Abstract
Replication of the flavivirus Kunjin virus is associated with virus-induced membrane structures within the cytoplasm of infected cells; these membranes appear as packets of vesicles associated with the sites of viral RNA synthesis and as convoluted membranes (CM) and paracrystalline arrays (PC) containing the components of the virus-specified protease (E. G. Westaway, J. M. Mackenzie, M. T. Kenney, M. K. Jones, and A. A. Khromykh, J. Virol. 71:6650–6661, 1997). To determine the cellular origins of these membrane structures, we compared the immunolabelling patterns of several cell markers in relation to these sites by immunofluorescence and immunoelectron microscopy. A marker for the trans-Golgi membranes and the trans-Golgi network, 1,4-galactosyltransferase (GalT), was redistributed to large foci in the cytoplasm of Kunjin virus-infected cells, partially coincident with immunofluorescent foci associated with the putative sites of viral RNA synthesis. As determined by immunoelectron microscopy, the induced vesicle packets contained GalT, whereas the CM and PC contained a specific protein marker for the intermediate compartment (ERGIC53). A further indicator of the role of cellular organelles in their biogenesis was the observation that the Golgi apparatus-disrupting agent brefeldin A prevented further development of immunofluorescent foci of induced membranes if added before the end of the latent period but that once formed, these membrane foci were resistant to brefeldin A dispersion. Reticulum membranes emanating from the induced CM and PC were also labelled with the rough endoplasmic reticulum marker anti-protein disulfide isomerase and were obviously redistributed during infection. This is the first report identifying trans-Golgi membranes and the intermediate compartment as the apparent sources of the flavivirus-induced membranes involved in events of replication.
The genus Flavivirus is the best characterized within the Flaviviridae in regard to replication and provides a useful reference for the molecular biology of the Hepacivirus and Pestivirus genera. The RNA positive-strand genome of 11 kb has one long open reading frame giving rise to a single polyprotein comprising three structural proteins and seven nonstructural (NS) proteins in the gene order NH2-C-prM-E-NS1-NS2A-NS2B-NS3-NS4A-NS4B-NS5-COOH. NS3 protein contains motifs for the viral (serine) protease and helicase-associated activities, and NS5 protein contains motifs for methyltransferase (a capping enzyme) and RNA polymerase (4, 18, 34). Our previous studies with Kunjin virus (KUN) (an agent of Australian encephalitis) have defined for the first time many of the major features of flavivirus replication, e.g., the absence of subgenomic RNA, the role of double-stranded RNA (dsRNA) as a recycling template for RNA synthesis, identification and precise boundaries of all of the NS proteins (including new cleavage sites), and immunolocalization of the NS proteins and the core protein (5, 7, 29, 39–41, 48, 49). We are now exploring the role of cell membranes in KUN replication.
Flavivirus RNA replication occurs within the cytoplasm of infected cells in association with prominent virus-induced membrane structures which are separable by sedimentation from cellular membranes and retain RNA-dependent RNA polymerase (RDRP) activity after detergent treatment (6, 14). Membrane fractionation followed by detergent treatment and sedimentation through sucrose density gradients has been used to purify the KUN replication complex away from the structural proteins (6). Ultrastructural analyses of these fractions revealed that all of the characteristic flavivirus-induced membranes were associated with the purified RDRP activity, and electrophoretic separation of the associated radiolabelled proteins remaining after detergent treatment revealed a profile of NS3, NS1, NS2A, and NS2B/NS4A; NS5 was apparently degraded during the detergent treatment, but RDRP activity was retained (6). Furthermore, a replicon or subgenomic KUN RNA deficient in the structural genes but retaining the first 60 nucleotides of the core protein gene was able to replicate within transfected cells, also indicating that only the NS proteins were required for RNA replication (17).
We prepared a complete suite of polyclonal antibodies to the KUN NS proteins and core protein for defining their subcellular and ultrastructural locations, and we showed for the first time specific associations of NS proteins with unique flavivirus-induced membranes in infected Vero cells (29, 48, 49). These membranes were first described many years ago (20), but no known role in flavivirus replication has been attributed to them (6). We discovered by immunogold labelling of cryosections of infected cells that KUN NS2B and NS3 (the viral protease complex) and NS4A were colocalized in cytoplasmic membranes described as convoluted membranes (CM) and paracrystalline arrays (PC) (29, 49). In contrast, NS1, NS3, NS2A, and NS4A were colocalized in a separate unique cytoplasmic site defined as vesicle packets (VP) (29, 49), first described in dengue 2 virus (DEN2) infections (27, 28). Antibodies to dsRNA, the putative template for viral RNA synthesis, were also colocalized specifically within VP, indicating that the VP enclose the viral replication complex (28, 29, 49). Our recent studies have shown that the cytoplasmic foci identified with anti-dsRNA antibodies coincided precisely by immunofluorescence (IF) with the location of nascent RNA after pulse-labelling of infected cells with bromouridine (50). The KUN core and NS4B proteins were associated with proliferated endoplasmic reticulum (ER) membranes and translocated to the nucleus during infection (48). These results showed that the flavivirus nonstructural proteins migrate to induced membrane sites with apparently specific functions in the virus replication cycle.
There is an increasing interest in the origins of the ultrastructural locations of the sites of RNA replication of positive-strand RNA animal viruses. For example, poliovirus induces an accumulation of small vesicles within the cytoplasm of infected cells by inhibiting the fusion of ER vesicles to the Golgi apparatus (8, 9). Accumulation of these vesicles leads to the formation of rosettes containing the poliovirus proteins responsible for RNA replication (1, 2). Immunogold labelling and biochemical analyses of these vesicles indicated they were derived from autophagic vacuoles (35). Togaviruses appear to modify endosomes or lysosomes for replication of their RNA, inducing large vacuoles enclosing small bilayered vesicles containing the viral RNA (10, 13, 21, 30).
In order to define the intracellular origins of the flavivirus-induced membranes associated with specific NS proteins, we compared the distributions in the cytoplasm of dsRNA and NS3 with various protein markers associated with specific cellular compartments by IF and cryoimmunoelectron microscopy (cryo-IEM). Antibodies to NS3 provided a useful marker probe, because NS3 is located both in VP (with dsRNA) and in CM and PC (with no dsRNA) (49). The results show that the source of induced membranes in flavivirus-infected cells is more complex than that observed in the replication of poliovirus or the togaviruses.
MATERIALS AND METHODS
Cells and virus.
Vero cells were grown in medium 199 (Gibco) supplemented with 5% fetal calf serum. In maintenance medium (Eagle’s minimum essential medium), serum was replaced with 0.1% bovine serum albumin. KUN strain MRM 61C was grown in Vero cells as previously described (49).
Antibodies.
A guinea pig polyclonal antibody recognizing dsRNA in cells was generously provided by Jia Yee Lee (Macfarlane Burnett Centre for Medical Research, Melbourne, Australia) (21). Antibodies as specified were generously provided by the following investigators: monoclonal antibodies to ERGIC53 (36) and to Giantin (23) by H.-P. Hauri (University of Basel, Basel, Switzerland) and to protein disulfide isomerase (PDI) (ID3) (45) by S. Fuller (European Molecular Biology Laboratory, Heidelberg, Germany), rabbit polyclonal antibodies to 1,4-galactosyltransferase (GalT) (46) by E. Berger (University of Zurich, Zurich, Switzerland) and to human Lamp1 (93/B) (11) by M. Fukuda (La Jolla Cancer Research Foundation, La Jolla, Calif.), and goat polyclonal antibodies to mannose-6-phosphate receptor (MPR300, Zi I-2) by A. Hille (Department of Biochemistry, Georg August University, Göttingen, Germany). Antibodies specific for mouse, rabbit, guinea pig, or goat immunoglobulin G (IgG) and conjugated to fluorescein isothiocyanate (FITC) or Texas red were purchased from Jackson ImmunoResearch, West Grove, Pa.
IF.
Vero cells on coverslips were infected with KUN at a multiplicity of infection (MOI) of 2 to 5 and processed after fixation for indirect IF at 24 h postinfection (p.i.) unless stated otherwise. The standard fixation method was acetone treatment of cell monolayers at −20°C for 30 s (48); however, incubation of cells with 95% methanol for 10 min at 20°C before acetone fixation was necessary for visualization of anti-PDI antibodies. Dual-labelling experiments used FITC- and Texas red-conjugated species-specific anti-IgG antibodies. An oil immersion lens was used with epifluorescent lighting, and images were obtained either by using a confocal microscope (Bio-Rad MRC-600) or by photography onto Kodak color film with a Nikon E600 microscope. Images were merged and processed on an IBM computer by using Adobe Photoshop and Powerpoint software.
RIP of cell lysates.
Subconfluent cell monolayers were infected with KUN at an MOI of 5. At 24 h p.i. the cells were radiolabelled with 50 μCi of [35S]methionine-cysteine (Tran35S-label; ICN) per ml for 4 h in the presence of actinomycin D. The cells were subsequently harvested in radioimmunoprecipitation (RIP) assay buffer (10 mM Tris [pH 7.4], 150 mM NaCl, 0.1% sodium dodecyl sulfate [SDS], 1% Triton X-100, 1% sodium deoxycholate) and clarified by low-speed centrifugation at 4°C. The supernatant was used for RIP with antibodies to GalT and NS3 as previously described (49). Isolated proteins were resolved on an SDS–12.5% polyacrylamide gel that was fixed and incubated with Amplify (Amersham), and labelled proteins were visualized by autoradiography.
IEM.
Subconfluent cell monolayers were infected with KUN at an MOI of 5. At 24 h p.i. the cells were harvested in 2% trypsin and resuspended in 4% paraformaldehyde–0.1% glutaraldehyde in 0.1 M sodium phosphate buffer (pH 7.4). The cells were then washed in 0.1 M phosphate buffer (pH 7.4) and embedded in 20% gelatin before cryofixation (27). Ultrathin cryosections were collected on a 19:1 mix of 2.3 M sucrose–2% methylcellulose for immunolabelling with protein A-gold (batch 9705; Utrecht University, Utrecht, Netherlands) or goat anti-mouse IgG–gold (Biocell, Cardiff, United Kingdom) as described previously (28, 48). For dual-labelling experiments the protocols of Geuze et al. (12) and Slot et al. (38) were used with protein A-gold of different sizes (see also reference 28).
RESULTS
Association of the putative intracellular site of KUN RNA synthesis with markers for the trans-Golgi region, IC, and RER.
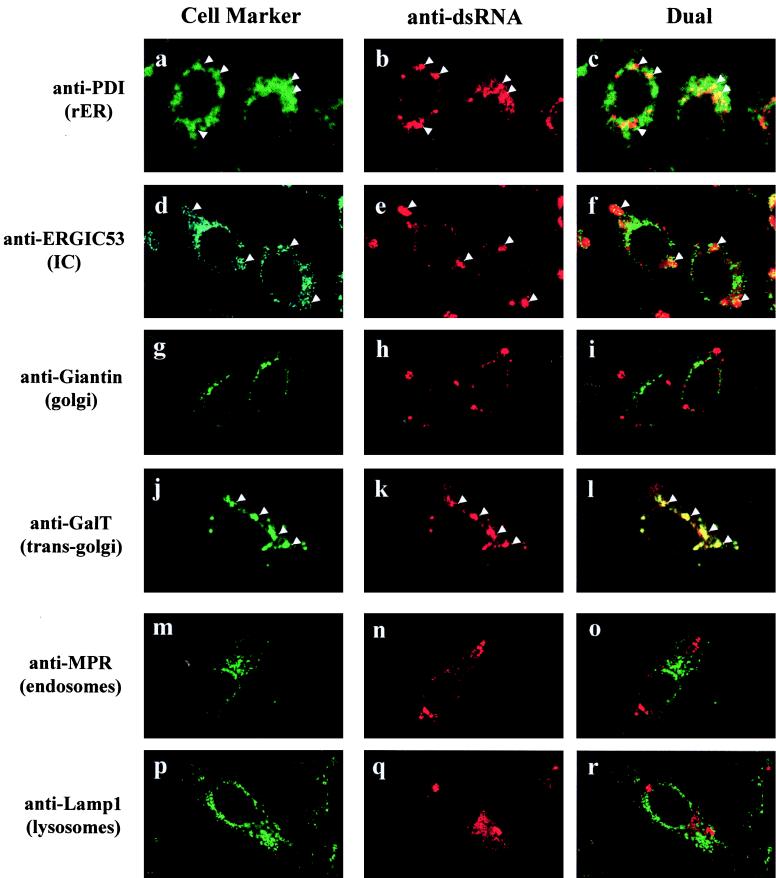
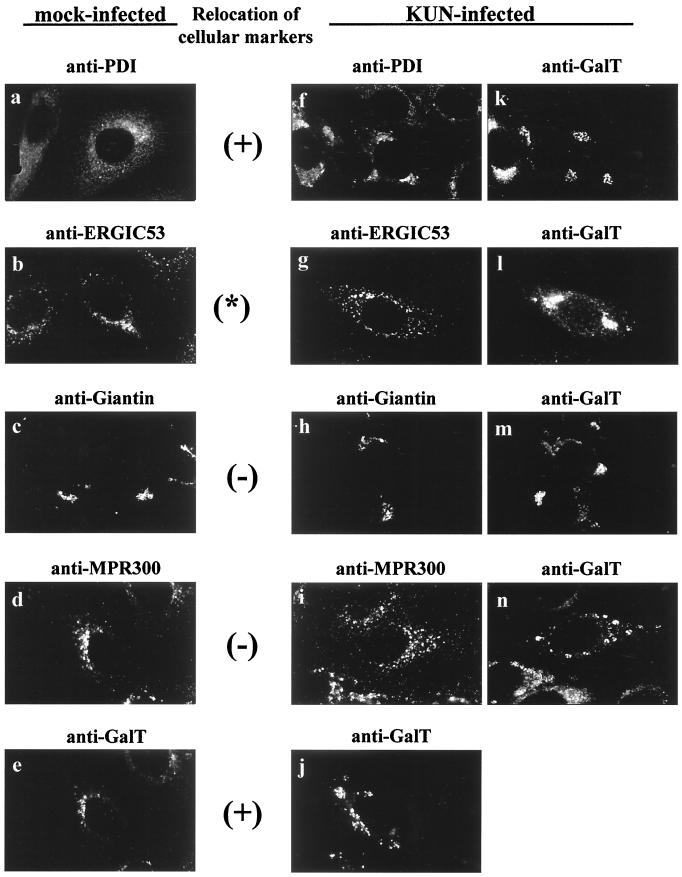
Previously we have shown that KUN RNA synthesis in infected cells appears to occur in cytoplasmic foci readily identifiable by IF with antibodies to dsRNA (29, 48, 49), which colocalize precisely with antibodies able to detect nascent viral RNA pulse-labelled with bromouridine (50). In order to probe by IF the cellular origins of membranes associated with the dsRNA foci, we performed dual-labelling experiments at 24 h p.i. with anti-dsRNA antibodies together with antibodies to various cellular compartment markers (Fig. 1). This initial screening provided an overview of the general distribution of the cellular markers compared with the apparent intracellular sites of KUN RNA synthesis. The labelling pattern of GalT, a marker for the trans-Golgi membranes and trans-Golgi network, appeared to be largely coincident with the cytoplasmic foci observed with anti-dsRNA antibodies (Fig. 1j to l). Both anti-GalT and anti-dsRNA antibodies stained as discrete foci scattered throughout the cytoplasm (often perinuclear), which is typical of dsRNA staining during the late stages of KUN infection (29, 32, 48, 49). Variation in the distribution of foci defined by anti-dsRNA antibodies, as seen in Fig. 1, is commonly observed during flavivirus infection, possibly due to differences in the stages of the replication cycle or the responses of individual cells not synchronously infected (29, 48, 49). Interestingly, the other components of the Golgi apparatus (i.e., cis- and medial-Golgi immunostained with anti-Giantin) were not coincident with the dsRNA foci (Fig. 1g to i), indicating that the dsRNA foci were not directly associated with the Golgi apparatus itself. We also observed some coincidental staining with markers for the rough ER (RER) (anti-PDI) and possibly the intermediate compartment (IC) (anti-ERGIC53) (Fig. 1a to f), suggesting that some elements of these compartments were also localized to the dsRNA foci. However, the coincidental labelling of anti-dsRNA with anti-PDI and anti-ERGIC53 was much less than that observed for anti-GalT. In contrast, markers for endosomes (anti-MPR300) and lysosomes (anti-Lamp1) did not appear to be coincident with the KUN dsRNA foci (Fig. 1m to r).
FIG. 1.
Markers for the RER, IC, and trans-Golgi region are associated by IF with the putative site of KUN RNA synthesis. KUN-infected Vero cells were fixed with cold acetone at 24 h p.i. and processed for indirect IF with anti-dsRNA antibodies and Texas red conjugates (b, e, h, k, n, and q) for comparisons in the same cell with FITC labelling of antibodies to various cellular compartments (a, d, g, j, m, and p), as indicated. Apparent partial coincidence in the dual labels was observed as a yellow hue in panels c, f, and l (arrowheads). No coincidence with dsRNA foci is apparent for the markers of the cis- and medial-Golgi (Giantin), endosomes (MPR300), and lysosomes (Lamp1). The actual specificities of all of the primary antibodies are described in the first section of Results.
In summary, the cytoplasmic dsRNA foci coincided with a marker for the trans-Golgi membranes and trans-Golgi network by IF, suggesting that the sites of KUN RNA synthesis are derived from this compartment or from membranes containing GalT. In addition, some elements of the RER and possibly the IC were also observed to be partially overlapping with dsRNA foci.
GalT, a resident protein from the trans-Golgi membranes and the trans-Golgi network, is also found in induced VP, the putative site of viral RNA synthesis.
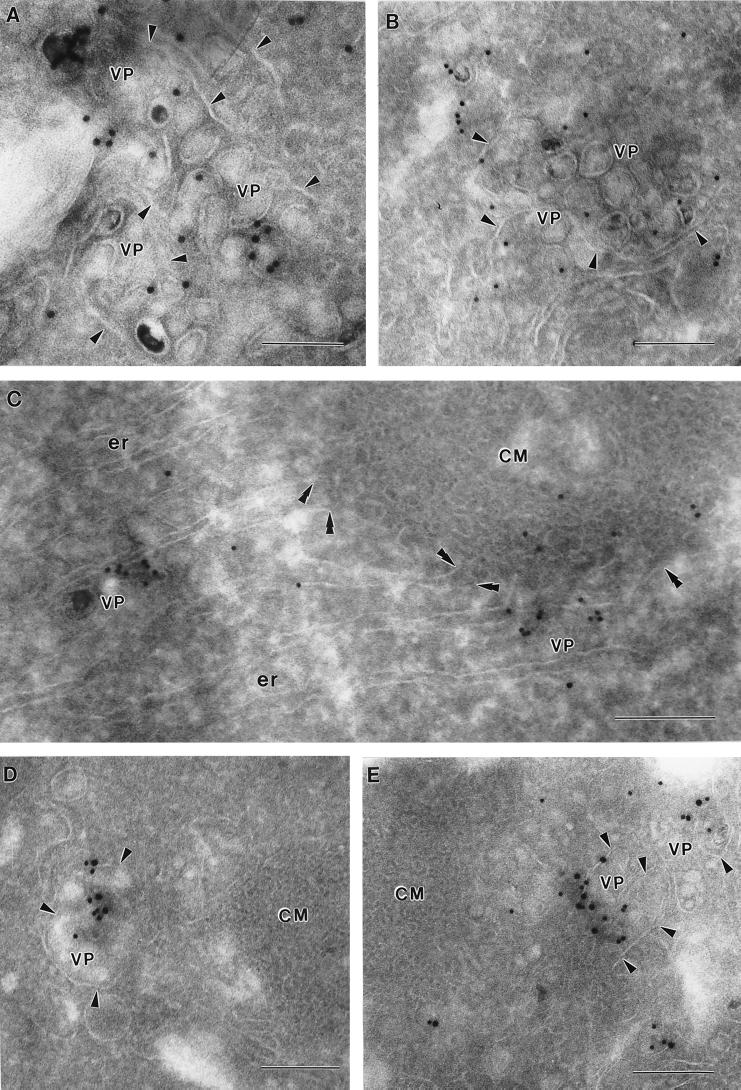
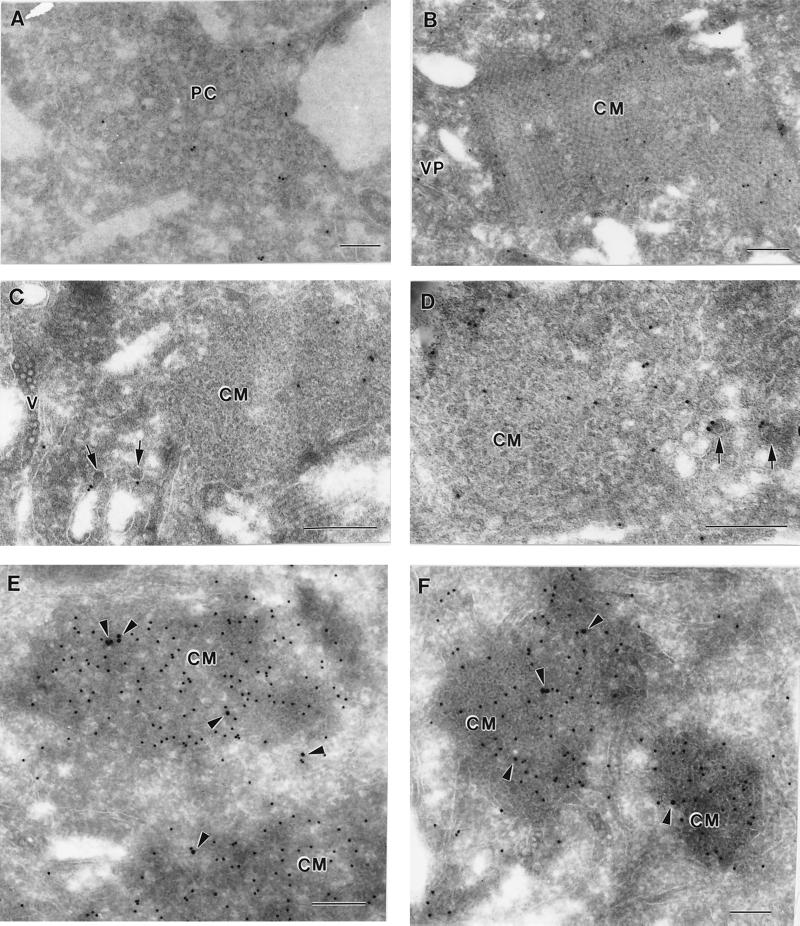
In order to explore the relationship of the GalT marker to the ultrastructure of KUN-induced membranes, cryosections of KUN-infected cells were immunolabelled with anti-GalT antibodies and 10-nm-diameter gold particles at 24 h p.i. (Fig. 2). Gold particles were enriched within clusters of virus-induced vesicles often seen bounded by a surrounding membrane; these structures have been recently identified as VP in DEN2- and KUN-infected cells and contain the putative dsRNA template, NS3, and other NS proteins associated with the flavivirus replication complex (27–29, 49). Anti-GalT antibodies appeared to label the membranes of individual vesicles, but they were seldom associated with the membrane surrounding the VP (Fig. 2A and B) and were more enriched on some vesicles than on others (compare Fig. 2E). Another collection of induced membranes, previously described as CM (6, 20, 48, 49), were observed in close proximity to the VP (Fig. 2C to E); however, the bounding membrane of VP was often absent or obscured at the region of contact with CM (Fig. 2D and E). Generally, anti-GalT antibodies were not observed within either CM (Fig. 2D and E) or PC (data not shown) structures, although some gold particles were observed within the outermost region of the CM structure adjacent to a probable VP enriched with anti-GalT antibodies (Fig. 2C). Interestingly, ER membranes could be observed apparently emanating from the CM (Fig. 2C), possibly connecting also to the VP, suggesting that the CM may in part be continuous with the ER. (Unfortunately, visualization of ribosomes is difficult in cryosections [27], but note the anti-PDI labelling of connecting ER in Fig. 6.) Anti-GalT antibodies were also observed to immunolabel vesicles adjacent to the Golgi apparatus. Dual-labelling experiments with both anti-dsRNA and anti-GalT antibodies showed that the enrichment of GalT labelling within the VP coincided precisely with that of dsRNA, possibly upon individual vesicles within the packet (Fig. 2D and E). In single-labelling experiments, anti-dsRNA antibodies labelled only the VP (results not shown). Interestingly, anti-GalT seldom labelled VP in the absence of dsRNA labelling, suggesting that GalT protein was closely associated with replication of the viral RNA rather than merely a structural component throughout the membranes of the VP.
FIG. 2.
GalT, a marker for the trans-Golgi membranes and trans-Golgi network, is located within KUN-induced VP. Infected Vero cells were harvested at 24 h p.i. and processed for cryo-IEM and immunolabelling. Ultrathin cryosections were cut and probed with antibodies to GalT and protein A–10-nm-diameter gold particles. Enrichment of antibodies to GalT was observed in all sections (A to E) within the virus-induced VP, which appear to be at an early poorly defined stage of development in panel C. Also in panel C, double arrowheads indicate continuities between ER membranes and the CM structures. In panels D and E, cryosections were dual labelled with anti-GalT antibodies (15-nm-diameter gold particles) and anti-dsRNA antibodies (10-nm-diameter gold particles), showing a close association between GalT and the putative viral RNA template within VP. Note that the VP vary in size and membrane definition, and the bounding membrane enclosing the VP is indicated by single arrowheads. Bars, 200 nm.
FIG. 6.
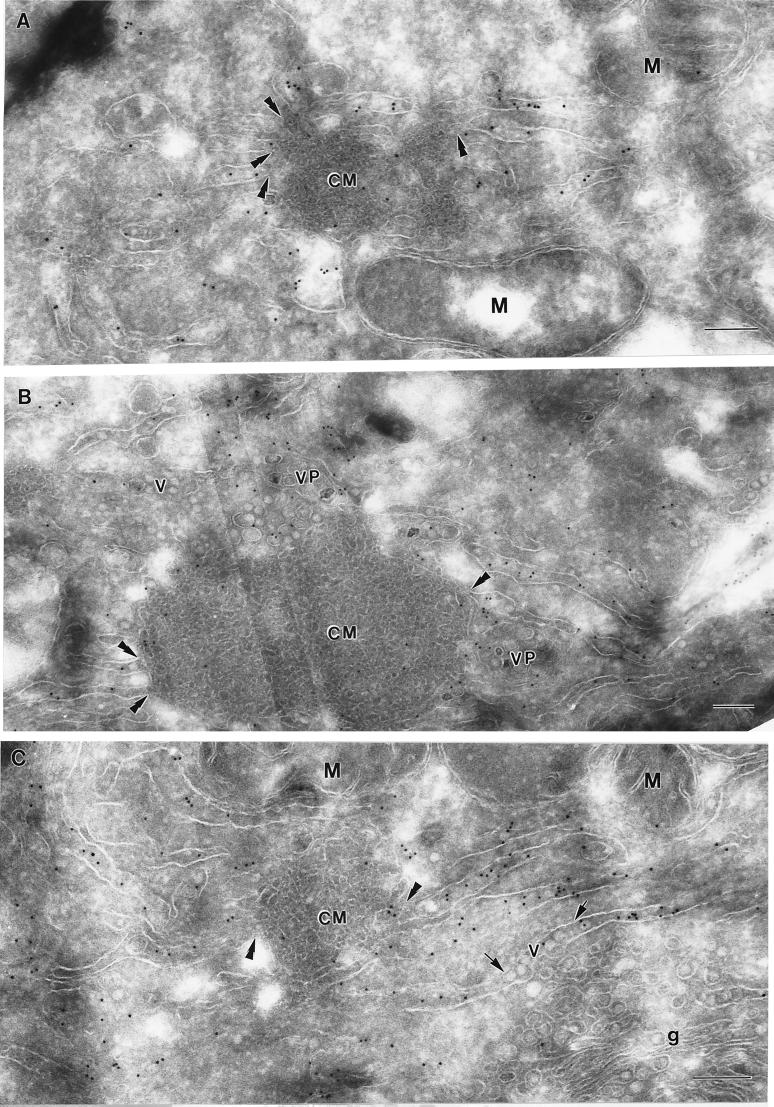
ER membranes emanating from the CM are heavily labelled with anti-PDI antibodies, indicative of the RER, which often appear to be continuous with the outer convoluted membranes (double arrowheads). Virions have accumulated in PDI-labelled ER (arrows in panel C) that often appear to be distended. VP are also evident adjacent to the CM and membranes labelled with PDI (B). M, mitochondria; v, virus particles; g, Golgi bodies. Bars, 200 nm.
The results in this section show that GalT (a marker protein for the trans-Golgi region) is enriched on individual vesicles within the VP and suggest that the assumed intracellular site of KUN RNA synthesis may contain membranes derived from the trans-Golgi region. GalT was not significantly enriched in the morphologically distinct CM, which contain the viral protease complex NS2B-NS3 (49).
GalT is associated with the viral proteins proposed to constitute the KUN replication complex.
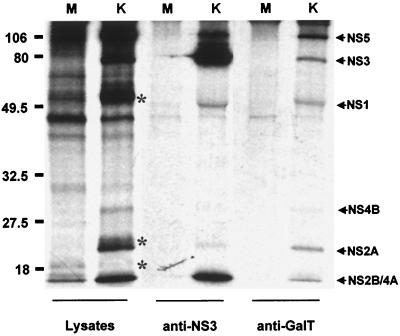
To further show that GalT was associated with the KUN replication complex, we performed RIP analysis of infected-cell lysates with anti-GalT antibodies (Fig. 3). All of the KUN NS proteins previously shown to be colocalized with the putative replication site of KUN RNA were coprecipitated with anti-GalT antibodies, viz., NS5, NS3, NS1, NS2A, and NS4A (Fig. 3, lane 6) (29, 49). A similar protein profile was obtained previously by RIP of KUN-infected cell lysates with anti-dsRNA antibodies (49), and it was deficient in the structural proteins E, prM, and C, as in the anti-GalT immunoprecipitation (compare lanes 2 and 6 in Fig. 3). Interestingly, a similar selective profile of viral proteins was observed in radiolabelled active replication complexes sedimented after detergent treatment (6). GalT protein could not be visualized in lanes 5 and 6 of Fig. 3, possibly because the inclusion of actinomycin D during the metabolic labelling period tends to inhibit translation of some cellular proteins. Anti-NS3 antibodies coprecipitated the same nonstructural proteins, with enrichment of NS3 and NS2B/NS4A (lane 4), which are colocalized in CM and PC induced membranes (29, 49).
FIG. 3.
GalT is closely associated by coimmunoprecipitation with the KUN nonstructural proteins NS5, NS3, NS1, NS2A, and NS4A, which are proposed to constitute the flavivirus replication complex (29, 49). Infected Vero cells were radiolabelled (in the presence of actinomycin D) from 24 to 28 h p.i. and harvested in RIP assay buffer for RIP with either anti-NS3 (lanes 3 and 4) or anti-GalT (lanes 5 and 6) antibodies. Lanes 1 and 2 represent the initial lysates before RIP. Specific KUN nonstructural proteins are indicated by arrows, and the KUN structural proteins E, prM, and C (notably absent in the immunoprecipitate in lane 6) are indicated by asterisks in lane 2. Lanes M, mock-infected cell lysates; lanes K, KUN-infected cell lysates. Proteins were separated on an SDS–12.5% polyacrylamide gel and visualized by autoradiography. Sizes of protein markers, indicated on the left, are expressed in kilodaltons.
In summary, we have provided very strong evidence that GalT, and perhaps membranes from the trans-Golgi region, not only were located within the VP but also appeared to be associated closely with the KUN NS proteins and the proposed template involved in viral RNA replication.
Redistribution of GalT in KUN-infected Vero cells.
Because of the presence in infected cells of large masses of virus-induced membranes associated with KUN NS proteins (29, 49) and the observations that anti-GalT antibodies, but not anti-Giantin antibodies, were associated with these membrane masses by IF (Fig. 1), we next compared the staining patterns of GalT with other cell markers within mock- and KUN-infected Vero cells at 24 h p.i. Figure 4 shows that the distributions of markers for the RER (anti-PDI) and trans-Golgi region (anti-GalT) in infected cells were profoundly changed from their distributions in mock-infected cells, notably in apparent aggregation of membranes, whereas the distributions of the other cellular markers remained relatively unaffected (compare panels a to e with panels f to j). Thus, anti-GalT label in mock-infected cells stained mainly as small juxtanuclear foci (Fig. 4e), but that in KUN-infected cells appeared mainly as large perinuclear inclusion bodies and discrete foci within the cytoplasm (Fig. 4j to n), similar to those coincident with dsRNA foci in Fig. 1. However, comparison of Giantin and GalT staining in infected cells (Fig. 4h and m) showed that not all of the cellular GalT appeared to redistribute away from the Golgi complex, as some coincidence with Giantin staining in the perinuclear region was retained. In contrast, we observed total colocalization of GalT and Giantin by dual labelling in mock-infected cells (results not shown). Because the general distribution of anti-Giantin antibodies remained similar in mock- and virus-infected cells (Fig. 4c and h), these results suggest that KUN infection did not disrupt the cis or medial components of the Golgi apparatus. In accord with the results on overlapping localization with dsRNA in Fig. 1, we also observed strong partial colocalization between anti-GalT and anti-PDI (Fig. 4f and k). In mock-infected Vero cells, anti-PDI labelling displayed a typically fine reticular staining pattern within the cytoplasm (Fig. 4a), but within KUN-infected cells, the reticular pattern became denser in the perinuclear region and included cytoplasmic foci of various sizes (Fig. 4f). A similar redistribution of ER-associated ribosomes was detected previously by IF in KUN-infected Vero cells (32).
FIG. 4.
GalT, PDI, and to a lesser extent the IC marker ERGIC53 appear to be redistributed in cytoplasm after infection of Vero cells with KUN. (f to n) Infected cells at 24 h p.i. dual labelled for IF with both anti-GalT antibodies (j to n) and antibodies to other cell compartment markers (f to i). Evidence suggesting relocation was based on comparisons of the relative distribution of each marker in mock-infected (a to e) and KUN-infected (f to j) cells, as discussed in the text. Obvious signs of redistribution of GalT and PDI are indicated with a plus sign, and the asterisk between the anti-ERGIC53 panels indicates a small amount of redistribution.
In summary, KUN infection caused a major redistribution of the markers for the trans-Golgi region (GalT) and the RER (PDI), but not of those for the Golgi apparatus or other cellular compartments. Some minor overlap of GalT labelling could also be seen with anti-ERGIC53, similar to that observed with anti-dsRNA. These results indicate that the profound ultrastructural changes due to the appearance of induced membranes in infected cells (29, 49) are associated with the visible changes seen by IF in the distribution of cellular markers, especially of elements of the trans-Golgi region, the RER, and, to a small extent, the IC.
Immunogold labelling shows that virus-induced CM and PC structures are apparently derived from the IC and are directly connected to the RER.
In order to explore the cellular origins of infected-cell compartments not associated with dsRNA, namely, the CM and PC, we immunolabelled KUN-infected cryosections with antibodies to the IC marker ERGIC53 (Fig. 5) and to PDI (see Fig. 6), because of their close association with both anti-dsRNA and anti-GalT antibodies by IF. In single-labelling experiments, anti-ERGIC53 antibodies labelled CM and PC structures reasonably well but did not detectably label a VP or apparent arrays of virions (Fig. 5A to D). The morphological distinction between the CM and the PC structures is not always clear because of their apparent interconversion, as discussed previously (49). The CM in Fig. 5B and E appear to be undergoing such a transition to or from PC. The immunogold-labelled ERGIC53 was slightly enriched within the CM compared to the distribution throughout the cell. Within the CM and PC the distribution of anti-ERGIC53 antibodies was random but appeared to be localized mainly to the internal membranes. Anti-ERGIC53 antibodies also labelled the bounding membranes of small vesicles with electron-dense centers, ranging from 50 to 60 nm in diameter, within the cytoplasm (Fig. 5C and D). These vesicles are similar in appearance to those immunolabelled with the same antibody by Schweizer et al. (36) on the cis face of the Golgi apparatus and therefore may be transport vesicles associated with the IC. Very little if any background labelling by anti-ERGIC53 antibodies was observed over mitochondria or nuclei. Dual-labelling experiments confirmed that ERGIC53 was associated with CM structures heavily labelled, as expected (49), with anti-NS3 antibodies (Fig. 5E and F). It should be noted that during the dual-labelling experiments, the anti-ERGIC53 antibody labelled less efficiently than in single-labelling experiments. Thus, the immunogold labelling results indicate that the CM and PC may be derived from or incorporate a marker from the IC.
FIG. 5.
KUN-induced CM and PC structures are labelled with a marker associated with the IC. Infected cells were harvested and processed for cryo-IEM and immunolabelling. (A to D) Ultrathin cryosections were cut and probed with antibodies to the IC marker ERGIC53 and labelled with protein A–10-nm-diameter gold particles. Both PC and CM were randomly but specifically labelled in panels A and B, respectively; they appear to be interconvertible structures (see text), and hence their labelling as either CM or PC is sometimes subjective. The arrows in panels C and D indicate small cytoplasmic vesicles (possibly elements of the IC) labelled with anti-ERGIC53 antibodies in close proximity to virus-induced CM also labelled with anti-ERGIC53 antibodies. (E and F) Cryosections were dual labelled with antibodies to ERGIC53 (15-nm-diameter gold) and NS3 (10-nm-diameter gold), and coincidental labelling of both antibodies within CM is highlighted with arrowheads. V, accumulated virus particles within distended ER (C). Bars, 200 nm.
In contrast to the anti-ERGIC53 antibodies, anti-PDI antibodies localized primarily to reticulum membranes emanating from the CM (Fig. 6). In some cryosections the labelled ER membranes appeared to be continuous with the CM, suggesting that the RER and modified IC membranes are continuous (see also Fig. 2C). Minor anti-PDI labelling was observed variably within the CM structures, but this may represent transient trafficking of PDI within the IC, as has been observed by others (33, 37, 45). PDI labelling was at times associated with reticulum membranes containing virus particles (Fig. 6C), similar to observations with resin-embedded sections of flavivirus-infected cells, in which virus particles often appear within hypertrophied RER connecting to CM or PC (31). We observed minimal labelling by PDI antibody on either mitochondria or nuclei.
The results from this section and the other immunolabelling experiments provide evidence that the IC is a likely source of or is transported to the induced CM and PC and that these structures appear to be continuous with reticulum membranes immunolabelled with anti-PDI antibodies.
Effect of BFA on virus-induced membrane formation.
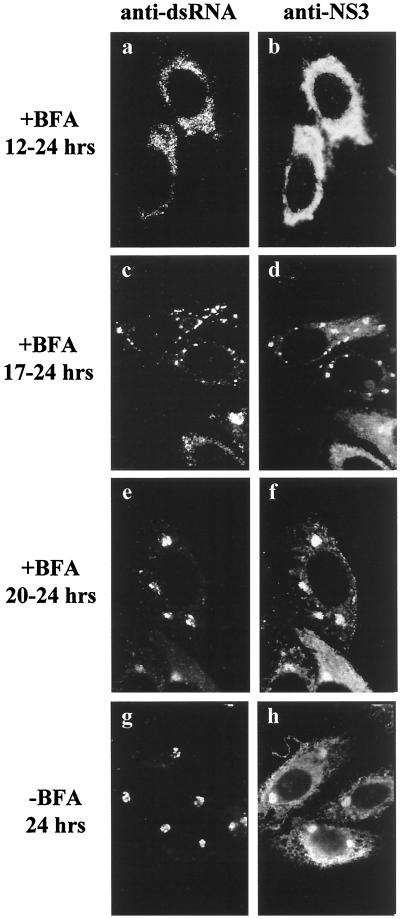
Flavivirus replication requires a relatively long latent period of 13 to 15 h (47); during this time, both viral RNA and protein syntheses obviously must occur, albeit at a low level. The characteristic virus-induced membranes are not clearly formed until about the end of the latent period (49), and the present results indicate an involvement of some of the Golgi apparatus-associated membranes in this process. To investigate the effect of the Golgi apparatus-disrupting agent brefeldin A (BFA) (24, 25) on virus-induced membrane formation, we incubated KUN-infected Vero cells with BFA at different times p.i. KUN-infected cells immunolabelled at 24 h p.i. without any prior incubation with BFA showed coincident discrete foci by IF within the cytoplasm when labelled with anti-dsRNA and anti-NS3 antibodies (Fig. 7g and h), in accord with our previous report (49). When BFA was added before the virus-induced membranes were clearly formed, i.e., from 12 h p.i., the staining patterns of dsRNA were dispersed or diffuse (Fig. 7a and b), similar to those reported previously at 8 h p.i. (49). When we treated infected cells just after the end of the latent period, i.e., from 17 to 24 h p.i., small discrete foci labelled with anti-dsRNA were observed scattered throughout the cytoplasm (Fig. 7c), similar to those observed in untreated cells at 16 h p.i. (49), and many of these were dual labelled with anti-NS3 antibodies (Fig. 7d). Incubation of infected cells with BFA from 20 to 24 h p.i. produced little or no difference in labelling patterns by IF compared to untreated infected cells at 24 h p.i., but the dsRNA foci were larger than those observed after treatments commencing earlier (compare Fig. 7c and d with Fig. 7e and f).
FIG. 7.
Effect of the Golgi apparatus-disrupting agent BFA on the formation of KUN-induced cytoplasmic foci as detected by IF. Infected cells were incubated with (a to f) or without (g and h) BFA (10 μg/ml) at different times p.i., as indicated, and the relative distributions of anti-dsRNA (a, c, e, and g) and anti-NS3 (b, d, f, and h) antibodies were analyzed after acetone fixation and subsequent dual labelling with Texas red- and FITC-conjugated antibodies, respectively.
Clearly, treatment with BFA prevented subsequent membrane induction when BFA was added during the latent period, i.e., before the formation of the characteristic foci of NS3 and dsRNA as observed by IF, indicating that the induced membranes were derived from the Golgi region. However, once formed, these virus-induced membrane structures appeared to be very stable to BFA treatment (Fig. 7c to f), but any further development was inhibited.
GalT remains within the dsRNA foci during BFA treatment of KUN-infected cells.
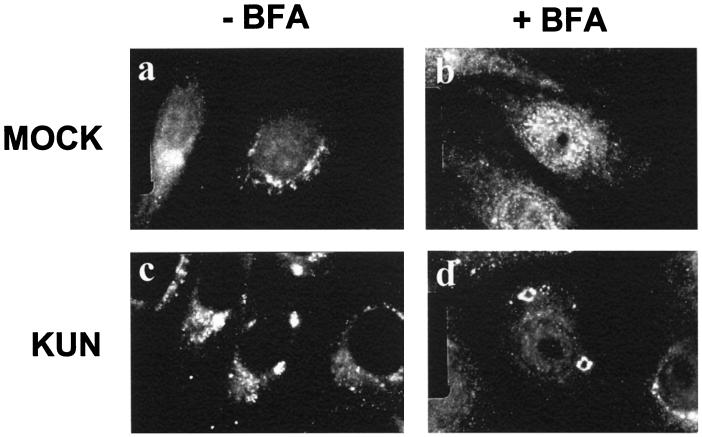
We showed above that the KUN-induced cytoplasmic foci containing the putative replication sites were resistant to dispersion by BFA (Fig. 7). Because these sites containing dsRNA were also labelled with anti-GalT antibodies by IF (Fig. 1) and by cryo-IEM (Fig. 2), we investigated the effect of BFA treatment from 20 to 24 h p.i. on the distribution of GalT within infected cells (Fig. 8). As a control, we also treated mock-infected cells with BFA for 4 h; in this case anti-GalT antibodies exhibited a diffuse cytoplasmic staining in the presence of BFA, as expected (25, 44), compared to the perinuclear accumulation in the absence of BFA (compare Fig. 8a and b). In contrast, in KUN-infected cells at 24 h p.i., GalT remained within large cytoplasmic foci irrespective of BFA treatment (compare Fig. 8c and d).
FIG. 8.
Stable association of GalT within cytoplasmic foci in KUN-infected cells after treatment with BFA. Vero cells were either mock infected or infected with KUN for 20 h, and BFA was then added to the medium and left for an additional 4 h. The cells were fixed and probed with anti-GalT antibodies and an FITC conjugate. Within mock-infected cells GalT was immunostained in the perinuclear region in the absence of BFA (a) but appeared dispersed throughout the cytoplasm upon addition of BFA (b). In contrast, GalT remained within large cytoplasmic foci in KUN-infected cells in both the presence and absence of BFA (c and d).
These results show that while BFA treatment dispersed the trans-Golgi membranes in mock-infected cells, the KUN-induced foci observed by IF were resistant to dispersion by BFA and retained GalT as well as dsRNA and NS3 in association with the putative replication complex.
DISCUSSION
Our previous results on immunolocalization of replication sites in KUN-infected cells established that the putative dsRNA template was associated uniquely in VP with several NS proteins, viz., NS1, NS3, NS5, NS2A, and NS4A, and that NS3 with its protease cofactor NS2B and NS4A was located also in the CM and PC, the putative site of proteolytic cleavages (29, 49). During this study, we have further characterized the intracellular sites of KUN replication by IF and cryo-IEM, focusing on the origins of the associated membranes. We showed that antibodies to dsRNA and to a marker for the trans-Golgi region (GalT) labelled coincidentally within both IF foci and the virus-induced VP (Fig. 1 and 2). In contrast, the CM and PC structures but not the VP were labelled with antibodies to the IC marker ERGIC53 (Fig. 5), indicating a clear distinction between the possible origins of these induced membranes involved in apparently different functions, namely, proteolytic processing of viral proteins and viral RNA replication, respectively. In addition, anti-PDI antibodies heavily labelled reticulum membranes emanating from the CM and PC that in some cryosections appeared to be continuous with the virus-induced CM and PC (Fig. 2C and 6). Thus, during KUN infection, GalT largely dissociated from other Golgi apparatus-associated compartments (Fig. 1 and 4), and RER appeared to proliferate in association with the CM and PC structures. This report indicates for the first time the likely origins of the unique cytoplasmic membranes induced during the flavivirus replication cycle, and we can now attribute different cell markers to them, as well as their previously established different constellations of viral markers (29, 49).
We found that GalT was enriched within the virus-induced VP as determined by cryo-IEM (Fig. 2) and was observed by IF to have partially and specifically redistributed to areas proximal to or overlapping with the locations of the IC and RER markers ERGIC53 and PDI, respectively, and away from the other Golgi components and endosomes (Fig. 4). An important question is whether formation of the VP in KUN-infected cells requires redistribution of GalT protein either alone or in conjunction with membranes and other proteins from the trans-Golgi region. Dual IF with an antibody to TGN46 (kindly donated by V. Ponnambalam, University of Dundee, Dundee, United Kingdom), a resident protein of the trans-Golgi network, and anti-dsRNA showed coincidental staining (data not shown), suggesting the VP are truly derived from trans-Golgi associated membranes and are not formed by redistribution of GalT alone to other membrane sites. An involvement of GalT-containing vesicles with the RER has been observed in HeLa cells after treatment with BFA (44), and those authors showed by cryo-IEM that BFA induced clusters of GalT-positive vesicles adjacent to or, at times, apparently continuous with RER membranes. The size and morphology of these vesicles are similar to those of vesicles labelled with GalT in KUN VP. Therefore it is possible that KUN infection may be “hijacking” vesicles from the retrograde pathway from the trans-Golgi region to the RER. However, we cannot discount the possibility that newly synthesized GalT maybe redistributed to the VP directly from the RER. The origin of the flavivirus VP obviously differs from the modified endosomes or lysosomes comprising cytopathic vacuole type I of alphaviruses, where RNA synthesis occurs (10, 30), and from the aggregated low-density smooth membranes at the site of synthesis of poliovirus RNA (1, 2).
Within uninfected cells, the IC represents a compartment separate from the RER based on the localization therein of different protein markers (37), yet the RER and IC appear to be physically connected during expression of some viral glycoproteins (15, 19). We observed a similar phenomenon in KUN-infected Vero cells by using cryo-IEM and specific labelling with anti-PDI antibodies of RER (Fig. 6) and with antiERGIC53 antibodies of CM and PC (Fig. 5). The occasional observation of PDI labelling within the CM and PC suggests that these membrane structures may retain some IC properties, because PDI is thought to recycle from the IC via its C-terminal KDEL motif to the RER (33). It should be noted that the viral proteins resident within the CM and PC, namely, NS2B, NS3, and NS4A, are sequentially translated and cleaved by the viral protease and do not appear to contain any obvious motifs associated with selective retention or retrieval within the IC or RER. Interestingly, membrane structures with a similar morphology have been observed during infection of cells with vesicular stomatitis virus (26) and mouse hepatitis virus (19) and after overexpression of the rubella virus E1 glycoprotein (15, 16). These similar membrane arrays all appear to contain specific cell markers associated with the IC, including ERGIC53 (16), and are frequently observed to be continuous with the RER, analogous to the associations of KUN CM and RER. It may be that in some cases protein expression associated with enveloped viruses accentuates the proliferation of this cellular compartment.
To investigate the dynamics involved in membrane formation, we incubated KUN-infected Vero cells with BFA at different times p.i. BFA is a known Golgi apparatus-disrupting agent, and its effects lead to the redistribution of Golgi proteins, including GalT, by retrograde transport to the ER (24, 25, 44). When BFA was added from 12 h, i.e., during the KUN latent period, no induction of the characteristic large foci representing sites of virus replication was observed by IF with either anti-dsRNA or anti-NS3 antibodies (Fig. 7). This result supports the proposal that VP are derived at least in part from Golgi apparatus-associated compartments. When BFA was added after the latent period, at about 17 h, the previously induced membranes appeared to be stable (Fig. 7 and 8). BFA restricts anterograde protein transport between the ER and Golgi apparatus, leading to recycling of proteins between the ER and IC (25). Therefore, the inhibitory effects of BFA on membrane induction during KUN infection suggest that movement of cell or viral proteins from the RER to the Golgi apparatus is required for membrane induction, rather than just accumulation of viral proteins within the ER and IC. In our previous studies we showed that the KUN cytoplasmic foci labelled with antibodies to NS proteins or to dsRNA were also resistant to disruption by detergent and vinblastine sulfate (32, 48, 49). The only previous study of the effects of BFA on flavivirus replication in Vero cells focused primarily on transport of the structural glycoproteins and maturation of the virion (42).
Flaviviruses encode three glycoproteins within their genomes, prM, E, and NS1 (34). NS1 is a nonstructural glycoprotein containing a large amount of mannose and complex glycans and exists in its mature form as a dimer in infected cells (51). NS1 has been proposed to play a role early in yellow fever virus RNA replication (22), and we have observed that NS1 is associated with the putative template dsRNA in VP in both KUN (49)- and DEN2 (28)-infected cells. An association of GalT with NS1 in VP may be relevant to terminal glycosylation of NS1 by GalT and to possible targeting of NS1 to VP membranes. A central role for trans-Golgi membranes in flavivirus replication is also supported by their content of the cellular protein furin, which plays a role in the final maturation of the flavivirus virion (43).
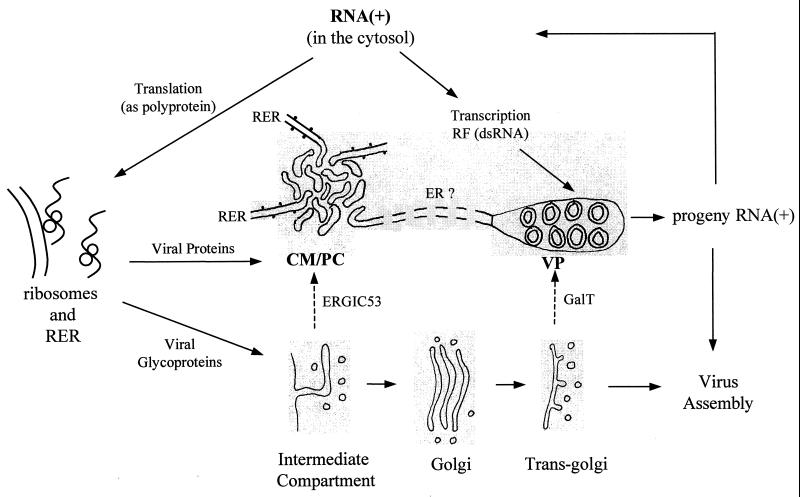
The present results show that infection of Vero cells with KUN leads to a dramatic rearrangement of intracellular membranes containing markers for the RER, the trans-Golgi region, and, to a lesser extent, the IC. The rearranged or induced structures in some cryosections appear to be physically connected and have defined roles in the flavivirus replication cycle. Recently, we suggested that KUN infection induces virus replication factories in which translation, proteolytic cleavage, RNA synthesis, and possibly virus assembly are all occurring within each defined area of largely induced membranes in order to ensure a more efficient process (49). Our present model of flavivirus replication (Fig. 9) proposes that late in infection the viral RNA genome is replicated asymmetrically and semiconservatively in a replicative intermediate by using a dsRNA template (replicative form) (5) in the VP, from where it is subsequently exported to the cytoplasm for translation, transcription into the replicative form, or virus assembly. Translation of the viral RNA occurs on the RER, where the glycoprotein products prM, E, and NS1 are translocated during synthesis into the ER lumen. Signal peptidase cleavages occur within the lumen, while the majority of the remaining cleavages in the nonstructural region are performed by the virus-encoded protease NS2B/NS3 (3, 4), possibly within the CM and PC (49), which appear to be IC derived. Individual NS proteins either remain within the CM and PC (NS2B, NS3, and NS4A), migrate to the nucleus (NS4B), or are further transported to VP (NS1, NS3, NS5, NS2A, and NS4A) for participation in continual replication of the RNA (29, 48, 49).
FIG. 9.
Proposed model of the flavivirus replication cycle, showing the involvement late in infection of the cytosol, cell organelles, and induced membranes identified as CM, which appear to be interconvertible to PC, and as VP. Movements of viral RNA and proteins are indicated by solid arrows, and movements of the cell membrane markers GalT and ERGIC53 to induced membranes are indicated by broken arrows. Replication occurs in the following sequence: positive-strand RNA [RNA(+)] is translated from one long open reading frame, and the polyprotein is cleaved by signal peptidases in the lumen or by the viral protease NS2B-NS3 in the induced CM and PC (49). The RNA polymerase NS5 plus NS1 and unknown cofactors copy specifically RNA(+) into the dsRNA template or replicative form (RF) (5), which enters the induced VP and assembles with NS1-NS2A-NS3-NS4A-NS5 to form the replication complex (29, 49). Progeny RNA(+) is released to repeat the cycle or for assembly into virus particles, which accumulate in vesicles of the adjoining ER.
A goal in future work will be to determine whether vesicular transport occurs within or between the virus-induced membranes and how the individual virus-encoded proteins are transported to discrete compartments. Complementary studies on the immunolocalization of KUN NS proteins in purified induced membrane fractions, and details of how these membranes are induced (presumably by nonstructural proteins interacting with specific membrane proteins), should present further avenues of investigation. We are now well placed to pursue such studies and thus further define the molecular and cellular events involved in flavivirus replication.
ACKNOWLEDGMENTS
We thank S. Fuller, H.-P. Hauri, E. Berger, A. Hille, M. Fukuda, and J.-Y. Lee for generously providing antibodies. We also thank R. Parton, J. Stow, and A. Khromykh for critical reading of the manuscript and for helpful discussions.
Funding for this work was provided by the National Health and Medical Research Council of Australia.
Footnotes
SASVRC publication 100.
REFERENCES
- 1.Bienz K, Egger D, Pasamontes L. Association of polioviral proteins of the P2 genomic region with the viral replication complex and virus-induced membrane synthesis as visualized by electron microscopic immunochemistry and autoradiography. Virology. 1987;160:220–226. doi: 10.1016/0042-6822(87)90063-8. [DOI] [PubMed] [Google Scholar]
- 2.Bienz K, Egger D, Troxler M, Pasamontes L. Structural organization of poliovirus RNA replication is mediated by viral proteins of the P2 genomic region. J Virol. 1990;64:1156–1163. doi: 10.1128/jvi.64.3.1156-1163.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cahour A, Falgout B, Lai C J. Cleavage of the dengue virus polyprotein at the NS3/NS4A and NS4B/NS5 junctions is mediated by viral protease NS2B/NS3, whereas NS4A/NS4B may be processed by a cellular protease. J Virol. 1992;66:1535–1542. doi: 10.1128/jvi.66.3.1535-1542.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chambers T J, Grakoui A, Rice C M. Processing of the yellow fever virus nonstructural polyprotein: a catalytically active NS3 proteinase domain and NS2B are required for cleavages at dibasic sites. J Virol. 1991;65:6042–6050. doi: 10.1128/jvi.65.11.6042-6050.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chu P W G, Westaway E G. Replication strategy of Kunjin virus: evidence for recycling role of replicative form RNA as a template in semiconservative and asymmetric replication. Virology. 1985;140:68–79. doi: 10.1016/0042-6822(85)90446-5. [DOI] [PubMed] [Google Scholar]
- 6.Chu P W G, Westaway E G. Molecular and ultrastructural analysis of heavy membrane fractions associated with the replication of Kunjin virus RNA. Arch Virol. 1992;125:177–191. doi: 10.1007/BF01309636. [DOI] [PubMed] [Google Scholar]
- 7.Coia G, Parker M D, Speight G, Byrne M E, Westaway E G. Nucleotide and complete amino acid sequences of Kunjin virus: definitive gene order and characteristics of the virus-specified proteins. J Gen Virol. 1988;69:1–21. doi: 10.1099/0022-1317-69-1-1. [DOI] [PubMed] [Google Scholar]
- 8.Doedens J R, Kirkegaard K. Inhibition of cellular protein secretion by poliovirus proteins 2B and 3A. EMBO J. 1995;14:894–907. doi: 10.1002/j.1460-2075.1995.tb07071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doedens J R, Giddings T H, Jr, Kirkegaard K. Inhibition of endoplasmic reticulum-to-Golgi traffic by poliovirus protein 3A: genetic and ultrastructural analysis. J Virol. 1997;71:9054–9064. doi: 10.1128/jvi.71.12.9054-9064.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Froshauer S, Kartenbeck J, Helenius A. Alphavirus RNA replicase is located on the cytosolic surface of endosomes and lysosomes. J Cell Biol. 1988;107:2075–2086. doi: 10.1083/jcb.107.6.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fukuda M, Viitala J, Matteson J, Carlsson S R. Cloning of cDNAs encoding human lysosomal membrane glycoproteins, h-lamp-1 and h-lamp-2. Comparison of their deduced amino acid sequences. J Biol Chem. 1988;263:18920–18928. [PubMed] [Google Scholar]
- 12.Geuze H J, Slot J W, van der Ley P A, Scheffer R C T. Use of colloidal gold particles in double-labeling immunoelectron microscopy of ultrathin frozen tissue sections. J Cell Biol. 1981;89:653–665. doi: 10.1083/jcb.89.3.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grimley P M, Berezesky I K, Friedman R. Cytoplasmic structures associated with an arbovirus infection: loci of viral ribonucleic acid synthesis. J Virol. 1968;2:1326–1338. doi: 10.1128/jvi.2.11.1326-1338.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grun J B, Brinton M A. Separation of functional West Nile virus replication complexes from intracellular membrane fragments. J Gen Virol. 1988;69:3121–3127. doi: 10.1099/0022-1317-69-12-3121. [DOI] [PubMed] [Google Scholar]
- 15.Hobman T C, Woodward L, Farquhar M G. The rubella virus E1 glycoprotein is arrested in a novel post-ER, pre-golgi compartment. J Cell Biol. 1992;118:795–811. doi: 10.1083/jcb.118.4.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hobman T C, Zhao B, Chan H, Farquhar M G. Immunoisolation and characterization of a subdomain of the endoplasmic reticulum that concentrates proteins involved in COPII vesicle biogenesis. Mol Biol Cell. 1998;9:1265–1278. doi: 10.1091/mbc.9.6.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khromykh A A, Westaway E G. Subgenomic replicons of the flavivirus Kunjin: construction and applications. J Virol. 1997;71:1497–1505. doi: 10.1128/jvi.71.2.1497-1505.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koonin E V. Computer-assisted identification of a putative methyltransferase domain in NS5 protein of flaviviruses and lambda 2 protein of reovirus. J Gen Virol. 1993;74:733–740. doi: 10.1099/0022-1317-74-4-733. [DOI] [PubMed] [Google Scholar]
- 19.Krijnse Locker J, Ericsson M, Rottier P J, Griffiths G. Characterization of the budding compartment of mouse hepatitis virus: evidence that transport from the RER to the golgi complex requires only one vesicular transport step. J Cell Biol. 1994;124:55–70. doi: 10.1083/jcb.124.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leary K, Blair C D. Sequential events in the morphogenesis of Japanese encephalitis virus. J Ultrastruct Res. 1980;72:123–129. doi: 10.1016/s0022-5320(80)90050-7. [DOI] [PubMed] [Google Scholar]
- 21.Lee J-Y, Marshall J A, Bowden D S. Characterization of rubella virus replication complexes using antibodies to double-stranded RNA. Virology. 1994;200:307–312. doi: 10.1006/viro.1994.1192. [DOI] [PubMed] [Google Scholar]
- 22.Lindenbach B D, Rice C M. trans-complementation of yellow fever virus NS1 reveals a role in early RNA replication. J Virol. 1997;71:9608–9617. doi: 10.1128/jvi.71.12.9608-9617.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Linstedt A D, Foguet M, Renz M, Seelig H P, Glick B S, Hauri H-P. A C-terminally-anchored golgi protein is inserted into the endoplasmic reticulum and then transported to the golgi apparatus. Proc Natl Acad Sci USA. 1995;92:5102–5105. doi: 10.1073/pnas.92.11.5102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lippincott-Schwartz J, Yuan L C, Bonifacino J S, Klausner R D. Rapid redistribution of golgi proteins into the ER in cells treated with brefeldin A: evidence for membrane recycling from the golgi to ER. Cell. 1989;56:801–813. doi: 10.1016/0092-8674(89)90685-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lippincott-Schwartz J, Donaldson J G, Schweizer A, Berger E G, Hauri H-P, Yuan L C, Klausner R D. Microtubule-dependent retrograde transport of proteins into the ER in the presence of brefeldin A suggests an ER recycling pathway. Cell. 1990;60:821–836. doi: 10.1016/0092-8674(90)90096-w. [DOI] [PubMed] [Google Scholar]
- 26.Lotti L V, Torrisi M-R, Pascale M C, Bonatti S. Immunocytochemical analysis of the transfer of vesicular stomatitis virus G glycoprotein from the intermediate compartment to the golgi complex. J Cell Biol. 1992;118:43–50. doi: 10.1083/jcb.118.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mackenzie J M, Jones M K, Young P R. Improved membrane preservation of flavivirus-infected cells with cryosectioning. J Virol Methods. 1996;56:67–75. doi: 10.1016/0166-0934(95)01916-2. [DOI] [PubMed] [Google Scholar]
- 28.Mackenzie J M, Jones M K, Young P R. Immunolocalization of the dengue virus nonstructural glycoprotein NS1 suggests a role in viral RNA replication. Virology. 1996;220:232–240. doi: 10.1006/viro.1996.0307. [DOI] [PubMed] [Google Scholar]
- 29.Mackenzie J M, Khromykh A A, Jones M K, Westaway E G. Subcellular localization and some biochemical properties of the flavivirus Kunjin nonstructural proteins NS2A and NS4A. Virology. 1998;245:203–215. doi: 10.1006/viro.1998.9156. [DOI] [PubMed] [Google Scholar]
- 30.Magliano D, Marshall J A, Bowden D S, Vardaxis N, Meanger J, Lee J-Y. Rubella virus replication complexes are virus-modified lysosomes. Virology. 1998;240:57–63. doi: 10.1006/viro.1997.8906. [DOI] [PubMed] [Google Scholar]
- 31.Murphy F A. Morphology and morphogenesis. In: Monath T, editor. St. Louis encephalitis. Washington, D.C: American Public Health Association; 1980. pp. 65–103. [Google Scholar]
- 32.Ng M L, Pedersen J S, Toh B H, Westaway E G. Immunofluorescent sites in Vero cells infected with the flavivirus Kunjin. Arch Virol. 1983;78:177–190. doi: 10.1007/BF01311313. [DOI] [PubMed] [Google Scholar]
- 33.Pelham H R B. Evidence that luminal ER proteins are sorted from secreted proteins in a post-ER compartment. EMBO J. 1988;7:913–918. doi: 10.1002/j.1460-2075.1988.tb02896.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rice C M, Lenches E M, Eddy S R, Shin S J, Sheets R L, Strauss J H. Nucleotide sequence of yellow fever virus: implications for flavivirus gene expression and evolution. Science. 1985;229:726–733. doi: 10.1126/science.4023707. [DOI] [PubMed] [Google Scholar]
- 35.Schlegel A, Giddings T H, Jr, Ladinsky M S, Kirkegaard K. Cellular origin and ultrastructure of membranes induced during poliovirus infection. J Virol. 1996;70:6576–6588. doi: 10.1128/jvi.70.10.6576-6588.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schweizer A, Fransen J A M, Bachi T, Ginsel L, Hauri H-P. Identification, by monoclonal antibody, of a 53-kD protein associated with a tubulo-vesicular compartment at the cis-side of the golgi apparatus. J Cell Biol. 1988;107:1643–1653. doi: 10.1083/jcb.107.5.1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schweizer A, Matter K, Ketcham C M, Hauri H P. The isolated ER-golgi intermediate compartment exhibits properties that are different from ER and cis-golgi. J Cell Biol. 1991;113:45–54. doi: 10.1083/jcb.113.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Slot J W, Geuze H J, Gigengack S, Lienhard G E, James D E. Immuno-localization of the insulin regulatable glucose transporter in brown adipose tissue of the rat. J Cell Biol. 1991;113:123–135. doi: 10.1083/jcb.113.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Speight G, Westaway E G. Positive identification of NS4A, the last of the hypothetical nonstructural proteins of flaviviruses. Virology. 1989;170:299–301. doi: 10.1016/0042-6822(89)90383-8. [DOI] [PubMed] [Google Scholar]
- 40.Speight G, Westaway E G. Carboxy-terminal analysis of nine proteins specified by the flavivirus Kunjin: evidence that only the intracellular core protein is truncated. J Gen Virol. 1989;70:2209–2214. doi: 10.1099/0022-1317-70-8-2209. [DOI] [PubMed] [Google Scholar]
- 41.Speight G, Coia G, Parker M D, Westaway E G. Gene mapping and positive identification of the nonstructural proteins NS2A, NS2B, NS3, NS4B and NS5 of the flavivirus Kunjin and their cleavage sites. J Gen Virol. 1988;69:23–34. doi: 10.1099/0022-1317-69-1-23. [DOI] [PubMed] [Google Scholar]
- 42.Sreenivasan V, Ng K L, Ng M L. Brefeldin A affects West Nile virus replication in Vero cells but not C6/36 cells. J Virol Methods. 1993;45:1–17. doi: 10.1016/0166-0934(93)90135-e. [DOI] [PubMed] [Google Scholar]
- 43.Stadler K, Allison S L, Schalich J, Heinz F X. Proteolytic activation of tick-borne encephalitis virus by furin. J Virol. 1997;71:8475–8481. doi: 10.1128/jvi.71.11.8475-8481.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stous G J, Berger E G, van Kerkhof P, Bosshart H, Berger B, Geuze H J. Brefeldin A induces a microtubule-dependent fusion of galactosyltransferase-containing vesicles with the rough endoplasmic reticulum. Biol Cell. 1991;71:25–31. doi: 10.1016/0248-4900(91)90048-r. [DOI] [PubMed] [Google Scholar]
- 45.Vaux D, Tooze J, Fuller S. Identification by anti-idiotype antibodies of an intracellular membrane protein that recognizes a mammalian endoplasmic reticulum retention signal. Nature. 1990;345:495–502. doi: 10.1038/345495a0. [DOI] [PubMed] [Google Scholar]
- 46.Watzele G, Bachofner R, Berger E G. Immunocytochemical localization of the golgi apparatus using protein-specific antibodies to galactosyltransferases. Eur J Cell Biol. 1991;56:451–458. [PubMed] [Google Scholar]
- 47.Westaway E G. Proteins specified by group B togaviruses in mammalian cells during productive infections. Virology. 1973;51:454–465. doi: 10.1016/0042-6822(73)90444-3. [DOI] [PubMed] [Google Scholar]
- 48.Westaway E G, Khromykh A A, Kenney M T, Mackenzie J M, Jones M K. Proteins C and NS4B of the flavivirus Kunjin translocate independently into the nucleus. Virology. 1997;234:31–41. doi: 10.1006/viro.1997.8629. [DOI] [PubMed] [Google Scholar]
- 49.Westaway E G, Mackenzie J M, Kenney M T, Jones M K, Khromykh A A. Ultrastructure of Kunjin virus-infected cells: co-localization of NS1 and NS3 with double-stranded RNA, and of NS2B with NS3, in virus-induced membrane structures. J Virol. 1997;71:6650–6661. doi: 10.1128/jvi.71.9.6650-6661.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Westaway E G, Khromykh A A, Mackenzie J M. Nascent flavivirus RNA colocalized in situ with double-stranded RNA in stable replication complexes. Virology. 1999;258:108–117. doi: 10.1006/viro.1999.9683. [DOI] [PubMed] [Google Scholar]
- 51.Winkler G, Randolph V B, Cleaves G R, Ryan T E, Stollar V. Evidence that the mature form of the flavivirus nonstructural protein NS1 is a dimer. Virology. 1988;162:187–196. doi: 10.1016/0042-6822(88)90408-4. [DOI] [PubMed] [Google Scholar]