al., 2024a, 2024b), developing embryos (Park et al., 2020; Zhang et al., 2020a), respiratory system (Lu et al., 2021), and locomotion function (Zhang et al., 2020b). RNA sequencing (RNA-seq) has also provided a better understanding of the detrimental effects of MP on organisms, revealing that MP exposure can induce an inflammatory response in the lungs of mice and alter gene expression related to the immune system and programmed cell death (Lu et al., 2021). Exposure to di-(2-ethylhexyl) phthalate (DEHP)-contaminated MPs affects the oxidative response, lipid metabolism, and immune response in mice (Deng et al., 2020). Recent studies have also highlighted genotoxic effects of MP, associated with oxidative stress, inflammation, and DNA repair disruption (Jin et al., 2018; Lei et al., 2018; Tagorti & Kaya, 2022).
The detrimental effects of MP exposure appear to extend beyond specific organs or tissues, posing a systemic threat to the entire organism (Deng et al., 2017). Various studies have explored the relationship among environmental pollutants, sleep, and longevity (Bushey et al., 2010; Chuang et al., 2018; Yu et al., 2019). A recent cohort analysis of the UK Biobank linked sleep quality to accelerated biological aging resulting from air pollution (i.e., PM2.5 and NO2) (Gao et al., 2022). However, direct evidence regarding the impact of MP exposure as inert pollutants on broader health aspects, such as sleep and lifespan, remains limited. Due to ethical and technical constraints, animal studies are indispensable for such research. Drosophila melanogaster, commonly known as the fruit fly, inhabits diverse environments, ranging from tropical to temperate climates (Skevington & Dang, 2002). This species shares many conserved functions with mammals, including gastrointestinal and reproductive functions. Fruit flies are attracted to rotting fruits and vegetables, which may be contaminated with MPs via plant uptake or airborne deposition (Azeem et al., 2021; Conti et al., 2020). Additionally, fruit fly larvae develop in the soil until the pupal stage, likely encountering MPs from contaminated soil (Ashburner et al., 1986; Wang et al., 2019b). Thus, D. melanogaster is an ideal organism for assessing MP toxicity due to its short life cycle, high reproductive rate, ease of handling, and extensive genetic tools, making it a highly tractable and powerful model for investigating molecular and cellular mechanisms (Aime & Adamantidis, 2022; Jiang & Pan, 2022; Ma et al., 2022; Wan et al., 2023; Wei et al., 2023).
In the present study, we evaluated the systemic toxicity of MPs in D. melanogaster, focusing on general health concepts, such as sleep, lifespan, and fecundity. The flies were exposed to food mixed with two concentrations of MPs: 1×MPs (0.02 mg/mL) and 10×MPs (0.2 mg/mL) (EFSA Panel on Contaminants in the Food Chain, 2016; Matthews et al., 2021; Zhong et al., 2022). Exposure periods covered different developmental stages, ranging from 2 days to 10 weeks. Results indicated that MP exposure led to intestinal damage, disrupted sleep patterns, reduced ovary size, shortened lifespan, and genotoxic effects, as revealed by RNA-seq analysis.
MATERIALS AND METHODS
Drosophila melanogaster stock and maintenance
Wild-type Canton-S flies were maintained in an environment with a temperature of 25°C and relative humidity of 60% under a 12 h light/dark cycle. All experiments were performed under these conditions. The flies were divided into three groups, with the control group raised on standard cornmeal-dextrose-yeast-agar food (per batch: 4.4 L of H2O, 24 g of agar, 80 g of yeast, 280 g of cornmeal, 200 g of sucrose, 40 g of soymeal, and 12 g of methylparaben). Female flies were used for most experiments unless otherwise specified.
Microplastics (MPs)
Spherical MPs were purchased from Cospheric (Cat# FMG-Green Fluorescent Polymer Microspheres 1.3 g/cc 1–5 μm–500 mg, USA) (Barboza et al., 2018; Bringer et al., 2020; Lu et al., 2021). The MP size ranged from 1 to 5 μm, with excitation and emission wavelengths of 450 nm and 520 nm, respectively. MPs were added to the standard food at 60°C before solidification and stored in the dark (wrapped in foil paper). Flies were raised on standard food or standard food containing 0.02 mg/mL MP (1×MP group, particle concentration: 3.68×106/mL) or 0.2 mg/mL MP (10×MP group, particle number concentration: 3.68×107/mL), respectively (EFSA Panel on Contaminants in the Food Chain, 2016; Marsden et al., 2019; Matthews et al., 2021; Panacek et al., 2011; Posgai et al., 2011; World Health Organization, 2022; Zhang et al., 2020b; Zhong et al., 2022).
Histological analysis of guts and ovaries
Whole adult flies were anesthetized with CO2 and visualized under bright-field or fluorescent light using a fluorescence microscope (MF43-N, Mshot, China). Brains, guts, and ovaries were dissected at room temperature (24°C) in 1×phosphate-buffered saline (PBS). Fresh samples for immediate quantification of gut leakage and ovary size were imaged without fixation. Samples for qualitative analysis were fixed in 4% paraformaldehyde (Cat# 1.17701.018, GHTECH, China) for 30 min at 4°C, followed by 3×5 min washing in 1×PBS with 0.5% Triton (0.5% PBST). The fixed samples were then stained with 4’,6-diamidino-2-phenylindole (DAPI, 1 μg/mL, Cat# C1005, Beyotime, China) for 5 min at room temperature. After rinsing with 0.5% PBST for 3×5 min, samples were mounted in VECTASHIELD antifade mounting medium (Cat# H-1000, Vector Laboratories, USA) and imaged using a 5×objective lens and an Apotome fluorescence microscope (Image 2Z, Zeiss, Germany). Images were analyzed using ZEN imaging (black edition) (https://www.zeiss.com/microscopy/en/products/software/zeiss-zen.html, Zeiss, Germany).
Dye exclusion method using trypan blue
Virgin flies aged 0–2 days were raised on standard food with 1×MPs, 10×MPs, or no MPs (control) for 6 days. On day 7, all groups were starved for 16 h prior to trypan blue treatment, then fed 2 mol/L sucrose solution with 10% trypan blue stain (Cat# 15-250-061, Gibco, USA) using 0.3 mm capillary tubes (Shanghai Changcheng, China) for 2 h. Subsequently, crops and guts were dissected in 1×PBS and imaged at 3×objective under a stereomicroscope (MZ101, Mshot, China) and digital camera (YH-1600, Yuehe, China). ImageJ (v.1.52P, https://imagej.nih.gov/ij/download.html) was used for image quantification. The width of the trypan blue-positive area and the cross-section diameter of each gut sample were measured, with the ratio used to evaluate the level of gut leakage.
Sleep assays and sleep changes
Flies were raised on standard cornmeal food at 25°C. Virgin flies (0–1 day old) were collected and individually placed in 65 mm×5 mm glass tubes containing 5% sucrose and 2% agar food at one end. These tubes, each holding a single fly, were loaded into the DAM2 system (TriKinetics, USA) at 25°C under a 12 h light/dark (LD) cycle. After 2 days of entrainment, baseline sleep was recorded for 2 days. On day 3, immediately after lights-on, flies were transferred to new tubes containing fresh food with 1×MPs, 10×MPs, or no MPs (control), and sleep was recorded for another 6 days. Sleep profiles were plotted in 30 min bins over the entire recording period. Sleep parameters, including total sleep, maximum episode length, mean episode length, number of episodes, and activity while awake, were analyzed for both the light and dark periods (LP and DP, respectively). Each parameter after 5 days of MP exposure was quantified as the change on day 8, normalized to the average of the baseline days (∆=day 8−(day 1+day 2)/2). Analysis was performed using DAMFileScan113 and SCAMP 2019v2 (TriKinetics; https://www.trikinetics.com/) in MATLAB (RRID: SCR_001622).
Lifespan assay
Thirty virgin flies aged 0–1 day were placed into a vial containing standard cornmeal food with 1×MPs, 10×MPs, or no MPs (control). Virgin flies were used to exclude the possible influence of mating experience and oviposition on lifespan, thus ensuring baseline consistency. Flies were transferred to fresh food tubes every 2–3 days. Eight vial replicates were established for each treatment group. The number of living flies in each vial was recorded when the transfer occurred until all flies in each vial died.
Ovary size evaluation
Virgin flies aged 0–3 days were raised on three kinds of food containing 1×MPs, 10×MPs, or no MPs (control). Ovaries were collected and dissected in 1×PBS every 7 days from days 0 to 28, then imaged using a stereomicroscope and digital camera under the same settings as described above. Each ovary from a single fly was outlined as the region of interest (ROI) and measured using ImageJ (v.1.52P) (https://imagej.nih.gov/ij/download.html). The average ROI area from a pair of ovaries from a single fly was quantified for analysis.
Female fecundity assay
Forty virgin female flies (F0) and 40 male flies were allowed to mate overnight, with 10 females and 10 males per group selected and transferred to a Petri dish the following morning for egg collection for 2 h. Each dish contained 5% sucrose and 2% agar medium with yeast paste on top. To determine the effects of MPs on female fecundity during development, approximately 2 000 eggs were collected and distributed evenly into 48 vials with either standard food or 1×MP food until adulthood (F1). After eclosion, 15 F1 virgin females were collected and allowed to mate with 12 males without MP exposure, then transferred to a Petri dish for female fecundity tests for 6 consecutive days. Egg number was counted every 24 h using a microscope (MZ101, Mshot, China). Six control F1 and 1×MP-treated F1 group replicates were established for analysis.
To assess the effects of MPs on fertilization ability, 2–3-day-old virgins were raised on standard cornmeal food containing 1×MPs, 10×MPs, or no MPs (control) for 5 days. On day 6, male flies were introduced to mate with the virgin females for 2 days. On day 8, the females were transferred to a Petri dish for egg collection over a 24 h period.
To evaluate the effects of MPs on reproductive capacity, 2–3-day-old mated females were raised on standard cornmeal food containing 1×MPs, 10×MPs, or no MPs (control) for 7 days. On day 8, the females were transferred to a Petri dish for egg collection over a 24 h period.
Each Petri dish contained 2% yeast solution on top of an apple juice-agar medium to provide better contrast for the eggs, which were imaged using a digital camera (VARIO-SUMMILUX-H 1:1.9-2.4/17-80 ASPH, Leica, Germany). Egg number was counted using the Cell Counter plugin in ImageJ (v.1.52P) (https://imagej.nih.gov/ij/download.html). Sixteen replicates were established for each group for analysis.
RNA-seq analysis
Virgin female flies aged 0–5 days were divided into 1×MP and control groups. Each group initially consisted of four vials containing 40 flies each. Flies were raised for 48 days, with transfer to new vials containing fresh food with/without 1×MPs every 2–3 days. Brain and ovary samples were first dissected in ice-cold adult hemolymph-like saline solution (AHL; 3.156 g of NaCl (Cat# 7647-14-5, Macklin, China), 1.712 g of sucrose (Cat# 57-50-1, Sigma-Aldrich, USA), 0.946 g of trehalose (Cat# 6138-23-4, Sigma-Aldrich, USA), 0.834 g of MgCl2-6H2O (Cat# 7791-18-6, Sigma-Aldrich, USA), 0.596 g of HEPES (Cat# 7365-45-9, Sigma-Aldrich, USA), 0.186 g of KCl (Cat# 7447-40-7, Sigma-Aldrich, USA), 0.168 g of NaHCO3 (Cat# 144-55-8,Sigma-Aldrich, USA), 0.147 g of CaCl2 (Cat# 10035-04-8, Macklin, China), and 0.069 g of NaH2PO4-H2O (Cat# 10049-21-5, Macklin, China) in 500 mL; pH 7.5), then transferred to a centrifuge tube in liquid nitrogen. The brains of 40 flies from either the control or 1×MP group were pooled together as one sample for RNA extraction. Three samples from the control group and two samples from the 1×MP group with high-quality RNA (RNA integrity number, RIN>7.0) were included for further RNA-seq analysis. Similarly, the ovaries of 40 flies from either the control group or 1×MP group were pooled together as one sample. Four samples from the control group and three samples from the 1×MP group were included for further RNA-seq analysis.
RNA extraction, library construction, and sequencing of samples were completed by Hangzhou Lianchuan Biotechnology (China). Total RNA was extracted using Trizol reagent (Cat# 15596018, Thermo Fisher, USA) following the manufacturer’s procedures. Total RNA quantity and purity were analyzed using a Bioanalyzer 2100 and RNA 6000 Nano LabChip Kit (Cat# 5067-1511, Agilent, USA), with high-quality RNA samples (RIN>7.0) used to construct the sequencing library. After extraction, mRNA was purified from the total RNA (5 μg) using Dynabeads Oligo (dT) (Cat# 61002, Thermo Fisher, USA), with two rounds of purification. The mRNA was then fragmented into short fragments using a Magnesium RNA Fragmentation Module (Cat# 6150S NEB, USA). The cleaved RNA fragments were reverse-transcribed to create cDNA using SuperScript™ II Reverse Transcriptase (Cat# 18064014, Invitrogen, USA), which was then used to synthesize U-labeled second-stranded DNA with Escherichia coli DNA polymerase I (Cat# M0209S, NEB, USA), RNase H (Cat# 0297S, NEB, USA), and dUTP Solution (Cat# R0133, Thermo Fisher, USA). An A-base was then added to the blunt ends of each strand for ligation to the indexed adapters. Each adapter contained a T-base overhang for ligation of the adapter to the A-tailed fragmented DNA. Dual-index adapters were ligated to the fragments, and size selection was performed with AMPureXP beads (Cat# A63882, Beckman Coulter, USA). After heat-labile UDG enzyme (Cat# 0280S, NEB, USA) treatment of the U-labeled second-stranded DNA, the ligated products were amplified by polymerase chain reaction (PCR) under the following conditions: initial denaturation at 95°C for 3 min; eight cycles of denaturation at 98°C for 15 s, annealing at 60°C for 15 s, extension at 72°C for 30 s; and final extension at 72°C for 5 min. The average insert size for the final cDNA libraries was 300±50 bp. Finally, 2×150 bp paired-end sequencing (PE150) was performed using the Illumina NovaSeq™ 6000 platform (LC-Bio Technologies, China) following the vendor’s recommended protocols. To obtain high-quality clean reads, reads were filtered using Cutadapt (v.1.9, https://cutadapt.readthedocs.io/en/stable/) and sequence quality was verified using FastQC (v.0.11.9, http://www.bioinformatics.babraham.ac.uk/projects/fastqc/). Differentially expressed gene (DEG) analysis between two different groups was performed using DESeq2 (https://bioconductor.org/packages/release/bioc/html/DESeq2.html) (Love et al., 2014; Sahraeian et al., 2017) based on |log2 fold-change|≥1 and Q-value<0.05. The DEGs were then subjected to Gene Ontology (GO) enrichment analysis (Ashburner et al., 2000; Gene Ontology Consortium, 2021). Data analysis and visualization were performed using R (https://www.r-project.org/) and the OmicStudio platform of LC-Bio Technologies (China; https://www.omicstudio.cn/tool).
Statistical analysis
All statistical analyses were performed using GraphPad Prism v.8 (RRID:SCR_002798) (GraphPad Software, USA). Data are presented as mean±standard error of the mean (SEM) with individual values. For normally distributed data, one-way analysis of variance (ANOVA) followed by Bonferroni’s multiple comparison test or student t-test was applied, while for non-normally distributed data, the Kruskal-Wallis test followed by Dunn’s multiple comparison test was used. Significance was set to P<0.05. All statistical analyses are summarized in Supplementary Tables S1, S2.
RESULTS
MPs were detected in the digestive system
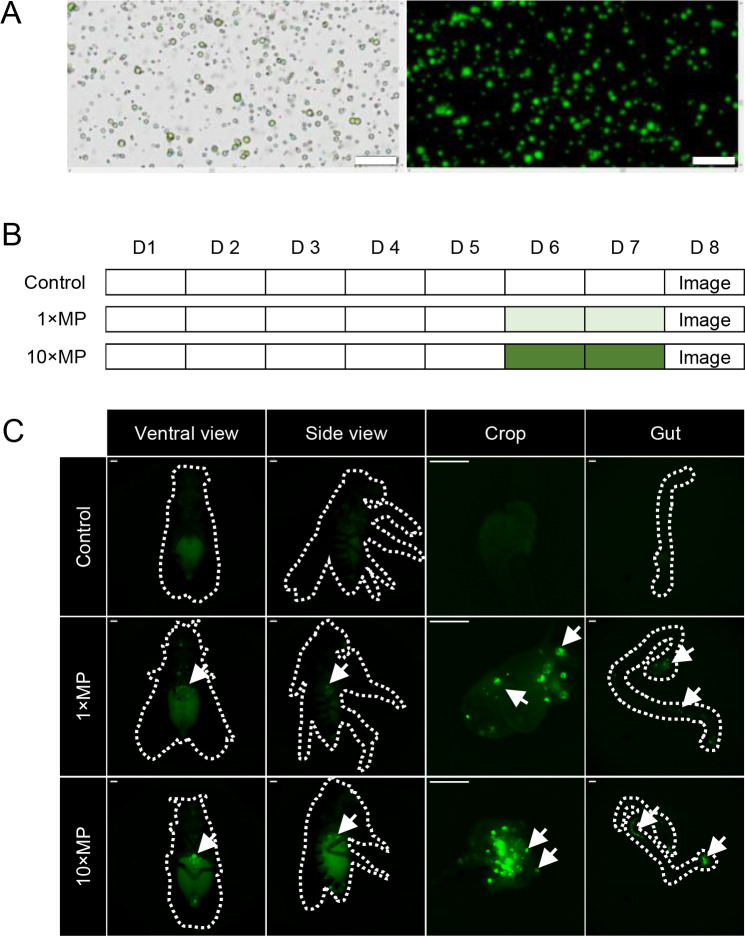
The MP particles used to treat flies were first verified using bright-field and fluorescence microscopy (Figure 1A). The green fluorescent microspheres ranged in size from 1 to 5 µm, with a density of 1.3 g/cc, zeta potential of 23.14 mV, and softening point and decomposition temperature of 290°C, as described in our previous study (Lu et al., 2021). To confirm whether the MPs mixed in food were ingested and retained in the digestive system, flies were exposed to standard cornmeal food with 1×MPs, 10×MPs, or standard food alone (control) for 2 days (Figure 1B). Fluorescence signals were examined in the whole body, followed by collection and further examination of the digestive organs. After 2 days of exposure, MPs were observed in the thorax and abdomen, with strong signals detected in the crops and guts (Figure 1C).
Figure 1.
Microplastics (MP) were ingested and retained in the digestive system
A: MPs used for exposure were characterized under light and fluorescent microscopy. Scale bars: 20 µm. B: Flies of the treatment groups were raised on standard food for 5 days and exposed to MPs at two doses: 1×MPs (0.02 mg/mL MP mixed in food) and 10×MPs (0.2 mg/mL mixed in food) from days 6 to 7. Control and MP-treated groups were sampled on day 8 and subjected to imaging analysis (D represents day). C: Fluorescence-labeled MPs were detected in crops and guts after 2 days of MP treatment (dashed lines indicate outlines of flies, arrows indicate MP with fluorescence; n=5–7 per group. Scale bars: 200 µm).
MP exposure caused gut damage in adult flies
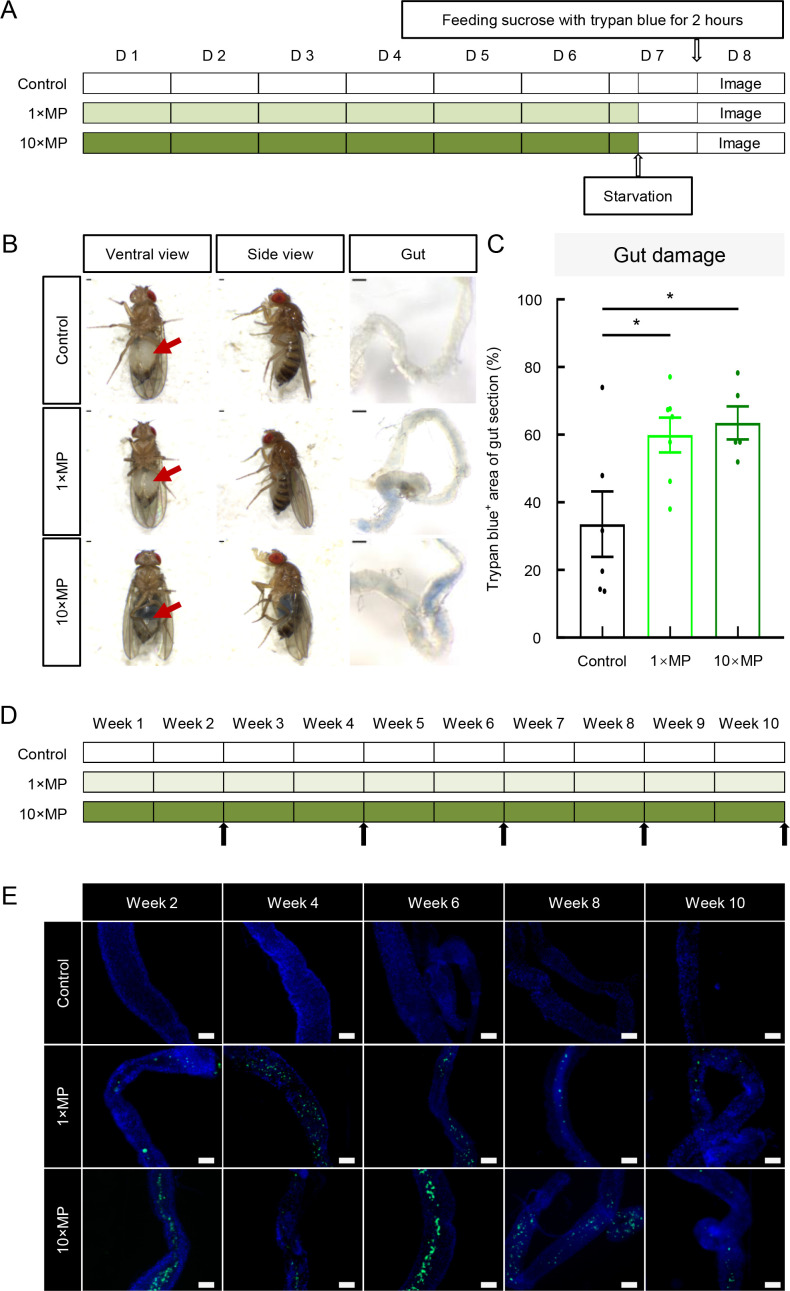
Previous research has shown that MP exposure leads to gut damage in fly larvae (Zhang et al., 2020b). To examine whether MP exposure also causes gut damage in adult flies, adult flies were exposed to standard food or MP-mixed food at two doses (1×MPs and 10×MPs) from days 1–7. The trypan blue dye exclusion method, which selectively stains dead tissues or cells (Strober, 2001) (Figure 2A), revealed visible trypan blue in the abdomens of intact flies exposed to 1×MPs and 10×MPs but not in control flies (Figure 2B). The ratio of trypan blue-positive areas in gut sections exhibited a significant increase in the 1×MP and 10×MP groups compared to the controls (Figure 2B, C), suggesting that MP-induced gut damage occurred as early as 7 days of treatment in adult flies. To determine how long MP remain in the digestive system, flies were chronically exposed to MP for up to 10 weeks (Figure 2D). Results showed that MPs were robustly detected in gut samples collected every 2 weeks over the 10 week period (Figure 2E). These findings demonstrate that MPs mixed in food are ingested, cause significant gut damage, and remain in the digestive system.
Figure 2.
Intestinal cell damage was detected in MP-treated groups using trypan blue dye exclusion method
A: Adult flies were exposed to standard food or MP mixed in food at two doses: 1×MPs and 10×MPs from days 1 to 7. On day 7, flies were starved for 16 h, then fed sucrose with trypan blue on day 8 for 2 h, with images taken 2 h later (D represents day). B: Gut leakage was revealed by trypan blue staining of gut walls of 1×MP and 10×MP treatment groups compared to controls (arrows indicate trypan blue in abdomen) . Scale bars: 200 µm. C: Quantitative analysis of trypan blue-colored proportion of gut section showed a significant increase of gut-damaged area in 1×MP and 10×MP groups compared to controls (n=5–7 per group, one-way ANOVA followed by Bonferroni’s post-hoc analysis, *: P<0.05). D: Following chronic MP exposure for 10 weeks, sub-groups of flies were dissected, and guts were imaged at the end of weeks 2, 4, 6, 8, and 10 (arrows indicate sampling and imaging time points). E: MPs were observed in the guts at all examined time points (n=3 per group). Scale bars: 100 μm.
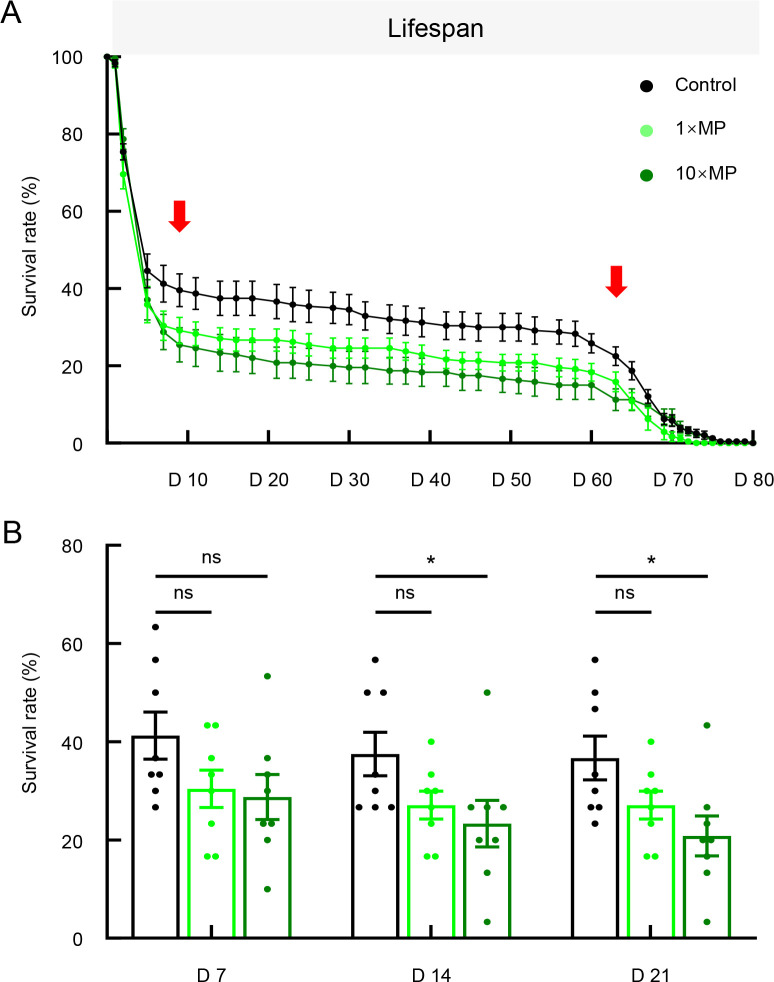
MP exposure reduced D. melanogaster lifespan
Previous studies have demonstrated an association between gut homeostasis and lifespan (Fan et al., 2018; Galenza & Foley, 2021). Given the observed gut damage following MP exposure, we hypothesized that continuous MP exposure would reduce lifespan. As expected, flies in the MP-treated groups exhibited a reduction in lifespan compared to flies in the control group (Figure 3A). We further quantified survival rates on days 7, 14, and 21 (Figure 3B). Although the 1×MP group exhibited a declining survival trend, the difference was not significant at any time point. In contrast, the 10×MP group showed significantly lower survival rates on days 14 and 21 compared to the control group. These findings indicate that MP exposure reduces lifespan, with the onset of death occurring as early as day 9 of treatment (Figure 3A).
Figure 3.
MP exposure reduced lifespan in flies
A: Both 1×MP and 10×MP treatments reduced lifespan. B: Quantitative analysis of the survival rate at different time points: days 7, 14, and 21 showed that 10×MP treatment resulted in a significant decrease in fly survival rate (n=8 tubes per group, 30 flies per tube initially; one-way ANOVA followed by Bonferroni’s post-hoc analysis, *: P<0.05; ns: Not significant). D represents day. Solid red arrows indicate range of significant differences between control and 10×MP groups.
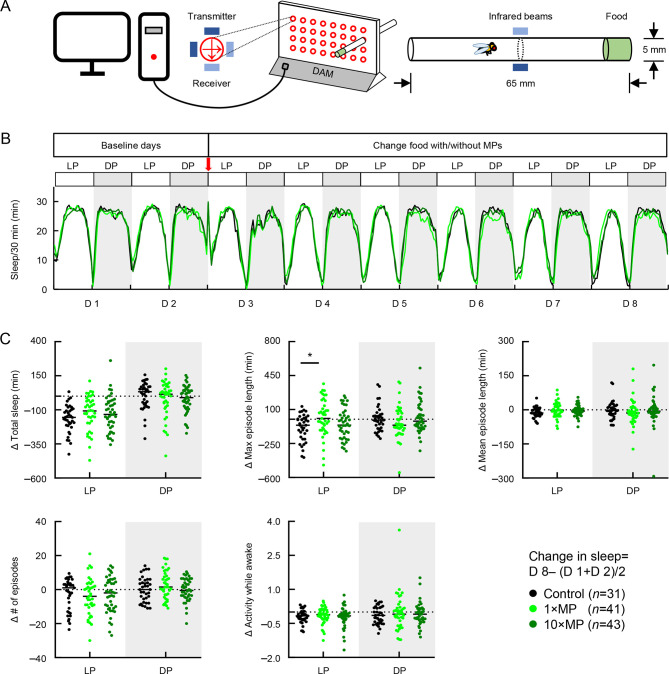
MP exposure disturbed sleep structure and altered brain gene expression
Gut function has been linked to sleep regulation (Tang et al., 2022; Vaccaro et al., 2020). To investigate whether MP-induced gut damage leads to abnormal sleep, flies were placed in a D. melanogaster activity monitor system (DAM2) for continuous observation under standard, 1×MP, or 10×MP food conditions (Figure 4A). Results indicated that the sleep profiles showed no significant differences among the groups before or after MP exposure (Figure 4B). On day 8, after 5 days of exposure, the daytime maximum sleep episode length was significantly increased in the 1×MP group compared to the control, while no significant change was observed in the 10×MP group (Figure 4C, middle panel). Furthermore, no significant changes in total sleep, mean episode duration, number of episodes, or locomotion were detected (Figure 4C). These findings suggest that MP exposure can disturb sleep structure by increasing daytime maximum sleep episode length, supporting the link between gut function and daytime sleepiness.
Figure 4.
MP exposure disturbed sleep structure in flies
A: Schematic of D. melanogaster activity monitoring system. Sleep activity was detected by recording crossing changes in infrared beams at the tube center. B: Adult flies were raised on standard food from days 1–2, then transferred to standard food or MP mixed in food at two doses: 1×MPs and 10×MPs from days 3–8. Sleep profile of total sleep with/without MP food across 8 days (D represents day). C: Quantitative analysis of changes in sleep amount, maximum episode length, mean episode length, number of episodes, and activity while awake during daytime (LP, light period) and nighttime (DP, dark period) showed that 1×MP treatment resulted in a significant increase in maximum episode length during daytime (n=31–43 per group; one-way ANOVA followed by Bonferroni’s post-hoc analysis, *: P<0.05).
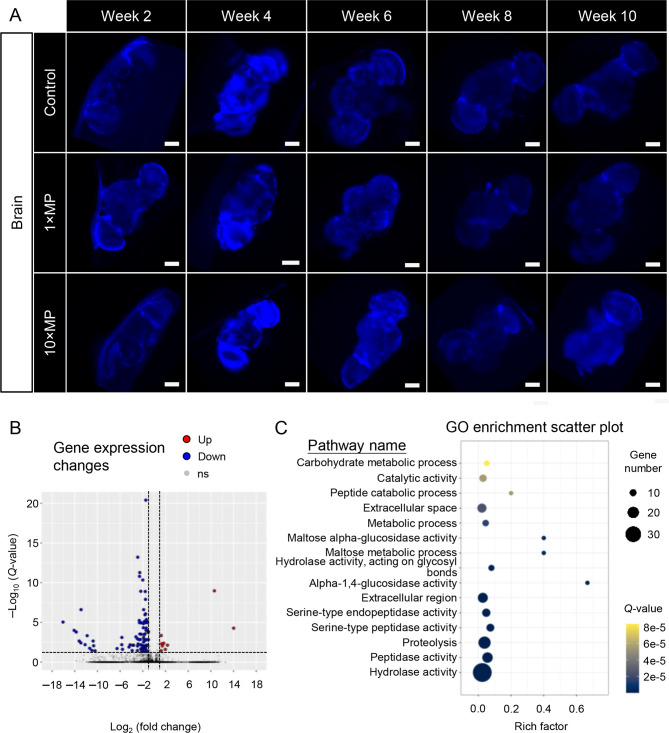
Although no accumulation of MP signals was detected in fly brains over the 10 week period (Figure 5A), 48 days of MP exposure led to changes in gene expression in the brain. Notably, transcriptomic analysis identified 98 DEGs, including nine up-regulated and 89 down-regulated genes, which were subjected to GO enrichment analysis (Figure 5B, C; Table 1). Results showed that MP exposure significantly altered the expression of genes related to hydrolase activity, peptidase activity, serine-type peptidase activity, proteolysis, and carbohydrate metabolism. Despite the absence of MP signals in the brain, our findings suggest potential involvement in regulating the brain-gut axis through pathways that modulate neuropeptide and metabolite levels and stability, influence the balance of energy supply and consumption, and participate in cell signaling and the release of inflammatory mediators during inflammatory responses.
Figure 5.
No MPs were detected in brains after 10 weeks of MP treatment, but altered brain gene expression was observed
A: Adult flies were exposed to standard food or MP mixed in food at two doses. Treatment continued for 10 weeks, and sub-groups of flies were dissected and imaged at the end of weeks 2, 4, 6, 8, and 10. No MPs were observed in fly brains at any time point (scale bars=100 μm). B: MP exposure led to differential gene expression in fly brains. C: Gene Ontology (GO) enrichment analysis showed brain pathways altered by MP exposure (rich factor indicates degree of pathway enrichment score. Q-value represents significance of enriched pathway).
Table 1. Pathway-related genes shown in GO scatter plot of the brain.
| Terms | Q-value | Genes |
| Hydrolase activity | 3.88E-15 | Mal-A1, CG12374, CG31198, LysD, Jon25Biii, Bace, Alp10, βTry, Cht9, Jon99Ci, Jon25Bi, LManVI, LManV, Jon99Cii, primo-2, CG6295, mus312, CG15255, CG31343, εTry, Alp9, CG10472, CG18180, Jon99Ciii, CG3841, CG8773, CG15534, Alp2, CG31233, CG6048, LysP, Jon74E, Jon99Fi, Mal-A2, Phae1, CG6696, CG6283 |
| Peptidase activity | 2.12E-12 | CG31198, Bace, βTry, Jon99Ci, Jon25Bi, Jon99Cii, CG15255, CG31343, εTry, CG10472, CG18180, Jon99Ciii, CG8773, CG31233, CG6048, Jon74E, Jon99Fi, CG6696 |
| Proteolysis | 3.65E-12 | CG12374, CG31198, Jon25Biii, Bace, βTry, Jon99Ci, Jon25Bi, Jon99Cii, CG15255, CG31343, εTry, CG10472, CG18493, CG18180, Jon99Ciii, CG8773, CG31233, CG6048, Jon74E, Jon99Fi, Phae1, CG6696 |
| Serine-type peptidase activity | 1.60E-09 | βTry, Jon99Ci, Jon25Bi, Jon99Cii, εTry, CG10472, CG18493, CG18180, Jon99Ciii, CG6048, Jon74E, Jon99Fi |
| Serine-type endopeptidase activity | 3.26E-08 | Jon25Biii, βTry, Jon99Ci, Jon25Bi, Jon99Cii, εTry, CG10472, CG18180, Jon99Ciii, CG6048, Jon74E, Jon99Fi, Phae1 |
| Extracellular region | 3.08E-07 | Obp56e, yellow-d, βTry, Cht9, CG6295, Obp99a, CG10725, Diedel, εTry, CG34426, Ag5r, Cp36, lectin-37Da, Mur18B, Lsp1α, js, CG6283 |
| Alpha-1,4-glucosidase activity | 3.27E-07 | Mal-A1, Mal-A7, Mal-A4, Mal-A2 |
| Hydrolase activity, acting on glycosyl bonds | 1.09E-06 | Mal-A1, LysD, Cht9, LManVI, LManV, CG15534, LysP, Mal-A2 |
| Maltose metabolic process | 3.15E-06 | Mal-A1, Mal-A7, Mal-A4, Mal-A2 |
| Maltose alpha-glucosidase activity | 3.15E-06 | Mal-A1, Mal-A7, Mal-A4, Mal-A2 |
| Metabolic process | 1.56E-05 | Mal-A1, LysD, Cht9, LManVI, LManV, CG15534, Ugt35E2, LysP, Mal-A2 |
| Extracellular space | 2.37E-05 | Obp56e, CG12374, LysD, βTry, Cht9, Listericin, Jhbp12, Obp99a, Diedel, εTry, Ag5r, CG15534, CG14913, LysP, Lsp1α |
| Peptide catabolic process | 5.39E-05 | CG31198, CG31343, CG8773, CG31233 |
| Catalytic activity | 5.50E-05 | Mal-A1, LysD, Mal-A7, Alp10, LManVI, LManV, Alp9, Alp2, Mal-A4, LysP, Mal-A2 |
| Carbohydrate metabolic process | 8.35E-05 | Mal-A1, Mal-A7, Cht9, LManVI, LManV, Mal-A4, Mal-A2 |
| Metalloaminopeptidase activity | 1.12E-04 | CG31198, CG31343, CG8773, CG31233 |
| Peptide binding | 1.24E-04 | CG31198, CG31343, CG8773, CG31233 |
| Metallopeptidase activity | 1.43E-04 | CG31198, CG15255, CG31343, CG8773, CG31233, CG6696 |
| Aminopeptidase activity | 4.72E-04 | CG31198, CG31343, CG8773, CG31233 |
| Alpha-glucosidase activity | 1.02E-03 | Mal-A1, Mal-A2 |
| Carbohydrate binding | 1.08E-03 | LManVI, LManV, CG15818, lectin-37Da, CG14500 |
| Alkaline phosphatase activity | 1.14E-03 | Alp10, Alp9, Alp2 |
| Phosphatidylserine 1-acylhydrolase activity | 2.81E-03 | CG6295, CG6283 |
| 1-acyl-2-lysophosphatidylserine acylhydrolase activity | 2.81E-03 | CG6295, CG6283 |
| Chitin binding | 3.94E-03 | Cht9, CG10725, CG34426, Mur18B, js |
| Phospholipase A1 activity | 5.41E-03 | CG6295, CG6283 |
| Cytolysis | 8.56E-03 | LysD, LysP |
| Alpha-mannosidase activity | 8.56E-03 | LManVI, LManV |
| Rhythmic behavior | 9.95E-03 | tim, vri |
| Mannose metabolic process | 9.95E-03 | LManVI, LManV |
| Cell wall macromolecule catabolic process | 1.40E-02 | LysD, LysP |
| Chitin metabolic process | 1.63E-02 | CG10725, CG34426, Mur18B, js |
| Protein deglycosylation | 2.31E-02 | LManVI, LManV |
| Endopeptidase activity | 2.31E-02 | Jon25Biii, Jon99Ci, Jon25Bi |
| Lysozyme activity | 2.52E-02 | LysD, LysP |
| Defense response to Gram-positive bacterium | 2.99E-02 | LysD, Listericin, LysP |
| Obsolete nuclear telomeric heterochromatin | 2.99E-02 | SuUR |
| Protein localization to chromosome | 2.99E-02 | SuUR |
| Photoperiodism | 2.99E-02 | tim |
| Negative regulation of transcription regulatory region DNA binding | 2.99E-02 | tim |
| Aspartic-type endopeptidase activity involved in amyloid precursor protein catabolic process | 2.99E-02 | Bace |
| Neuromuscular junction development, skeletal muscle fiber | 2.99E-02 | Act57B |
| Neutral L-amino acid transmembrane transporter activity | 2.99E-02 | NAAT1 |
| Neutral amino acid transport | 2.99E-02 | NAAT1 |
| L-amino acid transport | 2.99E-02 | NAAT1 |
| D-amino acid transport | 2.99E-02 | NAAT1 |
| D-amino acid transmembrane transporter activity | 2.99E-02 | NAAT1 |
| Imaginal disc-derived wing hair organization | 3.07E-02 | vri, qua |
| Cell surface | 3.11E-02 | Alp10, Alp9, Alp2 |
| Chitinase activity | 3.80E-02 | LysD, Cht9 |
| Phosphatase activity | 3.86E-02 | Alp10, Alp9, Alp2 |
| Actin filament | 4.84E-02 | Act57B, qua |
| Negative regulation of DNA-templated DNA replication | 4.84E-02 | SuUR |
| Amyloid precursor protein catabolic process | 4.84E-02 | Bace |
| Non-membrane spanning protein tyrosine phosphatase activity | 4.84E-02 | primo-2 |
| Slx1-Slx4 complex | 4.84E-02 | mus312 |
| Homologous recombination | 4.84E-02 | mus312 |
| 3'-flap endonuclease activity | 4.84E-02 | mus312 |
MP exposure impacted ovarian size and response
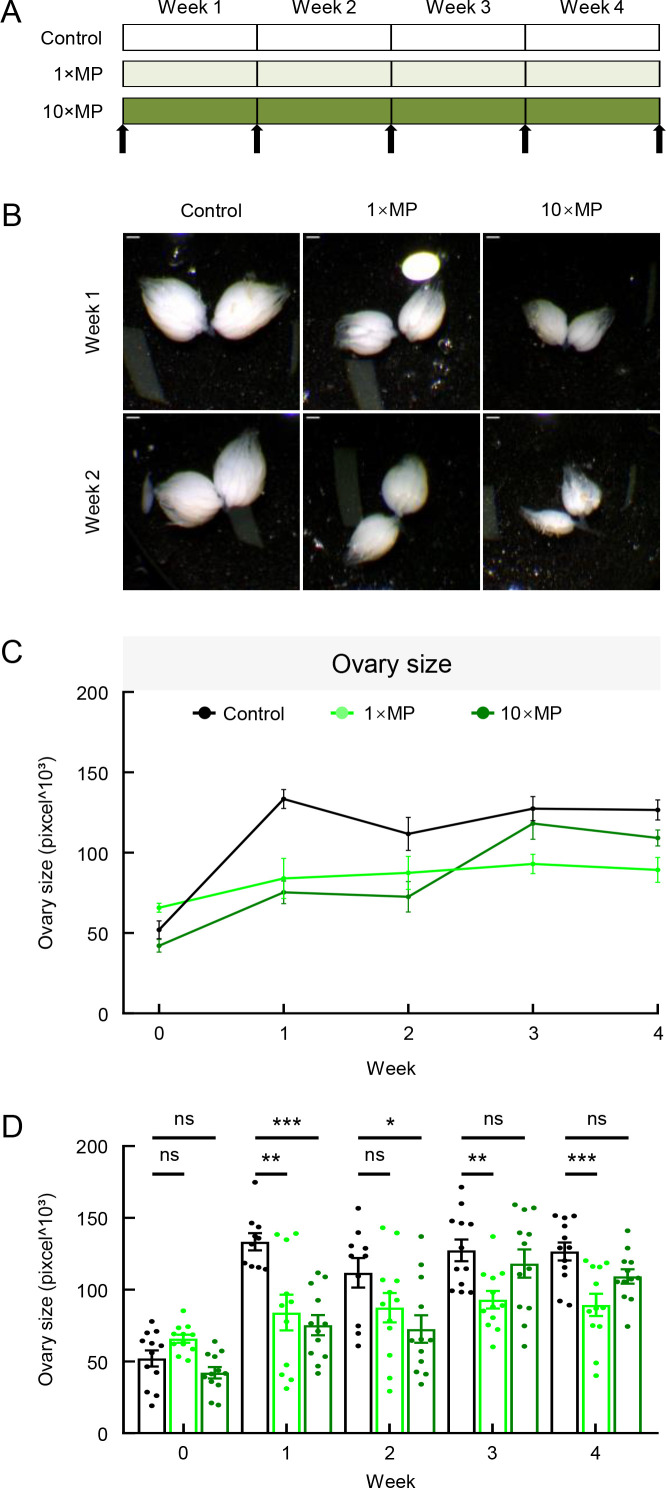
Previous studies have reported MP penetration in rodent ovaries (An et al., 2021). To examine the potential presence and impact of MPs in fruit fly ovaries, flies were exposed to varying durations and concentrations of MPs, with the ovaries then dissected and measured at weeks 0, 1, 2, 3, and 4 (Figure 6A). At baseline (week 0), ovary sizes were similar across all groups. In the control group, ovary size increased during the first week and remained at a comparable level until the end of week 4 (Figure 6C, D). In contrast, MP-treated flies had significantly smaller ovaries compared to the control group, with notable size reductions observed at weeks 1, 3, and 4 for the 1×MP group and weeks 1 and 2 for the 10×MP group (Figure 6C, D), suggesting that MP exposure inhibits ovarian development.
Figure 6.
MP exposure resulted in a reduction in ovary size
A: Adult flies were exposed to standard food or MP mixed in food at two doses: 1×MPs and 10×MPs. Treatment continued for 4 weeks, and sub-groups of flies were dissected and imaged at weeks 0, 1, 2, 3, and 4 (arrows indicate dissection and imaging time points). B, C: 1×MP and 10×MP treatments both led to a reduction in ovary size. Scale bars: 200 µm. D: Quantitative analysis showed significant differences in 1×MP group at weeks 1, 3, and 4 and in 10×MP group at week 1 compared to controls (n=10–12 per group; one-way ANOVA followed by Bonferroni’s post-hoc analysis, *: P<0.05; **: P<0.01; ***: P<0.001; ns: Not significant).
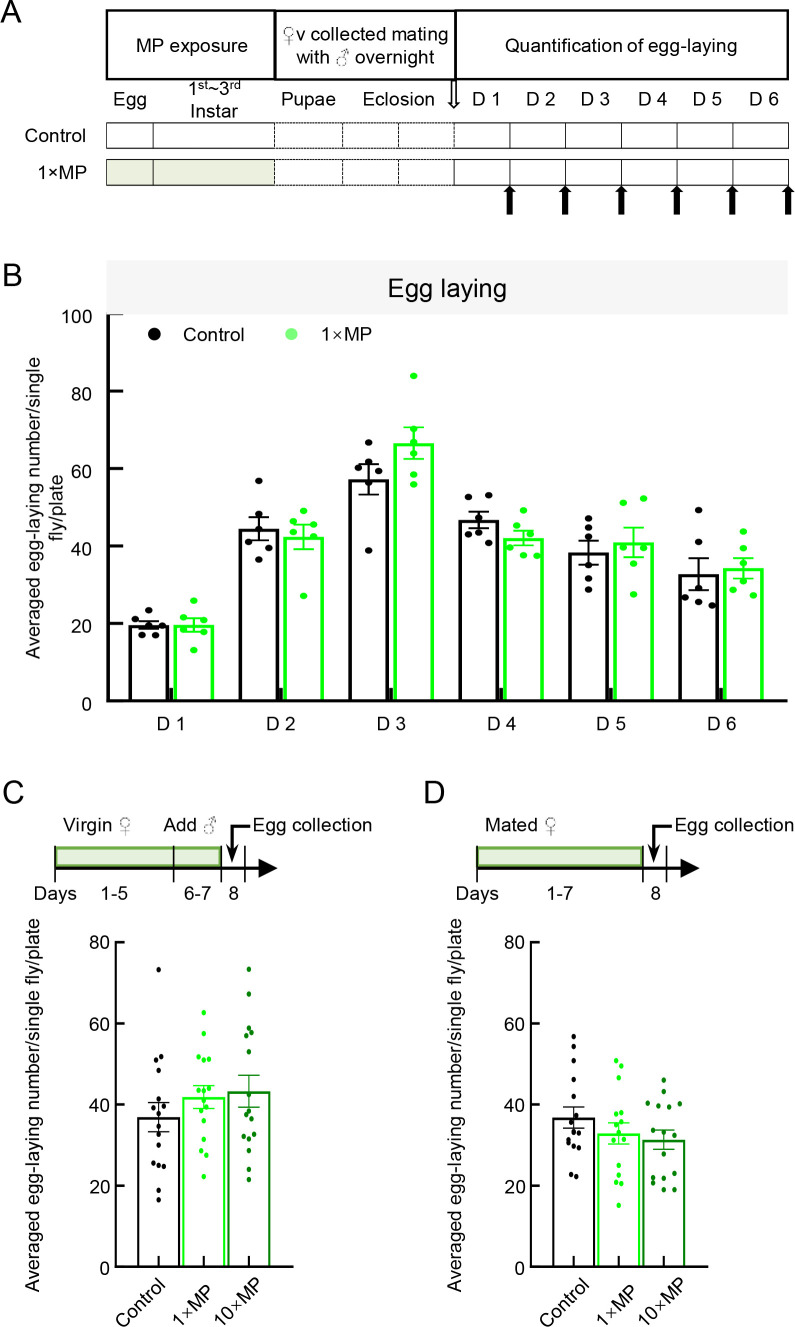
Mendes & Mirth (2016) showed that different nutrient conditions significantly influence ovary size plasticity and ovariole number. An egg-laying assay was conducted to assess the impacts of MP exposure on female fecundity. Regardless of whether MP exposure occurred in larvae during development (Figure 7A, B), adult virgin females (Figure 7C), or adult mated females (Figure 7D), no significant difference in the number of eggs laid was observed between the MP-treated and control groups (Figure 7B–D).
Figure 7.
Female fecundity showed resistance to exposure to 0.02 mg/mL and 0.2 mg/mL MP mixed in food
A: To test the effect of MP exposure during development on female fecundity, embryos were exposed to 1×MPs until eclosion, with virgin flies mated with untreated males used for fertility tests. B: A 6-day egg-laying assay showed that MP treatment had no effect on egg-laying number (n=6 plates per group, 15 females per plate; Student t-test). C: To test the effect of MP exposure during adulthood on female fecundity of virgin flies, virgin flies were exposed to standard food or MP mixed in food at two doses: 1×MPs and 10×MPs from days 1–7. On day 6, untreated male flies were introduced and allowed to mate with females for 2 days. On day 8, females were transferred to a Petri dish for egg collection and images were taken and counted 24 h later. D: To test the effect of MP exposure during adulthood on female fecundity of mated flies, mated untreated female flies were exposed to standard food or MP mixed in food at two doses: 1×MPs and 10×MPs from days 1–7. On day 8, flies were transferred to a Petri dish for egg collection and images were taken and counted 24 h later. C, D: Quantitative analysis of number of eggs laid after MP exposure during adulthood in virgin and mated flies showed no significant difference between the control and MP-treated groups (n=16 per group, one-way ANOVA followed by Bonferroni’s post-hoc analysis). D represent day.
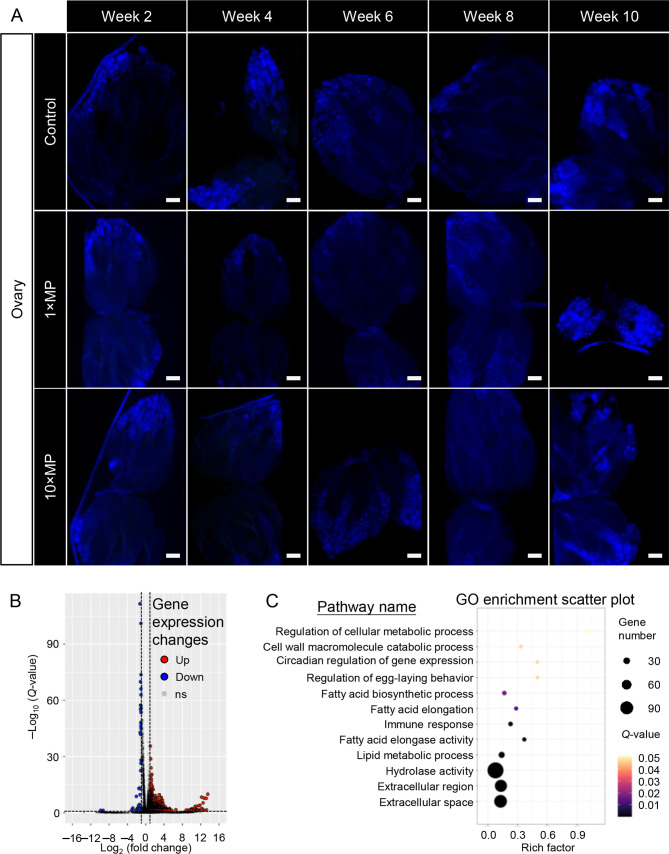
To determine why ovary size decreased despite normal egg-laying ability, we first tested for MP penetration over a 10 week period. Results showed that, unlike in rodents, no MP signals were detected in the ovaries of fruit flies (Figure 8A).
Figure 8.
No MPs were observed in the ovaries after 10 weeks of treatment, but ovarian gene expression was altered
A: Adult flies were exposed to standard food or MP mixed in food at two doses: 1×MPs and 10×MPs. Treatment continued for 10 weeks, and sub-groups of flies were dissected and imaged at the end of weeks 2, 4, 6, 8, and 10. No MPs were observed in the ovaries at any time point (scale bars=100 μm; n=3–4 per group). B: MP exposure led to differential gene expression in the fly ovaries. C: GO enrichment analysis showed pathways altered in the ovaries following MP exposure (rich factor indicates degree of pathway enrichment score. Q-value represents significance of enriched pathway).
For further insight into the impact of MP exposure, ovarian samples from flies exposed to 1×MPs for 48 days were subjected to transcriptomic analysis. Notably, 732 DEGs were identified, including 688 up-regulated and 44 down-regulated genes, which were subjected to GO enrichment analysis (Figure 8B, C). Results showed that MP exposure altered the expression of genes involved in extracellular region/space, hydrolase activity, lipid metabolism, fatty acid elongation and biosynthesis, and regulation of egg-laying behavior (Figure 8C; Table 2). Despite the absence of MPs in the ovary, the proximity of the gut to the ovary suggests that MPs leaking from the gut may affect ovarian development. These findings indicate that MP exposure can affect the extracellular matrix (ECM) surrounding the ovary and disrupt ovarian development.
Table 2. Pathway-related genes shown in GO scatter plot of the ovaries.
| Terms | Q-value | Genes |
| Extracellular space | 5.97E-15 | ImpL2, mfas, CG10433, Swim, CG17633, Ance, Timp, gbb, retinin, CG8562, PGRP-LB, Ag5r2, Obp99c, Mmp1, CG6959, SPARC, CG5162, CG3868, CG42342, CG5867, PGRP-LC, NLaz, nec, CG5945, Adgf-D, CG18585, LysP, CG8093, PGRP-SC1a, CG5397, Est-6, Ag5r, CG7142, PGRP-LA, Ance-4, Spn43Ab, CG14820, Spn42Dd, CG33127, CG8560, LysX, CG16799, Spn77Ba, Npc2e, Spn47C, CG17919, etaTry, CCHa2, CG15534, CG9400, Apoltp, Ilp7, CG18628, CG4546, CG31997, Fst, PGRP-SC2, CG44008, CG16820, Peritrophin-15a, upd3, LysS, iotaTry, CG3604, Tep3, CG9673, PGRP-SC1b, CG42780, CG9672, CG14661, Vm32E, Npc2h, BG642312, daw, upd1, Spn28Dc, CG15533, CG18067, Gasp, CG32984, ssp7, Phk-3, CG31207, serp, Spn42Dc, tsl, CG8539, Pde1c |
| Extracellular region | 9.18E-15 | ImpL2, Fs, CG18749, CG17633, Ance, Timp, gbb, retinin, Nplp3, PGRP-LB, CG6283, Ag5r2, Obp99c, Vm26Aa, CG4666, Vm26Ab, Yp2, CG5162, Yp1, Vm34Ca, CG42397, CG42486, Muc26B, bou, lectin-37Da, PGRP-SC1a, CG5177, Gbp2, Idgf1, Est-6, EbpIII, Yp3, Ag5r, Npc2g, CG6277, Spn42Dd, Muc68E, yellow-k, Glt, l (2)34Fc, CG14526, Spn77Ba, CG18258, fon, shf, CG32284, etaTry, CCHa2, CG15293, CG9400, Ilp7, NimB3, spheroide, ckd, PGRP-SC2, yellow-d, CG3505, upd3, dec-1, iotaTry, Tep3, CG13075, Lectin-galC1, PGRP-SC1b, Vm32E, Npc2h, CG6933, daw, NimB2, upd1, Spn28Dc, fat-spondin, Gasp, yellow-c, grass, Phk-3, yellow-d2, CG10116, Nplp4, serp, tsl, CG7298 |
| Hydrolase activity | 9.15E-06 | alpha-Man-IIb, Swim, Jon25Biii, CG17633, laza, Ance, Jon99Fii, CG10477, CG8562, PGRP-LB, LManVI, CG4822, Mipp1, CG10472, Jon99Fi, CG6283, nrv2, lambdaTry, Mmp1, CG30100, alpha-Est7, CG11911, Yp2, FASN2, CG16749, CG5162, LManV, CG17571, eya, PGRP-LC, Yp1, CG15255, CG14120, CG7378, CG31343, CG33346, CG43110, Adgf-D, CG18585, LysP, CG33082, Mal-A2, CG9449, CG8093, PGRP-SC1a, CG10827, Jon65Aii, CG5397, CG5177, hiro, Est-6, mag, Yp3, CG11842, Jon65Ai, CG5171, CG7142, CG9701, PGRP-LA, Ance-4, CG18180, CG14820, alpha-Est8, CG6277, Amy-p, LManI, Jon44E, Decay, CG33127, CG8560, Glt, LysX, CG16799, CG30002, CG3819, CG10970, CG9676, CG14526, Mal-A3, CG4653, CG18258, etaTry, CG15534, kappaTry, spheroide, CG31198, PGRP-SC2, CG3505, CG11841, CG30371, CG11912, LysS, iotaTry, CG11147, CG9673, CG9649, CG9780, PGRP-SC1b, CG4301, CG9672, CG6067, Phae2, CG31326, CG1809, DNaseII, Nlg1, alpha-Est2, CG15533, grass, CG31200, CG31265, Jon66Ci, CG42335, Btnd, CG10116, Vha100-5, serp, CG8539, Pde1c |
| Lipid metabolic process | 2.24E-04 | CG8303, CG30008, CG5326, CG9743, melt, CG16904, CG6283, CG5162, CG18609, NLaz, Fad2, CG8093, mag, GstE14, CG9458, CG6277, CG18258, CG10097, CG6660, Npc1b, eloF, CG17562, FarO, CG15531, Cyt-b5-r |
| Immune response | 1.16E-03 | Swim, PGRP-LB, Thor, PGRP-LC, nec, PGRP-SC1a, PGRP-LA, PGRP-SC2, upd3, PGRP-SC1b, CG15529 |
| Fatty acid elongase activity | 1.16E-03 | CG30008, CG5326, CG16904, CG18609, CG9458, CG6660, eloF |
| Fatty acid elongation | 1.16E-02 | CG30008, CG5326, CG16904, CG18609, CG9458, eloF |
| Fatty acid biosynthetic process | 1.58E-02 | CG30008, CG5326, CG9743, CG16904, CG18609, Fad2, CG9458, CG6660, eloF, CG15531 |
| Cell wall macromolecule catabolic process | 4.39E-02 | LysP, LysX, CG16799, LysS |
| Circadian regulation of gene expression | 4.39E-02 | nej, cry, Clk |
| Regulation of egg-laying behavior | 4.39E-02 | Gadd45, Drip, Est-6 |
| Regulation of cellular metabolic process | 4.76E-02 | hry, melt |
DISCUSSION
The distribution and concentration of MPs in the environment have been extensively documented (Fu et al., 2020; Yu et al., 2016), and their potential adverse effects have been studied in various organs and tissues using animal models, including both invertebrates and vertebrates (Prokić et al., 2021). In the present study, we used D. melanogaster as a terrestrial animal model to study the impacts of chronic MP exposure on sleep, lifespan, and reproduction. We used two doses of MPs (0.02 mg/mL and 0.2 mg/mL mixed in food) previously reported to adversely affect normal fly development and mating behavior (Panacek et al., 2011; Posgai et al., 2011; Zhang et al., 2020b).
Following dietary exposure to MPs, fluorescence-labeled MP particles were detected in the digestive system of the flies, with retention in the crops and guts. Gut leakage and cell damage were revealed by trypan blue staining. The detrimental impact of MPs on gut physiology is consistent with findings in adult zebrafish (Jin et al., 2018, 2019; Qiao et al., 2019), marine medaka (Kang et al., 2021), mice (Lu et al., 2018), larval flies (Demir et al., 2022), and adult flies (Zhang et al., 2020b), albeit with MP concentrations approximately 100 times higher than those used in the current study. Ingested MPs have also been detected in other organs, including the liver (Collard et al., 2017) and placenta (Ragusa et al., 2021), suggesting that ingested MPs may potentially affect other organs beyond the digestive system.
The gut serves as a crucial interface between the body and environment, initiating the pathophysiological processes of various multisystem diseases. In addition to gut damage, MP exposure significantly increased the length of daytime sleep episodes without affecting locomotion ability. This subtle yet significant impact on daytime sleep episode length should not be neglected, as both sleep quantity and quality are vital for overall physiological health (Pilcher et al., 1997; Wesselius et al., 2018). For instance, patients with obstructive sleep apnea exhibit markedly higher levels of intestinal barrier markers (Li et al., 2021), suggesting a link between gut impairment and sleep abnormalities. The “gut-brain axis” is associated with sleep via the immune response, endocrine regulation, and neural modulation (Sgro et al., 2022; Tang et al., 2022). Here, transcriptome GO analysis revealed that MP exposure altered molecular pathways related to hydrolase, peptidase, and serine-type peptidase activity, as well as proteolysis and carbohydrate metabolism, in the brain. These observations suggest that MP exposure may affect the regulation of the brain-gut axis, altering the levels and stability of neuropeptides and metabolites, as well as the balance of energy supply and consumption. Previous studies have shown that changes in sleep duration and quality can affect protein hydrolysis (Piovezan et al., 2015), while protein hydrolysates can alleviate stress and enhance sleep (Qian et al., 2021). GO enrichment analysis indicated that the hydrolase and proteolysis pathways may play a role in sleep structure modulation. GO analysis also indicated that MP exposure down-regulated carbohydrate metabolism, consistent with previous research showing that mice on a carbohydrate-restricted diet exhibit decreased survival and accelerated aging, along with alterations in gut microbiota that promote inflammation (He et al., 2020). MP exposure may trigger an inflammatory response, increasing the duration of sleep episodes (Besedovsky et al., 2019). Our transcriptomic analysis demonstrated that MP exposure altered the expression of achl, a gene involved in signal transduction from neurons to immune tissues. The up-regulation of achl, which inhibits the expression of immune response genes (Li et al., 2017), may decrease the resistance of flies, negatively impacting immune function and overall physiological state, including lifespan. However, several recent studies have indicated that exposure to higher MP concentrations can significantly shorten the total amount of sleep in D. melanogaster (Jin et al., 2024; Tang et al., 2023). These differing results may be due to the dose effect, but further research is needed to clarify the underlying mechanisms.
All living organisms are continuously exposed to MPs, which are widely distributed in the environment and negatively impact organisms across their entire lives. Our assays on flies revealed that both MP doses caused a significant decrease in lifespan. Although no significant dose-dependent effect of MP treatment was detected, there was a trend towards further reduction in lifespan under the 10×MP dose compared to the 1×MP dose, in alignment with recent findings at even higher doses (1 000× and 2 000×) (Kauts et al., 2023). Previous studies have shown that exposure to polystyrene microbeads reduces growth rates and lifespan in monogonont rotifers (Jeong et al., 2016), while ingestion of high concentrations of polyethylene MPs increases mortality rates in sea bass larvae (Mazurais et al., 2015). Comparable adverse effects on lifespan have also been observed in Caenorhabditis elegans larvae after 3 days of MP exposure (Shang et al., 2020), as well as in flies exposed to MPs for 28 days (El Kholy & Al Naggar, 2023; Urbisz et al., 2024). However, to the best of our knowledge, the present study is the first to demonstrate the detrimental effects of MP exposure on D. melanogaster lifespan (up to 80 days), highlighting potentially sensitive and resistant windows. Although direct evidence linking MP exposure to human lifespan is lacking, the urgency to address environmental MP pollutants remains critical. Further studies are needed to elucidate the cellular and molecular mechanisms underlying MP-induced shortened lifespan. Our findings of gut damage from MP exposure, as revealed by trypan blue staining, suggest a possible link between gut homeostasis and lifespan (Fan et al., 2018; Galenza & Foley, 2021). Several animal models have reported disturbed gut microbiota linked to the adverse effects of MP exposure (Fackelmann & Sommer, 2019; Jin et al., 2018; Lu et al., 2018, 2019). For instance, Newell & Douglas (2014) demonstrated that axenic flies show longer developmental stages and elevated glucose and triglyceride levels. Gould et al. (2018) reported that fly lifespan and fecundity are impacted by gut microbiome diversity. The intestine, a plastic organ influenced by both external and internal factors (Colombani & Andersen, 2020), coordinates physiological activities. Altered gut microbiota from intermittent fasting is associated with lifespan extension in flies via target of rapamycin (TOR)-independent pathways (Catterson et al., 2018). Reactive oxygen species (ROS) may be another candidate pathway by which MP exposure reduces lifespan, as ROS accumulation in the gut contributes to sleep dysfunction and death (Vaccaro et al., 2020). Proteolysis, a hallmark of aging, is disrupted by MP exposure, leading to increased oxidative stress, cellular damage, DNA damage, and aging processes, ultimately impacting lifespan (Korovila et al., 2017). Our GO enrichment analyses identified proteolysis as one of the top significant pathways, providing molecular insight into the MP-induced reduction in survival rates.
Beyond individual lifespan, fecundity is essential for species reproduction and population survival. The effects of MP exposure on animal reproductive systems have been extensively studied, including zebrafish (Qiang & Cheng, 2021), male mice (Deng et al., 2021; Hou et al., 2021; Jin et al., 2021), and female rats (An et al., 2021). In our study, we observed a decrease in ovary size in flies after just one week of MP exposure, which persisted during the chronic 4 week MP exposure period. Consistent with previous studies (Goodman et al., 2021), cell morphology was altered in cells exposed to MPs. Transcriptomic analysis also suggested that MP exposure altered ECM gene expression. Fly ovaries can regenerate three types of cells, namely, germline (GSC), somatic (SSC) and escort stem cells (ESC) (Kirilly & Xie, 2007). The ECM plays a pivotal role in maintaining and shaping the ovary and organizing ovarian stem cell niches (Díaz-Torres et al., 2021; Pearson et al., 2016). GO analysis also revealed that MP exposure altered fatty acid biosynthesis and elongation, consistent with findings of increased unsaturated lipid concentrations in the ovaries of aged female flies (Li et al., 2022). Our results suggest MP exposure can alter ovarian structure by shifting unsaturated lipid biosynthesis and modifying the surrounding ECM. Ovarian development is influenced by nutritional conditions. For example, third instar larvae fed with 20% sucrose food exhibit decreased ovariole number compared to those fed with standard food (Mendes & Mirth, 2016). The smaller ovary size observed in our study upon MP exposure may be due to limited nutrient absorption resulting from MP-induced gut damage. Moreover, structural interaction between the ovary and parts of the midgut and hindgut housed in the abdomen may contribute to this effect. Gut leakage can initiate local immune responses, indirectly affecting the ovaries, as supported by GO enrichment in the immune response pathway. Although GO analysis showed MP exposure altered genes related to circadian regulation and egg-laying behavior, no significant changes in egg-laying were detected, even at distinct developmental stages. This finding aligns with previous studies using MP concentrations 1 000 times higher than ours (Jin et al., 2024). However, several earlier studies have reported impaired female reproductive function, possibly due to a dose effect (Shen et al., 2021; Tang et al., 2023). As such, further investigation is needed to clarify how different MP concentrations impact reproductive functions and to explore potential generational/cross-generational impacts. Another study in flies reported a reduction in egg-laying after 28 days of MP exposure (Urbisz et al., 2024). We speculate that these differences in results may be attributable to an interactive effect of age and accumulated MP impact, as egg-laying typically peaks around 8–10 days after eclosion (Aigaki & Ohba, 1984), and no detectable MP entry into the ovary was observed in our study, even after 10 weeks of exposure.
Our study has several limitations. Although we observed a decrease in ovary size after 4 weeks of MP exposure, the underlying mechanisms and subtle effects on reproduction, including potential impacts on future generations, require further investigation. Additionally, efforts should be made to explore the link between reduced lifespan and diminished ovaries. Furthermore, the combined effects of MPs with potentially accumulated organic pollutants or heavy metals could exacerbate the adverse impacts on lifespan and reproduction, given the release of phthalate esters (Deng et al., 2020), polychlorinated biphenyls (Nor & Koelmans, 2019), and Cd, Pb, and Zn heavy metals (Yan et al., 2020) from MPs and other small particles (Alaraby et al., 2020; Anand et al., 2019). Reported MP concentrations in the natural environment also vary widely, ranging from 2.5×10–5 to 121 mg/mL (EFSA Panel on Contaminants in the Food Chain, 2016), with fresh/drinking water concentrations ranging from 3.75×10–5 to 0.162 mg/mL (Marsden et al., 2019) and indoor and outdoor air concentrations ranging from 16.7×10–3 to 1×103 f/mL (Li et al., 2020; Wright & Kelly, 2017). Further studies are needed to investigate the impacts of different MP exposure concentrations and provide a more realistic understanding of animal responses.
SUPPLEMENTARY DATA
Supplementary data to this article can be found online.
Acknowledgments
COMPETING INTERESTS
The authors declare that they have no competing interests.
AUTHORS’ CONTRIBUTIONS
W.Y. and Z.J.L.: Conceptualization, Investigation, Methodology, Formal analysis, Visualization, Writing-original draft. Z.Y.L.: Investigation, Resources. S.Q.J.: Investigation, Funding acquisition. W.K.F.T.: Investigation and editing. Z.Q.M.: Formal analysis, Writing-review & editing. L.L. and C.L.: Conceptualization, Writing-original draft, review & editing, Supervision, Project administration, Funding acquisition. All authors read and approved the final version of the manuscript.
Funding Statement
This work was supported by the Key Collaborative Research Program of the Alliance of International Science Organizations (ANSO-CR-KP-2021-12 to L.L.), National Natural Science Foundation of China (32071009, 32371063, 82341248 to C.L., 31971072 and 32171154 to L.L.), Guangdong Basic and Applied Basic Research Foundation (2024A1515011500 to C.L.), and Shenzhen Science Technology and Innovative Commission (SZSTI JCYJ20180508152336419 to L.L. and GJHZ20200731095406021 to S.J.)
Contributor Information
Chang Liu, Email: chang.liu3@siat.ac.cn.
Lei Li, Email: saralilei@siat.ac.cn.
DATA AVAILABILITY
The raw metagenomics sequencing reads can be downloaded from the NCBI (PRJNA1101743), China National Center for Bioinformation (PRJCA025077), and Science Data Bank databases (DOI:10.57760/sciencedb.j00139.00127).
References
- Aigaki T, Ohba S. 1984. Individual analysis of age-associated changes in reproductive activity and lifespan of Drosophila virilis. Experimental Gerontology, 19 (1): 13–23.
- Aime M, Adamantidis AR Sleep to survive predators. Neuroscience Bulletin. 2022;38(9):1114–1116. doi: 10.1007/s12264-022-00875-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alaraby M, Demir E, Domenech J, et al. 2020. In vivo evaluation of the toxic and genotoxic effects of exposure to cobalt nanoparticles using Drosophila melanogaster. Environmental Science: Nano, 7 (2): 610–622.
- An R, Wang XF, Yang L, et al Polystyrene microplastics cause granulosa cells apoptosis and fibrosis in ovary through oxidative stress in rats. Toxicology. 2021;449:152665. doi: 10.1016/j.tox.2020.152665. [DOI] [PubMed] [Google Scholar]
- Anand AS, Gahlot U, Prasad DN, et al Aluminum oxide nanoparticles mediated toxicity, loss of appendages in progeny of Drosophila melanogaster on chronic exposure. Nanotoxicology. 2019;13(7):977–989. doi: 10.1080/17435390.2019.1602680. [DOI] [PubMed] [Google Scholar]
- Ashburner M, Ball CA, Blake JA, et al Gene ontology: tool for the unification of biology. Nature Genetics. 2000;25(1):25–29. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner M, Carson HL, Thompson Jr JN. 1986. The Genetics and Biology of Drosophila. vol. 3e. London: Academic Press.
- Azeem I, Adeel M, Ahmad MA, et al. 2021. Uptake and accumulation of nano/microplastics in plants: a critical review. Nanomaterials (Basel), 11 (11): 2935.
- Barboza LGA, Vieira LR, Branco V, et al Microplastics increase mercury bioconcentration in gills and bioaccumulation in the liver, and cause oxidative stress and damage in Dicentrarchus labrax juveniles. Scientific Reports. 2018;8(1):15655. doi: 10.1038/s41598-018-34125-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besedovsky L, Lange T, Haack M The sleep-immune crosstalk in health and disease. Physiological Reviews. 2019;99(3):1325–1380. doi: 10.1152/physrev.00010.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bringer A, Cachot J, Prunier G, et al Experimental ingestion of fluorescent microplastics by pacific oysters, Crassostrea gigas, and their effects on the behaviour and development at early stages. Chemosphere. 2020;254:126793. doi: 10.1016/j.chemosphere.2020.126793. [DOI] [PubMed] [Google Scholar]
- Bushey D, Hughes KA, Tononi G, et al. 2010. Sleep, aging, and lifespan in Drosophila. BMC Neuroscience, 11 : 56.
- Catterson JH, Khericha M, Dyson MC, et al. 2018. Short-term, intermittent fasting induces long-lasting gut health and TOR-independent lifespan extension. Current Biology, 28 (11): 1714–1724. e4.
- Chuang HC, Su TY, Chuang KJ, et al Pulmonary exposure to metal fume particulate matter cause sleep disturbances in shipyard welders. Environmental Pollution. 2018;232:523–532. doi: 10.1016/j.envpol.2017.09.082. [DOI] [PubMed] [Google Scholar]
- Collard F, Gilbert B, Compère P, et al Microplastics in livers of European anchovies (Engraulis encrasicolus, L. ) Environmental Pollution. 2017;229:1000–1005. doi: 10.1016/j.envpol.2017.07.089. [DOI] [PubMed] [Google Scholar]
- Colombani J, Andersen DS The Drosophila gut: A gatekeeper and coordinator of organism fitness and physiology. WIREs Developmental Biology. 2020;9(6):e378. doi: 10.1002/wdev.378. [DOI] [PubMed] [Google Scholar]
- Conti GO, Ferrante M, Banni M, et al Micro- and nano-plastics in edible fruit and vegetables. The first diet risks assessment for the general population. Environmental Research. 2020;187:109677. doi: 10.1016/j.envres.2020.109677. [DOI] [PubMed] [Google Scholar]
- Demir FT, Akkoyunlu G, Demir E. 2022. Interactions of ingested polystyrene microplastics with heavy metals (Cadmium or Silver) as environmental pollutants: a comprehensive in vivo study using Drosophila melanogaster. Biology (Basel), 11 (10): 1470.
- Deng YF, Yan ZH, Shen RQ, et al Microplastics release phthalate esters and cause aggravated adverse effects in the mouse gut. Environment International. 2020;143:105916. doi: 10.1016/j.envint.2020.105916. [DOI] [PubMed] [Google Scholar]
- Deng YF, Yan ZH, Shen RQ, et al Enhanced reproductive toxicities induced by phthalates contaminated microplastics in male mice (Mus musculus) Journal of Hazardous Materials. 2021;406:124644. doi: 10.1016/j.jhazmat.2020.124644. [DOI] [PubMed] [Google Scholar]
- Deng YF, Zhang Y, Lemos B, et al Tissue accumulation of microplastics in mice and biomarker responses suggest widespread health risks of exposure. Scientific Reports. 2017;7:46687. doi: 10.1038/srep46687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz-Torres A, Rosales-Nieves AE, Pearson JR, et al. 2021. Stem cell niche organization in the Drosophila ovary requires the ECM component Perlecan. Current Biology, 31 (8): 1744–1753. e5.
- EFSA Panel on Contaminants in the Food Chain Presence of microplastics and nanoplastics in food, with particular focus on seafood. EFSA Journal. 2016;14(6):e04501. [Google Scholar]
- El Kholy S, Al Naggar Y. 2023. Exposure to polystyrene microplastic beads causes sex-specific toxic effects in the model insect Drosophila melanogaster. Scientific Reports, 13 (1): 204.
- Eriksen M, Lebreton LCM, Carson HS, et al Plastic pollution in the world's oceans: more than 5 trillion plastic pieces weighing over 250, 000 tons afloat at sea. PLoS One. 2014;9(12):e111913. doi: 10.1371/journal.pone.0111913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fackelmann G, Sommer S Microplastics and the gut microbiome: How chronically exposed species may suffer from gut dysbiosis. Marine Pollution Bulletin. 2019;143:193–203. doi: 10.1016/j.marpolbul.2019.04.030. [DOI] [PubMed] [Google Scholar]
- Fan XL, Gaur U, Yang MY Intestinal homeostasis and longevity: drosophila gut feeling. Advances in Experimental Medicine and Biology. 2018;1086:157–168. doi: 10.1007/978-981-13-1117-8_10. [DOI] [PubMed] [Google Scholar]
- Fu DD, Chen CM, Qi HY, et al Occurrences and distribution of microplastic pollution and the control measures in China. Marine Pollution Bulletin. 2020;153:110963. doi: 10.1016/j.marpolbul.2020.110963. [DOI] [PubMed] [Google Scholar]
- Galenza A, Foley E. 2021. A glucose-supplemented diet enhances gut barrier integrity in Drosophila. Biology Open, 10 (3): bio056515.
- Gao X, Huang NH, Guo XB, et al Role of sleep quality in the acceleration of biological aging and its potential for preventive interaction on air pollution insults: Findings from the UK Biobank cohort. Aging Cell. 2022;21(5):e13610. doi: 10.1111/acel.13610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman KE, Hare JT, Khamis ZI, et al Exposure of human lung cells to polystyrene microplastics significantly retards cell proliferation and triggers morphological changes. Chemical Research in Toxicology. 2021;34(4):1069–1081. doi: 10.1021/acs.chemrestox.0c00486. [DOI] [PubMed] [Google Scholar]
- Gould AL, Zhang V, Lamberti L, et al Microbiome interactions shape host fitness. Proceedings of the National Academy of Sciences of the United States of America. 2018;115(51):E11951–E11960. doi: 10.1073/pnas.1809349115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann NB, Huffer T, Thompson RC, et al Are we speaking the same language? Recommendations for a definition and categorization framework for plastic debris. Environmental Science & Technology. 2019;53(3):1039–1047. doi: 10.1021/acs.est.8b05297. [DOI] [PubMed] [Google Scholar]
- He CQ, Wu QM, Hayashi N, et al Carbohydrate-restricted diet alters the gut microbiota, promotes senescence and shortens the life span in senescence-accelerated prone mice. The Journal of Nutritional Biochemistry. 2020;78:108326. doi: 10.1016/j.jnutbio.2019.108326. [DOI] [PubMed] [Google Scholar]
- Ho YW, Lim JY, Yeoh YK, et al. 2022. Preliminary Findings of the High Quantity of Microplastics in Faeces of Hong Kong Residents. Toxics, 10 (8).
- Hou BL, Wang FY, Liu T, et al Reproductive toxicity of polystyrene microplastics: In vivo experimental study on testicular toxicity in mice. Journal of Hazardous Materials. 2021;405:124028. doi: 10.1016/j.jhazmat.2020.124028. [DOI] [PubMed] [Google Scholar]
- Jeong CB, Won EJ, Kang HM, et al Microplastic size-dependent toxicity, oxidative stress induction, and p-JNK and p-p38 activation in the monogonont rotifer (Brachionus koreanus) Environmental Science & Technology. 2016;50(16):8849–8857. doi: 10.1021/acs.est.6b01441. [DOI] [PubMed] [Google Scholar]
- Jiang XY, Pan YF Neural control of action selection among innate behaviors. Neuroscience Bulletin. 2022;38(12):1541–1558. doi: 10.1007/s12264-022-00886-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin H, Xu YF, Kong FH, et al. 2024. Chronic exposure to polytetrafluoroethylene microplastics caused sex-specific effects in the model insect, Drosophila melanogaster (Diptera: Drosophilidae). Journal of Economic Entomology.
- Jin HB, Ma T, Sha XX, et al Polystyrene microplastics induced male reproductive toxicity in mice. Journal of Hazardous Materials. 2021;401:123430. doi: 10.1016/j.jhazmat.2020.123430. [DOI] [PubMed] [Google Scholar]
- Jin YX, Lu L, Tu WQ, et al Impacts of polystyrene microplastic on the gut barrier, microbiota and metabolism of mice. Science of the Total Environment. 2019;649:308–317. doi: 10.1016/j.scitotenv.2018.08.353. [DOI] [PubMed] [Google Scholar]
- Jin YX, Xia JZ, Pan ZH, et al Polystyrene microplastics induce microbiota dysbiosis and inflammation in the gut of adult zebrafish. Environmental Pollution. 2018;235:322–329. doi: 10.1016/j.envpol.2017.12.088. [DOI] [PubMed] [Google Scholar]
- Kang HM, Byeon E, Jeong H, et al. 2021. Different effects of nano- and microplastics on oxidative status and gut microbiota in the marine medaka Oryzias melastigma. Journal of Hazardous Materials, 405 : 124207.
- Kauts S, Mishra Y, Yousuf S, et al Toxicological profile of polyethylene terephthalate (PET) microplastic in ingested Drosophila melanogaster (Oregon R+) and its adverse effect on behavior and development. Toxics. 2023;11(9):782. doi: 10.3390/toxics11090782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirilly D, Xie T The Drosophila ovary: an active stem cell community. Cell Research. 2007;17(1):15–25. doi: 10.1038/sj.cr.7310123. [DOI] [PubMed] [Google Scholar]
- Korovila I, Hugo M, Castro JP, et al Proteostasis, oxidative stress and aging. Redox Biology. 2017;13:550–567. doi: 10.1016/j.redox.2017.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei LL, Wu SY, Lu SB, et al. 2018. Microplastic particles cause intestinal damage and other adverse effects in zebrafish Danio rerio and nematode Caenorhabditis elegans. Science of the Total Environment, 619 – 620 : 1–8.
- Li JJ, Terry EE, Fejer E, et al. 2017. Achilles is a circadian clock-controlled gene that regulates immune function in Drosophila. Brain, Behavior, and Immunity, 61 : 127–136.
- Li QJ, Xu T, Zhong H, et al Impaired intestinal barrier in patients with obstructive sleep apnea. Sleep and Breathing. 2021;25(2):749–756. doi: 10.1007/s11325-020-02178-y. [DOI] [PubMed] [Google Scholar]
- Li YJ, Bagheri P, Chang P, et al Direct imaging of lipid metabolic changes in Drosophila ovary during aging using DO-SRS microscopy. Frontiers in Aging. 2022;2:819903. doi: 10.3389/fragi.2021.819903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YW, Shao LY, Wang WH, et al Airborne fiber particles: Types, size and concentration observed in Beijing. Science of the Total Environment. 2020;705:135967. doi: 10.1016/j.scitotenv.2019.135967. [DOI] [PubMed] [Google Scholar]
- Liao ZL, Ji XL, Ma Y, et al Airborne microplastics in indoor and outdoor environments of a coastal city in Eastern China. Journal of Hazardous Materials. 2021;417:126007. doi: 10.1016/j.jhazmat.2021.126007. [DOI] [PubMed] [Google Scholar]
- Lim XZ Microplastics are everywhere - but are they harmful? Nature. 2021;593(7857):22–25. doi: 10.1038/d41586-021-01143-3. [DOI] [PubMed] [Google Scholar]
- Lin Z, Li Z, Ji S, et al Size-dependent deleterious effects of nano- and microplastics on sperm motility. Toxicology. 2024a;506:153834. doi: 10.1016/j.tox.2024.153834. [DOI] [PubMed] [Google Scholar]
- Lin Z, Li Z, Ji S, et al Microplastics from face mask impairs sperm motility. Mar Pollut Bull. 2024b;203:116422. doi: 10.1016/j.marpolbul.2024.116422. [DOI] [PubMed] [Google Scholar]
- Love MI, Huber W, Anders S Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biology. 2014;15(12):550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu K, Lai KP, Stoeger T, et al Detrimental effects of microplastic exposure on normal and asthmatic pulmonary physiology. Journal of Hazardous Materials. 2021;416:126069. doi: 10.1016/j.jhazmat.2021.126069. [DOI] [PubMed] [Google Scholar]
- Lu L, Luo T, Zhao Y, et al Interaction between microplastics and microorganism as well as gut microbiota: A consideration on environmental animal and human health. Science of the Total Environment. 2019;667:94–100. doi: 10.1016/j.scitotenv.2019.02.380. [DOI] [PubMed] [Google Scholar]
- Lu L, Wan ZQ, Luo T, et al. 2018. Polystyrene microplastics induce gut microbiota dysbiosis and hepatic lipid metabolism disorder in mice. Science of the Total Environment, 631 – 632 : 449–458.
- Lu YF, Zhang Y, Deng YF, et al Uptake and accumulation of polystyrene microplastics in zebrafish (Danio rerio) and toxic effects in liver. Environmental Science & Technology. 2016;50(7):4054–4060. doi: 10.1021/acs.est.6b00183. [DOI] [PubMed] [Google Scholar]
- Ma BX, Wang RC, Liu YH, et al. 2022. Serotonin signaling modulates sexual receptivity of virgin female Drosophila. Neuroscience Bulletin, 38 (11): 1277–1291.
- Mallik A, Xavier KAM, Naidu BC, et al Ecotoxicological and physiological risks of microplastics on fish and their possible mitigation measures. Science of the Total Environment. 2021;779:146433. doi: 10.1016/j.scitotenv.2021.146433. [DOI] [PubMed] [Google Scholar]
- Marsden P, Koelmans AA, Bourdon-Lacombe J, et al. 2019. Microplastics in drinking water. Geneva: World Health Organization.
- Matthews S, Xu EG, Dumont ER, et al. 2021. Polystyrene micro- and nanoplastics affect locomotion and daily activity of Drosophila melanogaster. Environmental Science: Nano, 8 (1): 110–121.
- Mazurais D, Ernande B, Quazuguel P, et al Evaluation of the impact of polyethylene microbeads ingestion in European sea bass (Dicentrarchus labrax) larvae. Marine Environmental Research. 2015;112:78–85. doi: 10.1016/j.marenvres.2015.09.009. [DOI] [PubMed] [Google Scholar]
- Mendes CC, Mirth CK. 2016. Stage-specific plasticity in ovary size is regulated by insulin/insulin-like growth factor and ecdysone signaling in Drosophila. Genetics, 202 (2): 703–719.
- Newell PD, Douglas AE. 2014. Interspecies interactions determine the impact of the gut microbiota on nutrient allocation in Drosophila melanogaster. Applied and Environmental Microbiology, 80 (2): 788–796.
- Nor NHM, Koelmans AA Transfer of PCBs from microplastics under simulated gut fluid conditions is biphasic and reversible. Environmental Science & Technology. 2019;53(4):1874–1883. doi: 10.1021/acs.est.8b05143. [DOI] [PubMed] [Google Scholar]
- Nor NHM, Kooi M, Diepens NJ, et al Lifetime accumulation of microplastic in children and adults. Environmental Science & Technology. 2021;55(8):5084–5096. doi: 10.1021/acs.est.0c07384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panacek A, Prucek R, Safarova D, et al. 2011. Acute and chronic toxicity effects of silver nanoparticles (NPs) on Drosophila melanogaster. Environmental Science & Technology, 45 (11): 4974–4979.
- Park EJ, Han JS, Park EJ, et al Repeated-oral dose toxicity of polyethylene microplastics and the possible implications on reproduction and development of the next generation. Toxicology Letters. 2020;324:75–85. doi: 10.1016/j.toxlet.2020.01.008. [DOI] [PubMed] [Google Scholar]
- Pearson JR, Zurita F, Tomás-Gallardo L, et al ECM-regulator timp is required for stem cell niche organization and cyst production in the Drosophila ovary. PLoS Genetics. 2016;12(1):e1005763. doi: 10.1371/journal.pgen.1005763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilcher JJ, Ginter DR, Sadowsky B Sleep quality versus sleep quantity: relationships between sleep and measures of health, well-being and sleepiness in college students. Journal of Psychosomatic Research. 1997;42(6):583–596. doi: 10.1016/S0022-3999(97)00004-4. [DOI] [PubMed] [Google Scholar]
- Piovezan RD, Abucham J, Dos Santos RVT, et al The impact of sleep on age-related sarcopenia: Possible connections and clinical implications. Ageing Research Reviews. 2015;23:210–220. doi: 10.1016/j.arr.2015.07.003. [DOI] [PubMed] [Google Scholar]
- Posgai R, Cipolla-Mcculloch CB, Murphy KR, et al Differential toxicity of silver and titanium dioxide nanoparticles on Drosophila melanogaster development, reproductive effort, and viability: size, coatings and antioxidants matter. Chemosphere. 2011;85(1):34–42. doi: 10.1016/j.chemosphere.2011.06.040. [DOI] [PubMed] [Google Scholar]
- Prokić MD, Gavrilović BR, Radovanović TB, et al Studying microplastics: Lessons from evaluated literature on animal model organisms and experimental approaches. Journal of Hazardous Materials. 2021;414:125476. doi: 10.1016/j.jhazmat.2021.125476. [DOI] [PubMed] [Google Scholar]
- Qian JJ, Zheng L, Su GW, et al Identification and screening of potential bioactive peptides with sleep-enhancing effects in bovine milk casein hydrolysate. Journal of Agricultural and Food Chemistry. 2021;69(38):11246–11258. doi: 10.1021/acs.jafc.1c03937. [DOI] [PubMed] [Google Scholar]
- Qiang LY, Cheng JP Exposure to polystyrene microplastics impairs gonads of zebrafish (Danio rerio) Chemosphere. 2021;263:128161. doi: 10.1016/j.chemosphere.2020.128161. [DOI] [PubMed] [Google Scholar]
- Qiao RX, Sheng C, Lu YF, et al Microplastics induce intestinal inflammation, oxidative stress, and disorders of metabolome and microbiome in zebrafish. Science of the Total Environment. 2019;662:246–253. doi: 10.1016/j.scitotenv.2019.01.245. [DOI] [PubMed] [Google Scholar]
- Ragusa A, Svelato A, Santacroce C, et al Plasticenta: First evidence of microplastics in human placenta. Environment International. 2021;146:106274. doi: 10.1016/j.envint.2020.106274. [DOI] [PubMed] [Google Scholar]
- Sahraeian SME, Mohiyuddin M, Sebra R, et al Gaining comprehensive biological insight into the transcriptome by performing a broad-spectrum RNA-seq analysis. Nature Communications. 2017;8(1):59. doi: 10.1038/s41467-017-00050-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwabl P, Koppel S, Konigshofer P, et al Detection of various microplastics in human stool: a prospective case series. Annals of Internal Medicine. 2019;171(7):453–457. doi: 10.7326/M19-0618. [DOI] [PubMed] [Google Scholar]
- Sgro M, Kodila ZN, Brady RD, et al Synchronizing our clocks as we age: the influence of the brain-gut-immune axis on the sleep-wake cycle across the lifespan. Sleep. 2022;45(3):zsab268. doi: 10.1093/sleep/zsab268. [DOI] [PubMed] [Google Scholar]
- Shang X, Lu JW, Feng C, et al. 2020. Microplastic (1 and 5 μm) exposure disturbs lifespan and intestine function in the nematode Caenorhabditis elegans. Science of the Total Environment, 705 : 135837.
- Shen J, Liang BY, Zhang DK, et al. 2021. Effects of PET microplastics on the physiology of Drosophila. Chemosphere, 283 : 131289.
- Skevington JH, Dang PT Exploring the diversity of flies (Diptera) Biodiversity. 2002;3(4):3–27. doi: 10.1080/14888386.2002.9712613. [DOI] [Google Scholar]
- Strober W. 2001. Trypan blue exclusion test of cell viability. Current Protocols in Immunology, Appendix 3 : Appendix 3B.
- Tagorti G, Kaya B. 2022. Genotoxic effect of microplastics and COVID-19: The hidden threat. Chemosphere, 286 (Pt 3): 131898.
- Tang H, Zhong LC, Xu YF, et al Polypropylene microplastics affect the physiology in Drosophila model. Bulletin of Entomological Research. 2023;113(3):355–360. doi: 10.1017/S0007485322000633. [DOI] [PubMed] [Google Scholar]
- Tang MS, Song XR, Zhong WW, et al Dietary fiber ameliorates sleep disturbance connected to the gut-brain axis. Food & Function. 2022;13(23):12011–12020. doi: 10.1039/d2fo01178f. [DOI] [PubMed] [Google Scholar]
- The Gene Ontology Consortium The Gene Ontology resource: enriching a GOld mine. Nucleic Acids Research. 2021;49(D1):D325–D334. doi: 10.1093/nar/gkaa1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbisz AZ, Malota K, Chajec Ł, et al. 2024. Size-dependent and sex-specific negative effects of micro- and nano-sized polystyrene particles in the terrestrial invertebrate model Drosophila melanogaster. Micron, 176 : 103560.
- Vaccaro A, Dor YK, Nambara K, et al. 2020. Sleep loss can cause death through accumulation of reactive oxygen species in the gut. Cell, 181 (6): 1307–1328. e15.
- Van Raamsdonk LWD, Van Der Zande M, Koelmans AA, et al Current insights into monitoring, bioaccumulation, and potential health effects of microplastics present in the food Chain. Foods. 2020;9(1):72. doi: 10.3390/foods9010072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Sebille E, Wilcox C, Lebreton L, et al A global inventory of small floating plastic debris. Environmental Research Letters. 2015;10(12):124006. doi: 10.1088/1748-9326/10/12/124006. [DOI] [Google Scholar]
- Wan XL, Shen P, Shi K, et al. 2023. A neural circuit controlling virgin female aggression induced by mating-related cues in Drosophila. Neuroscience Bulletin, 39 (9): 1396–1410.
- Wang J, Li YJ, Lu L, et al Polystyrene microplastics cause tissue damages, sex-specific reproductive disruption and transgenerational effects in marine medaka (Oryzias melastigma) Environmental Pollution. 2019a;254:113024. doi: 10.1016/j.envpol.2019.113024. [DOI] [PubMed] [Google Scholar]
- Wang J, Liu XH, Li Y, et al Microplastics as contaminants in the soil environment: A mini-review. Science of the Total Environment. 2019b;691:848–857. doi: 10.1016/j.scitotenv.2019.07.209. [DOI] [PubMed] [Google Scholar]
- Wei YB, Zhang H, Liu Y Charting normative brain variability across the human lifespan. Neuroscience Bulletin. 2023;39(2):362–364. doi: 10.1007/s12264-022-00952-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesselius HM, Van Den Ende ES, Alsma J, et al Quality and quantity of sleep and factors associated with sleep disturbance in hospitalized patients. JAMA Internal Medicine. 2018;178(9):1201–1208. doi: 10.1001/jamainternmed.2018.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. 2022. Dietary and inhalation exposure to nano- and microplastic particles and potential implications for human health. Geneva: WHO.
- Wright SL, Kelly FJ Plastic and human health: a micro issue? Environmental Science & Technology. 2017;51(12):6634–6647. doi: 10.1021/acs.est.7b00423. [DOI] [PubMed] [Google Scholar]
- Wright SL, Ulke J, Font A, et al Atmospheric microplastic deposition in an urban environment and an evaluation of transport. Environment International. 2020;136:105411. doi: 10.1016/j.envint.2019.105411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan W, Hamid N, Deng S, et al Individual and combined toxicogenetic effects of microplastics and heavy metals (Cd, Pb, and Zn) perturb gut microbiota homeostasis and gonadal development in marine medaka (Oryzias melastigma) Journal of Hazardous Materials. 2020;397:122795. doi: 10.1016/j.jhazmat.2020.122795. [DOI] [PubMed] [Google Scholar]
- Yu HJ, Chen PP, Gordon SP, et al The association between air pollution and sleep duration: a cohort study of freshmen at a university in Beijing, China. International Journal of Environmental Research and Public Health. 2019;16(18):3362. doi: 10.3390/ijerph16183362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu XB, Peng JP, Wang JD, et al Occurrence of microplastics in the beach sand of the Chinese inner sea: the Bohai Sea. Environmental Pollution. 2016;214:722–730. doi: 10.1016/j.envpol.2016.04.080. [DOI] [PubMed] [Google Scholar]
- Zhang N, Li YB, He HR, et al You are what you eat: Microplastics in the feces of young men living in Beijing. Science of the Total Environment. 2021;767:144345. doi: 10.1016/j.scitotenv.2020.144345. [DOI] [PubMed] [Google Scholar]
- Zhang R, Wang M, Chen XP, et al Combined toxicity of microplastics and cadmium on the zebrafish embryos (Danio rerio) Science of the Total Environment. 2020a;743:140638. doi: 10.1016/j.scitotenv.2020.140638. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Wolosker MB, Zhao YP, et al. 2020b. Exposure to microplastics cause gut damage, locomotor dysfunction, epigenetic silencing, and aggravate cadmium (Cd) toxicity in Drosophila. Science of the Total Environment, 744 : 140979.
- Zhong LC, Jin H, Tang H, et al Intake of polyamide microplastics affects the behavior and metabolism of Drosophila. Chemosphere. 2022;308:136485. doi: 10.1016/j.chemosphere.2022.136485. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data to this article can be found online.
Data Availability Statement
The raw metagenomics sequencing reads can be downloaded from the NCBI (PRJNA1101743), China National Center for Bioinformation (PRJCA025077), and Science Data Bank databases (DOI:10.57760/sciencedb.j00139.00127).