Abstract
Background
Gestational diabetes mellitus (GDM) is associated with increased long-term risk of cardiovascular disease but the cardiovascular structural and functional changes that contribute to risk are not well understood.
Objectives
The purpose of this study was to determine whether GDM is associated with adverse cardiac remodeling and endothelial dysfunction a decade after delivery, independent of type 2 diabetes.
Methods
Women with deliveries between 2008 and 2009 were initially selected from a prospective clinical cohort. Pregnancy history was chart abstracted and a follow-up study visit was conducted at 8 to 10 years postpartum. Cardiac structure and function were assessed with echocardiography. Endothelial function was measured with peripheral arterial tonometry and glycocalyx analysis.
Results
Among 254 women assessed at an average age of 38 years, 53 (21%) had prior GDM. At follow-up, women with GDM had more incident prediabetes or diabetes (58% vs 20% without GDM), more impairment in peripheral arterial tonometry (reactive hyperemia 1.58 vs 1.95; P = 0.01) and reduced perfusion, a marker of glycocalyx assessment (red blood cell filling 0.70 ± 0.04 vs 0.72 ± 0.05; P < 0.01). Despite adjustment for demographic and reproductive characteristics, women with GDM had great septal wall thickness by 8% (95% CI: 2.3%-14.7%) and worse diastology with higher E/E’ by 11% (95% CI: 1.1%-21.5%). After additional adjustment for diabetes and prediabetes, several parameters remained significantly impaired.
Conclusions
Having GDM within the past decade was associated with more adverse cardiac structure/function and vascular endothelial function. Some, but not all, risks may be mediated through the development of prediabetes or type 2 diabetes. Enhanced preventive efforts are needed to mitigate cardiovascular risk among women with GDM.
Key words: echocardiogram, endothelial function, gestational diabetes, pregnancy
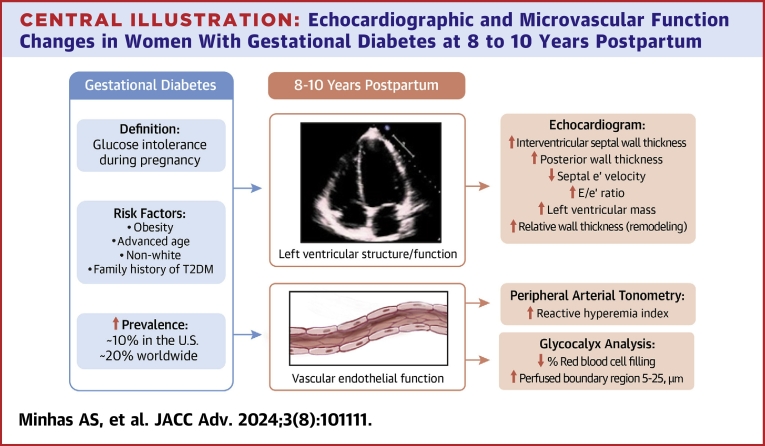
Central Illustration
Gestational diabetes mellitus (GDM) is among the most common adverse pregnancy outcomes and affects 17 to 20% of pregnancies worldwide.1,2 It is characterized by glucose intolerance developing during pregnancy and is associated with multiple maternal and fetal complications.2,3 Within the first 10 years postpartum, 15 to 30% of women with GDM develop type 2 diabetes mellitus (T2DM), likely due to persistent pancreatic beta-cell dysfunction.4, 5, 6, 7, 8 Women with GDM are also twice as likely to develop chronic hypertension and hyperlipidemia.9, 10, 11
GDM is known to be associated with increased cardiovascular disease (CVD) risk, but it is unclear whether this risk is independent of future development of diabetes or prediabetes, with studies reporting mixed results.9 A recent study demonstrated that women with GDM had increased coronary artery calcification risk several years after GDM pregnancy, even with normoglycemia, suggesting that atherosclerotic CVD risk is present in women with GDM independent of future development of prediabetes or T2DM.12
Several pathophysiologic processes are proposed as potential explanations for increased CVD risk in women with GDM. Alterations in left ventricular (LV) structure (increased wall thickness) and mechanics (adverse diastology) have been documented.13, 14, 15 Vascular endothelial dysfunction is also implicated. Endothelial dysfunction precedes atherosclerosis and predicts cardiovascular events.16,17 Data regarding endothelial dysfunction in women with GDM within 2 decades postpartum are primarily in small studies (<20 women), are mixed, and do not account for obesity or subsequent dysglycemia.18, 19, 20, 21, 22, 23 Comparing women with vs without glucose intolerance during pregnancy, the largest study to date evaluating endothelial function (n = 38), via flow-mediated dilatation, did not find any differences at 6 years postpartum. Notably, this study was limited by modest sample size and inclusion of milder gestational glucose impairment rather than true GDM alone, which may have contributed to the null results.24
Subclinical cardiac structural/functional changes and microvascular dysfunction can identify women at highest risk for future CV events who may benefit the most from targeted interventions. We conducted a cross-sectional study in a prospective cohort of women with GDM-affected pregnancies to assess echocardiographic and vascular parameters at 8 to 10 years postpartum. We hypothesized that women with GDM would have adverse LV structural and functional changes and impaired microvascular function in the first decade postpartum and that these impairments would be independent of the development of future diabetes and prediabetes.
Methods
Study source
Women (defined as biologic sex) were enrolled from the Magee Obstetric Maternal and Infant database at the University of Pittsburgh, Pittsburgh, Pennsylvania. The protocol for enrollment of this original cohort has been described previously.25,26 Briefly, women (n = 4,048) with placental pathology samples obtained for clinical indications between 2008 and 2009 were originally included. Among the women who were eligible (alive, nonpregnant, and without chronic hypertension or diabetes before the index pregnancy), 498 were subsequently enrolled; the remaining either declined or were unable to be contacted.25 Index pregnancy refers to that which made the participant eligible for study enrollment. Participants were enrolled in an ongoing substudy evaluating the association of placental vascular lesions, adverse pregnancy outcomes, and CVD risk factors.25 Among this group, 254 further underwent 2-dimensional echocardiogram and microvascular function testing with peripheral arterial tonometry (PAT) and glycocalyx analysis. By design, women selected for the smaller imaging substudy were overenrolled for placental maternal vascular malperfusion lesions and therefore the occurrence of adverse pregnancy outcomes was high. Women with prepregnancy chronic hypertension or diabetes were excluded. All women with available echocardiograms or microvascular testing were included in our study. This study was approved by the University of Pittsburgh Institutional Review Board (STUDY19110278) and the Johns Hopkins University Institutional Review Board (IRB00272184).
Study procedures
Women were assessed at a mean of 9 years after delivery. The full protocol describing the methods has been published previously.27 Briefly, women underwent standardized blood pressure, height, and weight measurements. Blood was collected for fasting laboratory testing. GDM and other pregnancy comorbidities were determined via electronic medical record chart abstraction of physician diagnoses using International Classification of Diseases-9 or -10 codes at discharge. At the study visit, a diagnosis of chronic hypertension was established if the average of three blood pressure readings was ≥130/80 mm Hg or the participant reported use of antihypertensive medications. T2DM was diagnosed by hemoglobin A1c result (≥6.5%) or self-report (approximately 96% of participants were diagnosed with T2DM based on the hemoglobin A1c result). Prediabetes was diagnosed by hemoglobin A1c result (≥5.7%-<6.5%). Nine participants did not have a hemoglobin A1c performed and were excluded from analyses.
Participants underwent standard transthoracic 2-dimensional echocardiograms by dedicated research sonographers. All measurements were performed in accordance with the American Society of Echocardiography guidelines.28,29 LV remodeling was assessed by relative wall thickness (RWT = [2 × posterior LV wall thickness]/LV diastolic diameter), with an abnormal RWT defined as >0.42.28 Diastolic function was also assessed. Lower septal/lateral e’ velocity and higher E/e’ ratio were indicative of worse diastolic function.
For microvascular assessment, EndoPAT (Itamar Medical, Ltd) was used. EndoPAT is an Food and Drug Administration-approved device which records the pulse amplitude in the participant’s fingertips at rest and during reactive hyperemia induced by brachial artery cuff occlusion and release.30 The primary measurement obtained is the net response, or the reactive hyperemia pulse amplitude tonometry index (RHI), which is a marker of microvascular endothelial function and predicts future cardiovascular events.30, 31, 32
Additional assessment of microvascular function was performed using fully automated, commercially available sidestream dark field videomicroscopy and software (GlycoCheck) to examine the sublingual microcirculation endothelial glycocalyx, the delicate luminal surface layer at the interface of the endothelial cell and circulating blood cells.33, 34, 35, 36, 37 This is a reproducible noninvasive method that uses a handheld sidestream dark field videomicroscopy camera to record red blood cell (RBC) flow in the capillaries under the tongue.36,38 The microvascular density is estimated by total length of perfused microvessels/mm2 identified in the area recorded. A lower value of the density suggests rarefaction of the microvasculature.36,39 The RBC filling is the proportion of identified microvessel segments occupied by RBC with a lower value suggesting less vascular perfusion. The glycocalyx is the extracellular matrix that lines the luminal surface of endothelial cells, provides barrier function to protect endothelial cells, and regulates vascular function and homeostasis.33 The median diameter of the glycocalyx (μm) can be measured. The perfused boundary region (PBR) reflects the depth of the glycocalyx penetrable by RBCs flowing through the vessel lumen. An increase in lateral movement of RBCs into the glycocalyx, measured as an increased PBR in vessel segments of 5 to 25 um diameter, is associated with dysfunctional glycocalyx and appears to correlate with early atherosclerosis and diabetes complications.40, 41, 42, 43, 44, 45 The four glycocalyx measurements used in this study are microvascular density, RBC filling (marker of perfusion), median microvessel diameter, and PBR 5 to 25, which is a measure of the depth of RBC penetration into the glycocalyx and is an indicator of glycocalyx integrity (higher penetration indicate glycocalyx damage or dysfunction).
Statistical analysis
We compared clinical measurements, biomarkers, and imaging parameters among women with a prior history of GDM and without GDM. Symmetrically distributed variables were reported as mean (SD) and skewed continuous variables were reported as medians (25th-75th percentile). Comparisons for normally distributed continuous variables were made using Student’s t-test and for non-normally distributed variables with Wilcoxon rank sum test. Categorical variables were presented as absolute numbers (percentages) and comparisons performed with chi-squared test.
We used multivariable linear regression to evaluate the association of echocardiographic and microvascular function parameters with GDM history, with non-normally distributed continuous variables natural log-transformed prior to regression (E/E’ ratio, E/A ratio, LV mass, RWT, microvascular density, RBC filling, and median diameter). Log-transformed variables were back transformed for inclusion in the table for ease of interpretation. Regression diagnostics were performed for all models. Model 1 was adjusted for age, race, and any history of preeclampsia and preterm birth in the index pregnancy. Model 2 included the variables in Model 1 plus current body mass index (BMI), hypertension, T2DM, and prediabetes.
We then compared echocardiographic and microvascular function parameters in cross-categories of women by GDM and prediabetes/T2DM status to evaluate for progressive impairment in echocardiographic and microvascular parameters with worsening glycemic status. Categories were defined as follows: 1) women without GDM in the index pregnancy and no incident prediabetes or T2DM; 2) women with GDM and no incident prediabetes or T2DM; 3) women with no GDM but incident prediabetes or T2DM; and 4) women with GDM and either incident prediabetes or T2DM. Analysis of variance and Kruskal-Wallis tests were used for normally or non-normally distributed continuous variables, respectively. Pairwise comparisons were also performed for evaluating differences among groups 2 to 4 compared to group 1 (no GDM and no incident prediabetes or T2DM). For all analyses, a P value of <0.05 was considered statistically significant. All analyses were performed using Stata Statistical Software, Version 16 (StataCorp).
Results
Demographics and baseline characteristics
Among 254 included women, 53 (20%) had a pregnancy with GDM. At the follow-up visit, time since delivery, age, race, and current BMI were comparable between women with and without GDM, while systolic and diastolic blood pressure were higher (Table 1). Women with GDM had higher prepregnancy BMI and preeclampsia. As expected, they were also more likely to have incident prediabetes or diabetes at follow-up.
Table 1.
Characteristics of Study Participants According to GDM Status
| Non-GDM (n = 201) | GDM Cases (n = 53) | P Value | |
|---|---|---|---|
| Demographics | |||
| Age at follow-up visit, y | 38.1 ± 5.9 | 39.8 ± 5.3 | 0.07 |
| Race/ethnicity | 0.86 | ||
| White | 131 (65%) | 32 (60%) | - |
| Black | 68 (34%) | 20 (38%) | - |
| Other | 2 (1%) | 1 (2%) | - |
| Education, y | 15.2 ± 2.5 | 14.9 ± 2.6 | 0.38 |
| Years since index delivery | 8.5 (9.0-9.7) | 9.3 (8.5-9.9) | 0.56 |
| Pregnancy history | |||
| Preterm birth in index pregnancy | 43 (21%) | 11 (21%) | 0.92 |
| Gestational hypertension in index pregnancy | 16 (8%) | 3 (6%) | 0.57 |
| Preeclampsia in any pregnancy | 30 (15%) | 15 (28%) | 0.023 |
| Prepregnancy BMI, kg/m2 | 26 (22-31) | 29 (25-37) | <0.001 |
| Placental maternal vascular malperfusion lesions | 79 (39%) | 18 (34%) | 0.51 |
| Current clinical findings | |||
| Systolic BP | 113 (106-122) | 119 (110-127) | 0.014 |
| Diastolic BP | 74 (68-81) | 78 (72-85) | 0.013 |
| BMI, kg/m2 | 30 (24-36) | 30 (27-37) | 0.40 |
| Fat percent (%) | 40 (31-46) | 41 (35-46) | 0.21 |
| Hypertension | 60 (30%) | 23 (45%) | 0.041 |
| Diabetes | 8 (4%) | 16 (32%) | <0.001 |
| Prediabetes | 33 (17%) | 17 (34%) | 0.008 |
| Cardiovascular risk factors | |||
| High-sensitivity C-reactive protein, mg/L | 0.20 (0.09-0.49) | 0.43 (0.16-1.41) | 0.001 |
| Hemoglobin A1c, % | 5.3 (5.1-5.6) | 5.8 (5.3-6.4) | <0.001 |
| Glucose, mg/dL | 82 (77-93) | 100 (88-115) | <0.001 |
| Low-density lipoprotein, mg/dL | 100 (78-123) | 89 (71-128) | 0.36 |
| Very low-density lipoprotein, mg/dL | 15 (11-23) | 21 (16-29) | <0.001 |
| Total cholesterol, mg/dL | 173 (150-201) | 168 (144-204) | 0.80 |
| Triglycerides, mg/dL | 76 (56-117) | 105 (79-147) | <0.001 |
| High-density lipoprotein, mg/dL | 54 (44-63) | 50 (44-59) | 0.11 |
| Insulin, mIU/L | 6 (3-12) | 9 (5-17) | 0.018 |
| HOMA-IR | 1.2 (0.6-2.4) | 2.2 (1.2-4.3) | 0.002 |
BMI = body mass index; BP = blood pressure; GDM = gestational diabetes mellitus; HOMA-IR = homeostatic model assessment for insulin resistance.
Values are mean, n (%), or median (IQR).
Imaging outcomes
Interventricular septal and LV posterior wall thickness were significantly greater in women with GDM. Diastology parameters (septal e’, E/e’, and E/A) were also more unfavorable, and LV mass and RWT were higher (worse) in women with GDM (Central Illustration, Table 2). Unadjusted RHI, RBC filling, and PBR 5 to 25 were significantly more impaired in women with GDM compared to women without GDM (Table 2).
Central Illustration.
Echocardiographic and Microvascular Function Changes in Women With Gestational Diabetes at 8 to 10 Years Postpartum
Women with gestational diabetes have evidence of subclinical cardiovascular disease at 8 to 10 years postpartum. Echocardiograms reveal increased left ventricular wall thickness and impaired diastolic function parameters. Vascular endothelial dysfunction is also noted. Peripheral arterial tonometry testing and glycocalyx analysis both demonstrate endothelial dysfunction, with unfavorable measures in women with gestational diabetes abbreviation as in Figure 1.
Table 2.
Echocardiogram Parameters and Endothelial Function Tests at the 9-Year Follow-Up Visit Among Women With and Without GDM During Pregnancy
| Non-GDM | GDM | P Value | |
|---|---|---|---|
| Echocardiogram | n = 201 | n = 33 | |
| IVS thickness, cm | 0.92 ± 0.16 | 1.02 ± 0.17 | <0.001 |
| LV posterior wall thickness, cm | 0.91 ± 0.14 | 0.99 ± 0.15 | 0.004 |
| LV EF, % | 63 ± 5 | 63 ± 6 | 0.53 |
| Septal e’ velocity, cm/s | 10.0 ± 2.4 | 9.1 ± 2.3 | 0.036 |
| Lateral e’ velocity, cm/s | 12 (11-14) | 11.5 (10-13) | 0.10 |
| E/e’ ratio | 7.1 (5.9-8.4) | 7.8 (6.4-8.9) | 0.034 |
| E/A ratio | 1.3 (1.1-1.6) | 1.1 (1.0-1.3) | 0.018 |
| LV mass, g | 143 (119-167) | 148 (138-176) | 0.026 |
| RWT | 0.39 (0.34-0.44) | 0.45 (0.37-0.50) | 0.022 |
| Peripheral arterial tonometry | n = 108 | n = 20 | |
| Reactive hyperemia index | 1.95 (1.48-2.44) | 1.58 (1.32-1.70) | 0.011 |
| Glycocalyx analysis | n = 161 | n = 48 | |
| Microvascular density, segments/mm2 | 397 ± 128 | 364 ± 129 | 0.11 |
| RBC filling, %RBC filling | 0.72 ± 0.05 | 0.70 ± 0.04 | 0.006 |
| Median diameter, μm | 8.9 (8.3-9.5) | 9.0 (8.2-9.7) | 0.77 |
| PBR 5-25, μm | 2.04 ± 0.23 | 2.11 ± 0.21 | 0.040 |
EF = ejection fraction; GDM = gestational diabetes mellitus; IVS = interventricular septum; LV = left ventricle; PBR = perfused boundary region; RBC = red blood cell; RWT = relative wall thickness (abnormal >0.42).
Values are mean ± SD or median (25th-75th percentile). Glycocheck analysis of the sublingual microcirculation and glycocalyx was used to compare density, perfusion (RBC filling), median diameter (P50), and penetration of RBCs into the glycocalyx of vessel segments of 5 to 25 μm diameter (perfused boundary region or PBR 5-25). Disruption of the glycocalyx results in greater penetration of RBCs toward the vessel wall.
In adjusted linear regression Model 1 (adjusted for age, race, and any history of preeclampsia and preterm birth in index pregnancy), interventricular septal/LV posterior wall thickness, E/e’ ratio, and RWT remained higher among women with GDM. Specifically, interventricular septal wall thickness was 8% higher and posterior wall thickness was 6% higher. These results suggest that wall thickness, E/e’, and RWT are all higher in women with gestational diabetes, supporting impairment in diastolic parameters. After adjustment for additional cardiovascular risk factors (BMI, hypertension, T2DM, and prediabetes), findings of greater wall thickness were attenuated but E/e’ ratio remained impaired (Table 3). Similarly, parameters of endothelial function, including RHI, microvascular density, RBC filling, and PBR 5 to 25 were less favorable in women with GDM compared to without. Most of these associations persisted following adjustment for CVD risk factors in Model 2 (all remained significantly impaired in GDM except PBR 5-25) (Table 3). These results support endothelial dysfunction among women with GDM that is independent of CVD risk factors.
Table 3.
Unadjusted and Adjusteda β Coefficients and Percent Differences (95% CIs) for Echocardiographic Parameters and Microvascular Function Comparing Women With and Without GDM
| Unadjusted | Model 1 | Model 2 | |
|---|---|---|---|
| Echocardiogram | |||
| IVS thickness, cm | 0.10 (0.04-0.16) P = 0.001 |
0.08 (0.02-0.14) P = 0.006 |
0.03 (−0.03, 0.09) P = 0.38 |
| LV posterior wall thickness, cm | 0.08 (0.02-0.13) P = 0.004 |
0.05 (0.00-0.11) P = 0.048 |
0.016 (−0.04, 0.07) P = 0.57 |
| LV EF, % | −0.65 (−2.67, 1.38) P = 0.53 |
−1.03 (−3.13, 1.06) P = 0.33 |
−1.14 (−3.54, 1.27) P = 0.35 |
| Septal e’ velocity, cm/s | −0.96 (−1.85, −0.06) P = 0.036 |
−0.74 (−1.63, −0.08) P = 0.10 |
−0.36 (−1.31, 0.60) P = 0.46 |
| Lateral e’ velocity, cm/sb | 0.92 (0.85-1.00) P = 0.05 |
0.95 (0.88-1.00) P = 0.22 |
1.00 (0.91-1.09) P = 0.99 |
| E/e’ ratiob | 1.13 (1.03-1.23) P = 0.011 |
1.11 (1.01-1.21) P = 0.028 |
1.11 (1.00-1.23) P = 0.043 |
| E/A ratiob | 0.90 (0.81-0.99) P = 0.042 |
0.95 (0.86-1.05) P = 0.29 |
1.01 (0.90-1.13) P = 0.83 |
| LV massb | 1.12 (1.01-1.23) P = 0.024 |
1.09 (0.99-1.20) P = 0.07 |
1.05 (0.95-1.15) P = 0.33 |
| RWTb | 1.09 (1.02-1.17) P = 0.011 |
1.08 (1.00-1.16) P = 0.037 |
1.01 (0.93-1.09) P = 0.78 |
| Peripheral arterial tonometry | |||
| Reactive hyperemia index | 0.83 (0.71-0.97) P = 0.018 |
−0.23 (−0.38, −0.07) P = 0.005 |
−0.17 (−0.35, 0.00) P = 0.056 |
| Glycocalyx analysis | |||
| Microvascular density,b segments/mm2 | 0.91 (0.81-1.01) P = 0.09 |
−0.12 (−0.23, −0.004) P = 0.042 |
−0.14 (−0.27, −0.01) P = 0.031 |
| RBC filling,b %RBC filling | 0.97 (0.95-0.99) P = 0.006 |
−0.03 (−0.05, −0.01) P = 0.007 |
−0.03 (−0.05, −0.01) P = 0.017 |
| Median diameter,b μm | 1.00 (0.96-1.04) P = 0.97 |
0.01 (−0.03, 0.05) P = 0.72 |
0.01 (−0.03, 0.05) P = 0.67 |
| PBR 5-25, μm | 0.08 (0.00-0.15) P = 0.040 |
0.08 (0.00-0.15) P = 0.05 |
0.08 (−0.01, 0.17) P = 0.08 |
Values are β coefficient (95% CI).
Abbreviations as in Table 2.
Model 1 includes adjustment for age, race, and any history of preeclampsia and preterm birth in index pregnancy; Model 2 includes prior covariates plus body mass index at follow-up visit, hypertension, type 2 diabetes mellitus, and prediabetes
Given non-normal distribution, natural log-transformed values were used in regression models and then transformed back for easier interpretation.
Cross-categorization by GDM history and current glycemic status
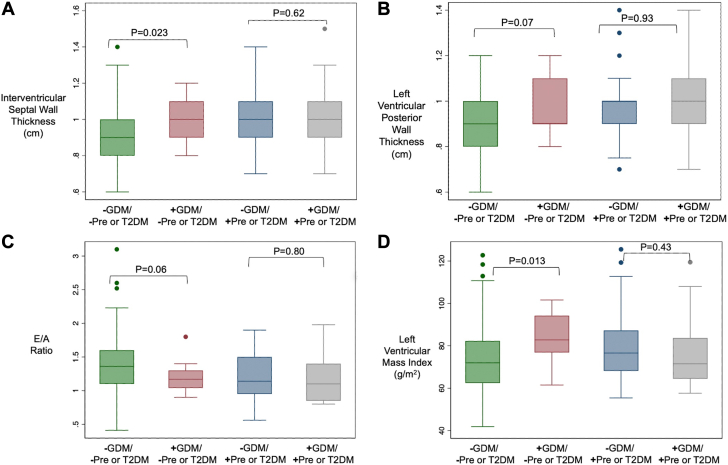
As an additional exploratory analysis, we evaluated progressive impairment in echocardiographic and microvascular function with worsening glycemic status. For this, groups were analyzed according to history of GDM and current glycemic status in order of worsening status: 1) no GDM, no T2DM/prediabetes; 2) GDM, no T2DM/prediabetes; 3) no GDM or T2DM/prediabetes; and 4) both GDM and T2DM/prediabetes. Several differences were seen among echocardiographic parameters across the groups. Notably, women with GDM without progression to prediabetes/T2DM had higher interventricular septal wall thickness and higher LV mass. Women with both GDM and incident prediabetes or T2DM had the greatest impairment in echocardiographic structural and functional variables compared to the reference (group 1, free of dysglycemia) (Supplemental Table 1, Figure 1). Microvascular function parameters trended in a similar direction but did not meet statistical significance thresholds.
Figure 1.
Comparison of Echocardiographic and Endothelial Function Parameters Across Subgroups by GDM and Current Glycemic Status
Women with GDM even in the absence of incident prediabetes or type 2 diabetes (red boxes) have significantly increased interventricular septal wall thickness (A) and higher left ventricular mass index (D), and nearly significantly increased left ventricular posterior wall thickness (B) and elevated E/A ratio (C), compared to women without GDM (green boxes). In the presence of prediabetes and type 2 diabetes, history of GDM does not result in any significant differences in echocardiographic parameters (blue and gray boxes in all panels). GDM = gestational diabetes mellitus; T2DM = type 2 diabetes mellitus.
Discussion
As expected, women with GDM were more likely to develop prediabetes or T2DM (∼60%) within a decade after delivery. They were also more likely to have adverse cardiac remodeling (thicker LV walls) and impaired diastolic parameters. Furthermore, they had impairments in microvascular function, as assessed by two separate methods. While some parameters were attenuated, several echocardiographic and vascular function parameters remained impaired after adjustment for traditional cardiovascular risk factors, suggesting that worsening cardiovascular health status may be a mediator for some, but not all, of the increased CV risk with GDM. Notably, the average age of women studied was young (mean 38 years), but those with prior GDM already had evidence of more adverse cardiac structural/functional and peripheral microvascular changes compared to similarly aged women without GDM history.
Prior studies evaluating postpartum echocardiographic changes in those with GDM are limited. One study of older women demonstrated similar findings of LV remodeling and impaired diastolic parameters that remained after adjusting for T2DM at 20 years after pregnancy.14 In our stratified analyses, we demonstrated that the largest absolute increase in wall thickness and LV mass and decrease in septal/lateral e’ velocity occurred with GDM history, even without development of prediabetes and T2DM. Notably, the additional insult of incident prediabetes or T2DM had a relatively lower impact in the absolute values. These findings support GDM as an independent risk factor for cardiac structural/functional changes and suggest that women with GDM, including those with normoglycemia after delivery, may have similar cardiac changes of hypertrophy and diastolic abnormalities as seen in diabetic cardiomyopathy.46
Importantly, our data indicate that these abnormalities develop in very young women (mean age <40 years) within 10 years of GDM pregnancy (versus 20 years in the prior study) and that these abnormalities persist despite adjustment for preeclampsia which is itself associated with worse cardiovascular outcomes.27 The adverse effects of elevated glucose levels, insulin resistance, and increased systemic inflammation that occur during a pregnancy complicated by GDM may contribute to the cardiac changes seen in the decade after delivery. The association between GDM and cardiac structural and function changes may in part be due to worsening insulin resistance (women with GDM have worsening HOMA), higher BMI, and higher blood pressure. This is supported by some attenuation in our results after adjusting for these traditional cardiovascular risk factors. However, elevated risk remains for women with GDM despite adjustment for traditional factors so these do not explain the entire risk.
Additionally, we demonstrate that women with GDM have reduced microvascular function as assessed by two separate tests, PAT and glycocalyx assessment. Impaired vascular function with GDM has previously been reported with other methods.11,22,47,48 However, we find that several of these microvascular changes are independent of incident diabetes and prediabetes (lower microvascular density and RBC filling). These findings suggest that postpartum endothelial and microvascular dysfunction may be a mechanism for increased maternal CVD risk. This may in part be driven by obesity. Pro-inflammatory cytokines and adipokines released from adipose tissue can have deleterious effects on long-term vascular health.49 In our study, women with GDM were more likely to have higher prepregnancy BMI, suggesting potential longer exposure duration to metabolically active visceral adipose tissue but this should be considered directly in future studies.
Unique strengths of this study include extensive cardiovascular phenotyping including echocardiograms, PAT, and glycocalyx analysis of women with GDM, and availability of extensive clinically confirmed pregnancy history including placental pathology. Prior notable studies performed evaluating the contribution of GDM to long-term CVD have been limited by the use of self-reported GDM.12,14
Study Limitations
Limitations of the current study include lack of 6-week postpartum glucose tolerance testing to confirm resolution of GDM. Additionally, sample size is modest and may explain why some parameters were not found to be statistically significantly impaired among women with GDM though they trended in an unfavorable direction. We also did not have detailed information such as dietary patterns and physical activity which could affect cardiovascular parameters. Future larger confirmatory studies should be performed aiming to capture granular information with long-term follow-up post-GDM pregnancy.
Conclusions
Pregnancy provides a unique window to identify individuals at increased risk for future CVD. Lifestyle modifications remain a key focus to help reduce both GDM risk and subsequent development of cardiometabolic disorders, including obesity, hypertension, and diabetes. Interventions targeting weight loss and increasing physical activity in the postpartum period are paramount, but effective strategies and identification of highest risk individuals have been limited.50 Both PAT and glyocalyx analysis are fast, noninvasive methods with low risk for adverse events and could potentially detect women at highest risk for future CVD in a research or clinical setting. Prospective studies should be performed that can follow women with GDM for the development of CVD, specifically evaluating the role of echocardiographic and microvascular abnormalities as potential risk mediators and future targets for intervention.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: Women with gestational diabetes have increased LV wall thickness, unfavorable diastology, and adverse microvascular function within a decade after delivery. These abnormal findings are present independently of incident T2DM and prediabetes.
TRANSLATIONAL OUTLOOK: Preventive efforts should be enhanced to mitigate the long-term risks of gestational diabetes beyond maintenance of normoglycemia. Additional research should be performed to investigate the role of targeting subclinical markers to reduce the risk of frank CVD after pregnancy complicated by gestational diabetes.
Funding support and author disclosures
This work was funded by the American Heart Association Go Red for Women Strategically Focused Research Network grants 16SFRN27870000, 16SFRN28930000 Window Study, and 16SFRN28340000 Glycocalyx pathways linking pregnancy profile with microvascular dysfunction postpartum and completed with collaboration between Johns Hopkins University School of Medicine and the Magee-Women’s Research Institute. Dr Minhas was supported by NIH K23HL171886 and NIH KL2TR003099. Dr Countouris was funded through the National Institutes of Health (NHLBI T32 Training Grant HL129964). Dr Ndumele was supported by American Heart Association Cardiometabolic Strategically Focused Research Network Grant 20SFRN35120152. Dr Selvin was supported by NIH/NHLBI grant K24 HL152440. Dr Michos was supported by the Amato Fund for Women’s Cardiovascular Health at Johns Hopkins University. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For a supplemental table, please see the online version of this paper.
Supplementary data
References
- 1.Fong A., Serra A., Herrero T., Pan D., Ogunyemi D. Pre-gestational versus gestational diabetes: a population based study on clinical and demographic differences. J Diabetes Complicat. 2014;28:29–34. doi: 10.1016/j.jdiacomp.2013.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McIntyre H.D., Catalano P., Zhang C., Desoye G., Mathiesen E.R., Damm P. Gestational diabetes mellitus. Nat Rev Dis Primers. 2019;5:47. doi: 10.1038/s41572-019-0098-8. [DOI] [PubMed] [Google Scholar]
- 3.Anon ACOG Practice Bulletin No. 190: gestational diabetes mellitus. Obstet Gynecol. 2018;131:e49–e64. doi: 10.1097/AOG.0000000000002501. [DOI] [PubMed] [Google Scholar]
- 4.Kim C., Newton K.M., Knopp R.H. Gestational diabetes and the incidence of type 2 diabetes: a systematic review. Diabetes Care. 2002;25:1862–1868. doi: 10.2337/diacare.25.10.1862. [DOI] [PubMed] [Google Scholar]
- 5.Lee A.J., Hiscock R.J., Wein P., Walker S.P., Permezel M. Gestational diabetes mellitus: clinical Predictors and long-term risk of developing type 2 diabetes: a retrospective cohort study using survival analysis. Diabetes Care. 2007;30:878–883. doi: 10.2337/dc06-1816. [DOI] [PubMed] [Google Scholar]
- 6.Feig D.S., Zinman B., Wang X., Hux J.E. Risk of development of diabetes mellitus after diagnosis of gestational diabetes. CMAJ. 2008;179:229–234. doi: 10.1503/cmaj.080012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bellamy L., Casas J.-P., Hingorani A.D., Williams D. Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. Lancet. 2009;373:1773–1779. doi: 10.1016/S0140-6736(09)60731-5. [DOI] [PubMed] [Google Scholar]
- 8.Vounzoulaki E., Khunti K., Abner S.C., Tan B.K., Davies M.J., Gillies C.L. Progression to type 2 diabetes in women with a known history of gestational diabetes: systematic review and meta-analysis. BMJ. 2020;369 doi: 10.1136/bmj.m1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Minhas A.S., Ying W., Ogunwole S.M., et al. The association of adverse pregnancy outcomes and cardiovascular disease: current Knowledge and future directions. Curr Treat Options Cardio Med. 2020;22:61. doi: 10.1007/s11936-020-00862-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaul P., Savu A., Nerenberg K.A., et al. Impact of gestational diabetes mellitus and high maternal weight on the development of diabetes, hypertension and cardiovascular disease: a population-level analysis. Diabet Med. 2015;32:164–173. doi: 10.1111/dme.12635. [DOI] [PubMed] [Google Scholar]
- 11.Brewster S., Zinman B., Retnakaran R., Floras J.S. Cardiometabolic Consequences of gestational dysglycemia. J Am Coll Cardiol. 2013;62:677–684. doi: 10.1016/j.jacc.2013.01.080. [DOI] [PubMed] [Google Scholar]
- 12.Gunderson E.P., Sun B., Catov J.M., et al. Gestational diabetes history and glucose tolerance after pregnancy associated with coronary artery Calcium in women during Midlife: the CARDIA study. Circulation. 2021;143:974–987. doi: 10.1161/CIRCULATIONAHA.120.047320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oliveira A.P., Calderon I.M., Costa R.A., Roscani M.G., Magalhães C.G., Borges V.T. Assessment of structural cardiac abnormalities and diastolic function in women with gestational diabetes mellitus. Diabetes Vasc Dis Res. 2015;12:175–180. doi: 10.1177/1479164114563302. [DOI] [PubMed] [Google Scholar]
- 14.Appiah D., Schreiner P.J., Gunderson E.P., et al. Association of gestational diabetes mellitus with left ventricular structure and function: the CARDIA study. Dia Care. 2016;39:400–407. doi: 10.2337/dc15-1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Freire C.M.V., do Carmo Pereira Nunes M., Melo Barbosa M., et al. Gestational diabetes: a Condition of early diastolic abnormalities in young women. J Am Soc Echocardiogr. 2006;19:1251–1256. doi: 10.1016/j.echo.2006.04.021. [DOI] [PubMed] [Google Scholar]
- 16.Reddy K.G., Nair R.N., Sheehan H.M., Hodgson J.M.C.B. Evidence that selective endothelial dysfunction may occur in the absence of angiographic or ultrasound atherosclerosis in patients with risk factors for atherosclerosis. J Am Coll Cardiol. 1994;23:833–843. doi: 10.1016/0735-1097(94)90627-0. [DOI] [PubMed] [Google Scholar]
- 17.Anderson T.J., Gerhard M.D., Meredith I.T., et al. Systemic nature of endothelial dysfunction in atherosclerosis. Am J Cardiol. 1995;75:71B–74B. doi: 10.1016/0002-9149(95)80017-m. [DOI] [PubMed] [Google Scholar]
- 18.Banerjee M., Anderson S.G., Malik R.A., Austin C.E., Cruickshank J.K. Small artery function 2 years postpartum in women with altered glycaemic distributions in their preceding pregnancy. Clin Sci (Lond) 2012;122:53–61. doi: 10.1042/CS20110033. [DOI] [PubMed] [Google Scholar]
- 19.Carpenter M.W. Gestational diabetes, pregnancy hypertension, and late vascular disease. Diabetes Care. 2007;30:S246–S250. doi: 10.2337/dc07-s224. [DOI] [PubMed] [Google Scholar]
- 20.Hannemann M.M., Liddell W.G., Shore A.C., Clark P.M., Tooke J.E. Vascular function in women with previous gestational diabetes mellitus. J Vasc Res. 2002;39:311–319. doi: 10.1159/000065543. [DOI] [PubMed] [Google Scholar]
- 21.Fakhrzadeh H., Alatab S., Sharifi F., et al. Carotid intima media thickness, brachial flow mediated dilation and previous history of gestational diabetes mellitus: Intima media thickness and previous GDM. J Obstet Gynaecol Res. 2012;38:1057–1063. doi: 10.1111/j.1447-0756.2011.01829.x. [DOI] [PubMed] [Google Scholar]
- 22.Anastasiou E., Lekakis J.P., Alevizaki M., et al. Impaired Endothelium-dependent Vasodilatation in women with previous gestational diabetes. Diabetes Care. 1998;21:2111–2115. doi: 10.2337/diacare.21.12.2111. [DOI] [PubMed] [Google Scholar]
- 23.Pleiner J., Mittermayer F., Langenberger H., et al. Impaired vascular nitric oxide bioactivity in women with previous gestational diabetes. Wien Klin Wochenschr. 2007;119:483–489. doi: 10.1007/s00508-007-0838-8. [DOI] [PubMed] [Google Scholar]
- 24.Brewster S., Floras J., Zinman B., Retnakaran R. Endothelial function in women with and without a history of glucose intolerance in pregnancy. J Diabetes Res. 2013;2013:1–9. doi: 10.1155/2013/382670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Catov J.M., Muldoon M.F., Gandley R.E., et al. Maternal vascular lesions in the Placenta predict vascular impairments a decade after delivery. Hypertension. 2022;79:424–434. doi: 10.1161/HYPERTENSIONAHA.121.18394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Catov J.M., Peng Y., Scifres C.M., Parks W.T. Placental pathology measures: can they be rapidly and reliably integrated into large-scale perinatal studies? Placenta. 2015;36:687–692. doi: 10.1016/j.placenta.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 27.Countouris M.E., Villanueva F.S., Berlacher K.L., Cavalcante J.L., Parks W.T., Catov J.M. Association of hypertensive disorders of pregnancy with left ventricular remodeling later in Life. J Am Coll Cardiol. 2021;77:1057–1068. doi: 10.1016/j.jacc.2020.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lang R.M., Badano L.P., Mor-Avi V., et al. Recommendations for cardiac Chamber Quantification by Echocardiography in Adults: an Update from the American Society of Echocardiography and the European association of cardiovascular imaging. J Am Soc Echocardiogr. 2015;28:1–39.e14. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 29.Nagueh S.F., Smiseth O.A., Appleton C.P., et al. Recommendations for the evaluation of left ventricular diastolic function by Echocardiography: an Update from the American Society of Echocardiography and the European association of cardiovascular imaging. J Am Soc Echocardiogr. 2016;29:277–314. doi: 10.1016/j.echo.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 30.Axtell A.L., Gomari F.A., Cooke J.P. Assessing endothelial Vasodilator function with the Endo-PAT 2000. J Vis Exp. 2010:2167. doi: 10.3791/2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heitzer T., Schlinzig T., Krohn K., Meinertz T., Münzel T. Endothelial dysfunction, oxidative stress, and risk of cardiovascular events in patients with coronary artery disease. Circulation. 2001;104:2673–2678. doi: 10.1161/hc4601.099485. [DOI] [PubMed] [Google Scholar]
- 32.Rubinshtein R., Kuvin J.T., Soffler M., et al. Assessment of endothelial function by non-invasive peripheral arterial tonometry predicts late cardiovascular adverse events. Eur Heart J. 2010;31:1142–1148. doi: 10.1093/eurheartj/ehq010. [DOI] [PubMed] [Google Scholar]
- 33.Rovas A., Lukasz A.-H., Vink H., et al. Bedside analysis of the sublingual microvascular glycocalyx in the emergency room and intensive care unit – the GlycoNurse study. Scand J Trauma Resusc Emerg Med. 2018;26:16. doi: 10.1186/s13049-018-0483-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bol M.E., Beurskens D.M.H., Delnoij T.S.R., et al. Variability of microcirculatory measurements in Critically Ill patients. Shock. 2020;54:9–14. doi: 10.1097/SHK.0000000000001470. [DOI] [PubMed] [Google Scholar]
- 35.Weissgerber T.L., Garcia-Valencia O., Milic N.M., et al. Early Onset preeclampsia is associated with glycocalyx Degradation and reduced microvascular perfusion. J Am Heart Assoc. 2019;8 doi: 10.1161/JAHA.118.010647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eickhoff M.K., Winther S.A., Hansen T.W., et al. Assessment of the sublingual microcirculation with the GlycoCheck system: reproducibility and examination conditions. PLoS One. 2020;15 doi: 10.1371/journal.pone.0243737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Valerio L., Peters R.J., Zwinderman A.H., Pinto-Sietsma S.-J. Reproducibility of sublingual microcirculation parameters obtained from sidestream darkfield imaging. PLoS One. 2019;14 doi: 10.1371/journal.pone.0213175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brands J., Hubel C.A., Althouse A., Reis S.E., Pacella J.J. Noninvasive sublingual microvascular imaging reveals sex-specific reduction in glycocalyx barrier properties in patients with coronary artery disease. Physiol Rep. 2020;8 doi: 10.14814/phy2.14351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wadowski P.P., Schörgenhofer C., Rieder T., et al. Microvascular rarefaction in patients with cerebrovascular events. Microvasc Res. 2022;140 doi: 10.1016/j.mvr.2021.104300. [DOI] [PubMed] [Google Scholar]
- 40.Noble M.I.M., Drake-Holland A.J., Vink H. Hypothesis: arterial glycocalyx dysfunction is the first step in the atherothrombotic process. QJM. 2008;101:513–518. doi: 10.1093/qjmed/hcn024. [DOI] [PubMed] [Google Scholar]
- 41.Nieuwdorp M., Mooij H.L., Kroon J., et al. Endothelial glycocalyx damage coincides with microalbuminuria in type 1 diabetes. Diabetes. 2006;55:1127–1132. doi: 10.2337/diabetes.55.04.06.db05-1619. [DOI] [PubMed] [Google Scholar]
- 42.Broekhuizen L.N., Lemkes B.A., Mooij H.L., et al. Effect of sulodexide on endothelial glycocalyx and vascular permeability in patients with type 2 diabetes mellitus. Diabetologia. 2010;53:2646–2655. doi: 10.1007/s00125-010-1910-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nieuwdorp M., Meuwese M.C., Mooij H.L., et al. Measuring endothelial glycocalyx dimensions in humans: a potential novel tool to monitor vascular vulnerability. J Appl Physiol. 2008;104:845–852. doi: 10.1152/japplphysiol.00440.2007. [DOI] [PubMed] [Google Scholar]
- 44.Goedhart P.T., Khalilzada M., Bezemer R., Merza J., Ince C. Sidestream Dark Field (SDF) imaging: a novel stroboscopic LED ring-based imaging modality for clinical assessment of the microcirculation. Opt Express. 2007;15 doi: 10.1364/oe.15.015101. [DOI] [PubMed] [Google Scholar]
- 45.Butler M.J., Down C.J., Foster R.R., Satchell S.C. The Pathological Relevance of increased endothelial glycocalyx permeability. Am J Pathol. 2020;190:742–751. doi: 10.1016/j.ajpath.2019.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pofi R., Giannetta E., Galea N., et al. Diabetic Cardiomiopathy progression is Triggered by miR122-5p and Involves extracellular matrix. JACC Cardiovasc Imaging. 2021;14:1130–1142. doi: 10.1016/j.jcmg.2020.10.009. [DOI] [PubMed] [Google Scholar]
- 47.Stanhewicz A.E., Schlarmann R.L., Brustkern K.M., Jalal D.I. Oxidative stress contributes to reductions in microvascular endothelial- and nitric oxide-dependent dilation in women with a history of gestational diabetes. J Appl Physiol. 2022;133:361–370. doi: 10.1152/japplphysiol.00189.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jensen L.A., Chik C.L., Ryan E.A. Review of gestational diabetes mellitus effects on vascular structure and function. Diabetes Vasc Dis Res. 2016;13:170–182. doi: 10.1177/1479164115624681. [DOI] [PubMed] [Google Scholar]
- 49.McElwain C.J., Tuboly E., McCarthy F.P., McCarthy C.M. Mechanisms of endothelial dysfunction in pre-eclampsia and gestational diabetes mellitus: Windows into future cardiometabolic health? Front Endocrinol. 2020;11:655. doi: 10.3389/fendo.2020.00655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stith B.J., Buls S.M., Keim S.A., et al. Moms in motion: weight loss intervention for postpartum mothers after gestational diabetes: a randomized controlled trial. BMC Pregnancy Childbirth. 2021;21:461. doi: 10.1186/s12884-021-03886-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.