Abstract
Recent approval of the dual glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptor agonist, tirzepatide, for the management of type 2 diabetes mellitus (T2DM) has reinvigorated interest in exploitation of GIP receptor (GIPR) pathways as a means of metabolic disease management. However, debate has long surrounded the use of the GIPR as a therapeutic target and whether agonism or antagonism is of most benefit in management of obesity/diabetes. This controversy appears to be partly resolved by the success of tirzepatide. However, emerging studies indicate that prolonged GIPR agonism may desensitise the GIPR to essentially induce receptor antagonism, with this phenomenon suggested to be more pronounced in the human than rodent setting. Thus, deliberation continues to rage in relation to benefits of GIPR agonism vs antagonism. That said, as with GIPR agonism, it is clear that the metabolic advantages of sustained GIPR antagonism in obesity and obesity-driven forms of diabetes can be enhanced by concurrent GLP-1 receptor (GLP-1R) activation. This narrative review discusses various approaches of pharmacological GIPR antagonism including small molecule, peptide, monoclonal antibody and peptide-antibody conjugates, indicating stage of development and significance to the field. Taken together, there is little doubt that interesting times lie ahead for GIPR agonism and antagonism, either alone or when combined with GLP-1R agonists, as a therapeutic intervention for the management of obesity and associated metabolic disease.
Keywords: diabetes, glucose-dependent insulinotropic polypeptide, glucagon-like peptide-1, obesity, polypharmacy, satiety
Introduction
Glucose-dependent insulinotropic polypeptide (GIP) is a 42-amino acid polypeptide hormone secreted from intestinal K-cells of the duodenum and proximal jejunum (Buchan et al. 1978). GIP was discovered in 1969, through collaboration between John Brown and Raymond Pederson at University of British Colombia, Vancouver, alongside Viktor Mutt and Erik Jorpes from Karolinska Institutet, Stockholm (Brown et al. 1969, 1970, 1982). Thus, GIP was recognised by endocrinologists over a decade prior to another closely related, but now more widely renowned gut-derived hormone, glucagon-like peptide-1 (GLP-1) (Müller et al. 2019). Like GLP-1, GIP is released into the circulation in response to ingestion of macronutrients, it is degraded by dipeptidyl peptidase-4 (DPP-4) and accounts for a major part of the ‘incretin-effect’ by enhancing glucose-stimulated insulin secretion (GSIS) (Pederson et al. 1975). GIP exerts its effects on GSIS via binding at GIP receptors (GIPR) on beta cells and activation of cyclic adenosine monophosphate (cAMP) plus associated signal transduction pathways (Ding & Gromada 1997). Additionally, GIP and its receptor are evidenced within the brain, with GIPR expression in the hypothalamus being implicated in the modulation of food intake and satiety, particularly in the arcuate, paraventricular, and dorsomedial nuclei regions (Adriaenssens et al. 2019, Samms et al. 2020). In addition, GIPR signalling is also demonstrated within circumventricular organs (CVOs), including the area postrema and nucleus tractus solitarius of the dorsal vagal complex (DVC) (Adriaenssens et al. 2019, 2023), which are not enclosed by the blood–brain barrier (BBB). Importantly, despite complex interplay between neuronal circuitry within these brain regions, distinct outcomes following GIPR agonism have been confirmed. For example, while hypothalamic GIPRs supress food intake, it appears GIPRs in the DVC may be implicated in taste avoidance (Adriaenssens et al. 2023). Use of fluorescently tagged GLP-1 mimetics indicate that exogenous peptides are not thought to cross the BBB but can interact with CVOs (Secher et al. 2014), with the same likely to be true for GIP-based compounds. Moreover, GIP possesses peripheral actions to improve insulin action and modulates lipid metabolism that influences overall energy balance whilst also potentially reducing energy intake (Samms et al. 2020).
Given the aforementioned biological consequences of GIPR modulation (Fig. 1), it is clear that this signalling pathway holds theoretical promise for the treatment of both type 2 diabetes mellitus (T2DM) and obesity, as has been witnessed to profound effect with GLP-1 (Nauck et al. 2021a). This is especially relevant since GIP appears to be quantitatively the most important incretin hormone in both rodents and humans (Gault et al. 2003a, Holst 2019). When we consider that GIP was discovered over a decade prior to GLP-1, it begs the question: Why has the success of GLP-1R mimetics not been emulated or even preceded by GIPR mimetics? The rise of GIP from enterogastrone to major metabolic hormone makes an interesting story (Marks 2020), but the answer is largely due to the well-known insensitivity of humans with obesity and obesity-driven T2DM to the insulinotropic and glucose-lowering actions of GIP (Nauck et al. 2021b). Such insensitivity can be reversed by lowering blood glucose using insulin, sulphonylureas or DPP-4 inhibitors (Højberg et al. 2009, Irwin et al. 2010, Stensen et al. 2022). However, the situation has not been helped by the debate spanning several decades on whether GIPR agonism or antagonism is most beneficial (Irwin & Flatt 2009a, Campbell et al. 2022). This review will focus on current evidence that supports the therapeutic promise of GIP and GIPR antagonism and the various approaches taken to impart this.
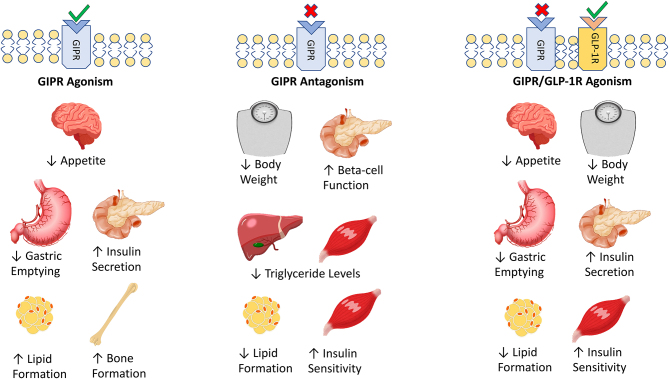
Figure 1.
A summary of the tissue-specific benefits of GIPR agonism and antagonism. In addition, the impact of combined GIPR antagonism combined with GLP-1R agonism is considered. Agonism is indicated by green ticks at the GPCR while antagonism is indicated by red crosses. Increases in the therapeutic effect in each instance are indicated by upward arrows and decreases and indicated by downward arrows.
GIPR antagonism
The above-mentioned actions of GIP relate primarily to receptor agonism, with positive effects on insulin secretion (Ding & Gromada 1997) and satiety (Samms et al. 2020, Fig. 1) having clear potential benefits in obesity and diabetes (Flatt 2008, Irwin & Flatt 2009b). However, it is well established that circulating levels of GIP are elevated in human obesity and obesity-driven forms of T2DM (Ebert & Creutzfeldt 1980, Salera et al. 1982), with studies using obese rodents reporting expansion of intestinal K-cell mass and elevated circulating GIP in genetically inherited obesity (Flatt et al. 1983) and following prolonged exposure to a high-fat, high-calorie diet (Bailey et al. 1986).
As such, it has been demonstrated that GIPR-null mice are protected against high-fat-fed-induced obesity and insulin resistance (Miyawaki et al. 2002), indicating a role for the GIPR in the onset of obesity (Flatt 2008). In addition, specific destruction of GIP-secreting K-cells in mice also safeguards against diet-induced obesity and ameliorates insulin resistance (Althage et al. 2008). Furthermore, various methods to specifically inhibit GIP secretion in rodents are demonstrated to alleviate obesity and insulin resistance (Nasteska et al. 2014, Kanemaru et al. 2020, Murata et al. 2021). Moreover, it is understood that fat is a powerful stimulus for the secretion of GIP which acts at adipocytes. In vitro and ex vivo studies indicate this increases fat storage (Fig. 1) via upregulation of lipoprotein lipase (LPL) activity (Kim et al. 2007) and is associated with phosphorylation of cAMP-response element binding protein (CREB) and nuclear localisation of cAMP-responsive CREB coactivator 2 (TORC2) in human adipocytes (Kim et al. 2010). GIPR agonism promotes fatty acid uptake (Killion et al. 2020a), insulin-induced free fatty acid incorporation into adipocytes (Møller et al. 2016) and inhibits lipolysis (Getty-Kaushik et al. 2006), whilst improving blood flow to the adipose tissue (Asmar et al. 2019). GIPR agonism is also directly implicated in adipocyte growth, with studies in cultured human omental preadipocytes highlighting proliferative actions alongside a reduction in pro-apoptotic transcription factors such as Bcl-2-associated death promoter (BAD) (Chen et al. 2021). Importantly, a GIPR antagonist was reported to annul these preadipocyte proliferative effects (Chen et al. 2021). However, full in vivo characterisation of the mechanisms involved is currently lacking within the literature.
Accordingly, in humans, polymorphisms of the GIPR that lead to perturbed activity are linked to reduced body mass index (Lyssenko et al. 2011, Kizilkaya et al. 2021). Notably, alterations in G protein coupling and subsequent intracellular signalling cascades with several of these GIPR variants directly mirror consequences of GIPR antagonism (Kizilkaya et al. 2021). Additionally, GIP is implicated in increasing cytokine penetration into adipocytes to drive insulin-resistance within these peripheral tissues (Timper et al. 2013). Whether this effect is direct or indirect has been debated recently (Campbell et al. 2022), but either way it is clear that GIP exerts important effects on adipocyte biology (English et al. 2020). In this regard, the literature, based on numerous independent and diverse observations, highlights a clear role for GIPR activation in the development of obesity, grounding the concept of GIPR antagonism as a potential approach to alleviate insulin resistance and excessive weight gain (Irwin et al. 2020). While no such therapy has yet made it to clinic, a number of approaches have been employed to impart GIPR blockade including small molecule, immune-neutralisation and peptidic that will be discussed herein.
Small molecule GIPR antagonism
When considering the extensive body of work linked to the discovery and development of small-molecular weight GLP-1R modulators, including recent work with danuglipron in phase 2 clinical trials (Saxena et al. 2023), it is perhaps surprising that a similar literature search in relation to the GIPR heralds much fewer results. Small molecules remain a mainstay of drug development owing to excellent oral bioavailability and reduced production costs when compared to biologics (Beck et al. 2022). The desire to generate medications suitable for oral administration is likely the largest driver here, as evident with the retrofitting of Novo Nordisk’s GLP-1R agonist semaglutide, co-formulated with sodium N-(8-(2-hydroxybenzoyl) amino caprylate (SNAC), to prevent destruction of the peptide within the stomach and promote gastric absorption (Bucheit et al. 2020). While this formulation of semaglutide, marketed as Rybelsus®, represents a significant success in generation of an orally available direct GLP-1R modulator, it is important to note that much greater quantities of peptide are required for oral delivery compared to injectable formulations (14 mg daily vs. 2.4 mg weekly, as respective maximal dosages), which is likely influencing global shortages of the peptide (Whitley et al. 2023).
Thus, the appetite for small molecule incretin modulators remains high within the pharmaceutical industry. In the case of GIPR antagonists, only one such agent is described in the literature, termed SKL-14959 (Nakamura et al. 2012, Table 1), while no similar GIPR agonist small molecule can be sourced in the literature at the time of writing. SKL-14959 is a potent GIPR antagonist with a molecular weight of less than 400 Daltons and an IC50, in relation to cAMP downregulation of 2.9 uM (Nakamura et al. 2012). Although SKL-14959 may not be entirely selective, with activity at GLP-1R and the glucagon receptor indicated at concentrations above 3100 and 1000 nM, respectively (Nakamura et al. 2012). When evaluated in the acute setting in normal mice, SKL-14959 increased circulating triglyceride levels and reduced LPL and hepatic lipase (HPL) activity following an oil tolerance test (Nakamura et al. 2012), which would be indicative of reduced lipid uptake and storage. Additionally, SKL-14959 also effectively countered the actions of exogenously delivered GIP in terms of reducing GSIS during a glucose tolerance test (Nakamura et al. 2012).
Table 1.
A summary of GIPR antagonists with compound classifications, structures, stage of development and supporting references where available. Amino acid sequences are provided for peptide-based antagonists using single-letter abbreviations. ‘Pal’ indicates a C-16 palmitic acid attachment, ‘γE-C16’ indicates a palmitic acid attached via gamma-glutamic acid, ‘Aib’ indicates inclusion of the unnatural amino acid, 2-aminoisobutyric acid. ‘mGIPAb’ is a murine monoclonal antibody while ‘hGIPR’ is human based. Cases in which structures are not available are denoted ‘N/A’.
| Compound name | Compound classification | Structure | Stage of development | Company/institution | Reference |
|---|---|---|---|---|---|
| SKL-1459 | Small molecule | N/A | Preclinical | Sanwa Kagaku Kenkyusho Co. | Nakamura et al. (2012, 2018) |
| GIP(3–42) | Peptide | EGTFISDYSIAMDKIHQQDFVNWLLAQKGKKNDWKHNITQ | Preclinical | University of Copenhagen | Deacon et al. (2006), Hansen et al. (2016) |
| GIP(3–30)NH2 | Peptide | EGTFISDYSIAMDKIHQQDFVNWLLAQK-NH2 | Preclinical with acute study in humans | University of Copenhagen | Deacon et al. (2006), Hansen et al. (2016), Sparre-Ulrich et al. (2017), Gasbjerg et al. (2018) |
| GIP(7–30) | Peptide | EGTFISDYSIAMDKIHQQDFVNWLLAQK | Preclinical | Boston University | Tseng et al. (1999) |
| GIP(4–42) | Peptide | GTFISDYSIAMDKIHQQDFVNWLLAQKGKKNDWKHNITQ | Preclinical | Ulster University | Kerr et al. (2011) |
| GIP(5–42) | Peptide | TFISDYSIAMDKIHQQDFVNWLLAQKGKKNDWKHNITQ | Preclinical | Ulster University | Kerr et al. (2011) |
| GIP(6–42) | Peptide | FISDYSIAMDKIHQQDFVNWLLAQKGKKNDWKHNITQ | Preclinical | Ulster University | Kerr et al. (2011) |
| GIP(7–42) | Peptide | ISDYSIAMDKIHQQDFVNWLLAQKGKKNDWKHNITQ | Preclinical | Ulster University | Kerr et al. 2011 |
| GIP(8–42) | Peptide | SDYSIAMDKIHQQDFVNWLLAQKGKKNDWKHNITQ | Preclinical | Ulster University | Kerr et al. (2011) |
| (Pro3)GIP | Peptide | YAPGTFISDYSIAMDKIHQQDFVNWLLAQKGKKNDWKHNITQ | Preclinical | Ulster University | Gault et al. (2002) |
| (Ala3)GIP | Peptide | YAAGTFISDYSIAMDKIHQQDFVNWLLAQKGKKNDWKHNITQ | Preclinical | Ulster University | Gault et al. (2007a,b, c) |
| (Phe3)GIP | Peptide | YAFGTFISDYSIAMDKIHQQDFVNWLLAQKGKKNDWKHNITQ | Preclinical | Ulster University | Gault et al. (2007a,b, c) |
| (Tyr3)GIP | Peptide | YAYGTFISDYSIAMDKIHQQDFVNWLLAQKGKKNDWKHNITQ | Preclinical | Ulster University | Gault et al. (2007a, b, c ) |
| (Hyp3)GIP | Peptide | YAOGTFISDYSIAMDKIHQQDFVNWLLAQKGKKNDWKHNITQ | Preclinical | Ulster University | O’Harte et al. (2006) |
| (Hyp3)GIP(K16Pal) | Peptide | YAOGTFISDYSIAMDK-[Pal]-IHQQDFVNWLLAQKGKKNDWKHNITQ | Preclinical | Ulster University | O’Harte et al. (2006) |
| GIP(3–30)-Cex-K40Pal | Peptide | EGTFISDYSIAMDKIHQQDFVNWLLAQKPSSGAPPPSK[Pal] | Preclinical | Ulster University | Pathak et al. (2015a) |
| GIP(6–30)-Cex-K40Pal | Peptide | EGTFISDYSIAMDKIHQQDFVNWLLAQKPSSGAPPPSK[Pal] | Preclinical | Ulster University | Pathak et al. (2015b) |
| Pro3GIP(3–30)-Cex-K40Pal | Peptide | PGTFISDYSIAMDKIHQQDFVNWLLAQKPSSGAPPPSK[Pal] | Preclinical | Ulster University | Pathak et al. (2015a) |
| [Nα-Ac,L14,R18,E21] hGIP(5–31)-K11(γE-C16) | Peptide | TFISDYK-[γE-C16]-IALDKIRQQEFVNWLLAQKG | Preclinical | Novo Nordisk | Yang et al. (2022) |
| NαAc,K10[γEγE-C16], Arg18,hGIP(5–42) | Peptide | TFISDKSIALDK-[γE-C16]-IRQQEFVNWLLAQKGKKNDWKHNITQ | Preclinical | Novo Nordisk | Yang et al. (2022) |
| GIPg013 | Monoclonal antibody | N/A | Preclinical | MedImmune / AstraZeneca | Ravn et al. (2013) |
| GIPmAb | Monoclonal antibody | N/A | Preclinical | Case Western Reserve University | Boylan et al. (2015) |
| hGIPR-Ab | Monoclonal antibody | N/A | Preclinical | Amgen | Killion et al. (2018) |
| mGIPR-Ab/P1 | Peptide-Monoclonal antibody conjugate | [mGIPAb]-GGGGG-H-Aib-EGTFTSDVSSYLE-Aib-QAAKEFIAWLVKGGG | Preclinical | Amgen | Lu et al. (2021) |
| hGIPR-Ab/P1 | Peptide-Monoclonal antibody conjugate | [hGIPAb]-GGGGG-H-Aib-EGTFTSDVSSYLE-Aib-QAAKEFIAWLVKGGG | Preclinical | Amgen | Lu et al. (2021) |
| AMG133 | Peptide-Monoclonal antibody conjugate | N/A | Phase 2 to be completed 2025 | Amgen | Clinical Trial Identifier: NCT05669599 |
When assessed in the chronic setting over a 96-day dosing period in high-fat-diet-induced obese (DIO) mice, daily SKL-14959 administration reduced body mass by approximately 7%, an effect that appeared to be independent of food intake (Nakamura et al. 2018). Lack of SKL-14959 induced effects on feeding may potentially highlight inability of the molecule to penetrate the BBB and appetite controlling regions within the hypothalamus. In support of observations in the acute setting (Nakamura et al. 2012), triglyceride levels in liver, muscle and gastrointestinal muscle were reduced (Nakamura et al. 2018), although as the authors concede there is no report of GIPR expression in liver or muscle tissue in rodents (Usdin et al. 1993), indicating a likely indirect effect. That said, LPL activity was also reduced that would be linked to a reduction in tissue lipid uptake.
It is unclear why further study of SKL-14959 has not been pursued, especially given promising weight reducing effects in DIO rodents. The compound, which is of unknown structure, appears to be more tolerable than previous attempts of small molecule development against related G protein-coupled receptor (GPCR) targets such as glucagon, which despite clear benefits on diabetes in clinical trials (Cheng et al. 2020) were ultimately shelved due to hepatic impairment (Lafferty et al. 2022). However, it is noteworthy that despite almost identical binding affinities of SKL-14959 and (Pro3)GIP for the GIPR, a peptidic GIPR modulator (Gault et al. 2002) did not impart as significant a hyperglycaemic effect when administered daily to normal mice over a period of 11 days (Irwin et al. 2004), which is certainly more attractive when considering the target population for a GIPR antagonist treatment. This may have been an important consideration in the halting of development of SKL-14959. Moreover, (Pro3)GIP exerted antihyperglycaemic actions in genetically obese diabetic (ob/ob) as well as DIO mice (Gault et al. 2005, 2007b , Irwin et al. 2007a , McClean et al. 2007). That said, there are recent notable species-specific effects of GIPR modulating peptides that also need to be considered when interpreting effects of (Pro3)GIP (Sparre-Ulrich et al. 2016, Fig. 2), which will be considered in more detail next. Interestingly, 4-hydroxybenzoic acid 2-bromobenzylidene hydrazide (4H2BH) is a small-molecular-weight compound that has been reported to inhibit both GIPR and glucagon receptor activity (Franklin et al. 2011), with obvious dual benefits for obesity-related diabetes. However, 4H2BH has not been progressed beyond experiments assessing the impact a single injection in rodents, perhaps suggesting issues with pharmacokinetics and/or safety of this compound.
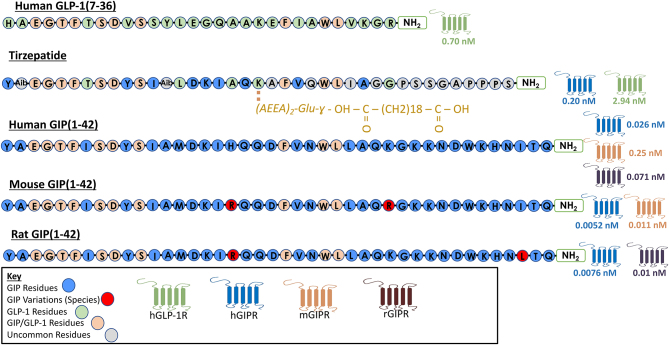
Figure 2.
A peptidic structure analysis of glucagon-like peptide-1 (GLP-1) (7–36), the dual GIP/GLP-1 receptor co-agonist tirzepatide and glucose-dependent insulinotropic peptide (GIP) (1–42). Structures for human, mouse and rat GIP(1-42) are provided. Amino acid residues are indicated by single-letter abbreviations. Residues shared with GIP(1–42) are shaded in blue, shared with GLP-1(7–36) are shaded in green, residues shared with both GLP-1 and GIP are indicated in orange and those unique to tirzepatide are shaded in grey. Additionally, species variations between GIP(1-42) are indicated in red. A 20-carbon fatty acid modification, namely eicosanedioic acid, is linked to Glu20 with the full structure provided in gold lettering. ‘Aib’ residues indicate inclusion of 2-aminoisobutyric acid, a non-naturally occurring amino acid. Potency at human GLP-1 and GIP receptors (hGLP-1R and hGIPR, respectively) as well as at mouse and rat GIP receptors (mGIPR and rGIPR) are provided, where appropriate, based on EC50 values provided within the literature for each peptide (Sparre-Ulrich et al. 2016, Willard et al. 2020).
GIPR immune-neutralisation
A number of studies have indicated that immunisation against GIP peptides, as a means to stimulate active endogenous antibody generation, is an efficacious method of improving obesity-related diabetes in rodents (Fulurija et al. 2008, Irwin et al. 2009a,b, 2012, Montgomery et al. 2010, Wolfe et al. 2023), although suitability of this approach in humans is yet to be determined. Indeed, given the various important physiological actions of GIP (Fig. 1), it may be anticipated that side effects could occur with this approach. Thus, more recent developments have employed administration of GIP monoclonal antibodies as a less permanent method of GIPR blockade, which should decrease side effects risk.
Passive immunity against GIP via administration of monoclonal antibodies (MABs) targeting endogenous GIP, or the GIPR, have proven effective in various studies, either alone or when combined with GLP-1R agonism. While GIP antibodies such as GIPg013 have been developed primarily as a research tool to assess the biological roles and actions of native GIP (Ravn et al. 2013, Table 1), others have been investigated as potential pharmacological interventions in obesity. For example, when a GIP MAB, namely GIP mAb (Table 1), targeting the last 17 residues of the C-terminus of murine GIP, was injected once weekly for 17 weeks in normal mice prior to exposure to a high-fat diet, these mice had a remarkable 47% weight loss when compared to untreated control animals (Boylan et al. 2015). This was associated with reductions in subcutaneous, abdominal and hepatic fat, with obvious improvements in overall metabolism (Boylan et al. 2015). Moreover, these initial findings have been endorsed using human GIPR MABs (hGIPR-Ab; Table 1) in non-human primates (NHP), indicating real potential for translation to the human setting (Killion et al. 2018). However, antibody monotherapy in NHPs elicited only a very modest weight reduction of 2%, but when combined with the GLP-1R mimetic dulaglutide, a 15% body weight reduction was observed, which was significantly beyond the 9% reduction achieved with dulaglutide monotherapy (Killion et al. 2018).
The apparent synergy between GLP-1R mimetics and GIPR antagonists has been exploited elsewhere in the pursuit of GIP MABs for obesity management (Fig. 1). An exciting new direction is the conjugation of GIPR MABs to peptidic GLP-1R agonists to create a unimolecular dual-acting compound. As such, a recent study by Lu and colleagues reports the successful development of murine and human- based GIPR MABs conjugated to GLP-1(7–37) analogues via a flexible (GGGGS)3 linker (Lu et al. 2021, Table 1). Administration of the murine-based compound, mGIPR-Ab/P1, over 18 days in DIO mice elicited a 29% reduction in body weight which was associated with reductions in hyperinsulinaemia and cholesterol (Lu et al. 2021). Moreover, when the antibody component was delivered alone, a body weight reduction of only 1% was observed, corroborating previous findings of Killion and colleagues on the synergy of this combination therapy approach (Killion et al. 2018). Encouragingly, the humanised version of this molecule, hGIPR-Ab/P1, elicited a 14% body weight reduction following 6 weeks administration in obese NHPs although alterations in insulin and cholesterol were less evident than in the corresponding rodent study (Lu et al. 2021, Table 1). Mechanistic in vitro studies with hGIPR-Ab/P1 indicate a 100-fold increase in cAMP generation when exposed to cells expressing both the GIPR and GLP-1R, when compared to cells expressing one or the other receptor, manifesting in upregulated insulin secretion from INS1 832/3 beta cells (Lu et al. 2021). It is thought that dimerisation of the GIPR and GLP-1R in tissues that co-express these receptors allows hGIPR-Ab/P1 to bind simultaneously to both targets and elicit a heightened effect (Whitaker et al. 2012, Lu et al. 2021). While Lu and colleagues have evidenced this in pancreatic tissues, where GIPR and GLP1-R expression is known to be abundant (Irwin & Flatt 2009a), further work is required to confirm the phenomenon in other metabolically relevant sites such as the hypothalamus.
The excellent transition of this dual GIP MAB and GLP-1R agonist approach to NHPs validates appraising this paradigm in the human setting (Fig. 1). Recently, Amgen have completed a phase 1 trial of a GLP-1R mimetic–GIPR antibody conjugate molecule, termed AMG133, which elicited a 15% reduction in body weight over 85 days in its cohort of participants with obesity when administered at the highest dose of 420 mg monthly (Véniant et al. 2024, clinical trial identifier: NCT04478708). Excitingly, for subjects receiving either of the highest doses of AMG133 (280 or 420 mg, respectively), 10% body weight was maintained after 150 days of withdrawal (Véniant et al. 2024). However, given the lack of comparison against tirzepatide and given the relatively small cohort size, excitement will have to remain tempered for now. A phase 2 study investigating the compound in obese individuals with or without T2DM is currently underway and the results are awaited with great anticipation, estimated to be published in early 2025 (clinical trial identifier: NCT05669599), with hope that answers around the potential for more sustained weight loss with AMG133 can be answered through this trial.
Peptide-derived GIPR antagonism
Although notable success to annul GIPR signalling has been made with small molecule GIPR modulators and immune neutralisation, this would appear to be more achievable and probably safer through utilising peptide-based ligands of the endogenous receptor. Thus, peptide screening processes such as alanine scanning, or in silico molecular conformational software, allows determination of important amino acid residues for peptide activity as well as receptor recognition and binding (Lee et al. 2019). In the case of GIP, modification to central amino acid residues is better tolerated for maintaining GIPR agonistic properties than those at the N-terminus (Alaña et al. 2006, Venneti et al. 2011, Table 1; Fig. 2), with the N-terminus being fundamental for GIPR agonist activity (Hinke et al. 2001, Kerr et al. 2011, Hansen et al. 2016). It is perhaps not surprising that the naturally occurring DPP-4 cleavage products of full length and truncated GIP, namely GIP(3–42) and GIP(3–30) respectively, are antagonists of the GIPR when employed at supraphysiological concentrations (Parker et al. 2006, Hansen et al. 2016, Table 1). However, at normal circulating concentrations, neither metabolite is thought to have an appreciable impact upon GIPR function and overall metabolism (Deacon et al. 2006). Interestingly, further N-terminally truncated GIP metabolites have also been established to possess GIPR antagonistic properties, including GIP(4–42), GIP(5–42), GIP(6–42), GIP(7–42) and GIP(8–42) (Kerr et al. 2011, Table 1).
The C-terminally truncated GIP(1–30), found in intestinal K-cells (Fujita et al. 2010), has been shown to have similar potency as GIP(1–42) in acute and longer-term studies (Fujita et al. 2010, Gault et al. 2011). The fragment form, GIP(7–30)NH2, was the earliest GIPR antagonist used and effectively demonstrated the importance of GIPR signalling in the insulin response to oral glucose in rats (Tseng et al. 1999). However, of the various truncated metabolites, GIP(3–30) is believed to be a highly effective naturally occurring GIPR antagonist (Hansen et al. 2016), being superior to GIP(3–42) in terms of inhibiting GIP-induced insulin, glucagon and somatostatin release in vitro and in the perfused rat pancreas (Sparre-Ulrich et al. 2017). Indeed, GIP(3–30)NH2 was the first GIPR antagonist to be utilised in human studies and shown to reduce the GSIS effects of GIP by 82% in healthy volunteers (Gasbjerg et al. 2018). Interestingly, no influence on circulating lipid levels was observed following acute GIP(3–30)NH2 infusion, but this may be linked to use of a single infusion and the fact that volunteers were healthy (Gasbjerg et al. 2018).
Interestingly, whilst the aforementioned peptidic GIPR antagonists have either N- or C-terminal truncation of the GIP amino acid sequence, or a combination of both, an analogue of GIP(1–42), namely DPP-4 resistant (Pro3)GIP, appeared to break this mould (Gault et al. 2002, Table 1). (Pro3)GIP effectively antagonised cAMP-stimulatory action of GIP in vitro with an IC50 of 2.6 µM whilst also impeding GIP-induced insulin secretion during a glucose tolerance test (GTT) in ob/ob mice (Gault et al. 2002). In addition, related Glu3-substituted analogues of GIP(1–42) were also shown to possess postulated GIPR inhibitory actions (Table 1; O’Harte et al. 2006, Gault et al. 2007a). More intensive study of (Pro3)GIP followed, which demonstrated that non-fasting glucose and plasma insulin levels as well as GSIS were not impacted following 11-days once-daily administration of the peptide in non-diabetic mice, thereby suggesting possible compensation by endogenous GLP-1 (Irwin et al. 2004). Indeed, GIPR knock-out mice exhibit increased islet sensitivity to GLP-1 (Pamir et al. 2003), with once daily injection of (Pro3)GIP for 50 days in DIO mice increasing circulating total GLP-1 concentrations (McClean et al. 2007). Additional investigations with (Pro3)GIP revealed prominent amelioration of insulin resistance and substantial improvements of overall metabolism in ob/ob (Gault et al. 2005, Irwin et al. 2007a) as well as DIO mice (Gault et al. 2007b). Interestingly, benefits were largely absent in streptozotocin (STZ)-treated insulin-deficient mice (McClean et al. 2008a), suggesting positive effects to be insulin dependent. Encouraging effects on metabolism were also observed when (Pro3)GIP was combined with other therapies, such as PYY(3–36) (Irwin et al. 2007b), cannabinoid CB1 receptor antagonism (Irwin et al. 2008), and cholecystokinin (CCK) receptor activation (Irwin et al. 2013) as well as GLP-1R agonism (Irwin et al. 2009b), in keeping with observations of the marked benefits of AMG133 noted previously. Although (Pro3)GIP analogues with a protracted duration of biological action have also been characterised (Gault et al 2007a, McClean et al. 2008b), subsequent study revealed differences in the affinity of (Pro3)GIP for human and rodent GIPRs and the occurrence of noteworthy species-specific effects of GIP peptides as described further next.
Species specificity of GIP peptides
There are small, but seemingly important, differences in the sequence of human and rodent GIP, with the human GIP sequence specifically containing His18, Lys30 and Ile40 amongst its 42 amino acid residues, which are substituted with Arg18 and Arg30 in mouse GIP and then Arg18 and Leu40 in rat GIP (Bailey 2020, Fig. 2). In agreement, a recent report further highlights physiologically important species- and population-specific evolutionary conservation of the GIP peptide amino acid sequence (Lindquist et al. 2022, Fig. 2), although there is less certainty around importance of conservation of the GIPR sequence (Irwin 2020). Indeed (Pro3)GIP, that is based on the human GIP amino acid sequence, was shown to display greater affinity for human than mouse or rat GIPRs in transfected cell lines (Sparre-Ulrich et al. 2016, Fig. 2), being considered as a low potency GIPR agonist as opposed to a full antagonist (Sparre-Ulrich et al. 2016). This suggests that diminished GIP action rather than total GIPR blockade may be sufficient to impart the positive effects in obesity (Gault et al. 2005, 2007b, Irwin et al. 2007a, McClean et al. 2007). Indeed, this fits well with the clear benefits of GIP immune-neutralisation described earlier, that is unlikely to induce total blockade of GIP action. Interspecies variations of the human, rat and mouse GIP(3–30) sequences have been additionally confirmed through their GIPR antagonist capabilities, with each peptide recognised as a true competitive GIPR antagonist only within their respective parent systems (Gabe et al. 2018, Perry et al. 2019). Furthermore, whilst the GIPg013 GIPR antibody reported earlier effectively antagonised mouse, rat dog, and human GIPRs, a closely related GIPR antibody, Gipg133, had no GIPR antagonist activity at mouse and rat GIPRs (Ravn et al. 2013).
Despite concerns over species variation (Fig. 2), human (Pro3)GIP(3–30)-based peptides have since been described that possess full GIPR antagonist activity in rodent systems (Pathak et al. 2005, Table 1). This discovery shadowed initial findings with a GIP(3–30) based-peptide, namely GIP(3–30)-Cex-K40PAL, that combines GIP(3–30) with the nine C-terminal residues of the GLP-1R agonist, exendin(1–39) (Pathak et al. 2015a, Table 1), where this C-terminal extension, Cex, was previously demonstrated to improve metabolic stability and reduce renal clearance of GLP-1 peptides (Simonsen et al. 2013). An additional C-terminal lysine residue was also attached to the molecule at position 40, K40PAL, to facilitate attachment of a C-16 fatty acid that prolongs the bioactivity profile (Pathak et al. 2015a, Table 1). Modification to the C-terminus of GIP has previously been reported to interfere less with ligand-receptor binding (Hinke et al. 2001). As an additional means to ensure adequate receptor engagement, the molecule was also N-terminally capped with phenyl lactic acid to preserve the helical structure (Doig & Baldwin 1995, Pathak et al. 2015a). Importantly, GIP(3–30)-Cex-K40PAL and the related Pro3GIP(3–30)-Cex-K40PAL molecules were determined to effectively antagonise the actions of native GIP with nanomolar potency, specifically in terms of cAMP recruitment in human GIPR-transfected Chinese Hamster Lung cells in addition to a reduction of GIP-induced insulin secretion from rodent BRIN-BD11 cells (Pathak et al. 2015a). More significantly, the GIPR antagonist peptides were then assessed in DIO mice and shown to induce sustained weight loss, counter insulin resistance and improve glycaemic control following once daily injection for 21 days (Pathak et al. 2015a). Indeed, Pro3GIP(3–30)-Cex-K40PAL was the better performing of the two peptides and body weight at study termination in this group of mice was not significantly different from non-obese controls. It is also interesting to note that these mice presented with a reduction of fat mass, but no obvious impact on lean mass, indicating an appropriate manner of weight loss (Pathak et al. 2015a), that may not be the case with some GLP-1 mimetics (Lafferty et al. 2023). Generally speaking, the phenotype induced by GIP(3–30)-Cex-K40PAL and Pro3GIP(3–30)-Cex-K40PAL were similar to those observed previously with Pro3GIP (McClean et al. 2007).
Finally, a related GIPR antagonist peptide, namely GIP(6–30)Cex-K40PAL, exhibited profound benefits on metabolism in diabetic db/db mice, but particularly so when added to liraglutide treatment (Pathak et al. 2015b), further supporting the notion of the significant therapeutic promise of combined GIPR antagonism and GLP-1 agonism (Fig. 1). Interestingly, there is a school of thought that GIPR antagonism can impart beta-cell resting benefits (Gault et al. 2005, Tanday et al. 2022), to help protect chronically over-activated beta cells and prevent their apoptosis (Fig. 1). In this respect, it is notable that GIP(6–30)Cex-K40PAL and liraglutide were administered sequentially in db/db mice by Pathak and colleagues to impart scheduled periods of beta-cell rest and activation (Pathak et al. 2015b), which may represent a treatment paradigm worthy of further consideration.
More recently another acylated GIPR antagonist peptide has been reported in the literature, termed (Nα-Ac, L14, R18, E21) hGIP(5–31)-K11(γE-C16) (Yang et al. 2022, Table 1). This GIP(5–31) analogue seems to have directly arisen from an earlier reported C-terminally intact GIP analogue, namely NαAc, K10(γEγE-C16), R18, hGIP(5–42), which was studied in a head-to-head comparison with GIPR agonist peptides (Mroz et al. 2019, Table 1). (Nα-Ac, L14, R18, E21) hGIP(5–31)-K11(γE-C16) was shown to exert modest reductions of food intake and body weight following 27 days administration in DIO mice (Yang et al. 2022). More interestingly, when combined with the GLP-1R agonist, semaglutide, these mice displayed increased appetite suppression and body weight loss as well as a modest improvement of glucose tolerance when compared to semaglutide monotherapy (Yang et al. 2022), in good support of previous observations utilising this GLP-1R agonism and GIPR antagonism treatment paradigm (Pathak et al. 2015b; Fig. 1). To date, none of these longer acting, acylated GIPR antagonist peptides have been evaluated in humans, but the use of suitably characterised humanised versions may represent the next major advancement in this area of research.
Does prolonged GIPR agonism equate to antagonism?
Considerable excitement has surrounded the emergence and clinical approval of tirzepatide, the first-in-class GIPR/GLP-1R co-agonist peptide. Tirzepatide was shown to elicit up to 10% body weight reduction and substantial decrease in waist circumference following 12 weeks administration in T2DM patients during phase 2 trials (Frías et al. 2021), manifesting in an average weight loss of 11.2 kg in the group receiving the highest dose of tirzepatide compared to an average weight loss of 5.7 kg in participants receiving semaglutide. There may be a further benefit in this combination owing to a proposed anti-emetic effect of GIPR activation at the area postrema (Borner et al. 2021), which is the vomiting centre of the brain and could alleviate nauseating side effects of GLP-1R agonists.
Despite these marked benefits, the precise mechanism of action of tirzepatide remains unclear. The peptide amino acid sequence bears a striking resemblance to human GIP, in keeping with the strong preference of tirzepatide towards the GIPR over the GLP-1R (Willard et al. 2020, Fig. 2). Fascinatingly, there is a growing body of evidence suggesting that prolonged GIPR activation desensitises the GIPR in vitro, essentially then mimicking GIPR antagonism (Campbell 2021, Gasbjerg et al. 2023b). This desensitisation is postulated to be the result of reduced GIPR recycling to the cell-membrane surface following initial activation and internalisation, as evidenced in 3T3-L1 adipocytes (Mohammad et al. 2014), as well as primary rodent adipose tissue exposed to a long-acting GIPR agonist for 24 h (Killion et al. 2020a). While ex vivo confirmation of GIPR desensitisation following prolonged GIPR agonism in adipocytes indicates relevance of this phenomenon in vivo (Killion et al. 2020a), comparable data in other tissues is currently lacking. Moreover, prolonged GIPR agonism exerted clear benefits on bone strength and composition in insulin-resistant high-fat-fed mice (Vyavahare et al. 2020), as well as improving cognition (Siano et al. 2011), suggesting lack of receptor desensitisation in these tissues. This is strengthened by studies in type 2 diabetic patients, where GIPR agonism clearly reduces bone resorption (Christensen et al. 2020) despite postulated beta-cell insensitivity to GIP in this population (Nauck et al. 1993). However, the desensitisation perspective is supported by a study comparing administration of a long-acting GIPR agonist and a GIPR mAb in DIO mice, where both approaches elicited almost identical reductions in body weight when employed as monotherapy (< 5%) or when combined with liraglutide (~20%) (Killion et al. 2020a). Moreover, recent evidence appears to indicate that the human GIPR may be more prone to desensitisation through internalisation than the murine GIPR (Gasbjerg et al 2023a), that may be an important consideration when evaluating the translational applicability of GIPR antagonists.
Further to this, biased agonism at the level of GLP-1R has also been suggested with tirzepatide (Xiao et al 2023) that leads to favoured cAMP generation over β-arrestin recruitment for GLP-1R but not GIPR, activation (Gasbjerg et al 2023b). However, this needs to be considered in the context that tirzepatide binds with greater preference to GIP rather than GLP-1 receptors (Willard et al. 2020). GIPR desensitisation also appears to hold more weight to its argument than the theory of incretin receptor compensation, which surmises that when either incretin receptor is knocked out, sensitivity for the opposite hormone is improved (Campbell 2021), as is its therapeutic effect. However, double incretin-receptor knock-out mice also display reduced weight gain when exposed to a high-fat diet (Hansotia et al. 2007). Thus, while a compensatory phenomenon may play a partial role when antagonising a singular receptor, it is unlikely to be the primary mechanism at play when considering the therapeutic benefits GIPR antagonism in combination with GLP-1R agonism.
Further research and mode of action of tirzepatide
The remarkable story of tirzepatide has substantially reinvigorated interest in the GIPR as a drug target. However, more research is needed to determine the molecular action of this dual GIP/GLP-1 analogue and to assess whether GIPR agonism in this context equates to antagonism. Our laboratory at Ulster has been a strong advocate for the exploitation of GIP therapeutics since the late 1990s, providing substantial evidence for beneficial metabolic effects of both agonism and antagonism of the GIPR (Gault et al. 2003b,c, Irwin & Flatt 2009a). This position appeared counterintuitive to many (Meier & Nauck 2004, Seino et al. 2010), but may now have greater appeal, given that the action of tirzepatide is believed to possibly involve desensitisation of the GIPR (Gasbjerg et al. 2023a). Thus, as evident from our early preclinical studies (Gault et al. 2005, McClean et al. 2007), diminished action or antagonism of GIP substantially decreases obesity-driven insulin resistance by depleting liver triglycerides, reducing adiposity, and thereby substantially diminishing insulin demand with the induction of beneficial beta-cell rest and decreased circulating insulin. As noted above, subsequent studies using our next generation of GIP antagonist peptides, namely GIP(3–30)-Cex-K40PAL, Pro3GIP(3–30)-Cex-K40PAL or GIP(6–30)Cex-K40PAL, evoked remarkably similar effects (Pathak et al. 2015a,b). This scenario clearly contrasts with the more obvious and predominantly insulin-releasing incretin effects triggered by GIP agonism, which would predominate if tirzepatide acted simply as a dual GIP/GLP-1 agonist. Another mechanistic pathway postulated recently by Gasbjerg and colleagues is that tirzepatide acts solely as a GLP-1R super agonist (Gasbjerg et al. 2023b), but this possibility seems less appealing based on the more impressive effects of tirzepatide over those of GLP-1 mimetics in both humans and animal models with obesity-diabetes (Coskun et al. 2018, Frias et al. 2020b). Additionally, the aforementioned binding preference of tirzepatide towards the GIPR, and high sequence homology with native GIP, would tend to cast aspersions on this argument (Willard et al. 2020, Fig. 2). Future acute studies in man looking at the efficacy of tirzepatide alone and in combination with the GIPR antagonist GIP(3–30) or the GLP-1R antagonist exendin(9–39) can be expected to help to resolve this issue and shape the development of future GIP-incorporating compounds.
Conclusion
The resounding therapeutic success of the GIPR/GLP-1R co-agonist, tirzepatide, has seen a resurgence of interest in GIPR modulation for the management of T2DM and obesity (Nauck & Müller 2023). While it appears that the pendulum has begun to swing in favour of GIPR agonism over GIPR antagonism for imparting metabolic benefits, significant debate remains given recent evidence that prolonged GIPR activation may lead to GIPR desensitisation (Killion et al. 2020a,b), particularly in the human setting (Gasbjerg et al 2023a). This becomes even more relevant given the receptor preference of tirzepatide towards the GIPR (Willard et al. 2020). In addition to this, it is apparent that metabolic advantages of GIPR antagonism can be enhanced through concomitant GLP-1R activation (Fig. 1), as evidenced by preclinical studies (Gault et al. 2005, 2007a, b, 2011, Irwin et al. 2009a,b, Pathak et al. 2015a,b) and progression of the conjugated MAB GIPR antagonist/GLP-1R agonist therapy, AMG133 (Véniant et al. 2024), to phase 2 clinical trials. Finally, the organ-specific effects of GIPR agonism and antagonism must be ascertained, particularly in relation to desensitisation given the wealth of evidence supporting a central mechanism for GIPR-agonist-induced reductions in food intake (Seino et al. 1997, Adriaenssens et al. 2019, Samms et al. 2020), but with no current evidence supporting a role for desensitisation of GIPR in appetite-regulating centres of the brain through prolonged exposure. Thus, with continuing development on GIP/GLP-1R co-agonists modalities (Lafferty et al. 2023), it is hoped that clarity can be ascertained as to whether GIPR agonism or antagonism has the greatest role to play in management of obesity and related metabolic diseases.
Declaration of interests
PRF, VAG and NI are named on patents filed by Ulster University for the exploitation of incretin-based drugs and other peptide therapeutics. RAL, VAG, PRF and NI are shareholders in Dia Beta Labs Ltd., an Ulster University spinout developing peptide therapeutics for the management of metabolic disease.
Funding
This work did not receive any specific grant from any funding agency in the public, commercial, or not-for-profit sector.
References
- Adriaenssens AE, Biggs EK, Darwish T, Tadross J, Sukthankar T, Girish M, Polex-Wolf J, Lam BY, Zvetkova I, Pan W, et al.2019Glucose-dependent insulinotropic polypeptide receptor-expressing cells in the hypothalamus regulate food intake. Cell Metabolism 30987–996.e6. ( 10.1016/j.cmet.2019.07.013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adriaenssens A, Broichhagen J, de Bray A, Ast J, Hasib A, Jones B, Tomas A, Burgos NF, Woodward O, Lewis J.et al.2023Hypothalamic and brainstem glucose-dependent insulinotropic polypeptide receptor neurons employ distinct mechanisms to affect feeding. JCI Insight 8e164921. ( 10.1172/jci.insight.164921) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alaña I Parker JC Gault VA Flatt PR O’Harte FPM Malthouse JPG & Hewage CM. 2006NMR and alanine scan studies of glucose-dependent insulinotropic polypeptide in water. Journal of Biological Chemistry 28116370–16376. ( 10.1074/jbc.M510414200) [DOI] [PubMed] [Google Scholar]
- Althage MC Ford EL Wang S Tso P Polonsky KS & Wice BM. 2008Targeted ablation of glucose-dependent insulinotropic polypeptide-producing cells in transgenic mice reduces obesity and insulin resistance induced by a high fat diet. Journal of Biological Chemistry 28318365–18376. ( 10.1074/jbc.M710466200) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asmar M Asmar A Simonsen L Dela F Holst JJ & Bülow J. 2019GIP-induced vasodilation in human adipose tissue involves capillary recruitment. Endocrine Connections 8806–813. ( 10.1530/EC-19-0144) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey CJ.2020GIP analogues and the treatment of obesity-diabetes. Peptides 125170202. ( 10.1016/j.peptides.2019.170202) [DOI] [PubMed] [Google Scholar]
- Bailey CJ Flatt PR Kwasowski P Powell CJ & Marks V. 1986Immunoreactive gastric inhibitory polypeptide and K cell hyperplasia in obese hyperglycaemic (ob/ob) mice fed high fat and high carbohydrate cafeteria diets. Acta Endocrinologica 112224–229. ( 10.1530/acta.0.1120224) [DOI] [PubMed] [Google Scholar]
- Beck H Härter M Haß B Schmeck C & Baerfacker L. 2022Small molecules and their impact in drug discovery: a perspective on the occasion of the 125th anniversary of the Bayer Chemical Research Laboratory. Drug Discovery Today 271560–1574. ( 10.1016/j.drudis.2022.02.015) [DOI] [PubMed] [Google Scholar]
- Borner T Geisler CE Fortin SM Cosgrove R Alsina-Fernandez J Dogra M Doebley S Sanchez-Navarro MJ Leon RM Gaisinsky Jet al. 2021GIP receptor agonism attenuates GLP-1 receptor agonist-induced nausea and emesis in preclinical models. Diabetes 702545–2553. ( 10.2337/db21-0459) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boylan MO Glazebrook PA Tatalovic M & Wolfe MM. 2015Gastric inhibitory polypeptide immunoneutralization attenuates development of obesity in mice. American Journal of Physiology, Endocrinology and Metabolism 309E1008–E1018. ( 10.1152/ajpendo.00345.2015) [DOI] [PubMed] [Google Scholar]
- Brown JC.1982Gastric Inhibitory Polypeptide, Monographs on Endocrinology, Vol. 24, pp. 1–88. Berlin, Germany: Springer-Verlag. [PubMed] [Google Scholar]
- Brown JC Pederson RA Jorpes E & Mutt V. 1969Preparation of highly active enterogastrone. Canadian Journal of Physiology and Pharmacology 47113–114. ( 10.1139/y69-020) [DOI] [PubMed] [Google Scholar]
- Brown JC Mutt V & Pederson RA. 1970Further purification of a polypeptide demonstrating enterogastrone activity. Journal of Physiology 20957–64. ( 10.1113/jphysiol.1970.sp009155) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchan AMJ Polak JM Capella C Solcia E & Pearse AGE. 1978Electronimmunocytochemical evidence for the K cell localization of gastric inhibitory polypeptide (GIP) in man. Histochemistry 5637–44. ( 10.1007/BF00492251) [DOI] [PubMed] [Google Scholar]
- Bucheit JD Pamulapati LG Carter N Malloy K Dixon DL & Sisson EM. 2020Oral semaglutide: a review of the first oral glucagon-like peptide 1 receptor agonist. Diabetes Technology and Therapeutics 2210–18. ( 10.1089/dia.2019.0185) [DOI] [PubMed] [Google Scholar]
- Campbell JE.2021Targeting the GIPR for obesity: to agonize or antagonize? Potential mechanisms. Molecular Metabolism 46101139. ( 10.1016/j.molmet.2020.101139) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell JE, Beaudry JL, Svendsen B, Baggio LL, Gordon AN, Ussher JR, Wong CK, Gribble FM, D’Alessio DA, Reimann F, et al.2022GIPR is predominantly localized to nonadipocyte cell types within white adipose tissue. Diabetes 711115–1127. ( 10.2337/db21-1166) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, He X, Guo Y, Liu L, Li H, Tan J, Feng W, Guan H, Cao X, Xiao H, et al.2021Glucose-dependent insulinotropic polypeptide modifies adipose plasticity and promotes beige adipogenesis of human omental adipose-derived stem cells. FASEB Journal 35e21534. ( 10.1096/fj.201903253R) [DOI] [PubMed] [Google Scholar]
- Cheng C Jabri S Taoka BM & Sinz CJ. 2020Small molecule glucagon receptor antagonists: an updated patent review (2015–2019). Expert Opinion on Therapeutic Patents 30509–526 ( 10.1080/13543776.2020.1769600) [DOI] [PubMed] [Google Scholar]
- Christensen MB Lund AB Jørgensen NR Holst JJ Vilsbøll T & Knop FK. 2020Glucose-dependent insulinotropic polypeptide (GIP) reduces bone resorption in patients with type 2 diabetes. Journal of the Endocrine Society 4bvaa097. ( 10.1210/jendso/bvaa097) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coskun T, Sloop KW, Loghin C, Alsina-Fernandez J, Urva S, Bokvist KB, Cui X, Briere DA, Cabrera O, Roell WC, et al.2018LY3298176, a novel dual GIP and GLP-1 receptor agonist for the treatment of type 2 diabetes mellitus: from discovery to clinical proof of concept. Molecular Metabolism 183–14. ( 10.1016/j.molmet.2018.09.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deacon CF Plamboeck A Rosenkilde MM de Heer J & Holst JJ. 2006GIP-(3–42) does not antagonize insulinotropic effects of GIP at physiological concentrations. American Journal of Physiology, Endocrinology and Metabolism 291E468–E475. ( 10.1152/ajpendo.00577.2005) [DOI] [PubMed] [Google Scholar]
- Ding WG & Gromada J. 1997Protein kinase A-dependent stimulation of exocytosis in mouse pancreatic beta-cells by glucose-dependent insulinotropic polypeptide. Diabetes 46615–621. ( 10.2337/diab.46.4.615) [DOI] [PubMed] [Google Scholar]
- Doig AJ & Baldwin RL. 1995N- and C-capping preferences for all 20 amino acids in alpha-helical peptides. Protein Science 41325–1336. ( 10.1002/pro.5560040708) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert R & Creutzfeldt W. 1980Gastric inhibitory polypeptide. Clinics in Gastroenterology 9679–698. ( 10.1016/S0300-5089(2100478-8) [DOI] [PubMed] [Google Scholar]
- English A Craig SL Flatt PR & Irwin N. 2020Individual and combined effects of GIP and xenin on differentiation, glucose uptake and lipolysis in 3T3-L1 adipocytes. Biological Chemistry 4011293–1303. ( 10.1515/hsz-2020-0195) [DOI] [PubMed] [Google Scholar]
- Flatt PR.2008Dorothy Hodgkin Lecture 2008. Gastric inhibitory polypeptide (GIP) revisited: a new therapeutic target for obesity-diabetes? Diabetic Medicine 25759–764. ( 10.1111/j.1464-5491.2008.02455.x) [DOI] [PubMed] [Google Scholar]
- Flatt PR Bailey CJ Kwasowski P Swanston-Flatt SK & Marks V. 1983Abnormalities of GIP in spontaneous syndromes of obesity and diabetes in mice. Diabetes 32433–435. ( 10.2337/diab.32.5.433) [DOI] [PubMed] [Google Scholar]
- Franklin ZJ McDonnell B Montgomery IA Flatt PR & Irwin N. 2011Dual modulation of GIP and glucagon action by the low molecular weight compound 4-hydroxybenzoic acid 2-bromobenzylidene hydrazide. Diabetes, Obesity and Metabolism 13742–749. ( 10.1111/j.1463-1326.2011.01401.x) [DOI] [PubMed] [Google Scholar]
- Frias JP Nauck MA Van J Benson C Bray R Cui X Milicevic Z Urva S Haupt A & Robins DA. 2020Efficacy and tolerability of tirzepatide, a dual glucose-dependent insulinotropic peptide and glucagon-like peptide-1 receptor agonist in patients with type 2 diabetes: a 12-week, randomized, double-blind, placebo-controlled study to evaluate different dose-escalation regimens. Diabetes, Obesity and Metabolism 22938–946. ( 10.1111/dom.13979) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frías JP, Davies MJ, Rosenstock J, Pérez Manghi FC, Fernández Landó L, Bergman BK, Liu B, Cui X, Brown K. & SURPASS-2 Investigators 2021Tirzepatide versus semaglutide once weekly in patients with type 2 diabetes. New England Journal of Medicine 385503–515. ( 10.1056/NEJMoa2107519) [DOI] [PubMed] [Google Scholar]
- Fujita Y Asadi A Yang GK Kwok YN & Kieffer TJ. 2010Differential processing of pro-glucose-dependent insulinotropic polypeptide in gut. American Journal of Physiology, Gastrointestinal and Liver Physiology 298G608–G614. ( 10.1152/ajpgi.00024.2010) [DOI] [PubMed] [Google Scholar]
- Fulurija A Lutz TA Sladko K Osto M Wielinga PY Bachmann MF & Saudan P. 2008Vaccination against GIP for the treatment of obesity. PLoS One 3e3163. ( 10.1371/journal.pone.0003163) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabe MBN Sparre-Ulrich AH Pedersen MF Gasbjerg LS Inoue A Bräuner-Osborne H Hartmann B & Rosenkilde MM. 2018Human GIP(3-30)NH2 inhibits G protein-dependent as well as G protein-independent signaling and is selective for the GIP receptor with high-affinity binding to primate but not rodent GIP receptors. Biochemical Pharmacology 15097–107. ( 10.1016/j.bcp.2018.01.040) [DOI] [PubMed] [Google Scholar]
- Gasbjerg LS, Christensen MB, Hartmann B, Lanng AR, Sparre-Ulrich AH, Gabe MBN, Dela F, Vilsbøll T, Holst JJ, Rosenkilde MM, et al.2018GIP(3-30)NH2 is an efficacious GIP receptor antagonist in humans: a randomised, double-blinded, placebo-controlled, crossover study. Diabetologia 61413–423. ( 10.1007/s00125-017-4447-4) [DOI] [PubMed] [Google Scholar]
- Gasbjerg LS Rasmussen R Dragan A Lindquist P Melchiorsen J Schiellerup S Tordrup E Gadgaard S Kizilkaya H Hartmann Bet al. 2023aAltered desensitization and internalization patterns of rodent versus human GIP receptors – a major drug discovery challenge. Authorea [epub]. ( 10.22541/au.168837507.72959473/v1) [DOI] [PubMed] [Google Scholar]
- Gasbjerg LS Rosenkilde MM Meier JJ Holst JJ & Knop FK. 2023bThe importance of glucose-dependent insulinotropic polypeptide receptor activation for the effects of tirzepatide. Diabetes, Obesity and Metabolism 253079–3092. ( 10.1111/dom.15216) [DOI] [PubMed] [Google Scholar]
- Gault VA O’Harte FP Harriott P & Flatt PR. 2002Characterization of the cellular and metabolic effects of a novel enzyme-resistant antagonist of glucose-dependent insulinotropic polypeptide. Biochemical and Biophysical Research Communications 2901420–1426. ( 10.1006/bbrc.2002.6364) [DOI] [PubMed] [Google Scholar]
- Gault VA O’Harte FP Harriott P Mooney MH Green BD & Flatt PR. 2003aEffects of the novel (Pro3)GIP antagonist and exendin(9-39)amide on GIP- and GLP-1-induced cyclic AMP generation, insulin secretion and postprandial insulin release in obese diabetic (ob/ob) mice: evidence that GIP is the major physiological incretin. Diabetologia 46222–230. ( 10.1007/s00125-002-1028-x) [DOI] [PubMed] [Google Scholar]
- Gault VA Flatt PR & O’Harte FPM. 2003bGlucose-dependent insulinotropic polypeptide analogues and their therapeutic potential for the treatment of obesity-diabetes. Biochemical and Biophysical Research Communications 308207–213. ( 10.1016/s0006-291x(0301361-5) [DOI] [PubMed] [Google Scholar]
- Gault VA O’Harte FP & Flatt PR. 2003cGlucose-dependent insulinotropic polypeptide (GIP): anti-diabetic and anti-obesity potential? Neuropeptides 37253–263. ( 10.1016/j.npep.2003.09.002) [DOI] [PubMed] [Google Scholar]
- Gault VA Irwin N Green BD McCluskey JT Greer B Bailey CJ Harriott P O’Harte FP & Flatt PR. 2005Chemical ablation of gastric inhibitory polypeptide receptor action by daily (Pro3)GIP administration improves glucose tolerance and ameliorates insulin resistance and abnormalities of islet structure in obesity-related diabetes. Diabetes 542436–2446. ( 10.2337/diabetes.54.8.2436) [DOI] [PubMed] [Google Scholar]
- Gault VA Hunter K Irwin N Green BD Greer B Harriott P O’Harte FP & Flatt PR. 2007aCharacterisation and biological activity of Glu3 amino acid substituted GIP receptor antagonists. Archives of Biochemistry and Biophysics 461263–274. ( 10.1016/j.abb.2007.03.001) [DOI] [PubMed] [Google Scholar]
- Gault VA McClean PL Cassidy RS Irwin N & Flatt PR. 2007bChemical gastric inhibitory polypeptide receptor antagonism protects against obesity, insulin resistance, glucose intolerance and associated disturbances in mice fed high-fat and cafeteria diets. Diabetologia 501752–1762. ( 10.1007/s00125-007-0710-4) [DOI] [PubMed] [Google Scholar]
- Gault VA Hunter K Irwin N Greer B Green BD Harriott P O’Harte FP & Flatt PR. 2007cCharacterisation and glucoregulatory actions of a novel acylated form of the (Pro3)GIP receptor antagonist in type 2 diabetes. Biological Chemistry 388173–179. ( 10.1515/BC.2007.019) [DOI] [PubMed] [Google Scholar]
- Gault VA Porter DW Irwin N & Flatt PR. 2011Comparison of sub-chronic metabolic effects of stable forms of naturally occurring GIP(1–30) and GIP(1–42) in high-fat fed mice. Journal of Endocrinology 208265–271. ( 10.1530/JOE-10-0419) [DOI] [PubMed] [Google Scholar]
- Getty-Kaushik L Song DH Boylan MO Corkey BE & Wolfe MM. 2006Glucose-dependent insulinotropic polypeptide modulates adipocyte lipolysis and reesterification. Obesity 141124–1131. ( 10.1038/oby.2006.129) [DOI] [PubMed] [Google Scholar]
- Hansen LS Sparre-Ulrich AH Christensen M Knop FK Hartmann B Holst JJ & Rosenkilde MM. 2016N-terminally and C-terminally truncated forms of glucose-dependent insulinotropic polypeptide are high-affinity competitive antagonists of the human GIP receptor. British Journal of Pharmacology 173826–838. ( 10.1111/bph.13384) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansotia T Maida A Flock G Yamada Y Tsukiyama K Seino Y & Drucker DJ. 2007Extrapancreatic incretin receptors modulate glucose homeostasis, body weight, and energy expenditure. Journal of Clinical Investigation 117143–152. ( 10.1172/JCI25483) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinke SA Manhart S Pamir N Demuth H W Gelling R Pederson RA & McIntosh CH. 2001Identification of a bioactive domain in the amino-terminus of glucose-dependent insulinotropic polypeptide (GIP). Biochimica et Biophysica Acta 1547143–155. ( 10.1016/s0167-4838(0100181-9) [DOI] [PubMed] [Google Scholar]
- Højberg PV Vilsbøll T Rabøl R Knop FK Bache M Krarup T Holst JJ & Madsbad S. 2009Four weeks of near-normalisation of blood glucose improves the insulin response to glucagon-like peptide-1 and glucose-dependent insulinotropic polypeptide in patients with type 2 diabetes. Diabetologia 52199–207. ( 10.1007/s00125-008-1195-5) [DOI] [PubMed] [Google Scholar]
- Holst JJ.2019The incretin system in healthy humans: the role of GIP and GLP-1. Metabolism: Clinical and Experimental 9646–55. ( 10.1016/j.metabol.2019.04.014) [DOI] [PubMed] [Google Scholar]
- Irwin DM.2020Molecular evolution of GIP and exendin and their receptors. Peptides 125170158. ( 10.1016/j.peptides.2019.170158) [DOI] [PubMed] [Google Scholar]
- Irwin N & Flatt PR. 2009aTherapeutic potential for GIP receptor agonists and antagonists. Best Practice & Research. Clinical Endocrinology & Metabolism 23499–512. ( 10.1016/j.beem.2009.03.001) [DOI] [PubMed] [Google Scholar]
- Irwin N & Flatt PR. 2009bEvidence for beneficial effects of compromised gastric inhibitory polypeptide action in obesity-related diabetes and possible therapeutic implications. Diabetologia 521724–1731. ( 10.1007/s00125-009-1422-8) [DOI] [PubMed] [Google Scholar]
- Irwin N Gault VA Green BD Greer B McCluskey JT Harriott P O’Harte FP & Flatt PR. 2004Effects of short-term chemical ablation of the GIP receptor on insulin secretion, islet morphology and glucose homeostasis in mice. Biological Chemistry 385845–852. ( 10.1515/BC.2004.110) [DOI] [PubMed] [Google Scholar]
- Irwin N McClean PL O’Harte FP Gault VA Harriott P & Flatt PR. 2007aEarly administration of the glucose-dependent insulinotropic polypeptide receptor antagonist (Pro3)GIP prevents the development of diabetes and related metabolic abnormalities associated with genetically inherited obesity in ob/ob mice. Diabetologia 501532–1540. ( 10.1007/s00125-007-0692-2) [DOI] [PubMed] [Google Scholar]
- Irwin N Hunter K & Flatt PR. 2007bComparison of the metabolic effects of GIP receptor antagonism and PYY(3–36) receptor activation in high fat fed mice. Peptides 282192–2198. ( 10.1016/j.peptides.2007.08.008) [DOI] [PubMed] [Google Scholar]
- Irwin N Hunter K & Flatt PR. 2008Comparison of independent and combined chronic metabolic effects of GIP and CB1 receptor blockade in high-fat fed mice. Peptides 291036–1041. ( 10.1016/j.peptides.2008.01.006). [DOI] [PubMed] [Google Scholar]
- Irwin N McClean PL Patterson S Hunter K & Flatt PR. 2009aActive immunisation against gastric inhibitory polypeptide (GIP) improves blood glucose control in an animal model of obesity-diabetes. Biological Chemistry 39075–80. ( 10.1515/BC.2009.002) [DOI] [PubMed] [Google Scholar]
- Irwin N McClean PL Hunter K & Flatt PR. 2009bMetabolic effects of sustained activation of the GLP-1 receptor alone and in combination with background GIP receptor antagonism in high fat-fed mice. Diabetes, Obesity and Metabolism 11603–610. ( 10.1111/j.1463-1326.2009.01036.x) [DOI] [PubMed] [Google Scholar]
- Irwin N Gault V & Flatt PR. 2010Therapeutic potential of the original incretin hormone glucose-dependent insulinotropic polypeptide: diabetes, obesity, osteoporosis and Alzheimer’s disease? Expert Opinion on Investigational Drugs 191039–1048. ( 10.1517/13543784.2010.513381) [DOI] [PubMed] [Google Scholar]
- Irwin N Montgomery IA & Flatt PR. 2012Evaluation of the long-term effects of gastric inhibitory polypeptide-ovalbumin conjugates on insulin resistance, metabolic dysfunction, energy balance and cognition in high-fat-fed mice. British Journal of Nutrition 10846–56. ( 10.1017/S0007114511005228) [DOI] [PubMed] [Google Scholar]
- Irwin N Montgomery IA O’Harte FP Frizelle P & Flatt PR. 2013Comparison of the independent and combined metabolic effects of subchronic modulation of CCK and GIP receptor action in obesity-related diabetes. International Journal of Obesity 371058–1063. ( 10.1038/ijo.2012.179) [DOI] [PubMed] [Google Scholar]
- Irwin N Gault VA O’Harte FPM & Flatt PR. 2020Blockade of gastric inhibitory polypeptide (GIP) action as a novel means of countering insulin resistance in the treatment of obesity-diabetes. Peptides 125170203. ( 10.1016/j.peptides.2019.170203) [DOI] [PubMed] [Google Scholar]
- Kanemaru Y Harada N Shimazu-Kuwahara S Yamane S Ikeguchi E Murata Y Kiyobayashi S Hatoko T & Inagaki N. 2020Absence of GIP secretion alleviates age-related obesity and insulin resistance. Journal of Endocrinology 24513–20. ( 10.1530/JOE-19-0477) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr BD Flatt AJS Flatt PR & Gault VA. 2011Characterization and biological actions of N-terminal truncated forms of glucose-dependent insulinotropic polypeptide. Biochemical and Biophysical Research Communications 404870–876. ( 10.1016/j.bbrc.2010.12.077) [DOI] [PubMed] [Google Scholar]
- Killion EA, Wang J, Yie J, Shi SDH, Bates D, Min X, Komorowski R, Hager T, Deng L, Atangan L, et al.2018Anti-obesity effects of GIPR antagonists alone and in combination with GLP-1R agonists in preclinical models. Science Translational Medicine 10eaat3392. ( 10.1126/scitranslmed.aat3392) [DOI] [PubMed] [Google Scholar]
- Killion EA, Chen M, Falsey JR, Sivits G, Hager T, Atangan L, Helmering J, Lee J, Li H, Wu B, et al.2020aChronic glucose-dependent insulinotropic polypeptide receptor (GIPR) agonism desensitizes adipocyte GIPR activity mimicking functional GIPR antagonism. Nature Communications 114981. ( 10.1038/s41467-020-18751-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killion EA Lu SC Fort M Yamada Y Véniant MM & Lloyd DJ. 2020bGlucose-dependent insulinotropic polypeptide receptor therapies for the treatment of obesity, do agonists = antagonists? Endocrine Reviews 41bnz002. ( 10.1210/endrev/bnz002) [DOI] [PubMed] [Google Scholar]
- Kim SJ Nian C & McIntosh CH. 2007Activation of lipoprotein lipase by glucose-dependent insulinotropic polypeptide in adipocytes. A role for a protein kinase B, LKB1, and AMP-activated protein kinase cascade. Journal of Biological Chemistry 2828557–8567. ( 10.1074/jbc.M609088200) [DOI] [PubMed] [Google Scholar]
- Kim SJ Nian C & McIntosh CH. 2010GIP increases human adipocyte LPL expression through CREB and TORC2-mediated trans-activation of the LPL gene. Journal of Lipid Research 513145–3157. ( 10.1194/jlr.M006841) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kizilkaya HS Sørensen KV Kibsgaard CJ Gasbjerg LS Hauser AS Sparre-Ulrich AH Grarup N & Rosenkilde MM. 2021Loss of function glucose-dependent insulinotropic polypeptide receptor variants are associated with alterations in BMI, bone strength and cardiovascular outcomes. Frontiers in Cell and Developmental Biology 9749607. ( 10.3389/fcell.2021.749607) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafferty RA Flatt PR & Irwin N. 2023GLP-1/GIP analogs: potential impact in the landscape of obesity pharmacotherapy. Expert Opinion on Pharmacotherapy 24587–597. ( 10.1080/14656566.2023.2192865) [DOI] [PubMed] [Google Scholar]
- Lafferty RA McShane LM Franklin ZJ Flatt PR O’Harte FPM & Irwin N. 2022Sustained glucagon receptor antagonism in insulin-deficient high-fat-fed mice. Journal of Endocrinology 25591–101. ( 10.1530/JOE-22-0106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AC Harris JL Khanna KK & Hong JH. 2019A comprehensive review on current advances in peptide drug development and design. International Journal of Molecular Sciences 202383. ( 10.3390/ijms20102383) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist P Gasbjerg LS Mokrosinski J Holst JJ Hauser AS & Rosenkilde MM. 2022The location of missense variants in the human gip gene is indicative for natural selection. Frontiers in Endocrinology 13891586. ( 10.3389/fendo.2022.891586) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu SC, Chen M, Atangan L, Killion EA, Komorowski R, Cheng Y, Netirojjanakul C, Falsey JR, Stolina M, Dwyer D, et al.2021GIPR antagonist antibodies conjugated to GLP-1 peptide are bispecific molecules that decrease weight in obese mice and monkeys. Cell Reports. Medicine 2100263. ( 10.1016/j.xcrm.2021.100263) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyssenko V, Eliasson L, Kotova O, Pilgaard K, Wierup N, Salehi A, Wendt A, Jonsson A, De Marinis YZ, Berglund LM, et al.2011Pleiotropic effects of GIP on islet function involve osteopontin. Diabetes 602424–2433. ( 10.2337/db10-1532) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks V.2020The early history of GIP 1969–2000: from enterogastrone to major metabolic hormone. Peptides 1251–7. ( 10.1016/j.peptides.2020.170276) [DOI] [PubMed] [Google Scholar]
- McClean PL Irwin N Cassidy RS Holst JJ Gault VA & Flatt PR. 2007GIP receptor antagonism reverses obesity, insulin resistance, and associated metabolic disturbances induced in mice by prolonged consumption of high-fat diet. American Journal of Physiology, Endocrinology and Metabolism 293E1746–E1755. ( 10.1152/ajpendo.00460.2007) [DOI] [PubMed] [Google Scholar]
- McClean PL Gault VA Irwin N McCluskey JT & Flatt PR. 2008aDaily administration of the GIP-R antagonist (Pro3)GIP in streptozotocin-induced diabetes suggests that insulin-dependent mechanisms are critical to anti-obesity-diabetes actions of (Pro3)GIP. Diabetes, Obesity and Metabolism 10336–342. ( 10.1111/j.1463-1326.2007.00712.x) [DOI] [PubMed] [Google Scholar]
- McClean PL Irwin N Hunter K Gault VA & Flatt PR. 2008b(Pro(3))GIP[mPEG]: novel, long-acting, mPEGylated antagonist of gastric inhibitory polypeptide for obesity-diabetes (diabesity) therapy. British Journal of Pharmacology 155690–701. ( 10.1038/bjp.2008.317) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier JJ & Nauck MA. 2004GIP as a potential therapeutic agent? Hormone and Metabolic Research 36859–866. ( 10.1055/s-2004-826176) [DOI] [PubMed] [Google Scholar]
- Miyawaki K, Yamada Y, Ban N, Ihara Y, Tsukiyama K, Zhou H, Fujimoto S, Oku A, Tsuda K, Toyokuni S, et al.2002Inhibition of gastric inhibitory polypeptide signaling prevents obesity. Nature Medicine 8738–742. ( 10.1038/nm727) [DOI] [PubMed] [Google Scholar]
- Mohammad S Patel RT Bruno J Panhwar MS Wen J & McGraw TE. 2014A naturally occurring GIP receptor variant undergoes enhanced agonist-induced desensitization, which impairs GIP control of adipose insulin sensitivity. Molecular and Cellular Biology 343618–3629. ( 10.1128/MCB.00256-14) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Møller CL, Vistisen D, Færch K, Johansen NB, Witte DR, Jonsson A, Pedersen O, Hansen T, Lauritzen T, Jørgensen ME, et al.2016Glucose-dependent insulinotropic polypeptide is associated with lower low-density lipoprotein but unhealthy fat distribution, independent of insulin: the ADDITION-PRO Study. Journal of Clinical Endocrinology and Metabolism 101485–493. ( 10.1210/jc.2015-3133) [DOI] [PubMed] [Google Scholar]
- Montgomery IA Irwin N & Flatt PR. 2010Active immunization against (Pro(3))GIP improves metabolic status in high-fat-fed mice. Diabetes, Obesity and Metabolism 12744–751. ( 10.1111/j.1463-1326.2010.01228.x) [DOI] [PubMed] [Google Scholar]
- Mroz PA Finan B Gelfanov V Yang B Tschöp MH DiMarchi RD & Perez-Tilve D. 2019Optimized GIP analogs promote body weight lowering in mice through GIPR agonism not antagonism. Molecular Metabolism 2051–62. ( 10.1016/j.molmet.2018.12.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller TD, Finan B, Bloom SR, D’Alessio D, Drucker DJ, Flatt PR, Fritsche A, Gribble F, Grill HJ, Habener JF, et al.2019Glucagon-like peptide 1 (GLP-1). Molecular Metabolism 3072–130. ( 10.1016/j.molmet.2019.09.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata Y, Harada N, Kishino S, Iwasaki K, Ikeguchi-Ogura E, Yamane S, Kato T, Kanemaru Y, Sankoda A, Hatoko T, et al.2021Medium-chain triglycerides inhibit long-chain triglyceride-induced GIP secretion through GPR120-dependent inhibition of CCK. iScience 24102963. ( 10.1016/j.isci.2021.102963) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T Tanimoto H Mizuno Y Tsubamoto Y & Noda H. 2012Biological and functional characteristics of a novel low-molecular weight antagonist of glucose-dependent insulinotropic polypeptide receptor, SKL-14959, in vitro and in vivo. Diabetes, Obesity and Metabolism 14511–517. ( 10.1111/j.1463-1326.2011.01555.x) [DOI] [PubMed] [Google Scholar]
- Nakamura T Tanimoto H Mizuno Y Okamoto M Takeuchi M Tsubamoto Y & Noda H. 2018Gastric inhibitory polypeptide receptor antagonist, SKL-14959, suppressed body weight gain on diet-induced obesity mice. Obesity Science and Practice 4194–203. ( 10.1002/osp4.164) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasteska D Harada N Suzuki K Yamane S Hamasaki A Joo E Iwasaki K Shibue K Harada T & Inagaki N. 2014Chronic reduction of GIP secretion alleviates obesity and insulin resistance under high-fat diet conditions. Diabetes 632332–2343. ( 10.2337/db13-1563) [DOI] [PubMed] [Google Scholar]
- Nauck MA & Müller TD. 2023Incretin hormones and type 2 diabetes. Diabetologia 661780–1795. ( 10.1007/s00125-023-05956-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauck MA Heimesaat MM Orskov C Holst JJ Ebert R & Creutzfeldt W. 1993Preserved incretin activity of glucagon-like peptide 1 [7–36 amide] but not of synthetic human gastric inhibitory polypeptide in patients with type-2 diabetes mellitus. Journal of Clinical Investigation 91301–307. ( 10.1172/JCI116186) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauck MA Quast DR Wefers J & Meier JJ. 2021aGLP-1 receptor agonists in the treatment of type 2 diabetes - state-of-the-art. Molecular Metabolism 46101102. ( 10.1016/j.molmet.2020.101102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauck MA Quast DR Wefers J & Pfeiffer AFH. 2021bThe evolving story of incretins (GIP and GLP-1) in metabolic and cardiovascular disease: a pathophysiological update. Diabetes, Obesity and Metabolism 23(Supplement 3) 5–29. ( 10.1111/dom.14496) [DOI] [PubMed] [Google Scholar]
- O’Harte FPM Hunter K Gault VA Irwin N Green BD Greer B Harriott P Bailey CJ & Flatt PR. 2006Antagonistic effects of two novel GIP analogs, (Hyp3)GIP and (Hyp3)GIPLys16PAL, on the biological actions of GIP and longer-term effects in diabetic ob/ob mice. American Journal of Physiology, Endocrinology and Metabolism 292E1674–E1682. ( 10.1152/ajpendo.00391.2006) [DOI] [PubMed] [Google Scholar]
- Pamir N Lynn FC Buchan AM Ehses J Hinke SA Pospisilik JA Miyawaki K Yamada Y Seino Y McIntosh CH & Pederson RA. 2003Glucose-dependent insulinotropic polypeptide receptor null mice exhibit compensatory changes in the enteroinsular axis. American Journal of Physiology Endocrinology & Metabolism284E931-E939. ( 10.1152/ajpendo.00270.2002) [DOI] [PubMed] [Google Scholar]
- Parker JC Lavery KS Irwin N Green BD Greer B Harriott P O’Harte FP Gault VA & Flatt PR. 2006Effects of sub-chronic exposure to naturally occurring N-terminally truncated metabolites of glucose-dependent insulinotrophic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1), GIP(3-42) and GLP-1(9-36)amide, on insulin secretion and glucose homeostasis in ob/ob mice. Journal of Endocrinology 19193–100. ( 10.1677/joe.1.06904) [DOI] [PubMed] [Google Scholar]
- Pathak V Gault VA Flatt PR & Irwin N. 2015aAntagonism of gastric inhibitory polypeptide (GIP) by palmitoylation of GIP analogues with N- and C-terminal modifications improves obesity and metabolic control in high fat fed mice. Molecular and Cellular Endocrinology 401120–129. ( 10.1016/j.mce.2014.10.025) [DOI] [PubMed] [Google Scholar]
- Pathak V Vasu S Gault VA Flatt PR & Irwin N. 2015bSequential induction of beta cell rest and stimulation using stable GIP inhibitor and GLP-1 mimetic peptides improves metabolic control in C57BL/KsJ db/db mice. Diabetologia 582144–2153. ( 10.1007/s00125-015-3653-1) [DOI] [PubMed] [Google Scholar]
- Pederson RA Schubert HE & Brown JC. 1975Gastric inhibitory polypeptide. Its physiologic release and insulinotropic action in the dog. Diabetes 241050–1056. ( 10.2337/diab.24.12.1050) [DOI] [PubMed] [Google Scholar]
- Perry RA Craig SL Ng MT Gault VA Flatt PR & Irwin N. 2019Characterisation of glucose-dependent insulinotropic polypeptide receptor antagonists in rodent pancreatic beta cells and mice. Clinical Medicine Insights. Endocrinology and Diabetes 121179551419875453. ( 10.1177/1179551419875453) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravn P, Madhurantakam C, Kunze S, Matthews E, Priest C, O’Brien S, Collinson A, Papworth M, Fritsch-Fredin M, Jermutus L, et al.2013Structural and pharmacological characterization of novel potent and selective monoclonal antibody antagonists of glucose-dependent insulinotropic polypeptide receptor. Journal of Biological Chemistry 28819760–19772. ( 10.1074/jbc.M112.426288) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salera M Giacomoni P Pironi L Cornia G Capelli M Marini A Benfenati F Miglioli M & Barbara L. 1982Gastric inhibitory polypeptide release after oral glucose: relationship to glucose intolerance, diabetes mellitus, and obesity. Journal of Clinical Endocrinology and Metabolism 55329–336. ( 10.1210/jcem-55-2-329) [DOI] [PubMed] [Google Scholar]
- Samms RJ Coghlan MP & Sloop KW. 2020How may GIP enhance the therapeutic efficacy of GLP-1? Trends in Endocrinology and Metabolism 31410–421. ( 10.1016/j.tem.2020.02.006) [DOI] [PubMed] [Google Scholar]
- Saxena AR Frias JP Gorman DN Lopez RN Andrawis N Tsamandouras N & Birnbaum MJ. 2023Tolerability, safety and pharmacodynamics of oral, small-molecule glucagon-like peptide-1 receptor agonist danuglipron for type 2 diabetes: a 12-week, randomized, placebo-controlled, phase 2 study comparing different dose-escalation schemes. Diabetes, Obesity and Metabolism 252805–2814. ( 10.1111/dom.15168) [DOI] [PubMed] [Google Scholar]
- Secher A, Jelsing J, Baquero AF, Hecksher-Sørensen J, Cowley MA, Dalbøge LS, Hansen G, Grove KL, Pyke C, Raun K, et al.2014The arcuate nucleus mediates GLP-1 receptor agonist liraglutide-dependent weight loss. Journal of Clinical Investigation 1244473–4488. ( 10.1172/JCI75276) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seino Y Fukushima M & Yabe D. 2010GIP and GLP-1, the two incretin hormones: similarities and differences. Journal of Diabetes Investigation 18–23. ( 10.1111/j.2040-1124.2010.00022.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siano A Húmpola MV Rey MC Simonetta A & Tonarelli GG. 2011Interaction of acylated and substituted antimicrobial peptide analogs with phospholipid-polydiacetylene vesicles. Correlation with their biological properties. Chemical Biology and Drug Design 7885–93. ( 10.1111/j.1747-0285.2011.01099.x) [DOI] [PubMed] [Google Scholar]
- Simonsen L Holst JJ Madsen K & Deacon CF. 2013The C-terminal extension of exendin-4 provides additional metabolic stability when added to GLP-1, while there is minimal effect of truncating exendin-4 in anaesthetized pigs. Regulatory Peptides 18117–21. ( 10.1016/j.regpep.2012.12.012) [DOI] [PubMed] [Google Scholar]
- Sparre-Ulrich AH Hansen LS Svendsen B Christensen M Knop FK Hartmann B Holst JJ & Rosenkilde MM. 2016Species-specific action of (Pro3)GIP - a full agonist at human GIP receptors, but a partial agonist and competitive antagonist at rat and mouse GIP receptors. British Journal of Pharmacology 17327–38. ( 10.1111/bph.13323) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparre-Ulrich AH Gabe MN Gasbjerg LS Christiansen CB Svendsen B Hartmann B Holst JJ & Rosenkilde MM. 2017GIP(3-30)NH2 is a potent competitive antagonist of the GIP receptor and effectively inhibits GIP-mediated insulin, glucagon, and somatostatin release. Biochemical Pharmacology 13178–88. ( 10.1016/j.bcp.2017.02.012) [DOI] [PubMed] [Google Scholar]
- Stensen S Gasbjerg LS Rosenkilde MM Vilsbøll T Holst JJ Hartmann B Christensen MB & Knop FK. 2022Endogenous glucose-dependent insulinotropic polypeptide contributes to sitagliptin-mediated improvement in β-cell function in patients with type 2 diabetes. Diabetes 712209–2221. ( 10.2337/db22-0059) [DOI] [PubMed] [Google Scholar]
- Tanday N Lafferty RA Flatt PR & Irwin N. 2022Beneficial metabolic effects of recurrent periods of beta-cell rest and stimulation using stable neuropeptide Y1 and glucagon-like peptide-1 receptor agonists. Diabetes, Obesity and Metabolism 242353–2363. ( 10.1111/dom.14821) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timper K, Grisouard J, Sauter NS, Herzog-Radimerski T, Dembinski K, Peterli R, Frey DM, Zulewski H, Keller U, Müller B, et al.2013Glucose-dependent insulinotropic polypeptide induces cytokine expression, lipolysis, and insulin resistance in human adipocytes. American Journal of Physiology, Endocrinology and Metabolism 304E1–E13. ( 10.1152/ajpendo.00100.2012) [DOI] [PubMed] [Google Scholar]
- Tseng CC Zhang XY & Wolfe MM. 1999Effect of GIP and GLP-1 antagonists on insulin release in the rat. American Journal of Physiology 276E1049–E1054. ( 10.1152/ajpendo.1999.276.6.E1049) [DOI] [PubMed] [Google Scholar]
- Usdin TB Mezey E Button DC Brownstein MJ & Bonner TI. 1993Gastric inhibitory polypeptide receptor, a member of the secretin-vasoactive intestinal peptide receptor family, is widely distributed in peripheral organs and the brain. Endocrinology 1332861–2870. ( 10.1210/endo.133.6.8243312) [DOI] [PubMed] [Google Scholar]
- Véniant MM, Lu SC, Atangan L, Komorowski R, Stanislaus S, Cheng Y, Wu B, Falsey JR, Hager T, Thomas VA, et al.2024A GIPR antagonist conjugated to GLP-1 analogues promotes weight loss with improved metabolic parameters in preclinical and phase 1 settings. Nature Metabolism 6290–303. ( 10.1038/s42255-023-00966-w) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venneti KC Malthouse JPG O’Harte FPM & Hewage CM. 2011Conformational, receptor interaction and alanine scan studies of glucose-dependent insulinotropic polypeptide. Biochimica et Biophysica Acta 1814882–888. ( 10.1016/j.bbapap.2011.04.002) [DOI] [PubMed] [Google Scholar]
- Vyavahare SS Mieczkowska A Flatt PR Chappard D Irwin N & Mabilleau G. 2020GIP analogues augment bone strength by modulating bone composition in diet-induced obesity in mice. Peptides 125170207. ( 10.1016/j.peptides.2019.170207) [DOI] [PubMed] [Google Scholar]
- Whitaker GM Lynn FC McIntosh CH & Accili EA. 2012Regulation of GIP and GLP1 receptor cell surface expression by N-glycosylation and receptor heteromerization. PLoS One 7e32675. ( 10.1371/journal.pone.0032675) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitley HP Trujillo JM & Neumiller JJ. 2023Special report: potential strategies for addressing GLP-1 and dual GLP-1/GIP receptor agonist shortages. Clinical Diabetes 41467–473. ( 10.2337/cd23-0023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willard FS, Douros JD, Gabe MB, Showalter AD, Wainscott DB, Suter TM, Capozzi ME, van der Velden WJ, Stutsman C, Cardona GR, et al.2020Tirzepatide is an imbalanced and biased dual GIP and GLP-1 receptor agonist. JCI Insight 5e140532. ( 10.1172/jci.insight.140532) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe MM Apovian CM & Boylan MO. 2023Glucose-dependent insulinotropic polypeptide monoclonal antibodies prevent and treat obesity in wild-type and hyperphagic mice. Obesity 311499–1504. ( 10.1002/oby.23758) [DOI] [PubMed] [Google Scholar]
- Xiao J El Eid L Buenaventura T Boutry R Bonnefond A Jones B Rutter GA Froguel P & Tomas A. 2023Control of human pancreatic beta cell kinome by glucagon-like peptide-1 receptor biased agonism. Diabetes, Obesity and Metabolism 252105–2119. ( 10.1111/dom.15083) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B, Gelfanov VM, El K, Chen A, Rohlfs R, DuBois B, Kruse Hansen AM, Perez-Tilve D, Knerr PJ, D’Alessio D, et al.2022Discovery of a potent GIPR peptide antagonist that is effective in rodent and human systems. Molecular Metabolism 66101638. ( 10.1016/j.molmet.2022.101638) [DOI] [PMC free article] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a