Abstract
Cancer is the disease that poses the greatest threat to human health today. Among them, hepatocellular carcinoma (HCC) is particularly prominent due to its high recurrence rate and extremely low five-year postoperative survival rate. In addition to surgical treatment, radiotherapy, chemotherapy, and immunotherapy are the main methods for treating HCC. Due to the natural drug resistance of chemoradiotherapy and targeted drugs, satisfactory results have not been achieved in terms of therapeutic efficacy and cost. AMP-Activated Protein Kinase (AMPK) is a serine/threonine protein kinase. It mainly coordinates the metabolism and transformation of energy between cells, which maintaining a balance between energy supply and demand. The processes of cell growth, proliferation, autophagy, and survival all involve various reaction of cells to energy changes. The regulatory role of AMPK in cellular energy metabolism plays an important role in the occurrence, development, treatment, and prognosis of HCC. Here, we reviewed the latest progress on the regulatory role of AMPK in the occurrence and development of HCC. Firstly, the molecular structure and activation mechanism of AMPK were introduced. Secondly, the emerging regulator related to AMPK and tumors were elaborated. Next, the multitasking roles of AMPK in the occurrence and development mechanism of HCC were discussed separately. Finally, the translational implications and the challenges of AMPK-targeted therapies for HCC treatment were elaborated. In summary, these pieces of information suggest that AMPK can serve as a promising specific therapeutic target for the treatment of HCC.
Keywords: Hepatocellular carcinoma, AMPK, regulator, multitasking roles, challenges and limitations
Introduction
According to the new data released by GLOBOCAN 2020, there are about 906,000 new cases of liver cancer (ranked 6th) and about 830,000 deaths (ranked 3rd) per year [1]. Hepatocellular carcinoma (HCC) is the main type of liver cancer, accounting for nearly 90% of the overall liver cancer cases [2,3]. The progress of HCC is mostly caused by viral hepatitis that cannot be cured, non-alcoholic fatty liver disease that poorly managed, and long-term exposure to aflatoxins and alcohol [4,5]. In recent years, non-alcoholic steatohepatitis has been a rapidly increasing risk factor and is expected to become the main cause of HCC in economically developed regions in the near future [6]. Currently, the main treatment methods for HCC patients are surgical resection and liver transplantation [6]. However, the low applicable rate of surgery and the high recurrence rate after surgery seriously affect the survival rate of HCC patients [7]. In addition, radiotherapy, chemotherapy and targeted drugs for HCC have not achieved satisfactory results in terms of therapeutic efficacy and cost due to their natural drug resistance [8]. Therefore, it is urgent to explore new targets for the treatment of HCC from the field of molecular biology in order to develop targeted treatment strategies.
AMP-Activated Protein Kinase (AMPK), a serine/threonine protein kinase, is a key hub for regulating cellular energy metabolism [9]. It mainly coordinates the metabolism and conversion of the intercellular, and maintains the balance of energy supply and demand [10]. The processes of cell growth, proliferation, autophagy, and survival all involve various reactions of cells to energy changes [11]. The regulatory role of AMPK in cellular energy metabolism plays an important role in the occurrence, development, treatment, and prognosis of tumors [12].
The liver is an important metabolic organ in the human body [13]. The imbalance of liver metabolism is considered to be the mechanism of continuous deterioration after liver tissue injury [14]. Coordinating intercellular energy metabolism and maintaining a balance between energy supply and demand is an innovative idea for the treatment of HCC. As an energy sensor for eukaryotic cells, the dysfunction of AMPK may be an important process in tumorigenesis and development [12]. However, the role of AMPK in tumor metabolism is still elusive to a large extent. Therefore, this article reviews the latest advances of AMPK in tumor, especially in HCC, with a focus on how AMPK participates in the occurrence and development of HCC.
Structure and the activation of AMPK
Approximately only about 2% of the genes in the entire genome encode protein kinases in higher eukaryotes [15,16], while it is these protein kinases that regulate the function of thousands of other proteins in cells. Therefore, the regulatory effect of protein kinases is known as the “life of switch”. Protein kinases can be classified into two ways: one is based on catalytic domains (CAMK, MAPK, CK1, AGC, AKT and Other) [17,18]; The second is based on substrates (tyrosine kinases, serine/threonine kinases, histidine kinases and aspartate/glutamate kinases) [19].
AMPK, a serine/threonine protein kinase, is widely present in eukaryotes, mainly coordinating intercellular metabolism and converting intercellular energy [20]. It is a key hub for regulating cellular energy metabolism [21]. Activating AMPK, on the one hand, shuts down the synthetic metabolic pathway that consumes adenosine triphosphate (ATP), and on the other hand, initiates the catabolic metabolic pathway that produces ATP [22]. This can regulate the body energy metabolism and maintain a balance between energy supply and demand [23]. Various cellular reactions to energy changes are involved, including cell growth, proliferation, autophagy, survival, cell polarity, cell motility, and so on [11]. This energy changes of AMPK exerts a dual function of promoting or inhibiting tumor development and plays an important role in the development/progression of cancer cells [24].
Overall molecular structure of AMPK
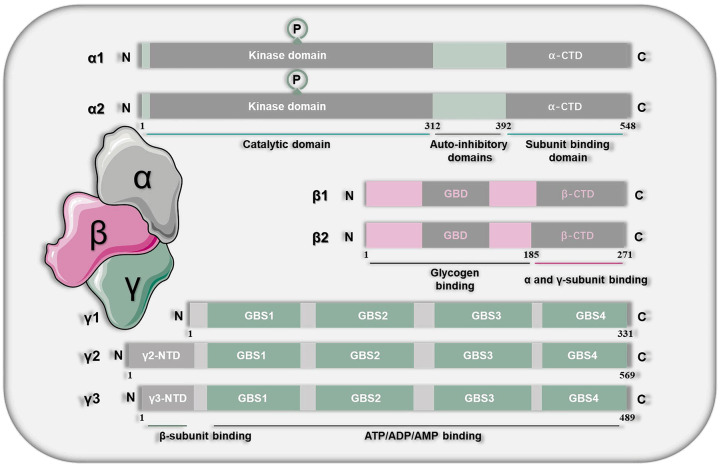
AMPK, a heterotrimeric protein, is composed of α, β and γ subunits. Among them, the α subunit plays a catalytic role, β and γ play a regulatory role. Each of the subunits has 2/3 isoforms encoded by genes (α1 and α2; β1 and β2; γ1, γ2 and γ3) [25] (Figure 1). In theory, the different isomers of α, β and γ can form various possible combinations, with a total of 12 types of combinations [26]. Among them, α1, α2, γ1 and γ2 subunits are mainly expressed in liver tissue [27,28].
Figure 1.
The overall molecular structure of AMPK. AMPK is composed of α, β and γ subunits. The α subunit plays a catalytic role, β and γ play a regulatory role. Each of the subunits has 2/3 isoforms encoded by genes (α1 and α2; β1 and β2; γ1, γ2 and γ3). The subunit α contains 548 amino acids, which can be divided into a N-terminal of the catalytic domain, an intermediate auto-inhibitory domain, and a C-terminal of the subunit binding domain. The N-terminus is the core site of catalysis, containing a typical catalytic domain of serine/threonine protein kinase. The self-inhibitory domain can reduce AMPK activity at low levels of AMP. The subunit β is like a bracket of α and γ subunits, which can connect α and γ subunits together. The subunit γ can be referred to as regulatory subunit, which can bind to regulatory substances such as ATP, AMP, or ADP.
The subunit α contains 548 amino acids, which can be divided into a N-terminal of the catalytic domain (1-312AA), an intermediate auto-inhibitory domain (312-392AA), and a C-terminal of the subunit binding domain (392-548AA) [27]. The N-terminus is the core site of catalysis, containing a typical catalytic domain of serine/threonine protein kinase. The self-inhibitory domain can reduce AMPK activity at low levels of adenosine monophosphate (AMP). The C-terminus contains allosteric binding sites that participate in binding with AMP. There are 8 sites in subunit α that can be phosphorylated, among which the threonine 172 (Thr172) site and its phosphorylation play an important role in regulating AMPK activity [29]. The total AMPK and active AMPK proteins detected in the study usually refer to the subunit α [30].
The subunit β is like a bracket of α and γ subunits, which can connect α and γ subunits together. Following the highly variable N-terminal (1-185AA) of the subunit β is the C-terminal sequence (186-271AA), which has two conserved structural domains: Kinase Interacting Sequence (KIS) and Association with SNF1 kinase Complex (ASC) [31,32]. The ASC domain is necessary for the formation of stable and active heterotrimeric proteins [33], while KIS does not interact with other subunits of the kinase [34]. The KIS domain is closely related to the N-isoamylase domains, which is a functional glycogen binding domain on β subunit. Its function may be related to the regulation of AMPK by glycogen [35].
The subunit γ can be referred to as regulatory subunit, which can bind to regulatory substances such as ATP, AMP, or adenosine diphosphate (ADP) [36]. The N-terminal of the subunit γ varies greatly in size and sequence. Compared with γ1, the N-terminal of γ2 and γ3 is longer. The subunit γ contains four serially repeated cystathionine-beta-synthase domains [37]. Among the three isoforms of the subunit γ, one is linked with nucleotide, while the others are bind with the regulatory sites of the activating nucleotide AMP and the inhibiting nucleotide ATP [38]. It can enable AMPK to sensitively detect the AMP: ATP ratio [39]. The three isoforms in turn modulate the activity of AMPK’s kinase domain in its α subunit [40].
Activation mechanism of AMPK
As an important energy metabolism regulatory pathway, AMPK can sense cellular energy status in various ways and can be activated under appropriate conditions [41]. There are multiple mechanisms for the activation of AMPK, among which the most common is triggered by AMP (or ADP), known as classical mechanisms. Other mechanisms that do not rely on AMP (or ADP) are called non-classical mechanisms [42-44].
There are three classical mechanisms triggered by AMP [45,46]: (1) The binding of AMP and subunit γ leads to conformational activation of AMPK. (2) Promoting the phosphorylation of Thr172 residues on subunit α through upstream kinases (LKB1 complex). (3) Inhibiting the dephosphorylation of Thr172 residues on subunit α through protein phosphatases (PP2A, PP2C). The bind of adenine nucleotide and AMPK-γ subunit is also known as canonical inputs [43]. The latter that activation by ligands that bind between the α and β subunits is referred to as non-canonical input [43]. All three classical mechanisms are due to the direct binding of AMP to AMPK, rather than directly to the upstream kinases or protein phosphatases [47].
In addition, other stimuli activate AMPK through mechanisms that do not involve AMP (or ADP), known as non-classical mechanisms. For example, the activation by the Ca2+/calmodulin-dependent protein kinase kinase (CaMKK) pathway, which is independent of changes in adenine nucleotides [48]. By increasing the hormones of intracellular Ca2+, then regulating calmodulin dependent protein kinase CaMKKβ, it can realize phosphorylation of Thr172 to activate AMPK [43]. In addition, the tetrahydrofolate analogues (such as pemetrexed and methotrexate) catalyze 5-amino-1-beta-D-ribofuranosylimidazole-4-carboxamide monophosphate (ZMP) by inhibiting tetrahydrofolate utilization enzymes, causing the accumulation of ZMP in cells. Which combines with the subunit γ to activate AMPK [43,49]. Most other activators (such as glycolysis inhibitor 2-deoxyglucose, the anti-diabeticx drugs metformin and phenformin) indirectly activate AMPK by inhibiting ATP synthesis [43,50].
Emerging regulator related to tumors
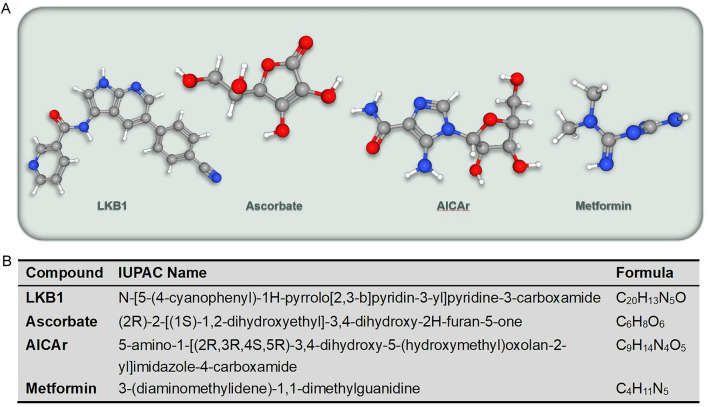
The formation of tumors is the result of multiple factors and multiple genes involved in multiple stages of development [51]. Among them, the imbalance of cellular energy induced damage to the intracellular environment is the beginning of all adverse events [26]. AMPK, as the central guardian of maintaining energy homeostasis, participates in regulating the occurrence and development of tumors by perceiving cellular energy imbalance and coordinating different cellular processes [26]. The important role of AMPK in regulating tumorigenesis and development has been emphasized (Table 1). The regulation of AMPK mainly includes two modes: activators and inhibitors. Almost all studies related to AMPK modulators have only described activators. Several specific activators have been described, including the serine/threonine kinase liver kinase B1 (LKB1), Ca2+/CaMKK, ascorbate, 5-Aminoimidazole-4-carboxamide ribonucleoside (AICAr) and Metformin (Figure 2).
Table 1.
List of regulators of AMPK related to tumors
| Regulator | Subject | Function | Model | Reference |
|---|---|---|---|---|
| LKB1 | HDAC11 | Maintain HCC stemness | Human HCC tissues, cell lines and KO mice | [58] |
| PRMT5 | Promote esophageal squamous cell carcinoma proliferation and metastasis | Human Esophageal squamous cell carcinoma tissue samples | [59] | |
| SIK1/SIK3 | Mediate key tumor suppressive effects in NSCLC | NSCLC cell lines and mouse models | [60] | |
| Ca2+/CaMKK | n-3 PUFA | Protect human HCC from developing steatosis | FFA4 deficient mice, Hep3B and HepG2 cells | [62] |
| Palbociclib | Regulate intracellular lipids in mammary tumor cells | FM3A mouse mammary tumor cells | [63] | |
| - | Promote autophagy in HCC cells | Human HCC cell lines | [64] | |
| NCAPD2 | Inhibit autophagy to promote colorectal cancer | Human colorectal cancer tissues | [65] | |
| Ascorbate | - | Enhance chemosensitivity of ovarian cancer | Mouse models with ovarian cancer | [70] |
| Reduce toxicity of chemotherapy | ||||
| Erastin | Slow tumor growth in pancreatic cancer xenografts | Human/mouse pancreatic cancer cell lines | [71] | |
| AICAr | - | Inhibit the growth of prostate cancer cells | Human prostate cancer cell lines | [73] |
| Enhance the chemical sensitivity of prostate cancer cells | ||||
| - | Enhance the sensitivity of prostate cancer cells to radiotherapy | Human prostate cancer cell lines | [74] | |
| PFKFB3 | Enhance the cytotoxicity on colorectal cancer cells | Human colorectal cancer cell lines | [76] | |
| Metformin | - | Disrupt protein synthesis and inhibits cell growth and proliferation | Human colorectal cancer cell lines | [87] |
| DMU-212 | Activate autophagy and cell apoptosis | lung cancer cell lines | [89] |
Figure 2.
Several specific activators of AMPK related to tumors (A) and its IUPAC name of the compound (B).
LKB1
LKB1, also known as serine/threonine kinase 11-STK11, is a key upstream activator of AMPK [52]. LKB1 is located on human chromosome 19p13.3 and was initially identified as a tumor suppressor gene [53].
Research has found that LKB1 is one of the most common mutated genes in human cancer. Among various subtypes of non-small cell lung cancer, LKB1 is the second most common tumor suppressor, with at least 17-23% of cases experiencing mutations or genomic deletions [54]. LKB1 undergoes somatic mutations in 20% of cervical cancer, which is the first known recurrent genetic change in cancer caused by human papillomavirus [55]. The expression of LKB1 is reduced in gastric cancer tissue, and the recovery of LKB1 expression reduces the survival ability of tumor cells and improves their sensitivity to anticancer drugs [56]. The loss of LKB1 increases the invasiveness and migration ability of breast cancer cells, while the activation of LKB1 reduces the formation of master cells and the expression of pluripotent factors [57]. Besides, abnormal expression of LKB1 is associated with poor prognosis in HCC [58]. LKB1/AMPK is essential for HDAC11 to regulate glycolysis, which maintains HCC stemness [58]. LKB1/AMPK/mechanistic target of rapamycin (mTOR) is involved in Protein Arginine Methyltransferase 5 promoting proliferation and metastasis of esophageal squamous cell carcinoma [59]. And the AMPK mediates key tumor-suppressive effects of LKB1 in non-small cell lung cancer [60].
LKB1, as an important upstream activator of AMPK, synergistically controls the growth of cancer cells in response to changes in the intracellular environment, which may become a new target and drug for cancer [61].
Ca2+/CaMKK
Ca2+/CaMKK is an activation kinase of downstream kinase AMPK. It responds to an increase in intracellular Ca2+concentration through the phosphorylation of its activation-loop Thr residues, playing an important role in many Ca2+-dependent pathways.
Omega-3 polyunsaturated fatty acids protect human hepatoma cells to avoid developing steatosis through Free Fatty Acid receptor 4 (FFA4), and the signaling cascade involves CaMKK/AMPK [62]. Ca2+/CaMKK/AMPK participates in the process of Palbociclib stimulating LPL secretion to regulate lipid levels in breast tumor cells [63]. Ca2+/CAMKK/AMPK is involved in a positive feedback loop between mitochondrial fission and cytoplasmic calcium signaling. This feedback loop significantly promotes the overall autophagy process of HCC cells [64]. In addition, research has also confirmed that the Ca2+/CAMKK2/AMPK/mTORC1 pathway participates in Non-SMC condensin I complex subunit D2 (NCAPD2) inhibition of autophagy regulation to promote colorectal cancer [65].
Ascorbate
Ascorbate, also known as vitamin C, is a polyhydroxy compound with anti-tumor cell survival properties. Laboratory studies have confirmed that millimolar vitamin C concentration can inhibit the viability of tumor cells [66]. Although this conclusion is currently controversial [67]. However, experiments have shown that ascorbate can improve the response of advanced non-small cell lung cancer to platinum chemotherapy [68]. Moreover, ascorbate can inhibit cancer metastasis through a peroxide mediated mechanism [69].
Recent studies have found that the downstream mechanism of ascorbate-induced cancer cell death may be related to the AMPK/mTOR pathway [70]. Research has shown that millimolar ascorbate can induce deoxyribonucleic acid (DNA) damage and the depletion of cellular ATP, activate the ataxia telangiectasia mutated/adenosine phosphate activated protein kinase pathway, and lead to mammalian rapamycin target inhibition and death in ovarian cancer cells.
In addition, vitamin C can also make pancreatic cancer cells sensitive to Erastin-induced ferroptosis by activating AMPK/Nrf2/HMOX1 pathway [71]. The combination of vitamin C and erastin can significantly slow tumor growth in pancreatic cancer xenografts.
AICAr
AICAr, a nucleoside analogue, is one of the most commonly used pharmacological modulators of AMPK activity [72].
Research has confirmed that AICAr can inhibit the growth of prostate cancer cells, induce cell apoptosis, weaken the cell migration, invasion, and EMT-related protein expression, and enhance the chemical sensitivity of prostate cancer cells to paclitaxel by regulating the AMPK/mTOR - dependent pathway [73].
AICAr can enhance the sensitivity of prostate cancer cells to radiotherapy [74]. AICAr can synergistically enhance the clonogenic killing capacity, spheroid growth inhibition and pro-apoptotic effect of X-rays on prostate cancer cells. The mechanism of radiosensitization may involve cell cycle regulation. This also suggests that the activation of AMPK combined with radiotherapy can target metabolic active and invasive tumors, which providing new ideas for tumor treatment.
In addition, AICAr suppresses cell proliferation and synergizes with decitabine in myelodysplastic syndrome through DNA damage induction [75]. AICAr enhances the cytotoxicity of PFKFB3 inhibitors on colorectal cancer cells in an AMPK signal independent manner [76]. The combination of AICAr and anti-mouse IL-10 mAb restores the function of the Tfh cells within the tumor in the 4T1 mouse model [77]. AICAr induces AMPK-independent cell death in human adult T-cell leukemia/lymphoma associated cell lines, and exhibits anti-tumor activity [78].
However, some scholars have summarized and found that many AICAr effects previously attributed to AMPK activation are actually unrelated to AMPK [72]. This also reminds scholars to dialectically view AICAr as a related research mechanism for AMPK activation.
Metformin
Metformin, a biguanide antidiabetic drug, is one of the most commonly used oral drugs to treat type 2 diabetes [79]. In recent years, studies have found that the use of metformin alone or in combination with other drugs has brought many unexpected benefits to various cancers [80-83], cardiovascular diseases [84], liver diseases [85], obesity [86], and more. Among them, the anti-tumor properties of metformin are related to its direct and indirect regulation of cellular metabolism [79]. The direct regulation is mediated by AMPK-dependent and -independent pathways. Metformin activates AMPK, leading to the inhibition of mTOR signaling, which disrupts protein synthesis and inhibits cell growth and proliferation [87]. Metformin can also inhibit mTORC1 in an AMPK-independent manner, which is a key regulator for cell growth [88]. Inhibiting mTORC1 can integrate intracellular and extracellular stimuli to achieve anticancer effects. Additionally, metformin can activate autophagy and cell apoptosis through AMPK-independent pathways, thereby inhibiting the development of cancer [89].
However, a recent randomized double-blind phase II trial on the anti-tumor activity of metformin has encountered a setback [90]. In order to slow down biochemical recurrence caused by androgen deprivation therapy or delay the progression of advanced prostate cancer, this experiment utilizes metformin to demonstrate anti-tumor activity through mTOR inhibition secondary AMPK-activation. However, adding metformin to androgen deprivation therapy did not reduce the risk of metabolic syndrome associated with androgen deprivation therapy or differences in prostate-specific antigen response. Therefore, further validation is needed to determine whether the AMPK activator metformin can delay the progression of all tumors.
Multitasking roles in HCC
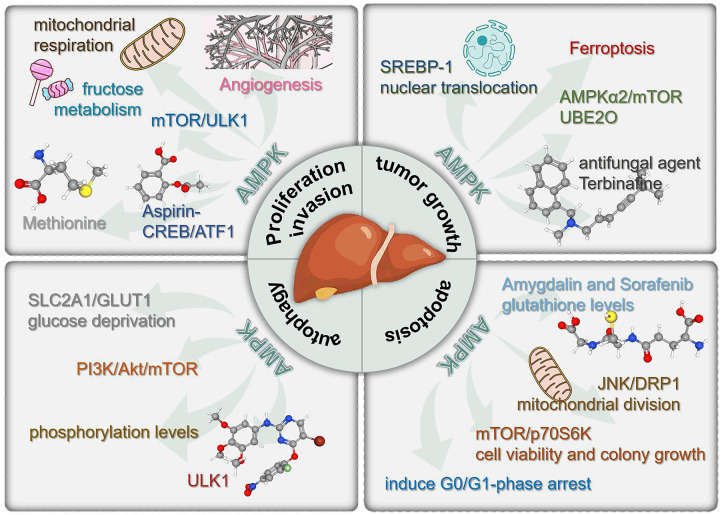
With the development of molecular biology, the role of energy metabolism and the balance of energy supply and demand in the occurrence and development of cancer has been paid more and more attention. AMPK is a serine/threonine protein kinase that primarily coordinating intercellular metabolism and converting intercellular energy [91]. Based on this, people have always believed that AMPK can inhibit cancer by slowing cellular metabolism. Therefore, AMPK can serve as a tumor inhibitor. In addition to basic researches, a large number of clinical trials have also found that many anticancer agents can activate AMPK, which also proves the above viewpoint [90,92,93]. However, recent studies have shown that in some cases, AMPK can also promote tumor growth and metastasis. In 2019, Reuben Shaw published a research result in the journal Cell Metabolism [94], which confirmed that advanced cancer can trigger AMPK cell re-circulating signals, engulf cell debris, and provide the necessary nutrition for the growth of large lung tumors. In some cases, blocking AMPK can prevent the growth of the most common advanced lung cancer tumors. In 2020, Lin HK published a study in the journal Molecular Cell [95], which found that the activity of AMPK in the metastatic foci was significantly higher in the lung metastasis of breast cancer mice than in the tumor in situ. Interference with AMPK α1 can eliminate lung metastasis of cancer, while the growth of the primary tumor is not affected by this. This suggests that overexpression of AMPK is associated with shorter metastasis free survival in breast cancer patients. Lots of exciting studies have shown that the regulatory effect of AMPK on cellular energy metabolism has a dual function of promoting or inhibiting tumor development (Figure 3). So, is there any controversies and conflicting views on the role of AMPK in HCC? The answer is completely affirmative.
Figure 3.
Schematic diagram of the multitasking roles of AMPK in regulating the progression of HCC.
Promoting proliferation and invasion
Angiogenesis is the foundation of tumor growth and metastasis [96]. Active angiogenesis in HCC leads to a high incidence of hematogenous metastasis [97,98]. Research by Zhuang SM et al. [99] has confirmed that AMPK signaling in tumor endothelial cells from HCC can be activated by upregulated fructose metabolism in the tumor hypoxic microenvironment. The activation of AMPK signaling can upregulate mitochondrial respiration, thereby enhancing the migration and proliferation ability of tumor endothelial cells from HCC, and ultimately promoting HCC angiogenesis and metastasis.
Zhang et al. [100] found that the AMPK/mTOR/Unc-51-like kinase 1 (ULK1) axis is the main pathway for Phosphoserine phosphatase (PSPH) to induce autophagy, promote cell proliferation and invasion in Huh7 cells. PSPH affects the AMPK/mTOR/ULK1 signaling pathway in Huh7 cells by activating LKB1 and the transforming growth factor beta-activated kinase 1 (TAK1). Inhibition of AMPK can abolish the effect of PSPH overexpression on the behavior of Huh7 cells. Through this experiment, it can be seen that AMPK can promote the proliferation and invasion of HCC cells, and inhibit cell apoptosis.
Methionine, an essential amino acid required for cell growth and development, is mainly metabolized in the liver [101]. Studies have found that inhibition of AMPK strongly impairs cell growth, cell migration, and colony formation at high methionine concentrations [102]. This shows that AMPK plays an important role in methionine supplementation to reduce the tumor aggressiveness of HCC cells.
Blocking the AMPK-mediated cAMP-PKA-CREB/ATF1 signaling pathway and combining it with Aspirin can enhance the efficacy of inhibiting HCC [103]. Studies have found that Aspirin can induce CREB/ATF1 phosphorylation in HCC cells, which greatly reduces the anti-HCC effect of Aspirin. Inhibition of AMPK abolishes the induction of CREB/ATF1 phosphorylation by Aspirin [103]. This in turn increases the sensitivity of HCC to aspirin chemotherapy. These data indicate that the inhibition of AMPK-mediated signaling axis can significantly improve the efficacy of Aspirin assisted HCC chemotherapy. And the inhibition of AMPK has an anti HCC effect.
Inhibiting tumor growth
Sterol regulatory element-binding protein 1 (SREBP-1) is an important transcription factor that regulates lipid metabolism [104]. It is an independent prognostic indicator for overall survival and disease-free survival in HCC patients [105]. AMPK can interact with SREBP1, inhibiting its processing and nuclear translocation [106]. In the context of HCC and liver physiology, the AMPK activator AICAR has been shown to negatively regulate hepatic transcription and processing of SREBP-1, and help reduce HCC cell proliferation and liver tumorigenesis in mice [105].
Ferroptosis is an iron-dependent form of programmed cell death driven by excessive membrane peroxidation for cell apoptosis [105,107]. Zhao Lei et al. [108] found that in orthotopic HCC animal models, AMPK and Iron level in HCC cells can be significantly activated by sora@Fe-MIL on average. The activation of the AMPK pathway simultaneously increases intracellular iron levels, enhances the ferroptosis induction effect of sorafenib, and significantly inhibits the growth of primary in situ tumor.
The AMPKα2/mTOR axis is important for ubiquitin-conjugating enzyme E2O (UBE2O) to promote the proliferation and invasion of HCC cell [109]. UBE2O is an oncogene which is highly expressed in HCC. In HCCLM3 cells, the overexpression of UBE2O significantly reduces AMPKα2 and increases p-mTOR, leading to the adverse clinical outcomes of patients. Silencing AMPKα2 reverses the inactivation of the mTOR pathway induced by UBE2O downregulation. The high expression of UBE2O promotes HCC cell proliferation, migration, and invasion by reducing the stability of AMPKα2 and activating the mTOR pathway.
Studies have also found that the antifungal agent Terbinafine has the effect of inhibiting the tumor growth of HCC [110]. This effect is mainly achieved by activating AMPK and inhibiting mTORC1 signaling. In addition, Terbinafine alone or in combination with Sorafenib was found to delay tumor progression and significantly prolong the survival of tumor-bearing mice. This expands the thinking of AMPK in the research on inhibiting the progression of HCC.
Regulating autophagy
Autophagy is a lysosomal dependent self-digestion process [111]. During nutrient deficiency, soluble proteins and organelles in the cytoplasm are degraded into amino acids through autophagy, providing substrates for energy metabolism that maintain metabolic balance [112]. AMPK is an important upstream regulatory factor in autophagy [113]. Promoting autophagy is the main mechanism by which AMPK inhibits the occurrence of HCC [114,115]. Intervention in AMPK activity can regulate autophagy intensity, and inhibition of autophagy can counteract the protective effect of activated AMPK on HCC occurrence [115,116].
The AMPK-ULK1 pathway is vital for Isoginkgetin (ISO)-induced autophagy by inhibiting the expression of SLC2A1/GLUT1 [117]. It was found that the phosphorylation of PRKAA/AMPKα and ULK1 in HepG2 cells increased in a dose-dependent manner after ISO treatment. SLC2A1/GLUT1 deficiency can lead to glucose deprivation, which in turn activates the AMPK-ULK1 pathway. So, ISO may induce autophagy by promoting cell starvation and activating the AMPK-ULK1 axis.
The AMPK and PI3K/Akt/mTOR pathways are important components for Fisetin to inhibit autophagy in HepG2 cells [118]. The study exposed HepG2 cells to different concentrations of Fisetin and determined autophagic flux formation and ATP levels by assay, confirming that fisetin inhibits autophagy by activating PI3K/Akt/mTOR and regulating the AMPK signaling pathway.
It was found that in metformin-induced autophagy, AMPK expression was activated and phosphorylation levels of mTOR and p70 S6 kinases were inhibited [119]. Metformin was confirmed to induce autophagy in human hepatoma cells through the AMPK-mTOR signaling pathway.
In vitro experiments have also confirmed that curcumin can inhibit the growth of HepG2 cells through autophagy of the AMPK/ULK1 pathway [120].
Modulating apoptosis
The inhibition of AMPK leads to inhibition of mTOR, thereby inducing apoptosis of HepG2 cells. Tarek et al. [121] found that the combination of Amygdalin and Sorafenib could lead to the highest glutathione levels, induce pro-autophagic genes such as AMPK and high-mobility group box 1, and inhibit the mTOR and BCL2 anti-apoptotic genes. They employed in vitro and in silico synthesis methods to find that Amygdalin binds to the active site of the AMPK enzyme, thereby inhibiting its activity. This inhibition of AMPK ultimately leads to inhibition of mTOR, which induces apoptosis in HepG2 cells.
Pseudolaric acid B (PAB) triggers apoptosis in HCC cells by activating the AMPK/JNK/DRP1/mitochondrial division pathway [122]. PAB is known to inhibit the viability of Hepa1-6 cells and induce apoptosis in a dose-dependent manner. It disrupts mitochondrial membrane potential and impairs ATP production. PAB activates AMPK, and compound C inhibits AMPK attenuates PAB-stimulated JNK activation and blocks DRP1-dependent mitochondrial division and apoptosis. Our in vivo data confirm that PAB inhibits tumor growth and induces apoptosis by inducing the AMPK/JNK/DRP1/mitochondrial division signaling pathway in a syngeneic mouse model of HCC.
Isisercetin-induces apoptosis and autophagy in HCC cells through the AMPK/mTOR/p70S6K signaling pathway [123]. ISO exposure was found to inhibit cell viability and colony growth, activate the apoptotic pathway, and trigger dysregulated autophagy by activating the AMPK/mTOR/p70S6K pathway. Autophagy inhibition reverses ISO-induced upregulation of AMPK phosphorylation and downregulation of mTOR and p70S6K phosphorylation.
The AMPK and p53/p21 axis are involved in Hemistepsin A to inhibit human HCC cells proliferation and induce G0/G1-phase arrest, cell senescence, and apoptosis [124]. In addition, the AMPK/STAT3 axis and MARCH1 are participate in Sinomenine to arrest the HCC cells cycle at the G0/G1-phase, induce apoptosis, and inhibit the proliferation of HCC cells [125].
New insights and translational implications
HCC accounts for more than 85% of malignant liver tumors and is one of the deadliest diseases of the liver [126,127]. The development of HCC is a multi-step process of intratumoral heterogeneity involving the change of tumor microenvironment, the imbalance of cell signal transduction pathways, and the disorder in energy metabolism patterns [128,129]. AMPK is an important kinase that regulates energy homeostasis [130]. In previous reports, AMPK has played an important role as a eukaryotic energy sensor in liver metabolism and HCC development. Although AMPK activation may be beneficial for HCC cells to cope with various metabolic stress responses, under certain conditions, AMPK activation has anti-tumor and anticancer functions, and may mediate the inhibitory effect of mitochondrial inhibitors on HCC cell growth [131]. Further research on new AMPK small molecule activators and AMPK gene deletions will clarify the translational significance of AMPK-targeted therapies for HCC.
AMPK activators and AMPK gene knockout techniques have been widely applied in basic experiments to regulate the progression of HCC. These drugs and technologies activate the AMPK pathway through different mechanisms. LKB1/AMPK is essential for HDAC11 to regulate glycolysis, which maintains HCC stemness [58]. Ca2+/CAMKK/AMPK is involved in a positive feedback loop between mitochondrial fission and cytoplasmic calcium signaling. This feedback loop significantly promotes the overall autophagy process of HCC cells [64]. sora@Fe-MIL, a unique sorafenib nanocomposite, can increase iron levels in HCC cells and activate AMPK. This can enhance the ferroptosis induction effect of sorafenib, thereby increasing the sensitivity of positive HCC cells to sorafenib [108]. Nicotinamide mononucleotide (NMN) can activate the AMPK and inhibit the mTOR signaling pathway, thereby activating autophagy and ferroptosis of HCC cells in 75%-85% of primary liver, and inhibiting HCC progression [132]. The interaction between liver cancer related protein TD26 and nSREBP1 can block AMPK mediated inhibition of SREB1. TD26 relieves AMPK inhibition and promotes increased adipogenesis in liver cancer [106]. The pharmacological inhibitor compound C of AMPK can significantly upregulate the expression of PD-1 in Tregs. AMPK gene knockout can more effectively inhibit T cell proliferation, promote the release of IL-10 and transforming growth factor - β. The above suggests that AMPK can downregulate PD-1 in regulatory T cells, thereby promoting anti-tumor immunity [133]. AMPK inhibitors (compound C) inhibit 3-Epi-betulinic acid 3-O-β-D-glucopyranoside (eBAG) induced AMPK phosphorylation and reduce eBAG induced cell death. This indicates that AMPK is an important mediator for eBAG induced autophagic cell death. In addition, eBAG inhibits tumor growth in xenograft liver tumor models by regulating AMPK-mTOR-S6 signaling pathway [134]. The treatment of type II diabetes with metformin activated AMPK can not only inhibit the proliferation of HCC cells, but also enhance the chemosensitivity of hepatocarcinoma cells to cisplatin [135-137]. Nucleotide bound oligomeric domain 2 (NOD2), a recognized innate immune sensor, can initiate a powerful immune response against pathogens [138]. NOD2 plays a role as a tumor suppressor and chemotherapy modulator in HCC cells by directly activating the AMPK pathway [139]. Currently, resistance to anti-tumor drugs remains the main challenge in the treatment of HCC [140]. Sorafenib (SOR) is a first-line treatment for liver cancer. CXCR3 induces metabolic alteration in SOR-resistance HCC cells by downregulating AMPK pathway activity and lipid peroxidation, as well as upregulating adipocyte cytokine levels [141]. The activation of the MPK pathway by the AMPK activator metformin enhances the sensitivity of HCC to in vivo SOR therapy. These findings confirm the important role of AMPK in the development of resistance in HCC cells to SOR therapy.
AMPK activators and AMPK gene knockout techniques have been extensively studied in laboratory researches related to HCC. However, in clinical trials, it is still in its infancy. At present, there are very few clinical trial results available for reference.
Challenges and limitations
The development of HCC is a multi-step process, with complex interactions between altered signaling pathways, tumor microenvironment, and diverse genetic backgrounds, leading to high heterogeneity of tumors [128]. Intervention in HCC heterogeneity may provide new avenues for its treatment. With the advancement of multi omics and gene editing technologies, the exploration of molecular pathways for the development of HCC has deepened, promoting the identification of various biomarkers. Effective biomarkers can accurately screen potential beneficiaries, which will help improve the success rate of clinical trials and also avoid exposing patients with low likelihood of benefit to unnecessary safety risks [142]. The dual role of AMPK as an important energy converter in the development of HCC determines its potential as an important biomarker in the treatment process. However, current clinical trials worldwide have not delved into them, and potential biomarkers for AMPK-targeted therapies response still need to be further explored.
Clarifying the specific role of AMPK in physiological processes such as HCC cell cycle regulation, apoptosis, and tumorigenesis will be the key to AMPK-targeted therapies. In terms of technology alone, post-transcriptional gene silencing technology can also improve the functional research of AMPK, in addition to the application of AMPK activators and AMPK gene knockout technology. By detecting the expression level of AMPK genes, the functional status of AMPK in HCC can be understood. RNAi is a technical tool for gene function research [143]. Well-designed miRNAs can provide long-term silencing with reduced side effects and safer to use [144]. Studies have confirmed that targeting the miR-AMPK pathway has the potential to treat NAFLD/NASH [145]. However, the application and research of this technology in the HCC field are still in the early stages of development. Therefore, this method needs further improvement in future research.
As is well known, the therapeutic targets for drugs that act on the signaling pathways may lead to drug resistance, tumor progression, immunologic suppression, and new infections [146]. Therefore, AMPK-targeted therapies for HCC will inevitably cause side effects. The application of nanomaterials will effectively improve the accuracy of drug design, synthesis, and delivery procedures [147]. Future research needs to consider how to improve the efficiency of AMPK-targeted therapies for HCC while reducing potential adverse reactions.
Conclusions and perspectives
In this review, we summarized the latest evidence on the multitasking roles of AMPK in the occurrence and development of tumors, especially HCC. AMPK serves as a key hub in regulating cellular energy metabolism. It can regulate the biological functions of various cancer cells by shutting down the synthetic metabolic pathway that consumes ATP or activating the catabolic pathways that produces ATP. These biological functions include tumor cell invasion, proliferation, autophagy, apoptosis, etc.
The metabolic balance effects of AMPK have been elucidated in vivo and in vitro models of HCC. Previous studies have shown that the regulatory role of AMPK in tumor development is initiated by multiple activators and conducted through different signaling pathways and molecules. Here the latest research progress of AMPK in the occurrence and development of tumors, especially HCC were summarized. The AMPK-specific activators that have been widely used in the field of cancer in recent years were also recapitulated. At the same time, some potential regulatory pathways of AMPK involved in HCC are reviewed from the perspectives of proliferation, invasion, autophagy, and apoptosis. And during the review process, it was found that there are conflicting views on the role of AMPK in the occurrence and development of HCC. Through a detailed summary, this article analyzes and summarizes the dual functions of AMPK in promoting or inhibiting tumor development in the energy metabolism regulation process of HCC cells. This provides a balanced perspective for objectively evaluating the role of AMPK in HCC. Then, the new insights and translational significance of AMPK-targeted therapies for HCC were discussed. The challenges and limitations faced by AMPK-targeted therapies for HCC were elaborated. The study of AMPK targeting HCC can fully utilize RNA interference technology to search for key biomarkers at the genetic level. New technologies such as nanomaterials can also be flexibly applied to improve the accuracy of drug design, synthesis, and delivery procedures. These ideas will help promote the clinical translation of AMPK-targeted therapies for HCC.
Although various regulatory mechanisms of AMPK have been elucidated, there are still some questions that need to be answered. For example, due to the diversity and abundance of activators, the complexity of regulatory mechanisms, and multiple regulatory pathways, the precise role of AMPK in the pathogenesis and prognosis of HCC remains difficult to determine. And targeting AMPK for anti-cancer is not easy. In some cases, simply increasing or maintaining cellular AMPK activity may not be advisable. Therefore, further research is needed to regulate AMPK for the treatment of HCC. Therefore, in order to develop targeted treatment strategies, further research on AMPK is still needed to identify new targets for the treatment of HCC, especially targets related to metabolic regulation.
Acknowledgements
This work was supported by the Anhui Provincial Natural Science Foundation (grant number: 2308085MH293) and the University research program of Anhui University of Traditional Chinese Medicine (grant number: 2023AH040098).
Disclosure of conflict of interest
None.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Chen J, Gao G, Zhang Y, Dai P, Huang Y. Comprehensive analysis and validation of SNX7 as a novel biomarker for the diagnosis, prognosis, and prediction of chemotherapy and immunotherapy response in hepatocellular carcinoma. BMC Cancer. 2023;23:899. doi: 10.1186/s12885-023-11405-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sun YF, Wu HL, Shi RF, Chen L, Meng C. KIF15 promotes proliferation and growth of hepatocellular carcinoma. Anal Cell Pathol (Amst) 2020;2020:6403012. doi: 10.1155/2020/6403012. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 4.Lin CH, Kuo JC, Li D, Koenig AB, Pan A, Yan P, Bai XF, Lee RJ, Ghoshal K. AZD5153, a bivalent BRD4 inhibitor, suppresses hepatocarcinogenesis by altering BRD4 chromosomal landscape and modulating the transcriptome of HCC cells. Front Cell Dev Biol. 2022;10:853652. doi: 10.3389/fcell.2022.853652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pan A, Truong TN, Su YH, Dao DY. Circulating biomarkers for the early diagnosis and management of hepatocellular carcinoma with potential application in resource-limited settings. Diagnostics (Basel) 2023;13:676. doi: 10.3390/diagnostics13040676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Armakolas A, Dimopoulou V, Nezos A, Stamatakis G, Samiotaki M, Panayotou G, Tampaki M, Stathaki M, Dourakis S, Koskinas J. Cellular, molecular and proteomic characteristics of early hepatocellular carcinoma. Curr Issues Mol Biol. 2022;44:4714–4734. doi: 10.3390/cimb44100322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Y, Zou H, Zheng Z, Liu Z, Hu H, Wu W, Wang T. Advances in the study of bioactive nanoparticles for the treatment of HCC and its postoperative residual cancer. Int J Nanomedicine. 2023;18:2721–2735. doi: 10.2147/IJN.S399146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sang N, Zhong X, Gou K, Liu H, Xu J, Zhou Y, Zhou X, Liu Y, Chen Z, Zhou Y, Li Y, Tao L, Su N, Zhou L, Qiu J, Yang X, Zuo Z, Fu L, Zhang J, Li D, Li C, Sun Q, Lei J, Li R, Yang S, Cen X, Zhao Y. Pharmacological inhibition of LSD1 suppresses growth of hepatocellular carcinoma by inducing GADD45B. MedComm (2020) 2023;4:e269. doi: 10.1002/mco2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li J, Wang Y, Chen D, Liu-Bryan R. Oral administration of berberine limits post-traumatic osteoarthritis development and associated pain via AMP-activated protein kinase (AMPK) in mice. Osteoarthritis Cartilage. 2022;30:160–171. doi: 10.1016/j.joca.2021.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yao Z, Guo J, Du B, Hong L, Zhu Y, Feng X, Hou Y, Shi A. Effects of Shenling Baizhu powder on intestinal microflora metabolites and liver mitochondrial energy metabolism in nonalcoholic fatty liver mice. Front Microbiol. 2023;14:1147067. doi: 10.3389/fmicb.2023.1147067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou Q, Kim SH, Pérez-Lorenzo R, Liu C, Huang M, Dotto GP, Zheng B, Wu X. Phenformin promotes keratinocyte differentiation via the calcineurin/NFAT pathway. J Invest Dermatol. 2021;141:152–163. doi: 10.1016/j.jid.2020.05.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li HB, Chen JK, Su ZX, Jin QL, Deng LW, Huang G, Shen JN. Cordycepin augments the chemosensitivity of osteosarcoma to cisplatin by activating AMPK and suppressing the AKT signaling pathway. Cancer Cell Int. 2021;21:706. doi: 10.1186/s12935-021-02411-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shi B, Qian J, Miao H, Zhang S, Hu Y, Liu P, Xu L. Mulberroside A ameliorates CCl4-induced liver fibrosis in mice via inhibiting pro-inflammatory response. Food Sci Nutr. 2023;11:3433–3441. doi: 10.1002/fsn3.3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Musso G, Cassader M, De Michieli F, Rosina F, Orlandi F, Gambino R. Nonalcoholic steatohepatitis versus steatosis: adipose tissue insulin resistance and dysfunctional response to fat ingestion predict liver injury and altered glucose and lipoprotein metabolism. Hepatology. 2012;56:933–942. doi: 10.1002/hep.25739. [DOI] [PubMed] [Google Scholar]
- 15.Atkinson EL, Iegre J, Brear PD, Zhabina EA, Hyvönen M, Spring DR. Downfalls of chemical probes acting at the kinase ATP-site: CK2 as a case study. Molecules. 2021;26:1977. doi: 10.3390/molecules26071977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bai Y, Li L, Dong B, Ma W, Chen H, Yu Y. Phosphorylation-mediated PI3K-Art signalling pathway as a therapeutic mechanism in the hydrogen-induced alleviation of brain injury in septic mice. J Cell Mol Med. 2022;26:5713–5727. doi: 10.1111/jcmm.17568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu K, Zhai X, Huang S, Jiang L, Yu Z, Huang J. Protein kinases: potential drug targets against schistosoma japonicum. Front Cell Infect Microbiol. 2021;11:691757. doi: 10.3389/fcimb.2021.691757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.García-Aranda M, Redondo M. Protein kinase targets in breast cancer. Int J Mol Sci. 2017;18:2543. doi: 10.3390/ijms18122543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maeda S. Second era of molecular-targeted cancer therapies in dogs. J Vet Med Sci. 2023;85:790–798. doi: 10.1292/jvms.23-0204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang K, Li Y. Signaling pathways and targeted therapeutic strategies for polycystic ovary syndrome. Front Endocrinol (Lausanne) 2023;14:1191759. doi: 10.3389/fendo.2023.1191759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aderinto N, Abdulbasit MO, Tangmi ADE, Okesanya JO, Mubarak JM. Unveiling the growing significance of metabolism in modulating immune cell function: exploring mechanisms and implications; a review. Ann Med Surg (Lond) 2023;85:5511–5522. doi: 10.1097/MS9.0000000000001308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feng Y, Jia B, Shen Z. Metformin and bladder cancer: drug repurposing as a potential tool for novel therapy: a review. Medicine (Baltimore) 2022;101:e31635. doi: 10.1097/MD.0000000000031635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu Y, Wang J. Ferroptosis, a rising force against renal fibrosis. Oxid Med Cell Longev. 2022;2022:7686956. doi: 10.1155/2022/7686956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shen Q, Yang H, Kong QP, Li GH, Li L. Metabolic modeling identifies a novel molecular type of glioblastoma associated with good prognosis. Metabolites. 2023;13:172. doi: 10.3390/metabo13020172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mehmood T, Pichyangkura R, Muanprasat C. Chitosan oligosaccharide prevents afatinib-induced barrier disruption and chloride secretion through modulation of AMPK, PI3K/AKT, and ERK signaling in T84 cells. Polymers (Basel) 2022;14:4255. doi: 10.3390/polym14204255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hsu CC, Peng D, Cai Z, Lin HK. AMPK signaling and its targeting in cancer progression and treatment. Semin Cancer Biol. 2022;85:52–68. doi: 10.1016/j.semcancer.2021.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fang C, Pan J, Qu N, Lei Y, Han J, Zhang J, Han D. The AMPK pathway in fatty liver disease. Front Physiol. 2022;13:970292. doi: 10.3389/fphys.2022.970292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang TH, Sun J, Zhou SS, Gao J, Liu Y. Identification of direct activator of adenosine monophosphate-activated protein kinase (AMPK) by structure-based virtual screening and molecular docking approach. Int J Mol Sci. 2017;18:1408. doi: 10.3390/ijms18071408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li Q, Wu J, Huang J, Hu R, You H, Liu L, Wang D, Wei L. Paeoniflorin ameliorates skeletal muscle atrophy in chronic kidney disease via AMPK/SIRT1/PGC-1α-mediated oxidative stress and mitochondrial dysfunction. Front Pharmacol. 2022;13:859723. doi: 10.3389/fphar.2022.859723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gąssowska-Dobrowolska M, Kolasa A, Beversdorf DQ, Adamczyk A. Alterations in cerebellar microtubule cytoskeletal network in a valproicacid-induced rat model of autism spectrum disorders. Biomedicines. 2022;10:3031. doi: 10.3390/biomedicines10123031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Coccetti P, Nicastro R, Tripodi F. Conventional and emerging roles of the energy sensor Snf1/AMPK in saccharomyces cerevisiae. Microb Cell. 2018;5:482–494. doi: 10.15698/mic2018.11.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moreno D, Towler MC, Hardie DG, Knecht E, Sanz P. The laforin-malin complex, involved in Lafora disease, promotes the incorporation of K63-linked ubiquitin chains into AMP-activated protein kinase beta subunits. Mol Biol Cell. 2010;21:2578–2588. doi: 10.1091/mbc.E10-03-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Subotić A, Swinnen E, Demuyser L, De Keersmaecker H, Mizuno H, Tournu H, Van Dijck P. A bimolecular fluorescence complementation tool for identification of protein-protein interactions in Candida albicans. G3 (Bethesda) 2017;7:3509–3520. doi: 10.1534/g3.117.300149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ramon M, Ruelens P, Li Y, Sheen J, Geuten K, Rolland F. The hybrid four-CBS-domain KINβγ subunit functions as the canonical γ subunit of the plant energy sensor SnRK1. Plant J. 2013;75:11–25. doi: 10.1111/tpj.12192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ma Y, Li J. Metabolic shifts during aging and pathology. Compr Physiol. 2015;5:667–686. doi: 10.1002/cphy.c140041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saunders H, Dias WB, Slawson C. Growing and dividing: how O-GlcNAcylation leads the way. J Biol Chem. 2023;299:105330. doi: 10.1016/j.jbc.2023.105330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rajan P, Natraj P, Ranaweera SS, Dayarathne LA, Lee YJ, Han CH. Anti-adipogenic effect of the flavonoids through the activation of AMPK in palmitate (PA)-treated HepG2 cells. J Vet Sci. 2022;23:e4. doi: 10.4142/jvs.21256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jiang X, Tan HY, Teng S, Chan YT, Wang D, Wang N. The role of AMP-activated protein kinase as a potential target of treatment of hepatocellular carcinoma. Cancers (Basel) 2019;11:647. doi: 10.3390/cancers11050647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chauhan AS, Zhuang L, Gan B. Spatial control of AMPK signaling at subcellular compartments. Crit Rev Biochem Mol Biol. 2020;55:17–32. doi: 10.1080/10409238.2020.1727840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yan Y, Zhou XE, Xu HE, Melcher K. Structure and physiological regulation of AMPK. Int J Mol Sci. 2018;19:3534. doi: 10.3390/ijms19113534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Healy JE, Gearhart CN, Bateman JL, Handa RJ, Florant GL. AMPK and ACCchange with fasting and physiological condition in euthermic and hibernating golden-mantled ground squirrels (Callospermophilus lateralis) Comp Biochem Physiol A Mol Integr Physiol. 2011;159:322–331. doi: 10.1016/j.cbpa.2011.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hardie DG, Ross FA, Hawley SA. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol. 2012;13:251–262. doi: 10.1038/nrm3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hardie DG, Schaffer BE, Brunet A. AMPK: an energy-sensing pathway with multiple inputs and outputs. Trends Cell Biol. 2016;26:190–201. doi: 10.1016/j.tcb.2015.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Flores K, Siques P, Brito J, Arribas SM. AMPK and the challenge of treating hypoxic pulmonary hypertension. Int J Mol Sci. 2022;23:6205. doi: 10.3390/ijms23116205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lei Y, Huang Y, Wen X, Yin Z, Zhang Z, Klionsky DJ. How cells deal with the fluctuating environment: autophagy regulation under stress in yeast and mammalian systems. Antioxidants (Basel) 2022;11:304. doi: 10.3390/antiox11020304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hawley SA, Ross FA, Russell FM, Atrih A, Lamont DJ, Hardie DG. Mechanism of activation of AMPK by cordycepin. Cell Chem Biol. 2020;27:214–222. e214. doi: 10.1016/j.chembiol.2020.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hardie DG. Keeping the home fires burning: AMP-activated protein kinase. J R Soc Interface. 2018;15:20170774. doi: 10.1098/rsif.2017.0774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yamada E, Lee TW, Pessin JE, Bastie CC. Targeted therapies of the LKB1/AMPK pathway for the treatment of insulin resistance. Future Med Chem. 2010;2:1785–1796. doi: 10.4155/fmc.10.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lu Y, Yuan T, Min X, Yuan Z, Cai Z. AMPK: potential therapeutic target for vascular calcification. Front Cardiovasc Med. 2021;8:670222. doi: 10.3389/fcvm.2021.670222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhao M, Finlay D, Kwong E, Liddington R, Viollet B, Sasaoka N, Vuori K. Cell adhesion suppresses autophagy via Src/FAK-mediated phosphorylation and inhibition of AMPK. Cell Signal. 2022;89:110170. doi: 10.1016/j.cellsig.2021.110170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kong D, Li G, Yang Z, Cheng S, Zhang W, Feng L, Zhang K. Identification of an ACK1/TNK2-based prognostic signature for colon cancer to predict survival and inflammatory landscapes. BMC Cancer. 2022;22:84. doi: 10.1186/s12885-021-09165-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cho HJ, Lee J, Yoon SR, Lee HG, Jung H. Regulation of hematopoietic stem cell fate and malignancy. Int J Mol Sci. 2020;21:4780. doi: 10.3390/ijms21134780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gu GL, Zhang Z, Zhang YH, Yu PF, Dong ZW, Yang HR, Yuan Y. Detection and analysis of common pathogenic germline mutations in Peutz-Jeghers syndrome. World J Gastroenterol. 2021;27:6631–6646. doi: 10.3748/wjg.v27.i39.6631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhou W, Yan LD, Yu ZQ, Li N, Yang YH, Wang M, Chen YY, Mao MX, Peng XC, Cai J. Role of STK11 in ALK-positive non-small cell lung cancer. Oncol Lett. 2022;23:181. doi: 10.3892/ol.2022.13301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shackelford DB, Shaw RJ. The LKB1-AMPK pathway: metabolism and growth control in tumour suppression. Nat Rev Cancer. 2009;9:563–575. doi: 10.1038/nrc2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jiang S, Chen R, Yu J, Li N, Ke R, Luo L, Zou J, Zhang J, Zhang K, Lu N, Huang D. Clinical significance and role of LKB1 in gastric cancer. Mol Med Rep. 2016;13:249–256. doi: 10.3892/mmr.2015.4508. [DOI] [PubMed] [Google Scholar]
- 57.Bort A, Sánchez BG, Spínola E, Mateos-Gómez PA, Rodríguez-Henche N, Díaz-Laviada I. The red pepper’s spicy ingredient capsaicin activates AMPK in HepG2 cells through CaMKKβ. PLoS One. 2019;14:e0211420. doi: 10.1371/journal.pone.0211420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bi L, Ren Y, Feng M, Meng P, Wang Q, Chen W, Jiao Q, Wang Y, Du L, Zhou F, Jiang Y, Chen F, Wang C, Tang B, Wang Y. HDAC11 regulates glycolysis through the LKB1/AMPK signaling pathway to maintain hepatocellular carcinoma stemness. Cancer Res. 2021;81:2015–2028. doi: 10.1158/0008-5472.CAN-20-3044. [DOI] [PubMed] [Google Scholar]
- 59.Chen YR, Li HN, Zhang LJ, Zhang C, He JG. Protein arginine methyltransferase 5 promotes esophageal squamous cell carcinoma proliferation and metastasis via LKB1/AMPK/mTOR signaling pathway. Front Bioeng Biotechnol. 2021;9:645375. doi: 10.3389/fbioe.2021.645375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hollstein PE, Eichner LJ, Brun SN, Kamireddy A, Svensson RU, Vera LI, Ross DS, Rymoff TJ, Hutchins A, Galvez HM, Williams AE, Shokhirev MN, Screaton RA, Berdeaux R, Shaw RJ. The AMPK-related kinases SIK1 and SIK3 mediate key tumor-suppressive effects of LKB1 in NSCLC. Cancer Discov. 2019;9:1606–1627. doi: 10.1158/2159-8290.CD-18-1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li N, Huang D, Lu N, Luo L. Role of the LKB1/AMPK pathway in tumor invasion and metastasis of cancer cells (Review) Oncol Rep. 2015;34:2821–2826. doi: 10.3892/or.2015.4288. [DOI] [PubMed] [Google Scholar]
- 62.Kang S, Huang J, Lee BK, Jung YS, Im E, Koh JM, Im DS. Omega-3 polyunsaturated fatty acids protect human hepatoma cells from developing steatosis through FFA4 (GPR120) Biochim Biophys Acta Mol Cell Biol Lipids. 2018;1863:105–116. doi: 10.1016/j.bbalip.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 63.Fujii T, Kamishikiryo J, Morita T. Palbociclib regulates intracellular lipids in mammary tumor cells by secreting lipoprotein lipase. Pharmacol Rep. 2022;74:503–512. doi: 10.1007/s43440-022-00365-0. [DOI] [PubMed] [Google Scholar]
- 64.Huang Q, Cao H, Zhan L, Sun X, Wang G, Li J, Guo X, Ren T, Wang Z, Lyu Y, Liu B, An J, Xing J. Mitochondrial fission forms a positive feedback loop with cytosolic calcium signaling pathway to promote autophagy in hepatocellular carcinoma cells. Cancer Lett. 2017;403:108–118. doi: 10.1016/j.canlet.2017.05.034. [DOI] [PubMed] [Google Scholar]
- 65.Jing Z, He X, Jia Z, Sa Y, Yang B, Liu P. NCAPD2 inhibits autophagy by regulating Ca(2+)/CAMKK2/AMPK/mTORC1 pathway and PARP-1/SIRT1 axis to promote colorectal cancer. Cancer Lett. 2021;520:26–37. doi: 10.1016/j.canlet.2021.06.029. [DOI] [PubMed] [Google Scholar]
- 66.Chen P, Reed G, Jiang J, Wang Y, Sunega J, Dong R, Ma Y, Esparham A, Ferrell R, Levine M, Drisko J, Chen Q. Pharmacokinetic evaluation of intravenous vitamin C: a classic pharmacokinetic study. Clin Pharmacokinet. 2022;61:1237–1249. doi: 10.1007/s40262-022-01142-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Courtes MG, Baudoux N, Astaras C, Fernandez E. Does high-dose intravenous vitamin C has anti-cancer activity? Rev Med Suisse. 2022;18:1002–1006. doi: 10.53738/REVMED.2022.18.782.1002. [DOI] [PubMed] [Google Scholar]
- 68.Furqan M, Abu-Hejleh T, Stephens LM, Hartwig SM, Mott SL, Pulliam CF, Petronek M, Henrich JB, Fath MA, Houtman JC, Varga SM, Bodeker KL, Bossler AD, Bellizzi AM, Zhang J, Monga V, Mani H, Ivanovic M, Smith BJ, Byrne MM, Zeitler W, Wagner BA, Buettner GR, Cullen JJ, Buatti JM, Spitz DR, Allen BG. Pharmacological ascorbate improves the response to platinum-based chemotherapy in advanced stage non-small cell lung cancer. Redox Biol. 2022;53:102318. doi: 10.1016/j.redox.2022.102318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.O’Leary BR, Alexander MS, Du J, Moose DL, Henry MD, Cullen JJ. Pharmacological ascorbate inhibits pancreatic cancer metastases via a peroxide-mediated mechanism. Sci Rep. 2020;10:17649. doi: 10.1038/s41598-020-74806-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ma Y, Chapman J, Levine M, Polireddy K, Drisko J, Chen Q. High-dose parenteral ascorbate enhanced chemosensitivity of ovarian cancer and reduced toxicity of chemotherapy. Sci Transl Med. 2014;6:222ra18. doi: 10.1126/scitranslmed.3007154. [DOI] [PubMed] [Google Scholar]
- 71.Liu Y, Huang P, Li Z, Xu C, Wang H, Jia B, Gong A, Xu M. Vitamin C sensitizes pancreatic cancer cells to erastin-induced ferroptosis by activating the AMPK/Nrf2/HMOX1 pathway. Oxid Med Cell Longev. 2022;2022:5361241. doi: 10.1155/2022/5361241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Višnjić D, Lalić H, Dembitz V, Tomić B, Smoljo T. AICAr, a widely used AMPK activator with important AMPK-independent effects: a systematic review. Cells. 2021;10:1095. doi: 10.3390/cells10051095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Su CC, Hsieh KL, Liu PL, Yeh HC, Huang SP, Fang SH, Cheng WC, Huang KH, Chiu FY, Lin IL, Huang MY, Li CY. AICAR induces apoptosis and inhibits migration and invasion in prostate cancer cells through an AMPK/mTOR-dependent pathway. Int J Mol Sci. 2019;20:1647. doi: 10.3390/ijms20071647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rae C, Mairs RJ. AMPK activation by AICAR sensitizes prostate cancer cells to radiotherapy. Oncotarget. 2019;10:749–759. doi: 10.18632/oncotarget.26598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liu J, Liang L, Li X, Peng YL, Zhang J, Wang XL, Liu J, Nie L. AICAR suppresses cell proliferation and synergizes with decitabine in myelodysplastic syndrome via DNA damage induction. Biotechnol Lett. 2021;43:1131–1142. doi: 10.1007/s10529-021-03112-2. [DOI] [PubMed] [Google Scholar]
- 76.Yan S, Yuan D, Li Q, Li S, Zhang F. AICAR enhances the cytotoxicity of PFKFB3 inhibitor in an AMPK signaling-independent manner in colorectal cancer cells. Med Oncol. 2021;39:10. doi: 10.1007/s12032-021-01601-y. [DOI] [PubMed] [Google Scholar]
- 77.Khamaru P, Chakraborty S, Bhattacharyya A. AMPK activator AICAR in combination with anti-mouse IL10 mAb restores the functionality of intra-tumoral Tfh cells in the 4T1 mouse model. Cell Immunol. 2022;382:104639. doi: 10.1016/j.cellimm.2022.104639. [DOI] [PubMed] [Google Scholar]
- 78.Aikawa A, Kozako T, Kato N, Ohsugi T, Honda SI. Anti-tumor activity of 5-aminoimidazole-4-carboxamide riboside with AMPK-independent cell death in human adult T-cell leukemia/lymphoma. Eur J Pharmacol. 2023;961:176180. doi: 10.1016/j.ejphar.2023.176180. [DOI] [PubMed] [Google Scholar]
- 79.Lv Z, Guo Y. Metformin and its benefits for various diseases. Front Endocrinol (Lausanne) 2020;11:191. doi: 10.3389/fendo.2020.00191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Brown JR, Chan DK, Shank JJ, Griffith KA, Fan H, Szulawski R, Yang K, Reynolds RK, Johnston C, McLean K, Uppal S, Liu JR, Cabrera L, Taylor SE, Orr BC, Modugno F, Mehta P, Bregenzer M, Mehta G, Shen H, Coffman LG, Buckanovich RJ. Phase II clinical trial of metformin as a cancer stem cell-targeting agent in ovarian cancer. JCI Insight. 2020;5:e133247. doi: 10.1172/jci.insight.133247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Huang X, Sun T, Wang J, Hong X, Chen H, Yan T, Zhou C, Sun D, Yang C, Yu T, Su W, Du W, Xiong H. Metformin reprograms tryptophan metabolism to stimulate CD8+ T-cell function in colorectal cancer. Cancer Res. 2023;83:2358–2371. doi: 10.1158/0008-5472.CAN-22-3042. [DOI] [PubMed] [Google Scholar]
- 82.Cunha Júnior AD, Bragagnoli AC, Costa FO, Carvalheira JBC. Repurposing metformin for the treatment of gastrointestinal cancer. World J Gastroenterol. 2021;27:1883–1904. doi: 10.3748/wjg.v27.i17.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cejuela M, Martin-Castillo B, Menendez JA, Pernas S. Metformin and breast cancer: where are we now? Int J Mol Sci. 2022;23:2705. doi: 10.3390/ijms23052705. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 84.Bu Y, Peng M, Tang X, Xu X, Wu Y, Chen AF, Yang X. Protective effects of metformin in various cardiovascular diseases: clinical evidence and AMPK-dependent mechanisms. J Cell Mol Med. 2022;26:4886–4903. doi: 10.1111/jcmm.17519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jin P, Jiang J, Zhou L, Huang Z, Qin S, Chen HN, Peng L, Zhang Z, Li B, Luo M, Zhang T, Ming H, Ding N, Li L, Xie N, Gao W, Zhang W, Nice EC, Wei Y, Huang C. Disrupting metformin adaptation of liver cancer cells by targeting the TOMM34/ATP5B axis. EMBO Mol Med. 2022;14:e16082. doi: 10.15252/emmm.202216082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yerevanian A, Soukas AA. Metformin: mechanisms in human obesity and weight loss. Curr Obes Rep. 2019;8:156–164. doi: 10.1007/s13679-019-00335-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Orang A, Ali SR, Petersen J, McKinnon RA, Aloia AL, Michael MZ. A functional screen with metformin identifies microRNAs that regulate metabolism in colorectal cancer cells. Sci Rep. 2022;12:2889. doi: 10.1038/s41598-022-06587-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lin H, Ao H, Guo G, Liu M. The role and mechanism of metformin in inflammatory diseases. J Inflamm Res. 2023;16:5545–5564. doi: 10.2147/JIR.S436147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhao XP, Zheng XL, Huang M, Xie YJ, Nie XW, Nasim AA, Yao XJ, Fan XX. DMU-212 against EGFR-mutant non-small cell lung cancer via AMPK/PI3K/Erk signaling pathway. Heliyon. 2023;9:e15812. doi: 10.1016/j.heliyon.2023.e15812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mahalingam D, Hanni S, Serritella AV, Fountzilas C, Michalek J, Hernandez B, Sarantopoulos J, Datta P, Romero O, Pillai SMA, Kuhn J, Pollak M, Thompson IM. Utilizing metformin to prevent metabolic syndrome due to androgen deprivation therapy (ADT): a randomized phase II study of metformin in non-diabetic men initiating ADT for advanced prostate cancer. Oncotarget. 2023;14:622–636. doi: 10.18632/oncotarget.28458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Urban C, Hayes HV, Piraino G, Wolfe V, Lahni P, O’Connor M, Phares C, Zingarelli B. Colivelin, a synthetic derivative of humanin, ameliorates endothelial injury and glycocalyx shedding after sepsis in mice. Front Immunol. 2022;13:984298. doi: 10.3389/fimmu.2022.984298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang S, Lin Y, Xiong X, Wang L, Guo Y, Chen Y, Chen S, Wang G, Lin P, Chen H, Yeung SJ, Bremer E, Zhang H. Low-dose metformin reprograms the tumor immune microenvironment in human esophageal cancer: results of a phase II clinical trial. Clin Cancer Res. 2020;26:4921–4932. doi: 10.1158/1078-0432.CCR-20-0113. [DOI] [PubMed] [Google Scholar]
- 93.Subbiah V, Coleman N, Piha-Paul SA, Tsimberidou AM, Janku F, Rodon J, Pant S, Dumbrava EEI, Fu S, Hong DS, Zhang S, Sun M, Jiang Y, Roszik J, Song J, Yuan Y, Meric-Bernstam F, Naing A. Phase I study of mTORC1/2 inhibitor sapanisertib (CB-228/TAK-228) in combination with metformin in patients with mTOR/AKT/PI3K pathway alterations and advanced solid malignancies. Cancer Res Commun. 2024;4:378–387. doi: 10.1158/2767-9764.CRC-22-0260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Eichner LJ, Brun SN, Herzig S, Young NP, Curtis SD, Shackelford DB, Shokhirev MN, Leblanc M, Vera LI, Hutchins A, Ross DS, Shaw RJ, Svensson RU. Genetic analysis reveals AMPK is required to support tumor growth in murine kras-dependent lung cancer models. Cell Metab. 2019;29:285–302. e287. doi: 10.1016/j.cmet.2018.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cai Z, Li CF, Han F, Liu C, Zhang A, Hsu CC, Peng D, Zhang X, Jin G, Rezaeian AH, Wang G, Zhang W, Pan BS, Wang CY, Wang YH, Wu SY, Yang SC, Hsu FC, D’Agostino RB Jr, Furdui CM, Kucera GL, Parks JS, Chilton FH, Huang CY, Tsai FJ, Pasche B, Watabe K, Lin HK. Phosphorylation of PDHA by AMPK drives TCA cycle to promote cancer metastasis. Mol Cell. 2020;80:263–278. e267. doi: 10.1016/j.molcel.2020.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lei Y, Cai S, Zhang CD, Li YS. The biological role of extracellular vesicles in gastric cancer metastasis. Front Cell Dev Biol. 2024;12:1323348. doi: 10.3389/fcell.2024.1323348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hou CY, Ma CY, Lin YJ, Huang CL, Wang HD, Yuh CH. WNK1-OSR1 signaling regulates angiogenesis-mediated metastasis towards developing a combinatorial anti-cancer strategy. Int J Mol Sci. 2022;23:12100. doi: 10.3390/ijms232012100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhu Y, Liu W, Wang Z, Wang Y, Tan C, Pan Z, Wang A, Liu J, Sun G. ARHGEF2/EDN1 pathway participates in ER stress-related drug resistance of hepatocellular carcinoma by promoting angiogenesis and malignant proliferation. Cell Death Dis. 2022;13:652. doi: 10.1038/s41419-022-05099-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fang JH, Chen JY, Zheng JL, Zeng HX, Chen JG, Wu CH, Cai JL, Wang ZY, Zhuang SM. Fructose metabolism in tumor endothelial cells promotes angiogenesis by activating AMPK signaling and mitochondrial respiration. Cancer Res. 2023;83:1249–1263. doi: 10.1158/0008-5472.CAN-22-1844. [DOI] [PubMed] [Google Scholar]
- 100.Zhang J, Wang E, Zhang L, Zhou B. PSPH induces cell autophagy and promotes cell proliferation and invasion in the hepatocellular carcinoma cell line Huh7 via the AMPK/mTOR/ULK1 signaling pathway. Cell Biol Int. 2021;45:305–319. doi: 10.1002/cbin.11489. [DOI] [PubMed] [Google Scholar]
- 101.Stüwe SH, Goetze O, Arning L, Banasch M, Schmidt WE, Schöls L, Saft C. Hepatic mitochondrial dysfunction in Friedreich ataxia. BMC Neurol. 2011;11:145. doi: 10.1186/1471-2377-11-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tripodi F, Badone B, Vescovi M, Milanesi R, Nonnis S, Maffioli E, Bonanomi M, Gaglio D, Tedeschi G, Coccetti P. Methionine supplementation affects metabolism and reduces tumor aggressiveness in liver cancer cells. Cells. 2020;9:2491. doi: 10.3390/cells9112491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhang H, Yang S, Wang J, Jiang Y. Blockade of AMPK-mediated cAMP-PKA-CREB/ATF1 signaling synergizes with aspirin to inhibit hepatocellular carcinoma. Cancers (Basel) 2021;13:1738. doi: 10.3390/cancers13071738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yu Y, Xu Z, Zhou H, Xu R, Xu J, Liu W, Wu Y, Qiu Y, Zhang G, Huang X, Chen Y. RBP7 functions as a tumor suppressor in HR + breast cancer by inhibiting the AKT/SREBP1 pathway and reducing fatty acid. Cancer Cell Int. 2024;24:118. doi: 10.1186/s12935-024-03299-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Su F, Koeberle A. Regulation and targeting of SREBP-1 in hepatocellular carcinoma. Cancer Metastasis Rev. 2024;43:673–708. doi: 10.1007/s10555-023-10156-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wang C, Tong Y, Wen Y, Cai J, Guo H, Huang L, Xu M, Feng M, Chen X, Zhang J, Wu H, Kong X, Xia Q. Hepatocellular carcinoma-associated protein TD26 interacts and enhances sterol regulatory element-binding protein 1 activity to promote tumor cell proliferation and growth. Hepatology. 2018;68:1833–1850. doi: 10.1002/hep.30030. [DOI] [PubMed] [Google Scholar]
- 107.He Y, Dong Y, Chen Y, Zhang G, Zhang H, Lei G, Du Y, Chen X, Ye Y, Liu H. Multi-omics characterization and therapeutic liability of ferroptosis in melanoma. Signal Transduct Target Ther. 2022;7:268. doi: 10.1038/s41392-022-01067-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhu J, Zhao S, Zhu Y, Ma S, Han T, Xu F, Li H, Wang B, Guo Z, Chen D, Qu Y, Tian Z, Zhao J, Liu L. Sorafenib sensitization in tumor therapy by iron overload and AMPK activation. Nano Research. 2024;17:6386–6399. [Google Scholar]
- 109.Shi Z, Liu R, Lu Q, Zeng Z, Liu Y, Zhao J, Liu X, Li L, Huang H, Yao Y, Huang D, Xu Q. UBE2O promotes hepatocellular carcinoma cell proliferation and invasion by regulating the AMPKα2/mTOR pathway. Int J Med Sci. 2021;18:3749–3758. doi: 10.7150/ijms.63220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhang EB, Zhang X, Wang K, Zhang F, Chen TW, Ma N, Ni QZ, Wang YK, Zheng QW, Cao HJ, Xia J, Zhu B, Xu S, Ding X, Wang X, Li Z, Cheng S, Xie D, Li JJ. Antifungal agent Terbinafine restrains tumor growth in preclinical models of hepatocellular carcinoma via AMPK-mTOR axis. Oncogene. 2021;40:5302–5313. doi: 10.1038/s41388-021-01934-y. [DOI] [PubMed] [Google Scholar]
- 111.Chop M, Ledo C, Nicolao MC, Loos J, Cumino A, Rodriguez Rodrigues C. Hydatid fluid from Echinococcus granulosus induces autophagy in dendritic cells and promotes polyfunctional T-cell responses. Front Cell Infect Microbiol. 2024;14:1334211. doi: 10.3389/fcimb.2024.1334211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhou L, Wang J, Hou H, Li J, Li J, Liang J, Li J, Niu X, Hou R, Zhang K. Autophagy inhibits inflammation via down-regulation of p38 MAPK/mTOR signaling cascade in endothelial cells. Clin Cosmet Investig Dermatol. 2023;16:659–669. doi: 10.2147/CCID.S405068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cheng C, Wang T, Song Z, Peng L, Gao M, Hermine O, Rousseaux S, Khochbin S, Mi JQ, Wang J. Induction of autophagy and autophagy-dependent apoptosis in diffuse large B-cell lymphoma by a new antimalarial artemisinin derivative, SM1044. Cancer Med. 2018;7:380–396. doi: 10.1002/cam4.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lai HY, Tsai HH, Yen CJ, Hung LY, Yang CC, Ho CH, Liang HY, Chen FW, Li CF, Wang JM. Metformin resensitizes sorafenib-resistant HCC cells through AMPK-dependent autophagy activation. Front Cell Dev Biol. 2021;8:596655. doi: 10.3389/fcell.2020.596655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kim YW, Bak SB, Lee WY, Bae SJ, Lee EH, Yang JH, Kim KY, Song CH, Kim SC, Yun UJ, Park KI. Systemic and molecular analysis dissect the red ginseng induction of apoptosis and autophagy in HCC as mediated with AMPK. J Ginseng Res. 2023;47:479–491. doi: 10.1016/j.jgr.2023.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yang X, Liu Y, Li M, Wu H, Wang Y, You Y, Li P, Ding X, Liu C, Gong J. Predictive and preventive significance of AMPK activation on hepatocarcinogenesis in patients with liver cirrhosis. Cell Death Dis. 2018;9:264. doi: 10.1038/s41419-018-0308-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yao J, Tang S, Shi C, Lin Y, Ge L, Chen Q, Ou B, Liu D, Miao Y, Xie Q, Tang X, Fei J, Yang G, Tian J, Zeng X. Isoginkgetin, a potential CDK6 inhibitor, suppresses SLC2A1/GLUT1 enhancer activity to induce AMPK-ULK1-mediated cytotoxic autophagy in hepatocellular carcinoma. Autophagy. 2023;19:1221–1238. doi: 10.1080/15548627.2022.2119353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sundarraj K, Raghunath A, Panneerselvam L, Perumal E. Fisetin inhibits autophagy in HepG2 cells via PI3K/Akt/mTOR and AMPK pathway. Nutr Cancer. 2021;73:2502–2514. doi: 10.1080/01635581.2020.1836241. [DOI] [PubMed] [Google Scholar]
- 119.Gao C, Fang L, Zhang H, Zhang WS, Li XO, Du SY. Metformin induces autophagy via the AMPK-mTOR signaling pathway in human hepatocellular carcinoma cells. Cancer Manag Res. 2020;12:5803–5811. doi: 10.2147/CMAR.S257966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chen Y, Li Q, Ren S, Chen T, Zhai B, Cheng J, Shi X, Song L, Fan Y, Guo D. Investigation and experimental validation of curcumin-related mechanisms against hepatocellular carcinoma based on network pharmacology. J Zhejiang Univ Sci B. 2022;23:682–698. doi: 10.1631/jzus.B2200038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.El-Sewedy T, Salama AF, Mohamed AE, Elbaioumy NM, El-Far AH, Albalawi AN, Elmetwalli A. Hepatocellular carcinoma cells: activity of amygdalin and sorafenib in targeting AMPK/mTOR and BCL-2 for anti-angiogenesis and apoptosis cell death. BMC Complement Med Ther. 2023;23:329. doi: 10.1186/s12906-023-04142-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Liu Z, Wang N, Meng Z, Lu S, Peng G. Pseudolaric acid B triggers cell apoptosis by activating AMPK/JNK/DRP1/mitochondrial fission pathway in hepatocellular carcinoma. Toxicology. 2023;493:153556. doi: 10.1016/j.tox.2023.153556. [DOI] [PubMed] [Google Scholar]
- 123.Shui L, Wang W, Xie M, Ye B, Li X, Liu Y, Zheng M. Isoquercitrin induces apoptosis and autophagy in hepatocellular carcinoma cells via AMPK/mTOR/p70S6K signaling pathway. Aging (Albany NY) 2020;12:24318–24332. doi: 10.18632/aging.202237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Baek SY, Hwang UW, Suk HY, Kim YW. Hemistepsin A inhibits cell proliferation and induces G0/G1-phase arrest, cellular senescence and apoptosis via the AMPK and p53/p21 signals in human hepatocellular carcinoma. Biomolecules. 2020;10:713. doi: 10.3390/biom10050713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yang W, Feng Q, Li M, Su J, Wang P, Wang X, Yin Y, Wang X, Zhao M. Sinomenine suppresses development of hepatocellular carcinoma cells via inhibiting MARCH1 and AMPK/STAT3 signaling pathway. Front Mol Biosci. 2021;8:684262. doi: 10.3389/fmolb.2021.684262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Li Y, Chen R, Yang J, Mo S, Quek K, Kok CH, Cheng XD, Tian S, Zhang W, Qin JJ. Integrated bioinformatics analysis reveals key candidate genes and pathways associated with clinical outcome in hepatocellular carcinoma. Front Genet. 2020;11:814. doi: 10.3389/fgene.2020.00814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Guan R, Guo W, Hong W, Lin Y, Zou X, Shi N, Yang D, Zhou Y, Jian Z, Jin H, Lin W, Yu M. Identification of aberrantly methylated differentially CpG sites in hepatocellular carcinoma and their association with patient survival. Front Oncol. 2020;10:1031. doi: 10.3389/fonc.2020.01031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Gu Y, Huang H, Tong Q, Cao M, Ming W, Zhang R, Zhu W, Wang Y, Sun X. Multi-view radiomics feature fusion reveals distinct immuno-oncological characteristics and clinical prognoses in hepatocellular carcinoma. Cancers (Basel) 2023;15:2338. doi: 10.3390/cancers15082338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Shi F, Cao S, Zhu Y, Yu Q, Guo W, Zhang S. High expression of DHX9 promotes the growth and metastasis of hepatocellular carcinoma. J Clin Lab Anal. 2021;35:e24052. doi: 10.1002/jcla.24052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kim BJ, Bak SB, Bae SJ, Jin HJ, Park SM, Kim YR, Jung DH, Song CH, Kim YW, Kim SC, Lee WY, Park SD. Protective effects of red ginseng against tacrine-induced hepatotoxicity: an integrated approach with network pharmacology and experimental validation. Drug Des Devel Ther. 2024;18:549–566. doi: 10.2147/DDDT.S450305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Cairns J, Ly RC, Niu N, Kalari KR, Carlson EE, Wang L. CDC25B partners with PP2A to induce AMPK activation and tumor suppression in triple negative breast cancer. NAR Cancer. 2020;2:zcaa039. doi: 10.1093/narcan/zcaa039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sun Z, Liu L, Liang H, Zhang L. Nicotinamide mononucleotide induces autophagy and ferroptosis via AMPK/mTOR pathway in hepatocellular carcinoma. Mol Carcinog. 2024;63:577–588. doi: 10.1002/mc.23673. [DOI] [PubMed] [Google Scholar]
- 133.Pokhrel RH, Acharya S, Ahn JH, Gu Y, Pandit M, Kim JO, Park YY, Kang B, Ko HJ, Chang JH. AMPK promotes antitumor immunity by downregulating PD-1 in regulatory T cells via the HMGCR/p38 signaling pathway. Mol Cancer. 2021;20:133. doi: 10.1186/s12943-021-01420-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Liu M, Liu X, He K, Jian Y, Li Y, Guo J, Yang J, Xu Z, Kang W. 3-Epi-betulinic acid 3-O-β-D-glucopyranoside (eBAG) induces autophagy by activation of AMP-activated protein kinase in hepatocellular carcinoma. Food Sci Hum Wellness. 2024;13:1453–1464. [Google Scholar]
- 135.Chen S, Cao Q, Wen W, Wang H. Targeted therapy for hepatocellular carcinoma: challenges and opportunities. Cancer Lett. 2019;460:1–9. doi: 10.1016/j.canlet.2019.114428. [DOI] [PubMed] [Google Scholar]
- 136.Dong H, Huang J, Zheng K, Tan D, Chang Q, Gong G, Zhang Q, Tang H, Sun J, Zhang S. Metformin enhances the chemosensitivity of hepatocarcinoma cells to cisplatin through AMPK pathway. Oncol Lett. 2017;14:7807–7812. doi: 10.3892/ol.2017.7198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zheng L, Yang W, Wu F, Wang C, Yu L, Tang L, Qiu B, Li Y, Guo L, Wu M, Feng G, Zou D, Wang H. Prognostic significance of AMPK activation and therapeutic effects of metformin in hepatocellular carcinoma. Clin Cancer Res. 2013;19:5372–5380. doi: 10.1158/1078-0432.CCR-13-0203. [DOI] [PubMed] [Google Scholar]
- 138.Oura K, Morishita A, Hamaya S, Fujita K, Masaki T. The roles of epigenetic regulation and the tumor microenvironment in the mechanism of resistance to systemic therapy in hepatocellular carcinoma. Int J Mol Sci. 2023;24:2805. doi: 10.3390/ijms24032805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ma X, Qiu Y, Sun Y, Zhu L, Zhao Y, Li T, Lin Y, Ma D, Qin Z, Sun C, Han L. NOD2 inhibits tumorigenesis and increases chemosensitivity of hepatocellular carcinoma by targeting AMPK pathway. Cell Death Dis. 2020;11:174. doi: 10.1038/s41419-020-2368-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Xue D, Han J, Liang Z, Jia L, Liu Y, Tuo H, Peng Y. Current perspectives on the unique roles of exosomes in drug resistance of hepatocellular carcinoma. J Hepatocell Carcinoma. 2022;9:99–112. doi: 10.2147/JHC.S351038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ren Y, Gu YK, Li Z, Xu GZ, Zhang YM, Dong MX, Wang Y, Zhou XB. CXCR3 confers sorafenib resistance of HCC cells through regulating metabolic alteration and AMPK pathway. Am J Transl Res. 2020;12:825–836. [PMC free article] [PubMed] [Google Scholar]
- 142.Zhou T, Zhang J. Types and progress of clinical trial design for breast cancer: a narrative review. Transl Breast Cancer Res. 2023;4:20. doi: 10.21037/tbcr-23-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Peng X, Ma X, Lu S, Li Z. A versatile plant rhabdovirus-based vector for gene silencing, miRNA expression and depletion, and antibody production. Front Plant Sci. 2020;11:627880. doi: 10.3389/fpls.2020.627880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zhang F, Zhou Y, Ding J. The current landscape of microRNAs (miRNAs) in bacterial pneumonia: opportunities and challenges. Cell Mol Biol Lett. 2022;27:70. doi: 10.1186/s11658-022-00368-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sun H, Kemper JK. MicroRNA regulation of AMPK in nonalcoholic fatty liver disease. Exp Mol Med. 2023;55:1974–1981. doi: 10.1038/s12276-023-01072-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Li Y, Jia Y, Wang X, Shang H, Tian Y. Protein-targeted degradation agents based on natural products. Pharmaceuticals (Basel) 2022;16:46. doi: 10.3390/ph16010046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Traldi F, Resmini M. Impact of protein corona formation on the thermoresponsive behavior of acrylamide-based nanogels. Biomacromolecules. 2024;25:1340–1350. doi: 10.1021/acs.biomac.3c01405. [DOI] [PMC free article] [PubMed] [Google Scholar]