Abstract
Phytophthora infestans is a major oomycete plant pathogen, responsible for potato late blight, which led to the Irish Potato Famine from 1845–1852. Since then, potatoes resistant to this disease have been bred and deployed worldwide. Their resistance (R) genes recognize pathogen effectors responsible for virulence and then induce a plant response stopping disease progression. However, most deployed R genes are quickly overcome by the pathogen. We use targeted sequencing of effector and R genes on herbarium specimens to examine the joint evolution in both P. infestans and potato from 1845–1954. Currently relevant effectors are historically present in P. infestans, but with alternative alleles compared to modern reference genomes. The historic FAM-1 lineage has the virulent Avr1 allele and the ability to break the R1 resistance gene before breeders deployed it in potato. The FAM-1 lineage is diploid, but later, triploid US-1 lineages appear. We show that pathogen virulence genes and host resistance genes have undergone significant changes since the Famine, from both natural and artificial selection.
Subject terms: Population genetics, Plant evolution, Effectors in plant pathology
The joint evolution in Phytophthora infestans and potato over a one hundred period since the Famine is examined based on targeting sequencing of pathogen effector genes and host resistance genes from historic outbreak samples present in plant herbaria.
Introduction
In 1843, a devastating plant disease struck US potatoes and two years later, in 1845, the pathogen spread to Europe, causing a destructive potato disease1. This epidemic (1845–1852) had profound consequences in Ireland, leading to the Irish Famine which resulted in the death of about 1 million people and the emigration of another 1 million people, as potatoes were a staple food2. The Irish Potato Famine left lasting effects as Ireland’s population never fully recovered to pre-Famine levels and millions lost land that had been farmed for generations2. The responsible plant pathogen was identified in 1846 by M. J. Berkeley and subsequently renamed Phytophthora infestans in 1876 by Anton de Bary2–4. The pathogen is an Oomycete and can reproduce by clonal and sexual methods5. The clonal lineage of P. infestans that caused the Famine was named FAM-1 and was subsequently displaced by the US-1 lineage in the 1930s–1950s6.
Phytophthora infestans continues to threaten potato and tomato production worldwide, necessitating the use of expensive fungicides for management5,6. Over the past 180 years, extensive efforts have focused on developing resistant cultivars of Solanum tuberosum to counter the disease7. Despite discovering several resistance (R) genes in wild Solanaceous relatives, achieving durable resistance to the pathogen has remained challenging8,9. Like other plant pathogens, P. infestans employs effector proteins to facilitate colonization of host plants10,11. Many of these effectors contain a specific amino acid motif known as the RXLR motif (Arginine – Any Amino Acid – Leucine – Arginine)12. In response, host plants have evolved R proteins that recognize these RXLR effector proteins, triggering an immune response that halts disease progression10,11. Effectors recognized by R genes are referred to as “avirulence factors,” and this gene-for-gene response system accounts for the specific resistance of certain host genotypes against distinct pathogen strains13.
All varieties planted in Ireland at the time of the Famine were highly susceptible to disease2. Shortly after the onset of the Irish Famine, efforts were made to select disease-resistant potatoes or import new varieties with resistance14. Although early efforts did not yield significant progress, breeding endeavors led to more resistant varieties, such as the Champion variety that survived the late blight outbreak in 187914–16. Notable introductions included the “Rough Purple Chili” variety from Chile, which carries a distinctive genetic deletion useful for identifying Chilean sources of Solanum cultivars17,18. However, the pathogen continued to overcome deployed host resistance to the point where little effective resistance was observed by 192916.
Initial explorations of close-wild relatives of potato from South America and Mexico by John Lindley and others were susceptible to late blight15.Subsequently, identification of late blight resistant accessions in S. demissum from Mexico facilitated the breeding of cultivated potatoes in the early part of the 20th century16,19,20.
Although 11 R genes from S. demissum were effective against various races of P. infestans, their introduction into domestic potato varieties proved challenging and resistance was short lived21,22. Phytophthora infestans was able to overcome the introduction of R genes from wild hosts in most instances20,21. Most R genes provide protection against specific races of P. infestans, and some races of the pathogen exhibit virulence on all 11 originally described R genes23. Despite recent identification of more than 20 additional R genes in the genomic era of the 21st century, achieving durable resistance to P. infestans has remained elusive21,24. Recently, the stacking of multiple R genes into transgenic potatoes from several wild species of potato has shown promise for late blight resistance in field trials conducted in Africa25. Genetic control, including R genes, is essential in managing P. infestans26. While genome sequencing of P. infestans has predicted over 500 putative effectors, only twelve avirulence effectors have been cloned and characterized (Table 1)26–29. Many effectors exhibit signs of rapid evolutionary adaptation, contributing to resistance breakdown in the potato late blight pathosystem8,27.
Table 1.
Summary of well-described R-gene and effector pairs analyzed in this study that are cloned or not cloned and the reference where they are reported
| R genea | Solanum spp.b | Status | Reference | Effectorc | Status | Reference |
|---|---|---|---|---|---|---|
| R1 | S. demissum | Cloned | 75 | Avr1 | Cloned | 76 |
| R2 | S. demissum | Cloned | 44 | Avr2 | Cloned | 77 |
| R3a | S. demissum | Cloned | 78 | Avr3a | Cloned | 79 |
| R3b | S. demissum | Cloned | 80 | Avr3b | Cloned | 81 |
| R4 | S. demissum | Not cloned | 82 | Avr4 | Cloned | 83 |
| R8 | S. demissum | Cloned | 84 | Avr8 | Cloned | 81,84 |
| R10 | S. demissum | Not cloned | 85 | Avr10 | Cloned | 36 |
| Rpi-blb2 | S. bulbocastanum | Cloned | 86 | Avr-blb2 | Cloned | 87 |
| Rpi-smira1 | S. tuberosum cultivar ‘Sárpo Mira’ | Not cloned | 81 | Avr-smira1 | Cloned | 81 |
| Rpi-vnt1.1 | S. venturii | Cloned | 88 | Avr-vnt1 | Cloned | 76,89 |
| N/A | 90 | PexRD24 | Cloned | 90 |
aEleven S. demissum resistance (R) genes designated R1-R11 are distinguished in a potato differential set by Black and Mastenbroek R1, R3, and R10, and to a lesser extent R2 and R4, have widely been used for introgression in European breeding programs11,19. Note R gene names are italicized.
bSolanum spp. host Latin names are italicized.
cNote that Avr-blb1 was not successfully baited with the enrichment sequencing and it is not included in the data analysis. Effector gene names are italicized
Mycological herbarium specimens containing both host (potato) and pathogen (P. infestans) genetic material have been preserved since the time of the Irish Famine1,30–32. Sequencing of P. infestans from these specimens has demonstrated the presence of many currently known effectors, and the number of RXLR effectors appears to have increased over time33,34. However, information regarding the evolution of RXLR effectors of P. infestans in response to host R gene deployment over time has not been examined. Understanding the evolutionary pathways of specific known effectors could provide valuable insights into understanding the durability of deployed effectors and their associated R genes.
Both R gene identification in S. tuberosum and RXLR gene identification in P. infestans have been achieved through targeted enrichment sequencing35–38. This sequencing technique allows the number of reads of interest to be maximized by focusing on specific genes or genomic regions36,38. Targeted enrichment sequencing employs bait sequences, which consist of 60–80 base pair oligonucleotides closely aligned with the loci of interest (Fig. 1). These baits enrich the sequencing library for the genomic fragments of interest, which are then sequenced, resulting in a set of reads containing a disproportionate number of the targeted loci36,38.
Fig. 1. Graphic outline of sample processing.

Each sample included in this study was a dried Solanum specimen with both host and pathogen DNA. Extracted DNA was enriched for both host R genes and pathogen RXLR genes before sequencing.
The goals of this study were fourfold: 1) to utilize targeted enrichment sequencing to simultaneously sequence both host R genes and pathogen effectors in P. infestans infected Solanum species from herbarium specimens from 1845–1954; 2) to analyze and document changes in avirulence factors of the pathogen in response to the deployment of resistance genes; 3) to investigate the temporal changes in the abundance of R genes and RXLR genes since the time of the Irish Famine; 4) to assess the evolution and diversity within R genes and RXLR effector genes over the sampling period.
Results
Genome sequencing and enrichment of targeted genomic regions
Baited enrichment sequencing was used to sequence DNA from 29 historic P. infestans infected Solanum specimens collected between 1845 and 1954 from herbaria (Table 2). The trimmed sequence reads were aligned to the whole genomes of both the Solanum host and P. infestans. Over the whole genomes, approximately 61% of reads aligned to the Solanum tuberosum SolTub3.0 genome, while 20% of reads aligned to the P. infestans 1306 genome, indicating a balanced distribution between the two genomes despite the predominance of host tissue in most samples (Supplementary Table 1B). Assembly and local alignment of unmapped reads revealed homology to other Solanum spp. or Phytophthora genomes, along with the presence of minor amounts of sequences from other microorganisms.
Table 2.
Chronological list of historical herbarium specimens used in this study, including their herbarium source, host, collector, date of collection, country, and the SSR genotype of P. infestans (when available)
| Sample Name | Herbariuma | Hostb | Collector | Date | Country (City) | SSr Genotypec |
|---|---|---|---|---|---|---|
| H246 FH-822447 | FH | S. tuberosum | M. Desmazieres | 1845 | France | |
| K47 K-M178122 | K | S. tuberosum | M.J. Berkeley | 1846 | Britain | FAM_1 |
| K8 K-M177514 | K | S. tuberosum | D. Moore | 1847 | Ireland | |
| H256 FH-822286 | FH | S. tuberosum | M. J. Berkeley | 1849 | Ireland | FAM_1 |
| K53 K-M 185583a | K | S. tuberosum | M. J. Berkeley | 1852 | Germany | |
| K10 K-M177506 | K | S. tuberosum | M. J. Berkeley | 1853 | Britain | |
| US0186686 | BPI | S. tuberosum | J. B. Ellis | 1855 | New York | FAM-1 |
| K52 K-M 185583b | K | S. tuberosum | M. J. Berkeley | 1855 | Germany | |
| K41 K-M177512 | K | S. tuberosum | M. J. Berkeley | 1879 | Britain | FAM-1 |
| K81 K-M1851619 | K | S. tuberosum | C. Spegazzini | 1879 | Italy | FAM-1 |
| US0186680 | BPI | S. tuberosum | W. Trelease | 1880 | WI, Madison | FAM-1 |
| US0186932 | BPI | S. tuberosum | F. L. Harvey | 1880 | ME, Orono | FAM-1 |
| US0186856 | BPI | S. tuberosum | G. Linhart | 1882 | Hungary | FAM-1 |
| K48 K-M185632 | K | S. verrucossum | P. Sydow | 1887 | Germany | |
| US0186842 | BPI | S. nigrum | P. Sydow | 1896 | Germany, Berlin | FAM-1 |
| H227 FH-822278 | FH | S. tuberosum | F. Bucholtz | 1909 | USSR | FAM-1 |
| US0186979 | BPI | S. tuberosum | G.R. Lyman | 1915 | PA, Bath | FAM-1 |
| H281 FH-822354 | FH | S. tuberosum | R. Thaxter | 1916 | ME, Kittery Pt | FAM-1 |
| US0186868 | BPI | S. tuberosum | G. P. Clinton | 1918 | CT, Milford | FAM-1 |
| US0186928 | BPI | S. tuberosum | G.F. Gravatt | 1934 | AL, Wrangel | FAM-1 |
| US0186860 | BPI | S. tuberosum | A. D. McDonnell | 1935 | CT, Preston | |
| US0186929 | BPI | S. tuberosum | K. Starcs | 1935 | Latvia | FAM-1 |
| US0186841 | BPI | S. nigrum | R. Sprague | 1937 | OR, Astoria | FAM-1 |
| US0186843 | BPI | S. sarachoides | M.W. Gardner | 1943 | CA, San Mateo Co. | US-1 |
| US0186972 | BPI | S. tuberosum | J. S. Niederhauser | 1948 | Mexico, Chihuahua | NDc |
| H287 FH-822365 | FH | S. tuberosum | B. O. Saville | 1948 | Canada | US-1 |
| K126 K-M185339 | K | S. tuberosum | J.H.H. | 1952 | Britain | US-1 |
| IMI53089 | IMI | S. tuberosum | T. A. Russell | 1953 | Fr Cameroon | US-1 |
| US0186956 | BPI | S. tuberosum | S.C. Litzenberger | 1954 | Nicaragua, Santa Maria | FAM-1 |
a29 specimens sampled from the collections housed at: K, the Royal Botanic Gardens Kew Mycological Herbarium; BPI: the USDA National Fungus Collection, Beltsville, MD; IMI: CABI Bioscience, Egham UK; and FH, The Farlow Herbarium, Harvard University, Cambridge, MA. For FH and K, the sample number includes our internal specimen number (top) and herbarium specimen number.
bSolanum spp host Latin names are italicized.
bSSR genotype were done using 12 plex microsatellites as defined from Saville et al, 202140. Empty cells indicate specimens were not genotyped previously by SSR genotyping. cND signifies that the sample did not match any known genotype and is a recombinant.
Across the entire P. infestans 1306 reference genome 521,699 SNPs were detected. The ratio of reference to alternate alleles at biallelic SNP sites was utilized to estimate the ploidy of P. infestans in each sample. Sixteen historic samples were classified as diploid and three (US0186943, H287, K126) were classified as triploid (Supplementary Fig. 1). All specimens genotyped as FAM-1 were diploid while US-1 genotypes were triploid. The ploidy assignment for the remaining ten samples was more challenging, with three appearing diploid with low confidence, one showing characteristics between triploid and diploid (IMI53089, US-1), and six remaining unresolved due to the limited number of high-quality biallelic SNPs.
Similarly, across the entire S. tuberosum SolTub3.0 reference genome, a total of 9,767,138 SNPs were detected. Although histograms of allelic fractions at biallelic SNP sites suggested tetraploid characteristics as expected of cultivated potato, the similarity was not statistically significant (Supplementary Fig. 2).
The analysis of enrichment sequencing data revealed that, on average, 30.15% of sequenced bait reads mapping to the P. infestans 1306 reference genome corresponded to the targeted bait regions, resulting in an average coverage depth of 77X across the RXLR genome (Supplementary Table 1A). Within the targeted RXLR genome sequenced a total of 5459 single nucleotide polymorphisms (SNPs), 423 insertions, and 262 deletions were observed, amounting to 6144 variants across 388,942 base pairs. The ratio of nonsynonymous to synonymous variants in the RXLR genome was 1.74. For Solanum spp., 22.73% of reads mapping to the SolTub3.0 genome aligned to the targeted R gene bait regions, with an average coverage depth of 248X (Supplementary Table 1B). Within the targeted R genome sequenced a total of 33,454 SNPs, 796 insertions, and 658 deletions were observed, resulting in 34,908 variants across 301,133 base pairs. The ratio of nonsynonymous to synonymous variants in the R genome was 1.90. In addition to the R genes, alleles at the StCDF1 gene and the plastid trnV-UAC/ndhC spacer indicate that a few historic potato samples were able to form tubers during long days while most samples have a Chilean origin (Supplemental Discussion 1).
Effectors and their associated R genes
Effectors and R genes were compared among four time periods (1845–1852, 1853–1883, 1884–1924 and 1925–1954). Note that Avr-blb1 was not successfully baited so its presence could not be tracked in our data set of P. infestans. Among the 11 other known cloned effectors (Table 1), all except Avr3b were detected in most of the historic samples at all time periods (Supplementary Fig. 3). Interestingly, Avr3b was first found in herbarium samples collected in 1948 (US0186972) and 1954 (US0186956). Notably, sample US0186956 collected in 1954 from Nicaragua was a FAM-1 genotype, while sample US0186972 collected from Mexico did not match FAM-1 or US-1 and was likely a recombinant since both clonal and sexual reproduction were reported in Mexico at that time (Table 2)34. Our findings suggest a Central American/Mexican origin of Avr3b.
While coverage of the seven well-described R genes showed consistency across samples, the depth of coverage varied compared to the RXLR genes (Supplementary Fig. 4). This variability was particularly pronounced in the last period of sampling (1925–1954).
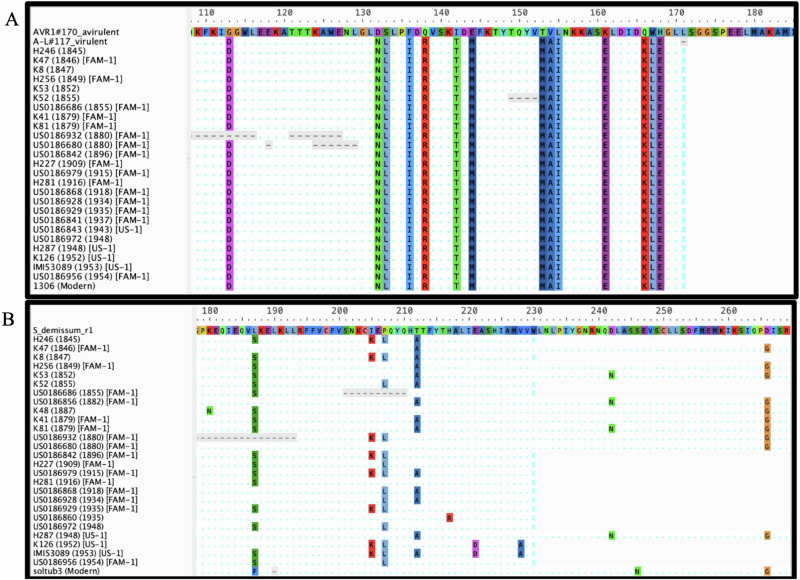
A lack of selection on the effector Avr1 by R1 is indicated by the fact that all samples, regardless of variation in the host R1 gene within the same samples, exhibited identical sequences of Avr1 (Fig. 2). All the samples of both FAM-1 and US-1 lineages had the AL virulent resistant breaking allele which is recognized by R1 (Fig. 2A).
Fig. 2. Amino acid alignment of Avr1 and R1.
A Amino acid alignment of a 100 amino acid excerpt of Avr1 from P. infestans. The first line is the Avr1 avirulent allele which is recognized by R1. The second line is the AL virulent, resistance-breaking allele which escapes detection by R1. The subsequent amino acid sequences are from high coverage samples analyzed in this study. All historic samples show a premature stop codon (indicated by x) leading to the virulent allele and are thus able to overcome R1. B Alignment of a 100 amino acid region excerpted from late blight resistance gene R1 cloned from Solanum demissum and R1 homologs in our samples and the reference genome. The functional S. demissum allele is shown on the top line, followed chronologically by the samples analyzed in this study. The final line is the R1 homolog in the reference SolTub3.0 genome. Several samples have premature stop codons (indicated by x) signifying a truncation of the R1 protein as compared to S. demissum.
Investigation of allelic variation in Avr3a reveals the Avr3aKI allele in all samples (Supplementary Fig. 5A). Therefore, the P. infestans identified in these herbarium samples would be sensitive to R3a mediated resistance and thus not able to overcome that host R gene. Alignment of the entire Avr2 gene showed all samples had the avirulent allele, with the exception of the 1948 Mexican sample US0186972 which had one polymorphism which corresponds to the virulent allele (Supplementary Fig. 5B), and thus able to overcome R2. Alignment of historic Solanum R2 genes compared to the S. demissum R2 allele which provides resistance to late blight, showed all samples had a premature stop codon except for K8 (Supplementary Fig. 5C).
Phylogenetic analysis was performed for the regions containing the described effector gene Avr8 and R gene R8 and the phylogenies was compared (Supplementary Fig. 6). The Avr8 alleles were differentiated based on mutations, and US-1 genotypes formed a distinct cluster. In contrast, alleles of R8 did not cluster together according to P. infestans genotype.
An ancestral recombination graph of Avr10 demonstrated that samples identified as the US-1 genotype formed a distinct cluster, and more recent samples (US0186956 and US0186972) shared a coalescent ancestor with the modern reference genome (1306) (Supplementary Fig. 7).
RXLR Genome Expansion
The RXLR genome of historic P. infestans displayed overall stability until the emergence of the US-1 lineage (in our sample set in1948), at which point genome expansion of numerous additional effectors was observed (Fig. 3). For FAM-1 lineages, an average of 664 covered effectors were found, compared to an average of 675 covered effectors for US-1 lineages. The coverage in the Solanum host R genome appeared to be more variable over time, with less apparent trends across different loci, a reflection of the different breeding efforts for S. tuberosum with time (Fig. 4). We assessed RXLR genome expansion by counting the number of RXLRs with coverage, normalized for overall coverage depth, and data indicated an expansion in the size of the RXLR genome over the period in which the herbarium specimens were collected (Supplementary Fig. 8A, R2 = 0.2182).
Fig. 3. Coverage of all baited RXLR loci in the Phytophthora infestans 1306 reference genome.
The center is a phylogeny showing the relationships between all of the baited RXLR loci. Cloned effectors are marked with a black circle on the phylogeny and a flag at the edge of the figure. The 25 samples with an average RXLR coverage depth greater than 5X are represented in concentric rings. The samples are presented chronologically, with the oldest herbarium sample (H246) in the innermost ring and the most recent specimen (US0186956) in the outermost ring. The major regions of effectome expansion during the spread of the US-1 lineage are boxed in black.
Fig. 4. Coverage of all baited R loci in the Solanum tuberosum SolTub3.0 reference genome.
The center is a phylogeny showing the relationships between all of the baited R loci. Cloned R genes are marked with a black circle on the phylogeny and a flag at the edge of the figure. Additionally, StCDF1, a locus related to potato day length toleration, is included and flagged. All 29 samples are represented in concentric rings. The samples are presented chronologically, with the oldest herbarium sample (H246) in the innermost ring and the most recent herbarium sample (US0186956) in the outermost ring.
Furthermore, the analysis revealed additional RXLR and R genes that did not align with the reference genome but were retrieved from the targeted baiting process. These unmapped reads were assembled into contigs and compared to the NCBI nucleotide database, revealing alignments to wild Solanum species and R/RXLR loci (Supplementary Fig. 8B–E). This suggests the presence of R and RXLR genes in historic samples that were not annotated in the modern reference genomes, with an increasing trend of gene alignments to wild Solanum species over time, coinciding with the introduction of wild Solanum species in breeding programs.
RXLR and R gene diversity
The well-described R genes exhibited varying numbers of segregating sites (137 to 293), and Tajima’s D values ranged from −0.712 (R8) to 0.663 (Rpi-vnt1) (Supplementary Table 2). As for the well-described RXLR genes, the number of segregating sites ranged from 0 to 22, and Tajima’s D values were negative or null for all loci except Avr10 (0.419). Several RXLR genes displayed little diversity, despite some samples predating the putative introduction of R genes. Other RXLR genes showed negative Tajima’s D values, indicating the potential influence of natural selection.
Nucleotide diversity statistics were also calculated for sample cohorts within each sampling period: Famine Period (1845–1852), Post Famine Period (1853–1883), Turn of the Century (1884–1924), and Plant Breeding Period (1925–1954), as well as overall (Supplementary Fig. 9). For RXLR genes, numbers of pairwise differences, nucleotide diversity and number of segregating sites were higher in the Plant Breeding Period than earlier time periods. Tajima’s D was consistently negative for the RXLR genome throughout the entire sampling period (all four eras), indicating an abundance of rare polymorphisms in the dataset. This could potentially be due to an expansion in population size after a bottleneck, or positive selection on the RXLR genome. Tajima’s D for the R genome was lowest during the Post Famine Period, indicating negative selection pressures during that time (Supplementary Fig. 9B).
Discussion
Simultaneous targeted sequencing of Solanum and Phytophthora infestans
Enrichment sequencing has been utilized for both Phytophthora infestans effectors and Solanum spp. R genes in previous studies35–38. However, this study combines enrichments of both the pathogen and host simultaneously in historic late blight specimens, allowing for the combined analysis of gene families in both species that evolved in response to each other.
One benefit of enrichment sequencing is that, by using baits to enrich the sequencing library for loci of interest, more sequencing resources can be allocated to the regions of the genome of interest. For example, RXLR genes constitute less than 1% of the Phytophthora infestans genome, yet, in this study, an average of 30.15% of all reads mapped to the reference genome of P. infestans to annotated RXLR genes (Supplementary Table 1)36. Ancient DNA samples are also challenging to work with due to their degraded and impure nature39. Ancient DNA is often highly fragmented, and in addition to the host and pathogen tissue in the sampled herbarium specimens, contaminants such as other microorganisms and bacteria are present39. Enrichment sequencing using small target baits improved our efficacy in obtaining the genes of interest. The samples used in this study were found to contain DNA from other microorganisms, but this DNA was present in lesser amounts compared to the targeted host and pathogen DNA and filtered out. In some samples, host leaf tissue is more prevalent than pathogen mycelial tissue. In a traditional sequencing experiment, this would result in many more reads aligning to the S. tuberosum reference rather than the P. infestans reference. However, the dual enrichment strategy employed here allowed us to capture targeted regions of both genomes successfully, even when host and pathogen were present in unequal amounts.
An increase in P. infestans ploidy since the Irish potato famine
Based on the allelic fractions at biallelic SNPs, the P. infestans genomes in the herbarium specimens in this study were a mixture of diploids and triploids. Older lineages belonging to the FAM-1 genotype were all diploid, while more recent mid-20th century US-1 lineages were triploid, consistent with previous published findings from more time-limited datasets34,40,41. For some lineages, determining ploidy was not straightforward, partly due to a lack of high-quality biallelic SNPs for certain samples. However, sample IMI53089 appeared to be between the triploid and diploid predicted states, which could be a result of aneuploidy (Supplementary Fig. 1). Recent work on chromosome-level assemblies of P. infestans indicates that aneuploids are very common29. More extensive sequencing coverage across all chromosomes could provide enough SNPs to determine ploidy at the chromosome level instead of the genome level. Additionally, the ploidy of Solanum spp. samples could not be confidently assigned. Perhaps sequencing a broader number of these historic host genomes might alleviate this challenge.
Historic effectors and their associated R genes show significant variation
Consistent with previous studies, we found many RXLR genes to be historically present in all samples33,34. Homologs of several R genes were also identified. Out of 11 well-described RXLR genes, only Avr3b was absent from some samples (Supplementary Fig. 3). Samples from the final time (1925–1954) displayed more variation in effector presence or absence than other time periods, with partial absence of Avr-blb2 in some samples (Supplementary Fig. 3). Seven well-described R genes were at least partially present in all samples. However, although there is coverage of these genes, implying the presence of some of the gene structures in historic samples, their functionality may differ due to allelic variation. For example, all Avr3a alleles in this study contained the KI allele, indicating the avirulent form of the effector and sensitivity to R3a resistance (Supplementary Fig. 5)42. By comparing the Avr2 region in all our samples to known virulent and avirulent alleles of Avr2, it is evident that all historic P. infestans in the samples tested were sensitive to R2 resistance, with the exception of one more recent sample from 1948, US0186972, which appears to have a combination of both alleles43. This may represent an intermediate state between resistance breaking and sensitivity for this gene. It is notable that John Niederhauser, a World Food Prize recipient, collected this sample when he worked in Mexico in 1948. Intensive germplasm screening for development of late blight-resistant potato cultivars was underway there for many years, fueling evolution in the P. infestans effector genome. Similarly, some R genes exhibited incomplete or partial coverage and were predicted nonfunctional. For example, the resistance gene R2 exhibited 154 segregating sites in samples we tested. The only sample that did not have a premature stop codon compared to the S. demissum R2 allele was sample K8, collected in Ireland in 184744. From this, we can infer that sample K8 may have had a functional R2 protein, while all other samples were missing a significant part of the protein after translation, impacting functionality45.
The most intriguing RXLR-R gene pair observed in this study was Avr1 and R1, a resistance gene from S. demissum (Fig. 2)19,46–48. None of the Solanum spp. sampled in this experiment had a complete copy of the R1 gene from S. demissum, with SNPs and deletions occurring throughout the gene (Fig. 2B). However, all P. infestans samples exhibited the resistance-breaking, virulent allele of Avr1 (Fig. 2A) This implies that although R1 resistance had not yet been deployed in cultivated potatoes during the Famine Period, the pathogen already possessed the ability to overcome the first resistance gene breeders would deploy later in the 20 th century. This could be attributed to the pathogen’s prior exposure to this resistance gene in wild hosts. Although none of the samples had the complete R1 resistance gene, they were also not clonally identical. We found high nucleotide diversity in the Solanum tuberosum loci we sequenced in this experiment, indicating that the Solanum germplasm grown during and shortly after the Famine was more diverse than previously reported2,14,16. The widespread prevalence of the virulent Avr1 allele in our sample set demonstrates that even if R1 resistance had been introduced earlier into cultivated potatoes, the pathogen would have been able to overcome such resistance quickly, at least in the case of R1. In fact, John Lindley reported in his species description paper of S. demissum in 1848 that S. demissum collected in Mexico at the time was susceptible to disease15.
Natural and artificial selection in the RXLR and R genomes
Phylogenies of the well-described RXLR and R genes exhibit correlations with other characteristics. For example, a phylogeny of Avr8 shows that P. infestans genotyped as US-1 grouped together, indicating the evolution of a shared allele of Avr8 in the US-1 genotypes. In contrast, a phylogeny inferred from R8 sequences did not exhibit the same pattern (Supplementary Fig. 6). This suggests that the evolved Avr8 allele unique to US-1 was successful on different Solanum host R8 alleles.
Ancestral recombination graphs (ARGs) of RXLR genes also provide evidence for the evolution of the RXLR genome. For example, the ARG of Avr10 shows no recombination events, with all nodes in the tree representing coalescent events back to a common ancestor (Supplementary Fig. 7). However, the US-1 lineages clustered together in this ARG, indicating that the differences between Avr10 in US-1 and FAM-1 were significant enough to cause divergence between these lineages. Many single SNPs were observed in Avr10 in the FAM-1 lineage over time and no recombination was observed between FAM-1 and US-1. Notably, the more recent US-1 lineages also grouped with the reference genome.
Interestingly, there is low variation in the presence and absence of R gene content between samples (Fig. 4). However, changes in the presence and absence of RXLR genes become apparent in more recent samples, particularly those collected from 1937 and onwards (Fig. 3). This coincides with the time suggested by plant breeder Glendinning, Salaman and others of the emergence of more structured potato breeding programs in the United States and Europe, aimed at combating late blight and other potato diseases14,19,20,22,49. It is possible that P. infestans RXLR genomes were evolving to overcome newly introduced R genes during this period, which would explain the changes in RXLR gene coverage. The more recent US-1 genotype also emerged during this time; but both the US-1 and FAM-1 lineages have undergone shifts in RXLR genome content in the mid-twentieth century33,50. It is interesting that potato breeder Redcliffe Salaman noted in his breeding trials: “…in the autumn of 1932 our hopes were considerably dashed when certain of the immune seedlings, then growing in the open, showed signs of being attacked by Blight. In 1936 the attack was more serious, but it occurred a month later than that affecting the field crops in the neighborhood. It was thought probable that our ‘immune’ stocks were succumbing to some new form or biotype of P. infestans”51. Salaman was observing infection by the US-1 genotype, that appeared in the field during that time.
When comparing the number of covered effectors across the sample set while controlling for coverage depth, it becomes evident that the size of the RXLR genome has increased over time, consistent with previous findings (Supplementary Fig. 8)33. This expansion could be attributed to the spread of additional P. infestans lineages, as well as the ongoing evolution of the RXLR genome in response to plant breeding efforts. Analysis of unmapped contigs from each sample also reveals the presence of additional R and RXLR genes not found in their respective reference genomes. Further characterization and description of these additional R and RXLR loci could provide insights into the historic pan-genome of both R and RXLR genes.
Following the Irish Potato Famine, there was significant interest in identifying potato varieties resistant to late blight, either through selection or importation of new germlasm3,20,22,49. These early efforts were succeeded by the establishment of the US Potato Breeding Program in 1929, along with other initiatives aimed at incorporating late blight resistance from wild relatives into cultivated potato20,22,49. The artificial selection conducted by potato breeders to enhance the potato R genome is evident in these historic genomes, which exhibit strong signs of R gene selection (dN/dS > 1). Consequently, natural selection appears to have driven a high rate of nonsynonymous to synonymous substitutions in the P. infestans RXLR genome during this same period. This suggests that reports of P. infestans rapidly overcoming introduced resistance genes may be attributed, at least in part, to selection among effector genes. Homologs of several early-discovered R genes (R1, R2, and R3b) in our samples demonstrate high nucleotide diversity and many segregating sites, highlighting the allelic variety among the Solanum genome in our samples (Supplementary Table 2).
In this study, we have shown that both the RXLR genome and R genome have undergone significant changes since the Irish Famine, both from artificial and natural selection. Changes in the R genome have been reflected in the RXLR genome, such as the polymorphisms observed in Avr2. We could not do gene function experiments with historic genomes. Moving forward, it will be crucial to characterize the functionality and identify the causal mutations for avirulence or virulence in more modern-day P. infestans effector genes to better understand the historic RXLR genome. Given the limited characterization of only a few RXLR and R genes, it remains challenging to interpret the significance of historic mutations. Additionally, our sample set is constrained to what was collected historically and spans a wide range of locations and collection years, limiting our understanding of changes in localized host and pathogen populations. However, exploring these samples has provided a snapshot into the host pathogens arms race that has characterized late blight resistance breeding for the last century and a half after the Irish Famine.
Methods
Sample selection and DNA extraction
Twenty-nine historic herbarium specimens were selected for enrichment sequencing (Table 2). Each specimen consisted of dried leaf tissue of a Solanum host species (mostly S. tuberosum) that was infected with Phytophthora infestans. Specimens were collected and deposited in various herbaria from 1845–1954 and were chosen for this study based on their original collection date and host.
DNA extraction was performed on each sample using a QIAGEN DNeasy Plant Mini Kit following the manufacturer’s instructions (Qiagen, Valencia, CA). All DNA extractions were conducted in a dedicated laboratory solely used for ancient P. infestans DNA work, with no prior use for modern P. infestans samples. Storage of ancient samples and laboratory supplies for working with ancient samples were kept separate from modern P. infestans samples in an ancient DNA lab. Every precaution was taken to prevent cross-contamination among the ancient DNA samples, and there was no physical contact between specimens.
After completion of the DNA extractions, DNA quantity and quality were assessed using a QUBIT machine with the High Sensitivity Double-Stranded DNA Kit following the manufacturer’s instructions (Invitrogen, Waltham, MA). In cases where the DNA concentration was too low for sequencing, extractions were repeated. A target of 1 µg of DNA was set as the baseline for all samples. For each historic herbarium specimen, at least two DNA extractions were combined to increase the DNA quantity.
Enrichment sequencing
Two bait libraries were designed based on the genes of interest (Daicel Arbor Biosciences). For the pathogen, Phytophthora infestans, a list of known RXLR genes was compiled from the T30-4 reference genome as well as a handful of other published RXLR genes28. From this list of 568 known RXLR reference genes, 8105 bait sequences, each approximately 80 nucleotides in length, were designed for RXLR target capture (https://github.com/allisoncoomber/targeted_sequencing_baits). For the host, Solanum spp., a list of 117 R genes for late blight resistance (and one for day length tolerance, StCDF1) was compiled from the SolTub3.0 genome and other published resources involving potato wild relatives. Using these 117 input R genes, 8647 baits, each 80 nucleotides in length, were generated (https://github.com/allisoncoomber/targeted_sequencing_baits). Both sets of baits were designed to match the target input genes (listed RXLRs and R genes) as well as any highly similar loci. The bait design focused on the regions of interest, excluding repeat-rich regions. Additionally, a Blast search was conducted against the relevant genomes to ensure that there were not a high number of off-target alignment sites.
All 29 samples were enriched with both bait libraries (Fig. 1). This allowed for the concentration of both Phytophthora infestans RXLR genes and Solanum spp. R genes in the sequencing library for each sample. Samples were sequenced on a NovaSeq with 150 bp paired end reads by Arbor Biosciences myReads (Ann Arbor, MI).
Read trimming and alignment
Raw reads received in FASTQ format were trimmed using TrimGalore, a wrapper around Cutadapt and FastQC52–54. TrimGalore was run in –paired mode, ensuring that both reads in a pair met all requirements. Adapters were removed along with reads less than 20 base pairs in length. A short 20 basepair minimum length was chosen because of the highly fragmented nature of the ancient DNA in these samples. A quality cutoff of 20 Phred score was used in trimming with the “—nextseq” flag within Cutadapt to prevent the G overcalling that can occur with the NovaSeq platform. FastQC results for the trimmed reads were collated with MultiQC and manually reviewed55.
The trimmed reads were then aligned to both reference genomes. For the pathogen, Phytophthora infestans assembly Pinf1306_UCR_1 (PRJNA868814) was used as the reference genome29. For the host, Solanum tuberosum assembly SolTub_3.0 (PRJNA63145) was used as the reference genome56. The BWA-MEM algorithm from the Burrow-Wheelers Aligner was employed for aligning the reads to each respective genome57. From this point onward, the alignments were divided into two cohorts: one containing the pathogen alignments and the other containing the host alignments. Within each cohort, all 29 samples were kept separate. Subsequent steps were conducted in parallel for each cohort.
Variant calling
To appropriately group reads for downstream variant calling, read groups were added to each alignment using Picard AddReadGroups58. The read groups specified the sample, experiment, and library for each alignment. All alignments were then sorted using SAMTools59. Picard MarkDuplicates was then used to identify any PCR duplicates from library preparation58. Any duplicates that were identified as derived from amplification and not biological duplicates were ignored during variant calling.
The program mapDamage was utilized to rescale the quality scores of all alignments based on post-mortem DNA damage patterns60. This limits the DNA damage associated with ancient samples from influencing the alignments by reducing the quality score of the alignment where post-mortem damage is expected.
Haplotypes were called using GATK HaplotypeCaller in ERC mode to produce GVCF (Genomic Variant Call Format) files containing genotype likelihoods61. All GCVF haplotype call files were combined using GATK GenomicsDBI to build an indexed database. Joint genotyping for each cohort was then performed with GATK GenotypeGVCFs, resulting in one combined VCF file of genotypes for each cohort (pathogen and host-aligned reads, respectively).
Each resultant VCF file was split into separate files for INDELs and SNPs using GATK SelectVariants. INDEL and SNP variants for both cohorts were individually filtered using GATK VariantFiltration to retain only high-quality variants. Filtered INDEL and SNP VCF files were then combined, removing, or collapsing any overlapping INDELs. Finally, variants were annotated using custom-built databases for each reference with SNPEff62.
Coverage analysis
To assess the success of the bait libraries in retrieving targeted DNA from these degraded samples, the SAMTools suite was used to evaluate coverage. SAMTools view, flagstat, depth, and bedcov were executed for each rescaled, deduplicated alignment file to determine the number of reads from the sequencing libraries that aligned to each reference genome. BED files listing the regions of the genomes containing the target genes were utilized in combination with SAMTools to determine the number of reads aligning to the baited loci.
The BEDTools suite was employed to generate files listing the coverage depth at each site from the P. infestans alignments for regions of all samples corresponding to well-described RXLR genes63. The same process was repeated with the S. tuberosum alignments, but this time focusing on R genes instead of RXLR genes. The depths at each position within these important genes were plotted using a custom Python script.
Additional BED files were generated corresponding to all known R genes in S. tuberosum and all known RXLR genes in P. infestans using genome annotations and conducting BLAST searches for additional homologs. BEDTools was used to generate coverage depth files for all known R genes and RXLR genes, respectively, for each sample. A custom Python script was written to summarize these files and generate circular coverage diagrams based on the percent coverage of each R gene or RXLR gene.
Assembly and characterization of unmapped reads
We sought to investigate if there were RXLR genes or R genes which were present in these historic samples but not the modern reference genome. We expected reads from such genes would be enriched and sequenced by our experimental design, but not mapped to the reference genome. Therefore, reads that did not align to either the host or the pathogen reference genome were collected for each sample. These reads were assembled into contigs using SPAdes de novo assembler64. A Blast search was performed to compare these contigs to the NCBI nucleotide database using local Blast65. The Blast results were manually filtered to identify the closest match to each contig. Matches were summarized at the genus level based on descriptions. If the genus was Phytophthora or Solanum, the match was further examined to determine if the region of interest was a potential RXLR or R gene based on the description. For all contigs where the genus was a non-target organism, a taxonomic pie chart was created in Microsoft Excel. The number of unmapped contigs in each sample with specific characteristics (all unmapped, matching wild Solanum, matching RXLR genes, and matching R genes) were summarized into plots using a custom Python script.
Ploidy analysis
The Phytophthora infestans data from each sample was analyzed to determine the ploidy of the pathogen isolate infecting the leaf in each sample. GATK VariantsToTable was used to extract a table of all high-quality SNPs. A custom Python script was written to filter out high coverage, high quality, biallelic SNPs. Histograms of allelic fractions for each polymorphism at all high coverage biallelic SNP sites were then plotted, with fitted lines.
The program nQuire was also employed to estimate the ploidy of each P. infestans sample based on the frequency distribution at biallelic sites66. The nQuire models and ploidy estimates were compared with the histograms generated by the custom Python script to determine ploidy.
The same process was repeated for the Solanum spp. data for each sample to evaluate host ploidy.
Selection analysis
Alternate FASTAs were generated for all samples for all loci in the R genome, RXLR genome, and Solanum spp. gene StCDF1. The annotated, filtered variants previously called were used by BCFTools consensus to apply any changes to the reference genome for each sample. Low coverage masks were generated for each sample to replace any poorly covered regions with “N.”
A total of 709 RXLR genes and 137 R genes (including StCDF1) were included in selection analysis. DendroPY was used to calculate average number of pairwise differences, nucleotide diversity, number of segregating sites, Tajima’s D, and Watterson’s Theta for each locus in both datasets67. A custom Python script was developed to separate the samples by collection date and plot the resulting selection statistics. SNPEff was also run on reduced VCF files containing only variants in the RXLR genome and R genome datasets for further analysis. Selection statistics for the well described R genes and RXLR genes were calculated and summarized across all time periods.
The selection tests used included: average number of pairwise differences, nucleotide diversity, Tajima’s D, number of segregating sites, and Watterson’s theta. Average number of pairwise differences calculates the average number of differences in nucleotide pairs between sequences in a population sample. It provides insight into the genetic diversity within a population, where a higher number indicates greater diversity. Nucleotide diversity is also a measure of genetic variation, measuring the average number of nucleotide differences per site between two DNA sequences randomly chosen from a population. Tajima’s D is a statistical test used to detect departures from neutrality in DNA sequence data. Positive Tajima’s D values may indicate balancing selection or population subdivision, while negative values may suggest purifying selection or population expansion. Number of segregating sites measures the number of variable sites in a population sample, where different alleles are present at a particular nucleotide position. Watterson’s Theta estimates the population mutation rate based on the number of segregating sites. This provides an estimate of the long-term effective population size and mutation rate in a population.
Additionally, alternate FASTA files for specific loci of interest, such as well-described RXLR genes, R genes, and StCDF1, were visualized. These sequences were aligned against modern copies of these genes to investigate potential polymorphisms using Muscle68. Alignments were visualized in AliView69.
RXLR genome size
To estimate the size of the RXLR genome for each sample, the differences in coverage were controlled. Samples with less than 10X coverage for the RXLR genome were excluded from the analysis. The remaining samples were down sampled to the same level of coverage as the lowest coverage sample (13.55X). The number of covered loci in the RXLR genome in each down-sampled sample was summed and plotted.
Phylogenetics
The alternate FASTA files were aligned and converted into PHYLIP format for each of the well-described RXLR effectors and R genes (Table 1). RAxML with 1000 bootstrap replicates was used to infer maximum likelihood trees from each of these alignments70. The resulting phylogenetic trees were visualized with Mesquite71. The alternate FASTA files of all genes in the R genome and RXLR genome were also generated, aligned, and concatenated into partitioned PHYLIP alignments. RAxML with 1000 bootstrap replicates was used to infer phylogenies from these alignments as well70. The phylogenetic trees were visualized and colored with Mesquite71.
To compare the topologies of the well-described RXLR and R gene pairs, Visual TreeCmp was used to generate statistics comparing the two trees in each case72.
Ancestral recombination graphs
From the generated alternate FASTA files, the homolog of RXLR gene Avr10 was extracted from all high coverage samples as well as the reference genome. This collection of Avr10 homologs was used as input for the program kwarg to generate ancestral recombination graphs of this locus73. The kwarg analysis used 1000 replicates and the minimum recombinant tree was visualized using Graphviz74.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary information
Acknowledgements
Funding for salaries for AC provided by NSF AgBioFews Training Grant Number, 2018-1966, JR Co-PI and salaries for AS, sequencing and supplies funded by Grip4PSI Grant Number 5572998, JR Co-PI, respectively. Additional research funds for AC work from a Seed Grant from the Triangle Center for Evolutionary Medicine and Grip4PSI Grant Number 5572998 JR Co-PI. Appreciation is expressed to Dr. Ross Whetten, Dr. Justin Whitehill, Dr. David Rasmussen, Dr. Jeff Thorne and Dr. Ignazio Carbone from NC State University, Ingo Hein, James Hutton Institute and Dr. Michael Martin, Norwegian Institute of Science and Technology for advice on the computational methods and workflows used to analyze data in this work.
Author contributions
A.C. and J.R. conceived the idea for the work. A.C., A.S., and J.R. edited and authored the paper. A.C. and A.S. performed the experiments. A.C. developed computational workflows and analyzed the data. J.R. is a senior author, and A.C. is the first author of the paper. Reprints and permissions information is available at www.nature.com/reprints. Readers are welcome to comment on the online version of the paper.
Peer review
Peer review information
Nature Communications thanks Guillaume Besnard, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Data availability
The sequence datasets generated in this study has been deposited in the NCBI database under the Sequence Read Archive project PRJNA1035512 https://www.ncbi.nlm.nih.gov/sra. The GitHub link contains sequences of the baits used. https://github.com/allisoncoomber/targeted_sequencing_baits. A source file of data is included here.
Code availability
The software used to analyze the data is cited in “Methods” section and reference lists and no custom code was written.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-024-50749-4.
References
- 1.Ristaino, J. B. Tracking historic migrations of the Irish potato famine pathogen, Phytophthora infestans. Microbes Infect.4, 1369–1377 (2002). 10.1016/S1286-4579(02)00010-2 [DOI] [PubMed] [Google Scholar]
- 2.Bourke, A. ‘The Visitation Of God?‘ the Potato And The Great Irish Famine. (Lilliput Press Ltd, 1993).
- 3.De Bary, A. Researches into the nature of the potato fungus, Phytophthora infestans. J. Bot. Paris14, 105–126 (1876). [Google Scholar]
- 4.Berkeley, M. J. Observations, botanical and physiological, on the potato murrain. J. Hort. Soc. 1, 9–34 (1846).
- 5.Fry, W. E. et al. Five reasons to consider phytophthora infestans a reemerging pathogen. Phytopathology105, 966–981 (2015). 10.1094/PHYTO-01-15-0005-FI [DOI] [PubMed] [Google Scholar]
- 6.Ristaino, J. B., Cooke, D. E., Acuña, I. & Muñoz, M. The Threat of Late Blight to Global Food Security. Emerging Plant Diseases and Global Food Security (eds, Ristaino, J. and Records, A.) 101–132 (American Phytopathological Society Press, 2020).
- 7.Nowicki, M., Foolad, M. R., Nowakowska, M. & Kozik, E. U. Potato and tomato late blight caused by Phytophthora infestans: an overview of pathology and resistance breeding. Plant Dis.96, 4–17 (2012). 10.1094/PDIS-05-11-0458 [DOI] [PubMed] [Google Scholar]
- 8.Leesutthiphonchai, W., Vu, A. L., Ah-Fong, A. M. V. & Judelson, H. S. How does Phytophthora infestans evade control efforts? modern insight into the late blight disease. Phytopathology108, 916–924 (2018). 10.1094/PHYTO-04-18-0130-IA [DOI] [PubMed] [Google Scholar]
- 9.Paluchowska, P., Śliwka, J. & Yin, Z. Late blight resistance genes in potato breeding. Planta255, 127 (2022). 10.1007/s00425-022-03910-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andersen, E. J., Ali, S., Byamukama, E., Yen, Y. & Nepal, M. P. Disease resistance mechanisms in plants. Genes9, 339 (2018). 10.3390/genes9070339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vleeshouwers, V. G. et al. Understanding and exploiting late blight resistance in the age of effectors. Annu. Rev. Phytopathol.49, 507–531 (2011). 10.1146/annurev-phyto-072910-095326 [DOI] [PubMed] [Google Scholar]
- 12.Wawra, S. et al. The RxLR motif of the host targeting effector AVR3a of Phytophthora infestans is cleaved before secretion. Plant Cell29, 1184–1195 (2017). 10.1105/tpc.16.00552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flor, H. Inheritance of pathogenicity in Melampsora lini. Phytopathology32, 653–669 (1942). [Google Scholar]
- 14.Glendinning, D. R. Potato introductions and breeding up to the early 20th century. N. Phytologist94, 479–505 (1983). 10.1111/j.1469-8137.1983.tb03460.x [DOI] [Google Scholar]
- 15.Lindley, J. Notes on the wild potato. J. R. Hort. Soc.3, 65–72 (1848). [Google Scholar]
- 16.Salaman, R. N. Potato Varieties. (Cambridge University Press, 1926).
- 17.Powell, W., Baird, E., Duncan, N. & Waugh, R. Chloroplast DNA variability in old and recently introduced potato cultivars. Ann. Appl. Biol.123, 403–410 (1993). 10.1111/j.1744-7348.1993.tb04102.x [DOI] [Google Scholar]
- 18.Hosaka, K. Distribution of the 241 bp deletion of chloroplast DNA in wild potato species. Am. J. Pot. Res.79, 119–123 (2002). 10.1007/BF02881520 [DOI] [Google Scholar]
- 19.Black, W. XVII—inheritance of resistance to blight (Phytophthora infestans) in potatoes: inter-relationships of genes and strains. Proc. R. Soc. Edinb., Sect. B: Bio. Sci.64, 312–352 (1951). [DOI] [PubMed] [Google Scholar]
- 20.Reddick, D. Problems in breeding for disease resistance. Chron. Botanica6, 73–77 (1940). [Google Scholar]
- 21.Fry, W. Phytophthora infestans: the plant (and R gene) destroyer. Mol. Plant Pathol.9, 385–402 (2008). 10.1111/j.1364-3703.2007.00465.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reddick, D. & Mills, W. Building up virulence in Phytophthora infestans. Am. Pot. J.15, 29–34 (1938). 10.1007/BF02895549 [DOI] [Google Scholar]
- 23.Stewart, H., Bradshaw, J. & Pande, B. The effect of the presence of R‐genes for resistance to late blight (Phytophthora infestans) of potato (Solanum tuberosum) on the underlying level of field resistance. Plant Pathol.52, 193–198 (2003). 10.1046/j.1365-3059.2003.00811.x [DOI] [Google Scholar]
- 24.Stefańczyk, E., Sobkowiak, S., Brylińska, M. & Śliwka, J. Expression of the potato late blight resistance gene Rpi-phu1 and Phytophthora infestans effectors in the compatible and incompatible interactions in potato. Phytopathology107, 740–748 (2017). 10.1094/PHYTO-09-16-0328-R [DOI] [PubMed] [Google Scholar]
- 25.Ghislain, M. et al. Stacking three late blight resistance genes from wild species directly into African highland potato varieties confers complete field resistance to local blight races. Plant Biotech. J.17, 1119–1129 (2019). 10.1111/pbi.13042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Varshney, R. K. & Tuberosa, R. Translational genomics in crop breeding for biotic stress resistance: an introduction. Transl. Genomics Crop Breed.: |Biot. Stress1, 1–9 (2013). [Google Scholar]
- 27.Win, J. et al. Adaptive evolution has targeted the C-terminal domain of the RXLR effectors of plant pathogenic oomycetes. Plant Cell19, 2349–2369 (2007). 10.1105/tpc.107.051037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haas, B. J. et al. Genome sequence and analysis of the Irish potato famine pathogen Phytophthora infestans. Nature461, 393–398 (2009). 10.1038/nature08358 [DOI] [PubMed] [Google Scholar]
- 29.Matson, M. E., Liang, Q., Lonardi, S. & Judelson, H. S. Karyotype variation, spontaneous genome rearrangements affecting chemical insensitivity, and expression level polymorphisms in the plant pathogen Phytophthora infestans revealed using its first chromosome-scale assembly. PLoS Pathog.18, e1010869 (2022). 10.1371/journal.ppat.1010869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.May, K. J. & Ristaino, J. B. Identity of the mtDNA haplotype (s) of Phytophthora infestans in historical specimens from the Irish potato famine. Mycol. Res.108, 471–479 (2004). 10.1017/S0953756204009876 [DOI] [PubMed] [Google Scholar]
- 31.Ristaino, J. B. The importance of mycological and plant herbaria in tracking plant killers. Front. Ecol. Evol.7, 521 (2020). 10.3389/fevo.2019.00521 [DOI] [Google Scholar]
- 32.Ristaino, J. B., Groves, C. T. & Parra, G. R. PCR amplification of the Irish potato famine pathogen from historic specimens. Nature411, 695–697 (2001). 10.1038/35079606 [DOI] [PubMed] [Google Scholar]
- 33.Martin, M. D. et al. Reconstructing genome evolution in historic samples of the Irish potato famine pathogen. Nat. Commun.4, 2172 (2013). 10.1038/ncomms3172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yoshida, K. et al. The rise and fall of the Phytophthora infestans lineage that triggered the Irish potato famine. elife2, e00731 (2013). 10.7554/eLife.00731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Witek, K. et al. Accelerated cloning of a potato late blight–resistance gene using RenSeq and SMRT sequencing. Nat. Biotechnol.34, 656–660 (2016). 10.1038/nbt.3540 [DOI] [PubMed] [Google Scholar]
- 36.Thilliez, G. J. et al. Pathogen enrichment sequencing (PenSeq) enables population genomic studies in oomycetes. N. Phytologist221, 1634–1648 (2019). 10.1111/nph.15441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lin, X. et al. Identification of Avramr1 from Phytophthora infestans using long read and cDNA pathogen‐enrichment sequencing (PenSeq). Mol. Plant Pathol.21, 1502–1512 (2020). [DOI] [PMC free article] [PubMed]
- 38.Jupe, F. et al. Resistance gene enrichment sequencing (Ren Seq) enables reannotation of the NB‐LRR gene family from sequenced plant genomes and rapid mapping of resistance loci in segregating populations. Plant J.76, 530–544 (2013). 10.1111/tpj.12307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Orlando, L. et al. Ancient DNA analysis. Nat. Rev. Meth. Prim.1, 14 (2021). 10.1038/s43586-020-00011-0 [DOI] [Google Scholar]
- 40.Saville, A. C. & Ristaino, J. B. Global historic pandemics caused by the FAM-1 genotype of Phytophthora infestans on six continents. Scien. Rep.11, 12335 (2021). 10.1038/s41598-021-90937-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Knaus, B. J., Tabima, J. F., Shakya, S. K., Judelson, H. S. & Grünwald, N. J. Genome-wide increased copy number is associated with emergence of dominant clones of the irish potato famine pathogen Phytophthora infestans. mBio.11, e00326 (2020). [DOI] [PMC free article] [PubMed]
- 42.Bos, J. I., Chaparro-Garcia, A., Quesada-Ocampo, L. M., Gardener, B. B. M. & Kamoun, S. Distinct amino acids of the Phytophthora infestans effector AVR3a condition activation of R3a hypersensitivity and suppression of cell death. Mol. Plant-Microbe Inter.22, 269–281 (2009). 10.1094/MPMI-22-3-0269 [DOI] [PubMed] [Google Scholar]
- 43.Yang, L.-N. et al. The Phytophthora infestans AVR2 effector escapes R2 recognition through effector disordering. Mol. Plant-Microbe Inter.33, 921–931 (2020). 10.1094/MPMI-07-19-0179-R [DOI] [PubMed] [Google Scholar]
- 44.Lokossou, A. A. et al. Exploiting knowledge of R/Avr genes to rapidly clone a new LZ-NBS-LRR family of late blight resistance genes from potato linkage group IV. Mol. Plant-Microbe Inter.22, 630–641 (2009). 10.1094/MPMI-22-6-0630 [DOI] [PubMed] [Google Scholar]
- 45.Black, W., Mastenbroek, C., Mills, W. & Peterson, L. C. A proposal for an international nomenclature of races of Phytophthora infestans and of genes controlling immunity in Solanum demissum derivatives. Euphytica2, 173–179 (1953). 10.1007/BF00053724 [DOI] [Google Scholar]
- 46.Malcolmson, J. F. & Black, W. New R genes in Solanum demissum Lindl. and their complementary races of Phytophthora infestans (Mont.) de Bary. Euphytica15, 199–203 (1966). 10.1007/BF00022324 [DOI] [Google Scholar]
- 47.Du, Y. et al. RXLR effector diversity in Phytophthora infestans isolates determines recognition by potato resistance proteins; the case study AVR1 and R1. Stud. Mycol.89, 85–93 (2018). 10.1016/j.simyco.2018.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reddick, D. Frost-tolerant and blight-resistant Potatoes. Phytopathology20, 987–991 (1930).
- 49.Martin, M. D. et al. Genomic characterization of a South American Phytophthora hybrid mandates reassessment of the geographic origins of Phytophthora infestans. Mol. Biol. Evol.33, 478–491 (2016). 10.1093/molbev/msv241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Salaman, R. Potato Varieties, Past Present and Future. The History and Social Influence of the Potato. (Cambridge Univ. Press, 1949)
- 51.Ames, M. & Spooner, D. M. DNA from herbarium specimens settles a controversy about origins of the European potato. Am. J. Bot.95, 252–257 (2008). 10.3732/ajb.95.2.252 [DOI] [PubMed] [Google Scholar]
- 52.Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet. J.17, 10–12 (2011). 10.14806/ej.17.1.200 [DOI] [Google Scholar]
- 53.Krueger, F. Trim galore. A wrapper tool around cutadapt and fastqc to consistently apply quality and adapter trimming to FastQ fileshttps://github.com/FelixKrueger/TrimGalore (2015).
- 54.Andrews, S. FastQC: a quality control tool for high throughput sequence data. http://www.bioinformatics.babraham.ac.uk/projects/fastqc (2010).
- 55.Ewels, P., Magnusson, M., Lundin, S. & Käller, M. MultiQC: summarize analysis results for multiple tools and samples in a single report. Bioinformatics32, 3047–3048 (2016). 10.1093/bioinformatics/btw354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xu, X. et al. Genome sequence and analysis of the tuber crop potato. Nature475, 189–195 (2011). 10.1038/nature10158 [DOI] [PubMed] [Google Scholar]
- 57.Li, H. Aligning sequence reads, clone sequences and assembly contigs with BWA-MEM. arXiv:1303.3997 (2013).
- 58.Broad Institute. Picard toolkit. Broad Institute, GitHub repository. https://broadinstitute.github.io/picard/ (2019).
- 59.Danecek, P. et al. Twelve years of SAMtools and BCFtools. Gigascience10, giab008 (2021). 10.1093/gigascience/giab008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jónsson, H., Ginolhac, A., Schubert, M., Johnson, P. L. & Orlando, L. mapDamage2. 0: fast approximate Bayesian estimates of ancient DNA damage parameters. Bioinformatics29, 1682–1684 (2013). 10.1093/bioinformatics/btt193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Van der Auwera, G. A. & O’Connor, B. D. Genomics In The Cloud: Using Docker, GATK, and WDL in Terra. (O’Reilly Media, 2020).
- 62.Cingolani, P. et al. A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: SNPs in the genome of Drosophila melanogaster strain w1118; iso-2; iso-3. fly6, 80–92 (2012). 10.4161/fly.19695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Quinlan, A. R. BEDTools: the swiss‐army tool for genome feature analysis. Curr. Protoc. Bioinforma.47, 11.12. 11–11.12. 34 (2014). 10.1002/0471250953.bi1112s47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Prjibelski, A., Antipov, D., Meleshko, D., Lapidus, A. & Korobeynikov, A. Using SPAdes De novo assembler. Curr. Protoc. Bioinforma.70, e102 (2020). 10.1002/cpbi.102 [DOI] [PubMed] [Google Scholar]
- 65.Camacho, C. et al. BLAST+: architecture and applications. BMC Bioinforma.10, 421 (2009). 10.1186/1471-2105-10-421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Weiß, C. L., Pais, M., Cano, L. M., Kamoun, S. & Burbano, H. A. nQuire: a statistical framework for ploidy estimation using next generation sequencing. BMC Bioinforma.19, 1–8 (2018). 10.1186/s12859-018-2128-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sukumaran, J. & Holder, M. T. DendroPy: a python library for phylogenetic computing. Bioinformatics26, 1569–1571 (2010). 10.1093/bioinformatics/btq228 [DOI] [PubMed] [Google Scholar]
- 68.Edgar, R. C. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 32, 1792–1797 (2004). 10.1093/nar/gkh340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Larsson, A. AliView: a fast and lightweight alignment viewer and editor for large datasets. Bioinformatics30, 3276–3278 (2014). 10.1093/bioinformatics/btu531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stamatakis, A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics30, 1312–1313 (2014). 10.1093/bioinformatics/btu033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Maddison, W. & Maddison, D. Mesquite 2. a modular system for evolutionary analysis. Version 2.1. https://api.semanticscholar.org/CorpusID:116015395 (2007).
- 72.Goluch, T., Bogdanowicz, D. & Giaro, K. Visual treeCmp: comprehensive comparison of phylogenetic trees on the web. Meth. Ecol. Evol.11, 494–499 (2020). 10.1111/2041-210X.13358 [DOI] [Google Scholar]
- 73.Ignatieva, A., Lyngsø, R. B., Jenkins, P. A. & Hein, J. KwARG: parsimonious reconstruction of ancestral recombination graphs with recurrent mutation. Bioinformatics37, 3277–3284 (2021). 10.1093/bioinformatics/btab351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ellson, J., Gansner, E. R., Koutsofios, E., North, S. C. & Woodhull, G. Graphviz and dynagraph—static and dynamic graph drawing tools. Graph drawing software, 127–148 (2004).
- 75.Ballvora, A. et al. The R1 gene for potato resistance to late blight (Phytophthora infestans) belongs to the leucine zipper/NBS/LRR class of plant resistance genes. Plant J.30, 361–371 (2002). 10.1046/j.1365-313X.2001.01292.x [DOI] [PubMed] [Google Scholar]
- 76.Vleeshouwers, V. G. et al. Effector genomics accelerates discovery and functional profiling of potato disease resistance and Phytophthora infestans avirulence genes. PLoS one3, e2875 (2008). 10.1371/journal.pone.0002875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gilroy, E. M. et al. Presence/absence, differential expression and sequence polymorphisms between PiAVR2 and PiAVR2‐like in Phytophthora infestans determine virulence on R2 plants. N. Phytologist191, 763–776 (2011). 10.1111/j.1469-8137.2011.03736.x [DOI] [PubMed] [Google Scholar]
- 78.Huang, S. et al. Comparative genomics enabled the isolation of the R3a late blight resistance gene in potato. Plant J.42, 251–261 (2005). 10.1111/j.1365-313X.2005.02365.x [DOI] [PubMed] [Google Scholar]
- 79.Armstrong, M. R. et al. An ancestral oomycete locus contains late blight avirulence gene Avr3a, encoding a protein that is recognized in the host cytoplasm. Proc. Nat. Acad. Sci. USA102, 7766–7771 (2005). 10.1073/pnas.0500113102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li, G. et al. Cloning and characterization of R3b; members of the R3 superfamily of late blight resistance genes show sequence and functional divergence. Mol. Plant-Microbe Inter.24, 1132–1142 (2011). 10.1094/MPMI-11-10-0276 [DOI] [PubMed] [Google Scholar]
- 81.Rietman, H. et al. Qualitative and quantitative late blight resistance in the potato cultivar Sarpo Mira is determined by the perception of five distinct RXLR effectors. Mol. Plant-Microbe Inter.25, 910–919 (2012). 10.1094/MPMI-01-12-0010-R [DOI] [PubMed] [Google Scholar]
- 82.van Poppel, P. M., Huigen, D. J. & Govers, F. Differential recognition of Phytophthora infestans races in potato R4 breeding lines. Phytopathology99, 1150–1155 (2009). 10.1094/PHYTO-99-10-1150 [DOI] [PubMed] [Google Scholar]
- 83.Van Poppel, P. M. et al. The Phytophthora infestans avirulence gene Avr4 encodes an RXLR-dEER effector. Mol. Plant-Microbe Inter.21, 1460–1470 (2008). 10.1094/MPMI-21-11-1460 [DOI] [PubMed] [Google Scholar]
- 84.Vossen, J. H. et al. The Solanum demissum R8 late blight resistance gene is an Sw-5 homologue that has been deployed worldwide in late blight resistant varieties. Theor. Appl. Genet.129, 1785–1796 (2016). 10.1007/s00122-016-2740-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bradshaw, J. E., Bryan, G. J., Lees, A. K., McLean, K. & Solomon-Blackburn, R. M. Mapping the R10 and R11 genes for resistance to late blight (Phytophthora infestans) present in the potato (Solanum tuberosum) R-gene differentials of Black. Theor. Appl. Genet.112, 744–751 (2006). 10.1007/s00122-005-0179-9 [DOI] [PubMed] [Google Scholar]
- 86.van der Vossen, E. A. et al. The Rpi‐blb2 gene from Solanum bulbocastanum is an Mi‐1 gene homolog conferring broad‐spectrum late blight resistance in potato. Plant J.44, 208–222 (2005). 10.1111/j.1365-313X.2005.02527.x [DOI] [PubMed] [Google Scholar]
- 87.Oh, S.-K. et al. In planta expression screens of Phytophthora infestans RXLR effectors reveal diverse phenotypes, including activation of the Solanum bulbocastanum disease resistance protein Rpi-blb2. Plant Cell21, 2928–2947 (2009). 10.1105/tpc.109.068247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Foster, S. J. et al. Rpi-vnt1. 1, a Tm-22 homolog from Solanum venturii, confers resistance to potato late blight. Mol. Plant-microbe Inter.22, 589–600 (2009). 10.1094/MPMI-22-5-0589 [DOI] [PubMed] [Google Scholar]
- 89.Pel, M. A. Mapping, isolation and characterization of genes responsible for late blight resistance in potato. (Wageningen University and Research, 2010).
- 90.Ellson, J., Gansner, E. R., Koutsofios, E., North, S. C. & Woodhull, G. Graphviz and Dynagraph — Static and Dynamic Graph Drawing Tools. In Graph Drawing Software. Mathematics and Visualization (eds Jünger, M. & Mutzel, P.) 127–148 (Springer, 2004).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The sequence datasets generated in this study has been deposited in the NCBI database under the Sequence Read Archive project PRJNA1035512 https://www.ncbi.nlm.nih.gov/sra. The GitHub link contains sequences of the baits used. https://github.com/allisoncoomber/targeted_sequencing_baits. A source file of data is included here.
The software used to analyze the data is cited in “Methods” section and reference lists and no custom code was written.