ABSTRACT
Background and hypothesis
Published literature suggests that sleep duration and quality may be affected in adults with chronic kidney disease. However, the relationship between these two entities remains a matter of debate. The objective of this systematic review and meta-analysis is to assess the effect of sleep duration and quality on chronic kidney disease.
Methods
A systematic review of the Medline/PubMed, Embase, Cochrane Library, and CINAHL databases was conducted for articles pertaining to the association between sleep duration and quality on chronic kidney disease. The main outcome was the hazard/risk ratio of chronic kidney disease in patients of varying sleep durations and quality.
Results
In total, 42 studies (2 613 971 patients) with a mean age of 43.55 ± 14.01 years were included in the meta-analysis. Compared with a reference range of 7 to 8 hours of sleep, short sleep durations of ≤4 hours (RR 1.41, 95% CI: 1.16 to 1.71, P < 0.01), ≤5 hours (RR 1.46, 95% CI: 1.22 to 1.76, P < 0.01), ≤6 hours (RR 1.18, 95% CI: 1.09 to 1.29, P < 0.01), and ≤7 hours (RR 1.19, 95% CI: 1.12 to 1.28, P < 0.01) were significantly associated with an increased risk of incident chronic kidney disease. Long sleep durations of ≥8 hours (RR 1.15, 95% CI: 1.03 to 1.28, P < 0.01) and ≥9 hours (RR 1.46, 95% CI: 1.28 to 1.68, P < 0.01) were also significantly associated with an increased risk of incident chronic kidney disease. Meta-regression did not find any significant effect of age, gender, geographical region, and BMI and an association with sleep duration and risk of incident chronic kidney disease.
Conclusion
Both short and long sleep durations were significantly associated with a higher risk of chronic kidney disease. Interventions targeted toward achieving an optimal duration of sleep may reduce the risk of incident chronic kidney disease.
Keywords: kidney failure, sleep hygiene, sleep quality
KEY LEARNING POINTS.
What was known:
Short and long sleep durations may be associated with an increased risk of chronic kidney disease, however, this relationship remains unclear.
This study adds:
This analysis of pooled data sought to investigate the relationship between sleep duration and the risk of chronic kidney disease. From 39 studies including 2 525 312 patients, short and long sleep durations were associated with a higher risk of developing chronic kidney disease.
Potential impact:
Interventions aimed at ensuring an optimal duration of sleep may be beneficial in reducing the risk of developing chronic kidney disease.
INTRODUCTION
Chronic kidney disease (CKD) is a pervasive and escalating health issue affecting 10%–15% of the world's adult population [1]. Its prevalence continues to rise due to the widespread adoption of sedentary lifestyles and longer life expectancies resulting from improved medical knowledge and healthcare [2, 3]. The burden on human health and healthcare systems alike cannot be overstated, with an increasing number of patients suffering from severe complications such as magnified risks of cardiovascular disease, organ failure necessitating replacement therapy, and multi-factor mortality [4]. As the name suggests, this progressive condition traditionally develops as we age and over the course of many years, influenced by cumulative effects of both genetic and environmental factors, which range from abrupt disturbances in physiology and function, to more subtle and often overlooked parts of daily life [5]. Sleep is one such risk factor that has gradually risen as a contributor to not just kidney disease, but overall health as well, alongside other crucial factors such as diet and exercise [6].
The prevalence of sleep disturbances varies by demographics, but can affect up to 20%–40% of people worldwide [7]. In recent times, many studies have documented myriad downstream effects and associations of sleep duration and quality with various aspects of health [8]. The relationship between sleep and kidney disease has also garnered increasing attention, with the emergence of a few large cohort studies [9, 10]. In general, the association of shortened and prolonged sleep durations, as well as poor sleep quality, with CKD is supported by a substantial body of research [9, 10, 11]. However, there are some contradictory findings, and further objective clarity is needed to fully characterize this complex relationship, especially in different socio-cultural contexts [9, 11, 12].
The proposed mechanisms and pathophysiology underlying this relationship are diverse and many. Certain diseases, such as obstructive sleep apnea and other sleep disorders, can lead to disturbances in both sleep duration and quality. These are processes that are detrimental to kidney function such as sympathetic activation, nocturnal hypertension, hypoxia, oxidative stress, inflammation, and glomerular endothelial dysfunction [13, 14, 15]. Insufficient sleep, insomnia, and other disorders affecting circadian rhythm have also been linked to poor cognitive and emotional function, downstream hormone imbalances such as hypercortisolism, and other risk factors for CKD such as aspects of metabolic syndrome [16]. Furthermore, CKD and declining renal function itself can also impact sleep, with an increased prevalence of restless leg syndrome and other potential effects of uremia and other deranged electrolytes, adding to the complexity of CKD management [12, 17]. Patients on renal replacement therapy often report poorer sleep quality compared with healthy controls, along with poorer sleep efficiency and fragmentation index [18]. Various modalities of renal replacement therapy have their treatment-related burdens for the patient, which may affect their sleep. Apart from anemia and fluid and toxin accumulation, patients can also suffer from pain, pruritus, and psychological problems [19, 20].
In summary, CKD and sleep are intricate and interrelated conditions that require integrated management to optimal outcomes in patients [9, 11, 12]. However, the contributing value of poor sleep to the increasing incidence of CKD, and therefore its role in the prevention and management of CKD remains unclear [9, 10, 12]. In a world on the cusp of a paradigm shift toward preventive medicine, the understanding and education of upstream risk factors must improve, and the need to optimize an aspect as fundamental and integral to human life as sleep remains a topmost priority and, indeed, necessity [9]. This systematic review and meta-analysis aims to provide an updated overview of the current body of knowledge around the relationship between sleep and the risk of CKD, with the hypothesis that there is a significant impact of poor sleep on the risk of CKD, which merely awaits objective characterization for appropriate intervention. The authors believe this study will provide much-needed clarity and guidance on this important frontier of health, driving progress toward improved CKD prevention and management strategies.
MATERIALS AND METHODS
Data sources and searches
The protocol for this review was registered with PROSPERO (CRD42023420030). With reference to the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) guidelines, a search was conducted on Medline/PubMed, Embase, the Cochrane Register for Controlled Trials (CENTRAL), and the Cumulative Index of Nursing and Allied Health (CINAHL) databases for studies published from inception to 19 June 2023 [21]. The search strategy used a combination of free text words and medical subject heading terms (Supplement 1). The reference lists of systematic reviews and included articles and the gray literature were also screened manually to identify additional studies for a comprehensive search. Contact with authors of included studies was made where feasible to collect supplementary data.
Study selection
Two blinded authors (M.S. and T.T.) independently screened titles and abstracts to check the eligibility for inclusion using the online platform Rayyan, with disputes being resolved through consensus from a third independent author (J.H.K.) [22]. Reviewers then assessed the full texts of shortlisted studies against the following pre-defined inclusion and exclusion criteria.
The inclusion criteria were (i) observational studies that investigated the association between sleep duration and CKD in participants aged 18 years or older, (ii) full-text studies, (iii) studies published in a peer-reviewed journal, and (iv) studies published in the English language. Participants with CKD were defined as individuals with an estimated glomerular filtration rate (eGFR) <60 ml/min/1.73 m2 over 3 months or more, irrespective of cause, in accordance with the published definitions of the National Kidney Foundation Kidney Disease Outcomes Quality Initiative (KDIQI) [23]. For the outcome of incident CKD, studies were eligible if they included patients without a diagnosis of CKD, not on hemodialysis or peritoneal dialysis, or not receiving renal transplant at baseline. For the outcome of prevalent CKD, studies were eligible if they included patients who had a diagnosis of CKD, not on hemodialysis or peritoneal dialysis, or not receiving renal transplant at baseline. The included studies employed subjective and objective sleep measurements, which comprised subjective sleep questionnaires such as the Pittsburgh Sleep Quality Index (PSQI), and objective sleep measurements such as polysomnography.
The exclusion criteria were (i) studies including participants <18 years old, (ii) animal studies, (iii) case reports, (iv) in vitro studies, and (v) reviews.
Data collection
Data from included articles were collected by two blinded, independent reviewers (M.S. and T.T.) in duplicate onto a structured proforma specifically designed for the study and piloted beforehand on a sample of selected studies. Disagreement was resolved by discussion and consensus with a third reviewer (J.H.K.). The data collection sheet contained key characteristics of studies, according to the Population, Intervention, Comparison, Outcome, Study Type framework [24, 25]. Data collected included geographical region; sample size, inclusion and exclusion criteria; baseline characteristics of participants such as mean age, gender, ethnicity, and mean BMI; comorbidities such as diabetes, hypertension, hyperlipidemia, coronary artery disease, and smoking status CKD definition; details of how CKD was assessed; stage of CKD stage; summary measures of incident; and prevalent CKD across various sleep durations and sleep quality as measured with the PSQI, with corresponding 95% confidence intervals, publication year, year of study completion, and study design. The PSQI is a self-rated questionnaire that assesses sleep quality and disturbances over a 1-month time interval, with seven components involving subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleeping medication, and daytime dysfunction [26].
Risk of bias assessment
Two blinded, independent reviewers (M.S. and T.T.) conducted the risk of bias assessment of included articles using the Risk of Bias in Non-randomized Studies of Exposures (ROBINS-E) tool. The ROBINS-E is designed specifically to assess the risk of bias of cohort studies on the premises of confounding, measurement of exposure, selection of participants, post-exposure interventions, missing data, measurement of outcome, and selection of the reported result [27]. Exclusion of low-quality studies was performed only during sensitivity analyses in an objective to explore the result's heterogeneity. Otherwise, all studies were retained independently of their quality, following Glass's approach [28].
Publication bias
Publication bias was assessed by visual inspection of the respective funnel plots [29]. The asymmetry of funnel plots was further assessed using Egger's linear regression method and Begg's test [30]. Missing studies were imputed using the trim-and-fill method [31].
Statistical analysis
All analysis was conducted in R Studio (version 4.2.2) using the meta package [32]. Descriptive statistics were presented as means and standard deviations for continuous variables and counts for categorical variables. When studies reported medians and interquartile ranges, these were converted to means and standard deviations using the methods of Wan et al. [33] A conventional pairwise meta-analysis was done in risk ratios. The meta-analysis compared CKD risk for short (<7 hours) and long (>8 hours), versus referent sleep duration (mostly 7 to 8 hours). Where the baseline category differed from 7 to 8 hours, the baseline category was switched using the generalized least squares method [34]. In studies where multiple categories of short or long sleep were available, a single estimate was obtained using the generalized least squares method [34]. Random-effects models were used in all analysis regardless of heterogeneity as recent evidence suggests that it provides more robust outcome measures compared to the alternative fixed effects models [35]. The Hartung–Knapp method was also implemented to adjust the confidence interval of the overall estimate [36].
Statistical heterogeneity was assessed via I2 and Cochran Q test values, where an I2 value of <25% represented low heterogeneity and an I2 value ≥25% represented moderate to high heterogeneity [37, 38]. A Cochran Q test with a P value of ≤0.10 was considered significant for heterogeneity.
Where 10 or more studies were available for a particular outcome, additional analyses were conducted to evaluate potential sources of heterogeneity between studies [39]. Apart from subgroup analyses, univariate random-effects meta-regression were conducted, and effect moderators were confirmed using permutation testing with 1000 iterations to eliminate spurious results [40, 41]. Statistical significance was considered for outcomes with a P ≤ 0.05. Leave-out-one influence analyses were performed to examine the influence of individual studies on the overall findings. Cumulative meta-analyses were performed ranked by year published, to examine the stability of published data over time.
Certainty of evidence
The quality of pooled evidence was evaluated using the Grading of Recommendations Assessment, Development and Evaluations (GRADE) framework [42]. The GRADE framework rates each study on the basis of study design, consistency, directness, risk of bias, precision, and publication bias. For each outcome, the level of evidence was rated as high, moderate, low, or very low.
RESULTS
Literature search
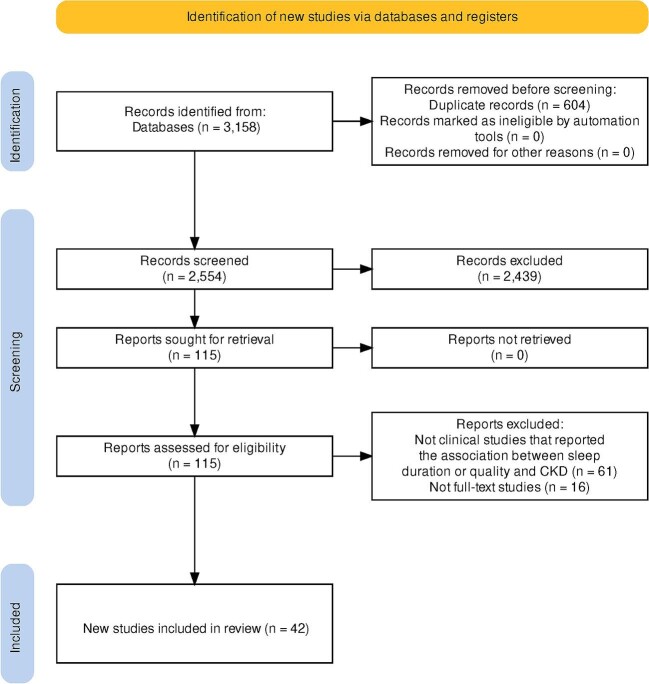
In the initial search, 3158 articles were included after removal of duplicates, of which 115 were selected for full-text review: 42 articles met the final inclusion criteria [9–11, 12, 43–79, 80], 21 studies were prospective cohort studies, and 21 studies were cross-sectional studies. The inter-rate reliability as assessed by Cohen's kappa was 0.98 [81]. Figure 1 shows the PRISMA flow diagram that summarizes the study selection process.
Figure 1:
PRISMA flow diagram.
Study characteristics
The total sample size was 2 613 971 participants. The mean age of the participants was 43.55 ± 14.01 years, and 73% of patients were male. The included studies were completed between 1998 and 2020. Three studies measured sleep duration and quality by polysomnography or actigraphy, whereas 39 studies measured sleep duration and quality by self-reported sleep questionnaire. Table 1 and 2 contains the summary of the key characteristics for included articles. Of the 43 included studies, 25 were rated at low risk of bias, 10 at moderate risk of bias, and seven at high risk of bias (Supplement 2).
Table 1:
Characteristics of included studies.
| Author | Study design | Inclusion criteria | Geographical region | Stage of CKD | Method of diagnosis of CKD | Method of measurement of sleep duration and quality |
|---|---|---|---|---|---|---|
| Agarwal et al. [12] | Cohort study | Patients with CKD or on hemodialysis | North America | 3 to 5 | eGFR measurements | Actigraphy |
| Bo et al. [9] | Cohort study | Patients from a standard medical examination program conducted by the MJ Health Management Institution (Taipei, Taiwan) from 1996 to 2014 | Asia | 3 to 5 | eGFR measurements | Self-reported questionnaire |
| Cha et al. [11] | Cohort study | Patients from the KoGES Study | Asia | 3 to 5 | eGFR measurements | Self-reported questionnaire |
| Chang et al. [10] | Cohort study | Patients from the KHANES Survey over 19 years old with information on sleep parameters | Asia | 3 to 5 | eGFR measurements | Self-reported questionnaire |
| Choi et al. [43] | Cross-sectional study | Patients from the KoGES study | Asia | 3 to 5 | eGFR measurements | Self-reported questionnaire |
| Cohen et al. [44] | Cross-sectional study | Predialysis patients with CKD | North America | 3 to 5 | eGFR measurements | Self-reported questionnaire |
| Del Brutto et al. [45] | Cross-sectional study | Atahualpa residents aged ≥60 years who were offered a brain MRI | South America | 3 to 5 | eGFR measurements | Self-reported questionnaire |
| Fang et al. [46] | Cross-sectional study | Patients from the KHANES Survey | Asia | 3 to 5 | eGFR measurements | Self-reported questionnaire |
| Fujibayashi et al. [47] | Cross-sectional study | Patients who underwent annual health checkups between May 2006 to August 2010 | Asia | 3 to 4 | eGFR measurements | Self-reported questionnaire |
| Geng et al. [48] | Cohort study | Patients from the Singapore Chinese Health Study with information on daily sleep duration | Asia | 5 | eGFR measurements | Self-reported questionnaire |
| Gu et al. [49] | Cohort study | Adult Patients from the KHANES with type 2 diabetes mellitus | Asia | 3 to 5 | eGFR measurements | Self-reported questionnaire |
| Hirano et al. [50] | Cohort study | Patients from the St Luke's International Hospital in Tokyo | Asia | 3 to 5 | eGFR measurements | Self-reported questionnaire |
| Jean-Lous et al. [51] | Cross-sectional study | Patients from the National Health Interview Survey | North America | 3 to 5 | eGFR measurements | Self-reported questionnaire |
| Jiang et al. [52] | Cohort study | Patients from the China Health and Retirement Longitudinal Study | Asia | 3 to 5 | Self-reported | Self-reported questionnaire |
| Karatas et al. [53] | Cohort study | Patients who were predialysis or undergoing hemodialysis | Africa | 3 to 5 | eGFR measurements | Self-reported questionnaire |
| Kim et al. [54] | Cohort study | Patients from the Kangbuk Samsung Health Study | Asia | 3 to 5 | eGFR measurements | Self-reported questionnaire |
| Kucuk et al. [55] | Cohort study | Patients with CKD, undergoing hemodialysis, peritoneal dialysis, or post-renal transplant | Asia | 4 to 5 | eGFR measurements | Self-reported questionnaire |
| Lee et al. [56] | Cohort study | Patients with CKD or ESRD | North America | 3 to 5 | eGFR measurements | Self-reported questionnaire |
| Li et al. [57] | Cross-sectional study | Patients from the Kailuan cohort study | Asia | 3 to 5 | eGFR measurements | Self-reported questionnaire |
| Lu et al. [58] | Cross-sectional study | Patients from the 2013, 2014 and 2016 Behavioral Risk Factor Surveillance System | North America | 3 to 5 | Self-reported | Self-reported questionnaire |
| Meng et al. [59] | Cross-sectional study | Cross-sectional study from the Tianjin Metabolic Diseases Hospital | Asia | 3 to 5 | eGFR measurements | Self-reported questionnaire |
| Nagai et al. [60] | Cross-sectional study | Patients from the Jichi Medical School Ambulatory Blood Pressure Monitoring study | Asia | 3 to 5 | eGFR measurements | Self-reported questionnaire |
| Nakajima et al. [61] | Cohort study | Patients from the NAFLD in Gifu Area, Longitudinal Analysis study | Asia | 3 to 5 | eGFR measurements | Self-reported questionnaire |
| Nishimura et al. [62] | Cohort study | Patients with type 2 diabetes | Asia | 3 to 5 | eGFR measurements | Polysomnography |
| Petrov et al. [63] | Cohort study | Patients from the 2009–2012 National Health and Nutrition Examination Survey | Asia | 1 to 5 | eGFR measurements | Self-reported questionnaire |
| Pinto et al. [64] | Cross-sectional study | Patients with CKD compared with healthy controls | Asia | 5 | eGFR measurements | Self-reported questionnaire |
| Platinga et al. [65] | Cross-sectional study | Patients from the National Health and Nutrition Examination Survey 2005–2008 | North America | 3 to 4 | eGFR measurements | Self-reported questionnaire |
| Sabbatini et al. [66] | Cross-sectional study | Kidney graft recipients and patients on hemodialysis, compared with healthy controls | South America | 5 | eGFR measurements | Self-reported questionnaire |
| Salifu et al. [67] | Cross-sectional study | Cross-sectional survey of the National Health Interview Survey | North America | 3 to 5 | Self-reported | Self-reported questionnaire |
| Sasaki et al [68] | Cross-sectional study | Patients who underwent annual health checkups between April 2003 and March 2004 | North America | 3 to 5 | eGFR measurements | Self-reported questionnaire |
| Sun et al. [69] | Cross-sectional study | Patients from the 2011 and 2015 surveys of the CHARLS | Europe | 3 to 5 | eGFR measurements | Self-reported questionnaire |
| Tan et al. [70] | Cross-sectional study | Patients from the Singapore Malay Eye Study and Singapore Indian Eye Study | North America | 3 to 5 | eGFR measurements | Self-reported questionnaire |
| Tu et al. [71] | Cohort study | Patients with CKD not on dialysis | Asia | 3 to 5 | eGFR measurements | Self-reported questionnaire |
| Unruh et al. [72] | Cohort study | Patients undergoing hemodialysis, compared with healthy controls | Asia | 5 | eGFR measurements | Polysomnography |
| Wang et al. [73] | Cross-sectional study | Patients from CHARLS | Asia | 3 to 5 | Self-reported | Self-reported questionnaire |
| Warsame et al. [74] | Cross-sectional study | Patients from the NHANES cohort aged ≥60 years of age with measured serum creatine and at least one assessment of cognitive function | Asia | 3 to 5 | eGFR measurements | Self-reported questionnaire |
| Wu et al. [75] | Cross-sectional study | Participants from the NHANES | North America | 3 to 5 | eGFR measurements | Self-reported questionnaire |
| Xu et al. [76] | Cohort study | Patients from the CHARLS study | Asia | 3 to 5 | eGFR measurements | Self-reported questionnaire |
| Yamamoto et al. [77] | Cohort study | Patients who underwent annual health checkups between April 2006 and 2010 | Asia | 3 to 5 | eGFR measurements | Self-reported questionnaire |
| Ye et al. [78] | Cross-sectional study | Patients from the risk evaluation of cancers in Chinese diabetic individuals: a longitudinal (REACTION) study |
North America | 3 to 5 | eGFR measurements | Self-reported questionnaire |
| Yu et al. [79] | Cross-sectional study | Patients from the KHANES >19 years old with information on sleep parameters | North America | 3 to 5 | eGFR measurements | Self-reported questionnaire |
| Zhang et al. [80] | Cohort study | Patients from the UK Biobank | Asia | 3 to 5 | eGFR measurements | Self-reported questionnaire |
CHARLS, China Health and Retirement Longitudinal Study; DM, diabetes mellitus; HTN, hypertension; HLD, hyperlipidemia; KoGES, Korea Genome and Epidemiology Study; KHANES, Korea National Health and Nutritional Examination; NHANES, National Health and Nutrition Examination Survey; NR, not reported
Table 2:
Characteristics of included patients.
| Author | Total population | Number with CKD | Mean age (years) | Male (%) | Mean BMI (kg/m2) | Obesity (%) | DM (%) | HTN (%) | HLD (%) | CAD (%) | Smoking (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Agarwal et al. [12] | 280 | 261 | 61.10 | 83 | 29.2 | NR | 47.00 | NR | NR | NR | NR |
| Bo et al. [9] | 194 039 | 8519 | 38.20 | 51 | 22.8 | NR | NR | NR | NR | 0.00 | 23.00 |
| Cha et al. [11] | 2837 | 642 | 55.40 | 50 | NR | 31.00 | 34.91 | 11.66 | NR | 0.00 | 40.70 |
| Chang et al. [10] | 26 249 | 963 | 39.30 | 55 | 22.1 | 24.80 | 3.20 | 13.38 | 11.05 | NR | NR |
| Choi et al. [43] | 1360 | 125 | 60.00 | 40 | 24.5 | NR | 12.10 | 37.90 | 24.00 | 7.60 | 11.30 |
| Cohen et al. [44] | 153 | 92 | 61.50 | 51 | NR | NR | 46.10 | NR | NR | NR | NR |
| Del Brutto et al. [45] | 314 | 182 | 71.10 | 31 | NR | NR | NR | NR | NR | NR | NR |
| Fang et al. [46] | 17 418 | 963 | 51.30 | 44 | 23.9 | NR | 24.50 | 24.50 | NR | NR | 39.48 |
| Fujibayashi et al. [47] | 25 493 | 2748 | 48.50 | 74 | 23.1 | 24.90 | NR | NR | NR | 2.20 | 55.20 |
| Geng et al. [48] | 63 147 | 1143 | 56.40 | 44 | 23.1 | NR | 8.90 | 23.60 | NR | 0.00 | 31.30 |
| Gu et al. [49] | 5930 | 742 | 58.70 | 49 | 24.8 | NR | 100.00 | 60.00 | 29.80 | 0.00 | 48.10 |
| Hirano et al. [50] | 76 705 | 4214 | 45.80 | 49 | NR | 19.20 | 2.00 | 7.70 | 4.60 | 0.00 | 38.70 |
| Jean-Lous et al. [51] | 123 967 | 305 | 42.00 | 50 | NR | 27.70 | 8.20 | 24.10 | NR | 3.00 | 42.40 |
| Jiang et al. [52] | 11 677 | 560 | 58.70 | 45 | 23.4 | NR | NR | NR | NR | 0.00 | 69.90 |
| Karatas et al. [53] | 240 | 149 | 52.80 | 52 | NR | NR | NR | NR | NR | NR | NR |
| Kim et al. [54] | 241 607 | 644 | 40.60 | 56 | 24.5 | 39.40 | 6.10 | 16.50 | NR | 1.60 | 61.40 |
| Kucuk et al. [55] | 140 | 140 | 43.00 | 51 | NR | NR | NR | NR | NR | NR | NR |
| Lee et al. [56] | 500 | 500 | 60.00 | 60 | 28.6 | NR | 8.66 | 50.40 | NR | 55.10 | NR |
| Li et al. [57] | 11 040 | 425 | 53.70 | NR | 25.0 | NR | 26.00 | 67.30 | NR | 1.35 | 44.20 |
| Lu et al. [58] | 1 191 768 | 40 866 | NR | 51 | NR | NR | NR | NR | NR | NR | NR |
| Meng et al. [59] | 336 | 102 | 52.70 | 53 | 26.7 | NR | NR | NR | NR | NR | 37.00 |
| Nagai et al. [60] | 514 | 194 | 72.30 | 37 | 24.6 | NR | 14.70 | NR | 21.10 | 0.00 | 21.30 |
| Nakajima et al. [61] | 2201 | 2201 | 42.10 | 54 | 22.0 | NR | NR | NR | NR | 0.00 | 63.00 |
| Nishimura et al. [62] | 462 | 303 | 58.50 | 86 | 27.0 | NR | 100.00 | 68.60 | NR | NR | 23.40 |
| Petrov et al. [63] | 8030 | 601 | 46.40 | 51 | NR | 33.90 | 7.70 | 31.00 | NR | 3.10 | NR |
| Pinto et al. [64] | 39 | 20 | 54.90 | NR | NR | NR | NR | NR | NR | NR | NR |
| Platinga et al. [65] | 9110 | 1805 | 47.20 | 50 | NR | 33.80 | 8.30 | 42.00 | NR | 8.50 | 19.90 |
| Sabbatini et al. [66] | 715 | 546 | 40.80 | 60 | 23.6 | NR | NR | NR | NR | NR | NR |
| Salifu et al. [67] | 128 479 | 2480 | 59.60 | 45 | NR | 36.70 | 34.20 | 68.00 | NR | 22.60 | 54.50 |
| Sasaki et al. [68] | 3600 | 182 | 47.00 | 78 | 23.1 | NR | 0.00 | 0.00 | 0.00 | 0.00 | 63.40 |
| Sun et al. [69] | 11 339 | 887 | 58.80 | 47 | 23.8 | 10.72 | 11.60 | 37.90 | NR | NR | 39.28 |
| Tan et al. [70] | 1258 | 268 | 64.60 | 49 | 27.4 | 67.50 | 100.00 | 91.50 | NR | 20.50 | 11.10 |
| Tu et al. [71] | 326 | 326 | 57.87 | 73 | 26.5 | NR | 53.60 | 69.70 | NR | NR | 16.60 |
| Unruh et al. [72] | 184 | 46 | 62.70 | 72 | 28.0 | NR | 32.60 | NR | NR | 32.60 | 56.50 |
| Wang et al. [73] | 4003 | NR | 52.48 | 43 | 24.1 | NR | 5.30 | 18.70 | NR | 8.20 | 36.60 |
| Warsame et al. [74] | 3215 | 1061 | NR | 46 | 27.9 | NR | 23.10 | 75.40 | NR | 8.80 | 50.10 |
| Wu et al. [75] | 8269 | 223 | 48.50 | 49 | 30.0 | NR | 13.80 | 37.90 | 32.20 | 5.74 | NR |
| Xu et al. [76] | 4188 | 346 | 58.90 | 43 | 23.7 | NR | 8.00 | NR | NR | 12.20 | 28.60 |
| Yamamoto et al. [77] | 6834 | 550 | 34.00 | 50 | 22.0 | NR | 0.50 | 2.40 | 1.70 | 0.30 | 19.10 |
| Ye et al. [78] | 33 850 | NR | 58.10 | 33 | 24.7 | 15.10 | 22.80 | 60.30 | NR | NR | 15.20 |
| Yu et al. [79] | 19 994 | NR | 52.50 | 52 | 24.0 | NR | 7.00 | 1.10 | 8.00 | NR | 0.90 |
| Zhang et al. [80] | 370 671 | 6365 | 56.40 | 45 | 29.2 | NR | 8.90 | NR | NR | NR | 45.80 |
DM, diabetes mellitus; HTN, hypertension; HLD, hyperlipidemia; NR, not reported
Incident CKD
Meta-analysis for short sleep duration
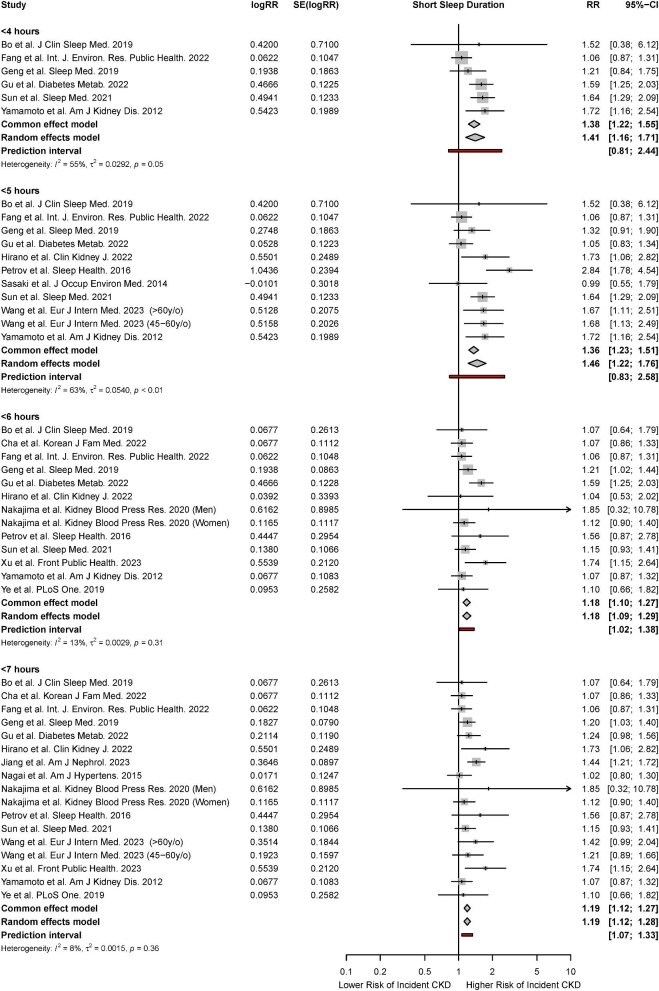
There were 14 studies that reported the association of short sleep duration with the risk of incident CKD [9, 11, 46, 48, 49, 50, 52, 60, 61, 63, 69, 76, 77, 78]. Based on the random-effects model, short sleep durations of ≤4 hours (HR, 1.41; 95% CI, 1.16 to 1.71; P < 0.01, I2 = 55%), ≤5 hours (HR, 1.46; 95% CI, 1.22 to 1.76; P < 0.01, I2 = 63%), ≤6 hours (HR, 1.18; 95% CI, 1.09 to 1.29; P < 0.01, I2 = 13%), and ≤7 hours (HR, 1.19; 95% CI, 1.12 to 1.28; P < 0.01, I2 = 8%) were associated with a significantly increased risk of incident CKD. Significant associations were observed across both fixed- and random-effects models (Fig. 2).
Figure 2:
Forest plot for association of short sleep duration with a risk of incident CKD.
Given that the meta-analysis of short sleep duration and risk of incident CKD contained sufficient studies for further analyses, meta-regression was also performed to examine the influence of study-level covariates on the risk of incident CKD. Meta-regression found that the percentage of male gender was a statistically significant effect moderator of the association of sleep duration ≤4 hours and risk of incident CKD, accounting for 67.22% of heterogeneity and leaving 43.72% of residual heterogeneity. The pooled RR increased by a factor of 0.97 (95% CI, 0.60 to 4.41) per 1% increase in number of male participants. The bubble plot is shown in Supplement 3. Other characteristics including mean age, year of study completion, percentage of male participants, percentage of obese participants, and mean BMI were not significant effect moderators of the risk of incident CKD for the other classifications of sleep duration (Supplement 4).
For all outcomes, although visual inspection suggested the possibility of funnel plot asymmetry, this was not shown by Egger's test. Trim and fill resulted in minimal change to the pooled effect sizes. For all analyses, leave-one-out influence analysis showed that no single study had a drastic change on the pooled risk ratio, and cumulative meta-analysis showed a significant and stable pooled effect size (Supplements 5 and 6).
Meta-analysis for long sleep duration
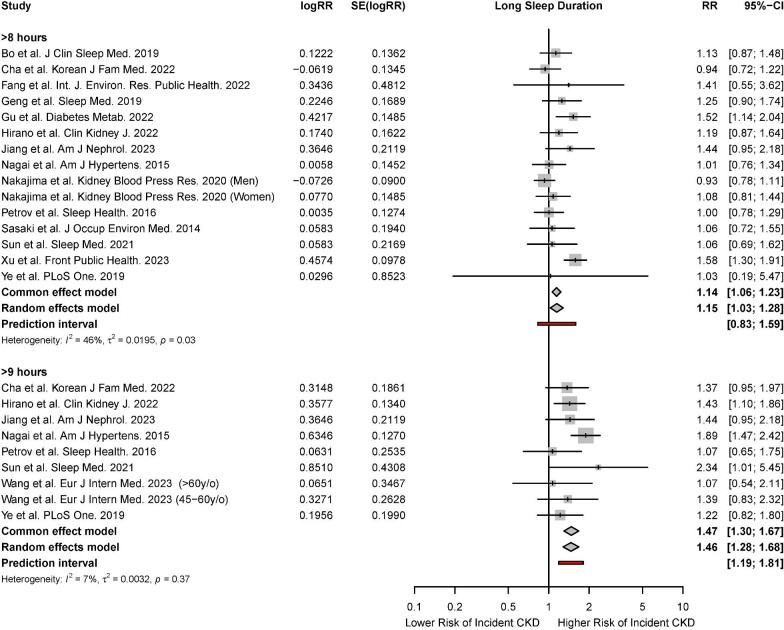
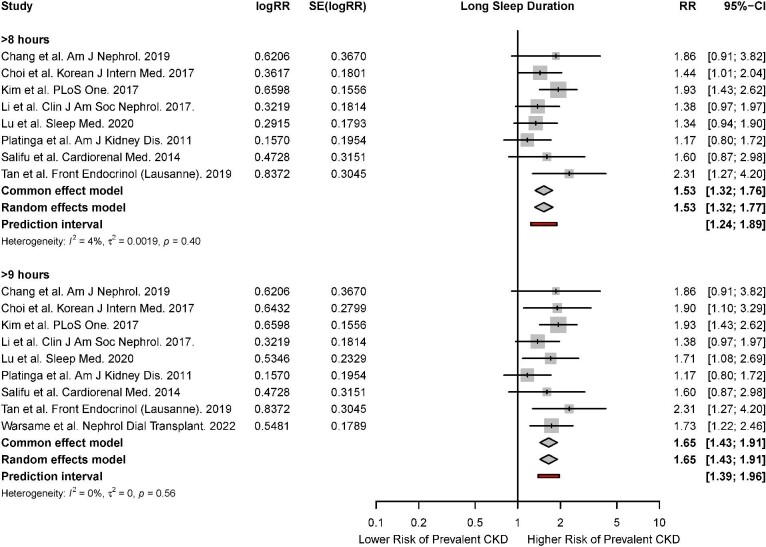
There were 14 studies that reported the association of long sleep duration with incident CKD [9, 11, 46, 48, 49, 50, 52, 60, 61, 63, 68, 69, 76, 78]. Based on the random-effects model, long sleep durations of ≥8 hours (HR, 1.15; 95% CI, 1.03 to 1.28; P < 0.01, I2 = 46%) and ≥9 hours (HR, 11.46; 95% CI, 1.28 to 1.68; P < 0.01, I2 = 7%) were associated with a significantly increased risk of incident CKD (Fig. 3).
Figure 3:
Forest plot for association of long sleep duration with risk of incident CKD.
Meta-regression was also performed for sleep durations ≥8 hours to examine the influence of study-level covariates on the risk of incident CKD. Meta-regression found that mean age, year of study completion, percentage of male participants, percentage of obese participants, and mean BMI were not significant effect moderators of the risk of incident CKD (Supplement 4).
For sleep durations ≥8 hours, while visual inspection suggested the possibility of funnel plot asymmetry, this was not shown by Egger's test. Trim and fill resulted in minimal change to the pooled effect sizes. For all analyses, leave-one-out influence analysis showed that no single study had a drastic change on the pooled risk ratio, and cumulative meta-analysis showed a significant and stable pooled effect size (Supplements 5 and 6).
Prevalent CKD
Meta-analysis for short sleep duration
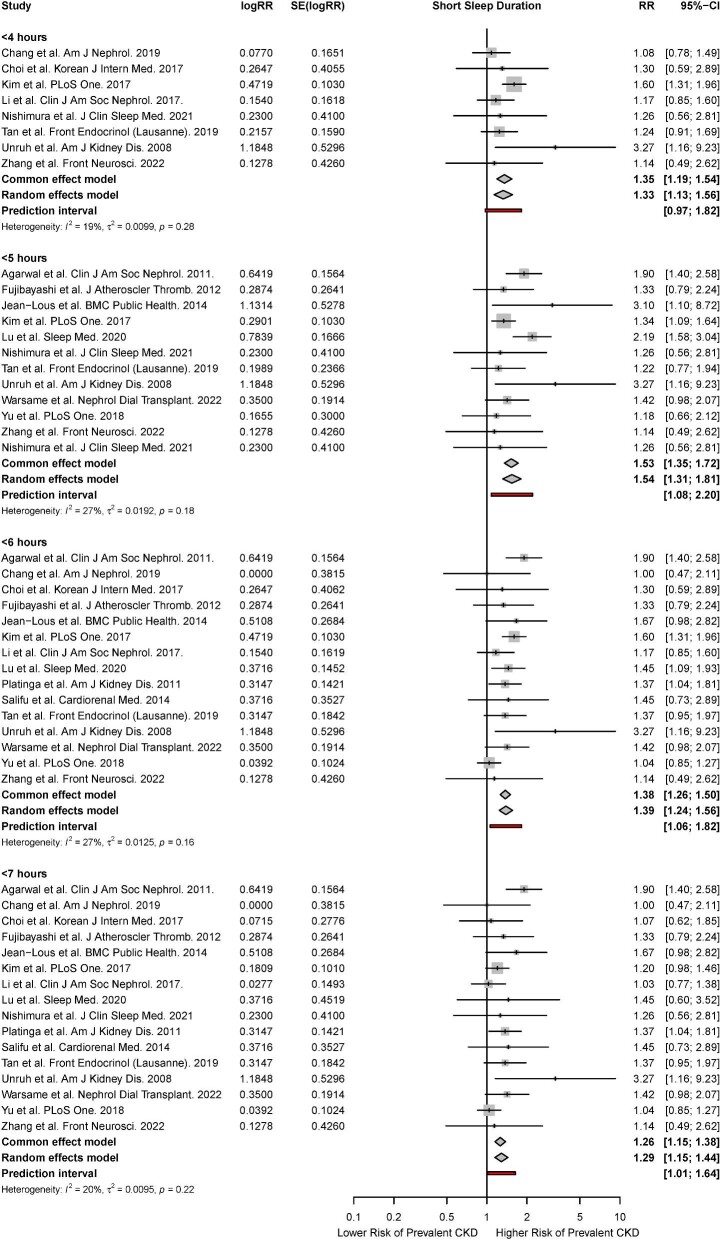
There were 16 studies that reported the association of short sleep duration with the risk of prevalent CKD [10, 12, 43, 47, 51, 54, 57, 58, 62, 65, 67, 70, 72, 74, 79, 80]. Based on the random-effects model, short sleep durations of ≤4 hours (RR, 1.33; 95% CI, 1.13 to 1.56; P < 0.01, I2 = 19%), ≤5 hours (RR, 1.54; 95% CI, 1.31 to 1.81; P < 0.01, I2 = 27%), ≤6 hours (RR, 1.39; 95% CI, 1.24 to 1.56; P < 0.01, I2 = 27%), and ≤7 hours (RR, 1.29; 95% CI, 1.16 to 1.44; P < 0.01, I2 = 20%) were associated with a significantly increased risk of prevalent CKD. Significant associations were observed across both fixed- and random-effects models (Fig. 4). Meta-regression found that mean age, year of study completion, percentage of male participants, percentage of obese participants, and mean BMI were not significant effect moderators of the risk of prevalent CKD (Supplement 4). Subgroup analyses stratified by study methodology found that the association of short sleep duration with prevalent CKD remained significant across cohort and cross-sectional studies. Subgroup analyses also did not find any significant differences in association of short sleep duration with prevalent CKD between studies that used objective and subjective measures of sleep duration (Supplement 8).
Figure 4:
Forest plot for association of short sleep duration with risk of prevalent CKD.
Meta-analysis for long sleep duration
There were nine studies that reported the association of long sleep duration with the risk of prevalent CKD [10, 43, 54, 57, 58, 65, 67, 70, 74]. Based on the random-effects model, long sleep durations of ≥8 hours (RR, 1.53; 95% CI, 1.32 to 1.77; P < 0.01, I2 = 4%) and ≥9 hours (RR, 1.65; 95% CI, 1.43 to 1.91; P < 0.01, I2 = 0%) were associated with a significantly increased risk of prevalent CKD. Significant associations were observed across both fixed- and random-effects models (Fig. 5).
Figure 5:
Forest plot for association of long sleep duration with risk of prevalent CKD.
Meta-analysis for sleep quality
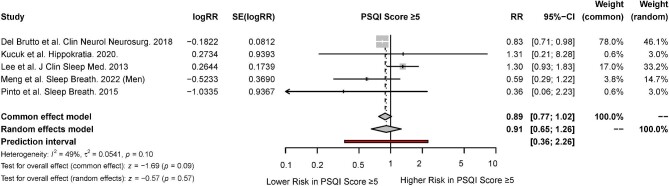
There were five studies that reported the association of sleep quality with CKD [45, 55, 56, 59, 64]. Based on the random-effects model, poor sleep quality as indicated by a PSQI score of ≥5 was not associated with a significantly increased risk of CKD (RR, 0.91; 95% CI, 0.65 to 1.26; P < 0.57, IP = 49%) (Fig. 6).
Figure 6:
Forest plot for association of sleep quality with risk of CKD.
GRADE quality of evidence
The certainty of evidence for the association of sleep duration and quality with CKD were assessed using the GRADE framework. The results of this assessment are shown in Supplement 9.
DISCUSSION
In this systematic review and meta-analysis of 2 525 312 participants, short sleep duration and long sleep duration versus a reference range of 7 to 8 hours was associated with an increased risk of CKD. This association remained consistent across sleep durations of <4, 5, 6, and 7 hours for short sleep durations, and >8 and 9 hours for long sleep durations. Meta-regression found that the percentage of male participants and percentage of Caucasian participants were significant effect moderators of the association between short sleep duration and risk of CKD. The results of the meta-analysis were robust across different sensitivity analyses, including leave-one-out, cumulative, and trim-and-fill analyses. In 2020, Hao et al. investigated the relationship between sleep duration and the risk of CKD [82]. Our meta-analysis updates this review and further provides meta-analyses of the association of sleep quality with the risk of CKD. Given that insomnia and poor sleep quality often co-occur with cardiovascular diseases, psychiatric illness, and impaired social functioning that may complicate the course of CKD, identification of patients with short or excessive sleep duration may allow for early intervention to minimize delays and improve clinical outcomes and quality of life.
This meta-analysis provides robust evidence that both inadequate and excessive sleep may increase the risk of CKD in adults, with these findings being supported by previous works. Published literature has proposed several mechanisms to explain the influence of sleep deprivation on renal pathophysiology. First, the sympathetic nervous system is often overactive in situations of reduced sleep duration, which has been shown to be a risk factor for the development of CKD [83, 84]. Second, sleep deprivation predisposes to overactivity of the renin–angiotensin aldosterone system, with the resulting chronobiological alterations playing an important role in the progression of CKD [85]. Third, sleep deprivation results in elevated high-sensitivity C-reactive protein concentrations, which predisposes people to a chronic inflammatory state and increases the risk of cardiovascular illness, including CKD [86, 87]. Fourth, uremia is commonly implicated in the pathogenesis of poor sleep quality in patients with CKD. Chronic uremia is associated with neuropathy and myopathy, which predisposes people to upper airway dilator muscle dysfunction and sleep apnea [88, 89]. Peripheral neuropathy, which may arise either secondary to uremia or from the underlying cause of CKD such as diabetes, may also predispose patients to develop restless leg syndrome or periodic limb movements [90, 91]. Taken together, these findings suggest a strong pathophysiological basis for the link between accumulated sleep deficit and the risk of CKD. Nonetheless, it is plausible that among the studies that reported the prevalence of CKD, CKD itself may contribute to poor sleep [17]. Further research with larger prospective studies may be warranted to confirm the effect of poor sleep on the risk of CKD.
With regards to shift work and its influence on the risk of CKD, Sasaki et al. found no significant association of shift work with the risk of CKD [68]. However, short sleep duration was associated with a significantly higher risk of CKD in shift workers but not in non-shift workers, suggesting that shift work may be an effect modifier of the association between sleep duration and CKD. The basis for this association may be the disrupted circadian rhythm in shift workers, which contributes to the development of cardiovascular disease and other metabolic disorders [92, 93]. Shift work has been found to enhance the association between short sleep duration and obesity. While shift work may be associated with deleterious lifestyle factors and job strain which predisposes to the development of CKD, Sasaki et al. found that the risk of CKD in shift workers with short sleep duration was significantly increased even after adjustment for lifestyle factors, such as smoking status, alcohol consumption, exercise, and job strain [94, 95]. These results indicate that the interaction of shift work and short sleep duration may accelerate the development of CKD.
Interestingly, the meta-regression revealed that the percentage of male participants was a significant effect moderator of the association between short sleep duration and the risk of CKD, specifically among ≤4 hours of sleep. An increased percentage of male participants increased the risk ratio of developing CKD, among these subsets of sleep duration. Whereas published epidemiological data suggests a female preponderance for CKD, kidney function was found to decline faster in men than women. Possible reasons include unhealthier lifestyles in men, the damaging effects of testosterone, and the protective effects of estrogen on renal function [96]. Furthermore, modifiable risk factors including BMI and plasma glucose accelerate CKD progression in a greater extent in men than woman. Taken together, the interplay of these hormonal and lifestyle factors, coupled with the existing influence of short sleep duration, may result in the apparent increase in risk of CKD among male patients compared with female patients.
The meta-analysis found that long sleep duration was associated with an increased risk of CKD. This association remained significant across ≥8, ≥9, and ≥10 hours of sleep. Published literature suggests that the association between long sleep duration and CKD is multifactorial, being an interplay of physical inactivity, systemic inflammation, immune dysfunction, and disease status. For instance, long sleep duration has been associated with higher CRP and interleukin-6 levels, which are markers of a pro-inflammatory state [97]. Correspondingly, those who sleep for long periods often experience sleep fragmentation, excessive daytime sleepiness, poor sleep quality, and a sedentary lifestyle [98, 99]. The findings of this meta-analysis also support those reported by Hao et al. in 2020, which found a significant association between sleep >8 or 9 hours and the risk of CKD [82]. However, it should be noted that there were limited data pertaining to the association of sleep duration ≥10 hours and the risk of CKD, with only two studies being identified. Further primary studies investigating the risk of CKD in this subset of participants may be useful in confirming this apparent association between long sleep duration and the risk of CKD.
Interestingly, the meta-analysis did not find any significant association between poor sleep quality and the risk of CKD. Within the included studies, a PSQI cutoff score of 5 was selected as being indicative of poor sleep quality. The PSQI has demonstrated good sensitivity and specificity in validation studies, although it remains a subjective classification of sleep quality [100]. There is a well-established link between poor sleep quality and cardiovascular and psychiatric illness, given the mutually dependent interactions of the kidney–heart–brain crosstalk. The progression of CKD may create a vicious cycle of sleep disorders, CKD, and CKD-related complications. However, there is a lack of sufficient robust evidence for the relationship between poor sleep quality and CKD, as seen from the small number of studies identified in this systematic review. The causal relationship between sleep quality and CKD also remains debatable, as poor sleep quality may be a manifestation of major underlying factors, including a heavy burden of concomitant disease or inadequate dialysis. Within the included studies for the meta-analysis of sleep quality, there were patients who had concomitant sleep disorders, such as sleep apnea and restless leg syndrome. These sleep disorders may be effect modifiers of the relationship between sleep quality and the risk of CKD. Further well-conducted studies will be useful in elucidating this relationship between poor sleep quality and the risk of CKD.
The strengths of this study lie in the large number of systematically included studies from diverse settings. Through a rigorous prespecified protocol of systematic searching, bias assessment, and quality grading according to international guidelines, these help to enhance the generalizability of these findings. Of the 39 included studies, only seven studies were found to be of high risk of bias, and their exclusion did not change the findings to of the meta-analysis significantly. Meta-regression was able to adequately explain the observed heterogeneity in the meta-analysis, and the pooled effect sizes were robust to subgroup analyses, meta-regression, influence, and cumulative and small-study analyses. Overall, minimal evidence of publication bias was found.
There were appreciable limitations of this meta-analysis. First, the observational nature of the included studies does not permit causal conclusion due to the inability to exclude residual confounding. Second, there were unfortunately no data available for patients on transplant. Therefore, subgroup analyses comparing patients undergoing and not undergoing renal transplants could not be performed. Third, there were limited data available pertaining to the association of sleep quality with the risk of CKD, with only five studies identified in this respect. Further well-conducted studies will be useful in identifying possible associations between sleep quality and the risk of CKD. Fourth, the conduct of meta-regression was limited by the availability of published literature and could only be performed among studies that reported data on the particular covariate. Aggregate or ecological bias could not be excluded due to the assumption of linearity. Fifth, there was a lack of randomized trials identified in the literature search, which may reduce the overall strength of the meta-analysis and increase the effect of confounding factors and bias. Therefore, causal relationships cannot be inferred from this meta-analysis. The power of the meta-regression is also relatively small in the presence of confounding factors, which may increase the chance of false positive results, therefore the results of the meta-regression should be interpreted with caution.
CONCLUSION
In this systematic review and meta-analysis of 39 studies involving 2 525 312 participants, both short and long sleep durations were significantly associated with a higher risk of CKD. This association remained significant across various sleep durations, including ≤4, ≤5, ≤6, and ≤7 hours of sleep. Similar associations were also seen for ≥8, ≥9, and ≥10 hours of sleep. Meta-regression revealed that mean age, percentage of male patients, and year of study completion did not significantly alter the pooled effect sizes. Poor sleep quality as indicated by a PSQI score of ≥5 was not associated with a higher risk of CKD. This study highlights the relationships between sleep duration and CKD and adds to the growing evidence base suggesting the interplay between both entities. Physicians treating patients with sleep disorders should be aware of these associations and adopt targeted interventions to improve clinical outcomes and quality of life in these patients.
Supplementary Material
Acknowledgements
All authors have made substantial contributions to all the following: (i) the conception and design of the study, or acquisition of data, or analysis and interpretation of data; (ii) drafting the article or revising it critically for important intellectual content; and (iii) final approval of the version to be submitted. No writing assistance was obtained in the preparation of the manuscript. The manuscript, including related data, figures and tables has not been previously published and the manuscript is not under consideration elsewhere.
Contributor Information
Jin Hean Koh, Yong Loo Lin School of Medicine, National University of Singapore, Singapore, Singapore.
Brian Sheng Yep Yeo, Yong Loo Lin School of Medicine, National University of Singapore, Singapore, Singapore.
Timothy Wei En Tan, Yong Loo Lin School of Medicine, National University of Singapore, Singapore, Singapore.
Mark Yong Siang See, Lee Kong Chian School of Medicine, Nanyang Technological University, Singapore, Singapore.
Adele Chin Wei Ng, Department of Otorhinolaryngology-Head and Neck Surgery, Singapore General Hospital, Singapore, Singapore.
Shaun Ray Han Loh, Department of Otorhinolaryngology-Head and Neck Surgery, Singapore General Hospital, Singapore, Singapore; Singhealth Duke-NUS Sleep Centre, Duke-NUS Medical School, Singapore, Singapore.
Joshua Gooley, Singhealth Duke-NUS Sleep Centre, Duke-NUS Medical School, Singapore, Singapore.
Chieh Suai Tan, Department of Renal Medicine, Singapore General Hospital, Singapore, Singapore.
Song Tar Toh, Department of Otorhinolaryngology-Head and Neck Surgery, Singapore General Hospital, Singapore, Singapore; Singhealth Duke-NUS Sleep Centre, Duke-NUS Medical School, Singapore, Singapore.
FUNDING
No funding was required for this study.
DATA AVAILABILITY STATEMENT
All data are available from Medline/Pubmed, Embase, the Cochrane Library and CINAHL.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
REFERENCES
- 1. Hill NR, Fatoba ST, Oke JL et al. Global prevalence of chronic kidney disease—a systematic review and meta-analysis. PLoS ONE 2016;11:e0158765. 10.1371/journal.pone.0158765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Burgos-Calderón R, Depine S, Aroca-Martínez G. Population kidney health. A new paradigm for chronic kidney disease management. Int J Environ Res Public Health 2021;18:6786. doi: 10.3390/ijerph18136786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pike M, Taylor J, Kabagambe E et al. The association of exercise and sedentary behaviours with incident end-stage renal disease: the Southern Community Cohort Study. BMJ Open 2019;9:e030661. 10.1136/bmjopen-2019-030661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hamrahian SM, Falkner B. Hypertension in chronic kidney disease. Adv Exp Med Biol 2017;956:307–25. 10.1007/5584_2016_84 [DOI] [PubMed] [Google Scholar]
- 5. Glassock RJ, Rule AD. Aging and the kidneys: anatomy, physiology and consequences for defining chronic kidney disease. Nephron 2016;134:25–29. 10.1159/000445450 [DOI] [PubMed] [Google Scholar]
- 6. Bo Y, Yeoh EK, Guo C et al. Sleep and the risk of chronic kidney disease: a cohort study. J Clin Sleep Med 2019;15:393–400. 10.5664/jcsm.7660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Matsumoto T, Chin K. Prevalence of sleep disturbances: sleep disordered breathing, short sleep duration, and non-restorative sleep. Respir Investig 2019;57:227–37. 10.1016/j.resinv.2019.01.008 [DOI] [PubMed] [Google Scholar]
- 8. Tao F, Cao Z, Jiang Y et al. Associations of sleep duration and quality with incident cardiovascular disease, cancer, and mortality: a prospective cohort study of 407,500 UK biobank participants. Sleep Med 2021;81:401–9. 10.1016/j.sleep.2021.03.015 [DOI] [PubMed] [Google Scholar]
- 9. Bo Y, Yeoh E-K, Guo C et al. Sleep and the risk of chronic kidney disease: a cohort study. J Clin Sleep Med 2019;15:393–400. 10.5664/jcsm.7660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chang Y-C, Chang C-H, Huang Y-T et al. Sleep duration and proteinuria progression: a population-based cohort study. Am J Nephrol 2019;49:41–51. 10.1159/000495847 [DOI] [PubMed] [Google Scholar]
- 11. Cha YJ, Kim JY, Cho E et al. Impact of sleep duration on decline in kidney function in adult patients with hypertension: a community-based prospective cohort study. Korean J Fam Med 2022;43:312–18. 10.4082/kjfm.21.0164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Agarwal R, Light RP. Sleep and activity in chronic kidney disease: a longitudinal study. Clin J Am Soc Nephrol: CJASN 2011;6:1258–65. 10.2215/CJN.10581110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Turek NF, Ricardo AC, Lash JP. Sleep disturbances as nontraditional risk factors for development and progression of CKD: review of the evidence. Am J Kidney Dis 2012;60:823–33. 10.1053/j.ajkd.2012.04.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yeo BSY, Koh JH, Ng ACW et al. The association of obstructive sleep apnea with blood and cerebrospinal fluid biomarkers of Alzheimer's dementia—a systematic review and meta-analysis. Sleep Med Rev 2023;70:101790. 10.1016/j.smrv.2023.101790 [DOI] [PubMed] [Google Scholar]
- 15. Yeo BSY, Koh JH, Tan BKJ et al. Improved inflammatory and cardiometabolic profile after soft-tissue sleep surgery for obstructive sleep apnea: a systematic review and meta-analysis. JAMA Otolaryngol Head Neck Surg 2022;148:862–9. 10.1001/jamaoto.2022.2285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Depner CM, Stothard ER, Wright KP Jr. Metabolic consequences of sleep and circadian disorders. Curr Diab Rep 2014;14:507. 10.1007/s11892-014-0507-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Maung SC, El Sara A, Chapman C et al. Sleep disorders and chronic kidney disease. WJN 2016;5:224–32. 10.5527/wjn.v5.i3.224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Eloot S, Holvoet E, Dequidt C et al. The complexity of sleep disorders in dialysis patients. Clin Kidney J 2021;14:2029–36. 10.1093/ckj/sfaa258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Koch BC, Nagtegaal JE, Hagen EC et al. Different melatonin rhythms and sleep-wake rhythms in patients on peritoneal dialysis, daytime hemodialysis and nocturnal hemodialysis. Sleep Med 2010;11:242–6. 10.1016/j.sleep.2009.04.006 [DOI] [PubMed] [Google Scholar]
- 20. Roumelioti ME, Argyropoulos C, Pankratz VS et al. Objective and subjective sleep disorders in automated peritoneal dialysis. Can J Kidney Health Dis 2016;3:93. 10.1186/s40697-016-0093-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Page MJ, McKenzie JE, Bossuyt PM et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ouzzani M, Hammady H, Fedorowicz Z et al. Rayyan—a web and mobile app for systematic reviews. Syst Rev 2016;5:210. 10.1186/s13643-016-0384-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Levey AS, Eckardt K-U, Tsukamoto Y et al. Definition and classification of chronic kidney disease: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 2005;67:2089–100. 10.1111/j.1523-1755.2005.00365.x [DOI] [PubMed] [Google Scholar]
- 24. Saaiq M, Ashraf B. Modifying “Pico” question into “Picos” model for more robust and reproducible presentation of the methodology employed in a scientific study. World J Plast Surg 2017;6:390–2. [PMC free article] [PubMed] [Google Scholar]
- 25. Schardt C, Adams MB, Owens T et al. Utilization of the PICO framework to improve searching PubMed for clinical questions. BMC Med Inform Decis Mak 2007;7:16. 10.1186/1472-6947-7-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Buysse DJ, Reynolds CF 3rd, Monk TH et al. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res 1989;28:193–213. 10.1016/0165-1781(89)90047-4 [DOI] [PubMed] [Google Scholar]
- 27. ROBINS-E Development Group (Higgins J MR, Rooney A, Taylor K, Thayer K, Silva R, Lemeris C, Akl A, Arroyave W, Bateson T, Berkman N, Demers P, Forastiere F, Glenn B, Hróbjartsson A, Kirrane E, LaKind J, Luben T, Lunn R, McAleenan A, McGuinness L, Meerpohl J, Mehta S, Nachman R, Obbagy J, O'Connor A, Radke E, Savović J, Schubauer-Berigan M, Schwingl P, Schunemann H, Shea B, Steenland K, Stewart T, Straif K, Tilling K, Verbeek V, Vermeulen R, Viswanathan M, Zahm S, Sterne J). ROBINS-E. https://www.google.com/url?q=https%3A%2F%2Fwww.riskofbias.info%2Fwelcome%2Frobins-e-tool&sa=D&sntz=1&usg=AOvVaw2qCjudHw8UZxaWbDb_78Ir.
- 28. GV. G . Meta-analysis at 25. Accessed 1 July, 2023; https://www.scirp.org/reference/referencespapers?referenceid=590931
- 29. Sedgwick P. What is publication bias in a meta-analysis? BMJ 2015;351:h4419. 10.1136/bmj.h4419 [DOI] [PubMed] [Google Scholar]
- 30. Egger M, Smith GD, Schneider M et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629. 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sterne JAC, Sutton AJ, Ioannidis JPA et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 2011;343:d4002. 10.1136/bmj.d4002 [DOI] [PubMed] [Google Scholar]
- 32. Balduzzi S, Rücker G, Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evid Based Mental Health 2019;22:153–60. 10.1136/ebmental-2019-300117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wan X, Wang W, Liu J et al. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 2014;14:135. 10.1186/1471-2288-14-135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Berrington A, Cox DR. Generalized least squares for the synthesis of correlated information. Biostatistics 2003;4:423–31. 10.1093/biostatistics/4.3.423 [DOI] [PubMed] [Google Scholar]
- 35. Tufanaru C, Munn Z, Stephenson M et al. Fixed or random effects meta-analysis? Common methodological issues in systematic reviews of effectiveness. Int J Evid Based Healthc 2015;13:196–207. 10.1097/XEB.0000000000000065 [DOI] [PubMed] [Google Scholar]
- 36. van Aert RCM, Jackson D. A new justification of the Hartung-Knapp method for random-effects meta-analysis based on weighted least squares regression. Res Synth Methods 2019;10:515–27. 10.1002/jrsm.1356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Higgins JP, Thompson SG, Deeks JJ et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fletcher J. What is heterogeneity and is it important? BMJ 2007;334:94–96. 10.1136/bmj.39057.406644.68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Deeks JJ, Higgins JPT, Altman DG, on behalf of the Cochrane Statistical Methods G. Analysing data and undertaking meta-analyses. Cochrane Handbook for Systematic Reviews of Interventions 2019;8:241–84. 10.1002/9781119536604.ch10 [DOI] [Google Scholar]
- 40. Thompson SG, Higgins JPT. How should meta-regression analyses be undertaken and interpreted? Stat Med 2002;21:1559. 10.1002/sim.1187 [DOI] [PubMed] [Google Scholar]
- 41. Higgins JPT, Thompson SG. Controlling the risk of spurious findings from meta-regression. Stat Med 2004;23:1663–82. 10.1002/sim.1752 [DOI] [PubMed] [Google Scholar]
- 42. Guyatt GH, Oxman AD, Vist GE et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924. 10.1136/bmj.39489.470347.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Choi H, Kim HC, Lee JY et al. Sleep duration and chronic kidney disease: the Korean Genome and Epidemiology Study (KoGES)-Kangwha study. Korean J Intern Med 2017;32:323–34. 10.3904/kjim.2015.400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cohen SD, Patel SS, Khetpal P et al. Pain, sleep disturbance, and quality of life in patients with chronic kidney disease. Clin J Am Soc Nephrol: CJASN 2007;2:919–25. 10.2215/CJN.00820207 [DOI] [PubMed] [Google Scholar]
- 45. Del Brutto OH, Mera RM. Understanding the direction of the relationship between white matter hyperintensities of vascular origin, sleep quality, and chronic kidney disease—results from the Atahualpa Project. Clin Neurol Neurosurg 2018;165:10–14. 10.1016/j.clineuro.2017.12.019 [DOI] [PubMed] [Google Scholar]
- 46. Fang Y, Son S, Yang J et al. Perturbation of circadian rhythm is associated with increased prevalence of chronic kidney disease: results of the Korean Nationwide Population-Based Survey. Int J Environ Res Public Health 2022;19:5732. 10.3390/ijerph19095732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Fujibayashi K, Fukuda H, Yokokawa H et al. Associations between healthy lifestyle behaviors and proteinuria and the estimated glomerular filtration rate (eGFR). JAT 2012;19:932–40. 10.5551/jat.12781 [DOI] [PubMed] [Google Scholar]
- 48. Geng T-T, Jafar TH, Yuan J-M et al. Sleep duration and risk of end-stage renal disease: the Singapore Chinese Health Study. Sleep Med 2019;54:22–7. 10.1016/j.sleep.2018.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gu K-M, Min SH, Cho J. Sleep duration and mortality in patients with diabetes: results from the 2007-2015 Korea national health and nutrition examination survey. Diabetes Metab 2022;48:101312. 10.1016/j.diabet.2021.101312 [DOI] [PubMed] [Google Scholar]
- 50. Hirano K, Komatsu Y, Shimbo T et al. Longitudinal relationship between long sleep duration and future kidney function decline. Clin Kidney J 2022;15:1763–9. 10.1093/ckj/sfac107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Jean-Louis G, Williams NJ, Sarpong D et al. Associations between inadequate sleep and obesity in the US adult population: analysis of the national health interview survey (1977-2009). BMC Public Health 2014;14:290. 10.1186/1471-2458-14-290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Jiang B, Tang D, Dai N et al. Association of self-reported nighttime sleep duration with chronic kidney disease: china health and retirement longitudinal study. Am J Nephrol 2023;54:249–57. 10.1159/000531261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Karatas A, Canakci E, Turkmen E. Comparison of sleep quality and quality of life indexes with sociodemographic characteristics in patients with chronic kidney disease. Niger J clin Prac 2018;21:1461–67. 10.4103/njcp.njcp_146_18 [DOI] [PubMed] [Google Scholar]
- 54. Kim C-W, Chang Y, Sung E et al. Sleep duration and quality in relation to chronic kidney disease and glomerular hyperfiltration in healthy men and women. PLoS ONE 2017;12:e0175298. 10.1371/journal.pone.0175298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kucuk O, Kaynar K, Arslan FC et al. Comparison of mental health, quality of sleep and life among patients with different stages of chronic kidney disease and undergoing different renal replacement therapies. Hippokratia 2020;24:51–8. [PMC free article] [PubMed] [Google Scholar]
- 56. Lee J, Nicholl DDM, Ahmed SB et al. The prevalence of restless legs syndrome across the full spectrum of kidney disease. J Clin Sleep Med 2013;9:455–59. 10.5664/jcsm.2664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Li J, Huang Z, Hou J et al. Sleep and CKD in Chinese adults: a cross-sectional study. Clin J Am Soc Nephrol: CJASN 2017;12:885–92. 10.2215/CJN.09270816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Lu C, Liao B, Nie J et al. The association between sleep duration and chronic diseases: a population-based cross-sectional study. Sleep Med 2020;73:217–22. 10.1016/j.sleep.2020.05.005 [DOI] [PubMed] [Google Scholar]
- 59. Meng L, Ding Y, Li J et al. Impact of inflammatory markers on the relationship between sleep quality and diabetic kidney disease. Sleep Breath 2022;26:157–65. 10.1007/s11325-021-02380-6 [DOI] [PubMed] [Google Scholar]
- 60. Nagai M, Hoshide S, Takahashi M et al. Sleep duration, kidney function, and their effects on cerebral small vessel disease in elderly hypertensive patients. Am J Hypertens 2015;28:884–93. 10.1093/ajh/hpu243 [DOI] [PubMed] [Google Scholar]
- 61. Nakajima H, Hashimoto Y, Okamura T et al. Association between sleep duration and incident chronic kidney disease: a population-based cohort analysis of the NAGALA study. Kidney Blood Press Res 2020;45:339–49. 10.1159/000504545 [DOI] [PubMed] [Google Scholar]
- 62. Nishimura A, Kasai T, Matsumura K et al. Obstructive sleep apnea during rapid eye movement sleep in patients with diabetic kidney disease. J Clin Sleep Med 2021;17:453–60. 10.5664/jcsm.8924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Petrov ME, Buman MP, Unruh ML et al. Association of sleep duration with kidney function and albuminuria: NHANES 2009-2012. Sleep Health 2016;2:75–81. 10.1016/j.sleh.2015.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Pinto AR, da Silva NC, Pinato L. Analyses of melatonin, cytokines, and sleep in chronic renal failure. Sleep Breath 2016;20:339–44. 10.1007/s11325-015-1240-9 [DOI] [PubMed] [Google Scholar]
- 65. Plantinga L, Lee K, Inker LA et al. Association of sleep-related problems with CKD in the United States, 2005-2008. Am J Kidney Dis 2011;58:554–64. 10.1053/j.ajkd.2011.05.024 [DOI] [PubMed] [Google Scholar]
- 66. Sabbatini M, Crispo A, Pisani A et al. Sleep quality in renal transplant patients: a never investigated problem. Nephrol Dial Transplant 2005;20:194–8. 10.1093/ndt/gfh604 [DOI] [PubMed] [Google Scholar]
- 67. Salifu I, Tedla F, Pandey A et al. Sleep duration and chronic kidney disease: analysis of the national health interview survey. Cardiorenal Med 2014;4:210–16. 10.1159/000368205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Sasaki S, Yoshioka E, Saijo Y et al. Short sleep duration increases the risk of chronic kidney disease in shift workers. J Occup Environ Med 2014;56:1243–48. 10.1097/JOM.0000000000000322 [DOI] [PubMed] [Google Scholar]
- 69. Sun H, Qin K, Zou C et al. The association of nighttime sleep duration and quality with chronic kidney disease in middle-aged and older Chinese: a cohort study. Sleep Med 2021;86:25–31. 10.1016/j.sleep.2021.08.007 [DOI] [PubMed] [Google Scholar]
- 70. Tan NYQ, Chan J, Cheng CY et al. Sleep duration and diabetic kidney disease. Front Endocrinol 2019;9:808. 10.3389/fendo.2018.00808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Tu C-Y, Chou Y-H, Lin Y-H et al. Sleep and emotional disturbance in patients with non-dialysis chronic kidney disease. J Formos Med Assoc 2019;118:986–94. 10.1016/j.jfma.2018.10.016 [DOI] [PubMed] [Google Scholar]
- 72. Unruh ML, Sanders MH, Redline S et al. Subjective and objective sleep quality in patients on conventional thrice-weekly hemodialysis: comparison with matched controls from the sleep heart health study. Am J Kidney Dis 2008;52:305–13. 10.1053/j.ajkd.2008.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Wang Y, Jiang G, Hou N et al. Effects and differences of sleep duration on the risk of new-onset chronic disease conditions in middle-aged and elderly populations. Eur J Intern Med 2023;107:73–80. 10.1016/j.ejim.2022.11.005 [DOI] [PubMed] [Google Scholar]
- 74. Warsame F, Chu NM, Hong J et al. Sleep duration and cognitive function among older adults with chronic kidney disease—results from the National Health and Nutrition Examination Survey (2011-2014). Nephrol Dial Transp 2022;7:1636–44. 10.1093/ndt/gfac325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Wu C-C, Wang H-E, Liu Y-C et al. Sleeping, smoking, and kidney diseases: evidence from the NHANES 2017-2018. Front Med 2021;8:745006. 10.3389/fmed.2021.745006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Xu S, Jin J, Dong Q et al. Association between sleep duration and quality with rapid kidney function decline and development of chronic kidney diseases in adults with normal kidney function: the China health and retirement longitudinal study. Front Public Health 2022;10:1072238. 10.3389/fpubh.2022.1072238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Yamamoto R, Nagasawa Y, Iwatani H et al. Self-reported sleep duration and prediction of proteinuria: a retrospective cohort study. Am J Kidney Dis 2012;59:343–55. 10.1053/j.ajkd.2011.08.032 [DOI] [PubMed] [Google Scholar]
- 78. Ye Y, Zhang L, Yan W et al. Self-reported sleep duration and daytime napping are associated with renal hyperfiltration and microalbuminuria in an apparently healthy Chinese population. PLoS ONE 2019;14:e0214776. 10.1371/journal.pone.0214776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Yu JH, Han K, Kim NH et al. U-shaped association between sleep duration and urinary albumin excretion in Korean adults: 2011-2014 Korea National Health and Nutrition Examination Survey. PLoS ONE 2018;13:e0192980. 10.1371/journal.pone.0192980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Zhang H, Wang B, Chen C et al. Sleep patterns, genetic susceptibility, and incident chronic kidney disease: a prospective study of 370 671 participants. Front Neurosci 2022;16:725478. 10.3389/fnins.2022.725478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. McHugh ML. Interrater reliability: the kappa statistic. Biochem Med 2012;22:276–82. 10.11613/BM.2012.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Hao Q, Xie M, Zhu L et al. Association of sleep duration with chronic kidney disease and proteinuria in adults: a systematic review and dose-response meta-analysis. Int Urol Nephrol 2020;52:1305–20. 10.1007/s11255-020-02488-w [DOI] [PubMed] [Google Scholar]
- 83. Neumann J, Ligtenberg G, Klein II et al. Sympathetic hyperactivity in chronic kidney disease: pathogenesis, clinical relevance, and treatment. Kidney Int 2004;65:1568–76. 10.1111/j.1523-1755.2004.00552.x [DOI] [PubMed] [Google Scholar]
- 84. Basta M, Chrousos GP, Vela-Bueno A et al. Chronic insomnia and the stress system. Sleep Med Clin 2007;2:279–91. 10.1016/j.jsmc.2007.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Kobori H, Navar LG. Urinary angiotensinogen as a novel biomarker of intrarenal renin-angiotensin system in chronic kidney disease. Int Rev Thromb 2011;6:108–16. [PMC free article] [PubMed] [Google Scholar]
- 86. Chiang JK. Short duration of sleep is associated with elevated high-sensitivity C-reactive protein level in Taiwanese adults: a cross-sectional study. J Clin Sleep Med 2014;10:743–9. 10.5664/jcsm.3862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Meier-Ewert HK, Ridker PM, Rifai N et al. Effect of sleep loss on C-reactive protein, an inflammatory marker of cardiovascular risk. J Am Coll Cardiol 2004;43:678–83. 10.1016/j.jacc.2003.07.050 [DOI] [PubMed] [Google Scholar]
- 88. Beecroft JM, Hoffstein V, Pierratos A et al. Pharyngeal narrowing in end-stage renal disease: implications for obstructive sleep apnoea. Eur Respir J 2007;30:965. 10.1183/09031936.00161906 [DOI] [PubMed] [Google Scholar]
- 89. Abuyassin B, Sharma K, Ayas Najib T et al. Obstructive sleep apnea and kidney disease: a potential bidirectional relationship? J Clin Sleep Med 2015;11:915–24. 10.5664/jcsm.4946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Jiménez-Jiménez FJ, Alonso-Navarro H, García-Martín E et al. Association between restless legs syndrome and peripheral neuropathy: a systematic review and meta-analysis. Euro J of Neurol 2021;28:2423–42. 10.1111/ene.14840 [DOI] [PubMed] [Google Scholar]
- 91. Ning P, Mu X, Yang X et al. Prevalence of restless legs syndrome in people with diabetes mellitus: a pooling analysis of observational studies. eClinMed 2022;46:101357. 10.1016/j.eclinm.2022.101357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Drake CL, Roehrs T, Richardson G et al. Shift work sleep disorder: prevalence and consequences beyond that of symptomatic day workers. Sleep 2004;27:1453–62. 10.1093/sleep/27.8.1453 [DOI] [PubMed] [Google Scholar]
- 93. Ohayon MM, Smolensky MH, Roth T. Consequences of shiftworking on sleep duration, sleepiness, and sleep attacks. Chronobiol Int 2010;27:575–89. 10.3109/07420521003749956 [DOI] [PubMed] [Google Scholar]
- 94. Frost P, Kolstad HA, Bonde JP. Shift work and the risk of ischemic heart disease—a systematic review of the epidemiologic evidence. Scand J Work Environ Health 2009;35:163–79. 10.5271/sjweh.1319 [DOI] [PubMed] [Google Scholar]
- 95. Tenkanen L, Sjöblom T, Härmä M. Joint effect of shift work and adverse life-style factors on the risk of coronary heart disease. Scand J Work Environ Health 1998;24:351–7. 10.5271/sjweh.355 [DOI] [PubMed] [Google Scholar]
- 96. Carrero JJ, Hecking M, Chesnaye NC et al. Sex and gender disparities in the epidemiology and outcomes of chronic kidney disease. Nat Rev Nephrol 2018;14:151–64. 10.1038/nrneph.2017.181 [DOI] [PubMed] [Google Scholar]
- 97. Irwin MR, Olmstead R, Carroll JE. Sleep disturbance, sleep duration, and inflammation: a systematic review and meta-analysis of cohort studies and experimental sleep deprivation. Biol Psychiatry 2016;80:40–52. 10.1016/j.biopsych.2015.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Youngstedt SD, Kripke DF. Long sleep and mortality: rationale for sleep restriction. Sleep Med Rev 2004;8:159–74. 10.1016/j.smrv.2003.10.002 [DOI] [PubMed] [Google Scholar]
- 99. Pilcher JJ, Ginter DR, Sadowsky B. Sleep quality versus sleep quantity: relationships between sleep and measures of health, well-being and sleepiness in college students. J Psychosom Res 1997;42:583. [DOI] [PubMed] [Google Scholar]
- 100. Buysse DJ, Hall ML, Strollo PJ et al. Relationships between the Pittsburgh Sleep Quality Index (PSQI), Epworth Sleepiness Scale (ESS), and clinical/polysomnographic measures in a community sample. J Clin Sleep Med 2008; 04:563–71. 10.5664/jcsm.27351 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are available from Medline/Pubmed, Embase, the Cochrane Library and CINAHL.