Summary
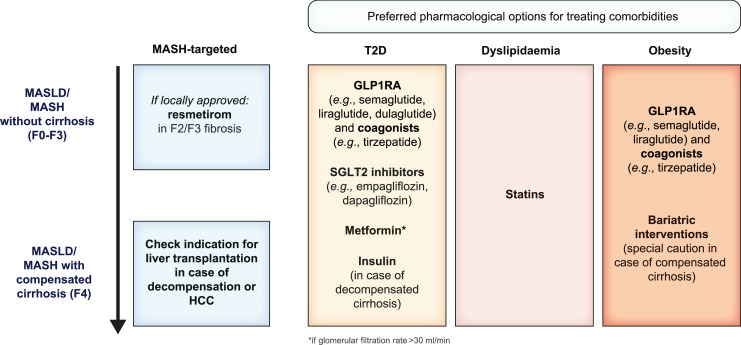
Metabolic dysfunction-associated steatotic liver disease (MASLD), previously termed non-alcoholic fatty liver disease (NAFLD), is defined as steatotic liver disease (SLD) in the presence of one or more cardiometabolic risk factor(s) and the absence of harmful alcohol intake. The spectrum of MASLD includes steatosis, metabolic dysfunction-associated steatohepatitis (MASH, previously NASH), fibrosis, cirrhosis and MASH-related hepatocellular carcinoma (HCC). This joint EASL-EASD-EASO guideline provides an update on definitions, prevention, screening, diagnosis and treatment for MASLD. Case-finding strategies for MASLD with liver fibrosis, using non-invasive tests, should be applied in individuals with cardiometabolic risk factors, abnormal liver enzymes, and/or radiological signs of hepatic steatosis, particularly in the presence of type 2 diabetes (T2D) or obesity with additional metabolic risk factor(s). A stepwise approach using blood-based scores (such as FIB-4) and, sequentially, imaging techniques (such as transient elastography) is suitable to rule-out/in advanced fibrosis, which is predictive of liver-related outcomes. In adults with MASLD, lifestyle modification – including weight loss, dietary changes, physical exercise and discouraging alcohol consumption – as well as optimal management of comorbidities – including use of incretin-based therapies (e.g. semaglutide, tirzepatide) for T2D or obesity, if indicated – is advised. Bariatric surgery is also an option in individuals with MASLD and obesity. If locally approved and dependent on the label, adults with non-cirrhotic MASH and significant liver fibrosis (stage ≥2) should be considered for a MASH-targeted treatment with resmetirom, which demonstrated histological effectiveness on steatohepatitis and fibrosis with an acceptable safety and tolerability profile. No MASH-targeted pharmacotherapy can currently be recommended for the cirrhotic stage. Management of MASH-related cirrhosis includes adaptations of metabolic drugs, nutritional counselling, surveillance for portal hypertension and HCC, as well as liver transplantation in decompensated cirrhosis.
Introduction
Metabolic dysfunction-associated steatotic liver disease (MASLD) has become the most common chronic liver disease, and its prevalence will likely continue to rise. The presence of MASLD is tightly linked to type 2 diabetes (T2D), obesity and other cardiometabolic risk factors. MASLD is associated with an increased risk of cardiovascular events, chronic kidney disease, hepatic and extrahepatic malignancies, and liver-related outcomes, including liver failure and hepatocellular carcinoma (HCC). Therefore, the high socio-economic burden of MASLD poses a global health challenge that needs to be addressed by medical societies and policymakers [1].
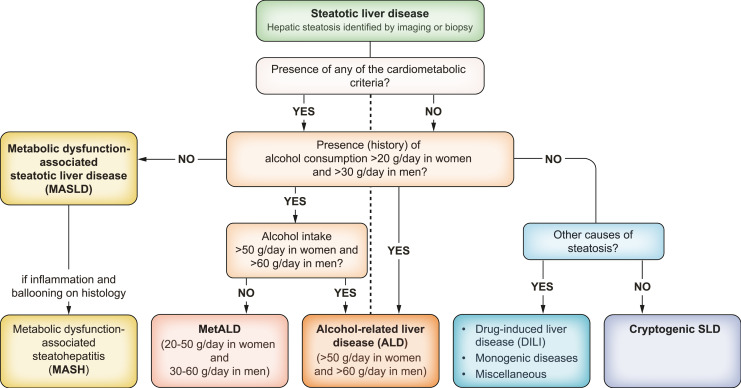
MASLD is defined as the presence of excess triglyceride storage in the liver in the presence of at least one cardiometabolic risk factor. The term MASLD comprises different conditions, including isolated liver steatosis (metabolic dysfunction-associated steatotic liver, MASL), metabolic dysfunction-associated steatohepatitis (MASH), as well as fibrosis and cirrhosis. MASH is characterised by histological features of hepatocellular ballooning and lobular inflammation. MASLD replaces the old term non-alcoholic fatty liver disease (NAFLD) and is embedded in the new consensus definition of steatotic liver disease (SLD). Besides MASLD, SLD also includes MASLD with moderate (increased) alcohol intake (MetALD), alcohol-related liver disease (ALD), specific aetiologies of SLD (e.g. drug-induced, monogenic diseases) and cryptogenic SLD (Fig. 1) [2].
Fig. 1.
Flow-chart for SLD and its sub-categories [2]. SLD, diagnosed histologically or by imaging, has many potential aetiologies. MASLD is defined as the presence of hepatic steatosis in conjunction with (at least) one cardiometabolic risk factor and no other discernible cause. The quantity of alcohol intake, the drinking pattern, and the type of alcohol consumed should be assessed in all individuals with SLD using detailed medical history, psychometric instruments and/or validated biomarkers. ALD, alcohol-related liver disease; DILI, drug-induced liver disease; MASH, metabolic dysfunction-associated steatohepatitis; MASLD, metabolic dysfunction-associated steatotic liver disease; MetALD, MASLD with moderate (increased) alcohol consumption; SLD, steatotic liver disease.
The current Clinical Practice Guidelines (CPGs) for the diagnosis, treatment, and follow-up of individuals with MASLD have been generated as a joint effort by the European Association for the Study of the Liver (EASL), European Association for the Study of Diabetes (EASD) and European Association for the Study of Obesity (EASO). They update the multi-society NAFLD CPG released in 2016 [3].
Intensified research efforts in recent years have significantly expanded our understanding of the pathophysiology and natural course of the disease. This has culminated in improved diagnostic tools and novel therapeutic options, which is reflected in the expanded scope of the current CPG. The availability of improved treatment options underlines the need to identify at-risk individuals with MASLD early, as we now possess the tools to positively influence the course of the disease, which is expected to prevent relevant clinical events.
These CPGs are targeted at health care providers involved in the care of individuals with (or at risk of) MASLD. They provide a framework for the early identification of affected individuals, risk stratification and therapeutic management including non-pharmacological and pharmacological treatment. Furthermore, they provide guidance on the management of end-stage MASLD and MASLD in the setting of advanced liver disease and liver transplantation.
The purpose of this document is to assist physicians, affected and at-risk individuals, healthcare providers and health-policymakers from Europe and worldwide in the decision-making process, by providing evidence-based data, which also takes into consideration the burden of clinical management for the healthcare system. The recommendations are intended to guide clinical practice in circumstances where all possible resources and therapies are available. Thus, users should adapt the recommendations to their local regulations, availability of resources, infrastructure, and cost-benefit strategies.
Preamble
The nomenclature of SLD and definition of MASLD were established in June 2023, following an international, multi-society guided Delphi process [2]. The diagnosis of MASLD requires the presence of at least one cardiometabolic risk factor in an individual with documented steatosis. This has raised concerns as to whether evidence generated under the NAFLD definition would still apply to individuals with MASLD. Several re-examinations from existing cohort studies support that NAFLD-related findings can be fully extrapolated to individuals with MASLD. As an example, analyses of a large tertiary care NAFLD cohort and the population-based Nutrition Examination Survey (NHANESIII) data found a nearly complete overlap between NAFLD and MASLD populations, with 99.8% accordance in the NAFLD cohort, while only 5.3% of individuals with NAFLD in the NHANESIII database did not fulfil the MASLD criteria [4]. In addition, clinical characteristics were almost identical, and non-invasive tests showed equal accuracy and cut-offs for both definitions [4]. Finally, long-term follow-up showed similar mortality rates, with slightly higher mortality in MASLD compared to NAFLD [4]. Therefore, we have transferred the evidence on NAFLD to the MASLD population and use the term MASLD interchangeably. Notably, MetALD represents a distinct entity to which our recommendations and statements generated with the “pure” NAFLD definition may not apply.
Methods
The EASL Governing Board initiated these CPGs in September 2021. A multi-disciplinary committee of experts was selected by EASL, EASD and EASO, whose members were primarily involved in the management of MASLD. The development of these CPGs followed a standard operating procedure set out by EASL and meets the international standards for CPGs set out by the Guidelines International Network [5]. The committee defined the objectives, the key issues and identified the guidelines’ key questions and developed them following the PICO format: P – patient, problem, or population, I – intervention, C – comparison, control or comparator, O – outcome. PICO questions were vetted through a simplified Delphi process by an international 46-member panel, including clinicians, patient representatives, and other stakeholders competent in the field of MASLD beyond the CPG panel and the governing boards of EASL, EASD and EASO. Every PICO question that did not reach >75% agreement in the first round of the Delphi process was revised; the revised questions were then submitted for approval by the Delphi panellists in a second round. Once the final PICO questions had been determined, a systematic review of the literature was conducted on the most important scientific databases (PubMed, Scopus, Embase, Google Scholar) by performing a free-text search. The levels of evidence were developed by applying the Oxford Centre for Evidence-based Medicine system (Table 1) [6]. The strength of recommendations reflects the quality (grade) of underlying evidence (Table 2). The committee members submitted contributions to specific PICO questions, which were integrated into the final document and discussed between the panel members to achieve unanimous consensus for each of the recommendations. The Wilson and Jungner criteria served as a framework for evaluating the appropriateness and effectiveness of screening programs and the implementation of population-based screening initiatives [7]. In cases where the committee determined guidance to be necessary despite a lack of available supporting literature, a recommendation was developed based on expert opinion and consensus.
Table 1.
Grades of recommendation
| Grade | Wording | Criteria |
|---|---|---|
| Strong | Must, shall, should, is recommended Shall not, should not, is not recommended |
Evidence, consistency of studies, risk-benefit ratio, individual preferences, ethical obligations, feasibility |
| Weak or open | Can, may, is suggested May not, is not suggested |
Table 2.
Level of Evidence based on the Oxford Centre for Evidence-based Medicine (adapted)
| Level | Criteria | Simple model for high, intermediate and low evidence |
|---|---|---|
| 1 | Systematic Reviews (SR) (with homogeneity) of randomised controlled trials (RCT) | Further research is unlikely to change our confidence in the estimate of benefit and risk |
| 2 | Randomised controlled trials (RCT) or observational studies with dramatic effects; Systematic Reviews (SR) of lower quality studies (i.e. non-randomised, retrospective) |
|
| 3 | Non-randomised controlled cohort/follow-up study/control arm of randomised trial (systematic review is generally better than an individual study) | Further research (if performed) is likely to have an impact on our confidence in the estimate of benefit and risk and may change the estimate |
| 4 | Case-series, case-control, or historically controlled studies (systematic review is generally better than an individual study) | |
| 5 | Expert opinion (mechanism-based reasoning) | Any estimate of effect is uncertain |
*Level may be graded down based on study quality, imprecision, indirectness (study does not match questions), because of inconsistency between studies, or because the absolute effect size is very small; Level may be graded up if there is a large or very large effect size.
The draft statements and recommendations of the CPG panel were then sent to the Delphi panel for consensus agreement. All suggestions and recommendations reached the threshold of 75% agreement, but some questions, recommendations and statements were adjusted following well-justified comments by the Delphi panel. The process involved multiple rounds of questioning and feedback until a consensus or convergence of opinions was achieved, and the feedback was incorporated into the final consensus recommendations and statements. The strength of consensus was defined according to the percentage of agreement by the Delphi panel members where ≥95% agreement was classified as strong consensus and 75-95% were classified as consensus. Neutral votes were not counted when calculating the consensus.
Definition, Prevalence and Natural Course
Is the presence of steatotic liver in the general population an important factor in identifying individuals at risk for liver-related outcomes, independent of the presence of other hepatotoxic factors?
Recommendations
The incidental finding of steatosis should prompt assessment of the potential aetiology of SLD, alongside tests for the presence of advanced fibrosis, as this could determine the risk of liver-related and/or cardiovascular outcomes and appropriate care (LoE 3, strong recommendation, strong consensus).
MASLD, ALD and MetALD are the most common causes of SLD, but other causes such as drug-induced liver disease and monogenic SLD should be considered, depending on the context (LoE 3, strong recommendation, strong consensus).
General population-based screening for SLD is not advised (LoE 3, strong recommendation, strong consensus).
Statement
While the presence of steatotic liver in the general population is not independently associated with liver-related outcomes, the stage of liver fibrosis and persistently elevated liver enzymes are associated with liver-related outcomes (LoE 3, strong consensus).
Hepatic steatosis is the hallmark of MASLD, defined as the presence of hepatic steatosis in conjunction with at least one cardiometabolic risk factor (Table 3) and no other discernible cause. MASLD and ALD (alcohol intake >50 g/day for females and >60 g/day for males) comprise the most common causes of SLD. A new category, requiring further characterisation, termed MetALD, describes those with MASLD who consume greater amounts of alcohol (20-50 g/day for females and 30-60 g/day for males, respectively), but do not meet the criteria for ALD. Notably, the history of alcohol consumption is an important factor as the current drinking pattern may not necessarily reflect previous drinking behaviour. Importantly, despite sharing the same prevalence of cardiometabolic risk factors, MetALD is associated with a higher risk of all-cause mortality, underpinning MetALD as a distinct subclass of SLD with poorer prognosis [8]. Therefore, diagnostic and treatment recommendations provided for MASLD cannot be extended to the MetALD population. A proposal for the simplified diagnostic work-up of a case of SLD is outlined in Fig. 1.
Table 3.
Cardiometabolic risk factors in the definition of MASLD [2]
| Metabolic risk factor | Adult criteria |
|---|---|
| Overweight or Obesity | Body mass index ≥25 kg/m2 (≥23 kg/m2 in people of Asian ethnicity) |
| Waist circumference • ≥94 cm in men and ≥80 cm in women (Europeans) • ≥90 cm in men and ≥80 cm in women (South Asians and Chinese) • ≥85 cm in men and ≥90 cm in women (Japanese) | |
| Dysglycaemia or type 2 diabetes | Prediabetes: HbA1c 39-47 mmol/mol (5.7-6.4%) or fasting plasma glucose 5.6-6.9 mmol/L (100-125 mg/dl) or 2-h plasma glucose during OGTT 7.8-11 mmol/L (140-199 mg/dl) orType 2 diabetes: HbA1c ≥48 mmol/mol (≥6.5%) or fasting plasma glucose ≥7.0 mmol/L (≥126 mg/dl) or 2-h plasma glucose during OGTT ≥11.1 mmol/L (≥200 mg/dl) orTreatment for type 2 diabetes |
| Plasma triglycerides | ≥1.7 mmol/L (>150 mg/dl) or lipid-lowering treatment |
| HDL-cholesterol | ≤1.0 mmol/L (<39 mg/dl) in men and ≤1.3 mmol/L (<50 mg/dl) in women or lipid-lowering treatment |
| Blood pressure | ≥130/85 mmHg or treatment for hypertension |
HbA1c, glycated haemoglobin; HDL, high-density lipoprotein; OGTT, oral glucose tolerance test.
Other causes of SLD (Table 4) should be considered when the most common risk factors have been excluded (Fig. 1]. Familial aggregation of SLD supports a genetic disease, particularly for early-onset SLD [9]. Individuals with steatosis but without cardiometabolic risk factors and no known cause may have “possible MASLD” – requiring further tests of insulin-resistance – or cryptogenic SLD [2]. The identification of liver steatosis should prompt an assessment for the presence of liver fibrosis through non-invasive tests (see below).
Table 4.
SLD due to aetiologies other than MASLD, MetALD or ALD
| Condition | Clinical/lab/histological findings | Diagnostic criteria |
|---|---|---|
| Hepatitis C virus-associated steatotic liver (genotype 3) | Low triglycerides, HCV genotype 3 | HCV antibody with reflex testing HCV RNA and HCV genotype |
| Drug-induced Liver Disease (DILI) | Mostly microvescicular SLD | Investigate for drug intake: • Corticosteroids • Tamoxifen • Amiodarone • Irinotecan • Methotrexate • Lomitapide • Valproate • 5-Fluorouracil Liver biopsy for confirmation |
| Hypobetalipoproteinaemia | Low triglycerides and cholesterol, fat malabsorption, vitamin A deficiency | ApoB level, genetic testing (APOB, MTTP, PCSK-9, targeted panel sequencing) |
| Lipodystrophy | Accumulation of fat in the visceral area and in the muscle (generically inherited or induced by HAART therapy) | CT scan or MRI, targeted panel sequencing for congenital lipodystrophies, MRI |
| LAL deficiency (Wolman disease, cholesteryl ester storage disease-CESD) | Elevated LDL-C and triglycerides, low HDL-C, hypersplenism, advanced fibrosis in young age, predominately microvesicular steatosis | Enzyme assay, genetic testing (LIPA) |
| Pregnancy associated | HELLP syndrome Acute onset |
Elevated liver enzymes and low platelets, haemolysis, SLD at abdominal ultrasound |
| Wilson disease | Younger age, neuropsychiatric symptoms, low ceruloplasmin | 24-h urine copper excretion; quantitative copper on liver biopsy, genetic testing (ATP7B) |
| Nutrient deficiency/malnutrition | Parenteral nutrition, bypass surgeries, bariatric surgery, anorexia | Nutrient levels |
| Celiac disease | Diarrhoea, iron deficiency, vitamins deficiency | Tissue transglutaminase IgA, duodenal biopsy |
| Endocrine diseases | Hypothyroidism, PCOS, growth hormone (GH) deficiency, panhypopituitarism (primary or secondary) | TSH, fT4, fT3, endocrine testing |
| Other inherited metabolic conditions | Early age and severe onset, absence of triggering factors, systemic involvement, positive history of advanced disease in first degree relatives | Targeted panel sequencing, whole exome sequencing (WES) |
ALD, alcohol-related liver disease; ApoB, apolipoprotein B; fT3, free triiodothyronine; fT4, free thyroxine; HCV, hepatitis C virus; HAART, highly active antiretroviral therapy; HELLP, haemolysis, elevated liver enzymes and low platelets; LAL, lysosomal acid lipase; MASLD, metabolic dysfunction-associated steatotic liver disease; PCOS, polycystic ovary syndrome; SLD, steatotic liver disease; TSH, thyroid-stimulating hormone.
As in any other liver diseases, multiple aetiologies of steatosis can coexist in the same individual and are likely associated with variations in natural history and therapeutic responses. Notably, MASLD may accelerate the progression of liver disease in individuals with ALD and chronic hepatitis B, and synergistically induce cirrhosis or HCC development [8, 10, 11].
The estimated global prevalence of MASLD in the general population has risen from 25% in 2016 [12] to currently more than 30%, and the incidence is continually increasing [12–14]. It has been estimated that approximately 10–30% of persons with isolated steatosis progress to steatohepatitis and advanced liver disease, but the risk is much higher in the presence of T2D (42-65% have steatosis) [15, 16]. Still, in a Swedish cohort study, the cumulative incidence of major adverse liver outcomes over ten years in individuals with T2D increased with the number of components of the metabolic syndrome, but was still <2% in those presenting with all 5 components [17]. Of note, the presence of steatosis in the general population is not associated with a clinically meaningful increase in the risk of liver-related outcomes, which strongly argues against population-based screening for SLD. In a Swedish population-based cohort of 10,568 adults with biopsy-confirmed MASLD and 49,925 matched general population comparators, mortality rates from cirrhosis and HCC were modestly elevated in simple steatosis (absolute rate differences, 1.2 and 0.7/1,000 person-years [PY], respectively), but these rates increased progressively in MASH without fibrosis (3.0 and 1.3/1,000 PY, respectively), non-cirrhotic fibrosis (5.5 and 2.5/1,000 PY, respectively) and cirrhosis (22.3 and 5.5/1,000 PY, respectively) [18]. Furthermore, no suitable tests for population-based screening for SLD are currently available and the presence of steatosis per se would not necessarily prompt treatment for liver disease.
Elevation of liver enzymes, namely aminotransferases, is associated with increased liver-related mortality. Notably, lower thresholds than the ones currently in place have been proposed [19–21]. Thus, an individual is considered to have elevated liver enzymes when alanine aminotransferase (ALT) is >33 U/L in males and >25 U/L in females. However, individuals with MASLD and normal aminotransferase levels can still have significant steatohepatitis and develop advanced fibrosis or cirrhosis [22] and the risk of liver-related outcomes, i.e. mortality, hospitalisation, and HCC is increased with worsening of liver fibrosis [23].
While MASLD is not associated with liver-related outcomes in the general population, it may be associated with an increased risk of extrahepatic outcomes (hazard ratio [HR] 1.34) [24], and the risk increases with the number of cardiometabolic risk factors [15]. Although some studies indicated a higher risk of cardiovascular disease-related mortality (HR 1.30) [25, 26], others did not confirm this result in the general population, but only in those with biopsy-proven MASH [24] or other risk factors like T2D [15]. Normal-weight individuals with SLD have a higher mortality risk despite having a lower incidence of cirrhosis and T2D, while the incidence of cardiovascular disease and cancer is similar [27]. Overall, individuals with MASLD have a higher risk of non-fatal cardiovascular disease (HR 1.40) [25], coronary heart disease (odds ratio [OR] 1.33)[28], heart failure (OR 1.5) [29], chronic kidney disease (HR 1.43), T2D and diabetes-related peripheral polyneuropathy (HRs 2.19 and 2.48, respectively) and obstructive sleep apnoea (OSA, HR 2.22) [26].
In the general population, MASLD is not associated with increased overall cancer-related mortality [24], but it is associated with higher risk of HCC and certain extrahepatic cancers, mostly thyroid and gastrointestinal [30].
Which risk factors and comorbidities have the greatest impact on the natural history of the hepatic disease including hepatocellular carcinoma in MASLD?
Statements
Type 2 diabetes and obesity (particularly abdominal obesity) are the metabolic diseases with the strongest impact on the natural history of MASLD, including progression to MASLD/MASH-related advanced fibrosis, cirrhosis and hepatocellular carcinoma (LoE 2, strong consensus).
Males aged >50 years, postmenopausal women, and individuals with multiple cardiometabolic risk factors are at increased risk of progressive fibrosis and the development of cirrhosis and its complications (LoE 2, strong consensus).
Compared to matched control populations, individuals with MASLD have increased all-cause mortality of 17.05/1,000 PY (range 10.31–28.05). In individuals with MASH, liver-related mortality is as high as 25.6/1,000 PY (range, 6.3–103.8) [31], with fibrosis stage being the strongest predictor for liver-related mortality and HCC risk in biopsy-proven MASLD [32, 33]. Fibrosis progression in turn is mostly influenced by older age (though this may be more related to duration of exposure), post-menopausal state, Hispanic ethnicity, the presence and severity of cardiometabolic risk factors, as well as environmental and genetic factors [34]. While the strongest modifiable factor (alcohol) and genetic background are discussed later, this section will focus on the relative impact of cardiometabolic comorbidities on the progression to cirrhosis and its complications (ascites, hepatic encephalopathy, oesophageal varices bleeding) including HCC.
MASLD is closely linked to and often precedes the development of cardiometabolic risk factors, in particular T2D [33]. Conversely, having several cardiometabolic risk factors confers a greater risk of progressive liver damage and major adverse liver outcomes [17, 35]. Age (>50 years), insulin resistance and multiple cardiometabolic risk factors all increase the probability of MASH, severe fibrosis/cirrhosis and both overall and liver-related mortality [36]. Nevertheless, the relative impact of each cardiometabolic risk factor is not the same: obesity and particularly T2D are the most important determinants of the risk of cirrhosis and HCC. Furthermore, MASLD is impacted by socioeconomic inequities, which are related to greater obesity prevalence, lower nutritional quality and lower physical activity [37–39].
Obesity
The presence, duration and severity of obesity are associated with an increased risk of disease progression in MASLD. According to the WHO, body mass index (BMI) cut-offs of 25-29.9 kg/m2 and ≥30 kg/m2 define overweight and obesity, respectively, in non-Asians [40], while BMI cut-offs are lower for Asians (23-24.9 kg/m2 for overweight and ≥25 kg/m2 for obesity) [41] (see Table 3]. Visceral fat distribution, i.e. abdominal obesity, mediates the majority of the cardiometabolic risk [42]. Waist circumference is a crude index of abdominal obesity and visceral fat accumulation, although the definition of cut-offs is sex- and population-dependent [42]. The current cut-offs of ≥94 cm in men and ≥80 cm in women for Caucasians (and adjusted for other ethnicities) are associated with an increased cardiometabolic risk [40, 42] and increased risk for MASLD.
Prospective studies with paired liver biopsies showed that weight gain of >5 kg, higher insulin resistance and more pronounced hepatic steatosis during follow-up were associated with the progression of fibrosis [43]. In the Million Women Study, the adjusted relative risk of cirrhosis increased by 1.3 for each 5-unit rise in BMI of women followed-up for 6.2 years [44].
Overweight or obesity in individuals with compensated cirrhosis at baseline are associated with a higher risk of clinical decompensation, independently of liver function, portal pressure and underlying aetiology of liver disease [45]. Furthermore, obesity is associated with a significantly increased risk of HCC development and HCC-related mortality [46]. This association was found in persons with cryptogenic cirrhosis and alcohol-related cirrhosis but not in individuals with liver diseases of other aetiologies [47]. The early onset of obesity has a meaningful impact on the development of HCC, as shown in a case-control study comparing 622 individuals with newly diagnosed HCC and 660 healthy controls, where obesity in early adulthood was associated with HCC development [48].
Type 2 Diabetes Mellitus (T2D)
The presence and duration of T2D is the major determinant of fibrosis progression and HCC development in people with MASLD [49]. MASLD is highly prevalent in individuals with T2D [50], and T2D is associated with an increased risk of liver fibrosis, assessed by vibration-controlled transient elastography (VCTE) and/or magnetic resonance (MR)-based techniques [51], or with the prevalence of advanced (F3-F4) fibrosis on histology in biopsy-proven MASLD/MASH, ranging from 30 to 38% [49, 52]. Furthermore, in a study on 447 adult participants with MASLD and paired liver biopsies >1 year apart, individuals with T2D had a significantly higher cumulative incidence of fibrosis progression at 4 years (24% vs. 20%), 8 years (60% vs. 50%), and 12 years (93% vs. 76%) (p <0.005), with an adjusted HR of 1.69 (95% confidence interval [CI], 1.17–2.43; p <0.005) on multivariate analysis [53].
In addition, T2D is also associated with poor outcomes in individuals with biopsy-proven MASH and compensated cirrhosis, including a 4-fold increased risk of death and an approximately 2-fold increased risk of liver-related outcomes, including HCC, over a median follow-up of 5 years [54]. Another study reported a 4-fold increased risk of HCC in individuals with T2D and MASH-related cirrhosis followed for 47 months [55]. T2D was also found to be the strongest independent risk factor for the development of HCC in a large European population-based study that included 136,703 individuals with MASLD and a low prevalence of advanced fibrosis as assessed by FIB-4 [56]. Furthermore, the HR for HCC significantly increased from 1.07 in the absence of T2D to 8.36 in the presence of T2D [57].
Different subtypes/endotypes/clusters of T2D are associated with different risks of disease progression [58, 59], with particularly high risk for MASLD/MASH progression in those individuals with severe insulin resistance [60]. Simple clinical variables can be used to determine diabetes endotypes and are available at https://diabetescalculator.ddz.de/diabetescluster/. Such pathophysiological heterogeneity can already be observed in individuals before the onset of T2D [61]. Of note, the effect of T2D on HCC risk is not unique to MASLD but also extends to other aetiologies.
Hypertension and Dyslipidaemia
Individuals with MASLD have a high rate of dyslipidaemia as well as hypertension [33]. Hypertension per se has been associated with fibrosis progression in a large meta-analysis [62] and in a large retrospective study of 271,906 individuals with MASLD; those with both hypertension and dyslipidaemia had a 1.8-fold higher risk of progression to cirrhosis or HCC compared to individuals with no cardiometabolic risk factor [57].
Impact of Multiple Cardiometabolic Risk Factors
The risk of disease progression and HCC clearly increases in the presence of multiple metabolic risk factors. In a large US cohort, individuals with only one cardiometabolic risk factor (e.g., hypertension, dyslipidaemia, or obesity) had a low risk of progression to cirrhosis or HCC, but each additional metabolic trait led to a stepwise increase in this risk, with T2D having the strongest association [57]. In a population-based study [63], the cumulative incidence of moderate-to-advanced liver fibrosis estimated by VCTE was 2.8% and 1.9%, respectively, over a median period of around 4 years. This incidence was higher in MASLD (7.1% for liver stiffness measurement [LSM] ≥8.0 kPa and 5% for LSM ≥9.2 kPa) and dysglycaemia (6.2% for LSM ≥8.0 kPa and 4.7% for LSM ≥9.2 kPa) subgroups. In the global cohort, dysglycaemia, abdominal obesity and atherogenic dyslipidaemia were independent determinants of progression to moderate-to-advanced liver fibrosis.
Obstructive Sleep Apnoea (OSA) and Polycystic Ovary Syndrome (PCOS)
Both OSA and PCOS are associated with MASLD, and several studies suggest OSA is also associated with more advanced MASLD/MASH histology [64, 65], while only one study reported an association between PCOS and MASH severity or advanced fibrosis [66]. However, the available evidence does not support a strong effect of OSA and PCOS on the risk of liver disease progression or HCC.
Menopausal Status
Menopausal status is associated with approximately 2.4-fold higher odds of MASLD [67]. Women aged >50 years have increased odds of advanced fibrosis due to MASLD even after adjustment for covariates [68]. The risk of severe fibrosis is elevated even in normal-weight post-menopausal women with MASLD compared to normal-weight pre-menopausal women with MASLD [69]. The association of menopause with severe fibrosis is, in part, mediated by older age and change in body fat composition.
Ethnicity
In the US, the prevalence of steatohepatitis with or without T2D is highest in the Hispanic population [70]. It is inherently difficult to dissect the impact of genetic, cultural, socioeconomic and ethnic factors on MASLD progression. However, a meta-analysis of 34 studies reported that the prevalence and severity of MASLD differs among ethnic groups in the US [71].
Smoking
Smoking has been associated with an increased risk of HCC independent of aetiology [72] as well as in MASLD specifically [73]. In a meta-analysis of 81 studies, the pooled OR for HCC development was 1.55 (95% CI: 1.46 to 1.65) in current smokers and 1.39 (95% CI: 1.26 to 1.52) in former smokers [74]. In addition, the overall adverse health effects further support smoking cessation in individuals with MASLD.
Does any alcohol consumption in adults with non-cirrhotic or cirrhotic MASLD have an adverse effect on the natural course of liver disease?
Statements
Accumulating evidence shows that alcohol consumption and metabolic risk factors have modifying effects on the onset and progression of chronic liver disease which are independent and can be synergistic (LoE 2, strong consensus).
The presumed beneficial health effects of moderate alcohol consumption are inconsistent across studies and emerging evidence does not support a protective effect of light to moderate amounts of alcohol, particularly in individuals with cardiometabolic risk factors (LoE 3, strong consensus).
Recommendations
The amount, pattern and history of alcohol intake should be documented in all individuals with SLD (LoE 3, strong recommendation, strong consensus).
Alcohol intake may be qualitatively and quantitatively assessed by validated instruments and/or specific biomarkers in individuals with SLD (Table 5) (LoE 3, open recommendation, strong consensus).
Individuals with SLD, particularly those with moderate or high alcohol intake, should be discouraged from consuming alcohol (LoE 3, strong recommendation, consensus).
All alcohol consumption should be stopped completely and permanently in individuals with advanced fibrosis or cirrhosis (LoE 3, strong recommendation, strong consensus).
Table 5.
Tools to quantify alcohol consumption and identify alcohol use disorders [75]
| Psychometric instruments | Biomarkers |
|---|---|
|
Indirect alcohol markers:
|
AST/ALT, aspartate/alanine aminotransferase; CDT, carbohydrate-deficient transferrin; GGT, gamma-glutamyltransferase; MCV, mean corpuscular volume.
The consequences of alcohol consumption in people with MASLD are multidimensional, including considerations regarding liver-related events, overall mortality, cancer occurrence and cardiovascular outcomes, in particular coronary artery disease. Most studies are fraught with uncertainties around the amounts of alcohol consumed [76, 77]. While earlier studies found that any level of alcohol consumption is deleterious for overall health [78], particularly because of increased cancer incidence, more recent analyses from the Global Burden of Disease Study have nuanced that interpretation [79]. The impact of alcohol consumption depends on background disease rates, which vary by region, age, sex, and year. In young adults, thresholds of healthy alcohol consumption are close to zero. In older populations facing a high burden of cardiovascular disease [79], small amounts of alcohol consumption are associated with improved health outcomes [79, 80]. Even if the validity of the J-shaped relative risk curve has been debated [81, 82], these findings suggest that the population-average risk is a synthesis of risks for diverse health outcomes (e.g. heart disease, cancer, injuries), which have differently shaped risk relationships with alcohol consumption, and are more or less relevant to different sociodemographic groups [83]. Moreover, patterns of drinking are an important consideration since irregular heavy drinking and binge drinking might offset protective effects, in particular for coronary artery disease [82], and have been shown to be an independent risk factor for liver-specific outcomes [8].
Equally important may be the possible synergy between alcohol consumption and the presence of metabolic risk factors for liver disease progression [76, 84], with the strongest effect for central obesity [85]. This corroborates older findings of BMI as an independent risk factor for fibrosis in individuals with ALD [86]. After adjustment for different confounders, the increased risk of liver-related mortality in overweight or obese men starts at 15 drinks per week (roughly 30 g of alcohol per day)[87]. While this supra-additive effect has been well documented at the general population or cohort level, at the individual level the relative contribution of alcohol vs. metabolic risk factors cannot currently be predicted. This is a clear limitation when issuing general recommendations for safe levels of alcohol consumption in individuals with MASLD.
In people with non-cirrhotic MASLD the evidence for low or moderate alcohol consumption is conflicting. Earlier cohort and cross-sectional studies on individuals with non-cirrhotic MASLD showed no effect or even protective effects of low-moderate alcohol consumption on overall mortality, MASLD and steatohepatitis [88–91]. However, this has been challenged by emerging data from longitudinal studies [92]. Hence, an emerging body of evidence now suggests that any level of alcohol consumption, even within recommended limits, is associated with worsening of liver outcomes in MASLD and that moderate levels of alcohol are associated with a doubling of incident liver disease [93]. Recent meta-analyses found no protective effects against cirrhosis at any level of drinking when compared to long-term abstainers [94]. In women, cirrhosis risk increases with moderate alcohol consumption (starting at one to two drinks per day) and is higher when alcohol is consumed daily [95] and outside meals [96]. However, in men, there is some evidence for a threshold effect at higher daily levels, although precise estimates of this threshold are not available [94]. Alcohol consumption may also increase the risk of HCC in persons with obesity, with a synergistic interaction even after adjustment for multiple carcinogenic confounders [97]. The interaction of alcohol with metabolic risk factors increases the risk of HCC [98]. Finally, the evidence for cardiovascular protection is conflicting in individuals with MASLD [99], with a documented lack of protection towards subclinical atherosclerotic markers or lesions [100].
Very few data are available specifically for MASLD-related cirrhosis. In a large series of individuals with alcohol-related cirrhosis, even comparatively low levels of consumption (1-6 glasses per week) were associated with reduced overall survival and increased occurrence of hepatic decompensation, thus supporting total abstinence in individuals with compensated cirrhosis [101]. In a retrospective longitudinal study of 195 individuals with MASH-related cirrhosis, alcohol consumption was an independent predictor of HCC occurrence [102]. Any level of alcohol consumption, including social drinking, was associated with an increased risk of HCC development vs. abstinence [102]. Conversely, obesity increases the risk of HCC in individuals with alcohol-related cirrhosis [47]. Therefore, we recommend discouraging alcohol consumption in all individuals with SLD, particularly in those with moderate (4-7 drinks per week for women or 4-14 drinks per week for men) and high (>7 drinks per week for women, >14 drinks per week for men) alcohol consumption.
Prevention
In the general population or high-risk groups, can non-pharmacological measures be recommended to prevent the development of MASLD and its adverse complications, including hepatocellular carcinoma?
Recommendation
In the general population, non-pharmacological measures should be recommended to prevent the development of MASLD and its complications, including hepatocellular carcinoma, and preventive measures should be reinforced in high-risk groups (LoE 3, strong recommendation, strong consensus).
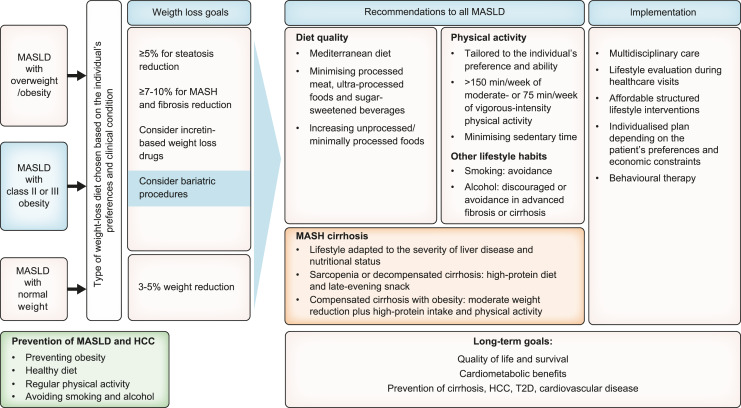
As outlined above, obesity and T2D are strong risk factors for the development and progression of MASLD and are associated with liver-related outcomes including HCC. In addition, an unhealthy diet and a sedentary lifestyle increase the risk of MASLD. Therefore, there is substantial potential to prevent MASLD through lifestyle interventions. Observational prospective studies show an inverse association of MASLD development with the Mediterranean diet or similar healthy dietary patterns [103, 104], and a direct association with unhealthy patterns [105]. Moreover, improved diet quality (see below) has been associated with a lower risk of new-onset MASLD [103]. Prospective studies have also shown that better adherence to healthy eating patterns may decrease the risk of developing HCC [106–109]. With regard to specific nutrients or foods, prospective studies showed that sugar-sweetened beverages and red meat consumption (unprocessed and processed) were associated with an increased risk of developing MASLD (in a dose-dependent manner), [110, 111], liver cancer and liver disease-related mortality [112, 113].
There is evidence that smoking is related to MASLD, liver fibrosis and liver cancer [114]. Furthermore, physical activity is related to reduced risk of MASLD [115], HCC and liver-related mortality [116]. Altogether, a healthy lifestyle has been associated with a reduced risk of HCC [117, 118].
The high availability of low-cost, ultra-processed, high-sugar food and drinks, and the marketing thereof, have been identified as important factors in promoting obesity and associated diseases, including MASLD. Recognising the potential of public policies to prevent obesity and MASLD, we would like to echo the recommendations laid out by the EASL-Lancet Liver Commission in 2021 [1]. Public measures may include a unified European approach in regulating unhealthy food and beverage marketing, subsidising the availability of high-quality healthy food, public educational programmes to increase food literacy and health awareness, and the promotion of industry-led food reformulations [1]. Particularly front-of-package nutrition labelling may help enable people to make conscious food choices and judge the quality of food [119].
Screening, Case-Finding, Diagnosis and Monitoring
Should a policy of screening for MASLD at risk of fibrotic disease (or fibrosis progression) in primary care or at the non-hepatology specialist level be implemented in the general population or only in individuals with cardiometabolic risk factors?
Which at-risk individuals should undergo case-finding for MASLD at risk of fibrotic disease (or fibrosis progression) in the primary care (or other specialty) setting to reduce hepatic complications of MASLD?
Recommendations
Healthcare providers may consider case-finding strategies for MASLD with liver fibrosis in individuals with cardiometabolic risk factors (Table 3], abnormal liver enzymes, and/or radiological signs of hepatic steatosis (LoE 3, weak recommendation, consensus).
Healthcare providers should look for MASLD with liver fibrosis either in individuals with (A) type 2 diabetes or (B) abdominal obesity and ≥1 additional metabolic risk factor(s) (Table 3] or (C) abnormal liver function tests (LoE 3, strong recommendation, consensus).
Statement
Early diagnosis of fibrosis and subsequent appropriate management can potentially prevent progression to cirrhosis and its complications and may justify screening in these populations at risk (LoE 3, strong consensus).
In deciding whether a medical condition warrants screening, the Wilson and Jungner criteria are often applied [7]. While there is no doubt that MASLD is highly prevalent [120], the absolute risk of liver-related events from MASLD in the general population is very low [121]. So far, no randomised-controlled trial (RCT) has demonstrated that MASLD screening improves clinical outcomes in either the general population or hospital setting.
The natural history of MASLD is relatively well-defined, though there is substantial individual variability in disease trajectories [120]. It takes decades for MASLD to progress to cirrhosis and hepatic decompensation [62]. The risk of future liver-related events starts to increase at fibrosis stage 2 [122, 123]. Although HCC may develop in non-cirrhotic MASLD, cirrhosis remains the key risk factor for HCC [124]. If one can prevent MASLD from progressing to cirrhosis, theoretically most liver-related events can be prevented.
Various professional societies have recommended clinical care pathways with an emphasis on the use of non-invasive tests (NITs) and the liaison between primary care/non-hepatology and hepatology settings [125–128]. These pathways all start with simple fibrosis scores comprising routine clinical and laboratory parameters and should be practical in most settings. These tests should be acceptable to most individuals and can be performed repeatedly at an affordable cost. A recent RCT at general medical and diabetes clinics showed that automated fibrosis score calculation followed by reminder messages in the electronic health system could increase the referral of individuals with increased fibrosis scores to hepatologists for specific fibrosis testing (from 3% to 33%) [129]. In individuals in whom life expectancy is determined by extrahepatic factors (e.g. advanced age, malignancies, advanced cardiovascular disease), case-finding strategies for MASLD with fibrosis are not recommended.
One key gap in screening or case-finding is how the diagnosis may change disease management and improve clinical outcomes. One may argue that regardless of the diagnosis of MASLD, healthcare providers should advocate lifestyle changes in persons with metabolic risk factors. However, in the minority who are diagnosed with cirrhosis, surveillance for HCC and varices may potentially improve outcomes. The introduction of specific drug treatments for MASH, if able to reduce progression to cirrhosis and/or prevent liver-related outcomes, will further tip the balance in favour of case-finding.
There have been positive cost-effectiveness studies on MASLD or fibrosis screening in the general population [130], and among individuals diagnosed with SLD [131], metabolic syndrome [132], and T2D [133]. However, one study modelling the primary care setting suggests that screening with the NAFLD fibrosis score (NFS) is not cost-effective [134]. Likewise, another study suggests that screening by abdominal ultrasound followed by liver biopsy is not cost-effective in individuals with T2D [135], though this approach deviates from usual practice. Importantly, most evidence was accrued in the US healthcare system and results on cost-effectiveness may not easily be extrapolated to healthcare systems in European countries.
Finally, there is initial evidence that first-degree relatives of individuals with advanced liver fibrosis due to MASLD are at increased risk of both MASLD (2- to 3-fold higher) and advanced liver fibrosis (∼12-fold higher), independently of metabolic risk factors [9, 136].
In the adult population with MASLD, are selected non-invasive scores and imaging modalities more useful than liver enzyme testing for the detection of MASLD with fibrosis?
In adults with MASLD or at-risk individuals, are clinical care pathways based on the sequential application of non-invasive scores and imaging cost-effective for the identification and management of individuals with MASLD at risk of fibrotic disease (or of fibrosis progression) compared to referral based on physician’s discretion?
Recommendations
In adults with MASLD, non-invasive scores based on combinations of blood tests or combinations of blood tests with imaging techniques measuring mechanical properties and/or hepatic fat content should be used for the detection of fibrosis since their diagnostic accuracy is higher than standard liver enzyme testing (alanine [ALT] and aspartate aminotransferase [AST]) (LoE 2, strong recommendation, strong consensus).
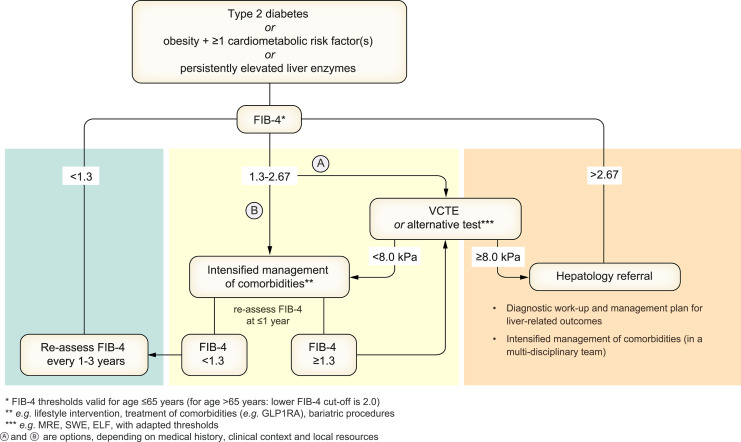
In adults with MASLD, a multi-step approach is recommended (detailed in Fig. 2 and below): First, an established non-patented blood-based score, such as FIB-4, should be used. Thereafter, established imaging techniques, such as liver elastography, are recommended as a second step to further clarify the fibrosis stage if fibrosis is still suspected or in high-risk groups (LoE 2, strong recommendation, strong consensus).
Tests of specific collagen-related blood constituents (e.g. ELF) may serve as an alternative to imaging to identify advanced liver fibrosis (LoE 2, open recommendation, consensus).
Clinical care pathways may be adopted based on the sequential application of non-invasive scores and imaging tests in adults with MASLD or at-risk individuals, recognising that most adults with MASLD are seen in non-hepatology settings (LoE 2, weak recommendation, strong consensus).
Fig. 2.
Proposed strategy for non-invasive assessment of the risk for advanced fibrosis and liver-related outcomes in individuals with metabolic risk factors or signs of SLD. Individuals with (A) T2D or (B) abdominal obesity and ≥1 additional cardiometabolic risk factor(s) or (C) persistently elevated liver enzymes should undergo a multi-step diagnostic process, as indicated in the figure, to identify individuals with MASLD and advanced fibrosis. The algorithm can also be applied in case of incident finding of steatosis. This strategy is intended to identify individuals at risk of developing liver-related outcomes. ELF, enhanced liver fibrosis; FIB-4, fibrosis-4 index; GLP1RA, glucagon-like peptide-1 receptor agonist; MRE, magnetic resonance elastography; SLD, steatotic liver disease; SWE, shear wave elastography; VCTE, vibration-controlled transient elastography.
Non-invasive methods for determining the grade of fibrosis are mainly based on the examination of blood components or on imaging methods that mostly reflect mechanical tissue properties. Importantly, these methods lack power in the general population and therefore are only useful in selected cohorts of individuals with MASLD. Furthermore, test performance is highly dependent on the background prevalence of the condition to be tested. Therefore, as most tests were developed and validated in a referral centre setting, they should only be used in a primary care setting when specifically validated for this purpose.
It has been shown that a combination of values from blood tests and anthropometric data enables a better prediction of fibrosis than single liver enzyme values (ALT and/or AST). The following scores have been described in the literature and were tested in several studies for their predictive power for fibrosis:
FIB-4 (Fibrosis-4 index) = age x AST/(platelet count x √(ALT)) (age in years, ALT and AST in U/L, and platelet count in 109/L) [137].
APRI (AST to platelet ratio index) = (AST/TopNormal AST) x (100/platelet count) [138].
NFS (NAFLD fibrosis score) = -1.675 + 0.037 × age + 0.094 × BMI + 1.13 × impaired fasting glucose (yes = 1, no = 0) + 0.99 × AST/ALT - 0.013 × platelet count - 0.66 × albumin (age in years; BMI in kg/m2; AST and ALT in (U/L); platelet count in 109/L and albumin in g/dl) [139].
FIB-4 is the most widely established and available tool. However, its ability to detect fibrosis is limited in the intermediate range (1.3-2.67), in the elderly and in individuals with T2D [140]. FIB-4 as a single test may therefore result in a high number of false positives, especially in lower prevalence populations. Notably, in individuals older than 65 years, a different lower FIB-4 cut-off of 2.0 applies. Both FIB-4 and the NFS have moderate accuracy for predicting fibrosis stages ≥F3 with AUROCs of about 0.77 and 0.75 for FIB-4 and NFS, respectively [141]. Furthermore, both FIB-4 and the NFS perform poorly in individuals younger than 35 years [142]. Recently, machine-learning techniques have been applied to develop optimised scores from multi-parametric inputs. Derived scores (such as FIB-6) cannot be defined in closed formulae but may have improved diagnostic value [143].
Several scores, including the LiverRisk, SAFE and MAF-5 score are currently being developed for the population-based setting [144–146]. Future studies will need to address how these scores perform regarding accuracy, in sequential testing and regarding cost-effectiveness.
Tests based on components of collagen formation can provide additional evidence of fibrosis:
The ELF™ (enhanced liver fibrosis) test produces a single score based on quantitative measurements of three serum markers of extracellular collagen metabolism [147]. ELF = 2.278 + 0.851 × In(hyaluronic acid [HA]) + 0.751 × In(amino-terminal propeptide of type III procollagen [PIIINP]) + 0.394 × In(tissue inhibitor of metalloproteinase 1 [TIMP-1]) as measured on the ADVIA Centaur XP/XPT system, Atellica IM Analyzer and Atellica CI Analyzer. In a meta-analysis of 63 studies, ELF showed a relatively high performance in detecting significant fibrosis, advanced fibrosis or cirrhosis (AUROCs 0.811, 0.812 and 0.810, respectively) [148].
ADAPT – including age, presence of diabetes, PRO-C3, and platelet count – has recently shown relatively high performance in identifying MASLD with advanced fibrosis in the tertiary hepatology care setting [149], and in ruling out advanced fibrosis in low-risk populations [150].
Fibrosis leads to modified mechanical properties of the liver, which can be assessed using imaging techniques, such as ultrasound- and MR-based elastography [151].
Special ultrasound devices enable liver transient elastography. With the vibration-controlled transient elastography (VCTE), liver stiffness measurement (LSM) and controlled attenuation parameter (CAP) values are determined which allow for a relatively reliable estimation of the degree of fibrosis and steatosis, respectively [152, 153]. Other ultrasound-based methods implementable with common ultrasound devices are two-dimensional shear wave elastography and point shear wave elastography [154]. They show a similar ability to assess the degree of fibrosis as VCTE [155, 156]. Unfortunately, adults with class 2 obesity cannot be reliably examined with many of these ultrasound techniques [157].
MR elastography (MRE) for assessment of liver stiffness requires specialised hardware to generate mechanical waves and dedicated acquisition techniques, which are only available at a few sites [154]. Several substantial comparative studies have shown that MRE provides at least equal quality in fibrosis staging as ultrasound-based elastography techniques [158, 159]. Advantages of MRE for the diagnosis of advanced fibrosis were reported recently [160].
Another, more indirect MRI-based method for MASH and fibrosis diagnostics called LiverMultiScan can be performed with common MRI units without an elastography unit. Since intracellular and extracellular areas of the liver differ in their T1 relaxation times, a ‘corrected T1’ (cT1) map can be generated from T1 values (with correction for effects of iron by parallel T2* measurement). Resulting values provide a good estimate of the relative proportion of extracellular space and thus inflammatory activity and degree of fibrosis [161, 162]. However, low availability and high costs limit its wider use. Whether its performance exceeds that of PDFF-based measurement of liver lipid content remains to be determined.
Quantitative assessment of liver lipid content is helpful for the grading of liver steatosis and for monitoring the effects of an intervention. MRI can be used to quantify the triglyceride content (usually expressed as proton density fat fraction [PDFF]) in the liver and is the non-invasive gold standard for hepatic lipid quantification in MASLD [163, 164]. It is important to note that the percentage of PDFF is not directly comparable to the percentage of steatosis on histology. The latter percentage indicates the proportion of hepatocytes that are macroscopically fatty, whereas PDFF provides an estimate of the volume fraction of lipids in the liver (different from histological grade of steatosis) [165]. Localised 1H-magnetic resonance spectroscopy (MRS) can also be used to assess hepatic lipid content and is even more sensitive than imaging, especially for accurate quantification of low-lipid content [166]. Methods for lipid quantification in the liver are now available on most clinical MRI units. Ultrasound-based CAP values provide a good estimate of the liver steatosis grade [167]. While CT scans obtained in clinical routine (usually performed for other purposes) can provide a rough estimate of liver steatosis and thereby suggest SLD [163], this method is not suitable for proper assessment of steatosis.
Combined scores for fibrosis diagnosis that use blood analyses and imaging results (elastography and steatosis evaluation) have been proposed and tested in recent years:
Which one of these scores has superior diagnostic performance is currently under investigation [172, 173] but may depend on the population studied (e.g. diabetes alone) [172].
Recommended Strategy in Adults with Suspected MASLD
The presence of MASLD and advanced fibrosis should be evaluated in individuals with (A) T2D or (B) abdominal obesity and ≥1 additional metabolic risk factor(s) or (C) persistently elevated liver enzymes (Fig. 2]. A multi-step process is recommended to identify individuals with advanced fibrosis. First, a FIB-4 test should be performed. As depicted in Fig. 2, individuals with a relevant risk profile should follow different pathways depending on the result of this test, owing to a potentially high number of individuals in this group with unidentified advanced fibrosis [49, 52]. If FIB-4 is below 1.3, these individuals can be assumed to be at low risk of advanced fibrosis and may be re-assessed every 1-3 years. However, despite the high negative predictive value, clinicians should recognise that FIB-4 will miss around 10% of individuals with advanced fibrosis, and it has not been formally demonstrated that repeating FIB-4 over time is effective in picking up the remaining individuals. If FIB-4 is >1.3 (or >2.0 in individuals aged >65), the risk for advanced fibrosis is increased. However, due to the low predictive value, the high number of false positive results may create a high subsequent testing burden. Thus, two options are recommended in individuals with FIB-4 scores between 1.3 and 2.67, depending on medical history, clinical context, and local resources. One option is that individuals proceed to liver elastography (e.g. VCTE) as a second step to clarify the stage of fibrosis; this option is particularly suggested in individuals with FIB-4 values close to 2.67 or in high-risk conditions. An alternative option is that individuals with FIB-4 values between 1.3 and 2.67 undergo a 1-year intervention of lifestyle change and intensified management of cardiometabolic risk factors. If the re-tested FIB-4 level is still elevated after 1 year, elastography is recommended as the second step to clarify the stage of fibrosis. Blood tests for specific collagen blood components (e.g. ELF) can be used as an alternative to elastography or a supplementary method for detecting advanced liver fibrosis. This stepwise approach demonstrated practicability to identify individuals at risk of developing liver-related events in early follow-up [174]. This approach also serves to stratify individuals into clinical care pathways (e.g. general practitioner, diabetologist, hepatology specialist referral) according to their risk of developing liver-related outcomes [175].
In adults with MASLD, should non-invasive scores, circulating biomarkers, liver stiffness measurement, and imaging methods replace liver biopsy for the diagnosis of metabolic dysfunction-associated steatohepatitis (MASH) and/or advanced fibrosis?
Recommendation
Blood biomarker-derived scores and elastography should be used to exclude advanced fibrosis, while elastography is better suited to predict advanced fibrosis (LoE 2, strong recommendation, consensus).
Statements
None of these non-invasive methods can assess relevant microscopic features of MASLD such as ballooning or lobular inflammation (LoE 2, strong consensus).
Some blood biomarker-based scores may help to identify individuals with MASH at risk of disease progression (LoE 3, consensus).
Blood biomarker-derived scores and elastography can help in risk stratification for clinical outcomes, as observational studies have identified thresholds related to liver-related outcomes and mortality (LoE 3, strong consensus).
In most cases, liver biopsy is not required for clinical management of individuals with MASLD; however, liver biopsy is still required for the definite diagnosis of steatohepatitis and can help to rule out alternative causes of liver disease (LoE 1, strong consensus).
Non-invasively obtained blood-based biomarkers (such as FIB-4 and ELF) and measurements of liver stiffness (VCTE or MRE) are suitable for reliably detecting advanced fibrosis with positive and negative predictive values strongly dependent on the chosen cut-off values and the prevalence of fibrosis of different stages in the studied population. Sensitivity generally increases with increasing degree of fibrosis.
The different approaches measure different properties or processes in the liver. This is important when interpreting their results (Table 6): AST and ALT enzymes and derived scores indicate (if other causes of their elevation can be ruled out) hepatic inflammation or hepatocyte injury; ELF and ADAPT indicate increased collagen metabolism; while elastography methods are sensitive to the amount of existing (cross-linked) extracellular collagen structures (see Table 6] [140].
Table 6.
Targets of different non-invasive techniques (selection) and suggested thresholds for ruling out/in certain features of MASLD
| Non-invasive Test | Biological processes reflected | Rule-out cut-off | Rule-in cut-off | Prediction of liver-related outcomes |
|---|---|---|---|---|
| Primary target: Hepatic steatosis | ||||
| US scan – standard | Lipid content | N/A | N/A | + |
| VCTE: CAP (Controlled attenuation parameter) [167] | Lipid content | S1: 248 dB/m S2: 268 dB/m S3: 280 dB/m |
? | |
| MRI – MRI-PDFF ([164]) | Lipid content | S1: 5% S2: 11-18% S3: 16-23% |
+ | |
| Primary target: Hepatic fibrosis | ||||
| AST/ALT ratio [153, 176] | Stress to hepatocytes | F3: 0.8 | F3: 1.0 | + |
| FIB-4 [141, 159, 176] | Stress to hepatocytes, hypersplenism | F2: 0.66-0.89 F3: 1.3 |
F2: 2.67 F3: 2.67 |
++ |
| APRI [159, 176] | Stress to hepatocytes, hypersplenism | F3: 0.5 | F3: 1.5 | ++ |
| NFS [139, 176] | Stress to hepatocytes, hypersplenism, metabolic burden | F3: -1.455 | F3: 0.676 | ++ |
| ELF [148, 177] | Collagen metabolism | F3: 7.7 | F3: 9.8 | +++ |
| ADAPT [150] | Collagen metabolism, hypersplenism, metabolic burden | F3: 4.46 | F3: 7.15 | ? |
| VCTE: LSM (liver stiffness) [157, 176, 177] | Fibrosis, extracellular volume fraction | F3: 8 kPa | F3: 12 kPa | +++ |
| US – 2D-SWE [156] | Fibrosis, extracellular volume fraction | F3: 8 kPa | F3: 10.5 kPa | +++ |
| MRI – MRE [171, 178] | Fibrosis, extracellular volume fraction | F2: 3.14 kPa F3: 3.53 kPa F4: 4.45 kPa |
+++ | |
| MEFIB [170, 171] | Stress to hepatocytes, fibrosis, hypersplenism | F2: MRE <3.3 kPa and FIB-4 <1.6 | F2: MRE ≥3.3 kPa and FIB-4 ≥1.6 | +++ |
| Primary target: “At-risk MASH” | ||||
| FAST [169, 176] | Stress to hepatocytes, fibrosis, lipid content | 0.35 | 0.67 | ++ |
| MAST [168] | Stress to hepatocytes, fibrosis, lipid content | 0.165 | 0.242 | ++ |
| Corrected T1 [161] | Extracellular volume fraction, (fibrosis) | 825 ms | 875 ms | ++ |
| NIS2+ [179] | Stress to hepatocytes, fibrosis, extracellular matrix remodelling | 0.46 | 0.68 | ? |
ADAPT, age, presence of diabetes, PRO‐C3, and platelet count; ALT, alanine aminotransferase, APRI, AST to platelet ratio index; AST, aspartate aminotransferase; CAP, controlled attenuation parameter; ELF, enhanced liver fibrosis; F1-F4, fibrosis stage (F2: moderate fibrosis, F3: severe fibrosis, F4: cirrhosis); FAST, FibroScan-AST, MAST, MRI-AST; MEFIB, MRE combined with FIB-4, MRE, magnetic resonance elastography; NFS, NAFLD fibrosis score; PDFF, proton density fat fraction; S1–S3, stage of steatosis (S1: mild (<10% hepatocytes), S2: moderate (10–30% hepatocytes), S3: severe (>30% hepatocytes) steatosis); SWE, shear-wave elastography; VCTE, vibration-controlled transient elastography; US, ultrasound.
The predictive value of the test/procedure for liver-related outcomes (e.g. cirrhosis complications, HCC, liver-related death) is qualitatively depicted (+ low, ++ moderate, +++ high, ? unknown).
Merged cells represent non-invasive techniques with single cut-offs.
These non-invasive methods are also useful for monitoring the course of disease and the effect of therapeutic interventions (degree of steatosis and stage of fibrosis). However, when interpreting changes, variability in results under similar conditions needs to be considered. This variability may be lower when using the same methods/devices/labs for individual follow-up studies [140].
Although all non-invasive methods (in contrast to the limited sample by biopsy) can provide information on the entire liver (even spatially resolved in the case of imaging), no histological characteristics of the tissue can be assessed. Only liver biopsy allows for an assessment of microscopic features (ballooning, lobular inflammation, Mallory bodies, microvesicular vs. macrovesicular steatosis, staging of fibrosis), including the presence of MASH. However, the presence of steatohepatitis (independent of fibrosis stage) may not impact treatment decisions and therefore, a liver biopsy is usually not required for the clinical diagnosis and treatment of MASLD. Still, liver biopsies may be considered essential as part of clinical studies (e.g. to determine the NAFLD activity score [NAS]) or to rule out other diseases (e.g. autoimmune hepatitis).
In adults with MASLD, should non-invasive scores, circulating biomarkers, liver stiffness measurement, and imaging techniques be used as a surrogate for liver biopsy to monitor progression of MASH and predict liver-related outcomes?
Recommendations
In adults with MASLD, sequential assessment with non-invasive tools may assist in ruling out fibrosis progression (LoE 3, weak recommendation, strong consensus).
In adults with MASLD, non-invasive tools can help predict the risk of overall and liver-related events and mortality (LoE 2, weak recommendation, strong consensus).
In a retrospective longitudinal study with paired liver biopsies (median time interval: 2.6 years), the increase over time of APRI, FIB-4 and NFS was significantly associated with one-stage fibrosis progression (cross-validated C-statistic >0.80). FIB-4 and NFS had high negative predictive values (around 90%), but suboptimal positive predictive values for predicting progression to advanced fibrosis [180]. Six retrospective cohort studies on individuals with biopsy-proven MASLD showed that NFS and FIB-4 predict the occurrence of liver-related events (AUROCs from 0.72 to 0.86) and overall mortality (AUROCs from 0.67 to 0.82) [181, 182], with a similar or lower accuracy compared to histology. Other studies have shown that FIB-4 can predict liver-related mortality in biopsy-proven or clinically diagnosed MASLD [183], APRI >1.5 predicted HCC occurrence in Asian individuals with an ultrasound diagnosis of MASLD [184], and NFS predicted cardiovascular mortality in the NHANES cohort [185]. Retrospective analyses have provided further evidence of the potential of FIB-4, NFS and ELF to predict progression to cirrhosis and liver-related events [186, 187]. Importantly, the diagnostic accuracy of FIB-4 and NFS for monitoring MASLD progression has not been extensively tested in the therapeutic setting.
Evidence also exists for the ability of elastography techniques such as LSM by transient elastography to predict outcomes in MASLD (Table 6]. Several observational studies showed that LSM had good accuracy for liver-related events, liver-related and overall mortality [176, 183, 188]. Similarly, baseline LSM was an independent risk factor for developing HCC, hepatic decompensation and liver-related death in individuals with advanced MASLD-related fibrosis/compensated cirrhosis [189]. In longitudinal studies with serial VCTE measurements, changes in LSM (20% increase, stable, 20% decrease) correlated with the risk of liver-related events (including HCC) and liver-related death [176, 189]. In a retrospective analysis in persons with advanced fibrosis included in clinical trials, an LSM cut-off of ≥16.6 kPa (HR 3.99, 95% CI 2.6-5.98) and a ≥5.5 kPa increase (and ≥20%; HR 1.98, 95% CI 1.20-3.26) predicted the development of cirrhosis, whereas LSM ≥30.7 kPa predicted liver-related events (HR 10.13, 4.38-23.41) [190]. A recent individual participant data meta-analysis including 2,518 participants, with a median follow-up of 57 months, showed that time-dependent AUCs at 5 years were 0.72 for histology, 0.76 for LSM-VCTE, 0.74 for FIB-4, and 0.70 for NFS. All these tests were significant predictors of the primary outcome (overall mortality and liver-related outcomes) [191].
Of note, VCTE measurements are highly variable [192], with a coefficient of variation of up to 60% in those with cirrhosis [193]. Furthermore, high VCTE measurements >7.9 kPa may not be reproducible at subsequent measurements in about one-third of individuals [194]. In addition, despite a clear correlation between longitudinal changes of FIB-4 and risk of clinical outcomes, up to 50% of individuals with liver events may have persistently low FIB-4 [195]. Furthermore, only a very small proportion of individuals developed liver-related events, despite one-third of the population having intermediate or high FIB-4 on ≥1 occasion [195]. The coefficient of variation of individual NITs can hence be substantial and needs to be considered when proposing them as markers of disease evolution, particularly on an individual basis.
Recent retrospective studies also evaluated the clinical meaning of dynamic changes in FIB-4 and LSM (as measured by VCTE) on long-term outcomes. A large population-based Swedish study in individuals with available FIB-4 at two time points within 5 years reported that progression from a low or intermediate to a high-risk group was associated with an increased risk of developing severe liver disease (HR 7.99 and 8.64, respectively) [196]. Along this line, a retrospective analysis of 533 cases with compensated advanced chronic liver disease related to MASLD demonstrated that changes in LSM independently predicted the occurrence of hepatic decompensation, HCC, overall mortality, and liver-related mortality [189]. Further prospective studies are needed to optimise the cut-offs for risk stratification and to evaluate the impact of changes in non-invasive scores and LSM on long-term outcomes.
In adults with MASLD, does genetic testing (alone or in combination) provide an additional advantage over other non-invasive scores and imaging in predicting risk of liver disease development, severity, progression and liver-related outcomes, or response to specific therapeutic approaches?
Recommendations
Clinicians in specialised centres may consider assessing the genetic risk profile (e.g. PNPLA3 p.I148M variant and/or polygenic risk scores) to personalise risk stratification, but this concept should be evaluated in larger prospective studies (LoE 3, open recommendation, consensus).
Genetic risk variants can be evaluated in clinical studies for stratification of disease risk progression and sub-phenotyping of MASLD (LoE 3, open recommendation, strong consensus).
Clinicians can consider referring individuals with a strong family history of severe disease in first degree relatives or early presentation with a severe phenotype, especially in the absence of metabolic triggers (and/or e.g. in individuals with normal body weight), for the evaluation of coexisting, treatable, genetic causes of liver disease by next-generation sequencing approaches (LoE 4, open recommendation, consensus).
Inherited factors play a major role in the development and progression of MASLD, synergising with the metabolic causes of the disease [197]. Overall evidence suggests that the PNPLA3 p.I148M and TM6SF2 p.E167K variants are major risk factors for progressive MASLD, and genotyping helps to non-invasively predict progressive MASH, cirrhosis and HCC when considered alongside clinical factors [198–200], but accuracy is suboptimal for prediction of liver disease severity and progression at the individual level. Mendelian randomisation studies strongly suggest that these genetic risk factors are major drivers of MASLD. Notably, the impact of the PNPLA3 variant is larger in post-menopausal women than in men [201, 202].
Besides PNPLA3 p.I148M and TM6SF2 p.E167K, additional variants, including common risk variants in MBOAT7, GCKR, GPAM, protective variants in HSD17B13, APOE and MTARC1, and rare variants (e.g. in APOB, MTTP, CIDEB and ATG7), have been robustly associated with the risk of progressive MASLD [203–209].
Comprehensive polygenic risk scores are superior to PNPLA3 and TM6SF2 alone for risk prediction of progressive MASLD, especially in individuals with metabolic triggers, and the benefits of their use over clinical risk factors alone becomes more evident for long-term prediction [200, 210, 211]. Such scores are not yet commonly available in clinical practice but should be validated in prospective studies.
Initial data suggest that individuals with (a) strong family history, (b) early disease onset, or (c) lack of accruing factors may benefit from a comprehensive genetic evaluation (e.g. whole-exome sequencing or targeted panel sequencing) that may identify strong genetic determinants of SLD with potential implications for treatment and family counselling [136, 212–215]. Using next generation sequencing (NGS), a refined diagnosis (e.g. monogenic SLD) can currently be reached in up to one-third of individuals [212, 214, 216].
PNPLA3 p.I148M is associated with a distinct pathogenesis, and may predict response to some therapeutic approaches and side effects of drugs, e.g. liver damage related to long-acting insulin-induced lipid accumulation [217], but no clinical recommendation can be made presently. Genetic variants in PNPLA3 and HSD17B13 can be targeted by RNA interference therapies that are under evaluation in clinical studies on individuals with MASLD carrying at-risk genotypes, but there is not yet any clinical indication for genotyping outside the clinical research setting [218, 219].
Is the assessment of metabolic abnormalities (e.g. insulin sensitivity/resistance) useful for risk stratification or management of adults with MASLD?
In adults with MASLD, should diagnostic procedures be performed for associated comorbidities (e.g., cardiovascular diseases, diabetes, dyslipidaemia or obesity)?
Recommendations
Clinicians should assess associated comorbidities (e.g., type 2 diabetes, dyslipidaemia, hypertension, kidney disease, sleep apnoea, polycystic ovary syndrome) and cardiovascular risk in adults with MASLD (LoE 2, strong recommendation, strong consensus).
At initial diagnosis of MASLD and at regular follow-up intervals, laboratory tests and physical examinations for related comorbidities are recommended (Table 7) (LoE 2, strong recommendation, strong consensus).
Adults with MASLD should be encouraged to participate in extrahepatic cancer screening according to current guidelines, based on their exposure to obesity and type 2 diabetes as risk factors for extrahepatic malignancies (LoE 3, strong recommendation, strong consensus).
Assessment of insulin resistance (e.g., using the homeostasis model assessment of insulin resistance [HOMA-IR] or estimates derived from the oral glucose tolerance test) may be considered to clarify metabolic dysfunction in adults with (suspected) MASLD and without an established diagnosis of type 2 diabetes (LoE 3, weak recommendation, consensus).
Table 7.
Diagnostic procedures to identify relevant comorbidities of MASLD
| Comorbidity | Assessment/parameter | Ref. |
|---|---|---|
| Obesity | Body mass index Waist circumference Waist to height ratio Further investigations*: Body composition analysis (if available) TSH and free thyroxine (if suspicion of hypothyroidism) |
[220, 221] |
| Type 2 diabetes or Insulin resistance | Fasting plasma glucose HbA1c Oral glucose tolerance test, 2-h post-load glucose Fasting plasma insulin and/or C-peptide HOMA-IR Further investigations*: Insulin resistance indices from oral glucose tolerance test or mixed meal tests |
[222, 223] |
| Dyslipidaemia | Fasting plasma triglycerides Fasting plasma total, LDL and HDL cholesterol Once in a lifetime: measurement of lipoprotein (a) Further investigations*: Non-esterified fatty acids Apolipoprotein B |
[224] |
| Kidney disease | Creatinine in plasma and urine Albumin in serum and urine Estimated glomerular filtration rate (eGFR) |
|
| Cardiovascular disease | Fasting plasma uric acid Serum high-sensitivity C-reactive protein (hsCRP) Serum ferritin Systolic and diastolic blood pressure Further investigations*: 24-h ambulatory blood pressure monitoring Echocardiography for heart failure Serum NT-ProBNP Transferrin saturation |
[25, 26] |
| Atherosclerosis | Complete blood count; Platelets Elevated lipoprotein (a) is an independent causal risk factor for atherosclerotic cardiovascular disease Further investigations*: Fibrinogen Homocysteine Von Willebrand factor antigen Carotid artery intima media thickness EchoDoppler plaque instability Coronary artery calcification |
[25, 26] |
| Obstructive sleep apnoea | Neck circumference Epworth score Further investigations*: Sleep studies Overnight pulse oximetry Polisomnography CPAP trial |
[64] |
| PCOS | Sex hormones: LH, FSH, testosterone, SHBG Ovarian ultrasound |
[66] |
FSH, follicle-stimulating hormone; HDL, high-density lipoprotein; HOMA-IR, homeostatic model assessment of insulin resistance; hsCRP, high-sensitivity CRP; LDL, low-density lipoprotein; LH, luteinising hormone; NT-proBNP, N-terminal pro-brain natriuretic peptide; PCOS, polycystic ovary syndrome; SHBG, sex hormone binding globulin; TSH, thyroid-stimulating hormone. *According to clinical evaluation and a priori probability.
Insulin resistance, defined as impaired insulin action in its target tissues, is tightly linked to the pathogenesis of SLD and results from genetic risk and acquired factors, mainly hypercaloric nutrition, gut dysbiosis and specifically adipose tissue dysfunction, which is characterised by redistribution of non-esterified fatty acids and subsequent imbalance of the release of anti- and proinflammatory cytokines. This favours hepatic lipid storage and leads to hepatic insulin resistance with reduced postprandial glycogen storage and increased glucose production [33, 225]. Thereby, insulin resistance also associates with cardiometabolic risk factors and represents a key feature of the metabolic syndrome, particularly obesity and T2D [225, 226]. The presence of MASLD is associated with a more than two-fold increased risk of an incident diagnosis of T2D, and this risk is increased with more advanced stages of MASLD [227]. Of note, the prevalence of MASLD correlates with the degree of insulin resistance even within T2D [228]. While the laborious hyperinsulinemic-normoglycemic clamp test represents the gold-standard method, insulin resistance can also be measured clinically with the HOMA-IR index from fasting insulin or C-peptide and fasting glucose concentrations, including in people with T2D (unless on insulin treatment) [222, 223, 228]. Given the central role of adipose tissue, the adipose tissue insulin resistance index (Adipo-IR) even predicts the severity of hepatic fibrosis [229]. However, the interpretation of HOMA-IR and Adipo-IR requires standardised measurement of insulin and/or C-peptide concentrations.
Presence, duration and severity of excess weight are associated with an increased risk of disease progression in MASLD [230]. EASO advocates for the use of the adiposity-based chronic disease concept for diagnosis, treatment and prevention of comorbidities [231], underscoring a complications-centred approach with total body fat and, in particular, visceral fat distribution determining the majority of the risk [232]. Evaluation of the hepato-adipo-cardio-renal axes is particularly important. For instance, MASLD is linked to an increased risk of chronic kidney disease, especially if fibrosis is present. Glomerular hyperfiltration is considered an early marker of both renal and liver disease, with its identification prompting risk factor management and preventive measures. In a large meta-analysis including 1,222,032 individuals, the risk of incident chronic kidney disease (CKD) stage ≥3 was 1.45-fold higher in individuals with MASLD, independent of demographic and CKD risk factors [233]. Still, there is uncertainty as to whether there is a direct causal link between MASLD and CKD as both diseases share common risk factors [234].
Cardiovascular disease is consistently and independently associated with circulating non-esterified fatty acid concentrations and higher intima media thickness, warranting carotid ultrasound examination in adults with increased non-esterified fatty acids [235] and contrast-enhanced ultrasound to assess plaque vulnerability. NT-proBNP (N-terminal pro-brain natriuretic peptide) has been shown to be independently associated with cardiovascular mortality in the general population [236]. The combined use of NT-proBNP and FIB-4 is helpful in risk-stratification of adults since they are independently associated with higher mortality in MASLD. While the clinical relevance of comorbidities in MASLD development is well established, strategies for their thorough assessment have not yet been outlined. Table 7 summarises the main comorbidities to screen for, available laboratory tests and complementary examinations according to clinical evaluation and a priori probability.
In adults with non-cirrhotic MASLD or MASH, is surveillance indicated for early detection of hepatocellular carcinoma?
Recommendations
In adults with non-cirrhotic MASLD or MASH in the absence of severe fibrosis (i.e. those with fibrosis stage <F3) assessed by non-invasive markers or liver biopsy, surveillance for early detection of hepatocellular carcinoma is currently not recommended (LoE 3, weak recommendation, consensus).
In adults with non-cirrhotic MASLD or MASH in the presence of severe fibrosis (F3) assessed by non-invasive markers or liver biopsy, surveillance may be considered based on an individual risk assessment (LoE 4, weak recommendation, strong consensus).
There is currently no consensus on whether surveillance for HCC is beneficial in non-cirrhotic MASLD or MASH. The risk factors and natural history of HCC development in the context of MASLD are not as well-defined as for other aetiologies of liver disease, and robust data on the incidence of HCC in MASLD, particularly in individuals without cirrhosis, is lacking. Nevertheless, several studies have shown that individuals with MASLD without cirrhosis may develop HCC, albeit rarely [237]. In a US Veterans Administration cohort [124] including 1,500 individuals with HCC from 2005 to 2010, metabolic syndrome was particularly prevalent in the subgroup who had no cirrhosis. In the same cohort, the risk of HCC in the absence of cirrhosis was 5-fold higher in individuals with MASLD than in those with chronic hepatitis C. In a tertiary centre for HCC referral in Northern England [238], individuals with HCC due to MASLD had a lower prevalence of cirrhosis (77.2%) than those with other aetiologies. Similar observations have been confirmed in a European study [239] and two Japanese studies, where cirrhosis was absent in 38% and 49% of individuals with MASLD-related HCC, respectively [240, 241].
Effective surveillance is challenging in individuals without cirrhosis. In a retrospective analysis of individuals with MASLD who were diagnosed with HCC between 2003 and 2012, those with HCC on a non-cirrhotic liver more frequently had a larger nodule size and/or a greater rate of HCC recurrence than those with cirrhosis [242]. Similarly, in another case series of 44 individuals who developed MASLD-related HCC between 2010 and 2012, only one individual without cirrhosis (out of six) underwent liver resection, while the late diagnosis impeded curative treatments in the others [243].
The utility and applicability of HCC surveillance depends on several factors: incidence of HCC in target populations, availability of efficient diagnostic tests at acceptable cost, and availability and effectiveness of treatments. Cost-effectiveness analyses indicate that HCC screening should be considered for individuals with HCC risk exceeding 1.0–1.5% per year [244, 245]. The annual incidence rates of HCC in MASH-related cirrhosis cohorts range from 0.2 to 2.6% [246], while the HCC incidence in individuals without cirrhosis is very low, ranging from 0.08 to 0.63 per 1,000 PY [247–251]. A systematic review and a meta-analysis concluded that the risk estimate is likely too low to justify routine screening in MASLD without evidence of advanced fibrosis [248]. A recent study updated these figures and found a low incidence of HCC at 10 years (2.7%) in those with MASLD without cirrhosis [249]. Importantly, cost-effectiveness is dependent on the healthcare system, and as most studies are derived from the US, their results cannot easily be extrapolated to European healthcare systems. In addition, it may be questioned whether the incidence cut-offs of 1.0-1.5%/year still hold true. Overall, there still is insufficient evidence to warrant HCC surveillance in individuals with MASLD without cirrhosis.
Accumulating evidence suggests that NITs may be useful to identify those individuals with MASLD without cirrhosis at highest risk of HCC development. In the national Veterans Affairs study, a FIB-4 >2.67, suggestive of advanced fibrosis (F3/F4), was associated with an increased risk of HCC (0.39/1,000 vs. 0.04/1,000 PY in individuals with persistently low FIB-4) in the absence of cirrhosis [251]. In another retrospective cohort study, the annual incidence of HCC was 2.8 per 1,000 PY with FIB-4 >2.67 and 0.7 per 1,000 PY with FIB-4 <1.30 [250]. In a study of histologically confirmed MASLD in Sweden, the overall HCC rate varied with the advancing stage of MASLD (per 1,000 PY): 0.8 in simple steatosis, 1.2 in MASH without fibrosis, 2.3 in fibrosis (F1-F3) without cirrhosis, and 6.2 in cirrhosis [54]. Overall, the incidence of HCC in those with MASLD and earlier stages of fibrosis (stage 0–2) is extremely low and determinants of risk have not been well quantified.
Individuals with F3 fibrosis have an intermediate risk of developing HCC that is lower than that of individuals with cirrhosis, but not negligible. Adults with MASLD and F3 fibrosis are difficult to stage reliably in a non-invasive manner, making HCC surveillance decisions harder and less favourable from a cost-effectiveness standpoint. Studies in MASLD indicate an elevated HCC risk with FIB-4 >3.25 (risk >1%/year) [251] or change of LSM over time in individuals with histologic diagnosis of F3-F4 fibrosis and/or baseline LSM >10 kPa (HR 1.72) [189] in individuals without cirrhosis. Further studies focusing on cost analysis are warranted in individuals with MASLD without cirrhosis but with high FIB-4 or LSM to confirm the need for surveillance in this intermediate-risk group of individuals.
Metabolic syndrome itself and T2D have also been reported to be associated with an increased risk of HCC in large cohort studies [46, 252], but the increase in absolute HCC risk attributable to metabolic factors (e.g. T2D, obesity) or genetic factors in individuals with non-cirrhotic MASLD is very small.
Should hepatocellular carcinoma monitoring programmes be implemented in all adults with MASH-related cirrhosis, or should they be implemented based on risk stratification?
Recommendations
According to current guidelines, hepatocellular carcinoma monitoring programmes should be applied to individuals with MASLD-related cirrhosis (LoE 3, strong recommendation, strong consensus).
Risk stratification can help in optimising strategies for monitoring individuals at higher risk of hepatocellular carcinoma (Table 8) (LoE 4, weak recommendation, strong consensus).
As ultrasound-based surveillance has a low sensitivity for detection of hepatocellular carcinoma at an early-stage, particularly in adults with MASLD-related cirrhosis and obesity, alpha-fetoprotein (AFP) measurement can be combined with ultrasound in individuals at high risk (LoE 3, open recommendation, consensus).
Cross-sectional imaging by MRI may be undertaken in selected adults at high risk with persistent poor visualisation at ultrasound, particularly in individuals with dysplastic or regenerative nodules(LoE 3, open recommendation, strong consensus).
Table 8.
Factors associated with a higher risk of HCC occurrence in non-cirrhotic MASLD
The approach to HCC monitoring in adults with MASH-related cirrhosis involves a combination of risk stratification and individualised decision-making. According to current EASL guidelines [256, 257], risk-based surveillance divides individuals with cirrhosis into three groups: the high-risk group must undergo surveillance, and more costly screening tools than ultrasound are justified; the low-risk group may not need surveillance at all; and the remaining intermediate-risk group should be offered surveillance. The main problem is how to perform an effective risk-stratification for HCC risk in persons with MASLD and cirrhosis.
Cirrhosis in MASLD is under-recognised compared to other aetiologies [258], with less than one in four individuals with cirrhosis undergoing HCC surveillance [259]. These failures need to be addressed in future clinical and research efforts, as outlined in the previous section.
EASL recommends semi-annual abdominal ultrasound as the primary strategy for HCC surveillance [256]. However, individuals with MASLD have increased odds of persistent poor visualisation with a pooled sensitivity of 45% for ultrasound alone and increased risk of false positive or indeterminate results [260, 261]. Cross-sectional abdominal imaging is increasingly used for HCC surveillance in clinical practice, although supported by limited data. According to the prospective PRIUS study, MRI had significantly higher sensitivity (85.7% vs. 26.2%) and specificity (97.0% vs. 94.4%) than ultrasound for early HCC detection [262]. However, this comes at high financial costs with incremental cost-effectiveness ratios of $14,474/life year and $25,202/quality-adjusted life year at an annual HCC incidence of 3%. [263]. Currently, MRI is reserved for selected individuals in whom ultrasound visualisation is inadequate, particularly if they have a high estimated risk of developing HCC.
Alpha-fetoprotein (AFP) is the only biomarker with sufficient evidence to support its use in clinical practice, in combination with ultrasound. In a meta-analysis of 13,367 individuals, the combined use of AFP and ultrasound was reported to increase the sensitivity for detection of early HCC from 45% to 63% [260]. Notably, changes in AFP values across serial measurements are superior to single AFP values for the detection of early-stage HCC [264].
NITs for fibrosis prediction may help in risk stratification. The NAFLD fibrosis score (NFS) had a reported c-index of up to 0.9 for the prediction of HCC in a longitudinal study of 1,173 European individuals with MASLD [187]. Another model incorporated genetic variants in a risk score that predicted HCC independently of classical risk factors, but the AUROC when used alone was only 0.65 [210].
Longitudinal changes in NITs can be more informative. In a recent study, the annual risk of HCC was as high as 2.5% in individuals with cirrhosis and persistently high FIB-4 values, whereas it was below 0.3% in individuals who had a cirrhosis diagnosis but persistently low FIB-4 values. High FIB-4 (>2.67) at baseline and at 3 years was linked to a >50-fold higher risk of HCC compared to persistently low FIB-4 (<1.45) values [265].
Although still not recommended in the guidelines, accumulating data also suggest that LSM may have a role in the prediction of liver-related outcomes. The analysis of 1,039 individuals with MASLD with a histologic diagnosis of F3-F4 fibrosis and/or an LSM >10 kPa showed that the change in LSM over time predicted the occurrence of HCC (HR 1.72; 95% CI 1.01-3.02) [189].
Interest in algorithms combining demographic and clinical variables with blood-based biomarkers has increased (Table 9). However, none of these calculators has been validated in phase III/IV studies.
Table 9.
Proposed tools for HCC risk-stratification in MASLD-related cirrhosis
| NITs | Formula/model variables/data | Study cohorts | Performance | Reference |
|---|---|---|---|---|
| HCC risk score | age + sex + platelet count + albumin + AST/√ALT available at: www.hccrisk.com |
7,068 individuals with MASLD-related cirrhosis (407 incident HCC) Mean FU: 3.7 years |
Derivation cohort, C-index = 0.749 Validation cohort, C-index = 0.718 |
[266] |
| aMAP | (0.06 × age (years) + 0.89 × sex (M = 1; F = 0) + 0.48 × [(log10 bilirubin (µmol/L) × 0.66 + albumin (g/L) × −0.085] – 0.01 × platelets (×103/mm3) + 7.4)/14.77 × 100 | Overall individuals, n = 17,374 NVH validation cohort, n = 720 Total: 1,389 individuals with MASLD Median FU: 4.61 years F3-F4, n = 243 (17.5%) |
NHV cohort: Overall, C-index = 0.85 Cirrhosis, C-index = 0.61 Overall, C-index = 0.887 F3-F4, C-index = 0.754 |
[267] [268] |
| GALAD | -10.08 + 0.09 × age (years) + 1.67 × gender (M = 1, F = 0) + 2.34 × log10 AFP (ng/ml) + 0.04 × AFP-L3 (%) + 1.33 × log10 DCP (ng/ml) | 389 individuals with MASH (28 incident HCC) Median FU: 167 months Cirrhosis, n = 77 (19.6%) |
Higher GALAD score in individuals who developed HCC vs. individuals HCC-free as early as 1.5 years before HCC diagnosis | [269] |
| HEDS study | Risk factor associated to HCC development in individuals with cirrhosis: Male gender (OR = 2.47; 95% CI 1.54–4.07; p <0.001) Years with cirrhosis (OR = 1.06; 95% CI 1.02–1.1; p = 0.004), Family h/o of liver cancer (OR = 2.69; 95% CI 1.11–5.86; p = 0.02) Age (OR = 1.17; 95% CI 1.03–1.33; p = 0.02) Obesity (OR = 1.7; 95% CI, 1.08–2.73; p = 0.02) AST (OR = 1.54; 95% CI 0.97–2.42; p = 0.06) AFP (OR = 1.32; 95% CI 0.97–1.77; p = 0.07) Albumin (OR = 0.7; 95% CI 0.46–1.07; p = 0.10) |
Total: 1,325 individuals with cirrhosis (95 incident HCC) Median FU: 2.2 years MASLD, n = 327 (24.9%); 19 incident HCC |
Performance of the multivariate set of risk factors: C-index = 0.73 | [270] |
| THRI | age + etiology + gender + platelets Age: <45 years = 0 points; 45–60 years, 50 points; >60 years, 100 points Etiology: autoimmune/HCV SVR, 0 points; other, 36 points; MASLD, 54 points; HCV/HBV, 97 points Gender: Female = 0 points; Male = 80 points Platelets: >200 = 0 points; 140–200 = 20 points; 80–130 = 70 points; <80 = 89 points Total: 0–366 points |
Derivation cohort: 2,079 individuals with cirrhosis MASLD, n = 111 (5.3%) Total: 2,491 individuals with cirrhosis MASLD, n = 1,182 (48%) |
10-year HCC incidence: low-risk (<120) = 3%; medium-risk [121–176, 180–219, 222, 223, 225–247] = 10% high-risk (>240) = 32% C-index = 0.69 |
[271] [272] |
| LiverRisk score | Linear regression model using age (years), blood glucose, cholesterol, AST, ALT, GGT and platelets Available at: https://www.liverriskscore.com |
Derivation cohort: 14,726 participants without known liver disease from the general population undergoing transient elastography for assessment of liver fibrosis. Two external validation cohorts: 4,370 and 3,999 individuals |
8-year risk of HCC in the high-risk group = 4.4% 8-year risk of HCC development in the two lower risk groups ≤0.1% |
[144] |
AFP, alpha-fetoprotein; DCP, Des-γ-carboxy prothrombin; FU, follow-up; NHV, non-viral hepatitis; NIT, non-invasive test; OR, odds ratio.
Ultimately, the decision to implement an HCC monitoring programme is a collaborative effort between the affected individual and their healthcare provider. Factors to consider include the individual's overall health, the presence of other medical conditions, familial history of HCC along with the individual’s personal preferences.
Treatment of MASLD: General Considerations
In adults with MASLD, which of the following – reduction of steatosis, resolution of MASH, improvement of fibrosis, stabilisation of fibrosis, prevention of progression to cirrhosis – is the most relevant therapeutic target for improving liver-related outcomes?
Statements
In adults with MASLD and advanced fibrosis or cirrhosis, regression of fibrosis has been associated with a reduced risk of liver-related outcomes (LoE 2, strong consensus).
Improvement in disease activity and resolution of steatohepatitis have been associated with regression of fibrosis (LoE 2, strong consensus).
Reduction of steatosis has been associated with histological improvements (particularly necro-inflammation) in some pharmacological intervention studies (LoE 2, strong consensus).
Since improved mortality has not been demonstrated for any of these treatment-induced histological changes, further long-term follow-up studies are needed to demonstrate that halting disease progression and/or reduction of steatosis, resolution of steatohepatitis or regression of fibrosis translate into a reduced risk of clinical outcomes (LoE 3, strong consensus).
The goal of any disease management is to obtain clinically meaningful benefit. For MASLD this has mainly focused on liver-related outcomes, although the potential impact on other related diseases (e.g., cardiovascular disease, extrahepatic malignancy) and quality of life are increasingly being explored (including in interventional trials) as part of a more holistic approach. Liver-related outcomes usually refer to cirrhosis decompensation, decline in liver function, occurrence of HCC and liver transplantation [273]. In trials assessing clinical outcomes in non-cirrhotic MASH, progression to cirrhosis is also generally included as a liver-related outcome.
The heterogeneous, fluctuating, and slow natural history of the disease requires long-term studies to demonstrate the effects of an intervention on clinical outcomes, and such studies are currently limited. Therefore, several surrogate endpoints have been defined, including improvements or absence of deterioration in histological features, as well as, more recently, imaging-based features or other NITs. These are likely to translate into a reduction of liver-related outcomes. However, to date, these surrogates have not been unambiguously linked to true clinical benefits (e.g., liver-related mortality) [274]. As per regulatory guidance, the primary endpoints in registrational phase III clinical trials for non-cirrhotic MASH are: (a) resolution of steatohepatitis without worsening of fibrosis and (b) regression of at least 1 stage in fibrosis without worsening of steatohepatitis [275]. These endpoints are considered likely reasonable surrogates for clinical benefit.
Despite the emphasis on diagnosis and treatment of fibrosis, only one study has shown fibrosis regression to translate into a reduced risk of liver-related events [177]. Several studies have shown that changes in histologically assessed activity are linked with changes in fibrosis over time. In a natural history cohort of 445 individuals with 4.9 years mean follow-up between paired liver biopsies from the NASH CRN, improvement in the NAS over time was associated with fibrosis regression, and worsening of the NAS was associated with fibrosis progression [276]. In a retrospective analysis of 441 individuals with paired biopsies from two clinical trials conducted by the NASH CRN, resolution of steatohepatitis was the strongest predictor of fibrosis regression [277].
In several studies and recent meta-analyses, changes in MRI-PDFF-assessed steatosis correlated with histological endpoints. In particular, in several studies, a relative reduction in MRI-PDFF of ≥30% was associated with a high likelihood of resolution of steatohepatitis (OR 5.45) [278], although this may be dependent on specific modes of action, might not be generalisable, and potentially depends on the mode-of-action of the investigated intervention. A recent study of 100 paired biopsies also suggests that this endpoint correlates with fibrosis regression (adjusted OR of 6.46), but further validation is needed [279].
In adults with MASLD, should non-invasive scores, serum markers, liver stiffness measurements, and imaging be used as a substitute for liver biopsy for monitoring therapeutic responses?
Statements
Non-invasive tests have been linked with histologically assessed treatment response, but the most appropriate non-invasive test may depend on the type of intervention and patient-related factors (LoE 2, strong consensus).
Longitudinal changes in non-invasive test results have been correlated with changes in the risk of adverse outcomes on a cohort or population level (LoE 3, consensus).
In the setting of randomised controlled trials and depending on the mode of intervention, changes of non-invasive markers (e.g., MRI-PDFF relative reduction by ≥30%, ALT reduction by ≥17 U/L) have been associated with resolution of steatohepatitis (LoE 2, strong consensus).
Liver biopsy is not suited for monitoring disease evolution or response to therapy in routine clinical practice due to its invasiveness and procedure-related limitations (LoE 5, strong consensus).
Recommendations
At the individual level, non-invasive tests may be repeatedly used to assess fibrosis progression in a tailored fashion but may provide limited information about treatment response (LoE 5, weak recommendation, strong consensus).
In individual cases and in clinical trials, liver biopsy can be used to monitor disease progression or response to treatment (LoE 1, open recommendation, strong consensus).
Many studies have used NITs to provide evidence supporting the observed efficacy of an intervention (vs. placebo) on histological endpoints. However, few studies have linked longitudinal changes in NITs with changes in fibrosis or other histological endpoints. Furthermore, for most NITs, we lack a validated threshold for the magnitude of change that is predictive of histological responses or clinical outcomes on the individual level.
Secondary analyses from RCTs investigating NIT responses to pharmacological treatments and their relation to histological outcomes have shown that reductions in Pro-C3 levels are associated with histological response to the fibroblast growth factor (FGF)19 analogue aldafermin, the FGF21 analogue pegozafermin and the chemokine receptor CCR2/5 inhibitor cenicriviroc [280–282], while reductions in ELF scores were shown to be associated with histological response to aldafermin [280].
The most extensive analysis in that regard is from the REGENERATE trial of obeticholic acid [283], which analysed NIT responses between histological responders and non-responders. Some improvements in NITs were related to the treatment arm but not per se to histological response, whereas others, e.g. VCTE or ELF, correlated better with changes in fibrosis stage regardless of the treatment arm, suggesting that they reflect histological changes regardless of a drug’s mode of action.
While a relative reduction in MRI-PDFF-measured liver lipid content of ≥30% has been shown to predict MASH resolution in several studies [278, 284], it also predicted fibrosis regression in one study [279] though this was not unanimously confirmed [285, 286]. A secondary analysis of the NASH CRN FLINT trial identified a decrease in ALT at week 24 as a predictor of response (namely a decrease in NAS of ≥2 and no worsening of fibrosis) [287]. An ALT decrease of ≥17 U/L yielded the highest accuracy to separate responders from non-responders (OR >10). In a combined analysis of the simtuzumab and selonsertib trials in MASH-related cirrhosis [177], a change in ELF of 0.5 was related to clinical outcomes but how this related to fibrosis regression or other histological endpoints was not reported. MRE has been proposed as an alternative to VCTE, but despite numerical differences, changes in MRE over time were not significantly different between histological responders and non-responders in the selonsertib phase II trial [285]. cT1 decreased more in responders (NAS decrease of ≥2 or fibrosis regression) than in non-responders in the aldafermin ALPINE trial but the optimal cut-off to separate responders from non-responders is unclear [280]. Promising data have also been reported for FAST [288].
In adults with MASLD, can the management of liver disease and extrahepatic comorbidities within multidisciplinary teams involving hepatologists and other specialists improve clinical outcomes?
Recommendation
Given the multidirectional connections between MASLD and cardiometabolic comorbidities, a multidisciplinary approach is recommended to ensure all components are appropriately targeted to improve both liver-related and extrahepatic outcomes (LoE 3, strong recommendation, strong consensus).
Treatment of cardiometabolic comorbidities may modify disease progression and contribute to a reduction in liver-related events (e.g. statins, aspirin, renin-angiotensin-aldosterone modulators) [289, 290]. Anti-diabetic and anti-obesity treatments such as pioglitazone and glucagon-like peptide 1 receptor agonists can have a hepatic benefit (e.g., phase II data on pioglitazone and glucagon-like peptide 1 receptor agonists [GLP1RAs]) as discussed in the treatment section below. The correlation between the cardiovascular system and the liver is complex, with conflicting data on the independent contribution of MASLD/MASH to cardiovascular disease [291, 292]. These considerations support the need for the appropriate and optimal treatment of all these co-morbidities which may be best delivered by a broad multidisciplinary team.
Treatment of MASLD: Non-Pharmacological Therapy
In adults with MASLD, what is the efficacy of dietary and behavioural therapy-induced weight loss on histologically/non-invasively assessed liver damage/fibrosis and liver-related outcomes compared with no intervention?
Recommendations
In adults with MASLD, dietary and behavioural therapy-induced weight loss should be recommended to improve liver injury, as assessed histologically or non-invasively (LoE 1, strong recommendation, strong consensus).
In adults with MASLD and overweight, dietary and behavioural therapy-induced weight loss should aim at a sustained reduction of ≥5% to reduce liver fat, 7-10% to improve liver inflammation, and ≥10% to improve fibrosis (LoE 2, strong recommendation, strong consensus).
Statement
Further follow-up studies are needed to determine the long-term effectiveness of dietary and behavioural therapy-induced weight loss (including its magnitude) on clinical liver-related outcomes and liver-related mortality (LoE 3, strong consensus).
It has repeatedly been demonstrated in clinical trials that weight reduction achieved by caloric restriction, either with or without increased physical activity, leads to improvements in MASLD biomarkers, including liver enzymes, steatosis, MASH, and fibrosis [293–295] (Fig. 3). There is a dose-dependent association between the amount of weight loss and the extent of improvement in biomarkers of liver damage [294]. However, evidence for an effect of weight reduction by lifestyle modification on advanced fibrosis or cirrhosis is insufficient, owing to the minority of individuals with advanced fibrosis in most clinical trials and the lack of subgroup analyses [296]. A stringent interventional trial with histological endpoints suggested a bodyweight reduction of ≥5% is required to reduce liver lipid content, 7-10% to improve inflammation, and ≥10% to improve fibrosis [296]. However, a limited proportion of individuals achieve a weight reduction of ≥5% [296, 297]. In addition, long-term adherence to behavioural changes is often insufficient, as seen in obesity trials [298]. There is limited data on long-term dietary interventions in MASLD since study durations range from 2-24 months [299–301]. Only a few studies have performed 12-24-month follow-ups, showing a maximal weight loss at 6 months, followed by a gradual weight regain to a net weight loss of about 5% at 12-24 months and partial regain of liver lipid content and stiffness [302–304]. These data emphasise the importance of accessible and affordable long-term structured lifestyle modification programmes, including diet, physical activity, and behavioural therapy, for individuals with MASLD, and highlight the need for longer-term (≥2 years) RCTs on lifestyle interventions.
Fig. 3.
Lifestyle management algorithm for MASLD. Note: Behavioural therapy includes: self-monitoring, clinicians providing affected individuals with self-efficacy and motivation, setting realistic negotiable goals, and overcoming barriers. Examples of unprocessed/minimally processed foods: vegetables, fruits (not juice), low-fat dairy, nuts, olive oil, legumes, unprocessed fish and poultry. Overweight/obesity: Overweight: BMI of 25–29.9 kg/m2 (non-Asian) or 23–24.9 (Asian), Obesity: ≥30 kg/m2 (non-Asian) ≥25 kg/m2 (Asian). Class II obesity: BMI ≥35 kg/m2 (non-Asian) or BMI ≥30 kg/m2 (Asian). Normal weight: BMI<25 kg/m2 (non-Asian) or <23 kg/m2 (Asian). BMI, body-mass index; HCC, hepatocellular carcinoma; MASH, metabolic dysfunction-associated steatohepatitis; MASLD, metabolic dysfunction-associated steatotic liver disease; T2D, type 2 diabetes.
There are multiple beneficial dietary approaches to lose weight and improve MASLD. Hypocaloric low-carbohydrate diets and low-fat diets appear to be similarly effective in reducing liver lipid content and related biomarkers [305, 306]. However, the Mediterranean diet seems to have added value for liver lipid reduction and cardiometabolic health and may be easier to maintain in the long-term [301, 303]. There is currently insufficient evidence on the efficacy or safety of very low carbohydrate ketogenic diets – characterised by extreme carbohydrate restriction to <20-50 g/day (10-25% of total calories) and high fat and protein contents – in individuals with MASLD [307], taking into consideration potential cardiovascular, kidney and other side effects [308]. Time-restricted eating, also called intermittent fasting, is a new dietary strategy in which calories are consumed in a defined time window [309]. There is currently very little evidence for a beneficial effect of time-restricted eating over regular caloric restriction on hepatic lipid content in individuals with MASLD [299, 304, 310]. The long-term adherence can be improved by taking into account the individual’s preferences, clinical, cultural, and economic characteristics. In a Cochrane systematic review of RCTs in people with MASLD, with follow-up periods of 2–24 months, data were sparse regarding the effects of lifestyle interventions on any clinical outcome (death, liver-related complications, and liver cancer) [311]. Long-term, large RCTs are needed to test the effect of lifestyle interventions on clinical outcomes.
In adults with MASLD, is changing diet quality effective in reducing histologically/non-invasively assessed liver damage/fibrosis and liver-related outcomes compared with no intervention?
Recommendation
In adults with MASLD, improving diet quality (similar to the Mediterranean dietary pattern), limiting the consumption of ultra-processed food (rich in sugars and saturated fat) and avoiding sugar-sweetened beverages should be recommended to improve histologically or non-invasively assessed liver injury (LoE 2, strong recommendation, strong consensus).
Statement
There is little evidence that improving diet quality beneficially impacts clinical liver-related outcomes (LoE 3, consensus).
In a meta-analysis of observational studies and clinical trials, the Mediterranean diet has repeatedly been shown to provide hepatic and cardiovascular health benefits [312, 313], even without weight loss [314]. The Mediterranean diet is characterised by high intakes of olive oil, vegetables, fruits, nuts and seeds, legumes, whole grains, fish, and seafood. Notably, the Mediterranean diet advocates the reduction of sugars and refined carbohydrates, saturated fat, ultra-processed foods, and red and processed meat, which are all related to MASLD risk in observational studies [105, 300, 315–317]. Saturated fat has been shown to have a negative effect on liver steatosis in several short-term clinical trials [305]. Several observational and cross-sectional studies have shown the harmful association between MASLD and high intake of red and processed meat [318–320]. Intake of added sugars, especially fructose, plays a major role in MASLD development [305]. Evidence from RCTs and a large prospective observational study shows an association of dietary added sugars, especially in the form of sugar-sweetened beverages, with increased liver steatosis, the incidence and prevalence of MASLD [321–323], and potentially the risk of MASH [324]. A prospective cohort study showed a dose-response association between soft drink consumption and MASLD; a consumption of ≥4 servings per week was related to a 45% increased risk of developing MASLD [111]. Similarly, an unhealthy diet is also related to the risk of liver cancer. In an observational study, high intake of red meat, saturated fat, cholesterol, and refined sugars are associated with an increased liver cancer risk [106]. In contrast, the Mediterranean diet or similar healthy eating patterns are associated with a lower risk of liver cancer [106]. Adherence to healthier eating patterns has also been associated with lower risk of all-cause, cardiovascular- and cancer-related mortality in US adults with MASLD in an observational study [325]. Importantly, dietary guidance to improve cardiovascular health advocates the same dietary principles [326].
In adults with MASLD, are physical activity and exercise effective at reducing histologically/non-invasively assessed liver damage/fibrosis and liver-related outcomes compared with no intervention?
Recommendation
In adults with MASLD, physical activity and exercise should be recommended to reduce steatosis, tailored to the individual’s preference and ability (preferably >150 min/week of moderate or 75 min/week of vigorous-intensity physical activity) (LoE 1, strong recommendation, strong consensus).
Statement
In comparison to the well-documented cardiometabolic benefits, there is less robust evidence for benefits of physical activity and exercise on histological outcomes, non-invasively assessed liver damage/fibrosis and liver-related clinical outcomes (LoE 5, strong consensus).
Sedentary behaviour is an independent predictor of MASLD [327] and is associated with a greater risk of MASLD progression [328], while several RCTs and meta-analyses have demonstrated that exercise alone, without dietary interventions or significant weight loss, reduces liver steatosis in individuals with MASLD [329]. An RCT showed that a 3-month aerobic exercise regimen (three 90-minute sessions/week) reduced liver steatosis and liver stiffness, independent of weight loss [330].
Many RCTs tested the effects of different types of exercise training programmes, including aerobic training, resistance training, high-intensity interval training, or combined, with varying frequency and length of sessions and intensities. Most were effective for steatosis reduction, without a single optimal exercise prescription being identified for individuals with fibrosis [331, 332]. A meta-analysis of 19 RCTs that compared two types of aerobic training, high-intensity interval training and traditional moderate-intensity continuous training, showed that both exercise types were equally effective for liver fat reduction [333].
Owing to limited clinical trial data, it is currently unclear whether exercise training can independently improve liver histology [334, 335]. However, RCTs in individuals with MASLD show that exercise improves quality of life, cardiorespiratory fitness, reduces visceral fat, and improves muscle mass and function even in advanced liver disease [336, 337]. Furthermore, several observational studies have demonstrated that regular increased physical activity is associated with reduced risk for liver fibrosis (measured by non-invasive markers), cirrhosis, all-cause mortality [338–340] and HCC [341]. Using data from the UK Biobank cross-sectional study, physical activity measured using accelerometery was inversely related with hepatic fibro-inflammation measured by MRI [342]. Therefore, increased physical activity, exercise and reduction in sedentary behaviour, independent of weight loss, has hepatic and cardiometabolic benefit and should be routinely recommended [328, 343]. The combination of aerobic plus resistance training is preferred [328]. Aerobic activity (30–60 min) on most days of the week can lead to a small amount of weight and fat loss, improvement in cardiometabolic parameters, maintenance of fat-free mass during weight loss and weight maintenance after weight loss [298]. Physical activity concentrated within 1 to 2 days of the week, termed the “weekend warrior” pattern, was also associated with a lower risk of cardiovascular outcomes [344].
In adults with MASLD who are normal weight, are diet and exercise interventions effective in reducing histologically/non-invasively assessed liver damage/fibrosis and liver-related outcomes in comparison with no intervention?
Recommendation
In normal-weight adults with MASLD, diet and exercise interventions should be recommended to reduce liver fat (LoE 3, strong recommendation, strong consensus).
Statement
In normal-weight adults with MASLD, there is currently no evidence regarding the beneficial effect of diet and/or exercise on liver histology, fibrosis and liver-related clinical outcomes (LoE 5, consensus).
MASLD can develop in individuals with normal BMI within the ethnic-specific cut-offs [345]. Normal-weight MASLD is defined as the presence of MASLD in an individual with a BMI <25 kg/m2 (non-Asian ethnicity) or <23 kg/m2 (Asian ethnicity) [346].
Normal-weight individuals with MASLD have a higher prevalence of metabolic alterations including insulin resistance, greater visceral obesity, and decreased muscle mass compared to normal-weight controls [347, 348]. In an RCT of a 12-month lifestyle intervention programme, a 3-5% weight reduction led to remission of MASLD (measured by 1H-MRS) among 50% of the individuals without obesity. Moreover, individuals defined as non-obese were more likely than individuals with obesity to maintain weight reduction and normal liver enzymes over long-term (6-year) follow-up [349]. Similarly, in a large cohort study that included 2,383 normal-weight adults with MASLD, weight reduction over a median follow-up of 3 years was associated with MASLD resolution (measured by abdominal ultrasound) in a dose-dependent manner [350]. A few small observational studies have indicated that dietary fructose and sugar-sweetened beverage consumption is higher in individuals with MASLD and no obesity [346, 347]; thus, these individuals may benefit from reducing their intake and improving diet quality.
In adults with MASLD, are nutraceuticals (food supplements, herbal products, gut microbiota-modifying agents) effective to reduce histologically/non-invasively assessed liver damage/fibrosis and liver-related outcomes compared with no intervention?
Recommendation
In adults with MASLD, nutraceuticals cannot be recommended since there is insufficient evidence of their effectiveness in reducing histologically/non-invasively assessed liver damage/fibrosis and liver-related outcomes in MASLD, nor of their safety (LoE 2, open recommendation, strong consensus).
Statement
In adults with MASLD, coffee consumption has been associated with improvements in liver damage and reduced liver-related clinical outcomes in observational studies (LoE 4, strong consensus).
A Cochrane meta-analysis of 202 RCTs for people with MASLD assessed the benefits and harms of nutritional supplements for treatment of MASLD. Follow-up ranged from 1 month to 28 months. The evidence indicates considerable uncertainty about effects of interventions on all clinical outcomes (liver transplantation, liver decompensation, HCC, and mortality), as well as on serious adverse events. High-quality comparative RCTs with adequate follow-up are needed [351].
Microbiome-centred therapies such as engineered bacteria, postbiotics, and phages have mainly been tested in preclinical models. The effectiveness and safety of microbiome-based treatments must be evaluated through rigorous pharmacological studies and larger RCTs in individuals with MASLD [352]. In a meta-analysis of RCTs that investigated the effects of probiotics on MASLD, probiotic treatment reduced ALT, AST and liver steatosis, but the included studies had a number of limitations (e.g. short-term, small sample size, limited assessment of liver outcomes) [353].
Coffee consumption – caffeinated or not – has been shown to have a protective association with MASLD in several observational studies of varying quality. In a meta-analysis of various types of observational studies, the risk of MASLD and fibrosis was lower among those who drank coffee compared to those who did not. The results were stronger and more consistent for fibrosis than for steatosis [354]. Similarly, an earlier meta-analysis showed that intake of ≥3 cups of coffee per day (vs. <2 per day) was related to reduced risk of MASLD [355]. In a nationally representative cross-sectional study, ≥3 cups of coffee daily were independently associated with lower LSM but not with steatosis (measured by CAP) [356]. In addition, in prospective observational studies, coffee consumption was inversely related to death from chronic liver disease and HCC [357, 358]. Since observational studies are prone to unmeasured or residual confounding, and RCTs investigating the hepatic effects of coffee consumption are scarce and negative or inconclusive [359, 360], no firm conclusions can be drawn.
Treatment of MASLD: Pharmacological Therapy
In adults with MASH, is there sufficient evidence to recommend prescription of existing non-glucose-lowering drugs to reduce histologically/non-invasively assessed liver damage/fibrosis and liver-related outcomes compared to no pharmacological intervention?
Recommendations
If approved locally and dependent on the label, adults with non-cirrhotic MASH with significant liver fibrosis (stage ≥2) should be considered for treatment with resmetirom as a MASH-targeted therapy, as this treatment demonstrated histological efficacy on steatohepatitis and fibrosis in a large phase III registrational trial with an acceptable safety and tolerability profile (LoE 2, strong recommendation, consensus).
Treatment with resmetirom, if approved locally, may be considered for individuals with MASLD who are non-cirrhotic and with documentation of either: (A) advanced fibrosis; (B) at-risk steatohepatitis with significant fibrosis (by liver biopsy, when available, or by non-invasive panels validated for that purpose); or (C) risk of adverse liver-related outcomes (e.g., by elastography- or biomarker-defined thresholds) (LoE 3, open recommendation, consensus).
No MASH-targeted pharmacotherapy can currently be recommended for adults with MASH at the cirrhotic stage (LoE 5, weak recommendation, strong consensus).
Given the lack of robust demonstration of histological efficacy on steatohepatitis and liver fibrosis derived from large phase III trials and potential long-term risks, vitamin E cannot be recommended as a MASH-targeted therapy (LoE 2, weak recommendation, strong consensus).
Statement
For individuals with MASLD undergoing therapy with resmetirom, data on sustainability of histological benefits, individual prediction of response, liver-related outcomes and long-term safety are not currently available (LoE 5, strong consensus).
Liver-Directed Thyroid Hormone Receptor Agonists
The incidence of clinical and subclinical hypothyroidism appears to be higher in individuals with MASLD or MASH relative to age-matched controls, and low thyroid function is associated with more severe outcomes [361, 362]. Thyroid hormones reduce hepatic steatosis by stimulating hepatic lipophagy and mitochondrial biogenesis, and by inhibiting hepatic lipogenesis. They can also interfere with fibrogenesis by inhibiting TGF-β signalling [363, 364]. Thyromimetics that are selective for the β subtype (liver expressed) of the thyroid hormone receptor have been evaluated as a treatment for MASH. Resmetirom is an orally active, liver-directed, thyroid hormone receptor agonist with high selectivity for the β1 receptor [365]. Results of a registrational, phase III trial of resmetirom in individuals with non-cirrhotic MASH (mostly fibrosis stages 2 and 3) of 1 year duration have been reported [366]. In the US, this led to the accelerated approval of resmetirom in March 2024. Resmetirom performed better than placebo as it improved both disease activity (resolution of steatohepatitis) and fibrosis. Progression of fibrosis in individuals with stage 2 fibrosis was lower than in the placebo arm. Liver enzymes and serum lipids were also significantly reduced while the effects on glycaemic control and body weight were neutral. Side effects were mostly gastrointestinal with good overall safety and tolerability. Predictive criteria of response and optimal duration of therapy are currently unknown. The phase III trial is continuing to determine if longer treatment results in improved clinical outcomes [367], including preventing the progression to cirrhosis. A trial exploring clinical outcomes in a cirrhotic population is also ongoing.
The MAESTRO-NASH phase III registrational trial of resmetirom included individuals with at-risk MASH defined histologically by active steatohepatitis (NAS ≥4) and significant fibrosis (stage 2 or 3). While individuals selected for pharmacotherapy would ideally fit the same histological profile as those included in the registrational trial(s), it is anticipated that liver biopsy will be used sparingly in clinical practice, and a liver biopsy is not required by the drug label in the US. Therefore alternative non-invasive panels with high predictive value validated for the detection of at-risk MASH (e.g. NIS2+ [179, 368, 369], FAST [169, 370], or MRI-based panels [168, 170, 173, 371, 372]) or those with well-validated thresholds to define risk of advanced fibrosis or liver-related outcomes (e.g. VCTE-LSM ≥10 kPa [174, 191], MRE ≥5 kPa [178, 373], or ELF ≥9.8 [177, 374, 375]) could play an important role in selecting individuals for pharmacotherapy (Table 6], as long as thresholds for a high likelihood of cirrhosis are not met. Notably, resmetirom significantly improved MRI-PDFF and liver stiffness measurements in the MAESTRO-NAFLD phase III trial that did not require a liver biopsy for study inclusion [376].
In the US, resmetirom is given at a daily dose of 80 mg in individuals with a body weight <100 kg and at 100 mg in those with a body weight ≥100 kg (dose reduction is advised with concomitant use of moderate CYP2C8 inhibitors such as clopidogrel). At these doses, the most common side effects were diarrhoea (up to 33%), nausea (up to 22%), pruritus (up to 11%) and vomiting (up to 11%) [366]. Individuals receiving resmetirom should be monitored for gastrointestinal side effects and thyroid hormone function. Circulating sex hormone-binding globulin (SHGB) levels have been suggested as a surrogate for target engagement.
Importantly, evidence is currently limited to 52-week histological outcome data. This raises uncertainty as to whether responses will be sustained in the long-term. Similarly, there is currently no evidence to provide confident guidance on when to stop treatment, particularly considering that about 70-80 % of participants did not respond to treatment according to histological criteria. In the MAESTRO-NASH trial, a ≥30 % reduction in hepatic lipid content by MRI-PDFF and a 120% increase in SHGB were associated with a positive treatment response [366]. Linked to the lack of long-term data, there is uncertainty regarding long-term safety and effectiveness of resmetirom. Particularly the effects on the pituitary-thyroid hormone axis and increases in SHGB levels warrant close monitoring for thyroid, gonadal or bone disease [377].
Many individuals who may be eligible for treatment with resmetirom will already be receiving other pharmacological treatments, e.g. GLP1RA, which raises the question of how to integrate resmetirom into combination treatments. About 14% of participants in the MAESTRO-NASH trial were on GLP1RA treatment, with stable dosage in the 6 months and stable body weight in the 3 months preceding screening liver biopsy [366]. Therefore, it appears reasonable to use resmetirom according to the same criteria in individuals already receiving GLP1RA treatment. Given the burden of the disease at current epidemiological estimates and corresponding financial strains on healthcare systems, future cost-effectiveness studies are warranted.
Currently, resmetirom is the only MASH-targeting drug with positive results from a registrational phase III clinical trial. However, considering the expected evolution of MASH-targeted treatment options in coming years [378], recommendations will need to be continuously updated to reflect the latest evidence.
Vitamin E
Vitamin E is a lipid-soluble vitamin acting as a peroxyl radical scavenger with antioxidant, anti-inflammatory, and anti-apoptotic properties. It reduces de novo lipogenesis and therefore contributes to a reduction in liver lipid content. Higher dietary intake of vitamin E, as measured by serum alpha tocopherol levels, was associated with reduced mortality from several chronic conditions (cardiovascular diseases, stroke, cancer) [379, 380], suggesting that current levels of dietary intake are insufficient [380]. Phenome-wide analyses in the general population suggested that increased dietary vitamin E intake protects against MASLD, both clinically and radiologically defined, particularly in individuals with T2D [381]. Nonetheless, the impact of vitamin E supplementation on cardiovascular mortality or prostate cancer is still not settled and clinical intervention studies have shown no benefit [382–384]. For individuals with MASH and bridging fibrosis or cirrhosis, case-control studies have shown that long-term exposure to vitamin E is associated with decreased risk of death, transplant and hepatic decompensation [385]. In the largest RCT to date, vitamin E supplementation (800 IU daily over 2 years) in individuals with non-diabetic MASH resulted in improvement in both steatosis and disease activity, which was corroborated by a reduction in liver enzymes [386]. Smaller studies have suggested reduction in liver enzymes but there is currently no clear data on fibrosis improvement and no large phase III trial has been performed.
Ursodeoxycholic Acid
Ursodeoxycholic acid (UDCA) is a natural hydrophobic bile acid with wide hepatoprotective effects including antioxidant, immunomodulatory and anti-apoptotic properties. There are three larger, placebo-controlled trials of UDCA in MASH differing in the dose of UDCA used [387–389] and only two of them report histological endpoints [387, 388]. Despite several limitations and methodological differences, there is a strong indication of biochemical efficacy (ALT reduction) and a good safety profile, but no proof of histological efficacy. A synthetic UDCA derivative, 24-norursodeoxycholic acid (norucholic acid), has shown anti-cholestatic, anti-inflammatory and anti-fibrotic properties in preclinical models [390] and is being tested for MASH with initial results showing improvement in ALT and liver steatosis [391].
Obeticholic Acid
Obeticholic acid (OCA) is an oral, synthetic analogue of chenodeoxycholic acid designed to have a much stronger, nanomolar, potency as a FXR (farnesoid X receptor) agonist than the native bile acid [392]. The drug is approved at a 5 or 10 mg daily dose for second-line therapy in primary biliary cholangitis [393] and was developed for MASH at a higher dose (25 mg daily), based on a phase II placebo-controlled trial showing improvement in fibrosis and liver enzymes after 18 months of treatment [394]. These results were confirmed in a large phase III registrational trial of individuals with MASH and significant fibrosis (cirrhosis excluded) both at the interim analysis of 931 individuals [395] and at a subsequent analysis on 1,607 individuals by a different, consensus, pathologists’ panel [396]. At 25 mg daily, OCA achieved both a higher proportion of fibrosis improvement and a lower proportion of worsening than placebo. Despite improved disease activity (hepatocellular ballooning and lobular inflammation) there was no significant difference in resolution of steatohepatitis. Dose-related pruritus and increases in LDL cholesterol are expected class effects of FXR agonists [397, 398] but additional concerns over the risk-benefit ratio (including hepatotoxicity and hepatic events) resulted in a denial of accelerated approval, leading to discontinuation of the clinical outcome phase of the registrational trial and of the development programme in MASH.
Omega-3 Polyunsaturated Fatty Acids
Omega-3 polyunsaturated fatty acids have hepatic anti-inflammatory and insulin-sensitising effects but are decreased in the livers of individuals with MASH [399]. However, supplementation with eicosapentaenoic acid (in ethyl ester formulation) did not show any histological efficacy vs. placebo in RCTs [400, 401]. Studies with icosabutate, a structurally engineered omega 3 fatty acid with distinct intracellular distribution and metabolism [402] are ongoing.
Statins
MASLD induces atherogenic dyslipidaemia and statin therapy is therefore often indicated to prevent cardiovascular events [403]. The safety of statins has been well established in individuals with MASLD [403, 404] with no increased risk of hepatotoxicity [405], yet many individuals with MASLD are undertreated [406]. Case-control studies have shown that statin intake is associated with a reduced risk of MASLD, MASH and liver fibrosis [407], as well as a reduction in the risk of hepatic decompensation, mortality and HCC in individuals with cirrhosis [408]. Nonetheless the efficacy of statins, specifically for treating MASH, cannot be established, since there are no large RCTs of statins with histological endpoints. The same holds true for fibrates and ezetimibe. Silymarin (an extract of milk thistle) may improve liver enzymes but the few, small, RCTs available [409, 410] did not document histological improvement.
In adults with MASH, is there sufficient evidence to recommend prescription of existing glucose-lowering drugs to reduce histologically/non-invasively assessed liver damage/fibrosis and liver-related outcomes compared to no pharmacological intervention?
Recommendations
In the absence of a formal demonstration of histological improvement in large, well conducted, phase III trials, glucagon-like peptide 1 receptor agonists (GLP1RA) cannot currently be recommended as MASH-targeted therapies (LoE 5, strong recommendation, strong consensus).
GLP1RAs are safe to use in MASH (including compensated cirrhosis) and should be used for their respective indications, namely type 2 diabetes and obesity, as their use improves cardiometabolic outcomes (LoE 2, strong recommendation, strong consensus).
Where available, pioglitazone is safe to use in adults with non-cirrhotic MASH but given the lack of robust demonstration of histological efficacy on steatohepatitis and liver fibrosis in large phase III trials, pioglitazone cannot be recommended as a MASH-targeted therapy (LoE 2, weak recommendation, consensus).
There is insufficient evidence to recommend the use of sodium-glucose cotransporter-2 (SGLT2) inhibitors or metformin as MASH-targeted therapies; however, they are safe to use in MASLD and should be used for their respective indications, namely type 2 diabetes, heart failure and chronic kidney disease (LoE 3, strong recommendation, strong consensus).
Statements
In case of substantial weight loss induced by GLP1RAs, a hepatic histological benefit could be expected, although this has not been extensively documented so far (LoE 2, strong consensus).
There is insufficient evidence to support using any other glucose-lowering drug class as MASH-targeted therapies (LoE 5, strong consensus).
Incretin Mimetics
Glucagon-like peptide-1 receptor agonists (GLP1RAs), single or dual (i.e., glucose-dependent insulinotropic polypeptide [GIP]-GLP1RAs), are approved for the treatment of T2D, with some also approved for obesity (liraglutide, semaglutide, and tirzepatide); these incretin mimetics have shown beneficial effects on cardiovascular and renal outcomes [411]. Their different actions include potentiation of prandial insulin secretion, as well as an inhibition of appetite and increased satiety, mediated both centrally and through reduced gastric motility, which mainly accounts for the weight-loss effects [412]. Other hormones or their analogues potentiate the anorexigenic effects of GLP1 (GIP, glucagon, cagrilintide) or have additional peripheral effects such as increasing lipolysis, lipid oxidation and energy expenditure and are now being developed as dual or triple co-agonists that can induce a similar magnitude of weight loss as bariatric surgery [413]. Some of the studies performed in T2D or obesity documented a reduction in liver enzymes and hepatic lipid content, reinforcing the rationale to test co-agonists in MASH [411].
While an initial study with liraglutide indicated a histological benefit in MASH [414], drugs that are being developed for MASH now include semaglutide, and dual GLP1-GIP (e.g. tirzepatide), dual GLP1-glucagon (e.g. cotadutide, survodutide, efinopegdutide) or triple GLP1-GIP-glucagon (e.g. retatrutide) agonists. The largest available trial on semaglutide in MASH (vs. placebo over an 18-month treatment period) demonstrated resolution of steatohepatitis but no fibrosis improvement [415]. A large registrational, phase III study with semaglutide is ongoing. Combining semaglutide with lipogenesis inhibitors may provide additional benefit [416, 417] and such approaches are being tested in larger trials. Histology data are not yet available for the newer dual and triple agonists. Tirzepatide (GLP1-GIP RA) has been shown to significantly reduce both liver and visceral fat in those with T2D, in association with major weight loss (comparable to bariatric surgery) [418], and promising results on steatohepatitis resolution from a phase II study in MASH have been communicated. Dual GLP1-glucagon RAs (cotadutide and efinopegdutide) have also been shown to improve liver steatosis, liver enzymes and indexes of fibrosis in individuals with MASLD [419, 420]. Weight-loss effects of survodutide are promising [421] as are the preliminary histology data from a phase IIb trial [422].
Case-control studies have suggested that exposure to GLP1RAs or SGLT2 inhibitors in people with T2D is associated with a reduction in liver-related outcomes [423, 424] although the only available pilot trial of semaglutide in individuals with cirrhosis did not demonstrate a histological improvement [425].
Sodium-Glucose Co-Transporter-2 Inhibitors
SGLT2 inhibitors are approved for T2D, with some (empagliflozin, dapagliflozin) also approved for chronic kidney disease and heart failure because of their beneficial effect on cardiovascular and renal outcomes [426]. They induce renal glucosuria, weight loss, blood pressure reduction, and protection from major cardiovascular outcomes, including heart failure. The weight loss is due to renal energy loss and reduction in fat mass, with reductions in both visceral and abdominal subcutaneous adipose tissue [427]. Controlled clinical trials with liver histological endpoints are currently not available. Trials in people with T2D (not all with MASLD and some excluding high ALT values) have shown a moderate reduction in liver lipid content with empagliflozin [428, 429], dapagliflozin [430] and licogliflozin [431]. Reductions in ALT were shown with empagliflozin [428] and licogliflozin [431].
Peroxisome Proliferator-Activated Receptor Agonists
In several RCTs, pioglitazone, a thiazolidinedione which mainly activates peroxisome proliferator-activated receptor (PPAR)γ, has been shown to improve histological features of steatohepatitis [386, 432–434], without a clear effect on fibrosis regression even after prolonged (3-year) therapy [433]. However, no large, international, phase III trial has been conducted and pioglitazone has been withdrawn from the market in several European countries. The drug has beneficial effects on insulin sensitivity, glycaemic control, serum lipids and prevention of cardiovascular events in individuals with T2D [435, 436] but the side effect profile (weight gain, pedal oedema, haemodilution, bone loss in post-menopausal women and a debate around the risk of bladder cancer) has limited its development for MASH [437]. Pioglitazone R-enantiomers lacking PPARγ activity but retaining non-genomic effects through inhibition of the mitochondrial pyruvate carrier have shown preliminary biochemical and antifibrotic responses with improved side effect profiles [438]. A phase IIb trial showed a dose-dependent histological improvement of steatohepatitis and fibrosis with the pan-PPAR agonist lanifibranor [439], though side effects were reminiscent of PPARγ agonists, namely a 2.5% increase in weight, pedal oedema and mild anaemia. A large registrational, phase III trial is ongoing. Saroglitazar, a dual PPARα/γ agonist has been shown to improve insulin resistance, liver steatosis and liver enzymes [440] and is approved in India for the treatment of T2D and MASH [441]. Trials with liver histological endpoints are ongoing.
Metformin
Small and uncontrolled initial trials of metformin have shown an ALT reduction and an insulin-sensitising effect [442, 443], but were not followed by sufficiently large and well-conducted RCTs. Currently, there is no evidence that metformin alone can improve histology in MASH. As far as clinical outcomes, there is some indication from observational and case-control studies that, in people with T2D and MASLD-related advanced fibrosis or cirrhosis, metformin may improve transplant-free survival (but not the risk of hepatic decompensation), and reduce the risk of primary liver and extrahepatic cancer [444, 445]. Thus, metformin should not be discontinued in those individuals with cirrhosis (unless discontinuation is required due to hepatic decompensation or renal failure), as this could increase mortality [446].
Fig. 4 summarises our recommended choice of pharmacological treatment options in individuals with MASH, depending on comorbidities and stage of disease.
Fig. 4.
Treatment recommendations beyond lifestyle modification in MASLD/MASH. The recommended choice of pharmacological treatment options in individuals with MASLD/MASH is dependent on comorbidities and stage of disease. BMI, body mass index; GLP1RA, glucagon-like peptide 1 receptor agonist; HCC, hepatocellular carcinoma; MASH, metabolic dysfunction-associated steatohepatitis; MASLD, metabolic dysfunction-associated steatotic liver disease; SGLT2, sodium-glucose cotransporter 2; T2D, type 2 diabetes.
In adults with MASH, is there sufficient evidence to recommend prescription of existing weight-loss agents to reduce histologically/non-invasively assessed liver damage/fibrosis and liver-related outcomes compared to no pharmacological intervention?
Recommendation
Non-incretin-based weight-loss agents are not recommended as MASH-targeted therapies (LoE 5, strong recommendation, strong consensus).
Controlled trials (with histological endpoints or liver-related outcomes) of weight-loss agents other than incretin hormone analogues [447] (e.g., orlistat, phentermine-topiramate, naltrexone-bupropion) have either not been performed or have been inconclusive [448].
Treatment of MASLD: Surgical and Endoscopic Therapy
In adults with MASLD and obesity, are bariatric/metabolic surgery procedures or endoscopic weight-loss interventions effective to reduce histologically/non-invasively assessed liver damage and liver-related outcomes compared with no intervention?
Recommendations
In adults with non-cirrhotic MASLD who have an approved indication, bariatric surgery should be considered, because it can induce long-term beneficial effects on the liver and is associated with remission of type 2 diabetes and improvement of cardiometabolic risk factors (LoE 3, strong recommendation, strong consensus).
In adults with MASLD-related compensated advanced chronic liver disease/compensated cirrhosis who have an approved indication, bariatric surgery can be considered but careful evaluation (indication, type of surgery, presence of clinically significant portal hypertension) by a multidisciplinary team with experience in bariatric surgery in this particular population is required (LoE 4, weak recommendation, strong consensus).
Metabolic/bariatric endoscopic procedures require further validation as MASH-targeted therapy and cannot currently be recommended (LoE 4, weak recommendation, strong consensus).
The most common bariatric surgery procedures include purely gastric components like gastric banding (either adjustable or nonadjustable), sleeve gastrectomy and vertical banded gastroplasty, or techniques that divert gastric content distally into the small intestine (gastric with diversion) like Roux-en-Y gastric bypass, biliopancreatic diversion or one anastomosis gastric bypass. Indications for bariatric surgery are BMI ≥40 kg/m2, or BMI ≥35–40 kg/m2 in the presence of associated comorbidities, or BMI ≥30–35 kg/m2 if people have T2D and/or hypertension with poor control despite optimal medical therapy [449]. In the Asian population, the threshold is lower since clinical obesity is recognised in individuals with BMI >25 kg/m2 [41].
Many prospective studies have shown that bariatric surgery induces stable weight loss, remission of T2D [450], improvement in cardiovascular risk [451], and a reduction in cancer (including liver cancer) risk [452]. In line with data from prospective studies, the large retrospective SPLENDOR study found significantly lower rates of adverse liver-related outcomes and major adverse cardiovascular events in individuals who underwent metabolic surgery compared to non-surgical controls [453]. Two meta-analyses that included more than 30 studies and enrolled more than 3,700 individuals with MASLD/MASH undergoing bariatric surgery showed that Roux-en-Y gastric bypass was associated with the most individuals achieving improvement in steatohepatitis, and had a greater impact on MASLD histology compared with other procedures [454, 455]. Roux-en-Y gastric bypass improved or resolved liver fibrosis in 30% of individuals [454]. Interestingly, the percentage of individuals with improved steatosis and hepatic fibrosis was higher in Asian countries [455]. However, in a study with control biopsies after surgery, advanced fibrosis (bridging fibrosis or cirrhosis) persisted in 47% of individuals sometimes even 5 years or more post-surgery and despite significant weight loss [456]. A better understanding of weight loss-dependent and -independent effects on hepatic fibrosis is warranted.
In an observational study, MASH was resolved in 84% of 180 individuals with class 2 obesity and MASH 5 years after Roux-en-Y gastric bypass (66%), sleeve gastrectomy (12%) or gastric banding (22%) [457]. The BRAVES multicentre, open-label, randomised study demonstrated histological resolution of MASH without worsening of fibrosis in 55% of those assigned to Roux-en-Y gastric bypass or sleeve gastrectomy at 1-year follow-up vs. 15% in the lifestyle modification group in the intention-to-treat analysis [458]. In this open-label RCT, fibrosis improvement by ≥1 stage without worsening of MASH after 1 year was achieved in 37% and 39% of patients after Roux-en-Y gastric bypass and sleeve gastrectomy, respectively, vs. 23% after lifestyle modification. However, the majority of study participants had mild fibrosis [458]. In this RCT, about 6% of participants had severe adverse events related to surgery [458]. Endoscopic bariatric/metabolic therapies for weight loss are more affordable and associated with a lower risk of complications. These endoscopic procedures include intragastric balloon, endoscopic sleeve gastroplasty, aspiration device, transpyloric shuttle, Botox injection, duodenal jejunal bypass liner, duodenal mucosa resurfacing, incisionless magnetic anastomosis system, and primary obesity surgery endoluminal. A meta-analysis that included 33 studies with 1,710 individuals reporting liver-related endpoints (e.g., NITs, liver fibrosis, steatosis) showed a significant improvement in parameters related to liver steatosis and fibrosis with various endoscopic bariatric therapies [459]. However, most included studies were retrospective, with few histology data.
End-Stage Liver Disease and Liver Transplantation
In adults with MASH-related cirrhosis, should dietary and lifestyle recommendations be adapted to the severity of liver disease, nutritional status, and sarcopenia?
Recommendations
In adults with MASH cirrhosis, it is recommended that dietary and lifestyle recommendations be adapted to the severity of liver disease, nutritional status and the presence of sarcopenia/sarcopenic obesity (LoE 2, strong recommendation, strong consensus).
In adults with sarcopenia, sarcopenic obesity or decompensated cirrhosis, it is recommended that a high-protein diet is provided, as well as a late-evening snack. (LoE 2, strong recommendation, consensus).
Moderate weight reduction can be suggested in adults with compensated cirrhosis and obesity, with an emphasis on high protein intake and physical activity to maintain muscle mass and reduce the risk of sarcopenia (LoE 3, weak recommendation, strong consensus).
Among individuals with cirrhosis awaiting liver transplantation, malnutrition and sarcopenia (a progressive decline in skeletal muscle mass and function [460]) are prevalent. Sarcopenia affects 50-60% of individuals [461] and is associated with higher rates of wait-list complications, morbidity, and mortality [462, 463]. Sarcopenic obesity, the state of decreased muscle mass in the setting of increased fat mass, occurs mainly in MASH-related cirrhosis and is found in 20–35% of individuals with cirrhosis pre-and-post liver transplant [460, 464]. Obesity and sarcopenic obesity are risk factors for clinical decompensation and worsen prognosis [45, 464]. Evaluation for sarcopenia includes the skeletal muscle index or psoas muscle area at the third lumbar vertebra, if a CT scan has been performed, and the measurement of frailty using tools like hand grip or liver frailty index and other diagnostic procedures summarised in the joint European Society for Clinical Nutrition and Metabolism (ESPEN)-EASO consensus statement [465].
Nutritional intervention improves nutritional status, hepatic encephalopathy, survival, and quality of life in people with cirrhosis [466, 467]. In an RCT among individuals with decompensated cirrhosis, a 6-month dietitian-supported home-based intensive high-calorie, protein-rich nutrition therapy was associated with improvement in frailty, sarcopenia and, among treatment-adherent individuals, liver disease scores and survival [468]. The EASL CPGs on nutrition in chronic liver disease provide a comprehensive review of the recommended nutritional intake in individuals with cirrhosis [463]. The approach of the majority of nutritional interventions in cirrhosis is to supply at least 35 kcal/kg of body weight/day, with a daily recommended protein intake of 1.2–1.5 g/kg of body weight/day (sufficiently rich in branched-chain amino acids) to prevent or reverse muscle mass loss [460, 461, 463] (Table 10). In individuals with compensated cirrhosis and obesity, a reduction in body weight through lifestyle interventions, including moderate caloric restriction and supervised moderate-intensity physical exercise, has been shown to reduce portal pressure and may prevent clinical decompensation [45, 469]. For individuals with cirrhosis and obesity, weight-loss interventions require special attention to avoid sarcopenia [470].
Table 10.
Summary of protein, energy, and dietary pattern recommendations for adults with cirrhosis as indicated by medical associations’ Practice Guidance/Guidelines. In addition, individuals with cirrhosis must abstain from alcohol
| Society/Association | EASL 2019 [463] | ESPEN 2019 [461] and Joint ESPEN/UEG guideline 2023 [470] | AASLD 2021 [460] | |||
|---|---|---|---|---|---|---|
| BMI Status† |
Mixed BMIs | Obese (BMI >30 kg/m2) | Mixed BMIs | Obese (BMI >30 kg/m2) | Non-obese | Obese (non-hospitalised, clinically stable) |
| Daily energy | 35 kcal/kg actual BW (in nonobese individuals) | >5–10% WR, moderately hypocaloric diet (-500–800 kcal/d) | 30-35 kcal/kg only for DC. Regular energy requirements in CC |
WR. No need for increased energy intake |
≥35 kcal/kg body weight/day | 25-35 kcal/kg/day for individuals with BMI 30-40 kg/m2, and 20-25 kcal/kg/day for individuals with BMI ≥40 kg/m2. WR if medically required, under the supervision of a multidisciplinary team. Caution applied to prescribing weight loss in decompensated cirrhosis. |
| Daily protein | 1.2–1.5 g/kg actual BW | >1.5 g/kg IBW | 1.2 g/kg (for non-malnourished individuals with CC) to 1.5 g/kg (for malnourished and/or sarcopenic cirrhosis) | Individuals with overweight or obesity and compensated cirrhosis: 1.2 g/kg ABW/d. Individuals with overweight or obesity and compensated cirrhosis undergoing weight-loss programs: 1.2-1.5 g/kg ABW/d. Individuals with overweight or obesity and compensated cirrhosis and malnutrition or sarcopenia: 1.5 g/kg ABW/d. | 1.2-1.5 g/kg IBW For individuals with cirrhosis who are critically ill, a target of 1.2-2.0 g/kg IBW |
Intake of target protein (1.2-1.5 g/kg/day) and physical activity are required to reduce the loss of muscle contractile function and muscle mass that can occur with weight loss. |
| Meal patterns | Split food intake into 3 main meals and 3 snacks | Three to five meals a day and a late evening snack | Maximum interval of 3-4 hours between nutritional intake while awake. To minimise nocturnal fasting time, an early breakfast and/or late-evening snack recommended |
|||
| Dietary protein source in case of HE | Individuals may tolerate animal protein (meat) less well than vegetable protein (beans, peas, etc.) and dairy proteins | In individuals who are protein “intolerant”, vegetable proteins should be used | A diverse range of protein sources, including vegetable and dairy products, should be encouraged. | |||
ABW, adjusted body weight; BMI, body mass index; CC, compensated cirrhosis; DC, decompensated cirrhosis; HE, hepatic encephalopathy; IBW, ideal body weight; WR, weight reduction.
ABW = reference body weight (in which BMI = 25) + 0.33*(actual body weight - reference body weight).
†In a case of fluid retention, body weight should be corrected by evaluating the individual’s dry weight.
To prevent accelerated starvation and the related proteolysis, there is a need to shorten fasting intervals between meals by eating every 4-6 hours and having a late-evening snack [463]. A late-evening snack containing complex carbohydrates and protein reduces lipid oxidation, improves nitrogen balance, reduces skeletal muscle proteolysis, increases muscle mass, reduces hepatic encephalopathy and improves quality of life [471, 472].
Physical activity and exercise are anabolic stimuli that can improve muscle mass and function. Consistent benefits of exercise demonstrated in RCTs include reversal of sarcopenia and improvements in aerobic capacity, muscle mass and strength, performance measures, health-related quality of life and hepatic venous pressure gradient [473, 474].
In adults with MASH-related cirrhosis, how should pharmacologic interventions for diabetes and lipid control or cardiovascular prevention be adapted to the severity of the liver condition?
Recommendations
Metformin can be used in adults with compensated cirrhosis and preserved renal function but should not be used in adults with decompensated cirrhosis, especially when there is concomitant renal impairment, because of the risk of lactic acidosis (LoE 3, strong recommendation, strong consensus).
Sulfonylureas should be avoided in adults with hepatic decompensation because of the risk of hypoglycaemia (LoE 4, weak recommendation, strong consensus).
GLP1 receptor agonists can be used in adults with Child-Pugh class A cirrhosis, according to its indication (LoE 2, weak recommendation, strong consensus).
SGLT2 inhibitors can be used in adults with Child-Pugh class A and B cirrhosis (LoE 4, weak recommendation, consensus).
Statins can be used in adults with chronic liver disease, including those with compensated cirrhosis; they should be used in adults according to cardiovascular risk guidelines to reduce cardiovascular events (LoE 1, strong recommendation, strong consensus).
Metformin improves ALT but not histological steatosis, inflammation and fibrosis in individuals with MASLD [475]. However, observational data suggest a potential protective effect against HCC [476, 477]. Metformin may cause lactic acidosis through impairment of oxidative phosphorylation [478]. The risk of metformin-associated lactic acidosis is increased in individuals with renal impairment and hepatic decompensation, especially when both are present [479].
The risk of sulfonylurea-induced hypoglycaemia is increased in individuals with advanced liver disease. Gliclazide has significant hepatic metabolism. Hepatotoxicity has also been reported for glibenclamide and is rarely seen with gliclazide [480, 481].
SGLT2 inhibitors increase glycosuria. Apart from an improvement in blood glucose, they reduce bodyweight and blood pressure, and have been shown to have beneficial cardiovascular effects, prevent progression of renal disease, and potentially even improve ALT and MRI-measured intrahepatic triglyceride content [482]. Drug exposure to empagliflozin and dapagliflozin increased by 67-75% in individuals with Child-Pugh class C cirrhosis. Drug exposure to canagliflozin increased by 96-111% in individuals with Child-Pugh class B cirrhosis, and the drug has not been studied in individuals with Child-Pugh class C cirrhosis. SGLT2 inhibitors should be used with caution or avoided in people with severe renal impairment.
Data on the use of GLP1RAs in advanced liver disease are limited. In a small RCT of 71 participants with compensated MASH-related cirrhosis, semaglutide at a dose of 2.4 mg weekly was well tolerated and improved steatosis, liver enzymes, bodyweight and HbA1c [425]. Future studies should also scrutinise the impact of GLP1RAs on adipose tissue and skeletal muscle mass, especially as sarcopenia is a risk factor for mortality in individuals with cirrhosis.
Statins are important treatments to prevent cardiovascular events. Multiple observational studies suggest a benefit of statins on the prevention of HCC and/or cirrhotic complications [483]. ALT elevation may be observed in up to 3% of individuals during statin treatment, but severe liver injury is rare, and liver fibrosis progression has not been observed [484]. There are few studies on the use of statins in individuals with decompensated cirrhosis. One RCT testing simvastatin in individuals with variceal haemorrhage failed to show an impact on rebleeding and suggested an improvement in overall survival, and the drug was safe in this high-risk population [485]. However, using high-dose statins in decompensated cirrhosis confers an increased risk of severe adverse events. In a multicentre European clinical trial in individuals with Child-Pugh class B or C cirrhosis, 19% of those receiving simvastatin 40 mg daily developed liver toxicity and rhabdomyolysis [486].
In adults with MASLD, can non-invasive scores, serum markers, liver stiffness measurements, and/or imaging replace hepatic venous pressure gradient (HVPG) and endoscopy in identifying individuals with clinically significant portal hypertension and varices requiring treatment, respectively?
Recommendations
Liver stiffness measurement (LSM) by vibration-controlled transient elastography (VCTE) ≤15 kPa plus platelet count ≥150 × 109/L may be used to rule out clinically significant portal hypertension (CSPH) in adults with MASLD (LoE 3, weak recommendation, strong consensus).
If CSPH is present, non-selective beta-blockers may be started unless contraindicated (LoE 3, weak recommendation, strong consensus).
In adults with compensated advanced chronic liver disease but LSM ≥20 kPa and/or platelet count <150 × 109/L, an upper gastrointestinal endoscopy should be performed to screen for varices unless they already fulfil the criteria to initiate non-selective beta-blockers (LoE 3, strong recommendation, strong consensus).
Statement
The threshold of LSM ≥25 kPa to rule in CSPH is only applicable to non-obese (BMI <30 kg/m2) adults with MASLD; while obesity can confound LSM, current evidence is insufficient to suggest the optimal non-invasive test to rule in CSPH in adults with MASLD and obesity (LoE 3, strong consensus).
According to the Baveno VII criteria, one may exclude CSPH when LSM is <15 kPa and platelet count is normal at ≥150 × 109/L, and rule in CSPH when LSM is >25 kPa [487]. The LSM cut-off of 25 kPa has been used to rule in CSPH in individuals with various liver diseases such as chronic viral hepatitis and MASLD [487]. However, both underweight and obesity have been shown to confound LSM, resulting in false-positive diagnoses of CSPH [488]. The ANTICIPATE-NASH model, a function of LSM, platelet count and BMI, may help in assessing the risk of CSPH and liver-related events in individuals with compensated MASH-related cirrhosis and guide shared decision making on endoscopic surveillance [489] (available online: https://www.bcn-liverhuvh.com/resources).
The PREDESCI trial showed that the use of non-selective beta-blockers (NSBBs) in individuals with CSPH could reduce not only variceal haemorrhage but also other decompensating events [490]. NSBBs and endoscopic variceal ligation are both acceptable treatments for primary prophylaxis against the first episode of variceal haemorrhage [487]. Therefore, if an individual has CSPH and can tolerate NSBBs, it is reasonable to start NSBBs without first performing upper gastrointestinal endoscopy.
In case of contraindications or NSBB intolerance, the Baveno VI criteria can be used to select individuals for upper gastrointestinal endoscopy to screen for varices. Several studies have confirmed a low missed rate of <5% for varices needing treatment when LSM is <20 kPa and platelet count is ≥150 × 109/L [491]. This notion has been confirmed in a multicentre cohort of individuals with MASH-related cirrhosis [492].
Spleen stiffness measurement by VCTE has been shown to correlate with hepatic venous pressure gradient and predict the presence of varices needing treatment [493, 494]. There are, however, two caveats. First, data in individuals with MASLD are limited. Second, the existing data largely used the 50 Hz probe, which was not designed for spleen stiffness measurement. The current dedicated probe for spleen stiffness measurement uses ultrasound at a frequency of 100 Hz [495]. Because the measurement of shear wave velocity is influenced by the frequency of ultrasound, future studies should define optimal cut-offs for the interpretation of spleen stiffness measurement.
In adults with MASLD who are candidates for liver transplantation, should the evaluation of (cardiometabolic) comorbidities in the pre- and post-transplant phase be different from that of individuals with liver disease of other aetiologies?
Statement
Adults with MASLD are at increased risk of major cardiovascular events in the pre-, peri- and post-transplant phase (LoE 2, strong consensus).
Recommendations
Adults with MASLD who are candidates for liver transplantation should be evaluated by a multidisciplinary team for cardiovascular and metabolic comorbidities to mitigate the risk of major cardiovascular events in the pre-, peri- and post-transplant phase (LoE 3, strong recommendation, strong consensus).
A comprehensive screening for comorbidities in adults with MASLD before liver transplantation (Table 11), including a stepwise and risk-adjusted cardiac work-up algorithm (Fig. 5), may help to optimise management of adults with MASLD before, during and after liver transplantation (LoE 5, weak recommendation, strong consensus).
Table 11.
Screening and management for comorbidities in individuals with MASLD before liver transplantation. Modified from [496, 497]
| Condition | Recommendation |
|---|---|
| Type 2 diabetes | • Screen for impaired fasting glucose (IFG) or glucose tolerance (IGT) and/or T2D (OGTT, HbA1c) • Achieve glycaemic control before LT • Preferentially use weight-lowering (e.g. SGLT2 inhibitors, GLP1RA) or weight-neutral (e.g. metformin) diabetes medication, considering risk of other diabetes complications, if liver and/or renal function allow this |
| Nutrition | • Assess nutritional status before LT • Assess alcohol consumption • Healthy diet, physical exercise and lifestyle modification (including weight reduction in individuals with obesity) represent pillars in pre-LT management |
| Cardiovascular | • Pre-LT cardiovascular risk stratification is mandatory • Risk-adapted algorithm of cardiac work-up should be followed (see Fig. 5) • LT candidates with cardiovascular risk should be managed with goal-directed medical management (e.g., statins, anti-platelet agents, beta blockers, RAAS blockers), based on the stage of cirrhosis and renal function |
| Kidney | • Kidney function should be adequately monitored before LT • Comedications need to be adjusted (or replaced) dependent on kidney function |
| Malignancies | • Screening for pre-LT malignancies should follow the same protocols applied to individuals with non-MASLD related cirrhosis (including gastrointestinal and genital cancers) |
GLP1RA, glucagon-like peptide-1 receptor agonist; HbA1c, glycated haemoglobin; LT, liver transplantation; MASLD, metabolic dysfunction-associated steatotic liver disease; OGTT, oral glucose tolerance test; RAAS, renin-angiotensin-aldosterone system; SGLT2, sodium-glucose cotransporter-2.
Fig. 5.
Cardiovascular work-up algorithm in the evaluation of individuals with MASLD before liver transplantation. Adults with MASLD who are candidates for liver transplantation should be evaluated by a multidisciplinary team using a stepwise and risk-adjusted cardiac work-up algorithm to mitigate the risk of major cardiovascular events in the pre-, peri- and post-transplant phase (modified from [496, 497]). CCTA, coronary computed tomography angiography; CV, cardiovascular; DSE, dobutamine stress echocardiography; ECG, electrocardiogram; LT, liver transplantation; TTE, transthoracic echocardiography. *Indicates suboptimal sensitivity in high-risk populations.
Adult liver transplant candidates with MASLD have different characteristics compared to candidates with other aetiologies of liver disease. In a large analysis of 68,950 adults undergoing first liver transplantation between 2002 and 2016 using the European Liver Transplant Registry database, individuals transplanted for MASH were more likely to have HCC, were older, and had a higher BMI [498]. Infections (24%) and cardio/cerebrovascular complications (5.3%) were the commonest causes of death in individuals with MASH without HCC [498]. Independent risk factors for death in individuals transplanted for MASH without HCC were recipient age, model for end-stage liver disease score and obesity [498]. Individuals with MASLD and T2D are at particular risk for all-cause mortality and deaths due to cardiac and renal causes following liver transplantation [499]. MASLD/MASH is independently associated with cardiovascular diseases, renal and thyroid dysfunction, OSA, and a hypercoagulable state, which can affect perioperative outcomes [500]. The risk of major (including fatal) cardiovascular events appears to be particularly high in the first year after transplantation, especially in the perioperative period [501]. Despite the overall higher rate of risk factors in adults with MASLD, long-term graft- and recipient survival after liver transplantation do not differ in many retrospective and registry analyses [498, 502, 503], supporting that candidates with MASLD can be safely transplanted, if properly managed [496].
Although MASH is considered an independent risk factor for cardiovascular events similar to other traditional risk factors, there is insufficient evidence to support a fundamentally different approach to the pre-transplant cardiovascular risk assessment, as stated in the ILTS consensus statement [504]. Therefore, a stepwise and risk-adjusted approach to meticulously assess the presence of cardiovascular diseases in liver transplant candidates with MASLD seems appropriate (Fig. 5]. Scores such as the CAR-OLT (cardiovascular risk in orthotopic liver transplantation) risk score have been suggested to support identifying liver transplant candidates at particular risk for cardiovascular diseases, who warrant further cardiological investigations [505]. Since many individuals with MASLD do not achieve the target heart rate with (physical) cardiopulmonary exercise, pharmacological stress tests will be the test of choice in most individuals. Myocardial perfusion scintigraphy is also suitable to exclude relevant coronary artery disease in liver transplant candidates [506]. However, a meta-analysis found low sensitivities of 28% for stress echocardiography and 61% for myocardial perfusion scintigraphy for detection of coronary artery disease in liver transplant candidates [507]. Therefore, non-invasive angiography with coronary computed tomography angiography (CCTA) may be an alternative in liver transplant candidates, particularly in those with MASLD who are at high risk of significant coronary artery disease [496]. In individuals with positive signals on stress tests or CCTA, or for whom there is clinical suspicion of coronary artery disease, coronary angiography and, if indicated, revascularisation, should be performed [504]. At present, there are no prospective randomised-controlled diagnostic trials demonstrating superior outcomes with any (specific) preoperative screening strategy in individuals with MASLD undergoing liver transplantation [504].
In potential liver transplant recipients with MASH and severe obesity, do pharmacologic treatments, endoscopic interventions, and bariatric surgery for weight loss improve outcomes before and after transplantation?
Recommendations
Adults with obesity and end-stage MASLD listed for liver transplantation should undergo therapeutic interventions aimed at weight reduction without worsening sarcopenia as this will improve peri-operative outcomes (LoE 3, strong recommendation, strong consensus).
Implementation of dietary modification and supervised physical exercise should be the first line management approach with the objective of reducing BMI <40 kg/m2 and ideally <35 kg/m2 (LoE 1, strong recommendation, strong consensus).
In adults with end-stage MASLD listed for liver transplantation, pharmacological weight-loss strategies may be considered after careful risk-benefit assessment (e.g. presence of sarcopenia, liver function impairment) (LoE 4, weak recommendation, consensus).
In adults with compensated cirrhosis and without clinically significant portal hypertension, sleeve gastrectomy prior to liver transplantation may be considered as an alternative option to dietary or pharmacological weight loss (LoE 3, open recommendation, strong consensus).
In case of decompensated cirrhosis, bariatric surgery is contraindicated and needs to be discussed in the context of considering liver transplantation (LoE 4, open recommendation, strong consensus).
Statement
Weight loss and optimised treatment of comorbidities before transplantation may confer a benefit in terms of cardiovascular morbidity, as well as long-term survival and reduced recurrence of severe MASLD after liver transplantation (LoE 3, strong consensus).
While the impact of obesity on overall and graft survival is controversial [508], candidates for liver transplantation with obesity have higher waitlist mortality and lower transplant rates [509], and are less likely to be evaluated for liver transplantation [510]. Currently BMI-based criteria are no longer a contraindication [504]. Increased risk for cardiometabolic complications in individuals with obesity has led to the claim that, in select individuals with obesity and MASLD-related cirrhosis, obesity should be managed prior to liver transplantation, though a target BMI or the benefit of weight loss have not been formally established.
Optimisation of medical treatment of comorbidities [511], implementation of dietary modifications and supervised physical exercise should be performed according to recommended guidelines and the recommendations outlined above, as no indication for the utility of specific measures for individuals with MASH is available [504]. Although thresholds for optimal weight reduction before liver transplant have not been established, achieving a BMI <40 kg/m2 appears ideal [504] and any weight loss is desirable. The overall benefit of weight reduction and improvements in weight-related comorbidities on peri-operative outcomes has been established [512]. The benefit on cardiovascular complications and overall survival is predictable based on the epidemiological and natural history data but has not been formally demonstrated. No data are available on the effectiveness of such measures on the incidence of recurrent or de novo MASH after liver transplantation.
GLP1RAs, as the most promising weight-loss agents, have not been studied in liver transplant candidates. Although their tolerability profile is well established, these drugs cannot be recommended until the impact of severe hepatic impairment on drug pharmacokinetics is well determined.
Endoscopic bariatric techniques such as intragastric balloons are now recommended as a weight-loss intervention in individuals with obesity who have failed a trial of conventional weight-loss strategies [513]. Gastric or oesophageal varices and clinically significant portal hypertension are an absolute contraindication [514, 515] although successful placement of these balloons (16.5% weight loss and 11% portal pressure reduction) after variceal eradication by band ligation has been reported in individuals with preserved liver function [516]. Very small series have been reported to date, providing proof of principle of feasibility in select individuals with mean weight loss of 11% at 6 months [517].
Bariatric surgery is the best studied technique for achieving weight loss in liver transplant candidates but should only be considered after the failure of conservative measures [515]. Individuals with decompensated cirrhosis have a 10-fold higher risk of death after bariatric surgery than those with compensated cirrhosis [518] and are thus not good candidates for this procedure. In individuals with compensated cirrhosis, portal hypertension should be assessed pre-operatively, and bariatric surgery should be avoided in individuals with clinically significant portal hypertension (>10 mmHg), unless performed in highly selected individuals and highly experienced centres [470]. Adjustable gastric bands should be avoided as should Roux-en-Y Gastric Bypass (as it can induce malabsorption of immunosuppressive medication and blocks endoscopic access to the biliary tree) leaving endoscopic sleeve gastrectomy as an acceptable procedure [515]. In a series of 32 liver transplant candidates with well compensated cirrhosis (a third with MASLD alone) sleeve gastrectomy induced a median weight loss of 31 kg at 1 year after the procedure, with low morbidity, and allowed 88% of individuals to proceed with liver transplantation [519].
In individuals with decompensated cirrhosis listed for liver transplantation, combined liver transplantation and sleeve gastrectomy can be completed successfully [520, 521]. The procedure is reserved for individuals who despite targeted medical weight-loss interventions do not achieve a BMI <35-40 kg/m2 at the time of liver transplantation [522]. Complications specifically related to laparoscopic sleeve gastrectomy are very rare and long-term benefits have been reported with durable weight loss (35-38%) [520, 521, 523] and fewer metabolic complications (IR, hypertension, T2D and metabolic syndrome and less anti-hypertensive and lipid-lowering medication) [520, 521]. However, logistical requirements severely limit feasibility in most transplant centres.
In adults who received liver transplantation due to MASLD-related end-stage liver disease, can non-pharmacologic or pharmacologic measures reduce the risk of MASLD recurrence and improve long-term outcomes compared with no intervention?
Statements
In adults transplanted for MASLD-related end-stage liver disease, there is a high risk of recurrence of MASLD after liver transplantation, especially in adults with several metabolic risk factors (LoE 3, strong consensus).
Adults transplanted for MASLD-related end-stage liver disease are also at risk of cardiovascular events and kidney disease which can negatively impact long-term survival (LoE 2, strong consensus).
No specific issues related to MASLD are known to alter choice of medication or target values; the risk of recurrence of severe, fibrotic steatohepatitis reinforces the need to obtain optimal control of cardiometabolic risk factors (LoE 5, strong consensus).
The benefit of controlling weight and obesity-related comorbidities on recurrence of MASLD post-liver transplant and on progression to advanced fibrosis is expected but needs to be demonstrated in dedicated trials (LoE 5, strong consensus).
Recommendations
In adults transplanted for MASLD-related end-stage liver disease, therapeutic interventions to control obesity and related cardiometabolic complications are recommended (LoE 3, strong recommendation, strong consensus).
After liver transplantation, standard non-pharmacological dietary and lifestyle interventions should be universally implemented; pharmacological management of hypertension, type 2 diabetes and lipid disorders should be implemented according to general clinical guidelines (LoE 3, strong recommendation, strong consensus).
GLP1 receptor agonists may be considered to control weight and obesity-related comorbidities, although specific trials in transplant recipients are needed (LoE 5, weak recommendation, strong consensus).
Weight gain occurs rapidly after liver transplantation (in the first 6 months), frequently leading to a high prevalence of overweight and obesity (58% and 21%, respectively, at 3 years) [524]. In addition, immunosuppressive medications may have a deleterious impact on metabolic function [525]. Obesity after liver transplantation is independently associated with a 2-fold higher mortality risk [526]. Cardiovascular complications are the second most common cause of non-hepatic mortality in liver transplant recipients [527], and those with T2D [528] and chronic kidney disease [529] are at the highest risk for cardiovascular events.
In this setting, both recurrence of MASLD or de novo MASLD after liver transplantation are common concerns. Unfortunately, available retrospective studies have major shortcomings, such as imprecise adjudication of pre-transplant aetiologies of terminal liver disease, no data on alcohol use post liver transplantation and no follow-up protocol biopsies. In a multicentric retrospective study of 150 individuals specifically transplanted for MASLD-related cirrhosis, 5-year recurrence rates for metabolic syndrome, steatosis, steatohepatitis and advanced fibrosis (stages 3 and 4) were 86%, 80%, 60% and 20%, respectively [530]. The presence of ≥1 metabolic risk factor considerably increased the risk of developing advanced fibrosis. Descriptive data on de novo MASH (i.e. steatohepatitis occurring in liver transplant recipients transplanted for diseases other than MASLD) is even more scarce and conflicting [530, 531], with a blurry distinction from recurrent MASLD due to imprecise adjudication of MASLD as a primary cause of transplant. While some studies reported that de novo MASH may be less severe histologically (advanced fibrosis, steatohepatitis) and less durable than recurrent MASH [530], the largest study with long follow-up documented advanced fibrosis/cirrhosis in 20% of cases 3 years after liver transplantation [532]. Besides the risk of fibrosis progression, individuals transplanted for MASH-related cirrhosis are at increased risk of cardiovascular events [501, 504], kidney disease [533], and extrahepatic neoplasms [534].
Management of obesity and control of comorbidities (arterial hypertension, T2D and dyslipidaemia in particular) is critical for reducing cardiovascular risk and improving long-term survival after liver transplantation. Optimal blood pressure control is associated with a 42% relative reduction in all-cause mortality and a 35% reduction in cardiovascular events, stroke in particular [535]. Unfortunately, real-life data show that a minority of transplanted individuals actually achieve long-term optimal blood pressure control (defined as <140/<90 mmHg): 29% at 5-years post-transplant and less than 10% in those at high risk [535]. Statins, including high-intensity statins (atorvastatin, rosuvastatin), can be used pending careful titration and regular follow-up as they lack significant drug-drug interactions with immunosuppressive regimens [536]. GLP1RAs at dosages used for weight loss have not been studied specifically in liver transplant recipients. Data for dulaglutide in solid organ transplant recipients with T2D has shown modest weight loss (-4 kg) and improved glycaemic control [537]. There is no theoretical concern about specific safety issues or efficacy of newer GLP1RAs, although the possibility exists that nausea and vomiting during dose escalation could alter the absorption of immunosuppressive drugs. Finally, early steroid withdrawal, immunosuppression minimisation and switching between different classes of immunosuppressants are recommended by international guidelines to decrease the metabolic complications of immunosuppression [538].
Delayed sleeve gastrectomy following liver transplantation (2-year median delay) has been reported in a small series [539] without mortality or graft loss and with a body weight loss of 20% with improvement in diabetes control and lesser diabetes medication. Comorbid conditions may resolve to the same extent as in non-transplanted individuals undergoing sleeve gastrectomy and no changes in immunosuppressive regimens were necessary [540]. However, reported experience has been minimal so far and technical difficulties (due to adhesions) may limit surgical feasibility. The advent of new anti-obesity drugs may relegate bariatric surgery to a second-line therapy.
Future Directions
Despite the enormous advances in the field, many important areas on the management of MASLD require further evidence to refine our clinical practice. Some of these areas, where further research is pressingly needed, are listed in Box 1. As MetALD has become a formally recognised entity, it is important to describe its natural course and to revisit the safety limits of alcohol consumption and whether these limits should differ by the severity of liver disease and other clinical factors. The best approach to detect and quantify alcohol consumption should also be defined. For all individuals with MASLD as well as for the general population, the choice of non-invasive tests and settings of assessment may be further optimised, particularly in the setting of general population-based screening and/or specific subgroups (e.g. T2D, individuals >65 years of age, ethnicity, MetALD, individuals undergoing therapeutic interventions). The best target population and best tools for HCC surveillance should be better defined, especially as HCC development in MASLD individuals without cirrhosis is well recognised. Regarding treatment of MASLD, the relevance of personalised lifestyle interventions for maintaining healthy behaviour and preventing liver-related outcomes needs to be prospectively validated. The efficacy of treating cardiometabolic comorbidities as well as the efficacy of MASH-targeting therapies in individuals with MetALD needs to be thoroughly assessed, with a focus on liver-related outcomes (or their respective surrogates). With the registration of resmetirom and potentially other agents in the future, it is important to define non-response and stopping rules as well as to ascertain their long-term metabolic (e.g. bone density, endocrine functions), cardiovascular and oncological (e.g. extrahepatic malignancy) safety. The potential additive or synergistic effects of combining drugs intended to treat MASH and/or cardiometabolic comorbidities needs to be prospectively assessed. All pharmacological agents receiving accelerated approval for the treatment of MASH must demonstrate beneficial effects on clinical outcomes (i.e. mortality, decompensation, liver transplantation, HCC) in the extended period of phase III clinical trials and real-world studies. In addition, it is important to develop effective pharmacological treatments for individuals with MASH-related cirrhosis.
Box 1.
Key research agenda in MASLD (selected topics).
MASLD, metabolic dysfunction-associated steatotic liver disease; MetALD, MASLD with moderate alcohol consumption; NIT, non-invasive test; SLD, steatotic liver disease.
Appendix. Delphi round agreement on the recommendations of the present clinical practice guidelines
| Recommendation/statement | Consensus |
|---|---|
| Definition, prevalence and natural course | |
| The incidental finding of steatosis should prompt assessment of the potential aetiology of SLD, alongside tests for the presence of advanced fibrosis, as this could determine the risk of liver-related and/or cardiovascular outcomes and appropriate care (LoE 3, strong recommendation). | 100% |
| MASLD, ALD and MetALD are the most common causes of SLD, but other causes such as drug-induced liver disease and monogenic SLD should be considered, depending on the context (LoE 3, strong recommendation). | 98% |
| General population-based screening for SLD is not advised (LoE 3, strong recommendation). | 95% |
| While the presence of steatotic liver in the general population is not independently associated with liver-related outcomes, the stage of liver fibrosis and persistently elevated liver enzymes are associated with liver-related outcomes (LoE 3). | 98% |
| Type 2 diabetes and obesity (particularly abdominal obesity) are the metabolic diseases with the strongest impact on the natural history of MASLD, including progression to MASLD/MASH-advanced fibrosis, cirrhosis and hepatocellular carcinoma (LoE 2). | 98% |
| Males aged >50 years, postmenopausal women, and individuals with multiple cardiometabolic risk factors are at increased risk of progressive fibrosis and the development of cirrhosis and its complications (LoE 2). | 95% |
| Accumulating evidence shows that alcohol consumption and metabolic risk factors have modifying effects on the onset and progression of chronic liver disease which are independent and can be synergistic (LoE 2). | 96% |
| The presumed beneficial health effects of moderate alcohol consumption are inconsistent across studies and emerging evidence does not support a protective effect of light to moderate amounts of alcohol, particularly in individuals with cardiometabolic risk factors (LoE 3). | 100% |
| The amount, pattern and history of alcohol intake should be documented in all individuals with SLD (LoE 3, strong recommendation). | 100% |
| Alcohol intake may be qualitatively and quantitatively assessed by validated instruments and/or specific biomarkers in individuals with SLD (Table 5) (LoE 3, open recommendation). | 97% |
| Individuals with SLD, particularly those with moderate or high alcohol intake, should be discouraged from consuming alcohol (LoE 3, strong recommendation). | 91% |
| All alcohol consumption should be stopped completely and permanently in individuals with advanced fibrosis or cirrhosis (LoE 3, strong recommendation). | 100% |
| Prevention | |
| In the general population, non-pharmacological measures should be recommended to prevent the development of MASLD and its complications, including hepatocellular carcinoma, and preventive measures should be reinforced in high-risk groups (LoE 3, strong recommendation). | 96% |
| Screening, case-finding, diagnosis and monitoring | |
| Healthcare providers may consider case-finding strategies for MASLD with liver fibrosis in individuals with cardiometabolic risk factors (Table 3), abnormal liver enzymes, and/or radiological signs of hepatic steatosis (LoE 3, weak recommendation). | 91% |
| Healthcare providers should look for MASLD with liver fibrosis either in individuals with (A) type 2 diabetes or (B) abdominal obesity and ≥1 additional metabolic risk factor(s) (Table 3) or (C) abnormal liver function tests (LoE 3, strong recommendation). | 89% |
| Early diagnosis of fibrosis and subsequent appropriate management can potentially prevent progression to cirrhosis and its complications and may justify screening in these populations at risk (LoE 3). | 95% |
| In adults with MASLD, non-invasive scores based on combinations of blood tests or combinations of blood tests with imaging techniques measuring mechanical properties and/or hepatic fat content should be used for the detection of fibrosis since their diagnostic accuracy is higher than standard liver enzyme testing (alanine [ALT] and aspartate aminotransferase [AST]) (LoE 2, strong recommendation). | 97% |
| In adults with MASLD, a multi-step approach is recommended (detailed in Figure 2 and below): First, an established non-patented blood-based score such as FIB-4 should be performed. Thereafter, established imaging techniques such as liver elastography are recommended as a second step to further clarify the fibrosis stage if fibrosis is still suspected or in high-risk groups (LoE 2, strong recommendation). | 100% |
| Tests of specific collagen-related blood constituents (e.g. ELF) may serve as an alternative to imaging to identify advanced liver fibrosis (LoE 2, open recommendation). | 86% |
| Clinical care pathways may be adopted based on the sequential application of non-invasive scores and imaging tests in adults with MASLD or at-risk individuals, recognising that most adults with MASLD are seen at non-hepatology settings (LoE 2, weak recommendation). | 98% |
| Blood biomarker-derived scores and elastography should be used to exclude advanced fibrosis, while elastography is better suited to predict advanced fibrosis (LoE 2, strong recommendation). | 92% |
| None of these non-invasive methods can assess relevant microscopic features of MASLD such as ballooning or lobular inflammation (LoE 2). | 96% |
| Some blood biomarker-based scores may help to identify individuals with MASH at risk of disease progression (LoE 3). | 89% |
| Blood biomarker-derived scores and elastography can help in risk stratification for clinical outcomes, as observational studies have identified thresholds related to liver-related outcomes and mortality (LoE 3). | 100% |
| In most cases, liver biopsy is not required for clinical management of individuals with MASLD; however, liver biopsy is still required for the definite diagnosis of steatohepatitis and can help to rule out alternative causes of liver disease (LoE 1). | 100% |
| In adults with MASLD, sequential assessment with non-invasive tools may assist in ruling out fibrosis progression (LoE 3, weak recommendation). | 95% |
| In adults with MASLD, non-invasive tools can help predict the risk of overall and liver-related events and mortality (LoE 2, weak recommendation). | 97% |
| Clinicians in specialised centres may consider assessing the genetic risk profile (e.g. PNPLA3 p.I148M variant and/or polygenic risk scores) for personalising risk stratification, but this concept should be evaluated in larger prospective studies (LoE 3, open recommendation). | 92% |
| Genetic risk variants can be evaluated in clinical studies for stratification of disease risk progression and sub-phenotyping of MASLD (LoE 2, open recommendation). | 98% |
| Clinicians can consider referring individuals with a strong family history of severe disease in first degree relatives or early presentation with a severe phenotype, especially in the absence of metabolic triggers (and/or e.g. in individuals with normal body weight), for the evaluation of coexisting, treatable, genetic causes of liver disease by next-generation sequencing approaches (LoE 4, open recommendation). | 90% |
| Clinicians should assess associated comorbidities (e.g., type 2 diabetes, dyslipidaemia, hypertension, kidney disease, sleep apnoea, polycystic ovary syndrome) and cardiovascular risk in adults with MASLD (LoE 2, strong recommendation). | 100% |
| At initial diagnosis of MASLD and at regular follow-up intervals, laboratory tests and physical examinations for related comorbidities are recommended (Table 7) (LoE 2, strong recommendation). | 100% |
| Adults with MASLD should be encouraged to participate in extrahepatic cancer screening according to current guidelines, based on their exposure to obesity and type 2 diabetes as risk factors for extrahepatic malignancies (LoE 3, strong recommendation). | 96% |
| Assessment of insulin resistance (e.g., using the homeostasis model assessment of insulin resistance [HOMA-IR] or estimates derived from the oral glucose tolerance test) may be considered to clarify metabolic dysfunction in adults with (suspected) MASLD and without an established diagnosis of type 2 diabetes (LoE 3, weak recommendation). | 92% |
| In adults with non-cirrhotic MASLD or MASH in the absence of severe fibrosis (i.e. those with fibrosis stage <F3) assessed by non-invasive markers or liver biopsy, surveillance for early detection of hepatocellular carcinoma is currently not recommended (LoE 3, weak recommendation). | 86% |
| In adults with non-cirrhotic MASLD or MASH in the presence of severe fibrosis (F3) assessed by non-invasive markers or liver biopsy, surveillance may be considered based on an individual risk assessment (LoE 4, weak recommendation). | 95% |
| According to current guidelines, hepatocellular carcinoma monitoring programmes should be applied to individuals with MASLD-related cirrhosis (LoE 3, strong recommendation). | 100% |
| Risk stratification can help in optimising strategies for monitoring individuals at higher risk of hepatocellular carcinoma (Table 8) (LoE 4, weak recommendation). | 100% |
| As ultrasound-based surveillance has a low sensitivity for detection of hepatocellular carcinoma at an early-stage, particularly in adults with MASLD cirrhosis and obesity, alpha-fetoprotein (AFP) measurement can be combined with ultrasound in individuals at high risk (LoE 3, open recommendation). | 93% |
| Cross-sectional imaging by MRI may be undertaken in selected adults at high risk with persistent poor visualisation at ultrasound, particularly in individuals with dysplastic or regenerative nodules (LoE 3, open recommendation). | 100% |
| Treatment of MASLD: General considerations | |
| In adults with MASLD and advanced fibrosis or cirrhosis, regression of fibrosis has been associated with a reduced risk of liver-related outcomes (LoE 2). | 95% |
| Improvement in disease activity and resolution of steatohepatitis have been associated with regression of fibrosis (LoE 2). | 98% |
| Reduction of steatosis has been associated with histological improvements (particularly necro-inflammation) in some pharmacological intervention studies (LoE 2). | 98% |
| Since improved mortality has not been demonstrated for any of these treatment-induced histological changes, further long-term follow-up studies are needed to demonstrate that halting disease progression and/or reduction of steatosis, resolution of steatohepatitis or regression of fibrosis translate into a reduced risk of clinical outcomes (LoE 3). | 95% |
| Non-invasive tests have been linked with histologically assessed treatment response, but the most appropriate non-invasive test may depend on the type of intervention and patient-related factors (LoE 2). | 100% |
| Longitudinal changes in non-invasive test results have been correlated with changes in the risk of adverse outcomes on a cohort or population level (LoE 3). | 92% |
| In the setting of randomised controlled trials and depending on the mode of intervention, changes of non-invasive markers (e.g., MRI-PDFF relative reduction by ≥30%, ALT reduction by ≥17 U/L) have been associated with resolution of steatohepatitis (LoE 2). | 98% |
| Liver biopsy is not suited for monitoring disease evolution or response to therapy in routine clinical practice due to its invasiveness and procedure-related limitations (LoE 5). | 95% |
| At the individual level, non-invasive tests may be repeatedly used to assess fibrosis progression in a tailored fashion but may provide limited information about treatment response (LoE 5, weak recommendation). | 95% |
| In individual cases and in clinical trials, liver biopsy can be used to monitor disease progression or response to treatment (LoE 1, open recommendation). | 100% |
| Given the multidirectional connections between MASLD and cardiometabolic comorbidities, a multidisciplinary approach is recommended to ensure all components are appropriately targeted to improve both liver-related and extrahepatic outcomes (LoE 3, strong recommendation). | 100% |
| Treatment of MASLD: Non-pharmacological therapy | |
| In adults with MASLD, dietary and behavioural therapy-induced weight loss should be recommended to improve liver injury, as assessed histologically or non-invasively (LoE 1, strong recommendation). | 100% |
| In adults with MASLD and overweight, dietary and behavioural therapy-induced weight loss should aim at a sustained reduction of ≥5% to reduce liver fat, 7-10% to improve liver inflammation, and ≥10% to improve fibrosis (LoE 2, strong recommendation). | 100% |
| Further follow-up studies are needed to determine the long-term effectiveness of dietary and behavioural therapy-induced weight loss (including its magnitude) on clinical liver-related outcomes and liver-related mortality (LoE 3). | 100% |
| In adults with MASLD, improving diet quality (similar to the Mediterranean dietary pattern), limiting the consumption of ultra-processed food (rich in sugars and saturated fat) and avoiding sugar-sweetened beverages should be recommended to improve histologically or non-invasively assessed liver injury (LoE 2, strong recommendation). | 95% |
| There is little evidence that improving diet quality beneficially impacts clinical liver-related outcomes (LoE 3). | 93% |
| In adults with MASLD, physical activity and exercise should be recommended to reduce steatosis, tailored to the individual’s preference and ability (preferably >150 min/week of moderate or 75 min/week of vigorous-intensity physical activity) (LoE 1, strong recommendation). | 97% |
| In comparison to the well-documented cardiometabolic benefits, there is less robust evidence for benefits of physical activity and exercise on histological outcomes, non-invasively assessed liver damage/fibrosis and liver-related clinical outcomes (LoE 5). | 96% |
| In normal-weight adults with MASLD, diet and exercise interventions should be recommended to reduce liver fat (LoE 3, strong recommendation). | 100% |
| In normal-weight adults with MASLD there is currently no evidence regarding the beneficial effect of diet and/or exercise on liver histology, fibrosis and liver-related clinical outcomes (LoE 5). | 92% |
| In adults with MASLD, nutraceuticals cannot be recommended since there is insufficient evidence of their effectiveness in reducing histologically/non-invasively assessed liver damage/fibrosis and liver-related outcomes in MASLD, nor of their safety (LoE 2, open recommendation). | 98% |
| In adults with MASLD, coffee consumption has been associated with improvements in liver damage and reduced liver-related clinical outcomes in observational studies (LoE 4). | 95% |
| Treatment of MASLD: Pharmacological therapy | |
| If approved locally and dependent on the label, adults with non-cirrhotic MASH with significant liver fibrosis (stage ≥2) should be considered for treatment with resmetirom as a MASH-targeted therapy, as this treatment demonstrated histological efficacy on steatohepatitis and fibrosis in a large phase III registrational trial with an acceptable safety and tolerability profile (LoE 2, strong recommendation). | 88% |
| Treatment with resmetirom, if approved locally, may be considered for individuals with MASLD who are non-cirrhotic and with documentation of either: (A) advanced fibrosis; (B) at-risk steatohepatitis with significant fibrosis (by liver biopsy, when available, or by non-invasive panels validated for that purpose); or (C) risk of adverse liver-related outcomes (e.g., by elastography- or biomarker-defined thresholds) (LoE 3, open recommendation). | 89% |
| No MASH-targeted pharmacotherapy can currently be recommended for adults with MASH at the cirrhotic stage (LoE 5, weak recommendation). | 95% |
| Given the lack of robust demonstration of histological efficacy on steatohepatitis and liver fibrosis derived from large phase III trials and potential long-term risks, vitamin E cannot be recommended as a MASH-targeted therapy (LoE 2, weak recommendation). | 100% |
| For individuals with MASLD undergoing therapy with resmetirom, data on sustainability of histological benefits, individual prediction of response, liver-related outcomes and long-term safety are currently not available (LoE 5). | 100% |
| In the absence of a formal demonstration of histological improvement in large, well conducted, phase III trials, glucagon-like peptide 1 receptor agonists (GLP1RA) cannot currently be recommended as MASH-targeted therapies (LoE 5, strong recommendation). | 98% |
| GLP1RAs are safe to use in MASH (including compensated cirrhosis) and should be used for their respective indications, namely type 2 diabetes and obesity, as their use improves cardiometabolic outcomes (LoE 2, strong recommendation). | 98% |
| Where available, pioglitazone is safe to use in adults with non-cirrhotic MASH but given the lack of robust demonstration of histological efficacy on steatohepatitis and liver fibrosis in large phase III trials, pioglitazone cannot be recommended as a MASH-targeted therapy (LoE 2, weak recommendation). | 88% |
| There is insufficient evidence to recommend the use of sodium-glucose cotransporter-2 (SGLT2) inhibitors or metformin as MASH-targeted therapies; however, they are safe to use in MASLD and should be used for their respective indications, namely type 2 diabetes, heart failure and chronic kidney disease (LoE 3, strong recommendation). | 100% |
| In case of substantial weight loss induced by GLP1RAs, a hepatic histological benefit could be expected, although this has not been extensively documented so far (LoE 2). | 98% |
| There is insufficient evidence to support using any other glucose-lowering drug class as MASH-targeted therapies (LoE 5). | 100% |
| Non-incretin-based weight-loss agents are not recommended as MASH-targeted therapies (LoE 5, strong recommendation). | 98% |
| Treatment of MASLD: Surgical and endoscopic therapy | |
| In adults with non-cirrhotic MASLD who have an approved indication, bariatric surgery should be considered because it can induce long-term beneficial effects on the liver and is associated with remission of type 2 diabetes and improvement of cardiometabolic risk factors (LoE 3, strong recommendation). | 98% |
| In adults with MASLD-related compensated advanced chronic liver disease/compensated cirrhosis who have an approved indication, bariatric surgery can be considered but careful evaluation (indication, type of surgery, presence of clinically significant portal hypertension) by a multidisciplinary team with experience in bariatric surgery in this particular population is required (LoE 4, weak recommendation). | 100% |
| Metabolic/bariatric endoscopic procedures require further validation as MASH-targeted therapy and cannot currently be recommended (LoE 4, weak recommendation). | 100% |
| End-stage liver disease and liver transplantation | |
| In adults with MASH cirrhosis, it is recommended that dietary and lifestyle recommendations be adapted to the severity of liver disease, nutritional status and the presence of sarcopenia/sarcopenic obesity (LoE 2, strong recommendation). | 100% |
| In adults with sarcopenia, sarcopenic obesity or decompensated cirrhosis, it is recommended that a high-protein diet is provided, as well as a late-evening snack (LoE 2, strong recommendation). | 93% |
| Moderate weight reduction can be suggested in adults with compensated cirrhosis and obesity, with an emphasis on high protein intake and physical activity to maintain muscle mass and reduce the risk of sarcopenia (LoE 3, weak recommendation). | 100% |
| Metformin can be used in adults with compensated cirrhosis and preserved renal function but should not be used in adults with decompensated cirrhosis, especially when there is concomitant renal impairment, because of the risk of lactic acidosis (LoE 3, strong recommendation). | 100% |
| Sulfonylureas should be avoided in adults with hepatic decompensation because of the risk of hypoglycaemia (LoE 4, weak recommendation). | 98% |
| GLP1 receptor agonists can be used in adults with Child-Pugh class A cirrhosis, according to its indication (LoE 2, weak recommendation). | 98% |
| SGLT2 inhibitors can be used in adults with Child-Pugh class A and B cirrhosis (LoE 4, weak recommendation). | 92% |
| Statins can be used in adults with chronic liver disease, including those with compensated cirrhosis; they should be used in adults according to cardiovascular risk guidelines to reduce cardiovascular events (LoE 1, strong recommendation). | 98% |
| Liver stiffness measurement (LSM) by vibration-controlled transient elastography (VCTE) ≤15 kPa plus platelet count ≥150 × 109/L may be used to rule out clinically significant portal hypertension (CSPH) in adults with MASLD (LoE 3, weak recommendation). | 95% |
| If CSPH is present, non-selective beta-blockers may be started unless contraindicated (LoE 3, weak recommendation). | 97% |
| In adults with compensated advanced chronic liver disease but LSM ≥20 kPa and/or platelet count <150 × 109/L, an upper gastrointestinal endoscopy should be performed to screen for varices unless they already fulfil the criteria to initiate non-selective beta-blockers (LoE 3, strong recommendation). | 98% |
| The threshold of LSM ≥25 kPa to rule in CSPH is only applicable to non-obese (BMI <30 kg/m2) adults with MASLD; while obesity can confound LSM, current evidence is insufficient to suggest the optimal non-invasive test to rule in CSPH in adults with MASLD and obesity (LoE 3). | 97% |
| Adults with MASLD are at increased risk for major cardiovascular events in the pre-, peri- and post-transplant phase (LoE 2). | 100% |
| Adults with MASLD who are candidates for liver transplantation should be evaluated by a multidisciplinary team for cardiovascular and metabolic comorbidities to mitigate the risk of major cardiovascular events in the pre-, peri- and post-transplant phase (LoE 3, strong recommendation). | 100% |
| A comprehensive screening for comorbidities in adults with MASLD before liver transplantation (Table 11), including a stepwise and risk-adjusted cardiac work-up algorithm (Fig. 5), may help to optimise management of adults with MASLD before, during and after liver transplantation (LoE 5, weak recommendation). | 100% |
| Adults with obesity and end-stage MASLD listed for liver transplantation should undergo therapeutic interventions aimed at weight reduction without worsening sarcopenia as this will improve peri-operative outcomes (LoE 3, strong recommendation). | 98% |
| Implementation of dietary modification and supervised physical exercise should be the first line management approach with the objective of reducing BMI <40 kg/m2 and ideally <35 kg/m2 (LoE 1, strong recommendation). | 100% |
| In adults with end-stage MASLD listed for liver transplantation, pharmacological weight-loss strategies may be considered after careful risk-benefit assessment (e.g. presence of sarcopenia, liver function impairment) (LoE 4, weak recommendation). | 86% |
| In adults with compensated cirrhosis and without clinically significant portal hypertension, sleeve gastrectomy prior to liver transplantation may be considered as an alternative option to dietary or pharmacological weight loss (LoE 3, open recommendation). | 97% |
| In case of decompensated cirrhosis, bariatric surgery is contraindicated and needs to be discussed in the context of considering liver transplantation (LoE 4, open recommendation). | 100% |
| Weight loss and optimised treatment of comorbidities before transplantation may confer a benefit in terms of cardiovascular morbidity, as well as long-term survival and reduced recurrence of severe MASLD after liver transplantation (LoE 3). | 100% |
| In adults transplanted for MASLD-related end-stage liver disease, there is a high risk of recurrence of MASLD after liver transplantation, especially in adults with several metabolic risk factors (LoE 3). | 100% |
| Adults transplanted for MASLD-related end-stage liver disease are also at risk of cardiovascular events and kidney disease which can negatively impact long-term survival (LoE 2). | 100% |
| No specific issues related to MASLD are known to alter choice of medication or target values; the risk of recurrence of severe, fibrotic steatohepatitis reinforces the need to obtain optimal control of cardiometabolic risk factors (LoE 5). | 100% |
| The benefit of controlling weight and obesity-related comorbidities on recurrence of MASLD post-liver transplant and on progression to advanced fibrosis is expected but needs to be demonstrated in dedicated trials (LoE 5). | 100% |
| In adults transplanted for MASLD-related end-stage liver disease, therapeutic interventions to control obesity and related cardiometabolic complications are recommended (LoE 3, strong recommendation). | 100% |
| After liver transplantation, standard non-pharmacological dietary and lifestyle interventions should be universally implemented; pharmacological management of hypertension, type 2 diabetes and lipid disorders should be implemented according to general clinical guidelines (LoE 3, strong recommendation). | 100% |
| GLP1 receptor agonists may be considered to control weight and obesity-related comorbidities, although specific trials in transplant recipients are needed (LoE 5, weak recommendation). | 100% |
Abbreviations
AASLD, American Association for the Study of Liver Diseases; ALD, alcohol-related liver disease; BMI, body mass index; CAP, controlled attenuation parameter; CCTA, coronary computed tomography angiography; CKD, chronic kidney disease; CPGs, clinical practice guidelines; CSPH, clinically significant portal hypertension; EASD, European Association for the Study of Diabetes; EASL, European Association for the Study of the Liver; EASO, European Association for the Study of Obesity; ELF, enhanced liver fibrosis; FXR, farnesoid X receptor; GIP, glucose-dependent insulinotropic polypeptide; GLP1RA, glucagon-like peptide-1 receptor agonist; HCC, hepatocellular carcinoma; HR, hazard ratio; HVPG, hepatic venous pressure gradient; IFG, impaired fasting glucose; LSM, liver stiffness measurement; MASLD, metabolic dysfunction-associated steatotic liver disease; MASH, metabolic dysfunction-associated steatohepatitis; MR, magnetic resonance; NAFLD, non-alcoholic fatty liver disease; NAS, NAFLD activity score; NFS, NAFLD fibrosis score; NIT, non-invasive tests; NSBBs, non-selective beta blockers; OR, odds ratio; OSA, obstructive sleep apnoea; PCOS, polycystic ovary syndrome; PDFF, proton density fat fraction; PY, person-years; RCT, randomised-controlled trial; SGLT2, sodium-glucose cotransporter-2; SHBG, sex hormone-binding globulin; SLD, steatotic liver disease; TSH, thyroid-stimulating hormone; VCTE, vibration-controlled transient elastography.
Acknowledgements
The authors would like to thank the members of the Delphi Panel of this Clinical Practice Guideline for their valuable contribution:
Quentin Anstee, Marco Arrese, Heike Bantel, Giulia Besutti, Jérôme Boursier, Christopher Byrne, Ali Canbay, Cyrielle Caussy, Helena Cortez-Pinto, Mattias Ekstedt, Mirto Foletto, Jacob George, Liana Gheorghe, Isabel Graupera, Hannes Hagström, Kate Hallsworth, Onno Holleboom, Achim Kautz, Marko Korenjak, Karoline Lackner, Christos Lionis, Giulio Marchesini, Juris J. Meier, Juan M. Mendive, Luca Miele, Geltrude Mingrone, J. Bernadette Moore, Philip Newsome, George Papatheodoridis, Valerie Paradis, Gianluca Perseghin, Ralph Peterli, Salvatore Petta, Manuel Romero-Gomez, Jörn M. Schattenberg, Silvia Sookoian, Wendy Spearman, Norbert Stefan, Maja Thiele, Dina Tiniakos, Emmanouil Tsochatzis, Bernard Van Beers, José Willemse, Yusuf Yilmaz, and Volkan Yumuk. The authors would also like to thank the external reviewers and the EASL, EASD and EASO Governing Boards for their valuable contribution to the review process.
Conflict of Interest Statement
Luca Valenti has disclosed speaking engagements with Viatris, Novo Nordisk, and GSK; consulting roles with Novo Nordisk, Pfizer, Boehringer Ingelheim, Resalis, and MSD; and received unrestricted grant support from Gilead. Elisabetta Bugianesi serves as a consultant for Novo Nordisk, Boehringer Ingelheim, Eli Lilly, Pfizer, Bristol-Myers Squibb, and MSD. Amalia Gastaldelli has acted as a consultant for Boehringer Ingelheim, Eli Lilly and Company, and Metadeq Diagnostics; participated in advisory boards for Boehringer Ingelheim, Merck Sharp & Dohme, Novo Nordisk, Metadeq Diagnostics, and Pfizer; and received speaker's honorarium or other fees from Eli Lilly and Company, Merck Sharp & Dohme, Novo Nordisk, and Pfizer. Frank Tacke’s lab has received research funding from Gilead, AstraZeneca, and MSD (funding to the institution); he has received honoraria for consulting or lectures from AstraZeneca, Gilead, AbbVie, BMS, Boehringer, Intercept, Falk, Inventiva, MSD, GSK, Orphalan, Merz, Pfizer, Alnylam, Novo Nordisk, Sanofi, and Novartis. Michael Roden has participated in consulting for and/or scientific advisory boards of BMS, Boehringer Ingelheim Pharma, Echosens, Eli Lilly, Madrigal, MSD, Novo Nordisk, as well as in clinical trials supported by Boehringer Ingelheim, Novo Nordisk, and Nutricia/Danone. Paul Horn received grants from Novo Nordisk (through the University of Birmingham) and MSD (through Charité - Universitätsmedizin Berlin); speaking fees from Orphalan; and travel support from IPSEN. Hannele Yki-Järvinen declares no conflicts of interest regarding the present work. Shira Zelber-Sagi received travel support for attending a conference and honoraria for a lecture presentation in this conference by AbbVie, a one-time consulting fee by SIEMENS, and holds an unpaid leadership role as Chair-elect of the EASL public health committee. Vincent Wong has served as a consultant or advisory board member for AbbVie, Boehringer Ingelheim, Echosens, Gilead Sciences, Intercept, Inventiva, Novo Nordisk, Pfizer, Sagimet Biosciences, TARGET PharmaSolutions, and Visirna; a speaker for Abbott, AbbVie, Echosens, Gilead Sciences, Novo Nordisk, and Unilab; received a research grant from Gilead Sciences, and is a co-founder of Illuminatio Medical Technology. Gema Frühbeck received payment of honoraria for attendance to Advisory Boards from Lilly, and Novo Nordisk as well as payment of honoraria for lectures as Member of the OPEN Spain Initiative. Sven Francque holds a senior clinical investigator fellowship from the Research Foundation Flanders (FWO) and has acted as consultant for Astellas, Falk Pharma, Genfit, Gilead Sciences, GlympsBio, Janssens Pharmaceutica, Inventiva, Merck Sharp & Dome, Pfizer, Roche. He has acted as consultant for Abbvie, Actelion, Aelin Therapeutics, AgomAb, Aligos Therapeutics, Allergan, Alnylam, Astellas, Astra Zeneca, Bayer, Boehringer Ingelheim, Bristoll-Meyers Squibb, CSL Behring, Coherus, Echosens, Dr. Falk Pharma, Eisai, Enyo, Galapagos, Galmed, Genetech, Genfit, Genflow Biosciences, Gilead Sciences, Intercept, Inventiva, Janssens Pharmaceutica, PRO.MED.CS Praha, Julius Clinical, Madrigal, Medimmune, Merck Sharp & Dome, Mursla Bio, NGM Bio, Novartis, Novo Nordisk, Promethera, Roche, Siemens Healthineers, and lectured for for Abbvie, Allergan, Bayer, Eisai, Genfit, Gilead Sciences, Janssens Cilag, Intercept, Inventiva, Merck Sharp & Dome, Novo Nordisk, Promethera, Siemens. Dror Dicker received grants, personal fees, and nonfinancial support from NovoNordisk and Eli Lilly, and personal fees and nonfinancial support from Boehringer Ingelheim outside the submitted work. Vlad Ratziu serves as a consultant for Novo-Nordisk, Madrigal, Boehringer-Ingelheim, GSK, and 89 Bio; and has a grant to institution from MSD. Fritz Schick declares no conflict of interest pertaining to this article. Roberto Vettor has received honoraria from Novo Nordisk, Eli Lilly and AstraZeneca, and has served on advisory boards for Novo Nordisk and Eli Lilly.
Please refer to the accompanying ICMJE disclosure forms for further details.
Supplementary Material.
References
- 1. Karlsen TH, Sheron N, Zelber-Sagi S, Carrieri P, Dusheiko G, Bugianesi E, et al. The EASL-Lancet Liver Commission: protecting the next generation of Europeans against liver disease complications and premature mortality. Lancet. 2022;399(10319):61–116. [DOI] [PubMed] [Google Scholar]
- 2. Rinella ME, Lazarus JV, Ratziu V, Francque SM, Sanyal AJ, Kanwal F, et al. A multi-society Delphi consensus statement on new fatty liver disease nomenclature. J Hepatol. 2023;79(6):1542–56. [DOI] [PubMed] [Google Scholar]
- 3. European Association for the Study of the Liver . EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64(6):1388–402. [DOI] [PubMed] [Google Scholar]
- 4. Younossi ZM, Paik JM, Stepanova M, Ong J, Alqahtani S, Henry L. Clinical profiles and mortality rates are similar for metabolic dysfunction-associated steatotic liver disease and non-alcoholic fatty liver disease. J Hepatol. 2024;80(5):694–701. [DOI] [PubMed] [Google Scholar]
- 5. Cornberg M, Tacke F, Karlsen TH, European Association for the Study of the L . Clinical Practice Guidelines of the European Association for the study of the Liver - Advancing methodology but preserving practicability. J Hepatol. 2019;70(1):5–7. [DOI] [PubMed] [Google Scholar]
- 6. The Oxford Levels of Evidence 2”. Oxford Centre for Evidence-Based Medicine. Available from: https://www.cebm.ox.ac.uk/resources/levels-of-evidence/ocebm-levels-of-evidence]. [Google Scholar]
- 7. Wilson JM, Jungner YG. [Principles and practice of mass screening for disease]. Bol Oficina Sanit Panam. 1968;65(4):281–393. [PubMed] [Google Scholar]
- 8. Israelsen M, Torp N, Johansen S, Hansen CD, Hansen ED, Thorhauge K, et al. Validation of the new nomenclature of steatotic liver disease in patients with a history of excessive alcohol intake: an analysis of data from a prospective cohort study. Lancet Gastroenterol Hepatol. 2024;9(3):218–28. [DOI] [PubMed] [Google Scholar]
- 9. Ebrahimi F, Hagstrom H, Sun J, Bergman D, Shang Y, Yang W, et al. Familial coaggregation of MASLD with hepatocellular carcinoma and adverse liver outcomes: Nationwide multigenerational cohort study. J Hepatol. 2023;79(6):1374–84. [DOI] [PubMed] [Google Scholar]
- 10. Choi HSJ, Brouwer WP, Zanjir WMR, de Man RA, Feld JJ, Hansen BE, et al. Nonalcoholic Steatohepatitis Is Associated With Liver-Related Outcomes and All-Cause Mortality in Chronic Hepatitis B. Hepatology (Baltimore, Md). 2020;71(2):539–48. [DOI] [PubMed] [Google Scholar]
- 11. Chiang DJ, McCullough AJ. The impact of obesity and metabolic syndrome on alcoholic liver disease. Clin Liver Dis. 2014;18(1):157–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Younossi ZM, Golabi P, Paik JM, Henry A, Van Dongen C, Henry L. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): a systematic review. Hepatology (Baltimore, Md). 2023;77(4):1335–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Quek J, Chan KE, Wong ZY, Tan C, Tan B, Lim WH, et al. Global prevalence of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in the overweight and obese population: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2023;8(1):20–30. [DOI] [PubMed] [Google Scholar]
- 14. Le MH, Le DM, Baez TC, Wu Y, Ito T, Lee EY, et al. Global incidence of non-alcoholic fatty liver disease: A systematic review and meta-analysis of 63 studies and 1,201,807 persons. J Hepatol. 2023;79(2):287–95. [DOI] [PubMed] [Google Scholar]
- 15. Golabi P, Paik JM, Kumar A, Al Shabeeb R, Eberly KE, Cusi K, et al. Nonalcoholic fatty liver disease (NAFLD) and associated mortality in individuals with type 2 diabetes, pre-diabetes, metabolically unhealthy, and metabolically healthy individuals in the United States. Metabolism. 2023;146:155642. [DOI] [PubMed] [Google Scholar]
- 16. En Li Cho E, Ang CZ, Quek J, Fu CE, Lim LKE, Heng ZEQ, et al. Global prevalence of non-alcoholic fatty liver disease in type 2 diabetes mellitus: an updated systematic review and meta-analysis. Gut. 2023;72(11):2138–48. [DOI] [PubMed] [Google Scholar]
- 17. Shang Y, Grip ET, Modica A, Skroder H, Strom O, Ntanios F, et al. Metabolic Syndrome Traits Increase the Risk of Major Adverse Liver Outcomes in Type 2 Diabetes. Diabetes Care. 2024;47(6):978–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Simon TG, Roelstraete B, Khalili H, Hagstrom H, Ludvigsson JF. Mortality in biopsy-confirmed nonalcoholic fatty liver disease: results from a nationwide cohort. Gut. 2021;70(7):1375–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Prati D, Taioli E, Zanella A, Della Torre E, Butelli S, Del Vecchio E, et al. Updated definitions of healthy ranges for serum alanine aminotransferase levels. Ann Intern Med. 2002;137(1):1–10. [DOI] [PubMed] [Google Scholar]
- 20. Valenti L, Pelusi S, Bianco C, Ceriotti F, Berzuini A, Iogna Prat L, et al. Definition of Healthy Ranges for Alanine Aminotransferase Levels: A 2021 Update. Hepatol Commun. 2021;5(11):1824–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kwo PY, Cohen SM, Lim JK. ACG Clinical Guideline: Evaluation of Abnormal Liver Chemistries. Am J Gastroenterol. 2017;112(1):18–35. [DOI] [PubMed] [Google Scholar]
- 22. Portillo-Sanchez P, Bril F, Maximos M, Lomonaco R, Biernacki D, Orsak B, et al. High Prevalence of Nonalcoholic Fatty Liver Disease in Patients With Type 2 Diabetes Mellitus and Normal Plasma Aminotransferase Levels. J Clin Endocrinol Metab. 2015;100(6):2231–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hagstrom H, Kechagias S, Ekstedt M. Risk for hepatic and extra-hepatic outcomes in nonalcoholic fatty liver disease. J Intern Med. 2022;292(2):177–89. [DOI] [PubMed] [Google Scholar]
- 24. Liu Y, Zhong GC, Tan HY, Hao FB, Hu JJ. Nonalcoholic fatty liver disease and mortality from all causes, cardiovascular disease, and cancer: a meta-analysis. Sci Rep. 2019;9(1):11124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mantovani A, Csermely A, Petracca G, Beatrice G, Corey KE, Simon TG, et al. Non-alcoholic fatty liver disease and risk of fatal and non-fatal cardiovascular events: an updated systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2021;6(11):903–13. [DOI] [PubMed] [Google Scholar]
- 26. Xiao J, Ng CH, Chan KE, Fu C, Tay P, Yong JN, et al. Hepatic, Extra-hepatic Outcomes and Causes of Mortality in NAFLD - An Umbrella Overview of Systematic Review of Meta-Analysis. J Clin Exp Hepatol. 2023;13(4):656–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wijarnpreecha K, Li F, Lundin SK, Suresh D, Song MW, Tao C, et al. Higher mortality among lean patients with non-alcoholic fatty liver disease despite fewer metabolic comorbidities. Aliment Pharmacol Ther. 2023;57(9):1014–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Toh JZK, Pan XH, Tay PWL, Ng CH, Yong JN, Xiao J, et al. A Meta-Analysis on the Global Prevalence, Risk factors and Screening of Coronary Heart Disease in Nonalcoholic Fatty Liver Disease. Clin Gastroenterol Hepatol. 2022;20(11):2462–73 e10. [DOI] [PubMed] [Google Scholar]
- 29. Mantovani A, Petracca G, Csermely A, Beatrice G, Bonapace S, Rossi A, et al. Non-alcoholic fatty liver disease and risk of new-onset heart failure: an updated meta-analysis of about 11 million individuals. Gut. 2023;72(2):372–380. [DOI] [PubMed] [Google Scholar]
- 30. Mantovani A, Petracca G, Beatrice G, Csermely A, Tilg H, Byrne CD, Targher G. Non-alcoholic fatty liver disease and increased risk of incident extrahepatic cancers: a meta-analysis of observational cohort studies. Gut. 2022;71(4):778–88. [DOI] [PubMed] [Google Scholar]
- 31. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology (Baltimore, Md). 2016;64(1):73–84. [DOI] [PubMed] [Google Scholar]
- 32. Ekstedt M, Hagstrom H, Nasr P, Fredrikson M, Stal P, Kechagias S, Hultcrantz R. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology (Baltimore, Md). 2015;61(5):1547–54. [DOI] [PubMed] [Google Scholar]
- 33. Loomba R, Friedman SL, Shulman GI. Mechanisms and disease consequences of nonalcoholic fatty liver disease. Cell. 2021;184(10):2537–64. [DOI] [PubMed] [Google Scholar]
- 34. Younossi ZM. Non-alcoholic fatty liver disease - A global public health perspective. J Hepatol. 2019;70(3):531–44. [DOI] [PubMed] [Google Scholar]
- 35. Golabi P, Otgonsuren M, de Avila L, Sayiner M, Rafiq N, Younossi ZM. Components of metabolic syndrome increase the risk of mortality in nonalcoholic fatty liver disease (NAFLD). Medicine (Baltimore). 2018;97(13):e0214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Le MH, Devaki P, Ha NB, Jun DW, Te HS, Cheung RC, Nguyen MH. Prevalence of non-alcoholic fatty liver disease and risk factors for advanced fibrosis and mortality in the United States. PLoS One. 2017;12(3):e0173499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Vilar-Gomez E, Nephew LD, Vuppalanchi R, Gawrieh S, Mladenovic A, Pike F, et al. High-quality diet, physical activity, and college education are associated with low risk of NAFLD among the US population. Hepatology (Baltimore, Md). 2022;75(6):1491–506. [DOI] [PubMed] [Google Scholar]
- 38. Giammarino AM, Qiu H, Bulsara K, Khan S, Jiang Y, Da BL, et al. Community Socioeconomic Deprivation Predicts Nonalcoholic Steatohepatitis. Hepatol Commun. 2022;6(3):550–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Koutny F, Aigner E, Datz C, Gensluckner S, Maieron A, Mega A, et al. Relationships between education and non-alcoholic fatty liver disease. Eur J Intern Med. 2023;118:98–107. [DOI] [PubMed] [Google Scholar]
- 40. WHO . Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. 2000;894:i-xii, 1–253. [PubMed] [Google Scholar]
- 41. Haam JH, Kim BT, Kim EM, Kwon H, Kang JH, Park JH, et al. Diagnosis of Obesity: 2022 Update of Clinical Practice Guidelines for Obesity by the Korean Society for the Study of Obesity. J Obes Metab Syndr. 2023;32(2):121–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120(16):1640–5. [DOI] [PubMed] [Google Scholar]
- 43. Ekstedt M, Franzen LE, Mathiesen UL, Thorelius L, Holmqvist M, Bodemar G, Kechagias S. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology (Baltimore, Md). 2006;44(4):865–73. [DOI] [PubMed] [Google Scholar]
- 44. Liu B, Balkwill A, Reeves G, Beral V, Million Women Study C. Body mass index and risk of liver cirrhosis in middle aged UK women: prospective study. BMJ. 2010;340:c912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Berzigotti A, Garcia-Tsao G, Bosch J, Grace ND, Burroughs AK, Morillas R, et al. Obesity is an independent risk factor for clinical decompensation in patients with cirrhosis. Hepatology (Baltimore, Md: ). 2011;54(2):555–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Borena W, Strohmaier S, Lukanova A, Bjorge T, Lindkvist B, Hallmans G, et al. Metabolic risk factors and primary liver cancer in a prospective study of 578,700 adults. Int J Cancer. 2012;131(1):193–200. [DOI] [PubMed] [Google Scholar]
- 47. Nair S, Mason A, Eason J, Loss G, Perrillo RP. Is obesity an independent risk factor for hepatocellular carcinoma in cirrhosis? Hepatology (Baltimore, Md). 2002;36(1):150–5. [DOI] [PubMed] [Google Scholar]
- 48. Hassan MM, Abdel-Wahab R, Kaseb A, Shalaby A, Phan AT, El-Serag HB, et al. Obesity Early in Adulthood Increases Risk but Does Not Affect Outcomes of Hepatocellular Carcinoma. Gastroenterology. 2015;149(1):119–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ajmera V, Cepin S, Tesfai K, Hofflich H, Cadman K, Lopez S, et al. A prospective study on the prevalence of NAFLD, advanced fibrosis, cirrhosis and hepatocellular carcinoma in people with type 2 diabetes. J Hepatol. 2023;78(3):471–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Younossi ZM, Golabi P, de Avila L, Paik JM, Srishord M, Fukui N, et al. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: A systematic review and meta-analysis. J Hepatol. 2019;71(4):793–801. [DOI] [PubMed] [Google Scholar]
- 51. Stefan N, Cusi K. A global view of the interplay between non-alcoholic fatty liver disease and diabetes. Lancet Diabetes Endocrinol. 2022;10(4):284–96. [DOI] [PubMed] [Google Scholar]
- 52. Castera L, Laouenan C, Vallet-Pichard A, Vidal-Trecan T, Manchon P, Paradis V, et al. High Prevalence of NASH and Advanced Fibrosis in Type 2 Diabetes: A Prospective Study of 330 Outpatients Undergoing Liver Biopsies for Elevated ALT, Using a Low Threshold. Diabetes Care. 2023;46(7):1354–62. [DOI] [PubMed] [Google Scholar]
- 53. Huang DQ, Wilson LA, Behling C, Kleiner DE, Kowdley KV, Dasarathy S, et al. Fibrosis Progression Rate in Biopsy-Proven Nonalcoholic Fatty Liver Disease Among People With Diabetes Versus People Without Diabetes: A Multicenter Study. Gastroenterology. 2023;165(2):463–72 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Simon TG, Roelstraete B, Sharma R, Khalili H, Hagstrom H, Ludvigsson JF. Cancer Risk in Patients With Biopsy-Confirmed Nonalcoholic Fatty Liver Disease: A Population-Based Cohort Study. Hepatology (Baltimore, Md). 2021;74(5):2410–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yang JD, Ahmed F, Mara KC, Addissie BD, Allen AM, Gores GJ, Roberts LR. Diabetes Is Associated With Increased Risk of Hepatocellular Carcinoma in Patients With Cirrhosis From Nonalcoholic Fatty Liver Disease. Hepatology (Baltimore, Md). 2020;71(3):907–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Alexander M, Loomis AK, van der Lei J, Duarte-Salles T, Prieto-Alhambra D, Ansell D, et al. Risks and clinical predictors of cirrhosis and hepatocellular carcinoma diagnoses in adults with diagnosed NAFLD: real-world study of 18 million patients in four European cohorts. BMC Med. 2019;17(1):95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kanwal F, Kramer JR, Li L, Dai J, Natarajan Y, Yu X, et al. Effect of Metabolic Traits on the Risk of Cirrhosis and Hepatocellular Cancer in Nonalcoholic Fatty Liver Disease. Hepatology (Baltimore, Md). 2020;71(3):808–19. [DOI] [PubMed] [Google Scholar]
- 58. Deutsch AJ, Ahlqvist E, Udler MS. Phenotypic and genetic classification of diabetes. Diabetologia. 2022;65(11):1758–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Herder C, Roden M. A novel diabetes typology: towards precision diabetology from pathogenesis to treatment. Diabetologia. 2022;65(11):1770–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Schon M, Prystupa K, Mori T, Zaharia OP, Bodis K, Bombrich M, et al. Analysis of type 2 diabetes heterogeneity with a tree-like representation: insights from the prospective German Diabetes Study and the LURIC cohort. Lancet Diabetes Endocrinol. 2024;12(2):119–31. [DOI] [PubMed] [Google Scholar]
- 61. Wagner R, Heni M, Tabak AG, Machann J, Schick F, Randrianarisoa E, et al. Pathophysiology-based subphenotyping of individuals at elevated risk for type 2 diabetes. Nat Med. 2021;27(1):49–57. [DOI] [PubMed] [Google Scholar]
- 62. Singh S, Allen AM, Wang Z, Prokop LJ, Murad MH, Loomba R. Fibrosis progression in nonalcoholic fatty liver vs nonalcoholic steatohepatitis: a systematic review and meta-analysis of paired-biopsy studies. Clin Gastroenterol Hepatol. 2015;13(4):643–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Julian MT, Ballesta S, Pera G, Perez-Montes de Oca A, Soldevila B, Caballeria L, et al. Abdominal obesity and dsyglycemia are risk factors for liver fibrosis progression in NAFLD subjects: A population-based study. Front Endocrinol (Lausanne). 2022;13:1051958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Asfari MM, Niyazi F, Lopez R, Dasarathy S, McCullough AJ. The association of nonalcoholic steatohepatitis and obstructive sleep apnea. Eur J Gastroenterol Hepatol. 2017;29(12):1380–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Aron-Wisnewsky J, Minville C, Tordjman J, Levy P, Bouillot JL, Basdevant A, et al. Chronic intermittent hypoxia is a major trigger for non-alcoholic fatty liver disease in morbid obese. J Hepatol. 2012;56(1):225–33. [DOI] [PubMed] [Google Scholar]
- 66. Sarkar M, Terrault N, Chan W, Cedars MI, Huddleston HG, Duwaerts CC, et al. Polycystic ovary syndrome (PCOS) is associated with NASH severity and advanced fibrosis. Liver Int. 2020;40(2):355–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Jaroenlapnopparat A, Charoenngam N, Ponvilawan B, Mariano M, Thongpiya J, Yingchoncharoen P. Menopause is associated with increased prevalence of nonalcoholic fatty liver disease: a systematic review and meta-analysis. Menopause. 2023;30(3):348–54. [DOI] [PubMed] [Google Scholar]
- 68. Yang JD, Abdelmalek MF, Pang H, Guy CD, Smith AD, Diehl AM, Suzuki A. Gender and menopause impact severity of fibrosis among patients with nonalcoholic steatohepatitis. Hepatology (Baltimore, Md). 2014;59(4):1406–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Yoneda M, Thomas E, Sumida Y, Eguchi Y, Schiff ER. The influence of menopause on the development of hepatic fibrosis in nonobese women with nonalcoholic fatty liver disease. Hepatology (Baltimore, Md). 2014;60(5):1792. [DOI] [PubMed] [Google Scholar]
- 70. Harrison SA, Gawrieh S, Roberts K, Lisanti CJ, Schwope RB, Cebe KM, et al. Prospective evaluation of the prevalence of non-alcoholic fatty liver disease and steatohepatitis in a large middle-aged US cohort. J Hepatol. 2021;75(2):284–91. [DOI] [PubMed] [Google Scholar]
- 71. Rich NE, Oji S, Mufti AR, Browning JD, Parikh ND, Odewole M, et al. Racial and Ethnic Disparities in Nonalcoholic Fatty Liver Disease Prevalence, Severity, and Outcomes in the United States: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2018;16(2):198–210 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Petrick JL, Campbell PT, Koshiol J, Thistle JE, Andreotti G, Beane-Freeman LE, et al. Tobacco, alcohol use and risk of hepatocellular carcinoma and intrahepatic cholangiocarcinoma: The Liver Cancer Pooling Project. Br J Cancer. 2018;118(7):1005–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Yoo JJ, Park MY, Cho EJ, Yu SJ, Kim SG, Kim YJ, et al. Smoking Increases the Risk of Hepatocellular Carcinoma and Cardiovascular Disease in Patients with Metabolic-Associated Fatty Liver Disease. J Clin Med. 2023;12(9):3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Abdel-Rahman O, Helbling D, Schob O, Eltobgy M, Mohamed H, Schmidt J, et al. Cigarette smoking as a risk factor for the development of and mortality from hepatocellular carcinoma: An updated systematic review of 81 epidemiological studies. J Evid Based Med. 2017;10(4):245–54. [DOI] [PubMed] [Google Scholar]
- 75. European Association for the Study of the Liver; European Association for the Study of the Liver . EASL Clinical Practice Guidelines: Management of alcohol-related liver disease. J Hepatol. 2018;69(1):154–81. [DOI] [PubMed] [Google Scholar]
- 76. Åberg F, Byrne CD, Pirola CJ, Männistö V, Sookoian S. Alcohol consumption and metabolic syndrome: Clinical and epidemiological impact on liver disease. J Hepatol. 2023;78(1):191–206. [DOI] [PubMed] [Google Scholar]
- 77. Staufer K, Huber-Schonauer U, Strebinger G, Pimingstorfer P, Suesse S, Scherzer TM, et al. Ethyl glucuronide in hair detects a high rate of harmful alcohol consumption in presumed non-alcoholic fatty liver disease. J Hepatol. 2022;77(4):918–30. [DOI] [PubMed] [Google Scholar]
- 78. Global Burden of Disease Study . Alcohol use and burden for 195 countries and territories, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2018;392(10152):1015–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Global Burden of Disease Study . Population-level risks of alcohol consumption by amount, geography, age, sex, and year: a systematic analysis for the Global Burden of Disease Study 2020. Lancet. 2022;400(10347):185–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Thun MJ, Peto R, Lopez AD, Monaco JH, Henley SJ, HeathCW, Jr., Doll R. Alcohol consumption and mortality among middle-aged and elderly U.S. adults. N Engl J Med. 1997;337(24):1705–14. [DOI] [PubMed] [Google Scholar]
- 81. Chikritzhs T, Fillmore K, Stockwell T. A healthy dose of scepticism: four good reasons to think again about protective effects of alcohol on coronary heart disease. Drug Alcohol Rev. 2009;28(4):441–4. [DOI] [PubMed] [Google Scholar]
- 82. Bagnardi V, Zatonski W, Scotti L, La Vecchia C, Corrao G. Does drinking pattern modify the effect of alcohol on the risk of coronary heart disease? Evidence from a meta-analysis. J Epidemiol Community Health. 2008;62(7):615–9. [DOI] [PubMed] [Google Scholar]
- 83. Rehm J, Gmel GE Sr., Gmel G, Hasan OSM, Imtiaz S, Popova S, et al. The relationship between different dimensions of alcohol use and the burden of disease-an update. Addiction. 2017;112(6):968–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Åberg F, Färkkilä M, Männistö V. Interaction Between Alcohol Use and Metabolic Risk Factors for Liver Disease: A Critical Review of Epidemiological Studies. Alcohol Clin Exp Res. 2020;44(2):384–403. [DOI] [PubMed] [Google Scholar]
- 85. Aberg F, Helenius-Hietala J, Puukka P, Farkkila M, Jula A. Interaction between alcohol consumption and metabolic syndrome in predicting severe liver disease in the general population. Hepatology (Baltimore, Md). 2018;67(6):2141–9. [DOI] [PubMed] [Google Scholar]
- 86. Naveau S, Giraud V, Borotto E, Aubert A, Capron F, Chaput JC. Excess weight risk factor for alcoholic liver disease. Hepatology (Baltimore, Md). 1997;25(1):108–11. [DOI] [PubMed] [Google Scholar]
- 87. Hart CL, Morrison DS, Batty GD, Mitchell RJ, Davey Smith G. Effect of body mass index and alcohol consumption on liver disease: analysis of data from two prospective cohort studies. BMJ. 2010;340:c1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Younossi ZM, Stepanova M, Ong J, Yilmaz Y, Duseja A, Eguchi Y, et al. Effects of Alcohol Consumption and Metabolic Syndrome on Mortality in Patients With Nonalcoholic and Alcohol-Related Fatty Liver Disease. Clin Gastroenterol Hepatol. 2019;17(8):1625–33.e1. [DOI] [PubMed] [Google Scholar]
- 89. Hajifathalian K, Torabi Sagvand B, McCullough AJ. Effect of Alcohol Consumption on Survival in Nonalcoholic Fatty Liver Disease: A National Prospective Cohort Study. Hepatology (Baltimore, Md). 2019;70(2):511–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Sookoian S, Castaño GO, Pirola CJ. Modest alcohol consumption decreases the risk of non-alcoholic fatty liver disease: a meta-analysis of 43 175 individuals. Gut. 2014;63(3):530–2. [DOI] [PubMed] [Google Scholar]
- 91. Dunn W, Sanyal AJ, Brunt EM, Unalp-Arida A, Donohue M, McCullough AJ, Schwimmer JB. Modest alcohol consumption is associated with decreased prevalence of steatohepatitis in patients with non-alcoholic fatty liver disease (NAFLD). J Hepatol. 2012;57(2):384–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Aberg F, Puukka P, Salomaa V, Mannisto S, Lundqvist A, Valsta L, et al. Risks of Light and Moderate Alcohol Use in Fatty Liver Disease: Follow-Up of Population Cohorts. Hepatology (Baltimore, Md). 2020;71(3):835–48. [DOI] [PubMed] [Google Scholar]
- 93. Jarvis H, O'Keefe H, Craig D, Stow D, Hanratty B, Anstee QM. Does moderate alcohol consumption accelerate the progression of liver disease in NAFLD? A systematic review and narrative synthesis. BMJ Open. 2022;12(1):e049767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Roerecke M, Vafaei A, Hasan OSM, Chrystoja BR, Cruz M, Lee R, et al. Alcohol Consumption and Risk of Liver Cirrhosis: A Systematic Review and Meta-Analysis. Am J Gastroenterol. 2019;114(10):1574–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Askgaard G, Grønbæk M, Kjær MS, Tjønneland A, Tolstrup JS. Alcohol drinking pattern and risk of alcoholic liver cirrhosis: a prospective cohort study. J Hepatol. 2015;62(5):1061–7. [DOI] [PubMed] [Google Scholar]
- 96. Simpson RF, Hermon C, Liu B, Green J, Reeves GK, Beral V, Floud S. , et al. , . Alcohol drinking patterns and liver cirrhosis risk: analysis of the prospective UK Million Women Study. Lancet Public Health. 2019;4(1):e41–e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Loomba R, Yang HI, Su J, Brenner D, Barrett-Connor E, Iloeje U, Chen CJ. Synergism between obesity and alcohol in increasing the risk of hepatocellular carcinoma: a prospective cohort study. Am J Epidemiol. 2013;177(4):333–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Marrero JA, Fontana RJ, Fu S, Conjeevaram HS, Su GL, Lok AS. Alcohol, tobacco and obesity are synergistic risk factors for hepatocellular carcinoma. J Hepatol. 2005;42(2):218–24. [DOI] [PubMed] [Google Scholar]
- 99. Kashiwagi K, Yamaguchi A, Shiba S, Taniki N, Inoue N, Takaishi H, et al. Moderate alcohol consumption is not associated with subclinical cardiovascular damage but with hepatic fibrosis in non-alcoholic fatty liver disease. Alcohol. 2020;89:1–7. [DOI] [PubMed] [Google Scholar]
- 100. VanWagner LB, Ning H, Allen NB, Ajmera V, Lewis CE, Carr JJ, et al. Alcohol Use and Cardiovascular Disease Risk in Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology. 2017;153(5):1260–72.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Louvet A, Bourcier V, Archambeaud I, d'Alteroche L, Chaffaut C, Oberti F, et al. Low alcohol consumption influences outcomes in individuals with alcohol-related compensated cirrhosis in a French multicenter cohort. J Hepatol. 2023;78(3):501–12. [DOI] [PubMed] [Google Scholar]
- 102. Ascha MS, Hanouneh IA, Lopez R, Tamimi TA-R, Feldstein AF, Zein NN. The incidence and risk factors of hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. Hepatology (Baltimore, Md). 2010;51(6):1972–8. [DOI] [PubMed] [Google Scholar]
- 103. Ma J, Hennein R, Liu C, Long MT, Hoffmann U, Jacques PF, et al. Improved Diet Quality Associates With Reduction in Liver Fat, Particularly in Individuals With High Genetic Risk Scores for Nonalcoholic Fatty Liver Disease. Gastroenterology. 2018;155(1):107–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Maskarinec G, Namatame LA, Kang M, Buchthal SD, Ernst T, Monroe KR, et al. Differences in the association of diet quality with body fat distribution between men and women. Eur J Clin Nutr. 2020;74(10):1434–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Zhang S, Gan S, Zhang Q, Liu L, Meng G, Yao Z, et al. Ultra-processed food consumption and the risk of non-alcoholic fatty liver disease in the Tianjin Chronic Low-grade Systemic Inflammation and Health Cohort Study. Int J Epidemiol. 2022;51(1):237–49. [DOI] [PubMed] [Google Scholar]
- 106. Ma Y, Yang W, Simon TG, Smith-Warner SA, Fung TT, Sui J, et al. Dietary Patterns and Risk of Hepatocellular Carcinoma Among U.S. Men and Women. Hepatology (Baltimore, Md). 2019;70(2):577–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Bogumil D, Park SY, Le Marchand L, Haiman CA, Wilkens LR, Boushey CJ, Setiawan VW. High-Quality Diets Are Associated With Reduced Risk of Hepatocellular Carcinoma and Chronic Liver Disease: The Multiethnic Cohort. Hepatol Commun. 2019;3(3):437–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Liu Y, Yang W, VoPham T, Ma Y, Simon TG, Gao X, et al. Plant-Based and Animal-Based Low-Carbohydrate Diets and Risk of Hepatocellular Carcinoma Among US Men and Women. Hepatology (Baltimore, Md). 2021;73(1):175–85. [DOI] [PubMed] [Google Scholar]
- 109. Luu HN, Neelakantan N, Geng TT, Wang R, Goh GB, Clemente JC, et al. Quality diet indexes and risk of hepatocellular carcinoma: Findings from the Singapore Chinese Health Study. Int J Cancer. 2021;148(9):2102–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Kim MN, Lo CH, Corey KE, Luo X, Long L, Zhang X, et al. Red meat consumption, obesity, and the risk of nonalcoholic fatty liver disease among women: Evidence from mediation analysis. Clin Nutr. 2022;41(2):356–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Zhang S, Gu Y, Bian S, Lu Z, Zhang Q, Liu L, et al. Soft drink consumption and risk of nonalcoholic fatty liver disease: results from the Tianjin Chronic Low-Grade Systemic Inflammation and Health (TCLSIH) cohort study. Am J Clin Nutr. 2021;113(5):1265–74. [DOI] [PubMed] [Google Scholar]
- 112. Ma Y, Yang W, Li T, Liu Y, Simon TG, Sui J, et al. Meat intake and risk of hepatocellular carcinoma in two large US prospective cohorts of women and men. Int J Epidemiol. 2019;48(6):1863–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Zhao L, Zhang X, Coday M, Garcia DO, Li X, Mossavar-Rahmani Y, et al. Sugar-Sweetened and Artificially Sweetened Beverages and Risk of Liver Cancer and Chronic Liver Disease Mortality. JAMA. 2023;330(6):537–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Marti-Aguado D, Clemente-Sanchez A, Bataller R. Cigarette smoking and liver diseases. J Hepatol. 2022;77(1):191–205. [DOI] [PubMed] [Google Scholar]
- 115. Schneider CV, Zandvakili I, Thaiss CA, Schneider KM. Physical activity is associated with reduced risk of liver disease in the prospective UK Biobank cohort. JHEP Rep. 2021;3(3):100263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Simon TG, Kim MN, Luo X, Yang W, Ma Y, Chong DQ, et al. Physical activity compared to adiposity and risk of liver-related mortality: results from two prospective, nationwide cohorts. J Hepatol. 2020;72(6):1062–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Luu HN, Behari J, Goh GB, Wang R, Jin A, Thomas CE, et al. Composite Score of Healthy Lifestyle Factors and Risk of Hepatocellular Carcinoma: Findings from a Prospective Cohort Study. Cancer Epidemiol Biomarkers Prev. 2021;30(2):380–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Zhang YB, Pan XF, Chen J, Cao A, Zhang YG, Xia L, et al. Combined lifestyle factors, incident cancer, and cancer mortality: a systematic review and meta-analysis of prospective cohort studies. Br J Cancer. 2020;122(7):1085–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Andreeva VA, Egnell M, Touvier M, Galan P, Julia C, Hercberg S. International evidence for the effectiveness of the front-of-package nutrition label called Nutri-Score. Cent Eur J Public Health. 2021;29(1):76–9. [DOI] [PubMed] [Google Scholar]
- 120. Wong VW, Ekstedt M, Wong GL, Hagstrom H. Changing epidemiology, global trends and implications for outcomes of NAFLD. J Hepatol. 2023;79(3):842–52. [DOI] [PubMed] [Google Scholar]
- 121. Lin H, Yip TC, Zhang X, Li G, Tse YK, Hui VW, et al. Age and the relative importance of liver-related deaths in nonalcoholic fatty liver disease. Hepatology (Baltimore, Md). 2023;77(2):573–84. [DOI] [PubMed] [Google Scholar]
- 122. Taylor RS, Taylor RJ, Bayliss S, Hagstrom H, Nasr P, Schattenberg JM, et al. Association Between Fibrosis Stage and Outcomes of Patients With Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. Gastroenterology. 2020;158(6):1611–25 e12. [DOI] [PubMed] [Google Scholar]
- 123. Sanyal AJ, Van Natta ML, Clark J, Neuschwander-Tetri BA, Diehl A, Dasarathy S, et al. Prospective Study of Outcomes in Adults with Nonalcoholic Fatty Liver Disease. N Engl J Med. 2021;385(17):1559–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Mittal S, El-Serag HB, Sada YH, Kanwal F, Duan Z, Temple S, et al. Hepatocellular Carcinoma in the Absence of Cirrhosis in United States Veterans is Associated With Nonalcoholic Fatty Liver Disease. Clin Gastroenterol Hepatol. 2016;14(1):124–31 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Kanwal F, Shubrook JH, Adams LA, Pfotenhauer K, Wai-Sun Wong V, Wright E, et al. Clinical Care Pathway for the Risk Stratification and Management of Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology. 2021;161(5):1657–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Cusi K, Isaacs S, Barb D, Basu R, Caprio S, Garvey WT, et al. American Association of Clinical Endocrinology Clinical Practice Guideline for the Diagnosis and Management of Nonalcoholic Fatty Liver Disease in Primary Care and Endocrinology Clinical Settings: Co-Sponsored by the American Association for the Study of Liver Diseases (AASLD). Endocr Pract. 2022;28(5):528–62. [DOI] [PubMed] [Google Scholar]
- 127. Rinella ME, Neuschwander-Tetri BA, Siddiqui MS, Abdelmalek MF, Caldwell S, Barb D, et al. AASLD Practice Guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Hepatology (Baltimore, Md). 2023;77(5):1797–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Pryke R, Guha IN. Time to focus on chronic liver diseases in the community: A review of primary care hepatology tools, pathways of care and reimbursement mechanisms. J Hepatol. 2023;78(3):663–71. [DOI] [PubMed] [Google Scholar]
- 129. Zhang X, Yip TC, Wong GL, Leow WX, Liang LY, Lim LL, et al. Clinical care pathway to detect advanced liver disease in patients with type 2 diabetes through automated fibrosis score calculation and electronic reminder messages: a randomised controlled trial. Gut. 2023;72(12):2364–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Serra-Burriel M, Graupera I, Toran P, Thiele M, Roulot D, Wai-Sun Wong V, et al. Transient elastography for screening of liver fibrosis: Cost-effectiveness analysis from six prospective cohorts in Europe and Asia. J Hepatol. 2019;71(6):1141–51. [DOI] [PubMed] [Google Scholar]
- 131. Vilar-Gomez E, Lou Z, Kong N, Vuppalanchi R, Imperiale TF, Chalasani N. Cost Effectiveness of Different Strategies for Detecting Cirrhosis in Patients With Nonalcoholic Fatty Liver Disease Based on United States Health Care System. Clin Gastroenterol Hepatol. 2020;18(10):2305–14 e12. [DOI] [PubMed] [Google Scholar]
- 132. Phisalprapa P, Supakankunti S, Charatcharoenwitthaya P, Apisarnthanarak P, Charoensak A, Washirasaksiri C, et al. Cost-effectiveness analysis of ultrasonography screening for nonalcoholic fatty liver disease in metabolic syndrome patients. Medicine (Baltimore). 2017;96(17):e6585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Noureddin M, Jones C, Alkhouri N, Gomez EV, Dieterich DT, Rinella ME, et al. , . Nashnet. Screening for Nonalcoholic Fatty Liver Disease in Persons with Type 2 Diabetes in the United States Is Cost-effective: A Comprehensive Cost-Utility Analysis. Gastroenterology. 2020;159(5):1985–7 e4. [DOI] [PubMed] [Google Scholar]
- 134. Tapper EB, Hunink MG, Afdhal NH, Lai M, Sengupta N. Cost-Effectiveness Analysis: Risk Stratification of Nonalcoholic Fatty Liver Disease (NAFLD) by the Primary Care Physician Using the NAFLD Fibrosis Score. PLoS One. 2016;11(2):e0147237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Corey KE, Klebanoff MJ, Tramontano AC, Chung RT, Hur C. Screening for Nonalcoholic Steatohepatitis in Individuals with Type 2 Diabetes: A Cost-Effectiveness Analysis. Dig Dis Sci. 2016;61(7):2108–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Tamaki N, Ahlholm N, Luukkonen PK, Porthan K, Sharpton SR, Ajmera V, et al. Risk of advanced fibrosis in first-degree relatives of patients with nonalcoholic fatty liver disease. J Clin Invest. 2022;132(21):e162513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Vallet-Pichard A, Mallet V, Nalpas B, Verkarre V, Nalpas A, Dhalluin-Venier V, et al. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology (Baltimore, Md). 2007;46(1):32–6. [DOI] [PubMed] [Google Scholar]
- 138. Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, Lok AS. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology (Baltimore, Md). 2003;38(2):518–26. [DOI] [PubMed] [Google Scholar]
- 139. Angulo P, Hui JM, Marchesini G, Bugianesi E, George J, Farrell GC, et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology (Baltimore, Md). 2007;45(4):846–54. [DOI] [PubMed] [Google Scholar]
- 140. European Association for the Study of the Liver; Clinical Practice Guideline Panel; Chair:; EASL Governing Board representative:; Panel members: . EASL Clinical Practice Guidelines on non-invasive tests for evaluation of liver disease severity and prognosis - 2021 update. J Hepatol. 2021;75(3):659–89. [DOI] [PubMed] [Google Scholar]
- 141. Vali Y, Lee J, Boursier J, Petta S, Wonders K, Tiniakos D, et al. Biomarkers for staging fibrosis and non-alcoholic steatohepatitis in non-alcoholic fatty liver disease (the LITMUS project): a comparative diagnostic accuracy study. Lancet Gastroenterol Hepatol. 2023;8(8):714–25. [DOI] [PubMed] [Google Scholar]
- 142. McPherson S, Hardy T, Dufour JF, Petta S, Romero-Gomez M, Allison M, et al. Age as a Confounding Factor for the Accurate Non-Invasive Diagnosis of Advanced NAFLD Fibrosis. Am J Gastroenterol. 2017;112(5):740–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Anushiravani A, Alswat K, Dalekos GN, Zachou K, Ormeci N, Al-Busafi S, et al. Multicenter validation of FIB-6 as a novel machine learning non-invasive score to rule out liver cirrhosis in biopsy-proven MAFLD. Eur J Gastroenterol Hepatol. 2023;35(11):1284–8. [DOI] [PubMed] [Google Scholar]
- 144. Serra-Burriel M, Juanola A, Serra-Burriel F, Thiele M, Graupera I, Pose E, et al. Development, validation, and prognostic evaluation of a risk score for long-term liver-related outcomes in the general population: a multicohort study. Lancet. 2023;402(10406):988–96. [DOI] [PubMed] [Google Scholar]
- 145. van Kleef LA, Francque SM, Prieto-Ortiz JE, Sonneveld MJ, Sanchez-Luque CB, Prieto-Ortiz RG, et al. Maf-5 Predicts Liver Fibrosis Risk and Outcome in the General Population with Metabolic Dysfunction. Gastroenterology. 2024. doi: [DOI] [PubMed] [Google Scholar]
- 146. Sripongpun P, Kim WR, Mannalithara A, Charu V, Vidovszky A, Asch S, et al. The steatosis-associated fibrosis estimator (SAFE) score: A tool to detect low-risk NAFLD in primary care. Hepatology (Baltimore, Md). 2023;77(1):256–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Rosenberg WM, Voelker M, Thiel R, Becka M, Burt A, Schuppan D, et al. Serum markers detect the presence of liver fibrosis: a cohort study. Gastroenterology. 2004;127(6):1704–13. [DOI] [PubMed] [Google Scholar]
- 148. Hinkson A, Lally H, Gibson H, Jones R, Rowe IA, Shinkins B, Parker R. Meta-analysis: Enhanced liver fibrosis test to identify hepatic fibrosis in chronic liver diseases. Aliment Pharmacol Ther. 2023;57(7):750–62. [DOI] [PubMed] [Google Scholar]
- 149. Daniels SJ, Leeming DJ, Eslam M, Hashem AM, Nielsen MJ, Krag A, et al. ADAPT: An Algorithm Incorporating PRO-C3 Accurately Identifies Patients With NAFLD and Advanced Fibrosis. Hepatology (Baltimore, Md). 2019;69(3):1075–86. [DOI] [PubMed] [Google Scholar]
- 150. Eslam M, Wong GL, Hashem AM, Chan HL, Nielsen MJ, Leeming DJ, et al. A Sequential Algorithm Combining ADAPT and Liver Stiffness Can Stage Metabolic-Associated Fatty Liver Disease in Hospital-Based and Primary Care Patients. Am J Gastroenterol. 2021;116(5):984–93. [DOI] [PubMed] [Google Scholar]
- 151. Ozturk A, Olson MC, Samir AE, Venkatesh SK. Liver fibrosis assessment: MR and US elastography. Abdom Radiol (NY). 2022;47(9):3037–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Mikolasevic I, Orlic L, Franjic N, Hauser G, Stimac D, Milic S. Transient elastography (FibroScan(®)) with controlled attenuation parameter in the assessment of liver steatosis and fibrosis in patients with nonalcoholic fatty liver disease - Where do we stand? World J Gastroenterol. 2016;22(32):7236–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Wong VW, Vergniol J, Wong GL, Foucher J, Chan HL, Le Bail B, et al. Diagnosis of fibrosis and cirrhosis using liver stiffness measurement in nonalcoholic fatty liver disease. Hepatology (Baltimore, Md). 2010;51(2):454–62. [DOI] [PubMed] [Google Scholar]
- 154. Anstee QM, Castera L, Loomba R. Impact of non-invasive biomarkers on hepatology practice: Past, present and future. J Hepatol. 2022;76(6):1362–78. [DOI] [PubMed] [Google Scholar]
- 155. Pirmoazen AM, Khurana A, El Kaffas A, Kamaya A. Quantitative ultrasound approaches for diagnosis and monitoring hepatic steatosis in nonalcoholic fatty liver disease. Theranostics. 2020;10(9):4277–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Cassinotto C, Boursier J, Paisant A, Guiu B, Irles-Depe M, Canivet C, et al. Transient Versus Two-Dimensional Shear-Wave Elastography in a Multistep Strategy to Detect Advanced Fibrosis in NAFLD. Hepatology (Baltimore, Md). 2021;73(6):2196–205. [DOI] [PubMed] [Google Scholar]
- 157. Kwok R, Tse YK, Wong GL, Ha Y, Lee AU, Ngu MC, et al. Systematic review with meta-analysis: non-invasive assessment of non-alcoholic fatty liver disease--the role of transient elastography and plasma cytokeratin-18 fragments. Aliment Pharmacol Ther. 2014;39(3):254–69. [DOI] [PubMed] [Google Scholar]
- 158. Selvaraj EA, Mózes FE, Jayaswal ANA, Zafarmand MH, Vali Y, Lee JA, et al. Diagnostic accuracy of elastography and magnetic resonance imaging in patients with NAFLD: A systematic review and meta-analysis. J Hepatol. 2021;75(4):770–85. [DOI] [PubMed] [Google Scholar]
- 159. Xiao G, Zhu S, Xiao X, Yan L, Yang J, Wu G. Comparison of laboratory tests, ultrasound, or magnetic resonance elastography to detect fibrosis in patients with nonalcoholic fatty liver disease: A meta-analysis. Hepatology (Baltimore, Md). 2017;66(5):1486–501. [DOI] [PubMed] [Google Scholar]
- 160. Imajo K, Honda Y, Kobayashi T, Nagai K, Ozaki A, Iwaki M, et al. Direct Comparison of US and MR Elastography for Staging Liver Fibrosis in Patients With Nonalcoholic Fatty Liver Disease. Clin Gastroenterol Hepatol. 2022;20(4):908–17.e11. [DOI] [PubMed] [Google Scholar]
- 161. Andersson A, Kelly M, Imajo K, Nakajima A, Fallowfield JA, Hirschfield G, et al. Clinical Utility of Magnetic Resonance Imaging Biomarkers for Identifying Nonalcoholic Steatohepatitis Patients at High Risk of Progression: A Multicenter Pooled Data and Meta-Analysis. Clin Gastroenterol Hepatol. 2022;20(11):2451–61.e3. [DOI] [PubMed] [Google Scholar]
- 162. Pavlides M, Banerjee R, Sellwood J, Kelly CJ, Robson MD, Booth JC, et al. Multiparametric magnetic resonance imaging predicts clinical outcomes in patients with chronic liver disease. J Hepatol. 2016;64(2):308–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Starekova J, Hernando D, Pickhardt PJ, Reeder SB. Quantification of Liver Fat Content with CT and MRI: State of the Art. Radiology. 2021;301(2):250–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Gu J, Liu S, Du S, Zhang Q, Xiao J, Dong Q, Xin Y. Diagnostic value of MRI-PDFF for hepatic steatosis in patients with non-alcoholic fatty liver disease: a meta-analysis. Eur Radiol. 2019;29(7):3564–73. [DOI] [PubMed] [Google Scholar]
- 165. Qadri S, Vartiainen E, Lahelma M, Porthan K, Tang A, Idilman IS, et al. Marked difference in liver fat measured by histology vs. magnetic resonance-proton density fat fraction: A meta-analysis. JHEP Rep. 2024;6(1):100928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Machann J, Thamer C, Schnoedt B, Stefan N, Haring HU, Claussen CD, et al. Hepatic lipid accumulation in healthy subjects: a comparative study using spectral fat-selective MRI and volume-localized 1H-MR spectroscopy. Magn Reson Med. 2006;55(4):913–7. [DOI] [PubMed] [Google Scholar]
- 167. Karlas T, Petroff D, Sasso M, Fan JG, Mi YQ, de Ledinghen V, et al. Individual patient data meta-analysis of controlled attenuation parameter (CAP) technology for assessing steatosis. J Hepatol. 2017;66(5):1022–30. [DOI] [PubMed] [Google Scholar]
- 168. Noureddin M, Truong E, Gornbein JA, Saouaf R, Guindi M, Todo T, et al. MRI-based (MAST) score accurately identifies patients with NASH and significant fibrosis. J Hepatol. 2022;76(4):781–7. [DOI] [PubMed] [Google Scholar]
- 169. Newsome PN, Sasso M, Deeks JJ, Paredes A, Boursier J, Chan WK, et al. FibroScan-AST (FAST) score for the non-invasive identification of patients with non-alcoholic steatohepatitis with significant activity and fibrosis: a prospective derivation and global validation study. Lancet Gastroenterol Hepatol. 2020;5(4):362–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Jung J, Loomba RR, Imajo K, Madamba E, Gandhi S, Bettencourt R, et al. MRE combined with FIB-4 (MEFIB) index in detection of candidates for pharmacological treatment of NASH-related fibrosis. Gut. 2021;70(10):1946–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Ajmera V, Kim BK, Yang K, Majzoub AM, Nayfeh T, Tamaki N, et al. Liver Stiffness on Magnetic Resonance Elastography and the MEFIB Index and Liver-Related Outcomes in Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis of Individual Participants. Gastroenterology. 2022;163(4):1079–89 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Castera L, Garteiser P, Laouenan C, Vidal-Trecan T, Vallet-Pichard A, Manchon P, et al. Prospective head-to-head comparison of non-invasive scores for diagnosis of fibrotic MASH in patients with type 2 diabetes. J Hepatol. 2024. [DOI] [PubMed] [Google Scholar]
- 173. Kim BK, Tamaki N, Imajo K, Yoneda M, Sutter N, Jung J, et al. Head-to-head comparison between MEFIB, MAST, and FAST for detecting stage 2 fibrosis or higher among patients with NAFLD. J Hepatol. 2022;77(6):1482–90. [DOI] [PubMed] [Google Scholar]
- 174. Boursier J, Hagstrom H, Ekstedt M, Moreau C, Bonacci M, Cure S, et al. Non-invasive tests accurately stratify patients with NAFLD based on their risk of liver-related events. J Hepatol. 2022;76(5):1013–20. [DOI] [PubMed] [Google Scholar]
- 175. Abeysekera KWM, Valenti L, Younossi Z, Dillon JF, Allen AM, Nourredin M, et al. Implementation of a liver health check in people with type 2 diabetes. Lancet Gastroenterol Hepatol. 2024;9(1):83–91. [DOI] [PubMed] [Google Scholar]
- 176. Lin H, Lee HW, Yip TC, Tsochatzis E, Petta S, Bugianesi E, et al. Vibration-Controlled Transient Elastography Scores to Predict Liver-Related Events in Steatotic Liver Disease. JAMA. 2024: 331(15):1287–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Sanyal AJ, Anstee QM, Trauner M, Lawitz EJ, Abdelmalek MF, Ding D, et al. Cirrhosis regression is associated with improved clinical outcomes in patients with nonalcoholic steatohepatitis. Hepatology (Baltimore, Md). 2022;75(5):1235–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Liang JX, Ampuero J, Niu H, Imajo K, Noureddin M, Behari J, et al. An individual patient data meta-analysis to determine cut-offs for and confounders of NAFLD-fibrosis staging with magnetic resonance elastography. J Hepatol. 2023;79(3):592–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Harrison SA, Ratziu V, Magnanensi J, Hajji Y, Deledicque S, Majd Z, et al. NIS2+™, an optimisation of the blood-based biomarker NIS4® technology for the detection of at-risk NASH: A prospective derivation and validation study. J Hepatol. 2023;79(3):758–67. [DOI] [PubMed] [Google Scholar]
- 180. Siddiqui MS, Yamada G, Vuppalanchi R, Van Natta M, Loomba R, Guy C, et al. Diagnostic accuracy of noninvasive fibrosis models to detect change in fibrosis stage. Clin Gastroenterol Hepatol. 2019;17(9):1877–85 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Angulo P, Bugianesi E, Bjornsson ES, Charatcharoenwitthaya P, Mills PR, Barrera F, et al. Simple noninvasive systems predict long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology. 2013;145(4):782–9 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Hagstrom H, Nasr P, Ekstedt M, Stal P, Hultcrantz R, Kechagias S. Accuracy of noninvasive scoring systems in assessing risk of death and liver-related endpoints in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2019;17(6):1148–56 e4. [DOI] [PubMed] [Google Scholar]
- 183. Boursier J, Vergniol J, Guillet A, Hiriart JB, Lannes A, Le Bail B, et al. Diagnostic accuracy and prognostic significance of blood fibrosis tests and liver stiffness measurement by FibroScan in non-alcoholic fatty liver disease. J Hepatol. 2016;65(3):570–8. [DOI] [PubMed] [Google Scholar]
- 184. Kawamura Y, Arase Y, Ikeda K, Seko Y, Imai N, Hosaka T, et al. Large-scale long-term follow-up study of Japanese patients with non-alcoholic Fatty liver disease for the onset of hepatocellular carcinoma. Am J Gastroenterol. 2012;107(2):253–61. [DOI] [PubMed] [Google Scholar]
- 185. Kim D, Kim WR, Kim HJ, Therneau TM. Association between noninvasive fibrosis markers and mortality among adults with nonalcoholic fatty liver disease in the United States. Hepatology (Baltimore, Md). 2013;57(4):1357–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Younossi ZM, Anstee QM, Wai-Sun Wong V, Trauner M, Lawitz EJ, Harrison SA, et al. The Association of Histologic and Noninvasive Tests With Adverse Clinical and Patient-Reported Outcomes in Patients With Advanced Fibrosis Due to Nonalcoholic Steatohepatitis. Gastroenterology. 2021;160(5):1608–19 e13. [DOI] [PubMed] [Google Scholar]
- 187. Younes R, Caviglia GP, Govaere O, Rosso C, Armandi A, Sanavia T, et al. Long-term outcomes and predictive ability of non-invasive scoring systems in patients with non-alcoholic fatty liver disease. J Hepatol. 2021;75(4):786–94. [DOI] [PubMed] [Google Scholar]
- 188. Munteanu M, Pais R, Peta V, Deckmyn O, Moussalli J, Ngo Y, et al. Long-term prognostic value of the FibroTest in patients with non-alcoholic fatty liver disease, compared to chronic hepatitis C, B, and alcoholic liver disease. Aliment Pharmacol Ther. 2018;48(10):1117–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Petta S, Sebastiani G, Vigano M, Ampuero J, Wai-Sun Wong V, Boursier J, et al. Monitoring Occurrence of Liver-Related Events and Survival by Transient Elastography in Patients With Nonalcoholic Fatty Liver Disease and Compensated Advanced Chronic Liver Disease. Clin Gastroenterol Hepatol. 2021;19(4):806–15 e5. [DOI] [PubMed] [Google Scholar]
- 190. Loomba R, Huang DQ, Sanyal AJ, Anstee QM, Trauner M, Lawitz EJ, et al. Liver stiffness thresholds to predict disease progression and clinical outcomes in bridging fibrosis and cirrhosis. Gut. 2023;72(3):581–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Mozes FE, Lee JA, Vali Y, Alzoubi O, Staufer K, Trauner M, et al. Performance of non-invasive tests and histology for the prediction of clinical outcomes in patients with non-alcoholic fatty liver disease: an individual participant data meta-analysis. Lancet Gastroenterol Hepatol. 2023;8(8):704–13. [DOI] [PubMed] [Google Scholar]
- 192. Nascimbeni F, Lebray P, Fedchuk L, Oliveira CP, Alvares-da-Silva MR, Varault A, et al. Significant variations in elastometry measurements made within short-term in patients with chronic liver diseases. Clin Gastroenterol Hepatol. 2015;13(4):763-71 e1-6. [DOI] [PubMed] [Google Scholar]
- 193. Bradley CR, Cox EF, Palaniyappan N, Aithal GP, Francis ST, Guha IN. Variability of noninvasive MRI and biological markers in compensated cirrhosis: insights for assessing disease progression. Eur Radiol Exp. 2022;6(1):52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Chow JC, Wong GL, Chan AW, Shu SS, Chan CK, Leung JK, et al. Repeating measurements by transient elastography in non-alcoholic fatty liver disease patients with high liver stiffness. J Gastroenterol Hepatol. 2019;34(1):241–8. [DOI] [PubMed] [Google Scholar]
- 195. Hagstrom H, Talback M, Andreasson A, Walldius G, Hammar N. Repeated FIB-4 measurements can help identify individuals at risk of severe liver disease. J Hepatol. 2020;73(5):1023–9. [DOI] [PubMed] [Google Scholar]
- 196. Hagstrom H, Talback M, Andreasson A, Walldius G, Hammar N. Ability of noninvasive scoring systems to identify individuals in the population at risk for severe liver disease. Gastroenterology. 2020;158(1):200–14. [DOI] [PubMed] [Google Scholar]
- 197. Trepo E, Valenti L. Update on NAFLD genetics: From new variants to the clinic. J Hepatol. 2020;72(6):1196–209. [DOI] [PubMed] [Google Scholar]
- 198. Chen VL, Oliveri A, Miller MJ, Wijarnpreecha K, Du X, Chen Y, et al. PNPLA3 Genotype and Diabetes Identify Patients With Nonalcoholic Fatty Liver Disease at High Risk of Incident Cirrhosis. Gastroenterology. 2023;164(6):966–77 e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Liu YL, Patman GL, Leathart JB, Piguet AC, Burt AD, Dufour JF, et al. Carriage of the PNPLA3 rs738409 C >G polymorphism confers an increased risk of non-alcoholic fatty liver disease associated hepatocellular carcinoma. J Hepatol. 2014;61(1):75–81. [DOI] [PubMed] [Google Scholar]
- 200. Pelusi S, Baselli G, Pietrelli A, Dongiovanni P, Donati B, McCain MV, et al. Rare Pathogenic Variants Predispose to Hepatocellular Carcinoma in Nonalcoholic Fatty Liver Disease. Sci Rep. 2019;9(1):3682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Cherubini A, Ostadreza M, Jamialahmadi O, Pelusi S, Rrapaj E, Casirati E, et al. Interaction between estrogen receptor-alpha and PNPLA3 p.I148M variant drives fatty liver disease susceptibility in women. Nat Med. 2023;29(10):2643–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Rosso C, Caviglia GP, Birolo G, Armandi A, Pennisi G, Pelusi S, et al. Impact of PNPLA3 rs738409 Polymorphism on the Development of Liver-Related Events in Patients With Nonalcoholic Fatty Liver Disease. Clin Gastroenterol Hepatol. 2023;21(13):3314–21 e3. [DOI] [PubMed] [Google Scholar]
- 203. Mancina RM, Dongiovanni P, Petta S, Pingitore P, Meroni M, Rametta R, et al. The MBOAT7-TMC4 Variant rs641738 Increases Risk of Nonalcoholic Fatty Liver Disease in Individuals of European Descent. Gastroenterology. 2016;150(5):1219–30 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Abul-Husn NS, Cheng X, Li AH, Xin Y, Schurmann C, Stevis P, et al. A Protein-Truncating HSD17B13 Variant and Protection from Chronic Liver Disease. N Engl J Med. 2018;378(12):1096–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Dongiovanni P, Stender S, Pietrelli A, Mancina RM, Cespiati A, Petta S, et al. Causal relationship of hepatic fat with liver damage and insulin resistance in nonalcoholic fatty liver. J Intern Med. 2018;283(4):356–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Baselli GA, Jamialahmadi O, Pelusi S, Ciociola E, Malvestiti F, Saracino M, et al. Rare ATG7 genetic variants predispose patients to severe fatty liver disease. J Hepatol. 2022;77(3):596–606. [DOI] [PubMed] [Google Scholar]
- 207. Jamialahmadi O, Mancina RM, Ciociola E, Tavaglione F, Luukkonen PK, Baselli G, et al. Exome-Wide Association Study on Alanine Aminotransferase Identifies Sequence Variants in the GPAM and APOE Associated With Fatty Liver Disease. Gastroenterology. 2021;160(5):1634–46 e7. [DOI] [PubMed] [Google Scholar]
- 208. Verweij N, Haas ME, Nielsen JB, Sosina OA, Kim M, Akbari P, et al. Germline Mutations in CIDEB and Protection against Liver Disease. N Engl J Med. 2022;387(4):332–44. [DOI] [PubMed] [Google Scholar]
- 209. Haas ME, Pirruccello JP, Friedman SN, Wang M, Emdin CA, Ajmera VH, et al. Machine learning enables new insights into genetic contributions to liver fat accumulation. Cell Genom. 2021;1(3):100066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Bianco C, Jamialahmadi O, Pelusi S, Baselli G, Dongiovanni P, Zanoni I, et al. Non-invasive stratification of hepatocellular carcinoma risk in non-alcoholic fatty liver using polygenic risk scores. J Hepatol. 2021;74(4):775–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. De Vincentis A, Tavaglione F, Jamialahmadi O, Picardi A, Antonelli Incalzi R, Valenti L, et al. A Polygenic Risk Score to Refine Risk Stratification and Prediction for Severe Liver Disease by Clinical Fibrosis Scores. Clin Gastroenterol Hepatol. 2022;20(3):658–73. [DOI] [PubMed] [Google Scholar]
- 212. Vilarinho S, Ajmera V, Zheng M, Loomba R. Emerging Role of Genomic Analysis in Clinical Evaluation of Lean Individuals With NAFLD. Hepatology (Baltimore, Md). 2021;74(4):2241–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Hakim A, Zhang X, DeLisle A, Oral EA, Dykas D, Drzewiecki K, et al. Clinical utility of genomic analysis in adults with idiopathic liver disease. J Hepatol. 2019;70(6):1214–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Pelusi S, Ronzoni L, Malvestiti F, Bianco C, Marini I, D'Ambrosio R, et al. Clinical exome sequencing for diagnosing severe cryptogenic liver disease in adults: A case series. Liver Int. 2022;42(4):864–70. [DOI] [PubMed] [Google Scholar]
- 215. Pelusi S, Ronzoni L, Rondena J, Rosso C, Pennisi G, Dongiovanni P, et al. Prevalence and Determinants of Liver Disease in Relatives of Italian Patients With Advanced MASLD. Clin Gastroenterol Hepatol. 2024;S1542-3565(24)00046-6. [DOI] [PubMed] [Google Scholar]
- 216. Zheng M, Hakim A, Konkwo C, Deaton AM, Ward LD, Alnylam Human G, et al. Advancing diagnosis and management of liver disease in adults through exome sequencing. EBioMedicine. 2023;95:104747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Pillai S, Duvvuru S, Bhatnagar P, Foster W, Farmen M, Shankar S, et al. The PNPLA3 I148M variant is associated with transaminase elevations in type 2 diabetes patients treated with basal insulin peglispro. Pharmacogenomics J. 2018;18(3):487–93. [DOI] [PubMed] [Google Scholar]
- 218. Mak LY, Gane E, Schwabe C, Yoon KT, Heo J, Scott R, et al. A phase I/II study of ARO-HSD, an RNA interference therapeutic, for the treatment of non-alcoholic steatohepatitis. J Hepatol. 2023;78(4):684–92. [DOI] [PubMed] [Google Scholar]
- 219. Cherubini A, Casirati E, Tomasi M, Valenti L. PNPLA3 as a therapeutic target for fatty liver disease: the evidence to date. Expert Opin Ther Targets. 2021;25(12):1033–43. [DOI] [PubMed] [Google Scholar]
- 220. Gibson S, Ashwell M. A simple cut-off for waist-to-height ratio (0.5) can act as an indicator for cardiometabolic risk: recent data from adults in the Health Survey for England. Br J Nutr. 2020;123(6):681–90. [DOI] [PubMed] [Google Scholar]
- 221. Salmon-Gomez L, Catalan V, Fruhbeck G, Gomez-Ambrosi J. Relevance of body composition in phenotyping the obesities. Rev Endocr Metab Disord. 2023;24(5):809–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Isokuortti E, Zhou Y, Peltonen M, Bugianesi E, Clement K, Bonnefont-Rousselot D, et al. Use of HOMA-IR to diagnose non-alcoholic fatty liver disease: a population-based and inter-laboratory study. Diabetologia. 2017;60(10):1873–82. [DOI] [PubMed] [Google Scholar]
- 223. Gastaldelli A. Measuring and estimating insulin resistance in clinical and research settings. Obesity (Silver Spring). 2022;30(8):1549–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Thomas PE, Vedel-Krogh S, Nordestgaard BG. Measuring lipoprotein(a) for cardiovascular disease prevention - in whom and when? Curr Opin Cardiol. 2024;39(1):39–48. [DOI] [PubMed] [Google Scholar]
- 225. Xourafa G, Korbmacher M, Roden M. Inter-organ crosstalk during development and progression of type 2 diabetes mellitus. Nat Rev Endocrinol. 2024;20(1):27–49. [DOI] [PubMed] [Google Scholar]
- 226. Bril F, Sanyal A, Cusi K. Metabolic Syndrome and Its Association with Nonalcoholic Steatohepatitis. Clin Liver Dis. 2023;27(2):187–210. [DOI] [PubMed] [Google Scholar]
- 227. Mantovani A, Petracca G, Beatrice G, Tilg H, Byrne CD, Targher G. Non-alcoholic fatty liver disease and risk of incident diabetes mellitus: an updated meta-analysis of 501 022 adult individuals. Gut. 2021;70(5):962–9. [DOI] [PubMed] [Google Scholar]
- 228. Zaharia OP, Strassburger K, Strom A, Bonhof GJ, Karusheva Y, Antoniou S, et al. Risk of diabetes-associated diseases in subgroups of patients with recent-onset diabetes: a 5-year follow-up study. Lancet Diabetes Endocrinol. 2019;7(9):684–94. [DOI] [PubMed] [Google Scholar]
- 229. Kalavalapalli S, Leiva EG, Lomonaco R, Chi X, Shrestha S, Dillard R, et al. Adipose Tissue Insulin Resistance Predicts the Severity of Liver Fibrosis in Patients With Type 2 Diabetes and NAFLD. J Clin Endocrinol Metab. 2023;108(5):1192–201. [DOI] [PubMed] [Google Scholar]
- 230. Fabbrini E, Sullivan S, Klein S. Obesity and nonalcoholic fatty liver disease: biochemical, metabolic, and clinical implications. Hepatology (Baltimore, Md). 2010;51(2):679–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Frühbeck G, Busetto L, Dicker D, Yumuk V, Goossens GH, Hebebrand J, et al. The ABCD of Obesity: An EASO Position Statement on a Diagnostic Term with Clinical and Scientific Implications. Obes Facts. 2019;12(2):131–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Kim DH, Park BJ, Kim W, Jung Y, Kim YJ, Yoon JH, et al. Visceral fat as a strong and independent risk factor of nonalcoholic fatty liver disease. J Hepatol. 2008;48:S350. [Google Scholar]
- 233. Mantovani A, Petracca G, Beatrice G, Csermely A, Lonardo A, Schattenberg JM, et al. Non-alcoholic fatty liver disease and risk of incident chronic kidney disease: an updated meta-analysis. Gut. 2022;71(1):156–62. [DOI] [PubMed] [Google Scholar]
- 234. Byrne CD, Targher G. NAFLD as a driver of chronic kidney disease. J Hepatol. 2020;72(4):785–801. [DOI] [PubMed] [Google Scholar]
- 235. Huang Y, Wang Y, Xiao Z, Yao S, Tang Y, Zhou L, et al. The association between metabolic dysfunction-associated steatotic liver disease, cardiovascular and cerebrovascular diseases and the thickness of carotid plaque. BMC Cardiovasc Disord. 2023;23(1):554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Ciardullo S, Cannistraci R, Muraca E, Zerbini F, Perseghin G. Liver fibrosis, NT-ProBNP and mortality in patients with MASLD: A population-based cohort study. Nutr Metab Cardiovasc Dis. 2024;34(4):963–971. [DOI] [PubMed] [Google Scholar]
- 237. Stine JG, Wentworth BJ, Zimmet A, Rinella ME, Loomba R, Caldwell SH, Argo CK. Systematic review with meta-analysis: risk of hepatocellular carcinoma in non-alcoholic steatohepatitis without cirrhosis compared to other liver diseases. Alimentary Pharmacology & Therapeutics. 2018;48(7):696–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Dyson J, Jaques B, Chattopadyhay D, Lochan R, Graham J, Das D, et al. Hepatocellular cancer: the impact of obesity, type 2 diabetes and a multidisciplinary team. J Hepatol. 2014;60(1):110–7. [DOI] [PubMed] [Google Scholar]
- 239. Pais R, Fartoux L, Goumard C, Scatton O, Wendum D, Rosmorduc O, Ratziu V. Temporal trends, clinical patterns and outcomes of NAFLD-related HCC in patients undergoing liver resection over a 20-year period. Aliment Pharmacol Ther. 2017;46(9):856–63. [DOI] [PubMed] [Google Scholar]
- 240. Tokushige K, Hashimoto E, Horie Y, Taniai M, Higuchi S. Hepatocellular carcinoma in Japanese patients with nonalcoholic fatty liver disease, alcoholic liver disease, and chronic liver disease of unknown etiology: report of the nationwide survey. J Gastroenterol. 2011;46(10):1230–7. [DOI] [PubMed] [Google Scholar]
- 241. Yasui K, Hashimoto E, Komorizono Y, Koike K, Arii S, Imai Y, et al. Characteristics of patients with nonalcoholic steatohepatitis who develop hepatocellular carcinoma. Clin Gastroenterol Hepatol. 2011;9(5):428–33. [DOI] [PubMed] [Google Scholar]
- 242. Mohamad B, Shah V, Onyshchenko M, Elshamy M, Aucejo F, Lopez R, et al. Characterization of hepatocellular carcinoma (HCC) in non-alcoholic fatty liver disease (NAFLD) patients without cirrhosis. Hepatology International. 2016;10(4):632–9. [DOI] [PubMed] [Google Scholar]
- 243. Perumpail RB, Wong RJ, Ahmed A, Harrison SA. Hepatocellular Carcinoma in the Setting of Non-cirrhotic Nonalcoholic Fatty Liver Disease and the Metabolic Syndrome: US Experience. Dig Dis Sci. 2015;60(10):3142–8. [DOI] [PubMed] [Google Scholar]
- 244. Parikh ND, Singal AG, Hutton DW, Tapper EB. Cost-Effectiveness of Hepatocellular Carcinoma Surveillance: An Assessment of Benefits and Harms. Am J Gastroenterol. 2020;115(10):1642–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Sarasin FP, Giostra E, Hadengue A. Cost-effectiveness of screening for detection of small hepatocellular carcinoma in western patients with Child-Pugh class A cirrhosis. Am J Med. 1996;101(4):422–34. [DOI] [PubMed] [Google Scholar]
- 246. Orci LA, Sanduzzi-Zamparelli M, Caballol B, Sapena V, Colucci N, Torres F, et al. Incidence of Hepatocellular Carcinoma in Patients With Nonalcoholic Fatty Liver Disease: A Systematic Review, Meta-analysis, and Meta-regression. Clin Gastroenterol Hepatol. 2022;20(2):283–92 e10. [DOI] [PubMed] [Google Scholar]
- 247. Ioannou GN. Epidemiology and risk-stratification of NAFLD-associated HCC. J Hepatol. 2021;75(6):1476–84. [DOI] [PubMed] [Google Scholar]
- 248. White DL, Kanwal F, El–Serag HB. Association Between Nonalcoholic Fatty Liver Disease and Risk for Hepatocellular Cancer, Based on Systematic Review. Clinical Gastroenterology and Hepatology. 2012;10(12):1342–59.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Reig M, Gambato M, Man NK, Roberts JP, Victor D, Orci LA, Toso C. Should Patients With NAFLD/NASH Be Surveyed for HCC? Transplantation. 2019;103(1):39–44. [DOI] [PubMed] [Google Scholar]
- 250. Behari J, Gougol A, Wang R, Luu HN, Paragomi P, Yu Y-C, et al. Incidence of hepatocellular carcinoma in nonalcoholic fatty liver disease without cirrhosis or advanced liver fibrosis. Hepatology Communications. 2023;7(7):e00183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251. Kanwal F, Kramer JR, Mapakshi S, Natarajan Y, Chayanupatkul M, Richardson PA, et al. Risk of Hepatocellular Cancer in Patients With Non-Alcoholic Fatty Liver Disease. Gastroenterology. 2018;155(6):1828–37 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Simon TG, King LY, Chong DQ, Nguyen LH, Ma Y, VoPham T, et al. Diabetes, metabolic comorbidities, and risk of hepatocellular carcinoma: Results from two prospective cohort studies. Hepatology (Baltimore, Md). 2018;67(5):1797–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Chen Y, Wang X, Wang J, Yan Z, Luo J. Excess body weight and the risk of primary liver cancer: an updated meta-analysis of prospective studies. Eur J Cancer. 2012;48(14):2137–45. [DOI] [PubMed] [Google Scholar]
- 254. Dongiovanni P, Romeo S, Valenti L. Hepatocellular carcinoma in nonalcoholic fatty liver: role of environmental and genetic factors. World J Gastroenterol. 2014;20(36):12945–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255. Ajmera VH, Terrault NA, Harrison SA. Is moderate alcohol use in nonalcoholic fatty liver disease good or bad? A critical review. Hepatology (Baltimore, Md). 2017;65(6):2090–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256. European Association for the Study of the Liver; European Association for the Study of the Liver . EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182–236. [DOI] [PubMed] [Google Scholar]
- 257. European Association for the Study of the Liver . EASL Policy Statement: risk-based surveillance for hepatocellular carcinoma among patients with cirrhosis. 2023. Available from: https://easl.eu/publication/easl-policy-statement-risk-based/.
- 258. Walker M, El-Serag HB, Sada Y, Mittal S, Ying J, Duan Z, et al. Cirrhosis is under-recognised in patients subsequently diagnosed with hepatocellular cancer. Aliment Pharmacol Ther. 2016;43(5):621–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. Wolf E, Rich NE, Marrero JA, Parikh ND, Singal AG. Use of Hepatocellular Carcinoma Surveillance in Patients With Cirrhosis: A Systematic Review and Meta-Analysis. Hepatology (Baltimore, Md). 2021;73(2):713–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260. Tzartzeva K, Obi J, Rich NE, Parikh ND, Marrero JA, Yopp A, et al. Surveillance Imaging and Alpha Fetoprotein for Early Detection of Hepatocellular Carcinoma in Patients With Cirrhosis: A Meta-analysis. Gastroenterology. 2018;154(6):1706–18 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261. Atiq O, Tiro J, Yopp AC, Muffler A, Marrero JA, Parikh ND, et al. An assessment of benefits and harms of hepatocellular carcinoma surveillance in patients with cirrhosis. Hepatology (Baltimore, Md). 2017;65(4):1196–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262. Kim SY, An J, Lim YS, Han S, Lee JY, Byun JH, et al. MRI With Liver-Specific Contrast for Surveillance of Patients With Cirrhosis at High Risk of Hepatocellular Carcinoma. JAMA Oncol. 2017;3(4):456–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263. Kim HL, An J, Park JA, Park SH, Lim YS, Lee EK. Magnetic Resonance Imaging Is Cost-Effective for Hepatocellular Carcinoma Surveillance in High-Risk Patients With Cirrhosis. Hepatology (Baltimore, Md). 2019;69(4):1599–613. [DOI] [PubMed] [Google Scholar]
- 264. Hughes DM, Berhane S, Emily de Groot CA, Toyoda H, Tada T, Kumada T, et al. Serum Levels of α-Fetoprotein Increased More Than 10 Years Before Detection of Hepatocellular Carcinoma. Clinical Gastroenterology and Hepatology. 2021;19(1):162–70.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265. Cholankeril G, Kramer JR, Chu J, Yu X, Balakrishnan M, Li L, et al. Longitudinal changes in fibrosis markers are associated with risk of cirrhosis and hepatocellular carcinoma in non-alcoholic fatty liver disease. J Hepatol. 2023;78(3):493–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266. Ioannou GN, Green P, Kerr KF, Berry K. Models estimating risk of hepatocellular carcinoma in patients with alcohol or NAFLD-related cirrhosis for risk stratification. J Hepatol. 2019;71(3):523–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267. Fan R, Papatheodoridis G, Sun J, Innes H, Toyoda H, Xie Q, et al. aMAP risk score predicts hepatocellular carcinoma development in patients with chronic hepatitis. J Hepatol. 2020;73(6):1368–78. [DOI] [PubMed] [Google Scholar]
- 268. Toyoda H, Fujii H, Iwaki M, Hayashi H, Oeda S, Hyogo H, et al. Validation of Noninvasive Markers for HCC Risk Stratification in 1389 Patients With Biopsy-proven NAFLD. Gastro Hep Advances. 2023;2(8):1093–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269. Best J, Bechmann LP, Sowa JP, Sydor S, Dechene A, Pflanz K, et al. GALAD Score Detects Early Hepatocellular Carcinoma in an International Cohort of Patients With Nonalcoholic Steatohepatitis. Clin Gastroenterol Hepatol. 2020;18(3):728–35 e4. [DOI] [PubMed] [Google Scholar]
- 270. Reddy KR, McLerran D, Marsh T, Parikh N, Roberts LR, Schwartz M, et al. Incidence and Risk Factors for Hepatocellular Carcinoma in Cirrhosis: The Multicenter Hepatocellular Carcinoma Early Detection Strategy (HEDS) Study. Gastroenterology. 2023;165(4):1053–63 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271. Sharma SA, Kowgier M, Hansen BE, Brouwer WP, Maan R, Wong D, et al. Toronto HCC risk index: A validated scoring system to predict 10-year risk of HCC in patients with cirrhosis. J Hepatol. 2017;S0168-8278(17)32248-1. [DOI] [PubMed] [Google Scholar]
- 272. Astrom H, Ndegwa N, Hagstrom H. External validation of the Toronto hepatocellular carcinoma risk index in a Swedish population. JHEP Rep. 2021;3(5):100343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273. Siddiqui MS, Harrison SA, Abdelmalek MF, Anstee QM, Bedossa P, Castera L, et al. Case definitions for inclusion and analysis of endpoints in clinical trials for nonalcoholic steatohepatitis through the lens of regulatory science. Hepatology (Baltimore, Md). 2018;67(5):2001–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274. Sanyal AJ, Castera L, Wong VW. Noninvasive Assessment of Liver Fibrosis in NAFLD. Clin Gastroenterol Hepatol. 2023;21(8):2026–39. [DOI] [PubMed] [Google Scholar]
- 275. Rinella ME, Tacke F, Sanyal AJ, Anstee QM; participants of the AASLD/EASL Workshop . Report on the AASLD/EASL joint workshop on clinical trial endpoints in NAFLD. J Hepatol. 2019;71(4):823–33. [DOI] [PubMed] [Google Scholar]
- 276. Kleiner DE, Brunt EM, Wilson LA, Behling C, Guy C, Contos M, et al. Association of Histologic Disease Activity With Progression of Nonalcoholic Fatty Liver Disease. JAMA Netw Open. 2019;2(10):e1912565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277. Brunt EM, Kleiner DE, Wilson LA, Sanyal AJ, Neuschwander-Tetri BA; Nonalcoholic Steatohepatitis Clinical Research Network . Improvements in Histologic Features and Diagnosis Associated With Improvement in Fibrosis in Nonalcoholic Steatohepatitis: Results From the Nonalcoholic Steatohepatitis Clinical Research Network Treatment Trials. Hepatology (Baltimore, Md). 2019;70(2):522–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278. Stine JG, Munaganuru N, Barnard A, Wang JL, Kaulback K, Argo CK, et al. Change in MRI-PDFF and Histologic Response in Patients With Nonalcoholic Steatohepatitis: A Systematic Review and Meta-Analysis. Clin Gastroenterol Hepatol. 2021;19(11):2274–83 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279. Tamaki N, Munaganuru N, Jung J, Yonan AQ, Loomba RR, Bettencourt R, et al. Clinical utility of 30% relative decline in MRI-PDFF in predicting fibrosis regression in non-alcoholic fatty liver disease. Gut. 2022;71(5):983–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280. Harrison SA, Rossi SJ, Paredes AH, Trotter JF, Bashir MR, Guy CD, et al. NGM282 Improves Liver Fibrosis and Histology in 12 Weeks in Patients With Nonalcoholic Steatohepatitis. Hepatology (Baltimore, Md). 2020;71(4):1198–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281. Ratziu V, Sanyal A, Harrison SA, Wong VW, Francque S, Goodman Z, et al. Cenicriviroc Treatment for Adults With Nonalcoholic Steatohepatitis and Fibrosis: Final Analysis of the Phase 2b CENTAUR Study. Hepatology (Baltimore, Md). 2020;72(3):892–905. [DOI] [PubMed] [Google Scholar]
- 282. Loomba R, Sanyal AJ, Kowdley KV, Bhatt DL, Alkhouri N, Frias JP, et al. Randomized, Controlled Trial of the FGF21 Analogue Pegozafermin in NASH. N Engl J Med. 2023;389(11):998–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283. Rinella ME, Dufour JF, Anstee QM, Goodman Z, Younossi Z, Harrison SA, et al. Non-invasive evaluation of response to obeticholic acid in patients with NASH: Results from the REGENERATE study. J Hepatol. 2022;76(3):536–48. [DOI] [PubMed] [Google Scholar]
- 284. Harrison SA, Bashir MR, Guy CD, Zhou R, Moylan CA, Frias JP, et al. Resmetirom (MGL-3196) for the treatment of non-alcoholic steatohepatitis: a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2019;394(10213):2012–24. [DOI] [PubMed] [Google Scholar]
- 285. Jayakumar S, Middleton MS, Lawitz EJ, Mantry PS, Caldwell SH, Arnold H, et al. Longitudinal correlations between MRE, MRI-PDFF, and liver histology in patients with non-alcoholic steatohepatitis: Analysis of data from a phase II trial of selonsertib. J Hepatol. 2019;70(1):133–41. [DOI] [PubMed] [Google Scholar]
- 286. Bril F, Barb D, Lomonaco R, Lai J, Cusi K. Change in hepatic fat content measured by MRI does not predict treatment-induced histological improvement of steatohepatitis. J Hepatol. 2020;72(3):401–10. [DOI] [PubMed] [Google Scholar]
- 287. Loomba R, Sanyal AJ, Kowdley KV, Terrault N, Chalasani NP, Abdelmalek MF, et al. Factors Associated With Histologic Response in Adult Patients With Nonalcoholic Steatohepatitis. Gastroenterology. 2019;156(1):88–95 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288. Wai-Sun Wong V, Anstee QM, Nitze LM, Geerts A, George J, Nolasco V, et al. FibroScan-aspartate aminotransferase (FAST) score for monitoring histological improvement in non-alcoholic steatohepatitis activity during semaglutide treatment: post-hoc analysis of a randomised, double-blind, placebo-controlled, phase 2b trial. EClinicalMedicine. 2023;66:102310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289. Malehmir M, Pfister D, Gallage S, Szydlowska M, Inverso D, Kotsiliti E, et al. Platelet GPIbalpha is a mediator and potential interventional target for NASH and subsequent liver cancer. Nat Med. 2019;25(4):641–55. [DOI] [PubMed] [Google Scholar]
- 290. Simon TG, Henson J, Osganian S, Masia R, Chan AT, Chung RT, Corey KE. Daily Aspirin Use Associated With Reduced Risk For Fibrosis Progression In Patients With Nonalcoholic Fatty Liver Disease. Clin Gastroenterol Hepatol. 2019;17(13):2776–84 e4, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291. Alexander M, Loomis AK, van der Lei J, Duarte-Salles T, Prieto-Alhambra D, Ansell D, et al. Non-alcoholic fatty liver disease and risk of incident acute myocardial infarction and stroke: findings from matched cohort study of 18 million European adults. BMJ. 2019;367:l5367, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292. Anstee QM, Mantovani A, Tilg H, Targher G. Risk of cardiomyopathy and cardiac arrhythmias in patients with nonalcoholic fatty liver disease. Nat Rev Gastroenterol Hepatol. 2018;15(7):425–39. [DOI] [PubMed] [Google Scholar]
- 293. Fernandez T, Vinuela M, Vidal C, Barrera F. Lifestyle changes in patients with non-alcoholic fatty liver disease: A systematic review and meta-analysis. PLoS One. 2022;17(2):e0263931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294. Koutoukidis DA, Koshiaris C, Henry JA, Noreik M, Morris E, Manoharan I, et al. The effect of the magnitude of weight loss on non-alcoholic fatty liver disease: A systematic review and meta-analysis. Metabolism. 2021;115:154455. [DOI] [PubMed] [Google Scholar]
- 295. Haigh L, Kirk C, El Gendy K, Gallacher J, Errington L, Mathers JC, Anstee QM. The effectiveness and acceptability of Mediterranean diet and calorie restriction in non-alcoholic fatty liver disease (NAFLD): A systematic review and meta-analysis. Clin Nutr. 2022;41(9):1913–31. [DOI] [PubMed] [Google Scholar]
- 296. Vilar-Gomez E, Martinez-Perez Y, Calzadilla-Bertot L, Torres-Gonzalez A, Gra-Oramas B, Gonzalez-Fabian L, et al. Weight Loss Through Lifestyle Modification Significantly Reduces Features of Nonalcoholic Steatohepatitis. Gastroenterology. 2015;149(2):367–78.e5. [DOI] [PubMed] [Google Scholar]
- 297. Malespin MH, Barritt ASt, Watkins SE, Schoen C, Tincopa MA, Corbin KD, et al. Weight Loss and Weight Regain in Usual Clinical Practice: Results From the TARGET-NASH Observational Cohort. Clin Gastroenterol Hepatol. 2022;20(10):2393–5 e4, [DOI] [PubMed] [Google Scholar]
- 298. Wharton S, Lau DCW, Vallis M, Sharma AM, Biertho L, Campbell-Scherer D, et al. Obesity in adults: a clinical practice guideline. CMAJ. 2020;192(31):E875–E91, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299. Ezpeleta M, Gabel K, Cienfuegos S, Kalam F, Lin S, Pavlou V, et al. Effect of alternate day fasting combined with aerobic exercise on non-alcoholic fatty liver disease: A randomized controlled trial. Cell Metab. 2023;35(1):56–70.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300. Younossi ZM, Zelber-Sagi S, Henry L, Gerber LH. Lifestyle interventions in nonalcoholic fatty liver disease. Nat Rev Gastroenterol Hepatol. 2023;20(11):708–22. [DOI] [PubMed] [Google Scholar]
- 301. Kawaguchi T, Charlton M, Kawaguchi A, Yamamura S, Nakano D, Tsutsumi T, et al. Effects of Mediterranean Diet in Patients with Nonalcoholic Fatty Liver Disease: A Systematic Review, Meta-Analysis, and Meta-Regression Analysis of Randomized Controlled Trials. Semin Liver Dis. 2021;41(3):225–34. [DOI] [PubMed] [Google Scholar]
- 302. Marin-Alejandre BA, Cantero I, Perez-Diaz-Del-Campo N, Monreal JI, Elorz M, Herrero JI, et al. Effects of two personalized dietary strategies during a 2-year intervention in subjects with nonalcoholic fatty liver disease: A randomized trial. Liver Int. 2021;41(7):1532–44. [DOI] [PubMed] [Google Scholar]
- 303. Yaskolka Meir A, Rinott E, Tsaban G, Zelicha H, Kaplan A, Rosen P, et al. Effect of green-Mediterranean diet on intrahepatic fat: the DIRECT PLUS randomised controlled trial. Gut. 2021;70(11):2085–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304. Wei X, Lin B, Huang Y, Yang S, Huang C, Shi L, et al. Effects of Time-Restricted Eating on Nonalcoholic Fatty Liver Disease: The TREATY-FLD Randomized Clinical Trial. JAMA Netw Open. 2023;6(3):e233513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305. Yki-Järvinen H, Luukkonen PK, Hodson L, Moore JB. Dietary carbohydrates and fats in nonalcoholic fatty liver disease. Nat Rev Gastroenterol Hepatol. 2021;18(11):770–86. [DOI] [PubMed] [Google Scholar]
- 306. Hansen CD, Gram-Kampmann EM, Hansen JK, Hugger MB, Madsen BS, Jensen JM, et al. Effect of Calorie-Unrestricted Low-Carbohydrate, High-Fat Diet Versus High-Carbohydrate, Low-Fat Diet on Type 2 Diabetes and Nonalcoholic Fatty Liver Disease : A Randomized Controlled Trial. Ann Intern Med. 2023;176(1):10–21. [DOI] [PubMed] [Google Scholar]
- 307. Zelber-Sagi S, Grinshpan LS, Ivancovsky-Wajcman D, Goldenshluger A, Gepner Y. One size does not fit all; practical, personal tailoring of the diet to NAFLD patients. Liver Int. 2022;42(8):1731–50. [DOI] [PubMed] [Google Scholar]
- 308. Crosby L, Davis B, Joshi S, Jardine M, Paul J, Neola M, Barnard ND. Ketogenic Diets and Chronic Disease: Weighing the Benefits Against the Risks. Front Nutr. 2021;8:702802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309. Rynders CA, Thomas EA, Zaman A, Pan Z, Catenacci VA, Melanson EL. Effectiveness of intermittent fasting and time-restricted feeding compared to continuous energy restriction for weight loss. Nutrients. 2019;11(10):2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310. Holmer M, Lindqvist C, Petersson S, Moshtaghi-Svensson J, Tillander V, Brismar TB, et al. Treatment of NAFLD with intermittent calorie restriction or low-carb high-fat diet - a randomised controlled trial. JHEP Rep. 2021;3(3):100256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311. Buzzetti E, Linden A, Best LM, Madden AM, Roberts D, Chase TJG, et al. Lifestyle modifications for nonalcohol-related fatty liver disease: a network meta-analysis. Cochrane Database Syst Rev. 2021;6(6):CD013156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312. Hassani Zadeh S, Mansoori A, Hosseinzadeh M. Relationship between dietary patterns and non-alcoholic fatty liver disease: A systematic review and meta-analysis. J Gastroenterol Hepatol. 2021;36(6):1470–8. [DOI] [PubMed] [Google Scholar]
- 313. Gepner Y, Shelef I, Komy O, Cohen N, Schwarzfuchs D, Bril N, et al. The beneficial effects of Mediterranean diet over low-fat diet may be mediated by decreasing hepatic fat content. J Hepatol. 2019;71(2):379–88. [DOI] [PubMed] [Google Scholar]
- 314. Properzi C, O'Sullivan TA, Sherriff JL, Ching HL, Jeffrey GP, Buckley RF, et al. Ad Libitum Mediterranean and Low-Fat Diets Both Significantly Reduce Hepatic Steatosis: A Randomized Controlled Trial. Hepatology (Baltimore, Md). 2018;68(5):1741–54. [DOI] [PubMed] [Google Scholar]
- 315. He K, Li Y, Guo X, Zhong L, Tang S. Food groups and the likelihood of non-alcoholic fatty liver disease: a systematic review and meta-analysis. Br J Nutr. 2020;124(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316. Peng X, Li J, Zhao H, Lai J, Lin J, Tang S. Lifestyle as well as metabolic syndrome and non-alcoholic fatty liver disease: an umbrella review of evidence from observational studies and randomized controlled trials. BMC Endocr Disord. 2022;22(1):95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317. Liu Z, Huang H, Zeng Y, Chen Y, Xu C. Association between ultra-processed foods consumption and risk of non-alcoholic fatty liver disease: a population-based analysis of NHANES 2011-2018. Br J Nutr. 2023;130(6):996–1004. [DOI] [PubMed] [Google Scholar]
- 318. Etemadi A, Sinha R, Ward MH, Graubard BI, Inoue-Choi M, Dawsey SM, Abnet CC. Mortality from different causes associated with meat, heme iron, nitrates, and nitrites in the NIH-AARP Diet and Health Study: population based cohort study. BMJ. 2017;357:j1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319. Zelber-Sagi S, Ivancovsky-Wajcman D, Fliss Isakov N, Webb M, Orenstein D, Shibolet O, Kariv R. High red and processed meat consumption is associated with non-alcoholic fatty liver disease and insulin resistance. J Hepatol. 2018;68(6):1239–46. [DOI] [PubMed] [Google Scholar]
- 320. Noureddin M, Zelber-Sagi S, Wilkens LR, Porcel J, Boushey CJ, Le Marchand L, et al. Diet Associations With Nonalcoholic Fatty Liver Disease in an Ethnically Diverse Population: The Multiethnic Cohort. Hepatology (Baltimore, Md). 2020;71(6):1940–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321. Simons N, Veeraiah P, Simons P, Schaper NC, Kooi ME, Schrauwen-Hinderling VB, et al. Effects of fructose restriction on liver steatosis (FRUITLESS); a double-blind randomized controlled trial. Am J Clin Nutr. 2021;113(2):391–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322. Geidl-Flueck B, Hochuli M, Nemeth A, Eberl A, Derron N, Kofeler HC, et al. Fructose- and sucrose- but not glucose-sweetened beverages promote hepatic de novo lipogenesis: A randomized controlled trial. J Hepatol. 2021;75(1):46–54. [DOI] [PubMed] [Google Scholar]
- 323. Park WY, Yiannakou I, Petersen JM, Hoffmann U, Ma J, Long MT. Sugar-Sweetened Beverage, Diet Soda, and Nonalcoholic Fatty Liver Disease Over 6 Years: The Framingham Heart Study. Clin Gastroenterol Hepatol. 2022;20(11):2524–32 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324. Mosca A, Nobili V, De Vito R, Crudele A, Scorletti E, Villani A, et al. Serum uric acid concentrations and fructose consumption are independently associated with NASH in children and adolescents. J Hepatol. 2017;66(5):1031–6. [DOI] [PubMed] [Google Scholar]
- 325. Vilar-Gomez E, Vuppalanchi R, Gawrieh S, Pike F, Samala N, Chalasani N. Significant Dose-Response Association of Physical Activity and Diet Quality With Mortality in Adults With Suspected NAFLD in a Population Study. Am J Gastroenterol. 2023;118(9):1576–91. [DOI] [PubMed] [Google Scholar]
- 326. Lichtenstein AH, Appel LJ, Vadiveloo M, Hu FB, Kris-Etherton PM, Rebholz CM, et al. 2021 Dietary Guidance to Improve Cardiovascular Health: A Scientific Statement From the American Heart Association. Circulation. 2021;144(23):e472–e87. [DOI] [PubMed] [Google Scholar]
- 327. Kim D, Vazquez-Montesino LM, Li AA, Cholankeril G, Ahmed A. Inadequate Physical Activity and Sedentary Behavior Are Independent Predictors of Nonalcoholic Fatty Liver Disease. Hepatology (Baltimore, Md). 2020;72(5):1556–68. [DOI] [PubMed] [Google Scholar]
- 328. Stine JG, Long MT, Corey KE, Sallis RE, Allen AM, Armstrong MJ, et al. American College of Sports Medicine (ACSM) International Multidisciplinary Roundtable report on physical activity and nonalcoholic fatty liver disease. Hepatol Commun. 2023;7(4):e0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329. Baker CJ, Martinez-Huenchullan SF, D'Souza M, Xu Y, Li M, Bi Y, et al. Effect of exercise on hepatic steatosis: Are benefits seen without dietary intervention? A systematic review and meta-analysis. J Diabetes. 2021;13(1):63–77, [DOI] [PubMed] [Google Scholar]
- 330. Oh S, Tsujimoto T, Kim B, Uchida F, Suzuki H, Iizumi S, et al. Weight-loss-independent benefits of exercise on liver steatosis and stiffness in Japanese men with NAFLD. JHEP Rep. 2021;3(3):100253, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331. Hallsworth K, Fattakhova G, Hollingsworth KG, Thoma C, Moore S, Taylor R, et al. Resistance exercise reduces liver fat and its mediators in non-alcoholic fatty liver disease independent of weight loss. Gut. 2011;60(9):1278–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332. Hashida R, Kawaguchi T, Bekki M, Omoto M, Matsuse H, Nago T, et al. Aerobic vs. resistance exercise in non-alcoholic fatty liver disease: A systematic review. J Hepatol. 2017;66(1):142–52. [DOI] [PubMed] [Google Scholar]
- 333. Sabag A, Barr L, Armour M, Armstrong A, Baker CJ, Twigg SM, et al. The Effect of High-intensity Interval Training vs Moderate-intensity Continuous Training on Liver Fat: A Systematic Review and Meta-Analysis. J Clin Endocrinol Metab. 2022;107(3):862–81. [DOI] [PubMed] [Google Scholar]
- 334. O'Gorman P, Naimimohasses S, Monaghan A, Kennedy M, Melo AM, Ni Fhloinn D, et al. Improvement in histological endpoints of MAFLD following a 12-week aerobic exercise intervention. Aliment Pharmacol Ther. 2020;52(8):1387–98. [DOI] [PubMed] [Google Scholar]
- 335. Chen G, Banini BA, Do A, Gunderson C, Zaman S, Lim JK. Exercise Does Not Independently Improve Histological Outcomes in Biopsy-Proven Non-Alcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. Genes (Basel). 2023;14(9):1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336. Duarte-Rojo A, Ruiz-Margain A, Montano-Loza AJ, Macias-Rodriguez RU, Ferrando A, Kim WR. Exercise and physical activity for patients with end-stage liver disease: Improving functional status and sarcopenia while on the transplant waiting list. Liver Transpl. 2018;24(1):122–39. [DOI] [PubMed] [Google Scholar]
- 337. Abdelbasset WK, Tantawy SA, Kamel DM, Alqahtani BA, Soliman GS. A randomized controlled trial on the effectiveness of 8-week high-intensity interval exercise on intrahepatic triglycerides, visceral lipids, and health-related quality of life in diabetic obese patients with nonalcoholic fatty liver disease. Medicine (Baltimore). 2019;98(12):e14918, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338. Kim D, Murag S, Cholankeril G, Cheung A, Harrison SA, Younossi ZM, Ahmed A. Physical Activity, Measured Objectively, Is Associated With Lower Mortality in Patients With Nonalcoholic Fatty Liver Disease. Clin Gastroenterol Hepatol. 2021;19(6):1240–7.e5, [DOI] [PubMed] [Google Scholar]
- 339. Chun HS, Lee M, Lee HA, Oh SY, Baek HJ, Moon JW, et al. Association of Physical Activity With Risk of Liver Fibrosis, Sarcopenia, and Cardiovascular Disease in Nonalcoholic Fatty Liver Disease. Clin Gastroenterol Hepatol. 2023;21(2):358–69.e12. [DOI] [PubMed] [Google Scholar]
- 340. Henry A, Paik JM, Austin P, Eberly KE, Golabi P, Younossi I, et al. Vigorous physical activity provides protection against all-cause deaths among adults patients with nonalcoholic fatty liver disease (NAFLD). Aliment Pharmacol Ther. 2023;57(6):709–22. [DOI] [PubMed] [Google Scholar]
- 341. DiJoseph K, Thorp A, Harrington A, Schmitz KH, Chinchilli VM, JG. S. Physical Activity and Risk of Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis. Dig Dis Sci. 2023;68(3):1051–9. [DOI] [PubMed] [Google Scholar]
- 342. Sherry AP, Willis SA, Yates T, Johnson W, Razieh C, Sargeant JA, et al. Physical activity is inversely associated with hepatic fibro-inflammation: A population-based cohort study using UK Biobank data. JHEP Rep. 2023;5(1):100622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343. Francque SM, Marchesini G, Kautz A, Walmsley M, Dorner R, Lazarus JV, et al. Non-alcoholic fatty liver disease: A patient guideline. JHEP Rep. 2021;3(5):100322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344. Khurshid S, Al-Alusi MA, Churchill TW, Guseh JS, Ellinor PT. Accelerometer-Derived “Weekend Warrior” Physical Activity and Incident Cardiovascular Disease. JAMA. 2023;330(3):247–52, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345. Ye Q, Zou B, Yeo YH, Li J, Huang DQ, Wu Y, et al. Global prevalence, incidence, and outcomes of non-obese or lean non-alcoholic fatty liver disease: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2020;5(8):739–52, [DOI] [PubMed] [Google Scholar]
- 346. Long MT, Noureddin M, Lim JK. AGA Clinical Practice Update: Diagnosis and Management of Nonalcoholic Fatty Liver Disease in Lean Individuals: Expert Review. Gastroenterology. 2022;163(3):764–74 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347. Younes R, Bugianesi E. NASH in Lean Individuals. Semin Liver Dis. 2019;39(1):86–95. [DOI] [PubMed] [Google Scholar]
- 348. Sookoian S, Pirola CJ. Systematic review with meta-analysis: risk factors for non-alcoholic fatty liver disease suggest a shared altered metabolic and cardiovascular profile between lean and obese patients. Aliment Pharmacol Ther. 2017;46(2):85–95, [DOI] [PubMed] [Google Scholar]
- 349. Wong VW, Wong GL, Chan RS, Shu SS, Cheung BH, Li LS, et al. Beneficial effects of lifestyle intervention in non-obese patients with non-alcoholic fatty liver disease. J Hepatol. 2018;69(6):1349–56, [DOI] [PubMed] [Google Scholar]
- 350. Sinn DH, Kang D, Cho SJ, Paik SW, Guallar E, Cho J, Gwak GY. Weight change and resolution of fatty liver in normal weight individuals with nonalcoholic fatty liver disease. Eur J Gastroenterol Hepatol. 2021;33(1S Suppl 1):e529–e34. [DOI] [PubMed] [Google Scholar]
- 351. Komolafe O, Buzzetti E, Linden A, Best LM, Madden AM, Roberts D, et al. Nutritional supplementation for nonalcohol-related fatty liver disease: a network meta-analysis. Cochrane Database Syst Rev. 2021;7(7):CD013157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352. Koning M, Herrema H, Nieuwdorp M, Meijnikman AS. Targeting nonalcoholic fatty liver disease via gut microbiome-centered therapies. Gut Microbes. 2023;15(1):2226922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353. Tang Y, Huang J, Zhang WY, Qin S, Yang YX, Ren H, et al. Effects of probiotics on nonalcoholic fatty liver disease: a systematic review and meta-analysis. Therap Adv Gastroenterol. 2019;12:1756284819878046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354. Hayat U, Siddiqui AA, Okut H, Afroz S, Tasleem S, Haris A. The effect of coffee consumption on the non-alcoholic fatty liver disease and liver fibrosis: A meta-analysis of 11 epidemiological studies. Ann Hepatol. 2021;20:100254. [DOI] [PubMed] [Google Scholar]
- 355. Chen YP, Lu FB, Hu YB, Xu LM, Zheng MH, Hu ED. A systematic review and a dose-response meta-analysis of coffee dose and nonalcoholic fatty liver disease. Clin Nutr. 2019;38(6):2552–7. [DOI] [PubMed] [Google Scholar]
- 356. Niezen S, Mehta M, Jiang ZG, Tapper EB. Coffee Consumption Is Associated With Lower Liver Stiffness: A Nationally Representative Study. Clin Gastroenterol Hepatol. 2022;20(9):2032–40 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357. Kennedy OJ, Fallowfield JA, Poole R, Hayes PC, Parkes J, Roderick PJ. All coffee types decrease the risk of adverse clinical outcomes in chronic liver disease: a UK Biobank study. BMC Public Health. 2021;21(1):970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358. Bhurwal A, Rattan P, Yoshitake S, Pioppo L, Reja D, Dellatore P, Rustgi V. Inverse Association of Coffee with Liver Cancer Development: An Updated Systematic Review and Meta-analysis. J Gastrointestin Liver Dis. 2020;29(3):421–8. [DOI] [PubMed] [Google Scholar]
- 359. Mansour A, Mohajeri-Tehrani MR, Samadi M, Qorbani M, Merat S, Adibi H, et al. Effects of supplementation with main coffee components including caffeine and/or chlorogenic acid on hepatic, metabolic, and inflammatory indices in patients with non-alcoholic fatty liver disease and type 2 diabetes: a randomized, double-blind, placebo-controlled, clinical trial. Nutr J. 2021;20(1):35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360. Linden-Torres E, Zambrano-Galvan G, Sahebkar A, Rios-Mier M, Simental-Mendia LE. Coffee consumption has no effect on circulating markers of liver function but increases adiponectin concentrations: A systematic review and meta-analysis of randomized controlled trials. Nutr Res. 2022;106:24–34. [DOI] [PubMed] [Google Scholar]
- 361. Kim D, Kim W, Joo SK, Bae JM, Kim JH, Ahmed A. Subclinical Hypothyroidism and Low-Normal Thyroid Function Are Associated With Nonalcoholic Steatohepatitis and Fibrosis. Clin Gastroenterol Hepatol. 2018;16(1):123–31 e1. [DOI] [PubMed] [Google Scholar]
- 362. Hatziagelaki E, Paschou SA, Schon M, Psaltopoulou T, Roden M. NAFLD and thyroid function: pathophysiological and therapeutic considerations. Trends Endocrinol Metab. 2022;33(11):755–68. [DOI] [PubMed] [Google Scholar]
- 363. Ritter MJ, Amano I, Hollenberg AN. Thyroid Hormone Signaling and the Liver. Hepatology (Baltimore, Md). 2020;72(2):742–52, [DOI] [PubMed] [Google Scholar]
- 364. Alonso-Merino E, Martín Orozco R, Ruíz-Llorente L, Martínez-Iglesias OA, Velasco-Martín JP, Montero-Pedrazuela A, et al. Thyroid hormones inhibit TGF-β signaling and attenuate fibrotic responses. Proceedings of the National Academy of Sciences. 2016;113(24):E3451–E60, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365. Kelly MJ, Pietranico-Cole S, Larigan JD, Haynes NE, Reynolds CH, Scott N, et al. Discovery of 2-[3,5-dichloro-4-(5-isopropyl-6-oxo-1,6-dihydropyridazin-3-yloxy)phenyl]-3,5-dio xo-2,3,4,5-tetrahydro[1,2,4]triazine-6-carbonitrile (MGL-3196), a Highly Selective Thyroid Hormone Receptor beta agonist in clinical trials for the treatment of dyslipidemia. J Med Chem. 2014;57(10):3912–23. [DOI] [PubMed] [Google Scholar]
- 366. Harrison SA, Bedossa P, Guy CD, Schattenberg JM, Loomba R, Taub R, et al. A Phase 3, Randomized, Controlled Trial of Resmetirom in NASH with Liver Fibrosis. N Engl J Med. 2024;390(6):497–509. [DOI] [PubMed] [Google Scholar]
- 367. Harrison SA, Ratziu V, Anstee QM, Noureddin M, Sanyal AJ, Schattenberg JM, et al. Design of the phase 3 MAESTRO clinical program to evaluate resmetirom for the treatment of nonalcoholic steatohepatitis. Aliment Pharmacol Ther. 2024;59(1):51–63. [DOI] [PubMed] [Google Scholar]
- 368. Harrison SA, Ratziu V, Boursier J, Francque S, Bedossa P, Majd Z, et al. NIS4™ for non-invasive diagnosis of nonalcoholic steatohepatitis and liver fibrosis. Lancet Gastroenterol Hepatol. 2020;5(11):970–85. [DOI] [PubMed] [Google Scholar]
- 369. Sanyal AJ, Shankar SS, Yates KP, Bolognese J, Daly E, Dehn CA, et al. Diagnostic performance of circulating biomarkers for non-alcoholic steatohepatitis. Nat Med. 2023;29(10):2656–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370. Ravaioli F, Dajti E, Mantovani A, Newsome PN, Targher G, Colecchia A. Diagnostic accuracy of FibroScan-AST (FAST) score for the non-invasive identification of patients with fibrotic non-alcoholic steatohepatitis: a systematic review and meta-analysis. Gut. 2023;72(7):1399–409. [DOI] [PubMed] [Google Scholar]
- 371. Tamaki N, Imajo K, Sharpton S, Jung J, Kawamura N, Yoneda M, et al. Magnetic resonance elastography plus Fibrosis-4 versus FibroScan-aspartate aminotransferase in detection of candidates for pharmacological treatment of NASH-related fibrosis. Hepatology (Baltimore, Md). 2022;75(3):661–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372. Truong E, Gornbein JA, Yang JD, Noureddin N, Harrison SA, Alkhouri N, Noureddin M. MRI-AST (MAST) Score Accurately Predicts Major Adverse Liver Outcome, Hepatocellular Carcinoma, Liver Transplant, and Liver-Related Death. Clin Gastroenterol Hepatol. 2023;21(10):2570–7.e1. [DOI] [PubMed] [Google Scholar]
- 373. Gidener T, Ahmed OT, Larson JJ, Mara KC, Therneau TM, Venkatesh SK, et al. Liver Stiffness by Magnetic Resonance Elastography Predicts Future Cirrhosis, Decompensation, and Death in NAFLD. Clin Gastroenterol Hepatol. 2021;19(9):1915–24 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374. Sanyal AJ, Harrison SA, Ratziu V, Abdelmalek MF, Diehl AM, Caldwell S, et al. The Natural History of Advanced Fibrosis Due to Nonalcoholic Steatohepatitis: Data From the Simtuzumab Trials. Hepatology (Baltimore, Md). 2019;70(6):1913–27. [DOI] [PubMed] [Google Scholar]
- 375. Harrison SA, Wong VW, Okanoue T, Bzowej N, Vuppalanchi R, Younes Z, et al. Selonsertib for patients with bridging fibrosis or compensated cirrhosis due to NASH: Results from randomized phase III STELLAR trials. J Hepatol. 2020;73(1):26–39. [DOI] [PubMed] [Google Scholar]
- 376. Harrison SA, Taub R, Neff GW, Lucas KJ, Labriola D, Moussa SE, et al. Resmetirom for nonalcoholic fatty liver disease: a randomized, double-blind, placebo-controlled phase 3 trial. Nat Med. 2023;29(11):2919–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377. Cusi K. Selective Agonists of Thyroid Hormone Receptor Beta for the Treatment of NASH. N Engl J Med. 2024;390(6):559–61. [DOI] [PubMed] [Google Scholar]
- 378. Ratziu V, Tacke F. At the dawn of potent therapeutics for fatty liver disease - introducing the miniseries on promising pharmacological targets for NASH. J Hepatol. 2023;79(2):261–2. [DOI] [PubMed] [Google Scholar]
- 379. Huang J, Weinstein SJ, Yu K, Männistö S, Albanes D. Relationship Between Serum Alpha-Tocopherol and Overall and Cause-Specific Mortality. Circ Res. 2019;125(1):29–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380. Jayedi A, Rashidy-Pour A, Parohan M, Zargar MS, Shab-Bidar S. Dietary Antioxidants, Circulating Antioxidant Concentrations, Total Antioxidant Capacity, and Risk of All-Cause Mortality: A Systematic Review and Dose-Response Meta-Analysis of Prospective Observational Studies. Adv Nutr. 2018;9(6):701–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381. Scorletti E, Creasy KT, Vujkovic M, Vell M, Zandvakili I, Rader DJ, et al. Dietary Vitamin E Intake Is Associated With a Reduced Risk of Developing Digestive Diseases and Nonalcoholic Fatty Liver Disease. Am J Gastroenterol. 2022;117(6):927–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382. Fortmann SP, Burda BU, Senger CA, Lin JS, Whitlock EP. Vitamin and mineral supplements in the primary prevention of cardiovascular disease and cancer: An updated systematic evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2013;159(12):824–34. [DOI] [PubMed] [Google Scholar]
- 383. Bjelakovic G, Nikolova D, Gluud C. Meta-regression analyses, meta-analyses, and trial sequential analyses of the effects of supplementation with beta-carotene, vitamin A, and vitamin E singly or in different combinations on all-cause mortality: do we have evidence for lack of harm? PLoS One. 2013;8(9):e74558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384. Myung SK, Ju W, Cho B, Oh SW, Park SM, Koo BK, Park BJ, et al. Efficacy of vitamin and antioxidant supplements in prevention of cardiovascular disease: systematic review and meta-analysis of randomised controlled trials. Bmj. 2013;346:f10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385. Vilar-Gomez E, Vuppalanchi R, Gawrieh S, Ghabril M, Saxena R, Cummings OW, Chalasani N. Vitamin E Improves Transplant-Free Survival and Hepatic Decompensation Among Patients With Nonalcoholic Steatohepatitis and Advanced Fibrosis. Hepatology (Baltimore, Md). 2020;71(2):495–509. [DOI] [PubMed] [Google Scholar]
- 386. Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, et al. Pioglitazone, Vitamin E, or Placebo for Nonalcoholic Steatohepatitis. N Engl J Med. 2010;362(18):1675–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387. Lindor KD, Kowdley KV, Heathcote EJ, Harrison ME, Jorgensen R, Angulo P, et al. Ursodeoxycholic acid for treatment of nonalcoholic steatohepatitis: results of a randomized trial. Hepatology (Baltimore, Md). 2004;39(3):770–8, [DOI] [PubMed] [Google Scholar]
- 388. Leuschner UF, Lindenthal B, Herrmann G, Arnold JC, Rossle M, Cordes HJ, et al. High-dose ursodeoxycholic acid therapy for nonalcoholic steatohepatitis: a double-blind, randomized, placebo-controlled trial. Hepatology (Baltimore, Md). 2010;52(2):472–9, [DOI] [PubMed] [Google Scholar]
- 389. Ratziu V, de Ledinghen V, Oberti F, Mathurin P, Wartelle-Bladou C, Renou C, et al. A randomized controlled trial of high-dose ursodesoxycholic acid for nonalcoholic steatohepatitis. J Hepatol. 2011;54(5):1011–9. [DOI] [PubMed] [Google Scholar]
- 390. Zhu C, Boucheron N, Müller AC, Májek P, Claudel T, Halilbasic E, et al. 24-Norursodeoxycholic acid reshapes immunometabolism in CD8(+) T cells and alleviates hepatic inflammation. J Hepatol. 2021;75(5):1164–76, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391. Traussnigg S, Schattenberg JM, Demir M, Wiegand J, Geier A, Teuber G, et al. Norursodeoxycholic acid versus placebo in the treatment of non-alcoholic fatty liver disease: a double-blind, randomised, placebo-controlled, phase 2 dose-finding trial. Lancet Gastroenterol Hepatol. 2019;4(10):781–93, [DOI] [PubMed] [Google Scholar]
- 392. Adorini L, Pruzanski M, Shapiro D. Farnesoid X receptor targeting to treat nonalcoholic steatohepatitis. Drug Discov Today. 2012;17(17-18):988–97. [DOI] [PubMed] [Google Scholar]
- 393. Nevens F, Andreone P, Mazzella G, Strasser SI, Bowlus C, Invernizzi P, et al. A Placebo-Controlled Trial of Obeticholic Acid in Primary Biliary Cholangitis. N Engl J Med. 2016;375(7):631–43. [DOI] [PubMed] [Google Scholar]
- 394. Neuschwander-Tetri BA, Loomba R, Sanyal AJ, Lavine JE, Van Natta ML, Abdelmalek MF, et al. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet. 2015;385(9972):956–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395. Younossi ZM, Ratziu V, Loomba R, Rinella M, Anstee QM, Goodman Z, et al. Obeticholic acid for the treatment of non-alcoholic steatohepatitis: interim analysis from a multicentre, randomised, placebo-controlled phase 3 trial. Lancet. 2019;394(10215):2184–96. [DOI] [PubMed] [Google Scholar]
- 396. Sanyal AJ, Ratziu V, Loomba R, Anstee QM, Kowdley KV, Rinella ME, et al. Results from a new efficacy and safety analysis of the REGENERATE trial of obeticholic acid for treatment of pre-cirrhotic fibrosis due to non-alcoholic steatohepatitis. J Hepatol. 2023;79(5):1110–20. [DOI] [PubMed] [Google Scholar]
- 397. Ratziu V, Rinella ME, Neuschwander-Tetri BA, Lawitz E, Denham D, Kayali Z, et al. EDP-305 in patients with NASH: A phase II double-blind placebo-controlled dose-ranging study. J Hepatol. 2022;76(3):506–17. [DOI] [PubMed] [Google Scholar]
- 398. Ratziu V, Harrison SA, Loustaud-Ratti V, Bureau C, Lawitz E, Abdelmalek M, et al. Hepatic and renal improvements with FXR agonist vonafexor in individuals with suspected fibrotic NASH. J Hepatol. 2023;78(3):479–92. [DOI] [PubMed] [Google Scholar]
- 399. Puri P, Baillie RA, Wiest MM, Mirshahi F, Choudhury J, Cheung O, et al. A lipidomic analysis of nonalcoholic fatty liver disease. Hepatology (Baltimore, Md). 2007;46(4):1081–90. [DOI] [PubMed] [Google Scholar]
- 400. Sanyal AJ, Abdelmalek MF, Suzuki A, Cummings OW, Chojkier M; EPE-A Study Group . No significant effects of ethyl-eicosapentanoic acid on histologic features of nonalcoholic steatohepatitis in a phase 2 trial. Gastroenterology. 2014;147(2):377–84 e1, [DOI] [PubMed] [Google Scholar]
- 401. Argo CK, Patrie JT, Lackner C, Henry TD, de Lange EE, Weltman AL, et al. Effects of n-3 fish oil on metabolic and histological parameters in NASH: a double-blind, randomized, placebo-controlled trial. J Hepatol. 2015;62(1):190–7, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402. Fraser DA, Wang X, Lund J, Nikolic N, Iruarrizaga-Lejarreta M, Skjaeret T, et al. A structurally engineered fatty acid, icosabutate, suppresses liver inflammation and fibrosis in NASH. J Hepatol. 2022;76(4):800–11. [DOI] [PubMed] [Google Scholar]
- 403. Athyros VG, Tziomalos K, Gossios TD, Griva T, Anagnostis P, Kargiotis K, et al. Safety and efficacy of long-term statin treatment for cardiovascular events in patients with coronary heart disease and abnormal liver tests in the Greek Atorvastatin and Coronary Heart Disease Evaluation (GREACE) Study: a post-hoc analysis. Lancet. 2010;376(9756):1916–22. [DOI] [PubMed] [Google Scholar]
- 404. Bril F, Portillo Sanchez P, Lomonaco R, Orsak B, Hecht J, Tio F, Cusi K. Liver Safety of Statins in Prediabetes or T2DM and Nonalcoholic Steatohepatitis: Post Hoc Analysis of a Randomized Trial. J Clin Endocrinol Metab. 2017;102(8):2950–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405. Chalasani N, Aljadhey H, Kesterson J, Murray MD, Hall SD. Patients with elevated liver enzymes are not at higher risk for statin hepatotoxicity. Gastroenterology. 2004;126(5):1287–92, [DOI] [PubMed] [Google Scholar]
- 406. Blais P, Lin M, Kramer JR, El-Serag HB, Kanwal F. Statins Are Underutilized in Patients with Nonalcoholic Fatty Liver Disease and Dyslipidemia. Dig Dis Sci. 2016;61(6):1714–20, [DOI] [PubMed] [Google Scholar]
- 407. Ayada I, van Kleef LA, Zhang H, Liu K, Li P, Abozaid YJ, et al. Dissecting the multifaceted impact of statin use on fatty liver disease: a multidimensional study. EBioMedicine. 2023;87:104392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408. El-Serag HB, Johnson ML, Hachem C, Morgana RO. Statins are associated with a reduced risk of hepatocellular carcinoma in a large cohort of patients with diabetes. Gastroenterology. 2009;136(5):1601–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409. Wah Kheong C, Nik Mustapha NR, Mahadeva S. A Randomized Trial of Silymarin for the Treatment of Nonalcoholic Steatohepatitis. Clin Gastroenterol Hepatol. 2017;15(12):1940–9.e8. [DOI] [PubMed] [Google Scholar]
- 410. Navarro VJ, Belle SH, D'Amato M, Adfhal N, Brunt EM, Fried MW, et al. Silymarin in non-cirrhotics with non-alcoholic steatohepatitis: A randomized, double-blind, placebo controlled trial. PLoS One. 2019;14(9):e0221683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411. Newsome PN, Ambery P. Incretins (GLP-1 receptor agonists and dual/triple agonists) and the liver. J Hepatol. 2023;79(6):1557–65. [DOI] [PubMed] [Google Scholar]
- 412. Drucker DJ. GLP-1 physiology informs the pharmacotherapy of obesity. Mol Metab. 2022;57:101351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413. Nogueiras R, Nauck MA, Tschop MH. Gut hormone co-agonists for the treatment of obesity: from bench to bedside. Nat Metab. 2023;5(6):933–44. [DOI] [PubMed] [Google Scholar]
- 414. Armstrong MJ, Gaunt P, Aithal GP, Barton D, Hull D, Parker R, et al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet. 2016;387(10019):679–90. [DOI] [PubMed] [Google Scholar]
- 415. Newsome PN, Buchholtz K, Cusi K, Linder M, Okanoue T, Ratziu V, et al. A Placebo-Controlled Trial of Subcutaneous Semaglutide in Nonalcoholic Steatohepatitis. N Engl J Med. 2021;384(12):1113–24. [DOI] [PubMed] [Google Scholar]
- 416. Alkhouri N, Herring R, Kabler H, Kayali Z, Hassanein T, Kohli A, et al. Safety and efficacy of combination therapy with semaglutide, cilofexor and firsocostat in patients with non-alcoholic steatohepatitis: A randomised, open-label phase II trial. J Hepatol. 2022;77(3):607–18. [DOI] [PubMed] [Google Scholar]
- 417. Loomba R, Noureddin M, Kowdley KV, Kohli A, Sheikh A, Neff G, et al. Combination Therapies Including Cilofexor and Firsocostat for Bridging Fibrosis and Cirrhosis Attributable to NASH. Hepatology (Baltimore, Md). 2021;73(2):625–43. [DOI] [PubMed] [Google Scholar]
- 418. Gastaldelli A, Cusi K, Fernandez Lando L, Bray R, Brouwers B, Rodriguez A. Effect of tirzepatide versus insulin degludec on liver fat content and abdominal adipose tissue in people with type 2 diabetes (SURPASS-3 MRI): a substudy of the randomised, open-label, parallel-group, phase 3 SURPASS-3 trial. Lancet Diabetes Endocrinol. 2022;10(6):393–406. [DOI] [PubMed] [Google Scholar]
- 419. Nahra R, Wang T, Gadde KM, Oscarsson J, Stumvoll M, Jermutus L, et al. Effects of Cotadutide on Metabolic and Hepatic Parameters in Adults With Overweight or Obesity and Type 2 Diabetes: A 54-Week Randomized Phase 2b Study. Diabetes Care. 2021;44(6):1433–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420. Romero-Gomez M, Lawitz E, Shankar RR, Chaudhri E, Liu J, Lam RLH, et al. A phase IIa active-comparator-controlled study to evaluate the efficacy and safety of efinopegdutide in patients with non-alcoholic fatty liver disease. J Hepatol. 2023;79(4):888–97. [DOI] [PubMed] [Google Scholar]
- 421. Bluher M, Rosenstock J, Hoefler J, Manuel R, Hennige AM. Dose-response effects on HbA(1c) and bodyweight reduction of survodutide, a dual glucagon/GLP-1 receptor agonist, compared with placebo and open-label semaglutide in people with type 2 diabetes: a randomised clinical trial. Diabetologia. 2024;67(3):470–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422. A Phase 2 Randomized Trial of Survodutide in MASH and Fibrosis A; Sanyal J., Bedossa P., Fraessdorf M., Neff G.W., Lawitz E., Bugianesi E., Anstee Q.M., Ajaz Hussain S., Newsome P.N., Ratziu V., Hosseini-Tabatabaei A., Schattenberg J.M., Noureddin M., Alkhouri N., and Younes R., for the NCT04771273 Trial Investigators*. N Engl J Med. 2024;(in press). [DOI] [PubMed] [Google Scholar]
- 423. Simon TG, Patorno E, Schneeweiss S. Glucagon-Like Peptide-1 Receptor Agonists and Hepatic Decompensation Events in Patients With Cirrhosis and Diabetes. Clin Gastroenterol Hepatol. 2022;20(6):1382–93.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424. Yen FS, Hou MC, Cheng-Chung Wei J, Shih YH, Hsu CY, Hsu CC, Hwu CM. Glucagon-like Peptide-1 Receptor Agonist Use in Patients With Liver Cirrhosis and Type 2 Diabetes. Clin Gastroenterol Hepatol. 2023;22(4):902–903. [DOI] [PubMed] [Google Scholar]
- 425. Loomba R, Abdelmalek MF, Armstrong MJ, Jara M, Kjær MS, Krarup N, et al. Semaglutide 2·4 mg once weekly in patients with non-alcoholic steatohepatitis-related cirrhosis: a randomised, placebo-controlled phase 2 trial. Lancet Gastroenterol Hepatol. 2023;8(6):511–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426. Brown E, Heerspink HJL, Cuthbertson DJ, Wilding JPH. SGLT2 inhibitors and GLP-1 receptor agonists: established and emerging indications. Lancet. 2021;398(10296):262–76. [DOI] [PubMed] [Google Scholar]
- 427. Ridderstråle M, Andersen KR, Zeller C, Kim G, Woerle HJ, Broedl UC, et al. Comparison of empagliflozin and glimepiride as add-on to metformin in patients with type 2 diabetes: a 104-week randomised, active-controlled, double-blind, phase 3 trial. Lancet Diabetes Endocrinol. 2014;2(9):691–700. [DOI] [PubMed] [Google Scholar]
- 428. Kuchay MS, Krishan S, Mishra SK, Farooqui KJ, Singh MK, Wasir JS, et al. Effect of Empagliflozin on Liver Fat in Patients With Type 2 Diabetes and Nonalcoholic Fatty Liver Disease: A Randomized Controlled Trial (E-LIFT Trial). Diabetes Care. 2018;41(8):1801–8. [DOI] [PubMed] [Google Scholar]
- 429. Kahl S, Gancheva S, Straßburger K, Herder C, Machann J, Katsuyama H, et al. Empagliflozin Effectively Lowers Liver Fat Content in Well-Controlled Type 2 Diabetes: A Randomized, Double-Blind, Phase 4, Placebo-Controlled Trial. Diabetes Care. 2020;43(2):298–305. [DOI] [PubMed] [Google Scholar]
- 430. Latva-Rasku A, Honka MJ, Kullberg J, Mononen N, Lehtimäki T, Saltevo J, et al. The SGLT2 Inhibitor Dapagliflozin Reduces Liver Fat but Does Not Affect Tissue Insulin Sensitivity: A Randomized, Double-Blind, Placebo-Controlled Study With 8-Week Treatment in Type 2 Diabetes Patients. Diabetes Care. 2019;42(5):931–7. [DOI] [PubMed] [Google Scholar]
- 431. Harrison SA, Manghi FP, Smith WB, Alpenidze D, Aizenberg D, Klarenbeek N, et al. Licogliflozin for nonalcoholic steatohepatitis: a randomized, double-blind, placebo-controlled, phase 2a study. Nat Med. 2022;28(7):1432–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432. Aithal GP, Thomas JA, Kaye PV, Lawson A, Ryder SD, Spendlove I, et al. Randomized, placebo-controlled trial of pioglitazone in nondiabetic subjects with nonalcoholic steatohepatitis. Gastroenterology. 2008;135(4):1176–84. [DOI] [PubMed] [Google Scholar]
- 433. Cusi K, Orsak B, Bril F, Lomonaco R, Hecht J, Ortiz-Lopez C, et al. Long-Term Pioglitazone Treatment for Patients With Nonalcoholic Steatohepatitis and Prediabetes or Type 2 Diabetes Mellitus: A Randomized Trial. Ann Intern Med. 2016;165(5):305–15. [DOI] [PubMed] [Google Scholar]
- 434. Belfort R, Harrison SA, Brown K, Darland C, Finch J, Hardies J, et al. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med. 2006;355(22):2297–307. [DOI] [PubMed] [Google Scholar]
- 435. Dormandy JA, Charbonnel B, Eckland DJ, Erdmann E, Massi-Benedetti M, Moules IK, et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet. 2005;366(9493):1279–89. [DOI] [PubMed] [Google Scholar]
- 436. Kernan WN, Viscoli CM, Furie KL, Young LH, Inzucchi SE, Gorman M, et al. Pioglitazone after Ischemic Stroke or Transient Ischemic Attack. N Engl J Med. 2016;374(14):1321–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437. Staels B, Butruille L, Francque S. Treating NASH by targeting peroxisome proliferator-activated receptors. J Hepatol. 2023;79(5):1302–16. [DOI] [PubMed] [Google Scholar]
- 438. Harrison SA, Thang C, Bolze S, Dewitt S, Hallakou-Bozec S, Dubourg J, et al. Evaluation of PXL065 - deuterium-stabilized (R)-pioglitazone in patients with NASH: A phase II randomized placebo-controlled trial (DESTINY-1). J Hepatol. 2023;78(5):914–25. [DOI] [PubMed] [Google Scholar]
- 439. Francque SM, Bedossa P, Ratziu V, Anstee QM, Bugianesi E, Sanyal AJ, et al. A Randomized, Controlled Trial of the Pan-PPAR Agonist Lanifibranor in NASH. N Engl J Med. 2021;385(17):1547–58. [DOI] [PubMed] [Google Scholar]
- 440. Gawrieh S, Noureddin M, Loo N, Mohseni R, Awasty V, Cusi K, et al. Saroglitazar, a PPAR-α/γ Agonist, for Treatment of NAFLD: A Randomized Controlled Double-Blind Phase 2 Trial. Hepatology (Baltimore, Md). 2021;74(4):1809–24. [DOI] [PubMed] [Google Scholar]
- 441. Goyal O, Nohria S, Goyal P, Kaur J, Sharma S, Sood A, Chhina RS. Saroglitazar in patients with non-alcoholic fatty liver disease and diabetic dyslipidemia: a prospective, observational, real world study. Sci Rep. 2020;10(1):21117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442. Nair S, Diehl AM, Wiseman M, FarrGH, Jr., Perrillo RP. Metformin in the treatment of non-alcoholic steatohepatitis: a pilot open label trial. Aliment Pharmacol Ther. 2004;20(1):23–8. [DOI] [PubMed] [Google Scholar]
- 443. Loomba R, Lutchman G, Kleiner DE, Ricks M, Feld JJ, Borg BB, et al. Clinical trial: pilot study of metformin for the treatment of non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2009;29(2):172–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444. Vilar-Gomez E, Vuppalanchi R, Desai AP, Gawrieh S, Ghabril M, Saxena R, et al. Long-term metformin use may improve clinical outcomes in diabetic patients with non-alcoholic steatohepatitis and bridging fibrosis or compensated cirrhosis. Aliment Pharmacol Ther. 2019;50(3):317–28. [DOI] [PubMed] [Google Scholar]
- 445. Fujiwara N, Friedman SL, Goossens N, Hoshida Y. Risk factors and prevention of hepatocellular carcinoma in the era of precision medicine. J Hepatol. 2018;68(3):526–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 446. Zhang X, Harmsen WS, Mettler TA, Kim WR, Roberts RO, Therneau TM, et al. Continuation of metformin use after a diagnosis of cirrhosis significantly improves survival of patients with diabetes. Hepatology (Baltimore, Md). 2014;60(6):2008–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 447. Grunvald E, Shah R, Hernaez R, Chandar AK, Pickett-Blakely O, Teigen LM, et al. AGA Clinical Practice Guideline on Pharmacological Interventions for Adults With Obesity. Gastroenterology. 2022;163(5):1198–225. [DOI] [PubMed] [Google Scholar]
- 448. Harrison SA, Fecht W, Brunt EM, Neuschwander-Tetri BA. Orlistat for overweight subjects with nonalcoholic steatohepatitis: A randomized, prospective trial. Hepatology (Baltimore, Md). 2009;49(1):80–6. [DOI] [PubMed] [Google Scholar]
- 449. Di Lorenzo N, Antoniou SA, Batterham RL, Busetto L, Godoroja D, Iossa A, et al. Clinical practice guidelines of the European Association for Endoscopic Surgery (EAES) on bariatric surgery: update 2020 endorsed by IFSO-EC, EASO and ESPCOP. Surg Endosc. 2020;34(6):2332–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 450. Panunzi S, Carlsson L, De Gaetano A, Peltonen M, Rice T, Sjostrom L, et al. Determinants of Diabetes Remission and Glycemic Control After Bariatric Surgery. Diabetes Care. 2016;39(1):166–74. [DOI] [PubMed] [Google Scholar]
- 451. Elsaid MI, Li Y, Bridges JFP, Brock G, Minacapelli CD, Rustgi VK. Association of Bariatric Surgery With Cardiovascular Outcomes in Adults With Severe Obesity and Nonalcoholic Fatty Liver Disease. JAMA Netw Open. 2022;5(10):e2235003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452. Sjöholm K, Carlsson LMS, Svensson P-A, Andersson-Assarsson JC, Kristensson F, Jacobson P, et al. Association of Bariatric Surgery With Cancer Incidence in Patients With Obesity and Diabetes: Long-term Results From the Swedish Obese Subjects Study. Diabetes Care. 2022;45(2):444–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 453. Aminian A, Al-Kurd A, Wilson R, Bena J, Fayazzadeh H, Singh T, et al. Association of Bariatric Surgery With Major Adverse Liver and Cardiovascular Outcomes in Patients With Biopsy-Proven Nonalcoholic Steatohepatitis. JAMA. 2021;326(20):2031–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 454. Fakhry TK, Mhaskar R, Schwitalla T, Muradova E, Gonzalvo JP, Murr MM. Bariatric surgery improves nonalcoholic fatty liver disease: a contemporary systematic review and meta-analysis. Surg Obes Relat Dis. 2019;15(3):502–11. [DOI] [PubMed] [Google Scholar]
- 455. Zhou H, Luo P, Li P, Wang G, Yi X, Fu Z, et al. Bariatric Surgery Improves Nonalcoholic Fatty Liver Disease: Systematic Review and Meta-Analysis. Obes Surg. 2022;32(6):1872–83. [DOI] [PubMed] [Google Scholar]
- 456. Pais R, Aron-Wisnewsky J, Bedossa P, Ponnaiah M, Oppert JM, Siksik JM, et al. Persistence of severe liver fibrosis despite substantial weight loss with bariatric surgery. Hepatology (Baltimore, Md). 2022;76(2):456–68. [DOI] [PubMed] [Google Scholar]
- 457. Lassailly G, Caiazzo R, Ntandja-Wandji LC, Gnemmi V, Baud G, Verkindt H, et al. Bariatric Surgery Provides Long-term Resolution of Nonalcoholic Steatohepatitis and Regression of Fibrosis. Gastroenterology. 2020;159(4):1290–301 e5. [DOI] [PubMed] [Google Scholar]
- 458. Verrastro O, Panunzi S, Castagneto-Gissey L, De Gaetano A, Lembo E, Capristo E, et al. Bariatric-metabolic surgery versus lifestyle intervention plus best medical care in non-alcoholic steatohepatitis (BRAVES): a multicentre, open-label, randomised trial. Lancet. 2023;401(10390):1786–97. [DOI] [PubMed] [Google Scholar]
- 459. Ren M, Zhou X, Zhang Y, Mo F, Yang J, Yu M, Ji F. Effects of Bariatric Endoscopy on Non-Alcoholic Fatty Liver Disease: A Comprehensive Systematic Review and Meta-Analysis. Front Endocrinol (Lausanne). 2022;13:931519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 460. Lai JC, Tandon P, Bernal W, Tapper EB, Ekong U, Dasarathy S, Carey EJ. Malnutrition, Frailty, and Sarcopenia in Patients With Cirrhosis: 2021 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology (Baltimore, Md). 2021;74(3):1611–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 461. Plauth M, Bernal W, Dasarathy S, Merli M, Plank LD, Schütz T, Bischoff SC. ESPEN guideline on clinical nutrition in liver disease. Clinical nutrition. 2019;38(2):485–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 462. van Vugt JLA, Alferink LJM, Buettner S, Gaspersz MP, Bot D, Murad SD, et al. A model including sarcopenia surpasses the MELD score in predicting waiting list mortality in cirrhotic liver transplant candidates: a competing risk analysis in a national cohort. J Hepatol. 2018;68(4):707–14. [DOI] [PubMed] [Google Scholar]
- 463. European Association for the Study of the Liver; European Association for the Study of the Liver . EASL Clinical Practice Guidelines on nutrition in chronic liver disease. J Hepatol. 2019;70(1):172–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 464. Montano-Loza AJ, Angulo P, Meza-Junco J, Prado CM, Sawyer MB, Beaumont C, et al. Sarcopenic obesity and myosteatosis are associated with higher mortality in patients with cirrhosis. J Cachexia Sarcopenia Muscle. 2016;7(2):126–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 465. Donini LM, Busetto L, Bischoff SC, Cederholm T, Ballesteros-Pomar MD, Batsis JA, et al. Definition and diagnostic criteria for sarcopenic obesity: ESPEN and EASO consensus statement. Clin Nutr. 2022;41(4):990–1000. [DOI] [PubMed] [Google Scholar]
- 466. Iwasa M, Iwata K, Hara N, Hattori A, Ishidome M, Sekoguchi-Fujikawa N, et al. Nutrition therapy using a multidisciplinary team improves survival rates in patients with liver cirrhosis. Nutrition. 2013;29(11):1418–21. [DOI] [PubMed] [Google Scholar]
- 467. Sharma BC, Maharshi S, Sachdeva S, Mahajan B, Sharma A, Bara S, et al. Nutritional therapy for persistent cognitive impairment after resolution of overt hepatic encephalopathy in patients with cirrhosis: A double-blind randomized controlled trial. J Gastroenterol Hepatol. 2023;38(11):1917–25. [DOI] [PubMed] [Google Scholar]
- 468. Meena BL, Taneja S, Tandon P, Sahni N, Soundararajan R, Gorsi U, et al. Home-based intensive nutrition therapy improves frailty and sarcopenia in patients with decompensated cirrhosis: A randomized clinical trial. J Gastroenterol Hepatol. 2023;38(2):210–8. [DOI] [PubMed] [Google Scholar]
- 469. Berzigotti A, Albillos A, Villanueva C, Genesca J, Ardevol A, Augustin S, et al. Effects of an intensive lifestyle intervention program on portal hypertension in patients with cirrhosis and obesity: The SportDiet study. Hepatology (Baltimore, Md: ). 2017;65(4):1293–305. [DOI] [PubMed] [Google Scholar]
- 470. Bischoff SC, Ockenga J, Eshraghian A, Barazzoni R, Busetto L, Campmans-Kuijpers M, et al. Practical guideline on obesity care in patients with gastrointestinal and liver diseases - Joint ESPEN/UEG guideline. Clin Nutr. 2023;42(6):987–1024. [DOI] [PubMed] [Google Scholar]
- 471. Plank LD, Gane EJ, Peng S, Muthu C, Mathur S, Gillanders L, et al. Nocturnal nutritional supplementation improves total body protein status of patients with liver cirrhosis: a randomized 12-month trial. Hepatology (Baltimore, Md: ). 2008;48(2):557–66. [DOI] [PubMed] [Google Scholar]
- 472. Tsien CD, McCullough AJ, Dasarathy S. Late evening snack: exploiting a period of anabolic opportunity in cirrhosis. J Gastroenterol Hepatol. 2012;27(3):430–41. [DOI] [PubMed] [Google Scholar]
- 473. Jamali T, Raasikh T, Bustamante G, Sisson A, Tandon P, Duarte-Rojo A, Hernaez R. Outcomes of Exercise Interventions in Patients With Advanced Liver Disease: A Systematic Review of Randomized Clinical Trials. Am J Gastroenterol. 2022;117(10):1614–20. [DOI] [PubMed] [Google Scholar]
- 474. Aamann L, Dam G, Borre M, Drljevic-Nielsen A, Overgaard K, Andersen H, et al. Resistance Training Increases Muscle Strength and Muscle Size in Patients With Liver Cirrhosis. Clin Gastroenterol Hepatol. 2020;18(5):1179–87 e6. [DOI] [PubMed] [Google Scholar]
- 475. Josef P, Ali I, Ariel P, Alon M, Nimer A. Relationship between retinal vascular caliber and coronary artery disease in patients with non-alcoholic fatty liver disease (NAFLD). Int J Environ Res Public Health. 2013;10(8):3409–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 476. Tsai PC, Kuo HT, Hung CH, Tseng KC, Lai HC, Peng CY, et al. Metformin reduces hepatocellular carcinoma incidence after successful antiviral therapy in patients with diabetes and chronic hepatitis C in Taiwan. J Hepatol. 2023;78(2):281–92. [DOI] [PubMed] [Google Scholar]
- 477. Zhang X, Wong GL, Yip TC, Tse YK, Liang LY, Hui VW, et al. Angiotensin-converting enzyme inhibitors prevent liver-related events in nonalcoholic fatty liver disease. Hepatology (Baltimore, Md). 2022;76(2):469–82. [DOI] [PubMed] [Google Scholar]
- 478. Owen MR, Doran E, Halestrap AP. Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem J. 2000;348(Pt 3):607–14. [PMC free article] [PubMed] [Google Scholar]
- 479. Yip TC, Chan RNC, Wong VW, Tse YK, Liang LY, Hui VW, et al. Association of metformin use on metabolic acidosis in diabetic patients with chronic hepatitis B-related cirrhosis and renal impairment. Health Sci Rep. 2021;4(3):e352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 480. Khan R, Foster GR, Chowdhury TA. Managing diabetes in patients with chronic liver disease. Postgrad Med. 2012;124(4):130–7. [DOI] [PubMed] [Google Scholar]
- 481. Chitturi S, George J. Hepatotoxicity of commonly used drugs: nonsteroidal anti-inflammatory drugs, antihypertensives, antidiabetic agents, anticonvulsants, lipid-lowering agents, psychotropic drugs. Semin Liver Dis. 2002;22(2):169–83. [DOI] [PubMed] [Google Scholar]
- 482. Hsiang JC, Wong VW. SGLT2 Inhibitors in Liver Patients. Clin Gastroenterol Hepatol. 2020;18(10):2168–72 e2. [DOI] [PubMed] [Google Scholar]
- 483. Zhang J, Fu S, Liu D, Wang Y, Tan Y. Statin can reduce the risk of hepatocellular carcinoma among patients with nonalcoholic fatty liver disease: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2023;35(4):353–8. [DOI] [PubMed] [Google Scholar]
- 484. Ekstedt M, Franzen LE, Mathiesen UL, Holmqvist M, Bodemar G, Kechagias S. Statins in non-alcoholic fatty liver disease and chronically elevated liver enzymes: a histopathological follow-up study. J Hepatol. 2007;47(1):135–41. [DOI] [PubMed] [Google Scholar]
- 485. Abraldes JG, Villanueva C, Aracil C, Turnes J, Hernandez-Guerra M, Genesca J, et al. Addition of Simvastatin to Standard Therapy for the Prevention of Variceal Rebleeding Does Not Reduce Rebleeding but Increases Survival in Patients With Cirrhosis. Gastroenterology. 2016;150(5):1160–70 e3. [DOI] [PubMed] [Google Scholar]
- 486. Pose E, Napoleone L, Amin A, Campion D, Jimenez C, Piano S, et al. Safety of two different doses of simvastatin plus rifaximin in decompensated cirrhosis (LIVERHOPE-SAFETY): a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Gastroenterol Hepatol. 2020;5(1):31–41. [DOI] [PubMed] [Google Scholar]
- 487. de Franchis R, Bosch J, Garcia-Tsao G, Reiberger T, Ripoll C, Baveno VIIF. Baveno VII - Renewing consensus in portal hypertension. J Hepatol. 2022;76(4):959–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 488. Wong GL, Chan HL, Choi PC, Chan AW, Lo AO, Chim AM, Wong VW. Association between anthropometric parameters and measurements of liver stiffness by transient elastography. Clin Gastroenterol Hepatol. 2013;11(3):295–302. e1-3. [DOI] [PubMed] [Google Scholar]
- 489. Pons M, Augustin S, Scheiner B, Guillaume M, Rosselli M, Rodrigues SG, et al. Noninvasive Diagnosis of Portal Hypertension in Patients With Compensated Advanced Chronic Liver Disease. Am J Gastroenterol. 2021;116(4):723–32. [DOI] [PubMed] [Google Scholar]
- 490. Villanueva C, Albillos A, Genesca J, Garcia-Pagan JC, Calleja JL, Aracil C, et al. beta blockers to prevent decompensation of cirrhosis in patients with clinically significant portal hypertension (PREDESCI): a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2019;393(10181):1597–608. [DOI] [PubMed] [Google Scholar]
- 491. Stafylidou M, Paschos P, Katsoula A, Malandris K, Ioakim K, Bekiari E, et al. Performance of Baveno VI and Expanded Baveno VI Criteria for Excluding High-Risk Varices in Patients With Chronic Liver Diseases: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2019;17(9):1744–55 e11. [DOI] [PubMed] [Google Scholar]
- 492. Petta S, Sebastiani G, Bugianesi E, Vigano M, Wong VW, Berzigotti A, et al. Non-invasive prediction of esophageal varices by stiffness and platelet in non-alcoholic fatty liver disease cirrhosis. J Hepatol. 2018;69(4):878–85. [DOI] [PubMed] [Google Scholar]
- 493. Hu X, Huang X, Hou J, Ding L, Su C, Meng F. Diagnostic accuracy of spleen stiffness to evaluate portal hypertension and esophageal varices in chronic liver disease: a systematic review and meta-analysis. Eur Radiol. 2021;31(4):2392–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 494. Dajti E, Ravaioli F, Zykus R, Rautou PE, Elkrief L, Grgurevic I, et al. Accuracy of spleen stiffness measurement for the diagnosis of clinically significant portal hypertension in patients with compensated advanced chronic liver disease: a systematic review and individual patient data meta-analysis. Lancet Gastroenterol Hepatol. 2023;8(9):816–28. [DOI] [PubMed] [Google Scholar]
- 495. Zhang X, Song J, Zhang Y, Wen B, Dai L, Xi R, et al. Baveno VII algorithm outperformed other models in ruling out high-risk varices in individuals with HBV-related cirrhosis. J Hepatol. 2023;78(3):574–83. [DOI] [PubMed] [Google Scholar]
- 496. Tsochatzis EA, Watt KD, VanWagner LB, Verna EC, Berzigotti A. Evaluation of recipients with significant comorbidity - Patients with cardiovascular disease. J Hepatol. 2023;78(6):1089–104. [DOI] [PubMed] [Google Scholar]
- 497. Burra P, Becchetti C, Germani G. NAFLD and liver transplantation: Disease burden, current management and future challenges. JHEP Rep. 2020;2(6):100192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 498. Haldar D, Kern B, Hodson J, Armstrong MJ, Adam R, Berlakovich G, et al. Outcomes of liver transplantation for non-alcoholic steatohepatitis: A European Liver Transplant Registry study. J Hepatol. 2019;71(2):313–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 499. Lee DU, Han J, Lee KJ, Kwon J, Fan GH, Jung D, Urrunaga NH. The clinical implications of pre-liver transplant diabetes on post-liver transplant outcomes in patients with NASH: analysis of the UNOS database. Hepatol Int. 2022;16(6):1448–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 500. Sharma S, Stine JG, Verbeek T, Bezinover D. Management of Patients With Non-alcoholic Steatohepatitis Undergoing Liver Transplantation: Considerations for the Anesthesiologist. J Cardiothorac Vasc Anesth. 2022;36(8 Pt A):2616–27. [DOI] [PubMed] [Google Scholar]
- 501. Vanwagner LB, Bhave M, Te HS, Feinglass J, Alvarez L, Rinella ME. Patients transplanted for nonalcoholic steatohepatitis are at increased risk for postoperative cardiovascular events. Hepatology (Baltimore, Md). 2012;56(5):1741–50. [DOI] [PubMed] [Google Scholar]
- 502. Verna EC, Phipps MM, Halazun KJ, Markovic D, Florman SS, Haydel BM, et al. Outcomes in liver transplant recipients with nonalcoholic fatty liver disease-related HCC: results from the US multicenter HCC transplant consortium. Liver Transpl. 2023;29(1):34–47. [DOI] [PubMed] [Google Scholar]
- 503. Zhou GP, Jiang YZ, Sun LY, Zhu ZJ. Clinical evidence of outcomes following liver transplantation in patients with nonalcoholic steatohepatitis: An updated meta-analysis and systematic review. Int J Surg. 2022;104:106752. [DOI] [PubMed] [Google Scholar]
- 504. Tsochatzis E, Coilly A, Nadalin S, Levistky J, Tokat Y, Ghobrial M, et al. International Liver Transplantation Consensus Statement on End-stage Liver Disease Due to Nonalcoholic Steatohepatitis and Liver Transplantation. Transplantation. 2019;103(1):45–56. [DOI] [PubMed] [Google Scholar]
- 505. VanWagner LB, Ning H, Whitsett M, Levitsky J, Uttal S, Wilkins JT, et al. A point-based prediction model for cardiovascular risk in orthotopic liver transplantation: The CAR-OLT score. Hepatology (Baltimore, Md). 2017;66(6):1968–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 506. Reddy ST, Thai NL, Oliva J, Tom KB, Dishart MK, Doyle M, et al. Cardio-hepatic risk assessment by CMR imaging in liver transplant candidates. Clin Transplant. 2018;32(5):e13229. [DOI] [PubMed] [Google Scholar]
- 507. Soldera J, Camazzola F, Rodriguez S, Brandao A. Cardiac stress testing and coronary artery disease in liver transplantation candidates: Meta-analysis. World J Hepatol. 2018;10(11):877–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 508. Spengler EK, O'Leary JG, Te HS, Rogal S, Pillai AA, Al-Osaimi A, et al. Liver Transplantation in the Obese Cirrhotic Patient. Transplantation. 2017;101(10):2288–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 509. Segev DL, Thompson RE, Locke JE, Simpkins CE, Thuluvath PJ, Montgomery RA, Maley WR. Prolonged waiting times for liver transplantation in obese patients. Ann Surg. 2008;248(5):863–70. [DOI] [PubMed] [Google Scholar]
- 510. Halegoua-De Marzio DL, Wong SY, Fenkel JM, Doria C, Sass DA. Listing Practices for Morbidly Obese Patients at Liver Transplantation Centers in the United States. Exp Clin Transplant. 2016;14(6):646–9. [DOI] [PubMed] [Google Scholar]
- 511. Pais R, Cariou B, Noureddin M, Francque S, Schattenberg JM, Abdelmalek MF, et al. A proposal from the liver forum for the management of comorbidities in non-alcoholic steatohepatitis therapeutic trials. J Hepatol. 2023;79(3):829–41. [DOI] [PubMed] [Google Scholar]
- 512. European Association for the study of Liver Diseases . EASL Clinical Practice Guidelines: Liver transplantation. J Hepatol. 2016;64(2):433–85. [DOI] [PubMed] [Google Scholar]
- 513. Muniraj T, Day LW, Teigen LM, Ho EY, Sultan S, Davitkov P, et al. AGA Clinical Practice Guidelines on Intragastric Balloons in the Management of Obesity. Gastroenterology. 2021;160(5):1799–808. [DOI] [PubMed] [Google Scholar]
- 514. Neto MG, Silva LB, Grecco E, de Quadros LG, Teixeira A, Souza T, et al. Brazilian Intragastric Balloon Consensus Statement (BIBC): practical guidelines based on experience of over 40,000 cases. Surg Obes Relat Dis. 2018;14(2):151–9. [DOI] [PubMed] [Google Scholar]
- 515. Patton H, Heimbach J, McCullough A. AGA Clinical Practice Update on Bariatric Surgery in Cirrhosis: Expert Review. Clin Gastroenterol Hepatol. 2021;19(3):436–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 516. Vijayaraghavan R, Sarin SK, Bharadwaj A, Anand L, Maiwall R, Choudhury A, et al. Intragastric Balloon in Obese Compensated Nonalcoholic Steatohepatitis Cirrhosis Patients Is Safe and Achieves Significant Weight Reduction at 6-Months. Dig Dis Sci. 2023;68(3):1035–41. [DOI] [PubMed] [Google Scholar]
- 517. Watt KD, Heimbach JK, Rizk M, Jaruvongvanich P, Sanchez W, Port J, et al. Efficacy and Safety of Endoscopic Balloon Placement for Weight Loss in Patients With Cirrhosis Awaiting Liver Transplantation. Liver Transpl. 2021;27(9):1239–47. [DOI] [PubMed] [Google Scholar]
- 518. Mosko JD, Nguyen GC. Increased perioperative mortality following bariatric surgery among patients with cirrhosis. Clin Gastroenterol Hepatol. 2011;9(10):897–901. [DOI] [PubMed] [Google Scholar]
- 519. Sharpton SR, Terrault NA, Posselt AM. Outcomes of Sleeve Gastrectomy in Obese Liver Transplant Candidates. Liver Transpl. 2019;25(4):538–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 520. Zamora-Valdes D, Watt KD, Kellogg TA, Poterucha JJ, Di Cecco SR, Francisco-Ziller NM, et al. Long-term outcomes of patients undergoing simultaneous liver transplantation and sleeve gastrectomy. Hepatology (Baltimore, Md). 2018;68(2):485–95. [DOI] [PubMed] [Google Scholar]
- 521. Gunturu NS, Castillo-Larios R, Bowers S, Edwards M, Burns J, Perry D, Elli EF. Combined Sleeve Gastrectomy with Liver Transplant in Patients with Obesity: a Feasibility Study. Obes Surg. 2022;32(11):3600–4. [DOI] [PubMed] [Google Scholar]
- 522. Heimbach JK, Watt KD, Poterucha JJ, Ziller NF, Cecco SD, Charlton MR, et al. Combined liver transplantation and gastric sleeve resection for patients with medically complicated obesity and end-stage liver disease. Am J Transplant. 2013;13(2):363–8. [DOI] [PubMed] [Google Scholar]
- 523. Tariq N, Saharia A, Nwokedi U, Hobeika MJ, Mobley CM, Hsu D, et al. Combined liver transplantation and sleeve gastrectomy: Report of a brief-interval staged approach. Liver Transpl. 2023;29(4):422–30. [DOI] [PubMed] [Google Scholar]
- 524. Anastácio LR, Ferreira LG, de Sena Ribeiro H, Lima AS, Vilela EG, Toulson Davisson Correia MI. Body composition and overweight of liver transplant recipients. Transplantation. 2011;92(8):947–51. [DOI] [PubMed] [Google Scholar]
- 525. Montano-Loza AJ, Rodríguez-Perálvarez ML, Pageaux GP, Sanchez-Fueyo A, Feng S. Liver transplantation immunology: Immunosuppression, rejection, and immunomodulation. J Hepatol. 2023;78(6):1199–215. [DOI] [PubMed] [Google Scholar]
- 526. van Son J, Stam SP, Gomes-Neto AW, Osté MCJ, Blokzijl H, van den Berg AP, et al. Post-transplant obesity impacts long-term survival after liver transplantation. Metabolism. 2020;106:154204. [DOI] [PubMed] [Google Scholar]
- 527. Fatourou EM, Tsochatzis EA. Management of metabolic syndrome and cardiovascular risk after liver transplantation. Lancet Gastroenterol Hepatol. 2019;4(9):731–41. [DOI] [PubMed] [Google Scholar]
- 528. VanWagner LB, Serper M, Kang R, Levitsky J, Hohmann S, Abecassis M, et al. Factors Associated With Major Adverse Cardiovascular Events After Liver Transplantation Among a National Sample. Am J Transplant. 2016;16(9):2684–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 529. VanWagner LB, Montag S, Zhao L, Allen NB, Lloyd-Jones DM, Das A, et al. Cardiovascular Disease Outcomes Related to Early Stage Renal Impairment After Liver Transplantation. Transplantation. 2018;102(7):1096–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 530. Vallin M, Guillaud O, Boillot O, Hervieu V, Scoazec JY, Dumortier J. Recurrent or de novo nonalcoholic fatty liver disease after liver transplantation: natural history based on liver biopsy analysis. Liver Transpl. 2014;20(9):1064–71. [DOI] [PubMed] [Google Scholar]
- 531. Balitzer D, Tsai JH, Gill RM. Clinicopathologic features of de novo non-alcoholic steatohepatitis in the post-transplant setting. Diagn Pathol. 2022;17(1):65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 532. Galvin Z, Rajakumar R, Chen E, Adeyi O, Selzner M, Grant D, et al. Predictors of De Novo Nonalcoholic Fatty Liver Disease After Liver Transplantation and Associated Fibrosis. Liver Transpl. 2019;25(1):56–67. [DOI] [PubMed] [Google Scholar]
- 533. Musso G, Gambino R, Tabibian JH, Ekstedt M, Kechagias S, Hamaguchi M, et al. Association of non-alcoholic fatty liver disease with chronic kidney disease: a systematic review and meta-analysis. PLoS Med. 2014;11(7):e1001680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 534. Gitto S, de Maria N, di Benedetto F, Tarantino G, Serra V, Maroni L, et al. De-novo nonalcoholic steatohepatitis is associated with long-term increased mortality in liver transplant recipients. Eur J Gastroenterol Hepatol. 2018;30(7):766–73. [DOI] [PubMed] [Google Scholar]
- 535. VanWagner LB, Holl JL, Montag S, Gregory D, Connolly S, Kosirog M, et al. Blood pressure control according to clinical practice guidelines is associated with decreased mortality and cardiovascular events among liver transplant recipients. Am J Transplant. 2020;20(3):797–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 536. Azhie A, Sheth P, Hammad A, Woo M, Bhat M. Metabolic Complications in Liver Transplantation Recipients: How We Can Optimize Long-Term Survival. Liver Transpl. 2021;27(10):1468–78. [DOI] [PubMed] [Google Scholar]
- 537. Singh P, Pesavento TE, Washburn K, Walsh D, Meng S. Largest single-centre experience of dulaglutide for management of diabetes mellitus in solid organ transplant recipients. Diabetes Obes Metab. 2019;21(4):1061–5. [DOI] [PubMed] [Google Scholar]
- 538. Charlton M, Levitsky J, Aqel B, OʼGrady J, Hemibach J, Rinella M, et al. International Liver Transplantation Society Consensus Statement on Immunosuppression in Liver Transplant Recipients. Transplantation. 2018;102(5):727–43. [DOI] [PubMed] [Google Scholar]
- 539. Morris MC, Jung AD, Kim Y, Lee TC, Kaiser TE, Thompson JR, et al. Delayed Sleeve Gastrectomy Following Liver Transplantation: A 5-Year Experience. Liver Transpl. 2019;25(11):1673–81. [DOI] [PubMed] [Google Scholar]
- 540. Tsamalaidze L, Stauffer JA, Arasi LC, Villacreses DE, Franco JSS, Bowers S, Elli EF. Laparoscopic Sleeve Gastrectomy for Morbid Obesity in Patients After Orthotopic Liver Transplant: a Matched Case-Control Study. Obes Surg. 2018;28(2):444–50. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.