Abstract
Transfer RNAs (tRNAs) are the most highly modified cellular RNAs, both with respect to the proportion of nucleotides that are modified within the tRNA sequence and with respect to the extraordinary diversity in tRNA modification chemistry. However, the functions of many different tRNA modifications are only beginning to emerge. tRNAs have two general clusters of modifications. The first cluster is within the anticodon stem-loop including several modifications essential for protein translation. The second cluster of modifications is within the tRNA elbow, and roles for these modifications are less clear. In general, tRNA elbow modifications are typically not essential for cell growth, but nonetheless several tRNA elbow modifications have been highly conserved throughout all domains of life. In addition to forming modifications, many tRNA modifying enzymes have been demonstrated or hypothesized to also play an important role in folding tRNA acting as tRNA chaperones. In this review, we summarize the known functions of tRNA modifying enzymes throughout the lifecycle of a tRNA molecule, from transcription to degradation. Thereby, we describe how tRNA modification and folding by tRNA modifying enzymes enhance tRNA maturation, tRNA aminoacylation, and tRNA function during protein synthesis, ultimately impacting cellular phenotypes and disease.
Keywords: RNA, transfer RNA (tRNA), RNA modification, RNA methylation, RNA processing, RNA binding protein, precursor tRNA (pre-tRNA), aminoacyl tRNA synthetase, ribosome, protein synthesis, RNA folding, RNA structure
Transfer RNAs (tRNAs) are essential molecules that act as physical adapters between the genetic code and amino acids in every cell. Almost all tRNAs feature a cloverleaf secondary structure that folds into an L-shaped tertiary structure (Fig. 1A). Decades of ongoing research has established that all tRNAs contain modifications, which play a variety of roles in tRNA structure and function, and which are introduced by a diverse set of dedicated tRNA modifying enzymes (1, 2, 3, 4). Underlying the abundance and energy investment of tRNA modification within cells, genes encoding tRNA modifying enzymes can account for 1% of the protein coding genes in an organism. For example, the model organism Escherichia coli has ∼4300 protein coding genes, of which 59 encode enzymes involved in the formation of 28 tRNA modifications (Fig. 1B). Highlighting the importance of tRNA modification, mutations within tRNA sequences that prevent modification or within the genes encoding tRNA modifying enzymes have been implicated in a growing number of human diseases (5, 6, 7).
Figure 1.
tRNA structure and modification.A, cloverleaf secondary structure (left) and L-shaped tertiary structure (right) typical of the majority of tRNAs. B, locations and identities of all modifications in Escherichia coli tRNAs. Common modifications are denoted by color, as indicated in the legend, which also indicates the abbreviations for these common modifications. Modifications are systematically abbreviated with letters preceding the nucleotide indicating a base modification. Superscript numbers indicate the position of the nucleotide where the modification is found. Letters after the nucleotide indicate modification to the ribose sugar. More explanation for the abbreviation of tRNA modifications is excellently summarized in (1). Multiple different modifications can occur at positions 32, 34, and 37 in different tRNAs, as shown in tables. Abbreviations not included in the legend but present in the table are as follows: acp–aminocarboxypropyl, I–inosine, ac–acetyl, k–lysidine, (c)mnm–(carboxy)methylaminomethyl, Se–selenium, Q–queuosine, ho–hydroxy, cmo–carboxymethoxy, mcmo–methoxycarbonylmethoxy, i–isopentyl, (c)t–(cyclic)threonylcarbamoyl. C, locations of modifications within the E. coli tRNAPhe structure (82) as a typical tRNA to demonstrate clusters of modifications in the tRNA elbow and in the tRNA anticodon stem-loop. Modifications are colored as per the legend in panel B. D, examples of some of the modifications found within tRNAs. Atoms that constitute each modification are indicated in red.
Within the L-shaped three-dimensional tRNA structure, two clusters of tRNA modifications are apparent (Fig. 1C) (1, 8). The first cluster of tRNA modifications is found within the tRNA anticodon stem-loop. In this region, modifications often play direct functional roles during protein translation (9, 10). For this reason, several modifications found within this region are essential for cell viability. The second cluster of tRNA modifications is evident in the elbow of the L-shaped tertiary structure, which is also referred to as the tRNA body (8). In general, modifications within this region fine-tune tRNA structure and stability (1).
Modifications to tRNA are often simple reactions, such as isomerization of the uracil base to form pseudouridine, reduction of uridine to form dihydrouridine, methylation at various atoms of the nucleobase or ribose sugar, or exchange of oxygen for sulfur during thiolation; however, some reactions are more complex and involve more than one enzyme, forming bulky hypermodifications (Fig. 1D). Most often, tRNA hypermodifications are found within the tRNA anticodon loop, especially at position 34, which is the wobble position that base pairs to the nucleotide at the third position of an mRNA codon during translation, and at the conserved purine at position 37, which is adjacent to the anticodon. Certain modifications to the tRNA elbow are found in many tRNAs across all domains of life, suggesting a conserved function. These include dihydrouridine (D) at various positions within the D arm, 5-methyluridine (m5U, also known as ribothymidine, rT) 54, pseudouridine (Ψ) 55, 1-methyladenosine (m1A) 58, 2ʹ-O-methylguanosine (Gm) 18, and 7-methylguanosine (m7G) 46. In contrast, modifications within the tRNA anticodon loop are often found in only a handful of tRNAs and display more variation between different organisms, suggesting possible roles for certain modifications in environmental adaptation. In the model organism E. coli, all tRNA modifications are thought to be mapped, and with the identification of the TapT enzyme in 2019, all enzymes responsible for E. coli modifications have been identified (11, 12, 13). In contrast, complete mapping of tRNA modifications in most organisms has yet to be accomplished and novel modifications and modifying enzymes remain to be discovered.
The majority of tRNA modifying enzymes have been found to be nonessential for cells grown in ideal laboratory conditions (14); however, many cellular phenotypes have been identified for tRNA modification enzyme knockout (KO) strains in stress conditions, suggesting an importance for these enzymes in maximizing cellular fitness (Table 1). Moreover, deletion of two or more modification enzymes oftentimes results in more severe growth defects, emphasizing the likely redundant nature of tRNA modifications and modifying enzymes (15). In addition to the catalytic role of modifying tRNAs, certain tRNA modification enzymes may also play an important role in folding tRNAs, acting as tRNA chaperones (16, 17, 18). Thus, tRNA modifying enzymes not only affect the modification status of tRNAs, but also tRNA folding. tRNA interacts with a variety of factors during its lifecycle (Figs. 2 and 3) and tRNA modification status and folding affect several of these interactions. In this review, we summarize the precise roles of tRNA modifying enzymes and tRNA modifications with respect to the tRNA lifecycle. In particular, we focus on how modifications and the responsible enzymes collaborate to affect tRNA maturation and function during translation.
Table 1.
Stress-related growth phenotypes for tRNA modification deletion strains
| tRNA modification | tRNA modifying enzyme | Organism | Phenotype | Ref. |
|---|---|---|---|---|
| Ψ55 | TruB | Escherichia coli | Reduced fitness in coculture competition |
(17, 86, 174) |
| Impaired recovery from heat shock | ||||
| Ψ55 | TruB | T. thermophilus | Slow growth at 50 °C (cold for thermophilic model organism) | (175) |
| m5U54 | TrmA | E. coli | Reduced fitness in coculture competition | (16, 176) |
| Increased growth in the presence of hygromycin | ||||
| m5U54 | Trm2 | Saccharomyces cerevisiae | Increased growth in the presence of hygromycin | (176) |
| m7G46 | TrmB | E. coli, Pseudomonas aeruginosa, Acinetobacter baumannii, Colletotrichum lagenarium | Slow growth in presence of H2O2 | (177, 178, 179, 180) |
| m7G46 | TrmB | T. thermophilus | Slow growth at 80 °C | (181) |
| m7G46 | Trm8/Trm82 | S. cerevisiae | Slow growth at 38 °C in minimal media with 2% glycerol; augmented temperature sensitive growth in absence of trm4, pus7, or dus3 genes | (15, 182) |
| Ψ38/39 | Pus3 | S. cerevisiae | Slow growth at 37 °C | (183) |
| t6A37 | Tsc2/Tsc3 | S. cerevisiae | Several phenotypes, including slow growth at 37 °C and slow growth in presence of TOR inhibitors | (117) |
| ncm5/mcm534 | Elongator complex | S. cerevisiae and Schizosaccharomyces pombe | Several phenotypes, including slow growth and temperature sensitivity | (184) |
| D16 | DusC | E. coli | Slow growth at cold temperature (24 °C), exacerbated by loss of TmcA (forms ac4C) | (185) |
| m5C34 and m7G46 | Nsun2 and METTL1 | Homo sapiens (HeLa cells) | Increased sensitivity to 5-fluorouracil | (186) |
| m1A9 | Trm10 | S. cerevisiae | Increased sensitivity to 5-fluorouracil, particularly at 38 °C (along with additional tRNA modifying enzymes) | (187) |
Figure 2.
General lifecycle of a tRNA. Following transcription of pre-tRNA by RNA polymerase (1), tRNA undergoes several maturation steps (2) including 5ʹ and 3ʹ end processing, intron splicing, modification of many nucleotides, and RNA folding, giving rise to a mature tRNA. Mature tRNA is then aminoacylated to form aminoacyl-tRNA (aa-tRNA), which binds EF-Tu•GTP, forming the ternary complex for delivery to the ribosome (3). Various ribonucleases target tRNA to degrade aberrantly matured tRNA (4) or to cleave tRNA into functional tRNA-derived small RNAs (tDR) (5). Finally, tRNAs also participate in a variety of nontranslation alternative processes (6).
Figure 3.
Examples of tRNA-protein complex structures illustrating the various interactions of tRNAs throughout its lifecycle. In all cases, tRNA is colored black and is in roughly the same orientation to demonstrate how different proteins interact with various surfaces of the tRNA. From left to right on the top row: yeast unbound tRNA (PDB: 4TNA) (237); Pyrococcus horikoshii ArcTGT homodimer colored in shades of red bound to two tRNAVal molecules to modify G15 with tRNA adopting the lambda form. The second tRNA molecule is shown in gray (1J2B) (89); Archaeoglobus fulgidus TiaS bound to tRNAIle2 for modifying C34 (PDB: 3AMT) (238); Escherichia coli RlmN bound to tRNAGlu for modification of A37 (PDB: 5HR7) (239); E. coli DusC bound to tRNATrp to modify U16 (PDB ID: 4YCP) (240) and Thermus thermophilus Dus bound to tRNAPhe to modify U20 (PDB: 3B0V) (241) displaying how different dihydrouridine synthases target different tRNA residues. The second line contains the METTL1 (dark blue) and WDR4 (light blue) heterodimer bound to tRNA to modify G46 (PDB: 8EG0) (242); Thermus aquaticus EF-Tu bound to tRNACys (PDB: 1B23) (243); yeast AspRS bound to tRNA (PDB: 1ASZ) (244); human mitochondrial protein only RNase P in complex with pre-tRNATyr. RNase P is colored in dark blue, TRMT10C is colored in light blue, and MRPP3 is colored in indigo (PDB: 7ONU) (43); and Thermotoga maritima RNase P in complex with tRNAPhe (PDB: 3Q1Q) (245). The protein component is colored in pink and the RNA in gray. PDB, Protein Data Bank.
tRNA maturation overview
tRNAs are transcribed as precursor (pre)-tRNAs, which undergo a multienzyme maturation process prior to fulfilling diverse functions (Fig. 2). These maturation events are not strictly temporally defined, and the order can vary based on both organism and tRNA identity. In eukaryotes, the subcellular localization of tRNA processing components largely dictates the tRNA processing order. For example, in yeast many tRNAs are transcribed in the nucleolus whereas others may be transcribed in the nucleoplasm (19). In other eukaryotes, the details regarding the location of tRNA transcription and maturation remain unexplored. Nonetheless, tRNAs subsequently encounter different processing enzymes within the nucleus and cytoplasm, followed by potential retrograde import to the nucleus and reexport back to the cytoplasm (2, 20). Following transcription, the 5ʹ leader and 3ʹ trailer sequences are removed from the pre-tRNA by various endonucleases and exonucleases (21, 22, 23, 24). With the exception of several bacteria, the tRNA genes from many organisms lack the 3ʹ-CCA tail necessary for aminoacylation (25). As such, this sequence must be posttranscriptionally added by the tRNA nucleotidyltransferase (26). Certain tRNAs contain an intron which is usually located within the tRNA anticodon stem-loop (2, 27, 28). Eukaryotic and archaeal tRNA introns require several enzymes to excise the intron and repair the tRNA; however, the details of these reactions differ between organisms, and the definitive cellular location of tRNA splicing remains to be determined for several cell types (29, 30, 31). For example, whereas it has been postulated that tRNA splicing occurs in the nucleus in Xenopus oocytes and human cells (32, 33), more recent evidence using endogenous factors suggests splicing of pre-tRNA in mammalian cells occurs in the cytoplasm (34). Moreover, the tRNA splicing machinery is localized to the cytoplasmic surface of mitochondria in yeast (35). Bacterial tRNAs rarely contain introns, but when present they are self-spliced without the need for proteins (36).
Ribose and base modifications in tRNA can occur at all steps of tRNA maturation. Again, the intracellular localization of the responsible modifying enzyme plays a role in whether a modification will occur during the early or late steps of tRNA maturation, and in many cases, it may be that a tRNA is modified by a given enzyme at the first available opportunity within the cell, rather than in a strict order (37). In the two sections below, we will note instances of collaboration between tRNA modifying enzymes and crosstalk of modifications themselves with other enzymes involved in tRNA maturation, with a focus on how early tRNA modifying enzymes affect later acting modification enzymes.
Cleavages of pre-tRNA: interplay between modifying enzymes and tRNA processing
Decades ago, it was first shown that many tRNA modifications occur prior to tRNA end processing and tRNA intron splicing in Xenopus oocytes (32, 38, 39). As such, it is unsurprising that crosstalk exists between tRNA modifying enzymes and other enzymes involved in tRNA maturation. Even a link between tRNA transcription and modification has been identified as changes in RNA polymerase III activity correlate with the activity of the tRNA dimethyltransferase Trm1 (40). In yeast, the 5ʹ end is almost always removed prior to the 3ʹ trailer, but in E. coli, an initial cleavage event at the 3ʹ end often precedes 5ʹ processing by RNase P (23, 24, 27, 28). Although RNase P is usually a ribonucleoprotein, certain organisms and organelles instead rely on an endonuclease comprised solely of proteins and thus called protein only RNase P (41) (Fig. 3). Interestingly metazoan mitochondrial protein only RNase P is a multienzyme complex composed of TRMT10C (also known as MRPP1), MRPP2, and PRORP (also known as MRPP3), wherein TRMT10C is a tRNA methyltransferase responsible for N1-methylation of purines at position 9 (42). Within this RNase P complex, TRMT10C forms contacts with all four tRNA arms, in addition to MRPP2 and PRORP (Fig. 3) (43). Presence of TRMT10C is necessary for efficient cleavage by RNase P by properly orientating PRORP and pre-tRNA, consequently enhancing the rate of nuclease activity (44). For certain pre-tRNA substrates, prior binding of SAM by TRMT10C may enhance pre-tRNA binding and cleavage (45); however, the methyltransferase activity of TRMT10C does not contribute to tRNA binding or activity of protein only RNase P (46). TRMT10C can methylate independently of PRORP, but for certain pre-tRNAs the presence of PRORP may increase the rate of methylation (45). Although the functional interdependence (if any) between the two tRNA maturation activities of TRMT10C remains unclear (44, 46), it has been hypothesized that TRMT10C may function as a platform for coordinating tRNA maturation events, including methylation, 5ʹ cleavage, in addition to 3ʹ processing by RNase Z and the CCA adding enzyme (47). In a similar manner, the plant single-subunit protein only RNase P has been found to interact with the dimethyltransferases TRM1A and TRM1B and these methyltransferases seem to additionally interact with RNase Z (48). In contrast to the role of TRMT10C in human mitochondrial RNase P, TRM1A/TRM1B interacts with plant RNase P in an indirect, tRNA-dependent manner, which nonetheless highlights coordination between tRNA modifying enzymes and tRNA processing enzymes as a theme during tRNA maturation.
Intron splicing occurs independently of tRNA end processing and modifications (27). Although several tRNA modification enzymes are insensitive to the presence or absence of tRNA introns, others absolutely rely on intron presence (or absence) for catalysis, as summarized in (49). For example, pseudouridylation of U35 in tRNATyr by Pus1 within various eukaryotes absolutely requires intron presence but does not require the intron to be of a certain size or sequence (50, 51, 52), and C5-methylation of yeast tRNAPhe at cytosine 40 requires an intron, but is not sensitive to the presence of the D or T arms (53). As far as currently known, the activity of the tRNA splicing machinery is unaffected by tRNA modifying enzymes.
Early tRNA modifications affect the activity of later acting tRNA modifying enzymes
Approximately 10% of all nucleosides within tRNA are posttranscriptionally modified by a large set of dedicated tRNA modifying enzymes. Each tRNA contains its own unique set of modifications, but certain modifications, including m5U54, Ψ55, m7G46, and D at several positions, are found in many tRNAs (8). As suggested above, tRNA modifying enzymes may introduce their modification at any time during tRNA maturation (32, 38, 39), although a growing number of studies suggest that the modification process is at least somewhat ordered, with certain modifications appearing prior to tRNA end processing and splicing and others forming only after the pre-tRNA is processed. However, it is unlikely that tRNA modification follows a strict sequential order, as many tRNA modifying enzymes can effectively modify in vitro transcribed (i.e. unmodified) tRNA. Several instances of tRNA modifications positively and negatively affecting the activity of enzymes introducing other modifications have been uncovered and have been referred to as “modification crosstalk,” “modification circuits,” or “modification networks” (54, 55, 56, 57, 58). Determining the relative temporal order of tRNA modification is a challenging task because tRNA modifications are introduced quickly and because there are several technical difficulties. For example, it is necessary to overcome challenges in purifying specific tRNA isoacceptors, obstacles of quantitatively distinguishing sites of modification (e.g. distinguishing sites of several pseudouridines), and the need for sophisticated expertise and equipment to monitor many chemically distinct nucleotides in one experiment. Most often, modification crosstalk has been uncovered by extracting total tRNA or a specific tRNA isoacceptor from deletion strains lacking one (or more) tRNA modification enzyme. This tRNA is then monitored for changes in the steady-state abundance of other modifications, typically by using either high-performance LC/MS or various next-generation sequencing techniques (59). In other cases, in vitro studies have been used to determine how the presence/absence of modifications affects tRNA binding and modification by modifying enzymes; this approach can determine the mechanisms underlying tRNA modification crosstalk. Known instances of tRNA modifications positively and negatively affecting the activities of tRNA modifying enzymes that have been examined using these and similar assays are summarized in Table 2. As several reviews have explained modification circuits with an emphasis on tRNA anticodon stem-loop modifications (54, 55, 56, 57, 58), we summarize here recent techniques that have advanced our knowledge regarding the temporal placement of tRNA modifications with a focus on particular modifications within the tRNA variable and T loops: m7G46, m5U54, Ψ55, and m1A58.
Table 2.
Interactions between tRNA modifications and tRNA modifying enzymes
| Initial modification (enzyme) | Affected modification (enzyme) | Interaction+/−a | Organisms | Evidence | Ref. |
|---|---|---|---|---|---|
| m1A58 (TRM6/TRM61) | m5U54 (TRMT2A) | + | H. sapiens (HEK293FT) | LC/MS analysis of TRM6 mutant cell lines | (66) |
| m1A58 (TrmI) | s2m5U54 (TtuABC, IscS) | + | T. thermophilus | LC/MS and enzymatic analysis of KO strain | (188) |
| Ψ55 (Pus4) | m5U54 (Trm2), | + | S. cerevisiae | LC/MS analysis of KO strain | (60) |
| m1A58 (Trm6/Trm61) | |||||
| Ψ55 (TruB) | Gm18 (TrmH) m5s2U54 (TtuABC, IscS), | − | T. thermophilus | LC/MS and enzymatic analysis of KO strain | (175) |
| m1A58 (TrmI) | |||||
| Ψ55 (TruB) | m5U54 (TrmA) | + | E. coli | Increased affinity of TrmA to Ψ55 tRNA in vitro | (74) |
| m5U54 (Trm2) | m1A58 (Trm6/Trm61) | + | S. cerevisiae | LC/MS analysis of KO strain | (60) |
| m5U54 (TrmA), s4U8 (ThiI), m7G46 (TrmB) | Ψ55 (TruB) | − | E. coli | Decreased affinity and activity of TruB for singly modified tRNA in vitro | (74) |
| m7G46 (TrmB) | acp3U47 (TapT) | + | E. coli | LC/MS and primer extension analysis of KO strain | (13) |
| m7G46 (TrmB) | Gm18 (TrmH) m1G37 (TrmD) | + | T. thermophilus | LC/MS and enzymatic assays of KO strain | (181) |
| i6A37 (MiaA) | (C/U)m34 (TrmL) | + | E. coli | LC/MS of bulk and specific tRNAs from ΔmiaA strains; in vitro activity assays | (189, 190) |
| t6A37 (TsaBDE) | L34 (TilS) | + | E. coli | Increased TilS activity in vitro | (191) |
| t6A37 (KEOPS) | mcm5s2U34 (Elongator) | - | S. cerevisiae | HPLC analysis of KO strains | (117) |
| i6A37 (MOD5) or t6A37 (KEOPS) | m3C32 (Trm140) | + | S. cerevisiae | tRNA-HySeq and LC/MS analysis of KO strains; tRNA pulldowns; in vitro methylation assays | (109, 192) |
| S. pombe | |||||
| t6A37 (Sua5/KEOPS) | m3C32 (METTL2A) | + | H. sapiens (cell line HEK293T) | In vitro methylations assays | (108) |
| m1G37 (TrmD) | mcmo5U34 (CmoM) | + | E. coli | Faster in vitro methylation | (73) |
| Q34 (Tgt) | m5C38 (Dnmt2) | + | S. pombe | In vivo bisulfite sequencing of KO strains | (142, 193) |
| D. discoideum | |||||
| H. sapiens (HeLa and HCT116 cell lines) | |||||
| Cm32 and Gm34 (Trm7 and Trm732 or Trm734) | yW37 (yeast, Tyw1-4) or o2yW37 (humans, unknown) | + | S. cerevisiae | LC/MS and biochemical characterization of tRNA from KO strains | (194, 195, 196, 197) |
| S. pombe | |||||
| Mus musculus | |||||
| H. sapiens | |||||
| m3C32 (Trm140) | m3U32 (ADAT2/3) | + | T. brucei | In vitro modification assays show U32 methylation must occur prior to C-to-U editing and Trm140 must be present for editing reaction to occur | (198) |
| Deamination of m3C32 to m3U32 | I34 (ADAT2/3) | + | T. brucei | More efficient inosine formation in vitro | (199) |
| One or more of the full modification set | D20 (Dus2) | + | S. cerevisiae | Tighter binding of modified tRNA and faster oxidation of Dus2 | (200) |
Abbreviation: KO, knockout.
+ indicates modification whose presence is stimulated by the enzyme identified in the first column. - indicates a modification whose presence is repressed by the enzyme identified in the first column.
Several studies have identified that T arm modifications, in particular m5U54 and Ψ55, generally tend to be among the earliest introduced during tRNA maturation. Recently, a time-resolved NMR assay was developed to follow the modification of a stable-isotope labeled in vitro transcribed tRNAPhe molecule within yeast cell extract (60). This experiment identified that Ψ55 is introduced early and quickly into tRNA, followed by m5U54 (over a long period of time) and m7G46 (over a short period of time), followed by m2G10, m5C49, and D16. Finally, m1A58 was the last modification to be detected within tRNAPhe (m22G26 and m5C40 were not observed within the incubation timeframe). Although this experiment is able to detect many modifications at one time in a cell-like environment, disadvantages include loss of cellular compartmentalization, meaning tRNA may encounter cytoplasmic enzymes sooner than it would within the cell. An interesting finding of this study is the apparently late introduction of the m1A58 modification. Considering the enzymes responsible for m1A58 (Trm6/Trm61) are localized to the nucleus (61), it would be surprising for m1A58 to be one of the last modifications to be introduced. Moreover, methylation of A58 in initiator tRNA is well-known to protect initiator tRNAMet from nuclear decay (61, 62, 63), again suggesting this modification to be introduced early. To reconcile these differences, the interdependence of the early modifications m5U54 and Ψ55 on m1A58 was examined using yeast KOs (60), and the effect of these early modifications on Trm6/Trm61 activity was studied with partially modified tRNAs in vitro (64). Presence of m5U54 and Ψ55 increases m1A58 content in bulk tRNA in the cell, and previous introduction of Ψ55 is necessary for efficient formation of m1A58 in in vitro transcribed elongator tRNA, but interestingly not initiator tRNA (60, 64). In vitro, m5U54 additionally stimulates Trm6/Trm61 activity (64). Importantly, as both Trm2 (m5U54) and Pus4 (Ψ55) are localized to the nucleus in yeast, it is feasible that these enzymes modify tRNA prior to Trm6/Trm61.
Mass spectrometry has long been used to detect tRNA modifications, and recently nucleic acid isotope labeling coupled mass spectrometry was developed to examine the temporal order of tRNA modification (65). This assay uses stable isotope pulse-chase labeling of HEK293 cultures to observe tRNA modification in newly transcribed tRNA. Using tRNAPhe, Ψ55 found to also be very quickly incorporated in human tRNA, followed in this case by m5U54, m1A58, m5C49, and then m7G46. In the anticodon stem-loop, m1G37 is introduced quickly and slowly converted to yW37, and Cm and Gm are introduced following m1G37 (65). Interestingly, in another study, HEK293FT cells lacking Trm6 (and therefore m1A58) showed decreased m5U/m5Um content at position 54 in tRNAPhe and tRNALys (66).
Finally, recent advances within the field of tRNA sequencing have made strides toward distinguishing many tRNA modifications (67, 68). In comparison to the experiments described above, next-generation sequencing approaches are advantageous as high-throughput studies, which allow for study of all tRNA species individually without extracting specific isoacceptors or examining only overall trends using bulk tRNA. Unlike Illumina sequencing, Nanopore sequencing can directly sequence RNA without the need for complementary DNA preparation and additionally can distinguish several modifications from canonical nucleotides during this process. Recently, Nano-tRNA-seq recapitulated the requirement of the yeast pus4 gene (catalyzing Ψ55) for efficient m5U54 and m1A58 formation (67). As Nanopore sequencing is a single-molecule method that can determine the presence of modifications within individual tRNAs, this technique is likely to uncover more tRNA modification networks, as has recently been accomplished for rRNA (59, 67, 69). In contrast to Nanopore sequencing, Illumina RNA sequencing requires short reads and thus RNA is usually fragmented prior to sequencing. Because of this step, Illumina sequencing is generally regarded to not be a single molecule method, and only the proportion of modifications in a population can be reported. However, since tRNAs are small (76–90 nucleotides), the entire length can be covered by a single Illumina sequencing read, especially due to advances in overcoming tRNA folding and bulky modifications during complementary DNA preparation (70, 71). Thus, a recent computational pipeline termed single-read analysis of crosstalks was developed to treat Illumina tRNA sequencing reads in a “pseudo-single molecular manner” to examine interdependencies between tRNA modification, aminoacylation, and fragmentation (72). Application of this technique to previously published Illumina tRNA sequencing datasets confirmed several modification crosstalks in tRNA, and identified new interdependencies, several of which involve the m1A58 modification (72).
The studies described above have identified several interdependencies between the introduction of tRNA modifications in vivo, particularly in yeast and humans. While the results above determined the presence of tRNA modification circuits in vivo, we are still lacking an understanding of the mechanisms that give rise to several of these interdependences. A few studies have addressed this by using partially modified tRNA and purified enzymes to compare the binding and affinity of these enzymes for unmodified and partially modified tRNA (64, 73, 74, 75). By studying the binding and modification preferences of the bacterial enzymes responsible for m7G46, m5U54, and Ψ55 (TrmB, TrmA, and TruB, respectively), we uncovered that TruB has a tighter affinity and faster modification rate for unmodified tRNA compared to tRNA that contains one or more additional modifications (74), suggesting the early introduction of Ψ55 is likely to also occur in bacteria. The affinity of TrmA was increased for tRNA containing Ψ55; however, methylation of this Ψ55 tRNA was slower than unmodified tRNA. It is plausible that m5U54 acts early, but potentially following Ψ55 introduction in bacteria. In vivo studies will need to be accomplished to determine the preferred order of modification in bacteria. Finally, corroborating the introduction of m7G somewhere during the middle of tRNA maturation, TrmB did not have a binding or modification preference for tRNA containing or lacking modifications (74), and in vivo studies suggest a requirement of m7G46 formation for later incorporation of acp3U47 in bacterial tRNA (13). Taken together, in vivo detection of modifications combined with enzymatic studies are likely to uncover more tRNA modification circuits and their mechanisms in the future.
Many tRNA modifying enzymes affect tRNA folding and structural dynamics
Although the vast majority of tRNAs adopt the canonical L-shaped tertiary structure in the absence of any modifications, tRNA modifying enzymes have been shown to enhance tRNA folding and structural dynamics through two independent processes: (1) the chemistry of the modified nucleotide altering tRNA structure or dynamics or (2) the act of the tRNA modifying enzyme binding and accessing its target base while locally unfolding the tRNA structure, providing potentially misfolded tRNA a second chance at correctly folding. Each of these topics has been recently reviewed elsewhere (16, 17, 18). Below, we will briefly summarize how tRNA modifying enzymes impact tRNA structure.
Only very few tRNAs require modification in order to adopt the canonical tRNA structure and these instances primarily involve mitochondrial tRNAs, which often feature an atypical secondary structure, sometimes lacking canonical tRNA features such as the D and/or T arms (76). In particular, unmodified human mitochondrial tRNALys primarily adopts an extended hairpin conformation secondary structure, but introduction of m1A9 shifts the structural equilibrium to the canonical L-shape fold by disrupting a base-pair between A9 and U64 (77). Similarly, unmodified mitochondrial tRNAAsp exists in several conformations in vitro, but presence of its native modifications (m1A9, m1G10, Ψ27, and Q34) stabilizes the canonical cloverleaf structure (78). Also, m1A9 has been shown to be important for the overall structure of T arm-less mitochondrial tRNAs from nematodes (79). Finally, m2,2G at different positions within the tRNA D arm functions to restrict the folding of tRNAs that can fold into multiple conformations (80, 81).
In contrast to the examples above, the vast majority of tRNAs do not strictly require any modifications to adopt the canonical L-shaped tertiary structure (82). However, various biophysical techniques have demonstrated how modifications subtly affect tRNA structure and thermodynamics (83). For example, several tRNA body modifications collectively contribute to the stability of tRNASer as demonstrated by loss of tRNA thermal stability in their absence (75), NMR studies have shown the contribution of m1A58 for folding of the tRNA elbow for yeast tRNAiMet (64), CD spectrometry has demonstrated Ψ has enhanced base-stacking propensity compared to uridine (84), and molecular dynamics simulations have shown various hypermodifications at position 37 result in different nucleotide glycosidic and backbone conformations compared to unmodified tRNA (85). Thus, modifications play subtle, but important roles to enhance the structure and dynamics of tRNA.
In addition to the role of modifications in augmenting tRNA structure, the enzymes that modify tRNAs themselves influence tRNA folding independently of their modification activity. Following decades-old research showing expression of catalytically inactive TruB rescues the coculture growth defect of ΔtruB E. coli (86), TruB was the first modification enzyme proven to be a tRNA chaperone (17). Upon binding tRNA, TruB disrupts tertiary interactions between the D and T arms, thus providing a potentially misfolded tRNA multiple chances at refolding and obtaining its correct fold (17). Subsequently, bacterial TrmA was additionally characterized to be a tRNA chaperone (16). So far, E. coli TrmA and TruB have been the only tRNA modifying enzymes categorized as tRNA chaperones; however, likely several more tRNA chaperones are yet to be discovered (18), including TrmA and TruB’s eukaryotic homologs, Trm2 and Pus4. Evidence supporting this possibility includes the observation that the expression of catalytically inactive Trm2 rescues accumulation of tRNASer variants (87), and that the bovine mitochondrial Trm2 homolog lacks a key catalytic residue vital for methylation suggesting tRNA binding (and possibly folding) is more conserved than tRNA modification by Trm2 (88). Additional tRNA modifying enzymes that disturb tRNA tertiary interactions and are likely to act as tRNA chaperones include ArcTGT, which remodels tRNA from its typical L-shaped structure into an alternative structure, termed the “lambda (λ)” form (see tRNA bound to ArcTGT, Fig. 3) in order to access its target base (89). Finally, a catalytically inactive variant of the methyltransferase Trm1 can rescue pre-tRNA maturation in Schizosaccharomyces pombe and functions redundantly with the tRNA chaperone La, suggesting Trm1 may also act as a tRNA chaperone (90, 91, 92).
tRNA modifications affect tRNA cellular stability
In general, tRNAs are thought to be very stable within cells, with half-lives similar to that of ribosomal RNA (2). However, tRNAs are subjected to different quality control mechanisms during their lifecycle, wherein defective (pre-)tRNAs are repaired or degraded. These mechanisms have been best studied in S. cerevisiae, where two main tRNA decay pathways have been identified: nuclear surveillance and rapid tRNA decay (RTD). In tRNA nuclear surveillance, pre-tRNAiMet lacking m1A58 or elongator pre-tRNAs with unprocessed 3ʹ ends are bound by the TRAMP complex. TheTrf4 subunit of this complex subsequently polyadenylates the tRNA 3ʹ end, followed by degradation via 3ʹ-to-5ʹ exonucleolytic cleavage by Rrp6 as part of the nuclear exosome (93). Whereas the nuclear surveillance pathway primarily monitors pre-tRNAs in the nucleus, the RTD pathway instead acts predominantly on mutation-containing or hypomodified cytoplasmic tRNAs with unstable acceptor/T stems using a different machinery to degrade tRNAs in the 5ʹ-to-3ʹ direction. Here, Met22 and the exonucleases Xrn1 and Rat1 work together to degrade tRNAs with acceptor stems that leave the 5ʹ tRNA termini exposed (94).
Although these decay pathways act on hypomodified tRNAs, the exonucleases involved in these processes are hypothesized to recognize their substrates based on an aberrant or unstable tRNA fold, thus explaining why not all hypomodified tRNAs are degraded and why the RTD pathway is most active in yeast grown at high temperatures (95). Interestingly, overexpression of a single specific tRNA is often sufficient to suppress phenotypes associated with RTD. For example, yeast lacking the methyltransferases trm8 and trm4 are slow growing at high temperatures (15). Although both methyltransferases have a wide range of substrate tRNAs in yeast, strikingly, examination of tRNA abundance revealed only tRNAVal(AAC) levels are decreased, and the phenotype associated with this strain can be suppressed by overexpressing this tRNA. This trend has also been demonstrated for other tRNA modification combinations, with other examples listed in Table 3. Thus, it may be that not all tRNAs require all modifications; instead, certain modifications may be more important in specific tRNAs than others (96).
Table 3.
tRNA modifications known to influence the cellular stability of one or more tRNAs
| Modification enzyme (modification) | Affected tRNA(s) | Organism, exacerbating conditions, mechanism (if known) | Ref. |
|---|---|---|---|
| m7G46 (Trm8/Trm82) and m5C48/49 (Trm4) | tRNAVal(AAC) and tRNACys(GCA) to a smaller extent | S. cerevisiae, heat stress (37 °C); RTD | (15) |
| m7G46 (Trm8/Trm82) and D47 (Dus3) | tRNAVal(AAC) and tRNACys(GCA) to a smaller extent | S. cerevisiae, heat stress (37 °C); RTD | (15) |
| m7G46 (Trm8/Trm82) and Ψ13 (Pus7) | tRNAVal(AAC) | S. cerevisiae, heat stress (37 °C); RTD | (15) |
| m7G46 (Trm8/Trm82) | tRNATyr(GUA) and tRNAPro(AGG) to a smaller extent | S. pombe, heat stress (38 °C); RTD | (201) |
| m7G46 (METTL1/WDR4) and m5C48/49 (NSUN2) | tRNAVal(AAC) | H. sapiens (HeLa cell line) exposed to 5-fluorouracil; mechanism unknown | (186) |
| m7G46 (TrmB) | tRNAPhe, tRNAIle | T. thermophilus, heat stress (80 °C) | (181) |
| ac4C12 (Tan1) and Um44 (Trm44) | tRNASer(CGA) and tRNASer(UGA); tRNALeu(GAG) to a smaller extent | S. cerevisiae, particularly at high temperatures; exacerbated when grown in glycerol; RTD | (202) |
| m2,2G26 (Trm1) and m5C48/49 (Trm4) | tRNASer(CGA) and tRNASer(UGA) | S. cerevisiae, particularly upon heat stress; RTD | (203) |
| m2,2G26 (Trm1) | Several Trm1 substrate tRNAs | S. pombe, particularly when sla1 (La protein) is also deleted | (92) |
| m1A58 (Trm6/Trm61) | pre-tRNAiMet | S. cerevisiae (RTD and nuclear surveillance) and S. pombe (RTD), particularly in heat stress | (61, 62, 63) |
| m7G46 (METTL1/WDR4) | Several METTL1 substrate tRNAs | H. sapiens (glioblastoma multiforme cell line LNZ308) | (100) |
| s4U8 (ThiI) | Several ThiI substrate tRNAs | Vibrio cholerae during stationary phase; bacterial RNA degradosome (RNase E) | (97) |
| s4U8 (ThiI) and m5U54 (TrmA) | tRNATyr | V. cholerae during stationary phase; bacterial RNA degradosome (RNase E) | (97) |
| s4U8 (ThiI) and Ψ55 (TruB) | tRNATyr | V. cholerae during stationary phase; bacterial RNA degradosome (RNase E) | (97) |
| m1G9 (Trm10) | tRNATrp | S. cerevisiae, 5-fluorouracil; unknown (mediated by Met22, but Xrn1 and Rat1 nucleases are not involved) | (204) |
| m5C38 (Dnmt2) and m5C34 (NSun2) | tRNAAsp(GTC) and tRNAGly(GCC) | M. musculus embryonic stem cells (MEFs); mechanism unknown | (205) |
In contrast to yeast, the mechanisms of tRNA decay in other organisms including bacteria and mammals are less studied. Work in Vibrio cholerae has demonstrated that several different tRNAs lacking elbow modifications including s4U8, m5U54, and Ψ55 are subject to decay by the RNA degradosome, which is comprised of the helicase RhlB, enolase, the polynucleotide phosphorylase PNPase, and RNase E (97). Furthermore, KO or knockdown of METTL1 and/or WDR4 in different human cells lines results in decreased abundances of many tRNAs that normally contain m7G by an unknown decay mechanism (98, 99, 100). Overexpression of METTL1 conversely increases the cellular abundance of several m7G-containing tRNAs, which has been implicated in driving several types of cancer through altering translation in a codon-biased manner (98, 99, 100). These examples of bacterial and human tRNA degradation appear to affect tRNAs more broadly than the yeast instances described earlier wherein only a small number of specific tRNAs are targeted in the absence of modification enzymes (Table 3).
tRNA modifications during canonical tRNA function: protein translation
The canonical role of tRNAs is to bring amino acids to the ribosome for protein synthesis, which we will briefly discuss here. First, tRNAs are aminoacylated by their cognate aminoacyl-tRNA (aa-tRNA) synthetase (aaRS) to form aa-tRNA. Subsequent steps depend on the identity of the tRNA, but as the majority of tRNAs are elongator tRNAs, we focus here on elongator tRNAs and the specific case of initiator tRNA is addressed in a dedicated section. Bacterial elongation factor Tu (EF-Tu, eEF1A in eukaryotes) in its GTP state binds an aa-tRNA to form the EF-Tu•aa-tRNA•GTP ternary complex, which delivers aa-tRNA to the ribosomal acceptor (A) site. Following peptide bond formation between the peptidyl-tRNA in the peptidyl (P) site and the aa-tRNA, bacterial elongation factor G (EF-G, eEF2 in eukaryotes) catalyzes translocation, wherein the uncharged tRNA moves to the exit (E) site and peptidyl-tRNA moves to the P site. When the ribosome encounters a stop codon (UAG, UGA, or UAA), bacterial release factors 1 or 2 (RF1 or RF2) promote hydrolysis of the peptidyl-tRNA, and release factor 3 help RF1 or RF2 dissociate from the ribosome.
Unsurprisingly, tRNA modifications within the anticodon or adjacent to the anticodon within the tRNA anticodon stem loop have been shown to play a variety of direct and sometimes essential roles during translation, which will be discussed below. However, tRNA modifications within the tRNA elbow can also impact this process. Here, we summarize known roles for tRNA modifications during tRNA aminoacylation, elongation/initiation factor binding, translation initiation, and translation elongation. Presumably, tRNA modifications do not play a role in translation termination as a protein-based process.
tRNA modifications affect tRNA aminoacylation
To maintain the fidelity of protein synthesis, it is paramount that aminoacyl-tRNA synthetases charge only their cognate tRNA and not other similar tRNA species. Although seemingly not a common theme for tRNA aminoacylation, there are cases where tRNA modifications act as determinants or antideterminants for aaRS, which has been recently reviewed in (101). In particular, it is well-known that lysidine (L) 34 determines the aminoacylation and codon identity of tRNAIle2 as an isoleucyl-rather than methionyl-tRNA (102). Moreover, in vitro aminoacylation of unmodified tRNAs often tends to be inefficient (103), suggesting a role for modifications in fine-tuning the affinity of tRNA for its cognate synthetase or enhancing the esterification reaction. Finally, as discussed above, proper tRNA folding is a prerequisite for tRNA charging; thus, the tRNA chaperone activity of certain modification enzymes is likely to increase the proportion of aminoacylated tRNAs in cells (16, 17, 18, 104) which we recently confirmed for E. coli TrmA and TruB (105). Interestingly, the aaRS SerRS displays crosstalk with the tRNA methylating enzyme Trm140 (yeast) and its homolog METTL6 (humans), whereby SerRS immunoprecipitates Trm140/METTL6 in a tRNA-dependent manner, and SerRS presence increases the tRNASer methylase activity of these enzymes both in vitro and in vivo (106, 107, 108, 109), suggesting a codependence of these processes.
In Table 4, we have summarized tRNA modifications that are known to affect the activity of certain aaRS. For the majority of cases, these effects have been determined by comparing the steady-state kinetic parameters for aaRS with native (fully modified), unmodified, or partially modified tRNAs in vitro.
Table 4.
tRNA modifications affecting aminoacylation
| Modification (enzyme) | Affected aaRS | Affected tRNA | Organism | Evidence/mechanism | Ref. |
|---|---|---|---|---|---|
| mnm5s2U34 (MnmE, MnmG; MnmA) | GluRS | tRNAGlu | E. coli | In vitro aminoacylation kinetics | (206, 207, 208, 209, 210) |
| LysRS | tRNALys | ||||
| GlnRS | tRNAGln | ||||
| L34 (aka k2C, TilS) | IleRS | tRNAIleCAU | E. coli | tRNAIleCAU lacking L34 is efficiently aminoacylated by MetRS and not IleRS. L34 modification serves as a positive determinant for IleRS and a negative determinant for MetRS | (102) |
| MetRS | tRNAIleCAU | E. coli | |||
| I34 (Tad2/3) | IleRS | tRNAIleIAU | S. cerevisiae | Introduction of only I34 increases the kcat of aminoacylation 12-fold compared to an unmodified transcript | (103) |
| t6A37 (TsaBDE) | IleRS | tRNAIleGAU | E. coli | Faster aminoacylation (∼25-fold) of tRNAIleGAUin vitro by E. coli IleRS when t6A37 is present | (191) |
| agm2C34 (TiaS) | IleRS | tRNAIleagm2CAU | archaea (Haloarcula marismortui) | Analog of lysidine; similarly is responsible for Ile identity | (211, 212) |
| m1G37 (TrmD) | ProRS | tRNAProCGG | E. coli | 17-fold reduction in catalytic efficiency for tRNAProCGG aminoacylation by ProRS | (213) |
| m1G37 (Trm5) | ArgRS | tRNAAsp | S. cerevisiae | methylation prevents misacylation of tRNAAsp by ArgRS by reducing vmax and increasing KM | (214) |
| Ψ35 (Pus1) | TyrRS | tRNATyr | S. cerevisiae | In vitro aminoacylation kinetics | (215) |
| Ψ34/Ψ36 (Pus1) | IleRS | tRNAIleΨAΨ | S. cerevisiae | 40-fold decrease in catalytic efficiency of transcript compared to native tRNA (additional native tRNA modifications may also be involved) | (103) |
| yW37 (Trm7) | PheRS | tRNAPhe | S. cerevisiae and S. pombe | Decreased cellular aminoacylation for Δtrm7 cells compared to wildtype when grown in minimal media | (72, 216) |
| m1A9 (unknown) | PheRs | mt-tRNAPhe | Ascaris suum | Decreased in vitro aminoacylation for at least these two T-armless tRNAs | (79) |
| MetRS | mt-tRNAMet | ||||
| m2,2G26 (Trm1) | SerRS | tRNASer(CGA/UGA) | S. pomble | ∼25% decrease for in vivo aminoacylation levels | (92) |
| Ψ31 (Pus6) | MetRS | tRNAeMet | S. cerevisiae | Pus6 presence increases amount of tRNAMet immunoprecipitated by MetRS | (217) |
Translation initiation: t6A37 restricts codon reading by initiator tRNA
Unlike EF-Tu/eEF1A, which binds all elongator aa-tRNAs, initiation factor 2 (IF2, bacteria; eIF2, eukaryotes) is specific to binding only initiator tRNAs (fMet-tRNAfMet, bacteria; Met-tRNAiMet, eukaryotes). In vitro transcribed mammalian tRNAiMet has the ability to be aminoacylated and form a complex with eIF2•GTP, but a comparison to the interactions with fully modified tRNAiMet has yet to be conducted (110). Although modifications to tRNAiMet do not appear to affect binding to eIF2, in plants and fungi, presence of a 2′-O-phosphoribosyl modification of the tRNAiMet ribose at position 64 formed by Rit1 acts as a steric block preventing initiator tRNA from binding to eEF1A (111, 112). Absence of this modification allows Met-tRNAiMet to bind eEF1 in vivo (113). In contrast, the sequence of vertebrate tRNAiMet itself acts to prevent elongation factor binding in a modification-independent manner (114). In bacteria, IF2 first binds the ribosome to then recruit fMet-tRNAfMet. To the best of our knowledge, all studies of bacterial translation initiation in vitro have used native fMet-tRNAfMet (115), and no studies examining the role of modifications for bacterial initiation have been conducted.
Mammalian unmodified tRNAiMet can effectively form 43S preinitiation and 48S initiation complexes in vitro, and fully assembled 80S ribosomes produce methionyl-puromycin at similar speeds in vitro with unmodified and modified initiator tRNAs (110). Thus, modifications to tRNAiMet do not seem to be essential for translation initiation; however, at least one tRNA modification works to fine-tune this step. Several lines of in vivo evidence suggest the highly conserved t6A37 modification is important for restricting translation initiation to AUG codons in eukaryotes (116, 117, 118). Interestingly, t6A37 is found in eukaryotic initiator tRNAs, but is noticeably absent in bacterial tRNAfMet, even though this modification is present in nearly all other tRNAs with an ANN anticodon in bacteria (N = any nucleotide). Unlike eukaryotic tRNAiMet, which rarely decodes non-AUG codons, bacterial tRNAfMet effectively decodes GUG and UGG codons, supporting the role of t6A37 of preventing initiation at non-AUG codons in eukaryotes (117).
Several roles for tRNA modifications during translation elongation
Before delivery to the ribosome, elongator tRNAs must form a ternary complex with EF-Tu/eEF1A. Since EF-Tu/eEF1A must bind virtually all aa-tRNAs regardless of sequence, it is unsurprising that mutations in the tRNA sequence, that do not perturb tRNA tertiary structure, do not greatly affect Phe-tRNAPhe affinity for EF-Tu•GTP (119). In a similar manner, Phe-tRNAPhe lacking modifications binds EF-Tu•GTP with only an approximately 1.5-fold lower affinity compared to fully modified Phe-tRNAPhe (120). Likewise, affinities of unmodified Gly-tRNAGly, Val-tRNAVal, Ala-tRNAAla, and Gln-tRNAGln binding EF-Tu are no more than three-fold lower compared to their modified counterparts (121, 122, 123). Presumably, EF-Tu•GTP displays a similar lack of preference between other modified and unmodified aa-tRNA species, and eEF1A probably behaves in a similar manner to EF-Tu. As EF-Tu only contacts the tRNA acceptor stem and T arm (Fig. 3), which do not harbor many modifications, the small increases in affinity observed for elongation factors in binding modified tRNAs can likely be attributed to tRNA modification enzymes fine-tuning the L-shaped tRNA tertiary structure and tRNA dynamics. Interestingly, unusual T-armless tRNAs in nematode mitochondria require m1A9 for effective binding of EF-Tu (79). For the special case of selenocysteine translation at UGA codons, the selenocysteine tRNA-specific elongation factor (SelB) is specific for binding only Sec-tRNASec (124). The affinities of apo SelB for modified and unmodified Sec-tRNASec are almost identical, suggesting SelB does not require modifications for tRNA binding (125).
Once part of a ternary complex, tRNAs bind to the ribosome in a complex, multistep process. In brief, a translating ribosome reversibly samples aa-tRNA•EF-Tu•GTP ternary complexes, forming initial binding complexes. If the ribosome next identifies a cognate codon:anticodon pair, GTPase activation and subsequent GTP hydrolysis by EF-Tu is triggered, followed by the release of inorganic phosphate. Dissociation of EF-Tu may proceed or follow accommodation of aa-tRNA in the ribosomal A site, which is then followed either by proofreading, wherein aa-tRNA may be ejected from the ribosome, or rapid peptide bond formation (126, 127). The next step of elongation is translocation, which prepares the ribosome for another round of elongation (Fig. 4).
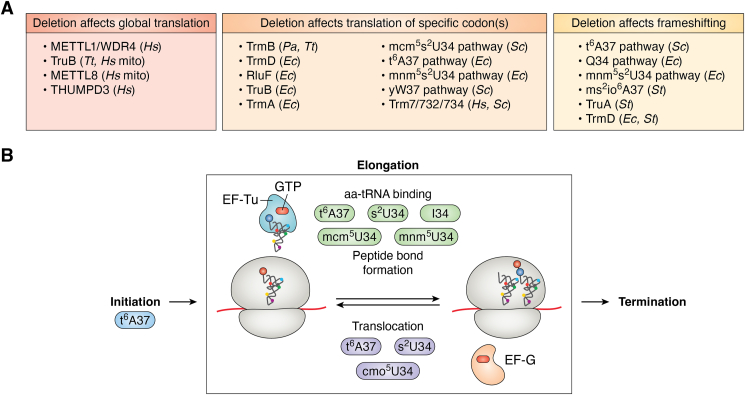
Figure 4.
Summary of the roles of modifications during different steps of translation.A, tRNA modifying enzymes whose absence in vivo affects global translation, specific codon translation, or frameshifting. Model organism used in each study is indicated in parenthesis. Hs: Homo sapiens, Tt: Thermus thermophilus, Pa: Pseudomonas aeruginosa, Ec: Escherichia coli, Sc: Saccharomyces cerevisiae, St: Salmonella typhimurium, mito: mitochondria. B, general schematic of the overall steps of translation. Modifications found to play roles within each step are highlighted within colored boxes.
tRNA modifications have been shown to play a multitude of roles during translation elongation, including aiding tRNA binding to the ribosome, enhancing decoding of mRNA codons, restricting decoding to only cognate codons, preventing proofreading at cognate codons, aiding in translocation, and enhancing or suppressing various forms of ribosomal frameshifting, and several of these processes have been reviewed extensively elsewhere (9, 10, 128). In the paragraphs below, we will detail general roles for tRNA modifications in translation and the approaches used to study these. Roles for several specific modifications are summarized in Table 5.
Table 5.
Examples of tRNA modifications influencing translation elongation
| Modification (enzymes) | Codons and/or tRNAs affected; model organism | Experiments | Ref. |
|---|---|---|---|
| mcm5s2U34 (ELP and URM1 pathway) | AAA, GAA, and CAA; S. cerevisiae | Proteome analysis, tandem-codon translation reporter assays, in vitro translation assays | (218) |
| s2U moiety of mcm5s2U34 (URM1 pathway) | tRNALysUUU reading AAA codon; S. cerevisiae | In vitro translation kinetics | (219) |
| mcm5(s2)U34 (Trm9) | AGA, AGG, and GAA codons; S. cerevisiae | Tandem-codon translation reporter assays, proteomics | (134, 220) |
| Ψ35 (RluF) | tRNATyrGUA; E. coli | Tandem-codon translation reporter assays | (221) |
| m7G46 (TrmB) | tRNAPheGAA reading UUU and UUC, tRNAAspGUC reading GAC and GAU codons; P. aeruginosa | Tandem-codon translation reporter assays | (178) |
| m7G46 (METTL1) | Global translation in human HuCCT1 and RBE cell lines, particularly for codons read by m7G modified tRNAs | Polysome profiling, ribosome profiling, and pulse-labeling | (98) |
| mnm5U34 (MnmE/G), m1G37 (TrmD), t6A37 (TsaBCDE) | E. coli tRNAGluUUC, tRNAProUUC; both tRNAIleGAU; and tRNAAsnGUU, respectively | In vitro translation (PURExpress system) | (222) |
| Cm32 and Gm34 (Trm7) | Human, mouse and yeast global translation, Phe UUU and UUC codons particularly affected | Polysome profiling, tRNA overexpression, ribosome profiling | (195, 197, 223) |
| yW37 (Tyw2-4) | tRNAPheGAA reading UUU | In vitro translation kinetics | (141) |
| t6A37 and mnm5U34 (TdcBCDE, MnmEG) | tRNALys anticodon stem loop; E. coli | In vitro ribosomal A site binding and translocation | (224) |
| cmo5U34 (CmoA, CmoB) | tRNAVal1 anticodon stem loop reading GUU; E. coli | In vitro ribosomal A site binding and translocation | (224) |
| Ψ55 (TRUB1) | Global mitochondrial translation, H. sapiens (HeLa cell line) | Comparison of specific protein abundance by Western blot | (225) |
| m7G (TrmB) | Global translation, T. thermophilus under heat stress | 35S-pulse labeling | (181) |
| Ψ55 (TruB) | Global translation, T. thermophilus under cold stress | 35S-pulse labeling | (175) |
| m5C34, 48 (Trm4) | TTG codons; S. cerevisiae | Tandem-codon translation reporter assays, proteomics | (226) |
| m3C32 (METTL8) | Global translation, H. sapiens HCT116 cell line; mitochondrial translation, particularly at codons read by tRNATyr and tRNASer, HAP1 human cells | Polysome profiling shows subtle affects; ribosome profiling displays stalls at A and/or P site | (107) |
| m3C32 (METTL6) | Translation efficiency of several genes, mouse ESCs | Ribosome profiling | (227, 228) |
| m5C38 (Dnmt2) and m5C34 (NSun2) | Global translation in MEFs and mouse embryos | 35S-pulse labeling and polysome profiling | (205) |
| m2G6 (THUMPD3) | Global translation in human HEK293T cells | Polysome profiling | (229) |
| Q34 (TGT) | Global translation, especially at Q34-decoded codons; HeLa cells | Polysome profiling, ribosome profiling | (142) |
| m1G37 (TrmD) | Prevents tRNAPro decoding CC(C/U)-C/U slippery sequences from frameshifting; additionally prevents ribosomal stalling at select codons; E. coli | Structural studies, reporter assays, in vitro ribosome binding and translation studies, ribosome profiling | (230, 231, 232, 233, 234) |
| I34 (ADAT2/3) | Altered translation for a subset of mRNAs | Ribosome profiling, polysome profiling | (235, 236) |
Of course, all maturation processes affected by tRNA modifications can influence translation (e.g. modifications that lead to lower concentrations of cellular tRNA or decreased aminoacylation levels), but modifications themselves additionally play roles in protein synthesis. Traditional in vivo experiments to examine the role of tRNA modifications on translation generally compare a tRNA modifying enzyme KO to its corresponding WT strain, (e.g. pulse-labeling, translation reporter experiments, and examination of single protein expression by Western blotting) and are often unable to distinguish between a direct role of a modification in translation and the effects of the modification for a previous step of tRNA maturation. In contrast, in vitro assays using purified ribosomes and translation components and differently modified tRNA can often demonstrate direct roles for tRNA modifications in translation and narrow down the affected step in translation. As many tRNA modification enzymes modify only select tRNAs and the codon content of mRNAs often plays a role in fine-tuning translation efficiency, it is important to examine how codons are affected differently by the loss of a tRNA modification enzyme. High-throughput experiments to examine translation such as ribosome profiling (129, 130) can distinguish mRNA codons (and thus their corresponding tRNAs) that are most affected by modification enzyme deletion and provide clues into how translation is altered by examining whether ribosome pausing occurs when the codon is in the A site or P site. For example, ribosome profiling has been systematically accomplished for 57 yeast deletion strains lacking genes involved in tRNA modification (131). Similarly, ribosome-bound tRNA capture (132) can be used to identify which tRNAs are bound to ribosomes, whether they are in the A or P site, which modifications ribosome-bound tRNAs contain, and how this compares to the overall cellular tRNA population. Further, by comparing the proteome between a tRNA modifying enzyme KO and its corresponding WT, codon-bias can be examined for upregulated and downregulated proteins to identify codons that are potentially affected by tRNA modification (105, 133, 134).
Since non-Watson-Crick base-pairs are tolerated at the “wobble” position between the third nucleotide of a codon and the first nucleotide (N34) of a tRNA anticodon, modifications at the wobble position in tRNAs are frequently found to restrict, enhance, or rarely, change decoding. In particular, uridines at position 34 are modified at a high frequency and have been extensively studied and reviewed in (10, 135, 136). Likewise, the deamination of adenosine forming the edited based inosine (I) is common at the wobble position. With the ability to base pair with A, C, and U bases, I34 expands base pairing capacity and is essential for the decoding of certain codons in bacteria and eukaryotes (137, 138, 139, 140). Modifications adjacent to the anticodon, especially at the conserved purine 37, often play roles in stabilizing the codon:anticodon base-pairing interaction by enhancing base-stacking (141). By optimizing the speed of translation, anticodon arm modifications can contribute to protein folding, leading to accumulation of aggregated proteins in the absence modifications including Q34, mcm5U34, and t6A37 (10, 117, 133, 142). Although the impact of many anticodon stem-loop modifications on translation have been studied, it remains unknown whether tRNA modifying enzymes that target nucleotides outside of the tRNA anticodon (i.e. in the tRNA elbow) directly contribute to translation. By enhancing the structure, folding, and dynamics of the tRNA elbow, these modifications may promote tRNA movement through the ribosome. Supporting this notion, several tRNA elbow modifications have been indirectly implicated in protein synthesis (Table 5).
Alternative roles of tRNAs and tRNA modifying enzymes
tRNAs across all domains of life participate in roles outside of protein synthesis. Noncanonical roles for aminoacylated tRNAs include the delivery of amino acids for biosynthesis of amino acids (143), antibiotics (144), tetrapyrroles (145), and various components of the cell wall (146). Additionally, aa-tRNAs deliver destabilizing amino acids for N-terminal proteolytic tagging by the N-end rule pathway to regulate the half-life of proteins in cells (147). Uncharged tRNAs regulate gene expression by acting as nutrient sensors, for example in the bacterial stringent response and in amino acid starvation conditions in eukaryotes (148). Moreover, tRNAs inhibit intrinsic apoptosis by binding cytochrome c (149). To the best of our knowledge, tRNA modifications have not been shown to play direct roles in the processes described above to date. Conversely, roles for tRNA modifications in virus biology have been defined. For example, retroviruses, including HIV, utilize a tRNA containing the m1A58 modification formed by TRMT6/TRMT61 as a primer for viral reverse transcription (150). Using HIV-1 as a model, it has been shown m1A58 is necessary to generate the so-called “plus-strand strong-stop” (151) during reverse transcription, which is required for proper plus strand synthesis and viral genome integration (66, 152, 153, 154, 155). Further supporting the role of m1A58 by TRMT6/TRMT61 in HIV replication, TRMT6 is required for efficient accumulation of viral proteins (66). Additional tRNA modifications have been implicated in viral pathogenesis by altering translation in the host (156, 157, 158, 159).
In addition to noncanonical functions for full-length mature aminoacylated and nonaminoacylated tRNAs described above, the specific fragmentation of tRNAs gives rise to tRNA-derived small RNAs (tDRs, also known by other names including tRNA fragments) (160, 161). tDRs are formed by several ribonucleases including angiogenin, and are often upregulated in cellular stress conditions (162). An assortment of tDRs of different types and lengths play a variety of functions in each domain of life in various cellular processes including ribosome biogenesis and gene silencing by binding to complementary nucleic acids and to various proteins (163, 164, 165). Several tRNA modification enzymes have been shown to regulate the biogenesis of tDRs; these cases have been extensively reviewed in (161, 162) and will only be briefly summarized here. In most or all cases identified to date, the presence of a tRNA modification and its corresponding enzyme limits tDR production (162), suggesting tRNA modifying enzymes impart a protective effect against nucleases. For example, loss of m19A by TRMT10A increases formation of 5ʹ-tRNAGln fragments in TRMT10A-deficient patient lymphoblasts (166) and 2′O-methylation of C34 by the methyltransferase fibrillarin as part of a C/D box small nucleolar ribonucleoprotein complex prevents tRNAMet(CAT) cleavage by angiogenin in HAP1 cells (167). The mechanism(s) by which tRNA modifying enzymes control tDR biosynthesis remain unknown but could either be due to binding of the tRNA modifying enzyme regulating the accessibility of tRNA for nucleases or by tRNA modification or folding status acting as determinants for tDR-forming nucleases.
Finally, some tRNA modifying enzymes may have moonlighting activities in other cellular functions. Most interestingly, at least two tRNA modifying enzymes, Mod5 and Pus4, can exist in a prion form in yeast as [MOD5+] and [BIG+] (Better In Growth), respectively (168, 169). [MOD5+] cells have decreased i6A37 modification compared to nonprion state WT cells, but [BIG+] cells seem to have similar Ψ55 levels as their counterparts although an increase in translation elongation factor TEF1/TEF2 mRNA pseudouridylation is observed (168, 169). Interestingly, aggregation of Mod5 protein in vitro is stimulated by tRNA binding (170). The Mod5 human homolog TRIT1 additionally forms amyloid fibers in vitro and in vivo (171). The implications on tRNA modifying enzyme prion formation and tRNA modification has yet to be described.
Conclusions and perspectives
In summary, we have described how tRNA modifying enzymes affect tRNAs at all stages of the tRNA lifecycle by modifying and folding tRNAs. Together, the research summarized here suggests that deletion of a single tRNA modification enzyme often affects more than the loss of the given modification; instead, the entire tRNA modification status can be altered and the tRNA folding and structural dynamics may be affected. Some hypomodified and potentially misfolded tRNA may be targeted for degradation, affecting cellular tRNA abundance. Alternatively, hypomodified tRNA may be improperly recognized by its interacting factors, which may alter tRNA processing, aminoacylation, translation and/or other noncanonical tRNA functions.
Therefore, comprehensive awareness of the molecular functions of tRNA modifying enzymes is vital to further our understanding of the roles of tRNA modifying enzymes in a variety of organisms, in genetic diseases and cancer (6). Knowing the underlying mechanisms of diseases and whether they are a result of changes in tRNA levels, aminoacylation or other tRNA functions provides clues to developing targeted therapies, e.g. by aiming to increase tRNA concentrations or the concentrations of factors interacting with tRNAs. Furthermore, knowledge of the consequences of including or excluding modifications in tRNA is crucial for potential therapeutic development and optimization of tRNA molecules as future medicines for diseases (172, 173). We anticipate that the engineering of tRNA modifications will play a critical role in designing efficient suppressor tRNAs for read-through of premature stop codons or in creating tRNAs delivering noncanonical amino acids to the ribosome. In brief, our understanding of the complex functions of tRNA modifying enzymes for the tRNA lifecycle has sufficiently advanced to enable exciting new opportunities in developing new therapeutics and biotechnological applications.
Conflict of interest
The authors declare that they have no conflicts of interest with the contents of this article.
Acknowledgments
Author contributions
S. K. S. and U. K. conceptualization; S. K. S. and U. K. writing–review and editing; S. K. S. data curation; S. K. S. project administration; S. K. S. visualization; S. K. S. writing–original draft; U. K. supervision; U. K. funding acquisition.
Funding and additional information
A version of this review was included as an introductory chapter in the PhD thesis of S. K. S. This work was supported by the Natural Sciences and Engineering Research Council of Canada [U. K.: Discovery Grant RGPIN-2020-04965 and Discovery Accelerator Supplement RGPAS-202-00010].
Reviewed by members of the JBC Editorial Board. Edited by Karin Musier-Forsyth
Contributor Information
Sarah K. Schultz, Email: sarah.schultz@umanitoba.ca.
Ute Kothe, Email: ute.kothe@umanitoba.ca.
References
- 1.Lorenz C., Lünse C.E., Mörl M. tRNA modifications: impact on structure and thermal adaptation. Biomolecules. 2017;7:35. doi: 10.3390/biom7020035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Phizicky E.M., Hopper A.K. The life and times of a tRNA. RNA. 2023;29:898–957. doi: 10.1261/rna.079620.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Crécy-Lagard V., Jaroch M. Functions of bacterial tRNA modifications: from ubiquity to diversity. Trends Microbiol. 2021;29:41–53. doi: 10.1016/j.tim.2020.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grosjean H. Springer Berlin Heidelberg; Berlin, Heidelberg: 2005. Modification and Editing of RNA: Historical Overview and Important Facts to Remember in Fine-tuning of RNA Functions by Modification and Editing. [Google Scholar]
- 5.Torres A.G., Batlle E., Ribas de Pouplana L. Role of tRNA modifications in human diseases. Trends Mol. Med. 2014;20:306–314. doi: 10.1016/j.molmed.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 6.Suzuki T. The expanding world of tRNA modifications and their disease relevance. Nat. Rev. Mol. Cell Biol. 2021;22:375–392. doi: 10.1038/s41580-021-00342-0. [DOI] [PubMed] [Google Scholar]
- 7.Pereira M., Francisco S., Varanda A.S., Santos M., Santos M.A.S., Soares A.R. Impact of tRNA modifications and tRNA-modifying enzymes on proteostasis and human disease. Int. J. Mol. Sci. 2018;19:3738. doi: 10.3390/ijms19123738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boccaletto P., Stefaniak F., Ray A., Cappannini A., Mukherjee S., Purta E., et al. MODOMICS: a database of RNA modification pathways. 2021 update. Nucleic Acids Res. 2022;50:D231–D235. doi: 10.1093/nar/gkab1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith T.J., Giles R.N., Koutmou K.S. Anticodon stem-loop tRNA modifications influence codon decoding and frame maintenance during translation. Semin. Cell Dev. Biol. 2023;154:105–113. doi: 10.1016/j.semcdb.2023.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ranjan N., Rodnina M.V. tRNA wobble modifications and protein homeostasis. Translation (Austin) 2016;4 doi: 10.1080/21690731.2016.1143076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Crécy-Lagard V., Ross R.L., Jaroch M., Marchand V., Eisenhart C., Brégeon D., et al. Survey and validation of tRNA modifications and their corresponding genes in Bacillus subtilis sp subtilis strain 168. Biomolecules. 2020;10:977. doi: 10.3390/biom10070977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takakura M., Ishiguro K., Akichika S., Miyauchi K., Suzuki T. Biogenesis and functions of aminocarboxypropyluridine in tRNA. Nat. Commun. 2019;10:5542. doi: 10.1038/s41467-019-13525-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meyer B., Immer C., Kaiser S., Sharma S., Yang J., Watzinger P., et al. Identification of the 3-amino-3-carboxypropyl (acp) transferase enzyme responsible for acp3U formation at position 47 in Escherichia coli tRNAs. Nucleic Acids Res. 2020;48:1435–1450. doi: 10.1093/nar/gkz1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Phizicky E.M., Hopper A.K. tRNA biology charges to the front. Genes Dev. 2010;24:1832–1860. doi: 10.1101/gad.1956510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alexandrov A., Chernyakov I., Gu W., Hiley S.L., Hughes T.R., Grayhack E.J., et al. Rapid tRNA decay can result from lack of nonessential modifications. Mol. Cell. 2006;21:87–96. doi: 10.1016/j.molcel.2005.10.036. [DOI] [PubMed] [Google Scholar]
- 16.Keffer-Wilkes L.C., Soon E.F., Kothe U. The methyltransferase TrmA facilitates tRNA folding through interaction with its RNA-binding domain. Nucleic Acids Res. 2020;48:7981–7990. doi: 10.1093/nar/gkaa548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keffer-Wilkes L.C., Veerareddygari G.R., Kothe U. RNA modification enzyme TruB is a tRNA chaperone. Proc. Natl. Acad. Sci. U. S. A. 2016;113:14306–14311. doi: 10.1073/pnas.1607512113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Porat J., Kothe U., Bayfield M.A. Revisiting tRNA chaperones: new players in an ancient game. RNA. 2021;27:543–559. doi: 10.1261/rna.078428.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thompson M., Haeusler R.A., Good P.D., Engelke D.R. Nucleolar clustering of dispersed tRNA genes. Science. 2003;302:1399–1401. doi: 10.1126/science.1089814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chatterjee K., Nostramo R.T., Wan Y., Hopper A.K. tRNA dynamics between the nucleus, cytoplasm and mitochondrial surface: location, location, location. Biochim. Biophys. Acta Gene Regul. Mech. 2018;1861:373–386. doi: 10.1016/j.bbagrm.2017.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakanishi K., Nureki O. Recent progress of structural biology of tRNA processing and modification. Mol. Cells. 2005;19:157–166. [PubMed] [Google Scholar]
- 22.Mohanty B.K., Kushner S.R. Enzymes involved in posttranscriptional RNA metabolism in gram-negative bacteria. Microbiol. Spectr. 2018;6 doi: 10.1128/microbiolspec.RWR-0011-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kufel J., Tollervey D. 3′-processing of yeast tRNATrp precedes 5′-processing. RNA. 2003;9:202–208. doi: 10.1261/rna.2145103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mörl M., Marchfelder A. The final cut. The importance of tRNA 3′-processing. EMBO Rep. 2001;2:17–20. doi: 10.1093/embo-reports/kve006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hartmann R.K., Gössringer M., Späth B., Fischer S., Marchfelder A. The making of tRNAs and more - RNase P and tRNase Z. Prog. Mol. Biol. Transl. Sci. 2009;85:319–368. doi: 10.1016/S0079-6603(08)00808-8. [DOI] [PubMed] [Google Scholar]
- 26.Xiong Y., Steitz T.A. A story with a good ending: tRNA 3′-end maturation by CCA-adding enzymes. Curr. Opin. Struct. Biol. 2006;16:12–17. doi: 10.1016/j.sbi.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 27.O'Connor J.P., Peebles C.L. In vivo pre-tRNA processing in Saccharomyces cerevisiae. Mol. Cell Biol. 1991;11:425–439. doi: 10.1128/mcb.11.1.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li Z., Deutscher M.P. Maturation pathways for E. coli tRNA precursors: a random multienzyme process in vivo. Cell. 1996;86:503–512. doi: 10.1016/s0092-8674(00)80123-3. [DOI] [PubMed] [Google Scholar]
- 29.Lopes R.R., Kessler A.C., Polycarpo C., Alfonzo J.D. Cutting, dicing, healing and sealing: the molecular surgery of tRNA. Wiley Interdiscip Rev. RNA. 2015;6:337–349. doi: 10.1002/wrna.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schmidt C.A., Matera A.G. tRNA introns: presence, processing, and purpose. Wiley Interdiscip Rev. RNA. 2020;11 doi: 10.1002/wrna.1583. [DOI] [PubMed] [Google Scholar]
- 31.Gerber J.L., Köhler S., Peschek J. Eukaryotic tRNA splicing - one goal, two strategies, many players. Biol. Chem. 2022;403:765–778. doi: 10.1515/hsz-2021-0402. [DOI] [PubMed] [Google Scholar]
- 32.Nishikura K., De Robertis E.M. RNA processing in microinjected Xenopus oocytes. Sequential addition of base modifications in the spliced transfer RNA. J. Mol. Biol. 1981;145:405–420. doi: 10.1016/0022-2836(81)90212-6. [DOI] [PubMed] [Google Scholar]
- 33.Paushkin S.V., Patel M., Furia B.S., Peltz S.W., Trotta C.R. Identification of a human endonuclease complex reveals a link between tRNA splicing and pre-mRNA 3′ end formation. Cell. 2004;117:311–321. doi: 10.1016/s0092-8674(04)00342-3. [DOI] [PubMed] [Google Scholar]
- 34.Akiyama Y., Lyons S.M., Abe T., Anderson P.J., Ivanov P. Cytoplasmic processing of human transfer RNAs. bioRxiv. 2022 doi: 10.1101/2022.04.28.489951. [preprint] [DOI] [Google Scholar]
- 35.Yoshihisa T., Yunoki-Esaki K., Ohshima C., Tanaka N., Endo T. Possibility of cytoplasmic pre-tRNA splicing: the yeast tRNA splicing endonuclease mainly localizes on the mitochondria. Mol. Biol. Cell. 2003;14:3266–3279. doi: 10.1091/mbc.E02-11-0757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reinhold-Hurek B., Shub D.A. Self-splicing introns in tRNA genes of widely divergent bacteria. Nature. 1992;357:173–176. doi: 10.1038/357173a0. [DOI] [PubMed] [Google Scholar]
- 37.Hopper A.K. Transfer RNA post-transcriptional processing, turnover, and subcellular dynamics in the yeast Saccharomyces cerevisiae. Genetics. 2013;194:43–67. doi: 10.1534/genetics.112.147470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grosjean H., Droogmans L., Giégé R., Uhlenbeck O.C. Guanosine modifications in runoff transcripts of synthetic transfer RNA-Phe genes microinjected into Xenopus oocytes. Biochim. Biophys. Acta. 1990;1050:267–273. doi: 10.1016/0167-4781(90)90179-6. [DOI] [PubMed] [Google Scholar]
- 39.Koski R.A., Clarkson S.G. Synthesis and maturation of Xenopus laevis methionine tRNA gene transcripts in homologous cell-free extracts. J. Biol. Chem. 1982;257:4514–4521. [PubMed] [Google Scholar]
- 40.Arimbasseri A.G., Blewett N.H., Iben J.R., Lamichhane T.N., Cherkasova V., Hafner M., et al. RNA polymerase III output is functionally linked to tRNA dimethyl-G26 modification. PLoS Genet. 2015;11 doi: 10.1371/journal.pgen.1005671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Holzmann J., Frank P., Löffler E., Bennett K.L., Gerner C., Rossmanith W. RNase P without RNA: identification and functional reconstitution of the human mitochondrial tRNA processing enzyme. Cell. 2008;135:462–474. doi: 10.1016/j.cell.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 42.Bhatta A., Hillen H.S. Structural and mechanistic basis of RNA processing by protein-only ribonuclease P enzymes. Trends Biochem. Sci. 2022;47:965–977. doi: 10.1016/j.tibs.2022.05.006. [DOI] [PubMed] [Google Scholar]
- 43.Bhatta A., Dienemann C., Cramer P., Hillen H.S. Structural basis of RNA processing by human mitochondrial RNase P. Nat. Struct. Mol. Biol. 2021;28:713–723. doi: 10.1038/s41594-021-00637-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vilardo E., Toth U., Hazisllari E., Hartmann R.K., Rossmanith W. Cleavage kinetics of human mitochondrial RNase P and contribution of its non-nuclease subunits. Nucleic Acids Res. 2023;51:10536–10550. doi: 10.1093/nar/gkad713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Karasik A., Fierke C.A., Koutmos M. Interplay between substrate recognition, 5′ end tRNA processing and methylation activity of human mitochondrial RNase P. RNA. 2019;25:1646–1660. doi: 10.1261/rna.069310.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vilardo E., Nachbagauer C., Buzet A., Taschner A., Holzmann J., Rossmanith W. A subcomplex of human mitochondrial RNase P is a bifunctional methyltransferase--extensive moonlighting in mitochondrial tRNA biogenesis. Nucleic Acids Res. 2012;40:11583–11593. doi: 10.1093/nar/gks910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reinhard L., Sridhara S., Hällberg B.M. The MRPP1/MRPP2 complex is a tRNA-maturation platform in human mitochondria. Nucleic Acids Res. 2017;45:12469–12480. doi: 10.1093/nar/gkx902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Arrivé M., Bruggeman M., Skaltsogiannis V., Coudray L., Quan Y.F., Schelcher C., et al. A tRNA-modifying enzyme facilitates RNase P activity in Arabidopsis nuclei. Nat. Plants. 2023;9:2031–2041. doi: 10.1038/s41477-023-01564-0. [DOI] [PubMed] [Google Scholar]
- 49.Grosjean H., Szweykowska-Kulinska Z., Motorin Y., Fasiolo F., Simos G. Intron-dependent enzymatic formation of modified nucleosides in eukaryotic tRNAs: a review. Biochimie. 1997;79:293–302. doi: 10.1016/s0300-9084(97)83517-1. [DOI] [PubMed] [Google Scholar]
- 50.Johnson P.F., Abelson J. The yeast tRNATyr gene intron is essential for correct modification of its tRNA product. Nature. 1983;302:681–687. doi: 10.1038/302681a0. [DOI] [PubMed] [Google Scholar]
- 51.Choffat Y., Suter B., Behra R., Kubli E. Pseudouridine modification in the tRNA(Tyr) anticodon is dependent on the presence, but independent of the size and sequence, of the intron in eucaryotic tRNA(Tyr) genes. Mol. Cell Biol. 1988;8:3332–3337. doi: 10.1128/mcb.8.8.3332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van Tol H., Beier H. All human tRNATyr genes contain introns as a prerequisite for pseudouridine biosynthesis in the anticodon. Nucleic Acids Res. 1988;16:1951–1966. doi: 10.1093/nar/16.5.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jiang H.Q., Motorin Y., Jin Y.X., Grosjean H. Pleiotropic effects of intron removal on base modification pattern of yeast tRNAPhe: an in vitro study. Nucleic Acids Res. 1997;25:2694–2701. doi: 10.1093/nar/25.14.2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Han L., Phizicky E.M. A rationale for tRNA modification circuits in the anticodon loop. RNA. 2018;24:1277–1284. doi: 10.1261/rna.067736.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Barraud P., Tisné C. To be or not to be modified: miscellaneous aspects influencing nucleotide modifications in tRNAs. IUBMB Life. 2019;71:1126–1140. doi: 10.1002/iub.2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li J., Zhu W.Y., Yang W.Q., Li C.T., Liu R.J. The occurrence order and cross-talk of different tRNA modifications. Sci. China Life Sci. 2021;64:1423–1436. doi: 10.1007/s11427-020-1906-4. [DOI] [PubMed] [Google Scholar]
- 57.Sokołowski M., Klassen R., Bruch A., Schaffrath R., Glatt S. Cooperativity between different tRNA modifications and their modification pathways. Biochim. Biophys. Acta Gene Regul. Mech. 2018;1861:409–418. doi: 10.1016/j.bbagrm.2017.12.003. [DOI] [PubMed] [Google Scholar]
- 58.Hori H. Regulatory factors for tRNA modifications in extreme- thermophilic bacterium Thermus thermophilus. Front. Genet. 2019;10:204. doi: 10.3389/fgene.2019.00204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Motorin Y., Marchand V. Analysis of RNA modifications by second- and third-generation deep sequencing: 2020 update. Genes (Basel) 2021;12:278. doi: 10.3390/genes12020278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Barraud P., Gato A., Heiss M., Catala M., Kellner S., Tisne C. Time-resolved NMR monitoring of tRNA maturation. Nat. Commun. 2019;10:3373. doi: 10.1038/s41467-019-11356-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Anderson J., Phan L., Cuesta R., Carlson B.A., Pak M., Asano K., et al. The essential Gcd10p-Gcd14p nuclear complex is required for 1-methyladenosine modification and maturation of initiator methionyl-tRNA. Genes Dev. 1998;12:3650–3662. doi: 10.1101/gad.12.23.3650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tasak M., Phizicky E.M. Initiator tRNA lacking 1-methyladenosine is targeted by the rapid tRNA decay pathway in evolutionarily distant yeast species. PLoS Genet. 2022;18 doi: 10.1371/journal.pgen.1010215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kadaba S., Krueger A., Trice T., Krecic A.M., Hinnebusch A.G., Anderson J. Nuclear surveillance and degradation of hypomodified initiator tRNAMet in S. Cerevisiae. Genes Dev. 2004;18:1227–1240. doi: 10.1101/gad.1183804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yared M.J., Yoluç Y., Catala M., Tisné C., Kaiser S., Barraud P. Different modification pathways for m1A58 incorporation in yeast elongator and initiator tRNAs. Nucleic Acids Res. 2023 doi: 10.1093/nar/gkad722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Heiss M., Hagelskamp F., Marchand V., Motorin Y., Kellner S. Cell culture NAIL-MS allows insight into human tRNA and rRNA modification dynamics in vivo. Nat. Commun. 2021;12:389. doi: 10.1038/s41467-020-20576-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fukuda H., Chujo T., Wei F.Y., Shi S.L., Hirayama M., Kaitsuka T., et al. Cooperative methylation of human tRNA3Lys at positions A58 and U54 drives the early and late steps of HIV-1 replication. Nucleic Acids Res. 2021;49:11855–11867. doi: 10.1093/nar/gkab879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lucas M.C., Pryszcz L.P., Medina R., Milenkovic I., Camacho N., Marchand V., et al. Quantitative analysis of tRNA abundance and modifications by nanopore RNA sequencing. Nat. Biotechnol. 2023;42:72–86. doi: 10.1038/s41587-023-01743-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Thomas N.K., Poodari V.C., Jain M., Olsen H.E., Akeson M., Abu-Shumays R.L. Direct nanopore sequencing of individual full length tRNA strands. ACS Nano. 2021;15:16642–16653. doi: 10.1021/acsnano.1c06488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bailey A.D., Talkish J., Ding H., Igel H., Duran A., Mantripragada S., et al. Concerted modification of nucleotides at functional centers of the ribosome revealed by single-molecule RNA modification profiling. Elife. 2022;11 doi: 10.7554/eLife.76562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cozen A.E., Quartley E., Holmes A.D., Hrabeta-Robinson E., Phizicky E.M., Lowe T.M. ARM-seq: AlkB-facilitated RNA methylation sequencing reveals a complex landscape of modified tRNA fragments. Nat. Methods. 2015;12:879–884. doi: 10.1038/nmeth.3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zheng G., Qin Y., Clark W.C., Dai Q., Yi C., He C., et al. Efficient and quantitative high-throughput tRNA sequencing. Nat. Methods. 2015;12:835–837. doi: 10.1038/nmeth.3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hernandez-Alias X., Katanski C.D., Zhang W., Assari M., Watkins C.P., Schaefer M.H., et al. Single-read tRNA-seq analysis reveals coordination of tRNA modification and aminoacylation and fragmentation. Nucleic Acids Res. 2023;51 doi: 10.1093/nar/gkac1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Masuda I., Takase R., Matsubara R., Paulines M.J., Gamper H., Limbach P.A., et al. Selective terminal methylation of a tRNA wobble base. Nucleic Acids Res. 2018;46 doi: 10.1093/nar/gky013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schultz S.K., Kothe U. tRNA elbow modifications affect the tRNA pseudouridine synthase TruB and the methyltransferase TrmA. RNA. 2020;26:1131–1142. doi: 10.1261/rna.075473.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nomura Y., Ohno S., Nishikawa K., Yokogawa T. Correlation between the stability of tRNA tertiary structure and the catalytic efficiency of a tRNA-modifying enzyme, archaeal tRNA-guanine transglycosylase. Genes Cells. 2016;21:41–52. doi: 10.1111/gtc.12317. [DOI] [PubMed] [Google Scholar]
- 76.Suzuki T., Nagao A., Suzuki T. Human mitochondrial tRNAs: biogenesis, function, structural aspects, and diseases. Annu. Rev. Genet. 2011;45:299–329. doi: 10.1146/annurev-genet-110410-132531. [DOI] [PubMed] [Google Scholar]
- 77.Helm M., Giegé R., Florentz C. A Watson-Crick base-pair-disrupting methyl group (m1A9) is sufficient for cloverleaf folding of human mitochondrial tRNALys. Biochemistry. 1999;38:13338–13346. doi: 10.1021/bi991061g. [DOI] [PubMed] [Google Scholar]
- 78.Messmer M., Pütz J., Suzuki T., Suzuki T., Sauter C., Sissler M., et al. Tertiary network in mammalian mitochondrial tRNAAsp revealed by solution probing and phylogeny. Nucleic Acids Res. 2009;37:6881–6895. doi: 10.1093/nar/gkp697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sakurai M., Ohtsuki T., Watanabe K. Modification at position 9 with 1-methyladenosine is crucial for structure and function of nematode mitochondrial tRNAs lacking the entire T-arm. Nucleic Acids Res. 2005;33:1653–1661. doi: 10.1093/nar/gki309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Urbonavicius J., Armengaud J., Grosjean H. Identity elements required for enzymatic formation of N2,N2-dimethylguanosine from N2-monomethylated derivative and its possible role in avoiding alternative conformations in archaeal tRNA. J. Mol. Biol. 2006;357:387–399. doi: 10.1016/j.jmb.2005.12.087. [DOI] [PubMed] [Google Scholar]
- 81.Steinberg S., Cedergren R. A correlation between N2-dimethylguanosine presence and alternate tRNA conformers. Rna. 1995;1:886–891. [PMC free article] [PubMed] [Google Scholar]
- 82.Byrne R.T., Konevega A.L., Rodnina M.V., Antson A.A. The crystal structure of unmodified tRNAPhe from Escherichia coli. Nucleic Acids Res. 2010;38:4154–4162. doi: 10.1093/nar/gkq133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Motorin Y., Helm M. tRNA stabilization by modified nucleotides. Biochemistry. 2010;49:4934–4944. doi: 10.1021/bi100408z. [DOI] [PubMed] [Google Scholar]
- 84.Davis D.R. Stabilization of RNA stacking by pseudouridine. Nucleic Acids Res. 1995;23:5020–5026. doi: 10.1093/nar/23.24.5020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Seelam Prabhakar P., Takyi N.A., Wetmore S.D. Posttranscriptional modifications at the 37th position in the anticodon stem-loop of tRNA: structural insights from MD simulations. RNA. 2021;27:202–220. doi: 10.1261/rna.078097.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gutgsell N., Englund N., Niu L., Kaya Y., Lane B.G., Ofengand J. Deletion of the Escherichia coli pseudouridine synthase gene truB blocks formation of pseudouridine 55 in tRNA in vivo, does not affect exponential growth, but confers a strong selective disadvantage in competition with wild-type cells. RNA. 2000;6:1870–1881. doi: 10.1017/s1355838200001588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Johansson M.J., Byström A.S. Dual function of the tRNA(m(5)U54)methyltransferase in tRNA maturation. RNA. 2002;8:324–335. doi: 10.1017/s1355838202027851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Powell C.A., Minczuk M. TRMT2B is responsible for both tRNA and rRNA m(5)U-methylation in human mitochondria. RNA Biol. 2020;17:451–462. doi: 10.1080/15476286.2020.1712544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ishitani R., Nureki O., Fukai S., Kijimoto T., Nameki N., Watanabe M., et al. Crystal structure of archaeosine tRNA-guanine transglycosylase. J. Mol. Biol. 2002;318:665–677. doi: 10.1016/S0022-2836(02)00090-6. [DOI] [PubMed] [Google Scholar]
- 90.Porat J., Vakiloroayaei A., Remnant B.M., Talebi M., Cargill T., Bayfield M.A. Crosstalk between the tRNA methyltransferase Trm1 and RNA chaperone La influences eukaryotic tRNA maturation. J. Biol. Chem. 2023;299 doi: 10.1016/j.jbc.2023.105326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Copela L.A., Chakshusmathi G., Sherrer R.L., Wolin S.L. The La protein functions redundantly with tRNA modification enzymes to ensure tRNA structural stability. RNA. 2006;12:644–654. doi: 10.1261/rna.2307206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Vakiloroayaei A., Shah N.S., Oeffinger M., Bayfield M.A. The RNA chaperone La promotes pre-tRNA maturation via indiscriminate binding of both native and misfolded targets. Nucleic Acids Res. 2017;45:11341–11355. doi: 10.1093/nar/gkx764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Megel C., Morelle G., Lalande S., Duchêne A.M., Small I., Maréchal-Drouard L. Surveillance and cleavage of eukaryotic tRNAs. Int. J. Mol. Sci. 2015;16:1873–1893. doi: 10.3390/ijms16011873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Whipple J.M., Lane E.A., Chernyakov I., D'Silva S., Phizicky E.M. The yeast rapid tRNA decay pathway primarily monitors the structural integrity of the acceptor and T-stems of mature tRNA. Genes Dev. 2011;25:1173–1184. doi: 10.1101/gad.2050711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Payea M.J., Sloma M.F., Kon Y., Young D.L., Guy M.P., Zhang X., et al. Widespread temperature sensitivity and tRNA decay due to mutations in a yeast tRNA. RNA. 2018;24:410–422. doi: 10.1261/rna.064642.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Phizicky E.M., Alfonzo J.D. Do all modifications benefit all tRNAs? FEBS Lett. 2010;584:265–271. doi: 10.1016/j.febslet.2009.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kimura S., Waldor M.K. The RNA degradosome promotes tRNA quality control through clearance of hypomodified tRNA. Proc. Natl. Acad. Sci. U. S. A. 2019;116:1394–1403. doi: 10.1073/pnas.1814130116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ma J., Han H., Huang Y., Yang C., Zheng S., Cai T., et al. METTL1/WDR4-mediated m(7)G tRNA modifications and m(7)G codon usage promote mRNA translation and lung cancer progression. Mol. Ther. 2021;29:3422–3435. doi: 10.1016/j.ymthe.2021.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dai Z., Liu H., Liao J., Huang C., Ren X., Zhu W., et al. N(7)-Methylguanosine tRNA modification enhances oncogenic mRNA translation and promotes intrahepatic cholangiocarcinoma progression. Mol. Cell. 2021;81:3339–3355.e3338. doi: 10.1016/j.molcel.2021.07.003. [DOI] [PubMed] [Google Scholar]
- 100.Orellana E.A., Liu Q., Yankova E., Pirouz M., De Braekeleer E., Zhang W., et al. METTL1-mediated m(7)G modification of Arg-TCT tRNA drives oncogenic transformation. Mol. Cell. 2021;81:3323–3338.e3314. doi: 10.1016/j.molcel.2021.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Giegé R., Eriani G. The tRNA identity landscape for aminoacylation and beyond. Nucleic Acids Res. 2023;51:1528–1570. doi: 10.1093/nar/gkad007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Muramatsu T., Nishikawa K., Nemoto F., Kuchino Y., Nishimura S., Miyazawa T., et al. Codon and amino-acid specificities of a transfer RNA are both converted by a single post-transcriptional modification. Nature. 1988;336:179–181. doi: 10.1038/336179a0. [DOI] [PubMed] [Google Scholar]
- 103.Senger B., Auxilien S., Englisch U., Cramer F., Fasiolo F. The modified wobble base inosine in yeast tRNAIle is a positive determinant for aminoacylation by isoleucyl-tRNA synthetase. Biochemistry. 1997;36:8269–8275. doi: 10.1021/bi970206l. [DOI] [PubMed] [Google Scholar]
- 104.Bhaskaran H., Rodriguez-Hernandez A., Perona J.J. Kinetics of tRNA folding monitored by aminoacylation. RNA. 2012;18:569–580. doi: 10.1261/rna.030080.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Schultz S.K., Katanski C.D., Halucha M., Peña N., Fahlman R.P., Pan T., et al. Modifications in the T arm of tRNA globally determine tRNA maturation, function, and cellular fitness. Proc. Natl. Acad. Sci. U. S. A. 2024;121 doi: 10.1073/pnas.2401154121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bohnsack K.E., Kleiber N., Lemus-Diaz N., Bohnsack M.T. Roles and dynamics of 3-methylcytidine in cellular RNAs. Trends Biochem. Sci. 2022;47:596–608. doi: 10.1016/j.tibs.2022.03.004. [DOI] [PubMed] [Google Scholar]
- 107.Xu L., Liu X., Sheng N., Oo K.S., Liang J., Chionh Y.H., et al. Three distinct 3-methylcytidine (m(3)C) methyltransferases modify tRNA and mRNA in mice and humans. J. Biol. Chem. 2017;292:14695–14703. doi: 10.1074/jbc.M117.798298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mao X.L., Li Z.H., Huang M.H., Wang J.T., Zhou J.B., Li Q.R., et al. Mutually exclusive substrate selection strategy by human m3C RNA transferases METTL2A and METTL6. Nucleic Acids Res. 2021;49:8309–8323. doi: 10.1093/nar/gkab603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Han L., Marcus E., D'Silva S., Phizicky E.M. S. cerevisiae Trm140 has two recognition modes for 3-methylcytidine modification of the anticodon loop of tRNA substrates. Rna. 2017;23:406–419. doi: 10.1261/rna.059667.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pestova T.V., Hellen C.U. Preparation and activity of synthetic unmodified mammalian tRNAi(Met) in initiation of translation in vitro. RNA. 2001;7:1496–1505. doi: 10.1017/s135583820101038x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Förster C., Chakraburtty K., Sprinzl M. Discrimination between initiation and elongation of protein biosynthesis in yeast: identity assured by a nucleotide modification in the initiator tRNA. Nucleic Acids Res. 1993;21:5679–5683. doi: 10.1093/nar/21.24.5679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Basavappa R., Sigler P.B. The 3 A crystal structure of yeast initiator tRNA: functional implications in initiator/elongator discrimination. EMBO J. 1991;10:3105–3111. doi: 10.1002/j.1460-2075.1991.tb07864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Aström S.U., Byström A.S. Rit1, a tRNA backbone-modifying enzyme that mediates initiator and elongator tRNA discrimination. Cell. 1994;79:535–546. doi: 10.1016/0092-8674(94)90262-3. [DOI] [PubMed] [Google Scholar]
- 114.Drabkin H.J., Estrella M., Rajbhandary U.L. Initiator-elongator discrimination in vertebrate tRNAs for protein synthesis. Mol. Cell Biol. 1998;18:1459–1466. doi: 10.1128/mcb.18.3.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Milón P., Rodnina M.V. Kinetic control of translation initiation in bacteria. Crit. Rev. Biochem. Mol. Biol. 2012;47:334–348. doi: 10.3109/10409238.2012.678284. [DOI] [PubMed] [Google Scholar]
- 116.Daugeron M.C., Lenstra T.L., Frizzarin M., El Yacoubi B., Liu X., Baudin-Baillieu A., et al. Gcn4 misregulation reveals a direct role for the evolutionary conserved EKC/KEOPS in the t6A modification of tRNAs. Nucleic Acids Res. 2011;39:6148–6160. doi: 10.1093/nar/gkr178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Thiaville P.C., Legendre R., Rojas-Benítez D., Baudin-Baillieu A., Hatin I., Chalancon G., et al. Global translational impacts of the loss of the tRNA modification t(6)A in yeast. Microb. Cell. 2016;3:29–45. doi: 10.15698/mic2016.01.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.El Yacoubi B., Hatin I., Deutsch C., Kahveci T., Rousset J.P., Iwata-Reuyl D., et al. A role for the universal Kae1/Qri7/YgjD (COG0533) family in tRNA modification. EMBO J. 2011;30:882–893. doi: 10.1038/emboj.2010.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nazarenko I.A., Harrington K.M., Uhlenbeck O.C. Many of the conserved nucleotides of tRNA(Phe) are not essential for ternary complex formation and peptide elongation. EMBO J. 1994;13:2464–2471. doi: 10.1002/j.1460-2075.1994.tb06531.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Harrington K.M., Nazarenko I.A., Dix D.B., Thompson R.C., Uhlenbeck O.C. In vitro analysis of translational rate and accuracy with an unmodified tRNA. Biochemistry. 1993;32:7617–7622. doi: 10.1021/bi00081a003. [DOI] [PubMed] [Google Scholar]
- 121.Achenbach J., Jahnz M., Bethge L., Paal K., Jung M., Schuster M., et al. Outwitting EF-Tu and the ribosome: translation with d-amino acids. Nucleic Acids Res. 2015;43:5687–5698. doi: 10.1093/nar/gkv566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Schrader J.M., Chapman S.J., Uhlenbeck O.C. Tuning the affinity of aminoacyl-tRNA to elongation factor Tu for optimal decoding. Proc. Natl. Acad. Sci. U. S. A. 2011;108:5215–5220. doi: 10.1073/pnas.1102128108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Liu J.C., Liu M., Horowitz J. Recognition of the universally conserved 3′-CCA end of tRNA by elongation factor EF-Tu. RNA. 1998;4:639–646. doi: 10.1017/s1355838298980013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Commans S., Böck A. Selenocysteine inserting tRNAs: an overview. FEMS Microbiol. Rev. 1999;23:335–351. doi: 10.1111/j.1574-6976.1999.tb00403.x. [DOI] [PubMed] [Google Scholar]
- 125.Paleskava A., Konevega A.L., Rodnina M.V. Thermodynamic and kinetic framework of selenocysteyl-tRNASec recognition by elongation factor SelB. J. Biol. Chem. 2010;285:3014–3020. doi: 10.1074/jbc.M109.081380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Pape T., Wintermeyer W., Rodnina M.V. Complete kinetic mechanism of elongation factor Tu-dependent binding of aminoacyl-tRNA to the A site of the E. coli ribosome. EMBO J. 1998;17:7490–7497. doi: 10.1093/emboj/17.24.7490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Rodnina M.V., Gromadski K.B., Kothe U., Wieden H.J. Recognition and selection of tRNA in translation. FEBS Lett. 2005;579:938–942. doi: 10.1016/j.febslet.2004.11.048. [DOI] [PubMed] [Google Scholar]
- 128.Duechler M., Leszczyńska G., Sochacka E., Nawrot B. Nucleoside modifications in the regulation of gene expression: focus on tRNA. Cell Mol. Life Sci. 2016;73:3075–3095. doi: 10.1007/s00018-016-2217-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ingolia N.T., Ghaemmaghami S., Newman J.R., Weissman J.S. Genome-wide analysis in vivo of translation with nucleotide resolution using ribosome profiling. Science. 2009;324:218–223. doi: 10.1126/science.1168978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ingolia N.T. Genome-wide translational profiling by ribosome footprinting. Methods Enzymol. 2010;470:119–142. doi: 10.1016/S0076-6879(10)70006-9. [DOI] [PubMed] [Google Scholar]
- 131.Chou H.J., Donnard E., Gustafsson H.T., Garber M., Rando O.J. Transcriptome-wide analysis of roles for tRNA modifications in translational regulation. Mol. Cell. 2017;68:978–992.e974. doi: 10.1016/j.molcel.2017.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Chen C.W., Tanaka M. Genome-wide translation profiling by ribosome-bound tRNA capture. Cell Rep. 2018;23:608–621. doi: 10.1016/j.celrep.2018.03.035. [DOI] [PubMed] [Google Scholar]
- 133.Nedialkova D.D., Leidel S.A. Optimization of codon translation rates via tRNA modifications maintains proteome integrity. Cell. 2015;161:1606–1618. doi: 10.1016/j.cell.2015.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Deng W., Babu I.R., Su D., Yin S., Begley T.J., Dedon P.C. Trm9-Catalyzed tRNA modifications regulate global protein expression by codon-biased translation. PLoS Genet. 2015;11 doi: 10.1371/journal.pgen.1005706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Schaffrath R., Leidel S.A. Wobble uridine modifications-a reason to live, a reason to die? RNA Biol. 2017;14:1209–1222. doi: 10.1080/15476286.2017.1295204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Armengod M.E., Meseguer S., Villarroya M., Prado S., Moukadiri I., Ruiz-Partida R., et al. Modification of the wobble uridine in bacterial and mitochondrial tRNAs reading NNA/NNG triplets of 2-codon boxes. RNA Biol. 2014;11:1495–1507. doi: 10.4161/15476286.2014.992269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Agris P.F., Eruysal E.R., Narendran A., Väre V.Y.P., Vangaveti S., Ranganathan S.V. Celebrating wobble decoding: half a century and still much is new. RNA Biol. 2018;15:537–553. doi: 10.1080/15476286.2017.1356562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Gerber A.P., Keller W. An adenosine deaminase that generates inosine at the wobble position of tRNAs. Science. 1999;286:1146–1149. doi: 10.1126/science.286.5442.1146. [DOI] [PubMed] [Google Scholar]
- 139.Wolf J., Gerber A.P., Keller W. tadA, an essential tRNA-specific adenosine deaminase from Escherichia coli. EMBO J. 2002;21:3841–3851. doi: 10.1093/emboj/cdf362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Torres A.G., Piñeyro D., Filonava L., Stracker T.H., Batlle E., Ribas de Pouplana L. A-to-I editing on tRNAs: biochemical, biological and evolutionary implications. FEBS Lett. 2014;588:4279–4286. doi: 10.1016/j.febslet.2014.09.025. [DOI] [PubMed] [Google Scholar]
- 141.Konevega A.L., Soboleva N.G., Makhno V.I., Semenkov Y.P., Wintermeyer W., Rodnina M.V., et al. Purine bases at position 37 of tRNA stabilize codon-anticodon interaction in the ribosomal A site by stacking and Mg2+-dependent interactions. RNA. 2004;10:90–101. doi: 10.1261/rna.5142404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Tuorto F., Legrand C., Cirzi C., Federico G., Liebers R., Müller M., et al. Queuosine-modified tRNAs confer nutritional control of protein translation. EMBO J. 2018;37:e99777. doi: 10.15252/embj.201899777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sheppard K., Yuan J., Hohn M.J., Jester B., Devine K.M., Söll D. From one amino acid to another: tRNA-dependent amino acid biosynthesis. Nucleic Acids Res. 2008;36:1813–1825. doi: 10.1093/nar/gkn015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Shepherd J., Ibba M. Direction of aminoacylated transfer RNAs into antibiotic synthesis and peptidoglycan-mediated antibiotic resistance. FEBS Lett. 2013;587:2895–2904. doi: 10.1016/j.febslet.2013.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Chery M., Drouard L. Plant tRNA functions beyond their major role in translation. J. Exp. Bot. 2023;74:2352–2363. doi: 10.1093/jxb/erac483. [DOI] [PubMed] [Google Scholar]
- 146.Dare K., Ibba M. Roles of tRNA in cell wall biosynthesis. Wiley Interdiscip. Rev. RNA. 2012;3:247–264. doi: 10.1002/wrna.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Tasaki T., Sriram S.M., Park K.S., Kwon Y.T. The N-end rule pathway. Annu. Rev. Biochem. 2012;81:261–289. doi: 10.1146/annurev-biochem-051710-093308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Raina M., Ibba M. tRNAs as regulators of biological processes. Front. Genet. 2014;5:171. doi: 10.3389/fgene.2014.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hou Y.M., Yang X. Regulation of cell death by transfer. RNA Antioxid. Redox Signal. 2013;19:583–594. doi: 10.1089/ars.2012.5171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wang S., Li H., Lian Z., Deng S. The role of RNA modification in HIV-1 infection. Int. J. Mol. Sci. 2022;23:7571. doi: 10.3390/ijms23147571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Roth M.J., Schwartzberg P.L., Goff S.P. Structure of the termini of DNA intermediates in the integration of retroviral DNA: dependence on IN function and terminal DNA sequence. Cell. 1989;58:47–54. doi: 10.1016/0092-8674(89)90401-7. [DOI] [PubMed] [Google Scholar]
- 152.Gilboa E., Mitra S.W., Goff S., Baltimore D. A detailed model of reverse transcription and tests of crucial aspects. Cell. 1979;18:93–100. doi: 10.1016/0092-8674(79)90357-x. [DOI] [PubMed] [Google Scholar]
- 153.Burnett B.P., McHenry C.S. Posttranscriptional modification of retroviral primers is required for late stages of DNA replication. Proc. Natl. Acad. Sci. U. S. A. 1997;94:7210–7215. doi: 10.1073/pnas.94.14.7210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Auxilien S., Keith G., Le Grice S.F., Darlix J.L. Role of post-transcriptional modifications of primer tRNALys,3 in the fidelity and efficacy of plus strand DNA transfer during HIV-1 reverse transcription. J. Biol. Chem. 1999;274:4412–4420. doi: 10.1074/jbc.274.7.4412. [DOI] [PubMed] [Google Scholar]
- 155.Ben-Artzi H., Shemesh J., Zeelon E., Amit B., Kleiman L., Gorecki M., et al. Molecular analysis of the second template switch during reverse transcription of the HIV RNA template. Biochemistry. 1996;35:10549–10557. doi: 10.1021/bi960439x. [DOI] [PubMed] [Google Scholar]
- 156.Maynard N.D., Macklin D.N., Kirkegaard K., Covert M.W. Competing pathways control host resistance to virus via tRNA modification and programmed ribosomal frameshifting. Mol. Syst. Biol. 2012;8:567. doi: 10.1038/msb.2011.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Jungfleisch J., Böttcher R., Talló-Parra M., Pérez-Vilaró G., Merits A., Novoa E.M., et al. CHIKV infection reprograms codon optimality to favor viral RNA translation by altering the tRNA epitranscriptome. Nat. Commun. 2022;13:4725. doi: 10.1038/s41467-022-31835-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.D Oliviera A., Dai X., Mottaghinia S., Geissler E.P., Etienne L., Zhang Y., et al. Recognition and cleavage of human tRNA methyltransferase TRMT1 by the SARS-CoV-2 main protease. bioRxiv. 2023 doi: 10.1101/2023.02.20.529306. [preprint] [DOI] [Google Scholar]
- 159.Zhang K., Eldin P., Ciesla J.H., Briant L., Lentini J.M., Ramos J., et al. Proteolytic cleavage and inactivation of the TRMT1 tRNA modification enzyme by SARS-CoV-2 main protease. bioRxiv. 2024 doi: 10.1101/2023.02.10.527147. [preprint] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Holmes A.D., Chan P.P., Chen Q., Ivanov P., Drouard L., Polacek N., et al. A standardized ontology for naming tRNA-derived RNAs based on molecular origin. Nat. Methods. 2023;20:627–628. doi: 10.1038/s41592-023-01813-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kuhle B., Chen Q., Schimmel P. tRNA renovatio: rebirth through fragmentation. Mol. Cell. 2023;83:3953–3971. doi: 10.1016/j.molcel.2023.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Su Z., Wilson B., Kumar P., Dutta A. Noncanonical roles of tRNAs: tRNA fragments and beyond. Annu. Rev. Genet. 2020;54:47–69. doi: 10.1146/annurev-genet-022620-101840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Li Z., Stanton B.A. Transfer RNA-derived fragments, the underappreciated regulatory small RNAs in microbial pathogenesis. Front. Microbiol. 2021;12 doi: 10.3389/fmicb.2021.687632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Chen Q., Zhang X., Shi J., Yan M., Zhou T. Origins and evolving functionalities of tRNA-derived small RNAs. Trends Biochem. Sci. 2021;46:790–804. doi: 10.1016/j.tibs.2021.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Fu M., Gu J., Wang M., Zhang J., Chen Y., Jiang P., et al. Emerging roles of tRNA-derived fragments in cancer. Mol. Cancer. 2023;22:30. doi: 10.1186/s12943-023-01739-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Cosentino C., Toivonen S., Diaz Villamil E., Atta M., Ravanat J.L., Demine S., et al. Pancreatic β-cell tRNA hypomethylation and fragmentation link TRMT10A deficiency with diabetes. Nucleic Acids Res. 2018;46:10302–10318. doi: 10.1093/nar/gky839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Vitali P., Kiss T. Cooperative 2′-O-methylation of the wobble cytidine of human elongator tRNA(Met)(CAT) by a nucleolar and a Cajal body-specific box C/D RNP. Genes Dev. 2019;33:741–746. doi: 10.1101/gad.326363.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Suzuki G., Shimazu N., Tanaka M. A yeast prion, Mod5, promotes acquired drug resistance and cell survival under environmental stress. Science. 2012;336:355–359. doi: 10.1126/science.1219491. [DOI] [PubMed] [Google Scholar]
- 169.Garcia D.M., Campbell E.A., Jakobson C.M., Tsuchiya M., Shaw E.A., DiNardo A.L., et al. A prion accelerates proliferation at the expense of lifespan. Elife. 2021;10:e60917. doi: 10.7554/eLife.60917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Read D.F., Waller T.J., Tse E., Southworth D.R., Engelke D.R., Smaldino P.J. Aggregation of Mod5 is affected by tRNA binding with implications for tRNA gene-mediated silencing. FEBS Lett. 2017;591:1601–1610. doi: 10.1002/1873-3468.12627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Waller T.J., Read D.F., Engelke D.R., Smaldino P.J. The human tRNA-modifying protein, TRIT1, forms amyloid fibers in vitro. Gene. 2017;612:19–24. doi: 10.1016/j.gene.2016.10.041. [DOI] [PubMed] [Google Scholar]
- 172.Anastassiadis T., Köhrer C. Ushering in the era of tRNA medicines. J. Biol. Chem. 2023;299 doi: 10.1016/j.jbc.2023.105246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Coller J., Ignatova Z. tRNA therapeutics for genetic diseases. Nat. Rev. Drug Discov. 2023;23:108–125. doi: 10.1038/s41573-023-00829-9. [DOI] [PubMed] [Google Scholar]
- 174.Kinghorn S.M., O'Byrne C.P., Booth I.R., Stansfield I. Physiological analysis of the role of truB in Escherichia coli: a role for tRNA modification in extreme temperature resistance. Microbiology (Reading) 2002;148:3511–3520. doi: 10.1099/00221287-148-11-3511. [DOI] [PubMed] [Google Scholar]
- 175.Ishida K., Kunibayashi T., Tomikawa C., Ochi A., Kanai T., Hirata A., et al. Pseudouridine at position 55 in tRNA controls the contents of other modified nucleotides for low-temperature adaptation in the extreme-thermophilic eubacterium Thermus thermophilus. Nucleic Acids Res. 2011;39:2304–2318. doi: 10.1093/nar/gkq1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Jones J.D., Franco M.K., Tardu M., Smith T.J., Snyder L.R., Eyler D.E., et al. Conserved 5-methyluridine tRNA modification modulates ribosome translocation. bioRxiv. 2023 doi: 10.1101/2023.11.12.566704. [preprint] [DOI] [Google Scholar]
- 177.Schultz S.K., Meadows K., Kothe U. Molecular mechanism of tRNA binding by the Escherichia coli N7 guanosine methyltransferase TrmB. J. Biol. Chem. 2023;299 doi: 10.1016/j.jbc.2023.104612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Thongdee N., Jaroensuk J., Atichartpongkul S., Chittrakanwong J., Chooyoung K., Srimahaeak T., et al. TrmB, a tRNA m7G46 methyltransferase, plays a role in hydrogen peroxide resistance and positively modulates the translation of katA and katB mRNAs in Pseudomonas aeruginosa. Nucleic Acids Res. 2019;47:9271–9281. doi: 10.1093/nar/gkz702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.McGuffey J.C., Jackson-Litteken C.D., Di Venanzio G., Zimmer A.A., Lewis J.M., Distel J.S., et al. The tRNA methyltransferase TrmB is critical for Acinetobacter baumannii stress responses and pulmonary infection. mBio. 2023;14 doi: 10.1128/mbio.01416-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Takano Y., Takayanagi N., Hori H., Ikeuchi Y., Suzuki T., Kimura A., et al. A gene involved in modifying transfer RNA is required for fungal pathogenicity and stress tolerance of Colletotrichum lagenarium. Mol. Microbiol. 2006;60:81–92. doi: 10.1111/j.1365-2958.2006.05080.x. [DOI] [PubMed] [Google Scholar]
- 181.Tomikawa C., Yokogawa T., Kanai T., Hori H. N7-Methylguanine at position 46 (m7G46) in tRNA from Thermus thermophilus is required for cell viability at high temperatures through a tRNA modification network. Nucleic Acids Res. 2010;38:942–957. doi: 10.1093/nar/gkp1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Alexandrov A., Grayhack E.J., Phizicky E.M. tRNA m7G methyltransferase Trm8p/Trm82p: evidence linking activity to a growth phenotype and implicating Trm82p in maintaining levels of active Trm8p. RNA. 2005;11:821–830. doi: 10.1261/rna.2030705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Lecointe F., Simos G., Sauer A., Hurt E.C., Motorin Y., Grosjean H. Characterization of yeast protein Deg1 as pseudouridine synthase (Pus3) catalyzing the formation of psi 38 and psi 39 in tRNA anticodon loop. J. Biol. Chem. 1998;273:1316–1323. doi: 10.1074/jbc.273.3.1316. [DOI] [PubMed] [Google Scholar]
- 184.Karlsborn T., Tükenmez H., Mahmud A.K., Xu F., Xu H., Byström A.S. Elongator, a conserved complex required for wobble uridine modifications in eukaryotes. RNA Biol. 2014;11:1519–1528. doi: 10.4161/15476286.2014.992276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Ikeuchi Y., Kitahara K., Suzuki T. The RNA acetyltransferase driven by ATP hydrolysis synthesizes N4-acetylcytidine of tRNA anticodon. EMBO J. 2008;27:2194–2203. doi: 10.1038/emboj.2008.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Okamoto M., Fujiwara M., Hori M., Okada K., Yazama F., Konishi H., et al. tRNA modifying enzymes, NSUN2 and METTL1, determine sensitivity to 5-fluorouracil in HeLa cells. PLoS Genet. 2014;10 doi: 10.1371/journal.pgen.1004639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Gustavsson M., Ronne H. Evidence that tRNA modifying enzymes are important in vivo targets for 5-fluorouracil in yeast. RNA. 2008;14:666–674. doi: 10.1261/rna.966208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Shigi N., Suzuki T., Terada T., Shirouzu M., Yokoyama S., Watanabe K. Temperature-dependent biosynthesis of 2-thioribothymidine of Thermus thermophilus tRNA. J. Biol. Chem. 2006;281:2104–2113. doi: 10.1074/jbc.M510771200. [DOI] [PubMed] [Google Scholar]
- 189.Benítez-Páez A., Villarroya M., Douthwaite S., Gabaldón T., Armengod M.E. YibK is the 2′-O-methyltransferase TrmL that modifies the wobble nucleotide in Escherichia coli tRNA(Leu) isoacceptors. RNA. 2010;16:2131–2143. doi: 10.1261/rna.2245910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Zhou M., Long T., Fang Z.P., Zhou X.L., Liu R.J., Wang E.D. Identification of determinants for tRNA substrate recognition by Escherichia coli C/U34 2′-O-methyltransferase. RNA Biol. 2015;12:900–911. doi: 10.1080/15476286.2015.1050576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Thiaville P.C., El Yacoubi B., Köhrer C., Thiaville J.J., Deutsch C., Iwata-Reuyl D., et al. Essentiality of threonylcarbamoyladenosine (t(6)A), a universal tRNA modification, in bacteria. Mol. Microbiol. 2015;98:1199–1221. doi: 10.1111/mmi.13209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Arimbasseri A.G., Iben J., Wei F.Y., Rijal K., Tomizawa K., Hafner M., et al. Evolving specificity of tRNA 3-methyl-cytidine-32 (m3C32) modification: a subset of tRNAsSer requires N6-isopentenylation of A37. RNA. 2016;22:1400–1410. doi: 10.1261/rna.056259.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Müller M., Hartmann M., Schuster I., Bender S., Thüring K.L., Helm M., et al. Dynamic modulation of Dnmt2-dependent tRNA methylation by the micronutrient queuine. Nucleic Acids Res. 2015;43:10952–10962. doi: 10.1093/nar/gkv980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Guy M.P., Phizicky E.M. Conservation of an intricate circuit for crucial modifications of the tRNAPhe anticodon loop in eukaryotes. RNA. 2015;21:61–74. doi: 10.1261/rna.047639.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Guy M.P., Podyma B.M., Preston M.A., Shaheen H.H., Krivos K.L., Limbach P.A., et al. Yeast Trm7 interacts with distinct proteins for critical modifications of the tRNAPhe anticodon loop. RNA. 2012;18:1921–1933. doi: 10.1261/rna.035287.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Guy M.P., Shaw M., Weiner C.L., Hobson L., Stark Z., Rose K., et al. Defects in tRNA anticodon loop 2′-O-methylation are implicated in nonsyndromic X-linked intellectual disability due to mutations in FTSJ1. Hum. Mutat. 2015;36:1176–1187. doi: 10.1002/humu.22897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Nagayoshi Y., Chujo T., Hirata S., Nakatsuka H., Chen C.W., Takakura M., et al. Loss of Ftsj1 perturbs codon-specific translation efficiency in the brain and is associated with X-linked intellectual disability. Sci. Adv. 2021;7:eabf3072. doi: 10.1126/sciadv.abf3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Rubio M.A., Gaston K.W., McKenney K.M., Fleming I.M., Paris Z., Limbach P.A., et al. Editing and methylation at a single site by functionally interdependent activities. Nature. 2017;542:494–497. doi: 10.1038/nature21396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Rubio M.A., Ragone F.L., Gaston K.W., Ibba M., Alfonzo J.D. C to U editing stimulates A to I editing in the anticodon loop of a cytoplasmic threonyl tRNA in Trypanosoma brucei. J. Biol. Chem. 2006;281:115–120. doi: 10.1074/jbc.M510136200. [DOI] [PubMed] [Google Scholar]
- 200.Rider L.W., Ottosen M.B., Gattis S.G., Palfey B.A. Mechanism of dihydrouridine synthase 2 from yeast and the importance of modifications for efficient tRNA reduction. J. Biol. Chem. 2009;284:10324–10333. doi: 10.1074/jbc.M806137200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.De Zoysa T., Phizicky E.M. Hypomodified tRNA in evolutionarily distant yeasts can trigger rapid tRNA decay to activate the general amino acid control response, but with different consequences. PLoS Genet. 2020;16 doi: 10.1371/journal.pgen.1008893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Kotelawala L., Grayhack E.J., Phizicky E.M. Identification of yeast tRNA Um(44) 2′-O-methyltransferase (Trm44) and demonstration of a Trm44 role in sustaining levels of specific tRNA(Ser) species. RNA. 2008;14:158–169. doi: 10.1261/rna.811008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Dewe J.M., Whipple J.M., Chernyakov I., Jaramillo L.N., Phizicky E.M. The yeast rapid tRNA decay pathway competes with elongation factor 1A for substrate tRNAs and acts on tRNAs lacking one or more of several modifications. RNA. 2012;18:1886–1896. doi: 10.1261/rna.033654.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Bowles I.E., Jackman J.E. A tRNA-specific function for tRNA methyltransferase Trm10 is associated with a new tRNA quality control mechanism in Saccharomyces cerevisiae. RNA. 2024;30:171–187. doi: 10.1261/rna.079861.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Tuorto F., Liebers R., Musch T., Schaefer M., Hofmann S., Kellner S., et al. RNA cytosine methylation by Dnmt2 and NSun2 promotes tRNA stability and protein synthesis. Nat. Struct. Mol. Biol. 2012;19:900–905. doi: 10.1038/nsmb.2357. [DOI] [PubMed] [Google Scholar]
- 206.Sylvers L.A., Rogers K.C., Shimizu M., Ohtsuka E., Söll D. A 2-thiouridine derivative in tRNAGlu is a positive determinant for aminoacylation by Escherichia coli glutamyl-tRNA synthetase. Biochemistry. 1993;32:3836–3841. doi: 10.1021/bi00066a002. [DOI] [PubMed] [Google Scholar]
- 207.Tamura K., Himeno H., Asahara H., Hasegawa T., Shimizu M. In vitro study of E.coli tRNA(Arg) and tRNA(Lys) identity elements. Nucleic Acids Res. 1992;20:2335–2339. doi: 10.1093/nar/20.9.2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Fahlman R.P., Dale T., Uhlenbeck O.C. Uniform binding of aminoacylated transfer RNAs to the ribosomal A and P sites. Mol. Cell. 2004;16:799–805. doi: 10.1016/j.molcel.2004.10.030. [DOI] [PubMed] [Google Scholar]
- 209.Seno T., Agris P.F., Söll D. Involvement of the anticodon region of Escherichia coli tRNAGln and tRNAGlu in the specific interaction with cognate aminoacyl-tRNA synthetase. Alteration of the 2-thiouridine derivatives located in the anticodon of the tRNAs by BrCN or sulfur deprivation. Biochim. Biophys. Acta. 1974;349:328–338. doi: 10.1016/0005-2787(74)90120-8. [DOI] [PubMed] [Google Scholar]
- 210.Madore E., Florentz C., Giegé R., Sekine S., Yokoyama S., Lapointe J. Effect of modified nucleotides on Escherichia coli tRNAGlu structure and on its aminoacylation by glutamyl-tRNA synthetase. Predominant and distinct roles of the mnm5 and s2 modifications of U34. Eur. J. Biochem. 1999;266:1128–1135. doi: 10.1046/j.1432-1327.1999.00965.x. [DOI] [PubMed] [Google Scholar]
- 211.Ikeuchi Y., Kimura S., Numata T., Nakamura D., Yokogawa T., Ogata T., et al. Agmatine-conjugated cytidine in a tRNA anticodon is essential for AUA decoding in archaea. Nat. Chem. Biol. 2010;6:277–282. doi: 10.1038/nchembio.323. [DOI] [PubMed] [Google Scholar]
- 212.Köhrer C., Srinivasan G., Mandal D., Mallick B., Ghosh Z., Chakrabarti J., et al. Identification and characterization of a tRNA decoding the rare AUA codon in Haloarcula marismortui. RNA. 2008;14:117–126. doi: 10.1261/rna.795508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Clifton B.E., Fariz M.A., Uechi G.I., Laurino P. Evolutionary repair reveals an unexpected role of the tRNA modification m1G37 in aminoacylation. Nucleic Acids Res. 2021;49:12467–12485. doi: 10.1093/nar/gkab1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Pütz J., Florentz C., Benseler F., Giegé R. A single methyl group prevents the mischarging of a tRNA. Nat. Struct. Biol. 1994;1:580–582. doi: 10.1038/nsb0994-580. [DOI] [PubMed] [Google Scholar]
- 215.Bare L.A., Uhlenbeck O.C. Specific substitution into the anticodon loop of yeast tyrosine transfer RNA. Biochemistry. 1986;25:5825–5830. doi: 10.1021/bi00367a072. [DOI] [PubMed] [Google Scholar]
- 216.Han L., Guy M.P., Kon Y., Phizicky E.M. Lack of 2′-O-methylation in the tRNA anticodon loop of two phylogenetically distant yeast species activates the general amino acid control pathway. PLoS Genet. 2018;14 doi: 10.1371/journal.pgen.1007288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Levi O., Arava Y.S. Pseudouridine-mediated translation control of mRNA by methionine aminoacyl tRNA synthetase. Nucleic Acids Res. 2021;49:432–443. doi: 10.1093/nar/gkaa1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Rezgui V.A., Tyagi K., Ranjan N., Konevega A.L., Mittelstaet J., Rodnina M.V., et al. tRNA tKUUU, tQUUG, and tEUUC wobble position modifications fine-tune protein translation by promoting ribosome A-site binding. Proc. Natl. Acad. Sci. U. S. A. 2013;110:12289–12294. doi: 10.1073/pnas.1300781110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Ranjan N., Rodnina M.V. Thio-modification of tRNA at the wobble position as regulator of the kinetics of decoding and translocation on the ribosome. J. Am. Chem. Soc. 2017;139:5857–5864. doi: 10.1021/jacs.7b00727. [DOI] [PubMed] [Google Scholar]
- 220.Begley U., Dyavaiah M., Patil A., Rooney J.P., DiRenzo D., Young C.M., et al. Trm9-catalyzed tRNA modifications link translation to the DNA damage response. Mol. Cell. 2007;28:860–870. doi: 10.1016/j.molcel.2007.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Addepalli B., Limbach P.A. Pseudouridine in the anticodon of Escherichia coli tRNATyr(QΨA) is catalyzed by the dual specificity enzyme RluF. J. Biol. Chem. 2016;291:22327–22337. doi: 10.1074/jbc.M116.747865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Hibi K., Amikura K., Sugiura N., Masuda K., Ohno S., Yokogawa T., et al. Reconstituted cell-free protein synthesis using in vitro transcribed tRNAs. Commun. Biol. 2020;3:350. doi: 10.1038/s42003-020-1074-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Pintard L., Lecointe F., Bujnicki J.M., Bonnerot C., Grosjean H., Lapeyre B. Trm7p catalyses the formation of two 2′-O-methylriboses in yeast tRNA anticodon loop. EMBO J. 2002;21:1811–1820. doi: 10.1093/emboj/21.7.1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Phelps S.S., Malkiewicz A., Agris P.F., Joseph S. Modified nucleotides in tRNA(Lys) and tRNA(Val) are important for translocation. J. Mol. Biol. 2004;338:439–444. doi: 10.1016/j.jmb.2004.02.070. [DOI] [PubMed] [Google Scholar]
- 225.Jia Z., Meng F., Chen H., Zhu G., Li X., He Y., et al. Human TRUB1 is a highly conserved pseudouridine synthase responsible for the formation of Ψ55 in mitochondrial tRNAAsn, tRNAGln, tRNAGlu and tRNAPro. Nucleic Acids Res. 2022;50:9368–9381. doi: 10.1093/nar/gkac698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Chan C.T., Pang Y.L., Deng W., Babu I.R., Dyavaiah M., Begley T.J., et al. Reprogramming of tRNA modifications controls the oxidative stress response by codon-biased translation of proteins. Nat. Commun. 2012;3:937. doi: 10.1038/ncomms1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Ignatova V.V., Kaiser S., Ho J.S.Y., Bing X., Stolz P., Tan Y.X., et al. METTL6 is a tRNA m(3)C methyltransferase that regulates pluripotency and tumor cell growth. Sci. Adv. 2020;6 doi: 10.1126/sciadv.aaz4551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Schöller E., Marks J., Marchand V., Bruckmann A., Powell C.A., Reichold M., et al. Balancing of mitochondrial translation through METTL8-mediated m(3)C modification of mitochondrial tRNAs. Mol. Cell. 2021;81:4810–4825.e4812. doi: 10.1016/j.molcel.2021.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Yang W.Q., Xiong Q.P., Ge J.Y., Li H., Zhu W.Y., Nie Y., et al. THUMPD3-TRMT112 is a m2G methyltransferase working on a broad range of tRNA substrates. Nucleic Acids Res. 2021;49:11900–11919. doi: 10.1093/nar/gkab927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Hoffer E.D., Hong S., Sunita S., Maehigashi T., Gonzalez R.L.J., Whitford P.C., et al. Structural insights into mRNA reading frame regulation by tRNA modification and slippery codon-anticodon pairing. Elife. 2020;9:e51898. doi: 10.7554/eLife.51898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Nguyen H.A., Hoffer E.D., Dunham C.M. Importance of a tRNA anticodon loop modification and a conserved, noncanonical anticodon stem pairing in tRNACGGProfor decoding. J. Biol. Chem. 2019;294:5281–5291. doi: 10.1074/jbc.RA119.007410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Gamper H.B., Masuda I., Frenkel-Morgenstern M., Hou Y.M. Maintenance of protein synthesis reading frame by EF-P and m(1)G37-tRNA. Nat. Commun. 2015;6:7226. doi: 10.1038/ncomms8226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Masuda I., Hwang J.Y., Christian T., Maharjan S., Mohammad F., Gamper H., et al. Loss of N(1)-methylation of G37 in tRNA induces ribosome stalling and reprograms gene expression. Elife. 2021;10:e70619. doi: 10.7554/eLife.70619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Masuda I., Matsubara R., Christian T., Rojas E.R., Yadavalli S.S., Zhang L., et al. tRNA methylation is a global determinant of bacterial multi-drug resistance. Cell Syst. 2019;8:302–314.e308. doi: 10.1016/j.cels.2019.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Torres A.G., Rodríguez-Escribà M., Marcet-Houben M., Santos Vieira H.G., Camacho N., Catena H., et al. Human tRNAs with inosine 34 are essential to efficiently translate eukarya-specific low-complexity proteins. Nucleic Acids Res. 2021;49:7011–7034. doi: 10.1093/nar/gkab461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Lyu X., Yang Q., Li L., Dang Y., Zhou Z., Chen S., et al. Adaptation of codon usage to tRNA I34 modification controls translation kinetics and proteome landscape. PLoS Genet. 2020;16 doi: 10.1371/journal.pgen.1008836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Hingerty B., Brown R.S., Jack A. Further refinement of the structure of yeast tRNAPhe. J. Mol. Biol. 1978;124:523–534. doi: 10.1016/0022-2836(78)90185-7. [DOI] [PubMed] [Google Scholar]
- 238.Osawa T., Kimura S., Terasaka N., Inanaga H., Suzuki T., Numata T. Structural basis of tRNA agmatinylation essential for AUA codon decoding. Nat. Struct. Mol. Biol. 2011;18:1275–1280. doi: 10.1038/nsmb.2144. [DOI] [PubMed] [Google Scholar]
- 239.Schwalm E.L., Grove T.L., Booker S.J., Boal A.K. Crystallographic capture of a radical S-adenosylmethionine enzyme in the act of modifying tRNA. Science. 2016;352:309–312. doi: 10.1126/science.aad5367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Byrne R.T., Jenkins H.T., Peters D.T., Whelan F., Stowell J., Aziz N., et al. Major reorientation of tRNA substrates defines specificity of dihydrouridine synthases. Proc. Natl. Acad. Sci. U. S. A. 2015;112:6033–6037. doi: 10.1073/pnas.1500161112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Yu F., Tanaka Y., Yamashita K., Suzuki T., Nakamura A., Hirano N., et al. Molecular basis of dihydrouridine formation on tRNA. Proc. Natl. Acad. Sci. U. S. A. 2011;108:19593–19598. doi: 10.1073/pnas.1112352108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Ruiz-Arroyo V.M., Raj R., Babu K., Onolbaatar O., Roberts P.H., Nam Y. Structures and mechanisms of tRNA methylation by METTL1-WDR4. Nature. 2023;613:383–390. doi: 10.1038/s41586-022-05565-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Nissen P., Thirup S., Kjeldgaard M., Nyborg J. The crystal structure of Cys-tRNACys-EF-Tu-GDPNP reveals general and specific features in the ternary complex and in tRNA. Structure. 1999;7:143–156. doi: 10.1016/s0969-2126(99)80021-5. [DOI] [PubMed] [Google Scholar]
- 244.Cavarelli J., Eriani G., Rees B., Ruff M., Boeglin M., Mitschler A., et al. The active site of yeast aspartyl-tRNA synthetase: structural and functional aspects of the aminoacylation reaction. EMBO J. 1994;13:327–337. doi: 10.1002/j.1460-2075.1994.tb06265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Reiter N.J., Osterman A., Torres-Larios A., Swinger K.K., Pan T., Mondragón A. Structure of a bacterial ribonuclease P holoenzyme in complex with tRNA. Nature. 2010;468:784–789. doi: 10.1038/nature09516. [DOI] [PMC free article] [PubMed] [Google Scholar]