Abstract
Stability problems have limited the uptake of cationic olefin metathesis catalysts in chemical biology. Described herein are anionic catalysts that improve water-solubility, robustness, and compatibility with biomolecules such as DNA. A sulfonate tag is installed on the cyclic (alkyl)(amino) carbene (CAAC) ligand platform, chosen for resistance to degradation by nucleophiles, base, water, and β-elimination. Hoveyda–Grubbs catalysts bearing the sulfonated CAAC ligands deliver record productivity in metathesis of unprotected carbohydrates and nucleosides at neutral pH. Decomposed catalyst has negligible impact on metathesis selectivity, whereas N-heterocyclic carbene (NHC) catalysts degrade rapidly in water and cause extensive C=C migration.
Keywords: olefin metathesis, ruthenium, aqueous metathesis, chemical biology, sulfonates, anionic ligand, isomerization
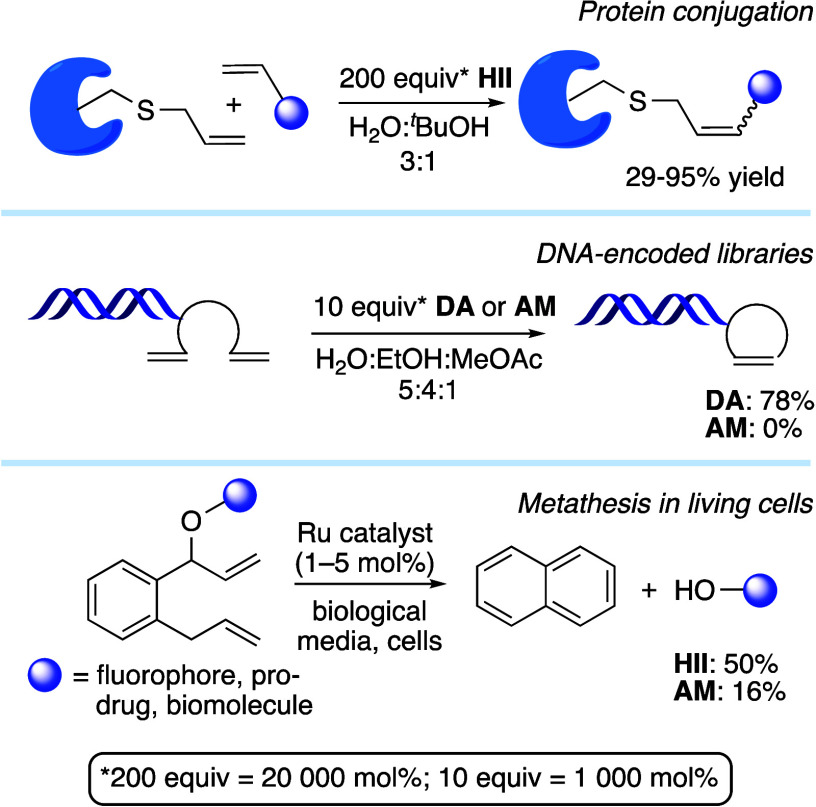
Olefin metathesis catalysts capable of modifying unprotected biomolecules hold great potential as a tool that bridges chemistry and biology.1−4 Modification of peptides or proteins (Figure 1) has seen much study.4c,5 Other, emerging applications include drug discovery via DNA-encoded libraries (DEL),6,7 and in vivo metathesis in blood8 or living cells.9−11 Most of these applications require metathesis in water. Surprising, therefore, is the widespread preference for neutral catalysts such as HII (the second-generation Hoveyda–Grubbs catalyst; Chart 1a),4−7 despite the availability of water-soluble analogues (Chart 1b).12−15 In several comparative studies, water-soluble catalysts (e.g., Aquamet, AM; Ru-1) proved less effective than HII in mixed organic-aqueous media,5,9,6,16 notwithstanding superior phase homogeneity.6
Figure 1.
Selected applications of olefin metathesis in chemical biology. For catalysts, see Chart 1.
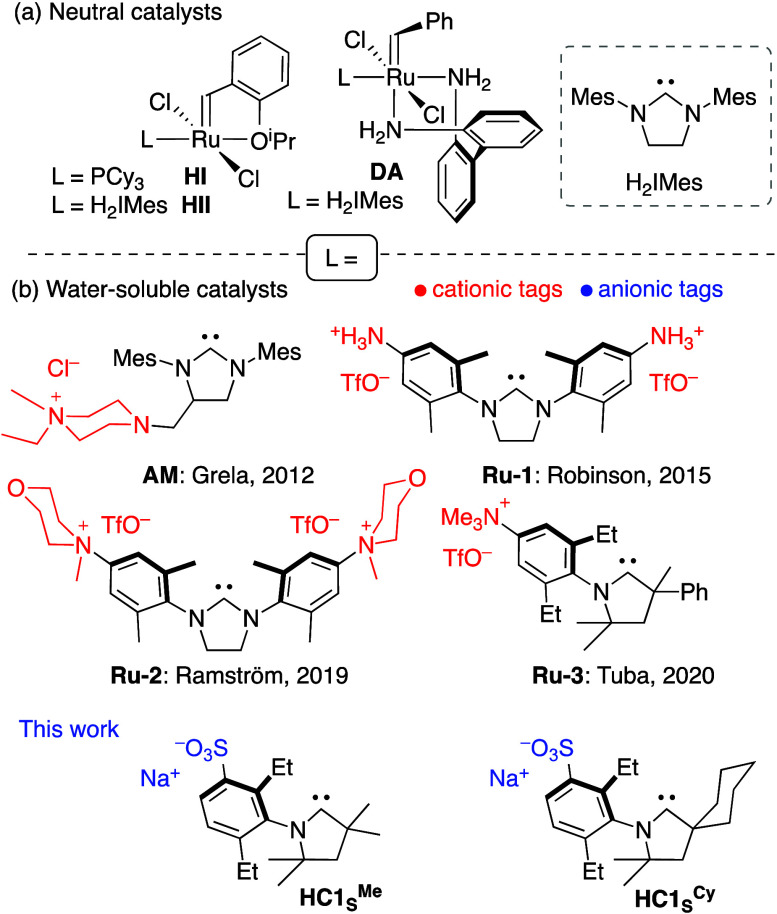
Chart 1. (a) Neutral Metathesis Catalysts Cited in Figure 1; (b) Charged, Water-Soluble Catalysts That Enable Metathesis in Water.
We questioned whether the ubiquitous reliance on cationic tags for water-solubility17,18 (see Chart 1b) might have unforeseen negative impacts. The failure of cationic AM in DEL applications, accompanied by DNA degradation, has been attributed to electrostatic attraction between the ammonium group and the negatively charged phosphate backbone of DNA.6 As a more general hazard, cationic ligands may increase the acidity of ligated water in the [Ru]–OH2 (aqua complexes) formed in water,19−22 accelerating decomposition into metathesis-inactive23 Ru-hydroxides. Anionic tags offer a compelling alternative, reinforced by their potential to enhance water-solubility via participation in extended H-bonding networks.24,25 Maximizing catalyst concentrations in water is crucial in chemical biology, which has been characterized as a race between metathesis and decomposition.4c
In undertaking catalyst redesign, we therefore prioritized anionic charge. We chose weakly basic sulfonate tags,26 which confer high solubility, remain anionic over a wide pH range,27 and show low affinity with ruthenium unless driven by chelation26a,26c or silver salts.26b Two recent studies of Suzuki–Miyaura coupling in water describe the compatibility of sulfonate-tagged phosphines with DNA.28,29 A further criterion was installation on a cyclic alkyl amino carbene (CAAC) ligand. CAACs are privileged ligands in metathesis relative to their phosphine and N-heterocyclic carbene (NHC) predecessors. Within Hoveyda-class catalysts, CAACs improve resistance to degradation by (inter alia) water, nucleophiles, Brønsted base, and β-elimination.30 Ammonium-functionalized Ru-3 (Chart 1b) is the sole charged CAAC catalyst reported to date.31 Here we describe anionic CAAC catalysts that deliver record performance in the metathesis of terminal olefins in water, including unprotected nucleoside and carbohydrate derivatives.
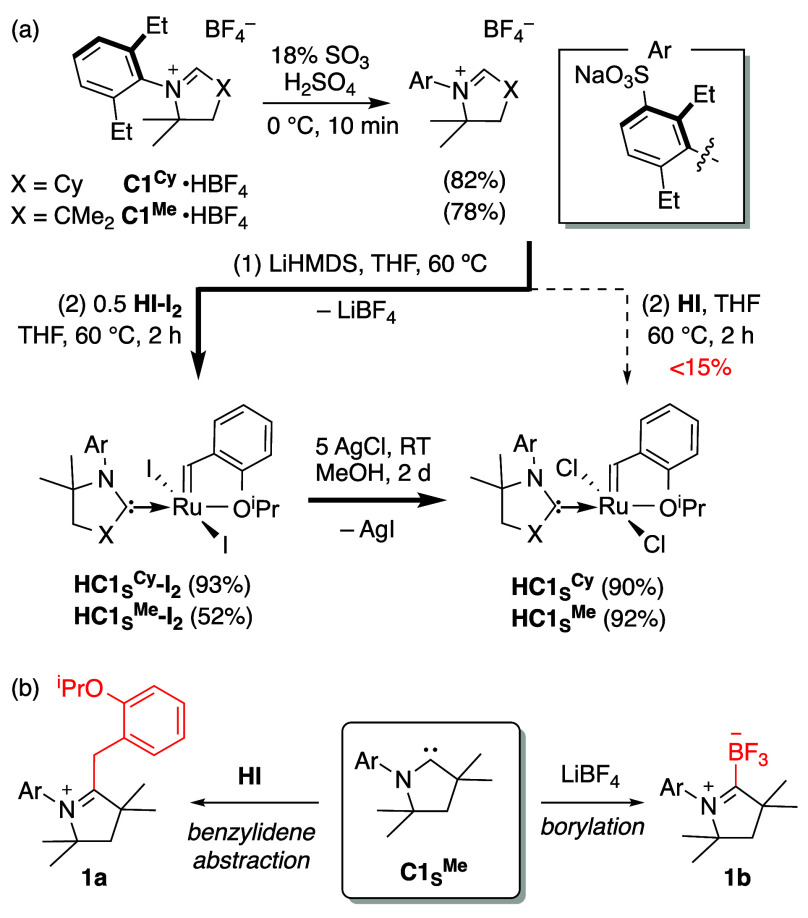
Direct m-sulfonation of the known CAAC•HBF4 salts with fuming sulfuric acid, via protocols established in NHC chemistry,32 offers the most direct entry to the anionic carbenes (Scheme 1a, top). Monosulfonated products were obtained in ca. 80% yield for proligands bearing a single aromatic ring. Salts bearing a second aromatic group yielded a mixture of polysulfonated products, and were not pursued.
Scheme 1. (a) Synthesis of Sulfonate-Tagged CAAC Ligands and Catalysts; (b) Side-Reactions Resulting in Consumption of C1SMe.
The major electronic and solubility properties that differentiate the anionic carbenes from their predecessors have important synthetic consequences. First, transmetalation cannot be used to install these ligands. Strong Ag–CAAC binding impedes carbene transfer even for neutral CAAC ligands,33 a problem exacerbated for anionic carbenes.34 Instead, we attempted a modified version of the original route to neutral CAAC catalysts, in which the iminium salt is deprotonated with strong base, and the resulting CAAC is added to HI (Scheme 1a, dashed arrow).35,36 Both steps were carried out at 60 °C, to maximize the THF-solubility of the CAAC-sulfonates. Extensive decomposition resulted, however, and the target catalysts were isolated in <15% yield. The low yields may be due in part to abstraction of the benzylidene ligand by the carbene (Scheme 1b, left),37 as suggested by NMR and mass spectrometric evidence for 1a.
To impede benzylidene abstraction, we turned to a “back-door” synthetic strategy developed in parallel work.38 This involves installation of the CAAC ligand on the di-iodide analogue of HI,39 in which the bulky iodide ligands limit access of the free carbene to the [Ru]=CHAr carbon.38 Following CAAC ligation, the desired chloride complex can be safely generated via anion exchange. The success of this approach is shown in Scheme 1a (bold arrow). Installation of the sulfonated CAAC ligands on HI-I2 was complete within 2 h, affording the cyclohexyl C1SCy derivative in 93% yield after chromatography. The smaller C1SMe analogue was isolated in lower yields (52%), consistent with more facile participation in side-reactions. In addition to attack on the benzylidene functionality, these include the recently uncovered borylation by the BF4– counteranion (Scheme 1b, right).40 Formation of 1b (Figure S9) accounts for the initially puzzling requirement for excess CAAC.
The final synthetic step, exchange of the iodide ligands for chloride, was carried out in methanol, in which the iodide complexes are fully soluble. Chlorination by AgCl proved more efficient than NaCl, a reflection of the very small dissociation constant for the AgI product.41 The limited solubility of AgCl in methanol retards exchange, but reaction was complete after 2 days (Tables S2, S3), affording the chloride complexes in >90% isolated yield. Somewhat unexpectedly, the new catalysts are soluble in CH2Cl2, as well as methanol, acetone, water and to some extent THF (see Table S4). As anticipated, the smaller catalyst shows higher water-solubility.
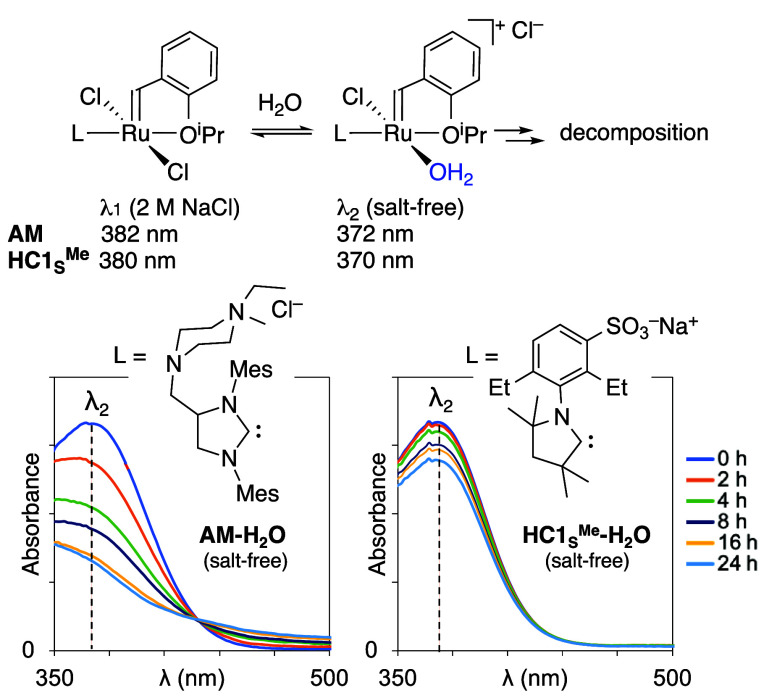
With the sulfonated CAAC catalysts in hand, our first priority was to examine their water-tolerance relative to AM. The stability of metathesis complexes is conventionally assessed by NMR analysis, via integration of the alkylidene signal against an internal standard over time. In D2O, such experiments are precluded by rapid exchange-averaging, which causes the benzylidene signals to broaden into the baseline. Electronic spectroscopy offers an invaluable alternative, particularly given the diagnostic colors of the precatalysts relative to their Ru(II) decomposition products. Prior spectrophotometric studies revealed the rapid degradation of AM and related catalysts in water,19−22 and the role of chloride ion in retarding decomposition. We examined the stability of the small CAAC complex HC1SMe relative to AM, in the expectation that reduced size would correlate with increased vulnerability.30c
Consistent with the literature reports,19,20AM-H2O forms immediately on dissolving AM in water in the absence of NaCl. The aqua complex decays over 2 h at RT (Figure 2, left). Aquation of HC1SMe under these conditions is likewise immediate, as judged from the observation of a single absorption band at 370 nm (Figure 2, right; cf. λmax = 380 nm in 2 M NaCl(aq); Figure S18). The aqua species is significantly more water-stable than its AM analogue, however, undergoing only 20% loss over 24 h at RT. The capacity of the anionic CAAC ligand to retard the decomposition cascade holds promise for aqueous metathesis, to which we now turn.
Figure 2.
Aquation of AM and HC1SMe in degassed water, and rapid ensuing degradation of AM.
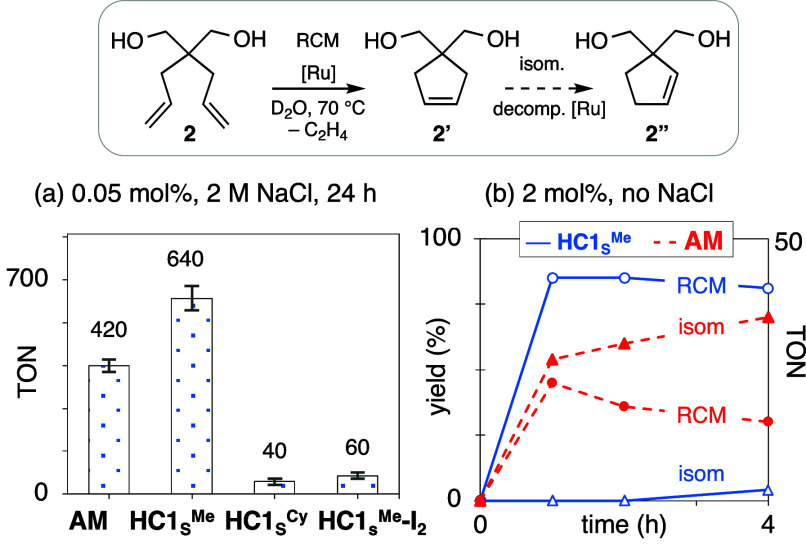
To benchmark the performance of the new catalysts in water, we examined the ring-closing metathesis (RCM) of diol 2 (Figure 3a). The maximum turnover number (TON) reported for RCM of 2 in water is 650 ± 35 for an HII derivative embedded in a lipophilic streptavidin pocket,42 or 210 for AM in buffered water,43 at ca. 40 °C. In experiments with HC1SMe and AM, we observed TONs of 640 and 420, respectively, albeit at a higher temperature (70 °C). The iodide catalyst HC1SMe-I2 and cyclohexyl catalyst HC1SCy were much less productive (TON 60 and 40, respectively; Figure 3a), probably due to their steric bulk, as well as poorer solvation. Of note, related NHC-iodide catalysts exhibit outstanding tolerance for trace water, delivering very high TONs despite reacting more slowly than the chloride analogues.30d The much poorer performance seen for HC1SMe-I2 in bulk water reinforces the point that any factors that retard metathesis have a major negative impact in aqueous metathesis, where decomposition rates are significantly faster.
Figure 3.
(a) RCM productivity of water-soluble catalysts (Table S5): TONs based on yield of 2′ at 24 h. No 2″ observed. (b) Reaction profile in the absence of NaCl, showing extensive isomerization for AM.
In the absence of NaCl, metathesis productivity and selectivity decline sharply, and catalyst loadings must be increased 40-fold to achieve high conversions of 2. As shown in Figure 3b, AM is particularly affected. At 4 h, the yield of 2′ is only 30% (TON 15), the balance being isomerization product 2″. Olefin isomerization is well established as the major side-reaction for metathesis of terminal olefins in organic solvents.44 It clearly remains a major problem in bulk water for NHC catalysts, notwithstanding the different decomposition pathways and Ru speciation.23 In striking contrast, the CAAC catalyst induces negligible isomerization in water.45 This has important implications for metathesis in chemical biology, where high excesses of the ruthenium species are required to drive metathesis.
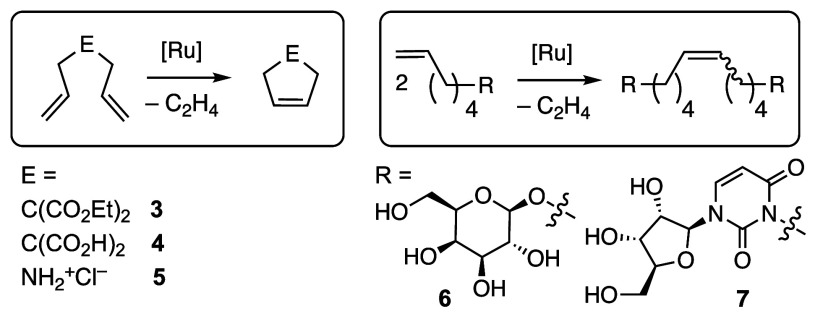
While the TON of 640 for HC1SMe in the presence of NaCl (Figure 3a) is excellent for metathesis in water, it is dramatically lower than the TON of >11,000 achieved by the same catalyst in RCM of diethyl diallylmalonate 3 in CH2Cl2 even at RT (Table 1, entry 1). Competitive binding of water and olefin may be an intrinsic limitation to metathesis productivity in water, by analogy to the competitive inhibition suggested for THF.44 Consistent with this hypothesis, yields in RCM of 2 are slightly higher in 1:1 D2O-tBuOH than neat D2O (Table S6).
Table 1. Performance of Sulfonate Catalysts in Metathesisa.
| entry | substrate | Ru cat | mol % | t (h) | yield (%) | TON |
|---|---|---|---|---|---|---|
| 1 | 3a | HC1SMe | 0.005 | 24 | 56 | 11,200 |
| 2 | 4 | HC1SMe | 0.5 | 24 | 35 | 70 |
| 0.5 | 24 | 49b | 98b | |||
| 3 | 5 | HC1SMe | 0.1 | 24 | 8 | 80 |
| 1 | 24 | 100 | 100 | |||
| 4 | 6 | HC1SMe | 1 | 24 | 100 | 100c |
| 0.1 | 24 | 51 | 510 | |||
| 5 | 7 | HC1SCy | 0.1 | 4 | 70 | 700 |
| 0.05 | 4 | 42 | 840 | |||
| 1 | 4 | 100 | 100 | |||
| HC1SMe | 0.1 | 4 | 100 | 1,000d | ||
| 0.05 | 4 | 76 | 1,520 |
In CH2Cl2 (substrate 3; RT); or D2O with 2 M NaCl (all others); at 70 °C except where noted. Yields within ±2% in replicate runs. Optimal performance requires inert atmosphere: see Table S5.
At pH 3.
Cf. 2% yield at RT.
Cf. 9% yield after 24 h at RT with 1 mol % HC1SMe.
RCM of diallylmalonic acid 4 in D2O was also attempted (Table 1, entry 2). Yields are limited to ca. 35% at pH 7, perhaps owing to chelation of anionic carboxylate: they increase to 50% at pH 3.
The efficacy of the CAAC–NaCl combination in suppressing isomerization was examined more closely in experiments with diallylamine hydrochloride 5 (Table 1, entry 3). The latter substrate constitutes a highly sensitive probe of C=C migration, owing to the metathesis-inactivity of its N-vinylamine isomer. Cyclization of 5 was quantitative at 1 mol % HC1SMe, a catalyst loading 5-fold lower than that routinely required.19 At 0.1 mol %, RCM yields dropped to ca. 10%, but again, no isomerization was evident even after 24 h. The CAAC complex thus stands out in enabling selective metathesis in water.
A final set of experiments focused on metathesis of unprotected biomolecules, an area of tremendous opportunity, with few reports to date.4−7,15,16 Metathesis of protected carbohydrate substrates has enabled advances in applications ranging from antimicrobial therapeutics to tissue engineering.46,47 The Ramström group recently reported metathetical coupling of unprotected carbohydrates in neat water, without cosolvents or privileged coupling partners.15 Up to 81% dimerization of β-d-galactopyranoside 6 was described on use of 5 mol % of NHC catalyst Ru-2, when acid was used to suppress C=C migration.48 In comparison, HC1SMe enables quantitative dimerization at neutral pH with 1 mol % Ru, or ca. 50% yield at 0.1 mol % (Table 1, entry 4). Given the susceptibility of these long-chain substrates to isomerization, and the limitations of NMR detection, we reanalyzed the higher-loading experiment by mass spectrometry. A small M–14 peak was evident, indicating ca. 5% isomerization prior to metathesis. In comparison, RCM of related mannosides by Ru-2 in the absence of acid caused nearly 90% isomerization at 60 °C, with the desired dimer being formed in just 5% yield.15
Finally, motivated by the explosion in interest in oligonucleotide therapeutics,49 and the enhanced activity demonstrated for nucleoside dimers accessed via click chemistry,50 we examined metathetical coupling of uridine-tagged 7 (Table 1, entry 5). Prior examples of nucleoside metathesis employ protected glycosylamines in organic solvent, and proceed in low yields.51 Coupling of 7 is the first reported example of the metathesis of an unprotected nucleoside. Both HC1SMe and HC1SCy dimerized 7 quantitatively, at loadings of 0.1 or 1 mol %, respectively; at 0.05 mol % HC1SMe, yields reached 76%. A limitation, however, is the need for elevated temperatures. At RT, <10% dimer is observed even at 1 mol % Ru, perhaps because the electron-withdrawing sulfonate ligand exacerbates the slow propagation characteristic of CAAC catalysts.30b,52 Nevertheless, the TON of 1,520 for nucleoside substrate 7 is the highest yet reported for metathesis of terminal olefins in water. The improvement over RCM of the simpler substrates 2 and 5 may reflect the extended “tether length” to the terminal olefin (the incipient site of Ru installation), which is beneficial in related contexts.14,15
Water-soluble ammonium catalysts for olefin metathesis, although long established, have seen little use for challenging reactions in chemical biology. The surprising preference for neutral, organic-soluble catalysts is driven by higher metathesis productivity, which outweighs the challenges in achieving phase homogeneity. The foregoing describes novel, negatively charged catalysts that are set to change this picture. A CAAC ligand bearing an anionic sulfonate tag improves water-tolerance, solubility, and metathesis performance. The small CAAC catalyst HC1SMe delivers record productivity, at neutral pH, for coupling of unprotected carbohydrates and nucleosides. The TON of 1,520 for nucleoside dimerization is the highest yet reported for metathesis of terminal olefins in water. Importantly, isomerization is also negligible. In contrast, decomposed AM causes extensive isomerization, indicating that this unwanted side-reaction remains a threat for NHC catalysts even in aqueous media. The selectivity of the CAAC catalyst for metathesis in water thus represents a key additional asset. Redesign to enable ambient-temperature operation would expand opportunities in chemical biology. Such “next-generation” catalysts are now being pursued in our laboratories.
Acknowledgments
This work was funded by the Research Council of Norway (RCN projects 288135 and 331967) and the Natural Sciences and Engineering Research Council of Canada (NSERC). We thank Apeiron Synthesis for gifts of AM and C1Cy•HBF4.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acscatal.4c02811.
Experimental details, NMR and UV–vis spectra (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Scinto S. L.; Bilodeau D. A.; Hincapie R.; Lee W.; Nguyen S. S.; Xu M.; am Ende C. W.; Finn M. G.; Lang K.; Lin Q.; Pezacki J. P.; Prescher J. A.; Robillard M. S.; Fox J. M. Bioorthogonal Chemistry. Nat. Rev. Methods Primers 2021, 1, 1–30. 10.1038/s43586-021-00028-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozhkin B.; Ward T. R. Bioorthogonal strategies for the in vivo synthesis or release of drugs. Bioorg. Med. Chem. 2021, 45, 116310. 10.1016/j.bmc.2021.116310. [DOI] [PubMed] [Google Scholar]
- Levin E.; Ivry E.; Diesendruck C. E.; Lemcoff N. G. Water in N-Heterocyclic Carbene-Assisted Catalysis. Chem. Rev. 2015, 115, 4607–4692. 10.1021/cr400640e. [DOI] [PubMed] [Google Scholar]
- For recent reviews of olefin metathesis in chemical biology, see:; a Matsuo T. Functionalization of Ruthenium Olefin-Metathesis Catalysts for Interdisciplinary Studies in Chemistry and Biology. Catalysts 2021, 11, 359. 10.3390/catal11030359. [DOI] [Google Scholar]; b Messina M. S.; Maynard H. D. Modification of Proteins using Olefin Metathesis. Mater. Chem. Front. 2020, 4, 1040–1051. 10.1039/C9QM00494G. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Isenegger P. G.; Davis B. G. Concepts of Catalysis in Site-Selective Protein Modifications. J. Am. Chem. Soc. 2019, 141, 8005–8013. 10.1021/jacs.8b13187. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Sabatino V.; Ward T. R. Aqueous Olefin Metathesis: Recent Developments and Applications. Beilstein J. Org. Chem. 2019, 15, 445–468. 10.3762/bjoc.15.39. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Vinogradova E. V. Organometallic Chemical Biology: An Organometallic Approach to Bioconjugation. Pure Appl. Chem. 2017, 89, 1619–1640. 10.1515/pac-2017-0207. [DOI] [Google Scholar]
- a Thomson A. L.; Gleeson E. C.; Belgi A.; Jackson W. R.; Izgorodina E. I.; Robinson A. J. Negating coordinative cysteine and methionine residues during metathesis of unprotected peptides. Chem. Commun. 2023, 59, 6917–6920. 10.1039/D3CC01476B. [DOI] [PubMed] [Google Scholar]; b Thomson A. L.; Robinson A. J.; Belgi A. Synthesis of Cystine-Stabilised Dicarba Conotoxin EpI: Ring-Closing Metathesis of Sidechain Deprotected, Sulfide-Rich Sequences. Mar. Drugs 2023, 21, 390. 10.3390/md21070390. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Gleeson E. C.; Jackson W. R.; Robinson A. J. Ring Closing Metathesis of Unprotected Peptides. Chem. Commun. 2017, 53, 9769–9772. 10.1039/C7CC04100D. [DOI] [PubMed] [Google Scholar]; d Cochrane S. A.; Huang Z.; Vederas J. C. Investigation of the ring-closing metathesis of peptides in water. Org. Biomol. Chem. 2013, 11, 630–639. 10.1039/C2OB26938D. [DOI] [PubMed] [Google Scholar]
- Monty O. B. C.; Nyshadham P.; Bohren K. M.; Palaniappan M.; Matzuk M. M.; Young D. W.; Simmons N. Homogeneous and Functional Group Tolerant Ring-Closing Metathesis for DNA-Encoded Chemical Libraries. ACS Comb. Sci. 2020, 22, 80–88. 10.1021/acscombsci.9b00199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X.; Fan L.; Phelps C. B.; Davie C. P.; Donahue C. P. Ruthenium Promoted On-DNA Ring-Closing Metathesis and Cross-Metathesis. Bioconjugate Chem. 2017, 28, 1625–1629. 10.1021/acs.bioconjchem.7b00292. [DOI] [PubMed] [Google Scholar]
- Nasibullin I.; Yoshioka H.; Mukaimine A.; Nakamura A.; Kusakari Y.; Chang T.-C.; Tanaka K. Catalytic olefin metathesis in blood. Chem. Sci. 2023, 14, 11033–11039. 10.1039/D3SC03785A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatino V.; Rebelein J. G.; Ward T. R. ″Close-to-Release″: Spontaneous Bioorthogonal Uncaging Resulting from Ring-Closing Metathesis. J. Am. Chem. Soc. 2019, 141, 17048–17052. 10.1021/jacs.9b07193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toussaint S. N. W.; Calkins R. T.; Lee S.; Michel B. W. Olefin Metathesis-Based Fluorescent Probes for the Selective Detection of Ethylene in Live Cells. J. Am. Chem. Soc. 2018, 140, 13151–13155. 10.1021/jacs.8b05191. [DOI] [PubMed] [Google Scholar]
- Schunck N. S.; Mecking S. In vivo Olefin Metathesis in Microalgae Upgrades Lipids to Building Blocks for Polymers and Chemicals. Angew. Chem., Int. Ed. 2022, 61, e202211285 10.1002/anie.202211285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skowerski K.; Szczepaniak G.; Wierzbicka C.; Gulajski L.; Bieniek M.; Grela K. Highly active catalysts for olefin metathesis in water. Catal. Sci. Technol. 2012, 2, 2424–2427. 10.1039/c2cy20320k. [DOI] [Google Scholar]
- Nagyházi M.; Turczel G.; Balla Á.; Szálas G.; Tóth I.; Gál G. T.; Petra B.; Anastas P. T.; Tuba R. Towards Sustainable Catalysis - Highly Efficient Olefin Metathesis in Protic Media Using Phase Labelled Cyclic Alkyl Amino Carbene (CAAC) Ruthenium Catalysts. ChemCatChem. 2020, 12, 1953–1957. 10.1002/cctc.201902258. [DOI] [Google Scholar]
- Wang Z. J.; Jackson W. R.; Robinson A. J. A simple and practical preparation of an efficient water soluble olefin metathesis catalyst. Green Chem. 2015, 17, 3407–3414. 10.1039/C5GC00252D. [DOI] [Google Scholar]
- Timmer B. J. J.; Ramström O. Acid-Assisted Direct Olefin Metathesis of Unprotected Carbohydrates in Water. Chem. Eur. J. 2019, 25, 14408–14413. 10.1002/chem.201903155. [DOI] [PubMed] [Google Scholar]
- As a counter-example, AM enabled stapling of an unprotected peptide in water, albeit at a loading of 40 mol% (ca. 2 turnovers), where HII failed. See:; Masuda S.; Tsuda S.; Yoshiya T. Ring-Closing Metathesis of Unprotected Peptides in Water. Org. Biomol. Chem. 2018, 16, 9364–9367. 10.1039/C8OB02778A. [DOI] [PubMed] [Google Scholar]
- Olszewski T. K.; Bieniek M.; Skowerski K. Ruthenium-Based Complexes Bearing Quaternary Ammonium Tags as Versatile Catalysts for Olefin Metathesis: From the Discovery to Practical Applications. Org. Process Res. Dev. 2020, 24, 125–145. 10.1021/acs.oprd.9b00483. [DOI] [Google Scholar]
- Jana A.; Grela K. Forged and fashioned for faithfulness-ruthenium olefin metathesis catalysts bearing ammonium tags. Chem. Commun. 2018, 54, 122–139. 10.1039/C7CC06535C. [DOI] [PubMed] [Google Scholar]
- Matsuo T.; Yoshida T.; Fujii A.; Kawahara K.; Hirota S. Effect of Added Salt on Ring-Closing Metathesis Catalyzed by a Water-Soluble Hoveyda-Grubbs Type Complex To Form N-Containing Heterocycles in Aqueous Media. Organometallics 2013, 32, 5313–5319. 10.1021/om4005302. [DOI] [Google Scholar]
- Foster J. C.; Grocott M. C.; Arkinstall L. A.; Varlas S.; Redding M. J.; Grayson S. M.; O’Reilly R. K. It is Better with Salt: Aqueous Ring-Opening Metathesis Polymerization at Neutral pH. J. Am. Chem. Soc. 2020, 142, 13878–13885. 10.1021/jacs.0c05499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church D. C.; Takiguchi L.; Pokorski J. K. Optimization of Ring-Opening Metathesis Polymerization (ROMP) under Physiologically Relevant Conditions. Polym. Chem. 2020, 11, 4492–4499. 10.1039/D0PY00716A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jongkind L. J.; Rahimi M.; Poole D.; Ton S. J.; Fogg D. E.; Reek J. N. H. Protection of Ruthenium Olefin Metathesis Catalysts by Encapsulation in a Self-assembled Resorcinarene Capsule. ChemCatChem. 2020, 12, 4019–4023. 10.1002/cctc.202000111. [DOI] [Google Scholar]
- Goudreault A. Y.; Walden D. M.; Nascimento D. L.; Botti A. G.; Steinmann S. N.; Michel C.; Fogg D. E. Hydroxide-Induced Degradation of Olefin Metathesis Catalysts: A Challenge for Metathesis in Alkaline Media. ACS Catal. 2020, 10, 3838–3843. 10.1021/acscatal.9b05163. [DOI] [Google Scholar]
- Scheu R.; Rankin B. M.; Chen Y.; Jena K. C.; Ben-Amotz D.; Roke S. Charge Asymmetry at Aqueous Hydrophobic Interfaces and Hydration Shells. Angew. Chem., Int. Ed. 2014, 53, 9560–9563. 10.1002/anie.201310266. [DOI] [PubMed] [Google Scholar]
- A computational study suggests a further potential advantage. Greater differences between the transition-state barriers for metathesis, vs decomposition, were calculated for HII catalysts with a negatively charged borate tag on the NHC backbone, vs analogues with a cationic tag or neutral HII. See:; Jawiczuk M.; Marczyk A.; Mlodzikowska-Pienko K.; Trzaskowski B. Impact of the Carbene Derivative Charge on the Decomposition Rates of Hoveyda-Grubbs-like Metathesis Catalysts. J. Phys. Chem. A 2020, 124, 6158–6167. 10.1021/acs.jpca.0c03096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulfonate ligands have been little explored in olefin metathesis. For examples, see:; a Bashir O.; Piche L.; Claverie J. P. 18-Electron Ruthenium Phosphine Sulfonate Catalysts for Olefin Metathesis. Organometallics 2014, 33, 3695–3701. 10.1021/om500212x. [DOI] [Google Scholar]; b Teo P.; Grubbs R. H. Facile Synthesis of Effcient and Selective Ruthenium Olefin Metathesis Catalysts with Sulfonate and Phosphate Ligands. Organometallics 2010, 29, 6045–6050. 10.1021/om1007924. [DOI] [Google Scholar]; c Monfette S.; Fogg D. E. Ruthenium Metathesis Catalysts Containing Chelating Aryloxide Ligands. Organometallics 2006, 25, 1940–1944. 10.1021/om050952j. [DOI] [Google Scholar]; d Lynn D. M.; Mohr B.; Grubbs R. H.; Henling L. M.; Day M. W. Water-Soluble Ruthenium Alkylidenes: Synthesis, Characterization, and Application to Olefin Metathesis polymerization in Protic Solvents. J. Am. Chem. Soc. 2000, 122, 6601–6609. 10.1021/ja0003167. [DOI] [Google Scholar]
- Shaughnessy K. H. Hydrophilic Ligands and Their Application in Aqueous-Phase Metal-Catalyzed Reactions. Chem. Rev. 2009, 109, 643–710. 10.1021/cr800403r. [DOI] [PubMed] [Google Scholar]
- Ding Y.; DeLorey J. L.; Clark M. A. Novel Catalyst System for Suzuki-Miyaura Coupling of Challenging DNA-Linked Aryl Chlorides. Bioconjugate Chem. 2016, 27, 2597–2600. 10.1021/acs.bioconjchem.6b00541. [DOI] [PubMed] [Google Scholar]
- Li J.-Y.; Huang H. Development of DNA-Compatible Suzuki-Miyaura Reaction in Aqueous Media. Bioconjugate Chem. 2018, 29, 3841–3846. 10.1021/acs.bioconjchem.8b00676. [DOI] [PubMed] [Google Scholar]
- For the resistance of CAAC catalysts to degradation of the metallacyclobutane intermediate via β-elimination, see:; a Nascimento D. L.; Fogg D. E. Origin of the Breakthrough Productivity of Ruthenium-CAAC Catalysts in Olefin Metathesis (CAAC = Cyclic Alkyl Amino Carbene). J. Am. Chem. Soc. 2019, 141, 19236–19240. 10.1021/jacs.9b10750. [DOI] [PubMed] [Google Scholar]; b Occhipinti G.; Nascimento D. L.; Foscato M.; Fogg D. E.; Jensen V. R. The Janus Face of High Trans-Effect Carbenes in Olefin Metathesis: Gateway to Both Productivity and Decomposition. Chem. Sci. 2022, 13, 5107–5117. 10.1039/D2SC00855F. [DOI] [PMC free article] [PubMed] [Google Scholar]; For their improved stability to trace water, vs NHC analogues, see:; c Blanco C. O.; Fogg D. E. Water-Accelerated Decomposition of Olefin Metathesis Catalysts. ACS Catal. 2023, 13, 1097–1102. 10.1021/acscatal.2c05573. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Blanco C.; Sims J.; Nascimento D. L.; Goudreault A. Y.; Steinmann S. N.; Michel C.; Fogg D. E. The Impact of Water on Ru-Catalyzed Olefin Metathesis: Potent Deactivating Effects Even at Low Water Concentrations. ACS Catal. 2021, 11, 893–899. 10.1021/acscatal.0c04279. [DOI] [PMC free article] [PubMed] [Google Scholar]; For their resistance to nucleophiles and base, see:; e Nascimento D. L.; Reim I.; Foscato M.; Jensen V. R.; Fogg D. E. Challenging Metathesis Catalysts with Nucleophiles and Bronsted Base: The Stability of State-of-the-Art Catalysts to Attack by Amines. ACS Catal. 2020, 10, 11623–11633. 10.1021/acscatal.0c02760. [DOI] [PMC free article] [PubMed] [Google Scholar]; For resistance to O2, see:; Ton S. J.; Fogg D. E. The Impact of Oxygen on Leading and Emerging Ru-Carbene Catalysts for Olefin Metathesis: An Unanticipated Correlation Between Robustness and Metathesis Activity. ACS Catal. 2019, 9, 11329–11334. 10.1021/acscatal.9b03285. [DOI] [Google Scholar]
- Ru-3 showed outstanding performance in the cross-metathesis of unsaturated phospholipids in methanol, but has not yet been deployed for ring-closing or cross-metathesis of terminal olefins in water. See: ref (13).
- Czégéni C. E.; Papp G.; Kathó Á.; Joó F. Water-soluble gold(I)-NHC complexes of sulfonated IMes and SIMes and their catalytic activity in hydration of alkynes. J. Mol. Catal. A 2011, 340, 1–8. 10.1016/j.molcata.2011.03.009. [DOI] [Google Scholar]
- The low lability of Ag-CAAC adducts relative to Ag-NHCs precluded CAAC transfer to [RuCl2(p-cymene)]2. See:; Romanov A. S.; Bochmann M. Synthesis, structures and photoluminescence properties of silver complexes of cyclic (alkyl)(amino)carbenes. J. Organomet. Chem. 2017, 847, 114–120. 10.1016/j.jorganchem.2017.02.045. [DOI] [Google Scholar]
- Attempted transmetalation of an anionic Ag-NHC adduct with RuCl2(p-cymene)(PPh3) yielded only heterobimetallic Ru-Ag products. See:; a Planer S.; Frosch J.; Koneczny M.; Trzybiński D.; Woźniak K.; Grela K.; Tamm M. Heterobimetallic Coinage Metal-Ruthenium Complexes Supported by Anionic N-Heterocyclic Carbenes. Chem. - Eur. J. 2021, 27, 15218–15226. 10.1002/chem.202102553. [DOI] [PMC free article] [PubMed] [Google Scholar]; A computational study attributed failure to the greater Ag-CNHC bond strength of the anionic carbene. See:; b Marczyk A.; Trzaskowski B. Ruthenium Metathesis Catalysts Bearing Anionic N-Heterocyclic Carbenes: A Computational Study on Failed Approaches to Their Synthesis. Organometallics 2023, 42, 689–695. 10.1021/acs.organomet.3c00068. [DOI] [Google Scholar]
- Marx V. M.; Sullivan A. H.; Melaimi M.; Virgil S. C.; Keitz B. K.; Weinberger D. S.; Bertrand G.; Grubbs R. H. Cyclic Alkyl Amino Carbene (CAAC) Ruthenium Complexes as Remarkably Active Catalysts for Ethenolysis. Angew. Chem., Int. Ed. 2015, 54, 1919–1923. 10.1002/anie.201410797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- In Skowerski’s improved two-step protocol, the ruthenium reagent is added only once the base is consumed, to limit unintended Ru-base reactions. See:; Gawin R.; Tracz A.; Chwalba M.; Kozakiewicz A.; Trzaskowski B.; Skowerski K. Cyclic Alkyl Amino Ruthenium Complexes-Efficient Catalysts for Macrocyclization and Acrylonitrile Cross Metathesis. ACS Catal. 2017, 7, 5443–5449. 10.1021/acscatal.7b00597. [DOI] [Google Scholar]
- Signals for the benzyliminium product are evident in the 1H-13C HMBC-NMR spectrum: see Figure S8. Abstraction of the benzylidene ligand from HII by nucleophiles has precedent. See:; a Ireland B. J.; Dobigny B. T.; Fogg D. E. Decomposition of a Phosphine-Free Metathesis Catalyst by Amines and Other Nitrogen Bases: Metallacyclobutane Deprotonation as a Major Deactivation Pathway. ACS Catal. 2015, 5, 4690–4698. 10.1021/acscatal.5b00813. [DOI] [Google Scholar]; For nucleophilic attack by a small carbene at the [Ru]=CHR carbon, see:; b Rufh S.; Goudreault A. Y.; Foscato M.; Jensen V. R.; Fogg D. E. Rapid Decomposition of Olefin Metathesis Catalysts by a Truncated N-Heterocyclic Carbene (NHC): Unprecedentedly Efficient Catalyst Quenching and NHC Vinylation. ACS Catal. 2018, 8, 11822–11826. 10.1021/acscatal.8b03123. [DOI] [Google Scholar]
- da Silva P. H. L.Synthesis of Ruthenium Metathesis Catalysts: Small Cyclic (Alkyl)(Amino)Carbene and Fluorophore-Tagged Ligands for Frontier Applications in Olefin Metathesis. M.Sc. Thesis, University of Ottawa, Ottawa, 2023. [Google Scholar]
- Blanco C.; Nascimento D. L.; Fogg D. E. Routes to High-Performing Ruthenium-Iodide Catalysts for Olefin Metathesis: Phosphine Lability Is Key to Efficient Halide Exchange. Organometallics 2021, 40, 1811–1816. 10.1021/acs.organomet.1c00253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boisvert E.-J. Y.; Ramos Castellanos R.; Ferguson M. J.; Fogg D. E. Abstraction of Trifluoroborane from Tetrafluoroborate: Li+-Assisted Borylation of Nucleophilic Carbenes. ChemCatChem. 2024, e202401003 10.1002/cctc.202401003. [DOI] [Google Scholar]
- The dissociation constant for AgI in MeOH at 25 °C is 5 orders of magnitude lower than that for AgCl: AgI 10–18, AgCl 10–13. See: Salomon M. In Physical Chemistry of Organic Solvent Systems; Covington A., Ed.; Springer: New York, 1973; p 161. [Google Scholar]
- Jeschek M.; Reuter R.; Heinisch T.; Trindler C.; Klehr J.; Panke S.; Ward T. R. Directed evolution of artificial metalloenzymes for in vivo metathesis. Nature 2016, 537, 661–665. 10.1038/nature19114. [DOI] [PubMed] [Google Scholar]
- Grimm A. R.; Sauer D. F.; Davari M. D.; Zhu L. L.; Bocola M.; Kato S.; Onoda A.; Hayashi T.; Okuda J.; Schwaneberg U. Cavity Size Engineering of a beta-Barrel Protein Generates Efficient Biohybrid Catalysts for Olefin Metathesis. ACS Catal. 2018, 8, 3358–3364. 10.1021/acscatal.7b03652. [DOI] [Google Scholar]
- van Lierop B. J.; Lummiss J. A. M.; Fogg D. E., Ring-Closing Metathesis. In Olefin Metathesis-Theory and Practice; Grela K., Ed.; Wiley: Hoboken, NJ, 2014; pp 85–152. [Google Scholar]
- For the limited isomerization induced by CAAC catalysts in organic solvent, see:; Butilkov D.; Frenklah A.; Rozenberg I.; Kozuch S.; Lemcoff N. G. Highly Selective Olefin Metathesis with CAAC-Containing Ruthenium Benzylidenes. ACS Catal. 2017, 7, 7634–7637. 10.1021/acscatal.7b02409. [DOI] [Google Scholar]
- Merrett K.; Liu W.; Mitra D.; Camm K. D.; McLaughlin C. R.; Liu Y.; Watsky M. A.; Li F.; Griffith M.; Fogg D. E. Synthetic Neoglycopolymer-Recombinant Human Collagen Hybrids as Biomimetic Crosslinking Agents in Corneal Tissue Engineering. Biomaterials 2009, 30, 5403–5408. 10.1016/j.biomaterials.2009.06.016. [DOI] [PubMed] [Google Scholar]
- Smith B. A. H.; Bertozzi C. R. The clinical impact of glycobiology: targeting selectins, Siglecs and mammalian glycans. Nat. Rev. Drug Discovery 2021, 20, 217–243. 10.1038/s41573-020-00093-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- This yield corresponds to the sum of the E/Z isomers reported in the final entry of Table 1 in ref (15).
- Kulkarni J. A.; Witzigmann D.; Thomson S. B.; Chen S.; Leavitt B. R.; Cullis P. R.; van der Meel R. The current landscape of nucleic acid therapeutics. Nat. Nanotechnol. 2021, 16, 630–643. 10.1038/s41565-021-00898-0. [DOI] [PubMed] [Google Scholar]
- Baraniak D.; Ruszkowski P.; Baranowski D.; Framski G.; Boryski J. Nucleoside dimers analogs containing floxuridine and thymidine with unnatural linker groups: synthesis and cancer line studies. Part III. Nucleosides, Nucleotides Nucleic Acids 2019, 38, 980–1005. 10.1080/15257770.2019.1641206. [DOI] [PubMed] [Google Scholar]
- Amblard F.; Nolan S. P.; Agrofoglio L. A. Metathesis strategy in nucleoside chemistry. Tetrahedron 2005, 61, 7067–7080. 10.1016/j.tet.2005.04.040. [DOI] [Google Scholar]
- Ou X.; Occhipinti G.; Boisvert E.-J. Y.; Jensen V. R.; Fogg D. E. Mesomeric Acceleration Counters Slow Initiation of Ruthenium-CAAC Catalysts for Olefin Metathesis. ACS Catal. 2023, 13, 5315–5325. 10.1021/acscatal.2c03828. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.