Abstract
Background
Chronic kidney disease–associated pruritus (CKD-ap) is a common complication that negatively affects the quality of life. Difelikefalin has emerged as a novel FDA-approved drug to manage CKD-ap. This systematic review and meta-analysis will assess the efficacy and safety of Difelikefalin versus placebo to manage CKD-ap.
Methods
PubMed, Scopus, WOS, Central, and Embase were systematically searched until November 2023. RevMan was used to perform meta-analysis. Quality assessment was conducted using the Cochrane RoB 2.0 tool. Results were reported as risk ratio (RR) and mean difference (MD) with a 95% confidence interval (CI). PROSPERO ID: (CRD42023485979).
Results
Five RCTs with a total of 896 participants were included. Difelikefalin significantly decreased the weekly mean WI-NRS score (MD: −0.99 [−1.22, −0.75], p ˂ .00001), 5-D itch scale total score (MD: −1.51 [−2.26, −0.76], p > .0001), and Skindex-10 total score (MD: −7.39 [−12.51, −2.28], p = .005), but showed significantly higher adverse events (RR: 1.26 [1.03, 1.55], p = .03), versus placebo. However, there was no significant difference between both groups in serious adverse events (RR: 1.42 [0.78, 2.57], p = .25) or death (RR: 0.81 [0.19, 3.34], p = .77).
Conclusion
Difelikefalin appears to be a promising agent for the management of CKD-induced pruritus in patients with end-stage renal disease. However, evidence is still underpowered due to the paucity of the current data; therefore, more robust RCTs are required to confirm the benefit of Difelikefalin.
Keywords: Difelikefalin, pruritus, hemodialysis, systematic review, meta-analysis
1. Introduction
End-stage renal disease (ESRD) affects about four million people worldwide, with the majority being treated with lifetime hemodialysis (HD) therapy [1]. The latter is likely to be increasingly used due to improvements in dialysis technology and patient access [1]. This may attract attention toward optimizing the quality of care in the ESRD population, notably preventing disease-related complications. Chronic kidney disease–associated pruritus (CKD-aP), previously known as uremic pruritus, is a common complication among patients with ESRD that can lead to severe quality-of-life alterations, anxiety, and depression [2].
Previous data from real-world observational studies have revealed that up to 80% of hemodialyzed subjects may experience CKD-aP, which results in moderate to severe itch in nearly 40% of them [3]. Additionally, in another analysis of two large retrospective studies, six out of every 10 dialysis-dependent patients reported some level of itching [4]. The international Dialysis Outcomes and Practice Patterns Study (DOPPS) study demonstrated that although the prevalence of CKD-aP is decreasing among HD patients, it remains largely underestimated. Thus, in this study, 18% of symptomatic patients did not receive treatment, while 17% did not declare their itching to health-care staff. Furthermore, the prevalence of pruritus was underestimated by most medical directors [5].
Difelikefalin (DFK) is a novel drug approved by the FDA in 2021 to manage moderate to severe pruritus among patients with CKD [6]. This was after the evidence from randomized controlled trials (RCTs), including a phase III RCT that supported the superiority of DFK compared to placebo in reducing itching symptoms [7,8]. DFK acts by selectively binding to κ-opioid receptors (KOR) that are located in the peripheral nervous system and immune cells, with negligible or absent action on the central nervous system (CNS). Consequently, it is believed that the anti-prurigenic effects of DFK may be related to neurological and inflammatory modulation of itch-triggering pathways [9–11]. With the emergence of recent trials on DFK for CKD-aP in the setting of HD [12–14], there is a need for comprehensive evaluations to examine the available evidence of the drug’s efficacy and safety profile. Therefore, we conducted this systematic review and meta-analysis to evaluate the safety and efficacy of DFK to manage CKD-ap.
2. Methodology
2.1. Protocol registration
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement guidelines [15] and the Cochrane Handbook of Systematic Reviews and Meta-analysis [16]. The protocol was registered on PROSPERO with ID: (CRD42023485979).
2.2. Search strategy
A systematic literature search was conducted on five databases (PubMed, Web of Science, Central, Scopus, and Embase) until November 2023. The following search terms were used: (1) Difelikefalin OR Korsuva Combined with (2) ‘Renal Dialyses’ OR ‘Renal Dialysis’ OR Hemodialysis OR Hemodialyses OR ‘Extracorporeal Dialyses’ OR ‘Extracorporeal Dialysis’ OR ‘Chronic Renal Insufficiencies’ OR ‘Chronic Renal Insufficiency’ OR ‘Chronic Kidney Insufficiency’ OR ‘Chronic Kidney Insufficiencies’ OR ‘Chronic Kidney Diseases’ OR ‘Chronic Kidney Disease’ OR ‘Chronic Renal Diseases’ OR ‘Chronic Renal Disease’), only English-written studies were considered with no other Search restriction. The final search strategy and terms are detailed in Table S1.
2.3. Eligibility criteria
A PICOS (Population, Intervention, Comparator, Outcome, and Study design) approach was used to construct the eligibility criteria: (P), hemodialysis patients with pruritis; (I), DFK (0.5 μg/kg, selected upon previous phase II RCTs); (C), placebo; (O), our primary outcomes included mean change from baseline in the weekly WI-NRS score (Worst Itch Intensity Numerical Rating Scale, it is a validated 11-point scale with scores ranging from 0 to 10, with higher scores indicating greater itch intensity), mean change from baseline in 5-D itch scale total score (The 5-D itch scale score assesses five domains of itch and their impact (duration, degree, direction, disability, and distribution). Scores on the 5-D itch scale range from 5 to 25, with higher scores indicating worse itch-related QOL), and mean change from baseline in the Skindex-10 scale total score (assesses 3 domains related to itch (disease, mood/emotional distress, and social functioning), scores range from 0 to 60, with higher scores indicating worse itch-related QoL). Secondary outcomes included ≥3-point improvement from baseline in weekly mean WI-NRS score, ≥4-point improvement from baseline in weekly mean WI-NRS score, Patient Global Impression of Change (PGIC) (assessed according to the patient’s overall impression of changes in itch in comparison to their impression during the run-in period), and adverse events; and (S), (RCTs).
We excluded uncontrolled trials, observational studies, case reports, animal experimental studies, protocols, conference abstracts, reviews, and studies published in any language other than English.
2.4. Study selection
Covidence online software was used for the screening process. After the duplicates were removed manually and automatically by Covidence, four reviewers (R.R, M.K., M.H., and A.A.) independently screened titles and abstracts, then they reviewed the full-text articles according to the predefined inclusion and exclusion criteria to reach the final included studies. Any conflicts were solved by A.S. in discussion with M.A. after reviewing the full text.
2.5. Data extraction
Four reviewers (R.R, M.K., M.H., and A.A.) independently extracted data using a pre-designed extraction sheet as follows: summary characteristics (protocol registration (NCT), study design/phase, blinding Status, country, total participants, DFK (dose, route and frequency), treatment duration, main inclusion criteria, primary outcome, and follow-up duration); baseline characteristics (number of patients in each group, age, gender (male), dry weight, time since diagnosis of ESRD, time since initiation of dialysis, duration of pruritus, cause of chronic kidney disease, baseline use of anti-pruritic medication, baseline WI-NRS score, baseline 5-D itch scale total score, baseline Skindex-10 scale total score); efficacy data (mean change from baseline in the weekly WI-NRS score, mean change from baseline in 5-D itch scale total score, mean change from baseline in Skindex-10 scale total score, ≥3 points improvement from baseline in weekly mean WI-NRS score, ≥4 points improvement from baseline in weekly mean WI-NRS score, and PGIC); and safety data (any adverse events, any serious adverse events, adverse events leading to discontinuation, death, dizziness, somnolence, nausea, vomiting, diarrhea, and constipation).
2.6. Risk of bias and certainty of evidence
Four reviewers (R.R, M.K., M.H., and A.A.) independently evaluated the quality of the included studies using the Cochrane risk of bias 2.0 tool, which assessed five domains as follows: randomization process, deviations from intended interventions, missing outcome data, outcome measurements, and selection of the reported results. Each domain had a rating of ‘low risk’, ‘high risk’, or ‘some concerns’. Reasons for each rating were recorded. A discrepancy check was performed to compare the reviewers’ assessments. Any conflicts were resolved by A.S. and M.A., who made the final judgment after reviewing the full text.
To appraise the certainty of evidence, we utilized the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) guidelines [17,18]. We considered inconsistency, imprecision, indirectness, publication bias, and risk of bias. The evaluation was carried out for each outcome, and the decisions made were justified and documented.
2.7. Data analysis
Cochrane Review Manager software (Rev Man) v5.4 was used to perform this meta-analysis. A fixed-effects model was applied through pooled analysis unless there was heterogeneity, where a random-effects model was applied. Dichotomous outcomes were reported as risk ratio (RR), while mean difference (MD) was used to report continuous outcomes, all with a 95% confidence interval (CI). Heterogeneity was detected by the Chi-square test with an alpha level below 0.1 or by the I-square test exceeding 50%. In this case, sensitivity analysis was conducted repetitively by omitting one study at a time to identify the origin of the heterogeneity.
3. Results
3.1. Study results and study selection
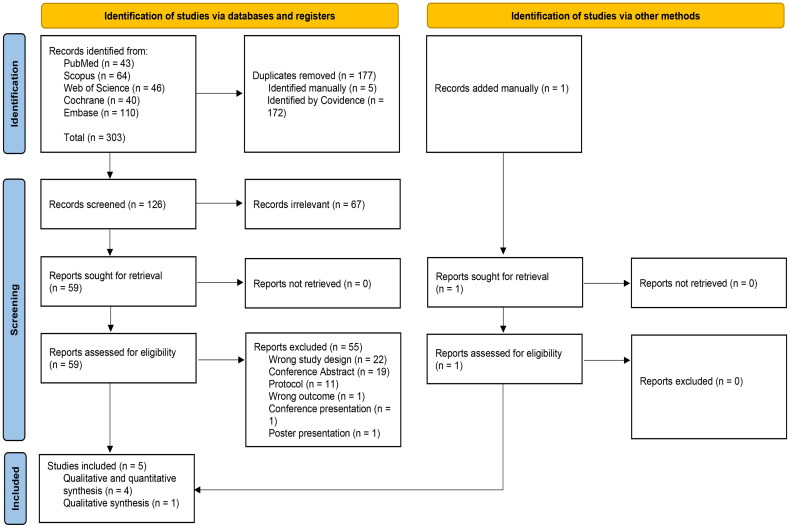
A total of 303 studies were imported while searching databases. After removing 177 duplicates, one hundred and twenty-six studies underwent title and abstract screening. The resulting 59 studies proceeded with full-text screening for eligibility. After excluding 55 irrelevant articles, five RCTs were included for qualitative analysis, with only four RCTs included in the quantitative analysis. Finally, we manually included Narita et al. [13] from the New England Journal of Medicine Evidence (NEJM Evidence), which is a novel journal (Figure 1).
Figure 1.
PRISMA flow chart of the screening process.
3.2. Characteristics of included studies
Five RCTs [7,8,12–14] with a total of 896 participants were included in the qualitative analysis: 445 in the DFK group and 451 in the placebo group. Four RCTs [7,8,13,14] with a total of 763 participants were included in the meta-analysis, excluding only Yosipovitch et al. [12], due to different routes of administration of DFK (oral) and different doses. All studies were multicentric; three were conducted in the United States and two in Japan. Most participants were males (65.6%). The age distribution appeared similar across studies, with mean age (year) ranging from 57 to 69 in the DFK group and from 56.8 to 65.6 in the placebo group. The summary and baseline characteristics are illustrated in more detail in Tables 1 and 2, respectively.
Table 1.
Summary characteristics of the included RCTs.
| Study ID | Study design/phase | Blinding status | Country | Total number of participants | DFK |
Duration of treatment | Main inclusion criteria | Primary outcome | Follow-up duration | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Dose | Route | Frequency | |||||||||
| Fishbane et al. [8] | Multicenter RCT/phase 3 | Double-blinded | United States | 378 | 0.5 μg/kg | Intravenous | Three times per week for 12 weeks | 12 Weeks | Eligible patients were adults (≥18 years of age) with ESKD who had been undergoing hemodialysis at least three times per week for at least 3 months and who had moderate-to-severe pruritus. | The percentage of patients who had an improvement (decrease) of at least 3 points from baseline at week 12 in the weekly mean score on the daily WI-NRS. | 14 Weeks |
| Fishbane et al. [7] | Multicenter RCT/phase 2 | Double-blinded | United States | 89 | 0.5 μg/kg | Intravenous | Thrice weekly after each hemodialysis session for 8 weeks | 8 Weeks | Male or female adults ≥18 years; patients with ESKD who have been on hemodialysis 3 times per week for at least 3 months before screening; persistent pruritus during the month before screening, with weekly mean WI-NRS score over the 7 days before randomization >4 | Change from baseline at week 8 in the weekly mean of the 24-h daily WI-NRS score. | 8 Weeks |
| Narita et al. [14] | Multicenter RCT/Phase 2 | Double-blinded | Japan | 124 | 0.5 μg/kg | Intravenous | Three times a week at the end of each hemodialysis session for 8 weeks | 8 Weeks | Japanese male or female patients with ESKD and moderate to severe pruritus who were 20 years or older and undergoing maintenance hemodialysis 3 times a week for at least 12 weeks were enrolled in this trial. | Change from baseline in the weekly mean WI-NRS score at week 8. | 8 Weeks |
| Narita et al. [13] | Multicenter RCT/Phase 3 | Double-blinded | Japan | 178 | 0.5 μg/kg | Intravenous | Three times per week intravenously for 6 weeks | 6 Weeks | Japanese patients with ESKD of either sex with moderate to severe pruritus, 20 years of age who were undergoing maintenance hemodialysis three times a week for 12 weeks were enrolled in this trial. | Change from baseline in the WI-NRS score at week 4. | 4 Weeks |
| Yosipovitch et al. [12] | Multicenter RCT/phase 2 | Double-blinded | United States | 133 | 0.5 mg | Oral | Once daily for 12 weeks | 12 Weeks | The study enrolled adults (≥18 years) with CKD. Subjects who were not receiving HD had moderate renal impairment (stage 3 NDD-CKD) or severe renal impairment (stage 4–5 NDD-CKD). Subjects undergoing HD were receiving HD 3 times per week for ≥3 months before screening. had moderate-to-severe pruritus. |
Change from baseline in the weekly mean of the daily WI-NRS scores at week 12. | 12 Weeks |
Note: RCT: randomized controlled trial; ESKD: end stage kidney disease; WI-NRS: Worst Itch Intensity Numerical Rating Scale; CKD: chronic kidney disease; HD: hemodialysis; NDD; non-dialysis dependent.
Table 2.
Baseline characteristics of the participants.
| Study ID | Number of participants in each group |
Age (years), mean (SD) |
Gender (male), N. (%) |
Dry weight (kg), mean (SD) |
Time since diagnosis of ESKD (years), mean (SD) |
Time since initiation of dialysis (years), mean (SD) |
Duration of pruritus (years), mean (SD) |
Cause of chronic kidney disease no. (%) |
Baseline use of anti-pruritic medication, N. (%) |
Baseline scores, mean (SD) |
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diabetes |
Hypertension |
Glomerulonephritis |
Cystic disease |
Other |
WI-NRs score |
5-D itch scale total score |
Skindex-10 scale total score |
|||||||||||||||||||||||||
| DFK | Placebo | DFK | Placebo | DFK | Placebo | DFK | Placebo | DFK | Placebo | DFK | Placebo | DFK | Placebo | DFK | Placebo | DFK | Placebo | DFK | Placebo | DFK | Placebo | DFK | Placebo | DFK | Placebo | DFK | Placebo | DFK | Placebo | DFK | Placebo | |
| Fishbane et al. [8] | 189 | 188 | 58.2 (11.2) | 56.8 (13.9) | 112 (59.3) | 118 (62.8) | 85.9 (20.3) | 85.0 (21.1) | 4.7 (3.9) | 5.7 (5.2) | 4.4 (4) | 4.7 (4.2) | 3.2 (3.2) | 3.5 (3.4) | 108 (57.1) | 95 (50.5) | 59 (31.2) | 73 (38.8) | 9 (4.8) | 7 (3.7) | 3 (1.6) | 3 (1.6) | 10 (5.3) | 10 (5.3) | 72 (38.1) | 78 (41.5) | 7.1 (1.4) | 7.3 (1.6) | 16.9 (3.5) | 17.9 (3.5) | 36.2 (14.4) | 38.3 (15.4) |
| Fishbane et al. [7] | 44 | 45 | 57 (12.75) | 60 (14.25) | 26 (59.1) | 28 (62.2) | 83.5 (20.9) | 81.0 (19.8) | 5.9 (4.9) | 6.6 (5.4) | 5.4 (4.9) | 5.9 (4.9) | 4.7 (3.9) | 4.4 (4.7) | 24 (54.5) | 21 (46.7) | 21 (47.7) | 21 (46.7) | 6 (13.6) | 5 (11.1) | 2 (4.5) | 0 | 2 (4.5) | 1 (2.2) | 20 (45.5) | 18 (40.0) | 7.1 (1.4) | 6.8 (1.5) | 17.3 (3.6) | 17.2 (3.1) | 35.1 (13.4) | 35.5 (12.4) |
| Narita et al. [14] | 61 | 63 | 65.6 (11.4) | 64.1 (12.7) | 45 (74) | 43 (68) | 59.98 (11.22) | 60.63 (12.71) | NA | NA | 6.7 (7.2) | 6.8 (6.1) | 4.5 (4.4) | 4.3 (4.4) | 32 (52.5) | 27 (42.9) | NA | NA | 11 (18) | 10 (15.9) | 0 | 3 (4.8) | 4 (6.6) | 8 (12.7) | NA | NA | 6.83 (1.4) | 6.53 (1.31) | NA | NA | NA | NA |
| Narita et al. [13] | 85 | 88 | 64.4 (10.5) | 64.1 (12.7) | 74 (87) | 72 (82) | 62.64 (11.63) | 63.33 (12.94) | NA | NA | 8.4 (7.6) | 7.9 (6.7) | 5.7 (5.5) | 4.6 (3.8) | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | 6.57 (1.29) | 6.40 (1.28) | 15.7 (2.8) | 15.4 (3.0) | NA | NA |
| Yosipovitch et al. [12] | 66 | 67 | 69.0 (12.0) | 65.6 (12.1) | 33 (50.0) | 37 (55.2) | 84.0 (18.3) | 87.8 (18.7) | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | 24 (36.4) | 28 (41.8) | 7.0 (1.2) | 7.0 (1.1) | 16.2 (3.1) | 16.8 (3.1) | 33.1 (14.3) | 34.9 (14.0) |
Note: NA: not available; SD: standard deviation; ESKD: end stage kidney disease; WI-NRS: Worst Itch Intensity Numerical Rating Scale.
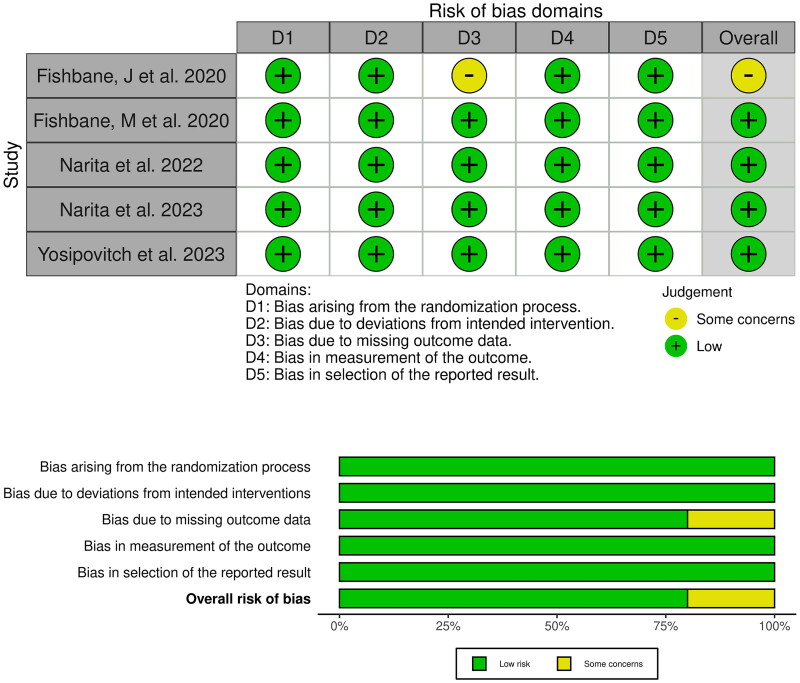
3.3. Risk of bias and certainty of evidence
Four RCTs [7,12–14] showed an overall low risk of bias, and only 1 RCT [8] showed an overall some concerns. The risk of bias is illustrated in more detail in Figure 2. Certainty of evidence is demonstrated in detail in a GRADE evidence profile (Table 3).
Figure 2.
Quality assessment of risk of bias in the included trials. The upper panel presents a schematic representation of risks (low = red, unclear = yellow, and high = red) for specific types of biases of each of the studies in the review. The lower panel presents risks (low = red, unclear = yellow, and high = red) for the subtypes of biases of the combination of studies included in this review.
Table 3.
GRADE evidence profile.
| Certainty assessment |
Summary of findings |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Participants (studies) follow-up | Risk of bias | Inconsistency | Indirectness | Imprecision | Publication bias | Overall certainty of evidence | Study event rates (%) |
Relative effect (95% CI) | Anticipated absolute effects |
||
| With [placebo] | With [difelikefalin] | Risk with [placebo] | Risk difference with [difelikefalin] | ||||||||
| Mean change from baseline in the weekly NRS score (week 4) | |||||||||||
| 752 (4 RCTs) | Not serious | Not serious | Not serious | Not serious | None | ⊕⊕⊕⊕High | 381 | 371 | – | – | MD 0.98 lower (1.28 lower to 0.68 lower) |
| Mean change from baseline in the weekly NRS score (week 8) | |||||||||||
| 578 (3 RCTs) | Not serious | Not serious | Not serious | Not serious | None | ⊕⊕⊕⊕ High | 292 | 286 | – | – | MD 1 lower (1.39 lower to 0.61 lower) |
| Mean change from baseline in 5-D itch scale total score | |||||||||||
| 749 (4 RCTs) | Not serious | Seriousa | Not serious | Seriousb | None | ⊕⊕◯◯ Low | 379 | 370 | – | – | MD 1.51 lower (2.26 lower to 0.76 lower) |
| Mean change from baseline in Skindex-10 scale total score | |||||||||||
| 467 (2 RCTs) | Not serious | Seriousa | Not serious | Seriousb | None | ⊕⊕◯◯ Low | 234 | 233 | – | – | MD 7.39 lower (12.51 lower to 2.28 lower) |
| ≥3 point improvement from baseline in weekly mean NRS score | |||||||||||
| 602 (3 RCTs) | Not serious | Seriousa | Not serious | Very seriousb | None | ⊕◯◯◯ Very low | 90/309 (29.1%) | 139/293 (47.4%) | RR 1.61 (1.13 to 2.31) | 291 per 1000 | 178 more per 1000 (from 38 more to 382 more) |
| ≥4 point improvement from baseline in weekly mean NRS score | |||||||||||
| 602 (3 RCTs) | Not serious | Seriousa | Not Serious | Very seriousb | None | ⊕◯◯◯ Very low | 60/309 (19.4%) | 109/293 (37.2%) | RR 1.94 (1.21 to 3.11) | 194 per 1000 | 183 more per 1000 (from 41 more to 410 more) |
| Patient global impression of change (PGIC) | |||||||||||
| 381 (3 RCTs) | Not serious | Not serious | Not serious | Seriousc | None | ⊕⊕⊕◯ Moderate | 62/193 (32.1%) | 108/188 (57.4%) | RR 1.79 (1.42 to 2.27) | 321 per 1000 | 254 more per 1000 (from 135 more to 408 more) |
| Adverse events - any adverse events | |||||||||||
| 768 (4 RCTs) | Not serious | Seriousa | Not serious | Seriousb | None | ⊕⊕◯◯ Low | 220/385 (57.1%) | 264/383 (68.9%) | RR 1.26 (1.03 to 1.55) | 571 per 1000 | 149 more per 1000 (from 17 more to 314 more) |
| Adverse events - any serious adverse events | |||||||||||
| 768 (4 RCTs) | Not serious | Not serious | Not serious | Very seriousb | None | ⊕⊕◯◯ Low | 57/385 (14.8%) | 74/383 (19.3%) | RR 1.42 (0.78 to 2.57) | 148 per 1000 | 62 more per 1000 (from 33 fewer to 232 more) |
| Adverse events - adverse events leading to discontinuation | |||||||||||
| 768 (4 RCTs) | Not serious | Not serious | Not serious | Very seriousb | None | ⊕⊕◯◯ Low | 15/385 (3.9%) | 28/383 (7.3%) | RR 1.80 (0.97 to 3.35) | 39 per 1000 | 31 more per 1000 (from 1 fewer to 92 more) |
| Adverse events - death | |||||||||||
| 768 (4 RCTs) | Not serious | Not serious | Not serious | Very seriousb | none | ⊕⊕◯◯ Low | 4/385 (1.0%) | 3/383 (0.8%) | RR 0.81 (0.19 to 3.34) | 10 per 1000 | 2 fewer per 1000 (from 8 fewer to 24 more) |
Note: CI: confidence interval; MD: mean difference; RR: risk ratio. Boldface is used to highlight the most important or statistically significant results/findings.
aI-square >50%.
bWide conference interval & the number of events is less than 300 events.
cThe number of events is less than 300 events.
3.4. Efficacy outcomes
3.4.1. WI-NRS score, 5-D itch scale total score, and SKindex-10 total score
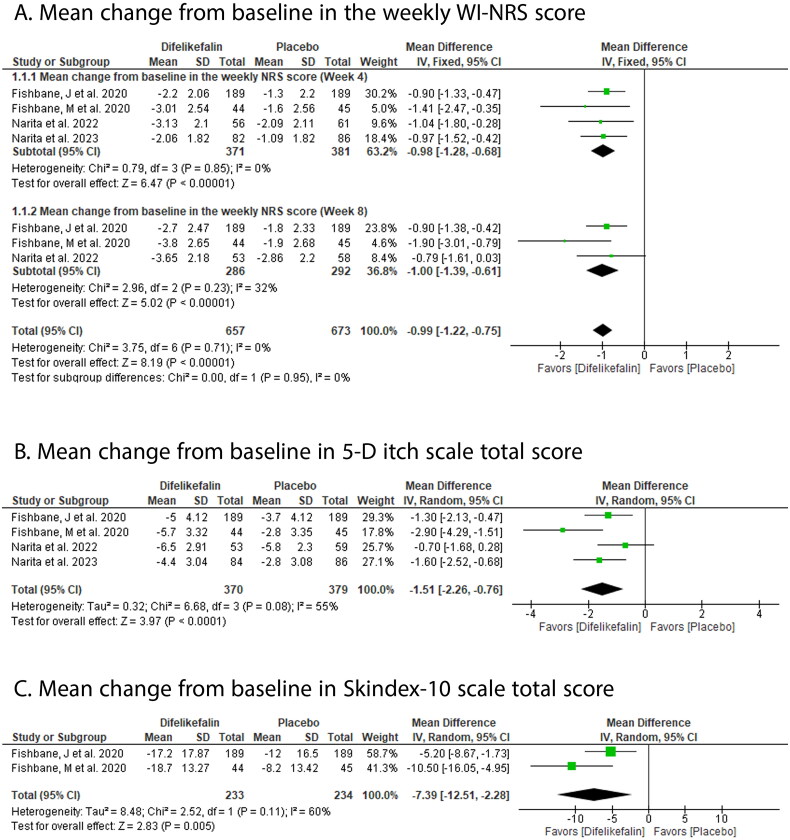
DFK significantly decreased the weekly mean WI-NRS score compared with placebo at week 4 (MD: −0.98 with 95% CI [−1.28, −0.68], p ˂ .00001) and week 8 (MD: −1.00 with 95% CI [−1.39, −0.61], p ˂ .00001) (Figure 3(A)); 5-D itch scale total score (MD: −1.51 with 95% CI [−2.26, −0.76], p > .0001) (Figure 3(B)); and Skindex-10 total score (MD: −7.39 with 95% CI [−12.51, −2.28], p = .005) (Figure 3(C)).
Figure 3.
Forest plot of the efficacy outcomes (A-mean change from baseline in the weekly WI-NRS score, B-mean change from baseline in 5-D itch scale total score, C-mean change from baseline in Skindex-10 scale total score). MD: mean difference; CI: confidence interval.
Pooled studies were homogenous in weekly mean WI-NRS score at week 4 (p = .85, I2 = 0%) and week 8 (p = .23, I2 =32%). However, they were heterogenous in 5-D itch scale total score (p = .08, I2 = 55%) and Skindex-10 total score (p = .11, I2 = 60%).
In Yosipovitch et al. oral DFK significantly decreased weekly mean WI-NRS score versus placebo at week 4 with 1.0 mg (p = .005), but it was not effective with 0.25 mg (p = .100) and 0.5 mg (p = .102). However, at week 8, oral DFK significantly reduced the weekly mean WI-NRS score with 0.25 mg (p = .045), 0.5 mg (p = .043), and 1.0 mg (p = .013). Finally, at week 12, oral DFK significantly reduced the weekly mean WI-NRS score with 1.0 mg (p = .018), but it was not effective with 0.25 mg (p = .146) and 0.5 mg (p = .269). However, there was no significant difference between oral DFK and placebo in 5-D itch scale score with 0.25 mg (p = .515), 0.5 mg (p = .099), and 1.0 mg (p = .070), or SKindex-10 total score with 0.25 mg (p = .580), 0.5 mg (p = .335), and 1.0 mg (p = .116).
3.4.2. WI-NRS score improvement and PGIG
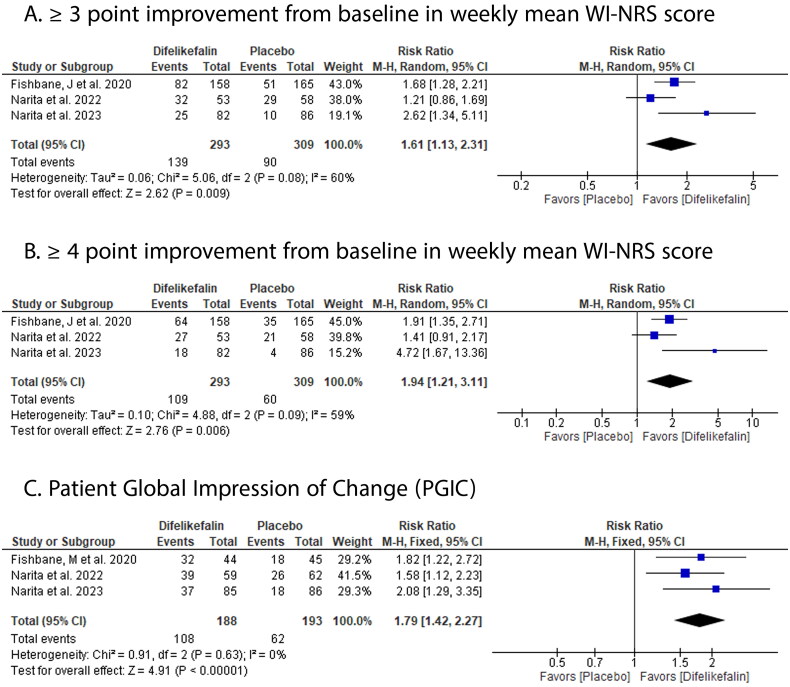
DFK showed significantly higher improvement in weekly mean WI-NRS score versus placebo by ≥ 3 points (RR: 1.61 with 95% CI [1.13, 2.31], p = .009) (Figure 4(A)) and ≥ 4 points (RR: 1.94 with 95% CI [1.21, 3.11], p = .006) (Figure 4(B)), and higher PGIG (RR: 1.79 with 95% CI [1.42, 2.27], p ˂ .00001) (Figure 4(C)).
Figure 4.
Forest plot of the efficacy outcomes (A- ≥3-point improvement from baseline in weekly mean WI-NRS score, B- ≥4-point improvement from baseline in weekly mean WI-NRS score, C- patient global impression of change). RR: risk ratio; CI: confidence interval.
Pooled studies were homogenous in PGIG (p = .63, I2 = 0%) but were heterogenous in improvement in weekly mean WI-NRS score by ≥ 3 points (p = .08, I2 = 60%) and ≥ 4 points (p = .09, I2 = 59%).
In Yosipovitch et al. oral DFK achieved improvement in weekly mean WI-NRS score by ≥ 3 points with 1.0 mg (72.1%) versus placebo (57.9%), and ≥ 4 points with 0.25 mg (55.3%), 0.5 mg (45%), and 1.0 mg (64.8%) versus placebo (49.8%). Also, DFK was significantly higher in PGIC versus placebo with 0.5 mg (p = .001) and 1.0 mg (p = .007), but there was no significant difference with 0.25 mg.
3.5. Safety outcomes
3.5.1. Adverse events
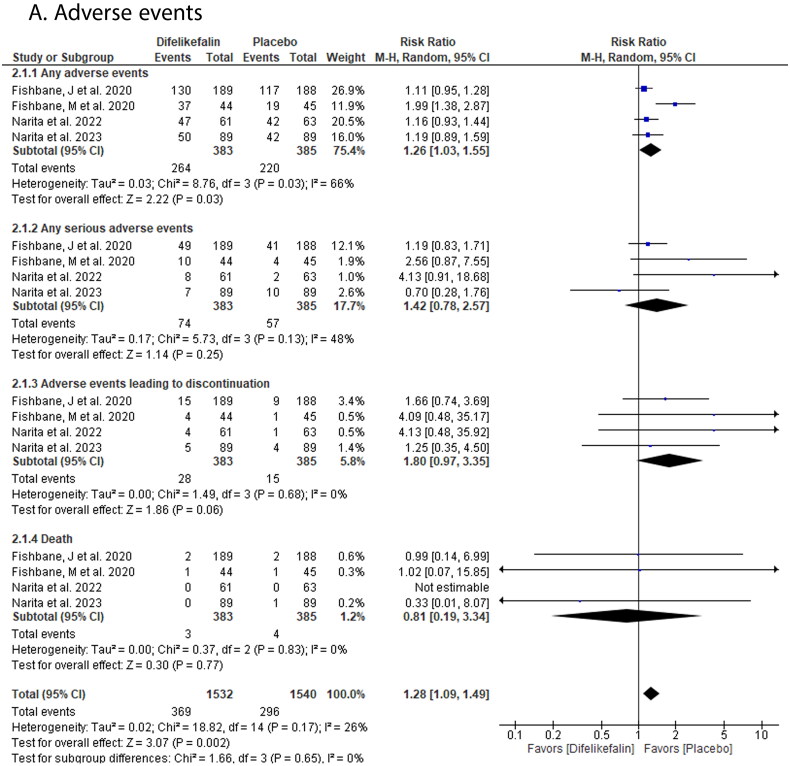
DFK showed a significantly higher incidence of any adverse events compared with placebo (RR: 1.26 with 95% CI [1.03, 1.55], p = .03). However, there was no significant difference between both groups in the incidence of serious adverse events (RR: 1.42 with 95% CI [0.78, 2.57], p = .25), adverse events leading to discontinuation (RR: 1.80 with 95% CI [0.97, 3.35], p = .06), and death (RR: 0.81 with 95% CI [0.19, 3.34], p = .77) (Figure 5).
Figure 5.
Forest plot of the safety outcomes (A- adverse events). RR: risk ratio; CI: confidence interval.
Pooled studies were homogenous in serious adverse events (p = .13, I2 = 48%), adverse events leading to discontinuation (p = .68, I2 = 0%), and death (p = .83, I2 = 0%). However, they were heterogenous in adverse events (p = .03, I2 = 66%).
In Yosipovitch et al. the incidence of adverse events with oral DFK was (50.7%) with 0.25 mg, (51.5%) with 0.5 mg, and (58.2%) with 1.0 mg versus (50.7%) with placebo. Also, serious adverse events occurred in (13%) with 0.25 mg, (13.6%) with 0.5 mg, and (13.4%) with 1.0 mg versus (7.5%) with placebo. There were adverse events leading to discontinuation of oral DFK with 0.25 mg (5.8%), 0.5 mg (7.6%), and 1.0 mg (11.9%) versus placebo (1.5%). Deaths occurred with 1.0 mg oral DFK by (1.5%) versus (4.5%) with placebo; however, no deaths were reported with 0.25 mg or 0.5 mg.
3.5.2. Specific adverse events
DFK showed a significantly higher incidence of dizziness (RR: 2.68 with 95% CI [1.23, 5.82], p = .01) and diarrhea (RR: 3.40 with 95% CI [1.54, 7.52], p = .003) compared with placebo. However, there was no significant difference between both groups in the incidence of somnolence (RR: 1.23 with 95% CI [0.38, 3.94], p = .73), nausea (RR: 4.45 with 95% CI [0.76, 25.87], p = .10), constipation (RR: 8.12 with 95% CI [1.03, 64.19], p = .05) and vomiting (RR: 1.32 with 95% CI [0.57, 3.03], p = .51) (Figure S1).
Pooled studies were homogenous in dizziness (p = .21, I2 = 33%), somnolence (p = .88, I2 = 0%), nausea (p = .22, I2 = 33%), vomiting (p = .40, I2 = 0%), diarrhea (p = .22, I2 = 34%), and constipation (p = .92, I2 = 0%).
In Yosipovitch et al. oral DFK caused dizziness with 0.5 mg (3%) and 1.0 mg (7.5%) versus no dizziness with 0.25 mg and placebo. Constipation occurred with oral DFK 0.25 mg (2.9%), 0.5 mg (3%), 1.0 mg (6%), and with placebo (3%). Also, diarrhea occurred in (2.9%) of oral DFK 0.25 mg, (4.5%) with 0.5 mg, and (6%) with 1.0 mg versus (1.5%) with placebo.
4. Discussion
In the present systematic review and meta-analysis, we showed that DFK has an overall good efficacy and safety profile among hemodialysis patients affected by CKD-aP. Thus, DFK treatment was significantly effective in inducing a reduction in all itching scores, with the most notable MD for the SKindex-10 total score. The latter was shown to be correlated with the severity of itching subjective experience as well as the mental and physical health-related QoL in hemodialyzed patients [19], which indicates that DFK anti-pruritic action can cause improvement in the QoL, a key element in the treatments’ objectives of ESRD. Fishbane et al. reported simultaneous improvement in sleep disturbances in the DFK-treated group, which would highly contribute to QoL positive changes [7]. On the other hand, the DFK-treated group was more likely to develop non-severe side effects, with dizziness and diarrhea being the drug-specific events.
Our results are consistent with those of the previous systematic review carried out by Wala et al. in which the authors concluded that DFK can effectively lead to significant amelioration in WI-NRS score, SKindex-10, and 5-D itch scale while being well tolerable by CKD patients [20]. Nonetheless, this 2022 study described the results of three trials only without providing a pooled analysis of the drug-related outcomes. In another 2022 study, Topf et al. evaluated the results of two pivotal, phase 3 open-label clinical trials (KALM-1 and KALM-2). They reported a rapid and sustainable reduction in different itch scales, notably the WI-NRS, Skindex-10 and, 5-D Itch scale with DFK therapy [21].
The pathogenesis of CKD-aP is complex and still not well understood. Multiple CKD-related homeostatic alterations are believed to contribute to the activations of pruritogenic pathways. These include metabolic changes such as hyperuricemia, calcium/phosphate imbalance, and anemia, systemic and skin inflammation with hyperactivation of mast cells and Th1 cells (oversecreting IL-2, IL-6, and TNF-α), skin xerosis and microangiopathy, peripheral and central neuropathy (μ-opioid overexpression and κ-opioid downregulation) as well as psychological anomalies [22,23]. The levels of KOR were shown to be lower in the skin of hemodialysis patients suffering from uremic pruritus [24]. However, the exact molecular way by which CKD interferes with the KOR system remains unknown and needs to be explored by future animal studies.
There are several mechanisms by which it is believed that KOR agonists interfere with itching pathways. One very likely mechanism is the interruption of itching signals in the peripheral afferent sensitive fibers (i.e., pruriceptive C-fibers containing itch‐sensory neurons), particularly at the dorsal root ganglion where KOR are condensely distributed [25]. Nonetheless, in a recent experiment, it was shown that DFK acts mainly on large-diameter, myelinated mechanoreceptors (e.g., Aβ-fibers) rather than small-diameter, unmyelinated pruriceptive C-fibers [11]. Based on these findings, the authors suggested an indirect anti-pruritic action of DFK via the mechanoreceptors-mediated modulation of ascending pruritic signals from these C-fibers. They also demonstrated that DFK suppresses itch and scratching behavior independently of inflammation in a murine model of atopic dermatitis [11]. However, it is hard to exclude the anti-inflammatory potential of DFK, especially since peripheral KOR signaling was shown to inhibit neurogenic inflammation, nociceptor sensitization by inflammatory mediators, and subsequently, pain and itch behaviors [26].
Moreover, in subjects treated with DFK, there was a correlation between itch intensity reduction and reductions in serum levels of inflammatory markers, including IFNγ, IL-2, and GM-CSF [27]. Also, it was previously established that CKD-aP may involve inflammatory triggers such as Th1-cells, CRP, and IL-6 [28]. This makes the anti-inflammatory action a typical mechanism of potentially effective drugs on CKD-aP, notably DFK. Importantly, KOR are less expressed in the skin of patients with CKD-aP [24], suggesting that KOR action deficiency in these patients may play a role in the pathogenesis of itching. In a recent experiment, Nguyen et al. revealed that medullary KOR neurons (in the rostral ventromedial medulla) inhibit pain and itch through a descending circuit [29]; however, it is unlikely that DFK displays such an anti-pruritic effect due to its uniquely hydrophilic nature which significantly limits its bioavailability in the central nervous system [30].
DFK has peripheral selectivity with restricted CNS penetration, which may explain the absence of psychiatric, sedative, and respiratory depressant side effects usually seen with KOR agonists or other opioid agents. This may also prevent drug dependency, which is a key advantage of DFK. A study of polydrug users has indicated that DFK presents a low potential for abuse [31]. Since DFK is deprived of central action, its side effects are likely due to peripheral mechanisms. KOR are present in the peripheral vestibular system. Notably, in a previous study, KOR agonism resulted in inhibitory, presynaptic input to hair cells within the axolotl vestibular afferent neurons in addition to inducing postsynaptic facilitation of the afferent response mediated by mu-opioid receptors (MOR) [32]. This may alter the adequate perception of balance with position changes or reduce vestibule reactivity, which patients may interpret as dizziness.
At the level of the GI tract of mice, KOR were identified in the myenteric and submucosal neurons, fibers in the muscle layer, blood vessels, and mucosa [33]. Opioid receptor agonists have multiple effects on the GI tract, including reduction of tonic/segmental contractions and impairment of peristalsis by inhibition of the release of acetylcholine and substance P, as well as decrease of GI secretion by inhibition of the activity of acetylcholine and VIP containing neurons [34]. In particular, KOR agonists were reported to inhibit smooth muscle contraction in human and animal enteric tissue [35] and modulate GI motility [36,37], which would lead to constipation among patients receiving DFK therapy. Nevertheless, with the complexity, subtypes variety, and possible non-canonical function of the enteric opioid system, the paradoxical response to KOR agonists manifesting as transit hypermotility and/or hypersecretion is not surprising.
4.1. Clinical practice and future research
The currently used treatments to manage CKD-aP frequently fail to provide the desirable symptomatic relief. Thus, in this context, there is a real unmet need to find effective drugs with acceptable safety profiles. Oral antihistamines, the most commonly prescribed drugs for CKD-aP, have only been investigated in few trials; therefore, their effectiveness is not confirmed [38]. Gabapentinoids such as gabapentin and pregabalin are more extensively tested in the trials of CKD-ap, demonstrating overall positive effects on symptom reductions; however, this is contrasted by high rates of side effects that frequently cause treatment discontinuation [39]. Central opioid receptor agonists (e.g., naltrexone, nalfurafine hydrochloride) show conflicting results in dialysis patients with the potential of neuropsychiatric adverse effects such as insomnia and somnolence, especially with nalfurafine hydrochloride [38,39].
Although DFK seems to have a better safety profile than all the previous drug classes due to its CNS-free action, head-to-head comparisons are needed to explore whether DFK would be the better option. Also, it is necessary to provide more evaluations of higher dose tolerability during DFK therapy as the available data remains insufficient in this regard.
The higher incidence of side effects observed with DFK, although overall mild (particularly dizziness and diarrhea), suggests the need for further evaluation of the drug’s safety profile. Since the follow-up period in all studies did not exceed 12 weeks, the longer-term safety outcomes of DFK are unclear. Notably, the assessment of serious side effects and mortality requires more extensive evaluations. In four RCTs, DFK was given to hemodialysis patients intravenously, while only in the Yosipovitch et al. study oral forms used [12]. Intravenous DFK was administered three times per week versus once daily for oral DFK. Given the potential need for long-term use of DFK to achieve a meaningful reduction in CKD-ap, the administration mode may play an important role in patients’ adherence to the drug. Also, it is unknown if the two forms are equivalent in terms of efficacy and safety. This issue might be worthy of investigation by future studies. Finally, it would be interesting to explore if the CKD stage (i.e., with versus without hemodialysis) and dialysis modality (hemodialysis versus peritoneal dialysis) can impact the outcomes of DFK therapy.
4.2. Limitations
Our study has several limitations. First, the small number of the included studies. Thus, we included only five RCTs with a total of 896 participants, which considerably limits the robustness and generalizability of the findings. Second, three out of five of the included RCTs were phase II, which would provide less optimal quality of evidence. As promising results from phase II studies frequently do not translate into positive phase III [40,41]. Third, the evaluated data were those of short-term effects (≤12 weeks), whereas CKD-aP is likely to be a chronic symptom; thereby, identifying effective drugs requires long-term follow-up of treatment benefits. Also, with such a short monitoring period, the safety analysis of DFK was incomplete; thereby, it remains in the short term, only to be interpreted as preliminary and far from being conclusive. Fourth, the presence of numerous heterogeneities affects most efficacy endpoints. Fifth, since all studies focused on the improvement of pruritus scores as the efficacy outcome, examination of the effects of DFK on QoL is lacking and is yet to be investigated. Finally, the clinical advantage of DFK cannot be fully determined without head-to-head comparisons with other anti-pruritic agents used in the same context of hemodialysis.
5. Conclusion
DFK appears to be a promising agent for the management of CKD-induced pruritus in patients with ESRD undergoing hemodialysis. However, evidence is still underpowered due to the paucity of the current data. Therefore, the current results need to be interpreted carefully, and more multicenter large-scale RCTs are necessary to confirm the efficacy and safety of DFK among the hemodialysis population.
Supplementary Material
Funding Statement
The authors received no funding for this study.
Authors contributions
M.A. conceived the idea. A.S. and M.A. designed the research workflow. A.S., M.A., and I.E. searched the databases. R.R, M.K., M.H., and A.A. screened the retrieved records, extracted relevant data, and assessed the risk of bias, I.E. in discussion with A.S. and M.A. resolved any conflicts. A.S. performed the analysis. A.S., I.E., and Y.K. wrote the final manuscript. M.T. and M.A. supervised the project. All authors have read and agreed to the final version of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- 1.Bello AK, Okpechi IG, Osman MA, et al. Epidemiology of haemodialysis outcomes. Nat Rev Nephrol. 2022;18(6):378–395. doi: 10.1038/s41581-022-00542-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim D, Pollock C.. Epidemiology and burden of chronic kidney disease-associated pruritus. Clin Kidney J. 2021;14(Suppl 3):i1–i7. doi: 10.1093/ckj/sfab142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pisoni RL, Wikström B, Elder SJ, et al. Pruritus in haemodialysis patients: international results from the Dialysis Outcomes and Practice Patterns Study (DOPPS). Nephrol Dial Transplant. 2006;21(12):3495–3505. doi: 10.1093/ndt/gfl461. [DOI] [PubMed] [Google Scholar]
- 4.Ramakrishnan K, Bond TC, Claxton A, et al. Clinical characteristics and outcomes of end-stage renal disease patients with self-reported pruritus symptoms. Int J Nephrol Renovasc Dis. 2013;7:1–12. doi: 10.2147/IJNRD.S52985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rayner HC, Larkina M, Wang M, et al. International comparisons of prevalence, awareness, and treatment of pruritus in people on hemodialysis. Clin J Am Soc Nephrol. 2017;12(12):2000–2007. doi: 10.2215/CJN.03280317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Urquhart L. FDA new drug approvals in Q2 2021. Nat Rev Drug Discov. 2021;20(8):578. doi: 10.1038/d41573-021-00126-3. [DOI] [PubMed] [Google Scholar]
- 7.Fishbane S, Mathur V, Germain MJ, et al. Randomized controlled trial of difelikefalin for chronic pruritus in hemodialysis patients. Kidney Int Rep. 2020;5(5):600–610. doi: 10.1016/j.ekir.2020.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fishbane S, Jamal A, Munera C, et al. A phase 3 trial of difelikefalin in hemodialysis patients with pruritus. N Engl J Med. 2020;382(3):222–232. doi: 10.1056/NEJMoa1912770. [DOI] [PubMed] [Google Scholar]
- 9.Rastogi A, Fishbane S, Lerma E.. Difelikefalin for the treatment of moderate-to-severe pruritus associated with chronic kidney disease on hemodialysis. Expert Rev Clin Pharmacol. 2023;16(5):387–400. doi: 10.1080/17512433.2023.2197209. [DOI] [PubMed] [Google Scholar]
- 10.Viscusi ER, Torjman MC, Munera CL, et al. Effect of difelikefalin, a selective kappa opioid receptor agonist, on respiratory depression: a randomized, double-blind, placebo-controlled trial. Clin Transl Sci. 2021;14(5):1886–1893. doi: 10.1111/cts.13042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tamari M, Zamidar L, Ver Heul AM, et al. Difelikefalin suppresses itch and reduces scratching independent of inflammation in a murine model of atopic dermatitis. J Allergy Clin Immunol. 2023;152(4):927–932. doi: 10.1016/j.jaci.2023.06.022. [DOI] [PubMed] [Google Scholar]
- 12.Yosipovitch G, Awad A, Spencer RH, et al. A phase 2 study of oral difelikefalin in subjects with chronic kidney disease and moderate-to-severe pruritus. J Am Acad Dermatol. 2023;89(2):261–268. doi: 10.1016/j.jaad.2023.03.051. [DOI] [PubMed] [Google Scholar]
- 13.Narita I, Tsubakihara Y, Takahashi N, et al. Difelikefalin for hemodialysis patients with pruritus in Japan. NEJM Evid. 2023;2(11):EVIDoa2300094. [DOI] [PubMed] [Google Scholar]
- 14.Narita I, Tsubakihara Y, Uchiyama T, et al. Efficacy and safety of difelikefalin in japanese patients with moderate to severe pruritus receiving hemodialysis: a randomized clinical trial. JAMA Netw Open. 2022;5(5):e2210339. doi: 10.1001/jamanetworkopen.2022.10339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Higgins JPT, Thomas J, Chandler J, et al. Cochrane handbook for systematic reviews of interventions. Version 6. Cochrane; 2019. doi: 10.1002/9781119536604. [DOI] [Google Scholar]
- 17.Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guyatt GH, Oxman AD, Kunz R, et al. What is “quality of evidence” and why is it important to clinicians? BMJ. 2008;336(7651):995–998. doi: 10.1136/bmj.39490.551019.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lopes MB, Karaboyas A, Sukul N, et al. Utility of a single itch-related question and the Skindex-10 questionnaire for assessing pruritus and predicting health-related quality of life in patients receiving hemodialysis. Kidney Med. 2022;4(6):100476. doi: 10.1016/j.xkme.2022.100476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wala K, Szepietowski JC.. Difelikefalin in the treatment of chronic kidney disease-associated pruritus: a systematic review. Pharmaceuticals. 2022;15(8):934. doi: 10.3390/ph15080934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Topf J, Wooldridge T, McCafferty K, et al. Efficacy of difelikefalin for the treatment of moderate to severe pruritus in hemodialysis patients: pooled analysis of KALM-1 and KALM-2 phase 3 studies. Kidney Med. 2022;4(8):100512. doi: 10.1016/j.xkme.2022.100512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schricker S, Kimmel M.. Unravelling the pathophysiology of chronic kidney disease-associated pruritus. Clin Kidney J. 2021;14(Suppl 3):i23–i31. doi: 10.1093/ckj/sfab200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Verduzco HA, Shirazian S.. CKD-associated pruritus: new insights into diagnosis, pathogenesis, and management. Kidney Int Rep. 2020;5(9):1387–1402. doi: 10.1016/j.ekir.2020.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wieczorek A, Krajewski P, Kozioł-Gałczyńska M, et al. Opioid receptors expression in the skin of haemodialysis patients suffering from uraemic pruritus. J Eur Acad Dermatol Venereol. 2020;34(10):2368–2372. doi: 10.1111/jdv.16360. [DOI] [PubMed] [Google Scholar]
- 25.Kim BS, Inan S, Ständer S, et al. Role of kappa-opioid and mu-opioid receptors in pruritus: peripheral and central itch circuits. Exp Dermatol. 2022;31(12):1900–1907. doi: 10.1111/exd.14669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Snyder LM, Chiang MC, Loeza-Alcocer E, et al. Kappa opioid receptor distribution and function in primary afferents. Neuron. 2018;99(6):1274.e6–1288.e6. doi: 10.1016/j.neuron.2018.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shirazian S, Spencer RH, Kilfeather SA.. 137 Reduction of pruritus by difelikefalin correlates with reductions in markers for pruritus and inflammation in subjects undergoing hemodialysis. Am J Kidney Dis. 2022;79(4):S42. [Google Scholar]
- 28.Kimmel M, Alscher DM, Dunst R, et al. The role of micro-inflammation in the pathogenesis of uraemic pruritus in haemodialysis patients. Nephrol Dial Transplant. 2006;21(3):749–755. doi: 10.1093/ndt/gfi204. [DOI] [PubMed] [Google Scholar]
- 29.Nguyen E, Smith KM, Cramer N, et al. Medullary kappa-opioid receptor neurons inhibit pain and itch through a descending circuit. Brain. 2022;145(7):2586–2601. doi: 10.1093/brain/awac189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Albert-Vartanian A, Boyd MR, Hall AL, et al. Will peripherally restricted kappa-opioid receptor agonists (pKORAs) relieve pain with less opioid adverse effects and abuse potential? J Clin Pharm Ther. 2016;41(4):371–382. doi: 10.1111/jcpt.12404. [DOI] [PubMed] [Google Scholar]
- 31.Shram MJ, Spencer RH, Qian J, et al. Evaluation of the abuse potential of difelikefalin, a selective kappa-opioid receptor agonist, in recreational polydrug users. Clin Transl Sci. 2022;15(2):535–547. doi: 10.1111/cts.13173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vega R, Soto E.. Opioid receptors mediate a postsynaptic facilitation and a presynaptic inhibition at the afferent synapse of axolotl vestibular hair cells. Neuroscience. 2003;118(1):75–85. doi: 10.1016/s0306-4522(02)00971-5. [DOI] [PubMed] [Google Scholar]
- 33.Bagnol D, Mansour A, Akil H, et al. Cellular localization and distribution of the cloned mu and kappa opioid receptors in rat gastrointestinal tract. Neuroscience. 1997;81(2):579–591. doi: 10.1016/s0306-4522(97)00227-3. [DOI] [PubMed] [Google Scholar]
- 34.De Luca A, Coupar IM.. Insights into opioid action in the intestinal tract. Pharmacol Ther. 1996;69(2):103–115. doi: 10.1016/0163-7258(95)02053-5. [DOI] [PubMed] [Google Scholar]
- 35.Hughes PA, Costello SP, Bryant RV, et al. Opioidergic effects on enteric and sensory nerves in the lower GI tract: basic mechanisms and clinical implications. Am J Physiol Gastrointest Liver Physiol. 2016;311(3):G50–G513. doi: 10.1152/ajpgi.00442.2015. [DOI] [PubMed] [Google Scholar]
- 36.Ramabadran K, Bansinath M, Turndorf H, et al. Stereospecific inhibition of gastrointestinal transit by kappa opioid agonists in mice. Eur J Pharmacol. 1988;155(3):329–331. doi: 10.1016/0014-2999(88)90524-9. [DOI] [PubMed] [Google Scholar]
- 37.Bansinath M, Ramabadran K, Turndorf H, et al. Kappa-opiate agonist-induced inhibition of gastrointestinal transit in different strains of mice. Pharmacology. 1991;42(2):97–102. doi: 10.1159/000138779. [DOI] [PubMed] [Google Scholar]
- 38.Ko MJ, Peng YS, Wu HY.. Uremic pruritus: pathophysiology, clinical presentation, and treatments. Kidney Res Clin Pract. 2023;42(1):39–52. doi: 10.23876/j.krcp.21.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Combs SA, Teixeira JP, Germain MJ.. Pruritus in kidney disease. Semin Nephrol. 2015;35(4):383–391. doi: 10.1016/j.semnephrol.2015.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zia MI, Siu LL, Pond GR, et al. Comparison of outcomes of phase II studies and subsequent randomized control studies using identical chemotherapeutic regimens. J Clin Oncol. 2005;23(28):6982–6991. doi: 10.1200/JCO.2005.06.679. [DOI] [PubMed] [Google Scholar]
- 41.Liang F, Wu Z, Mo M, et al. Comparison of treatment effect from randomised controlled phase II trials and subsequent phase III trials using identical regimens in the same treatment setting. Eur J Cancer. 2019;121:19–28. doi: 10.1016/j.ejca.2019.08.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.