Abstract
Purpose
Although younger age has been negatively associated with persistence to adjuvant endocrine therapy (ET), factors contributing to non-persistence remain poorly understood. We assessed factors associated with non-persistence to ET and described the 5-year trajectories of quality of life (QoL) and symptoms in young women (≤40 years) with hormone receptor-positive breast cancer (BC).
Methods
We retrieved data on clinical characteristics and non-persistence from the medical annual records in the European cohort of the “Helping Ourselves, Helping Others: The Young Women's BC Study” (IBCSG 43-09 HOHO). Women completed surveys at baseline, biannually for three years, and annually for another seven years. Data collection included sociodemographic information, QoL aspects assessed by the Cancer Rehabilitation Evaluation System-Short Form and symptoms assessed by the Breast Cancer Prevention Trial symptom scales. Cox regression models were applied to identify factors associated with non-persistence.
Results
The cumulative risk of interrupting ET within 5 years was 27.7 % (95 % CI, 21.5–35.2). The QoL subscale scores remained stable over 5 years, with slight improvements in the physical subscale. Hot flashes decreased (p < 0.001), while vaginal problems intensified (p < 0.001) over time. Being married without children and having difficulties interacting and communicating with the medical team were significantly associated with non-persistence.
Conclusions
Discussing the desire to conceive with partnered childless women and establishing a good relationship with the medical team may be important in addressing the non-persistence in young BC survivors. As recent data suggests the safety of pausing ET to conceive, this approach may be a reasonable future option to limit non-persistence.
Keywords: Persistence, Adjuvant endocrine therapy, Breast cancer, Young women, Quality of life
Highlights
-
•
Persistence to endocrine treatment in young women is poorly understood.
-
•
Quality of life remained stable over 5 years.
-
•
Hot flashes decreased and vaginal problems intensified over time.
-
•
Women being married without children were less likely to persist.
-
•
Good communicating with the medical team is important for persistence.
1. Introduction
Breast cancer (BC) is the most common female malignancy [1], with approximately 4 % of BC diagnoses occurring in women <40 years in the US in 2019 [2] and in the EU in 2020 [3]. BC in younger women is characterized by less favorable outcomes [[4], [5], [6], [7], [8]]. For women with hormone receptor-positive (HR+) disease, 5–10 years of adjuvant endocrine therapy (ET) can reduce the risk of recurrence and death [9,10]. To ensure this risk reduction, adherence to ET (i.e., the extent to which an individual takes the medication as prescribed) as well as persistence with ET (i.e., continuing to take the medication for the prescribed duration [11]) are critical. Overall, rates of adherence and persistence to 5 years of adjuvant ET reported in the literature range from 41 to 72 % and 31–73 %, respectively [12,13]. These suboptimal rates can impact disease outcomes in general [14] and may be exacerbated in younger women, who have higher-risk disease and a longer life expectancy than older women [15].
To understand why women with early BC stop taking ET, many studies have investigated factors associated with adherence and persistence. Side effects are a key factor in treatment discontinuation [12,[16], [17], [18], [19]]. Toivonen et al. [20] found a consistent negative association between adherence to ET and the incidence and severity of treatment side effects, particularly arthralgia and cognitive changes, and the number of symptoms. However, most of these associations were tested in univariable models and worse side effects were less likely to be associated with adherence to ET in studies with retrospective or cross-sectional than with prospective designs [20]. An individual's ability to cope with side effects also appears to be important for continuing to take medication [19,21]. There is no systematic evidence on the relationship between (health-related) quality of life (QoL) and adherence [20]. A couple of studies indicate that better QoL is associated with higher ET adherence or persistence [22,23], and early negative changes in QoL during aromatase inhibitor (AI) therapy are associated with treatment discontinuation [24].
Other factors negatively associated with adherence and persistence to ET include financial issues (increasing out-of-pocket costs, worse financial status) [12,18,25], switching medication [12], follow-up care by general practitioner (vs. specialist) [12,16], increased number of hospitalizations [16], depression [18,20], comorbidities [18], and lack of or insufficient social support [12,[16], [17], [18],20]. Factors likely to enhance the continuation of treatment include good patient-physician relationship [[16], [17], [18],20], higher self-efficacy [[16], [17], [18],20], sufficient knowledge about treatment objectives and support received [17,21], and positive attitudes toward ET [20].
Overall, younger age has been negatively associated with adherence and persistence [12,15,26], but knowledge of the specific correlates of persistence to ET in young women (i.e., aged 40 or younger) is rather scarce. A recent comprehensive review of 147 studies showed that the current evidence is predominantly based on postmenopausal women [27]. An exception is the North American multicenter prospective cohort study “Helping Ourselves, Helping Others: The Young Women's Breast Cancer Study” (HOHO-YWS; NCT01468246). In this study, among the 607 women who initiated ET, 20 % were not persistent [28]. Correlates of higher odds of non-persistence included younger age, being married, and reporting more weight problems, whereas receipt of chemotherapy, and greater symptom burden (i.e., hot flashes and vaginal symptoms) were associated with lower odds of non-persistence.
In the present report, we used data from the European HOHO-YWS companion study (IBCSG 43-09 HOHO [29]) to assess factors associated with non-persistence to ET and to investigate the association of QoL and symptom trajectories with non-persistence over a 5-year period among young survivors [29].
2. Participants and methods
IBCSG 43-09 HOHO is a longitudinal cohort study including women diagnosed with early/advanced BC at the age of 40 years or younger. Women were enrolled in 18 institutions in Italy and Switzerland <6 months after diagnosis, between July 2009 and January 2016. Participating women gave informed consent and completed a comprehensive survey at baseline (time of enrollment) and every 6 months for the first 3 years, then yearly for an additional 7 years. Medical data on disease outcome, treatment, and comorbidities were collected by the treating physicians at annual follow-up visits. The European survey was a shorter version of the US HOHO-YWS cohort questionnaire to consider cultural differences and to increase women's long-term engagement in survey completion. The Europa Donna advocacy group of Southern Switzerland assisted in this process to ensure that the survey reflected all issues relevant to young BC survivors. The study was not designed as a comparative multiethnic/country survey due to limited resources.
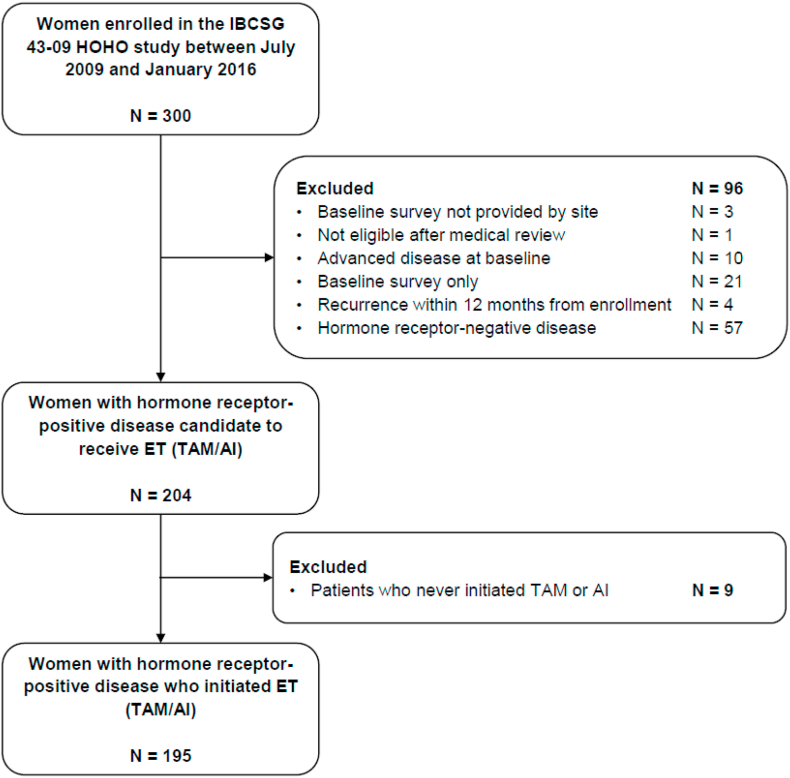
The present analysis included women with HR + disease who initiated tamoxifen (TAM) or AI ± luteinizing hormone-releasing hormone analogue (LHRHa) (n = 195, Fig. 1). Data on exposure was retrieved annually from the medical records. Our primary outcome was non-persistence, defined as interruption of ET for more than 90 days or indefinitely, over a 5-year period from the start of ET. The outcome date was the 90th day of interruption. Women who did not interrupt ET during the considered period, or who did so for less than 90 days, were censored at the date of recurrence, last follow-up, or 5 years after ET initiation, whichever occurred first.
Fig. 1.
Participant flow chart.
Self-reported socio-demographic information from the survey included country of enrollment, education, marital/relationship status, parity, employment status, self-perceived financial situation, having a first-degree relative with breast/ovarian cancer and perceived decision-making for ET. Clinical characteristics collected from baseline medical records included type of surgery, chemotherapy, tumor grade, stage, and HER2 positivity.
Symptoms were assessed longitudinally by the Breast Cancer Prevention Trial (BCPT) symptom scales [30,31]. The BCPT evaluates commonly reported physical and psychological symptoms after BC. We selected four symptom scales including hot flashes (2 items), vaginal problems (2 items), musculoskeletal pain (3 items), and weight problems (3 items). Women indicated how much each symptom bothered them in the past 4 weeks on a 5-point severity scale (0–4). Scores for each scale were calculated by averaging the items. Higher scores indicate greater bothering by symptoms.
QoL aspects and rehabilitation needs of women who have been diagnosed with cancer were assessed with the validated Cancer Rehabilitation Evaluation System-Short Form (CARES-SF), a multidimensional instrument with 6 subscales. For this analysis we only considered four subscales: physical (physical changes and disruption of daily activities caused by the disease); medical interaction (problems interacting and communicating with the medical team); psychosocial (psychosocial issues, communication and relationship problems, except with partners); sexual (problems related to interest and performance of sexual activity) [32]. Body image was measured by the subscale of the CARES psycho-social summary scale [33], which includes three questions: 1) I am uncomfortable with the changes in my body; 2) I am embarrassed to show my body to others because of my illness; 3) I am uncomfortable showing my scars to others. For all CARES and CARES-SF items, respondents were asked to rate how much each statement applied to them (scale range 0–4). Higher scores indicate greater difficulty and a poorer QoL.
2.1. Statistical analysis
Socio-demographic and clinical characteristics were analyzed using descriptive statistics. Categorical variables were reported with absolute and relative frequencies, continuous variables with median and interquartile range (IQR). The cumulative risk function of ET interruption (non-persistence) from ET initiation was estimated using the Kaplan-Meier method. Univariable and multivariable Cox regression models were applied to identify factors (fixed at baseline or time-dependent) associated with non-persistence. For the multivariable analysis, we selected variables based on a combination of statistical significance (p-value <0.10), strength of association (HR > 1.25 or <0.80), and clinical/biological relevance. Linear mixed-effects models were fitted to evaluate the trend in CARES-SF domain scores and BCPT symptom scales through the 5-year period. Analyses were conducted using the SAS software v. 9.4 (SAS Institute, Cary, NC).
3. Results
3.1. Sample characteristics
Among the 195 women included in the analysis, the median time between diagnosis and completion of the baseline survey was 2.5 months (IQR 1.6–3.7 months). Median follow-up was 5.7 years (IQR 4.9–7.3 years). Table 1 summarizes the study population characteristics at enrollment. The median age was 37 years (IQR 34–39), 66.6 % were married or in a stable relationship, and 50.2 % had children prior to BC diagnosis. More than one-third (37.9 %) of the women had a university education, 83.6 % were employed, and 45.6 % felt financially comfortable. Forty-six percent of women had undergone a mastectomy, and 65.1 % received chemotherapy.
Table 1.
Women's socio-demographic and clinical characteristics at enrollment in the HOHO study (N = 195).
| N | % | |
|---|---|---|
| Country of enrollment | ||
| Italy | 141 | 72.3 |
| Switzerland | 54 | 27.7 |
| Age at baseline <35 years | 63 | 32.3 |
| Age at baseline, median (IQR) | 36.8 (33.9–39.3) | |
| University education | 74 | 37.9 |
| Married, or in a stable relationship, and parity at baseline | ||
| Married No | Parity Yes | 10 | 5.1 |
| Married Yes | Parity Yes | 88 | 45.1 |
| Married No | Parity No | 55 | 28.2 |
| Married Yes | Parity No | 42 | 21.5 |
| Employed at baseline | 163 | 83.6 |
| Financial comfort at baseline | ||
| Enough money for special things | 89 | 45.6 |
| Enough money to pay bills but little spare money for extras | 55 | 28.2 |
| Money to pay bills but only after cutting back/difficulty paying bills | 41 | 21.0 |
| Missing/unknown | 10 | 5.1 |
| First-degree relative with breast or ovarian cancer at baseline | 85 | 43.6 |
| Received/receiving chemotherapy at baseline | 127 | 65.1 |
| Underwent mastectomy at baseline/after neo-adjuvant treatment | 90 | 46.2 |
| pT | ||
| 1 | 105 | 53.8 |
| 2 | 66 | 33.8 |
| 3/4 | 10 | 5.1 |
| Xa | 14 | 7.2 |
| pN | ||
| 0 | 90 | 46.2 |
| 1 | 62 | 31.8 |
| 2/3 | 30 | 15.4 |
| Xa | 13 | 6.7 |
| Grade | ||
| 1 | 11 | 5.6 |
| 2 | 91 | 46.7 |
| 3 | 85 | 43.6 |
| Unknown | 8 | 4.1 |
| HER2 positive | 48 | 24.6 |
Received neoadjuvant treatment.
Eighty percent of women (n = 156) received TAM first, and 20 % (n = 39) started on an AI with LHRHa (except for 6 patients who had bilateral oophorectomy). During treatment 14.9 % (n = 29) of women switched therapy. Of those receiving TAM (n = 156), 21 (13.5 %) switched to an AI, and of those receiving an AI (n = 39), 8 (20.5 %) switched to TAM.
Thirty-five percent of women (n = 69) reported that the decision whether to take ET was shared with their doctor, 34.9 % (n = 68) reported that it was mainly a doctor's decision, and 18.5 % (n = 36) that it was their own decision. In 22 cases (11.3 %), the information on decision-making was missing.
3.2. Non-persistence to ET
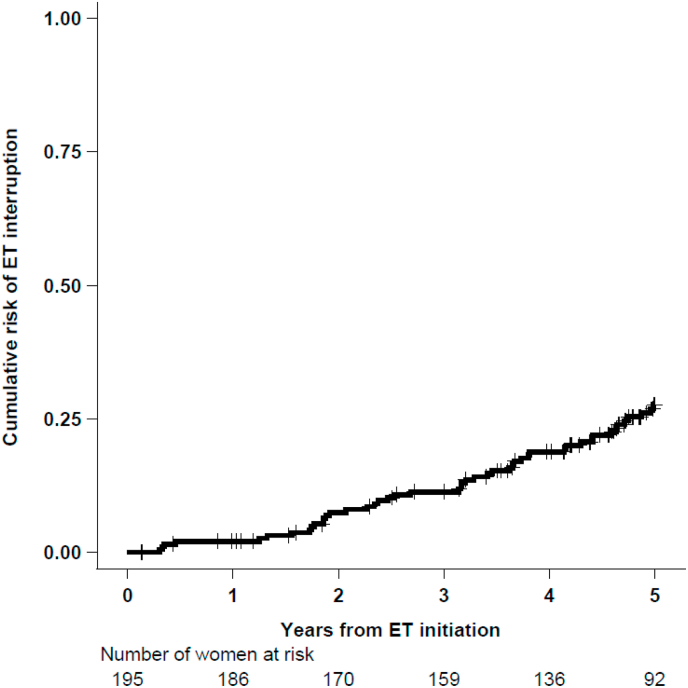
The cumulative probability of interrupting ET was 18.8 % (95 % CI, 13.8–25.3) within 4 years, and 27.7 % (95 % CI, 21.5–35.2) within 5 years (Fig. 2). Over a 5-year period from ET initiation, 47 women (24.1 %) have interrupted ET for more than 90 days or indefinitely (non-persistence, event of interest). 17 women were censored due to recurrence (4 loco-regional, 10 distant and 3 s primary events occurring after a median of 2.5 years from ET initiation), 39 women were censored because the last follow-up was before 5 years, while 92 women reached 5 years of treatment and were censored at their 5-year date from ET initiation.
Fig. 2.
Cumulative risk of ET interruption (non-persistence) over time.
Among the 47 non-persistent women, interruption occurred on average 3.0 years after ET initiation (IQR 1.9–4.2). Thirty-seven women interrupted TAM (22 in combination with LHRHa), 10 interrupted AI (in combination with LHRHa). Reasons for interruption of ET were: medical/patient decision not otherwise specified (n = 31), desire to become pregnant (n = 11, 4 of whom were enrolled in the POSITIVE trial [34], which evaluated the temporary interruption of adjuvant ET to attempt pregnancy in young women with BC), and toxicity (n = 5). Among those who switched ET (n = 29), only 6 women interrupted ET thereafter. Five interrupted AI (4 for patient/medical decision, 1 for toxicity), one interrupted TAM for patient/medical decision after having switched two times (TAM > AI > TAM).
Among the subgroup of women without children at enrollment (n = 97), 33 women (34.0 %) interrupted ET compared to only 14 of 98 women (14.3 %) with children before diagnosis. Only nine women, all childless before diagnosis, had children during follow-up. All of them interrupted TAM, in two cases due to medical or patient decision, and in seven cases because of a desire to become pregnant.
3.3. Quality of life and symptom burden over 5 years
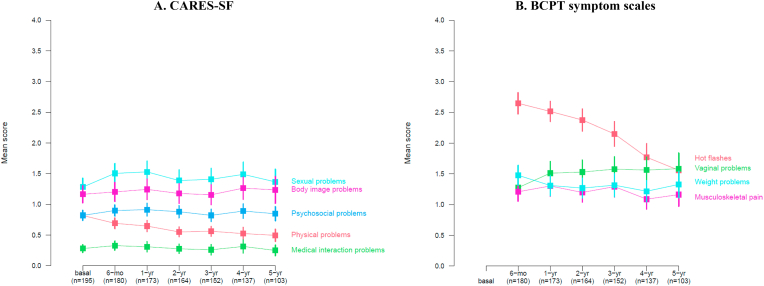
The trajectories of the QoL and symptom scales are shown in Fig. 3 (panels A and B). Overall, the scores of the medical (p-value = 0.76), psychosocial (p-value = 0.26), sexual (p-value = 0.47), and body image (p-value = 0.23) CARES-SF subscales remained quite stable over the 5-year observation period. Small improvements were observed for the physical subscale (p-value<0.001). Hot flashes improved over time (p-value<0.001), while vaginal problems worsened continuously up to five years of treatment (p-value<0.001). An increase in weight gain was reported after 4 years (p-value = 0.09), while musculoskeletal pain fluctuated between improvement and worsening from year to year (p-value = 0.82).
Fig. 3.
Trajectories of CARES-SF domain scores (panel A) and BCPT symptom scales (panel B) over 5-years of follow-up A.
Note: mean and corresponding 95 % confidence intervals for CARES-SF domain scores at baseline and during follow-up (lower scores indicating lower problems) and BCPT symptom scales at follow-up (lower scores indicating less symptom burden).
3.4. Factors associated with ET non-persistence
Table 2 shows factors (fixed at baseline or time-dependent) associated with non-persistence. In univariable analysis, women who were younger than 35 years and those who were married without children at enrollment, were more likely to interrupt ET compared with women older than 35 years or those who were married and had already children. In addition, women who reported more problems interacting and communicating with the medical team (CARES-SF medical interaction subscale) were more likely not to complete 5 years of treatment. In multivariable analyses, being married without children (vs. being married) and having more problems related to medical interactions remained significantly associated with non-persistence. University education, receipt of chemotherapy, type of surgery (mastectomy vs. breast-conserving surgery), all the symptom scales and the remaining CARES-SF subscales were not associated with non-persistence in either univariable or multivariable analyses (Table 2).
Table 2.
Univariable and multivariable analysis of factors associated with ET interruption (non-persistence).
| Univariable analysis |
Multivariable analysis |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| N. of patients | Person-years | N. of events | HR | 95 % CI | p-value | HR | 95 % CI | p-value | |
| Socio-demographic and clinical variables | |||||||||
| Country of enrollment | |||||||||
| Italy | 141 | 580 | 30 | 1.00 | 1.00 | ||||
| Switzerland | 54 | 211 | 17 | 1.57 | 0.87–2.84 | 0.14 | 1.42 | 0.75–2.67 | 0.28 |
| Age at baseline <35 years | |||||||||
| No | 132 | 539 | 24 | 1.00 | 1.00 | ||||
| Yes | 63 | 253 | 23 | 2.12 | 1.20–3.76 | 0.01 | 1.26 | 0.66–2.39 | 0.48 |
| University education | |||||||||
| No | 116 | 472 | 24 | 1.00 | 1.00 | ||||
| Yes | 74 | 298 | 22 | 1.47 | 0.82–2.62 | 0.19 | 1.19 | 0.65–2.19 | 0.57 |
| Married or in a stable relationship and parity (both time-dependent) | |||||||||
| Married No | Parity Yes | 46 | 1 | 0.54 | 0.07–4.17 | 0.56 | 0.39 | 0.05–3.05 | 0.37 | |
| Married Yes | Parity Yes | 373 | 13 | 1.00 | 1.00 | |||||
| Married No | Parity No | 192 | 8 | 1.22 | 0.51–2.94 | 0.66 | 0.89 | 0.35–2.31 | 0.82 | |
| Married Yes | Parity No | 175 | 25 | 4.45 | 2.27–8.71 | <0.001 | 3.52 | 1.65–7.50 | 0.001 | |
| Received/receiving chemotherapy | |||||||||
| No | 68 | 277 | 16 | 1.00 | |||||
| Yes | 127 | 515 | 31 | 1.03 | 0.56–1.88 | 0.93 | |||
| Underwent mastectomy at baseline/after neo-adjuvant treatment | |||||||||
| No | 105 | 414 | 30 | 1.00 | 1.00 | ||||
| Yes | 90 | 378 | 17 | 0.60 | 0.33–1.09 | 0.10 | 0.64 | 0.34–1.18 | 0.15 |
| Health-related quality of life variables (continuous time-dependent) | |||||||||
| CARES-SF physical scale (+0.1) | 1.01 | 0.96–1.06 | 0.82 | ||||||
| CARES-SF medical scale (+0.1) | 1.06 | 1.02–1.10 | 0.002 | 1.05 | 1.01–1.08 | 0.01 | |||
| CARES-SF psychosocial scale (+0.1) | 1.00 | 0.95–1.04 | 0.84 | ||||||
| CARES-SF sexual scale (+0.1) | 1.01 | 0.98–1.03 | 0.62 | ||||||
| CARES-SF body image scale (+0.1) | 1.00 | 0.97–1.03 | 0.94 | ||||||
| BCPT hot flashes (+0.1) | 1.01 | 0.99–1.04 | 0.32 | ||||||
| BCPT vaginal problems (+0.1) | 1.01 | 0.99–1.04 | 0.24 | ||||||
| BCPT musculoskeletal pain (+0.1) | 1.00 | 0.97–1.03 | 0.92 | ||||||
| BCPT weight problems (+0.1) | 1.00 | 0.97–1.03 | 0.95 | ||||||
Abbreviations: HR, hazard ratio; CI, confidence interval.
4. Discussion
Adherence and persistence to adjuvant ET in women with HR + BC is crucial to ensure the expected benefit in terms of clinical outcomes. Accordingly, ET adherence and persistence have been important research topics over the years. In our study we focused on young women with BC, a subgroup poorly represented in the literature on ET adherence and persistence, yet potentially most affected by both the negative effects of ET (e.g., premature menopause and the associated reduced fertility [35]) and worse disease outcomes. In our sample, the cumulative risk of interrupting ET within 5 years was 27.7 %. The corresponding US HOHO-YWS cohort reported that 20 % of women were not persistent within 5 years of diagnosis [28], while in the Suppression of Ovarian Function Trial (SOFT) and Tamoxifen and Exemestane Trial (TEXT) 19.8 % of women <35 years of age discontinued all protocol-assigned therapy prematurely [36]. Interpretation of these data is complicated by differences in age categories, methods of adherence/persistence assessment, and timing between studies.
In our sample, women who were married or in a stable relationship but did not have children during the observation period had a higher risk of non-persistence than married women with children. Although fertility concerns were associated with non-initiation and early discontinuation of ET in reproductive-age BC survivors [37], the extensive literature on factors associated with adherence and persistence does not particularly emphasize this aspect, probably because most of the existing evidence is based on studies in postmenopausal women [27]. The desire to become pregnant seems to be an important factor to consider when evaluating persistence to ET in young women. Although the impact of anti-cancer treatments on fertility in young women with BC is modest [38], pregnancy is contraindicated while receiving ET [9]. The POSITIVE trial showed no clear worsening of BC outcomes in the short term in young women with HR + BC who temporarily stopped ET to attempt pregnancy. Although longer-term follow-up is needed to confirm the safety of this strategy, the “POSITIVE approach” may help motivate young patients to continue with ET [39].
The trajectories of the CARES-SF psychosocial, sexual, and body image subscales were stable over the 5-year period, with slight improvements observed for the physical subscale. In the US HOHO-YWS cohort, the CARES physical, body image, and sexual subscales improved significantly over the 5-year period after BC surgery [40]. Analysis of global QoL indicators in SOFT and TEXT in women younger than 35 at baseline showed minimal changes over the 5-year treatment period [36]. In our study the physical, psychosocial, body image and sexual subscales were not significantly associated with non-persistence. Previous studies with shorter follow-up and predominantly including postmenopausal women showed that the better the QoL, the more likely patients are to continue taking ET [22,23]. On the other hand, a systematic review found that no QoL factor, assessed by two or more independent studies, was consistently associated with adherence [20].
The trajectories of symptom burden varied by symptom, with hot flashes becoming less severe and vaginal problems becoming more bothersome over time. These changes in symptom severity were not significantly associated with non-persistence. Similarly, in the HOHO-YWS cohort, greater hot flash and vaginal symptom burden was associated with lower odds of non-persistence [28]. For younger women, the survival benefits of ET may be more relevant, and they may therefore be more willing to endure the negative consequences of ET, although they describe side effects with a greater intensity than older women [41]. They may also have a high self-efficacy and positive decisional balance, two factors consistently associated with adherence [20,27]. Moreover, coping with the daily challenges of side effects is weighed against the perceived risk of cancer recurrence, which may lead to fear of recurrence if treatment is stopped [42].
We also observed that difficulty in interacting and communicating with the healthcare team was significantly associated with a higher risk of non-persistence. The patient-healthcare provider relationship has previously been identified as a critical factor in adherence and persistence [17,25]. In a study by Berkowitz et al., about one third of the patients (2353 women, 54 men) reported that their side effects were dismissed or minimized by their healthcare team [43]. Another study showed that the ability to ask questions and understand information was significantly associated with increased persistence [44]. In an era of diminishing resources for follow-up care, interactive digital support for monitoring symptoms and medication adherence may be a promising approach to foster communication with healthcare professionals [[45], [46], [47]].
A major limitation of our study is that we assessed only a selection of the many factors reported in the literature to influence non-persistence. Other factors such as social support, self-efficacy, attitudes towards ET, sexual activity, or knowledge of treatment goals may also play a role in young women's decisions to continue with ET. Although we found statistically significant changes in some of the symptom scales, it is difficult to interpret their clinical relevance because there is no information available on meaningful score changes for the scales used. In addition, 21 women (7%) who only completed the baseline questionnaire were not included in the analysis: these women could be those experiencing more symptoms and worse QoL and thus a potential bias in the interpretation of the results cannot be excluded.
5. Conclusions
Our results show that most women continued with ET despite side effects. Women who were partnered and childless were more likely to discontinue ET. This finding suggests that the desire and timing of pregnancy should be a priority when discussing ET with patients, considering individual factors and available risk data [39]. The initial results of the POSITIVE study are reassuring, and, if confirmed in the long term, temporary discontinuation of ET to pursue pregnancy may help to reduce non-persistence in young women who wish to become mothers. Establishing a good relationship and communication with the medical team could also motivate young patients to persist with prescribed therapies.
Funding sources
This work was supported by the Frontier Science & Technology Research Foundation, Southern Europe (FSE), Chiasso, Switzerland and the ETOP IBCSG Partners Foundation, Bern, Switzerland.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
CRediT authorship contribution statement
Eleonora Pagan: Writing – review & editing, Writing – original draft, Methodology, Formal analysis. Monica Ruggeri: Writing – review & editing, Writing – original draft, Project administration, Data curation, Conceptualization. Nadia Bianco: Writing – review & editing, Data curation. Eraldo Oreste Bucci: Writing – review & editing, Data curation. Rossella Graffeo: Writing – review & editing, Data curation. Markus Borner: Writing – review & editing, Data curation. Monica Giordano: Writing – review & editing, Data curation. Lorenzo Gianni: Writing – review & editing, Data curation. Manuela Rabaglio: Writing – review & editing, Data curation. Andrea Freschi: Writing – review & editing, Data curation. Elisabetta Cretella: Writing – review & editing, Data curation. Elena Seles: Writing – review & editing, Data curation. Alberto Farolfi: Writing – review & editing, Data curation. Edda Simoncini: Writing – review & editing, Data curation. Mariangela Ciccarese: Writing – review & editing, Data curation. Daniel Rauch: Writing – review & editing, Data curation. Adolfo Favaretto: Writing – review & editing, Data curation. Friedemann Honecker: Writing – review & editing, Data curation. Rossana Berardi: Writing – review & editing, Data curation. Alessandra Franzetti-Pellanda: Writing – review & editing, Data curation. Shari Gelber: Writing – review & editing, Methodology, Conceptualization. Ann H. Partridge: Writing – review & editing, Conceptualization. Aron Goldhirsch: Conceptualization. Vincenzo Bagnardi: Writing – review & editing, Writing – original draft, Formal analysis, Conceptualization. Olivia Pagani: Writing – review & editing, Writing – original draft, Data curation, Conceptualization. Karin Ribi: Writing – review & editing, Writing – original draft, Conceptualization.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:Eraldo Oreste Bucci reports a relationship with AstraZeneca R&D that includes: consulting or advisory and travel reimbursement. Eraldo Oreste Bucci reports a relationship with Astellas Pharma Europe Ltd that includes: travel reimbursement. Eraldo Oreste Bucci reports a relationship with Bristol Myers Squibb Co that includes: travel reimbursement. Eraldo Oreste Bucci reports a relationship with Eli Lilly and Company that includes: travel reimbursement. Eraldo Oreste Bucci reports a relationship with Merck Serono that includes: travel reimbursement. Eraldo Oreste Bucci reports a relationship with Roche that includes: travel reimbursement. Eraldo Oreste Bucci reports a relationship with Takeda Oncology that includes: travel reimbursement. Lorenzo Gianni reports a relationship with AstraZeneca that includes: consulting or advisory. Lorenzo Gianni reports a relationship with Seagen Inc that includes: consulting or advisory. Lorenzo Gianni reports a relationship with Pfizer and Novartis that includes: travel reimbursement. Alberto Farolfi reports a relationship with from Janssen Oncology, GSK-Tesaro, Astrazeneca, Clovis that includes: paid expert testimony. Rossana Berardi reports a relationship with AZ, BI, Novartis, MSD, Otsuka, Lilly, Roche, Amgen, GSK, EISAI, Seage that includes: consulting or advisory. Anne Patridge reports a relationship with Wolters Kluwer, Novartis that includes: equity or stocks and funding grants. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The IBCSG 43-09 HOHO European protocol was developed and conducted by the Frontier Science & Technology Research Foundation, Southern Europe (FSE) and the International Breast Cancer Study Group (IBCSG) with the financial support of the Fondazione Leonardo, Lugano Switzerland, Pink Ribbon Switzerland and private donations. We thank the patients, physicians, nurses, and trial coordinators who participated in the HOHO study.
References
- 1.Arnold M., et al. Current and future burden of breast cancer: global statistics for 2020 and 2040. Breast. 2022;66:15–23. doi: 10.1016/j.breast.2022.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DeSantis C.E., et al. Breast cancer statistics, 2019. CA Cancer J Clin. 2019;69(6):438–451. doi: 10.3322/caac.21583. [DOI] [PubMed] [Google Scholar]
- 3.Ecis - European cancer information System - incidence and Mortality. 2020. https://ecis.jrc.ec.europa.eu/explorer.php?$0-0$1-All$2-All$4-1,2$3-0$6-0,85$5-2008,2008$7-7$CEstByCountry$X0_8-3$X0_19-AE27$X0_20-No$CEstBySexByCountry$X1_8-3$X1_19-AE27$X1_-1-1$CEstByIndiByCountry$X2_8-3$X2_19-AE27$X2_20-No$CEstRelative$X3_8-3$X3_9-AE27$X3_19-AE27$CEstByCountryTable$X4_19-AE27 [Google Scholar]
- 4.Fredholm H., et al. Long-term outcome in young women with breast cancer: a population-based study. Breast Cancer Res Treat. 2016;160(1):131–143. doi: 10.1007/s10549-016-3983-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Partridge A.H., et al. Subtype-dependent relationship between young age at diagnosis and breast cancer survival. J Clin Oncol. 2016;34(27):3308–3314. doi: 10.1200/JCO.2015.65.8013. [DOI] [PubMed] [Google Scholar]
- 6.Fu J., et al. Young patients with hormone receptor-positive breast cancer have a higher long-term risk of breast cancer specific death. J Breast Cancer. 2019;22(1):96–108. doi: 10.4048/jbc.2019.22.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paluch-Shimon S., et al. ESO-ESMO fifth international consensus guidelines for breast cancer in young women (BCY5) Ann Oncol. 2022;33(11):1097–1118. doi: 10.1016/j.annonc.2022.07.007. [DOI] [PubMed] [Google Scholar]
- 8.Heer E., et al. Global burden and trends in premenopausal and postmenopausal breast cancer: a population-based study. Lancet Glob Health. 2020;8(8):e1027–e1037. doi: 10.1016/S2214-109X(20)30215-1. [DOI] [PubMed] [Google Scholar]
- 9.Burstein H.J., et al. Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer: ASCO clinical practice Guideline focused update. J Clin Oncol. 2019;37(5):423–438. doi: 10.1200/JCO.18.01160. [DOI] [PubMed] [Google Scholar]
- 10.Cardoso F., et al. Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up†. Ann Oncol. 2019;30(8):1194–1220. doi: 10.1093/annonc/mdz173. [DOI] [PubMed] [Google Scholar]
- 11.Cramer J.A., et al. Medication compliance and persistence: terminology and definitions. Value Health. 2008;11(1):44–47. doi: 10.1111/j.1524-4733.2007.00213.x. [DOI] [PubMed] [Google Scholar]
- 12.Murphy C.C., et al. Adherence to adjuvant hormonal therapy among breast cancer survivors in clinical practice: a systematic review. Breast Cancer Res Treat. 2012;134(2):459–478. doi: 10.1007/s10549-012-2114-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yussof I., et al. Factors influencing five-year adherence to adjuvant endocrine therapy in breast cancer patients: a systematic review. Breast. 2022;62:22–35. doi: 10.1016/j.breast.2022.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hershman D.L., et al. Early discontinuation and non-adherence to adjuvant hormonal therapy are associated with increased mortality in women with breast cancer. Breast Cancer Res Treat. 2011;126(2):529–537. doi: 10.1007/s10549-010-1132-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sopik V. International variation in breast cancer incidence and mortality in young women. Breast Cancer Res Treat. 2021;186(2):497–507. doi: 10.1007/s10549-020-06003-8. [DOI] [PubMed] [Google Scholar]
- 16.Moon Z., et al. Barriers and facilitators of adjuvant hormone therapy adherence and persistence in women with breast cancer: a systematic review. Patient Prefer Adherence. 2017;11:305–322. doi: 10.2147/PPA.S126651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lambert L.K., et al. Patient-reported factors associated with adherence to adjuvant endocrine therapy after breast cancer: an integrative review. Breast Cancer Res Treat. 2018;167(3):615–633. doi: 10.1007/s10549-017-4561-5. [DOI] [PubMed] [Google Scholar]
- 18.Paranjpe R., et al. Identifying adherence barriers to oral endocrine therapy among breast cancer survivors. Breast Cancer Res Treat. 2019;174(2):297–305. doi: 10.1007/s10549-018-05073-z. [DOI] [PubMed] [Google Scholar]
- 19.Peddie N., et al. The impact of medication side effects on adherence and persistence to hormone therapy in breast cancer survivors: a qualitative systematic review and thematic synthesis. Breast. 2021;58:147–159. doi: 10.1016/j.breast.2021.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Toivonen K.I., et al. Potentially modifiable factors associated with adherence to adjuvant endocrine therapy among breast cancer survivors: a systematic review. Cancers. 2020;13(1) doi: 10.3390/cancers13010107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.AlOmeir O., Patel N., Donyai P. Adherence to adjuvant endocrine therapy among breast cancer survivors: a systematic review and meta-synthesis of the qualitative literature using grounded theory. Support Care Cancer. 2020;28(11):5075–5084. doi: 10.1007/s00520-020-05585-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hershman D.L., et al. Psychosocial factors related to non-persistence with adjuvant endocrine therapy among women with breast cancer: the Breast Cancer Quality of Care Study (BQUAL) Breast Cancer Res Treat. 2016;157(1):133–143. doi: 10.1007/s10549-016-3788-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pinheiro L.C., et al. Investigating associations between health-related quality of life and endocrine therapy underuse in women with early-stage breast cancer. J Oncol Pract. 2017;13(5):e463–e473. doi: 10.1200/JOP.2016.018630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kadakia K.C., et al. Patient-reported outcomes and early discontinuation in aromatase inhibitor-treated postmenopausal women with early stage breast cancer. Oncol. 2016;21(5):539–546. doi: 10.1634/theoncologist.2015-0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stanton A.L., Petrie K.J., Partridge A.H. Contributors to nonadherence and nonpersistence with endocrine therapy in breast cancer survivors recruited from an online research registry. Breast Cancer Res Treat. 2014;145(2):525–534. doi: 10.1007/s10549-014-2961-3. [DOI] [PubMed] [Google Scholar]
- 26.Partridge A.H., et al. Nonadherence to adjuvant tamoxifen therapy in women with primary breast cancer. J Clin Oncol. 2003;21(4):602–606. doi: 10.1200/JCO.2003.07.071. [DOI] [PubMed] [Google Scholar]
- 27.Yang S., et al. Investigation of factors affecting adherence to adjuvant hormone therapy in early-stage breast cancer patients: a comprehensive systematic review. J Breast Cancer. 2023;Aug;26(4):309–333. doi: 10.4048/jbc.2023.26.e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosenberg S.M., et al. Adjuvant endocrine therapy non-initiation and non-persistence in young women with early-stage breast cancer. Breast Cancer Res Treat. 2023;197(3):547–558. doi: 10.1007/s10549-022-06810-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ruggeri M., et al. Fertility concerns, preservation strategies and quality of life in young women with breast cancer: baseline results from an ongoing prospective cohort study in selected European Centers. Breast. 2019;47:85–92. doi: 10.1016/j.breast.2019.07.001. [DOI] [PubMed] [Google Scholar]
- 30.Stanton A.L., Bernaards C.A., Ganz P.A. The BCPT symptom scales: a measure of physical symptoms for women diagnosed with or at risk for breast cancer. J Natl Cancer Inst. 2005;97(6):448–456. doi: 10.1093/jnci/dji069. [DOI] [PubMed] [Google Scholar]
- 31.Terhorst L., et al. Evaluation of the psychometric properties of the BCPT Symptom Checklist with a sample of breast cancer patients before and after adjuvant therapy. Psycho Oncol. 2011;20(9):961–968. doi: 10.1002/pon.1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schag C.A., Ganz P.A., Heinrich R.L. CAncer Rehabilitation Evaluation System--short form (CARES-SF). A cancer specific rehabilitation and quality of life instrument. Cancer. 1991;68(6):1406–1413. doi: 10.1002/1097-0142(19910915)68:6<1406::aid-cncr2820680638>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 33.Ganz P.A., et al. The CARES: a generic measure of health-related quality of life for patients with cancer. Qual Life Res. 1992;1(1):19–29. doi: 10.1007/BF00435432. [DOI] [PubMed] [Google Scholar]
- 34.Partridge A.H., et al. Interrupting endocrine therapy to attempt pregnancy after breast cancer. N Engl J Med. 2023;388(18):1645–1656. doi: 10.1056/NEJMoa2212856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Llarena N.C., et al. Impact of fertility concerns on tamoxifen initiation and persistence. J Natl Cancer Inst. 2015;107(10) doi: 10.1093/jnci/djv202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saha P., et al. Treatment efficacy, adherence, and quality of life among women younger than 35 Years in the international breast cancer study group TEXT and SOFT adjuvant endocrine therapy trials. J Clin Oncol. 2017;35(27):3113–3122. doi: 10.1200/JCO.2016.72.0946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Llarena N.C., et al. Impact of fertility concerns on tamoxifen initiation and persistence. JNCI: Journal of the National Cancer Institute. 2015;107(10) doi: 10.1093/jnci/djv202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Poorvu P.D., et al. Cancer treatment-related infertility: a critical review of the evidence. JNCI Cancer Spectr. 2019;3(1):pkz008. doi: 10.1093/jncics/pkz008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lipsyc-Sharf M., Partridge A.H. Fertility and sexual health in young women with early-stage breast cancer. Surg Oncol Clin N Am. 2023;32(4):747–759. doi: 10.1016/j.soc.2023.05.012. [DOI] [PubMed] [Google Scholar]
- 40.Rosenberg S.M., et al. Association of breast cancer surgery with quality of life and psychosocial well-being in young breast cancer survivors. JAMA Surg. 2020;155(11):1035–1042. doi: 10.1001/jamasurg.2020.3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jacobs J.M., et al. The patient's voice: adherence, symptoms, and distress related to adjuvant endocrine therapy after breast cancer. Int J Behav Med. 2020;27(6):687–697. doi: 10.1007/s12529-020-09908-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Toivonen K.I., et al. A survey of potentially modifiable patient-level factors associated with self-report and objectively measured adherence to adjuvant endocrine therapies after breast cancer. Patient Prefer Adherence. 2021;15:2039–2050. doi: 10.2147/PPA.S319087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Berkowitz M.J., et al. How patients experience endocrine therapy for breast cancer: an online survey of side effects, adherence, and medical team support. J Cancer Surviv. 2021;15(1):29–39. doi: 10.1007/s11764-020-00908-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cluze C., et al. Adjuvant endocrine therapy with tamoxifen in young women with breast cancer: determinants of interruptions vary over time. Ann Oncol. 2012;23(4):882–890. doi: 10.1093/annonc/mdr330. [DOI] [PubMed] [Google Scholar]
- 45.Bergqvist J., Lundström S., Wengström Y. Patient interactive digital support for women with adjuvant endocrine therapy in order to increase compliance and quality of life. Support Care Cancer. 2021;29(1):491–497. doi: 10.1007/s00520-020-05476-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Graetz I., et al. Use of a web-based app to improve breast cancer symptom management and adherence for aromatase inhibitors: a randomized controlled feasibility trial. J Cancer Surviv. 2018;12(4):431–440. doi: 10.1007/s11764-018-0682-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gernier F., et al. Impact of web application support versus standard management on adherence with adjuvant hormone therapy in patients treated for breast cancer: the WEBAPPAC study. BMC Cancer. 2023;23(1):736. doi: 10.1186/s12885-023-11242-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.