Abstract
Background
Transcatheter aortic valve replacement (TAVR) is an important treatment option for patients with severe symptomatic aortic stenosis. It is important to identify predictors of excellent outcomes (good clinical outcomes, more time spent at home) after TAVR that are potentially amenable to improvement.
Objectives
The purpose of the study was to use machine learning to identify potentially modifiable predictors of clinically relevant patient-centered outcomes after TAVR.
Methods
We used data from 8,332 TAVR cases (January 2016-December 2021) from 21 hospitals to train random forest models with 57 patient characteristics (demographics, comorbidities, surgical risk score, lab values, health status scores) and care process parameters to predict the end point, a composite of parameters that designated an excellent outcome and included no major complications (in-hospital or at 30 days), post-TAVR length of stay of 1 day or less, discharge to home, no readmission, and alive at 30 days. We used recursive feature elimination with cross-validation and Shapley Additive Explanation feature importance to identify parameters with the highest predictive values.
Results
The final random forest model retained 29 predictors (15 patient characteristics and 14 care process components); the area under the curve, sensitivity, and specificity were 0.77, 0.67, and 0.73, respectively. Four potentially modifiable predictors with relatively high Shapley Additive Explanation values were identified: type of anesthesia, direct movement to stepdown unit post-TAVR, time between catheterization and TAVR, and preprocedural length of stay.
Conclusions
This study identified four potentially modifiable predictors of excellent outcome after TAVR, suggesting that machine learning combined with hospital-level data can inform modifiable components of care, which could support better delivery of care for patients undergoing TAVR.
Key words: artificial intelligence, cardiology, care process, patient-centered, random forest, TAVR
Central Illustration
Aortic stenosis (AS) is the most common valve disease in the United States,1 affecting 4.4% of adults aged ≥65 years.2 Severe AS occurs when there is a hemodynamically significant narrowing of the aortic valve. Untreated AS is correlated with high morbidity and mortality.3,4
Transcatheter aortic valve replacement (TAVR) is an alternative to surgical aortic valve replacement for patients with severe AS.3 Results of the recent PARTNER 3 trial suggest that TAVR is becoming the preferred choice for candidates of all risk levels in patients aged ≥65 years.5 Despite recent advances in technology and techniques, complications leading to extended lengths of stay or readmission are common post-TAVR.6 As mortality rates for most cardiovascular procedures have declined over the past 2 to 3 decades, there has been greater emphasis on understanding quality of care among patients who survive these interventions.
Existing evidence has examined factors impacting outcomes post-TAVR but has not identified a combination of pre- and peri-procedural levers that can help achieve outcomes that matter most to patients. Based on a recent study, patients care about being able to do specific day-to-day activities, maintaining independence, reducing pain, symptoms and suffering, and staying alive.7 These goals can be translated to concrete outcomes, and achieving these patient goals can be termed a “Tier-1” outcome. With the increasing volume of TAVR procedures, more data are available that can be leveraged to improve patient outcomes.8
Machine learning (ML) techniques use algorithms to detect patterns in large, complex datasets to make predictions. ML has the potential to make novel or more accurate predictions than traditional statistical models.9 ML holds promise for cardiovascular medicine, as an increasing volume of high-quality data can be strategically combined with the drive for greater efficiencies in health care systems and the growing demand for personalized care.8
Previous ML models have identified parameters that are predictive of patient risk levels for transcatheter aortic valve implantation/TAVR5,10,11 and percutaneous coronary intervention.12 However, these studies have predominantly identified nonmodifiable patient characteristics as predictors of patient outcomes. Using ML to identify predictors that are modifiable at the clinical point of care would create opportunities for health care providers and systems to improve their delivery of care to patients.
In this study, we used a large multicenter dataset to develop a random forest (RF) ML model to identify modifiable care process predictors of Tier-1 outcomes 30-days post-TAVR. We present the accuracy of the RF model and identify the most important modifiable care process predictors, including how they influenced patient outcomes in our dataset.
Methods
Data source
This retrospective study utilized de-identified data from the Biome multicenter data repository (Biome Analytics), which aggregates clinical and financial data from 21 hospitals in the United States with TAVR programs. This repository included 11 teaching and 10 nonteaching hospitals, comprising public and private institutions. Thirteen of the hospitals were located on the West Coast. Twenty of the hospitals conducted more than 50 TAVR procedures per year, and 13 of the hospitals conducted over 100 TAVRs annually.
Clinical data fields were those defined by the National Cardiovascular Data Registry and Transcatheter Valve Therapy Registry.13 Other data fields were derived from line-item cost accounting data provided by member hospitals.
Study population
The study population included all TAVR cases from January 2016 through December 2021 at member hospitals. Cases with missing data in ≥1 model predictors were excluded. To be included, cases were required to satisfy the following criteria: “Elective” as the admission status, transfemoral access, not valve-in-valve TAVR, procedure was not aborted, length of stay (LOS) was not an outlier (LOS outlier defined as case with admit or discharge dates that was null, discharge date before the admit date, or post-TAVR LOS >100 days), the discharge location was not null, and there was matched administrative/hospital cost data. LOS was defined as the time from admission to discharge. Discharge status was defined as the discharge status recorded during the index event.
Study outcomes
The study’s end point was a composite outcome designed to indicate whether a case had an excellent outcome at 30 days. The components of the composite outcome were defined based on parameters that are clinically relevant and that matter to patients: ability to do a specific activity (proxy measure: discharged ≤1 day postprocedure), maintaining independence (proxy measure: discharged home, no readmission within 30 days of procedure), reducing/eliminating pain or symptoms (proxy measure: no major complications), and staying alive.7
To qualify as an excellent outcome, termed “Tier-1,” a patient must have met all of the following criteria: 1) No major complications in-hospital or within 30 days of procedure, including acute kidney injury, stroke, hemorrhage, or vascular complications. Occurrence of any of these complications disqualified the patient from a Tier-1 outcome; 2) No pacemaker insertion in-hospital or within 30 days of procedure; 3) A postprocedural LOS of ≤1 day; 4) Discharged to home; 5) No readmission within 30 days of procedure; and 6) Alive at 30 days.
The outcome parameters were binary, and the outcome was defined to ensure that components can be assessed using registry data. All other cases were designated as Not Tier-1.
Potential predictors
Potential predictor variables were identified based on expert clinical opinion and the experience of member hospitals. A total of 57 potential predictors were identified (Supplemental Table 1). Each parameter was classified as a patient characteristic or a process characteristic potentially amenable to improvement. Some process characteristics were potentially modifiable, such as day of the week on which the procedure occurs in the case of an elective procedure, and some were not modifiable (eg, procedure duration).
Study design and model development
Cases were randomly divided (70:30) into a development set and a test set. To address high rates of missing observations for two potentially important features (potential predictors), imputation was performed. For the Kansas City Cardiomyopathy Questionnaire-12 score, the median value was imputed for missing values (5.8%). For the five-meter walk test, missing values (10.1%) were randomly imputed to either the maximum value (60 seconds) or the median, with probability based on the proportion of the maximum value in observed cases. Categorical predictors were coded ordinally, with a value of 1 corresponding to the lowest likelihood of a Tier-1 outcome.
The RF model was implemented in Python version 3.8 using the scikit-learn library version 1.01.14 Each forest contained 100 trees, and the threshold for classification of a TAVR case to Tier-1 was a predicted Tier-1 outcome by ≥50 trees. RF model hyperparameters were selected using grid search with bootstrap samples and 3-fold cross-validation. Hyperparameters searched were max_depth (maximum = 15), max_features (sqrt or log2), min samples_leaf (maximum = 5), and min_samples_split (maximum = 10). Other hyperparameters were at default values. Predictors were introduced into the RF model in stages. In the baseline stage, 33 patient characteristics were included, and the recursive feature elimination with cross-validation procedure was used to eliminate parameters with very low predictive power. At the next stage, 24 process characteristics were introduced, and 29 total predictors were retained after the recursive feature elimination with cross-validation elimination in the final model (Supplemental Table 2).
To evaluate the RF model’s predictive power, sensitivity, specificity, positive predictive value, and negative predictive value were calculated using the test set. As an additional validation check, a logistic regression model was estimated in Python using the same outcome and predictor variables as the final RF model. Receiver-operator characteristic curves and the total area under the curve (AUC) were calculated for RF and logistic regression models. A calibration curve was constructed for the RF model to compare the predicted vs actual proportion of Tier-1 cases in the test set for each of the 20 bins. Shapley Additive Explanation (SHAP) values were calculated for each TAVR case for each parameter in the RF model and summed across test set cases to show both the magnitude and direction of each parameter to the model’s overall predictions. As a confirming diagnostic, the Gini feature importance was calculated for each parameter in the RF model.
Model development and reporting were conducted in accordance with the critical questions posed by van Smeden et al15 and the standards outlined by the Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis Initiative.16
Results
Dataset characteristics
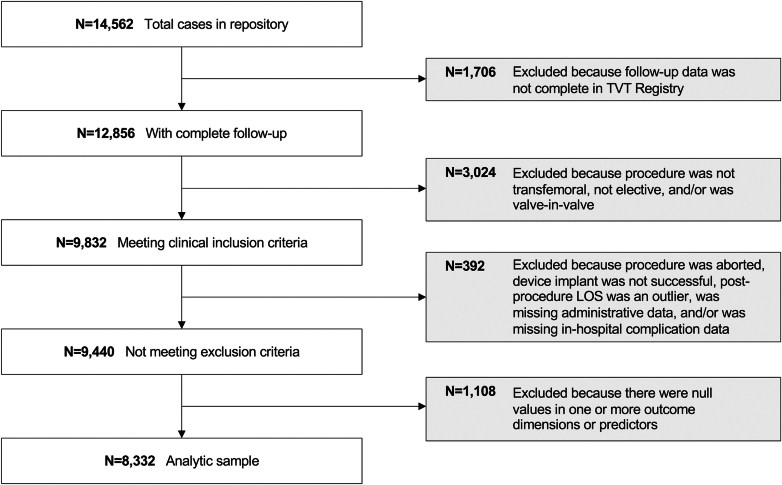
A total of 14,562 TAVR cases were identified from the Biome multicenter data repository (Biome Analytics) (Figure 1). After exclusion, the total number of cases included in the analysis was 8,332. Patient characteristics and process components were similar across development and test sets (Table 1). The mean age of patients in the overall cohort was 79.5 ± 8.5 years, and 56% were male. The mean Society of Thoracic Surgeons (STS) risk score was 4.0 ± 3.7.
Figure 1.
CONSORT Diagram
Study attrition. LOS = length of stay; TVT = transcatheter valve therapy.
Table 1.
Patient Characteristics, Process Components, and Outcomes Across Development and Test Sets
| Overall Cohort (N = 8,332) | Development Set (n = 5,832) | Test Set (n = 2,500) | P Value | |
|---|---|---|---|---|
| Patient characteristics | ||||
| Age (y) | 79.53 ± 8.52 | 79.5 ± 8.54 | 79.61 ± 8.48 | 0.60 |
| Female | 3,662 (44) | 2,575 (44.2) | 1,087 (43.5) | 0.57 |
| STS risk score | 4.04 ± 3.65 | 4.02 ± 3.6 | 4.07 ± 3.75 | 0.56 |
| Preprocedural KCCQ-12 Overall Score | 51.99 ± 24.56 | 51.95 ± 24.62 | 52.11 ± 24.44 | 0.79 |
| Glomerular filtration rate (mL/min) | 66.02 ± 26.29 | 65.94 ± 26.14 | 66.23 ± 26.64 | 0.64 |
| Body mass index (kg/m2) | 27.81 ± 6.52 | 27.85 ± 6.48 | 27.71 ± 6.59 | 0.36 |
| Platelets (per μL) | 208,602 ± 71,218 | 208,882 ± 72,506 | 207,949 ± 68,128 | 0.58 |
| Body surface area (m2) | 1.32 ± 0.47 | 1.32 ± 0.47 | 1.32 ± 0.47 | 0.66 |
| Five-meter walk test (s) | 14.41 ± 18.36 | 14.22 ± 18.18 | 14.85 ± 18.75 | 0.15 |
| Ejection fraction (%) | 58.19 ± 12.54 | 58.18 ± 12.51 | 58.21 ± 12.6 | 0.93 |
| Conduction defect | 2,804 (33.7) | 1,942 (33.3) | 862 (34.5) | 0.30 |
| Atrial fibrillation/flutter | 2,651 (31.8) | 1,859 (31.9) | 792 (31.7) | 0.86 |
| Diabetes mellitus | 2,819 (33.8) | 1,975 (33.9) | 844 (33.8) | 0.93 |
| Prior stroke | 790 (9.5) | 548 (9.4) | 242 (9.7) | 0.69 |
| Prior pacemaker | 939 (11.3) | 662 (11.4) | 277 (11.1) | 0.72 |
| Acute coronary syndrome | 181 (2.2) | 122 (2.1) | 59 (2.4) | 0.44 |
| Prior cardiogenic shock | 23 (0.3) | 15 (0.3) | 8 (0.3) | 0.62 |
| Hostile chest | 393 (4.7) | 266 (4.6) | 127 (5.1) | 0.31 |
| Chronic lung disease | 1,914 (23) | 1,326 (22.7) | 588 (23.5) | 0.44 |
| Prior coronary artery bypass graft | 1,048 (12.6) | 706 (12.1) | 342 (13.7) | 0.05 |
| Prior percutaneous coronary intervention | 2,146 (25.8) | 1,484 (25.5) | 662 (26.5) | 0.32 |
| Prior cardiac arrest | 16 (0.2) | 10 (0.2) | 6 (0.2) | 0.51 |
| Infective endocarditis | 38 (0.5) | 31 (0.5) | 7 (0.3) | 0.12 |
| Current or recent smoker within 1 y | 362 (4.3) | 247 (4.2) | 115 (4.6) | 0.45 |
| Currently on dialysis | 309 (3.7) | 206 (3.5) | 103 (4.1) | 0.19 |
| Immunocompromised | 575 (6.9) | 406 (7) | 169 (6.8) | 0.74 |
| Transient ischemic attack | 599 (7.2) | 426 (7.3) | 173 (6.9) | 0.53 |
| Hypertension | 7,372 (88.5) | 5,158 (88.4) | 2,214 (88.6) | 0.88 |
| Peripheral arterial disease | 1,409 (16.9) | 982 (16.8) | 427 (17.1) | 0.79 |
| Number of previous cardiac surgeries | 0.17 ± 0.42 | 0.16 ± 0.42 | 0.18 ± 0.44 | 0.06 |
| Heart failure hospitalization in the past 2 weeks | 6,525 (78.3) | 4,558 (78.2) | 1,967 (78.7) | 0.59 |
| Previous implantable cardioverter-defibrillator | 183 (2.2) | 132 (2.3) | 51 (2) | 0.52 |
| Prior aortic valve procedure | 151 (1.8) | 96 (1.7) | 55 (2.2) | 0.08 |
| Process characteristics | ||||
| Anesthesia type: moderate sedation | 4,593 (55.1) | 3,215 (55.1) | 1,378 (55.1) | 1.00 |
| Cardiopulmonary bypass | 11 (0.1) | 8 (0.1) | 3 (0.1) | 0.84 |
| Carotid ultrasound | 352 (4.2) | 254 (4.4) | 98 (3.9) | 0.37 |
| Time elapsed between diagnostic catheterization and procedure (days) | 46.7 ± 65.23 | 46.13 ± 64.22 | 47.95 ± 67.51 | 0.24 |
| Closure device used | 7,166 (86.0) | 5,013 (86.0) | 2,153 (86.12) | 0.84 |
| Contrast volume (mL) | 97.9 ± 53.6 | 97.5 ± 53.4 | 98.9 ± 54.1 | 0.30 |
| Direct to step-down (bypass ICU) | 2,237 (26.9) | 1,555 (26.7) | 682 (27.3) | 0.56 |
| 5-m walk documentation compliance | 7,487 (89.9) | 5,249 (90) | 2,238 (89.5) | 0.5 |
| Foley catheter | 575 (6.9) | 409 (7) | 166 (6.6) | 0.54 |
| Intraprocedure inotrope positive | 2,151 (25.8) | 1,506 (25.8) | 645 (25.8) | 0.98 |
| KCCQ-12 documentation compliance | 7,854 (94.3) | 5,497 (94.3) | 2,357 (94.3) | 0.97 |
| Preprocedural length of stay (d) | 0.49 ± 7.22 | 0.55 ± 8.56 | 0.35 ± 1.65 | 0.24 |
| Mechanical assist device placed at start of procedure | 36 (0.4) | 23 (0.4) | 13 (0.5) | 0.42 |
| Pulmonary function testing | 1,318 (15.8) | 915 (15.7) | 403 (16.1) | 0.62 |
| Preprocedure testing | 718 (8.6) | 509 (8.7) | 209 (8.4) | 0.58 |
| Procedure day of the week | 0.90 | |||
| Monday | 1,799 (21.6) | 1,258 (21.6) | 541 (21.6) | |
| Tuesday | 1,315 (15.8) | 920 (15.8) | 395 (15.8) | |
| Wednesday | 2,703 (32.4) | 1,906 (32.7) | 797 (31.9) | |
| Thursday | 1,714 (20.6) | 1,186 (20.3) | 528 (21.1) | |
| Friday | 785 (9.4) | 549 (9.4) | 236 (9.4) | |
| Weekend | 16 (0.2) | 13 (0.2) | 3 (0.1) | |
| Procedure duration (min) | 0.91 ± 0.85 | 0.9 ± 0.82 | 0.94 ± 0.9 | 0.04 |
| Procedural start time (h) | 10.69 ± 2.8 | 10.66 ± 2.79 | 10.75 ± 2.83 | 0.18 |
| Sentinel Protect System | 1,671 (20.1) | 1,161 (19.9) | 510 (20.4) | 0.61 |
| Swan-Ganz catheterization | 540 (6.5) | 379 (6.5) | 161 (6.4) | 0.92 |
| TAVR and percutaneous coronary intervention during admission | 192 (2.3) | 157 (2.7) | 35 (1.4) | <0.01 |
| Valve sheath access method (percutaneous) | 8,249 (99) | 5,777 (99.1) | 2,472 (98.9) | 0.46 |
| Procedure location | 0.21 | |||
| Catheter lab | 2,608 (31.3) | 1,832 (31.4) | 776 (31) | |
| Hybrid cath lab suite | 2,499 (30) | 1,780 (30.5) | 719 (28.8) | |
| Hybrid OR suite | 3,225 (38.7) | 2,220 (38.1) | 1,005 (40.2) | |
| Valve type: balloon expandable | 6,965 (83.6) | 4,860 (83.3) | 2,105 (84.2) | 0.33 |
Values are mean ± SD or n (%). P values < 0.05 are indicated in bold.
ICU = intensive care unit; KCCQ12 = Kansas City Cardiomyopathy Questionnaire 12; OR = operating room; STS = Society of Thoracic Surgeons; TAVR = transcatheter aortic valve replacement.
Approximately half of the overall cohort had a Tier-1 outcome (n = 3,793, 45.5%) (Supplemental Table 3). The most common reason a patient was not classified with a Tier-1 outcome was LOS >1 day (n = 3,943, 86.9% of all Non-Tier-1 cases). Other common reasons were ≥1 complications (N = 1,618, 35.7% of all Non-Tier-1 cases) and readmission within 30 days (n = 525, 11.6% of all Non-Tier-1 cases).
Random forest algorithm performance evaluation
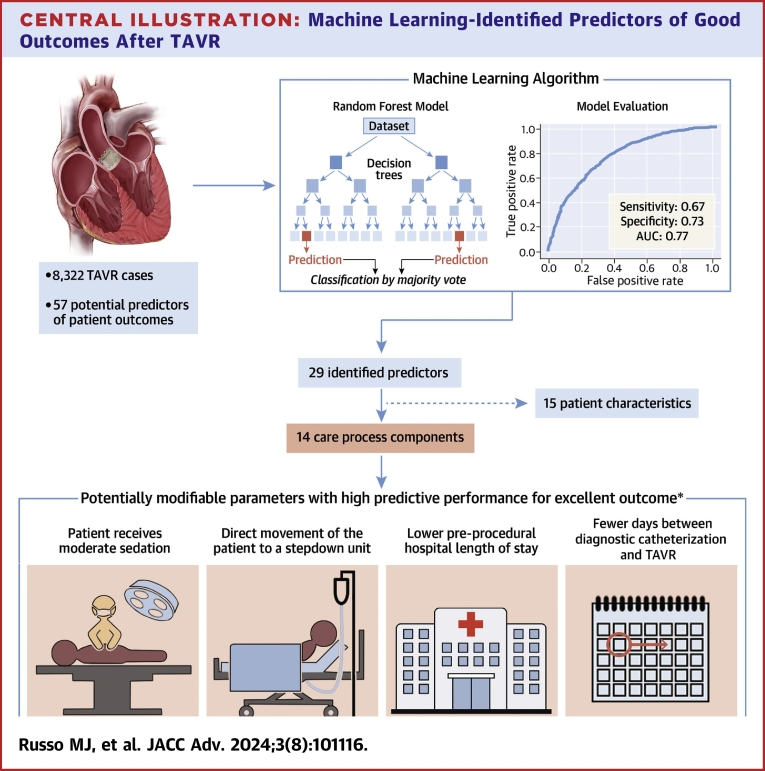
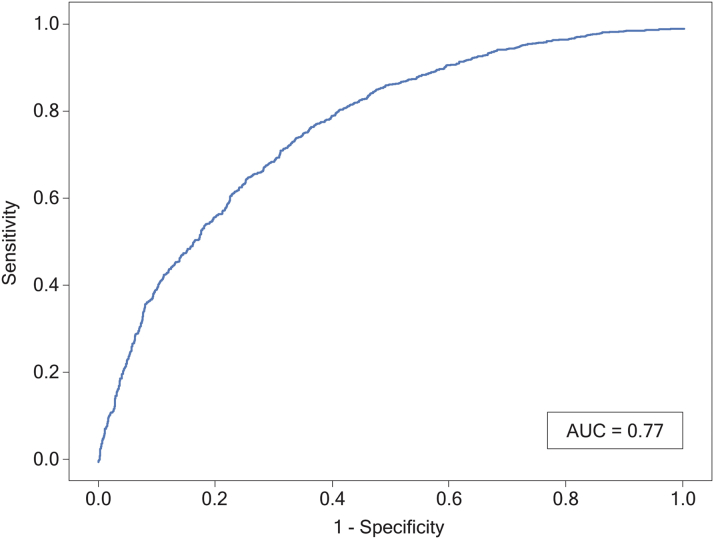
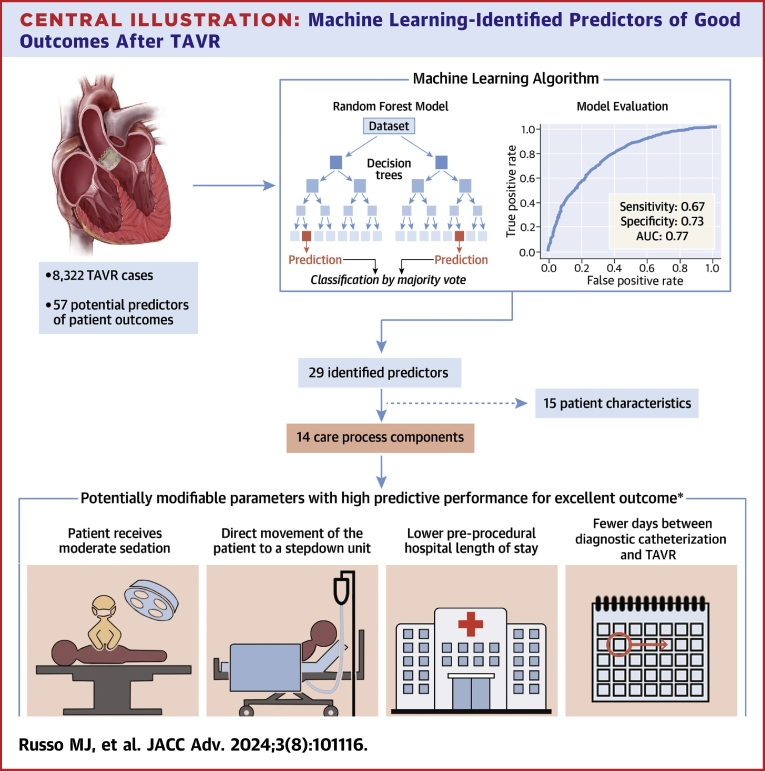
The final RF model with 29 predictors had good sensitivity (0.67) and specificity (0.73) for outcome differentiation, with an AUC of 0.77 (Figure 2). Positive predictive value and negative predictive value were 0.67 and 0.73, respectively. Of the 29 predictors, 15 were patient characteristics and 14 were care process components, of which four were potentially modifiable at the point of care and contributed heavily toward achieving the Tier-1 outcome (Central Illustration).
Figure 2.
Receiver-Operating Characteristic Curve in the Prediction of Outcomes After TAVR
The model had good discriminatory ability (AUC: 0.77) at predicting 30-day good patient outcome after TAVR. Good outcome was defined as no major complications within 30 days, postprocedural LOS ≤1 day, no pacemaker insertion, discharge to home, no readmission within 30 days, and alive at 30 days. AUC = area under the curve; LOS = length of stay; TAVR = transcatheter aortic valve replacement.
Central Illustration.
Machine Learning-Identified Predictors of Good Outcomes After TAVR
Random forest model determined components of the care process that can be modified to potentially improve patient outcomes. ∗Excellent outcome = no major complications in-hospital or within 30 days AND no pacemaker insertion in-hospital or within 30 days AND postprocedural LOS ≤1 day AND discharged to home AND no readmission within 30 days of procedure AND alive at 30 days. AUC = area under the curve; LOS = length of stay; TAVR = transcatheter aortic valve replacement.
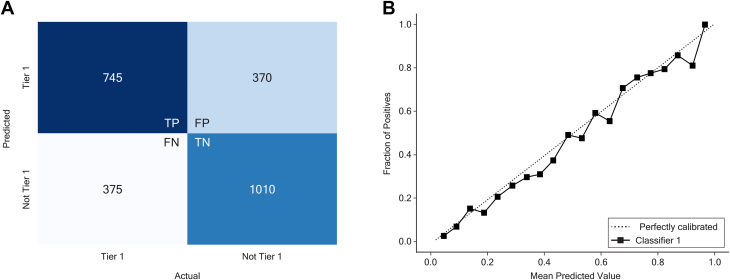
When evaluated on the test set of 2,500 patients, the false positive rate and false negative rate were each 15% (Figure 3A), and good calibration was achieved between predicted and actual outcomes (Figure 3B). The logistic regression model constructed with the same parameters had AUC of 0.77 (Supplemental Table 4, Supplemental Figure 1).
Figure 3.
Random Forest Model Performance on the Test Set
(A) The model predicted outcomes correctly in the majority of cases in the test set. (B) Good calibration was achieved between predicted and actual outcomes. FN= false negative; FP = false positive; TP = true negative; TN = true negative.
Assessment of predictor importance
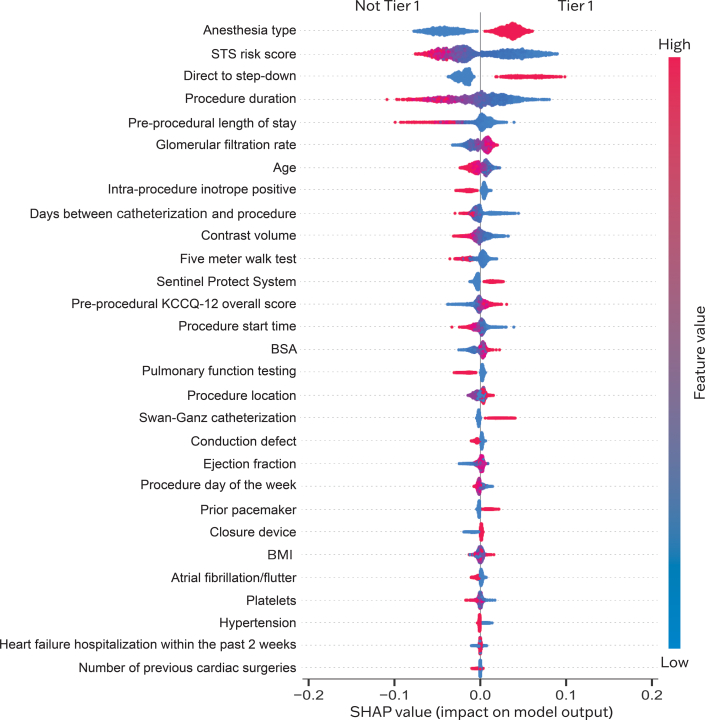
From the SHAP summary plot, we identified four highly impactful modifiable predictors: receipt of moderate sedation, direct movement of the patient to a stepdown unit, lower preprocedural LOS, and fewer days between diagnostic catheterization and TAVR that increased the likelihood of a Tier-1 outcome (Figure 4). These four predictors also had the highest Gini feature importance among modifiable parameters in the final RF model.
Figure 4.
Shapley Additive Explanation (SHAP) Value Plot
Predictors are ranked on the y-axis from top to bottom in descending order of their importance. The x-axis represents SHAP values. The plot is red, where the value of the feature is high, and blue, where the value of the feature is low. Binary variables are encoded as Low for No, High for Yes. Categorical variables are encoded with higher values being those expected to positively influence Tier-1 outcome. BMI = body mass index; BSA = body surface area; KCCQ-12 = Kansas City Cardiomyopathy Questionnaire-12; STS = Society of Thoracic Surgeons.
Discussion
In this study, we describe a ML algorithm that uses patient-level data to predict excellent (Tier 1) outcomes following TAVR with high sensitivity and specificity. Modifiable parameters with the highest predictive importance for excellent outcomes were the type of anesthesia used (with moderate sedation being predictive of good outcomes after TAVR), patient movement directly to a stepdown unit postprocedure, minimal time between diagnostic catheterization and the procedure, and shorter preprocedural LOS. These results demonstrate that patient-level data can help predict excellent outcomes following TAVR. The goal of our model was to leverage these patient-level data to inform heart team and hospital efforts to improve modifiable parameters of care, given the observed variation in those parameters—and TAVR outcomes—across hospitals.
The current study is unique in that the predefined outcome (Tier-1/Not Tier-1) is a composite of parameters of clinical and patient-centered importance. Existing algorithms like the STS risk score calculate the risk of mortality and morbidity but do not measure other outcomes that matter to patients. The Tier-1 composite outcome accounts for traditional outcomes like mortality but also includes additional outcomes important to patients including survival, short LOS, no major complications (those associated with worse survival or quality of life at 1 year17), and no readmission.
In defining Tier-1, the focus was to ensure the inclusion of components that are clinically relevant and matter to patients while focusing on variables that can be captured in administrative or registry data. Patient-centered care has been identified as a key component of health care quality in the cardiovascular disease space.18,19 Strategies such as shared decision-making that elevate patient priorities have been growing in importance to health care providers, facilities, payers, and patients, particularly in the preparation of patients for surgery.18, 19, 20 In cardiovascular surgery, patient-centered preparation may reduce disparities in outcomes.21 Our study reflects this important shift in priorities by including parameters of importance to patients in our definition of a good outcome post-TAVR (eg, survival, short LOS, no major complications, and no readmission).
This work is also notable because of the large dataset used for development and testing of the random forest model. In a recent review of ML prediction in cardiovascular diseases, many algorithms were developed based on sample sizes of less than 1,000.22 We used a dataset of 8,332 cases after exclusions, which, to our knowledge, is the largest dataset used in machine-learning predictions of outcomes post-TAVR to date.5,23,24 ML has previously been used to determine predictors of mortality,5,10,23 risk of bleeding,24 and need for pacemaker implantation25 post-TAVR, but these identified only nonmodifiable predictors such as patient comorbidities that are not amenable to care process improvement. Two previous studies identified potentially modifiable parameters as predictors of outcomes post-TAVR (fluid and electrolyte disturbance, procedures done before the weekend, valve type, and conscious sedation), but they were smaller in size than the current study and used traditional multivariate logistic regression.6,26
ML has the potential to advance cardiovascular patient care by identifying novel patterns in large, complex datasets.27 Our model identified several modifiable process characteristics discussed below and, similar to other studies,28, 29, 30 had predictive power comparable to standard linear models. These results demonstrate that detecting patterns in the TAVR care process by applying ML algorithms to patient-level data can potentially improve patient outcomes. The reporting of SHAP values may be particularly helpful in enhancing the interpretability of ML models for clinicians. Nevertheless, in the absence of significance testing for individual variables that traditional regression models provide, it is especially important to consider the potential clinical significance of individual predictors, both when choosing which predictors to include in an ML model and in interpreting the results.
The type of anesthesia used during the procedure was the most important predictor in our ML model, with general anesthesia predicting worse outcomes. This aligns with previous findings that showed use of moderate sedation was associated with lower health care resource utilization, reduced 30-day mortality, and increased odds of being discharged home while having the same procedural success as with general anesthesia. Findings from the 3M TAVR study also suggest that modification of certain aspects of the TAVR approach can increase the efficiency of the procedure as well as lead to cost savings.31 There is a movement toward using a minimalist, risk-stratified clinical approach to TAVR that will maintain effectiveness and safety while reducing health care resource use and costs.32 Such pathways may include use of local anesthesia rather than general anesthesia. These modifications have been associated with shorter procedure times, earlier discharge, and/or lower readmission rates among patients undergoing TAVR.32, 33, 34, 35, 36
Conscious sedation in patients receiving TAVR has increased over time, ranging from 33.4% in 2016 to 64.1% in 2019.37 As the physical and hemodynamic stresses of the procedure have lessened, the intensity of anesthetic support required has declined as well. Transitioning from general anesthesia to conscious sedation may further enable TAVR cases to move from operating rooms to catheterization laboratories and improve postprocedural recovery times. Although the overall prevalence of moderate sedation increased in later years of the study period, its utilization still varies widely across hospitals, suggesting opportunities for improvement in many locations. Thus, findings from this study align with existing evidence emphasizing the value of conscious sedation during TAVR.
Our model also identified that patients who moved directly to a stepdown unit after their procedure rather than to the intensive care unit (ICU) were more likely to have excellent outcomes, controlling for potential risk of poor postprocedure clinical status. Interestingly, the correlation between STS risk score (measuring underlying risk) and direct-to-stepdown was quite small (−0.06). Other risk factors for ICU admission, including atrial fibrillation/flutter, presence of a conduction defect, and recent heart failure hospitalization, are also included in the model. Stepdown units provide an intermediate level of care for patients with needs falling between the general ward and the ICU.38,39 Previous studies have shown benefits associated with availability of a stepdown unit, including improved efficiencies of patient flow,38 reductions in ICU congestion,40 improved patient outcomes after ICU care,41 and significantly reduced adjusted hospital mortality.42 The results presented here provide new evidence supporting the benefits of a stepdown unit for patients undergoing TAVR.
Lower preprocedural LOS was also associated with a higher likelihood of excellent outcomes. Although the average preprocedure LOS observed in this study was a relatively short 0.49 days (Table 1), the standard deviation was 7.22 days, indicating that a substantial number of patients had very long stays before their TAVR procedure. These patients are the suggested targets of performance improvement efforts. Elective cardiac patients are at risk of physical deterioration in the preprocedural period.43,44 In one study, 45% of patients reported that their health suffered in the wait period before cardiac surgery,44 suggesting that a “time to therapy” concept might apply to TAVR. Further, patients with AS presenting with acute heart failure benefit from the prompt decrease in left ventricular afterload known to occur post-TAVR,45 supporting the hypothesis that efficacy of TAVR may be time-dependent, especially in certain subgroups of patients. Various groups have shown that strategic use of the preprocedural period is associated with improved quality of life,46 shorter postoperative LOS,43 lower likelihood of admission to rehabilitation,47 and lower overall resource utilization.48 The effect of preprocedural intervention has not been studied in TAVR patients, but a clinical trial is ongoing to investigate the safety and efficacy of prehabilitation in high-risk patients undergoing TAVR.49 It is possible that the patients in this study benefited from being discharged to home prior to TAVR, as well as from avoiding a long period of hospitalization before their procedure. These results emphasize the need for health care providers to optimize the use of the preprocedural period. Decreasing the preprocedural LOS by discharging patients to home prior to surgery is a potential avenue for future research.
Existing evidence has shown that longer time from catheterization to TAVR (wait times) impacts post-TAVR prognosis, and the current study underscores this. A recent study found that increased wait times were associated with an increase in 1-year mortality by 2% per week after referral for TAVR.50 Such evidence highlights the need for strategies to minimize delays in access to TAVR and prompt identification of high-risk patients who require faster processing. It has been suggested that treatment delay beyond 1 month should be avoided and that patients and physicians should proceed with aortic valve replacement on a semi-urgent rather than an elective basis.51
To help understand when during the care pathway the heart team can intervene, we identified process factors that can be potentially modified before the procedure, namely preprocedural LOS and time from catheterization to TAVR. Practice guidelines could be targeted to implement strategies to reduce the time from catheterization to TAVR, whereas hospital-specific interventions are needed to target preprocedural LOS. Variables that can be modified during or after the procedure are anesthesia type and direct transfer to stepdown.
Study Limitations
Our study has several limitations, including the potential for unmeasured confounding. Specifically, patient outcomes post-TAVR are likely influenced by factors other than the 57 features included in this study, as we were limited to variables captured in the National Cardiovascular Data Registry and Transcatheter Valve Therapy Registry. We imputed missing values for two registry fields, which may reduce the potential predictive power of those variables in our models. We were unable to adjust for provider preference when studying predictors or account for variables that may have been influenced by hospital-specific protocols or provider preferences. Second, this was a retrospective study of all TAVRs performed at hospitals that shared data with Biome Analytics, so it does not represent all patients who underwent TAVR in the United States during the study period. The Biome dataset is derived from 21 hospitals, 13 of which are located on the West Coast, and thus the data are not necessarily geographically representative of the entire United States. Future external validation should be performed to assess the broader generalizability of these findings.
Conclusions
Using ML and a large dataset of 8,332 TAVR cases, we identified 29 predictors of patient outcomes post-TAVR, including four highly impactful predictors that are potentially modifiable components of the care process. These findings suggest that ML algorithms can be leveraged in value-based care arrangements such as pay-for-performance and outcomes-based contracting, offering insights into risk stratification, quality of care, and process optimization. Future work is warranted to evaluate these findings in other datasets and to evaluate the clinical potential of ML models for the TAVR care process.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: We used a large dataset and a ML approach to identify features of the care process that may be modified to optimize patient outcomes post-TAVR. This model was comparable to a traditional logistic regression model and showed high sensitivity and specificity, indicating that it could assist clinical decision-making to achieve patient-centered outcomes.
TRANSLATIONAL OUTLOOK: Future studies are warranted to test these results on additional datasets, individually score each of the variables included in the composite outcome, or explore the potential for a per-case risk score to aid in preprocedural planning.
Funding support and author disclosures
This research was funded by Edwards Lifesciences. Dr Russo received research grants from Edwards Lifesciences and has served as a consultant for Abbott, Boston Scientific, and Edwards Lifesciences. Dr Elmariah has received institutional research grants and consulting fees from Edwards Lifesciences and Medtronic. Dr Kaneko is on the advisory board for Edwards Lifesciences, Abbott, and Johnson and Johnson; and is a consultant for Medtronic. Dr Daniels is the founder and CEO of Solo Pace, Inc. Dr Makkar is a consultant for and has received research grants from Edwards Lifesciences, Abbott, Medtronic, and Boston Scientific. Drs Chikermane, Thompson, Clancy, and Benuzillo report employment with Edwards Lifesciences. Drs Clancy and Thompson are shareholders at Edwards Lifesciences. Drs Lawrence and Pawlikowski are employees at Biome Analytics. Dr Luck is a consultant to Edwards Lifesciences; and a shareholder and board member at Biome Analytics.
Acknowledgments
The authors thank Emily Farrar, PhD, Sheena Hemnani, MS, and Sarah Colton of Boston Strategic Partners, Inc for their editorial contributions and assistance with manuscript preparation.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental tables and a figure, please see the online version of this paper.
Supplementary data
References
- 1.Carabello B.A. Introduction to aortic stenosis. Circ Res. 2013;113:179–185. doi: 10.1161/CIRCRESAHA.113.300156. [DOI] [PubMed] [Google Scholar]
- 2.Durko A.P., Osnabrugge R.L., Van Mieghem N.M., et al. Annual number of candidates for transcatheter aortic valve implantation per country: current estimates and future projections. Eur Heart J. 2018;39:2635–2642. doi: 10.1093/eurheartj/ehy107. [DOI] [PubMed] [Google Scholar]
- 3.Mc Morrow R., Kriza C., Urbán P., et al. Assessing the safety and efficacy of TAVR compared to SAVR in low-to-intermediate surgical risk patients with aortic valve stenosis: an overview of reviews. Int J Cardiol. 2020;314:43–53. doi: 10.1016/j.ijcard.2020.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goody P.R., Hosen M.R., Christmann D., et al. Aortic valve stenosis. Arterioscler Thromb Vasc Biol. 2020;40:885–900. doi: 10.1161/ATVBAHA.119.313067. [DOI] [PubMed] [Google Scholar]
- 5.Hernandez-Suarez D.F., Kim Y., Villablanca P., et al. Machine learning prediction models for in-hospital mortality after transcatheter aortic valve replacement. JACC Cardiovasc Interv. 2019;12:1328–1338. doi: 10.1016/j.jcin.2019.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arbel Y., Zivkovic N., Mehta D., et al. Factors associated with length of stay following trans-catheter aortic valve replacement - a multicenter study. BMC Cardiovasc Disord. 2017;17:137. doi: 10.1186/s12872-017-0573-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coylewright M., Palmer R., O'Neill E.S., Robb J.F., Fried T.R. Patient-defined goals for the treatment of severe aortic stenosis: a qualitative analysis. Health Expect. 2016;19:1036–1043. doi: 10.1111/hex.12393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim J. Big data, health informatics, and the future of cardiovascular medicine. J Am Coll Cardiol. 2017;69:899–902. doi: 10.1016/j.jacc.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 9.Rajula H.S.R., Verlato G., Manchia M., Antonucci N., Fanos V. Comparison of conventional statistical methods with machine learning in medicine: diagnosis, drug development, and treatment. Medicina (Kaunas) 2020;56:455. doi: 10.3390/medicina56090455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Penso M., Pepi M., Fusini L., et al. Predicting long-term mortality in TAVI patients using machine learning techniques. J Cardiovasc Dev Dis. 2021;8:44. doi: 10.3390/jcdd8040044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mamprin M., Lopes R.R., Zelis J.M., et al. Machine learning for predicting mortality in transcatheter aortic valve implantation: an inter-center cross validation study. J Cardiovasc Dev Dis. 2021;8:65. doi: 10.3390/jcdd8060065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zack C.J., Senecal C., Kinar Y., et al. Leveraging machine learning techniques to forecast patient prognosis after percutaneous coronary intervention. JACC Cardiovasc Interv. 2019;12:1304–1311. doi: 10.1016/j.jcin.2019.02.035. [DOI] [PubMed] [Google Scholar]
- 13.Carroll J.D., Mack M.J., Vemulapalli S., et al. STS-ACC TVT registry of transcatheter aortic valve replacement. J Am Coll Cardiol. 2020;76:2492–2516. doi: 10.1016/j.jacc.2020.09.595. [DOI] [PubMed] [Google Scholar]
- 14.Pedregosa F., Varoquaux G., Gramfort A., et al. Scikit-learn: machine learning in Python. J Mach Learn Res. 2011;12:2825–2830. [Google Scholar]
- 15.van Smeden M., Heinze G., Van Calster B., et al. Critical appraisal of artificial intelligence-based prediction models for cardiovascular disease. Eur Heart J. 2022;43:2921–2930. doi: 10.1093/eurheartj/ehac238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Collins G.S., Reitsma J.B., Altman D.G., Moons K.G.M. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD) Circulation. 2015;131:211–219. doi: 10.1111/1471-0528.13244. [DOI] [PubMed] [Google Scholar]
- 17.Arnold S.V., Cohen D.J., Dai D., et al. Predicting quality of life at 1 year after transcatheter aortic valve replacement in a real-world population. Circ Cardiovasc Qual Outcomes. 2018;11 doi: 10.1161/CIRCOUTCOMES.118.004693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Okunrintemi V., Nasir K. Optimizing patient-reported experiences for cardiovascular disease: current landscape and future opportunities. Methodist Debakey Cardiovasc J. 2020;16:220–224. doi: 10.14797/mdcj-16-3-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Okunrintemi V., Spatz E.S., Di Capua P., et al. Patient-provider communication and health outcomes among individuals with atherosclerotic cardiovascular disease in the United States: medical expenditure panel survey 2010 to 2013. Circ Cardiovasc Qual Outcomes. 2017;10 doi: 10.1161/CIRCOUTCOMES.117.003635. [DOI] [PubMed] [Google Scholar]
- 20.Mihalj M., Carrel T., Urman R.D., Stueber F., Luedi M.M. Recommendations for preoperative assessment and shared decision-making in cardiac surgery. Curr Anesthesiol Rep. 2020;10:185–195. doi: 10.1007/s40140-020-00377-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Turkson-Ocran R.N., Ogunwole S.M., Hines A.L., Peterson P.N. Shared decision making in cardiovascular patient care to address cardiovascular disease disparities. J Am Heart Assoc. 2021;10 doi: 10.1161/JAHA.120.018183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krittanawong C., Virk H.U.H., Bangalore S., et al. Machine learning prediction in cardiovascular diseases: a meta-analysis. Sci Rep. 2020;10 doi: 10.1038/s41598-020-72685-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Agasthi P., Ashraf H., Pujari S.H., et al. Artificial intelligence trumps TAVI(2)-SCORE and CoreValve Score in predicting 1-year mortality post-transcatheter aortic valve replacement. Cardiovasc Revasc Med. 2021;24:33–41. doi: 10.1016/j.carrev.2020.08.010. [DOI] [PubMed] [Google Scholar]
- 24.Navarese E.P., Zhang Z., Kubica J., et al. Development and validation of a practical model to identify patients at risk of bleeding after TAVR. JACC Cardiovasc Interv. 2021;14:1196–1206. doi: 10.1016/j.jcin.2021.03.024. [DOI] [PubMed] [Google Scholar]
- 25.Truong V.T., Beyerbach D., Mazur W., et al. Machine learning method for predicting pacemaker implantation following transcatheter aortic valve replacement. Pacing Clin Electrophysiol. 2021;44:334–340. doi: 10.1111/pace.14163. [DOI] [PubMed] [Google Scholar]
- 26.Akinseye O.A., Shahreyar M., Nwagbara C.C., et al. Modifiable predictors of in-hospital mortality in patients undergoing transcatheter aortic valve replacement. Am J Med Sci. 2018;356:135–140. doi: 10.1016/j.amjms.2018.04.008. [DOI] [PubMed] [Google Scholar]
- 27.Krittanawong C., Zhang H., Wang Z., Aydar M., Kitai T. Artificial intelligence in precision cardiovascular medicine. J Am Coll Cardiol. 2017;69:2657–2664. doi: 10.1016/j.jacc.2017.03.571. [DOI] [PubMed] [Google Scholar]
- 28.Christodoulou E., Ma J., Collins G.S., et al. A systematic review shows no performance benefit of machine learning over logistic regression for clinical prediction models. J Clin Epidemiol. 2019;110:12–22. doi: 10.1016/j.jclinepi.2019.02.004. [DOI] [PubMed] [Google Scholar]
- 29.Lynam A.L., Dennis J.M., Owen K.R., et al. Logistic regression has similar performance to optimised machine learning algorithms in a clinical setting: application to the discrimination between type 1 and type 2 diabetes in young adults. Diagn Progn Res. 2020;4:6. doi: 10.1186/s41512-020-00075-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tennenhouse L.G., Marrie R.A., Bernstein C.N., Lix L.M. Machine-learning models for depression and anxiety in individuals with immune-mediated inflammatory disease. J Psychosom Res. 2020;134 doi: 10.1016/j.jpsychores.2020.110126. [DOI] [PubMed] [Google Scholar]
- 31.Butala N.M., Wood D.A., Li H., et al. Economics of minimalist transcatheter aortic valve replacement: results from the 3M-TAVR economic study. Circ Cardiovasc Interv. 2022;15 doi: 10.1161/CIRCINTERVENTIONS.122.012168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lauck S.B., Wood D.A., Baumbusch J., et al. Vancouver transcatheter aortic valve replacement clinical pathway: minimalist approach, standardized care, and discharge criteria to reduce length of stay. Circ Cardiovasc Qual Outcomes. 2016;9:312–321. doi: 10.1161/CIRCOUTCOMES.115.002541. [DOI] [PubMed] [Google Scholar]
- 33.Bonaros N., Petzina R., Cocchieri R., et al. Transaortic transcatheter aortic valve implantation as a first-line choice or as a last resort? An analysis based on the ROUTE registry. Eur J Cardio Thorac Surg. 2017;51:919–926. doi: 10.1093/ejcts/ezw406. [DOI] [PubMed] [Google Scholar]
- 34.Kolte D., Khera S., Sardar M.R., et al. Thirty-day readmissions after transcatheter aortic valve replacement in the United States: insights from the nationwide readmissions database. Circ Cardiovasc Interv. 2017;10 doi: 10.1161/CIRCINTERVENTIONS.116.004472. [DOI] [PubMed] [Google Scholar]
- 35.McCarthy F.H., Spragan D.D., Savino D., et al. Outcomes, readmissions, and costs in transfemoral and alterative access transcatheter aortic valve replacement in the US Medicare population. J Thorac Cardiovasc Surg. 2017;154:1224–12232.e1. doi: 10.1016/j.jtcvs.2017.04.090. [DOI] [PubMed] [Google Scholar]
- 36.McNeely C., Zajarias A., Robbs R., Markwell S., Vassileva C.M. Transcatheter aortic valve replacement outcomes in nonagenarians stratified by transfemoral and transapical approach. Ann Thorac Surg. 2017;103:1808–1814. doi: 10.1016/j.athoracsur.2017.02.056. [DOI] [PubMed] [Google Scholar]
- 37.Butala N.M., Chung M., Secemsky E.A., et al. Conscious sedation versus general anesthesia for transcatheter aortic valve replacement: variation in practice and outcomes. JACC Cardiovasc Interv. 2020;13:1277–1287. doi: 10.1016/j.jcin.2020.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Prin M., Wunsch H. The role of stepdown beds in hospital care. Am J Respir Crit Care Med. 2014;190:1210–1216. doi: 10.1164/rccm.201406-1117PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Plate J.D.J., Leenen L.P.H., Houwert M., Hietbrink F. Utilisation of intermediate care units: a systematic review. Crit Care Res Pract. 2017;2017 doi: 10.1155/2017/8038460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim S.H., Chan C.W., Olivares M., Escobar G.J. Association among ICU congestion, ICU admission decision, and patient outcomes. Crit Care Med. 2016;44:1814–1821. doi: 10.1097/CCM.0000000000001850. [DOI] [PubMed] [Google Scholar]
- 41.Lekwijit S., Chan C.W., Green L.V., Liu V.X., Escobar G.J. The impact of step-down unit care on patient outcomes after ICU discharge. Crit Care Explor. 2020;2 doi: 10.1097/CCE.0000000000000114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Capuzzo M., Volta C., Tassinati T., et al. Hospital mortality of adults admitted to intensive care units in hospitals with and without intermediate care units: a multicentre European cohort study. Crit Care. 2014;18:551. doi: 10.1186/s13054-014-0551-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Waite I., Deshpande R., Baghai M., et al. Home-based preoperative rehabilitation (prehab) to improve physical function and reduce hospital length of stay for frail patients undergoing coronary artery bypass graft and valve surgery. J Cardiothorac Surg. 2017;12:91. doi: 10.1186/s13019-017-0655-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mulgan R., Logan R.L. The coronary bypass waiting list: a social evaluation. N Z Med J. 1990;103:371–372. [PubMed] [Google Scholar]
- 45.Heidenreich P.A., Bozkurt B., Aguilar D., et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American heart association Joint committee on clinical practice guidelines. Circulation. 2022;145:e895–e1032. doi: 10.1161/CIR.0000000000001063. [DOI] [PubMed] [Google Scholar]
- 46.Steinmetz C., Bjarnason-Wehrens B., Baumgarten H., et al. Prehabilitation in patients awaiting elective coronary artery bypass graft surgery - effects on functional capacity and quality of life: a randomized controlled trial. Clin Rehabil. 2020;34:1256–1267. doi: 10.1177/0269215520933950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cabilan C.J., Hines S., Munday J. The effectiveness of prehabilitation or preoperative exercise for surgical patients: a systematic review. JBI Database System Rev Implement Rep. 2015;13:146–187. doi: 10.11124/jbisrir-2015-1885. [DOI] [PubMed] [Google Scholar]
- 48.Boreskie K.F., Hay J.L., Kehler D.S., et al. Prehabilitation: the right medicine for older frail adults anticipating transcatheter aortic valve replacement, coronary artery bypass graft, and other cardiovascular care. Clin Geriatr Med. 2019;35:571–585. doi: 10.1016/j.cger.2019.07.006. [DOI] [PubMed] [Google Scholar]
- 49.Harvey J. 2022. Accessed July 11, 2024. Prehabilitation for patients undergoing transcatheter aortic valve replacement (TAVR-Prehab) ( NCT03107897) https://cdn.clinicaltrials.gov/large-docs/97/NCT03107897/Prot_SAP_000.pdf. [Google Scholar]
- 50.Roule V., Rebouh I., Lemaitre A., et al. Impact of wait times on late postprocedural mortality after successful transcatheter aortic valve replacement. Sci Rep. 2022;12:5967. doi: 10.1038/s41598-022-09995-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Malaisrie S.C., McDonald E., Kruse J., et al. Mortality while waiting for aortic valve replacement. Ann Thorac Surg. 2014;98:1564–1571. doi: 10.1016/j.athoracsur.2014.06.040. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.