Abstract
Rice grain is widely consumed as a staple food, providing essential nutrition for households, particularly marginalized families. It plays a crucial role in ensuring food security, promoting human nutrition, supporting good health, and contributing to global food and nutritional security. Addressing the diverse quality demands of emerging diverse and climate-risked population dietary needs requires the development of a single variety of rice grain that can meet the various dietary and nutritional requirements. However, there is a lack of concrete definition for rice grain quality, making it challenging to cater to the different demands. The lack of sufficient genetic study and development in improving rice grain quality has resulted in widespread malnutrition, hidden hunger, and micronutrient deficiencies affecting a significant portion of the global population. Therefore, it is crucial to identify genetically evolved varieties with marked qualities that can help address these issues. Various factors account for the declining quality of rice grain and requires further study to improve their quality for healthier diets. We characterized rice grain quality using Lancastrians descriptor and a multitude of intrinsic and extrinsic quality traits. Next, we examined various components of rice grain quality favored in the Asia–Pacific region. This includes preferences by different communities, rice industry stakeholders, and value chain actors. We also explored the biological aspects of rice grain quality in the region, as well as specific genetic improvements that have been made in these traits. Additionally, we evaluated the factors that can influence rice grain quality and discussed the future directions for ensuring food and nutritional security and meeting consumer demands for grain quality. We explored the diverse consumer bases and their varied preferences in Asian-Pacific countries including India, China, Nepal, Bhutan, Vietnam, Sri Lanka, Pakistan, Thailand, Cambodia, Philippines, Bangladesh, Indonesia, Korea, Myanmar and Japan. The quality preferences encompassed a range of factors, including rice head recovery, grain shape, uniform size before cooking, gelatinization, chalkiness, texture, amylose content, aroma, red-coloration of grain, soft and shine when cooked, unbroken when cooked, gelatinization, less water required for cooking, gelatinization temperature (less cooking time), aged rice, firm and dry when cooked (gel consistency), extreme white, soft when chewed, easy-to-cook rice (parboiled rice), vitamins, and minerals. These preferences were evaluated across high, low, and medium categories. A comprehensive analysis is provided on the enhancement of grain quality traits, including brown rice recovery, recovery rate of milled rice, head rice recovery, as well as morphological traits such as grain length, grain width, grain length–width ratio, and grain chalkiness. We also explored the characteristics of amylose, gel consistency, gelatinization temperature, viscosity, as well as the nutritional qualities of rice grains such as starch, protein, lipids, vitamins, minerals, phytochemicals, and bio-fortification potential. The various factors that impact the quality of rice grains, including pre-harvest, post-harvest, and genotype considerations were explored. Additionally, we discussed the future direction and genetic strategies to effectively tackle these challenges. These qualitative characteristics represent the fundamental focus of regional and national breeding strategies employed by different countries to meet consumer preference. Given the significance of rice as a staple food in Asia–Pacific countries, it is primarily consumed domestically, with only a small portion being exported internationally. All the important attributes must be clearly defined within specific parameters. It is crucial for geneticists and breeders to develop a rice variety that can meet the diverse demands of consumers worldwide by incorporating multiple desirable traits. Thus, the goal of addressing global food and nutritional security, and human healthy can be achieved.
Graphical Abstract
Supplementary Information
The online version contains supplementary material available at 10.1186/s12284-024-00725-9.
Keywords: Food security, Nutritional security, Functionality, Protein, Flavor, Structure–functional relationship
Introduction
Rice (Oryza sativa L.) serves as a fundamental food source for a significant portion of the global population, nourishing over 3.5 billion individuals on our planet. The majority of production and consumption is dominated by Asian countries, with China and India alone accounting for approximately 55% (Kong et al. 2015). Amongst the various cereal crops, rice alone accounts for 20% of the recommended calorie intake for the global population. Rice is an important food source for a large portion of the world's population. Understanding the connection between the quality of rice grains and crop yield is an important step towards addressing issues such as food scarcity, malnutrition, and ensuring the well-being of millions of consumers. Micro-nutrient deficiencies are a global concern, affecting preschool-aged children, women of reproductive age, and impoverished working communities. Insufficient intake of essential nutrients like iron, zinc, vitamins, and folic acid can lead to various health issues such as a compromised immune system, stunted growth, decreased productivity, and a deficiency in micronutrients (Stevens et al. 2022). At present, rice researchers, geneticists and breeders are heavily involved in the development of rice varieties that have high yields. Advancements in high-yielding rice breeding have nearly reached a plateau, raising concerns about meeting the changing dietary demands for a balanced green revolution that considers both yield and quality (Chen et al. 2019; Wu et al. 2020).
The quality of rice grains is an essential factor that influences the perception of its food value among consumers in developing countries. However, there is a prevailing concept of rice grain quality in the rice seed industry that lacks clarity, along with a measurement technique that is not well-defined. The perception of rice grain quality can vary among consumers, influenced by factors such as human civilizations, history, socio-cultural norms, and geographical background (Cuevas et al. 2017). This highlights the importance of developing universal "rice grain quality traits" that can meet the needs of the evolving population with changing dietary requirements. Through thorough research into market values and industry trends, rice grain qualities are determined by considering consumer needs, market demand, and the sustainability of rice food value chain (Demont et al. 2017; My et al. 2018). The profile of a rice product encompasses a range of characteristics that enhance its effectiveness, and it is through these attributes that consumers express their preferences (Lancaster 1966). The quality of rice can be classified into two categories: intrinsic and extrinsic quality traits (Demont and Ndour 2015). The quality of the grain is determined by its shape, size, purity, uniformity, head rice, softness, taste, cooking and eating properties, nutrition, aroma, color, and cleanliness. Additionally, branding, packaging, and labelling are also important factors that contribute to overall rice grain quality (Demont et al. 2017; My et al. 2018).
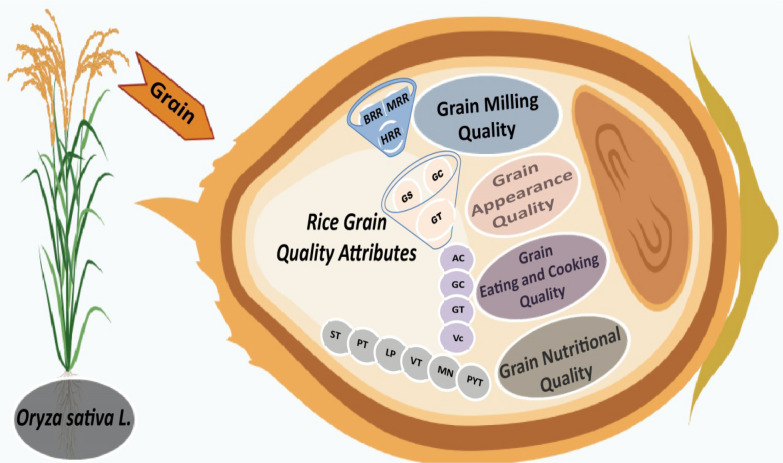
The quality of rice grain is influenced by a combination of genetic and environmental factors, which can be categorized into appearance, milling, nutritional content, and cooking properties. The evaluation of various physico-chemical properties of rice grain quality is conducted in a comprehensive manner during the processing and cooking stages (Long et al. 2023). The quality of rice milling is assessed based on factors such as milled rice, and head rice recovery rate. Appearance quality takes into account grain shape, chalkiness, and transparency. Nutritional quality is determined by the levels of starch, protein, lipids, minerals, vitamins, and phytochemicals. Cooking-eating quality is evaluated based on amylose content, gel consistency, and gelatinization temperature (Zhang et al. 2016a, b; Fig. 1). Rice milling quality generally enhances maximum yield recovery without grain breakage. The quality of rice milling is determined by a complex genetic control mechanism, which is heavily influenced by various genetic factors (Zhou et al. 2020). The quality of rice milling has a direct impact on its appearance, including factors such as grain shape, chalkiness, and translucency. These characteristics play a significant role in determining consumer preferences and marketability. The appealing qualities of rice grains, such as their well-proportioned shape, delicate chalkiness, clear translucency, and radiant appearance, greatly appeal to consumers (Gong et al. 2023). The quality of rice grain plays a marked role in determining the sensory characteristics associated with cooking and eating. The eating-cooking quality is a comprehensive reflection of the preferences consumers have for grain quality which include a fine texture, penetrating aroma, cleanliness, non-sticky elasticity, excellent taste, and a smooth consistency (Zhou et al. 2020; Fitzgerald et al. 2009a, b). The nutritional quality of rice is classified into grain proteins, lipids, vitamins, minerals, and phytochemicals. The genetics of external and internal characteristics related to milling, appearance, eating-cooking, and nutritional quality remains largely unexplored; despite that fact that significant progress has been made in rice grain quality characterization and use of molecular markers. In spite of these advancements in enhancing the quality of rice grain, only a few key genes have been thoroughly studied in this regard.
Fig. 1.
Qualitative characteristic of rice grains. Grain milling quality—brown rice recovery (BRR), milled rice recovery (MRR) and head rice recovery (HRR). Grain appearance quality—grain size (GS), grain chalkiness (GC), grain transparency (GT). Grain eating and cooking quality—amylose content (AC), gel consistency (GC), gelatinization temperature (GT), and viscosity (VC). Grain nutritional quality—starch (ST), protein (PT), lipids (LP), vitamins (VT), minerals (MN) and phytochemicals (PYT), respectively
In this review, we have primarily summarized the (i) comprehensive preferences for rice grain quality in countries across the Asia–Pacific region. (ii) definition of consumer preferences for rice grain quality, (iii) measurement and characterization of rice grain quality, (iv) genetic analysis of rice grain quality traits, (v) genetic enhancement of preferred rice grain quality traits, and (vi) factors influencing grain quality traits and future directions for global food and nutrition security. We conducted a thorough literature review to assess the grain quality attributes. These aspects encompass: (i) the biological mechanism of consumer quality traits, (ii) consumer preferences, (iii) participants in the value chain, and (iv) advancements in genetic research. All the studies cited in this review are based on published research articles, peer reviews, expert opinions, technical reports and general consumer perceptions. Experts have consistently reviewed each section and verified the similar qualitative characteristics with a reference to Custodio et al. (2019) analysis of quality preferences in Southeast Asia and South Asia. This review provides valuable insights into consumer demand, product design strategies, genetic improvement directions, role of rice in food and nutritional security worldwide.
Biological Niches of Rice Grain Quality
Rice grains are influenced by a variety of ecological and socio-cultural factors that ultimately impact the quality of rice. Rice cultivation varies depending on the climate, with indica rice being grown in tropical conditions and japonica rice thriving in temperate climates (Maclean et al. 2013). Paddy rice is a result of crossbreeding between Oryza sativa L. and the African rice cultivar, O. glaberrima. The rice cultivars (O. sativa) have two subspecies; indica and japonica species. Traditionally, japonica rice is shorter in length and is primarily enjoyed in Japan, Korea, and the northern regions of China. The grains of japonica rice have lower amylose content, resulting in a soft and moist consistency after cooking. On the other hand, indica rice has higher amylose content, leading to a firmer and drier consistency after cooking. The best rice for the world is a non-glutinous variety with amylose content of over 10%. In addition, it is worth noting that indica is widely recognized as the top rice cultivar worldwide, encompassing all rice-growing regions (Kovach et al. 2009). According to Jamjod et al. (2017), the majority of Thailand's growing regions are dedicated to cultivating jasmine and non-fragrance indica rice, taking into account their specific agro-ecological conditions. In addition, the HoM Mali aromatic rice variety stands out for its premium quality, displaying resistance to milling breaks, a shiny appearance, and a translucent texture. According to the Prom-U-Thai and Rerkasem (2020), the rice varieties RD15, RD6, and TRKB are responsible for a significant portion, approximately 55%, of rice production in Thailand. Production of Basmati rice is primarily limited to specific regions in India and Pakistan, while parboiled rice is mainly cultivated in South Indian regions. Glutinous rice, on the other hand, is commonly grown in Laos, Thailand, the Dai ethnic group, the Tai people of Myanmar, and the southern and western parts of China. A study by Hansen et al. (2002), examines the rapid evolution of taste buds in Chinese consumers, specifically those who consume a diet primarily consisting of rice and wheat. Another study by Wailes and Chavez (2012) focuses on the demand for rice among African population. It is unclear how much the consumption habits of the non-rice eating and rice eating communities have shifted over time.
Perceptions of Rice Grain Quality Characteristics in Asia Pacific Countries
The demand for high-quality rice is increasing due to various factors such as population growth, socio-cultural norms and traditions, and changing consumer lifestyles. Rice plays a significant role in consumers' lives, whether it's for special occasions, festivals, or everyday meals. As societies evolve and people migrate or immigrate, the cost and variety of rice options also come into play. Rice has become a staple that reflects the fashion and preferences of a society. In order to establish a clear roadmap for a unified grain quality, it is crucial to thoroughly analyze and understand the rice food value chain in countries across the Asia–Pacific region. Thus, this review discusses the different perceptions of grain quality among consumers in Southeast Asia and South Asia. The quality of rice varies greatly depending on the specific time and context in which it is produced. The majority of the population consumes white milled rice, taking into account its nutritional aspects (Custodio et al. 2019). The nutritional quality of food remains a mystery, as it is influenced by the evolving preferences of the growing population. These preferences may be rooted in long-standing eating habits, suggesting a connection to ancient civilizations. On the other hand, in Southeast Asia, the demand for rice is mainly driven by its high nutritional value, smooth texture, and aromatic fragrance (Custodio et al. 2016; Wangcharoen et al. 2016). The characteristics of high-quality grains are consistent shape and size, a glossy appearance, lack of chalkiness, translucency, long and slender when uncooked, and plump and firm when cooked.
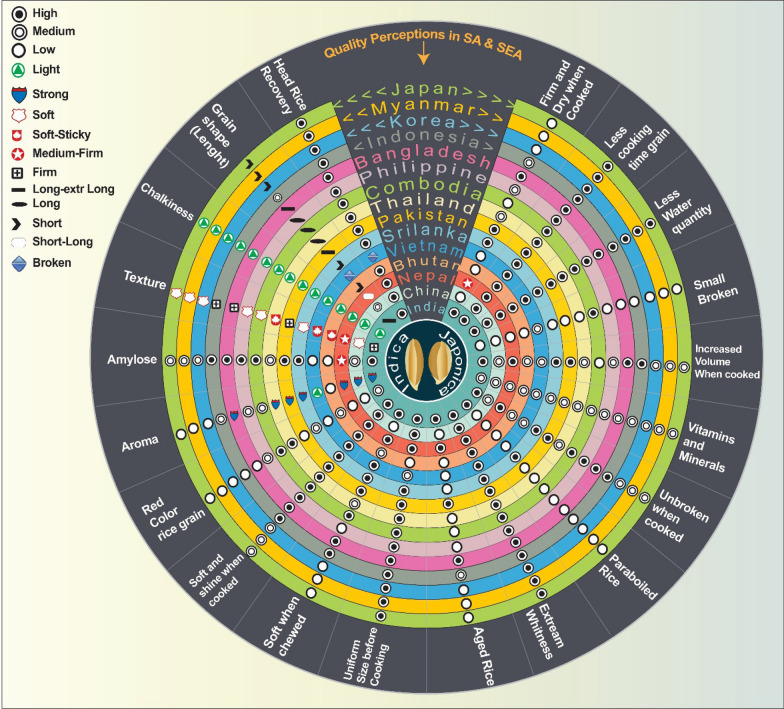
Ultimately, within rural communities in Asia–Pacific countries, the quality of rice grains can be classified as high, medium, or low. Top-notch rice should have a larger volume when cooked, which not only increases the length of the grains but also provides a satisfying feeling of fullness. On the other hand, lower quality rice may have impurities, a dry and rough texture, and a reduced cooking quality, often resulting in broken rice. In an urbanized communities, there are striking similarities to rural areas, with the exception of the availability of premium rice (high) and lower quality rice (poor) (Custodio et al. 2019). These findings align with the typical preferences of South Asian consumers, except for a change in preference for medium grain shaped rice (Custodio et al. 2016; Juliano and Villareal 1993). This change could be attributed to the rise of parboiled traditional rice, known for its firm and dry texture and high amylose content (Graham 2002; Custodio et al. 2016, 2019). In general, the perception of grain quality in South Asian countries is consistent, with some minor differences depending on the traditional and socio-cultural dietary practices (Custodio et al. 2019; Fig. 2).
Fig. 2.
Rice grain quality preferences in Asia Pacific countries (India, China, Nepal, Bhutan, Vietnam, Sri Lanka, Pakistan, Thailand, Cambodia, Philippine, Bangladesh, Indonesia, Korea, Myanmar, Japan). The quality perceptions indicators are recorded as low, medium, high, light, strong, soft, soft sticky, firm, medium-firm, short, long, extra-long, and broken rice
Genetic Basis of Rice Grain Quality
Milling Qualities
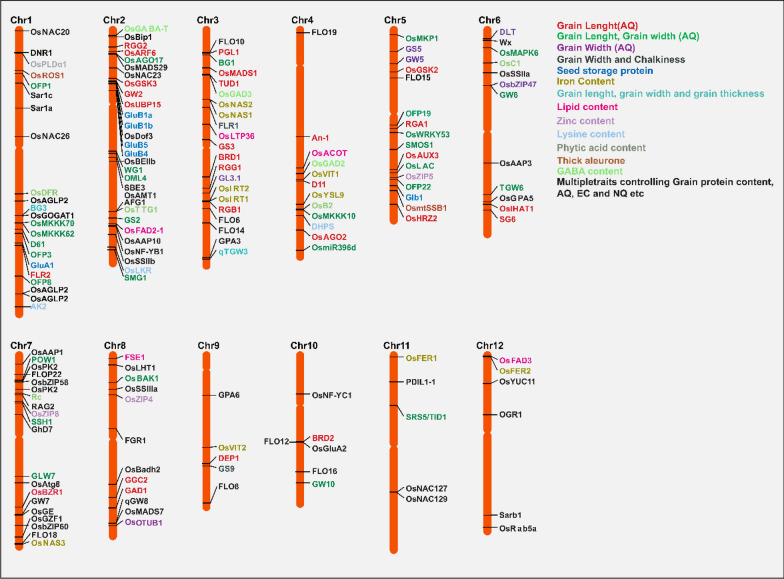
The quality parameters of rice milling are assessed during the paddy processing phase, which involves the production of full brown rice. This is then followed by the recovery of milled rice (bran de-husked) and head rice (without broken grains). The milling qualities are closely linked to the evolution of rice grain shape and chalkiness. Typically, grain milling qualities are achieved with shorter, round grains and reduced chalkiness. On the other hand, longer and slender grains with higher chalkiness yield poorer milling qualities (Gong et al. 2020). The yield of milled rice is closely linked to the attributes of rice milling. The mill processing unit, grain moisture content (14%), and the procedures and storage facilities all have a significant impact on it. Research on functional genetics and genomics for milling qualities have made significant progress in quantitative traits loci (QTL) mapping for desirable traits (Fig. 3; Gong et al. 2020; Deng et al. 2022).
Fig. 3.
Genes cloned in milling, appearance, eating and cooking, and nutritional quality attributes in rice. All the genes cloned for particular traits are widely presented based on specific chromosomal location. Only the gene names were provided with their trait legends besides the chromosome. Same color represents-same genes identified for the same traits
A recent study by Deng et al. (2022) provided valuable insights into the molecular basis of rice milling attributes through a genome-wide association study (GWAS). However, this study primarily highlights the crucial role of the Wx gene (which has been previously characterized) in controlling the recovery of head rice by manipulating the layers of unstructured starch granules. The study also highlights the interplay between various factors such as amylose content, milling quality, grain shape, and chalkiness, which are all influenced by the Wx gene. In future, it is important to prioritize the genetic characterization of milling quality characters and the identification of desirable major/minor QTLs/genes for enhancing milling quality parameters (Fig. 3).
Appearance Qualities
The quality of rice grain appearance is determined by its visible physical characteristics, which greatly influence consumers during the marketing of rice grains. The quality of rice grain appearance can be evaluated based on the shape of the grains, chalkiness, level of transparency, and grain color (Zhou et al. 2020).The shape of grains is an important factor in determining the yield of rice grains. It is characterized by its length, width, and the ratio between the two (Li et al. 2022). In the past, there was significant research on various indica and japonica rice cultivars, focusing on their morphological and agronomical characteristics (Zhou et al. 2020; Li et al. 2022). Extensive studies have been conducted in the past few decades to analyze significant QTLs and genes (Fig. 3). The heritability of grain shape in rice is significant, and researchers have successfully identified several genes with major genetic effects that are responsible for determining grain shape. Huang et al. (2013a, b) identified minor effect genes/QTLs that play a role in shaping rice grains.
Aroma
The aroma of rice plays a significant role in defining its sensory quality and has a significant impact on its market value. The rice aroma content is determined by the inactivation of the badh2 gene, which is found on chromosome 8 (Chen et al. 2006). So far, over 10 genes derived from badh2 gene family have been identified to have an impact on aromatic compounds in rice. These genes include badh2-E7, badh2-E2, badh2-E4-5, badh2-p-50UTR, and badh2-p. They play a crucial role in determining the aroma of rice (Gong et al. 2023; Fig. 3). It is worth noting that a smaller set of genes has been identified through integrated map-based cloning, such as Osbadh1, OsGly, and OsP5CS. The annotated genes are found in different loci and may play a role in increasing the intensity of 2-AP and influencing aroma in rice (Zafar and Jianlong 2023).
Pericarp Color
The pericarp of rice grains can come in various shades, including red, brown, purple, or black. Colored rice is rich in phenolic compounds such as anthocyanin and proanthocyanidin, which have biological functions in preventing oxidative stress, improving hyperlipidemia, and preventing obesity. The red rice pericarp exhibits distinct characteristics due to the presence of two genes, Rc and Rd, which are associated with its domestication. A recent study by Xie et al. (2024) revealed the presence of multiple alleles of the Rc gene. These alleles encode a bHLH protein and are associated with a red pericarp phenotype.
Interestingly, the researchers found that a 14-base pair deletion in the gene's reading frame which is capable of restoring its functionality. The Rd gene plays a vital role in catalyzing the conversion of dihydroflavonol into leucoanthocyanidin by encoding a dihydroflavonol-4-reductase (DFR). The Rd gene alone does not contribute to color production, but it does play a role in the assimilation of proanthocyanidin in grain coloration (Sweeney et al. 2006; Furukawa et al. 2007). The genotype Rc-rd results in brown-colored rice grains, while both rc-rd and Rc-Rd genotypes produce white-colored rice grains (Furukawa et al. 2007). Colored rice grains are highly regarded by consumers as a potent source of nutrition and an effective remedy for various emerging diseases. The traditional colored or brown rice grains are excellent sources of fiber and numerous vitamins, thanks to the bran layers that remain intact with the endosperm during processing. According to Veni (2019), colored rice grains are a valuable source of low-fat, healthy carbohydrates, as well as a reservoir of iron and zinc.
Grain Length
Several genes associated with grain length have been previously identified. One of these genes, QTL-GS3, was specifically identified for its role in rice grain length and has been successfully cloned on chromosome 3. The negative regulatory mechanism was discovered through functional characterization of two NILs, Minghui 63 (large grain) and Chuan 7 (small grain). When comparing the sequence of GS3 in different varieties for grain length, it was observed that long grain cultivars have a cysteine codon (TGC) in the termination region, which leads to premature termination. The main reason for the larger grain size is a truncation of about 178 amino acids at the C-terminal region of the protein (Fan et al. 2006). The GS3 contains four functional domains: one localized in the N-terminal region, another in the trans-membrane domain, a third in TNFR/NGFR, and the last one in the C-terminal region. These domains exhibit allelic variations due to natural fluctuations (Mao et al. 2010). The GS3 gene plays a crucial role in determining grain length in various cultivars. Different allelic variations of GS3 have been identified, such as GS3-1 in indica-derived cultivars with medium-length grain shape, GS3-2 in japonica-derived cultivars with short grain length, GS3-3 in Minghui 63-derived cultivars with long grains, and GS3-4 in Chuan 7-derived cultivars with extremely short grains. These allelic variations are linked to the termination of OSR function and the loss of functional domains on the C-terminal in GS3-4.
A recent report by Sun et al. (2018) found that GS3 is present in the G protein subunits and plays a role in controlling grain length through the G-protein pathway. The study highlights a regulatory mechanism in GS3 that affects grain length, which involves the competitive binding pattern of G-protein subunits, along with the involvement of DEP1 and GGC2. Interestingly, GS3 also inhibits the function of DEP1 and GGC2, resulting in the development of smaller grains. The GL3.1/qGL3 loci is responsible for decoding a protein phosphatase kelch (PPKL) family member, which in turn leads to an increase in grain length by negatively affecting cell numbers and grain glumes (longitudinally). This position is closely related to GS3 and the improvement of grain length through an enhancer gene (Zhang et al. 2012; Gao et al. 2015). In addition, the GS3 location has a wide-ranging impact on the GL3.3 gene, which encodes kinase activity similar to GSK3/SHAGGY. The genotypes that possess both the gs3 and gl3.1 genes exhibit a marked increase in grain size compared to the genotypes that only carry one of these genes (Xia et al. 2018; Fig. 3).
Grain Width
The width of rice grain has been extensively studied and characterized over the past few decades. For instance, GW2 was identified as the first QTL and was successfully cloned on chromosome 2. This QTL encodes the RING-type E3-ubiquitin ligase enzyme (Song et al. 2007). A deletion of a base pair in the 4th exon of gene GW2 results in the premature termination of the stop codon, leading to the production of a truncated protein and development of a long-grain trait. The loss of gene GW2 leads to an increase in cell numbers within the spikelet hull, resulting in improved rice grain weight, grain width, and overall yield. The qSW5/GW5 are considered significant QTLs for grain width, located on chromosome 5 (Weng et al. 2008).
Recent studies have revealed that the GW5 gene is responsible for decoding a protein that is bound to the membrane and contains a domain motif similar to IQ calmodulin (Duan et al. 2017; Liu et al. 2017). The GW5 gene has the potential to regulate grain weight by inhibiting the kinase activity of GSK2 on the OsBZR1 and DLT through BR signaling pathways (Liu et al. 2017). The GS5 gene is a significant genetic factor that plays a dominant role in determining the size of rice grains on chromosome 5. It exerts extensive control over grain size by positively regulating grain filling, width, and weight. The GS5 enzyme was decoded by Li et al. (2011). The heightened expression of the GS5 gene hinders the coordination between OsBAK1-7 and OsMSBP1, effectively suppressing the extracellular leucine-rich repeat (LRR) domain of OsBAK1-7. This approach, effectively circumvents OsBAK1-7, as it undergoes endocytosis in concert with OsMSBP1 (Liu et al. 2015; Fig. 3).
Grain Length and Width
The correlation between grain length and width is negative, and it necessitates a well-balanced genetic mechanism to synchronize these traits. The GW8/GW7 QTLs play a crucial role in determining the ratio of grain length to width. These QTLs are found on chromosome 7 and 8, and they have a significant impact on regulating the shape of grains. GW8 is a significant QTL for grain width, playing a positive role in regulating grain width through the decoding of OsSPL16 protein. It is also responsible for promoting cell proliferation (Wang et al. 2012). GW8 has been reported to cause malfunction in the promoter of GW7, resulting in the inhibition of its expression (Wang et al. 2015).
Studies have shown that certain QTLs, including GW7/GL7/GLG7, play a key role in regulating the ratio of grain length to width. This regulation is achieved through the decoding of a TRM motif protein, which is similar to the Arabidopsis LONGIFOLIA proteins (Wang et al. 2015; Zhou et al. 2015). There is an up-regulation of GW7 at the location of copy number variation (CNVs), which leads to the development of slender grains without any loss in yield. Therefore, GW8-GW7 is designed to showcase a beneficial approach for improving the quality of rice grains. A study by Zhao et al. (2018) found GS9a as a negative regulator for grain length/width ratio. This regulator is found on chromosome 9 and has been found to contribute to both horizontal cell multiplication and vertical cell elongation. The GS9 allele is associated with the enhancement of rice slender grain, in contrast to the standard GS9 allele which does not exhibit any alterations in grain thickness or weight (Zhao et al. 2021; Fig. 3).
Grain Chalkiness
Grain chalkiness occurs when starch granules and protein bodies are arranged loosely and asymmetrically, resulting in an opaque grain appearance (Lisle et al. 2000). In the world of rice, chalkiness content is seen as a negative trait that has a significant impact on various aspects such as milling, appearance, cooking, and nutritional value. This, in turn, can potentially decrease market demand and hinder the value chain process (Fitzgerald et al. 2009b). The level of chalkiness content in rice endosperm is primarily influenced by environmental factors, particularly temperature. As a result, optimizing this content can be quite challenging due to its complex nature. Several QTLs/genes related to chalkiness have been documented, but only two significant QTLs, Chalk5 and WCR1, located on chromosome 5 and 1, have been successfully cloned and characterized. Chalk5 plays an important role in enhancing grain chalkiness (Li et al. 2014). It is responsible for decoding an enzyme called vacuolar H+-trans-located pyro-phosphatase. An increased expression of the Chalk5 gene causes an increase in H+ absorption, which disrupts the pH balance and development of the endosperm-bounded-membrane, as well as the protein body's formation. This ultimately leads to the development of air spaces and an increase in their quantity within the rice endosperm. The spatial distributions of storage proteins and air space can contribute to the development of chalkiness content (Li et al. 2014). A QTL for white core rate in rice grains, which involves an F-box protein, has been reported to play a negative role in regulating the chalkiness of rice grains (Wu et al. 2022). The chalkiness content of rice endosperm is determined through the synthesis of reactive oxygen species (ROS) and inhibited programmed cell death (PCD). In addition, a functional SNP (A/G) located in the promoter region of WCR1 is strongly linked to variations in nucleotides and the WCR phenotype (Fig. 3; Wu et al. 2022).
Eating and Cooking Qualities
The primary focus of current breeding is to enhance the quality of rice grains in terms of flavor, aroma, and taste, as experienced by individuals. The quality of the rice grains is showcased through their exceptional taste, enticing aroma, clear appearance, sparkling visibility, both sticky and non-sticky texture, as well as their elasticity and flexibility. Additionally, the grains remain soft even after cooling (Fitzgerald et al. 2009). The current methods for testing and evaluating indicators for eating and cooking qualities are insufficient in providing a comprehensive definition. Nevertheless, the evaluation of eating and cooking quality often takes into account factors such as amylose content, gel consistency, and gelatinization temperature (Tian et al. 2009). The starch content plays a crucial role in determining the eating and cooking quality of rice grains, as it covers the majority of the rice endosperm. There are two classifications based on their molecular structure: amylose and amylopectin. According to Duan and Sun (2005), the amylose content is considered to be the main factor in determining the eating and cooking quality. The formation of the rice grain's soft, fluffy, and smooth texture is attributed to the optimal amylose content, which ranges from 14 to 20%. The gelatinization temperature results in a smooth and sticky texture, creating a coarse paste when the rice grain is cooked. The gelatinization temperature affects the structure of rice starch, as well as its bio-synthesis, gel consistency, and cooking time. The lower gelatinization temperature of rice grain effectively shortens cooking time by slowing down water absorption. All these factors are significantly and consistently linked to the quality of eating and cooking. According to Zhang et al. (2020), there is a negative correlation between amylose content and gel consistency, while gel consistency shows a positive correlation with gelatinization temperature. Through a thorough analysis of eating and cooking quality, it is evident that the rice endosperm serves as a storage site for starch and proteins. The dimension of rice grains has a significant impact on its eating and cooking quality. Furthermore, the influence of starch and proteins on the quality of rice when it comes to eating and cooking is not well understood (Pfister and Sciences 2016). A comprehensive explanation of the process of starch biosynthesis was recently published by Li et al. (2019) and Huang et al. (2021a, b). Currently, our focus is on showcasing the qualitative inheritance of key genes that play significant roles in enhancing the superior qualities of rice grains. The endosperm of cereals contains a well-preserved mechanism for starch biosynthesis, which involves several important enzymes such as ADP-AGPase, GBSS, SSS, SBE, and DBE (Huang et al. 2021a, b). The synthesis of starch granules is primarily regulated by ADP, with AGPase serving as the main substrate. After synthesis, ADPG is transported to the amyloplast for the deposition of amylose and amylopectin (Pfister and Sciences 2016). GBSS gene and different isoforms of SS, SBE, and DBE are responsible for the synthesis of amylose and amylopectin (Pfister and Sciences 2016). The composition of both amylose and amylopectin involves a multitude of enzymes and iso-enzymes, leading to the intricate genetic characterization of their eating and cooking quality (Tian et al. 2009). Only a limited number of QTLs/genes were successfully identified in the elite rice eating and cooking quality. An important gene (Wx) and its various alleles play a significant role in the synthesis of amylose content. This gene is located on chromosome 6 and has a major impact on the cooking quality of rice grains (Gong et al. 2023). A study conducted by Su et al. (2011) found that the qGC6 and Wx gene share the same locus on chromosome 6, specifically in relation to gel consistency. The inverse relationship between gelatinization temperature and amylose content suggests that the Wx gene has a broad impact on the eating and cooking quality of rice (Gong et al. 2023). The gelatinization temperature is partly regulated by the ALK gene, which is found in close proximity to the Wx gene loci on chromosome 6. The ALK gene encodes an SSIIa enzyme that plays a crucial role in determining the branching composition of amylopectin chains. This enzyme also has an impact on starch granule structure, gelatinization temperature, and the overall eating and cooking quality of starch (Gao et al. 2011). In conclusion, the genes Wx and ALK have a significant impact on the eating and cooking quality of rice. The Wx gene plays a role in regulating amylose content, gel consistency, and partly, gelatinization temperature. On the other hand, the ALK gene regulates amylose content, gel consistency, and paste texture (Gong et al. 2023).
Nutritional Qualities
The quality of nutrients in rice grains is determined by their ability to support consumer health, growth, development, reproduction, and overall physiological well-being. It is evident that the evolving populations with changing dietary preferences rely heavily on consuming brown or milled rice. The nutrient compositions of brown/milled rice exhibit significant variations in terms of starch, protein, lipid, minerals, vitamins, and phytochemicals. Nevertheless, enhancing the nutrient density in rice cultivars would be a valuable resource for populations striving to address issues of food and nutritional security (Zhao et al. 2020; Fig. 3).
Grain Protein Content
Rice grains are rich in protein, along with a significant amount of starch. The protein content of rice crops typically ranges from 5 to 16%. In addition, it has been found that indica rice has a protein content that is approximately 2% to 3% higher than japonica rice (Alam et al. 2023). Rice proteins comprise albumin, globulin, prolamin, and glutelin. Out of all the proteins, one stands out with a higher proportion—glutelin protein; which plays an essential role in regulating high-lysine content and enhancing digestibility (He et al. 2021). The variations in glutelin protein composition will affect the nutritional quality of rice grains. At present, the main focus is on rice grain protein to develop rice cultivars with high nutritional value. However, it has been observed that higher grain protein content tends to negatively impact the eating and cooking quality of rice. According to Long et al. (2023), there is an inverse relationship between the nutritional and cooking qualities. Numerous QTLs have been identified in relation to protein content (Zhao et al. 2022; Alam et al. 2023). However, protein content can be influenced by environmental factors, which is why most studies do not focus on data from a single environment. Grain protein is highly influenced by environmental factors. So far, researchers have successfully cloned and studied the functions of two important QTLs, namely qPC1 and qGPC-10, which play a crucial role in enhancing the protein content of rice grains (Peng et al. 2014; Yang et al. 2019a, b). The qPC1 plays a key role in synthesizing starch and grain proteins by functionally decoding an amino acid transporter called OsAAP6 and culminates in increased grain protein content (Peng et al. 2014). The qGPC-10 gene encodes a precursor of OsGluA2 and plays a role in positively regulating the protein content of rice grains. The qGPC-10 gene has been found to enhance the glutelin content in the protein fraction, resulting in an increase in the protein content of rice grains (Yang et al. 2019a, b). The expression of the OsAPP6 and OsGluA2 genes is responsible for the reduced protein content in rice grains. Typically, high protein content can lead to the development of a high density starch granules, which in turn forms a compact structure in the rice endosperm. This compact structure reduces water imbibition, ultimately resulting in poor palatability (Martin and Fitzgerald 2002). As a result, rice breeders face a significant challenge in achieving a balance between high-nutrition and high eating-cooking quality in a single variety (Fig. 3).
Lipids
Lipids play a crucial role in the structure of starch granules, forming strong connections with both amylose and non-starch lipids. The distribution is primarily concentrated in the rice bran, as highlighted by Choudhury and Juliano (1980). The lipid concentration in rice grains ranged from 0.89 to 2.16% (Azudin and Morrison 1986), and primarily consists of palmitic acid, oleic acid, and linoleic acid. Among all lipids, only oleic acid and linoleic acid hold significant importance for human consumption (Li et al. 2022; Ren et al. 2023). Lipids play a significant role in influencing the taste, cooking properties, and nutritional value of rice. The bonding relationship between amylose and amylopectin is often intricate, involving glycolipids and phospholipids. In a recent study by Concepcion et al. (2020), certain factors can affect the texture of cooked rice, such as starch expansion and gelatinization temperature. In addition, the aroma of rice is primarily influenced by unsaturated fatty acids, which impacts its nutritional and cooking qualities (Concepcion et al. 2018). There have been a limited genetic studies on the synthesis of rice grain oil concentration. A QTL was identified for grain nutrition, specifically related to nutrition-eating-taste (NET), which is believed to play a crucial role in lipid-nutrient metabolism. A study by Yang et al. (2022) examined the impact of various amino acids, vitamins, and polyphenol compounds on the taste of rice. Recent GWASs have identified several candidate genes, including PAL6, LIN6, MYR2, ARA6, and OsLP1, that regulate rice oil/lipid metabolism under natural variations (Zhou et al. 2021; Hong et al. 2023; Fig. 3).
Vitamins
Vitamins have a tendency to be absorbed by the human body and are divided into two categories: water soluble and fat soluble (fat soluble vitamins include A, D, E, and K). Rice grains contain a range of essential vitamins including B1, B2, B3, B4, B6, B7, B9, and E, while they do not contain vitamins A, C, and D (Champagne et al. 2004). Vitamin A deficiency is a common issue among rice-consuming communities, as rice grain lacks β-carotene, which is a precursor to vitamin A (Burkhardt et al. 1997). At present, malnutrition and hidden hunger pose significant health risks to populations in countries with poor diets. These conditions are linked to diseases such as blindness, xerophthalmia, and keratomalacia (Ye et al. 2000). A significant amount of research has been dedicated to the synthesis of vitamins within the rice grain. The geranyl–geranyl diphosphate substrate (GGPP) and iso-prenoid, a common precursor (C20) play major roles in the production of carotenoid C40. Phytoene synthase (psy) enzyme is responsible for β-carotene synthesis in rice grains by incorporating two molecules of GGPP.
A recent revolutionary breakthrough in rice breeding was the introduction of golden rice, developed by integrating a prototype GR1 using a phytoene synthase gene (psy) derived from daffodil. The phytoene desaturase gene (crtl) and lycopene cyclase (lcy) genes were obtained from E. uredovora (Ye et al. 2000). Additionally, the lcy gene was modified to produce β-carotene in golden rice (Schaub et al. 2005). Nevertheless, golden rice has certain limitations when it comes to synthesizing β-carotene at extremely high levels. To overcome this, the psy gene has been replaced with its maize homologous gene, resulting in a significant increase in pro-vitamin A to 23 folds/37 (mg/g) in golden rice. This improved version has been named golden rice 2 (Tang et al. 2009). Staxanthin is a red-colored keto-group carotenoid that plays a central role in antioxidant function. The production of astaxanthin is delayed in higher plants. The genes related to the biosynthetic pathway of β-carotenes include sPaCrtI, sZmPSY1, sHpBHY, and sCrBKT and play crucial roles in enhancing the astaxanthin content in rice grains (Zhu et al. 2018). Other important genes in this pathway include phytoene desaturase, phytoene synthase, β-carotene hydroxylase, and β-carotene keto-lase. A recent metabolomic study by Tian et al. (2019) discovered that certain genes, including tHMG1, ZmPSY1, and PaCRTI, have significant impact on increasing carotenoid levels in rice grains. There were reports of natural variation in the levels of Vitamin B1 (thiamine) and vitamin B6 (pyridoxine) in milled rice. The THIC gene is responsible for encoding vitamin B1, while PDX1.3a–c and PDX2 are responsible for encoding vitamin B6. The genes exhibit a unique enzymatic activity that is synthesized within rice leaves (Mangel et al. 2022; Fig. 3).
Macro and Micro Nutrients (Mineral Elements)
Nutrients play important roles in supporting the growth, development, and reproductive system of plants, including rice grains. The acquisition of nutrients in the plant system is regulated by photosynthesis, which involves the utilization of water and carbon dioxide in the presence of sunlight. In addition to hydrogen and carbon, the remaining 15 mineral elements play essential role in the development of crops (White and Brown 2010). The mineral elements were categorized into two groups: macro and micro nutrients (White and Brown 2010). The macro nutrients include sulfur (S), phosphorus (P), magnesium (Mg), calcium (Ca), potassium (K), nitrogen (N), oxygen (O), carbon (C), and hydrogen (H), while the micro nutrients consist of molybdenum (Mo), nickel (Ni), copper (Cu), zinc (Zn), manganese (Mn), boron (B), iron (Fe), and chlorine (Cl). Out of all the minerals, zinc and iron are particularly important for the health benefits they provide to humans (Wirth et al. 2009). Iron deficiency can lead to various health issues, such as anemia and impaired growth, particularly in newborns and expectant mothers (Ritchie and Roser 2017). A multitude of genetic studies have explored increasing iron and zinc content in rice grains. A study by Paul et al. (2012) identified OsGlb for globulin, OsGluB1 and OsGluA2 for glutelin, and Osfer2 genes for regulating iron and zinc content in rice grains. The transport mechanism of iron content is based on chelation in low iron conditions and reduction in high iron conditions, converting Fe+++ to Fe++. The chelation mechanism is facilitated by the NAS enzyme to release phytosiderophores, which are involved in iron deficit zone (Kok et al. 2018). Thus, the intake of iron into the plant system is regulated by both the reduction and chelation systems working together. It is worth noting that iron content in rice grains has been significantly enhanced by the efficient utilization of three candidate genes, namely OsNAS1, OsNAS2, and OsNAS3 (Lee et al. 2012; Kok et al. 2018). The expression of the OsYSL2 gene has a significant impact on the iron content of rice grains. A study by Bashir et al. (2013) highlights the significant impact of two vacuolar genes, namely OsVIT1 and OsVIT2, on the quantitative enhancement of iron and zinc content through iron transport. A novel gene, SoyferH2, which plays a role in improving iron levels, primarily regulated by OsGluB1 and OsGlb promoters, and were combined with three specific genes, HvNAS1, HvNAAT-A, and HvNAAT-B. These genes are responsible for decoding mugineic acid to increase the iron content in milled rice (Masuda et al. 2013a, b).
Another critical element to consider is zinc, which plays a vital role in regulating over 300 enzymes involved in the metabolism of carbohydrates, lipids, proteins, and nucleic acids (Swamy et al. 2016). Malnutrition is a significant issue in regions where milled rice is commonly consumed, with zinc deficiency being a major contributing factor (Rao et al. 2020). According to the Dietary Reference Intake, it is important for adults to consume an adequate amount of zinc, which ranges from 7 to 13 mg/g/, in order to maintain a healthy lifestyle. Insufficient zinc supply can lead to various health issues such as imbalanced appetite, slowed growth, weakened immune function, weight and hair loss, digestive problems, eye and skin problems, delayed wound healing, and mental disorders (Prasad 2004). There has been significant progress in genetic research on zinc content in recent years. Increasing the density of zinc content in rice grains can be achieved through zinc bio-fortification, which is an alternative approach. OsZIPs, OsIRTs, OsNASs genes were cloned and their zinc and iron content regulatory mechanisms were studied. The zinc and iron content in rice grains during the filling stage is quantitatively increased by certain genes (Das et al. 2020). The uptake of zinc occurs through the root system of rice and then transported from the vegetative parts to the endosperm (Yin et al. 2016). Zinc uptake in rice endosperm is facilitated by the transfer of zinc from vegetative parts (Wu et al. 2010). A limited number of rice metal transporter genes (ZIP family) have been identified and are observed to be up-regulated in zinc deficient environments (Tan et al. 2015). In another study by Aung et al. (2013), it was discovered that the overexpression of gene OsYSL2, belonging to the NAS family genes, has the potential to enhance zinc and iron content in rice. In a study by Tan et al. (2015), it was observed that OsIRT and MxIRT genes had a positive impact on the levels of iron and zinc. A zinc bio-fortified rice has been developed in Bangladesh by Harvest Plus and the International Rice Research Institute (IRRI), which boast of increased zinc content by 60% to meet daily zinc requirements (Goldstein 2018). A similar collaboration with Indian research institutions has also resulted in a rice variety in India with a zinc content of 28 mg/kg (Rao et al. 2020). A study by Trijatmiko et al. (2016) reported the successful insertion of the OsNAS2 and SferH-1 genes into rice, without causing any negative effects on the quality or quantity of the crop (Fig. 3).
Bio-fortifications of Rice Grains
Essentially, the bio-fortification of staple food crops aims to enhance the nutrient profiles. Ensuring a sufficient supply of nutrients for staple crops is crucial for addressing the needs of a growing population and promoting food and nutritional security. An innovative approach has been developed to enhance the nutritional value of rice grains by simultaneously increasing the levels of zinc, iron, and β-carotene through a multi-gene stacking system. As part of this approach, researchers successfully created a transgenic line by increasing the levels of zinc, iron, and β-carotene using specific genes found in maize (PHYTOENE SYNTHASE or ZmPSY), bacteria (CAROTENE DESATURASE or CRTI), beans (FERRITIN or PvFERRITIN), and Arabidopsis (nicotianamine synthase1 or AtNAS1) (Zhu et al. 2020). This is the latest development in metabolic engineering, where genes are extracted from a variety of pooled metabolic pathways. The goal was to create a plant system capable of meeting the nutritional needs of humans (Fig. 3).
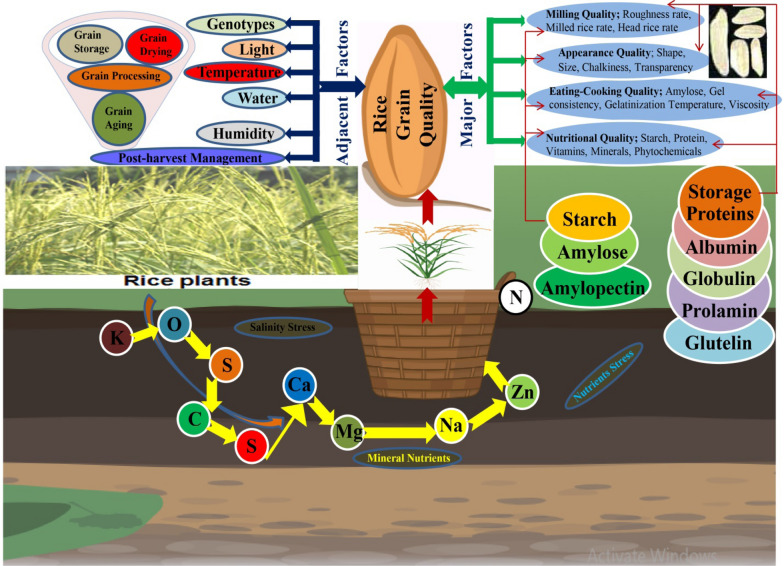
Factors Influencing Rice Grain Quality
Pre-harvest Factors
Ecological Factors
It is widely recognized that various environmental factors and agronomic practices have significant impacts on the quality characteristics of rice grains. The phenotypic performance of a genotype is influenced by various factors, including the genotype itself, environment, and interaction between genotype and environment. It has been noted that growing environment conditions also play a significant role in this regard (Abebe et al. 2023). The regulation of aroma genes is influenced by various environmental factors such as water, soil, light, and temperature (Sakthivel et al. 2009). As a result, aromatic rice varieties are limited to specific growing regions. Many aromatic rice varieties lose their distinctive aroma when grown outside of their native environment. Many important traits of rice are determined by multiple genes, resulting in quantitative characteristics. Improving the quality of rice grains can be quite challenging due to their susceptibility to environmental factors. This makes it difficult to enhance grain quality using traditional breeding methods. Environmental factors such as temperature extremes, drought, and salinity stress can have a significant impact on various aspects of rice, including grain yield, milling quality, appearance quality, eating-cooking quality, and nutritional quality (Sreenivasulu et al. 2015). It has been established that drought stress during the flowering period have minimal impact on the morphological and nutritional grain quality traits, except for an increase in grain chalkiness (Yang et al. 2019a, b). Decreased temperatures during the flowering period have the potential to improve milling and nutritional characteristics, but they may also reduce the eating-cooking quality parameter of late growing rice varieties (Huang et al. 2021a, b). Salinity has a negative impact on various quality traits of rice grains. It leads to a decrease in head rice recovery and amylose content, while increasing the chalkiness rate (Zheng et al. 2023). Several studies have demonstrated that crop production is significantly influenced by environmental factors. In addition to environmental factors, genetic factors play a significant role in regulating grain length, amylose content, grain width, and aspect ratio (Fitzgerald et al. 2009a, b). Environmental factors have a significant impact on various grain quality characteristics, including brown rice, milled rice, head rice, grain chalkiness, grain chalkiness level, grain length, grain width, aspect ratio, gel consistency, amylose content, and protein content. The protein content and eating-cooking quality traits of rice are primarily influenced by ecological factors and cultivation practices (Lou et al. 2023).
Field Management Practices
The field management practices encompass the various cultural practices carried out throughout the entire rice growing cycle, including pre-harvest activities. Research has shown that this particular practice has minimal impact on the quality and yield of rice grains when compared to other factors such as genetics and the environment. In addition, the rate of nitrogen application has a significant impact on both the yield and quality of rice grains. There is a strong correlation between nitrogenous fertilizers and the milling and nutritional quality traits of rice. In addition, there is a negative correlation between appearance and eating-quality (Zhou et al. 2018). A comprehensive study has indicated that the application rate of nitrogen plays a crucial role in altering both the grain yield and quality. In a recent study, it has been found that applying a moderate nitrogen rate (210–260 kg/ha) can have positive effects on the milling and nutritional quality, as well as the eating-cooking qualities of japonica species (Liang et al. 2021). The impact of nitrogen application on eating-cooing quality parameters has been thoroughly studied, revealing its ability to modify protein content and starch characteristics. Increasing the nitrogen levels in the endosperm can have a positive impact on its texture when cooked. This is due to a decrease in amylopectin branching and an increase in protein content, resulting in a more compact texture. However, it is important to note that this may also lead to a decrease in palatability levels (Lou et al. 2023). In addition to the zinc (Zn) and iron (Fe) levels in the rice grains, the cooking characteristics are influenced by various other mineral contents found in the rice grain. Multiple studies have shown a strong correlation between mineral elements and amino acid and grain protein content. In addition, gel consistency of grains showed a clear correlation with K, C, and Mn contents of the rice grain. The amylose content was greatly affected by the levels of K, sodium (Na), Mg, Cu, and Mn. The mineral uptake and sequestration in the rice plant system is a highly intricate process that can be significantly influenced by soil fertility, fertilizer usage, and changing climate.
Post-harvest Factors
Drying Facilities
Ensuring the quality of rice grain is the primary focus of flexible rice consumption, particularly during off-seasons. Several post-harvest techniques are utilized to maintain the quality of rice grains, including drying, storage, and processing. The drying temperature for storing rice grain typically falls between 40 and 60 °C. The recommended storage moisture levels range from 11 o 15% at temperatures of 16–20 °C, with a relative humidity of 60%, allowing for storage of up to 12 months (Müller et al. 2022). The drying process of rice involves the transfer of heat from the drying air to the surface mass of rice grains. In order to effectively dry rice grains, it is important to ensure that the water vapor pressure of the grains is higher than the temperature of the drying air. If the pressure is lower, it can lead to an increase in moisture content. According to Müller et al. (2022), maintaining an equal vapor pressure between grains and drying air is crucial for improving storage conditions. This is because when the moisture content of rice grains is reduced, it can lead to shrinkage and cracks, resulting in breakage and fissures. The heat applied to rice grains lead to evaporation and an increase in internal pressure, resulting in improved drying efficiency and time savings. The storage environment's temperature has a significant impact on the composition of starch, the breakdown of starch, and the color of rice grains (Donlao et al. 2018). By raising the drying temperature in storage conditions, the density of rice starch can be enhanced, and thus improving gelatinization and reduced amylose retrogradation. It is important to maintain storage conditions at a temperature below 70 °C. This is because as the temperature gradually increases from 40 to 100 °C, the crystallization of starch decreases from 21.97 to 20.35%. This decrease also affects the amylopectin chains (Ramos et al. 2019). The interactions between starch, amylose, amylopectin, and gelatinization play a significant role in the cooking and nutritional quality of rice grains (Fig. 4).
Fig. 4.
Factors influencing rice grain quality
Storage Facilities
Rice grain storage chambers are meticulously designed with a focus on structural integrity and are equipped with state-of-the-art facilities. They offer storage services specifically for rice grains/seeds (Kudra 1996). Rice grains are traditionally stored in various types of containers, such as mud, structured warehouses, aerated chambers, and other manually designed storage chambers. The quality of rice grains can be significantly influenced by the storage conditions over time, whether it is for a few months or even years (Singh et al. 2006). In rural areas of Asia–Pacific region, rice consumers often store rice grains for extended periods of time, ranging from 6 months to a year or even longer. The reasoning behind this approach is that using aged rice grains enhances the taste and texture of the cooked rice, resulting in a larger quantity. Many people tend to prefer consuming rice grains stored around 1 year old. The aged rice grains possess exceptional qualities in terms of their eating, cooking, and nutritional value. Storing rice for extended periods can lead to a decline in its quality and development of unwanted flavors, as microorganisms tend to grow over time.
Declining rice grain quality is linked to the respiratory system of the rice grains. Storage time, temperature, and relative humidity are crucial factors that can be affected by the concentration of O2 and CO2. These factors have the potential to impact rice grain metabolism and the respiration system (Singh and Fielke 2017). According to Huang et al. (2013a, b), the decomposition of 1 g of dry matter requires 1.07 g of O2 under aerobic conditions and results in the production of 1.47 g of CO2, which can be used to calculate the dry matter loss. The subtle changes in moisture and temperature have an impact on the composition of starch, protein, and lipids in rice grains. Consequently, these changes can affect eating, cooking, and nutritional quality (Yanxia et al. 2018). Storing the rice grains at a temperature below 4 °C helps to bring back their original characteristics, with only slight adjustments in moisture content, moisture activity, and grain appearance (Klaykruayat et al. 2020). Nevertheless, the color of rice grains primarily corresponds to the growth of microorganisms on the surface (Atungulu 2018: Fig. 4).
Rice Grain Processing Facilities
White rice is widely consumed worldwide, and it is produced by removing the bran layer from the rice endosperm during the milling process (Runge et al. 2019). Friction between the grains and milling roller can lead to inconsistencies in grain size, shape, and the surface of coarse rice, as well as rice breakage (Zeng et al. 2018). Quality of milling is directly influenced by water absorption of rice grains, leading to a smooth texture, tenderness, and increased gelatinization rate (Müller et al. 2022). The quality of polished rice is determined by the lipid content, appearance, and uniform coloration and shine of the rice grain surface (Rodríguez-Arzuaga et al. 2016). The brown rice undergoes a similar process, with the exception of removing the intact aleuronic layer on the surface of the rice grains culminating in higher nutritional properties (Fig. 4).
Rice Grain Aging
Storing rice grains for a specific duration before consumption is known as rice aging. In South Asian countries like India, Nepal, Bangladesh, Sri Lanka, and Pakistan, aged rice is highly favored for its superior quality attributes. Curiously, when given the option between aged and fresh rice, most consumers tend to prefer aged rice over the fresh variety. This suggests that aged rice is a more desirable trait for these Asian consumers (Bhattacharya 2011); while many consumers in East Asia prefer fresh rice grains. The aging process affects size, water absorption, cooking properties, texture, and stickiness, when compared to freshly harvested rice (Chrastil 1990). In addition, the aging process of rice enhances tensile strength, softness, sensory quality, adhesion, cohesiveness, hardness, rough surface, and resistance to grinding (Chrastil 1990; Zhou et al. 2002). One significant disadvantage of aged rice is its lengthy storage period, which typically lasts around 3–4 months or even longer under natural conditions (Saikrishna et al. 2018). The process of rice aging occurs between the post-harvest stage and consumption. Aging involves a range of physiochemical properties (Singh et al. 2006). In general, the process of rice aging leads to an increase in rice volume and a quick absorption of water; in addition to enhancing the texture properties, paste, color, aroma, chemical compositions, and eating-cooking quality (Zhou et al. 2003). The aging process of rice involves the manipulation of proteins and cell wall lipids, as well as the oxidation of lipids and interactions between proteins and starch (Sodhi et al. 2003). During the aging process of rice, various changes occur in the proteins and lipids. The lipids in rice consist of fatty acid compounds and are strongly linked to amylase, carbonyl, and hydro-peroxides. These connections lead to the oxidation of proteins and the condensation of volatile compounds. According to Sodhi et al. (2003), rice grain aging can affect the expansion of starch granules and alter the texture of cooked rice. The process of rice aging involves the manipulation of cooking quality through the swelling or puffing of starch granules, resulting in gelatinization. This process leads to the production of starch molecules and loosened amylose granules (Nardi and Bellopede 1997). Nevertheless, a reaction between insoluble starch and protein, resulting in a subpar cooking quality was reported by Ohno et al. (2005), where aged rice has a slightly harder and non-sticky texture compared to fresh milled rice. This difference in texture is believed to be closely related to protein content manipulations, particularly protein oxidation on the outer surface of the rice grain.
Adeboye et al. (2015) investigated genetic variations of 10 rice varieties in a controlled environment with a temperature of 55 °C, relative humidity of 72 ± 2%, and a duration of 72 h. The findings indicate a notable decline in genetic integrity as rice grain age, resulting in decreased germination rate (Zhang et al. 2016a, b). Lipid peroxidation is a significant process that leads to seed deterioration through the catalytic action of the LOX3 enzyme (Xu et al. 2015). Through proteomic analysis, it has been established that the LOX3 enzyme has a negative impact on rice grain longevity. In recent studies, researchers have utilized SSR markers to assess the genetic diversity in aging rice. The findings indicate a direct correlation between various factors such as polymorphic alleles, gene diversity index, polymorphic ratio bands, effective allele numbers, specific individual plant number, Shannon index, and seed germination rate. Notably, an optimal germination rate of up to 60% was observed (Zhou et al. 2023). However, as of now, no QTLs/genes have been identified or successfully cloned. This opens up a new research area for scholars to explore the crucial QTLs/genes linked to the aging of rice (Fig. 4).
Assumptions and Implications for Future Nutritional Demand and Food Security
It is widely recognized that the quality of rice grains is a complex characteristic that has implications for growing populations. The rice grain industry have been experiencing significant growth on a regularly basis, with a primary focus on increasing yield and maintaining quality standards. Undoubtedly, there has been significant progress and remarkable achievements breeding for rice yield. However, the enhancement of rice grain quality attributes still lags behind. As the global population continues to grow with changing dietary requirements, there is an increasing demand for high-quality grain to meet the rising living standards. Nevertheless, it is crucial to re-evaluate rice grain yield quality, taking into account the current market conditions and the increasing demands of a growing population. A thorough analysis is required to gather extensive data on rice quality perceptions in order to effectively address the needs of the food value chain. This review offers a comprehensive analysis of available literature research on consumer choices, farmers' and industrial requirements, and the genetic improvement of various quality traits in rice grains. These traits include milling, appearance, eating–cooking, and nutritional quality. Our findings revealed that there is a broad consumer perception of rice grain quality that is time-and location-specific. Consumers' preferences are constantly changing based on time, location, culture, lifestyle, and societal trends. These preferences can be influenced by occasions, festivals, gatherings, and even migration or immigration. Additionally, factors like the cost of rice, rice options, fashion, and society also play a role in shaping consumer choices. Existing rice market provides a diverse selection of rice quality characteristics, taking into account the preferences and purchasing behavior of consumers. The overall quality ratings for rice range from very poor to poor, poor to middle class, middle class to good, good to premium, and premium to high. The first class is of low quality and is aimed at impoverished communities. The second class is of average quality, intended for those slightly above the poverty line. The middle-class community is catered to with classes of moderate to good quality grain rice. The upper middle class can access classes of good to premium quality. Lastly, the extremely affluent society can avail classes of premium to high quality. Developing a single rice variety that can possess all the quality characters is a complex task, given the diverse demands for rice quality. In order to address the increasing demand from a changing population, it is crucial to establish a standardized and high-quality rice variety. This can be achieved by integrating regional and national rice breeding targets to develop a branded elite rice variety or germplasm with improved characteristics. The rice value chain actors from countries such as India, China, Nepal, Bhutan, Vietnam, Sri Lanka, Pakistan, Thailand, Cambodia, Philippines, and Bangladesh can enhance the quality of rice through packaging, labelling, and branding to facilitate international trade.
The japonica cultivar dominates the biological niches of rice grain quality. The indica and japonica rice cultivars have successfully adapted to both tropical and temperate geographical conditions. The japonica grain type has a shorter and medium length with lower amylose content. It is primarily consumed in Japan, Korea, and the northern part of China. Instead, the indica rice grain type is slender and long with higher amylose content. It is mainly consumed in Southeast and South Asiatic regions. The non-sticky variety of rice is considered the highest quality and is led by indica rice cultivars. India is the dominant producer of indica type of rice, accounting for 90% of the total production. Similarly, Thailand has a strong hold on the production of Jasmine and non-fragrance indica type of rice, with a market share of 98%, thanks to their favorable agro-ecological conditions. It is unclear how much the consumption habits of non-rice eating communities within the current developing population of Asian migrants/immigrants have influenced the shift between indica and japonica rice types.
There is a lack of research on the genetic enhancement of rice grain quality traits. The recent advancements in enhancing milling, appearance, eating-cooking and nutritional grain quality indicators highlight the need for a more comprehensive rice product profile. This can be achieved by incorporating all the quality evaluation standards that are relevant to rice grain quality. Given the complex nature of quality attributes, their expression is influenced by a combination of genetic factors, including gene interaction and epistasis, as well as environmental conditions and crop production. Improving the milling qualities of rice, such as brown rice recovery, milled rice recovery, and head rice recovery, as well as appearance qualities like grain length, grain width, grain length–width ratio, and grain chalkiness, is essential for enhancing consumer preferences in Asia Pacific countries. Additionally, enhancing the eating and cooking qualities of rice, including amylose, gel consistency, gelatinization temperature, viscosity, and nutritional qualities such as starch, protein, lipids, vitamins, minerals, phytochemicals, and bio-fortification, can vigilantly contribute to meeting consumer demands. The genetic studies for all these component traits have yet to be explored, which means that developing varieties with this genetic background could meet the consumer demand for seed, food, nutrition, health, and contribute to global peace and security.
The quality of rice grains can be affected by various factors such as the facilities used for drying, storage, processing, and the aging process. A lack of proper drying facility results in the retention of excess moisture in the rice grain, creating an ideal environment for the growth of microorganisms and leading to rapid quality deterioration. Many farming communities face the challenge of inadequate grain storage conditions, leading to the rapid growth of micro-organisms and pests. This can result in the production of unhealthy grain and a decline in the quality of food consumed. Due to the inclusion of broken rice grain in grain processing, many families in poor communities are unable to access high-quality grain with proper shape. As a result, they heavily rely on consuming broken rice, which lacks sufficient nutrition. This has led to the prevalence of malnutrition and hidden hunger within these communities. Aged rice grain consumption is a popular trend in many Asian countries. Many rural communities choose to consume aged rice grains, considering them to be of superior quality and a symbol of prestige. On the other hand, the absence of advanced storage facilities and limited storage duration significantly compromises the quality of stored rice grains. Often, consumers in impoverished communities are left with no alternative but to purchase spoiled rice grains. This unfortunate situation leads to numerous health risks, nutrient imbalances, and ultimately disrupts food security. A strategic plan must be devised to address these challenges.
Concluding Remarks and Future Outlook
Approximately 10% of the global population, which amounts to 828 million people, goes to bed daily without access to food. Existing breeding objectives aim to maximize the multiplication of nutrients and compounds for optimal results. Incorporating plant breeding into mainstream practices in developing rice varieties that meet consumer demand and preferences will not only ensures nutritious rice grain and quality; but its impact on food consumption in the Asia–Pacific region.
Humanity faces a multitude of daunting challenges that put lives at risk. One potential solution to ensure that the remaining populations have access to nutritious foods is to develop varieties that offer both high grain yield and exceptional grain quality. Here, we present a strategy for the development of a single rice variety that can meet the high-quality traits required by teeming and changing consumer requirements across the Asia–Pacific region. Our comprehensive review presented significant findings regarding the subpar quality of rice grains to various stakeholders, including consumers, policy makers, breeders, and research centers. Developing a single variety with desirable traits will meet the diverse needs of consumers. It is crucial for the top rice research organizations to prioritize the establishment of a globally recognized standard for rice grain quality, encompassing a wide range of quality parameters. The food value chain undergoes a shift towards a demand-driven phase, driven by modernization and the impact of international trade. The food value chain actors have the potential to meet the needs and desires of their consumers in terms of supply, prices, and meeting expectations. Geneticists should focus on addressing consumer preferences for rice grain quality across different market segments, including local, regional, and international markets. This will encourage policy makers to prioritize the well-being and security of populations that rely on rice seed, food, and nutrition. This will markedly encourage them to invest in research and development at various levels—local, regional, national, and international—in order to assess and address food security (Fig. 5).
Fig. 5.
Future breeding strategies to combine multiple grain superiority and a roadmap for feeding maximum growing populations with high nutritious rice
Supplementary Information
Author Contributions
The conceptualization, methodology and techniques were developed by AM, and HY; data collection, analysis, and visualization was performed by AM, LG, WA, and OR; manuscript writing was done by AM, HY, LG and JKA; the rest of the authors have reviewed, edited and approved it for publication.
Funding
This work was supported by grants from STI 2030-Major Project 2023ZD04069, National Natural Science Foundation of China (U21A20211), AgroST Project (NK20220501) and earmarked Fund of China Agriculture Research System (CARS-01-01).
Availability of Data and Materials
Data availability is not applicable for this manuscript, no data has been generated or analyzed under these investigations.
Declarations
Competing intersts
All authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abebe D, Mohammed W, Tesfaye A (2023) Genotype X environment interaction and stability analysis in upland rice (Oryza sativa L.) varieties in Ethiopia. J Crop Sci Biotechnol 26:51–62 10.1007/s12892-022-00161-5 [DOI] [Google Scholar]
- Adeboye KA, Adabale OW, Adetumbi JA, Ayo-Vaughan MA, Daniel IO (2015) SSR analysis of genetic changes during artificial ageing of rice seeds stored under gene bank management. Plant Breed Seed Sci 71:37–45 10.1515/plass-2015-0020 [DOI] [Google Scholar]
- Alam M, Tan X, Zhang H, Lou G, Yang H, Zhou Y, Hussain A, Bhantana P, Jiang G, He Y (2023) QTL mining and validation of grain nutritional quality characters in rice (Oryza sativa L.) using two introgression line populations. Agriculture 13:1725 10.3390/agriculture13091725 [DOI] [Google Scholar]
- Aung MS, Masuda H, Kobayashi T, Nakanishi H, Yamakawa T, Nishizawa NK (2013) Iron biofortification of Myanmar rice. Front Plant Sci 4:1–14 10.3389/fpls.2013.00158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atungulu GG, Olatunde G, Wilson S (2018) Engineering methods to reduce aflatoxin contamination of cornin on-farm bin drying and storage systems. Drying Technol 36(8):932–951 10.1080/07373937.2017.1365726 [DOI] [Google Scholar]
- Bashir K, Takahashi R, Nakanishi H, Nishizawa NK (2013) The road to micronutrient biofortification of rice: progress and prospects. Front Plant Sci 4:15 10.3389/fpls.2013.00015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azudin MN, Morrison WR (1986) Non-starch lipids and starch lipids in milled rice. J Cereal Sci 4:23–31 10.1016/S0733-5210(86)80004-2 [DOI] [Google Scholar]
- Bhattacharya KR (2011) Rice quality: a guide to rice properties and analysis. Elsevier, Amsterdam [Google Scholar]
- Burkhardt PK, Beyer P, Wünn J, Klöti A, Armstrong GA, Schledz M, von Lintig J, Potrykus I (1997) Transgenic rice (Oryza sativa) endosperm expressing daffodil (Narcissus pseudonarcissus) phytoene synthase accumulates phytoene, a key intermediate of provitamin A biosynthesis. Plant J 11:1071–1078 10.1046/j.1365-313X.1997.11051071.x [DOI] [PubMed] [Google Scholar]
- Champagne ET, Wood DF, Juliano BO, Donald B (2004) The rice grain and its gross composition. Rice Chem Technol 3:77–107 10.1094/1891127349.004 [DOI] [Google Scholar]
- Chen S, Juan Wu, Yang Yi, Shi W, Mingliang Xu (2006) The fgr gene responsible for rice fragrance was restricted within 69 kb. Plant Sci 171:505–514 10.1016/j.plantsci.2006.05.013 [DOI] [PubMed] [Google Scholar]
- Chen E, Huang X, Tian Z, Wing RA, Han B (2019) The genomics of Oryza species provides insights into rice domestication and heterosis. Annu Rev Plant Biol 70:639–665 10.1146/annurev-arplant-050718-100320 [DOI] [PubMed] [Google Scholar]
- Choudhury NH, Juliano BO (1980) Effect of amylose content on the lipids of mature rice grain. J Phytochem 19:1385–1389 10.1016/0031-9422(80)80179-8 [DOI] [Google Scholar]
- Chrastil J (1990) Chemical and physicochemical changes of rice during storage at different temperatures. J Cereal Sci 11:71–85 10.1016/S0733-5210(09)80182-3 [DOI] [Google Scholar]
- Concepcion JCT, Ouk S, Riedel A, Calingacion M, Zhao D, Ouk M, Garson MJ, Fitzgerald MA (2018) Quality evaluation, fatty acid analysis and untargeted profiling of volatiles in Cambodian rice. Food Chem 240:1014–1021 10.1016/j.foodchem.2017.08.019 [DOI] [PubMed] [Google Scholar]
- Concepcion JCT, Calingacion M, Garson MJ, Fitzgerald MA (2020) Lipidomics reveals associations between rice quality traits. Metabolomics 16:1–16 10.1007/s11306-020-01670-6 [DOI] [PubMed] [Google Scholar]
- Cuevas RP, de Guia A, Demont M (2017) Developing a framework of gastronomic systems research to unravel drivers of food choice. Int J Gastron Food Sci 9:88–99 10.1016/j.ijgfs.2017.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Custodio MC, Demont M, Laborte A, Ynion J (2016) Improving food security in Asia through consumer-focused rice breeding. Glob Food Secur 9:19–28 10.1016/j.gfs.2016.05.005 [DOI] [Google Scholar]
- Custodio MC, Cuevas RP, Ynion J, Laborte AG, Velasco ML, Demont M (2019) Rice quality: how is it defined by consumers, industry, food scientists, and geneticists. Trends Food Sci Technol 92:122–137 10.1016/j.tifs.2019.07.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das P, Adak S, Arun LM (2020) Genetic manipulation for improved nutritional quality in rice. Front Genet 11:776 10.3389/fgene.2020.00776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demont M, Ndour M (2015) Upgrading rice value chains: experimental evidence from 11 African markets. Global Food Security 5:70–76 10.1016/j.gfs.2014.10.001 [DOI] [Google Scholar]
- Demont M, Fiamohe R, Thierry Kinkpe A (2017) Comparative advantage in demand and the development of rice value chains in West Africa. World Dev 96:578–590 10.1016/j.worlddev.2017.04.004 [DOI] [Google Scholar]
- Deng Z, Liu Y, Gong C, Chen B, Wang T (2022) Waxy is an important factor for grain fissure resistance and head rice yield as revealed by a genome-wide association study. J Exp Bot 73:6942–6954 10.1093/jxb/erac330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donlao N, Matsushita Y, Ogawa Y (2018) Influence of postharvest drying conditions on resistant starch content and quality of non-waxy long-grain rice (Oryza sativa L.). Drying Technol 36(8):952–964 10.1080/07373937.2017.1366505 [DOI] [Google Scholar]
- Duan M, Sun SSM (2005) Profiling the expression of genes controlling rice grain quality. Plant Mol Biol 59:165–178 10.1007/s11103-004-7507-3 [DOI] [PubMed] [Google Scholar]
- Duan P, Xu J, Zeng D, Zhang B, Geng M, Zhang G, Huang K, Huang L, Xu R, Ge S (2017) Natural variation in the promoter of GSE5 contributes to grain size diversity in rice. Mol Plant 10:685–694 10.1016/j.molp.2017.03.009 [DOI] [PubMed] [Google Scholar]
- Fan C, Xing Y, Mao H, Lu T, Han B, Xu C, Li X, Zhang Q (2006) GS3, a major QTL for grain length and weight and minor QTL for grain width and thickness in rice, encodes a putative transmembrane protein. Theor Appl Genet 112:1164–1171 10.1007/s00122-006-0218-1 [DOI] [PubMed] [Google Scholar]
- Fitzgerald MA, Bergman CJ, Resurreccion AP, Möller J, Jimenez R, Reinke RF, Martin M, Blanco P, Molina F, Chen M-H (2009a) Addressing the dilemmas of measuring amylose in rice. Cereal Chem 86:492–498 10.1094/CCHEM-86-5-0492 [DOI] [Google Scholar]
- Fitzgerald MA, McCouch SR, Hall RD (2009b) Not just a grain of rice: the quest for quality. Trends Plant Sci 14:133–139 10.1016/j.tplants.2008.12.004 [DOI] [PubMed] [Google Scholar]
- Furukawa T, Maekawa M, Oki T, Suda I, Iida S, Shimada H, Takamure I, Kadowaki K (2007) ’The Rc and Rd genes are involved in proanthocyanidin synthesis in rice pericarp. Plant J 49:91–102 10.1111/j.1365-313X.2006.02958.x [DOI] [PubMed] [Google Scholar]
- Gao Z, Zeng D, Cheng F, Tian Z, Guo L, Su Y, Yan M, Jiang H, Dong G, Huang Y (2011) ALK, the key gene for gelatinization temperature, is a modifier gene for gel consistency in rice F. J Integr Plant Biol 53:756–765 [DOI] [PubMed] [Google Scholar]
- Gao X, Zhang X, Lan H, Huang Ji, Wang J, Zhang H (2015) The additive effects of GS3 and qGL3 on rice grain length regulation revealed by genetic and transcriptome comparisons. BMC Plant Biol 15:1–13 10.1186/s12870-015-0515-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein P (2018) HarvestPlus talks zinc rice with farmers in southeastern Bangladesh
- Gong D, Xu X, Wu L, Dai G, Zheng W, Xu Z (2020) Effect of biochar on rice starch properties and starch-related gene expression and enzyme activities. Sci Rep 10:16917 10.1038/s41598-020-73888-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong D, Zhang X, He F, Chen Y, Li R, Yao J, Zhang M, Zheng W, Yu G (2023) Genetic improvements in rice grain quality: a review of elite genes and their applications in molecular breeding. Agronomy 13:1375 10.3390/agronomy13051375 [DOI] [Google Scholar]
- Graham R (2002) A proposal for IRRI to establish a grain quality and nutrition research center. Cereal Chemistry
- Hansen J, Fuller F, Gale F, Crook F, Wailes E, Moore M (2002) China’s japonica rice market: growth and competitiveness. In: Rice situation and outlook yearbook, ERS/USDA 32–37
- He W, Wang L, Lin Q, Yu F (2021) Rice seed storage proteins: biosynthetic pathways and the effects of environmental factors. J Integr Plant Biol 63:1999–2019 10.1111/jipb.13176 [DOI] [PubMed] [Google Scholar]
- Hong J, Rosental L, Xu Y, Xu D, Orf I, Wang W, Hu Z, Su S, Bai S, Ashraf M (2023) Genetic architecture of seed glycerolipids in Asian cultivated rice. Plant Cell Environ 46:1278–1294 10.1111/pce.14378 [DOI] [PubMed] [Google Scholar]
- Huang R, Jiang L, Zheng J, Wang T, Wang H, Huang Y, Hong Z (2013b) Genetic bases of rice grain shape: so many genes, so little known. Trends Plant Sci 18:218–226 10.1016/j.tplants.2012.11.001 [DOI] [PubMed] [Google Scholar]
- Huang L, Tan H, Zhang C, Li Q, Liu Q (2021a) Starch biosynthesis in cereal endosperms: an updated review over the last decade. Plant Commun 2:100237 10.1016/j.xplc.2021.100237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang M, Cao J, Liu Y, Zhang M, Hu L, Xiao Z, Chen J, Cao F (2021b) Low-temperature stress during the flowering period alters the source–sink relationship and grain quality in field-grown late-season rice. J Agron Crop Sci 207:833–839 10.1111/jac.12542 [DOI] [Google Scholar]
- Huang H, Danao M-G, Rausch K, Singh V (2013a) Diffusion and production of carbon dioxide in bulk corn at various temperatures and moisture content. American Society of Agricultural and Biological Engineers, Kansas City, Missouri, July 21–July 24, 2013, 1
- Jamjod S, Yimyam N, Lordkaew S, Prom-U-Thai C, Rerkasem B (2017) Characterization of on-farm rice germplasm in an area of the crop’s center of diversity. Chiang Mai J Sci 16:85–98 [Google Scholar]
- Juliano BO, Villareal CP (1993) Grain quality evaluation of world rices. Internatioinal Rice Research Institute [Google Scholar]
- Klaykruayat S, Mahayothee B, Khuwijitjaru P, Nagle M, Müller J (2020) Influence of packaging materials, oxygen and storage temperature on quality of germinated parboiled rice. LWT 121:108926 10.1016/j.lwt.2019.108926 [DOI] [Google Scholar]
- Kok ADX, Yoon LL, Sekeli R, Yeong WC, Yusof ZNB, Song KL, Shah F, Iqbal A (2018) Rice crop: current developments
- Kong X, Kasapis S, Bao J (2015) Viscoelastic properties of starches and flours from two novel rice mutants induced by gamma irradiation. Food Sci Technol 60:578–582 [Google Scholar]
- Kovach MJ, Calingacion MN, Fitzgerald MA, McCouch SR (2009) The origin and evolution of fragrance in rice (Oryza sativa L.). Proc Natl Acad Sci 106:14444–14449 10.1073/pnas.0904077106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudra DT (1996) On-farm drying and storage systems. Dry Technol 14:477–478 10.1080/07373939608917110 [DOI] [Google Scholar]
- Lancaster KJ (1966) A new approach to consumer theory. J Polit Econ 74:132–157 10.1086/259131 [DOI] [Google Scholar]
- Lee S, Kim Y-S, Jeon US, Kim Y-K, Schjoerring JK, An G (2012) Activation of rice nicotianamine synthase 2 (OsNAS2) enhances iron availability for biofortification. Mol Cells 33:269–275 10.1007/s10059-012-2231-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Fan C, Xing Y, Jiang Y, Luo L, Sun L, Shao D, Xu C, Li X, Xiao J (2011) Natural variation in GS5 plays an important role in regulating grain size and yield in rice. Nat Genet 43:1266–1269 10.1038/ng.977 [DOI] [PubMed] [Google Scholar]
- Li Y, Fan C, Xing Y, Yun P, Luo L, Yan B, Peng B, Xie W, Wang G, Li X (2014) Chalk5 encodes a vacuolar H+-translocating pyrophosphatase influencing grain chalkiness in rice. Nat Genet 46:398–404 10.1038/ng.2923 [DOI] [PubMed] [Google Scholar]
- Li K, Zhang T, Sui Z, Narayanamoorthy S, Jin C, Li S, Corke H (2019) Genetic variation in starch physicochemical properties of Chinese foxtail millet (Setaria italica Beauv.). Int J Biol Macromol 133:337–345 10.1016/j.ijbiomac.2019.04.022 [DOI] [PubMed] [Google Scholar]
- Li P, Chen Y-H, Lu J, Zhang C-Q, Liu Q-Q, Li Q-F (2022) Genes and their molecular functions determining seed structure, components, and quality of rice. Rice 15:18 10.1186/s12284-022-00562-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang H, Gao S, Ma J, Zhang T, Wang T, Zhang S, Wu Z (2021) Effect of nitrogen application rates on the nitrogen utilization, yield and quality of rice. Food Nutr Sci 12:13–27 [Google Scholar]
- Lisle AJ, Martin M, Fitzgerald MA (2000) Chalky and translucent rice grains differ in starch composition and structure and cooking properties. Cereal Chem 77:627–632 10.1094/CCHEM.2000.77.5.627 [DOI] [Google Scholar]
- Liu L, Tong H, Xiao Y et al (2015) Activation of Big Grain1 significantly improves grain size by regulating auxin transport in rice. Proc Natl Acad Sci USA 112:11102–11107 10.1073/pnas.1512748112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Chen J, Zheng X, Wu F, Lin Q, Heng Y, Tian P, Cheng Z, Yu X, Zhou K (2017) GW5 acts in the brassinosteroid signalling pathway to regulate grain width and weight in rice. Nat Plants 3:1–7 10.1038/nplants.2017.43 [DOI] [PubMed] [Google Scholar]
- Long X, Guan C, Wang L, Jia L, Fu X, Lin Q, Huang Z, Liu C (2023) Rice storage proteins: focus on composition, distribution, genetic improvement and effects on rice quality. Rice Sci 30(3):207–221 10.1016/j.rsci.2023.03.005 [DOI] [Google Scholar]
- Lou G, Bhat MA, Tan X, Wang Y, He Y (2023) Research progress on the relationship between rice protein content and cooking and eating quality and its influencing factors. Seed Biol 2:16 10.48130/SeedBio-2023-0016 [DOI] [Google Scholar]
- Maclean J, Hardy B, Hettel G (2013) Rice almanac: source book for one of the most important economic activities on earth (IRRI)
- Mangel N, Fudge JB, Gruissem W, Fitzpatrick TB, Vanderschuren H (2022) Natural variation in vitamin B1 and vitamin B6 contents in rice germplasm. Front Plant Sci 13:856880 10.3389/fpls.2022.856880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao H, Sun S, Yao J, Wang C, Yu S, Xu C, Li X, Zhang Q (2010) Linking differential domain functions of the GS3 protein to natural variation of grain size in rice. Proc Natl Acad Sci 107:19579–19584 10.1073/pnas.1014419107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin M, Fitzgerald MA (2002) Proteins in rice grains influence cooking properties. J Cereal Sci 36:285–294 10.1006/jcrs.2001.0465 [DOI] [Google Scholar]
- Masuda H, Aung MS, Nishizawa NK (2013a) Iron biofortification of rice using different transgenic approaches. Rice 6:1–12 10.1186/1939-8433-6-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda H, Kobayashi T, Ishimaru Y, Takahashi M, Aung MS, Nakanishi H, Mori S, Nishizawa NK (2013b) Iron-biofortification in rice by the introduction of three barley genes participated in mugineic acid biosynthesis with soybean ferritin gene. Front Plant Sci 4:132 10.3389/fpls.2013.00132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller A, Nunes MT, Maldaner V, Coradi PC, de Moraes RS, Martens S, Leal AF, Pereira VF, Marin CK (2022) Rice drying, storage and processing: effects of post-harvest operations on grain quality. Rice Sci 29:16–30 10.1016/j.rsci.2021.12.002 [DOI] [Google Scholar]
- My NHD, Demont M, Van Loo EJ, de Guia A, Rutsaert P, Tuan TH, Verbeke W (2018) What is the value of sustainably-produced rice? Consumer evidence from experimental auctions in Vietnam. Food Policy 79:283–296 10.1016/j.foodpol.2018.08.004 [DOI] [Google Scholar]
- Nardi S, Del Lungo T, Bellopede M (1997) Rice quality assessment: comparison between a traditional method and the hot water soluble amylose determination to evaluate cooking loss. Ital J Food Sci 9:57–64 [Google Scholar]
- Ohno T, Ohisa N (2005) Studies on textural and chemical changes in aged rice grains. Food Sci Technol Res 11:385–389 10.3136/fstr.11.385 [DOI] [Google Scholar]
- Paul S, Ali N, Gayen D, Datta SK, Datta K (2012) Molecular breeding of Osfer2 gene to increase iron nutrition in rice grain. GM Crops Food 3:310–316 10.4161/gmcr.22104 [DOI] [PubMed] [Google Scholar]
- Peng Bo, Kong H, Li Y, Wang L, Zhong M, Sun L, Gao G, Zhang Q, Luo L, Wang G (2014) OsAAP6 functions as an important regulator of grain protein content and nutritional quality in rice. Nat Commun 5:1–12 10.1038/ncomms5847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfister B, Zeeman SC (2016) Formation of starch in plant cells. Cell Mol Life Sci 73:2781–2807 10.1007/s00018-016-2250-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad AS (2004) Zinc deficiency: its characterization and treatment. Metals Ions Biol Syst 41:103–137 [PubMed] [Google Scholar]
- Prom-U-Thai C, Rerkasem B (2020) Rice quality improvement-a review. Agron Sustain Dev 40:28 10.1007/s13593-020-00633-4 [DOI] [Google Scholar]
- Ramos AH, Rockenbach BA, Ferreira CD, Gutkoski LC, de Oliveira M (2019) Characteristics of flour and starch isolated from red rice subjected to different drying conditions. Starch-Stärke 71:1800257 10.1002/star.201800257 [DOI] [Google Scholar]
- Ren D, Ding C, Qian Q (2023) Molecular bases of rice grain size and quality for optimized productivity. Sci Bull 68:314–350 10.1016/j.scib.2023.01.026 [DOI] [PubMed] [Google Scholar]
- Ritchie H, Roser M (2017) Micronutrient deficiency. Our world in data
- Rodríguez-Arzuaga M, Cho S, Billiris MA, Siebenmorgen T, Seo H-S (2016) Impacts of degree of milling on the appearance and aroma characteristics of raw rice. J Sci Food Agric 96:3017–3022 10.1002/jsfa.7471 [DOI] [PubMed] [Google Scholar]
- Runge J, Heringer OA, Ribeiro JS, Biazati LB (2019) Multi-element rice grains analysis by ICP OES and classification by processing types. Food Chem 271:419–424 10.1016/j.foodchem.2018.07.162 [DOI] [PubMed] [Google Scholar]
- Saikrishna A, Dutta S, Subramanian V, Moses JA, Anandharamakrishnan C (2018) Ageing of rice: a review. J Cereal Sci 81:161–170 10.1016/j.jcs.2018.04.009 [DOI] [Google Scholar]
- Sakthivel K, Sundaram RM, Shobha Rani N, Balachandran SM, Neeraja CN (2009) Genetic and molecular basis of fragrance in rice. Biotechnol Adv 27:468–473 10.1016/j.biotechadv.2009.04.001 [DOI] [PubMed] [Google Scholar]
- Sanjeeva Rao D, Neeraja CN, Madhu Babu P, Nirmala B, Suman K, Subba Rao LV, Surekha K, Raghu P, Longvah T, Surendra P (2020) Zinc biofortified rice varieties: challenges, possibilities, and progress in India. Front Nutr 7:26 10.3389/fnut.2020.00026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaub P, Al-Babili S, Drake R, Beyer P (2005) Why is golden rice golden (yellow) instead of red? Plant Physiol 138:441–450 10.1104/pp.104.057927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh CB, Fielke JM (2017) Recent developments in stored grain sensors, monitoring and management technology. IEEE Instrum Meas Mag 20:32–55 10.1109/MIM.2017.7951690 [DOI] [Google Scholar]
- Singh N, Kaur L, Sandhu KS, Kaur J, Nishinari K (2006) Relationships between physicochemical, morphological, thermal, rheological properties of rice starches. Food Hydrocolloids 20:532–542 10.1016/j.foodhyd.2005.05.003 [DOI] [Google Scholar]
- Sodhi NS, Singh N, Arora M, Singh J (2003) Changes in physico-chemical, thermal, cooking and textural properties of rice during aging. J Food Process Preserv 27:387–400 10.1111/j.1745-4549.2003.tb00525.x [DOI] [Google Scholar]
- Song X-J, Huang W, Shi M, Zhu M-Z, Lin H-X (2007) A QTL for rice grain width and weight encodes a previously unknown RING-type E3 ubiquitin ligase. Nat Genet 39:623–630 10.1038/ng2014 [DOI] [PubMed] [Google Scholar]
- Sreenivasulu N, Butardo VM Jr, Misra G, Cuevas RP, Anacleto R, Kavi Kishor PB (2015) Designing climate-resilient rice with ideal grain quality suited for high-temperature stress. J Exp Bot 66:1737–1748 10.1093/jxb/eru544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens GA, Beal T, Mbuya MNN, Luo H, Neufeld LM (2022) Micronutrient deficiencies among preschool-aged children and women of reproductive age worldwide: a pooled analysis of individual-level data from population-representative surveys. Lancet Glob Health 10:1590–1599 10.1016/S2214-109X(22)00367-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Y, Rao Y, Hu S, Yang Y, Gao Z, Zhang G, Liu J, Hu J, Yan M, Dong G (2011) Map-based cloning proves qGC-6, a major QTL for gel consistency of japonica/indica cross, responds by Waxy in rice (Oryza sativa L.). Theor Appl Genet 123:859–867 10.1007/s00122-011-1632-6 [DOI] [PubMed] [Google Scholar]
- Sun S, Wang L, Mao H, Shao L, Li X, Xiao J, Ouyang Y, Zhang Q (2018) A G-protein pathway determines grain size in rice. Nat Commun 9:851 10.1038/s41467-018-03141-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swamy BPM, Rahman MA, Inabangan-Asilo MA, Amparado A, Manito C, Chadha-Mohanty P, Reinke R, Slamet-Loedin IH (2016) Advances in breeding for high grainzinc in rice. Rice 9(1):1–16 10.1186/s12284-016-0122-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney MT, Thomson MJ, Pfeil BE, McCouch S (2006) Caught red-handed: Rc encodes a basic helix-loop-helix protein conditioning red pericarp in rice. Plant Cell 18:283–294 10.1105/tpc.105.038430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan S, Han R, Li P, Yang G, Li S, Zhang P, Wang W-B, Zhao W-Z, Yin L-P (2015) Over-expression of the Mx IRT1 gene increases iron and zinc content in rice seeds. Transgenic Res 24:109–122 10.1007/s11248-014-9822-z [DOI] [PubMed] [Google Scholar]
- Tang G, Qin J, Dolnikowski GG, Russell RM, Grusak MA (2009) Golden rice is an effective source of vitamin A. Am J Clin Nutr 89:1776–1783 10.3945/ajcn.2008.27119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Z, Qian Q, Liu Q, Yan M, Liu X, Yan C, Liu G, Gao Z, Tang S, Zeng D (2009) Allelic diversities in rice starch biosynthesis lead to a diverse array of rice eating and cooking qualities. Proc Natl Acad Sci 106:21760–21765 10.1073/pnas.0912396106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Y-S, Wang Bo, Peng R-H, Xu J, Li T, Fu X-Y, Xiong A-S, Gao J-J, Yao Q-H (2019) Enhancing carotenoid biosynthesis in rice endosperm by metabolic engineering. Plant Biotechnol J 17:849 10.1111/pbi.13059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trijatmiko KR, Dueñas C, Tsakirpaloglou N, Torrizo L, Arines FM, Adeva C, Balindong J, Oliva N, Sapasap MV, Borrero J (2016) Biofortified indica rice attains iron and zinc nutrition dietary targets in the field. Sci Rep 6:19792 10.1038/srep19792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veni BK (2019) Nutrition profiles of different colored rice: a review. J Pharmacogn Phytochem 8:303–305 [Google Scholar]
- Wailes EJ, Chavez EC (2012) International rice baseline with deterministic and stochastic projections. 2012–2021
- Wang S, Wu K, Yuan Q, Liu X, Liu Z, Lin X, Zeng R, Zhu H, Dong G, Qian Q (2012) Control of grain size, shape and quality by OsSPL16 in rice. Nat Genet 44:950–954 10.1038/ng.2327 [DOI] [PubMed] [Google Scholar]
- Wang S, Li S, Liu Q, Wu K, Zhang J, Wang S, Wang Y, Chen X, Zhang Y, Gao C (2015) The OsSPL16-GW7 regulatory module determines grain shape and simultaneously improves rice yield and grain quality. Nat Genet 47:949–954 10.1038/ng.3352 [DOI] [PubMed] [Google Scholar]
- Wangcharoen W, Phanchaisri C, Daengpok W, Phuttawong R, Hangsoongnern T, Phanchaisri B (2016) Consumer acceptance test and some related properties of selected KDML 105 rice mutants. J Food Sci Technol 53:3550–3556 10.1007/s13197-016-2334-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng J, Gu S, Wan X, Gao H, Guo T, Su N, Lei C, Zhang X, Cheng Z, Guo X (2008) Isolation and initial characterization of GW5, a major QTL associated with rice grain width and weight. Cell Res 18:1199–1209 10.1038/cr.2008.307 [DOI] [PubMed] [Google Scholar]
- White PJ, Brown PH (2010) Plant nutrition for sustainable development and global health. Ann Bot 105:1073–1080 10.1093/aob/mcq085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirth J, Poletti S, Aeschlimann B, Yakandawala N, Drosse B, Osorio S (2009) Rice endosperm iron biofortification by targeted and synergistic action of nicotianamine synthase and ferritin. Plant Biotechnol 6:31–644 [DOI] [PubMed] [Google Scholar]
- Wu C, Lu L, Yang X, Feng Y, Wei Y, Hao H, Stoffella PJ, He Z (2010) Uptake, translocation, and remobilization of zinc absorbed at different growth stages by rice genotypes of different Zn densities. J Agric Food Chem 58:6767–6773 10.1021/jf100017e [DOI] [PubMed] [Google Scholar]
- Wu K, Wang S, Song W, Zhang J, Wang Y, Liu Q, Yu J, Ye Y, Li S, Chen J (2020) Enhanced sustainable green revolution yield via nitrogen-responsive chromatin modulation in rice. Science 367:2046 10.1126/science.aaz2046 [DOI] [PubMed] [Google Scholar]
- Wu B, Yun P, Zhou H, Xia D, Gu Y, Li P, Yao J, Zhou Z, Chen J, Liu R (2022) Natural variation in WHITE-CORE RATE 1 regulates redox homeostasis in rice endosperm to affect grain quality. Plant Cell 34:1912–1932 10.1093/plcell/koac057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia D, Zhou H, Liu R, Dan W, Li P, Wu B, Chen J, Wang L, Gao G, Zhang Q (2018) GL3. 3, a novel QTL encoding a GSK3/SHAGGY-like kinase, epistatically interacts with GS3 to produce extra-long grains in rice. Mol Plant 11:754–756 10.1016/j.molp.2018.03.006 [DOI] [PubMed] [Google Scholar]
- Xie L, Wu D, Fang Y, Ye C, Zhu Q H, Fan L (2024) Population genomic analysis unravels the evolutionary roadmap of pericarp color in rice. Plant Commun 5:3 10.1016/j.xplc.2023.100778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Wei Y, Zhu Y, Lian L, Xie H, Cai Q, Zhang J (2015) Antisense suppression of LOX3 gene expression in rice endosperm enhances seed longevity. Plant Biotechnol Jl 13(4):526–539 10.1111/pbi.12277 [DOI] [PubMed] [Google Scholar]
- Yang X, Wang B, Chen L, Li P, Cao C (2019a) The different influences of drought stress at the flowering stage on rice physiological traits, grain yield, and quality. Sci Rep 9:3742 10.1038/s41598-019-40161-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Guo M, Sun S, Zou Y, Yin S, Liu Y, Tang S, Gu M, Yang Z, Yan C (2019b) Natural variation of OsGluA2 is involved in grain protein content regulation in rice. Nat Commun 10:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Yang Z, Liu C, Shen Z, Zhan S, Lyu C, Zhang Y, Li F, Shi K (2022) The NET locus determines the food taste, cooking and nutrition quality of rice. Sci Bull 67:2045–2049 10.1016/j.scib.2022.09.023 [DOI] [PubMed] [Google Scholar]
- Yanxia A, Xianqing Z, Zhang Y (2018) Changes in physicochemical, cooking and sensory characteristics of rice shifted from low-temperature storage. Grain Oil Sci Technol 1:8–14 10.3724/SP.J.1447.GOST.2018.18018 [DOI] [Google Scholar]
- Ye X, Al-Babili S, Kloti A, Zhang J, Lucca P, Beyer P, Potrykus I (2000) Engineering the provitamin A (β-carotene) biosynthetic pathway into (carotenoid-free) rice endosperm. Science 287:303–305 10.1126/science.287.5451.303 [DOI] [PubMed] [Google Scholar]
- Yin H, Gao X, Stomph T, Li L, Zhang F, Zou C (2016) Zinc concentration in rice (Oryza sativa L.) grains and allocation in plants as affected by different zinc fertilization strategies. Commun Soil Sci Plant Anal 47:761–768 10.1080/00103624.2016.1146891 [DOI] [Google Scholar]
- Zafar S, Jianlong X (2023) Recent advances to enhance nutritional quality of rice. Rice 30:523–536 [Google Scholar]
- Zeng Y, Jia F, Meng X, Han Y, Xiao Y (2018) The effects of friction characteristic of particle on milling process in a horizontal rice mill. Adv Powder Technol 29:1280–1291 10.1016/j.apt.2018.02.021 [DOI] [Google Scholar]
- Zhang X, Wang J, Huang J, Lan H, Wang C, Yin C, Wu Y, Tang H, Qian Q, Li J (2012) Rare allele of OsPPKL1 associated with grain length causes extra-large grain and a significant yield increase in rice. Proc Natl Acad Sci 109:21534–21539 10.1073/pnas.1219776110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Zhou L, Zhu Z, Lu H, Zhou X, Qian Y, Li Q, Lu Y, Gu M, Liu Q (2016a) Characterization of grain quality and starch fine structure of two japonica rice (Oryza sativa) cultivars with good sensory properties at different temperatures during the filling stage. J Agric Food Chem 64:4048–4057 10.1021/acs.jafc.6b00083 [DOI] [PubMed] [Google Scholar]
- Zhang Y-X, Xu H-H, Liu S-J, Li N, Wang W-Q, Møller IM, Song S-Q (2016b) Proteomic analysis reveals different involvement of embryo and endosperm proteins during aging of Yliangyou 2 hybrid rice seeds. Front Plant Sci 7:1394 10.3389/fpls.2016.01394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Yang Y, Chen Z, Chen F, Pan L, Lu Y, Li Q, Fan X, Sun Z, Liu Q (2020) Characteristics of grain physicochemical properties and the starch structure in rice carrying a mutated ALK/SSIIa gene. J Agric Food Chem 68:13950–13959 10.1021/acs.jafc.0c01471 [DOI] [PubMed] [Google Scholar]
- Zhao DS, Li QF, Zhang CQ, Zhang C, Yang QQ, Pan LX, Ren XY, Lu J, Gu MH, Liu QQ (2018) GS9 acts as a transcriptional activator to regulate rice grain shape and appearance quality. Nat Commun 9:1240 10.1038/s41467-018-03616-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M, Lin Y, Chen H (2020) Improving nutritional quality of rice for human health. Theor Appl Genet 133:1397–1413 10.1007/s00122-019-03530-x [DOI] [PubMed] [Google Scholar]
- Zhao D, Liu J, Ding A, Zhang T, Ren X, Zhang L, Li Q, Fan X, Zhang C, Liu Q (2021) Improving grain appearance of erect-panicle japonica rice cultivars by introgression of the null gs9 allele. J Integr Agric 20:2032–2042 10.1016/S2095-3119(21)63659-6 [DOI] [Google Scholar]
- Zhao L, Zhao C-F, Zhou L-H, Yao S, Zhao Q-Y, Chen T, Zhu Z, Zhang Y-D, Wang C-L (2022) Mapping QTLs for rice (Oryza sativa L.) grain protein content via chromosome segment substitution lines. Cereal Res Commun 50:699–708 10.1007/s42976-021-00237-y [DOI] [Google Scholar]
- Zheng C, Liu C, Liu L, Tan Y, Sheng X, Yu D, Sun Z, Sun X, Chen J, Yuan D (2023) Effect of salinity stress on rice yield and grain quality: a meta-analysis. Eur J Agron 144:126765 10.1016/j.eja.2023.126765 [DOI] [Google Scholar]
- Zhou Z, Robards K, Helliwell S, Blanchard C (2002) Ageing of stored rice: changes in chemical and physical attributes. J Cereal Sci 35:65–78 10.1006/jcrs.2001.0418 [DOI] [Google Scholar]
- Zhou Z, Robards K, Helliwell S (2003) Effect of rice storage on pasting properties of rice flour. Food Res Int 36:625–634 10.1016/S0963-9969(03)00013-9 [DOI] [Google Scholar]
- Zhou Y, Miao J, Gu H, Peng X, Leburu M, Yuan F, Gu H, Gao Y, Tao Y, Zhu J (2015) Natural variations in SLG7 regulate grain shape in rice. Genetics 201:1591–1599 10.1534/genetics.115.181115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou C, Huang Y, Jia B, Wang Y, Wang Y, Xu Q, Li R, Wang S, Dou F (2018) Effects of cultivar, nitrogen rate, and planting density on rice-grain quality. Agronomy 8:246 10.3390/agronomy8110246 [DOI] [Google Scholar]
- Zhou H, Xia D, He Y (2020) Rice grain quality—traditional traits for high quality rice and health-plus substances. Mol Breed 40:1–17 10.1007/s11032-019-1080-6 [DOI] [Google Scholar]
- Zhou H, Xia D, Li P, Ao Y, Xu X, Wan S, Li Y, Wu B, Shi H, Wang K (2021) Genetic architecture and key genes controlling the diversity of oil composition in rice grains. Mol Plant 14:456–469 10.1016/j.molp.2020.12.001 [DOI] [PubMed] [Google Scholar]
- Zhou H, Li S, Liu J, Hu J, Le S, Li M (2023) Identification and analysis of the genetic integrity of different types of rice resources through SSR markers. Sci Rep 13:2428 10.1038/s41598-023-29514-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Q, Zeng D, Yu S, Cui C, Li J, Li H, Chen J, Zhang R, Zhao X, Chen L (2018) From golden rice to aSTARice: bioengineering astaxanthin biosynthesis in rice endosperm. Mol Plant 11:1440–1448 10.1016/j.molp.2018.09.007 [DOI] [PubMed] [Google Scholar]
- Zhu Q, Wang B, Tan J, Liu T, Li L, Liu Y-G (2020) Plant synthetic metabolic engineering for enhancing crop nutritional quality. Plant Commun 1:100017 10.1016/j.xplc.2019.100017 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data availability is not applicable for this manuscript, no data has been generated or analyzed under these investigations.