Abstract
Objective:
Offspring of parents with Huntington’s disease (HD) are faced with substantial levels of chronic uncontrollable and unpredictable stress. These stressors may place them at heightened risk of psychological distress and negative effects on executive functioning. This study investigated working memory, secondary control coping strategies (e.g., cognitive reappraisal, acceptance, distraction), and symptoms of anxiety/depression in offspring at-risk for HD.
Method:
Adolescent (ages 10-19) and young adult (ages 20-29) offspring (n = 33) [mean (SD) age = 19.12(6.01) years; 61% female] of parents with HD were recruited in a Huntington Disease Society of America Level 1 Center of Excellence. Participants completed self-report measures of coping and neuropsychiatric symptoms (i.e. anxiety, depression) and a standardized working memory assessment. Pearson correlations and path analyses were used to test associations.
Results:
Participant scores on the working memory assessment were significantly lower compared to normative data, and scores on a mixed anxiety/depression scale revealed a significant elevation compared to normative data. Working memory, secondary control coping, and symptoms of anxiety/depression were significantly correlated. Analyses of the full model revealed the total indirect effect of working memory on anxiety/depression through secondary control coping was significant [β = −0.20].
Conclusions:
Secondary control coping skills are an important factor in understanding the relationship between working memory and symptoms of anxiety/depression in offspring of parents with HD. Future longitudinal research is needed to establish the direction of these associations. Working memory and coping skills represent potential targets for intervention to reduce risk for anxiety/depression in this population.
Keywords: Huntington’s disease, working memory, coping, anxiety and depression
Introduction
Huntington’s disease (HD) is an autosomal dominant neurodegenerative disease caused by an expanded cytosine-adenine-guanine (CAG) repeat on the HTT gene. Each offspring of a parent with HD has a 50% chance of carrying the genetic mutation and all of those who inherit the genetic mutation will eventually develop the disease (The Huntington’s Disease Collaborative Research Group, 1993). Regardless of gene status, offspring are exposed to substantial levels of stress that heighten the risk for neuropsychiatric symptoms, especially anxiety and depression (Keenan et al., 2007; Vamos et al., 2007). Therefore, it is important to understand the skills that offspring need to effectively cope with HD-related stress and the neurocognitive resources that are needed to enact these coping skills. Previous research has identified coping skills that are important for offspring to cope with other types of parental disorders, including parental depression (e.g., Compas et al., 2010), and the importance of executive function skills that are associated with the ability to cope (Grech et al., 2016). However, these processes have not been assessed in offspring of parents with HD, an especially vulnerable population. The current study focused on two factors uniquely important in understanding the impact of stress in offspring of parents with HD— the ability to cope with stress and the proficiency of working memory, a type of executive function.
Offspring of parents with HD are faced with chronic, uncontrollable and unpredictable stress, including observing the onset and progression of HD symptoms in a parent, caring for a parent, experiencing ramifications of neuropsychiatric symptoms of a parent, and realizing their own risk of developing HD (Kavanaugh, 2014; Keenan et al., 2007; Kjoelaas et al., 2020; Maxted et al., 2014). Ultimately, much of this stress demands an ability to dynamically adapt to both static and fluctuating stressors. Coping strategies that focus on the ability to adapt to a problem, rather than trying to change a source of stress, are characterized as secondary control coping and include skills such as acceptance, cognitive reappraisal, positive thinking and distraction (Compas et al., 2017; Connor-Smith et al., 2000). Secondary control coping skills are well suited to manage uncontrollable stress and may be particularly important for coping with HD-related stress (Downing et al., 2012). For example, offspring of parents with HD may reappraise a parent’s disease by focusing on the positive aspects of the time they have together, or identify ways that the disease has brought their family closer together. Prior research has shown that teaching secondary control coping skills as part of a cognitive behavioral intervention can reduce risk for anxiety and depression in adolescents of parents with major depressive disorder (e.g., Compas et al., 2010, 2015), suggesting that learning these skills may be a promising avenue for intervention with offspring of parents with HD.
The use of complex cognitive coping strategies is predicated on intact and efficient executive function including inhibitory control, cognitive flexibility, and working memory (Andreotti et al., 2013; Miyake & Friedman, 2012). For example, working memory skills require holding, updating, and manipulating information in a short time frame (e.g., recalling a sequence of numbers and reporting them in reverse order). Standardized tests of working memory measure the ability to manipulate information held in short term memory under “cold” conditions (i.e., non-emotional conditions). These skills can then be drawn upon to manipulate information (e.g., to think about a stressor in a different way through reappraisal) under conditions of stress and heightened emotional arousal (i.e., under “hot” conditions). For example, cognitive reappraisal, a type of secondary control coping, requires thinking about a stressful situation and simultaneously viewing it from an alternative perspective. Therefore, impairments in working memory may undermine the ability to utilize coping strategies that are most effective in the face of chronic, uncontrollable stress. Further, there is evidence that chronic stress directly impairs executive function skills and may indirectly impair the ability to use secondary control coping skills and other complex cognitive forms of coping (e.g., Liston, McEwen, & Casey, 2009).
Evidence for the association between working memory (and other aspects of executive function), secondary control coping skills, and emotional and behavioral problems has been established in other types of pediatric health populations (e.g., Campbell et al., 2009; Prussien et al., 2018; Robinson et al., 2015). For example, secondary control coping significantly mediated the relationship between a composite of standardized working memory assessment and total problem behaviors in a sample of adolescents with leukemia (Campbell et al., 2009). Similarly, a study using functional neuroimaging in survivors of childhood brain tumors found that increased brain activation in response to a working memory N-back task was associated with greater use of secondary control coping strategies and better psychosocial functioning (Robinson et al., 2015).
There is evidence of impairment in working memory skills in individuals with HD (Lahr et al., 2018), as well as in individuals with disorders of anxiety and depression (Moran, 2016; Snyder, 2013). Recent research has begun to examine brain development and cognitive function in individuals with juvenile HD and adolescent offspring at risk for HD (Lee et al., 2018a; van der Plas et al., 2019). However, no studies to our knowledge have examined processes of coping and working memory in adolescent or young adult offspring of parents with HD. Understanding the possible impairments in working memory skills is especially important in offspring at risk for HD as problems with working memory may undermine the ability to efficiently cope with the stress presented by their parents’ disease. Furthermore, an inability to manage levels of stress may ultimately place these offspring at greater risk for anxiety, depression, and other neuropsychiatric problems.
The present study investigated the associations among working memory, the use of secondary control coping strategies, and symptoms of anxiety and depression in a sample of adolescent and young adult offspring of parents with HD. The primary hypotheses were: (1) Offspring will have reduced working memory proficiency, but higher levels of anxiety and depression compared with normative data. (2) Working memory, secondary control coping skills, and symptoms of anxiety and depression will be related at the bivariate level. Specifically, (2a) working memory skills will be negatively associated with symptoms of anxiety and depression; (2b) working memory skills will be positively associated with secondary control coping skills; (2c) secondary control coping skills will be negatively associated with symptoms of anxiety and depression. (3) Finally, there will be an indirect association between working memory and symptoms of anxiety and depression through secondary control coping. Participants’ age, levels of stress related to their parents’ HD, and disease characteristics of their parents’ HD (i.e., CAG repeat length) were controlled for in supplemental analyses. Given the high levels of stress in this population regardless of their genetic status, the ethical statutes preventing all offspring under the age of 18 from pursuing genetic testing (Huntington’s Disease Society of America, 2009), and the fact that many young adult offspring choose not to be tested, it is important to study the relationship between working memory, coping, and symptoms of anxiety and depression in all offspring of parents with HD regardless of their gene status.
Method
Participants
Participants (n = 33) included 19 adolescent and 14 young adult offspring ages 10 to 29 years [mean (SD) age = 19.12(6.01) years; 61% female]. The majority of the sample identified as White or Caucasian (n = 32) and only one participant identified as Black, or African American. Parents with HD in the sample ranged in age from 32 to 58 years [mean (SD) age = 45.58 (9.24) years; 42% female] and had a mean CAG repeat length of 44.42 (2.57). All parents with HD in the sample identified as White or Caucasian. Although HD can affect individuals of all ethnic groups, it occurs much more frequently among those of European descent (Bates et al., 2015), which is representative of the current sample. Due to the ethical guidelines precluding individuals under age 18 years from undergoing genetic testing, the researchers were blind to the genetic status of all adolescents in the current sample. Of the individuals 18 years and older in the current sample who could pursue genetic testing, the researchers were only aware of the genetic status of a small subset (n = 5).
Procedure
Families were recruited through a Huntington Disease Society of America Level 1 Center of Excellence (COE) between October 2018 and December 2019. Eligibility requirements included (1) participants must use English as their primary language, (2) parents with HD must be part of the Huntington’s Disease COE Clinic, (3) parents with HD may range in disease severity (i.e., pre-symptomatic, prodromal, manifest), (4) parents with HD must be over 30 years of age, and (5) have at least one offspring in the second two decades of life (10-19 [adolescent] or 20-29 [young adult]). Informed consent and assent were obtained from all parents and offspring prior to study enrollment and participation. This study was reviewed and approved by the Institutional Review Board. The Medical Director of the COE oversaw recruitment of eligible families and a member of the clinical team at the COE made the initial study introduction.
Measures
To assess demographics, coping strategies, and levels of neuropsychiatric symptoms in the sample, all participants were asked to complete questionnaire data via REDCap, a secure online database (Harris et al., 2009).
Demographics.
Demographic data for parents and offspring included information on age, sex, ethnic group, and education level. Disease characteristics (i.e. number of CAG repeats on the huntingtin gene) for parents with HD were extracted from medical records.
Stress and coping.
The Responses to Stress Questionnaire-Huntington’s Disease Version (RSQ-HD; Ciriegio et al., 2019; Connor-Smith et al., 2000) is a self-report measure that was used to identify sources of stress and coping strategies in response to stress from being in a family impacted by HD. First participants were provided with a list of 10 representative stressors related to HD (e.g., fear about my future, getting genetically tested, having to take on more caretaking responsibilities, feeling isolated or different from my peers) and asked to indicate if they had experienced any of these stressors in the previous 6 months. This portion of the RSQ serves primarily as a cue to help respondents identify specific sources of stress that will be the focus of the subsequent coping items. A sum of the total number of stressor items endorsed was calculated for each participant. The second portion of the RSQ includes 57-items reflecting coping and automatic responses to stressors, where each item is rated on a 4-point scale. Proportion scores are computed to identify five factors: primary control engagement coping, secondary control engagement coping, disengagement coping, involuntary engagement, and involuntary disengagement. Because of the uncontrollable nature of stress faced by offspring of parents with HD, the current study focused on secondary control coping skills that include acceptance (e.g., I realize I just have to live with things the way they are), cognitive reappraisal (e.g., I think about the things I’m learning from being from a family impacted by HD, or that something good will come from it), positive thinking (e.g., I tell myself that I can get through this, or I will be okay), and distraction (e.g., I keep my mind off of the stressful parts of HD by doing something else). The RSQ has demonstrated excellent internal consistency, test-retest reliability, and convergent and construct validity (Compas et al., 2017). Internal consistency for the secondary control coping scale in the current sample was α = .85.
Anxiety and depression symptoms.
Affective problems were assessed using the Youth Self Report (YSR; Achenbach & Rescorla, 2001) and the Adult Self Report (ASR; Achenbach & Rescorla, 2003) from the Achenbach System of Empirically Based Assessment (ASEBA). The ASEBA instruments are empirically driven, developmentally appropriate tools that assess psychopathology across the lifespan. These instruments have been developed and well validated in individuals ages 1 ½−90+ years, both in the U.S. and in international samples (Achenbach et al., 2017). Offspring less than 18 years completed the YSR and offspring 18 years and older completed the ASR. The YSR and ASR are self-report measures that yield both empirically based syndrome scales and DSM-V oriented scales. These measures have been shown to have excellent internal consistency, test-retest reliability and construct validity. The normative samples for the YSR and ASR are representative of the US population, providing adequate data on levels of emotional and behavioral problems in adolescents and young adults (Achenbach et al., 2002). The current study focused on the Mixed Anxious/Depressed Syndrome Scale as a broad indicator of affective problems and this scale can be calculated in both the YSR and ASR. Ten items are included in both the child/adolescent and adult versions to reflect developmental continuities in some types of symptoms (e.g., sad, unhappy, depressed), while there are also various unique items in the child/adolescent and adult versions (3 and 8 items, respectively) to reflect developmental differences in other symptoms (e.g., afraid of going to school vs. poor relations with the opposite sex). Internal consistency for the Mixed Anxious/Depressed Syndrome Scale in the current sample was α = .84 for the YSR and α = .90 for the ASR.
Working memory skills.
Working memory was assessed in the sample using the List Sorting Working Memory Task, a subtest of the NIH Toolbox Cognition Battery (Gershon et al., 2010; Tulsky et al., 2014). The List Sorting Working Memory Task requires the immediate recall and sequencing of various orally and visually presented stimuli. Pictures of different foods and animals are displayed with accompanying audio recording (e.g., “elephant”), and the participant is asked to recall aloud to the test administrator the items in size order from smallest to biggest, first within a single dimension (either animals or food, 1-List) and then on two dimensions (foods and then animals, 2-List). A task score is calculated by summing the total number of items correctly recalled and sequenced on the 1- and 2-Lists. Raw scores can range from 0-26 and are converted to fully adjusted T-scores (M = 50, SD =10), which compares the individual’s score to the NIH Toolbox nationally representative normative sample while adjusting for age, sex, race, ethnicity, and educational attainment. This task has been shown to be developmentally appropriate and recommended for ages 7-85 years old (Tulsky et al., 2013, 2014). Administration of the NIH Toolbox Cognition Battery was completed electronically on an iPad and conducted by trained research assistants during in-person study visits at the COE.
Statistical Analyses
Working memory and symptoms of anxiety and depression mean T-scores in the current sample were compared to nationally representative normative T-scores using independent sample t-tests. Bivariate associations between working memory, coping, and symptoms of anxiety and depression were identified using Pearson correlational analyses in SPSS (26th edition). Additionally, bivariate associations between working memory, coping, and symptoms of anxiety and depression with offspring age, sex, levels of stress (i.e., total sum of stressors on the RSQ), and a disease characteristic of their parents’ HD (i.e., CAG repeats) were examined. Statistical power analyses indicated that with a sample of n = 33, there was adequate power (β = .80, α = .05) to detect correlations of r > .361. In order to evaluate both the direct association between working memory and symptoms of anxiety and depression and the indirect association between working memory and symptoms of anxiety and depression accounted for by secondary control coping, Model 4 of the PROCESS macro (v3.4) for SPSS was used. PROCESS macro is an Ordinary Least Squares and logistic regression path analysis-modeling tool that describes the total direct (path c, c’) and indirect (path ab) effects through standardized regression coefficients and uses listwise deletion (Hayes, 2017). Current analyses were conducted with a 95% confidence interval for all effects. Offspring age, levels of stress, and CAG repeats of the offspring’s parent with HD were added as covariates in supplementary analyses of direct and indirect associations.
Results
The range, means, and standard deviations of measures are reported in Table 1. In support of the first hypothesis, adolescents and young adults obtained a mean T-score of 46.36 (SD = 9.71) on the NIH Toolbox List Sorting Working Memory Task. This reflects a significant difference in working memory functioning compared to normative data, t(32) = −2.2, p = .04, and represents a small effect (d = 0.36). Also consistent with the first hypothesis, the sample’s mean T score on the Mixed Anxiety and Depression Scale on the YSR and ASR was 57.93 (SD = 7.97) revealing a large effect for elevated neuropsychiatric symptoms compared to normative data, t(29) = 5.4, p < .001, d = 0.79.
Table 1.
Descriptive statistics for measures of offspring working memory, coping, symptoms of anxiety and depression, and levels of stress.
| Range | M | SD | |
|---|---|---|---|
| NIH Toolbox List Sorting Working Memory Task | 19 – 64 | 46.36 | 9.71 |
| Secondary Control Coping | .12 - .37 | .26 | .06 |
| Mixed Anxiety and Depression | 50 – 78 | 57.93 | 7.97 |
| Levels of Stress | 0 – 9 | 2.84 | 2.16 |
Scores for the NIH Toolbox List Sorting Working Memory Task and the Mixed Anxiety and Depression Scale from the YSR/ASR are standardized T-scores (M = 50, SD = 10). Secondary control coping scores as measured on the RSQ-HD are reported in ratio scores. Levels of stress is a sum of the total number of stressor items endorsed on the RSQ-HD (max score = 10)
Support for the second hypothesis was found in bivariate correlation analyses (see Table 2). Working memory, as measured on the List Sorting Working Memory Task, was significantly correlated with secondary control coping on the RSQ (r = .40, p < .05), and secondary control coping was significantly correlated with anxiety/depression on the YSR/ASR (r = −.60, p < .01). Further, there was an association between working memory and symptoms of anxiety/depression (r = −.36, p = .051) that was consistent with the hypothesis and approached significance. Levels of stress, as measured by the total number of stressor items endorsed on the RSQ, was significantly correlated with secondary control coping (r = −.38, p = .03). Parental CAG repeat length was significantly correlated with offspring age (r = −.62, p < .001). Sex of the offspring was not found to be significantly correlated with any measures.
Table 2.
Correlations between offspring age, sex, working memory, coping, symptoms of anxiety and depression, levels of stress, and parental CAG repeat length
| 1. | 2. | 3. | 4. | 5. | 6. | |
|---|---|---|---|---|---|---|
| 1. Age | — | |||||
| 2. Sex | .13 | — | ||||
| 3. NIH Toolbox List Sorting Working Memory Task | .02 | −.27 | — | |||
| 4. Secondary Control Coping | .31 + | .00 | .40 * | — | ||
| 5. Mixed Anxiety and Depression | −.15 | .24 | −.36 + | −.60 ** | — | |
| 6. Levels of Stress | −.02 | .09 | −.12 | −.38 * | .09 | — |
| 7. CAG repeat length in parents with HD | −.62 ** | −.20 | −.06 | −.20 | −.18 | .35 + |
p < 0.01 (2-tailed)
p < 0.05 (2-tailed)
p < 0.10 (2-tailed)
Finally, the third hypothesis was confirmed (see Figure 1). Analyses of the full model using PROCESS revealed the direct association between working memory and anxiety/depression (path c) was negative and approaching significance (β = −.36, p = .051). The direct association between working memory and secondary control coping (path a) was significant (β = .38, p < .05). In the full model, the effect of secondary control coping on anxiety/depression (path b) remained significant (β = −.54, p < .01), while the effect of working memory was no longer significant when coping was included in the model (path c’; β = −.15, n.s.). The total indirect effect of working memory on anxiety/depression through secondary control coping (path ab) was significant [β = −0.20 (SE = 0.09; 95% CI = −0.42 to −0.06)].
Figure 1.
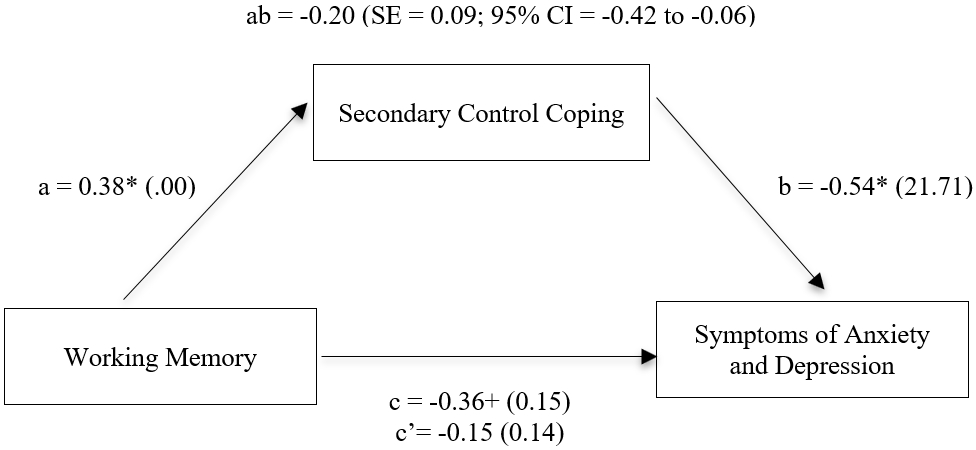
Direct and indirect associations of working memory and symptoms of anxiety and depression through secondary control coping without covariates. Standardized path coefficients with standard errors in the parentheses are given.
** p < 0.01; * p < 0.05; + p < 0.10
Supplemental analyses were conducted in which offspring age, levels of HD-related stress in offspring, and CAG repeat length in parents with HD were included as covariates in the PROCESS analyses. The same pattern of significant paths was found when age of the offspring was included in the model as a covariate [path a, β = .34, p = .05; path b, β = −.58, p = <.05; path c, β = −.35, p = .06; path c’, β = −.15, n.s.; path ab, β = −.20 (SE = 0.10; 95% CI = −0.44 to −0.005)]. Age was a significant predictor of secondary control coping in the model (β = .38, p = .03), but was not associated with symptoms of anxiety and depression. In the model with levels of stress included as a covariate, the total indirect effect of working memory on symptoms of anxiety and depression through secondary control coping remained significant [path a, β = .34, p = .06; path b, β = −.58, p = <.05; path c, β = −.36, p = .06; path c’, β = −.16, n.s.; path ab, β = −.19 (SE = 0.10; 95% CI = −0.43 to −0.02)]. Levels of stress was not a significant predictor of any measures in the full model. Furthermore, when parental CAG repeat length was included in the model as a covariate, the pattern of significant paths once again remained the same [path a, β = .37, p = .05; path b, β = −.61, p = <.05; path c, β = −.29, p = n.s.; path c’, β = −.06, n.s.; path ab, β = −.23 (SE = 0.12; 95% CI = −0.48 to −0.02)].
Discussion
Offspring of parents with HD are faced with myriad of chronic, uncontrollable and unpredictable stressors related to the progression of the disease in a parent, and uncertainty and concerns about their own risk. Previous studies investigating other groups of adolescents and young adults faced with parental or familial illness have found that executive function skills and the skills used to cope with stress are important factors in understanding the association between stress and psychiatric symptoms (Prussien et al., 2018; Reising et al., 2018; Robinson et al., 2015). The current study extended this research to examine the potential role of working memory and secondary control coping skills as factors associated with symptoms of anxiety and depression in adolescents and young adults at risk for HD. Support was found for all three hypotheses. Most importantly, evidence was found for an indirect association of working memory with anxiety and depressive symptoms through secondary control coping.
Consistent with the first hypothesis, this sample of offspring of parents with HD had scored significantly lower than the normative sample on a standardized measure of working memory and significantly higher than the normative sample on a standardized measure of symptoms of anxiety and depression. With regard to working memory, the observed level of problems was small in magnitude (d = .36). This finding is consistent with previous studies that have found variation in levels of cognitive function among gene expanded and gene non-expanded offspring of parents with HD (Lee et al., 2018b). It is noteworthy that the levels of problems in working memory in the current study were found in a sample of offspring of parents with HD that includes both gene positive and gene negative individuals. The small effect may be due to the inclusion of both gene expanded and non-expanded individuals in our sample. Furthermore, with regard to symptoms of anxiety and depression, the observed elevation in the current study was large in magnitude (d = .79). Findings from prior studies of affective symptoms in individuals at risk for and at various stages of HD have been mixed. For example, in a study of at-risk, prodromal, and symptomatic individuals with HD, Chisholm et al. (2012) found that levels of depressive symptoms in individuals at-risk for HD were comparable to a sample of healthy controls (Chisholm et al., 2012). In contrast, the current study used a measure of an empirically-derived syndrome of mixed symptoms of anxiety and depression and compared scores of the offspring of parents with HD with national normative data. The findings from the current study suggest that symptoms of anxiety and depression may be heightened in at-risk offspring of parents with HD as a consequence of the high levels of stress faced by these individuals.
Support was also found for the second hypothesis. Working memory skills were significantly positively associated with secondary control coping. This finding supports the hypothesis that working memory skills may be an important foundation for the ability to utilize complex coping strategies that are most effective in the face of chronic, uncontrollable stress. The current findings also supported the hypothesis that secondary control coping would be negatively associated with symptoms of anxiety/depression. Finally, the direct association between working memory and symptoms of anxiety and depression was negative, as hypothesized, and approached significance (r = −.36, p = .051). These findings indicate that adolescents and young adults who engaged more in the use of acceptance, cognitive reappraisal, and distraction when coping with HD-related stressors had fewer symptoms of anxiety and depression.
In support of the third and primary hypothesis we found a significant indirect association between working memory and symptoms of anxiety and depression through secondary control coping. This suggests that secondary control coping is a unique important factor in understanding the relationship between working memory and symptoms of anxiety and depression in offspring of parents with HD. Furthermore, impairment in working memory may contribute to difficulties in using secondary control coping skills to deal with the stress of HD, which in turn is related to increased levels of anxiety and depression. These findings remained significant in supplemental analyses that included offspring age, levels of HD-related stress, and parental CAG repeat length as covariates. Taken together, these findings indicate two potential targets for cognitive and behavioral interventions that may be beneficial in aiding the psychological well-being of adolescent and young adult offspring of parents with HD: bolstering cognitive executive function skills, and promoting the use of complex cognitive coping strategies such as secondary control coping skills. These targets of intervention may be especially important, as there are currently no available biologic interventions to slow the decline of functioning individuals at-risk for HD.
The current study has several strengths. We employed a direct, performance-based measure of working memory from the NIH Toolbox Cognitive Battery, along with well-validated measures of secondary control coping skills (the RSQ), and anxiety and depression (the YSR/ASR), all with extensive normative data across age, sex, race, ethnicity and education to allow for the interpretation of levels of these symptoms in adolescents and young adults. This sample included a wide age range of offspring of parents with HD expanding across adolescence and young adulthood.
We also note several limitations that can be addressed in future research studies. First, due to the small sample size there was relatively low statistical power that could only detect medium to large effects and limits the generalizability of the findings. Second, because it is cross-sectional, our study could not provide definitive conclusions about the directions of the associations among working memory, secondary control coping, and symptoms of anxiety and depression. A longitudinal design in which working memory is measured at an initial time point, followed by the measurement of coping at a second time point, and symptoms of anxiety/depression at a third follow-up could be used to establish temporal precedence and provide a more rigorous test of mediation. Third, the current findings are based on our full sample, which included both gene positive and gene negative offspring of parents with HD. However, none of the adolescents and only a small portion of the young adults had undergone genetic testing at the time of the study. Future research that includes genetic testing data on offspring of parents with HD will be important to allow for comparison of those individuals with and without genetic mutations. Fourth, we examined an empirically derived syndrome of mixed anxiety/depression; future research could measure these symptoms separately to determine if there are effects that may be unique to anxiety as compared with depression. Fifth, we examined only one aspect of executive function, working memory, and future research should examine other domains of executive functioning including inhibitory control and cognitive flexibility. Finally, randomized controlled trials to test interventions to enhance working memory skills (e.g., Jordan et al., 2019) or coping skills (e.g., Compas et al., 2010) or both (e.g., Bettis et al., 2017) would provide evidence for the role of these skills in reducing risk for neuropsychiatric symptoms in offspring of parents with HD.
In summary, the current study provides evidence for elevated symptoms of anxiety and depression in a sample of offspring of parents with HD. Levels of anxiety and depression were significantly associated with both difficulties in working memory and use of complex cognitive coping skills. Further, we found evidence for an indirect path from working memory skills to the use of secondary control coping strategies, which in turn were related to lower levels of symptoms of anxiety and depression. These findings provide a basis for future longitudinal and intervention research in this population.
Key Points.
Question: How are working memory and coping with stress related to symptoms of anxiety and depression in adolescent and young adult offspring of parents with Huntington’s disease (HD)?
Findings: Support was found for the indirect association of working memory with symptoms of anxiety and depressive through secondary control coping (e.g., acceptance, cognitive reappraisal) in offspring of parents with HD.
Importance: Secondary control coping skills are an important factor in understanding the relationship between working memory and symptoms of anxiety/depression in offspring of parents with HD.
Next Steps: Future research should examine these associations using a longitudinal design and test working memory and skills for coping with stress as two potential targets for intervention that may reduce the risk of symptoms of anxiety and depression in offspring of parents with HD.
References
- Achenbach T, Dumenci L, & Rescorla L (2002). Ten-Year Comparisons of Problems and Competencies for National Samples of Youth: Self, Parent, and Teacher Reports. Journal of Emotional and Behavioral Disorders, 10(4), 194–203. [Google Scholar]
- Achenbach TM, Ivanova MY, & Rescorla LA (2017). Empirically based assessment and taxonomy of psychopathology for ages 1½–90+ years: Developmental, multi-informant, and multicultural findings. In Comprehensive Psychiatry (Vol. 79, pp. 4–18). W.B. Saunders. 10.1016/j.comppsych.2017.03.006 [DOI] [PubMed] [Google Scholar]
- Achenbach T, & Rescorla L (2001). Manual for the ASEBA School-Age Forms and Profiles. University of Vermont, Research Center for Children, Youth & Families. [Google Scholar]
- Achenbach T, & Rescorla L (2003). Manual for the ASEBA Adult Forms and Profiles. University of Vermont, Research Center for Children, Youth & Families. [Google Scholar]
- Andreotti C, Thigpen JE, Dunn MJ, Watson K, Potts J, Reising MM, Robinson KE, Rodriguez EM, Roubinov D, Luecken L, & Compas BE (2013). Cognitive reappraisal and secondary control coping: associations with working memory, positive and negative affect, and symptoms of anxiety/depression. Anxiety, Stress & Coping, 26(1), 20–35. 10.1080/10615806.2011.631526 [DOI] [PubMed] [Google Scholar]
- Bates GP, Dorsey R, Gusella JF, Hayden MR, Kay C, Leavitt BR, Nance M, Ross CA, Scahill RI, Wetzel R, Wild EJ, & Tabrizi SJ (2015). Huntington disease. In Nature Reviews Disease Primers (Vol. 1). Nature Publishing Group. 10.1038/nrdp.2015.5 [DOI] [PubMed] [Google Scholar]
- Bettis AH, Coiro MJ, England J, Murphy LK, Zelkowitz RL, Dejardins L, Eskridge R, Hiber Adery L, Yarboi J, Pardo D, & Compas BE (2017). Comparison of two approaches to prevention of mental health problems in college students: Enhancing coping and executive function skills. Journal of American College Health, 65(5), 313–322. 10.1080/07448481.2017.1312411 [DOI] [PubMed] [Google Scholar]
- Campbell LK, Scaduto M, van Slyke D, Niarhos F, Whitlock JA, & Compas BE (2009). Executive function, coping, and behavior in survivors of childhood acute lymphocytic leukemia. Journal of Pediatric Psychology, 34(3), 317–327. 10.1093/jpepsy/jsn080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisholm LZ, Flavin KT, Paulsen JS, & Ready R (2012). Psychological well-being in persons affected by Huntington’s disease: A comparison of at-risk, prodromal, and symptomatic groups. Journal of Health Psychology, 18(3), 408–418. 10.1177/1359105312444646 [DOI] [PubMed] [Google Scholar]
- Ciriegio A, Hale L, Pfalzer A, McDonell K, Riordan H, Moroz S, Claassen D, & Compas B (2019). Understanding the Effects of Stress in Families Impacted by Huntington’s Disease: Associations of Coping and Psychiatric Symptoms. Annual Huntington Study Group Meeting. [Google Scholar]
- Compas BE, Champion JE, Forehand R, Cole DA, Reeslund KL, Fear J, Hardcastle EJ, Keller G, Rakow A, Garai E, Merchant MJ, & Roberts L (2010). Coping and parenting: Mediators of 12-month outcomes of a family group cognitive-behavioral preventive intervention with families of depressed parents. Journal of Consulting and Clinical Psychology, 78(5), 623–634. 10.1037/a0020459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compas BE, Forehand R, Thigpen J, Hardcastle E, Garai E, McKee L, Keller G, et al. (2015). Efficacy and moderators of a family group cognitive-behavioral preventive intervention for children of parents with depression. Journal of Consulting and Clinical Psychology, 83(3), 541–553. 10.1037/a0039053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compas BE, Jaser SS, Bettis AH, Watson KH, Gruhn MA, Dunbar JP, Williams E, & Thigpen JC (2017). Coping, emotion regulation, and psychopathology in childhood and adolescence: A meta-analysis and narrative review. Psychological Bulletin, 143(9), 939–991. 10.1037/bul0000110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor-Smith JK, Compas BE, Wadsworth ME, Thomsen AH, & Saltzman H (2000). Responses to stress in adolescence: Measurement of coping and involuntary stress responses. Journal of Consulting and Clinical Psychology, 68(6), 976–992. 10.1037/0022-006X.68.6.976 [DOI] [PubMed] [Google Scholar]
- Downing NR, Williams JK, Leserman AL, & Paulsen JS (2012). Couples’ coping in prodromal huntington disease: A mixed methods study. Journal of Genetic Counseling, 21(5), 662–670. 10.1007/s10897-012-9480-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grech LB, Kiropoulos LA, Kirby KM, Butler E, Paine M, & Hester R (2016). Coping Mediates and Moderates the Relationship Between Executive Functions and Psychological Adjustment in Multiple Sclerosis. Neuropsychology, 30(3), 361–376. 10.1037/neu0000256.supp [DOI] [PubMed] [Google Scholar]
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, & Conde JG (2009). Research electronic data capture (REDCap)-A metadata-driven methodology and workflow process for providing translational research informatics support. Journal of Biomedical Informatics, 42(2), 377–381. 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes AF (2017). Introduction to Mediation, Moderation, and Conditional Process Analysis Second Edition. The Guilford Press. [Google Scholar]
- Huntington’s Disease Society of America. (2009). Genetic Testing Huntington’s Disease: Family Guide Series. [Google Scholar]
- Jordan LC, Siciliano RE, Cole DA, Lee CA, Patel NJ, Murphy LK, Markham LK, Prussien KV, Gindville MC, & Compas BE (2019, in press). Cognitive training in children with hypoplastic left heart syndrome: A pilot randomized trial. Progress in Pediatric Cardiology. [Google Scholar]
- Kavanaugh M. (2014). Children and Adolescents Providing Care to a Parent with Huntington’s Disease: Disease Symptoms, Caregiving Tasks and Young Carer Well-Being. Child and Youth Care Forum, 43(6), 675–690. 10.1007/s10566-014-9258-x [DOI] [Google Scholar]
- Keenan KF, Miedzybrodzka Z, van Teijlingen E, McKee L, & Simpson SA (2007). Young people’s experiences of growing up in a family affected by Huntington’s disease. Clinical Genetics, 71(2), 120–129. 10.1111/j.1399-0004.2006.00702.x [DOI] [PubMed] [Google Scholar]
- Kjoelaas S, Tillerås KH, & Feragen KB (2020). The Ripple Effect: A Qualitative Overview of Challenges When Growing Up in Families Affected by Huntington’s Disease. Journal of Huntington’s Disease, 1–13. 10.3233/jhd-190377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahr J, Minkova L, Tabrizi SJ, Stout JC, Klöppel S, & Scheller E (2018). Working Memory-Related Effective Connectivity in Huntington’s Disease Patients. Frontiers in Neurology, 9. 10.3389/fneur.2018.00370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JK, Conrad A, Epping E, Mathews K, Magnotta V, Dawson JD, & Nopoulos P (2018a). Effect of Trinucleotide Repeats in the Huntington’s Gene on Intelligence. EBioMedicine, 31, 47–53. 10.1016/j.ebiom.2018.03.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JK, Conrad A, Epping E, Mathews K, Magnotta V, Dawson JD, & Nopoulos P (2018b). Effect of Trinucleotide Repeats in the Huntington’s Gene on Intelligence. EBioMedicine, 31, 47–53. 10.1016/j.ebiom.2018.03.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liston C, McEwen B, & Casey BJ (2009). Psychosocial stress reversibly disrupts prefrontal processing and attentional control. Proceedings of the National Academy of Sciences of the United States of America, 106(3), 912–917. 10.1073/pnas.0807041106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxted C, Simpson J, & Weatherhead S (2014). An exploration of the experience of Huntington’s disease in family dyads: An interpretative phenomenological analysis. Journal of Genetic Counseling, 23(3), 339–349. 10.1007/s10897-013-9666-3 [DOI] [PubMed] [Google Scholar]
- Miyake A, & Friedman NP (2012). The nature and organization of individual differences in executive functions: Four general conclusions. Current Directions in Psychological Science, 21(1), 8–14. 10.1177/0963721411429458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran TP (2016). Anxiety and Working Memory Capacity: A Meta-Analysis and Narrative Review. Psychological Bulletin, 142(8), 831–864. 10.1037/bul0000051.supp [DOI] [PubMed] [Google Scholar]
- Prussien K. v., DeBaun MR, Yarboi J, Bemis H, McNally C, Williams E, & Compas BE (2018). Cognitive function, coping, and depressive symptoms in children and adolescents with sickle cell disease. Journal of Pediatric Psychology, 43(5), 543–551. 10.1093/jpepsy/jsx141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reising MM, Bettis AH, Dunbar JP, Watson KH, Gruhn M, Hoskinson KR, & Compas BE (2018). Stress, coping, executive function, and brain activation in adolescent offspring of depressed and nondepressed mothers. Child Neuropsychology, 24(5), 638–656. 10.1080/09297049.2017.1307950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson KE, Pearson MM, Cannistraci CJ, Anderson AW, Kuttesch JF, Wymer K, Smith SE, Park S, & Compas BE (2015). Functional neuroimaging of working memory in survivors of childhood brain tumors and healthy children: Associations with coping and psychosocial outcomes. Child Neuropsychology, 21(6), 779–802. 10.1080/09297049.2014.924492 [DOI] [PubMed] [Google Scholar]
- Snyder HR (2013). Major depressive disorder is associated with broad impairments on neuropsychological measures of executive function: A meta-analysis and review. Psychological Bulletin, 139(1), 81–132. 10.1037/a0028727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Huntington’s Disease Collaborative Research Group. (1993). A Novel Gene Containing a Trinucleotide Repeat that is Expanded and Unstable on Huntington’s Disease Chromosomes. Cell, 72, 971–983. [DOI] [PubMed] [Google Scholar]
- Tulsky DS, Carlozzi N, Chiaravalloti ND, Beaumont JL, Kisala PA, Mungas D, Conway K, & Gershon R (2014). NIH Toolbox Cognition Battery (NIHTB-CB): List Sorting Test to Measure Working Memory. Journal of the International Neuropsychological Society, 20(6), 599–610. 10.1017/S135561771400040X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulsky DS, Carlozzi NE, Chevalier N, Espy KA, Beaumont JL, & Mungas D (2013). NIH toolbox cognition battery (CB): Measuring working memory. Monographs of the Society for Research in Child Development, 78(4), 70–87. 10.1111/mono.12035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vamos M, Hambridge J, Edwards M, & Conaghan J (2007). The Impact of Huntington’s Disease on Family Life. Psychosomatics, 48(5), 400–404. [DOI] [PubMed] [Google Scholar]
- van der Plas E, Langbehn DR, Conrad AL, Koscik TR, Tereshchenko A, Epping EA, Magnotta VA, & Nopoulos PC (2019). Abnormal brain development in child and adolescent carriers of mutant huntingtin. Neurology, 93(10), E1021–E1030. 10.1212/WNL.0000000000008066 [DOI] [PMC free article] [PubMed] [Google Scholar]


